- Twin Cities
University of Minnesota
- Bachelor's Degrees
- Master's Degrees
- Doctorate Degrees
- Certificates
- Coursera Online Courses
- Licensing Programs
- Post-Secondary Enrollment Options (PSEO)
- Credit Online Courses
- Professional Development Online Courses
- Student Stories
- Health and Well-being
- Learn Online

Master of Science in Clinical Research

- Program Website
- Contact Department
- On-Campus Component No
- Cost $1,593.00 per credit (resident, part-time), $2,465.00 per credit (nonresident, part-time)
- Total Credits 38
- Credential Graduate Degree
- Application Deadlines See program website
- Campus Twin Cities
- College School of Public Health
- Department Epidemiology and Community Health
The MS in Clinical Research program is a graduate degree as well as a career development path for physician-scientists, clinical scholars, and biomedical researchers.
The program trains you to conduct patient-oriented research, directly interacting with human subjects to better understand disease, the development of therapeutic interventions, and the conduct of clinical trials. You will learn how to conduct epidemiological and behavioral studies and understand issues related to outcomes-based research, and also develop grant writing and data analytic skills.
Sample Courses
- PUBH 6301 – Fundamentals of Clinical Research
- PUBH 6307 – Clinical Epidemiology
- PUBH 7420 – Clinical Trials
- PUBH 6450 – Biostatistics I
Online Catalog
Career Outlook
There is increasing demand for well-trained individuals who can design, implement, and interpret clinical research studies. Graduates go on to be academic physicians and principal investigators at major academic institutions around the country. Some of our graduates are industry leaders.
Boston University Academics
Boston University
- Campus Life
- Schools & Colleges
- Degree Programs
- Search Academics
- MS in Clinical Research
For more information, please visit the Graduate Medical Sciences website .
The Master of Science in Clinical Research is a rigorous program that meets the needs of health professionals engaged in the full spectrum of patient-oriented research. This flexible degree program is designed for a variety of professionals, including physicians who will plan and oversee translational research and clinical trials; research nurses; study coordinators; managers in clinical research and site management organizations (CROs and SROs); and professionals in the pharmaceutical, biotechnology, and medical device industries.
Learning Objectives
Upon completion of the MS in Clinical Research , students are expected to:
- Demonstrate the ability to design and conduct clinical research, analyze results, and answer a research question.
- Demonstrate the ability to read and critique the clinical research literature.
- Present clinical research findings (from literature or their own research) to peers.
Our Mission
Inspire, Instruct, Innovate. The MS in Clinical Research program is dedicated to the discovery, development, and application of knowledge as it pertains to all areas of clinical research. Our mission is to foster an engaging and effective educational environment that promotes the pursuit of outstanding teaching and learning through formal classroom and practical training. With established collaborative relationships with pharmaceutical, biotech, and academic institutions, students are provided with unique opportunities to pursue clinical research in areas that are of personal and professional interest.
We hope that the information you receive about our program encourages you to pursue your graduate degree in clinical research with us. If you are interested, click here to get your application started. We accept applications for both September and January start dates.
Degree Requirements
The program consists of three components:
- Minimum of 32 graduate credits; 22 required and 10 elective
- Clinical research practicum; hands-on involvement in a clinical research project
- Capstone Project; clinical research project resulting in a written research paper
Master of Science in Clinical Research degree candidates are required to complete all of the following:
A minimum of 32 credits at the graduate level across four semesters. These must include the following 22 credits of required coursework:
- GMS CI 631 Management of Clinical Trials (4 cr), spring semester
- GMS CI 640 OL Regulatory and Compliance Issues (4 cr), spring semester
- GMS CI 670 Biostatistics with Computing (4 cr), fall semester
- GMS CI 675 Designing Clinical Research Studies (4 cr), fall semester
- GMS CI 790 Seminar in Clinical Research (2 cr), spring semester
- GMS CI 794/795 Practicum in Clinical Research (2 cr), fall or spring semester
- GMS CI 804/805 Research (2 cr), fall or spring semester
A minimum of 10 credits in elective coursework: A wide variety of courses offered in Graduate Medical Sciences will count toward elective credit. A minimum of 10 credits must be taken as electives or directed study. Up to 4 credits across two semesters may be taken as the practicum or for research. Students who have completed one or more of the required courses before matriculation may acquire “advanced standing” for that requirement. “Advanced standing” means that the student may waive the requirement but would need to replace the course requirement credits by taking an elective course(s). The student would not need to retake the course requirement. To waive a course requirement, students must speak to their academic advisor and complete/submit a “Petition for Approval of Advanced Standing.” No transfer credits from other BU departments or institutions will be accepted.
Completion of a minimum of 240 hours of a practicum in clinical research is required for the degree. The goal of the practicum component is to provide the student hands-on exposure to clinical research. The student will work with a mentor and will be actively involved in the development, execution, and evaluation of a clinical research project or projects. During the practicum, it is expected that the student will be exposed to some or all of the following: clinical research planning, protocol preparation, interaction with Institutional Review Boards, regulatory requirements, selection of subjects/patients for the clinical trial, study monitoring, and data analysis. The practicum may be completed with a mentor who is actively conducting clinical research studies within a clinical research or hospital setting. It may also be performed under the direction of a clinical research professional within a drug, device, or biotechnology company, a clinical research organization (CRO), or site management organization (SMO) actively involved in clinical trials.
Capstone Project
Students in the MSCR program are required to complete a capstone project that provides a culminating experience and applies the principles and methods learned in the coursework to a real-life clinical study.
The goal of the capstone project is to demonstrate the student’s understanding of the clinical research process from both a theoretical and a practical point of view. Students conduct their capstone research in a wide variety of settings, including academic medical centers and local drug or device companies.
Students generally identify their capstone mentor and develop their capstone proposal while they are completing their coursework or practicum. The capstone project must involve the analysis and interpretation of data. Students are encouraged but are not required to conduct primary data collection. Once the final draft is approved, the student gives a short oral presentation on their capstone project to the readers, capstone mentor, MSCR students and faculty members, and any other interested parties. The purpose of the oral presentation is to demonstrate the student’s ability to (1) describe clearly the capstone topic, methods, and results, (2) demonstrate their understanding of study design and analytic principles and methods, and (3) place their research into a clinical context.
Additional Information
Students are required to abide by the rules and regulations of Graduate Medical Sciences:
- Credit toward a degree will only be obtained from a passing grade (A to B–).
- Grades of I and C+ or lower are interpreted as failures. A student receiving such grades in a total of 8 credit hours may be terminated. A student receiving a failing grade will not be permitted to take a makeup examination.
- A degree candidate, after completing all departmental course requirements, must register each regular semester as a continuing student and pay the continuing student fee until all remaining degree requirements are completed.
Please visit the Graduate Medical Sciences website at bumc.bu.edu/gms to view detailed administrative policies and procedures.
Study Options
The MSCR program can be completed on either a part-time or full-time basis depending on the student’s goal. Most of the courses take place in the late afternoons or early evenings to accommodate those who work during the day.
The program is designed so that a full-time student may complete their coursework in one academic year, including summer. Practicum and capstone components of the program should begin near completion of the coursework and the time frame for finishing them will be determined on a student-by-student basis by the program director or assistant director. A full-time student is enrolled in 12–18 credits per academic semester (fall and spring).
Part-time students must register for at least 4 but not more than 11 credits each academic semester until all course requirements are fulfilled.
Continuing Student
Students who have completed all departmental course requirements (32 credits) must register each subsequent semester as continuing students until all requirements for the degree have been completed.
Nondegree Option
A number of individuals with an accredited bachelor’s degree or its international equivalent, who are uncertain as to whether they want to enroll in MSCR, have the option of taking a few of the MSCR courses as a nondegree student, and may then make their decision about completing the MSCR application process. We allow the transfer toward the MSCR degree of up to 2 MSCR courses (8 credits) taken as a nondegree student. Applicants must submit a copy of the Application for Admission, indicating the specific objectives of the studies/courses sought. In addition, the applicant must submit (with the application) the nonrefundable application fee. Nondegree applicants are not eligible for University sources of financial aid or aid that requires matriculation in a degree program.
Related Bulletin Pages
- Graduate Medical Sciences Courses
- Abbreviations and Symbols
Beyond the Bulletin
- GMS Admissions
- Financial Assistance
- The Vesalius Certificate
- Anatomy & Neurobiology
- Behavioral Neuroscience
- Biochemistry
- MD/PhD in Bioinformatics
- Biomedical Forensic Sciences
- Biomedical Research Technologies
- Biomedical Sciences (PiBS)
- Dual MS in Medical Sciences and Clinical Research
- MD/MS in Clinical Research
- Hybrid Graduate Certificate Program in Clinical Research
- Online Graduate Certificate Program in Clinical Research
- Forensic Anthropology
- Genetic Counseling
- Genetics & Genomics
- Health Care Emergency Management
- Health Professions Education
- Medical Anthropology & Cross-Cultural Practice
- Medical Sciences
- Mental Health Counseling & Behavioral Medicine Program
- Microbiology
- Molecular & Translational Medicine
- Neuroscience
- Nutrition & Metabolism
- Oral Biology
- Oral Health Sciences
- Pathology & Laboratory Medicine
- Pharmacology & Experimental Therapeutics
- Physician Assistant
- Physiology or Biophysics
- Departments
- BU Medical Campus Library
- Graduate Medical Sciences Student Organization (GMSSO)
Terms of Use
Note that this information may change at any time. Read the full terms of use .
related websites
- Graduate Medical Sciences
Accreditation
Boston University is accredited by the New England Commission of Higher Education (NECHE).

