
Snapsolve any problem by taking a picture. Try it in the Numerade app?


Electrolysis
Required practical 3, core practicals.

Aims of Experiment
Investigate what happens when two different aqueous solutions are electrolysed using inert electrodes.
In this experiment you will:
- use a low voltage power supply and carbon rod electrodes to pass a current through two different salt solutions
- identify the element formed at the positive and negative electrodes for each solution
- add extra detail to a basic electrochemical diagram (provided)
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Each group will need:
low voltage supply (0‐12 V) connecting leads crocodile clips two small test tubes (gas collecting) matches/splints
blue litmus paper 100 ml beaker stop watch 2 graphite rods copper chloride solution sodium chloride solution
Experiment Set-up
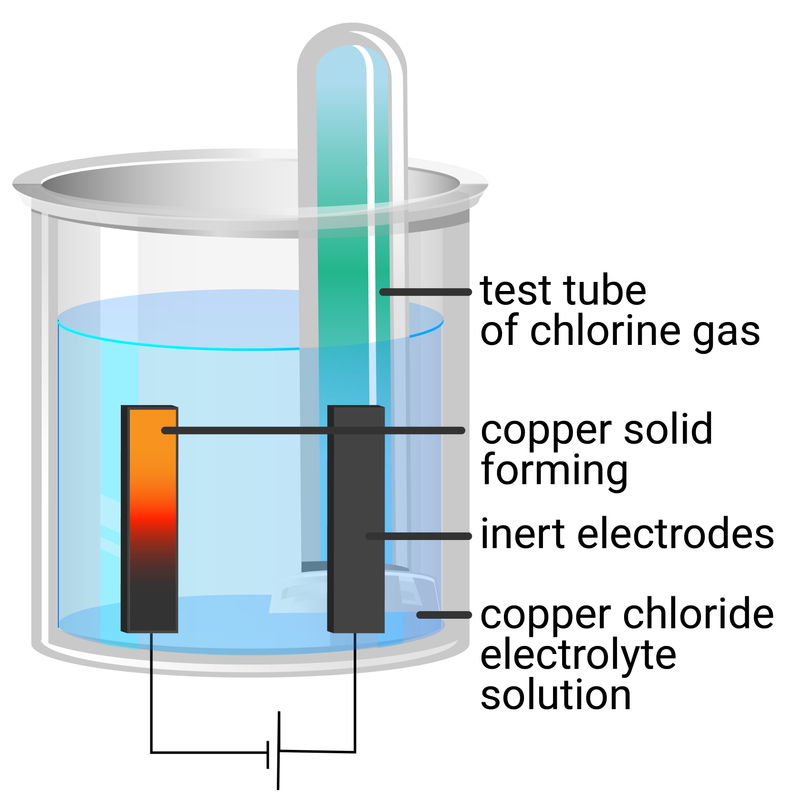
Copper chloride solution.
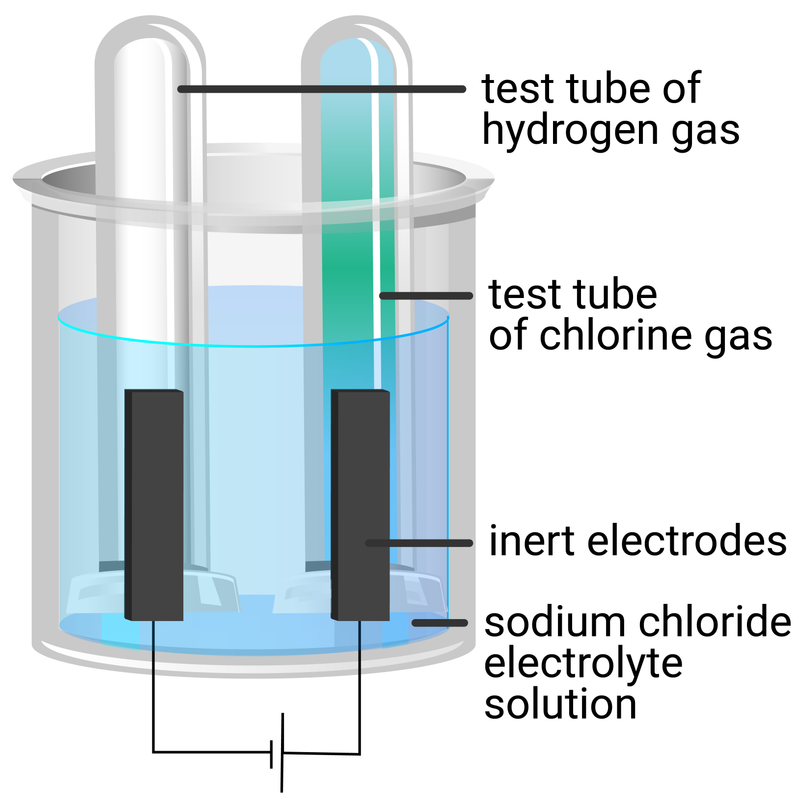
sodium chloride solution
- use a measuring cylinder to add 40 ml of copper chloride solution into a beaker
- place two graphite rods into the copper sulfate solution - attaching one electrode to the negative terminal of a dc supply, and the other electrode to the positive terminal
- place two small test tubes over each electrode to collect any gases produced
- turn on the power supply and observe what happens at each electrode
- test any gas produced by holding a piece of blue litmus next to the electrode, or by holding a lit splint next to it
- record your observations and the results of your tests
- rinse the electrochemical cell apparatus and collect a new set of electrodes
- repeat steps 1‒6 using sodium chloride solution
Results and Analysis
Descibe the tests for both hydrogen and chlorine.
