New Report Highlights Diabetes Research Advances and Achievements

Today, the American Diabetes Association® (ADA) released its 2023 Research Report , highlighting investments in advancing diabetes research and clinical practice. ADA research grants focused on innovative projects with high impact and helped researchers establish collaborative networks to move their innovations into the hands of people living with diabetes.
“Research at the ADA is the engine that drives clinical advances by catapulting them into practice. 2023 has brought many prominent achievements. We are incredibly proud of our legacy of highlighting science and eager to build on this research to move even closer to a world free of diabetes and all its burdens,” said Charles “Chuck” Henderson, the ADA’s chief executive officer.
The report highlights include:
- Support behavioral and mental health of people with diabetes
- Tackle the epidemic of youth-onset type 2 diabetes
- Improve the lives of women living with diabetes
- Increased investment in early career researchers by expanding funding opportunities for postdoctoral fellowship awards to ensure these researchers can stay within the field of diabetes.
- Takeaways from the 2023 Scientific Sessions, where researchers from all over the world shared the latest progress and study results with the global diabetes community.
- Identify and address disparities in access and outcomes for Hispanic/Latino communities
- Implement virtual interventions for those living with type 1 diabetes
- Improve outcomes for the deaf community through specially designed diabetes self-management education and support (DSMES)
In addition, the report provides an update on the Pathway to Stop Diabetes® (Pathway) program, which pairs talented early-career scientists with mentorship from world-renowned diabetes scientists to drive research innovation free from traditional project constraints. This year, through the Pathway program, ADA dedicated over $4.8 million dollars in new grant funding to support breakthroughs in translation and clinical science, technology, care, and potential cures in the field of diabetes.
To learn more about the ADA’s research findings and ongoing areas of study, visit professional.diabetes.org .
About the American Diabetes Association The American Diabetes Association (ADA) is the nation’s leading voluntary health organization fighting to bend the curve on the diabetes epidemic and help people living with diabetes thrive. For 83 years, the ADA has driven discovery and research to treat, manage, and prevent diabetes while working relentlessly for a cure. Through advocacy, program development, and education we aim to improve the quality of life for the over 136 million Americans living with diabetes or prediabetes. Diabetes has brought us together. What we do next will make us Connected for Life ® . To learn more or to get involved, visit us at diabetes.org or call 1-800-DIABETES (1-800-342-2383). Join the fight with us on Facebook ( American Diabetes Association ), Spanish Facebook ( Asociación Americana de la Diabetes ), LinkedIn ( American Diabetes Association ), Twitter ( @AmDiabetesAssn ), and Instagram ( @AmDiabetesAssn ).

Contact Virginia Cramer for press-related questions.
Donate Today and Change Lives!
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Research Areas

