Looking for our teaching and curriculum resources?
Education has the power to transform people’s lives. We want every young person in the UK to have the best possible education outcomes and to gain the knowledge and skills necessary to thrive in our society. We fund education research to i nform and drive the change needed to make this happen.
Learn more about our interest in Education research

Our goal is to find ways to improve educational outcomes through policy change and interventions that are grounded in robust evidence.
We fund research and development projects relating to education across all life stages – from early years through school, to further and higher education and vocational learning. We want to understand young people’s pathways as they move through these stages, and how they acquire skills and capabilities.
Many young people are disadvantaged in the education system, by factors such as special educational needs, disability, socio-economic background and location. Through the research we fund, we help to understand and address these disadvantages. We also aim to improve the quality of teaching and learning, and to understand and support teachers.
We recognise that education is not just what happens in the classroom – we also want to understand the wider influences on people’s education and chances in life, such as the role of families and informal learning.
Why education needs research
Pupils are on average three months behind on learning as a result of the COVID-19 crisis, and teachers estimate almost half need intensive catch-up support. The quality of teaching and learning has a major influence on educational achievement. Research can help to understand how effective learning takes place, and how teaching practice and education policy can help pupils achieve their potential.
Women who are the first in their family to graduate from university earn 7% less in their mid-20s compared to female graduates whose parents attended university. In contrast, first generation male graduates tend not to face a similar pay penalty. Our research helps to understand the relationships between educational achievement and labour market success, as well as many other spheres such as health and well-being, civic engagement and parenting.
The majority of graduating BTEC students – who are more likely to be from disadvantaged backgrounds – achieve at least a 2:1 , demonstrating that for many students, these qualifications lead to university success. Research helps us to understand the factors that influence educational achievement, such as family background, where you live, and the choices of provision and pathways that are available. Research also sheds light on why these relationships exist and how disadvantages might be mitigated.
Widespread closures of schools, colleges and universities in response to COVID-19 led to a rapid transition to fully-remote distance learning for the majority of students. There is debate about how to build robust remote learning capabilities and infrastructure into the education system and more high-quality research into distance learning in schools and colleges, outside the context of the pandemic, is required.
What do we fund in education?
We are currently funding research, development and analysis projects, with a focus on:
- Skills and capabilities that equip children and young people for life and work, both within and beyond educational institutions.
- Teaching quality, particularly projects that improve practice through evidence-based interventions and those that harness digital technologies to improve teaching, learning, parental engagement and child development.
- Young people’s pathways, with an increased focus on young people following non-HE routes.
- Educational disadvantage, including special educational needs, physical disabilities, mental health issues, socio-economic disadvantage, geographical disadvantage and looked after children.
- Direct interventions that improve young people’s lives and align with the four priorities identified above and which are grounded in evidence.
Students and teachers
One of the objectives of our work in education is to address the shortage of research and quantitative skills across social science students in the UK. Our student programmes provide opportunities for students, particularly those from disadvantaged backgrounds, to develop their skills and confidence in quantitative and scientific methods.
Since 1962, the Foundation has also supported major curriculum projects and the creation of a wealth of teaching resources across STEM subjects and beyond.
For the first time, we have comprehensive analysis of public spending on education, around £95 billion. We now know how spending is targeted at different stages of education, how it has changed over time, and what is driving those changes. In 2021, this annual analysis from the IFS revealed school spending per pupil in 2024 will be about the same level as in 2010, despite extra spending. Schools serving more disadvantaged pupils have seen even larger cuts over the last decade. Our education spending analysis has attracted significant media coverage, increasing pressure on the government to take action.
In 2019, the DfE published a Recruitment and Retention Strategy to address issues with teacher supply. The Strategy echoed Nuffield-funded research from the NFER, which called for a greater emphasis on teacher retention as well as recruitment. The NFER research has also led to the DfE setting up a new policy area on part-time and flexible working, which has resulted in guidance, case studies and resources for schools.
We have awarded over £1.3 million for research into the impact of COVID-19 on education. Findings from the IFS and NFER contributed to government policy changes around school closures, home learning, technology provision, support for disadvantaged children and catch-up and recovery programmes. ASK Research also highlighted that pupils at special schools faced particular challenges. These projects provided vital evidence that pupils in low-income families and those with special educational needs were the worst hit by learning disruption.
Education topics
Early years
Primary education
Secondary education
Post-16 further education
Higher education
Education projects
12 of 286 results
Education | 2021 – 2021
The skills imperative 2035: Essential skills for tomorrow’s workforce
Education | 2025 – 2026 New
Investigating performance across Key Stage 2 maths topics
Education | 2024 – 2028
Teaching improvement through data and evaluation (TIDE)
Education | 2024 – 2026 New
Long-term outcomes of high-achieving disadvantaged children
Exploring academic selection and grammar schools in northern ireland.
Education | 2024 – 2026
Pupil school mobility: types, pathways and implications for education
Education | 2024 – 2024 New
Early years digital media literacy review
Can digital parenting interventions benefit early language development, developing a classroom intervention to improve conversation skills, vocabulary for reading: the power of words, spending across different stages of education, a feasibility and pilot trial of the early years library.
Education | 2024 – 2025 New
Teacher recruitment & retention challenges in England
Latest in education, the nuffield foundation’s response to the department for education’s advanced british standard consultation, project launch: teaching improvement through data and evaluation, connecting mental health and education, the future of work and skills: from place-based to place-led, neli preschool in ellergreen nursery school and childcare centre, neli preschool improves children’s language skills , neli case study: uxendon manor primary school, josh hillman: a level reform plans , funding call: addressing the growing send challenge, teacher crisis: the latest data, funding for research into the future of schools, funding opportunity: cost of living crisis, nuffield early language intervention in the making, addressing the social and economic impacts of the covid-19 pandemic, study of school breaktimes inspires campaigners, policy makers and researchers, new opportunities for post-16 maths education, improving access to university through nuffield research placements, what’s behind the teacher workforce crisis a look at current challenges and future solutions, improving reading attainment in primary & early secondary, lessons from the pandemic: family well-being, childcare and gender, the skills imperative 2035: essential skills for tomorrow’s workforce launch event, child conduct problems and socio-economic status, teacher supply after the pandemic, stay up to date.
Get the latest news about the Nuffield Foundation and the work we fund through our email newsletter.
Follow us on social media.
Search for all ‘education’ related content.
Spend your summer holiday enhancing your university application and career prospects on a Nuffield Research Placement.
Q-Step is a major strategic programme designed to promote a step-change in quantitative social science education and training.
We aim to improve people’s lives by funding research that informs social policy, primarily in Education , Welfare and Justice . We also fund student programmes that give young people skills and confidence in science and research.
We are an open, collaborative and engaged funder that offers more than money. Through connecting the individual projects we fund, we strengthen their collective impact and give voice to an overarching narrative.
Privacy and cookie policy
Privacy overview.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
Education Prizes 2024: Give someone the recognition they deserve! Nominate before 19 June

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Education Prizes 2024: Give someone the recognition they deserve! Nominate before 19 June
Delve into a wide range of chemical concepts and processes with this collection of over 200 step-by-step practicals
Developed by the Nuffield Foundation and the Royal Society of Chemistry, each resource contains detailed information for teachers and technicians.
‘Dissolving’ polystyrene in acetone
In association with Nuffield Foundation
Investigate what happens to polystyrene when it is placed in propanone (acetone) in this demonstration. Includes kit list and safety instructions.

‘Magic’ writing with colour changing reactions
Reveal invisible messages or pictures drawn with aqueous solutions by spraying them with suitable reagents in this demonstration. Includes kit list and safety instructions.

A chromate–dichromate equilibrium
Try this class practical to investigate an equilibrium between chromate(VI), dichromate(VI) and hydrogen ions. Includes kit list and safety instructions.
A controlled explosion using hydrogen and air
Show how a hydrogen–air mixture can gain explosive properties using a plastic drink bottle in this demonstration. Includes kit list and safety instructions.

A hydrogen powered rocket
Try this spectacular demonstration to make a rocket using a plastic drink bottle fuelled by hydrogen and air. Includes kit list and safety instructions.

A microscale acid–base titration
Use microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical. Includes kit list and safety instructions.
A microscale oxidation of alcohols
Use this practical to investigate the oxidation reactions of various alcohols with acidified potassium dichromate. Includes kit list and safety instructions.
A red–blue oscillating reaction
Use this practical or demonstration to provide a visual illustration of an oscillating reaction and redox equilibria. Includes kit list and safety instructions.

A reversible reaction of hydrated copper(II) sulfate
A class practical which investigates the reversible reaction of hydrated copper(II) sulfate. Includes kit list and safety instructions.
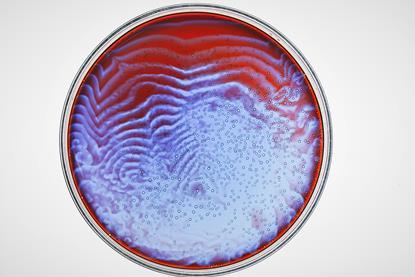
A simple oscillating reaction
Use this demonstration to illustrate an oscillating reaction as bromate ions oxidise malonic acid to carbon dioxide. Includes kit list and safety instructions.

A solid–solid reaction between lead nitrate and potassium iodide
Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide.

A spontaneous exothermic reaction
Illustrate the reaction between glycerol and potassium manganate(VII) to produce flames and steam in this demonstration. Includes kit list and safety instructions.

A test to distinguish between ethanol and methanol
A class practical to distinguish between methanol and ethanol using the iodoform reaction. Includes kit list, safety instructions, procedure and teaching notes.

