Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
Contributor Disclosures
Please read the Disclaimer at the end of this page.
TYPE 1 DIABETES OVERVIEW
Type 1 diabetes mellitus is a chronic medical condition that occurs when the pancreas, an organ in the abdomen, produces very little or no insulin ( figure 1 ). Insulin is a hormone that helps the body to use glucose for energy. Glucose is a sugar that comes, in large part, from foods we eat. Insulin allows glucose to enter cells in the body where it is needed and stores excess glucose for later use. It has other important actions as well. Without insulin, blood glucose (sugar) levels become too high, and over time, this will harm the body.
Type 1 diabetes requires regular blood sugar monitoring and treatment with insulin. Treatment, lifestyle adjustments, and self-care can control blood sugar levels and minimize the risk of disease-related complications.
Type 1 diabetes often begins in childhood or young adulthood, but it can develop at any age. In the United States, Canada, and Europe, type 1 diabetes accounts for 5 to 10 percent of all cases of diabetes.
Other topics that discuss type 1 diabetes are available:
● (See "Patient education: Type 1 diabetes: Insulin treatment (Beyond the Basics)" .)
● (See "Patient education: Glucose monitoring in diabetes (Beyond the Basics)" .)
● (See "Patient education: Type 1 diabetes and diet (Beyond the Basics)" .)
● (See "Patient education: Hypoglycemia (low blood glucose) in people with diabetes (Beyond the Basics)" .)
● (See "Patient education: Care during pregnancy for patients with type 1 or 2 diabetes (Beyond the Basics)" .)
DIABETES CARE DURING THE COVID-19 PANDEMIC
COVID-19 stands for "coronavirus disease 2019." It is an infection caused by a virus called SARS-CoV-2. The virus first appeared in late 2019 and has since spread throughout the world.
People with certain underlying health conditions, including diabetes, are at increased risk of severe illness if they get COVID-19. COVID-19 infection can also lead to severe complications of diabetes, including diabetic ketoacidosis (DKA). Getting vaccinated is the best way to lower the risk of severe illness.
THE IMPACT OF TYPE 1 DIABETES
Being diagnosed with type 1 diabetes can be a frightening and overwhelming experience, and it is common to have questions about why it developed, what it means for long-term health, and how it will affect everyday life.
For most people, the first few months after being diagnosed are filled with emotional highs and lows. You and your family can use this time to learn as much as possible so that diabetes-related care (eg, blood glucose [sugar] testing, continuous glucose monitoring, medical appointments, daily insulin, nutrition, foot care) becomes a part of your routine. (See "Patient education: Glucose monitoring in diabetes (Beyond the Basics)" and "Patient education: Preventing complications from diabetes (Beyond the Basics)" .)
In addition, you should talk with members of your diabetes health care team about resources that are available for medical as well as psychological support. This might include group classes; meetings with a nutritionist, social worker, or nurse educator; and other educational resources, such as books, websites, or magazines. Several of these resources are listed below. (See 'Where to get more information' below.)
Despite the risks associated with type 1 diabetes, most people can lead active lives and continue to enjoy the foods and activities they enjoyed before being diagnosed with diabetes. Diabetes does not mean an end to special occasion foods like birthday cake. With a little advanced planning, most people with diabetes can enjoy exercise in almost any form.
TYPE 1 DIABETES CAUSES
Type 1 diabetes usually develops when the immune system destroys the insulin-producing cells (called the beta cells) in the pancreas. This is called an autoimmune response. The cause of this abnormal immune response is being studied.
This process occurs over many months or years, and there may be no symptoms of diabetes. High blood sugar and its associated symptoms (frequent urination, thirst) do not usually occur until more than 90 percent of the cells that make insulin have been destroyed.
Type 1 diabetes can develop in people with a family history of type 1 diabetes, but it also develops in people with no family history of diabetes. In either case, people who develop diabetes have one or more genes that make them susceptible to the disease. Environmental factors and exposure to certain viruses might trigger the autoimmune response.
Close relatives (children, siblings) of a person with type 1 diabetes have an increased risk of developing type 1 diabetes, compared with a person with no family history (5 to 6 percent versus 0.4 percent, respectively). Genetic testing can help to determine if a family member is at risk of developing diabetes. However, these tests are currently only available to people who participate in a clinical research trial.
Family members at greatest risk for developing type 1 diabetes have high levels of substances called "pancreatic autoantibodies" circulating in their blood, which can indicate that the body has started to destroy its own insulin-producing cells. These blood tests are not routinely obtained, but they are measured in research programs. One such program available across the United States is called TrialNet (www.trialnet.org/our-research/risk-screening).
TYPE 1 DIABETES DIAGNOSIS
The diagnosis of diabetes is based on your symptoms and blood tests.
Symptoms — Most people with type 1 diabetes have symptoms of high blood sugar levels (hyperglycemia) at the time of diagnosis. These include:
● Excessive thirst
● Feeling tired
● Needing to urinate frequently
● Blurred vision
● Feeling hungry
● Unintentional weight loss
● Frequent yeast infections or urinary tract infections
● Slowly healing wounds
Less commonly, there are symptoms of a serious problem called diabetic ketoacidosis (DKA). People with DKA have symptoms of high blood sugar (see above), as well as nausea and vomiting, belly pain, breathing rapidly, feeling sluggish, having trouble paying attention, and sometimes coma. DKA is a medical emergency and must be treated promptly.
Blood tests — Simple, inexpensive blood tests are used to diagnose diabetes. Having a higher than normal blood sugar, as well as the symptoms described above, usually means that you have diabetes.
Type 1 versus type 2 diabetes — In youth, it is usually easy for a health care provider to tell if you have type 1 or type 2 diabetes. In this situation, your health care team will treat you as if you have type 1 while awaiting the results of further blood tests. However, with adult-onset type 1 diabetes, there are many situations where it is not clear if the person has type 1 or 2 diabetes. In this situation, additional blood testing may be ordered.
TYPE 1 DIABETES TREATMENT
Treatment of diabetes requires a team approach, including you and your family and health care providers (doctor, nurse, diabetes educator, dietitian), and sometimes other clinicians (mental health professionals, podiatrist, etc). The treatment of type 1 diabetes is discussed separately. (See "Patient education: Type 1 diabetes: Insulin treatment (Beyond the Basics)" .)
COMPLICATIONS OF TYPE 1 DIABETES
Keeping your blood sugar near normal can reduce your risk of long-term complications, such as eye and kidney diseases and problems with the nerves in different parts of your body. This means that you will need to check your blood sugar several times per day or use a continuous glucose monitoring device, give insulin shots or use an insulin pump, monitor what you eat, and see your diabetes care team on a regular basis. You should also have your eyes checked by an eye care professional and practice good foot care (see "Patient education: Foot care for people with diabetes (Beyond the Basics)" ). Your diabetes care provider will check your kidney function regularly.
People with type 1 diabetes are at increased risk of cardiovascular disease, which can cause heart attack, chest pain, stroke, and even death. (See "Patient education: Preventing complications from diabetes (Beyond the Basics)" .)
However, you can substantially lower your risk of cardiovascular disease by:
● Not smoking (see "Patient education: Quitting smoking (Beyond the Basics)" )
● Managing high blood pressure and high cholesterol with diet, exercise, and medications (see "Patient education: High cholesterol and lipids (Beyond the Basics)" and "Patient education: High blood pressure treatment in adults (Beyond the Basics)" )
● Keeping your glycated hemoglobin (A1C) at 7 percent or lower
● Taking a low-dose aspirin every day (for adults with cardiovascular disease and sometimes those who are at increased risk of cardiovascular disease)
PREGNANCY AND TYPE 1 DIABETES
Women with type 1 diabetes are usually able to become pregnant and have a healthy baby. However, it is important to carefully control blood sugar levels before and during pregnancy to minimize the risk of complications. A full discussion of this topic is available separately. (See "Patient education: Care during pregnancy for patients with type 1 or 2 diabetes (Beyond the Basics)" .)
WHERE TO GET MORE INFORMATION
Your health care provider is the best source of information for questions and concerns related to your medical problem.
This article will be updated as needed on our website ( www.uptodate.com/patients ). Related topics for patients, as well as selected articles written for health care professionals, are also available. Some of the most relevant are listed below.
Patient level information — UpToDate offers two types of patient education materials.
The Basics — The Basics patient education pieces answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials.
Patient education: Type 1 diabetes (The Basics) Patient education: Hemoglobin A1C tests (The Basics) Patient education: Preparing for pregnancy when you have diabetes (The Basics) Patient education: Diabetic ketoacidosis (The Basics) Patient education: Latent autoimmune diabetes in adults (The Basics) Patient education: Diabetes and infections (The Basics) Patient education: Foot care for people with diabetes (The Basics) Patient education: Diabetic foot ulcer (The Basics) Patient education: Checking your blood sugar at home (The Basics) Patient education: Keeping track of your blood sugar (The Basics) Patient education: Blood glucose tests (The Basics) Patient education: Kidney disease caused by diabetes (The Basics) Patient education: Friedreich ataxia (The Basics)
Beyond the Basics — Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are best for patients who want in-depth information and are comfortable with some medical jargon.
Patient education: Type 1 diabetes: Insulin treatment (Beyond the Basics) Patient education: Glucose monitoring in diabetes (Beyond the Basics) Patient education: Type 1 diabetes and diet (Beyond the Basics) Patient education: Hypoglycemia (low blood glucose) in people with diabetes (Beyond the Basics) Patient education: Care during pregnancy for patients with type 1 or 2 diabetes (Beyond the Basics) Patient education: Preventing complications from diabetes (Beyond the Basics) Patient education: Quitting smoking (Beyond the Basics) Patient education: High cholesterol and lipids (Beyond the Basics) Patient education: High blood pressure treatment in adults (Beyond the Basics)
Professional level information — Professional level articles are designed to keep doctors and other health professionals up-to-date on the latest medical findings. These articles are thorough, long, and complex, and they contain multiple references to the research on which they are based. Professional level articles are best for people who are comfortable with a lot of medical terminology and who want to read the same materials their doctors are reading.
Amylin analogs for the treatment of diabetes mellitus Glucose monitoring in the ambulatory management of nonpregnant adults with diabetes mellitus Classification of diabetes mellitus and genetic diabetic syndromes Clinical presentation, diagnosis, and initial evaluation of diabetes mellitus in adults Exercise guidance in adults with diabetes mellitus Measurements of chronic glycemia in diabetes mellitus General principles of insulin therapy in diabetes mellitus Glycemic management and vascular complications in type 1 diabetes mellitus Pregestational (preexisting) diabetes mellitus: Antenatal glycemic control Pancreatic beta cell function Management of blood glucose in adults with type 1 diabetes mellitus Hypoglycemia in adults with diabetes mellitus Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus Nutritional considerations in type 1 diabetes mellitus Diabetic kidney disease: Pathogenesis and epidemiology Overview of general medical care in nonpregnant adults with diabetes mellitus Pancreas and islet transplantation in diabetes mellitus
The following organizations also provide reliable health information.
● National Library of Medicine
( www.nlm.nih.gov/medlineplus/healthtopics.html )
● National Institute of Diabetes and Digestive and Kidney Diseases
( www.niddk.nih.gov )
● American Diabetes Association (ADA)
(800)-DIABETES (800-342-2383)
( www.diabetes.org )
● The Endocrine Society
( www.endo-society.org )
● Hormone Health Network
( www.hormone.org/diseases-and-conditions/diabetes , available in English and Spanish)
● Juvenile Diabetes Research Foundation
( www.jdrf.org )
● Glu, an online community of the T1D Exchange
( www.myglu.org/ )
● US Centers for Disease Control and Prevention
( www.cdc.gov/diabetes/basics/type1.html , available in English and Spanish)
● Type 1 Diabetes TrialNet: Pathway to Prevention
( www.trialnet.org/our-research/risk-screening )

PARITA PATEL, MD, AND ALLISON MACEROLLO, MD
Am Fam Physician. 2010;81(7):863-870
A more recent article on diabetes mellitus is available .
See related editorial on page 843 .
Author disclosure: Nothing to disclose.
Based on etiology, diabetes is classified as type 1 diabetes mellitus, type 2 diabetes mellitus, latent autoimmune diabetes, maturity-onset diabetes of youth, and miscellaneous causes. The diagnosis is based on measurement of A1C level, fasting or random blood glucose level, or oral glucose tolerance testing. Although there are conflicting guidelines, most agree that patients with hypertension or hyperlipidemia should be screened for diabetes. Diabetes risk calculators have a high negative predictive value and help define patients who are unlikely to have diabetes. Tests that may help establish the type of diabetes or the continued need for insulin include those reflective of beta cell function, such as C peptide levels, and markers of immune-mediated beta cell destruction (e.g., autoantibodies to islet cells, insulin, glutamic acid decarboxylase, tyrosine phosphatase [IA-2α and IA-2β]). Antibody testing is limited by availability, cost, and predictive value.
