- Sign In to save searches and organize your favorite content.
- Not registered? Sign up

Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Patient Case Studies and Panel Discussion: Leukemia – Rare and Emerging Subtypes
- Get Citation Alerts
- Download PDF to Print
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
- Patient Case Study 1
In the first case study, a 77-year-old woman presented with multiple nodular lesions and plaques on her face, chest, and back. She had a history of type 2 diabetes, stage 3 hypertension, hyperlipidemia, coronary heart disease, cerebral infarction, glaucoma, lens extracapsular extraction and posterior chamber intraocular lens implantation, Sjögren syndrome, rheumatoid arthritis, and left axillary vein and brachial vein thrombosis.
She had previously received a conventional therapy of Chinese medicine, but her condition did not improve. Her clinicians performed a bone marrow biopsy and an aspiration biopsy of a nodule on the right side of her face, and immunostaining results revealed the following immunophenotype: CD4+, CD123+, CD43+, CD56+, with Ki-67 level of 30% to 40%.
The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm, which is a rare blood cancer in the myeloid malignancies family. Andrew D. Zelenetz, MD, PhD, Memorial Sloan Kettering Cancer Center, noted that this disease used to be classified as a variant of acute lymphoblastic leukemia (ALL) and has a distinctive immunophenotype and clinical appearance, characterized by purple skin lesions.
He said a helpful tool for remembering the immunophenotype of this disease is to think “123456”: CD123, CD4, and CD56. Conversely, Nitin Jain, MD, The University of Texas MD Anderson Cancer Center, noted that although this rule of thumb can be helpful, it is important to keep in mind that approximately 10% of patients with this malignancy are actually CD56-negative.
Daniel A. Pollyea, MD, MS, University of Colorado Cancer Center, emphasized the unique phenotypic expression pattern in this malignancy, and the risk of cytopenias due to bone marrow involvement. “Certainly there are patients with bone marrow involvement who don't have cytopenias and have predominant expression of these skin manifestations,” he said. “But I think the CD123 is really the key, because this is a very, very difficult diagnosis to make, and that can be the linchpin.” He added that CD123 expression status is important to know not only for diagnostic purposes but also from a therapeutic perspective. However, many clinical pathologists do not possess the capabilities to test for CD123, so if a diagnosis of blastic plasmacytoid dendritic cell neoplasm is even being entertained, a discussion with a pathologist regarding testing for CD123 is critical.
The nodule on the right side of the patient’s face was surgically excised, and she was treated with gemcitabine, nedaplatin (a second-generation platinum drug used in China that is not approved by the FDA; it is similar to carboplatin and cisplatin), and bleomycin. The patient experienced an initial response to therapy but subsequently developed additional nodular lesions on her arm.
According to Dr. Pollyea, regardless of what transpired with this particular patient, surgical resection of skin lesions did not have a role in this case. “Typically, if the disease is going to respond, the skin lesions are very, very sensitive,” he said. “So there are issues with wound healing if you perform a large resection.”
The panel then discussed tagraxofusp-erzs, a recently approved drug for the treatment of this disorder that has been shown to be highly effective. 1 Dr. Pollyea noted that the mechanism of action of this drug is “quite brilliant.”
“You're taking one of nature's most potent toxins and delivering it directly to a cell population of critical importance in this disease, and potentially the precursor or primitive population of the disease,” he said.
A trial of tagraxofusp treatment in patients with blastic plasmacytoid dendritic cell neoplasms led to durable responses and high complete response rates, particularly in the first-line setting (72%). 1 In relapsed/refractory disease, it was less effective, but “still very effective,” according to Dr. Zelenetz, with a complete response rate of 38%. However, significant toxicity was seen, with capillary leak syndrome a fatal toxicity.
Jae Park, MD, Memorial Sloan Kettering Cancer Center, noted that because of the limited clinical experience with this agent, it is critical to administer the drug in an inpatient setting whenever possible and to closely monitor any patient-related physical changes, including weight fluctuations, kidney function, and respiratory status.
William G. Wierda, MD, PhD, The University of Texas MD Anderson Cancer Center, agreed, adding that he actually treated patients with this compound on a clinical trial before its approval. “During the trial, we were closely monitoring daily weight, albumin, and [liver function], and making daily adjustments in dosing based on what was happening with patients clinically,” he said. “So it's important to be very familiar with the prescribing information.”
Given this particular patient’s age, history, and comorbidities, stem cell transplantation was not an option. However, according to Dr. Park, allotransplant should be considered in these cases whenever possible, and earlier rather than later. “Even with a good response, it becomes difficult to continue this regimen,” he said. “And after [patients] relapse, there are very few treatment options available.”
- Patient Case Study 2
A 28-year-old woman presented with fatigue and lymphadenopathy. Her initial WBC count was 11.1 k/uL with 40% blasts, and she showed hypercellular bone marrow. Her immunophenotype included the following: 88.0% CD45+/–, CD34+, CD19+, CD10+ (variable), CD20– (∼4% of cells stain), sCD22+, CD13–, CD33–, CD38+, CD56–, CD2+/–, CD3–, CD4–, CD8–, CD7–, CD5–, CD117, HLA-DR+, sIg light chain–, cCD79a+, cCD22+, MPO–, cIgM+, and TdT+. After noting the complexity of the patient’s immunophenotype, Dr. Pollyea emphasized the importance of working with a skilled hematopathologist in cases such as this.
The patient was diagnosed with B-cell ALL and treated with the CALGB 10403 regimen. 2 At day 30, bone marrow biopsy showed residual disease with 16% blasts by flow. As her next course of treatment, the patient received blinatumomab for one cycle.
Dr. Jain agreed that this was a reasonable next step, but added that an additional cycle of chemotherapy would also have been feasible. Although the patient was high-risk, he would not yet say treatment had failed after only one treatment cycle.
“I think on the adult side we have to take our cues from the pediatricians who have been so incredibly successful with this disease,” said Dr. Pollyea. “And CALGB 10403 is a regimen that attempts to apply the pediatric regimens to an adolescent/young adult population.” 2
He added that pediatricians tend to stick to protocol, and the protocol for this particular regimen allows for a more extended induction period. “So at this point you should have a lot of concerns about this patient, but I think the protocol allows you to continue.”
About 4 weeks after starting blinatumomab, the patient experienced complete remission confirmed by bone marrow biopsy. She also received 6 cycles of intrathecal chemotherapy throughout the course of her treatment and showed no evidence of central nervous system involvement.
A month later, she presented with enlarged lymph nodes in her groin and neck, and bone marrow biopsy confirmed 63% blasts with an ALL phenotype. A same-day inguinal lymph node biopsy was consistent with lymphoblastic leukemia involvement.
Although the patient experienced a complete remission initially, Dr. Park noted that minimal residual disease (MRD) status was never confirmed. This factor is critical in assessing a patient’s depth of remission, and MRD-positive patients should receive additional therapy sooner rather than later to get to MRD-negative status, he said.
Dr. Jain said that additional diagnostic testing in the form of RNA sequencing would be appropriate in this case, but noted a caveat of the limited availability of this type of testing. The patient underwent next-generation sequencing (NGS), which revealed the following: DIAPH1-PDGFRB fusion; CDKN2A/B - p14 ARF loss exon 1 and CDKN2b loss; PIK3R1 splice site 1746-2A>6; and TP53 N288fs*60.
According to Dr. Park, interpreting NGS data can be difficult, and misinterpretation can lead to the wrong choice of treatment. This again underlines the importance of consulting with a skilled pathologist or other experienced ALL expert to assist in interpreting mutation profiles.
The patient was determined to have Ph-like ALL (a newly recognized entity of Ph-negative ALL with a poor prognosis) and was enrolled in the KTE-CA19 CAR-T (axicabtagene ciloleucel [axi-cel]) trial ( ClinicalTrials.gov identifier: NCT02614066). She received cytoreductive chemotherapy with hyperCVAD part A before apheresis for CAR-T generation, and experienced favorable cytoreduction (she received fludarabine/cyclophosphamide for lymphodepletion). She then received a post–CAR-T infusion and showed no response; her blast count increased from 0.42 to 80.35 within a week.
“This is just a tough case,” said Dr. Park, noting the unusually refractory nature of the disease. “Initial response rates to CAR-T cell therapy are approximately 80%, so she’s already in the very unlucky 20% of cases,” he said.
Dr. Jain described 2 subtypes of Ph-like ALL: approximately half are CRLF2 -rearranged, 3 and these patients should ideally be referred to a clinical trial. The other half are nonrearranged, 3 and these patients should be referred for RNA sequencing to determine fusion genes.
No response was seen to further treatment, and the patient chose to continue care in hospice.
According to Dr. Zelenetz, incorporation of comprehensive genetic analysis and fluorescence in situ hybridization testing is important to identify high-risk patients (such as those with Ph-like phenotype) and plan for allogeneic hematopoietic stem cell transplantation (alloHSCT) or referral to clinical trials as early as possible.
MRD assessment by flow and/or NGS is critical to assess depth of response, modification of therapy, and candidacy for early alloHSCT. Dr. Park noted that both gene sequencing tests are validated, so patient preference should take priority.
Incorporation of tyrosine kinase inhibitors (TKIs) in Ph-like ALL is being investigated in clinical trials, and patients with this disease should be referred earlier rather than later, added Dr. Zelenetz. “But the nuance to that is understanding how to integrate TKIs into this entity, which is going to be dependent on understanding the mechanisms involved in the disease,” he said. “It won’t be just one TKI [that everyone receives]; it's much more complicated than that, unfortunately.”
Dr. Jain added that although Ph-like ALL has been established as high risk in the setting of chemotherapy, its classification remains to be determined in the new era of targeted therapies. “Some emerging data suggest that blinatumomab, inotuzumab, and CAR-T-cell therapy may overcome the negative prognostication of Ph-like ALL,” he said. “So those are some data we’ll hopefully see at the ASH Annual Meeting.”
Jarrod Holmes, MD, Annadel Medical Group, also participated in the panel discussion.
Pemmaraju N , Lane AA , Sweet KL , et al. . Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm . N Engl J Med 2019 ; 380 : 1628 – 1637 .
- Search Google Scholar
- Export Citation
Stock W , Luger SW , Advani AS , et al. . A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403 . Blood 2016 ; 133 : 1548 – 1559 .
Jain N , Roberts KG , Jabbour E , et al. . Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults . Blood 2017 ; 129 : 572 – 581 .
Disclosures: Dr. Zelenetz has disclosed that he receives research support from Genentech/Roche, Gilead, MEI, and BeiGene; he has been a consultant for Celegene/JUNO, Genentech/Roche, Gilead, BeiGene, Pharmacyclics, Jansen, Amgen, Astra‐Zeneca, Novartis, and MEI Pharma; and he is on the Scientific Advisory Board of the Lymphoma Research Foundation and Adaptive Biotechnologies. Dr. Jain has disclosed that he is a consultant for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; receives grant/research support from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Genentech, Inc., Incyte Corporation, Adaptive Biotechnologies, ADC Therapeutics, Cellectis, Precision Biosciences, Servier, Verastem, Pfizer, Inc., and Pharmacyclics; is a scientific advisor for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; and has received honoraria from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics. Dr. Park has disclosed that he receives grant/research support from Amgen Inc., Genentech, Inc., Incyte Corporation, Juno Therapeutics, Inc., Kite Pharma, Novartis Pharmaceuticals Corporation, and Servier; and is a scientific advisor for from Amgen Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, Incyte Corporation, Kite Pharma, Novartis Pharmaceuticals Corporation, Allogene Therapeutics, Autolus Therapeutics plc, and Takeda Pharmaceuticals North America, Inc. Dr. Pollyea has disclosed that he is a scientific advisor for AbbVie, Inc., Agios, Inc., Celgene Corporation, Daiichi-Sankyo Co., Forty Seven, Inc., Janssen Pharmaceutica Products, LP, Pfizer Inc., and Takeda Pharmaceuticals North America, Inc. Dr. Wierda has disclosed that he is a consultant for Genzyme Corporation and receives grant/research support from AbbVie, Inc., Acerta Pharma, Genentech, Inc., Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, Juno Therapeutics, Inc., Karyopharm Therapeutics, Kite Pharma, Cyclacel Pharmaceuticals, Inc., GlaxoSmithKline/Novartis Pharmaceuticals Corporation, Loxo Oncology, Inc., miRagen Therapeutics, Inc., Oncternal Therapeutics, Inc., Xencor, Inc., Pharmacyclics, and Sunesis Pharmaceuticals, Inc. Dr. Holmes has disclosed that he has no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
Article Sections
Article information.
- Get Permissions
- Similar articles in PubMed
Google Scholar
Related articles.
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [66.249.64.20|162.248.224.4]
- 162.248.224.4
Character limit 500 /500
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Development and Effects of Leukemia Nursing Simulation Based on Clinical Reasoning
Associated data.
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
While the effects of simulation education and the importance of the clinical reasoning process have been well-reported, an acute myelocytic leukemia (AML) patient-care simulation program has yet to be formulated exclusively for the clinical experience of students. This study developed and subsequently applied a simulation program based on clinical reasoning for AML to improve the learning outcomes and describe the learning experience for nursing students. Following a mixed-methods framework, the program’s effects on students’ knowledge were quantitatively measured, while their learning experience was qualitatively measured using self-reflection through journal writing. Differences in the pre- and post-scores between the experimental and control groups were statistically significant for theoretical knowledge and clinical performance. In addition, content analysis of both groups’ journals revealed three themes: (1) transformation into a self-directed learner for understanding the clinical situation, (2) increased awareness of clinical reasoning ability, and (3) embodiment of the clinical reasoning process. Standardizing the developed program’s scenarios prompted the participants’ compliance and engagement, and effectively achieved the learning outcomes. This simulation program aided the assessment of nursing intervention’s effectiveness and suggested objective criteria according to clinical reasoning. Similar programs involving other clinical cases, not exclusive to leukemia, should be developed and evaluated.
1. Introduction
The Fourth Industrial Revolution is a recent new-generation technological revolution in information and communication [ 1 ]. Thus, current nursing education requires a new paradigm to allow various learning experiences for practical application of the students’ clinical practice [ 2 ] and keep pace with the rapid technological advancements. One factor that will lead to nursing education change in the Fourth Industrial Revolution era is education using simulation [ 3 , 4 ]. Educational simulations can help nursing students enhance clinical reasoning skills, gain experience through practice, improve self-confidence, and gradually develop their vision for what constitutes excellent care [ 5 ]. Thinking like an expert nurse requires a form of engaged moral reasoning informed by generalized knowledge and rational processes and enhanced through expert guidance and coaching [ 6 ].
Although various scenarios involving important diseases in which direct clinical practice is necessary, the number of programs remains sorely lacking. Acute myelocytic leukemia (AML) is a disease in which hematopoietic stem cells become malignant cells, proliferate in the bone marrow, spread to the blood, and invade the liver, spine, and lymph glands. As a result, anemia, white blood cell reduction, platelet reduction, and leukocytosis occur [ 7 , 8 ]. Diseases like AML require a simulation operation scenario as their sudden onset nature with poor prognosis can make nursing care and clinical observation difficult for students [ 7 ]. Nursing performance should cope with various realistic situations based on understanding and professional knowledge of the physical and psychological complex state of AML patients [ 8 ]. Despite the pressing need for AML simulation education, scenarios involving routine and skin cancer patient care, including chemotherapy [ 9 , 10 , 11 ], and gynecological cancer patient care, are currently prioritized for development and use in nursing education [ 12 , 13 ]. Therefore, if direct education on AML nursing is not provided, nursing college students may work with limited clinical competence and confidence in AML nursing.
A nurse’s ability to perform clinical reasoning results in rational clinical decisions; thus, improving patient care performance and quality [ 14 ]. Clinical reasoning is a critical thinking strategy that verifies and analyzes patient-related data, applies a nursing process to solve the patient’s problem, makes a nursing diagnosis, and establishes a nursing plan accordingly [ 15 ]. Therefore, a high level of reasoning ability is related to patient well-being and safety [ 16 ], and needs to be educated as a core competency of nursing students. The American Association of Colleges of Nursing (AACN) [ 17 ] confirms and emphasizes the importance of clinical reasoning in undergraduate education, describing it as an essential competency for integrative problem-solving.
Clinical reasoning competency can be improved through knowledge acquisition via lectures and repeated clinical practice experience [ 15 , 18 ]. However, clinical practice to promote learners’ clinical reasoning capabilities is limited due to the rising level of medical consumers’ rights, changes in the health care environment, and the Covid-19 pandemic [ 19 ]. In addition, simulation effectively improves learner confidence, clinical judgment, and decision-making skills that are part of clinical reasoning [ 5 ]. Therefore, the proposal for an education method using high-fidelity simulation (HFS) to supplement clinical practice limitations [ 15 , 20 ] is meaningful.
The study aimed to (1) develop the simulation program based on clinical reasoning for AML; (2) identify the level and differences of nursing students’ self-confidence, theoretical knowledge, and clinical performance in learning (learning outcomes) after simulation application; and (3) describe the simulation program’s learning experience of nursing students after application of simulation. The improvement of learning outcomes was hypothesized based on Jeffries et al.’s simulation model [ 21 ].
2. Materials and Methods
2.1. study design and participants.
Following a mixed-methods framework, the clinical reasoning–based simulation program’s effects on students’ knowledge were quantitatively measured, while their learning experience was qualitatively measured. The study’s participants were undergraduate students from a nursing college in South Korea. As AML patients require complex nursing monitoring and management, students’ theoretical understanding of AML must be reinforced with application scenarios for a more holistic approach in caring for such patients.
The researchers verbally assured the participants of the study’s purpose and process. Participants subsequently submitted signed written and informed consent after a full explanation about their participation. Students who had taken hematology classes from their adult nursing theory courses, who had physical and psychological difficulties participating in education-related team activities, declined to be photographed and video recorded, and disagreed with the confidentiality terms, were excluded from the study. The matched pair method of the G Power 3.19.2 program (Heinrich-Heine-University, Dusseldorf, Germany) was used to calculate the number of participants, with an effect size of 0.5, α = 0.05, and power of 0.95 to achieve a total of 45 for the experimental and control groups, respectively. A total of 96 students (48 in both experimental and control groups) were initially selected after considering the exclusion rate. From this number, two students were subsequently excluded due to issues with personal schedules, while three others had insufficient responses. The final number of participants was 91 (45 in the experimental group and 46 in the control group).
2.2. Scenario Development Process
The International Association for Clinical Simulation and Learning (INACSL) Standard of Best Practice [ 22 ] was used as a basis for designing the simulation operation class and the leukemia patient treatment guideline for the scenario setup. The design also included clinical reasoning, which involves identifying the signs/symptoms (S), etiological factors (E), and the problem (P) [ 23 ].
Content validation was conducted via email: an expert panel composed of two clinical registered nurses (hematology/oncology), four nursing educators (two responsible for the simulation and two specialized in adult nursing), and one internist sent their opinion on scenario appropriateness and errors. After a pilot test, the final simulation scenario was confirmed following edits and supplements. Table 1 shows the simulation operating process of the developed scenario. Supplementary Table S1 provides more details on the scenario.
Simulation set: Continuous bleeding in the mouth after brushing teeth.
Note. 1 absolute neutrophil count, 2 antibiotic susceptibility testing, 3 blood pressure, 4 bodyweight, 5 C-reactive protein, 6 electrocardiogram, 7 heart rate, 8 gargling, 9 intravenous, 10 normal saline, 11 platelet concentrates, 12 respiratory rate, 13 situation, background, assessment, recommendation; 14 oxygen saturation as detected by the pulse oximeter, 15 temperature.
2.3. Measurement
2.3.1. quantitative study tool.
For the quantitative analysis, the researchers conducted a survey to examine three factors: self-confidence, theoretical knowledge, and clinical performance. Questions were formulated for each factor in relation to nursing process performance for AML patient care. Two adult nursing educators and two registered nurses validated the questions for each factor in clinical practice. For clinical performance, both students and researchers conducted the evaluation.
The researchers identified seven questions regarding core competency to evaluate self-confidence in terms of nursing process performance for leukemia patients. The self-confidence level was graded from 1 (“Not at all”) to 5 (“Very likely”) for each question. Total scores ranged from 7 to 35, lowest to highest; a higher score indicated a higher nursing process performance for leukemia patients. Cronbach’s α was 0.818 for this factor.
A 10-item multiple-choice questionnaire evaluated the students’ theoretical knowledge regarding leukemia patient care. Questions with a content validity index (CVI) score of 0.8 or higher were used. Each correct answer received 1 point, while incorrect and missing answers received 0 points. The score distribution was from 0 to 10. Similar to self-confidence, a higher score indicated higher knowledge of the nursing process for leukemia patients.
Twenty questions for situational activities that the participants performed were identified. For performable clinical activities, 2 points were given for “Well done”, 1 point for “Average”, and 0 for “Poorly done” or “Not done”. The score distribution was from 0 to 40. A higher score indicated a higher nursing process performance for leukemia patients. The Cronbach’s α used was 0.924.
2.3.2. Qualitative Study Tool
The experimental group documented their experiences through reflection journals, which were collected and used as qualitative data. The reflection journal is a document organized by the learner for reviewing the lesson, which allows the learner to consolidate all learning points and reflections during the learning process and enables the educator to diagnose educational difficulties [ 24 ]. The nursing students were asked the following reflection questions:
- What do you think your team did best in this scenario?
- Write down any points you think are lacking in this scenario.
- Write what you learned or felt through this scenario.
2.4. Study Procedure
The simulation was conducted in the university’s high-fidelity patient simulation training center, and data were collected from November 27 through 14 December 2018. A one-week gap in the pre- and post-study evaluations of the control and the experimental group were done to prevent diffusion. The detailed study process is shown in Figure 1 .