A thermometric titration
Use this class practical to practise locating end-points in titration by measuring temperature during the reaction. Includes kit list and safety instructions.
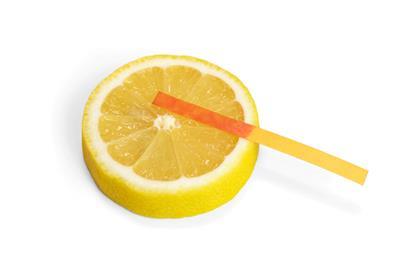
Acid or alkali? Acidic or alkaline? A litmus paper test
Test a variety of substances to see if they are acidic or alkaline, using litmus paper as the indicator. Includes kit list and safety instructions.

Addition polymerisation with phenylethene
Use this practical or demonstration as an example of addition polymerisation using phenylethene to form polyphenylethene. Includes kit list and safety instructions.

Allotropes of sulfur
Use this practical to explore the changes in the colour and consistency of sulfur as you heat it, melt it and eventually boil it. Includes kit list and safety instructions.

Ammonia fountain demonstration
Try this experiment to make a miniature chemical fountain using only soluble ammonia and atmospheric pressure. Includes kit list and safety instructions.

Ammonium dichromate volcano
Try this demonstration to create a mini volcanic eruption illustrating the decomposition of ammonium dichromate. Includes kit list and safety instructions.
An equilibrium using copper(II) and ammonia
Try this practical to explore an equilibrium involving copper(II) ions, with copper(II) sulfate, ammonia and sulfuric acid. Includes kit list and safety instructions.

Anodising aluminium
Explore an application of electrolysis in this demonstration by anodising aluminium to improve corrosion resistance. Includes kit list and safety instructions.

Burning money: what makes combustion happen?
Surprise your students by soaking a piece of paper (or an old £5 note) in ethanol and water and igniting it. Includes kit list and safety instructions.

Cannon fire
Increase the rate of burning with the inclusion of oxygen, in this loud exothermic practical

Carbon filtration and activated charcoal
Try this practical to remove objectionable tastes and odours from water using carbon in the form of activated charcoal. Includes kit list and safety instructions.

Catalysing the reaction of sodium thiosulfate and hydrogen peroxide
Illustrate the effect of a catalyst as sodium thiosulfate is oxidised by hydrogen peroxide in this demonstration. Includes kit list and safety instructions.

Catalysis of a sodium thiosulfate and iron(III) nitrate reaction
Investigate the effect of transition metal catalysts on the reaction between iron(III) nitrate and sodium thiosulfate. Includes kit list and safety instructions.

Catalysis of the reaction between zinc and sulfuric acid
Compare the rate of reaction between zinc and sulfuric acid with copper as a catalyst in this simple class practical. Includes kit list and safety instructions.

Catalysts for the thermal decomposition of potassium chlorate
Try this demonstration to investigate the effectiveness of various catalysts for the decomposition of potassium chlorate. Includes kit list and safety instructions.

Catalytic oxidation of potassium sodium tartrate
Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. Includes kit list and safety instructions.

Chemiluminescence of luminol: a cold light experiment
Show how the energy of a chemical reaction can be given out as light. Includes kit list and safety instructions.

Chromatography of sweets | 11–14 years
In association with Nuffield Foundation , By Holly Walsh and Sandrine Bouchelkia
Try this class practical to carry out chromatography using dye from different coloured M&M’s®. Includes kit list and safety instructions.

Colourful reactions using ammonia solution
Turn ammonia solution red, white or blue by adding phenolphthalein, lead nitrate or copper(II) sulfate in this demonstration. Includes kit list and safety instructions.

Colourimetric determination of copper ore
Use this practical to introduce students to the determination of copper ore by colourimetry using copper(II) sulfate. Includes kit list and safety instructions.

Combustion of ethanol
Illustrate the large energy changes that take place during the combustion of alcohols with this spectacular demonstration. Includes kit list and safety instructions.

Combustion of hydrogen in air
Try this demonstration or class experiment to investigate how varying amounts of fuel and oxygen affect combustion. Includes kit list and safety instructions.

Comparing heat energy from burning alcohols
Investigate the amounts of heat energy produced by the combustion of different alcohols in this class experiment. Includes kit list and safety instructions.

Comparing light- and heavy-duty detergents
Try this set of experiments to compare the effects of light- and heavy-duty detergents with different pH values. Includes kit list and safety instructions.

Comparing the melting points of solder, tin and lead
Test the melting points of lead, tin and solder to investigate solder as a solid mixture and alloy in this practical. Includes kit list and safety instructions.

Corrosion in different atmospheric conditions
Try this practical to test the corrosion of metals in dry air, moist air and air polluted by acidic sulfur dioxide. Includes kit list and safety instructions.

Cracking hydrocarbons in liquid paraffin with a catalyst
Model the industrial process of cracking larger hydrocarbons to produce smaller alkanes in this demonstration or class practical. Includes kit list and safety instructions.
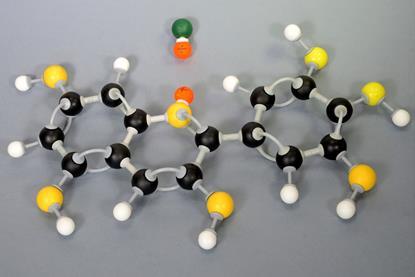
Cracking hydrocarbons on a microscale
Use this microscale experiment to illustrate hydrocarbon cracking using paraffin, bromine water and aluminium oxide. Includes kit list and safety instructions.
Dehydration of ethanol to form ethene
Use this class practical or demonstration to produce ethene gas as an example of an unsaturated hydrocarbon. Includes kit list and safety instructions.

Detecting starch in food on a microscale
Test different foodstuffs for the presence of starch using iodine in this microscale class practical. Includes kit list and safety instructions.

Detergents, soaps and surface tension
A series of brief experiments on the effects of detergents and soaps on the surface tension of purified and hard water. Includes kit list and safety instructions.

Determining relative molecular mass by weighing gases
Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. Includes kit list and safety instructions.

Determining the relative atomic mass of magnesium
Use this practical to determine the relative atomic mass of magnesium using its reaction with hydrochloric acid. Includes kit list and safety instructions.

Determining the relative molecular mass of butane
Use this demonstration to calculate the relative molecular mass of butane using simple apparatus. Includes kit list and safety instructions.

Diffusion in liquids
Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Includes kit list and safety instructions.

Diffusion of gases: ammonia and hydrogen chloride
A demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. Includes kit list and safety instructions.

Displacement reactions between metals and their salts
Students will investigate competition reactions of metals and determine a reactivity series of the four metals used. Includes kit list and safety instructions.

Displacement reactions of non-metals
Investigate a displacement series of non-metals using oxygen and chlorine in this class practical or demonstration. Includes kit list and safety instructions.

Dissolved substances in tap water and seawater
Compare the solids and gases dissolved in tap water and seawater in this class practical and demonstration. Includes kit list and safety instructions.

Distribution of iodine between two immiscible solvents
Use this class experiment or demonstration to create an equilibrium distribution using iodine in two immiscible solvents. Includes kit list and safety instructions.

Dyeing three colours from the same dye bath
Show how dyeing involves chemical interactions between dyes and the molecular nature of different fibres in this demonstration. Includes kit list and safety instructions.

Electrolysis of brine
Use this colourful practical to introduce students to the electrolysis of brine, or sodium chloride solution. Includes kit list and safety instructions.

Electrolysis of copper(II) sulfate solution
Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Includes kit list and safety instructions.

Electrolysis of molten lead(II) bromide
Introduce your students to the study of electrolysis through the production of metallic lead and bromine in this demonstration. Includes kit list and safety instructions.

Electrolysis of molten zinc chloride
Try this demonstration to show how an ionic salt will conduct electricity when molten but not when solid. Includes kit list, video and safety instructions.

Emulsifiers in the kitchen
Test a range of common ingredients to see which ones stabilise an oil and water emulsion in this class practical. Includes kit list and safety instructions.

Endothermic solid–solid reactions
Observe an endothermic reaction between two solids in this demonstration or class experiment. Includes kit list and safety instructions.

Energy content in foods
Try this class experiment to investigate how much energy different foods contain. Includes kit list and safety instructions.

Equilibria involving carbon dioxide in aqueous solution
Use this demonstration or class practical to illustrate changes to equilibria in carbonated soda water. Includes kit list and safety instructions.

Estimating the concentration of bleach
Compare the chlorine content and concentration of sodium hypochlorite in different bleaches in this class practical. Includes kit list and safety instructions.

Exothermic metal displacement reactions
Try this class experiment to explore what happens when different metals are added to a copper(II) sulfate solution. Includes kit list and safety instructions.

Exothermic metal–acid reactions
Use this class practical to explore the temperature changes resulting from adding different metals to hydrochloric acid. Includes kit list and safety instructions.

Exothermic or endothermic? Classifying reactions
Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Includes kit list and safety instructions.
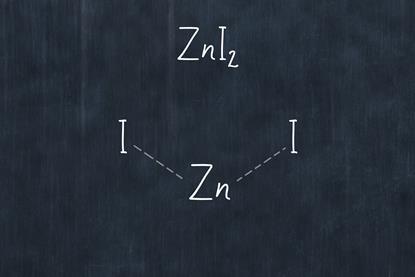
Exothermic redox reaction of zinc with iodine
Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound.

Exploding a tin can using methane
Use this demonstration to illustrate how methane can create an explosive mixture with the oxygen in air. Includes kit list and safety instructions.

Exploding bubbles of hydrogen and oxygen
Create a small explosion in this demonstration by electrolysing water to produce hydrogen and oxygen bubbles. Includes kit list, video and safety instructions.

Extracting copper from copper(II) carbonate
Use this practical to produce copper from copper(II) carbonate, modelling the extraction of copper from malachite. Includes kit list and safety instructions.

Extracting iodine from seaweed
Discover how ribbon seaweed (or kelp) can be used as a source of iodine in this demonstration or class experiment. Includes kit list and safety instructions.

Extracting iron from breakfast cereal
Try this class practical or demonstration to extract food-grade iron from breakfast cereals using neodymium magnets. Includes kit list and safety instructions.

