- How To Find Articles with Databases
- How To Evaluate Articles
- How To Read A Scientific Paper
- How To Interpret Data
- How To Write A Lab Report
- How To Write A Scientific Paper
- Get More Help
- Reference: Encyclopedia, Handbooks & Dictionaries
- Research Tools: Databases, Protocols & Citation Locators
- E-Journal Lists by Subject
- Scholarly vs Popular
- Search Tips
- Open Resources
- E-Journal lists by subject
- Develop a Research Question

Writing Lab Reports
Writing lab reports follows a straightforward and structured procedure. It is important to recognize that each part of a lab report is important, so take the time to complete each carefully. A lab report is broken down into eight sections: title, abstract, introduction, methods and materials, results, discussion, conclusion, and references.
- Ex: "Determining the Free Chlorine Content of Pool Water"
- Abstracts are a summary of the experiment as a whole and should familiarize the reader with the purpose of the research.
- Abstracts will always be written last, even though they are the first paragraph of a lab report.
- Not all lab reports will require an abstract. However, they are often included in upper-level lab reports and should be studied carefully.
- Why was the research done or experiment conducted?
- What problem is being addressed?
- What results were found?
- What are the meaning of the results?
- How is the problem better understood now than before, if at all?
Introduction
- The introduction of a lab report discusses the problem being studied and other theory that is relevant to understanding the findings.
- The hypothesis of the experiment and the motivation for the research are stated in this section.
- Write the introduction in your own words. Try not to copy from a lab manual or other guidelines. Instead, show comprehension of the experiment by briefly explaining the problem.
Methods and Materials
- Ex: pipette, graduated cylinder, 1.13mg of Na, 0.67mg Ag
- List the steps taken as they actually happened during the experiment, not as they were supposed to happen.
- If written correctly, another researcher should be able to duplicate the experiment and get the same or very similar results.
- The results show the data that was collected or found during the experiment.
- Explain in words the data that was collected.
- Tables should be labeled numerically, as "Table 1", "Table 2", etc. Other figures should be labeled numerically as "Figure 1", "Figure 2", etc.
- Calculations to understand the data can also be presented in the results.
- The discussion section is one of the most important parts of the lab report. It analyzes the results of the experiment and is a discussion of the data.
- If any results are unexpected, explain why they are unexpected and how they did or did not effect the data obtained.
- Analyze the strengths and weaknesses of the design of the experiment and compare your results to other similar experiments.
- If there are any experimental errors, analyze them.
- Explain your results and discuss them using relevant terms and theories.
- What do the results indicate?
- What is the significance of the results?
- Are there any gaps in knowledge?
- Are there any new questions that have been raised?
- The conclusion is a summation of the experiment. It should clearly and concisely state what was learned and its importance.
- If there is future work that needs to be done, it can be explained in the conclusion.
- If using any outside sources to support a claim or explain background information, those sources must be cited in the references section of the lab report.
- In the event that no outside sources are used, the references section may be left out.
Other Useful Sources
- The Lab Report
- Sample Laboratory Report #2
- Some Tips on Writing Lab Reports
- Writing a Science Lab Report
- << Previous: How To Interpret Data
- Next: How To Write A Scientific Paper >>
- Last Updated: Mar 8, 2024 2:26 PM
- URL: https://guides.libraries.indiana.edu/STEM
Social media
- Instagram for Herman B Wells Library
- Facebook for IU Libraries
Additional resources
Featured databases.
- Resource available to authorized IU Bloomington users (on or off campus) OneSearch@IU
- Resource available to authorized IU Bloomington users (on or off campus) Academic Search (EBSCO)
- Resource available to authorized IU Bloomington users (on or off campus) ERIC (EBSCO)
- Resource available to authorized IU Bloomington users (on or off campus) Nexis Uni
- Resource available without restriction HathiTrust Digital Library
- Databases A-Z
- Resource available to authorized IU Bloomington users (on or off campus) Google Scholar
- Resource available to authorized IU Bloomington users (on or off campus) JSTOR
- Resource available to authorized IU Bloomington users (on or off campus) Web of Science
- Resource available to authorized IU Bloomington users (on or off campus) Scopus
- Resource available to authorized IU Bloomington users (on or off campus) WorldCat
IU Libraries
- Diversity Resources
- About IU Libraries
- Alumni & Friends
- Departments & Staff
- Jobs & Libraries HR
- Intranet (Staff)
- IUL site admin
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
APA Sample Paper: Experimental Psychology

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Post-lockdown teaching support
- Get the print issue
- RSC Education

- More from navigation items
Smartphone spectroscopy

Help students grasp the Beer–Lambert law by going back to basics with spectrophotometry

Source: All images © Shutterstock
Let students use their smartphones in class – and demonstrate the Beer–Lambert law
Measuring the concentration of analytes and understanding the Beer–Lambert law are an important part of many chemistry curriculums. Thanks to recent breakthroughs in manufacturing and design, spectrophotometers are smaller and more accurate than ever before. As a result, they have become sealed devices or ‘black boxes’ to protect their accuracy and functionality. This offers little or no opportunity for students to see inside a spectrophotometer to investigate what it does and how it works.
There are numerous reports of home-made spectrophotometers designed by teachers and students , with impressive results. But what if you do not have access to the necessary electronics to build your own device or you do not feel comfortable working with raw electronic components?
Take out your smartphone.
This is what I did during the first wave of the Covid-19 pandemic, when RSC education coordinators made short videos showcasing experiments students can do at home. The smartphone spectroscopy video deconstructs a common spectrophotometer into its simplest components: a light source with a filter, a sample holder and a detector.
This is what I did during the first wave of the Covid-19 pandemic, when RSC education coordinators made short videos showcasing experiments students can do at home. The smartphone spectroscopy video ( LINK ) deconstructs a common spectrophotometer into its simplest components: a light source with a filter, a sample holder and a detector.
Do it yourself
Here’s how you can adapt the experiment for teaching the Beer–Lambert law.
You can set the activity for students to try at home or use it as a hands-on classroom experiment.
For the filters, use green sweet wrappers (Quality Street for example) or cut up a green plastic drink bottle. The blackcurrant squash drink samples are purple so they will absorb green light (green is opposite purple on the colour wheel).
Download this
Download the instructions for this simple activity as MS Word or pdf .
This activity is just as effective in the school lab as it is at home, making it a great activity for remote learning for any age. 16 – 18 students can use their results to plot a graph and manipulate logarithmic equations to find the concentration of an unknown dilution of squash.
Download all
Download the instructions for this simple activity from the Education in Chemistry website: LINK .
For increased accuracy, use a small torch with a narrow beam and use identical containers such narrow drinking glasses or narrow sweet boxes (eg Tic Tac boxes). This will keep the path length of the light constant. Keep the volume of water constant too so that the only variable is the amount of blackcurrant squash added to each sample. Enclosing the entire set-up in a large box provides more consistent and accurate results as it reduces the influence of outside light. It’s important to discuss constants and variables with students, particularly when setting up experiments.
You can download a light meter smartphone app or purchase a light meter from a camera store. Sometimes called lux meters, there are many free versions available. Apps like the Arduino Science Journal and Phyphox also include a light meter function. Place the light source and filter on one side of the sample and the light meter or smartphone camera on the other side directly across from the light.
The Beer–Lambert law
The squash drink absorbs the energy of a photon of light, which reduces the transmission of the light as it passes through the sample. In dilute samples, most of the green light from the torch passes through the drink and into the light meter or smartphone camera, giving a large lux number (high transmittance). In concentrated samples there is more squash drink available to absorb the light so less light passes through to the detector, giving a smaller lux number (low transmittance).
The lux number measured by the light meter for each sample is directly proportional to the transmittance. Using a blank sample of water as the incident light, the transmittance can be calculated for each sample. Percentage transmittance (% T ) is equal to 100 multiplied by the lux number of the transmitted light ( I ) divided by the lux number of the incident light ( I 0 ):
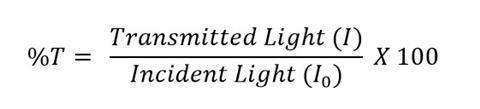
The transmittance values can be converted to absorbance for use with the Beer–Lambert Law and the following equation: Absorbance = 2 – log 10 (% T ). Although the results obtained from this set-up are less accurate than commercial devices, the experiment still demonstrates the Beer–Lambert law very well.
In the lab, students’ working memories can become overloaded due to the pressures of time, having to follow instructions and recall theory. By stripping spectrophotometry and the Beer–Lambert law back to basics, you can help your students conceptualise the theory more quickly and reduce misconceptions. This set-up does not replace spectrophotometers, but it will help students understand the inner workings of these devices at a fundamental level.
John O’Donoghue
Join an online teacher support workshop with RSC education coordinators about this approach to spectroscopy and find out more about using the experiment in your class on 11 February 2021. Sign up today .

More from John O'Donoghue

Christmas, community and chemistrees
Encouraging scientific thinking in the classroom lab

The science of smartwatches
- Analytical chemistry
- Applying scientific method
- Maths skills
- Observing and measuring
- Practical skills and safety
- Recording data
- Spectroscopy
Related articles

2021-01-18T13:27:00Z By John O'Donoghue
How wearable tech uses chemistry to help monitor your health – with spectroscopy, battery technology and smart materials

How chemists are stopping paint drip
2020-07-20T09:04:00Z By Rowan Frame
Analytical chemistry is playing an important role in discovering why some 20th century paintings have started to liquefy

Infrared (IR) spectroscopy: Uses of IR spectroscopy
Five out of five
Infrared spectroscopy is a valuable technique in analytical chemistry. Learn about how spectra arise and the instruments used to measure them
2 readers' comments
Only registered users can comment on this article., more from ideas.

How to run a successful Chemistry Olympiad club
2024-06-05T07:00:00Z By Lynne Maxwell
Discover effective approaches and essential resources to prepare learners for this competition (and others), recommended by two heads of science

5 ways to use structure strips to scaffold learning
2024-05-08T05:08:00Z By Kristy Turner
Boost your students’ ability to digest topics and write independently with these margin-sized prompts


Escape the classroom: and revise chemistry knowledge
2024-05-03T09:21:00Z By Hayley Russell
Challenge your students to break out of the lab and prepare for exams
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud
The Chemistry Lab Experiment
To find the molarity of the unknown acid, first we had to create a standard solution, the solution we created was Sodium Hydroxide (NaOH). We wanted a 0. 1 molar solution of sodium hydroxide so to get this we had to dissolve 4g of NaOH into 1000cm? of water, but we didn’t want 1000cm? we wanted 250cm? so to work out how much sodium hydroxide would be needed you need to do the same equation to the number of grams (g) than with the volume of water, so to get 1000cm? down to 250cm?
You divide it by 4, so you divide 4 by 4 which gives you 1, so one gram of NaOH is needed to make a 0.
1 molar solution in 250cm? of water. Next is making the solution, the equipment needed to make this standard solution is: a balance, beaker, volumetric flask, glass rod, wash bottle. And the ingredients for the solution are NaOH and distilled water. To make NaOH solution is to measure out 1g of sodium hydroxide and place on a scrap piece of paper which is on the balance, it isn’t essential that you get exactly 1g just approximately 1g.
Then put some distilled water into a beaker enough to dissolve the sodium hydroxide, transfer the sodium hydroxide from the paper to the beaker and dissolve by swirling and stirring. Once dissolved transfer this solution to a volumetric flask, and wash out the beaker and glass rod which was used to stir the solid NaOH into the water, now add distilled water to the volumetric flask, up until the bottom of the meniscus is on the 250cm?line and shake and mix it up a little, then you have made your solution.

