- Search Menu
- Advance Articles
- Thematic Issues
- Clinical Practice Guidelines
- Supplements
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Journal of Clinical Endocrinology & Metabolism
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
What does this mean for those with pcos, introduction, materials and methods, acknowledgments, author contributions, disclosures, data availability, recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome.
This article is simultaneously published in Fertility and Sterility, Journal of Clinical Endocrinology and Metabolism, European Journal of Endocrinology and Human Reproduction .
Participants of the International PCOS Network are listed in the Appendix.
- Article contents
- Figures & tables
- Supplementary Data
Helena J Teede, Chau Thien Tay, Joop J E Laven, Anuja Dokras, Lisa J Moran, Terhi T Piltonen, Michael F Costello, Jacky Boivin, Leanne M Redman, Jacqueline A Boyle, Robert J Norman, Aya Mousa, Anju E Joham, on behalf of the International PCOS Network, Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome, The Journal of Clinical Endocrinology & Metabolism , Volume 108, Issue 10, October 2023, Pages 2447–2469, https://doi.org/10.1210/clinem/dgad463
- Permissions Icon Permissions
What is the recommended assessment and management of those with polycystic ovary syndrome (PCOS), based on the best available evidence, clinical expertise, and consumer preference?
International evidence-based guidelines address prioritized questions and outcomes and include 254 recommendations and practice points, to promote consistent, evidence-based care and improve the experience and health outcomes in PCOS.
The 2018 International PCOS Guideline was independently evaluated as high quality and integrated multidisciplinary and consumer perspectives from six continents; it is now used in 196 countries and is widely cited. It was based on best available, but generally very low to low quality, evidence. It applied robust methodological processes and addressed shared priorities. The guideline transitioned from consensus based to evidence-based diagnostic criteria and enhanced accuracy of diagnosis, whilst promoting consistency of care. However, diagnosis is still delayed, the needs of those with PCOS are not being adequately met, evidence quality was low and evidence-practice gaps persist.
The 2023 International Evidence-based Guideline update reengaged the 2018 network across professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all stages. Extensive evidence synthesis was completed. Appraisal of Guidelines for Research and Evaluation-II (AGREEII)-compliant processes were followed. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was applied across evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength and diversity and inclusion were considered throughout.
This summary should be read in conjunction with the full Guideline for detailed participants and methods. Governance included a six-continent international advisory and management committee, five guideline development groups, and paediatric, consumer, and translation committees. Extensive consumer engagement and guideline experts informed the update scope and priorities. Engaged international society-nominated panels included paediatrics, endocrinology, gynaecology, primary care, reproductive endocrinology, obstetrics, psychiatry, psychology, dietetics, exercise physiology, obesity care, public health and other experts, alongside consumers, project management, evidence synthesis, statisticians and translation experts. Thirty-nine professional and consumer organizations covering 71 countries engaged in the process. Twenty meetings and five face-to-face forums over 12 months addressed 58 prioritized clinical questions involving 52 systematic and 3 narrative reviews. Evidence-based recommendations were developed and approved via consensus across five guideline panels, modified based on international feedback and peer review, independently reviewed for methodological rigour, and approved by the Australian Government National Health and Medical Research Council (NHMRC).
The evidence in the assessment and management of PCOS has generally improved in the past five years, but remains of low to moderate quality. The technical evidence report and analyses (∼6000 pages) underpins 77 evidence-based and 54 consensus recommendations, with 123 practice points. Key updates include: i) further refinement of individual diagnostic criteria, a simplified diagnostic algorithm and inclusion of anti-Müllerian hormone (AMH) levels as an alternative to ultrasound in adults only; ii) strengthening recognition of broader features of PCOS including metabolic risk factors, cardiovascular disease, sleep apnea, very high prevalence of psychological features, and high risk status for adverse outcomes during pregnancy; iii) emphasizing the poorly recognized, diverse burden of disease and the need for greater healthcare professional education, evidence-based patient information, improved models of care and shared decision making to improve patient experience, alongside greater research; iv) maintained emphasis on healthy lifestyle, emotional wellbeing and quality of life, with awareness and consideration of weight stigma; and v) emphasizing evidence-based medical therapy and cheaper and safer fertility management.
Overall, recommendations are strengthened and evidence is improved, but remain generally low to moderate quality. Significantly greater research is now needed in this neglected, yet common condition. Regional health system variation was considered and acknowledged, with a further process for guideline and translation resource adaptation provided.
The 2023 International Guideline for the Assessment and Management of PCOS provides clinicians and patients with clear advice on best practice, based on the best available evidence, expert multidisciplinary input and consumer preferences. Research recommendations have been generated and a comprehensive multifaceted dissemination and translation programme supports the Guideline with an integrated evaluation program.
This effort was primarily funded by the Australian Government via the National Health Medical Research Council (NHMRC) (APP1171592), supported by a partnership with American Society for Reproductive Medicine, Endocrine Society, European Society for Human Reproduction and Embryology, and the European Society for Endocrinology. The Commonwealth Government of Australia also supported Guideline translation through the Medical Research Future Fund (MRFCRI000266). HJT and AM are funded by NHMRC fellowships. JT is funded by a Royal Australasian College of Physicians (RACP) fellowship. Guideline development group members were volunteers. Travel expenses were covered by the sponsoring organizations. Disclosures of interest were strictly managed according to NHMRC policy and are available with the full guideline, technical evidence report, peer review and responses ( www.monash.edu/medicine/mchri/pcos ). Of named authors HJT, CTT, AD, LM, LR, JBoyle, AM have no conflicts of interest to declare. JL declares grant from Ferring and Merck; consulting fees from Ferring and Titus Health Care; speaker's fees from Ferring; unpaid consultancy for Ferring, Roche Diagnostics and Ansh Labs; and sits on advisory boards for Ferring, Roche Diagnostics, Ansh Labs, and Gedeon Richter. TP declares a grant from Roche; consulting fees from Gedeon Richter and Organon; speaker's fees from Gedeon Richter and Exeltis; travel support from Gedeon Richter and Exeltis; unpaid consultancy for Roche Diagnostics; and sits on advisory boards for Roche Diagnostics. MC declares travels support from Merck; and sits on an advisory board for Merck. JBoivin declares grants from Merck Serono Ltd.; consulting fees from Ferring B.V; speaker's fees from Ferring Arzneimittell GmbH; travel support from Organon; and sits on an advisory board for the Office of Health Economics. RJN has received speaker's fees from Merck and sits on an advisory board for Ferring. AJoham has received speaker's fees from Novo Nordisk and Boehringer Ingelheim. The guideline was peer reviewed by special interest groups across our 39 partner and collaborating organizations, was independently methodologically assessed against AGREEII criteria and was approved by all members of the guideline development groups and by the NHMRC.
Building on the 2018 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome (PCOS), this Guideline updates and expands clinical questions, aiming to ensure that women with PCOS receive optimal, evidence-based care that meets their needs and improves health outcomes. The guideline and translation program were developed with full consumer participation at all stages including priority topics and outcomes for those with PCOS. The aim is to support women and their healthcare providers to optimize diagnosis, assessment and management of PCOS. There is an emphasis on improved education and awareness of healthcare professionals, partnership in care, and empowerment of women with PCOS. Personal characteristics, preferences, culture and values are considered, in addition to resource availability across different settings. With effective translation, the Guideline will address priorities identified by women with PCOS, upskill healthcare professionals, empower consumers, improve care and outcomes, identify key research gaps, and promote vital future research.
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting reproductive-aged women, with impacts across the lifespan from adolescence to post menopause. PCOS prevalence is between 10% to 13% as confirmed in the guideline process ( 1 , 2 ). PCOS aetiology is complex; clinical presentation is heterogeneous with reproductive, metabolic, and psychological features ( 1 , 2 ). Women internationally experience delayed diagnosis and dissatisfaction with care ( 3-5 ). Clinical practice in the assessment and management of PCOS remains inconsistent, with ongoing key evidence-practice gaps. Following on from the 2018 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome ( 6 , 7 ), independently evaluated as high quality, this extensive update integrates current literature with previous systematic reviews and extends to new clinical questions prioritized by consumers. Ultimately, we aim to update, extend and translate rigorous, comprehensive evidence-based guidelines for diagnosis, assessment and treatment, to improve the lives of those with PCOS worldwide.
To do so, the Guideline leverages substantive government and society investment and brings together extensive consumer engagement and international collaboration with leading societies and organizations, multidisciplinary experts, and primary care representatives. This comprehensive evidence-based Guideline is constructed from a rigorous, Appraisal of Guidelines for Research and Evaluation-II (AGREEII)-compliant, evidence-based guideline development process. It provides a single source of international evidence-based recommendations to guide clinical practice with the opportunity for adaptation in relevant health systems. Together with an extensive translation program, the aim is to reduce worldwide variation in care and promote high quality clinical service provision to improve health outcomes and quality of life in women with PCOS. The Guideline is supported by a multifaceted international translation programme with co-designed resources to enhance the skills of healthcare professionals and to empower women with PCOS, with an integrated comprehensive evaluation program. Here, we summarize recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of PCOS.
Best practice evidence-based guideline development methods were applied and are detailed in the full Guideline and the technical report, which are available online ( www.monash.edu/medicine/mchri/pcos ) ( 8 ). In brief, extensive healthcare professional and consumer or patient engagement informed the Guideline priority areas. International society-nominated panels from across three leading entities, four partner organizations and thirty-two collaborating entities included consumers and experts in paediatrics, endocrinology, gynaecology, primary care, reproductive endocrinology, psychology, dietetics, exercise physiology, sleep, bariatric/ metabolic surgery, public health, other co-opted experts, project management, evidence synthesis and translation. Governance included an international advisory and a management committee, five guideline development groups (GDGs) with 56 members, and paediatric, consumer, and translation committees. The five GDGs covered i) Screening, diagnostic and risk assessment and life stage; ii) Psychological features and models of care; iii) Lifestyle management; iv) Management of nonfertility features; and v) Assessment and management of infertility. The leading entities; the Australian National Health and Medical Research Council (NHMRC) Centres for Research Excellence in Women's Health in Reproductive Life and in Polycystic Ovary Syndrome, led by Monash University, partnered with the American Society for Reproductive Medicine, the Endocrine Society, the European Society of Endocrinology and the European Society of Human Reproduction and Embryology and collaborated with 32 other entities. With international meetings over 12 months fifty-five prioritized clinical questions involved 52 systematic and three narrative reviews, generating evidence-based and consensus recommendations with accompanying practice points. Committee members nominated by partner and collaborator organizations provided international peer review, and independent experts reviewed methods which were then submitted to NHMRC for independent review. The target audience includes multidisciplinary healthcare professionals, consumers or patients, policy makers, and educators. The Guideline includes a focus on equity, cultural and ethnic diversity, avoidance of stigma and inclusivity (see full guideline for details).
Processes aligned with all elements of the AGREE-II tool for quality guideline assessment ( 9 ), with extensive evidence synthesis and meta-analysis. Integrity assessment was integrated into guideline evidence synthesis processes and followed the Research Integrity in Guideline Development (RIGID) framework, with studies assessed against criteria from the Research Integrity Assessment (RIA) tool and the Trustworthiness in RAndomised Controlled Trials (TRACT) checklist ( 10-12 ). Evidence synthesis methods are outlined in the full guideline and followed best practice ( 9 , 13 , 14 ) Guideline recommendations are presented by category, terms used, evidence quality and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework considerations. Category includes evidence-based (sufficient evidence in PCOS) or consensus (insufficient evidence in PCOS, also evidence in general or relevant populations was considered) recommendations and accompanying practice points (implementation considerations) ( Table 1 ).
Categories of PCOS guideline recommendations
Abbreviation: PCOS, polycystic ovary syndrome.
The terms include “should”, “could” and “should not”, which are informed by the nature of the recommendation (evidence or consensus), the GRADE framework and the evidence quality and are independent descriptors reflecting GDG judgement. They refer to overall interpretation and practical application of the recommendation, balancing benefits and harms. “Should” is used where benefits of the recommendation exceed harms and where the recommendation can be trusted to guide practice. Conditional recommendations are reflected using the terms “could” or “should/could consider” which are used where evidence quality was limited or available studies demonstrate little clear advantage of one approach over another, or the balance of benefits to harms was unclear. “Should not” applies when there is a lack of appropriate evidence, or harms may outweigh benefits.
Evidence quality was categorized according to the GRADE framework, with judgments about the quality of the included studies and/or synthesized evidence incorporating risk of bias, inconsistency, indirectness, imprecision and any other considerations (eg, publication bias) that may influence evidence quality. These judgments considered study number and design, statistical data and importance of outcomes ( Table 2 ). The quality of evidence reflects the confidence that the estimate of the effect is adequate to support each recommendation ( 13 ), largely determined by the expert evidence synthesis team. GRADE acknowledges that evidence quality is a continuum; any discrete categorization involves some arbitrary decisions; nevertheless, the advantages of simplicity, transparency, and clarity outweigh these limitations ( 13 ).
Quality (certainty) of evidence categories (adapted from GRADE)
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
The GRADE framework enabled structured and transparent consideration across evidence quality, feasibility, acceptability, cost, implementation, and ultimately recommendation strength ( 13 ) and was completed at face to face guideline group meetings for all clinical questions ( Table 3 ) ( 15 ).
The grading of recommendations, assessment, development, and evaluation (GRADE) framework recommendation strength
Notably, certainty of evidence varied across outcomes within each question. Here evidence certainty reflects the lowest certainty for the critical outcomes. Evidence was often stronger for the top ranked outcome, and high quality randomized controlled trials (RCTs) were often present, despite overall low quality of evidence. These nuances were considered by the GDG for all question as per the technical report, with any apparent discrepancy between recommendation strength and evidence certainty justified in the full Guideline. Finally, we note that this is a living Guideline with annual evidence review in rapidly evolving areas.
The recommendations ( Table 4 ) apply the category, descriptive terms, GRADE of the recommendations and the quality of the evidence. The full Guideline, technical evidence and administrative reports are available online ( www.monash.edu/medicine/mchri/pcos ). The Guideline outlines the clinical need for the question, the clinical question, the evidence summary, the recommendations and practice points, and a summary of the justification developed by the GDGs using the GRADE framework. Extensive international peer review from across the 39 organizations was then considered by each GDG and recommendations were reconsidered applying the GRADE framework if justified. The comprehensive evidence reviews, profiles, and GRADE frameworks supporting each recommendation can be found in the Technical Report. The administrative report on guideline development, disclosure of interest process and declarations, peer review feedback and responses can also be found online. Here, we present the evidence-based and consensus recommendations and practice points ( Table 4 ). This summary, the full Guideline and technical reports are supported by a comprehensive co-designed translation program to optimize dissemination and impact with resources freely available online ( www.monash.edu/medicine/mchri/pcos ).
Recommendations for the assessment and management of polycystic ovary syndrome (PCOS). © Monash University on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life, 2023.
See Table 1 for the definition of CR, EBR, and PP.
© International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash University (monash.edu/medicine/mchri/pcos), 2023, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This image/content is not covered by the terms of the Creative Commons licence of this publication. For permission re reuse, please contact the rights holder.
Two algorithms are provided to support recommendations on diagnosis ( Fig. 1 ) and infertility management ( Fig. 2 ).

Algorithm 1—Diagnostic algorithm for polycystic ovary syndrome (PCOS). © Monash University on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life, 2023. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash University ( monash.edu/medicine/mchri/pcos ), 2023, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This image/content is not covered by the terms of the Creative Commons licence of this publication. For permission re reuse, please contact the rights holder. * Exclusion of other causes = TSH, prolactin, 17-OH progesterone, FSH or if clinically indicated exclude other causes (eg, Cushing's syndrome, adrenal tumours). For hypogonadotrophic hypogonadism, usually due to low body fat or intensive exercise, exclude clinically and with LH and FSH levels. TSH, thyroid stimulating hormone.

Algorithm 2—Infertility algorithm for polycystic ovary syndrome (PCOS). © Monash University on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life, 2023. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023, Helena Teede et al. Monash University ( monash.edu/medicine/mchri/pcos ), 2023, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This image/content is not covered by the terms of the Creative Commons licence of this publication. For permission re reuse, please contact the rights holder. Central blue pathway follows best practice evidence and is preferred.
The International Evidence-based Guideline for the Assessment and Management of PCOS and the related translation program aims to provide a high quality, reliable source of international evidence-based recommendations to guide consistent clinical practice and to empower women with evidence-based information. All recommendations were formulated after an assessment of the best available evidence, multidisciplinary clinical expertise, consumer preferences and structured review by five GDGs. The guideline provides 77 evidence-based and 54 consensus recommendations, with 123 practice points underpinned by a technical report on evidence synthesis and GRADE detailed considerations (∼6000 pages). The evidence has generally improved over the past five years but remains of low to moderate quality, requiring significant research investment into this neglected, yet common condition.
Key recommendations and updates include that PCOS should be diagnosed using the 2018 International Evidence-based Guideline criteria, which built on the consensus based 2003 Rotterdam criteria. This requires the presence of two of the following: i) clinical/biochemical hyperandrogenism; ii) ovulatory dysfunction; and iii) polycystic ovaries on ultrasound; and here in 2023, alternatively anti-Müllerian hormone (AMH) can now be used instead of ultrasound. Exclusion of other aetiologies. Importantly, where irregular menstrual cycles and hyperandrogenism are present, diagnosis is simplified and ultrasound or AMH are not required for diagnosis. In adolescents, both hyperandrogenism and ovulatory dysfunction are required, with ultrasound and AMH not recommended due to poor specificity. AMH was highlighted as a rapidly evolving area in 2018 and evidence is now strong enough to make this new recommendation. This will significantly change practice and offers women a low cost, convenient option, without evidence of overdiagnosis.