- © Copyright
- Mobile Version
Programs in Clinical Research
1 year | full-time, 2 years | part-time, boston university medical campus.
Boston University’s programs in Clinical Research aim to meet the needs of health professionals engaged in the full-spectrum of patient-oriented research. We offer both master’s degree and certificate programs to individuals seeking careers in clinical research in industry or academic settings.
Clinical Research program offerings:
- Master of Science in Clinical Research (MSCR)
- Online Graduate Certificate
- Dual Degree in Medical Sciences and Clinical Research
- Dual Degree in Medicine and Clinical Research
About the MS in Clinical Research Program (MSCR)
Located in Boston’s South End at the Boston University Chobanian & Avedisian School of Medicine, the Master of Science in Clinical Research Program (MSCR) has been in existence since 2001 and is ranked #1 by College Choice among universities that offer an MS in Clinical Research. As an entity of BU CAMED, students are provided with opportunities and are exposed to resources that are part of the Chobanian & Avedisian School of Medicine , the School of Public Health , the Goldman School of Dental Medicine , Boston Medical Center , two VA Administrations, and BioSquare. All of this offers students endless opportunities for personal, academic and professional development.
The Master’s in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations. Other courses cover how basic science discoveries translate into clinical research and new therapies. The total course requirement is 32 credit hours: 22 hours are required curriculum, including practicum and research credits; 10 hours are elective courses. Students will also complete a research “practicum”; a hands-on involvement in a clinical research project under a scientific mentor. The final requirement for the degree is to conduct clinical research, write, and present a capstone project.
Who is this program designed for?
The Master’s in Clinical Research program is designed for anyone interested in a career in clinical research. This includes those with MD or PhD degrees interested in becoming independent principal investigators, as well as those with bachelors or masters degrees who seek advancement in a research career in industry or academic settings.
Online Graduate Clinical Research Certificate
If you are not ready to commit to our Master’s program, we also offer an Online Graduate Certificate in Clinical Research. This certificate program involves 4 courses across two semesters, and can be completed in one academic year.
Learning Objectives
Upon completion of the Master’s in Clinical Research program, students are expected to:
- Demonstrate the ability to design and conduct clinical research, analyze results and answer a research question.
- Demonstrate the ability to read and critique the clinical research literature.
- Present clinical research findings (from literature or their own research) to peers.
Our Mission
Inspire, Instruct, Innovate … The MS in Clinical Research program is dedicated to the discovery, development, and application of knowledge as it pertains to all areas of clinical research. Our mission is to foster an engaging and effective educational environment that promotes the pursuit of outstanding teaching and learning through formal classroom and practical training. With established collaborative relationships with pharmaceutical, biotech, and academic institutions, students are provided with unique opportunities to pursue clinical research in areas that are of personal and professional interest.
We hope that the information you receive about our program encourages you to pursue your graduate degree in clinical research with us. If you are interested, click here to get your application started.
If you would like to visit our campus or have any questions about our program, please contact Stacey Hess Pino at [email protected] . We look forward to meeting and/or speaking with you!
Janice Weinberg, ScD Director, MS in Clinical Research; P rofessor, Department of Biostatistics
Stacey Hess Pino, MS, MS Assistant Director, MS in Clinical Research; Director, Online Graduate Certificate Program in Clinical Research; Assistant Professor, Department of Medical Sciences & Education
View the latest COVID-19 operational updates | Learn more
School of Public Health
A nationally top-ranked institution that offers a superior array of advanced degrees and is home to world-renowned research and training centers.
- Degrees & Programs
- Master of Science (MS)
Clinical Research MS
- Epidemiology & Community Health Student Services [email protected]
- Director of Graduate Studies Dr. Kamakshi Lakshminarayan, MD, PhD
Join the next generation of clinical and translational researchers.
The Clinical Research MS program trains the next generation of researchers and serves as a career development path for physician-scientists, clinical scholars, and biomedical researchers. Students learn to conduct patient-oriented research and clinical trials, as well as develop therapeutic interventions. Our curriculum will deepen your expertise in epidemiologic and behavioral studies, outcomes-based research, grant writing, and data analytics.
- Program Brochure
Alumni Profile
Ina Park, MS '07 is the 2018 recipient of the Emerging Leader Award.

Advantages of the Program
- Career and impact focused. The MS program provides career development for physician-scientists, clinical scholars, and biomedical researchers. The certificate provides training for research coordinators and project managers.
- Integrated health sciences. The U of M’s infrastructure of six health sciences schools, gives students the ability to learn from and work with a diverse array of health experts.
- Personal attention: The Director of Graduate Studies is a practicing clinician as well as an NIH–AHRQ–funded researcher who has completed a career development award and is available to mentor and advise students on academic career development.
- Flexible curriculum. Students tailor the program with courses that fit their training needs. All courses are offered online.
Connect with Us
- Request More Information
- Prospective Students
- Current Students

Contact 420 Delaware St. S.E. Minneapolis, MN 55455
612-626-3500 [email protected]
- Education Home
- Medical Education Technology Support
- Graduate Medical Education
- Medical Scientist Training Program
- Public Health Sciences Program
- Continuing Medical Education
- Clinical Performance Education Center
- Center for Excellence in Education
- Research Home
- Biochemistry & Molecular Genetics
- Biomedical Engineering
- Cell Biology
- Microbiology, Immunology, & Cancer Biology (MIC)
- Molecular Physiology & Biological Physics
- Neuroscience
- Pharmacology
- Public Health Sciences
- Office for Research
- Clinical Research
- Clinical Trials Office
- Funding Opportunities
- Grants & Contracts
- Research Faculty Directory
- Cancer Center
- Cardiovascular Research Center
- Carter Immunology Center
- Center for Behavioral Health & Technology
- Center for Brain Immunology & Glia
- Center for Diabetes Technology
- Center for Immunity, Inflammation & Regenerative Medicine
- Center for Public Health Genomics
- Center for Membrane & Cell Physiology
- Center for Research in Reproduction
- Myles H. Thaler Center for AIDS & Human Retrovirus Research
- Child Health Research Center (Pediatrics)
- Division of Perceptual Studies
- Research News: The Making of Medicine
- Core Facilities
- Virginia Research Resources Consortium
- Center for Advanced Vision Science
- Charles O. Strickler Transplant Center
- Keck Center for Cellular Imaging
- Institute of Law, Psychiatry & Public Policy
- Translational Health Research Institute of Virginia
- Clinical Home
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopaedic Surgery
- Otolaryngology
- Physical Medicine & Rehabilitation
- Plastic Surgery, Maxillofacial, & Oral Health
- Psychiatry & Neurobehavioral Sciences
- Radiation Oncology
- Radiology & Medical Imaging
- UVA Health: Patient Care
- Diversity Home
- Diversity Overview
- Student Resources
- GME Trainee Resources
- Faculty Resources
- Community Resources
Master of Science in Clinical Research (MS-CR)
Apply today.
The Master of Science in Clinical Research Program in the Department of Public Health Sciences is an interdisciplinary graduate degree designed to meet the changing needs of the current health care field, particularly the increasing need for trained professionals with well-developed quantitative and analytic skills.
The MS-CR program provides training to health and medical professionals with significant relevant experience who have a physician mentor and desire/need quantitative and analytic skills in patient-oriented and translational research, as well as more traditional clinical investigation. Using an interdisciplinary blend of biostatistics, epidemiology, clinical trial design, medical informatics, and health services research, the MS-CR program equips clinical researchers with the statistical and data management tools needed to conduct translational clinical and comparative effectiveness studies in medical care. Students complete a minimum 31-credit curriculum that includes core courses, specialized coursework, and a final independent research project, co-mentored by a PHS faculty member and the student’s research advisor. Since its inception in 1997, the MS-CR program has graduated over 150 students, most of whom were physicians in training.
The experience is one of intensive study aimed toward giving students the analytical skills needed in all areas of clinical research.
- MS-CR Curriculum
Dual MD – MS CR Program
- MS-CR Class Schedules – Spring and Fall
Program History
Launched in 1997, the MS-CR program subsumed the existing Master of Science in Epidemiology program, which was created in 1981. The new program builds on the structure and courses previously offered in the Epidemiology program, while offering a breadth and diversity of courses not previously possible. The MS in Clinical Research is administered by the Department of Public Health Sciences in the School of Medicine.
Program Administration

