Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 May 2024

Kidney stone growth through the lens of Raman mapping
- John W. Robinson 1 ,
- William W. Roberts 2 , 3 &
- Adam J. Matzger 1 , 4
Scientific Reports volume 14 , Article number: 10834 ( 2024 ) Cite this article
514 Accesses
Metrics details
- Biomineralization
- Imaging studies
- Renal calculi
Bulk composition of kidney stones, often analyzed with infrared spectroscopy, plays an essential role in determining the course of treatment for kidney stone disease. Though bulk analysis of kidney stones can hint at the general causes of stone formation, it is necessary to understand kidney stone microstructure to further advance potential treatments that rely on in vivo dissolution of stones rather than surgery. The utility of Raman microscopy is demonstrated for the purpose of studying kidney stone microstructure with chemical maps at ≤ 1 µm scales collected for calcium oxalate, calcium phosphate, uric acid, and struvite stones. Observed microstructures are discussed with respect to kidney stone growth and dissolution with emphasis placed on < 5 µm features that would be difficult to identify using alternative techniques including micro computed tomography. These features include thin concentric rings of calcium oxalate monohydrate within uric acid stones and increased frequency of calcium oxalate crystals within regions of elongated crystal growth in a brushite stone. We relate these observations to potential concerns of clinical significance including dissolution of uric acid by raising urine pH and the higher rates of brushite stone recurrence compared to other non-infectious kidney stones.
Similar content being viewed by others
GeoBioMed perspectives on kidney stone recurrence from the reactive surface area of SWL-derived particles

Multicolor imaging of calcium-binding proteins in human kidney stones for elucidating the effects of proteins on crystal growth

Human kidney stones: a natural record of universal biomineralization
Introduction.
Chemical mapping using Raman microscopy has long been used to better understand the growth and function of biomineral systems including bones 1 , 2 , teeth 3 , 4 , and, more recently, kidney stones (urinary calculi) 5 , 6 , 7 , 8 . Compositional and/or structural maps of urinary calculi have been produced using micro computed tomography (µCT) 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , fluorescence microscopies 6 , 7 , 8 , electron microscopies 6 , 7 , 16 , 17 , optical microscopies 6 , 7 , 8 , 18 , multi-photon spectroscopy 19 , infrared microscopy 20 , 21 , 22 , and Raman microscopy 5 , 6 , 7 , 8 . Micro computed tomography allows complete volume imaging of intact stones but has limited voxel resolution of 2–5 µm depending on stone size 14 . Fluorescence microscopies allow imaging on similar length scales compared to Raman microscopy 6 , 7 , 8 ; however, autofluorescence in kidney stones is primarily sensitive to organic components whereas Raman spectroscopy provides direct information about mineral composition 6 , 7 , 8 . Electron microscopy has much higher resolution (~ 1 nm) than other techniques but requires significant sample preparation and high vacuum that can dehydrate certain minerals over time. Optical microscopies, including circular and crossed polarized light 6 , 7 , 8 , 18 , require stone destruction in the form of thin sectioning. Multiphoton spectroscopy provides mineral phase identification but requires specialized laser systems 19 . Reflectance-mode infrared microscopy offers similar vibrational information as Raman spectroscopy but spatial resolution is limited to > 5 µm by the diffraction limit of infrared light 22 or the size of attenuated total reflectance probes 23 . In contrast, commercially available Raman microscopes can map with spatial resolution < 1 µm while providing direct identification of minerals through their vibrational signatures.
Vibrational spectroscopy has a long history of use for analysis of urinary stones 24 , 25 , 26 and infrared spectroscopy of ground stone samples is commonly used to identify bulk components for clinical purposes 25 . In contrast, use of Raman spectroscopy for compositional analysis of urinary stones has largely been confined to use in research settings 24 , 27 , 28 , 29 . Early work by Daudon identified the potential utility of Raman microprobe analysis for identification of small features involved in nucleation of urinary stones 24 . A recent review by Lucas, Bazin, and Daudon noted opportunities for using Raman spectroscopy to study various pathological biomineralizations and highlighted the mapping of kidney stones at 5 µm spatial resolution 5 , 21 , 28 . As we demonstrate in this work, achieving 0.5–1 µm scale Raman mapping allows insight into kidney stone growth that would be missed by µCT.
Raman mapping of biological samples is often difficult due to autofluorescence caused by biomolecules and heating damage caused by high-powered lasers. Biomolecule autofluorescence may be avoided by use of longer wavelengths for excitation but with a concomitant reduction in Raman intensity proportional to λ -4 and a degradation of achievable spatial resolution. With Raman scattering, longer wavelength lasers often necessitate longer exposure times and/or higher laser power to achieve the same signal/noise as shorter wavelength lasers. In kidney stones, this increased exposure duration increases the risk of sample damage by mineral decomposition or carbonization of intercrystalline biomolecules.
A common technique to reduce autofluorescence is to rely on photobleaching induced by the lasers used for Raman scattering with autofluorescence reduced by a factor of 80% in bone samples using a 532 nm laser 30 . Unlike the apatite found in bone, certain minerals found in kidney stones, like brushite (CaHPO 4 ·2H 2 O) and struvite (MgNH 4 PO 4 ·6H 2 O), will dehydrate under intense radiation and cannot be laser photobleached without risking sample damage. As an alternative to photobleaching, a hydrogen peroxide + light treatment has recently been shown to reduce autofluorescence in biological samples 31 . This approach has been adapted here to improve signal to noise in Raman spectra for kidney stones allowing mapping of majority brushite and struvite stones with a 532 nm laser. The improved sensitivity has allowed us to observe calcium oxalate and apatite crystals with ~ 1 µm dimensions within brushite stones and struvite stones for the first time.
Here we report results of Raman mapping for six samples taken from five different kidney stones. The six most common minerals found in kidney stones are represented: calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), hydroxyapatite (HAp), uric acid (UA), brushite, and struvite. Each sample is mapped at 1 µm or 0.5 µm spatial resolution, an order of magnitude improvement over previous studies using Raman mapping 5 . This enables observation of micrometer-sized features that appear related to changes in kidney stone growth. The crystal orientation of COM is correlated to changes in Raman peak intensity allowing basic orientational mapping of COM when using polarized lasers for Raman excitation. The origin of minor constituents is discussed in relation to crystallization conditions including < 10 µm wide rings found in urinary calculi that are otherwise formed of steady growth of a single mineral. We present an example of depth profiling in a struvite stone and discuss its potential application in the study of laser lithotripsy.
Results and discussion
Raman spectra for samples of COM, COD, HAp, UA, brushite, and struvite powders are shown in Fig. 1 . These were used subsequently for least-squares fitting of mapping data. Prominent peaks useful for mineral identification are listed in Table 1 along with vibrational mode assignments, if available. Separate spectra for each mineral are included in the Supporting Information (Figs. S1 – S7 ). Brushite and struvite both weakly fluoresce when irradiated with 532 nm light which results in the rising background seen in Fig. 1 .
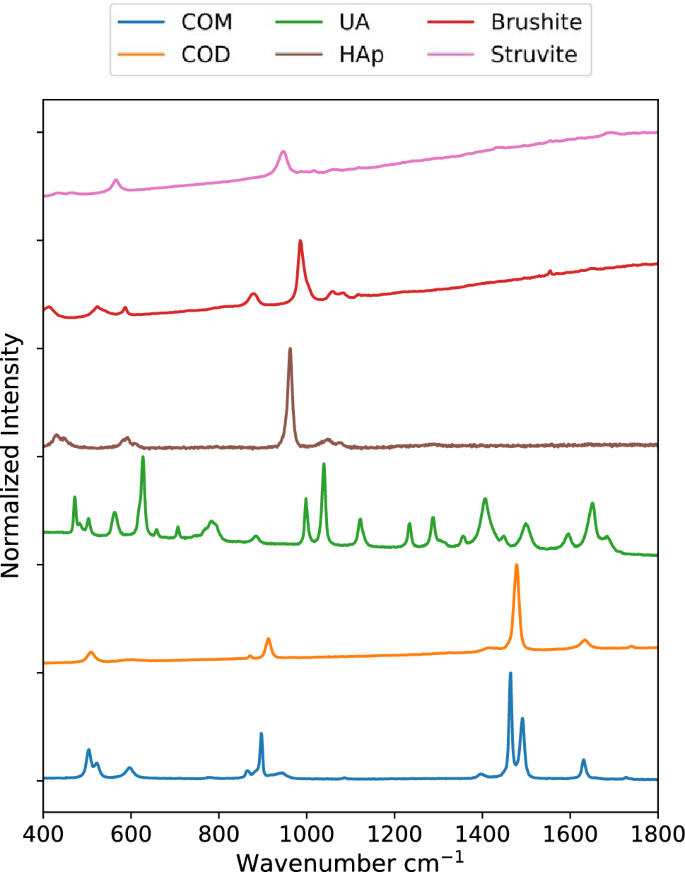
Raman spectra for common kidney stone minerals. Raman spectra were collected using powders of calcium oxalate monohydrate, calcium oxalate dihydrate, uric acid, hydroxyapatite, brushite, and struvite minerals from 400 to 1800 cm −1 . The uric acid spectrum was collected using a 785 nm laser. All others were collected using a 532 nm laser.
Calcium oxalate monohydrate stone
The two most prominent peaks in COM (Fig. 1 ) at 1464 and 1491 cm −1 originate from the stretching of carboxylate groups in the oxalate ion of COM 32 . The relative intensity of each depends on the orientation of COM with respect to laser polarization. In certain orientations, only one of the two carboxylate stretches may be obvious in the collected Raman spectrum (Figure S8). COM spectra with either the 1464 or 1491 cm −1 dominating appear qualitatively similar to the Raman spectrum of COD with its single carboxylate peak at 1476 cm -1 . This similarity is close enough that least squares methods can erroneously identify COD as a major presence when powdered COM and COD are used for fitting (Figure S9 ). Similar misidentification seems to appear in the literature in Supplementary Fig. 6 of Sivaguru 6 in which we would identify all three presented spectra as COM based on the positions of oxalate stretching peaks from 1460 to 1495 cm −1 and oxalate bending peaks at 890–920 cm −1 6 . For this reason, we recommend COM be identified in maps using at least two different spectra: one with the 1464 cm −1 peak dominant and a second with the 1491 cm −1 peak dominant. At ≈2 cm −1 wavenumber resolution, this is sufficient to consistently distinguish COM and COD and allows contrast of regions with differing COM orientation as shown in Fig. 2 .
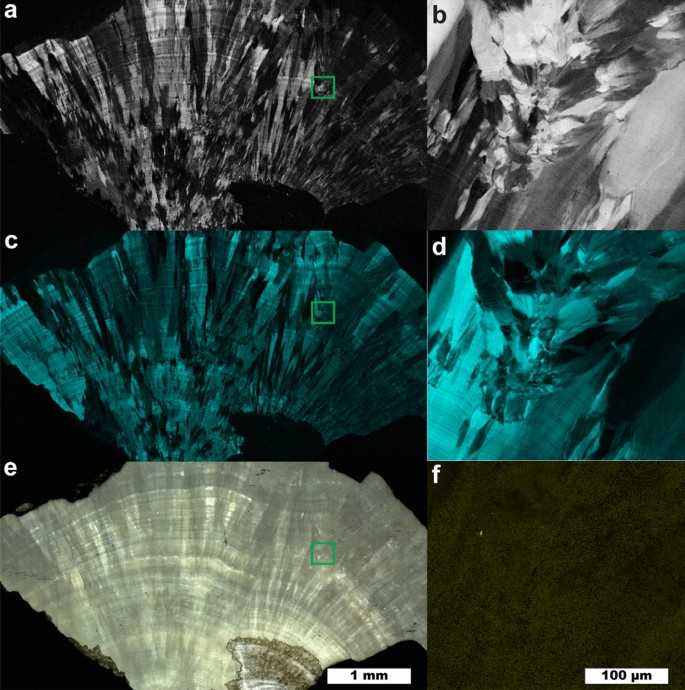
Raman map of a COM stone with orientation differences. ( a ) 5 µm spatial resolution map of COM with 1464 cm −1 carboxylate stretch dominant. ( b ) 1 µm spatial resolution map of inset in ( a ). ( c ) 5 µm spatial resolution map of COM with 1491 cm −1 carboxylate stretch dominant. ( d ) 1 µm spatial resolution map of inset in ( c ). ( e ) White light image. ( f ) 1 µm spatial resolution map of COD, inset from ( e ).
Figure 2 shows a large fragment from a COM stone that exhibits layered radial growth in the manner of type 1a stones according the classification of Daudon 11 , 17 . Using the diagenetic classifications outlined in Sivaguru et al. this fragment primarily exhibits “cortex COM” (COM c ): COM with microscale and nanoscale layering due to embedding of biomolecules during growth 6 , 7 . COM c typically forms during later stone growth and coats other minerals (COM, COD, or apatite) that form the nidus of growth. COM c layering is exhibited over the entire region of the fragment in Fig. 2 indicating this fragment lacks the original center of the stone.
At several points there are changes in the radius of curvature in the growth layers indicating disruption in evenly layered growth. Figures 2 b, c, d and f focus on one such region revealing a lack of ≤ 1 µm layering that is characteristic of COM c and the presence of a ~ 3 µm COD crystal (Fig. 2 f). This indicates COD nucleated or agglomerated near this site, potentially alongside COM. The lack of clear crystal facets prevents identification of the original phases present. COM c layers show continued growth around the disruptive COM/COD agglomerations, eventually coating them entirely.
Uric acid stone
Figure 3 shows a map of a predominantly uric acid stone at 10 µm and 1 µm spatial resolutions showing a cross section of an entire UA stone and the fine structure near a fracture, respectively. The interior of the uric acid stone shows larger UA crystals with numerous cracks or voids present. A lack of sharp uric acid crystal facets and the prominent voids near the center of the stone indicates the kidney stone nidus may have been composed of a less-dense mineral than uric acid. Dissolution of kinetically favored uric acid dihydrate followed by reprecipitation as uric acid is consistent with the observed porosity at the center of the stone. Uric acid dihydrate begins to convert to anhydrous uric acid in < 24 h both in air and in water 36 , 37 , 38 . As these samples have been stored in air for > 4 years, uric acid dihydrate is expected to have decomposed leaving poorly attached anhydrous uric acid that would have been removed during sample preparation.
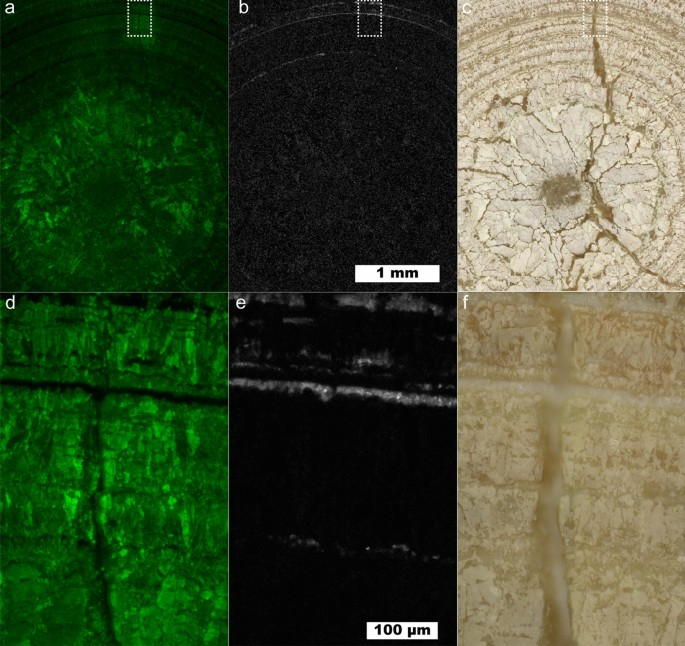
Uric acid kidney stone containing layers of COM. ( a ) Map showing intensity of uric acid at 10 µm spatial resolution. ( b ) Map of COM at 10 µm spatial resolution using the 1464 cm −1 peak. ( c ) White light image for ( a ) and ( b ). ( d ) 1 µm spatial resolution map of inset in ( a ). ( e ) 1 µm spatial resolution map of inset in ( b ). ( f ) White light image for ( d ) and ( e ).
A layered uric acid structure predominates in the outer regions of the stone. The radially concentric growth around the entire circumference of the stone indicates consistent exposure to urine. In the layered zone several ≤ 10 µm thick layers of COM are present. These COM layers maintain the concentric growth pattern of the uric acid suggesting COM is also deposited during consistent exposure to urine much like the COM c observed in type 1a stones 6 , 7 , 11 , 17 . COM may have nucleated directly on the UA stone surface in an example of crystal pseudoseeding as there are known epitaxial relationships between UA and other kidney stone minerals 39 .
While > 5 µm layers of COM within uric acid can be observed by µCT, the presence of thinner, broken layers like the innermost ring of COM seen in Fig. 3 e may be difficult to detect. Identifying the presence of 1 µm thick layers is important in the understanding of uric acid stone growth as even thin layers of COM not observable by µCT could act as protective barriers against uric acid dissolution and excretion. Urine pH plays a pivotal role in uric acid solubility with an exponential increase at approximately pH 5.5 40 . In contrast, COM solubility is low and relatively stable in saline solution at physiological pH 41 , 42 . Thus, even if urine pH is not consistently low, uric acid stones could persist in vivo due to the protective barrier provided by COM across a wide range of physiological pH.
The ability of thin COM layers to prevent uric acid dissolution will depend on a number of additional factors including their mechanical stability, completeness of coverage, and dissolution/mixing efficiency at coverage gaps. There are reports of partially successful and failed applications of dissolution therapy to mixed-phase uric acid stones containing calcium minerals 43 , 44 . In-vitro dissolution experiments with carefully selected human stone samples or appropriate artificial models will be necessary to understand the effect, if any, of thin COM layers on uric acid dissolution.
Brushite stone
The brushite stone pictured in Fig. 4 shows two distinct brushite stone morphologies: a “classic” brushite layer 15 of elongated radial crystals between two regions of more compact brushite that resembles type 1a stones 12 . The even, layered growth in the compact brushite regions indicate continuous exposure to urine much like the layers of COM c in type 1a stones. In the compact regions, there is one concentric layer of COD and one concentric layer of HAp. Otherwise, the compact regions are formed almost entirely from brushite. HAp is typically associated with higher pH solutions than brushite with HAp having a lower solubility above pH 4.1 46 , 47 Despite its thermodynamic stability, other forms of calcium phosphate are typically favored kinetically, including brushite 48 . This suggests the HAp layer observed resulted from a period in which the outer brushite layer was exposed to solution allowing dissolution and reprecipitation as HAp.
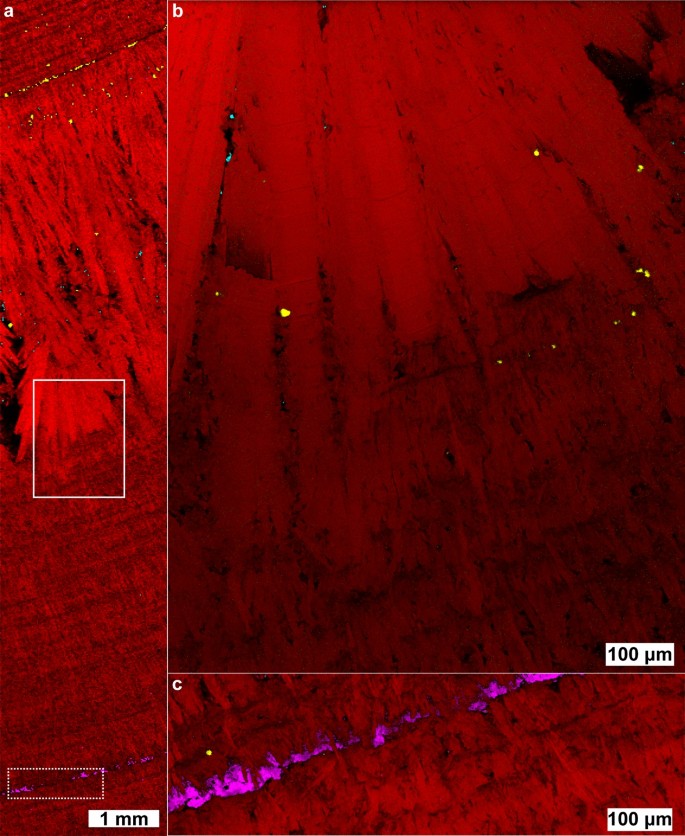
Raman chemical image of a predominantly brushite kidney stone. The stone contains brushite (red), COM (cyan), COD (yellow), and apatite (magenta). ( a ) 10 µm spatial resolution map along the length of a brushite stone. ( b ) 1 µm spatial resolution map of solid inset. ( c ) 1 µm spatial resolution map of hydroxyapatite band (dashed inset). White light images are shown in Figure S10 .
The COD band in Fig. 4 a occurs at the start of non-compact crystallization of brushite. Unlike more compact regions, scattered COM and COD crystals are apparent in the layer of radially elongated brushite crystals. The apparent co-precipitation of COM, COD, and brushite indicates urine was supersaturated with respect to all three minerals during growth of elongated brushite. As calcium is the common ion among the three minerals, the elongated, radial bundles of brushite may be an indicator of high calcium availability in urine which has been observed in brushite stone forming patients 49 . COD is also more commonly associated with higher calcium concentrations than COM 50 giving additional evidence for a mechanism of increased calcium concentration rather than a simultaneous increase in both oxalate and phosphate at the onset of elongated brushite formation. The higher relative area occupied by COD crystals at the onset of elongated crystal growth indicates a higher ratio of COD/brushite growth rate. The decrease in relative calcium oxalate/brushite area toward the outer regions of elongated growth suggests the supersaturation ratio \(\frac{{S}_{CaOx}}{{S}_{Brushite}}\) decreased over time leading to a lower ratio of CaOx/brushite growth rates. Oxalate is the limiting ion in calcium oxalate precipitation in typical urine with a concentration an order of magnitude lower than calcium 51 . In contrast, phosphate concentration is approximately an order of magnitude higher than calcium in typical urine which likely explains the large, continuous growth of radial brushite bundles with isolated crystals of calcium oxalate forming as additional oxalate is excreted by the kidneys.
It has been noted that faster-growing stones are more likely to reoccur in patients and brushite stones reoccur more often than other non-infectious stones 52 . However, no morphological distinction has been made between radially bundled brushite and compact brushite with both being identified as type 4d 11 , 52 . Given the potential for mechanistic differences between compact brushite and radially bundled brushite, we believe it would be beneficial to subdivide the classification of brushite stones by morphology for future research into recurrence.
Mixed calcium oxalates and hydroxyapatite stone
Figure 5 shows a 1 µm spatial resolution map of a mixed oxalate and apatite stone (the process for building the overlayed map is described in the Supporting Information). Similar samples were studied in detail by Sivaguru et al. 6 , 7 and we refer readers to their work for a more detailed discussion of stone formation mechanisms. This stone sample is notable for its relatively open structure with large, open pores among the crystals that were filled by cyanoacrylate during sample preparation. Several structures of interest are outlined in Fig. 5 : mixed apatite and calcite (A), a COM crystal surrounded by a COD crystal (B), a large 100 µm scale hexagonal platelet of COM (C), and a cut single crystal of COM exhibiting layered growth (D). Calcium carbonate is rare in kidney stones and crystalluria 53 , 54 indicating the calcite we observe is likely the result of laser damage during Raman imaging. Though calcium carbonate may also form during laser lithotripsy, evidence from low-resolution maps strongly suggest damage from the Raman laser (Figs. S11 , S13 ). Calcium carbonate in the preliminary low-resolution map has a spectrum closer to that of amorphous calcium carbonate whereas spatially averaged spectra from Fig. 5 match more closely with calcite (Figure S13 ). Had calcium carbonate been present prior to Raman laser irradiation it likely would have converted to calcite over time or during initial grinding and polishing of the sample. This apparent phase change between maps suggests formation of amorphous calcium carbonate due to heating of calcium oxalate to > 400 °C 55 , 56 followed by conversion to calcite. Thus presence of calcium carbonate as detected by Raman spectroscopy should be treated with caution.
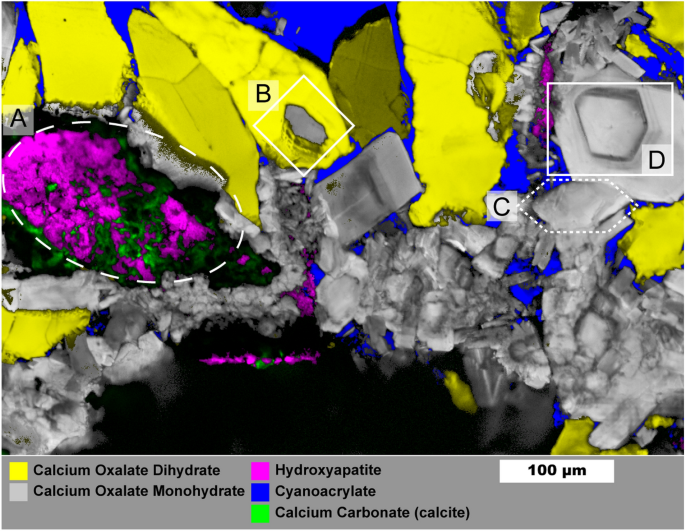
Raman image of mixed calcium oxalate and apatite stone fragment. Mapping was performed with 1 µm spatial resolution. Region ( A ) shows mixed apatite originating from in vivo growth and calcium carbonate that formed from laser damage during prior Raman analysis. Region ( B ) shows COM plates growing from COD dissolution. Region ( C ) shows an example of a large COM hexagonal plate. Region ( D ) Large COM crystal with apparent layering of organic matter. Maps for individual mineral components are shown in Figure S12 .
The COM crystal in (B) is surrounded entirely by COD which has been observed previously 7 . The COD-COM transition typically requires aqueous solution 57 , 58 . This appears to be a diagenetic COD-COM transition at the surface of the COD crystal as the COM morphology. The appearance of layering is similar to in-vitro examples of COM growth on COD that we have observed in attempts at growing calcium oxalate stones (Figure S14 ). Region (C) shows a ≈ 100 µm long crystal with hexagonal plate morphology that can be induced by additives like citrate or by high ionic strength solutions which both inhibit growth perpedicular to the charged (100) planes of COM 59 , 60 .
The crystal in region (D) shows a cut perpendicular to the (010) axis 59 and, despite having the morphology of a COM single crystal, shows layered variation with visually darker layers having greater autofluorescence (Figure S15 ). Such layering suggests crystal (D) crystals did not grow in a constant environment and may have grown through deposition of amorphous calcium oxalate 61 , 62 that converted to COM upon contact with existing crystals leading to a single-crystal-like structure while allowing incorporation of protein.
Mixed oxalates
Figure 6 shows another ~ 1 mm fragment from the same stone as that shown in Fig. 5 . No HAp was observed in this fragment and much of the COM in Fig. 6 has a radial structure with layering. Three small, irregular veins of COD are present and all are surrounded by the cyanoacrylate glue used for embedding. As COM has a lower solubility than COD at room temperature, calcium and oxalate ions from dissolved COD appear to be reprecipitating on the radial COM bundles thereby replacing COM as described by Sivaguru 7 The voids are left due to differences in density as COD (1.9 g/cm 3 ) is less dense than the more stable COM (2.2 g/cm 3 ).
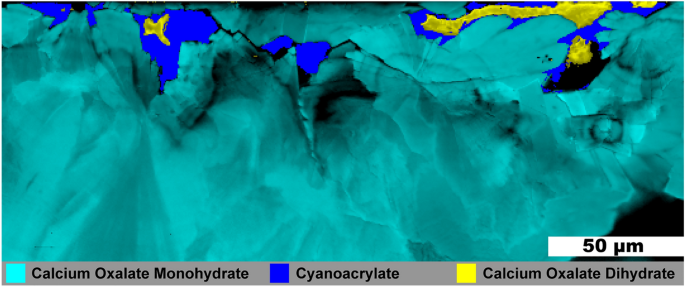
Raman image of a mixed calcium oxalate stone fragment. 0.5 µm spatial resolution map of calcium oxalate dihydrate and calcium oxalate monohydrate from a mixed calcium oxalate + apatite stone. White light image is shown in Figure S16 .
Struvite stone
A stone formed predominantly of struvite is imaged in Fig. 7 . This stone shows a concentric layered structure suggesting even growth. The inset in Fig. 7 a focuses on a region with mixed struvite and apatite. At the low irradiance settings used to avoid sample damage for struvite stones, these two minerals are difficult to distinguish as their most prominent peaks are centered at 945 and 962 cm −1 with full-width at half maximum of 21 and 11 cm −1 for each peak, respectively. Additionally, carbonate substitution in apatite structures shifts the phosphate ν 1 peak to lower wavenumbers 63 increasing the signal/noise required to resolve the two peaks. In lieu of higher irradiance, spatial averaging was used to confirm presence of apatite when signal/noise was too low in individual spectra.

