

Research & Development
At Merck Animal Health, we’re focused on innovation. It’s the lifeblood of our industry and the path to tomorrow’s breakthrough products.
Guided by our customers, we aim to offer products that are more than just effective tools for the treatment and control of animal disease. And because our customers come first, we strive to develop products that help improve the health of animals overall, and that benefit their owners as well.

We need to invest heavily in research today if we are to see scientific advances tomorrow. Our robust portfolio of veterinary medicines and vaccines reflects our unwavering commitment to research and development.
We have hundreds of ongoing projects focused on everything from combating parasites and infections to improving animal health and performance.
Our research includes the vital field of biologics, where we’re focused on using new technologies that will address a range of diseases. This includes zoonotic diseases (diseases that affect both humans and animals).
As a leading animal health company, we are committed to bringing the latest technologies to our research efforts. We are focused on advancing veterinary medicine through novel solutions in key therapeutic areas. We have an exceptional track record of discovering and developing first- and best-in-class opportunities in small molecules, vaccine technologies and biotechnologies, such as monoclonal antibodies.
One of our proudest innovations is the SPHEREON technology we developed to freeze-dry live poultry vaccines into small, highly soluble particles (spheres) that make it easier to administer in large batch sizes, among other advances.

We conduct our critical research with multiple research sites focused on both vaccine (biological) and pharmaceutical research. We also maintain research operations focused on local issues and respond quickly to changing market needs.

In today’s competitive, ever-changing business environment, no company can afford to do it alone. We achieve our ambitious goals through internal discovery efforts and co-innovation partnerships with pharmaceutical and crop science companies as well as academic institutions. Furthermore, we invest in adjacent technology opportunities through a dedicated innovation fund.
We also collaborate closely with our colleagues in Merck Human Health to share best practices and leverage the power of our combined research effort.
You are now leaving Merck Animal Health, a website maintained by Merck & Co., Inc.
Merck is known as MSD outside the United States and Canada. This link will take you to a website intended for those living outside the United States and Canada.
You are now leaving this website. By continuing, you will be directed to a site intended only for residents of the United States and Canada.
We are called MSD everywhere, except in the United States and Canada where we are known as Merck & Co Inc, Kenilworth, NJ USA.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.20(9); 2022 Sep

A guide to open science practices for animal research
Kai diederich.
German Federal Institute for Risk Assessment, German Centre for the Protection of Laboratory Animals (Bf3R), Berlin, Germany
Kathrin Schmitt
Philipp schwedhelm, bettina bert, céline heinl.
Translational biomedical research relies on animal experiments and provides the underlying proof of practice for clinical trials, which places an increased duty of care on translational researchers to derive the maximum possible output from every experiment performed. The implementation of open science practices has the potential to initiate a change in research culture that could improve the transparency and quality of translational research in general, as well as increasing the audience and scientific reach of published research. However, open science has become a buzzword in the scientific community that can often miss mark when it comes to practical implementation. In this Essay, we provide a guide to open science practices that can be applied throughout the research process, from study design, through data collection and analysis, to publication and dissemination, to help scientists improve the transparency and quality of their work. As open science practices continue to evolve, we also provide an online toolbox of resources that we will update continually.
Open science has become a buzzword in the scientific community that too often misses the practical application for individual researchers. This Essay, provides a guide to choosing the most appropriate tools to make animal research more transparent.
Introduction
Over the past decade, the quality of published scientific literature has been repeatedly called into question by the failure of large replication studies or meta-analyses to demonstrate sufficient translation from experimental research into clinical successes [ 1 – 5 ]. At the same time, the open science movement has gained more and more advocates across various research areas. By sharing all of the information collected during the research process with colleagues and with the public, scientists can improve collaborations within their field and increase the reproducibility and trustworthiness of their work [ 6 ]. Thus, the International Reproducibility Networks have called for more open research [ 7 ].
However, open science practices have not been adopted to the same degree in all research areas. In psychology, which was strongly affected by the so-called reproducibility crisis, the open science movement initiated real practical changes leading to a broad implementation of practices such as preregistration or sharing of data and material [ 8 – 10 ]. By contrast, biomedical research is still lagging behind. Open science might be of high value for research in general, but in translational biomedical research, it is an ethical obligation. It is the responsibility of the scientist to transparently share all data collected to ensure that clinical research can adequately evaluate the risks and benefits of a potential treatment. When Russell and Burch published “The Principles of Humane Experimental Technique” in 1959, scientists started to implement their 3Rs principle to answer the ethical dilemma of animal welfare in the face of scientific progress [ 11 ]. By replacing animal experiments wherever possible, reducing the number of animals to a strict minimum, and refining the procedures where animals have still to be used, this ethical dilemma was addressed. However, in recent years, whether the 3Rs principle is sufficient to fully address ethical concerns about animal experiments has been questioned [ 12 ].
Most people tolerate the use of animals for scientific purposes only under the basic assumption that the knowledge gained will advance research in crucial areas. This implies that performed experiments are reported in a way that enables peers to benefit from the collected data. However, recent studies suggest that a large proportion of animal experiments are never actually published. For example, scientists working within the European Union (EU) have to write an animal study protocol for approval by the competent authorities of the respective country before performing an animal experiment [ 13 ]. In these protocols, scientists have to describe the planned study and justify every animal required for the project. By searching for publications resulting from approved animal study protocols from 2 German University Medical Centers, Wieschowski and colleagues found that only 53% of approved protocols led to a publication after 6 years [ 14 ]. Using a similar approach, Van der Naald and colleagues determined a publication rate of 60% at the Utrecht Medical Center [ 15 ]. In a follow-up survey, the respective researchers named so-called “negative” or null-hypothesis results as the main cause for not publishing outcomes [ 15 ]. The current scientific system is shaped by publishers, funders, and institutions and motivates scientists to publish novel, surprising, and positive results, revealing one of the many structural problems that the numerous efforts towards open science initiatives are targeting. Non-publication not only strongly contradicts ethical values, but also it compromises the quality of published literature by leading to overestimation of effect sizes [ 16 , 17 ]. Furthermore, publications of animal studies too often show poor reporting that strongly impairs the reproducibility, validity, and usefulness of the results [ 18 ]. Unfortunately, the idea that negative or equivocal findings can also contribute to the gain of scientific knowledge is frequently neglected.
So far, the scientific community using animals has shown limited resonance to the open science movement. Due to the strong controversy surrounding animal experiments, scientists have been reluctant to share information on the topic. Additionally, translational research is highly competitive and researchers tend to be secretive about their ideas until they are ready for publication or patent [ 19 , 20 ]. However, this missing openness could also point to a lack of knowledge and training on the many open science options that are available and suitable for animal research. Researchers have to be convinced of the benefits of open science practices, not only for science in general, but also for the individual researcher and each single animal. Yet, the key players in the research system are already starting to value open science practices. An increasing number of journals request open sharing of data, funders pay for open access publications and institutions consider open science practices in hiring decisions. Open science practices can improve the quality of work by enabling valuable scientific input from peers at the early stages of research projects. Furthermore, the extended communication that open science practices offer can draw attention to research and help to expand networks of collaborators and lead to new project opportunities or follow-up positions. Thus, open science practices can be a driver for careers in academia, particularly those of early career researchers.
Beyond these personal benefits, improving transparency in translational biomedical research can boost scientific progress in general. By bringing to light all the recorded research outputs that until now have remained hidden, the publication bias and the overestimation of effect sizes can be reduced [ 17 ]. Large-scale sharing of data can help to synthesize research outputs in preclinical research that will enable better decision-making for clinical research. Disclosing the whole research process will help to uncover systematic problems and support scientists in thoroughly planning their studies. In the long run, we predict that the implementation of open science practices will lead to the use of fewer animals in unintentionally repeated experiments that previously showed unreported negative results or in the establishment of methods by avoiding experimental dead ends that are often not published. More collaborations and sharing of materials and methods can further reduce the number of animal experiments used for the implementation of new techniques.
Open science can and should be implemented at each step of the research process ( Fig 1 ). A vast number of tools are already provided that were either directly conceptualized for animal research or can be adapted easily. In this Essay, we provide an overview of open science tools that improve transparency, reliability, and animal welfare in translational in vivo biomedical research by supporting scientists to clearly communicate their research and by supporting collaborative working. Table 1 lists the most prominent open science tools we discuss, together with their respective links. We have structured this Essay to guide you through which tools can be used at each stage of the research process, from planning and conducting experiments, through to analyzing data and communicating the results. However, many of these tools can be used at many different steps. Table 1 has been deposited on Zenodo and will be updated continuously [ 21 ].

