
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


10: Vitamin C Analysis (Experiment)
- Last updated
- Save as PDF
- Page ID 95879

- Santa Monica College
- To standardize a \(\ce{KIO3}\) solution using a redox titration.
- To analyze an unknown and commercial product for vitamin C content via titration.
- To compare your results for the commercial product with those published on the label.
Note: You will need to bring a powdered or liquid drink, health product, fruit samples, or other commercial sample to lab for vitamin C analysis. You will need enough to make 500 mL of sample for use in 3-5 titrations. Be sure the product you select actually contains vitamin C (as listed on the label or in a text or website) and be sure to save the label or reference for comparison to your final results. Be careful to only select products where the actual vitamin C content in mg or percent of RDA (recommended daily allowance) is listed. The best samples are lightly colored and/or easily pulverized.
The two reactions we will use in this experiment are:
\[\ce{KIO3(aq) + 6 H+(aq) +5 I- (aq)→ 3 I2(aq) + 3 H2O(l) + K+(aq) } \quad \quad \text{generation of }\ce{I2} \label{1}\]
\[\underbrace{\ce{C6H8O6(aq)}}_{\text{vitamin C(ascorbic acid)}}\ce{ + I2(aq) →C6H6O6(aq) +2 I- (aq) + 2 H+(aq) } \quad \quad \text{oxidation of vitamin C}\label{2}\]
Reaction \ref{1} generates aqueous iodine, \(\ce{I2}\) ( aq ). This is then used to oxidize vitamin C (ascorbic acid, \(\ce{C6H8O6}\)) in reaction \ref{2}. Both of these reactions require acidic conditions and so dilute hydrochloric acid, \(\ce{HCl}\) ( aq ), will be added to the reaction mixture. Reaction one also requires a source of dissolved iodide ions, \(\ce{I^-}\) ( aq ). This will be provided by adding solid potassium iodide, \(\ce{KI}\) ( s ), to the reaction mixture.
This is a redox titration. The two relevant half reactions for reaction \ref{2} above are:
Reduction half reaction for Iodine at pH 5:
\[\ce{I2 +2e^{⎯} → 2I^{⎯}}\]
Oxidation half reaction for vitamin C (\(\ce{C6H8O6}\)) at pH 5:
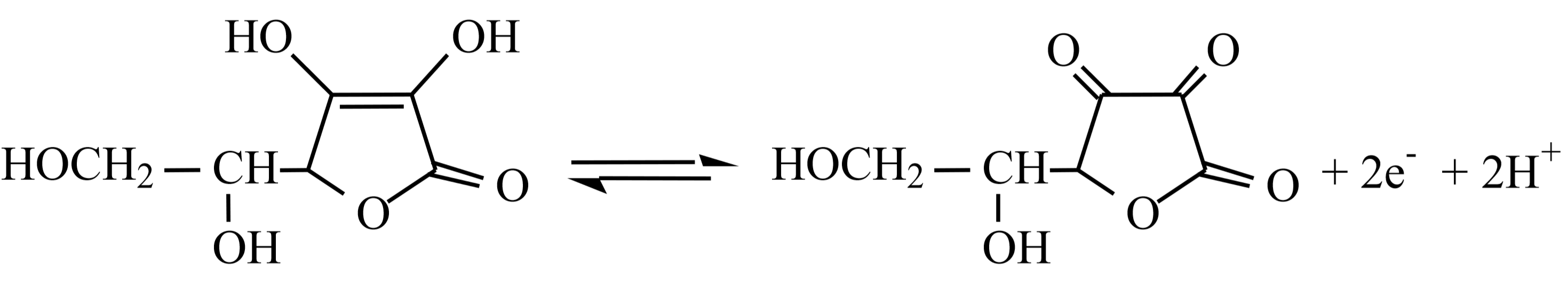
A few drops of starch solution will be added to help determine the titration endpoint. When the vitamin C (ascorbic acid) is completely oxidized, the iodine, \(\ce{I2}\) ( aq ), will begin to build up and will react with the iodide ions, \(\ce{I^-}\) ( aq ), already present to form a highly colored blue \(\ce{I3^-}\)-starch complex, indicating the endpoint of our titration.
Vitamin C: An Important Chemical Substance
Vitamin C , known chemically as ascorbic acid , is an important component of a healthy diet. The history of Vitamin C revolves around the history of the human disease scurvy, probably the first human illness to be recognized as a deficiency disease. Its symptoms include exhaustion, massive hemorrhaging of flesh and gums, general weakness and diarrhea. Resultant death was common. Scurvy is a disease unique to guinea pigs, various primates, and humans. All other animal species have an enzyme which catalyzes the oxidation of L- gluconactone to L-ascorbic acid, allowing them to synthesize Vitamin C in amounts adequate for metabolic needs.
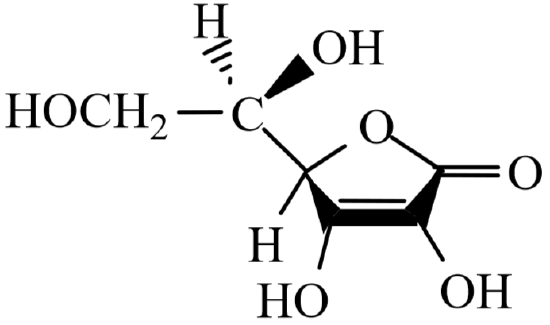
L-Ascorbic Acid -- Vitamin C
As early as 1536, Jacques Cartier, a French explorer, reported the miraculous curative effects of infusions of pine bark and needles used by Native Americans. These items are now known to be good sources of ascorbic acid. However, some 400 years were to pass before Vitamin C was isolated, characterized, and synthesized. In the late 1700's, the British Navy ordered the use of limes on ships to prevent scurvy. This practice was for many years considered to be quackery by the merchant marines, and the Navy sailors became known as “Limeys”. At that time scurvy aboard sailing vessels was a serious problem with often up to 50% of the crew dying from scurvy on long voyages.
The RDA ( Recommended Daily Allowance ) for Vitamin C put forward by the Food and Nutrition Board of the National Research Counsel is 60 mg/day for adults. It is recommended that pregnant women consume an additional 20 mg/day. Lactating women are encouraged to take an additional 40 mg/day in order to assure an adequate supply of Vitamin C in breast milk. Medical research shows that 10 mg/day of Vitamin C will prevent scurvy in adults. There has been much controversy over speculation that Vitamin C intake should be much higher than the RDA for the prevention of colds and flu. Linus Pauling, winner of both a Nobel Prize in Chemistry and the Nobel Peace Prize, has argued in his book, Vitamin C and the Common Cold , that humans should be consuming around 500 mg of Vitamin C a day (considered by many doctors to be an excessive amount) to help ward off the common cold and prevent cancer.
Vitamin C is a six carbon chain, closely related chemically to glucose. It was first isolated in 1928 by the Hungarian-born scientist Szent-Gyorgi and structurally characterized by Haworth in 1933. In 1934, Rechstein worked out a simple, inexpensive, four-step process for synthesizing ascorbic acid from glucose. This method has been used for commercial synthesis of Vitamin C. Vitamin C occurs naturally primarily in fresh fruits and vegetables.
Table 1: Vitamin C content of some foodstuffs
From Roberts, Hollenberg, and Postman, General Chemistry in the Laboratory .
Work in groups of three, dividing the work into three parts (standardization, unknown analysis, and food products) among your group members and then compare data if you are to finish in one period. Work carefully: your grade for this experiment depends on the accuracy and precision of each of your final results.
Materials and Equipment
You will need the following additional equipment for this experiment: 3 Burets, 1 Mortar and pestle, 1 Buret stand
Avoid contact with iodine solutions, as they will stain your skin. Wear safety glasses at all times during the experiment.
WASTE DISPOSAL : You may pour the blue colored titrated solutions into the sink. However, all unused \(\ce{KIO3}\) (after finishing parts A-C) must go in a waste container for disposal. This applies to all three parts of the experiment.
Proper Titration Techniques
Using a Buret
Proper use of a buret is critical to performing accurate titrations. Your instructor will demonstrate the techniques described here.
- Rinsing: Always rinse a buret (including the tip) before filling it with a new solution. You should rinse the buret first with deionized water, and then twice with approximately 10-mL aliquots of the solution you will be using in the buret. Be sure to swirl the solution to rinse all surfaces. If you are using an acid or base solution be careful to avoid spilling the solution on hands or clothing.
- Filling: Mount the buret on a buret stand. Be sure that the tip fits snuggly into the buret and is pressed all the way in. If the tip is excessively loose, exchange it for a tighter fitting one. Using a funnel rinsed in the same manner as the buret, fill the buret with the titrant to just below the 0.00 mL mark. There is no need to fill the buret to exactly 0.00 mL since you will use the difference between the ending and starting volumes to determine the amount delivered. When the buret is full, remove the funnel as drops remaining in or around the funnel can creep down and alter your measured volume. If you overfill the buret, drain a small amount into an empty beaker. Do not re-use this "extra" solution as it may have been contaminated by the beaker or diluted slightly by any water present in the beaker. Always pour fresh solution into the buret.
- Removing Air Bubbles: Often air bubbles will be trapped in the tip of a newly filled buret. These can be difficult to see and troublesome as they alter the measured volume when they escape. To remove air bubbles hold the buret over an open beaker and open the stopcock fully to allow solution to flow out of the buret. Your instructor will demonstrate this technique. Refill the buret as necessary.
- Reading the Buret: You should always read the volume in a buret from the bottom of the meniscus viewed at eye level (see Figure 1). A black or white card held up behind the buret helps with making this reading. Burets are accurate to ±0.02 mL and all readings should be recorded to two decimal places. Be sure to record both the starting and ending volumes when performing a titration. The difference is the volume delivered.
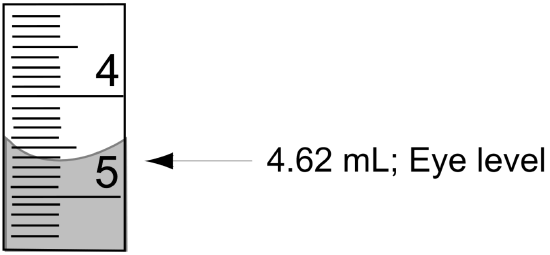
Figure 1: Reading a Buret
Good Titration Techniques
Throughout your scientific careers you will probably be expected to perform titrations; it is important that you learn proper technique. In performing a titration generally an indicator that changes color is added to a solution to be titrated (although modern instruments can now perform titrations automatically by spectroscopically monitoring the absorbance). Add titrant from the buret dropwise , swirling between drops to determine if a color change has occurred. Only if you know the approximate end-point of a titration should you add titrant faster, but when you come within a few milliliters of the endpoint you should begin to slow down and add titrant dropwise.
As you become proficient in performing titrations you will get a "feeling" for how much to open the stopcock to deliver just one drop of titrant. Some people become so proficient that they can titrate virtually "automatically" by allowing the titrant to drip out of the buret dropwise while keeping a hand on the stopcock, and swirling the solution with the other hand. If you do this, be sure that the rate at which drops are dispensed is slow enough that you can stop the flow before the next drop forms! Overshooting an end-point by even one drop is often cause for having to repeat an entire titration. Generally, this will cost you more time than you will gain from a slightly faster droping rate.
Refill the buret between titrations so you won’t go below the last mark. If a titration requires more than the full volume of the buret, you should either use a larger buret or a more concentrated titrant. Refilling the buret in the middle of a trial introduces more error than is generally acceptable for analytical work.
Set-up and Preparation of Equipment
- Clean and rinse a large 600-mL beaker using deionized water. Label this beaker “standard \(\ce{KIO3}\) solution.”
- From the large stock bottles of ~0.01 M \(\ce{KIO3}\) obtain about 600 mL of \(\ce{KIO3}\) solution. This should be enough \(\ce{KIO3}\) for your group for all three parts of the experiment including rinsings. The reason for collecting one beaker of stock is there is no guarantee that different batches of \(\ce{KIO3}\) from the stockroom will have the same exact molarity. By having one beaker of stock you ensure that all your trials come from the same solution. (If you run out of stock or spill this solution accidentally you will need to repeat part A on the new solution).
- Clean and rinse three burets once with deionized water and then twice with small (5-10 ml) aliquots of standard \(\ce{KIO3}\) from your large beaker. Pour the rinsings into a waste beaker.
- Fill each of the burets (one for each part of the experiment) with \(\ce{KIO3}\) from your beaker. Remove any air bubbles from the tips. The starting volumes in each of the burets should be between 0.00 mL and 2.00 mL. If you use a funnel to fill the burets be sure it is cleaned and rinsed in the same way as the burets and removed from the buret before you make any readings to avoid dripping from the funnel into the buret.
Each of the following parts should be performed simultaneously by different members of your group. You do not have enough time to do these sequentially and finish in one lab period.
Part A: Standardization of your \(\ce{KIO3}\) solution
The \(\ce{KIO3}\) solution has an approximate concentration of about ~0.01 M. You will need to determine exactly what the molarity is to three significant figures. Your final calculated results for each trial of this experiment should differ by less than ± 0.0005 M. Any trials outside this range should be repeated. You will need to calculate in advance how many grams of pure Vitamin C powder (ascorbic acid, \(\ce{C6H8O6}\)) you will need to do this standardization (this is part of your prelaboratory exercise). Remember that your buret holds a maximum of 50.00 mL of solution and ideally you would like to use between 25-35 mL of solution for each titration (enough to get an accurate measurement, but not more than the buret holds).
- Calculate the approximate mass of ascorbic acid you will need and have your instructor initial your calculations on the data sheet.
- Weigh out approximately this amount of ascorbic acid directly into a 250-mL Erlenmeyer flask. Do not use another container to transfer the ascorbic acid as any loss would result in a serious systematic error. Record the mass added in each trial to three decimal places in your data table. It is not necessary that you weigh out the exact mass you calculated, so long as you record the actual mass of ascorbic acid added in each trial for your final calculations.
- Dissolve the solid ascorbic acid in 50-100 mL of deionized water in an Erlenmeyer flask.
- Add approximately 0.5-0.6 g of \(\ce{KI}\), 5-6 mL of 1 M \(\ce{HCl}\), and 3-4 drops of 0.5% starch solution to the flask. Swirl to thoroughly mix reagents.
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark blue color does not fade after 20 seconds of swirling.
- Calculate the molarity of this sample. Repeat the procedure until you have three trials where your final calculated molarities differ by less than ± 0.0005 M.
Part B: Vitamin C Unknown (internal control standard)
- Obtain two Vitamin C tablets containing an unknown quantity of Vitamin C from your instructor.
- Weigh each tablet and determine the average mass of a single tablet.
- Grind the tablets into a fine powder using a mortar and pestle.
- Weigh out approximately 0.20-0.25 grams of the powdered unknown directly into a 250-mL Erlenmeyer flask. Do not use another container to transfer the sample as any loss would result in a serious systematic error. Record the mass added in each trial to three decimal places in your data table.
- Dissolve the sample in about 100 mL of deionized water and swirl well. Note that not all of the tablet may dissolve as commercial vitamin pills often use calcium carbonate (which is insoluble in water) as a solid binder.
- Add approximately 0.5-0.6 g of \(\ce{KI}\), 5-6 mL of 1 M \(\ce{HCl}\), and 2-3 drops of 0.5% starch solution to the flask before beginning your titration. Swirl to mix.
- Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the mass of unknown slightly in subsequent trials.
- Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of Vitamin C contained in each tablet. Be sure to use the average molarity for \(\ce{KIO3}\) determined in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Part C: Fruit juices, foods, health-products, and powdered drink mixes
Solids samples
- Pulverize solid samples (such as vitamin pills, cereals, etc.) with a mortar and pestle. Powdered samples (such as drink mixes) may be used directly.
- Weigh out enough powdered sample, so that there will be about 100 mg of ascorbic acid (according to the percentage of the RDA or mg/serving listed by the manufacturer) in each trial.
- Add the sample to a 250-mL Erlenmeyer flask containing 50-100 mL of water. (Note: If your sample is highly colored, you might want to dissolve the KI in the water before adding the mix, so that you can be sure it dissolves).
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling.
- Calculate the milligrams of ascorbic acid per gram of sample. Be sure to use the average molarity determined for the \(\ce{KIO3}\) in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Liquid samples
- If you are using a pulpy juice, strain out the majority of the pulp using a cloth or filter.
- Using a graduated cylinder, measure out at least 100 mL of your liquid sample. Record the volume to three significant figures (you will calculate the mass of ascorbic acid per milliliter of juice).
- Add this liquid to an Erlenmeyer flask.
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling. With juices it sometimes takes a little longer for the blue color to fade, in which case the endpoint is where the color is permanent.
- Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the volume of unknown slightly in subsequent trials.
- Calculate the milligrams of ascorbic acid per milliliter of juice. Be sure to use the average molarity determined for the \(\ce{KIO3}\) in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Pre-laboratory Assignment: Vitamin C Analysis
- If an average lemon yields 40 mL of juice, and the juice contains 50 mg of Vitamin C per 100 mL of juice, how many lemons would one need to eat to consume the daily dose of Vitamin C recomended by Linus Pauling? Show all work.
- Why are \(\ce{HCl}\), \(\ce{KI}\), and starch solution added to each of our flasks before titrating in this experiment? What is the function of each?
- \(\ce{HCl}\):
- \(\ce{KI}\):
- A label states that a certain cold remedy contains 200% of the US Recommended Daily Allowance (RDA) of Vitamin C per serving, and that a single serving is one teaspoon (about 5 mL). Calculate the number of mg of Vitamin C per serving and per mL for this product. Show all work.
- Based on the balanced reactions \ref{1} and \ref{2} for the titration of Vitamin C, what is the mole ratio of \(\ce{KIO3}\) to Vitamin C from the combined equations?
_______ moles \(\ce{KIO3}\) : _______ moles Vitamin C (ascorbic acid)
- Assuming that you want to use about 35 mL of \(\ce{KIO3}\) for your standardization titration in part A, about how many grams of ascorbic acid should you use? (you will need this calculation to start the lab). Show all work.
Hint: you will need to use the approximate \(\ce{KIO3}\) molarity given in the lab instructions and the mole ratio you determined in the prior problem.
Lab Report: Vitamin C Analysis
Mass of ascorbic acid to be used for standardization of ~0.01 M \(\ce{KIO3}\): __________ g ______Instructor’s initials
Supporting calculations:
Standardization Titration Data:
*All values should be with in ±0.0005 M of the average; trials outside this range should be crossed out and a fourth trial done as a replacement. Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
- Average Molarity of \(\ce{KIO3}\):
"Internal Control Sample" (unknown) code:
Mass of Tablet 1:
Mass of Tablet 2:
Average mass:
Control Standard (Unknown) Titration Data:
* Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
- ____________mg/g
- ____________mg/tablet
Name of Sample Used: ________________________________________________________
- Briefly describe the sample you chose to examine and how you prepared it for analysis. You may continue on the back if necessary:
Part C Titration Data:
*Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
Average ascorbic acid :
- What is the concentration of Vitamin C listed on the packaging by the manufacturer or given in the reference source? This can be given in units of %RDA, mg/g, mg/mL, mg/serving, or %RDA per serving. Be sure to include the exact units cited.
- Manufacturer’s claim: ____________________________ (value and units)
- Serving Size (if applicable): ________________________ (value and units)
____________ mg / g or mL
- If your reference comes from a text book or the internet give the citation below. If it comes from a product label please remove the label and attach it to this report.
- Using your average milligrams of Vitamin C per gram or milliliter of product from part C as the "correct" value, determine the percent error in the manufacturer or text’s claim (show calculations)?
- What can you conclude about the labeling of this product or reference value? How do you account for any discrepancies? Does the manufacturer or reference overstate or understate the amount of Vitamin C in the product? If so, why might they do this? Explain below. Use the back of this sheet if necessary.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Vitamin C - an Update on Current Uses and Functions
Introductory Chapter: Vitamin C
Submitted: 03 December 2018 Published: 03 January 2019
DOI: 10.5772/intechopen.83392
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Vitamin C - an Update on Current Uses and Functions
Edited by Jean Guy LeBlanc
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
1,162 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Author Information
Jean guy leblanc *.
- CERELA-CONICET, San Miguel de Tucumán, Argentina
*Address all correspondence to: [email protected], [email protected]
1. Vitamins
The word vitamin was originally coined to describe amines that are essential for life. It is now known that although not all vitamins are amines, there are organic micronutrients that mean that they must be consumed in small quantities for adequate growth and are required in numerous metabolic reactions to maintain homeostasis. There are 13 vitamins that are recognized by all researchers, and these can be classified as either being soluble in fats (fat soluble) (including vitamins A (retinols and carotenoids), D (cholecalciferol), E (tocopherols and tocotrienols), and K (quinones)) or soluble in water (water soluble) (including vitamin C (ascorbic acid) and the B group vitamins). B group vitamins include the following: vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folic acid or folate), and vitamin B12 (cobalamins).
2. Vitamin deficiencies
Although all 13 vitamins are present in a wide variety of foods, deficiencies are still very common in all parts of the world. There is no magic food that contains all the vitamins; the only way to avoid deficiencies is to consume a variety of foods, which are the bases of all the nutritional guidelines, or consume dietary supplements. In addition to malnutrition, certain diseases and treatments have been shown to affect vitamin absorption or bioavailability. Furthermore, pregnant women and children have a greater need for vitamins because of their increased metabolism during cell replication.
3. Vitamin C
Vitamin C, also known as ascorbic acid, is mainly present in fruits and vegetables; citrus fruits, tomatoes and potatoes are the principal exogenous source of this vitamin. The consumption of such foods is important since the human body does not have the ability to produce this essential micronutrient. Because it is water soluble, it can easily be lost by cooking and long-term storage; fortunately, most fruits and vegetables that contain large amounts of vitamin C are consumed fresh without cooking. However, it is well known that most people do not consume the recommended five servings of fruits and vegetables that would be necessary for them to fulfill their daily recommended intake of vitamin C that is around 200 mg. Because of this problem, it is now common that ascorbic acid be used as a dietary supplement, which can easily be added to foods or consumed directly in capsules or part of multivitamin preparations.
Even though it is almost unheard of that people still can be affected by scurvy today, which is directly caused by vitamin C deficiency, its early symptoms are very common. These include fatigue, inflammatory problems (especially of the gums), depression, joint pain, and anemia. Besides an inadequate ingestion of the vitamin, other causes have been linked to vitamin C deficiency such as smoking (direct and passive), malnutrition (inadequate ingestion of eating unbalanced diets), certain drugs, and malabsorption caused by certain diseases.
The consumption of vitamin C supplements are nowadays very common, not only to prevent deficiencies but also to ensure the wide range of beneficial health effects that have been reported to be associated with the consumption of this vitamin. These include having an active role in immunity, reason for which is that many consume ascorbic acid when they have a common cold, and also because it has been stated that it can play a role in cancer (in its prevention and treatment), cardiovascular diseases, and age-related diseases such a cataracts, among others. Vitamin C is also the most commonly used antioxidant substance in foods because of its safety. This property has made it the object of numerous studies where it is used as adjunctive treatments in many bacterial and virus infections and cancer treatments, another reason that has made it the vitamin of choice by consumers to improve their general health.
4. Conclusions
Even though the role of vitamin C has been known since the early 1930s and a series of interesting studies have been performed in the 1970s, only recently researchers have been actively studying and demonstrating its role and function in the treatment and prevention of many diseases. These studies will be the key to providing the scientific basis that explains why this simple but important vitamin possesses such a wide range of positive biological activities.
Acknowledgments
The authors would like to thank the Consejo Nacional de Investigaciones Científicas y Técnicas and the Agencia Nacional de Promoción Científica y Tecnológica for their financial support.
Conflict of interest
No conflict of interests exists with the publication of this chapter.
© 2019 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Published: 10 May 2019
By Nermin M. Yussif
3094 downloads
By Fadime Eryılmaz Pehlivan
1861 downloads
By Philippe Humbert, Loriane Louvrier, Philippe Saas ...
2276 downloads
Log In | Join AACT | Renew Membership
AACT Member-Only Content
You have to be an AACT member to access this content, but good news: anyone can join!
- AACT member benefits »
- Forgot User Name or Password?
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
Vitamin C Quality Control Mark as Favorite (10 Favorites)
LESSON PLAN in Concentration , Reduction , Redox Reaction , Accuracy , Dimensional Analysis , Measurements , Significant Figures , Titrations , Indicators , Oxidation , Error Analysis , Chemical Technical Professionals . Last updated July 11, 2023.
In this lesson, students will learn about a career in the skilled technical workforce, develop skills utilized in a quality control lab, and obtain data that may not have a clear “right answer.” For example, though many over-the-counter medications and vitamins state the amount of active ingredient, any individual tablet may have between 97 to 103% of the stated label claim. In addition, any products past the expiry date may have less due to potential decomposition. Students practice scientific communication by reporting their findings in a professional manner.
Grade Level
High School
NGSS Alignment
This lesson will help prepare your students to meet the performance expectations in the following standards:
- HS-PS1-2 : Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
- HS-PS1-7 : Use mathematical representation to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
- Using Mathematics and Computational Thinking
- Analyzing and Interpreting Data
- Planning and Carrying Out Investigations
- Engaging in Argument from Evidence
- Obtaining, Evaluating, and Communicating Information
By the end of this lesson, students should be able to:
- Explain the role of chemical technicians in a quality control lab.
- Prepare solutions and conduct titrations.
- Analyze titration data and communicate results and quality control recommendations in a technical memo.
Chemistry Topics
This lesson supports students’ understanding of:
- Chemical reactions
- Oxidation-reduction reactions
- Data analysis and significant figures
Teacher Preparation :
- 30-45 minutes to prepare solutions
- 30 minutes to standardize iodine solution
- 30 minutes for the introductory activity on Chemistry and Quality Control (can be assigned as homework as a pre-lab assignment)
- 60-90 minutes for lab activity (depends on whether students complete 2 or more titrations per analysis)
- 100 mL volumetric flask
- 100-mL & 10-mL graduated cylinders
- two 125-mL Erlenmeyer flasks
- 50-mL burette, ring stand and burette clamp
- 1-mL volumetric pipette and pipette bulb
- Deionized or distilled water
- 1000 mg Vitamin C tablets are recommended, see teacher notes for more detail; do not use multivitamins as the other ingredients may interfere with the titration.
- 1% starch solution (3 mL per lab group, see teacher notes for preparation details)
- 2% Iodine-Potassium Iodide Solution (referred to throughout this lesson as “iodine solution;” see teacher notes for preparation and standardization details)
- Ascorbic acid stock solution , about 1 mg/mL (20-30 mL total for teacher standardization, or 10-20 mL per lab group if students standardize iodine solution; see teacher notes)
- Always wear safety goggles when handling chemicals in the lab.
- Iodine solutions may stain, so gloves and lab coat or apron may be worn.
- Students should wash their hands thoroughly before leaving the lab.
- When students complete the lab, instruct them how to clean up their materials and dispose of any chemicals.
- Do not consume lab solutions, even if they are otherwise edible products.
- Food in the lab should be considered a chemical not for consumption.
Teacher Notes
- The National Science Board estimates that there are over 16 million jobs in the Skilled Technical Workforce (STW) that don’t require a 4-year college degree, but require STEM knowledge such as the use of chemicals, application of arithmetic and algebra, and the knowledge of quality control and other techniques for manufacturing goods. This lesson was developed as part of a content writing team to support the ACS Strategic Initiative on Fostering a Skilled Technical Workforce, with the goal of increasing awareness of and appreciation for STW opportunities in the chemistry enterprise at the high school level.
- This lesson plan introduces students to quality control (QC) in pharmaceuticals and nutritional supplements. Students practice using some of the skills needed by chemical technicians, including preparing solutions, conducting chemical analyses, and preparing a report to summarize results.
- Lilly Quality Control Laboratories Help Ensure High Quality Medicines from Eli Lilly and Company
- Chemical Technician Career Video from Career One Stop
- Lab Technician | What I do & how much I make | Part 1 from Khan Academy
- For the lab portion of this lesson, 1000 mg vitamin C tablets are recommended. Lower dose vitamins could be used and would take less time and titrant to analyze, but care should be taken to ensure that amounts are not too small for students to measure or have too few significant figures for meaningful data analysis. Be sure it is not a multivitamin, as other ingredients may interfere with titration data.
- In this lab, ascorbic acid (vitamin C) is directly titrated with iodine (iodimetry). The titrant iodine is reduced and the ascorbic acid analyte is oxidized as shown below. A starch solution is used as an indicator, as it forms a dark blue-black complex with I 2 once all the ascorbic acid is consumed. This color change marks the endpoint of the reaction.