Raman mapping images of a predominantly struvite kidney stone. Small amounts of apatite (magenta) are interspersed among struvite (green). ( a ) 10 µm/pixel survey map of stone. ( b ) White light image of inset in ( a ). ( c ) Raman map of inset in ( a ).
Apatite was observed mainly near or within larger voids in the struvite layers. Unlike the case of calcium oxalates within “classic” brushite stones shown in Fig. 4 , there is no clear difference in struvite morphology between regions containing apatite and those without. This suggests that the environment for struvite crystallization remained relatively constant during stone formation and that apatite either formed in urine and aggregated onto the growing struvite stone 64 or formed by dissolution and conversion of struvite in the presence of calcium ions 65 , 66 . As with the COD-to-COM conversion, the voids could be formed during the dissolution and conversion process as hydroxyapatite (3.2 g/cm 3 ) is more dense than struvite (1.7 g/cm 3 ).
Volume Raman mapping
Figure 8 shows the results of confocal volume imaging the region marked by the white box in Fig. 7 b. Volume imaging confirms the observation that apatite is associated with voids that are exposed at the surface of the struvite stone (Fig. 8 , region a). Volume imaging also revealed the presence of sub-surface apatite in region (b) of Fig. 8 which appears as a relatively brighter band in optical images (Fig. 7 b). Apatite in kidney stones typically takes the form of aggregated spherules 67 with diameters on the order of 10 µm. We expect these small spherules are more effective at backscattering light in optical images than organized crystal growths because refractive index will vary on shorter length scales in the aggregated spherulites increasing opportunities reflection, multiple refraction, or other forms of scattering. Relating optical cues to chemical and structural features is potentially valuable for practical application by urologists as optical differences may help urologists focus on specific mineral types. Further testing with ex-vivo lithotripsy is required to understand specific relationships between optical cues and ablation behavior.
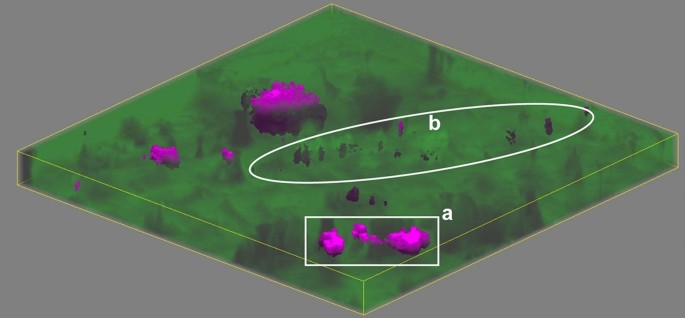
A volume map of a predominantly struvite stone. The area corresponds to the region marked in Fig. 7 b. Raman volume imaging was conducted with a nominal resolution of 1 µm 3 voxels. Struvite and apatite are represented by green and magenta, respectively, with higher saturation indicating a closer fit by least squares. Sides of the image are 250 µm long and depth is ≈ 13.3 µm for 20 µm of stage movement and the refractive index of struvite (≈ 1.5).
Applying Raman mapping to lithotripsy research
Raman mapping and volume imaging have the potential to enable experiments that image kidney stone composition and structure before and after ex-vivo laser ablation of stones. While scattering by individual crystals within a kidney stone may prevent volume imaging to the ≈ 400 µm deep craters 68 caused by 1 J laser pulses, surgical lasers operating in “dusting” mode or with a fiber standoff distance produce shallower features that can be fully imaged. One open question in laser lithotripsy is whether free urine or water within voids contributes significantly to stone ablation. Volume imaging Raman spectroscopy followed by laser ablation of sample stones has the potential to offer insight into this potential mechanism as mineral composition, void structure, and fluorescent molecule presence can be correlated to features of ablated particles and the craters that remain.
We have demonstrated the ability of Raman microscopy to produce 0.5–1 µm spatial resolution chemical maps of kidney stone samples. Hydrogen peroxide combined with light was used to decrease autofluorescence enabling Raman spectroscopy without the need for extensive photobleaching. High-resolution chemical maps were used to identify potential mechanisms of kidney stone growth for the six most common stone minerals: COM, COD, HAp, uric acid, brushite, and struvite. Mapping a type 1a COM stone revealed COD agglomeration likely plays a role in disrupting concentric layer growth. In contrast, thin concentric layers of COM within uric acid stones were observed to form without disrupting the concentric layering of uric acid itself. This suggests nucleation of COM occurred at the surface of the uric acid stone, a potential example nucleation by pseudoseeding. A brushite stone with distinct regions of compact, layered growth or interlocking radial bundles showed greater presence of calcium oxalates among the void-prone, interlocking radial bundles of brushite. This suggested high calcium concentrations contributed to simultaneous precipitation of all three minerals. Observations of mixed COM, COD, and HAp stones show evidence for COD dissolution with subsequent recrystallization as COM. Large regions with COM single crystal morphologies exhibited apparent layering of organic material. Apatite in struvite stones was primarily found near or within void spaces suggesting formation during dissolution of struvite into more stable and denser apatitic forms. Volume mapping with 1 µm 3 voxel resolution was performed to a depth of ≈13.3 µm on a struvite kidney stone for the first time.
With simultaneous structural and compositional analysis, Raman mapping can be used to understand both physical and chemical structure of kidney stones more completely than other techniques. Future directions for Raman imaging include observing chemical changes in both ablated sediment and exposed craters after laser ablation of previously mapped areas. Relationships between laser lithotripsy conditions and efficient stone destruction can then be identified and optimized to decrease time needed for surgery while avoiding thermal damage to surrounding tissues. With volume imaging, Raman microscopy can be used to study the importance of laser ablation mechanisms like the vaporization of urine within pores. The improvement in Raman spatial resolution allowed identification of previously unobserved features including ≈1 μm thick COM layers in uric acid stones and the co-localization of ≤ 5 μm calcium oxalate crystals with brushite crystals of specific, elongated morphology. Application of Raman imaging to a wider variety of stones will lead to new insights into kidney stone growth benefitting both scientists seeking to better understand pathological biomineralization and clinicians seeking to improve treatment of kidney stone disease.
Kidney stone samples were selected from a de-identified library of human stones for Raman mapping. For polishing, smaller fragments (< 5 mm) were embedded in cyanoacrylate glue or CrystalBond 509 in depressions milled at the center of 1 inch outer diameter acrylic holders. Crystalbond 509 heated to 125 °C was preferred for minerals with higher decomposition temperatures (COM, UA, Hap) while cyanoacrylate was preferred for minerals that would decompose on contact with melted CrystalBond 509. 69 , 70 Larger fragments were polished by embedding in CrystalBond 509 (COM, UA, HAp) or CrystalBond 555 (COD, brushite, struvite) to allow for dissolution and removal of the embedding medium after polishing. CrystalBond 555 was melted at a temperature of 70 °C before embedding, below the temperature at which significant decomposition is expected to occur for brushite, struvite, and COD.
Samples were leveled by hand using wetted sandpaper of progressively finer grits ending at grit size J2500 which has an average particle size of 5.5 µm. Sandpaper was placed on float glass to maintain a flat grinding surface. Water was used for wetting sandpaper for samples embedded in cyanoacrylate and CrystalBond 509. Light mineral oil was used for samples embedded in CrystalBond 555.
After grinding, samples were polished consecutively using aqueous alumina suspensions of 1 µm and 0.3 µm nominal diameter on a low-nap cloth (Alpha-A Cloth, Ted Pella) backed by float glass. For struvite and brushite samples embedded in CrystalBond 555, aqueous alumina suspensions were mixed with mineral oil which coated the water-soluble CrystalBond 555 thereby preventing its dissolution during polishing. Mineral oil from grinding and polishing was removed by soaking samples in hexanes or petroleum ether overnight followed by rinsing. Soaking and rinsing was repeated at least three times.
After the first polishing, and prior to Raman mapping, kidney stone samples containing predominantly inorganic minerals were treated with hydrogen peroxide and light to deactivate autofluorescent organic material using a method adapted from Yakubovskaya et al. 31 Samples were covered with 6% H 2 O 2 (aq), typically 1 mL, and placed in a petri dish to prevent evaporation. A high-power rectangular array of white LEDs (GS Vitec LT-V9-15) was placed 6 cm from the sample and irradiation was carried out for 1 h. After irradiation the sample was gently rinsed with deionized water and allowed to air dry. Samples were re-polished after peroxide treatment with 0.3 µm alumina suspension as before. We attempted treatment of uric acid samples in the same manner as the inorganic minerals but observed no reduction in autofluorescence. Uric acid fluoresces strongly at 532 nm though Raman signal can still be observed with sufficient exposure time. As uric acid is an antioxidant known to scavenge hydroxyl radicals in the body, chemical bleaching may not be effective on uric acid stones 71 , 72 .
Raman mapping was performed using Renishaw InVia microscopes. Samples containing predominantly inorganic minerals were mapped using a 532 nm laser. High confocality mode was used for better fluorescence rejection when using the 532 nm laser unless otherwise noted. Uric acid stones were mapped using a 785 nm laser as autofluorescence could not be reduced by the hydrogen peroxide + light treatment. Standard confocality mode was used for uric acid stones. Surface-following contour maps for each sample were generated by manually focusing on a minimum of four points in the flat-polished regions of the sample. For the highest spatial resolution maps of 0.5 µm for COM, COD, or HAp, typical scan parameters were 0.1 s exposure for a scan speed of 5 µm/s and a laser power of ≈50 mW at the sample with a spot diameter of ≈0.5 µm for a 50 × objective. The resulting power density is ≈20 W/µm 2 . For struvite and brushite samples laser power was lowered to ≈25 mW at the sample surface, exposure times were decreased to 0.05 µs, and scan speeds increased to 20 µm/s for a power density of 2.5 W/µm 2 . The lowered irradiance applied to brushite and struvite prevented laser-induced damage on all polished and peroxide-treated samples tested. Higher irradiance may be used successfully though performing initial mapping at lower irradiance to test for damage is recommended.
Data availability
Raw images in the manuscript and supporting information are provided individually in the electronic supporting materials. Tabulated data for Raman spectra plotted in the manuscript and supporting information are provided as CSV files in the electronic supporting materials.
Timlin, J. A., Carden, A. & Morris, M. D. Chemical microstructure of cortical bone probed by Raman Transects. Appl. Spectrosc. 53 (11), 1429–1435 (1999).
Article ADS CAS Google Scholar
Carden, A. & Morris, M. D. Application of vibrational spectroscopy to the study of mineralized tissues (review). J. Biomed. Opt 5 (3), 259–268. https://doi.org/10.1117/1.429994 (2000).
Article ADS CAS PubMed Google Scholar
Gupta, S. D. et al. Mineralization of dental tissues and caries lesions detailed with raman microspectroscopic imaging. Analyst 146 (5), 1705–1713. https://doi.org/10.1039/D0AN01938K (2021).
Article ADS PubMed Google Scholar
Wentrup-Byrne, E., Armstrong, C. A., Armstrong, R. S. & Collins, B. M. Fourier transform Raman microscopic mapping of the molecular components in a human tooth. J. Raman Spectrosc. 28 (2–3), 151–158. https://doi.org/10.1002/(SICI)1097-4555(199702)28:2/3%3c151::AID-JRS71%3e3.0.CO;2-5 (1997).
3.0.CO;2-5" data-track-action="article reference" href="https://doi.org/10.1002%2F%28SICI%291097-4555%28199702%2928%3A2%2F3%3C151%3A%3AAID-JRS71%3E3.0.CO%3B2-5" aria-label="Article reference 4" data-doi="10.1002/(SICI)1097-4555(199702)28:2/3 3.0.CO;2-5">Article ADS CAS Google Scholar
Castiglione, V. et al. Raman chemical imaging, a new tool in kidney stone structure analysis: Case-study and comparison to fourier transform infrared spectroscopy. PLoS ONE 13 (8), e0201460. https://doi.org/10.1371/journal.pone.0201460 (2018).
Article CAS PubMed PubMed Central Google Scholar
Sivaguru, M. et al. Human kidney stones: A natural record of universal biomineralization. Nat. Rev. Urol. 18 (7), 404–432. https://doi.org/10.1038/s41585-021-00469-x (2021).
Article CAS PubMed Google Scholar
Sivaguru, M. et al. Geobiology reveals how human kidney stones dissolve in vivo. Sci. Rep. 8 (1), 13731. https://doi.org/10.1038/s41598-018-31890-9 (2018).
Article ADS CAS PubMed PubMed Central Google Scholar
Todorov, L. G. et al. GeoBioMed perspectives on kidney stone recurrence from the reactive surface area of SWL-derived particles. Sci. Rep. 12 (1), 18371. https://doi.org/10.1038/s41598-022-23331-5 (2022).
Zarse, C. A. et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urol. 4 (1), 15. https://doi.org/10.1186/1471-2490-4-15 (2004).
Article PubMed PubMed Central Google Scholar
Manzoor, M. A. P., Agrawal, A. K., Singh, B., Mujeeburahiman, M. & Rekha, P.-D. Morphological characteristics and microstructure of kidney stones using synchrotron radiation μCT reveal the mechanism of crystal growth and aggregation in mixed stones. PLOS ONE 14 (3), e0214003. https://doi.org/10.1371/journal.pone.0214003 (2019).
Corrales, M., Doizi, S., Barghouthy, Y., Traxer, O. & Daudon, M. Classification of stones according to Michel Daudon: A narrative review. Eur. Urol. Focus 7 (1), 13–21. https://doi.org/10.1016/j.euf.2020.11.004 (2021).
Article PubMed Google Scholar
Williams, J. C., Lingeman, J. E., Daudon, M. & Bazin, D. Using micro computed tomographic imaging for analyzing kidney stones. Comptes Rendus. Chimie 25 (S1), 1–12. https://doi.org/10.5802/crchim.89 (2021).
Article CAS Google Scholar
Williams, J. C., Lingeman, J. E., Coe, F. L., Worcester, E. M. & Evan, A. P. Micro-CT imaging of Randall’s plaques. Urolithiasis 43 (1), 13–17. https://doi.org/10.1007/s00240-014-0702-z (2015).
Williams, J. C., McAteer, J. A., Evan, A. P. & Lingeman, J. E. Micro-computed tomography for analysis of urinary calculi. Urol. Res. 38 (6), 477–484. https://doi.org/10.1007/s00240-010-0326-x (2010).
Williams, J. C., Worcester, E. & Lingeman, J. E. What can the microstructure of stones tell us?. Urolithiasis 45 (1), 19–25. https://doi.org/10.1007/s00240-016-0944-z (2017).
Keller, E. X. et al. Fragments and dust after holmium laser lithotripsy with or without “Moses technology”: How are they different?. J. Biophotonics 12 (4), e201800227. https://doi.org/10.1002/jbio.201800227 (2019).
Daudon, M. et al. Examination of Whewellite kidney stones by scanning electron microscopy and powder neutron diffraction techniques. J. Appl. Crystallogr. 42 (1), 109–115. https://doi.org/10.1107/S0021889808041277 (2009).
Al-Atar, U. et al. Mechanism of calcium oxalate monohydrate kidney stones formation: Layered spherulitic growth. Chem. Mater. 22 (4), 1318–1329. https://doi.org/10.1021/cm901751g (2010).
Gleeson, M. et al. Kidney stone classification using multimodal multiphoton microscopy. ACS Photonics 10 (10), 3594–3604. https://doi.org/10.1021/acsphotonics.3c00651 (2023).
Anderson, J. C., Williams, J. C., Evan, A. P., Condon, K. W. & Sommer, A. J. Analysis of urinary calculi using an infrared microspectroscopic surface reflectance imaging technique. Urol. Res. 35 (1), 41–48. https://doi.org/10.1007/s00240-006-0077-x (2007).
Bazin, D. et al. Combining field effect scanning electron microscopy, deep UV fluorescence, Raman, classical and synchrotron radiation fourier transform infra-red spectroscopy in the study of crystal-containing kidney biopsies. Comptes Rendus Chimie 19 (11), 1439–1450. https://doi.org/10.1016/j.crci.2015.03.001 (2016).
Valido, H. et al. Calcium oxalate kidney stones, where is the organic matter?: A synchrotron based infrared microspectroscopy study. J. Biophotonics https://doi.org/10.1002/jbio.202000303 (2020).
Article Google Scholar
Sofińska-Chmiel, W. et al. Chemical studies of multicomponent kidney stones using the modern advanced research methods. Molecules 28 (16), 6089. https://doi.org/10.3390/molecules28166089 (2023).
Daudon, M., Protat, M. F., Reveillaud, R. J. & Jaeschke-Boyer, H. Infrared spectrometry and Raman microprobe in the analysis of urinary calculi. Kidney Int. 23 (6), 842–850. https://doi.org/10.1038/ki.1983.104 (1983).
Cloutier, J., Villa, L., Traxer, O. & Daudon, M. Kidney stone analysis: “Give Me Your Stone, I Will Tell You Who You Are!”. World J. Urol. 33 (2), 157–169. https://doi.org/10.1007/s00345-014-1444-9 (2015).
Estepa, L. & Daudon, M. Contribution of Fourier transform infrared spectroscopy to the identification of urinary stones and kidney crystal deposits. Biospectroscopy 3 (5), 347–369. https://doi.org/10.1002/(SICI)1520-6343(1997)3:5%3c347::AID-BSPY3%3e3.0.CO;2-# (1997).
3.0.CO;2-#" data-track-action="article reference" href="https://doi.org/10.1002%2F%28SICI%291520-6343%281997%293%3A5%3C347%3A%3AAID-BSPY3%3E3.0.CO%3B2-%23" aria-label="Article reference 26" data-doi="10.1002/(SICI)1520-6343(1997)3:5 3.0.CO;2-#">Article CAS Google Scholar
Tamosaityte, S. et al. Raman spectroscopy as a non-destructive tool to determine the chemical composition of urinary sediments. Comptes Rendus. Chimie 25 (S1), 73–82. https://doi.org/10.5802/crchim.121 (2022).
Lucas, I. T., Bazin, D. & Daudon, M. Raman opportunities in the field of pathological calcifications. Comptes Rendus. Chimie 25 (S1), 83–103. https://doi.org/10.5802/crchim.110 (2022).
Cui, X. et al. Analysis and classification of kidney stones based on Raman spectroscopy. Biomed. Opt. Express. 9 (9), 4175–4183. https://doi.org/10.1364/BOE.9.004175 (2018).
Golcuk, K. et al. Is photobleaching necessary for Raman imaging of bone tissue using a green laser?. Biochim. Biophys. Acta Biomembr. 1758 (7), 868–873. https://doi.org/10.1016/j.bbamem.2006.02.022 (2006).
Yakubovskaya, E., Zaliznyak, T., Martínez Martínez, J. & Taylor, G. T. Tear down the fluorescent curtain: A new fluorescence suppression method for Raman microspectroscopic analyses. Sci. Rep. 9 (1), 15785. https://doi.org/10.1038/s41598-019-52321-3 (2019).
Petit, I. et al. Vibrational signatures of calcium oxalate polyhydrates. ChemistrySelect 3 (31), 8801–8812. https://doi.org/10.1002/slct.201801611 (2018).
Ulian, G., Valdrè, G., Corno, M. & Ugliengo, P. The vibrational features of hydroxylapatite and Type A carbonated apatite: A first principle contribution. Am. Mineralogist 98 (4), 752–759. https://doi.org/10.2138/am.2013.4315 (2013).
Casciani, F. & Condrate, R. A. Sr. The vibrational spectra of brushite, CaHPO 4 ·2H 2 O. Spectrosc. Lett. 12 (10), 699–713. https://doi.org/10.1080/00387017908069196 (1979).
Stefov, V., Šoptrajanov, B., Kuzmanovski, I., Lutz, H. D. & Engelen, B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. III. Spectra of protiated and partially deuterated magnesium ammonium phosphate hexahydrate. J. Mol. Struct. 752 (1), 60–67. https://doi.org/10.1016/j.molstruc.2005.05.040 (2005).
Zellelow, A. Z., Kim, K.-H., Sours, R. E. & Swift, J. A. Solid-state dehydration of uric acid dihydrate. Cryst. Growth Des. 10 (1), 418–425. https://doi.org/10.1021/cg9010218 (2010).
Izatulina, A. R., Gurzhiy, V. V., Krzhizhanovskaya, M. G., Chukanov, N. V. & Panikorovskii, T. L. Thermal behavior and phase transition of uric acid and its dihydrate form, the common biominerals uricite and tinnunculite. Minerals 9 (6), 373. https://doi.org/10.3390/min9060373 (2019).
Presores, J. B. & Swift, J. A. Solution-mediated phase transformation of uric acid dihydrate. CrystEngComm 16 (31), 7278–7284. https://doi.org/10.1039/C4CE00574K (2014).