Explain why copper was deposited at the cathode for copper(II) chloride, but hydrogen gas was produced for sodium chloride.
Higher Tier - Write half equations for each of the electrodes in both experiments.
Exam Question and Model Answer
A student conducts an investigation to find out what is produced during the electrolysis of sodium sulfate. Describe how the student could carry out an investigation, and show (using ions) what is given off at each electrode.
Level 1 (1-2 marks)
Add sodium sulfate solution to a beaker, and connect two electrodes to a power supply. Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode. hydrogen gas will form at the negative electrode oxygen gas will form at the positive electrode
Level 2 (3-4 marks)
Wearing safety glasses, add sodium sulfate solution to a beaker, and connect two inert electrodes (make sure they do not touch) to a power supply. Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode. Any gases produced can be collected in the test tubes and tested (squeaky pop test - hydrogen, relight a glowing splint - oxygen). hydrogen gas will form at the negative electrode oxygen gas will form at the positive electrode
Level 3 (5-6 marks)
Wearing safety glasses, add sodium sulfate solution to a beaker, and connect two inert electrodes (make sure they do not touch) to a power supply. Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode. Any gases produced can be collected in the test tubes and tested (squeaky pop test - hydrogen, relight a glowing splint - oxygen). hydrogen gas will form at the negative electrode: 2H + (aq) + 2e - → H 2 (g) oxygen gas will form at the positive electrode: 4OH - (aq) → 2H 2 O(l) + O 2 (g) + 4e -
- No category
Electrolysis Y10 Test
Related documents