Extracting metals with charcoal
Try this class practical to illustrate the idea of competition reactions between metals and carbon. Includes kit list and safety instructions.

Extraction of iron on a match head
Try this practical as a small scale example of metal extraction, reducing iron(III) oxide with carbon on a match head. Includes kit list and safety instructions.

Fat-pan fires and the conditions for combustion
Use this demonstration to illustrate the conditions required to start combustion, and how to put out a pan fire safely. Includes kit list and safety instructions.

Fermentation of glucose using yeast | 14–16 years
In association with Nuffield Foundation , By Neil Goalby
Use this class practical to investigate the fermentation of glucose by yeast and test for ethanol. Includes kit list, safety instructions, questions and answers

Finding the formula of copper(II) oxide
Use this class practical with your students to deduce the formula of copper(II) oxide from its reduction by methane. Includes kit list and safety instructions.

Finding the formula of hydrated copper(II) sulfate
In this experiment students will measure the mass of hydrated copper(II) sulfate before and after heating and use mole calculations to find the formula.

Flame colours: a demonstration
Explore how different elements rect when exposed to a flame, and discuss how alkali metals, alkaline earth metals, and metal salts change the colour of fire.

Floating and sinking bubbles
Make bubbles of carbon dioxide, hydrogen or methane in this demonstration exploring density, diffusion and solubility. Includes kit list and safety instructions.

Generating, collecting and testing gases
Read our standard guidance on generating, collecting and testing gases during practical experiments, including carbon dioxide, hydrogen, oxygen and chlorine.

Halogen reactions with iron wool
Illustrate an exothermic redox reaction by heating iron wool with chlorine, bromine and iodine with this demonstration. Includes kit list and safety instructions.

Halogens in aqueous solution and their displacement reactions
Explore the chemical properties of halogens using this demonstration or class experiment. Includes kit list and safety instructions.

Handling liquid bromine and preparing bromine water
Find out how to handle liquid bromine and prepare bromine water safely using these health, safety and technical notes.

Heating chocolate and egg
Use this practical to introduce students to physical and chemical changes and the safe use of Bunsen burners. Includes kit list and safety instructions.

Heating copper in air
Explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. Includes kit list and safety instructions.

Heating group 1 metals in air and in chlorine
Use this demonstration to illustrate the reactions of lithium, sodium and potassium in air and in chlorine. Includes kit list, video and safety instructions.

How can hardness in water be removed?
Investigate the effects of three treatments for softening hard water in this class practical and demonstration. Includes kit list and safety instructions.

How much oxygen is used when iron wool rusts?
Try this practical to investigate how much oxygen is used in rusting and calculate the percentage of oxygen in air. Includes kit list and safety instructions.

Hydrogels in plant water storage crystals
Investigate plant water storage crystals as one application of hydrogels in this fun class practical. Includes kit list and safety instructions.

Identifying polymers by density
Investigate and identify a variety of polymers used in everyday materials by testing their density in this practical. Includes kit list and safety instructions.

Identifying the products of combustion
Illustrate the presence of water and carbon dioxide in the products of hydrocarbon combustion in this demonstration. Includes kit list and safety instructions.

Identifying the products of electrolysis
Try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. Includes kit list and safety instructions.

Indicators and dry ice demonstration
Create bubbles, ‘fog’ and a colour change adding dry ice to alkaline ammonia or sodium hydroxide solution in this demonstration. Includes kit list and safety instructions.

Investigating hydrogels in nappies and hair gel
Investigate hydrogels as polymeric smart materials in this series of activities using nappies and hair gel. Includes kit list and safety instructions.

Investigating the role of water in acidity
Try this practical or demonstration to explore the importance of water for acidity using hydrogen chloride and methylbenzene. Includes kit list and safety instructions.

Investigating the solubility of lead halides
Encourage students to make and test predictions about the pattern of solubility among lead halides in this practical. Includes kit list and safety instructions.

Iodine clock reaction demonstration method
Use this iodine clock reaction demonstration to introduce your students to rates of reaction and kinetics. Includes kit list and safety instructions.

Iron and sulfur reaction
This demonstration or class experiment shows the exothermic reaction of iron and sulphur. Includes kit list and safety instructions.

Leaf chromatography
Try this class practical to use paper chromatography to separate and investigate the pigments in a leaf. Includes kit list and safety instructions.

Liquefying chlorine gas
Use this demonstration to produce liquid chlorine and compare it with bromine and iodine in their condensed state. Includes kit list and safety instructions.

Making a crystal garden
Create chemical gardens with your students by growing crystals of coloured silicates in this class practical. Includes kit list and safety instructions.

Making a pH indicator using red cabbage
Try this class practical to make a pH indicator from red cabbage with your students. Includes kit list and safety instructions.

Making a photographic print using silver chloride
Try this practical or demonstration to create a photographic image of an object using light sensitive silver chloride. Includes kit list and safety instructions.

Making and testing ammonia
In this experiment, students make ammonia, investigate its solubility in water and test its alkaline nature. Includes kit list and safety instructions.

Making esters from alcohols and acids
Investigate the reactions between a range of alcohols and acids by producing a variety of esters in this class experiment. Includes kit list and safety instructions.

Making glass
Try this class practical to make samples of glass using lead oxide, zinc oxide and boric acid. Includes kit list and safety instructions.

Making glue from milk
Try this class practical to prepare a polymer glue from milk using the protein casein. Includes kit list and safety instructions.

Making magnesium carbonate: the formation of an insoluble salt in water
Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Includes kit list and safety instructions.

Making plastic from potato starch
Try this class practical to make a plastic using potato starch and investigate the effects of adding a ‘plasticiser’. Includes kit list and safety instructions.

Making rayon
Use this demonstration to produce rayon fibres in the classroom using cotton wool or filter paper. Includes kit list and safety instructions.

Making soaps and detergents using castor oil
Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Includes kit list and safety instructions.

Making solder as an alloy of tin and lead
Try this practical to make solder by heating together the metals tin and lead before investigating the alloy’s properties. Includes kit list and safety instructions.


Melting and freezing stearic acid
In this class practical students take the temperature of stearic acid at regular intervals as they heat and cool it. Includes kit list and safety instructions.

Microscale extraction of copper
Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series. Includes kit list and safety instructions.

Microscale reactions of positive ions with sodium hydroxide
Try this microscale practical exploring the reactions of various positive ions with sodium hydroxide. Includes kit list and safety instructions.

Microscale oxidation of cyclohexanol by potassium dichromate(VI)
Use this quick class experiment to observe the oxidation of cyclohexanol to produce cyclohexanone. Includes kit list and safety instructions.

Microscale preparation of ethyl benzoate
Try this class practical to prepare the ester ethyl benzoate on a microscale by warming ethanol and benzoic acid. Includes kit list and safety instructions.

Modelling alloys with plasticine
Try this class activity to explore how alloying can be used to change the properties of a metal. Includes kit list and teaching notes.

Modelling the greenhouse effect
Use this demonstration to illustrate the greenhouse effect and the role of carbon dioxide as a greenhouse gas. Includes kit list and safety instructions.
Neutralising an acidic solution
Use this simple practical to illustrate the pH and temperature changes as an acidic solution is neutralised. Includes kit list and safety instructions.

Oxidation of ethanol
In this class practical, ethanol is oxidised by acidified sodium dichromate to form ethanal and then ethanoic acid. Includes kit list and safety instructions.

Phenol-methanal polymerisiation
Make Bakelite in class and investigate its properties using phenol, methanal and ethanoic acid in this demonstration. Includes kit list and safety instructions.

Preferential discharge of cations during electrolysis
Use this practical to show that metal cations are preferentially discharged, in relation to the metal’s position in the reactivity series. Includes kit list and safety instructions.

Preparing a soluble salt by neutralisation
In this practical, students react alkaline ammonia with sulfuric acid to form the soluble salt ammonium sulfate. Includes kit list and safety instructions.

Preparing an insoluble salt in a precipitation reaction
Produce an insoluble salt precipitate by reacting two soluble metal salts together in this class experiment. Includes kit list and safety instructions.

Preparing and using cobalt chloride indicator papers
Make your own cobalt chloride indicator papers, which can be used to test for the presence of water. Includes kit list and safety instructions.

Preparing salts by neutralisation of oxides and carbonates
Try these class experiments to illustrate the production of soluble salts from insoluble metal oxides and carbonates. Includes kit list and safety instructions.

Preventing rust
Try this practical to test methods for preventing rust on iron nails, including painting, greasing and sacrificial protection. Includes kit list and safety instructions.

Properties of alkali metal compounds
Try this class practical to explore the physical and chemical properties of various alkali metal compounds. Includes kit list and safety instructions.

Purifying an impure solid
Purify alum as an example of obtaining a pure chemical from an impure sample in this class practical. Includes kit list and safety instructions.

PVA polymer slime
In this fun class experiment student will make slime by adding borax solution to PVA. Includes kit list and safety instructions.
Quantitative electrolysis of aqueous copper(II) sulfate
Use this demonstration to find the value of the Faraday constant from electrolysis of copper(II) sulfate solution. Includes kit list and safety instructions.

Rate of evaporation
Use this class practical to measure and compare the rate of evaporation of propanone under different conditions. Includes kit list and safety instructions.

Rate of reaction of potassium manganate(VII) and oxalic acid
Investigate the effect of surface area or concentration on rate of reaction using oxalic acid in rhubarb and potassium manganate(VII). Includes kit list and safety instructions.

Reacting aluminium and iodine
Illustrate the spectacular reaction between aluminium and iodine with water as a catalyst in this demonstration. Includes kit list and safety instructions.

Reacting copper(II) oxide with sulfuric acid
Illustrate the reaction of an insoluble metal oxide with a dilute acid to produce crystals of a soluble salt in this class practical. Includes kit list and safety instructions.

Reacting magnesium with copper(II) oxide
Illustrate reduction, oxidation and the relative reactivity of magnesium and copper(II) oxide in this demonstration. Includes kit list and safety instructions.