Frincu, M. C., Fogarty, C. E. & Swift, J. A. Epitaxial relationships between uric acid crystals and mineral surfaces: A factor in urinary stone formation. Langmuir 20 (16), 6524–6529. https://doi.org/10.1021/la049091u (2004).
Wang, Z. & Königsberger, E. Solubility equilibria in the uric acid-sodium urate–water system. Thermochim. Acta 310 (1), 237–242. https://doi.org/10.1016/S0040-6031(97)00230-X (1998).
Königsberger, E., Tromans, A., May, P. M. & Hefter, G. Solubility of calcium oxalate monohydrate in concentrated electrolyte solutions. J. Chem. Eng. Data 66 (1), 840–847. https://doi.org/10.1021/acs.jced.0c00925 (2021).
Ibis, F. et al. A combined experimental and modelling study on solubility of calcium oxalate monohydrate at physiologically relevant pH and temperatures. Crystals 10 (10), 924. https://doi.org/10.3390/cryst10100924 (2020).
Gridley, C. M., Sourial, M. W., Lehman, A. & Knudsen, B. E. Medical dissolution therapy for the treatment of uric acid nephrolithiasis. World J. Urol. 37 (11), 2509–2515. https://doi.org/10.1007/s00345-019-02688-9 (2019).
Vermeulen, C. W. & Fried, F. A. Observations on dissolution of uric acid calculi. J. Urol. 94 (3), 293–296. https://doi.org/10.1016/S0022-5347(17)63618-8 (1965).
Pramanik, R., Asplin, J. R., Jackson, M. E. & Williams, J. C. Protein content of human apatite and brushite kidney stones: Significant correlation with morphologic measures. Urol. Res. 36 (5), 251–258. https://doi.org/10.1007/s00240-008-0151-7 (2008).
Brown, W. E., Patel, P. R. & Chow, L. C. Formation of CaHPO 4 2H 2 O from enamel mineral and its relationship to caries mechanism. J. Dent. Res. 54 (3), 475–481. https://doi.org/10.1177/00220345750540031001 (1975).
Brown, P. W. Phase relationships in the ternary system CaO─P 2 O 5 ─H 2 O at 25°C. J. Am. Ceram. Soc. 75 (1), 17–22. https://doi.org/10.1111/j.1151-2916.1992.tb05435.x (1992).
Abbona, F., Christensson, F., Angela, M. F. & Madsen, H. E. L. Crystal habit and growth conditions of brushite, CaHPO 4 ⋅ 2H 2 O. J. Cryst. Growth 131 (3), 331–346. https://doi.org/10.1016/0022-0248(93)90183-W (1993).
Siener, R., Netzer, L. & Hesse, A. Determinants of brushite stone formation: A case-control study. PLOS ONE 8 (11), e78996. https://doi.org/10.1371/journal.pone.0078996 (2013).
Bazin, D. et al. Hyperoxaluria Is related to whewellite and hypercalciuria to weddellite: What happens when crystalline conversion occurs?. Comptes Rendus Chimie 19 (11), 1492–1503. https://doi.org/10.1016/j.crci.2015.12.011 (2016).
Sarigul, N., Korkmaz, F. & Kurultak, İ. A new artificial urine protocol to better imitate human urine. Sci. Rep. 9 (1), 20159. https://doi.org/10.1038/s41598-019-56693-4 (2019).
Daudon, M., Jungers, P., Bazin, D. & Williams, J. C. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis 46 (5), 459–470. https://doi.org/10.1007/s00240-018-1043-0 (2018).
Werness, P. G., Bergert, J. H. & Smith, L. H. Crystalluria. J. Cryst. Growth 53 (1), 166–181. https://doi.org/10.1016/0022-0248(81)90063-4 (1981).
Article ADS Google Scholar
Frochot, V. et al. Advances in the identification of calcium carbonate urinary crystals. Clin. Chim. Acta 515 , 1–4. https://doi.org/10.1016/j.cca.2020.12.024 (2021).
Hourlier, D. Thermal decomposition of calcium oxalate: Beyond appearances. J. Therm. Anal. Calorim. 136 (6), 2221–2229. https://doi.org/10.1007/s10973-018-7888-1 (2019).
Rak, J., Skurski, P., Gutowski, M. & Błażejowski, J. Thermodynamics of the thermal decomposition of calcium oxalate monohydrate examined theoretically. J. Therm. Anal. Calorim. 43 (1), 239–246. https://doi.org/10.1007/bf02635991 (1995).
Izatulina, A. R. et al. Hydrated calcium oxalates: Crystal structures, thermal stability, and phase evolution. Cryst. Growth Des. 18 (9), 5465–5478. https://doi.org/10.1021/acs.cgd.8b00826 (2018).
Echigo, T., Kimata, M., Kyono, A., Shimizu, M. & Hatta, T. Re-investigation of the crystal structure of whewellite [Ca(C 2 O 4 )·H 2 O] and the dehydration mechanism of caoxite [Ca(C 2 O 4 )·3H 2 O]. Mineralogical Mag. 69 (1), 77–88. https://doi.org/10.1180/0026461056910235 (2005).
Millan, A. Crystal growth shape of whewellite polymorphs: Influence of structure distortions on crystal shape. Cryst. Growth Des. 1 (3), 245–254. https://doi.org/10.1021/cg0055530 (2001).
Robinson, J. W., Ghani, K. R., Roberts, W. W. & Matzger, A. J. Near-infrared absorption coefficients in kidney stone minerals and their relation to crystal structure. J. Phys. Chem. C 127 (1), 759–767. https://doi.org/10.1021/acs.jpcc.2c07475 (2023).
Hajir, M., Graf, R. & Tremel, W. Stable amorphous calcium oxalate: Synthesis and potential intermediate in biomineralization. Chem. Commun. 50 (49), 6534–6536. https://doi.org/10.1039/C4CC02146K (2014).
Ihli, J. et al. Precipitation of amorphous calcium oxalate in aqueous solution. Chem. Mater. 27 (11), 3999–4007. https://doi.org/10.1021/acs.chemmater.5b01642 (2015).
Awonusi, A., Morris, M. D. & Tecklenburg, M. M. J. Carbonate assignment and calibration in the Raman spectrum of apatite. Calcif. Tissue Int. 81 (1), 46–52. https://doi.org/10.1007/s00223-007-9034-0 (2007).
Prywer, J., Sadowski, R. R. & Torzewska, A. Aggregation of struvite, carbonate apatite, and proteus mirabilis as a key factor of infectious urinary stone formation. Cryst. Growth Des. 15 (3), 1446–1451. https://doi.org/10.1021/cg5018032 (2015).
Qin, L., Putnis, C. V. & Wang, L. Facet-specific dissolution-precipitation at struvite-water interfaces. Cryst. Growth Des. https://doi.org/10.1021/acs.cgd.1c00400 (2021).
Kurtulus, G. & Tas, A. C. Transformations of neat and heated struvite (MgNH 4 PO 4 ⋅ 6H 2 O). Mater. Lett. 65 (19), 2883–2886. https://doi.org/10.1016/j.matlet.2011.06.086 (2011).
Racek, M., Racek, J. & Hupáková, I. Scanning electron microscopy in analysis of urinary stones. Scand. J. Clin. Lab. Investig. 79 (3), 208–217. https://doi.org/10.1080/00365513.2019.1578995 (2019).
Frank, D. S., Aldoukhi, A. H., Roberts, W. W., Ghani, K. R. & Matzger, A. J. Polymer-mineral composites mimic human kidney stones in laser lithotripsy experiments. ACS Biomater. Sci. Eng. 5 (10), 4970–4975. https://doi.org/10.1021/acsbiomaterials.9b01130 (2019).
Frost, R. L., Weier, M. L. & Erickson, K. L. Thermal decomposition of struvite. J. Therm. Anal. Calorim. 76 (3), 1025–1033. https://doi.org/10.1023/B:JTAN.0000032287.08535.b3 (2004).
Bayuseno, A. P. & Schmahl, W. W. thermal decomposition of struvite in water: Qualitative and quantitative mineralogy analysis. Environ. Technol. 41 (27), 3591–3597. https://doi.org/10.1080/09593330.2019.1615558 (2020).
Ames, B. N., Cathcart, R., Schwiers, E. & Hochstein, P. uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. 78 (11), 6858–6862. https://doi.org/10.1073/pnas.78.11.6858 (1981).
Simic, M. G. & Jovanovic, S. V. Antioxidation mechanisms of uric acid. J. Am. Chem. Soc. 111 (15), 5778–5782. https://doi.org/10.1021/ja00197a042 (1989).
Download references
Acknowledgements
We would like to thank Boston Scientific Corporation for funding and the Rackham Graduate School at the University of Michigan for a fellowship to JWR.
Author information
Authors and affiliations.
Department of Chemistry, University of Michigan, Ann Arbor, MI, 48109, USA
John W. Robinson & Adam J. Matzger
Division of Endourology, Department of Urology, University of Michigan, Ann Arbor, MI, 48109, USA
William W. Roberts
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, 48109, USA
Macromolecular Science and Engineering Program, University of Michigan, Ann Arbor, MI, 48109, USA
Adam J. Matzger
You can also search for this author in PubMed Google Scholar
Contributions
JWR developed and carried out experimental work and wrote the initial draft of this manuscript. AJM and WWR provided advisory support. All authors reviewed and aided in editing of the manuscript.
Corresponding author
Correspondence to Adam J. Matzger .
Ethics declarations
Competing interests.
WWR is a paid consultant for Boston Scientific.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Robinson, J.W., Roberts, W.W. & Matzger, A.J. Kidney stone growth through the lens of Raman mapping. Sci Rep 14 , 10834 (2024). https://doi.org/10.1038/s41598-024-61652-9
Download citation
Received : 29 February 2024
Accepted : 08 May 2024
Published : 12 May 2024
DOI : https://doi.org/10.1038/s41598-024-61652-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hyperspectral imaging
- Vibrational spectroscopy
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Association between daily sitting time and kidney stones based on the National Health and Nutrition Examination Survey (NHANES) 2007-2016: A cross-sectional study
Affiliation.
- 1 Department of Urology and Institute of Urology (Laboratory of Reconstructive Urology), West China Hospital, Sichuan University, Chengdu, Sichuan, China.
- PMID: 38768465
- DOI: 10.1097/JS9.0000000000001560
Background: Kidney stones are among the most common urological conditions affecting approximately 9% of the world population. Although some unhealthy diets and unhealthy lifestyles are reportedly risk factors for kidney stone, the association between daily sitting time and kidney stone has not been explored.
Materials and methods: This large-scale, cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES) database 2007-2016. Kidney stone history and daily sitting time were retrieved from the questionnaire and 24-hour recall interviews. Logistic regression and subgroup analysis were conducted to investigate the association. The analysis was further stratified by vigorous recreational activity.
Results: A total of 19188 participants aged ≥20 years with complete information were included in this study. The overall prevalence of kidney stone was 9.6%. Among participants without vigorous recreational activity, a trend towards an increasing prevalence of kidney stone was observed with increased daily sitting time. However, the trend was not observed in individuals who participated in vigorous recreational activity, as they experienced a decreased risk of kidney stone despite having a daily sitting time of 6 to 8 hours (crude model OR=0.659, 95% CI: 0.457 to 0.950, P=0.028), indicating that vigorous recreational activity may partially attenuate the detrimental effect of prolonged sitting time.
Conclusion: Our study revealed an increasing trend of prevalence of kidney stone with increased daily sitting time among the population not performing vigorous recreational activity despite the difference was nonsignificant. Vigorous recreational activity may modify the association between daily sitting time and kidney stone. More prospective cohort studies are warranted to further examine this association.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
- Patient Care & Health Information
- Diseases & Conditions
- Kidney stones
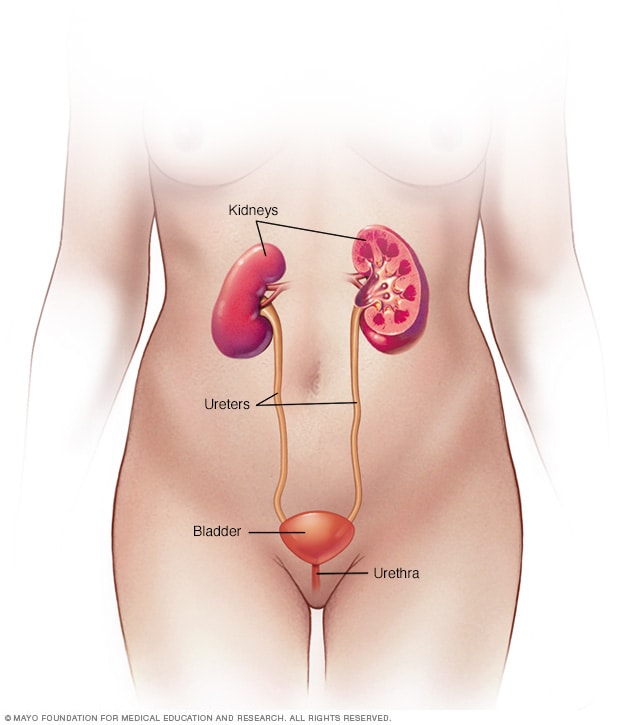
Female urinary system
Your urinary system includes the kidneys, ureters, bladder and urethra. The urinary system removes waste from the body through urine. The kidneys are located toward the back of the upper abdomen. They filter waste and fluid from the blood and produce urine. Urine moves from the kidneys through narrow tubes to the bladder. These tubes are called the ureters. The bladder stores urine until it's time to urinate. Urine leaves the body through another small tube called the urethra.
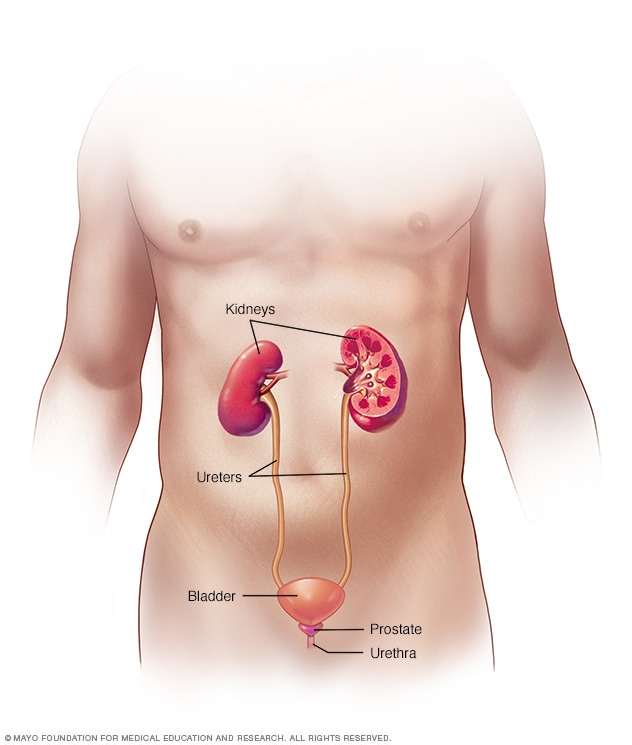
Male urinary system
Kidney stones (also called renal calculi, nephrolithiasis or urolithiasis) are hard deposits made of minerals and salts that form inside your kidneys.
Diet, excess body weight, some medical conditions, and certain supplements and medications are among the many causes of kidney stones. Kidney stones can affect any part of your urinary tract — from your kidneys to your bladder. Often, stones form when the urine becomes concentrated, allowing minerals to crystallize and stick together.
Passing kidney stones can be quite painful, but the stones usually cause no permanent damage if they're recognized in a timely fashion. Depending on your situation, you may need nothing more than to take pain medication and drink lots of water to pass a kidney stone. In other instances — for example, if stones become lodged in the urinary tract, are associated with a urinary infection or cause complications — surgery may be needed.
Your doctor may recommend preventive treatment to reduce your risk of recurrent kidney stones if you're at increased risk of developing them again.
Products & Services
- A Book: Mayo Clinic Book of Home Remedies
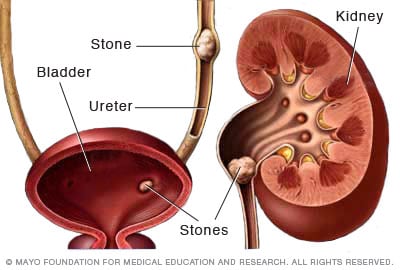
Kidney stones form in your kidneys. As stones move into your ureters — the thin tubes that allow urine to pass from your kidneys to your bladder — signs and symptoms can result. Signs and symptoms of kidney stones can include severe pain, nausea, vomiting, fever, chills and blood in your urine.
A kidney stone usually will not cause symptoms until it moves around within the kidney or passes into one of the ureters. The ureters are the tubes that connect the kidneys and bladder.
If a kidney stone becomes lodged in the ureters, it may block the flow of urine and cause the kidney to swell and the ureter to spasm, which can be very painful. At that point, you may experience these symptoms:
- Severe, sharp pain in the side and back, below the ribs
- Pain that radiates to the lower abdomen and groin
- Pain that comes in waves and fluctuates in intensity
- Pain or burning sensation while urinating
Other signs and symptoms may include:
- Pink, red or brown urine
- Cloudy or foul-smelling urine
- A persistent need to urinate, urinating more often than usual or urinating in small amounts
- Nausea and vomiting
- Fever and chills if an infection is present
Pain caused by a kidney stone may change — for instance, shifting to a different location or increasing in intensity — as the stone moves through your urinary tract.
When to see a doctor
Make an appointment with your doctor if you have any signs and symptoms that worry you.
Seek immediate medical attention if you experience:
- Pain so severe that you can't sit still or find a comfortable position
- Pain accompanied by nausea and vomiting
- Pain accompanied by fever and chills
- Blood in your urine
- Difficulty passing urine
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Kidney stones often have no definite, single cause, although several factors may increase your risk.
Kidney stones form when your urine contains more crystal-forming substances — such as calcium, oxalate and uric acid — than the fluid in your urine can dilute. At the same time, your urine may lack substances that prevent crystals from sticking together, creating an ideal environment for kidney stones to form.
Types of kidney stones
Knowing the type of kidney stone you have helps determine its cause, and may give clues on how to reduce your risk of getting more kidney stones. If possible, try to save your kidney stone if you pass one so that you can bring it to your doctor for analysis.
Types of kidney stones include:
Calcium stones. Most kidney stones are calcium stones, usually in the form of calcium oxalate. Oxalate is a substance made daily by your liver or absorbed from your diet. Certain fruits and vegetables, as well as nuts and chocolate, have high oxalate content.
Dietary factors, high doses of vitamin D, intestinal bypass surgery and several metabolic disorders can increase the concentration of calcium or oxalate in urine.
Calcium stones may also occur in the form of calcium phosphate. This type of stone is more common in metabolic conditions, such as renal tubular acidosis. It may also be associated with certain medications used to treat migraines or seizures, such as topiramate (Topamax, Trokendi XR, Qudexy XR).
- Struvite stones. Struvite stones form in response to a urinary tract infection. These stones can grow quickly and become quite large, sometimes with few symptoms or little warning.
- Uric acid stones. Uric acid stones can form in people who lose too much fluid because of chronic diarrhea or malabsorption, those who eat a high-protein diet, and those with diabetes or metabolic syndrome. Certain genetic factors also may increase your risk of uric acid stones.
- Cystine stones. These stones form in people with a hereditary disorder called cystinuria that causes the kidneys to excrete too much of a specific amino acid.
Risk factors
Factors that increase your risk of developing kidney stones include:
- Family or personal history. If someone in your family has had kidney stones, you're more likely to develop stones, too. If you've already had one or more kidney stones, you're at increased risk of developing another.
- Dehydration. Not drinking enough water each day can increase your risk of kidney stones. People who live in warm, dry climates and those who sweat a lot may be at higher risk than others.
- Certain diets. Eating a diet that's high in protein, sodium (salt) and sugar may increase your risk of some types of kidney stones. This is especially true with a high-sodium diet. Too much salt in your diet increases the amount of calcium your kidneys must filter and significantly increases your risk of kidney stones.
- Obesity. High body mass index (BMI), large waist size and weight gain have been linked to an increased risk of kidney stones.
- Digestive diseases and surgery. Gastric bypass surgery, inflammatory bowel disease or chronic diarrhea can cause changes in the digestive process that affect your absorption of calcium and water, increasing the amounts of stone-forming substances in your urine.
- Other medical conditions such as renal tubular acidosis, cystinuria, hyperparathyroidism and repeated urinary tract infections also can increase your risk of kidney stones.
- Certain supplements and medications, such as vitamin C, dietary supplements, laxatives (when used excessively), calcium-based antacids, and certain medications used to treat migraines or depression, can increase your risk of kidney stones.
Kidney stones care at Mayo Clinic
- Goldman L, et al., eds. Nephrolithiasis. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Kidney stones. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/urologic-diseases/kidney-stones. Accessed Jan. 20, 2020.
- McKean SC, et al., eds. Kidney stones. In: Principles and Practice of Hospital Medicine. 2nd ed. McGraw-Hill Education; 2017. https://accessmedicine.mhmedical.com/. Accessed Jan. 20, 2020.
- What are kidney stones? American Urological Association. https://www.urologyhealth.org/urologic-conditions/kidney-stones. Accessed Jan. 20, 2020.
- Kellerman RD, et al. Nephrolithiasis. In: Conn's Current Therapy 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Braswell-Pickering EA. Allscripts EPSi. Mayo Clinic. Nov. 3, 2021.
- Curhan GC, et al. Diagnosis and acute management of suspected nephrolithiasis in adults. https://www.uptodate.com/search/contents. Accessed Jan. 20, 2020.
- Yu ASL, et al., eds. Diagnostic kidney imaging. In: Brenner & Rector's The Kidney. 11th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Fontenelle LF, et al. Kidney stones: Treatment and prevention. American Family Physician. 2019. https://www.aafp.org/afp/2019/0415/p490.html. Accessed Jan. 20, 2020.
- Preminger GM. Options in the management of renal and ureteral stones in adults. https://www.uptodate.com/search/contents. Accessed Jan. 20, 2020.
- Preventing Kidney Stones
Associated Procedures
- Computerized tomography (CT) urogram
- Intravenous pyelogram
- Percutaneous nephrolithotomy
News from Mayo Clinic
- Mayo Clinic Minute: Advances in minimally invasive kidney stone surgery May 07, 2024, 04:15 p.m. CDT
- Preventing kidney stones before they form Oct. 11, 2023, 01:59 p.m. CDT
- Mayo Clinic Minute: Where is the kidney stone belt? July 03, 2023, 02:00 p.m. CDT
- Mayo Clinic Minute: What can you eat to avoid kidney stones? March 30, 2023, 03:30 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Advertisement
Automatic Detection and Scoring of Kidney Stones on Noncontrast CT Images Using S.T.O.N.E. Nephrolithometry: Combined Deep Learning and Thresholding Methods
- Research Article
- Published: 27 October 2020
- Volume 23 , pages 436–445, ( 2021 )
Cite this article