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
Log In | Join AACT | Renew Membership
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
- AACT member benefits »
- Forgot User Name or Password?
Hydrolysis of Salts Mark as Favorite (27 Favorites)
LAB in Net Ionic Equation , Indicators , Strong vs Weak , Salts . Last updated May 11, 2021.
In this lab, students will observe the hydrolysis of several salt samples. They will first predict which solutions are acidic, basic or neutral, and then discover the pH of each through the use of indicators. Students will share and compile their experimental results, as well as have an opportunity to determine the net-ionic equations for each reaction.
Grade Level
High School
AP Chemistry Curriculum Framework
This lab supports the following units, topics and learning objectives:
- TRA -1.B: Represent changes in matter with a balanced chemical or net ionic equation: a. For physical changes. b. For given information about the identity of the reactants and/or product. c. For ions in a given chemical reaction.
- TRA-2.B: Identify species as Brønsted-Lowry acids, bases, and/or conjugate acid-base pairs, based on proton-transfer involving those species.
- SPQ-5.C : Identify the qualitative effect of changes in pH on the solubility of a salt.
- SAP-9.B: Calculate pH and pOH based on concentrations of all species in a solution of a strong acid or a strong base.
- SAP-9.E: Explain results from the titration of a mono- or polyprotic acid or base solution, in relation to the properties of the solution and its components.
- SAP-10.A : Explain the relationship between the predominant form of a weak acid or base in solution at a given pH and the pKa of the conjugate acid or the pKb of the conjugate base.
NGSS Alignment
This lab will help prepare your students to meet the performance expectations in the following standards:
- Analyzing and Interpreting Data
- Obtaining, Evaluating, and Communicating Information
By the end of this lab, students should be able to
- Explain that the pH of a solution containing a dissolved salt may be acidic, basic or neutral.
- Use pH paper and universal indicator to determine if a solution is acidic, basic or neutral.
- Describe the meaning of the term hydrolysis .
- Write a net-ionic equation for a dissolved salt in water.
Chemistry Topics
This lab supports students’ understanding of
- Acids & Bases
- Strong vs. Weak Acids/Bases
- Net Ionic Equations
Teacher Preparation : 30-45 minutes
Lesson : 60 minutes
- 100ml beakers (3 per group)
- Glass stir rods
- pH Hydrion paper
- Distilled water
- Sodium Chloride, NaCl
- Sodium Acetate, NaC 2 H 3 O 2
- Aluminum Chloride, AlCl 3
- Sodium Oxalate, Na 2 C 2 O 4
- Sodium Carbonate, Na 2 CO 3
- Potassium Nitrate, KNO 3
- Ammonium Chloride, NH 4 Cl
- Sodium Bicarbonate, NaHCO 3
- Zinc Sulfate, ZnSO 4
- Copper (II) Sulfate, CuSO 4
- Sodium Phosphate, Na 3 PO 4
- Potassium Chlorate, KClO 3
- Labeling tape and markers
- Universal Indicator
- Large poster paper or white board
- Always wear safety goggles and apron when handling chemicals in this lab. All salts can be irritants to skin and eyes.
- Students should wash their hands thoroughly before leaving the lab.
- When students complete the lab, instruct them how to clean up their materials and dispose of any chemicals.
- Potassium chlorate is a strong oxidizing agent; do not heat or poke with the spatula.
- Sodium oxalate is a poison. Be sure to wash your hands and wipe down the counters well after the lab.
Teacher Notes
- In this lab students will observe and record the pH of various salt solutions. I then coach them to write net ionic equations for each solution in an attempt to explain what is happening to the salts in water.
- For the lab, students are divided into four research groups, each of which is assigned three salts, for a total of twelve salts (none which are used in prior examples). Students predict whether each of their assigned salts will be acidic, neutral, or basic in water, then measure the pH of each with Hydrion paper and universal indicator.
- Each group then presents their findings to the class on large poster paper or a white board, showing the net ionic equations and pH levels they recorded. These presentations are key, as each group must explain both their predictions for each assigned salt and whether or not the prediction was supported by the data. Also, students must attempt to explain any data that does not match their predictions or otherwise does not make sense.
- The net ionic equations on the posters serve as the models for all students to examine as part of their inquiry experience. As their fellow students present, students ask clarifying questions, and record their findings.
- During this lab, the students usually observe pH readings shifted to the acidic end of the scale. As the presentations continue, they gradually realize that this is true across all of the groups, and eventually someone realizes that everyone assumed that the deionized water has a pH of 7. This usually leads to a student excitedly jumping up and testing the pH of the water, which is always slightly acidic due to dissolved carbon dioxide from the air. This allows students to experience first-hand the value of communicating with other groups; in this case, they discover an unexpected variable affecting all of their data.
- Engagement in this lab is enhanced by the fact that they are responsible for the predictions, data collection, presentations and, most importantly, discussion of discrepant events.