Proficient in: Chemistry
“ She followed all my directions. It was really easy to contact her and respond very fast as well. ”
In my solution it wasn’t 1g, I weighed 0. 99g. The next stage is to calculate the molarity of your solution.
To work out the moles it is moles= grams ? relative molecular mass (RMM) so for my solution it will be 0. 99? 40 (40 is the RMM of sodium hydroxide, this is calculated by adding the mass of each atom in the compound together, so for NaOH it is Na=23 O=16 and H=1. 23+16+1=40 this is where the 40 comes from.) 0. 99 ? 40= 0. 02475 rounded to 4 decimal places is 0. 0248 that is the molarity of the 250cm? but molarity is always measured in 1000cm? so now you have to times 0. 0248 by 4, 0. 0248 x 4= 0. 992, and that is the final molarity of your solution so my molarity is 0. 992M.
Now is to titrate you solution with the unknown acid, to do this you need: a clamp, a beaker for acid, a beaker for your standard solution and another beaker for waste, a conical flask, 50ml burette, 25ml pipette.
Once all the equipment has been set up you now need to add your unknown solution into the burette and leave the tap open and put the waste beaker under it to make sure there is no air bubbles in the burette, turn the tap off and fill the burette up, now take the pipette filler and fill up your pipette with your standard solution and put that in the conical flask, add a colour indicator to the conical flask and put the conical flask under the burette open the tap, and you are looking for the first colour change that lasts for approximately 10 seconds, repeat the titration until you have 3 results within .
1 of each other. In my titrations I did 4, the first result was 22. 6ml used, the second was 23. 1ml, third was 22. 7ml and the final one was 22. 8ml. Now the calculation for the molarity of the acid can be solved. The first step in working out the concentration of the unknown acid is balancing the equation. The equation for our experiment is: NaOH + HCl i NaCl + H2O and this equation is already balanced because there is 1 atom of Na on each side, 1 atom of O on each side, 2 atoms of H on either side and 1 atom of Cl on each side.
So this reaction is a 1:1 reaction. The reasons this is a 1:1 reaction can be found in the periodic table, the RMM of each side of the equation has to be the same and to work this out you need the atomic mass, Na=23, O=16, H=1 (x2) and Cl=35. The atomic mass is the larger of the two numbers on the periodic table found with an element. The total of these atomic masses is 76.
And it is exactly the same on the other side it is just that the compounds are different, this is due to the groups on the periodic table that they are in and that determines the bonds between atoms. The equation to work out the concentration of the unknown acid is: moles x 1000 ? average titration. The average titration is all the titration results added together and divided by 4, but we are going to discard the 23. 1ml result because it isn’t close enough to the other three so is recognised as an anomaly, so (22.6 + 22. 7 +22. 8)? 3 = 22. 7cm? so now using the equation you can work out the concentration of the acid. (0. 0248 x 1000)? 22. 7 = 0. 1093, the actual concentration of the acid was 0. 0984. My predicted concentration is 0. 0109 above the actual concentration this could be due to inaccuracies with the measuring of the mass of NaOH to begin with also wrongly measuring the amount of my standard solution was used to titrate the acid.
Cite this page
The Chemistry Lab Experiment. (2017, Aug 12). Retrieved from https://paperap.com/the-chemistry-lab-experiment/
"The Chemistry Lab Experiment." PaperAp.com , 12 Aug 2017, https://paperap.com/the-chemistry-lab-experiment/
PaperAp.com. (2017). The Chemistry Lab Experiment . [Online]. Available at: https://paperap.com/the-chemistry-lab-experiment/ [Accessed: 7 Jun. 2024]
"The Chemistry Lab Experiment." PaperAp.com, Aug 12, 2017. Accessed June 7, 2024. https://paperap.com/the-chemistry-lab-experiment/
"The Chemistry Lab Experiment," PaperAp.com , 12-Aug-2017. [Online]. Available: https://paperap.com/the-chemistry-lab-experiment/. [Accessed: 7-Jun-2024]
PaperAp.com. (2017). The Chemistry Lab Experiment . [Online]. Available at: https://paperap.com/the-chemistry-lab-experiment/ [Accessed: 7-Jun-2024]
- Stpm Chemistry Experiment 2 Pages: 6 (1504 words)
- Chemistry Popcorn Lab Results Pages: 2 (355 words)
- Example Of A Chemistry Lab Report About Volumetric Analysis Pages: 1 (216 words)
- Chemistry Flame Test Lab Report Pages: 2 (354 words)
- Organic Chemistry Boiling Point Lab Report Pages: 1 (247 words)
- Ap Chemistry Lab Report Pages: 4 (915 words)
- Chemistry Lab Report Pages: 2 (387 words)
- Density Lab Report Chemistry Pages: 4 (1134 words)
- Ib Chemistry Lab Report Pages: 7 (2042 words)
- Chemistry Lab Report Conductimetric Pages: 1 (273 words)