PHS students take a break in front of UVA’s Old Medical School Building. (Photo taken by Abby Sacks.)
- Director Academic Programs & Registrar: Tracey C. Brookman
- Education Program Chair: Ruth Gaare Bernheim, J.D., M.P.H.
- Consulting & Collaboration
- MPH Values, Mission and Goals
- MPH Program Competencies
- MPH Program Faculty and Staff
- MPH Newsletters
- Data-Driven Solutions
- History of the MPH Program
- Collaborative Community Partnerships
- Population Health Equity
- MD/MS-CR Program
- Certificate of Public Health Sciences
- Prospective GPH Students
- 4 + 1 Undergraduate – Public Health Program
- PHS Instructional Faculty
- PHS Education Committees
- Classroom Directions
- New and International Student Information
- Frequently Asked Questions
- Faculty & Administration
- Research Programs & Affiliated Centers
- Submit PHS News & Events
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
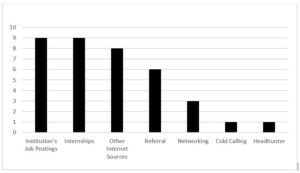
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
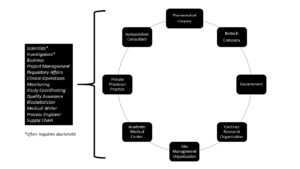
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
Master of Science in Clinical Investigation
Foundational skills for success in clinical and translational research..

- School School of Medicine
- Duration 35 credits
- Format Online
- Enrollment Fall
- Tuition $65,502 total
About the Program
Vanderbilt’s Master of Science in Clinical Investigation (MSCI) program trains investigators in the techniques and processes used in clinical and translational research. This program provides a direct, mentored experience in clinical investigation and, through didactic work, provides trainees with a strong foundation in study design, biostatistics with R, biomedical ethics, human genetics, drug and device development, and genomics. The program typically takes two years to complete. Graduates successfully compete for grants such as the NIH K23, VA Career Development Award, NIH R01, and major foundation grants.
Facts & Stats
- 99% Program completion rate
- $109.7M+ Foundation grants awarded to MSCI trainees since 2000
- $160M+ K and R federal awards to MSCI alumni and students
Key Takeaways
- Mentored research apprenticeship
- Didactic work covering the essentials of study design, biostatistics, ethics, drug development, and data analysis
- Final thesis based research project resulting in a first-authored manuscript to a peer-reviewed journal, or a completed proposal for a major federal or foundational grant
- Career path development
Who Should Enroll?
- Board-eligible physicians enrolled in a fellowship
- Residents with protected time for research
- Medical students with protected time for research
- Vanderbilt faculty members with the consent of their Department Chair
- Post-Doctoral PhDs anticipating a career in clinical and translational research
- PhD candidates in the Nursing School anticipating a career in clinical and translational research
- International students are eligible to enroll (ineligible if in the US on a student visa at the time of enrollment)
Completion of the Vanderbilt MSCI program has been critical in my journey to becoming a successful physician-scientist. I completed the program amid the COVID pandemic, which one could imagine afforded several challenges, each of which was seamlessly navigated by the MSCI leadership team—Eric, Arnita, and Alp. The faculty is world-class and very approachable. I could attempt to list all the skills I learned through the program, but I think most importantly, I learned to be an ethical and thoughtful physician-scientist equipped with the knowledge to answer research questions. Donald Lynch, MD, MSCI Assistant Professor, Medicine, Cardiovascular Health and Disease, University of Cincinnati
Master of Science in Clinical Research
100% Online
Our online master’s degree program is designed to broaden your skillset and expand your network. Flexibility of taking your classes from anywhere. Become even more competitive with our dual degree options. Enhance your leadership opportunities in the pharmaceutical and biotechnology industries.
Apply Schedule Appointment Request Information
Curriculum & Outcomes
The MS in Clinical Research curriculum provides foundational and advanced knowledge in Clinical Research including drug development, clinical trial operations, study design, data management, regulatory affairs, medical ethics, scientific writing, and biostatistical literacy. Students can enroll full-time to complete the program in two years; or, choose a part-time option.
Graduates are prepared for leadership positions in pharmaceutical and biotechnology industries, government agencies, medical institutions, academic institutions and hospitals. Within these fields, graduates are qualified to work as clinical investigators, clinical research associates (in-house or remote), clinical research coordinators, data research coordinators, project support, project managers, data managers, safety specialists, regulatory managers, and many other positions.
View Admissions Requirements View curriculum
Our Faculty
Faculty members in the Department of Clinical Research are recognized experts, published scientists, committed mentors and proven professionals. They bring a combination of scholarly theory and real-world experience to the courses.
Online Degree Program
This 100% online program will allow students to excel academically as well as professionally in the exciting and growing field of clinical research with the same intensity and quality of seated classes without the time and travel constraints of a traditional classroom.
Master of Health Sciences in Clinical Research Training
- Financial Information
- Requirements
Department Chair: David C. Page, PhD Program Director: Steven C. Grambow, PhD Co-Directors: Kevin P. Weinfurt, PhD; John W. Williams, MD, MHSc Senior Program Coordinator: Gail D. Ladd
Website: biostat.duke.edu/education-and-training/clinical-research-training-program
This Duke University School of Medicine program provides formal academic training in the quantitative and methodological principles of clinical research. In contrast to a public health degree which focuses on epidemiology, this program is designed primarily for clinical fellows who are training for academic careers. The program offers formal courses in research design, statistical analysis, medical genomics, research management, scientific communication, research project development, and responsible conduct of research. Students who complete a prescribed course of study in the training program are awarded a Master of Health Sciences in Clinical Research degree by the School of Medicine.
The Clinical Research Training Program is offered by core faculty from the Department of Biostatistics and Bioinformatics and other clinical and basic science departments within the School of Medicine.
Basic Science Research Track (BSRT) Degree Option
The Basic Science Research Track (BSRT) is an optional customized curriculum for degree candidates designed specifically for physician-scientists. The track prepares individuals for careers as physician-scientists across a range of discovery sciences. The curriculum includes coursework that prepares researchers to perform rigorous basic science; manage, analyze, and present data; oversee a laboratory; and successfully compete for research funding.
This track requires eighteen (18) credits of graded coursework and eighteen (18) credits for an approved research project. Five (5) courses (241, 253, 275, 245/276, and 279) are required for all BSRT candidates. Students may choose from other offered courses for the remaining required credits. Trainees begin work on the required research project during their first year to provide a deep immersion in basic and laboratory research methods. The second year includes electives and a required course on scientific communications to lay the foundation for a successful career in basic research. The second year incorporates a successful defense of the research project.
Certificate (Academic Core in Clinical Research Certificate)
The certificate option leads to the Academic Core in Clinical Research Certificate awarded by the Duke University School of Medicine. Applicants must successfully complete the five (5) required core courses which constitute the foundation of the full degree program (CRP 241, 242, 245, 253, and 254). Students who complete the certificate may convert their status to degree-seeking and apply completed coursework toward degree requirements. Students must change status prior to receiving their certificate in order to become a degree candidate. If a student elects to pursue a degree program, a certificate will not be awarded. If a student is awarded a certificate and later decides to seek the degree, they are required to re-apply to the program and contact Gail Ladd ([email protected]).
The courses in the program are also available to qualified individuals who want to acquire specific skills but who may not want to pursue a master’s degree. In addition to clinical fellows, such individuals include faculty members, post-doctoral fellows, other trainees, and health professionals at Duke and NIH. This option allows the flexibility of taking various combinations of courses subject only to constraints imposed by course prerequisites. Students who are interested i seeking a certificate or degree may contact Gail Ladd ( [email protected] ) for additional information.
Non-program
The courses in the program are also available to qualified individuals who are enrolled in other Duke credit-bearing degree programs. To register for CRTP courses, please contact Gail Ladd ( [email protected] ) for permission and to verify eligibility.
CRTP does not permit auditing.