- Generally, titrations should be run in triplicate, but time may limit students to fewer. It is helpful to have students share data (for example, via a shared Google Sheets) to allow more meaningful (and realistic) error analysis.
- For the final report, students can research the recommended daily amounts and upper limits for Vitamin C themselves, or you can provide the following information. While many students may know the problem with too little Vitamin C (scurvy), they may not know that too much Vitamin C can cause diarrhea or stomach cramps, and increase the risk of kidney stones. If time permits, a discussion about the importance of quality control as it pertains to these recommendations could help students further appreciate the relevance and real-world impact of QC careers.
- At the end of the final report, students are asked to submit raw data and full calculations as appendices. You could have them just submit the lab handout for this section, or, to make it more formal, you could have them neatly write or type up their data tables and calculations.
- The scenario can be modified to test fruit juices (see Ballentine, 1941 in “Further reading about techniques” section below). Light-colored juices must be used in order to see the endpoint.
- To increase the challenge for a more advanced class, you could remove the SOP section from the student handout and have the students research methods to measure ascorbic acid and write their own procedure. There are two methods of using iodine to oxidize ascorbic acid (iodometry and iodimetry) that differ only in how the reagents are combined.
- Another option to increase the level of difficulty of this lesson is to remove the guided calculations and have students independently determine how to analyze the results.
Standardization of Iodine Solution with Ascorbic Acid
- You can use 2% Tincture of Iodine Solution from a drug store (which contains 1.8 to 2.2 grams of I 2 /100 mL solution with an approximate molarity of 0.08 M I 2 ), or Lugol solution (1% iodine solution which contains 1 g I 2 /100 mL of solution with an approximate molarity of 0.04 M I 2 ). In either case, it must be standardized relative to ascorbic acid (see below).
- The procedures in the student document assume that time is limited, and that the teacher standardizes the iodine solution in advance and provides the conversion factor to the students. Alternatively, provide the standardization procedures (below) to the class, and each lab group can perform one titration (or more, if time allows) and contribute a data point for a group calibration. This standardization step would be a valuable addition to help students understand another example of the roles and responsibilities of skilled technical workers if time allows.
- Ascorbic acid oxidizes quickly in the presence of oxygen. The standard solution should preferably be prepared on the day of the lab but may be prepared the day before if stored in a brown bottle or a bottle wrapped in aluminum foil and refrigerated. If the teacher is completing the standardization and providing students with a conversion factor, the entire standardization procedure can occur the day before/when the ascorbic acid solution is fresh.
- The 1% starch solution is the indicator that will identify the endpoint of the titration when excess iodine reacts with starch and turns the solution blue-black. The exact concentration is not important, and the solution can be prepared by dissolving 1 g of soluble starch in 100 mL of distilled or deionized water (water should be heated for easier dissolving), or by spraying ironing starch into distilled or deionized water. More detailed instructions are available here .
- Procedure to standardize the iodine solution:
- Using a 100-mL volumetric flask, dissolve 0.10 g of ascorbic acid in distilled or deionized water to prepare a stock solution with a concentration of ~1 mg/mL of solution. Record the exact mass of ascorbic acid used and label the concentration of the stock solution $\ce{{$\frac{mg~asorbic~acid} {100~mL~solution} $}}$. (It should be approximately 100 mg ascorbic acid/100 mL solution.)
- Using a volumetric pipette, transfer 10 mL of the ascorbic acid solution to an Erlenmeyer flask.
- Using the 10-mL graduated cylinder, add 1 mL of 1% starch solution to the ascorbic acid solution in the flask.
- Fill the burette with the iodine solution and record the initial volume to the nearest 0.01 mL (or as appropriate for the particular burettes used).
- Add the iodine solution by drops to the flask, gently swirling to mix, until the dark blue color of the iodine-starch complex persists.
- Record the final volume and determine conversion factor $\ce{{$\frac{mg~asorbic~acid} {100~mL~solution} $}}$ as shown below.
Calculations and Sample Data for Standardization
- Use the most precise balances available for best results.
- Mass of ascorbic acid: 0.103 g ascorbic acid = 103 mg ascorbic acid
- Concentration of ascorbic acid stock solution from step 1: $\ce{{$\frac{103~mg~asorbic~acid} {100~mL~solution} $}}$
- Determine the mass, in mg, of ascorbic acid in 10 mL solution:
Trial 1 & 2:
$\ce{10.0~mL~of~ascorbic~acid~solution * {$\frac{103~mg~ascorbic~acid}{100~mL~solution}$} = 10.3~mg~of~ascorbic~acid}$
- For each trial, determine how many mL of iodine solution needed to react with the mass of ascorbic acid in 10 mL solution.
Trial 1: 3.36 mL reacted with 10.3 mg of ascorbic acid
Trial 2: 3.42 mL reacted with 10.3 mg of ascorbic acid
- Calculate a conversion factor that relates 1 mL of iodine solution to mass of ascorbic acid.
$\ce{{$\frac{10.3~mg~ascorbic~acid}{3.36~mL~solution}$} = {$\frac{3.07~mg~ascorbic~acid}{1.00~mL~solution}$}}$
$\ce{{$\frac{10.3~mg~ascorbic~acid}{3.36~mL~solution}$} = {$\frac{3.01~mg~ascorbic~acid}{1.00~mL~solution}$}}$
Average Conversion Factor: 1 mL iodine solution = 3.04 mg ascorbic acid (provide this value to the students)
- If desired, you can have the students calculate molarities of the solutions instead of mg/mL, but this gravimetric conversion factor is more direct and is more likely to be used in a QC laboratory.
Further reading about techniques
- Ballentine, R. (1941). Determination of Ascorbic Acid in Citrus Fruit Juices. Industrial and Engineering Chemistry, Analytical Edition, 13(2), 89. doi: doi.org/10.1021/i560090a011
- Moore, C. E. (1948). The determination of vitamin C as a means of teaching iodimetry. Journal of Chemical Education, 25(12), 671. doi: doi.org/10.1021/ed025p671
- Cesar R. Silva, J. A. (1999). Ascorbic Acid as a Standard for Iodometric Titrations. Journal of Chemical Education, 76(10), 1421-1422. doi: doi.org/10.1021/ed076p1421
- This resource provides a very useful reference for use and care of burettes.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cochrane Database Syst Rev
Vitamin C supplementation for the primary prevention of cardiovascular disease
Vitamin C is an essential micronutrient and powerful antioxidant. Observational studies have shown an inverse relationship between vitamin C intake and major cardiovascular events and cardiovascular disease (CVD) risk factors. Results from clinical trials are less consistent.
To determine the effectiveness of vitamin C supplementation as a single supplement for the primary prevention of CVD.
Search methods
We searched the following electronic databases on 11 May 2016: the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; MEDLINE (Ovid); Embase Classic and Embase (Ovid); Web of Science Core Collection (Thomson Reuters); Database of Abstracts of Reviews of Effects (DARE); Health Technology Assessment Database and Health Economics Evaluations Database in the Cochrane Library. We searched trial registers on 13 April 2016 and reference lists of reviews for further studies. We applied no language restrictions.
Selection criteria
Randomised controlled trials of vitamin C supplementation as a single nutrient supplement lasting at least three months and involving healthy adults or adults at moderate and high risk of CVD were included. The comparison group was no intervention or placebo. The outcomes of interest were CVD clinical events and CVD risk factors.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted the data and assessed the risk of bias.
Main results
We included eight trials with 15,445 participants randomised. The largest trial with 14,641 participants provided data on our primary outcomes. Seven trials reported on CVD risk factors. Three of the eight trials were regarded at high risk of bias for either reporting or attrition bias, most of the 'Risk of bias' domains for the remaining trials were judged as unclear, with the exception of the largest trial where most domains were judged to be at low risk of bias.
The composite endpoint, major CVD events was not different between the vitamin C and placebo group (hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.89 to 1.10; 1 study; 14,641 participants; low‐quality evidence) in the Physicians Health Study II over eight years of follow‐up. Similar results were obtained for all‐cause mortality HR 1.07, 95% CI 0.97 to 1.18; 1 study; 14,641 participants; very low‐quality evidence, total myocardial infarction (MI) (fatal and non‐fatal) HR 1.04 (95% CI 0.87 to 1.24); 1 study; 14,641 participants; low‐quality evidence, total stroke (fatal and non‐fatal) HR 0.89 (95% CI 0.74 to 1.07); 1 study; 14,641 participants; low‐quality evidence, CVD mortality HR 1.02 (95% 0.85 to 1.22); 1 study; 14,641 participants; very low‐quality evidence, self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA) HR 0.96 (95% CI 0.86 to 1.07); 1 study; 14,641 participants; low‐quality evidence, self‐reported angina HR 0.93 (95% CI 0.84 to 1.03); 1 study; 14,641 participants; low‐quality evidence.
The evidence for the majority of primary outcomes was downgraded (low quality) because of indirectness and imprecision. For all‐cause mortality and CVD mortality, the evidence was very low because more factors affected the directness of the evidence and because of inconsistency.
Four studies did not state sources of funding, two studies declared non‐commercial funding and two studies declared both commercial and non‐commercial funding.
Authors' conclusions
Currently, there is no evidence to suggest that vitamin C supplementation reduces the risk of CVD in healthy participants and those at increased risk of CVD, but current evidence is limited to one trial of middle‐aged and older male physicians from the USA. There is limited low‐ and very low‐quality evidence currently on the effect of vitamin C supplementation and risk of CVD risk factors.
Plain language summary
Vitamin C supplementation to prevent cardiovascular disease
Cardiovascular diseases (CVD) are a group of conditions affecting the heart and blood vessels. CVD is a global burden and varies between regions, and this variation has been linked in part to dietary factors. Such factors are important because they can be modified to help with CVD prevention and management.This review assessed the effectiveness of vitamin C supplementation as a single supplement at reducing cardiovascular death, all‐cause death, non‐fatal endpoints (such as heart attacks, strokes and angina) and CVD risk factors in healthy adults and adults at high risk of CVD .
Study characteristics
We searched scientific databases for randomised controlled trials (clinical trials where people are allocated at random to one of two or more treatments) looking at the effects of vitamin C supplementation in healthy adults or those at high risk of developing CVD. We did not include people who already had CVD (e.g. heart attacks and strokes). The evidence is current to May 2016.
Key results
Eight trials fulfilled our inclusion criteria. One large trial looked at the effects of vitamin C supplements on the risk of major CVD events (fatal and non‐fatal) and found no beneficial effects. This trial was however conducted in middle‐aged and older male doctors in the USA and so its not certain that the effects are the same in other groups of people. Seven trials looked at the effects of vitamin C supplements on CVD risk factors. We could not combine these trials as there was lots of missing information and differences between the trials in terms of the participants recruited, the dose of vitamin C and the duration of trials. Overall, there were inconsistent effects of vitamin C supplements on lipid levels and blood pressure and more research is needed. Four of the included studies did not mention sources of funding of the study, two had non‐commercial (grants) funding and two had both commercial (industries) and non‐commercial funding (grants).
Quality of the evidence
The evidence was of low or very low quality for major CVD events (myocardial infraction, stroke, angina and coronary artery bypass grafting), all‐cause mortality and CVD mortality. The evidence was of low quality because it was not applicable to the general population (included only USA male physicians) and limited studies of vitamin C on the prevention of CVD.
Summary of findings
Summary of findings for the main comparison.
1 Middle‐aged US male physicians and is therefore not highly applicable to the decision context (downgraded by one for indirectness). 2 Small number of included studies (n = 1) for these outcomes (downgraded by one for imprecision). 3 8 years follow‐up (timeframe) may not be sufficient to detect mortality (downgraded by one for indirectness).
Description of the condition
Cardiovascular disease (CVD) remains the number one cause of death globally ( WHO 2011a ). CVDs are the result of disorders of the heart and blood vessels and include cerebrovascular disease, coronary heart disease (CHD), and peripheral arterial disease (PAD) ( WHO 2011b ). In 2008, an estimated 17.3 million people died from CVDs, representing 30% of all global deaths. Of these deaths, an estimated 7.3 million were due to CHD and 6.2 million were due to stroke ( WHO 2011a ). Over 80% of CVD deaths occur in low‐ and middle‐income countries, and the number of CVD deaths is expected to increase to 23.3 million by 2030 ( Mathers 2006 ; WHO 2011a ).
One of the main mechanisms thought to cause CVD is atherosclerosis, in which the arteries become narrowed by plaques or atheromas ( NHS 2012 ). Atherosclerosis can cause CVD when the arteries are completely blocked by a blood clot or when blood flow is restricted by a narrowed artery, limiting the amount of blood and oxygen that can be delivered to organs or tissue ( British Heart Foundation 2012 ). Whilst arteries may naturally become harder and narrower with age, this process may be accelerated by factors such as smoking, high cholesterol, high blood pressure, obesity, a sedentary lifestyle, and ethnicity ( NHS 2012 ). Prevention of CVD by targeting modifiable factors remains a key public health priority. Diet plays a major role in the aetiology of many chronic diseases including CVD, thereby contributing to a significant geographical variability in morbidity and mortality rates across different countries and populations worldwide ( WHO 2003 ). A number of dietary factors have been found to be associated with CVD risk, such as a low consumption of fruit and vegetables ( Begg 2007 ), a high intake of saturated fat ( Siri‐Tarino 2010 ) and a high consumption of salt ( He 2011 ). Dietary factors are important since they can be modified in order to lower CVD risk, making them a prime target for interventions aimed at primary prevention and management of CVD.
Description of the intervention
The intervention examined in this review is vitamin C supplementation as a single ingredient. No limit was placed on the dose or frequency at which vitamin C is taken. Vitamin C (ascorbic acid or ascorbate) is an essential micronutrient that acts as a powerful water‐soluble antioxidant, reducing oxidative stress. It cannot be synthesised in the body and is acquired primarily through the consumption of fruit, vegetables, supplements, fortified beverages, and fortified breakfast or 'ready‐to‐eat' cereals ( Frei 1989 ; WHO 2006 ).
Adults need 40 mg/day of vitamin C, which can be obtained from a healthy diet. Supplementation of vitamin C up to 1000 mg per day is unlikely to cause side effects ( NHS choices 2015 ), whereas larger amounts can cause stomach pain, diarrhoea and flatulence. The pharmacokinetics of vitamin C are complex where the relationship between the amount ingested and plasma and tissue levels is dependent on absorption, tissue transport, renal reabsorption and excretion and rate of utilisation ( Levine 2011 ). The dose concentration curve is sigmoidal with its steep portion between 30 mg and 100 mg of vitamin C daily. At doses greater than 100 mg/day, plasma concentrations reach a plateau between 70 μmol/L and 80 μmol/L. At doses greater than 400 mg/day, further increases in plasma concentrations are minimal ( Levine 2011 ).
Data on the adverse effects of vitamin C supplementation show that these are relatively rare. A survey of 9328 patients who used high‐dose intravenous vitamin C during the preceding 12 months revealed that only 101 had side effects, mostly minor, including lethargy/fatigue in 59 patients, change in mental status in 21 patients and vein irritation/phlebitis in six patients ( Padayatty 2010 ). In a recent meta‐analysis of the effects of vitamin C supplementation, alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) on blood pressure ( Juraschek 2012 ), few trials (six of 29) reported adverse effects, however details of these were not provided in the paper.
How the intervention might work
Population‐based observational studies have shown an inverse association between plasma vitamin C concentrations and vitamin C intake with blood pressure ( McCarron 1984 ; Moran 1993 ). Observational studies have also shown an inverse relationship between vitamin C intake and mortality due to CVD ( Jacques 1995 ; Simon 1998 ). However, the results from randomised controlled trials (RCTs) have not observed beneficial effects of vitamin C supplementation in the prevention of cardiovascular events ( Cook 2007 ; The Physicians Health Study II ), or mortality outcomes ( Bjelakovic 2007 ).
Low‐density lipoprotein (LDL) cholesterol in the blood may be importantly atherogenic only after oxidative modification, which allows it to be taken up by macrophages in the artery walls. These macrophages, which are attracted to regions where oxidised LDL is being taken up, become loaded with cholesterol (and are then described as "foam cells" in the artery walls), leading to the development of "fatty streaks". Oxidised LDL can also be cytotoxic. Antioxidants such as vitamin C can protect LDL from oxidative modification and may help avoid CVD ( Steinberg 1989 ).
A recent review has summarised the important functions of vitamin C in the vascular bed in support of endothelial cells ( May 2013 ). These functions include increasing the synthesis and deposition of type IV collagen in the basement membrane, stimulating endothelial proliferation, inhibiting apoptosis, scavenging radical species, and sparing endothelial cell‐derived nitric oxide to help modulate blood flow. Endothelial dysfunction is an early sign of inflammatory disease such as atherosclerosis and vitamin C could have a part to play in preventing these early stages.
In the early stages of atherosclerosis, monocytes adhere to the walls of the endothelium, causing the vessel walls to thicken and lose their elasticity. Research has shown that vitamin C supplementation can reduce the rate of monocyte adhesion to the endothelial cell wall. A study looked at the effects of vitamin C (250 mg per day, six weeks duration) in healthy adults with normal and below‐average plasma vitamin C concentration at baseline. Before the study, participants with below average levels of vitamin C had 30% greater monocyte adhesion than normal, putting them at higher risk for atherosclerosis. After six weeks of vitamin C supplementation, the rate of monocyte adhesion fell by 37% ( Woollard 2002 ).
Furthermore, intercellular adhesion molecule‐1 (ICAM‐1) is an inducible surface glycoprotein that mediates the adhesion of monocytes to the endothelium. The researchers went on to demonstrate that the same dose and duration of vitamin C supplementation was able to reduce monocyte ICAM‐1 expression by 50% in participants with below‐average plasma vitamin C concentration ( Rayment 2003 ). Vitamin C supplementation might improve nitric oxide bioactivity ( Huang 2000 ), as well as endothelial function of brachial and coronary arteries, as suggested by short‐term interventions among high‐risk individuals ( Grebe 2006 ; McNulty 2007 ; Silvestro 2002 ; Solzbach 1997 ).
Why it is important to do this review
A systematic review of the effects of individual vitamins and minerals, and multivitamins, on clinical endpoints has been conducted ( Fortmann 2013 ). This review was conducted for the US Task Force for Preventative Services. The authors found two trials of vitamin C supplementation reporting clinical endpoints relevant for CVD prevention, where no effect of the intervention was found. In terms of effects on CVD risk factors, from preliminary searching of the Cochrane Library, we identified five systematic reviews in the Database of Abstracts of Reviews of Effects (DARE), which assessed the effects of vitamin C supplementation on blood pressure ( Juraschek 2012 ; McRae 2006a ; Ness 1997 ), low‐density lipid (LDL) cholesterol and triglycerides ( McRae 2008 ), and total cholesterol ( McRae 2006b ). Only two of these included only RCTs ( Juraschek 2012 ; McRae 2008 ), the reminder include also non‐randomised experimental studies and observational studies. The first review of RCTs covered both primary and secondary prevention and the effects of vitamin C supplementation alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) in trials between two and 26 weeks duration ( Juraschek 2012 ). The authors concluded that vitamin C supplementation reduced systolic and diastolic blood pressure in short‐term trials. The second review concluded that supplementation with at least 500 mg/day of vitamin C, for a minimum of four weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, the lack of quality assessment and analysis of statistical heterogeneity, and the small sample sizes of the included trials, limit the reliability of the authors' conclusions ( McRae 2008 ).
For the current review we examined evidence from RCTs of vitamin C as a single supplement in the general population and those at moderate to high risk of CVD. This review will update and build on the existing systematic reviews discussed above by assessing vitamin C supplementation (as a single supplement only) in populations relevant for the primary prevention of CVD, in trials of at least three months duration and assessing a wider range of outcomes.
To determine the effectiveness of vitamin C supplementation as a single supplement for the primary prevention of cardiovascular disease (CVD).
Criteria for considering studies for this review
Types of studies.
All randomised controlled trials (RCTs) including cross‐over trials,studies reported as full‐text, those published as abstract only, and unpublished data were eligible for inclusion.
Types of participants
Healthy adults (18 years old or over) from the general population and those at moderate to high risk of CVD (e.g. hypertension, hyperlipidaemia, overweight/obesity). As the review focuses on the primary prevention of CVD, we excluded those who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), and those with angina or angiographically‐defined coronary heart disease (CHD). If participants were at high risk of CVD they were included if less than 25% of participants had CVD at baseline.
We also planned to exclude trials involving participants with type 2 diabetes, although this is a major risk factor for CVD, as interventions for the treatment and management of type 2 diabetes are covered by reviews registered with the Cochrane Metabolic and Endocrine Disorders Group.
Types of interventions
The intervention was vitamin C supplements alone as a single ingredient. No limit was placed on the dose or frequency of vitamin C taken. Trials were only considered where the comparison group was placebo or no intervention. Multifactorial intervention studies (including other additional interventions such as dietary changes and exercise) were not included in this review, in order to avoid confounding. If there had been a sufficient number of trials, we also planned to stratify results by dose of vitamin C.
Types of outcome measures
We included studies with follow‐up periods of at least three months. Follow‐up is considered to be the time elapsed since the start of the intervention.
Primary outcomes
Major cardiovascular events, cardiovascular mortality, all‐cause mortality.
- Non‐fatal endpoints such as MI, CABG, PTCA, angina, or angiographically‐defined CHD, stroke, carotid endarterectomy, peripheral arterial disease (PAD)
Secondary outcomes
- Changes in blood pressure (BP) (systolic (SBP) and diastolic (DBP) and blood lipids (total cholesterol, high‐density lipid (HDL) cholesterol, low‐density lipid (LDL) cholesterol, triglycerides)
- Occurrence of type 2 diabetes as a major CVD risk factor
- Validated health‐related quality of life measures
Adverse effects
Search methods for identification of studies, electronic searches.
We identified trials through systematic searches of the following bibliographic databases on 11 May 2016:
- Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2016, Issue 4 of 12)
- Health Technology Assessment (HTA) in the Cochrane Library (2016, Issue 2 of 4)
- Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library (2015, Issue 2 of 4)
- NHS Economic Evaluation Database (NEED) in the Cochrane Library (2015, Issue 2 of 4)
- MEDLINE (Ovid, 1946 to April week 4 2016)
- Embase Classic and Embase (Ovid, 1947 to 2016 Week 19)
- Web of Science Core Collection (Thomson Reuters, 1970 to 11 May 2016)
We used Medical subject headings (MeSH) or equivalent and text word terms. Searches were designed in accordance with the Cochrane Heart Group methods and guidance.
The search strategies are detailed in Appendix 1 . The Cochrane sensitivity‐maximising RCT filter ( Lefebvre 2011 ) was applied to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked reference lists of reviews for additional studies. We searched ClinicalTrials.gov (http://www.clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry platform (ICTRP) search portal (http://apps.who.int/trialsearch/) for ongoing trials on 13 April 2016 using the search terms Vitamin C OR ascorbic acid AND cardio*. Where necessary we contacted authors for any additional information.
Selection of studies
Two review authors (LA, LH, NF, RW, OG or KR) independently screened for inclusion titles and abstracts of all the studies we identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and two review authors (LA, LH, NF, RW, OG or KR) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third author (KR/SS). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram ( Figure 1 ) and ' Characteristics of excluded studies ' table.