Diabetes affects an estimated 38.4 million people in the United States and is the eighth leading cause of death. Diabetes is characterized by the body’s inability to produce and/or respond appropriately to insulin. These defects result in persistent elevation of blood glucose levels and other metabolic abnormalities, which in turn lead to the development of disease complications, such as heart disease and stroke, blindness, kidney failure, and lower limb amputation. In addition to increasing the risk for these complications, diabetes also doubles the risk for many forms of cancer, some forms of dementia, hearing loss, erectile dysfunction, urinary incontinence, and many other common diseases.
- Type 1 diabetes affects approximately 6 percent of adults and the majority of children and youth with diagnosed diabetes.
- Type 2 diabetes is the most common form of the disease, accounting for about 90 to 94 percent of diagnosed diabetes cases in U.S. adults. Type 2 diabetes is also increasingly being diagnosed in children and adolescents, and disproportionately affects individuals from racial and ethnic minority populations.
- Prediabetes affects an estimated 97.6 million adults in the United States. Individuals with prediabetes are at high risk of developing type 2 diabetes.
- Gestational diabetes affects a significant proportion of pregnant persons. In addition to placing the pregnant person and their child at risk for complications during childbirth, gestational diabetes increases their future risk for type 2 diabetes.
The NIDDK supports basic, clinical, and translational research to combat diabetes and its associated complications. For example, NIDDK-supported researchers are:
- studying genetic and environmental factors that contribute to the development and progression of diabetes;
- identifying ways to improve diabetes health equity and reduce diabetes health disparities ;
- studying ways to preserve insulin-producing cells of the pancreas;
- identifying new methods to improve blood glucose monitoring and insulin delivery in type 1 diabetes;
- examining behavioral approaches to prevent type 2 diabetes and to enhance diabetes self-management;
- conducting clinical trials testing new prevention and treatment strategies for diabetes and its complications; and
- uncovering the fundamental cellular and molecular pathways underlying development of diabetes and its complications to develop new and more personalized approaches to prevention and management.
The NIDDK also administers the Special Statutory Funding Program for Type 1 Diabetes Research, which is a special appropriation dedicated to supporting research on type 1 diabetes and its complications. More information on the Program and the research it supports is available on the Type 1 Diabetes Research Special Statutory Funding Program website .
In addition, NIDDK has congressional authorization for the National Diabetes Information Clearinghouse , which provides services via the NIDDK Health Information Center. NIDDK responds to questions and provides health information about diabetes to people with diabetes and to their families, health professionals, and the public.
Research Updates and News
- Bariatric surgery provides long-term blood glucose control, type 2 diabetes remission
- Celebrating the 50th Anniversary of Diabetes Research Centers
- Diabetes in America now available
- Islet Transplantation for Treating Difficult-to-Manage Type 1 Diabetes in Adults
- The Special Diabetes Program: 25 Years of Advancing Type 1 Diabetes Research
View More News Items
Select Landmark Studies
- Diabetes Prevention Program (DPP)
- Blood Glucose Control Studies for Type 1 Diabetes: DCCT and EDIC
To achieve its mission, NIDDK supports, conducts, coordinates, and plans research. NIDDK also provides data and samples from NIDDK-funded studies and explains research findings to health professionals and the public.
Support Research
NIDDK invests in basic, clinical and translational research and training at colleges, universities and other institutions.
- Bioengineering, Biotechnology, and Imaging as applied to Diabetes, Metabolic, and Endocrine Diseases
- Clinical Research in Type 1 Diabetes
- Clinical Research in Type 2 Diabetes
- Diabetes and Metabolism HIV/AIDS
- Diabetes Genetics and Genomics
- Diabetes, Endocrine, and Metabolic Disease Translational Research
- Diabetes: Treatment, Prevention, and Complications
Conduct Research
NIDDK investigators conduct biomedical research and training in the Institute's laboratories and clinical facilities in Maryland and Arizona.
- Diabetes, Endocrinology, and Obesity Branch
- Laboratory of Biochemistry and Genetics
- Laboratory of Biological Modeling
- Laboratory of Bioorganic Chemistry
- Laboratory of Cell and Molecular Biology
Coordinate & Plan Research
NIDDK takes multiple approaches to research planning and priority setting.
Meetings & Workshops
There are no upcoming related meetings or workshops at this time.
Strategic Plans & Reports
- Diabetes in America
- NIDDK Strategic Plan for Research
- Diabetes in America, 3rd Edition
- Special Statutory Funding Program for Type 1 Diabetes Research: Progress Report
- Special Statutory Funding Program for Type 1 Diabetes Research: Evaluation Report
Provide Access to Research Resources
NIDDK makes publicly supported resources, data sets, and studies available to researchers.
Provide Health Information
NIDDK provides patient education information, practice tools for diagnosis and treatment, and statistics.
- Diabetes Statistics
- Health Information about Diabetes
- Diabetes Discoveries & Practice Blog
- Diabetes for Health Professionals
- Advanced Health Information Search
New cause of diabetes discovered, offering potential target for new classes of drugs to treat the disease
Researchers at Case Western Reserve University and University Hospitals have identified an enzyme that blocks insulin produced in the body -- a discovery that could provide a new target to treat diabetes.
Their study, published Dec. 5 in the journal Cell, focuses on nitric oxide, a compound that dilates blood vessels, improves memory, fights infection and stimulates the release of hormones, among other functions. How nitric oxide performs these activities had long been a mystery.
The researchers discovered a novel "carrier" enzyme (called SNO-CoA-assisted nitrosylase, or SCAN) that attaches nitric oxide to proteins, including the receptor for insulin action.
They found that the SCAN enzyme was essential for normal insulin action, but also discovered heightened SCAN activity in diabetic patients and mice with diabetes. Mouse models without the SCAN enzyme appeared to be shielded from diabetes, suggesting that too much nitric oxide on proteins may be a cause of such diseases.
"We show that blocking this enzyme protects from diabetes, but the implications extend to many diseases likely caused by novel enzymes that add nitric oxide," said the study's lead researcher Jonathan Stamler, the Robert S. and Sylvia K. Reitman Family Foundation Distinguished Professor of Cardiovascular Innovation at the Case Western Reserve School of Medicine and president of Harrington Discovery Institute at University Hospitals. "Blocking this enzyme may offer a new treatment."
Given the discovery, next steps could be to develop medications against the enzyme, he said.
The research team included Hualin Zhou and Richard Premont, both from Case Western Reserve School of Medicine and University Hospitals, and students Zack Grimmett and Nicholas Venetos from the university's Medical Science Training Program.
Many human diseases, including Alzheimer's, cancer, heart failure and diabetes, are thought to be caused or accelerated by nitric oxide binding excessively to key proteins. With this discovery, Stamler said, enzymes that attach the nitric oxide become a focus.
With diabetes, the body often stops responding normally to insulin. The resulting increased blood sugar stays in the bloodstream and, over time, can cause serious health problems. Individuals with diabetes, the Centers for Disease Control reports, are more likely to suffer such conditions as heart disease, vision loss and kidney disease.
But the reason that insulin stops working isn't well understood.
Excessive nitric oxide has been implicated in many diseases, but the ability to treat has been limited because the molecule is reactive and can't be targeted specifically, Stamler said.
"This paper shows that dedicated enzymes mediate the many effects of nitric oxide," he said. "Here, we discover an enzyme that puts nitric oxide on the insulin receptor to control insulin. Too much enzyme activity causes diabetes. But a case is made for many enzymes putting nitric oxide on many proteins, and, thus, new treatments for many diseases."
- Diseases and Conditions
- Hormone Disorders
- Chronic Illness
- Alzheimer's
- Huntington's Disease
- Disorders and Syndromes
- Nitrous oxide
- Diabetes mellitus type 1
- Diabetes mellitus type 2
- Drug discovery
- Nitrogen oxide
Story Source:
Materials provided by Case Western Reserve University . Note: Content may be edited for style and length.
Journal Reference :
- Hua-Lin Zhou, Zachary W. Grimmett, Nicholas M. Venetos, Colin T. Stomberski, Zhaoxia Qian, Precious J. McLaughlin, Puneet K. Bansal, Rongli Zhang, James D. Reynolds, Richard T. Premont, Jonathan S. Stamler. An enzyme that selectively S-nitrosylates proteins to regulate insulin signaling . Cell , 2023; DOI: 10.1016/j.cell.2023.11.009
Cite This Page :
Explore More
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
Trending Topics
Strange & offbeat.
- Open access
- Published: 08 May 2024
Advances and challenges of the cell-based therapies among diabetic patients
- Ramin Raoufinia 1 , 2 ,
- Hamid Reza Rahimi 2 ,
- Ehsan Saburi 2 &
- Meysam Moghbeli ORCID: orcid.org/0000-0001-9680-0309 2
Journal of Translational Medicine volume 22 , Article number: 435 ( 2024 ) Cite this article
284 Accesses
Metrics details
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in clinical settings, highlighting their strengths and limitations. We also discussed immunomodulatory strategies employed in cell therapies. Therefore, this review highlights key progresses that pave the way to design transformative treatments to improve the life quality among diabetic patients.
Diabetes mellitus poses a formidable global public health challenge due to its rapid growing prevalence and associated morbidity, disability, and mortality [ 1 ]. According to the International Diabetes Federation, over 537 million adults aged 20–79 had diabetes worldwide in 2021 that is expected to rise to around 783 million cases by 2045 [ 2 ]. Obesity, unhealthy diets, physical inactivity as well as genetic and epigenetic predispositions are important risk factors of diabetes [ 3 , 4 , 5 ]. Diabetes is typically classified into type 1 diabetes mellitus (T1DM), gestational diabetes mellitus (GDM), and type 2 diabetes mellitus (T2DM) [ 2 ]. T1DM primarily arises from autoimmune-related damage of insulin-secreting beta cells, resulting in severe hyperglycemia and ketoacidosis [ 6 ]. In contrast, T2DM generally has a more gradual onset characterized by insulin resistance along with diminished compensatory insulin secretion from pancreatic beta cell dysfunction [ 7 ]. Diabetes is associated with macrovascular complications such as heart disease and stroke, as well as microvascular issues in eyes, kidneys, and nervous system [ 8 ]. Cancer is also a leading cause of diabetes-related death, and dementia-associated mortality has risen in recent decades [ 9 , 10 , 11 , 12 ]. Cell therapy involves transferring autologous or allogenic cellular material into patients [ 13 ]. The global market size of cell therapy is estimated to grow from $9.5 billion in 2021 to $23 billion by 2028 [ 14 ]. It combines stem and non-stem cell therapies consisting of unicellular or multicellular preparations. Cell therapies typically use autologous or allogenic cells via injection and infusion [ 15 ]. In the present review, we discussed the recent advances in cell-based therapy of diabetes, from foundational islet transplantation to regenerative strategies to highlight key developments that improve the effective treatments for diabetic patients.
Cell replacement therapy for diabetes
Pancreatic transplantation was firstly used in 1966 to treat type 1 diabetes using whole organ transplants. During the 1970s–80s, segmental pancreatic grafts were combined with techniques to divert digestive secretions away from transplanted cells. Three main techniques emerged; simultaneous pancreas-kidney transplants, pancreas transplants following kidney transplants, and pancreatic transplants. International collaboration on tracking outcomes began in 1980 with the formation of several pancreatic transplant registries and associations. However, whole organ transplantation was faced with several challenges including organ rejection, vascular complications, limited organ availability, and the effects of lifelong immunosuppression [ 16 , 17 ]. Islet cell transplantation was explored as an alternative, however isolating and transplanting pancreatic islets proved difficult due to donor availability, rejection, and immunosuppression side effects. Recent research has focused on stem cell sources that could reconstitute immune tolerance and preserve beta cell function such as mesenchymal stem cells, bone marrow cells, and embryonic stem cells [ 18 ]. A novel stem cell therapy called VX-880 was developed using proprietary technology to grow insulin-producing beta cells from allogeneic stem cells. Clinical trials began in 2021 after FDA approval to deliver the cells intrahepatically under immune suppression. A second approach called VX-264 encapsulates the same cells, avoiding immunosuppression but requiring surgical implantation [ 17 ]. In 2023, FDA approved the first allogeneic pancreatic islet cell therapy called Lantidra for adults with type 1 diabetes experiencing severe hypoglycemia. Approval was based on two studies where 21–30% of participants no longer required insulin one year post-treatment, with benefits lasting over five years in some cases. However, this treatment have mild and serious adverse events that are associated with treatment dose and the methods of islet cell infusion [ 19 , 20 ].
Emerging strategies for cell delivery via microencapsulation and biological devices in clinical trials
Alginate capsules as cell delivery systems.
A seminal investigation conducted in 1994 demonstrated the successful transplantation of alginate-encapsulated islets into the peritoneum of kidney transplant patients who were receiving immunosuppression therapy. Remarkably, these patients achieved insulin independence for up to nine months [ 21 ]. However, subsequent trials conducted without immunosuppression yielded inconsistent outcomes. In a study conducted in 2006, islets were encapsulated in triple-layer alginate capsules and implanted intraperitoneally in type 1 diabetes (T1D) patients. There was a positive correlation between the encapsulation and insulin production that reduced exogenous insulin requirements during one year. Despite this progress, the entry of cytokines remained a potential concern [ 22 ]. Another study employed the single-layer barium-alginate capsules that sustained insulin production for up to 2.5 years [ 23 ]. It has been reported that the microneedle, comprising a calcium alginate frame with polydopamine-coated poly-lactic-co-glycolic acid microspheres encapsulating insulin, enables light-triggered insulin release. Microneedle provided a suitable insulin dose to maintain blood glucose levels in line with daily fluctuations. These results established the efficacy and safety of the developed microneedle for diabetes treatment [ 24 ]. Another therapeutic approach explored the encapsulation of pancreatic islets with mesenchymal stem cells (MSCs) and decellularized pancreatic extracellular matrix (ECM). ECM derived from the pancreas supported islet cell growth and maintenance to enhance insulin expression [ 25 ]. Sodium alginate and hyaluronic acid were incorporated due to their roles in collagen production, wound healing, and physical crosslinking. The 3D porous membranes allowed optimal water and oxygen transfer while diverting excess exudate from diabetic wounds. Hydrogel accelerated re-epithelization, while decreased inflammation, indicating potential as the diabetic wound dressings [ 26 ]. Additionally, the incorporation of specific ECM components, such as collagen IV and RGD, into alginate-based microcapsules significantly improved the survival, insulin secretion, and longevity of microencapsulated islets [ 27 ].
Encaptra® device from ViaCyte
In contrast to microencapsulation techniques, ViaCyte developed a semipermeable pouch method named Encaptra, which contains pancreatic precursor cells derived from the embryonic stem cells [ 28 ]. In the initial trial conducted in 2014, the “VC-01” device was implanted in T1D individuals without the use of immunosuppression [ 29 ]. The trial confirmed the safety of the device; however, the occurrence of hypoxia induced cellular necrosis [ 30 ]. The device was modified as “VC-02” with larger pores, and two trials (NCT03162926, NCT03163511) demonstrated promising outcomes, including increased fasting C-peptide levels and a 20% reduction in insulin requirements during one year in the majority of participants [ 31 ]. In order to eliminate the necessity for immunosuppressants, ViaCyte collaborated with Gore to develop an expanded polytetrafluoroethylene (ePTFE) device with both immuno-isolating and pro-angiogenic properties [ 32 ]. This device (NCT04678557) aimed to prevent immune cell attachment and T-cell activation [ 33 ]. Additionally, ViaCyte is exploring the integration of CRISPR technology to modify stem cells, specifically by eliminating β2-microglobulin expression and PD-L1 up regulation. It is hypothesized that these genetic modifications will further hinder immune cell attachment and T-cell activation [ 30 , 34 ].
Semipermeable device from Semma therapeutics
Semma Therapeutics, which has been acquired by Vertex, pioneered the utilization of differentiated stem cell-derived islet cell clusters in clinical trials. Semma houses these cells between two semipermeable polyvinylidene fluoride membranes and is designed for subcutaneous implantation (NCT04786262) [ 31 , 35 ]. Vertex reported a significant breakthrough by infusing differentiated beta cells via the portal vein in a participant who was receiving immunosuppressants. This approach led to substantial C-peptide production and improved glycemic control during 90 days [ 36 ].
βAir device from Beta O2
Beta O2’s innovative βAir device utilizes an alginate-PTFE membrane complex to encapsulate islets, providing partial immunoisolation while ensuring a continuous supply of oxygen, which is crucial for optimal islet function [ 37 , 38 ]. The βAir device that was seeded with human islets was subcutaneously implanted in T1D individuals (NCT02064309). Although, low insulin levels were produced for up to eight weeks, there was not any reduction in the required exogenous insulin [ 37 ]. While, increasing the number of islets could potentially enhance their function, it is important to note that the continuous reliance on oxygen poses a risk of infection, despite efforts to optimize the survival of encapsulated islets [ 39 , 40 ].
Cell pouch™ device from Sernova
Sernova has developed the Cell Pouch device, which offers pre-vascularized polypropylene chambers for islet transplantation without the need for immunoprotection. The device consists of multiple cylindrical chambers that are prefilled with PTFE plugs, which are then removed after implantation to create the empty space [ 41 ]. In a 2012 trial (NCT01652911), islets were placed in the vascularized pouches of three recipients who were also receiving immunosuppression that resulted in a transient increase in C-peptide levels [ 41 ]. In a 2018 trial (NCT03513939), immunosuppression was administered after implantation and islet introduction. This trial reported sustained C-peptide production for up to nine months in two recipients, along with improved glycemic control [ 42 ]. Regarding the limitations of immunosuppression, Sernova is exploring the possibility of encapsulating islets in hydrogel as an alternative approach [ 43 ].
Shielded living therapeutics™ from Sigilon Therapeutics
Sigilon has developed the Shielded Living Therapeutics sphere, which consists of cell clusters enclosed within an alginate-TMTD coating [ 44 ]. Preclinical studies demonstrated that murine islet transplants encapsulated within these spheres maintained normoglycemia for a period of six months [ 45 ]. In a 2020 trial conducted for hemophilia (NCT04541628), the spheres were evaluated for their ability to express Factor VIII [ 46 ]. However, the trial was paused due to the development of antibodies in the third recipient receiving the highest cell doses. While, preclinical studies have shown promising efficacy, there are safety concerns regarding the TMTD coating that need to be addressed before these spheres can be used for human islet transplantation as a treatment for diabetes [ 31 ]. Emerging technologies have been investigated in clinical trials for delivering insulin-producing islets or stem cell-derived beta cells via microencapsulation or use of implantable biological devices (Table 1). Optimizing encapsulation and developing alternative implantable devices moves the field toward delivering safe and effective islet replacement without chronic immunosuppression dependency that represented an important new frontier for the cell-based treatment of diabetes. However, continued refining will be required to fully realize this promising vision and using these preclinical concepts in clinic.
Immunoengineering strategies: biomaterials for modulating immune responses
Islet encapsulation aims to prevent immune responses toward transplant antigens. However, foreign body response (FBR) against biomaterials induces inflammation around encapsulated islets that obstructs oxygen/nutrient access and causes graft failure [ 31 ]. Extensive research revealed biomaterial properties profoundly influence FBR severity, with high purity/biocompatibility moderating inflammation [ 47 ]. Deeper understanding of biomaterial immunobiology enabled developing immune-modulating constructs to steer host interactions. By altering topology/chemistry to hinder nonspecific binding and cell adhesion, these “immune-evasive biomaterials” intended to attenuate xenograft rejection at inception [ 44 ]. Both innate and adaptive immune responses have crucial roles in the context of pancreatic islet transplantation. These responses encompass the activation of tissue macrophages and neutrophils following injury, leading to the release of inflammatory cytokines that subsequently activate antigen-presenting cells (APCs), CD8 + T cells, CD4 + T cells, and cytotoxic T lymphocytes (Fig. 1 ). Zwitterionic polymers conferred anti-fouling attributes but crosslinking limitations constrained their application [ 48 ]. Novel mild zwitterionization introduced alginate modifications that prolonged prevention of fibrotic overgrowth by mitigating initial responses [ 49 , 50 , 51 ]. The prevention of graft rejection following islet cell transplantation necessitates the systemic administration of immunosuppressive agents. While, these agents effectively suppress immune responses, their continuous use exposes patients to an increased risk of infection and cancer. To mitigate these concerns, an alternative approach involving the localized delivery of immunosuppressants at the transplantation site has emerged. This localized delivery system offers several advantages, including targeted drug delivery, reduced systemic exposure, and potentially reduces the immunosuppressants doses [ 52 ]. Polymeric carriers dispersed cyclosporine A continuously at the graft site to dynamically tamp down proinflammatory cascades and T-cell activation [ 53 , 54 ]. TGF-β/IL-10 co-delivery at the microencapsulation interface hindered innate antigen presentation, obstructing adaptive response priming [ 55 , 56 ]. Regulatory T-cells emerged as the potent immunomodulators when coated on islets to improve insulin production in vitro [ 57 ]. Similarly, recombinant Jagged-1 surface patterning increased regulatory lymphocytes in vitro while enhancing glycemic oversight in vivo [ 58 ]. Targeting proinflammatory effector T-cells or presenting their Fas ligand death receptor improved long-term viability when combined with rapamycin prophylaxis [ 52 , 59 ]. Immobilizing thrombomodulin or urokinase mitigated local inflammation, with the latter conferring lifelong xenotransplant survival [ 60 ]. Peptides recognizing IL-1 receptors provided robust protection from destabilizing proinflammatory cytokines [ 61 ]. Leukemia inhibiting factor improved islet performance over polyethylene glycol encapsulation alone by inducing regulatory T-cell lineages [ 62 ]. Silk scaffolds facilitated IL-4/dexamethasone emancipation that meaningfully decreased immune reactions to grafts [ 63 ]. Therefore, the localized delivery of immunosuppressants at the transplantation site represents a promising strategy for islet cell transplantation. Compared to systemic administration, local delivery can achieve targeted immune modulation only at the graft location while reducing drug exposure throughout the body. This localized approach aims to sufficiently suppress the immune response to prevent rejection, while limiting negative side effects that may occur from systemic immunosuppression. A variety of biomaterials and surface modification strategies have been developed and investigated for the local delivery of immunosuppressive agents and immunomodulatory cytokines [ 64 , 65 , 66 ]. Understanding how biomaterial properties influence the immune response is critical to design biomaterials that can modulate inflammation and improve islet graft survival through localized immunomodulation.
Cell-based therapy through the integration of additive manufacturing techniques
Additive manufacturing utilizes computer modeling to fabricate complex 3D structures on-site with minimal post-processing. Common methods for the biomedical application are fused filament fabrication (FFF), stereolithography (SLA), and bioprinting [ 67 ]. FFF is a layer-by-layer technique that extrudes heated thermoplastics [ 68 ]. Commonly used feedstocks include acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA). Other thermoplastics that have been utilized with FDM include thermoplastic polyurethane (TPU), polycarbonate (PC), polystyrene (PS), polyetherimide (PEI), polycaprolactone (PCL), polyaryletherketone (PAEK), and polyetheretherketone (PEEK), with the latter demonstrating high strength and heat tolerance. A major advantage of FDM is its ability to fabricate multi-material objects through continuous printing and alteration of the build material. In addition to typical polymers like PC and polystyrene (PS), FDM can print composites reinforced with glass, metals, ceramics, and bioresorbable polymers via integration of the constituent powders with a binding matrix. This enables enhanced control over the experimental component fabrication. While, ceramic and metal filaments traditionally contain the corresponding powder mixed with a binder, FDM provides versatility in the functional prototype construction from a wide range of thermoplastic feedstocks using precise and additive layer manufacture [ 68 , 69 , 70 , 71 , 72 ]. It provides geometric reproducibility and reduced variability compared to traditional techniques. FFF prints served as scaffolds for the transplanted cells [ 67 ]. However, minimum feature size is limited to ? ∼ 250 μm by nozzle diameter [ 68 ]. SLA employs light-curable liquid resins and achieves higher 50–150 μm resolution than FFF but with restricted material choices. Bone grafts and surgical guides are common applications [ 67 ]. Incorporating biomaterials like hydroxyapatite has expanded utility, though processing is required to mitigate cytotoxicity. Additive manufacturing can address limitations in oxygen transport, cell/material placement control and vasculature formation, and clinically translatable insulin-secreting implants [ 67 ]. Therefore, additive manufacturing technologies have the potential to enhance various aspects of the cell-based transplant design, from improving nutrient transport through optimized implant geometry to achieving precision integration of therapeutic agents (Table 2).
Enhancing nutrient transport through optimization of implant geometry
Tissue engineering for the islet transplantation requires maximizing nutrient transport [ 73 , 74 ]. Traditional scaffold fabrication introduces macroporosity but lacks precision that results in inflammation [ 67 ]. Cell encapsulation provides immunoprotection by limiting interactions between transplanted cells and the host immune system. However, this protective barrier also poses challenges for the efficient transport of essential nutrients, including oxygen, to the encapsulated cells. Modifying the geometries of encapsulation devices using conventional methods to enhance oxygen delivery has proven to be inconsistently challenging [ 67 ], so that novel approaches are required to address these challenges. Additive manufacturing allows customizing biomaterial scaffolds with defined geometries and micropore sizes to improve transport [ 75 , 76 , 77 , 78 , 79 ]. The 3D printed PLA scaffolds with islets have successful vascularization and cellular survival after subcutaneous transplantation [ 80 , 81 ]. Interlocking toroidal hydrogel-elastomer constructs also increased surface area and cell viability [ 82 , 83 , 84 ].
Enhancing vascularization and engraftment
Rich host vascularization of transplant devices is essential to support long-term islet survival through efficient nutrient delivery and insulin kinetics. Early platforms modified bulk material properties to promote vessel infiltration and anastomoses [ 85 , 86 , 87 , 88 , 89 ]. Additive manufacturing can further optimize microscale geometry to both accelerate host vessel connections and control intra-device vasculature homogeneity beyond traditional fabrication. Initial work reproduced macroscale vessels but scales were diverged from cell-based therapies [ 73 , 90 , 91 , 92 ]. Leveraging Additive manufacturing designed structures guided vessel formation in vitro and in vivo [ 80 , 89 , 93 ]. Shifting to bioprinting complex branching conduits in supportive hydrogels facilitated clinical translation for diverse cell therapies [ 94 , 95 , 96 , 97 , 98 ]. Researchers focused on developing a 3D scaffold platform to improve the transplantation outcomes of islet cells in T1D. The scaffold featured a heparinized surface and immobilized vascular endothelial growth factor (VEGF) to enhance vascularization. Scaffold effectively promoted angiogenesis and facilitated the growth of new blood vessels. Additionally, encapsulated islets within the scaffold had functional responses to glucose stimuli. These findings suggested that the developed scaffold platform holds potential for successful extra-hepatic islet transplantation, offering new possibilities for T1D treatment [ 99 ]. Research on vascularization of islets via additive manufacturing techniques has primarily focused on the fundamental discoveries. In one study, engineered pseudo islets (EPIs) were created by combining the mouse insulin-secreting beta cells with rat heart microvascular endothelial cells. EPIs demonstrated extensive outgrowth of capillaries into the surrounding matrix. Although, EPIs containing both cell types that underwent capillarization maintained viability and function over time in culture, non-vascularized EPIs lacking endothelial cells could not sustain viability or functionality long-term. This supported the potential for inducing angiogenesis within bioengineered islet constructs. Future work may combine patient-specific stem cell-derived human beta cells with endothelial cells using this approach to promote long-term graft survival for treating type 1 diabetes [ 98 ]. While, large-scale 3D printed vascularized structures are currently limited for the islet transplantation, advancements in leveraging additive manufacturing for the optimization vascularization conditions through the pore sizes and material choices, may facilitate translation to β-cell therapy in type 1 diabetes.
Precision placement of cells and matrix for enhanced control
Beyond distributing biomaterials, additive manufacturing enables micro-level cell and protein control. For islet transplantation, optimal cellular distribution and supportive extracellular matrix niche reduce rapid dysfunction and apoptosis [ 100 , 101 , 102 ]. Traditional techniques heterogeneously load cells after fabrication or struggle with incomplete encapsulation [ 103 , 104 ]. Bioprinting allows in situ encapsulation and printing of multiple cell types and matrix components while dictating 3D placement and dimensions [ 105 , 106 ]. Islet transplant research prints hydrogel-encapsulated clusters surrounded by supportive cells and doped with immune modulators to improve the transplant environment [ 107 ]. Progress in bioprinting offers consistency and defines physical/chemical graft properties beyond traditional fabrication.
Achieving controlled integration of therapeutic agents for enhanced efficacy
In addition to the cell and matrix placement, additive manufacturing enables precision therapeutic integration. Incorporating therapeutics aims to recapitulate the in vivo environment through angiogenesis, islet health promotion, and immunomodulation [ 67 , 108 ]. Growth factors promote vessel formation and insulin secretion while decrease apoptosis [ 108 , 109 , 110 , 111 ]. Local immunomodulators regulate the immune system in a specific site of the body. They decrease inflammation and promote the successful integration of transplanted cells or tissues by minimizing the need for widespread immune suppression in whole body [ 67 ]. Traditional homogeneous delivery methods restrict the ability to customize the spatial distribution of substances and pose a risk of harmful effects on transplants or hosts [ 112 ]. The use of discreet gradients in bioprinting can offer precise physiological signals. By combining traditional drug release methods with AM, it becomes possible to create tissues that exhibit distinct therapeutic localization. Bioprinted composites have the ability to release factors with gradients throughout the entire construct that enables a more comprehensive and targeted approach in tissue engineering [ 112 , 113 , 114 ].
Cell based gene therapy
Gene therapy holds great promise for diabetes management, offering innovative approaches to deliver and manipulate the insulin gene in various tissues. Viral methods, such as lentivirus, adenovirus, and adeno-associated virus (AAV), along with non-viral techniques like liposomes and naked DNA, have been utilized to deliver the insulin gene to target tissues [ 115 ]. This section aims to provide an overview of important studies in the field of gene therapy for diabetes management, emphasizing advancements in insulin gene delivery and manipulation (Table 3).
Enteroendocrine K-cells and pancreatic β-cells
Enteroendocrine K-cells in the intestines and pancreatic β-cells share similarities in their production of glucose-dependent insulinotropic polypeptide (GIP) and their regulatory mechanisms. Understanding these similarities offers insights into T2D management and improving glucose homeostasis. However, attempts to reverse diabetes effectively through K-cell transplantation have been unsuccessful. Nevertheless, research on gene editing techniques has shown promising results in management of the diabetes mellitus [ 116 , 117 ]. AAV vectors have been employed to co-express insulin and glucokinase genes in skeletal muscles, demonstrating long-term effectiveness in achieving normo-glycemia without exogenous insulin [ 118 , 119 ].
Gene editing techniques
Gene editing techniques using AAV vectors effectively improved normo-glycemia in animal models. Co-expression of insulin and glucokinase in transgenic mice increased glucose absorption and regulated insulin production. Duodenal homeobox 1 (PDX1) gene transfer via AAV2 in a humanized liver mouse model also led to insulin secretion and glycemic control [ 120 ]. Adenovirus-mediated transfection of hepatic cells with neurogenin 3 (NGN3) resulted in insulin production and trans-differentiation of oval cell populations [ 121 , 122 ]. Targeting specific promoters in liver cells such as phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), albumin, and insulin-like growth factor binding protein-1 (IGFBP-1) enhanced hepatic insulin gene therapy [ 123 , 124 ]. AAV-mediated overexpression of SIRT1 reduced inflammation, hypoxia, apoptosis and improved neural function in the retina of diabetic db/db mice [ 125 ]. Another study developed a plasmid expressing a single-strand insulin analogue for intramuscular injection using a specialized gene delivery technique. A single administration provided sustained insulin expression for 1.5 months and effectively regulated blood glucose levels without immune responses or tissue damage in diabetic mice.
Non-viral gene delivery methods
Non-viral approaches have also key roles in achieving glycemic control. The combination of insulin fragments with DNA plasmid, administered via intravenous injection improved normo-glycemia for extended periods. DNA transposon facilitated gene integration into the host chromosome that addressed the short-term liver expression. Additionally, the co-injection of DNA plasmid containing insulin with furin significantly enhanced insulin production within muscles [ 126 ]. Non-viral plasmids were engineered to carry proinsulin and pancreatic regenerating genes to ameliorate streptozotocin-induced T1DM [ 127 ]. The pVAX plasmid vectors prolonged therapeutic effects in achieving normo-glycemia without the need for further treatment [ 127 ]. Bioreducible cationic polymers, such as poly-(cystamine bisacrylamide-diamino hexane) (p(CBA-DAH)), have been employed to deliver RAE-1 to pancreatic islets, resulting in improved insulin levels [ 128 ]. Furthermore, ex vivo gene transfer and autologous grafts have shown promising outcomes in animal models. The introduction of the human insulin gene into pancreatic or liver cells followed by autologous grafts improved insulin secretion, glycemic control, and alleviated the diabetic complications in pigs. However, gene silencing eventually occurred, necessitating a deeper understanding of the underlying mechanisms [ 128 , 129 ].
Stem cell based therapy in diabetes
