Periodic Table of Element Groups
- Periodic Table
- Chemical Laws
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties. This is what is meant by periodicity or periodic table trends .
There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths , alkali metals, alkaline earth, halogens, and noble gasses.

Groups in the Periodic Table of Elements
Click on an element to read about the chemical and physical properties of the group to which that element belongs.
Alkali Metals
- Less dense than other metals
- One loosely bound valence electron
- Highly reactive, with reactivity increasing moving down the group
- The largest atomic radius of elements in their period
- Low ionization energy
- Low electronegativity
Alkaline Earth Metals
- Two electrons in the valence shell
- Readily form divalent cations
- Low electron affinity
Transition Metals
The lanthanides (rare earth) and actinides are also transition metals. The basic metals are similar to transition metals but tend to be softer and to hint at nonmetallic properties. In their pure state, all of these elements tend to have a shiny, metallic appearance. While there are radioisotopes of other elements, all of the actinides are radioactive.
- Very hard, usually shiny, ductile, and malleable
- High melting and boiling points
- High thermal and electrical conductivity
- Form cations (positive oxidation states)
- Tend to exhibit more than one oxidation state
Metalloids or Semimetals
- Electronegativity and ionization energy intermediate between that of metals and nonmetals
- May possess a metallic luster
- Variable density, hardness, conductivity, and other properties
- Often make good semiconductors
- Reactivity depends on the nature of other elements in the reaction
The halogens and noble gases are nonmetals, although they have their own groups, too.
- High ionization energy
- High electronegativity
- Poor electrical and thermal conductors
- Form brittle solids
- Little if any metallic luster
- Readily gain electrons
The halogens exhibit different physical properties from each other but do share chemical properties.
- Extremely high electronegativity
- Very reactive
- Seven valence electrons, so elements from this group typically exhibit a -1 oxidation state
Noble Gases
The noble gasses have complete valence electron shells, so they act differently. Unlike other groups, noble gasses are unreactive and have very low electronegativity or electron affinity.
Click on the element symbol in the table for further information.
- Alkali Metal
- Alkaline Earth
- Transition Metal
- Basic Metal
- Periodic Table Definition in Chemistry
- How to Use a Periodic Table of Elements
- Chemical Element Charges Table
- List of Periodic Table Groups
- Introduction to the Periodic Table
- Periodic Table Study Guide - Introduction & History
- What Are the Properties of Nonmetals?
- Periodic Table for Kids
- Element Families of the Periodic Table
- How Is the Periodic Table Organized Today?
- The Difference Between an Element Group and Period
- Metals: List of Elements
- Metals Versus Nonmetals - Comparing Properties
- What Are the Parts of the Periodic Table?
- The Periodic Properties of the Elements
- Family Definition in Chemistry
Module 2: Atoms, Molecules, and Ions
The periodic table, learning outcomes.
- State the periodic law and explain the organization of elements in the periodic table
- Predict the general properties of elements based on their location within the periodic table
- Identify metals, nonmetals, and metalloids by their properties and/or location on the periodic table
As early chemists worked to purify ores and discovered more elements, they realized that various elements could be grouped together by their similar chemical behaviors. One such grouping includes lithium (Li), sodium (Na), and potassium (K): These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. A second grouping includes calcium (Ca), strontium (Sr), and barium (Ba), which also are shiny, good conductors of heat and electricity, and have chemical properties in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and K are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of two of their atoms to one oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Germany (1870) independently recognized that there was a periodic relationship among the properties of the elements known at that time. Both published tables with the elements arranged according to increasing atomic mass. But Mendeleev went one step further than Meyer: He used his table to predict the existence of elements that would have the properties similar to aluminum and silicon, but were yet unknown. The discoveries of gallium (1875) and germanium (1886) provided great support for Mendeleev’s work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev’s contributions to the development of the periodic table are now more widely recognized (Figure 1).

Figure 1. (a) Dimitri Mendeleev is widely credited with creating (b) the first periodic table of the elements. (credit a: modification of work by Serge Lachinov; credit b: modification of work by “Den fjättrade ankan”/Wikimedia Commons)
You can view the transcript for “The Periodic Table: Crash Course Chemistry #4” here (opens in new window) .
By the twentieth century, it became apparent that the periodic relationship involved atomic numbers rather than atomic masses. The modern statement of this relationship, the periodic law , is as follows: the properties of the elements are periodic functions of their atomic numbers . A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows, called periods or series , and 18 vertical columns, called groups . Groups are labeled at the top of each column. In the United States, the labels traditionally were Roman numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common. For the table to fit on a single page, parts of two of the rows, a total of 14 columns, are usually written below the main body of the table.
Figure 2. Elements in the periodic table are organized according to their properties.
Many elements differ dramatically in their chemical and physical properties, but some elements are similar in their behaviors. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, good conductors of heat and electricity—shaded yellow); nonmetals (elements that appear dull, poor conductors of heat and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
The elements can also be classified into the main-group elements (or representative elements ) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row elements are actinides ; Figure 3). The elements can be subdivided further by more specific properties, such as the composition of the compounds they form. For example, the elements in group 1 (the first column) form compounds that consist of one atom of the element and one atom of hydrogen. These elements (except hydrogen) are known as alkali metals , and they all have similar chemical properties. The elements in group 2 (the second column) form compounds consisting of one atom of the element and two atoms of hydrogen: These are called alkaline earth metals , with similar properties among members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, also known as inert gases ). The groups can also be referred to by the first element of the group: For example, the chalcogens can be called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both group 1 and group 17 elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.
Figure 3. The periodic table organizes elements with similar properties into groups.
Example 1: Naming Groups of Elements
Atoms of each of the following elements are essential for life. Give the group name for the following elements:
- alkaline earth metal
- alkali metal
Check Your Learning
Give the group name for each of the following elements:
In studying the periodic table, you might have noticed something about the atomic masses of some of the elements. Element 43 (technetium), element 61 (promethium), and most of the elements with atomic number 84 (polonium) and higher have their atomic mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes (you will learn more about radioactivity in the nuclear chemistry module ). An average atomic weight cannot be determined for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even exist in nature. The number in square brackets is the atomic mass number (and approximate atomic mass) of the most stable isotope of that element.