For the Student
This lab explores how dissolved salts affect the pH of aqueous solutions. When a salt dissolves in water, the solution is not always neutral. This is because water molecules can act either as a proton (H + ) donors or proton acceptors. Salts may cause the formation of H + and OH - ions by the reaction of one of its ions with water. This reaction is called hydrolysis (“water-breaking”) .
A salt that is formed from a weak acid and a strong base will generally form water solutions that are basic. The basic anion accepts a proton from a water molecule forming a weak acid and leaving OH - ions from the water molecules in solution. The solution will be basic due to the increase of OH - ion concentration. For example, the salt NaNO 2 can be formed from the weak acid HNO 2 and the strong base NaOH. If NaNO 2 is added to water, the nitrite ion, NO 2 - , reacts with water to form hydroxide ions:
NO 2 - + H 2 O ⇌ HNO 2 + OH -
The sodium ions do not react with water, since NaOH is strong and remains completely dissociated in water. Hence sodium nitrite is basic in water.
Likewise, salts that are formed from a strong acid and a weak base generally form water solutions that are acidic. The acidic cation donates a proton to the water molecule, forming a weak base and creating H 3 O + ions in solution. For example, the salt NH 4 NO 3 can be formed from the strong acid HNO 3 and the weak base NH 3 (in water). The nitrate ions do not react with water, since HNO 3 is a strong acid. However, the ammonium ions react with water to form hydronium ions. Hence ammonium nitrate is acidic in water:
NH 4 + + H 2 O ⇌ NH 3 + H 3 O +
In addition, water molecules may accept protons from the hydrated metallic cations; imagine the water molecules surrounding the metal ions being replaced by hydroxide ions.
For metal(II) ions: M(H 2 O) 6 2+ + H 2 O ⇌ M(H 2 O) 5 (OH) + + H 3 O + For metal (III) ions: M(H 2 O) 6 3+ + H 2 O ⇌ M(H 2 O) 5 (OH) +2 + H 3 O +
The purpose of this experiment is to observe hydrolysis and to discover which salt solutions are neutral, acidic, or basic. Your job is to predict whether each salt will be acidic, basic or neutral in solution, test your predictions, and then report back to the class.
- 100ml beakers
- Salts (see chart)
- Wash your hands thoroughly before leaving the lab.
- Follow the teacher’s instructions for cleanup of materials and disposal of chemicals.
Engage (Pre-lab)
You are part of a research team. Each team has been assigned three salts to investigate. For each of your assigned salts, predict whether the salt will be acidic, basic or neutral in water.
- Write your predictions for each salt, as well as your reasons for your predictions, in complete sentences below. (Leave some space after each one for revisions if necessary when you get to the “Explain” section of the lab).
Explore (Procedure)
- Label a beaker clearly with the name and number of each salt.
- Mix a small amount (pea-sized scoop) of each salt in about 30-50 mL of deionized water in a 100ml beaker and stir with the glass stirring rod. Do not stir with the metal spatula.
- Add 3-4 drops of universal indicator. Note the color. Add more indicator as needed to see a definite color. Compare the colors with this chart . Record the color in the data table provided.
- Determine the approximate pH by using a glass stir rod to place a drop of the solution on pH hydrion paper. Record your data and observations in the “Data Collection” table provided below.
- Keep your beakers to use as exhibits in your reports. Cover with Parafilm.
- Rinse off your stir rods and spatulas.
- Throw away used pH paper.
Explain (Analysis)
- For each salt, compare your predictions (Engage/pre-lab question) with your results. Write a sentence or two about how your predictions are similar or different from your results for each reaction. If they are different, also explain why you think the pH’s turned out as they did.
- Write the net ionic equations for each ion of each of your assigned salts in water. (Equations for the other salts will be completed after the class presentations) Add only one proton to basic anions. Make sure your equations agree with your results (hydronium ions produced if it was acidic, hydroxide ions produced if it was basic, no net ionic equation if it was neutral).
Elaborate (Presentations)
- Your predictions and explanations
- Your observations
- Your revisions to your predictions, with explanations
- Your equations (written large on poster paper/)
- As other groups report, record their findings in the space provided in your data table. Pay attention!
- Ask questions of the reporting groups. Challenge them to explain their predictions and final equations.
- Clean-Up: Once everyone has reported, pour your solutions down the sink, remove labels and rinse well with water. Wash your hands and wipe down the counters well.
- Fill out the Data Collection table below for all of the salts. Only move one proton to make the parents!
- Go back and write the net ionic equations for both ions of every salt of this lab in water (all twelve ) in the table provided. If there is no Net Ionic Equation, write “No NIE”.
- In the space after the Data Collection table write a paragraph about what you learned from this lab. Use your results as well as results from other groups as examples. How could this lab be improved?

Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
Education Prizes 2024: Give someone the recognition they deserve! Nominate before 19 June

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Education Prizes 2024: Give someone the recognition they deserve! Nominate before 19 June
Displacement reactions between metals and their salts
In association with Nuffield Foundation
- Four out of five
- No comments
Some metals are more reactive than others. Students will investigate competition reactions of metals and determine a reactivity series of the four metals used
In this experiment, a strip of metal is added to a solution of a compound of another metal. A more reactive metal displaces (pushes out) a less reactive metal from its compound. There are many ways of carrying out this series of reactions. The one described here uses a spotting tile but the same procedure could be adapted for use with test tubes. The advantages of the spotting tile method include:
- very small quantities of chemicals are used.
- the whole set of experiments is displayed together, making comparison easier.
- clearing-up afterwards is simple and avoids metal deposits being left in sinks.
Video support and linked resources
A version of this experiment is included in our Reactivity of metals video (from 10:35), along with supporting resources , including illustrated technician notes , integrated instructions , worksheets, a structure strip and more.
Careful thought needs to be given to distribution of the chemicals to the class. Solutions could be distributed in test tubes, or in small bottles fitted with droppers for sharing between several pairs of students. Metals could be issued in sets. The teacher should keep control of the magnesium ribbon, dispensing short lengths when required.
There should be no flames alight so that students are not tempted to burn pieces of magnesium and the teacher should be alert to the possibility of pieces of magnesium being removed from the laboratory.
The experiment should take about 30 minutes.
- Eye protection
- Spotting tile, with at least 16 depressions (or two smaller tiles)
- Dropping (teat) pipette
- Beaker (100 cm 3 )
- Felt tip pen or other means of labelling
Access to about 5 cm 3 each of the following 0.1 M metal salt solutions (note 1):
- Copper(II) sulfate (or nitrate(V))
- Lead(II) nitrate (TOXIC, DANGEROUS FOR THE ENVIRONMENT)
- Magnesium sulfate (or nitrate(V))
- Zinc sulfate (or nitrate(V))
- Copper foil
- Lead foil (TOXIC, DANGEROUS FOR THE ENVIRONMENT)
- Magnesium ribbon
Chemical notes
- Solutions may be dispensed in 5 cm 3 beakers to each pair of students or in small bottles fitted with droppers to groups of students.
- Metals should be approximately 1 cm lengths or squares of ribbon or foil cleaned with emery paper and as similar in size as possible.
Health, safety and technical notes
- Read our standard health and safety guidance
- Copper(II) sulfate solution, CuSO 4 (aq) – see CLEAPSS Hazcard HC027c .
- Lead nitrate(V) solution, Pb(NO 3 ) 2 (aq), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC057a .
- Magnesium sulfate solution, MgSO 4 (aq) – see CLEAPSS Hazcard HC059b .
- Zinc sulfate solution, ZnSO 4 (aq) – see CLEAPSS Hazcard HC108b .
- Copper foil, Cu(s) – see CLEAPSS Hazcard HC026 .
- Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059a . Do NOT leave in a place where pupils would have potentially unsupervised access.
- Zinc foil, Zn(s) – see CLEAPSS Hazcard HC017 .
- Lead foil, Pb(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056 .
- Using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Label this row with the name of the solution. Rinse the pipette well with water afterwards.
- Do this for each metal ion solution in turn, rinsing the pipette when you change solution.
- Put a piece of each metal in each of the solutions, using the illustration as a guide.
- Over the next few minutes observe which mixtures have reacted and which have not.
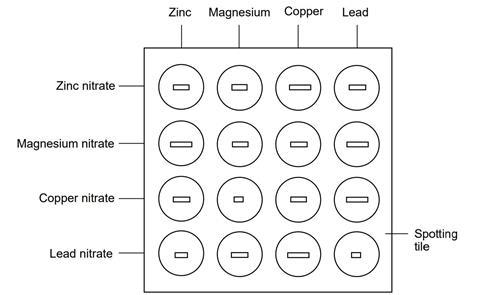
Source: Royal Society Chemistry
A guide for the set-up of the spotting tile.
Teaching notes
Remind the class that they are looking for cases where one metal displaces another. Some of the solutions are slightly acidic so that bubbles of hydrogen are sometimes seen. Explain that this does not count as displacement of one metal by another.
It might be best to get the class to tell you what they think the order of reactivity is while they still have the evidence in front of them, so that apparent discrepancies can be resolved.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- Practical experiments
- Reactions and synthesis
Specification
- 2.1.4 explain and describe the displacement reactions of metals with other metal ions in solution;
- 2.1.5 collect and/or analyse experimental data to predict where an unfamiliar element should be placed in the reactivity series or make predictions about how it will react;
- Oxidising and reducing agents.
- The electrochemical series as a series of metals arranged in order of their ability to be oxidised (reactions, other than displacement reactions, not required).
- Mandatory experiment 1.2 - Redox reactions of group VII elements - halogens as oxidising agents (reactions with bromides, iodides, Fe²⁺ and sulfites). Displacement reactions of metals (Zn with Cu²⁺, Mg with Cu²⁺). (Half equations only required e.g. 2Br⁻…
- Reduction involves the loss of oxygen.
- Identify the substances which are oxidised or reduced in terms of gain or loss of oxygen.
- A more reactive metal can displace a less reactive metal from a compound.
- Explain reduction and oxidation in terms of loss or gain of oxygen, identifying which species are oxidised and which are reduced.
- 4.2 Explain displacement reactions as redox reactions, in terms of gain or loss of electrons
- 4.3 Explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the…
- 4.5 Explain oxidation as the gain of oxygen and reduction as the loss of oxygen
- C3.2.1 deduce an order of reactivity of metals based on experimental results including reactions with water, dilute acid and displacement reactions with other metals
- C4.5.2 explain reduction and oxidation in terms of loss or gain of oxygen, identifying which species are oxidised and which are reduced
- C1.1.13 explain oxidation in terms of gain of oxygen
- C3.3a explain reduction and oxidation in terms of loss or gain of oxygen, identifying which species are oxidised and which are reduced
- C4.1f deduce an order of reactivity of metals based on experimental results
- C4.4.1 explain reduction and oxidation in terms of loss or gain of oxygen, identifying which species are oxidised and which are reduced
- C4.1e deduce an order of reactivity of metals based on experimental results
- (c) the relative reactivities of metals as demonstrated by displacement (e.g. iron nail in copper(II) chloride solution) and competition reactions (e.g. thermit reaction)
Related articles