Efforts are ongoing to develop standardized processes for donor and recipient selection/allocation to increase pancreas utilization [ 130 , 131 , 132 , 133 ]. Techniques for isolating pancreatic islets are being optimized to become more standardized and consistent. Noninvasive imaging technologies allow the monitoring of the transplanted islets without surgery [ 134 , 135 ]. Biomarkers could also evaluate how immunomodulation strategies are working [ 136 , 137 , 138 ]. Researchers are also exploring alternative transplant sites in the body beyond just the liver, to see if the other locations may better support islet graft survival and function. Together, these areas of refinement aim to improve the safety and reliability of islet transplantation procedures as a potential therapy for diabetes [ 139 ]. Bioengineering approaches are being developed to optimize the islet transplantation microenvironment using biomaterials which enhance islet engraftment and function through engineered extracellular niches [ 140 , 141 ]. For example, encapsulation techniques aim to protect pancreatic islets against immune reponse by enclosing them within semipermeable hydrogel polymer capsules [ 142 , 143 ]. This localized immunoisolation strategy utilizes biomaterials like alginate to create a physical barrier preventing immune cell contact while still allowing nutrient and oxygen diffusion. Researchers concurrently seek alternative unlimited cellular sources to address limited islet availability. Mesenchymal stem cells possess immunomodulatory properties and their adjuvant delivery, either early in disease onset or simultaneously with islet transplantation, has shown promising signs of improving outcomes in preclinical investigations. By dampening inflammatory responses and favoring regenerative processes, stem cells may help to establish a more tolerogenic transplant environment. These bioengineering and cell therapy approaches offer potential pathways towards eliminating the exogenous insulin requirement [ 144 , 145 ]. A variety of stem cell types have therapeutic potential for diabetes (Fig. 2 ). Pluripotent stem cells possess immense promise for overcoming the limitations of islet transplantation. Human embryonic stem cells and induced pluripotent stem cells are especially attractive candidates due to their unique ability to both self-renew indefinitely and differentiate into any cell type. This makes them an ideal source of replacement pancreatic beta cells. Significant research effort across academic and industrial laboratories has led to advancement in differentiation protocols that can convert pluripotent stem cells into functional beta-like cells in vitro. However, establishing consistent, well-characterized cellular production methods that comply with stringent safety and efficacy standards remains a priority for clinical translation. Ongoing work aims to generate therapeutic stem cell-derived beta cell replacements exhibiting stable, glucose-responsive insulin secretion comparable to primary islets. Although, technological and regulatory hurdles still must be cleared, pluripotent stem cells have the greatest potential to finally solve the problem of limited cell availability and provide an unlimited source of transplantable tissue suitable for widespread treatment of diabetes [ 145 , 146 , 147 , 148 ]. There are currently six registered clinical trials evaluating the use of human pluripotent stem cells for the T1D treatment. All trials except one use PEC-01 cells, which consist of a mixture of pancreatic endoderm and polyhormonal cell population derived from CyT49 stem cells that are fully committed to endocrine differentiation upon implantation [ 149 ]. The initial trial implanted PEC-01 cells within an encapsulation device, hypothesizing no need for immunosuppression. While, well-tolerated with minor adverse effects, insufficient engraftment occurred due to foreign body responses that eliminated the cells [ 150 ]. The trial transitioned in 2017 to use an open encapsulation device that required immunosuppression. Subcutaneous engraftment, differentiation of cells into islet-like clusters, and glucose-responsive insulin production provided the first evidence that pancreatic progenitor cells can survive, mature, and function as the endocrine cells in humans. Potential benefits on stimulated C-peptide levels and glycemic control were observed in one patient [ 151 , 152 ]. Two reports in late 2021 described results in 17 patients receiving PEC-01 cells in an open device. Engraftment and insulin expression occurred in the majority, glucose-responsive secretion in over one-third, and various glycemic improvements were observed at six months. Explanted tissues contained heterogeneous pancreatic compositions including mature beta cells, with no teratoma formation and mild adverse effects related to surgery/immunosuppression. VX-880 uses fully differentiated insulin-producing stem cell-derived islet cells in phase 1/2 trial evaluating portal infusion and different doses requiring immunosuppression. Preliminary results suggest early engraftment and insulin secretion. The manin challenge was controlling immune rejection without systemic immunosuppression [ 149 ]. Several strategies are being explored to address the challenges of immune rejection in stem cell therapies for diabetes. They include generating stem cell lines that are universally compatible through HLA silencing, developing milder regimens of immunosuppression, and refining encapsulation and containment approaches to protect transplanted cells toward immune response. Establishing standardized stem cell banks is also an area of investigation [ 153 , 154 ]. Xenotransplantation using gene-edited porcine islets remains an exciting avenue of research given advances to improve engraftment and reduce immunogenicity in preclinical studies [ 155 ]. Novel approaches continue to emerge as well, such as decellularization techniques, 3D bioprinting of tissue constructs, and creating interspecies chimeras. Rapid evolution of cell-based therapies across both academic and commercial sectors is promising to restore normoglycemic control in diabetic cases. Refinement of existing methods and development of new strategies hold potential to perform a safe and effective cell replacement without reliance on systemic immunosuppression. Stem cell and regenerative therapies may ultimately manage diabetes through restored endogenous insulin production [ 156 ]. Recently a meta analysis evaluated the safety and efficacy of MSC-based therapy for diabetes in humans. This comprehensive analysis was conducted on 262 patients across six trials that met the inclusion criteria within the last five years. The results reveal that treatment with MSCs significantly reduced the dosage of anti-diabetic drugs over a 12-months. Following treatment, HbAc1 levels decreased by an average of 32%, fasting blood glucose levels decreased by an average of 45%, and C-peptide levels showed a decrease of 38% in two trials and an increase of 36% in four trials. Notably, no severe adverse events were reported across all trials. Therefore, it can be concluded that MSC therapy for type 2 diabetes is safe and effective [ 157 ].
Advances in islet transplantation and stem cell-derived Beta cells
Limited number of the islet transplantation donors highlights the importance of cell therapy in diabetes. Although, higher islet numbers from multiple donors increase the success, limited pancreas availability restricts widespread use [ 158 ]. Using multiple donors also increases rejection risk, while isolation of the islets can cause tissue damage [ 159 ]. To overcome these challenges, researchers have explored the differentiation of stem cells into beta cells in vitro to generate an unlimited supply of insulin-producing cells with standardized and characterized products. Genetic engineering techniques have also been investigated to confer advantages such as stress resistance or immune evasion [ 158 ]. ViaCyte has developed a stem cell-derived pancreatic progenitor called PEC-01, which has the ability to mature into endocrine cells in rodent models. To protect the transplanted cells from immune response, retrieval encapsulation devices were also created [ 160 , 161 , 162 ]. In an initial human clinical trial conducted in 2014 (NCT02239354), the Encaptra device was utilized with the aim of providing complete immunoprotection of transplanted cells through the use of a cell-impermeable membrane. Although, the PEC-Encap product showed reliable tolerance and minimal adverse effects, the trial was stopped due to the inadequate engraftment of functional products. While, a few endocrine cells were observed, fibrosis around the capsule led to graft loss and supression of the insulin secretion. To address this challenge, a more recent development called the PEC-Direct device was introduced, which featured openings in the membrane to facilitate vascularization, thereby improving nutrient exchange and supporting cell viability. However, since host cells could infiltrate the device, immunosuppression was necessary following the transplantation [ 163 , 164 , 165 ]. Protocols were developed to generate clusters of stem cell-derived beta cells that secreted glucose-responsive insulin. These clusters, referred to SC-islets, also contained other endocrine cells, including glucagon-producing cells. SC-islets improved glycemic control in diabetic mice and nonhuman primates [ 146 , 166 , 167 , 168 ]. In a trial conducted in 2017 (NCT03163511), the transplantation of progenitor cells resulted in the maturation of endocrine cells, and glucose-responsive C-peptide secretion was observed 6–9 months post-transplantation. Notably, the majority of these mature endocrine cells exhibited glucagon-positive characteristics. The porous regions housing the endocrine cells allowed for the infiltration of host vessels to facilitate vascularization. However, non-cellular regions were isolated by the presence of fibrosis [ 164 , 165 ]. Although, there was not a sufficient levels of circulating C-peptide in these trials, the findings underscored the significance of promoting vascularization and minimizing fibrotic reactions [ 164 , 169 ]. Vertex conducted a human trial in 2021 (NCT04786262) involving the transplantation of half-dose VX-880 cells (SC-islets) without a device to avoid previous problems, which necessitated immunosuppression. Preliminary results reported improved glycemic control, although it took longer to achieve the same outcome compared to rodent models [ 158 ]. Overall, progresses in islet transplantation and stem cell-derived beta cells pave the way for overcoming the limitations of traditional approaches. Further research and refinements are also required to achieve consistent and clinically significant outcomes in the treatment of diabetes.
Chalenges and limitations
Cell-based therapies have been significantly progressed for diabetes; however, there are still several challenges that need to be overcome. Clinical trials investigating encapsulation devices and islet transplantation techniques have provided valuable insights but face several obstacles including oxygenation, host immune responses, and insufficient long-term engraftment success. Immunoengineering of biomaterials and additive manufacturing for the development of 3D islet structures aim to modulate inflammation and promote graft revascularization. Nevertheless, achieving consistent normalization of blood glucose levels without exogenous insulin remains a challenge in human studies. In the field of gene therapy and stem cell differentiation, research focuses on genetically-modified or progenitor-derived insulin-secreting β-like cells to optimize protocols that ensure safety and functionality. The main challenge is to establish stable and functional cells capable of permanently restoring normoglycemia without the need for external intervention. One major barrier is the immune response, which targets allogeneic and xenogeneic islet grafts. Although, local immunotherapy minimizes the systemic effects, evading graft destruction through biomaterials without the requirement of immune suppression remains a significant challenge. The translation of precision 3D islet constructs and genetically reprogrammed cells also necessitates scalable manufacturing processes to ensure consistent function and long-term safety across batches. When critically appraising progress in the field of cell-based diabetes treatments, it is imperative to consider the regulatory, ethical, economic, and safety factors that shape translational applications. At the regulatory level, oversight bodies play a pivotal role in establishing standards to ensure patient welfare while enabling therapeutic innovation. FDA oversees clinical trials and product approvals in the United States (US), while in Europe the EMA provides parallel regulatory guidance. Within the US, organizations like the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) govern organ and cell allocation protocols [ 17 , 170 ]. However, as regenerative approaches diverge from traditional organ transplantation, regulatory pathways require ongoing harmonization between the agencies and jurisdictions. Continual dialogue between researchers, oversight boards, and policymakers will be crucial to streamline guidelines in a patient-centric manner that balances safety, efficacy, and timely access to cutting-edge therapies. For instance, as stem cell-derived beta cells and 3D bioprinted tissue constructs emerge, traditional drug and device frameworks may not adequately address product characterization and manufacturing complexities for these advanced therapeutic products [ 67 ]. Within clinics, maintaining compliance with evolving regulations impacts research directives and ultimately patients’ access to the novel treatments. Addressing informed consent, clinical trial design, and privacy protections for sensitive health data are also paramount from an ethical perspective [ 128 , 129 ]. Autonomy and agency of research participants in decision-making related to experimental therapies demand prudency. Equitable accessibility of new treatment options also warrants attention to avoid certain populations facing undue barriers. Cell sourcing presents ethical issues depending on derivation from embryonic, fetal or adult tissues. Logistical matters like shipping and processing stem cell-derived islets prior to transplantation necessitate scrutiny. Tumorigenic potential of the undifferentiated pluripotent stem cells should be optimized through rigorous preclinical testing. Transitioning therapies between animal and early human investigations necessitates well-characterized cellular products showing consistent safety and glucose-responsive insulin secretion profiles comparable to pancreatic islets. Long-term animal model data substantiating lack of malignant transformation following transplantation aids allaying ethical safety concerns as the therapies progress clinically. Researchers carefully screen new concepts to prevent side effects in participants while pursuing curative goals. In terms of economic costs, islet and stem cell transplant procedures remain prohibitively expensive for broad applicability despite promising clinical signals. The field requires sustained study to validate techniques, track long-term outcomes, assess healthcare costs offsets from mitigating diabetes’ debilitating complications, and establish cost-benefit ratios for national reimbursement paradigms. Public-private partnerships may accelerate large, interventional trials and longitudinal research to precisely quantify the cellular therapies’ safety profiles and real-world efficacies compared to intensive management versus costs of intensive diabetes care. Ongoing developments like 3D bioprinting offer catalytic manufacturing potential fundamentally recalibrating economics by enhancing yields, standardizing procedures, and reducing costs through scale. By thoroughly and sensitively examining regulatory frameworks, informed consent processes, risks and benefits, as well as financial considerations at both micro and macro levels, researchers, oversight boards and broader stakeholder networks can advance cell-based therapies towards delivering life-changing benefits for all communities. A multidisciplinary, conscientious approach balances progress against patient welfare. A combination of multiple strategies may help to overcome these limitations. For instance, gene-modified islets integrated within vascularized biomaterial implants or sequenced therapies have promising results to prime grafts in pro-regenerative environments before transplantation. Collaboration across disciplines offers hope that refined individualized therapies may eventually achieve durable insulin independence through functional pancreatic cell or tissue engraftment, not only for diabetes but also for chronic pancreatitis. Regarding, ongoing progresses in unraveling these barriers, cell replacement approaches have the potential to improve diabetes management.
Conclusions
This review provides a comprehensive overview of the advances, challenges, and future directions in various cell-based therapeutic approaches for the treatment of diabetes. Significant progresses have been achieved in microencapsulation design, immunomodulation, tissue constructs, genetic and cellular reprogramming techniques, as well as initial clinical translation. However, the complete restoration of normoglycemia without the need for lifelong immunosuppression is still considered as a significant therapeutic challenge. Therefore, addressing the transplant environment of the hostile nature, developing minimally invasive delivery methods, and overcoming limitations in engraftment efficiency and longevity are crucial issues for the future researches. Through the sustained multidisciplinary efforts for the improvement of existing strategies and establishing novel paradigms, achieving durable insulin independence can be a realistic goal for all diabetic cases through the personalized cell replacement or regeneration.
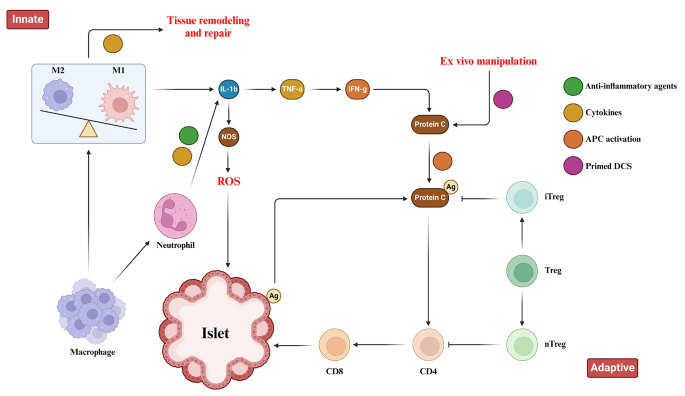
Immune Responses toward pancreatic islets following transplantation. This figure illustrates the immune responses, including the innate and adaptive immunity that are triggered upon pancreatic islet transplantation. Immune response begins with the activation of tissue macrophages and neutrophils in response to injury. Subsequent, release of inflammatory cytokines stimulates antigen-presenting cells (APCs), CD4 + T cells, CD8 + T cells, and cytotoxic T lymphocytes to orchestrate the immune response
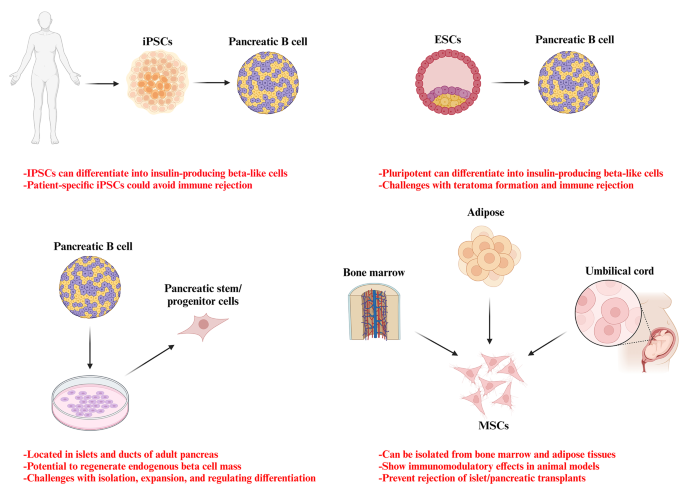
Potential stem cell sources for the treatment of diabetes
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acrylonitrile butadiene styrene
Activate antigen-presenting cells
Adeno-associated virus
Duodenal homeobox 1
Engineered pseudo islets
Expanded polytetrafluoroethylene
Extracellular matrix
Foreign body response
Fused filament fabrication
Gestational diabetes mellitus
Glucose 6-phosphatase
Insulin-like growth factor binding protein-1
Mesenchymal stem cells
Neurogenin 3
Organ Procurement and Transplantation Network
Phosphoenolpyruvate carboxykinase
Polyaryletherketone
Polycaprolactone
Polycarbonate
Polyetheretherketone
Polyetherimide
Poly-lactic acid
Polystyrene
Stereolithography
Thermoplastic polyurethane
Type 1 diabetes
Type 1 diabetes mellitus
Type 2 diabetes mellitus
United Network for Organ Sharing
United States
Vascular endothelial growth factor
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Article Google Scholar
Cho NH, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Article CAS PubMed Google Scholar
Moghbeli M, Naghibzadeh B, Ghahraman M, Fatemi S, Taghavi M, Vakili R, et al. Mutations in HNF1A gene are not a Common cause of familial young-onset diabetes in Iran. Indian J Clin Biochem. 2018;33(1):91–5.
Akhlaghipour I, Bina AR, Mogharrabi MR, Fanoodi A, Ebrahimian AR, Khojasteh Kaffash S, et al. Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population. Hum Genomics. 2022;16(1):11.
Article CAS PubMed PubMed Central Google Scholar
Moghbeli M, Khedmatgozar H, Yadegari M, Avan A, Ferns GA, Ghayour Mobarhan M. Cytokines and the immune response in obesity-related disorders. Adv Clin Chem. 2021;101:135–68.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Reviews Endocrinol. 2020;16(7):349–62.
Article CAS Google Scholar
Siqueira ISLd, Alves Guimarães R, Mamed SN, Santos TAP, Rocha SD, Pagotto V, et al. Prevalence and risk factors for self-report diabetes mellitus: a population-based study. Int J Environ Res Public Health. 2020;17(18):6497.
Free radical research.
Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol. 2022;13:800995.
Mojarrad M, Moghbeli M. Genetic and molecular biology of bladder cancer among Iranian patients. Mol Genet Genomic Med. 2020;8(6):e1233.
Article PubMed PubMed Central Google Scholar
Moghbeli M. Genetic and molecular biology of breast cancer among Iranian patients. J Transl Med. 2019;17(1):218.
Abbaszadegan MR, Moghbeli M. Genetic and molecular origins of colorectal Cancer among the iranians: an update. Diagn Pathol. 2018;13(1):97.
Kim I. A brief overview of cell therapy and its product. J Korean Association Oral Maxillofacial Surg. 2013;39(5):201.
Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philosophical Trans Royal Soc B: Biol Sci. 2015;370(1680):20150017.
El-Kadiry AE-H, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med. 2021;8:756029.
Squifflet J-P, Gruessner R, Sutherland D. The history of pancreas transplantation: past, present and future. Acta Chir Belg. 2008;108(3):367–78.
Article PubMed Google Scholar
Parums DV. First Regulatory approval for allogeneic pancreatic islet Beta cell infusion for adult patients with type 1 diabetes Mellitus. Med Sci Monitor: Int Med J Experimental Clin Res. 2023;29:e941918–1.
Yang L, Hu Z-M, Jiang F-X, Wang W. Stem cell therapy for insulin-dependent diabetes: are we still on the road? World J Stem Cells. 2022;14(7):503.
Affan M, Dar MS. Donislecel-the first approved pancreatic islet cell therapy medication for type 1 diabetes: a letter to the editor. Ir J Med Sci (1971-). 2023:1–2.
Harris E. FDA greenlights first cell therapy for adults with type 1 diabetes. JAMA. 2023.
Soon-Shiong P, Heintz R, Merideth N, Yao Q, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet (London England). 1994;343(8903):950–1.
Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–8.
Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–9.
Weng L, Wang X, Liu H, Yu Z, Liu S. Light-responsive microneedle array with tunable insulin release function for painless and on-demand anti-diabetic therapy. Mater Lett. 2023:135684.
Okcu A, Yazir Y, Şimşek T, Mert S, Duruksu G, Öztürk A, et al. Investigation of the effect of pancreatic decellularized matrix on encapsulated islets of Langerhans with mesenchymal stem cells. Tissue Cell. 2023;82:102110.
Khaliq T, Sohail M, Minhas MU, Mahmood A, Munir A, Qalawlus AHM, et al. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int J Pharm. 2023;643:123244.
Kuwabara R, Qin T, Llacua LA, Hu S, Boekschoten MV, de Haan BJ, et al. Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta Biomater. 2023;158:151–62.
Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem cell Res. 2014;12(3):807–14.
Dufrane D, van Steenberghe M, Goebbels R-M, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–8.
Pullen LC. Stem cell–derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Wiley Online Library; 2018. pp. 1581–2.
Dang HP, Chen H, Dargaville TR, Tuch BE. Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes. J Cell Mol Med. 2022;26(18):4756–67.
Viacyte. ViaCyte and gore enter clinical phase agreement based on novel membrane technology for PEC-encap product candidate. 2020.
Viacyte. viacyte announces initiation of phase 2 study of encapsulated cell therapy for type 1 diabetes patients 2021 2021. https://viacyte.com/press-releases/viacyte‐announces‐initiation‐of‐phase‐2‐study‐of‐encapsulated‐cell‐ther‐apy‐for‐type‐1‐diabetes‐patients/ .
Hodgson J. Drug pipeline 3Q23—ERT, bispecifics and CRISPR in sickle cell disease. Nat Biotechnol. 2023;41(11):1498–500.
Pagliuca F. Pre-clinical proof-of-Concept in two lead programs in type 1 diabetes. International Socety for Stem Cell Research; 2019.
Jones PM, Persaud SJ. β-cell replacement therapy for type 1 diabetes: closer and closer. Diabet Med. 2022;39(6).
Carlsson P-O, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–44.
Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42(13):918–22.
Evron Y, Colton CK, Ludwig B, Weir GC, Zimermann B, Maimon S, et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. 2018;8(1):6508.
Cao R, Avgoustiniatos E, Papas K, de Vos P, Lakey JR. Mathematical predictions of oxygen availability in micro-and macro‐encapsulated human and porcine pancreatic islets. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;108(2):343–52.
Gala-Lopez B, Pepper A, Dinyari P, Malcolm A, Kin T, Pawlick L, et al. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch–preliminary experience. CellR4. 2016;4(5):e2132.
Google Scholar
Sernova Corp Presents Positive Preliminary. Safety and Efficacy Data in its Phase I/II Clinical Trial for Type-1 Diabetes: Biospace. https://www.biospace.com/article/sernova‐corp‐presents‐positive‐preliminary‐safety‐and‐efficacy‐data‐in‐its‐phase‐i‐ii‐clinical‐trial‐for‐type‐1‐diabetes/ .
Bachul PJ, Perez-Gutierrez A, Juengel B, Golab K, Basto L, Perea L et al. 306-OR: modified approach for improved isllotransplantation into prevascularized sernova cell pouch device: preliminary results of the phase i/ii clinical trial at University of Chicago. Diabetes. 2022;71(Supplement_1).
Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–52.
Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Shapiro AD, Konkle BA, Croteau SE, Miesbach WA, Hay CRM, Kazmi R, et al. First-in-human phase 1/2 clinical trial of SIG-001, an innovative shielded cell therapy platform, for hemophilia Α. Blood. 2020;136:8.
Taraballi F, Sushnitha M, Tsao C, Bauza G, Liverani C, Shi A, et al. Biomimetic tissue engineering: tuning the immune and inflammatory response to implantable biomaterials. Adv Healthc Mater. 2018;7(17):1800490.
Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, et al. A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings. Adv Healthc Mater. 2017;6(4):1601091.
Liu Q, Chiu A, Wang L-H, An D, Zhong M, Smink AM, et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat Commun. 2019;10(1):5262.
Noverraz F, Montanari E, Pimenta J, Szabó L, Ortiz D, Gonelle-Gispert C, et al. Antifibrotic effect of ketoprofen-grafted alginate microcapsules in the transplantation of insulin producing cells. Bioconjug Chem. 2018;29(6):1932–41.
Jeon SI, Jeong J-H, Kim JE, Haque MR, Kim J, Byun Y, et al. Synthesis of PEG-dendron for surface modification of pancreatic islets and suppression of the immune response. J Mater Chem B. 2021;9(11):2631–40.
Derakhshankhah H, Sajadimajd S, Jahanshahi F, Samsonchi Z, Karimi H, Hajizadeh-Saffar E, et al. Immunoengineering Biomaterials in Cell-based therapy for type 1 diabetes. Tissue Eng Part B: Reviews. 2022;28(5):1053–66.
Piemonti L, Maffi P, Nano R, Bertuzzi F, Melzi R, Mercalli A, et al. Treating diabetes with islet transplantation: lessons from the Milan experience. Transplantation, Bioengineering, and regeneration of the endocrine pancreas. Elsevier; 2020. pp. 645–58.
Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24(10):3927.
Chen X, Liu H, Li H, Cheng Y, Yang L, Liu Y. In vitro expansion and differentiation of rat pancreatic duct-derived stem cells into insulin secreting cells using a dynamic three-dimensional cell culture system. Genet Mol Res. 2016;15(2).
Becker MW, Simonovich JA, Phelps EA. Engineered microenvironments and microdevices for modeling the pathophysiology of type 1 diabetes. Biomaterials. 2019;198:49–62.
Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A. 2013;19(11–12):1465–75.
Izadi Z, Hajizadeh-Saffar E, Hadjati J, Habibi-Anbouhi M, Ghanian MH, Sadeghi-Abandansari H, et al. Tolerance induction by surface immobilization of Jagged-1 for immunoprotection of pancreatic islets. Biomaterials. 2018;182:191–201.
McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81.
Chen H, Teramura Y, Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly (ethylene glycol)-conjugated phospholipid. J Controlled Release. 2011;150(2):229–34.
Su J, Hu B-H, Lowe WL Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–14.
Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, et al. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS ONE. 2012;7(12):e50265.
Kumar M, Nandi SK, Kaplan DL, Mandal BB. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production. ACS Biomaterials Sci Eng. 2017;3(10):2443–56.
Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–49.
Shi Y, Zhao YZ, Jiang Z, Wang Z, Wang Q, Kou L, et al. Immune-Protective formulations and process strategies for improved survival and function of transplanted islets. Front Immunol. 2022;13:923241.
Zhang S, Yang H, Wang M, Mantovani D, Yang K, Witte F, et al. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation. 2023;4(6):100503.
CAS PubMed PubMed Central Google Scholar
Accolla RP, Simmons AM, Stabler CL. Integrating Additive Manufacturing techniques to improve cell-based implants for the treatment of type 1 diabetes. Adv Healthc Mater. 2022;11(13):e2200243.
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. ACS; 2014.
Bol RJ, Šavija B. Micromechanical models for FDM 3D-Printed polymers: a review. Polymers. 2023;15(23):4497.
Paul S. Finite element analysis in fused deposition modeling research: a literature review. Measurement. 2021;178:109320.
Monaldo E, Ricci M, Marfia S. Mechanical properties of 3D printed polylactic acid elements: experimental and numerical insights. Mech Mater. 2023;177:104551.
Anoop M, Senthil P. Microscale representative volume element based numerical analysis on mechanical properties of fused deposition modelling components. Materials Today: Proceedings. 2021;39:563 – 71.
McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences. 2006;103(31):11461-6.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences. 2012;109(11):4245-50.
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RM, del Saenz L, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11):597.
Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D. Paving the way for successful islet encapsulation. Drug Discovery Today. 2019;24(3):737–48.
Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–8.
Song S, Roy S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: cells, biomaterials, and devices. Biotechnol Bioeng. 2016;113(7):1381–402.
Zhi ZL, Kerby A, King AJF, Jones PM, Pickup JC. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 2012;55(4):1081–90.
Farina M, Chua CYX, Ballerini A, Thekkedath U, Alexander JF, Rhudy JR, et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials. 2018;177:125–38.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D et al. 3D printed vascularized device for Subcutaneous Transplantation of Human islets. Biotechnol J. 2017;12(9).
Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater Horiz. 2019;6(6):1197–206.
Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–104.
Ernst AU, Wang LH, Ma M. Interconnected toroidal hydrogels for islet encapsulation. Adv Healthc Mater. 2019;8(12):1900423.
Liang J-P, Accolla RP, Jiang K, Li Y, Stabler CL. Controlled release of anti-inflammatory and proangiogenic factors from macroporous scaffolds. Tissue Eng Part A. 2021;27(19–20):1275–89.
Pedraza E, Brady A-C, Fraker CA, Molano RD, Sukert S, Berman DM, et al. Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2013;22(7):1123–35.
Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32(26):6045–51.
Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomedical Mater Res Part B: Appl Biomaterials. 2018;106(5):1788–98.
Liu X, Jakus AE, Kural M, Qian H, Engler A, Ghaedi M, et al. Vascularization of natural and synthetic bone scaffolds. Cell Transplant. 2018;27(8):1269–80.
Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG. Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A. 2015;21(5–6):1055–65.
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–800.
Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proceedings of the National Academy of Sciences. 2017;114(35):9337-42.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J. 2017;12(9):1700169.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14(13):2202–11.
Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68.
Gao Q, Liu Z, Lin Z, Qiu J, Liu Y, Liu A, et al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomaterials Sci Eng. 2017;3(3):399–408.
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344.
Hospodiuk M, Dey M, Ayan B, Sosnoski D, Moncal KK, Wu Y, et al. Sprouting angiogenesis in engineered pseudo islets. Biofabrication. 2018;10(3):035003.
Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, et al. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater. 2016;5(13):1606–16.
Dionne KE, Colton CK, Lyarmush M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21.
de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, Van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–75.
Thomas F, Wu J, Contreras JL, Smyth C, Bilbao G, He J, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130(2):333–8.
Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: more than a membrane story. World J Transplantation. 2016;6(1):69.
Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48.
Pati F, Jang J, Ha D, Won Kim S, Rhie J, Shim J, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53.
Hu S, Martinez-Garcia FD, Moeun BN, Burgess JK, Harmsen MC, Hoesli C, et al. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater Sci Engineering: C. 2021;123:112009.
Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Delivery Translational Res. 2015;5:125–36.
Kooptiwut S, Kaewin S, Semprasert N, Sujjitjoon J, Junking M, Suksri K, et al. Estradiol prevents high glucose-induced β-cell apoptosis by decreased BTG2 expression. Sci Rep. 2018;8(1):12256.
Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34(23):5792–801.
Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, et al. Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep. 2016;14(3):2644–50.
Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003.
Liu YY, Yu HC, Liu Y, Liang G, Zhang T, Hu QX. Dual drug spatiotemporal release from functional gradient scaffolds prepared using 3 D bioprinting and electrospinning. Polym Eng Sci. 2016;56(2):170–7.
Freeman FE, Pitacco P, van Dommelen LH, Nulty J, Browe DC, Shin J-Y, et al. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv. 2020;6(33):eabb5093.
Wong MS, Hawthorne WJ, Manolios N. Gene therapy in diabetes. Self Nonself. 2010;1(3):165.
Ahmad Z, Rasouli M, Azman AZF, Omar AR. Evaluation of insulin expression and secretion in genetically engineered gut K and L-cells. BMC Biotechnol. 2012;12:1–9.
Tudurí E, Bruin JE, Kieffer TJ. Restoring insulin production for type 1 diabetes. J Diabetes. 2012;4(4):319–31.
Romer AI, Sussel L. Pancreatic islet cell development and regeneration. Current opinion in endocrinology, diabetes, and obesity. 2015;22(4):255.
Jaén ML, Vilà L, Elias I, Jimenez V, Rodó J, Maggioni L, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol therapy-methods Clin Dev. 2017;6:1–7.
Li H, Li X, Lam KS, Tam S, Xiao W, Xu R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J Biomed Sci. 2008;15:487–97.
Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–42.
Abed A, Critchlow C, Flatt PR, McClenaghan NH, Kelly C. Directed differentiation of progenitor cells towards an islet-cell phenotype. Am J Stem Cells. 2012;1(3):196.
PubMed PubMed Central Google Scholar
Zhao M, Amiel SA, Ajami S, Jiang J, Rela M, Heaton N, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE. 2008;3(7):e2666.
Handorf AM, Sollinger HW, Alam T. Genetic engineering of surrogate β cells for treatment of type 1 diabetes mellitus. J Diabetes Mellitus. 2015;5(04):295–312.
Grant MB, Adu-Agyeiwaah Y, Vieira CP, Asare-Bediako B, Hammer SS, Calzi SL, et al. Intravitreal administration of AAV2-SIRT1 reverses diabetic retinopathy (DR) in a murine model of type 2 diabetes (T2D). Investig Ophthalmol Vis Sci. 2022;63(7):2310.
Yoon J-W, Jun H-S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002;8(2):62–8.
Hou W-R, Xie S-N, Wang H-J, Su Y-Y, Lu J-L, Li L-L, et al. Intramuscular delivery of a naked DNA plasmid encoding proinsulin and pancreatic regenerating III protein ameliorates type 1 diabetes mellitus. Pharmacol Res. 2011;63(4):320–7.
Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J Controlled Release. 2012;162(3):606–11.
Dezashibi HM, Shabani A. A Mini-review of Current Treatment approaches and Gene Therapy as potential interventions for diabetes Mellitus types 1. Adv Biomed Res. 2023;12:219.
Vantyghem M-C, de Koning EJ, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274–85.
Hudson A, Bradbury L, Johnson R, Fuggle S, Shaw J, Casey J, et al. The UK pancreas allocation scheme for whole organ and islet transplantation. Am J Transplant. 2015;15(9):2443–55.
Cornateanu SM, O’Neill S, Dholakia S, Counter CJ, Sherif AE, Casey JJ, et al. Pancreas utilization rates in the UK–an 11-year analysis. Transpl Int. 2021;34(7):1306–18.
Nordheim E, Lindahl JP, Carlsen RK, Åsberg A, Birkeland KI, Horneland R, et al. Patient selection for islet or solid organ pancreas transplantation: experiences from a multidisciplinary outpatient-clinic approach. Endocr Connections. 2021;10(2):230–9.
Arifin DR, Bulte JW. In vivo imaging of pancreatic islet grafts in diabetes treatment. Front Endocrinol. 2021;12:640117.
Murakami T, Fujimoto H, Inagaki N. Non-invasive beta-cell imaging: visualization, quantification, and beyond. Front Endocrinol. 2021;12:714348.
Piemonti L, Everly MJ, Maffi P, Scavini M, Poli F, Nano R, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–64.
Anteby R, Lucander A, Bachul PJ, Pyda J, Grybowski D, Basto L, et al. Evaluating the prognostic value of islet autoantibody monitoring in islet transplant recipients with long-standing type 1 diabetes mellitus. J Clin Med. 2021;10(12):2708.
Buron F, Reffet S, Badet L, Morelon E, Thaunat O. Immunological monitoring in beta cell replacement: towards a pathophysiology-guided implementation of biomarkers. Curr Diab Rep. 2021;21:1–11.
Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74.
Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis. 2018;14(4):163–8.
Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019;199:40–51.
Basta G, Montanucci P, Calafiore R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Invest. 2021;12(3):301–9.
Samojlik MM, Stabler CL. Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in type 1 diabetes. Acta Biomater. 2021;133:87–101.
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, et al. Placenta derived mesenchymal stem cells transplantation in type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metabolic Disorders. 2021;20:1179–89.
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Sambathkumar R, Migliorini A, Nostro MC. Pluripotent stem cell-derived pancreatic progenitors and β-like cells for type 1 diabetes treatment. Physiology. 2018;33(6):394–402.
Sordi V, Monaco L, Piemonti L. Cell therapy for type 1 diabetes: from islet transplantation to stem cells. Hormone Res Paediatrics. 2022;96(6):658–69.
Henry RR, Pettus J, Wilensky J, SHAPIRO AJ, Senior PA, Roep B et al. Initial clinical evaluation of VC-01TM combination product—a stem cell–derived islet replacement for type 1 diabetes (T1D). Diabetes. 2018;67(Supplement_1).
Shapiro A, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus JH et al. Insulin expression and glucose-responsive circulating C-peptide in type 1 diabetes patients implanted subcutaneously with pluripotent stem cell-derived pancreatic endoderm cells in a macro-device. David and Donner, Thomas W and Bellin, Melena D and Hsueh, Willa and Pettus, Jeremy H and Wilensky, Jon S and Daniels, Mark and Wang, Richard M and Kroon, Evert J and Brandon, Eugene Paul and D’Amour, Kevin A and Foyt, Howard, Insulin Expression and Glucose-Responsive Circulating C-Peptide in Type. 2019;1.
Keymeulen B, Jacobs-Tulleneers-Thevissen D, Kroon EJ, Jaiman MS, Daniels M, Wang R et al. 196-LB: stem cell–derived islet replacement therapy (VC-02) demonstrates production of C-peptide in patients with type 1 diabetes (T1D) and hypoglycemia unawareness. Diabetes. 2021;70(Supplement_1).
Piemonti L. Felix dies natalis, insulin… ceterum autem censeo beta is better. Acta Diabetol. 2021;58(10):1287–306.
Sordi V, Pellegrini S, Piemonti L. Immunological issues after stem cell-based β cell replacement. Curr Diab Rep. 2017;17:1–8.
Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transpl. 2020;25(5):449–56.
Edgar L, Pu T, Porter B, Aziz J, La Pointe C, Asthana A, et al. Regenerative medicine, organ bioengineering and transplantation. J Br Surg. 2020;107(7):793–800.
Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 Diabetes- A literature review. Diabetes Metab Syndr Obes. 2023;16:769–77.
Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30(5):530–48.
Paraskevas S, Maysinger D, Wang R, Duguid WP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6.
Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–6.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell–derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Translational Med. 2015;4(10):1214–22.
Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28(12):2047–61. e5.
Dolgin E, Diabetes. Encapsulating the problem. Nature. 2016;540(7632):S60–2.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38(4):460–70.
Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol. 2019;21(2):263–74.
Shapiro AJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2(12).
Witkowski P, Anteby R, Olaitan OK, Forbes RC, Niederhaus S, Ricordi C, et al. Pancreatic islets Quality and Potency cannot be verified as required for drugs: reflection on the FDA Review of a biological license application for human islets. Transplantation. 2021;105(12):e409–10.
Download references
Acknowledgements
Author information, authors and affiliations.
Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran
Ramin Raoufinia
Department of Medical Genetics and Molecular Medicine, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Ramin Raoufinia, Hamid Reza Rahimi, Ehsan Saburi & Meysam Moghbeli
You can also search for this author in PubMed Google Scholar
Contributions
RR, HRR, and ES were involved in search strategy and drafting. MM revised, designed, and supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Meysam Moghbeli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Raoufinia, R., Rahimi, H.R., Saburi, E. et al. Advances and challenges of the cell-based therapies among diabetic patients. J Transl Med 22 , 435 (2024). https://doi.org/10.1186/s12967-024-05226-3
Download citation
Received : 14 February 2024
Accepted : 22 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12967-024-05226-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cell therapy
- Translplantation
- Immunosuppression
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Research Projects