How to Become a Clinical Research Physician

Renee Whitmore
Editor-in-Chief
Share this on:

Ready to start your journey?
In this article, we will be covering…
If you’re ready to transition from practicing traditional medical health care to a more strategic role within the medical field, becoming a clinical research physician may just be the next health care challenge you’re seeking.
As a clinical research physician, you will meet and discuss basic medical science, examine strategies, and analyze commercial, regulatory, and economic factors that impact local, regional, national, and even international health care.
Pursuing a job as a clinical research physician can mean working in the corporate health care sector, with health insurance policy-creation, with either in-state, local or federal government regulations, or for a global organization like the World Health Organization (WHO). Clinical research physicians typically begin their careers with a traditional four-year bachelor’s degree , medical school, residency, and then an actual hands-on career in the medical specialty of their choice. After a combination of medical certification and academic experience, paired with clinical medical work, a physician can explore transitioning to a clinical research physician role.
According to Payscale , the median pay for clinical research physicians is about $189,231 and the US Bureau of Labor and Statistics indicates that physicians in practice made a median income of $208,000. The best part? Once you move on to the corporate world, you can make even more.

What Education and Experience Do You Need to Become a Clinical Research Physician?
Any aspiring clinical research physician will typically need a bachelor’s degree in a physical or life science, a four-year degree from a medical school, and, depending on your specialty, three to seven years of an internship and residency program. After residency, years of clinical medical practice as a physician are required before applying for a position as a clinical research physician.
In addition to working for an international corporation, a global health care organization like the World Health Organization, or a transition into academia, clinical research physicians can transition into the medical writing field, work as a consultant for media outlets, or as a legal consultant for court cases.
Post-doctoral research work, medical fellowships, and pharmaceutical training or internships are not required but can be helpful in your job search.
Why Do Clinical Research Physicians Need Clinical and Medical Experience?
Whether a clinical research physician transitions into a consulting or laboratory research position, corporate health care, health insurance policy-creation, or moves to a state, local, or federal government regulation position, medical experience is required. Hands-on practice in patient assessment, diagnostics, and treatment provides the basis for success in a non-clinical setting.
While clinical research is typically part of the coursework and certification process of obtaining a bachelor’s degree or PhD in this field of study, working with and assisting existing clinical research scientists provides an extra level of research experience.

If you enter clinical practice with an idea of what type of clinical research physician position you eventually would like to secure, specializing in a similar field is beneficial. The combination of specialized medical training, building researching skills, and focused study can also elevate your marketability as a clinical research physician.
If you already have hands-on clinical experience as a health care provider and are intrigued by the strategic, planning, and/or regulatory side of medicine, it might be time for you to consider transitioning into a role as a clinical research physician.
While some of these positions are laboratory-based and focus primarily on research, data collection, and results analysis, there are numerous other options in this career path that offer work in the corporate, nonprofit, and governmental arenas.

Want to continue learning?

How to Start a Civil Engineering Firm

Most Affordable Online Health Care Administration Degrees

How Much Does a Sports Medicine Physician Make?