- Yingpu Cui 1 ,
- Zhaonan Sun 1 ,
- Shuai Ma 1 ,
- Weipeng Liu 2 ,
- Xiangpeng Wang 2 ,
- Xiaodong Zhang 1 &
- Xiaoying Wang ORCID: orcid.org/0000-0001-9822-961X 1
1437 Accesses
23 Citations
3 Altmetric
Explore all metrics
To develop and validate a deep learning and thresholding-based model for automatic kidney stone detection and scoring according to S.T.O.N.E. nephrolithometry.
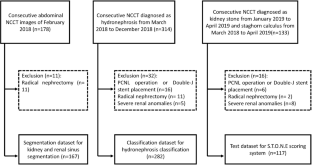
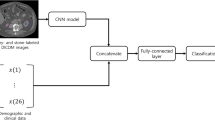
Abdominal noncontrast computed tomography (NCCT) images were retrospectively archived from February 2018 to April 2019 for three parts: a segmentation dataset ( n = 167), a hydronephrosis classification dataset ( n = 282), and test dataset ( n = 117). The model consisted of four steps. First, the 3D U-Nets for kidney and renal sinus segmentation were developed. Second, the deep 3D dual-path networks for hydronephrosis grading were developed. Third, the thresholding methods were used to detect and segment stones in the renal sinus region. The stone size, CT attenuation, and tract length were calculated from the segmented stone region. Fourth, the stone’s location was determined. The stone detection performance was estimated with sensitivity and positive predictive value (PPV). The hydronephrosis grading and stone size, tract length, number of involved calyces, and essence grading were estimated with the area under the curve (AUC) method and linear-weighted κ statistics, respectively.
The stone detection algorithm reached a sensitivity of 95.9 % (236/246) and a PPV of 98.7 % (236/239). The hydronephrosis classification algorithm achieved an AUC of 0.97. The scoring model results showed good agreement with radiologist results for the stone size, tract length, number of involved calyces, and essence grading ( κ = 0.95, 95 % confidence interval [CI]: 0.92, 0.98; κ = 0.97, 95 % CI: 0.95, 1.00; κ = 0.95, 95 % CI: 0.92, 0.98; and κ = 0.97, 95 % CI: 0.94, 1.00), respectively.
Conclusions
The scoring model was constructed that can automatically detect and score stones in NCCT images.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Automatic Urinary Stone Detection System for Abdominal Non-Enhanced CT Images Reduces the Burden on Radiologists
Development and external validation of a machine learning-based model to classify uric acid stones in patients with kidney stones of Hounsfield units < 800
Detection of urinary tract calculi on CT images reconstructed with deep learning algorithms
Abbreviations.
Three dimensional
Computed tomography
Noncontrast computed tomography
Percutaneous nephrolithotomy
Receiver operating characteristic curve
Area under the curve
Positive predictive value
Confidence interval
Park S (2007) Medical management of urinary stone disease. Expert Opin Pharmacother 8:1117–1125
Article CAS Google Scholar
Scales CD, Tasian GE, Schwaderer AL, Goldfarb DS, Star RA, Kirkali Z (2016) Urinary stone disease: advancing knowledge, patient care, and population health. Clin J Am Soc Nephrol 11:1305–1312
Article Google Scholar
Allison SJ (2014) Stones: ultrasonography and computed tomography: performance in detection of kidney stones. Nat Rev Nephrol 10:611
Turk C, Petrik A, Sarica K et al (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69:475–482
De S, Autorino R, Kim FJ et al (2016) Corrigendum re: “Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis” [Eur Urol 2015;67:125-37]. Eur Urol 69:e85
Okhunov Z, Friedlander JI, George AK, Duty BD, Moreira DM, Srinivasan AK, Hillelsohn J, Smith AD, Okeke Z (2013) S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology 81:1154–1159
Noureldin YA, Elkoushy MA, Andonian S (2015) External validation of the S.T.O.N.E. nephrolithometry scoring system. Can Urol Assoc J 9:190–195
Kambadakone AR, Eisner BH, Catalano OA, Sahani DV (2010) New and evolving concepts in the imaging and management of urolithiasis: urologists’ perspective. Radiographics 30:603–623
Okhunov Z, Helmy M, Perez-Lansac A, Menhadji A, Bucur P, Kolla SB, Cho JS, Osann K, Lusch A, Landman J (2013) Interobserver reliability and reproducibility of s.T.o.N.e. nephrolithometry for renal calculi. J Endourol 27:1303–1306
Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A (2017) Deep learning: a primer for radiologists. Radiographics 37:2113–2131
Demehri S, Kalra MK, Rybicki FJ, Steigner ML, Lang MJ, Houseman EA, Curhan GC, Silverman SG (2011) Quantification of urinary stone volume: attenuation threshold-based CT method-a technical note. Radiology 258:915–922
Duan X, Wang J, Qu M, Leng S, Liu Y, Krambeck A, McCollough C (2012) Kidney stone volume estimation from computerized tomography images using a model based method of correcting for the point spread function. J Urol 188:989–995
Hoffmann U, Kwait DC, Handwerker J, Chan R, Lamuraglia G, Brady TJ (2003) Vascular calcification in ex vivo carotid specimens: precision and accuracy of measurements with multi-detector row CT. Radiology 229:375–381
Jacobs C, van Rikxoort EM, Scholten ET, de Jong PA, Prokop M, Schaefer-Prokop C, van Ginneken B (2015) Solid, part-solid, or non-solid?: classification of pulmonary nodules in low-dose chest computed tomography by a computer-aided diagnosis system. Investig Radiol 50:168–173
Fernbach SK, Maizels M, Conway JJ (1993) Ultrasound grading of hydronephrosis-introduction to the system used by the society-for-fetal-urology. Pediatr Radiol 23:478–480
Maizels M, Keays M, Snyder H, Leonard M (2008) Reliability assessment of Society for Fetal Urology ultrasound grading system for hydronephrosis-discussion. J Urol 180:1683–1683
Eisner BH, Kambadakone A, Monga M, Anderson JK, Thoreson AA, Lee H, Dretler SP, Sahani DV (2009) Computerized tomography magnified bone windows are superior to standard soft tissue windows for accurate measurement of stone size: an in vitro and clinical study. J Urol 181:1710–1715
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
Ward AD, Hamarneh G, Ashry R, Schweitzer ME (2007) 3D shape analysis of the supraspinatus muscle: a clinical study of the relationship between shape and pathology. Acad Radiol 14:1229–1241
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-Net: learning dense volumetric segmentation from sparse annotation. Springer International Publishing, Cham, pp. 424–432
Zhu WT, Liu CC, Fan W, Xie XH (2018) DeepLung: deep 3D dual path nets for automated pulmonary nodule detection and classification. 2018 Ieee Winter Conference on Applications of Computer Vision (Wacv 2018) 2018-:673-681
Ziemba JB, Li P, Gurnani R, Kawamoto S, Fishman EK, Fung G, Ludwig WW, Stoianovici D, Matlaga BR (2018) A user-friendly application to automate CT renal stone measurement. J Endourol 32:685–691
Jendeberg J, Geijer H, Alshamari M, Liden M (2018) Prediction of spontaneous ureteral stone passage: automated 3D-measurements perform equal to radiologists, and linear measurements equal to volumetric. Eur Radiol 28:2474–2483
Selby MG, Vrtiska TJ, Krambeck AE, McCollough CH, Elsherbiny HE, Bergstralh EJ, Lieske JC, Rule AD (2015) Quantification of asymptomatic kidney stone burden by computed tomography for predicting future symptomatic stone events. Urology 85:45–50
Oliveira B, Torres HR, Queiros S, Morais P, Vilaca JL (2018) Segmentation of kidney and renal collecting system on 3D computed tomography images 2018 IEEE 6th International Conference on Serious Games and Applications for Health (SeGAH),
Papalia R, Abreu ALD, Panebianco V et al (2015) Novel kidney segmentation system to describe tumour location for nephron-sparing surgery. World J Urol 33:865–871
Langkvist M, Jendeberg J, Thunberg P, Loutfi A, Liden M (2018) Computer aided detection of ureteral stones in thin slice computed tomography volumes using convolutional neural networks. Comput Biol Med 97:153–160
Download references
Author information
Authors and affiliations.
Department of Radiology, Peking University First Hospital, 8, Xishiku Street, Xicheng District, Beijing, 100034, China
Yingpu Cui, Zhaonan Sun, Shuai Ma, Xiaodong Zhang & Xiaoying Wang
Beijing Smart Tree Medical Technology Co. Ltd., Beijing, China
Weipeng Liu & Xiangpeng Wang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Xiaoying Wang .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. For this type of study, formal consent is not required.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
(DOCX 732 kb)
Rights and permissions
Reprints and permissions
About this article
Cui, Y., Sun, Z., Ma, S. et al. Automatic Detection and Scoring of Kidney Stones on Noncontrast CT Images Using S.T.O.N.E. Nephrolithometry: Combined Deep Learning and Thresholding Methods. Mol Imaging Biol 23 , 436–445 (2021). https://doi.org/10.1007/s11307-020-01554-0
Download citation
Received : 21 July 2020
Revised : 06 September 2020
Accepted : 13 October 2020
Published : 27 October 2020
Issue Date : June 2021
DOI : https://doi.org/10.1007/s11307-020-01554-0