The Division of Diabetes Translation (DDT) conducts and supports studies, often in collaboration with partners, to develop and apply sound science to reduce the burden of diabetes and to address the research needs of DDT programs and the diabetes community.
Analyses from various studies, such as LEAD and SEARCH, to help improve diabetes surveillance and guide decision-making.
Research, including the Diabetes Prevention Program Outcome Study (DPPOS), that evaluates the success of diabetes interventions and strategies.
Reviews of the effect of type 2 diabetes-related health policies on various populations, such as the NEXT-D2 study.
Collection of the most recent publications and access to archived ones.
- US Diabetes Surveillance System
- Chronic Kidney Disease Surveillance System
- Vision and Eye Health Surveillance System
- Diabetes State Burden Toolkit
- Diabetes Prevention Impact Toolkit
To receive updates about diabetes topics, enter your email address:
- Diabetes Home
- State, Local, and National Partner Diabetes Programs
- National Diabetes Prevention Program
- Native Diabetes Wellness Program
- Chronic Kidney Disease
- Vision Health Initiative
- Heart Disease and Stroke
- Overweight & Obesity
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Curing Diabetes
Our research progress.

Nanotechnology and combined strategies to enhance local immunomodulation for treatment of Type 1 Diabetes
In the quest to enhance the lives of those suffering from T1D, Dr. Diana Velluto, Research Assistant Professor in the Department of Surgery at the Diabetes Research Institute, University of Miami School of Medicine, is making incredible advances. Her work centers on the use of innovative nanotechnology to transform how we approach diabetes treatment.

First-ever Dynamic Analysis of Pancreatic Regeneration in Human T1D
A groundbreaking technique has been established which has allowed scientists from the Diabetes Research Institute (DRI) at the University of Miami Miller School of Medicine to culture live sections of the pancreas for nearly two weeks. The scientists have used the living pancreas sections to chart the first dynamic single-cell map of the regenerative pathways of the pancreas.

New Discoveries Offer Hope in the Fight Against T1D
In a momentous breakthrough, researchers at the Diabetes Research Institute (DRI) have revealed pivotal revelations that may transform our strategy for averting and postponing the emergence of type 1 diabetes (T1D).

Revolutionizing Transplantation: Re-igniting a New Beacon of Hope in Immunotherapy
Another significant step is being achieved by researchers at the DRI on the lengthy journey to bring islet cell transplantation closer to reality for individuals with type 1 diabetes.

How Do Research Discoveries Translate into Clinical Cures?
Scientific research drives discovery into novel biological pathways that regulate normal bodily functions and can indicate alterations that may play a role in autoimmunity, cancer and other diseases. Understanding of the cells, molecules and genes involved in these pathways can lead to the development of drugs, cell therapies, devices and other technologies that might enable prevention, modification, or reversal of disease. But how do scientists take laboratory findings and translate them into people?

Inside Our Labs: New T1D Therapies
August 2022 – Dr. Giacomo Lanzoni is an Assistant Professor at the Diabetes Research Institute, University of Miami Miller School of Medicine. His research is focused on developing stem cell-based therapies for Type 1 Diabetes. In this disease, insulin-producing pancreatic islet beta cells are lost due to an autoimmune attack.

Inside Our Labs: Islet Transplantation
June 2022 – The Diabetes Research Institute (DRI) at the University of Miami Miller School of Medicine in Miami, Florida first began performing clinical islet transplantation in 1985. Since then, our center has blossomed into one of the largest independent centers worldwide, with 57 transplant recipients totaling 101 islet infusions since the year 2000.

Inside Our Labs: Treg Therapy and other Autoimmune Therapies
May 2022 – Dr. Bayer’s lab focuses on Treg therapy and other autoimmune therapies. The main overarching goal of their current work is to better understand autoimmune-targeted treatments and how it can lead to diabetes reversal or late-stage development treatment. They focus on understanding the mechanisms by which the immune system provides immune regulation to maintain health and prevent disease.
Inside Our Labs: Mechanisms of Islet Transplant Immune Tolerence
April 2022 – Dr. Abdulreda’s DRIF funded project entitled “Mechanisms of Islet Transplant Immune Tolerance” aims to understand immune pathways that contribute to graft rejection or immune tolerance. Currently, islet transplantation requires continual immunosuppressive treatment to reduce the chance of islet graft rejection.

COVID-19, Diabetes and Mesenchymal Stem Cells: Groundbreaking Findings Bring New Hope
February 2022 – At the DRI, we have been studying Mesenchymal Stem Cells and their immunomodulatory properties for more than a decade. These cells can function as potent inhibitors of inflammation, can modulate immunity, and can stimulate Regulatory T cells (Tregs). Intense studies are ongoing at our Institute to use these cells to fend off autoimmunity in type 1 diabetes and to limit progression of diabetes complication.

2020 Long-Term Culture of Human Pancreatic Slices Reveals Regeneration of Insulin-Producing Cells
DRI scientists developed a method allowing for the long-term culture of “pancreatic slices” to study the regeneration of the human pancreas in real time. The results, published Nature Communications, demonstrate for the first time that extended cultures of near-intact human pancreatic tissue retain the ability of the live organ to replenish insulin-producing beta cells. The use of this system as a model to study pancreatic regeneration could have important therapeutic implications for the treatment of diabetes.

2019 Long-Term Islet Transplant Recipients Show Near-Normal Glucose Control
Using continuous glucose monitoring (CGM), DRI scientists show that a small group of islet transplant recipients who were insulin independent for an average of 10 years have blood sugar levels that are similar to those without type 1 diabetes.

2019 Engineered ‘Suicide Genes’ Prevent Tumors in Stem-Cell Derived Beta Cells
For the first time, DRI scientists engineer a stem cell line containing two ‘suicide genes’ that induce cell death in all but the desired insulin-producing cells. This double fail-safe approach, published in Stem Cell Reports , opens the door to advancing cell-replacement therapies for people living with type 1 diabetes.