Reacting zinc and copper(II) oxide
Illustrate competition reactions using the exothermic reaction between copper(II) oxide and zinc in this class demonstration. Includes kit list and safety instructions.

Reactions of chlorine with water or halide ions
Generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. Includes kit list and safety instructions.

Reactions of chlorine, bromine and iodine with aluminium
Try this demonstration to produce some spectacular exothermic redox reactions by reacting aluminium with halogens. Includes kit list and safety instructions.

Reactions of metals with acids producing salts
Explore the production of hydrogen gas and salts when metals react with acids in this class experiment. Includes kit list and safety instructions.

Reactivity trends of the alkali metals
Use this experiment to demonstrate the trend in reactivity down group 1 of the Periodic Table, exploring the physical and chemical properties of the alkali metals.

Rechargeable cells: the lead–acid accumulator
Use this practical to demonstrate the chemistry behind rechargeable batteries, using a lead–acid accumulator cell. Includes kit list and safety instructions.

Recovering water from a solution using a condenser
Use this demonstration to show how pure water can be recovered from copper sulfate solution using a condenser. Includes kit list and safety instructions.

Recovering water from copper(II) sulfate solution
Try this practical to introduce students to aqueous solutions by distilling water from copper(II) sulfate solution. Includes kit list and safety instructions.

Separating salts from seawater
Try this simple practical to show that seawater contains a mixture of different salts. Includes kit list and safety instructions.

Separating sand and salt by filtering and evaporation
Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Includes kit list and safety instructions.

Silver and lead halides
Try this practical or demonstration to produce silver and lead halides in a series of precipitation reactions. Includes kit list and safety instructions.

Sodium ethanoate ‘stalagmite’
Quickly grow your own ‘stalagmite’ from a supersaturated solution of sodium ethanoate in this demonstration. Includes kit list and safety instructions.

Solubility patterns of halogen anions
Try this microscale practical to identify and explain patterns in the solubility of fluoride, chloride, bromide and iodide anions. Includes kit list and safety instructions.
Solubility trends of metal halides
Investigate patterns in the solubility of halides of silver and some Group 1 and 2 metals in this microscale practical. Includes kit list and safety instructions.

Sulfuric acid as a dehydrating agent
Try these two demonstrations to illustrate the difference between dehydration and drying using sulfuric acid. Includes kit list and safety instructions.

Supercooling and the energetics of freezing
Explore what happens when a liquid is supercooled using sodium thiosulfate in this class practical. Includes kit list and safety instructions.
Testing for catalase enzymes
Try this class experiment to detect the presence of enzymes as they catalyse the decomposition of hydrogen peroxide. Includes kit list and safety instructions.

Testing the pH of different solutions
Use this practical to reinforce students’ understanding of pH by preparing and testing acidic and alkaline solutions. Includes kit list and safety instructions.

Testing the pH of oxides
Use this class practical to investigate the pH of different metal and non-metal oxides using a universal indicator. Includes kit list and safety instructions.

Testing the hardness of water
Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions.

The ‘blue bottle’ experiment
In this demonstration, the redox indicator Methylene blue can be oxidised many times by shaking. Includes kit list and safety instructions.

The ‘breathalyser’ reaction | 16–18 years
In association with Nuffield Foundation , By Tim Jolliff and Sandrine Bouchelkia
Try this demonstration to recreate an early ‘breathalyser’ test, passing ethanol vapour through potassium dichromate. Includes kit list and safety instructions

The ‘Old Nassau’ or Halloween clock reaction
Illustrate dramatic colour changes as a result of redox and precipitation reactions in this vivid demonstration. Includes kit list and safety instructions.

The ‘whoosh’ bottle demonstration
This exciting demonstration is a combustion reaction where a mixture of alcohol and air in a large bottle is ignited. Includes kit list and safety instructions.

The acidic reactions of ethanoic acid
Explore the properties of ethanoic acid as a weak organic acid in this class experiment. Includes kit list and safety instructions.

The change in mass when magnesium burns
A class practical to measure the change in mass when magnesium burns and to find the formula of magnesium oxide. Includes kit list and safety instructions.

The chemistry of cooking potatoes
Use this class practical to investigate what happens to potatoes and potato cells when they are boiled. Includes kit list and safety instructions.
The combustion of iron wool
Try this quick teacher demonstration to demonstrate the increase in mass as iron wool is heated in air. Includes kit list and safety instructions.

The composition and formula of water
Try this demonstration to determine the formula of water through the reaction of copper(II) oxide with hydrogen. Includes kit list and safety instructions.
The conversion of alcohols to halogenoalkanes
Try this practical or demonstration to produce bromoethane in a substitution reaction between ethanol and phosphorus tribromide. Includes kit list and safety instructions.

The cornflour ‘bomb’
Create a small explosion inside a tin can using cornflour in this demonstration, illustrating energy transformation. Includes kit list and safety instructions.

The density of carbon dioxide
Illustrate the higher density of carbon dioxide relative to air by pouring it over a lighted candle in this demonstration. Includes kit list and safety instructions.

The density of ice
Demonstrate to students what happens as ice cubes floating on oil start to melt and the density of the water changes. Includes kit list and safety instructions.

The effect of concentration and temperature on an equilibrium | Le Chatelier’s principle
Try this demonstration to illustrate how changing chlorine concentration or temperature shifts the position of an equilibrium. Includes kit list and safety instructions.
The effect of concentration on equilibrium | Le Chatelier’s principle
Illustrate the reversible reaction between bismuth(III) oxychloride and bismuth(III) chloride in this demonstration. Includes kit list and safety instructions.

The effect of pressure and temperature on equilibrium | Le Chatelier’s principle
Try this demonstration to explore the effects of pressure and temperature on an equilibrium mixture with your students. Includes kit list and safety instructions.

The equilibrium between two coloured cobalt species
In this demonstration the equilibrium between two different coloured cobalt species is disturbed. Le Chatelier’s principle is used to predict a colour change.

The formation of a sol
Convert a rusty-brown precipitate of iron(III) hydroxide into a cherry-red iron(III) oxide sol in this demonstration. Includes kit list and safety instructions.

The fractional distillation of crude oil
Try this class practical or demonstration to simulate the industrial fractional distillation of crude oil. Includes kit list and safety instructions.

The migration of ions during electrolysis of potassium manganate(VII)
Try this class practical to investigate the migration of ions during electrolysis as evidence for the ionic model. Includes kit list and safety instructions.

The properties of alcohols
Ethanol and propan-1-ol are tested for pH, reaction with sodium, combustion and oxidation with acidified dichromate(VI) solution. Includes kit list and safety instructions.
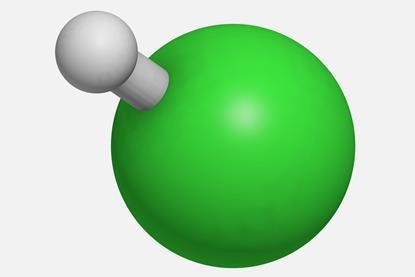
The properties of hydrogen chloride
Use this demonstration and practical to investigate properties of hydrogen chloride, such as its solubility in water. Includes kit list and safety instructions.
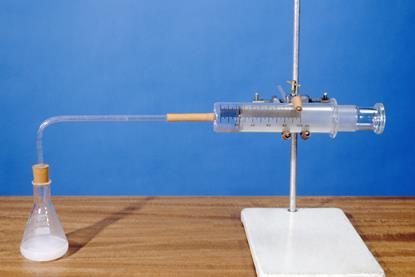
The rate of reaction of magnesium with hydrochloric acid
A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Includes kit list and safety instructions.

The reaction of aluminium and copper(II) sulfate
Try this practical or demonstration to illustrate the displacement of copper from copper sulfate using aluminium foil, with kit list and safety instructions.

The reaction of carbon dioxide with water
Form a weak acid from the reaction of carbon dioxide with water in this class practical. Includes kit list and safety instructions.

The reaction of ethyne with chlorine
Try this teacher demonstration with your students to illustrate the spontaneous reaction of ethyne and chlorine. Includes kit list and safety instructions.

The reactivity of iron
Illustrate iron’s position in the reactivity series by heating it with copper and magnesium oxides in this practical. Includes kit list and safety instructions.

The silver mirror test with Tollens’ reagent
Try this practical to explore the mirror-making reaction between silver nitrate (Tollens’ reagent) and glucose. Includes kit list, video and safety instructions.

The structure and properties of chocolate
Investigate how melting chocolate changes its structure and affects properties like taste, texture and melting point. Includes kit list and safety instructions.

The sublimation of air freshener | 11–14 years
In association with Nuffield Foundation , By Dorothy Warren
Use this experiment to demonstrate sublimation, showing how solid air freshener changes directly from a solid to a gas. Includes kit list and safety instructions.

The thermal properties of water
Explore water’s boiling point, specific heat capacity and thermal conductivity in this demonstration. Includes kit list and safety instructions.

The thermite reaction between aluminium and iron(III) oxide
Illustrate a highly exothermic thermite reaction resulting in molten iron in this teacher demonstration. Includes kit list and safety instructions.

Thermal decomposition of calcium carbonate
A class practical on the thermal decomposition of calcium carbonate. Includes kit list and safety instructions.

Thermal decomposition of nitrates: ‘writing with fire’
Make an invisible message ‘glow’ by applying a lighted splint to filter paper treated with sodium nitrate in this demonstration. Includes kit list and safety instructions.

Thermal decomposition of metal carbonates
Use this class practical to compare the thermal stabilities of carbonates of reactive and less reactive metals. Includes kit list and safety instructions.

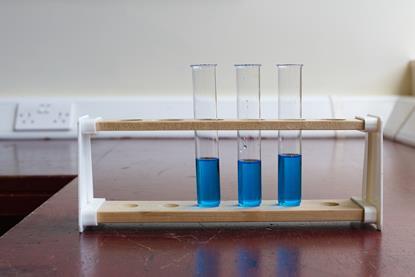
Titrating sodium hydroxide with hydrochloric acid
Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.