Key Concepts and Summary
The discovery of the periodic recurrence of similar properties among the elements led to the formulation of the periodic table, in which the elements are arranged in order of increasing atomic number in rows known as periods and columns known as groups. Elements in the same group of the periodic table have similar chemical properties. Elements can be classified as metals, metalloids, and nonmetals, or as a main-group elements, transition metals, and inner transition metals. Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
Metal or Nonmetal?
1. (a) metal, inner transition metal; (b) nonmetal, representative element; (c) metal, representative element; (d) nonmetal, representative element; (e) metal, transition metal; (f) metal, inner transition metal; (g) metal, transition metal; (h) nonmetal, representative element; (i) nonmetal, representative element; (j) metal, representative element
Identifying Elements
- noble gases
- alkaline earth metals
- alkali metals
- the noble gas in the same period as germanium
- the alkaline earth metal in the same period as selenium
- the halogen in the same period as lithium
- the chalcogen in the same period as cadmium
- the halogen in the same period as the alkali metal with 11 protons
- the alkaline earth metal in the same period with the neutral noble gas with 18 electrons
- the noble gas in the same row as an isotope with 30 neutrons and 25 protons
- the noble gas in the same period as gold
- the alkali metal with 11 protons and a mass number of 23
- the noble gas element with and 75 neutrons in its nucleus and 54 electrons in the neutral atom
- the isotope with 33 protons and 40 neutrons in its nucleus
- the alkaline earth metal with 88 electrons and 138 neutrons
- the chalcogen with a mass number of 125
- the halogen whose longest-lived isotope is radioactive
- the noble gas, used in lighting, with 10 electrons and 10 neutrons
- the lightest alkali metal with three neutrons
1. (a) He; (b) Be; (c) Li; (d) O
3. (a) krypton, Kr; (b) calcium, Ca; (c) fluorine, F; (d) tellurium, Te
5. (a) [latex]{}_{11}^{23}\text{Na}[/latex] ; (b) [latex]{}_{54}^{129}\text{Xe}[/latex] ; (c) [latex]{}_{33}^{73}\text{As}[/latex] ; (d) [latex]{}_{88}^{226}\text{Ra}[/latex]
actinide: inner transition metal in the bottom of the bottom two rows of the periodic table
alkali metal: element in group 1
alkaline earth metal: element in group 2
chalcogen: element in group 16
group: vertical column of the periodic table
halogen: element in group 17
inert gas: (also, noble gas) element in group 18
inner transition metal: (also, lanthanide or actinide) element in the bottom two rows; if in the first row, also called lanthanide, of if in the second row, also called actinide
lanthanide: inner transition metal in the top of the bottom two rows of the periodic table
main-group element: (also, representative element) element in columns 1, 2, and 12–18
metal: element that is shiny, malleable, good conductor of heat and electricity
metalloid: element that conducts heat and electricity moderately well, and possesses some properties of metals and some properties of nonmetals
noble gas: (also, inert gas) element in group 18
nonmetal: element that appears dull, poor conductor of heat and electricity
period: (also, series) horizontal row of the period table
periodic law: properties of the elements are periodic function of their atomic numbers.
periodic table: table of the elements that places elements with similar chemical properties close together
pnictogen: element in group 15
representative element: (also, main-group element) element in columns 1, 2, and 12–18
series: (also, period) horizontal row of the period table
transition metal: element in columns 3–11
- Chemistry 2e. Provided by : OpenStax. Located at : https://openstax.org/ . License : CC BY: Attribution . License Terms : Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- The Periodic Table: Crash Course Chemistry #4. Authored by : CrashCourse. Located at : https://youtu.be/0RRVV4Diomg . License : Other . License Terms : Standard YouTube License
advanced search
- browse resources
- filing cabinet
- submit a resource
- about the PSRC
Website Detail Page
Primary details, user comments (1), more than meets the eye.
Author: Mary Salit Posted: September 16, 2012 at 1:27PM Source: The Physics Front collection
There is some unique content beneath the shiny interface and pretty graphics here. Almost every element has a link to a podcast (and a transcript) describing its history and uses in an approachable narrative form, and links to videos and "resources." The resources in particular seem like material that could be incorporated into a lesson plan rather than simply used as reference material. In many cases they outline activities which could be done in a laboratory session or as a demonstration. In other cases, such as the reaction of rubidium with water (a little dangerous for an in class demo) they feature videos of the experiment instead. It is the resources section which differentiates this site from similar ones, and makes it useful for teachers, not just students preparing reports.
- Standards (4)
AAAS Benchmark Alignments (1993 Version)
4. the physical setting.
- 4D (6-8) #1. All matter is made up of atoms, which are far too small to see directly through a microscope. The atoms of any element are alike but are different from atoms of other elements. Atoms may stick together in well-defined molecules or may be packed together in large arrays. Different arrangements of atoms into groups compose all substances.
- 4D (6-8) #5. Scientific ideas about elements were borrowed from some Greek philosophers of 2,000 years earlier, who believed that everything was made from four basic substances: air, earth, fire, and water. It was the combinations of these "elements" in different proportions that gave other substances their observable properties. The Greeks were wrong about those four, but now over 100 different elements have been identified, some rare and some plentiful, out of which everything is made. Because most elements tend to combine with others, few elements are found in their pure form.
- 4D (6-8) #6. There are groups of elements that have similar properties, including highly reactive metals, less-reactive metals, highly reactive nonmetals (such as chlorine, fluorine, and oxygen), and some almost completely nonreactive gases (such as helium and neon). An especially important kind of reaction between substances involves combination of oxygen with something elseÑas in burning or rusting. Some elements don't fit into any of the categories; among them are carbon and hydrogen, essential elements of living matter.
- 4D (9-12) #1. Atoms are made of a positive nucleus surrounded by negative electrons. An atom's electron configuration, particularly the outermost electrons, determines how the atom can interact with other atoms. Atoms form bonds to other atoms by transferring or sharing electrons.