Prevention, timely diagnosis, and treatment are important in patients with diabetes mellitus. Many of the complications associated with diabetes, such as nephropathy, retinopathy, neuropathy, cardiovascular disease, stroke, and death, can be delayed or prevented with appropriate treatment of elevated blood pressure, lipids, and blood glucose. 1 – 4
In 1997, the American Diabetes Association (ADA) introduced an etiologically based classification system and diagnostic criteria for diabetes, 5 which were updated in 2010. 1 Type 2 diabetes accounts for approximately 90 to 95 percent of all persons with diabetes in the United States, and its prevalence is increasing in adults worldwide. 6 With the rise in childhood obesity, type 2 diabetes is increasingly being diagnosed in children and adolescents. 6
The risk of diabetes is increased in close relatives suggesting a genetic predisposition, although no direct genetic link has been identified. 7 Type 1 diabetes accounts for 5 to 10 percent of persons with diabetes 6 and is characterized by insulin deficiency that is typically an autoimmune-mediated condition.
Latent autoimmune diabetes in adults includes a heterogenous group of conditions that are phenotypically similar to type 2 diabetes, but patients have autoantibodies that are common with type 1 diabetes. Diagnostic criteria include age of 30 years or older; no insulin treatment for six months after diagnosis; and presence of autoantibodies to glutamic acid decarboxylase, islet cells, tyrosine phosphatase (IA-2α and IA-2β), or insulin.
Patients with maturity-onset diabetes of youth typically present before 25 years of age, have only impaired insulin secretion, and have a monogenetic defect that leads to an autosomal dominant inheritance pattern. These patients are placed in a subcategory of having genetic defects of beta cell. 8
The old terminology of prediabetes has now been replaced with “categories of increased risk for diabetes.” This includes persons with impaired fasting glucose, impaired glucose tolerance, or an A1C level of 5.7 to 6.4 percent. 1 , 9 , 10
Diagnostic Criteria and Testing
The 1997 ADA consensus guidelines lowered the blood glucose thresholds for the diagnosis of diabetes. 5 This increased the number of patients diagnosed at an earlier stage, although no studies have demonstrated a reduction in long-term complications. Data suggest that as many as 5.7 million persons in the United States have undiagnosed diabetes. 6 Table 1 compares specific diagnostic tests for diabetes. 11 – 14
TESTS TO DIAGNOSE DIABETES
Blood Glucose Measurements . The diagnosis of diabetes is based on one of three methods of blood glucose measurement ( Table 2 ) . 1 Diabetes can be diagnosed if the patient has a fasting blood glucose level of 126 mg per dL (7.0 mmol per L) or greater on two separate occasions. The limitations of this test include the need for an eight-hour fast before the blood draw, a 12 to 15 percent day-to-day variance in fasting blood glucose values, and a slightly lower sensitivity for predicting microvascular complications. 15 , 16
Diabetes can also be diagnosed with a random blood glucose level of 200 mg per dL (11.1 mmol per L) or greater if classic symptoms of diabetes (e.g., polyuria, polydipsia, weight loss, blurred vision, fatigue) are present. Lower random blood glucose values (140 to 180 mg per dL [7.8 to 10.0 mmol per L]) have a fairly high specificity of 92 to 98 percent; therefore, patients with these values should undergo more definitive testing. A low sensitivity of 39 to 55 percent limits the use of random blood glucose testing. 15
The oral glucose tolerance test is considered a first-line diagnostic test. Limitations include poor reproducibility and patient compliance because an eight-hour fast is needed before the 75-g glucose load, which is followed two hours later by a blood draw. 17 The criterion for diabetes is a serum blood glucose level of greater than 199 mg per dL (11.0 mmol per L).
In 2003, the ADA lowered the threshold for diagnosis of impaired fasting glucose to include a fasting glucose level between 100 and 125 mg per dL (5.6 and 6.9 mmol per L). Impaired glucose tolerance continues to be defined as a blood glucose level between 140 and 199 mg per dL (7.8 and 11.0 mmol per L) two hours after a 75-g load. Patients meeting either of these criteria are at significantly higher risk of progression to diabetes and should be counseled on effective strategies to lower their risk, such as weight loss and exercise. 1 , 9
A1C . A1C measurement has recently been endorsed by the ADA as a diagnostic and screening tool for diabetes. 1 One advantage of using A1C measurement is the ease of testing because it does not require fasting. An A1C level of greater than 6.5 percent on two separate occasions is considered diagnostic of diabetes. 18 Lack of standardization has historically deterred its use, but this test is now widely standardized in the United States. 19 A1C measurements for diagnosis of diabetes should be performed by a clinical laboratory because of the lack of standardization of point-of-care testing. Limitations of A1C testing include low sensitivity, possible racial disparities, and interference by anemia and some medications. 15
TESTS TO IDENTIFY TYPE OF DIABETES
Tests that can be used to establish the etiology of diabetes include those reflective of beta cell function (e.g., C peptide) and markers of immune-mediated beta cell destruction (e.g., insulin, islet cell, glutamic acid decarboxylase, IA-2α and IA-2β autoantibodies). Table 3 presents the characteristics of these tests. 20 – 27
C peptide is linked to insulin to form proinsulin and reflects the amount of endogenous insulin. Patients with type 1 diabetes have low C peptide levels because of low levels of endogenous insulin and beta cell function. Patients with type 2 diabetes typically have normal to high levels of C peptide, reflecting higher amounts of insulin but relative insensitivity to it. In a Swedish study of patients with clinically well-defined type 1 or 2 diabetes, 96 percent of patients with type 2 diabetes had random C peptide levels greater than 1.51 ng per mL (0.50 nmol per L), whereas 90 percent of patients with type 1 diabetes had values less than 1.51 ng per mL. 20 In the clinically undefined population, which is the group in which the test is most often used, the predictive value is likely lower.
Antibody testing is limited by availability, cost, and predictive value, especially in black and Asian patients. Prevalence of any antibody in white patients with type 1 diabetes is 85 to 90 percent, 5 whereas the prevalence in similar black or Hispanic patients is lower (19 percent in both groups in one study). 28 In persons with type 2 diabetes, the prevalence of islet cell antibody is 4 to 21 percent; glutamic acid decarboxylase antibody, 7 to 34 percent; IA-2, 1 to 2 percent; and any antibody, 11.6 percent. 24 , 25 , 29 In healthy persons, the prevalence of any antibody marker is 1 to 2 percent 30 ; thus, overlap of the presence of antibodies in various types of diabetes and patients limits the utility of individual tests.
As with any condition, a rationale for screening should first be established. Diabetes is a common disease that is associated with significant morbidity and mortality. It has an asymptomatic stage that may be present for up to seven years before diagnosis. The disease is treatable, and testing is acceptable and accessible to patients. Early treatment of diabetes that was identified primarily by symptoms improves microvascular outcomes. 31 However, it is not clear whether universal screening reduces diabetes-associated morbidity and mortality. Table 4 presents screening guidelines from several organizations. 1 , 8 , 32 – 38
TYPE 1 DIABETES
Screening for type 1 diabetes is not recommended because there is no accepted treatment for patients who are diagnosed in the asymptomatic phase. The Diabetes Prevention Trial identified a group of high-risk patients based on family history and positivity to islet cell antibodies. However, treatment did not prevent progression to type 1 diabetes in these patients. 39
TYPE 2 DIABETES
Medications and lifestyle interventions may reduce the risk of diabetes, although 20 to 30 percent of patients with type 2 diabetes already have complications at the time of presentation. 40 Although a recent analysis suggests that screening for and treating impaired glucose tolerance in persons at risk of diabetes may be cost-effective, the data on screening for type 2 diabetes are less certain. 41 It is unclear whether the early diagnosis of type 2 diabetes through screening programs, with subsequent intensive interventions, provides an incremental benefit in final health outcomes compared with initiating treatment after clinical diagnosis.
Guidelines differ regarding who should be screened for type 2 diabetes. The U.S. Preventive Services Task Force (USPSTF) recommends limiting screening to adults with a sustained blood pressure of greater than 135/80 mm Hg. 34 , 42 The American Academy of Family Physicians concurs, but specifically includes treated and untreated patients. 43 The Canadian Task Force on Preventive Health Care recommends screening all patients with hypertension or hyperlipidemia. 33 The ADA recommends screening a much broader patient population based on risk. 1
There are several questionnaires to predict a patient's risk of diabetes. The Diabetes Risk Calculator was developed using data from the National Health and Nutrition Examination Survey III and incorporates age, height, weight, waist circumference, ethnicity, blood pressure, exercise, history of gestational diabetes, and family history. 13 , 14 For diagnosis of diabetes, it has a positive predictive value (PPV) of 14 percent and a negative predictive value (NPV) of 99.3 percent. The tool is most valuable in helping define which patients are very unlikely to have diabetes. 13
GESTATIONAL DIABETES
Whether patients should be screened for gestational diabetes is unclear. The USPSTF states that there is insufficient evidence to recommend for or against screening. 34 The ADA and the American College of Obstetricians and Gynecologists recommend risk-based testing, although most women require testing based on these inclusive guidelines. 36 The Glucola test is the most commonly used screening test for gestational diabetes and includes glucose testing one hour after a 50-g oral glucose load. An abnormal Glucola test result (i.e., blood glucose level of 140 mg per dL or greater) should be confirmed with a 75-g or 100-g oral glucose tolerance test. Whether screening and subsequent treatment of gestational diabetes alter clinically important perinatal outcomes is unclear. Untreated gestational diabetes is associated with a higher incidence of macrosomia and shoulder dystocia. 44 A randomized controlled trial found that treatment led to a reduction in serious perinatal complications, with a number needed to treat of 34. Treatment did not reduce risk of cesarean delivery or admission to the neonatal intensive care unit, however. 44
New-Onset Symptomatic Hyperglycemia
Patients may initially present with diabetic ketoacidosis or hyperglycemic hyperosmolar state ( Table 5 ) , 45 both of which are initially managed with insulin because they are essentially insulin deficiency states. Both groups of patients may present with polyuria, polydipsia, and signs of dehydration. Diagnostic criteria of diabetic ketoacidosis include a blood glucose level greater than 250 mg per dL (13.9 mmol per L), pH of 7.3 or less, serum bicarbonate level less than 18 mEq per L (18 mmol per L), and moderate ketonemia. However, significant ketosis has also been shown to occur in up to one third of patients with hyperglycemic hyperosmolar state. 46
Although diabetic ketoacidosis typically occurs in persons with type 1 diabetes, more than one half of newly diagnosed black patients with unprovoked diabetic ketoacidosis are obese and many display classic features of type 2 diabetes—most importantly with a measurable insulin reserve. 47 Thus, the presentation does not definitively determine the type of diabetes a patient has. Presence of antibodies, particularly glutamic acid decarboxylase antibody, predicts a higher likelihood of lifelong insulin requirement. There is, however, an overlap of presence of antibodies in type 1 and type 2 diabetes, and among patients with type 2 diabetes who may not require insulin. 48
A Swedish population-based study showed that among the 9.3 percent of young adults with newly diagnosed diabetes that could not be classified as type 1 or type 2, the presence of glutamic acid decarboxylase antibody was associated with a need for insulin within three years (odds ratio = 18.8; 95% confidence interval, 1.8 to 191). 26 The PPV for insulin treatment was 92 percent in those with the antibody. It should be noted that among patients who were negative for antibodies, 51 percent also needed insulin within three years. In contrast, the United Kingdom Prospective Diabetes Study found that only 5.7 percent of patients without glutamic acid decarboxylase antibody eventually needed insulin therapy, giving the test an NPV of 94 percent. 25 With these conflicting data, clinical judgment using a patient's phenotype, history, presentation, and selective laboratory testing is the best way to manage patients with diabetes.
American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [published correction appears in Lancet . 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183-1197.
Centers for Disease Control and Prevention. 2007 national diabetes fact sheet. http://www.cdc.gov/diabetes/pubs/factsheet07.htm. Accessed July 8, 2009.
Tuomi T. Type 1 and type 2 diabetes: what do they have in common?. Diabetes. 2005;54(suppl 2):S40-S45.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69.
Knowler WC, Barrett-Connor E, Fowler SE, et al.; for the Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
Tuomilehto J, Lindström J, Eriksson JG, et al.; for the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350.
Tabaei BP, Herman WH. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care. 2002;25(11):1999-2003.
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275-278.
Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31(5):1040-1045.
Mochan E, Ebell M. Risk-assessment tools for detecting undiagnosed diabetes. Am Fam Physician. 2009;80(2):175-178.
Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93(7):2447-2453.
Petersen PH, Jørgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and HbA1C for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl. 2005;240:51-60.
Ko GT, Chan JC, Woo J, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998;35(pt 1):62-67.
International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334.
Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE for the NGSP Steering Committee. The National Glycohemoglobin Standardization Program: a five-year progress report. Clin Chem. 2001;47(11):1985-1992.
Berger B, Stenström G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest. 2000;60(8):687-693.
Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436-472.
Sabbah E, Savola K, Kulmala P, et al. Diabetes-associated autoanti-bodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. The Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1999;84(5):1534-1539.
Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48(1):150-157.
Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group [published correction appears in Lancet . 1998;351(9099):376]. Lancet. 1997;350(9087):1288-1293.
Bottazzo GF, Bosi E, Cull CA, et al. IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71) [published correction appears in Diabetologia . 2005;48(6):1240]. Diabetologia. 2005;48(4):703-708.
Törn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as type 1 diabetes on clinical grounds. Diabetes Metab Res Rev. 2000;16(6):442-447.
Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies—a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood Diabetes in Finland Study Group. Diabetologia. 1998;41(4):424-429.
Avilés-Santa L, Maclaren N, Raskin P. The relationship between immune-mediated type 1 diabetes mellitus and ethnicity. J Diabetes Complications. 2004;18(1):1-9.
