
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.6: De Broglie’s Matter Waves
- Last updated
- Save as PDF
- Page ID 4524

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Describe de Broglie’s hypothesis of matter waves
- Explain how the de Broglie’s hypothesis gives the rationale for the quantization of angular momentum in Bohr’s quantum theory of the hydrogen atom
- Describe the Davisson–Germer experiment
- Interpret de Broglie’s idea of matter waves and how they account for electron diffraction phenomena
Compton’s formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie’s hypothesis of matter waves . In 1926, De Broglie’s hypothesis, together with Bohr’s early quantum theory, led to the development of a new theory of wave quantum mechanics to describe the physics of atoms and subatomic particles. Quantum mechanics has paved the way for new engineering inventions and technologies, such as the laser and magnetic resonance imaging (MRI). These new technologies drive discoveries in other sciences such as biology and chemistry.
According to de Broglie’s hypothesis, massless photons as well as massive particles must satisfy one common set of relations that connect the energy \(E\) with the frequency \(f\), and the linear momentum \(p\) with the wavelength \(λ\). We have discussed these relations for photons in the context of Compton’s effect. We are recalling them now in a more general context. Any particle that has energy and momentum is a de Broglie wave of frequency \(f\) and wavelength \(\lambda\):
\[ E = h f \label{6.53} \]
\[ \lambda = \frac{h}{p} \label{6.54} \]
Here, \(E\) and \(p\) are, respectively, the relativistic energy and the momentum of a particle. De Broglie’s relations are usually expressed in terms of the wave vector \(\vec{k}\), \(k = 2 \pi / \lambda\), and the wave frequency \(\omega = 2 \pi f\), as we usually do for waves:
\begin{aligned} &E=\hbar \omega \label{6.55}\\ &\vec{p}=\hbar \vec{k} \label{6.56} \end{aligned}
Wave theory tells us that a wave carries its energy with the group velocity . For matter waves, this group velocity is the velocity \(u\) of the particle. Identifying the energy E and momentum p of a particle with its relativistic energy \(mc^2\) and its relativistic momentum \(mu\), respectively, it follows from de Broglie relations that matter waves satisfy the following relation:
\[ \lambda f =\frac{\omega}{k}=\frac{E / \hbar}{p / \hbar}=\frac{E}{p} = \frac{m c^{2}}{m u}=\frac{c^{2}}{u}=\frac{c}{\beta} \label{6.57} \]
where \(\beta = u/c\). When a particle is massless we have \(u=c\) and Equation \ref{6.57} becomes \(\lambda f = c\).
Example \(\PageIndex{1}\): How Long are de Broglie Matter Waves?
Calculate the de Broglie wavelength of:
- a 0.65-kg basketball thrown at a speed of 10 m/s,
- a nonrelativistic electron with a kinetic energy of 1.0 eV, and
- a relativistic electron with a kinetic energy of 108 keV.
We use Equation \ref{6.57} to find the de Broglie wavelength. When the problem involves a nonrelativistic object moving with a nonrelativistic speed u , such as in (a) when \(\beta=u / c \ll 1\), we use nonrelativistic momentum p . When the nonrelativistic approximation cannot be used, such as in (c), we must use the relativistic momentum \(p=m u=m_{0} \gamma u=E_{0} \gamma \beta/c\), where the rest mass energy of a particle is \(E_0 = m c^2 \) and \(\gamma\) is the Lorentz factor \(\gamma=1 / \sqrt{1-\beta^{2}}\). The total energy \(E\) of a particle is given by Equation \ref{6.53} and the kinetic energy is \(K=E-E_{0}=(\gamma-1) E_{0}\). When the kinetic energy is known, we can invert Equation 6.4.2 to find the momentum
\[ p=\sqrt{\left(E^{2}-E_{0}^{2}\right) / c^{2}}=\sqrt{K\left(K+2 E_{0}\right)} / c \nonumber \]
and substitute into Equation \ref{6.57} to obtain
\[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}} \label{6.58} \]
Depending on the problem at hand, in this equation we can use the following values for hc :
\[ h c=\left(6.626 \times 10^{-34} \: \mathrm{J} \cdot \mathrm{s}\right)\left(2.998 \times 10^{8} \: \mathrm{m} / \mathrm{s}\right)=1.986 \times 10^{-25} \: \mathrm{J} \cdot \mathrm{m}=1.241 \: \mathrm{eV} \cdot \mu \mathrm{m} \nonumber \]
- For the basketball, the kinetic energy is \[ K=m u^{2} / 2=(0.65 \: \mathrm{kg})(10 \: \mathrm{m} / \mathrm{s})^{2} / 2=32.5 \: \mathrm{J} \nonumber \] and the rest mass energy is \[ E_{0}=m c^{2}=(0.65 \: \mathrm{kg})\left(2.998 \times 10^{8} \: \mathrm{m} / \mathrm{s}\right)^{2}=5.84 \times 10^{16} \: \mathrm{J} \nonumber \] We see that \(K /\left(K+E_{0}\right) \ll 1\) and use \(p=m u=(0.65 \: \mathrm{kg})(10 \: \mathrm{m} / \mathrm{s})=6.5 \: \mathrm{J} \cdot \mathrm{s} / \mathrm{m} \): \[ \lambda=\frac{h}{p}=\frac{6.626 \times 10^{-34} \: \mathrm{J} \cdot \mathrm{s}}{6.5 \: \mathrm{J} \cdot \mathrm{s} / \mathrm{m}}=1.02 \times 10^{-34} \: \mathrm{m} \nonumber \]
- For the nonrelativistic electron, \[ E_{0}=mc^{2}=\left(9.109 \times 10^{-31} \mathrm{kg}\right)\left(2.998 \times 10^{8} \mathrm{m} / \mathrm{s}\right)^{2}=511 \mathrm{keV} \nonumber \] and when \(K = 1.0 \: eV\), we have \(K/(K+E_0) = (1/512) \times 10^{-3} \ll 1\), so we can use the nonrelativistic formula. However, it is simpler here to use Equation \ref{6.58}: \[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}}=\frac{1.241 \: \mathrm{eV} \cdot \mu \mathrm{m}}{\sqrt{(1.0 \: \mathrm{eV})[1.0 \: \mathrm{eV}+2(511 \: \mathrm{keV})]}}=1.23 \: \mathrm{nm} \nonumber \] If we use nonrelativistic momentum, we obtain the same result because 1 eV is much smaller than the rest mass of the electron.
- For a fast electron with \(K=108 \: keV\), relativistic effects cannot be neglected because its total energy is \(E = K = E_0 = 108 \: keV + 511 \: keV = 619 \: keV\) and \(K/E = 108/619\) is not negligible: \[ \lambda=\frac{h}{p}=\frac{h c}{\sqrt{K\left(K+2 E_{0}\right)}}=\frac{1.241 \: \mathrm{eV} \cdot \mu \mathrm{m}}{\sqrt{108 \: \mathrm{keV}[108 \: \mathrm{keV}+2(511 \: \mathrm{keV})]}}=3.55 \: \mathrm{pm} \nonumber \].
Significance
We see from these estimates that De Broglie’s wavelengths of macroscopic objects such as a ball are immeasurably small. Therefore, even if they exist, they are not detectable and do not affect the motion of macroscopic objects.
Exercise \(\PageIndex{1}\)
What is de Broglie’s wavelength of a nonrelativistic proton with a kinetic energy of 1.0 eV?