Insulin resistance is recognized as a key feature of PCOS, yet routinely available measures of insulin resistance are inaccurate and clinical measurement is not currently recommended. Once diagnosed, assessment and management should address reproductive, metabolic, cardiovascular, dermatologic, sleep, and psychological features. A lifelong health plan is recommended including a focus on healthy lifestyle, prevention of excess weight gain, optimization of fertility and preconception risk factors, and prevention and treatment of diverse clinical features. These include metabolic risk factors, diabetes, cardiovascular disease, and sleep disorders, which are all increased in PCOS. PCOS should be considered a high-risk condition in pregnancy with women identified and monitored. An increased premenopausal risk of endometrial cancer should also be recognized, whilst absolute risks remain low.
Symptoms of depression and anxiety are significantly increased and should be screened for in all women with PCOS, with psychological assessment and therapy as indicated. Greater awareness of psychological features including eating disorders and impacts on body image and quality of life is needed.
Dissatisfaction with PCOS diagnosis and care is high and significant improvement in education and awareness is strongly recommended for women and healthcare professionals including high quality, evidence-based resources. Shared decision making and self-empowerment are fundamental and integrated models of care should be codesigned, funded and evaluated.
Supported healthy lifestyle remains vital throughout the lifespan in PCOS, with a strong focus on overall health, prevention of weight gain and, if required, on weight management. Recognizing the benefits of many diet and physical activity regimens, there is no one specific regimen that has benefits over others in PCOS. Weight bias and stigma should be minimized and healthcare professionals should seek permission to weigh women, with explanation of weight-related risks.
Combined oral contraceptive pills are the first line pharmacological treatment for menstrual irregularity and hyperandrogenism, with no specific recommended preparation and a preference for lower ethinyl estradiol dose preparations and those with less side-effects. Metformin is recommended primarily for metabolic features and has greater efficacy than inositol, which offers limited clinical benefits in PCOS. Metformin is not routinely recommended for use in pregnant women with PCOS. Mechanical laser therapy is effective for hair reduction in some subgroups, whilst anti-androgens have a limited role where other therapies are ineffective or contraindicated. Anti-obesity agents and bariatric/metabolic surgery may be considered based on general population guidelines, balancing potential for benefits and side effects.
Letrozole is the preferred first line pharmacological infertility therapy, with clomiphene in combination with metformin; gonadotrophins or ovarian surgery primarily having a role as second line therapy. In vitro fertilization (IVF) could be offered, potentially with in vitro maturation, as third line therapy, where other ovulation induction therapies have failed and in the absence of an absolute indication for IVF in women with PCOS and anovulatory infertility. Given the underlying risk for pregnancy complications in PCOS, single embryo transfer should be preferred.
Overall, evidence in PCOS is low to moderate quality. Based on high prevalence and significant health impact, greater priority, education, models of care, funding, and research are recommended. Guideline translation will be extensive including multilingual education outputs and evidence-based resources for consumers (the ASKPCOS app), healthcare professionals and policy makers.
The guideline recommendations are protected under copyright, however a clear process for adaption of guideline recommendations to regional context is available by contacting the author for correspondence online ( www.monash.edu/medicine/mchri/pcos ). The translation program will be free and internationally accessible, building on the existing range of codesigned resources including the patient focused, evidence-based PCOS APP (AskPCOS), used in 186 countries and based on a rigorously developed question prompt list. Multi-faceted patient codesigned resources will aim to enhance health literacy with comprehensive PCOS-related health information available in multiple formats and in 15–20 languages. Internationally accessible resources include education modules for healthcare professionals at different career stages and disciplines, healthcare professional accredited courses, practice resources and tools, webinars with international expert panels, and e-health information resources that will be available online ( www.monash.edu/medicine/mchri/pcos ). Importantly, the Guideline and translation of the Guideline is expected to improve patient experiences through the provision of timely and accurate diagnosis, of accessible evidence-based information and of improved multi-disciplinary support. Ultimately, this international initiative may serve as an exemplar for large scale collaborative engagement, pooling of resources, avoidance of duplication and inconsistency with consensus-based statements, and codesign of best quality consistent guidelines with processes for local adaption and healthcare impact. Key elements include extensive collaboration, broad stakeholder representation, consumer partnership, distributive leadership, adequate funding, robust project management and governance, adherence to best practice and integrated comprehensive translation, and evaluation. We sincerely thank the partner and collaborating organizations, consumer groups and members of the GDGs for their substantive commitment to the international partnership to optimize health outcomes for women with this common, heterogeneous, and much neglected condition.
We gratefully acknowledge contribution of our partners and collaborating organizations:
The Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL) (APP1171592), Centre for Research Excellence in Polycystic Ovary Syndrome (CRE PCOS) (APP1078444) and the members of these Centres who coordinated this international guideline effort.
Our partner and co-funding organizations are:
American Society for Reproductive Medicine (ASRM)
Endocrine Society (ENDO)
European Society for Endocrinology (ESE)
European Society of Human Reproduction and Embryology (ESHRE)
Collaborating and engaged societies and consumer providing in-kind support include:
Androgen Excess and Polycystic Ovary Syndrome Society (AEPCOS)
Asia Pacific Paediatric Endocrine Society (APPES)
Asia Pacific Initiative on Reproduction (ASPIRE)
Australia and New Zealand Society for Paediatric Endocrinology and Diabetes (ANZSPED)
Australian Diabetes Society (ADS)
Brazilian Society of Endocrinology and Metabolism (SBEM)
British Fertility Society (BFS)
Canadian Society of Endocrinology and Metabolism (CSEM)
Dietitians Association Australia (DA)
Endocrine Society Australia (ESA)
European Society for Paediatric Endocrinology (ESPE)
Exercise and Sports Science Australia (ESSA)
Fertility Society Australia and New Zealand (FSA)
International Federation of Fertility Societies (IFFS)
International Federation of Gynecology and Obstetrics (FIGO)
International Society of Endocrinology (ISE)—40 partner societies
Italian Society of Gynaecology and Obstetrics
Japanese Society for Paediatric Endocrinology (JSPE)
Latin American Society for Paediatric Endocrinology (SLEP)
Nordic Federation of Societies of Obstetrics and Gynaecology (NFOG)
PCOS Challenge Inc: The National Polycystic Ovary Syndrome Association
PCOS Society of India
PCOS Vitality
Paediatric Endocrine Society (PES)
Royal Australasian College of Physicians (RACP)
Royal Australian New Zealand College of Obstetricians and Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Radiologists (RANZCR)
Royal College of Obstetricians and Gynaecologists (RCOG)
Society for Endocrinology
South African Society of Gynaecology and Obstetrics (SASOG)
Victorian Assisted Reproductive Technology Association (VARTA)
Other relevant organizations are welcome to apply to partner in guideline translation.
The Australian National Health Medical Research Council (NHMRC) (APP1171592) primarily funded this work. The American Society for Reproductive Medicine, Endocrine Society, the European Society of Human Reproduction and Embryology and the European Society for Endocrinology provided partnership funding. Collaborating organizations provided in-kind support. The Commonwealth Government of Australia also supported Guideline Translation through the Medical Research Future Fund (MRFCRI000266). HJT and AM are funded by NHMRC fellowships and CTT by an RACP fellowship.
HJT led the guidelines from funding, engaging partners, coordinating processes, prioritizing clinical questions, co-chairing guideline meetings, coordinating peer review responses and leading writing, approval and publication processes. Listed authors held senior leadership roles as chair or deputy chair of the five GDGs or leadership of the evidence team with roles from the management committee, chair/ co-chair of GDG or the early career evidence network, involvement at all stages, responding to feedback, providing input into and endorsing the guideline. All other included authors were actively engaged as partner nominees and multidisciplinary GDG or consumer experts. The evidence synthesis network was led by CTT AM, across search strategies, training, Covidence processes, quality appraisal and GRADE, meta-analysis, evidence integrity processes (with BM) and preparing the technical report. The listed members of this network led evidence synthesis across the clinical questions and had input into the technical report.
Disclosures of interest were declared at the outset and updated throughout the guideline process, aligned with National Health Medical Research Council (NHMRC) guideline processes. These are available online ( www.monash.edu/medicine/mchri/pcos ). Of named authors HJT, CTT, AD, LM, LR, JBoyle, AM have no conflicts of interest to declare. JL declares grant from Ferring and Merck; consulting fees from Ferring and Titus Health Care; speaker's fees from Ferring; unpaid consultancy for Ferring, Roche Diagnostics and Ansh Labs; and sits on advisory boards for Ferring, Roche Diagnostics, Ansh Labs, and Gedeon Richter. TP declares a grant from Roche; consulting fees from Gedeon Richter and Organon; speaker's fees from Gedeon Richter and Exeltis; travel support from Gedeon Richter and Exeltis; unpaid consultancy for Roche Diagnostics; and sits on advisory boards for Roche Diagnostics. MC declares travels support from Merck; and sits on an advisory board for Merck. JBoivin declares grants from Merck Serono Ltd.; consulting fees from Ferring B.V; speaker's fees from Ferring Arzneimittell GmbH; travel support from Organon; and sits on an advisory board for the Office of Health Economics. RJN has received speaker's fees from Merck and sits on an advisory board for Ferring. AJoham has received speaker's fees from Novo Nordisk and Boehringer Ingelheim.
All data extracted and analyzed in the guideline is available in a repository and can be accessed via https://doi.org/10.26180/23625288.v1
Members of the PCOS Network:
The international advisory panel, guideline technical team, paediatric, consumer and translation committees, the Indigenous cultural advisor and the extended early career support network who assisted with evidence synthesis, can be found online ( www.monash.edu/medicine/mchri/pcos ).
Guideline Development Members and Key Contributors (in Addition to Listed Authors)
Wiebke Arlt, University of Birmingham, UK
Ricardo Azziz, University of Alabama at Birmingham, USA
Adam Balen, Leeds Teaching Hospital; British Fertility Society, UK
Lisa Bedson, Repromed, Australia
Lorna Berry, Polycystic Ovary Syndrome Association of Australia, Australia
Jacky Boivin, Cardiff University, UK
Leah Brennan, Latrobe University, Australia
Wendy Brown, Monash University, Australia
Tania Burgert, University Missouri—Kansas School of Medicine, USA
Maureen Busby, PCOS Vitality, Ireland
Carolyn Ee, Western Sydney University, Australia
Rhonda M. Garad, Monash University, Australia
Melanie Gibson-Helm, Te Tātai Hauora o Hine, Victoria University of Wellington; NZ
Cheryce Harrison, Monash University, Australia
Roger Hart, The University of Western Australia; City Fertility, Australia
Kim Hopkins, PCOS Challenge: National Polycystic Ovary Syndrome Association, USA
Angelica Lindén Hirschberg, Karolinska Institutet, Karolinska University Hospital, Sweden
Tuong Ho, HOPE Research Centre, My Duc Hospital, Vietnam
Kathleen Hoeger, University of Rochester, USA
Cailin Jordan, Genea Hollywood Fertility, Australia
Richard S. Legro, Penn State Clinical and Translational Institute, USA
Rong Li, Peking University Third Hospital, China
Marla Lujan, Cornell University, USA
Ronald Ma, Chinese University of Hong Kong, Hong Kong /China
Darren Mansfield, Monash and Epworth Health, Monash University, Australia
Kate Marsh, Northside Nutrition & Dietetics, Australia
Edgar Mocanu, Rotunda Hospital, Ireland
Ben Mol, Monash University, Australia
Rachel Mormon, Verity—PCOS Charity, UK
Sharon Oberfield, Columbia University Medical Center, USA
Malika Patel, University of Cape Town; Groote Schuur Hospital, South Africa
Loyal Pattuwage, Cochrane Australia, Monash University, Australia
Alexia Peña, The Robinson Research Institute at the University of Adelaide, Australia
Leanne Redman, Pennington Biomedical Research Center, USA
Luk Rombauts, Monash University, Australia
Daniela Romualdi, Fondazione Policlinico Universitario Agostino Gemelli, Italy
Duru Shah, PCOS Society of India; Centre for Women's Health and Fertility, India
Poli Mara Spritzer, Federal University of Rio Grande Do Sul, Brazil
Elisabet Stener-Victorin, Karolinska Institutet, Sweden
Fahimeh Ramezani Tehrani, Shahid Beheshti University of Medical Sciences, Iran
Shakila Thangaratinam, University of Birmingham, UK
Mala Thondan, Harp Family Medical, Australia
Eszter Vanky, Norwegian University of Science and Technology; Norway
Chandrika Wijeyaratne, University of Colombo, Sri Lanka
Selma Witchel, Children's Hospital of Pittsburgh of UPMC, University of Pittsburgh, USA
Dongzi Yang, Reproductive Medical Centre, Sun Yat-Sen Memorial Hospital, China
Bulent Yildiz, Hacettepe University, Turkey
International Early Career Evidence Synthesis Network Leads
Simon Alesi, Monash University, Australia
Snigdha Alur-Gupta, University of Rochester, USA
Jodie Avery, University of Adelaide, Australia
Mahnaz Bahri Khomami, Monash University, Australia
Jamie Benham, University of Calgary, Canada
Hugh Bidstrup, Australian Catholic University, Australia
Su Jen Chua, Monash University, Australia
Laura Cooney, University of Wisconsin, USA
Thisara Coster, Monash University, Australia
Victoria Fitz, Harvard University, USA
Madeline Flanagan, Monash University, Australia
Maria Forslund, University of Gothenburg, Sweden
Geranne Jiskoot, Erasmus MC, Netherlands
Maryam Kazemi, Icahn School of Medicine at Mount Sinai, USA
Punith Kempegowda, University of Birmingham, UK
Yvonne Louwers, Erasmus MC, Netherlands
Johanna Melin, University of Helsinki, Finland
Eka Melson, University of Leicester, UK
Yitayeh Belsti Mengistu, Monash University, Australia
Negar Naderpoor, Monash University, Australia
Adriana Neven, Monash University, Australia
Hester Pastoor, Erasmus MC, Netherlands
Thais Rocha, University of Birmingham, UK
Angelo Sabag, Western Sydney University, Australia
Anuradhaa Subramanian, University of Birmingham, UK
Katrina Tan, Monash Health, Australia
Teede H , Deeks A , Moran L . Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan . BMC Med . 2010 ; 8 ( 1 ): 41 .
Google Scholar
Azziz R , Carmina E , Chen Z , et al. Polycystic ovary syndrome . Nat Rev Dis Primers . 2016 ; 2 ( 1 ): 16057 .
Gibson-Helm M , Teede H , Dunaif A , Dokras A . Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome . J Clin Endo & Metab . 2017 ; 102 : 604 ‐ 612 .
Dokras A , Saini S , Gibson-Helm M , Schulkin J , Cooney L , Teede H . Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome . Fertil Steril . 2017 ; 107 ( 6 ): 1380 ‐ 1386.e1 .
Teede H , Gibson-Helm M , Norman RJ , Boyle J . Polycystic ovary syndrome: perceptions and attitudes of women and primary health care physicians on features of PCOS and renaming the syndrome . J Clin Endocrinol Metab . 2014 ; 99 ( 1 ): E107 ‐ E111 .
Teede J Helena , Marie Misso , Michael Costello , et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018 www.monash.edu/medicine/mchri/pcos
Teede HJ , Misso ML , Costello MF , et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome . Hum Reprod . 2018 ; 33 ( 9 ): 1602 ‐ 1618 .
Misso ML , Teede HJ . Evidence based guideline (EBG) development: A practical guide. In: Ilic D, ed. Knowledge Transfer: Practices, Types and Challenges . Nova Science Publishers, Inc. ; 2012 : 141 ‐ 174 .
Google Preview
Brouwers MC , Kho ME , Browman GP , et al. AGREE II: advancing guideline development, reporting and evaluation in health care . CMAJ . 2010 ; 182 ( 18 ): E839 ‐ E842 .
Mousa A , Tay CT , Teede H . Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Monash University. Report; 2023. https://doi.org/10.26180/23625288.v1
Weibel S , Popp M , Reis S , Skoetz N , Garner P , Sydenham E . Identifying and managing problematic trials: A research integrity assessment tool for randomized controlled trials in evidence synthesis . Res Synth Methods . 2023 ; 14 ( 3 ): 357 ‐ 369 .
Mol BW , Lai S , Rahim A , et al. Checklist to assess Trustworthiness in RAndomised Controlled Trials (TRACT checklist): concept proposal and pilot . Res Integrity Peer Rev . 2023 ; 8 ( 1 ): 6 .
National Health and Medical Research Council . NHMRC levels of evidence and grades for recommendations for developers of guidelines. Australia ; 2009 .
National Health and Medical Research Council . NHMRC standards and procedures for externally developed guidelines. Australia ; 2007 .
GRADE working group . Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines .
Author notes
Email alerts, citing articles via.
- About The Journal of Clinical Endocrinology & Metabolism
- About the Endocrine Society
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1945-7197
- Print ISSN 0021-972X
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement
A better understanding of PCOS offers fresh hope for new treatments
New insights into polycystic ovary syndrome are revealing more about the causes of this common but misunderstood whole-body condition, and these could lead to new treatments
By Alice Klein
26 January 2023

I WAS 19, my face raging with acne, when my dermatologist started asking me questions that seemed to have nothing to do with my skin. “Are your periods regular? Do you have any excess body hair?” he asked. “You may have polycystic ovary syndrome,” he concluded. I had no idea what he was talking about. “It can make it difficult to have children,” he said as he saw me out.
Reeling, I went to my family doctor, who ordered blood tests and an ultrasound of my ovaries that confirmed I had polycystic ovary syndrome, or PCOS. But she admitted she didn’t know much about it, leaving me confused and miserable about this mysterious condition I had suddenly been saddled with.