Application of open science practices at each step of the research process can maximize the impact of performed animal experiments. The implementation of these practices will lead to less time pressure at the end of a project. Due to the connection of most of these open science practices, spending more time in the planning phase and during the conduction of experiments will save time during the data analysis and publication of the study. Indeed, consulting reporting guidelines early on, preregistering a statistical plan, and writing down crucial experimental details in an electronic lab notebook, will strongly accelerate the writing of a manuscript. If protocols or even electronic lab notebooks were made public, just citing these would simplify the writing of publications. Similarly, if a data management plan is well designed before starting data collection, analyzing, and depositing data in a public repository, as is increasingly required, will be fast. NTS, non-technical summary.
A copy of this table has been deposited at Zenodo and will be updated continuously 10.5281/zenodo.6497559 .
DOAJ, Directory of Open Access Journals; DOI, digital object identifier; EDA, Experimental Design Assistant; MGI, Mouse Genome Informatics; RRID, Research Resource Identifier.
Planning the study
Transparent practices can be adopted at every stage of the research process. However, to ensure full effectivity, it is highly recommended to engage in detailed planning before the start of the experiment. This can prevent valuable time from being lost at the end of the study due to careless decisions being made at the beginning. Clarifying data management at the start of a project can help avoiding filing chaos that can be very time consuming to untangle. Keeping clear track of a project and study design will also help if new colleagues are included later on in the project or if entire project parts are handed over. In addition, all texts written on the rationale and hypothesis of the study or method descriptions, or design schemes created during the planning phase can be used in the final publications ( Fig 1 ). Similarly, information required for preregistration of animal studies or for reporting according to the ARRIVE guidelines are an extension of the details required for ethical approval [ 22 , 23 ]. Thus, the time burden within the planning phase is often overestimated. Furthermore, the thorough planning of experiments can avoid the unnecessary use of animals by preventing wrong avenues from being pursued.
Implementing open scientific practices at the beginning of a project does not mean that the idea and study plan must be shared immediately, but rather is critical for making the entire workflow transparent at the end of the project. However, optional early sharing of information can enable peers to give feedback on the study plan. Studies potentially benefit more from this a priori input than they would from the classical a posteriori peer-review process.
Most people perceive guidelines as advice that instructs on how to do something. However, it is sometimes useful to consider the term in its original meaning; “the line that guides us”. In this sense, following guidelines is not simply fulfilling a duty, but is a process that can help to design a sound research study and, as such, guidelines should be consulted at the planning stage of a project. The PREPARE guidelines are a list of important points that should be thought-out before starting a study involving animal experiments in order to reduce the waste of animals, promote alternatives, and increase the reproducibility of research and testing [ 24 ]. The PREPARE checklist helps to thoroughly plan a study and focuses on improving the communication and collaboration between all involved participants of the study (i.e., animal caretakers and scientists). Indeed, open science begins with the communication within a research facility. It is currently available in 33 languages and the responsible team from Norecopa, Norway’s 3R-center, takes requests for translations into further languages.
The UK Reproducibility Network has also published several guiding documents (primers) on important topics for open and reproducible science. These address issues such as data sharing [ 25 ], open access [ 26 ], open code and software [ 27 ], and preprints [ 28 ], as well as preregistration and registered reports [ 27 ]. Consultation of these primers is not only helpful in the relevant phases of the experiment but is also encouraged in the planning phase.
Although the ARRIVE guidelines are primarily a reporting guideline specifically designed for preparing a publication containing animal data, they can also support researchers when planning their experiments [ 22 , 23 ]. Going through the ARRIVE website, researchers will find tools and explanations that can support them in planning their experiments [ 29 ]. Consulting the ARRIVE checklist at the beginning of a project can help in deciding what details need to be documented during conduction of the experiments. This is particularly advisable, given that compliance to ARRIVE is still poor [ 18 ].
Experimental design
To maximize the validity of performed experiments and the knowledge gained, designing the study well is crucial. It is important that the chosen animal species reflects the investigated disease well and that basic characteristics of the animal, such as sex or age, are considered carefully [ 30 ]. The Canadian Institutes of Health Research provides a collection of resources on the integration of sex and gender in biomedical research with animals, including tips and tools for researchers and reviewers [ 31 ]. Additionally, it is advisable to avoid unnecessary standardization of biological and environmental factors that can reduce the external validity of results [ 32 ]. Meticulous statistical planning can further optimize the use of animals. Free to use online tools for calculating sample sizes such as G*Power or the inVivo software package for R can further support animal researchers in designing their statistical plan [ 33 , 34 ]. Randomization for the allocation of groups can be supported with specific tools for scientists like Research Randomizer, but also by simple online random number generators [ 35 ]. Furthermore, it might be advisable when designing the study to incorporate pathological analyses into the experimental plan. Optimal planning of tissue collection, performance of pathological procedures according to accepted best practices, and use of optimal pathological analysis and reporting methods can add some extra knowledge that would otherwise be lost. This can improve the reproducibility and quality of translational biomedicine, especially, but not exclusively, in animal studies with morphological endpoints. In all animal studies, unexpected deaths in experimental animals can occur and be the cause of lost data or missed opportunities to identify health problems [ 36 , 37 ].
To support researchers in designing their animal research, the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) has also developed the Experimental Design Assistant (EDA) [ 38 , 39 ]. This online tool helps researchers to better structure in vivo research by creating detailed schemes of the study design. It provides feedback on the entered design, drawing researcher’s attention to crucial decisions in the project. The resulting schemes can be used to transparently share the study design by uploading it into a study preregistration, enclosing it in a grant application, or submitting it with a final manuscript. The EDA can be used for different study designs in diverse scenarios and helps to communicate researcher plans to others [ 40 ]. The EDA might be particularly of interest to clarify very complex study designs involving multiple experimental groups. Working with the EDA might appear rather complex in the beginning, but the NC3R provides regular webinars that can help to answer any questions that arise.
Preregistration
Preregistration is an effective tool to improve the quality and transparency of research. To preregister their work, scientists must determine crucial details of the study before starting any experiment. Changes occurring during a study can be outlined at the end. A preregistered study plan should include at least the hypothesis and determine all the parameters that are known in advance. A description of the planned study design and statistical analysis will enable reviewers and peers to better retrace the workflow. It can prevent the intentional use of the flexibility of analysis to reach p -values under a certain significance level (e.g., p-hacking or HARKing (Hypothesizing After Results are Known)). With preregistration, scientists can also claim their idea at an early stage of their research with a citable individual identifier that labels the idea as their own. Some open preregistration platforms also provide a digital object identifier (DOI), which makes the registered study citable. Three public registries actively encourage the preregistration of animal studies conducted around the world: OSF registry, preclinicaltrials.eu, and animalstudyregistry.org [ 41 – 45 ]. Scientists can choose the registry according to their needs. Preregistering a study in a public registry supports scientists in planning their study and later to critically reevaluate their own work and assess its limitations and potentials.
As an alternative to public registries, researchers can also submit their study plan to one of hundreds of journals already publishing registered reports, including many journals open to animal research [ 8 ]. A submitted registered report passes 2 steps of peer review. In the first step, reviewers comment on the idea and the study design. After an “in-principle-acceptance,” researchers can conduct their study as planned. If the authors conduct the experiments as described in the accepted study protocol, the journal will publish the final study regardless of the outcome. This might be an attractive option, especially for early career researchers, as a manuscript is published at the beginning of a project with the guarantee of a future final publication.
The benefits of preregistration can already be observed in clinical research, where registration has been mandatory for most trials for more than 20 years. Preregistration in clinical research has helped to make known what has been tested and not just what worked and was published, and the implementation of trial registration has strongly reduced the number of publications reporting significant treatment effects [ 46 ]. In animal research, with its unrealistically high percentage of positive results, preregistration seems to be particularly worthwhile.
Research data management
To get the most out of performed animal experiments, effective sharing of data at the end of the study is essential. Sharing research data optimally is complex and needs to be prepared in advance. Thus, data management can be seen as one part of planning a study thoroughly. Many funders have recognized the value of the original research data and request a data management plan from applicants in advance [ 25 , 47 ]. Various freely available tools such as DMPTool or DMPonline already exist to design a research data management plan that complies to the requirements of different funders [ 48 , 49 ]. The data management plan defines the types of data collected and describes the handling and names responsible persons throughout the data lifecycle. This includes collecting the data, analyzing, archiving, and sharing it. Finally, a data management plan enables long-term access and the possibility for reuse by peers. Developing such a plan, whether it is required by funders or not, will later simplify the application of the FAIR data principle (see section on the FAIR data principle). The Longwood Medical Area Research Data Management Working Group from the Harvard Medical School developed a checklist to assist researchers in optimally managing their data throughout the data lifecycle [ 50 ]. Similarly, the Joint Information Systems Committee (JISC) provides a great research data management toolkit including a checklist for researchers planning their project [ 51 ]. Consulting this checklist in the planning phase of a project can prevent common errors in research data management.
Non-technical project summary
One instrument specifically conceived to create transparency on animal research for the general public is the so-called non-technical project summary (NTS). All animal protocols approved within the EU must be accompanied by these comprehensible summaries. NTSs are intended to inform the public about ongoing animal experiments. They are anonymous and include information on the objectives and potential benefits of the project, the expected harm, the number of animals, the species, and a statement of compliance with the requirements of the 3Rs principle. However, beyond simply informing the public, NTSs can also be used for meta-research to help identify new research areas with an increased need for new 3R technologies [ 52 , 53 ]. NTSs become an excellent tool to appropriately communicate the scientific value of the approved protocol and for meta-scientists to generate added value by systematically analyzing theses summaries if they fulfill a minimum quality threshold [ 54 , 55 ]. In 2021, the EU launched the ALURES platform ( Table 1 ), where NTSs from all member states are published together, opening the opportunities for EU-wide meta-research. NTSs are, in contrast to other open science practices, mandatory in the EU. However, instead of thinking of them as an annoying duty, it might be worth thoroughly drafting the NTS to support the goals of more transparency towards the public, enabling an open dialogue and reducing extreme opinions.
Conducting the experiments
Once the experiments begin, documentation of all necessary details is essential to ensure the transparency of the workflow. This includes methodological details that are crucial for replicating experiments, but also failed attempts that could help peers to avoid experiments that do not work in the future. All information should be stored in such a way that it can be found easily and shared later. In this area, many new tools have emerged in recent years ( Table 1 ). These tools will not only make research transparent for colleagues, but also help to keep track of one’s own research and improve internal collaboration.
Electronic laboratory notebooks
Electronic laboratory notebooks (ELNs) are an important pillar of research data management and open science. ELNs facilitate the structured and harmonized documentation of the data generation workflow, ensure data integrity, and keep track of all modifications made to the original data based on an audit trail option. Moreover, ELNs simplify the sharing of data and support collaborations within and outside the research group. Methodological details and research data become searchable and traceable. There is an extensive amount of literature providing advice on the selection and the implementation process of an ELN depending on the specific needs and research area and its discussion would be beyond the scope of this Essay [ 56 – 58 ]. Some ELNs are connected to a laboratory information management system (LIMS) that provides an animal module supporting the tracking of animal details [ 59 ]. But as research involving animals is highly heterogeneous, this might not be the only decision point and we cannot recommend a specific ELN that is suitable for all animal research.
ELNs are already established in the pharmaceutical industry and their use is on the rise among academics as well. However, due to concerns around costs for licenses, data security, and loss of flexibility, many research institutions still fear the expenses that the introduction of such a system would incur [ 56 ]. Nevertheless, an increasing number of academic institutions are implementing ELNs and appreciating the associated benefits [ 60 ]. If your institution already has an ELN, it might be easiest to just use the option available in the research environment. If not, the Harvard Medical School provides an extensive and updated overview of various features of different ELNs that can support scientists in choosing the appropriate one for their research [ 61 ]. There are many commercial ELN products, which may be preferred when the administrative workload should be outsourced to a large extent. However, open-source products such as eLabFTW or open BIS provide a greater opportunity for customization to meet specific needs of individual research institutions [ 62 – 64 ]. A huge number of options are available depending on the resources and the features required. Some scientists might prefer generic note taking tools such as Evernote or just a simple Word document that offers infinite flexibility, but specific ELNs can further support good record keeping practice by providing immutability, automated backups, standardized methods, and protocols to follow. Clearly defining the specific requirements expected might help to choose an adequate system that would improve the quality of the record compared to classical paper laboratory notebooks.
Sharing protocols
Adequate sharing of methods in translational biomedical sciences is key to reproducibility. Several repositories exist that simplify the publication and exchange of protocols. Writing down methods at the end of the project bears the risk that crucial details might be missing [ 65 ]. On protocols.io, scientists can note all methodological details of a procedure, complete them with uploaded documents, and keep them for personal use or share them with collaborators [ 66 ]. Authors can also decide at any point in time to make their protocol public. Protocols published on protocols.io receive a DOI and become citable; they can be commented on by peers and adapted according to the needs of the individual researcher. Protocol.io files from established protocols can also be submitted together with some context and sample datasets to PLOS ONE , where it can be peer-reviewed and potentially published [ 67 , 68 ]. Depending on the affiliation of the researchers to academia or industry and on an internal or public sharing of files, protocols.io can be free of charge or come with costs. Other journals also encourage their authors to deposit their protocols in a freely accessible repository, such as protocol exchange from Nature portfolio [ 69 ]. Another option might be to separately submit a protocol that was validated by its use in an already published research article to an online and peer-reviewed journal specific for research protocols, such as Bio-Protocol. A multitude of journals, including eLife and Science already collaborate with Bio-Protocol and recommend authors to publish the method in Bio-Protocol [ 70 ]. Bio-Protocol has no submission fees and is freely available to all readers. Both protocols.io and Bio-Protocol allow the illustration of complex scientific methods by uploading videos to published protocols. In addition, protocols can be deposited in a general research repository such as the Open Science Framework (OSF repository) and referenced in appropriate publications.
Sharing critical incidents
Sharing critical or even adverse events that occur in the context of animal experimentation can help other scientists to avoid committing the same mistakes. The system of sharing critical incidents is already established in clinical practice and helps to improve medical care [ 71 , 72 ]. The online platform critical incident reporting system in laboratory animal science (CIRS-LAS) represents the first preclinical equivalent to these clinical systems [ 73 ]. With this web-based tool, critical incidents in animal research can be reported anonymously without registration. An expert panel helps to analyze the incident to encourage an open dialogue. Critical incident reporting is still very marginal in animal research and performed procedures are very variable. These factors make a systemic analysis and a targeted search of incidence difficult. However, it may be of special interest for methods that are broadly used in animal research such as anesthesia. Indeed, a broad feed of this system with data on errors occurring in standard procedures today could help avoid critical incidences in the future and refine animal experiments.
Sharing animals, organs, and tissue
When we think about open science, sharing results and data are often in focus. However, sharing material is also part of a collaborative and open research culture that could help to greatly reduce the number of experimental animals used. When an animal is killed to obtain specific tissue or organs, the remainder is mostly discarded. This may constitute a wasteful practice, as surplus tissue can be used by other researchers for different analyses. More animals are currently killed as surplus than are used in experiments, demonstrating the potential for sharing these animals [ 74 , 75 ].
Sharing information on generated surplus is therefore not only economical, but also an effective way to reduce the number of animals used for scientific purposes. The open-source software Anishare is a straightforward way for breeders of genetically modified lines to promote their surplus offspring or organs within an institution [ 76 ]. The database AniMatch ( Table 1 ) connects scientists within Europe who are offering tissue or organs with scientists seeking this material. Scientists already sharing animal organs can support this process by describing it in publications and making peers aware of this possibility [ 77 ]. Specialized research communities also allow sharing of animal tissue or animal-derived products worldwide that are typically used in these fields on a collaborative basis via the SEARCH-framework [ 78 , 79 ]. Depositing transgenic mice lines into one of several repositories for mouse strains can help to further minimize efforts in producing new transgenic lines and most importantly reduce the number of surplus animals by supporting the cryoconservation of mouse lines. The International Mouse Strain Resource (IMSR) can be used to help find an adequate repository or to help scientists seeking a specific transgenic line find a match [ 80 ].
Analyzing the data
Animal researchers have to handle increasingly complex data. Imaging, electrophysiological recording, or automated behavioral tracking, for example, produce huge datasets. Data can be shared as raw numerical output but also as images, videos, sounds, or other forms from which raw numerical data can be generated. As the heterogeneity and the complexity of research data increases, infinite possibilities for analysis emerge. Transparently reporting how the data were processed will enable peers to better interpret reported results. To get the most out of performed animal experiments, it is crucial to allow other scientists to replicate the analysis and adapt it to their research questions. It is therefore highly recommended to use formats and tools during the analysis that allow a straightforward exchange of code and data later on.
Transparent coding
The use of non-transparent analysis codes have led to a lack of reproducibility of results [ 81 ]. Sharing code is essential for complex analysis and enables other researchers to reproduce results and perform follow-up studies, and citable code gives credit for the development of new algorithms ( Table 1 ). Jupyter Notebooks are a convenient way to share data science pipelines that may use a variety of coding languages, including like Python, R or Matlab, and also share the results of analyses in the form of tables, diagrams, images, and videos. Notebooks contain source code and can be published or collaboratively shared on platforms like GitHub or GitLab, where version control of source code is implemented. The data-archiving tool Zenodo can be used to archive a repository on GitHub and create a DOI for the archive. Thereby contents become citable. Using free and open-source programming language like R or Python will increase the number of potential researchers that can work with the published code. Best practice for research software is to publish the source code with a license that allows modification and redistribution.
Choice of data visualization
Choosing the right format for the visualization of data can increase its accessibility to a broad scientific audience and enable peers to better judge the validity of the results. Studies based on animal research often work with very small sample sizes. Visualizing these data in histograms may lead to an overestimation of the outcomes. Choosing the right dot plots that makes all recorded points visible and at the same time focusses on the summary instead of the individual points can further improve the intuitive understanding of a result. If the sample size is too low, it might not be meaningful to visualize error bars. A variety of freely available tools already exists that can support scientists in creating the most appropriate graphs for their data [ 82 ]. In particular, when representing microscopy results or heat maps, it should be kept in mind that a large part of the population cannot perceive the classical red and green representation [ 83 ]. Opting for the color-blind safe color maps and checking images with free tools such as color oracle ( Table 1 ) can increase the accessibility of graphs. Multiple journals have already addressed flaws in data visualization and have introduced new policies that will accelerate the uptake of transparent representation of results.
Publication of all study outcomes
Open science practices have received much attention in the past few years when it comes to publication of the results. However, it is important to emphasize that although open science tools have their greatest impact at the end of the project, good study preparation and sharing of the study plan and data early on can greatly increase the transparency at the end.
The FAIR data principle
To maximize the impact and outcome of a study, and to make the best long-term use of data generated through animal experiments, researchers should publish all data collected during their research according to the FAIR data principle. That means the data should be findable, accessible, interoperable, and reusable. The FAIR principle is thus an extension of open access publishing. Data should not only be published without paywalls or other access restrictions, but also in such a manner that they can be reused and further processed by others. For this, legal as well as technical requirements must be met by the data. To achieve this, the GoFAIR initiative has developed a set of principles that should be taken into account as early as at the data collection stage [ 49 , 84 ]. In addition to extensively described and machine-readable metadata, these principles include, for example, the application of globally persistent identifiers, the use of open file formats, and standardized communication protocols to ensure that humans and machines can easily download the data. A well-chosen repository to upload the data is then just the final step to publish FAIR data.
FAIR data can strongly increase the knowledge gained from performed animal experiments. Thus, the same data can be analyzed by different researchers and could be combined to obtain larger sample sizes, as already occurs in the neuroimaging community, which works with comparable datasets [ 85 ]. Furthermore, the sharing of data enables other researchers to analyze published datasets and estimate measurement reliabilities to optimize their own data collection [ 86 , 87 ]. This will help to improve the translation from animal research into clinics and simultaneously reduce the number of animal experiment in future.
Reporting guidelines
In preclinical research, the ARRIVE guidelines are the current state of the art when it comes to reporting data based on animal experiments [ 22 , 23 ]. The ARRIVE guidelines have been endorsed by more than 1,000 journals who ask that scientists comply with them when reporting their outcomes. Since the ARRIVE guidelines have not had the expected impact on the transparency of reporting in animal research publications, a more rigorous update has been developed to facilitate their application in practice (ARRIVE 2.0 [ 23 ]). We believe that the ARRIVE guidelines can be more effective if they are implemented at a very early stage of the project (see section on guidelines). Some more specialized reporting guidelines have also emerged for individual research fields that rely on animal studies, such as endodontology [ 88 ]. The equator network collects all guidelines and makes them easily findable with their search tool on their website ( Table 1 ). MERIDIAN also offers a 1-stop shop for all reporting guidelines involving the use of animals across all research sectors [ 89 ]. It is thus worth checking for new reporting guidelines before preparing a manuscript to maximize the transparency of described experiments.
Identifiers
Persistent identifiers for published work, authors, or resources are key for making public data findable by search engines and are thus a prerequisite for compliance to FAIR data principles. The most common identifier for publications will be a DOI, which makes the work citable. A DOI is a globally unique string assigned by the International DOI Foundation to identify content permanently and provide a persistent link to its location on the Internet. An ORCID ID is used as a personal persistent identifier and is recommendable to unmistakably identify an author ( Table 1 ). This will avoid confusions between authors with the same name or in the case of name changes or changes of affiliation. Research Resource Identifiers (RRID) are unique ID numbers that help to transparently report research resources. RRID also apply to animals to clearly identify the species used. RRID help avoid confusion between different names or changing names of genetic lines and, importantly, make them machine findable. The RRID Portal helps scientists find a specific RRID or create one if necessary ( Table 1 ). In the context of genetically altered animal lines, correct naming is key. The Mouse Genome Informatics (MGI) Database is the authoritative source of official names for mouse genes, alleles, and strains ([ 90 ]).
Preprint publication
Preprints have undergone unprecedented success, particularly during the height of the Coronavirus Disease 2019 (COVID-19) pandemic when the need for rapid dissemination of scientific knowledge was critical. The publication process for scientific manuscripts in peer-reviewed journals usually requires a considerable amount of time, ranging from a few months to several years, mainly due to the lengthy review process and inefficient editorial procedures [ 91 , 92 ]. Preprints typically precede formal publication in scientific journals and, thus, do not go through a peer review process, thus, facilitating the prompt open dissemination of important scientific findings within the scientific community. However, submitted papers are usually screened and checked for plagiarism. Preprints are assigned a DOI so they can be cited. Once a preprint is published in a journal, its status is automatically updated on the preprint server. The preprint is linked to the publication via CrossRef and mentioned accordingly on the website of the respective preprint platform.
After initial skepticism, most publishers now allow papers to be posted on preprint servers prior to submission. An increasing number of journals even allow direct submission of a preprint to their peer review process. The US National Institutes of Health and the Wellcome Trust, among other funders, also encourage prepublication and permit researchers to cite preprints in their grant applications. There are now numerous preprint repositories for different scientific disciplines. BioASAP provides a searchable database for preprint servers that can help in identifying the one that best matches an individual’s needs [ 93 ]. The most popular repository for animal research is bioRxiv, which is hosted by the Cold Spring Harbor Laboratory ( Table 1 ).
The early exchange of scientific results is particularly important for animal research. This acceleration of the publication process can help other scientists to adapt their research or could even prevent animal experiments if other scientists become aware that an experiment has already been done before starting their own. In addition, preprints can help to increase the visibility of research. Journal articles that have a corresponding preprint publication have higher citation and Altmetric counts than articles without preprint [ 94 ]. In addition, the publication of preprints can help to combat publication bias, which represents a major problem in animal research [ 16 ]. Since journals and readers prioritize cutting-edge studies with positive results over inconclusive or negative results, researchers are reluctant to invest time and money in a manuscript that is unlikely to be accepted in a high-impact journal.
In addition to the option of publishing as preprint, other alternative publication formats have recently been introduced to facilitate the publication of research results that are hard to publish in traditional peer-reviewed journals. These include micro publications, data repositories, data journals, publication platforms, and journals that focus on negative or inconclusive results. The tool fiddle can support scientists in choosing the right publication format [ 95 , 96 ].
Open access publication
Publishing open access is one of the most established open science strategies. In contrast to the FAIR data principle, the term open access publication refers usually to the publication of a manuscript on a platform that is accessible free of charge—in translational biomedical research, this is mostly in the form of a scientific journal article. Originally, publications accessible free of charge were the answer to the paywalls established by renowned publishing houses, which led to social inequalities within and outside the research system. In translational biomedical research, the ethical aspect of urgently needed transparency is another argument in favor of open access publication, as these studies will not only be findable, but also internationally readable.
There are different ways of open access publishing; the 2 main routes are gold open access and green open access. Numerous journals offer now gold open access. It refers to the immediate and fully accessible publication of an article. The Directory of Open Access Journals (DOAJ) provides a complete and updated list for high-quality, open access, and peer-reviewed journals [ 97 ]. Charité–Universitätsmedizin Berlin offers a specific tool for biomedical open access journals that supports animal researchers to choose an appropriate journal [ 49 ]. In addition, the Sherpa Romeo platform is a straightforward way to identify publisher open access policies on a journal-by-journal basis, including information on preprints, but also on licensing of articles [ 51 ]. Hybrid open access refers to openly accessible articles in otherwise paywalled journals. By contrast, green open access refers to the publication of a manuscript or article in a repository that is mostly operated by institutions and/or universities. The publication can be exclusively on the repository or in combination with a publisher. In the quality-assured, global Directory of Open Access Repositories (openDOAR), scientists can find thousands of indexed open access repositories [ 49 ]. The publisher often sets an embargo during which the authors cannot make the publication available in the repository, which can restrict the combined model. It is worth mentioning that gold open access is usually more expensive for the authors, as they have to pay an article processing charge. However, the article’s outreach is usually much higher than the outreach of an article in a repository or available exclusively as subscription content [ 98 ]. Diamond open access refers to publications and publication platforms that can be read free of charge by anyone interested and for which no costs are incurred by the authors either. It is the simplest and fairest form of open access for all parties involved, as no one is prevented from participating in scientific discourse by payment barriers. For now, it is not as widespread as the other forms because publishers have to find alternative sources of revenue to cover their costs.
As social media and the researcher’s individual public outreach are becoming increasingly important, it should be remembered that the accessibility of a publication should not be confused with the licensing under which the publication is made available. In order to be able to share and reuse one’s own work in the future, we recommend looking for journals that allow publications under the Creative Commons licenses CC BY or CC BY-NC. This also allows the immediate combination of gold and green open access.
Creative commons licenses
Attributing Creative Commons (CC) licenses to scientific content can make research broadly available and clearly specifies the terms and conditions under which people can reuse and redistribute the intellectual property, namely publications and data, while giving the credit to whom it deserves [ 49 ]. As the laws on copyright vary from country to country and law texts are difficult to understand for outsiders, the CC licenses are designed to be easily understandable and are available in 41 languages. This way, users can easily avoid accidental misuse. The CC initiative developed a tool that enables researchers to find the license that best fits their interests [ 49 ]. Since the licenses are based on a modular concept ranging from relatively unrestricted licenses (CC BY, free to use, credit must be given) to more restricted licenses (CC BY-NC-ND, only free to share for non-commercial purposes, credit must be given), one can find an appropriate license even for the most sensitive content. Publishing under an open CC license will not only make the publication easy to access but can also help to increase its reach. It can stimulate other researchers and the interested public to share this article within their network and to make the best future use of it. Bear in mind that datasets published independently from an article may receive a different CC license. In terms of intellectual property, data are not protected in the same way as articles, which is why the CC initiative in the United Kingdom recommends publishing them under a CC0 (“no rights reserved”) license or the Public Domain Mark. This gives everybody the right to use the data freely. In an animal ethics sense, this is especially important in order to get the most out of data derived from animal experiments.
Data and code repositories
Sharing research data is essential to ensure reproducibility and to facilitate scientific progress. This is particularly true in animal research and the scientific community increasingly recognizes the value of sharing research data. However, even though there is increasing support for the sharing of data, researchers still perceive barriers when it comes to doing so in practice [ 99 – 101 ]. Many universities and research institutions have established research data repositories that provide continuous access to datasets in a trusted environment. Many of these data repositories are tied to specific research areas, geographic regions, or scientific institutions. Due to the growing number and overall heterogeneity of these repositories, it can be difficult for researchers, funding agencies, publishers, and academic institutions to identify appropriate repositories for storing and searching research data.
Recently, several web-based tools have been developed to help in the selection of a suitable repository. One example is Re3data, a global registry of research data repositories that includes repositories from various scientific disciplines. The extensive database can be searched by country, content (e.g., raw data, source code), and scientific discipline [ 49 ]. A similar tool to help find a data archive specific to the field is FAIRsharing, based at Oxford University [ 102 ]. If there is no appropriate subject-specific data repository or one seems unsuitable for the data, there are general data repositories, such as Open Science Framework, figshare, Dryad, or Zenodo. To ensure that data stored in a repository can be found, a DOI is assigned to the data. Choosing the right license for the deposited code and data ensures that authors get credit for their work.
Publication and connection of all outcomes
If scientists have used all available open science tools during the research process, then publishing and linking all outcomes represents the well-deserved harvest ( Fig 2 ). At the end of a research process, researchers will not just have 1 publication in a journal. Instead, they might have a preregistration, a preprint, a publication in a journal, a dataset, and a protocol. Connecting these outcomes in a way that enables other scientists to better assess the results that link these publications will be key. There are many examples of good open science practices in laboratory animal science, but we want to highlight one of them to show how this could be achieved. Blenkuš and colleagues investigated how mild stress-induced hyperthermia can be assessed non-invasively by thermography in mice [ 103 ]. The study was preregistered with animalstudyregistry.org , which is referred to in their publication [ 104 ]. A deviation from the originally preregistered hypothesis was explained in the manuscript and the supplementary material was uploaded to figshare [ 105 ].