Study process.
2.5. Analysis Methods
SPSS Win 20.0 (SPSS Inc., Chicago, IL, USA) was used in quantitative data analysis, calculating the participants’ basic demographics, frequency, percentage average, and standard deviation for each question. The chi-square test was used to analyze the participants’ basic demographics, prior theoretical knowledge, clinical performance, and self-confidence. Comparisons between the experimental and control groups regarding self-confidence, theoretical knowledge, and clinical performance were performed using a t-test. Cronbach’s α coefficient was used to validate the measurement reliability.
Experts in content analysis with sufficient understanding of the current study, but not directly involved in program operation, carried out the qualitative data collection. The collected data were subjected to the content analysis method developed by Krippendorff [ 25 ]. An investigator read all reflection journals and extracted core ideas and concepts on the students’ learning experiences during the simulation program. The extracted data were subsequently categorized through interconnection and abstraction. To verify the categories’ credibility, the investigators returned to the original data following these categories, reading, and analyzing them as a whole. Two qualitative research experts reviewed the analyzed results for feasibility.
2.6. Training of Research Assistants
To prevent bias in the results, the investigator did not participate in simulation operation and data collection. Four research assistants (one simulation instructor, one simulation operator, and two data collectors) were trained to perform these tasks instead. The simulation instructor had more than seven years of clinical experience and more than two years of simulation operation experience, while the simulation operator possessed more than two years of clinical experience and five years of experience in simulation operation. Both instructor and operator were trained twice for 2 h each training by the investigator on scenarios and research-related matters. In addition, the instructor participated in a >8 h simulation-related conference. Two data collectors had two training sessions for 1 h each on how to fill out the survey and precautions. Moreover, their understanding of each question item was also confirmed. The investigator conducted debriefing at the end of the simulation.
3.1. Homogeneity Verification
No statistically significant difference regarding the basic demographics between the experimental and control groups was found ( Table 2 ).
General characteristics and homogeneity of experimental and control groups ( n = 91).
3.2. The Scenario’s Effect
After pre- and post-study scores analyses, statistically significant differences in self-confidence ( p < 0.001), theoretical knowledge ( p = 0.001), and clinical performance ( p < 0.001) were found in the experimental group. However, those in the control group showed statistically significant differences in self-confidence ( p = 0.019) and clinical performance ( p = 0.002). Upon comparing the pre- and post-study scores between the groups, there were statistically significant differences in theoretical knowledge ( p = 0.014) and clinical performance ( p = 0.020), but not in self-confidence ( Table 3 ).
Mean score comparisons between experimental group and control group per variable ( n = 91).
3.3. Content Analysis of Reflection Journals
As a result of analyzing the reflection journal, three main themes and eight sub-themes were derived. The learning experiences of clinical reasoning–based simulation were shown as a process of transformation to a self-directed learner for understanding the clinical situation, increased awareness of clinical reasoning ability, and embodiment of the clinical reasoning process. Details are shown in Table 4 .
Students’ simulation learning experience process based on clinical reasoning.
4. Discussion
This study developed a patient with leukemia–care simulation program based on clinical reasoning for nursing students. Clinical reasoning is a cognitive process that uses critical thinking strategies in clinical situations as well as a problem-solving strategy that identifies and diagnoses actual and potential problems of patients based on patient-related data [ 26 , 27 ]. To meet the complex needs of AML patients, nursing students must demonstrate excellent clinical reasoning and judgment [ 28 ].
Specifically, the program was designed to teach theoretical knowledge, and clinical performance related to the subject provided by prior learning and participate in simulation scenarios and debriefings based on clinical reasoning. Because the study design characteristics of such simulations have been seen to play a mediating role in reinforcing learners’ nursing competence [ 29 ], the simulations based on clinical reasoning were effective in achieving the learning outcomes in nursing leukemia patients. It also enabled the study participants to focus on problem-solving by identifying the patients’ main complaints and establishing and applying nursing intervention priorities according to the nursing process scenario flow. Moreover, the program aided the participants in acquiring practical application of the clinical reasoning process effectively.
Unlike simulation scenarios developed in previous studies, the standardized cues allowed participants to avoid deviating from program flow and engaging them in the scenario. However, because there is very little research on simulation programs based on clinical reasoning and/or evaluating their effectiveness regarding standardized cues [ 30 ], it is necessary to develop more diverse clinical cases to run the education programs.
Although diseases like AML can lead to swift death due to infection or internal bleeding if no appropriate treatment is applied [ 7 ], nursing education exclusively focuses on basic knowledge and techniques regarding cancer patients [ 31 ]. Previous simulation programs focused more on primary patient care, such as skin assessment, pain management, chemotherapy [ 9 , 10 , 11 ]. This study was specifically designed to comprehensively check the patient’s condition by constructing a clinical reasoning–based scenario as well as identifying and diagnosing a patient’s actual and potential problems. The simulation program developed in this study can be suitable for training systematic thinking to effectively cope with clinical situations requiring complex therapeutic approaches, such as AML, in a safe environment.
Unlike Kweon’s [ 32 ] study, there was no statistically significant difference in self-confidence between the experimental and control groups post-operation of the simulation. Self-confidence directly affected nursing performance, leading to clinical performance improvement [ 33 ]; yet even if the simulation scenario was developed in accordance with the learners’ level, sufficient understanding was still required to boost confidence. In addition, analysis of the reflection journals showed that the participants’ self-motivation and confidence increased due to the motivation and encouragement of instructors and the stimulation of their curiosity. Therefore, in the process of acquiring clinical reasoning and making it one’s own, it cannot be said that the operation of the clinical reasoning–based nursing simulation was not effective in improving confidence because the self-confidence can be answered in the self-report questionnaire while recognizing one’s shortcomings. Moreover, interventions targeting these factors are thus necessary to enhance the learners’ self-confidence. Sufficient preliminary preparation and positive support of instructors, as well as and sufficient pre-learning and repeated learning situations, should thus be provided to learners.
The experimental group’s theoretical knowledge showed a statistically significant increase after the simulation, consistent with prior studies’ results [ 34 , 35 ]. As the current study holistically applied the clinical reasoning process to the scenario flow and debriefing, the cognitive learning effect increased. As the experimental group also underwent self-directed learning through pre-simulation videos and hands-on learning, the effects of learning increased more compared to the control. The participants recognized and tried to supplement their lack of knowledge, becoming self-directed learners by recognizing the importance of repeated learning. These results show that simulation-based clinical reasoning is effective not only as an alternative to clinical practices but also for theoretical education. However, further research is necessary to determine the learning effects’ longevity and its effective utilization in clinical practices.
In the clinical performance evaluation, the participants’ self-evaluation was significantly lower than that of the operator. The participants were also found to be comparing their clinical performance with the nurses’ core competencies in the journal analysis. If they were unable to answer patient queries or assess the patient’s condition accurately, they associated the results with a clinical performance and underappreciated themselves. The simulation operator should, therefore, disclose full information to the participants. Additionally, the facilitator should provide continuous positive feedback and standardized cues to support participants, eliminating the frustration and enabling them to complete the process. The journals also demonstrated how incorporating clinical reasoning enabled the integration of theoretical knowledge and clinical practice. This change appeared as students became aware of the clinical reasoning–based simulation situation.
The learners were initially unable to understand how the clinical reasoning was applied. However, after the current study program, they recognized and perceived nursing assessment, diagnosis, and intervention according to clinical reasoning and evaluation as part of the overall nursing workflow. It shows that the developed simulation program effectively systematized nursing performance and enhanced work efficiency.
Generalizing the study’s results remain challenging due to the limited study population. While measures were taken to prevent the spread of the study content between groups, it remains difficult to state that the spread effects were deterred entirely. Having used a self-reported measurement tool cannot also completely remove errors due to the respondent’s subjective interpretation.
Unexplored topics, such as the comparisons in educational effects of the various clinical cases, the utilization degree of the knowledge and clinical performance skills acquired from the simulation, and the evaluation of their effects limit the study. Subsequently, future studies can identify the causes behind the absence of change in self-confidence despite improvement in clinical performance level and determine the reason behind the difference in clinical performance evaluation between facilitator(s) and students. The feasibility of replacing the theoretical curriculum with the simulation can also be researched in future works.
5. Conclusions
The current study developed a standardized simulation scenario involving AML and evaluated its effect using quantitative and qualitative data. After the simulation program’s operation, statistically significant effects on the participants’ theoretical knowledge and clinical performance were found. Analysis of the journals showed that the participants experienced transformation, becoming self-directed learners and having increased awareness of the clinical reasoning ability. Incorporating clinical reasoning also allowed them to integrate both theoretical knowledge and clinical practice. Compared to the existing lecture-based method, results show that the developed program would be a valuable tool to enhance knowledge and clinical performance—the objective of clinical education.
Through this study’s result, simulation education was proposed to be applied to nursing students and clinical nurses. As the program developed was related to medical diagnosis and nursing diagnosis, it would help assess the nursing intervention’s effectiveness and suggest objective criteria according to clinical reasoning. It is proposed to be used in the new nurses’ residency program or as a refresher education program for experienced nurses to cope with complex and diverse medical environments.
Acknowledgments
The study’s authors would like to express their heartfelt appreciation to the clinical registered nurses, nursing educators, and the internist who assisted in scenario development, the instructors and operators who assisted during the scenario operation, and the study participants. This study would not have been possible without their valuable time and support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18084190/s1 , Table S1: Overview of nursing simulation about acute myelocytic leukemia (AML).
Author Contributions
Conceptualization, A.J.; methodology, A.J.; formal analysis, A.J. and S.K.; writing—original draft preparation, M.S. and S.K.; writing—review and editing, A.J. and S.K.; project administration, A.J.; funding acquisition, A.J. All authors have read and agreed to the published version of the manuscript.
This study was supported by the National Research Foundation of Korea (NRF 2017R1C1B5017463) and research fund from Nambu University, 2021.
Institutional Review Board Statement
The study was conducted according to the Declaration of Helsinki guidelines and obtained from the bioethics committee of the Korea National Institute for Bioethics Policy, designated by the Ministry of Health and Welfare (IRB No. PO1-201811-13-005; Date: 23 November 2018).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acute Lymphocytic Leukemia

