- Skip to main content
- Skip to main navigation
- Awards & Honors
- News by topic
- News archives
- E-Newsletter
- Tuesday Newsday
- UCSC Magazine
- Administrative Messages
Home / 2024 / May / Tiny target discovered on RNA to short-circuit inflammation

New study discovers tiny target on RNA to short-circuit inflammation
Paper details high-throughput process for rapid screening, identification of mysterious ‘long non-coding RNA’
May 24, 2024
By Mike Peña

UC Santa Cruz researchers have discovered a peptide in human RNA that regulates inflammation and may provide a new path for treating diseases such as arthritis and lupus. The team used a screening process based on the powerful gene-editing tool CRISPR to shed light on one of the biggest mysteries about our RNA–the molecule responsible for carrying out genetic information contained in our DNA.
This peptide originates from within a long non-coding RNA (lncRNA) called LOUP. According to the researchers, the human genome encodes over 20,000 lncRNAs, making it the largest group of genes produced from the genome. But despite this abundance, scientists know little about why lncRNAs exist or what they do. This is why lncRNA is sometimes referred to as the "dark matter of the genome."
The study , published May 23 in the Proceedings of the National Academy of Sciences (PNAS), is one of the very few in the existing literature to chip away at the mysteries of lncRNA. It also presents a new strategy for conducting high-throughput screening to rapidly identify functional lncRNAs in immune cells. The pooled-screen approach allows researchers to target thousands of genes in a single experiment, which is a much more efficient way to study uncharacterized portions of the genome than traditional experiments which focus on one gene at a time.
The research was led by immunologist Susan Carpenter, a professor and Sinsheimer Chair of UC Santa Cruz's Molecular, Cell, and Developmental Biology Department . She studies the molecular mechanisms involved in protection against infection. Specifically, she focuses on the processes that lead to inflammation to determine the role that lncRNAs play in these pathways.
"Inflammation is a central feature of just about every disease," she said. "In this study, my lab focused on trying to determine which lncRNA genes are involved in regulating inflammation."
This meant studying lncRNAs in a type of white blood cell known as a monocyte. They used a modification of the CRISPR/Cas9 technology, called CRISPR inhibition (CRISPRi), to repress gene transcription and find out which of a monocyte's lncRNAs play a role in whether it differentiates into a macrophage—another type of white blood cell that's critical to a well-functioning immune response.
In addition, the researchers used CRISPRi to screen macrophage lncRNA for involvement in inflammation. Unexpectedly, they located a region that is multifunctional and can work as an RNA as well as containing an undiscovered peptide that regulates inflammation.
Understanding that this specific peptide regulates inflammation gives drugmakers a target to block the molecular interaction behind that response in order to suppress it, Carpenter said. "In an ideal world, you would design a small molecule to disrupt that specific interaction, instead of, say, targeting a protein that might be expressed throughout the body," she explained. "We're still a long way from targeting these pathways with that level of precision, but that’s definitely the goal. There's a lot of interest in RNA therapeutics right now."
Co-authors of the study from UC Santa Cruz include Haley Halasz, Eric Malekos, Sergio Covarrubias, Samira Yitiz, Christy Montano, Lisa Sudek, and Sol Katzman, along with researchers at UCSF and MIT. The research was supported with funding from the National Institute of General Medical Sciences (R35GM137801 to Carpenter) and the National Institute of Allergy and Infectious Diseases (F31AI179201 to Malekos).
University News
- University News Home
- Monthly Newsletter
Other News & Events
- Campus Calendars

- UCSC Chancellor
- Press Releases
- Contacts for Reporters
- Send us an email
- Report an accessibility barrier
- Land Acknowledgment
- Accreditation
Last modified: May 24, 2024 128.114.113.87
Premium Content

- MIND, BODY, WONDER
The end of inflammation? New approach could treat dozens of diseases.
Cancer, aging, and severe COVID-19 have all been linked to damage from inflammation. Now scientists are flipping their focus to find new drugs that may revolutionize treatments.
Growing up in Atlanta, Georgia, Lauren Finney Harden had always had allergies. But after she moved to New York City for her first job in 2007, inflammation “just exploded” throughout her body.
“I had insane full-body rashes and strange gastro issues. I’d get massive burps that made me feel like I needed to throw up, but nothing would come up but air,” she says. Eventually, she was diagnosed with lupus, a disease in which the immune system attacks the body’s own tissues and organs. She was put on a drug called prednisone, a corticosteroid that tamps down inflammation.
But the cure, at times, felt worse than the disease. “I looked four months pregnant all the time,” Finney Harden says, “and I’d get cold sores every other week; my body could not fight off anything.”
Finney Harden’s experience is unfortunately a common one with traditional autoimmune treatments like prednisone. A broad immunosuppressant , prednisone works by disabling the production of pro-inflammatory molecules that are crucial for the body to mount an immune defense. So while prednisone—and drugs like it—are adept at quickly snuffing out inflammation, they leave the body vulnerable to any bug it encounters, and they can come with toxic side effects.
“Simply stopping inflammation is not enough to return tissue to its normal state,” says Ruslan Medzhitov , a professor of immunobiology at Yale School of Medicine. This approach ignores the other side of the inflammation coin: resolution. Resolving inflammation is an active, highly choreographed process for rebuilding tissue and removing the dead bacteria and cells. When that process is disrupted, inflammatory diseases arise.
In the early 2000s researchers began to recognize the role of inflammation in conditions as varied as Alzheimer’s , cancer , diabetes , and heart disease , prompting them to recast inflammation as the unifying explanation for a myriad of ailments, including those we develop as we age. Even aging itself, and its associated pathologies, is driven by persistent inflammation .
For Hungry Minds
“Until relatively recently, we believed that inflammation just stopped,” says Molly Gilligan, an internal medicine resident at Columbia University who studies how the immune system impacts cancer development. Immunologists thought that products of inflammation—molecules that trigger it and dead cells and tissue—are eventually metabolized, or spontaneously dissipate on their own.
The reality is more complicated, and recognizing that could have game-changing effects on how we treat a wide swath of diseases.
Why is inflammation dangerous?
Inflammation evolved to serve an important function: It rids our bodies of stuff that doesn’t belong, including foreign invaders like bacteria and viruses, tumor cells, and irritants like splinters.
“A classic example of inflammatory onset is the bee sting—the site becomes hot, red, swollen, and painful,” says Derek Gilroy , a professor of immunology at University College London. This response comes from a series of biological changes: blood vessels dilate to deliver white blood cells to the site of injury, making tissues turn red. Fluid also floods the site, causing swelling. The molecules that trigger these vascular transformations precipitate the itching, pain, and fever associated with inflammation.
White blood cells, the body’s first responders, then swarm and kill the invaders. Under normal circumstances, this carnage is contained, with the initial inflammatory response subsiding within 24 to 48 hours.
When inflammation becomes chronic, though, the chemical weapons deployed by front-line immune cells often damage healthy tissue, and our bodies become collateral damage. The price exacted includes worn joints , damaged neurons , scarred kidneys , and more. Autoimmune diseases like rheumatoid arthritis and lupus, characterized by pain and worsening disability, have long been associated with persistent inflammation.
In extreme cases, such as the cytokine storms associated with sepsis or severe COVID-19, inflammation can destroy and disable multiple organs , leading to catastrophic system failure and death.
What happens after inflammation?
Medzhitov likens an infection to a broken pipe that has flooded an office with water. Fixing the pipe might stop water from streaming in, but it doesn’t restore the office to its previous, functional state. Similarly, inflammation has a clean-up phase known as resolution, and it proceeds in a series of highly coordinated steps.
Like inflammation’s onset, its resolution is orchestrated by an army of signaling molecules. Among the most intensely studied are the specialized pro-resolving mediators, or SPMs, which were discovered in the 1990s by Charles Serhan , a professor of anesthesia at Harvard Medical School. Serhan was inspired by his postdoctoral mentor, Bengt Samuelsson , who uncovered how fatty molecules called lipids trigger inflammation. Serhan was searching for similar molecules when he identified lipoxin. But to his surprise, rather than inciting inflammation, lipoxin seemed to hamper it.
Over the next several years, Serhan and his colleagues identified additional SPMs. These molecules are derived from essential fatty acids such as those omega-3s famously found in cold-water fish like salmon and sardines. But they are difficult to study in the lab. “One of the main challenges is that they have short half-lives, so the body metabolizes them very quickly,” Gilligan says. Because of this, researchers who work on them often turn to synthetic versions of the molecules, or mimetics, which are simpler, more stable, and cheaper to produce.
Catherine Godson , a professor of molecular medicine at University College Dublin, has long been interested in diabetes, given its impact on global public health as the most common cause of kidney failure. When she learned of SPMs, she was excited by the idea of encouraging resolution to treat diabetics, a “population with a particularly high risk for infection.”
In mice with diabetic kidney disease , scarring from kidney inflammation gradually destroys the organ. Her team is testing the therapeutic potential of a lipoxin mimetic in these and other animal models. They’ve also looked at the mimetic’s effect in human tissue in lab cell cultures taken from patients with atherosclerosis, an inflammatory disease of the blood vessel wall. In both cases, inflammatory factors plummeted when the mimetic was introduced; for the mice, the kidneys recovered their function in a stunning reversal of established disease.
Gilroy notes, however, that the story on SPMs is incomplete. “While lipoxins are present at levels in the body that indicate that they’re important in resolution, other SPMs such as resolvins require more evaluation,” he says.
You May Also Like

Is long COVID forever? A new study has clues.

