- Case report
- Open access
- Published: 10 August 2020

Non-classical presentation of vitamin D deficiency: a case report
- Mohanad Kamaleldin Mahmoud Ibrahim 1 &
- Mustafa Khidir Mustafa Elnimeiri 2
Journal of Medical Case Reports volume 14 , Article number: 126 ( 2020 ) Cite this article
7351 Accesses
3 Citations
22 Altmetric
Metrics details
Vitamin D is a fat-soluble vitamin; vitamin D is essential to sustain health and it protects against osteoporosis. It is crucial to the human body’s physiology in terms of muscular movement and neurological signal transmission, and to the immune system in defense against invading pathogens.
Case presentation
This was a case of a 26-year-old Sudanese woman who presented with a 2-year history of anosmia, recurrent nasal polyps, back pain, and chronic fatigue. She was diagnosed as having a case of vitamin D deficiency and responded well to treatment.
There is an association between vitamin D deficiency and recurrent allergic nasal conditions.
Peer Review reports
Vitamin D is a fat-soluble vitamin; it is naturally present in some foods and as dietary supplements. It is also produced endogenously through exposure to ultraviolet rays from sunlight. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation. The first occurs in the liver and produces 25-hydroxyvitamin D (25(OH)D), also known as calcidiol. The second occurs in the kidney and forms the physiologically active 1,25-dihydroxy vitamin D (1,25(OH) 2 D), also known as calcitriol [ 1 ].
Vitamin D is found in cells throughout the body; vitamin D is essential to sustain health and it protects against osteoporosis. It is crucial to the human body’s physiology in terms of muscular movement and neurological signal transmission, and to the immune system in defense against invading pathogens [ 2 ].
Although there are different methods and criteria for defining vitamin D levels, the criteria Holick proposed have been widely accepted. In this proposal, vitamin D deficiency is defined as blood level of less than 20 ng/ml; insufficiency of vitamin D is defined as blood levels ranging between 20 and 29.9 ng/ml and sufficiency if greater than or equal to 30 ng/ml [ 3 ]. About one billion people globally have vitamin D deficiency and 50% of the population has vitamin D insufficiency. The majority of affected people with vitamin D deficiency are the elderly, obese patients, nursing home residents, and hospitalized patients. Vitamin D deficiency arises from multiple causes including inadequate dietary intake and inadequate exposure to sunlight. Certain malabsorption syndromes such as celiac disease, short bowel syndrome, gastric bypass, some medications and cystic fibrosis may also lead to vitamin D deficiency [ 4 ].
Vitamin D deficiency is now more prevalent than ever and should be screened in high-risk populations. Many conflicting studies now show an association between vitamin D deficiency and cancer, cardiovascular disease, diabetes, autoimmune diseases, and neuropsychiatric disorders [ 5 , 6 ].
This was a case of a 26-year-old Sudanese woman, married, who has a 3-year-old boy. This woman presented to our ear, nose, and throat (ENT) department complaining of anosmia for the past 2 years. She had a history of two functional endoscopic sinus surgeries (FESSs) for nasal polyps: the first one was 6 years ago and the second one was 3 years prior to presentation. She complained of being highly sensitive to different irritants including dust, weather change, perfumes, and pets.She also stated that she attended more than three different physicians due to generalized fatigue and getting tired easily after simple daily activity in addition to sleeping for more than 10 hours a day.She attended an orthopedic clinic for unspecified lower back pain that was not related to any type of trauma or physical activity; a lumbosacral magnetic resonance imaging (MRI) was done and revealed no abnormal findings.She mentioned that she is known to be anxious most of the time and aggressive toward simple reactions from her family members. She had no psychiatric history and was not using any medications.
She was not known to be diabetic or hypertensive or to have any chronic illnesses; she was not on any regular medication. She is a housewife of high socioeconomic status; she is well educated, graduated from dental school with a bachelor’s degree, but currently not employed. She has never consumed tobacco or alcohol; she practiced regular cardio exercises.On examination, she looked healthy, well, not pale or jaundiced. Her pulse rate was 74/minute and her blood pressure was 118/70. Her body mass index (BMI) was 26.8. All systems examinations were normal except for bilateral nasal polyps. Complete blood count (CBC), renal function test (REF), electrolyte, liver function test (LFT), thyroid function test (TFT), urine analysis (general urine test), antinuclear antibody (ANA), and rheumatoid factor (RF) were all normal. An imaging profile included lumbo-sacral MRI, a computed tomography (CT) scan of her sinuses, and electrocardiogram (ECG), which were normal except for bilateral nasal polyps and severe sinusitis that looked allergic to fungi in nature.She underwent FESS surgery to remove the polyps and clean out her sinuses; up to 6 weeks after surgery she used nasal steroids (mometasone furoate 0.005%) two times a day, but her symptoms regarding anosmia were not improved. MRI of her brain and a CT scan of her sinuses were done and both revealed normal features. A vitamin D deficiency was suggested and the laboratory results revealed a low vitamin D level of 7 ng/ml. Treatment with vitamin D supplement was prescribed at 50,000 international units (IU) weekly for 8 weeks and then 1000 IU maintenance dose daily, she was advised to take food rich in vitamin D and get exposed to sunlight for 20 minutes three times a week after the loading dose of supplement. She was at regular follow-up for 6 months; at rates of weekly for the first month, every 2 weeks for the second month, and monthly for the rest of the follow-up period. At each visit, she was assessed with clinical history and examination. It was noticed that the symptoms of tiredness, sleeping, anosmia, and back pain were dramatically improving during that period. At the 6 months follow-up, her blood level of vitamin D was normal, she described her condition as free from all symptoms, and she returned back to normal physical activity.
Discussion and conclusions
This was a non-classical case of vitamin D deficiency of a 26-year-old woman who presented with chronic anosmia and recurrent nasal polyps. She was diagnosed as having a case of vitamin D deficiency and responded well to vitamin D replacement therapy. This case correlated an association between decreased levels of vitamin D and recurrent nasal polyps that led in time to chronic anosmia as a result of chronic high sensitivity reactions triggered by our patient’s autoimmune system. The literature links chronic rhinosinusitis with nasal polyps (CRSwNP) with asthma and allergic rhinitis, but the cellular and molecular mechanisms that contribute to the clinical symptoms are not fully understood. Sinonasal epithelial cell barrier defects, increased exposure to pathogenic and colonized bacteria, and dysregulation of the host immune system are all thought to play prominent roles in disease pathogenesis [ 7 ].
Despite all the previous surgical and medical interventions over the past 6 years, our patient’s condition did not improve and she still complained of anosmia. A study revealed that this patient was experiencing excessive allergic reactions that led to recurrent nasal polyps. It is well known that classical clinical effects of vitamin D deficiency are bones and musculoskeletal-related disorders, several lines of evidence demonstrate the effects of vitamin D on pro-inflammatory cytokines, regulatory T cells, and immune responses, with a conflicting interpretation of the effects of vitamin D on allergic diseases [ 8 ].
The working diagnosis was suggested in relation to some musculoskeletal symptoms and chronic fatigue especially when the imaging profile for her lower back and all routine investigations were normal. It has been suggested that clinicians should routinely test for hypovitaminosis D in patients with musculoskeletal symptoms, such as bone pain, myalgias, and generalized weakness which might be misdiagnosed as fibromyalgia and chronic fatigue [ 9 ]. The most common causes of anosmia were assessed as well and they were negative, these included sinonasal diseases, post infectious disorder, and post-traumatic disorder, and congenital defects and disorders caused by neurodegenerative disease [ 10 ].
Thus blood level for vitamin D was requested and the results were of low D level.
In the past history of the previous nasal polyps surgeries, our patient noted that there was no anosmia and her main complaints were classic complaints of sinusitis, including sneezing, nasal blockage and headache. Soon after surgery her symptoms improved except for the allergy-related symptoms, despite usage of inhaled steroids spray. She stated that, at the last time, the presentation was different since it was only anosmia, indicating that there was significant inflammation that affected the smell receptors around the olfactory epithelium. After the last nasal polyps and sinuses drainage surgery, the symptoms related to allergic reactions, including chronic sneezing, did not improve for up to 6 weeks and she was still suffering from hyposmia, although that was a fair postoperative period for recovery.
The symptoms of anosmia and sneezing, and other systematic symptoms, gradually started to improve after vitamin D supplements, indicating that the main reason behind her symptoms was vitamin D deficiency. She was followed up for up to 6 months after establishment of vitamin D supplements and at the last follow-up she had a normal sense of smell, and she was free from back pain, fatigue, and allergy-related symptoms.
This was a non-classical presentation as our patient was young and she did not have alkaline phosphatase, calcium, and phosphorus abnormalities [ 11 ] that are expected in cases of vitamin D deficiency.
This case revealed an association between decreased levels of vitamin D and recurrent nasal polyps that led to anosmia as a result of hypersensitive reactions produced by the body’s systems.
Although vitamin D deficiency is prevalent, measurement of serum 25(OH)D level is expensive, and universal screening is not supported. However, vitamin D testing may benefit those at risk for severe deficiency.
It is highly recommended to consider vitamin D deficiency among all patients with unspecified symptoms or in cases of non-diagnosed disorder regardless of the presenting complaint.
In conclusion, there is an association between vitamin D deficiency and recurrent allergic nasal conditions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic rhinosinusitis with nasal polyps
Computed tomography
Electrocardiogram
Ear, nose, and throat
Functional endoscopic sinus surgery
Body mass index
Complete blood count
Renal function test
Liver function test
Thyroid function test
Antinuclear antibody
Rheumatoid factor
International unit
National Institutes of Health. Vitamin D: fact sheet for health professionals. 2020. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#en2 . Accessed 10 Apr 2020.
Google Scholar
National Institutes for Health (NIH). Vitamin D fact sheet for consumers. 2019. https://ods.od.nih.gov/factsheets/VitaminD-Consumer/ . Accessed 20 Dec 2019.
Kuriacose R, Olive KE. Vitamin D insufficiency/deficiency management. South Med J. 2014;107(2):66–70. https://doi.org/10.1097/SMJ.0000000000000051 .
Article CAS PubMed Google Scholar
Sizar O, Khare S, Givler A. Vitamin D deficiency. Treasure Island: Stat Pearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK532266/ . PMID: 30335299. Accessed 20 Dec 2019.
Wlliam B, Fatme A, Meis M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D 3 supplementation can have important patient and public health benefits. Eur J Clin Nutr. 2020;74:366–76. https://doi.org/10.1038/s41430-020-0564-0 .
Article CAS Google Scholar
Hanmin W, Weiwen C, Dongqing L, Xiaoe Y, Xiaode Z, Nancy O, et al. Vitamin D and chronic diseases. Aging Dis. 2017;8(3):346–53. https://doi.org/10.14336/AD.2016.1021 .
Article Google Scholar
Whitney W, Ropert P, Robert C. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(04):565–72. https://doi.org/10.1016/j.jaip.2016.04.012 .
Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. https://doi.org/10.4065/mcp.2010.0567 .
Article CAS PubMed PubMed Central Google Scholar
Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752–8. https://doi.org/10.4065/mcp.2010.0138 .
Article PubMed PubMed Central Google Scholar
Sanne B, Elbrich M, Duncan B, Antje W, Veronika S, Joel D, et al. Anosmia: a clinical review. Chem Senses. 2017;42(7):513–23. https://doi.org/10.1093/chemse/bjx025 .
Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis. BMJ Case Rep. 2014; https://doi.org/10.1136/bcr-2014-204558 .
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Community Medicine and Epidemiology, Faculty of Medicine, Ibn Sina University, Khartoum, Sudan
Mohanad Kamaleldin Mahmoud Ibrahim
Preventive Medicine and Epidemiology, Alneelain University, Khartoum, Sudan
Mustafa Khidir Mustafa Elnimeiri
You can also search for this author in PubMed Google Scholar
Contributions
MI analyzed and interpreted the findings of the case report and was the major contributor in writing the manuscript. ME reviewed the report and added valuable comments. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohanad Kamaleldin Mahmoud Ibrahim .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from Albasar Institutional Review Board.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ibrahim, M.K.M., Elnimeiri, M.K.M. Non-classical presentation of vitamin D deficiency: a case report. J Med Case Reports 14 , 126 (2020). https://doi.org/10.1186/s13256-020-02454-1
Download citation
Received : 26 March 2020
Accepted : 08 July 2020
Published : 10 August 2020
DOI : https://doi.org/10.1186/s13256-020-02454-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vitamin D deficiency
- Nasal polyps
- Chronic fatigability
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Summary of Recommendation
- USPSTF Assessment of Magnitude of Net Benefit
- Practice Considerations
- Update of Previous USPSTF Recommendation
- Supporting Evidence
- Research Needs and Gaps
- Recommendations of Others
- Article Information
See the Figure for a more detailed summary of the recommendations for clinicians. See the Practice Considerations section for additional information regarding the I statement. USPSTF indicates US Preventive Services Task Force.
USPSTF indicates US Preventive Services Task Force.
US Preventive Services Task Force (USPSTF) Grades and Levels of Evidence
- USPSTF Review: Screening for Vitamin D Deficiency in Adults JAMA US Preventive Services Task Force April 13, 2021 This systematic review to support the 2021 US Preventive Services Task Force Recommendation Statement on screening for vitamin D deficiency summarizes published evidence on the benefits and harms of screening and interventions for vitamin D deficiency in asymptomatic, community-dwelling adults. Leila C. Kahwati, MD, MPH; Erin LeBlanc, MD, MPH; Rachel Palmieri Weber, PhD; Kayla Giger, BS; Rachel Clark, BA; Kara Suvada, BS; Amy Guisinger, BS; Meera Viswanathan, PhD
- USPSTF 2021 Recommendations on Screening for Asymptomatic Vitamin D Deficiency in Adults JAMA Editorial April 13, 2021 Sherri-Ann M. Burnett-Bowie, MD, MPH; Anne R. Cappola, MD, ScM
- Patient Information: Screening for Vitamin D Deficiency in Adults JAMA JAMA Patient Page April 13, 2021 This JAMA Patient Page summarizes the US Preventive Services Task Force’s 2021 recommendation that current evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults (I statement). Jill Jin, MD, MPH
- USPSTF Still Finds Insufficient Evidence to Support Screening for Vitamin D Deficiency JAMA Network Open Editorial April 13, 2021 Erin D. Michos, MD, MHS; Rita R. Kalyani, MD, MHS; Jodi B. Segal, MD, MPH
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
US Preventive Services Task Force. Screening for Vitamin D Deficiency in Adults : US Preventive Services Task Force Recommendation Statement . JAMA. 2021;325(14):1436–1442. doi:10.1001/jama.2021.3069
Manage citations:
© 2024
- Permissions
Screening for Vitamin D Deficiency in Adults : US Preventive Services Task Force Recommendation Statement
- Editorial USPSTF 2021 Recommendations on Screening for Asymptomatic Vitamin D Deficiency in Adults Sherri-Ann M. Burnett-Bowie, MD, MPH; Anne R. Cappola, MD, ScM JAMA
- Editorial USPSTF Still Finds Insufficient Evidence to Support Screening for Vitamin D Deficiency Erin D. Michos, MD, MHS; Rita R. Kalyani, MD, MHS; Jodi B. Segal, MD, MPH JAMA Network Open
- US Preventive Services Task Force USPSTF Review: Screening for Vitamin D Deficiency in Adults Leila C. Kahwati, MD, MPH; Erin LeBlanc, MD, MPH; Rachel Palmieri Weber, PhD; Kayla Giger, BS; Rachel Clark, BA; Kara Suvada, BS; Amy Guisinger, BS; Meera Viswanathan, PhD JAMA
- JAMA Patient Page Patient Information: Screening for Vitamin D Deficiency in Adults Jill Jin, MD, MPH JAMA
Importance Vitamin D is a fat-soluble vitamin that performs an important role in calcium homeostasis and bone metabolism and also affects many other cellular regulatory functions outside the skeletal system. Vitamin D requirements may vary by individual; thus, no one serum vitamin D level cutpoint defines deficiency, and no consensus exists regarding the precise serum levels of vitamin D that represent optimal health or sufficiency.
Objective To update its 2014 recommendation, the US Preventive Services Task Force (USPSTF) commissioned a systematic review on screening for vitamin D deficiency, including the benefits and harms of screening and early treatment.
Population Community-dwelling, nonpregnant adults who have no signs or symptoms of vitamin D deficiency or conditions for which vitamin D treatment is recommended.
Evidence Assessment The USPSTF concludes that the overall evidence on the benefits of screening for vitamin D deficiency is lacking. Therefore, the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults cannot be determined.
Recommendation The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults. (I statement)
See the Summary of Recommendation figure.
Quiz Ref ID Vitamin D is a fat-soluble vitamin that performs an important role in calcium homeostasis and bone metabolism and also affects many other cellular regulatory functions outside the skeletal system. 1 - 3 Vitamin D requirements may vary by individual; thus, no one serum vitamin D level cutpoint defines deficiency, and no consensus exists regarding the precise serum levels of vitamin D that represent optimal health or sufficiency. According to the National Academy of Medicine, an estimated 97.5% of the population will have their vitamin D needs met at a serum level of 20 ng/mL (49.9 nmol/L) and risk for deficiency, relative to bone health, begins to occur at levels less than 12 to 20 ng/mL (29.9-49.9 nmol/L). 1 , 4 A report based on data from the 2014 National Health and Nutrition Examination Survey found that 5% of the population 1 year or older had very low 25-hydroxyvitamin D (25[OH]D) levels (<12 ng/mL) and 18% had levels between 12 and 19 ng/mL. 5
Quiz Ref ID The US Preventive Services Task Force (USPSTF) concludes that the overall evidence on the benefits of screening for vitamin D deficiency is lacking. Therefore, the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults cannot be determined ( Table ).
See the Figure , Table , and eFigure in the Supplement for more information on the USPSTF recommendation rationale and assessment. For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual. 6
Quiz Ref ID This recommendation applies to community-dwelling, nonpregnant adults who have no signs or symptoms of vitamin D deficiency, such as bone pain or muscle weakness, or conditions for which vitamin D treatment is recommended. This recommendation focuses on screening (ie, testing for vitamin D deficiency in asymptomatic adults and treating those found to have a deficiency), which differs from USPSTF recommendation statements on supplementation.
Quiz Ref ID Although there is insufficient evidence to recommend for or against screening for vitamin D deficiency, several factors are associated with lower vitamin D levels. Low dietary vitamin D intake may be associated with lower 25(OH)D levels. 7 Little or no UV B exposure (eg, because of winter season, high latitude, or sun avoidance) and older age are also associated with an increased risk for low vitamin D levels. 8 - 12 Obesity is associated with lower 25(OH)D levels, 13 and people who are obese have a 1.3- to 2-fold increased risk of being vitamin D–deficient, depending on the threshold used to define deficiency. 8 , 9 , 13 , 14 The exact mechanism for this finding is not completely understood.
Depending on the serum threshold used to define deficiency, the prevalence of vitamin D deficiency is 2 to 10 times higher in non-Hispanic Black persons than in non-Hispanic White persons, likely related to differences in skin pigmentation. 7 - 9 , 14 However, these prevalence estimates are based on total 25(OH)D levels, and controversy remains about whether this is the best measure of vitamin D status among different racial and ethnic groups.
A significant proportion of the variability in 25(OH)D levels among individuals is not explained by the risk factors noted above, which seem to account for only 20% to 30% of the variation in 25(OH)D levels. 11 , 15
Vitamin D deficiency is usually treated with oral vitamin D. There are 2 commonly available forms of vitamin D—vitamin D 3 (cholecalciferol) and vitamin D 2 (ergocalciferol). Both are available as either a prescription medication or an over-the-counter dietary supplement.
The prevalence of vitamin D deficiency varies based on how deficiency is defined. According to data from the 2011 to 2014 National Health and Nutrition Examination Survey, which used the liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay to measure 25(OH)D levels, 5% of the population 1 year or older had very low 25(OH)D levels (<12 ng/mL) and 18% had levels between 12 and 19 ng/mL. 5 (To convert 25[OH]D values to nmol/L, multiply by 2.496.)
In some observational studies, lower vitamin D levels have been associated with risk for fractures, falls, functional limitations, some types of cancer, diabetes, cardiovascular disease, depression, and death. 16 , 17 However, observations of these associations are inconsistent. This inconsistency may be because of different studies using different cutoffs to define a low vitamin D level or because vitamin D requirements and the optimal cutoff that defines a low vitamin D level or vitamin D deficiency may vary by individual or by subpopulation. For example, non-Hispanic Black persons have lower reported rates of fractures 18 despite having increased prevalence of lower vitamin D levels than White persons. 7 - 9 , 14 Further, it is unknown whether these associations are linked to causality.
The goal of screening for vitamin D deficiency would be to identify and treat it before associated adverse clinical outcomes occur. Total 25(OH)D level is currently considered the best marker of vitamin D status. 4 , 19 A variety of assays can be used to measure 25(OH)D levels; however, levels can be difficult to measure accurately, and assays may underestimate or overestimate 25(OH)D levels. Additionally, the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community-dwelling adults.
Screening may misclassify persons with a vitamin D deficiency because of the uncertainty about the cutoff for defining deficiency and the variability of available testing assays. Misclassification may result in overdiagnosis (leading to nondeficient persons receiving unnecessary treatment) or underdiagnosis (leading to deficient persons not receiving treatment).
Quiz Ref ID A rare but potential harm of treatment with vitamin D is toxicity, which is characterized by marked hypercalcemia as well as hyperphosphatemia and hypercalciuria. However, the 25(OH)D level associated with toxicity (typically >150 ng/mL) 20 is well above the level considered to be sufficient. In general, treatment with oral vitamin D does not seem to be associated with serious harms.
The prevalence of screening for vitamin D deficiency by primary care clinicians in the US has not been well studied. Data suggest that laboratory testing for vitamin D levels has increased greatly over the last several years or longer. One study reported a more than 80-fold increase in Medicare reimbursement volumes for vitamin D testing from 2000 to 2010. 21
The USPSTF has published recommendations on the use of vitamin D supplementation for the prevention of falls 22 and fractures 23 and vitamin supplementation for the prevention of cardiovascular disease or cancer. 24 These recommendations differ from the current recommendation statement in that they address vitamin D supplementation without first determining a patient's vitamin D status (ie, regardless of whether they have a deficiency).
This recommendation updates the 2014 USPSTF recommendation statement on screening for vitamin D deficiency in asymptomatic adults. In 2014, the USPSTF concluded that the evidence was insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency. 25 For the current recommendation statement, the USPSTF again concludes that the evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults.
To update its 2014 recommendation statement, the USPSTF commissioned a systematic review 26 , 27 of the evidence on screening for vitamin D deficiency, including the benefits and harms of screening and early treatment. The review focused on asymptomatic, community-dwelling, nonpregnant adults 18 years or older who do not have clinical signs of vitamin D deficiency or conditions that could cause vitamin D deficiency, or for which vitamin D treatment is recommended, and who were seen in primary care settings.
Total 25(OH)D levels can be measured by both binding and chemical assays. Serum total 25(OH)D levels are difficult to measure accurately, and different immunoassays can lead to underestimation or overestimation of total 25(OH)D levels. 19 LC-MS/MS is considered the reference assay. However, LC-MS/MS is a complicated process and is subject to variation and error, including interference from other chemical compounds. 19
In 2010, the National Institutes of Health Office of Dietary Supplements, in collaboration with other organizations, initiated the Vitamin D Standardization Program. 28 , 29 The primary goal of the program has been to promote the standardized measurement of 25(OH)D levels. Most of the trials reviewed for this recommendation precede this standardization program. When previously banked samples have been reassayed using these standardized methods, both upward and downward revisions of 25(OH)D levels have been observed, depending on the original assay that was used. 19 , 30 , 31
The USPSTF found no studies that directly evaluated the benefits of screening for vitamin D deficiency. The USPSTF did find 26 randomized clinical trials (RCTs) and 1 nested case-control study that reported on the effectiveness of treatment of vitamin D deficiency (variably defined as a level <20 ng/mL to <31.2 ng/mL) on a variety of health outcomes, including all-cause mortality, fractures, incidence of diabetes, cardiovascular events and cancer, falls, depression, physical function, and infection. 26 , 27
Eight RCTs and 1 nested case-control study reported on all-cause mortality in community-dwelling adults. Study duration ranged from 16 weeks to 7 years. In a pooled analysis of the 8 trials (n = 2006), there was no difference in all-cause mortality in persons randomized to vitamin D treatment compared with controls (relative risk [RR], 1.13 [95% CI, 0.39-3.28]). 26 , 27 In the Women’s Health Initiative (WHI) Calcium–Vitamin D nested case-control study, there was no association between treatment with vitamin D and calcium and all-cause mortality among participants with baseline vitamin D levels between 14 and 21 ng/mL and among participants with baseline levels less than 14 ng/mL. 32 , 33
Six RCTs reported on fracture outcomes in community-dwelling adults. Study duration ranged from 12 weeks to 7 years. A pooled analysis of the 6 trials (n = 2186) found no difference in the incidence of fractures among those randomized to vitamin D treatment compared with placebo (RR, 0.84 [95% CI, 0.58-1.21]). 26 The USPSTF found only 1 trial reporting on hip fracture in community-dwelling adults. In that study, only 1 hip fracture occurred, leading to a very imprecise effect estimate. 34 In the WHI Calcium–Vitamin D nested case-control study, there was no association between treatment with vitamin D and calcium and clinical fracture or hip fracture incidence. 32
Five RCTs reported on incident diabetes. Study duration ranged from 1 year to 7 years. A pooled analysis of the 5 trials (n = 3356) found no difference in the incidence of diabetes among participants randomized to vitamin D treatment compared with placebo (RR, 0.96 [95% CI, 0.80-1.15]). 26
For several outcomes, the USPSTF found inadequate evidence on the benefit of treatment of asymptomatic vitamin D deficiency. Limitations of the following evidence include few studies reporting certain outcomes and, for some outcomes, variable methods of ascertainment, variable reporting of outcomes, small study size, or short duration of follow-up.
Two trials, the Vitamin D and Omega-3 Trial (VITAL) (n = 2001 in trial subgroup) 35 and the Vitamin D Assessment Study (ViDA) (n = 1270 in trial subgroup), 36 reported on cardiovascular events. Both trials observed no statistically significant differences in cardiovascular events between the treatment and placebo groups among the subgroup of participants with serum vitamin D levels less than 20 ng/mL at baseline. VITAL had 5.3 years of follow-up, while the ViDA trial had only 3.3 years of follow-up. The ViDA trial also used a heterogeneous definition of cardiovascular events, which included venous thromboembolism, pulmonary embolism, inflammatory cardiac conditions, arrhythmias, and conduction disorders.
Two trials, VITAL 35 and a post hoc analysis of the ViDA trial, 37 and the WHI nested case-control study 38 , 39 reported on the effect of vitamin D treatment on the incidence of cancer. Both trials reported no difference in cancer incidence between participants randomized to treatment and placebo among the subgroup of participants with serum 25(OH)D levels less than 20 ng/mL at baseline. The ViDA trial had only 3 years of follow-up, which may be a short period to detect an effect on cancer incidence. In the WHI Calcium–Vitamin D nested case-control study, the adjusted odds ratios (ORs) for incident breast or colorectal cancer over 7 years of follow-up did not demonstrate a statistically significant association between exposure to active treatment and incidence of cancer among participants with vitamin D deficiency at baseline. 38 , 39
Nine trials reported fall outcomes in community-dwelling adults. 26 , 27 Some trials reported only falls, others only the number of participants who experienced 1 or more falls (ie, “fallers”), and some trials reported both outcomes. A pooled analysis of 6 trials found no association between vitamin D treatment and number of fallers (RR, 0.90 [95% CI, 0.75-1.08]), while a pooled analysis of 5 trials found a significant association between vitamin D treatment and falls (incidence rate ratio, 0.76 [95% CI, 0.57-0.94]). 26 , 27 However, heterogeneity was high in both analyses, ascertainment methods for falls and fallers were variable across studies, and the variable reporting of falls, fallers, or both outcomes raises the possibility of selective outcome reporting. One trial reported on the incidence of 2 or more falls, a different definition of “fallers” than in the trials included in the pooled analysis above. It found no significant difference between participants randomized to vitamin D or placebo among the subgroup of participants with baseline vitamin D levels less than 12 ng/mL (adjusted OR, 1.03 [95% CI, 0.59-1.79]) or among those with levels between 12 and 20 ng/mL (adjusted OR, 1.13 [95% CI, 0.87-1.48]). 40
Three trials reported depression outcomes. One, VITAL-DEP (Depression Endpoint Prevention), was an ancillary study to the VITAL trial. Among the subgroup of participants with baseline serum vitamin D levels less than 20 ng/mL (n = 1328), there was no difference in the change in Personal Health Questionnaire Depression Scale scores between those randomized to vitamin D compared with placebo over a median follow-up of 5.3 years. 41 The other 2 trials were relatively small and of short duration. Both reported no significant difference in depression measures between vitamin D treatment and placebo. 42 , 43 Two trials reporting on physical functioning measures reported conflicting results. 44 , 45 An unplanned subgroup analysis of 1 trial conducted in persons with impaired fasting glucose found no difference in incidence of a first urinary tract infection in participants with vitamin D deficiency who were treated with vitamin D compared with placebo. 46
As noted, the studies comprising the body of evidence cited above did not uniformly define vitamin D deficiency. Different studies enrolled participants with vitamin D levels that ranged from less than 20 ng/mL to less than 31.2 ng/mL. For those outcomes with sufficient data (mortality, fractures, and falls), findings were similar between studies using a lower threshold and studies using a higher threshold. 26 , 27
The USPSTF found no studies that directly evaluated the harms of screening for vitamin D deficiency. The USPSTF found 36 studies that reported adverse events and harms from treatment with vitamin D (with or without calcium) compared with a control group. The absolute incidence of adverse events varied widely across studies; however, the incidence of total adverse events, such as gastrointestinal symptoms, fatigue, musculoskeletal symptoms, and headaches, and serious adverse events was generally similar between treatment and control groups. In the 10 trials that reported incidence of kidney stones, there was only 1 case. 26 , 27
A draft version of this recommendation statement was posted for public comment on the USPSTF website from September 22, 2020, to October 19, 2020. Some comments requested the USPSTF to evaluate the evidence on or make a recommendation regarding vitamin D supplementation. In response, the USPSTF wants to clarify that this recommendation focuses on screening for vitamin D deficiency. The USPSTF does have separate recommendations that address vitamin D supplementation (ie, providing vitamin D to all persons without testing, and regardless of vitamin D level) for a variety of conditions. 22 - 24 In response to comments, the USPSTF also wants to clarify that this recommendation applies to asymptomatic, community-dwelling adults. It does not apply to persons in institutional or hospital settings, who may have underlying or intercurrent conditions that warrant vitamin D testing or treatment. The USPSTF also wants to clarify that it did not review the emerging evidence on COVID-19, the disease caused by the new coronavirus SARS-CoV-2, and vitamin D.
More studies are needed that address the following areas:
More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex.
More research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.
When vitamin D deficiency is better defined, studies on the benefits and harms of screening for vitamin D deficiency will be helpful.
No organization recommends population-based screening for vitamin D deficiency, and the American Society for Clinical Pathology recommends against it. 47 The American Academy of Family Physicians supports the USPSTF 2014 recommendation, which states that there is insufficient evidence to recommend screening the general population for vitamin D deficiency. 48 The Endocrine Society 49 and the American Association of Clinical Endocrinologists 50 recommend screening for vitamin D deficiency in individuals at risk. The Endocrine Society does not recommend population screening for vitamin D deficiency in individuals not at risk. 49
Corresponding Author: Alex H. Krist, MD, MPH, Virginia Commonwealth University, 830 E Main St, One Capitol Square, Sixth Floor, Richmond, VA 23219 ( [email protected] ).
Accepted for Publication: February 22, 2021.
The US Preventive Services Task Force (USPSTF) members: Alex H. Krist, MD, MPH; Karina W. Davidson, PhD, MASc; Carol M. Mangione, MD, MSPH; Michael Cabana, MD, MA, MPH; Aaron B. Caughey, MD, PhD; Esa M. Davis, MD, MPH; Katrina E. Donahue, MD, MPH; Chyke A. Doubeni, MD, MPH; John W. Epling Jr, MD, MSEd; Martha Kubik, PhD, RN; Li Li, MD, PhD, MPH; Gbenga Ogedegbe, MD, MPH; Douglas K. Owens, MD, MS; Lori Pbert, PhD; Michael Silverstein, MD, MPH; James Stevermer, MD, MSPH; Chien-Wen Tseng, MD, MPH, MSEE; John B. Wong, MD.
Affiliations of The US Preventive Services Task Force (USPSTF) members: Fairfax Family Practice Residency, Fairfax, Virginia (Krist); Virginia Commonwealth University, Richmond (Krist); Feinstein Institute for Medical Research at Northwell Health, New York, New York (Davidson); University of California, Los Angeles (Mangione); Albert Einstein College of Medicine, New York, New York (Cabana); Oregon Health & Science University, Portland (Caughey); University of Pittsburgh, Pittsburgh, Pennsylvania (Davis); University of North Carolina at Chapel Hill (Donahue); Mayo Clinic, Rochester, Minnesota (Doubeni); Virginia Tech Carilion School of Medicine, Roanoke (Epling Jr); George Mason University, Fairfax, Virginia (Kubik); University of Virginia, Charlottesville (Li); New York University, New York, New York (Ogedegbe); Stanford University, Stanford, California (Owens); University of Massachusetts Medical School, Worcester (Pbert); Boston University, Boston, Massachusetts (Silverstein); University of Missouri, Columbia (Stevermer); University of Hawaii, Honolulu (Tseng); Pacific Health Research and Education Institute, Honolulu, Hawaii (Tseng); Tufts University School of Medicine, Boston, Massachusetts (Wong).
Author Contributions: Dr Krist had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The USPSTF members contributed equally to the recommendation statement.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Authors followed the policy regarding conflicts of interest described at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures . All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings.
Funding/Support: The USPSTF is an independent, voluntary body. The US Congress mandates that the Agency for Healthcare Research and Quality (AHRQ) support the operations of the USPSTF.
Role of the Funder/Sponsor: AHRQ staff assisted in the following: development and review of the research plan, commission of the systematic evidence review from an Evidence-based Practice Center, coordination of expert review and public comment of the draft evidence report and draft recommendation statement, and the writing and preparation of the final recommendation statement and its submission for publication. AHRQ staff had no role in the approval of the final recommendation statement or the decision to submit for publication.
Disclaimer: Recommendations made by the USPSTF are independent of the US government. They should not be construed as an official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: We thank Howard Tracer, MD (AHRQ), who contributed to the writing of the manuscript, and Lisa Nicolella, MA (AHRQ), who assisted with coordination and editing.
Additional Information: The US Preventive Services Task Force (USPSTF) makes recommendations about the effectiveness of specific preventive care services for patients without obvious related signs or symptoms. It bases its recommendations on the evidence of both the benefits and harms of the service and an assessment of the balance. The USPSTF does not consider the costs of providing a service in this assessment. The USPSTF recognizes that clinical decisions involve more considerations than evidence alone. Clinicians should understand the evidence but individualize decision-making to the specific patient or situation. Similarly, the USPSTF notes that policy and coverage decisions involve considerations in addition to the evidence of clinical benefits and harms.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Everything counts!