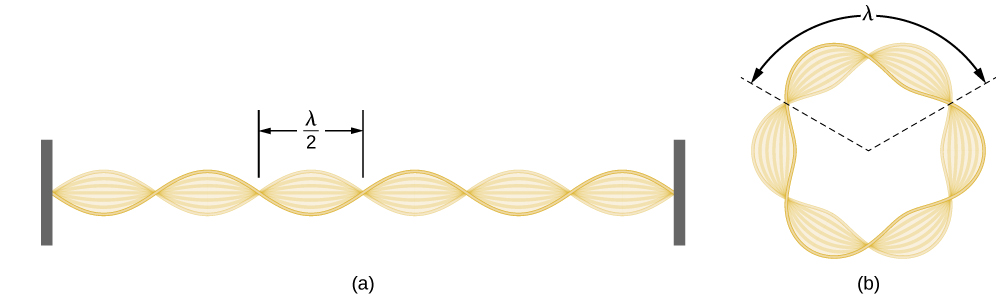
Using the concept of the electron matter wave, de Broglie provided a rationale for the quantization of the electron’s angular momentum in the hydrogen atom, which was postulated in Bohr’s quantum theory. The physical explanation for the first Bohr quantization condition comes naturally when we assume that an electron in a hydrogen atom behaves not like a particle but like a wave. To see it clearly, imagine a stretched guitar string that is clamped at both ends and vibrates in one of its normal modes. If the length of the string is l (Figure \(\PageIndex{1}\)), the wavelengths of these vibrations cannot be arbitrary but must be such that an integer k number of half-wavelengths \(\lambda/2\) fit exactly on the distance l between the ends. This is the condition \(l=k \lambda /2\) for a standing wave on a string. Now suppose that instead of having the string clamped at the walls, we bend its length into a circle and fasten its ends to each other. This produces a circular string that vibrates in normal modes, satisfying the same standing-wave condition, but the number of half-wavelengths must now be an even number \(k\), \(k=2n\), and the length l is now connected to the radius \(r_n\) of the circle. This means that the radii are not arbitrary but must satisfy the following standing-wave condition:
\[ 2 \pi r_{n}=2 n \frac{\lambda}{2} \label{6.59}. \]
If an electron in the n th Bohr orbit moves as a wave, by Equation \ref{6.59} its wavelength must be equal to \(\lambda = 2 \pi r_n / n\). Assuming that Equation \ref{6.58} is valid, the electron wave of this wavelength corresponds to the electron’s linear momentum, \(p = h/\lambda = nh / (2 \pi r_n) = n \hbar /r_n\). In a circular orbit, therefore, the electron’s angular momentum must be
\[ L_{n}=r_{n} p=r_{n} \frac{n \hbar}{r_{n}}=n \hbar \label{6.60} . \]
This equation is the first of Bohr’s quantization conditions, given by Equation 6.5.6 . Providing a physical explanation for Bohr’s quantization condition is a convincing theoretical argument for the existence of matter waves.
Example \(\PageIndex{2}\): The Electron Wave in the Ground State of Hydrogen
Find the de Broglie wavelength of an electron in the ground state of hydrogen.
We combine the first quantization condition in Equation \ref{6.60} with Equation 6.5.6 and use Equation 6.5.9 for the first Bohr radius with \(n = 1\).
When \(n=1\) and \(r_n = a_0 = 0.529 \: Å\), the Bohr quantization condition gives \(a_{0} p=1 \cdot \hbar \Rightarrow p=\hbar / a_{0}\). The electron wavelength is:
\[ \lambda=h / p = h / \hbar / a_{0} = 2 \pi a_{0} = 2 \pi(0.529 \: Å)=3.324 \: Å .\nonumber \]
We obtain the same result when we use Equation \ref{6.58} directly.
Exercise \(\PageIndex{2}\)
Find the de Broglie wavelength of an electron in the third excited state of hydrogen.
\(\lambda = 2 \pi n a_0 = 2 (3.324 \: Å) = 6.648 \: Å\)
Experimental confirmation of matter waves came in 1927 when C. Davisson and L. Germer performed a series of electron-scattering experiments that clearly showed that electrons do behave like waves. Davisson and Germer did not set up their experiment to confirm de Broglie’s hypothesis: The confirmation came as a byproduct of their routine experimental studies of metal surfaces under electron bombardment.
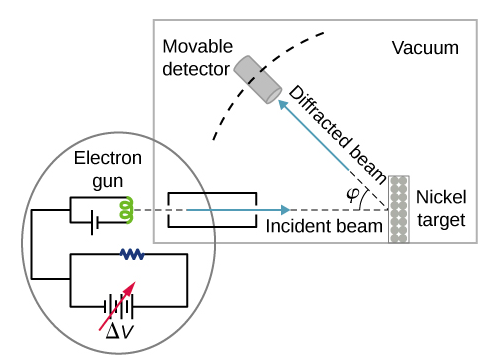
In the particular experiment that provided the very first evidence of electron waves (known today as the Davisson–Germer experiment ), they studied a surface of nickel. Their nickel sample was specially prepared in a high-temperature oven to change its usual polycrystalline structure to a form in which large single-crystal domains occupy the volume. Figure \(\PageIndex{2}\) shows the experimental setup. Thermal electrons are released from a heated element (usually made of tungsten) in the electron gun and accelerated through a potential difference ΔV, becoming a well-collimated beam of electrons produced by an electron gun. The kinetic energy \(K\) of the electrons is adjusted by selecting a value of the potential difference in the electron gun. This produces a beam of electrons with a set value of linear momentum, in accordance with the conservation of energy:
\[ e \Delta V=K=\frac{p^{2}}{2 m} \Rightarrow p=\sqrt{2 m e \Delta V} \label{6.61} \]
The electron beam is incident on the nickel sample in the direction normal to its surface. At the surface, it scatters in various directions. The intensity of the beam scattered in a selected direction φφ is measured by a highly sensitive detector. The detector’s angular position with respect to the direction of the incident beam can be varied from φ=0° to φ=90°. The entire setup is enclosed in a vacuum chamber to prevent electron collisions with air molecules, as such thermal collisions would change the electrons’ kinetic energy and are not desirable.
When the nickel target has a polycrystalline form with many randomly oriented microscopic crystals, the incident electrons scatter off its surface in various random directions. As a result, the intensity of the scattered electron beam is much the same in any direction, resembling a diffuse reflection of light from a porous surface. However, when the nickel target has a regular crystalline structure, the intensity of the scattered electron beam shows a clear maximum at a specific angle and the results show a clear diffraction pattern (see Figure \(\PageIndex{3}\)). Similar diffraction patterns formed by X-rays scattered by various crystalline solids were studied in 1912 by father-and-son physicists William H. Bragg and William L. Bragg. The Bragg law in X-ray crystallography provides a connection between the wavelength \(\lambda\) of the radiation incident on a crystalline lattice, the lattice spacing, and the position of the interference maximum in the diffracted radiation (see Diffraction ).