Turning ‘red wine’ into ‘water’
Use acidified potassium permanganate – or ‘red wine’ – to make ‘water’, ‘milk’ and ‘lemonade’ in this engaging demonstration. Includes kit list and safety instructions.

Turning copper coins into ‘silver’ and ‘gold’
Perform what looks like alchemy with ordinary copper coins in this teacher demonstration. Includes kit list and safety instructions.

Universal indicator ‘rainbow’
Try this demonstration to create a rainbow effect using universal indicator, hydrochloric acid and sodium hydroxide. Includes kit list and safety instructions.

Unsaturation in fats and oils
Use this class practical to investigate the amounts of unsaturated fats and oils in different foods. Includes kit list and safety instructions.

Urea-methanal polymerisation
Explore condensation polymerisation by creating and investigating the properties of a thermosetting polymer in this demonstration. Includes kit list and safety instructions.

Using indigestion tablets to neutralise an acid
Investigate and measure the neutralising effect of indigestion tablets on hydrochloric acid in this class practical. Includes kit list and safety instructions.

Water expands when it freezes
Use this demonstration to show that water expands when it freezes, showing students how it can break a bottle. Includes kit list and safety instructions.

What are the dissolved solids in seawater?
Analyse the salts that crystallise from evaporating seawater, illustrating cation and anion tests in this demonstration. Includes kit list and safety instructions.

What causes iron to rust?
Use this class experiment to help students investigate what conditions are needed for iron to rust. Includes kit list and safety instructions.

What ions cause hardness in water?
Investigate how different cations and anions in dissolved salts affect the formation of a lather in this experiment. Includes kit list and safety instructions.

What makes a substance acidic?
Try these experiments to investigate acidity and learn how the acidic properties of some substances require water. Includes kit list and safety instructions.

Where is carbon in the reactivity series?
Determine the position of carbon in the reactivity series by heating with metal oxides in this practical and demonstration. Includes kit list and safety instructions.

Which substances conduct electricity?
In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Includes kit list and safety instructions.

Yeast and the expansion of bread dough
Try this class practical to investigate how temperature affects yeast and the expansion of bread dough. Includes kit list and safety instructions.
- Contributors
- Email alerts
Site powered by Webvision Cloud