Application of open science practices can increase the reproducibility and visibility of a research project at the same time. By publishing different research outputs with more detailed information than can be included in a journal article, researchers enable peers to replicate their work. Reporting according to guidelines and using transparent visualization will further improve this reproducibility. The more research products that are generated, the more credit can be attributed. By communicating on social media or additionally publishing slides from delivered talks or posters, more attention can be raised. Additionally, publishing open access and making the work machine-findable makes it accessible to an even broader number of peers.
It might also be helpful to provide all resources from a project in a single repository such as Open Science Framework, which also implements other, different tools that might have been used, like GitHub or protocols.io.
Communicating your research
Once all outcomes of the project are shared, it is time to address the targeted peers. Social media is an important instrument to connect research communities [ 106 ]. In particular, Twitter is an effective way to communicate research findings or related events to peers [ 107 ]. In addition, specialized platforms like ResearchGate can support the exchange of practical experiences ( Table 1 ). When all resources related to a project are kept in one place, sharing this link is a straightforward way to reach out to fellow scientists.
With the increasing number of publications, science communication has become more important in recent years. Transparent science that communicates openly with the public contributes to strengthening society’s trust in research.
Conclusions
Plenty of open science tools are already available and the number of tools is constantly growing. Translational biomedical researchers should seize this opportunity, as it could contribute to a significant improvement in the transparency of research and fulfil their ethical responsibility to maximize the impact of knowledge gained from animal experiments. Over and above this, open science practices also bear important direct benefits for the scientists themselves. Indeed, the implementation of these tools can increase the visibility of research and becomes increasingly important when applying for grants or in recruitment decisions. Already, more and more journals and funders require activities such as data sharing. Several institutions have established open science practices as evaluation criteria alongside publication lists, impact factor, and h-index for panels deciding on hiring or tenure [ 108 ]. For new adopters, it is not necessary to apply all available practices at once. Implementing single tools can be a safe approach to slowly improve the outreach and reproducibility of one’s own research. The more open science products that are generated, the more reproducible the work becomes, but also the more the visibility of a study increases ( Fig 2 ).
As other research fields, such as social sciences, are already a step ahead in the implementation of open science practices, translational biomedicine can profit from their experiences [ 109 ]. We should thus keep in mind that open science comes with some risks that should be minimized early on. Indeed, the more open science practices become incentivized, the more researchers could be tempted to get a transparency quality label that might not be justified. When a study is based on a bad hypothesis or poor statistical planning, this cannot be fixed by preregistration, as prediction alone is not sufficient to validate an interpretation [ 110 ]. Furthermore, a boom of data sharing could disconnect data collectors and analysts, bearing the risk that researchers performing the analysis lack understanding of the data. The publication of datasets could also promote a “parasitic” use of a researcher’s data and lead to scooping of outcomes [ 111 ]. Stakeholders could counteract such a risk by promoting collaboration instead of competition.
During the COVID-19 pandemic, we have seen an explosion of preprint publications. This unseen acceleration of science might be the adequate response to a pandemic; however, the speeding up science in combination with the “publish or perish” culture could come at the expense of the quality of the publication. Nevertheless, a meta-analysis comparing the quality of reporting between preprints and peer-reviewed articles showed that the quality of reporting in preprints in the life sciences is at most slightly lower on average compared to peer-reviewed articles [ 112 ]. Additionally, preprints and social media have shown during this pandemic that a premature and overconfident communication of research results can be overinterpreted by journalists and raise unfounded hopes or fears in patients and relatives [ 113 ]. By being honest and open about the scope and limitations of the study and choosing communication channels carefully, researchers can avoid misinterpretation. It should be noted, however, that by releasing all methodological details and data in research fields such as viral engineering, where a dual use cannot be excluded, open science could increase biosecurity risk. Implementing access-controlled repositories, application programming interfaces, and a biosecurity risk assessment in the planning phase (i.e., by preregistration) could mitigate this threat [ 114 ].
Publishing in open access journals often involves higher publication costs, which makes it more difficult for institutes and universities from low-income countries to publish there [ 115 ]. Equity has been identified as a key aim of open science [ 116 ]. It is vital, therefore, that existing structural inequities in the scientific system are not unintentionally reinforced by open science practices. Early career researchers have been the main drivers of the open science movement in other fields even though they are often in vulnerable positions due to short contracts and hierarchical and strongly networked research environments. Supporting these early career researchers in adopting open science tools could significantly advance this change in research culture [ 117 ]. However, early career researchers can already benefit by publishing registered reports or preprints that can provide a publication much faster than conventional journal publications. Communication in social media can help them establish a network enabling new collaborations or follow-up positions.
Even though open science comes with some risks, the benefits easily overweigh these caveats. If a change towards more transparency is accompanied by the implementation of open science in the teaching curricula of the universities, most of the risks can be minimized [ 118 ]. Interestingly, we have observed that open science tools and infrastructure that are specific to animal research seem to mostly come from Europe. This may be because of strict regulations within Europe for animal experiments or because of a strong research focus in laboratory animal science along with targeted research funding in this region. Whatever the reason might be, it demonstrates the important role of research policy in accelerating the development towards 3Rs and open science.
Overall, it seems inevitable that open science will eventually prevail in translational biomedical research. Scientists should not wait for the slow-moving incentive framework to change their research habits, but should take pioneering roles in adopting open science tools and working towards more collaboration, transparency, and reproducibility.
Acknowledgments
The authors gratefully acknowledge the valuable input and comments from Sebastian Dunst, Daniel Butzke, and Nils Körber that have improved the content of this work.
Abbreviations
Funding statement.
The authors received no specific funding for this work.
Support the College of Veterinary Medicine

College of Veterinary Medicine
- Student Research
Undergraduate Summer Research Program
- Recent cohorts and projects
- Student Summer Research Projects
- Program at a glance
- Program benefits
- Program activities
- Where do our BRUSH scholars come from?
- Check Eligibility
- Faculty trainers for BRUSH students
- Testimonials
- Expectations of BRUSH scholars
- What’s next for BRUSH program alumni?
- Application process
- BRUSH Summer Research Program Flyer
- 2024 Brush Information Sessions
Both undergraduate and Doctor of Veterinary Medicine students work hard on their research projects each summer. Here are some fruits of their efforts: materials created to educate a general public on complex biomedical science work.