- Environment
- Information Science
- Social Issues
- Argumentative
- Cause and Effect
- Classification
- Compare and Contrast
- Descriptive
- Exemplification
- Informative
- Controversial
- Exploratory
- What Is an Essay
- Length of an Essay
- Generate Ideas
- Types of Essays
- Structuring an Essay
- Outline For Essay
- Essay Introduction
- Thesis Statement
- Body of an Essay
- Writing a Conclusion
- Essay Writing Tips
- Drafting an Essay
- Revision Process
- Fix a Broken Essay
- Format of an Essay
- Essay Examples
- Essay Checklist
- Essay Writing Service
- Pay for Research Paper
- Write My Research Paper
- Write My Essay
- Custom Essay Writing Service
- Admission Essay Writing Service
- Pay for Essay
- Academic Ghostwriting
- Write My Book Report
- Case Study Writing Service
- Dissertation Writing Service
- Coursework Writing Service
- Lab Report Writing Service
- Do My Assignment
- Buy College Papers
- Capstone Project Writing Service
- Buy Research Paper
- Custom Essays for Sale
Can’t find a perfect paper?
- Free Essay Samples
laboratory experiment
Updated 03 March 2023
Subject Biology , Scientific Method
Downloads 42
Category Literature , Science
Topic Experiment , Microbiology , Understanding
The primary goal of the lab experiment was to look into certain important processes in material science, such as recovery, recrystallization, and growth. The investigation was restricted to dislocation defects, or more precisely, line defects and grain boundaries. Heat Treatment, Metallographic Polishing, Metallographic Etching, Hardness Indentation Test, and Optical Microscopy were the five experimental procedures that were taken into consideration. Findings included the existence of a linear relationship between the material's hardness and the percentage reduction of the thickness of the material, as well as the fact that the hardness was consistent with the microstructure that was observed. Above all, the results compounded the understanding of the experiment’s main objectives that focused on measuring and comparing the hardness of the brass sample, the importance of thermal treatments in the restorative processing of materials that have been shaped by mechanical deformation, and a clear concise understanding of the detailed microstructural changes occurring during the three stages of annealing. Introduction (183) Material science delves into the processes of recovery, recrystallization, and grain growth of materials. The recovery process involves softening of the material at a lower temperature. Recrystallization, on the other hand, involves softening of the material at higher temperatures which eliminate most of the dislocations through nucleation of new and dislocation-free grains at the grain boundaries whereas growth of grains happens under higher temperatures and longer time. Engineering students find it imperative to understand the characteristics and properties of materials for purposes of advanced use observed in the ever-advancing engineering field that values and appreciates applications of technology. Within the context of this laboratory experiment, considerations are made of a metallic material that is to be work hardened followed by an investigation of the aforementioned processes at the center of material science. As such, this laboratory reports on an understating of the detailed microstructure changes that occur during the three stages of annealing, demonstrate the use of thermal treatments in the restorative processing of materials shaped by mechanical changes such as deformation, and the measure as well as a comparison of brass hardness. Experimental Procedures (383) Part I- Heat Treatment A brass metal prepared as shown in the figure below was subjected to a thermal treatment inducing a temperature of the gradient along its length. The brass sample was attached, aligned, and positioned as shown in the figure above taking into account the orientation. The brass was allowed to clear the rectangular slot in the bottom of the furnace to extend into the water bath and was quenched rapidly at the end of the annealing treatment. When brass sample was in position, power to the furnace was turned on beginning at full power ramping up to the target annealing temperature of 900°C. Once the temperature reaches the data acquisition program was started and data recorded. Decreasing water level was adjusted with a squirt bottle to avoid wetting the furnace. The temperature was held at 900°C for 15 minutes while adjusting the power source to compensate for the smaller variations. After 15 minutes, the sample was quenched by loosening the retention screw which allowed the brass to be submerged in water. The power was turned off, the furnace cooled and recording of data was stopped, and the brass sample recovered from the water bath. Part II – Metallographic Polishing The brass sample was mounted in the holding clamp and pressed lightly against the grinder in which a flat surface was obtained. The flat surface was then inspected for contours and abrasions through metallographic polishing. Part III – Metallographic Etching The sample was subjected to electro-polishing where a fine finish was achieved. The sample was then immediately washed using running water, rinsed with alcohol and then dried under hot air blower. Part IV – Hardness Indentation Test A series of Rockwell “A” hardness measurements were made at ⅛-inch intervals along the most severely worked edge of the brass sample and along the edge submerged in the water. The results were then recorded in the datasheet. Part V – Optical Microscopy The brass sample was mounted on a glass slide with clay using the specimen to level the surface. Data recorded in the datasheet for part IV, was then used in selecting five points on the edge to detect where hardness was different. A photograph of the microstructures at each of the identified points was sketched and their descriptions detailed. Results and Discussion (530) The laboratory experiment is limited to investigating dislocations and grain boundaries, the observed hardness increases as a percentage reduction of the metal’s thickness, which in turn increases the number of dislocations within the brass. Cold work provides an opportunity for the migration of the line defects or rather dislocations into configurations that orient the atoms to allow for plastic deformation. The more the brass sample is deformed, the more the number of dislocations which leads to an increase in energy. Since the dislocations occur randomly in different orientations, they meet while blocking each other’s movement, hence the metal becomes harder. The configurations that encourage acquisition of lower energy strains thus enable the metal sample to increase in hardness contrary to its former state. From the microstructure sketches, it is observable that the recrystallized grain size increases with increasing temperature. The explanation is that at a higher temperature, the dislocations move out of grains into the grain boundaries. It is at higher temperatures that recrystallization of new grains begins. The implication is that, at higher temperatures, the recrystallized grain sizes increase with an increase in temperatures since new grains start to grow larger compared to cold-worked edge. Nevertheless, recrystallization aims at increasing the grain sizes which in the end eliminates most of the dislocations by nucleating the strain-free grains. In this view, the hardness of the brass metal decreases with increasing grain sizes since a number of dislocations are made few. Fewer dislocations imply few different orientations whose movements if blocked makes the metal brass harder, thus, the hardness observed the decrease in hardness. If it is assumed that the temperature gradient is linear, then there is a temperature below which no recrystallization is observed. Recrystallization begins at higher temperatures that must be attained before the grain sizes begin to increase. In comparison to the tabulated recrystallization temperature of brass, the temperature should not be above 900°C since at 920°C the metal brass would melt. A plot of hardness against the length of the material which depicts the annealing temperature along the most deformed edge of brass shows that hardness readings are consistent with the observed microstructure. The microstructure is subdivided into five areas namely still worked area, recovery area, recrystallization area, the growth area, and cold-worked edge area. The area marked as the still cold-worked area is harder compared to other areas and is characterized with many dislocations that can readily undergo configuration and orientations upon deformation. In the recovery area, an annihilation of the brass metal allows the line defects to move. The movement is great where migration is out of the grains into the grain boundaries. The recovery area is observed to be softer. In the area marked recrystallization, small, new grains are observed to appear at the grain boundaries. In the growth area, grains that appear purple in color completely overcome the old grains that first appeared in the recrystallization area. In this area, it is the soft new grains that eat up the old grains facilitates by the presence of high thermal energy and enlarge to develop into the observed bigger grains. The fifth area is the cold worked edge. This area appears to be super-soft. Conclusion (137) In summary, the three objectives including: (1) to measure as well as compare the hardness of metallic sample, (2) to investigate the significance of the use of thermal treatments in the restorative processing of materials that have been shaped by mechanical deformation, and (3) to clearly understand the detailed microstructural changes occurring during the three stages of annealing have been achieved. Through the laboratory experiment, there has been insightful understanding as well as interpretation of the three main processes involved in material science that includes the recovery, recrystallization, and growth processes. From the experimental findings, the manner in which material hardening occurs after deformation can now be been well explained. The relationship between grain size and material strength has also been perfectly understood. Finally, various points characterizing the heat treatment process have been adequately observed and marked.
Deadline is approaching?
Wait no more. Let us write you an essay from scratch
Related Essays
Related topics.
Find Out the Cost of Your Paper
Type your email
By clicking “Submit”, you agree to our Terms of Use and Privacy policy. Sometimes you will receive account related emails.
Experimentation on Animals Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
Presenting the case, author’s rebuttal, works cited.
The debate about experimentation on animals, though well documented in literature, is still endeavoring to free itself from past controversies and current challenges.
This particular debate have attracted many advocates and critics, each advancing valid reasons as to whether it is morally, scientifically and logically right to subject animals to experimentation (Horner & Minifie 304). Experimentation on animals has indeed been very beneficial in medical fields.
However, it has been observed that animals suffer a great deal in the course of these experiments. It is against this background that this essay aims to expand on the debate about experimentation on animals with an aim to come up with a well-reasoned framework that could be used to offer direction on the appropriateness or inappropriateness of these experiments in modern times.
One of the reasons used by those who advocate for the use of animals in experiments is that these experiments progress important scientific knowledge that will in the long-term benefit humans as well as animals (Horner & Minifie 316). Indeed, supporters have for a very long time recognized the intrinsic value of conducting medical research with animals, especially in finding solutions to medical conditions that continue to affect mankind.
From a moral standpoint, advocates of using animals for biomedical research suggests that it is indeed morally wrong to permit people and animals to succumb to various forms of injuries and ailments when remedies and cures can be easily discovered through animal research (ILAR 1; Horner & Minifie 317).
However, critics of experimenting with animals argue that animals are subjected to a lot of pain and suffering in the course of coming up with scientific breakthroughs which in the long run may prove futile.
In this perspective, the critics argue that it is morally and spiritually wrong to cause pain and suffering for the benefit of mankind (Festing 569). In addition, the critics argue that universally acceptable benchmarks to adequately measure and control pain while subjecting animals to scientific experiments are non-existent.
Another reason espoused by supporters of experimenting with animals is that humans are susceptible to many of the same disease-causing organisms that affect animals. Current literature indeed demonstrates that “…humans have 65 infectious diseases in common with dogs, 50 with cattle, 46 with sheep and goats, 42 with pigs, 35 with horses, and 26 with fowl” (ILAR 4).
In addition, some communicable diseases such as rabies and malaria can be transmitted between animals and humans, not mentioning that other diseases such as hemophilia, diabetes, and epilepsy are common in both humans and animals. Animals are also vulnerable to a multiplicity of the same bacterial or viral infections as humans, such as anthrax and smallpox (ILAR 6).
Indeed, current literature reveals that some of the “…medical advances that have been dependent on the use of animals in their development include safe anesthetics, blood transfusions, penicillin and other antibiotics, vaccines against polio, measles and meningitis, and drugs to treat asthma, hypertension and leukemia” (Festing 570).
As such, advocates argue that it is imperative to use animals in biomedical experiments to have a better understanding of how these diseases evolve as well as their prevention and treatment modalities.
To expand on the above point, advocates of experimenting with animals propose that an animal is selected as an ‘animal model’ for biomedical studies only if it inherently shares similar characteristics with humans that are of relevance to the study (ILAR 6).
This, according to the advocates, should remove any pragmatic or moral concerns related to subjecting animals to the experiments for futile outcomes. Louis Pasteur, for instance, made use of dogs as an animal model for the purposes of studying rabies – a disease that is common in both humans and dogs.
His scientific experiment facilitated the development of a rabies vaccine primarily because dogs and humans can both develop rabies, not mentioning the fact that the immune systems of dogs and humans display similar reactions when exposed to the rabies vaccine (ILAR 6).
Critics, however, have argued that it serves no purpose to use animals as research subjects merely because they share the same diseases with humans (Horner & Minifie 318). On the contrary, scientists should use available knowledge on such diseases to search for treatment procedures using other non-animal or computer-generated models instead of struggling for a cure by subjecting another living creature to untold pain and suffering.
In addition, critics argue that the western, reductionist, scientific world is not necessary interested in discovering new forms of treatment through subjecting animals to biomedical research for the sake of mankind; rather, many scientists and organizations engage in animal experimentation in the pursuit of profit (Van Roten 539). This, according to the critics, is morally, legally and scientifically wrong.
The last reason advanced by proponents as to why experimentation on animals should continue is that animals pose minimal risks as compared to humans when it comes to testing the efficacy or efficiency of the scientific discoveries (Van Roten 538). This assertion goes hand in hand with the religious perspective of creation, which offers man dominion over all animal and plant species.
The argument also draws its strength from the moral paradigm that insinuates that it is not in the best interests of man to cause harm to fellow humans for the purpose of developing a treatment strategy aimed primarily at avoiding harm or destruction to penetrate through the realms of mankind.
In layman’s term, this assertion means that it serves no purpose to harm humans for the sake of coming up with a strategy aimed at preventing such harm. In consequence, animals come into the equation as the worthy alternatives not necessarily for man’s progression, but also for their own (Horner & Minifie 319). However, critics are quick to reject the notion of dominion of people over nature and animals, further stressing that animals have their own intrinsic value and rights that should be respected by all humans (Von Roten 539).
It is wrong to abandon experimenting on animals merely because critics and other animal activists argue that experimenting with animals in scientific research subjects them to a lot of pain and suffering. This is because the benefits accruing from such research not only benefit humans but also the animals that become inflicted by the same diseases that affect humans.
As much as it is known that some animals do suffer in research, the issue really should revolve around refining experimental processes aimed at curtailing animal pain and suffering through the use of proper restraint techniques, effective anesthetics, and acceptable dosing and euthanasia methodologies, among others (Horner & Minifie 319). It is important to note that animal experimentation progresses significant scientific knowledge aimed at benefiting both humans and animals.
The assertion by critics that it serves no purpose to use animals as research subjects merely because they share the same diseases with humans simply does not hold water. A world without vaccines, anesthetics and antibiotics is unimaginable, and these scientific breakthroughs came as a direct result of the interaction between scientists and animal research subjects (ILAR 6).
In addition, it should be realized that just as an individual undergo suffering when they become inflicted with diseases such as malaria or rabies, animals also do undergo a lot of suffering when they get inflicted by the same or common diseases. The best way forward, therefore, is to use the animals to come up with better treatment procedures for both animals and humans while maintaining the highest animal welfare standards to curtail suffering.
Lastly, it clearly serves no purpose for critics to equate animal rights with human rights in addition to rejecting the assertion on man’s domination over the animals (Von Roten 539). It is indeed true that animals have their own intrinsic values and rights which should of course be respected.
One of such right is that animals should not be subjected to unnecessary or avoidable pain and suffering, particularly for profit gain. But just as it is a violation of animal rights to cause pain and suffering to animals for profit gain on the part of humans, it is also morally unacceptable to let people suffer the consequences of diseases by not making use of animals in experiments aimed at developing superior treatment regimens to cure the ailments.
Claims and counterclaims have been floated in this paper in regards to the broad topic of experimentation on animals. From the discussion, it is evidently clear that the merits for undertaking animal experimentation for scientific gain, especially in-terms of developing treatments and cures for diseases that continue to affect both humans and animals, far outweighs the merits provided by critics against the practice.
The fact that animals should be treated with care, respect and dignity is unquestionable, and so is the fact that they should be used for bio-medical reasons so as to counteract the various forms of medical conditions affecting both humans and animals.
This conclusion synchronizes well with many public opinion polls that have dependably revealed that a majority of people around the world endorse the use of animals for scientific as well as medical gains (ILAR 1). However, it should be noted that such use should not cause unnecessary or avoidable pain and suffering to animals.
Festing, S. The Animal Research Debate. Political Quarterly 76.4 (2005): 568-572. Web.
Horner, J., & Minifie, F.D. Research Ethics 1: Responsible Conduct of Research (RCR) – Historical and Contemporary Issues Pertaining to Human and Animal Experimentation. Journal of Speech, Language & Hearing Research 54.1 (2011): 303-329. Web.
Institute for Laboratory Animal Research. Science, Medicine, and Animals. 2004. Web.
Von Roten, F.C. Mapping Perceptions of Animal Experimentation: Trend and Explanatory Factors. Social Science Quarterly 89.2 (2008): 537-549. Web.
- Ethics and Self-Experimentation Argument
- Louis Pasteur's Epidemiology and Vaccinations
- Laboratory Experiments on Animals: Argument Against
- The Singer Solution to World Poverty
- The Dangers of Using Cell Phone While Driving
- Save the Children (UNICEF)
- What the Founders Meant by the First Amendment?
- Performative Culture: Taiwan Pride
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2018, October 12). Experimentation on Animals. https://ivypanda.com/essays/experimentation-on-animals/
"Experimentation on Animals." IvyPanda , 12 Oct. 2018, ivypanda.com/essays/experimentation-on-animals/.
IvyPanda . (2018) 'Experimentation on Animals'. 12 October.
IvyPanda . 2018. "Experimentation on Animals." October 12, 2018. https://ivypanda.com/essays/experimentation-on-animals/.
1. IvyPanda . "Experimentation on Animals." October 12, 2018. https://ivypanda.com/essays/experimentation-on-animals/.
Bibliography
IvyPanda . "Experimentation on Animals." October 12, 2018. https://ivypanda.com/essays/experimentation-on-animals/.
Live revision! Join us for our free exam revision livestreams Watch now →
Reference Library
Collections
- See what's new
- All Resources
- Student Resources
- Assessment Resources
- Teaching Resources
- CPD Courses
- Livestreams
Study notes, videos, interactive activities and more!
Sociology news, insights and enrichment
Currated collections of free resources
Browse resources by topic
- All Sociology Resources
Resource Selections
Currated lists of resources
Exam Support
Example Answer for Question 5 Paper 1: A Level Sociology, June 2017 (AQA)
Last updated 9 Jun 2017
- Share on Facebook
- Share on Twitter
- Share by Email
Section B – Methods in Context: Q5 [20 marks]
By a field experiment, sociologists mean an experiment that is carried out within a natural social setting and which aims to control as many variables as possible (bearing in mind the importance of not artificially influencing the study). In the same way as with a laboratory experiment there is a control group and an experimental group upon whom the variables are tested. Positivist sociologists would favour the use of quantitative data as they are seeking to establish a cause-effect relationship between teacher labelling in classrooms and its effects on education. Because the data yielded from field experiments is quantitative it is seen from a theoretical perspective to be highly reliable and as such the sociologist is able to determine correlations between teacher labelling and the impact it has on the educational achievement of the pupils being labelled.
One advantage of using a field experiment to study the effects of teacher labelling (according to Item C) is the fact that it can be “conducted within a natural setting” rather than in an artificial laboratory environment. This could have a positive impact upon the validity of the study as both teachers and pupils are within a familiar social environment and it is possible for the researcher to control the variables to some extent to ensure consistency as classrooms are naturally a regulated environment. However there will still be some freedom which allows the researcher to observe natural behaviours (particularly if they carry out the experiment in a covert fashion) which allows a more valid picture of the effects of teacher labelling to be observed.
However due to the closed nature of classrooms, the researcher would need to approach a gatekeeper in order to gain access to the natural classroom setting. To help the researcher overcome issues relating to access they could approach the head teacher and gain informed consent as well as undergo a DBS check to work with children (this could be timely and costly) but this may have a negative impact on their research from an ethical perspective if they then went on to conceal their identity or the true nature of the research from either the pupils or the teachers participating as this would be an example of deception. To overcome this, the researcher could of course be honest with the participants but this raises the issue of impression management and social desirability as any participants that were aware of the presence of the researcher would possibly change their natural behaviour and would not demonstrate the true extent of labelling and the effect it has on pupils. The teacher for example would be highly aware of the need to behave in line with professional standards as otherwise their job might be at risk and so they would be unlikely to openly label students negatively in front of the researcher. Similarly according to Item C, “some pupils, teachers and parents may refuse to participate in a field experiment” – particularly if they feel intimidated by the presence of the researcher or if they have concerns about the anonymity of the study or the power held by the researcher. Students may see the researcher as part of the school hierarchy and therefore may moderate their behaviour to conform to the expectations of the school. This means the researcher is either unlikely to gain a valid picture of teacher labelling and its effect on pupils or they may have to act unethically to ensure the authenticity of their study.
There are also some significant ethical limitations of using field experiments to study teacher labelling which must be considered. Rosenthal and Jacobson (1968) famously conducted a field experiment in a Californian primary school which involved giving pupils an IQ test. Teachers were then told that this test had been used to identify the top 20% of pupils (known as the spurters due to their potential to develop) although in reality these pupils had been selected at random and not on the basis of their ability. After eight months, the pupils were re-tested and there was a significant difference between the performance of the “spurters” and the rest of the group which the sociologists put down to the impact of teacher labelling as this was the independent variable. Though this study did indeed provide extremely valid data illustrating the impact of teacher labelling on pupils’ achievement it also raised ethical issues. In the first instance, both the teachers and the pupils had experienced deception about the true nature of the research and as such had not been able to offer informed consent. There was also the issue of the psychological harm caused to the pupils which went directly against the school’s legal duty of care and also their objectives of raising achievement for all pupils. This suggests that field experiments are not a particularly useful method for a sociologist studying teacher labelling and its effect on students as they cause too many ethical objections.
However one significant advantage of the use of field experiments such as that conducted by Rosenthal and Jacobson was that it was highly reliable due to the relative simplicity of the method. As such within five years the research had been replicated numerous times and the original findings still stand. This would suggest that for a positivist sociologist the method of field experiments does in fact have its uses. However it is important to note from a practical perspective according to Item C that “field experiments tend to be small scale” due to restrictions on time and money available to conduct the research and this means they also have significant limitations in terms of representativeness and the ability to generalise about the effects of teacher labelling.
Based on the evidence however, sociologists would argue that although there are some clear advantages to using field experiments, in general the disadvantages outweigh the advantages. [~950 Words]
Please Note: These answers have been produced without the knowledge of the mark scheme and merely reflect my attempt at producing a model answer on the day of the exam.
- Methods in Context
You might also like
Teaching sociological skills: analysis, teaching sociological skills: application from an item, 100 women series.
30th October 2017
Education: Back to the Source - Rosenthal and Jacobson
Study Notes
Example Answer for Q5 Paper 1 (2019) AQA A Level Sociology
Methods in context: researching in-school factors.
Topic Videos
Research Methods: Observations
Research methods: official statistics, our subjects.
- › Criminology
- › Economics
- › Geography
- › Health & Social Care
- › Psychology
- › Sociology
- › Teaching & learning resources
- › Student revision workshops
- › Online student courses
- › CPD for teachers
- › Livestreams
- › Teaching jobs
Boston House, 214 High Street, Boston Spa, West Yorkshire, LS23 6AD Tel: 01937 848885
- › Contact us
- › Terms of use
- › Privacy & cookies
© 2002-2024 Tutor2u Limited. Company Reg no: 04489574. VAT reg no 816865400.
Essay on Science Laboratory
Science Laboratory
Science is a practical subject, teaching of which cannot be done properly only in theory form. For proper education of science, it is necessary to conduct various kinds of experimental works, which are practical in nature.
These practical functions cannot be carry out in absence of scientific apparatus and equipments. The place where various kinds of scientific apparatus and equipments are arranged in systematic manner is called science laboratory.
Science laboratory is central to scientific instructions and it forms essential component of science education.
ADVERTISEMENTS:
It is in this place that various kinds of practical works are carry out by the students. Without proper and well- equipped science laboratory, it is not possible to carry out the science teaching process effectively in any school or educational institution.
Students learn to handle various apparatus and to think independently in the laboratory, because of which it is considered to be one of an important place. When students carry out various kinds of experiments, then they draw conclusions from their studies, which raise their level of self confidence and develop scientific attitude among them.
These are considered to be main objectives of science teaching, for which it is considered by experts that without a well equipped and organised scientific laboratory, there cannot be any proper teaching of science. Students should be encouraged by the science teacher to make active parts in various experimental processes, as most of the achievements of modern science are due to the application of experimental methods.
If students get information or knowledge by playing active role in learning process then they gets permanent kind of information, because of which at school stage, practical work is considered to be more important for the students.
Although it has proven by the above discussion that science laboratories play very important functions, for which they are considered to be much important, but still need and importance of science laboratories can be explained in the following points:
i. In laboratory, it is possible to keep various scientific instruments and chemicals in safe and secure conditions, as without them, it is not possible to carry out any kind of experiment in any way.
ii. If there is proper of well equipped and properly arranged laboratory in the school, then students will get encouraged by it to take active part in the experimental processes as in such kind of laboratory, a congenial kind of atmosphere exist, which promote the interest of students in practical works.
iii. With the help of well equipped and organised laboratory, science teacher will get help in developing the scientific attitudes among the students to considerable extent.
iv. All the students have to carry out experiments collectively in the laboratory as often there is shortage of such facilities in schools. With such functions, spirit of co-operation and team work gets developed among the students and they begin to appreciate the work done by others. Not only this, through this, they also begin to appreciate the views and ideas of others, which help them in becoming successful in future life.
v. When students themselves get the opportunity to take part in experimental processes, then their area of experiences get widen and their level of intuitiveness also gets developed, as a result of which, they become people with wide mentality and open-mindedness.
Related Articles:
- What is Laboratory Method of Teaching Science?
- What are the Merits and Demerits of Laboratory Method of Teaching Science?
- What is the Importance of Practical Work in Science?
- Designing and Furnishing of Lecture cum Science Laboratory Room
Subscribe to the PwC Newsletter
Join the community, edit social preview.