Outstanding scientists elected as Fellows of the Royal Society
Over 90 exceptional researchers from across the world have this year been elected to the Fellowship of the Royal Society , the UK’s national academy of sciences.
Recognised for their invaluable contributions to science, the elected Fellows are leaders in their fields. They include the Nobel laureate, Professor Emmanuelle Charpentier; an Emmy winner, Dr Andrew Fitzgibbon (for his contributions to the 3D camera tracker software “boujou”); and the former Chief Medical Advisor to the US President, Professor Anthony Fauci.
Drawn from across academia, industry and wider society, the new intake spans disciplines as varied as pioneering treatments for Huntington’s Disease, developing the first algorithm for video streaming, generating new insights into memory formation, and studying the origins and evolution of our universe.
Sir Adrian Smith, President of the Royal Society, said:
“I am pleased to welcome such an outstanding group into the Fellowship of the Royal Society.
“This new cohort have already made significant contributions to our understanding of the world around us and continue to push the boundaries of possibility in academic research and industry.
“From visualising the sharp rise in global temperatures since the industrial revolution to leading the response to the Covid-19 pandemic, their diverse range of expertise is furthering human understanding and helping to address some of our greatest challenges.
“It is an honour to have them join the Fellowship.”
Statistics about this year’s intake of Fellows:
- 30% of this year’s intake of Fellows, Foreign Members and Honorary Fellows are women
- New Fellows have been elected from 23 UK institutions, including The University of Nottingham, British Antarctic Survey, University of Strathclyde and the Natural History Museum
- They have been elected from countries including Brazil, China, Japan, Mexico and Singapore
The full list of the newly elected Fellows and Foreign Members of the Royal Society is, in alphabetical order:
New Fellows
Professor Simon Aldridge FRS Professor of Chemistry, Inorganic Chemistry Laboratory, University of Oxford
Professor Sir John Aston Kt FRS Harding Professor of Statistics in Public Life at Statistical Laboratory, Department of Pure Mathematics and Mathematical Statistics, University of Cambridge
Professor Frances Balkwill OBE FMedSci FRS Professor of Cancer Biology, Centre for Tumour Microenvironment, Barts Cancer Institute, Queen Mary University of London
Dr David Bentley OBE FMedSci FRS Former Vice President and Chief Scientist, Illumina Inc
Dr David Bentley FRS Professor, Department of Biochemistry and Molecular Genetics and Co-Director, RNA Bioscience Initiative, Anschutz Medical School, University of Colorado Denver, USA
Professor Donna Blackmond FRS John C. Martin Endowed Chair in Chemistry, Department of Chemistry, Scripps Research, USA
Professor Sarah-Jayne Blakemore FBA FMedSci FRS Professor of Psychology and Cognitive Neuroscience, Department of Psychology, University of Cambridge
Professor Helen Blau FRS Donald E and Delia B Baxter Foundation Professor and Director, Baxter Laboratory for Stem Cell Biology, Stanford University School of Medicine, USA
Professor Martin Blunt FREng FRS Professor of Flow in Porous Media, Department of Earth Science and Engineering, Imperial College London
Professor Daniel Bradley FRS Professor of Population Genetics, Trinity College Dublin
Professor Emmanuel Breuillard FRS Professor of Pure Mathematics, Mathematical Institute, University of Oxford
Sir Philip Campbell FRS Editor Emeritus, Nature
Professor Brian Cantor CBE FREng FRS Visiting Professor, Department of Materials, University of Oxford and Professor and Senior Advisor, Brunel Centre for Advanced Solidification Technology (BCAST), Brunel University London
Professor Kenneth Carslaw FRS Professor of Atmospheric Science, School of Earth and Environment, University of Leeds
Dr Andrew Carter FRS Programme Leader, Structural Studies Division, MRC Laboratory of Molecular Biology
Professor Patrick Chinnery FMedSci FRS Professor of Neurology, Department of Clinical Neurosciences, University of Cambridge
Professor Yanick Crow FMedSci FRS Professor and Programme Leader, MRC Human Genetics Unit, University of Edinburgh and Institute Imagine, Université Paris, France
Professor Barry Dickson FRS Professorial Research Fellow, Queensland Brain Institute, Australia
Professor Jo Dunkley OBE FRS Professor of Physics and Astrophysical Sciences, Departments of Physics and Astrophysical Sciences, Princeton University, USA
Professor Aled Edwards FRS Temerty Nexus Chair in Health Innovation and Technology, Structural Genomics Consortium, University of Toronto, Canada
Professor Paul Elliott CBE FMedSci FRS Professor of Epidemiology and Public Health Medicine, Imperial College London
Dr Alan Evans FRS Distinguished James McGill Professor of Neurology, Departments of Neurology and Psychiatry, McGill University, Canada
Professor Rebecca Fitzgerald FMedSci FRS Professor of Cancer Prevention and Director, Early Cancer Institute, University of Cambridge
Dr Andrew Fitzgibbon FREng FRS Engineering Fellow, Graphcore Ltd
Professor Michael Garrett FRS Sir Bernard Lovell Chair of Astrophysics and Director of Jodrell Bank Centre for Astrophysics (JBCA), Department of Physics and Astronomy, University of Manchester
Professor Toby Gee FRS Professor, Department of Mathematics, Imperial College London
Professor Nigel Goldenfeld FRS Chancellor's Distinguished Professor of Physics, Department of Physics, University of California San Diego, USA
Professor Anjali Goswami FRS Research Leader in Evolutionary Biology, Natural History Museum, London and President of the Linnean Society of London
Professor Maria Harrison FRS William H. Crocker Professor, Boyce Thompson Institute for Plant Research and Adjunct Professor, Cornell University, USA
Professor Richard Hartley FRS Emeritus Distinguished Professor, College of Engineering, Computing and Cybernetics, The Australian National University, Australia
Professor Laura Herz FRS Professor of Physics, Department of Physics, University of Oxford
Professor David Hodell FRS Woodwardian Professor of Geology and Director, Godwin Laboratory for Palaeoclimate Research, Department of Earth Sciences, University of Cambridge and fellow of Clare College
Professor Saskia Hogenhout FRS Group Leader, John Innes Centre
Sir Peter Horby Kt FMedSci FRS Moh Family Foundation Professor of Emerging Infections and Global Health, Nuffield Department of Medicine and Director, Pandemic Sciences Institute, University of Oxford
Professor Richard Jardine FREng FRS Professor of Geomechanics, Department of Civil and Environmental Engineering, Imperial College London, Imperial College Proconsul and Visiting Professor, Zhejiang University, China
Professor Heidi Johansen Berg FRS Professor of Cognitive Neuroscience, Nuffield Department of Clinical Neurosciences, University of Oxford
Mr Simon Knowles FRS CTO and EVP engineering, Graphcore
Professor David Komander FRS Head, Ubiquitin Signalling Division, Walter and Eliza Hall Institute of Medical Research (WEHI) and Professor, Department of Medical Biology, University of Melbourne, Australia
Professor Daniela Kühn FRS Mason Professor of Mathematics, School of Mathematics, University of Birmingham
Professor Eric Lauga FRS Professor of Applied Mathematics, Department of Applied Mathematics and Theoretical Physics, University of Cambridge
Professor Chwee Lim FRS NUS Society Chair Professor, Institute for Health Innovation & Technology, National University of Singapore, Singapore and NUS Society Chair Professor, Department of Biomedical Engineering, College of Design and Engineering, National University of Singapore
Professor Duncan Lorimer FRS Professor of Physics and Astronomy, Department of Physics and Astronomy, West Virginia University, USA
Professor Douglas MacFarlane FRS Sir John Monash Distinguished Professor, School of Chemistry, Monash University, Australia
Professor Barbara Maher FRS Professor Emerita of Environmental Magnetism, Lancaster Environment Centre, Lancaster University
Professor George Malliaras FRS Prince Philip Professor of Technology, Department of Engineering, University of Cambridge
Professor Ivan Marusic FRS Pro Vice-Chancellor and Redmond Barry Distinguished Professor, University of Melbourne, Australia
Professor Tamsin Mather FRS Professor of Earth Sciences, Department of Earth Sciences, University of Oxford
Professor Stephen McGrath FRS Discovery Leader in Sustainable Soils and Crops, Rothamsted Research
Professor Patricia Monaghan FRS Regius Professor of Zoology, School of Biodiversity, One Health and Veterinary Medicine, University of Glasgow
Professor Graham Moore FRS Director, The John Innes Centre
Professor Francis Nimmo FRS Professor of Planetary Sciences, Department of Earth and Planetary Sciences, University of California Santa Cruz, USA
Professor Sarah Otto FRS Professor, Department of Zoology, University of British Columbia, Canada
Professor Adrian Owen OBE FRS Professor in Cognitive Neuroscience and Imaging, University of Western Ontario, Canada
Professor Lloyd Peck FRS Science Leader, British Antarctic Survey, Cambridge
Professor José Penadés FRS Professor of Microbiology, Centre for Bacterial Resistance Biology, Department of Infectious Disease, Imperial College London
Professor Sir Andrew Pollard FMedSci FRS Ashall Professor of Infection and Immunity, Director of the Oxford Vaccine Group and Consultant in Paediatric Infectious Disease, Department of Paediatrics, University of Oxford
Professor Oscar Randal-Williams FRS Sadleirian Professor of Pure Mathematics, Department of Pure Mathematics and Mathematical Statistics, University of Cambridge
Professor Keith Ridgway CBE FREng FRS Senior Executive – Manufacturing, University of Strathclyde
Professor Tom Rodden FRS Pro-Vice-Chancellor and Professor of Interactive Computing, School of Computer Science, Nottingham University
Professor Stuart Rowan FRS Barry L MacLean Professor of Molecular Engineering, Pritzker School of Molecular Engineering and Department of Chemistry, University of Chicago, USA and Chemical Sciences and Engineering Division, Argonne National Laboratory, USA
Mr Simon Segars FRS Former CEO, Arm Holdings PLC. Board member Dolby Labs Inc, Vodafone Group PLC, Edge Impulse Inc, and Board Chair, Silicon Quantum Computing Pty
Professor Yang Shi FRS Professor of Epigenetics and Member, Ludwig Institute for Cancer Research, University of Oxford
Professor Lorraine Symington FRS Harold S Ginsberg Professor of Microbiology and Immunology, Columbia University, USA
Professor Sarah Tabrizi FMedSci FRS Professor of Clinical Neurology and Neurogenetics, Department of Neurodegenerative Disease, UCL Queen Square Institute of Neurology, University College London
Professor Patrick Unwin FRS Professor of Chemistry and Head, Department of Chemistry, University of Warwick
Professor Mihaela van der Schaar FRS John Humphrey Plummer Professor of Machine Learning, Artificial Intelligence and Medicine, Departments of Applied Mathematics and Theoretical Physics, Engineering and Medicine, University of Cambridge
Professor Bart Vanhaesebroeck FRS Professor of Cell Signalling, Research Department of Oncology, Cancer Institute, Faculty of Medical Sciences, University College London
Professor Glynn Winskel FRS Professor of Computer and Information Science, University of Strathclyde
Professor William Wisden FMedSci FRS Chair of Molecular Neuroscience, Department of Life Sciences, Imperial College London
Professor Xiaodong Zhang FRS Professor, Faculty of Medicine, Imperial College London and The Francis Crick Institute
New Honorary Fellows
Professor Kwame Anthony Appiah FRS Silver Professor of Philosophy and Law, New York University, USA
Lord Anthony Hughes PC FRS Former Judge, UK Supreme Court
New Foreign Members
Professor Yakir Aharonov ForMemRS Distinguished Professor of Theoretical Physics, Institute for Quantum Studies and Faculty of Physics, Schmid College of Science, Chapman University, USA and Professor Emeritus, Tel Aviv University, Israel
Dr Adriaan Bax ForMemRS NIH Distinguished Investigator and Chief of the Section of Biophysical NMR Spectroscopy, National Institutes of Health, USA
Professor Rene Bernards ForMemRS Professor of Molecular Carcinogenesis, Division of Molecuar Carcinogenesis, The Netherlands Cancer Institute, Netherlands
Professor Emily A. Carter ForMemRS Associate Laboratory Director and Gerhard R Andlinger Professor in Energy and the Environment, Princeton Plasma Physics Laboratory and Princeton University, USA
Professor Emmanuelle Charpentier ForMemRS Scientific and Managing Director, Max Planck Unit for the Science of Pathogens, Germany
Professor Patrick Cramer ForMemRS President, Max Planck Society and Director, Department of Molecular Biology, Max Planck Institute for Multidisciplinary Sciences, Germany
Professor Ingrid Daubechies ForMemRS James B Duke Professor, Department of Mathematics and Department of Electrical and Computer Engineering, Duke University, USA
Professor Anthony Fauci ForMemRS Distinguished University Professor, Georgetown University School of Medicine, and the McCourt School of Public Policy
Professor Thomas Henzinger ForMemRS Professor, Institute of Science and Technology Austria
Professor Ruth Lehmann ForMemRS Director and President, Whitehead Institute and Professor, Department of Biology, Massachusetts Institute of Technology
Dr Susana Magallón ForMemRS Senior Research Scientist and Director, Institute of Biology, Universidad Nacional Autónoma de México (UNAM), Mexico
Professor Michael Mann ForMemRS Presidential Distinguished Professor, Department of Earth and Environmental Science, University of Pennsylvania, and Director, Penn Center for Science, Sustainability and the Media (PCSSM), University of Pennsylvania, USA
Professor Anthony Movshon ForMemRS University Professor, and Silver Professor of Neural Science and Psychology, New York University and Professor of Ophthalmology and of Neuroscience and Physiology, and Investigator, Neuroscience Institute, NYU Grossman School of Medicine, USA
Professor William Nix ForMemRS Professor Emeritus, Department of Materials Science and Engineering, Stanford University, USA
Professor Kyoko Nozaki ForMemRS Professor, Department of Chemistry and Biotechnology, Graduate School of Engineering, University of Tokyo, Japan
Professor Jian-Wei Pan ForMemRS Professor, Department of Modern Physics and Executive Vice President, University of Science and Technology of China (USTC), China
Dr Aviv Regev ForMemRS Executive Vice President and Global Head, Genentech Research and Early Development, Genentech/Roche, USA
Professor Ares Rosakis ForMemRS Theodore von Kármán Professor of Aeronautics and Professor of Mechanical Engineering, Division of Engineering and Applied Science, California Institute of Technology, USA
Professor Paul Schulze-Lefert ForMemRS Director, Max Planck Institute for Plant Breeding Research, Germany
Professor Erin Schuman ForMemRS Director, Max Planck Institute for Brain Research, Germany
Professor Mark H. Thiemens ForMemRS Distinguished Professor of Chemistry and Biochemistry and John Dove Isaacs Endowed Chair in Natural Philosophy for Physical Sciences, University of California San Diego, USA
Professor Cesar Victora ForMemRS Emeritus Professor and Director, International Center for Equity in Healths, Federal University of Pelotas, Brazil
Email updates
We promote excellence in science so that, together, we can benefit humanity and tackle the biggest challenges of our time.
Subscribe to our newsletters to be updated with the latest news on innovation, events, articles and reports.
What subscription are you interested in receiving? (Choose at least one subject)

If you would like to apply for a placement or register interest in hosting a placement, please use the apply options on our homepage.