Study flow diagram.
Data extraction and management
Two review authors (LA, LH, NF, RW) independently extracted study characteristics from included studies using a pre‐standardised data extraction form, and contacted chief investigators to request additional relevant information if necessary. We extracted details of the study design, participant characteristics, study setting, intervention (including dose and duration), and outcome data including details of outcome assessment, adverse effects, and methodological quality (randomisation, blinding, attrition) from each of the included studies. We resolved disagreements by consensus or by involving a third author (KR/SS). One author (NF) transferred data into the Review Manager ( RevMan 2012 ) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second author (KR/LA) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (NF, RW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). We resolved any disagreements by discussion or by involving another author (KR/SS). We assessed the risk of bias according to the following domains.
- Random sequence generation
- Allocation concealment
- Blinding of participants and personnel
- Blinding of outcome assessment
Incomplete outcome data
- Selective outcome reporting
- Other bias (bias due to problems not covered elsewhere, e.g. industry funding)
We graded each potential source of bias as having a 'low risk of bias', a 'high risk of bias' or an 'unclear risk of bias'. Studies were regarded as at high risk of bias if any of the domains listed above were regarded at high risk of bias.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and report any deviations from it in the ' Differences between protocol and review ' section of the systematic review.
Measures of treatment effect
Data were processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). We expressed dichotomous outcomes as hazard ratios (HRs), with 95% confidence intervals (CIs). For continuous outcomes, net changes were compared (i.e. intervention group minus control group differences) and a mean difference (MD) and 95% CIs calculated for each study.
Unit of analysis issues
Cross‐over trials.
We used data only from the first half of the trial as a parallel group design. We only considered risk factor changes (i.e. blood pressure, lipid levels) before patients crossed over to the other therapy and where the duration was a minimum of three months before cross‐over occurred.
Studies with multiple intervention groups
Data for the control group were used for each intervention group comparison. We reduced the weight assigned to the control group by dividing the control group number (N) by the number of intervention groups.
Cluster‐randomised trials
If identified, we intended to analyse cluster‐randomised trials using the unit of randomisation (cluster) as the number of observations. Where necessary, individual‐level means and standard deviations (SDs) adjusted for clustering would be utilised together with the number of clusters in the denominator, in order to weight the trials appropriately. We did not find any cluster‐RCTs that met the inclusion criteria for our review.
Dealing with missing data
We contacted investigators in order to verify key study characteristics and obtain missing numerical outcome data where possible.
Missing data were captured in the data extraction form and reported in the 'Risk of bias' table. If a trial collected an outcome measure at more than one time point, the longest period of follow‐up with 20% or fewer dropouts was utilised.
Assessment of heterogeneity
For each outcome, we conducted tests of heterogeneity using the Chi 2 test of heterogeneity and I 2 statistic. Where there was no heterogeneity, a fixed‐effect meta‐analysis was performed. If moderate to substantial heterogeneity was detected (40% to 100%), we looked for possible explanations for this (e.g. participants and intervention). If the source of heterogeneity could not be explained, we considered the following options: provide a narrative overview and not aggregate the studies at all or use a random‐effects model with appropriate cautious interpretation.
Assessment of reporting biases
Had there been sufficient studies (10 or more), we intended to plot the trial effect against the standard error and present the results as funnel plots ( Sterne 2011 ). Since asymmetry could be caused by a relationship between effect size and sample size or by publication bias, we planned to examine any observed effect for clinical heterogeneity and carry out additional sensitivity tests ( Sterne 2011 ). There were insufficient trials to conduct this analysis.
Data synthesis
Statistical analysis was carried out using the Cochrane Collaboration’s statistical software, ( RevMan 2012 ). Dichotomous data were entered as events and the number of participants and continuous data were entered as means and SDs. In the absence of moderate to substantial heterogeneity (40% to 100%) and provided that there were sufficient trials, we combined the results, using a fixed‐effect model. In the presence of substantial heterogeneity we plotted the effects for individual trials in the forest plot but have not pooled them statistically.
Subgroup analysis and investigation of heterogeneity
If there were sufficient trials (10 or more) we intended to stratify results by high risk of CVD versus the general population, and also by dose of vitamin C.
Sensitivity analysis
We planned to carry out sensitivity analyses with studies of six months or more follow‐up, and excluding studies at a high risk of bias. Studies were regarded as at high risk of bias if any of the domains in the risk of bias tool were regarded at high risk of bias.
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results. Two review authors (LA, KR) rated the quality for each outcome. We presented a summaries of the evidence in Table 1 , which provides key information about the best estimate of the magnitude of the effect, in relative terms for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). We presented results on the outcomes as described in Types of outcome measures .
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' ( Meader 2014 ) to help with standardisation of 'Summary of findings' tables ( Appendix 2 ).
Description of studies
Results of the search.
The searches generated 5555 hits after duplicates were removed. Screening of titles and abstracts identified 227 papers to go forward for formal inclusion and exclusion. Of these, nine randomised controlled trials (RCTs) met the inclusion criteria. There is one trial in abstract form awaiting classification. Details of the flow of studies through the review are shown in the PRISMA flow diagram in Figure 1 .
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies . Eight trials were included randomising a total of 15,445 participants. The largest trial recruited males only (14,641 randomised) ( The Physicians Health Study II ), six trials recruited male and female participants, and one trial did not specify the gender of participants ( Mostafa 1989 ). The trials varied in the participants recruited. Three trials recruited patients with hypercholesterolaemia ( ASAP Study ; Cerna 1992 ; Jacques 1995 ), one trial recruited patients with hypertension ( Schindler 2003 ), one trial recruited older participants aged 60 to 80 years, some with borderline or newly diagnosed hypertension ( Fotherby 2000 ), one trial recruited healthy young medical students aged 18 to 25 years ( Menne 1975 ), another recruited from a US University campus, but no details of age were provided ( Mostafa 1989 ), and the largest trial recruited US male physicians aged 50 years or older at the start of the study ( The Physicians Health Study II ), where some participants had CVD risk factors (see Characteristics of included studies ).
Two trials were conducted in Boston, MA, USA ( Jacques 1995 ; The Physicians Health Study II ). The remaining studies were conducted in Bratislava, Czechoslovakia ( Cerna 1992 ), the UK ( Fotherby 2000 ), South Africa ( Menne 1975 ), Mississippi, USA ( Mostafa 1989 ), Kuopio, Eastern Finland ( ASAP Study ), and for one trial this was unclear ( Schindler 2003 ).
The duration of the intervention and follow‐up periods varied considerably from three months to eight years. The trial with the longest intervention and follow‐up period was eight years ( The Physicians Health Study II ). This was followed by three years ( ASAP Study ); two years ( Schindler 2003 ), 18 months ( Cerna 1992 ), eight months ( Jacques 1995 ), six months ( Mostafa 1989 ), four months ( Menne 1975 ), and three months ( Fotherby 2000 ).
In five of the trials the dose of vitamin C supplementation was 500 mg/day ( ASAP Study ; Cerna 1992 ; Fotherby 2000 ; Mostafa 1989 ; The Physicians Health Study II ); in two trials the dose was 1 g/day ( Jacques 1995 ; Menne 1975 ), and in the remaining trial the dose was 2 g/day ( Schindler 2003 ).
Details of the trial awaiting assessment is presented in the Characteristics of studies awaiting classification table. This study is only available as an abstract and we are awaiting responses from the authors to our requests asking for further information.
Excluded studies
Details and reasons for exclusion for studies that closely missed the inclusion criteria are provided in the Characteristics of excluded studies table. Reasons for exclusion for the majority of studies included alternative designs (not RCTs), short‐term studies (< three months), and no relevant outcomes (see Figure 1 ).
Risk of bias in included studies
Details are presented for the included trial in the 'Risk of bias' tables in the Characteristics of included studies table and in Figure 2 ; Figure 3 .