How do you read organization’s silence over rise of Nazism?

Got milk? Does it give you problems?
“This is the first direct evidence we have that daily supplementation may reduce AD incidence, and what looks like more pronounced effect after two years of supplementation for vitamin D,” said Karen Costenbader, senior author of the study.
Michele Blackwell/Unsplash
Vitamin D supplements lower risk of autoimmune disease, researchers say
Haley Bridger
BWH Communications
Study of older adults is ‘first direct evidence’ of protection against rheumatoid arthritis, psoriasis, other conditions
In a new study, investigators from Brigham and Women’s Hospital found the people who took vitamin D, or vitamin D and omega-3 fatty acids, had a significantly lower rate of autoimmune diseases — such as rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, and psoriasis — than people who took a placebo.
With their findings published Wednesday in BMJ , the team had tested this in the large-scale vitamin D and omega-3 trial (VITAL), a randomized study which followed participants for approximately five years. Investigators found the people who took vitamin D, or vitamin D and omega-3 fatty acids had a significantly lower rate of AD than people who took a placebo.
“It is exciting to have these new and positive results for nontoxic vitamins and supplements preventing potentially highly morbid diseases,” said senior author Karen Costenbader of the Brigham’s Division of Rheumatology, Inflammation and Immunity. “This is the first direct evidence we have that daily supplementation may reduce AD incidence, and what looks like more pronounced effect after two years of supplementation for vitamin D. We look forward to honing and expanding our findings and encourage professional societies to consider these results and emerging data when developing future guidelines for the prevention of autoimmune diseases in midlife and older adults.”
“Now, when my patients, colleagues, or friends ask me which vitamins or supplements I’d recommend they take to reduce risk of autoimmune disease, I have new evidence-based recommendations for women age 55 years and older and men 50 years and older,” said Costenbader. “I suggest vitamin D 2000 IU a day and marine omega-3 fatty acids (fish oil), 1000 mg a day — the doses used in VITAL.”
VITAL is a randomized, double-blind, placebo-controlled research study of 25,871 men (age 50 and older) and women (age 55 and older) across the U.S., conducted to investigate whether taking daily dietary supplements of vitamin D3 (2000 IU) or omega-3 fatty acids (Omacor fish oil, 1 gram) could reduce the risk for developing cancer, heart disease, and stroke in people who do not have a prior history of these illnesses. Participants were randomized to receive either vitamin D with an omega-3 fatty acid supplement; vitamin D with a placebo; omega-3 fatty acid with a placebo; or placebo only. Prior to the launch of VITAL, investigators determined that they would also look at rates of AD among participants, as part of an ancillary study.
“Given the benefits of vitamin D and omega-3s for reducing inflammation, we were particularly interested in whether they could protect against autoimmune diseases,” said JoAnn Manson, co-author and director of the parent VITAL trial at the Brigham.
Participants answered questionnaires about new diagnoses of diseases, including rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, psoriasis, and inflammatory bowel disease, with space to write in all other new onset ADs. Trained physicians reviewed patients’ medical records to confirm reported diagnoses.
“Autoimmune diseases are common in older adults and negatively affect health and life expectancy. Until now, we have had no proven way of preventing them, and now, for the first time, we do,” said first author, Jill Hahn, a postdoctoral fellow at the Brigham. “It would be exciting if we could go on to verify the same preventive effects in younger individuals.”
Among patients who were randomized to receive vitamin D, 123 participants in the treatment group and 155 in the placebo group were diagnosed with confirmed AD (22 percent reduction). Among those in the fatty acid arm, confirmed AD occurred in 130 participants in the treatment group and 148 in the placebo group. Supplementation with omega-3 fatty acids alone did not significantly lower incidence of AD, but the study did find evidence of an increased effect after longer duration of supplementation.
The VITAL study included a large and diverse sample of participants, but all participants were older and results may not be generalizable to younger individuals who experience AD earlier in life. The trial also only tested one dose and one formulation of each supplement. The researchers note that longer follow-up may be more informative to assess whether the effects are long-lasting.
This study was funded by the National Institutes of Health grants R01 AR059086, U01 CA138962, R01 CA138962.
Share this article
You might like.
New study finds step-count and time are equally valid in reducing health risks

Medical historians look to cultural context, work of peer publications in wrestling with case of New England Journal of Medicine

Biomolecular archaeologist looks at why most of world’s population has trouble digesting beverage that helped shape civilization
Glimpse of next-generation internet
Physicists demo first metro-area quantum computer network in Boston
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 20, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Vitamin D deficiency tied to worse outcomes with early kidney disease
by Lori Solomon
Vitamin D deficiency is associated with increased risks for cardiovascular mortality and chronic kidney disease (CKD) progression in patients with early-stage disease, according to a study published online May 11 in the Journal of Endocrinological Investigation .
Yanhong Lin, from Southern Medical University in Guangzhou, China, and colleagues examined the effects of 25-hydroxyvitamin D (25[OH]D) deficiency on cardiovascular mortality and kidney outcomes in patients with early-stage CKD. The analysis included 9,229 adult patients with CKD (stages 1 to 3) from 19 medical centers across China (January 2000 to May 2021).
The researchers found that compared with patients having 25(OH)D ≥20 ng/mL, a there was a significantly higher risk for cardiovascular mortality (hazard ratio, 1.90) and CKD progression (hazard ratio, 2.20) as well as a steeper annual decline in estimated glomerular filtration rate (estimate, −7.87 percent per year) in those with serum 25(OH)D <10 ng/mL.
"In conclusion, 25(OH)D deficiency was common in patients with early-stage CKD," the authors write. "Vitamin D status should be closely monitored in patients with early CKD. Well-designed randomized clinical trials are needed to determine whether timely vitamin D supplementation can prevent cardiovascular events and loss of kidney function in patients with early-stage CKD and 25(OH)D deficiency."
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Study: Newborns whose mother spoke in a mix of languages during pregnancy are more sensitive to a range of sound pitches
3 hours ago
Study finds ultraviolet radiation may affect subcutaneous fat regulation, could lead to new obesity treatments

Regular fish oil supplement use might increase first-time heart disease and stroke risk
9 hours ago

Pedestrians may be twice as likely to be hit by electric/hybrid cars as petrol/diesel ones
Study finds jaboticaba peel reduces inflammation and controls blood sugar in people with metabolic syndrome
10 hours ago

Study reveals how extremely rare immune cells predict how well treatments work for recurrent hives
12 hours ago

Researchers find connection between PFAS exposure in men and the health of their offspring

Researchers develop new tool for better classification of inherited disease-causing variants

Specialized weight navigation program shows higher use of evidence-based treatments, more weight lost than usual care

Exercise bouts could improve efficacy of cancer drug
Related stories.

High systemic immune-inflammation index tied to higher mortality with peritoneal dialysis
Sep 22, 2023

Incident CVD, mortality risk heightened for at least one year after COVID-19
Feb 21, 2023

Peritonitis tied to cardiovascular mortality in peritoneal dialysis population
Dec 27, 2022
Tirzepatide improves kidney outcomes in T2DM with increased CV risk
Jun 6, 2022

Remote patient monitoring tied to better dialysis technique survival
Feb 23, 2024

Benefit of ICD attenuated in CKD patients receiving cardiac resynchronization
Sep 25, 2023
Recommended for you
Drug-like inhibitor shows promise in preventing flu

Hope for a cure for visceral leishmaniasis, an often fatal infectious disease
16 hours ago
Matcha mouthwash shown to inhibit bacteria that cause periodontitis
18 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 20 August 2019
Interventions and public health nutrition
The economic case for prevention of population vitamin D deficiency: a modelling study using data from England and Wales
- M. Aguiar 1 , 2 ,
- L. Andronis 1 , 3 ,
- M. Pallan 1 ,
- W. Högler 4 , 5 &
- E. Frew ORCID: orcid.org/0000-0002-5462-1158 1
European Journal of Clinical Nutrition volume 74 , pages 825–833 ( 2020 ) Cite this article
1829 Accesses
28 Citations
819 Altmetric
Metrics details
- Endocrinology
Vitamin D deficiency (VDD) affects the health and wellbeing of millions worldwide. In high latitude countries such as the United Kingdom (UK), severe complications disproportionally affect ethnic minority groups.
To develop a decision-analytic model to estimate the cost effectiveness of population strategies to prevent VDD.
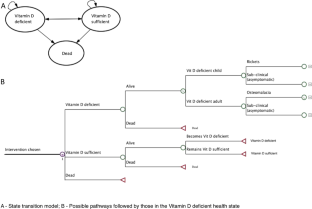
An individual-level simulation model was used to compare: (I) wheat flour fortification; (II) supplementation of at-risk groups; and (III) combined flour fortification and supplementation; with (IV) a ‘no additional intervention’ scenario, reflecting the current Vitamin D policy in the UK. We simulated the whole population over 90 years. Data from national nutrition surveys were used to estimate the risk of deficiency under the alternative scenarios. Costs incurred by the health care sector, the government, local authorities, and the general public were considered. Results were expressed as total cost and effect of each strategy, and as the cost per ‘prevented case of VDD’ and the ‘cost per Quality Adjusted Life Year (QALY)’.
Wheat flour fortification was cost saving as its costs were more than offset by the cost savings from preventing VDD. The combination of supplementation and fortification was cost effective (£9.5 per QALY gained). The model estimated that wheat flour fortification alone would result in 25% fewer cases of VDD, while the combined strategy would reduce the number of cases by a further 8%.
There is a strong economic case for fortifying wheat flour with Vitamin D, alone or in combination with targeted vitamin D3 supplementation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Global Perspective of the Vitamin D Status of African-Caribbean Populations: A Systematic Review and Meta-analysis

Official recommendations for vitamin D through the life stages in developed countries
Vitamin d deficiency 2.0: an update on the current status worldwide.
Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739s–48s. 2005/10/28
Article CAS Google Scholar
Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, et al. National diet and nutrition survey results from years 5 and 6 (combined) of the rolling programme (2012/2013–2013/2014). Public Health Engand and the Food Standards Agency; 2016.
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394–415. 2016/01/09
Uday S, Fratzl-Zelman N, Roschger P, Klaushofer K, Chikermane A, Saraff V, et al. Cardiac, bone and growth plate manifestations in hypocalcemic infants: revealing the hidden body of the vitamin D deficiency iceberg. BMC Pediatr. 2018;18:183.
Article Google Scholar
Maiya S, Sullivan I, Allgrove J, Yates R, Malone M, Brain C, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. 2008;94:581–4.
Patel JV, Chackathayil J, Hughes EA, Webster C, Lip GY, Gill PS. Vitamin D deficiency amongst minority ethnic groups in the UK: a cross sectional study. Int J Cardiol. 2013;167:2172–6. 2012/11/13
Uday S, Högler W. Prevention of rickets and osteomalacia in the UK: political action overdue. Arch Dis Child. 2018;103:901–906.
Brown LL, Cohen B, Tabor D, Zappalà G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health—expert panel meeting report. BMC Proc. 2018;12:6 https://doi.org/10.1186/s12919-018-0102-4.
Article PubMed PubMed Central Google Scholar
Vatanparast H, Nisbet C, Gushulak B. Vitamin D insufficiency and bone mineral status in a population of newcomer children in Canada. Nutrients. 2013;5:1561–72. https://www.ncbi.nlm.nih.gov/pubmed/23673607 .
Bärebring L, Schoenmakers I, Glantz A, Hulthén L, Jagner Å, Ellis J, et al. Vitamin D status during pregnancy in a multi-ethnic population-representative Swedish cohort. Nutrients. 2016;8:655.
Ramnemark A, Norberg M, Pettersson-Kymmer U, Eliasson M. Adequate vitamin D levels in a Swedish population living above latitude 63 N: the 2009 Northern Sweden MONICA study. Int J Circumpolar Health. 2015;74:27963.
Andersson Å, Björk A, Kristiansson P, Johansson G. Vitamin D intake and status in immigrant and native Swedish women: a study at a primary health care centre located at 60 N in Sweden. Food Nutr Res. 2013;57:20089.
Glerup H, Rytter L, Mortensen L, Nathan E. Vitamin D deficiency among immigrant children in Denmark. Eur J Pediatr. 2004;163:272–3.
O’Callaghan KM, Kiely ME. Ethnic disparities in the dietary requirement for vitamin D during pregnancy: considerations for nutrition policy and research. Proc Nutr Soc. 2018;77:164–73.
Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull. 2014;39:322–50. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4288313/ .
(SACN) SAC on N. Vitamin D and Health. Public Health England; 2016. https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition .
Uday S, Kongjonaj A, Aguiar M, Tulchinsky T, Högler W. Variations in infant and childhood vitamin D supplementation programmes across Europe and factors influencing adherence. Endocr Connect. 2017;6:667–75. https://www.ncbi.nlm.nih.gov/pubmed/28924002 .
Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, et al. National diet and nutrition survey results from years 1, 2, 3 and 4 (combined) of the rolling programme (2008/2009 –2011/2012). Public Health Engand and Food Standards Agency; 2014.
Basatemur E, Sutcliffe A. Incidence of hypocalcemic seizures due to vitamin D deficiency in children in the United Kingdom and Ireland. J Clin Endocrinol Metab. 2014;100:E91–5.
Julies P, Lynn RM, Pall K, Leoni M, Calder A, Mughal Z, et al. I16 Nutritional rickets presenting to secondary care in children (16 years)—a UK surveillance study. Arch Dis Child. 2018;103(Suppl 1):A202 LP–A203. http://adc.bmj.com/content/103/Suppl_1/A202.3.abstract .
Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–44. 2016/02/13
Darling AL, Hart KH, Macdonald HM, Horton K, Kang’Ombe AR, Berry JL, et al. Vitamin D deficiency in UK South Asian Women of childbearing age: a comparative longitudinal investigation with UK Caucasian women. Osteoporos Int. 2013;24:477–88.
Martin CA, Gowda U, Renzaho AMN. The prevalence of vitamin D deficiency among dark-skinned populations according to their stage of migration and region of birth: a meta-analysis. Nutrition. 2016;32:21–32.
van der Meer IM, Middelkoop BJC, Boeke AJP, Lips P. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: an overview. Osteoporos Int. 2011;22:1009–21. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3046351/ .
Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–32. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3447083/ .
Aguiar M, Andronis L, Pallan M, Högler W, Frew E. Preventing vitamin D deficiency (VDD): a systematic review of economic evaluations. Eur J Public Health. 2017;27:292–301. 2017/02/17
Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy. 2004;9:110–8.
Davis S, Stevenson M, Tappenden P, Wailoo AJ. NICE DSU Technical Support Document 15: Cost-effectiveness modelling using patient-level simulation. School of Health and Related Research (ScHARR), The University of Sheffield. 2014.
(NICE) NI for H and CE. Developing NICE guidelines: the manual [Internet]. 3rd ed. Process and methods [PMG20]. 2014. https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation .
Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Value Health. 2012;15:812–20.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11:6.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Statistics O for N. Census. UK: Data Services Census Support.
Allen RE, Dangour AD, Tedstone AE, Chalabi Z. Does fortification of staple foods improve vitamin D intakes and status of groups at risk of deficiency? A United Kingdom modeling study. Am J Clin Nutr. 2015;102:338–44.
Research NS, Laboratory MRCEW, London UC, School M. National Diet and Nutrition Survey Years 1–6, 2008/09-2013/14 [Internet]. UK Data Services; 2017. https://doi.org/10.5255/UKDA-SN-6533-7 .
Allen RE. Would fortification of more foods with vitamin D improve vitamin D intakes and status of groups at risk of deficiency in the UK? London School of Hygiene & Tropical Medicine; 2013.
Limited T FreeD: Vitamin D supplementation in Lewisham; 2014. http://www.therapyaudit.com/media/2015/05/lewisham-casestudy.pdf .
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD007146.pub3 .
Article PubMed Google Scholar
Aguiar M. Decision analytic modelling of the prevention of vitamin D deficiency in England and Wales. Birmingham, UK: University of Birmingham; 2018. http://etheses.bham.ac.uk/8120/ .
Food Standards Agency (FSA). Improving folate intakes of women of reproductive age and preventing neural tube defects: practical issues [Internet]. 2007. http://tna.europarchive.org/20120419000433/http://www.food.gov.uk/multimedia/pdfs/fsa070604.pdf .
NABIM. Statistics [Internet]; 2014. http://www.nabim.org.uk/statistics .
Roy S, Sherman A, Monari-Sparks MJ, Schweiker O, Hunter K. Correction of low vitamin d improves fatigue: effect of correction of low vitamin D in fatigue study (EViDiF Study). North Am J Med Sci. 2014;6:396–402. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4158648/ .
Santos AS, Guerra-Junior AA, Godman B, Morton A, Ruas CM. Cost-effectiveness thresholds: methods for setting and examples from around the world. Exp Rev Pharm Outcomes Res. 2018;18:277–88.
Google Scholar
Jentink J, van de Vrie-Hoekstra NW, de Jong-van den Berg LT, Postma MJ. Economic evaluation of folic acid food fortification in The Netherlands. Eur J Public Heal. 2008;18:270–4.
Bentley TGK, Weinstein MC, Willett WC, Kuntz KM. A cost-effectiveness analysis of folic acid fortification policy in the United States. Public Health Nutr. 2009;12:455–67.
Grosse SD, Berry RJ, Tilford JM, Kucik JE, Waitzman NJ. Retrospective assessment of cost savings from prevention. Am J Prev Med. 2016;50:S74–80.
Basatemur E, Hunter R, Horsfall L, Sutcliffe A, Rait G. Costs of vitamin D testing and prescribing among children in primary care. Eur J Pediatr. 2017;176:1405–9. http://www.ncbi.nlm.nih.gov/pubmed/28803270 .
Poole CD, Smith J, Davies JS. Cost-effectiveness and budget impact of Empirical vitamin D therapy on unintentional falls in older adults in the UK. BMJ Open. 2015;5:e007910.
Del Valle HB, Yaktine AL, Taylor CL, Ross AC. Dietary reference intakes for calcium and vitamin D. Washington (DC), US: National Academies Press; 2011.
Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:810–9. https://academic.oup.com/ajcn/article/105/4/810-819/4569717 .
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. https://doi.org/10.1136/bmj.i6583 . Accessed 18 Apr 2019.
(IOM) Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC), US: National Academies Press; 2011.
Lindsay A, Benoist B, Dary O, Hurrell R, WHO/FAO. Guidelines on food fortification with micronutrients. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations; 2006.
WHO/FAO. Evaluating the public health significance of micronutrient malnutrition. In: Guidelines on food fortification with micronutrients. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations; 2006. p. 39–92.
Urrutia-Pereira M, Solé D. Vitamin D deficiency in pregnancy and its impact on the fetus, the newborn and in childhood. Rev Paul Pediatr. 2015;33:104–13.
Holick MF. The influence of vitamin D on bone health across the life cycle. J Nutr. 2005;135:2726S–2727S. https://academic.oup.com/jn/article/135/11/2726S/4669897 .
Darnton-Hill I, Webb P, Harvey PWJ, Hunt JM, Dalmiya N, Chopra M, et al. Micronutrient deficiencies and gender: social and economic costs. Am J Clin Nutr. 2005;81:1198S–1205S. https://doi.org/10.1093/ajcn/81.5.1198 .
Article CAS PubMed Google Scholar
Bivins R. “The English Disease” or “Asian Rickets”? Medical responses to postcolonial immigration. Bull Hist Med. 2007;81:533.
Horton S. The economics of food fortification. J Nutr. 2006;136:1068–71. https://doi.org/10.1093/jn/136.4.1068 .
Filby A, Wood H, Jenks M, Taylor M, Burley V, Barbier M, et al. Examining the cost-effectiveness of moving the healthy start vitamin programme from a targeted to a universal offering: cost-effectiveness systematic review [Internet]. NICE, editor. Vol. July; 2015. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-guidelines/healthy-start-cost-effectiveness-review.pdf .
Download references
Acknowledgements
Dr. Sue Horton, School of Public Health and Health Systems, University of Waterloo, for initial advice on the economics of food fortification. Dr. Helena Pachón, Rollins School of Public Health, Emory University, for the insights on the practicalities of wheat flour fortification. The team at the Center for Health Economics Research and Evaluation, University Technology Sydney, particularly Dr. Phillip Haywood, as well as Dr. Kim Dalziel, Center for Health Policy, University of Melbourne, for the methodological advice. Smita Hanciles and Gwenda Scott from Lewisham Local Authority, UK, as well as Eleanor McGee from Birmingham Local Authority, UK, for the insights on supplementation alternatives and data access.
This research was funded by the College of Medical and Dental Sciences of the University of Birmingham, through an internal PhD studentship grant.
Author information
Authors and affiliations.
Institute of Applied Health Research, University of Birmingham, Birmingham, B15 2TT, UK
M. Aguiar, L. Andronis, M. Pallan & E. Frew
Collaboration for Outcomes Research and Evaluation, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, V6T 1Z3, Canada
Population, Evidence and Technologies, Division of Health Sciences, University of Warwick, Coventry, CV4 7AL, UK
L. Andronis
Department of Paediatrics and Adolescent Medicine, Faculty of Medicine, Johannes Kepler University, Linz, A-4040, Austria
Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, B15 2TT, UK
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to designing the research. MA conducted the research and analysed data supervised by LA, MP, WH and EF. All authors contributed substantially to writing the paper, while MA and EF had primary responsibility for final content. All authors have read and approved the final paper.
Corresponding author
Correspondence to E. Frew .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.

Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary material., rights and permissions.
Reprints and permissions
About this article
Cite this article.
Aguiar, M., Andronis, L., Pallan, M. et al. The economic case for prevention of population vitamin D deficiency: a modelling study using data from England and Wales. Eur J Clin Nutr 74 , 825–833 (2020). https://doi.org/10.1038/s41430-019-0486-x
Download citation
Received : 08 March 2019
Revised : 01 July 2019
Accepted : 15 July 2019
Published : 20 August 2019
Issue Date : May 2020
DOI : https://doi.org/10.1038/s41430-019-0486-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The evident and the hidden factors of vitamin d status in older people during covid-19 pandemic.
- Paula Schmidt Azevedo
- Ricardo Ambrosio Fock
- Sergio Rupp de Paiva
Nutrire (2021)
Prevalence of vitamin D deficiency and its associated risk factors among rural population of the northern part of the Persian Gulf
- Maryam Marzban
- Mohammadreza Kalantarhormozi
- Iraj Nabipour
BMC Endocrine Disorders (2021)
Serum 25-hydroxyvitamin D is negatively associated with severe periodontitis: a cross-sectional study
- Fangjing Zhou
BMC Oral Health (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Association of low vitamin D level and full-term early-onset neonatal sepsis; a case-control study
- Open access
- Published: 18 May 2024
- Volume 50 , article number 101 , ( 2024 )
Cite this article
You have full access to this open access article

- Shereen A. Mohamed ORCID: orcid.org/0000-0001-5943-9083 1 ,
- Nermin R. Kamel 1 ,
- Aya E. Fouda 1 ,
- Rabab E. Elhawary 2 &
- Mohamed A. Abdelmegeid 1
115 Accesses
1 Altmetric
Explore all metrics
Sepsis is one of the main causes of death in newborns worldwide. Vitamin D levels during fetal and neonatal periods have a significant role in the development of the immunological system. The study aims to evaluate the association between vitamin D levels and the risk of early-onset neonatal sepsis in full-term neonates in a developing country.
This case–control study was conducted at the Neonatal Intensive Care Units (NICUs) of Kasr Alainy Hospital, Cairo, Egypt. The study was composed of two groups; the sepsis group involved full-term neonates appropriate for gestational age with sepsis-related clinical signs. The control group included newborns with no signs of clinical/laboratory infection within 72 h of life. Blood samples were collected on admission during the first three days of life in both groups for the measurement of 25-hydroxyvitamin D levels, Complete Blood Count (CBC), C reactive protein (CRP), and blood culture.
Forty-five newborns with clinical and laboratory findings of early-onset neonatal sepsis within 72 h of life were enrolled, and the control group included forty-five newborns with no evidence of sepsis. Vitamin D levels in the sepsis group were significantly lower than in the control group. Apgar score at the first minute was significantly lower in the sepsis group. 57.8% of neonates with sepsis had positive blood cultures. There was a statistical difference between deficient, insufficient, and sufficient vitamin D levels regarding the duration of the NICU stay, which was longer in neonates with deficient vitamin D levels. CRP was significantly higher in neonates with deficient vitamin D levels. The area under the receiver operating characteristic curve for serum vitamin D in the prediction of neonatal sepsis was 0.76 at a cutoff < 19.7(ng/ml).
In the current study, full-term newborns with EOS had considerably lower vitamin D levels than healthy controls. Through appropriate vitamin supplementation of the mothers during pregnancy, it could be possible to ensure adequate vitamin D levels for newborns. This may contribute to the reduction of the risk of EOS, together with the other well-known preventive measures (i.e. breastfeeding and intrapartum antibiotic prophylaxis).
Similar content being viewed by others