Add a new code entry for this paper
Remove a code repository from this paper, mark the official implementation from paper authors, add a new evaluation result row, remove a task, add a method, remove a method, edit datasets, lola: llm-assisted online learning algorithm for content experiments.
3 Jun 2024 · Zikun Ye , Hema Yoganarasimhan , Yufeng Zheng · Edit social preview
In the rapidly evolving digital content landscape, media firms and news publishers require automated and efficient methods to enhance user engagement. This paper introduces the LLM-Assisted Online Learning Algorithm (LOLA), a novel framework that integrates Large Language Models (LLMs) with adaptive experimentation to optimize content delivery. Leveraging a large-scale dataset from Upworthy, which includes 17,681 headline A/B tests aimed at evaluating the performance of various headlines associated with the same article content, we first investigate three broad pure-LLM approaches: prompt-based methods, embedding-based classification models, and fine-tuned open-source LLMs. Our findings indicate that prompt-based approaches perform poorly, achieving no more than 65% accuracy in identifying the catchier headline among two options. In contrast, OpenAI-embedding-based classification models and fine-tuned Llama-3-8b models achieve comparable accuracy, around 82-84%, though still falling short of the performance of experimentation with sufficient traffic. We then introduce LOLA, which combines the best pure-LLM approach with the Upper Confidence Bound algorithm to adaptively allocate traffic and maximize clicks. Our numerical experiments on Upworthy data show that LOLA outperforms the standard A/B testing method (the current status quo at Upworthy), pure bandit algorithms, and pure-LLM approaches, particularly in scenarios with limited experimental traffic or numerous arms. Our approach is both scalable and broadly applicable to content experiments across a variety of digital settings where firms seek to optimize user engagement, including digital advertising and social media recommendations.
Code Edit Add Remove Mark official
Tasks edit add remove, datasets edit, results from the paper edit, methods edit add remove.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cambridge Open