Citation Formats
%T Visual Elements Periodic Table %D 2011 %I The Royal Society of Chemistry %C London %U https://www.rsc.org/periodic-table %O application/flash
%0 Electronic Source %D 2011 %T Visual Elements Periodic Table %I The Royal Society of Chemistry %V 2024 %N 2 June 2024 %9 application/flash %U https://www.rsc.org/periodic-table
The AIP Style presented is based on information from the AIP Style Manual .
The APA Style presented is based on information from APA Style.org: Electronic References .
The Chicago Style presented is based on information from Examples of Chicago-Style Documentation .
The MLA Style presented is based on information from the MLA FAQ .
- Shared Folders (1)
This resource is stored in a shared folder.
You must login to access shared folders.
Supplements
- Comments (1)
Make a Comment Relate this resource Contact us
Similar Materials
WebElements Periodic Table
Origin of the Periodic Table
Imagine the Universe: Time that Star! Lesson Plan on Periodic Behavior and…
Featured By
Physics To Go Aug 1 - Aug 16, 2006
- RMIT Australia
- RMIT Europe
- RMIT Vietnam
- RMIT Global
- RMIT Online
- Alumni & Giving

- What will I do?
- What will I need?
- Who will help me?
- About the institution
- New to university?
- Studying efficiently
- Time management
- Mind mapping
- Note-taking
- Reading skills
- Argument analysis
- Preparing for assessment
- Critical thinking and argument analysis
- Online learning skills
- Starting my first assignment
- Researching your assignment
- What is referencing?
- Understanding citations
- When referencing isn't needed
- Paraphrasing
- Summarising
- Synthesising
- Integrating ideas with reporting words
- Referencing with Easy Cite
- Getting help with referencing
- Acting with academic integrity
- Artificial intelligence tools
- Understanding your audience
- Writing for coursework
- Literature review
- Academic style
- Writing for the workplace
- Spelling tips
- Writing paragraphs
- Writing sentences
- Academic word lists
- Annotated bibliographies
- Artist statement
- Case studies
- Creating effective poster presentations
- Essays, Reports, Reflective Writing
- Law assessments
- Oral presentations
- Reflective writing
- Art and design
- Critical thinking
- Maths and statistics
- Sustainability
- Educators' guide
- Learning Lab content in context
- Latest updates
- Students Alumni & Giving Staff Library
Learning Lab
Getting started at uni, study skills, referencing.
- When referencing isn't needed
- Integrating ideas
Writing and assessments
- Critical reading
- Poster presentations
- Postgraduate report writing
Subject areas
For educators.
- Educators' guide
- The arrangement of the periodic table
The Periodic table is a visual representation of the elements. The way the elements are arranged reveals information about their properties.
\[\require{mhchem}\]
The periodic table arranges elements in order of increasing atomic number. The rows and columns in the periodic table are known as periods and groups, respectively.
The elements in each group have similar chemical properties due to the similarity in their valence electron configurations.
Example: Group \(\mathrm{1}\) elements
All of these elements have one \(s\) electron in their outermost (valence) shell. Group \(\mathrm{I}\) elements, except hydrogen, are soft metals and demonstrate high reactivity with water and oxygen.
Valence electrons and core electrons
Electrons located in the outermost shell (largest and highest-energy shell) of an atom are known as valence electrons. The rest of the electrons in the atom are known as core electrons. Valence electrons are accessible, thus participate in the chemical reactions. Core electrons are tightly bound to the nucleus and inaccessible.
Classification of the elements
Groups of elements, metals and non-metals.
Based on selected physical properties, elements are classified into metals and nonmetals. Metals are located on the left side of the periodic table, and nonmetals are located on the right.
Physical properties of metals - conductors of electricity and heat, exist as solids at room temperature (except Mercury), ductile, malleable, shiny appearance (metallic lustre), high density and high melting point.
Elements know as metalloids exhibit both metallic and nonmetallic properties. Metalloids are located between metals and nonmetals in the periodic table.
s,p,d,f blocks
The elements in the periodic table are categorised into \(s,p,d,f\) blocks based on the location of the last electron. The \(s\) block contains elements that have their last electron in \(s\) subshell. Similarly, \(p,d,f\) blocks have elements with their last electron located in \(p,d,f\) subshells, respectively.
Classification based on electron configurations of the elements
- The noble gases - The last electron located in the \(p\) subshell except \(\ce{He}\). All noble gases, except \(\ce{He}\) have electron configuration ending with \(p^{6}\) (completes the \(p\) subshell).
- The representative elements - The elements located in \(s\) block and the first five columns of \(p\) block belongs to the representative elements group. The last electron of these elements fills an \(s\) subshell partially or entirely or fills a \(p\) subshell partially.
- The transition elements - The elements located in the \(d\) block.
- The inner transition elements - The elements located in the \(f\) block.
Classification of the periodic table based on electron configuration of the elements.
Periodic table trends
Atomic radii.
Atomic radii increase from the top to the bottom of a group of the periodic table. As \(n\) increases when proceeding down a group, valence orbitals become larger, leading to an increase in atomic radii.
In contrast, from left to right of a period, atomic radii decrease. The effective nuclear charge (Z) increases when the atomic number (Z) increases across the period. As a result of the higher Z, orbitals become smaller.
Ionisation energy
The first ionisation energy is the minimum energy required to remove an electron from a neutral atom. The second and third ionisation energies are the energies necessary to remove more electrons from the atom.
First ionisation energy increases when moving from left to right across a period as larger Z makes electrons bound tightly. When proceeding down on a group, the first ionisation energy decreases as an electron in a higher energy level is easier to remove. The trend in the first ionisation energy is inverse of the atomic radii.
Electron affinity
Electron affinity is the amount of energy change when an electron is added to a gaseous phase atom to form an anion. The electron affinity becomes more negative when moving across a period from left to right.
Electronegativity
Electronegativity is the power of an atom in a molecule to attract electrons. The larger the value, the larger the electron attracting ability. Atoms with higher electronegativity form anions, whereas atoms with smaller electronegativity form cations. Electronegativity decreases from top to bottom and increases from left to right of the periodic table.