- Acute lymphocytic leukemia (ALL), also known as acute lymphoblastic leukemia, refers to an abnormal growth of lymphocyte precursors or lymphoblasts.
Table of Contents
- What is Acute Lymphocytic Leukemia?
Pathophysiology
Statistics and incidences, clinical manifestations, complications, assessment and diagnostic findings, medical management, pharmacologic therapy, surgical management, nursing assessment, nursing diagnosis, nursing care planning & goals, nursing interventions, discharge and home care guidelines, documentation guidelines, what is acute lymphocytic leukemia.
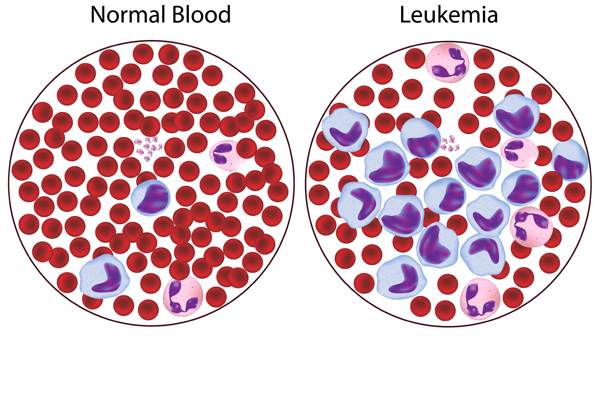
Acute leukemias have large numbers of immature leukocytes and overproduction of cells in the blast stage of maturation.
- Acute leukemia is a malignant proliferation of white blood cell precursors in bone marrow or lymph tissue, and their accumulation in peripheral blood, bone marrow, and body tissues.
- About 20% of leukemias are acute.
Pathogenesis isn’t clearly understood, but the pathophysiology may be explained by the following:
- Accumulation . Due to the precipitating factors, immature, non-functioning WBCs appear to accumulate first in the tissue where they originate (lymphocytes in lymph tissue, granulocytes in bone marrow).
- Infiltration . These immature WBCs then spill into the bloodstream and from there infiltrate other tissues.
- Malfunction . Eventually, this infiltration results in organ malfunction because of encroachment and hemorrhage .
- Schematic diagram and pathophysiology
One of the most common forms of acute leukemia is acute lymphocytic leukemia.
- Acute lymphocytic leukemia is more common in males than in females, in whites (especially in people of Jewish descent), in children ( between ages 2 and 5 ), and in people who live in urban and industrialized areas.
- 80% of all leukemias between 2 and 5 years old are ALL.
- Acute leukemias account for 20% of adult leukemias.
- Among children, however, it is the most common form of cancer.
- Incidence is 6 out of every 100, 000 people.
Research on predisposing factors isn’t conclusive but points to some combination of viruses, immunologic factors, genetic factors, and exposure to radiation and certain chemicals.
- Congenital disorders. Down syndrome , Bloom syndrome, Fanconi anemia , congenital agammaglobulinemia, and ataxia-telangiectasia usually predisposes to ALL.
- Familial tendency. Genetics also play a part in the development of ALL.
- Viruses. Viral remnants have been found in leukemic cells, so they are likely one of the causes of ALL.
Signs of acute lymphocytic leukemia may be gradual or abrupt.
- High fever . High fever accompanied by thrombocytopenia and abnormal bleeding (such as nosebleeds and gingival bleeding) manifests in the patient.
- Bruising. Easy bruising after minor trauma is a sign of leukemia.
- Dyspnea. A decrease in the mature blood components leads to dyspnea .
- Anemia. Anemia is present in ALL because of a decrease in mature RBCs.
- Fatigue . The patient experiences fatigue more frequently than normal.
- Tachycardia. As the oxygen-carrying component of the blood decreases, the body compensates by pumping out blood faster than normal.
Untreated, acute leukemia is invariably fatal, usually because of complications that result from leukemic cell infiltration of the bone marrow and vital organs.
- Infection. Immature WBCs are not fit to defend the body against pathogens, so infection is always a possible complication to watch out for.
- Organ malfunction. Encroachment or hemorrhage occurs when immature WBCs spill into the bloodstream and other tissues and eventually lead to organ or tissue malfunction.
The diagnosis of ALL can be confirmed with a combination of the following:
- Bone marrow aspiration . Typical clinical findings and bone marrow aspirate showing a proliferation of immature WBCs confirm ALL.
- Bone marrow biopsy. A bone marrow biopsy, usually of the posterior superior iliac spine, is part of the diagnostic workup.
- Blood counts. Blood counts show severe anemia , thrombocytopenia, and neutropenia.
- Differential leukocyte count. Differential leukocyte count determines cell type.
- Lumbar puncture . Lumbar puncture detects meningeal involvement.
- Uric acid levels. Elevated uric acid levels and lactic dehydrogenase levels are commonly found.
With treatment, the prognosis varies.
- Systemic chemotherapy . Systemic chemotherapy aims to eradicate leukemic cells and induce remission (less than 5% of blast cells in the marrow and peripheral blood are normal).
- Radiation therapy. Radiation therapy is given for testicular infiltrations .
- Platelet transfusion is performed to prevent bleeding and RBC transfusion to prevent anemia.
ALL chemotherapy includes the following drugs and also other drugs included in the treatment:
- Vincristine. Vincristine is an anti-cancer (antineoplastic or cytotoxic) chemotherapy drug and is classified as a plant alkaloid.
- Prednisone. This drug works is by altering the body’s normal immune system responses.
- Cytarabine. Cytarabine belongs to the category of chemotherapy called antimetabolites, wherein When the cells incorporate these substances into the cellular metabolism, they are unable to divide and they attack cells at very specific phases in the cycle.
- L-asparaginase. Asparaginase breaks down asparagine in the body, so since the cancer cells cannot make more asparagine, they die.
- Daunorubicin . Daunorubicin is classified as an antitumor antibiotic which is made from natural products produced by species of the soil fungus Streptomyces, and these drugs act during multiple phases of the cell cycle and are considered cell-cycle specific.
- Antibiotic, antifungal , and antivirals . These control infection, a common complication of acute leukemias.
Aggressive treatment may include surgical management through:
- Bone marrow transplant. Bone marrow transplant is a choice that can be considered for a patient with ALL.
- Stem cell transplant. Stem cell transplant in ALL is one of the latest development in the treatment of acute leukemias
Nursing Management
The care plan for the leukemic patient should emphasize comfort, minimize the adverse effects of chemotherapy, promote preservation of veins, manage complications, and provide teaching and psychological support.
The clinical picture varies with the type pf leukemia as well as the treatment implemented, so the following must be assessed:
- Health history. The health history may reveal a range of subtle symptoms reported by the patient before the problem is detectable on physical examination.
- Physical examination. A thorough, systematic assessment incorporating all body systems is essential.
- Laboratory results. The nurse also must closely monitor the results of laboratory studies and culture results need to be reported immediately.
Based on the assessment data, major nursing diagnoses for the patient with ALL may include:
- Risk for infection related to overproduction of immature WBCs.
- Risk for impaired skin integrity related to toxic effects of chemotherapy, alteration in nutrition , and impaired immobility.
- Imbalanced nutrition, less than body requirements , related to hypermetabolic state, anorexia , mucositis, pain, and nausea .
- Acute pain and discomfort related to mucositis, leukocyte infiltration of systemic tissues, fever, and infection.
- Hyperthermia related to tumor lysis or infection.
- Fatigue and activity intolerance related to anemia, infection, and deconditioning.
Main Article: 5 Leukemia Nursing Care Plans
The major goals for the patient may include:
- Absence of pain.
- Attainment and maintenance of adequate nutrition.
- Activity tolerance.
- Ability to provide self-care and to cope with the diagnosis and prognosis.
- Positive body image .
The interventions included in the care plan of the patient follows.
Before treatment:
- Education. The nurse should explain the disease course, treatment, and adverse effects.
- Infection. The nurse should teach the patient and his family how to recognize symptoms of infection such as fever, chills, cough , and sore throat .
- Bleeding. The nurse should educate the patient and the family how to recognize abnormal bleeding through bruising and petechiae and how to stop it with direct pressure and ice application.
- Promote good nutrition. The nurse should explain that chemotherapy causes weight loss and anorexia, so the patient must be encouraged to eat and drink high-calorie and high-protein foods and beverages.
- Rehabilitation. The nurse should help establish and appropriate rehabilitation program for the patient during remission.
Plan meticulous, supportive care:
- Meningeal leukemia. Watch out for meningeal leukemia ( confusion , lethargy, headache) and know how to manage care after intrathecal chemotherapy.
- Hyperuricemia. Prevent hyperuricemia, a possible result of rapid, chemotherapy-induced leukemia cell lysis through encouraging fluids to 2000 ml daily, giving acetazolamide and sodium bicarbonate tablets, and allopurinol.
- Infection control. Control infection by placing the patient in a private room and instituting neutropenic precautions.
- Skincare. Provide thorough skin care by keeping the patient’s skin and perianal area clean, applying mild lotions and creams to keep skin from cracking and drying, and thoroughly cleaning skin before all invasive skin procedures.
- Constipation . Prevent constipation by providing adequate hydration, a high-residue diet, stool softeners, and mild laxatives, and by encouraging walking .
- Mouth ulcers. Control mouth ulceration by checking often for obvious ulcers and gum swelling , and by providing frequent mouth care and saline rinses.
- Psychological support. Provide psychological support by establishing a trusting relationship to promote communication.
- Manage stress. Minimize stress by providing a calm, quiet atmosphere that is conducive to rest and relaxation .
Expected patient outcomes may include:
- Shows no evidence of infection.
- Experiences no bleeding.
- Attains optimal level of nutrition.
- Reports satisfaction with pain and comfort levels.
- Has less fatigue and increased activity.
- Copes with anxiety and grief .
- Absence of complications.
Most patients cope better when they have an understanding of what is happening to them.
- Education. Based on the patient’s education, literacy level, and interest, teaching of the patient and family should focus on the disease, its treatment, and certainly the resulting significant risk of infection and bleeding.
- Vascular access device. Management of a vascular access device can be taught to most patients or family members , and the nurses may need to provide follow-up care for the patient.
- Home care services. Coordination of home care services and instruction can help alleviate anxiety about managing the patient’s care at home.
The focus of documentation should include:
- Recent or current antibiotic therapy.
- Signs and symptoms of infectious process.
- Individual risk factors that may potentiate blood loss.
- Baseline vital signs, mentation, urinary output, and subsequent assessments.
- Results of laboratory tests or diagnostic procedures.
- Client’s description of response to pain, specifics of pain inventory, expectations of pain management , and acceptable level of pain.
- Caloric intake.
- Individual cultural or religious restrictions and personal preferences.
- Plan of care.
- Teaching plan.
- Responses to interventions, teaching, and actions performed.
- Attainment or progress toward desired outcome.
- Modifications to plan of care.
- Discharge needs.
- Specific referrals made.
3 thoughts on “Acute Lymphocytic Leukemia”
The content was very helpful 😊
Wow. I answered all the questions correctly. There are geniuses out here.
thank you to you all at nurse labs this write up was of great help to me. God bless you all
Leave a Comment Cancel reply
- Campus Directory
- Current Students
- Faculty & Staff