© Copyright 2024 Online Schools Report
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
- Doctor of Philosophy (PhD) in Clinical Investigation
Offered By: Graduate Training Programs in Clinical Investigation (GTPCI)
Onsite | Full-Time | 3 – 5 years
- MAS Application Fee Waiver Requirements
- Master of Arts (MA) in Geography and Environmental Engineering
- Master of Arts and Master of Science in Public Health (MA/MSPH)
- Master of Arts in Public Health Biology (MAPHB)
- Master of Bioethics (MBE)
- Mission, Vision, and Values
- Student Experience
- Program Outcomes
- For Hopkins Undergraduate Students
- Master of Health Science (MHS) - Department of Biochemistry and Molecular Biology
- Master of Health Science (MHS) - Department of Epidemiology
- Alumni Update
- MHS Combined with a Certificate Program
- Master of Health Science (MHS) - Department of Molecular Microbiology and Immunology
- Alumni Highlights
- Post-Baccalaureate Program in Environmental Health for Pre-Medicine Students
- Bachelor's/MHS in Health Economics and Outcomes Research
- MHS HEOR Careers
- Frequently Asked Questions
- Master of Health Science (MHS)
- Concurrent School-Wide Master of Health Science Program in Biostatistics
- Master of Health Science - Department of Population, Family and Reproductive Health
- Master of Health Science Online (MHS) - Department of Population, Family and Reproductive Health
- Careers in Health Economics
- Core Competencies
- Meet the Director
- What is Health Economics
- MPH Capstone Schedule
- Concentrations
- Online/Part-Time Format
- Requirements
Tuition and Funding
- Executive Board Faculty
- Master of Science (MS) in Geography and Environmental Engineering
- Independent Professional Project and Final Essay
- Program Objectives and Outcomes
- Internships
- Master of Science (ScM) - Department of Biochemistry and Molecular Biology
- Master of Science (ScM) - Department of Biostatistics
- Master of Science (ScM) - Department of Epidemiology
- Master of Science (ScM) - Department of Molecular Microbiology and Immunology
- ScM Faculty Advisers
- Master of Science in Engineering (MSE) in Geography and Environmental Engineering
- Bachelor's/MSPH in Health Policy
- FAQ for MSPH in Health Policy
- Field Placement Experience
- MSPH Capstone
- MSPH Practicum
- Required and Elective Courses
- Student Timeline
- Career Opportunities
- 38-Week Dietetics Practicum
- Completion Requirements
- MSPH/RD Program FAQ
- Program Goals
- Master's Essay Titles
- Application Fee Waiver Requirements
- Doctor of Philosophy (PhD) - Department of Biostatistics
- Doctor of Philosophy (PhD) - Department of Epidemiology
- Program Goals and Expectations
- Doctor of Philosophy (PhD) - Department of Molecular Microbiology and Immunology
- Doctor of Philosophy (PhD) - Department of Population, Family and Reproductive Health
- Track in Environmental Sustainability, Resilience, and Health
- Track in Exposure Sciences and Environmental Epidemiology
- Track in Health Security
- Track in Toxicology, Physiology and Molecular Mechanisms
- PhD in Geography and Environmental Engineering Faculty Advisers
- Recent Graduates and Dissertation Titles
- PhD Funding
- PhD TA Requirement
- Recent Dissertation Titles
- JHU-Tsinghua Doctor of Public Health
- Core Course Requirements
- Concentration in Women’s and Reproductive Health
- Custom Track
- Concentration in Environmental Health
- Concentration in Global Health: Policy and Evaluation
- Concentration in Health Equity and Social Justice
- Concentration in Health Policy and Management
- Concentration in Implementation Science
- Meet Current Students
- Combined Bachelor's / Master's Programs
- Concurrent MHS Option for BSPH Doctoral Students
- Concurrent MSPH Option for JHSPH Doctoral students
- Doctor of Medicine and Doctor of Philosophy (MD/PhD)
- Adolescent Health Certificate Program
- Bioethics Certificate Program
- Climate and Health Certificate Program
- Clinical Trials Certificate Program
- Community- Based Public Health Certificate Program
- Demographic Methods Certificate Program
- Environmental and Occupational Health Certificate Program
- Epidemiology for Public Health Professionals Certificate Program
- Evaluation: International Health Programs Certificate Program
- Food Systems, the Environment and Public Health Certificate Program
- Frequently Asked Questions for Certificate Programs
- Gender and Health Certificate Program
- Gerontology Certificate Program
- Global Digital Health Certificate Program
- Global Health Certificate Program
- Global Health Practice Certificate Program
- Health Communication Certificate Program
- Health Disparities and Health Inequality Certificate Program
- Health Education Certificate Program
- Health Finance and Management Certificate Program
- Health and Human Rights Certificate Program
- Healthcare Epidemiology and Infection Prevention and Control Certificate Program
- Humane Sciences and Toxicology Policy Certificate Program
- Humanitarian Health Certificate Program
- Implementation Science and Research Practice Certificate Program
- Injury and Violence Prevention Certificate Program
- International Healthcare Management and Leadership Certificate Program
- Leadership for Public Health and Healthcare Certificate Program
- Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Public Health Certificate Program
- Maternal and Child Health Certificate Program
- Mental Health Policy, Economics and Services Certificate Program
- Non-Degree Students General Admissions Info
- Pharmacoepidemiology and Drug Safety Certificate Program
- Population Health Management Certificate Program
- Population and Health Certificate Program
- Product Stewardship for Sustainability Certificate Program
- Public Health Advocacy Certificate Program
- Public Health Economics Certificate Program
- Public Health Informatics Certificate Program
- Public Health Practice Certificate Program
- Declaration of Intent - Public Health Preparedness
- Public Health Training Certificate for American Indian Health Professionals
- Public Mental Health Research Certificate Program
- Quality, Patient Safety and Outcomes Research Certificate Program
- Quantitative Methods in Public Health Certificate Program
- Requirements for Successful Completion of a Certificate Program
- Rigor, Reproducibility, and Responsibility in Scientific Practice Certificate Program
- Risk Sciences and Public Policy Certificate Program
- Spatial Analysis for Public Health Certificate Program
- Training Certificate in Public Health
- Tropical Medicine Certificate Program
- Tuition for Certificate Programs
- Vaccine Science and Policy Certificate Program
- Online Student Experience
- Online Programs for Applied Learning
- Barcelona Information
- Registration, Tuition, and Fees
- Agency Scholarship Application
- General Scholarship Application
- UPF Scholarship Application
- Course Evaluations
- Online Courses
- Registration
- General Institute Tuition Information
- International Students
- Directions to the Bloomberg School
- All Courses
- Important Guidance for ONSITE Students
- D.C. Courses
- Registration and Fees
- Cancellation and Closure Policies
- Application Procedures
- Career Search
- Current Activities
- Current Trainees
- Related Links
- Process for Appointing Postdoctoral Fellows
- Message from the Director
- Program Details
- Admissions FAQ
- Current Residents
- Elective Opportunities for Visiting Trainees
- What is Occupational and Environmental Medicine?
- Admissions Info
- Graduates by Year
- Compensation and Benefits
- How to Apply
- Academic Committee
- Course Details and Registration
- Tuition and Fees
- ONLINE SOCI PROGRAM
- Principal Faculty
- Johns Hopkins RAPID Psychological First Aid
- General Application
- JHHS Application
- Areas of Study
- Important Dates
- Our Faculty
- Welcome Letter
- Descripción los Cursos
- Programa en Epidemiología para Gestores de Salud, Basado en Internet
- Consultants
- Britt Dahlberg, PhD
- Joke Bradt, PhD, MT-BC
- Mark R. Luborsky, PhD
- Marsha Wittink, PhD
- Rebekka Lee, ScD
- Su Yeon Lee-Tauler, PhD
- Theresa Hoeft, PhD
- Vicki L. Plano Clark, PhD
- Program Retreat
- Mixed Methods Applications: Illustrations
- Announcements
- 2023 Call for Applications
- Jennifer I Manuel, PhD, MSW
- Joke Bradt, PhD
- Josiemer Mattei, PhD, MPH
- Justin Sanders, MD, MSc
- Linda Charmaran, PhD
- Nao Hagiwara, PhD
- Nynikka R. A. Palmer, DrPH, MPH
- Olayinka O. Shiyanbola, BPharm, PhD
- Sarah Ronis, MD, MPH
- Susan D. Brown, PhD
- Tara Lagu, MD, MPH
- Theresa Hoft, PhD
- Wynne E. Norton, PhD
- Yvonne Mensa-Wilmot, PhD, MPH
- A. Susana Ramírez, PhD, MPH
- Animesh Sabnis, MD, MSHS
- Autumn Kieber-Emmons, MD, MPH
- Benjamin Han, MD, MPH
- Brooke A. Levandowski, PhD, MPA
- Camille R. Quinn, PhD, AM, LCSW
- Justine Wu, MD, MPH
- Kelly Aschbrenner, PhD
- Kim N. Danforth, ScD, MPH
- Loreto Leiva, PhD
- Marie Brault, PhD
- Mary E. Cooley, PhD, RN, FAAN
- Meganne K. Masko, PhD, MT-BC/L
- PhuongThao D. Le, PhD, MPH
- Rebecca Lobb, ScD, MPH
- Allegra R. Gordon, ScD MPH
- Anita Misra-Hebert, MD MPH FACP
- Arden M. Morris, MD, MPH
- Caroline Silva, PhD
- Danielle Davidov, PhD
- Hans Oh, PhD
- J. Nicholas Dionne-Odom, PhD RN ACHPN
- Jacqueline Mogle, PhD
- Jammie Hopkins, DrPH, MS
- Joe Glass, PhD MSW
- Karen Whiteman, PhD MSW
- Katie Schultz, PhD MSW
- Rose Molina, MD
- Uriyoán Colón-Ramos, ScD MPA
- Andrew Riley, PhD
- Byron J. Powell, PhD, LCSW
- Carrie Nieman MD, MPH
- Charles R. Rogers, PhD, MPH, MS, CHES®
- Emily E. Haroz, PhD
- Jennifer Tsui, Ph.D., M.P.H.
- Jessica Magidson, PhD
- Katherine Sanchez, PhD, LCSW
- Kelly Doran, MD, MHS
- Kiara Alvarez, PhD
- LaPrincess C. Brewer, MD, MPH
- Melissa Radey, PhD, MA, MSSW
- Sophia L. Johnson, PharmD, MPH, PhD
- Supriya Gupta Mohile, MD, MS
- Virginia McKay, PhD
- Andrew Cohen, MD, PhD
- Angela Chen, PhD, PMHNP-BC, RN
- Christopher Salas-Wright, PhD, MSW
- Eliza Park MD, MS
- Jaime M. Hughes, PhD, MPH, MSW
- Johanne Eliacin, PhD, HSPP
- Lingrui Liu ScD MS
- Meaghan Kennedy, MD
- Nicole Stadnick, PhD, MPH
- Paula Aristizabal, MD
- Radhika Sundararajan, MD
- Sara Mamo, AuD, PhD
- Tullika Garg, MD MPH FACS
- Allison Magnuson, DO
- Ariel Williamson PhD, DBSM
- Benita Bamgbade, PharmD, PhD
- Christopher Woodrell MD
- Hung-Jui (Ray) Tan, MD, MSHPM
- Jasmine Abrams, PhD
- Jose Alejandro Rauh-Hain, MD
- Karen Flórez, DrPH, MPH
- Lavanya Vasudevan, PhD, MPH, CPH
- Maria Garcia, MD, MPH
- Robert Brady, PhD
- Saria Hassan, MD
- Scherezade Mama, DrPH
- Yuan Lu, ScD
- 2021 Scholars
- Sign Up for Our Email List
- Workforce Training
- Cells-to-Society Courses
- Course/Section Numbers Explained
- Pathway Program with Goucher College
- The George G. Graham Lecture
About the PhD in Clinical Investigation Program
The program is targeted toward internal physician postdoctoral fellows in clinical departments of the School of Medicine. It involves one year of full-time academic classroom work, followed by at least two years of mentored training in clinical research. The combination of a year of instruction and a year of clinicals allows students the scientific grounding for subsequent original research. This research effort is jointly mentored by faculty from the program and a mentor from the student’s SOM department. After fulfilling all requirements, a Doctor of Philosophy degree in Clinical Investigation is awarded by the Johns Hopkins Bloomberg School of Public Health.
Curriculum for the PhD in Clinical Investigation
Browse an overview of this program's requirements in the JHU Academic Catalogue and explore all course offerings in the Bloomberg School Course Directory .
Prerequisites for the PhD Degree
- Satisfactory completion of 90 credit hours of course work, including one year of full-time in-residency course work
- Five additional courses to be taken in the second or third years
- Continuous registration for the Research Forum and registration for Thesis Research each term
- Completion of a Comprehensive Exam at the end of the didactic year
- Satisfaction of all university requirements for the PhD, including completion of a Preliminary Oral Examination, Thesis Preparation, and Thesis Defense
Admissions Requirements
For general admissions requirements, please visit the How to Apply page. For our PhD specific application requirements, please see our How to Apply page.
This specific program also requires:
Prior Graduate Degree
Advanced medical degree: e.g., MD, MBBS, PhD
Prior Work Experience
Work with human subjects in clinical investigation
Standardized Test Scores
Standardized test scores are required for this program. This program accepts the following standardized test scores: USMLE and GRE or MCAT. Applications will be reviewed holistically based on all application components.
GTPCI is one of 60 national recipients of an NIH-sponsored CTSA KL2 Award to support institutional career development programs for physicians and dentists, encouraging them to become independent, patient-oriented clinical investigators. This Multi- disciplinary Clinical Research Career Development Program funds clinical research training for a broad group of physicians, dentists, and other scientists who have a doctorate in a health-related field, including pharmacy, nursing, epidemiology, and behavioral sciences. The Johns Hopkins KL2 program will provide career development support for junior faculty physicians or dentists from within Johns Hopkins Medical Institutions.
Information regarding the cost of tuition and fees can be found on the Bloomberg School's Tuition and Fees page.
Need-Based Relocation Grants Students who are admitted to PhD programs at JHU starting in Fall 2023 or beyond can apply to receive a $1500 need-based grant to offset the costs of relocating to be able to attend JHU. These grants provide funding to a portion of incoming students who, without this money, may otherwise not be able to afford to relocate to JHU for their PhD program. This is not a merit-based grant. Applications will be evaluated solely based on financial need. View more information about the need-based relocation grants for PhD students .
Questions about the program? We're happy to help.
Director Khalil Ghanem, MD, PhD
Academic Program Manager Cristina A. DeNardo, MEd 410-502-9734 [email protected]

- Graduate Certificate in Clinical Research
- Graduate Certificate in Healthcare Compliance
- Online Experience
Accelerate your career with a clinical research certificate program online