What is 'immune amnesia?' This long-term side effect of measles is newly relevant.

What your biological age can reveal about your health
Manipulating macrophages.
Scientists speculate that one way lipoxins and other pro-resolution molecules work is by interacting with immune cells called macrophages.
Because they’re so abundant during inflammation, macrophages have traditionally been thought of as pro-inflammatory cells, says Gerhard Krönke , an immunologist and rheumatologist at the University of Erlangen-Nürnberg. “But a paradigm shift in the last decade or so suggests that macrophages are pivotal players in the resolution of inflammation.”
Gilroy agrees, calling macrophages “linchpin cells at the juxtaposition of inflammation and resolution: It can go one way if we’re healthy and the other way if we’re not.”
Initially, when the danger posed by invaders is at its peak, the macrophages drawn to the area are inflammatory—secreting pro-inflammatory cytokines and amping up production of antimicrobial agents. But this balance shifts as the tide of the confrontation turns. After the number of viruses declines, the debris left behind—viral remnants, dead immune cells, and other waste—must be collected and cleared away before it sparks another cycle of inflammation. That’s when the macrophages switch gears.
Attracted by “eat me” signals expressed on the surface of dying cells, macrophages readily engulf and clear cellular corpses from the environment. But it’s not just about clearing the wreckage—this act also flips a genetic switch, reprogramming macrophages to restore balance to the system and heal the tissues.
“Macrophages start to produce factors that tell the local tissue, Don’t recruit any more inflammatory cells here, or, Let’s proliferate and start repairs there,” says Kodi Ravichandran , an immunologist at Washington University in St. Louis whose research focuses on how dead cells are cleared from the body.
Clearing away cellular debris
Now consensus is building that many of the illnesses attributed to inflammation—both chronic and acute—can be traced to a failure in resolution. Often that translates into a failure to clear away dead cells.
“If you knock out receptors in the macrophages of mice that recognize dying cells, for example, they become incapable of eating up these cells, resulting in a lupus-like disease,” with symptoms such as arthritis and skin rash, says Krönke.
A similar mechanism is at work in older people, says Gilroy. As we age, the body loses a protein that recognizes dying cells; this blocks macrophages’ ability to find and eat debris. Locked in a pro-inflammatory state, these macrophages continue to produce molecules that amplify the inflammatory response early on.
Perhaps COVID-19 has been more severe in older populations “because they’ve lost some of the pro-resolution pathways with age,” suggests Luke O’Neill , an immunologist at Trinity College Dublin. He notes that COVID-19 has also been problematic for people with genetic differences that impact immune function, resulting in overactive inflammatory responses or underactive pro-resolving ones. His group and others have demonstrated that macrophages primed for inflammatory action play a significant role in critical COVID-19 cases, and they are currently testing pro-resolving strategies to combat this effect.
Cancer’s course, too, is affected when inflammation fails to resolve. The soup of toxins, growth factors, and other inflammatory by-products that accompany inflammation spurs cancer’s growth and spread. Many conventional treatments end up exacerbating the problem, according to Dipak Panigrahy , an assistant professor of pathology at Beth Israel Deaconess Medical Center in Boston.
“Chemotherapy and radiation are like sledgehammers,” Panigrahy says. “They may kill the tumor, but the debris they create stimulates inflammation, which feeds circulating tumor cells that survive the treatment.”
A decade ago, Panigrahy was puzzling over this conundrum when he met Serhan at a conference on lipids in Cancún, Mexico. “I had just presented my research on cell death in cancer and how there’s no way to clear the resulting debris when I heard Serhan’s talk about how he discovered these lipids that eliminated debris,” he says. The two Boston-based researchers have shared a close collaboration ever since.
In proof-of-concept experiments conducted on mice, Panigrahy’s group was able to prevent tumors from recurring after surgery by dosing the animals with mimetics of resolvin, one of the pro-resolving mediators discovered in Serhan’s lab. Phase one clinical trials for pancreatic, brain, and colon cancers will begin this year, says Panigrahy.
Long COVID and inflammation
Although much work remains to decode its secrets, “long COVID likely results from a catastrophic failure of appropriate immune response and resolution,” Gilroy suggests.
Meg St. Esprit is part of a large cohort of COVID-19 survivors who continue to suffer symptoms months after the virus has passed. She and her family contracted the disease in November 2020, and for seven days the mother of four in Pittsburgh, Pennsylvania, was beset by a high fever and severe headaches. Debilitating fatigue, vertigo, and brain fog soon followed. But while her husband and children recovered, St. Esprit’s symptoms lingered, and new ones emerged.
Since her bout with COVID-19, she has developed blood clots and myocarditis—dangerous consequences of inflammation. It’s also as if her entire body has gone haywire. “Different parts of it regularly flare up now,” she says. “My thumb joints swell to twice their normal size, my knee puffs out like a grapefruit, and I’ve had hives more times than I can count.”
Drugs to tweak the natural inflammatory process would thus be a powerful tool in our arsenal for long COVID as well. Even now the hunt is on . O’Neill and colleagues, for example, are testing molecules in clinical trials that push macrophages to be pro-resolving. SPMs are being tested extensively in animal models of diseases like cancer and sepsis, and more modestly in small patient trials studying eczema and periodontal disease .
But Gilroy cautions that the answer may be more nuanced than anti-inflammatory versus pro-resolution, and that drugs targeting both approaches may be needed.
“It’s like driving a car at full speed,” he says. “In order to stop, you take your foot off the accelerator, which would be like dampening inflammation’s onset. And then you apply the brakes, or in other words, promote its resolution.”
Related Topics
- IMMUNE SYSTEM
- CORONAVIRUS

Now we know how COVID attacks your heart

COVID-19 can ruin your sleep in many different ways—here's why

Multiple COVID infections can lead to chronic health issues. Here’s what to know.