Association of Early-Onset Sepsis and Vitamin D Deficiency in Term Neonates
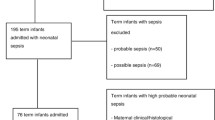
Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants
Is Lower Vitamin D Level Associated with Increased Risk of Neonatal Sepsis? A Prospective Cohort Study
Neonatal sepsis is a clinical term describing non-specific symptoms and signs of infection that may be proven with a positive culture [ 1 ]. The early-onset sepsis (EOS) occurs in the first three days of life [ 1 ]. Low and middle-income countries have high mortality rates among neonates with sepsis [ 2 ]. Also in high-income countries infections and sepsis represent a relevant cause of mortality and morbidity, especially in those peripheral and rural areas with low-density offers of second-level perinatal care [ 3 ].
Immature immunological systems predispose newborn infants to sepsis. The hypothesis relating vitamin D deficiency with the raised incidence of infections was assumed for over a century. Its levels during the prenatal and neonatal periods have been associated with the development of the immune system. Vitamin D levels may have an impact on the production of immunoglobulins, T cell lymphocyte activation, killer and T helper cell differentiation, neutrophil migration, and the production of antimicrobial peptides. Moreover, cord-blood 25-hydroxyvitamin D low levels have been related to the risk of early-onset neonatal sepsis [ 4 , 5 , 6 ].
Several studies were carried out to determine the relationship between low vitamin D concentrations and the likelihood of early-onset neonatal sepsis and sepsis indicators [ 5 , 7 , 8 , 9 , 10 , 11 ].
This study aims to analyze the association between different vitamin D levels and increased risk of early-onset neonatal sepsis in full-term neonates appropriate for gestational age (AGA) in Egypt.
This case–control study was conducted at Neonatal Intensive Care Units (NICUs) of Kasr ElAiny Hospital, Cairo University. All participants were enrolled within one year.
The study included full-term AGA neonates. The enrolled population was divided into two groups; the sepsis group included full-term AGA neonates with sepsis-related clinical signs; temperature instability, tachycardia or bradycardia, hypotension, need for supplemented oxygen, apnea, need for mechanical ventilation, abdominal distension, feeding intolerance, necrotizing enterocolitis, serum parameters other than C reactive protein (CRP): white blood cell count, absolute neutrophil count, platelets count (Table 1 shows the clinical and laboratory criteria for EOS) [ 12 ]. The control group included newborns showing no signs of infection or laboratory markers of infection within 72 h of birth.
Exclusion criteria included neonates admitted after 72 h of life, newborns with major congenital anomalies for possible renal and hepatic involvement that might influence vitamin D levels, and genetic diseases especially those affecting the immunological system [ 13 ]. Maternal and perinatal factors (urine tract infection, chorioamnionitis, premature rupture of membrane, meconium-stained amniotic fluid, prolonged labor, and neonates with small gestational age) were also excluded for increased risks of adverse effects, including neonatal sepsis [ 14 ].
For all participants, careful history was recorded including perinatal data (gestational age, mode of delivery, resuscitation maneuvers, and Apgar score), and postnatal ones (activity, suckling, and NICU admission).
A thorough clinical examination was necessary to find the clinical signs of sepsis, which included agitation, lethargy, hypotonia, pallor or mottled skin, hypothermia or hyperthermia, apnea or tachypnea, tachycardia or bradycardia, poor oral intake, jaundice, and intestinal obstruction [ 15 ]. Newborns were followed during their NICU stay period.
The following laboratory investigations were performed: CRP, blood culture, and complete blood count (CBC). Newborns fulfilling the inclusion criteria and admitted during their first three postnatal days of life in both groups at admission underwent blood sample collection and a level of 25_hydroxyvitamin D (25-OHD) test. After centrifugation, the serum was separated, and it was kept at -20 °C until testing. ELISA kit ( DiaMetra DKO146) was utilized according to the manufacturer. The vitamin D concentration in the sample was calculated through a calibration curve.
Vitamin D level was considered sufficient if it was more than 30 ng/ml, insufficient level laid between 20 to 30 ng/ml, and deficient level if less than 20 ng/ml.
Statistical analysis
The IBM SPSS (Statistical Package for Social Science) version 25 for Windows was used to code, compute, and analyze the data. Frequency tables (numbers and percentages) were used to display descriptive statistics for qualitative data. The Shapiro–Wilk test was used to determine the normality of the data for the quantitative variables, and the results were displayed as standard deviations (SD). The Median (minimum–maximum) was used for variables with non-normal distributions.
Chi-square test was used in analytical statistics to assess the relationship between categorical variables. If the Excel cell count in four-cell tables was less than 5, the Fisher Exact Test has been used instead. The Monte Carlo test was used if the expected cell count in more than four-cell tables was less than 5.
An independent sample t-test was used in two independent groups to investigate the association between normally distributed continuous variables. Two independent continuous variables that were not normally distributed were compared using the Mann–Whitney U test (z). Non-normally distributed data were correlated using the Spearman correlation method.
To ascertain whether there were any statistically significant differences between the means of two or more independent (unrelated) groups, the one-way analysis of variance (ANOVA) was utilized. Additionally, the Kruskal–Wallis H Test was employed to compare continuous variables with non-normal distribution across many groups.
To compute the sensitivity and specificity rates and, consequently, the positive and negative probability ratios of such variables, receiver operating characteristic (ROC) curves were used to evaluate the overall prediction accuracy of test parameters, as demonstrated by the area under the curve (AUC).
The prediction of sepsis' independent factors was carried out using binary stepwise logistic regression analysis. The forward Wald approach was used to add significant predictors from the univariate analysis to the regression model.
Generated odds ratios with a 95% confidence interval were adjusted odds ratios (AOR).
Results were deemed significant for all the statistical tests when the chance of error was less than or equal to 5% ( p ≤ 0.05).
This case–control study was conducted at Neonatal Intensive Care Units (NICUs) of Kasr Alainy Hospital of Cairo University, Egypt. Ninety newborns were enrolled and divided into two groups. The sepsis group included forty-five newborns with clinical and laboratory findings of EOS within 72 h of life. The control group included forty-five newborns with no signs of clinical-laboratory signs of infection within the same time window.
There was no statistically significant difference between the two groups regarding gestational age, gender, consanguinity, mode of delivery, Apgar score at 5 min, temperature, and hemoglobin level. However, there was a significant difference between the two groups regarding Apgar score at the first minute, total leucocytic count (significantly higher within the sepsis group), and platelet count (significantly lower within the newborns with sepsis) . Jaundice was the most prevalent clinical manifestation in the control group (88.9%), whereas respiratory distress predominated in the sepsis group (64.4%). CRP was increased within the sepsis group. Serum vitamin D levels were significantly lower in the cases with sepsis compared to the control ones ( p < 0.001) (Table 2 ).
Twenty-six (57.8%) neonates with sepsis had positive blood cultures, and 19(42.2%) had no growth results (Table 3 ).
Serum vitamin D level was statistically lower in septic neonates with respiratory distress. Newborns with poor feeding, respiratory difficulties, lethargy, cardiovascular symptoms of sepsis ( tachycardia or bradycardia & hypotension), and mottling had significantly lower vitamin D levels than those without ( p < 0.05). Even neonates with positive blood cultures had significantly lower vitamin D levels than newborns with negative blood cultures ( p = 0.001) (Table 4 ).
There was a significant difference between deficient vitamin D status (vit D level < 20 ng/ml.), insufficient status (vit D laid between 20—30 ng/ml.), and sufficient vitamin D status (vit D > 30 ng/ml.) groups regarding duration of NICU stay: it was longer in neonates with deficient vitamin D status (12 days vs 6.5 days in neonates with sufficient vitamin D status). Also, CRP was significantly higher in newborns with deficient vitamin D status, with a median value of 42 g/dl compared to 12 g/dl in newborns with sufficient vitamin D status (Table 5 ).
The AUC for serum vitamin D in the prediction of neonatal sepsis was 0.76 at a cutoff < 19.7(ng/ml). (Table 6 and Fig. 1 ).
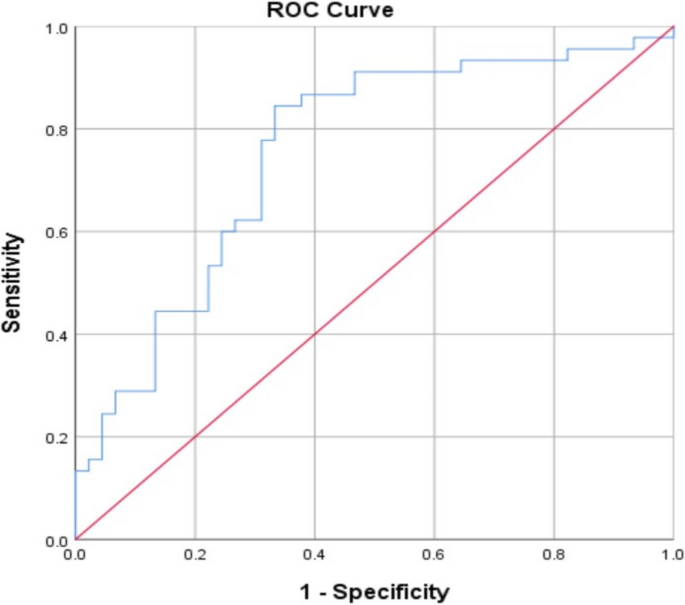
Receiver operating characteristic (ROC) curve for prediction of neonatal sepsis using serum vitamin D levels in the studied groups
The independent predictors of sepsis among the studied cases were serum Vitamin D level (AOR = 2.8), vitamin D status (Deficient < 20 ng/ml) (AOR = 4.5), presence of respiratory difficulties (AOR = 2.1), and mottling (AOR = 1.9). The overall percent prediction of sepsis by them combined was 75.6% (Table 7 ).
Vitamin D is essential to the optimum function of the immune system, especially the innate immune system which performs efficiently by stimulating the production of antimicrobial peptides in epithelial cells, neutrophils, and macrophages. Hence, vitamin D insufficiency may be considered a substantial risk factor for acquiring infections [ 16 ].
This study aimed to correlate the different levels of serum vitamin D with the development of EOS in full-term AGA newborns. In our research, there was no significant difference in the mode of delivery or sex between the cases and controls. This was in line with the findings of Kumar et al. [ 17 ]. The current study found newborns with sepsis having a statistically significant decrease in first-minute Apgar scores than controls. Similarly, other studies found significant associations between lower Apgar scores at the first and fifth minutes and the development of neonatal sepsis [ 18 , 19 , 20 ]. The innate immune system which is the first line of defense is immature at birth. In addition, the adaptive immune system is still not able to efficiently respond to T-cell-dependent antigens. In addition, the WHO classified low Apgar scores as an indicator of perinatal asphyxia . Thus, newborns with low Apgar scores may be at greater risk of infection [ 21 , 22 , 23 , 24 ].
Chen et al. reported that vitamin D administration during pregnancy led to a considerable decline in the amount of circulating maternal serum CRP,. This fact reveals the association between vitamin D deficiency and inflammation, infection, and septic shock [ 25 ]. The current study identified an association between low vitamin D levels and high neonatal serum CRP, in line with previous studies that found an inverse relationship between CRP levels in septic neonates and blood vitamin D levels [ 17 , 26 , 27 ]. While, Grzanka et al., concluded that there was no significant relationship between vitamin D concentrations and CRP and attributed this finding to the small sample size and single evaluation of vitamin D concentration [ 28 ].
In the current research, serum vitamin D levels in the sepsis group were considerably lower than in the control one. This result was consistent with other researches which concluded that full-term newborns with low serum vitamin D levels were more prone to the risk of infection [ 5 , 17 , 29 , 30 ]. According to Soliman et al., a multivariate logistic regression analysis used to predict neonatal sepsis showed that a decline in serum vitamin D levels was substantially related to the risk of sepsis in newborns [ 26 ]. Another study explained this association by the disruption of the macrophage function and the inflammatory cytokines generation that can result from a vitamin D deficit [ 7 ] .
We observed in the EOS newborns different statuses of vitamin D: 66% were in the vitamin D deficient range, 11% in the insufficient one, and 22.2% in the sufficient subgroup. Vitamin D insufficiency elevated the risk of EOS by 1.44 times, while vitamin D deficiency elevated the risk of EOS to 53.44 times. As well, Kumar et al. reported the mean serum vitamin D levels were considerably lower in neonates with EOS compared to healthy controls. Out of 100 neonates with EOS, 77.0% had vitamin D deficiency, 23.0% insufficiency, and none had appropriate levels, while in the one hundred healthy controls vitamin D levels were adequate in 31%, insufficient in 28%, and deficient in 41.0% [ 17 ].
The link between serum vitamin D levels and EOS in newborns without maternal risk factors has been furthermore investigated by Sarwade et al. They concluded that in preterm and full-term ill neonates, without maternal risk factors, severe vitamin D deficiency of less than 10 ng/ml was considerably correlated with culture-positive EOS in neonates in the first three days of life [ 31 ].
In this study, there was an inverse correlation between positive blood culture results and vitamin D levels. This was in agreement with Moromizato et al., who observed a higher rate of infection and culture positivity among vitamin D-deficient patients [ 32 ]. Also, mean vitamin D levels were lower among neonates who suffered septic shock confirmed by culture than those with negative cultures [ 16 ].
The results of blood cultures in this study reported that 57.8% of those within the sepsis group had positive blood cultures. These results are higher than those reported by Betty and Inderpreet who found that culture-proven sepsis occurred in only 21% of cases with sepsis [ 33 ]. The sensitivity of blood cultures in neonatal sepsis is low and depends on the timing and number of cultures taken, blood volume, technique, temperature, culture medium, and organism density. Furthermore, the implementation of peripartum maternal antimicrobial practice makes the diagnostic value of neonatal blood cultures unreliable [ 1 ]. In the current study, out of 26 patients with positive blood cultures, 65.4% and 26.9% had coagulase-negative staphylococci (CoNS) and klebsiella respectively. Lee et al. also reported that Gram-positive organisms were the most predominant organisms of EOS in Korea. CoNS was noted as the highest, followed by Staphylococcus aureus [ 34 ].
An inverse correlation between serum vitamin D levels and the duration of NICU stay was reported in the current study. In contrast, Saadat et al. found no correlation between vitamin D levels and the length of hospital stay [ 35 ].
This study concluded that a serum vitamin D level of 19.7 ng/mL had an AUC of 0.76 for the prediction of EOS with 84.4% sensitivity and 66.7% specificity. The cutoff value of vitamin D as a measure of the risk of developing neonatal sepsis was reported by Behera et al. to be 15.48 ng/ml, with 80% sensitivity and 99.9% specificity [ 36 ]. Additionally, Soliman et al. observed that vitamin D, at a cutoff point of 18.75 ng/ml, had a 100% sensitivity, and 80% specificity for the diagnosis of newborn sepsis [ 26 ]. Another case–control study which recruited sixty-two infants with EOS and the same number as a control group, revealed that vitamin D levels were considerably lower in EOS with a cutoff value of 25 ng/ml, 88.7% sensitivity, and 79% specificity [ 4 ].
In this study, the independent predictors of sepsis among the studied group were serum vitamin D level (AOR = 2.8), vitamin D deficient category (< 20 ng/ml) (AOR = 4.5), presence of respiratory difficulties (AOR = 2.1), and mottling (AOR = 1.9). The overall value of predicted sepsis by these factors combined was 75.6%. Also, it concluded that vitamin D deficiencies and insufficiencies considerably raise the risk of EOS.
Finally, there are few limitations to this study, the first of which is the absence of measurements of maternal serum vitamin D levels. Second, the sample size is small. Lastly, the lack of serum vitamin D level measurement after vitamin D administration to the septic neonates and assessing its correlation with clinical and laboratory parameters of neonatal sepsis hindered revealing its effect on immune modulation.
According to this study, full-term newborns with EOS had considerably lower vitamin D levels than healthy controls. However, this cannot be considered a diagnostic parameter (the diagnosis is still challenging for neonatologists) but a valid support to identify the subjects at major risk. Furthermore, vitamin D maternal supplementation, along with effective preventive measures (ie breastfeeding, intrapartum antibiotic prophylaxis for group B-streptococcal infection, etc.) may lower the risk of neonatal infections and improve morbidity and mortality rates [ 6 , 7 , 37 ].
Availability of data and materials
Data is available upon request.
Abbreviations
Adjusted odds ratios
Area under the curve
Complete blood count
Early-onset sepsis
Neonatal Intensive Care Units
Receiver operating characteristic
Standard deviations
Celik IH, Hanna M, Canpolat FE, Pammi M. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. 2022;91(2):337–50.
Article PubMed Google Scholar
Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Heal. 2022;10(5):e661–72.
Article CAS Google Scholar
Serra G, Miceli V, Albano S, Corsello G. Perinatal and newborn care in a two years retrospective study in a first level peripheral hospital in Sicily (Italy). Ital J Pediatr. 2019;45(1):1–8.
Article Google Scholar
Jeengar B, Gothwal S, Meena KK, Garg VK, Athwani V, Gupta ML, et al. Vitamin D levels and early onset sepsis in newborns. J Neonatol. 2021;35(2):64–9.
Cizmeci MN, Kanburoglu MK, Akelma AZ, Ayyildiz A, Kutukoglu I, Malli DD, et al. Cord-blood 25-hydroxyvitamin D levels and risk of early-onset neonatal sepsis: a case–control study from a tertiary care center in Turkey. Eur J Pediatr. 2015;174(6):809–15.
Article CAS PubMed Google Scholar
Myint AA. Serum vitamin D levels in term neonates with early onset sepsis. Pediatr Neonatal Biol Open Access. 2018;3(3):124.
Cetinkaya M, Cekmez F, Buyukkale G, Erener-Ercan T, Demir F, Tunc T, et al. Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants. J Perinatol. 2015;35(1):39–45.
Seliem MS, Abdel Haie OM, Mansour AI, Salama SSME. The relation between vitamin D level and increased risk for early-onset neonatal sepsis in full-term infants. Med Res J. 2016;15(1):16–21.
Tayel SI, Soliman SE, Elsayed HM. Vitamin D deficiency and vitamin D receptor variants in mothers and their neonates are risk factors for neonatal sepsis. Steroids. 2018;134:37–42.
Lee SY, Kim HE, An SH. The association between vitamin D levels and neonatal early-onset sepsis: a systematic review and meta-analysis. Korean J Clin Pharm. 2018;28(1):10–6.
Gamal TS, Madiha AAS, Hanan MK, Abdel-Azeem MEM, Marian GS. Neonatal and maternal 25-oh vitamin d serum levels in neonates with early-onset sepsis. Children. 2017;4(5):37.
Article PubMed PubMed Central Google Scholar
Gitto E, Karbownik M, Reiter RJ, Xian Tan D, Cuzzocrea S, Chiurazzi P, et al. Effects of melatonin treatment in septic newborns. Pediatr Res. 2001;50(6):756–60.
Serra G, Memo L, Antona V, Corsello G, Favero V, Lago P, et al. Jacobsen syndrome and neonatal bleeding: report on two unrelated patients. Ital J Pediatr. 2021;47(1):4–11.
Piro E, Serra G, Schierz IAM, Giuffrè M, Corsello G. Fetal growth restriction: A growth pattern with fetal, neonatal and long-term consequences. EuroMediterranean Biomed J. 2019;14(9):38–44.
Google Scholar
Odabasi IO, Bulbul A. Review neonatal sepsis. Sisli Etfal Hast Tip Bul. 2020;54(2):142–58.
Gupta DVK, Dhaneria DM. Study of serum vitamin d levels and its association with neonatal sepsis among newborns. Pediatr Rev Int J Pediatr Res. 2020;7(6):242–7.
Kumar A, Narang GS, Singh G, Virk N. Association of vitamin D deficiency with early onset sepsis in term neonates. Int J Contemp Pediatr. 2019;6(2):440.
Adatara P, Afaya A, Salia SM, Afaya RA, Konlan KD, Agyabeng-Fandoh E, et al. Risk factors associated with neonatal sepsis: a case study at a specialist Hospital in Ghana. Sci World J. 2019;2019:9369051.
Yismaw AE, Abebil TY, Biweta MA, Araya BM. Proportion of neonatal sepsis and determinant factors among neonates admitted in University of Gondar comprehensive specialized hospital neonatal Intensive care unit Northwest Ethiopia 2017. BMC Res Notes. 2019;12(1):1–5.
Rafi MA, Miah MMZ, Wadood MA, Hossain MG. Risk factors and etiology of neonatal sepsis after hospital delivery: A case-control study in a tertiary care hospital of Rajshahi, Bangladesh. PLoS One. 2020;15:e0242275.
Article CAS PubMed PubMed Central Google Scholar
Mamo SA, Teshome GS, Tesfaye T, Goshu AT. Perinatal asphyxia and associated factors among neonates admitted to a specialized public hospital in South Central Ethiopia: A retrospective cross-sectional study. PLoS One. 2022;17:1–14. https://doi.org/10.1371/journal.pone.0262619 .
Gutbir Y, Wainstock T, Sheiner E, Segal I, Sergienko R, Landau D, et al. Low Apgar score in term newborns and long-term infectious morbidity: a population-based cohort study with up to 18 years of follow-up. Eur J Pediatr. 2020;179(6):959–71.
Mitra DK, Mullany LC, Harrison M, Mannan I, Shah R, Begum N, et al. Incidence and risk factors of neonatal infections in a rural Bangladeshi population: a community-based prospective study. J Heal Popul Nutr. 2018;37(1):1–11.
Mustefa A, Abera A, Aseffa A, Abathun T, Degefa N, Tadesse H, et al. Prevalence of neonatal sepsis and associated factors amongst neonates admitted in arbaminch general hospital, arbaminch, southern Ethiopia, 2019. J Pediatr Neonatal Care. 2020;10(1):1–7.
Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients. 2014;6:2206–16.
Soliman Y, Sakr M, Emran T, El Samanoudy M. Impact of serum level of vitamin D on term neonates with early onset sepsis. Int J Med Arts. 2019;0(0):0–0.
Tao RX, Zhou QF, Xu ZW, Hao JH, Huang K, Mou Z, et al. Inverse correlation between vitamin D and C-reactive protein in newborns. Nutrients. 2015;7(11):9218–28.
Grzanka A, Machura E, Mazur B, Misiolek M, Jochem J, Kasperski J, et al. Relationship between vitamin D status and the inflammatory state in patients with chronic spontaneous urticaria. J Inflamm (Lond). 2014;11(1):2.