2021 Student Summer Research Projects
Read about and view 2021 summer research projects in "Of Mice-icles, Moo-Moo Munchies, Canine Athletes, and More."
2020 Student Summer Research Projects
Find examples of 2020 student work here , including everything from infographics to poetry.
2019 Student Summer Research Projects
Find examples of student work here , including videos, Venn diagrams, and more.
2018 Student Summer Research Projects
Alexander Zanetti; Better Breathing Through Bugs: How the Keys to Asthma Prevention May Lie in the Gut; Blog post
Allison Gerras; Investigating the Role of Biological Sex and Early Life Stress in Gastrointestinal Health; Infographic
Brady Stutzman; Research Project; Personal blog post
Brandon Frantz; Developmental Lung Disease: Understanding Early Death in Puppies; Infographic
Cailin Harro; Hepatocellular Carcinoma: Investigating Liver Cancer in Dogs; Infographic
Cassidy Harris; My Summer Research Project: The More You Know; Website
Chioma Ngene; Understanding the Metabolism of Mycobacterium Tuberculosis; Infographic
Chris Brennan; V(D)J Recombination: Why We're Able to Get Sick; Blog post
Hailee Butler; African Painted Dogs; Infographic
Jade Neverson; So...What Started This? Acetaminophen Overdose; Infographic
Jaquia White; PLASMIN: Reversing the Damage of Drug-Induced Liver Injury; Infographic
Jessica Kessler; Early Life Stress and the GI Tract; Infographic
Joanna Acosta Bencosme; Neutropils: Cows vs Humans; Infographic
Joe Faryean; Welcome to the Science Zone; Video
Jordan Pieczynski; Canine Estrous Cycle; Infographic
Kennedy Aldrich; Glycogen Depletion in Working Horses; Infographic
Liam Thomas; Searching for Blindness; Diagram
Lindsey Lund; Microsatellite Instability; Infographic
Makenzie McDowell; Microbiome, Probiotics, Shelter Kittens, and Research; Blog post
Marie Negron Camacho; Is All the Fat Bad?; Infographic
Michael Mark; Bronchopulmonary Dysplasia; Infographic
Peter Fowler; Michigan Tick Survey; Infographic
Samantha Gruenwald; Hot vs Cold: The Importance of Housing Temperatures for Mice in Research; Infographic
Sarah Marhofer; Creating a Universal Influenza Vaccine; Infographic
Sumana Prabhakar; AliveCor ECG Vet Application; Infographic
Terry Everett; Compare or Contrast: The Cytotoxic Effects of Iodinated Contrast Media on Renal Proximal Tubule Cells; Infographic
Zoe Williams; Myofibrillar Myopathy; Diagram
- Open access
- Published: 24 August 2011
Issues and special features of animal health research
- Christian Ducrot 1 ,
- Bertrand Bed'Hom 2 ,
- Vincent Béringue 3 ,
- Jean-Baptiste Coulon 4 ,
- Christine Fourichon 5 ,
- Jean-Luc Guérin 6 ,
- Stéphane Krebs 5 ,
- Pascal Rainard 7 ,
- Isabelle Schwartz-Cornil 3 ,
- Didier Torny 8 ,
- Muriel Vayssier-Taussat 9 ,
- Stephan Zientara 10 ,
- Etienne Zundel 11 &
- Thierry Pineau 12
Veterinary Research volume 42 , Article number: 96 ( 2011 ) Cite this article
52k Accesses
19 Citations
5 Altmetric
Metrics details
In the rapidly changing context of research on animal health, INRA launched a collective discussion on the challenges facing the field, its distinguishing features, and synergies with biomedical research. As has been declared forcibly by the heads of WHO, FAO and OIE, the challenges facing animal health, beyond diseases transmissible to humans, are critically important and involve food security, agriculture economics, and the ensemble of economic activities associated with agriculture. There are in addition issues related to public health (zoonoses, xenobiotics, antimicrobial resistance), the environment, and animal welfare.
Animal health research is distinguished by particular methodologies and scientific questions that stem from the specific biological features of domestic species and from animal husbandry practices. It generally does not explore the same scientific questions as research on human biology, even when the same pathogens are being studied, and the discipline is rooted in a very specific agricultural and economic context.
Generic and methodological synergies nevertheless exist with biomedical research, particularly with regard to tools and biological models. Certain domestic species furthermore present more functional similarities with humans than laboratory rodents.
The singularity of animal health research in relation to biomedical research should be taken into account in the organization, evaluation, and funding of the field through a policy that clearly recognizes the specific issues at stake. At the same time, the One Health approach should facilitate closer collaboration between biomedical and animal health research at the level of research teams and programmes.
Table of contents
1. introduction, 2. issues and special features of animal health research, 2.1. animal health and veterinary public health, 2.2. issues at stake in animal health, 2.3. importance of diseases, prioritization of issues at stake, 2.3.1. special features of diseases according to the types of animals, 2.3.2. prioritization of issues at stake, 2.3.3. issues at stake in animal health research, 3. special features of animal health research, 3.1. distinguishing features of the objectives, methods, and biological models, 3.2. special features of scientific questioning, 3.3. generic and methodological areas of convergence with human health, 4. relationships between animal health and human health research, 4.1. domestic animal models for human targeted research, 4.2. funding and evaluation of research, 4.3. parallels between research, surveillance of diseases and the pharmaceutical industry, 4.3.1. surveillance and control of diseases.
4.3.2. Pharmaceutical industry
4.4. The "One world, One Health" approach
5. Conclusion
Competing interests, authors' contributions, acknowledgements.
Understanding of animal health research, and the expectations of donors and research organizations, is changing. A growing number of actors consider such research from the limited perspective of the dangers and risks directly posed to human health by traditional and emerging animal diseases. Some furthermore consider health as an asset shared by all species, animal and human, that would be guaranteed by a single medicine guided by biomedical research. In this evolving context, a collective discussion on the special features of animal health research, the issues at stake and the specific contributions such research can provide to generic health research was deemed necessary. This article summarizes the results of this discussion, addressing the issues at stake at the global level. Presented in three sections, the first describes the challenges facing animal health and research on animal health, the majority of which are not related to zoonotic diseases. The second section describes the distinguishing features of animal health research that are related to scientific constraints, the manner by which the discipline is grounded in an agricultural and economic context, and the perspectives from which scientific questions are posed. The third section addresses the relationships between animal health and biomedical research. The conclusion proposes changes that would permit research to be adapted to the special features of the field while at the same time favouring partnerships with research on human health. This discussion deliberately was limited to livestock; pets and wild animals only are mentioned for purposes of comparison.
In animals, health may be defined as the absence of disease or the normal functioning of an organism and normal behaviour based on the observation of a certain number of individuals that determine the standard and thus health [ 1 ]. In production sectors, health also may be defined as the state allowing the highest productivity. However, this narrow definition often is enriched by the concept of a balance between the animal and its environment, and of the animal's physical welfare. This broader definition undoubtedly is linked to changes observed in the field of veterinary medicine, which is focussing increasingly on prevention rather than cure, and which takes the animal's environment into fuller account [ 2 ].
Animal diseases may be organized schematically into three categories. Multifactorial diseases are provoked by a set of risk factors linked in particular to livestock management, with at times the participation of pathogens widespread in livestock. Known as "production diseases", multifactorial diseases are present on a large majority of livestock farms with highly variable frequencies. The major epidemic diseases are highly contagious and impact livestock heavily (for example, foot-and-mouth disease, swine fever, highly pathogenic avian influenza); the challenge is to eradicate such diseases from a territory when possible, and their appearence in a totally susceptible population can have extensive health and economic consequences. Other transmissible infectious diseases are less contagious or have slighter impacts, and frequently are present in populations in an endemic manner. Among transmissible diseases are zoonotic diseases, which are those that can be transmitted to humans. Animals also may be healthy carriers of agents that are pathogenic for humans but which do not affect the health of the animal (for example, Salmonella and Campylobacter ).
In response to these challenges, and picking up on a framework produced by international bodies (World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), World organization for Animal Health (OIE)), WHO [ 3 ] currently defines veterinary public health as " the sum of all contributions to the physical, mental and social well-being of humans through an understanding and application of veterinary science ". In an editorial of the OIE bulletin [ 4 ], The Veterinary Services are stated as the " key players in the prevention and control of animal diseases and in the improvement of food security, nutrition, food safety, veterinary public health and market access for animals and products ". Veterinary public health activities thus include the control of animal diseases that have a direct impact on human health due their zoonotic character, as well as the control of all non-transmissible animal diseases capable of causing important production losses (safety of animal product supply) and disrupting markets (animal and products of animal origin).
There are four types of issues at stake in the field of animal health:
1/ Economic issues for a range of diseases that impact the economic viability of livestock farms (notably livestock diseases and endemic diseases that lead to production losses, prevention or treatment costs, disruption of the farm or the work of the livestock farmer) and animal production sectors (notably epidemic diseases due to their effect on production, the impact of health regulations on markets, and impediments to trade). In industrialized countries, these diseases weigh heavily on the overall economic competiveness of livestock farms, businesses, and animal production sectors. In developing countries, there are the added risks of food scarcity, capital dilution (insofar as cattle constitute standing capital, the only form of savings and social security for many people), and the loss of draught and labor power (leading to a reduction in overall agricultural efficiency).
2/ Public health issues , which concern three domains: zoonoses, infectious or parasitic diseases transmissible from animals to humans, whether contagious (for example, tuberculosis, brucellosis, certain influenza viruses), vectorial (West Nile disease, Rift Valley fever, Lyme disease), or food-borne (BSE, toxic food poisoning); resistance to antibiotics; and traces of medicine in animal products.
3/ Environmental issues related to the impact of agriculture; this involves the dumping of xenobiotics into the environment (medicine residues), the spread of resistance to antibiotics, and infectious diseases that can be transmitted between domestic and wild animals (such as bovine tuberculosis detected in wildlife).
4/ Animal welfare issues , which are related closely to changes of regulations in this domain. Diseases induce suffering and pain, the absence of which is one of the criteria chosen for recently proposed animal welfare evaluation tools [ 5 ].
In a recent report on the state of food and agriculture in the world focusing on livestock, the FAO [ 6 ] summarizes these different issues at stake as: "Animal diseases, and a lack of adequate food hygiene resulting in foodborne illnesses, are a problem for everyone because they can threaten human health, disrupt markets and trade, reduce productivity and deepen poverty. Improving the management of livestock with a view to preventing and controlling disease can provide significant economic, social, and human health benefits for the poor and for society at large" . Among the report's four key messages, it is noted that, " Livestock diseases pose systemic risks that must be addressed ."
For all of these diseases, while the issues at stake primarily concern agricultural farms, associated economic sectors also are involved: live animals, products of animal origin, agricultural inputs and services. Consumers and citizens are all concerned, as much by quantitative and qualitative food security as by public health. Livestock and agro-food sectors play a central role in industrialized countries, reaching 53% of the gross domestic product [ 7 ] (food safety, extensive economic activities linked to supplying the livestock sector which include the pharmaceutical industry, and the valorization and trade of agricultural products and food that often are very technologically advanced), as in developing countries (subsistence agriculture, food security, intake of quality protein). The economic issues involved in animal health, without even mentioning the risks of bioterrorism, therefore represent critical strategic challenges , even if they receive less media coverage than public health issues.
Furthermore, these different types of issues are not independent of each other . For example, the risk of the presence of medicine residues in animal products, as well as the risk of antibiotic resistance coming from the animal world, are both public health issues, and are both directly correlated to the frequency of enzootic diseases impacting the economic equilibrium of animal production chains; they thus pertain above all to the economic stakes involved in ensuring animal health.
For production animals, infectious and parasitic diseases predominate, even if metabolic and degenerative disorders naturally exist that most frequently are related to an insufficient control of production systems. In contrast, household pets and sports animals present a pathological profile very similar to humans (endocrinian disorders, cancers, degenerative neuro and osteoarticular diseases, obesity, aging). This leads to a more reduced presence of infectious and parasitic pathologies in favour of internal medicine, cancerology, and endocrinology, although antibiotic and anti-parasite medicines and vaccines together account for 75% of the consumption of medicine by pets. Lastly, non-captive wildlife constitutes a relatively new subject of animal health research, principally concerning major epidemiological reservoirs of potentially zoonotic agents (for example, bat lyssavirus and avian influenza) and sentinels of contamination and toxicologic pollution of the environment.
It is difficult to arrange the different challenges presented by animal diseases and their control into an order of priority. There are several ways to assess the importance of animal diseases. The first is to estimate their impact on zootechnical and economic performance . The average mortality rates of animals in Western European livestock systems can be significant for certain age groups, and may reach high levels in herds when pathology is poorly controlled. For example, the mortality of calves before weaning is on average 12%, that of dairy cows 3%, that of piglets before weaning 20% (including stillborns), with another 7% loss between the weaning and slaughter of pigs. The various costs of controlling disease are added to those of mortality. The current economic impact of mastitis in dairy cows in France may be assessed at 350 million €/year, principally due to reductions in productivity and longevity, reduced sale prices of milk and the costs of prevention measures and treatment. In poultry, coccidioses have a major impact; based on a British model [ 8 ], their global economic impact is estimated at over two billion dollars, principally due to their impact on production and feed efficiency. In the case of endemic diseases, economic losses remain usually limited in each farm, but the global economic impact is high due to the large number of farms affected [ 9 ]. The probability of epidemic diseases is lower but when present, they may induce very severe losses [ 10 ], even beyond the agronomic and agri food sectors.
OIE's list of notifiable diseases [ 11 ] includes infectious transmissible diseases deemed to be most damaging at the international level from an economic and public health point of view; among the 119 diseases listed, only 31 are zoonotic to one degree or another [ 12 ]. The declared priorities of international bodies (WHO, FAO, OIE) federated under the GLEWS [ 13 ] programme ( Global Early Warning and Response System for Major Animal Diseases, including Zoonoses ) for the surveillance and monitoring of animal diseases nevertheless derive from an approach first initiated by WHO that gave priority to zoonotic diseases. This is why the GLEWS list includes 6 non-zoonotic and 19 zoonotic diseases.
On the basis of vaccine production, it should be noted that almost all those used in the field of animal health protect against strictly animal pathogens. The rabies vaccine is one of the rare veterinary vaccines meant to protect humans. Certain other veterinary vaccines, such as for leptospira, target a zoonotic agent but are used mainly to protect pets, the exception being the New Zealand cattle vaccination programme that also aims to protect farmers; vaccines against zoonotic agents generally are not meant to protect animals in the name of public health.
Precise light was thrown on the subject by a bibliometric study covering the 2006-2009 period conducted under the European Era-Net EMIDA programme (Emerging Infectious Diseases of Animals) [ 14 ] which focused on infectious and parasitic diseases of production animals. The map generated by the study shows that animal health is situated at the intersection of other disciplinary fields such as human health, but also the health of wild animals and ecosystems, animal nutrition, animal genetics, and animal welfare. The study also demonstrates that barely 20% of the 12 000 publications on infectious diseases surveyed address zoonoses and food safety, and thus have a direct link to public health issues. This means that, in contrast, 80% of the publications address exclusively animal diseases presenting primarily economic, environmental, and animal welfare challenges. The distribution of research work on infectious and parasitic diseases at the international scale [ 15 ] according to the production animal species and pathogens involved is presented in Figure 1 .