These fish live beyond 100—and get healthier as they age

The Mediterranean diet has stood the test of time for a reason: It works
- Environment
- Paid Content
- Photography
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Inflammation in Health and Disease: New Insights and Therapeutic Avenues
Morena scotece.
1 Molecular Mechanisms of Cancer Program, Centro de Investigación del Cáncer (CIC), Instituto de Biología Molecular y Celular del Cáncer (IBMCC), CSIC-USAL, 37007 Salamanca, Spain; [email protected]
Javier Conde-Aranda
2 Molecular and Cellular Gastroenterology, Health Research Institute of Santiago de Compostela (IDIS), 15706 Santiago de Compostela, Spain
The inflammatory response is an adaptive mechanism that evolved to fight against infections and tissue damage. This complex mechanism is vital for the proper function of the organism, and because of that, is under tight regulation involving a complex network of factors, cells, and systems. Physiological inflammation is perfectly orchestrated to allow the mobilization of leukocytes from the circulation to the injured tissues for the removal of pathogens, tissue repair, and return to homeostasis. However, when the self-limited nature of the inflammatory response and the mechanisms of resolution fail, inflammation might become chronic, leading to the development of many disabling serious diseases such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), or psoriasis.
Knowledge of the molecular and cellular processes underlying chronic inflammation has substantially increased in recent decades. This resulted in enormous improvements in the treatments for immune-mediated inflammatory diseases, but also revealed the complexity of the cytokine networks and immune cell subtypes involved in non-resolving inflammatory responses. This fact could explain, in part, why certain groups of patients are totally or partially refractory to current therapies. Therefore, we cannot be satisfied with the available drugs, and more research focusing on deciphering the precise molecular pathways behind uncontrolled inflammation is mandatory for developing novel therapeutic approaches.
The 34 original and review articles included in this Special Issue contributed to the aim of describing and understanding new mechanisms that trigger inflammation and highlighted new putative treatments for different inflammatory conditions.
Neuroinflammation is a perfect example of the two faces of inflammation. While an acute inflammatory response is protective, chronic neuroinflammation leads to inflammatory conditions such as multiple sclerosis, and it also contributes to other neurological [ 1 ] and psychiatric disorders such as Alzheimer’s or Parkinson’s disease [ 2 ]. Microglia is pivotal for regulating central nervous system inflammation. Therefore, it would be interesting to develop therapeutic strategies conducted to mitigate their hyperactivation. Following that aim, Saliba et al. synthesized a new antagonist for the orphan G-protein coupled receptor 55, which decreased the production of prostaglandin E 2 and reduced the activation of pro-inflammatory signalling pathways [ 3 ]. In line with this, other receptors regulating microglia function are being explored. This is the case for some purinergic receptors such as P2Y receptors, whose actions on microglia and neuroinflammation were elegantly reviewed by Gómez Morillas et al. [ 4 ]. These data highlight that the isolation of new receptors that can be pharmaceutically targeted might be relevant for treating neuroinflammatory disorders. Actually, a similar approach is followed for other immune-mediated pathologies, in which the inhibition of various receptors such as sodium-glucose co-transporter 2 [ 5 ], angiotensin receptor [ 6 ], or toll-like receptors [ 7 ] is being tested for controlling the exacerbated inflammatory response during acute kidney injury, COVID-19 infection, heart failure, etc.
Focusing on autoimmune responses, Amend et al. [ 8 ] and Carvalheiro et al. [ 9 ] investigated the effects of IL-10 and angiopoietin-2 in the context of lupus and systemic sclerosis, respectively. The former shed light on the poorly understood role played by IL-10 in the development of lupus, revealing that IL-10 outcome depends on the complex interplay among the different immune cells and the inflammatory microenvironment. The latter investigated, for the first time, how angiopoietin-2 can increase the production of pro-inflammatory mediators by monocytes from systemic sclerosis patients, which could contribute to the development of fibrotic processes in the skin of those patients. Fibrosis is also a key feature in chronic kidney injury, and Leong et al. [ 10 ] functionally demonstrated in experimental acute kidney injury mouse models that cyclophilin inhibition protects from renal injury and fibrosis, an observation that is accompanied by reduced innate immune cell infiltration.
Inflammation significantly contributes to skin malignancies, as reviewed by Ansary et al. [ 11 ] and Razib Hossain et al. [ 12 ]. Interestingly, Yang et al. [ 13 ] demonstrated in vitro and in vivo the therapeutic potential of the natural small molecule neferine as an anti-inflammatory drug for atopic dermatitis. Moreover, Hathaway-Schrader et al. [ 14 ] showed new insights for a better comprehension of the immune evasion in melanoma cells.
Historically, inflammation-related research mainly comprised studies related to immune cells and inflammatory mediators. However, in recent years, more multidisciplinary and integrative approaches have been applied for the dissection of the inflammatory process [ 15 ]. This is the case for the article by Weiss et al. [ 16 ], which showed an interesting view of the participation of histone deacetylases in the activation of pro-inflammatory signalling cascades and mediators in cultured macrophages. Moreover, another work explored the methylation status of specific promoters for their participation in neuronal inflammatory diseases. Hypermethylated O 6 -methylguanine-DNA methyltransferase is commonly observed in brain tumours. However, Teuber-Hanselmann et al. [ 17 ] reported an original hypermethylation of that gene in inflammatory conditions affecting the central nervous system. Likewise, the participation of adipokines and obesity in regulating inflammation and cardiovascular disease [ 18 , 19 ] and the impact of the YAP/TAZ signalling in the immunomodulatory responses of tumours [ 20 ] have also been discussed. Of note, Götz et al. [ 21 ] demonstrated in vivo the relevance of the complement system in the recruitment of neutrophils and M2-polarised macrophages to ischemic tissues, which, most likely, are the most relevant players in the observed improvement of angiogenesis.
The impact of environmental factors such as pollutants, microparticles, or endocrine disruptors is gaining interest. The latter is not only relevant for their interference with the normal function of the endocrine system, but endocrine disruptors can also participate in the pathophysiology of non-alcoholic fatty liver disease (NAFLD) (as reviewed by Cano et al.) by modulating liver metabolism [ 22 ]. In the same way, the food additive titanium dioxide worsened experimental colitis in mice carrying IBD genetic risk mutations [ 23 ], suggesting that the ingestion of industrial compounds can be detrimental to patients with IBD with an increased genetic predisposition. Silicon dioxide is well-known for causing lung silicosis, and novel pathophysiological mechanisms and treatment options were reviewed by Adamcakova et al. [ 24 ]. Interestingly, Wang et al. [ 25 ] demonstrated the efficacy of a novel class of pharmacological compounds, namely SUL-151, in decreasing neutrophilia after cigarette smoke exposure in mice.
This Special Issue also contains translational studies demonstrating the association between high concentrations of IL-6 receptor antagonist and reduced carbohydrate disorders and NAFLD progression in obese individuals [ 26 ], the impairment of circulating monocytes and the aberrant production of pro-inflammatory mediators in spinal cord injury patients [ 27 ], and the existence of a fibroinflammatory signature in human follicular fluid of the female reproductive system [ 28 ]. Furthermore, Di Paola et al. [ 29 ] showed the effects of the thrombopoietin receptor agonist Eltrombopag in the differentiation of macrophages obtained from children with immune thrombocytopenia. Altogether, these data show, in the human setting, how inflammation can also be at play in conditions not typically recognised as immune-mediated diseases.
Finally, in this compilation of articles, we find very interesting reviews updating a wide variety of topics such as hypoalbuminemia as a surrogate of infections [ 30 ], the resolution of inflammation in IBD or pain [ 31 , 32 ], the impact of inflammation in liver tumorigenesis and alcohol disease [ 33 , 34 ], and nutraceutical supplementation in obesity-associated disorders [ 35 ]. Moreover, Rafael-Vidal et al. reviewed the potential use of calcineurin inhibitors to treat lupus nephritis [ 36 ], and the molecular and cellular mechanisms regulating osteoporosis after spinal cord injury were described by Shams et al. [ 37 ].
We hope the lectors will appreciate reading these papers. We thank all the authors and reviewers for their dedication and proactive participation that made the realisation of this remarkable Special Issue possible.
Funding Statement
The research of JCA is funded by the Instituto de Salud Carlos III through the ‘Miguel Servet’ Program and by the Fondo Social Europeo (FSE), CP19/00172 and PI21/00880.
Author Contributions
Conceptualization, M.S. and J.C.-A.; writing—original draft preparation, M.S. and J.C.-A.; writing—review and editing, M.S. and J.C.-A.; funding acquisition, J.C.-A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
New Alzheimer’s study suggests genetic cause of specific form of disease

Had a bad experience meditating? You’re not alone.
Rethinking life-support decisions

Harvard-led study IDs statin that may block pathway to some cancers
Cholesterol-lowering drug suppresses chronic inflammation that creates dangerous cascade
Tracy Hampton
MGH Communications
Statins, commonly used cholesterol-lowering drugs, may block a pathway that leads to the development of cancer from chronic inflammation, according to a new study led by investigators from Harvard-affiliated Mass General Cancer Center .
The team’s experiments showed that environmental toxins, such as those caused by exposure to allergens and chemical irritants, create a cascade effect that stimulates inflammation in the skin and pancreas that, when chronic, can result in cancer. Their findings suggest that using statins to suppress this pathway may have a protective effect.
The findings are published in Nature Communications.
In mice, pitavastatin suppressed environmentally induced inflammation in the skin and the pancreas and prevented the development of inflammation-related pancreatic cancers.
“Chronic inflammation is a major cause of cancer worldwide,” said senior author Shawn Demehri, a principal investigator at the Center for Cancer Immunology and Cutaneous Biology Research Center of Massachusetts General Hospital and an associate professor of dermatology at Harvard Medical School and the Bob and Rita Davis Family MGH Research Scholar 2023-2028 .
“We investigated the mechanism by which environmental toxins drive the initiation of cancer-prone chronic inflammation in the skin and pancreas. Furthermore, we examined safe and effective therapies to block this pathway in order to suppress chronic inflammation and its cancer aftermath,” Demehri said.
The study relied on cell lines, animal models, human tissue samples, and epidemiological data. The group’s cell-based experiments demonstrated that environmental toxins (such as exposure to allergens and chemical irritants) activate two connected signaling pathways called the TLR3/4 and TBK1-IRF3 pathways. This activation leads to the production of the interleukin-33 (IL-33) protein, which stimulates inflammation in the skin and pancreas that can contribute to the development of cancer.
When they screened a library of U.S. Food and Drug Administration-approved drugs, the researchers found that the statin pitavastatin effectively suppresses IL-33 expression by blocking the activation of the TBK1-IRF3 signaling pathway. In mice, pitavastatin suppressed environmentally induced inflammation in the skin and pancreas and prevented development of inflammation-related pancreatic cancers.
In human pancreas tissue samples, IL-33 was overexpressed in samples from patients with chronic pancreatitis (inflammation) and pancreatic cancer compared with normal pancreatic tissue. Also, in analyses of electronic health records data on more than 200 million people across North America and Europe, use of pitavastatin was linked to a significantly reduced risk of chronic pancreatitis and pancreatic cancer.
The findings demonstrate that blocking IL-33 production with pitavastatin may be a safe and effective preventive strategy to suppress chronic inflammation and the subsequent development of certain cancers.
“Next, we aim to further examine the impact of statins in preventing cancer development in chronic inflammation in liver and gastrointestinal tract and to identify other novel, therapeutic approaches to suppress cancer-prone chronic inflammation” said Demehri.
Research support was provided by the Burroughs Wellcome Fund, the LEO Foundation, the Sidney Kimmel Foundation, and the National Institutes of Health.
Share this article
You might like.
Findings eventually could pave way to earlier diagnosis, treatment, and affect search for new therapies

