Appointments at Mayo Clinic
Stem cells: what they are and what they do.
Stem cells offer promise for new medical treatments. Learn about stem cell types, current and possible uses, and the state of research and practice.
You've heard about stem cells in the news, and perhaps you've wondered if they might help you or a loved one with a serious disease. Here are some answers to frequently asked questions about stem cells.

What are stem cells?
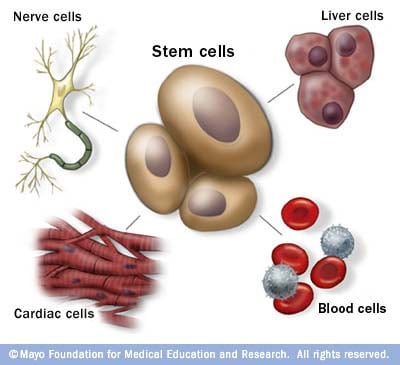
Stem cells: The body's master cells
Stem cells are the body's master cells. All other cells arise from stem cells, including blood cells, nerve cells and other cells.
Stem cells are a special type of cells that have two important properties. They are able to make more cells like themselves. That is, they self-renew. And they can become other cells that do different things in a process known as differentiation. Stem cells are found in almost all tissues of the body. And they are needed for the maintenance of tissue as well as for repair after injury.
Depending on where the stem cells are, they can develop into different tissues. For example, hematopoietic stem cells reside in the bone marrow and can produce all the cells that function in the blood. Stem cells also can become brain cells, heart muscle cells, bone cells or other cell types.
There are various types of stem cells. Embryonic stem cells are the most versatile since they can develop into all the cells of the developing fetus. The majority of stem cells in the body have fewer abilities to give rise to cells and may only help maintain and repair the tissues and organs in which they reside.
No other cell in the body has the natural ability to generate new cell types.
Why is there such an interest in stem cells?
Researchers are studying stem cells to see if they can help to:
- Increase understanding of how diseases occur. By watching stem cells mature into cells in bones, heart muscle, nerves, and other organs and tissue, researchers may better understand how diseases and conditions develop.
Generate healthy cells to replace cells affected by disease (regenerative medicine). Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease.
People who might benefit from stem cell therapies include those with leukemia, Hodgkin disease, non-Hodgkin lymphoma and some solid tumor cancers. Stem cell therapies also might benefit people who have aplastic anemia, immunodeficiencies and inherited conditions of metabolism.
Stem cells are being studied to treat type 1 diabetes, Parkinson's disease, amyotrophic lateral sclerosis, heart failure, osteoarthritis and other conditions.
Stem cells may have the potential to be grown to become new tissue for use in transplant and regenerative medicine. Researchers continue to advance the knowledge on stem cells and their applications in transplant and regenerative medicine.
Test new drugs for safety and effectiveness. Before giving drugs in development to people, researchers can use some types of stem cells to test the drugs for safety and quality. This type of testing may help assess drugs in development for toxicity to the heart.
New areas of study include the effectiveness of using human stem cells that have been programmed into tissue-specific cells to test new drugs. For the testing of new drugs to be accurate, the cells must be programmed to acquire properties of the type of cells targeted by the drug. Techniques to program cells into specific cells are under study.
Where do stem cells come from?
There are several sources of stem cells:
Embryonic stem cells. These stem cells come from embryos that are 3 to 5 days old. At this stage, an embryo is called a blastocyst and has about 150 cells.
These are pluripotent (ploo-RIP-uh-tunt) stem cells, meaning they can divide into more stem cells or can become any type of cell in the body. This allows embryonic stem cells to be used to regenerate or repair diseased tissue and organs.
- Adult stem cells. These stem cells are found in small numbers in most adult tissues, such as bone marrow or fat. Compared with embryonic stem cells, adult stem cells have a more limited ability to give rise to various cells of the body.
Adult cells altered to have properties of embryonic stem cells. Scientists have transformed regular adult cells into stem cells using genetic reprogramming. By altering the genes in the adult cells, researchers can make the cells act similarly to embryonic stem cells. These cells are called induced pluripotent stem cells (iPSCs).
This new technique may allow use of reprogrammed cells instead of embryonic stem cells and prevent immune system rejection of the new stem cells. However, scientists don't yet know whether using altered adult cells will cause adverse effects in humans.
Researchers have been able to take regular connective tissue cells and reprogram them to become functional heart cells. In studies, animals with heart failure that were injected with new heart cells had better heart function and survival time.
Perinatal stem cells. Researchers have discovered stem cells in amniotic fluid as well as umbilical cord blood. These stem cells can change into specialized cells.
Amniotic fluid fills the sac that surrounds and protects a developing fetus in the uterus. Researchers have identified stem cells in samples of amniotic fluid drawn from pregnant women for testing or treatment — a procedure called amniocentesis.
Why is there controversy about using embryonic stem cells?
The National Institutes of Health created guidelines for human stem cell research in 2009. The guidelines define embryonic stem cells and how they may be used in research and include recommendations for the donation of embryonic stem cells. Also, the guidelines state that embryonic stem cells from embryos created by in vitro fertilization can be used only when the embryo is no longer needed.
Where do these embryos come from?
The embryos being used in embryonic stem cell research come from eggs that were fertilized at in vitro fertilization clinics but never implanted in women's uteruses. The stem cells are donated with informed consent from donors. The stem cells can live and grow in special solutions in test tubes or petri dishes in laboratories.
Why can't researchers use adult stem cells instead?
Progress in cell reprogramming and the formation of iPSCs has greatly enhanced research in this field. However, reprogramming is an inefficient process. When possible, iPSCs are used instead of embryonic stem cells since this avoids the ethical issues about use of embryonic stem cells that may be morally objectionable for some people.
Although research into adult stem cells is promising, adult stem cells may not be as versatile and durable as are embryonic stem cells. Adult stem cells may not be able to be manipulated to produce all cell types, which limits how adult stem cells can be used to treat diseases.
Adult stem cells are also more likely to contain irregularities due to environmental hazards, such as toxins, or from errors acquired by the cells during replication. However, researchers have found that adult stem cells are more adaptable than was first thought.
What are stem cell lines, and why do researchers want to use them?
A stem cell line is a group of cells that all descend from a single original stem cell and are grown in a lab. Cells in a stem cell line keep growing but don't become specialized cells. Ideally, they remain free of genetic defects and continue to create more stem cells. Clusters of cells can be taken from a stem cell line and frozen for storage or shared with other researchers.
What is stem cell therapy (regenerative medicine), and how does it work?
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
Researchers grow stem cells in a lab. These stem cells are manipulated to specialize into specific types of cells, such as heart muscle cells, blood cells or nerve cells.
The specialized cells can then be implanted into a person. For example, if the person has heart disease, the cells could be injected into the heart muscle. The healthy transplanted heart muscle cells could then contribute to repairing the injured heart muscle.
Researchers have already shown that adult bone marrow cells guided to become heart-like cells can repair heart tissue in people, and more research is ongoing.
Have stem cells already been used to treat diseases?
Yes. Doctors have performed stem cell transplants, also known as bone marrow transplants, for many decades. In hematopoietic stem cell transplants, stem cells replace cells damaged by chemotherapy or disease or serve as a way for the donor's immune system to fight some types of cancer and blood-related diseases. Leukemia, lymphoma, neuroblastoma and multiple myeloma often are treated this way. These transplants use adult stem cells or umbilical cord blood.
Researchers are testing adult stem cells to treat other conditions, including some degenerative diseases such as heart failure.
What are the potential problems with using embryonic stem cells in humans?
For embryonic stem cells to be useful, researchers must be certain that the stem cells will differentiate into the specific cell types desired.
Researchers have discovered ways to direct stem cells to become specific types of cells, such as directing embryonic stem cells to become heart cells. Research is ongoing in this area.
Embryonic stem cells also can grow irregularly or specialize in different cell types spontaneously. Researchers are studying how to control the growth and development of embryonic stem cells.
Embryonic stem cells also might trigger an immune response in which the recipient's body attacks the stem cells as foreign invaders, or the stem cells might simply fail to function as expected, with unknown consequences. Researchers continue to study how to avoid these possible complications.
What is therapeutic cloning, and what benefits might it offer?
Therapeutic cloning, also called somatic cell nuclear transfer, is a way to create versatile stem cells independent of fertilized eggs. In this technique, the nucleus is removed from an unfertilized egg. This nucleus contains the genetic material. The nucleus also is removed from the cell of a donor.
This donor nucleus is then injected into the egg, replacing the nucleus that was removed, in a process called nuclear transfer. The egg is allowed to divide and soon forms a blastocyst. This process creates a line of stem cells that is genetically identical to the donor's cells — in essence, a clone.
Some researchers believe that stem cells derived from therapeutic cloning may offer benefits over those from fertilized eggs because cloned cells are less likely to be rejected once transplanted back into the donor. And it may allow researchers to see exactly how a disease develops.
Has therapeutic cloning in people been successful?
No. Researchers haven't been able to successfully perform therapeutic cloning with humans despite success in a number of other species.
Researchers continue to study the potential of therapeutic cloning in people.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Stem cell basics. National Institutes of Health. https://stemcells.nih.gov/info/basics/stc-basics/#stc-I. Accessed March 21, 2024.
- Lovell-Badge R, et al. ISSCR guidelines for stem cell research and clinical translation: The 2021 update. Stem Cell Reports. 2021; doi:10.1016/j.stemcr.2021.05.012.
- AskMayoExpert. Hematopoietic stem cell transplant. Mayo Clinic; 2024.
- Stem cell transplants in cancer treatment. National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant/. Accessed March 21, 2024.
- Townsend CM Jr, et al. Regenerative medicine. In: Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 21st ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed March 21, 2024.
- Kumar D, et al. Stem cell based preclinical drug development and toxicity prediction. Current Pharmaceutical Design. 2021; doi:10.2174/1381612826666201019104712.
- NIH guidelines for human stem cell research. National Institutes of Health. https://stemcells.nih.gov/research-policy/guidelines-for-human-stem-cell-research. Accessed March 21, 2024.
- De la Torre P, et al. Current status and future prospects of perinatal stem cells. Genes. 2020; doi:10.3390/genes12010006.
- Yen Ling Wang A. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. International Journal of Molecular Sciences. 2021; doi:10.3390/ijms22041769.
- Alessandrini M, et al. Stem cell therapy for neurological disorders. South African Medical Journal. 2019; doi:10.7196/SAMJ.2019.v109i8b.14009.
- Goldenberg D, et al. Regenerative engineering: Current applications and future perspectives. Frontiers in Surgery. 2021; doi:10.3389/fsurg.2021.731031.
- Brown MA, et al. Update on stem cell technologies in congenital heart disease. Journal of Cardiac Surgery. 2020; doi:10.1111/jocs.14312.
- Li M, et al. Brachyury engineers cardiac repair competent stem cells. Stem Cells Translational Medicine. 2021; doi:10.1002/sctm.20-0193.
- Augustine R, et al. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomedical Pharmacotherapy. 2021; doi:10.1016/j.biopha.2021.111425.
- Cloning fact sheet. National Human Genome Research Institute. https://www.genome.gov/about-genomics/fact-sheets/Cloning-Fact-Sheet. Accessed March 21, 2024.
- Dingli D (expert opinion). Mayo Clinic. Nov. 17, 2023.
Products and Services
- Sign up for Email: Get Your Free Resource – Coping with Cancer
- A Book: Living Medicine
- Give today to find cancer cures for tomorrow
- Acute lymphocytic leukemia
- Acute myelogenous leukemia
- Adjuvant therapy for cancer
- Amyloidosis
- Aplastic anemia
- Atypical cells: Are they cancer?
- Biopsy procedures
- Blood Cancers and Disorders
- Bone marrow transplant
- Cancer blood tests
- Myths about cancer causes
- Infographic: Cancer Clinical Trials Offer Many Benefits
- Cancer diagnosis: 11 tips for coping
- Cancer-related fatigue
- Cancer pain: Relief is possible
- Cancer risk: What the numbers mean
- Cancer surgery
- Cancer survival rate
- Cancer survivors: Care for your body after treatment
- Cancer survivors: Late effects of cancer treatment
- Cancer survivors: Managing your emotions after cancer treatment
- Cancer treatment myths
- Chemotherapy side effects: A cause of heart disease?
- Chronic lymphocytic leukemia
- Chronic myelogenous leukemia
- Curcumin: Can it slow cancer growth?
- What is type 1 diabetes? A Mayo Clinic expert explains
- Type 1 diabetes FAQs
- Cancer-related diarrhea
- DiGeorge syndrome (22q11.2 deletion syndrome)
- Eating during cancer treatment: Tips to make food tastier
- Epidermolysis bullosa
- Gaucher disease
- Heart cancer: Is there such a thing?
- High-dose vitamin C: Can it kill cancer cells?
- Hodgkin lymphoma (Hodgkin disease)
- Hodgkin's vs. non-Hodgkin's lymphoma: What's the difference?
- Low blood counts
- Measles Virus as a Cancer Fighter
- Monoclonal antibody drugs
- Mort Crim and Cancer
- Mouth sores caused by cancer treatment: How to cope
- Multiple myeloma
- Infographic: Multiple Myeloma
- Myelofibrosis
- Neuroblastoma
- No appetite? How to get nutrition during cancer treatment
- Non-Hodgkin's lymphoma
- Scleroderma
- Self-Image During Cancer
- Sickle cell anemia
- Sisters' Bone Marrow Transplant
- Small cell, large cell cancer: What this means
- Stem Cells 101
- Thalassemia
- Tumor vs. cyst: What's the difference?
- Type 1 diabetes
- Stem cell transplant
- How cancer spreads
- PICC line placement
- When cancer returns: How to cope with cancer recurrence
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Stem cells What they are and what they do
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.