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Only one study reported the method of random sequence generation which was regarded as at low risk of bias ( The Physicians Health Study II ); for the remaining eight studies this was unclear. No details were provided for the method of allocation concealment in all eight trials so this was judged to be at unclear risk of bias.
Five trials reported blinding participants and personnel and were judged to be at low risk of performance bias ( ASAP Study ; Fotherby 2000 ; Jacques 1995 ; Mostafa 1989 ; The Physicians Health Study II ). The remaining three studies were at unclear risk of performance bias as blinding or participants and personnel were not reported ( Cerna 1992 ; Menne 1975 ; Schindler 2003 ). Two trials were judged to be at low risk of detection bias as outcome assessors were blind to group allocation ( Fotherby 2000 ; The Physicians Health Study II ). For the remaining six trials blinding of outcome assessors was not stated and this was judged to be at unclear risk of bias ( ASAP Study ; Cerna 1992 ; Jacques 1995 ; Menne 1975 ; Mostafa 1989 ; Schindler 2003 ).
There was a low risk of attrition bias in three trials ( ASAP Study ; Jacques 1995 ; The Physicians Health Study II ). In one trial there was a high risk of attrition bias as no reasons for loss to follow‐up were given and the authors did not perform an intention‐to‐treat analysis ( Schindler 2003 ). For the remaining four studies this was judged as unclear ( Cerna 1992 ; Fotherby 2000 ; Menne 1975 ; Mostafa 1989 ).
Selective reporting
Two studies were judged to be at high risk of reporting bias ( ASAP Study ; Mostafa 1989 ). The first because no outcome data were provided for total cholesterol, LDL cholesterol, triglycerides or blood pressure ( ASAP Study ), the second because outcome data were not provided for the control group ( Mostafa 1989 ).
Other potential sources of bias
There was insufficient information to judge other potential sources of bias and all studies were regarded as unclear risk of bias.
Effects of interventions
See: Table 1
One study provided data for all our primary outcomes ( The Physicians Health Study II ). This was the largest study randomising 7329 US physicians to vitamin C supplementation and 7312 to the placebo group, with a mean follow‐up period of eight years. This trial was a factorial 2 x 2 trial of vitamin E and vitamin C supplementation and it is therefore possible to compare two vitamin C arms (active vitamin C and placebo vitamin E and active vitamin C and active vitamin E) with two non‐vitamin C arms (placebo vitamin C and active vitamin E and placebo vitamin C and E). The hazard ratios reported in this trial were adjusted for a number of variables including age, study cohort (the original Physicians study I or II), and vitamin E assignment. Results are presented below using the inverse variance method.
A composite measure of major cardiovascular events was the primary end point in this trial including non‐fatal myocardial infarction (MI), non‐fatal stroke, and cardiovascular mortality. Reported end points were confirmed in medical records and registers. The adjusted hazard ratios for this composite measure, all‐cause mortality, cardiovascular mortality, total MI (fatal and non‐fatal) and total stroke (fatal and non‐fatal) are presented below and graphically in Analysis 1.1 ; Analysis 1.3 ; Analysis 1.2 ; Analysis 1.4 ; Analysis 1.7 ) The authors of The Physicians Health Study II concluded that there was no evidence that vitamin C supplementation reduced the risk of major cardiovascular events and that these data provide no support for the use of vitamin C supplements for the prevention of cardiovascular disease (CVD) in middle‐aged and older men.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 1 Major cardiovascular event.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 2 Cardiovascular mortality.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 3 All‐cause mortality.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 4 Total myocardial infarction.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 7 Total stroke.
There was no effect of vitamin C supplementation on major cardiovascular events at eight years follow‐up hazard ratio (HR) 0.99 (95% confidence interval (CI) 0.89 to 1.10); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.1 ).
There was no substantial effect of vitamin C supplementation on all cardiovascular morality HR 1.02 (95% CI 0.85 to 1.22); 1 study; 14,641 participants; very low quality of evidence (graphically presented in Analysis 1.2 ).
Two deaths were reported in the ASAP Study , one in the vitamin C group (subarachnoid haemorrhage) and one in the placebo group (cardiac dysrhythmia). These data have not been incorporated in the meta‐analysis as the inverse variance method was used for the The Physicians Health Study II to take account of the adjusted hazard ratios for this study. In a separate analysis, incorporation of these two deaths had no effect on the estimate as the The Physicians Health Study II had 99.9% of the weight.
There was no effect of vitamin C supplementation on all‐cause morality at eight years follow‐up HR 1.07 (95% CI 0.97 to 1.18); 1 study; 14,641 participants; very low quality of evidence (graphically presented in Analysis 1.3 ).
Total myocardial infraction
There was no effect of vitamin C on total myocardial infarction (fatal and non‐fatal events) HR 1.04 (95% CI 0.87 to 1.24); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.4 ).
Self‐reported revascularisation
There was nor effect of vitamin C supplementation on revascularisation (coronary artery bypass grafting (CABG) and percutaneous transluminal coronary angioplasty (PTCA)) at eight years follow‐up HR 0.96, 95% CI 0.86 to 1.07; 1 study, 14,641 participants; low quality of evidence (graphically presented in Analysis 1.5 ).

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 5 Self‐reported CABG/PTCA.
Self‐reported angina
There were no considerable effects of vitamin C supplementation on self‐reported angina symptoms HR 0.93, 95% CI 0.84 to 1.03, 1 study, 14,641 participants, low quality of evidence (graphically presented in Analysis 1.6 ).

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 6 Self‐reported angina.
Total stroke
There was no effect of vitamin C supplementation on total stroke (fatal and non‐fatal events) at eight years follow‐up HR 0.89 (95% CI 0.74 to 1.07); 1 study; 14,641 participants; low quality of evidence (graphically presented in Analysis 1.7 ).
Cardiovascular risk factors
Seven studies ( ASAP Study ; Cerna 1992 ; Fotherby 2000 ; Jacques 1995 ; Menne 1975 ; Mostafa 1989 ; Schindler 2003 ) looked at our secondary outcomes, but only three provided clear outcome data that could be used in meta‐analyses ( Cerna 1992 ; Jacques 1995 ; Schindler 2003 ). These three trials looked at the effect of vitamin C on cholesterol (total cholesterol, HDL‐cholesterol and LDL‐cholesterol), however, due to significant heterogeneity between the trials (I 2 = 93% for total cholesterol, I 2 = 95% for LDL‐cholesterol, I 2 = 66% for HDL‐cholesterol, I 2 = 47% for triglycerides), meta‐analyses were not performed. Data from these three trials were not pooled but have been plotted to only show the graphical display. For three studies ( Cerna 1992 ; Jacques 1995 ; Schindler 2003 ), we imputed standard deviation differences from baseline to follow‐up as these data were not available in the papers. To do this, we followed the guidelines in the Cochrane Handbook for Systematic Reviews of interventions for obtaining standard deviations from standard errors ( Higgins 2011 , chapter 7.3.3) and have used a correlation coefficient in these calculations of 0.5 as recommended by Follman ( Follman 1992 ). Results are described narratively and the authors reports of the remaining four trials without usable data are also described below.
Blood pressure
One trial reported data that could be used in a meta‐analysis ( Analysis 1.8 ; Analysis 1.9 ). This trial reported no effects of vitamin C supplementation on either systolic blood pressure (SBP) (mean difference (MD) ‐1.00 mmHg, 95% CI ‐4.94 to 2.94; 16 participants) or diastolic blood pressure (DBP) (MD ‐2.00 mmHg, 95% CI ‐6.82 to 2.82; 16 participants) ( Schindler 2003 ). However, this study was extremely small, at high risk of attrition bias so results should be treated with caution. Antihypertensive medication was also used by some participants, which may have impacted on the results obtained.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 8 Systolic blood pressure (change from baseline, mmHg).

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 9 Diastolic blood pressure (change from baseline, mmHg).
No control group data were provided for one trial so these were not usable in a meta‐analysis ( Mostafa 1989 ).
One cross‐over trial reported results over the whole trial period and not in phases and so we were unable to incorporate the results in the meta‐analysis ( Fotherby 2000 ). The authors reported that clinic blood pressure did not change between the placebo and vitamin C stages. Daytime ambulatory blood pressure showed a smaller decrease in SBP of 2 ± 5.2 mmHg in comparison to DBP 1 ± 4.7 mmHg for 40 participants ( Fotherby 2000 ).
For one study, blood pressure was reported at baseline but not at follow‐up ( ASAP Study ).
Lipid levels
For total cholesterol ( Analysis 1.10 ), one of the three trials ( Cerna 1992 ), showed that there was a reduction in total cholesterol with vitamin C supplementation (MD ‐1.17 mmol/L. 95% CI ‐1.60 to ‐0.74; 1 study; 140 participants). The other two trials showed no evidence of an effect of vitamin C supplementation on total cholesterol (MD 0.02 mmol/L. 95% CI ‐0.18 to 0.22; 1 study; 138 participants) ( Jacques 1995 ) and (MD 0.05 mmol/L. 95% CI ‐0.14 to 0.24; 1 study; 16 participants) ( Schindler 2003 ). The reduction in lipid levels seen in the Cerna 1992 study compared to others may be attributable to the high baseline levels of total cholesterol and LDL‐cholesterol.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 10 Total cholesterol (change from baseline, mmol/L).
For LDL‐cholesterol ( Analysis 1.11 ), one trial ( Cerna 1992 ), showed that there was a significant reduction in LDL‐cholesterol with vitamin C supplementation (MD ‐1.27 mmol/L. 95% CI ‐1.67 to ‐0.87; 136 participants). The other two trials showed no evidence of effect of vitamin C supplementation on LDL‐cholesterol (MD 0.00 mmol/L. 95% CI ‐0.15 to 0.15; 1 study; 138 participants) ( Jacques 1995 ) and (MD 0.16 mmol/L. 95% CI ‐0.02 to 0.33; 1 study; 16 participants) ( Schindler 2003 ).

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 11 LDL‐cholesterol (change from baseline, mmol/L).
For HDL‐cholesterol ( Analysis 1.12 ), two trials showed no effect of vitamin C supplementation (MD 0.03 mmol/L. 95% CI ‐0.09 to 0.15; 1 study; 136 participants Cerna 1992 , and MD 0.00 mmol/L. 95% CI ‐0.06 to 0.06; 1 study; 138 participants Jacques 1995 ), whilst the third showed a decrease in HDL with the intervention (MD ‐0.13 mmol/L. 95% CI ‐0.23 to ‐0.03; 1 study; 16 participants Schindler 2003 ). This very small study was however regarded at high risk of attrition bias ( Schindler 2003 ). Data were also provided for the ASAP Study ( ASAP Study ) for men and women reported separately at three years follow‐up. We were unable to combine these data in the meta‐analysis as the numbers randomised to each group were unclear in the report. The mean HDL cholesterol increased in three years more among men who received vitamin C supplement than in men who received placebo (P = 0.025). In women, vitamin C had no effect on serum HDL cholesterol at three years.

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 12 HDL‐cholesterol (change from baseline, mmol/L).
Three trials looked at the effect of vitamin C on triglycerides ( Fotherby 2000 ; Jacques 1995 ; Schindler 2003 ) and heterogeneity between studies was 47%. One trial showed no effect of vitamin C supplementation on triglyceride levels (MD 0.05 mmol/L, 95% CI ‐0.14 to 0.24; 138 participants) ( Jacques 1995 ), whilst the other trial showed an increase in triglyceride levels with the intervention (MD 0.23 mmol/L, 95% CI 0.07 to 0.38, 16 participants) ( Schindler 2003 ). This very small study was however regarded at high risk of attrition bias ( Schindler 2003 ) ( Analysis 1.13 ). Pooling these studies using a random effects model favoured the placebo group but this did not reach statistical significance (MD 0.15 mmol/L, 95% CI ‐0.02 to 0.32) ( Analysis 1.13 ).