NASA Open Science Funding Opportunities
< Back to the Open-Source Science Initiative
NASA offers a number of opportunities in the Research Opportunities for Space and Earth Science (ROSES) for support of Open Science projects. Explore the list below of those that are currently open and ones that have been previously offered. Proposers should always consult NSPIRES for the most accurate and up-to-date information on NASA proposals. Questions about Open Science funding opportunities can be directed to [email protected].
F.14 High Priority Open-Source Science (HPOSS)
HPOSS supports new work to advance and streamline the open sharing of scientific information.
- Proposals for the development of new technology to increase the accessibility, inclusivity, or reproducibility of SMD research. This includes open source tools, software, frameworks, data formats, or libraries that will have a significant impact to the SMD science community.
- Proposals for the development of capacity building materials to support the adoption of open science practices. This includes curricula, tutorials, or other training materials that will be appropriate for one or more science disciplines supported by SMD.
- Awards of ~$100k support work for one year.
- Submit proposals for ROSES-24 at any point during the year, and they will be reviewed on a rolling basis.
- The ROSES-24 solicitation will be available through March 28, 2025, at which point the ROSES-25 solicitation will become available.
- Summaries of previously selected proposals are available under "Selections" on the HPOSS NSPIRES pages for ROSES-22 and ROSES-23 .
- Watch recording of HPOSS informational webinar and access the presentation slides .
F.8 Supplements for Open-Source Science (SOSS)
SOSS supports the addition of an open science component to an existing "parent" ROSES award.
- This could include making the results of the parent award more accessible, inclusive, and usable, or contributing back to relevant open science communities. This could also include cloud credits (through Amazon Web Services - AWS) to support activities from the parent award.
- ~$10K for cloud credits
- ~$50k otherwise
- Proposer must have an existing research proposal selected for funding by SMD with at least 15 months remaining in its period of performance at the time of the submission to SOSS.
- Submit proposals for ROSES-23 at any point during the year, and they will be reviewed on a rolling basis.
- The ROSES-23 solicitation will be available through March 29, 2024, at which point the ROSES-24 solicitation will become available.
Topical Workshops, Symposia, and Conferences (TWSC)
TWSC supports events including hackathons, un-conferences, and challenges that build open science skills.
- Rolling deadline.
- Events focused on Science Mission Directorate data, software, or open science practices
- Hackathons, un-conferences, and challenges that build open science skills
- Focused training in open science practices and principles
- Events MUST be openly available and allow for inclusive participation
- Potential proposers must confirm relevancy and availability of funds with a SMD program officer before preparing or submitting a proposal. TWSC has no dedicated funding, so proposers are directed to first contact a NASA division, office, or program that may have funding before preparing a proposal. See section 4.2.1 of the NOFO for information to include in an inquiry email and section 7.0 for contact information for Open Science TWSC inquiries.
F.7 Support for Open Source Tools, Frameworks, and Libraries (OSTFL)
Supports the improvement and sustainment of existing high-value, open-source tools, frameworks, and libraries that have significantly impacted the SMD science community.
- Foundational awards: cooperative agreements for up to five years for open-source tools, frameworks, and libraries that have a significant impact on two or more divisions of the SMD and
- Sustainment awards: grants or cooperative agreements of up to three years in duration for open-source tools, frameworks, and libraries that have significant impact in one or more divisions of the SMD.
- Notices of intent are requested by May 3, 2024, and proposals are due June 7, 2024.
- For previous examples of awards, see the 8 awards initially selected from the ROSES20 solicitation.
- A town hall on the ROSES24 F.7 OSTFL solicitation was held on April 2. Please review the slides and video recording .
F.14 Transform to Open Science Training (TOPS-T)
TOPS-T supports the development of discipline-specific open science curriculum and capacity building efforts for open science training.
- Not solicited in ROSES-24.
- $6.5M awarded over 3 years based on ROSES-22 solicitation. Read the news release and summaries of selected proposals .
F.19 Multidomain Reusable Artificial Intelligence Tools (MRAIT)
Solicits proposals that would enable critically needed machine learning tools to advance both Heliophysics and Earth Science research.
- Read summaries of selected proposals from the ROSES-22 solicitation
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Study explains why the brain can robustly recognize images, even without color
Press contact :, media download.

*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."

Previous image Next image
Even though the human visual system has sophisticated machinery for processing color, the brain has no problem recognizing objects in black-and-white images. A new study from MIT offers a possible explanation for how the brain comes to be so adept at identifying both color and color-degraded images.
Using experimental data and computational modeling, the researchers found evidence suggesting the roots of this ability may lie in development. Early in life, when newborns receive strongly limited color information, the brain is forced to learn to distinguish objects based on their luminance, or intensity of light they emit, rather than their color. Later in life, when the retina and cortex are better equipped to process colors, the brain incorporates color information as well but also maintains its previously acquired ability to recognize images without critical reliance on color cues.
The findings are consistent with previous work showing that initially degraded visual and auditory input can actually be beneficial to the early development of perceptual systems.
“This general idea, that there is something important about the initial limitations that we have in our perceptual system, transcends color vision and visual acuity. Some of the work that our lab has done in the context of audition also suggests that there’s something important about placing limits on the richness of information that the neonatal system is initially exposed to,” says Pawan Sinha, a professor of brain and cognitive sciences at MIT and the senior author of the study.
The findings also help to explain why children who are born blind but have their vision restored later in life, through the removal of congenital cataracts, have much more difficulty identifying objects presented in black and white. Those children, who receive rich color input as soon as their sight is restored, may develop an overreliance on color that makes them much less resilient to changes or removal of color information.
MIT postdocs Marin Vogelsang and Lukas Vogelsang, and Project Prakash research scientist Priti Gupta, are the lead authors of the study, which appears today in Science . Sidney Diamond, a retired neurologist who is now an MIT research affiliate, and additional members of the Project Prakash team are also authors of the paper.
Seeing in black and white
The researchers’ exploration of how early experience with color affects later object recognition grew out of a simple observation from a study of children who had their sight restored after being born with congenital cataracts. In 2005, Sinha launched Project Prakash (the Sanskrit word for “light”), an effort in India to identify and treat children with reversible forms of vision loss.
Many of those children suffer from blindness due to dense bilateral cataracts. This condition often goes untreated in India, which has the world’s largest population of blind children, estimated between 200,000 and 700,000.
Children who receive treatment through Project Prakash may also participate in studies of their visual development, many of which have helped scientists learn more about how the brain's organization changes following restoration of sight, how the brain estimates brightness, and other phenomena related to vision.
In this study, Sinha and his colleagues gave children a simple test of object recognition, presenting both color and black-and-white images. For children born with normal sight, converting color images to grayscale had no effect at all on their ability to recognize the depicted object. However, when children who underwent cataract removal were presented with black-and-white images, their performance dropped significantly.
This led the researchers to hypothesize that the nature of visual inputs children are exposed to early in life may play a crucial role in shaping resilience to color changes and the ability to identify objects presented in black-and-white images. In normally sighted newborns, retinal cone cells are not well-developed at birth, resulting in babies having poor visual acuity and poor color vision. Over the first years of life, their vision improves markedly as the cone system develops.
Because the immature visual system receives significantly reduced color information, the researchers hypothesized that during this time, the baby brain is forced to gain proficiency at recognizing images with reduced color cues. Additionally, they proposed, children who are born with cataracts and have them removed later may learn to rely too much on color cues when identifying objects, because, as they experimentally demonstrated in the paper, with mature retinas, they commence their post-operative journeys with good color vision.
To rigorously test that hypothesis, the researchers used a standard convolutional neural network, AlexNet, as a computational model of vision. They trained the network to recognize objects, giving it different types of input during training. As part of one training regimen, they initially showed the model grayscale images only, then introduced color images later on. This roughly mimics the developmental progression of chromatic enrichment as babies’ eyesight matures over the first years of life.
Another training regimen comprised only color images. This approximates the experience of the Project Prakash children, because they can process full color information as soon as their cataracts are removed.
The researchers found that the developmentally inspired model could accurately recognize objects in either type of image and was also resilient to other color manipulations. However, the Prakash-proxy model trained only on color images did not show good generalization to grayscale or hue-manipulated images.
“What happens is that this Prakash-like model is very good with colored images, but it’s very poor with anything else. When not starting out with initially color-degraded training, these models just don’t generalize, perhaps because of their over-reliance on specific color cues,” Lukas Vogelsang says.
The robust generalization of the developmentally inspired model is not merely a consequence of it having been trained on both color and grayscale images; the temporal ordering of these images makes a big difference. Another object-recognition model that was trained on color images first, followed by grayscale images, did not do as well at identifying black-and-white objects.
“It’s not just the steps of the developmental choreography that are important, but also the order in which they are played out,” Sinha says.
The advantages of limited sensory input
By analyzing the internal organization of the models, the researchers found that those that begin with grayscale inputs learn to rely on luminance to identify objects. Once they begin receiving color input, they don’t change their approach very much, since they’ve already learned a strategy that works well. Models that began with color images did shift their approach once grayscale images were introduced, but could not shift enough to make them as accurate as the models that were given grayscale images first.
A similar phenomenon may occur in the human brain, which has more plasticity early in life, and can easily learn to identify objects based on their luminance alone. Early in life, the paucity of color information may in fact be beneficial to the developing brain, as it learns to identify objects based on sparse information.
“As a newborn, the normally sighted child is deprived, in a certain sense, of color vision. And that turns out to be an advantage,” Diamond says.
Researchers in Sinha’s lab have observed that limitations in early sensory input can also benefit other aspects of vision, as well as the auditory system. In 2022, they used computational models to show that early exposure to only low-frequency sounds, similar to those that babies hear in the womb, improves performance on auditory tasks that require analyzing sounds over a longer period of time, such as recognizing emotions. They now plan to explore whether this phenomenon extends to other aspects of development, such as language acquisition.
The research was funded by the National Eye Institute of NIH and the Intelligence Advanced Research Projects Activity.
Share this news article on:
Related links.
- Project Prakash
- Department of Brain and Cognitive Sciences
Related Topics
- Brain and cognitive sciences
Related Articles
Scientists discover anatomical changes in the brains of the newly sighted

After a lifetime of blindness, newly sighted can immediately identify human locomotion
Early sound exposure in the womb shapes the auditory system
Vision is key to spatial skills
Previous item Next item
More MIT News

Understanding why autism symptoms sometimes improve amid fever
Read full story →

School of Engineering welcomes new faculty
Turning up the heat on next-generation semiconductors

Sarah Millholland receives 2024 Vera Rubin Early Career Award

A community collaboration for progress

MIT scholars will take commercial break with entrepreneurial scholarship
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
NSF Funded Expedition Project Uses AI to Rethink Computer Operating Systems