Davis TM, Wright AD, Mehta ZM, et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia. 2005;48(4):695-702.
Maclaren N, Lan M, Coutant R, et al. Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2beta predict immune-mediated (type 1) diabetes in relatives. J Autoimmun. 1999;12(4):279-287.
Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815-819.
American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the management of diabetes mellitus. http://www.aace.com/pub/pdf/guidelines/DMGuidelines2007.pdf .Accessed July 8, 2009.
Feig DS, Palda VA, Lipscombe L. Screening for type 2 diabetes mellitus to prevent vascular complications: updated recommendations from the Canadian Task Force on Preventive Health Care. CMAJ. 2005;172(2):177-180.
U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults. Recommendation statement. June 2008. http://www.ahrq.gov/clinic/uspstf08/type2/type2rs.htm#clinical . Accessed July 8, 2009.
Committee on Obstetric Practice. ACOG Committee Opinion No. 435: postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol. 2009;113(6):1419-1421.
American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces technical bulletin number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98(3):525-538.
Canadian Task Force on Preventive Health Care. Summary table of recommendations. Sreening for gestational diabetes mellitus. http://www.ctfphc.org/Tables/Ch02tab.htm . Accessed January 18, 2010.
U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus. Recommendation statement. May 2008. http://www.ahrq.gov/clinic/uspstf08/gestdiab/gdrs.htm . Accessed January 18, 2010.
Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685-1691.
Glucose tolerance and mortality: comparison of WHO American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617-621.
Gillies CL, Lambert PC, Abrams KR, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ. 2008;336(7654):1180-1185.
Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement [published correction appears in Ann Intern Med . 2008;149(2):147]. Ann Intern Med. 2008;148(11):846-854.
American Academy of Family Physicians. Recommendations for clinical preventive services. https://www.aafp.org/patient-care/clinical-recommendations/a-z.html . Accessed July 8, 2009.
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486.
Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectrum. 2002;15(1):28-36.
Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
Palmer JP, Hampe CS, Chiu H, Goel A, Brooks-Worrell BM. Is latent auto-immune diabetes in adults distinct from type 1 diabetes or just type 1 diabetes at an older age?. Diabetes. 2005;54(suppl 2):S62-S67.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2010 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Pathophysiology and Clinical Presentation
Pathophysiology:
Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). Nonimmune (type 1B diabetes), occurs secondary to other diseases and is much less common than autoimmune (type 1A). The destruction of beta cells in Type 1A diabetes results from the interaction of both genetic and environmental factors. Although the genetic susceptibility is not well understood, type 1 diabetes is most strongly associated with major histocompatibility complex (MHC), specifically histocompatibility leukocyte antigen (HLA) class II alleles (HLA-DQ and HLA-DR) (McCance & Heuther, 2014). Type 1 diabetes is less hereditary than type 2 but 7-13% of patients also have a first degree relative with type 1 diabetes (Mapes & Faulds, 2014). Environmental factors include viral infections (especially enteroviruses), exposure to infectious microorganisms (such as Helicobacter pylori ), exposure to cow’s milk proteins and a lack of vitamin D (McCance & Heuther, 2014).
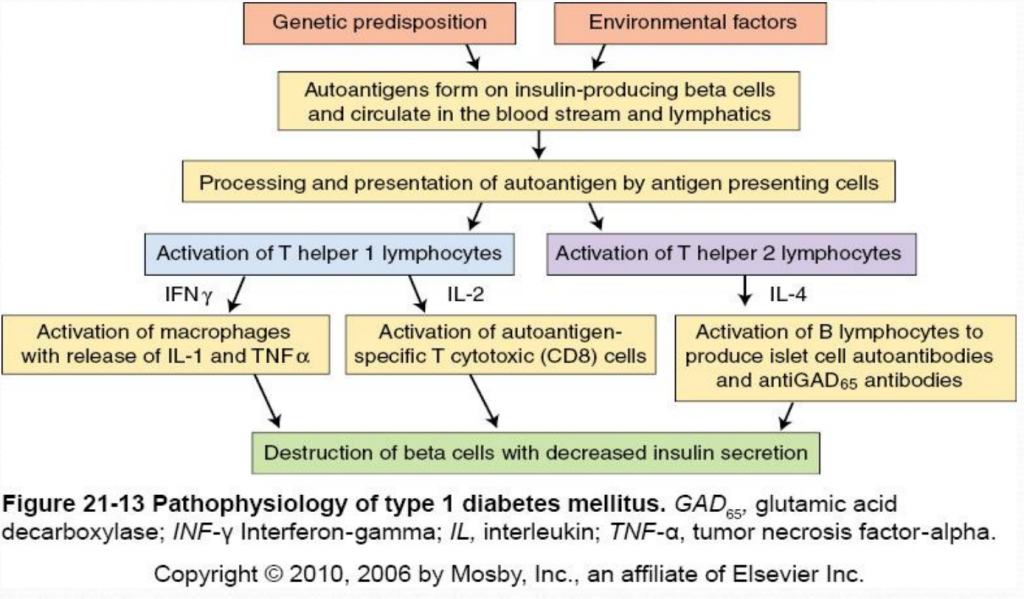
The destruction of insulin-producing beta cells in the pancreas starts with the formation of autoantigens. These autoantigens are ingested by antigen-presenting cells which activate T helper 1 (Th1) and T helper 2 (Th2) lmphocytes. Activated Th1 lymphocytes secrete interluekin-2 (IL-2) and interferon. IL-2 activates autoantigen-specific T cytotoxic lymphocytes which destroy islet cells through the secretion of toxic perforins and granzymes. Interferon activates macrophages and stimulates the release of inflammatory cytokines (including IL-1 and tumor necrosis factor [TNF]) which further destroy beta cells (McCance & Heuther, 2014). Activated Th2 lymphocytes produce IL-4 which stimulates B lymphocytes to proliferate and produce islet cell autoantibodies (ICAs) and anti-glutamic acid decarboxylase (antiGAD65) antibodies. AntiGAD65 is an enzyme that helps control the release of insulin from beta cells and can be used to determine the cause of diabetes (McCance & Heuther, 2014). Insulin autoantibodies [IAAs]) and zinc transporter 8 (Znt8) protein are also associated with type 1 diabetes mellitus. Despite it’s complicated pathophysiology, it is important to understand the destruction of beta cells in type 1 diabetes because it leads to a lack of insulin and amylin. Without insulin or amylin the body cannot promote glucose disappearance or limit glucose appearance from the bloodstream, respectively, resulting in hyperglycemia (Mapes & Faulds, 2014).
Clinical Presentation:
Type 1 diabetes does not present clinically until 80-90% of the beta cells have been destroyed (McCance & Heuther, 2014). Because insulin stimulates glucose uptake into tissues, stores glycose as glycogen, inhibits glucagon secretion and inhibits glucose production from the liver, the destruction of insulin-producing beta cells causes hyperglycemia (Mapes & Faulds, 2014). Type 1 diabetics may present with abrupt onset of diabetic ketoacidosis, polyuria, polyphagia, polydipsia, or rapid weight loss with marked hyperglycemia (Mapes & Faulds, 2014). To diagnose diabetes, patients must have an A1C level greater than 6.5% percent on two separate tests; the presence of ketones in the urine and/or autoantibodies in the blood can distinguish type 1 from type 2 diabetes (Mayo Clinic, 2014).

2 thoughts on “ Pathophysiology and Clinical Presentation ”
none for now
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- UBC Directories
- UBC Quick Links
- The University of British Columbia
- a place of mind
- Learn Pediatrics
- Diabetes: Approach to First Presentation
Click for pdf: Diabetes
General presentation
Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of pancreatic beta islet cells; whereas type 2 DM is the presence of high blood glucose with insulin resistance and relative insulin deficiency. Diabetes mellitus is a chronic condition that requires long-term follow-up and adequate patient (and parent) education to maintain good glycemic control to prevent long-term complications.
Epidemiology
Approximately 2/3 of all new diabetes diagnoses in patients less than 19 years of age in the United States are type 1 DM. Over 300,000 Canadians have type 1 DM, with a 3-5% increase each year; especially in children aged 5-9. Typically, the age of onset has a bimodal distribution, with the first peak in children 4-6 years old, and the second peak in children 10-14 years old (early puberty). Unlike other autoimmune diseases, the overall incidence appears to be equal in both genders. There is a higher risk of developing this condition in children with close relatives who have type 1 DM.
The incidence of type 2 DM has increased 10 fold in the last decade. There is an estimated 3600/100,000 cases of type 2 DM in Canadian adolescents and 1100/100,000 cases in Canadian children. This value may be as high as 1% in Canadian aboriginal youths and children. There is a strong association between increasing rates of obesity in the pediatric population and the development of type 2 DM.
Basic Physiology
In type 1 DM, there is autoimmune-mediated destruction of insulin-producing pancreatic beta cells that results in insulin deficiency. It is a progressive condition that occurs in genetically susceptible individuals. The autoimmune destruction can be triggered by various environmental agents. Some proposed environmental factors include pregnancy-related and perinatal influences, viruses, cow’s milk and cereals. There is a long latency period (where the patient is asymptomatic and euglycemic) between the onset of beta cell destruction and clinical presentation of diabetes mellitus. A large number of functional beta cells must be lost before clinical symptoms like hyperglycemia occurs.
Genetic polymorphisms in six genes have been shown to be associated with type 1 DM. Major Histocompatibility Complex genes and elsewhere in the genome all contribute to the risk, but only the HLA alleles seem to have a large effect.
The natural history has four stages:
- Preclinical autoimmune destruction of pancreatic beta cells
- Onset of clinical symptoms
- Transient remission
- Established diabetes with acute and chronic complications
Type 2 DM is a complex, multifactorial disease characterized by both relative insulin deficiency and insulin resistance with various environmental and behavioural risk factors. Increased hepatic glucose production, insulin resistance and progressive loss of glucose-stimulated insulin release all contribute to the development of hyperglycemia. In Type 2 DM, pancreatic beta cells retain the ability to produce insulin, but levels are not adequate to counteract the developing insulin resistance. The current theory is that insulin resistance develops first, followed and complicated by gradual destruction of beta cells. Insulin resistance worsens with obesity and physical inactivity; and improves with weight loss and increased physical exercise.
Puberty also plays a role in the development of type 2 DM in adolescents. During this period, insulin sensitivity is approximately 30% lower than that of preadolescents or adults, which results in hyperinsulinemia as a compensatory mechanism. In adolescents with both genetic predisposition and negative environmental contributors, this period of relative insulin resistance may result in a decompensated state (inadequate insulin secretion and glucose intolerance). The resulting hyperglycemic state may cause worsening abnormalities of insulin secretion and action, starting a vicious cycle that progress beyond the adolescent years.
Clinical Presentation
Childhood type 1 DM can present in the following ways:
Classic new onset:
Hyperglycemia without acidosis
Symptoms include:
- Can present as nocturia, bedwetting, daytime incontinence in a previously continent child
- Polydipsia – due to increased serum osmolality and hypovolemia
- Impaired glucose utilization in skeletal muscle and increased fat and muscle breakdown
Diabetic ketoacidosis
Similar symptoms but are usually more severe
- Clinical: polydipsia, polyuria, dehydration, hypotension, ketosis, etc.
- Metabolic: hyperglycemia, glycosuria, metabolic acidosis, ketonemia, etc.
Reported frequency varies between 15-67%
- Young children (<6) from low socioeconomic backgrounds are more likely to present with diabetic ketoacidosis
Silent Presentation
Diagnosis before onset of clinical symptoms
Typically occurs in children with a family member with type 1 DM (close monitoring)
Childhood type 2 DM can present in the following ways:
- Hyperglycemia, ketonuria, acidosis
- Frequency varies between 5-25%
Hyperosmolar hyperglycemic state
- Marked hyperglycemia (>33.3 mmol/L) and severe dehydration but no ketonuria
- Less common in adolescents
Symptomatic
- Due to hyperglycemia and include: polyuria, polydipsia, and nocturia
- Recent weight loss is less frequent
- Adolescent girls: vaginal discharge due to candida infection may be initial presentation
Asymptomatic
- Identified based on screening (for type 2 DM or urinalysis as part of a regular physical exam)
Questions to ask
Historical Investigation
Presenting condition:
- Have you been very thirsty? Do you drink a lot?
- Have you been urinating more than usual?
- Has the child had any bedwetting episodes?
- Has there been any recent weight loss?
- Have you been feeling tired lately?
- Have you noticed an increased appetite lately?
- Has the child had more frequent minor skin infections?
Predisposing factors:
- Have you had any viral infections recently?
- What kinds of exercise do you participate in on a regular basis? How frequent do you exercise? How long do you exercise each time?
- How many hours a day do you spend watching TV, using the computer, and play video games?
- What do you normally eat? What is the portion size? How many meals do you have per day? Do you normally eat out or home cooked meals? Do you eat as a family? Do you eat at the table or in front of the tv?
Family history:
- Are there any family members with insulin-dependent diabetes mellitus?
- Are there any family members with autoimmune conditions?
- Does your mother or father have diabetes?
- Are there any other family members with diabetes? (grandparents, aunts, uncles, brothers, sisters, etc.)