Comparison 1 Vitamin C supplementation versus no intervention or placebo, Outcome 13 Triglycerides (change from baseline, mmol/L).
One cross‐over trial reported results over the whole trial period and not in phases and so we were unable to incorporate the results in the meta‐analysis ( Fotherby 2000 ). For the total group of 40 participants, there was no difference between placebo and vitamin C phases of the study for mean total cholesterol (6.2 ± 0.9 versus 6.2 ± 1.0 mmol/L; 40 participants), LDL cholesterol (3.6 ± 0.8 versus 3.5 ± 0.9 mmol/L) and HDL cholesterol (1.53 ± 0.35 versus 1.56 ± 0.36 mmol/L). The authors did however find that when they stratified by sex, there was an increase in HDL cholesterol with vitamin C in women by 0.08 ± 0.36 mmol/L, but not in men ( Fotherby 2000 ).
For one study, total cholesterol, LDL cholesterol and triglycerides are reported at baseline but not at follow‐up ( ASAP Study ).
Type 2 diabetes
None of the included studies reported the occurrence of type 2 diabetes as a major CVD risk factor.
Health‐related quality of life
None of the included studies reported validated health‐related quality of life measures.
The Physicians Health Study II examined a series of adverse effects of both vitamin C and vitamin E supplementation compared to placebo. No differences were seen in any of the following outcomes: bleeding (because vitamin E may potentially inhibit platelet function), gastrointestinal tract symptoms (peptic ulcer, constipation, diarrhoea, gastritis, and nausea), fatigue, drowsiness, skin discolouration or rashes and migraine ( The Physicians Health Study II ). Adverse effects were also reported in the ASAP Study . These were described as death, serious adverse event and adverse event with no further details given. No differences were seen between the vitamin C and placebo groups.
None of the included studies reported costs.
Subgroup analyses
There were insufficient trials (less than 10) to stratify results by high risk of CVD versus the general population, and by dose of vitamin C.
Sensitivity analyses
There were insufficient trials to conduct sensitivity analyses.
Summary of main results
We included eight trials (15,445 participants randomised) from the 5555 papers screened. The largest trial with 14,641 participants provided data on our primary outcomes, cardiovascular disease (CVD) clinical events ( The Physicians Health Study II ). One smaller trial reported all‐cause mortality as adverse events. Seven trials reported on CVD risk factors. Three of these trials provided data in a useable format for meta‐analyses ( Cerna 1992 ; Jacques 1995 ; Schindler 2003 ); the remaining four did not ( ASAP Study ; Fotherby 2000 ; Menne 1975 ; Mostafa 1989 ). We attempted to contact authors to provide missing details but were unsuccessful despite repeated attempts. Heterogeneity in the three trials precluded meta‐analysis and we provide a narrative synthesis. Three trials were regarded at high risk of bias for reporting bias ( Schindler 2003 ), or attrition bias ( ASAP Study ; Mostafa 1989 ); most of the risk of bias domains for the remaining trials were judged as unclear, with the exception of the largest trial where most domains were judged to be at low risk of bias ( The Physicians Health Study II ).
The composite endpoint major CVD events was not different between the vitamin C and placebo group in the Physicians Health Study II and similar results were obtained for all‐cause mortality, total myocardial infarction (MI) (fatal and non‐fatal) and total stroke (fatal and non‐fatal). The authors of this trial concluded that vitamin C supplementation does not reduce the risk of major CVD events over eight years of follow‐up and should not be recommended for use in this group of participants ‐ middle‐aged and older men ( The Physicians Health Study II ). Adverse events were reported in this study and the ASAP Study with no significant differences between the vitamin C and placebo groups.
There were variable and inconsistent effects of vitamin C supplementation on CVD risk factors (lipid levels and blood pressure). None of the trials reported our other secondary outcomes occurrence of type 2 diabetes as a major risk factor for CVD, health‐related quality of life and costs.
Overall completeness and applicability of evidence
One large trial dominated this review and reports on our primary outcomes ( The Physicians Health Study II ). Whilst the study is adequately powered, the findings are limited to middle‐aged and older male physicians from the USA. The participants had variable baseline CVD risk but no significant effect modification was found between vitamin C and baseline risk.
Seven trials reported on CVD risk factors (lipid levels and blood pressure) with inconsistent findings. There were limitations in the available data as only three trials provided data in a useable format for meta‐analyses, and for the remaining studies we were unable to obtain additional information from the study authors. The findings to date for these outcomes are inconclusive.
We have presented the overall quality of the evidence for each primary outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results . All of the studies included in this review were randomised controlled trials (RCTs). There was no serious risk of bias detected for study limitations, inconsistency and publication bias. Grey literature was not searched but a comprehensive search across major databases and reference lists of relevant studies was carried out, therefore we could not formally assess these domains and they were not downgraded. The evidence for major CVD event, total MI, total stroke, self‐reported angina and self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA) was of low quality. The outcomes were downgraded by one for indirectness (the populations were poorly applicable) and downgraded by one for imprecision (small magnitude of the number of included studies < five studies). The evidence for all‐cause mortality and CVD mortality was very low quality. The outcomes were downgraded by two levels for indirectness (the populations were poorly applicable, and the timeframe was insufficient), and downgraded by one level for imprecision (small magnitude of the number of included studies < five studies). Overall, inconsistency was difficult to evaluate because one trial was evaluated and therefore heterogeneity and the forest plot can not be evaluated ( Table 1 ).
Potential biases in the review process
Although OpenGrey was not screened due to limited resources, a comprehensive search across major databases for interventions involving vitamin C supplementation was carried out for this review. In addition, the reference lists of systematic reviews were screened and authors contacted for information when needed. All screening, inclusion and exclusion and data abstraction were carried out independently by two review authors.
Multivitamins and mineral preparations including vitamin C were excluded from this review because it would not be possible to disentangle the specific effects of vitamin C. Multifactorial interventions were excluded to avoid confounding. This did however limit the number of trials that were eligible for inclusion.
Agreements and disagreements with other studies or reviews
In terms of clinical events, only one trial was identified to examine the effects of vitamin C on CVD events for primary prevention ( The Physicians Health Study II ). These results are however restricted to middle‐aged and older male physicians in the USA. One further smaller trial reported all‐cause mortality as adverse events ( ASAP Study ), with only one event reported in each of the vitamin C and placebo groups. Other trials have looked at the effects of vitamin C supplementation in women, but for secondary rather than primary prevention of CVD ( Cook 2007 ). There were similar findings in terms of major CVD events. A recent systematic review of the effects of individual vitamins and minerals, and multivitamins, on clinical endpoints has been conducted for the US Task Force for Preventative Services. The authors found two trials of vitamin C supplementation reporting clinical endpoints relevant for CVD prevention, where no effect of the intervention was found ( Fortmann 2013 ). These two trials are the same trials reported in the current review ( ASAP Study ; The Physicians Health Study II ).
We were unable to determine the effectiveness of vitamin C supplementation on major CVD risk factors (lipid levels and blood pressure) with the trials included in the current version of the review due to missing information, heterogeneity of participants, dose of supplementation, duration of intervention and follow‐up, and methodological quality.
Previous systematic reviews of RCTs have examined the effects of vitamin C supplementation alone and in combination with other agents (such as vitamin E, magnesium, zinc, selenium) in trials between two and 26 weeks duration ( Juraschek 2012 ). The authors concluded that vitamin C supplementation reduced systolic and diastolic blood pressure in short‐term trials. Another review of RCTs concluded that supplementation with at least 500 mg/day of vitamin C, for a minimum of four weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, the lack of quality assessment and analysis of statistical heterogeneity, and the small sample sizes of the included trials, limit the reliability of the authors' conclusions ( McRae 2008 ).
Implications for practice
Currently, there is no evidence to suggest that vitamin C supplementation reduces the risk of cardiovascular disease (CVD). However, the results of this review should be interpreted with caution as the evidence was rated as low quality mainly for indirectness (downgraded by one level) and imprecision (downgraded by one level) for major CVD event, total myocardial infarction (MI), total stroke, self‐reported angina and self‐reported coronary artery bypass grafting (CABG)/percutaneous transluminal coronary angioplasty (PTCA). The evidence was rated as very low quality mainly for very serious indirectness (downgraded by two levels) and imprecision (downgraded by one level) for all‐cause morality and CVD mortality. Inconsistency of the evidence was difficult to evaluate because only one trial was evaluated and therefore heterogeneity and the forest plot can not be evaluated.
Implications for research
Whilst a large adequately powered RCT reporting our primary outcomes clearly showed no effect of vitamin C supplementation on major CVD endpoints, these data are limited to middle‐aged and older male physicians from the USA. Future research should report the effect of vitamin C supplements on type 2 diabetes and use validated measures for quality of life. Future research should report economic data, costs of vitamin C supplements should be measured and reported as non of the trials reported costs. Future research and reports should provide adequate and transparent methodological details such as sequence generation, allocation concealment, blinding of outcomes assessors and report all outcomes. Higher‐quality trials are required as the current evidence is of low and very low methodological quality. There is limited evidence to date on the effects of vitamin C supplements on CVD risk factors.
Acknowledgements
We are grateful to Nicole Martin for conducting the searches for this review and for some duplicate screening. We are also grateful Cornelia Kunz and Mariana Dyakova at Warwick Medical School for their help with German and Russian translations.
Appendix 1. Search strategies
Cochrane library.
#1 MeSH descriptor: [Ascorbic Acid] this term only #2 ascorb* #3 vit* near/6 c #4 magnorbin #5 hybrin #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Cardiovascular Diseases] explode all trees #8 cardio* #9 cardia* #10 heart* #11 coronary* #12 angina* #13 ventric* #14 myocard* #15 pericard* #16 isch?em* #17 emboli* #18 arrhythmi* #19 thrombo* #20 atrial next fibrillat* #21 tachycardi* #22 endocardi* #23 (sick next sinus) #24 MeSH descriptor: [Stroke] explode all trees #25 (stroke or stokes) #26 cerebrovasc* #27 cerebral next vascular #28 apoplexy #29 (brain near/2 accident*) #30 ((brain* or cerebral or lacunar) near/2 infarct*) #31 MeSH descriptor: [Hypertension] explode all trees #32 hypertensi* #33 (peripheral next arter* next disease*) #34 ((high or increased or elevated) near/2 blood pressure) #35 MeSH descriptor: [Hyperlipidemias] explode all trees #36 hyperlipid* #37 hyperlip?emia* #38 hypercholesterol* #39 hypercholester?emia* #40 hyperlipoprotein?emia* #41 hypertriglycerid?emia* #42 MeSH descriptor: [Arteriosclerosis] explode all trees #43 MeSH descriptor: [Cholesterol] explode all trees #44 cholesterol #45 "coronary risk factor*" #46 MeSH descriptor: [Blood Pressure] this term only #47 "blood pressure" #48 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 #49 #6 and #48
1. Ascorbic Acid/ 2. ascorb*.tw. 3. (vit* adj6 c).tw. 4. magnorbin.tw. 5. hybrin.tw. 6. or/1‐5 7. exp Cardiovascular Diseases/ 8. cardio*.tw. 9. cardia*.tw. 10. heart*.tw. 11. coronary*.tw. 12. angina*.tw. 13. ventric*.tw. 14. myocard*.tw. 15. pericard*.tw. 16. isch?em*.tw. 17. emboli*.tw. 18. arrhythmi*.tw. 19. thrombo*.tw. 20. atrial fibrillat*.tw. 21. tachycardi*.tw. 22. endocardi*.tw. 23. (sick adj sinus).tw. 24. exp Stroke/ 25. (stroke or stokes).tw. 26. cerebrovasc*.tw. 27. cerebral vascular.tw. 28. apoplexy.tw. 29. (brain adj2 accident*).tw. 30. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 31. exp Hypertension/ 32. hypertensi*.tw. 33. peripheral arter* disease*.tw. 34. ((high or increased or elevated) adj2 blood pressure).tw. 35. exp Hyperlipidemias/ 36. hyperlipid*.tw. 37. hyperlip?emia*.tw. 38. hypercholesterol*.tw. 39. hypercholester?emia*.tw. 40. hyperlipoprotein?emia*.tw. 41. hypertriglycerid?emia*.tw. 42. exp Arteriosclerosis/ 43. exp Cholesterol/ 44. cholesterol.tw. 45. "coronary risk factor* ".tw. 46. Blood Pressure/ 47. blood pressure.tw. 48. or/7‐47 49. randomized controlled trial.pt. 50. controlled clinical trial.pt. 51. randomized.ab. 52. placebo.ab. 53. drug therapy.fs. 54. randomly.ab. 55. trial.ab. 56. groups.ab. 57. 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 58. exp animals/ not humans.sh. 59. 57 not 58 60. 6 and 48 and 59
1. ascorbic acid/ 2. ascorb*.tw. 3. (vit* adj6 c).tw. 4. magnorbin.tw. 5. hybrin.tw. 6. or/1‐5 7. exp cardiovascular disease/ 8. cardio*.tw. 9. cardia*.tw. 10. heart*.tw. 11. coronary*.tw. 12. angina*.tw. 13. ventric*.tw. 14. myocard*.tw. 15. pericard*.tw. 16. isch?em*.tw. 17. emboli*.tw. 18. arrhythmi*.tw. 19. thrombo*.tw. 20. atrial fibrillat*.tw. 21. tachycardi*.tw. 22. endocardi*.tw. 23. (sick adj sinus).tw. 24. exp cerebrovascular disease/ 25. (stroke or stokes).tw. 26. cerebrovasc*.tw. 27. cerebral vascular.tw. 28. apoplexy.tw. 29. (brain adj2 accident*).tw. 30. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 31. exp hypertension/ 32. hypertensi*.tw. 33. peripheral arter* disease*.tw. 34. ((high or increased or elevated) adj2 blood pressure).tw. 35. exp hyperlipidemia/ 36. hyperlipid*.tw. 37. hyperlip?emia*.tw. 38. hypercholesterol*.tw. 39. hypercholester?emia*.tw. 40. hyperlipoprotein?emia*.tw. 41. hypertriglycerid?emia*.tw. 42. exp Arteriosclerosis/ 43. exp Cholesterol/ 44. cholesterol.tw. 45. "coronary risk factor*".tw. 46. Blood Pressure/ 47. blood pressure.tw. 48. or/7‐47 49. 6 and 48 50. random$.tw. 51. factorial$.tw. 52. crossover$.tw. 53. cross over$.tw. 54. cross‐over$.tw. 55. placebo$.tw. 56. (doubl$ adj blind$).tw. 57. (singl$ adj blind$).tw. 58. assign$.tw. 59. allocat$.tw. 60. volunteer$.tw. 61. crossover procedure/ 62. double blind procedure/ 63. randomized controlled trial/ 64. single blind procedure/ 65. 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 66. (animal/ or nonhuman/) not human/ 67. 65 not 66 68. 49 and 67 69. limit 68 to embase
Web of Science
# 12 #11 AND #10 # 11 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) # 10 #9 AND #8 # 9 TS=(ascorb* or (vit* near/6 c) or magnorbin or hybrin) # 8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 7 TS=(hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) # 6 TS=("high blood pressure") # 5 TS=(hypertensi* OR "peripheral arter* disease*") # 4 TS=(stroke OR stokes OR cerebrovasc* OR cerebral OR apoplexy OR (brain SAME accident*) OR (brain SAME infarct*)) # 3 TS=("atrial fibrillat*" OR tachycardi* OR endocardi*) # 2 TS=(pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) # 1 TS=(cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*)
Appendix 2. Checklist to aid consistency and reproducibility of GRADE assessments
Data and analyses, comparison 1, characteristics of studies, characteristics of included studies [ordered by study id].
BMI: body mass index CVD: cardiovascular disease DBP: diastolic blood pressure HDL: high‐density lipoprotein ITT: intention‐to‐treat LDL: low‐density lipoprotein MI: myocardial infarction RCT: randomised controlled trial RDA: recommended daily allowance SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Differences between protocol and review.
Citation searches for key articles and OpenGrey searches were not conducted due to limited resources. Authors were contacted where necessary for additional information.
Contributions of authors
Review authors LA, NF, RW, LH, KR and OG screened titles and abstracts and assessed studies for formal inclusion and exclusion. LA, NF and RW abstracted data and assessed methodological rigour. NF wrote the first draft of the results and KR updated this section and wrote the other sections of the review. LA conducted the GRADE analysis, which was checked by KR and updated sections of the review. SS subject expertise, and assisted with the literature and writing the background for the review and assisted with interpretation of findings.
Sources of support
Internal sources.
- Warwick Medical School, University of Warwick, UK.
External sources
- NIHR Cochrane Programme Grant, UK.
- Karen Rees is also supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
None known.
References to studies included in this review
Asap study {published data only}.
- Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala‐Sarataho E, et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3‐year progression of carotid atherosclerosis . Journal of Internal Medicine 2000; 248 :377‐86. [ PubMed ] [ Google Scholar ]
- Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala‐Sarataho E, Voutilainen S, Rissanen TH, et al. Six‐year effect of combined vitamin C and E supplementation on atherosclerotic progression. The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study . Circulation 2003; 107 :947‐53. [ PubMed ] [ Google Scholar ]
Cerna 1992 {published data only}
- Cerna O, Ramacsay L, Ginter E. Plasma lipids, lipoproteins and atherogenic index in men and women administered vitamin C . Cor et Vasa 1992; 34 :246‐54. [ PubMed ] [ Google Scholar ]
Fotherby 2000 {published data only}
- Fotherby MD, Williams J, Ferns G. Effect of Vitamin C on Ambulatory Blood Pressure (Bp) in Older Hypertensive and Normotensive Persons . Age and Ageing . 1998; Vol. 27:5‐a.
- Fotherby MD, Williams JC, Forster LA, Craner P, Ferns GA. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons . Journal of Hypertension 2000; 18 :411‐5. [ PubMed ] [ Google Scholar ]
- Nicolaides A, Cesarone M R, Belcaro G, Sanctis M, Incandela L, Griffin M. Effect of vitamin c on atherosclerosis progression and cardiovascular events in a ten‐year randomized study (San Valentino) . Journal of the American College of Cardiology . 2002; Vol. 39:265.
Jacques 1995 {published data only}
- Jacques PF, Sulsky SI, Perrone GE, Jenner J, Schaefer EJ. Effect of vitamin C supplementation on lipoprotein cholesterol, apolipoprotein, and triglyceride concentrations . Annals of Epidemiology 1995; 5 :52‐9. [ PubMed ] [ Google Scholar ]
Menne 1975 {published data only}
- Menne IV, Grey PC, Kotze JP, Sommers DK, Brown JM, Spies JH. Ascorbic acid and blood lipid and uric acid levels of students . South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1975; 49 :2225‐8. [ PubMed ] [ Google Scholar ]
Mostafa 1989 {published data only}
- Mostafa S el‐D, Garner DD, Garrett L, Whaley RF, el‐Sekate M, Kiker M. Beneficial effects of vitamin C on risk factors of cardiovascular diseases . Journal of the Egyptian Public Health Association 1989; 64 ( 1‐2 ):123‐33. [ PubMed ] [ Google Scholar ]
Schindler 2003 {published data only}
- Schindler TH, Nitzsche EU, Munzel T, Olschewski M, Brink I, Jeserich M, et al. Coronary vasoregulation in patients with various risk factors in response to cold pressor testing: contrasting myocardial blood flow responses to short‐ and long‐term vitamin C administration . Journal of the American College of Cardiology 2003; 42 :814‐22. [ PubMed ] [ Google Scholar ]
The Physicians Health Study II {published data only}
- Christen WG, Gaziano M, Hennekens CH, for the steering committee of the Physicians Health Study II. Design of Physicians’ Health Study II—A randomized trial of beta‐carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials . Annals of Epidemiology 2000; 10 :125–34. [ PubMed ] [ Google Scholar ]
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial . JAMA 2008; 300 ( 18 ):2123‐33. [ PMC free article ] [ PubMed ] [ Google Scholar ]
References to studies excluded from this review
Bassuk 2004 {published data only}.
- Bassuk SS, Albert CM, Cook NR, Zaharris E, MacFadyen JG, Danielson E, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants . Journal of Women's Health 2004; 13 :99‐117. [ PubMed ] [ Google Scholar ]
Boushehri 2012 {published data only}
- Boushehri SN, Yusof RM, Taib MNM, Mirzaei K, Yazdekhasti N, Akbarzadeh S. Effect of vitamin supplementation on serum oxidised low‐density lipoprotein levels in male subjects with cardiovascular disease risk factors . Iranian Journal of Basic Medical Sciences 2012; 15 :958‐64. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Bunpo 2015 {published data only}
- Bunpo P, Anthony TG. Ascorbic acid supplementation does not alter oxidative stress markers in healthy volunteers engaged in a supervised exercise program . Applied Physiology, Nutrition, and Metabolism 2015; 41 ( 2 ):175‐80. [ PubMed ] [ Google Scholar ]
Calzada 1995 {published data only}
- Calzada C, Bruckdorfer KR, Rice‐Evans CA. The influence of antioxidant nutrients on platelet function in healthy volunteers . Atherosclerosis 1997; 128 :97‐105. [ PubMed ] [ Google Scholar ]
Dobson 1984 {published data only}
- Dobson HM, Muir MM, Hume R. The effect of ascorbic acid on the seasonal variations in serum cholesterol levels . Scottish Medical Journal 1984; 29 :176‐82. [ PubMed ] [ Google Scholar ]
Ghosh 1994 {published data only}
- Ghosh SK, Ekpo EB, Shah IU, Girling AJ, Jenkins C, Sinclair AJ. A double‐blind, placebo‐controlled parallel trial of vitamin C treatment in elderly patients with hypertension . Gerontology 1994; 40 :268‐72. [ PubMed ] [ Google Scholar ]
Jayachandran 2000 {published data only}
- Jayachandran M, Arivazhagan P, Panneerselvam C. Age‐associated plasma lipids, lipid peroxidation, and antioxidant systems in relation to vitamin C supplementation in humans . Journal of Anti‐Aging Medicine 2000; 3 :437‐45. [ Google Scholar ]
Osilesi 1991 {published data only}
- Osilesi O, Trout DL, Ogunwole JO, Glover EE. Blood pressure and plasma lipids during ascorbic acid supplementation in borderline hypertensive and normotensive adults . Nutrition Research 1991; 11 :405‐12. [ Google Scholar ]
Schutte 2004 {published data only}
- Schutte AE, Huisman HW, Oosthuizen W, Rooyen JM, Jerling JC. Cardiovascular effects of oral Supplementation of vitamin C, E and folic acid in young healthy males . International Journal for Vitamin and Nutrition Research 2004; 74 :285‐93. [ PubMed ] [ Google Scholar ]
Shidfar 2003 {published data only}
- Shidfar F, Keshavarz A, Jallali M, Miri R, Eshraghian M. Comparison of the effects of simultaneous administration of vitamin C and omega‐3 fatty acids on lipoproteins, apo A‐I, apo B, and malondialdehyde in hyperlipidemic patients . International Journal for Vitamin and Nutrition Research 2003; 73 :163‐70. [ PubMed ] [ Google Scholar ]
References to studies awaiting assessment
Nicolaides 2002 {published data only}.
- Nicolaides A, Cesarone MR, Belcaro G, Sanctis M, Incandela L, Griffin M. Effect of vitamin C on atherosclerosis progression and cardiovascular events in a ten‐year randomised study (San Valentino) . Journal of the American College of Cardiology 2002; 39 :265A. [ Google Scholar ]
Additional references
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez A. The Burden of Disease and Injury in Australia 2003 . Vol. Cat. no. PHE 82 , Canberra: Australian Institute of Health and Welfare, May 2007. [ Google Scholar ]
Bjelakovic 2007
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta‐analysis . JAMA 2007; 297 :842–57. [ PubMed ] [ Google Scholar ]
British Heart Foundation 2012
- British Heart Foundation 2012. Cardiovascular Disease . http://www.bhf.org.uk/heart‐health/conditions/cardiovascular‐disease.aspx (Accessed September 2013).
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study . Archives of Internal Medicine 2007; 167 :1610–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]