Acute Lymphocytic Leukemia Case Study
Leukemia is a cancer of white blood cells. In acute leukemia, the abnormal cells divide rapidly, quickly overtaking functional white and red blood cells. The most common form of cancer in children 0-14 years of age is acute lymphocytic leukemia (ALL). The survival rate in children has improved more than 50% in the last half century. Currently, there is a 65.3% overall survival rate; in children under 5 the survival rate increases to 90.4%. Come experience the cancer journey with 6-year-old Noah.

Module 6: Acute Lymphocytic Leukemia
Noah, 6 years old, was brought back to his pediatrician three weeks following a streptococcal throat infection...
Leukemia - Page 1
Upon receiving the results, the physician informed the stunned mother that her child had...
Leukemia - Page 2
A spinal tap was ordered to see if the leukemic cells had crossed the blood/brain barrier...
Leukemia - Page 3
A sputum culture was obtained and sent to the lab for gram stain, culture, and sensitivities...
Leukemia - Page 4
Noah was now in remission. Two weeks after achieving...
Leukemia - Page 5

Case Summary
Summary of the Case
Leukemia - Summary

Answers to Case Questions
Leukemia - Answers

Professionals
Health Professionals Introduced in Case
Leukemia - Professionals
Additional Links
Optional Links to Explore Further
Leukemia - Links
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies
Case Study: New Therapies for Acute Myeloid Leukemia
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 76-year-old woman presents to the emergency department following two weeks of progressive dyspnea and fatigue, and a new rash. Her medical history is significant for stage 2 chronic kidney disease, coronary artery disease, and diabetes.
Physical examination results are within normal limits, except for skin pallor and a petechial rash on the lower extremities bilaterally. She has an Eastern Cooperative Oncology Group (ECOG) performance status score of 1. Complete blood count with differential is significant for a white blood cell count of 18 × 10 9 /L with 40 percent circulating blasts, hemoglobin 6.7 g/dL, and platelet count of 20 × 10 9 /L. A bone marrow biopsy reveals a hypercellular marrow with 22 percent blasts, consistent with a diagnosis of acute myeloid leukemia (AML). Flow cytometry demonstrates CD33 negativity, and classic cytogenetic analysis revealed a normal karyotype. Molecular markers are pending for FLT3, IDH1, IDH2, and NPM1.
A pre-treatment echocardiogram is performed and is notable for mild global systolic dysfunction and a left-ventricular ejection fraction of 45 percent.
Which of the following is the most appropriate therapy?
- Gilternitinib
- Azacitidine and venetoclax
- Liposomal daunorubicin and cytarabine
- Gemtuzumab ozogamicin
Explanation
The best treatment option for this patient is azacitidine and venetoclax. Recently, the U.S. Food and Drug Administration (FDA) approved the BCL-2 inhibitor venetoclax in combination with a hypomethylating agent for patients with newly diagnosed AML who are 75 years or older, or those with comorbidities that preclude the use of intensive induction chemotherapy. 1 Approval was based on preliminary data published in February 2018 from a phase Ib study of 57 patients to evaluate the safety and efficacy of either azacitidine or decitabine in combination with venetoclax. 2
Eligibility criteria included previously untreated patients aged 65 years and older with AML who were ineligible for standard induction therapy, ECOG performance status of 0 to 2, and intermediate-risk or poor-risk cytogenetics. During dose escalation, oral venetoclax was administered daily in combination with either decitabine (days 1-5) or azacitidine (days 1-7). Results from this study population showed a complete remission (CR) or CR with incomplete marrow recovery (CRi) in 61 percent of patients. 2 A follow-up of the same clinical trial was recently published in January 2019 evaluating 145 patients. 3 This study demonstrated a CR + CRi rate at all doses of 67 percent, with notable responses in those with poor-risk cytogenetics and those who were at least 75 years old. The median duration of CR + CRi was 11.3 months, with a median overall survival of 17.5 months. 3
While this patient has newly diagnosed AML, her age and comorbidities, including CKD, borderline heart function, and diabetes, likely preclude her from being able to tolerate a standard induction chemotherapy regimen. 4 Gilteritinib (answer A) is an oral kinase inhibitor that was recently approved for treatment of relapsed or refractory AML, with a FLT3 mutation based on interim analysis of 138 patients in the ADMIRAL trial, showing CR or CRh in 21 percent of patients. 5 Answer D is incorrect because the liposomal form of daunorubicin and cytarabine is approved for indications of newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes, 6 which is not the case with this patient. Additionally, the left ventricular dysfunction is a relative contraindication to the danunorubicin. Ivosidenib is an IDH-1 inhibitor approved for patients with relapsed or refractory AML with a mutation in the IDH-1 gene. 7 While gemtuzumab ozogamicin (GO), an anti-CD33 monoclonal antibody, is well tolerated in older patients with newly diagnosed or relapsed AML, its approval is for treatment of CD33+ disease. 8 It would be an inappropriate choice for this patient because her flow cytometry demonstrated CD33 negativity. It is also not used as a single agent for initial induction therapy.
In summary, for older patients with newly diagnosed AML, a hypomethylating agent in combination with venetoclax should be considered when comorbidities preclude the use of standard induction chemotherapy.
Case study submitted by Nicole Held, DO, and Talha Badar, MD, of Medical College of Wisconsin, Milwaukee, WI.
Resources
- U.S. Food and Drug Administration FDA approves venetoclax in combination for AML in adults. . 2018.
- DiNardo CD, Pratz KW, Letai A, et al Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid luekaemia: a non-randomised, open-label, phase 1b study . Lancet Oncol. 2018 19:216-228.
- DiNardo CD, Pratz K, Pullarkat V, et al Venetoclax combined with decitabine or azacitidine in treatment-naïve, elderly patients with acute myeloid leukemia . Blood. 2019 133:7-17.
- Kantarjian H, O’brien S, Cortes J, et al Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome . Cancer. 2006 106:1090-1098.
- U.S. Food and Drug Administration FDA approves gilteritinib for relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation . 2018.
- Vyxeos (daunorubicin and cytarabine) package insert . Jazz Pharmaceuticals. 2017.
- U.S. Food and Drug Administration FDA approves first targeted treatment for patients with relapsed or refractory acute myeloid leukemia who have a certain genetic mutation . 2018.
- Sievers EL, Larson RA, Stadtmauer EA, et al Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse . J Clin Oncol. 2001 19:3244-3254.
American Society of Hematology. (1). Case Study: New Therapies for Acute Myeloid Leukemia. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia .
American Society of Hematology. "Case Study: New Therapies for Acute Myeloid Leukemia." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia (label-accessed May 19, 2024).
"American Society of Hematology." Case Study: New Therapies for Acute Myeloid Leukemia, 19 May. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/new-therapies-for-acute-myeloid-leukemia .
Citation Manager Formats
Leukemia Case Study (60 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Mr. Devito is a 48-year-old male who presents to his Primary Care Provider with left upper abdominal pain and complaints of weakness and fatigue. The nurse immediately notes how pale his skin is. A full set of vital signs reveals the following:
BP 142/90 mmHg
SpO 2 94% on Room Air
Temp 101.0°F
What furtner nursing assessments would you perform at this time?
- Heart and lung sounds
- Assess abdomen and review details of abdominal pain (OLDCARTS)
- Assess skin condition (color, quality, turgor, etc.)
- Peripheral perfusion (pulses, cap refill, etc.)
Upon further assessment, the nurse notes a palpable mass in the left upper quadrant, possibly an enlarged spleen, that is tender on palpation. The nurse also notes petechiae and bruising to the patient’s arms and legs. When questioned, the patient says “I seem to bruise so easily these days”. The patient’s lungs have diffuse crackles, heart sounds S1 and S2 present with no murmurs. The patient also reports a slight headache.
What laboratory or diagnostic tests do you anticipate the provider ordering?
- Complete Blood Count (to check for wbc – infection and reason for bruising)
- Full chemistry to ensure no electrolyte abnormalities or renal involvement
- Coagulation studies to determine cause of easy bruising
- Chest X-ray and sputum culture to identify source of infection
The provider orders a complete blood count, chemistry panel, and chest x-ray. The chest x-ray shows the patient has a slight pneumonia. He is sent home with a course of antibiotics while awaiting the test results.
The next day, the lab results show the following:
RBC 4.2 BUN 22
Hgb 8.4 Cr 0.9
Hct 25.2 K 3.9
WBC 144,000 Na 148
Plt 40,000 Ca 7.6
Based on the above lab results, what should the nurse be most concerned about?
- The patient has EXCESSIVE amounts of white blood cells. It would be expected for them to be slightly elevated because of the infection, but this is WAY beyond that.
- The patient is also anemic, with low platelets – this could explain the easy bruising
What do you believe may be going on, physiologically, with Mr. Devito?
- The excessive amounts of White Blood Cells, plus the easy bruising, anemia, and enlarged spleen point to some type of Leukemia.
- The body is excessively making immature, non-functioning white blood cells – hence the patient being susceptible to a pneumonia.
What further diagnostic testing should be performed to confirm a diagnosis?
- A bone marrow biopsy must be done to confirm a leukemia diagnosis
- The provider calls Mr. Devito and explains the results. They set an appointment for Mr. Devito to have a bone marrow biopsy. Biopsy results confirm Mr. Devito has Acute Myeloid Leukemia. Mr. Devito’s wife says “I don’t understand, I thought you said he just had pneumonia?”
How would you explain this to the patient’s wife?
- Leukemia causes the body to make a bunch of immature, non-functioning white blood cells. So when a patient gets an infection, like a respiratory infection, the body’s white blood cells can’t actually fight it off. So it’s common for patients to be prone to infections like pneumonia.
- Mr. Devito DID have pneumonia – but it was due to the poor immune response caused by the Leukemia.
Mr. Devito will be started on high-dose chemotherapy.
What education topics should be included in teaching for Mr. Devito and his wife?
- Mr. Devito will have a special port implanted in order to receive his chemotherapy
- Mr. Devito will likely also receive medications to manage the symptoms of the chemotherapy
- Mr. Devito may lose his hair, depending on the type of chemotherapy used, because chemo also kills healthy fast-growing cells
- Mr. Devito May experience something called neutropenia. This means he will be highly susceptible to infections. He should avoid having lots of visitors, avoid fresh flowers, and especially avoid being around anybody who is sick. He can even wear a mask in public if he so desires.
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Case Presentation: A 67-Year-Old Man with Chronic Lymphocytic Leukemia
John Allan, MD, presents and reviews the case of a 67-year-old man with chronic lymphocytic leukemia.