The Flaws and Human Harms of Animal Experimentation
Nonhuman animal (“animal”) experimentation is typically defended by arguments that it is reliable, that animals provide sufficiently good models of human biology and diseases to yield relevant information, and that, consequently, its use provides major human health benefits. I demonstrate that a growing body of scientific literature critically assessing the validity of animal experimentation generally (and animal modeling specifically) raises important concerns about its reliability and predictive value for human outcomes and for understanding human physiology. The unreliability of animal experimentation across a wide range of areas undermines scientific arguments in favor of the practice. Additionally, I show how animal experimentation often significantly harms humans through misleading safety studies, potential abandonment of effective therapeutics, and direction of resources away from more effective testing methods. The resulting evidence suggests that the collective harms and costs to humans from animal experimentation outweigh potential benefits and that resources would be better invested in developing human-based testing methods.
Introduction
Annually, more than 115 million animals are used worldwide in experimentation or to supply the biomedical industry. 1 Nonhuman animal (hereafter “animal”) experimentation falls under two categories: basic (i.e., investigation of basic biology and human disease) and applied (i.e., drug research and development and toxicity and safety testing). Regardless of its categorization, animal experimentation is intended to inform human biology and health sciences and to promote the safety and efficacy of potential treatments. Despite its use of immense resources, the animal suffering involved, and its impact on human health, the question of animal experimentation’s efficacy has been subjected to little systematic scrutiny. 2
Although it is widely accepted that medicine should be evidence based , animal experimentation as a means of informing human health has generally not been held, in practice, to this standard. This fact makes it surprising that animal experimentation is typically viewed as the default and gold standard of preclinical testing and is generally supported without critical examination of its validity. A survey published in 2008 of anecdotal cases and statements given in support of animal experimentation demonstrates how it has not and could not be validated as a necessary step in biomedical research, and the survey casts doubt on its predictive value. 3 I show that animal experimentation is poorly predictive of human outcomes, 4 that it is unreliable across a wide category of disease areas, 5 and that existing literature demonstrates the unreliability of animal experimentation, thereby undermining scientific arguments in its favor. I further show that the collective harms that result from an unreliable practice tip the ethical scale of harms and benefits against continuation in much, if not all, of experimentation involving animals. 6
Problems of Successful Translation to Humans of Data from Animal Experimentation
Although the unreliability and limitations of animal experimentation have increasingly been acknowledged, there remains a general confidence within much of the biomedical community that they can be overcome. 7 However, three major conditions undermine this confidence and explain why animal experimentation, regardless of the disease category studied, fails to reliably inform human health: (1) the effects of the laboratory environment and other variables on study outcomes, (2) disparities between animal models of disease and human diseases, and (3) species differences in physiology and genetics. I argue for the critical importance of each of these conditions.
The Influence of Laboratory Procedures and Environments on Experimental Results
Laboratory procedures and conditions exert influences on animals’ physiology and behaviors that are difficult to control and that can ultimately impact research outcomes. Animals in laboratories are involuntarily placed in artificial environments, usually in windowless rooms, for the duration of their lives. Captivity and the common features of biomedical laboratories—such as artificial lighting, human-produced noises, and restricted housing environments—can prevent species-typical behaviors, causing distress and abnormal behaviors among animals. 8 Among the types of laboratory-generated distress is the phenomenon of contagious anxiety. 9 Cortisone levels rise in monkeys watching other monkeys being restrained for blood collection. 10 Blood pressure and heart rates elevate in rats watching other rats being decapitated. 11 Routine laboratory procedures, such as catching an animal and removing him or her from the cage, in addition to the experimental procedures, cause significant and prolonged elevations in animals’ stress markers. 12 These stress-related changes in physiological parameters caused by the laboratory procedures and environments can have significant effects on test results. 13 Stressed rats, for example, develop chronic inflammatory conditions and intestinal leakage, which add variables that can confound data. 14
A variety of conditions in the laboratory cause changes in neurochemistry, genetic expression, and nerve regeneration. 15 In one study, for example, mice were genetically altered to develop aortic defects. Yet, when the mice were housed in larger cages, those defects almost completely disappeared. 16 Providing further examples, typical noise levels in laboratories can damage blood vessels in animals, and even the type of flooring on which animals are tested in spinal cord injury experiments can affect whether a drug shows a benefit. 17
In order to control for potential confounders, some investigators have called for standardization of laboratory settings and procedures. 18 One notable effort was made by Crabbe et al. in their investigation of the potential confounding influences of the laboratory environment on six mouse behaviors that are commonly studied in neurobehavioral experiments. Despite their “extraordinary lengths to equate test apparatus, testing protocols, and all possible features of animal husbandry” across three laboratories, there were systematic differences in test results in these labs. 19 Additionally, different mouse strains varied markedly in all behavioral tests, and for some tests the magnitude of genetic differences depended on the specific testing laboratory. The results suggest that there are important influences of environmental conditions and procedures specific to individual laboratories that can be difficult—perhaps even impossible—to eliminate. These influences can confound research results and impede extrapolation to humans.
The Discordance between Human Diseases and Animal Models of Diseases
The lack of sufficient congruence between animal models and human diseases is another significant obstacle to translational reliability. Human diseases are typically artificially induced in animals, but the enormous difficulty of reproducing anything approaching the complexity of human diseases in animal models limits their usefulness. 20 Even if the design and conduct of an animal experiment are sound and standardized, the translation of its results to the clinic may fail because of disparities between the animal experimental model and the human condition. 21
Stroke research presents one salient example of the difficulties in modeling human diseases in animals. Stroke is relatively well understood in its underlying pathology. Yet accurately modeling the disease in animals has proven to be an exercise in futility. To address the inability to replicate human stroke in animals, many assert the need to use more standardized animal study design protocols. This includes the use of animals who represent both genders and wide age ranges, who have comorbidities and preexisting conditions that occur naturally in humans, and who are consequently given medications that are indicated for human patients. 22 In fact, a set of guidelines, named STAIR, was implemented by a stroke roundtable in 1999 (and updated in 2009) to standardize protocols, limit the discrepancies, and improve the applicability of animal stroke experiments to humans. 23 One of the most promising stroke treatments later to emerge was NXY-059, which proved effective in animal experiments. However, the drug failed in clinical trials, despite the fact that the set of animal experiments on this drug was considered the poster child for the new experimental standards. 24 Despite such vigorous efforts, the development of STAIR and other criteria has yet to make a recognizable impact in clinical translation. 25
Under closer scrutiny, it is not difficult to surmise why animal stroke experiments fail to successfully translate to humans even with new guidelines. Standard stroke medications will likely affect different species differently. There is little evidence to suggest that a female rat, dog, or monkey sufficiently reproduces the physiology of a human female. Perhaps most importantly, reproducing the preexisting conditions of stroke in animals proves just as difficult as reproducing stroke pathology and outcomes. For example, most animals don’t naturally develop significant atherosclerosis, a leading contributor to ischemic stroke. In order to reproduce the effects of atherosclerosis in animals, researchers clamp their blood vessels or artificially insert blood clots. These interventions, however, do not replicate the elaborate pathology of atherosclerosis and its underlying causes. Reproducing human diseases in animals requires reproducing the predisposing diseases, also a formidable challenge. The inability to reproduce the disease in animals so that it is congruent in relevant respects with human stroke has contributed to a high failure rate in drug development. More than 114 potential therapies initially tested in animals failed in human trials. 26
Further examples of repeated failures based on animal models include drug development in cancer, amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI), Alzheimer’s disease (AD), and inflammatory conditions. Animal cancer models in which tumors are artificially induced have been the basic translational model used to study key physiological and biochemical properties in cancer onset and propagation and to evaluate novel treatments. Nevertheless, significant limitations exist in the models’ ability to faithfully mirror the complex process of human carcinogenesis. 27 These limitations are evidenced by the high (among the highest of any disease category) clinical failure rate of cancer drugs. 28 Analyses of common mice ALS models demonstrate significant differences from human ALS. 29 The inability of animal ALS models to predict beneficial effects in humans with ALS is recognized. 30 More than twenty drugs have failed in clinical trials, and the only U.S. Food and Drug Administration (FDA)–approved drug to treat ALS is Riluzole, which shows notably marginal benefit on patient survival. 31 Animal models have also been unable to reproduce the complexities of human TBI. 32 In 2010, Maas et al. reported on 27 large Phase 3 clinical trials and 6 unpublished trials in TBI that all failed to show human benefit after showing benefit in animals. 33 Additionally, even after success in animals, around 172 and 150 drug development failures have been identified in the treatment of human AD 34 and inflammatory diseases, 35 respectively.
The high clinical failure rate in drug development across all disease categories is based, at least in part, on the inability to adequately model human diseases in animals and the poor predictability of animal models. 36 A notable systematic review, published in 2007, compared animal experimentation results with clinical trial findings across interventions aimed at the treatment of head injury, respiratory distress syndrome, osteoporosis, stroke, and hemorrhage. 37 The study found that the human and animal results were in accordance only half of the time. In other words, the animal experiments were no more likely than a flip of the coin to predict whether those interventions would benefit humans.
In 2004, the FDA estimated that 92 percent of drugs that pass preclinical tests, including “pivotal” animal tests, fail to proceed to the market. 38 More recent analysis suggests that, despite efforts to improve the predictability of animal testing, the failure rate has actually increased and is now closer to 96 percent. 39 The main causes of failure are lack of effectiveness and safety problems that were not predicted by animal tests. 40
Usually, when an animal model is found wanting, various reasons are proffered to explain what went wrong—poor methodology, publication bias, lack of preexisting disease and medications, wrong gender or age, and so on. These factors certainly require consideration, and recognition of each potential difference between the animal model and the human disease motivates renewed efforts to eliminate these differences. As a result, scientific progress is sometimes made by such efforts. However, the high failure rate in drug testing and development, despite attempts to improve animal testing, suggests that these efforts remain insufficient to overcome the obstacles to successful translation that are inherent to the use of animals. Too often ignored is the well-substantiated idea that these models are, for reasons summarized here, intrinsically lacking in relevance to, and thus highly unlikely to yield useful information about, human diseases. 41
Interspecies Differences in Physiology and Genetics
Ultimately, even if considerable congruence were shown between an animal model and its corresponding human disease, interspecies differences in physiology, behavior, pharmacokinetics, and genetics would significantly limit the reliability of animal studies, even after a substantial investment to improve such studies. In spinal cord injury, for example, drug testing results vary according to which species and even which strain within a species is used, because of numerous interspecies and interstrain differences in neurophysiology, anatomy, and behavior. 42 The micropathology of spinal cord injury, injury repair mechanisms, and recovery from injury varies greatly among different strains of rats and mice. A systematic review found that even among the most standardized and methodologically superior animal experiments, testing results assessing the effectiveness of methylprednisolone for spinal cord injury treatment varied considerably among species. 43 This suggests that factors inherent to the use of animals account for some of the major differences in results.
Even rats from the same strain but purchased from different suppliers produce different test results. 44 In one study, responses to 12 different behavioral measures of pain sensitivity, which are important markers of spinal cord injury, varied among 11 strains of mice, with no clear-cut patterns that allowed prediction of how each strain would respond. 45 These differences influenced how the animals responded to the injury and to experimental therapies. A drug might be shown to help one strain of mice recover but not another. Despite decades of using animal models, not a single neuroprotective agent that ameliorated spinal cord injury in animal tests has proven efficacious in clinical trials to date. 46
Further exemplifying the importance of physiological differences among species, a 2013 study reported that the mouse models used extensively to study human inflammatory diseases (in sepsis, burns, infection, and trauma) have been misleading. The study found that mice differ greatly from humans in their responses to inflammatory conditions. Mice differed from humans in what genes were turned on and off and in the timing and duration of gene expression. The mouse models even differed from one another in their responses. The investigators concluded that “our study supports higher priority to focus on the more complex human conditions rather than relying on mouse models to study human inflammatory disease.” 47 The different genetic responses between mice and humans are likely responsible, at least in part, for the high drug failure rate. The authors stated that every one of almost 150 clinical trials that tested candidate agents’ ability to block inflammatory responses in critically ill patients failed.