2018 DRI Launches POSEIDON Study to Assess Impact of Omega-3 and Vitamin D in Type 1 Diabetes
Several scientific reports have suggested that high-dose omega-3 fatty acids and vitamin D may have a beneficial effect on autoimmune conditions, like type 1 diabetes. DRI researchers will formally test the effects of this intervention in children and adults newly diagnosed and in those with longer-standing T1D to evaluate any benefit on reducing inflammation, halting autoimmunity, and increasing insulin sensitivity and secretion.

2018 DRI Scientists Identify Unique Pancreatic Stem Cells
DRI scientists confirm the existence of progenitor cells (pancreatic stem cells) within the large ducts of the human pancreas that can be stimulated to develop into glucose-responsive beta cells. The findings, published in Cell Reports , could pave the way to regenerating a person’s own insulin-producing cells in type 1 diabetes patients.

2017 DRI Scientists Identify New Metabolic “Signature” as Potential Key Biomarker for Type 1 Diabetes Development
Currently, there is no good biomarker to detect whether the immune attack on the beta cells is underway. DRI researchers mapped out the biochemical changes that occur during type 1 diabetes onset, which is important not only for preventing the disease, but also for monitoring the recurrence of immune attack. This first-ever longitudinal study in experimental models was published in the Journal of Proteome Research .

2017 Novel Tissue-Engineered Islet Transplant Achieves Insulin Independence
DRI researchers report on the first trial participant to receive an islet transplant within a tissue-engineered scaffold, demonstrating that islets transplanted within this BioHub platform can successfully engraft and achieve insulin independence. The trial tests the omentum as an alternative transplant site. The one-year findings are published in the prestigious New England Journal of Medicine (NEJM) .

2016 Researchers Develop and Test “Biological” DRI BioHub Platform
The DRI develops and tests a bioengineered scaffold to house insulin-producing cells. This biological platform, which uses the recipient’s own plasma combined with thrombin, offers the opportunity to incorporate helper cells, nutrients, and local immune protection. These preclinical experiments are the basis for FDA submission for a Phase I/II clinical trial. The work is featured on the cover of the journal Diabetes .

2016 Novel Protocol Achieves 100 Percent Disease Remission in Experimental Models
DRI’s Dr. Allison Bayer and her team have developed a novel protocol demonstrating that adoptive Regulatory T cell (T reg) therapy can reverse the disease and reset autoimmunity in experimental models, achieving disease remission in 100 percent of the recipients. Importantly, the therapy was directed specifically at halting the destruction of the beta cells while the normal immune responses remained intact.

2015 DRI Researchers Convert Pancreatic Exocrine Cells into Insulin-Producing Endocrine Cells Using a Single Protein, BMP-7
Researchers successfully convert non-insulin producing cells into insulin-producing cells using a single agent, BMP-7 (bone morphogenetic protein-7), which is already clinically approved by the FDA. Their published findings in Diabetes demonstrate for the first time that non-endocrine tissue can be reprogrammed to respond to blood glucose without the use of any genetic manipulation, representing a safer method to increase the supply of islets for transplant into people with T1D.

2014 DRI Pioneers New Encapsulation Technology
DRI researchers demonstrate that their unique cell coating process allows efficient encapsulation of islets without compromising viability and function of the cells. The team’s novel method for “shrink wrapping” each cell had been designed to specifically address what are considered to be the limitations of traditional cell encapsulation strategies. The results of their study earn the cover position in Proceedings of the National Academy of Sciences .

2013 DRI Introduces Plans for BioHub Mini Organ
Scientists unveil their plan for the DRI BioHub, a bioengineered “mini organ” designed to mimic the native pancreas. The platform technology will contain thousands of insulin-producing cells that manage blood sugar levels in real time, plus other components that keep the cells healthy and viable long term. The DRI will focus on three primary areas: the development of a new transplant site; the development of a reliable supply of islets; and the ability to sustain the cells’ function without the need for harsh, systemic anti-rejection drugs.

2012 DRI and Collaborators Use Stem Cells to Eliminate Immunosuppression
Scientists from the Diabetes Research Institute (DRI) University of Miami Miller School of Medicine and DRI Federation center at Xiamen University (China) show that the use of mesenchymal stem cells (MSC) in kidney transplant recipients may replace a powerful anti-rejection drug. The results are published in the Journal of the American Medical Association (JAMA) .

2010 Researchers Identify Master Regulatory Genes in Pancreatic Islets
The DRI’s molecular biology team is the first to identify a disproportionately higher number of genes, called miR-7, in pancreatic islets compared to the non-islet tissue of the organ. These master regulatory genes are also found in fetal endocrine cells during development and play a central role in islet cell development, as well as in maintaining this endocrine function (as opposed to developing into other tissue). The findings are published in BMC Genomics .

2010 Study Shows Recurrence of T1D, Need for Multiple Strategies
Scientists show recurrence of type 1 diabetes may occur in patients after kidney-pancreas transplantation. Despite use of immunosuppression and continuous function of the kidney and exocrine portion of the transplanted pancreas, researchers identified the presence of autoimmune cells known to target insulin-producing cells. This study demonstrates the need to block the immune response to foreign tissue and prevent autoimmunity. The findings are published in Diabetes .

2010 DRI Performs Auto-Islet Transplant after Pancreas Trauma
DRI researchers report on the success of an islet auto-transplant performed after severe trauma. Walter Reed Army Medical Center surgeons remove the damaged pancreas from a soldier wounded in the Middle East. The tissue is sent to DRI where islets are isolated and sent back for transplant into the same patient, preventing diabetes. The findings, published in the New England Journal of Medicine , indicate that islet isolation and auto-transplant in cases of severe abdominal trauma can be performed using a remote processing center.

2008 Researchers Pioneer “Living Window” to Observe Islets in Real Time
DRI researchers develop novel method to monitor healthy islets in a living experimental model. For the first time, researchers can obtain real-time imaging of functioning islets transplanted in the anterior chamber of the eye. The clear “window” allows researchers to witness the islets in the same model over time as nerves and blood vessels develop and immune reactions occur. The work is featured on the cover of Nature Medicine .

2007 Invention Supplies Critical Oxygen, Increases Beta Cell Growth
Scientists at the DRI design and test a new cell culture device that closely mimics the natural oxygen environment, demonstrating a dramatic increase in beta cell development from an embryonic mouse pancreas. The findings were published in the journal Stem Cells .

2006 Discovery Shows that Human and Animal Islets Differ Dramatically
Scientists from the DRI’s islet physiology team discover that the internal structure of a human islet cell is dramatically different from the more often studied rodent islet – a striking finding that argues for the importance of studying human islets if medical research is to benefit people living with diabetes. The findings were published Proceedings of the National Academy of Sciences (PNAS) .

2006 Islet Transplantation Improves Patients’ Quality of Life
A Diabetes Research Institute study shows type 1 diabetes patients’ quality of life and sense of well-being improve following islet transplantation despite having to take harsh anti-rejection drugs. The findings were published in the American Journal of Transplantation .

2005 DRI Develops Safer Method for Turning Stem Cells into Insulin-Producing Cells
Scientists report for the first time that protein technology can be used to promote pancreatic cell differentiation. DRI’s Stem Cell and Molecular Biology teams use this technology to show how stem cells can be progressively educated along the pathway leading to functional beta cells. The findings, published in Diabetes , open a promising new avenue of research that might enable the development of more insulin-producing cells for transplant in the future.

2003 DRI Performs First Successful Islet Transplant in Asia
Espousing its philosophy of global collaboration, the Diabetes Research Institute was asked to send members of its clinical cell transplant team to Shanghai’s First People’s Hospital to assist the Chinese team with both the islet isolation and transplantation efforts. Receiving telephone guidance from the DRI’s senior faculty, the DRI team in China was able to overcome every technical obstacle encountered, despite the formidable distance, technology, and language barriers.

2001 New Islet Transplant Protocol Leads to Insulin Independence
Using a new combination of anti-rejection drugs and improved culture media for islets, the DRI’s cell transplant team performs a series of “islets alone” transplants in study participants with long-standing type 1 diabetes. The recipients are able to discontinue insulin therapy for more than a year following islet transplantation.

1999 DRI Study Results Ignite Global Interest in Islet Transplantation
Using monthly injections of a monoclonal antibody, DRI is the first to show that transplanted islets reverse diabetes in pre-clinical models without the need for any other anti-rejection drug. The recipients remain insulin independent for over one year post-transplant and emerging rejection episodes can be reversed using this antibody. Even after discontinuation of the antibody, many subjects remain off insulin with glucose responsiveness for several months. The results are published in Proceedings of the National Academy of Sciences.


1997 Researchers Identify Cells that Regulate Autoimmunity
The DRI’s immunogenetics team publishes new findings related to insulin production in the thymus and its role in the development of type 1 diabetes. The Nature Genetics paper describes how this type of insulin might play a key role in the immune system’s ability to recognize insulin molecules as “self”. Scientists believe that the amount of thymic insulin might determine either susceptibility to or protection from diabetes.

1995 Islet Transplant Patient Insulin Independent for Five Years
Biopsies show intact, functioning human islet cells in the liver of a patient who was completely insulin independent for five years following an islet transplant.

1990 Organ Transplant Patients Become Insulin Independent After Receiving Islets
Nine patients receive islet cells in conjunction with their multi-organ transplants. This study, published in The Lancet , demonstrates that islets can produce insulin independence in patients who had previously been pancreatectomized.

1988 Invention Expands the Number of Clinical Islet Transplantation Trials
Camillo Ricordi, M.D., develops an automated method for isolating large numbers of islets from a single donor pancreas. This technology leads to expansion of clinical trials in cell therapy for the treatment and cure of type 1 diabetes. The findings were published in the journal Diabetes .

1987 Researchers Discover New Method for Visualizing Islets, Improving Outcomes
DRI’s Rodolfo Alejandro, M.D., identifies a chemical that differentiates islets from non-islet tissue of the pancreas during the isolation process. This zinc-binding substance (dithizone) is absorbed only by islets, giving them a distinctive red color. This discovery enables researchers to optimize the cell separation process for improved clinical outcomes. It was also shown that the use of this substance does not interfere with islet function in vitro or in vivo. The findings were published in Transplantation .

1985 DRI Performs First Clinical Islet Transplants
Based upon results obtained in preclinical transplant models, DRI researchers begin the first human pilot clinical trial in patients with type 1 diabetes. The results of the pioneering study appeared in Advanced Models for the Therapy of Insulin-Dependent Diabetes .

1984 Successful Islet Transplant in Dogs Restores Natural Insulin Production
DRI researchers conduct the first successful transplant of healthy islets into dogs with diabetes, restoring long-term natural insulin-production and normalizing blood sugar levels. Previously, these experiments were only successful in the rodent model. The findings were published in the journal Diabetes .

1978 DRI Develops Gold-Standard Treatment for Pregnant Women with Type 1 Diabetes
Using newly developed self-glucose monitoring and individualized algorithms for intensive insulin therapy, DRI researchers demonstrate that tight blood sugar control in women with type 1 diabetes during pregnancy can result in successful, full-term deliveries with normal birth weights. The study results were published in Diabetes Care .

1975 DRI Researchers Reverse Diabetes in Rodents
Islet cells are successfully transplanted into rats with diabetes, restoring natural insulin production and normalizing blood sugar levels in laboratory animals. The results are published in the journal Diabetes.
back to top
Keep Up With Our Progress Toward A Cure & More
6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024
Collaborators.
- American Diabetes Association Professional Practice Committee : Nuha A ElSayed , Grazia Aleppo , Raveendhara R Bannuru , Dennis Bruemmer , Billy S Collins , Laya Ekhlaspour , Marisa E Hilliard , Eric L Johnson , Kamlesh Khunti , Ildiko Lingvay , Glenn Matfin , Rozalina G McCoy , Mary Lou Perry , Scott J Pilla , Sarit Polsky , Priya Prahalad , Richard E Pratley , Alissa R Segal , Jane Jeffrie Seley , Elizabeth Selvin , Robert C Stanton , Robert A Gabbay
- PMID: 38078586
- PMCID: PMC10725808 (available on 2025-01-01 )
- DOI: 10.2337/dc24-S006
The American Diabetes Association (ADA) "Standards of Care in Diabetes" includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, an interprofessional expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
© 2024 by the American Diabetes Association.
Publication types
- Diabetes Mellitus* / therapy
- Endocrinology*
- Hypoglycemia* / diagnosis
- Hypoglycemia* / prevention & control
- Reference Standards
- Societies, Medical
- Standard of Care
Grants and funding
- K23 DK121942/DK/NIDDK NIH HHS/United States
Logo Left Content

Logo Right Content
Stanford University School of Medicine blog
These are the tools for providing top-notch diabetes care to everyone
For 30 years, doctors have known that maintaining near-normal blood sugar has huge benefits for people with Type 1 diabetes.
A 1993 clinical trial found that participants who were taught methods for tightly managing their disease -- checking their blood sugar many times each day, making adjustments to insulin doses and receiving frequent help from their medical caregivers -- reduced their risk for long-term complications, including blindness, kidney failure and peripheral nerve damage, by 50% to 70%.
Yet since that trial, physicians have struggled to roll out intensive diabetes management programs to all patients. On average, patients across the U.S. still don't achieve the level of diabetes control that would minimize their long-term risks.

"It's not because people haven't been trying," said David Maahs , MD, PhD, a pediatric endocrinologist at Stanford Medicine Children's Health. "It's a complicated condition to take care of, and for the individual with diabetes and their family, it's constant work."
Stanford Medicine experts are making headway on the problem. A new scientific study published recently in Nature Medicine describes how the research team, led by Maahs and Priya Prahalad , MD, PhD, have implemented major advances in intensive diabetes management.
First, they tackled equity issues to ensure the latest diabetes technology got into the hands of every patient as soon as they were diagnosed. They also built artificial intelligence tools that gave diabetes caregivers the ability to quickly identify which patients most needed their help. Ultimately these steps enabled adolescent Type 1 diabetes patients to maintain better control of their blood sugar levels.
It's not because people haven't been trying. It's a complicated condition to take care of, and for the individual with diabetes and their family, it's constant work. David Maahs
Maahs talked about the methods used and the long-term ramifications. This interview was edited for length and clarity.
Your study built on recent technological advances that automate many tasks involved in living with diabetes. What are the advantages of the newer devices?
Patients can now wear continuous glucose monitors, which have a sensor inserted under the skin that reads a glucose value every 5 to 15 minutes. This is really helpful because you don't have to poke your finger six to 10 times a day to measure glucose levels, and the monitor can warn you if you're going low or high. If you're the parent of a child with Type 1 diabetes, you can get their glucose data from the cloud and onto your phone.
Another recent improvement in diabetes technology is that continuous glucose monitors can now communicate with an insulin pump. An algorithm helps control dosing to reduce or stop insulin if it predicts your blood sugar is going to go low, and it adds a bit more insulin if you're going high. You still have to give an insulin dose before you eat, but it really takes a lot of the burden out of managing Type 1 diabetes.
It's been a big challenge to get these improved diabetes devices into the hands of every U.S. patient; your earlier work shows that disadvantaged groups tend to be left behind. How did your new study tackle equity concerns?
We were testing the benefits of starting pediatric Type 1 diabetes patients on continuous glucose monitors as soon as possible after they were diagnosed, which we were usually able to do in the first week after diagnosis. Although insulin pumps were not a focus of this study, about half of our patients began using an insulin pump within a year of their diagnosis.

We all agreed that we wanted every new patient we saw to be included. If you look at earlier studies of diabetes technology, it tended to be tested in college-educated, white, privately insured people and not in other populations. We had to figure out how to meet challenges faced by less-advantaged patients. We learned that this went beyond the most obvious barriers we addressed, such as providing care in multiple languages.
For instance, at first, it seemed like some groups of patients were wearing their continuous glucose monitors less than we asked them to. But in fact, the transmission of their data to our system was incomplete because they had poor Wi-Fi access at home. That's an equity issue. We've been giving out devices to those who need them as part of the research so that everyone has enough internet connectivity to upload their data.
Also, at diagnosis, we sometimes can't tell whether someone has Type 1 or Type 2 diabetes. This happens more in minoritized populations, in youth who have an elevated body mass index, and these children are more likely to be publicly insured or non-English speakers. We made a conscious decision to include all these patients while we waited to learn the details of their diagnosis, so as not to miss anyone who might be eligible for our study.
We made a conscious decision to include (a diverse mix of) patients while we waited to learn the details of their diagnosis, so as not to miss anyone who might be eligible for our study. David Maahs
Close to 90% of our new Type 1 diabetes patients participated in this study, and a lot of those who chose not to participate enrolled instead in a different study of artificial pancreas technology, so we did quite a good job of including everyone. It was a very diverse population: About 35% of patients were publicly insured, and only about 40% were non-Hispanic white.
Getting the new technology to every patient was important. What else was needed to make sure all patients could succeed in managing their blood sugar levels?
The 1993 trial showed that it was really useful for patients to have frequent communication with their diabetes team. That can be hard to do with the resources of a typical diabetes clinic, where each diabetes educator has many patients to track.
To address this problem, we built an AI-powered data dashboard, which filters our patients' continuous glucose monitor data and puts it into a format that helps our team identify who is struggling. Instead of spending a lot of time manually evaluating their data, we can automatically rank which patients most need our help.
We look at the percentage of time the continuous glucose monitor has been worn, and if it's below a certain threshold, that's our first starting point. Sometimes people have lost their prescription for their CGM, continuous glucose monitor, and need a new one. We're able to reach out and help them.
If a patient is having too many low blood sugar readings, which are dangerous, that's another reason for them to go to the top of the list. Our diabetes educators can contact them to help adjust their insulin dosing. Likewise, if their average glucose is out of the target range less than 70% of the time, we can flag that they need some extra attention between their visits to the clinic.
On the other hand, if someone is wearing their monitor, they're not having low blood sugar readings and they are in the right blood sugar range most of the time, they're doing well. We'll check in with them at their quarterly clinic visit, but they don't need outreach in between. That knowledge helps our team shift its attention to the patients who most need it.
How did you build the algorithm that powers this dashboard?
The platform was developed at Stanford with systems design expert David Scheinker , PhD, and his SURF team ; they use tools such as machine learning and statistics to improve how health care is delivered. He teaches a class in which engineering undergraduates and grad students solve technical problems in health care.
We presented our concept to his class. Our problem was that each brand of diabetes equipment has a different system to share data. A diabetes educator with 10 patients might have had five or six different places to go look at their data, log in and so on. This made it extremely time-consuming to figure out which patients needed help.
More on diabetes
- NIH grant establishes Stanford Medicine as center of national diabetes training program
- 'Smart speaker' shows potential for better self-management of Type 2 diabetes
- Improved blood sugar control helps normalize diabetic teens' brains, Stanford-led study finds
- Managing type 1 diabetes: Voices of the underserved
Instead, our system has all the data in one place. It was built through an iterative process between our diabetes educators and the engineering team, so that ultimately the data is presented in the way that is most useful to the diabetes educators.
We published a study showing that the dashboard has shifted the diabetes educators' workload in a helpful way. They spend less time sifting through troves of data - something an algorithm can do perfectly - and more time talking to patients who need extra help between clinic visits.
How do you know that your approach worked?
Compared with our past patients who were diagnosed with Type 1 diabetes before this research began, our newer patients were more likely to reach their glucose targets after a year of living with the disease.
One treatment target for our patients, after a year with diabetes, was a glycosylated hemoglobin A1c measurement below 7%. This laboratory test assesses patients' blood sugar control over the prior three months, and a reading below 7% is the target for optimum health.
In our earlier data, 28% of patients met this target 12 months after diagnosis; now we have 64% of our patients meeting this goal. We also looked at how many people had very high A1c measurements, with values above 9%, and that measure has reduced dramatically. Similarly, by one year after diagnosis, our patients' blood sugar was in the target range 68% of the time.
We also have data showing that compared with our historic cohort, everyone received a similar benefit from our intensive approach to treatment, meaning that if you looked at people who had public versus private insurance, or were or were not English speakers, every group had similar improvements when we implemented our study. There are still some gaps between more- and less-privileged patients, so we still have work to do, but everyone benefited a similar amount. Often, when new medical technology becomes available, more privileged people get more benefit; it is very encouraging that we could buck that trend.
Image: habrovich ( stock.adobe.com )
Related posts
Kids fare better with early use of diabetes technology
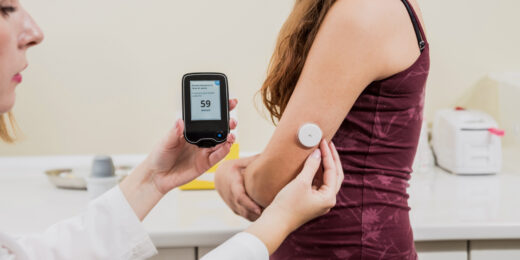
Why precision medicine leads to better diabetes care
Popular posts.
In the age of fentanyl, factual drug education can save teen lives
The efficiency of stem cell differentiation into functional beta cells for treating insulin-requiring diabetes: Recent advances and current challenges
- Published: 10 May 2024
Cite this article