Reacting copper(II) oxide with sulfuric acid
Illustrate the reaction of an insoluble metal oxide with a dilute acid to produce crystals of a soluble salt in this class practical. Includes kit list and safety instructions.

How to teach extraction of metals at 14–16
2024-04-09T07:20:00Z By Niall Begley
Solidify learners’ understanding of extraction processes with these tips, misconception busters and teaching ideas

Paracetamol book | Using thin-layer chromatography to investigate the reactions
In this activity you investigate the purity and identity of your laboratory prepared samples of nitrophenol or paracetamol using thin-layer chromatography
No comments yet
Only registered users can comment on this article., more from experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud

Salt Water Density Experiment

Introduction: Salt Water Density Experiment

Here's a brightly colored science experiment that not only looks cool, but allows students to develop their own understanding of density! I used this experiment for a freshman Physical Science class, but it could be adapted for many ages and situations. It's also an incredibly cheap experiment that can be done with virtually no lab equipment if necessary. Objectives: 1. Explain density using examples. 2. Identify and use laboratory equipment. Other potential objectives: 1. Measure mass. 2. Define mass. 3. Measure volume. 4. Define volume. 5. Calculate density. 6. Explain density conceptually. 7. Compare the densities of different materials. 8. Design an experiment to test a hypothesis.
Step 1: Materials

You can make this experiment anything you like. I used it very early in the year. Students had measured and timed things for the velocity unit, but I was still teaching measurement and introducing them to the lab. I usually included every skill they could possibly practice. Required: Pipets (Can be purchased online from all kinds of vendors. Example: http://www.flinnsci.com/store/Scripts/prodView.asp?idproduct=14269 About $25 for 400) You could substitute syringes, turkey basters, or some other device that would allow you to add the liquid to the bottom of a tube but pipets are so useful you might as well buy some if you don't have them. Useful (but could substitute): 250mL beakers (4-6 of these) Food coloring Salt (about 36g = 6 Tablespoons) Stirring rod Measuring spoons Test tube Substitutions: Cups or bowls instead of beakers Natural dyes instead of food coloring (Think beets for pink etc.) Sugar instead of salt (This is a little messier and if not well cleaned up more likely to be a problem but works just as well.) Spoons instead of stirring rods. Any kind of spoon that you use could be substituted for measuring spoons. You just need to know that you're adding a relatively standard amount more for each additional color. Optional: Electronic Scale or Triple Beam Balance for extension Test tube rack Test tube drying rack Test tube brush (for cleaning) These optional ones are more about teaching them about these pieces of laboratory equipment.
Step 2: Procedure

I usually demonstrate the procedure for students and then have them write a procedure and/or draw pictures of each step. Especially since I did it early in the year, I wanted them to practice writing their own procedures and then critically analyzing what could be improved to make the procedure understandable for someone else. You could also do this as a whole class to model what the process of writing a procedure "looks like" and then have them write their own later in the year. I usually rushed and did an imperfect job during the demonstration so they could "figure things out" but had the general idea of how to do it. I'd start by demoing really quickly adding the first and second layers to show that they would just mix and then do one "right" to show the value of doing it slowly and gently. Every student was able to do it by the end of class and it was a great way to start off their science experience with something that was a little challenging at first, but then successful. Make Salt Water Solutions: 1. Add 200mL of water to a 250mL beaker. 2. Add 1 Tablespoon of salt to the water. (Technically you should probably add the salt first and then the water.) 3. Add 4 drops of food coloring. (I let them add as much food coloring as they want and whatever creative colors they want to. Buy food coloring at the dollar store and it is really cheap.) 4. Stir with a stirring rod. 5. Repeat, increasing the amount of salt added by 1 Tablespoon for each successive solution. 6. Have one beaker of water with no salt for the least dense condition. Creating Layers: 1. Choose a volume of each color to add to the test tube. (2mL works well for small test tubes. 4 mL works well for larger ones. I'd try this out once before hand with your supplies. It's easier to only have to add one pipet of liquid.) 2. Add 4mL of the least dense solution any way that you want. 3. Add 4mL of the second least dense solution by very gently inserting the pipet tip into the liquid and to the very bottom of the tube. 4. Very slowly and gently release the solution. A new layer should form. Keep the bulb of the pipet squeezed. Very slowly and gently remove the pipet from the test tube. 5. Repeat with the remaining colors.
Step 3: Additional Ideas and Resources