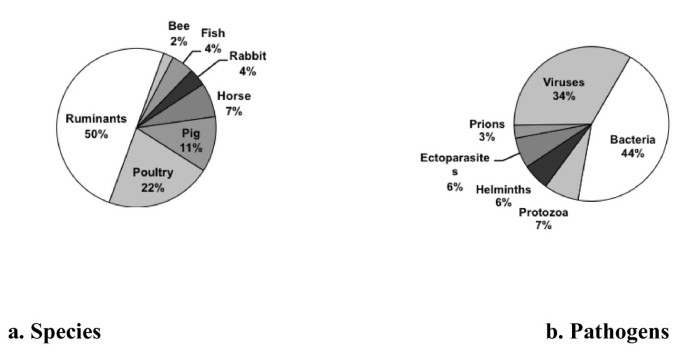
Distribution of publications on infectious and parasitic diseases in animal health according to the livestock species (a) and pathogens (b) involved . Analysis in the framework of the European Star-Idaz project [ 15 ] of 28 750 international scientific articles published on the subject from 2006 to June 2010.
At the European level, it should be noted that an effort to prioritize issues at stake and research involving over 50 infectious and parasitic animal diseases is led by a group of experts under the aegis of Discontools (Disease Control Tools) working with the European ETPGAH platform (European Technology Platform for Global Animal Health). The first outputs may be accessed online on the Discontools web site [ 16 ].
Given the breadth of the challenges related to animal health, numerous research questions need to be explored that touch upon different domains of biology and social sciences to broaden existing knowledge, with a continuum from basic to applied research. The questions involve knowledge of pathogens, the relationship between a host (infected animal) and a pathogenic agent, as well as the interaction of pathogens and hosts at the scale of animal populations. The research to be carried out thus aims to propose tools to control the exposure of domestic animals to pathogens, reinforce the resistance of hosts to pathogenic agents (notably through vaccination), and to treat sick animals. The containment and control of diseases through control and prevention programmes also requires assessments of economic and social impacts of health management plans.
In addition to such targetted research, there is a need for fundamental research geared to producing generic knowledge on animal models. The research undertaken in this field is enriching understanding of biology thanks to comparative biology. The diversity of the model species studied, the availability of experimental mechanisms and of biological material, as well as the mastery of particular infectious models, are all important assets for this research, which produces knowledge on living organisms that does not necessarily have an immediate application, but which may prove to be very useful in the future (example of innate immunity molecules-defensins, Toll receptors-identified in invertebrates that have vaccinal and immunomodulatory applications in humans and domestic animals).
Animal health research is distinguished by particular objectives, methods, biological models and scientific questions. However, there nevertheless are areas of generic and methodological convergence with biomedical research.
First of all, livestock farming is an economic activity whose end goal is to generate revenue. In this context, animal health is one of several factors that farmers must manage; they do so by minimizing their herds' exposure to health risks and by finding the least expensive way to limit the impact of disease [ 17 ]. In a given livestock system, diseases are closely linked to the way livestock are managed, notably to parameters related to the quality of housing, nutrition, hygiene, and to animal production levels. The intensification of livestock systems that has taken place in agriculture over the past fifty years has accentuated the tension between limiting inputs, increasing production, and the risk of disease.
Over time, questions regarding livestock health have moved beyond a sole objective of achieving economic gains by reducing disease frequency to addressing the sanitary quality of products of animal origin, reducing the use of xenobiotics, and animal welfare in the interest of public health and sustainable development. The multiplicity of the challenges leads to the question of how the best balance may be achieved between these different parameters. To continue working in this direction, animal health stakeholders, whether from the perspective of research or development, need to establish close ties with livestock sciences and agricultural professionals.
To take into account these elements, population medicine on farms will be needed, as well as research on diseases that specifically recognizes the close connections between health and animal production science. This implies in-depth collaboration with other animal science disciplines on one hand, and with the various stakeholders in the livestock world on the other. The only pertinent research is that carried out in close contact with the actual practices of farmers and animal sectors. For example, within an integrated agriculture framework, integrated research on livestock health management implies solid understanding of the livestock world, requires close collaboration between animal production, genetics, livestock economics, sociology and animal health disciplines, and relies on a partnership with livestock health stakeholders.
A second distinguishing feature of animal health research is the overwhelming predominance of infectious and parasitic diseases, at least for livestock, with a very large diversity of pathologies and a very large repertoire of pathogens involved [ 18 ]. Animal health research teams consequently are obliged to study a wide variety of pathogen families, developing in the process a pool of rare and precious skills in virology, bacteriology, parasitology, and medical entomology.
A third distinguishing feature of animal health research is related to the special genetic features of livestock animals. The evolution of animal species, which results in the diversity of species, takes much longer time than phases of domestication, which result in the diversity of breeds. The intensive selection practices implemented over the past fifty years has improved production considerably, but the cost has been a sharp drop in genetic diversity among livestock [ 19 , 20 ]. A distinguishing feature of livestock systems effectively is the possibility of human intervention to select animals for particular genetic traits, most often production (for example, quantity of milk) but also resistance to disease (for example, against scrapie). To understand the genetic foundations of susceptibility to infectious diseases, the duration of co-evolution, genetic diversity, and the respective evolutionary dynamics of hosts and pathogens therefore must be taken into account. The genetic improvement of the immune response is a complex selection objective. It generally either is directed against a single target (pathogen) that is constantly evolving (due to its rapid evolutionary dynamic), or seeks a better overall immunocompetence; in either case, there tends to a negative correlation with the selection of production traits.
Animals of economic importance include species that belong to very distinct animal clades such as fish, bees, chicken, pigs, goats, sheep and cattle. These clades diverged from each other hundreds of millions of years ago. Even within mammals, the Laurasiatheria superorder, which includes ruminants and pigs, and the Euarchontoglires superorder, which includes humans and mice, diverged from each other around 100 million years ago, rendering mice and human phylogenetically closer to each other (so called supra-primates) than they are to ruminants and pigs [ 21 ]. These millions of years of separated evolution generated specific anatomical, metabolic and physiological traits, as well as specific commensal-host and pathogen-host relationships. For example, fish show particularities linked to their aquatic environment with some pathogens entering via fins [ 22 ]; they present a more primitive immune system and their cells are highly permissive to DNA transfer, allowing highly efficient DNA vaccination [ 23 ].
Whereas the basic structures and the generation mechanisms of the T cell receptors and immunoglobulins are similar from teleost fish to higher mammals, each species presents particularities, such as specific isotypes (unlike humans, mice do not secrete IgD or IgG4) and specific mechanisms of antibody diversity generation (gene conversion in chicken, hyper somatic mutations in human and mice). Notably, cytokines are specific to some species; for example, those controlling the production of type I IFN in humans and probably pigs does not exist in mice. Across species, mother to offspring transmission of pathogens and of immunity is strongly dependent on developmental characteristics related to oviparity and variations in placentation modalities. Thus whereas baby mice acquire their immunoglobulin pool during pregnancy by translocation through the placenta, ruminants acquire their immunoglobulin pool at birth via the colostrum due to the relative impermeability of their placenta.
Most basic and applied research is conducted on laboratory mice, in which some human and domestic animal diseases have been experimentally adapted. In many instances, therapeutic and prophylactic treatments that are effective in laboratory mice do no work when transposed to human and veterinary species. This lack of transposition can be explained by the specific physiological traits mentioned above and by the artificial pathological mouse models used in the laboratories. It is very important for pathogen-host interactions and novel therapeutic and prophylactic treatments to be evaluated on the targeted veterinary species, thereby studying the effect in the actual host and consequently limiting a "mouse" bias as much as possible. Research and experiments on "target" species (fish, chicken, pigs, ruminants) therefore often is necessary, and presents an advantage because the research findings may be applied directly to the species without the extra step of validating an extrapolation based on an animal model, in contrast to research undertaken for biomedical applications.
Lastly, there are special features related to the types of actions taken for animal disease control and health management. Beyond vaccination and the protection of livestock, animal health rules covering contagious diseases include a range of control methods, including at times the slaughter of animals to eliminate those posing a risk for unaffected animals and humans. These practices lead to specific research questions regarding intervention mechanisms. At the top of this list is the need to update serological tools so that vaccinated animals may be distinguished from infected animals because disease control measures are different for these two categories of animals. Another priority is the set of questions regarding the comparative economic advantage of different control methods and the conditions by which they are appropriated by livestock farmers and public officials.
While the livestock world has many other distinguishing characteristics, these do not seem to have a notable impact on the manner by which animal health research is conducted.
In addition to the aspects discussed in the preceding sections, one of the main distinguishing features of animal health research are the scientific questions pursued, which are posed from the perspective of animal, and not human, health. Consequently, even in the case of zoonotic agents, the questions asked by animal health teams are not the same as those asked by biomedical teams. In the case of zoonotic vector agents, for example, Bartonella or Borrelia agents of Lyme disease, animal health research would focus on the role of animals as reservoirs of agents potentially pathogenic for humans, and on the elements that allow the development of an infectious agent in its host reservoir versus a human. Biomedical research, on the other hand, would focus on the development of an infectious agent in a human. For prion diseases, an animal health perspective leads to studying the diversity of strains found in the animal and to an attempt to decipher the interactions between the infectious strain and the host species. More broadly, studies of pathogenic agent/host interactions that are pursued from an animal health angle often prove to be fruitful from both a pure and applied perspective. This is due in particular to the genetic knowledge generated on the infected host and the possibility of implementing protocols with an experimental cohort with a defined genetic status. This is, for example, the case with the demonstration in sheep of the modulation of susceptibility to scrapie in connection with the polymorphism of the protein prion coding gene [ 24 ].
It thus would appear that, while working on the same agents and with the same tools, the questions pursued in animal health may be different from, and complementary to, those in human biology, and lead to the production of complementary knowledge. It follows that opportunities for collaboration between animal health and biomedical teams should be pursued, each having, through the questions they pursue and their "natural" partnership networks (hospitals versus farms or the environment), access to different and complementary types of samples. For example, collaboration could focus on comparing, with an epidemiological objective, Bartonella strains sampled from humans and different animal species.
In certain fields, research carried out in human biology and animal health use similar tools, and even the same models, to address research questions. When this is the case, notably in the framework of the study of zoonotic pathogens, the only difference lies in the nature of the questions explored.
In certain circumstances, the convergence continues up to point where the biomedical and animal health teams share the same questions, and then no evident distinguishing feature remains. The development of projects initially focused on animal health progressively may lead the teams involved to pose questions that are increasingly focussed on models shared with human biology. As an illustration, we may cite fundamental research approaches to the molecular mechanisms of the invasion of cells targeted by the influenza virus, or the biological origin of prions and the determinants of the species barrier modulating their transmission capacity. In such cases, it is easy to imagine that the same research could be conducted in research laboratories unrelated to animal health. However, an animal health perspective offers certain advantages, notably expertise for extensive experimental research in a confinement area, and special links maintained through collaborations with other scientists working notably in the fields of pathogenesis and animal genetics.
The discussion presented here was conducted in relation to human biology research work. A parallel approach could be envisioned in relation to work carried out on plant health. Such an analysis may elicit a certain community of tools and methods with animal health, an advantage of comparative biology, but apparently few shared issues at stake for the pathogens of interest.
Mice often prove to be an inadequate model in physiopathological, prophylactic, and therapeutic studies for humans. This is due to the reduced size of the species, physiological considerations, and the absence of a natural corresponding pathology. With regard to the latter point, it often is necessary to infect a mouse with the human pathogen agent, and thereby create an artificial model without pertinent symptoms. In certain situations, domestic species prove to be better study models for human-oriented research. Domestic species can be infected by viruses that have co-evolved with their host. These diseases present similarities in molecular and physiopathologic mechanisms to human disorders without being zoonotic. Pigs infected by an influenza virus that has adapted to pigs thus suffer an influenza syndrome resembling that found in humans infected with a human influenza virus. Young calves infected by a respiratory syncytial virus distinct from the human virus develop a broncho-pulmonary pathology close to that of a child. These animal disorders thus allow the development of therapeutic, vaccination, and diagnostic strategies that can be adapted or extrapolated to humans.
Furthermore, through evolutionary convergence, certain domestic species present more functional similarities to humans than mice: for example, sheep for respiratory pathology (immunologic study of asthma treatment), and pigs for skin structure (study of transcutaneous therapy or vaccination), cardio-vascular diseases, and the development of spontaneous melanoma where the progression of tumors resembles that observed in humans.
Lastly, domestic animals, due to their large size, allow immune functions to be studied in an original manner that would not be possible with mice. It thus is possible to catheterize lymphatic vessels in pigs, cows, and sheep to study baseline migrant leukocyte populations directly in the lymph during an infection or vaccination, enabling certain immune response features to be monitored in real time.
For these different reasons, in-depth knowledge of domestic animal physiopathologies and the existence of high performance animal experimentation platforms are useful for biomedical research. Overall, the diversity of models (animal species) studied, the foundation of comparative biology, is important to produce general knowledge that can have diverse applications, notably in human biology.
Compared to research on pathogens affecting public health, it is notoriously difficult to find funding for research dedicated to animal health that is focussed on non-zoonotic pathogens or to publish the results in high quality scientific journals. These difficulties seem to be inversely proportional to the genericity of the knowledge produced and to its potential biomedical contribution. For example, in a call for proposals on infectious disease research, an excellent project on a non-zoonotic pathogen will systematically be eclipsed by a project addressing a topic such as hemorrhagic fevers due to the evaluators' perception of the stakes involved. Similarily, numerous human health and scientific journals that have a high impact factor due to the larger size of the scientific community involved in human biology compared to animal health, rarely accept an article on non-zoonotic agents that effectively fall outside their domain.
This state of affairs is extremely important to take into consideration given the current imperative to obtain credit to finance research and the use of the "impact factor" criteria in the scientific evaluation of research teams. This point is even more critical as the apparent proximity of animal health and human biology sectors nevertheless does not render their objectives equivalent. An overly hasty approach to the question by evaluators who are ill-informed or insufficiently aware of the issues involved will lead them to apply criteria and indicators to animal health research that are appropriated from human biology and which are completely unsuitable, and indeed unfair, in the field of animal health. Research units that address both zoonotic and non-zoonotic pathogens face a delicate situation. Teams within the same unit are not in the same boat with regard to seeking funding and publication levels.