Follman 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response . Journal of Clinical Epidemiology 1992; 45 :769‐73. [ PubMed ] [ Google Scholar ]
Fortmann 2013
- Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O'Connor E, et al. Vitamin, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the U.S. Preventive Services Task Force . Rockville (MD): Agency for Healthcare Research and Quality (US) Nov 2013. [ PubMed ]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma . Proceedings of the National Academy of Sciences of the United States of America 1989; 86 :6377–81. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea . American Journal of Respiratory and Critical Care Medicine 2006; 173 :897–901. [ PubMed ] [ Google Scholar ]
- He F, Burnier M, MacGregor G. Nutrition in cardiovascular disease: salt in hypertension and heart failure . European Heart Journal 2011; 32 :3073‐80. [ PubMed ] [ Google Scholar ]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of interventions Version 5.1 [updated March 2011] . The Cochrane Library, The Cochrane Collaboration (2011). Available from www.cochrane‐handbook.org. [ Google Scholar ]
- Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric‐oxide synthase activity by increasing intracellular tetrahydrobiopterin . The Journal of Biological Chemistry 2000; 275 :17399–406. [ PubMed ] [ Google Scholar ]
Juraschek 2012
- Juraschek SP, Guallar E, Appel LJ, Miller ER. Effects of vitamin C supplementation on blood pressure: a meta‐analysis of randomized controlled trials . American Journal of Clinical Nutrition 2012; 95 ( 5 ):1079. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies . Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011) . The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org). [ Google Scholar ]
Levine 2011
- Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration‐function approach yields pharmacology and therapeutic discoveries . Advances in Nutrition 2011; 2 :78‐88. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030 . PLoS Medicine 2006; 3 ( 11 ):e442. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium . Antioxidants and Redox Signaling 2013; 19 ( 17 ):2068‐83. [ PMC free article ] [ PubMed ] [ Google Scholar ]
McCarron 1984
- McCarron DA, Morris CD, Henry HJ, Stanton JL. Blood pressure and nutrient intake in the United States . Science 1984; 224 :1392–8. [ PubMed ] [ Google Scholar ]
McNulty 2007
- McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease . Journal of Applied Physiology 2007; 102 :2040–5. [ PubMed ] [ Google Scholar ]
McRae 2006a
- McRae M P. Is vitamin C an effective antihypertensive supplement: a review and analysis of the literature . Journal of Chiropractic Medicine 2006; 5 ( 2 ):60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
McRae 2006b
- McRae M P. The efficacy of vitamin C supplementation on reducing total serum cholesterol in human subjects: a review and analysis of 51 experimental trials . Journal of Chiropractic Medicine 2006; 5 ( 1 ):2. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McRae MP. Vitamin C supplementation lowers serum low‐density lipoprotein cholesterol and triglycerides: a meta‐analysis of 13 randomised controlled trials . Journal of Chiropractic Medicine 2008; 7 ( 2 ):48. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation . Systemic Reviews 2014; 3 :83. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, et al. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects . American Journal of Clinical nutrition 1993; 57 :213–7. [ PubMed ] [ Google Scholar ]
- Ness AR, Chee D, Elliott P. Vitamin C and blood pressure: an overview . Journal of Human Hypertension 1997; 11 ( 6 ):343. [ PubMed ] [ Google Scholar ]
- NHS, 2012. Atherosclerosis . http://www.nhs.uk/conditions/Atherosclerosis/Pages/Introduction.aspx#commentCountLink Accessed September 2013.
NHS choices 2015
- Vitamin C . http://www.nhs.uk/Conditions/vitamins‐minerals/Pages/Vitamin‐C.aspx .
Padayatty 2010
- Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects . PLoS One 2010; 5 ( 7 ):e11414. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Rayment 2003
- Rayment SJ, Shaw J, Woollard KJ, Lunec J, Griffiths HR. Vitamin C supplementation in normal subjects reduces constitutive ICAM‐1 expression . Biochemical and Biophysical Research Communications 2003; 308 ( 2 ):339‐45. [ PubMed ] [ Google Scholar ]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) . Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Silvestro 2002
- Silvestro A, Scopacasa F, Oliva G, Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication . Atherosclerosis 2002; 165 :277–83. [ PubMed ] [ Google Scholar ]
- Simon JA, Hudes ES. Relation of serum ascorbic acid to serum lipids and lipoproteins in US adults . Journal of the American College of Nutrition 1998; 17 :250–5. [ PubMed ] [ Google Scholar ]
Siri‐Tarino 2010
- Siri‐Tarino P, Sun Q, Hu F, Krauss R. Saturated fat, carbohydrate, and cardiovascular disease . American Journal of Clinical Nutrition 2010; 91 :502‐9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Solzbach 1997
- Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients . Circulation 1997; 96 :1513–9. [ PubMed ] [ Google Scholar ]
Steinberg 1989
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modification of low‐ density lipoprotein that increases its atherogenicity . New England Journal of Medicine 1989; 320 :915‐924. [ PubMed ] [ Google Scholar ]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials . BMJ 2011; 343 :d4002. [ PubMed ] [ Google Scholar ]
- WHO Study Group. Diet, nutrition and the prevention of chronic diseases . World Health Organization, Geneva 2003; Vol. Report Series 916. [ PubMed ]
- WHO. Guidelines on food fortification with micronutrients . The World Health Organization, 2006. [ Google Scholar ]
- WHO. Global status report on non communicable diseases 2010 . Geneva: The World Health Organization, 2011. [ Google Scholar ]
- World Health Organization. Cardiovascular diseases (CVDs) . Factsheet Number 317 Updated March 2013.
Woollard 2002
- Woollard KJ, Loryman CJ, Meredith E, Bevan R, Shaw JA, Lunec J, et al. Effects of oral vitamin C on monocyte: endothelial cell adhesion in healthy subjects . Biochemical and Biophysical Research Communications 2002; 294 :1161‐8. [ PubMed ] [ Google Scholar ]
References to other published versions of this review
Flowers 2014.
- Flowers N, Wheelhouse R, Stranges S, Rees K. Vitamin C supplementation for the primary prevention of cardiovascular disease . Cochrane Database of Systematic Reviews 2014, Issue 5 . [DOI: 10.1002/14651858.CD011114] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Vitamin C is a water-soluble vitamin important for healthy skin, teeth, bones, and blood vessels. It is found especially in citrus fruits, tomatoes, potatoes, and green leafy vegetables. It is also called ascorbic acid and its chemical formula is C 6 H 8 O 6 . A deficiency of vitamin C in the diet causes scurvy. Vitamin C is an essential nutrient involved in the repair of tissue.
Vitamin C (Ascorbic acid) also promotes the healing of cuts, abrasions and wounds; helps fight infections; inhibits conversion of irritants in smog, tobacco smoke, and certain foods into cancer-causing substances; appears to lessen the risk of developing high blood pressure and heart disease; helps regulate cholesterol levels.
It was discovered in 1912, isolated in 1928, and first made in 1933. It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system. Vitamin C is available as a generic medication and over the counter. In some countries, ascorbic acid may be added to foods such as breakfast cereal.
Sources and Working of Vitamin C
Vitamin C is found in a wide variety of fruit and vegetables. Good sources are including:
- Oranges and orange juice
- Red and green peppers
- Strawberries
- Blackcurrants
- Brussels sprouts
Vitamin C helps to repair and regenerate tissues, protect against heart disease, aid in the absorption of iron, prevent scurvy, and decrease total and LDL (“bad”) cholesterol and triglycerides. Research indicates that vitamin C may help protect against a variety of cancers by combating free radicals, and helping neutralize the effects of nitrites, preservatives found in some packaged foods that may raise the risk of certain forms of cancer. Supplemental vitamin C may also lessen the duration and symptoms of a common cold, help delay or prevent cataracts, and support healthy immune function.
How Much Vitamin C We Need?
The safe upper limit for vitamin C is 2,000 milligrams a day, and there is a great track record with strong evidence that taking 500 milligrams daily is safe. While a cup of orange juice or a half-cup of red pepper would be enough to meet our RDA for Vitamin C, here are all the foods and beverages we had need to consume to reach 500 milligrams (mg):
- Cantaloupe, 1 cup (8 ounces): 59mg
- Orange juice, 1 cup: 97mg
- Broccoli, cooked, 1 cup: 74mg
- Red cabbage, 1/2 cup: 40mg
- Green pepper, 1/2 cup, 60mg
- Red pepper, 1/2 cup, 95mg
- Kiwi, 1 medium: 70mg
- Tomato juice, 1 cup: 45mg.
Benefits and Side Effects of Vitamin C
Vitamin C is often supplemented to reduce the symptoms of the common cold. It is unable to reduce the frequency of colds in a healthy population. Supplemental vitamin C is able to reduce the duration of a cold by 8-14% in any population, when it is taken as a daily preventative measure, or at the beginning of a cold.
Vitamin C is one of many antioxidants that can protect against damage caused by harmful molecules called free radicals, as well as toxic chemicals and pollutants like cigarette smoke. Free radicals can build up and contribute to the development of health conditions such as cancer, heart disease, and arthritis. Vitamin C is easily absorbed both in food and in pill form, and it can enhance the absorption of iron when the two are eaten together.
Vitamin C might raise blood sugar. In older women with diabetes, vitamin C in amounts greater than 300 mg per day increases the risk of death from heart disease. Taking less than 1,000mg of vitamin C supplements a day is unlikely to cause any harm.
In some people, vitamin C might cause nausea, vomiting, heartburn, stomach cramps, headache, and other side effects. The chance of getting these side effects increases the more vitamin C people take. Amounts higher than 2000 mg daily are POSSIBLY UNSAFE and may cause a lot of side effects, including kidney stones and severe diarrhea. In people who have had a kidney stone, amounts greater than 1000 mg daily greatly increase the risk of kidney stone recurrence.
Vitamin C content of a food sample such as fruit juice can be calculated by measuring the volume of the sample required to decolorize a solution of dichlorophenolindophenol (DCPIP) and then calibrating the results by comparison with a known concentration of vitamin C.
Reference: drweil.com , nhs.uk , examine.com , webmd.com , dictionary.com , wikipedia .
Strep Throat Symptoms
Cardiac psychology, environmental disease, bariatric surgery, there have been more reports of sharks in cape cod bay diving to take stripers strung on fishing lines, bounded rationality concept, dhaka stock exchange, treatment strategies to prevent kernicterus in neonatal jaundice, diagnosis, treatment and complications of hyperparathyroidism, multiplying fractions, latest post, electromagnetic forming (emf), magnetic bearing, retention ponds can substantially reduce tire particle pollution, asian monsoon transports ozone-depleting chemicals to the stratosphere, persian plateau revealed as critical hub for early human migration out of africa, forward converter.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
Take our Science Teaching Survey (15 minutes) and shape the future of education! Your insights matter.

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Take our Science Teaching Survey (15 mins) and shape the future of education!
Contemporary Chemistry
- 2 The chemistry behind fireworks
- 3 Crystal chemistry
- 4 Hydrogen fuel cells: the future of transport?
- 5 Investigating catalysts and what sank the Kursk
- 6 Shampoo and the chemistry of hair care
- 7 Lipid chemistry and dietary fats
- 8 Vitamins and the chemistry behind them
- 9 Star chemistry and molecules in outer space
- 10 Cleaning chemistry: soaps and detergents
- 11 Atoms and nanochemistry
Vitamins and the chemistry behind them
- No comments
From practical experiments to a directed activity related to text (DART), try these activities for 11–16 year olds to investigate the chemistry of vitamins
In this series of activities, students learn about a range of chemical ideas while investigating vitamins and vitamin supplements. Designed for 11–16 year olds, the resources give students the opportunity to conduct practical experiments, evaluate evidence for claims made about vitamins and engage with a variety of scientific and historical contexts. Stimulate your students to explore:
- The link between vitamin C and scurvy
- The structure and properties of vitamin C and how to test for it
- How vitamins were discovered
- Evidence for vitamin C preventing colds
- Whether vitamin supplements are effective
Each activity includes instructions for students, as well as editable worksheets and resources available for download.
1. Vitamin C and scurvy
In this directed activity related to text (DART), students read about the effects of scurvy, its historical prevalence among sailors and the discovery of a link between scurvy and vitamin C. The core of the activity is an extract from James Lind’s ’Of the Prevention of the Scurvy’, in which the Royal Navy surgeon describes an experiment conducted in 1747 to compare treatments for the disease.
After reading both texts, students answer a series of questions to check and consolidate their understanding, before considering how Lind could have improved his experiment and whether his conclusion was correct.
Download the resources
‘vitamin c and scurvy’ worksheet.
PDF | Editable Word document
2. Testing for vitamin C
Students conduct a practical experiment to compare the amounts of vitamin C in different fruit juices using a simple test with iodine solution. They then answer questions to reflect on what they have found out and compare their results with the labels on the fruit juice packaging.
The ‘Testing for vitamin C’ worksheet includes extension questions which relate the experiment to the DART, ’Vitamin C and scurvy’, as well as suggestions for further investigations to find out more about vitamin C.
An additional handout, ‘Did you know about vitamin C?’, provides further information about the chemical structure of vitamin C and how it used by the body.
‘Testing for vitamin C’ worksheet
‘did you know about vitamin c’ handout, plan a lesson around this activity.
Try this activity as part of a complete lesson plan for 14–16 year olds, measuring the amount of vitamin C in different fruit juices using a simple titration.
3. Catching a cold?
In this activity, students investigate the familiar claim that vitamin C helps to prevent or treat colds. They read about the work of Linus Pauling during the 1970s and evaluate the data he used to support this claim, identifying weaknesses in the original study and assessing how these might impact the conclusions we can draw from it.
An additional handout, ‘Did you know about Linus Pauling?’, provides background information about Pauling’s life and work.
‘Catching a cold?’ worksheet
‘did you know about linus pauling’ handout.
Discover a complete lesson plan for 14–16 year olds drawing on this activity to examine the evidence behind vitamins and vitamin supplements .
4. A cold survey
Students carry out a survey to determine whether vitamin C might help prevent colds. After answering the survey for themselves, students collate responses from the rest of the class, as well as responses collected from family and friends if there is sufficient time. They then work through a series of questions to analyse their results, identifying any patterns and drawing conclusions based on the available evidence.
‘A cold survey’ worksheet
5. are vitamins a waste of money.
Students work in groups to consider a range of views on vitamins pills and supplements. They rank four statements according to how strongly they agree with them, and provide reasons for their rankings, before presenting their decisions to the rest of the class. Together, students agree an answer to the question, ‘Are vitamins a waste of money?’, giving evidence in support.
An additional handout, ‘Did you know about Casimir Funk?’, introduces students to the scientist credited with the discovery of vitamins.
‘The pill thrill: are vitamins a waste of money?’ worksheet
‘did you know about casimir funk’ handout.
Use this activity as part of a complete lesson plan for 14–16 year olds, exploring whether vitamin pills work .
6. Key words
This handout provides information about terms relating to vitamins and the contexts used for these activities. Key words include ‘clinical trial’, ‘deficiency disease’, ‘double blind’, ‘placebo’, ‘RDI or RDA’ and ‘vitamins’.
‘Key words’ handout
Vitamin c and scurvy worksheet, testing for vitamin c worksheet, did you know about vitamin c handout, catching a cold worksheet, did you know about linus pauling handout, a cold survey worksheet, the pill thrill: are vitamins a waste of money worksheet, did you know about casimir funk handout, key words handout, additional information.
This activity was originally part of the Contemporary Chemistry website, compiled and published in 2004 with V. Kind’s Contemporary chemistry for schools and colleges .

The chemistry behind fireworks

Crystal chemistry

Hydrogen fuel cells: the future of transport?

Investigating catalysts and what sank the Kursk

Shampoo and the chemistry of hair care

Lipid chemistry and dietary fats

Star chemistry and molecules in outer space

Cleaning chemistry: soaps and detergents

Atoms and nanochemistry
- 11-14 years
- 14-16 years
- Practical experiments
- Applying scientific method
- Biological chemistry
Related articles

Why fermented foods are good for your gut – and your teaching
2024-04-15T05:30:00Z By Emma Davies
From kimchi to kefir, tuck into the complex chemistry of fermentation and its health potential

Crime-busting chemical analysis
2024-02-26T05:00:00Z By Kit Chapman
From dog detectives to AI, discover the cutting-edge advances in forensic science

Demonstrate concentration and density with a transition metal colloid cell
2024-02-19T10:06:00Z By Declan Fleming
Boost 11–14 learners’ understanding of diffusion and transition metal chemistry
No comments yet
Only registered users can comment on this article., more from resources.

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity

Metallic bonding | Structure strip | 14–16
By Kristy Turner
Describe the metallic bonding model and explain how this leads to particular properties in metals, with this scaffolded writing activity

Ionic bonding | Structure strip | 14–16
Understand the models and diagrams used to represent ionic bonding and their limitations, with this scaffolded writing activity
- Contributors
- Email alerts
Site powered by Webvision Cloud
- All Wellness
- All Skin Care
- Moisturizers
- Mineral Sunscreens
- Sunscreens for Kids
- Sunscreens for Dark Skin
- SPF Lip Balms
- Under Eye Patches
- All Hair Care
- Purple Shampoos
- Thinning Hair
- Head Shavers
- Hair Dryers
- All Oral Care
- Electric Toothbrushes
- Toothpastes
- Mouthwashes
- Water Flossers
- Meal Kit Delivery
- Gluten-Free Meal Kit Delivery
- Disposable Face Masks
- Air Purifiers
- Eco-Friendly Laundry Detergents
- Natural Deodorants
- Period Underwear
- All Fitness
- Exercise Bikes
- Walking Shoes
- Fitness Trackers
- Reusable Water Bottles
- Blackout Curtains
- Sound Machines
- Home & Kitchen
- All Home & Kitchen
- Kitchen Appliances & Tools
- All Kitchen Appliances & Tools
- Coffee Makers
- Kitchen Gadgets
- Small Home Appliances
- All Small Home Appliances
- Air Conditioners
- Space Heaters
- Humidifiers
- Bedding & Bath
- All Bedding & Bath
- Bath Towels
- Silk Pillowcases
- Duvet Inserts
- Office Chairs
- Standing Desks
- Desk Organizers
- Seat Cushions
- Under Desk Ellipticals
- All Outdoor
- Raised Garden Boxes
- Garden Hoses
- Beach Towels
- Solar Pool Covers
- Grilling Accessories
- Electronics
- All Electronics
- Wifi Routers
- Gaming Consoles
- Streaming Devices
- Instant Cameras
- Handheld Gaming Consoles
- 3D Printers
- All Headphones
- Noise Canceling
- Wireless Earbuds
- Smart Gadgets
- All Smart Gadgets
- Smart Watches
- Smart Bulbs
- Garage Door Openers
- All Computers
- Gaming Laptops
- Laptops for College Students
- Computer Monitors
- Ergonomic Keyboards
- Dog Carriers
- Litter Boxes
- Scratching Posts
- Cat Carriers
- All Pet Care
- Nail Clippers
- Flea & Tick
- All Luggage
- Lightweight
- Weekender Bags
- Accessories
- All Accessories
- Luggage Tags
- Travel Pillows
- Tech Gadgets
- Packing & Organization
- All Packing & Organization
- Packing Cubes
- Toiletry Bags
- Gift Guides
- All Gift Guides
- Valentine's Day
- All Valentine's Day
- For Any Loved Ones
- Mother's Day
- All Mother's Day
- Last Minute Gifts
- Best Mother's Day Gifts
- For Moms Who Have Everything
- Best from Amazon
- All Graduation
- For College Grads
- For High School Grads
- For Teachers
- Father's Day
- All Father's Day
- Best Father's Day Gifts
- For Dads Who Love Fishing
- Holiday Season & Christmas
- All Holiday Season & Christmas
- Gifts Under $25
- Practical Gifts
- Other occasions & loved ones
- All Other occasions & loved ones
- For Grandparents
- For Bridal Shower
- For New Parents
- For Any Occasion
- Deals & Sales
- All Deals & Sales
- Most Popular This Month
- Sales This Week
- New & Notable
- What to Buy This Month
- All Sleep Week
- Body Pillows
- Sleep Week Sales
- CNBC Select
- All CNBC Select
- Credit Cards
- Small Business
- Personal Finance
- Credit Monitoring
- Help for Low Credit Scores
- Sign up for the Select Newsletter
- Check out Shop TODAY
- Privacy Policy
- Do Not Sell My Personal Information
- Terms Of Service
- NBC News Sitemap
Follow Select
The 14 best vitamin C serums to brighten skin, according to dermatologists

Vitamin C, a powerful antioxidant, is a longstanding favorite of health professionals. When consumed in food or drink form, it can protect your entire body’s cells from damage; when applied topically, the best vitamin C serums deliver that protection directly to your skin cells. Research shows topical vitamin C can help prevent signs of aging, hyperpigmentation and cancer-causing UV damage.
To help you find the best vitamin C serum for your skin type and learn more about who best benefits from it, we spoke with three board-certified dermatologists and compiled a list of their recommendations to shop. We’re also highlighting effective products that have been tested by NBC Select staff.
SKIP AHEAD The best vitamin C serums | Benefits of vitamin C for skin | How to shop for vitamin C skin care products
Selected. Our top picks
How we picked the best vitamin C serums
You can find vitamin C in various skin care products, but our experts recommend working serums into your routine . “ Serums are lightweight and easier to layer with moisturizers, sunscreens, and cosmetic products,” says Dr. Erum Ilyas , M.D., FAAD, a board-certified dermatologist with the Schweiger Dermatology group in Pennsylvania. For a vitamin C serum to be effective, dermatologists say there are a few key things to consider:
- Type of vitamin C : According to our experts, the type of vitamin C will also determine how effective it is. Pure vitamin C, known as L ascorbic acid, is the most effective antioxidant when formulated correctly. But this is also the most irritating form, making it less than ideal for sensitive skin . Other forms of vitamin C are slightly less effective but gentler, including sodium ascorbyl palmitate (a fat-soluble molecule often found in creamier skin care products), and tetrahexyldecyl ascorbate (an oil-based form of vitamin C that penetrates skin deeply and is less irritating). Vitamin C products must be formulated at a low pH level (between 2.5 and 3.5) to effectively penetrate the skin, which some brands will list on the packaging. (Products with a low pH level are slightly acidic, which is one reason vitamin C can irritate. If you have sensitive skin, introduce a low-pH product slowly.)
- Concentration: Most vitamin C serums on the market are formulated with a concentration between 10% and 20%. “Beyond that, it’s just irritating and not necessarily more effective,” says Dr. Carmen Castilla , M.D., a board-certified dermatologist at the New York Dermatology Group in Manhattan.

select Retinol and retinoid products to target wrinkles, acne, discoloration and more
The best vitamin c serums in 2024.
To get the antioxidant benefits of vitamin C in your skin care routine, our experts recommend looking for a serum with a concentration between 10% and 20%. Below, they share their favorite formulas for all skin types, alongside other options tested by NBC Select staff.
Best overall vitamin C serum: Skinceuticals CE Ferulic
SkinCeuticals Vitamin C E Ferulic Serum
- Ferulic acid makes it potent
- Recommended by derms
- Smells like bacon water
All of our experts recommend Skinceuticals CE Ferulic, a serum containing 15% L-ascorbic acid. It’s often referred to as the gold standard in vitamin C serums by dermatologists. “It probably has the most evidence behind it,” says Castilla, referring to the clinical studies conducted by the brand that show a 41% reduction in oxidative damage in the skin. It’s also one of the more stable formulas on the market, thanks to the inclusion of vitamin E and ferulic acid, according to Dr. Sejal Shah , M.D., board-certified dermatologist and founder of Smarter Skin Dermatology in New York. “This combination makes vitamin C more potent,” says Ilyas, and helps prevent the L-ascorbic acid from oxidizing before it can reach your skin.
Lindsay Schneider , NBC Select commerce editor, says that when using this serum, she genuinely feels like it makes her skin look healthier, brighter and better. Zoe Malin , NBC Select associate updates editor, agrees. “This serum is very lightweight and it absorbs into my skin quickly,” says Malin. “That lets me move forward with the rest of my morning skin care routine a minute or two after I apply it.”
The one caveat our experts call out is the packaging: the dropper bottle means this serum is exposed to more air. According to the brand, however, the formula should remain stable and effective for up to six months after it’s opened.
Formula: Serum | Skin type: All | Key ingredients: Ferulic acid, vitamin E, vitamin C
Best budget-friendly vitamin C serum: CeraVe Skin Renewing Vitamin C Serum