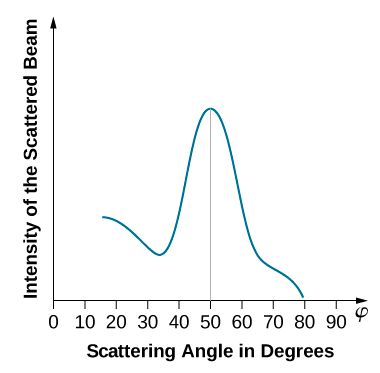
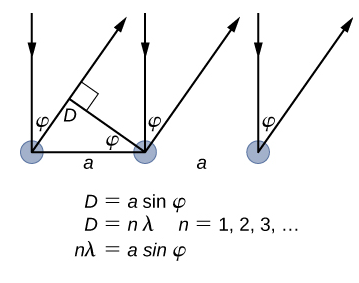
The lattice spacing of the Davisson–Germer target, determined with X-ray crystallography, was measured to be \(a=2.15 \: Å\). Unlike X-ray crystallography in which X-rays penetrate the sample, in the original Davisson–Germer experiment, only the surface atoms interact with the incident electron beam. For the surface diffraction, the maximum intensity of the reflected electron beam is observed for scattering angles that satisfy the condition nλ = a sin φ (see Figure \(\PageIndex{4}\)). The first-order maximum (for n=1) is measured at a scattering angle of φ≈50° at ΔV≈54 V, which gives the wavelength of the incident radiation as λ=(2.15 Å) sin 50° = 1.64 Å. On the other hand, a 54-V potential accelerates the incident electrons to kinetic energies of K = 54 eV. Their momentum, calculated from Equation \ref{6.61}, is \(p = 2.478 \times 10^{−5} \: eV \cdot s/m\). When we substitute this result in Equation \ref{6.58}, the de Broglie wavelength is obtained as
\[ \lambda=\frac{h}{p}=\frac{4.136 \times 10^{-15} \mathrm{eV} \cdot \mathrm{s}}{2.478 \times 10^{-5} \mathrm{eV} \cdot \mathrm{s} / \mathrm{m}}=1.67 \mathrm{Å} \label{6.62}. \]
The same result is obtained when we use K = 54eV in Equation \ref{6.61}. The proximity of this theoretical result to the Davisson–Germer experimental value of λ = 1.64 Å is a convincing argument for the existence of de Broglie matter waves.
Diffraction lines measured with low-energy electrons, such as those used in the Davisson–Germer experiment, are quite broad (Figure \(\PageIndex{3}\)) because the incident electrons are scattered only from the surface. The resolution of diffraction images greatly improves when a higher-energy electron beam passes through a thin metal foil. This occurs because the diffraction image is created by scattering off many crystalline planes inside the volume, and the maxima produced in scattering at Bragg angles are sharp (Figure \(\PageIndex{5}\)).

Since the work of Davisson and Germer, de Broglie’s hypothesis has been extensively tested with various experimental techniques, and the existence of de Broglie waves has been confirmed for numerous elementary particles. Neutrons have been used in scattering experiments to determine crystalline structures of solids from interference patterns formed by neutron matter waves. The neutron has zero charge and its mass is comparable with the mass of a positively charged proton. Both neutrons and protons can be seen as matter waves. Therefore, the property of being a matter wave is not specific to electrically charged particles but is true of all particles in motion. Matter waves of molecules as large as carbon \(C_{60}\) have been measured. All physical objects, small or large, have an associated matter wave as long as they remain in motion. The universal character of de Broglie matter waves is firmly established.
Example \(\PageIndex{3A}\): Neutron Scattering
Suppose that a neutron beam is used in a diffraction experiment on a typical crystalline solid. Estimate the kinetic energy of a neutron (in eV) in the neutron beam and compare it with kinetic energy of an ideal gas in equilibrium at room temperature.
We assume that a typical crystal spacing a is of the order of 1.0 Å. To observe a diffraction pattern on such a lattice, the neutron wavelength λ must be on the same order of magnitude as the lattice spacing. We use Equation \ref{6.61} to find the momentum p and kinetic energy K . To compare this energy with the energy \(E_T\) of ideal gas in equilibrium at room temperature \(T = 300 \, K\), we use the relation \(K = 3/2 k_BT\), where \(k_B = 8.62 \times 10^{-5}eV/K\) is the Boltzmann constant.
We evaluate pc to compare it with the neutron’s rest mass energy \(E_0 = 940 \, MeV\):
\[p = \frac{h}{\lambda} \Rightarrow pc = \frac{hc}{\lambda} = \frac{1.241 \times 10^{-6}eV \cdot m}{10^{-10}m} = 12.41 \, keV. \nonumber \]
We see that \(p^2c^2 << E_0^2\) and we can use the nonrelativistic kinetic energy:
\[K = \frac{p^2}{2m_n} = \frac{h^2}{2\lambda^2 m_n} = \frac{(6.63\times 10^{−34}J \cdot s)^2}{(2\times 10^{−20}m^2)(1.66 \times 10^{−27} kg)} = 1.32 \times 10^{−20} J = 82.7 \, meV. \nonumber \]
Kinetic energy of ideal gas in equilibrium at 300 K is:
\[K_T = \frac{3}{2}k_BT = \frac{3}{2} (8.62 \times 10^{-5}eV/K)(300 \, K) = 38.8 \, MeV. \nonumber \]
We see that these energies are of the same order of magnitude.
Neutrons with energies in this range, which is typical for an ideal gas at room temperature, are called “thermal neutrons.”
Example \(\PageIndex{3B}\): Wavelength of a Relativistic Proton
In a supercollider at CERN, protons can be accelerated to velocities of 0.75 c . What are their de Broglie wavelengths at this speed? What are their kinetic energies?
The rest mass energy of a proton is \(E_0 = m_0c^2 = (1.672 \times 10^{−27} kg)(2.998 \times 10^8m/s)^2 = 938 \, MeV\). When the proton’s velocity is known, we have β = 0.75 and \(\beta \gamma = 0.75 / \sqrt{1 - 0.75^2} = 1.714\). We obtain the wavelength λλ and kinetic energy K from relativistic relations.
\[\lambda = \frac{h}{p} = \frac{hc}{\beta \gamma E_0} = \frac{1.241 \, eV \cdot \mu m}{1.714 (938 \, MeV)} = 0.77 \, fm \nonumber \]
\[K = E_0(\gamma - 1) = 938 \, MeV (1 /\sqrt{1 - 0.75^2} - 1) = 480.1\, MeV \nonumber \]
Notice that because a proton is 1835 times more massive than an electron, if this experiment were performed with electrons, a simple rescaling of these results would give us the electron’s wavelength of (1835)0.77 fm = 1.4 pm and its kinetic energy of 480.1 MeV /1835 = 261.6 keV.
Exercise \(\PageIndex{3}\)
Find the de Broglie wavelength and kinetic energy of a free electron that travels at a speed of 0.75 c .
\(\lambda = 1.417 \, pm; \, K = 261.56 \, keV\)
6.5 De Broglie’s Matter Waves
Learning objectives.
By the end of this section, you will be able to:
- Describe de Broglie’s hypothesis of matter waves
- Explain how the de Broglie’s hypothesis gives the rationale for the quantization of angular momentum in Bohr’s quantum theory of the hydrogen atom
- Describe the Davisson–Germer experiment
- Interpret de Broglie’s idea of matter waves and how they account for electron diffraction phenomena
Compton’s formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie’s hypothesis of matter waves . In 1926, De Broglie’s hypothesis, together with Bohr’s early quantum theory, led to the development of a new theory of wave quantum mechanics to describe the physics of atoms and subatomic particles. Quantum mechanics has paved the way for new engineering inventions and technologies, such as the laser and magnetic resonance imaging (MRI). These new technologies drive discoveries in other sciences such as biology and chemistry.
According to de Broglie’s hypothesis, massless photons as well as massive particles must satisfy one common set of relations that connect the energy E with the frequency f , and the linear momentum p with the wavelength λ . λ . We have discussed these relations for photons in the context of Compton’s effect. We are recalling them now in a more general context. Any particle that has energy and momentum is a de Broglie wave of frequency f and wavelength λ : λ :
Here, E and p are, respectively, the relativistic energy and the momentum of a particle. De Broglie’s relations are usually expressed in terms of the wave vector k → , k → , k = 2 π / λ , k = 2 π / λ , and the wave frequency ω = 2 π f , ω = 2 π f , as we usually do for waves:
Wave theory tells us that a wave carries its energy with the group velocity . For matter waves, this group velocity is the velocity u of the particle. Identifying the energy E and momentum p of a particle with its relativistic energy m c 2 m c 2 and its relativistic momentum mu , respectively, it follows from de Broglie relations that matter waves satisfy the following relation:
where β = u / c . β = u / c . When a particle is massless we have u = c u = c and Equation 6.57 becomes λ f = c . λ f = c .