What emerges from this analysis is that, when research of equivalent scientific quality are considered together, work on non-zoonotic diseases are financed less easily, and are published in journals with a lower impact factor, than work on zoonotic animal diseases. In a similar fashion, research on animal diseases are financed and published less easily than human biomedical research. In the absence of specific corrective action, the existence of a "species barrier" in terms of funding and publication is endangering 80% of animal health research. It thus is absolutely necessary to act far upstream of national and international research programmes by ensuring that calls for research proposals specifically mention the issues at stake in animal health on one hand, and that research organizations for their part officially adopt a policy to recognize the stakes and scientific outputs that are specifically linked to animal health.
A parallel may be drawn between the domain of research and that of disease surveillance and control. OIE officials call attention to a school of thought circulating at the international level that suggests economies of scale would be possible if veterinary medicine services were regrouped with human health facilities in each country. Along the same lines, public services such as disease surveillance are perceived to be expendable variables that may be played with to cut costs in debt-ridden countries. In the same spirit, this school of thought also advocates that only animal diseases posing risks to humans should be considered important due to their zoonotic character. In such a logic of cost-cutting and the regrouping of animal and human health spheres, financial trade-offs naturally would favour human health priorities at the expense of veterinary services.
The OIE's strategy is to take the opposing view which holds that prevention costs less than resolving crises, and that quality prevention is based on national animal health systems that can ensure appropriate surveillance, early detection, transparency, and rapid response to animal disease outbreaks and on a durable network of veterinary services endowed with a specific budget. Thus in 2006, the OIE reiterated its affirmation that veterinary services were a global public good [ 25 ]. The disastrous consequences of cutbacks in public services, and the efficacy of the preventative and global approach taken by the OIE, is leading progressively to a swing of opinion in favour of this approach. This change is visible, for example, in the international documents debated during successive forums on the control of avian influenza [ 26 ].
4.3.2. Pharmaceutic industry
Most pharmaceutical companies have subsidiaries dedicated to animal health, which is related to the fact that economic scales between animal and human health cannot be compared; as an example, sales of a human vaccine may be 20 to 50 times higher than those of a veterinary vaccine. If a choice must be made between two very different vaccine projects, even if each is a priori profitable, the human vaccine automatically will be chosen over the veterinary vaccine. In the same manner, shared services will be put at the disposal of the human vaccine project given the higher economic stakes involved. Lastly, it also is more difficult to find public funding, and thus complementary private funding, for the development of vaccines against non-zoonotic pathogens than for human vaccines. A fusion between human and animal activities would translate into the disappearance of the animal sector, or into animal models being developed only when they have a direct interest for humans. In contrast, what is shared by animal and human vaccines is an ensemble of vaccine production technology, innovations in this field and preceding research on pathogen families, cytology, certain features of immunology, all knowledge that deserves to be shared between human and animal health in the form of cooperation.
4.4. The "One World, One Health" approach
As mentioned by the Director of the OIE in an editorial [ 27 ], the "One World, One Health" approach is indispensable in the sense that " the only way to prevent all these new hazards (zoonotics) is to adapt the existing systems of health governance at world, regional and national levels in a harmonised and coordinated manner ", but " the concept "One World, One Health" should not serve as a pretext for dangerous initiatives like trying to achieve economies of scale based on purely theoretical notions worthy of a sorcerer's apprentice, such as trying to merge the Veterinary Services and the Public Health Services ". Taking this perspective, it effectively is out of the question to merge services because each must assume its functions with the resources dedicated to it and the approaches suited to its particular mission; however, it is necessary to develop collaboration, cooperation, and synergies [ 28 ]. For the past few years, there has been a concensus on this issue among the OIE, FAO, and WHO. Different discussions are underway to define ways to implement this cooperation between organisations.
The present discussion, the opinion of experts, and a critical reading of the literature has led to the following observations.
International bodies (WHO, FAO, OIE) affirm that, over and above the threat of diseases that can be transmitted to humans (zoonotic diseases), the challenges facing the field of animal health are considerable. They concern food security, economics, agriculture and associated economic activities in both industrialized and developing countries. The challenges facing animal health, beyond those posed by zoonotic diseases, overlap with those of public health and the environment, notably regarding the use of xenobiotics and the development of antibiotic resistance.
The distinguishing features of animal health research are methodological and scientific in nature. They notably pertain to special biological features of domestic species and to the interaction between humans in their practice of livestock husbandry and animals in their biology and evolution. Animal biology generally does not pursue the same scientific questions as human biology, even when the same pathogens are being studied, and the discipline is rooted in a very specific agricultural and economic context. For animal health stakeholders, whether from the perspective of research or development, finding an optimal balance between the economic profitability of a farm, animal welfare, the maintenance of animal health and the quality of products of animal origin involves close collaboration between animal husbandry sciences and the agricultural profession.
Knowledge produced by comparative biology is fed by research conducted on animal species. For example, animal models are a source of generic knowledge due to their special evolutionary features and, in certain cases, their functional similarities with humans. The diversity of the model species studied and the control of particular infectious diseases contribute greatly to the production of knowledge about living organisms.
These observations present a strong case in favor of taking into account the uniqueness of animal health research, in terms of its organization, evaluation, and funding, compared to biomedical research. If this is not done, strictly biomedical priorities will lead to the elimination, sooner or later, of quality research on non-zoonotic animal diseases. A special "treatment" of this research thus is necessary with regard to the issues at stake; specially designed calls for proposals should be dedicated to the field, the field's journal corpus should be recognized as being different from that of biomedical research, and the research should be evaluated in the light of this specific corpus.
The "One Health" approach is important insofar as it argues that the management of health requires reinforced coordination between human and animal components and, in the same manner, in-depth collaboration between biomedical and animal health research. The organization of such collaboration can only reinforce the capacity of both groups to produce relevant science, and to realize the potential of research efforts and more global approaches integrating human and animal components in federated projects.
In terms of research, this collaboration may assume different forms and take place at different levels, ranging from cooperation between teams up to the organization of research and its funding. The questions explored in animal health and human biology regarding the same zoonotic pathogen frequently are complementary. They allow scientific collaborations to be built that can respond to more general questions, and notably to address the complexity of the biological systems of certain diseases. Another form of collaboration is the establishment of calls for joint public health and animal health proposals for research on pathogens whose study and control require combined research approaches. This has been the case for research on transmissible spongiform encephalopathies, with joint animal-human calls for projects and pluridisciplinary projects in the United Kingdom, Netherlands, Germany, France and the European Union. At a more general level, comparative biology represents a precious source of knowledge.
Baker JK, Greer WJ: Animal health: a layman's guide to disease control. 1980, IPP the Interstates Printers and publishers, Danville, Illinois
Google Scholar
Gunnarson S: Definition of health and disease in textbooks of veterinarian medicine. Animal production in Europe: The way forward in a changing world. Proc. 13th International Society of Animal Hygien, Saint-Malo, France. 2004, 105-109.
Future trends in veterinary public health. Report of a WHO Study Group. 907. WHO Technical Reports Series, World Health Organization, Geneva, 96 pp (2002), [Consulted 17 November 2010], [ http://whqlibdoc.who.int/trs/WHO_TRS_907.pdf ]
Editorial: Veterinary legislation is the foundation of any efficient animal health Policy. Bulletin n 4 OIE 77th General Session (2009). (consulted 31 May 2011), [ http://www.oie.int/en/publications-and-documentation/bulletins-online/ ]
Botreau R, Veissier I, Butterworth A, Bracke MBM, Keeling LJ: Definition of criteria for overall assessment of animal welfare. Anim Welf. 2007, 16: 225-228.
CAS Google Scholar
FAO-The State of Food and Agriculture 2009-Livestock in the Balance, 166pp. (consulted 9 June 2011), [ http://www.fao.org/docrep/012/i0680e/i0680e00.htm ]
Thornton PK: Livestock production: recent trends, future prospects. Philos Trans R Soc Lond B Biol Sci. 2010, 365: 2853-2867. 10.1098/rstb.2010.0134.
Article PubMed Central PubMed Google Scholar
Williams RB: A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int J Parasitol. 1999, 29: 1209-1229. 10.1016/S0020-7519(99)00086-7.
Article CAS PubMed Google Scholar
Seegers H, Fourichon C, Beaudeau F: Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003, 34: 475-491. 10.1051/vetres:2003027.
Article PubMed Google Scholar
Thompson D, Muriel P, Russell D, Osborne P, Bromley A, Rowland M, Creigh-Tyte S, Brown C: Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev Sci Tech. 2002, 21 (3): 675-687.
CAS PubMed Google Scholar
OIE listed diseases 2011. (consulted 31 May 2011), [ http://www.oie.int/eng/maladies/en_classification2010.htm ]
Ganière JP: Importance et hiérarchisation des zoonoses en France: le point de vue vétérinaire. Epidémiol et santé anim. 2004, 46: 27-32. (in French)
Global Early Warning and Response System for Major Animal Diseases including Zoonoses. (consulted 31 May 2011), [ http://www.glews.net/ ]
de Rycke J: World Research Landscape Mapping of European Research Organisations. A bibliometrical analysis of research output (2004-2008). EMIDA ERA-NET-Report of Wordpackage 2; 60 pp (2009). (Consulted 31 May 2011), [ http://www.emida-era.net/upload/pdf/Final%20WP2%20report%20090407.pdf ]
de Rycke J: World mapping of research institutions, topics and collaboration in the field of infectious diseases of animals; a bibliometric analysis using publication records extracted from the Web of Science (2006-2010). Report, 47 pp. 2010
Discontools: Disease Control Tools. (consulted 31 May 2011), [ http://www.discontools.eu/home/index ]
Ganière J-P, André-Fontaine G, Drouin P, Faye B, Madec F, Rosner G, Fourichon C, Wang B, Tillon J-P: L'écopathologie: une méthode d'approche de la santé en élevage. INRA Prod Anim. 1991, 4 (3): 247-256.
Timoney JF, Gillespie JH, Scott FW, Barlough JE: Microbiology and infectious diseases of domestic animals. 1988, Cornell University Press, New York, 8
Muir WM, Wong GK, Zhang Y, Wang J, Groenen MA, Crooijmans RP, Megens HJ, Zhang H, Okimoto R, Vereijken A, Jungerius A, Albers GA, Lawley CT, Delany ME, MacEachern S, Cheng HH: Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proc Natl Acad Sci USA. 2008, 105: 17312-17317. 10.1073/pnas.0806569105.
Article PubMed Central CAS PubMed Google Scholar
Taberlet P, Coissac E, Pansu J, Pompanon F: Conservation genetics of cattle, sheep, and goats. C R Biol. 2011, 334: 247-254. 10.1016/j.crvi.2010.12.007.
Nishihara H, Hasegawa M, Okada N: Pegasoferae, an unexpected mammalian clade revealed by tracking ancient retroposon insertions. Proc Natl Acad Sci USA. 2006, 103: 9929-9934. 10.1073/pnas.0603797103.
Harmache A, Leberre M, Droineau S, Giovannini M, Brémont M: Bioluminescence imaging of live infected salmonids reveals that the fin bases are the major portal of entry for Novirhabdovirus. J Virol. 2006, 80 (7): 3655-3659. 10.1128/JVI.80.7.3655-3659.2006.
Boudinot P, Blanco M, de Kinkelin P, Benmansour A: Combined DNA immunization with the glycoprotein gene of viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus induces double-specific protective immunity and nonspecific response in rainbow trout. Virology. 1998, 249 (2): 297-306. 10.1006/viro.1998.9322.
Goldmann W: PrP genetics in ruminant transmissible spongiform encephalopathies. Vet Res. 2008, 39 (4): 30-10.1051/vetres:2008010.
Editorial: Prevention, detection and monitoring of animal diseases, including those harmful to humans: veterinary services are the keystone of the global system. Bulletin n°3 Evaluation and support of Veterinary Services (2006). (consulted 31 May 2011), [ http://www.oie.int/en/publications-and-documentation/bulletins-online/ ]
The FAO-OIE-WHO Collaboration sharing responsibilities and coordinating global activities to address health risks at the animal-human-ecosystems interfaces. FAO-OIE-WHO Collaboration Concept Note on health risks at the human-animal interface (2010). (consulted 31 May 2011), [ http://www.oie.int/en/for-the-media/press-releases/detail/article/fao-oie-who-collaboration-concept-note-on-health-risks-at-the-human-animal-interface-is-available-on/ ]
Editorial: One World, One Health. OIE Bulletin n° 2 One World, One Health (2009). (Consulted 1 May 2011), [ http://www.oie.int/en/publications-and-documentation/bulletins-online/ ]
Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, Pfeiffer DU, Stärk K, Rweyemamu M: Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis. 2011, 11: 326-331. 10.1016/S1473-3099(10)70312-1.
Download references
The authors are deeply grateful to Claude Leclerc (Institut Pasteur), Alain Dehove, Elisabeth Erlacher-Vindel, Kasuaki Miyagishima (OIE), Jean-Christophe Audonnet, Michel Bublot, Catherine Charreyre, François Xavier Le Gros, Pascal Hudelet (Société Merial), for their contributions to this collective discussion, as well as Bernard Charley, Jean De Rycke, Michel Fougereau, Pierre Lekeux, Henri Salmon, Henri Seegers and Etienne Thiry for their critical reading of the initial report.
Author information
Authors and affiliations.
INRA, UR346 Epidémiologie animale, 63122, Saint Genès Champanelle, France
Christian Ducrot
INRA, UMR1313 Génétique Animale et Biologie Intégrative, 78352, Jouy-en-Josas Cedex, France
Bertrand Bed'Hom
INRA, UR892 Virologie et Immunologie Moléculaires, 78352, Jouy-en-Josas, France
Vincent Béringue & Isabelle Schwartz-Cornil
INRA, Département PHASE, 63122, Saint Genès Champanelle, France
Jean-Baptiste Coulon
ONIRIS-INRA, UMR1300 Bioagression, Épidémiologie et Analyse de risque, Atlanpole La Chantrerie, BP 40706, 44307, Nantes Cedex 3, France
Christine Fourichon & Stéphane Krebs
ENVT-INRA, UMR1225 IHAP Interactions hôtes-agents pathogènes, 23 chemin des Capelles, 31076, Toulouse Cedex, France
Jean-Luc Guérin
INRA, UR1282 IASP Infectiologie animale et santé publique, 37380, Nouzilly, France
Pascal Rainard
INRA, UMR1323 RiTME, 65 Boulevard de Brandebourg, 94205, Ivry-sur-Seine, France
Didier Torny
INRA, USC Bartonella et Tiques, ANSES, 23 Avenue du Général de Gaulle, F-94700, Maisons-Alfort, France
Muriel Vayssier-Taussat
ENVA-ANSES-INRA, UMR1161 Virologie, 7 avenue du Général de Gaulle, 94704, Maisons Alfort Cedex, France
Stephan Zientara
INRA, Département de santé animale, 37380, Nouzilly, France
Etienne Zundel
INRA, Département de santé animale, BP 93173, 31027, Toulouse Cedex 3, France
Thierry Pineau
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian Ducrot .
Additional information
The authors declare that they have no competing interests.
All authors participated in the collective discussion on the special issues of animal health research, search for bibliography and participated in the writing of the paper in their field of competence; more precisely, VB, JLG, PR, ISC, MVT, SZ and EZ were involved in the field of microbiology, ISC in immunology, CF and CD in epidemiology, BB in genetics, JBC and EZ in animal sciences, SK in economics, DT in sociology. CD, CF and SK were involved in the discussion with scientists from OIE, CD, CF and SZ with Société Merial, ISC, SZ and MVT with Institut Pasteur. TP and CD designed the work and defined the working group. CD chaired the discussions and coordinated the paper. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Ducrot, C., Bed'Hom, B., Béringue, V. et al. Issues and special features of animal health research. Vet Res 42 , 96 (2011). https://doi.org/10.1186/1297-9716-42-96
Download citation
Received : 08 June 2011
Accepted : 24 August 2011
Published : 24 August 2011
DOI : https://doi.org/10.1186/1297-9716-42-96
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Animal Health
- Avian Influenza
- Lyme Disease
- Animal Disease
Veterinary Research
ISSN: 1297-9716
- Submission enquiries: [email protected]
- General enquiries: [email protected]
ARC: supporting research projects on animal health and disease
Information on the Animal Health Research Club (ARC), which was established by the Biotechnology and Biological Sciences Research Council (BBSRC) in 2012.
Supporting high-quality, industrially relevant research projects on animal health and disease (PDF)
PDF , 546 KB
If you cannot open or read this document, you can ask for a different format.
Email [email protected] , telling us:
- the name of the document
- what format you need
- any assistive technology you use (such as type of screen reader).
Find out about our approach to the accessibility of our website .
ARC executive summary (PDF)
PDF , 171 KB
These documents provide information on ARC, which was established by BBSRC in 2012, and the research projects it has supported. They include:
- a booklet with case studies of proposals that have been funded by the club
- an executive summary.
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .

CGIAR RESEARCH INITIATIVES
Ilri is actively engaged in several cgiar initiatives and platforms..
- Climate adaptation and mitigation
- Environmental health and biodiversity
- Gender, equality, youth and social inclusion
- Nutrition, health and food security
- Poverty reduction, livelihoods and jobs
- Research compliance
- Communication and knowledge management
- Intellectual property and legal unit
- Data and research methods
- Animal and human health
- Feed and forage development
- Livestock genetics
- Policies, institutions and livelihoods
- Sustainable livestock systems
- Impact at scale
- CGIAR research initiatives
- Capacity development
- Centre for tropical livestock genetics and health
- ILRI Genebank
- Kapiti research station
- Mazingira centre
- One health centre
- Poultry facility
- The CGIAR AMR hub
- Bioscience facility
- Genomics platform

International Land Coalition Rangelands Initiative: Making rangelands secure

ACTIVE Feb 2028
Emerging public health threats in africa’s drylands.
- Burkina Faso
- Explore our work in the countries
- ILRI in the media


Policies and Design Processes to Enable Transformation
- Pereira, Laura
- Vrettos, Chris
- Cramer, Laura K.
- Drimie, Scott
- Muiderman, Karlijn
- Schapendonk, Frans
- Stringer, Lindsay C.
- Veeger, Marieke
- Vervoort, Joost M.
- Wamukoya, George

Opportunities to quantify resilience of dairy cattle to environmental stressors in Sub-Saharan Africa
- Oloo, Richard Dooso
- Ekine-Dzivenu, Chinyere C.
- Ojango, Julie M.K.
- Gebreyohanes, Gebregziabher
- Mrode, Raphael A.
- Okeyo Mwai, Ally
- Chagunda, Mizeck G.G.
- Publications
- Journal articles
- Presentations
- Infographics
- Browse archive

Feed the Future Animal Health Innovation Lab
The goal of the project is to enhance livestock health and productivity, household incomes, food security and human nutrition through East Coast fever control in Kenya.
The Feed the Future Animal Health Innovation Lab will develop local capacity in Kenya to carry out laboratory-based animal health research to improve East Coast fever vaccines and diagnostics and conduct social and economic evaluations of the effects of animal health interventions on human nutrition and wellbeing.
The project also leverages on the Transformation of Animal Health Services and Solutions in Low- and middle-income countries (TAHSSL) platform, an existing partnership of the International Livestock Research Institute, ClinGlobal and GALVmed, to conduct research and clinical studies to meet private-sector standards to facilitate product and market development.
- Improve the infection-and-treatment method of East Coast fever vaccination
- Improve East Coast fever diagnostic tools
- Improve the sub-unit vaccines, anti-sporozoite antibody-mediated vaccine solutions
- Integrate economics, gender and youth to quantify direct and indirect effects of East Coast fever vaccine innovations and interventions on human nutrition and wellbeing
- Assess maternal and child nutritional status in livestock-keeping households
- Build human and institutional capacity for animal health research
Expected outcomes
- Improved East Coast fever vaccines and diagnostics that are available, accessible and routinely used
- Internationally competitive research hub for animal health with extramural funding
- High-quality policy evidence to improve animal health and production
- High-quality scientific research produced by the PhD research fellows
- Animal health research support structures and laboratory infrastructure
- Integration of ethics, biosafety, biocontainment, Good Clinical Practices, Good Manufacturing Practices and gender modules into routine research training
- Enhanced engagement between participating animal health researchers and policymakers
- animal health
- capacity strengthening
- diagnostics
- food security
- human health
You may also like
Building resilience: Combatting Marek's disease in Ghana's chicken industry

Unlocking the power of the genome: A leap towards enhanced food nutrition security and species conservation

Strengthening disease surveillance in Vietnam: Insights from a ‘FarmVetCare’ mobile application training

Tony Barbet, ILRAD, University of Florida tickborne disease molecular biologist, dies

Integrating environmental and ecosystem health into One Health – choices, contexts and communities matter
ILRI and ICAR 2023 annual review of progress of collaborative projects

Available now: Issue 12 of ILRI South Asia Newsletter
Related publications.

Health of Ethiopian Animals for Rural Development project: Public–private partnership models
- Gizaw, Solomon
- Knight-Jones, Theodore J.D.

Innovation packages and scaling readiness workshop: Thermotolerant peste des petits ruminants vaccine
- Dione, Michel M.
- Jasada, Ijudai
- Maiga, Assoumane
- Sow, Ahmadou
- Zannou, Olivier
- Rekik, Mourad
- Hoek, Rein van der

Health of Ethiopian Animals for Rural Development (HEARD): Final project evaluation workshop report
- Desta, Hiwot

Health of Ethiopian Animals for Rural Development (HEARD) overall project brief

Health of Ethiopian Animals for Rural Development (HEARD) project final evaluation validation workshop: Draft report

Training report on mobile application of Farmvetcare for Sub-Department of Animal Health and Livestock Production in Lao Cai Province
- Thang Nguyen
- Phuong Nguyen
- Sinh Dang-Xuan
- Unger, Fred
- Hung Nguyen-Viet
Related Projects

ACTIVE Apr 2032
One health for humans, environment, animals and livelihoods (heal).

ACTIVE Dec 2025
Improving human health through sustainable value chains in human-animal-environmental interactions using ict in vietnam (ict4health).

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
About Grants
The lifecycle of grants and cooperative agreements consists of four phases: Pre-Award, Award, Post-Award, and Close Out.
Access to Data
The National Institute of Food and Agriculture is committed to serving its stakeholders, Congress, and the public by using new technologies to advance greater openness.

Access Data Gateway
The Data Gateway enables users to find funding data, metrics, and information about research, education, and extension projects that have received grant awards from NIFA.
View Resources Page
This website houses a large volume of supporting materials. In this section, you can search the wide range of documents, videos, and other resources.

Featured Webinar
Second annual virtual grants support technical assistance workshop.
Check out this five-day workshop in March 2024 workshop, designed to help you learn about NIFA grants and resources for grants development and management.
The National Institute of Food and Agriculture provides leadership and funding for programs that advance agriculture-related sciences.

Animal Health and Disease Research Capacity Program
The Animal Health and Disease (Section 1433) opportunity is a national-scope capacity program that distributes federal funds on a formula basis to all eligible entities, including all State Agricultural Experiment Stations (SAES) and AVMA accredited US Colleges or Schools of veterinary medicine.
Animal Health and Disease activities support research projects addressing the health and disease of agricultural animals. Applications may only be submitted by an accredited school or college of veterinary medicine or a state agricultural experiment station that conducts animal health and disease research in accordance with NARETPA section 1433(c). Notice that provides guidance on the process for adding eligible accredited colleges of veterinary medicine to the AHDR-33 capacity and infrastructure program.
Expected Program Outputs and Reporting Requirements Organizations receiving Animal Health and Disease funding from the National Institute of Food and Agriculture (NIFA), are required to submit the following reports:
- Annual AHDR Capacity Report
- Program/Project Initiation in the NIFA Reporting System (NRS). For more information on this reporting requirement please visit the NIFA Reporting System page .
- Program/Project progress reports. For more information on this reporting requirement please visit the NIFA Reporting System page .
- Project Financial Report.
- Financial Reporting. Awardees are required to submit a SF-425, Federal Financial Report annually no later than 120 days after the award anniversary date. The final SF-425 is due no later than 120 days after the termination date of the grant. The form should be submitted via ezFedGrants portal . All 1890 institutions questions relating to the SF-425 reports should be directed to [email protected] , all others should be directed to [email protected] .
Request the Administrative Manual for the Continuing Animal Health and Disease Research Program Report from USDA.
The Animal Health and Disease Research document "Essentials of a Project Proposal" is being updated and has been temporarily removed.
Your feedback is important to us.

- About Grants
- Forms & Deadlines
- Grants Policy
- News & Events

Interagency Collaborative Animal Research Education (ICARE) Project

The ICARE Project is a U.S. interagency initiative of the National Institutes of Health (NIH), U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), National Science Foundation (NSF), U.S. Department of Veterans Affairs (VA), National Aeronautics and Space Administration (NASA), and Biomedical Advanced Research and Development Authority (BARDA). This group of federal agencies is involved in the welfare of animals used in research, teaching, and testing in the US. Although federal animal care and use standards vary, all endorse local oversight for the humane care and use of animals by an IACUC.
The aim of the ICARE Project is to empower U.S. Institutional Animal Care and Use Committees (IACUCs) and their institutions to improve animal welfare and increase compliance with federal standards while minimizing regulatory burden. To accomplish this goal, the ICARE Project uses active learning pedagogy which has been shown to increase the effectiveness of adult education in the factual and theoretical understanding of scientific and ethical issues by engaging the learner in activities that require the application of high-level concepts.
Participants for all ICARE offerings are subject to the OLAW Code of Conduct .
Sign up for OLAW newsletters to be notified of upcoming events!
OLAW Conversations (Virtual)
OLAW Conversations is a discussion-based offering from OLAW, that is part of the ICARE Project, where information is presented by subject matter experts, and attendees add to the conversation by sharing their ideas, perspectives, and questions. Experts will also weigh in on participant discussion to offer insights and ensure regulatory compliance, where applicable. These sessions will not be recorded, and therefore, participation will be limited to those who register for the live events and transcripts will not be available. Participation is open to those involved in animal care and use programs, including IACUC members and staff, Institutional Officials, veterinarians, animal facilities personnel, administrators, consultants, and compliance and regulatory personnel. Registration is free. Please note that OLAW is unable to offer RACE or CPIA credit for these sessions at this time.
The views expressed by speakers and moderators do not necessarily reflect the official policies of the Department of HHS; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Resources from Past OLAW Conversations
The following resources have been posted with permission from the speaker.
ICARE Academies (In-Person)
ICARE Academies (IA) offer participants an understanding of research animal welfare and IACUC oversight issues through active learning and scientific teaching. Attendees will work in both large interactive sessions and small facilitated breakout groups. In all of the sessions, attendees will engage in learning activities such as interactive presentations, group work, and discussions. Participation is open to those involved in animal care and use programs, including IACUC members and staff, Institutional Officials, veterinarians, animal facilities personnel, administrators, consultants, and compliance and regulatory personnel. Please note that OLAW is unable to offer RACE or CPIA credit for programs at this time.
ICARE Academies Course Descriptions
IA Intro is a comprehensive 2-day workshop that introduces participants to the regulations and guidelines of the U.S. federal agencies that oversee animal welfare at research institutions. This course is appropriate for those IACUC and animal program personnel who are new to the program, newly promoted, or those who seek a review of federal standards.
IA Refining is a fast-paced 3-day workshop that provides participants with an understanding of animal welfare oversight concepts, enables them to correct misunderstandings of federal standards, and learn methods for more efficient and effective animal program and IACUC functioning. This course is recommended for those IACUC and animal program personnel who are familiar with federal animal welfare oversight standards.
Train the Trainer Institutes (TTI) teach institutional training personnel to use active learning in their training programs. Participants will engage in active learning applied to IACUC subject matter content and modules that address the scientific basis of active learning pedagogy.
Advanced Train the Trainer Institutes (ATTI) offer advanced training in the use of active learning, backward design, and scientific teaching applied to IACUC training. The ATTI is offered to individuals that have completed basic training at TTI.
Training Modules
Training modules developed by the participants of the Train the Trainer Institutes are tools that you can use to implement active learning training for IACUCs at your institution. Additional modules and supplemental materials will be added as they become available.
- ICARE Training Modules
Reference Materials

- Policy & Compliance
- Animals In NIH Research
Animals in NIH Research
Biomedical and behavioral research can involve working with cells in test tubes, computer modeling, laboratory animals, and clinical studies with people. Each kind of research plays a critical role in advancing our knowledge of health and disease. What we have learned from research with laboratory animals (also referred to as “animal models”) has provided the foundation for many safe and effective life-saving treatments for diseases and conditions affecting human health.
Laws, regulations, and policies dictate the requirements that protect animals used in federally funded research. These protections require consideration of non-animal alternatives to meet the scientific objectives. They ensure that the fewest numbers needed for thorough and repeatable results are chosen. They also outline standards that reflect the greatest commitment to animal care.
NIH's Commitment
NIH is committed to supporting ethically conducted, high quality research, which is guided by principles, regulations, and laws. Ensuring that animals receive the best possible care and treatment within the context of research is critical to achieving rigorous, reliable, and scientifically valid results.