Yang LR, Li H, Yang TY, Zhang T, Zhao RC. Relationship between Vitamin D deficiency and early-onset neonatal sepsis. Chinese J Contemp Pediatr. 2016;18(9):791–5.
Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15(1):84.
Sarwade A, Gosai M, Gohil J. Vitamin D levels in early onset neonatal sepsis without maternal risk factors: a case-control study. Vitam Miner. 2019;8(2):1–5.
Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107.
Chacko B, Sohi I. Early onset neonatal sepsis. Indian J Pediatr. 2005;72(1):23–6.
Lee SM, Chang M, Kim KS. Blood culture proven early onset sepsis and late onset sepsis in very-low-birth-weight infants in Korea. J Korean Med Sci. 2015;30 Suppl 1(Suppl 1):S67-74.
Saadat H, Mehrvari T, Goodarzi R, Kheiry F. The comparison of serum vitamin D level in the term neonates with and without sepsis in children hospital of Bandar Abbas city, Iran from 2016 to 2017. Hormozgan Med J. 2021;25(1):14–8.
Behera CK, Sahoo JP, Patra SD, Jena PK. Is lower vitamin D level associated with increased risk of neonatal sepsis? A prospective cohort study. Indian J Pediatr. 2020;87(6):427–32.
Aly H, Abdel-Hady H. Vitamin D and the neonate: an update. J Clin Neonatol. 2015;4(1):1.
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors funded this work.
Author information
Authors and affiliations.
Pediatrics Department, Kasr Alainy Faculty of Medicine, Cairo University, Cairo, Egypt
Shereen A. Mohamed, Nermin R. Kamel, Aya E. Fouda & Mohamed A. Abdelmegeid
Clinical Pathology Department, Kasr Alainy Faculty of Medicine, Cairo University, Cairo, Egypt
Rabab E. Elhawary
You can also search for this author in PubMed Google Scholar
Contributions
N.K., S.M. & R.E. conceived the study and conducted its design. S.M. & A.F. coordinated the implementation of the study. R. E was responsible for the laboratory investigations. A.F. helped to perform the statistical analysis, collected the data, and was responsible for the interpretation of laboratory data of patients. S.M. drafted the manuscript. R.E. and M. A. revised the design and shared in manuscript writing. The final manuscript was read and approved by all authors.
Corresponding author
Correspondence to Shereen A. Mohamed .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the Ethical Scientific Committee of the Faculty of Medicine, Cairo University (code: MS-319–2020) and per the Declaration of Helsinki for medical research involving human subjects. Informed consent was obtained from patients’ legal guardians before enrolling patients in the study.
Consent for publication
Competing interests.
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Mohamed, S.A., Kamel, N.R., Fouda, A.E. et al. Association of low vitamin D level and full-term early-onset neonatal sepsis; a case-control study. Ital J Pediatr 50 , 101 (2024). https://doi.org/10.1186/s13052-024-01665-2
Download citation
Received : 15 November 2023
Accepted : 28 April 2024
Published : 18 May 2024
DOI : https://doi.org/10.1186/s13052-024-01665-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vitamin D deficiency
- Neonatal Sepsis
- Find a journal
- Publish with us
- Track your research
Additive Interaction Between Insulin Resistance, Chronic Low-Grade Inflammation and Vitamin D Deficiency on the Risk of Type 2 Diabetes Mellitus: A Cohort Study
Affiliation.
- 1 Faculty of Medicine, University Blida 1, Algeria.
- PMID: 38739850
- DOI: 10.1080/27697061.2024.2352401
Objectives: The aim of this study was to explore, on an additive scale, the combined effect of the association between insulin resistance (IR), chronic low-grade inflammation (CLGI) and vitamin D deficiency (VDD) on the risk of type 2 Diabetes Mellitus (T2DM).
Methods: This is a cohort study, including 1484 non-diabetic subjects, followed for a period of four years. 25 hydroxy-vitamin D (25OHD), hypersensitive C-reactive protein (HsCRP) and triglyceride-glucose index were assessed. Based on VDD and CLGI, the population was subdivided into 4 exposure groups. Analysis was performed both in the case of IR and without IR. Cox proportional regression and additive interaction were applied to explore cumulative effects of exposure.
Results: At follow-up, 162 newly diagnosed cases of T2DM were identified. TYG index (RR = 4.0[2.8-5.6]), HsCRP (RR = 1.6 [1.4-1.7]) and 25OHD (RR = 0.96 [0.39-0.98]) were all significantly associated with the risk of T2DM ( p < 0.01). The highest excess risk was recorded in patients cumulating simultaneously IR, CLGI and VDD (RR= 8.4[3.6-19.8], p < 0.0001). The additive interaction was significant, the excess risk linked to the interaction RERI = 10.5[1.43-19.7], the proportion attributable to the combined effect: AP = 0.61[0.37-0.85], and the interaction was synergistic: synergy index: 2.8[1.42-5.69].
Conclusion: Baseline levels of TYG index, 25OHD and HsCRP are strongly predictive of future T2DM, and their joint effects are additive and synergistic. Interventional studies are therefore warranted in order to evaluate whether vitamin D supplementation, combined with appropriate anti-inflammatory therapies, is effective as a preventive strategy to reduce the risk of T2DM.
Keywords: 25OHD; HsCRP; Insulin-resistance; TYG Index; Type 2 Diabetes; additive interaction.
Disclaimer » Advertising
- HealthyChildren.org
Study Design
Participants, rickets screening procedures, laboratory methods and radiographic interpretations, case definition, statistical analysis, maternal and infant characteristics, effect of maternal vitamin d supplementation on biochemical rickets, subgroup analyses, infant bone biomarkers, radiographically confirmed rickets, conclusions, acknowledgments, maternal vitamin d supplementation and infantile rickets: secondary analysis of a randomized trial.
FUNDING: This work was supported in part by the Bill & Melinda Gates Foundation (OPP1066764). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Dr Lautatzis received salary support from the Canadian Pediatric Endocrine Group Fellowship Program and CIHR Canada Graduate Scholarship. The funding agencies were not involved in the design, implementation, analysis, or interpretation of the data.
CONFLICT OF INTEREST DISCLOSURES: There are no conflicts of interest to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Maria-Elena Lautatzis , Farhana K. Keya , Abdullah Al Mahmud , Ulaina Tariq , Carol Lam , Shaun K. Morris , Jennifer Stimec , Stanley Zlotkin , Tahmeed Ahmed , Jennifer Harrington , Daniel E. Roth; Maternal Vitamin D Supplementation and Infantile Rickets: Secondary Analysis of a Randomized Trial. Pediatrics 2024; e2023063263. 10.1542/peds.2023-063263
Download citation file:
- Ris (Zotero)
- Reference Manager
The role of maternal vitamin D supplementation in the prevention of infantile rickets is unknown, particularly in low- and middle-income countries without routine infant vitamin D supplementation. Through secondary analysis of a randomized, placebo-controlled trial in Bangladesh, we examined the dose-ranging effects of maternal vitamin D supplementation on the risk of biochemical rickets at 6 to 12 months of age.
Pregnant women ( n = 1300) were randomized into 5 groups: placebo, or vitamin D 4200 IU/week, 16 800 IU/week, or 28 000 IU/week from second trimester to delivery and placebo until 6 months postpartum; or 28 000 IU/week prenatally and until 6 months postpartum. Infants underwent biochemical rickets screening from 6 to 12 months of age ( n = 790). Relative risks (RR) and 95% confidence intervals (95% CI) of biochemical rickets were estimated for each group versus placebo.
Overall, 39/790 (4.9%) infants had biochemical rickets. Prevalence was highest in the placebo group (7.8%), and the risk was significantly lower among infants whose mothers received combined prenatal and postpartum vitamin D at 28 000 IU/week (1.3%; RR, 0.16; 95% CI, 0.03–0.72). Risks among infants whose mothers received only prenatal supplementation (4200 IU, 16 800 IU, 28 000 IU weekly) were not significantly different from placebo: 3.8% (RR, 0.48; 95% CI, 0.19–1.22), 5.8% (RR, 0.74; 95% CI, 0.33–1.69), and 5.7% (RR, 0.73; 95% CI, 0.32–1.65), respectively.
Maternal vitamin D supplementation (28 000 IU/week) during the third trimester of pregnancy until 6 months postpartum reduced the risk of infantile biochemical rickets. Further research is needed to define optimal postpartum supplementation dosing during lactation.
Maternal vitamin D supplementation during pregnancy and lactation modifies infant vitamin D status, but its effects on the risk of infantile rickets have not previously been established.
High-dose maternal vitamin D supplementation during the third trimester of pregnancy and up to 6-months postpartum reduced the risk of infantile rickets in Bangladesh. Maternal postpartum vitamin D supplementation may be an alternative to direct infant supplementation for rickets prevention.
Nutritional rickets is one of the most common causes of pediatric bone disease globally. 1 Biochemical abnormalities are detectable at an early stage of rickets across all age groups and have an important role in screening and diagnosis. 2 , – 6 Young infants with rickets often have a more subtle bony phenotype compared with older children given their lack of substantial weight bearing and may remain undiagnosed until later stages of the disease. However, the high metabolic demand for calcium resulting from rapid growth in infancy can lead to acute presentations of rickets with hypocalcemia before the emergence of other clinical or radiologic signs. 7 , – 9 Compared with older children, there may be substantial morbidity associated with infantile rickets given sequelae such as hypocalcemic seizures and, in rare cases, cardiomyopathy. 10 , – 12
Vitamin D deficiency is the predominant cause of nutritional rickets worldwide, particularly in infants. Maternal prenatal vitamin D status is the primary determinant of newborn vitamin D status. 13 , – 17 The major circulating metabolite of vitamin D, 25-hydroxyvitamin D (25(OH)D), crosses the placenta such that cord blood concentrations are highly correlated with maternal values at term. 18 However, the influence of maternal prenatal vitamin D status on infant vitamin D stores diminishes by 2 months of age and infants become dependent on other vitamin D sources. 19 In the Maternal Vitamin D for Infant Growth (MDIG) trial, 20 there was a dose-response effect of prenatal vitamin D supplementation on cord blood and infant vitamin D blood concentrations up to 3 months of age, as has been observed in other prenatal vitamin D supplementation trials. 21 , 22 Therefore, although deficiency in the early postnatal period may be caused primarily by maternal prenatal vitamin D deficiency, 23 vitamin D deficiency later in infancy is attributable to other risk factors. Because breast milk is a poor source of vitamin D if a lactating mother has inadequate vitamin D intake/status, prolonged breast feeding without vitamin D supplementation is an important cause of vitamin D deficiency in infants. However, adequate maternal intake of vitamin D during lactation can support vitamin D sufficiency in the breastfed infant. 24 For example, the MDIG trial demonstrated that continued maternal postpartum supplementation (28 000 IU/week) maintained infant 25(OH)D concentrations at or above 30 nmol/L up to 6 months of age. 20
The role of vitamin D in fetal calcium homeostasis is uncertain; whereas animal studies suggest transplacental transfer may be independent of prenatal maternal vitamin D status, some human studies have provided evidence that maternal prenatal vitamin D status affects fetal calcium accrual. 25 Immediately after delivery, vitamin D is required as an essential regulator of infant intestinal calcium absorption and bone mineral metabolism, similar to older children. 26 Therefore, it is plausible that maternal vitamin D supplementation in the prenatal and postpartum period would reduce the risk of infantile rickets by supporting fetal calcium accrual, neonatal vitamin D endowment, and infant vitamin D intake via breastmilk.
Although there is limited evidence establishing the effect of postpartum vitamin D supplementation in breastfeeding women on the risk of infantile rickets, 27 , 28 there have not been published trials examining prenatal supplementation alone or in combination with postpartum supplementation. Such evidence would be particularly relevant to many low- and middle-income countries such as Bangladesh, where there is a high burden of vitamin D deficiency among both women of child-bearing age and newborns and vitamin D supplementation in infants is not a routine practice. 17 , 29 , – 31 In this substudy of a randomized controlled trial, we aimed to estimate the effect of a range of doses of maternal vitamin D supplementation during pregnancy and continued supplementation during lactation, compared with placebo, on the risk of infantile biochemical rickets at 6 to 12 months of age in Dhaka, Bangladesh.
This study was based on secondary analyses of data from the MDIG trial, conducted in Dhaka, Bangladesh, from 2014 to 2018. This was a randomized double-blinded, placebo-controlled, dose-ranging trial of maternal vitamin D supplementation (from mid-gestation up to 6 months postpartum) for which the primary outcome was infant growth. 20 , 32 Briefly, 1300 generally healthy females 18 years of age or older were enrolled in the second trimester of pregnancy and randomized into 1 of 5 intervention groups: (1) placebo in prenatal and postpartum; (2) prenatal vitamin D3 (4200 IU/week) and placebo postpartum; (3) prenatal vitamin D3 (16 800 IU/week) and placebo postpartum; (4) prenatal vitamin D3 (28 000 IU/week) and placebo postpartum; or (5) vitamin D3 (28 000 IU/week) prenatal and to 6 months postpartum. Supplementation was administered weekly under direct supervision by trained study personnel either in the participant’s home or in the clinic. Participants in all groups were provided daily calcium (500 mg) and iron–folic acid supplements. Ethics approval for secondary use of the trial data for this sub-study was provided by the Research Ethics Board at the Hospital for Sick Children in Canada (REB #1000061259).
Individuals were excluded from the MDIG if there was history of medical conditions with altered vitamin D metabolism and/or hypercalcemia, were having a high-risk pregnancy, were unwilling to stop taking nonstudy vitamin D or calcium supplements or multivitamins containing calcium and/or vitamin D, or were currently being prescribed vitamin D supplements as part of a physician’s treatment plan for vitamin D deficiency. Infants in the MDIG cohort were eligible for biochemical screening at or after 6 months of age; those included in this substudy had at least 1 measurement of serum alkaline phosphatase (ALP) between 6 and 12 months of age ( Supplemental Fig 2 ). Infants with known disorders that affect calcium homeostasis or known skeletal dysplasia would have been excluded from the study, yet no such cases were identified.
Infants in the MDIG were born between June 2014 and February 2016. Systematic screening for rickets at 6-month follow-up visits was launched in May 2016. The biochemical screening panel included serum concentrations of ALP, calcium, and phosphate. Any of the initial parameters found to be outside of established reference ranges prompted a physician referral for assessment and treatment, facilitation of radiographs of wrists and/or knees and an extended laboratory panel (including parathyroid hormone [PTH] and 25(OH)D) that were managed according to the treating physician.
Infant serum calcium, phosphate, and ALP concentrations were measured using quantitative colorimetric assays (Beckman Coulter OSR60117, OSR6122, and OSR6104) at the Clinical Biochemistry Laboratory in Dhaka (icddr,b). Serum 25(OH)D concentrations were measured at the Analytical Facility for Bioactive Molecules (AFBM) in Toronto using high-performance liquid chromatography-tandem mass spectrometry, as previously described. 33 Infant intact PTH concentrations were quantified using a sandwich enzyme-linked immunosorbent assay kit (Immunotopic 60-3100) at AFBM. Clinical management by physicians in Dhaka was informed by local radiologist interpretations of wrist and/or knee radiographs, where available. However, if possible, wrist and/or knee radiographs obtained from children who screened positive for biochemical rickets were further reviewed using a standardized approach by a pediatric radiologist who was blinded to the clinical and laboratory data, as previously described. 20
Biochemical rickets is marked by an elevated ALP level, which is indicative of increased bone turnover; this is a nearly universal feature of rickets and usually the earliest biochemical abnormality. 34 A common compensatory response to hypocalcemia is an elevation in PTH, which promotes the mobilization of calcium from bones. The development of hypocalcemia and hypophosphatemia may occur as the disease progresses or in the presence of an inadequate PTH response. 35 , 36 However, there are no standardized cutoff points for these biochemical markers that define onset or stages of progression of rickets. Age-specific reference ranges must be used for these biochemical markers; ALP in particular is highly dependent on age and rate of bone growth. Here, we defined “biochemical rickets” as (1) ALP ≥ 450 U/L or (2) ALP ≥ 350 U/L plus at least 1 of the following: calcium ≤ 2.2 mmol/L or phosphate ≤ 1.6 mmol/L or PTH ≥ 6.9 pmol/L. The cutoffs for this definition were consensus-based among investigators. This definition used for analytical purposes differed slightly from the definition used to prompt clinical referral during the MDIG study because PTH was not available in real time as part of the initial screening panel.
Left skewing of ALP was noted with a higher-than-expected proportion of low values; of 790 infants in this substudy, 132 (17%) had ALP <90 U/L. These low values were distributed throughout the study period. Following an extensive review, no preanalytical factors were identified that might have artifactually lowered ALP. The distribution of other biochemical markers analyzed in the same samples were similarly distributed in the low ALP and non–low ALP groups (data not shown), ruling out overdilution as an explanation. Hypercalcemia was not observed in the infants with low ALP, making hereditary hypophosphatasia less likely. Malnutrition is known to decrease ALP production, 37 although we did not find differences in anthropometric parameters (weight for age z -score and height for age z -score at 6 months of age) between the low ALP and non–low ALP groups (data not shown). A set of serum samples ( n = 244) from infants in the MDIG across a wider age range than included in this study was tested at the AFBM laboratory at The Hospital for Sick Children using a different colorimetric assay (Alkaline Phosphatase Colorimetric Assay Kit; ab83369); 8.2% (20/244) were found to have ALP <90 IU/L compared with a frequency of 12% among all samples tested at the Clinical Biochemistry Laboratory (135/1085), suggesting that the high proportion of low values in this cohort was a reproducible finding.
Participant characteristics and biomarker concentrations were expressed as mean ± SD, median (25th and 75th percentiles), or frequencies and percentages. PTH was log-transformed because of right-skewing. Participant demographics across the 5 maternal vitamin D treatment arms were compared using analysis of variance for normally distributed continuous variables, Kruskal-Wallis for nonnormally distributed continuous variables, and χ-squared tests for categorical variables. To estimate the relative risk (RR) of infantile rickets in each prenatal and postnatal maternal vitamin D supplementation group, versus placebo, we used a modified Poisson regression with robust error variance. 38 Planned subgroup analyses included unadjusted regression models stratified by child sex, maternal vitamin D status at randomization (25(OH)D ≥30 nmol/L vs <30 nmol/L), and gestational age (term ≥ 37 weeks), respectively. All point estimates were presented with 95% confidence intervals (95% CI) and P values (α < 0.05 considered statistically significant). Data were analyzed using Stata version 16.1 (StataCorp 2019).
Characteristics of participants included in this substudy were similar across the 5 intervention groups ( Table 1 ), as previously reported for the MDIG trial. 20
Demographics and Characteristics of Participants, Stratified by Vitamin D Treatment Group
LAZ, length for age z-score; WAZ, weight for age z-score
Maternal prenatal vitamin D supplementation (second trimester to delivery); postnatal maternal supplementation (0–6 mo).
p value for Kruskal Wallis, Pearson χ 2 , or analysis of variance test.
Based on Intergrowth-21 growth standards, by gestational age, within first 48 h of life, n = 566.
Based on Intergrowth-21st growth standards, by gestational age, within first 48 h of life, n = 550.
Ever consumed a vitamin/supplement containing or possibly containing vitamin D from birth to 1 y.
Number of weeks a supplement containing or possibly containing vitamin D was consumed among infants with at least 1 wk of reported consumption from birth to 6 mo of age, median (interquartile range).
A total of 39 cases of biochemical rickets were identified among 790 infants who underwent biochemical screening. Of these 39 cases, 10 met the criteria based on ALP ≥450 U/L alone, 12 had ALP ≥350 U/L and phosphate ≤1.6 mmol/L as the only abnormalities, 14 had ALP ≥350 U/L and intact PTH ≥6.9 pmol/L as the only abnormalities, and 3 had more than 2 abnormalities.
The highest prevalence of rickets (7.9%) was found in the placebo group ( Table 2 ). The lowest prevalence (1.3%) was in the high-dose supplementation group in which mothers received 28 000 IU prenatally and up to 6 months postpartum; this corresponded to a significantly reduced risk of infantile biochemical rickets compared with placebo ( Table 2 ). High-dose vitamin D during the prenatal period alone (4200 IU/week, 16 800 IU/week, and 28 000 IU/week) did not have a significant effect on the risk of rickets, although there were fewer rickets cases identified in each of these groups compared with placebo ( Table 2 ).
RR of Rickets in Each Treatment Arm Compared With Placebo
RR, relative risk.
Poisson regression model with robust error variance used to obtain RR.
In an analysis restricted to infants born to women with baseline 25(OH)D <30 nmol/L during the second trimester of pregnancy ( n = 507), inferences were unchanged ( Fig 1 ). Inferences also remained the same in stratified analysis by sex (males or females), albeit more male than female infants were affected by rickets overall. Inferences remained the same when analysis was restricted to infants born at term ( ≥ 37 weeks’ gestation) ( Supplemental Tables 3 – 5 ).