Extension/Modification Ideas:
- Use a triple beam balance of electronic scale to teach or practice that skill
- Use hot and cold water instead.
- Compare salt and fresh water and connect with the environment.
- Have older students teach this to younger students.
- Use other liquids to make density columns. (Water, oil, alcohol, etc.)
- Make a much larger one as part of a science night.
- Let students try making different density salt solutions and try to figure out which ones work the best and how that relates to density.
Other Density Demos:
- Egg in salt water or tap water
- Ice in water or rubbing alcohol
Attachment: I attached a Word document that is a template for a guided lab report that you feel free to modify and use. Other Ideas and Resources: These are just some ideas and alternate extensions. Some additional references will many different versions of this same idea: DENSITY: SALTWATER COLUMN LAB TEACHER PREPARATION Explains a different way to prepare solutions and some "discussion" questions as a worksheet for students Liquid Rainbow Written from the standpoint of ocean science for elementary school. Contains a lot of "teacher lingo" (e.g. key concepts, big idea, objectives, etc.) GEMS: Discovering Density book This book costs $18, but I think is where I first saw the idea for this lab. The handouts are free online in Spanish. So that might make this a cool activity to do with students learning Spanish too! Steve Spangler 7-Layer Density Column This link provides a video showing a different density experiment with different types of liquids to see how their relative densities are different. They do use the word "heavier" instead of density. He uses a slightly different way of layering. NOAA Lesson Plan: Hot, Cold, Fresh and Salty Another Earth Science/Oceans connection. This lesson plan gives ideas about having students compare hot and cold water and fresh and salty water. These are extensions that would be great to add to a simpler lesson like the one I posted.
Step 4: Real World Connection

In research labs, human blood is carefully pipetted to form a layer on top of a substance called Ficoll-Paque (made by GE). When this tube is centrifuged, the red blood cells (the most dense) go to the bottom, the Ficoll is (the next most dense), then the white blood cells, and finally the plasma (the least dense). Labs use this technique to isolate different parts of the blood. For instance, if you want to isolate the white blood cells, you can remove the plasma layer (yellow) and then gently extract the thin cloudy white layer that contains the white blood cells. Wikipedia's Ficoll-Paque Article This page describes the basics of Ficoll Ficoll-Paque PLUS Manufacturer's Description A short paragraph from the manufactures that uses the word density multiple times Ficoll-Paque Instructional Video This video shows the layering of blood on top of the Ficoll very slowly and gently and the final layers that form
Step 5: Graphic Organizer Word Document
Someone let me know the Word document does not open currently. I tried uploading another version but it also says forbidden. Message me and I can try to send it to you.

Participated in the Education Contest

Recommendations

Text Contest

Paper and Cardboard Contest

Big and Small Contest


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Experiment_607_Single Displacement Reactions_1_1_1
- Last updated
- Save as PDF
- Page ID 303069
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Experiment 60 7 : Single Dis placement Reactions
Section 1: Purpose and Summary
Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid.
Determine the order of activity of metals and hydrogen based on observations.
Note: ‘Single displacement reactions’ are sometimes called ‘single replacement reactions’.
In this experiment, students will conduct different reactions between a metal and a salt of another metal. Students will also conduct reactions between metals and dilute acid solution. Based on observations, students will list the metals and hydrogen in order of activity.
Section 2: Safety Precautions and Waste Disposal
Safety Precautions:
Use of eye protection is recommended for all experimental procedures.
Waste Disposal:
While you are doing the experiment, pour your liquid waste into a beaker. Separate the solid metal pieces from the waste solution.
Metal pieces should be rinsed with water, and then they may be disposed of in proper solid waste container.
When you are finished with the experiment, collect the liquids into a waste beaker (liquid waste only). Transfer the liquid waste into the inorganic waste container in the fume hood.
Section 3: Procedure
Some combinations of reactants used in this experiment may react slowly or be difficult to detect at times. If you see no immediate visible result, set the test tube aside and allow it to stand for 5 to 10 minutes, then reexamine it.
To determine whether a reaction occurs or not, observe any bubble formation (gas evolution) in the solution or a change in appearance of the metallic solid (note the color and/or texture).
Part 1: Reaction of metal with aqueous metal salt solution
Part 2 : Reaction of metal with dilute acid solution
Post-lab Questions :
Write balanced molecular equation, complete ionic equation and net ionic equation for each reaction that you observed in this experiment.
Based on your observations, list Mg, Cu, Zn, Fe, and H in order of increasing activity (least to highest).
Aluminum lies below magnesium and above zinc in the reactivity series of metals. Predict its reaction with each of the metal solutions and dilute acid solution used in this experiment.