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Kidney stone
- Multidetector computed tomography
- Deep learning
- Image processing
- Computer-assisted
- Severity of illness index
- Find a journal
- Publish with us
- Track your research
Our bodies don’t just make gall and kidney stones – from saliva to tonsils, these are other ones to look out for
Senior Lecturer, School of Physiology, Pharmacology and Neuroscience, University of Bristol
Disclosure statement
Dan Baumgardt does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Bristol provides funding as a founding partner of The Conversation UK.
View all partners

Of all the body’s amazing abilities, perhaps one of the strangest is its capacity to make stones.
Many will have heard of kidney or gallstones , and be aware of the problems they can cause. But there are other, rarer types of stone in the body that can be found in the most unlikely places.
What are these body stones are made of? And what can we do to prevent them?
Kidney stones affect around one in ten people. They develop from mostly calcium and oxalate that is filtered from blood into our urine. ( Oxalates are naturally occurring compounds found in both plants and humans.) In larger amounts, the oxalate and calcium can crystallise and collect together to form a stone.
Kidney stones can vary considerably in size – from less than a millimetre across to centimetres or more . They can also form unusual shapes – if the stone builds up within the branching channels (calyces) of the kidney, it can take on the form of a deer’s antler. This is called a staghorn calculus .

These stones cause issues when they obstruct the ureters – the two tubes that transport urine from the kidneys to the bladder. If this happens, it can cause severe pain in the loins, as well as preventing normal urinary flow. This in turn can cause an infection, or urine accumulating in and around the kidney .
Another common condition is gallstones. These form inside either the gallbladder, or the biliary tree – the duct system that delivers bile to the gut to help break down fats. Gallstones form from either cholesterol or bile pigments , and can be singular or multiple .
But, like kidney stones, if gallstones work their way into a narrower space (like the common bile duct), they too can cause problems such as abdominal pain, infections and jaundice.
Rarer stone disorders
Stones, then, can develop from different bodily fluids. Take salivary stones , for instance.
Saliva is produced by glands that sit next to the ear and underneath the jaw and tongue. Once secreted into the mouth, it helps moisten food so it can be swallowed, as well as kicking off the digestion process. Salivary stones are made from many different elements , including calcium, magnesium and phosphate.
If salivary stones become stuck in the ducts, this can prevent the secretion of saliva into the mouth, causing pain and swelling. Stagnation of saliva might lead to bad breath, or a horrid taste in the mouth, especially if it triggers an infection of the salivary gland .
Stones can also be found in the tonsils. Located at the back of the mouth at the top of the throat, tonsils are masses of lymphoid tissue that are part of the body’s immune system. It’s ironic, then, that they so often get inflamed and infected.
The tonsils have cavities called crypts, in which morsels of food and saliva can lodge. The result is a tonsil stone , or tonsillolith.
These are often softer and less stony, but may harden with time and also come with their fair share of problems – mainly bad breath or recurrent infections.
Other body materials have the capacity to harden too, turning themselves into stone. Faeces, for instance, can become so hardened that it forms a stony mass called a faecolith .
And the debris, including sloughed skin, found in your belly button can also form a stone known as an omphalolith.
Read more: Navel gazing: checking your belly button can tell you a lot about your health
What can we do about stones?
Happily, there are some simple measures that might prevent these pesky stones forming, or help to get rid of them.
The most important is proper hydration . Drinking the correct amount of water dilutes urine, prevents constipation, and also reduces bacterial build up in the mouth, so can help avert many of these different stone types. In the case of tonsil stones, good oral hygiene including regular tooth brushing can help reduce the risk too.
Diet is also important, particularly for gallstones , which can be triggered by a high-fat diet and obesity. There are some risk factors that you can’t alter – such as being female or over 40, which raises the likelihood of gallstones forming. Avoiding calcium and oxalate-rich foods like dairy, spinach and rhubarb may help prevent kidney stones.
But what if you’ve already got a stone? If it’s made you poorly, removal by surgery or endoscopy may be necessary.
In the case of kidney stones, you can wait for the stones to pass through your system, down the ureter into the bladder and out – sometimes with an audible ping into the loo. A doctor may even ask you to sieve your urine using a tea strainer to try and catch the stone on its exit.
Salivary stones can sometimes be helped along by sucking on a lemon, which acts as a powerful stimulant for salivation –- creating a jet to clear the duct. Salivary and tonsil stones can also be gently prodded out using a blunt instrument.
In short, there are many different treatments available for the different types of body stone – and simple everyday measures that can help reduce the risk of them developing.

Research Fellow

Senior Research Fellow - Women's Health Services

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
The 4 Stages of Passing a Kidney Stone
Here’s what passing kidney stones feels like and what you can do to help things along
What Are Kidney Stones?
- What It Feels Like
When to Call a Healthcare Provider
- At-Home Care
- Medical Treatment
There are four stages of passing a kidney stone : The stone forms, leaves the kidney, reaches the bladder and causes pressure, and finally, exits the body.
Sometimes, a kidney stone will pass on its own, but this can be very painful. There are also times when you may need to seek medical care for help passing a kidney stone.
This article will cover the stages of passing a kidney stone, as well as what to know about the symptoms at each stage and when to seek medical attention.
supersizer / Getty Images
Your kidneys remove fluid and waste from your body. If you have too much salt, minerals, or chemicals in your body and not enough urine, the extra material can form crystals in your kidneys.
Other particles may attach to the crystals and form a hard object (a “stone”) that your body may try to pass.
Kidney stones are categorized by the main material they’re made from. The most common are calcium oxalate , uric acid, and cystine stones. Struvite stones are made of magnesium ammonium phosphate and are often associated with a urinary tract infection (UTI) . These stones can get large quickly.
How Common Are Kidney Stones?
About 1 out of every 10 people in the United States will have a kidney stone at some point in their life. They are more common in men than women. They also seem to be more common in white people than people of other races.
Stages of Passing a Kidney Stone
The development and natural passing of kidney stones can be broken down into four stages.
1) Kidney Stone Formation
Kidney stones can form if urine becomes concentrated because there is not enough water in the body. This allows crystals to form and attract other materials.
The creation of a kidney stone is not painful.
2) The Stone Leaves Kidney
The second stage of passing a kidney stone is when the stone has entered the tube that connects your kidneys to your bladder (ureter).
Pain can come in waves as the ureter spasms to try to pass the stone. The stone can get stuck at the junction of ureter and renal pelvis (ureteropelvic junction) or in the ureter itself, or near the junction of the ureter and the bladder (ureterovesical junction)
3) Bladder Pressure Builds
When the stone reaches the bladder , the pressure builds up in the organ. At this stage of passing a kidney stone, you will feel an urgent need to urinate frequently. Typically, the majority of intense pain is improved or resolved once the stone exits the ureter
4) The Kidney Stone Exits the Body
When you urinate, the kidney stone can be pushed out of the bladder and passed out of your body via the urethra. There is usually little or no pain during the last stage.
How Long Does It Take to Pass a Kidney Stone?
How long it takes to pass a kidney stone depends on how big it is. A stone less than 4 millimeters (mm) can pass in a week or two. Larger stones can take up to four to six weeks to pass.
What Does Passing a Kidney Stone Feel Like?
Most people associate the pain of passing a kidney stone with stage 4, or when it actually exits the body. But pain is most associated with stage 2 (when it leaves the kidney).
If the stone is small enough, it can go through all of the stages without you even realizing it. In other cases, the pain of passing a kidney stone can be severe. Some rank it as being worse than giving birth.
You may experience the following symptoms of larger kidney stones:
- Severe back, side, or abdominal pain (often on one side) that may come on suddenly
- Pain that comes in waves several times an hour
- Urgent need to urinate
- Pain when you urinate
- Blood in your urine
- Nausea or vomiting
- Fever and chills (This may be caused by a urinary tract infection, which is dangerous if it occurs at the same time as passing a kidney stone and warrants immediate medical attention.)
When kidney stones move in your body, they can cause intense pain that almost feels like someone is jabbing you with a knife.
What Do Kidney Stones Look Like When They Pass?
Kidney stones are pebble-like objects that vary in size. They can be as small as a grain of sand to as large as a ping-pong ball. Kidney stones can be smooth or have jagged sharp edges, and are usually yellow or brown.
If you have extreme pain, a fever, or are vomiting while passing a kidney stone, seek medical care right away.
Kidney stones can lead to infections that need to be treated. If you’re not sure what to do, call your provider. They may have you go to urgent care or the emergency room.
What Helps Kidney Stones Pass Quickly?
If you have a kidney stone, you might be able to pass it on your own. There are a few things you can do to help the process along:
- Stay hydrated. Drinking plenty of water or another hydrating fluid to help flush out a kidney stone is one of the most important things you can do.
- Avoid irritating drinks. Try to reduce how much coffee, tea, alcohol, and soda you drink while you’re trying to pass a kidney stone. Focus on drinking water. If you don’t like drinking plain water, try adding a little lemon. It contains citrate, a natural chemical that might help dissolve a stone.
- Think about your diet. Try not to snack on salty foods or add salt to your meals, as a high-sodium diet can be a risk factor for kidney stones. You also don’t want to eat too much protein, since it can also raise your risk. While kidney stones can form from calcium and oxalates, you don’t want to cut these nutrients out of your diet. You just want to be mindful of how much you consume.
- Get moving. If you’re in a lot of pain you might not want to move, but walking around can actually help the stone pass faster.
To help ease discomfort as you wait for a stone to fully pass:
- Use heat. A heating pad can help ease the pain of passing a kidney stone. Soaking in a warm bathtub might also be helpful.
- Take OTC painkillers. An over-the-counter pain reliever like Motrin (ibuprofen) may help with pain and inflammation. However, if you have severe kidney stone pain OTC pain medications may not be enough.
You may have some discomfort after you’ve passed the stone. If these at-home remedies do not help, call your provider.
How Can I Force a Kidney Stone to Pass at Home?
You can't reliably force a kidney stone to pass, but drinking plenty of water is the best way to encourage one to move through your system.
If You Cannot Pass a Kidney Stone at Home
If you end up calling your provider or going to the ER because you have a kidney stone that’s not passing, there are a few things they may try.
Sometimes, your provider might prescribe you a medication called Flomax (tamsulosin) to help pass a large kidney stone. However, research is not clear on whether the medication is always helpful for this purpose.
You might need to have a surgical procedure called shock wave lithotripsy that uses sound waves to break the stone up into smaller pieces, making it easier to pass. Surgeons can also use a special camera called ureteroscope (a narrow tube with a camera at the end) to get to the stone in the ureter or kidney and break it up using laser. You will be sedated during the procedure, so you won’t feel any pain or discomfort.
Surgeons can also use an endoscope (a narrow tube with a camera at the end) to get to the stone and break it up. You will be sedated during the procedure, so you won’t feel any pain or discomfort.
After the stone is taken out, it will go to a lab to see what it was made of. This can help you figure out if there are any steps you can take to reduce your chances of getting more kidney stones.
Can You Prevent Kidney Stones?
You can’t always prevent kidney stones. For example, if you already have kidney disease , you may not be able to avoid them.
You might also need to be on certain medications that can lead to the formation of kidney stones, including diuretics (which increase calcium excretion in the urine) and antacids (which can have stone-forming minerals in them).
But whether these apply to you or not, it's always worth taking steps to try to prevent kidney stones from occurring, including:
- Stay hydrated. Making sure you don’t get dehydrated is important if you’re prone to getting kidney stones.
- Watch your diet. Be aware of the foods you eat often and try to limit those high in protein, salt, and processed sugar. Eating citrus fruits is beneficial for preventing kidney stones.
Can Cranberry Juice Help Prevent Kidney Stones?
Research suggests that whether cranberry juice and cranberry extract supplements could help prevent kidney stones depends on the type of kidney stones you get. For example, if you get oxalate stones, drinking cranberry juice could actually make kidney stones more likely to form since it’s high in oxalates. However, other studies have shown the opposite.
There are four stages of passing a kidney stone: formation, moving into the ureter, reaching the bladder, and exiting the body in urine.
Kidney stones can be very painful, but once the stone passes you should feel much better. While you might be able to pass a kidney stone on your own at home, if you’re in extreme pain and have a fever, you should seek medical care.
If you can’t pass a kidney stone on your own, you might need to have it broken up or taken out surgically.
Khan SR, Pearle MS, Robertson WG, et al. Kidney stones . Nat Rev Dis Primers . 2016;2:16008. doi:10.1038/nrdp.2016.8
National Kidney Foundation. Kidney stones .
American Kidney Fund. Kidney stone causes, symptoms, treatment and prevention .
Urology of Greater Atlanta. What are the stages of passing a kidney stone? .
Urology Care Foundation. What are kidney stones? .
National Institute of Diabetes and Digestive and Kidney Disease. Symptoms & causes of kidney stones .
National Institute of Digestive and Diabetes and Kidney Disease. Definition and facts for kidney stones .
UMPC. How to treat kidney stones at home .
Mount Sinai. Kidney stones - self-care .
Urology Specialists of the Carolinas. How to pass kidney stones naturally .
Leonardo Ferreira Fontenelle, Thiago Dias Sarti. Kidney stones: treatment and prevention . American Family Physician . 2019;99(8):490-496.
Slattengren AH, Prasad S, Jarrett JB. PURLs: Kidney stones? It's time to rethink those meds . J Fam Pract . 2016;65(2):118-120.
National Kidney Foundation. 6 easy ways to prevent kidney stones .
Gamage KN, Jamnadass E, Sulaiman SK, Pietropaolo A, Aboumarzouk O, Somani BK. The role of fluid intake in the prevention of kidney stone disease: A systematic review over the last two decades . Turk J Urol . 2020;46(Supp. 1):S92-S103. doi:10.5152/tud.2020.20155
Gul Z, Monga M. Medical and dietary therapy for kidney stone prevention . Korean J Urol . 2014;55(12):775-779. doi:10.4111/kju.2014.55.12.775
McHarg T, Rodgers A, Charlton K. Influence of cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation . BJU Int . 2003;92(7):765-768. doi:10.1046/j.1464-410x.2003.04472.x
By Nancy LeBrun LeBrun is a Maryland-based freelance writer and award-winning documentary producer with a bachelor's degree in communications.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Nutr Res
- v.4(3); 2015 Jul