Example 6.11
How long are de broglie matter waves.
Depending on the problem at hand, in this equation we can use the following values for hc : h c = ( 6.626 × 10 −34 J · s ) ( 2.998 × 10 8 m/s ) = 1.986 × 10 −25 J · m = 1.241 eV · μ m h c = ( 6.626 × 10 −34 J · s ) ( 2.998 × 10 8 m/s ) = 1.986 × 10 −25 J · m = 1.241 eV · μ m
- For the basketball, the kinetic energy is K = m u 2 / 2 = ( 0.65 kg ) ( 10 m/s ) 2 / 2 = 32.5 J K = m u 2 / 2 = ( 0.65 kg ) ( 10 m/s ) 2 / 2 = 32.5 J and the rest mass energy is E 0 = m c 2 = ( 0.65 kg ) ( 2.998 × 10 8 m/s ) 2 = 5.84 × 10 16 J. E 0 = m c 2 = ( 0.65 kg ) ( 2.998 × 10 8 m/s ) 2 = 5.84 × 10 16 J. We see that K / ( K + E 0 ) ≪ 1 K / ( K + E 0 ) ≪ 1 and use p = m u = ( 0.65 kg ) ( 10 m/s ) = 6.5 J · s/m : p = m u = ( 0.65 kg ) ( 10 m/s ) = 6.5 J · s/m : λ = h p = 6.626 × 10 −34 J · s 6.5 J · s/m = 1.02 × 10 −34 m . λ = h p = 6.626 × 10 −34 J · s 6.5 J · s/m = 1.02 × 10 −34 m .
- For the nonrelativistic electron, E 0 = m c 2 = ( 9.109 × 10 −31 kg ) ( 2.998 × 10 8 m/s ) 2 = 511 keV E 0 = m c 2 = ( 9.109 × 10 −31 kg ) ( 2.998 × 10 8 m/s ) 2 = 511 keV and when K = 1.0 eV , K = 1.0 eV , we have K / ( K + E 0 ) = ( 1 / 512 ) × 10 −3 ≪ 1 , K / ( K + E 0 ) = ( 1 / 512 ) × 10 −3 ≪ 1 , so we can use the nonrelativistic formula. However, it is simpler here to use Equation 6.58 : λ = h p = h c K ( K + 2 E 0 ) = 1.241 eV · μ m ( 1.0 eV ) [ 1.0 eV+ 2 ( 511 keV ) ] = 1.23 nm . λ = h p = h c K ( K + 2 E 0 ) = 1.241 eV · μ m ( 1.0 eV ) [ 1.0 eV+ 2 ( 511 keV ) ] = 1.23 nm . If we use nonrelativistic momentum, we obtain the same result because 1 eV is much smaller than the rest mass of the electron.
- For a fast electron with K = 108 keV, K = 108 keV, relativistic effects cannot be neglected because its total energy is E = K + E 0 = 108 keV + 511 keV = 619 keV E = K + E 0 = 108 keV + 511 keV = 619 keV and K / E = 108 / 619 K / E = 108 / 619 is not negligible: λ = h p = h c K ( K + 2 E 0 ) = 1.241 eV · μm 108 keV [ 108 keV + 2 ( 511 keV ) ] = 3.55 pm . λ = h p = h c K ( K + 2 E 0 ) = 1.241 eV · μm 108 keV [ 108 keV + 2 ( 511 keV ) ] = 3.55 pm .
Significance
Check your understanding 6.11.
What is de Broglie’s wavelength of a nonrelativistic proton with a kinetic energy of 1.0 eV?
Using the concept of the electron matter wave, de Broglie provided a rationale for the quantization of the electron’s angular momentum in the hydrogen atom, which was postulated in Bohr’s quantum theory. The physical explanation for the first Bohr quantization condition comes naturally when we assume that an electron in a hydrogen atom behaves not like a particle but like a wave. To see it clearly, imagine a stretched guitar string that is clamped at both ends and vibrates in one of its normal modes. If the length of the string is l ( Figure 6.18 ), the wavelengths of these vibrations cannot be arbitrary but must be such that an integer k number of half-wavelengths λ / 2 λ / 2 fit exactly on the distance l between the ends. This is the condition l = k λ / 2 l = k λ / 2 for a standing wave on a string. Now suppose that instead of having the string clamped at the walls, we bend its length into a circle and fasten its ends to each other. This produces a circular string that vibrates in normal modes, satisfying the same standing-wave condition, but the number of half-wavelengths must now be an even number k , k = 2 n , k , k = 2 n , and the length l is now connected to the radius r n r n of the circle. This means that the radii are not arbitrary but must satisfy the following standing-wave condition:
If an electron in the n th Bohr orbit moves as a wave, by Equation 6.59 its wavelength must be equal to λ = 2 π r n / n . λ = 2 π r n / n . Assuming that Equation 6.58 is valid, the electron wave of this wavelength corresponds to the electron’s linear momentum, p = h / λ = n h / ( 2 π r n ) = n ℏ / r n . p = h / λ = n h / ( 2 π r n ) = n ℏ / r n . In a circular orbit, therefore, the electron’s angular momentum must be
This equation is the first of Bohr’s quantization conditions, given by Equation 6.36 . Providing a physical explanation for Bohr’s quantization condition is a convincing theoretical argument for the existence of matter waves.
Example 6.12
The electron wave in the ground state of hydrogen, check your understanding 6.12.
Find the de Broglie wavelength of an electron in the third excited state of hydrogen.
Experimental confirmation of matter waves came in 1927 when C. Davisson and L. Germer performed a series of electron-scattering experiments that clearly showed that electrons do behave like waves. Davisson and Germer did not set up their experiment to confirm de Broglie’s hypothesis: The confirmation came as a byproduct of their routine experimental studies of metal surfaces under electron bombardment.
In the particular experiment that provided the very first evidence of electron waves (known today as the Davisson–Germer experiment ), they studied a surface of nickel. Their nickel sample was specially prepared in a high-temperature oven to change its usual polycrystalline structure to a form in which large single-crystal domains occupy the volume. Figure 6.19 shows the experimental setup. Thermal electrons are released from a heated element (usually made of tungsten) in the electron gun and accelerated through a potential difference Δ V , Δ V , becoming a well-collimated beam of electrons produced by an electron gun. The kinetic energy K of the electrons is adjusted by selecting a value of the potential difference in the electron gun. This produces a beam of electrons with a set value of linear momentum, in accordance with the conservation of energy:
The electron beam is incident on the nickel sample in the direction normal to its surface. At the surface, it scatters in various directions. The intensity of the beam scattered in a selected direction φ φ is measured by a highly sensitive detector. The detector’s angular position with respect to the direction of the incident beam can be varied from φ = 0 ° φ = 0 ° to φ = 90 ° . φ = 90 ° . The entire setup is enclosed in a vacuum chamber to prevent electron collisions with air molecules, as such thermal collisions would change the electrons’ kinetic energy and are not desirable.
When the nickel target has a polycrystalline form with many randomly oriented microscopic crystals, the incident electrons scatter off its surface in various random directions. As a result, the intensity of the scattered electron beam is much the same in any direction, resembling a diffuse reflection of light from a porous surface. However, when the nickel target has a regular crystalline structure, the intensity of the scattered electron beam shows a clear maximum at a specific angle and the results show a clear diffraction pattern (see Figure 6.20 ). Similar diffraction patterns formed by X-rays scattered by various crystalline solids were studied in 1912 by father-and-son physicists William H. Bragg and William L. Bragg . The Bragg law in X-ray crystallography provides a connection between the wavelength λ λ of the radiation incident on a crystalline lattice, the lattice spacing, and the position of the interference maximum in the diffracted radiation (see Diffraction ).