Altered states of consciousness through yoga, mindfulness more common than thought and mostly beneficial, study finds — though clinicians ill-equipped to help those who struggle

Of the survivors within one study group, more than 40% recovered at least some independence.
When should Harvard speak out?
Institutional Voice Working Group provides a roadmap in new report
Had a bad experience meditating? You're not alone.
Finding right mix on campus speech policies.
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
- skip to Cookie Notice
- skip to Main Navigation
- skip to Main Content
- skip to Footer
- Find a Doctor
- Find a Location
- Appointments & Referrals
- Patient Gateway
- Español
- Leadership Team
- Quality & Safety
- Equity & Inclusion
- Community Health
- Education & Training
- Centers & Departments
- Browse Treatments
- Browse Conditions A-Z
- View All Centers & Departments
- Clinical Trials
- Cancer Clinical Trials
- Cancer Center
- Digestive Healthcare Center
- Heart Center
- Mass General for Children
- Neuroscience
- Orthopaedic Surgery
- Information for Visitors
- Maps & Directions
- Parking & Shuttles
- Services & Amenities
- Accessibility
- Visiting Boston
- International Patients
- Medical Records
- Billing, Insurance & Financial Assistance
- Privacy & Security
- Patient Experience
- Explore Our Laboratories
- Industry Collaborations
- Research & Innovation News
- About the Research Institute
- Innovation Programs
- Education & Community Outreach
- Support Our Research
- Find a Researcher
- News & Events
- Ways to Give
- Patient Rights & Advocacy
- Website Terms of Use
- Apollo (Intranet)
- Like us on Facebook
- Follow us on Twitter
- See us on LinkedIn
- Print this page
Press Release May | 30 | 2024
Statin Therapy May Prevent Cancer by Blocking Inflammatory Protein
Key takeaways.
- A team led by investigators from Mass General Cancer Center found that statins—commonly used cholesterol-lowering drugs—may block a pathway that leads to the development of cancer in the context of chronic inflammation
- The team’s experiments showed that environmental toxins, such as those caused by exposure to allergens and chemical irritants, create a cascade effect that stimulates inflammation in the skin and pancreas that can lead to cancer
- Their findings suggest that using statins to suppress this pathway may have a protective effect against the development of cancer that results from chronic inflammation
BOSTON, MA (May 30, 2024) — A new study led by investigators from Mass General Cancer Center , a founding member of the Mass General Brigham healthcare system, reveals that statins—commonly used cholesterol-lowering drugs—may block a particular pathway involved in the development of cancer that results from chronic inflammation. The findings are published in Nature Communications .
“Chronic inflammation is a major cause of cancer worldwide,” said senior author Shawn Demehri, MD, PhD, a principal investigator at the Center for Cancer Immunology and Cutaneous Biology Research Center of Massachusetts General Hospital and an associate professor of Dermatology at Harvard Medical School.
“We investigated the mechanism by which environmental toxins drive the initiation of cancer-prone chronic inflammation in the skin and pancreas,” says Demehri, who is also the Bob and Rita Davis Family MGH Research Scholar 2023-2028. “Furthermore, we examined safe and effective therapies to block this pathway in order to suppress chronic inflammation and its cancer aftermath.”
Demehri and his colleagues’ study relied on cell lines, animal models, human tissue samples and epidemiological data. The group’s cell-based experiments demonstrated that environmental toxins (such as exposure to allergens and chemical irritants) activate two connected signaling pathways called the TLR3/4 and TBK1-IRF3 pathways. This activation leads to the production of the interleukin-33 (IL-33) protein, which stimulates inflammation in the skin and pancreas that can contribute to the development of cancer.
When they screened a library of U.S. Food and Drug Administration–approved drugs, the researchers found that a statin, pitavastatin, effectively suppresses IL-33 expression by blocking the activation of the TBK1-IRF3 signaling pathway. In mice, pitavastatin suppressed environmentally-induced inflammation in the skin and the pancreas and prevented the development of inflammation-related pancreatic cancers.
In human pancreas tissue samples, IL-33 was over-expressed in samples from patients with chronic pancreatitis (inflammation) and pancreatic cancer compared with normal pancreatic tissue. Also, in analyses of electronic health records data on more than 200 million people across North America and Europe, use of pitavastatin was linked to a significantly reduced risk of chronic pancreatitis and pancreatic cancer.
The findings demonstrate that blocking IL-33 production with pitavastatin may be a safe and effective preventive strategy to suppress chronic inflammation and the subsequent development of certain cancers.
“Next, we aim to further examine the impact of statins in preventing cancer development in chronic inflammation in liver and gastrointestinal tract and to identify other novel, therapeutic approaches to suppress cancer-prone chronic inflammation” said Demehri.
Authorship: Jong Ho Park, Mahsa Mortaja, Heehwa G. Son, Xutu Zhao, Lauren M. Sloat, Marjan Azin, Jun Wang, Michael R. Collier, Krishna S. Tummala, Anna Mandinova, Nabeel Bardeesy, Yevgeniy R. Semenov, Mari Mino-Kenudson, and Shadmehr Demehri.
Disclosures: Disclosure forms provided by the authors are available with the full text of this article at https://doi.org/10.1038/s41467-024-48441-8 . Funding: Research support was provided by the Burroughs Wellcome Fund, the LEO Foundation, the Sidney Kimmel Foundation, and the National Institutes of Health.
Paper cited: Park JH et al. “Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression.” Nature Communications DOI: 10.1038/s41467-024-48441-8.

Shawn Demehri, MD, PhD
- Associate Professor of Dermatology, Harvard Medical School
- Director, High Risk Skin Cancer Clinic
- Press Release
Centers and Departments
- Research Institute
- Dermatology
- Krantz Family Center for Cancer Research
- MGH Research Scholar
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
About Massachusetts General Hospital
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The Mass General Research Institute conducts the largest hospital-based research program in the nation, with annual research operations of more than $1 billion and comprises more than 9,500 researchers working across more than 30 institutes, centers and departments. MGH is a founding member of the Mass General Brigham healthcare system.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 3, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Low-dose aspirin reduces inflammation caused by sleep loss
by American Academy of Sleep Medicine
A new study to be presented at the SLEEP 2024 annual meeting, held in Houston, Texas, June 1–5, found that low-dose acetylsalicylic acid, also known as aspirin, can reduce inflammatory responses to sleep restriction.
Results show that compared with placebo, preemptive administration of low-dose aspirin during sleep restriction reduced pro- inflammatory responses . Specifically, aspirin reduced interleukin-6 expression and COX-1/COX-2 double positive cells in lipopolysaccharide-stimulated monocytes, as well as C-reactive protein serum levels.
"The novelty of this study is that it investigated whether we can pharmacologically reduce the inflammatory consequences of sleep restriction," said lead author Larissa Engert, who has a doctorate in behavioral physiology and is a postdoctoral fellow in the department of neurology at Beth Israel Deaconess Medical Center and the division of sleep medicine at Harvard Medical School in Boston.
"We used a non-steroidal, anti-inflammatory drug because it has been shown to affect specific inflammatory pathways, which were previously shown to be dysregulated by experimental sleep restriction or sleep disturbances ."
The researchers collected data from 46 healthy adults in a randomized placebo-controlled crossover trial with three protocols—sleep restriction/aspirin, sleep restriction/placebo, and control sleep/placebo—each consisting of a 14-day at-home phase followed by an 11-day in-hospital stay.
In the sleep restriction/aspirin condition, participants took low-dose aspirin during the at-home phase and in-hospital stay. Each in-hospital stay started with two nights of an eight-hour sleep opportunity. Then, under the sleep restriction conditions, participants were exposed to five nights of a four-hour sleep opportunity, followed by three nights of recovery sleep.
The control sleep condition provided an eight-hour sleep opportunity throughout the in-hospital stay. Sleep and immunologic measures were assessed at baseline and various points throughout the study.
The data also reveal that the aspirin-induced reduction of inflammatory pathway activity in sleep-restricted participants was paralleled by decreased wake after sleep onset and increased sleep efficiency during recovery sleep, Engert noted.
"These findings show that it is possible to blunt inflammatory pathways activated by sleep restriction through preemptive administration of low-dose aspirin . This may foster the development of new therapeutics that specifically target those pathways, and do not exhibit the undesirable side effects associated with aspirin, such as bleeding and stroke.
"Such therapeutics could complement behavioral sleep improvement therapies to better prevent or control inflammation and its consequences in those experiencing periods of sleep deficiency," Engert said.
This study was supported by grants from the National Institutes of Health, German Research Foundation, and Sleep Research Society Foundation. The research abstract was published recently in an online supplement of the journal SLEEP and will be presented Monday, June 3, and Wednesday, June 5, during SLEEP 2024, the annual meeting of the Associated Professional Sleep Societies, a joint venture of the American Academy of Sleep Medicine and the Sleep Research Society.
Explore further
Feedback to editors

Scientists develop AI tool to predict how cancer patients will respond to immunotherapy
2 minutes ago

Neoadjuvant immunotherapy for early stage melanoma shows positive results
19 minutes ago
Targeting leukemia's survival route: A novel approach to overcoming leukemia recurrence
31 minutes ago
Study finds timing of brain waves shapes the words we hear

Study finds semaglutide associated with reduction in incidence and recurrence of alcohol-use disorder

New analytical tool can improve understanding of heritable human traits and diseases
2 hours ago
Q&A: Researchers determine that a subclass of stem cells replenish platelets more rapidly
New drug shows promise in easing chronic pain, study finds

Study finds people of color disproportionately dropped from Medicaid

More than meets the eye: Understanding how the brain controls social gaze
Related stories.