Nutritional Management of Kidney Stones (Nephrolithiasis)
Haewook han.
1 Department of Nephrology, Harvard Vanguard Medical Associate, Boston, MA 02115, USA.
Adam M. Segal
2 Harvard Vanguard Medical Associate, Clinical Instructor at Harvard Medical School, Boston, MA 02115, USA.
Julian L. Seifter
3 Harvard Vanguard Medical Associates; Brigham and Women's Hospital, Boston, MA 02115, USA.
Johanna T. Dwyer
4 Tufts University Friedman School of Nutrition and School of Medicine, Boston, MA 02111, USA.
The incidence of kidney stones is common in the United States and treatments for them are very costly. This review article provides information about epidemiology, mechanism, diagnosis, and pathophysiology of kidney stone formation, and methods for the evaluation of stone risks for new and follow-up patients. Adequate evaluation and management can prevent recurrence of stones. Kidney stone prevention should be individualized in both its medical and dietary management, keeping in mind the specific risks involved for each type of stones. Recognition of these risk factors and development of long-term management strategies for dealing with them are the most effective ways to prevent recurrence of kidney stones.
Introduction
Nephrolithiasis, or kidney stone, is the presence of renal calculi caused by a disruption in the balance between solubility and precipitation of salts in the urinary tract and in the kidneys. The incidence is at peak among white males age 20 and 30 years old. The National Health and Nutrition Examination Survey (NHANES) III (1988-1994) reported that there was a 5% prevalence of stone formation among adults in the United States and this represented a 4% increase from the NHANES II (1976-1980) [ 1 , 2 ]. Nephrolithiasis is considered to be a disease of affluence like obesity, hypertension, and type 2 diabetes because it is so prevalent in wealthy countries [ 3 , 4 ]. Urologic intervention is required in as many as 20% of patients with renal colic [ 5 ] and more than $2 billion is spent on treatment each year. The lifetime prevalence of kidney stones in the United States is 12% among men and 7% among women [ 6 , 7 ]. Kidney stones develop when urine becomes "supersaturated" with insoluble compounds containing calcium, oxalate (CaOx), and phosphate (CaP), resulting from dehydration or a genetic predisposition to over-excrete these ions in the urine. About 5-10% of Americans have this predisposition.
Kidney stone formation
When CaOx concentration is 4 times above the normal solubility a crystal starts to form. If the CaOx concentration is 7 to 11 times higher than normal solubility the nucleation begins. In low urine volume, the presence of high calcium, high oxalate the supersaturation (SS) of CaOx is increased. Citrate in the urine forms soluble complex with urinary Ca. If urine has low citrate concentration SSCaOx is promoted to form CaOx stone. If urine pH is > 6.5, proportion of divalent and trivalent ions are increased then SSCaP is favorable. The levels of urinary supersaturation of the different solutes determine the specific types of stones [ 8 , 9 , 10 ].
Kidney stones tend to recur. Approximately 50% people who form one stone form another within 10 years. The risk of recurrence ranges from 30-50% at 5 years in observational studies. The control groups in recent randomized controlled trials have a 2-5% annual recurrence rate after an incident calcium oxalate stone. Recurrence rates also depend on the stone type. When nuclei of uric acid form, they lower the metastable limit (e.g. susceptibility to perturbation) and favor further stone precipitation. Decreased supersaturation of the urine filtrate will decrease the risk of recurrence of kidney stone [ 11 ].
Types of Kidney Stones
Table 1 describes the various types of kidney stones and their prevalence. Approximately 70-80% of kidney stones are composed of calcium oxalate and calcium phosphate. Of the rest, 10% are struvite, 10% of uric acid; and less than 1% are composed of cystine or are diagnosed as drug-related stones. Calcium and uric acid stones are more common in men; women have more struvite stones. Figure 1 shows the appearance of the different types of stones.

Courtesy from Dr. J. Seifter, Harvard Medical School, Renal Division Brigham and Women's Hospital, Boston
Calcium stones
Most calcium stones are composed of calcium oxalate, either by itself or much more commonly in combination with calcium phosphate or calcium urate [ 9 , 13 ]. Hypercalciuria, low urine volume and hypocitraturia all predispose to the development of calcium stones. Hypercalciuria often occurs with diseases associated with hypercalcemia like hyperparathyroidism, malignancy, sarcoidosis and vitamin D excess [ 14 , 15 ]. When no other cause is found the hypercalcuria is known as "idiopathic hypercalciuria". Idiopathic hypercalciuria is familial and is likely a polygenic trait, although there are some rare monogenic causes of hypercalciuria and kidney stones such as Dent's disease, an X-linked disorder characterized by hypercalciuria, nephrocalcinosis, and the development of renal failure. Alkaline urine is a risk factor for the development calcium phosphate stones [ 11 , 16 , 17 ]. Another risk factor for calcium oxalate stone is hyperoxaluria, which occurs due to bowel disease (enteric hyperoxaluria) and genetic disorders of oxalate metabolism (primary hyperoxaluria) [ 18 ].
Dietary oxalate may be important in stone development; spinach, beets and rhubarb in particular, contain large amounts of oxalate and they may increase urinary oxalate excretion and predispose to the development of calcium oxalate stones. High dose vitamin C therapy can also lead to increased oxalate generation as vitamin C (ascorbic acid) is metabolized. Oxalate reabsorption in the colon is reduced by the formation of insoluble calcium oxalate [ 19 , 20 , 21 , 22 , 23 , 24 ].
This is very important in planning therapy because restricting dietary calcium results in less calcium being available in the intestinal lumen to bind the oxalate. This leads to increased oxalate absorption and therefore increased urinary oxalate excretion [ 21 , 25 , 26 , 27 ]. Therefore, dietary calcium should not be restricted in malabsorption syndromes such as small bowel disease, following surgical small bowel resection, jejuno-ileal bypass surgery and inflammatory bowel disease (IBD) in which there is malabsorption of fatty acids and bile salts. Intestinal calcium binds to fatty acids, causing less binding to oxalate. Non-absorbed bile salts in the colon will also cause increased colonic permeability to oxalate. Bariatric (weight loss) surgical techniques that create a malabsorptive state are being performed more frequently today than ever before. Calcium oxalate stone formation is an increasingly common complication with the more restrictive procedures, due to the highly restrictive forms of bariatric surgery such as the Roux-en-Y gastric bypass, sleeve gastrectomies and duodental switches with biliopancreatic diversion that generate malabsorption syndromes. Diarrheal losses cause volume depletion and decreased urine volume. Bicarbonate loss in the stool can cause a metabolic acidosis which can in turn lead to a low urinary pH and hypocitraturia (due to enhanced proximal reabsorption) which will predispose to the development uric acid and calcium oxalate stone formation [ 28 , 29 ].
Uric acid stones
Pure uric acid calculi are radiolucent on plain radiographs but visible on ultrasonography or computerized tomography (CT). These stones tend to form in individuals with hyperuricosuria. Approximately 15-20% of patients with uric acid stones have a history of gout [ 30 , 31 , 32 , 33 ]. A diet rich in animal protein, because of its high purine content, which produces uric acid in its catabolism, may increase the risk of uric acid stone formation [ 32 , 34 , 35 ]. At a urinary pH of less than 5.5, uric acid is poorly soluble, but solubility increases at a pH greater than 6.5.
Cystine stones
These stones tend to form only in patients with cystinuria, an autosomal recessive disorder affecting 1 in 15,000 adults in the USA that accounts for only 1% of patients with nephrolithiasis. In cystinuria, nephrolithiasis is the only clinical manifestation and it arises as a result of abnormal renal tubule transport which in turn leads to large amounts of urinary cystine excretion. Cystinuria occurs equally in males and females, although males are more severely affected. Stones begin to form in the 1st to 4th decades of life and tend to be large, multiple and bilateral. The diagnosis can be made by finding typical hexagonal crystals in the urine [ 36 , 37 ]. Urinary tract infection and obstruction are common, as is stone recurrence every 1-4 years.
Struvite stones
Struvite stones are also called triple phosphate stones, or infection stones. They form in the presence of upper urinary tract infections with urease-producing bacteria (most commonly Proteus and Klebsiella). Normal urine is undersaturated with ammonium phosphate; struvite stone formation occurs only when ammonia production is increased and the urine pH is elevated, which decreases the solubility of phosphate. Bacterial urease is essential for the development of struvite stones because it leads to an elevation in ammonium, carbonate and urinary pH all at the same time. In this setting phosphate combines with ammonium, magnesium and carbonate to form a stone composed of magnesium ammonium phosphate (struvite) and calcium carbonate-apatite.
Urease breaks down urinary urea into ammonia and carbon dioxide:
The ammonia produced by this reaction then combines with water:
Resulting in increased availability of ammonium in an alkaline urine.
Struvite stones commonly occur in patients with recurrent urinary tract infections, especially if they have abnormal urinary tract anatomy, or require frequent bladder catheterization. The stones may also occur on infected calcium, uric acid or cystine stones, especially after instrumental procedures. Struvite stones are three times more common in women than men, presumably because urinary tract infections are more common in women. They are typically very large and may be so large as to fill the renal pelvis (forming a "Staghorn calculus"). Their growth is rapid and they often grow back after surgical removal because infected fragments of stone have been left behind [ 38 , 39 , 40 ].
Clinical Diagnosis of Kidney Stones
Non-obstructing kidney stones produce no symptoms or signs apart from hematuria. However, the kidney stone may cause severe pain, usually accompanied by nausea, vomiting and hematuria (renal colic) when it passes into the ureter. Patients may also complain of urinary frequency and urgency. These signs and symptoms lead to many emergency department visits and hospitalization. The pattern of the pain from stone depends on its location: a stone in the upper ureter leads to pain in the flank that may radiate to the upper abdomen.
When the stone is in the lower ureter, pain may radiate to the ipsilateral testicle in men or labium in women. If the stone is lodged at the ureterovesical junction, the main symptoms will be urinary frequency or urgency. Symptoms quickly improve after passing the stone. On physical examination, the patient is often in excruciating pain, and is unable to achieve a comfortable position. Ipsilateral costovertebral angle tenderness may also be present.
Laboratory tests may show a leukocytosis which may be due to a stress response or infection. Serum creatinine is often elevated if the patient is volume depleted, or if there is bilateral ureteral obstruction or unilateral obstruction in a patient with a solitary kidney. The urinalysis will have red blood cells, white blood cells and occasionally crystals. However, because of the often non-specific physical examination and laboratory findings, imaging studies are critical in making the diagnosis.
Initial evaluation includes obtaining a non-contrast helical CT, which can accurately visualize the size and location of the stones. A kidney, ureter and bladder (KUB) film, although it is insensitive to uric acid stones since they are radiolucent and therefore are not visualized. However, it can visualize calcium - containing, struvite and cystine stones in the kidney or ureter. Complete ureteral obstruction and upper urinary tract infection (UTI) are indications for stone removal by extracorporeal shock wave lithotripsy (ESWL) or surgery [ 9 , 12 , 16 , 31 , 41 ].
Medical and nutrition evaluation of kidney stones
A comprehensive history should be taken by one of the health care providers, and the following items should be covered: prior kidney stones, composition of prior stones if known, dietary history including an estimate of typical daily fluid intake, social history including details regarding occupation and lifestyle, and family history.
The medical history should focus on identifying diseases that increase stone risk including conditions that lead to hypercalciuria, gout, chronic diarrhea and malabsorptive gastrointestinal disorders.
Interpretation of biochemical and urine tests
The urine sediment should be examined for crystals. A 24-hour urine collection should be performed to measure urine calcium, oxalate, uric acid, pH, volume, creatinine and citrate. Some laboratories calculate supersaturation values for calcium oxalate, calcium phosphate and uric acid and these are particularly helpful [ 12 , 42 , 43 ].
The 24-hour urine collection
The best way to evaluate stone risk is a 24-hour urine collection and analysis [ 8 , 12 ]. Two 24-hour urine collections are recommended for the initial evaluation for an accurate analysis and to determine variability [ 44 ]. The 24-hour urine collection should be several weeks after any procedures (i.e. 6-8 weeks after lithotripsy) in order to minimize the risk of result be being influenced by infection or presence of blood due to these causes. Infection can change the pH and citrate levels. It is very important that patients continue with their usual diet and activities during the collection period. The 24-hour urine creatinine excretion can give information about the adequacy of the urine collection. In general, adult males produce 18-24 mg creatinine/kg/d and females 15-20 mg/kg/d [ 9 , 12 ]. 24-hour urine collection is not accurate as the urinary creatinine levels will be higher than normal for over collection and lower than normal for under-collection [ 44 ].
Table 2 provides a summary of the normal values for the 24-hour urine collection and likely causes of abnormal values. The 24-hour urine sample should include volume, and the solutes calcium, phosphorus, oxalate, citrate, pH, and uric acid to provide an estimate of supersaturation and the risk of stone formation. Creatinine is tested to ensure full collection and to normalize solute excretion to the more constant amount of creatinine. Dietary factors include sulfates which are mostly from animal protein and sodium since they are related to calcium, potassium, and magnesium excretion. Urea nitrogen is used to estimate protein catabolic rate (PCR). The PCR is usually indicative of dietary protein intake in an individual who is not in a catabolic state. The relationship between urinary nitrogen appearance rate and estimated dietary protein intake is then calculated. The value of the 24-hour urine is to evaluate dietary nutrients and fluid intakes and to provide guidance for the patient's management. For example, normal urinary calcium levels are <250 mg/d for men and <200 mg/d for women. High urinary calcium may be caused by idiopathic hypercalciuria, or diet high in sodium or protein. Low urinary calcium is often due to malabsorption or underlying bone disease. A normal urinary oxalate level is 20-40 mg/d. High levels are due to high oxalate diet, increased endogenous production, high vitamin C consumption and irritable bowel disease. Normal urinary citrate levels are >450 mg/d for men and >550 mg/d for women. High animal protein diets and renal tubular acidosis (RTA) can increase acid production affecting urinary pH so that it declines citrate levels.
Range: courtesy from Litolink Corp, Chicago, IL, RTA: renal tubular acidosis, PCR: protein catabolic rate, CRI: chronic renal insufficiency, UA: uric acid, Cr: creatinine.
Nutrition assessment
The dietitian's role in nephrolithiasis care is very important. The dietitian should assess nutritional risk factors by dietary intake assessments and provide therapeutic recommendations based on dietary risks. Dietary assessment is very important both in treating and preventing stone formation. The dietitian should evaluate dietary intakes of calcium, oxalates, sodium, protein (both animal and plant), dietary supplements and fluid intake since these can either promote or inhibit stone formation, and plan the therapeutic diet based on those information. Fluid intake is particularly important to quantify.
There are several dietary assessment methods: the 24-hour recall, food record diet history and food frequency questionnaire. The dietary intakes should be reflected on the urinalysis and it is good way to evaluate the causes of kidney stones and to prevent recurrence. Food records provide information on intake of foods, beverages, and dietary supplements over specific periods. The most appropriate diet assessment for kidney stones is the food record during a 24-hour urine collection, as well as 1-2 days before the collection. The food record should be analyzed to evaluate intakes of protein, sodium, potassium, calcium, phosphorus, magnesium, uric acid, oxalate and fluid. Based on the food intake and urinalysis, the clinicians can provide the adequate medical and diet treatments.
Risk Factors for Kidney Stones
Major risk factors.
Risk factors for stone formation may be hereditary or disease related, such as idiopathic hypercalciuria, hyperoxalosis Dent's disease, medullary kidney disease, polycystic kidney disease, hyperparathyroidism, irritable bowel disease (IBD), renal tubular acidosis or sarcoidosis. Patients with a family history of nephrolithiasis have a 2.5 times greater risk of stone formation [ 42 ]. Other risk factors include environment and diet ( Table 3 ).
PKD: polycystic kidney disease, DM: diabetes mellitus, GI: gastrointestinal, Ox: oxalate, UA: uric acid, CaP: calcium phosphate, CaOx: calcium oxalate
Table 4 shows the conditions that favor stone formation. Urinary crystalloids can form nucleus on the existing surface and supersaturate urine. Low urinary magnesium causes decrease complex formation with urinary oxalate allowing free oxalate to be more available in the urine. Low urinary citrate also increases stone formation because citrate forms a complex with calcium so free calcium is more available for stone formation. High concentrations of uric acids in the urine will promote the nuclei to start stone formation. If the patient is dehydrated, he or she will have low urine output and therefore the urine can be supersaturated. Urine pH is very important for the formation of some types of stones. For example, low urine pH is favorable to formation of CaOx and uric acid stones while a high urine pH promotes CaP stone formation.
Dietary causes may also generate increased risks of various stones. High sodium intake increases urinary calcium excretion. High oxalate diets, large dose of vitamin C supplements (>1,000 mg/d) will increase urinary oxalate level. High protein diets (> 2.0 g/kg/d) can increase urinary calcium, decrease urine pH and also increase urinary uric acid level [ 14 ]. Therefore high protein diets can increase CaOx, and uric acid stone risks. Diuretics, such as furosemide can induce dehydration which can increase risk of supersaturation of solutes.
Environmental conditions such as heat may increase non-renal evaporative skin losses and by doing so they reduce urine volume and increase stone risk [ 24 , 45 , 46 , 47 , 48 , 49 , 50 , 51 ]. The most challenging aspect of encouraging patients to increase their fluid intakes is that they cannot wait for the normal thirst mechanism to urge them to drink because the hypothalamic- pituitary sensors/ neurons lead to increased antidiuretic hormone levels; therefore, the urine becomes more concentrated before the thirst mechanism is triggered and the urine becomes more dilute. One patient education method used is to remind people to drink fluid after each void.
Urine volume: a critical factor
Urine must be supersaturated with solutes to form a crystal, the first step to form a stone. Low fluid intake will lead to low urine output. When urine volume is low, the urine can theoretically be easily supersaturated with various solutes, such as calcium, oxalate, phosphorus, and uric acid. However, there are several inhibitors normally present in the urine to prevent crystallization of these solutes [ 52 , 53 , 54 , 55 ]. Only if the supersaturation is very high does the crystallization start. The most direct way for patients to decrease risks of supersaturation is to increase the urine volume with oral fluids to above 2.5 L/d of urine volume [ 56 ].
Hypercalciuria
Figure 2 shows a model of how idiopathic hypercalciuria occurs. Normal urine calcium excretion is less than 200-250 mg/d. If urine calcium excretion is higher than this, stone risk increases. To evaluate the stone risk, it is necessary to measure serum calcium, urinary calcium, oxalate, urine urea nitrogen (UUN), citrate, magnesium, creatinine, and volume ( Table 5 ). In idiopathic hypercalciuria, the serum calcium level is normal but urinary calcium is high because of increased absorption of calcium from the gastrointestinal tract. The increased absorption of calcium increases the ionized calcium level, decreases parathyroid hormone (PTH) secretion and decreases renal tubular reabsorption of calcium. The increased intestinal calcium absorption can be treated with 1, 25-dihydroxy vitamin D3. There is also evidence of reduced proximal tubular reabsorption of sodium and calcium in patients with idiopathic hypercalciuria, which leads to a negative calcium balance [ 57 ]. The combination of a low sodium diet and thiazide diuretics may lower urinary calcium excretion by increasing reabsorption of calcium.