The lattice spacing of the Davisson–Germer target, determined with X-ray crystallography, was measured to be a = 2.15 Å . a = 2.15 Å . Unlike X-ray crystallography in which X-rays penetrate the sample, in the original Davisson–Germer experiment, only the surface atoms interact with the incident electron beam. For the surface diffraction, the maximum intensity of the reflected electron beam is observed for scattering angles that satisfy the condition n λ = a sin φ n λ = a sin φ (see Figure 6.21 ). The first-order maximum (for n = 1 n = 1 ) is measured at a scattering angle of φ ≈ 50 ° φ ≈ 50 ° at Δ V ≈ 54 V , Δ V ≈ 54 V , which gives the wavelength of the incident radiation as λ = ( 2.15 Å ) sin 50 ° = 1.64 Å . λ = ( 2.15 Å ) sin 50 ° = 1.64 Å . On the other hand, a 54-V potential accelerates the incident electrons to kinetic energies of K = 54 eV . K = 54 eV . Their momentum, calculated from Equation 6.61 , is p = 2.478 × 10 −5 eV · s / m . p = 2.478 × 10 −5 eV · s / m . When we substitute this result in Equation 6.58 , the de Broglie wavelength is obtained as
The same result is obtained when we use K = 54 eV K = 54 eV in Equation 6.61 . The proximity of this theoretical result to the Davisson–Germer experimental value of λ = 1.64 Å λ = 1.64 Å is a convincing argument for the existence of de Broglie matter waves.
Diffraction lines measured with low-energy electrons, such as those used in the Davisson–Germer experiment, are quite broad (see Figure 6.20 ) because the incident electrons are scattered only from the surface. The resolution of diffraction images greatly improves when a higher-energy electron beam passes through a thin metal foil. This occurs because the diffraction image is created by scattering off many crystalline planes inside the volume, and the maxima produced in scattering at Bragg angles are sharp (see Figure 6.22 ).
Since the work of Davisson and Germer, de Broglie’s hypothesis has been extensively tested with various experimental techniques, and the existence of de Broglie waves has been confirmed for numerous elementary particles. Neutrons have been used in scattering experiments to determine crystalline structures of solids from interference patterns formed by neutron matter waves. The neutron has zero charge and its mass is comparable with the mass of a positively charged proton. Both neutrons and protons can be seen as matter waves. Therefore, the property of being a matter wave is not specific to electrically charged particles but is true of all particles in motion. Matter waves of molecules as large as carbon C 60 C 60 have been measured. All physical objects, small or large, have an associated matter wave as long as they remain in motion. The universal character of de Broglie matter waves is firmly established.
Example 6.13
Neutron scattering.
We see that p 2 c 2 ≪ E 0 2 p 2 c 2 ≪ E 0 2 so K ≪ E 0 K ≪ E 0 and we can use the nonrelativistic kinetic energy:
Kinetic energy of ideal gas in equilibrium at 300 K is:
We see that these energies are of the same order of magnitude.
Example 6.14
Wavelength of a relativistic proton, check your understanding 6.13.
Find the de Broglie wavelength and kinetic energy of a free electron that travels at a speed of 0.75 c .
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/university-physics-volume-3/pages/1-introduction
- Authors: Samuel J. Ling, Jeff Sanny, William Moebs
- Publisher/website: OpenStax
- Book title: University Physics Volume 3
- Publication date: Sep 29, 2016
- Location: Houston, Texas
- Book URL: https://openstax.org/books/university-physics-volume-3/pages/1-introduction
- Section URL: https://openstax.org/books/university-physics-volume-3/pages/6-5-de-broglies-matter-waves
© Jan 19, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Talk to our experts
1800-120-456-456
- De Broglie Equation

Introduction
The wave nature of light was the only aspect that was considered until Neil Bohr’s model. Later, however, Max Planck in his explanation of quantum theory hypothesized that light is made of very minute pockets of energy which are in turn made of photons or quanta. It was then considered that light has a particle nature and every packet of light always emits a certain fixed amount of energy.
By this, the energy of photons can be expressed as:
E = hf = h * c/λ
Here, h is Plank’s constant
F refers to the frequency of the waves
Λ implies the wavelength of the pockets
Therefore, this basically insinuates that light has both the properties of particle duality as well as wave.
Louis de Broglie was a student of Bohr, who then formulated his own hypothesis of wave-particle duality, drawn from this understanding of light. Later on, when this hypothesis was proven true, it became a very important concept in particle physics.
⇒ Don't Miss Out: Get Your Free JEE Main Rank Predictor 2024 Instantly! 🚀
What is the De Broglie Equation?
Quantum mechanics assumes matter to be both like a wave as well as a particle at the sub-atomic level. The De Broglie equation states that every particle that moves can sometimes act as a wave, and sometimes as a particle. The wave which is associated with the particles that are moving are known as the matter-wave, and also as the De Broglie wave. The wavelength is known as the de Broglie wavelength.
For an electron, de Broglie wavelength equation is:
λ = \[\frac{h}{mv}\]
Here, λ points to the wave of the electron in question
M is the mass of the electron
V is the velocity of the electron
Mv is the momentum that is formed as a result
It was found out that this equation works and applies to every form of matter in the universe, i.e, Everything in this universe, from living beings to inanimate objects, all have wave particle duality.
Significance of De Broglie Equation
De Broglie says that all the objects that are in motion have a particle nature. However, if we look at a moving ball or a moving car, they don’t seem to have particle nature. To make this clear, De Broglie derived the wavelengths of electrons and a cricket ball. Now, let’s understand how he did this.
De Broglie Wavelength
1. De Broglie Wavelength for a Cricket Ball
Let’s say,Mass of the ball = 150 g (150 x 10⁻³ kg),
Velocity = 35 m/s,
and h = 6.626 x 10⁻³⁴ Js
Now, putting these values in the equation
λ = (6.626 * 10 to power of -34)/ (150 * 10 to power of -3 *35)
This yields
λBALL = 1.2621 x 10 to the power of -34 m,
Which is 1.2621 x 10 to the power of -24 Å.
We know that Å is a very small unit, and therefore the value is in the power of 10−24−24^{-24}, which is a very small value. From here, we see that the moving cricket ball is a particle.
Now, the question arises if this ball has a wave nature or not. Your answer will be a big no because the value of λBALL is immeasurable. This proves that de Broglie’s theory of wave-particle duality is valid for the moving objects ‘up to’ the size (not equal to the size) of the electrons.
De Broglie Wavelength for an Electron
We know that me = 9.1 x 10 to power of -31 kg
and ve = 218 x 10 to power of -6 m/s
Now, putting these values in the equation λ = h/mv, which yields λ = 3.2 Å.
This value is measurable. Therefore, we can say that electrons have wave-particle duality. Thus all the big objects have a wave nature and microscopic objects like electrons have wave-particle nature.
E = hν = \[\frac{hc}{\lambda }\]
The Conclusion of De Broglie Hypothesis
From de Broglie equation for a material particle, i.e.,
λ = \[\frac{h}{p}\]or \[\frac{h}{mv}\], we conclude the following:
i. If v = 0, then λ = ∞, and
If v = ∞, then λ = 0
It means that waves are associated with the moving material particles only. This implies these waves are independent of their charge.


FAQs on De Broglie Equation
1.The De Broglie hypothesis was confirmed through which means?
De Broglie had not proved the validity of his hypothesis on his own, it was merely a hypothetical assumption before it was tested out and consequently, it was found that all substances in the universe have wave-particle duality. A number of experiments were conducted with Fresnel diffraction as well as a specular reflection of neutral atoms. These experiments proved the validity of De Broglie’s statements and made his hypothesis come true. These experiments were conducted by some of his students.
2.What exactly does the De Broglie equation apply to?