Current Stem Cell Research & Therapy
Impact Factor : 2.7
Indexed in: Scopus, SCI Expanded, MEDLINE/PubMed... View all
Volume 19 , Issues 11, 2024
This journal supports open access
Submission for General Articles
Submit to thematic issues.

Thematic Issue Issue: {[{issue.issue_title}]}
{[{issue.about_issue}]}
No Text Found
- Submit Abstracts
- Submit Manuscripts Online
- Thematic Issue Proposal
- Animated Abstract Submission

- About Journal
- Editorial Board
- Journal Insight
- Current Issue
- Volumes /Issues
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
- Announcements
- Forthcoming Thematic Issues
- Guest Editor Guidelines
- Editorial Management
- Ethical Guidelines for New Editors
- Reviewer Guidelines
- Abstract Ahead of Print 2
- Article(s) in Press 53
- Free Online Copy
- Most Cited Articles
- Most Accessed Articles
- Highlighted Article
- Most Popular Articles
- Editor's Choice
- Thematic Issues
- Open Access Articles
- Open Access Funding
- Library Recommendation
- Trial Requests
- Advertise With Us
- Meet the Executive Guest Editor(s)
- Brand Ambassador
- Author's Comment & Reviews
- New Journals 2023
- New Journals 2024
- Alert Subscription

- Health Conditions
- Health Products
What are stem cells, and why are they important?

Researchers have been looking for something that can help the body heal itself. Although studies are ongoing, stem cell research brings this notion of regenerative medicine a step closer. However, many of its ideas and concepts remain controversial. So, what are stem cells, and why are they so important?
Stem cells are cells that can develop into other types of cells. For example, they can become muscle or brain cells. They can also renew themselves by dividing, even after they have been inactive for a long time.
Stem cell research is helping scientists understand how an organism develops from a single cell and how healthy cells could be useful in replacing cells that are not working correctly in people and animals.
Researchers are now studying stem cells to see if they could help treat a variety of conditions that impact different body systems and parts.
This article looks at types of stem cells, their potential uses, and some ethical concerns about their use.