AUSTIN, Texas — Research that will use artificial intelligence to boost the performance and energy efficiency of computer operating systems will be led by a team from The University of Texas at Austin, thanks to a major grant from the U.S. National Science Foundation’s Expeditions in Computing program.
Today’s operating systems pose a significant barrier to a number of promised exciting innovations in computer hardware and applications — from personal assistant robots to autonomous vehicles to edge computing that could enable smart cities and less energy-intensive cloud computing. These operating systems often follow “one-size-fits-all” rules for how hardware resources get allocated between different applications running simultaneously. The inflexibility of these rules makes it hard to integrate new advancements, resulting in poor performance and inefficient use, a problem the UT research team plans to tackle by leveraging AI.
“Our project will employ AI-aided intelligent resource management and auto-adapt as new applications and hardware emerge,” said Aditya Akella, the Regents Chair in Computer Sciences #1 who is leading the project. “This will enable computing devices to be used at near-optimal efficiency while meeting the needs of arbitrary applications, and it will make computing infrastructure ‘self-driving’ by automating OS implementation and management.”
Akella said the project would extend beyond academics, bringing together not only computer scientists from UT, the Texas Advanced Computing Center, the University of Illinois at Urbana-Champaign, the University of Pennsylvania and the University of Wisconsin-Madison, but also industry partners from Amazon, Bosch, Cisco, Google, Microsoft and Broadcom.
“These partners collectively develop and run operating systems for much of the world’s computing infrastructure, and we will work with them to create the next-generation open-source intelligent and adaptive OS,” Akella said. “We believe that the project offers a timely opportunity to fundamentally change the trajectory of computing.”
He added that the new style of OS could help autonomous robots become “the smartphones of the 2030s and beyond.”
“The smartphone revolution was enabled by new OS frameworks (for example, iOS and Android) that enabled users to run arbitrary third-party apps that have come to determine how they interact with technology and essential services,” he said. “LDOS can similarly usher in the era of affordable personal robots and apps that support our day-to-day activities and improve society for all, particularly for aging and disadvantaged populations.”
In addition to the research initiative, the project will create new undergraduate and graduate curricula with modules, courses and certificates exploring the interplay of computer systems and AI. The project’s initiatives for broadening participation are designed to cultivate leadership among underrepresented groups in AI and prepare them for AI and computer systems technology and research careers. These initiatives seek to benefit hundreds to thousands of students each year.
This project has a natural synergy with UT’s Machine Learning Laboratory, where fundamental questions on new generative AI and machine learning techniques are being explored, and with the Center for Generative AI, which offers high-end GPU resources to train and evaluate new generative AI models.
Co-principal investigators from UT’s Department of Computer Science are Joydeep Biswas, Swarat Chaudhuri, Shuchi Chawla, Işıl Dillig, Daehyeok Kim, and Chris Rossbach. Co-PIs from UT’s Chandra Family Department of Electrical and Computer Engineering are Alex Dimakis and Sanjay Shakkottai.
The grant is for $12 million over five years, of which $9.3 million will come to UT. Expeditions awards represent some of the largest investments provided by the NSF’s Directorate for Computer and Information Science and Engineering. The full name of the project is NSF Expeditions in Computing: Learning Directed Operating System (LDOS) – A Clean-Slate Paradigm for Operating Systems Design and Implementation.
This new support further advances the University’s Year of AI , an initiative showcasing UT’s commitment to developing innovations and growing leaders to navigate the ever-evolving landscape brought about by AI.
Explore Latest Articles
May 20, 2024
Conquering Breast Cancer Using Supercomputers, Data, And Mathematical Modeling
Otters, Especially Females, Use Tools To Survive a Changing World

Artificial Intelligence Trained to Draw Inspiration From Images, Not Copy Them


IMAGES
VIDEO
COMMENTS
Nuffield Research Placements are funded by the Nuffield Foundation and delivered by STEM Learning. They are engaging, hands-on research projects, where students have the opportunity to make a meaningful contribution towards the work of a host organisation. They are a fantastic opportunity for students to apply skills and knowledge learned at ...
The Research Placements & Experience programme (formerly Nuffield Research Placements) provides engaging, hands-on projects, where Year 12 students have the opportunity to make a meaningful contribution towards the work of a host organisation through a well-supervised but independent placement collaboration relating to an area of science, quantitative social science, computing, technology ...
Nuffield Research Placements provide over 1,000 students each year with the opportunity to work ... (or equivalent) in science, technology, engineering, economics, geography, geology, economics, psychology, computing, statistics or maths. Be able to spend a few consecutive weeks over July and/or August to collaborate with your Project ...
Since 1962, the Foundation has supported major curriculum projects and the creation of a wealth of teaching resources across STEM subjects and beyond. Our work in education includes funding research and supporting its translation into policy and practice. The Foundation has supported some 60 major curriculum projects and countless smaller ones ...
Nuffield Research Placements. Programme guide for students . Introduction . Nuffield Research Placements (NRP), formerly known as Nuffield Science Bursaries, has supported approximately 20,000 post-16 students over the last 25 years to engage in real life research working alongside Science, Technology, Engineering and Maths ' (STEM ...
the Nuffield Research Placements (NRP) programme has been adapted so that an entirely virtual ... A well-supervised but independent research project relating to an area of science, quantitative social science, computing, technology, engineering or maths (or a combination!).
What is a Nuffield Research Placement? When? Summer holidays after Year 12 (in England, Wales and Northern Ireland) or S5 (in Scotland). How long? Four to six weeks. What? A well-supervised but independent research project relating to an area of science, quantitative social science, computing, technology, engineering or maths (or a combination ...
This summer, around 1,200 sixth formers and college students have undertaken Nuffield Research Placements in science (including social science), technology, engineering and maths (STEM) at workplaces across the UK.. Nuffield Research Placements change lives. They give students from more disadvantaged backgrounds, particularly those without a family history of higher education, the opportunity ...
Nuffield Research Placements provide over 1,000 students each year with the opportunity to work alongside ... Scottish Highers (or equivalent) in a science, technology, engineering, economics, geography, geology, social sciences (inc. psychology), computer, statistics or maths? ... Are you willing to undertake an onsite research project over 2 ...
The Nuffield Science Teaching Project was a programme to develop a better approach to teaching science in British secondary schools, under the auspices of the Nuffield Foundation. ... Journal of Research in Science Teaching 7.4 (December 1970) 283-302 (pdf, payment required).
The Nuffield project is the brainchild of the Nuffield Foundation set up by founder of Morris Motors, William Morris, Lord Nuffield in 1943; funding research for better social policy in education, welfare and justice. ... Institute, the foundation also seeks to provide opportunities for young people to develop skills and confidence in science ...
Published: Oct 11, 2021 3 min read. [email protected]. STEM Learning are now in their second year of delivering this highly regarded and prestigious programme thanks to ongoing funding from the Nuffield Foundation and support from UK Research and Innovation - and applications are now open for 2022! We hope to encourage Year 12/S5/Year 13 ...
Research. We want to improve people's life chances and identify ways to address disadvantage and inequality in our digitally driven society. Nuffield Foundation research provides independent evidence on topics that affect people's daily lives and their well-being.
What is a Nuffield Research Placement? Nuffield Research Placements give young people from disadvantaged backgrounds the opportunity to gain skills and confidence in science and research. ... A well-supervised but independent research project suitable for a 16/17 year-old. Projects must have broadly scientific, quantitative or technical content ...
The Nuffield Foundation is a charitable trust established in 1943 by William Morris, Lord Nuffield, the founder of Morris Motors Ltd. It aims to improve social well-being by funding research and innovation projects in education and social policy, and building research capacity in science and social science. Its current chief executive is Tim ...
Results so far - initial findings from the evaluation. Nuffield Research Placements have a positive impact on participants' access to STEM higher education courses. Nearly a third (32%) of participants enrolled in a STEM course in a Russell Group institution, compared to 25% of comparable pupils with similar demographic background and ...
Our goal is to find ways to improve educational outcomes through policy change and interventions that are grounded in robust evidence. We fund research and development projects relating to education across all life stages - from early years through school, to further and higher education and vocational learning. We want to understand young ...
Fermentation of glucose using yeast | 14-16 years. In association with Nuffield Foundation, By Neil Goalby. Use this class practical to investigate the fermentation of glucose by yeast and test for ethanol. Includes kit list, safety instructions, questions and answers.
Discover new research from across the sciences in our international, high impact journals. ... from news stories and blog posts to policy statements and projects. You can also find resources for teachers and history of science researchers. Blog. News. ... Nuffield Department of Medicine and Director, Pandemic Sciences Institute, University of ...
Register. Before you start your application, we need some basic information so we can stay in touch with you. Create a password. Email. The email address is not made public. It will only be used if you need to be contacted about your account or for opted-in notifications. Password. Confirm password.
Nuffield Research Placements provide over 1,000 students each year with the opportunity to work alongside professional scientists, technologists, engineers and mathematicians. Log in | Nuffield Research Placements
NASA offers a number of opportunities in the Research Opportunities for Space and Earth Science (ROSES) for support of Open Science projects. Explore the list below of those that are currently open and ones that have been previously offered. Proposers should always consult NSPIRES for the most accurate and up-to-date information on NASA proposals.
The Nuffield Primary Science materials were based on the findings of a research project called the Science Processes and Concept Exploration (SPACE). This was the first set of resources published in the UK for primary science teaching that was explicitly based on a constructivist view of learning. The research aims.
MIT postdocs Marin Vogelsang and Lukas Vogelsang, and Project Prakash research scientist Priti Gupta, are the lead authors of the study, which appears today in Science. Sidney Diamond, a retired neurologist who is now an MIT research affiliate, and additional members of the Project Prakash team are also authors of the paper. Seeing in black and ...
AUSTIN, Texas — Research that will use artificial intelligence to boost the performance and energy efficiency of computer operating systems will be led by a team from The University of Texas at Austin, thanks to a major grant from the U.S. National Science Foundation's Expeditions in Computing program.. Today's operating systems pose a significant barrier to a number of promised exciting ...
Quality Assured. Subject: Science Physics. This pack of Nuffield Physics activity sheets was designed to match the National Curriculum Science: Physics at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years. The pack includes student sheets for 97 different activities.
Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK Ian G. Mills Patrick G Johnston Centre for Cancer Research, Queen's University Belfast, Belfast, UK
Project Hail Mary is the latest science fiction novel by the creative mind of Andy Weir, who penned The Martian (Weir 2021). I was fortunate to contact Andy to have him answer a few questions about...
The largest category in our dataset was "what if" analyses—studies which used mathematical models to project the impact of masking on the contours of pandemics, epidemics, and outbreaks (204 - 212, 222, 223, 225 - 228). Such studies depend heavily on input parameters derived from mechanistic, observational, and experimental research.
A well-supervised but independent research project relating to an area of science, quantitative . social science, computing, technology, engineering or maths (or a combination!). ... • A personal statement outlining your interest in completing a Nuffield Research Placement.