Many of my friends have recounted similar experiences. Despite PCOS being the most common hormonal condition among women aged 18 to 45 and a leading cause of infertility, it has been hard for us to get a straight answer about what it actually is or what to do about it.
Seventeen years on from my diagnosis, however, the tide is turning. Researchers are finally piecing together the causes of PCOS and it is being taken seriously as a condition that doesn’t just affect the ovaries, but also has cardiovascular, metabolic and psychological repercussions. As a result, the condition is even set to get a different name later this year (see “Misleading moniker”). And what’s more, this clearer understanding is opening up routes to new treatments.
The first doctors to characterise PCOS were Irving Stein and Michael Leventhal at Northwestern University in Chicago. In 1935, they published a report on…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
Heavy or painful menstrual periods are linked to worse exam results
Subscriber-only
Autoimmune conditions linked to reactivated X chromosome genes
Father's gut microbiome may affect infant health, alpacas are the only mammals known to directly inseminate the uterus, popular articles.
Trending New Scientist articles
- Open access
- Published: 07 May 2024
Causal association between low vitamin D and polycystic ovary syndrome: a bidirectional mendelian randomization study
- Bingrui Gao 1 ,
- Chenxi Zhang 1 ,
- Deping Wang 1 , 2 ,
- Bojuan Li 1 ,
- Zhongyan Shan 1 ,
- Weiping Teng 1 &
- Jing Li ORCID: orcid.org/0000-0002-3681-4095 1
Journal of Ovarian Research volume 17 , Article number: 95 ( 2024 ) Cite this article
280 Accesses
Metrics details
Recent studies have revealed the correlation between serum vitamin D (VD) level and polycystic ovary syndrome (PCOS), but the causality and specific mechanisms remain uncertain.
We aimed to investigate the cause-effect relationship between serum VD and PCOS, and the role of testosterone in the related pathological mechanisms.
We assessed the causality between serum VD and PCOS by using genome-wide association studies (GWAS) data in a bidirectional two-sample Mendelian randomization (TS-MR) analysis. Subsequently, a MR mediation analysis was conducted to examine the mediating action of testosterone in the causality between serum VD and PCOS. Ultimately, we integrated GWAS data with cis-expression quantitative loci (cis-eQTLs) data for gene annotation, and used the potentially related genes for functional enrichment analysis to assess the involvement of testosterone and the potential mechanisms.
TS-MR analysis showed that individuals with lower level of serum VD were more likely to develop PCOS (OR = 0.750, 95% CI: 0.587–0.959, P = 0.022). MR mediation analysis uncovered indirect causal effect of serum VD level on the risk of PCOS via testosterone (OR = 0.983, 95% CI: 0.968–0.998, P = 0.025). Functional enrichment analysis showed that several pathways may be involved in the VD-testosterone-PCOS axis, such as steroid hormone biosynthesis and autophagy process.
Our findings suggest that genetically predicted lower serum VD level may cause a higher risk of developing PCOS, which may be mediated by increased testosterone production.
Introduction
Vitamin D (VD) is an essential fat-soluble steroid hormone that is necessary for calcium-phosphate metabolism, bone homeostasis, cell differentiation, and immune system function. The prevalence of VD deficiency (VDD) in the population has gradually increased over the past few decades. VDD is associated with various diseases, including cardiovascular disease, inflammation, dyslipidemia, weight gain, and infectious diseases [ 1 , 2 ]. Furthermore, mounting studies have indicated the potential link between the serum VD status and women's reproductive health. Firstly, the biological function of VD is mediated via intracellular VD receptors (VDRs), which are distributed among various tissues, encompassing hypothalamic, pituitary tissue, endometrium, and ovary [ 3 , 4 ]. Secondly, VD participates in regulating genes associated with ovarian and placental functions [ 5 , 6 ]. All evidences suggest that the serum VD plays a potentially significant role in female reproductive health.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder that effects women of reproductive age, with a global incidence ranging 20–25% [ 7 , 8 ]. PCOS will affect woman's endometrial function and oocyte competence [ 9 , 10 ], which leads to reproductive dysfunction in PCOS patients, including infertility, miscarriage, and pregnancy complications [ 11 , 12 , 13 ]. However, the exact pathogenesis of PCOS remains unclear. Prior observational studies have elucidated the correlation between the serum VD and the risk of PCOS. A recent study revealed that serum VD concentration were lower in women diagnosed with PCOS compared to body mass index (BMI)-matched control, suggesting that regardless of BMI, PCOS is correlated with reduced VD level [ 14 ]. However, these studies can only prove that there is a correlation between them, they cannot clarify the causality between them. In addition, hyperandrogenemia stands as one of the diagnostic criteria for PCOS and impacts 60–80% of patients [ 15 ]. Female are actually more sensitive to testosterone even though it is known as a male hormone [ 16 ]. Growing evidences showed that testosterone may play an important role between the serum VD level and the risk of PCOS. Hahn et al. illustrated an association between the serum VD level and the severity of hirsutism in individuals with PCOS [ 17 ]. The research conducted by Latic et al. indicates a negative correlation between serum VD level and testosterone production in patients with PCOS [ 18 ]. However, a study by Mesinovic et al. suggested no discernible correlation between the serum VD level and androgen production in individuals with PCOS [ 19 ]. Moreover, a large observational study by Gallea et al. also showcased the association between serum VD levels, insulin, and body weight among PCOS patients but not specifically with hyperandrogenemia [ 20 ]. The reason for these different results may be due to the fact that observational studies are susceptible to confounding factors as well as various biases [ 21 ]. Therefore, it is not clear whether testosterone production mediate the relationship between serum VD level and the risk of PCOS, due to the limitations of the study methodology.
In recent years, mendelian randomization (MR) analysis is widely used as an epidemiological method in medical research. Firstly, MR analysis can minimize the impact of confounding factors and various biases on the results by simulating randomized controlled trials (RCTs) at the genetic level, and secondly, MR analysis can also determine causality and reduce the impact of reverse causality on the results of the study [ 22 ].
Thus, in this study, we use the bidirectional two-sample MR (TS-MR) analysis to investigate the cause-effect relationship between the serum VD level and the risk of PCOS. Secondly, we perform the mediation MR analysis to test the mediating role of testosterone production between serum VD level and the risk of PCOS. Finally, we used the bioinformatics analysis to assess the possible biological functions and molecular mechanisms between them.
Materials and methods
Study design of mendelian randomization study.
Our study explored the cause-effect of serum VD level as an exposure on the risk of developing PCOS as an outcome trait and the effect of testosterone as a mediator between VD and PCOS through bidirectional TS-MR analysis, multivariable MR (MVMR) and mediator MR analysis (Fig. 1 ). In order to ensure the study's validity, the study needed to meet the three following crucial assumptions [ 23 ] (Fig. 1 C):1) the correlation assumption: instrumental variables (IVs) must be robustly correlated with the exposure factors; 2) the exclusion restriction assumption: IVs are not associated with potential confounders of the exposure or the outcome; and 3) the independence assumption: IVs do not influence the outcome variables through other pathways besides the exposure factors. This study followed guidelines of STROBE-MR [ 24 ] checklist (Table S 1 ).
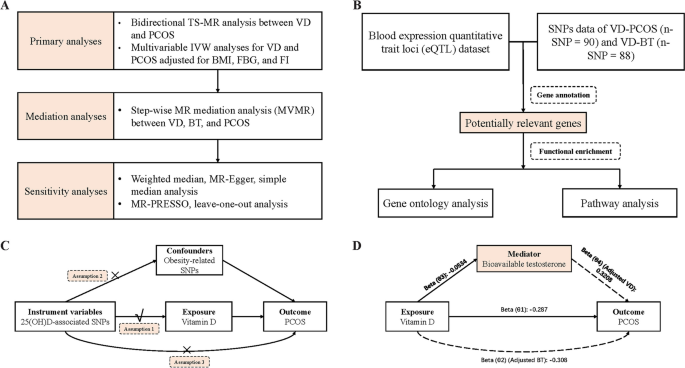
Flowchart of the study. A Flowchart of the MR study; ( B ) Flowchart of the Bioinformatics study; ( C ) Diagram of the MR assumptions of the association between VD and PCOS; ( D ) Illustrative diagram for the mediation MR analysis framework Abbreviations: MR, mendelian randomization; TS-MR, two-sample MR; VD, vitamin D; PCOS, polycystic ovary syndrome; IVW, inverse variance weighted; BMI, body mass index; FBG, fasting glucose; FI, fasting insulin; MVMR, multivariable MR; BT, bioavailable testosterone; SNPs, single-nucleotide polymorphisms
Data source and IVs selection of mendelian randomization study
We obtained data associated with VD from a large genome-wide association study (GWAS) that identified 143 loci among 417,580 participants which was conducted by Revez et al. in 2020 [ 25 ]. We accessed the summary data related to PCOS from a meta-analysis in the FinnGen and Estonian Biobank (EstBB), which included 3609 cases and 229,788 controls [ 7 ]. Summary data related to bioavailable testosterone (BT) were obtained from the UK Biobank (UKB). Data on serum fasting glucose (FBG) levels were obtained from a UKB GWAS we conducted in 340,002 British participants [ 26 ]. Summary data on circulating concentrations of fasting insulin (FI) were obtained from the MAGIC GWAS included 151,013 participants [ 27 ]. Pooled data related to BMI were acquired from a GWAS meta-analysis within the (GIANT) consortium, encompassing 681,275 participants [ 28 ]. Details of the GWAS database are summarized in Table S 2 .
In the bidirectional TS-MR analysis, Single-nucleotide polymorphisms (SNPs) with genome-wide significance ( P < 5 × 10 –8 ) were first selected. These SNPs were matched against the SNP-outcome GWAS database to exclude SNPs that could not be matched. To minimize the effects of linkage disequilibrium, we conducted a clumping process with an r 2 threshold of 0.001 and a clumping window of 10,000 kb and excluded these SNPs if present. Subsequently, we performed MR-PRESSO analysis immediately to demonstrate whether there was significant horizontal pleiotropy to exclude outlier SNPs [ 29 ]. To ensure that the IVs were not affected by confounding variables, we searched the PhenoScanner V2 [ 30 ] and deleted obesity-related SNPs associated with BMI and waist circumference (WC). Finally, 88 SNPs (VD on PCOS) and 2 SNPs (PCOS on VD) were used as IVs in the primary bidirectional TS-MR study, respectively. All SNPs exhibited an F statistic greater than 10. The variance explained for each SNP (R 2 ) was calculated using the widely-accepted formula [ 31 , 32 ]. We used the same method as above to screen the SNPs required in the MR mediation analysis. All the IVs SNPs are summarized in Table S 3 - 7 .
Statistic analysis of mendelian randomization study
Initially, the primary analysis aimed to explore the causal relationship between VD and PCOS. We used bidirectional TS-MR analysis to assess the causal relationship between VD and PCOS. In this, we used Cochran's Q test to assess the heterogeneity [ 33 ]; if there was no heterogeneity, we would use the fixed-effects inverse variance weighted (IVW) method, otherwise, we would use the random-effects IVW method [ 34 ]. Furthermore, considering that obesity, abnormal insulin levels, and abnormal glucose values are common in patients with PCOS, we adjusted genetically predicted BMI, FBG, and FI by MVMR to explore the direct causal effect between VD and PCOS. To make the results more robust.
Secondly, a stepwise MR analysis approach was used to examine whether there exist mediation effects of BT between VD and PCOS. To assess the direct causal effect between VD, BT, and PCOS, we performed an MVMR analysis using the MVMR R package [ 35 ]. Conditional F statistics were calculated for assessing the strength of the genetic instruments in MVMR analysis [ 36 ]. The product of the coefficients method [ 37 ] and the multivariate delta method [ 38 ] were used to calculate the indirect effects of VD on PCOS via mediator.
Sensitivity analysis of mendelian randomization study
The following tests were used as sensitivity analyses to assess the robustness of MR effect estimates to invalid genetic variants. Firstly, we conducted MR-Egger regression [ 39 , 40 ], weighted median [ 41 ], and weighted mode [ 42 ] methods. MR-Egger regression can detect and explain horizontal pleiotropy mainly through intercept tests [ 39 , 40 ]. Weighted median can yield impartial estimations even when over half of the information arise from flawed IVs [ 43 ]. We used weighted mode to divide SNPs into multiple subsets based on similar causal effects, and the estimates of causal effects were computed for the subset with the highest number of SNPs [ 42 ]. Secondly, the leave-one-out (LOO) analysis can test whether the results are affected by a single SNP [ 44 ]. Thirdly, as described above we performed MR-PRESSO analysis [ 29 ] to identify the presence of potential horizontal pleiotropic outliers in IVs that could lead to biased results, as well as searching for and removing obesity-related SNPs associated with BMI and WC from the PhenoScanner database [ 45 ].
All analyses were conducted using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). P values were considered significant at 0.05.
Bioinformatical analysis
We used the largest whole blood expression quantitative trait loci (eQTL) dataset from the eQTLGen consortium, which includes data on cis-eQTLs for 19,250 whole blood expressed genes from 31,684 individuals [ 46 ]. We combined SNPs data of VD-PCOS ( n -SNP = 90) and VD-BT ( n -SNP = 88) with cis-eQTLs data for gene annotation, respectively. Genes with P < 5*10 –8 and FDR < 0.05 were screened as potentially relevant genes for VD-PCOS and VD-BT.
Subsequently, we used these potentially relevant genes for bioinformatics analyses, including Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. GO analyses [ 47 ], including biological process (BP), molecular function (MF), and cellular composition (CC), are commonly used for large-scale functional enrichment studies. KEGG is a database that stores information about genomes, biological pathways, diseases, and drugs. We used the clusterProfiler package, org.Hs.eg.db package, and enrichplot package in the software R to perform GO and KEGG enrichment analyses of the potentially relevant genes. P < 0.05 for GO entries and KEGG pathways were considered significant.
Causal effect between serum vitamin D and polycystic ovary syndrome
In our bidirectional TS-MR analysis, the number of IVs of VD on PCOS and PCOS on VD were 90 and 2, respectively. The F-statistic values for each SNP were greater than 10 (Table S 3 ), indicating that the results were almost unaffected by weak instrumental bias. The result of fixed-effects IVW method (Cochran's Q statistic = 81.42, P = 0.704) indicated that genetically predicted higher level of VD led to a lower risk of developing PCOS after excluding obesity-associated SNPs ( n = 90 SNPs, OR = 0.750, 95% CI: 0.587–0.959, P = 0.022) (Table 1 ). MR-Egger, weighted median, and weighted mode methods all obtained similar magnitude and direction to IVW method (Table 1 ). The scatter plot demonstrates the inhibitory effect of individual SNP on PCOS (Fig. S 1 ). Since the MR-Egger P -intercept was greater than 0.05 (Table S 8 ) and the funnel plot (Fig. S 2 ) was roughly symmetrical, there was no indication of horizontal pleiotropy detected in the study. The results of the LOO analyses indicated that there were no potentially affecting SNPs in the main MR analyses (Fig. S 3 ). The result of the result of the MR-PRESSO test did not show any outlier SNPs. Nevertheless, the results of reverse TS-MR showed that genetically predicted risk of developing PCOS did not affect the VD level (fixed-IVW: n = 2 SNPs, OR = 1.004, 95% CI: 0.987–1.022, P = 0.640) (Table 1 ).
We subsequently explored the direct effect of the serum VD level on PCOS by MVMR methods, and the results of both Model 1 (adjusted BMI) and Model 2 (adjusted BMI, FBG, and FI) showed that the negative correlation between serum VD level and the risk of PCOS remained similar (Table 2 ). This confirms the robustness of the TS-MR results.
Mendelian randomization mediation analysis
After excluding the outlier SNPs and obesity-related SNPs, MVMR analysis (adjusted BT) revealed direct causal effects of serum VD level (OR: 0.735, 95% CI: 0.552–0.978; P = 0.035) on the risk of developing PCOS (Table 3 , Fig. 1 D). In the following steps of the MR mediation analysis, we found strong evidence for a causal effect of serum VD level (β: − 0.053, P = 0.026) on BT (Table 3 ). In addition to this, we also found a causal relationship between BT and PCOS (OR: 1.378, 95% CI: 1.123–1.691; P = 0.002) (Table 3 ).
Taken together, we found the potential mediation pathways between VD and PCOS: an indirect causal effect of VD on PCOS risk via BT (θ 3 × θ 4 ) (OR: 0.983, 95% CI: 0.968–0.998; P = 0.025) (Table 3 ). The pathway mediated 5.96% of the total causal effect of VD on PCOS risk. Detailed estimates of direct and indirect causal effects can be found in Table 3 .
Bioinformatics study
The results of the MR study suggested that reduced VD level may lead to the development of PCOS, and BT is a mediator between VD and PCOS, meaning that VD can ultimately influence the development of PCOS by affecting the production of testosterone. On the basis of the above studies, we collected IVs of VD-PCOS ( n -SNPs = 90) and VD-BT ( n -SNPs = 88) respectively, and combined these IVs with cis-eQTLs data for gene annotation respectively. Ultimately, 147 (VD-PCOS) and 164 (VD-BT) potentially relevant genes were annotated (Table S 9 - 10 ), respectively. We then used these genes to perform GO and KEGG analyses.