With the healthcare and social services sector poised to generate 45% of new U.S. jobs through 2032, developing expertise in clinical research can generate dynamic and promising career prospects. 1 Whether you're transitioning into clinical research from an unrelated field or advancing from a clinical healthcare role into management, Kent State University’s clinical research certificate program sharpens the core skills needed for these roles.
In this post, we'll identify key traits for success in clinical research, discuss how Kent State’s online Graduate Certificate in Clinical Research equips you for career growth and address common career change concerns.
Discovering Your Fit in Clinical Research
Clinical research is a field that thrives on diversity—of backgrounds, skills, and experiences. Whether you come from a healthcare background, the sciences, or fields less directly connected, like IT or management, your past experiences bring valuable perspectives. What's critical is your passion for advancing medical knowledge and improving patient outcomes.
Core skills that transition well into clinical research include meticulous attention to detail, analytical thinking, and robust interpersonal communication. These competencies help manage complex research protocols and collaborate effectively with diverse teams. If these abilities are part of your current skill set, you might already be more prepared for a career in clinical research than you realize.
Furthermore, being naturally curious and resilient are personal traits that complement professional skills in this field. Clinical researchers often face unforeseen challenges and complex questions requiring innovative solutions. Such traits help navigate these challenges and drive discoveries in medical research.
Evaluating these skills and traits against your current capabilities can be an illuminating first step to uncovering your potential in this dynamic field. The following sections will guide you on how to translate your existing skills into a flourishing career in clinical research with help from a flexible, robust educational program.
Empower Your Transition with a Clinical Research Certificate Program
Deciding to pursue a career in clinical research can be transformative, but making the leap requires solid, reliable support. Kent State's online clinical research certificate program provides that foundation, designed specifically to prepare individuals from diverse backgrounds for the challenges and opportunities in clinical research.
The program is structured to ease the burden of transition for busy professionals. It offers online coursework that allows students to balance their studies with personal and professional responsibilities. This format respects your current lifestyle and ensures that you can advance your education without sacrificing other areas of your life.
Clinical Research Skills for Clinical Healthcare Managers
Clinical care professionals, including nurses and physicians, can accelerate their careers through specialized clinical research training for healthcare professionals, enhancing their clinical research management skills and understanding of clinical studies. Available courses cover crucial topics like clinical research study design, clinical research results analysis, and effective clinical research grant writing.
Each course within the online clinical research certificate program delivers practical and theoretical knowledge for a successful career in clinical research. You will learn from experienced professionals who bring real-world insights into the classroom. Choose your focus from critical areas, including regulatory compliance, effective clinical management practices, and up-to-date methodologies. Design your curriculum, with help from Kent State’s esteemed faculty and dedicated staff, to equip yourself to excel in your niche of this dynamic industry.
Support Beyond the Virtual Classroom
Support continues beyond comprehensive curriculum choices . Kent State also offers practicum opportunities that help bridge the gap between academic and professional achievements, further enhancing your readiness for career advancement.
Additionally, the program encourages collaboration and networking among peers, which enhances your learning experience and builds a professional network that can be beneficial throughout your career. This community aspect fosters a sense of belonging and mutual support among students, which is vital as you navigate your new career path.
Addressing Your Career Change Concerns
Embarking on a new career path, particularly in a specialized field like clinical research, can evoke excitement and concern. At Kent State, the clinical research certificate program online addresses these concerns directly with a structured yet flexible learning environment designed for your success. Understanding that continuity in work and personal life is essential, the courses are delivered entirely online, with asynchronous course delivery. This format allows you to navigate your studies on your schedule, ensuring you do not have to put your professional life on hold.
A common concern for career changers is whether the investment in graduate-level education will lead to real job opportunities. Kent State carefully tailored the online clinical research certificate program to meet industry demands. The program not only educates you on the fundamentals but also on advanced topics that are immediately applicable in the workforce, such as biostatistics, clinical epidemiology, and regulatory affairs.
Moreover, the certificate program’s optional practicum, which you can complete locally, equips you with practical, hands-on experience under the guidance of experienced professionals, smoothing your transition into a new career role.
By addressing typical concerns head-on, Kent State's clinical research certificate program helps make your career transition as seamless and rewarding as possible. With a clear focus on educational quality and practical application, you are well-prepared to meet the challenges of a new role in this dynamic field.
Embrace a Future in Clinical Research with Confidence and Support
Earning Kent State’s online Graduate Certificate in Clinical Research opens the door to a future rich with opportunity. This program thoroughly prepares you with skills and practical experiences directly supporting your career goals. Contact an admissions outreach advisor to learn more about how the clinical research certificate program can help you shape your future.
- Retrieved on May 8, 2024, from bls.gov/news.release/pdf/ecopro.pdf
Return to Blog
Kent State University has engaged Everspring , a leading provider of education and technology services, to support select aspects of program delivery.
- Area of Interest
- Contact Info
- Get Started
What program are you interested in?
Master of Public Health
MS in Clinical Epidemiology
Bachelor of Science in Public Health
Psychiatry's new frontiers
This new issue of Stanford Medicine magazine reports on emerging research and innovative treatments to improve mental health.
Follow Stanford Medicine
Health Care
Healing through compassion and world-class science
Fueling discovery and innovation to advance human health
Empowering tomorrow's leading physicians and scientists
School Leadership
Academic Affairs
Continuing Medical Education
Faculty Development and Diversity
Faculty Profiles
MD Admissions
PhD Program
Dual-Degree Programs
Masters Programs
Community Engagement
Clinical Trials
High School & Undergraduate Programs
News, Events and Resources
Stanford Medicine leaders' message regarding Supreme Court ruling on race-conscious university admissions

About Stanford Medicine
A leader in the biomedical revolution, Stanford Medicine has a long tradition of leadership in pioneering research, creative teaching protocols and effective clinical therapies.

Night owl behavior could hurt mental health, sleep study finds
In a new, large-scale study of sleep behavior, Stanford Medicine scientists found that night owls don’t really thrive late at night.

Speech impairment in Parkinson’s
New research by Stanford Medicine scientists uncovers the brain connections that could be essential to preserving speech.

In the age of fentanyl, factual drug education can save teen lives
Toolkits designed by Stanford Medicine researchers are helping teens think critically about the choices they make around substance use.

Could the avian flu be our next pandemic threat?
What does it mean that H5N1 bird flu, also known as highly pathogenic avian influenza A, is spreading among dairy cows?
.png)
Symposium tackles AI’s role in medicine
Trust, human-centered AI and collaboration the focus of inaugural RAISE Health symposium.