Physical Examination
Do a complete physical exam with particular attention to the following:
- Assess hydration status
- Assess circulation: HR, BP, capillary refill
- Temperature: coexisting infection
- Use growth chart to check for weight loss
- Neck examination: look for thyroid abnormalities
- Respiratory: respiratory rate (hyperventilation – DKA), auscultation (respiratory infection), ketones on breath (DKA)
- Measure body weight and height, calculate BMI
- Measure lying and standing BP
- Inspect skin for acanthosisnigricans
- Examine feet to look for decreased sensation and circulation (pulses)
- Measure visual acuity
Differential diagnosis
- DM types 1 and 2
- Diabetes insipidus
- Urinary tract infection
- Malabsorption (e.g. Celiac disease)
- Secondary diabetes
- Maturity-onset diabetes of the young
Procedures for investigation
Diagnostic Criteria
- Fasting plasma glucose >7 mmol/L (no caloric intake for at least 8 hours)
- Symptoms of hyperglycemia, random venous plasma glucose >11.1 mmol/L
- Abnormal oral glucose tolerance test – plasma glucose >11.1 mmol/L measured 2 hours after a glucose load of 1.75 g/kg (max 75g)
- Glycated hemoglobin (A1C) ≥ 6.5%
Other Investigations
- Urinalysis for glucosuria and ketonuria
- Urinalysis for microalbuminemia
- Alemzadeh R, Wyatt DT. Section 6 – Diabetes mellitus in children. In: Kliegman: Nelson Textbook of Pediatrics. 18 th ed. Saunders, Pennsylvania. 2007
- Eisenbarth GS, McCulloch DK. Pathogenesis of type 1 diabetes mellitus. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Laffel L, Svoren B. Epidemiology, presentation, and diagnosis of type 2 diabetes mellitus in children and adolescents. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Levinson P, Nelson BA, Scherger JE. Diabetes mellitus type 1 in children. [Online]. 2007. Availabe from: FirstConsult, MDConsult. [cited 2011 Jan 15]
- Levitsky LL, Misra M. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- McCulloch DK, Robertson RP. Pathogenesis of type 2 diabetes mellitus. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2011
- Panagiotopoulos C. Type 2 diabetes in children and adolescents. BCMJ. 2004;46(9): 461-466
- Scherger JE, McIntire SDC, Escobar O, Heinzman DM. Diabetes mellitus type 2 in children. [Online]. 2007. Availabe from: FirstConsult, MDConsult. [cited 2011 Jan 15]
Acknowledgements
Written by: Ying Yao
Edited by: Dianna Louie
Last updated on November 10, 2011 @5:01 pm
Feedback: How useful was the above information?
Post comment click here to cancel reply..
You must be logged in to post a comment.
- Approach to the Child with a fever and rash
- Approach to Bradycardia
- Basics of cardiac pharmacology
- Approach to cardiac history taking
- Congestive heart failure in children
- Approach to Pediatric Hypertension
- Approach to Cyanotic Congenital Heart Disease in the Newborn
- Approach to Syncope: Is it Cardiac or Not?
- Approach to Pediatric Tachycardia
- Approach to Pediatric Chest Pain
- Approach to Cardiac Murmurs
- Normal Cardiac Physiology – Transition From Fetal to Neonatal
- Basic Physiology and Approach to Heart Sounds
- Approach to Pediatric ECGs
- Care of a Child with Turner Syndrome
- Approach to the Underweight Child
- Normal sexual maturity rating
- Hypothyroidism
- Approach to the Short Child
- Approach to Vomiting
- Suspected Foreign Body Ingestion
- Approach to a Mediastinal Mass
- Toxic Ingestion
- Nutritional Deficiencies
- Pharmacology of Common Agents Used in Gastrointestinal Conditions
- Approach to Pediatric Abdominal Pain
- Pediatric Gastrointestinal History Taking
- Approach to diarrhea
- Approach to a picky eater
- Constipation
- Hepatomegaly
- Splenomegaly
- Approach to Abdominal Mass
- Failure to Thrive
- Approach to Skin Lesions
- Common Paediatric Skin Conditions & Birthmarks
- Approach to the child with mental health concerns
- Child with a sore ear (Otalgia)
- To vaccinate or not to vaccinate
- Approach to a the Child with a Fever and Rash
- Approach to a Routine Adolescent Interview
- Sore Throat in Children – Clinical Considerations and Evaluation
- Approach to Strabismus
- Approach to Adolescent Substance Use
- Approach to the Child Who is Dry
- Conjunctivitis: Approach to the Child with a Red Eye
- Acne In Teens
- Pediatric Neck Mass
- Fetal Alcohol Spectrum Disorder
- To Circumcise or Not to Circumcise
- Approach to inborn errors of metabolism
- Infants of Diabetic Mothers
- Fever in the Newborn Period
- Neonatal Thrombocytopenia
- Consequences of Prematurity
- Respiratory Distress in the Newborn
- Approach to Neonatal Cyanosis
- Approach to the Child with IUGR/SGA
- Neonatal Jaundice
- Breastfeeding Problems
- Diaper Rash: Clinical Considerations and Evaluation
- The Pediatric Neurological History
- Evaluation of Pediatric Development (Normal)
- Closed Head Injury in Pediatrics
- Approach to the Ataxic Child
- Approach to the Child with a Seizure
- Basics to the Approach of Developmental Delay
- Lumbar Puncture in Pediatrics
- The Basics of Cerebral Palsy
- Approach to a Child with a Headache
- Febrile Seizures
- Approach to the Comatose Child
- Basics of Spina Bifida
- Principles of Pharmacotherapy in Neurology
- Easy Bleeding
- Non-Neonatal Jaundice
- Approach to Lymphadenopathy
- Tumor Lysis Syndrome
- Febrile Neutropenia
- Pediatric Neutropenia
- Blood Transfusion Reactions
- Approach to Sickle Cell Disease
- Iron-deficiency and Health Consequences in Children
- Easy Clotting
- Approach to Thrombocytopenia
- Approach to Pediatric Leukemias and Lymphomas
- Approach to Thalassemia
- Approach to Non-Accidental Injuries
- Common Pediatric Bone Diseases-Approach to Pathological Fractures
- Infant with an Abnormal Hip Exam
- Knock Kneed Children
- Elbow Injuries
- Approach to the Child with a Limp
- Pediatric Fractures
- Clearing the C - Spine
- Approach to a Child With a Cough
- Approach to Pediatric Hemoptysis
- Approach to Pediatric Dyspnea
- Introduction
- Systemic Exam
- Kidneys and Bladder
- Male Infants
- Female Infants
- Acute Assessment
- General exam
- Peripheral palpation
- Precordium palpation
- Auscultation
- General Inspection
- Genito-Urinary
- Musculoskeletal
- Neurological
- Mental Status Exam
- Cranial Nerve Exam
- Sensory Exam
- Cerebellar Exam
- Other Resources
- Physical Exam Setup
- Screening MSK Exam
- Focused MSK Exams
Emergency Procedures | Accessibility | Contact UBC | © Copyright The University of British Columbia
Diabetes Mellitus Clinical Presentation
During prediabetes and the early stages of diabetes, the majority of patients are asymptomatic. As a result, obtaining a diagnosis may be delayed for many years if routine screening measures for diabetes, such as laboratory work, are not performed during regular healthcare visits. Typically, patients with type 1 diabetes mellitus (T1DM) present with symptomatic hyperglycemia and sometimes with diabetic ketoacidosis (DKA). DKA is defined as an acute metabolic complication of diabetes distinguished by hyperglycemia, hyperketonemia, and metabolic acidosis. DKA occurs primarily in T1DM and is less common in T2DM; it can present with nausea, vomiting, and abdominal pain and cause cerebral edema, coma, and death. DKA may be the initial presentation in an estimated 25% of adults with T1DM. The most common symptoms associated with T1DM are polyuria, polydipsia, and polyphagia, along with lethargy, nausea, and blurred vision, all of which result from the hyperglycemia. The onset of symptoms may be abrupt. Polyuria is caused by osmotic diuresis secondary to hyperglycemia. In young children, severe nocturnal enuresis secondary to polyuria could be a warning sign of diabetes onset. In T1DM, thirst is a response to the hyperosmolar state and dehydration. The American Diabetes Association notes that adults with new-onset T1DM may present with a short duration of illness of 1 to 4 weeks or more, a gradually progressing process that can be misinterpreted as type 2 diabetes mellitus (T2DM). Patients with T1DM may also present with fatigue, weakness, and weight loss despite normal appetite. Patients with T2DM may present with symptomatic hyperglycemia but are frequently asymptomatic for diabetes, and T2DM is often discovered during routine checkups and laboratory testing. Classic symptoms of T2DM include polyuria, polydipsia, polyphagia, and weight loss. Other symptoms that may suggest hyperglycemia include blurred vision, lower-extremity paresthesia, and delayed wound healing. In some patients, initial symptoms may actually be indicative of diabetic complications (such as neuropathy, retinopathy, skin acanthosis, and recurring Candida infections), signifying that the T2DM has been present for some time. This highlights the need for routine screening, especially in high-risk patient populations. In some patients, a hyperosmolar hyperglycemic state occurs initially, particularly during a period of extreme stress or when glucose metabolism is further impaired by use of certain pharmacologic agents, such as corticosteroids. Since early diagnosis and clinical intervention are essential for preventing and/or reducing the complications associated with poorly controlled diabetes, patients should be reminded to maintain routine healthcare and seek medical care if they are experiencing any symptoms associated with hyperglycemia. The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
« Click here to return to Diabetes Awareness.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides constitutes appropriate attribution).
Permission is required from the Association for any commercial use or for reproduction in any print materials (contact [email protected] ).
Using the links to the slide deck will download the deck. You may need to check the folder for downloaded documents on your device to see/open the file.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Can Fam Physician
- v.66(6); 2020 Jun
Diabetic ketoacidosis as first presentation of type 1 diabetes mellitus in a young child
Diabetic ketoacidosis (DKA) is a life-threatening complication of hyperglycemia in patients with type 1 diabetes mellitus (T1DM). As a first presentation, overlapping signs of DKA and the respiratory infections that can trigger DKA, especially during cold and flu seasons, can complicate this diagnosis. Kussmaul breathing, characterized by tachypnea and increased depth of breath, is a classic sign of DKA; however, change in depth of breathing might not be apparent in younger children owing to their size. This case describes a toddler in respiratory distress, attending her primary care clinic with a 1-week history of upper respiratory tract symptoms. For family physicians, it is important to keep a differential diagnosis for respiratory distress in mind. If the examination is not consistent with more common presentations, then other causes should be considered.
A 22-month-old girl presented with her mother to the family doctor’s office for worsening dyspnea. Her mother described hearing a “barky” cough and reported symptoms consistent with a viral prodrome in the past week. She also had older siblings at home with upper respiratory tract infections. On examination she was tachypneic, using her accessory muscles to breathe, and was grunting. Auscultation of her chest revealed good air entry bilaterally with no adventitious sounds, and her oxygen saturation was normal. However, during the examination, despite receiving oxygen by facial mask and several doses of salbutamol, she became progressively lethargic. Therefore, emergency medical services transferred her to the emergency department with a provisional diagnosis of croup based on her initial clinical presentation and rapid clinical decline in the office. In the emergency department, results of bloodwork requested owing to her decreased level of consciousness revealed that she was profoundly hyperglycemic and she was treated according to the pediatric DKA protocol. She was admitted with a new diagnosis of T1DM and was referred for ongoing diabetes management.
Differential diagnosis
There are many differential diagnoses for Kussmaul breathing. In the context of T1DM, Kussmaul breathing is the result of metabolic acidosis. A focused differential diagnosis pertinent to the pediatric population should consider disorders from other systems ( Box 1 ).
Differential diagnoses for Kussmaul breathing in young children
- Heart failure
- Cardiogenic shock
Respiratory
- Infection—pneumonia, croup, bronchiolitis
- Uncontrolled asthma
- Upper airway obstruction (eg, foreign body)
Metabolic and endocrine
- Acidosis—MUDPILES (methanol, uremia, diabetic ketoacidosis, propylene glycol, paraldehyde, iron, isoniazid, lactic acidosis, ethylene glycol, salicylate drugs [eg, alcohol, ingested poisons, acetylsalicylic acid])
- Renal failure
- Inborn errors of metabolism
- Hyperthyroidism
Neurologic and psychological
- Intracranial pathology with increased intracranial pressure (eg, space-occupying lesion, bleeding, cerebral edema)
- Substantial head trauma
Type 1 diabetes mellitus is a common chronic condition in childhood. The age of onset for childhood T1DM has a bimodal distribution: one peak at 4 to 6 years of age and a second peak in early puberty (10 to 14 years of age). 1 In the United States, the incidence of T1DM in non-Hispanic white children is quoted to be 23.6 per 100 000 per year. 2 Among the provinces in Canada, incidence ranges from 36 per 100 000 in Newfoundland and Labrador 3 to 15 per 100 000 in Quebec. 4 A stressor (such as infection) on top of poor glycemic control can result in DKA.