In very broad terms, this applies to pretty much everything in the tangible universe. This means that people, non-living things, trees and animals, all of these come under the purview of the hypothesis. Any particle of any substance that has matter and has linear momentum also is a wave. The wavelength will be inversely related to the magnitude of the linear momentum of the particle. Therefore, everything in the universe that has matter, is applicable to fit under the De Broglie equation.
3.Is it possible that a single photon also has a wavelength?
When De Broglie had proposed his hypothesis, he derived from the work of Planck that light is made up of small pockets that have a certain energy, known as photons. For his own hypothesis, he said that all things in the universe that have to matter have wave-particle duality, and therefore, wavelength. This extends to light as well, since it was proved that light is made up of matter (photons). Hence, it is true that even a single photon has a wavelength.
4.Are there any practical applications of the De Broglie equation?
It would be wrong to say that people use this equation in their everyday lives, because they do not, not in the literal sense at least. However, practical applications do not only refer to whether they can tangibly be used by everyone. The truth of the De Broglie equation lies in the fact that we, as human beings, also are made of matter and thus we also have wave-particle duality. All the things we work with have wave-particle duality.
5.Does the De Broglie equation apply to an electron?
Yes, this equation is applicable for every single moving body in the universe, down to the smallest subatomic levels. Just how light particles like photons have their own wavelengths, it is also true for an electron. The equation treats electrons as both waves as well as particles, only then will it have wave-particle duality. For every electron of every atom of every element, this stands true and using the equation mentioned, the wavelength of an electron can also be calculated.
6.Derive the relation between De Broglie wavelength and temperature.
We know that the average KE of a particle is:
K = 3/2 k b T
Where k b is Boltzmann’s constant, and
T = temperature in Kelvin
The kinetic energy of a particle is ½ mv²
The momentum of a particle, p = mv = √2mK
= √2m(3/2)KbT = √2mKbT
de Broglie wavelength, λ = h/p = h√2mkbT
7.If an electron behaves like a wave, what should determine its wavelength and frequency?
Momentum and energy determine the wavelength and frequency of an electron.
8. Find λ associated with an H 2 of mass 3 a.m.u moving with a velocity of 4 km/s.
Here, v = 4 x 10³ m/s
Mass of hydrogen = 3 a.m.u = 3 x 1.67 x 10⁻²⁷kg = 5 x 10⁻²⁷kg
On putting these values in the equation λ = h/mv we get
λ = (6.626 x 10⁻³⁴)/(4 x 10³ x 5 x 10⁻²⁷) = 3 x 10⁻¹¹ m.
9. If the KE of an electron increases by 21%, find the percentage change in its De Broglie wavelength.
We know that λ = h/√2mK
So, λ i = h/√(2m x 100) , and λ f = h/√(2m x 121)
% change in λ is:
Change in wavelength/Original x 100 = (λ fi - λ f )/λ i = ((h/√2m)(1/10 - 1/21))/(h/√2m)(1/10)
On solving, we get
% change in λ = 5.238 %
Reset password New user? Sign up
Existing user? Log in
De Broglie Hypothesis
Already have an account? Log in here.
Today we know that every particle exhibits both matter and wave nature. This is called wave-particle duality . The concept that matter behaves like wave is called the de Broglie hypothesis , named after Louis de Broglie, who proposed it in 1924.
De Broglie Equation
Explanation of bohr's quantization rule.
De Broglie gave the following equation which can be used to calculate de Broglie wavelength, \(\lambda\), of any massed particle whose momentum is known:
\[\lambda = \frac{h}{p},\]
where \(h\) is the Plank's constant and \(p\) is the momentum of the particle whose wavelength we need to find.
With some modifications the following equation can also be written for velocity \((v)\) or kinetic energy \((K)\) of the particle (of mass \(m\)):
\[\lambda = \frac{h}{mv} = \frac{h}{\sqrt{2mK}}.\]
Notice that for heavy particles, the de Broglie wavelength is very small, in fact negligible. Hence, we can conclude that though heavy particles do exhibit wave nature, it can be neglected as it's insignificant in all practical terms of use.
Calculate the de Broglie wavelength of a golf ball whose mass is 40 grams and whose velocity is 6 m/s. We have \[\lambda = \frac{h}{mv} = \frac{6.63 \times 10^{-34}}{40 \times 10^{-3} \times 6} \text{ m}=2.76 \times 10^{-33} \text{ m}.\ _\square\]
One of the main limitations of Bohr's atomic theory was that no justification was given for the principle of quantization of angular momentum. It does not explain the assumption that why an electron can rotate only in those orbits in which the angular momentum of the electron, \(mvr,\) is a whole number multiple of \( \frac{h}{2\pi} \).
De Broglie successfully provided the explanation to Bohr's assumption by his hypothesis.
Problem Loading...
Note Loading...
Set Loading...
De Broglie Hypothesis
Does All Matter Exhibit Wave-like Properties?
- Quantum Physics
- Physics Laws, Concepts, and Principles
- Important Physicists
- Thermodynamics
- Cosmology & Astrophysics
- Weather & Climate
:max_bytes(150000):strip_icc():format(webp)/AZJFaceShot-56a72b155f9b58b7d0e783fa.jpg)
- M.S., Mathematics Education, Indiana University
- B.A., Physics, Wabash College
The De Broglie hypothesis proposes that all matter exhibits wave-like properties and relates the observed wavelength of matter to its momentum. After Albert Einstein's photon theory became accepted, the question became whether this was true only for light or whether material objects also exhibited wave-like behavior. Here is how the De Broglie hypothesis was developed.
De Broglie's Thesis
In his 1923 (or 1924, depending on the source) doctoral dissertation, the French physicist Louis de Broglie made a bold assertion. Considering Einstein's relationship of wavelength lambda to momentum p , de Broglie proposed that this relationship would determine the wavelength of any matter, in the relationship:
lambda = h / p
recall that h is Planck's constant
This wavelength is called the de Broglie wavelength . The reason he chose the momentum equation over the energy equation is that it was unclear, with matter, whether E should be total energy, kinetic energy, or total relativistic energy. For photons, they are all the same, but not so for matter.
Assuming the momentum relationship, however, allowed the derivation of a similar de Broglie relationship for frequency f using the kinetic energy E k :
f = E k / h
Alternate Formulations
De Broglie's relationships are sometimes expressed in terms of Dirac's constant, h-bar = h / (2 pi ), and the angular frequency w and wavenumber k :
p = h-bar * kE k
= h-bar * w
Experimental Confirmation
In 1927, physicists Clinton Davisson and Lester Germer, of Bell Labs, performed an experiment where they fired electrons at a crystalline nickel target. The resulting diffraction pattern matched the predictions of the de Broglie wavelength. De Broglie received the 1929 Nobel Prize for his theory (the first time it was ever awarded for a Ph.D. thesis) and Davisson/Germer jointly won it in 1937 for the experimental discovery of electron diffraction (and thus the proving of de Broglie's hypothesis).
Further experiments have held de Broglie's hypothesis to be true, including the quantum variants of the double slit experiment . Diffraction experiments in 1999 confirmed the de Broglie wavelength for the behavior of molecules as large as buckyballs, which are complex molecules made up of 60 or more carbon atoms.
Significance of the de Broglie Hypothesis
The de Broglie hypothesis showed that wave-particle duality was not merely an aberrant behavior of light, but rather was a fundamental principle exhibited by both radiation and matter. As such, it becomes possible to use wave equations to describe material behavior, so long as one properly applies the de Broglie wavelength. This would prove crucial to the development of quantum mechanics. It is now an integral part of the theory of atomic structure and particle physics.