The relative risk of biochemical rickets among varying doses of maternal prenatal and postpartum vitamin D supplementation compared with placebo using modified Poisson regression (blue bars). Subgroup analysis assessing the effect of maternal vitamin D supplementation on infantile rickets among women with vitamin D deficiency (25(OH)D <30 nmol) at baseline ( n = 507). The circles represent the effect estimates, with 95% confidence interval (CI) bars.
Serum calcium concentrations were highest in the combined supplementation group and lowest in the placebo group; however, these differences were not statistically significant ( Supplemental Fig 4 ). Phosphate concentrations were significantly higher and ALP concentrations were significantly lower in the combined supplementation group compared with placebo ( Supplemental Fig 4 ).
Of the 39 infants with biochemical rickets, 16 had radiographs of the wrist and/or knee available for review by the SickKids radiologist, of whom 4 were found to have radiographic findings of rickets, as previously reported. 20 Three of the 4 infants were in the placebo group, and the fourth was in the group administered 4200 IU/week prenatally. Mean ALP was higher at presentation for these infants, at 705 U/L, compared with mean 439 U/L for the other infants with biochemical rickets. All 4 infants were hypophosphatemic (serum phosphate <1.56 mmol/), and 1 was hypocalcemic (serum calcium <2.1 mmol/L). Radiographs were not available for all infants with biochemical rickets. In large part, this was because infants who met criteria of ALP ≥ 350 U/L and PTH ≥ 6.9 pmol/L were not flagged for imaging because PTH was not available in real time as part of the initial screening panels.
Combined prenatal and postpartum maternal supplementation (28 000; 28 000 IU/week) decreased the risk of biochemical rickets compared with placebo among infants 6 to 12 months of age. However, maternal prenatal supplementation alone at any dose, without postpartum continuation, did not significantly decrease the risk of biochemical rickets. Prenatal maternal vitamin D supplementation influences early postnatal infant 25(OH)D, but postpartum continuation was required to maintain 25(OH)D ≥30 nmol/L up to 6 months of age, as previously reported in the MDIG trial ( Supplemental Fig 3 ). 20 Therefore, the present findings strongly support the hypothesis that vitamin D deficiency (marked by inadequate circulating 25(OH)D), is an important cause of biochemical rickets in this infant population. As previously reported, all the cases of radiographically confirmed rickets were in the placebo and lowest-dose prenatal supplementation (4200 IU weekly prenatally) groups, further supporting the potential role of vitamin D in rickets prevention. However, we cannot rule out other causes of rickets in this setting; moreover, most infants with 25(OH)D <30 nmol/L did not have biochemical rickets, indicating that other contributing factors act in concert with vitamin D deficiency.
There were relatively more male infants affected by biochemical rickets in our study. It has been speculated that rickets may occur more frequently in boys because of greater linear bone growth and increased skeletal demands during times of rapid growth. Although not seen consistently, this phenomenon has been noted in several studies evaluating rickets in infancy. 39 , – 41 The present findings are consistent with evidence from 2 smaller randomized trials in India that previously found that there were fewer cases of biochemical rickets among infants of mothers who received postpartum supplementation. 27 , 28 Although it has been well established that infant 25(OH)D status can be influenced by maternal supplementation during lactation, the dose-response relationship remains uncertain. 24 , 42 Human milk is considered a poor source of vitamin D3 unless the lactating woman has high amount of vitamin D intake. 43 The transfer of the vitamin D parent compound (vitamin D3) is favored over 25(OH)D in the mammary gland, suggesting that the vitamin D concentration of breast milk is primarily affected by maternal vitamin D intake or cutaneous synthesis rather than maternal vitamin D status (ie, circulating 25(OH)D). 44 , 45 This distinction is important because the short half-life of vitamin D3 (12–24 hours) implies that an analogous dose of vitamin D is consumed by the infant soon after the corresponding maternal ingestion. 46 However, low daily doses of maternal vitamin D supplementation may not achieve sufficiently high circulating levels of vitamin D in breast milk to impact infant 25(OH)D, even if they prevent maternal vitamin D deficiency. 47 High-dose maternal supplementation, often greater than the Institute of Medicine–recommended upper limit of 4000 IU/day, 48 has been previously shown to have similar effects on breastfeeding infant 25(OH)D as daily infant vitamin D supplementation. 42 , 49 , 50 Further research involving direct comparison of various doses, including daily maternal dosing compared with intermittent weekly or bolus dosing regimens, is required to determine the minimum effective maternal postpartum dose to maintain 25(OH)D sufficiency in infants and in turn minimize the risk of rickets.
A strength of this study is that the randomized, dose-ranging, placebo-controlled design of the MDIG trial and the lack of routine infant supplementation permitted causal inferences regarding the effects of maternal vitamin D supplementation on the risk of biochemical rickets. However, several limitations of the study should be acknowledged. This is a substudy of a previous trial; the mother and infant pairs included were selected from the existing MDIG cohort based on data availability, which may have compromised the generalizability of the findings. Although the participants in this substudy were similar to the remainder of the MDIG cohort, it is possible that this cohort was not fully representative of the mothers and infants in the MDIG trial or of the general population in Dhaka. The biochemical case definition was useful for identifying early disease because infants with rickets may present without skeletal abnormalities; however, we lacked complete radiographic information for all the infants who met biochemical rickets criteria, and the longer term clinical significance of infantile biochemical rickets is uncertain. Because the diagnosis of biochemical rickets was based on cross-sectional biochemical evaluation starting at 6 months of age, we were unable to determine the precise age of onset of the abnormalities. Furthermore, a greater number of infants screened late in infancy or at older ages might have enabled us to describe the natural history of this process in the absence of routine supplementation or vitamin D treatment of those who screened positive in early infancy.
High-dose maternal postpartum vitamin D supplementation may serve as a viable public health strategy for rickets prevention by effectively increasing infant 25(OH)D status in conjunction with efforts to promote breastfeeding. Other low- and middle-income countries in South Asia that have similar burdens of maternal and infant vitamin D deficiency and do not have vitamin D supplementation programs could benefit from this strategy. Future studies should include comparisons of different doses of maternal postpartum supplementation and longer term follow-up including radiologic assessments and clinical outcomes.
We thank Huma Qamar of The Global Centre for Child Health, The Hospital for Sick Children, for her assistance with data organization and Talia Wolfe, former summer student at The Global Centre for Child Health, The Hospital for Sick Children, for her work on the initial data analysis.
Dr Roth is the principal investigator, conceptualized, designed, and supervised the study, drafted the initial manuscript, and critically reviewed and revised the manuscript; Dr Lautatzis designed the study, performed statistical analysis, drafted the initial manuscript, and critically reviewed and revised the manuscript; Dr Al Mahmud supervised data collection and field study activities in Dhaka and critically reviewed and revised the manuscript; Drs Ahmed and Keya contributed to local implementation of the study and data collection in Dhaka, and critically reviewed and revised the manuscript; Ms Tariq contributed to study design, performed statistical analysis, drafted the initial manuscript, and critically reviewed and revised the manuscript; Dr Harrington, Dr Zlotkin, Dr Lam, and Dr Morris contributed to study design, and critically reviewed and revised the manuscript; Dr Stimec provided expert review of radiographic data and critically reviewed and revised the manuscript; and all authors read and approved the final manuscript and agree to be accountable for all aspects of the work. The authors report no conflicts of interest or financial relationships relevant to this article to disclose.
Clinical Trial Registration: This trial has been registered at www.clinicaltrials.gov (identifier NCT01924013).
25-hydroxyvitamin D
95% confidence interval
Analytical Facility for Bioactive Molecules
alkaline phosphatase
Maternal Vitamin D for Infant Growth
parathyroid hormone
relative risk
Competing Interests

Supplementary data
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ann Gen Psychiatry