- Yunfei Luo 1 ,
- Peng Yu 1 &
- Jianping Liu 1
Explore all metrics
In recent years, the potential of stem cells (SCs) to differentiate into various types of cells, including β-cells, has led to a significant boost in development. The efficiency of this differentiation process and the functionality of the cells post-transplantation are crucial factors for the success of stem cell therapy in diabetes. Herein, this article reviews the current advances and challenges faced by stem cell differentiation into functional β-cells for diabetes treatment. In vitro, researchers have sought to enhance the differentiation efficiency of functional β-cells by mimicking the normal pancreatic development process, using gene manipulation, pharmacological and culture conditions stimulation, three-dimensional (3D) and organoid culture, or sorting for functional β-cells based on mature islet cell markers. Furthermore, in vivo studies have also looked at suitable transplantation sites, the enhancement of the transplantation microenvironment, immune modulation, and vascular function reconstruction to improve the survival rate of functional β-cells, thereby enhancing the treatment of diabetes. Despite these advancements, developing stem cells to produce functional β-cells for efficacious diabetes treatment is a continuous research endeavor requiring significant multidisciplinary collaboration, for the stem-cell-derived beta cells to evolve into an effective cellular therapy.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
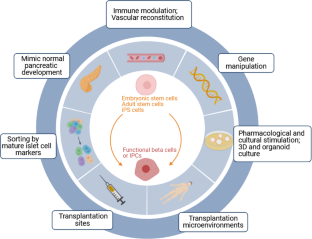
Abbreviations
insulin-producing cells
embryonic stem cells
nuclear transfer embryonic stem cells
cord blood stem cells
Umbilical cord blood mesenchymal stem cells
human amniotic fluid stem cells
adipose tissue-derived stem cells
islet-like cell aggregates
Muscle-derived stem/progenitor cells
induced pluripotent stem cells
human amniotic epithelial cells
human embryonic stem cell
human marrow stromal cells
epidermal growth factor
L. Wang, W. Peng, Z. Zhao, M. Zhang, Z. Shi, Z. Song, X. Zhang, C. Li, Z. Huang, X. Sun, L. Wang, M. Zhou, J. Wu, Y. Wang, Prevalence and treatment of diabetes in China, 2013–2018. JAMA 326 , 2498–2506 (2021). https://doi.org/10.1001/jama.2021.22208
Article PubMed PubMed Central Google Scholar
W. Bielka, A. Przezak, P. Molęda, E. Pius-Sadowska, B. Machaliński, Double diabetes-when type 1 diabetes meets type 2 diabetes: definition, pathogenesis and recognition. Cardiovasc. Diabetol. 23 , 62 (2024). https://doi.org/10.1186/s12933-024-02145-x
M. Lipsett, R. Aikin, M. Castellarin, S. Hanley, A.M. Jamal, S. Laganiere, L. Rosenberg, Islet neogenesis: a potential therapeutic tool in type 1 diabetes. Int. J. Biochem. Cell Biol. 38 , 498–503 (2006). https://doi.org/10.1016/j.biocel.2005.08.022
Article CAS PubMed Google Scholar
A. Cañibano-Hernández, L. Sáenz Del Burgo, A. Espona-Noguera, J. Ciriza, J.L. Pedraz, Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int. J. Pharm. 543 , 107–120 (2018). https://doi.org/10.1016/j.ijpharm.2018.03.041
J.B. Sneddon, Q. Tang, P. Stock, J.A. Bluestone, S. Roy, T. Desai, M. Hebrok, Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 22 , 810–823 (2018). https://doi.org/10.1016/j.stem.2018.05.016
Article CAS PubMed PubMed Central Google Scholar
G. Pan, Y. Mu, L. Hou, J. Liu, Examining the therapeutic potential of various stem cell sources for differentiation into insulin-producing cells to treat diabetes. Annales d’endocrinologie 80 , 47–53 (2019). https://doi.org/10.1016/j.ando.2018.06.1084
Article PubMed Google Scholar
J. Shen, Y. Cheng, Q. Han, Y. Mu, W. Han, Generating insulin-producing cells for diabetic therapy: existing strategies and new development. Ageing Res. Rev. 12 , 469–478 (2013). https://doi.org/10.1016/j.arr.2013.01.001
T. Farooq, K. Rehman, A. Hameed, M.S.H. Akash, Stem cell therapy and Type 1 Diabetes Mellitus: Treatment strategies and future perspectives. Adv. Exp. Med. Biol. 1084 , 95–107 (2019). https://doi.org/10.1007/5584_2018_195
H.S. Jun. Cell replacement and regeneration therapy for diabetes. Korean Diabetes J. 34 , 77–83 (2010). https://doi.org/10.4093/kdj.2010.34.2.77
S. Chen, K. Du, C. Zou, Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell. Res. Ther. 11 , 275 (2020). https://doi.org/10.1186/s13287-020-01793-6
A. Citro, A. Neroni, C. Pignatelli, F. Campo, M. Policardi, M. Monieri, S. Pellegrini, E. Dugnani, F. Manenti, M.C. Maffia, L. Valla, E. Kemter, I. Marzinotto, C. Olgasi, A. Cucci, A. Follenzi, V. Lampasona, E. Wolf, L. Piemonti, Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes. Nat. Commun. 14 , 878 (2023). https://doi.org/10.1038/s41467-023-36582-1
D. Kim, Y. Gu, M. Ishii, M. Fujimiya, M. Qi, N. Nakamura, T. Yoshikawa, S. Sumi, K. Inoue, In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cell. Pancreas 27 , e34–e41 (2003). https://doi.org/10.1097/00006676-200308000-00021
A. Rezania, J.E. Bruin, M.J. Riedel, M. Mojibian, A. Asadi, J. Xu, R. Gauvin, K. Narayan, F. Karanu, J.J. O’Neil, Z. Ao, G.L. Warnock, T.J. Kieffer, Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 61 , 2016–2029 (2012). https://doi.org/10.2337/db11-1711
J.J. Heit, S.K. Kim, Embryonic stem cells and islet replacement in diabetes mellitus. Pediatr. Diabetes 5 , 5–15 (2004). https://doi.org/10.1111/j.1399-543X.2004.00074.x
L. Sui, N. Danzl, S.R. Campbell, R. Viola, D. Williams, Y. Xing, Y. Wang, N. Phillips, G. Poffenberger, B. Johannesson, J. Oberholzer, A.C. Powers, R.L. Leibel, X. Chen, M. Sykes, D. Egli, Beta-cell replacement in mice using human Type 1 Diabetes nuclear transfer embryonic stem cells. Diabetes 67 , 26–35 (2018). https://doi.org/10.2337/db17-0120
P. Torre P., A.I. Flores, Current status and future prospects of perinatal stem cells. Genes. 12 (2020). https://doi.org/10.3390/genes12010006
K.C. Chao, K.F. Chao, Y.S. Fu, S.H. Liu, Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One 3 , e1451 (2008). https://doi.org/10.1371/journal.pone.0001451
S. Sarang, C. Viswanathan, Umbilical cord derived mesenchymal stem cells useful in insulin production - another opportunity in cell therapy. Int. J. Stem Cells 9 , 60–69 (2016). https://doi.org/10.15283/ijsc.2016.9.1.60
S.S. Kadam, M. Sudhakar, P.D. Nair, R.R. Bhonde, Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy 12 , 982–991 (2010). https://doi.org/10.3109/14653249.2010.509546
Y. Zhao, B. Lin, M. Dingeldein, C. Guo, D. Hwang, M.J. Holterman, New type of human blood stem cell: a double-edged sword for the treatment of type 1 diabetes. Transl. Res. 155 , 211–216 (2010). https://doi.org/10.1016/j.trsl.2010.01.003
M. El-Sherbiny, M.A. Eladl, A.V. Ranade, M. Guimei, H. Gabr, Functional beta-cells derived from umbilical cord blood mesenchymal stem cells for curing rats with streptozotocin-induced diabetes mellitus. Singapore Med. J. 61 , 39–45 (2020). https://doi.org/10.11622/smedj.2019120
X.P. Mu, L.Q. Ren, H.W. Yan, X.M. Zhang, T.M. Xu, A.H. Wei, J.L. Jiang, Enhanced differentiation of human amniotic fluid-derived stem cells into insulin-producing cells in vitro. J. Diabetes Investig. 8 , 34–43 (2017). https://doi.org/10.1111/jdi.12544
C.N. Street, S. Sipione, L. Helms, T. Binette, R.V. Rajotte, R.C. Bleackley, G.S. Korbutt, Stem cell-based approaches to solving the problem of tissue supply for islet transplantation in type 1 diabetes. Int. J. Biochem. Cell Biol. 36 , 667–683 (2004). https://doi.org/10.1016/j.biocel.2003.09.005
M. Li, S. Ikehara, Bone marrow stem cell as a potential treatment for diabetes. J. Diabetes Res. 2013 , 329596 (2013). https://doi.org/10.1155/2013/329596
E.M. Kang, P.P. Zickler, S. Burns, S.M. Langemeijer, S. Brenner, O.A. Phang, N. Patterson, D. Harlan, J.F. Tisdale, Hematopoietic stem cell transplantation prevents diabetes in NOD mice but does not contribute to significant islet cell regeneration once disease is established. Exp. Hematol. 33 , 699–705 (2005). https://doi.org/10.1016/j.exphem.2005.03.008
M.M. Gabr, M.M. Zakaria, A.F. Refaie, E.A. Abdel-Rahman, A.M. Reda, S.S. Ali, S.M. Khater, S.A. Ashamallah, A.M. Ismail, H.E.A. Ismail, N. El-Badri, M.A. Ghoneim, From human mesenchymal stem cells to insulin-producing cells: comparison between bone marrow- and adipose tissue-derived cells. Biomed Res Int 2017 , 3854232 (2017). https://doi.org/10.1155/2017/3854232
L.J. Yang, Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes. Autoimmun. Rev. 5 , 409–413 (2006). https://doi.org/10.1016/j.autrev.2005.10.009
A.B. Peck, J.G. Cornelius, M. Chaudhari, D. Shatz, V.K. Ramiya, Use of in vitro-generated, stem cell-derived islets to cure type 1 diabetes: how close are we? Ann. N. Y. Acad. Sci. 958 , 59–68 (2002). https://doi.org/10.1111/j.1749-6632.2002.tb02947.x
S. Kodama, D.L. Faustman, Routes to regenerating islet cells: stem cells and other biological therapies for type 1 diabetes. Pediatr. Diabetes 5 , 38–44 (2004). https://doi.org/10.1111/j.1399-543X.2004.00078.x
V. Chandra, G.S. Phadnis, P.D. Nair, R.R. Bhonde, Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells 27 , 1941–1953 (2009). https://doi.org/10.1002/stem.117
V. Chandra, G. Swetha, S. Muthyala, A.K. Jaiswal, J.R. Bellare, P.D. Nair, R.R. Bhonde, Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One 6 , e20615 (2011). https://doi.org/10.1371/journal.pone.0020615
H.P. Lin, T.M. Chan, R.H. Fu, C.P. Chuu, S.C. Chiu, Y.H. Tseng, S.P. Liu, K.C. Lai, M.C. Shih, Z.S. Lin, H.S. Chen, D.C. Yeh, S.Z. Lin, Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell Transplant. 24 , 521–532 (2015). https://doi.org/10.3727/096368915X686977
Z. Eydian, A. Mohammad Ghasemi, S. Ansari, A.N. Kamali, M. Khosravi, S. Momtaz, S. Riki, L. Rafighdoost, R. Entezari Heravi, Differentiation of multipotent stem cells to insulin-producing cells for treatment of diabetes mellitus: bone marrow- and adipose tissue-derived cells comparison. Mol. Biol. Rep. 49 , 3539–3548 (2022). https://doi.org/10.1007/s11033-022-07194-7
K.C. Lan, C.C. Wang, Y.P. Yen, R.S. Yang, S.H. Liu, D.C. Chan, Islet-like clusters derived from skeletal muscle-derived stem/progenitor cells for autologous transplantation to control type 1 diabetes in mice. Artif. Cells Nanomed. Biotechnol. 46 , S328–S335 (2018). https://doi.org/10.1080/21691401.2018.1492421
A.N. Balamurugan, B. Naziruddin, A. Lockridge, M. Tiwari, G. Loganathan, M. Takita, S. Matsumoto, K. Papas, M. Trieger, H. Rainis, T. Kin, T.W. Kay, S. Wease, S. Messinger, C. Ricordi, R. Alejandro, J. Markmann, J. Kerr-Conti, M.R. Rickels, C. Liu, X. Zhang, P. Witkowski, A. Posselt, P. Maffi, A. Secchi, T. Berney, P.J. O’Connell, B.J. Hering, F.B. Barton, Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999-2010. Am. J. Transplant. 14 , 2595–2606 (2014). https://doi.org/10.1111/ajt.12872
J. Fujikura, K. Hosoda, K. Nakao, Cell transplantation therapy for diabetes mellitus: endocrine pancreas and adipocyte. Endocr. J. 60 , 697–708 (2013). https://doi.org/10.1507/endocrj.ej13-0162
A. Soejitno, P.K. Prayudi, The prospect of induced pluripotent stem cells for diabetes mellitus treatment. Ther. Adv. Endocrinol. Metab. 2 , 197–210 (2011). https://doi.org/10.1177/2042018811420198
B. Soria, F.J. Bedoya, J.R. Tejedo, A. Hmadcha, R. Ruiz-Salmeron, S. Lim, F. Martin, Cell therapy for diabetes mellitus: an opportunity for stem cells? Cells Tissues Organs 188 , 70–77 (2008). https://doi.org/10.1159/000119407
L.N. Randolph, A. Bhattacharyya, X.L. Lian, Human beta cells generated from pluripotent stem cells or cellular reprogramming for curing diabetes. Regen. Eng. Transl. Med. 5 , 42–52 (2019). https://doi.org/10.1007/s40883-018-0082-y
X. Liu, Y. Wang, Y. Li, X. Pei, Research status and prospect of stem cells in the treatment of diabetes mellitus. Sci. China Life Sci. 56 , 306–312 (2013). https://doi.org/10.1007/s11427-013-4469-1
H.A. Russ, S. Efrat, Development of human insulin-producing cells for cell therapy of diabetes. Pediatr. Endocrinol. Rev. 9 , 590–597 (2011)
PubMed Google Scholar
M.M. Santosa, B.S. Low, N.M. Pek, A.K. Teo, Knowledge gaps in rodent pancreas biology: taking human pluripotent stem cell-derived pancreatic beta cells into our own hands. Front. Endocrinol. 6 , 194 (2015). https://doi.org/10.3389/fendo.2015.00194
Article Google Scholar
P. Wang, N.M. Fiaschi-Taesch, R.C. Vasavada, D.K. Scott, A. Garcia-Ocana, A.F. Stewart, Diabetes mellitus–advances and challenges in human beta-cell proliferation. Nat. Rev. Endocrinol. 11 , 201–212 (2015). https://doi.org/10.1038/nrendo.2015.9
S.G. Rane, J.H. Lee, H.M. Lin, Transforming growth factor-beta pathway: role in pancreas development and pancreatic disease. Cytokine Growth Factor Rev. 17 , 107–119 (2006). https://doi.org/10.1016/j.cytogfr.2005.09.003
A. Rezania, J.E. Bruin, P. Arora, A. Rubin, I. Batushansky, A. Asadi, S. O’Dwyer, N. Quiskamp, M. Mojibian, T. Albrecht, Y.H. Yang, J.D. Johnson, T.J. Kieffer, Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32 , 1121–1133 (2014). https://doi.org/10.1038/nbt.3033
M. Rhinn, P. Dollé, Retinoic acid signalling during development. Development 139 , 843–858 (2012). https://doi.org/10.1242/dev.065938
T.C. Schulz, H.Y. Young, A.D. Agulnick, M.J. Babin, E.E. Baetge, A.G. Bang, A. Bhoumik, I. Cepa, R.M. Cesario, C. Haakmeester, K. Kadoya, J.R. Kelly, J. Kerr, L.A. Martinson, A.B. McLean, M.A. Moorman, J.K. Payne, M. Richardson, K.G. Ross, E.S. Sherrer, X. Song, A.Z. Wilson, E.P. Brandon, C.E. Green, E.J. Kroon, O.G. Kelly, K.A. D’Amour, A.J. Robins, A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One 7 , e37004 (2012). https://doi.org/10.1371/journal.pone.0037004
J. Jensen, E.E. Pedersen, P. Galante, J. Hald, R.S. Heller, M. Ishibashi, R. Kageyama, F. Guillemot, P. Serup, O.D. Madsen, Control of endodermal endocrine development by Hes-1. Nat. Genet. 24 , 36–44 (2000). https://doi.org/10.1038/71657
A. Apelqvist, H. Li, L. Sommer, P. Beatus, D.J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, H. Edlund, Notch signalling controls pancreatic cell differentiation. Nature. 400 , 877–881 (1999). https://doi.org/10.1038/23716
L.C. Murtaugh, B.Z. Stanger, K.M. Kwan, D.A. Melton, Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl Acad. Sci. USA 100 , 14920–14925 (2003). https://doi.org/10.1073/pnas.2436557100
S.Y. Chun, D.L. Mack, E. Moorefield, S.H. Oh, T.G. Kwon, M.J. Pettenati, J.J. Yoo, P.D. Coppi, A. Atala, S. Soker, Pdx1 and controlled culture conditions induced differentiation of human amniotic fluid-derived stem cells to insulin-producing clusters. J. Tissue Eng. Regener. Med. 9 , 540–549 (2015). https://doi.org/10.1002/term.1631
Article CAS Google Scholar
W. Nishimura, H. Iwasa, M. Tumurkhuu, Role of the transintenance of pancreatic β-cells. Int. J. Mol. Sci. 23 (2022). https://doi.org/10.3390/ijms23094478
M.A. Huotari, P.J. Miettinen, J. Palgi, T. Koivisto, J. Ustinov, D. Harari, Y. Yarden, T. Otonkoski, ErbB signaling regulates lineage determination of developing pancreatic islet cells in embryonic organ culture. Endocrinology. 143 , 4437–4446 (2002). https://doi.org/10.1210/en.2002-220382
Y.M. Cho, J.M. Lim, D.H. Yoo, J.H. Kim, S.S. Chung, S.G. Park, T.H. Kim, S.K. Oh, Y.M. Choi, S.Y. Moon, K.S. Park, H.K. Lee, Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic beta-cell differentiation in human embryonic stem cells. Biochem. Biophys. Res. Commun. 366 , 129–134 (2008). https://doi.org/10.1016/j.bbrc.2007.11.112
B. Rajaei, M. Shamsara, L.M. Amirabad, M. Massumi, M.H. Sanati, Pancreatic Endoderm-derived from diabetic patient-specific induced pluripotent stem cell generates glucose-responsive insulin-secreting cells. J. Cell Physiol. 232 , 2616–2625 (2017). https://doi.org/10.1002/jcp.25459
M.F. Abazari, N. Nasiri, F. Nejati, S. Zare Karizi, M. Amini Faskhodi, E. Saburi, N. Aghapur, M.R. Mahdavi, A. Ardeshirylajimi, S.E. Enderami, F. Soleimanifar, Comparison of human-induced pluripotent stem cells and mesenchymal stem cell differentiation potential to insulin producing cells in 2D and 3D culture systems in vitro. J. Cell Physiol. 235 , 4239–4246 (2020). https://doi.org/10.1002/jcp.29298
B. Bose, S.P. Shenoy, S. Konda, P. Wangikar, Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes. Cell Biol. Int. 36 , 1013–1020 (2012). https://doi.org/10.1042/cbi20120210
J.H. Shim, S.E. Kim, D.H. Woo, S.K. Kim, C.H. Oh, R. McKay, J.H. Kim, Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 50 , 1228–1238 (2007). https://doi.org/10.1007/s00125-007-0634-z
A. El-Badawy, N. El-Badri, The cell cycle as a brake for beta-cell regeneration from embryonic stem cells. Stem Cell. Res. Ther. 7 , 9 (2016). https://doi.org/10.1186/s13287-015-0274-z
R. Faast, J. White, P. Cartwright, L. Crocker, B. Sarcevic, S. Dalton, Cdk6-cyclin D3 activity in murine ES cells is resistant to inhibition by p16(INK4a). Oncogene. 23 , 491–502 (2004). https://doi.org/10.1038/sj.onc.1207133
A. Calder, I. Roth-Albin, S. Bhatia, C. Pilquil, J.H. Lee, M. Bhatia, M. Levadoux-Martin, J. McNicol, J. Russell, T. Collins, J.S. Draper, Lengthened G1 phase indicates differentiation status in human embryonic stem cells. Stem Cells Dev. 22 , 279–295 (2013). https://doi.org/10.1089/scd.2012.0168
M.E. Bechard, C.V. Wright, New ideas connecting the cell cycle and pancreatic endocrine-lineage specification. Cell Cycle 16 , 301–303 (2017). https://doi.org/10.1080/15384101.2016.1256149
E. Stead, J. White, R. Faast, S. Conn, S. Goldstone, J. Rathjen, U. Dhingra, P. Rathjen, D. Walker, S. Dalton, Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 21 , 8320–8333 (2002). https://doi.org/10.1038/sj.onc.1206015
R.V. Mettus, S.G. Rane, Characterization of the abnormal pancreatic development, reduced growth and infertility in Cdk4 mutant mice. Oncogene. 22 , 8413–8421 (2003). https://doi.org/10.1038/sj.onc.1206888
T. Otonkoski, New tools for experimental diabetes research: Cellular reprogramming and genome editing. Ups J. Med. Sci. 121 , 146–150 (2016). https://doi.org/10.3109/03009734.2016.1149529
K.F. Leavens, J.R. Alvarez-Dominguez, L.T. Vo, H.A. Russ, A.V. Parent, Stem cell-based multi-tissue platforms to model human autoimmune diabetes. Mol. Metab. 66 , 101610 (2022). https://doi.org/10.1016/j.molmet.2022.101610
S. Balakrishnan, S. Dhavamani, C. Prahalathan, beta-Cell specific transcription factors in the context of diabetes mellitus and beta-cell regeneration. Mech. Dev. 163 , 103634 (2020). https://doi.org/10.1016/j.mod.2020.103634
Z.Y. Sun, T.Y. Yu, F.X. Jiang, W. Wang. Functional maturation of immature beta cells: A roadblock for stem cell therapy for type 1 diabetes. World J. Stem Cells. 26 (3), 193–207 (2021). https://doi.org/10.4252/wjsc.v13.i3.193
B. Rajaei, M. Shamsara, M.H. Sanati, In vitro generation of glucose-responsive insulin-secreting cells from PDX1-overexpressing human-induced Pluripotent stem cell derived from diabetic patient. ASAIO J. 64 , 819–826 (2018). https://doi.org/10.1097/MAT.0000000000000728
D. Dayer, M.R. Tabandeh, E. Moghimipour, M. Hashemi Tabar, A. Ghadiri, E. Allah Bakhshi, M. Orazizadeh, M.A. Ghafari, MafA overexpression: a new efficient protocol for in vitro differentiation of adipose-derived mesenchymal stem cells into functional insulin-producing cells. Cell J. 21 , 169–178 (2019). https://doi.org/10.22074/cellj.2019.5669
D. Gao, P. Dai, Z. Fan, J. Wang, Y. Zhang, The roles of different multigene combinations of Pdx1, Ngn3, Sox9, Pax4, and Nkx2.2 in the reprogramming of canine ADSCs into IPCs. Cell Transpl. 31 , 9636897221081483 (2022). https://doi.org/10.1177/09636897221081483
L. Velazco-Cruz, M.M. Goedegebuure, K.G. Maxwell, P. Augsornworawat, N.J. Hogrebe, J.R. Millman, SIX2 regulates human beta cell differentiation from stem cells and functional maturation in vitro. Cell Rep. 31 , 107687 (2020). https://doi.org/10.1016/j.celrep.2020.107687
A. Diane, N.A. Al-Shukri, U.M.R. Bin Abdul Mu, H.H. Al-Siddiqi, Beta-cell mitochondria in diabetes mellitus: a missing puzzle piece in the generation of hPSC-derived pancreatic beta-cells? J. Transl. Med. 20 , 163 (2022). https://doi.org/10.1186/s12967-022-03327-5
J.C. Davis, T.C. Alves, A. Helman, J.C. Chen, J.H. Kenty, R.L. Cardone, D.R. Liu, R.G. Kibbey, D.A. Melton, Glucose response by stem cell-derived beta cells in vitro is inhibited by a bottleneck in glycolysis. Cell Rep. 31 , 107623 (2020). https://doi.org/10.1016/j.celrep.2020.107623
L. Lu, L. Zhitao, C. Nannan, H. Mingzhu, H. Xiaoping, H. Dongsheng, P. Zongfu, L. Xiaoyang, Mitofusin2 promotes beta cell maturation from mouse embryonic stem cells via Sirt3/Idh2 activation. Stem Cells Int. 2022 , 1172795 (2022). https://doi.org/10.1155/2022/1172795
Q. Ma, Y. Xiao, W. Xu, M. Wang, S. Li, Z. Yang, M. Xu, T. Zhang, Z.N. Zhang, R. Hu, Q. Su, F. Yuan, T. Xiao, X. Wang, Q. He, J. Zhao, Z.J. Chen, Z. Sheng, M. Chai, H. Wang, W. Shi, Q. Deng, X. Cheng, W. Li, ZnT8 loss-of-function accelerates functional maturation of hESC-derived beta cells and resists metabolic stress in diabetes. Nat. Commun. 13 , 4142 (2022). https://doi.org/10.1038/s41467-022-31829-9
E. Yoshihara, C. O’Connor, E. Gasser, Z. Wei, T.G. Oh, T.W. Tseng, D. Wang, F. Cayabyab, Y. Dai, R.T. Yu, C. Liddle, A.R. Atkins, M. Downes, R.M. Evans, Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 586 , 606–611 (2020). https://doi.org/10.1038/s41586-020-2631-z
L.T. Lock, E.S. Tzanakakis, Stem/Progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng. 13 , 1399–1412 (2007). https://doi.org/10.1089/ten.2007.0047
J.R. Benthuysen, A.C. Carrano, M. Sander, Advances in beta cell replacement and regeneration strategies for treating diabetes. J Clin Invest. 126 , 3651–3660 (2016). https://doi.org/10.1172/JCI87439
S. Chen, M. Borowiak, J.L. Fox, R. Maehr, K. Osafune, L. Davidow, K. Lam, L.F. Peng, S.L. Schreiber, L.L. Rubin, D. Melton, A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 5 , 258–265 (2009). https://doi.org/10.1038/nchembio.154
T.K. Kim, J.S. Lee, H.S. Jung, T.K. Ha, S.M. Kim, N. Han, E.J. Lee, T.N. Kim, M.J. Kwon, S.H. Lee, M.K. Kim, B.D. Rhee, J.H. Park, Triiodothyronine induces proliferation of pancreatic β-cells through the MAPK/ERK pathway. Exp. Clin. Endocrinol. Diabetes 122 , 240–245 (2014). https://doi.org/10.1055/s-0034-1367060
S. Misiti, E. Anastasi, S. Sciacchitano, C. Verga Falzacappa, L. Panacchia, B. Bucci, D. Khouri, I. D’Acquarica, E. Brunetti, U. Di Mario, V. Toscano, R. Perfetti, 3,5,3’-Triiodo-L-thyronine enhances the differentiation of a human pancreatic duct cell line (hPANC-1) towards a beta-cell-Like phenotype. J. Cell Physiol. 204 , 286–296 (2005). https://doi.org/10.1002/jcp.20293
F.X. Jiang, K. Li, M. Archer, M. Mehta, E. Jamieson, A. Charles, J.E. Dickinson, M. Matsumoto, G. Morahan, Differentiation of IsleT progenitors regulated by Nicotinamide into Transcriptome-verified beta cells that ameliorate diabetes. Stem Cells 35 , 1341–1354 (2017). https://doi.org/10.1002/stem.2567
M.C. Gaudreau, R.R. Gudi, G. Li, B.M. Johnson, C. Vasu, Gastrin producing syngeneic mesenchymal stem cells protect non-obese diabetic mice from type 1 diabetes. Autoimmunity 55 , 95–108 (2022). https://doi.org/10.1080/08916934.2021.2012165
T. Dahan, O. Ziv, E. Horwitz, H. Zemmour, J. Lavi, A. Swisa, G. Leibowitz, F.M. Ashcroft, P. In’t Veld, B. Glaser, Y. Dor, Pancreatic β-cells express the fetal islet hormone gastrin in rodent and human diabetes. Diabetes. 66 , 426–436 (2017). https://doi.org/10.2337/db16-0641
X. Ma, Y. Lu, Z. Zhou, Q. Li, X. Chen, W. Wang, Y. Jin, Z. Hu, G. Chen, Q. Deng, W. Shang, H. Wang, H. Fu, X. He, X.H. Feng, S. Zhu, Human expandable pancreatic progenitor-derived beta cells ameliorate diabetes. Sci. Adv. 8 , eabk1826 (2022). https://doi.org/10.1126/sciadv.abk1826
E. Shin, T.Y. Kwon, Y. Cho, Y. Kim, J.H. Shin, Y.M. Han, ECM architecture-mediated regulation of β-cell differentiation from hESCs via Hippo-independent YAP activation. ACS Biomater. Sci. Eng. 9 , 680–692 (2023). https://doi.org/10.1021/acsbiomaterials.2c01054
S. Cechin, S. Alvarez-Cubela, J.A. Giraldo, R.D. Molano, S. Villate, C. Ricordi, A. Pileggi, L. Inverardi, C.A. Fraker, J. Dominguez-Bendala, Influence of in vitro and in vivo oxygen modulation on beta cell differentiation from human embryonic stem cells. Stem Cells Transl. Med. 3 , 277–289 (2014). https://doi.org/10.5966/sctm.2013-0160
B. Xu, D. Fan, Y. Zhao, J. Li, Z. Wang, J. Wang, X. Wang, Z. Guan, B. Niu, Three-dimensional culture promotes the differentiation of human dental pulp mesenchymal stem cells into insulin-producing cells for improving the diabetes therapy. Front. Pharmacol. 10 , 1576 (2019). https://doi.org/10.3389/fphar.2019.01576
J. Chmielowiec, M. Borowiak. In vitro differentiation and expansion of human pluripotent stem cell-derived pancreatic progenitors. Rev. Diabet. Stud. Spring 11 , 19–34 (2014). https://doi.org/10.1900/RDS.2014.11.19
K. Kalra, S.T. Chandrabose, T.S. Ramasamy, N. Kasim, Advances in the generation of functional beta-cells from induced pluripotent stem cells as a cure for diabetes mellitus. Curr. Drug Targets 19 , 1463–1477 (2018). https://doi.org/10.2174/1389450119666180605112917
Q. Wang, W. Donelan, H. Ye, Y. Jin, Y. Lin, X. Wu, Y. Wang, Y. Xi, Real-time observation of pancreatic beta cell differentiation from human induced pluripotent stem cells. Am. J. Transl. Res. 11 , 3490–3504 (2019)
CAS PubMed PubMed Central Google Scholar
F. Lebreton, V. Lavallard, K. Bellofatto, R. Bonnet, C.H. Wassmer, L. Perez, V. Kalandadze, A. Follenzi, M. Boulvain, J. Kerr-Conte, D.J. Goodman, D. Bosco, T. Berney, E. Berishvili, Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat. Commun. 10 , 4491 (2019). https://doi.org/10.1038/s41467-019-12472-3
X. Zhang, Z. Ma, E. Song, T. Xu, Islet organoid as a promising model for diabetes. Protein Cell 13 , 239–257 (2022). https://doi.org/10.1007/s13238-021-00831-0
C.H. Wassmer, F. Lebreton, K. Bellofatto, L. Perez, D. Cottet-Dumoulin, A. Andres, D. Bosco, T. Berney, V. Othenin-Girard, B. Martinez De Tejada, M. Cohen, C. Olgasi, A. Follenzi, E. Berishvili, Bio-engineering of pre-vascularized islet organoids for the treatment of Type 1 Diabetes. Transpl. Int. 35 , 10214 (2021). https://doi.org/10.3389/ti.2021.10214
F. Lebreton, K. Bellofatto, C.H. Wassmer, L. Perez, V. Lavallard, G. Parnaud, D. Cottet-Dumoulin, J. Kerr-Conte, F. Pattou, D. Bosco, V. Othenin-Girard, B. Martinez de Tejada, E. Berishvili, Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am. J. Transpl. 20 , 1600–6143 (Electronic), 1551–1561 (2020).
S. Pellegrini, V. Zamarian, V. Sordi, Strategies to improve the safety of iPSC-derived beta cells for beta cell replacement in diabetes. Transpl. Int. 35 , 10575 (2022). https://doi.org/10.3389/ti.2022.10575
A. Rezania, J.E. Bruin, J. Xu, K. Narayan, J.K. Fox, J.J. O’Neil, T.J. Kieffer, Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31 , 2432–2442 (2013). https://doi.org/10.1002/stem.1489
F.M. Docherty, K.A. Riemondy, R. Castro-Gutierrez, J.M. Dwulet, A.H. Shilleh, M.S. Hansen, S.P.M. Williams, L.H. Armitage, K.E. Santostefano, M.A. Wallet, C.E. Mathews, T.M. Triolo, R.K.P. Benninger, H.A. Russ, ENTPD3 marks mature stem cell-derived beta-cells formed by self-aggregation in vitro. Diabetes 70 , 2554–2567 (2021). https://doi.org/10.2337/db20-0873
T. van der Meulen, M.O. Huising, Maturation of stem cell-derived beta-cells guided by the expression of urocortin 3. Rev. Diabet. Stud. Spring 11 , 115–132 (2014). https://doi.org/10.1900/RDS.2014.11.115
D.H. Kassem, M.M. Kamal, L. El-Kholy Ael, H.O. El-Mesallamy, Association of expression levels of pluripotency/stem cell markers with the differentiation outcome of Wharton’s jelly mesenchymal stem cells into insulin producing cells. Biochimie 127 , 187–195 (2016). https://doi.org/10.1016/j.biochi.2016.05.019
H.E. Arda, L. Li, J. Tsai, E.A. Torre, Y. Rosli, H. Peiris, R.C. Spitale, C. Dai, X. Gu, K. Qu, P. Wang, J. Wang, M. Grompe, R. Scharfmann, M.S. Snyder, R. Bottino, A.C. Powers, H.Y. Chang, S.K. Kim, Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 23 , 909–920 (2016). https://doi.org/10.1016/j.cmet.2016.04.002
D. Balboa, D.G. Iworima, T.J. Kieffer, Human Pluripotent stem cells to model islet defects in diabetes. Front. Endocrinol. 12 , 642152 (2021). https://doi.org/10.3389/fendo.2021.642152
P. Augsornworawat, K.G. Maxwell, L. Velazco-Cruz, J.R. Millman, Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep. 32 , 108067 (2020). https://doi.org/10.1016/j.celrep.2020.108067
A. Veres, A.L. Faust, H.L. Bushnell, E.N. Engquist, J.H. Kenty, G. Harb, Y.C. Poh, E. Sintov, M. Gürtler, F.W. Pagliuca, Q.P. Peterson, D.A. Melton, Charting cellular identity during human in vitro β-cell differentiation. Nature 569 , 368–373 (2019). https://doi.org/10.1038/s41586-019-1168-5
F. Cayabyab, L.R. Nih, E. Yoshihara, Advances in pancreatic islet transplantation sites for the treatment of diabetes. Front. Endocrinol. 12 , 732431 (2021). https://doi.org/10.3389/fendo.2021.732431
T. Kasputis, D. Clough, F. Noto, K. Rychel, B. Dye, L.D. Shea, Microporous polymer scaffolds for the transplantation of embryonic stem cell derived pancreatic progenitors to a clinically translatable site for the treatment of Type I diabetes. ACS Biomater. Sci. Eng. 4 , 1770–1778 (2018). https://doi.org/10.1021/acsbiomaterials.7b00912
X. Li, Z. Wei, L. Wu, H. Lv, Y. Zhang, J. Li, H. Yao, H. Zhang, B. Yang, X. Xu, J. Jiang, Efficacy of Fe( 3 )O( 4 )@polydopamine nanoparticle-labeled human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in the treatment of streptozotocin-induced diabetes in rats. Biomater. Sci. 8 , 5362–5375 (2020). https://doi.org/10.1039/d0bm01076f
A. Ramzy, P.J. Belmonte, M.J.S. Braam, S. Ida, E.M. Wilts, M.K. Levings, A. Rezania, T.J. Kieffer, A century-long journey from the discovery of insulin to the implantation of stem cell-derived islets. Endocr. Rev. 44 , 222–253 (2023). https://doi.org/10.1210/endrev/bnac021
Tun SBB, M. Chua, R. Hasan, M. Köhler, X. Zheng, Y. Ali, M.H. Abdulreda, L. Juntti-Berggren, V.A. Barathi, P.O. Berggren, Islet transplantation to the anterior chamber of the Eye-A future treatment option for insulin-deficient Type-2 diabetics? A case report from a Nonhuman Type-2 diabetic primate. Cell Transpl. 29 , 963689720913256 (2020). https://doi.org/10.1177/0963689720913256
T.J.I. Kieffer. Progress towards a stem cell based therapy for diabetes. J. Stem Cells Regen. Med. 17 , 61 (2021). https://doi.org/10.46582/jsrm.1702010
N. Sakata, T. Aoki, G. Yoshimatsu, H. Tsuchiya, T. Hata, Y. Katayose, S. Egawa, M. Unno, Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab. Res. Rev. 30 , 1–10 (2014). https://doi.org/10.1002/dmrr.2463
J.D. Weaver, D.M. Headen, J. Aquart, C.T. Johnson, L.D. Shea, H. Shirwan, A.J. García, Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 3 , e1700184 (2017). https://doi.org/10.1126/sciadv.1700184
F. Van Hulle, K. De Groot, R. Hilbrands, U. Van de Velde, K. Suenens, G. Stangé, I. De Mesmaeker, D.L. De Paep, Z. Ling, B. Roep, P. Gillard, D. Pipeleers, B. Keymeulen, D. Jacobs-Tulleneers-Thevissen, Function and composition of pancreatic islet cell implants in omentum of type 1 diabetes patients. Am. J. Transpl. 22 , 927–936 (2022). https://doi.org/10.1111/ajt.16884
D.L. Faustman, M. Davis, Stem cells in the spleen: therapeutic potential for Sjogren’s syndrome, type I diabetes, and other disorders. Int. J. Biochem. Cell Biol. 42 , 1576–1579 (2010). https://doi.org/10.1016/j.biocel.2010.06.012
M.S. Remedi, C. Emfinger, Pancreatic beta-cell identity in diabetes. Diabetes Obes. Metab. 18 , 110–116 (2016). https://doi.org/10.1111/dom.12727 .
S. Yagihashi, W. Inaba, H. Mizukami, Dynamic pathology of islet endocrine cells in type 2 diabetes: beta-Cell growth, death, regeneration and their clinical implications. J. Diabetes Investig. 7 , 155–165 (2016). https://doi.org/10.1111/jdi.12424
P.A. Halban, Cell therapy for type 2 diabetes: is it desirable and can we get it? Diabetes Obes. Metab. 10 , 205–211 (2008). https://doi.org/10.1111/j.1463-1326.2008.00957.x
F. Fantuzzi, S. Toivonen, A.A. Schiavo, H. Chae, M. Tariq, T. Sawatani, N. Pachera, Y. Cai, C. Vinci, E. Virgilio, L. Ladriere, M. Suleiman, P. Marchetti, J.C. Jonas, P. Gilon, D.L. Eizirik, M. Igoillo-Esteve, M. Cnop, In depth functional characterization of human induced pluripotent stem cell-derived beta cells in vitro and in vivo. Front Cell Dev Biol 10 , 967765 (2022). https://doi.org/10.3389/fcell.2022.967765
N. Saber, J.E. Bruin, S. O’Dwyer, H. Schuster, A. Rezania, T.J. Kieffer, Sex differences in maturation of human embryonic stem cell-derived beta cells in mice. Endocrinology 159 , 1827–1841 (2018). https://doi.org/10.1210/en.2018-00048
M. Zhao, S.A. Amiel, S. Ajami, J. Jiang, M. Rela, N. Heaton, G.C. Huang, Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS One 3 , e2666 (2008). https://doi.org/10.1371/journal.pone.0002666
J.E. Bruin, A. Asadi, J.K. Fox, S. Erener, A. Rezania, T.J. Kieffer, Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Rep. 5 , 1081–1096 (2015). https://doi.org/10.1016/j.stemcr.2015.10.013
V. Sordi, L. Monaco, L. Piemonti, Cell therapy for type 1 diabetes: from islet transplantation to stem cells. Horm. Horm. Res. Paediatr. 96 , 658–669 (2022). https://doi.org/10.1159/000526618
S. Pellegrini, L. Piemonti, V. Sordi, Pluripotent stem cell replacement approaches to treat type 1 diabetes. Curr Opin Pharmacol 43 , 20–26 (2018). https://doi.org/10.1016/j.coph.2018.07.007
R.Y. Calne, M.A. Ghoneim, K.O. Lee, G.S. Uin, Gene and stem cell therapy for diabetes. Clin Transpl. 12 , 111–112 (2013).
Google Scholar
E. de Klerk, M. Hebrok, Stem cell-based clinical trials for Diabetes Mellitus. Front. Endocrinol. 12 , 631463 (2021). https://doi.org/10.3389/fendo.2021.631463
K. Kirk, E. Hao, R. Lahmy, P. Itkin-Ansari, Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell. Res. 12 , 807–814 (2014). https://doi.org/10.1016/j.scr.2014.03.003
A.A. Tomei, C. Villa, C. Ricordi, Development of an encapsulated stem cell-based therapy for diabetes. Expert Opin. Biol. Ther. 15 , 1321–1336 (2015). https://doi.org/10.1517/14712598.2015.1055242
B.E. Tuch, T.C. Hughes, M.D. Evans, Encapsulated pancreatic progenitors derived from human embryonic stem cells as a therapy for insulin-dependent diabetes. Diabetes Metab. Res. Rev. 27 , 928–932 (2011). https://doi.org/10.1002/dmrr.1274
A.J. Vegas, O. Veiseh, M. Gurtler, J.R. Millman, F.W. Pagliuca, A.R. Bader, J.C. Doloff, J. Li, M. Chen, K. Olejnik, H.H. Tam, S. Jhunjhunwala, E. Langan, S. Aresta-Dasilva, S. Gandham, J.J. McGarrigle, M.A. Bochenek, J. Hollister-Lock, J. Oberholzer, D.L. Greiner, G.C. Weir, D.A. Melton, R. Langer, D.G. Anderson, Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22 , 306–311 (2016). https://doi.org/10.1038/nm.4030
X. Wang, K.G. Maxwell, K. Wang, D.T. Bowers, J.A. Flanders, W. Liu, L.H. Wang, Q. Liu, C. Liu, A. Naji, Y. Wang, B. Wang, J. Chen, A.U. Ernst, J.M. Melero-Martin, J.R. Millman, M. Ma, A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci. Transl. Med. 13(2021). https://doi.org/10.1126/scitranslmed.abb4601
R. Anzalone, M. Lo Iacono, T. Loria, A. Di Stefano, P. Giannuzzi, F. Farina, G. La Rocca, Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. Rep. 7 , 342–363 (2011). https://doi.org/10.1007/s12015-010-9196-4
W. Zhang, Q. Ling, B. Wang, K. Wang, J. Pang, J. Lu, Y. Bi, D. Zhu, Comparison of therapeutic effects of mesenchymal stem cells from umbilical cord and bone marrow in the treatment of type 1 diabetes. Stem Cell. Res. Ther. 13 , 406 (2022). https://doi.org/10.1186/s13287-022-02974-1
Q. Huang, Y. Huang, J. Liu, Mesenchymal stem cells: an excellent candidate for the treatment of Diabetes Mellitus. Int J Endocrinol 2021 , 9938658 (2021). https://doi.org/10.1155/2021/9938658
T.Y. Yeung, K.L. Seeberger, T. Kin, A. Adesida, N. Jomha, A.M. Shapiro, G.S. Korbutt, Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One 7 , e38189 (2012). https://doi.org/10.1371/journal.pone.0038189
C. Fotino, C. Ricordi, V. Lauriola, R. Alejandro, A. Pileggi, Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev. Diabet. Stud. Summer 7 , 144–157 (2010). https://doi.org/10.1900/RDS.2010.7.144
Z. Azizi, R. Abbaszadeh, R. Sahebnasagh, A. Norouzy, E. Motevaseli, K. Maedler, Bone marrow mesenchymal stromal cells for diabetes therapy: touch, fuse, and fix? Stem Cell. Res. Ther. 13 , 348 (2022). https://doi.org/10.1186/s13287-022-03028-2
M. Haque, F. Lei, X. Xiong, J.K. Das, X. Ren, D. Fang, S. Salek-Ardakani, J.M. Yang, J. Song, Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight. 4 (2019). https://doi.org/10.1172/jci.insight.126471
Y.L. Sun, L.R. Shang, R.H. Liu, X.Y. Li, S.H. Zhang, Y.K. Ren, K. Fu, H.B. Cheng, B.H. Yahaya, Y.L. Liu, J.T. Lin, Therapeutic effects of menstrual blood-derived endometrial stem cells on mouse models of streptozotocin-induced type 1 diabetes. World J. Stem Cells 14 , 104–116 (2022). https://doi.org/10.4252/wjsc.v14.i1.104
E.J. Bassi, P.M. Moraes-Vieira, C.S. Moreira-Sa, D.C. Almeida, L.M. Vieira, C.S. Cunha, M.I. Hiyane, A.S. Basso, A. Pacheco-Silva, N.O. Camara, Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 61 , 2534–2545 (2012). https://doi.org/10.2337/db11-0844
D.A. Alagpulinsa, J.J.L. Cao, D. Sobell, M.C. Poznansky, Harnessing CXCL12 signaling to protect and preserve functional beta-cell mass and for cell replacement in type 1 diabetes. Pharmacol. Ther. 193 , 63–74 (2019). https://doi.org/10.1016/j.pharmthera.2018.08.011
E. Sintov, I. Nikolskiy, V. Barrera, J. Hyoje-Ryu Kenty, A.S. Atkin, D. Gerace, S.J. Ho Sui, K. Boulanger, D.A. Melton, Whole-genome CRISPR screening identifies genetic manipulations to reduce immune rejection of stem cell-derived islets. Stem Cell Rep. 17 , 1976–1990 (2022). https://doi.org/10.1016/j.stemcr.2022.08.002
G.G. Gornalusse, R.K. Hirata, S.E. Funk, L. Riolobos, V.S. Lopes, G. Manske, D. Prunkard, A.G. Colunga, L.A. Hanafi, D.O. Clegg, C. Turtle, D.W. Russell, HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35 , 765–772 (2017). https://doi.org/10.1038/nbt.3860
S.D. Sackett, S.J. Kaplan, S.A. Mitchell, M.E. Brown, A.L. Burrack, S. Grey, D. Huangfu, J. Odorico, Genetic engineering of immune evasive stem cell-derived islets. Transpl. Int. 35 , 10817 (2022). https://doi.org/10.3389/ti.2022.10817
H.M. Shahjalal, A. Abdal Dayem, K.M. Lim, T.I. Jeon, S.G. Cho, Generation of pancreatic beta cells for treatment of diabetes: advances and challenges. Stem Cell. Res. Ther. 9 , 355 (2018). https://doi.org/10.1186/s13287-018-1099-3
E. Hajizadeh-Saffar, Y. Tahamtani, N. Aghdami, K. Azadmanesh, M. Habibi-Anbouhi, Y. Heremans, N. De Leu, H. Heimberg, P. Ravassard, M.A. Shokrgozar, H. Baharvand, Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep. 30 , 9322 (2015). https://doi.org/10.1038/srep09322
M. Seno, M.C.I. Nostro, How can you choose the fate of iPSCs and stem cells, Regeneration or Carcinogenesis? A hypothetical insight.: II. Modelling human beta cell development with pluripotent stem cells. J. Stem Cells Regen. Med. 16 , 90–91 (2020). https://doi.org/10.46582/jsrm.1602013
J. Chen, H. Li, F. Addabbo, F. Zhang, E. Pelger, D. Patschan, H.C. Park, M.C. Kuo, J. Ni, G. Gobe, P.N. Chander, A. Nasjletti, M.S. Goligorsky, Adoptive transfer of syngeneic bone marrow-derived cells in mice with obesity-induced diabetes: selenoorganic antioxidant ebselen restores stem cell competence. Am. J. Pathol. 174 , 701–711 (2009). https://doi.org/10.2353/ajpath.2009.080606
K. Cierpka-Kmiec, A. Wronska, Z. Kmiec, In vitro generation of pancreatic beta-cells for diabetes treatment. I. beta-like cells derived from human pluripotent stem cells. Folia Histochem. Cytobiol. 57 , 1–14 (2019). https://doi.org/10.5603/FHC.a2019.0001
J. Homma, H. Sekine, T. Shimizu, Tricultured cell sheets develop into functional pancreatic islet tissue with a vascular network. Tissue Eng. A 29 , 211–224 (2023). https://doi.org/10.1089/ten.TEA.2022.0167
J. Yang, Y. Yan, X. Yin, X. Liu, I.V. Reshetov, P.A. Karalkin, Q. Li, R.L. Huang, Bioengineering and vascularization strategies for islet organoids: advancing toward diabetes therapy. Metabolism 152 , 155786 (2024). https://doi.org/10.1016/j.metabol.2024.155786
C. Bandeiras, A.J. Hwa, J.M.S. Cabral, F.C. Ferreira, S.N. Finkelstein, R.A. Gabbay, Economics of Beta-cell replacement therapy. Curr. Diab. Rep. 19 , 75 (2019). https://doi.org/10.1007/s11892-019-1203-9
G.G. Nair, E.S. Tzanakakis, M. Hebrok, Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy. Nat. Rev. Endocrinol. 16 , 506–518 (2020). https://doi.org/10.1038/s41574-020-0375-3
K. Wallner, R.G. Pedroza, I. Awotwe, J.M. Piret, P.A. Senior, Shapiro AMJ, C. McCabe, Stem cells and beta cell replacement therapy: a prospective health technology assessment study. BMC Endocr. Disord. 18 , 6 (2018). https://doi.org/10.1186/s12902-018-0233-7
C. Bandeiras, J.M.S. Cabral, R.A. Gabbay, S.N. Finkelstein, F.C. Ferreira, Bringing stem cell-based therapies for Type 1 diabetes to the clinic: early insights from bioprocess economics and cost-effectiveness analysis. Biotechnol. J. 14 , e1800563 (2019). https://doi.org/10.1002/biot.201800563
T.C. Schulz, Concise review: manufacturing of pancreatic endoderm cells for clinical trials in Type 1 diabetes. Stem Cells Transl. Med. 4 , 927–931 (2015). https://doi.org/10.5966/sctm.2015-0058
S. Sepyani, S. Momenzadeh, S. Safabakhsh, R. Nedaeinia, R. Salehi, Therapeutic approaches for Type 1 Diabetes: Promising cell-based approaches to achieve ultimate success. SLAS Discov. Adv. Life Sci. R & D 29 , 23–33 (2024). https://doi.org/10.1016/j.slasd.2023.11.002
N. Cuesta-Gomez, K. Verhoeff, I.T. Jasra, R. Pawlick, N. Dadheech, A.M.J. Shapiro, Characterization of stem-cell-derived islets during differentiation and after implantation. Cell Rep. 40 , 111238 (2022). https://doi.org/10.1016/j.celrep.2022.111238
C.J. Burns, S.J. Persaud, P.M. Jones, Diabetes mellitus: a potential target for stem cell therapy. Curr. Stem Cell. Res. Ther. 1 , 255–266 (2006). https://doi.org/10.2174/157488806776956832
A.L. Marquez-Aguirre, A.A. Canales-Aguirre, E. Padilla-Camberos, H. Esquivel-Solis, N.E. Diaz-Martinez, Development of the endocrine pancreas and novel strategies for beta-cell mass restoration and diabetes therapy. Braz. J. Med. Biol. Res. 48 , 765–776 (2015). https://doi.org/10.1590/1414-431X20154363
Z. Zhou, X. Zhu, H. Huang, Z. Xu, J. Jiang, B. Chen, H. Zhu, Recent progress of research regarding the applications of stem cells for treating Diabetes Mellitus. Stem Cells Dev. 31 , 102–110 (2022). https://doi.org/10.1089/scd.2021.0083
L. Yang, Z.M. Hu, F.X. Jiang, W. Wang, Stem cell therapy for insulin-dependent diabetes: Are we still on the road? World J. Stem Cells 14 , 503–512 (2022). https://doi.org/10.4252/wjsc.v14.i7.503
S. Bourgeois, T. Sawatani, A. Van Mulders, N. De Leu, Y. Heremans, H. Heimberg, M. Cnop, W. Staels, Towards a functional cure for diabetes using stem cell-derived beta cells: are we there yet? Cells. 10 (2021). https://doi.org/10.3390/cells10010191
Download references
This review was supported by grants from the National Nature Science Foundation of China (82160162, 81760150) and the key project of Jiangxi Provincial Natural Science Foundation (20202ACBL206008).
Author information
Authors and affiliations.
Department of Metabolism and Endocrinology, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Yunfei Luo, Peng Yu & Jianping Liu
You can also search for this author in PubMed Google Scholar
Contributions
Y.F.L. wrote the manuscript, P.Y. and J.P.L. supervised all stages of manuscript development. All authors have approved the final article.
Corresponding author
Correspondence to Jianping Liu .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Luo, Y., Yu, P. & Liu, J. The efficiency of stem cell differentiation into functional beta cells for treating insulin-requiring diabetes: Recent advances and current challenges. Endocrine (2024). https://doi.org/10.1007/s12020-024-03855-8
Download citation
Received : 14 March 2024
Accepted : 29 April 2024
Published : 10 May 2024
DOI : https://doi.org/10.1007/s12020-024-03855-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Functional β-cells
- Stem cell transplantation
- Find a journal
- Publish with us
- Track your research
- Asia Pacific
- Latin America
- Middle East & Africa
- North America
- Australia & New Zealand
Mainland China
- Hong Kong SAR, China
- Philippines
- Taiwan, China
- Channel Islands
- Netherlands
- Switzerland
- United Kingdom
- Saudi Arabia
- South Africa
- United Arab Emirates
- United States
From startups to legacy brands, you're making your mark. We're here to help.
- Innovation Economy Fueling the success of early-stage startups, venture-backed and high-growth companies.
- Midsize Businesses Keep your company growing with custom banking solutions for middle market businesses and specialized industries.
- Large Corporations Innovative banking solutions tailored to corporations and specialized industries.
- Commercial Real Estate Capitalize on opportunities and prepare for challenges throughout the real estate cycle.
- Community Impact Banking When our communities succeed, we all succeed. Local businesses, organizations and community institutions need capital, expertise and connections to thrive.
- International Banking Power your business' global growth and operations at every stage.
- Client Stories
Prepare for future growth with customized loan services, succession planning and capital for business equipment.
- Asset Based Lending Enhance your liquidity and gain the flexibility to capitalize on growth opportunities.
- Equipment Financing Maximize working capital with flexible equipment and technology financing.
- Trade & Working Capital Experience our market-leading supply chain finance solutions that help buyers and suppliers meet their working capital, risk mitigation and cash flow objectives.
- Syndicated Financing Leverage customized loan syndication services from a dedicated resource.
- Employee Stock Ownership Plans Plan for your business’s future—and your employees’ futures too—with objective advice and financing.
Institutional Investing
Serving the world's largest corporate clients and institutional investors, we support the entire investment cycle with market-leading research, analytics, execution and investor services.
- Institutional Investors We put our long-tenured investment teams on the line to earn the trust of institutional investors.
- Markets Direct access to market leading liquidity harnessed through world-class research, tools, data and analytics.
- Prime Services Helping hedge funds, asset managers and institutional investors meet the demands of a rapidly evolving market.
- Global Research Leveraging cutting-edge technology and innovative tools to bring clients industry-leading analysis and investment advice.
- Securities Services Helping institutional investors, traditional and alternative asset and fund managers, broker dealers and equity issuers meet the demands of changing markets.
- Financial Professionals
- Liquidity Investors
Providing investment banking solutions, including mergers and acquisitions, capital raising and risk management, for a broad range of corporations, institutions and governments.
- Center for Carbon Transition J.P. Morgan’s center of excellence that provides clients the data and firmwide expertise needed to navigate the challenges of transitioning to a low-carbon future.
- Corporate Finance Advisory Corporate Finance Advisory (“CFA”) is a global, multi-disciplinary solutions team specializing in structured M&A and capital markets. Learn more.
- Development Finance Institution Financing opportunities with anticipated development impact in emerging economies.
- Sustainable Solutions Offering ESG-related advisory and coordinating the firm's EMEA coverage of clients in emerging green economy sectors.
- Mergers and Acquisitions Bespoke M&A solutions on a global scale.
- Capital Markets Holistic coverage across capital markets.
- Capital Connect
- In Context Newsletter from J.P. Morgan
- Director Advisory Services
Accept Payments
Explore Blockchain
Client Service
Process Payments
Manage Funds
Safeguard Information
Banking-as-a-service
Send Payments
- Partner Network
A uniquely elevated private banking experience shaped around you.
- Banking We have extensive personal and business banking resources that are fine-tuned to your specific needs.
- Investing We deliver tailored investing guidance and access to unique investment opportunities from world-class specialists.
- Lending We take a strategic approach to lending, working with you to craft the fight financing solutions matched to your goals.
- Planning No matter where you are in your life, or how complex your needs might be, we’re ready to provide a tailored approach to helping your reach your goals.
Whether you want to invest on your own or work with an advisor to design a personalized investment strategy, we have opportunities for every investor.
- Invest on your own Unlimited $0 commission-free online stock, ETF and options trades with access to powerful tools to research, trade and manage your investments.
- Work with our advisors When you work with our advisors, you'll get a personalized financial strategy and investment portfolio built around your unique goals-backed by our industry-leading expertise.
- Expertise for Substantial Wealth Our Wealth Advisors & Wealth Partners leverage their experience and robust firm resources to deliver highly-personalized, comprehensive solutions across Banking, Lending, Investing, and Wealth Planning.
- Why Wealth Management?
- Retirement Calculators
- Market Commentary
Who We Serve
Explore a variety of insights.
Global Research
- Newsletters
Insights by Topic
Explore a variety of insights organized by different topics.
Insights by Type
Explore a variety of insights organized by different types of content and media.
- All Insights
We aim to be the most respected financial services firm in the world, serving corporations and individuals in more than 100 countries.