Sleep restriction tied to negative cognitive effects in teens with overweight, obesity
May 23, 2024

Study finds that better sleep is associated with lower loneliness
May 30, 2024

Restricting sleep may affect emotional reactions
Apr 8, 2020

Study shows a drink before bed can cause reductions in REM sleep
Jan 26, 2024
Insufficient sleep may lead to increased risk-taking behavior
Aug 25, 2017

Adults with a regular, healthy sleep schedule have a lower risk of death
Jun 5, 2023
Recommended for you

Could ultra-processed foods be associated with your insomnia?

The mind after midnight: Study shows disrupted sleep increases risk for suicide and homicide
May 29, 2024

The light or the content? What we know about screens and sleep disruption
May 28, 2024

Eating more fruits and vegetables may lead to optimal sleep duration
May 27, 2024

Social media-related nightmares: Study explores links between social media use, mental health and sleep quality
May 20, 2024
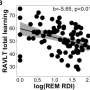
Research links sleep apnea severity during REM stage to verbal memory decline
May 14, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
March 7, 2017
New activity for anti-inflammatory drugs
At a glance.
- Researchers uncovered a previously unknown pathway of activity for NSAIDs (non-steroidal anti-inflammatory drugs).
- The findings could be used to expand uses for NSAIDs or design next-generation therapies.

Scientists sometimes find novel uses for old drugs. For example, the common pain reliever aspirin is now used by millions of people to help prevent heart attack, stroke, or certain cancers. Aspirin is a type of non-steroidal anti-inflammatory drug (NSAID). Because aspirin has found multiple uses, other NSAIDs might also have health benefits that haven’t yet been discovered.
Inflammation is the body’s normal reaction to injury or disease. The result is redness, swelling, pain, and warmth in the inflamed area of the body. Although the inflammatory response enables the body to heal after injury, long-term inflammation has been linked to the development of cancer and other diseases. The sudden widespread and exaggerated inflammation in sepsis can result in tissue damage, organ failure, and even death.
Previous research has revealed some of the molecular mechanisms involved in inflammation. Aspirin and other NSAIDs are known to have anti-inflammatory effects by inhibiting COX (cyclooxygenase) enzymes. These enzymes are pivotal in the inflammatory process.
A research team led by Dr. Hang Hubert Yin of the University of Colorado, Boulder, and the BioFrontiers Institute used high-throughput screening to test nearly 1,300 FDA-approved drugs for anti-inflammatory activity. The team focused on another group of enzymes called caspases that are known to be important for inflammation and thus might also serve as useful therapeutic targets. The work was funded by NIH’s National Institute of General Medical Sciences (NIGMS). Results appeared in Cell Chemical Biology on February 23, 2017.
The team first ranked the drugs by their ability to inhibit the activity of the caspase-4 enzyme. They found 27 compounds that inhibited caspase-4 activity to less than 25%. Of these, about half—and 8 of the top 10 most potent—were NSAIDs. Further tests of 9 selected NSAIDs showed that all except aspirin inhibited multiple caspases.
In human cells, the caspases were inhibited by the same NSAIDs in the same rank order as in the high-throughput screen. Fenbufen and indoprofen were the strongest inhibitors; naproxen and ibuprofen were weak inhibitors; and aspirin was ineffective. In additional experiments, the team found that the NSAID inhibition of caspase was independent of COX enzymes.
“NSAIDs like ibuprofen and aspirin are among the most prevalent pharmaceuticals worldwide, with over 30 billion doses taken annually in the United States alone. But their precise mechanisms of action are not entirely understood,” Yin says. “We provide the first evidence for a novel mechanism of action for NSAIDs.”
Caspases are known to play a role in inflammatory diseases such as rheumatoid arthritis and heart disease. This newly discovered mechanism of NSAID activity suggests future studies into how these drugs affect caspases in the human body. The results could inform strategies to fight inflammation with fewer side effects.
—by Geri Piazza
Related Links
- Drug Might Help Treat Sepsis
- Aspirin May Reduce Ovarian Cancer Risk
- Aspirin to Reduce Cancer Risk
- Chronic Inflammation
References: Non-steroidal Anti-inflammatory Drugs Are Caspase Inhibitors. Smith CE, Soti S, Jones TA, Nakagawa A, Xue D, Yin H. Cell Chem Biol . 2017 Feb 15. pii: S2451-9456(17)30033-8. doi: 10.1016/j.chembiol.2017.02.003. [Epub ahead of print]. PMID: 28238723.
Funding: NIH’s National Institute of General Medical Sciences (NIGMS).
Connect with Us
- More Social Media from NIH
New long COVID study uncovers high inflammation in patients as Senate calls for more research on 'crisis'
The study followed 113 patients at four different hospitals in Switzerland.
A new study in Science is shining a light on the continuing impact of long COVID, with research revealing further and continuing health concerns for some of the 16 million sufferers in the U.S.
Long COVID is a syndrome, or collection of symptoms, that continue or develop after an acute COVID-19 infection and can last weeks, months or years. There is no test to confirm if symptoms are related to long COVID. Some scientists suggest that long COVID is caused by overactive immune cells, but the exact cause remains unclear.
The study followed 113 patients at four different hospitals in Switzerland with mild and severe COVID-19 and found that 40 had symptoms of long COVID at six months, 22 of whom had persistent symptoms at 12 months.
Researchers looked at blood samples from the 40 who experienced long COVID symptoms, compared them to controls who were not infected with COVID-19, and found that those who had long COVID had evidence of inflammation (increased complement activity), blood cell dysregulation (hemolysis and platelet activation) and tissue injury in their blood.
MORE: Long COVID research opens door for further exploration on post-viral illness
The specific details from the small study may help provide "a basis for new diagnostic solutions," according to the researchers, for the condition with no known cure or FDA-approved treatments.
While these results finding evidence of inflammation in patients with long COVID symptoms are not entirely surprising nor specific to long COVID, they are a step forward in identifying the cause of long COVID.
It's more than just researchers, though, looking into developments in our understanding of the syndrome. The condition received renewed attention from the federal government last week, as the U.S. Senate Committee on Health, Education, Labor and Pensions convened a group of patients and experts to testify about the impacts of long COVID before a bipartisan group of Senators.

In the Senate's first-ever hearing on this topic, Sen. Tammy Baldwin said researchers and government officials need to "increase the sense of urgency" over understanding and treating the condition.
For Sen. Bernie Sanders, chairman of the committee, more needs to be done.
"We think we haven't done anywhere near enough, and we hope to turn that around," he said.
Medical experts testified at the hearing, telling the committee that the condition can emerge in patients of all ages and backgrounds, that the risk increases with multiple infections, and rates of long COVID are higher in minority communities.
"The burden of disease and disability from long COVID is on par with the burden of cancer and heart disease," Dr. Ziyad Al-Aly, M.D., a clinical epidemiologist at Washington University, said. "We must develop sustainable solutions to prevent repeated infections with SARS-CoV-2 and long COVID that would be embraced by the public."
Patients and Caregivers
Angela Meriquez Vazquez , a long COVID patient from California, testified that she has helped over 15,000 sufferers through online advocacy.
"We are living through the largest mass destabilizing event in modern history," she told the Senators.
MORE: America's gun violence problem by the numbers
As she told her own story, Meriquez Vazquez, a former runner, said she is currently on 12 medications. Although she said she has managed to continue working, and she has health care, the condition has forced her to work from home, lying down to minimize her symptoms.