Firstly, the potentially relevant genes of VD-PCOS were analyzed for enrichment. The results of GO analysis suggested that these genes were mainly related to androgen metabolic process, superoxide metabolic process, cell body membrane, and steroid dehydrogenase activity (Fig. 2 A). The KEGG analysis was mainly enriched in the process of autophagy, steroid biosynthesis, cytochrome P450 metabolic process, and vitamin digestion and absorption process (Fig. 2 C). Subsequently, potentially relevant genes associated with VD-BT were analyzed for enrichment. The results of GO analysis suggested that these genes were mainly associated with steroid metabolism, superoxide metabolism, autophagosome membrane, nuclear androgen receptor binding, and vitamin transmembrane transporter activity (Fig. 2 B), and the KEGG analysis was mainly enriched for autophagy, steroid biosynthesis, vitamin digestion and absorption, and cholesterol metabolism process (Fig. 2 C). All information of the enrichment analysis is shown in the additional file (Table S 11 -S 12 ).
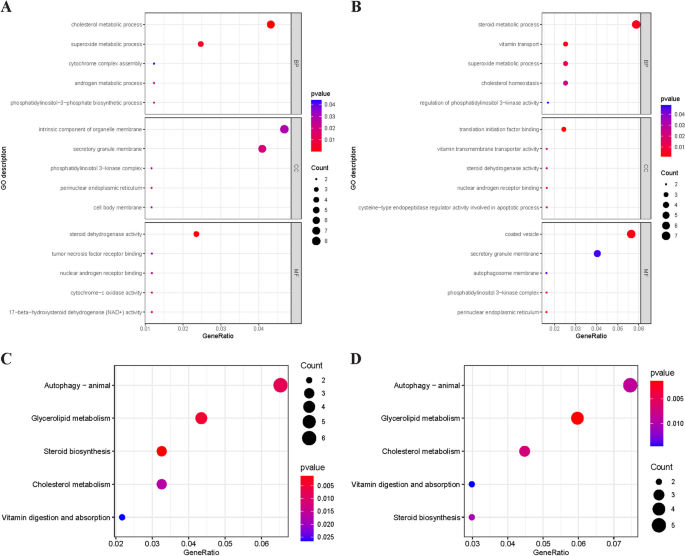
Gene Ontology and Kyoto Encyclopedia of the Genome pathway enrichment analysis of potentially relevant genes. A The GO enrichment analysis for potentially relevant genes related to VD and PCOS; ( B ) The GO enrichment analysis for potentially relevant genes related to VD and BT; ( C ). The KEGG pathway analysis for potentially relevant genes related to VD and PCOS; ( D ). The KEGG pathway analysis for potentially relevant genes related to VD and BT. Abbreviations: VD, vitamin D; PCOS, polycystic ovary syndrome; BT, bioavailable testosterone; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of the Genome
In our bidirectional TS-MR analysis, we found that higher serum VD level was causally associated with a lower risk of developing PCOS (OR = 0.750, 95% CI: 0.587–0.959, P = 0.022), whereas there was little evidence for a causal effect of the risk of PCOS on the effect of serum VD level. Furthermore, our MR mediation analysis confirmed that testosterone can act as one of the mediating factors between the causality of VD and PCOS (OR = 0.983, 95% CI: 0.968–0.998, P = 0.025). The mediating effect of testosterone was 5.96%. Ultimately, we utilized potentially relevant genes for GO and KEGG enrichment analysis to assess the involvement of testosterone and the potential biological and molecular mechanisms between them.
VD, a lipid-soluble vitamin, plays a pivotal role in numerous biological processes. Primarily synthesized endogenously through exposure to sunlight, it is also acquired, albeit to a lesser extent, from dietary sources [ 48 ]. VDD is considered a globally prevalent nutritional deficiency, with various studies reporting prevalence rates of 58–91% among infertile women [ 49 ]. A cross-sectional study encompassing 625 women diagnosed with PCOS and 217 control subjects revealed that Chinese women diagnosed with PCOS exhibited notably lower level of VD compared to their healthy [ 50 ]. The result from a large observational study conducted by Krul-Poel et al. similarly demonstrated significantly diminished level of VD among women within the PCOS group [ 51 ]. Recent research has demonstrated that women with PCOS exhibit lower serum concentrations of VD compared to BMI-matched controls. This implies that the level of VD is linked to PCOS irrespective of BMI [ 14 ]. Aligned with the outcomes of these observational studies, our research indicated that higher serum VD level serves as a protective factor for the risk of PCOS. To eliminate the influence of obesity as a potential confounder on the results, we excluded obesity-related SNPs in our TS-MR analysis. Subsequently, in our MVMR analyses, we adjusted for genetically predicted BMI, FBG, and FI to explore the direct causal relationship between VD and PCOS. These stringent measures significantly enhance the credibility and robustness of our findings.
The precise mechanism through which serum VD operates on PCOS remains elusive. Hyperandrogenemia stands as a pivotal diagnostic criterion for PCOS. Numerous past studies have concentrated on exploring the correlation between serum VD and hyperandrogenemia in PCOS, yet the conclusions drawn from these studies have not reached a consensus. A study conducted by Latic N et al. revealed a negative correlation between serum VD level and testosterone in PCOS patients. Additionally, Menichini et al. demonstrated a positive impact of VD supplementation (4000 IU) on total testosterone [ 52 ]. However, a study by Mesinovic et al. suggested no discernible correlation between serum VD and androgens in individuals with PCOS [ 19 ]. Moreover, a large observational study by Gallea et al. also showcased associations between serum VD level, insulin, and body weight among PCOS patients but not specifically with hyperandrogenemia [ 20 ]. The inconsistencies observed in these findings might stem from variations in race, sample sizes, seasonal disparities, and the lifestyles of the included subjects. Our study, employing Mendelian randomization, effectively mitigated the impact of sample size, seasonal fluctuations, and diverse lifestyles on the outcomes. Furthermore, our research focused solely on individuals of European ethnicity, and we excluded BMI-related SNPs when incorporating instrumental variables, thereby significantly reducing BMI's potential confounding effect on the results. These measures ensured the robustness and reliability of our findings. Our results suggest that testosterone acts as a mediator between serum VD and PCOS, implying that serum VD may potentially contribute to the development of PCOS by influencing testosterone production.
The mechanism by which serum VD ultimately contributes to the development of PCOS by affecting testosterone remains unclear, but possible explanation has been proposed. Serum VD heightens the activity of aromatase within the ovary, thereby fostering the conversion of androgens to estrogens, ultimately culminating in diminished androgens production [ 53 ]. Kinuta et al. demonstrated a marked reduction in aromatase activity within the ovaries of VDR knockout mice in contrast to the control group [ 54 ]. In addition, we performed bioinformatics analysis to explore more possible biological mechanisms. Firstly, the results of GO and KEGG analyses of potentially related genes of VD-PCOS showed that steroid biosynthetic process, androgen metabolic process, and nuclear androgen receptor binding process were the possible biological mechanisms between the causality of the serum VD level and PCOS. These results are consistent with the results of our bidirectional TS-MR analysis, demonstrating again that the serum VD can ultimately influence the development of PCOS by modulating testosterone production. Subsequently, we subjected potentially relevant genes associated with VD-BT to bioinformatics analysis. The results suggested that autophagy process and superoxide metabolism process might be the biological mechanism between serum VD and testosterone.
There are very few studies linking autophagy to PCOS, and the results of these studies suggest that the development of PCOS is closely related to the process of autophagy [ 55 ]. Texada et al. showed that autophagy can regulate steroid production by modulating cholesterol transport in endocrine cells [ 56 ]. In addition to this, the role of VD-mediated autophagy in disease has been extensively studied, and basic study by Hu et al. showed that VD can mediate the regulation of autophagy function through gastric epithelial cell VD receptors, which ultimately affects the pathogenic effects of H. pylori [ 57 ]. However, whether VD can mediate autophagy ultimately leading to PCOS remains unknown. The results of the bioinformatics study in this study suggest that autophagy is most likely one of the important mechanisms underlying the relationship between VD and PCOS.
Our study has proved that lower serum VD level causes higher prevalence of PCOS. The latter could have oocyte competence and endometrial function impaired [ 9 , 10 ], but also cause a few adverse outcomes related to reproduction, such as infertility, miscarriage, and premature delivery [ 12 , 13 ]. It has been found that VDD could decrease the rates of ovulation and success pregnancy in the PCOS patients, leading to less live birth [ 58 ]. In addition, It has been reported that serum VD level was independent predicting factor for live birth in the PCOS patients received ovulati0on induction [ 59 ]. Yasmine et al. have reported that endometrial thickness of PCOS patients maybe improved after VD administration [ 60 ]. A recent meta-analysis has shown that VD supplementation to PCOS women could decrease the occurrence rates of early miscarriage and premature delivery [ 53 ]. The nuclear receptor of VD (VDR) and 1,25(OH)2D3 membrane binding protein are expressed in both ovarian granulosa and theca cells [ 61 , 62 ]. It has been found that VD can regulate the expression of enzymes in the VDR and ovary, ultimately regulating ovarian function [ 63 ]. One study showed that VDR mRNA was significantly less expressed in granulosa cells of the women with PCOS [ 64 ]. It may cause PCOS patients to be more sensitive to VDD. Based on the above studies and ours, serum VD level need be monitored in the female population, especially in the women of reproductive age, and timely VD administration in PCOS patients would help to improve their reproductive function and pregnancy outcomes.
Our research has several advantages. Primarily, this study confirms the direct causal relationship of the serum VD level on the risk of PCOS through the utilization of the TS-MR analysis method. This method avoids the limitation commonly found in most observational studies, thereby fortifying the reliability and validity of our finding. Secondly, we ascertain the mediating function of testosterone in the relationship between serum VD and PCOS via MR mediation analysis, thus laying the groundwork for subsequent mechanistic studies. Finally, this is the first study to combine MR studies and bioinformatics analyses together to explore causal relationship and potential functional mechanisms between serum VD level, testosterone, and the risk of PCOS, which is quite different from other studies. Nonetheless, this study also has limitations. Firstly, our study failed to capture dietary and sun exposure information that may affect serum VD level. Secondly, the use of exclusively European data in a MR analysis may not be generalizable to other ethnic populations, albeit reducing the impact of ethnicity bias on the study outcomes. Finally, the absence of relevant data prevented us from independently exploring the relationship of serum VD 2 /D 3 with the risk of PCOS, warranting further investigation.
Conclusions
In conclusion, our studies confirm the causality between lower serum VD level and higher risk of PCOS. Furthermore, testosterone may act as a mediator between serum VD and PCOS. These findings emphasize the clinical importance of testing serum VD level and timely VD supplementation as possible primary prevention and treatment of PCOS.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- Polycystic ovary syndrome
Genome-wide association studies
Two-sample Mendelian randomization
Cis-expression quantitative loci
VD deficiency
VD receptors
Body mass index
- Mendelian randomization
Multivariable MR
Instrumental variables
Bioavailable testosterone
Fasting glucose
Fasting insulin
Single-nucleotide polymorphisms
Waist circumference
Inverse variance weighted
Leave one out
Gene ontology
Kyoto Encyclopedia of Genes and Genomes
Biological process
Molecular function
Cellular composition
Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–65.
Article CAS PubMed Google Scholar
Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89.
Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166:765–78.
Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102:460-468.e3.
Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. 2010;42:754–7.
Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19:2222–33.
Tyrmi JS, Arffman RK, Pujol-Gualdo N, Kurra V, Morin-Papunen L, Sliz E, et al. Leveraging Northern European population history: novel low-frequency variants for polycystic ovary syndrome. Hum Reprod. 2022;37:352–65.
Article PubMed Google Scholar
Bruni V, Capozzi A, Lello S. The Role of Genetics, Epigenetics and Lifestyle in Polycystic Ovary Syndrome Development: the State of the Art. Reprod Sci. 2022;29:668–79.
Palomba S, Daolio J, La Sala GB. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol Metab. 2017;28:186–98.
Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27:584–618.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97.
Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper Hum Reprod. 2021;36:2421–8.
CAS PubMed Google Scholar
Palomba S, De Wilde MA, Falbo A, Koster MPH, La Sala GB, Fauser BCJM. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21:575–92.
Bacopoulou F, Kolias E, Efthymiou V, Antonopoulos CN, Charmandari E. Vitamin D predictors in polycystic ovary syndrome: a meta-analysis. Eur J Clin Invest. 2017;47:746–55.
Lejman-Larysz K, Golara A, Baranowska M, Kozłowski M, Guzik P, Szydłowska I, et al. Influence of Vitamin D on the Incidence of Metabolic Syndrome and Hormonal Balance in Patients with Polycystic Ovary Syndrome. Nutrients. 2023;15:2952.
Article CAS PubMed PubMed Central Google Scholar
Durdiakova J, Ostatnikova D, Celec P. Testosterone and its metabolites–modulators of brain functions. Acta Neurobiol Exp (Warsz). 2011;71:434–54.
Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83.
Latic N, Erben RG. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int J Mol Sci. 2020;21:6483.
Mesinovic J, Teede HJ, Shorakae S, Lambert GW, Lambert EA, Naderpoor N, et al. The Relationship between Vitamin D Metabolites and Androgens in Women with Polycystic Ovary Syndrome. Nutrients. 2020;12:1219.
Gallea M, Granzotto M, Azzolini S, Faggian D, Mozzanega B, Vettor R, et al. Insulin and body weight but not hyperandrogenism seem involved in seasonal serum 25-OH-vitamin D3 levels in subjects affected by PCOS. Gynecol Endocrinol. 2014;30:739–45.
Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. Cardon L, editor. PLoS Med. 2007;4:e352.
Article PubMed PubMed Central Google Scholar
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89-98.
EPIC- InterAct Consortium, Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–52.
Article PubMed Central Google Scholar
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614.
Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. 2020;11:1647.
Mbatchou J, Barnard L, Backman J, Marcketta A, Kosmicki JA, Ziyatdinov A, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53:1097–103.
Chen J, Spracklen CN, Marenne G, Varshney A, Corbin LJ, Luan J, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021;53:840–60.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼ 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–9.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Mortada I. Hyperuricemia, Type 2 Diabetes Mellitus, and Hypertension: an Emerging Association. Curr Hypertens Rep. 2017;19:69.
Choi HK, McCormick N, Lu N, Rai SK, Yokose C, Zhang Y. Population Impact Attributable to Modifiable Risk Factors for Hyperuricemia. Arthritis Rheumatol. 2020;72:157–65.
Nakamura K, Sakurai M, Miura K, Morikawa Y, Yoshita K, Ishizaki M, et al. Alcohol intake and the risk of hyperuricaemia: a 6-year prospective study in Japanese men. Nutr Metab Cardiovasc Dis. 2012;22:989–96.
Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34:2926–40.
Article Google Scholar
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408.
Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med. 2021;40:5434–52.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48:713–27.
VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32.
MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304–14.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.
Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/27061298/ . [cited 2023 Feb 15].
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30–42.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–3.
Võsa U, Claringbould A, Westra H-J, Bonder MJ, Deelen P, Zeng B, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53:1300–10.
The Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:D1049–56.
Yuan C, Qian ZR, Babic A, Morales-Oyarvide V, Rubinson DA, Kraft P, et al. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J Clin Oncol. 2016;34:2899–905.
Cunningham TK, Allgar V, Dargham SR, Kilpatrick E, Sathyapalan T, Maguiness S, et al. Association of Vitamin D Metabolites With Embryo Development and Fertilization in Women With and Without PCOS Undergoing Subfertility Treatment. Front Endocrinol. 2019;10:13.
Shan C, Zhu Y, Yu J, Zhang Y, Wang Y, Lu N, et al. Low Serum 25-Hydroxyvitamin D Levels Are Associated With Hyperandrogenemia in Polycystic Ovary Syndrome: A Cross-Sectional Study. Front Endocrinol. 2022;13:894935.
Krul-Poel YHM, Koenders PP, Steegers-Theunissen RP, Ten Boekel E, Wee MMT, Louwers Y, et al. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): a cross-sectional study. Narayanan R, editor. PLOS One. 2018;13:e0204748.
Menichini D, Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol. 2020;36:1–5.
Yang M, Shen X, Lu D, Peng J, Zhou S, Xu L, et al. Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol. 2023;14:1148556.
Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–24.
Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17:2706–33.
Texada MJ, Malita A, Rewitz K. Autophagy regulates steroid production by mediating cholesterol trafficking in endocrine cells. Autophagy. 2019;15:1478–80.
Hu W, Zhang L, Li MX, Shen J, Liu XD, Xiao ZG, et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy. 2019;15:707–25.
Butts SF, Seifer DB, Koelper N, Senapati S, Sammel MD, Hoofnagle AN, et al. Vitamin D Deficiency Is Associated With Poor Ovarian Stimulation Outcome in PCOS but Not Unexplained Infertility. J Clin Endocrinol Metab. 2019;104:369–78.
Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D Status Relates to Reproductive Outcome in Women With Polycystic Ovary Syndrome: Secondary Analysis of a Multicenter Randomized Controlled Trial. J Clin Endocrinol Metab. 2016;101:3027–35.
Abuzeid Y. Impact of Vitamin D Deficiency on Reproductive Outcome in infertile anovulatory women with polycystic ovary syndrome: a systematic literature review. Curr Dev Nutr. 2020;4:nzaa067_001.
Li S, Qi J, Sun Y, Gao X, Ma J, Zhao S. An integrated RNA-Seq and network study reveals that valproate inhibited progesterone production in human granulosa cells. J Steroid Biochem Mol Biol. 2021;214:105991.
Hrabia A, Kamińska K, Socha M, Grzesiak M. Vitamin D3 Receptors and Metabolic Enzymes in Hen Reproductive Tissues. Int J Mol Sci. 2023;24:17074.
Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, et al. Vitamin D3 Regulates Follicular Development and Intrafollicular Vitamin D Biosynthesis and Signaling in the Primate Ovary. Front Physiol. 2018;9:1600.
Aghadavod E, Mollaei H, Nouri M, Hamishehkar H. Evaluation of Relationship between Body Mass Index with Vitamin D Receptor Gene Expression and Vitamin D Levels of Follicular Fluid in Overweight Patients with Polycystic Ovary Syndrome. Int J Fertil Steril. 2017;11:105–11.