The human body requires many different types of cells to function, but it does not produce every cell type fully formed and ready to use.
Scientists call a stem cell an “undifferentiated” cell because it can become any cell. In contrast, a blood cell, for example, is a “differentiated” cell because it has already formed into a specific kind of cell.
The sections below look at some types of stem cells in more detail.
Embryonic stem cells
Scientists extract embryonic stem cells from unused embryos left over from in vitro fertilization procedures. They do this by taking the cells from the embryos at the blastocyst stage , which is the phase in development before the embryo implants in the uterus.
These cells are undifferentiated cells that divide and replicate. However, they are also able to differentiate into specific types of cells.
Adult stem cells
There are two main types of adult stem cells: those in developed bodily tissues and induced pluripotent stem (iPS) cells.
Developed bodily tissues — such as organs, muscles, skin, and bone — include some stem cells . These cells can typically become differentiated cells based on where they exist. For example, a brain stem cell can only become a brain cell.
On the other hand, scientists manipulate iPS cells to make them behave more like embryonic stem cells for use in regenerative medicine. After collecting the stem cells, scientists usually store them in liquid nitrogen for future use. However, researchers have not yet been able to turn these cells into any kind of bodily cell.
Scientists are researching how to use stem cells to regenerate or treat the human body.
The list of conditions that stem cell therapy could help treat may be endless. Among other things, it could include conditions such as Alzheimer’s disease , heart disease , diabetes , and rheumatoid arthritis . Doctors may also be able to use stem cells to treat injuries in the spinal cord or other parts of the body.
They may do this in several ways, including the following.
Using stem cells in therapy
In some tissues, stem cells play an essential role in regeneration, as they can divide easily to replace dead cells. Scientists believe that knowing how stem cells work can help treat damaged tissue.
For instance, if someone’s heart contains damaged tissue, doctors might be able to stimulate healthy tissue to grow by transplanting laboratory-grown stem cells into the person’s heart. This could cause the heart tissue to renew itself.
One study suggested that people with heart failure showed some improvement 2 years after a single-dose administration of stem cell therapy. However, the effect of stem cell therapy on the heart is still not fully clear, and research is still ongoing.
Another investigation suggested that stem cell therapies could be the basis of personalized diabetes treatment. In mice and laboratory-grown cultures, researchers successfully produced insulin-secreting cells from stem cells derived from the skin of people with type 1 diabetes .
Study author Jeffrey R. Millman — an assistant professor of medicine and biomedical engineering at the Washington University School of Medicine in St. Louis, MO — said , “What we’re envisioning is an outpatient procedure in which some sort of device filled with the cells would be placed just beneath the skin.”
Millman hopes that these stem cell-derived beta cells could be ready for research in humans within 3–5 years.
Stem cells could also have vast potential in developing other new therapies.
Using stem cells in drug development
Another way that scientists could use stem cells is in developing and testing new drugs.
The type of stem cell that scientists commonly use for this purpose is the iPS cell. These are cells that have already undergone differentiation but which scientists have genetically “reprogrammed” using genetic manipulation, sometimes using viruses .
In theory, this allows iPS cells to divide and become any cell. In this way, they could act like undifferentiated stem cells.
For example, scientists want to grow differentiated cells from iPS cells to resemble cancer cells and use them to test anticancer drugs. This could be possible because conditions such as cancer, as well as some congenital disabilities, happen because cells divide abnormally.
However, more research is taking place to determine whether or not scientists really can turn iPS cells into any kind of differentiated cell and how they can use this process to help treat these conditions.
Do current stem cell therapies work?
In recent years, clinics have opened that offer different types of stem cell treatments. One 2016 study counted 570 of these clinics in the United States alone. They appear to offer stem cell-based therapies for conditions ranging from sports injuries to cancer.
However, most stem cell therapies are still theoretical rather than evidence-based. For example, researchers are studying how to use stem cells from amniotic fluid — which experts can save after an amniocentesis test — to treat various conditions.
The Food and Drug Administration (FDA) does allow clinics to inject people with their own stem cells as long as the cells are intended to perform only their normal function.
Aside from that, however, the FDA has only approved the use of blood-forming stem cells known as hematopoietic progenitor cells. Doctors derive these from umbilical cord blood and use them to treat conditions that affect the production of blood. Currently, for example, a doctor can preserve blood from an umbilical cord after a baby’s birth to save for this purpose in the future.
The FDA lists specific approved stem cell products, such as cord blood, and the medical facilities that use them on its website . It also warns people to be wary of undergoing any unproven treatments because very few stem cell treatments have actually reached the earliest phase of a clinical trial.
Ethical issues
Historically, the use of stem cells in medical research has been controversial. This is because when the therapeutic use of stem cells first came to the public’s attention in the late 1990s, scientists were only deriving human stem cells from embryos.
Many people disagree with using human embryonic cells for medical research because extracting them means destroying the embryo. This creates complex issues, as people have different beliefs about what constitutes the start of human life.
For some people, life starts when a baby is born, while for others, it starts when an embryo develops into a fetus. Meanwhile, other people believe that human life begins at conception, so an embryo has the same moral status and rights as a human child.
Former U.S. president George W. Bush had strong antiabortion views. He believed that an embryo should be considered a life and not be used for scientific experiments. Bush banned government funding for human stem cell research in 2001, but former U.S. president Barack Obama then revoked this order. Former U.S. president Donald Trump and current U.S. president Joe Biden have also gone back and forth with legislation on this.
However, by 2006, researchers had already started using iPS cells. Scientists do not derive these stem cells from embryonic stem cells. As a result, this technique does not have the same ethical concerns. With this and other recent advances in stem cell technology, attitudes toward stem cell research are slowly beginning to change.
However, other concerns related to using iPS cells still exist. This includes ensuring that donors of biological material give proper consent to have iPS cells extracted and carefully designing any clinical studies.
Researchers also have some concerns that manipulating these cells as part of stem cell therapy could lead to the growth of cancerous tumors.
Although scientists need to do much more research before stem cell therapies can become part of regular medical practice, the science around stem cells is developing all the time.
Scientists still conduct embryonic stem cell research, but research into iPS cells could help reduce some of the ethical concerns around regenerative medicine. This could lead to much more personalized treatment for many conditions and the ability to regenerate parts of the human body.
Learn more about stem cells, where they come from, and their possible uses here.
Last medically reviewed on July 7, 2021
- Medical Innovation
- Stem Cell Research
- Biology / Biochemistry
How we reviewed this article:
- Approved cellular and gene therapy products. (2021). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
- Bartunek, J., et al. (2020). Cardiopoietic stem cell therapy in ischaemic heart failure: Long-term clinical outcomes. https://onlinelibrary.wiley.com/doi/full/10.1002/ehf2.13031
- Brown, K. S., et al. (2019). The future state of newborn stem cell banking. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352006/
- Chatterjee, I., et al. (2016). Induced pluripotent stem (iPS) cell culture methods and induction of differentiation into endothelial cells. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539286/
- Dryden, J. (n.d.). Stem cells from diabetic patients coaxed to become insulin-secreting cells [News release]. https://medicine.wustl.edu/news/stem-cells-diabetic-patients-coaxed-develop-insulin-secreting-cells/
- Fact sheet on presidential executive order: Removing barriers to responsible scientific research involving human stem cells. (n.d.). https://obamawhitehouse.archives.gov/realitycheck/the-press-office/fact-sheet-presidential-executive-order
- FDA warns about stem cell therapies. (2019). https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies
- Millman, J. R., et al. (2016). Generation of stem cell-derived β-cells from patients with type 1 diabetes [Abstract]. https://pubmed.ncbi.nlm.nih.gov/27163171/
- Papapetrou, E. P. (2016). Patient-derived induced pluripotent stem cells in cancer research and precision oncology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5233709/
- Srivastava, M., et al. (2018). Amniotic fluid stem cells: A new era in regenerative medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783899/
- Stem cell basics. (n.d.). https://stemcells.nih.gov/info/basics/stc-basics/
- The White House. (2001). President discusses stem cell research [Press release]. https://georgewbush-whitehouse.archives.gov/news/releases/2001/08/20010809-2.html
- Turner, L., et al. (2016). Selling stem cells in the USA: Assessing the direct-to-consumer industry. https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(16)30157-6?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1934590916301576%3Fshowall%3Dtrue
- Update on changes to NIH requirements regarding proposed human fetal tissue research. (2021). https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-111.html?utm_source=dlvr.it&utm_medium=twitter
- What are stem cells? (n.d.). https://www.stanfordchildrens.org/en/topic/default?id=what-are-stem-cells-160-38
- Where do we get stem cells? (n.d.). http://stemcell.childrenshospital.org/about-stem-cells/adult-somatic-stem-cells-101/where-do-we-get-adult-stem-cells/
- Zhang, M., et al. (2016). Gene delivery and expression systems in induced pluripotent stem cells. https://link.springer.com/chapter/10.1007/978-981-10-1560-1_11
- Zheng, Y. L. (2016). Some ethical concerns about human induced pluripotent stem cells [Abstract]. https://pubmed.ncbi.nlm.nih.gov/26276162/
Share this article
Latest news
- How going to bed past midnight could affect your mental health
- Immunotherapy drug may help 'cure' advanced colorectal cancer without surgery
- Short bouts of exercise may boost efficacy of some types of cancer treatment
- Women with excess weight at ages 14 or 31 may have higher stroke risk
- Can eating more fruits and vegetables help with sleep?
Related Coverage
Stem cell transplants currently treat some cancers and blood and immune disorders. Researchers are also looking into other uses. Learn more here.
The use of stem cells, materials, or molecules to regenerate tissues and organs promises to revolutionize medicine. How long until this becomes…
Researchers have designed a type of stem cell that should be able to withhold transplantation into any recipient without facing rejection.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
How Does Stem Cell Therapy Work?
What are stem cells.
- How the Therapy Works
- What It Treats
Stem cell therapy is a type of regenerative medicine used to treat and study disease. It is used in cancer treatment and to reduce the risk of infection . Researchers are looking for other ways to use stem cells in medical therapies.
This article will cover stem cells, which conditions they treat, and their safety. It will also discuss stem cell therapy's side effects and risks.
Westend61 / Getty Images
Stem cells are unspecialized cells in the body. They can develop into any cell and in some cases can renew themselves an unlimited amount of times.
Stem cells are found in embryos and adult cells . There are two types of stem cells pluripotent and somatic.
Pluripotent stem cells are embryonic stem cells or induced pluripotent stem cells. These cells can become any of the cells in the body. Somatic stem cells, also known as adult stem cells can form tissue or an entire organ.
Stem cell therapy is the use of stem cells as a treatment for a condition.
Stem cells are given to people to replace cells that have been destroyed or have died. In the case of people with cancer , they may be used to help the body regain the ability to produce stem cells after treatment.
In people with multiple myeloma and certain types of leukemia, stem cell therapy is used to destroy cancer cells. This type of therapy is called graft-versus-cancer, the donor's white blood cells (WBCs) are used to destroy the cancerous tumor.
Stem Cell Treatment Cost
Stem cell treatments are very expensive. It is a new therapy and in some cases is still considered experimental. It's important to know how much insurance will cover before undergoing stem cell treatment. Some insurance companies have transplant case managers who can help navigate the process of getting insurance coverage and finding out what expenses may be like.
Learn more: Medicare and Stem Cell Therapy: What's Covered?
What Can Stem Cell Therapy Treat?
Stem cell therapy is a newer treatment that is still being researched. As a result, the Food and Drug Administration (FDA) has only approved it for certain cancers and conditions that affect the blood and immune system.
Conditions stem cell therapy is FDA-approved to treat are:
- Neuroblastoma
- Multiple myeloma
It is also used to reduce the risk of infection after stem cell transplantation in people with blood cancers.
Researchers are studying how stem cells can treat many other conditions than those listed above. There are stem cell clinical trials looking into using the therapy for neurodegenerative diseases like Parkinson's disease , Alzheimer's disease , multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS).
Companies that claim to use stem cells to treat other conditions are doing so illegally. Products that claim to treat arthritis, joint pain, or fight the signs of aging are not FDA-approved.
Types of Stem Cell Therapy
During stem cell therapy, stem cells are given through an intravenous (IV) line in the vein. The three places where blood-forming stem cells can come from are bone marrow, the umbilical cord, and blood. The transplants can be:
- Autologous : The stem cells are taken from the person who will be receiving the therapy.
- Allogeneic : The stem cells are donated by another person.
- Syngeneic: The stem cells come from an identical twin, if the person has one.
Is Stem Cell Therapy Safe?
While stem cell therapy has many great benefits there are risks to the therapy.
One of the greatest risks is graft-versus-host disease (GVHD). It occurs in one-half to one-third of allogeneic transplant recipients. This is when the body does not recognize the donor's WBCs and attacks them. This can cause problems throughout the body. Treatment involves medications to suppress the immune system to stop the body from attacking the donor cells.
Other potential risks to stem cell therapy include:
- Hepatic veno-occlusive disease
- Cancer relapse
- Post-transplant lymphoproliferative disorder (PTLD)
What Is the Future of Stem Cell Therapy?
The future of stem cell therapy is bright. Researchers are constantly looking to find out how stem cells can treat certain conditions and find new ways to use stem cells to treat and cure many diseases.
Stem cell therapy has been researched for over twenty years to find treatments for conditions like macular degeneration , glaucoma , stroke , and Alzheimer's disease .
Stem cell therapy is a newer medical treatment that uses stem cells to treat conditions like cancer. Some clinics sell stem cell therapy without FDA approval and this places the patient at a higher risk for side effects and poor outcomes. Talk to a trusted healthcare provider about where to find a reliable stem cell therapy provider.
American Cancer Society. How stem cell and bone marrow transplants are used to treat cancer .
National Institutes of Health. Stem cell basics .
Centers for Disease Control and Prevention. Stem cell and exosome products .
National Cancer Institute. Stem cell transplants in cancer treatment .
U.S. Food & Drug Administration. FDA approves cell therapy for patients with blood cancers to reduce risk of infection following stem cell transplantation .
Aly RM. Current state of stem cell-based therapies: an overview . Stem Cell Investig . 2020;7:8. doi:10.21037/sci-2020-001
American Cancer Society. Stem cell or bone marrow transplant side effects .
National Institutes of Health. Putting stem cell-based therapies in context .
By Patty Weasler, RN, BSN Weasler is a Wisconsin-based registered nurse with over a decade of experience in pediatric critical care.
- Utility Menu
GA4 tracking code
Our mission is to find cures for human diseases., harvard stem cell institute (hsci) scientists are working together across harvard schools, centers, and teaching hospitals, harnessing the power of stem cells to change medicine for the better..
Read more about HSCI
HSCI bridges the gaps in traditional research funding to encourage bold thinking and launch scientific careers.
Through our disease programs , we channel world-class resources, both intellectual and technological, toward some of the most prevalent, devastating diseases for which stem cell research holds promise.
In addition, our seed grants and junior faculty programs provide funding for innovative, early-stage projects in stem cell research. This allows up-and-coming scientists to pursue "high risk/high reward" avenues of research that might be difficult to fund from other sources.
- Blood Diseases
- Cardiovascular Diseases
- Kidney Disease
- Musculoskeletal Disease
- Nervous System Diseases
- Skin Diseases
- Fibrosis & Aging
- Stem Cells as Tools
All Research Areas

How a small fish could lead to better strategies to repair tendon tears
Hsci principal faculty jenna galloway, phd, is working to better understand the healing process after tendon tears with the hope of identifying new therapies that could help..

Jason Buenrostro lands MacArthur 'Genius Grant '
Buenrostro has been named a 2023 macarthur fellow for developing powerful new technologies that provide detailed views — right down to the single cell — of which genes get turned on and off in various contexts., 2024 call for applications: barry family hsci innovation award for early investigators, hsci community events, hsci faculty.

HSCI has been breaking down barriers to collaboration in stem cell science since 2004. We provide fertile ground for more than 350 research faculty and their labs, across the university’s schools, centers, teaching hospitals, and partner companies, to share knowledge and pursue bold new ideas.
With Harvard as a wellspring of discovery and a strong network that embraces new ways of working, we are better equipped than ever to change human health in ways that will benefit all of society.
Executive Committee
Principal faculty, affiliate faculty, from lab to clinic, company startups.
A key part of our mission is to move research out of the lab and into the clinic. Since our founding, we have been forging a clear path to translating discoveries into products that benefit patients. Now, we have the flexibility to organize people across institutions and sectors to tackle specific biological problems so we can make a lasting difference in people's lives.
HSCI faculty startups

Stem-cell research news delivered straight to your inbox.
Sign up for the HSCI newsletter
Chinese scientists cure diabetes using stem cells in world first

- The 59-year-old patient of 25 years received a transplant of pancreatic cells derived from his own stem cells in 2021. He is now insulin independent.
- This marks the world’s first successful use of stem cell-derived islet transplantation to cure diabetes .
- The achievement, published on Cell Discovery on April 30, comes after over a decade of research at Shanghai Changzheng Hospital.
- Diabetes is a major health threat, affecting 422 million people worldwide. While there is still no known cure for diabetes, methods of management include insulin injections and other medications.
- The patient reportedly suffered a significant decline in pancreatic islet function, which regulates blood sugar, after undergoing a kidney transplant in 2017. Since then, he had been dependent on daily multiple insulin injections.
- In July 2021, a team at the hospital led by researcher Yin Hao used the patient’s own blood cells to create stem cells, which were then converted into pancreatic islet cells.
- The transplant successfully eliminated the patient’s need for external insulin within 11 weeks. Oral medication was also gradually reduced and ultimately discontinued a year later.
- Follow-up exams showed restored pancreatic function and normal kidney function, suggesting that the patient has been cured .
- In 2023, the FDA approved a similar cell therapy treatment by a Chicago-based startup for type 1 diabetes.
- The Chinese researchers say more research is needed to confirm the long-term efficacy and broaden applicability of this treatment.

Asian Americans Have Higher Risk for Undiagnosed Heart Diseases Than White Americans

Incredible Dog in Singapore Has a Special Power That Helps Diabetic Patients

16-Year-Old Nearly Dies After Drinking Boba Tea EVERY DAY
- Happening Now
- San Francisco
- Los Angeles
- Orange County
- About NextShark
- AAPI Resources
Subscribe to NextShark's Newsletter
- UB Directory
- News Center >
- News Releases >
Most stem cells die after being injected into the brain. This new technique could change that

Injecting shear-thinning hydrogels (STH) into the brain protects the stem cells and results in more successful therapy. Courtesy of Stelios Andreadis and Fraser Sim
Research team uses shear-thinning hydrogels instead of saline solution; could lead to new therapies for MS, other neurological diseases
By Laurie Kaiser
Release Date: June 5, 2024

Stelios Andreadis

BUFFALO, N.Y. — When the myelin sheath that surrounds nerve fibers in the brain and spinal cord becomes damaged, a number of debilitating conditions can result that limit mobility, inhibit independence and reduce life expectancy. Multiple sclerosis (MS) is the most common demyelinating disease, affecting more than 2.5 million individuals globally every year.
Stem cell therapy to treat such diseases often has disappointing results because the transplanted cells die off before they can take effect. In fact, more than 95% of neural progenitor cells (NPCs) transplanted into individuals with a spinal cord injury die following injection. This is partly because the process of injecting the cells can damage them.
Two University at Buffalo researchers are working on a possible solution: injecting shear-thinning hydrogels (STH) into the brain, which protect the cells and result in more successful therapy.
Stelios Andreadis , PhD, SUNY Distinguished Professor in the Department of Chemical and Biological Engineering in the School of Engineering and Applied Sciences, and Fraser Sim , PhD, professor in the Department of Pharmacology and Toxicology in the Jacobs School of Medicine and Biomedical Sciences and director of UB’s Neuroscience Program were recently awarded a $2.9 million, five-year grant from the National Institute of Neurological Disorders and Stroke to further investigate this technology.
“STHs have emerged as promising candidates for the injection of Schwann cells and oligodendrocytes, the cells that form the myelin sheath in the brain and spinal cord,” said
Andreadis, who also directs UB’s Cell, Gene and Tissue Engineering (CGTE) Center, of which Sim is a member. “The work we plan to undertake has significant implications for regenerative medicine, as it has the potential to develop novel strategies to enhance stem cell delivery for treatment of devastating neurological diseases that remain intractable to current treatments.”
How shear-thinning hydrogels work
The hydrogels are called shear-thinning because once you put in them in a syringe and apply pressure, they turn into a liquid form, Andreadis explained.
“They change their viscosity in response to shear stress, and they can turn back into gel form when the force is removed, after the injection,” he said. “The fast transition from solid-like to fluid-like behavior, with increasing shear rate, is essential for successful injection and cell protection.”
The STHs are also designed to mimic the mechanical properties of the brain tissue such as stiffness. And the treatments are minimally invasive.
“We don’t open up the brain surgically,” Andreadis said, “but rather are using syringes to perform in what is called stereotactic surgery.”
Up until now, scientists have essentially put the stem cells into a simple saline solution before implanting them, Sim said.
“They just accepted the fact that a lot of cells will die when you transplant them,” said Sim, whose lab investigates the molecular control of cell fate and homeostasis of resident stem and progenitor cells in the human brain.
“With the hydrogel, we can introduce different factors that will allow the cells to overcome the inhibitory environment that’s present in MS lesions,” Sim said. “We think this will improve the outcome of cell therapy over the vanilla approach using cells in a saline solution.”

The researchers found that implanting the hydrogels into the brains of mice significantly improved the survival of the transplanted cells and enhanced nerve repair 12 weeks post-implantation. Courtesy of Stelios Andreadis and Fraser Sim
Testing on animal models that do not produce myelin
The two researchers began exploring STH technology a couple of years ago by transplanting human cells into the brains of a type of mouse that does not naturally produce myelin.
“The mouse’s condition models congenital hypomyelinating diseases in humans such Pelizaeus-Merzbacher disease, a rare and progressive degenerative central nervous system disorder,” Andreadis said. “We found that implanting the hydrogels significantly improved the survival of the transplanted cells and enhanced nerve repair in the brain 12 weeks post-implantation.”
The next step is to conduct testing on larger animal models with a brain size closer to that of humans. They are seeking answers to questions such as: How many cells do you need? Are the cells going in the parts of the brain where we want them to go? Are they migrating places that they’re not supposed to migrate?
“These are some of the issues we’ll be investigating in the next few years with support from the recent NIH research grant,” Andreadis said.
“This is a great opportunity to marry biomaterials science and engineering with neuroscience to develop a therapeutic strategy that can, hopefully, be brought to the clinic to treat devastating diseases and conditions such as MS,” Andreadis explained. “While there is currently no cure, we would like to develop a successful therapy that can limit the disease’s development and improve quality of life for MS patients and others who are suffering from neurological disorders.”
Sim said he has been grateful for the opportunity to combine his expertise with that of Andreadis.
“This project is a wonderful example of collaborative science,” he said. “Neither of us could do this work alone.”
The study, which will be published online and in print in an upcoming edition of Science Advances, was led by Ashis Kumar Podder, a graduate student in the Department of Chemical and Biological Engineering lab, and Mohamed Alaa Mohamed, PhD, a biomaterial chemist and postdoctoral fellow in the Department of Chemical and Biological Engineering. Contributors include Richard A. Seidman, PhD, a recent graduate of UB’s neuroscience program and current postdoctoral associate in the UB Department of Pharmacology and Toxicology; Georgios Tseropoulos, PhD, a recent graduate of UB’s chemical and biological engineering program and now a postdoctoral fellow at the University of Colorado; Jessie Polanco, who recently earned his PhD from UB’s neuroscience program, and Pedro Lei, PhD, assistant professor of research in the UB Department of Chemical and Biological Engineering.
Media Contact Information
Laurie Kaiser News Content Director Dental Medicine, Pharmacy Tel: 716-645-4655 [email protected]
Read the latest in your favorite channels.

Take UB With You. Wherever.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.39(12); 2014 Dec
Stem Cell Therapy: a Look at Current Research, Regulations, and Remaining Hurdles
Stem cell therapies offer great promise for a wide range of diseases and conditions. However, stem cell research—particularly human embryonic stem cell research—has also been a source of ongoing ethical, religious, and political controversy.