Wide differences have also become apparent in the regulation of the same genes, a point that is readily seen when observing differences between human and mouse livers. 48 Consistent phenotypes (observable physical or biochemical characteristics) are rarely obtained by modification of the same gene, even among different strains of mice. 49 Gene regulation can substantially differ among species and may be as important as the presence or absence of a specific gene. Despite the high degree of genome conservation, there are critical differences in the order and function of genes among species. To use an analogy: as pianos have the same keys, humans and other animals share (largely) the same genes. Where we mostly differ is in the way the genes or keys are expressed. For example, if we play the keys in a certain order, we hear Chopin; in a different order, we hear Ray Charles; and in yet a different order, it’s Jerry Lee Lewis. In other words, the same keys or genes are expressed, but their different orders result in markedly different outcomes.
Recognizing the inherent genetic differences among species as a barrier to translation, researches have expressed considerable enthusiasm for genetically modified (GM) animals, including transgenic mice models, wherein human genes are inserted into the mouse genome. However, if a human gene is expressed in mice, it will likely function differently from the way it functions in humans, being affected by physiological mechanisms that are unique in mice. For example, a crucial protein that controls blood sugar in humans is missing in mice. 50 When the human gene that makes this protein was expressed in genetically altered mice, it had the opposite effect from that in humans: it caused loss of blood sugar control in mice. Use of GM mice has failed to successfully model human diseases and to translate into clinical benefit across many disease categories. 51 Perhaps the primary reason why GM animals are unlikely to be much more successful than other animal models in translational medicine is the fact that the “humanized” or altered genes are still in nonhuman animals.
In many instances, nonhuman primates (NHPs) are used instead of mice or other animals, with the expectation that NHPs will better mimic human results. However, there have been sufficient failures in translation to undermine this optimism. For example, NHP models have failed to reproduce key features of Parkinson’s disease, both in function and in pathology. 52 Several therapies that appeared promising in both NHPs and rat models of Parkinson’s disease showed disappointing results in humans. 53 The campaign to prescribe hormone replacement therapy (HRT) in millions of women to prevent cardiovascular disease was based in large part on experiments on NHPs. HRT is now known to increase the risk of these diseases in women. 54
HIV/AIDS vaccine research using NHPs represents one of the most notable failures in animal experimentation translation. Immense resources and decades of time have been devoted to creating NHP (including chimpanzee) models of HIV. Yet all of about 90 HIV vaccines that succeeded in animals failed in humans. 55 After HIV vaccine gp120 failed in clinical trials, despite positive outcomes in chimpanzees, a BMJ article commented that important differences between NHPs and humans with HIV misled researchers, taking them down unproductive experimental paths. 56 Gp120 failed to neutralize HIV grown and tested in cell culture. However, because the serum protected chimpanzees from HIV infection, two Phase 3 clinical trials were undertaken 57 —a clear example of how expectations that NHP data are more predictive than data from other (in this case, cell culture) testing methods are unproductive and harmful. Despite the repeated failures, NHPs (though not chimpanzees or other great apes) remain widely used for HIV research.
The implicit assumption that NHP (and indeed any animal) data are reliable has also led to significant and unjustifiable human suffering. For example, clinical trial volunteers for gp120 were placed at unnecessary risk of harm because of unfounded confidence in NHP experiments. Two landmark studies involving thousands of menopausal women being treated with HRT were terminated early because of increased stroke and breast cancer risk. 58 In 2003, Elan Pharmaceuticals was forced to prematurely terminate a Phase 2 clinical trial when an investigational AD vaccine was found to cause brain swelling in human subjects. No significant adverse effects were detected in GM mice or NHPs. 59
In another example of human suffering resulting from animal experimentation, six human volunteers were injected with an immunomodulatory drug, TGN 1412, in 2006. 60 Within minutes of receiving the experimental drug, all volunteers suffered a severe adverse reaction resulting from a life-threatening cytokine storm that led to catastrophic systemic organ failure. The compound was designed to dampen the immune system, but it had the opposite effect in humans. Prior to this first human trial, TGN 1412 was tested in mice, rabbits, rats, and NHPs with no ill effects. NHPs also underwent repeat-dose toxicity studies and were given 500 times the human dose for at least four consecutive weeks. 61 None of the NHPs manifested the ill effects that humans showed almost immediately after receiving minute amounts of the test drug. Cynomolgus and rhesus monkeys were specifically chosen because their CD28 receptors demonstrated similar affinity to TGN 1412 as human CD28 receptors. Based on such data as these, it was confidently concluded that results obtained from these NHPs would most reliably predict drug responses in humans—a conclusion that proved devastatingly wrong.
As exemplified by the study of HIV/AIDS, TGN 1412, and other experiences, 62 experiments with NHPs are not necessarily any more predictive of human responses than experiments with other animals. The repeated failures in translation from studies with NHPs belie arguments favoring use of any nonhuman species to study human physiology and diseases and to test potential treatments. If experimentation using chimpanzees and other NHPs, our closest genetic cousins, are unreliable, how can we expect research using other animals to be reliable? The bottom line is that animal experiments, no matter the species used or the type of disease research undertaken, are highly unreliable—and they have too little predictive value to justify the resultant risks of harms for humans, for reasons I now explain.
The Collective Harms That Result from Misleading Animal Experiments
As medical research has explored the complexities and subtle nuances of biological systems, problems have arisen because the differences among species along these subtler biological dimensions far outweigh the similarities , as a growing body of evidence attests. These profoundly important—and often undetected—differences are likely one of the main reasons human clinical trials fail. 63
“Appreciation of differences” and “caution” about extrapolating results from animals to humans are now almost universally recommended. But, in practice, how does one take into account differences in drug metabolism, genetics, expression of diseases, anatomy, influences of laboratory environments, and species- and strain-specific physiologic mechanisms—and, in view of these differences, discern what is applicable to humans and what is not? If we cannot determine which physiological mechanisms in which species and strains of species are applicable to humans (even setting aside the complicating factors of different caging systems and types of flooring), the usefulness of the experiments must be questioned.
It has been argued that some information obtained from animal experiments is better than no information. 64 This thesis neglects how misleading information can be worse than no information from animal tests. The use of nonpredictive animal experiments can cause human suffering in at least two ways: (1) by producing misleading safety and efficacy data and (2) by causing potential abandonment of useful medical treatments and misdirecting resources away from more effective testing methods.
Humans are harmed because of misleading animal testing results. Imprecise results from animal experiments may result in clinical trials of biologically faulty or even harmful substances, thereby exposing patients to unnecessary risk and wasting scarce research resources. 65 Animal toxicity studies are poor predictors of toxic effects of drugs in humans. 66 As seen in some of the preceding examples (in particular, stroke, HRT, and TGN1412), humans have been significantly harmed because investigators were misled by the safety and efficacy profile of a new drug based on animal experiments. 67 Clinical trial volunteers are thus provided with raised hopes and a false sense of security because of a misguided confidence in efficacy and safety testing using animals.
An equal if indirect source of human suffering is the opportunity cost of abandoning promising drugs because of misleading animal tests. 68 As candidate drugs generally proceed down the development pipeline and to human testing based largely on successful results in animals 69 (i.e., positive efficacy and negative adverse effects), drugs are sometimes not further developed due to unsuccessful results in animals (i.e., negative efficacy and/or positive adverse effects). Because much pharmaceutical company preclinical data are proprietary and thus publicly unavailable, it is difficult to know the number of missed opportunities due to misleading animal experiments. However, of every 5,000–10,000 potential drugs investigated, only about 5 proceed to Phase 1 clinical trials. 70 Potential therapeutics may be abandoned because of results in animal tests that do not apply to humans. 71 Treatments that fail to work or show some adverse effect in animals because of species-specific influences may be abandoned in preclinical testing even if they may have proved effective and safe in humans if allowed to continue through the drug development pipeline.
An editorial in Nature Reviews Drug Discovery describes cases involving two drugs in which animal test results from species-specific influences could have derailed their development. In particular, it describes how tamoxifen, one of the most effective drugs for certain types of breast cancer, “would most certainly have been withdrawn from the pipeline” if its propensity to cause liver tumor in rats had been discovered in preclinical testing rather than after the drug had been on the market for years. 72 Gleevec provides another example of effective drugs that could have been abandoned based on misleading animal tests: this drug, which is used to treat chronic myelogenous leukemia (CML), showed serious adverse effects in at least five species tested, including severe liver damage in dogs. However, liver toxicity was not detected in human cell assays, and clinical trials proceeded, which confirmed the absence of significant liver toxicity in humans. 73 Fortunately for CML patients, Gleevec is a success story of predictive human-based testing. Many useful drugs that have safely been used by humans for decades, such as aspirin and penicillin, may not have been available today if the current animal testing regulatory requirements were in practice during their development. 74
A further example of near-missed opportunities is provided by experiments on animals that delayed the acceptance of cyclosporine, a drug widely and successfully used to treat autoimmune disorders and prevent organ transplant rejection. 75 Its immunosuppressive effects differed so markedly among species that researchers judged that the animal results limited any direct inferences that could be made to humans. Providing further examples, PharmaInformatic released a report describing how several blockbuster drugs, including aripiprazole (Abilify) and esomeprazole (Nexium), showed low oral bioavailability in animals. They would likely not be available on the market today if animal tests were solely relied on. Understanding the implications of its findings for drug development in general, PharmaInformatic asked, “Which other blockbuster drugs would be on the market today, if animal trials would have not been used to preselect compounds and drug-candidates for further development?” 76 These near-missed opportunities and the overall 96 percent failure rate in clinical drug testing strongly suggest the unsoundness of animal testing as a precondition of human clinical trials and provide powerful evidence for the need for a new, human-based paradigm in medical research and drug development.
In addition to potentially causing abandonment of useful treatments, use of an invalid animal disease model can lead researchers and the industry in the wrong research direction, wasting time and significant investment. 77 Repeatedly, researchers have been lured down the wrong line of investigation because of information gleaned from animal experiments that later proved to be inaccurate, irrelevant, or discordant with human biology. Some claim that we do not know which benefits animal experiments, particularly in basic research, may provide down the road. Yet human lives remain in the balance, waiting for effective therapies. Funding must be strategically invested in the research areas that offer the most promise.
The opportunity costs of continuing to fund unreliable animal tests may impede development of more accurate testing methods. Human organs grown in the lab, human organs on a chip, cognitive computing technologies, 3D printing of human living tissues, and the Human Toxome Project are examples of new human-based technologies that are garnering widespread enthusiasm. The benefit of using these testing methods in the preclinical setting over animal experiments is that they are based on human biology. Thus their use eliminates much of the guesswork required when attempting to extrapolate physiological data from other species to humans. Additionally, these tests offer whole-systems biology, in contrast to traditional in vitro techniques. Although they are gaining momentum, these human-based tests are still in their relative infancy, and funding must be prioritized for their further development. The recent advancements made in the development of more predictive, human-based systems and biological approaches in chemical toxicological testing are an example of how newer and improved tests have been developed because of a shift in prioritization. 78 Apart from toxicology, though, financial investment in the development of human-based technologies generally falls far short of investment in animal experimentation. 79
The unreliability of applying animal experimental results to human biology and diseases is increasingly recognized. Animals are in many respects biologically and psychologically similar to humans, perhaps most notably in the shared characteristics of pain, fear, and suffering. 80 In contrast, evidence demonstrates that critically important physiological and genetic differences between humans and other animals can invalidate the use of animals to study human diseases, treatments, pharmaceuticals, and the like. In significant measure, animal models specifically, and animal experimentation generally, are inadequate bases for predicting clinical outcomes in human beings in the great bulk of biomedical science. As a result, humans can be subject to significant and avoidable harm.
The data showing the unreliability of animal experimentation and the resultant harms to humans (and nonhumans) undermine long-standing claims that animal experimentation is necessary to enhance human health and therefore ethically justified. Rather, they demonstrate that animal experimentation poses significant costs and harms to human beings. It is possible—as I have argued elsewhere—that animal research is more costly and harmful, on the whole, than it is beneficial to human health. 81 When considering the ethical justifiability of animal experiments, we should ask if it is ethically acceptable to deprive humans of resources, opportunity, hope, and even their lives by seeking answers in what may be the wrong place. In my view, it would be better to direct resources away from animal experimentation and into developing more accurate, human-based technologies.
Aysha Akhtar , M.D., M.P.H., is a neurologist and preventive medicine specialist and Fellow at the Oxford Centre for Animal Ethics, Oxford, United Kingdom.
1. Taylor K, Gordon N, Langley G, Higgins W. Estimates for worldwide laboratory animal use in 2005 . Alternatives to Laboratory Animals 2008; 36 :327–42. [ PubMed ] [ Google Scholar ]
2. Systematic reviews that have been conducted generally reveal the unreliability and poor predictability of animal tests. See Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007;334:197. See also Pound P, Bracken MB. Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 2014;348:g3387. See Godlee F. How predictive and productive is animal research? BMJ 2014;348:g3719. See Benatar M. Lost in translation: Treatment trials in the SOD 1 mouse and in human ALS. Neurobiology Disease 2007; 26 :1–13 [ PubMed ] [ Google Scholar ] . And see Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: A systematic review of methylprednisolone . Alternatives to Laboratory Animals 2009; 37 :43–62. [ PubMed ] [ Google Scholar ]