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
We would like to express our sincere gratitude to the compilers of the GWAS summary dataset for their management of the data collection and data resources.
This work was supported by the General Program of National Natural Science Foundation of China (grant number No.81771741), Distinguished Professor at Educational Department of Liaoning Province (grant number No. [2014]187) to JL.
Author information
Authors and affiliations.
Department of Endocrinology and Metabolism, The Institute of Endocrinology, NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, 110000, P.R. China
Bingrui Gao, Chenxi Zhang, Deping Wang, Bojuan Li, Zhongyan Shan, Weiping Teng & Jing Li
Department of Endocrinology and Metabolism, Hongqi Hospital Affiliated to Mudanjiang Medical College, Mudanjiang, Heilongjiang, 157011, P.R. China
Deping Wang
You can also search for this author in PubMed Google Scholar
Contributions
Designed the study: Jing Li, Bingrui Gao; Collected data: Bingrui Gao, Chenxi Zhang; Performed statistical analyses: Bingrui Gao, Deping Wang, Bojuan Li; Drafted the manuscript: Bingrui Gao; Supervised the study and reviewed the manuscript: Jing Li, Zhongyan Shan, Weiping Teng.
Corresponding author
Correspondence to Jing Li .
Ethics declarations
Ethics approval and consent to participate.
Our analysis used publicly available genome-wide association study (GWAS) summary statistics. No new data were collected, and no new ethical approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
STROBE-MR Checklist; Table S2. Key characteristics of participating studies; Table S3. GWAS significant SNPs used as genetic instruments for VD level on PCOS; Table S4. GWAS significant SNPs used as genetic instruments for PCOS on VD level; Table S5. GWAS significant SNPs used as genetic instruments for VD level on BT; Table S6. GWAS significant SNPs used as genetic instruments for BT on PCOS; Table S7. GWAS significant SNPs used as genetic instruments for BT and VD level on PCOS; Table S8. Heterogeneity and directional pleiotropy test using MR-Egger intercepts; Table S9. Potentially relevant genes corresponding to IVs associated with VD and PCOS; Table S10. Potentially relevant genes corresponding to IVs associated with VD and PCOS; Table S11. GO and KEGG enrichment analysis for potentially relevant genes related to VD and PCOS; Table S12. GO and KEGG enrichment analysis for potentially relevant genes related to VD and BT; Figure S1. Scatter plot of the MR estimates for the association of VD level with PCOS; Figure S2. Funnel plot reveals overall heterogeneity of the impact of VD on PCOS; Figure S3. Leave-one-out analysis of the impact of the VD on PCOS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gao, B., Zhang, C., Wang, D. et al. Causal association between low vitamin D and polycystic ovary syndrome: a bidirectional mendelian randomization study. J Ovarian Res 17 , 95 (2024). https://doi.org/10.1186/s13048-024-01420-5
Download citation
Received : 23 February 2024
Accepted : 20 April 2024
Published : 07 May 2024
DOI : https://doi.org/10.1186/s13048-024-01420-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Testosterone
Journal of Ovarian Research
ISSN: 1757-2215
- General enquiries: [email protected]
Effects of different insulin sensitisers in the management of polycystic ovary syndrome: A systematic review and meta-analysis
Affiliations.
- 1 Monash Centre for Health Research and Implementation, Clinical and Molecular Medicine, Monash University, Melbourne, Victoria, Australia.
- 2 Department of Obstetrics and Gynecology, Helsinki University Hospital, University of Helsinki, Helsinki, Finland.
- 3 Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
- 4 Department of Obstetrics and Gynecology, Research Unit of Clinical Medicine and Medical Research Centre, Oulu University Hospital, Oulu, Finland.
- 5 Department of Woman and Child Health and Public Health, Fondazione Policlinico Universitario 'Agostino Gemelli' IRCCS, Rome, Italy.
- 6 Gynecological Endocrinology Unit, Division of Endocrinology, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
- 7 Discipline of Paediatrics, The University of Adelaide and Robinson Research Institute, North Adelaide, South Australia, Australia.
- 8 Department of Pediatrics, Division of Pediatric Endocrinology, UPMC Children's Hospital of Pittsburgh, Pittsburgh, Pennsylvania, USA.
- 9 Endocrine and Diabetes Units, Monash Health, Melbourne, Victoria, Australia.
- PMID: 37933831
- DOI: 10.1111/cen.14983
Objective: Characteristic features of polycystic ovary syndrome (PCOS) include insulin resistance and an increased risk for type 2 diabetes. To promote improved insulin sensitivity, insulin sensitisers have been used in PCOS. However, direct comparisons across these agents are limited. This study compared the effects of metformin, rosiglitazone and pioglitazone in the management of PCOS to inform the 2023 International Evidence-based PCOS Guideline.
Design: Systematic review and meta-analysis of the literature.
Patients: Women with PCOS and treatment with insulin sensitisers.
Measurements: Hormonal and clinical outcomes, as well as side effects.
Results: Of 1660 publications identified, 13 randomised controlled trials were included. Metformin was superior in lowering weight (mean difference [MD]: -4.39, 95% confidence interval [CI]: -7.69 to -1.08 kg), body mass index (MD: -0.95, 95% CI: -1.41 to -0.49 kg/m 2 ) and testosterone (MD: -0.10, 95% CI: -0.18 to -0.03 nmol/L) versus rosiglitazone, whereas there was no difference when comparing metformin to pioglitazone. Adding rosiglitazone or pioglitazone to metformin did not improve metabolic outcomes. However, rosiglitazone seemed superior to metformin in lowering lipid concentrations.
Conclusions: Metformin should remain the first-line insulin sensitising treatment in adults with PCOS for the prevention and management of weight and metabolic features. The addition of thiazolidinediones appears to offer little benefit.
Keywords: BMI; insulin resistance; metformin; pioglitazone; polycystic ovary syndrome; rosiglitazone; thiazolidinediones.
© 2023 John Wiley & Sons Ltd.
Publication types
- Meta-Analysis
- Systematic Review
- Research Support, Non-U.S. Gov't
- Diabetes Mellitus, Type 2* / drug therapy
- Hypoglycemic Agents / therapeutic use
- Insulin / therapeutic use
- Insulin Resistance*
- Metformin* / therapeutic use
- Pioglitazone / therapeutic use
- Polycystic Ovary Syndrome* / drug therapy
- Rosiglitazone / therapeutic use
- Thiazolidinediones* / therapeutic use
- Rosiglitazone
- Hypoglycemic Agents
- Pioglitazone
- Thiazolidinediones
Grants and funding
- National Health and Medical Research Council
- RACP Foundation
- Sigrid Juséliuksen Säätiö
- Novo Nordisk
- Academy of Finland
- Stiftelsen Handlanden Hjalmar Svenssons
- Iris foundation
- Orionin Tutkimussäätiö
- The Medical Society of Finland
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 11 February 2022
PCOS — a metabolic condition with health impacts on women and men
- Anju E. Joham 1 , 2 &
- Helena J. Teede 1 , 2
Nature Reviews Endocrinology volume 18 , pages 197–198 ( 2022 ) Cite this article
1493 Accesses
7 Citations
18 Altmetric
Metrics details
- Endocrine reproductive disorders
- Genetic association study
A new paper explores genetic risk factors for polycystic ovary syndrome (PCOS) and non-reproductive PCOS phenotypes in women and men. This work affirms that PCOS is indeed a metabolic disorder, that ovarian function is not required for cardiometabolic features and that this condition has implications for both men and women.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Teede, H. et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome. monash.edu . https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence-Based-Guidelines_20181009.pdf (2018).
Yilmaz, B., Vellanki, P., Ata, B. & Yildiz, B. O. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 110 , 523–533.e14 (2018).
Article Google Scholar
Yilmaz, B., Vellanki, P., Ata, B. & Yildiz, B. O. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 109 , 356–364.e32 (2018).
Lenarcik, A., Bidzińska-Speichert, B., Tworowska-Bardzińska, U. & Krępuła, K. Hormonal abnormalities in first-degree relatives of women with polycystic ovary syndrome (PCOS). Endokrynol. Pol. 62 , 129–133 (2011).
CAS PubMed Google Scholar
Zhu, J. et al. Evidence from men for ovary-independent effects of genetic risk factors for polycystic ovary syndrome. J. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgab838 (2021).
Gorsic, L. K., Dapas, M., Legro, R. S., Hayes, M. G. & Urbanek, M. Functional genetic variation in the anti-müllerian hormone pathway in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 104 , 2855–2874 (2019).
Dapas, M. et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 17 , e1003132 (2020).
Article CAS Google Scholar
Day, F. et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 14 , e1007813 (2018).
Berni, T. R., Morgan, C. L. & Rees, D. A. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J. Clin. Endocrinol. Metab. 106 , e3369–e3380 (2021).
Joo, Y. Y. et al. A polygenic and phenotypic risk prediction for polycystic ovary syndrome evaluated by phenome-wide association studies. J. Clin. Endocrinol. Metab. 105 , 1918–1936 (2020).
Download references
Author information
Authors and affiliations.
Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
Anju E. Joham & Helena J. Teede
Department of Endocrinology and Diabetes, Monash Health, Melbourne, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anju E. Joham .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Joham, A.E., Teede, H.J. PCOS — a metabolic condition with health impacts on women and men. Nat Rev Endocrinol 18 , 197–198 (2022). https://doi.org/10.1038/s41574-022-00636-z
Download citation
Published : 11 February 2022
Issue Date : April 2022
DOI : https://doi.org/10.1038/s41574-022-00636-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

This article is cited by
Alteration of plasma indoles in polycystic ovary syndrome.
- Xiaoqing Zhang
Reproductive Sciences (2024)
Sleep and obstructive sleep apnea in women with infertility
- Sally Ibrahim
- Reena Mehra
- Rebecca L. Flyckt
Sleep and Breathing (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Diabetes and Polycystic Ovary Syndrome (PCOS)
- Polycystic ovary syndrome (PCOS) is a condition that can impact fertility, and increase the risk of other chronic health conditions.
- More than half of people with PCOS develop type 2 diabetes by age 40.
- Find out the signs of PCOS, and what to do if you have it.

About PCOS and diabetes
PCOS is a condition where cysts (small sacs of fluid) develop on the ovaries. PCOS can cause irregular menstruation (periods), and is a common cause of infertility, affecting as many as 5 million people. In addition to infertility, it is a lifelong condition that can have other impacts.
People with PCOS often have insulin resistance . This is when their bodies make insulin, a key hormone in balancing blood sugar, but they can't use it effectively. Insulin resistance increases the risk of type 2 diabetes.
People with PCOS can develop serious health problems, especially if they have overweight, including:
- Type 2 diabetes
- Gestational diabetes (diabetes when pregnant)
- Heart disease —people with PCOS have a higher risk, which increases with age
- High blood pressure
- High LDL ("bad") cholesterol and low HDL ("good") cholesterol—increase the risk for heart disease
- Sleep apnea —a disorder that causes breathing to stop during sleep
PCOS is also linked to depression and anxiety, though the connection is not fully understood.
Symptoms of PCOS
The exact causes of PCOS aren't yet known. Imbalances in androgen levels (male reproductive hormones) may play an important part in PCOS. Family history of PCOS and overweight may also contribute.
Someone with PCOS may have few symptoms, while others may have them all. It's common for people to not find out they have PCOS until they are trying to get pregnant. PCOS often develops as young as age 11 or 12, around first menstruation. Symptoms include:
- Hair growth.
- Darkening of the skin in body creases, known as acanthosis nigricans.
- Irregular periods.
- Weight gain.
See your health care provider if you have these symptoms. Some people can have ovarian cysts without having PCOS.
If you're told you have PCOS, ask about getting tested for type 2 diabetes and how to manage the condition if you have it. Making healthy changes such as losing weight if you have overweight and increasing physical activity can lower your risk for type 2 diabetes . These behavior changes can also help you better manage diabetes if you have it to prevent or delay other health problems.
There are medicines that can help you ovulate, as well as reduce acne and hair growth. Make sure to talk with your health care provider about all your treatment options.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.
32 episodes
It can be ridiculously confusing living with PCOS, you're constantly reading conflicting advice. Each week, I, Clare Goodwin, will be helping to simplify this for you by sharing with you some actionable steps to take and breaking down the latest research so you can actually understand it. I'll also share some real life stories of women who've improved their PCOS symptoms to give you that weekly boost to just keep putting one foot in front of another. If you have PCOS and you want some real talk about what to do, hit that subscribe button now. Previously: The PCOS Nutritionist
PCOS Explained Clare Goodwin
- 4.8 • 205 Ratings
- MAY 15, 2024
Even this doctor struggled to get her PCOS under control, and what did help
In this gritty conversation, Clare interviews Dr Stephanie, a physician who shares her personal and professional journey dealing with Polycystic Ovary Syndrome (PCOS). Stephanie shares incredible advice around how we should view our doctors or primary care providers' advice on our own health journey, and how it ultimately is our own decision about the steps we move forward with. Stephanie also shares with us great tips for how to advocate for ourselves with a diagnosis, or how to feel co...
Falling pregnant within 3 months by making the lifestyle changes that matter: Cindy's story
When we are first diagnosed with PCOS we are often lead to believe that 'you will struggle to get pregnant', and we hear this time and time again. But, is this really the case?We spoke with our patient, Cindy about her experience with getting diagnosed in her late 20's, struggling to conceive then proceeding to fall pregnant naturally (a month before she was due to start fertility treatment!).Cindy is a doer and knew she had to take charge as soon as she got diagnosed which lead her to find t...
Losing weight and improving PCOS symptoms all came down to this...
We can often get caught up trying so hard to do everything perfectly, giving 100%, but as soon as we're not hitting that mark, we feel like we've failed, or as our PCOS patient Lily puts it, 'fallen off the wagon'.After struggling with the healthcare system, Lily took matters into her own hands and started our program. Lily followed the Ovie approach, a holistic lifestyle intervention that, within the space of a few months, helped her lose the stubborn weight she'd been trying so hard to addr...
- 1 hr 16 min
I wish I knew this at the start of my PCOS journey
In this episode, we're going deep on PCOS. PCOS affects about 1 in 8 women, but there's a lot of confusion out there about it.We discuss how genetics and lifestyle play a role, and we also touch on the common misconceptions with PCOS, and discuss it’s not just about what's happening in your ovaries; things like insulin resistance, stress, inflammation, and thyroid problems are all key drivers of this syndrome. We also highlight why what works for one person, might not work for another.But her...
- MAY 8, 2024
Fannie's fertility journey, and how she got the answers she needed
In this episode Clare sits down with Fannie to discuss her PCOS journey, starting from her teenage years dealing with irregular periods and uncertain diagnoses. She shares the impact of PCOS on her life, including symptoms like weight gain, sugar cravings, and insulin resistance. The conversation also delves into the significant role of understanding her blood work, particularly iron levels and their relation to insulin resistance and fertility. Her journey underscores the importanc...
- JAN 24, 2024
How we're approaching New Year goals differently
Each year when January comes about some may think that we need to create all these New Year's resolutions so that this year is going to be our best, only to run out of steam before January is even over (we've been there too). Instead we believe in setting sustainable intentions and goals that you can work towards gradually so you don't feel totally overwhelmed.Today, The Ovie team discuss how we're approaching the New Year and what kind of person we want to be not just for 2024 but for life.I...
- © 2024 PCOS Explained
Customer Reviews
205 Ratings
I appreciate all of the info you being to educate us!!
Glad it exists but not enough info
I’m glad there’s a podcast like this, dedicated to pcos that doesn’t seem to endorse the pseudo-science that other similar podcasts do. But there also isn’t enough info in these podcasts. I haven’t really heard actual advice given, and it seems like they do that on purpose to tempt you to buy the protocol, which I find really predatory. The lack of ACTUAL details about this protocol online and in their own social media makes me think that it wouldn’t be any different than most of the reasonable advice given elsewhere for free (balance protein and slow-digesting carbs, exercise, and take well studied supplements like inositol).
Best PCOS Podcast
I’ve listened to all the PCOS podcasts out there, and this one is the best! Evidence based informed that’s presented in easily digestible episodes. I love Clare and how she educates woman with empowerment.
Top Podcasts In Education
You might also like.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Polycystic ovary syndrome (pcos) treatment global strategic research report 2024-2030: north america and europe dominates, asia-pacific to exhibit fastest growth.
Polycystic Ovary Syndrome (PCOS) Treatment Market
Dublin, May 17, 2024 (GLOBE NEWSWIRE) -- The "Polycystic Ovary Syndrome (PCOS) Treatment - Global Strategic Business Report" report has been added to ResearchAndMarkets.com's offering. Global Polycystic Ovary Syndrome (PCOS) Treatment Market to Reach $3.8 Billion by 2030 The global market for Polycystic Ovary Syndrome (PCOS) Treatment estimated at US$2.6 Billion in the year 2023, is projected to reach a revised size of US$3.8 Billion by 2030, growing at a CAGR of 4.8% over the analysis period 2023-2030.
Recent market activities highlight a competitive landscape with players categorized as strong, active, niche, or trivial in 2023. Key market trends and drivers include the rising incidence of PCOS, advancements in PCOS management, and the growing prevalence of obesity, which is a significant risk factor for PCOS. These factors collectively spur the demand for PCOS drugs and drive market expansion.
The global market for Polycystic Ovary Syndrome (PCOS) treatment is poised for steady growth, driven by rising awareness and the increasing incidence of PCOS. Insulin-Sensitizing Agents, one of the segments analyzed in the report, is projected to record 4.5% CAGR and reach US$1.4 Billion by the end of the analysis period. Growth in the Oral Contraceptives segment is estimated at 5.7% CAGR for the next 8-year period.