EP: 1 . Case Presentation: A 67-Year-Old Man with Chronic Lymphocytic Leukemia
Ep: 2 . testing and risk stratification in cll, ep: 3 . first-line therapy options in cll, ep: 4 . chronic lymphocytic leukemia: resonate-2 trial, ep: 5 . comparing btk inhibitors in cll treatment, ep: 6 . treatment approaches for high-risk chronic lymphocytic leukemia, ep: 7 . the future of chronic lymphocytic leukemia treatment.
John Allan, MD: Welcome, everyone. Thank you for joining, I’m John Allan. I'm an assistant professor of medicine here at Weill Cornell Medicine, in New York City.
I'm a lymphoma physician. I have a specialization in CLL [chronic lymphocytic leukemia], and treating patients with CLL, and we'll be going through a case and I’ll be giving you my insights on my thoughts about the case and how I might manage the patient potentially differently or the same. I would like to start out by presenting the case so everyone is on the same page here. This is a 67-year-old male with a diagnosis of chronic lymphocytic leukemia. He's presenting to his primary care physician with complaints of fatigue and night sweats. In terms of past medical history, he's relatively healthy, except that he takes an over-the-counter antacid every few days, a couple times a week, for a sensitive stomach.
On a physical exam, he has a large mobile lymph node, these are bilaterally, in the cervical chain. No palpable spleen or liver, palpable liver is noted. Laboratory findings are consistent with a white blood cell count of a 102,000, lymphocytes are 79,000 of those 102. Hemoglobin is slightly low but relatively OK, and preserved at 11.4 grams per deciliter. His platelets are normal at 180,000. His neutrophil count is normal at 1.9. His LDH [lactate dehydrogenase] is actually very elevated, at 1470. And cytogenetics are showing a chromosome 11q deletion and IgHV [immunoglobulin heavy chain] unmutated status.
I presume this was sent off not by the primary care, but by his hematologist. And a beta-2 macroglobulin was also obtained and that's 3.0, right on the threshold of being abnormal. He's staged as a Rai Stage I, and the patient initiated treatment, and was started on ibrutinib once daily.
John Allan, MD: So, this case is a somewhat common presentation that we see, especially with a patient with these molecular abnormalities. Average age diagnosis of patients as a reminder is about 70 to 71 years of age, so the patient is well within that nice bell-shaped curve. He was relatively healthy, feeling well, up until very recently when these symptoms started.
The disease has been progressive relatively quickly, and associated with these B symptoms with a very elevated white blood cell count, along with the fevers and night sweats. Also note is that the LDH is rather elevated. The high end of normal, at least in my lab, is around 230 or 220. This patient is at 1470.
Additionally, the cytogenetics are identifying relatively high-risk features with the deletion 11q and IgHV unmutated status. This is somebody that I am concerned about. I do agree with the treatment decision to initiate therapy. A few things to note, deletion 11q, it's considered high risk feature, and patients do present a little bit differently with an 11q than some of the other patients with CLL.
Many times, the 11q patients have rather proliferative disease at diagnosis and they can present with a lot of bulky adenopathy. While this patient doesn't have technically bulky adenopathy, and really what's palpable is only about a centimeter and a half, it's very possible that with deep within the abdomen something that we're not palpating he can have a very large lymph node, 6 to 8 centimeters or larger.
Typically, that's associated with signs and symptoms, but given the fact that these fevers, night sweats and rather proliferative disease with an elevated LDH together with these high-risk features, this is someone I might be concerned about even with a transformation as an initial presentation of their CLL and would need to work up and rule that out appropriately.
With that said, the patient was started on ibrutinib, and overall is a good therapy. And so, those are the big, noteworthy aspects of this case, and can be a rather typical presentation for a patient with deletion 11q molecular features like this patient.
John Allan, MD: One other aspect of this case that's unique is that the patient is on over-the-counter antacids, and this is a relatively common thing. Many patients are takig either over-the-counter antacids like Pepcid [famotidine], Prilosec [omeprazole], etc.
I'm sorry, those are more PPIs [proton pump inhibitors] but acid suppressing medications either H2 [histamine2] blockers calcium absorbing, calcium carbonate like medications and or proton pump inhibitors.
And so, this is not just unique to CLL patients, this is just unique to older patients and patient population in general, where in the US specifically there is a lot of PPI use, whether it's over-the-counter or prescribed.
Ultimately, in clinical trials with BTK [Bruton’s tyrosine kinase] inhibitors and targeted agents we do see about 40% to 45% of patients on some type of acid suppressive medication. This is a very common scenario and it may have some relevance based on differentiating between BTKi's at this point in time.
Transcript Edited for Clarity
Initial Presentation
- A 67-year-old man presented to PCP with complaints of fatigue and night sweats
- PMH: patient takes OTC antiacid tablets a few times a week for a “sensitive” stomach
- PE: Enlarged mobile lymph nodes bilaterally (~1.5 cm), no palpable spleen or liver
- Laboratory findings:
- WBC; 102 X 109/L
- Lymphocytes; 79 X 109/L
- Hb; 11.4 g/dL
- Platelets; 180 X 109/L
- ANC; 1,900/mm3
- LDH 1470 U/L
- Cytogenetics; del(11q), IgVH-unmutated
- beta2M, 3.0 mg/L
- Rai Stage I
Treatment Plan
- Patient was started on ibrutinib

Understanding the Role of Zanubrutinib for the Treatment of CLL
Current frontline treatment options for patients with chronic lymphocytic leukemia have grown over the past year, specifically with the approval of zanubrutinib.

FDA Clears IND of PRAME TCR-IL-15 NK Program for R/R Myeloid Malignancies
The FDA issued a ‘safe to proceed’ for the investigational new drug application for PRAME TCR/IL-15 NK for relapsed or refractory myeloid malignancies.

Exciting Activity in Chronic Lymphocytic Leukemia
In season 4, episode 7 of Targeted Talks, Cyrus M. Khan, MD, discusses the latest FDA activity in the chronic lymphocytic leukemia space.

Real-World Analysis Shows Promising Outcomes, Safety With Ibrutinib in CLL
A 2-year follow-up study showed that ibrutinib maintained efficacy and safety among patients with chronic lymphocytic leukemia, highlighting its importance in the field.

Considering the Durability of Zanubrutinib in Relapsed/Refractory CLL
During a Case-Based Roundtable® event, Marc S. Hoffmann, MD, discussed his viewpoints on the use of Bruton tyrosine kinase inhibitors for patients with relapsed/refractory chronic lymphocytic leukemia and the efficacy behind zanubrutinib in the second article of a 2-part series.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Lablogatory
A blog for medical laboratory professionals