CeraVe Vitamin C Serum
- Budget friendly
- Closed packaging
- Includes ceramides
- A bit small
For an affordable alternative, all three of our experts also recommend this drugstore serum. In addition to 10% L-ascorbic acid, CeraVe’s serum also has ceramides, which strengthen and support the skin barrier, and hyaluronic acid , a humectant that helps skin attract and retain moisture. Castilla particularly likes its packaging: “It’s in a squirty bottle, which is a little bit better at preventing oxidation,” she says.
Formula: Serum | Skin type: Sensitive, acne-prone | Key ingredients: Hyaluronic acid, ceramides, vitamin C
Best acne-fighting vitamin C serum: La Roche Posay 10% Pure Vitamin C Serum
La Roche-Posay 10% Pure Vitamin C Serum
- Extra exfoliation
- Good for fading spots
- Not for sensitive skin
Another dermatologist-recommended drugstore vitamin C serum, this 10% L-ascorbic acid formula also has salicylic acid . This can be a pro or a con, depending on your skin type, according to our experts. If your skin can tolerate it, the exfoliative benefits of salicylic acid can work together with vitamin C to fade dark spots faster, says Ilyas. If your skin is sensitive, however, the combination may be too irritating, according to Shah.
Formula: Serum | Skin type: Not for sensitive | Key ingredients: Salicylic acid, vitamin C
Best vitamin C serum for acne scars: Kiehl’s Clearly Corrective Dark Spot Corrector

Kiehl's Clearly Corrective Dark Spot Correcting Serum
- Good for hyperpigmentation
- Targets acne scars
- Includes salicylic acid
- Not great for sensitive skin
Shah also likes the vitamin C and salicylic acid combo found in this Kiehl’s formula, which specifically aims to target dark spots, acne scars and hyperpigmentation . It has a stabilized form of vitamin C called 3-O-ethyl ascorbic acid, which is more easily absorbed by the skin, according to research published by the National Institutes of Health. You can use it both morning and night after cleansing.
Formula: Serum | Skin type: Combo | Key ingredients: Salicylic acid, vitamin C (3-O-ethyl ascorbic acid)
Best vitamin C serum for oily skin: U Beauty Resurfacing Compound

U Beauty Resurfacing Compound
- Gentler vitamin C
- Helps with collagen production
- Good for oily skin
U Beauty’s resurfacing compound is a serum that combines retinol , glycolic acid, and hyaluronic acid with vitamin C to target hyperpigmentation, fine lines, and uneven skin texture, according to the brand. It uses a gentler, more stable form of vitamin C called sodium ascorbyl phosphate or SAP. “Its penetration [into the skin] isn’t as good as L-ascorbic acid, but SAP has been shown to help with collagen production and it can also be a little bit better for oily acne prone skin types,” says Castilla. Apply the resurfacing compound on dry skin right after cleansing and massage into skin before layering on other products.
Formula: Serum | Skin type: Combo, mature | Key ingredients: Retinol, glycolic acid, hyaluronic acid, vitamin C
Best vitamin C serum for dry skin: Sunday Riley C.E.O. 15% Vitamin C Brightening Serum

Sunday Riley C.E.O. 15% Vitamin C Brightening Serum
- Little to no smell
- Hydrates moisture barrier
- On the expensive side
NBC Select associate reporter Bianca Alvarez is a huge fan of this serum, which she says has a creamy, lotion-like consistency that she loves for her dry skin. “This is by far my absolute favorite vitamin C option I’ve tried,” she says. Along with the 15% THD ascorbate (vitamin C) it uses to fight dark spots and signs of aging, this serum also uses glycolic acid and saccharide isomerate extract to gently exfoliate while strengthening the skin’s moisture barrier, according to the brand. The packaging is designed with a pump for easier application — plus, Alvarez loves that it doesn’t have any off-putting smells, unlike other serums she’s tried.
Formula: Creamy serum | Skin type: All | Key ingredients: Saccharide isomerate extract, glycolic acid, vitamin C (THD ascorbate)
Best vitamin C serum for sensitive skin: Mad Hippie Vitamin C Serum

Mad Hippie Vitamin C Serum
- Lower price point
- Includes vitamin E
Mad Hippie’s vitamin C serum also uses sodium ascorbyl phosphate, but comes at a much lower price point. “This one is really good for addressing hyperpigmentation,” says Castilla. Like the top-rated (and much more expensive) Skinceuticals CE Ferulic serum, Mad Hippie’s version also pairs brightening vitamin C with stabilizing vitamin E and ferulic acid; when combined, research shows this trio of antioxidants can double a serum’s ability to protect skin against sun damage. To top things off, it also hydrating thanks to ingredients like hyaluronic acid and aloe, according to the brand.
Formula: Serum | Skin type: Combo | Key ingredients: Vitamin E, ferulic acid, hyaluronic acid, aloe
Best vitamin C serum for redness: The Inkey List 15% Vitamin C + EGF Serum

The Inkey List 15% Vitamin C + EGF Serum
- Plant-derived peptide
You should use this stabilized vitamin C serum (which is converted to its active, i.e. strongest, free-radical neutralizing form once applied, says Shah) after cleansing and before moisturizer and SPF, according to the brand. In addition to the 15% ascorbyl glucoside (vitamin C), Shah likes that it has a plant-derived peptide designed to support skin elasticity.
Formula: Serum | Skin type: Combo | Key ingredients: Probiotics, vitamin C (ascorbyl glucoside)
Best anti-aging vitamin C serum: Dr. Loretta Anti-Aging Repair Serum

Dr. Loretta Anti-Aging Repair Serum
- Packaging is opaque
- Helps collagen production
- A bit expensive
This formula has tetrahexyldecyl ascorbate, a powerful but less irritating form of vitamin C, which penetrates more deeply into skin than L-ascorbic acid. “It also has glycolipids, which are helpful for collagen production,” says Castilla. (Collagen is the most plentiful protein in your body and is responsible for giving strength, structure and elasticity to your skin, according to the Cleveland Clinic .) As a bonus, this serum comes in an opaque, pump bottle to help reduce oxidation and maintain the formula’s effectiveness.
Formula: Serum | Skin type: Combo | Key ingredients: Glycolipids, vitamin C (tetrahexyldecyl ascorbate)
Best vitamin C lotion: Revision Skincare Vitamin C Lotion

Revision Skincare Vitamin C Lotion
- Good for acne-prone skin
- Hydrating and moisturizing
Shah recommends this serum-moisturizer hybrid, formulated with 15 percent tetrahexyldecyl ascorbate. This stable lotion comes in a squeeze bottle and is formulated with squalane , an oil with anti-inflammatory benefits , making it particularly good for those with sensitive or acne-prone skin . It comes in two concentrations: 15% and 30%. If vitamin C is a new addition to your skin care routine or your skin gets irritated easily, start with a lower concentration and make sure your skin can tolerate it before trying a formula with 30% vitamin C.
Formula: Serum-moisturizer | Skin type: Acne-prone | Key ingredients: Squalane, vitamin C (tetrahexyldecyl ascorbate)
Best vitamin C serum for beginners: Paula’s Choice C15 Super Booster

Paula’s Choice C15 Super Booster
- Can mix with moisturizer
- Includes ferulic acid
You can use this 15% L-ascorbic acid formula, which comes recommended by Castilla, on its own as serum, or as a brightening booster by adding a few drops to your favorite moisturizer , according to the brand. Like many of the other serums recommended by our experts, it’s formulated with stabilizing antioxidants ferulic acid and vitamin C.
Formula: Serum | Skin type: Combo | Key ingredients: Ferulic acid, vitamin C
Most versatile vitamin C serum: 107 Beauty Soseo Vinbiome Vitamin C Serum

107 Beauty Soseo Vinbiome Vitamin C Serum
- Targets redness
- Supports skin's microbiome
This multitasking serum, which comes recommended by Shah, targets pigmentation, redness, and skin elasticity. Formulated with 15% ethel ascorbic acid, a stabilized form of vitamin C, it also includes probiotics , which studies show support skin health by protecting the skin microbiome and boosting skin’s immune response.
Formula: Serum | Skin type: Combo, dry | Key ingredients: Probiotics, vitamin C (ethel ascorbic acid)
Best vitamin C serum with niacinamide: Summer Fridays CC Me Serum

Summer Fridays CC Me Serum
- Pump bottle
- Rich, lightweight texture
- Easy to spread over skin
- Takes a minute to settle
“I love this serum because it has a rich texture, yet isn’t heavy at all,” says Malin. “It spreads so well on my skin, and the pump bottle makes it easy to control how much serum I’m using at one time.” The serum is formulated with two types of vitamin C — ascorbyl glucoside and 3-O-ethyl ascorbic acid — as well as niacinamide (vitamin B3), an ingredient that brightens the skin and has anti-inflammatory properties. The serum also contains squalane to boost hydration.
Formula: Serum | Skin type: All, combo, dry, oily | Key ingredients: Vitamin C (ascorbyl glucoside and 3-O-ethyl ascorbic acid), niacinamide, squalane
Best hydrating vitamin C serum: Mara Chlorella + Reishi Sea Vitamin C Serum

Mara Chlorella + Reishi Sea Vitamin C Serum
- Long-lasting
- Smells fresh
- Helps fade dark spots
- Slightly oily feel
Since a small drop of this serum is enough to cover your face, Malin says one bottle lasted her about 10 months, making the higher price point worth it in the long run. “It has a luxurious texture and over time, I noticed that my skin tone looked more even and dark spots from acne scars faded, making them easier to cover with concealer,” she says. The serum is hydrating thanks to a proprietary type of algae, which stimulates natural hyaluronic acid synthesis, according to the brand. It’s made with THD ascorbate and 14 vitamin c-rich botanicals like fruits and spices, as well as adaptogens like reishi, chlorella and ginseng, which reduce puffiness and inflammation.
Formula: Serum | Skin type: All, combo, dry, oily | Key ingredients: Vitamin C (THD ascorbate), adaptogens

select Does your skin care routine actually need a toner? Experts weigh in.
What are the benefits of vitamin c for your skin.
When used as a consistent part of your skin care routine, vitamin C can have many benefits. Below, we summarized the most convincing benefits, according to our experts:
- Vitamin C helps protect your skin from sun and other harmful substances. “The most convincing evidence we have for vitamin C [in skin care] is the prevention of free radical damage,” says Castilla. In scientific terms, free radicals are molecules with an unpaired electron, making them unstable and capable of damaging cells. Antioxidants like vitamin C neutralize those radicals by donating an electron, according to Castilla. Sunlight is a common source of free radical exposure as is the pollution your skin is exposed to every day, says Shah. That exposure can “accelerate skin damage and aging through what we call oxidative stress,” she says. Vitamin C protects skin, preventing the breakdown of collagen and elastin.
- It’s effective at reducing hyperpigmentation. Vitamin C can also protect your skin from unwanted dark spots. “It inhibits an enzyme called tyrosinase in the skin to reduce hyperpigmentation ,” says Ilyas. It works best as a treatment for dark spots caused by sun damage and conditions like melasma , she adds. “Not all pigment is the same and I do not always find that acne scars respond consistently as well to vitamin C,” she says.
- Vitamin C can help reduce redness. Inflammation is often the culprit behind skin redness and dryness. Vitamin C can help treat these issues by inhibiting a pro-inflammatory signaling molecule, says Castilla.
- It can boost your skin’s collagen production. Your skin needs vitamin C to produce collagen , the primary building block for skin and other connective tissues, that’s responsible for skin elasticity and firmness. “ Collagen hydroxylase , an enzyme in the skin that is responsible for collagen formation, is dependent on Vitamin C to function,” says Ilyas.
How to shop for a vitamin C serum
Not all vitamin C skin care products are created equally. You’ll often see vitamin C skin care products in acid form — at specific concentrations, though, it can be irritating, especially for sensitive skin. Vitamin C is also a highly unstable ingredient, according to experts — the same chemical properties that allow it to work as an active antioxidant in your body also make it susceptible to oxidation, which will make it inactive in your skin. That’s why our experts recommend using it in the form of a serum, as they are lightweight and easy to layer with other products.
To get the most out of your serum, they recommend understanding the type and concentration of vitamin C in any formula, being mindful of how packaging can impact a serum’s effectiveness, and looking for companion ingredients that support the skin barrier.
Type of vitamin C
You’ll commonly find vitamin C incorporated into a topical product in two ways: in its active form as L-ascorbic acid, or in an inactive form that needs to convert into L-ascorbic acid on the skin, says Shah.
In controlled settings, L-ascorbic acid is the most effective, says Castilla, but in reality the effectiveness of a vitamin C serum may depend on a variety of factors, including your skin type. “L-ascorbic acid can be very unstable so whatever bottle that you get, you have to use it very quickly or it will oxidize,” she says. “Secondly, it has to be formulated at a low pH, meaning it’s very acidic and it can be very irritating for a lot of skin types, especially rosacea-prone skin types.”
Other types of vitamin C — such as 3-O-ethyl ascorbic acid, sodium ascorbyl phosphate, and tetrahexyldecyl ascorbate — are more stable. Shah particularly likes tetrahexyldecyl ascorbate since it converts to L-ascorbic acid really well and also penetrates the skin really well, she says.
Concentration
The dermatologists we spoke to recommend looking for products with a concentration of vitamin C between 10% and 20%. “Beyond 20%, it’s just irritating and not necessarily more effective,” says Castilla.
Exposure to light and air are disastrous for the unstable molecules found in vitamin C serums, according to our experts. “What vitamin C does for free radicals is contribute electrons to the free radicals [to stabilize them],” says Castilla. “Once that happens, then the vitamin C is no longer active.”When vitamin C molecules are exposed to oxygen, “it loses those electrons and it becomes an inactive form of vitamin C,” she explains.
Packaging and the way you store your serum are key to its effectiveness. Ilyas recommends serums in opaque or amber colored glass bottles, which block light exposure. And while many top vitamin C serums come in a glass bottle with a dropper, Castilla says those with airless pump bottles will stay active longer. Store your serum away from the sun and don’t hoard it — this unstable ingredient is best used quickly.
Companion ingredients
“Many worthy topical vitamin C-containing serums will often be combined with ferulic acid and vitamin E because this combination can increase the effectiveness of vitamin C,” says Ilyas.
Castilla also recommends pairing your vitamin C with sunscreen . “Sunscreens help block free radical damage, but not all of it,” she says. “Pairing sunscreen with a vitamin C doesn’t improve the UVA/UVB protection, but [the vitamin C] protects against the free radicals that are being produced.”
Frequently asked questions
“Vitamin C is a safe and reasonable product for most skin types,” says Ilyas, but how and when you add it to your skin care routine depends on your skin type and the type of vitamin C you choose. Ilyas usually recommends using vitamin C starting in your 30s because that’s when pigmentation changes associated with light exposure tend to reveal themselves, she says.
If you have sensitive skin, Castilla recommends looking for a formula made with a vitamin C derivative like sodium ascorbyl phosphate or tetrahexyldecyl ascorbate to avoid irritation.
You can use a vitamin C serum either your morning or evening routine, though most formulations are meant for morning use, says Castilla. When applied in the a.m., vitamin C can help fight free radical damage from UV light and the other oxidative stressors your skin is exposed to during the day. If you want to use vitamin C serum twice a day, she recommends sticking with a formula with a lower concentration (10% to 15%) to avoid irritation.
Meet our experts
At NBC Select, we work with experts who have specialized knowledge and authority based on relevant training and/or experience. We also take steps to ensure all expert advice and recommendations are made independently and without undisclosed financial conflicts of interest.
- Dr. Carmen Castilla , M.D., is a board-certified dermatologist at the New York Dermatology Group in Manhattan specializing in both medical and cosmetic dermatology.
- Dr. Sejal Shah , M.D., FAAD, is a board-certified dermatologist and founder of Smarter Skin Dermatology in New York. She specializes in cosmetic dermatology and treating skin of color.
- Dr. Erum Ilyas , M.D., FAAD, is a board-certified dermatologist with the Schweiger Dermatology group in Pennsylvania. Her expertise includes pediatric and medical dermatology and skin cancer treatment.
Why trust NBC Select?
Macaela MacKenzie is a journalist and former Glamour editor who has covered beauty and wellness treatments for over a decade. For this article, MacKenzie spoke to three dermatologists about the best ways to prevent and treat melasma and highlighted their top skin care picks and the rationale behind them.
Catch up on NBC Select's in-depth coverage of personal finance , tech and tools , wellness and more, and follow us on Facebook , Instagram and Twitter to stay up to date.
Macaela MacKenzie is a journalist covering women’s equality, wellness, and the gender gap and is the author of “Money, Power, Respect”.
15 Vitamin C Serums for Seriously Glowing Skin
An essential supplement and a powerful skincare ingredient.

Every item on this page was chosen by an ELLE editor. We may earn commission on some of the items you choose to buy.
A universally effective ingredient, vitamin C has cropped up in your favorite formulas, from facial cleansers to face masks to moisturizers , and wrinkle creams . “Vitamin C is a topical antioxidant that has many beneficial properties,” says celebrity dermatologist Dr. Karan Lal . “It reduces hyperpigmentation and acts as a cofactor for many cellular processes to maintain naturally glowing skin. Most of all, it scavenges free radicals that we come in contact with every single day.”
In addition to enhancing skin with a touch of radiance, vitamin C yields the power to make skin appear more youthful. According to fellow dermatologist Dr. Omar Ibrahimi , “[it’s] a key molecule for optimizing skin health and is an important and necessary cofactor (supporting molecule) that is required for the production of collagen, which is our skin’s main building block and currency for youthful skin.”
However, there’s one caveat that you should keep in mind while selecting a vitamin C serum for yourself. Some vitamin C serums are quite potent due to high concentration levels, so it’s essential to pay attention to this. “Vitamin C can be irritating at concentrations at or above 20 percent,” says Dr. Lal. Especially if you’re new to the vitamin C world, Dr. Lal recommends choosing a serum that includes a 10 percent concentration level. Overall, though, no matter your tolerance, Dr. Ibrahimi says to look for vitamin C formulas with L-ascorbic acid, the true active form of vitamin C. “L-ascorbic acid is the best option for most, as it is the most bioactive vitamin C compound,” he says.
Our top picks for vitamin C serums in 2024

Best Overall
Medik8 super c ferulic.

Best for Beginners
Cerave skin renewing vitamin c serum.

Best Antioxidant-Rich Formula
Alastin c-radical defense antioxidant serum.

SkinCeuticals C E Ferulic

Best for Brightening Skin
Paula's choice 25% vitamin c + glutathione clinical serum.

Best for Hyperpigmentation
Obagi medical medical professional-c serum 20%.

Best for Fine Lines
Olehenriksen banana bright 15% vitamin c dark spot serum.

Best for Acne-Prone Skin
La roche-posay pure vitamin c face serum.

Best Time-Release Formula
Ustawi vitamin c antioxidant serum.