In September 2014, the Sanford Stem Cell Clinical Center at the University of California, San Diego (UCSD) Health System announced the launch of a groundbreaking clinical trial to assess the safety of neural stem cell–based therapy in patients with chronic spinal cord injury. Researchers hope that the transplanted stem cells will develop into new neurons that replace severed or lost nerve connections and restore at least some motor and sensory function. 1
Two additional clinical trials at UCSD are testing stem cell–derived therapy for type-1 diabetes and chronic lymphocytic leukemia, the most common form of blood cancer. 1
These three studies are significant in that they are among the first efforts in stem cell research to make the leap from laboratory to human clinical trials. While the number of patients involved in each study is small, researchers are optimistic that as these trials progress and additional trials are launched, a greater number of patients will be enrolled. UCSD reports that trials for heart failure, amyotrophic lateral sclerosis, and blindness are in planning stages. 1
The study of stem cells offers great promise for better understanding basic mechanisms of human development, as well as the hope of harnessing these cells to treat a wide range of diseases and conditions. 2 However, stem cell research— particularly human embryonic stem cell (hESC) research, which involves the destruction of days-old embryos—has also been a source of ongoing ethical, religious, and political controversy. 2
The Politics of Progress
In 1973, the Department of Health, Education, and Welfare (now the Department of Health and Human Services) placed a moratorium on federally funded research using live human embryos. 3 , 4 In 1974, Congress adopted a similar moratorium, explicitly including in the ban embryos created through in vitro fertilization (IVF). In 1992, President George H.W. Bush vetoed legislation to lift the ban, and in 2001, President George W. Bush issued an executive order banning federal funding on stem cells created after that time. 3 , 4 Some states, however, have permitted their limited use. New Jersey, for example, allows the harvesting of stem cells from cloned human embryos, whereas several other states prohibit the creation or destruction of any human embryos for medical research. 3 , 4
In 2009, shortly after taking office, President Barack Obama lifted the eight-year-old ban on federally funded stem cell research, allowing scientists to begin using existing stem cell lines produced from embryos left over after IVF procedures. 5 (A stem cell line is a group of identical stem cells that can be grown and multiplied indefinitely.)
The National Institutes of Health (NIH) Human Embryonic Stem Cell Registry 6 lists the hESCs eligible for use in NIH-funded research. At this writing, 283 eligible lines met the NIH’s strict ethical guidelines for human stem cell research pertaining to the embryo donation process. 7 For instance, to get a human embryonic stem cell line approved, grant applicants must show that the embryos were “donated by individuals who sought reproductive treatment and who gave voluntary written consent for the human embryos to be used for research purposes.” 8 The ESCs used in research are not derived from eggs fertilized in a woman’s body. 9
Because of the separate legislative ban, it is still not possible for researchers to create new hESC lines from viable embryos using federal funds. Federal money may, however, be used to research lines that were derived using private or state sources of funding. 5
While funding restrictions and political debates may have slowed the course of stem cell research in the United States, 10 the field continues to evolve. This is evidenced by the large number of studies published each year in scientific journals on a wide range of potential uses across a variety of therapeutic areas. 11 – 13
The Food and Drug Administration (FDA) has approved numerous stem cell–based treatments for clinical trials. A 2013 report from the Pharmaceutical Research and Manufacturers of America lists 69 cell therapies as having clinical trials under review with the FDA, including 15 in phase 3 trials. The therapeutic categories represented in these trials include cardiovascular disease, skin diseases, cancer and related conditions, digestive disorders, transplantation, genetic disorders, musculoskeletal disorders, and eye conditions, among others. 14
Still, the earliest stem cell therapies are likely years away. To date, the only stem cell–based treatment approved by the FDA for use in this country is for bone marrow transplantation. 15 As of 2010 (the latest year for which data are available), more than 17,000 blood cancer patients had had successful stem cell transplants. 16
A Brief Stem Cell Timeline
Research on stem cells began in the late 19th century in Europe. German biologist Ernst Haeckel coined the term stem cell to describe the fertilized egg that becomes an organism. 17

In the U.S., the study of adult stem cells took off in the 1950s when Leroy C. Stevens, a cancer researcher based in Bar Harbor, Maine, found large tumors in the scrotums of mice that contained mixtures of differentiated and undifferentiated cells, including hair, bone, intestinal, and blood tissue. Stevens and his team concluded that the cells were pluripotent, meaning they could differentiate into any cell found in a fully grown animal. Stem cell scientists are using that carefully documented research today. 17
In 1968, Robert A. Good, MD, PhD, at the University of Minnesota, performed the first successful bone marrow transplant on a child suffering from an immune deficiency. Scientists subsequently discovered how to derive ESCs from mouse embryos and in 1998 developed a method to take stem cells from a human embryo and grow them in a laboratory. 17
Why Stem Cells?
Many degenerative and currently untreatable diseases in humans arise from the loss or malfunction of specific cell types in the body. 9 While donated organs and tissues are often used to replace damaged or dysfunctional ones, the supply of donors does not meet the clinical demand. 18 Stem cells seemingly provide a renewable source of replacement cells and tissues for transplantation and the potential to treat a myriad of conditions.
Stem cells have two important and unique characteristics: First, they are unspecialized and capable of renewing themselves through cell division. When a stem cell divides, each new cell has the potential either to remain a stem cell or to differentiate into other kinds of cells that form the body’s tissues and organs. Stem cells can theoretically divide without limit to replenish other cells that have been damaged. 9
Second, under certain controlled conditions, stem cells can be induced to become tissue- or organ-specific cells with special functions. They can then be used to treat diseases affecting those specific organs and tissues. While bone marrow and gut stem cells divide continuously throughout life, stem cells in the pancreas and heart divide only under appropriate conditions. 9
Embryonic Versus Adult Stem Cells
There are two main types of stem cells: 1) embryonic stem cells (ESCs), found in the embryo at very early stages of development; and 2) somatic or adult stem cells (ASCs), found in specific tissues throughout the body after development. 9
The advantage of embryonic stem cells is that they are pluripotent—they can develop into any of the more than 200 cell types found in the body, providing the potential for a broad range of therapeutic applications. Adult stem cells, on the other hand, are thought to be limited to differentiating into different cell types of their tissue of origin. 9 Blood cells, for instance, which come from adult stem cells in the bone marrow, can specialize into red blood cells, but they will not become other cells, such as neurons or liver cells.
A significant advantage of adult stem cells is that they offer the potential for autologous stem cell donation. In autologous transplants, recipients receive their own stem cells, reducing the risk of immune rejection and complications. Additionally, ASCs are relatively free of the ethical issues associated with embryonic stem cells and have become widely used in research.
Induced Pluripotent Stem Cells
Representing a relatively new area of research, induced pluripotent stem cells (iPSCs) are adult stem cells that have been genetically reprogrammed back to an embryonic stem cell–like state. The reprogrammed cells function similarly to ESCs, with the ability to differentiate into any cell of the body and to create an unlimited source of cells. So iPSCs have significant implications for disease research and drug development.
Pioneered by Japanese researchers in 2006, iPSC technology involves forcing an adult cell, such as a skin, liver, or stomach cell, to express proteins that are essential to the embryonic stem cell identity. The iPSC technology not only bypasses the need for human embryos, avoiding ethical objections, but also allows for the generation of pluripotent cells that are genetically identical to the patient’s. Like adult cells, these unlimited supplies of autologous cells could be used to generate transplants without the risk of immune rejection. 9
In 2013, researchers at the Spanish National Cancer Research Centre in Madrid successfully reprogrammed adult cells in mice, creating stem cells that can grow into any tissue in the body. Prior to this study, iPSCs had never been grown outside Petri dishes in laboratories. 19 And, in July 2013, Japan’s health minister approved the first use of iPSCs in human trials. The Riken Center for Developmental Biology will use the cells to attempt to treat age-related macular degeneration, a common cause of blindness in older people. The small-scale pilot study would test the safety of iPSCs transplanted into patients’ eyes. 20
The Promise of iPSCs
According to David Owens, PhD, Program Director of the Neuroscience Center at NIH’s National Institute of Neurological Disorders and Stroke (NINDS), one of the fundamental hurdles to using stem cells to treat disease is that scientists do not yet fully understand the diseases themselves, that is, the genetic and molecular signals that direct the abnormal cell division and differentiation that cause a particular condition. “You want that before you propose a therapeutic,” he says, “because you want a firm, rational basis for what you’re trying to do, what you’re trying to change.”
Although most of the media attention around stem cells has focused on regenerative medicine and cell therapy, researchers are finding that iPSCs, in particular, hold significant promise as tools for disease modeling. 21 , 22 A major barrier to research is often inaccessibility of diseased tissue for study. 23 Because iPSCs can be derived directly from patients with a given disease, they display all of the molecular characteristics associated with the disease, thereby serving as useful models for the study of pathological mechanisms.
“The biggest payoff early on will be using these cells as a tool to understand the disease better,” says Dr. Owens. For instance, he explains that creating dopamine neurons from iPSC lines could help scientists more closely study the mechanisms behind Parkinson’s disease. “If we get a better handle on the disorders themselves, then that will also help us generate new therapeutic targets.” Recent studies show the use of these patient-specific cells to model other neurodegenerative disorders, including Alzheimer’s and Huntington’s diseases. 24 – 26
In addition to using iPSC technology, it is also possible to derive patient-specific stem cell lines using an approach called somatic cell nuclear transfer (SCNT). This process involves adding the nuclei of adult skin cells to unfertilized donor oocytes. As reported in spring 2014, a team of scientists from the New York Stem Cell Foundation Research Institute and Columbia University Medical Center used SCNT to create the first disease-specific embryonic stem cell line from a patient with type-1 diabetes. The insulin-producing cells have two sets of chromosomes (the normal number in humans) and could potentially be used to develop personalized cell therapies. 27
Many Hurdles Ahead
The development of iPSCs and related technologies may help address the ethical concerns and open up new possibilities for studying and treating disease, but there are still barriers to overcome. One major obstacle is the tendency of iPSCs to form tumors in vivo . Using viruses to genomically alter the cells can trigger the expression of cancer-causing genes, or oncogenes. 28
Much more research is needed to understand the full nature and potential of stem cells as future medical therapies. It is not known, for example, how many kinds of adult stem cells exist or how they evolve and are maintained. 9
Some of the challenges are technical, Dr. Owens explains. For instance, generating large enough numbers of a cell type to provide the amounts needed for treatment is difficult. Some adult stem cells have a very limited ability to divide, making it difficult to multiply them in large numbers. Embryonic stem cells grow more quickly and easily in the laboratory. This is an important distinction because stem cell replacement therapies require large numbers of cells. 29
Also, says Dr. Owens, stem cell transplants present immunological hurdles: “If you do introduce cells into a tissue, will they be rejected if they’re not autologous cells? Or, you might have immunosuppression with the individual who received the cells, and then there are additional complications involved with that. That’s still not entirely clear.”
Such safety issues need to be addressed before any new stem cell–based therapy can advance to clinical trials with real patients. According to Dr. Owens, the preclinical testing stage typically takes about five years. This would include assessment of toxicity, tumorigenicity, and immunogenicity of the cells in treating animal models for disease. 30
“Those are things we have to continually learn about and try to address. It will take time to understand them better,” Dr. Owens says. Asked about the importance of collaboration in overcoming the scientific, regulatory, and financial challenges that lie ahead, he says, “It’s unlikely that one entity could do it all alone. Collaboration is essential.”
Research and Clinical Trials
Ultimately, stem cells have huge therapeutic potential, and numerous studies are in progress at academic institutions and biotechnology companies around the country. Studies at the NIH span multiple disciplines, notes Dr. Owens, who oversees funding for stem cell research at NINDS. ( Figure 1 shows the recent history of NIH funding for stem cell research.) He describes one area of considerable interest as the promotion of regeneration in the brain based on endogenous stem cells. Until recently, it was believed that adult brain cells could not be replaced. However, the discovery of neurogenesis in bird brains in the 1980s led to startling evidence of neural stem cells in the human brain, raising new possibilities for treating neurodegenerative disorders and spinal cord injuries. 31
“It’s a fascinating idea,” says Dr. Owens. “It’s unclear still what the functions of those cells are. They could probably play different roles in different species, but just the fundamental properties themselves are very interesting.” He cites a number of NINDS-funded studies looking at those basic properties.
In another NIH-funded study, Advanced Cell Technology (ACT), a Massachusetts-based biotechnology company, is testing the safety of hESC-derived retinal cells to treat patients with an eye disease called Stargardt’s macular dystrophy. A second ACT trial is testing the safety of hESC-derived retinal cells to treat age-related macular degeneration patients. 32 , 33
In April 2014, scientists at the University of Washington reported that they had successfully regenerated damaged heart muscles in monkeys using heart cells created from hESCs. The research, published in the journal Nature , was the first to show that hESCs can fully integrate into normal heart tissue. 34
The study did not answer every question and had its complications—it failed to show whether the transplanted cells improved the function of the monkeys’ hearts, and some of the monkeys developed arrhythmias. 34 , 35 Still, the researchers are optimistic that it will pave the way for a human trial before the end of the decade and lead to significant advances in treating heart disease. 29
In May 2014, Asterias Biotherapeutics, a California-based biotechnology company focused on regenerative medicine, announced the results of a phase 1 clinical trial assessing the safety of its product AST-OPC1 in patients with spinal cord injuries. 36 The study represents the first-in-human trial of a cell therapy derived from hESCs. Results show that all five subjects have had no serious adverse events associated with the administration of the cells, with the AST-OPC1 itself, or with the immunosuppressive regimen. A phase 1/2a dose-escalation study of AST-OPC1 in patients with spinal cord injuries is awaiting approval from the FDA. 37
The FDA itself has a team of scientists studying the potential of mesenchymal stem cells (MSCs), adult stem cells traditionally found in the bone marrow. Multipotent stem cells, MSCs differentiate to form cartilage, bone, and fat and could be used to repair, replace, restore, or regenerate cells, including those needed for heart and bone repair. 38
Publicly available information about federally and privately funded clinical research studies involving stem cells can be found at http://clinicaltrials.gov . However, the FDA cautions that the information provided on that site is supplied by the product sponsors and is not reviewed or confirmed by the agency.
“The biggest payoff early on will be using these cells as a tool to understand the disease better. If we get a better handle on the disorders themselves, then that will also help us generate new therapeutic targets.” —David Owens, PhD, Program Director, Neuroscience Center, National Institute of Neurological Disorders and Stroke
Global Research Efforts
Stem cell research policy varies significantly throughout the world as countries grapple with the scientific and social implications. In the European Union, for instance, stem cell research using the human embryo is permitted in Belgium, Britain, Denmark, Finland, Greece, the Netherlands, and Sweden; however, it is illegal in Austria, Germany, Ireland, Italy, and Portugal. 39
In those countries where cell lines are accessible, research continues to create an array of scientific advances and widen the scope of stem cell application in human diseases, disorders, and injuries. For example, in February 2014, Cellular Biomedicine Group, a China-based company, released the six-month follow-up data analysis of its phase 1/2a clinical trial for ReJoin, a human adipose-derived mesenchymal precursor cell (haMPC) therapy for knee osteoarthritis. The study, which tested the safety and efficacy of intra-articular injections of autologous haMPCs to reduce inflammation and repair damaged joint cartilage, showed knee pain was significantly reduced and knee mobility was improved. 40 And the journal Stem Cell Research & Therapy reported that researchers at the University of Adelaide in Australia recently completed a project showing stem cells taken from teeth could form “complex networks of brain-like cells.” Although the cells did not grow into full neurons, the researchers say that it will happen given time and the right conditions. 41
The Regulation of Stem Cells
In February 2014, the U.S. Court of Appeals for the District of Columbia Circuit upheld a 2012 ruling that a patient’s stem cells for therapeutic use fall under the aegis of the FDA. 42 The appeals case involved the company Regenerative Sciences, which was using patients’ MSCs in its Regenexx procedure to treat orthopedic problems. 43
The FDA’s Center for Biologics Evaluation and Research (CBER) regulates human cells, tissues, and cellular and tissue-based products (HCT/P) intended for implantation, transplantation, infusion, or transfer into a human recipient, including hematopoietic stem cells. Under the authority of Section 361 of the Public Health Service Act, the FDA has established regulations for all HCT/Ps to prevent the transmission of communicable diseases. 44
The Regenexx case highlights an ongoing debate about whether autologous MSCs are biological drugs subject to FDA approval or simply human cellular and tissue products. Some medical centers collect, concentrate, and reinject MSCs into a patient to treat osteoarthritis but do not add other agents to the injection. The FDA contends that any process that includes culturing, expansion, and added growth factors or antibiotics requires regulation because the process constitutes significant manipulation. Regenerexx has countered that the process does not involve the development of a new drug, which could be given to a number of patients, but rather a patient’s own MSCs, which affects just that one patient.
Ensuring the safety and efficacy of stem cell–based products is a major challenge, says the FDA. Cells manufactured in large quantities outside their natural environment in the human body can potentially become ineffective or dangerous and produce significant adverse effects such as tumors, severe immune reactions, or growth of unwanted tissue. Even stem cells isolated from a person’s own tissue can potentially present these risks when put into an area of the body where they could not perform the same biological function that they were originally performing. Stem cells are immensely complex, the FDA cautions—far more so than many other FDA-regulated products—and they bring with them unique considerations for meeting regulatory standards.
To date, no U.S. companies have received FDA approval for any autologous MSC therapy, although a study is ongoing to assess the feasibility and safety of autologous MSCs for osteoarthritis. 45 One of the major risks with MSCs is that they could potentially lead to cancer or differentiation into bone or cartilage. 46
What’s Next
The numerous stem cell studies in progress across the globe are only a first step on the long road toward eventual therapies for degenerative and life-ending diseases. Because of their unlimited ability to self-renew and to differentiate, embryonic stem cells remain, theoretically, a potential source for regenerative medicine and tissue replacement after injury or disease. However, the difficulty of producing large quantities of stem cells and their tendency to form tumors when transplanted are just a few of the formidable hurdles that researchers still face. In the meantime, the shorter-term payoff of using these cells as a tool to better understand diseases has significant implications.
Social and ethical issues around the use of embryonic stem cells must also be addressed. Many nations, including the U.S., have government-imposed restrictions on either embryonic stem cell research or the production of new embryonic stem cell lines. Induced pluripotent stem cells offer new opportunities for development of cell-based therapies while also providing a way around the ethical dilemma of using embryos, but just how good an alternative they are to embryonic cells remains to be seen.
It is clear that many challenges must be overcome before stem cells can be safely, effectively, and routinely used in the clinical setting. However, their potential benefits are numerous and hold tremendous promise for an array of new therapies and treatments.
Acknowledgments
The authors wish to thank the FDA staff for their support in writing this article and Rachael Conklin, Consumer Safety Officer, Consumer Affairs Branch, Division of Communication and Consumer Affairs, Center for Biologics Evaluation and Research, for her help in organizing the comments provided by FDA staff.
Tackling the hurdle of tumor formation in stem cell therapies
Pluripotent stem cells (PSCs) are a type of stem cells capable of developing into various cell types. Over the past few decades, scientists have been working towards the development of therapies using PSCs. Thanks to their unique ability to self-renew and differentiate (mature) into virtually any given type of tissue, PSCs could be used to repair organs that have been irreversibly damaged by age, trauma, or disease.
However, despite extensive efforts, regenerative therapies involving PSCs still have many hurdles to overcome. One being the formation of tumors (via the process of tumorigenesis) after the transplantation of PSCs. Once the PSCs differentiate into a specific type for stem cell therapy, there is a high probability of tumor formation after differentiated stem cells are introduced to the target organ. For the success of PSC-based therapies, the need of the hour is to minimize the risk of tumorigenesis by identifying potentially problematic cells in cultures, prior to transplantation.