Hematology Case Study: 75 Year Old Man with Leukopenia
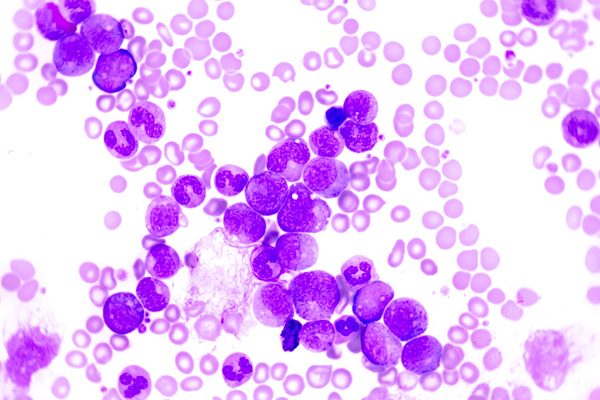
A 75 year old male first presented earlier this year with abnormal CBC results. The patient has a history of Type 2 diabetes, high blood pressure and atrial fibrillation. He was diagnosed with non-small-cell lung cancer (NSCLC) 6 years ago. His stage II NSCLC was completely removed with surgery. Surgery was followed up with adjuvant cisplatin-based chemotherapy to reduce the chance that the cancer would return. In June, he was referred to the hematology oncology department following consecutive CBCs that revealed leukopenia and thrombocytopenia. The CBC results from these specimens are shown below in Table 1.
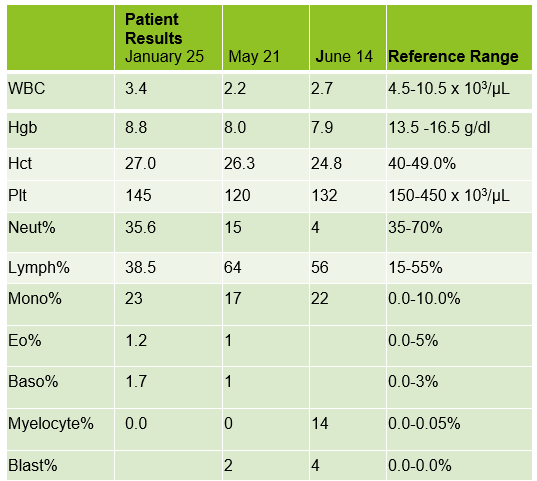
The peripheral blood sample from June was sent for flow cytometry. A leukemia/lymphoma phenotype was performed. Result comments noted proportionately decreased granulocytes with a left shift and 4% blasts. The blasts were CD34+, CD117+, HLA-DR+, CD13+ and CD33+ and were identified as myeloblasts. There were proportionately increased atypical monocytes with CD23 expression. Lymphocytes were also proportionately increased and included an increased population of CD57+, CD3+ T cells consistent with T-cell large granular (LGL) expansion. Immunophenotypic findings raised a concern for a myelodysplastic process. The hematologist discussed the findings with the patient and the patient was scheduled for a bone marrow biopsy. The procedure was performed 3 weeks later. CBC results on the day of the procedure are shown below in Table 2.
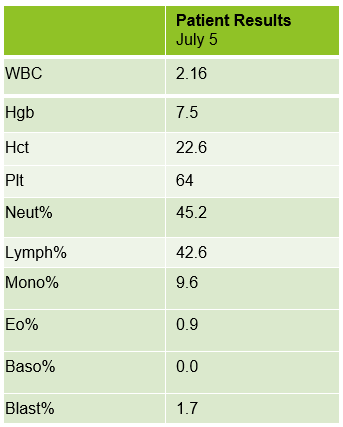
Bone marrow aspirate showed markedly increased myeloblasts (55%), consistent with acute myeloid leukemia (AML), nonacute promyelocytic leukemia (APL) type. The phenotype of the blasts was CD13+, CD33+, CD117+ and HLA-DR+. Blasts were negative for CD34. Several genomic variations were found in the specimen. These included variations in IDH2, SRSF2, STAG2 and ASXL1. Diagnosis: Increase in myeloblasts consistent with AML, nonAPL type.
In July, 20 days after the bone marrow procedure and AML diagnosis, the patient was scheduled to begin his first cycle of Azacitidine (Vidaza). Based on his critical hemoglobin, the patient received 1 unit of packed RBCs followed by his first Vidaza injections. This Cycle 1, Day 1 chemotherapy was well tolerated, and he returned home. The following day he returned for his second treatment. His CBC showed good response to the previous day’s transfusion and his Cycle 1, Day 2 Vidaza was administered without incident. However, that evening the patient presented to the ER with nausea, vomiting and nose bleeds. The patient was admitted to the hospital and received another RBC transfusion. For the next several days the patient continued to do poorly, requiring additional RBC transfusions, and the Vidaza treatments were deferred, then discontinued. The patient had several ER visits and hospital admissions with transfusions over the next 2 weeks. During this time, we saw his blast% on his differential peak at over 60%. The patient was transferred to the palliative care team with care and comfort measures. CBC results from Cycle 1, Day 1 and subsequent CBC results are shown below. Note the sharp increase in blasts over a 2-week period.
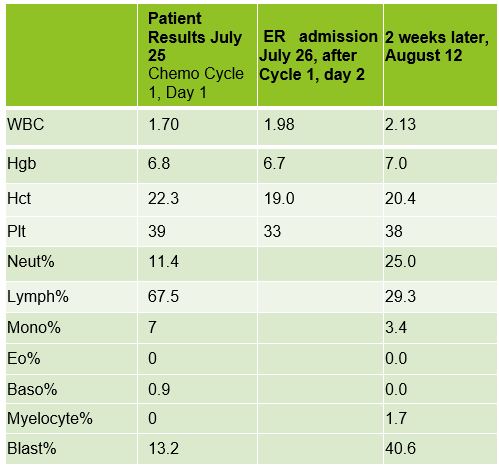
AML is the most common acute leukemia in adults. In AML with minimal differentiation, evidence of bone marrow failure is characterized by anemia, neutropenia, and thrombocytopenia. The median age for patients with AML in the US is 66-67, and those who are older than 55-65 at diagnosis often have challenges and lower odds for long term survival. These older patients tend to have poor tolerance to traditional aggressive chemotherapy because of other health issues. This patient was likely not a good candidate for strong chemotherapy because of his age and health history. In these more fragile patients, Vidaza may be used. Vidaza is a class of drug called a hypomethylating agent that works by switching off DNA methyltransferase. This switches on genes that stop the cancer cells growing and dividing. The goal is to reduce the number of abnormal blood cells and to control cell growth.
As you can see from the CBC results, the onset of this patient’s AML was very abrupt, and the disease progressed rapidly. He has several risk factors that made him more likely to be diagnosed with AML. Older age is a risk factor for AML, and AML is more common in males than females. He has a history of smoking which is a behavioral risk factor associated with AML. Additionally, patients with cancer who are treated with certain chemotherapy drugs are more likely to develop AML in the years following treatment. This patient was treated with cisplatin following lung cancer surgery. Cisplatin is an alkylating agent which has been linked to an increased risk of AML.
Also interesting is the note on the peripheral blood phenotype interpretation that a T-cell large granular lymphocyte (LGL) expansion was present. These are an increased population of CD57+, CD3+ T cells. LGL clones have been described in AML and a hallmark of this association is cytopenia, as is observed in this patient. The patient’s poor prognosis can partly be attributed to the p.Gly646TrfsTer12 alteration in the ASXL1 gene, identified in the bone marrow interpretation. This alteration is associated with decreased overall survival and poor prognosis which was observed in this patient.
I work in a hospital with a large hematology/oncology practice, and we see a lot of adult leukemia patients. Many of the patients we see regularly have Chronic Lymphocytic Leukemia (CLL). We feel like we get to know these patients, because even though we never see them, we see their CBCs every week, sometimes for many years. This was an interesting case because it reminded me of the sudden onset and rapid progression of AML. It was amazing to see the differentials change so dramatically in a matter of weeks. This patient is currently receiving care and comfort end of life measures.
Fattizzo, B, Bellani, V, et al. Large Granular Lymphocyte Expansion in Myeloid Diseases and Bone Marrow Failure Syndromes: Whoever Seeks Finds. Front. Oncol., Sec. Cancer Immunity and Immunotherapy. 01 October 2021.
Pratcorona M, Abbas S, Sanders MA, Koenders JE, et al.Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica. 2012 Mar;97(3):388-92. doi: 10.3324/haematol.2011.051532. Epub 2011 Nov 4. PMID: 22058207; PMCID: PMC3291593.
https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/types-treatment
Thomas XG, Dmoszynska A, Wierzbowska, et al. Results from a randomized phase III trial of decitabine versus supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed AML. Journal of Clinical Oncology 29:2011
Turgeon, Mary Louis. Clinical Hematology Theory and Procedures, 6 th ed, Jones and Bartlett Learning, 2017.