The reported frequency of DKA as the first presentation of childhood T1DM ranges from 15% to 67% in various studies. 5 More meaningfully, more than half of children younger than 3 years of age present with DKA as their first presentation of T1DM. 5 Presenting symptoms of DKA include polyuria and polydipsia, weight loss, fatigue, altered mental status, and respiratory distress (ie, Kussmaul breathing). 6 While polyuria and polydipsia frequently occur, Kussmaul breathing occurs in only 28% of DKA presentations. 7 In addition to physiologic stressors, other risk factors for developing DKA include female sex, no family predisposition, African American race, a younger age (< 5 years old), and other social factors including low socioeconomic status, limited access to medical services, and unstable family circumstances. 5 , 8 , 9
A 2016 case study reported a 7-week-old infant with DKA presenting to the emergency department with fever, altered mental status, and respiratory distress. In this situation, the workup included a capillary blood glucose reading and investigations into sources of infection. 10 This case study is consistent with reports that a preceding febrile illness is observed in 40% of DKA cases. 6
Our young patient presented with a viral prodrome and previous symptoms consistent with croup; however, the physical examination findings did not match the medical history. This case highlights the need for family physicians to be vigilant when past symptoms and the clinical examination appear discordant. During cold and flu season, family physicians might be lured into making an early diagnosis of respiratory infection in patients presenting with respiratory symptoms. Important yet simple questions probing for polyuria and polydipsia should be asked in young children presenting with respiratory issues to help identify whether DKA is a diagnostic consideration. Finally, consider performing point-of-care capillary blood glucose testing in children presenting with symptoms of respiratory distress and reduced level of consciousness to assess for DKA. Ketones can also be measured through urine dips or point-of-care capillary devices to support the diagnosis.
Editor’s key points
- ▸ Diabetic ketoacidosis (DKA) is a common presentation of type 1 diabetes mellitus in children younger than 3 years of age.
- ▸ Kussmaul breathing, characterized by tachypnea and increased depth of breath in response to metabolic acidosis, is a less common first presentation of DKA.
- ▸ During cold and flu season, DKA can be harder to identify because Kussmaul breathing might be mistaken as a sign of respiratory infection.
- ▸ Assessing for polyuria and polydipsia and performing point-of-care glucose and ketone testing in young children presenting with respiratory symptoms could help family physicians consider DKA and type 1 diabetes mellitus as potential diagnoses.
Competing interests
None declared
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de juin 2020 à la page e180 .
A case report: First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35-month-old girl
Affiliations.
- 1 Division of Pediatric Intensive Care Department of Pediatrics Shiraz University of Medical Sciences Shiraz Iran.
- 2 Division of Pediatric Metabolism and Endocrinology Department of Pediatrics Shiraz University of Medical Sciences Shiraz Iran.
- PMID: 34765201
- PMCID: PMC8572339
- DOI: 10.1002/ccr3.4984
Hyperglycemic hyperosmolar syndrome (HHS) is a rare complication of diabetes mellitus among pediatric patients. Since its treatment differs from diabetic ketoacidosis (DKA), hence, pediatricians should be aware of its diagnosis and management.
Keywords: case report; diabetes mellitus; hyperglycemic hyperosmolar syndrome (HHS); pediatric patients; rhabdomyolysis; thrombosis.
© 2021 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
Publication types
- Case Reports
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Symptoms of Diabetes
- Type 1 diabetes symptoms can develop in just a few weeks or months and can be severe.
- Type 2 diabetes symptoms can take years to develop.
- Gestational diabetes doesn’t usually have symptoms. Your doctor should test you for it between 24 and 28 weeks of pregnancy.

If you have any of the following diabetes symptoms, see your doctor about getting your blood sugar tested:
- Urinate (pee) a lot, often at night
- Are very thirsty
- Lose weight without trying
- Are very hungry
- Have blurry vision
- Have numb or tingling hands or feet
- Feel very tired
- Have very dry skin
- Have sores that heal slowly
- Have more infections than usual
Symptoms of type 1 diabetes
People who have type 1 diabetes may also have nausea, vomiting, or stomach pains. Type 1 diabetes can be diagnosed at any age. Symptoms can develop in just a few weeks or months and can be severe.
Symptoms of type 2 diabetes
Type 2 diabetes symptoms often take several years to develop. Some people don't notice any symptoms at all. Type 2 diabetes usually starts when you're an adult, though more and more children and teens are developing it. Because symptoms are hard to spot, it's important to know the risk factors for type 2 diabetes.
Symptoms of gestational diabetes
Gestational diabetes (diabetes during pregnancy) usually doesn't have any symptoms. If you're pregnant, your doctor should test you for gestational diabetes between 24 and 28 weeks of pregnancy. If needed, you can make changes like healthy eating and being active to protect your health and your baby's health. If those changes aren’t enough to manage your blood sugar, your doctor may prescribe medicine to help.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.

For Everyone
Health care providers, public health.
Expecting factors for inadequate glycemic control in children and adolescents with type 1 diabetes mellitus: a single center experience
- Research article
- Published: 18 May 2024
Cite this article

- Farah Sameer Yahya ORCID: orcid.org/0000-0002-4965-6073 1
Achieving an ideal glycemic control in children and adolescents with type 1 diabetes mellitus (T1DM) is both a difficult and challenging process. We aim to highlight the expected factors contributing to inadequate glycemic control in children and adolescents with T1DM in a sample of Iraqi children and adolescents.
This was a descriptive cross-sectional study that recruited 247 T1DM patients aged < 18 years & disease duration ≥ 1 year. Data collected included socio-demographic & clinical characteristics with recent HbA1c value. Each patient was examined for signs of puberty and any lipodystrophy at insulin injection sites. Factors studied using Independent-Samples T-Test, One way ANOVA & Multivariable logistic regression.
Of the 247 patients, 108 (43.7%) were males, and 139 (56.3%) were females. The mean & SD of the age of patients was 10.13 ± 3.85 years. The Mean & SD of the recent HbA1c level was 9.43 ± 2.56. HbA1c ≤ 7.5 was achieved in 27.1% of patients. Using Multivariable logistic regression to study the association between variable factors and inadequate glycemic control, showed a significant association with higher odds in terms of the older age of the patient, maternal illiteracy, presence of recurrent diabetic ketoacidosis (DKA) episodes, absence of carb counting, and presence of lipodystrophy. Higher HbA1c was also associated significantly with puberty, rural residency, poor socioeconomic status, DKA presentation, and using regular + NPH insulin regimen.
Conclusions
In the current study, inadequate glycemic control was induced by many factors, Strategies should be applied to control these factors to minimize the prospective risks of macro-vascular complications linked to T1DM in children & adolescents.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
The data supporting the conclusions of this study can be obtained from the corresponding author upon a reasonable request.
Al-agha AE, Alafif M, Abd-elhameed IA. Glycemic control, complications, and associated autoimmune diseases in children and adolescents with type 1 diabetes in Jeddah. Saudi Arabia. 2015;36:26–31. https://doi.org/10.15537/smj.2015.1.9829 .
Article Google Scholar
Turton JL, Raab R, Rooney KB. Low-carbohydrate diets for type 1 diabetes mellitus: a systematic review 2018:1–16.
Libman I, Haynes A, Lyons S, Pradeep P, Rwagasor E, Tung JY et al. ISPAD GUIDELINES ISPAD Clinical Practice Consensus guidelines 2022: definition, epidemiology, and classification of diabetes in children and adolescents 2022:1160–74. https://doi.org/10.1111/pedi.13454 .
Ogle GD, James S, Dabelea D, Pihoker C, Svennson J, Maniam J et al. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res Clin Pract. 2022;183:109083. https://doi.org/10.1016/j.diabres.2021.109083 .
EJ MDjb. IDF Diabetes Atlas. 10th Ed 2021. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf .
Zalzala SH, Al-lami FH, Fahad S. Epidemiological profile of type 1 diabetes among primary school children in Baghdad. Iraq 2020:13–6.
Chen J, Yin D, Dou K. Intensified glycemic control by HbA1c for patients with coronary heart disease and type 2 diabetes: a review of findings and conclusions 2023:1–16.
Lenters-Westra E, Schindhelm RK, Bilo HJ, Slingerland RJ. Haemoglobin A1c: historical overview and current concepts. Diabetes Res Clin Pract. 2013;99:75–84. https://doi.org/10.1016/j.diabres.2012.10.007 .
Article CAS PubMed Google Scholar
ADA. Estandares Para El Cuidado De La diabetes-2023. Diabtes Journals. 2023;46:1–298.
Google Scholar
Group TDC, CT of DI and C (DCCT/EDIC) SR. Intensive Diabetes Treatment and Cardiovascular Disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643. https://doi.org/10.1056/NEJMOA052187 .
Nevo-Shenker M, Shalitin S. The impact of hypo- and hyperglycemia on cognition and brain development in young children with type 1 diabetes. Horm Res Paediatr. 2021;94:115–23. https://doi.org/10.1159/000517352 .
Mcknight JA, Wild SH, Lamb MJE, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–50. https://doi.org/10.1111/DME.12676 .
Alassaf A, Odeh R, Gharaibeh L, Ibrahim S, Ajlouni K. Personal and clinical predictors of poor metabolic control in children with type 1 diabetes in Jordan. J Diabetes Res. 2019;2019:15–8. https://doi.org/10.1155/2019/4039792 .
Gesuita R, Skrami E, Bonfanti R, Cipriano P, Ferrito L, Frongia P, et al. The role of socio-economic and clinical factors on HbA1c in children and adolescents with type 1 diabetes: an Italian multicentre survey. Pediatr Diabetes. 2017;18:241–8. https://doi.org/10.1111/PEDI.12378 .
Hafidh K, Abdella NA. Glycemic control of adult patients with type 1 diabetes mellitus in Arabian Gulf countries; PREDICT. BMC Endocr Disord. 2022;22:1–10. https://doi.org/10.1186/s12902-022-00946-3 .
Article CAS Google Scholar
The Effect of Flexible Low. Glycemic diets on Glycemic Control in Children with type 1 diabetes. Diabetes Care 2001;24.
Gökşen D, Altinok YA, Özen S, Demir G, Darcan Ş. Effects of carbohydrate counting method on metabolic control in children with type 1 diabetes mellitus. JCRPE J Clin Res Pediatr Endocrinol. 2014;6:74–8. https://doi.org/10.4274/jcrpe.1191 .
Article PubMed Google Scholar
6th edition| IDF Diabetes Atlas. n.d. https://diabetesatlas.org/atlas/sixth-edition/ (accessed January 27, 2024).
White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV, et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and complications Trial (DCCT). J Pediatr. 2001;139:804–12. https://doi.org/10.1067/mpd.2001.118887 .
Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: a 4-year longitudinal study. J Pediatr Psychol. 2009;34:254–70. https://doi.org/10.1093/jpepsy/jsn079 .
Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, Siminerio L. Brief report: trajectories of glycemic control over early to middle adolescence. J Pediatr Psychol. 2010;35:1161–7. https://doi.org/10.1093/jpepsy/jsq011 .
Article PubMed PubMed Central Google Scholar
Luyckx K, Seiffge-Krenke I. Continuity and change in glycemic control trajectories from adolescence to emerging adulthood: relationships with family climate and self-concept in type 1 diabetes. Diabetes Care. 2009;32:797–801. https://doi.org/10.2337/dc08-1990 .
Frey MA, Templin T, Ellis D, Gutai J, Podolski CL. Predicting metabolic control in the first 5 yr after diagnosis for youths with type 1 diabetes: the role of ethnicity and family structure. Pediatr Diabetes. 2007;8:220–7. https://doi.org/10.1111/J.1399-5448.2007.00260.X .
Neylon OM, O’Connell MA, Skinner TC, Cameron FJ. Demographic and personal factors associated with metabolic control and self-care in youth with type 1 diabetes: a systematic review. Diabetes Metab Res Rev. 2013;29:257–72. https://doi.org/10.1002/DMRR.2392 .
Bandarian F, Sharifnejad Tehrani Y, Peimani M, Namazi N, Saeedi Moghaddam S, Esmaeili S, et al. National and sub-national burden and trend of type 1 diabetes in 31 provinces of Iran, 1990–2019. Sci Rep. 2023;13:1–10. https://doi.org/10.1038/s41598-023-31096-8 .
Ghazaiean M, Najafi B, Zamanfar D. Risk factors for suboptimal glycemic control in pediatrics with type 1 diabetes mellitus: a cross– sectional study. Sci Rep 2024:1–12. https://doi.org/10.1038/s41598-024-57205-9 .
Flores Monar GV, Islam H, Puttagunta SM, Islam R, Kundu S, Jha SB, et al. Association between Type 1 diabetes Mellitus and Celiac Disease: Autoimmune disorders with a Shared genetic background. Cureus. 2022;14:1–10. https://doi.org/10.7759/cureus.22912 .
Rewers MJ, Pillay K, de Beaufort C, Craig ME, Hanas R, Acerini CL, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15:102–14. https://doi.org/10.1111/pedi.12190 .
Taha Z, Eltoum Z, Washi S. Predictors of glucose control in children and adolescents with type 1 diabetes: results of a cross-sectional study in Khartoum, Sudan. Open Access Maced J Med Sci. 2018;6:2035–9. https://doi.org/10.3889/oamjms.2018.423 .
Niba LL, Aulinger B, Mbacham WF, Parhofer KG. Predictors of glucose control in children and adolescents with type 1 diabetes: results of a cross-sectional study in Cameroon. BMC Res Notes. 2017;10:1–10. https://doi.org/10.1186/s13104-017-2534-8 .
Djonou C, Tankeu AT, Dehayem MY, Tcheutchoua DN, Mbanya JC, Sobngwi E. Glycemic control and correlates in a group of sub saharan type 1 diabetes adolescents. BMC Res Notes. 2019;12:1–5. https://doi.org/10.1186/s13104-019-4054-1 .