Key takeaways
- J.P. Morgan Research forecasts that the GLP-1 market will exceed $100 bn by 2030, driven equally by diabetes and obesity usage.
- Total GLP-1 users in the U.S. may number 30 mn by 2030 — or around 9% of the overall population.
- The increasing appetite for obesity drugs will have myriad implications, boosting sectors such as biotech and creating headwinds for industries such as food and beverage.
Ozempic. Wegovy. Mounjaro. Zepbound. Originally developed to treat diabetes, these GLP-1 agonists — now also popularly known as obesity drugs — have been making headlines for their weight-loss effects. According to the Centers for Disease Control and Prevention (CDC), the prevalence of obesity in the U.S. has grown from 30.5% over 1999–2000 to 41.9% over 2017–2020.
What’s whetting the consumer appetite for these weight-loss drugs, and what does this mean for sectors ranging from biotech to food?
“The newest generations of GLP-1s and combos lead to 15–25+% weight loss on average, well above prior generations of products.”
Chris Schott
Senior Analyst covering the U.S. Diversified Biopharma sector, J.P. Morgan
What are GLP-1 agonists?
Glp-1 agonists are a class of medications used to treat type 2 diabetes (t2d). besides helping to lower blood sugar levels, they also suppress appetite and reduce calorie intake — fueling their growing popularity as obesity drugs. , “glp-1s have been used to treat t2d since 2005, starting with the approval of byetta, with follow-on products continually improving on efficacy. the most recent, ozempic and mounjaro, offer significant advantages over previous products and have accelerated class growth,” said chris schott, a senior analyst covering the u.s. diversified biopharma sector at j.p. morgan. “indeed, the newest generations of glp-1s and combos lead to 15–25+% weight loss on average, well above prior generations of products.” .
What’s driving the increase in appetite for obesity drugs?
Originally developed to treat diabetes, GLP-1 agonists — or obesity drugs — have risen in popularity thanks to their weight-loss effects.
J.P. Morgan Research forecasts the GLP-1 market will exceed $100 bn by 2030, fueled equally by diabetes and obesity usage.
Total GLP-1 users in the U.S. may number 30 mn by 2030 — or around 9% of the overall population.
This could lead to a paradigm shift in health care and also impact other sectors, from biotech to food.
What’s the market for obesity drugs?
J.p. morgan research forecasts the glp-1 category will exceed $100 bn by 2030, driven equally by diabetes and obesity usage. , today, glp-1s are used by around 10-12% of t2d patients in the u.s. “we model glp-1 usage expanding to around 35% of diabetics in the u.s. in 2030 and would not be surprised to see upside to this number, especially as outcomes data continues to emerge,” schott noted. “in addition, we forecast that around 15 mn obese patients will be on glp-1s by the end of the decade.” overall, total glp-1 users in the u.s. may number 30 mn by 2030 — or around 9% of the population. , the glp-1 landscape is currently dominated by two major players: u.s.-based eli lilly and denmark-based novo nordisk. “we expect the obesity market to largely be a duopoly between both companies, with modest share attributed to later entrants,” schott said. “while demand could continue to outstrip supply for the next several years, we do see these issues resolving in the longer term with more plants coming online and more competitive oral options becoming available.” , the u.s. obesity market is expanding rapidly .
The U.S. obesity market is forecast to reach $44 bn in 2030 — up from just $0.5 bn in 2020.
Sector implications
What this means for … health care.
The growing popularity of GLP-1s could transform how obesity is viewed and managed. “We believe this marks the beginning of a paradigm shift in the way that obesity is treated, with physicians moving to a weight-centric treatment of multiple co-morbidities associated with the condition. We expect this to drive substantial uptake of GLP-1s,” said Richard Vosser, Head of European Pharma & Biotech at J.P. Morgan. For instance, GLP-1s may aid in the management of cardiovascular disease and heart failure, which around 9 mn obese patients suffer from.
Likewise, the rise of GLP-1s will shape diabetes treatment. “In diabetes, we see growth of GLP-1s driven by a shift in medical guidelines, including those proposed by the American Diabetes Association and European Association for the Study of Diabetes, which place weight management and assessment of co-morbidities profile on par with glycaemic control,” Vosser added.
What this means for … biotech
With the GLP-1 market proving to be highly lucrative, new biotech firms will seek to enter the drug race. “Naturally, with a class so potentially unprecedentedly large, we expect many biotechs across the market cap range to be motivated to participate. Even capturing a small share of such a large market could be very interesting for some companies, and these efforts may also lead to attractive partnering opportunities,” said Jessica Fye, a Senior Analyst covering the Large-Cap Biotechnology sector at J.P. Morgan.
There is also scope for biotech firms to explore, through clinical trials, how certain medications work in tandem with GLP-1s. “For example, we believe companies investigating drugs targeting certain cardiovascular indications may want to consider planning to generate data that includes patients on GLP-1s,” said Anupam Rama, a Senior Analyst covering the U.S. Biotechnology sector at J.P. Morgan. “All in all, we see GLP-1s as an exciting category, with biotechs angling for a slice of the pie.”
What this means for … medtech
How will GLP-1s impact other technologies used to treat diabetes and obesity? Despite the recent fall in medtech share prices, J.P. Morgan Research does not see an imminent threat to devices such as insulin pumps and continuous glucose monitors (CGMs). “In fact, we anticipate CGM utilization could increase as they will be vital to track progress and determine if GLP-1s are actually working,” said Robbie Marcus, a Senior Analyst covering the U.S. Medical Supplies & Devices sector at J.P. Morgan. “Plus, the weight loss benefit from CGMs, while not quite comparable to that of GLP-1s, is nevertheless material, especially considering how much more affordable they are.”
Weight loss surgery will also continue to be in demand due to its superior and more sustainable clinical outcomes. “Even those patients who opt for drugs will most likely still undergo bariatric surgery down the line due to the low adherence rates and high recurrence of weight gain from GLP-1s,” Marcus noted.
Overall, the outlook is still positive for the medtech industry. “With medtech now trading at a slight discount to the S&P 500 vs. a 15–25% historical premium, we think a reasonable amount of GLP-1 risk is already priced in,” Marcus said. “While GLP-1s will be a huge drug class, medtech volumes can also increase over time. We think both can live side by side and don’t see them as mutually exclusive.”
What this means for … insurance
In the U.S., many insurance providers are scaling back on coverage of GLP-1s due to the high costs involved. As of November 2023, a month’s supply of Zepbound is priced around $1,060, while a month’s supply of Wegovy is around $1,350.
However, J.P. Morgan Research expects coverage to eventually improve, especially for obesity treatment. “Coverage for obesity currently far lags that for T2D, and this will likely remain the biggest debate in the class for some time,” Schott noted. “We estimate current coverage at only around 40%, but this will likely reach the 80% range by the end of the decade, driven by a series of outcomes studies that we expect will show broad health benefits from losing weight.”
In the life insurance space, companies that cover mortality risk will benefit the most from GLP-1s to the extent that the treatment of diabetes, obesity and related co-morbidities translates to longer life spans for the insured population. “In financial terms, higher life expectancies would allow life insurers to earn more premium income and higher investment income on reserves, as mortality claims are deferred,” said Jimmy Bhullar, Head of the U.S. Insurance research team at J.P. Morgan.
On the other hand, GLP-1s could have a negative impact on life insurers that cover longevity risk through products such as structured settlements and pension risk transfer (PRT) plans, or lapse-supported policies such as long-term care and universal life with secondary guarantees (ULSG), where insurer economics deteriorate the longer the policy stays in force. “This is because longer life spans would translate to more benefits paid in the future,” Bhullar noted.
What this means for … food and beverage
GLP-1s could have a significant impact on food and beverage consumption. The advent of GLP-1 use for appetite suppression has been a key factor in the median larger-cap U.S. food producers underperforming the S&P 500 by nearly 40% year to date. “We have seen a number of trends and possible disruptions come and go in consumer staples over the years, but never one quite like GLP-1s,” said Ken Goldman, Lead Equity Research Analyst for the U.S. Food Producers and Food Retailers sectors at J.P. Morgan.
Using data from alternative data provider Numerator, J.P. Morgan Research has found that current GLP-1 users purchased around 8% less food — including snacks, soft drinks and high=carb products — for at-home consumption over the last 12 months compared with the average consumer. Food intake could decrease by -3% in North America by 2030E, though the figure could be higher for packaged foods. While European food companies derive up to 30–40% of their sales in North America, many of them have broad category and regional exposures, mitigating potential headwinds.
“Overall, we think that if GLP-1s start to make a meaningful difference in consumption patterns, grocers will be hurt less than packaged food companies,” Goldman said. “This is especially as they sell a lot of higher-margin fresh food, which could offset much of the impact on the center store and snacking in particular.”
Related insights