About the Program
Medical Laboratory Sciences is an ideal degree path for those interested in a career in healthcare. Not only will you learn the science behind clinical diagnostics but how to perform and interpret these analyses with accuracy and precision, like a detective. The unique 3+1 design of our program means that after building a robust interdisciplinary science foundation on campus, you will spend your final year at one of our medical affiliates building advanced clinical competence and diagnostic skills. Pursuing this undergraduate path means as a professional you will play a critical role on the patient care team providing vital information for the accurate diagnosis and treatment of disease. This is because you will attain expertise across a multitude of disciplines such as hematology, microbiology, immunohematology and more that play a significant role in the treatment and resolution of illness and disease. Along with this expertise, earning national board certification ahead of or near graduation puts you in high demand across a diverse collection of career fields such as medical practice, biomedical research, governmental, and public health agencies to name a few. In addition, Medical Laboratory Sciences will also afford you an enriched foundation should you seek to further your studies in the areas of medicine, biochemistry, toxicology, epidemiology, management, and more in professional or graduate school.
School of Health Sciences Website
Medical Laboratory Sciences Major Change (CODO) Requirements
Degree Requirements
120 credits required, departmental/program major course requirements (94 credits), required major courses (62 credits).
- AGRY 32000 - Genetics Credits: 3.00
- BCHM 30700 - Biochemistry Credits: 3.00 ♦
- BIOL 20300 - Human Anatomy And Physiology Credits: 4.00 ♦
- BIOL 20400 - Human Anatomy And Physiology Credits: 4.00 ♦
- BIOL 22100 - Introduction To Microbiology Credits: 4.00
- CHM 11500 - General Chemistry Credits: 4.00 ♦
- CHM 11600 - General Chemistry Credits: 4.00 ♦
- CHM 25500 - Organic Chemistry For The Life Sciences I Credits: 3.00 ♦
- CHM 25501 - Organic Chemistry For The Life Sciences Laboratory I Credits: 1.00 ♦
- CHM 25600 - Organic Chemistry For The Life Sciences II Credits: 3.00 ♦
- CHM 25601 - Organic Chemistry For The Life Sciences Laboratory II Credits: 1.00 ♦
- HSCI 10100 - Introduction To The Health Sciences Professions Credits: 2.00
- HSCI 13000 - Introduction To Medical Laboratory Science Credits: 1.00
- HSCI 13100 - Introduction To Medical Terminology Credits: 3.00
- HSCI 20100 - Principles Of Public Health Science Credits: 3.00 (satisfies Science, Technology & Society for core)
- HSCI 20200 - Essentials Of Environmental, Occupational, And Radiological Health Sciences Credits: 3.00
- HSCI 33300 - Introduction To Immunology Credits: 3.00
- STAT 30100 - Elementary Statistical Methods Credits: 3.00 ♦
- English Selective - select any 20000 level or above ENGL course - Credit Hours: 3.00
- Science Selective - select a total of 7 credits from Science Selective List - Credit Hours: 7.00
Clinical Year (32 credits)
A cumulative GPA of at least 3.00 and a minimum science (CHM, BIOL, PHYS, MA) GPA of at least 2.75 is required to apply for admission into the clinical year.
Student must have at least 88 credits completed prior to the start of the clinical year.
(Course title and number of credits per course listed below vary by clinical location. Clinical year includes coursework in Chemistry, Hematology, Serology, Immunohematology, Microbiology, Urinalysis, and special topics such as: Laboratory Management, Parasitology, etc. The course titles and credits may vary depending on the affiliate site, but will adhere to the overall total of 32 credits at the 40000 level.
- HSCI 45100 - Clinical Biochemistry Credits: 1.00 to 10.00
- HSCI 45200 - Clinical Chemistry Credits: 1.00 to 10.00
- HSCI 45300 - Clinical Hematology Credits: 1.00 to 10.00
- HSCI 45400 - Clinical Immunohematology Credits: 1.00 to 10.00
- HSCI 45500 - Clinical Microbiology Credits: 1.00 to 10.00
- HSCI 45700 - Clinical Parasitology Credits: 1.00 to 10.00
- HSCI 45800 - Clinical Serology Credits: 1.00 to 10.00
- HSCI 46000 - Clinical Urinalysis Credits: 1.00 to 10.00
- HSCI 46500 - Introduction To Laboratory Education And Management Credits: 1.00 to 3.00
- HSCI 49000 - Special Topics Credits: 1.00 to 8.00 Approved Titles (Basic Lab Skills I),(Basic Lab Skills II),(Basic Lab Skills)
- Any PATH prefix course
Other Departmental/Program Course Requirements (23-24 credits)
- BIOL 11000 - Fundamentals Of Biology I Credits: 4.00 ♦ (satisfies Science for core)
- BIOL 11100 - Fundamentals Of Biology II Credits: 4.00 ♦ (satisfies Science for core)
- COM 11400 - Fundamentals Of Speech Communication Credits: 3.00 ♦ (satisfies Oral Communication for core)
- MA 16010 - Applied Calculus I Credits: 3.00 ♦ (satisfies Quantitative Reasoning for core)
- ENGL 10600 - First Year Composition With Conferences Credits: 4.00 ♦ (satisfies Written Communication and Information Literacy for core) or
- ENGL 10800 - First Year Composition Credits: 3.00 ♦ (satisfies Written Communication and Information Literacy for core)
- Human Cultures: Behavioral & Social Sciences - Credit Hours: 3.00 (satisfies Behavioral & Social Sciences for core)
- Human Cultures: Humanities - Credit Hours: 3.00 (satisfies Humanities for core)
Electives (2-3 credits)
- An Ethics course (such as PHIL 11100 Ethics or PHIL 27000 Environmental Ethics) is highly recommended for elective credit.
GPA Requirements
- A cumulative GPA of at least 3.00 and a minimum science (CHM, BIOL, PHYS, MA) GPA of at least 2.75 is required for admission into the clinical year.
- 2.0 GPA required for graduation
Pass/No Pass Policy
- A student may elect the Pass/Not-Pass grading option for elective courses only, unless an academic unit requires that a specific departmental course/s be taken Pass/Not-Pass. Students may elect to take University Core Curriculum courses Pass / Not-Pass; however, some major Plans of Study require courses that also fulfill UCC foundational outcomes. In such cases, students may not elect the Pass/Not-Pass option. A maximum of 24 credits of elective courses under the Pass/Not-pass grading option can be used toward graduation requirements. For further information, students should refer to the College of Health and Human Sciences Pass / Not-Pass Policy.
University Requirements
University core requirements, for a complete listing of university core course selectives, visit the provost’s website ..
- Human Cultures: Behavioral/Social Science (BSS)
- Human Cultures: Humanities (HUM)
- Information Literacy (IL)
- Oral Communication (OC)
- Quantitative Reasoning (QR)
- Science #1 (SCI)
- Science #2 (SCI)
- Science, Technology, and Society (STS)
- Written Communication (WC)
Civics Literacy Proficiency Requirement
The civics literacy proficiency activities are designed to develop civic knowledge of purdue students in an effort to graduate a more informed citizenry. for more information visit the civics literacy proficiency website..
Students will complete the Proficiency by passing a test of civic knowledge, and completing one of three paths:
- Attending six approved civics-related events and completing an assessment for each; or
- Completing 12 podcasts created by the Purdue Center for C-SPAN Scholarship and Engagement that use C-SPAN material and completing an assessment for each; or
- Earning a passing grade for one of these approved courses (or transferring in approved AP or departmental credit in lieu of taking a course).
Upper Level Requirement
- Resident study at Purdue University for at least two semesters and the enrollment in and completion of at least 32 semester hours of coursework required and approved for the completion of the degree. These courses are expected to be at least junior-level (30000+) courses.
- Students should be able to fulfill most , if not all , of these credits within their major requirements; there should be a clear pathway for students to complete any credits not completed within their major.
Additional Information
- Most Medical Laboratory Sciences students graduate in August.
- 3 years plus 1 year clinical (application required for clinical).
Sample 4-Year Plan
Fall 1st year.
- BIOL 11000 - Fundamentals Of Biology I Credits: 4.00 ♦
- CHM 11500 - General Chemistry Credits: 4.00 ♦
- MA 16010 - Applied Calculus I Credits: 3.00 ♦
- ENGL 10600 - First Year Composition With Conferences Credits: 4.00 ♦ or
- ENGL 10800 - First Year Composition Credits: 3.00 ♦
16-17 Credits
Spring 1st year.
- BIOL 11100 - Fundamentals Of Biology II Credits: 4.00 ♦
- COM 11400 - Fundamentals Of Speech Communication Credits: 3.00 ♦
- Human Cultures: Humanities Selective - Credit Hours: 3.00
Fall 2nd Year
Spring 2nd year.
- HSCI 20100 - Principles Of Public Health Science Credits: 3.00
- Human Cultures: Behavioral & Social Sciences- Credit Hours: 3.00
Fall 3rd Year
- Science Selective - Credit Hours: 3.00-4.00
- Science Selective - Credit Hours: 3.00
13-14 Credits
Spring 3rd year.
- English Selective (select any 20000 level or above ENGL course) - Credit Hours: 3.00
- Elective - Credit Hours: 2.00-3.00
14-15 Credits
Fall 4th year.
- HSCI Clinical Courses - 10000-59999 - Credit Hours: 16.00
Spring 4th Year
Pre-requisite information.
For pre-requisite information, click here .
World Language Courses
World Language proficiency requirements vary by program. The following list is inclusive of all world languages PWL offers for credit; for acceptable languages and proficiency levels, see your advisor. (ASL-American Sign Language; ARAB-Arabic; CHNS-Chinese; FR-French; GER-German; GREK-Greek(Ancient); HEBR-Hebrew(Biblical); HEBR-Hebrew(Modern); ITAL-Italian; JPNS-Japanese; KOR-Korean; LATN-Latin; PTGS=Portuguese; RUSS-Russian; SPAN-Spanish)
Critical Course
The ♦ course is considered critical.
In alignment with the Degree Map Guidance for Indiana’s Public Colleges and Universities, published by the Commission for Higher Education (pursuant to HEA 1348-2013), a Critical Course is identified as “one that a student must be able to pass to persist and succeed in a particular major. Students who want to be nurses, for example, should know that they are expected to be proficient in courses like biology in order to be successful. These would be identified by the institutions for each degree program.”
The student is ultimately responsible for knowing and completing all degree requirements. Consultation with an advisor may result in an altered plan customized for an individual student. The myPurduePlan powered by DegreeWorks is the knowledge source for specific requirements and completion.
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Outreach Research Coordinator
- Columbia University Medical Center
- Opening on: May 30 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $62,400.00 - $70,000.00
Position Summary
Responsibilities
- Work as part of a team to organize and deliver a range of innovative community outreach and engagement activities throughout the New York City boroughs that draw in and involve local churches, residents, and partners.
- The candidate will be pivotal in church recruitment and subject follow-up for departmental research projects.
- Work in different environments (both office-based and in the field) at times best suited to the community (including some evenings and weekends).
- Follow recruited participants through consent, enrollment, scheduling study visits, and follow-up calls.
- Work collaboratively with community health workers to plan and implement study screening events.
- Develop sustainable and positive relationships with a diverse population, including residents, community leaders, local groups, organizations, and local authorities.
- Build and sustain church and community partnerships to support participant recruitment for InTOuCH and the CHURCH Trial Attend all CHURCH Trial recruitment events to support participant outreach efforts
- Performs other duties/special projects as assigned.
Minimum Qualifications
- Bachelor’s Degree preferred, or equivalent combination of education, training, and experience
Preferred Qualifications
- A minimum of three (3) years of related work experience.
- Community engagement and clinical trials diversity, especially working with churches
- Bachelor’s degree in public health, communications, or a related public health field
- Proficiency in Microsoft Word, Excel, and PowerPoint
- Strong organizational, communication (both oral and written) and interpersonal skills.
- Must be able to work independently and in a team.
- Flexibility in hours (i.e., will work some evenings and weekends
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Programmer / Data Analyst
Assistant director, engagement, assistant director of enrollment management systems and slate.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .

COMMENTS
The MSCR MD/MS Program is a one-year articulated degree program that allows interested UCLA Medical Students to complete the Master of Science in Clinical Research Program (MSCR) during their Discovery Year (Year 3). The MSCR MD/MS Program leads to a Master of Science in Clinical Research graduate degree and is designed to develop physician ...
The Master of Science in Clinical Research is a rigorous program that meets the needs of individuals engaged in the full spectrum of clinical research. Our mission is to provide you with a high-quality education and a personalized, hands-on research experience. Our curriculum prepares you to enter the workforce as competently trained clinical ...
Department. The MS in Clinical Research program is a graduate degree as well as a career development path for physician-scientists, clinical scholars, and biomedical researchers. The program trains you to conduct patient-oriented research, directly interacting with human subjects to better understand disease, the development of therapeutic ...
Application Requirements. Applicants to the Master of Science in Clinical Research program must have earned a bachelor's degree from an accredited college or university prior to matriculation. To apply to the Master of Science in Clinical Research program, please submit the following: Completed online application; Official transcripts from all institutions of higher learning attended
The Master of Science in Clinical Research is a rigorous program that meets the needs of health professionals engaged in the full spectrum of patient-oriented research. This flexible degree program is designed for a variety of professionals, including physicians who will plan and oversee translational research and clinical trials; research nurses; study coordinators; managers in clinical ...
The Master of Science in Clinical Research (MSCR) program is an interdisciplinary research degree program housed within the Graduate School of Biomedical Sciences at the Icahn School of Medicine at Mount Sinai. The program is designed to impart the knowledge and skills needed for a successful career as a principal investigator and collaborator ...
The online Master's in Clinical Research for Health Professionals at Drexel is a 36-semester credit (12 course) program. It prepares health professionals, like you, with an extensive study and understanding of the scientific, ethical, and regulatory aspects of clinical research. Many students complete the requirements of the online clinical ...
The Master's in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations.
Join the next generation of clinical and translational researchers. The Clinical Research MS program trains the next generation of researchers and serves as a career development path for physician-scientists, clinical scholars, and biomedical researchers. Students learn to conduct patient-oriented research and clinical trials, as well as ...
The Master of Science in Clinical Research Program in the Department of Public Health Sciences is an interdisciplinary graduate degree designed to meet the changing needs of the current health care field, particularly the increasing need for trained professionals with well-developed quantitative and analytic skills. The MS-CR program provides ...
Developing Knowledge for Health-Related Research. Penn State's Master of Science in Clinical Research is a 30-credit degree program that provides the knowledge and insight required for careers in health-related research. The program includes graduate-level course work in biostatistics, epidemiology and health services research, and provides ...
This cutting-edge master's degree program provides world-class training in the methods and conduct of medical discovery for future leaders in biomedical research. MMSCI is a two-year, full-time program with a new three-year, part-time option available to Boston area students who meet the criteria to enroll in a part-time, residential program.
Our programs prepare the next generation of health care professionals and physician-scientists to advance evidence-based clinical practice through rigorous research. ... Whether you are considering a PhD in Clinical Research, a master's degree (MSCR), an MD/MSCR dual degree, or our certificate program, we provide an academically rigorous and ...
After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate). The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry.
About the Program. Vanderbilt's Master of Science in Clinical Investigation (MSCI) program trains investigators in the techniques and processes used in clinical and translational research. This program provides a direct, mentored experience in clinical investigation and, through didactic work, provides trainees with a strong foundation in study design, biostatistics with R, biomedical ethics ...
The MS in Clinical Research curriculum provides foundational and advanced knowledge in Clinical Research including drug development, clinical trial operations, study design, data management, regulatory affairs, medical ethics, scientific writing, and biostatistical literacy. Students can enroll full-time to complete the program in two years; or ...
In contrast to a public health degree which focuses on epidemiology, this program is designed primarily for clinical fellows who are training for academic careers. The program offers formal courses in research design, statistical analysis, medical genomics, research management, scientific communication, research project development, and ...
Looking to earn a masters degree in clinical research? Our online program offers three tracks (Clinical Research Management, Academic Clinical Research Management, and Drug Safety and Pharmacovigilance) as well as provides hands-on practical experience. Our courses include topics around patient engagement, medical affairs, decentralized trials, international regulations, and more. We are also ...
MSCR MS Program. The MSCR MS Program, leading to a Master of Science in Clinical Research graduate degree, is designed to develop physician scientists interested in clinical research or biomedical informatics to:. Design and conduct clinical research (clinical trials and observational studies) Successfully compete for funding (e.g., foundation grants, NIH K23, or R01's in clinical research)
Any aspiring clinical research physician will typically need a bachelor's degree in a physical or life science, a four-year degree from a medical school, and, depending on your specialty, three to seven years of an internship and residency program. After residency, years of clinical medical practice as a physician are required before applying ...
It involves one year of full-time academic classroom work, followed by at least two years of mentored training in clinical research. The combination of a year of instruction and a year of clinicals allows students the scientific grounding for subsequent original research. ... Prior Graduate Degree. Advanced medical degree: e.g., MD, MBBS, PhD ...
How to Become a Clinical Research Physician. To become a clinical research physician, you typically need a medical degree in clinical research or pharmaceutical medicine and prior work experience as a clinical research assistant. Important career skills include strong analytical, scientific, and organizational abilities, as well as extensive ...
25. University of Texas Rio Grande Valley Edinburg, TX Online Master of Science in Health Sciences in Clinical Laboratory Sciences. Website The University of Texas at Rio Grande Valley gives hospital and laboratory workers a leg up as they climb the corporate ladder, thanks to its convenient and affordable clinical research management master's degree program.
The following describes the research opportunities available to David Geffen School of Medicine students over the course of their medical school years. Including UCLA's MD/PhD or Medical Scientist Training Program and a few dual degree programs such as MD/ MBA, MD/MPH, and MD/MPP. The length of an MD/PhD program is 8 years while adding a ...
With the healthcare and social services sector poised to generate 45% of new U.S. jobs through 2032, developing expertise in clinical research can generate dynamic and promising career prospects. 1 Whether you're transitioning into clinical research from an unrelated field or advancing from a clinical healthcare role into management, Kent State University's clinical research certificate ...
AboutStanford Medicine. A leader in the biomedical revolution, Stanford Medicine has a long tradition of leadership in pioneering research, creative teaching protocols and effective clinical therapies. Facts & Figures: Stanford School of Medicine Performance Metrics. (released March 1, 2023) News.
Medical Laboratory Sciences is an ideal degree path for those interested in a career in healthcare. Not only will you learn the science behind clinical diagnostics but how to perform and interpret these analyses with accuracy and precision, like a detective. The unique 3+1 design of our program means that after building a robust ...
May provide supporting role for more complex studies under the direction of the PI, Clinical Research Coordinator and/or Clinical Research Assistant Lead. Experience and Education. Minimum Requirements ... Associate's degree in medical or science related field and 1 year of experience, or Bachelor's degree in medical or science related ...
Bachelor's degree in public health, communications, or a related public health field ... Grade 103 544016 Columbia University Medical Center New York United States Columbia University Medical Center Obstetrics and Gynecology Administrative Support, General Administration, Research (Lab and Non-Lab) Full Time. The Clinical Research Coordinator ...
The top-paying states for LPNs are: California: $76,580. Rhode Island: $75,470. Washington: $75,410. Alaska: $74,260. Massachusetts: $73,400. By enrolling in online medical assistant to LPN programs, you can increase your earning potential and take advantage of job opportunities in high-paying states and industries.