Adapted from practice guideline at department of nephrology, Harvard Vanguard Medical Associate.
Hyperoxaluria
The most common type of stones are CaOx (75%) and a high urinary excretion of oxalate is a risk factor for them. Dietary sources of oxalate include spinach, rhubarb, beets and some berries. Oxalate is also created from endogenous metabolism of glycine, hydroxyproline and ascorbic acid. A low oxalate diet is recommended for the prevention of CaOx stones; however, a recent study showed that dietary oxalate had little effect on urinary oxalate excretion although vitamin C intake was highly correlated with urinary oxalate excretion [ 58 ].
Increased urinary oxalate excretion has also been noted in patients with diabetes [ 57 , 59 ]. Patients with IBD have a high prevalence of CaOx stones with hypocalciuria due probably to their negative calcium balance. Negative calcium balance can also cause secondary hyperparathyroidism to maintain normal calcium levels in blood, and a calcium supplement may be effective for preventing CaOx stone risk by decreasing the hyperparathyroidism. The timing of calcium supplements is important and patients should take supplements at the meal time to bind oxalate from dietary sources. Individuals who have had restrictive bariatric surgery have a high risk of hyperoxaluria due to malabsorption and increased reabsorption of oxalate, which may increase hyperoxaluria [ 29 , 60 ]. Recent studies by Jiang et al. suggest that enteric colonization with Oxalobacter formigenes, which uses oxalate as a main energy source, reduces the risk of CaOx stone recurrence among individuals whose intakes of calcium were low [ 61 ]. In contrast, there is insufficient evidence to support the use of probiotics to reduce stone risk at this time [ 62 ].
Hypocitraturia
The urine is usually supersaturated with solutes, especially CaOx; however, the level tends to be less than the 10 times the level of concentration to form the CaOx crystals due to the presence of citrate in the urine. Citrate in the urine binds with urinary calcium to form a soluble compound and this increases the urine pH. CaOx stone formation is favored by a low urine pH; therefore, citrate can help prevent CaOx stone formation. The most common form of citrate prescribed is potassium citrate. The normal value of urinary citrate for males is >450 mg/d and for females >550 mg/d. Renal tubular acidosis and chronic diarrhea can also cause decreased citrate in the urine. However calcium, oxalate and urine pH should be checked before initiation of citrate treatment [ 63 ]. If urine pH increases later with citrate treatment, the risk of CaP stone formation rises. In patients who have IBD with high urinary oxalate, and low urinary sodium levels because of malabsorption and gastrointestinal loss of sodium, sodium citrate is more beneficial than potassium citrate, and it should be used. However, sodium citrate can increase urinary calcium excretion and therefore it may increase the risk of CaOx stones.
Urine pH is an important factor in the formation of kidney stones. A low urine pH can promote CaOx and uric acid stones, and a high urine pH can increase the risk of CaP stones. Urine pH is affected by the acid and alkaline ash from the diet, and before the advent of effective urinary acidifying and alkalinizing agents, it was necessary to rely on diet to alter urinary pH, although this could rarely be accomplished effectively. The practice has been largely outdated by the advent of better acidifying and alkalinizing agents. The mineral salt that predominates in foods determines whether the residue or 'ash' is acidic or alkaline. The minerals producing alkaline ash are sodium, potassium, magnesium, and calcium. Acid-forming minerals are sulfur, chlorine, and phosphorus. High animal protein diets which have high purine content and sulfur containing amino acids can reduce urine pH and will lead to an increased risk of uric acid stones. An alkaline ash diet which is high in citrate, mostly from fruits and vegetables, can increase urine pH and citrate excretion. Today alkali therapy is preferable because an alkaline ash diet is difficult to follow for most patients although an alkaline ash urine is preferable for the certain type of stone risk. However, for other stones, the reverse may be true. A high pH without alkali therapy may increase the risk of struvite stones from a UTI.
The prevalence of uric acid stones is about 5% of total kidney stone disease. The main determinant of uric acid stones is urine pH. A low urine pH has more insoluble uric acids concentration; therefore, the risk of uric acid stone is higher. Measurements of urinary calcium, uric acid, and post-prandial urine pH are used to assess the uric acid stone. The average adult consumes about 2 mg of purine/kg/d, which produces 200-300 mg of uric acid daily. Endogenous production of uric acid is about 300 mg/d. In some studies, uric acid excretion is 5.6 mg/kg/d [ 32 ] and total excretion of uric acid is less than 800 mg/d. Dietary consumption of purine varies daily among individuals. Kessler et al. conducted a cross-sessional study by using bicarbonate-rich mineral water and various types of juices on uric acid stone formation and found that black current juice decreased uric acid stone risk the most, by increasing the urine pH [ 64 , 65 ]. Ingestion of alcohol can also affect urinary uric acid excretion, and excesses should be avoided. If patients have gout, allopurinol is usually prescribed along with low purine diet to reduce blood uric acid and uricosuria [ 32 , 34 ].
Dietary risk factors
Several dietary factors can increase risk of the stone formation, including sodium, protein, potassium, calcium, magnesium and other nutrients. These constituents can be modified depending on the types of different stone risks. Foods that produce acid-ash after being metabolized in the body can affect the lowering of urinary pH whereas alkaline-ash foods can increase urinary pH. The specific diets are based on urine pH, urinary uric acid and types of stones ( Table 6 ).
Modified from reference [ 66 ].
Dietary sodium restriction alone decreases urinary calcium excretion [ 54 , 67 ]. Proximal tubular calcium reabsorption is increased on a low sodium diet (2,000-3,000 mg/d) and this in turn decreases the SSCaOx. In addition of thiazide diuretics, calcium reabsorption is enhanced and further decreases hypercalciuria. However, addition of thiazide can lead to volume depletion; although ion exchange and volume status will come to the steady state in a few days. If the patient continues to consume a high sodium diet, sodium will reach the distal nephron and increase the excretion of calcium and potassium along with citrate, resulting in a change in the urinary pH that will eventually increase the risk of stone formation. Therefore, after analyzing the 24-hour urine, stone risk is high, prescribing a low sodium diet will help avoid inappropriate thiazide use for patients with CaOx stones. Patients with IBD usually have low urinary sodium levels and low urinary citrate, and so use of sodium citrate instead of potassium is beneficial to improve fluid status from gastrointestinal losses and increase urine volume.
Potassium is abundant in most fruits and vegetables. However, if the patient has low urinary citrate and low urine pH, potassium citrate is commonly used along with such a diet to further improve hypocitruria. Monitoring 24-hour urinary excretion of potassium is important to evaluate compliance to diet and medications. Taylor et al. analyzed the 24-hour urine with the Diet Approaches to Stop Hypertension (DASH) diet and found that diets conforming more closely to DASH had decreased risk of stone formation [ 68 , 69 ]. Because high DASH score foods are high in potassium, magnesium, and phosphorus, these may increase urine pH, resulting in a decrease in SSCaOx and uric acid in the urine as well as increased urine volume and citrate [ 68 , 69 ]. If patients have chronic kidney disease and take angiotensin converting enzyme inhibitors (ACEI) as antihypertensive medications, serum potassium level should be monitored closely.
There are few markers of 24-hour urine to evaluate dietary protein intake, production and excretion. Urea nitrogen appearance (UNA) and PCR are measurements of daily protein intake that are calculated per kg body weight. In normal healthy steady state, intake of protein can be equivalent to protein catabolism; therefore, PCR determines the nitrogen balance from 24-hour urine urea concentration:
Patients who have acute, or chronic infection are usually malnourished and experience catabolism, and have more nitrogen in the urine. Therefore, this formula should not be used to evaluate protein intake. For patients without active stress of illness, the PCR can guide protein recommendations for patients to prevent further stone risks.
The ammonium (NH 4 ) level should be low in patients who are prescribed alkali therapy or who present with RTA. Monitoring citrate, an indicator of urine acidity, can identify these problems. Patients who take alkali therapy especially with low citrate levels have low urinary ammonium levels with higher pHs and therefore the risk of uric acids or CaOx can be substantially decreased.
High ammonium and sulfate are indicators of a high protein diet, especially one which is high in animal protein [ 30 , 70 ]. A high protein diet (>2.0 g/kg/d) can reduce urine pH; therefore, a moderate to low protein diet should be advised (0.8-1.4 g/kg/d). Currently most common and popular weight loss diets promote consumption of large amounts of protein but such a reducing diet is not recommended for the patients who have a history of kidney stones. This high protein diet regimen increases hypercalciuria, lowers pH of urine and increases uric acid levels, which increase kidney stone risk [ 14 ]. Massey et al. conducted a study to monitor the effect of stone risk in beef vs. plant protein and concluded that a moderate amount of protein intake of either type had the same effects in reducing CaOx stone risk [ 71 ]. The amount of protein seemed to be a more important factor in that study. Recently, an epidemiological study showed that animal protein intake was not independently associated with the incidence of nephrolithiasis among a large cohort of postmenopausal women [ 72 ]. However, the evaluation of stone risk varies by individuals and is complicated. Therefore, the recommendation of a usual protein intake remains until the scientific evidence to change this recommendation is provided.
Approximately 20% of dietary calcium is absorbed under normal conditions. There is substantial evidence that a higher calcium diet is associated with lower kidney stone formation, because the higher calcium intake will bind oxalate in the gut if it is consumed with meals thereby reducing oxalate absorption. Patients who consumed a diet with a normal calcium intake (i.e., 1,200 mg/d) plus a low animal protein intake had a 51% lower incidence of recurrent stones than patients who consumed low (400 mg/d) calcium diets [ 20 ]. Although data to date on taking calcium supplements does not show that they are theoretically effective in reducing stone risk, taking a calcium supplement with meals is beneficial because calcium can bind with the dietary oxalate and thus it is not absorbed.
Magnesium forms a complex with oxalate and decreases SSCaOx in the urine, which can reduce the risk of stone formation [ 68 , 69 ]. The DASH diet, which is high in magnesium, showed a decrease in stone risk by increasing pH and lowering SSCaOx [ 13 , 68 , 69 , 73 ]. Magnesium can also bind with oxalate in the gastrointestinal tract to reduce oxalate absorption; however, a magnesium supplement is not recommended especially patients with chronic kidney disease because magnesium is accumulated in the blood in advanced kidney disease. Decreased urinary magnesium may be a sign of malabsorption, malnutrition, small bowel disease or laxative abuse. Hypomagnesemia is not a risk factor for stone formation.
Vitamin C is metabolized to dehydroascorbic acid and then converted to oxalate which is then excreted in the urine; therefore, a high vitamin C intake can be a risk for stone formation by increasing endogenous oxalate. A recent observational study showed that consumption of more than 1,000 mg/d vitamin C was associated with a 40% higher risk of stone formation in men than in those who consumed the Dietary Reference Intake (DRI) for vitamin C [ 74 ].
Other dietary factors
Citrate consumption can increase urine pH, and also increases citrate concentration in the urine. Citrate also decreases SSCaOx due to its capacity to form a complex with calcium ions and inhibit crystallization of CaOx [ 63 ]. However, citrate may increase the risk of CaP stones. A clinical trial conducted by Koff et al. used potassium citrate and lemonade for 21 stone patients, and showed that potassium citrate increased urine pH with increased urinary citrate level but lemonade did not have an effect on urinary pH or citrate levels except for increasing urine volume [ 75 ].
Phytates are present in whole grains and legumes and they can inhibit CaOx stone formation. Some studies have shown an inverse correlation with phytate intake and the risk of kidney stone formation in women [ 74 , 76 , 77 , 78 ].
Treatment of Kidney Stones
Management in the acute setting.
Urgent surgical intervention is indicated in a patient with an obstructed, infected urinary tract, worsening renal function, intractable pain or vomiting or obstruction of a solitary or transplanted kidney. Analgesia is essential and parenteral Non-Steroid Anti-Inflammatory Drugs (NSAIDs: Ketorolac) are as effective as narcotics. NSAIDS are less likely to cause nausea, but should be avoided if the patient has impaired renal function. Pain is due to renal capsule dilatation, and so intractable pain may require decompression of the obstruction. Volume expansion with intravenous fluids is important in correcting the volume depletion that may have occurred from decreased intake and/ or vomiting and it may also increase the likelihood of stone passage by increasing urine production.
If urgent intervention is not required, the treating physician needs to decide if the stone can be passed spontaneously. The likelihood of spontaneous passage decreases as the size of the stone increases and stones >5-6 mm are not likely to pass spontaneously.
Patients who are having repeated stone attacks should be instructed to strain their urine and to submit the stone for composition analysis. Repeated imaging (plain abdominal radiography (KUB) for radiopaque stones and CT for radiolucent stones) is warranted to confirm stone passage. If follow-up imaging reveals no movement after a month, urologic intervention is generally warranted [ 79 ].
Surgical treatment
Larger and more proximal ureteral stones are less likely to pass spontaneously and usually require urologic evaluation. If the stone does not pass rapidly, the patient can be sent home with oral analgesia and instructions to return for fever or uncontrollable pain. Most urologists wait a few days before intervening unless there is a possible infection, low likelihood of spontaneous passage or unrelenting pain. Infection in the setting of obstruction is a surgical emergency and mandates emergency drainage.
Extracorporeal shock wave lithotripsy (ESWL) is a non-surgical procedure using shock waves to fragment stones into small pieces which pass spontaneously several days or weeks later. Obese patients may not be effectively treated with ESWL. Cystine stones are very hard and are often not effectively treated with ESWL ( Figure 3 ).