Healthcare Conference
Highlights from the annual J.P. Morgan Healthcare Conference, the industry’s biggest gathering.

Moving the fitness industry in a new direction
February 10, 2020
How is digital disruption affecting the future of fitness?

Leveraging cutting-edge technology and innovative tools to bring clients industry-leading analysis and investment advice.
This communication is provided for information purposes only. Please read J.P. Morgan research reports related to its contents for more information, including important disclosures. JPMorgan Chase & Co. or its affiliates and/or subsidiaries (collectively, J.P. Morgan) normally make a market and trade as principal in securities, other financial products and other asset classes that may be discussed in this communication.
This communication has been prepared based upon information, including market prices, data and other information, from sources believed to be reliable, but J.P. Morgan does not warrant its completeness or accuracy except with respect to any disclosures relative to J.P. Morgan and/or its affiliates and an analyst's involvement with any company (or security, other financial product or other asset class) that may be the subject of this communication. Any opinions and estimates constitute our judgment as of the date of this material and are subject to change without notice. Past performance is not indicative of future results. This communication is not intended as an offer or solicitation for the purchase or sale of any financial instrument. J.P. Morgan Research does not provide individually tailored investment advice. Any opinions and recommendations herein do not take into account individual client circumstances, objectives, or needs and are not intended as recommendations of particular securities, financial instruments or strategies to particular clients. You must make your own independent decisions regarding any securities, financial instruments or strategies mentioned or related to the information herein. Periodic updates may be provided on companies, issuers or industries based on specific developments or announcements, market conditions or any other publicly available information. However, J.P. Morgan may be restricted from updating information contained in this communication for regulatory or other reasons. Clients should contact analysts and execute transactions through a J.P. Morgan subsidiary or affiliate in their home jurisdiction unless governing law permits otherwise.
This communication may not be redistributed or retransmitted, in whole or in part, or in any form or manner, without the express written consent of J.P. Morgan. Any unauthorized use or disclosure is prohibited. Receipt and review of this information constitutes your agreement not to redistribute or retransmit the contents and information contained in this communication without first obtaining express permission from an authorized officer of J.P. Morgan. Copyright 2023 JPMorgan Chase & Co. All rights reserved.
You're now leaving J.P. Morgan
J.P. Morgan’s website and/or mobile terms, privacy and security policies don’t apply to the site or app you're about to visit. Please review its terms, privacy and security policies to see how they apply to you. J.P. Morgan isn’t responsible for (and doesn’t provide) any products, services or content at this third-party site or app, except for products and services that explicitly carry the J.P. Morgan name.
- Open access
- Published: 09 May 2024
Low ankle–brachial index is associated with higher cardiovascular mortality in individuals with nonalcoholic fatty liver disease
- Guang Xiong 1 ,
- Liuqing Guo 1 ,
- Liwei Li 2 &
- Min Liang 1
European Journal of Medical Research volume 29 , Article number: 276 ( 2024 ) Cite this article
32 Accesses
Metrics details
Background and aims
Ankle brachial index (ABI) is a risk factor for cardiovascular mortality, but it is unclear whether ABI is associated with cardiovascular mortality in patients with nonalcoholic fatty liver disease (NAFLD). The current study aimed to evaluate the association between ABI with cardiovascular and all-cause mortality in patients with NAFLD.
We performed a cohort study using the data of the1999–2004 National Health and Nutrition Examination Survey data of adults. Mortality data were followed up to December 2015. NAFLD was defined by the hepatic steatosis index or the US fatty liver index. ABI was classified into three groups: ABI ≤ 0.9 (low value); 0.9 < ABI ≤ 1.1 (borderline value); ABI greater than 1.1 (normal value).
We found that low ABI was associated with an increased risk of cardiovascular mortality in patients with NAFLD (HR: 2.42, 95% CI 1.10–5.33 for low value ABI vs normal value ABI, P for trend = 0.04), and the relationship was linearly and negatively correlated in the range of ABI < 1.4. However, low ABI was not associated with all-cause mortality in patients with NAFLD. Stratified by cardiovascular disease, ABI remains inversely correlated with cardiovascular mortality in NAFLD patients without cardiovascular disease. Stratified by diabetes, ABI is inversely correlated with cardiovascular mortality in NAFLD patients regardless of diabetes status.
Conclusions
Low ABI is independently associated with higher cardiovascular mortality in NAFLD cases. This correlation remains significant even in the absence of pre-existing cardiovascular disease or diabetes.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease globally, paralleling the rise in global obesity and Type 2 diabetes mellitus [ 1 , 2 ]. It is estimated that its prevalence is around 25% worldwide, gradually becoming a significant public health issue [ 1 ]. NAFLD is closely associated with metabolic disorders, and cardiovascular mortality (CVM) has been identified as the most common cause of death among NAFLD patients [ 3 , 4 , 5 , 6 ].
The ankle–brachial index (ABI) is a simple, non-invasive measurement method, calculated as the systolic blood pressure (SBP) ratio of the ankle artery to the brachial artery [ 7 ]. ABI is also a risk factor for arteriosclerosis and cardiovascular mortality, associated with increased CVM in populations with CVD, diabetes, and renal insufficiency [ 8 , 9 , 10 ]. However, the specific relationship between ABI and mortality in NAFLD patients has not been fully explored. Given the high metabolic burden and increased cardiovascular risk in NAFLD patients, ABI appears to be a valuable prognostic tool for this group. Identifying low ABI in NAFLD patients could help stratify risk, guide management strategies, and potentially intervene early to reduce mortality risk. Given its non-invasive nature and ease of measurement, ABI can be measured quickly by properly trained professionals within primary healthcare facilities [ 7 ]. With a high prevalence of NAFLD and the majority of NAFLD patients receiving care in primary health settings [ 11 ], routine ABI measurements is likely to offer significant benefits for individuals with NAFLD.
Therefore, this study aims to explore the predictive value of ABI for all-cause mortality and cardiovascular mortality in NAFLD patients. We seek to elucidate the utility of ABI in predicting mortality risk among NAFLD patients and its potential role in improving patient prognosis through better risk stratification.
Study sample
The participants in this study were recruited from the NHANES which is a non-institutionalized stratified probability sampling survey [ 12 ]. Because the ankle–brachial index was only studied from 1999 to 2004, we combined data from 1999 to 2004 to create a sample of 31,126 subjects. Of them, 15,332 were over the age of 20. We also excluded 8928 subjects who met the following criteria: (1) excessive drinking (> 14 and > 21 standard drinks weekly in women and men, respectively) ( n = 362); (2) seropositive for hepatitis B or C virus ( n = 293); (3) taking medications that can affect hepatic steatosis ( n = 495); (4) pregnant women ( n = 822); (5) missing ABI data ( n = 6499); (6) ABI > 1.4 (high ABI, not the focus of our study) ( n = 43); (7) missing the information required to define NAFLD by hepatic steatosis index (HSI, details were described below) ( n = 414). After excluding the aforementioned population, there were 6404 people left, 3649 of whom were classified as having NAFLD (HSI > 36) and had their ABI measured.
Due to incomplete covariate data, we further excluded 2070 individuals. This group comprised those with missing waist circumference ( n = 30), missing high density lipoprotein-cholesterol (HDL-cholesterol) ( n = 2), missing low density lipoprotein-cholesterol (LDL-cholesterol) ( n = 2030), missing triglycerides ( n = 2), missing fasting blood glucose ( n = 3), and missing fasting insulin ( n = 3). Considering the potential bias introduced by excluding these participants, we conducted a comparison of baseline characteristics between the population before and after exclusion (Table 1 ). The results indicated that the majority of variables showed no significant differences, particularly LDL levels, which remained consistent across both groups. Additionally, we excluded 33 subjects due to missing sampling weight data and 6 subjects due to missing mortality data. The total number of participants with NAFLD defined by HSI was 1540 (Fig. 1 ). We used the same method to screen the study sample of NAFLD diagnosed based on the US fatty liver index (USFLI, details were described below) for sensitivity analysis, and the sample of USFLI eventually included 1026 individuals (Supplementary Fig. 1).
Flow diagram of participants defined by hepatic steatosis index in the study
Measurement of ABI
ABI measurements were taken while the subjects were supine (parks Mini lab IV, model 3100) [ 13 ]. The subjects' SBP was measured in the ankle (posterior tibial artery) and right arm (brachial artery) in this study [ 13 ]. We measured SBP in the left arm if the subject had any condition on the right arm that could not be measured or might affect the precision of the measurement [ 13 ]. Subjects under the age of 60 were measured twice at each site, whereas subjects over the age of 60 were measured once at each site [ 13 ]. The mean SBP in bilateral ankles was then divided by the mean arm SBP to obtain left and right ABI separately (values of subjects aged 60 and over were calculated by the one-time value) [ 13 ]. The lower one of the left and right ABI values was then taken as the individual ABI [ 13 ].
Definition of NAFLD
For individual patients, especially in tertiary care settings, imaging studies are preferred, but for larger-scale research, serum biomarkers are favored due to the feasibility issues related to the availability and cost of imaging [ 14 , 15 ]. NHANES is a large-scale population health survey that lacks liver ultrasonography for steatosis in most of its cycles. Therefore, we opted for non-invasive models based on serological indicators to define NAFLD. For sensitivity analysis, we used two non-invasive methods to define NAFLD: the HSI (hepatic steatosis index) and the USFLI (US fatty liver index) [ 16 , 17 ]. NAFLD is defined as having HSI > 36 and USFLI ≥ 30, respectively. Both methods were obtained and validated in a non-institutionalized large-scale population health examination, and they all performed well in terms of diagnostic performance [ 16 , 17 ]. The HSI, with an area under the receiver-operating curve of 0.812, diagnoses NAFLD at values > 36 and excludes it at < 30, with sensitivities and specificities of 93.1% and 92.4%, respectively [ 16 ]. We used these two methods to diagnose NAFLD after excluding subjects with other chronic liver diseases (details have been described above) [ 18 ].
HSI = 8 × (alanine aminotransferase)/(aspartate aminotransferase) + BMI (+ 2,if DM; + 2,if female),
USFLI = (e −0.8073*non−Hispanic black+0.3458*Mexican American+0.0093*age+0.6151*loge(gamma−glutamyl transferase) +0.0249*waist circumference+1.1792*loge(insulin) +8242*loge(glucose) −14.7812 ) / (1 + e −0.8073*non−Hispanic black+0.3458*Mexican American+0.0093*age+0.6151*loge(gamma−glutamyl transferase) +0.0249*waist circumference+1.1792*loge(insulin) +8242*loge(glucose) −14.7812 ) * 100.
NHANES provided us with sociodemographic information, examination data, laboratory data, and questionnaire data [ 12 ]. Covariates were selected based on risk factors identified in previous studies [ 19 , 20 ]. Additionally, research focusing on risk factors for mortality among NAFLD patients was also consulted [ 21 ]. The preliminary covariates to be included are: age, gender, ethnicity, education level, marital status, economic status, body mass index (BMI), waist circumference, hypertension, diabetes, cardiovascular disease, and physical activity. Additionally, total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, triglycerides, fasting blood glucose, and fasting insulin levels are also considered. There were three levels of education: high school and below, high school and equal education, and college and above. Non-Hispanic White, Mexican American, non-Hispanic Black and others were the race categories. Meanwhile, this work classified marital status as married/living with a partner and other status. According to the ratio of family income to poverty guideline, the economic situation was classified as ≤ 1.0, > 1.0, or unknown. Smoking habits were divided into three categories: never smokers (smoking < 100 cigarettes in their lifetime), current smokers (still smoking, > 100 cigarettes in their lifetime), and ex-smokers (ever smoking, > 100 cigarettes in their lifetime). Diabetes was defined as subjects who met at least one of the criteria listed below [ 22 ]. The criteria were as follows: (1) a diagnosis of diabetes; (2) use of diabetes medications or insulin; (3) fasting blood glucose ≥ 126 mg/dL; and (4) hemoglobin A1c ≥ 6.5%. Hypertension was defined as meeting one or more of the following criteria [ 23 ]: (1) a history of hypertension; (2) SBP ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg; and (3) use of blood pressure medication. Cardiovascular disease (CVD) is defined as being informed by a health professional or a physician of the following conditions: coronary heart disease, congestive heart failure, heart attack, angina/angina pectoris, and stroke. Furthermore, this study divided physical activity into three levels based on the metabolic equivalent task level (MET-min: metabolic equivalent-minute) of moderate-to-vigorous physical activity (MVPA) per week [ 24 , 25 ], which were as follows: (1) low (< 600 MET-min MVPA/week); (2) moderate (600–1500 MET-min MVPA /week); and (3) high (> 1500 MET-min MVPA /week).
Each NHANES subject's death status was linked with NDI (National Death Index) death data, and they were all followed up on until December 31, 2015 [ 26 ]. ICD-10 was used to determine the cause of death: UCOD_113 (underlying cause of death 113) codes 55–64 and 70 are attributed to CVD [ 26 ].
Statistical analysis
Since the complex sampling survey design was used in the NHANES, this work used appropriate stratification, clustering, and sample weight to reflect the overall situation of the population in accordance with the NHANES analysis guidelines [ 27 ]. The baseline data were presented as the weighted mean ± standard error or weighted frequency (95% confidence interval). To compare the differences of continuous variables, the Kruskal–Wallis test or one-way ANOVA was used, and the Chi-square test was used to compare the differences between classified variables. Considering the multitude of covariates, we conducted collinearity analysis and removed certain covariates to mitigate the risk of overadjustment in the constructing the survival analysis model and to enhance the study's power. Given that NAFLD was defined using the HSI and USFLI, adjusting for the components within these two formulas could increase collinearity. Consequently, we removed specific components for each model (for the model based on HSI, BMI was removed; for the model based on USFLI, ethnicity, age, waist circumference, blood glucose, and fasting insulin were excluded). Furthermore, by calculating the Variance Inflation Factor values within the model, we identified and cautiously reduced covariates causing collinearity, which led to the further elimination of TC. Based on the criteria listed below, this study classified ABI into three groups [ 7 ]: (1) ABI ≤ 0.9 (low value); (2) 0.9 < ABI ≤ 1.1 (borderline value); (3) ABI greater than 1.1 (normal value). ABI was included in the regression model analysis as a continuous and a categorical variable, respectively, and we used multivariate Cox regression to investigate the relationship between ABI and mortality. When ABI is converted into a categorical variable, we enter the model with the median value of ABI in each group as a continuous variable to test the linear trend. In the fully adjusted model, the linear relationship between ABI and mortality was also evaluated using restricted cubic spline functions of three knots (the 5th, 50th, and 95th percentiles of ABI). This study further examined the relationship between ABI and mortality stratified by with and without cardiovascular disease, and the interactions of CVD and ABI were tested. In this study, all tests were two-sided, with a difference of P < 0.05 indicating significance. All statistical analyses were conducted using R 4.2.3 ( http://www.R-project.org ; The R Foundation).
In total, 1540 people took part in this study. The baseline patient characteristics are depicted in Table 2 . Compared to subjects with normal ABI, subjects with abnormal ABI (low or borderline value) were more likely to be older, male, non-Hispanic white, and living alone. They also had a lower level of education, a smoking habit, a lower level of physical activity, a lower level of alanine aminotransferase, a lower level of aspartate aminotransferase, and diseases such as CVD, hypertension, and diabetes. BMI, economic status, triglyceride, HDL-cholesterol, fasting insulin, gamma glutamyltransferase, and fasting blood glucose were not significantly different between the three groups, but LDL-cholesterol and TC were. The baseline patient characteristics of the sample defined by USFLI are shown in supplementary Table 1.
Over the median 13.25-year follow-up period, 330 subjects died (72 died of CVD). Table 3 shows the relationships between ABI and mortality in NAFLD patients. After controlling for demographic variables and smoking status (model 1), decreasing ABI had no effect on all-cause mortality. Even after adjusting for physical activity, coexisting diseases (hypertension, diabetes, cardiovascular disease), and waist circumference within model 2, ABI was not related to all-cause mortality. Furthermore, ABI was still not significantly related to all-cause mortality after including the metabolic confounders (HDL-cholesterol, LDL-cholesterol, triglycerides, fasting blood glucose, and fasting insulin) in the multivariable model (model 3). We used ABI as a continuous variable for sensitivity analysis and converted it to per 0.1 ABI (to make hazard ratio not too small). Similarly, no associations between ABI and all-cause mortality were found in any of the three models. When all of the above analyses were performed on the NAFLD sample defined by USFLI, the results were largely consistent (supplementary Table 2).
Then, in NAFLD cases, we conducted analyses of the relationships between ABI and CVM. Reduced ABI was associated with an increased risk of CVM in model 1 (HR: 3.09, 95% CI 1.38–6.93 for low ABI, P for trend = 0.02), and the results in model 2 were similar (HR: 2.35, 95% CI 1.03–5.37 for low ABI, P for trend = 0.03). After full adjustment, lower ABI was still associated with an increased risk of CVM (HR: 2.42, 95% CI 1.10–5.33, P for trend = 0.04). When ABI was used as a continuous variable in the sensitivity analysis, it was found to be negatively correlated with cardiovascular mortality in all models. Every 0.1 reduction in ABI increased the risk of cardiovascular mortality by 18% in the fully adjusted model (HR: 0.82, 95% CI 0.71–0.93). We used the restricted cubic spline function to confirm the linear relationship between ABI and cardiovascular mortality (Fig. 2 ). The results above were supported by a linear negative correlation between ABI and cardiovascular mortality ( P for nonlinearity = 0.85). When the analyses were repeated using the NAFLD sample defined by USFLI, the results were nearly identical to those obtained using the NAFLD sample defined by HSI (Supplementary Table 2 and Supplementary Fig. 2).
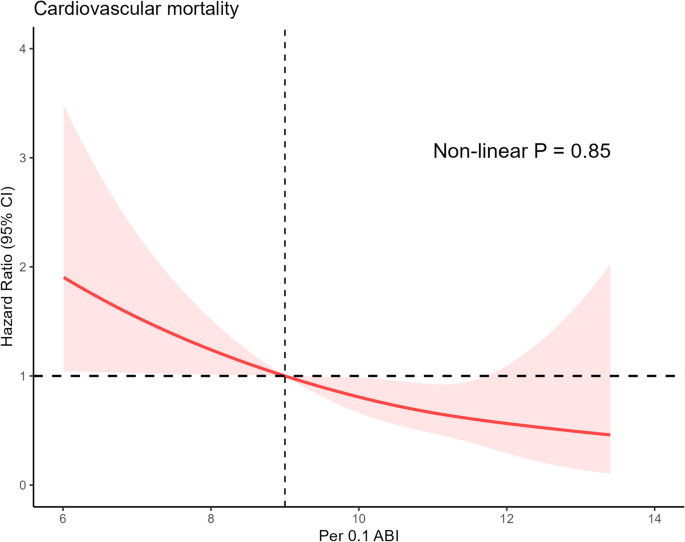
Association between per 0.1 ABI and cardiovascular mortality among patients with NAFLD defined by hepatic steatosis index. The red sold line represents the estimated hazard ratios, and the red shaded area represents the 95% confidence intervals. The restricted cubic spline function was adjusted for age, gender, ethnicity, education level, marital status, Family income-to-poverty ratio, smoking status, waist circumference, hypertension, diabetes, cardiovascular disease and physical activity, high density lipoprotein-cholesterol, low density lipoprotein-cholesterol, triglyceride, fasting blood glucose and fasting insulin. ABI was converted into per 0.1 ABI after an increase of 10 times. ABI: ankle–brachial index; NAFLD: nonalcoholic fatty liver disease
Given that CVD is a risk factor for CVM and all-cause mortality, this study investigated the relationship of ABI level with mortality among NAFLD cases based on the presence of cardiovascular disease at baseline (Table 4 ). The interactions between ABI and CVD were also investigated (Table 4 ). In all three models, ABI was found to be unrelated to all-cause mortality in patients with NAFLD, regardless of the presence of CVD. In all multivariate models, there was no interaction between CVD and ABI. The outcome variable was then changed to cardiovascular mortality for analysis. In multivariable model 1, ABI was negatively correlated with cardiovascular mortality in individuals with CVD (HR: 0.73, 95% CI 0.61–0.86). After adjusting for additional risk factors, the correlations between ABI and cardiovascular mortality in models 2 and 3 remained consistent with those in model 1 (HR: 0.76, 95% CI 0.61–0.95 for model 2, HR: 0.68, 95% CI 0.51–0.91 for model 3). ABI was also negatively correlated with cardiovascular mortality in people who did not have CVD, and the correlations were identical in all three models (HR: 0.80, 95% CI 0.63–1.00 for model 1, HR: 0.83, 95% CI 0.72–0.95 for model 2, HR: 0.80, 95% CI 0.65–0.99 for model 3).
We used NAFLD defined by the USFLI as a sample again for sensitivity analysis, and the results are shown in Supplementary Table 3. In Model 1, ABI was inversely correlated with all-cause mortality, irrespective of the presence or absence of CVD. After adjusting for further risk factors (Models 2 and 3), we discovered no links between ABI and all-cause mortality in NAFLD patients with or without CVD, which is consistent with observations made in NAFLD samples defined by HSI. When the relationship between ABI and cardiovascular mortality was examined, it was discovered that there was no correlation between ABI and CVM in CVD patients using the fully adjusted model (HR: 0.71, 95% CI 0.45–1.14 for model 3). However, after full adjustment, ABI was negatively correlated with cardiovascular mortality among individuals without CVD (HR: 0.61, 95% CI 0.48–0.77 for model 3), which was similar to the analysis based on HSI-defined samples. Furthermore, no interaction between CVD and ABI was found ( P = 0.50 for model 3).
Given the association between diabetes and low ABI, we examined the relationship between ABI and CVM as well as all-cause mortality among NAFLD patients, stratified by the presence of diabetes at baseline (Table 5 ). In Model 1, ABI was inversely associated with all-cause mortality in NAFLD patients with diabetes (HR: 0.81, 95% CI 0.70–0.94), but there was no significant association between ABI and all-cause mortality in NAFLD patients without diabetes. After adjusting for additional risk factors, ABI was not significantly related to all-cause mortality in NAFLD patients, regardless of the presence or absence of diabetes (Models 2 and 3). In the analysis of CVM, ABI was inversely associated with cardiovascular mortality in NAFLD patients, irrespective of their diabetes status. Sensitivity analysis using the sample of USFLI-defined NAFLD showed results that were generally consistent with those defined by HSI (Supplementary Table 4).
Our study provides novel insights into relationship between the ABI and mortality among patients with NAFLD, a patient group hitherto less studied in this context. We discovered a pronounced negative linear correlation between ABI and CVM within this cohort, a trend that persists even in the absence of CVD. Contradictory findings were observed regarding the relationship between low ABI and CVM among NAFLD patients with existing CVD. ABI was inversely related to cardiovascular mortality among NAFLD patients, regardless of diabetes status. Moreover, low ABI was not associated with all-cause mortality in patients with NAFLD.
NAFLD patients often carry a high metabolic burden and frequently present with multiple risk factors for CVD [ 6 , 28 ]. CVD is currently the leading cause of death among patients with NAFLD. Although the ankle–brachial index (ABI) was initially developed to detect lower extremity arterial occlusions, any such occlusion usually indicates the presence of systemic atherosclerosis, a precursor to cardiovascular disease [ 7 , 29 , 30 ]. Low ABI was predictive of an increased risk of CVM in a study involving 5748 participants [ 31 ], supporting the observation in our study that low ABI is associated with increased CVM risk. Importantly, our research extends this association to patients with NAFLD. ABI, in addition to indicating potential cardiovascular disease, is also associated with many traditional CVD risk factors [ 29 , 30 ]. Therefore, we speculate that NAFLD patients with low ABI, even without previous CVD, face an increased future risk of developing CVD, which in turn elevates their risk of CVM.
We discovered a different relationship between ABI and CVM among NAFLD cases with CVD in samples defined by two different panels [ 16 , 17 ]. As previously reported, CVD cases with low ABI are associated with an increased CVM risk [ 32 , 33 , 34 ], and NAFLD individuals are also associated with an increased CVM risk [ 3 , 5 , 6 ]. Therefore, it is reasonable to assume that NAFLD patients may have an increased CVM risk when they have CVD and a low ABI. This hypothesis aligns with findings in samples defined by the HSI but not in those defined by the USFLI, where no correlation was observed. The discrepancy may be attributed to the significantly smaller sample size for the CVD subgroup in the USFLI-defined samples, potentially introducing bias in the analysis. Although the correlation was not statistically significant in these cases, the observed hazard ratios suggest a trend consistent with the results from HSI-defined samples, warranting further investigation in larger cohorts.
Our analysis reveals that regardless of diabetes status, ABI is inversely associated with cardiovascular mortality in patients with NAFLD. This finding underscores the value of ABI as a predictive marker for cardiovascular mortality in NAFLD patients, even in the absence of diabetes. Low ABI is linked to conventional CVD risk factors [ 29 , 30 ], and diabetes stands as a strong indicator of cardiovascular mortality [ 35 ]. Consequently, it is not surprising that individuals with NAFLD who also suffer from diabetes and low ABI are at a substantially heightened risk for CVM. Notably, while the risk increase is more pronounced in the diabetic cohort, a low ABI also signifies elevated risk of cardiovascular mortality in non-diabetic individuals. These results consistently highlight the importance of ABI as an indicator for assessing the risk of cardiovascular mortality in NAFLD patients, irrespective of their diabetic status.
Furthermore, our research distinguished the impact of low ABI on CVM from all-cause mortality among NAFLD patients. Given that the primary cause of death in NAFLD patients is CVD, and ABI serves as an indicator of cardiovascular risk, it is logical to surmise a correlation between ABI and CVM in NAFLD. However, the predictive value of ABI for all-cause mortality appears to be limited. While low ABI has been associated with all-cause mortality in certain populations in some studies, these findings are not universally representative [ 36 , 37 ]. Our research did not confirm a significant link between ABI and all-cause mortality in the NAFLD cohort, a discrepancy that could be attributed to in the study populations. Additionally, causes of death in NAFLD patients extend beyond CVD to include liver-related and cancer-related fatalities [ 18 ]. Presently, there is no direct evidence to suggest an association between ABI and these causes of death. This lack of association might also explain why our study did not find a correlation between low ABI and all-cause mortality.
Interestingly, the study by Ciardullo et al. also utilized the NHANES database to explore the relationship between ABI and NAFLD mortality [ 38 ]. While their findings regarding CVM were consistent with ours, they contrasted with our results on all-cause mortality. This discrepancy could be attributed to the distinct diagnostic models for NAFLD, differences in covariates, and the differing methods used for group categorization. Ciardullo et al. used a single non-invasive model, the fatty liver index, to define NAFLD, whereas our study enhanced robustness by utilizing two different non-invasive models, HSI and USFLI [ 16 , 17 , 38 , 39 ]. In survival analysis, the model we constructed included covariates that differed from theirs, with the most significant difference being our inclusion of LDL-cholesterol, a well-known and influential risk factor in CVM [ 40 ]. Additionally, our approach to ABI categorization was more detailed. We divided NAFLD patients into more refined groups based on ABI ranges and explored dose–response relationships to determine if there were any inflection points. Ciardullo et al. conducted their analysis on the overall NAFLD population, whereas our study additionally performed stratified analyses based on the presence of diabetes or CVD [ 38 ]. Our results independently linked low ABI with an increased risk of CVM in NAFLD patients without previous CVD or diabetes. Considering that CVD and diabetes are significant risk factors for CVM, the risks associated with CVM are often underestimated in patients without these conditions. Thus, our findings highlight the predictive value of ABI for CVM in NAFLD patients without pre-existing CVD or diabetes. Moreover, through dose–response curves, we further explored the relationship between ABI levels and CVM, confirming a linear association between ABI and CVM in NAFLD patients. While the study by Ciardullo et al. primarily focused on the relationship between peripheral arterial disease (indicated by ABI < 0.90) and mortality in NAFLD patients, our research not only considers ABI as a categorical variable, but also analyzes it as a linear variable to more comprehensively evaluate its potential value in stratifying CVM risk among NAFLD patients [ 38 ].
ABI offers several advantages as a tool for predicting cardiovascular risk. It can be measured quickly by properly trained professionals within primary healthcare facilities, and it is a non-invasive examination [ 7 , 41 ]. Our research indicates that low ABI is associated with an increased risk of CVM in patients with NAFLD. Therefore, ABI screening can be utilized as a valuable tool within primary healthcare settings to identify NAFLD patients at high risk for CVM. This approach may facilitate early intervention, potentially improving health outcomes for this population group.
Our research has some advantages. The data in this study have been tracked for more than ten years, and the non-institutional complex sampling stratified design is used in this study, which can better represent the general population in the United States. Second, the collection of biochemical and questionnaire data was conducted by trained personnel in a standardized and homogeneous manner. Third, we performed sensitivity analysis and adjusted many potential covariates to make the results more credible. However, there are some limitations to this work that should be mentioned. Since the NAFLD diagnosis is based on a non-invasive model that has not been validated by histology, the accuracy of the NAFLD diagnosis was limited. These two models, on the other hand, have been validated by ultrasound in a large population and are reliable non-invasive models [ 16 , 17 ]. Second, due to the limitation of data on liver-related causes of death, we are unable to assess the associations between low ABI and liver-related mortality. Third, due to the small sample size, we were unable to further stratify the analysis by the degree of ABI reduction and further investigate the relationship between ABI in different ranges and cardiovascular mortality.
In conclusion, low ABI is independently associated with an increased risk of cardiovascular mortality in individuals with NAFLD. This correlation remains significant even in the absence of pre-existing cardiovascular disease or diabetes. However, ABI is unrelated to all-cause mortality. Routine ABI screening in patients with NAFLD may help in early identification of individuals at high risk of cardiovascular mortality, potentially enabling earlier intervention for these individuals. Nevertheless, more evidence is required to support this approach.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm .
Abbreviations
- Ankle–brachial index
- Nonalcoholic fatty liver disease
Hazard ratio
Confidence interval
Cardiovascular mortality
Systolic blood pressure
Cardiovascular disease
National Health and Nutrition Examination Survey
Hepatic steatosis index
US fatty liver index
Diastolic blood pressure
International Classification of Diseases
Metabolic equivalent-minute
Moderate-to-vigorous physical activity
Physical activity
National Death Index
Body mass index
Triglyceride
High density lipoprotein
Low density lipoprotein
Total cholesterol
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Article PubMed Google Scholar
Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, Henry L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–8.
Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–8.
Fujii H, Iwaki M, Hayashi H, Toyoda H, Oeda S, Hyogo H, Kawanaka M, Morishita A, Munekage K, Kawata K, et al. Clinical outcomes in biopsy-proven nonalcoholic fatty liver disease patients: a multicenter registry-based cohort study. Clin Gastroenterol Hepatol. 2023;21(2):370–9.
Article CAS PubMed Google Scholar
Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12.
Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67(5):1726–36.
Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909.
Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208.
Adragao T, Pires A, Branco P, Castro R, Oliveira A, Nogueira C, Bordalo J, Curto JD, Prata MM. Ankle–brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transp. 2012;27(1):318–25.
Article Google Scholar
Hanssen NM, Huijberts MS, Schalkwijk CG, Nijpels G, Dekker JM, Stehouwer CD. Associations between the ankle-brachial index and cardiovascular and all-cause mortality are similar in individuals without and with type 2 diabetes: nineteen-year follow-up of a population-based cohort study. Diabetes Care. 2012;35(8):1731–5.
Article CAS PubMed PubMed Central Google Scholar
Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–69.
National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm . Accessed Dec 2021
National Health and Nutrition Examination Survey, Lower Extremity Disease Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/1999-2000/manuals/le.pdf . Accessed Dec 2021
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73.
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Digest Liver Dis. 2010;42(7):503–8.
Article CAS Google Scholar
Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41(1):65–76.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808.
Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, Avezum A, Bengtsson Boström K, Chifamba J, Gulec S, et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Health. 2019;7(6):e748–60.
Kim D, Murag S, Cholankeril G, Cheung A, Harrison SA, Younossi ZM, Ahmed A. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19(6):1240-1247.e1245.
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-s28.
Google Scholar
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
International Physical Activity Questionnaire. https://sites.google.com/site/theipaq/questionnaire_links . Accessed Dec 2021
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
National Center for Health Statistics. 2015 Public-Use Linked Mortality Files. https://www.cdc.gov/nchs/data-linkage/mortality-public.htm . Accessed December 2021
National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/99-10-analytic-guidelines.pdf . Accessed Dec 2021
Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33(10):1190–200.
Stone K, Fryer S, Faulkner J, Meyer ML, Heffernan K, Kucharska-Newton A, Zieff G, Paterson C, Matsushita K, Hughes TM, et al. Associations of lower-limb atherosclerosis and arteriosclerosis with cardiovascular risk factors and disease in older adults: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2022;340:53–60.
Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–43.
O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113(3):388–93.
Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88(3):837–45.
Agnelli G, Cimminiello C, Meneghetti G, Urbinati S. Low ankle-brachial index predicts an adverse 1-year outcome after acute coronary and cerebrovascular events. J Thromb Haemostasis. 2006;4(12):2599–606.
Busch MA, Lutz K, Röhl JE, Neuner B, Masuhr F. Low ankle-brachial index predicts cardiovascular risk after acute ischemic stroke or transient ischemic attack. Stroke. 2009;40(12):3700–5.
Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, Barker L. Trends in death rates among US adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35(6):1252–7.
Article PubMed PubMed Central Google Scholar
Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–61.
Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109(6):733–9.
Ciardullo S, Bianconi E, Cannistraci R, Parmeggiani P, Marone EM, Perseghin G. Peripheral artery disease and all-cause and cardiovascular mortality in patients with NAFLD. J Endocrinol Invest. 2022;45(8):1547–53.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Abdullah SM, Defina LF, Leonard D, Barlow CE, Radford NB, Willis BL, Rohatgi A, McGuire DK, de Lemos JA, Grundy SM, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138(21):2315–25.
Mohler ER 3rd, Treat-Jacobson D, Reilly MP, Cunningham KE, Miani M, Criqui MH, Hiatt WR, Hirsch AT. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;9(4):253–60.
Download references
Acknowledgements
We would like to thank the staff of the National Health and Nutrition Examination Survey for their contributions.
There is no funding for this study.
Author information
Authors and affiliations.
Department of Geriatric Endocrinology and Metabolism, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Nanning, 530021, Guangxi, China
Guang Xiong, Liuqing Guo & Min Liang
Department of Gastroenterology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, China
You can also search for this author in PubMed Google Scholar
Contributions
G.X. and M.L had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: G.X. , L.G. and M.L. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: G.X. , M.L. Critical review of the manuscript for important intellectual content: All authors. Statistical analysis: G.X., L.L, and L.G. Study supervision: M.L. All authors reviewed the manuscript.
Corresponding author
Correspondence to Min Liang .
Ethics declarations
Ethics approval and consent to participate.
The studies involving human participants were reviewed and approved by National Center for Health Statistics. The participants provided their written informed consent to participate in this study.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
40001_2024_1878_moesm1_esm.jpg.
Supplementary Material 1. Supplementary Fig. 1. Flow diagram of participants defined by US fatty liver index in the study.
40001_2024_1878_MOESM2_ESM.jpg
Supplementary Material 2. Supplementary Fig. 2. Association between per 0.1 ABI and cardiovascular mortality among patients with NAFLD defined by US fatty liver index. The red sold line represents the estimated hazard ratios, and the red shaded area represents the 95% confidence intervals. The restricted cubic spline function was adjusted for age, gender, ethnicity, education level, marital status, Family income-to-poverty ratio, smoking status, waist circumference, hypertension, diabetes, cardiovascular disease and physical activity, high density lipoprotein-cholesterol, low density lipoprotein-cholesterol, triglyceride, fasting blood glucose and fasting insulin. ABI was converted into per 0.1 ABI after an increase of 10 times. ABI: ankle–brachial index; NAFLD, nonalcoholic fatty liver disease.
Supplementary Material 3. Supplementary Table 1. Characteristics of Study Population defined by US fatty liver index.
40001_2024_1878_moesm4_esm.docx.
Supplementary Material 4. Supplementary Table 2. Multivariate Hazard Ratio for Mortality based on the ABI among Individuals with NAFLD defined by US Fatty Liver Index.
40001_2024_1878_MOESM5_ESM.docx
Supplementary Material 5. Supplementary Table 3. Multivariate Hazard Ratio for Mortality based on the ABI among Individuals with NAFLD defined by US Fatty Liver Index (Stratified by the presence of baseline cardiovascular disease).
40001_2024_1878_MOESM6_ESM.docx
Supplementary Material 6. Supplementary Table 4. Multivariate Hazard Ratio for Mortality based on the ABI among Individuals with NAFLD defined by US Fatty Liver Index (Stratified by the presence of baseline diabetes).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xiong, G., Guo, L., Li, L. et al. Low ankle–brachial index is associated with higher cardiovascular mortality in individuals with nonalcoholic fatty liver disease. Eur J Med Res 29 , 276 (2024). https://doi.org/10.1186/s40001-024-01878-5
Download citation
Received : 02 February 2024
Accepted : 01 May 2024
Published : 09 May 2024
DOI : https://doi.org/10.1186/s40001-024-01878-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Type 1 diabetes articles from across Nature Portfolio
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads to the inability to regulate blood sugar levels. Patients are usually treated by insulin-replacement therapy.
Latest Research and Reviews
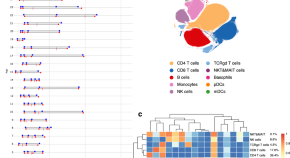
Distinct cellular immune responses in children en route to type 1 diabetes with different first-appearing autoantibodies
Previous studies have reported heterogeneity in the progression to clinical type 1 diabetes in children who develop either insulin- or glutamic acid decarboxylase-specific antibodies as their first autoantibodies. Here, the authors show that children who later develop disease have distinct characteristics in early immune responses, which are dependent on the type of autoantibodies that appear first.
- Inna Starskaia
- Milla Valta
- Riitta Lahesmaa
Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes
In a prospective study, a team-based approach combining continuous glucose monitoring with a technology-assisted remote patient monitoring program improved glycemia in a diverse cohort of children, adolescents and young adults with newly diagnosed type 1 diabetes.
- Priya Prahalad
- David Scheinker
- David M. Maahs
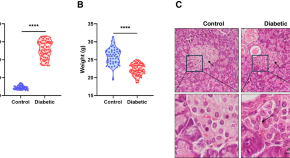
Nicotinamide Mononucleotide improves oocyte maturation of mice with type 1 diabetes
- Fucheng Guo
- Xiaoling Zhang
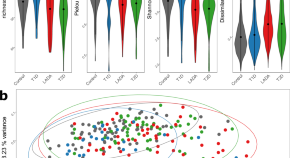
Characterization of the gut bacterial and viral microbiota in latent autoimmune diabetes in adults
- Casper S. Poulsen
- Mette K. Andersen
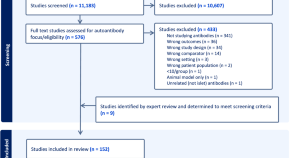
Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review
Felton et al. conduct a systematic review to determine the utility of islet autoantibodies as biomarkers of type 1 diabetes heterogeneity. They find that islet autoantibodies are most likely to be useful for patient stratification prior to clinical diagnosis.
- Jamie L. Felton
- Maria J. Redondo
- Paul W. Franks
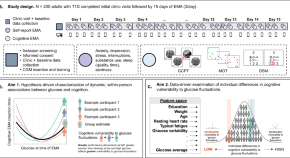
Dynamic associations between glucose and ecological momentary cognition in Type 1 Diabetes
- Z. W. Hawks
- L. T. Germine
News and Comment
Reply to ‘slowly progressive insulin dependent diabetes mellitus in type 1 diabetes endotype 2’.
- Noel G. Morgan
Slowly progressive insulin-dependent diabetes mellitus in type 1 diabetes endotype 2
- Tetsuro Kobayashi
- Takashi Kadowaki
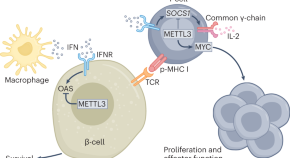
METTL3 restrains autoimmunity in β-cells
Activation of innate immunity has been linked to the progression of type 1 diabetes. A study now shows that overexpression of METTL3, a writer protein of the m 6 A machinery that modifies mRNA, restrains interferon-stimulated genes when expressed in pancreatic β-cells, identifying it as a promising therapeutic target.
- Balasubramanian Krishnamurthy
- Helen E. Thomas
Type 1 diabetes mellitus: a brave new world
One hundred years after the Nobel prize was bestowed on Banting and McLeod for the ‘discovery’ of insulin, we are again seeing major evolutions in the management of type 1 diabetes mellitus, with the prospect of achieving disease control beyond mere management now becoming real. Here, we discuss the latest, most notable developments.
- Pieter-Jan Martens
- Chantal Mathieu

β-cells protected from T1DM by early senescence programme
- Olivia Tysoe
Antivirals in the treatment of new-onset T1DM
- Claire Greenhill
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Recent Advances. ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it.
Documenting current trends in diabetes treatment and risk-factor control may inform public health policy and planning. We conducted a cross-sectional analysis of data from adults with diabetes in t...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Diabetes articles from across Nature Portfolio. Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing ...
Making insulin-resistant cells more sensitive to insulin is another goal of novel therapeutics for diabetes. In preclinical research, insulin sensitivity has been improved with gene therapy ...
Diabetes remains a substantial public health issue. Type 2 diabetes, which makes up the bulk of diabetes cases, is largely preventable and, in some cases, potentially reversible if identified and managed early in the disease course. However, all evidence indicates that diabetes prevalence is increasing worldwide, primarily due to a rise in obesity caused by multiple factors. Preventing and ...
The American Diabetes Association (ADA) is the nation's leading voluntary health organization fighting to bend the curve on the diabetes epidemic and help people living with diabetes thrive. For 83 years, the ADA has driven discovery and research to treat, manage, and prevent diabetes while working relentlessly for a cure.
This core challenge, of recognizing the limitations of the current models of care, is poignantly captured by this reflection on patients' real-world experience of living with type 2 diabetes in ...
Current Diabetes Reports provides in-depth review articles contributed by international experts on the most significant developments in the field. By presenting clear, insightful, balanced reviews that emphasize recently published papers of major importance, the journal elucidates current and emerging approaches to the diagnosis, treatment, management, and prevention of diabetes.
The Diabetes Research Institute houses teams of scientists, engineers, and clinicians with the expertise required to tackle diabetes from many angles. This integration of medicine and technology drives the vision behind the DRI strategy, a comprehensive, multidisciplinary approach to cure diabetes. The strategy builds upon decades of cure ...
Nanotechnology in diabetes research has played several roles in improving the outcome of diabetic management in diabetics through the deployment of novel nanotechnology-based glucose measurement and insulin ... The current advancement in technology in induced pluripotent stem cell research is to allow the use of ones' stem cells for ...
Current Approaches for Diabetes Management: What Are We Missing? ... of diabetes and their complex interplays with genetics and gut environment is a crucial factor that warrants further research in the development of more efficient and individualized therapy approaches for disease treatment. The use of multidrug combination therapy in diabetes ...
Diabetes Action is a nonprofit organization that supports research to prevent, treat, and cure type 1 and type 2 diabetes and their complications. Learn about the studies it is currently funding, such as BCG vaccine, gut microbiota, and sympathetic nervous system.
Journal of Diabetes Research publishes articles related to type 1 and type 2 diabetes. Topics include etiology, pathogenesis, management, and prevention of diabetes, as well as associated complications such as nephropathy. ... Data from the current study indicate that SLC25A28 overexpression promotes diet-induced obesity and accelerates lipid ...
The NIDDK supports basic, clinical, and translational research to combat diabetes and its associated complications. For example, NIDDK-supported researchers are: studying genetic and environmental factors that contribute to the development and progression of diabetes; identifying ways to improve diabetes health equity and reduce diabetes health ...
New research shows how diabetes rates in young people may rise by 2060. Read the summary. An Additional 12 Million US Adults Become Eligible for Diabetes Screening. New USPSTF and ADA guidelines lower the age for prediabetes and type 2 diabetes screening to 35. This study examined if testing practices aligned with guidelines and which ...
The research team included Hualin Zhou and Richard Premont, both from Case Western Reserve School of Medicine and University Hospitals, and students Zack Grimmett and Nicholas Venetos from the ...
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in ...
Research Projects. Print. The Division of Diabetes Translation (DDT) conducts and supports studies, often in collaboration with partners, to develop and apply sound science to reduce the burden of diabetes and to address the research needs of DDT programs and the diabetes community.
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). ... Research Open Access 27 Apr 2024 npj Digital Medicine.
June 2022 - The Diabetes Research Institute (DRI) at the University of Miami Miller School of Medicine in Miami, Florida first began performing clinical islet transplantation in 1985. Since then, our center has blossomed into one of the largest independent centers worldwide, with 57 transplant recipients totaling 101 islet infusions since the ...
The American Diabetes Association (ADA) "Standards of Care in Diabetes" includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, an interprofessional ...
A new scientific study published recently in Nature Medicine describes how the research team, led by Maahs and Priya Prahalad, MD, PhD, have implemented major advances in intensive diabetes management. First, they tackled equity issues to ensure the latest diabetes technology got into the hands of every patient as soon as they were diagnosed.
In recent years, the potential of stem cells (SCs) to differentiate into various types of cells, including β-cells, has led to a significant boost in development. The efficiency of this differentiation process and the functionality of the cells post-transplantation are crucial factors for the success of stem cell therapy in diabetes. Herein, this article reviews the current advances and ...
Cell therapy fails to slow early type 1 diabetes, but safety is established. T olerance is the holy grail in calming autoimmune disease, a truce in the immune system's faulty battle against the ...
J.P. Morgan Research forecasts that the GLP-1 market will exceed $100 bn by 2030, driven equally by diabetes and obesity usage. Total GLP-1 users in the U.S. may number 30 mn by 2030 — or around 9% of the overall population. The increasing appetite for obesity drugs will have myriad implications, boosting sectors such as biotech and creating ...
Ankle brachial index (ABI) is a risk factor for cardiovascular mortality, but it is unclear whether ABI is associated with cardiovascular mortality in patients with nonalcoholic fatty liver disease (NAFLD). The current study aimed to evaluate the association between ABI with cardiovascular and all-cause mortality in patients with NAFLD. We performed a cohort study using the data of the1999 ...
Type 1 diabetes articles from across Nature Portfolio. Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing ...