Advances in Motion

Clinical Trials
At any given time, there are approximately 1,200 clinical trials taking place at Massachusetts General Hospital, the largest hospital-based research enterprise in the United States.
Mass General conducts innovative clinical trials and research studies to increase the understanding of health and disease and advance the fields of medicine and clinical care. We invite patients and the community to participate in clinical trials for access to a wide variety of promising new diagnostics and therapies.
Search by Therapeutic Area Search Cancer Center Trials Healthy Volunteers Program
Latest Mass General Clinical Trials
In Orthopaedics
In Cardiovascular
Subscribe to the latest updates from Advances in Motion
Thank you for subscribing!
Error: Please enter a valid email address.
Email Address Submit
- Giving to Mass General Brigham
- Search the Site Site-wide search 0 items available in list reset search Search Close Search
Mass General Brigham
Hospital Research Institutes
As a world-recognized leader in research, Mass General Brigham is home to one of the largest hospital system-based research enterprises in the U.S.

Mass General Brigham’s location in the Massachusetts biotech and academic medical communities creates enhanced collaborations with partners from academia, industry, and venture capital to help stimulate the development of our discoveries into lifesaving therapies for our patients.
The greatest minds at our hospitals have a history of pushing the boundaries of medicine and leading innovative science for over 200 years. We have a legacy of medical achievement, with numerous medical firsts and 13 Nobel Laureates.
Our hospital-based medical research institutes
Click to view research institutes at Mass General Research Institute
- Brigham Research Institute (opens in new tab)
- Schoen Adams Research Institute at Spaulding Rehabilitation (opens in new tab)
- Mass Eye and Ear (opens in new tab)
- McLean Hospital (opens in new tab)
- Newton-Wellesley Hospital (opens in new tab)
$2 billion in research activities
World-class innovators find the therapies and technologies of tomorrow
From the bench to the bedside
At Mass General Brigham, our researchers are changing the world, while also providing care for patients. Our physician/scientists work to uncover the effects of new treatments and medications for conditions big and small to improve patient care. In the lab, in the clinic, and at the bedside, these experts lead the field.
Our research publications
Advances in Motion provides information about the latest breakthroughs, research and clinical advances from Massachusetts General Hospital and Mass Eye & Ear.
On a Mission offers the latest research, innovation and clinical news from Brigham and Women Hospital researchers and clinicians

Featured articles

Also of interest
Massachusetts General Hospital Biostatistics
Our Research
MGH Biostatistics leads and enables research in the design and conduct of clinical trials, observational data methods, and methods for precision medicine applications. Collectively, the MGH Biostatistics faculty members have expertise in methods for clinical trials and observational studies, clustered and repeated measures data, missing data, time-to-event outcomes, causal inference, Bayesian analysis, machine learning, genetic epidemiology, -omics applications, implementation science, and precision medicine.
In 2021, MGH Biostatistics faculty contributed to 297 published manuscripts and collaborated on 186 research projects across dozens of Divisions and Departments. The examples below highlight some of our major collaborative research efforts.
Select Collaborations
Amyotrophic lateral sclerosis (als).

MGH Biostatistics partners with the Sean M. Healey & AMG Center for ALS at Mass General and Tackle ALS to lead the first platform trial initiative for amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease). ALS is a neurological disease with limited treatment options.
A “platform trial” is a clinical trial in which multiple treatments are evaluated simultaneously using specialized statistical tools. New drug regimens can be added as they become available, decreasing or eliminating the lengthy start-up and execution times of traditional trial models. The platform model, already proven successful in the cancer field, will greatly accelerate therapy development of effective and breakthrough treatments for people with ALS by allowing investigators to test more drugs, increase patient access to trials and reduce the cost by quickly and efficiently evaluating the effectiveness of multiple therapies. The focus is on the disease, rather than any individual experimental agent, and the platform remains open long-term until successful cures are found.
MGH biostatisticians play prominent roles in a wide range of clinical and translational research at the MGH Cancer Center (MGHCC) and through the Biostatistics Core of the Dana-Farber/Harvard Cancer Center (DF/HCC).
- We have been instrumental in developing and reporting clinical trials for adult and pediatric cancers, ranging from early phase to randomized studies in local and multi-center protocols. Among the contributions to advances in novel and innovative therapies, we have maintained notably long-term collaborations in radiation oncology, especially the Proton Research Program .
- In addition to the therapeutic studies, we are involved in clinical trials initiated by the Cancer Outcomes Research & Education Program (CORE), as emphasis increasingly shifts to the quality of cancer care and the relevance of patient-oriented outcome. We also conduct biostatistics clinics as part of the Annual Workshop on Methods in Supportive Oncology Research hosted by the CORE leadership for junior investigators from around the world.
- Our contributions to translational research involve the design and analysis of preclinical models as well as human studies, including discovery and validation of prognostic and predictive biomarkers. In particular, MGH biostatisticians play key roles in the DF/HCC SPOREs for Glioma , ovarian , and gastrointestinal cancers .
- MGH Biostatistics has a robust research program in early detection of cancer, originally focused on ovarian cancer and recently expanded to include renal cell carcinoma (kidney), hepatocellular carcinoma (liver), lung cancer, and prostate cancer . NCI-sponsored research includes biomarker discovery and validation, algorithm development, and clinical diagnostic and screening studies for early detection of ovarian cancer in the Early Detection Research Network and lung cancer screening through the Liquid Biopsy Consortium . Additionally, we collaborate with the Center for Innovation in Early Cancer Detection at MGH.

As the Data Resource Core for RECOVER, a four-year, multimillion dollar project, MGH Biostatistics and MGB will provide expertise on study design and facilitate the collection and analysis of complex data across multiple cohort studies. The team is led by Andrea Foulkes, ScD, Chief of MGH Biostatistics, Elizabeth Karlson, MD, MS Director of Rheumatic Disease Epidemiology at Brigham and Women’s Hospital, and Shawn Murphy, MD, PhD, Chief Research Information Officer at Mass General Brigham, and includes complementary teams from Harvard Medical School and Harvard T.H. Chan School of Public Health.
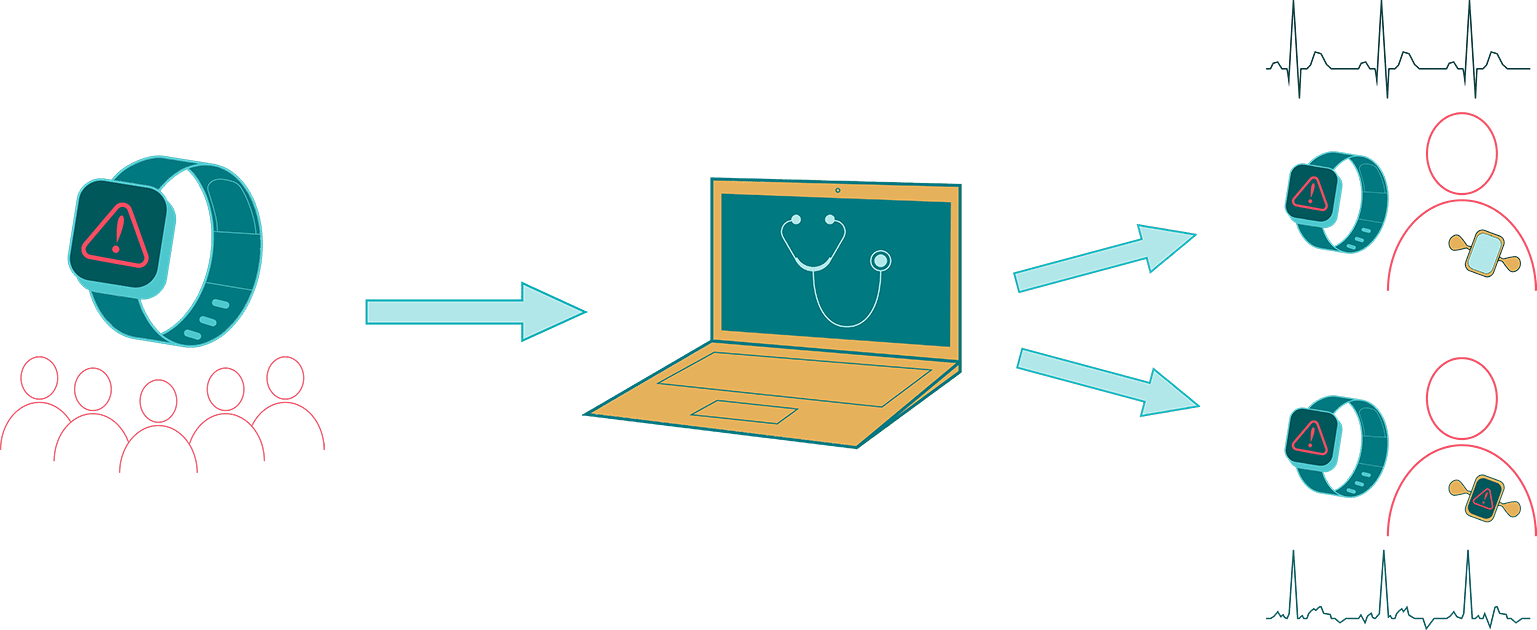
Fitbit Heart Study

The study assesses the positive predictive value of an irregular heart rhythm detection during the ECG patch monitor period and also examines the validity of irregular pulse tachograms during subsequent heart rhythm detections, self-reported AF diagnoses and treatments, and relations between irregular heart rhythm detections and AF episode duration. The Fitbit Heart Study results will provide critical insights into the use of consumer wearable technology for AF detection and for characterizing the nature of AF episodes detected using consumer-based PPG technology. Advancing research on innovative and accessible technology, like wearable Fitbit devices, will lead to more tools that help improve health outcomes and reduce the impact of AF on a large scale.
In 2020, t he PETAL Network redirected some of its effort to COVID-19 and completed a randomized controlled clinical trial of hydroxychloroquine in hospitalized patients with COVID-19 . It also launched multicenter observational retrospective and prospective stud ies designed to collect comprehensive data on hospitalized patients with COVID-19, including imaging, biospecimens, and long-term outcomes. In addition , the PETAL Network is a collaborator in both the ACTIV-3 and ACTIV-4 platform trials.
Featured Publication
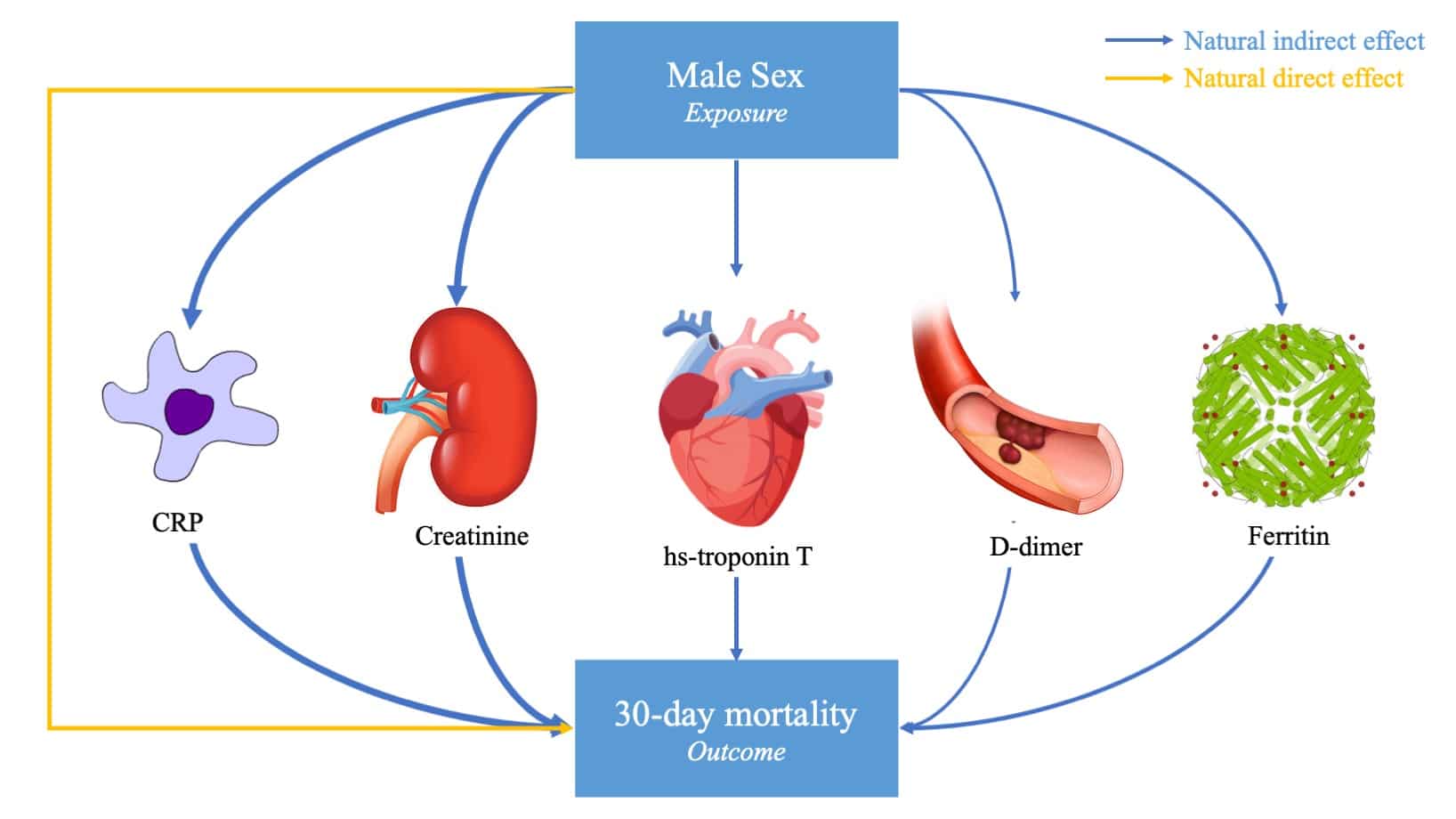
Biomarkers of cardiac injury, renal injury, and inflammation are strong mediators of sex-associated death in COVID-19
Heidi S. Lumish, Eunyoung Kim, Caitlin Selvaggi , Tingyi Cao , Aakriti Gupta, Andrea S. Foulkes and Muredach P. Reilly
Frontiers in Cardiovascular Medicine
MGH Biostatistics 125 Nashua Street, Suite 1040 Boston, MA 02114
E-mail : biostat@mgh.harvard.edu X (Formerly Twitter): @mghbiostat
- News & Events


NEPTCC → Research → Clinical Studies
Research Studies
→ About Us
Clinical Research Studies
Physicians at the Neuroendocrine and Pituitary Tumor Clinical Center at Massachusetts General Hospital are conducting several exciting research studies aimed at understanding and addressing medical issues related to pituitary disorders and learning more about the mechanisms of anorexia nervosa and applying novel treatments that will help improve future clinical treatments.
Our goal is to learn and create novel treatments that will help improve clinical care for patients with neuroendocrine disorders and neuroendocrine malignancies. Research at the Neuroendocrine and Pituitary Clinical Center at Mass General is divided into four major research areas including:
- Pathogenesis and regulation of human pituitary adenomas
- Pathophysiology of neuroendocrine disorders
- Relationship between nutritional disorders and neuroendocrine factors
- Development of novel therapies for patients with pituitary and hypothalamic disorders
Research Studies Available
→ Newsletter Archive
For over two decades, the Massachusetts General Hospital Neuroendocrine and Pituitary Clinical Center has provided a multidisciplinary approach to patients with pituitary and hypothalamic disorders.
We are actively recruiting participants for several research studies described on this site and we hope that you will consider participating. We encourage you to investigate the educational material provided regarding pituitary disorders, and please do not hesitate to contact us if you would like further information.
Patients may qualify for research studies in the Neuroendocrine Unit. We are currently accepting the following categories of patients for screening to determine study eligibility. Depending on the study, subjects may receive free testing, medication and/or stipends.
The Neuroendocrine Unit is involved in many different research studies. Types of studies and enrollment status changes frequently, so please call our office (617-726-3870) or check our webpage ( pituitary.mgh.harvard.edu ) for more information about potential studies which may not be listed here.

The Massachusetts General Hospital Translational and Clinical Research Centers (TCRC) supports the conduct of patient-oriented research projects and serves as a human research laboratory in which investigators can perform their funded projects. It is composed of two major units: the Clinical Research Center, a federally funded part of the Harvard Clinical Translation Science Center, directed largely to support NIH and foundation-sponsored research and the Translational Research Center, which is specially directed to work with investigators performing industry-sponsored human research.
Administration
Our experienced pool of research coordinators can assist in all study start-up, recruitment, and execution activities
Nursing Services
Our highly trained nursing staff can support a wide array of clinical activities and advise on protocol development
Laboratory Services
The TCRC’s onsite laboratory is dedicated to timely sample preparation, shipping, and storage according to protocol needs
Metabolic & Nutrition Services
Nutrition staff at the TCRC have extensive research training to support metabolic and nutrition protocols
Medical Research Officer Program
The Medical Research Officer can assist with activities such as PI oversight responsibilities and protocol development
Project Consulting Services
We can provide a wide array of services, such as protocol generation, program development and management

Clinical Trials Network and Institute
- Protocol Design
- SAFER Interviews
- Clinical Medical Monitoring
- SPCD Design
Making Your Clinical Trial Programs SUCCESSFUL – Smaller and Faster
Uniting academic leading science with experienced clinical trials leadership and implementation
Reducing placebo response impact and sample size requirements through Sequential Parallel Comparison Design
Optimizing the patient selection process using SAFER Interviews
Driving superior site training for top quality research team-building
Providing world-class staff, researchers, consultants, and expert advisory boards
Put CTNI’s Unique Capabilities to Work for You
If your aim is to reach a new state-of-the-art for studies into brain science, genetics, and the neurobiology of psychiatric disorders, it’s time to partner with CTNI. We are your best path to faster publishable results and world-class research excellence.
Sequential Parallel Comparison Design
Unique protocol designs and details such as the Sequential Parallel Comparison Design, a proven strategy that cuts placebo response by half,reduces sample size requirements, and increases the power of studies by making participant data more informative
Experienced Investigators
Principal investigators and site investigators with deep experience in managing and conducting industry sponsored clinical trials
World-Class Consultation
World-class scientific consultation and advisory boards for delivery of the most authoritative guidance available
Advanced Methods & Training
Advanced subject quality methods and rater training ensure both patients and sites are the best fit to your unique needs
Internationally Recognized Research Staff
Research staff improves your range and depth of expertise in studies across CNS disorders:
Anxiety · Depression · Bipolar disorder · Women’s mental health · Schizophrenia · Obsessive-Compulsive Disorder · ADHD · Geriatrics/Alzheimer’s disease · Pharmacogenetics · Neuroimaging · Pediatric psychopharmacology

Raising the Bar In Clinical Research & Innovation

One Bowdoin Square 9th Floor Boston, MA 02114
- PRIVACY POLICY
- MASSACHUSETTS GENERAL HOSPITAL HOME
© 2024 Copyright MGH Clinical Trials Network and Institute. Website design by Tomo360
This site uses cookies. By continuing to browse the site, you are agreeing to our use of cookies.

Translational Research Center
MGH is founding the Translational Research Center (TRC) to conduct clinical research trials that derisk new drug and medical device translational opportunities. Under the management of the Translational Management Group, the TRC executes proof-of-concept studies that quickly demonstrate the therapeutic benefit to patients or eliminate compounds with unexpected toxicity or other liabilities
TRC key features:
- In-patient facility on MGH main campus
- 18 to 20 beds, 4 exam rooms, 2 procedure rooms
- Adjacent to the current Clinical Research Center (CRC)
- Expert staff with over 20 years experience in the conduct of complex clinical designs
- Access to research pharmacy, metabolic kitchen, clinical laboratories, & MGH core facilities
- Ready access to state-of-the-art MGH imaging centers
- Core Competencies
- Human Systems Modeling
- Harvard Medical School Systems Biology
- Harvard/MIT Health Sciences & Technology program
- Partners Biobank
- Phenotyping
- Clinical Research Program
- Clinical Research Center
- Harvard Catalyst
- Disease-specific clinical investigators
- Broad Institute
- Partners Personalized Medicine – CLIA certified genetics lab
- Martinos Center - clinical
- MGH Systems Biology – preclinical animal models
- Contract and alliance management
- Clinical trial management
- Data management
- Regulatory Operations
- Clinical Operations
Clinical Trial Designs
- Patient-based studies of molecular mechanism where intensive or novel phenotyping is critical
- Target engagement investigations with PET ligand or state-of-the-art imaging
- Enrichment studies in pre-selected patients based on phenotypic information or genotype recorded in Partners EMR
- Studies to develop biomarkers or new assay read-outs that could be developed in conjunction with an MGH investigator
- Collaborative trials between company scientists and medical investigators that facilitate clinical learning
- Proof-of-concept studies that quickly demonstrate the therapeutic benefit to patients or eliminate compounds with toxicity or other liabilities
- Clinical Trials
A Study to Train and Evaluate Machine-learning Models for Medical Imaging
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
- Scottsdale/Phoenix, Arizona: 22-007739

About this study
The purpose of this study is to evaluate machine learning (ML)/AI models used for medical imaging.
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Inclusion Criteria:
- Radiologists who indicate a willingness to participate in the study.
- Patients referred to get a radiological scan for a clinical indication for which we have a machine learning model, restricted to the category of models defined below.
Exclusion Criteria
- Radiologists who do not indicate a willingness to participate in the study.
- Patients not referred to get a radiological scan for a clinical indication for which we have a machine learning model, restricted to the category of the models defined below.
Eligibility last updated 10/25/22. Questions regarding updates should be directed to the study team contact.
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- skip to Cookie Notice
- skip to Main Navigation
- skip to Main Content
- skip to Footer
- Find a Doctor
- Find a Location
- Appointments & Referrals
- Patient Gateway
- Español
- Leadership Team
- Quality & Safety
- Equity & Inclusion
- Community Health
- Education & Training
- Centers & Departments
- Browse Treatments
- Browse Conditions A-Z
- View All Centers & Departments
- Clinical Trials
- Cancer Clinical Trials
- Cancer Center
- Digestive Healthcare Center
- Heart Center
- Mass General for Children
- Neuroscience
- Orthopaedic Surgery
- Information for Visitors
- Maps & Directions
- Parking & Shuttles
- Services & Amenities
- Accessibility
- Visiting Boston
- International Patients
- Medical Records
- Billing, Insurance & Financial Assistance
- Privacy & Security
- Patient Experience
- Explore Our Laboratories
- Industry Collaborations
- Research & Innovation News
- About the Research Institute
- Innovation Programs
- Education & Community Outreach
- Support Our Research
- Find a Researcher
- News & Events
- Ways to Give
- Patient Rights & Advocacy
- Website Terms of Use
- Apollo (Intranet)
Mass General Cancer Center
- Like us on Facebook
- Follow us on Twitter
- See us on LinkedIn
- Print this page
Our Research
- 617-726-5130
- Browse research labs
- Search clinical trials

Krantz Family Center for Cancer Research
The Krantz Family Center for Cancer Research is the scientific engine driving basic and translational research at the Mass General Cancer Center. With a dedicated faculty of over 50 independent investigators and 500 students, postdoctoral scientists, research scientists and technologists, the Krantz Center is committed both to fundamental discovery and to its application in cancer.
Empowering Researchers to Tackle the Major Unsolved Challenges in Oncology
Learn how a transformative gift from philanthropists Jason and Keely Krantz is about to revolutionize cancer research at Mass General Cancer Center.
Explore More Research at Mass General Cancer Center
Mass General Cancer Center is committed to understanding the mechanisms by which the immune system works to kill cancer. In these videos, Marcela Maus, MD, PhD, Noopur Raje, MD and Matthew Frigault, MD discuss the power & evolution of cellular therapies .
A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment.
Drs. Noopur Raje, Marcela Maus and Matthew Frigault discuss the power and evolution of immune therapies, specifically, CAR T-Cell therapy in treating cancer.
Cancer Outcomes Research and Education Program
The Cancer Outcomes Research and Education Program (CORE) conducts innovative research and educational programs to improve the experience and outcomes of patients and caregivers across the continuum of cancer care.
Henri and Belinda Termeer Center for Targeted Therapies
The Termeer Center offers a comprehensive translational research program to speed the discovery and delivery of new targeted therapies to patients with early and advanced stage cancers.

Cancer Early Detection
The Cancer Early Detection and Diagnostics Clinic is a multidisciplinary collaboration working to bridge the gap between clinical care and research in cancer early detection.

View Research Areas & Resources at Mass General Cancer Center
Browse Research Labs
Browse Research Areas
Biomarker Discovery Lab | Cancer Early Detection and Diagnostics Clinic | Cancer Outcomes Research Program | Cellular Immunotherapy Program | Cellular Therapeutics & Transplantation Laboratory | Center for Cancer Immunology | Immune Monitoring Laboratory | Krantz Family Center for Cancer Research | Radiation Oncology Research | Severe Immunotherapy Complications | Termeer Center for Targeted Therapies | Therapeutic Intralesional Program | Tumor Imaging Metrics Core | Warshaw Institute for Pancreatic Cancer Research
Oncology Advances in Motion
View innovative research and new advances in clinical care from Mass General Cancer Center physicians and researchers. Subscribe here for monthly emails.
Research Funding
American Cancer Society Institutional Research Grant
Research at Mass General Cancer Center
Our commitment to eradicating cancer is fueled by scientific investigation as part of the largest hospital-based research program in the United States.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services

Many families take patients off life support too soon after traumatic brain injuries: study
M any patients who died after traumatic brain injuries may have survived and recovered if their families had waited to take them off life support , a new study found.
Researchers from Massachusetts General Hospital, Harvard Medical School and other universities analyzed "potential clinical outcomes" for patients with traumatic brain injury (TBI) who were removed from life support, according to a press release.
The study included 1,392 patients who were treated in 18 trauma centers across the U.S. over a 7½-year period.
HUNDREDS OF RURAL HOSPITALS ARE IN DANGER OF SHUTTING DOWN, STUDY FINDS: ‘AT RISK OF CLOSURE'
Using a mathematical model, the researchers compared patients for whom life support was withdrawn to similar patients who were kept on life support.
Among the group for whom life support was not withdrawn, more than 40% recovered at least some independence, according to a press release.
READ ON THE FOX NEWS APP
The researchers also discovered that the notion of remaining in a vegetative state was an "unlikely outcome" six months after injury.
When designing the study, the team didn’t know what to expect, according to study author Yelena Bodien, PhD, of the Department of Neurology’s Center for neurotechnology and neurorecovery at Massachusetts General Hospital.
HOME HOSPITAL CARE BRINGS ‘PHENOMENAL’ BENEFITS TO PATIENTS AND PROVIDERS, STUDY FINDS
"Our anecdotal experience was that some families are told their loved ones had no chance for recovery, they would never walk, talk, work or have a meaningful relationship again — yet they chose not to discontinue life support and their loved one made a remarkable recovery ," she told Fox News Digital.
"On the other hand, clinicians are under a lot of pressure to make early prognoses and do not want to commit someone to a life that would never be acceptable to them, so it could be that those patients who died after life support was withdrawn would have had very significant impairments otherwise."
"I think there are two stories here," said Bodien.
"One is that some patients with traumatic brain injury who died because life support was withdrawn may have recovered, but the other is that many would have died even if life support was continued."
A patient’s prognosis after severe traumatic brain injury is highly uncertain, she noted. "Sometimes patients with the most devastating injuries survive and make meaningful recoveries ."
The problem, Bodien said, is that health care providers lack the tools required to determine which patients with devastating injuries will recover, to what extent they will recover — and how long that will take.
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, was not involved in the research but said it was a "very important" study.
"Previous research shows a high-level recovery from mild TBI and a significant recovery percentage even with moderate to severe injury," Siegel told Fox News Digital.
HEAD INJURY ASSOCIATED WITH DOUBLED MORTALITY RATE, 30-YEAR STUDY REVEALS
"After head trauma, the brain may swell, and the use of mannitol and steroids and even sometimes surgery — where the top of the skull is removed — can be used to decrease pressure on the brain and increase chance of a full recovery," he continued.
Rehabilitation is also crucial, Siegel added.
"All of these tools should be given a chance to work in most cases."
Based on the study findings, Bodien recommended that clinicians should be "very cautious" with "irreversible decisions" like withdrawing life support in the days following traumatic brain injury .
"Families should also be aware of our results so that they can advocate for delaying a decision to discontinue life support if this is aligned with what they believe their loved one would want," she added.
There were some limitations to the study, Bodien said.
"The sample size of the study was small, which made it difficult to find an adequate number of participants who did not have life support discontinued and were clinically similar, or ‘matched,’ to those who had life support discontinued," she told Fox News Digital.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Among the participants who did not have life support discontinued, the researchers were not able to follow all of them for a six-month period.
Another limitation is that the researchers used clinical variables that were available on the day of, or the day after, hospitalization — but sometimes decisions to discontinue life support are made several days later.
"There are many considerations that may lead to a decision to discontinue life support after traumatic brain injury that we were unable to factor into our analyses," she continued.
"For example, personal beliefs, religion and advanced directives could all affect decision-making but were not captured in our study."
Bodien also noted that the Harvard study was focused on traumatic brain injury and cannot be generalized to other injuries and illnesses.
For more Health articles, visit www.foxnews.com/health .
Original article source: Many families take patients off life support too soon after traumatic brain injuries: study

- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Clinical Research Risks, Climate Change, and Human Health
- 1 Division of Clinical Public Health, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada
- 2 Program for Ethics and Care Ecologies (PEaCE), Hamilton Health Sciences, Toronto, Ontario, Canada
- 3 Department of Global Health and Social Medicine, King’s College London, London, United Kingdom
- Editor's Note Climate Change and Health—A New JAMA Series Mary M. McDermott, MD; Kirsten Bibbins-Domingo, PhD, MD, MAS JAMA
- Viewpoint Patient-Centered Climate Action and Health Equity Aaron S. Bernstein, MD, MPH; Kristin L. Stevens, MS; Howard K. Koh, MD, MPH JAMA
- Medical News & Perspectives How Climate Change Is Already Affecting Health Jennifer Abbasi JAMA
- Medical News & Perspectives Climate Change Takes a Toll on Mental Health in Africa Melissa Suran, PhD, MSJ JAMA
- JAMA Insights Introduction to JAMA Climate Change and Health Series Kristie L. Ebi, PhD, MPH; Jeremy J. Hess, MD, MPH JAMA
For clinical research to be deemed ethically acceptable, it is necessary that a study have a favorable benefit-risk ratio. 1 This requirement is met when risks and harms are minimized, potential benefits are maximized, and the potential benefits outweigh the potential risks. 1
- Editor's Note Climate Change and Health—A New JAMA Series JAMA
Read More About
D’Souza J , Samuel G. Clinical Research Risks, Climate Change, and Human Health. JAMA. 2023;330(23):2247–2248. doi:10.1001/jama.2023.23724
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Clinical Consequences of Occult Free Valproate Toxicity in Critically Ill Adult Patients: A Multicenter Retrospective Cohort Study
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Andrew J Webb
- For correspondence: [email protected]
- ORCID record for Richard R Riker
- ORCID record for Natasha D Lopez
- ORCID record for Melanie Z Goodberlet
- ORCID record for Michael J Schontz
- ORCID record for Kaylee K Marino
- ORCID record for Sacha N Uljon
- ORCID record for Eric S Rosenthal
- Info/History
- Supplementary material
- Preview PDF
Background and Objectives Valproate has wide pharmacokinetic variability and a narrow therapeutic index. Its protein binding is unpredictable, particularly among critically ill patients who may experience unexpectedly elevated free concentrations. We sought to identify the clinical consequences and determinants of occult free valproate toxicity in critically ill adults.
Methods We conducted a multicenter retrospective cohort study of adult patients admitted to an intensive care unit (ICU) who were receiving valproate and had concurrent total and free valproate concentrations measured. We examined whether valproate concentrations were independently associated with adverse drug effects (ADEs) including thrombocytopenia, hepatotoxicity, hyperammonemia, and pancreatic injury. Determinants of occult toxicity were also identified using logistic mixed-effects models, adjusting for age, weight, albumin, propofol and aspirin use, and blood urea nitrogen (BUN). Occult toxicity was defined as a free valproate concentration that was discordant with total concentration (e.g., supratherapeutic free concentration associated with therapeutic total concentration).
Results 311 unique patients (mean age 58 [±17] years, 36% female, 31% non-white, and 29% on valproate prior to admission) with 550 concurrent free and total valproate concentration pairs met inclusion criteria. The median (IQR) total valproate concentration was 46 mcg/mL (34-63) and the median free valproate concentration was 17 mcg/ml (11-23); median free fraction was 35% (25-63%). Eighty-four percent of total valproate concentrations represented occult free toxicity; a therapeutic total with a supratherapeutic free valproate concentration was the most common pattern (32% of concentration pairs). Each 2.5 mcg/mL increase in free valproate concentration was associated with thrombocytopenia (adjusted unit odds 1.15, 95% CI 1.05-1.26) and hepatotoxicity (adjusted unit odds 1.11, 95% CI 1.05-1.18). Albumin concentration (adjusted odds; aOR 0.17, 95% CI 0.08-0.36), BUN (aOR 1.36, 95% CI 1.09-1.70), and propofol exposure (aOR 3.06, 95% CI 1.38-6.79) were associated with occult toxicity.
Conclusion Free valproate concentrations should be measured in critically ill patients because it is associated with ADEs and is often underrepresented by total concentrations. Most critically ill patients are at risk, especially those with hypoalbuminemia, uremia, and lipid exposure.
Competing Interest Statement
A.J. Webb has received consulting fees from Acasti Pharma Inc. D.J. Gagnon is a clinical specialist in neurosciences for Lexicomp and is supported by NIGMS grant 1P20GM139745. C.S. Brown has received consulting fees from Trevena Pharmceuticals and grant funding from Astra Zeneca. R.R. Riker is supported by NIH-NIGMS grant 1P20GM139745. S.F. Zafar is supported by NIH-NINDS grants (1R01NS131347, 5K23NS114201, 1R01AG082693, and 1R21NS137117). E.S Rosenthal has received consulting fees from UCB, Inc. and Ceribell, Inc. and is supported by DoD grants (W81XWH-18-DMRDP-PTCRA) and NIH-NINDS and NIH-OD grants (R01NS117904, K23NS105950, R01NS113541, U54NS100064, OT2OD032701).
Funding Statement
D.J. Gagnon is supported by NIGMS grant 1P20GM139745. R.R. Riker is supported by NIH-NIGMS grant 1P20GM139745. S.F. Zafar is supported by NIH-NINDS grants (1R01NS131347, 5K23NS114201, 1R01AG082693, and 1R21NS137117). E.S Rosenthal is supported by DoD grants (W81XWH-18-DMRDP-PTCRA) and NIH-NINDS and NIH-OD grants (R01NS117904, K23NS105950, R01NS113541, U54NS100064, OT2OD032701).
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The study was deemed exempt by the Massachusetts General Brigham Institutional Review Board and the need for informed consent was waived (2023P000131).
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Conflicts of Interest: A.J. Webb has received consulting fees from Acasti Pharma Inc. D.J. Gagnon is a clinical specialist in neurosciences for Lexicomp and is supported by NIGMS grant 1P20GM139745. C.S. Brown has received consulting fees from Trevena Pharmceuticals and grant funding from Astra Zeneca. R.R. Riker is supported by NIH-NIGMS grant 1P20GM139745. S.F. Zafar is supported by NIH-NINDS grants (1R01NS131347, 5K23NS114201, 1R01AG082693, and 1R21NS137117). E.S Rosenthal has received consulting fees from UCB, Inc. and Ceribell, Inc. and is supported by DoD grants (W81XWH-18-DMRDP-PTCRA) and NIH-NINDS and NIH-OD grants (R01NS117904, K23NS105950, R01NS113541, U54NS100064, OT2OD032701).
The formatting of the abstract has been adjusted to include line breaks and bold headings.
Data Availability
Anonymized data not published within this article will be made available upon reasonable request from any qualified investigator after completion of a data use agreement.
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Addiction Medicine (324)
- Allergy and Immunology (629)
- Anesthesia (166)
- Cardiovascular Medicine (2391)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (380)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (843)
- Epidemiology (11786)
- Forensic Medicine (10)
- Gastroenterology (703)
- Genetic and Genomic Medicine (3759)
- Geriatric Medicine (350)
- Health Economics (636)
- Health Informatics (2403)
- Health Policy (935)
- Health Systems and Quality Improvement (902)
- Hematology (341)
- HIV/AIDS (783)
- Infectious Diseases (except HIV/AIDS) (13330)
- Intensive Care and Critical Care Medicine (769)
- Medical Education (366)
- Medical Ethics (105)
- Nephrology (400)
- Neurology (3517)
- Nursing (199)
- Nutrition (528)
- Obstetrics and Gynecology (677)
- Occupational and Environmental Health (665)
- Oncology (1828)
- Ophthalmology (538)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (234)
- Palliative Medicine (66)
- Pathology (447)
- Pediatrics (1035)
- Pharmacology and Therapeutics (426)
- Primary Care Research (423)
- Psychiatry and Clinical Psychology (3186)
- Public and Global Health (6161)
- Radiology and Imaging (1283)
- Rehabilitation Medicine and Physical Therapy (750)
- Respiratory Medicine (830)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (324)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (146)

- Prospective Students
- Master’s Students
- Doctoral Students
- Faculty & Staff
- International Students
- Women & Gender
- Black, Indigenous & People of Color
- Students with Disabilities
- Exploring What You Can Do with Your Friedman Degree
- Sustainable Agriculture & Food Systems
- Food & Nutrition Policy
- Nutrition Interventions & Communication
- International Development, Food Security & Humanitarian Response
- Biological and Clinical Research
- Epidemiology & Data Sciences
- Business Management, Innovation & Entrepreneurship
- Deciding Your Next Steps
- Resumes, CVs & Cover Letters
- Networking & Personal Branding
- Internships
- Jobs & Fellowships
- Interviewing
- Labor Market Insights & Navigating Job Offers
- For Employers
- Contact & Location
Massachusetts General Hospital
Post-doctoral research fellow, brain nutrition lab.
- Share This: Share Post-Doctoral Research Fellow, Brain Nutrition Lab on Facebook Share Post-Doctoral Research Fellow, Brain Nutrition Lab on LinkedIn Share Post-Doctoral Research Fellow, Brain Nutrition Lab on X
GENERAL SUMMARY/ OVERVIEW STATEMENT: The McCance Center for Brain Health in the Department of Neurology at the Massachusetts General Hospital (MGH) is a Harvard-affiliated Center in Boston well-poised to the discovery of novel tools and interventions that promote brain health. Our mission is to maximize brain health, optimize mental vitality, and prevent age-related brain disease. The McCance Center Clinical Trials Unit (CTU) is building capacity for conducting state-of-the-art interventions ranging from small compounds, repurposed drugs and natural products to multi-domain lifestyle interventions for the prevention of neurological disorders (i.e., Alzheimer’s and Parkinson’s disease, vascular dementia, etc). The McCance Center Brain Nutrition Laboratory (BNL) is hiring a Post-Doctoral Research Fellow in Brain Nutrition to leverage existing datasets to identify modifiable risk factors for mitigation through interventional studies in high-risk populations. Current McCance Center faculty, staff, and affiliates are interdisciplinary, including expertise in nutrition, genetics, neurology, psychiatry, exercise, sleep, data sciences, clinical, epidemiological and basic research. We are looking for a Research Fellow with interests in growing and developing expertise in nutritional neuroscience to join our team.
PRINCIPAL DUTIES AND RESPONSIBILITIES: • Leverage existing datasets with blood, CSF, neuroimaging biomarkers, and post-mortem human brain tissue available for clinical research. • Conduct longitudinal statistical analysis of repeated measures of brain health, disease incidence, and dimension reduction techniques applied to exposure and outcome measures in nutrition and neurology. • Draft technical reports of research findings for peer review publication in appropriate journals. • Develop grant proposals with suitable preliminary studies sections to compete for federal, foundation and industry research grants.
SKILLS & COMPETENCIES REQUIRED: Candidates will have attained a Doctoral degree (i.e., PhD, ND, MD, ScD, or equivalent per NIH definition) in areas that may include nutritional neuroepidemiology, medicine, epidemiology, or nutritional biochemistry/biology with experience in human investigations. • Clinical research experience including clinical trials or epidemiological studies in aging and Alzheimer’sdisease. • Independent in the statistical analysis of longitudinal biomarker studies in aging and dementia • Early indication of publications leveraging existing datasets, preferably using biomarkers is an asset • Recruitment success of research participants is an asset. • Signs of early funding success is an asset • Aspiration to publish impactful peer-reviewed scientific articles leading to successful career development award
EDUCATION: • Scientific Doctoral degree (i.e., PhD, ScD, or equivalent) in areas including nutritional epidemiology, molecular epidemiology, medicine, nutritional biochemistry/biology with experience in human investigations. • Clinical Doctoral degree (i.e., MD, ND, or equivalent) in medicine with MPH in epidemiology and biostatistics will be considered.
FURTHER INFORMATION: Apply Here

- Services and Fees
- Five Pillars
- Course Catalog (OpenCourses)
- Suggested Courses
- Popular Courses
- Getting Started on OpenCourses
- Preparing a BBB Session
- Accessing Course Participant List
- Accessing Course Completion Results
- Accessing Course Evaluation Results
- Contact CCRE
- Research Navigator
- LabArchives
- Research Information Science & Computing
- MGH Intranet
- Research Institute Intranet
- Responsible Conduct of Research
- MGH Clinical Resources and Environment
- Research Match
- Testimonials
For Researchers
- Supporting clinical and translational research at Mass General through specific centers and units
- Nurturing the next generation of Mass General clinical investigators
- Increasing Mass General’s overall clinical research funding
- Expanding Mass General’s pool of clinical research mentors
- Providing hands-on support to overcome the logistical challenges faced by clinical investigators
Free Services Provided to Investigators
- Consultations with DCR faculty (6-8 hours)
- IND/IDE submissions and project management
- Assistance with subject recruitment
- Identification of potential funding sources
- Budgeting for clinical studies
- Help with National Institutes of Health (NIH) and Patient Centered Outcomes Research (PCORI) applications
Services Available on a Fee Basis
- Project Management
- IRB new application submission
Your Privacy
Privacy overview.

IMAGES
VIDEO
COMMENTS
Search for clinical trials and research studies currently seeking participants at Massachusetts General Hospital and our partner hospitals. See all studies at Mass General that are recruiting or will start recruiting soon. Search open clinical trials throughout Mass General Brigham by therapeutic area.
Your tax-deductible gift to the Mass General Research Institute will provide crucial support to our scientists as they continue to work on ways to diagnose, treat and prevent disease. Research at Massachusetts General Hospital began over 200 years ago. Today, the Mass General Research Institute is home to the largest hospital-based research ...
The Mass General Brigham Biobank is a large research program designed to help researchers understand how people's health is affected by their genes, lifestyle, and environment. By participating in the Biobank, you can help us better understand, treat, and even prevent the diseases that might affect your health and the health of future ...
Mass General Brigham. 399 Revolution Drive, Suite 760. Somerville, MA 02145. [email protected]. The Clinical Trials Office provides contracting, budgeting and Clinical Trial Management Systems (CTMS) services to our member-hospital investigators and their industry sponsors conducting Phase I-IV clinical trials. Read more.
At any given time, there are approximately 1,200 clinical trials taking place at Massachusetts General Hospital, the largest hospital-based research enterprise in the United States. Mass General conducts innovative clinical trials and research studies to increase the understanding of health and disease and advance the fields of medicine and ...
20-177 Phase I. A First-in-Human, Phase 1a/1b, Open-Label, Dose-Escalation and Expansion Study to Investigate the Safety, Pharmacokinetics, and Antitumor Activity of the RAF Dimer Inhibitor BGB-3245 in Patients with Advanced or Refractory Tumors. Mass General Cancer Center offers patients access to a wide variety of clinical trials of promising ...
Research Navigator Office. If you would like to speak with someone about participating in research or joining a research study at Mass General Brigham, you can contact the Research Navigator Office at 857-282-5370 to get answers to your questions. As a patient at Mass General Brigham, you have access to even more opportunities than the general ...
Support and resources. We at Mass General Brigham know our research-infused care is what sets us apart. Your breakthroughs drive medical innovation, expand our knowledge, and improve patient outcomes. We continue to provide support for the research activities of our member institutions and encourage research collaboration with external entities.
Services. DCR faculty provide a variety of free consultations to assist investigators and staff with specific projects. To request a consultation, please click the button below. Request a Consultation. The DCR's Center for Clinical Research Education (CCRE) provides support in curriculum design, development, and delivery.
Hospital Research Institutes. As a world-recognized leader in research, Mass General Brigham is home to one of the largest hospital system-based research enterprises in the U.S. Mass General Brigham's location in the Massachusetts biotech and academic medical communities creates enhanced collaborations with partners from academia, industry ...
The Division of Clinical Research (DCR) of the Mass General Research Institute works to promote science at the clinical—research interface. The division sponsors a total of 10 centers and 15 units, each of which has a specific mission to assist investigators conducting clinical research through education and consultation. Our mission is to ...
MGH biostatisticians play prominent roles in a wide range of clinical and translational research at the MGH Cancer Center (MGHCC) and through the Biostatistics Core of the Dana-Farber/Harvard Cancer Center (DF/HCC).. We have been instrumental in developing and reporting clinical trials for adult and pediatric cancers, ranging from early phase to randomized studies in local and multi-center ...
→ About Us. Clinical Research Studies. Physicians at the Neuroendocrine and Pituitary Tumor Clinical Center at Massachusetts General Hospital are conducting several exciting research studies aimed at understanding and addressing medical issues related to pituitary disorders and learning more about the mechanisms of anorexia nervosa and applying novel treatments that will help improve future ...
The Massachusetts General Hospital Translational and Clinical Research Centers (TCRC) supports the conduct of patient-oriented research projects and serves as a human research laboratory in which investigators can perform their funded projects. It is composed of two major units: the Clinical Research Center, a federally funded part of the Harvard Clinical Translation Science Center, directed ...
Research staff improves your range and depth of expertise in studies across CNS disorders: Anxiety · Depression · Bipolar disorder · Women's mental health · Schizophrenia · Obsessive-Compulsive Disorder · ADHD · Geriatrics/Alzheimer's disease · Pharmacogenetics · Neuroimaging · Pediatric psychopharmacology. Raising the Bar In ...
Suggested Courses for Further Skill Development. For Experienced Research Study Staff. This track is designed for clinical research coordinators or clinical research project managers with more than 2 years of clinical research experience. These individuals will have a fair understanding MGH policies and procedures, are very familiar with ...
The research protocol was approved by a central institutional review board and by ... Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011 ... Massachusetts General Hospital, Boston. Kimberly Stone, M.D. Stanford University ...
MGH is founding the Translational Research Center (TRC) to conduct clinical research trials that derisk new drug and medical device translational opportunities. Under the management of the Translational Management Group, the TRC executes proof-of-concept studies that quickly demonstrate the therapeutic benefit to patients or eliminate compounds ...
Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices. Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best. Study IDs.
The polo-like kinase 1 (PLK1) has emerged as a promising therapeutic target for KRAS-mutant colorectal cancer.In this work, preclinical data were generated that established a rationale to clinically assess the PLK1 inhibitor onvansertib, in combination with FOLFIRI/bevacizumab, in patients with KRAS-mutant metastatic colorectal cancer.In the phase Ib study, the combination proved to be ...
The primary outcome for 15 trials for Research Question 1 was QOL, 8-21 and eight studies identified QOL as the secondary outcomes. 8,10,11,13,22-25 In addition, four of the trials for Research Question 2, 18,26-28 four for Research Question 3, 26,29-31 two for Research Question 4, 15,21 and two other trials for the other research questions ...
Our commitment to eradicating cancer is fueled by scientific investigation as part of the largest hospital-based research program in the United States. Search clinical trials 617-726-5130. Mass General Cancer Center fosters innovation in all phases of cancer research, with a powerful synergy between laboratory scientists and bedside physicians.
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, was not involved in the research but said it was a "very important" study.
For clinical research to be deemed ethically acceptable, it is necessary that a study have a favorable benefit-risk ratio. 1 This requirement is met when risks and harms are minimized, potential benefits are maximized, and the potential benefits outweigh the potential risks. 1 In evaluating the risks of clinical research, institutional review boards (IRBs) and ethics committees concentrate ...
Yes I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. ... The details of the IRB/oversight body that provided approval or exemption for the research described are given below: The study was deemed exempt by the Massachusetts ...
The McCance Center for Brain Health in the Department of Neurology at the Massachusetts General Hospital (MGH) is a Harvard-affiliated Center in Boston well-poised to the discovery of novel tools and interventions that promote brain health. ... • Clinical research experience including clinical trials or epidemiological studies in aging and ...
A cornerstone initiative of the Massachusetts General Hospital Research Institute, the Division of Clinical Research works to increase the quantity, quality and efficiency of translating basic science advances into improved care for our patients. The Division of Clinical Research promotes clinical research by: The Centers and Units within the ...
Clinical trials at UAB were instrumental in the FDA approval of therapies including GLP-1 inhibitors (such as Ozempic) for obesity and diabetes, immunotherapies for cancer, and a potentially curative gene therapy for sickle cell. ... Growth with Purpose is to expand clinical research across Alabama, including in cancer, neurology and cardiology.
New analyses of the longest clinical trial yet of the weight-loss drug Wegovy are shedding light on how quickly it helps people lose weight, how long they sustain that weight loss and how safe the ...