IMAGES
VIDEO
COMMENTS
The hypothesis can then be used to make predictions, such as 'In the hydrolysis of copper chloride, the product at the positive electrode will be chlorine.'
A student makes a hypothesis that when different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal
A student makes a hypothesis: 'When different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal'. (a) Describe how you would test this hypothesis in the laboratory.
Revision notes on 4.3.5 Required Practical: Electrolysis of Aqueous Solutions for the AQA GCSE Chemistry syllabus, written by the Chemistry experts at Save My Exams.
To test the hypothesis 'When different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal', you would need to perform an electrolysis experiment with various salt solutions using inert electrodes. Unfortunately, I cannot draw a diagram here, but I can describe the setup.
'When different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal'.
A student makes a hypothesis: "When different salt solutions are electrolyzed with inert electrodes, the product at the negative electrode is always a metal." (a) Describe how you would test this hypothesis in the laboratory.
Add sodium sulfate solution to a beaker, and connect two electrodes to a power supply. Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode. hydrogen gas will form at the negative electrode.
'When different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal'.
A student investigated the hypothesis: The electrolysis of a salt solution produces a metal at the negative electrode and a gas at the positive electrode. Figure 4 shows the apparatus used.
Aqueous salt solutions, therefore, may be acidic, basic, or neutral, depending on the relative acid-base strengths of the salt's constituent ions. For example, dissolving ammonium chloride in water results in its dissociation, as described by the equation
In this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. Based on the results, students will classify each solution as strong electrolyte, weak electrolyte, or nonelectrolyte.
Electrolysis Y10 Test. advertisement. 1. A student makes a hypothesis: 'When different salt solutions are electrolysed with inert electrodes, the product at the. negative electrode is always a metal'. (a) Describe how you would test this hypothesis in the laboratory. You should:
Example 14.4.1 14.4. 1: pH of a Solution of a Salt of a Weak Base and a Strong Acid. Aniline is an amine that is used to manufacture dyes. It is isolated as aniline hydrochloride, [C6H5NH+3]Cl [ C 6 H 5 NH 3 +] Cl, a salt prepared by the reaction of the weak base aniline and hydrochloric acid.
To test this conjecture in a controlled environment, the initial step is to accumulate various kinds of mixed element solutions. Afterward, you would utilize inactive conductors to perform an electrical decomposition process on them.
In this lab students will observe and record the pH of various salt solutions. I then coach them to write net ionic equations for each solution in an attempt to explain what is happening to the salts in water.
A student makes a hypothesis: 'When different salt solutions are electrolysed with inert electrodes, the product at the negative electrode is always a metal'. (a)€€€€€Describe how you would test this hypothesis in the laboratory.
Displacement reactions between metals and their salts. Some metals are more reactive than others. Students will investigate competition reactions of metals and determine a reactivity series of the four metals used. In this experiment, a strip of metal is added to a solution of a compound of another metal. A more reactive metal displaces (pushes ...
Let students try making different density salt solutions and try to figure out which ones work the best and how that relates to density.
Study with Quizlet and memorize flashcards containing terms like A student intended to make a salt solution with a concentration of 10.0 grams of solute per liter of solution. When the student's solution was analyzed, it was found to contain 8.90 grams of solute per liter of solution.
In this experiment, students will conduct different reactions between a metal and a salt of another metal. Students will also conduct reactions between metals and dilute acid solution. Based on observations, students will list the metals and hydrogen in order of activity.
7 Copper nitrate solution is blue. . 8. Suggest why the blue colour of the copper nitrate solution fades during the electrolysis. [1 mark] Determine the number of atoms of copper produced when copper nitrate solution is electrolysed for 20 minutes at a current of 0.6 A. Give your answer to 3 significant figures.
Calculate the percentage change in mass of plant tissue. Plot, draw and interpret appropriate graphs. In this practical, you should take care to prepare your samples of potato carefully and record your measurements accurately. This practical can be carried out with either salt or sucrose solutions of at least five different concentrations.
Our findings indicate that suppressing GhGSTF9 in cotton led to a notably salt-sensitive phenotype, whereas heterologous overexpression in Arabidopsis plants decreases the accumulation of reactive oxygen species under salt stress, thereby enhancing salt stress tolerance.