Against this backdrop, a research team led by Atsushi Intoh and Akira Kurisaki from Nara Institute of Science and Technology, Japan, has recently achieved a breakthrough discovery regarding stem cell therapy and tumorigenesis. "Our findings present advancements that could bridge the gap between stem cell research and clinical application," says Intoh, talking about the potential of their findings. Their study was published in Stem Cells Translational Medicine and focuses on a membrane protein called EPHA2, which was previously found to be elevated in PSCs prior to differentiation by the team.
Through several experiments involving both mouse and human stem cell cultures, the researchers gained insights into the role of EPHA2 in preserving the potency of PSCs to develop into several cell types. They found that EPHA2 in stem cells is co-expressed with OCT4 -- a transcription factor protein which controls the expression of genes which are critically involved in the differentiation of embryonic stem cells. Interestingly, when the EPHA2 gene was knocked down from the cells, cultured stem cells spontaneously differentiated. These results suggest that EPHA2 plays a central role in keeping stem cells in an undifferentiated state.
The researchers thus theorized that EPHA2-expressing stem cells, which would fail to differentiate, might be responsible for tumorigenesis upon transplantation into the target organ.
To test this hypothesis, the researchers prepared PSC cultures and artificially induced their differentiation into liver cells. Using a magnetic antibody targeting EPHA2, they extracted EPHA2-positive cells from a group of cultures prior to transplantation into mice. Interestingly, the formation of tumors in mice receiving transplants from cultures from which EPHA2 had been removed was vastly suppressed.
Taken together, these results point to the importance of EPHA2 in emerging stem cell-based therapies. "EPHA2 conclusively emerges as a potential marker for selecting undifferentiated stem cells, providing a valuable method to decrease tumorigenesis risks after stem cell transplantation in regenerative treatments," remarks Kurisaki.
Further in-depth studies on this protein may lead to the development of protocols that make PSCs safer to use. Luckily, however, these findings pave the way towards a future where we will be able to finally restore damaged organs and even overcome degenerative conditions.
- Prostate Cancer
- Skin Cancer
- Brain Tumor
- Medical Topics
- Immune System
- Embryonic stem cell
- Bone marrow transplant
- Adult stem cell
- Stem cell treatments
- Somatic cell nuclear transfer
- Somatic cell
- Monoclonal antibody therapy
Story Source:
Materials provided by Nara Institute of Science and Technology . Note: Content may be edited for style and length.
Journal Reference :
- Atsushi Intoh, Kanako Watanabe-Susaki, Taku Kato, Hibiki Kiritani, Akira Kurisaki. EPHA2 is a novel cell surface marker of OCT4-positive undifferentiated cells during the differentiation of mouse and human pluripotent stem cells . Stem Cells Translational Medicine , 2024; DOI: 10.1093/stcltm/szae036
Cite This Page :
Explore More
- Marine Cyanobacteria Can Communicate
- 'Tweezer-Like' Bionic Tools Feel Right
- Odd Planet-Forming Disks Around Low-Mass Stars
- Toward Blood Stem Cell Self-Renewal
- Restored Hearing and Speech in Kids Born Deaf
- Babies and AI Both Learn Key Foundation Models
- Myelination May Drive Drug Addiction
- Freshwater On Earth 4 Billion Years Ago
- Extended Battle: 3,500-Year-Old Mycenaean Armor
- Oral Insulin Drops: Relief for Diabetes Patients
Trending Topics
Strange & offbeat.
- Published: 15 March 2010
Welcome to Stem Cell Research & Therapy
- Ann Donnelly 1 ,
- Surayya Johar 1 ,
- Timothy O'Brien 2 &
- Rocky S Tuan 3
Stem Cell Research & Therapy volume 1 , Article number: 1 ( 2010 ) Cite this article
18k Accesses
13 Citations
7 Altmetric
Metrics details
Welcome to the first issue of the international open access journal Stem Cell Research & therapy , edited by Professor Rocky Tuan, of the University of Pittsburgh, and Professor Timothy O'Brien, of the National University of Ireland, Galway.
Stem Cell Research & Therapy aims to be the major forum for translational research into stem cell therapies. The journal has a special emphasis on basic, translational, and clinical research into stem cell therapeutics, including animal models, and clinical trials.
Stem cell research for therapeutic purposes has largely used adult stem cell sources. Embryonic stem cell research has enormous potential and also has major hurdles to overcome, not the least of which are ethical in nature. Funding for research into embryonic stem cells has also been in a state of transition. The change in US policy and subsequent National Institutes of Health guidelines allowing funding for human embryonic research has moved the use of stem cells of embryonic origin back into the spotlight [ 1 ]. Although legislation throughout the world varies, the international research community is striving to disseminate critical knowledge and useful ideas to aid the progress of our expertise in this area, and our open access policy will promote this.
Why is stem cell research important?
Stem cell research has great potential in the treatment of as-of-yet incurable diseases, including Huntington disease and Parkinson disease, Alzheimer disease, and amyotrophic lateral sclerosis. Other, more chronic conditions such as congestive cardiac failure, diabetes, and osteoarthritis may also respond well to stem cell therapy.
With the knowledge that stem cells can be induced to differentiate into specialized cells and that they can influence the tissues around them, the potential of stem cells as a therapeutic option is great. Recent advances have demonstrated that adult somatic cells, called induced pluripotent stem cells, can be reprogrammed into becoming stem-like in their nature and behavior [ 2 ].
Research is currently focused on calibration of the process of cell reprogramming, ensuring the quality of induced pluripotent stem cells, and modification of the stem cell niche. Future research will increasingly consider quality control of stem cell manufacture, delivery to the target areas, and architectural aids to ensure optimum placement and exposure of the stem cells.
Another important aspect of stem cell therapeutics will be a focus on the bioengineering of materials necessary to deliver and support stem cells on their therapeutic journey. Combinations of stem cell therapy with gene therapy will also expand the therapeutic repertoire as the effectiveness of the stem cell product may be enhanced via genetic modification. Thus, combinations of stem cells, biomaterials, and gene therapy may augment the therapeutic outcome but will result in complex regulatory challenges.
The potential paracrine mode of the therapeutic action of stem cells is worthy of substantial attention. Understanding the mechanism whereby stem cells heal tissue by regulating and interacting with host cells may lead to the development of novel therapeutic paradigms that may not require the stem cell per se as the therapeutic agent.
How and what will we publish?
BioMed Central is launching Stem Cell Research & Therapy to provide a new forum to highlight the growing area of stem cell therapeutics. In this open access journal, our research content will be made freely available upon publication. This means that readers worldwide will have immediate and free access to original research, promoting the immediate and wide distribution of the most current developments in the field [ 3 ]. Under our open access license, authors retain copyright of their article, allowing them, and any third party, to re-use their work as long as the authors are given correct attribution [ 4 ]. To cover the costs of open access, authors of original research are asked to pay an article-processing charge once their article has been accepted for publication. This is a flat fee that includes the use of color figures, unlimited pages, and additional data sets. Indeed, authors can upload both audio and visual files alongside their manuscript at no extra cost. To ensure permanence and high visibility, research published in Stem Cell Research & therapy will also be deposited in several international bibliographic databases [ 5 ].
Stem Cell Research & Therapy will publish original research as well as regular commissioned articles. Our reviews will provide a comprehensive overview of specific topics, collating and discussing the ever-changing advances in the field. There will be a specific focus on the therapeutic elements of stem cell research. Commentaries and viewpoint articles will be speculative and allow authors to be more opinionated in their views. Readers are firmly encouraged to participate and can do so by submitting letters to the editor on articles published in Stem Cell Research & therapy and on any issue in a related area. Brief comments can also be posted online on any article by using the tools displayed on the article's webpage. These tools will also allow articles to be shared via 'social media' services such as Facebook and Twitter, reflecting the commitment of the journal to disseminating our articles widely via the most popular and modern means.
We welcome your contributions
Stem Cell Research & Therapy will provide a platform for translational research into stem cell therapy. We are delighted to introduce this much-needed journal to the stem cell research community, and we welcome your responses and submissions. The Editors-in-Chief, supported by a global Editorial Board [ 6 ], are committed to making this journal a success, and we look forward to receiving your contributions.
National Institutes of Health Guidelines on Human Stem Cell Research. [ http://stemcells.nih.gov/policy/2009guidelines.htm ]
Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006, 126: 663-676. 10.1016/j.cell.2006.07.024.
Article CAS PubMed Google Scholar
BioMed Central Open Access Charter. [ http://www.biomedcentral.com/info/about/charter ]
Frequently asked questions about BioMed Central's article-processing charges. [ http://www.biomedcentral.com/info/about/apcfaq ]
What is BioMed Central?. [ http://www.biomedcentral.com/info/ ]
Stem Cell Research and Therapy Editorial Board. [ http://www.stemcellres.com/edboard ]
Download references
Author information
Authors and affiliations.
BioMed Central, Floor 6, 236 Gray's Inn Road, London, WC1X 8HL, UK
Ann Donnelly & Surayya Johar
REMEDI - National Centre for Biomedical Engineering Science, National University of Ireland, Galway, Ireland
Timothy O'Brien
McGowan Institute for Regenerative Medicine, University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center, 450 Technology Drive, Suite 300, Pittsburgh, PA315219-3110, USA
Rocky S Tuan
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Ann Donnelly or Surayya Johar .
Additional information
Competing interests.
AD and SJ are employees of BioMed Central and receive fixed salaries. TO and RT are the Editors-in-Chief of Stem Cell Research & Therapy and receive an annual honorarium.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Donnelly, A., Johar, S., O'Brien, T. et al. Welcome to Stem Cell Research & Therapy . Stem Cell Res Ther 1 , 1 (2010). https://doi.org/10.1186/scrt1
Download citation
Published : 15 March 2010
DOI : https://doi.org/10.1186/scrt1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Scientists identify ‘missing piece’ required for blood stem cell self-renewal
Key takeways/summary.
- Blood stem cells – key to transplants that are used as life-saving treatments for blood cancers and blood and immune disorders – have the capacity to self-renew, but quickly lose their ability to do so in a lab dish.
- UCLA scientists have identified a protein that not only enables blood stem cells to self-renew in a lab dish, but also allows these expanded cells to function effectively after being transplanted into mouse models.
- The findings could help make blood stem cell transplants available to more people and improve the accessibility and safety of gene therapies that use these cells.
UCLA scientists have identified a protein that plays a critical role in regulating human blood stem cell self-renewal by helping them sense and interpret signals from their environment.
The study , published in Nature, brings researchers one step closer to developing methods to expand blood stem cells in a lab dish, which could make life-saving transplants of these cells more available and increase the safety of blood stem cell-based treatments, such as gene therapies.
Blood stem cells, also known as hematopoietic stem cells, have the ability to make copies of themselves via a process called self-renewal, and can differentiate to produce all the blood and immune cells found in the body. For decades, transplants of these cells have been used as life-saving treatments for blood cancers such as leukemia and various other blood and immune disorders.
However, blood stem cell transplants have significant limitations. Finding a compatible donor can be difficult, particularly for people of non-European ancestry, and the number of stem cells available for transplant can be too low to safely treat a person’s disease.
These limitations persist because blood stem cells that have been removed from the body and placed in a lab dish quickly lose their ability to self-renew. After decades of research, scientists have come achingly close to solving this problem.
“We’ve figured out how to produce cells that look just like blood stem cells and have all of their hallmarks, but when these cells are used in transplants, many of them still don’t work; there’s something missing,” said Dr. Hanna Mikkola , senior author of the new study and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
To pinpoint the missing piece that prevents these blood stem cell-like cells from being fully functional, Julia Aguade Gorgorio, the paper’s first and co-corresponding author, analyzed sequencing data to identify genes that are silenced when blood stem cells are placed in a lab dish. One such gene, MYCT1, which encodes a protein by the same name, stood out as being essential to these cells’ self-renewal capacity.
They found that MYCT1 regulates a process called endocytosis, which plays a key role in how blood stem cells take in the signals from their environment that tell them when to self-renew, when to differentiate and when to be quiet.
“When cells perceive a signal, they have to internalize it and process it; MYCT1 controls how fast and how efficiently blood stem cells perceive these signals,” said Aguade Gorgorio, an assistant project scientist in the Mikkola lab. “Without this protein, the signals from the cells’ environment turn from whispers into screams and the cells become stressed out and dysregulated.”
The researchers compare MYCT1 to the sensors in modern cars that monitor all nearby activity and selectively relay the most crucial information to drivers at the right time, aiding decisions like when to safely turn or change lanes. Without MYCT1, blood stem cells resemble anxious drivers who, used to relying on these sensors, suddenly find themselves lost without their guidance.
Next, the researchers used a viral vector to reintroduce MYCT1 to see if its presence could restore blood stem cell self-renewal in a lab dish. Restoration of MYCT1, they found, not only made the blood stem cells less stressed and enabled them to self-renew in culture but also allowed these expanded cells to function effectively after being transplanted into mouse models.
As a next step, the team will investigate why the silencing of the MYCT1 gene occurs, and then, how to prevent this silencing without the use of a viral vector, which would be safer for use in a clinical setting.
“If we can find a way to maintain MYCT1 expression in blood stem cells in culture and after transplant, it will open the door to maximize all these other remarkable advances in the field,” said Mikkola, who is a professor of molecular, cell and developmental biology in the UCLA College and a member of the UCLA Health Jonsson Comprehensive Cancer Center . “This would not only make blood stem cell transplants more accessible and effective but also improve the safety and affordability of gene therapies that utilize these cells.”
This work was supported by the National Institutes of Health, the Swiss National Science Foundation, the European Molecular Biology Organization, the UCLA Jonsson Cancer Center Foundation, the James B. Pendleton Charitable Trust, the McCarthy Family Foundation, the California Institute for Regenerative Medicine, the UCLA AIDS Institute, the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center, the Royal Society, the Wellcome Trust and the UCLA Broad Stem Cell Research Center Stem Cell Training Program.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 6, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study uncovers how cancer stem cells spread and resist treatment
by University of Oxford
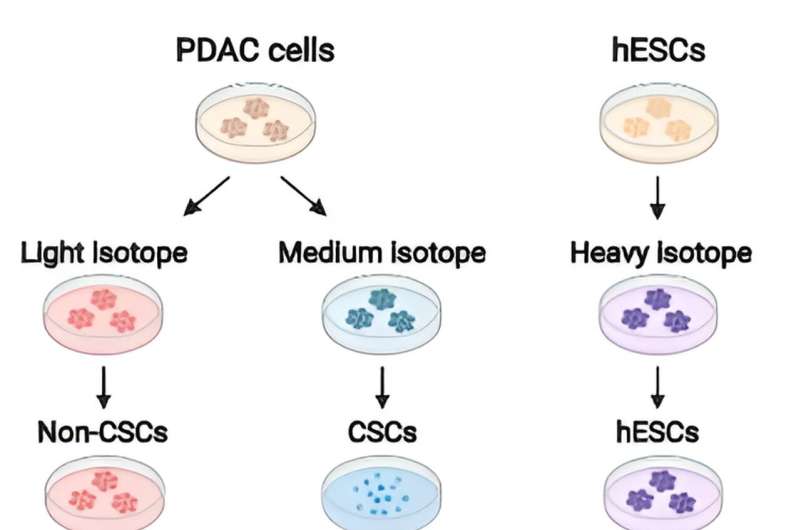
NDORMS researchers have identified a critical axis that controls the formation and behavior of cancer stem cells (CSCs), a subpopulation of cells that influence how lethal the cancer can be, its resistance to chemotherapy, and its ability to spread to other parts of the body.
The findings , published in Nature Communications , could pave the way for more effective cancer treatments.
The research, led by Associate Professor and CRUK career development fellow Siim Pauklin from NDORMS, focused on understanding the intricate interplay between cell-autonomous (mechanisms inside cells that function independently of signals coming from outside the cells) and non-cell-autonomous (external signals from outside cells) pathways that govern the stem cell-like characteristics of cancer stem cells (CSCs) in pancreatic cancer .
CSCs have also been discovered in the brain, breast, colon, esophagus, liver, lung, ovarian, prostate, stomach and thyroid cancers, among others.
Using a quantitative proteomic approach to analyze the set of proteins in the extracellular space (the secretome) of pancreatic CSCs, the research reveals how a complex axis involving the retinoblastoma (RB) proteins and the E2F transcription factors controls the production of signaling molecules that promote CSC formation and chemoresistance.
"We were surprised to find that the well-known cell cycle regulators pRb and E2F, which were previously thought to only have cell-autonomous effects, actually have a non-cell-autonomous role in controlling the secretion of key WNT ligands that drive the stem cell-like properties of CSCs," said Siim. "This work provides important insights into the non-cell autonomous mechanisms that allow cancer stem cells to thrive and evade therapy."
The researchers discovered that the E2F1 and E2F4 transcription factors induce the expression of WNT ligands, such as WNT7A, WNT7B, WNT10A, and WNT4, while the pRb and RBL2 proteins act to reduce their expression. This delicate balance is disrupted in cancer cells harboring KRAS mutations, leading to the aberrant secretion of these WNT ligands and the activation of the WNT/β-catenin signaling pathway in CSCs.
"The interplay between cell-autonomous transcriptional regulators and non-cell-autonomous signaling pathways is a crucial, yet often overlooked, aspect of cancer stem cell biology," Siim said. "Our findings highlight the importance of targeting these extracellular communication networks to effectively eliminate this resilient population of cells."
The study's insights could pave the way for the development of more effective cancer therapies, as targeting the identified pathway could potentially disrupt the self-renewal, chemoresistance, and metastatic potential of CSCs, which are often responsible for treatment failure and disease recurrence.
Explore further
Feedback to editors