Trending Reader Picks

What Americans think of Trump's verdict: POLL
- Jun 2, 9:01 AM

Chad Daybell sentenced in triple murder of family
- Jun 1, 1:38 PM

How Trump’s conviction will impact the election
- May 30, 5:23 PM
"Not since the emergence of the AIDS pandemic has there been such an imperative for large-scale change in healthcare, public health, and inequitable structures that bring exceptional risks of illness, suffering, disability, and mortality," Meriquez Vazzque said.
One of the Senators -- Republican Roger Marshall -- shared his own testimony, revealing to the committee that one of his loved ones "is one of the 16 million people" who has "suffered for two years" with the condition.
He told the committee his family member's illness is "like mono(nucleosis) that does not go away," adding that the person has seen 30 doctors in an attempt to find help.
Marshall said there needs to be more focus on treatments for long COVID at the Centers for Disease Control and Prevention.
"I'm frustrated that our CDC is more focused on vaccines than they are on treatments," he said.
Epidemiologists and Researchers weigh in
Dr. Al-Aly, while testifying, repeatedly called on our country's leaders and medical experts to come together to tackle the ongoing health crisis.
"We are the best nation on earth, and we can solve this," he said.
One of his proposed solutions is establishing a new multidisciplinary research institute to address infection-associated chronic conditions.
Research into the condition has been "slow," Dr. Charisse Madlock-Brown, Ph.D. from the University of Iowa, said at the hearing. She noted clinical trials are in the "experimental medicine" phase and pushed for more investment to identify proven treatments.
Sen. Tim Kaine said the National Institutes of Health has been provided more than $1 billion since 2020 to study long COVID, and he urged representatives from NIH to testify before the committee. In 2021, the NIH launched the Researching COVID to Enhance Recovery initiative to identify further risk factors and causes of long COVID.
"We can't take two years just to get 'geared up,'" he said.
According to the most recent information from the CDC , long COVID can cause up to 200 symptoms , including chronic fatigue, blood clots, gastrointestinal issues, brain fog and heart issues. Symptoms can last from months to years following a COVID infection. Risk factors for developing long COVID after a COVID-19 infection that have been identified include severe COVID-19 illness, underlying health conditions (such as asthma, diabetes, obesity or autoimmune diseases) and not getting the COVID-19 vaccine.
While the interest from the Senate and the new study in Science are promising, more research needs to be done to find the specific cause of why some people get long COVID from COVID-19, and others do not, and to find effective treatments.
Erin Hannon, MD, contributed to this report. Hannon is a resident physician in pediatrics from Columbia University/New York-Presbyterian Hospital, and a member of the ABC News Medical Unit.
Related Topics
- Coronavirus

What Trump's conviction could mean for his rights
- Jun 1, 2:31 PM

Shiloh Jolie-Pitt petitions to change last name
- Jun 1, 12:39 PM
ABC News Live
24/7 coverage of breaking news and live events

Vistusertib improves pulmonary inflammation and fibrosis by modulating inflammatory/oxidative stress mediators via suppressing the mTOR signalling
- Taslim B. Shaikh
- Yogesh Chandra
- Ramakrishna Sistla

Anti-inflammatory peptide therapeutics and the role of sulphur containing amino acids (cysteine and methionine) in inflammation suppression: A review
- Catherin Ann Biji
- Akshad Balde
- Rasool Abdul Nazeer
Exendin-4 blockade of T1R2/T1R3 activation improves Pseudomonas aeruginosa-related pneumonia in an animal model of chemically induced diabetes
Sirt1 inhibits macrophage polarization and inflammation in gouty arthritis by inhibiting the MAPK/NF-κB/AP-1 pathway and activating the Nrf2/HO-1 pathway
- Shiquan Shuai
Extracellular histones promote TWIK2-dependent potassium efflux and associated NLRP3 activation in alveolar macrophages during sepsis-induced lung injury
- Zongmei Wen
Tissue-resident C1q + macrophages exert anti-aging potential through the Sirt1 pathway
- Lingjuan Zhu
Infliximab modifies CD74-mediated lymphatic abnormalities and adipose tissue alterations in creeping fat of Crohn’s disease
- Weigang Shu
- Yongheng Wang
- Xiaolei Wang
Identification of diagnostic markers related to inflammatory response and cellular senescence in endometriosis using machine learning and in vitro experiment
- Pusheng Yang

Surface PD-1 expression in T cells is suppressed by HNRNPK through an exonic splicing silencer on exon 3
- Jiayun Wang
- Lingyan Yan

A signaling network map of Lipoxin (LXA4): an anti-inflammatory molecule
- G. P. Suchitha
- Rex Devasahayam Arokia Balaya
- Shobha Dagamajalu

A single dose of angiotensin-(1–7) resolves eosinophilic inflammation and protects the lungs from a secondary inflammatory challenge
- Giselle Santos Magalhaes
- Juliana Fabiana Gregorio
- Vanessa Pinho
Tumor necrosis factor receptor-associated factor 5 protects against intimal hyperplasia by regulation of macrophage polarization via directly targeting PPARγ
- Wen-Lin Cheng
- Sheng-ping Chao
PLD2 deletion ameliorates sepsis-induced cardiomyopathy by suppressing cardiomyocyte pyroptosis via the NLRP3/caspase 1/GSDMD pathway
Viperin inhibits interferon-γ production to promote Mycobacterium tuberculosis survival by disrupting TBK1-IKKε-IRF3-axis and JAK-STAT signaling
- Xinying Zhou
Integration of multiomics analyses reveals unique insights into CD24-mediated immunosuppressive tumor microenvironment of breast cancer
- Hongxia Zhu
- Taolan Zhang

Impacts of liver macrophages, gut microbiota, and bile acid metabolism on the differences in iHFC diet-induced MASH progression between TSNO and TSOD mice
- Naoya Igarashi
- Kaichi Kasai
- Yoshinori Nagai
Acute lung injury: a view from the perspective of necroptosis
- Jinyan Dong
- Weihong Liu
Macrophage-enriched novel functional long noncoding RNAs LRRC75A-AS1 and GAPLINC regulate polarization and innate immune responses
- Araceli Valverde
- Raza Ali Naqvi
- Afsar R. Naqvi

Tryptophan catabolism via the kynurenine pathway regulates infection and inflammation: from mechanisms to biomarkers and therapies
- Jingpu Zhang
Transcriptomic analysis reveals molecular characterization and immune landscape of PANoptosis-related genes in atherosclerosis
- Zhipeng Zheng

Quercetin and Kaempferol inhibit HMC-1 activation via SOCE/NFATc2 signaling and suppress hippocampal mast cell activation in lipopolysaccharide-induced depressive mice
Consistent efficacy outcomes between phase 2 and phase 3 trials in Crohn’s disease or ulcerative colitis in adults: a meta-analysis
- Qingwei Jiang

γδ T cells in oral diseases
Recognition of Mycobacterium tuberculosis by macrophage Toll-like receptor and its role in autophagy
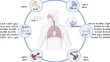
Immunopathogenesis of urticaria: a clinical perspective on histamine and cytokine involvement
- Rudranil Bhowmik
- Md. Adil Shaharyar
- Sanmoy Karmakar
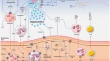
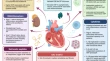
Inflammation in heart failure: pathophysiology and therapeutic strategies
- Jacinthe Boulet
- Vikas S. Sridhar
- Michel White
Causal role of myeloid cells in Parkinson’s disease: Mendelian randomization study
- Jiajun Chen

Bakuchicin alleviates ovalbumin-induced allergic asthma by regulating M2 macrophage polarization
- Yeon-Yong Kim
- Seungwon Jeong
- Soyoung Lee

Identification of a novel ADCC-related gene signature for predicting the prognosis and therapy response in lung adenocarcinoma
- Liangyu Zhang

Author Correction: Porphyromonas gingivalis promotes the progression of oral squamous cell carcinoma by stimulating the release of neutrophil extracellular traps in the tumor immune microenvironment
- Zhi-chen Guo
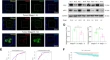
Tricarboxylic acid cycle metabolites: new players in macrophage
- Bing-Bing Cui
- Jing-Yi Wang
Bridging the divide: unveiling mutual immunological pathways of cancer and pregnancy
- Teodora Maria Toadere
- Andra Ţichindeleanu
- Iuliana Nenu
The NADase CD38 is a central regulator in gouty inflammation and a novel druggable therapeutic target
- Paulo Gil Alabarse
- Patricia Oliveira
- Ru Liu-Bryan

Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults
- Peixian Huang

Complex alterations in inflammatory pain and analgesic sensitivity in young and ageing female rats: involvement of ASIC3 and Nav1.8 in primary sensory neurons
- Diego N. Messina
- Emanuel D. Peralta
- Cristian G. Acosta

Long-chain acyl-CoA synthetase 4-mediated mitochondrial fatty acid metabolism and dendritic cell antigen presentation
- Fengxia Ding

Imbalance of T follicular helper cell subsets trigger the differentiation of pathogenic B cells in idiopathic membranous nephropathy
- Bishun Deng
- Huijie Huang