Why Animals are Used in Research
Learn about the unique and important roles of animals in biomedical and behavioral research, and how and why they are selected.

How Animals Have Helped Improve Public Health
Learn about how NIH-supported research using animals has contributed to important biomedical advances, including improving our knowledge, health, and society.
Why Properly Designed Experiments are Critical to Animal Research and Advancing Public Health
Learn about NIH’s efforts to ensure that the research it supports involving animals is of the highest quality, rigorous, scientifically valid, and reproducible.

Ensuring the Care of Research Animals
Learn about the laws, regulations, and policies that ensure the greatest commitment to the humane care and use of animals in NIH-supported research.

Adoption of Laboratory Animals after Research
Learn about steps research institutions can take to develop post-research animal adoption programs.

When Are Alternatives to Animals Used in Research
Learn about when it is possible and scientifically appropriate to conduct research that uses non-animal alternative methods. Research is being developed to advance the use of new and known alternatives to animals in research.
This page last updated on: August 19, 2022
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

U.S. Government Accountability Office
Animal Use in Research: NIH Should Strengthen Oversight of Projects It Funds at Foreign Facilities
The National Institutes of Health provided $2.2 billion in contracts or grants in FYs 2011-2021 to foreign organizations for research involving animals. Foreign facilities conducting this research must follow U.S. or international animal welfare standards.
NIH relies on annual reports submitted by funding recipients to monitor the foreign research facilities' compliance with these standards. But NIH doesn't verify the reliability of the information in these reports.
We recommended that NIH take steps, such as visiting foreign facilities, to ensure that it has reliable information about the welfare of the animals used in research that it funds.
What GAO Found
From fiscal year 2011 through fiscal year 2021, the National Institutes of Health (NIH) provided approximately $2.2 billion in contracts or grants to foreign organizations for research projects involving animals. NIH contracts, totaling about $1.9 billion, supported foreign animal research projects in 10 countries. About 90 percent of this contract funding supported research in the Netherlands, Denmark, and the United Kingdom. NIH grants, totaling about $318 million, supported foreign animal research projects in 44 countries. About 66 percent of this grant funding supported research in Australia, Canada, and the United Kingdom.
Examples of Animals That May Be Used in NIH-Funded Foreign Animal Research

Foreign facilities must meet several requirements to perform NIH-funded animal research. For example, the facilities are required to comply with U.S. policy governing animal care and use or provide evidence that acceptable standards for the humane care and use of animals will be met. Each facility must also commit to follow international principles for animal care and use and must certify compliance with relevant laws, regulations, and policies in the country where it operates. NIH has proposed two additional requirements for foreign facilities. These include a requirement that each facility submit an annual report affirming either that there was no reportable noncompliance with animal care and use standards during the year or that it notified NIH of any noncompliance.
NIH has established processes for oversight of foreign animal research, but it does not verify the reliability of annually reported information it uses to monitor compliance for ongoing projects. Before it awards funding, NIH takes some steps to identify, assess, and mitigate any animal welfare risks it identifies. After projects begin, NIH has processes to investigate and, if necessary, remediate any reported noncompliance with animal care and use standards. In addition, NIH uses annual reports submitted by award recipients to monitor foreign research facilities' ongoing compliance with these standards. However, because the award recipients self-generate the information in the annual reports, there are risks that any animal welfare issues may be misrepresented. Yet NIH does not take steps, such as conducting site visits or requiring third-party verification, to ascertain the reliability of this information. As a result, NIH may be missing opportunities to identify and respond to possible instances of noncompliance with animal care and use standards at foreign research facilities.
Why GAO Did This Study
The Department of Health and Human Services' NIH awards over 84 percent of its $45 billion annual budget to support research projects conducted by external organizations. Such projects include laboratory research involving the use of live vertebrate animals in foreign facilities. NIH funds these projects through agreements with domestic and foreign award recipients.
GAO was asked to examine NIH's oversight of the foreign animal research projects it funds. This report (1) identifies the amount of funding NIH awarded to foreign institutions in fiscal years 2011 through 2021 for animal research projects overseas, (2) describes the requirements that foreign facilities must meet to be eligible to perform animal research for NIH-funded projects, and (3) examines NIH's procedures for overseeing the foreign animal research it funds.
GAO analyzed NIH documents and data, including policies and processes related to oversight of, and requirements for, foreign animal research. GAO also interviewed NIH officials and experts in animal research from several organizations.
Recommendations
GAO recommends that NIH take steps—such as conducting site visits or requiring third-party verification—to provide reasonable assurance that award recipients' annual self-reported project information is reliable and adequate to ensure the humane care and use of laboratory animals. The Department of Health and Human Services concurred with this recommendation.
Recommendations for Executive Action
Full report, gao contacts.
Steve Morris Director [email protected] (202) 512-3841
Latesha Love-Grayer Director [email protected] (202) 512-4409
Office of Public Affairs
Sarah Kaczmarek Acting Managing Director [email protected] (202) 512-4800

Phone Numbers
Routine and emergency care.
Companion Animal Hospital in Ithaca, NY for cats, dogs, exotics, and wildlife
Equine and Nemo Farm Animal Hospitals in Ithaca, NY for horses and farm animals
Cornell Ruffian Equine Specialists, on Long Island for every horse
Ambulatory and Production Medicine for service on farms within 30 miles of Ithaca, NY
Animal Health Diagnostic Center New York State Veterinary Diagnostic Laboratory
General Information
Cornell University College of Veterinary Medicine Ithaca, New York 14853-6401

Cornell Feline Health Center
Supporting cat health with information and health studies..

2016 - 2017 Funded Research Projects
The Cornell Feline Health Center Research Grant Program provides vital financial support to Cornell researchers investigating issues that affect feline health. Projects currently funded by the Cornell Feline Health Center range from studies of feline infectious peritonitis (FIP) to finding the genetic causes of certain inherited diseases.
Scientific research has made feline medicine what it is today, and it’s making a healthier, happier tomorrow possible for cats around the world. If you believe in the positive power of our work to make a difference, please consider making a donation to the Cornell Feline Health Center today.

Grant title: Targeting tec Kinase to enhance anti-tumor activity of feline CD8+ cells . Investigators: Avery August, Ph.D.
Searching for the causes of a common tumor In this study, Dr. Angela McCleary-Wheeler will investigate the role of a series of intracellular proteins, part of a metabolic pathway called the Hedgehog pathway, in feline oral squamous cell carcinoma, the most common oral tumor in cats.
Grant title: The role of hedgehog signaling in feline oral squamous cell carcinoma. Investigators: Angela L. McCleary-Wheeler, DVM, Ph.D., DACVIM , with co-investigator Jeanine Peters-Kennedy, DVM, DACVP
Mapping the feline brain This study is focused on using this advanced imaging technique to learn about normal feline brain structures, information that can be used to diagnose structural abnormalities in the brains of cats with a variety of diseases.
Grant title: Advanced magnetic resonance imaging in the normal feline brain. Investigator: Philippa J. Johnson, BVSc, MSc, CertVDI , with co-principle investigator Sofia Cerda-Gonzalez, DVM

Grant title: Analyzing feline sepsis: why are cats not small dogs? Investigators: Robert Goggs, BVSc, Ph.D. with co-investigators Marjorie Brooks,DVM , Dan Fletcher, DVM, Ph.D. , Bruce Kornreich, DVM, Ph.D . , and Jo-Annie Letendre, DVM
Exploring a possible genetic risk for diabetes This research team is using advanced molecular biological techniques to investigate the role of genetics in the development of a variety of feline blood cells and in the risk for the development of diabetes mellitus, a common endocrine disease in cats.
Grant title: Discovery of loci affecting blood phenotypes and diabetes risk in the domestic cats. Investigators: Adam Boyko, Ph.D. with co-investigator Tracy Stokol , BVSc, Ph.D.

IMAGES
VIDEO
COMMENTS
Assess the reproducibility and translatability of the project. 2. Legal issues Consider how the research is affected by relevant legislation for animal research and other areas, e.g. animal transport, occupational health and safety. Locate relevant guidance documents (e.g. EU guidance on project evaluation). 3.
SARS CoV-2 referred to as COVID19 has devastated human populations, and threatens agricultural and companion animals as well as wildlife, including endangered species. The role of animals as incidental hosts is largely unknown. This study will extend the understanding of SARS-CoV-2 and provide data for improved disease risk assessments for wild ...
2022-2023 Funded Research Projects. Congratulations to the seven recipients of our 2022-2023 internal grant awards! Learn more about their projects below. The goal of these internal grants is to provide research support for canine health studies across a diverse range of issues — including, but not limited to — aging, behavior, cancer ...
2022 Research Projects. Your support gives these species a chance. ... The goal of the study is to inform policy and future research that fosters human-tapir coexistence in a region that is both ecologically and economically important. ... the Wild Animal Health Fund, a program of the American Association of Zoo Veterinarians, is a 501(c)(3 ...
Research & Development. At Merck Animal Health, we're focused on innovation. It's the lifeblood of our industry and the path to. tomorrow's breakthrough products. Guided by our customers, we aim to offer products that are more than just effective tools for the treatment and control of animal disease. And because our customers come first ...
These research-based programs discover and develop diagnostics, vaccines, biotherapeutics, disease management systems, and farm biosecurity measures to control animal diseases. The following accomplishments highlight ARS advances in animal health research in FY 2019. Hyperlinked accomplishment titles point to active parent research projects.
Our Impact 137 Funded Projects 80 Different Species $1,639,815.01 Raised for Research Studies This is only the beginning. With support from you, we can ... veterinarians have applied for 461 grants from the Wild Animal Health Fund. ... Some animals go extinct because of health reasons and until we have the research to uncover the many unknowns ...
Leveraging artificial intelligence (AI) approaches in animal health (AH) makes it possible to address highly complex issues such as those encountered in quantitative and predictive epidemiology, animal/human precision-based medicine, or to study host × pathogen interactions. AI may contribute (i) to diagnosis and disease case detection, (ii) to more reliable predictions and reduced errors ...
Fig 1. Using open science practices throughout translational research studies. Application of open science practices at each step of the research process can maximize the impact of performed animal experiments. The implementation of these practices will lead to less time pressure at the end of a project.
Summer Research [email protected]. Undergraduate Summer Research Program. Select. Both undergraduate and Doctor of Veterinary Medicine students work hard on their research projects each summer. Here are some fruits of their efforts: materials created to educate a general public on complex biomedical science work.
In the rapidly changing context of research on animal health, INRA launched a collective discussion on the challenges facing the field, its distinguishing features, and synergies with biomedical research. As has been declared forcibly by the heads of WHO, FAO and OIE, the challenges facing animal health, beyond diseases transmissible to humans, are critically important and involve food ...
The Cornell Feline Health Center Research Grant Program provides vital financial support to Cornell researchers investigating issues that affect feline health. Projects currently funded by the Cornell Feline Health Center range from studies of feline GI disorders to feline cancer. Scientific research has made feline medicine what it is today, and it's making a healthier, happier tomorrow ...
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services. Information on the Animal Health Research Club (ARC), which was established by the Biotechnology and Biological Sciences Research Council (BBSRC) in 2012.
The project also leverages on the Transformation of Animal Health Services and Solutions in Low- and middle-income countries (TAHSSL) platform, an existing partnership of the International Livestock Research Institute, ClinGlobal and GALVmed, to conduct research and clinical studies to meet private-sector standards to facilitate product and ...
Animal Health and Disease activities support research projects addressing the health and disease of agricultural animals. Applications may only be submitted by an accredited school or college of veterinary medicine or a state agricultural experiment station that conducts animal health and disease research in accordance with NARETPA section 1433(c).
2021- 2022 Funded Research Projects: Is Campylobacter a hidden source of canine GI troubles? Dogs can have an upset stomach and diarrhea for many reasons—getting into the trash, a new type of kibble or too many table scraps. But Dr. Kevin Cummings suspects there may be another cause that veterinarians could be missing: a bacterium called Campylobacter jejuni, which is a common cause of food ...
2023 Research Projects. Your support gives these species a chance. Antiviral medications for elephants with EEHV. Elephant endotheliotropic herpesvirus (EEHV) is a devastating, highly fatal hemorrhagic disease that impacts both species of elephants. This study focuses on the effectiveness and dosage of medications to treat infected elephants ...
The ICARE Project is a U.S. interagency initiative of the National Institutes of Health (NIH), U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), National Science Foundation (NSF), U.S. Department of Veterans Affairs (VA), National Aeronautics and Space Administration (NASA), and Biomedical Advanced Research and Development Authority (BARDA).
REEport: Project Proposal Guidelines. For new Animal Health Projects. A Research Proposal is a research plan that must be submitted when a Hatch Regular or Animal Health Project is initiated. It must have clear and documented relevance to agricultural research. This proposal is NOT a detailed proposal like those submitted to NIH/NSF, etc.
Biomedical and behavioral research can involve working with cells in test tubes, computer modeling, laboratory animals, and clinical studies with people. Each kind of research plays a critical role in advancing our knowledge of health and disease. What we have learned from research with laboratory animals (also referred to as "animal models ...
In addition, we will be introducing other ideas for research projects in welfare biology and related areas soon. 1. Exploring methods to assess the welfare of animals living in the wild. During the last few decades scientists have increasingly shown interest in evaluating the wellbeing of animals through the development of welfare assessment ...
2021 - 2022 Funded Research Projects. A Non-Inferiority, Randomized Clinical Trial of Topical Dermatophytosis Treatments in Shelter Cats Receiving Oral Itraconazole. Dermatophytosis, also known as ringworm, is a fungal infection of the skin that can cause itchy red, scaly rashes that can negatively impact a cat's quality of life and that can ...
The National Institutes of Health provided $2.2 billion in contracts or grants in FYs 2011-2021 to foreign organizations for research ... Denmark, and the United Kingdom. NIH grants, totaling about $318 million, supported foreign animal research projects in 44 countries. About 66 percent of this grant funding supported research in Australia ...
Animal research is invaluable for tackling some of the most confounding human diseases, including neurodegenerative conditions, cancer, metabolic disorders, cardiovascular disease, and emerging infections. Designing and testing new therapies and interventions to improve health.
Medical Research Organizations (1) Medical Schools (183) Teaching Hospitals (124) Research & Technology (346) Basic Science (24) Clinical Science (24) Electronic Health Records (8) ... Increasing manmade and natural disasters require new thinking about the role of health care staff, effective triaging, community partnerships, and security.
The Cornell Feline Health Center Research Grant Program provides vital financial support to Cornell researchers investigating issues that affect feline health. Projects currently funded by the Cornell Feline Health Center range from studies of feline infectious peritonitis (FIP) to finding the genetic causes of certain inherited diseases.