3. Mathews RAJ. Medical progress depends on animal models—doesn’t it? Journal of the Royal Society of Medicine 2008; 101 :95–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
4. See Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philosophy, Ethics, and Humanities in Medicine 2009; 4 :2 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Wall RJ, Shani M. Are animal models as good as we think? Theriogenology 2008; 69 :2–9. [ PubMed ] [ Google Scholar ]
5. See note 3, Mathews 2008. See also Hartung T, Zurlo J. Food for thought… alternative approaches for medical countermeasures to biological and chemical terrorism and warfare . ALTEX 2012; 29 :251–60 [ PubMed ] [ Google Scholar ] . See Leist M, Hartung T. Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice . Archives in Toxicology 2013; 87 :563–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See Mak IWY, Evaniew N, Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment . American Journal in Translational Research 2014; 6 :114–18 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see Pippin J. Animal research in medical sciences: Seeking a convergence of science, medicine, and animal law . South Texas Law Review 2013; 54 :469–511. [ Google Scholar ]
6. For an overview of the harms-versus-benefits argument, see LaFollette H. Animal experimentation in biomedical research In: Beauchamp TL, Frey RG, eds. The Oxford Handbook of Animal Ethics . Oxford: Oxford University Press; 2011:812–18. [ Google Scholar ]
7. See Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases . Nature Medicine 2010; 16 :1210–14 [ PubMed ] [ Google Scholar ] . See Institute of Medicine. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. Washington, DC: The National Academies Press; 2013. And see Degryse AL, Lawson WE. Progress towards improving animal models for IPF . American Journal of Medical Science 2011; 341 :444–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
8. See Morgan KN, Tromborg CT. Sources of stress in captivity . Applied Animal Behaviour Science 2007; 102 :262–302 [ Google Scholar ] . See Hart PC, Bergner CL, Dufour BD, Smolinsky AN, Egan RJ, LaPorte L, et al. Analysis of abnormal repetitive behaviors in experimental animal models In Warrick JE, Kauleff AV, eds. Translational Neuroscience and Its Advancement of Animal Research Ethics . New York: Nova Science; 2009:71–82 [ Google Scholar ] . See Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience . American Journal of Primatology 2003; 60 :1–15 [ PubMed ] [ Google Scholar ] . And see Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress . Contemporary Topics in Laboratory Animal Science 2004; 43 :42–51. [ PubMed ] [ Google Scholar ]
9. Suckow MA, Weisbroth SH, Franklin CL. The Laboratory Rat . 2nd ed. Burlington, MA: Elsevier Academic Press; 2006, at 323.
10. Flow BL, Jaques JT. Effect of room arrangement and blood sample collection sequence on serum thyroid hormone and cortisol concentrations in cynomolgus macaques ( Macaca fascicularis ). Contemporary Topics in Laboratory Animal Science 1997;36:65–8.
11. See note 8, Balcombe et al. 2004.
12. See note 8, Balcombe et al. 2004.
13. Baldwin A, Bekoff M. Too stressed to work. New Scientist 2007;194:24.
14. See note 13, Baldwin, Bekoff 2007.
15. Akhtar A, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: A review . Reviews in the Neurosciences 2008; 19 :47–60. [ PubMed ] [ Google Scholar ]
16. See note 13, Baldwin, Bekoff 2007.
17. See note 15, Akhtar et al. 2008.
18. See Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias . Stroke 2004; 35 :1203–8 [ PubMed ] [ Google Scholar ] . See also O’ Neil BJ, Kline JA, Burkhart K, Younger J. Research fundamentals: V. The use of laboratory animal models in research. Academic Emergency Medicine 1999;6:75–82.
19. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment . Science 1999; 284 :1670–2, at 1670. [ PubMed ] [ Google Scholar ]
20. See Curry SH. Why have so many drugs with stellar results in laboratory stroke models failed in clinical trials? A theory based on allometric relationships. Annals of the New York Academy of Sciences 2003;993:69–74. See also Dirnagl U. Bench to bedside: The quest for quality in experimental stroke research . Journal of Cerebral Blood Flow & Metabolism 2006; 26 :1465–78 [ PubMed ] [ Google Scholar ] .
21. van der Worp HB, Howells DW, Sena ES, Poritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Medicine 2010; 7 :e1000245. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
22. See note 20, Dirnagl 2006. See also Sena E, van der Worp B, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends in Neurosciences 2007; 30 :433–9. [ PubMed ] [ Google Scholar ]
23. See Gawrylewski A. The trouble with animal models: Why did human trials fail? The Scientist 2007;21:44. See also Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations . Stroke 2009; 40 :2244–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
24. See note 23, Gawrylewski 2007. There is some dispute as to how vigorously investigators adhered to the suggested criteria. Nevertheless, NXY-059 animal studies were considered an example of preclinical studies that most faithfully adhered to the STAIR criteria. For further discussion see also Wang MM, Guohua X, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Translational Stroke Research 2013; 4 :3–14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
25. See note 24, Wang et al. 2013.
26. O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke . Annals of Neurology 2006; 59 :467–7 [ PubMed ] [ Google Scholar ] .
27. See note 5, Mak et al. 2014.
28. See note 5, Mak et al. 2014.
29. See Perrin S. Preclinical research: Make mouse studies work . Nature 2014; 507 :423–5 [ PubMed ] [ Google Scholar ] . See also, generally, Wilkins HM, Bouchard RJ, Lorenzon NM, Linseman DA. Poor correlation between drug efficacies in the mutant SOD1 mouse mode versus clinical trials of ALS necessitates the development of novel animal models for sporadic motor neuron disease. In: Costa A, Villalba E, eds. Horizons in Neuroscience Research. Vol. 5. Hauppauge, NY: Nova Science; 2011:1–39.
30. Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: A systematic assessment . Neurology 2006; 67 :20–7 [ PubMed ] [ Google Scholar ] .
31. Sinha G. Another blow for ALS . Nature Biotechnology 2013; 31 :185 [ Google Scholar ] . See also note 30, Traynor et al. 2006.
32. See Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, et al. Experimental models of traumatic brain injury: Do we really need a better mousetrap? Neuroscience 2005; 136 :971–89 [ PubMed ] [ Google Scholar ] . See also Xiong YE, Mahmood A, Chopp M. Animal models of traumatic brain injury . Nature Reviews Neuroscience 2013; 14 :128–42 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see commentary by Farber: Farber N. Drug development in brain injury. International Brain Injury Association ; available at http://www.internationalbrain.org/articles/drug-development-in-traumatic-brain-injury/ (last accessed 7 Dec 2014).
33. Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments . Neurotherapeutics 2010; 7 :115–26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
34. Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development in Alzheimer’s disease: An appraisal from 1984 to 2014 . Journal of Internal Medicine 2014; 275 :251–83 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
35. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases . Proceedings of the National Academy of Sciences USA 2013; 110 :3507–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
36. Palfreyman MG, Charles V, Blander J. The importance of using human-based models in gene and drug discovery . Drug Discovery World 2002. Fall:33–40 [ Google Scholar ] .
37. See note 2, Perel et al. 2007.
38. Harding A. More compounds failing phase I. The Scientist 2004 Sept 13; available at http://www.the-scientist.com/?articles.view/articleNo/23003/title/More-compounds-failing-Phase-I/ (last accessed 2 June 2014).
39. See note 5, Pippin 2013.
40. See note 5, Hartung, Zurlo 2012.
41. Wiebers DO, Adams HP, Whisnant JP. Animal models of stroke: Are they relevant to human disease? Stroke 1990; 21 :1–3. [ PubMed ] [ Google Scholar ]
42. See note 15, Akhtar et al. 2008.
43. See note 2, Akhtar et al. 2009.
44. Lonjon N, Prieto M, Haton H, Brøchner CB, Bauchet L, Costalat V, et al. Minimum information about animal experiments: Supplier is also important . Journal of Neuroscience Research 2009; 87 :403–7. [ PubMed ] [ Google Scholar ]
45. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception . Pain 1999; 80 :67–82. [ PubMed ] [ Google Scholar ]
46. Tator H, Hashimoto R, Raich A, Norvell D, Fehling MG, Harrop JS, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury . Journal of Neurosurgery: Spine 2012; 17 :157–229. [ PubMed ] [ Google Scholar ]
47. See note 35, Seok et al. 2013, at 3507.
48. Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse . Nature Genetics 2007; 39 :730–2 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
49. Horrobin DF. Modern biomedical research: An internally self-consistent universe with little contact with medical reality? Nature Reviews Drug Discovery 2003; 2 :151–4. [ PubMed ] [ Google Scholar ]
50. Vassilopoulous S, Esk C, Hoshino S, Funke BH, Chen CY, Plocik AM, et al. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism . Science 2009; 324 :1192–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
51. See Guttman-Yassky E, Krueger JG. Psoriasis: Evolution of pathogenic concepts and new therapies through phases of translational research . British Journal of Dermatology 2007; 157 :1103–15 [ PubMed ] [ Google Scholar ] . See also The mouse model: Less than perfect, still invaluable. Johns Hopkins Medicine ; available at http://www.hopkinsmedicine.org/institute_basic_biomedical_sciences/news_events/articles_and_stories/model_organisms/201010_mouse_model.html (last accessed 10 Dec 2014). See note 23, Gawrylewski 2007. See note 2, Benatar 2007. See note 29, Perrin 2014 and Wilkins et al. 2011. See Cavanaugh S, Pippin J, Barnard N. Animal models of Alzheimer disease: Historical pitfalls and a path forward. ALTEX online first; 2014 Apr 10. And see Woodroofe A, Coleman RA. ServiceNote: Human tissue research for drug discovery . Genetic Engineering and Biotechnology News 2007; 27 :18 [ Google Scholar ] .
52. Lane E, Dunnett S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: How close are we to the clinic? Psychopharmacology 2008; 199 :303–12. [ PubMed ] [ Google Scholar ]
53. See note 52, Lane, Dunnett 2008.
54. See note 5, Pippin 2013.
55. Bailey J. An assessment of the role of chimpanzees in AIDS vaccine research . Alternatives to Laboratory Animals 2008; 36 :381–428. [ PubMed ] [ Google Scholar ]
56. Tonks A. The quest for the AIDs vaccine . BMJ 2007; 334 :1346–8 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
57. Johnston MI, Fauci AS. An HIV vaccine—evolving concepts . New England Journal of Medicine 2007; 356 :2073–81. [ PubMed ] [ Google Scholar ]
58. See Rossouw JE, Andersen GL, Prentice RL, LaCroix AZ, Kooperberf C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy menopausal women: Principle results from the Women’s Health Initiative randomized controlled trial . JAMA 2002; 288 :321–33 [ PubMed ] [ Google Scholar ] . See also Andersen GL, Limacher A, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial . JAMA 2004; 291 :1701–12 [ PubMed ] [ Google Scholar ] .
59. Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine . Progress in Brain Research 2009; 175 :83. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
60. Allen A. Of mice and men: The problems with animal testing. Slate 2006 June 1; available at http://www.slate.com/articles/health_and_science/medical_examiner/2006/06/of_mice_or_men.html (last accessed 10 Dec 2014).
61. Attarwala H. TGN1412: From discovery to disaster . Journal of Young Pharmacists 2010; 2 :332–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
62. See Hogan RJ. Are nonhuman primates good models for SARS? PLoS Medicine 2006; 3 :1656–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Bailey J. Non-human primates in medical research and drug development: A critical review . Biogenic Amines 2005; 19 :235–55. [ Google Scholar ]
63. See note 4, Wall, Shani 2008.
64. Lemon R, Dunnett SB. Surveying the literature from animal experiments: Critical reviews may be helpful—not systematic ones . BMJ 2005; 330 :977–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
65. Roberts I, Kwan I, Evans P, Haig S. Does animal experimentation inform human health care? Observations from a systematic review of international animal experiments on fluid resuscitation . BMJ 2002; 324 :474–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
66. See note 60,Allen 2006. See also Heywood R. Target organ toxicity . Toxicology Letters 1981; 8 :349–58 [ PubMed ] [ Google Scholar ] . See Fletcher AP. Drug safety tests and subsequent clinical experience . Journal of the Royal Society of Medicine 1978; 71 :693–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
67. See note 60, Allen 2006. See note 5, Pippin 2013. See also Greek R, Greek J. Animal research and human disease . JAMA 2000; 283 :743–4 [ PubMed ] [ Google Scholar ] .
68. See note 60, Allen 2006. See also note 5, Leist, Hartung 2013.
69. Food and Drug Administration. Development & approval process (drugs); available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ (last accessed 7 Dec 2014). See also http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm (last accessed 7 Dec 2014).
70. Drug discovery pipeline. IRSF; available at http://www.rettsyndrome.org/research-programs/programmatic-overview/drug-discovery-pipeline (last accessed 24 Sept 2014).
71. See note 60, Allen 2006.
72. Follow the yellow brick road. Nature Reviews Drug Discovery 2003;2:167, at 167.
73. See note 5, Pippin 2013.
74. For data on aspirin, see Hartung T. Per aspirin as astra … Alternatives to Laboratory Animals 2009; 37 (Suppl 2 ):45–7 [ PubMed ] [ Google Scholar ] . See also note 5, Pippin 2013. For data on penicillin, see Koppanyi T, Avery MA. Species differences and the clinical trial of new drugs: A review . Clinical Pharmacology and Therapeutics 1966; 7 :250–70 [ PubMed ] [ Google Scholar ] . See also Schneierson SS, Perlman E. Toxicity of penicillin for the Syrian hamster . Proceedings of the Society for Experimental Biology and Medicine 1956; 91 :229–30. [ PubMed ] [ Google Scholar ]
75. See note 67, Greek, Greek 2000.
76. Oral bioavailability of blockbuster drugs in humans and animals. PharmaInformatic . available at http://www.pharmainformatic.com/html/blockbuster_drugs.html (last accessed 19 Sept 2014).
77. Sams-Dodd F. Strategies to optimize the validity of disease models in the drug discovery process . Drug Discovery Today 2006; 11 :355–63 [ PubMed ] [ Google Scholar ] .
78. Zurlo J. No animals harmed: Toward a paradigm shift in toxicity testing . Hastings Center Report 2012;42. Suppl:s23–6 [ PubMed ] [ Google Scholar ] .
79. There is no direct analysis of the amount of money spent on animal testing versus alternatives across all categories; however, in 2008 the Chronicle of Higher Education reported that funding of research involving animals (under basic research) of the National Institute of Health (NIH) remained steady at about 42 percent since 1990. See Monastersky R. Protesters fail to slow animal research. Chronicle of Higher Education 2008:54. In 2012, NIH director Francis Collins noted that the NIH’s support for basic research has held steady at 54 percent of the agency’s budget for decades. The remainder of the NIH’s budget is heavily funded toward clinical research, suggesting that preclinical human-based testing methods are much less funded. See also Wadman M. NIH director grilled over translational research centre. Nature News Blog 2012 Mar 20. Available at http://blogs.nature.com/news/2012/03/nih-director-grilled-over-translational-research-center.html (last accessed 5 Mar 2015). There is no data that suggests that the NIH’s funding of animal experimentation has decreased. A 2010 analysis estimates that at least 50 percent of the NIH’s extramural funding is directed into animal research; see Greek R, Greek J. Is the use of sentient animals in basic research justifiable? Philosophy, Ethics, and Humanities in Medicine 2010; 5 :14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
80. For a helpful discussion on animal pain, fear, and suffering, see DeGrazia D. Taking Animals Seriously: Mental Lives and Moral Status . New York: Cambridge University Press; 1996:116–23. [ Google Scholar ]
81. See Akhtar A. Animals and Public Health: Why Treating Animals Better Is Critical to Human Welfare . Hampshire, UK: Palgrave Macmillan; 2012:chap. 5.