The U.S. Market is Estimated at $820.5 Million, While China is Forecast to Grow at 6.4% CAGR
North America and Europe currently dominate the market, while the Asia-Pacific region is expected to exhibit the fastest growth. Insulin-sensitizing agents, particularly Metformin, lead the market due to their widespread prescription for PCOS management. Oral contraceptives are anticipated to register the fastest growth among treatment options.
The Polycystic Ovary Syndrome (PCOS) Treatment market in the U.S. is estimated at US$820.5 Million in the year 2023. China, the world's second largest economy, is forecast to reach a projected market size of US$563.7 Million by the year 2030 trailing a CAGR of 6.4% over the analysis period 2023 to 2030. Among the other noteworthy geographic markets are Japan and Canada, each forecast to grow at 3.4% and 4.6% respectively over the 2023-2030 period. Within Europe, Germany is forecast to grow at approximately 4% CAGR.
Key Attributes:
MARKET OVERVIEW
Competition
Polycystic Ovary Syndrome (PCOS) Treatment - Global Key Competitors Percentage Market Share in 2023 (E)
Polycystic Ovary Syndrome (PCOS) - A Prelude
Treatment Options Available
Global PCOS Treatment Market to Witness Steady Growth
North America and Europe Dominates, Asia-Pacific to Exhibit Fastest Growth
Insulin Sensitizing Agents Lead; Metformin - A Widely Prescribed Insulin-sensitizing Agent for PCOS
Oral Contraceptives to Register Fastest Growth
Recent Market Activity
Competitive Market Presence - Strong/Active/Niche/Trivial for Players Worldwide in 2023 (E)
MARKET TRENDS & DRIVERS
Rising Incidence of PCOS and Rising Awareness Drives Market Growth
Recent Advances in PCOS Management to Spur the Demand for PCOS Drugs
Growing Prevalence of Obesity - A Prime Risk Factor for PCOS Drives Market Growth
Obesity Prevalence Rate (%) in Select Countries for the Years 2019 and 2030P
Weight Loss Medications Seek Role in PCOS Treatment
Dip in Fertility Levels Propels the Demand for PCOS Treatment
Innovative Therapies Ensure Timely Treatment for Patients Suffering from Polycystic Ovary Syndrome
Ongoing Research and Development Initiatives Mitigates Risks Pertaining to Infertility and Various Other Pregnancy Related Complications
Use of Advanced Technologies for Treating Polycystic Ovary Syndrome Witnesses a Surge
Laparoscopic Ovarian Drilling and other Surgical Procedures Come to the Rescue When Drugs Fail
FOCUS ON SELECT PLAYERS (Total 38 Featured)
Kern Pharma S.L.
GlaxoSmithKline PLC
Abbott Laboratories, Inc.
Merck & Co., Inc.
Eli Lilly and Company
Cadila Pharmaceuticals Ltd.
Ferring Pharmaceuticals Inc
Agile Therapeutics, Inc.
Italfarmaco SpA
Crinetics Pharmaceuticals, Inc.
Glenmark Pharmaceuticals Inc.
Celmatix, Inc
For more information about this report visit https://www.researchandmarkets.com/r/17dmt5
About ResearchAndMarkets.com ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing
Hosna mohammad sadeghi.
1 Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 11369, Iran; moc.liamg@9731smansoh (H.M.S.); moc.oohay@adya_ileda (I.A.); ri.ca.smut.tneduts@ivasuoM-ts (T.M.); moc.liamg@57ilainadheizram (M.D.)
2 Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 11369, Iran
Daniela Calina
3 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania
Anca Oana Docea
4 Department of Toxicology, Faculty of Pharmacy, University of Medicine and Pharmacy, Petru Rares, 200349 Craiova, Romania; moc.liamg@00anaoad
Taraneh Mousavi
Marzieh daniali, shekoufeh nikfar.
5 Department of Pharmacoeconomics and Pharmaceutical Administration, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 11369, Iran; [email protected]
6 Personalized Medicine Research Center, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran 11369, Iran
7 Evidence-Based Evaluation of Cost-Effectiveness and Clinical Outcomes Group, Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 11369, Iran
Aristidis Tsatsakis
8 Department of Analytical and Forensic Medical Toxicology, Sechenov University, 119991 Moscow, Russia; moc.liamg@sikastastsira
9 Department of Forensic Sciences and Toxicology, Faculty of Medicine, University of Crete, 71003 Heraklion, Greece
10 Laboratory of Toxicology, Medical School, University of Crete, 70013 Heraklion, Greece
Mohammad Abdollahi
Polycystic ovary syndrome (PCOS) is an endocrine-gynecology disorder affecting many women of childbearing age. Although a part of the involved mechanism in PCOS occurrence is discovered, the exact etiology and pathophysiology are not comprehensively understood yet. We searched PubMed for PCOS pathogenesis and management in this article and ClinicalTrials.gov for information on repurposed medications. All responsible factors behind PCOS were thoroughly evaluated. Furthermore, the complete information on PCOS commonly prescribed and repurposed medications is summarized through tables. Epigenetics, environmental toxicants, stress, diet as external factors, insulin resistance, hyperandrogenism, inflammation, oxidative stress, and obesity as internal factors were investigated. Lifestyle modifications and complementary and alternative medicines are preferred first-line therapy in many cases. Medications, including 3-hydroxy-3-methyl-3-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, thiazolidinediones, sodium-glucose cotransporter-2 inhibitors, dipeptidyl peptidase-4 inhibitors, glucose-like peptide-1 receptor agonists, mucolytic agents, and some supplements have supporting data for being repurposed in PCOS. Since there are few completed clinical trials with a low population and mostly without results on PCOS repurposed medications, it would be helpful to do further research and run well-designed clinical trials on this subject. Moreover, understanding more about PCOS would be beneficial to find new medications implying the effect via the novel discovered routes.
1. Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder that impacts many women of the reproductive age worldwide [ 1 ]. This syndrome is often associated with enlarged and dysfunctional ovaries, excess androgen levels, resistance to insulin, etc. [ 2 ]. It is estimated that approximately every 1 in 10 women face PCOS before menopause and struggle with its complications [ 3 ].
Although the high ratio of luteinizing hormone (LH) to follicle-stimulating hormone (FSH) and increased frequency of gonadotropin-releasing hormone (GnRH) is known as the underlying causes of PCOS [ 4 ], the exact etiology and pathology have not been comprehensively well-known [ 4 , 5 ]. Evidence suggests the role of different external and internal factors, including insulin resistance (IR), hyperandrogenism (HA), environmental factors, genetic, and epigenetics. In addition, it is worth mentioning that PCOS increases the risk of further complications like cardiovascular diseases [ 5 , 6 ], type 2 diabetes mellitus [ 5 , 6 ], metabolic syndrome [ 6 ], depression, and anxiety [ 7 ].
To manage this condition, the most crucial step is to lose at least 5% of the weight; therefore, having a regular exercise plan and fat and sugar-free diets are also recommended to every woman with PCOS. Furthermore, in some cases, taking complementary and alternative medicine strategies with or without other treatments is preferable due to their prior beliefs, lower costs, etc.
Physicians tend to use (combined) oral contraceptives, antiandrogen agents, insulin sensitizers, and ovulation inducers [ 4 ]. Up until today, there is no United States Food and Drug Administration (USFDA) approved medication specifically for PCOS, and all mentioned medications are used off-label [ 8 ]. Apart from the essential need for improvement in the research and development of new drug molecules and new drug discovery, novel medications could be found with drug repurposing methods [ 9 ]. On this very spot, there are plenty of medications, previously approved by USFDA for indications rather than PCOS; and, today, there is a desire to implement them as the therapeutic options in the management of PCOS.
These agents vary from anti-diabetic medications such as pioglitazone, empagliflozin, sitagliptin, liraglutide to 3-hydroxy-3-methyl-3-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors like simvastatin and atorvastatin, as well as mucolytic drugs like N-acetyl cysteine.
Given that PCOS is a growing issue that is unfortunately followed by many unwanted complications and that available methods and medications are not 100% effective, it is essential to investigate its pathogenesis and find out new pharmacological targets carefully. This could be done through repositioning approaches, saving time and cost.
This review discusses PCOS’s definition, diagnosis, and etiology, focusing on the pathogenesis and management of this syndrome. Internal and external factors contributing to PCOS have been comprehensively studied, and several commonly prescribed medications with their complete drug information are provided. Subsequently, a couple of repurposed medications are mentioned thoroughly, reviewing the related clinical trials over the past five years.
PubMed, Google Scholar, ScienceDirect, TRIP database, and UpToDate were comprehensively searched for publications including PCOS relevant keywords in different areas, focusing on the new ones (since 2016) and excluding those with a language rather than English or animal studies. In addition, Clinicaltrials.gov was searched to find data about completed or running trials of repurposed drugs in PCOS over the past five years.
3. Diagnosis
PCOS is among the conditions that cannot be diagnosed with basic diagnostic tests, including blood tests, culture, and biopsy; thus, there is no certain test for PCOS diagnosis. Differential diagnosis is called excluding the relevant disorders according to the symptoms and narrowing the choices. In order to establish a differential diagnosis for PCOS, hyperprolactinemia, thyroid disease, Cushing’s syndrome, and hyperplasia of adrenal should be excluded based on the associated investigations [ 10 , 11 ]. Although considering past medical history, weight changes, and symptoms of insulin resistance might be helpful, pelvic examination, a transvaginal ultrasound, and measuring the level of hormones are among the most frequently recommended investigations [ 12 ]. According to the National Health Service (NHS), irregular or infrequent periods, high levels of androgenic hormones or symptoms, and scans showing polycystic ovaries are the specified criteria for PCOS [ 13 ]. In addition, Rotterdam PCOS diagnostic criteria in adults are the most commonly used method. In an ultrasound, the presence of two clinical or biochemical hyperandrogenism, ovulatory dysfunction, or polycystic ovaries would finalize a PCOS diagnosis [ 14 ].
4. Etiology and Risk Factors
4.1. external factors, 4.1.1. epigenetic mechanism.
Epigenetic refers to inheritable alterations in genome and gene expression without any changes in DNA sequence [ 15 , 16 ]. These changes involve adding or omitting chemical components on DNA or histone [ 17 ]. Increased LH activity is a seen phenomenon in PCOS women. It may relate to the problems in follicle development and HA, which are common among PCOS patients [ 18 ]. LH/choriogonadotropin receptor (LHCGR) is responsible for the steroidogenesis process in theca cells [ 19 ]. This receptor hypomethylation leads to higher gene expression and sensitivity to LH [ 18 , 20 ].
A study on PCOS patients approved that hypomethylated sites are related to overexpression of LHCGR [ 15 , 19 ] on theca cells surface [ 19 ]. In addition, epoxide hydrolase 1 (EPHX1) is an active enzyme in degrading aromatic compounds [ 15 , 19 , 21 ]. Its gene promoter hypomethylation [ 15 , 19 ] increases enzyme expression [ 15 ]. Overproduction of EPHX1 reduces the transformation of testosterone to estradiol, which can contribute to PCOS [ 15 ]. Furthermore, peroxisome proliferator-activated receptor gamma (PPAR-γ) plays a role in ovaries’ function [ 15 , 18 , 19 , 22 ]. Hypermethylation of PPARγ, hypomethylation of nuclear co-repressor 1 [ 19 , 22 ], and alteration in acetylation of histone deacetylase 3, for which both are PPARγ co-repressors [ 15 ], are observed in PCOS patients showing HA [ 15 , 19 , 22 ]. These alterations were noticed in PCOS women’s granulosa cells [ 18 , 23 ].
4.1.2. Environmental Toxicants
The United States Environmental Protection Agency (USEPA) defines endocrine-disrupting chemical (EDC) as “an exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behavior” [ 24 ].
EDCs may act as hormones’ agonists or antagonists in binding to their receptors [ 25 ]. EDCs are almost parts of everything we use in our daily life [ 21 ]. Their structures consist of phenols or halogens like chlorine and bromine, so they imitate steroid hormones’ actions [ 21 ]. Studies have approved the higher serum concentration of EDCs in PCOS suffering women [ 21 , 26 ]. Prolonged and continuous exposure to EDCs from prenatal to adolescence can cause susceptibility to PCOS [ 21 , 27 ].
As an example, bisphenol A (BPA) BPA is a synthetic compound used in polycarbonate plastics, epoxy resins [ 25 , 28 ], dental filling, food and drink packages [ 25 ], baby bottles, and polyvinyl chloride (PVC) [ 28 ], which affects metabolism through different pathways. BPA directly affects oogenesis [ 29 ] by interacting with estrogen receptor (ER) α and β, non-classical membrane ER, and G-protein coupled receptor 30 (GPCR30) [ 21 , 28 , 29 ]. It also triggers androgen secretion and restrains testosterone catabolism in theca cells [ 21 , 29 ].
Another effect of BPA on interstitial theca cells is the overproduction of androgens by dysregulation of 17β-hydroxylase (P450c17) [ 28 , 30 ], cholesterol side-chain cleavage enzyme (P450scc), and steroidogenic acute regulatory protein [ 30 ]. BPA’s influence on granulosa cells refers to reducing the expression of aromatase enzyme and production of estrogen [ 21 , 29 ]. Lastly, it disturbs the intrafollicular environment and damages the oocyte development and maturation [ 21 , 29 ]. BPA’s indirect effect on HA involves downregulation of testosterone 2a-hydroxylase and testosterone 6b-hydroxylase enzymes in liver level, and thus a higher concentration of testosterone [ 30 , 31 ].
In addition, BPA is a potent ligand for sex hormone-binding globulin (SHBG) and replaces testosterone; thereby, free testosterone concentration increases. Androgen and BPA have a two-way relation; high androgen inactivates the uridine diphosphate-glucuronosyl transferase enzyme and reduces BPA clearance in the liver. This process causes a high concentration of free BPA in blood and worsens its negative effects on the ovaries [ 21 , 29 , 30 , 31 ].
Additionally, it is believed that BPA may act as an obesogen [ 28 , 30 ]. Its obesogenic influence includes upregulation of adipogenesis-related genes [ 30 ], stimulation of adipocytes differentiation [ 28 , 30 ], potentiation of the accumulation of lipid in cells incorporated in medical syndrome, and triggering the conversion of target cells to adipocytes via phosphatidylinositol 3-kinase pathway [ 30 ].
Adipogenesis due to BPA happens because of the activation of the glucocorticoid receptor. Activation of the receptor upregulates the enzyme involved in the conversion of cortisone to cortisol, thus inducing adipogenesis [ 28 ]. Moreover, BPA prompts the release of interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) [ 30 , 31 ], both involving adiposity and IR [ 30 ]. In addition, it restrains the release of adiponectin [ 28 , 29 , 30 , 31 ] and the beneficial compound in protecting against IR [ 28 , 30 , 31 ].
It can also change glucose homeostasis [ 28 , 29 , 31 ] by directly influencing the pancreatic cells [ 29 ]. BPA causes a chronic increase in insulin and further IR in long exposure [ 30 ] by affecting the mitochondrial activity and metabolic pathways of β-pancreatic cells [ 28 ]. BPA reduces glucagon secretion by inhibiting the intracellular calcium ion fluctuating pattern with a lack of glucose condition [ 30 ].
Advanced glycation end products (AGEs), also called glycotoxins, are another chemical group affecting body health. AGEs are pro-inflammatory molecules [ 21 , 23 , 29 , 32 ] that interact with their surface receptor called RAGE (receptor for AGE) [ 21 , 23 , 29 ] and stimulate pro-inflammatory pathways and oxidative stress [ 21 , 23 , 29 , 32 ]. AGEs can be absorbed into the body as exogenous compounds or derived from nonenzymatic glycation and oxidation of proteins and lipids [ 21 ]. Increased concentration of AGEs in serum has been detected in PCOS patients [ 21 ]. AGEs interrupt pre-ovulatory follicles growth via ERK1/MAPK pathway and damage follicles by oxidative stress caused by interaction with RAGEs [ 21 ]. This interaction increases intracellular inflammatory molecules [ 21 ].
In vitro studies on 3T3-L1 cell lines showed that glycotoxins are likely to trigger adipogenesis [ 21 ]. On the other hand, a higher body mass index corresponds to a lower extent of soluble RAGEs, which is responsible for glycotoxin clearance and deposition of AGEs in the reproductive system, especially in ovaries [ 21 , 29 ]. This bilateral relation worsens inflammatory processes and metabolic syndrome in PCOS [ 21 ]. AGEs also play a role in IR [ 21 , 29 ]. These compounds disrupted glucose transport in the human granulosa KGN cell line [ 21 ] and reduced glucose uptake by adipocytes in previous research [ 21 , 29 ]. They also involve IR by causing oxidative stress, inflammation, and glycation of proteins, which considerably diminishes insulin sensitivity [ 21 ]. Moreover, increased concentration of AGEs changes the insulin signaling pathway and interferes with glucose transporter 4 (GLUT-4) translocation [ 23 ].
4.1.3. Physical and Emotional Stress
Although there is minimal information on the role of stress in PCOS, it is known that PCOS possesses adverse effects on self-esteem and mental health. Chronic stress results in hypertrophy and hyperplasia of adipocytes. This phenomenon happens as a result of glucocorticoids’ effect on pre-adipocytes maturation. Chronic stress is also associated with adipokine secretion, attraction, and activation of stromal fat immune cells [ 33 ].
In addition, it is responsible for making an inflammatory condition by leading to high levels of inflammatory cytokines like IL-6 and TNF-α, along with disrupting oxidant-antioxidant balance [ 33 ]. In addition, chronic stress plays a vital role in IR.