Macroscopic Objects and Wavelength
Though de Broglie's hypothesis predicts wavelengths for matter of any size, there are realistic limits on when it's useful. A baseball thrown at a pitcher has a de Broglie wavelength that is smaller than the diameter of a proton by about 20 orders of magnitude. The wave aspects of a macroscopic object are so tiny as to be unobservable in any useful sense, although interesting to muse about.
- Wave Particle Duality and How It Works
- de Broglie Equation Definition
- De Broglie Wavelength Example Problem
- Wave-Particle Duality - Definition
- Quantum Physics Overview
- Top 10 Weird but Cool Physics Ideas
- The Photoelectric Effect
- What the Compton Effect Is and How It Works in Physics
- Understanding the Heisenberg Uncertainty Principle
- Photoelectric Effect: Electrons from Matter and Light
- Erwin Schrödinger and the Schrödinger's Cat Thought Experiment
- What Is a Photon in Physics?
- Orbital Definition and Example
- How to Solve an Energy From Wavelength Problem
- A Brief History of Atomic Theory
- Photon Definition
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Class 12 Physics (India)
Course: class 12 physics (india) > unit 11, de broglie wavelength.
- Comparing de Broglie wavelengths: Solved example
- de Broglie wavelength
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.5: The de Broglie-Bohr Model for the Hydrogen Atom - Version 4
- Last updated
- Save as PDF
- Page ID 154850
- Frank Rioux
- College of Saint Benedict/Saint John's University
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
The 1913 Bohr model of the hydrogen atom was replaced by Schrödingerʹs wave mechanical model in 1926. However, Bohrʹs model is still profitably taught today because of its conceptual and mathematical simplicity, and because it introduced a number of key quantum mechanical ideas such as the quantum number, quantization of observable properties, quantum jump and stationary state.
Bohr calculated the manifold of allowed electron energies by balancing the mechanical forces (centripetal and electron‐nucleus) on an electron executing a circular orbit of radius R about the nucleus, and then arbitarily quantizing its angular momentum. Finally by fiat he declared that the electron was in a non‐radiating stationary state because an orbiting (accelerating) charge radiates energy and will collapse into the oppositely charge nucleus.
In 1924 de Broglie postulated wave‐particle duality for the electron and other massive particles, thereby providing the opportunity to remove some of the arbitariness from Bohrʹs model. For example, an electron possessing wave properties is subject to constructive and destructive interference. As will be shown this leads naturally to quantization of electron momentum and kinetic energy, and consequently a manifold of allowed energy states for the electron relative to the nucleus. The de Broglie‐Bohr model of the hydrogen atom presented here treats the electron as a particle on a ring with wave‐like properties.
\[ \lambda = \frac{h}{m_e v} \nonumber \]
de Broglie's hypothesis that matter has wave-like properties.
\[n \lambda = 2 \pi r \nonumber \]
The consequence of de Broglieʹs hypothesis; an integral number of wavelengths must fit within the circumference of the orbit. This introduces the quantum number which can have values 1,2,3,... The n = 4 electron state is shown below.
\[ m_e v = \frac{n h }{2 \pi r} \nonumber \]
Substitution of the first equation into the second equation reveals that momentum is quantized.
\[ T = \frac{1}{2} m_e v^2 = \frac{n^2 h^2}{8 \pi^2 m_e r^2} \nonumber \]
If momentum is quantized, so is kinetic energy.
\[ E = T + V = \frac{n^2 h^2}{8 \pi^2 m_e r^2} - \frac{e^2}{4 \pi \varepsilon_0 r} \nonumber \]
Which means that total energy is quantized. The second term is the electron‐proton electrostatic potential energy.
The quantum mechanical interpretation of these ʺBohr orbitsʺ is that they are stationary states. In spite of the fact that we use the expression kinetic energy, which implies electron motion, there is no motion. The electron occupies the orbit as a particle‐wave, it is not orbiting the nucleus. If it was orbiting in a classical sense it would radiate energy and quickly collapse into the nucleus. Clearly the stability of matter requires the quantum mechanical version of kinetic energy.
The ground state energy and orbit radius of the electron in the hydrogen atom is found by plotting the energy as a function of the orbital radius. The ground state is the minimum in the total energy curve. Naturally calculus can be used to obtain the same information by minimizing the energy with respect to the orbit radius. However, the graphical method has the virtue of illuminating the issue of atomic stability.
Fundamental constants: electron charge, electron mass, Planck's constant, vacuum permitivity.
\[ \begin{matrix} e = 1.6021777 (10)^{-19} \text{coul} & m_e = 9.10939 (10)^{-31} \text{kg} \\ h = 6.62608 (10)^{-34} \text{joule sec} & \varepsilon_0 = 8.85419 (10)^{-12} \frac{ \text{coul}^2}{ \text{joule m}} \end{matrix} \nonumber \]
Quantum number and conversion fact between meters and picometers and joules and attojoules.
\[ \begin{matrix} n = 1 & pm = 10^{-12} m & \text{ajoule} = 10^{-18} \text{joule} \end{matrix} \nonumber \]
\[ \begin{matrix} r = 20 pm,~20.5 pm,~ 500 pm & T(r) = \frac{n^2 h^2}{8 \pi^2 m_e r^2} & V(r) = - \frac{e^2}{4 \pi \varepsilon_0 r} & E(r) = T(r) + V(r) \end{matrix} \nonumber \]
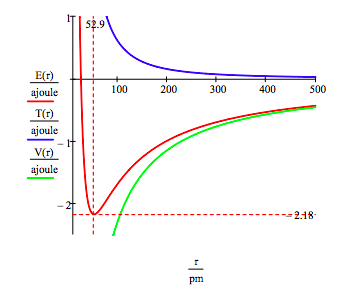
This figure shows that atomic stability involves a balance between potential and kinetic energy. The electron is drawn toward the nucleus by the attractive potential energy interaction (~ ‐1/R), but is prevented from collapsing into the nucleus by the extremely large kinetic energy (~1/R2) associated with small orbits.
As shown below, the graphical approach can also be used to find the electronic excited states.
\[ \begin{matrix} n = 2 & T(r) = \frac{n^2 h^2}{8 \pi^2 m_e r^2} & V(r) = - \frac{e^2}{4 \pi \varepsilon_0 r} & E(r) = T(r) + V(r) \end{matrix} \nonumber \]
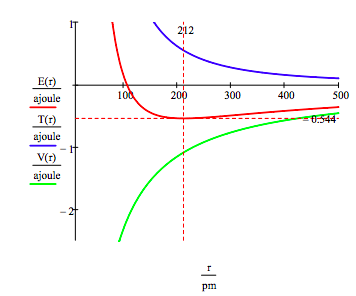
As mentioned earlier the manifold of allowed electron energies can also be obtained by minimizing the energy with respect to the orbit radius. This procedure yields,
\[ \begin{matrix} E_n = - \frac{m_e e^4}{2 \left(4 \pi \varepsilon_0 \right)^2 \hbar^2 } \frac{1}{n^2} & \text{and} & r_n = \frac{4 \pi \varepsilon_0 \hbar^2}{m_e e^2} n^2 \end{matrix} \nonumber \]
- Rotational Motion
Angular Momentum Of Electron
In this article, we will be learning about the angular momentum of electrons in detail. We will also be learning about De Broglie’s Explanation of the Quantization of Angular Momentum of Electrons.
What is Angular Momentum of Electron?
Bohr’s atomic model laid down various postulates for the arrangement of electrons in different orbits around the nucleus. According to Bohr’s atomic model, the angular momentum of electrons orbiting around the nucleus is quantized. He further added that electrons move only in those orbits where the angular momentum of an electron is an integral multiple of h/2. This postulate regarding the quantisation of angular momentum of an electron was later explained by Louis de Broglie. According to him, a moving electron in its circular orbit behaves like a particle-wave.