Vitamin D deficiency mediates the relationship between dietary patterns and depression: a case–control study
Gity sotoudeh.
1 Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
Firoozeh Raisi
2 Department of Psychiatry, Roozbeh Hospital and Psychiatry and Psychology Research Centre, Tehran University of Medical Sciences, Tehran, Iran
Maryam Amini
3 Department of Nutrition Research, Faculty of Nutrition Sciences and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Reza Majdzadeh
4 Knowledge Utilization Research Center and Community Based Participatory Research Center, Tehran University of Medical Sciences, Tehran, Iran
Mahdieh Hosseinzadeh
5 Department of Nutrition, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Fatemeh Khorram Rouz
6 Student Research Committee, Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Maryam Khosravi
7 Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Vakilabad Blv. Azadi Squre, Mashhad, Iran
8 Department of Public Health, North Khorasan University of Medical Sciences, Bojnurd, Iran
Associated Data
The datasets produced and analyzed during the current study are not publicly available, but they are available from the corresponding author on reasonable request.
Depression is a major contributor to disability-adjusted life years (DALY) lost in the world. Dietary patterns are widely used to investigate diet–disease relations. In the current study, the relationship between dietary patterns and depression was investigated. Besides, the role of serum vitamin D, zinc, magnesium, and total antioxidant capacity as potential mediatory variables was studied.
It was an individually matched case–control study in which 330 depressed and healthy subjects were recruited for the extraction of dietary patterns; psychiatrists diagnosed major depressive disorder, using the criteria of the Diagnostic and Statistical Manual of Mental Disorders. Serum vitamin D and aforementioned biomarkers were measured for a number of randomly selected depressed and healthy individuals. We conducted mediatory analysis by regression models.
Healthy and unhealthy dietary patterns were associated with the lower and higher odds of depression (OR 0.39, CI 0.17–0.92 and OR 2.6, CI 1.04–6.08), respectively. A significant relationship between serum vitamin D with depression after adjusting for potential confounders was observed as well (OR 0.93, CI 0.87–0.99). According to the mediatory analysis the unhealthy dietary patterns were related to depression via altering the serum vitamin D concentration.
This study showed that vitamin D deficiency mediates the relationship between unhealthy dietary patterns and depression. However, to get a clearer result further prospective studies are required.
Depression is a common heath problem all over the world which currently affects 264 million people [ 1 ]. It is a leading cause of disability worldwide and is a major contributor to DALY lost in the world [ 2 ]. Depression is highly prevalent in developed and developing countries. In the USA, it has been estimated that 6.3% of adult population are depressed [ 3 ]. Depression ranks the forth contributing factor for burden of disease and is anticipated to rank the second until 2020. In Iran, depression constitutes 35% to 45% of mental illnesses and about 8% to 20% of the population suffer from it [ 4 ]. There are some medications for treatment of depression such as drugs that increase serotonin concentration as tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs) and serotonin-specific reuptake inhibitors (SSRIs) which are reported to have either side effects or not to be effective enough [ 5 ].
Diet has long shown to contribute to the treatment of depression. Prior studies on diet–depression associations have mostly focused on nutrients [ 6 – 8 ], foods [ 9 ] and food groups [ 10 ] rather than dietary patterns. Due to the complex interactions between nutrients and foods, nutritional epidemiologists have suggested to deploy dietary pattern approach in investigating diet–disease relations [ 11 ]. This approach can provide a more comprehensive and new insight toward the diet–mental health relations [ 12 ]. Some studies have assessed the association between dominant dietary patterns and risk of depression [ 13 – 15 ] which most of them have been driven from food questioners and not biochemical assessment. Some potential biomarkers like 25(OH) D, total antioxidant capacity (TAC), zinc (Zn), and magnesium (Mg) in serum are reported to be significantly related to depression. For example, low levels of serum 25(OH) D were associated with depressive symptoms [ 16 ], and in a large cohort study it was concluded that hypovitaminosis D may increase vulnerability for depression [ 17 ]. According to another study vitamin D-deficient people had higher risk for depression [ 18 ] which was confirmed by a meta-analysis [ 19 ]. It is documented that vitamin D has some receptors in the hypothalamus [ 20 ], and plays an important role in brain development [ 21 ]. Furthermore, case–control studies have shown TAC concentration is lower in depressed people compared with their healthy counterparts, as well as a negative dose–response relation observed between depression severity and TAC [ 22 ]. According to several studies a negative relationship was seen between zinc and depression [ 23 , 24 ], and magnesium showed to have a protective effect in treatment of depression via glutamate system [ 25 ], neurotransmitter metabolism [ 23 ] and psychomotor function [ 26 ].
So far it is not established if biochemical ingredients mediate the relation between dietary patterns and depression. So, in the current study we aimed to investigate the mediatory role of all above-mentioned biomarkers in the relation of dietary patterns and depression.
We acknowledge that the paper has been compliance with STROBE checklist.
Participants and study design
A total of 110 depressed patients and 220 healthy individuals participated in this individually matched case–control observational study. Serum vitamin D, zinc, magnesium, and total antioxidant capacity were considered as potential mediatory variables, when evaluating the relationship between dietary patterns and depression. The patients were selected from two psychiatric clinics in Tehran. For recruitment of controls, we reached each patient’s residential area, and invited eligible people to participate in the study.
According to the criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV, the patients’ diseases were diagnosed by the psychiatrist as major depressive disorder [ 27 ]. No one in the case and control groups did not have history of depression in the past year. Individual matching between two groups was done, based on sex, age, and residential area. Each people with depression was matched with two people as control within 10-year age categories.
For evaluating the relationship between biochemical markers and depression, the sample size was calculated for each quantitative biochemical marker separately and the highest obtained sample size was 43 matched cases and controls (86 depressed and healthy people out of total cases and controls). They were randomly selected for biochemical analysis including serum levels of 25(OH) D, TAC, Zn, and Mg. It is notice worthy that in this study biases were minimized by matching, considering precise criteria for inclusion and exclusion, correct selection of the case and control participants and using new cases.
Inclusion criteria
People aged 18–65 years, residing in Tehran, having major depressive disorder with a maximum period of 3-month intervals from onset of five symptoms of depression to the beginning of the study were included in the case group. For inclusion of the control group, the criterion was the absence of major depressive disorder, based on Beck Depression Inventory questionnaire (BDI-II), standardized in Iran [ 28 ].
Exclusion criteria
People who suffered from cognitive impairment or other psychotic illnesses diagnosed by a psychiatrist; those who had severe depression or lacked ability to cooperate and answer the questions; took any anti-depression drugs or treatments; suffered from hormonal disorders like Addison’s, Cushing’s disease; had hyperthyroidism, hypothyroidism, and hyperparathyroidism; suffered from chronic diseases like cancer, heart disease, diabetes, stroke, fibromyalgia, kidney or liver failure, multiple sclerosis and Parkinson disease; had history of trauma, cuts, fractures, bleeding, burns, accidents and other similar events in the past 3 months that resulted in unconsciousness and hospitalization; suffered from chronic and infectious diseases like HIV, mononucleosis, tuberculosis, viral hepatitis and pneumonia in the past 2 weeks; people who were addicted to alcohol and/or drug at the time of the study or in the past 3 months; had BMI ≥ 40 kg/m 2 , pregnancy and lactation at the time of the study or in the past year, any type of special diet in the past 2 months, any type of special diet for more than 2 months in the past year; took vitamin D more than once in the last 6 months; took Zn and Mg within at least 2 previous months; and people who took other nutritional supplements continuously, by injection or orally in the past month.
After describing the aim of the study, the written informed consent form was signed by all the participants. The study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences. The ethics code was 19374-161-03-91.
Assessment of covariates
A demographic questionnaire was employed to collect general information and some confounders. Anthropometric measurements were obtained from all subjects with a precision of 100 g for weight and 0.5 cm for height. Dietary intakes of the subjects in the last 12 months were assessed using a valid and reliable semi-quantitative food frequency questionnaire (FFQ) [ 29 ]. Physical activity was measured by a valid questionnaire in Iran [ 30 ]. The questionnaire consisted of nine levels of activity from rest and sleep (MET = 0.9) to vigorous activity (MET ≥ 6), based on the metabolic equivalent task hours per day (MET-h/day). An M.Sc. holder in nutrition collected data of physical activity. Depression was diagnosed based on the fourth edition of DSM criteria by a psychotherapist. For quantitative measurement of anxiety as a confounder, the Iranian standardized Beck Anxiety Inventory or BAI-II [ 31 ] was utilized. We used standardized Beck Depression Inventory questionnaire or BDI-II [ 28 ] for screening controls.
Assessment of serum biomarkers
Blood samples were collected before patients took any antidepressant drugs. To measure biomarkers, 5 ml blood samples were collected from the subjects who fasted for 12 h, between 7 and 10 AM and transferred into tubes with no anticoagulant. After centrifuging for 20 min at 1500 g in room temperature, the serum was separated and stored at − 70 °C. Serum 25(OH) D was assay by Enzyme immunoassay (EIA) method (IDS, UK). We measured serum total antioxidant capacity (TAC) with 3-ethylbenzothiazoline-6-sulfonic acid as a peroxidase substrate suitable for using in ELISA procedures. Serum Mg and serum Zn were measured with colorimetric assay (0.05–5 mg/dl pars azemun) and chemistry methods (Selecta E, Vitalab, Netherland in µg/dl), respectively.
Statistical analysis
“Kolmogorov–Simonov test was applied to analyze the normality of covariates, followed by t test or Mann–Whitney test to compare variables in two groups. To compare qualitative variables Chi square was used. The exploratory factor analysis/principal component analysis was applied to determine the dietary patterns. According to the nutrient profiles and culinary recipes, food items of the FFQ were classified into 26 food groups. Food groups with factor loadings ≥ 0.3 were considered as important contributors to a dietary pattern. The factors were orthogonally converted using varimax rotation to improve interpretability. To identify whether a factor should be retained, the study factors were naturally interpreted in conjunction with eigenvalues that was equal to 1.5 and the scree plot was determined. The factor score for each person was calculated by summing the intakes of food groups weighted by his/her factor loading. The derived factors (two dietary patterns) were labeled based on our interpretation of the data and of the earlier literature. To identify the association of dietary patterns with other dependent variables, the calculated scores for each individual in each pattern were used as independent variables.
Finally, two dietary patterns, healthy (high in fruits, cruciferous, yellow, green leafy and other vegetables, low-fat dairies, whole grains, nuts, and olives) and unhealthy (high in refined grains and breads, high-fat dairy, solid oils, liquid oils and mayonnaise, pickles, snacks, soft drinks, industrial fruits and juice, red meats, poultry, processed meats, and sweets), were defined [ 32 , 33 ].
Then, dietary patterns were used to evaluate the association of depression with dietary patterns and to adjust the confounders in multiple logistic regression. Multiple logistic regression models were used to assess the mediatory role of blood biomarkers related to depression. The following criteria were used to seek mediatory role of a variable [ 34 ]:
- Significance of the relationship between dietary pattern(s) and depression;
- No longer significance of the relationship between dietary pattern and depression after adding the mediatory variable to the model. In other words, after adding the mediatory variable(s) into the model, the relationship between dietary pattern(s) and depression had to transfer into the relationship between the mediatory variable and depression. Therefore, a third model had to be designed for ensuring the significant relationship between the mediatory variable(s) and depression.
- Significance of the relationship between the mediatory variable and depression.
The mediatory analysis was performed after adjusting some confounding variables such as job, education, marital status, children number, energy intake, and so on. The mentioned covariates were related to both dietary patterns (as the independent variables) and depression (as the dependent variable) which indicated they were confounders. In this way, the mediatory variables were in the causal path of dietary pattern and depression [ 35 ].
All statistical analyses were carried out using SPSS (version 20; Chicago, IL).
According to Table 1 for some important variables including weight, height, age, energy intake, smoking, and hookah, there was not a significant difference between case and control group indicating the matching had been done correctly.
Table 1
Baseline characteristics of study population in case and control groups
Data were presented as frequencies and percentages for categorical variables and mean ± SD, median (Q1–Q3) for normally and non-normally distributed variables, respectively
* p values calculated by Chi square for categorical values and Independent samples t test or Mann–Whitney test for continuous values
Based on our published results [ 33 ], the healthy dietary pattern significantly was related to the lower odds ratio of depression (OR 0.39, CI 0.17–0.92), and the unhealthy dietary pattern significantly was related to the higher odds ratio of depression (OR 2.6, CI 1.04–6.08).
We observed a significant relationship between serum vitamin D (OR 0.93, CI 0.87–0.99) and TAC (OR 2.08, CI 1.17–3.72) with depression after adjustment for some potential confounders. However, there was no significant association between serum zinc and magnesium, and depression (Table 2 ).
Table 2
Compare of some serum biochemical factors in case and control groups
p < 0.05 values are in italic
a Total antioxidant capacity
c Magnesium
d Multiple logistic regression after adjusting for job, education, marital status, children number, smoking and hookah, depression history, unemployment history in past 5 year, tragic events in past 6 months, energy expenditure, and physical activity
e Multiple logistic regression after adjusting age, sex, non-depression drugs, smoking and hookah, history of depression, body mass index, energy expenditure, and physical activity
In addition, in mediatory analysis unhealthy dietary pattern was inversely related to depression via changing the serum level of vitamin D after adjusting for job, education, marital status, children number, smoking and hookah, depression history, unemployment history in past 5 year, tragic events in past 6 months, energy intake, and physical activity (Table 3 ).
Table 3
Logistic regression model for mediation analysis in the pathway of the relation of dietary patterns with depression
p ≤ 0.05 values are in italic
Model 1: Logistic regression model for studying of relationship between depression and dietary patterns
Model 2: Logistic regression for studying of relationship between healthy dietary pattern and depression with mediation variables
Model 3: Logistic regression for studying of relationship between mediation variables and depression
All of three models adjusted for job, education, marital status, children number, unemployment history in past 5 year, tragic events in past 6 months r, smoking and hookah, depression history, energy expenditure, and physical activity
* Confidence intervals and p values for Hosmer and Lemeshow test that is the criterion of the goodness-of-fit for Logistic regression were nonacceptable
Model 1 in Table 3 illustrates the significant relation between dietary patterns and depression. For hypothesis testing of mediatory role, vitamin D was added in regression in model 2 (the same Table). By adding this variable, the significant relation between both dietary patterns and depression eliminated. In other words, the relationship between dietary patterns and depression moved to the relationship between the mediatory variable and depression. Therefore, it can be concluded that vitamin D is an intermediate variable. For confirmation of the idea, we examined the relation of vitamin D with depression (Table 3 —model 3). This mediatory role could be established by the significant results of the latest model. It was concluded that only unhealthy dietary pattern is related to depression via the intermediary role of vitamin D. In other words, people who had an unhealthy diet, if their vitamin D was increased by one unit, their odds of depression would be reduced by 11%.
Complete mediation in which other exposures no longer affects outcome after intermediary variable was controlled. Based on the results, vitamin D was a complete mediator because after serum vitamin D had been entered the relationship between unhealthy dietary pattern and depression disappeared [ 36 ].
We repeated testing the model for TAC (Table 3 ) because TAC was significantly related to depression (Table 2 ). However, the goodness of fit for the logistic regression model (based on confidence intervals and p value for Hosmer and Lemeshow test), was not valid (Table 3 ). There was no significant association between depression and serum zinc and magnesium. Hence, we did not do mediatory analysis for them.
In our study, there was a significant relationship between depression and serum vitamin D as well as between the unhealthy dietary pattern and depression after adjustment for some potential confounders. There was also a mediatory role for vitamin D in the pathway between unhealthy dietary pattern and depression. Therefore, we concluded that if people on an unhealthy diet try to raise their serum vitamin D levels by consuming more vitamin D, the chance of depression will be reduced among them. To the best of our knowledge, it is the first study that evaluates mediatory role of serum vitamin D in the associations between dietary pattern and depression.
According to our findings hypovitaminosis D resulted in higher odds of depression. In accordance with the present study, Eyles et al. [ 37 ] showed that in rats whose mothers were vitamin D deficient their brain in terms of gross morphology, cellular proliferation, and growth factor signaling as well as expression of nerve growth factor was not developed properly. In contrast with the mentioned study, in a cross-sectional study conducted in middle-aged and elderly Chinese, depressive symptoms were not associated with 25(OH) D concentrations [ 38 ].
Several mechanisms have been proposed to explain the association between vitamin D and depression. Effects of active form of vitamin D (1, 25 dihydroxycholecalciferol) in brain tissue have been established by the detection of vitamin D receptors (VDR) in different parts of the brain [ 10 , 39 ] such as amygdale as the center of the limbic system that affects behavior and emotions [ 40 ]. Vitamin D has also several neuroprotective functions, for example calcitriol regulates concentrations of calcium in neurons that could decrease toxicity resulted from excess calcium [ 13 , 15 , 18 ]. However, more studies are needed to examine the long-term effect of vitamin D depletion on the brain.
We could not establish the mediatory roles of TAC in the pathway of the relationship between the dietary pattern and depression because the goodness-of-fit criterion for the Logistic regression models was not acceptable (logical confidence intervals and p values for the Hosmer–Lemeshow test). Similar to the current study, Gonoodi et al. [ 41 ] did not find any significant relation between serum Zn levels and depression score in 408 adolescent girls aged 12–18 years. There were other studies which reported serum Zn concentrations did not differ between depressed patients and healthy group that support our results [ 42 – 44 ]. A randomized clinical trial demonstrated the efficacy of zinc supplementation in treatment of depression [ 45 ]. Moreover, one meta-analyses confirmed an inverse association between serum Zn concentration and depression [ 46 ]. All depressed patients in our study were new cases and it is the probable reason we could not observe any relationship in this regard. In other words, there was not enough time for reduction of zinc in the newly recognized patients. Another possible cause can be justified by the fact that the populations in different studies were not the same.
Results concerning evaluating serum magnesium concentrations in depressive disorders were inconsistent. Some authors found higher levels of serum Mg in depressed patient compared to healthy group [ 47 , 48 ] which are in contrast to our finding. On the other hand, several studies reported an inverse association between serum Mg levels and depression [ 49 – 52 ]. However, it seems serum Mg levels may not be a proper indicator of depressive disorders [ 52 ].
Strengths and limitations
In the current study we recruited new cases of depression. In addition, we conducted mediatory analysis, considered all inclusion and exclusion criteria precisely and minimized selection bias in the control group by going to the residential area of each patient.
The most important limitation of our study goes back to the nature of case–control studies in which the chance of recall bias is high, as well as the temporal relationship between depression and dietary patterns cannot be realized in such studies. Another limitation was related to financial restrictions which forced us not to do biochemical measurements for all the participants.
Recommendations
Some oxidative stress biomarkers such as albumin, HDL cholesterol, and uric acid are likely to be associated with depression. Therefore, we highly recommend future studies which evaluate the mediatory role of the mentioned biomarkers in the relationship between dietary pattern and depression.
This study showed that Vitamin D deficiency mediates the relationship between unhealthy dietary patterns and depression. However, to confirm the finding further prospective studies are suggested.
Acknowledgements
We are appreciated Research Deputy of Tehran University of Medical Sciences.
Authors’ contributions
GS has made substantial contributions to conception and design. She revised the manuscript critically as well. FR has made substantial contributions to conception and recruitment. MA has made substantial contributions to revise the manuscript in all of terms to satisfy your valuable comments and criticisms. MH has made substantial contributions to interpretation of data, and has been involved in the drafting of the manuscript. RM has made substantial contributions to design and statistical analysis. FK has been involved in the drafting of the manuscript. MK has made substantial contributions to conception and design, recruitment, biochemical analysis, interpretation of data, and the drafting of the manuscript. All authors read and approved the final manuscript.
The Research Deputy of Tehran University of Medical (19374-161-03-91) supported financial resources of the study.
Availability of data and materials
Consent for publication.
The authors provided consent for publication.
Competing interests
We all authors declare that we have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gity Sotoudeh, Email: ri.ca.smut@hedotosg .
Firoozeh Raisi, Email: moc.liamg@isiarf .
Maryam Amini, Email: ri.ca.umbs@inimam .
Reza Majdzadeh, Email: [email protected] .
Mahdieh Hosseinzadeh, Email: [email protected] .
Fatemeh Khorram Rouz, Email: ri.ca.smum@159FRmarrohK .
Maryam Khosravi, Email: ri.ca.smum@mivarsohK .