Genetic correlations, shared risk genes and immunity landscapes between COVID-19 and venous thromboembolism: evidence from GWAS and bulk transcriptome data
- Langchao Yan
SS-31 inhibits mtDNA–cGAS–STING signaling to improve POCD by activating mitophagy in aged mice
- Yuanyuan Ma
- Shengjin Ge
Altered vacuole membrane protein 1 (VMP1) expression is associated with increased NLRP3 inflammasome activation and mitochondrial dysfunction
- Stephanie R. Zack
- Meghana Venkatesan
- Edward M. Campbell
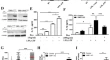
Interleukin-19 enhances eosinophil infiltration through upregulation of epithelium-derived RANTES expression via the ERK/NF-κB signalling pathway in patients with eosinophilic CRSwNP
- Zizhen Huang
- Gehua Zhang
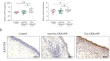
Deficiency of INPP4A promotes M2 macrophage polarization in eosinophilic chronic rhinosinusitis with nasal polyps
- Yingying Xu
- Xiaoting Tong
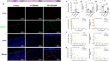
Inflammatory cell death PANoptosis is induced by the anti-cancer curaxin CBL0137 via eliciting the assembly of ZBP1-associated PANoptosome
- Zhi-Ya Zhou
- Xian-Hui He

Anti-CD20 treatment attenuates Th2 cell responses: implications for the role of lung follicular mature B cells in the asthmatic mice
- Jingling Li
- Hanxiang Nie
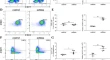
Challenges and opportunities in inflammatory bowel disease: from current therapeutic strategies to organoid-based models
- Lingjie Kong
- Chunyan Hua

Genetically proxied PCSK9 inhibition is associated with reduced psoriatic arthritis risk
- Jianfeng Li
CCL18, CHI3L1, ANG2, IL-6 systemic levels are associated with the extent of lung damage and radiomic features in SARS-CoV-2 infection
- Ilaria Ferrigno
- Laura Verzellesi
- Stefania Croci
Identification of inflammatory biomarkers in IgA nephropathy using the NanoString technology: a validation study in Caucasians
- Laurence Gaumond
- Caroline Lamarche
- Louis-Philippe Laurin
Identification of RRM2 as a key ferroptosis-related gene in sepsis
- Qingquan Liu