The angular momentum of an electron by Bohr is given by mvr or nh/2π (where v is the velocity, n is the orbit in which the electron is revolving, m is mass of the electron, and r is the radius of the nth orbit).
Among the various proposed models over the years, the Quantum Mechanical Model seems to best fit all properties. Watch the video to learn more about the features of the quantum mechanical model.

De Broglie’s Explanation to the Quantization of Anfgular Momentum of Electron:
The behaviour of particle waves can be viewed analogously to the waves travelling on a string. Particle waves can lead to standing waves held under resonant conditions. When a stationary string is plucked, a number of wavelengths are excited. On the other hand, we know that only those wavelengths survive which form a standing wave in the string, that is, which have nodes at the ends.
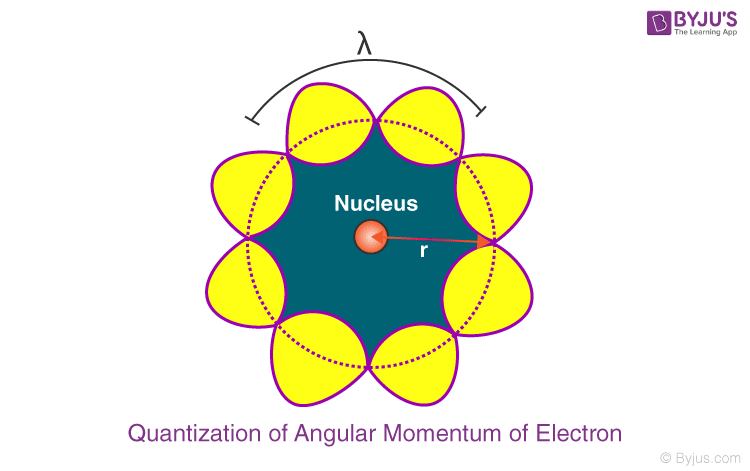
Thus, in a string, standing waves are formed only when the total distance travelled by a wave is an integral number of wavelengths. Hence, for any electron moving in k th circular orbit of radius r k , the total distance is equal to the circumference of the orbit, 2πr k .
Let this be equation (1).
λ is the de Broglie wavelength.
We know that de Broglie wavelength is given by:
p is electron’s momentum
h = Planck’s constant
Let this be equation (2).
Where mv k is the momentum of an electron revolving in the k th orbit. Inserting the value of λ from equation (2) in equation (1) we get,
Hence, de Broglie hypothesis successfully proves Bohr’s second postulate stating the quantization of angular momentum of the orbiting electron. We can also conclude that the quantized electron orbits and energy states are due to the wave nature of the electron.
Frequently Asked Questions – FAQs
Is it possible for electrons to have angular momentum, how to find the angular momentum of an electron, is the angular momentum of an electron quantised, what is bohr’s atomic model, who proposed the quantization of angular momentum.
To learn more physics concepts with the help of interactive and engaging video lessons, download BYJU’S – The Learning App.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

State de Broglie hypothesis.
Louis de broglie, in 1924, stated that a wave is associated with a moving particle (i.e. matter) and so named these waves as matter waves. he proposed that just like light has dual nature, electrons also have wave like properties. wavelength of a moving particle is given by λ = h p where h is the planck's constant and p is the momentum of moving particle..

IMAGES
VIDEO
COMMENTS
De Broglie's Hypothesis says that Matter consists of both the particle nature as well as wave nature. De Broglie wavelength λ is given as λ = h p, where p represents the particle momentum and can be written as: λ = h m v Where, h is the Planck's constant, m is the mass of the particle, and v is the velocity of the particle.; From the above relation, it can be said that the wavelength of the ...
Compton's formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie's hypothesis of matter waves.In 1926, De Broglie's hypothesis, together with Bohr's early ...
Compton's formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie's hypothesis of matter waves.In 1926, De Broglie's hypothesis, together with Bohr's early ...
CBSE Exam, class 12. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket
The wave which is associated with the particles that are moving are known as the matter-wave, and also as the De Broglie wave. The wavelength is known as the de Broglie wavelength. For an electron, de Broglie wavelength equation is: λ = \ [\frac {h} {mv}\] Here, λ points to the wave of the electron in question. M is the mass of the electron.
De Broglie successfully provided the explanation to Bohr's assumption by his hypothesis. Today we know that every particle exhibits both matter and wave nature. This is called wave-particle duality. The concept that matter behaves like wave is called the de Broglie hypothesis, named after Louis de Broglie, who proposed it in 1924.
The De Broglie hypothesis proposes that all matter exhibits wave-like properties and relates the observed wavelength of matter to its momentum. After Albert Einstein's photon theory became accepted, the question became whether this was true only for light or whether material objects also exhibited wave-like behavior. Here is how the De Broglie hypothesis was developed.
Instead what we do is to use electrons. Since electrons have a rest mass, unlike photons, they have a de Broglie wavelength which is really short, around 0.01 nanometers for easily achievable speeds. This means that a microscope using electron "matter waves" instead of photon light waves can see much smaller things. 4 comments.
Class 12 Physics https://www.youtube.com/@DynamicVidyapeeth/playlists?view=50&sort=dd&shelf_id=2Chapter 1, Electric Charges and Fields https://youtube.com/pl...
PG Concept Video | Wave Particle Duality | De Broglie's Hypothesis by Ashish Arora Students can watch all concept videos of class 12 Wave Particle Duality fo...
When de Broglie proposed this hypothesis in 1923, there was no scientific proof that the electron, whose corpuscular properties were widely established, might behave as radiant energy under certain conditions. ... Get all the important information related to the CBSE Class 12 Examination including the process of application, important calendar ...
The de Broglie‐Bohr model of the hydrogen atom presented here treats the electron as a particle on a ring with wave‐like properties. λ = h mev λ = h m e v. de Broglie's hypothesis that matter has wave-like properties. nλ = 2πr n λ = 2 π r. The consequence of de Broglieʹs hypothesis; an integral number of wavelengths must fit within ...
λ = h/mv k. Let this be equation (2). Where mvk is the momentum of an electron revolving in the k th orbit. Inserting the value of λ from equation (2) in equation (1) we get, 2πrk = kh/mv k. mvkrk = kh/2π. Hence, de Broglie hypothesis successfully proves Bohr's second postulate stating the quantization of angular momentum of the orbiting ...
The de Broglie hypothesis and the existence of matter waves has been confirmed for other elementary particles, neutral atoms and even molecules have been shown to be wave-like. ... The third class are matter waves which have a wavevector, ... The wave nature of the electron Nobel Lecture, 12, 1929; Tipler, Paul A. and Ralph A. Llewellyn (2003).
According to the de-Broglie hypothesis, a moving material particle behaves like a wave at times and like a particle at other times. Every moving material particle is connected with a wave. The de-Broglie wave or matter-wave is an unseen wave associated with a moving particle that propagates in the form of wave packets with the group velocity.
De Broglie proposed that a moving material particle of total energy E and momentum p has a wave associated with it (analogous to a photon). He suggested a relation between properties of the wave, like frequency and wavelength, with that of a particle, like energy and momentum. p = E c hv c h E c = hv c = h λ. Thus, the frequency and wavelength ...
Revision notes on 12.2.8 De Broglie's Hypothesis for the AQA A Level Physics syllabus, written by the Physics experts at Save My Exams. ... Dan graduated with a First-class Masters degree in Physics at Durham University, specialising in cell membrane biophysics. After being awarded an Institute of Physics Teacher Training Scholarship, Dan ...
State de Broglie hypothesis. Louis de Broglie, in 1924, stated that a wave is associated with a moving particle (i.e. matter) and so named these waves as matter waves. He proposed that just like light has dual nature, electrons also have wave like properties. where h is the Planck's constant and p is the momentum of moving particle.