Periodic trends
Source: Periodic table data comes from Periodic-Table-JSON , Attribution-ShareAlike 3.0 Unported ( CC BY-SA 3.0 ). Interactive Periodic table based on a version by Adrian Roselli.
- History of the periodic table
- Periodic table quiz
Still can't find what you need?
The RMIT University Library provides study support , one-on-one consultations and peer mentoring to RMIT students.
- Facebook (opens in a new window)
- Twitter (opens in a new window)
- Instagram (opens in a new window)
- Linkedin (opens in a new window)
- YouTube (opens in a new window)
- Weibo (opens in a new window)
- Copyright © 2024 RMIT University |
- Accessibility |
- Learning Lab feedback |
- Complaints |
- ABN 49 781 030 034 |
- CRICOS provider number: 00122A |
- RTO Code: 3046 |
- Open Universities Australia
Skip to Main Content
- My Assessments
- My Curriculum Maps
- Communities
- Workshop Evaluation
Share Suggestion
Visual elements: a visual interpretation of the table of elements, web-based resource, grade levels, course, subject.
- Printer Friendly Version
CONSTANCY AND CHANGE
Identify characteristic properties of matter that can be used to separate one substance from the other.
Differentiate between elements, compounds, and mixtures.
Identify groups of elements that have similar properties.
Explain how materials are characterized by having a specific amount of mass in each unit of volume ( density ).
Identify atoms as the basic building blocks of matter and that elements are composed of one type of atom.
Identify characteristics of elements derived from the periodic table.
Predict properties of elements using trends of the periodic table.
Identify properties of matter that depend on sample size.
Explain the unique properties of water ( polarity , high boiling point, forms hydrogen bonds , high specific heat ) that support life on Earth.
Differentiate between physical properties and chemical properties.
Differentiate between pure substances and mixtures; differentiate between heterogeneous and homogeneous mixtures.
Explain the relationship of an element’s position on the periodic table to its atomic number, ionization energy, electro-negativity, atomic size, and classification of elements.
Use electro-negativity to explain the difference between polar and non-polar covalent bonds
Description
Elements text - Dr. John Emsley, Science Writer in Residence at Cambridge University, editor of Chem@Cam , and acclaimed author of the following two books : The Elements and Molecules at an Exhibition (both Oxford University Press 1998).
Periodic Landscapes text - Dr Ann Prescott, Lecturer in Chemistry, Abertay University, Dundee, whose previous experience in the initiation of science/art exhibitions has been invaluable.
Animation - Alastair Wells, video maker & animator, Genetix, Glasgow.
Soundtracks - M.P.Lancaster, Memory Bank Studio, Glasgow.
Printed matter - David Wells, Portfolio Graphics, Glasgow.
Date Published
Insert template.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Mendeleev’s Periodic Table

Dmitri Mendeleev ‘s periodic table is the forerunner to the modern periodic table. It is a “ periodic ” table because it groups elements in rows and columns that showcase recurring properties, such as valence, electronegativity, and ionization energy.
- Mendeleev’s first periodic table in 1869 included the 63 known elements and spaces for three predicted, undiscovered elements. He revised and refined this table multiple times, as new data came to light.
- Dmitri Mendeleev did not invent the first periodic table. Instead, he devised a table that organizes elements by atomic weight and periodic properties and predicts properties of undiscovered elements.
- The key difference between Mendeleev’s table and the modern table is that the modern table orders elements by increasing atomic number. To be fair, the proton and atomic number were unknown in Mendeleev’s time and the difference only changes the position of a few elements.

The periodic table is one of the most important tools in chemistry, organizing elements based on their properties and atomic structures. Its development has been a long and complex process, with contributions from many scientists over the centuries. One of the most significant figures in this history is Dmitri Mendeleev, a Russian chemist who first proposed his version of the periodic table in 1869.
Before Mendeleev
Dmitri Mendeleev did not invent the first periodic table , but his table organizes elements much like the table we use today. Mendeleev’s work built on the discoveries of earlier chemists such as John Dalton and Antoine Lavoisier , who laid the foundations of modern chemistry. In the early 19th century, scientists began to investigate the properties of different elements and how they reacted with one another. This led to the development of atomic theory, which proposed that all matter was made up of tiny particles called atoms. By the mid-19th century, scientists had discovered around 60 elements, but there was no systematic way to organize them.
In 1863, John Newlands published a letter in Chemical News , organizing the elements by atomic weight according to his “Law of Octaves”, which is comparable to the modern octet rule . Lothar Meyer’s 1864 book pointed out the recurring or periodic properties in a periodic table organizing elements by their valences.
The story goes that Mendeleev saw the arrangement for his periodic table in a dream. His table orders the elements by increasing atomic weight, in rows and columns, with each column representing a group of elements with similar properties. Mendeleev’s 1869 table surpassed the work of Newlands and Meyer by including all of the 63 known elements and holding spaces for predicted undiscovered elements. The chemical and physical properties of undiscovered elements could be predicted based on periodicity. Specifically, Mendeleev’s periodic table predicted the properties of germanium , gallium, and scandium. Mendeleev also dealt with the lanthanides and actinides by placing them in two separate rows, which is the format that continues in the modern table.

Importance of Mendeleev’s Periodic Table
Mendeleev’s periodic table was a significant breakthrough in chemistry, providing a systematic way to organize the elements and predict their properties. For example, if you have a sample of sodium and understand its properties, you know the properties of lithium (above sodium on the table) and potassium (below sodium) without ever seeing them. All elements in that group (the alkali metals) are highly reactive shiny metals that burn in water and have a +1 oxidation state.
Mendeleev’s table was also a major step forward in understanding atomic structure, as it revealed patterns in the behavior of atoms that had not been noticed before. These patterns reflect the electron shells of atoms. Ultimately, chemical reactions involve interactions between electrons.
Mendeleev’s periodic table allowed scientists to make predictions about elements that had not yet been discovered. Knowing the properties of an unknown elements makes identifying and verifying it a simpler process.
Mendeleev’s Periodic Table vs the Modern Periodic Table
The difference between Mendeleev’s periodic table and the modern periodic table is that Mendeleev’s table ordered elements by increasing atomic weight while the modern table orders elements by increasing atomic number. The atomic weight is the sum of the protons and neutrons in an atom. In contrast, the atomic number is the number of protons. In a few cases, using atomic weight changes the order of elements. This is because of the isotope ratio of naturally occurring elements. But, the concepts of the proton and atomic number were not known in Mendeleev’s time.
Of course, the modern periodic table contains more elements than Mendeleev’s periodic table. The current table has 118 elements, while Mendeleev’s first table had 63.
Mendeleev’s periodic table was a significant breakthrough in chemistry because it offered a systematic way to organize the elements and predict their properties. Further, it allowed scientists to make predictions about elements that had not yet been discovered.
The modern periodic table is based on the concept of atomic number, instead of atomic weight. It includes more elements than Mendeleev’s table, yet still allows for predicting properties of undiscovered elements.
- Godfrey, Simon S. (2003). Dreams & Reality . Trafford Publishing. ISBN 1-4120-1143-4.
- Gordin, Michael (2004). A Well-Ordered Thing: Dmitrii Mendeleev and the Shadow of the Periodic Table . New York: Basic Books. ISBN 978-0-465-02775-0.
- Kak, Subhash (2004). “Mendeleev and the Periodic Table of Elements”. Sandhan . 4 (2): 115–123.
- doi: 10.48550/arXiv.physics/0411080
- Mendeleev, Dmitry Ivanovich; Jensen, William B. (2005). Mendeleev on the Periodic Law: Selected Writings, 1869–1905 . Mineola, New York: Dover Publications. ISBN 978-0-486-44571-7.
- Weeks, Mary Elvira (1956). The Discovery of the Elements (6th ed.). Easton, PA: Journal of Chemical Education.
Related Posts
- Browse Collection
- Shared Folders
- Get Involved
- For New Teachers
- Contact the Editors
- About Physics Front
The Physics Front is a free service provided by the AAPT in partnership with the NSF / NSDL .
Detail Page
Primary details, user comments (1), more than meets the eye.
Author: Mary Salit Posted: September 16, 2012 at 1:27PM
There is some unique content beneath the shiny interface and pretty graphics here. Almost every element has a link to a podcast (and a transcript) describing its history and uses in an approachable narrative form, and links to videos and "resources." The resources in particular seem like material that could be incorporated into a lesson plan rather than simply used as reference material. In many cases they outline activities which could be done in a laboratory session or as a demonstration. In other cases, such as the reaction of rubidium with water (a little dangerous for an in class demo) they feature videos of the experiment instead. It is the resources section which differentiates this site from similar ones, and makes it useful for teachers, not just students preparing reports.
- Standards (4)
AAAS Benchmark Alignments (1993 Version)
4. the physical setting.
- 4D (6-8) #1. All matter is made up of atoms, which are far too small to see directly through a microscope. The atoms of any element are alike but are different from atoms of other elements. Atoms may stick together in well-defined molecules or may be packed together in large arrays. Different arrangements of atoms into groups compose all substances.
- 4D (6-8) #5. Scientific ideas about elements were borrowed from some Greek philosophers of 2,000 years earlier, who believed that everything was made from four basic substances: air, earth, fire, and water. It was the combinations of these "elements" in different proportions that gave other substances their observable properties. The Greeks were wrong about those four, but now over 100 different elements have been identified, some rare and some plentiful, out of which everything is made. Because most elements tend to combine with others, few elements are found in their pure form.
- 4D (6-8) #6. There are groups of elements that have similar properties, including highly reactive metals, less-reactive metals, highly reactive nonmetals (such as chlorine, fluorine, and oxygen), and some almost completely nonreactive gases (such as helium and neon). An especially important kind of reaction between substances involves combination of oxygen with something elseÑas in burning or rusting. Some elements don't fit into any of the categories; among them are carbon and hydrogen, essential elements of living matter.
- 4D (9-12) #1. Atoms are made of a positive nucleus surrounded by negative electrons. An atom's electron configuration, particularly the outermost electrons, determines how the atom can interact with other atoms. Atoms form bonds to other atoms by transferring or sharing electrons.
This resource is part of 2 Physics Front Topical Units.
This interactive periodic table merges science and art to explore the elements in a visually striking way. The table is arranged in the traditional format, but each element is represented by a photograph or illustration relating to its origin or use. With a click, users can view extensive data and brief histories of 103 elements. Embedded animations for common elements complete the package.
- Physical Sciences K-8 Properties of Matter Unit
- Physics First Properties of Matter Unit
- Conceptual Physics Properties of Matter Unit
- Algebra-Based Physics Properties of Matter Unit
- AP/Calculus-Based Physics Properties of Matter Unit
This interactive periodic table merges science and art to explore the elements in a unique and visually striking way. It's arranged in the traditional format, but each element is represented by a photograph or illustration relating to its origin or use. With a click, users can view extensive data and brief histories of 103 elements. Animations have also been embedded for some of the more common elements
- Physical Sciences K-8 Elements and the Periodic Table Unit
- Physics First Elements and the Periodic Table Unit
- Conceptual Physics Elements and the Periodic Table Unit
- Algebra-Based Physics Elements and the Periodic Table Unit
- AP/Calculus-Based Physics Elements and the Periodic Table Unit
Citation Formats
%T Visual Elements Periodic Table %D 2011 %I The Royal Society of Chemistry %C London %U https://www.rsc.org/periodic-table %O application/flash
%0 Electronic Source %D 2011 %T Visual Elements Periodic Table %I The Royal Society of Chemistry %V 2024 %N 2 June 2024 %9 application/flash %U https://www.rsc.org/periodic-table
The AIP Style presented is based on information from the AIP Style Manual .
The APA Style presented is based on information from APA Style.org: Electronic References .
The Chicago Style presented is based on information from Examples of Chicago-Style Documentation .
The MLA Style presented is based on information from the MLA FAQ .
- Shared Folders (1)
This resource is stored in a shared folder.