Mohammad HA, Farghaly HS, Metwalley KA, Monazea EM, El-hafeez HAA. Original article predictors of glycemic control in children with type 1 diabetes mellitus in. Assiut-Egypt. 2012;16:796–802. https://doi.org/10.4103/2230-8210.100679 .
Clements MA, Lind M, Raman S, Patton SR, Lipska KJ, Fridlington AG, et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care. 2014;2:e000039. https://doi.org/10.1136/bmjdrc-2014-000039 .
Habteyohans BD, Hailu BS, Meseret F, Mohammed A, Berhanu Y, Alemu A, et al. Poor glycemic control and its associated factors among children with type 1 diabetes mellitus in Harar, eastern Ethiopia: a cross-sectional study. BMC Endocr Disord. 2023;23:1–12. https://doi.org/10.1186/s12902-023-01453-9 .
Enander R, Gundevall C, Strömgren A, Chaplin J, Hanas R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatr Diabetes. 2012;13:545–51. https://doi.org/10.1111/J.1399-5448.2012.00883.X .
Kalergis M, Pacaud D, Strychar I, Meltzer S, Jones PJH, Yale JF. Optimizing insulin delivery: assessment of three strategies in intensive diabetes management. Diabetes. Obes Metab. 2000;2:299–305. https://doi.org/10.1046/J.1463-1326.2000.00107.X .
Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014;2:133–40. https://doi.org/10.1016/S2213-8587(13)70144-X .
Trento M, Trinetta A, Kucich C, Grassi G, Passera P, Gennari S, et al. Carbohydrate counting improves coping ability and metabolic control in patients with type 1 diabetes managed by Group Care. J Endocrinol Investig 2011 342. 2010;34:101–5. https://doi.org/10.1007/BF03347038 .
Schmidt S, Meldgaard M, Serifovski N, Storm C, Christensen TM, Gade-Rasmussen B, et al. Use of an automated Bolus Calculator in MDI-Treated type 1 DiabetesThe BolusCal Study, a randomized controlled pilot study. Diabetes Care. 2012;35:984–90. https://doi.org/10.2337/DC11-2044 .
Article CAS PubMed PubMed Central Google Scholar
Scavone G, Manto A, Pitocco D, Gagliardi L, Caputo S, Mancini L, et al. Effect of carbohydrate counting and medical nutritional therapy on glycaemic control in type 1 diabetic subjects: a pilot study. Diabet Med. 2010;27:477–9. https://doi.org/10.1111/J.1464-5491.2010.02963.X .
Amiel S, Beveridge S, Bradley C, Gianfrancesco C, Heller S, James P, et al. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746. https://doi.org/10.1136/BMJ.325.7367.746 .
Holl RW, Swift PGF, Mortensen HB, Lynggaard H, Hougaard P, Aanstoot HJ, et al. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur J Pediatr. 2003;162:22–9. https://doi.org/10.1007/S00431-002-1037-2/METRICS .
Cengiz E, Danne T, Ahmad T, Ayyavoo A, Beran D, Ehtisham S, et al. ISPAD Clinical Practice Consensus guidelines 2022: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2022;23:1277–96. https://doi.org/10.1111/pedi.13442 .
Ashwell SG, Bradley C, Stephens JW, Witthaus E, Home PD. Unmodified human insulin in individuals with type 1 diabetes. Diabetes Care. 2008;31:1112–7. https://doi.org/10.2337/dc07-1183.Clinical .
Sharplin P, Gordon J, Peters JR, Tetlow AP, Longman AJ, Mcewan P. Switching from premixed insulin to glargine-based insulin regimen improves glycaemic control in patients with type 1 or type 2 diabetes: a retrospective primary care. -based Anal. 2009;8:1–8. https://doi.org/10.1186/1475-2840-8-9 .
Shibeshi MS, Daba AK, Meiso KM, Tadesse BT. Glycemic control among children and adolescents with diabetes in Southern Ethiopia: a cross-sectional study. BMC Endocr Disord. 2022;22:1–7. https://doi.org/10.1186/s12902-022-01070-y .
Download references
Acknowledgements
The author acknowledge all the staff members at pediatric Endocrinology and Diabetes Clinic in Al-Khansaa Teaching Hospital in Mosul City, Iraq, For there help & support during the period of data collection.
Note applicable.
Author information
Authors and affiliations.
Department of Pediatrics, College of Medicine, University of Mosul, Mosul, Iraq
Farah Sameer Yahya
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Farah Sameer Yahya .
Ethics declarations
Conflict of interest.
the named author declare that there was no conflict of interest associated with this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Yahya, F.S. Expecting factors for inadequate glycemic control in children and adolescents with type 1 diabetes mellitus: a single center experience. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01442-2
Download citation
Received : 23 February 2024
Accepted : 05 May 2024
Published : 18 May 2024
DOI : https://doi.org/10.1007/s40200-024-01442-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 1 diabetes mellitus
- Glycemic control
- Carb counting
- Lipodystrophy
- Find a journal
- Publish with us
- Track your research

How Do You Develop Diabetes Type 1? A Review By Doctors
Expert opinion from sarath chandr rellu, mbbs · 2 years of experience · india.
Diabetes type—1 is insulin-dependent diabetes mellitus, in which there is little or no insulin production from pancreas. Insulin is a hormone needed to allow sugar to enter cells to produce energy. It occurs in childhood, adolescents and may occur in adults also. Genetic and some viruses may cause diabetes type—1. Symptoms include increased thirst, increased hunger, weight loss.
Expert opinion from Rajesh Mishra
Mbbs · less than a year of experience · india.
Type 1 diabetes occurs when your immune system, the body's system for fighting infection, attacks and destroys the insulin-producing beta cells of the pancreas. Scientists think type 1 diabetes is caused by genes and environmental factors. These such as viruses, that might trigger the disease. It may be juvenile also.
Expert opinion from Daniel A. Foster
Do, mph · 16 years of experience · usa.
The exact cause is not known. Type 1 diabetes is a result of an auto-immune destruction of pancreatic islet cells that produce insulin. The risk factors include genetics, certain infections or pancreatic diseases.
→ Learn more about type 1 diabetes: See the causes, symptoms, treatment options and more.
→ See more questions and expert answers related to type 1 diabetes.
Disclaimer: This is for information purpose only, and should not be considered as a substitute for medical expertise. These are opinions from an external panel of individual doctors, and not to be considered as opinion of Microsoft. Please seek professional help regarding any health conditions or concerns.

Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Veillonella and Bacteroides are associated with gestational diabetes mellitus exposure and gut microbiota immaturity
Roles Formal analysis, Investigation, Methodology, Writing – original draft
Affiliation Laboratorio de Envejecimiento Saludable, Instituto Nacional de Medicina Genómica, Centro de Investigación Sobre Envejecimiento (CIE-CINVESTAV Sur), Ciudad de México, México
Roles Resources
Affiliation Hospital de la Niñez Oaxaqueña, Oaxaca, México
Affiliation Unidad de Bioquímica e Inmunología, Tecnológico Nacional de México-Instituto Tecnológico de Oaxaca, Oaxaca, México
Affiliation Centro de Investigación Facultad de Medicina UNAM-UABJO, Facultad de Medicina y Cirugía, Universidad Autónoma “Benito Juárez” de Oaxaca, Oaxaca, México
Roles Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing
* E-mail: [email protected] (NM-C); [email protected] (BP-G)
Affiliation Unidad de Vinculación Científica de la Facultad de Medicina UNAM en Instituto Nacional de Medicina Genómica, Ciudad de México, México
Roles Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing
- Fernanda Valdez-Palomares,
- Jaqueline Reyes Aguilar,
- Eduardo Pérez-Campos,
- Laura Pérez-Campos Mayoral,
- Noemi Meraz-Cruz,
- Berenice Palacios-González

- Published: May 14, 2024
- https://doi.org/10.1371/journal.pone.0302726
- Reader Comments
Dysbiosis during childhood impacts the configuration and maturation of the microbiota. The immaturity of the infant microbiota is linked with the development of inflammatory, allergic, and dysmetabolic diseases.
To identify taxonomic changes associated with age and GDM and classify the maturity of the intestinal microbiota of children of mothers with GDM and children without GDM (n-GDM).
Next-generation sequencing was used to analyze the V3–V4 region of 16S rRNA gene. QIIME2 and Picrust2 were used to determine the difference in the relative abundance of bacterial genera between the study groups and to predict the functional profile of the intestinal microbiota.
According to age, the older GDM groups showed a lower alpha diversity and different abundance of Enterobacteriaceae, Veillonella , Clostridiales , and Bacteroides . Regarding the functional profile, PWY-7377 and K05895 associated with Vitamin B12 metabolism were reduced in GDM groups. Compared to n-GDM group, GDM offspring had microbiota immaturity as age-discriminatory taxa in random forest failed to classify GDM offspring according to developmental age (OOB error 81%). Conclusion. Offspring from mothers with GDM have a distinctive taxonomic profile related to taxa associated with gut microbiota immaturity.
Citation: Valdez-Palomares F, Aguilar JR, Pérez-Campos E, Mayoral LP-C, Meraz-Cruz N, Palacios-González B (2024) Veillonella and Bacteroides are associated with gestational diabetes mellitus exposure and gut microbiota immaturity. PLoS ONE 19(5): e0302726. https://doi.org/10.1371/journal.pone.0302726
Editor: Michael Bader, Max Delbruck Centrum fur Molekulare Medizin Berlin Buch, GERMANY
Received: January 5, 2024; Accepted: April 10, 2024; Published: May 14, 2024
Copyright: © 2024 Valdez-Palomares et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Raw data are available in https://www.ncbi.nlm.nih.gov/bioproject/ with the accession number: PRJNA1085791.
Funding: Universidad Nacional Autónoma de México, PAPIIT-DGAPA grant IN221014, Noemi Meraz-Cruz Instituto Nacional de Medicina Genómica, INMEGEN grant 11/2016/I, Berenice Palacios-Gonzalez.
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Gestational diabetes mellitus (GDM) according to the American Diabetes Association is defined as “diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes before gestation” [ 1 ]. In Mexico, the prevalence of GDM has been increasing; currently, its incidence is 17.7% [ 2 ]. GDM during pregnancy increases the susceptibility of offspring to develop insulin resistance, obesity, and hypertension [ 3 , 4 ].
Early alteration of the childhood microbiota is associated with allergies, inflammation, and childhood obesity [ 5 – 7 ]. According to the Developmental Origin of Health and Disease (DOHaD) theory the intrauterine exposure to excessive energy may result in permanent physiological and metabolic alterations, increasing disease risk in adulthood to developing obesity and type 2 diabetes [ 8 – 13 ].
The intestinal microbiota of the newborn is particularly interesting because, due to rapid temporal variation, the bacterial communities in the intestine are remarkably unstable. Therefore, early childhood is a crucial time window where the child’s gut microbiota can be modified [ 14 , 15 ] in contrast to an adult individual’s "mature" microbiota, which seems relatively stable over time.
The aim of this study was to identify taxonomic changes associated with age and GDM and to classify the gut-microbiota maturity of offspring from mothers with GDM and the offspring from mothers without GDM (n-GDM)
Materials and methods
Study population.
The present is a cross-sectional study conducted in “C.S.T.III Dr. Gabriel Garzón Cossa" and “Hospital de la Niñez Oaxaqueña” located in Mexico. Offspring exposed and not exposed to GDM aged 0 to 30 months, either born vaginally or via C-section were included during routine hospital visit, only mothers that underwent a 75-g oral glucose tolerance test (OGTT) between 24- and 28-weeks’ gestation as part of screening protocol to determine GDM diagnosis were included. GDM was diagnosed according to the International Association of the Diabetes and Pregnancy Study Group criteria: Plasma glucose level ≥8.5 mmol/L following a 75 g OGTT [ 16 ]. A total of 40 infants were included: 26 infants not exposed to GDM (0–6 Months (n = 6), 7–12 Months (n = 10) and 13–30 Months (n = 10)) and 14 infants exposed to GDM (0–6 Months (n = 4), 7–12 Months (n = 5) and 13–30 Months (n = 5)). The 2009 WHO child growth standards (World Health Organization 2009) were used as a reference for children’s weight and length/height [ 17 ]. Maternal and infant characteristics and feeding practices (breastfeeding, formula, or mixed) were collected from medical records during interview and medical records (data were accessed for research purposes between the 9th of January 2017 and the 30th of November 2017). The following exclusion criteria were used: Infants with history of hospitalization and or use of antibiotics six months before the study, presence of chronic illness, gastrointestinal pathology, or diarrheal illness presented one month before the study. The study was performed according to the latest version of the Declaration of Helsinki and was approved by the Human Research Ethical Committee of Universidad Nacional Autónoma de México 079/2017/CEI-HGT. All parents or legal guardians and children provided written informed consent.
Anthropometric measurements
The children were weighed, for which their clothing, diapers, and ornaments were removed. Length was measured using an infantometer, placing the child in a supine position with the head resting against the headboard; the measurer slid the stirrup along the base until it was flat against the soles of the feet, and the measurement was recorded from the digital counter (accuracy ±1.0 mm). Head circumference was measured using Seca 121 tape (Seca 212). The anthropometric measurements were obtained using standardized procedures applied by trained personnel.