Flexible ureteroscopic stone removal, although invasive, is associated with a better chance of becoming stone free with a single procedure. It does have higher complication rates of ureteral injury or structure, though has become increasingly popular, because of the variety of devices that are available for stone removal including small diameter flexible ureteroscopes, ureteral access sheaths, holmium laser lithotripsy and stone baskets.
Percutaneous nephrostolithotomy is more invasive, but may be necessary for large stones or stones that cannot be removed cystoscopically. It is rare that a patient requires open ureterolithotomy or nephrolithotomy.
Medical and dietary treatment - preventive therapy
A recurrent stone former should undergo an evaluation for a treatable metabolic cause of kidney stones. This is guided by the results of the 24-hour urine collection.
Table 7 summarizes the dietary managements of kidney stones. Fluid intake is an essential component of treatment and should be adjusted so that urine output is greater than 2.5 L/day. Low urine citrate can be corrected using potassium citrate. Table 8 provides some general guidelines for treatment.
PTH: parathyroid hormone, RTA: renal tubular acidosis.
Adapted from reference [ 55 ].
National Kidney Foundation: Diet Guidelines for Kidney stone. Litolink Corp, Chicago, IL.
Treating calcium stones
Dietary sodium restriction is important as it is associated with a reduction in urine calcium excretion. Thiazide diuretics lead to increased serum calcium levels and reduced urine calcium levels and are therefore used in therapy for patients with hypercalciuria. Oxalate reabsorption in the colon is reduced by the formation of insoluble calcium oxalate. This is very important in therapy because restricting dietary calcium results in less calcium being available in the intestinal lumen to bind oxalate. This leads to increased oxalate absorption and therefore increased urinary oxalate excretion [ 25 ]. Table 9 shows the list of high oxalate foods.
Sources: references [ 80 , 81 , 82 , 83 ].
The high oxalate foods are considered to be healthy, with high fiber and nutrient dense in vitamins and minerals. Patients who have diabetes, hypertension and high blood cholesterol are often instructed to consume high oxalate foods such as fruits and vegetables. When patients develop kidney stones, they are instructed to change the diet to lower oxalate contents and therefore most patients are confused. The clinical dietitian should be able to advise the patients individualized diet to prevent kidney stone but to keep healthy diet.
Treating uric acid stones
As uric acid is more soluble in an alkaline urine, urine alkalinization is an important part of the treatment of uric acid stones. Patients should decrease their intake of animal proteins which helps decrease uric acid generation. Allopurinol, a xanthine oxidase inhibitor, is used to decrease the formation of uric acid.
Low purine diet is recommended if patient has elevated blood uric acid level [ 32 , 34 ]. A high protein diet (>2.0 g/kg) can cause a decrease in urine pH which can increase risk of uric acid stone therefore moderate amount of protein (0.8-1.4 g/kg/day) is recommended.
Treating cystine stones
There are no specific diet recommendations for cysteine stones except increasing fluid intake. Cystine solubility can be increased by alkalinization of the urine, although solubility only increases when the pH reaches 7-7.5. Thiol containing drugs like Penicillamine and Tiopronin may be given to patients who are unable to comply with increased fluid intake and urinary alkalinization or fail despite it. These drugs increase the solubility of cystine. Tiopronin is better tolerated than penicillamine which is associated with multiple side effects including rash, fever, serum sickness, epidermolysis and membranous nephropathy. Captopril is used because of its sulfhydryl group forms a thiol-cysteine disulfide bond that is more soluble than cysteine. However its efficacy is unclear. Low animal protein diet can lower the stone risk by lowering methionine which is precursor of cystine [ 84 ].
Treating Struvite stones
The preferred treatment of struvite stones is surgical removal because they are large. Antibiotic therapy is important and may slow stone growth. It is important to culture stone material to help direct antibiotic therapy. However low sodium diet can help prevention of struvite stone [ 84 ].
Kidney stones are common and recur frequently. Calcium oxalate stones are the most common. Urine supersaturation is increased with low urine volume and with increased urinary excretion of calcium, oxalate, phosphate, cysteine or uric acid. Citrate is the most common inhibitor of crystal formation and urinary pH is very important for preventing or treating different types of stones. There are many stone risk factors among which diet is very important one. Urgent stone removal and treatments are done by urology using surgery or ESWL. Medical and dietary treatments are most important ways to prevent recurrence of stone. Consumption of ample fluids is essential, and dietetic advice is helpful.
Conflict of interest: The authors have declared no conflict of interest.

Why do some people develop kidney stones
O n World Kidney Day, let's discuss an important topic that afflicts millions across the world. India accounts for 12% of the global prevalence of this health complication.
It's about kidney stones, which are very common in India and are relatively more common in North India.
Kidney stones, also known as renal stones, are solid deposits that form within the kidneys when minerals and salts in urine crystallize. These are often painful but sometimes can be silent and lead to renal failure, says Dr. Ahmed Kamaal, Senior Consultant, Urology, Amrita Hospital, Faridabad.
"Prevalence of renal stones in India is around 12% while recurrence rates of symptomatic stones are high at more than 50% within 5 years of a first episode, suggesting that identifiable high-risk group of patients may experience common pathways in the pathogenesis of stone formation that can be targeted for prevention," Dr. Kamaal says.
What causes kidney stones?
"Kidney stones have a variety of causes, and it is not always possible to pinpoint a cause in every case," says Dr. Kushal Banerjee, Senior Homeopath, Dr. Kalyan Banerjee’s Clinic.
Fault lies in what one eats
"Being overweight, lack of physical activity, not drinking adequate amounts of fluids, repeated urinary infections, taking excessive nutritional supplements, and excessive consumption of aerated beverages are some causes. Drugs like aspirin, some antacids, diuretics (drugs that remove fluid), certain antibiotics, and anti-epileptics may also cause kidney stones. A family history of kidney stones and a previous history of kidney stones also increase the risk of developing them. Certain congenital deformities of the excretory system may also increase chances of kidney stones," Dr. Banerjee adds.
He blames an unhealthy diet as another primary reason for kidney stone formation. He suggests that dietary causes of kidney stones are easily understood and are also easy to avoid. Excessive consumption of packaged food or a very high protein diet with low fiber should be avoided. If the patient has high levels of uric acid, it is important to avoid food items like spinach, aubergine, tomatoes, etc. While it is now being appreciated that a fixed quantity of fluids should not be advised for every individual it is important not to ignore thirst. Indiscriminate consumption of supplements and ‘protein shakes’ and similar concoctions can lead to the formation of kidney stones. Staying physically active, and consuming a balanced nutritious diet while avoiding junk food can keep the risk of forming kidney stones low.
Genetics, obesity, and underlying health conditions also trigger stone formation
Dr. Kamaal explains genetic links to kidney stone formation. "Genetic links to urolithiasis have been long established in certain heritable disorders, such as primary hyperoxaluria to AGXT gene, and Xanthinuria to XDH gene. Individuals with a family history of stone formation are more susceptible, suggesting a hereditary link to this condition," he explains. "Conditions such as hypercalciuria, hyperoxaluria, and hyperparathyroidism disrupt the normal balance of minerals in the body, increasing the likelihood of kidney stone development. Chronic urinary tract infections, cystic kidney diseases, and inflammatory bowel diseases can create an environment conducive to the development of kidney stones. Obese (BMI>30) people have RR (relative risk ) of 1.44 as compared to non-obese for stone formation, " he adds.
What causes recurrent kidney stone formation?
Recurrence of kidney stones occurs in many individuals despite treatment. More than 90% of the individuals who get treated for kidney stones develop another stone within 20-25 years.
Dr. Kamaal explains the reasons behind this. "Inadequate treatment can leave residual crystals, providing a foundation for the recurrence of kidney stones. Failure to make necessary lifestyle changes, such as improving dietary habits, maintaining proper hydration, and addressing metabolic abnormalities can contribute to the reformation of stones. Individuals with a family history of kidney stones may be more prone to recurrence due to inherited factors. Understanding the genetic component can aid in developing personalized preventive strategies," he explains.
"Adopting a healthy lifestyle plays a key role in preventing recurrent kidney stones. This includes maintaining a balanced diet, staying hydrated, avoiding excess salt and sugars, and engaging in regular physical activity," he suggests.
How to take care of the kidneys?
Taking care of kidneys is crucial for maintaining overall health and well-being, explains Dr Amit Langote, Head of the Nephrology Department, and Kidney Transplant, Medicover Hospitals, Navi Mumbai and shares important information on how to keep them kidneys.
- One often overlooked aspect of kidney health is the importance of staying hydrated. Adequate water intake helps to flush out toxins and waste products from the kidneys, reducing the risk of kidney stones and other complications.
- Eating a balanced diet rich in fruits, vegetables, and whole grains can provide essential nutrients that support kidney function.
- Maintaining a healthy weight is crucial for kidney health, as excess pounds can put a strain on these vital organs. For individuals with kidney disease, managing weight becomes even more essential to prevent complications and slow down the progression of the condition. Weight loss through a balanced diet and regular exercise can help reduce the risk of developing chronic kidney disease or other related conditions. Shedding extra weight can also alleviate symptoms for those already dealing with kidney issues, such as high blood pressure or diabetes. By focusing on achieving and maintaining a healthy weight, individuals can improve their overall quality of life and potentially extend the longevity of their kidneys.
- High blood pressure is a matter of concern when it comes to your kidney health. It is known to cause permanent damage to your kidneys. If your blood pressure is high, take medication, reduce salt intake, eat fruits and vegetables, and exercise regularly.
- Those with diabetes or prediabetes tend to have higher chances of suffering from kidney disease. When your blood sugar is high, your kidneys struggle to filter your blood. Too much stress on the kidney’s filtration can cause severe damage in the long run. In case you have diabetes, it’s the need of the hour to manage your blood sugar with diet and properly take medications or insulin as directed.
- Painkillers, while effective in providing relief from discomfort, can have detrimental effects on kidney health when used for long periods. One of the main concerns is that prolonged use of painkillers can lead to acute kidney injury or chronic kidney disease. This is because these medications put a strain on the kidneys by altering blood flow and damaging renal tissues over time. Furthermore, certain painkillers can cause issues such as electrolyte imbalances and reduced kidney function when used excessively. It's important to be mindful of not only the dosage but also the duration of using these medications to prevent any potential harm to your kidneys.
- Regular physical activity is another key factor in maintaining healthy kidneys. Exercise can help regulate blood pressure and improve overall circulation, which is crucial for proper kidney function.
- Avoid smoking and excessive consumption of alcohol to improve kidney health in the long run. By incorporating these simple tips into your daily routine, you can take proactive steps to safeguard your kidney health for years to come.
For more news like this visit TOI . Get all the Latest News , City News , India News , Business News , and Sports News . For Entertainment News , TV News , and Lifestyle Tips visit Etimes


IMAGES
VIDEO
COMMENTS
Kidney stone disease is common in the populations of industrialized countries. It is also considered to be a serious socio-medical problem (Pak 1998). Kidney stones are the products of a pathological biomineralization process in the urinary system (Bazin and Daudon 2012; Bazin et al. 2012) and are mostly mixtures of two or three or more components.
1. Introduction. Kidney stone disease, also known as nephrolithiasis or urolithiasis, is one of the oldest diseases known to medicine. It is estimated that 1-15% individuals suffer from kidney stone formation at some point during their lifetime, and the prevalence and incidence of kidney stone is reported to be increasing worldwide (1,2).A recent study concluded that the prevalence of kidney ...
Kidney stone disease is a crystal concretion formed usually within the kidneys. It is an increasing urological disorder of human health, affecting about 12% of the world population. It has been associated with an increased risk of end-stage renal failure. The etiology of kidney stone is multifactorial. The most common type of kidney stone is ...
Kidney stone disease (KSD) is a prevalent and usual disease, and its incidence is steadily rising worldwide. According to surveys, the prevalence of KSD in the U.S. is approximately 10% [].KSD has a high rate of recurrence, with a recurrence rate of about 50% within 5-10 years [].KSD is likely to cause a variety of complications, including pain, urinary tract obstruction, infection, and even ...
Kidney stone disease is a polygenic and multifactorial disorder with a worldwide distribution, and its incidence and prevalence are increasing. Although significant progress has been made in recent years towards identifying the specific factors that contribute to the formation of kidney stone, many …
Kidney Stone Pathophysiology, Evaluation and Management: Core Curriculum 2023. Shani Shastri, * Jiten Patel, * Kamalanathan K. Sambandam, and Eleanor D. Lederer. Kidney stone disease, also known as nephrolithiasis or urolithiasis, is a disorder in which urinary solutes precipitate to form aggregates of crystalline material in the urinary space.
Defining the kidney stone composition is important for determining a treatment plan, understanding etiology and preventing recurrence of nephrolithiasis, which is considered as a common, civilization disease and a serious worldwide medical problem. The aim of this study was to investigate the morphology and chemical composition of multicomponent kidney stones. The identification methods such ...
Figure 1. Raman spectra for common kidney stone minerals. Raman spectra were collected using powders of calcium oxalate monohydrate, calcium oxalate dihydrate, uric acid, hydroxyapatite, brushite ...
Background: Kidney stones are among the most common urological conditions affecting approximately 9% of the world population. Although some unhealthy diets and unhealthy lifestyles are reportedly risk factors for kidney stone, the association between daily sitting time and kidney stone has not been explored.
Kidney stone disease is a polygenic and multifactorial disorder with a worldwide distribution, and its incidence and prevalence are increasing. Although significant progress has been made in ...
For control and diagnosis of recurring and residual kidney stones, kidney stone analysis is critical. Meta-phylaxis requires careful stone analysis as a prerequisite. Web chemical analysis, thermogravimetry, polarization microscopy, powder X-ray diffraction (XRD), scanning electron microscopy (SEM), and spectroscopy are the current methods used ...
Stone diseases (gallstones and kidney stones) are extremely painful and often cause death. The prime aim of biomedical research in this area has been determination of factors resulting in stone formation inside the gallbladder and urinary tract. Many theories have been put forward to explain the mechanism of stone formation and their growth; however, their complete cycle of pathogenesis is ...
Results and conclusion: The study revealed a high prevalence of kidney stone is due to lo w fluid intake 72.07% (p=0.000), 49.84%. Life style modifications of smoking 36.03%, alcohol consumption ...
stone-formers with and without a family history of stones.9-11 Hypercalciuria and an excess risk of stone formation is seen in patients with Kidney stone disease: pathophysiology, investigation and medical treatment Renal colic accounts for about 1% of hos-pital admissions worldwide and is the reason for 80,000 emergency department
stones in kidney (Ebrahimi and Mariano, 2015). The compelling question is: why do physicians treat kidney stones? First of all, this disease is usually a painful condition where pain medication is a tempo-rary measure and a de nitive measure should be undertaken i.e stone removal. Moreover, pain medications are not e ective to alleviate pain.
The prevalence of kidney stone disease is increasing worldwide. The recurrence rate of urinary stones is estimated to be up to 50%. Nephrolithiasis is associated with increased risk of chronic and end stage kidney disease. Diet composition is considered to play a crucial role in urinary stone formation. There is strong evidence that an inadequate fluid intake is the major dietary risk factor ...
INTRODUCTION. Kidney stone disease (KSD) is a common clinical problem worldwide. It is basically the formation of stone in the kidney (nephrolithiasis), ureter (ureterolithiasis), or urinary bladder (cystolithiasis) by the successive physicochemical events of supersaturation, nucleation, aggregation, and finally retention [].The stone forms as a result of crystal deposition in the kidneys, and ...
The formation of kidney stone is also known as renal calculi or crystal. It is a serious though not life threatening disorder prevalent throughout the world. In medical terminology condition of having urinary calculi is termed as nephrolithiasis and urolithiasis where the root word "Lith" meaning "a stone" 1.
Kidney stones affect people of all races, cultures, and locations. Blood tests, urine tests, and scans are all utilized to diagnose this kidney stone. If the stone is not identified early on, the situation might get serious, and surgery may be required to remove the stone. Image processing is a very effective way to properly detect the stone.
The kidney stones are categorized into calcium, struvite or magnesium ammonium phosphate, uric acid or urate, cystine and other types of stones based on chemical composition and pathogenesis. Due ...
Kidney stone diseases and its occurrence is alarming in these days. Renal Calculus, also known as a kidney stone is a solid piece of material which is formed in the kidneys. Minerals in the urine are primary cause for production of renal calculus in kidneys. Kidney stones usually pass in the urine, a small stones may even pass without causing ...
Kidney stones (also called renal calculi, nephrolithiasis or urolithiasis) are hard deposits made of minerals and salts that form inside your kidneys. Diet, excess body weight, some medical conditions, and certain supplements and medications are among the many causes of kidney stones. Kidney stones can affect any part of your urinary tract ...
Purpose To develop and validate a deep learning and thresholding-based model for automatic kidney stone detection and scoring according to S.T.O.N.E. nephrolithometry. Procedures Abdominal noncontrast computed tomography (NCCT) images were retrospectively archived from February 2018 to April 2019 for three parts: a segmentation dataset (n = 167), a hydronephrosis classification dataset (n ...
Of all the body's amazing abilities, perhaps one of the strangest is its capacity to make stones. Many will have heard of kidney or gallstones, and be aware of the problems they can cause.But ...
Summary. There are four stages of passing a kidney stone: formation, moving into the ureter, reaching the bladder, and exiting the body in urine. Kidney stones can be very painful, but once the stone passes you should feel much better.
Manage blood stress: High blood pressure is the primary reason for kidney sickness. Get your blood kind checked often and paintings along with your medical doctor to hold it underneath and manage ...
Kidney stones can vary considerably in size, from less than a millimetre across to centimetres or more. They can also form unusual shapes - if the stone builds up within the branching channels ...
can help set your foundation for kidney stone prevention. After that, the below tips can aide in preventing calcium oxalate stones - the most common kidney stones. If you currently have a stone ...
The most appropriate diet assessment for kidney stones is the food record during a 24-hour urine collection, as well as 1-2 days before the collection. The food record should be analyzed to evaluate intakes of protein, sodium, potassium, calcium, phosphorus, magnesium, uric acid, oxalate and fluid.
India has a high prevalence of kidney stones. Renal stones are common and can lead to renal failure. Recurrence is frequent, but lifestyle changes, including diet and hydration, can help prevent it.