-Becky Socha, MS, MLS(ASCP) CM BB CM graduated from Merrimack College in N. Andover, Massachusetts with a BS in Medical Technology and completed her MS in Clinical Laboratory Sciences at the University of Massachusetts, Lowell. She has worked as a Medical Technologist for over 40 years and has taught as an adjunct faculty member at Merrimack College, UMass Lowell and Stevenson University for over 20 years. She has worked in all areas of the clinical laboratory, but has a special interest in Hematology and Blood Banking. She currently works at Mercy Medical Center in Baltimore, Md. When she’s not busy being a mad scientist, she can be found outside riding her bicycle.
Share this:
One thought on “hematology case study: 75 year old man with leukopenia”.
clinical data of fever, recent illness, history of drug intake, differential leucocytic count and white blood cells morpholog is needed
Leave a comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
- Introduction
- Conclusions
- Article Information
eTable 1. List of Codes Used for Identification of Medically Assisted Reproduction–Related Procedures
eTable 2. Grouping of ICD-10 Diagnostic Codes Used to Approximate the International Childhood Cancer Classification
eTable 3. Number of Cancers Among Children Born and Diagnosed in France Between 2010 and 2015 in the French National Registry of Childhood Cancers (RNCE) and in the Present Study
eTable 4. Birth Characteristics of Children Born in France in 2010 to 2021 by Cancer Status
eTable 5. Risk of Cancer in Children Born in France in 2010 to 2021 After Fresh Embryo Transfer Compared to Naturally Conceived Children
eTable 6. Risk of Cancer in Children Born in France in 2010 to 2021 After Frozen Embryo Transfer Compared to Naturally Conceived Children
eTable 7. Risk of Cancer in Children Born in France in 2010 to 2021 After Artificial Insemination Compared to Naturally Conceived Children
eTable 8. Number of Cases, Incidence Rate, and Risk of Cancer in Children Born After Natural Conception, Fresh Embryo Transfer, Frozen Embryo Transfer, or Artificial Insemination in Singletons Born in France in 2010 to 2021
eTable 9. Risk of Cancer in Children Born in France in 2010 to 2021 After Fresh Embryo Transfer, Frozen Embryo Transfer, or Artificial Insemination Compared to Naturally Conceived Children
eTable 10. Risk of Cancer in Children Born in France in 2010 to 2021 After Fresh Embryo Transfer, Frozen Embryo Transfer, or Artificial Insemination Compared to Naturally Conceived Children
eTable 11. Number of Children Born After Medically Assisted Reproduction in France Between 2010 and 2020 According to the French National Agency of Biomedicine (ABM) and in the Present Study
Data Sharing Statement
- Cancer Risk Among Children Born After Fertility Treatment JAMA Network Open Invited Commentary May 2, 2024 Marie Hargreave, PhD
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Rios P , Herlemont P , Fauque P, et al. Medically Assisted Reproduction and Risk of Cancer Among Offspring. JAMA Netw Open. 2024;7(5):e249429. doi:10.1001/jamanetworkopen.2024.9429
Manage citations:
© 2024
- Permissions
Medically Assisted Reproduction and Risk of Cancer Among Offspring
- 1 EPI-PHARE Scientific Interest Group in Epidemiology of Health Products, French National Agency for Medicines and Health Products Safety, French National Health Insurance, Saint-Denis, France
- 2 Epidemiology of Childhood and Adolescent Cancers, Centre for Research in Epidemiology and Statistics, French National Institute for Health and Medical Research (INSERM) Joint Research Unit (UMR) 1153, Université Paris-Cité, Paris, France
- 3 INSERM UMR 1231, Université Bourgogne Franche-Comté, Dijon, France
- 4 French National Registry of Childhood Cancers, Assistance Publique–Hôpitaux de Paris, Centre Hospitalier Régional Universitaire (CHU) Paul Brousse, Villejuif, France
- 5 French National Registry of Childhood Solid Tumours, CHU de Nancy, Nancy, France
- 6 Université Paris-Cité, Paris, France
- Invited Commentary Cancer Risk Among Children Born After Fertility Treatment Marie Hargreave, PhD JAMA Network Open
Question Does cancer risk differ among children born after fresh embryo transfer (ET), frozen embryo transfer (FET), or artificial insemination (AI) and children conceived naturally?
Findings In this cohort study of 8 526 306 children, the overall risk of cancer did not differ among children born after fresh ET, FET, or AI and children conceived naturally. However, an increased risk of leukemia was observed among children born after fresh ET (especially those with the longest follow-up) and among children born after FET (particularly acute lymphoblastic leukemia).
Meaning These findings suggest that the risk of leukemia among children conceived by FET or fresh ET should be further monitored.
Importance Cancer is a leading cause of death among children worldwide. Treatments used for medically assisted reproduction (MAR) are suspected risk factors because of their potential for epigenetic disturbance and associated congenital malformations.
Objective To assess the risk of cancer, overall and by cancer type, among children born after MAR compared with children conceived naturally.
Design, Setting, and Participants For this cohort study, the French National Mother-Child Register (EPI-MERES) was searched for all live births that occurred in France between January 1, 2010, and December 31, 2021 (and followed up until June 30, 2022). The EPI-MERES was built from comprehensive data of the French National Health Data System. Data analysis was performed from December 1, 2021, to June 30, 2023.
Exposure Use of assisted reproduction technologies (ART), such as fresh embryo transfer (ET) or frozen ET (FET), and artificial insemination (AI).
Main Outcomes and Measures The risk of cancer was compared, overall and by cancer type, among children born after fresh ET, FET, or AI and children conceived naturally, using Cox proportional hazards regression models adjusted for maternal and child characteristics at birth.
Results This study included 8 526 306 children with a mean (SD) age of 6.4 (3.4) years; 51.2% were boys, 96.4% were singletons, 12.1% were small for gestational age at birth, and 3.1% had a congenital malformation. There were 260 236 children (3.1%) born after MAR, including 133 965 (1.6%) after fresh ET, 66 165 (0.8%) after FET, and 60 106 (0.7%) after AI. A total of 9256 case patients with cancer were identified over a median follow-up of 6.7 (IQR, 3.7-9.6) years; 165, 57, and 70 were born after fresh ET, FET, and AI, respectively. The overall risk of cancer did not differ between children conceived naturally and those born after fresh ET (hazard ratio [HR], 1.12 [95% CI, 0.96 to 1.31]), FET (HR, 1.02 [95% CI, 0.78 to 1.32]), or AI (HR, 1.09 [95% CI, 0.86 to 1.38]). However, the risk of acute lymphoblastic leukemia was higher among children born after FET (20 case patients; HR 1.61 [95% CI, 1.04 to 2.50]; risk difference [RD], 23.2 [95% CI, 1.5 to 57.0] per million person-years) compared with children conceived naturally. Moreover, among children born between 2010 and 2015, the risk of leukemia was higher among children born after fresh ET (45 case patients; HR, 1.42 [95% CI, 1.06 to 1.92]; adjusted RD, 19.7 [95% CI, 2.8 to 43.2] per million person-years).
Conclusions and Relevance The findings of this cohort study suggest that children born after FET or fresh ET had an increased risk of leukemia compared with children conceived naturally. This risk, although resulting in a limited number of cases, needs to be monitored in view of the continuous increase in the use of ART.
Childhood cancer is a leading cause of death among children worldwide. Overall, 1800 cases occur every year in France among children aged younger than 15 years, with half occurring before age 6 years. 1 The early onset after birth and the embryologic characteristics of some cancers 2 suggest that the causes may take place around conception. Treatments involved in medically assisted reproduction (MAR), 3 including assisted reproduction technologies (ART; eg, fresh embryo transfer [ET] or frozen ET [FET]) and artificial insemination (AI), have been suggested as potential risk factors for childhood cancer. 4 - 6
Children born after MAR represent a growing population worldwide. 7 Compared with children conceived naturally, children born after MAR have an increased risk of adverse perinatal outcomes, including preterm birth, low birth weight, and fetal growth anomalies. 8 , 9 However, little is known about the medium-term and long-term effects of fertility treatments on child health. Previous studies on the risk of childhood cancer among children born after ART, most of which were based on relatively small cohorts of exposed children 10 - 18 or interview-based case-control study designs, 19 - 24 provided contrasting results. With regard to findings from large cohorts including 100 000 or more exposed children, 25 - 29 no increase in overall cancer risk has been reported, except 1 study 29 that reported a marginal association with ART. Nevertheless, positive associations have been reported when investigating specific cancer types. 25 - 29 In addition, previous studies that assessed the risk of childhood cancer by fresh ET or FET 12 , 15 , 17 , 25 - 28 were based on limited numbers of case patients exposed to each ART modality.
We aimed to assess the risk of cancer, overall and by cancer type, among children born after ART use (fresh ET or FET) or after AI compared with children conceived naturally, considering all children born in France between 2010 and 2021.
This cohort study was authorized by decree 2016-1871 from December 26, 2016, relating to the processing of personal data from the National Health Data System and French law Art. 1461-13 and 14. The EPI-PHARE Scientific Interest Group in Epidemiology of Health Products, which has permanent regulatory access to the data from the French National Health Data System (SNDS; in application of the provisions of Art. R. 1461-12 et seq. 9 of the French Public Health Code and French Data Protection Authority decision CNIL-2016-10 316), was exempted from participant consent and institutional review board approval. The study was registered in the EPI-PHARE SNDS-based registry under reference T-2020-10-272. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
This study was conducted using comprehensive data from the French National Mother-Child Register (EPI-MERES) for all children born alive in France between January 1, 2010, and December 31, 2021. The EPI-MERES database was built from the SNDS, 30 which provides comprehensive hospitalization and health insurance reimbursement data for more than 99% of the French population; it is an unselected register of all pregnancies from 2010 onward in France, identified by end-of-pregnancy diagnoses and procedure codes and linked to their offspring using a deterministic algorithm. Linkage of mother and infant records is available for 95% of children. As of today, the EPI-MERES includes data for more than 8.5 million liveborn children, accounting for 98% of all live births recorded in France since 2010. The EPI-MERES contains a wide set of information on mothers (medical history and sociodemographic characteristics), pregnancies (start and end dates, mode of delivery, and pregnancy outcome), and offspring (gestational age and birth weight, outpatient and inpatient health care use, and hospital discharge diagnoses and medical procedures coded according to the Classification Commune des Actes Médicaux [ CCAM ]). Patients with long-term diseases (LTDs), such as cancer, benefit from full coverage of their health expenditure, and the diagnosis is recorded in the Datamart Consommation Inter Régime (DCIR) using International Statistical Classification of Diseases, Tenth Revision ( ICD-10 ) codes. The DCIR is a French health care expenditure database that contains all of the individual data of health insurance beneficiaries. Data from the EPI-MERES have been used previously to conduct drug use and safety pharmacoepidemiologic studies. 31 - 34
Artificial insemination and ART are fully covered by French health insurance after medical validation and until a maternal age of 43 years. Procedures related to MAR and their dates were identified using CCAM codes for oocyte retrieval, ET, and AI (eTable 1 in Supplement 1 ). Children were considered as born after MAR when the conception date fell within 21 days of the MAR procedure recorded in the SNDS, and as naturally conceived otherwise. The recorded date of ET was retained as the ART procedure date. Children were considered as born after fresh ET when there were fewer than 21 days between the date of oocyte retrieval and the ET, and as born after FET when the delay was 21 days or longer.
Childhood cancers were identified using hospital discharge and LTD diagnoses, using algorithms constructed in collaboration with experts in cancer coding from the French National Registry of Childhood Cancers (RNCE). Children were considered case patients with cancer if they met at least 1 of the following criteria: (1) had at least 2 hospitalizations with a discharge diagnosis of cancer; (2) had at least 1 hospitalization with a discharge diagnosis of cancer in addition to 1 hospitalization for oncologic treatment (chemotherapy, radiotherapy, oncologic surgery, or bone marrow transplantation) or an LTD diagnosis of childhood cancer; or (3) had 1 hospitalization with a discharge diagnosis of cancer, followed by death within 6 months.
Cancer diagnoses were categorized into 12 groups as close as possible to those of the International Classification of Childhood Cancer, Third Edition ( ICCC-3 ; eTable 2 in Supplement 1 ), 35 using ICD-10 codes, in the absence of the morphology and topography codes of the International Classification of Diseases for Oncology, Third Edition ( ICD-O-3 ) required for ICCC-3 . For each child, the most frequently coded cancer diagnostic group among all their hospital stays was retained as the cancer diagnosis. Because of a lack of specificity, for malignant bone tumors and gonadic neoplasms, only case patients with identification of an oncologic treatment were retained. Because information available in the SNDS does not allow for the proper individual identification of same-sex siblings born of multiple pregnancies, only 1 case patient was retained in the case of identical cancer diagnosis in same-sex twins. Anonymized data provided by the RNCE for children born and diagnosed in the 2010 to 2015 period were used as the reference set for the distribution in large categories of cancer diagnoses (eTable 3 in Supplement 1 ).
Characteristics of children included year of birth, sex, singleton or multiple birth, birth weight (<1000, 1000-2499, 2500-3999, or ≥4000 g), gestational length (≤28, 29-32, 33-36, 37-41, or ≥42 weeks), and an indicator of fetal growth (small, adequate, or large for gestational age). Major congenital malformations were identified based on hospital discharge diagnoses within up to 12 months after birth, based on ICD-10 codes selected by the European Surveillance of Congenital Anomalies network. 36 Characteristics of mothers included maternal age at the index child birth (≤24, 25-29, 30-34, 35-39, or ≥40 years) and the French census-based deprivation index 37 of the municipality of residence at birth, used as a proxy for the household socioeconomic category. The French census-based deprivation index is categorized in quintiles, with the fifth quintile representing the most deprived municipalities.
Children contributed to person-years at risk from their date of birth until the date of first hospitalization with a discharge diagnosis of cancer, the date of death, or the end of the study on June 30, 2022, whichever occurred first. We compared the risk of cancer among children born after fresh ET, FET, or AI with that of children conceived naturally, taking all cancers together and by main cancer groups. The risk of leukemia was examined overall and by leukemia subtype (acute lymphoblastic leukemia [ALL] and acute myeloid leukemia [AML]).
Adjusted hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models including a priori selected covariates reported to be associated with childhood cancers and MAR in the literature (ie, sex, year of birth, multiple birth, maternal age at childbirth, and deprivation index). Additional models also adjusted for potential mediating factors including gestational length, birth weight, macrosomia, and congenital malformations. The percentages of missing data were small. To assess cancer risk among children followed over a longer period, complementary analyses restricted to children born between 2010 and 2015 were performed for most frequent childhood cancers. Partial likelihood tests were performed post hoc to determine whether the estimated HR differed across the various MAR modalities. Sensitivity analyses restricted to singletons were performed. Additional sensitivity analyses were performed using the first coded (instead of the most frequently coded) diagnosis group as the cancer diagnosis and restricting the case definition to children with chemotherapy exposure. The proportional hazards assumption was tested with Schoenfeld residuals, and there were no clear violations. Hazard ratios were not estimated for categories with fewer than 5 case patients. All P values were 2-sided and a significance level of .05 was applied. Data analysis was performed from December 1, 2021, to June 30, 2023, using SAS, version 9.4 (SAS Institute Inc).
The study cohort included 8 526 306 children, with a mean (SD) age of 6.4 (3.4) years. Boys comprised 51.2% of the cohort, and girls comprised 48.8%; 96.4% of children were singletons, 12.1% were small for gestational age at birth, and 3.1% had a congenital malformation. There were 260 236 children (3.1%) born after MAR, including 133 965 (1.6%) after fresh ET, 66 165 (0.8%) after FET, and 60 106 (0.7%) after AI. Although the number of births after fresh ET decreased from 2015 onward, a continuous increase in the number of children born after FET was observed over the study period and FET started to prevail over fresh ET in 2020. The annual number of children born after AI remained stable over the study period ( Figure ).
Compared with children conceived naturally, children born after MAR were more often part of a multiple birth, had lower birth weight and gestational age, were more often born small for gestational age, and were more often diagnosed with a congenital malformation. Children born after FET were more frequently born large for gestational age. Mothers whose children were born after MAR were older and their residence at birth was more often in the least deprived municipalities ( Table 1 ).
During a median follow-up of 6.7 (IQR, 3.7-9.6) years for children conceived naturally and 6.8 (IQR, 3.4-9.1) years, 4.4 (IQR, 2.4-7.3) years, and 6.5 (IQR, 3.6-9.4) years for children born after fresh ET, FET, and AI, respectively, 9256 case patients with cancer were identified. A total of 8964 cases were observed among children conceived naturally (incidence rate [IR], 164 per million person-years), 165 among children born after fresh ET (IR, 183 per million person-years), 57 among children born after FET (IR, 172 per million person-years), and 70 among children born after AI (IR, 179 per million person-years). Case patients with cancer more often were born preterm, were large for gestational age, and were diagnosed with congenital malformation compared with children without cancer (eTable 4 in Supplement 1 ). The overall risk of cancer did not differ between children conceived naturally and those born after fresh ET (HR, 1.12 [95% CI, 0.96 to 1.31]), FET (HR, 1.02 [95% CI, 0.78 to 1.32]), or AI (HR, 1.09 [95% CI, 0.86 to 1.38]) ( Table 2 ).