Fecal bacterial DNA isolation, libraries preparation and bioinformatic analysis
Fecal samples from infants aged 0–6 Months: (n = 6 not exposed to GDM; n = 4 exposed to GDM), aged 7–12 Months: (n = 10 not exposed to GDM; n = 5 exposed to GDM) and aged 13–30 Months: (n = 10 not exposed to GDM; n = 5 exposed to GDM) were collected from diapers or digital rectal examination, and placed in a sterile plastic container. The QIAamp PowerFecal Pro DNA kit (QIAGEN) was used to isolate bacterial DNA. DNA concentrations were determined using a NanoDrop V3.8.1 spectrophotometer. The 16s rRNA gene was amplified using primers 338F and 806R, targeting the V3-V4 hypervariable regions. The libraries were sequenced at the Sequencing Unit of the Instituto Nacional de Medicina Genómica using the Illumina Miseq platform (Illumina, San Diego, CA) [ 18 ]. Fastq reads were processed using the Quantitative Insights Into Microbial Ecology 2 (QIIME 2) [ 19 ]. The dada2 denoise-paired instruction was used for denoising quality, chimera checking, and clustering. For the 97% taxonomic assignment, the SILVA 16S reference database (version_138) and the classifiers naïve Bayes algorithm. A rooted phylogenetic tree was generated for further statistical analysis for α-diversity tests and Bray-Curtis for β-diversity tests. Microbial community metagenome prediction was performed with PICRUSt2 [ 20 ]. Microbiome Analyst was used to create the heat trees with mean abundance and the non-parametric Wilcoxon rank sum test corrected by Benjamin Hochberg (FD) ( http://www.microbiomeanalyst.ca ) [ 21 ]. Raw data are available in https://www.ncbi.nlm.nih.gov/bioproject/ with the accession number: PRJNA1085791.
Classification of gut-microbiota maturity by developmental stages using Random Forests
The random forest classification model used the ’randomForest’ R package. A rarefied ASV table served as input data; the model was trained on 26 non-GDM infants and validated on 10 non-GDM infants, built using the following parameters: ntree = 10,000 and mtry of p/3 ASVs randomly sampled at each split, in which p represents the number of ASVs. The model was further refined by applying tenfold cross-validation to estimate the minimal number of top-ranking age-discriminatory taxa required for prediction, including 15 ASVs to train the final model based on a mean decrease in Gini. The Random Forests algorithm was applied to classify healthy infants by chronologic age ranging from 0–6 months, >6–12 months, and >12–30 months, thereby identifying taxa that discriminate by chronologic age in healthy children and subsequently applied on 16 GDM offspring infants.
Statistical analysis
Continuous quantitative variables (anthropometric data) will be presented as the mean ± standard deviation (SD) for variables with parametric distribution and median and 25th and 75th percentiles for variables with non-parametric distribution. To evaluate the differences between the study groups, the one-way ANOVA or Kruskall Wallis test will be used according to the homoscedasticity criterion and the normality of the data. Statistical analyses will be performed in the GraphPad 9 statistical program and R ( https://www.r-project.org/ ) [ 22 , 23 ].
Population demographics and clinical characteristics
A total of 40 children were included: 26 children not exposed to GDM (n-GDM) and 14 children exposed to GDM (GDM) ( Table 1 ) . Regarding clinical parameters, there were no significant differences between the groups.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0302726.t001
Composition of the bacterial community of children exposed to GDM
From the Illumina 330 bp paired-end sequencing of the amplicon targeting the V3–V4 region of 16S rRNA gene, two thousand five hundred seventy-nine high-quality sequences among the 30 fecal samples from the participants with an average of 30,027 sequences per sample were generated. Chao1, Shannon, and Simpson index were used to describe alpha diversity ( Fig 1A–1C ). There were no significant differences in alpha diversity between n-GDM and GDM groups. To compare microbial communities’ composition, we calculated the beta diversity by Bray-Curtis index ( Fig 1D ). No significant difference was found between the groups.
(a) Chao index in n-GDM and GDM group, no significant differences were observed between the groups; (b) Shannon index in n-GDM and GDM group, no statistically significant differences were found between the groups; (c) Simpson index in n-GDM and GDM group, no differences were found among the group; (d) Bray-Curtis index in n-GDM and GDM groups, no significant differences were shown amongst the groups, n-GDM group (light blue circles) and GDM group (gray circles); (e) Phylum-level composition (% relative abundances) among the study groups. Firmicutes, coloured yellow, followed by Bacteroidetes, coloured orange; (f) Genus-level composition (% relative abundances) among the study groups, the most abundant taxa were Bacteroides , Akkermansia , Bifidobacterium , Blautia , Clostridium , Escherichia_Shigella , Faecalibacterium , Klebsiella , Parabacteroides , Streptococcus and Veillonella ; (g) Heat tree for pair-wise comparison. Those taxa that showed statistically significant differences were Veillonella of yellow colour, enrichment in the n-GDM group; and green-coloured Bacteroides enrichment in the GDM group.
https://doi.org/10.1371/journal.pone.0302726.g001
The predominant phyla were Firmicutes, with a mean abundance of 44%, followed by 24% of Bacteroidetes, Proteobacteria, which represented 26%, while Verrucomicrobia and Actinobacteria represented 1% and 5%, respectively ( Fig 1E ). Comparisons of the relative abundance of the taxa were made to determine differences at the taxonomic level between each age group ( Fig 1F ). The fecal microbiota of the GDM group had significantly lower (p = 0.007) proportions of Firmicutes, specifically in Veillonella genus, and significantly higher (p = 0.014) proportions of Bacteroidetes, specifically in Bacteroides , compared to n-GDM group ( Fig 1G ). Fecal microbiota according to type of delivery was also compared. In vaginally born infants, the predominant phyla were Bacteroidetes , while, Proteobacteria and Actinobacteria were main constituents in C-section infants ( S1A Fig ). No significant differences in alpha or beta diversity were detected among groups ( S1B and S1C Fig ), Enrichment in members of the Proteobacteria phyla: Gammaproteobacteria , Enterobacteriales , Enterobacteriaceae and Escherichia_Shigella genus was detected in C-section infants, while enrichment in the Bacteroidetes phylum members, Bacteroides , Bacteroidales , Bacteroidaceae and Bacteroides was detected in vaginally born infants ( S1D Fig ).
Influences of age-related GDM on the microbiota composition
The effect of age on the bacterial community composition was assessed. Regarding alpha diversity, the GDM group presented lower alpha diversity as age advanced ( Fig 2A–2C ). Interestingly, the GDM group remains at all alpha diversity metrics below the n-GDM groups. The beta diversity analysis ( Fig 2D ), calculated on the Bray-Curtis dissimilarity, revealed no statistically significant difference (F-value: 1.2489; R2: 0.14782; p-value: 0.088). Subsequently, the groups were divided according to age ranges: newborns to 6 months (Group 1), 7 to 12 months (Group 2), and 13 months to 24 months (Group 3). At the phyla level, no significant differences were found ( Fig 2E ). On the other hand, the GDM-1 group presented a lower abundance of Enterobacteriaceae (family) ( Fig 2F ). The GDM-1 and GDM2 groups showed a lower abundance of Veillonella (a genus belonging to the Clostridiales order) ( Fig 2F and 2G ), while the GDM3 group only showed a lower abundance in Clostridiales without reaching a difference in any specific genus ( Fig 2H ). The GDM-1 and GDM-3 groups also presented a higher abundance of Bacteroides (genus) ( Fig 2F and 2G ) .
(a) Chao index, (b) Shannon index, (c) Simpson index across the age, n-GDM group (light blue circles) and GDM group (gray circles), the GDM group presented lower alpha diversity as age advance; (d) Bray-Curtis index across the age in n-GDM and GDM groups, no significant differences were found between the groups (F-value: 1.2489; R2: 0.14782; p-value: 0.088), newborns to 6 months (n-GDM group, red circles; GDM group, dark purple), 7 to 12 months (n-GDM group, orange circles; GDM group, light purple) and 13 months to 24 months (n-GDM group, yellow circles; GDM group, gray circles); (e) Phylum-level composition (% relative abundances) among the study groups; Heat tree for pair-wise comparison, divided by age, (f) newborns to 6 months, (g) 7 to 12 months and (h) 13 months to 24 months. Those taxa that showed statistically significant differences are shown in colour. Taxa coloured yellow are enriched in the n-GDM group and those coloured green are enriched in the GDM group. The colour of each taxon indicates the log-2 ratio of the proportions observed in each condition.
https://doi.org/10.1371/journal.pone.0302726.g002
Age-related effects on microbial functional pathways
PICRUSt2 was employed to predict functional differences in fecal microbiota. The n-GDM microbiome was enriched for the KEGG pathway oxidoreductases (K05895 (cobK-cbiJ); pre-corrin-6A/cobalt-pre corrin-6A reductase; Vitamin B12 metabolism) and PWY-7377 (adenosylcobalamin biosynthesis I (anaerobic); cob(II) urinate a c-diamide biosynthesis I (early cobalt insertion)) ( Fig 3A and 3B ). These pathways were inversely related to age in n-GDM group. Vitamin 12 biosynthesis is mediated exclusively by the bacterial fermentation process, so a correlation matrix between the predicted abundance pathway and differentially expressed taxa was performed. PWY-7377 positively correlated with Veillonella , Clostridium sensu stricto ; Escherichia-Shigella and Streptococcus , meanwhile negatively correlated with Bacteroides ( Fig 3C ).
(a) KEGG Orthology (KO; K05895). Red indicates enrichment and green shows depletion; (b) Microbial metabolic pathway PWY-7377 Cob(II)yrinate a,c-diamide bio-synthesis I (early cobalt insertion) in n-GDM and GDM groups. p <0.05 are considered statistically significant differences. Statistically significant differences among the groups are shown with letters, where a>b>c>d; (c) Heatmap correlogram containing Pearson’s rho negative and positive correlation coefficients. Blue and red color denotes positive and negative correlation coefficients correlation, respectively. *p < 0.05. Fecal samples from infants aged 0–6 Months: (n-GDM1: n = 6; GDM1: n = 4), aged 7–12 Months: (n-GDM2: n = 10; GDM2: n = 5) and aged 13–30 Months: (n-GDM3: n = 10; GDM3: n = 5).
https://doi.org/10.1371/journal.pone.0302726.g003
GDM infants have immature gut microbiota in the early stages of development
To define the maturity of the microbiota, the Random Forests (RF) model resulting from healthy children was used, thus determining those taxa that help to distinguish the different stages of postnatal life. Rated lists of all bacterial taxa, in order of ’age-discriminative importance,’ were selected by considering those whose relative abundance values, when permuted, have the most considerable mean decrease in Gini. An increase in validation error was observed when including taxa beyond the top 15 ( Fig 4A ). Log-transformed counts of the top 15 relatives of age-discriminating taxa are shown in Fig 4B . RF model of 15 top discriminatory taxa accurately classified healthy offspring according to developmental age (OOB error 0%) ( Fig 4C ). The RF model was later applied with no further parameter optimization to classify GDM offspring according to periods of postnatal life. The results revealed that compared to healthy, GDM offspring had microbiota immaturity as age-discriminatory taxa in RF failed to classify developmental age (OOB error 81%) of GDM offspring ( Fig 4D ).
A top 15 age-discriminatory taxa random forest classification model was constructed with healthy offspring samples. (a) The number of features was selected by ten-fold cross-validation (b). The 15 features in the model and their abundance are shown the model was applied to healthy offspring (c) and to GDM offspring (d).
https://doi.org/10.1371/journal.pone.0302726.g004
GDM is a public health problem that increases the risk of maternal and perinatal morbidity and mortality and contributes to the development of long-term health complications in both mothers and offspring. GDM has been associated with changes in the intestinal microbiota both in the neonate and during pregnancy [ 24 – 27 ]. Interestingly, intestinal dysbiosis during the first stages of life is associated with long-term health status, which denotes the importance of the intestinal microbiota in the first years of life on the development of metabolic and immunological diseases [ 28 , 29 ]. In the present study, no changes in microbiota composition were observed when comparing children exposed to GDM with children not exposed to GDM. However, when the groups were separated by age, the offspring of mothers with GDM maintained lower alpha diversity. As mentioned above, the gut microbiota in early childhood (≤2 years) changes over time [ 30 ], both at the taxonomic level and in alpha and beta diversity metrics [ 31 ]. Offspring that were not exposed to DMG showed continuous development and maturation of the intestinal microbiota during the first years of life, unlike offspring from mothers exposed to DMG that presented lower alpha diversity, which has been associated with overall delay in microbial maturation and subsequent adverse health outcomes, including atopy and allergic diseases [ 32 , 33 ]. This result is consistent with other studies indicating that GDM is associated with lower diversity [ 34 , 35 ]. Unlike what Crusell et al. observed, alpha diversity remains decreased in the offspring of children exposed to GDM and does not show the recovery observed at nine months [ 34 ]. Furthermore, obese children have less diversity and richness [ 36 ], suggesting that in children with GDM, the immaturity of the gut microbiota could be a determining factor for the development of obesity in the future [ 37 ].