Stress triggers the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol [ 34 , 35 ]. Cortisol leads to IR by stimulating visceral fat accumulation, gluconeogenesis, and lipolysis [ 35 ]. Moreover, cortisol arouses glucose production in the liver [ 35 ]. Stress is also involved in enhancing insulin levels [ 34 ]. Other stress influences on PCOS may refer to inference with anti-mullerian hormone (AMH) and changing sex hormone levels [ 34 , 35 ].
4.1.4. Diet
Although nutrition contributions to PCOS is unclear, studies showed a relationship between some nutrient levels and PCOS indices.
Saturated fatty acids (SFAs) intake plays a role in PCOS by producing an inflammatory status [ 36 ] and reducing insulin sensitivity [ 37 ]. Taking SFAs induces inflammation by triggering an increase in TNF-α level in circulation and expressing a specific cytokine suppressor [ 36 ].
Vitamin D deficiency may exacerbate PCOS [ 37 , 38 ] or the comorbidities induced by PCOS [ 38 ]. Calcitriol upregulates insulin receptors at mRNA and protein levels. It also increases insulin sensitivity directly and indirectly. The direct effect occurs by activating PPAR-δ, the involved receptor in fatty acids metabolism in adipose tissue and skeletal muscle. The indirect impact is the regulation of intracellular calcium, which is vital for insulin-mediated signaling in fat and muscle [ 38 ]. On the other hand, vitamin D deficiency may result in insulin resistance by causing an inflammatory response [ 37 , 39 ]. Furthermore, vitamin D downregulates the AMH promoter [ 39 ].
4.2. Internal Factors
4.2.1. insulin resistance.
IR means an insufficient cells response to insulin [ 40 ]. IR is independent of patients’ adiposity, body fat topography, and androgen levels [ 18 , 41 ]; i.e., it has been reported in lean patients as well [ 18 , 42 ]. It should be mentioned that IR is tissue-selective in PCOS women [ 18 , 43 ], although skeletal muscles [ 18 , 43 , 44 ], adipose tissue, and liver lose their sensitivity to insulin, adrenal glands [ 18 , 43 ], and ovaries remain sensitive [ 18 , 28 , 43 , 45 ].
Insulin directly triggers androgens production in ovarian theca cells [ 32 , 44 , 46 , 47 , 48 ] and grow [ 48 ]. Insulin effectively stimulates ovarian follicle growth and hormone secretion by stimulating its receptors in the follicle membrane cells [ 49 ]. It also triggers ovarian P450c17 [ 18 , 23 , 50 ] and P450scc enzyme activity to promote ovarian steroidogenesis [ 18 , 51 ] and increases them with the synergistic effect of chorionic gonadotropin [ 52 ]. This hormone, as well as insulin-like growth factor 1 (IGF-1) [ 18 ], synergizes with luteinizing hormone [ 18 , 45 ]. Hyperinsulinemia increases LH-binding sites and androgen-producing response to LH [ 44 ]. LH and insulin interaction enhance steroidogenic acute regulatory enzyme and CYP450c17 mRNA expression [ 52 , 53 ]. CYP450c17 is involved in androgen production [ 23 , 44 ]. Likewise, IR independently enhances CYP17A1 activity, the productive enzyme in androstenedione and testosterone production [ 52 ].
On the other hand, hyperinsulinemia reduces hepatic SHBG [ 18 , 32 , 40 , 49 , 52 , 54 , 55 , 56 ], increasing free testosterone levels in blood [ 18 , 32 , 52 , 54 , 56 ]. In addition, hyperinsulinemia inhibits IGF-1 binding protein production in the liver. IGF-1 is responsible for triggering the production of androgens in thecal cells. Inhibition of the production of IGF-1 binding proteins leads to a higher concentration of this substance in blood circulation and then higher production of androgens in thecal cells [ 18 , 46 ]. Moreover, IGF-1 upregulation decreases a specific miRNA and thus accelerates granulosa cells apoptosis and inhibits folliculogenesis [ 52 ]. HA [ 46 ] and hyperinsulinemia [ 45 , 46 , 57 ] both play a role in stopping follicles growth [ 45 , 46 ]. This stoppage is attributed to menstrual irregularity, anovulatory sub-fertility, and amassing of immature follicles [ 46 ].
Furthermore, hyperinsulinemia contributes to PCOS by affecting the pituitary gland. Excessive insulin stimulates its receptors in the pituitary gland to release LH [ 49 ]. Accumulation of insulin stimulates GnRH and LH pulse secretion via influencing both amplitude and frequency [ 23 ]. Insulin’s indirect effect on PCOS is augmented by pituitary gonadotropin sensitivity to GnRH [ 18 ], and hyperinsulinemia increases GnRH neuron activity [ 58 ].
The insulin’s influence on adipose tissue and inflammation is another essential PCOS pathogenesis topic. Insulin stimulates adipogenesis and lipogenesis and inhibits lipolysis [ 42 ], resulting in fat accumulation [ 44 ]. IR leads to enhanced plasma levels of free fatty acids (FFAs), affecting the liver and adipose tissue [ 32 ]. Moreover, IR causes a reduction in omentin level independent of the patient’s body mass index (BMI). In addition, hyperglycemia can lead to inflammation by producing TNF-α from mononuclear cells (MNCs) [ 50 ].
4.2.2. Hyperandrogenism
Generally, hyperandrogenism (HA) reduces the SHBG level, leading to a higher concentration of free testosterone [ 18 , 59 ]. It was observed that PCOS women have higher concentrations of testosterone in plasma which can convert to estrone in adipose tissue. Increased alteration of estrone to estradiol affect follicle growth and increases the LH to FSH ratio causing ovulatory dysfunction [ 23 ].
HA can result in AMH upregulation, which inhibits ovulation and the development of follicles by a different mechanism. Furthermore, the IGF-II level is negatively related to androgen levels, and HA reduces IGF-II in follicular fluid. IGF-II positively relates to follicle diameters and estradiol concentration in follicular fluid [ 23 ]. In addition, HA increases LH indirectly [ 58 , 60 ]. Estradiol and progesterone are responsible for GnRH and LH secretion via negative feedback [ 58 , 61 , 62 ]. HA disrupts the negative feedback on secretion [ 18 , 23 , 61 , 62 ] resulting in increased LH levels [ 18 , 62 ]. Interaction of androgen and its receptor interferes with progesterone receptor transcription. Moreover, this receptor is involved in converting high levels of androgens to compounds that modulate the gamma-aminobutyric acid A (GABA A ). Modulation of the GABA A receptor triggers GnRH neurons and weakens the response to negative progesterone feedback [ 58 ]. In addition, it is assumed that androgens might decrease hepatic nuclear factor-4α (HNF-4α) levels by inhibiting lipid synthesis. HNF-4α stimulates SHBG expression by binding to its promoter [ 63 ].
HA contributes to other influential factors of PCOS, including IR, inflammation, and oxidative stress.
HA aggravates IR via different routes; it reduces the insulin sensitivity, expression of GLUT-4 and inhibits insulin degradation in the liver [ 23 , 32 ]. Moreover, HA increases a type of skeletal muscle fibers that have low insulin sensitivity [ 32 ]. On the other hand, HA worsens central adiposity, which is involved in IR [ 23 , 32 ]. Additionally, it was observed that testosterone increases inflammatory chemicals such as lipopolysaccharide-induced IL-6 in 3T3-L1 adipocytes by activating some signaling pathways [ 64 ]. One way androgen results in oxidative stress is by increasing MNC sensitivity to glucose and aggravating glucose-stimulated oxidative stress [ 65 ]. It is worth mentioning that dehydroepiandrosterone as an androgen decreases interferon-γ (IFN-γ), an essential regulator in normal ovarian physiology and cell function [ 64 ].
In addition, it should be mentioned that studies on PCOS women approved the resemblance of their fatty tissue to men, and hence the effect of HA on adipose tissue dysfunction [ 8 ]. In addition, HA is a cause of adipocyte hypertrophy and consequential damages to adipokine secretion [ 55 ].
4.2.3. Inflammation
Appropriate inflammation is a vital cause of oocyte growth and ovulation [ 66 ]. However, high levels of white blood cell [ 46 , 66 ], C-reactive protein (CRP) [ 4 , 46 , 50 , 66 , 67 ], and other inflammatory biomarkers in peripheral blood are associated with PCOS [ 4 , 46 , 66 , 67 , 68 ]. Inflammation is a cause of HA [ 44 , 69 ]. TNF-α is a pro-inflammatory chemical that can worsen IR. Contribution to IR happens due to interference of pro-inflammatory molecules with insulin signaling pathways [ 32 , 67 ] and reduction of GLUT-4 expression [ 23 ]. Some studies showed that the insulin receptor substrate (IRS) serine residue phosphorylation inhibits insulin receptor signaling [ 32 , 70 ]. This phenomenon results in the prevention of GLUT-4 translocation and glucose reuptake [ 70 ]. In addition, TNF-α showed the ability to prompt theca cells proliferation in vitro [ 71 ]. Furthermore, IL-1 hinders the FSH and LH receptors. Inhibition of these receptors leads to inhibition of follicular development and ovulation [ 66 ]. Both TNF-α and IL-1β inhibit activation of HNF-4α by different mechanisms [ 23 ]. In addition, NLRP3 inflammasomes induce follicular pyroptosis, ovarian fibrosis, and disturbance of follicular formation [ 66 ]. An increase in CRP level is another cause of IR in insulin-sensitive tissues. IR occurs because of increased pro-inflammatory factors secreted by the liver and monocytes. CRP stimulates this increase in secretion [ 72 ]. Moreover, another study approved the higher-than-normal level of IL-6 mRNA in granulosa cells [ 66 ].
4.2.4. Oxidative Stress
Oxidative stress (OS) is an imbalance between pro-oxidants and antioxidants [ 71 , 72 , 73 ]. Oxidative molecules include different chemicals such as reactive oxygen species (ROS) [ 73 , 74 , 75 ] (e.g., O 2− , H 2 O 2 , and OH − ) [ 76 ] and reactive nitrogen species (RNS) [ 74 , 75 ]. ROS plays a role in different mechanisms like signaling pathways [ 71 , 73 , 76 ], cell growth [ 71 , 73 ], and differentiation, as well as RNS [ 73 ]. RONS also acts on ovaries functions like steroidogenesis [ 67 , 77 ] and affects neurons responsible for feeding behavior to induce hunger [ 71 ]. Overproductions of oxidative chemicals cause various damage to vital molecules such as lipids, proteins, and DNA [ 73 , 74 , 75 , 77 ].
Increased OS has been seen in PCOS patients in different studies [ 74 , 78 , 79 ]. Increased levels of OS activate the nuclear factor-kappa B (NF-κB) [ 72 , 75 ]. NF-κB is involved in inflammatory pathways [ 75 ] and affects the production of pro-inflammatory cytokines like TNF-α and IL-6 [ 72 , 80 ]; the effect in IR and PCOS was explained above. A high level of OS also increases the release of TNF-α [ 77 ]. On the other hand, increased OS actuates some protein kinases that trigger serine/threonine phosphorylation instead of normal tyrosine phosphorylation of IRS. Thus, the insulin signaling pathway is inhibited, and OS leads to IR [ 67 ]. OS also plays a role in obesity. It increases mature adipocyte size and consequently stimulates pre-adipocyte proliferation and adipocyte differentiation. OS also imposes a major effect on obesity [ 71 ].
4.2.5. Obesity
Obesity is a key in low-grade chronic inflammation [ 72 ]. Accumulation of adipocytes in visceral fat leads to hypoxia and consequent necrosis, which causes inflammatory cytokines production [ 66 ]. Adipocyte death due to hypertrophy causes an inflammatory state [ 44 , 69 ]. The mononuclear cells of adipose tissue produce pro-inflammatory cytokines [ 6 , 44 , 81 ]. Excess abdominal fat is also responsible for the inflammatory condition [ 6 , 44 , 81 ].
Obesity also plays a role in hyperinsulinemia, IR, and HA occurrence. Visceral obesity arouses an increase in non-esterified fatty acids (NEFAs) levels in the blood. Skeletal muscles uptake NEFAs as the energy source instead of glucose. This hyperglycemia leads to a pancreas rapid reaction and hyperinsulinemia [ 55 ]. In addition, the lipolytic response of visceral fat to catecholamines causes lipotoxicity [ 44 ] and impairment of insulin clearance and activity [ 81 ].
FFA stimulates IRS-1 serine/threonine phosphorylation and reduces tyrosine phosphorylation. Increased FFAs reduce insulin and glucose uptake sensitivity in intramyocellular lipids [ 52 ]. Notably, that visceral fat is weightier in IR than abdominal [ 44 ] and subcutaneous fat [ 81 ] as the visceral fat lipolytic response to catecholamines is more severe [ 44 , 81 ]. The reason is the increased function of the β3 and higher expression of β1 and β2 receptors [ 81 ]. Moreover, the type 1 isoenzyme of 11β-hydroxysteroid dehydrogenase (11β-HSD) is involved in converting cortisone to active cortisol, which is highly expressed in adipose tissue, especially in adipose tissue visceral ones. Glucocorticoids reduce glucose uptake and insulin signaling in omental adipocytes [ 81 ]. In addition, visceral fat’s adiponectin secretion is less than subcutaneous fats, and this phenomenon leads to decreased adiponectin secretion in obesity [ 46 ].
In addition to all adipose tissue’s functions mentioned above, this tissue has endocrine function and secretes chemicals called adipokines or adipocytokines. Adipocytes produce leptin, a high concentration of which inhibits the expression of aromatase mRNA in granulosa cells—thus interrupting androgens to estrogen conversion [ 52 ]. In addition, it is suggested that increased leptin levels are related to the absence of folliculogenesis [ 81 ]. Moreover, adiponectin, secreted by adipocytes [ 52 ], has insulin-sensitizing, anti-diabetic, and anti-inflammatory effects [ 46 ]. The adiponectin insulin-sensitizing effect causes a reduction in FFA uptake and gluconeogenesis. It also plays a role in progesterone and estrogen production, ovulation, and decreased GnRH secretion [ 52 ]. Furthermore, adiponectin reduces LH secretion from the pituitary, triggers estradiol secretion in granulosa, and is associated with androgen production in ovaries [ 81 ]. Omentin-1, another adipose tissue secreted chemical, improves IGF-1-induced progesterone and estradiol secretion in different ways, including increasing the steroidogenic acute regulatory protein and CYP450 aromatase expression and enhancing IGF-1 receptor signaling [ 82 ].
Adipose tissue also has several enzymes responsible for converting androstenedione to testosterone and testosterone to dihydrotestosterone [ 45 ]. 17β-HSD converts androstenedione to testosterone [ 44 , 81 ] and estrone to estradiol [ 81 ]. This enzyme is expressed in adipose tissue [ 44 , 81 ]. As a result of this process, excess adiposity exacerbates HA [ 45 ].
Furthermore, the accumulation of lipid in non-adipose tissues, called lipotoxicity, causes oxidative/endoplasmic reticulum stress linked with inflammation and IR. Excess fatty acids in muscles and liver induce IR via serine phosphorylation of insulin receptor by diacylglycerol [ 83 ]. In addition, lipid accumulation in the liver diminishes HNF-4α levels leading to reduced SHBG production [ 63 ].
A summary of the most representative molecular mechanisms of PCOS pathogenesis is presented in Figure 1 .

Summarized scheme regarding the pathophysiology of PCOS. Abbreviations and symbols: ↑ (increased), ↓ (decreased), DNA (deoxyribonucleic acid), GnRH (Gonadotropin-releasing hormone), IL-6 (interleukin 6), IR (insulin resistance), LH (luteinizing hormone), PCOS (polycystic ovary syndrome), SHBG (sex hormone-binding globulin), TNF-α (tumor necrosis alpha).
5. Management
The management approach and selection of the best therapy option depend on the target patient and her priorities [ 4 ]. The complications may vary from seeking fertility, regulation of menstrual disturbances to weight reduction or relief from hyperandrogenic symptoms, including acne, hirsutism, or androgenic alopecia [ 84 ]. Indeed, the approach should be individualized for each person to meet the optimal result [ 8 ]. There is no one ideal treatment for all women diagnosed with PCOS, which leaves physicians no choice but symptomatic therapy [ 85 ].
5.1. Lifestyle Modification and Non-Pharmacological Approaches
5.1.1. weight loss.
Elevated androgenic hormone levels lead to weight gain in women with PCOS, mainly in the abdominal area. As a result, many PCOS women have an apple shape body instead of a pear shape [ 37 ]. The first step for women diagnosed with PCOS would be weight reduction and calorie intake restriction [ 86 ]. Many studies demonstrate that even a 5% to 10% reduction in weight can restore the regular menstruation cycle [ 87 ]. For obese women, it would be best if they could reach their normal range of body mass index (BMI). Along with weight loss, the level of free testosterone decreases, and the incidence of metabolic syndrome reduces [ 84 ].
5.1.2. Diet
As mentioned above, to achieve specific goals for each woman, the best diet or nutrient regimen would be the tailored one [ 87 ]. However, some suggestions may help choose what to eat more or less. An ideal diet would be rich in fibers and low in saturated fats and carbohydrates. There is a carbohydrate classification considering the blood glucose response they cause within 2 h: low and high glycemic index carbohydrates. Low glycemic index carbohydrates are at the top of our agenda; they include foods and vegetables like broccoli, raw carrot, lentils, soy, bran breakfast cereals, whole-grain bread, etc. Patients should also be aware that foods with a high glycemic index for prevention, white rice, cakes and cookies, fries or chips, and some fruits such as pineapple or watermelon are actual examples [ 37 ].
5.1.3. Exercise
Exercise and physical activity play a key role in weight reduction. They may be beneficial to improve insulin sensitivity [ 88 ]. Different studies suggest various times for exercise during the week, but the American Heart Association (AHA) recommends approximately 150 min of moderate or 75 min of vigorous and intense exercise per week [ 84 ]. Several studies show that exercise, with or without being on a diet, can resume ovulation in women with PCOS. Exercise probably can affect ovulation through modulation of the hypothalamic-pituitary-gonadal (HPG) axis. In overweight and obese women, exercise leads to lower insulin and free androgen levels, inducing the restoration of HPA regulation of ovulation [ 88 ].
5.2. Complementary and Alternative Medicine (CAM)
Current management and accessible medications are only moderately effective in PCOS, and there are still some cases left untreated despite non-pharmacological and pharmacological treatments. Some literature claims that pharmacologically based therapies are only effective in 60% of patients [ 64 ]. Recent studies have demonstrated that using complementary and alternative medicine (CAM) as adjunctive therapy may benefit the management [ 89 ]. Today, CAM is a well-known approach that has been used at least at one point in more than 70% of PCOS patients during their diseases [ 90 ]. Several manners can categorize it; according to the latest edition of the National Center for Complementary and Integrative Health (NCCIH), complementary approaches can be classified by their primary therapeutic input into three classes of nutritional, psychological, physical, or all of them in combination [ 68 ]. One of the significant merits of CAM is that people often tend to accept these methods due to their beliefs and cultures; this leads to their improved adherence or tolerance to the therapy. Taking a look at prior studies, various methods of CAM including traditional Chinese medicine (TCM), immunotherapy, diet therapy (herbal and medicinal foods, probiotics, and vitamin or supplementation therapy), psychotherapy, spa, yoga, Tai Chi, and oxygen therapy have been considered as effective strategies in reducing the severity of PCOS and its complications [ 89 , 91 , 92 , 93 , 94 , 95 ]. Two critical subgroups of CAM effective in PCOS management are discussed in the following sections.
5.2.1. Acupuncture
Acupuncture, a fundamental part of CAM, has been used in China for more than 3000 years [ 89 ]. It is a kind of sensory stimulation in which thin needles are placed into the skin and muscles. Acupuncture improves clinical manifestations of PCOS by activating somatic afferent nerves of the skin and muscles, modulating somatic and autonomic nervous system activity and endocrine/metabolic functions [ 91 ]. Within acupuncture, β-endorphin production increases, affecting the secretion of gonadotropin-releasing hormone, ovulation, and menstrual cycle. This means that acupuncture may induce ovulation and restore the menstrual cycle [ 64 ].
5.2.2. Supplementations
Apart from medications with USFDA approval, plenty of supplementation products has been shown to be effective in some women with PCOS. These products include vitamin D supplements, resveratrol, α-lipoic acid, omega-3, berberine, folic acid, myoinositol (MI), and d-chiro-inositol (DCI).
Vitamin D is effective in several studies, especially in cold seasons of the year. The deficiency of this vitamin is thought to be important in the pathogenesis of PCOS, so just the compensatory amount would be suggested [ 96 ].
Resveratrol is among the most recommended supplements for the treatment of PCOS. It is assumed to possess chemopreventive, anti-inflammatory and antioxidant, cardioprotective, and neuroprotective effects [ 97 ]. Resveratrol may play a beneficial role in PCOS by inhibiting HMG-CoA reductase expression and activity, just like statins [ 98 ]. Clinical use of this product has been shown to reduce IR and the risk of type 2 diabetes development [ 99 ].
Alpha-lipoic acid and omega-3 are the two supplements found to improve women’s lipid profile and insulin sensitivity through their anti-inflammatory and antioxidant properties [ 4 ].
Berberine is a nutraceutical compound with possible, desirable effects against IR and obesity, particularly against visceral adipose tissue (VAT) [ 100 ]. Folic acid is usually an agent given to PCOS women seeking fertility [ 24 ].
Last but not least, MI and DCI are other essential and well-studied supplements for PCOS treatment. MI has been demonstrated to improve the activity of insulin receptors and can potentially restore the ovulatory function in most women with PCOS [ 85 ]. Inositol influences intracellular metabolic processes; it activates key enzymes controlling glucose’s oxidative and nonoxidative metabolism. Studies conducted on PCOS women taken MI alone, DCI alone, and these combinations of the two showed that they cause increased frequency of ovulation, decreased need for FSH therapy for triggering the ovulation, and a significant improvement in the pregnancy rate [ 97 ].
5.3. Pharmacological Treatments
Before heading to pharmacological approaches, healthy lifestyle advice must be given to all women diagnosed with PCOS regardless of their weight, complaint, or anything else. This is because, in most cases, and especially in mild to moderate forms, women can solely benefit from diet and exercise [ 101 ]. However, the treatment would rely mainly on the patient’s choices and condition in others. If the patient does not want to get pregnant and complains mostly about her menstruation irregularity, combined oral contraceptives (COCs) or progestins are the drugs of choice. The physician can choose the best oral contraceptive with a look on other symptoms rather than menstruation irregularity; for example, Yasmin ® , Yaz ® , or some other agents can show antiandrogenic effects and can, on the other hand, result in the reduction of androgen production. As a result, they might be helpful in those with hirsutism and/or acne complications.
Metformin, from the biguanides category, is usually prescribed along with the first-choice drugs (COCs) to restore the ovulation cycle in PCOS women because of its insulin sensitivity-increasing properties. Metformin has an antihyperandrogenic effect in the short term too.
In other patients who just want relief from dermatological manifestations due to hyperandrogenism, agents such as aldosterone receptor antagonists (e.g., spironolactone) and 5-alpha reductases (e.g., finasteride) would be more beneficial. Therapy options change for those with infertility who should take agents for ovulation induction like clomiphene citrate and/or aromatase inhibitors [ 84 ].
Of course, there are lots of limitations and precautions, and not everyone can benefit from the agents mentioned above owing to their adverse effects or contraindications. Many COC agents cause nausea and vomiting as they try to stimulate the pregnancy situation for the body. In addition, depression, headaches, and migraine are commonly seen in those taking them. Metformin also causes nausea and vomiting in the first days of consumption which may not be tolerated in all patients and leads to abandonment of the therapy. Spironolactone, a widely used and prescribed agent for androgen-related complications, can cause hyperkalemia. Therefore, it is suggested to look up the adverse reactions or contraindications in reliable drug literature or ask the patient’s history of any possible reaction before the prescription.
The complete list of the routine medications used to treat PCOS and the step-by-step treatment pathway considering the patient’s complaints are provided here in Table 1 .
Commonly prescribed medications in PCOS.
Abbreviations: COC: Combined oral contraceptives; DHT: Dihydrotestosterone; ER: Estrogen receptor; FSH: Follicle-stimulating hormone; GnRH: Gonadotropin-releasing hormone; HD: High dose; IM: Intramuscular; LD: Low dose; LH: Luteinizing hormone; PCOS: Polycystic ovary syndrome; PMS: Premenstrual syndrome; Ref: Reference; XR: Extended-release.
5.4. Drug Repurposing in PCOS
Drug repurposing, or in other terms drug repositioning or drug re-tasking, actually means finding new indications in other diseases or conditions for a medication that has previously been in the market and has USFDA approval for a specific therapeutic goal [ 9 ]. Using this method has shortened the duration of the research and development process, given the thought that the medicines have passed pre-clinical and clinical, safety, and immunological tests. As mentioned before in this review, PCOS still does not have a single ideal pharmacological treatment, and doctors typically tend to cure patients’ symptoms with other agents. Taking a look at other drugs—mostly diabetes agents—may be helpful to recognize some new medications for women with PCOS-related complications.
Table 2 and Table 3 , respectively, present general information and clinical trials of drugs primarily approved for other indications (e.g., diabetes type II, hyperlipidemia, weight reduction, etc.), which are now being examined to see their potential effect in PCOS.
Repurposed medications for the treatment of PCOS.
Abbreviations: CYP3A4: Cytochrome P450 3A4; DPP-4: Dipeptidyl peptidase 4; GI: Gastrointestinal; GLP-1: Glucose-like peptide 1; HIV: Human immunodeficiency virus; HMG-CoA: β-Hydroxy-β-methylglutaryl-CoA; MEN-2: Multiple endocrine neoplasia syndrome type 2; MTC: Medullary thyroid carcinoma; NYHA: New York Heart Association; Ref: Reference; SGLT-2: Sodium-glucose cotransporter-2; USFDA: United States Food and Drug Administration.
Clinical trials of the repurposed medications for PCOS since 2016.
Abbreviations: a.m.: before noon; AC: active comparator; AEs: adverse effects; AMH: anti-mullerian hormone; BID: twice a day; BMI: body mass index; CA: crossover assignment; DB: double-blind; Exp: experimental; FSH: follicle stimulation hormone; g: gram; IM: intramuscular; IU: international unit; LH: luteinizing hormone; mcg: microgram; mg: milligram; NA: not available; OL: open-label; P: placebo; PA: parallel assignment; PC: placebo-controlled; PCOS: polycystic ovary syndrome; PO: per oral (by mouth); QB: quadruple blind; QD: once a day; R: randomized; Ref: reference; SB: single-blind; SC: subcutaneous; SGA: single group assignment; TB: triple blind; TID: three times a day; Tx: treatment; UTI: urinary tract infection.
6. Conclusions
Although the pathogenesis of PCOS is not fully understood, it is believed that different factors from epigenetic alterations to obesity, inflammation, and inactivity may aggravate this syndrome. Since there is still no certain medication or definite cure for this condition, the routine approach after advising on some lifestyle modification and supplementary tips is symptomatic therapy with plenty of agents, including contraceptives, oral antidiabetics, or antiandrogens. In terms of the repurposing, there is a good chance that other approved agents could exert beneficial effects on PCOS. Since the complete profiles of these agents are available, and their efficacy and safety have already been comprehensively studied, the pathway for finding novel treatments becomes a little more straightforward. However, there is still very much to discover and examine for a better understanding of the pathogenesis, and, as a result, targeting the mechanism by proper medication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas—that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
According to current research, PCOS increases the risk of endometrial cancer in women of all ages, although it has no effect on the risk of ovarian or breast cancer. ... Advanced diagnosis of polycystic ovary syndrome-new prediction models with standard parameters. Strowitzki T. Fertil Steril. 2021; 115:92-93. [Google Scholar] 2.
Introduction. Polycystic ovary syndrome (PCOS) is a complex endocrine disorder that is a leading cause of infertility in reproductive aged women [1, 2], and with an increasing incidence globally [3].The most widely accepted guidelines for a clinical diagnosis of PCOS requires the presence of a minimum of two out of three principal features: physical or biochemical signs of androgen excess ...
Polycystic ovary syndrome (PCOS) is a widely prevalent metabolic and endocrine disorder diagnosed in reproductive-aged females. ... and vitamin D deficiency are emerging risk factors of PCOS, paving the path for further research. 77. ... With new discoveries flow in tougher challenges, and it all comes down to selecting an effective solution ...
INTRODUCTION. Polycystic ovary syndrome (PCOS) is a complex condition defined by metabolic, fertility, and psychological consequences, with a prevalence of up to 20% among females of reproductive age[].It is diagnosed according to Rotterdam criteria (2 of the following): Oligo- or anovulation, clinical and/or biochemical hyperandrogenemia, or polycystic ovarian morphology on ultrasound[].
The 2023 International Guideline for the Assessment and Management of PCOS provides clinicians and patients with clear advice on best practice, based on the best available evidence, expert multidisciplinary input, and consumer preferences. Research recommendations have been generated, and a comprehe …
Polycystic ovary syndrome (PCOS) is a major public health problem affecting ~11-13% of women worldwide. In this Primer, Stener-Victorin et al. review the epidemiology and pathophysiology of PCOS ...
1. Introduction. Polycystic Ovarian Syndrome (PCOS) affects millions of women globally, and, depending on the diagnostic criteria used, PCOS has an estimated prevalence of between 7% to 17% of reproductive-aged women causing a range of symptoms such as irregular menstrual cycles, excessive hair growth, weight gain, and infertility [Citation 1].PCOS is characterized by a complex interplay of ...
Latest Research and Reviews. ... Research Highlights 27 Jul 2021 Nature Reviews Endocrinology. Volume: 17, P: 577. ... Polycystic ovary syndrome (PCOS) is a prevalent endocrine disorder ...
Polycystic ovary syndrome (PCOS) is the most common endocrinologic condition in women, affecting from 8% to 13% of reproductive-aged women (1, 2).It is an enigmatic condition that, while extremely common, creates challenges in its diagnosis and management, as leading symptoms may vary with age, and treatment may be tailored to specific requirements of individual need.
Polycystic ovary syndrome (PCOS) is a common disorder of unclear aetiology. ... (Box 2) and women with PCOS. Here, we summarize the latest research on the epigenetic alterations that accompany ...
Polycystic ovary syndrome (PCOS) is one of the most common reproductive endocrine disorders in women and despite this, diagnostic challenges, delayed diagnosis, and less-than-optimal treatment regimens plague the condition. ... University of Oulu PEDEGO Research Unit, Medical Research Centre, Oulu University Hospital, Oulu, Finland. PMID: 33211867
Following on from the 2018 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome (6, 7), independently evaluated as high quality, this extensive update integrates current literature with previous systematic reviews and extends to new clinical questions prioritized by consumers. Ultimately, we aim ...
Researchers are finally piecing together the causes of PCOS and it is being taken seriously as a condition that doesn't just affect the ovaries, but also has cardiovascular, metabolic and ...
PCOS remains inconsistent, with ongoing key practice evidence gaps. Following on from the 2018 International Evidence-based Guideline for the Assessment and Manage-ment of Polycystic Ovary Syndrome,6,7 independently evalu-ated as high quality, this extensive update integrates current literature with previous systematic reviews and extends to
Advances and challenges in PCOS understanding. The Rotterdam Consensus, held jointly by the European and the North American associations of reproductive medicine in 2003, defined the diagnostic criteria of PCOS which remain the most used worldwide for both individual diagnosis and research 7.It defined PCOS as the presence of any two of three features: hyperandrogenism (clinical or biochemical ...
Recent studies have revealed the correlation between serum vitamin D (VD) level and polycystic ovary syndrome (PCOS), but the causality and specific mechanisms remain uncertain. We aimed to investigate the cause-effect relationship between serum VD and PCOS, and the role of testosterone in the related pathological mechanisms. We assessed the causality between serum VD and PCOS by using genome ...
Study indicates PCOS may not be primarily a female reproductive disorder. New genetic research suggests men can develop characteristics of polycystic ovary syndrome (PCOS)—a common metabolic and reproductive disorder that affects women. The study was presented virtually at ENDO 2021, the Endocrine Society's annual meeting.
Assessing C reactive protein/albumin ratio as a new biomarker for polycystic ovary syndrome: a case-control study of women from Bahraini medical clinics. BMJ open , 8 (10), e021860. https://doi ...
CNN —. Polycystic ovary syndrome, known as PCOS, has long been known for symptoms such as missed periods or excess body hair. Now, new research has revealed another potential effect: cognitive ...
1 Monash Centre for Health Research and Implementation, Clinical and Molecular Medicine, Monash University, Melbourne, ... Objective: Characteristic features of polycystic ovary syndrome (PCOS) include insulin resistance and an increased risk for type 2 diabetes. To promote improved insulin sensitivity, insulin sensitisers have been used in PCOS.
A new paper explores genetic risk factors for polycystic ovary syndrome (PCOS) and non-reproductive PCOS phenotypes in women and men. This work affirms that PCOS is indeed a metabolic disorder ...
San Francisco, CA 94158. Phone: 415-885-3674. Fax: 415-353-7744. Email: [email protected]. Follow us on Instagram. The UCSF PCOS Multidisciplinary Clinic and Research Center leads the field in research, training, and patient care. Learn more about our PCOS research.
Polycystic ovary syndrome (PCOS) is a common hormonal condition that affects women of reproductive age. It usually starts during adolescence, but symptoms may fluctuate over time. PCOS can cause hormonal imbalances, irregular periods, excess androgen levels and cysts in the ovaries. Irregular periods, usually with a lack of ovulation, can make ...
PCOS is a condition where cysts (small sacs of fluid) develop on the ovaries. PCOS can cause irregular menstruation (periods), and is a common cause of infertility, affecting as many as 5 million people. In addition to infertility, it is a lifelong condition that can have other impacts. People with PCOS often have insulin resistance.
PCOS Explained on Apple Podcasts. 32 episodes. It can be ridiculously confusing living with PCOS, you're constantly reading conflicting advice. Each week, I, Clare Goodwin, will be helping to simplify this for you by sharing with you some actionable steps to take and breaking down the latest research so you can actually understand it.
Introduction. Polycystic Ovary Syndrome (PCOS) is one of the most common heterogeneous endocrine disorders in women of reproductive age, involving endocrine, reproductive, and metabolic systems. The underlying etiology of this disease is highly complicated with the key clinical manifestations of hyperandrogenemia (clinical and/or biochemical ...
The global market for Polycystic Ovary Syndrome (PCOS) Treatment estimated at US$2.6 Billion in the year 2023, is projected to reach a revised size of US$3.8 Billion by 2030, growing at a CAGR of ...
The global market for Polycystic Ovary Syndrome (PCOS) Treatment estimated at US$2.6 Billion in the year 2023, is projected to reach a revised size of US$3.8 Billion by 2030, growing at a CAGR of ...
Inside Mounjaro factory trying to meet 300% demand increase of drug used for weight loss. The few studies available about babies whose mothers took GLP-1s early in pregnancy haven't turned up ...
1. Introduction. Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder that impacts many women of the reproductive age worldwide [].This syndrome is often associated with enlarged and dysfunctional ovaries, excess androgen levels, resistance to insulin, etc. [].It is estimated that approximately every 1 in 10 women face PCOS before menopause and struggle with its complications [].