Leukemia was the most commonly diagnosed type of cancer, accounting for 29.4% of total cases. Among these case patients, 2635 were children conceived naturally (IR, 48 per million person-years), 52 were born after fresh ET (IR, 58 per million person-years), 23 were born after FET (IR, 69 per million person-years), and 19 were born after AI (IR, 49 per million person-years) ( Table 2 ). Acute lymphoblastic leukemia accounted for 79.1% of leukemia cases. Among these case patients, 2 083 were children conceived naturally (IR, 38 per million person-years), 39 were born after fresh ET (IR, 43 per million person-years), 20 were born after FET (IR, 60 per million person-years), and 16 were born after AI (IR, 41 per million person-years). Overall, the risk of leukemia for children born after MAR did not differ significantly from that of children conceived naturally (fresh ET: HR, 1.19 [95% CI, 0.90 to 1.56]; FET: HR, 1.42 [95% CI, 0.94 to 2.14; and AI: HR, 1.01 [95% CI, 0.64 to 1.58]). The risk of ALL was significantly increased after FET (HR, 1.61 [95% CI, 1.04 to 2.50]; adjusted risk difference [RD], 23.2 [95% CI, 1.5 to 57.0] per million person-years), but not after fresh ET (HR, 1.14 [95% CI, 0.83 to 1.57]) ( Table 2 ). The partial likelihood test was not statistically significant ( P = .07).
Besides leukemia, the most frequent groups of cancers were tumors of the central nervous system (CNS; n = 1875), embryonal tumors (n = 2481), and lymphomas (n = 823). Compared with children conceived naturally, the risk of each of these cancer groups did not differ among children born after fresh ET or among those born after FET. The risk of malignant CNS tumors tended to be higher among children born after AI, although estimates did not reach statistical significance (HR, 1.51 [95% CI, 0.92 to 2.38]; adjusted RD, 16.8 [95% CI, –2.6 to 45.5] per million person-years) ( Table 2 ).
In the analyses restricted to children born between 2010 and 2015, the median follow-up duration was longer and more homogeneous for the different modes of conception. These median durations were as follows: 9.4 (IQR, 7.8-10.9) years for natural conception, 9.4 (IQR, 7.9-10.9) years for fresh ET, 8.8 (IQR, 7.5-10.4) years for FET, and 9.3 (IQR, 7.9-10.8) years for AI. The risk of leukemia was significantly increased among children born after fresh ET (HR, 1.42 [95% CI, 1.06 to 1.92]; adjusted RD, 19.7 [95% CI, 2.8 to 43.2] per million person-years) but not after FET (HR, 1.27 [95% CI, 0.70 to 2.29]). The partial likelihood test was not statistically significant ( P = .06). The risk of ALL was increased, without reaching statistical significance, among children born after fresh ET (HR, 1.33 [95% CI, 0.93 to 1.89]; adjusted RD, 12.5 [95% CI, – 2.7 to 33.8] per million person-years) and FET (HR, 1.47 [95% CI, 0.79 to 2.74]; adjusted RD, 17.9 [95% CI, −8.0 to 66.1] per million person-years). No other association was observed ( Table 3 ).
Additional adjustments for birth characteristics and congenital malformations marginally modified the estimates (eTables 5-7 in Supplement 1 ). Results remained stable in sensitivity analyses restricted to singletons or based on alternative case definitions (eTables 8-10 in Supplement 1 ).
In this nationwide birth cohort study including more than 8.5 million children, the risk of any cancer was not increased among children born after MAR. However, the findings suggest that children born after fresh ET or FET had a higher risk of leukemia.
The absence of an overall association between cancer and MAR in this study is consistent with the findings of most large observational studies. 25 - 28 However, the grouping of all cancer types, although meaningful for detecting common risk factors, could miss subtype specificities. In line with our findings, 4 previous studies 13 , 17 , 27 , 28 reported an increased risk of leukemia among children born after ART. With regard to ART modalities such as fresh ET or FET, the literature is still sparse and results are conflicting. The data used in this study were obtained from one of the largest cohorts of children born after FET to date; thus, our results support the hypothesis of an increased risk of leukemia, mainly observable for ALL, with a limited number for AML. In our complementary analysis restricted to births between 2010 and 2015, the risk of leukemia was increased for children born after FET (although statistical significance was not reached because of reduced numbers of exposed case patients) and also for those born after fresh ET, probably as a result of more homogeneous follow-up between children born after fresh ET or FET and better coverage of the age range corresponding to the incidence peak of leukemia (2-6 years). Sargisian et al 28 conducted a study (median follow-up of 9.9 years) in Nordic countries, and they found an increased risk of leukemia among children born after FET vs children conceived naturally (HR, 2.22 [95% CI, 1.47 to 3.35]) but not among those born after fresh ET. In another study, Weng et al 17 found that the risk of leukemia was significantly increased among children born after fresh ET, but not among children born after FET. However, estimates were based on fewer than 5 exposed case patients by ART modality. A meta-analysis conducted by Zhang et al 38 reported no increased cancer risk among children born after ART based on 15 cohorts, but the authors found an increased risk of childhood cancer among children born after FET (pooled HR, 1.37 [95% CI, 1.04 to 1.81]) compared with children conceived naturally. However, the finding was not statistically significant when children born after fresh ET were considered as the reference group (pooled HR, 1.28 [95% CI, 0.96 to 1.69]), indicating that the increased incidence of cancer among children born after FET may be based on in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), or any other ART. Differences between studies might be explained by heterogeneity across them in terms of sample size, follow-up duration, and study period, because substantial changes in ART practices occurred over time within the same laboratory and among different laboratories (eg, culture conditions: culture media used and oxygen tension, or embryo freezing processes).
Our findings do not provide evidence of an increased risk of other types of childhood cancer among children born after MAR. Based on 19 exposed case patients, the nonsignificantly increased risk of CNS tumors after AI reported here warrants further investigation. Furthermore, the number of hepatic tumors among exposed children was very small (<5 after fresh ET and 0 after FET), and the overall risk of embryonal tumors was not increased among children born after MAR.
The etiologic mechanisms behind a possible increased risk of cancer among children born after MAR are not known, but explanatory hypotheses consider epigenetic disturbance as a potential pathway. Previous studies have found that procedures involved in MAR, such as IVF, ICSI, and ovarian stimulation, can induce epigenetic changes in human embryos, cord blood, and placentae. 39 , 40 This finding is also supported by studies that suggest an association between MAR-related procedures and imprinting disorders, 41 such as Beckwith-Weidemann syndrome, 42 a pediatric overgrowth disorder involving a predisposition to tumor development.
Our study has major strengths. It is based on one of the largest cohorts published to date and on comprehensive data covering the entire French population, and we used reliable and systematically collected data, with no potential for recall bias and selection bias. We performed analyses for the most frequent childhood cancer groups and by MAR modality, and we had reliable individual information on major confounders and potential mediating factors.
Our findings must be interpreted in light of some limitations. We used reimbursement and hospitalization data to identify exposure to MAR procedures and childhood cancer occurrence. With regard to exposure assessment, MAR-related procedures are expensive treatments that benefit from full insurance coverage in France; therefore, they typically are not subject to nondeclaration. Compared with reported numbers from the French Agency of Biomedicine over the same period, our study was able to identify more than 90% of liveborn children conceived by MAR, and the distribution by fresh ET, FET, or AI was comparable (eTable 11 in Supplement 1 ). Still, information on the type of ART (ie, ICSI) or cross-border fertility care was not available.
With regard to outcome assessment, most pediatric cancers are life-threatening pathologies requiring hospitalization for diagnosis and treatment and are very unlikely to be underdiagnosed in France. The algorithms used in our study were able to identify the most frequent groups of childhood cancers with numbers close to that of the RNCE except for tumors of the adrenal glands, peripheral nerves, and autonomic nervous system, which were underestimated in our study based on ICD-10 diagnoses. Conversely, the number of bone tumors, hepatic tumors, and malignant CNS tumors was higher than that of the registry, possibly reflecting inclusions of metastatic localizations as primary sites. Exposure or outcome misclassification might have attenuated the associations.
Additionally, information on child emigration was not available. Given the rarity of childhood cancers, the number of potential undetected cases because of child emigration is likely to be small. Child emigration could also lead to overestimation of the follow-up time. However, these potential misclassification biases might not differ by conception mode; therefore, they should not have substantially biased our estimates.
Many potential confounders have been taken into account. However, as with most studies on the effects of fertility treatments on child health, our study was unable to distinguish between possible treatment effects and parental conditions underlying infertility 43 that might be associated with an increased risk of cancer in offspring. Therefore, residual confounding cannot be completely ruled out.
Finally, although this study included a large nationwide cohort, the absolute number of children with cancer was relatively small. Risk assessment of childhood cancers is challenging given their rarity, and results based on small numbers may be subject to both lack of power or the potential for spurious associations. We did not adjust for multiple comparisons. Therefore, these findings must be interpreted with caution.
In this cohort study, the overall risk of cancer was not increased after MAR but our findings suggest that children born after FET or fresh ET may have an increased risk of leukemia. This risk, although resulting in a limited number of cases, needs to be monitored in view of the continuous increase in the use of ART.
Accepted for Publication: February 12, 2024.
Published: May 2, 2024. doi:10.1001/jamanetworkopen.2024.9429
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Rios P et al. JAMA Network Open .
Corresponding Author: Paula Rios, MD, PhD, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products, French National Agency for Medicines and Health Products Safety, French National Health Insurance, 42 Bd de la Libération, 93200 Saint-Denis, France ( [email protected] ).
Author Contributions: Dr Rios and Mr Herlemont had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Zureik, Clavel, and Dray-Spira contributed equally to this work.
Concept and design: Rios, Zureik, Clavel, Dray-Spira.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Rios, Herlemont, Zureik, Clavel.
Critical review of the manuscript for important intellectual content: Rios, Fauque, Lacour, Jouannet, Weill, Zureik, Clavel, Dray-Spira.
Statistical analysis: Rios, Clavel.
Supervision: Fauque, Lacour, Jouannet, Zureik, Clavel, Dray-Spira.
Conflict of Interest Disclosures: Dr Rios received grant funding from the French National Agency for Medicines and Health Products Safety during the conduct of the study. No other disclosures were reported.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
300+ Nursing Cheatsheets. Start Free Trial. "Would suggest to all nursing students . . . Guaranteed to ease the stress!". ~Jordan. Leukemia Case Study (60 min) is mentioned in these lessons. Check out this case study on leukemia & learn everything you will need to about to ace your NCLEX questions. View the online lesson today!
Blood. 2013; 121:4854-4860. A 47-year-old woman presents to the emergency department complaining of fatigue and shortness of breath. She reports a two-week history of worsening exercise tolerance and a rather abrupt onset of shortness of breath over the past several hours.
In addition, AML1-ETO fusion gene was found in the case diagnosed with AML-M2. Whether the occurrence of AML1-ETO gene is before lymphoma or not, is not known. AML1-ETO gene is the product of t (8;21) (q22;q22) translocation in AML patients. AML1-ETO keeps the function of DNA binding sites in AML1 and the ability to recruit relevant cofactors ...
Acute myeloid leukemia (AML) is the most common leukemia among the adult population and accounts for about 80% of all cases. It is characterized by clonal expansion of immature "blast cells" in the peripheral blood and bone marrow resulting in ineffective erythropoiesis and bone marrow failure. With recent advancements in the management guidelines, the cure rates have increased up to 15% ...
Nurse Tracy works on an inpatient oncology unit and is caring for Margie, a 60-year-old female with a history of chronic lymphocytic leukemia, or CLL.After settling Margie in her room, Nurse Tracy goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Margie's care by recognizing and analyzing cues, prioritizing hypotheses, generating solutions ...
Relapsed acute myeloid leukemia. Discussion of Management. Dr. Andrew M. Brunner: This 49-year-old man had a subtype of AML that had arisen de novo, with a normal male karyotype and mutations in ...
This study developed a patient with leukemia-care simulation program based on clinical reasoning for nursing students. Clinical reasoning is a cognitive process that uses critical thinking strategies in clinical situations as well as a problem-solving strategy that identifies and diagnoses actual and potential problems of patients based on ...
Acute leukemias have large numbers of immature leukocytes and overproduction of cells in the blast stage of maturation. Acute lymphocytic leukemia (ALL), also known as acute lymphoblastic leukemia, refers to an abnormal growth of lymphocyte precursors or lymphoblasts. Acute leukemia is a malignant proliferation of white blood cell precursors in ...
The healthcare provider may request additional blood tests to assess for the presence of leukemia cells through the following: Peripheral blood smear. Flow cytometry. 3. Prepare the patient for bone marrow aspiration/biopsy. The diagnosis of leukemia is confirmed by bone marrow aspiration and/or biopsy.
Leukemia is a cancer of white blood cells. In acute leukemia, the abnormal cells divide rapidly, quickly overtaking functional white and red blood cells. The most common form of cancer in children 0-14 years of age is acute lymphocytic leukemia (ALL). The survival rate in children has improved more than 50% in the last half century.
Case Study: New Therapies for Acute Myeloid Leukemia. A 76-year-old woman presents to the emergency department following two weeks of progressive dyspnea and fatigue, and a new rash. Her medical history is significant for stage 2 chronic kidney disease, coronary artery disease, and diabetes. Physical examination results are within normal limits ...
Learn all about Leukemia in our free nursing course. View the video lessons, cheat sheets and quiz questions to help you master Leukemia. Leukemia. Watch More! Unlock the full videos with a FREE trial ... Leukemia Case Study (60 min) Leukemia - Signs and Symptoms Nursing Mnemonic (ANT) Nursing Care Plan (NCP) for Leukemia.
Study with Quizlet and memorize flashcards containing terms like Select the 4 findings that require immediate attention. , What are some potential issues or expected complications that the client might be at risk for?, After the nurse's assessment of the chart and laboratory values, the client is at highest risk for developing --- related to --- and more.
April Peters is a 10-year-old female with acute lymphoblastic leukemia (ALL) who presents to the emergency department with a temperature of 38 degrees C. (101 F.) and a complaint of a sore throat. She has been receiving chemotherapy since her diagnosis three months ago.
with leukemia 3 months ago. Her mother states that she has a fever of 102 F and has not been responsive to acetaminophen. B ackground: Primary problem/diagnosis: Sore throat secondary to acute lymphoblastic leukemia. RELEVANT past medical history: Patient was diagnosed with acute lymphoblastic leukemia 3 months ago and receiving chemotherapy.
Colaboradores/as. Mary Roberts MSN, RN, Sam Gillespie, BSc, Jennifer Montague, PhD, Zachary Kevorkian, MSMI. Nurse Tracy works on an inpatient oncology unit and is caring for Margie, a 60-year-old female with a history of chronic lymphocytic leukemia, or CLL. After settling Margie in her room, Nurse Tracy goes through the steps of the Clinical ...
It is common for subtle changes in a child's health to go unnoticed by parents. Which nursing interventions should the nurse implement to decrease the parents' distress? - Ask the family if they would like to meet with another family experiencing a similar situation. - Provide answers to the family's questions about the upcoming treatment plan.
Nurse Tracy works on an inpatient oncology unit and is caring for Margie, a 60-year-old with a history of chronic lymphocytic leukemia, or CLL. In collaboration with the registered nurse, RN Lisa, Nurse Tracy goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Margie's care by recognizing and ...
Check out this case study on leukemia & learn everything you will need to about to ace your NCLEX questions. View the online lesson today! Leukemia Case Study (60 min) Start a free trial to unlock videos, outlines, cheatsheets, and quizzes. ... Leukemia - Signs and Symptoms (Mnemonic)
And so, those are the big, noteworthy aspects of this case, and can be a rather typical presentation for a patient with deletion 11q molecular features like this patient. John Allan, MD: One other aspect of this case that's unique is that the patient is on over-the-counter antacids, and this is a relatively common thing. Many patients are takig ...
Table 1. CBC results from a 75 year old male. The peripheral blood sample from June was sent for flow cytometry. A leukemia/lymphoma phenotype was performed. Result comments noted proportionately decreased granulocytes with a left shift and 4% blasts. The blasts were CD34+, CD117+, HLA-DR+, CD13+ and CD33+ and were identified as myeloblasts.
Acute lymphoblastic leukemia accounted for 79.1% of leukemia cases. Among these case patients, 2 083 were children conceived naturally (IR, 38 per million person-years), 39 were born after fresh ET (IR, 43 per million person-years), 20 were born after FET (IR, 60 per million person-years), and 16 were born after AI (IR, 41 per million person ...
Background/Objectives: Eosinophilic dermatosis of hematologic malignancy (EDHM) is a rare cutaneous disorder associated with various hematologic malignancies, most commonly chronic lymphocytic leukemia. Detailed clinicopathologic studies of EDHM are lacking and the pathogenesis remains enigmatic. Initially thought to be a hypersensitivity reaction to insect stings, subsequent reports have ...