Several studies suggest early colonization is essential for establishing and maturing the gut microbiota. During the early colonization of the newborn intestine, facultative anaerobic Proteobacteria gradually consume oxygen in the gastrointestinal tract, and, consequently, obligate anaerobes colonize the new environment [ 38 ]. Premature infants exhibit immaturity in the gut microbiota, characterized by the predominance of pathogenic bacteria within the Enterobacteriaceae family, persistently low diversity, and a scarcity of strictly anaerobic taxa, including Veillonella relative to appropriately growing [ 39 ]. Interestingly, in this study, the offspring of mothers with GDM showed a lower abundance, specifically of the taxon Veillonella , which, as mentioned above, is considered involved in developing the gut microbiota as seed species along with Streptococcus [ 40 ]. The above suggests that the presence of GDM affects the maturity of the intestinal microbiota.
In addition, the groups with GDM had a greater abundance of Bacteroides; Vatanen et al. demonstrated in vitro studies that Bacteroides species from newborns with increased susceptibility to type 1 diabetes and allergies produce a subtype of lipopolysaccharide (LPS) that inhibits the immunostimulatory activity of Escherichia coli LPS [ 41 ]. The authors also point out that in the first year of life, the metabolism of human milk oligosaccharides (HMO) is involved in the maintenance and/or establishment of an intestinal microbiota dominated by Bifidobacterium versus Bacteroides, presumably because both genera contend for the HMO as an energy source, the above is of clinical relevance to promote breastfeeding in children from pregnancies with GDM. Differential enrichment in Bacteroidetes - and Proteobacteria -related taxa according to type of delivery was detected in infants as previously reported by other authors [ 42 ]. However, the present RF classification model trained using data from infants with different type of delivery, was conducted to define chronological age and microbiota maturity in the offspring form healthy mothers regardless of the type of delivery. According to this, ASVs assigned to the discriminatory genera Escherichia_Shigella and Bacteroides in C-section and vaginally born infants were among top 15 age discriminatory taxa in n-GDM offspring group, therefore, discriminatory taxa for type of delivery can also define microbiota maturity.
On the other hand, in this study, the PWY-7377 pathway implicated in the biosynthesis of vitamin B12 (cobalamin) was inversely related to age in the n-GDM group and significantly correlated with the abundance of Veillonella . The synthesis of vitamin B12 is mainly carried out by three orders: Propionibacterales, Corynebacterales, Coriobacterales belonging to Actinobacteria, and the orders Clostridiales, Selenomonadales and Veillonellales ( Veillonella atypica and Veillonella parvula ) of Firmicutes [ 43 ]. Although cobalamin in mammals is synthesized exclusively by the gut microbiota, microbial vitamin B12 production plays a limited function due to its limited availability in the environment. Two issues prevent humans from obtaining significant levels of cobalamin from this source. First, of the total corrinoids in feces, less than 2% of cobalamin is produced by intestinal bacteria; Secondly, due to the absence of transporters of this vitamin in the colon [ 44 , 45 ]. Taking this into account, a possible explanation for our findings could come from vitamin B12-dependent reactions in bacteria. Vitamin B12 contributes to the microbiota as a modulator of intestinal microbial ecology. Since 80% of bacteria encode cobalamin-dependent enzymes [ 45 ]. Considering this information and our results, one hypothesis is that those prototrophic taxa, such as Veillonella , would help supply cobalamin to auxotrophic taxa during the first months of life. Kundra et al. demonstrated that even in the absence of B12, the presence of prototrophic bacteria could supply sufficient cobalamin for the remaining 80% of auxotrophic taxa [ 46 ]. Thus, the presence of Veillonella could help modulate the configuration of the microbiota in the early stages of life.
We found that GDM-exposed offspring had disrupted microbiota maturation characterized by persistently low diversity and reduced Veillonella compared to non-GDM-exposed infants. Finally, a random forest classification model was used to model the maturation of the microbiota of healthy infants, based on the approach of Subramanian et al. [ 47 ]. The model taxa included Bifidobacterium , Escherichia-Shigella , Bacteroides , Veillonella , Clostridium sensu stricto (group I), and Faecalibacterium . Interestingly, Bacteroides , Bifidobacterium , and Clostridium sensu stricto I are first colonizers of the infant intestine [ 48 ]. While Faecalibacterium prausnitzii is present at low abundance and, in some cases, absent during early childhood and with advancing age, its abundance increases until reaching the levels observed in adulthood [ 49 ], suggesting that GDM negatively affects the early establishment in the gut of early colonizers concerning health and delayed establishment of microbiota members that are expected to increase in abundance after the first year of life. However, limitations of the study, are the relatively small number of infants exposed and not exposed to GDM and the predictive limitations of our cross-sectional study, additional prospective studies are needed to replicate our findings and determine whether these signatures of microbiota maturation could apply to GDM-exposed offspring. However, limitations of the study, are the relatively small number of infants exposed and not exposed to GDM and the predictive limitations of a cross-sectional study, further prospective studies are needed to replicate our findings and determine whether these signatures of microbiota maturation could be applicable to GDM-exposed offspring.
In conclusion, children of mothers with GDM have a distinctive taxonomic profile related to the immaturity of the intestinal microbiota. Our results show the importance of the first months of life for the maturation and future configuration of the intestinal microbiota.
Supporting information
S1 fig. microbiota analysis in children not exposed and exposed to gdm stratified by pregnancy outcome..
(a) Phylum-level composition (% relative abundances) among the study groups. (b) Observed features, Chao and Shannon index in C-section and vaginally born infants, no significant differences were observed between the groups; (c) Bray-Curtis index in C-section and vaginally born infants, no significant differences were shown amongst the groups. (d) Heat tree for pair-wise comparison. Those taxa that showed statistically significant differences were members of Proteobacteria in C-section group (red) and members of Bacteroidetes in vaginally born infants group (blue). The colour of each taxon indicates the log-2 ratio of the proportions observed in each condition.
https://doi.org/10.1371/journal.pone.0302726.s001
- View Article
- PubMed/NCBI
- Google Scholar
- 17. Organization W.H., WHO child growth standards and the identification of severe acute malnutrition in infants and children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund. 2009, World Health Organization: Geneva, Switzerland.

IMAGES
VIDEO
COMMENTS
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
The most common symptoms of type 1 diabetes mellitus (DM) are polyuria, polydipsia, and polyphagia, along with lassitude, nausea, and blurred vision, all of which result from the hyperglycemia itself. ... Symptoms at the time of the first clinical presentation can usually be traced back several days to several weeks. However, beta-cell ...
Diabetes mellitus is taken from the Greek word diabetes, meaning siphon - to pass through and the Latin word mellitus meaning sweet. A review of the history shows that the term "diabetes" was first used by Apollonius of Memphis around 250 to 300 BC. Ancient Greek, Indian, and Egyptian civilizations discovered the sweet nature of urine in this condition, and hence the propagation of the word ...
Amylin analogs for the treatment of diabetes mellitus; Classification of diabetes mellitus and genetic diabetic syndromes; Clinical presentation, diagnosis, and initial evaluation of diabetes mellitus in adults ... It is an infection caused by a virus called SARS-CoV-2. The virus first appeared in late 2019 and has since spread throughout the ...
One hour, 180 mg per dL. Two hour, 155 mg per dL. Diabetes can also be diagnosed with a random blood glucose level of 200 mg per dL (11.1 mmol per L) or greater if classic symptoms of diabetes (e ...
Diabetes mellitus is characterized by abnormally high levels of sugar (glucose) in the blood. When the amount of glucose in the blood increases, e.g., after a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates muscle and fat cells to remove glucose from the blood and stimulates the liver to metabolize ...
Nevertheless, it can present in younger adults and teenagers as the first presentation of diabetes mellitus type 2 (T2DM). 1. HHS is diagnosed by the following criteria: plasma glucose more than 600 mg/dl, venous pH > 7.25, serum bicarbonate >15 mmol/L, small amount of ketonuria or its absence, effective serum osmolality >320 mOsm/kg, ...
Pathophysiology and Clinical Presentation. Pathophysiology: Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). Nonimmune (type 1B diabetes), occurs secondary to other diseases and is much less common than autoimmune (type ...
Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of pancreatic beta islet ...
General presentation. Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of ...
Type 1 diabetes mellitus (T1D) is a heterogeneous disorder characterized by autoimmune-mediated destruction of pancreatic beta cells that culminates in absolute insulin deficiency. T1D is most commonly diagnosed in children and adolescents, usually presents with symptomatic hyperglycemia, and imparts the immediate need for exogenous insulin ...
Abstract. This article reviews our current understanding of the etiology, presentation, and management of type 1 diabetes. The discussion includes a review of the natural history of diabetes, the complex relationship between genetic and environmental risk for type 1 diabetes, and current methods for prediction of type 1 diabetes.
DKA is defined as an acute metabolic complication of diabetes distinguished by hyperglycemia, hyperketonemia, and metabolic acidosis. DKA occurs primarily in T1DM and is less common in T2DM; it can present with nausea, vomiting, and abdominal pain and cause cerebral edema, coma, and death. DKA may be the initial presentation in an estimated 25% ...
Warning Signs and Symptoms - Can occur slowly over time. Blurred vision. Tingling or numbness in legs, feet or fingers. Recurring skin, gum or urinary tract infections. Drowsiness. Slow healing of cuts and bruises. Any symptoms that occur with Type 1 diabetes. Impact of Sugary Foods and.
agers as the first presentation of diabetes mellitus type 2 (T2DM).1 HHS is diagnosed by the following criteria: plasma glucose more than 600 mg/dl, venous pH > 7.25, serum bicarbonate >15 mmol/L, small amount of ketonuria or its absence, effective serum osmolality >320 mOsm/kg, and obtundation, combativeness, or seizures (in approx-
Abstract. People with type 1 diabetes (T1DM) usually present with classic symptoms and occasionally diabetic ketoacidosis. People with type 2 diabetes mellitus (T2DM) may be asymptomatic or present with classic symptoms. In the context of thirst, polydipsia and polyuria, the presence or absence of weight loss is an important diagnostic feature.
Diabetes is a serious, chronic disease that occurs either when the pancreas does not produce enough insulin (a hormone that regulates blood sugar, or glucose), or when the body cannot effectively use the insulin it produces. Diabetes is an important public health problem, one of four priority noncommunicable diseases
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides ...
Editor's key points. Diabetic ketoacidosis (DKA) is a common presentation of type 1 diabetes mellitus in children younger than 3 years of age. Kussmaul breathing, characterized by tachypnea and increased depth of breath in response to metabolic acidosis, is a less common first presentation of DKA. During cold and flu season, DKA can be harder ...
Hyperglycemic hyperosmolar syndrome (HHS) is a rare complication of diabetes mellitus among pediatric patients. Since its treatment differs from diabetic ketoacidosis (DKA), hence, pediatricians should be aware of its diagnosis and management. ... First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a ...
Symptoms of gestational diabetes. Gestational diabetes (diabetes during pregnancy) usually doesn't have any symptoms. If you're pregnant, your doctor should test you for gestational diabetes between 24 and 28 weeks of pregnancy. If needed, you can make changes like healthy eating and being active to protect your health and your baby's health.
Nevertheless, it can present in younger adults and teenagers as the first presentation of diabetes mellitus type 2 (T2DM). 1 HHS is diagnosed by the following criteria: plasma glucose more than 600 mg/dl, venous pH > 7.25, serum bicarbonate >15 mmol/L, small amount of ketonuria or its absence, effective serum osmolality >320 mOsm/kg, and ...
Type 1. Insulin Dependant Diabetes Mellitus (IDDM) is an autoimmune that occurs when the pancreas stops producing insulin. This happens because the person's immune system mistakenly destroys the producing beta cells in the pancreas, leading to the person dependant on insulin injections. This type of diabetes usually below the age of 15.
Type 1 diabetes is an autoimmune disease, caused by the destruction of pancreatic islet cells. hence, absolute insulin deficiency [].Affected individuals have hyperglycemia and are susceptible to acute complications from hypoglycemia and ketoacidosis [].Long-term morbidity caused by Vascular complications as well as mortality are real challenges for young people with type 1 diabetes mellitus ...
Type 2 diabetes (T2D) in youth is recognized as a pediatric disease, but few reports describe the characteristics during diagnosis. We describe the clinical presentation of 503 youth with T2D.
Expert opinion from Rajesh Mishra. MBBS · Less than a year of experience · India. Type 1 diabetes occurs when your immune system, the body's system for fighting infection, attacks and destroys ...
Notably, the prevalence of DM was associated with an increased incidence of males (p < 0.001), patients in the age group ≥70 years (p < 0.001), and those with a BMI categorization as obese (p < 0.001). The heart failure type was also significantly associated with DM, with a higher proportion of HFpEF observed in the DM group (p = 0.006).
Introduction. Gestational diabetes mellitus (GDM) according to the American Diabetes Association is defined as "diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes before gestation" [].In Mexico, the prevalence of GDM has been increasing; currently, its incidence is 17.7% [].GDM during pregnancy increases the susceptibility of offspring to ...
A 44-year-old male with poorly controlled diabetes mellitus type 2 on metformin presented to the Emergency Department with a two-day history of right-sided chest pain and shortness of breath. Remarkably, the patient had a 1 cm boil on his forehead (Figure 1) with minimal pus formation, which he had noticed a few days earlier. Upon presentation ...
Comparative Analysis by Diabetes Status and Ejection Fraction. When categorized by diabetes status and ejection fraction, the study identified significant differences in sex distribution, age, and prevalence of hypertension among the groups as presented in Table 3 (p = 0.011, p = 0.002, and p = 0.020, respectively). There was a notable ...