Mammalian STE20-like kinase 1 inhibits synoviocytes activation in rheumatoid arthritis through mitochondrial dysfunction mediated by SIRT3/mTOR axis
- Find a journal
- Publish with us
- Track your research
New research adds evidence to the benefits of ginger supplements for treating autoimmune diseases
Study looked at the impact of ginger supplements on people's white blood cell function.
New research has revealed a potentially important role ginger supplements can play in controlling inflammation for people living with autoimmune diseases.
The research published today in JCI Insight focused on studying the impact of ginger supplementation on a type of white blood cell called the neutrophil. The study was especially interested in neutrophil extracellular trap (NET) formation, also known as NETosis, and what it may mean for controlling inflammation.
The study found ginger consumption by healthy individuals makes their neutrophils more resistant to NETosis. This is important because NETs are microscopic spider web-like structures that propel inflammation and clotting, which contribute to many autoimmune diseases, including lupus, antiphospholipid syndrome and rheumatoid arthritis.
"There are a lot of diseases where neutrophils are abnormally overactive. We found that ginger can help to restrain NETosis, and this is important because it is a natural supplement that may be helpful to treat inflammation and symptoms for people with several different autoimmune diseases," said senior co-author Kristen Demoruelle, MD, PhD, associate professor of medicine at the University of Colorado School of Medicine on the University of Colorado Anschutz Medical Campus.
In a clinical trial, the researchers found that daily intake of a ginger supplement for seven days (20 mg of gingerols/day) by healthy volunteers boosted a chemical inside the neutrophil called cAMP. These high levels of cAMP then inhibited NETosis in response to various disease-relevant stimuli.
"Our research, for the first time, provides evidence for the biological mechanism that underlies ginger's apparent anti-inflammatory properties in people," said senior co-author Jason Knight, MD, PhD, associate professor in the Division of Rheumatology at the University of Michigan.
The researchers say that many people with inflammatory conditions are likely to ask their health care providers whether natural supplements could be helpful for them or they already take supplements, like ginger, to help manage symptoms. Unfortunately, the precise impact on disease is often unknown. The researchers hope that providing more evidence about ginger's benefits, including the direct mechanism by which ginger impacts neutrophils, will encourage health care providers and patients to more strategically discuss whether taking ginger supplements as part of their treatment plan could be beneficial.
"There are not a lot of natural supplements, or prescription medications for that matter, that are known to fight overactive neutrophils. We, therefore, think ginger may have a real ability to complement treatment programs that are already underway. The goal is to be more strategic and personalized in terms of helping to relieve people's symptoms," Knight adds.
As a next step, the researchers hope to use this study to unlock funding for clinical trials of ginger in patients with autoimmune and inflammatory diseases where neutrophils are overactive, such as lupus, rheumatoid arthritis, antiphospholipid syndrome and even COVID-19.
- Immune System
- Joint Health
- Veterinary Medicine
- Pests and Parasites
- Vaccination
- Inflammation
- Microorganism
- Biological pest control
Story Source:
Materials provided by University of Colorado Anschutz Medical Campus . Original written by Julia Milzer. Note: Content may be edited for style and length.
Journal Reference :
- Ramadan A. Ali, Valerie C. Minarchick, Miela Zahavi, Christine E. Rysenga, Kristin A. Sturm, Claire K. Hoy, Cyrus Sarosh, Jason S. Knight, M. Kristen Demoruelle. Ginger intake suppresses neutrophil extracellular trap formation in autoimmune mice and healthy humans . JCI Insight , 2023; 8 (18) DOI: 10.1172/jci.insight.172011
Cite This Page :
Explore More
- How Statin Therapy May Prevent Cancer
- Origins of 'Welsh Dragons' Exposed
- Resting Brain: Neurons Rehearse for Future
- Observing Single Molecules
- A Greener, More Effective Way to Kill Termites
- One Bright Spot Among Melting Glaciers
- Martian Meteorites Inform Red Planet's Structure
- Volcanic Events On Jupiter's Moon Io: High Res
- What Negative Adjectives Mean to Your Brain
- 'Living Bioelectronics' Can Sense and Heal Skin
Trending Topics
Strange & offbeat.
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Search Awards
- Recent Awards
- Presidential and Honorary Awards
- About Awards
- How to Manage Your Award
- Grant General Conditions
- Cooperative Agreement Conditions
- Special Conditions
- Federal Demonstration Partnership
- Policy Office Website
Please report errors in award information by writing to: [email protected] .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Acute inflammation articles from across Nature Portfolio
Acute inflammation is a short-term process occurring in response to tissue injury, usually appearing within minutes or hours. It is characterized by five cardinal signs: pain, redness, immobility (loss of function), swelling and heat.
Latest Research and Reviews
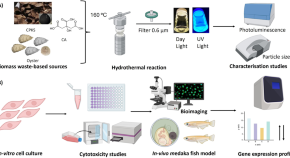
Biomass-derived carbon dots as fluorescent quantum probes to visualize and modulate inflammation
- Mahima Kumar
- Shanmugavel Chinnathambi
- Ganesh N. Pandian
The importance of murine phospho-MLKL-S345 in situ detection for necroptosis assessment in vivo
- Konstantinos Kelepouras
- Julia Saggau
- Gianmaria Liccardi
Post-resolution macrophages shape long-term tissue immunity and integrity in a mouse model of pneumococcal pneumonia
The post-resolution phase of inflammation is not simply a linear path towards cessation of immune response but rather a regulated process involving fluctuating immune activity. Here authors show a pivotal role for post-resolution macrophages in driving a wave of T cell recruitment and activation via prostaglandin E2 and α-integrin signalling during the resolution phase of murine pneumococcal pneumonia.
- Karen T. Feehan
- Hannah E. Bridgewater
- Derek W. Gilroy
Neutrophil–macrophage communication via extracellular vesicle transfer promotes itaconate accumulation and ameliorates cytokine storm syndrome
- Haixia Kang
Multimodal decoding of human liver regeneration
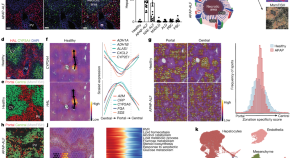
Harnessing single-nucleus RNA sequencing and spatial profiling, this work dissects unanticipated aspects of human liver regeneration to uncover a novel migratory hepatocyte subpopulation mediating wound closure following acute liver injury.
- K. P. Matchett
- J. R. Wilson-Kanamori
- N. C. Henderson
Apolipoprotein E controls Dectin-1-dependent development of monocyte-derived alveolar macrophages upon pulmonary β-glucan-induced inflammatory adaptation
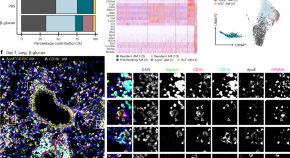
In this study, the authors suggest that in the lung ApoE is a checkpoint for monocyte-to-macrophage differentiation triggered by the dectin1–Card9 pathway.
- H. Theobald
- D. A. Bejarano
- A. Schlitzer
News and Comment
How the brain regulates inflammation.
- Alexandra Flemming
Maternal rhythms suppress neonatal inflammation
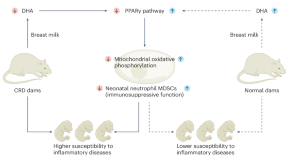
Maternal circadian rhythms influence the health of infants. Cui, Xu and colleagues find that disruption of maternal rhythms impairs neonatal immune cell function and aggravates neonatal inflammatory disorders, which can be rescued by the administration of docosahexaenoic acid (a metabolite found in breast milk).
- Markus Sperandio
- Christoph Scheiermann
Thirteen years to get from b to a: one of the neglected isoforms of IL-37 enters the stage
- Steven X. Cho
- Ina Rudloff
- Marcel F. Nold
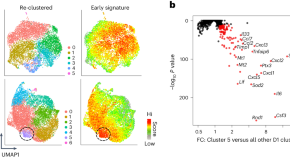
A distinct subtype of stromal cell orchestrates the inflammatory response to tissue injury
After acute injury to skeletal muscle, an ‘early responder’ subtype of stromal cells rapidly produces an array of inflammatory mediators. Disruption of this response causes abnormal accumulation of several adaptive lymphocyte populations, a prolongation of inflammation, and an effect on tissue regeneration.
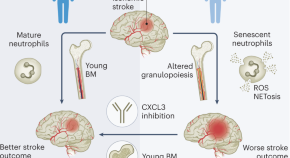
Inflammaging aggravates stroke pathology
In mice and humans, changes in neutrophil phenotypes and functionality during aging aggravate thromboinflammation in ischemic brain injury and determine the pathology associated with strokes. In mice, inhibition of CXCL3 signaling and rejuvenation of bone marrow offer ways of restricting brain injury and improving stroke outcomes.
- Christian Schulz
- Steffen Massberg
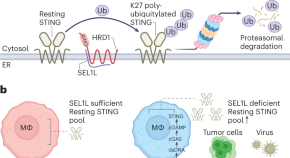
ERADication of STING limits inflammation
The cGAS–STING cytosolic double-stranded-DNA-sensing pathway provides protection against infection but also contributes to inflammatory pathology and thus must be tightly regulated. In this issue, Jie et al. find that endoplasmic-reticulum-associated degradation of the adaptor STING by SEL1L–HRD1 controls steady-state STING levels to limit STING-driven inflammation.
- Kevin MingJie Gao
- Katherine A. Fitzgerald
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
The new findings come amid intensifying efforts to understand the molecular underpinnings of exercises. Untangling the immune system's involvement in this process is but one aspect of these research efforts. "Our research suggests that with exercise, we have a natural way to boost the body's immune responses to reduce inflammation."
Inflammation is a biological response to harmful stimuli, such as pathogens, damaged cells or irradiation. It is a protective attempt by the organism to remove injurious stimuli and to initiate ...
UC Santa Cruz researchers have discovered a peptide in human RNA that regulates inflammation and may provide a new path for treating diseases such as arthritis and lupus. The team used a screening process based on the powerful gene-editing tool CRISPR to shed light on one of the biggest mysteries about our RNA.
New approach could treat dozens of diseases. Cancer, aging, and severe COVID-19 have all been linked to damage from inflammation. Now scientists are flipping their focus to find new drugs that may ...
Latest Research and Reviews Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression Interleukin-33 (IL-33) is a master initiator of cancer-prone chronic ...
Historically, inflammation-related research mainly comprised studies related to immune cells and inflammatory mediators. However, in recent years, more multidisciplinary and integrative approaches have been applied for the dissection of the inflammatory process [ 15 ].
Abstract. Although intermittent increases in inflammation are critical for survival during physical injury and infection, recent research has revealed that certain social, environmental and ...
Desai's new discovery suggests answers. Desai and his team say their research has identified a "keystone" mechanism responsible for age-related changes in the macrophages. These changes, the ...
Statins, commonly used cholesterol-lowering drugs, may block a pathway that leads to the development of cancer from chronic inflammation, according to a new study led by investigators from Harvard-affiliated Mass General Cancer Center. The team's experiments showed that environmental toxins, such as those caused by exposure to allergens and chemical irritants, create a cascade effect that ...
"Chronic inflammation is a major cause of cancer worldwide," said senior author Shawn Demehri, MD, PhD, a principal investigator at the Center for Cancer Immunology and Cutaneous Biology Research ...
Overview. Inflammation Research is a broad-spectrum journal focusing on all aspects of inflammation and related fields. Encompasses topics such as allergy and asthma, shock, pain, joint damage, skin disease, and clinical trials of relevant drugs. Presents original research, reviews, commentaries, and society proceedings.
Researchers discovered a new inflammatory disorder, called VEXAS, shared by men with diverse symptoms. The study demonstrates a new genomic approach to identify and classify inflammatory and other diseases. Instead of looking at similarities in symptoms, the team examined genomes to discover a new disease. AnnaStills / iStock / Getty Images Plus.
New evidence released today from a study of 31,245 patients already taking statin therapy indicates that inflammation may be a more powerful predictor of risk of future cardiovascular events ...
Funding: Research support was provided by the Burroughs Wellcome Fund, the LEO Foundation, the Sidney Kimmel Foundation, and the National Institutes of Health. Paper cited: Park JH et al. "Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression." Nature Communications DOI: 10.1038/s41467-024-48441-8.
The Journal of Inflammation Research is an international, peer-reviewed, open access journal that welcomes laboratory and clinical findings on the molecular basis, cell biology and pharmacology of inflammation. Meta-analyses will no longer be considered for publication. Specific topics covered by the journal include: The journal welcomes ...
Read the latest Research articles in Inflammation from Nature. ... A new humanized mouse model for COVID-19 demonstrates SARS-CoV-2 infection and subsequent activation of inflammasomes in human ...
Journal of Inflammation Research, Volume 17, Issue (2024) See all volumes and issues. ... New Classification of Rheumatoid Arthritis Based on Immune Cells and Clinical Characteristics. Jiaqian Wang, Yuan Xue & Liang Zhou. Pages: 3293-3305. Published online: 23 May 2024.
A new study to be presented at the SLEEP 2024 annual meeting, held in Houston, Texas, June 1-5, found that low-dose acetylsalicylic acid, also known as aspirin, can reduce inflammatory ...
Further research is needed to fully understand the link between brain inflammation, brain activity, and behavioral changes. "[T]his study suggests that the molecular mechanism behind many Long COVID-19 symptoms stems from this persistent inflammation while describing an animal model close enough to human biology to be useful in the design of ...
Caspases are known to play a role in inflammatory diseases such as rheumatoid arthritis and heart disease. This newly discovered mechanism of NSAID activity suggests future studies into how these drugs affect caspases in the human body. The results could inform strategies to fight inflammation with fewer side effects. —by Geri Piazza.
The brain can direct the immune system to an unexpected degree, capable of detecting, ramping up and tamping down inflammation, shows a new study in mice from researchers at Columbia's Zuckerman ...
A new study describes an anti-inflammatory signalling pathway mediated by TNFR2 and the signalling molecule 14-3-3ε and the effects of this pathway on macrophage polarization. ... New research ...
A new study in Science is shining a light on the continuing impact of long COVID, with research revealing further and continuing health concerns for some of the 16 million sufferers in the U.S ...
Mammalian STE20-like kinase 1 inhibits synoviocytes activation in rheumatoid arthritis through mitochondrial dysfunction mediated by SIRT3/mTOR axis. Inflammation Research is a broad-spectrum journal focusing on all aspects of inflammation and related fields. Encompasses topics such as allergy and asthma, ...
New research has revealed a potentially important role ginger supplements can play in controlling inflammation for people living with autoimmune diseases. The research focused on studying the ...
Nature Immunology 18 , 825 ( 2017) Cite this article. Evolving mechanistic and conceptual understanding of inflammation drives insight into human disease and new approaches for therapy ...
Research suggests asthma is a risk factor for cardiovascular disease. However, questions remain about the link between the chronic lung disorder and heart health. ... Heart Association showed that participants with persistent asthma had higher carotid plaque scores and higher levels of inflammatory biomarkers than people without asthma. A ...
This is a community-centered project which provides research training to participants and sets the foundation for future research in this emerging area. Epigenetic pathways act as channels between social factors and disease risk throughout life, and epigenetic modifications can impact inflammatory responses.
Acute inflammation articles from across Nature Portfolio. Acute inflammation is a short-term process occurring in response to tissue injury, usually appearing within minutes or hours. It is ...