Best for Skin Texture
Beautystat universal c skin refiner.
If you think your skin needs a vitamin C boost , look no further. We’ve perused thousands of customer reviews and consulted our personal collection of products to find the 15 best vitamin C serums for glowy skin.
Key ingredients : L-ascorbic acid, ferulic acid, vitamin E
Size : 1 fl oz
Vitamin C plays well with a number of ingredients, but none better than ferulic acid. Both vitamin C and ferulic acid are potent antioxidants that address discoloration and ward off free radicals that prompt premature aging . This Medik8 formula packages 30 percent (!) L-ascorbic acid and ferulic, along with vitamin E for added cushion.
Medik8 rating : 4.6/5 stars
An ELLE Editor says : “As a former vitamin C hater, I was skeptical to try a new formula but was pleasantly surprised by how well my skin behaved with Super C Ferulic (read my review here ). I have the most stubborn dark marks, so I knew I wouldn’t see any major transformation. However, after a few uses of this serum, my skin looked brighter and felt smoother, and some marks even lightened up. The consistency is light and absorbs fast, so no sticky residue here!” — Nerisha Penrose , ELLE.com beauty commerce editor
Key ingredients : l-ascorbic acid (vitamin C), hyaluronic acid, ceramides
What’s not to love about CeraVe? The brand is powered by gentle yet efficacious ingredients and backed by your favorite dermatologists. This vitamin C serum costs about as much as two bottles of orange juice, but the results last longer than the taste of the final sip. Brightening vitamin C works in tandem with nourishing ceramides and hyaluronic acid to whip dull, stressed skin into shape. Amazon rating : 4.5/5 stars Our expert says : “Who is a good candidate for vitamin C? In general, all skin types and skin tones can benefit from a vitamin C serum. I love CeraVe Skin Renewing Vitamin C Serum because it’s formulated with 10% l-ascorbic acid, which is the most active form of vitamin C, and in the right concentration to deliver peak benefits, like improved skin tone and texture. It’s also fragrance-free, non-comedogenic, and allergy-tested, so it’s effective yet gentle.” — Dr. Charles Puza
Key ingredients : Sodium ascorbate (vitamin C)
Thanks to this formula, an army of antioxidants takes on a slew of tasks to bring you closer to the skin of your dreams. By now, we know vitamin C corrects dullness and prevents any damage free radicals cause ; simultaneously, the antioxidant-powered serum improves elasticity, boosts collagen, and protects against external stressors that trigger the skin.
Alastin rating : 4.5/5 stars
An Alastin reviewer said : “Love, love, love this serum! It’s the only Vitamin C product I’ve found that isn’t oily and doesn’t sting. It has a wonderful silky texture that absorbs almost immediately. I’ve used this since it was introduced and my skin is brighter and more even-toned.”
Key ingredients : Antioxidants, hyaluronic Acid, vitamin C, vitamin E Size : 1 fl oz
Beloved by many a smooth-skinned celeb and knowledgeable dermatologists, this brightening serum contains vitamin C, vitamin E, and ferulic acid to replenish the skin. Because vitamin C can be very unstable in skincare, a dding ferulic acid helps it last longer –and work better.
Dermstore star rating : 4.7/5 stars
An ELLE editor said: “I can’t remember what life was like before the C E Ferulic acid became a mainstay in my morning routine, but it had to be as dull and lifeless as my skin at the time. As a former scar picker, this serum helped balance my tone and smooth my texture over time. Now, I’m not so ashamed to go makeup-free as I once was.” — Nerisha Penrose
Key ingredients : Vitamin C, glutathione, ergothioneine Size : 1 fl oz
With vitamin C, glutathione, and ergothioneine, this serum instantly brightens dull skin . Depending on your skin’s sensitivity, you can use the product alone or add a few drops to your favorite moisturizer for a more diffused but equally effective application.
Sephora star rating : 4.7/5 stars
A Sephora reviewer said: “ This product is absolutely amazing. I am always looking for vitamin C products and recently started trying Paula’s Choice products. I use 1-2 pumps all over my face and I think it is brightening up my skin tone and texture. I recommend it. I actually bought this for my sister and mom as well.”
Key ingredients: Antioxidants, hyaluronic acid, vitamin C Size: 1 fl oz
Obagi’s vitamin C serum is super lightweight, but it still makes a powerful impact on your skin. This L-ascorbic acid-infused blend effectively penetrates the skin while brightening hyperpigmentation and providing antioxidant protection from UV damage.
A Dermstore reviewer said: “Whenever anyone asks me about my skin, I always tell them I use this. It makes my face more hydrated, smoother, and radiant after use. It’s my amazing holy grail.”
Key ingredients : Vitamin C, phas, hyaluronic acid Size: 1 fl oz
Environmental damage is often the worst enemy to skin, as it causes irritation, damage, and even fine lines. Olehenriksen’s vitamin C-infused serum actively fights and protects against environmental stressors while reducing the appearance of wrinkles. You’ll notice a less inflamed , smoother, and happier complexion.
Sephora star rating : 4/5 stars
A Sephora reviewer said: “This is honestly a great vitamin C serum, it definitely brightens your face in the span of just wearing it for a few minutes. I noticed it helped smooth and clear my skin due to its 15 percent vitamin C.”
Key ingredients : Hyaluronic acid, vitamin C, salicylic acid
This serum does it all and then some; vitamin C is used to brighten and improve skin texture, hyaluronic acid adds moisture and plumpness to the skin, and salicylic acid reduces oiliness , which leads to breakouts. The result is radiant, glowing skin that’s hydrated and blemish-free.
Amazon star rating : 4.6/5 stars
An Amazon reviewer said: “I have hormonal acne that I thought would never go away. Plus, I’m in my late 20s and started to see signs of aging. I love this product. It helps with fine lines and wrinkles while also opening up your pores to release gunk. Fantastic.”
Key ingredients : Vitamin C, niacinamide, licorice root
This powerful yet gentle serum uses a version of time-released vitamin C in its formula, so the antioxidant effects of the ingredient last throughout the day as you wear it. It’s also made up of primarily naturally derived ingredients, so you can be sure you’re not putting your skin through too much stress.
Nordstrom star rating : 4.2/5 stars
A Nordstrom reviewer said: “ This serum is light yet hydrating. I love the fresh scent and texture, which melts into my skin. After several weeks of daily use, my skin feels firmer and more even. I use it once a day under my moisturizer. It is not sticky, either.”
Key ingredients : Vitamin C, squalane
This serum contains 20 percent potent, stable vitamin C to brighten, firm, and smooth textured skin for fast and effective results . It doesn’t mess around, so be ready to notice results faster than expected. Even Hailey Bieber , aka the queen of glazed doughnut skin, is a fan of its “incredible softening and brightening effects,” she said in a video interview .
Amazon star rating : 4.2/5 stars
An ELLE editor says: “This formula contains the highest percent of pure vitamin C allowed in over-the-counter topicals and has a patented encapsulated delivery system preventing oxidation. I never thought that vitamin C worked on my skin, and it often would cause my other serums to pill, so it’s a skin care step I often skipped—until this formula. I have been using this daily for about a month and notice the texture of my skin is smoother and more radiant.” — Danielle James , digital beauty director
Biossance Squalane + Vitamin C Dark Spot Serum

While vitamin C helps fade dark spots on the skin, the combination of squalane and natural extracts p rotects the skin from future photodamage . That means that over time, you’ll notice your skin tone becoming more even and supple—as did one of ELLE’s beauty writers, who now swears by the product .
Biossance star rating : 4.5/5 stars
A Biossance reviewer said: “Within a week or two of using this, I had a brown spot on my forehead go away that I never thought would disappear without going to a dermatologist. I have used every designer cream, but this one is all about the environment and works. So in love. Try this; you’ll be so happy!”
Sunday Riley C.E.O. 15% Vitamin C Brightening Serum

Key ingredients : Vitamin C Size : 1 fl oz
In the short term, this potent serum will help skin look radiant and glowy , like you just got a facial. But with continual use, the vitamin C and glycolic acid combo fades dark spots , hyperpigmentation, reduces pore visibility, and smooths out the skin’s textur e .
Nordstrom star rating : 4.4/5 stars
A Nordstrom reviewer said: “ At 51, I have a couple of age spots, and this serum has lightened them gradually. My skin is glowing. I use this with the Good Genes and have seen a dramatic result in reducing pore size.”
Dr. Barbara Sturm The Good C Vitamin C Serum

Key ingredients : Vitamin C, kakadu plum extract
This celeb-favorite skincare line created a gentle vitamin C serum perfect for anyone with reactive skin . Using a combination of synthetic and naturally-derived vitamin C, your skin will be red carpet ready and glowing in no time.
Nordstrom star rating : 3.6/5 stars
An ELLE editor said: “I like this formula for maintenance of my even skin tone. It plays well with my skincare routine and does not pill when I apply sunscreen as my last step. With only 5 percent Vitamin C, this isn’t the strongest of formulas, so if you are looking for something to get rid of hyperpigmentation and dark spots quickly, I’d suggest a formula with a minimum of 10 percent Vitamin C. However, if you have sensitive skin, this will be a great option for you.” — Danielle James
Vichy LiftActiv Vitamin C Serum

Key ingredients : Vitamin C, vitamin E
With 15 percent vitamin C, vitamin E, and a special antioxidant derived from pine bark, this anti-aging formula corrects and treats dullness, discoloration, and fine lines . With so many powerful ingredients in one bottle, you can probably cancel your next facial appointment.
Vichy star rating : 4.3/5 stars
A Vichy reviewer said: “I’ve only been using this for a week and am already seeing great results with reduced fine lines around my mouth and face. It feels rejuvenating when you use it, and I actually feel a tightening happening. My skin is brighter and more illuminated. I am turning 60 this year, so really looking for ways to battle these lines, and they look good!”
Omorovicza Daily Vitamin C Serum

Key ingredients : Vitamin C, hyaluronic acid
Omorovicza uses vitamin C to stimulate collagen in your skin , leading to a more plumped, youthful texture. The milky formula absorbs quickly while leaving your skin nourished and hydrated.
Nordstrom star rating : 3.8/5 stars
A Nordstrom reviewer said: “I really liked how lightweight the product went on. It felt airy and my skin absorbed it so quickly without feeling dry afterward. I was also really impressed with how lustrous my skin looked.”
.css-1wfsl5s{font-family:SaolDisplay,SaolDisplay-fallback,SaolDisplay-roboto,SaolDisplay-local,Georgia,Times,serif;font-weight:normal;margin-bottom:0.625rem;margin-top:0.625rem;text-align:center;}@media(max-width: 48rem){.css-1wfsl5s{font-size:2.375rem;line-height:1.2;}}@media(min-width: 48rem){.css-1wfsl5s{font-size:2.375rem;line-height:1.2;}}@media(min-width: 64rem){.css-1wfsl5s{font-size:3rem;line-height:1.2;letter-spacing:-0.0225rem;}}@media(min-width: 73.75rem){.css-1wfsl5s{font-size:3rem;line-height:1.2;}}.css-1wfsl5s b,.css-1wfsl5s strong{font-family:inherit;font-weight:bold;}.css-1wfsl5s em,.css-1wfsl5s i{font-style:italic;font-family:inherit;} Is vitamin C safe?

In addition to choosing the right formula strength for you, it’s important to note that “vitamin C is photolabile, which means it gets degraded with exposure to sunlight,” says Dr. Lal. “It also degrades upon exposure to air. For this reason, keep your vitamin C product in a dark place and make sure you tightly close your bottle to prevent breakdown of the product.”
Who is a good candidate for vitamin C?

While vitamin C has impressive benefits for the skin, it's important to understand whether your skin can tolerate it.
“In truth, everyone should be using a vitamin C product due to its reparative properties,” says Dr. Lal. “However, those with dry, sensitive skin, or acne should be careful and start out slow to prevent breakouts.” You can begin by patch testing, then gradually incorporate the serum into your routine to build up a tolerance. Lal also notes that serums are lightweight and usually combined with agents that allow for better penetration, whereas creams and lotions are meant to stay on top of the skin and work superficially. “Vitamin C serums take at least 12 weeks to take effect because it takes time for new cells to regenerate and for pigment to start clearing,” he adds.
When should you use vitamin C?

If vitamin C immediately conjures images of orange juice, then you already have your answer. “As a board-certified dermatologist, I consistently counsel my patients on the benefits of adding a vitamin C serum into their routine. I recommend applying a vitamin C serum each morning after cleansing the skin and before applying moisturizing and sunscreen,” Dr. Charles Puza says.
Can I use a vitamin C serum everyday?

Yes, as long as you’re not cocktailing too many actives and using sunscreen. If you have sensitive skin, patch testing is essential. “If you have sensitive skin, you might want to test the product on the side of your face or neck for a few days before widely applying it,” says Dr. Ibrahimi. “Try to use a derivative that tends to be better tolerated. I would also avoid using Vitamin C during or immediately after any laser or microneedling procedures as the skin barrier becomes more sensitive and there is a higher likelihood of irritation even in folks that do not have sensitive skin.”
Meet the Experts

Dr. Karan Lal is a double board-certified celebrity dermatologist with Schweiger Dermatology Group in New Jersey.
Dr. Omar Ibrahimi is a board-certified dermatologist and founder and medical director of the Connecticut Skin Institute .
Dr. Charles Puza is a board-certified dermatologist at MOMA Dermatology and CeraVe brand partner.

The 20 Best Face Exfoliators for Every Skin Type

The 10 Best Facials Worth the Splurge in 2024
10 Best Peptide Eye Creams for Smoother Under Eyes

10 Home Red Light Therapy Devices to Reduce Acne

The 12 Best Anti-Wrinkle Creams

What Are Best Growth Factor Serums?

Best Skin Care Benefits of Seaweed

16 Tinted Moisturizers for ‘No Makeup’ Makeup

12 Best Wrinkle Filler Serums

A Detailed Guide on Polyhydroxy Acids in Skin Care

Martha Stewart’s Go-To Beauty Products Are on Sale

IMAGES
VIDEO
COMMENTS
Vitamin C is a six carbon chain, closely related chemically to glucose. It was first isolated in 1928 by the Hungarian-born scientist Szent-Gyorgi and structurally characterized by Haworth in 1933. In 1934, Rechstein worked out a simple, inexpensive, four-step process for synthesizing ascorbic acid from glucose.
Researching Chemistry Vitamin C: Student Guide Page 3 Overview of the assignment and activities. Studying chemistry involves learning chemistry facts and concepts. It also involves developing particular skills. These include research skills, which may involve you in doing investigative experiments or researching information, perhaps from the ...
4. Helps with wound healing. Vitamin C encourages collagen growth, which is an important part of your body's healing process. "Collagen is a protein that keeps our skin looking young," Peart ...
Vitamin C Sample Preparation: Using an analytical balance, weigh a vitamin C "tablet" and place it in a mortar. Grind with a pestle into fine powder. Weigh approximately 0.10 g of the powder to a tenth of a milligram and transfer it quantitatively into a 125-mL Erlenmeyer flask. Record the weight in your notebook.
Vitamin C was named ascorbic acid to indicate its antiscorbutic (anti- scurvy) properties. Soon after its discovery, an industrial process was developed to produce ascorbic acid from glucose. Today it is the most produced vitamin in the world exceeding 100,000 tons per year (2).
Testing samples to determine vitamin C levels. Organise students into groups of three or four. Give each student a copy of 'Testing for vitamin C'. Assign each group to a set of equipment. Demonstrate the technique for testing the samples. Ensure there is a minimum of four samples to test in each group. Check for overlap - the same fruit ...
Introduction. Vitamin C, also known as L-ascorbic acid, is a water-soluble vitamin that is naturally present in some foods, added to others, and available as a dietary supplement. Humans, unlike most animals, are unable to synthesize vitamin C endogenously, so it is an essential dietary component [ 1 ].
3. Vitamin C. Vitamin C, also known as ascorbic acid, is mainly present in fruits and vegetables; citrus fruits, tomatoes and potatoes are the principal exogenous source of this vitamin. The consumption of such foods is important since the human body does not have the ability to produce this essential micronutrient.
Part III — Analysis of Vitamin C in Juice. Use a graduated cylinder to measure out a 25.0 mL sample of either pineapple or grapefruit juice. Add the juice to a 250 mL Erlenmeyer flask, and then add 20 mL of 0.5 M H2SO4. Add 1.00 g of KI and 25.00 mL of 0.0200 M KIO3 to the flask. Begin titrating with the thiosulfate solution.
50-mL burette, ring stand and burette clamp. 1-mL volumetric pipette and pipette bulb. Spatula. Deionized or distilled water. Vitamin C tablets (1 per group) 1000 mg Vitamin C tablets are recommended, see teacher notes for more detail; do not use multivitamins as the other ingredients may interfere with the titration.
Vitamin C is an essential micronutrient and powerful antioxidant. Observational studies have shown an inverse relationship between vitamin C intake and major cardiovascular events and cardiovascular disease (CVD) risk factors. ... and vitamin E assignment. Results are presented below using the inverse variance method. A composite measure of ...
Vitamin C. Vitamin C. Definition. Vitamin C is a water-soluble vitamin important for healthy skin, teeth, bones, and blood vessels. It is found especially in citrus fruits, tomatoes, potatoes, and green leafy vegetables. It is also called ascorbic acid and its chemical formula is C 6 H 8 O 6. A deficiency of vitamin C in the diet causes scurvy.
(vii) Determine the grams of vitamin C presented in the solution for each trial of the analysis. Molar mass of Vit C = 176 g/mol Trial 1: 176 g/mol x 0 moles = 0 g = 0 g Vit C Trial 2: 176 g/mol x 0 moles = 0 g = 0 g Vit C Trial 3: 176 g/mol x 0 moles = 0 g = 0 g Vit C (viii) calculate the % by mass of ascorbic acid in the vitamin C sample for ...
But here are some estimates of the vitamin C content in common citrus fruits: Orange: 70-90 milligrams (mg). Grapefruit: 80-100 mg. Lemon: 30-40 mg. Lime: 20-30 mg. "Just one orange or ...
Vitamins and the chemistry behind them. Bookmark. From practical experiments to a directed activity related to text (DART), try these activities for 11-16 year olds to investigate the chemistry of vitamins. In this series of activities, students learn about a range of chemical ideas while investigating vitamins and vitamin supplements.
The NaHCO₃ is omitted in an analysis of the sample. What happens to the Vitamin C amount? The Vitamin C amount will be too low. With no sodium carbonic acid, ascorbic acid will get oxidized --> less amount. Also, since some of the ascorbic acid may have been oxidized by the oxygen and not the iodate, less iodate will be added --> less Vitamin C.
15 of 15. Quiz yourself with questions and answers for Chapter 10 Quiz: The Water-Soluble Vitamins: B Vitamins and Vitamin C, so you can be ready for test day. Explore quizzes and practice tests created by teachers and students or create one from your course material.
Co Hg Of + I 3 + H₂O Co Hg O=+3 T +7 From the equation, vitimanc coures Ta- jon to I 2. Experimental Procedure, Part A.1. Determine the mass of solid KIO, required. Question: Experiment 30 Prelaboratory Assignment Vitamin C Analysis ever Date Lab Sec. Name Desk No. 1. Vitamin C is a reducing agent and antioxidant.
Experiment 43 Advance Study Assignment: Analysis for Vitamin C 1. Write a balanced equation for the reaction between I, and ascorbic acid. I oxigzrg redua the reducing agent. 2. A solution of I2 was standardized with ascorbic acid (Asc). Using a 0.1137-g sample of pure ascorbic acid, 26.75 mL of 1, were required to reach the starch end point. a.
View Module 12.docx from DIT 121 at Northern Virginia Community College. Module 12: Assignment - Vitamin C My daily recommendation (RDA) for vitamin C is 75 mg. Fruit and vegetable are the best
Vitamin C, a powerful antioxidant, is a longstanding favorite of health professionals. When consumed in food or drink form, it can protect your entire body's cells from damage; when applied ...
Key ingredients: L-ascorbic acid, ferulic acid, vitamin E . Size: 1 fl oz . Vitamin C plays well with a number of ingredients, but none better than ferulic acid. Both vitamin C and ferulic acid ...
Chemistry questions and answers. Experiment 30 Prelaboratory Assignment Vitamin C Analysis Nome A Lab Sec. Vitamin C is a reducing agent and antioxidant. Wat does it reduce in this experiment Expl Desk No. 1 Experimental Procedure, Part A.1. Determine the mass of solid KIO, required for the preparation of 250 ml of 0.01 M KIO, solution.
Experiment 30 345. Experiment 30 Prelaboratory Assignment Vitamin C Analysis in the It veduces 12 inte I Part A.1. Determine the mass of solid KIO, required for the preparation of 250 ml. of 001 M Experimental Procedure, A1 K10, solution. Repeat and record this calculatoin on the Report Sheet 3. Experimental Procedure, Part B.1.
Learn about 21 healthy foods full of vitamin K, a nutrient that helps your blood clot and supports your bones and heart. Cleveland Clinic experts explain.
MSN