You must login to access shared folders.
Supplements
- Comments (1)
Make a Comment Relate this resource Contact us
Similar Materials
WebElements Periodic Table
IPPEX Online: Interactive Matter Module
Featured By
Physics To Go Aug 1 - Aug 16, 2006
Log In | Join AACT | Renew Membership
AACT Member-Only Content
You have to be an AACT member to access this content, but good news: anyone can join!
- AACT member benefits »
- Forgot User Name or Password?
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
Elemental Art: A Visual Periodic Table Mark as Favorite (13 Favorites)
PROJECT in Introduction , Elements , History , Periodic Table , Atoms , Valence Electrons , Subatomic Particles , Electrons . Last updated January 03, 2024.
In this project students will research an assigned element from the Periodic Table and then create a poster that visually explains and expresses the element. The final posters can be arranged into a classroom Periodic Table.
Grade Level
Middle School
NGSS Alignment
This project will help prepare your students to meet the performance expectations in the following standards:
- Obtaining, Evaluating, and Communicating Information
By the end of this project, students should be able to
- Understand how the elements of the Periodic Table are organized.
- Recognize basic trends in the arrangement of elements.
- Differentiate between the subatomic particles in an atom.
Chemistry Topics
This project supports students’ understanding of
- Periodic Table
- Atomic Structure
- Subatomic Particles
Teacher Preparation : 15 minutes
Lesson : 2 days for research
- Chromebooks or computer lab time (for research)
- Construction paper or poster board
- Element Fact Sheet handout
- Colored paper (for creating the visual poster)
- Glue Sticks
- No specific safety precautions need to be observed for this activity.
Teacher Notes
- For review about the elements or the Periodic Table visit this website .
- If you’re planning on keeping the student projects to organize in the shape of a periodic table on the classroom wall or in the hallway, you might try coordinating with your school’s copy room or librarian to laminate the projects to make them more durable.
- Give students a list of resource website to use for their research and help direct their thinking. I normally post links to “useful” websites on my website or in Google Classroom.
- If finding paper supplies poses an issue, students can create their visual elements using PowerPoint or Google Slides.
- For high level students: instead of creating a poster, these students could create a short cartoon describing the element. Websites such as PowToon are reasonably priced and are safe for student use.
- For lower level students: give them a template for creating their project or further limit their research options.
- I normally post ALL student handouts in Google Classroom for students to complete.
- This could also be organized as a lapbook instead of a poster.
- For timing, I allow one day for completing the element fact sheet and 1 day for creating the poster.
- Analysis questions are provided for after the project is completed. Students can look at the collection of posters that are organized as the periodic table to complete this section.
- The analysis section will require that students are given a paper copy of the periodic table.
For the Student
Understanding the elements of the Periodic Table can seem like an impossible task. Some of the elements have crazy names (einsteinium or americium), while others have names you’ve heard before (oxygen or carbon). But why do you need to learn about the elements? Obviously, you need oxygen to breathe, but do some of the other elements really serve any purpose? For this project, you will be researching a specific element to present to the class. You will discover how your element is used and how it affects your daily life.
Prelab Questions
Define the following words:
- Energy Level (Electron Shell)
- Valence Electron
- Atomic Number
- Mass Number
After completing this project you will understand how the elements of the Periodic Table are organized and recognize the uses of different elements.
- Review the research questions below.
- Fill out the document as you complete your research and make sure you provide links to the website where you found the answer to each question.
- When your research is finished, submit it to your teacher for review.
- Begin brainstorming how to visually represent your element on a poster.
- When you are ready to begin constructing your poster, make sure you have a plan! You have a limited amount of time and supplies, so create a rough sketch of your layout and pictures before gluing or writing/drawing anything with markers.
- Make your work neat! Type your information and print it out if needed! Remember: these posters will be displayed in the hallway, so make sure your final product is something you’re proud of.
- You will complete the analysis section after everyone has completed their element poster.
Research Questions
Answer the following questions regarding your assigned element:
- What is the atomic number? (1 point)
- How many protons, electrons, and neutrons does an average atom have? (3 points)
- How many shells and valence electrons does an atom have? (2 points)
- What was it named after? (2 points)
- Where was it discovered? (2 points)
- Who discovered it? (2 points)
- When was it discovered? (1 point)
- Where can it be found? (3 points)
- Does the human body use it? Explain. (3 points)
- What are some of its main uses? Do we experience this element in everyday life? How? (7 points)
- Is it poisonous / hazardous? (3 points)
- In what compounds or forms is it commonly found? (5 points)
- How abundant is it? (4 points)
- In which group (column/family) can it be found on the periodic table? (1 point)
- What are some of its properties? (physical and chemical) (5 points)
- What state of matter is it commonly found? (1 point)
- What are at least 2 interesting facts about this element? (6 points)
- Include a drawing of its atomic structure/Bohr model (4 points)
Look at the collection of posters presented on the wall and use the information to answer the following questions:
- Explain any patterns or trends you notice in how the elements are arranged in terms of their atomic number and atomic mass.
- What do the atomic mass and atomic number of an element tell you?
- Color-code the provided Periodic Table by element category. Use blue for metals, red for non-metals, and yellow for metalloids. Do you notice any patterns? What are they?
- You discover a new element. You notice that the element is always bonded with chlorine and explodes when in contact with water. What group does this element most likely belong to?

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.3: Properties and Representations of Groups
- Last updated
- Save as PDF
- Page ID 151375

- Kathryn Haas
- Duke University
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Click here to see a lecture on this topic .
Group Multiplication
Now we will investigate what happens when we apply two symmetry operations in sequence. As an example, consider the \(NH_3\) molecule, which belongs to the \(C_{3v}\) point group. Consider what happens if we apply a \(C_3\)rotation (120˚ counter-clockwise) followed by a \(\sigma_v\) reflection (reflection over the \(\sigma_v\) axis) . We write this combined operation \(\sigma_v\)\(C_3\) (when written, symmetry operations operate on the thing directly to their right, just as operators do in quantum mechanics – we therefore have to work backwards from right to left from the notation to get the correct order in which the operators are applied). As we shall soon see, the order in which the operations are applied is important.
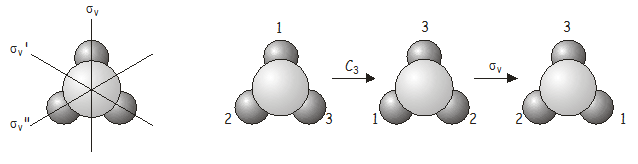
The combined operation \(\sigma_v\)\(C_3\) is equivalent to \(\sigma_v''\) (note the double prime on \(\sigma_v''\)!) , which is also a symmetry operation of the \(C_{3v}\) point group. Now let’s see what happens if we apply the operators in the reverse order, i.e., \(C_3\)\(\sigma_v\) is ( \(\sigma_v\) followed by \(C_3\) ).
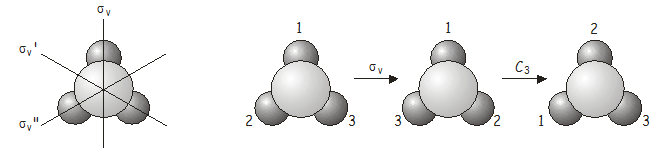
Again, the combined operation \(C_3\)\(\sigma_v\) is equivalent to another operation of the point group, this time \(\sigma_v'\) (note the single prime on \(\sigma_v'\)!) .
There are two important points that are illustrated by this example:
- The order in which two operations are applied is important. For two symmetry operations \(A\) and \(B\), \(AB\) is not necessarily the same as \(BA\), i.e. symmetry operations do not in general commute . In some groups the symmetry elements do commute; such groups are said to be Abelian .
- If two operations from the same point group are applied in sequence, the result will be equivalent to another operation from the point group. Symmetry operations that are related to each other by other symmetry operations of the group are said to belong to the same class . In \(NH_3\), the three mirror planes \(\sigma_v\) , \(\sigma_v'\) and \(\sigma_v''\) belong to the same class (related to each other through a \(C_3\) rotation), as do the rotations \(C_3^+\) and \(C_3^-\) (anticlockwise and clockwise rotations about the principal axis, related to each other by a vertical mirror plane).
Four Properties of Mathematical Groups
Now that we have explored some of the properties of symmetry operations and elements and their behavior within point groups, we are ready to introduce the formal mathematical definition of a group. The definitions below will be put into the context of molecular symmetry.
A mathematical group is defined as a set of elements (\(A_1\), \(A_2\), \(A_3\)...) together with a rule for forming combinations \(A_i\),\(A_j\)... For our purposes, \(A_1\), \(A_2\), \(A_3\), etc. are symmetry elements and \(A_i\), \(A_j\), etc. are symmetry operations described in a previous section . The elements of the group and the rule for combining them must satisfy the following four criteria.
- The group must include the identity \(E\) , which commutes with other members of the group. In other terms, \(E A_i= A_i \) for all the elements of the group. Application of the identity operation before or after another operation, \(A_i\), results in the same outcome as \(A_i\) alone.
- The elements must satisfy the group property that the combination of any pair of elements is also an element of the group. For example, in the \(C_{3v}\) point group, a C 3 rotation followed by a \(\sigma_v\) gives another operation that is already part of the group: a \(\sigma_v"\).
- Each symmetry operation \(A_i\) must have an inverse \(A_i^{-1}\), which is also an element of the group, such that \[A_i A_i^{-1} = A_i^{-1}A_i = E \nonumber \] The inverse \(g_i^{-1}\) effectively 'undoes’ the effect of the symmetry operation \(g_i\). For example, in the \(C_{3v}\) point group, the inverse of \(C_3^+\) is \(C_3^-\).
- The rule of combination must be associative \[(A_i A_j )(A_k) = A_i(A_jA_k) \nonumber \] Or \(A(BC)=(AB)C\). In other words, the order of operations should not matter.
Group theory is an important area in mathematics, and luckily for chemists the mathematicians have already done most of the work for us. Along with the formal definition of a group comes a comprehensive mathematical framework that allows us to carry out a rigorous treatment of symmetry in molecular systems and learn about its consequences.
Many problems involving operators or operations (such as those found in quantum mechanics or group theory) may be reformulated in terms of matrices. Any of you who have come across transformation matrices before will know that symmetry operations such as rotations and reflections may be represented by matrices. It turns out that the set of matrices representing the symmetry operations in a group obey all the conditions laid out above in the mathematical definition of a group, and using matrix representations of symmetry operations simplifies carrying out calculations in group theory. Before we learn how to use matrices in group theory, it will probably be helpful to review some basic definitions and properties of matrices.
*This page was adapted from here (click) .
Contributors and Attributions
Claire Vallance (University of Oxford)
Curated or created by Kathryn Haas
Visual Representation
- Reference work entry
- pp 3405–3410
- Cite this reference work entry

- Yannis Ioannidis 3
417 Accesses
Graphical representation
The concept of “representation” captures the signs that stand in for and take the place of something else [ 5 ]. Visual representation, in particular, refers to the special case when these signs are visual (as opposed to textual, mathematical, etc.). On the other hand, there is no limit on what may be (visually) represented, which may range from abstract concepts to concrete objects in the real world or data items.
In addition to the above, however, the term “representation” is often overloaded and used to imply the actual process of connecting the two worlds of the original items and of their representatives. Typically, the context determines quite clearly which of the two meanings is intended in each case, hence, the term is used for both without further explanation.
Underneath any visual representation lies a mapping between the set of items that are being represented and the set of visual elements that are used to represent them, i.e., to...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Recommended Reading
Card S.K., Mackinlay J.D., and Shneiderman B. Information visualization. In Readings in Information Visualization: Using Vision to Think, 1999, pp. 1–34.
Google Scholar
Card S.K., Mackinlay J.D., and Shneiderman B. Readings in Information Visualization: Using Vision to Think. Morgan Kaufman, Los Altos, CA, 1999.
Foley J.D., van Dam A., Feiner S.K., and Hughes J.F. Computer Graphics: Principles and Practice. Addison-Wesley, Reading, MA, 1990.
Haber E.M., Ioannidis Y., and Livny M. Foundations of visual metaphors for schema display. J. Intell. Inf. Syst., 3(3/4):263–298, 1994.
Article Google Scholar
Mitchell W. Representation. In Critical Terms for Literary Study,Lentricchia F and McLaughlin T. (eds.), 2nd edn., Chicago, IL. University of Chicago Press, 1995.
Tufte E.R. The Visual Display of Quantitative Information. Graphics Press, Cheshire, CO, 1983.
Tufte E.R. Envisioning Information. Graphics Press, Cheshire, CO, 1990.
Download references
Author information
Authors and affiliations.
University of Athens, Athens, Greece
Yannis Ioannidis
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
College of Computing, Georgia Institute of Technology, 266 Ferst Drive, 30332-0765, Atlanta, GA, USA
LING LIU ( Professor ) ( Professor )
Database Research Group David R. Cheriton School of Computer Science, University of Waterloo, 200 University Avenue West, N2L 3G1, Waterloo, ON, Canada
M. TAMER ÖZSU ( Professor and Director, University Research Chair ) ( Professor and Director, University Research Chair )
Rights and permissions
Reprints and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this entry
Cite this entry.
Ioannidis, Y. (2009). Visual Representation. In: LIU, L., ÖZSU, M.T. (eds) Encyclopedia of Database Systems. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-39940-9_449
Download citation
DOI : https://doi.org/10.1007/978-0-387-39940-9_449
Publisher Name : Springer, Boston, MA
Print ISBN : 978-0-387-35544-3
Online ISBN : 978-0-387-39940-9
eBook Packages : Computer Science Reference Module Computer Science and Engineering
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
visual representation of the elements grouped by similar properties a number written slightly smaller, below, and after an element's symbol indicating the number of atoms in a compound number written preceding a chemcial formula indicating the number of molecules
Features of the Periodic Table. Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
Figure 4.6.1 4.6. 1: The Periodic Table Showing the Elements in Order of Increasing Z. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right. The semimetals lie along a diagonal line separating the metals and nonmetals. An interactive Periodic table can be found Periodic Table of the Elements, LibreTexts.
chemical elements share similar properties. So they displayed these elements in the form of a table in which elements that had common properties were grouped in columns. This table, called the periodic table of the elements, was developed over the course of a century and a half and is now seen in chemistry text-books and wall charts around the ...
Alkali Metals. Less dense than other metals. One loosely bound valence electron. Highly reactive, with reactivity increasing moving down the group. The largest atomic radius of elements in their period. Low ionization energy. Low electronegativity.
The groups can also be referred to by the first element of the group: For example, the chalcogens can be called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both group 1 and group 17 elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.
Visual Elements Periodic Table. published by the The Royal Society of Chemistry. This is an online periodic table that presents the 103 elements in a visual manner. The elements are presented along with information on thier history, chemical, and physical properties. A unique image or animation associated with these properties is also given.
The Periodic table is a visual representation of the elements. The way the elements are arranged reveals information about their properties. ... The elements in each group have similar chemical properties due to the similarity in their valence electron configurations. Example: Group \(\mathrm{1}\) elements. Elements Electron configuration \(\ce ...
Visual Elements is an arts and science collaborative project supported by the Royal Society of Chemistry which aims to explore and reflect upon the diversity of elements that comprise matter in as unique and innovative manner as possible. Visual Elements aims to produce a new and vibrant visual assessment of the startling diversity of material ...
Features of the Periodic Table. Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of ...
Answer. 1.10: The Periodic Table and Periodic Properties is shared under a license and was authored, remixed, and/or curated by Kathryn A. Newton, Northern Michigan University. The Periodic Table of Elements categorizes like elements together. On the periodic table, elements that have similar properties are in the same groups (vertical).
Mendeleev's Periodic Table. Mendeleev's periodic table organizes elements by atomic weight and leaves gaps for undiscovered elements. Dmitri Mendeleev 's periodic table is the forerunner to the modern periodic table. It is a " periodic " table because it groups elements in rows and columns that showcase recurring properties, such as ...
periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the "periodic law" in their properties, in which elements in the same column (group) have similar properties.
The ADOMAH table by Valery Tsimmerman is a form of the left-step table that groups elements by their principal quantum number. The Physicist's Periodic Table of Elements by Timothy Stowe rearranges the standard table into both 3D-vertical (A) and 2D-horizontal (B) layouts. When merged with the work of Charles Janet and Eric Scerri (C) the ...
The definitive online periodic table reference site including technical data, and photographs and descriptions of thousands of samples of the chemical elements. Click any element below to see more. Newest samples 28 October, 2017
This group contains very reactive nonmetal elements. The noble gases are in group 8A. These elements also have similar properties to each other, the most significant property being that they are extremely unreactive, rarely forming compounds. We will learn the reason for this later, when we discuss how compounds form.
Representative Metals. are members of s block and parts of p block of the table Elements of group 1, (except hydrogen) 2, 13, (except boron) tin and lead of group 14, and bismuth of group 15 are ...
This interactive periodic table merges science and art to explore the elements in a unique and visually striking way. The table is arranged in the traditional format, but each element is represented by a photograph or illustration relating to its origin or use. With a click, users can view extensive data and brief histories of 103 elements.
If finding paper supplies poses an issue, students can create their visual elements using PowerPoint or Google Slides. For high level students: instead of creating a poster, these students could create a short cartoon describing the element. Websites such as PowToon are reasonably priced and are safe for student use.
The elements must satisfy the group property that the combination of any pair of elements is also an element of the group. For example, in the C3v C 3 v point group, a C 3 rotation followed by a σv σ v gives another operation that is already part of the group: a σv " σ v ". Each symmetry operation Ai A i must have an inverse A−1 i A i − ...
Underneath any visual representation lies a mapping between the set of items that are being represented and the set of visual elements that are used to represent them, i.e., to display them in some medium. In order for a visual representation to be useful, the mapping must satisfy certain properties: it must be expressive as well as effective ...