Magnesium is naturally present in a variety of foods, available as a supplement, and an ingredient in antacids and laxatives. The mineral plays an important role in assisting more than 300 enzymes to carry out various chemical reactions in the body such as building proteins and strong bones, and regulating blood sugar, blood pressure, and muscle and nerve functions. Magnesium also acts an electrical conductor that contracts muscles and makes the heart beat steadily.
More than half of the magnesium in our body is stored in bones, and the remaining in various tissues throughout the body.
Recommended Amounts
RDA: The Recommended Dietary Allowance (RDA) for adults 19-51+ years is 400-420 mg daily for men and 310-320 mg for women. Pregnancy requires about 350-360 mg daily and lactation, 310-320 mg.
UL: The Tolerable Upper Intake Level is the maximum daily intake unlikely to cause harmful effects on health. The UL for magnesium is 350 milligrams from supplements only. High-dose supplements can lead to diarrhea, nausea, and cramping in some people. Extra magnesium from food is safe because the kidneys will eliminate excess amounts in urine.
Magnesium and Health
Magnesium is a key factor in making several parts of the body run smoothly: the heart, bones, muscles, nerves, and others. Without enough magnesium, these areas malfunction. This is summarized in research, which finds that a magnesium deficiency or low magnesium diet leads to health problems. Although epidemiological studies show that higher magnesium diets are associated with lower rates of disease, results are mixed from clinical trials showing that magnesium supplementation can correct these conditions. It may be because a magnesium-rich diet is often higher in other nutrients, which collectively work together in disease prevention as opposed to a supplement containing a single nutrient.
For disease prevention, a good rule of thumb is to eat a daily diet that includes some magnesium-rich foods and take a supplement if directed by a physician to correct a deficiency if blood levels are low.
Magnesium is a component of bone; in fact 60% of the body’s magnesium is stored in bone. It is also involved with the activity of bone-building cells and the parathyroid hormone, which regulates calcium levels. Population studies have found an association of greater bone mineral density in men and women with higher magnesium diets. [1] A cohort study of 73,684 postmenopausal women from the Women’s Health Initiative found that a lower magnesium intake was associated with lower bone mineral density of the hip and total body, although the authors cautioned that their finding did not translate into an increased risk of fractures. [2] A meta-analysis of 24 observational studies examining fracture risk did not find that higher magnesium intakes were associated with a reduced risk of hip and total fractures. [3] Clinical trials have shown mixed results with the use of magnesium supplements to increase bone mineral density. More research is needed to see if and how much of a supplement can reduce fracture risk.
Magnesium is sometimes prescribed as a complementary treatment for migraine headaches, as clinical studies have found low magnesium levels in people suffering from this condition. [4] Randomized double-blind controlled trials have found that magnesium citrate and magnesium oxide supplements (about 500 mg/day) taken for up to 3 months were protective against migraines. [5] In a randomized double-blind clinical trial, 70 patients who were admitted to the emergency room with acute migraine headache were given either the usual IV treatment for migraine (dexamethasone/metoclopramide) or IV magnesium sulfate. The study found the magnesium to be more effective and faster-acting than the typical treatment. This was a one-time administration in an acute setting, so studies with longer follow-up are needed to confirm this benefit.
The National Headache Foundation suggests a daily dose of 400-600 mg of magnesium to reduce the frequency of migraine attacks; however because this is greater than the RDA, it may lead to side effects (e.g., muscle weakness, diarrhea) in some people and may not be safe with certain medical conditions. They recommend discussing the use of high-dosage magnesium supplements with a physician.
Magnesium assists with neurological pathways that, when not functioning correctly, are believed to lead to mood disorders like depression and anxiety. Several observational studies have linked lower magnesium levels with increased depression. [6,7] However, a small number of randomized clinical trials have not shown consistent results that magnesium supplementation is an effective treatment for depression. [7] The control groups in these trials, either given a placebo or an antidepressant medication, showed similar effects as the treatment group receiving magnesium supplements. The trials also tended to have a small number of participants with a short duration, ranging from 1-8 weeks. Longer trials with standardized depression rating scales are needed to better assess this connection.
High blood pressure is a risk factor for cardiovascular disease (CVD), and magnesium helps to regulate blood pressure. Studies have shown an association with magnesium deficiency and high blood pressure. However, the results of clinical studies are mixed on using magnesium supplements to lower blood pressure. Epidemiological studies have found an association of the DASH diet (including magnesium-rich fruits, vegetables, and low-fat dairy products) with decreased blood pressure, but DASH is also rich in potassium and calcium that may lower blood pressure, so it is not clear if magnesium or a combination of nutrients is protective. Other population studies have shown that higher magnesium intakes and/or higher blood levels of magnesium are associated with a lower risk of stroke and deaths from heart disease, although again it is difficult to separate out other nutrients in these same foods that are protective against CVD. [1]
The Food and Drug Administration has approved a health claim on food products or supplements containing magnesium to state, “ Consuming diets with adequate magnesium may reduce the risk of high blood pressure (hypertension). However, the FDA has concluded that the evidence is inconsistent and inconclusive.” [8]
Magnesium assists enzymes that regulate blood sugar and insulin activity. Prospective cohort studies show an association of diets low in magnesium with an increased risk of type 2 diabetes . [4] However, the results are mixed in clinical trials of magnesium supplements for people with diabetes, some finding an improvement in insulin sensitivity when correcting a magnesium deficiency, and others showing no change. Results are also mixed on the effectiveness of supplements in improving overall blood sugar control. Part of the reason may be differences in the study design of these clinical trials. The American Diabetes Association reports a lack of evidence at this time to recommend magnesium supplements to improve blood sugar control in people with diabetes. [9]
Food Sources
Magnesium is found in plant foods like legumes, dark green leafy vegetables, nuts , seeds, whole grains , and fortified cereals. It is also in fish, poultry, and beef.
- Almonds , peanuts, cashews
- Pumpkin seeds
- Peanut butter
- Beans (black, kidney)
- Soybeans, soymilk
- Cooked spinach, Swiss chard
- White potato with skin
- Oatmeal (instant, whole oats)
- Dark chocolate (at least 70%)
- Milk , yogurt
Supplements
A magnesium supplement may be prescribed if the body is having problems absorbing the nutrient. Over-the-counter magnesium supplements come in different forms; liquid types like magnesium citrate or chloride may be better absorbed than solid tablets like magnesium oxide and sulfate.
Magnesium can have a laxative effect at high doses; in fact, it is sold as a laxative in the form of magnesium hydroxide. Magnesium hydroxide is also an ingredient in some popular antacids to treat heartburn and upset stomach; it is important to be aware of the laxative effect when using magnesium hydroxide tablets for an upset stomach.
The interplay of magnesium and vitamin D
Research from the National Health and Nutrition Examination Survey (NHANES) data show that higher intakes of magnesium from food or supplements is associated with significantly reduced risks of vitamin D deficiency. [12]
Signs of Deficiency and Toxicity
Although magnesium is naturally found in a variety of foods and some fortified foods, some research suggests that magnesium levels may be lower in soils than in prior years, and food processing can reduce magnesium content from plant foods containing the mineral. [13] A low to moderate deficiency of magnesium is not likely to produce noticeable symptoms. The body also helps to preserve magnesium levels when stores are low by limiting the amount excreted in urine and absorbing more magnesium in the gut. [4] However, national dietary surveys show that most Americans of all ages eat less than recommended amounts. [14] Further, a normal blood level of magnesium may not accurately predict total magnesium levels in the body, as most of the mineral is stored in tissue and bones. Certain types of magnesium deficiency show a normal blood level. [15]
Severe deficiency occurs with a long-term low magnesium diet, malabsorption, and large losses from alcohol abuse or use of medications that deplete magnesium (some diuretics, proton pump inhibitors, and antibiotics).
- Fatigue, weakness
- Poor appetite
- Nausea, vomiting
- Numbness or tingling in skin
- Muscle cramps
- Abnormal heart rate
- Alcohol abuse. A long-term excessive intake of alcohol is associated with a poor diet low in magnesium, digestive upset that leads to malabsorption, and problems with various organs that can flush out too much magnesium through urine.
- Older ages. The elderly have lower magnesium intakes according to national dietary surveys. Aging also causes decreased absorption of magnesium in the gut and increased excretion in urine. Furthermore, older adults are more likely to be on medications for chronic diseases that can lower magnesium stores.
- Conditions with malabsorption. Diseases that interfere with digestion can lower the amount of magnesium absorbed. Most magnesium is absorbed in the largest segment of the small intestine, the ileum, which may be compromised in conditions like celiac and Crohn’s disease. Surgery that removes the ileum, which is sometimes needed with colon cancer, ulcerative colitis, or Crohn’s disease, further adds to a deficiency risk.
- Type 2 diabetes mellitus. Insulin resistance or uncontrolled diabetes can cause the kidneys to make extra urine to rid the body of high levels of blood sugar. This increased amount of urine may also flush out magnesium.
Toxicity is rare from food sources because the kidneys will remove excess magnesium in the urine. However toxic levels may occur with long-term use of high-dosage supplements. People with kidney disease have a higher risk of toxicity because their kidneys are not working properly and cannot flush out extra magnesium.
- Low mood, depression
- Muscle weakness
- Low blood pressure, abnormal heartbeat
- Heart attack
Did You Know?
Magnesium supplements are a popular remedy for leg and foot cramps, a bothersome condition that may jolt you awake in the middle of the night or during exercise. A deficiency of magnesium can certainly cause muscle cramping, but these supplements are often used whether or not people know they are truly deficient.
Evidence so far does not support this treatment for muscle cramps. A Cochrane review of seven randomized controlled trials looking at the effects of magnesium supplements on muscle cramps did not find a significant difference in intensity or duration of cramps in people using the supplements versus a placebo for one month. [16] Information was not available on the participants’ blood levels of magnesium at the start of the trials, so it is not known if the muscle cramps were related to a deficiency versus other factors.
Vitamins and Minerals
- National Institutes of Health Office of Dietary Supplements: Magnesium Fact Sheet for Health Professionals https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ . Accessed 9/2/2019.
- Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, LaCroix AZ, Wactawski-Wende J, Jackson RD. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr . 2014 Apr;99(4):926-33.
- Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos Int . 2016 Apr;27(4):1389-1399.
- Gröber U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients . 2015 Sep 23;7(9):8199-226
- Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr . 2013 May 1;4(3):378S-83S.
- Tarleton EK, Littenberg B. Magnesium intake and depression in adults. J Am Board Fam Med . 2015 Mar-Apr;28(2):249-56.
- Kirkland AE, Sarlo GL, Holton KF. The Role of Magnesium in Neurological Disorders. Nutrients . 2018 Jun 6;10(6).
- FDA Announces Qualified Health Claim for Magnesium and Reduced Risk of High Blood Pressure https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-qualified-health-claim-magnesium-and-reduced-risk-high-blood-pressure . Accessed 3/4/2023.
- Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS Jr. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care . 2013;36:3821-42.
- Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan L, Murff H. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. The American journal of clinical nutrition . 2018 Dec 1;108(6):1249-58.
- Sakaguchi Y. The emerging role of magnesium in CKD. Clinical and Experimental Nephrology . 2022 May;26(5):379-84.
- Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC medicine . 2013 Dec;11(1):1-4.
- Cazzola R, Della Porta M, Manoni M, Iotti S, Pinotti L, Maier JA. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon . 2020 Nov 1;6(11):e05390.
- Moshfegh A, Goldman J, Ahuja J, Rhodes D, LaComb R. What We Eat in America, NHANES 2005-2006: Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium. U.S. Department of Agriculture, Agricultural Research Service. 2009. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0506/usual_nutrient_intake_vitD_ca_phos_mg_2005-06.pdf . Accessed 9/2/2019.
- Razzaque MS. Magnesium: are we consuming enough?. Nutrients . 2018 Dec 2;10(12):1863.
- Garrison SR, Allan GM, Sekhon RK, Musini VM, Khan KM. Magnesium for skeletal muscle cramps. Cochrane Database Syst Rev . 2012 Sep 12;(9):CD009402.
Last reviewed March 2023
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.

COMMENTS
Background Vitamin D is a fat-soluble vitamin; vitamin D is essential to sustain health and it protects against osteoporosis. It is crucial to the human body's physiology in terms of muscular movement and neurological signal transmission, and to the immune system in defense against invading pathogens. Case presentation This was a case of a 26-year-old Sudanese woman who presented with a 2 ...
Ongoing studies will evaluate the effect of vitamin D supplementation in patients with severe vitamin D deficiency (ClinicalTrials.gov number, NCT03188796), other subgroups of patients that may be ...
A level of 25 hydroxy vitamin D (25-OH D) less than 12.5 nmol/L (5 ng/mL) is suggested for the diagnosis of rickets with a healthy maintenance level of approximately greater than 50 nmol/L (20 ng/mL) [ 1, 2 ]. It should be noted that newer data suggests a lower limit of 80 nmol/L may be a more acceptable level in adults [ 1, 2 ].
In the WHI Calcium-Vitamin D nested case-control study, the adjusted odds ratios (ORs) for incident breast or colorectal cancer over 7 years of follow-up did not demonstrate a statistically significant association between exposure to active treatment and incidence of cancer among participants with vitamin D deficiency at baseline. 38,39
In this issue of the Journal, LeBoff and colleagues 2 report findings from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL), 3 which extend the results of that trial; taken together ...
Reports on individuals diagnosed with myopathy and coexisting vitamin D deficiency noted a clinical improvement in symptoms following vitamin D supplementation ( 3 ). These myopathies have been associated with the findings of atrophic muscle fibers on biopsy. In this study, we present a case of a 17-year-old girl with prolonged worsening ...
Vitamin D (VitD) is a naturally occurring, fat-soluble vitamin which regulates calcium and phosphate homeostasis in the human body and is also known to have a neuroprotective role. VitD deficiency ...
Currently, the role of vitamin D supplementation, and the optimal vitamin D dose and status, is a subject of debate, because large interventional studies have been unable to show a clear benefit ...
Studies on vitamin/hormone D deficiency have received a vast amount of attention in recent years, particularly concerning recommendations, guidelines, and treatments. ... of vitamin D deficiency among athletes and the response to supplementation may be different depending on the degree of deficiency or the lack of it, as in the case of ...
This Review highlights results from large randomized clinical trials performed during the period 2017-2020 and Mendelian randomization studies on vitamin D levels. Together, findings indicate ...
It therefore seems that hypertension and an acceleration of atherosclerosis are linked to Vitamin D deficiency. A study of 3316 patients showed consistent increases in plasma renin ... whereas boiling eggs achieved a retention rate of 86-88%. In the case of baked bread, rye bread retained 69% of its Vitamin D3, which was less than the 85% ...
n Vitamin D and COVID-19. Methods: The present case-control study was conducted at tertiary care hospital, AIIMS, Patna, Bihar, India. Total 156 cases and 204 controls were enrolled in the study after obtaining informed consent. Categorization of the patients were done based on clinical severity and level of Vitamin D. The association between these categories with different variables were ...
Results. One case-control study, ten cross-sectional studies and three cohort studies with a total of 31 424 participants were analysed. Lower vitamin D levels were found in people with depression compared with controls (SMD = 0.60,95% Cl 0.23-0.97) and there was an increased odds ratio of depression for the lowest v. highest vitamin D categories in the cross-sectional studies (OR = 1.31, 95 ...
Of course, vitamin D deficiency should always be treated and some high-risk patients with malabsorption syndromes, osteoporosis, or taking medications that interfere with vitamin D metabolism will benefit from supplementation." ... Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case-control study of plasma 25-hydroxyvitamin D ...
In a new study, investigators from Brigham and Women's Hospital found the people who took vitamin D, or vitamin D and omega-3 fatty acids, had a significantly lower rate of autoimmune diseases — such as rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, and psoriasis — than people who took a placebo.. With their findings published Wednesday in BMJ, the team had ...
Vitamin D deficiency is associated with increased risks for cardiovascular mortality and chronic kidney disease (CKD) progression in patients with early-stage disease, according to a study ...
To better understand the role of vitamin D deficiency in the disease course of COVID-19, we undertook a retrospective case-control study in North West England. Objective: To examine whether hospitalization with COVID-19 is more prevalent in individuals with lower vitamin D levels.
The anti-inflammatory, antiviral and immune modulator effect of Vitamin D could be beneficial to COVID-19. Aim: To find out the possible association between Vitamin D and COVID-19. Methods: The present case-control study was conducted at tertiary care hospital, AIIMS, Patna, Bihar, India. Total 156 cases and 204 controls were enrolled in the ...
nalysis of 24 observational studies, grouping them according to the gestational age at the time of serum sampling, to investigate whether vitamin D deficiency in different periods of gestation has different effects on PTB and to provide an evidence-based basis for pregnant women to measure and supplement vitamin D. Methods: The databases PubMed-Medline, EMBASE, the Cochrane Library, Web of ...
Vitamin D is a fat-soluble vitamin that plays an important role in calcium homeostasis and bone metabolism. Vitamin D deficiency can lead to osteomalacia and rickets in children and osteomalacia in adults. The fortification of milk with vitamin D in the 1930s was effective in eradicating rickets in the world. However, subclinical vitamin D deficiency is still widely prevalent in both developed ...
Vitamin D deficiency (VDD) affects the health and wellbeing of millions worldwide. In high latitude countries such as the United Kingdom (UK), severe complications disproportionally affect ethnic ...
Background Sepsis is one of the main causes of death in newborns worldwide. Vitamin D levels during fetal and neonatal periods have a significant role in the development of the immunological system. The study aims to evaluate the association between vitamin D levels and the risk of early-onset neonatal sepsis in full-term neonates in a developing country. Methods This case-control study was ...
Objectives: The aim of this study was to explore, on an additive scale, the combined effect of the association between insulin resistance (IR), chronic low-grade inflammation (CLGI) and vitamin D deficiency (VDD) on the risk of type 2 Diabetes Mellitus (T2DM). Methods: This is a cohort study, including 1484 non-diabetic subjects, followed for a period of four years. 25 hydroxy-vitamin D (25OHD ...
The mean Vitamin D level for the case group was calculated to be 17.6 ± 7.2 ng/dL, while the mean Vitamin D level in the control group was 32.34 ± 13.79 ng/dL. The results of the study revealed that a majority of the participants in the case group, precisely 62.5%, were deficient in Vitamin D.
BACKGROUND:. The role of maternal vitamin D supplementation in the prevention of infantile rickets is unknown, particularly in low- and middle-income countries without routine infant vitamin D supplementation. Through secondary analysis of a randomized, placebo-controlled trial in Bangladesh, we examined the dose-ranging effects of maternal vitamin D supplementation on the risk of biochemical ...
This study showed that vitamin D deficiency mediates the relationship between unhealthy dietary patterns and depression. However, to get a clearer result further prospective studies are required. ... Furthermore, case-control studies have shown TAC concentration is lower in depressed people compared with their healthy counterparts, ...
Enzymes that produce and break down vitamin D require magnesium. [10] Magnesium also helps the liver and kidneys to metabolize vitamin D. Therefore, a deficiency of magnesium may reduce the body's ability to use vitamin D even if vitamin D supplements are taken, which may in turn negatively affect the absorption of calcium.