IMAGES
VIDEO
COMMENTS
Introduction. Your lab report introduction should set the scene for your experiment. One way to write your introduction is with a funnel (an inverted triangle) structure: Start with the broad, general research topic. Narrow your topic down your specific study focus. End with a clear research question.
Experimental reports (also known as "lab reports") are reports of empirical research conducted by their authors. You should think of an experimental report as a "story" of your research in which you lead your readers through your experiment. As you are telling this story, you are crafting an argument about both the validity and reliability of ...
A lab report is broken down into eight sections: title, abstract, introduction, methods and materials, results, discussion, conclusion, and references. Title. The title of the lab report should be descriptive of the experiment and reflect what the experiment analyzed. Ex: "Determining the Free Chlorine Content of Pool Water" Abstract
After you complete a science experiment you will need to gather all your data and write an essay explaining what you discovered. Your instructor may require a specific number of pages or a minimum word count, so the length of your essay will depend on assignment details. ... 1 Purdue Online Writing Lab: Essay Writing ; 2 Yale College ...
Prepare a 3% NaCl solution by dissolving 30 g of NaCl in 1000 mL of deionized water. Pour 250 mL of the 3% NaCl solution into each beaker. Submerge two samples of each aluminum alloy in separate beakers containing the NaCl solution. Use the stirring rods to stir the solutions gently to ensure uniformity.
Tips on Writing Laboratory Report Essay. Students who take science degrees will have to take a few lab courses where they are asked to do an experiment and write a research report to demonstrate their understanding. In the research report, students must explain about the experiment and describe whether their findings agree to the hypothesis.
Lab report essay is more than just recording the procedure and results of the laboratory experiment. You must demonstrate your understanding on the subject by discussing on the experiment in terms of the theoretical issues that you are studying in the course. Lab report essay accounts for a substantial amount of points in your course up to 25%.
Well, it was quite daunting a time for me, BUT—I have to admit that I learned a lot from messing up my project. Looking back, the nine months I spent on my EE was indeed an endurance experience. Here are my takeaways from almost failing my Biology EE. Some of them might be relevant to other EE subjects, but they mainly revolve around the ...
ELEC 2210 Experiment 1 (Rev. 05/18/2020) 1 ELEC 2210 - EXPERIMENT 1 Basic Digital Logic Circuits The objectives of this experiment: ... The experiments in this lab course will be performed on NI myDAQ, pictured in Figure 1, which is a low-cost portable data acquisition (DAQ) device for experimentation with linear, electronic, ...
conventional econometric techniques. Lab experiments provide the investigator with a means to. directly influence the set of prices, budget sets, information set, and actions available to actors, and thus measure the impact of these factors on behavior within the context of the laboratory.1.
Writing the Experimental Report: Methods, Results, and Discussion. Tables, Appendices, Footnotes and Endnotes. References and Sources for More Information. APA Sample Paper: Experimental Psychology. Style Guide Overview MLA Guide APA Guide Chicago Guide OWL Exercises. Purdue OWL. Subject-Specific Writing.
Although the results obtained from this set-up are less accurate than commercial devices, the experiment still demonstrates the Beer-Lambert law very well. In the lab, students' working memories can become overloaded due to the pressures of time, having to follow instructions and recall theory. By stripping spectrophotometry and the Beer ...
Lab Report On The Lab. buffer, pestle grinder, centrifuge, phenol-chloroform, a vortex, agarose gel and dye, PCR water, 1.5 mL tubes and RNase. PCR Amplication- This lab involves the amplification of the 18s rRNA or actin genes. The lab starts with getting the PCR tube, labeled as DNA. Then, 2.5 microliters of DNA template from the week before ...
The equation for our experiment is: NaOH + HCl i NaCl + H2O and this equation is already balanced because there is 1 atom of Na on each side, 1 atom of O on each side, 2 atoms of H on either side and 1 atom of Cl on each side. So this reaction is a 1:1 reaction. The reasons this is a 1:1 reaction can be found in the periodic table, the RMM of ...
The laboratory experiment is limited to investigating dislocations and grain boundaries, the observed hardness increases as a percentage reduction of the metal's thickness, which in turn increases the number of dislocations within the brass. ... On our website, students and learners can find detailed writing guides, free essay samples, fresh ...
The debate about experimentation on animals, though well documented in literature, is still endeavoring to free itself from past controversies and current challenges. We will write a custom essay on your topic. This particular debate have attracted many advocates and critics, each advancing valid reasons as to whether it is morally ...
In the same way as with a laboratory experiment there is a control group and an experimental group upon whom the variables are tested. Positivist sociologists would favour the use of quantitative data as they are seeking to establish a cause-effect relationship between teacher labelling in classrooms and its effects on education. Because the ...
Lab safety is very important if you are going to work in a science lab. The two things to always remember when working in a lab is safety and that lab is a privilege. The Lab is a privilege each student is lucky to have, without it the students would not be able to conduct experiments in the laboratory. One of the most important reasons why ...
Science Laboratory. Science is a practical subject, teaching of which cannot be done properly only in theory form. For proper education of science, it is necessary to conduct various kinds of experimental works, which are practical in nature. These practical functions cannot be carry out in absence of scientific apparatus and equipments.
870 Words2 Pages. Recommended: My laboratory experience. INTRODUCTION TO LAB The laboratory experience gives you the opportunity of seeing, touching, smelling, and hearing (but not tasting!) the structures and materials you learn about in class. What you do in the lab helps you correlate the text and lecture with study of real organisms.
For any lab report, use a professional font and size. For example, 12-point Times New Roman. Double-space the report. Include a page number, usually either in the top or bottom right corner of each page. Clearly separate specific sections of the report with headings and subheadings.
In the rapidly evolving digital content landscape, media firms and news publishers require automated and efficient methods to enhance user engagement. This paper introduces the LLM-Assisted Online Learning Algorithm (LOLA), a novel framework that integrates Large Language Models (LLMs) with adaptive experimentation to optimize content delivery.
Our numerical experiments on Upworthy data show that LOLA outperforms the standard A/B testing method (the current status quo at Upworthy), pure bandit algorithms, and pure-LLM approaches, particularly in scenarios with limited experimental traffic or numerous arms. ... PAPERS. 696. This Journal is curated by: Lucian A. Bebchuk at Harvard Law ...
The use of nonpredictive animal experiments can cause human suffering in at least two ways: (1) by producing misleading safety and efficacy data and (2) by causing potential abandonment of useful medical treatments and misdirecting resources away from more effective testing methods.