What's going on in our brains when we plan? Study uncovers how mental simulations rely on stored memories

Researchers discover new therapeutic target for the treatment of melanoma resistant to targeted therapies

Excess nutrient signals in cells lead to premature aging in animals, study shows

Metabolic parameters found to be similar in children born via frozen versus fresh embryo transfer, research shows
16 hours ago
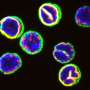
Researchers identify key differences in inner workings of immune cells
Researchers detail molecular pathway that impacts pancreatic cancer progression and treatment response
Epstein-Barr virus and brain cross-reactivity: Possible mechanism for multiple sclerosis detected
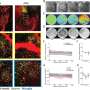
Precision laser surgery cuts focal epileptic seizure spread

Researchers find flavor restrictions affect tobacco buyers differently depending on socioeconomic status
17 hours ago

Younger children are more commonly diagnosed with ADHD than their older classmates, says new study
Related stories.
New target identified for pancreatic cancer treatment
Sep 26, 2023

Therapeutically harnessing cancer stem cell-derived exosomes
Jan 3, 2024
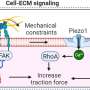
Mechanical constraints guide spatial pattern of glioblastoma cancer stem cells
Apr 2, 2024
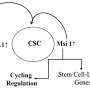
Musashi 1 in breast cancer: Implications for dormancy and survival in bone marrow
May 16, 2023
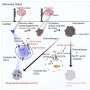
New way to kill breast cancer stem cells that have ancestral features and resist chemotherapy
Nov 24, 2023
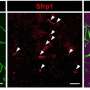
Study links protein secreted by blood vessels to drug-resistant cancer
May 15, 2024
Recommended for you

'Artificial lymph node' used to treat cancer in mice
18 hours ago

New combination therapy shows promise for bladder cancer patients unresponsive to standard treatment
Clinical study shows zebrafish avatars of cancer patients have high predictive power
19 hours ago

Engineered bacteria deliver chemotherapy directly to tumors
20 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Adding stem cells to a kidney transplant could get patients off anti-rejection drugs, trial finds
A novel approach to organ transplantation allowed patients to wean off anti-rejection drugs after two years, according to the results of a phase 3 clinical trial presented Monday.
The drugs, called immunosuppressants, are an essential part of any transplant recipient’s life: They help ensure that the immune system doesn’t attack the donated organ as a “foreign” object, leading to rejection. In doing so, however, they come with a host of toxic side effects, including harming the donated organ and increasing the recipient’s risk of infection and cancer.
And they must be taken for life.
“The holy grail of organ transplantation is achieving tolerance,” said Dr. Dorry Segev, a transplant surgeon at NYU Langone Health in New York City. That is, to be able to transplant people without the need for immunosuppressive drugs.
“When we say tolerance, we’re basically saying can the body accept someone else’s kidney without rejecting the kidney,” said Dr. Sanjeev Akkina, medical director of the kidney transplant program at Loyola Medicine in suburban Chicago. “The idea that you can actually be off of anti-rejection drugs for the rest of that life — there’s a lot of benefit in that.”
Akkina is one of the researchers involved with a phase 3 trial in organ transplant patients that uses stem cells taken from the organ donor in an attempt to wean the recipients off of these drugs. The results, presented Monday at the American Transplant Congress in Philadelphia, found that after two years, 16 of 19 patients were able to come off their anti-rejection drugs entirely. The three other patients had to go back on the drugs due to either a rejection episode or worsening kidney function.
The trial, funded by biotechnology company Medeor Therapeutics, is centered around MDR-101, a stem cell therapy that is derived from the person donating the organ. The findings have not yet been published in a peer-reviewed journal.
“These are excellent results,” said Dr. Jeffrey Veale, a urologist at UCLA Health who specializes in kidney transplantation . Not only were a majority of the patients able to stay off of the drugs for two years, the authors also showed a significant benefit in quality of life for the patients, added Veale, who was not involved with the research.
Kidneys are the most commonly transplanted organs in the United States with around 25,000 kidney transplants performed in recent years, according to the Organ Procurement and Transplantation Network . Around 10% of them, however, are repeat procedures after a transplanted kidney fails.
One reason this happens, Veale said, is because anti-rejection drugs can actually damage the kidney to the point that a new one is needed. If patients are able to ultimately get off of these drugs, the transplanted kidney should theoretically last a lifetime.
“That would expand the pool of organs available because you wouldn’t have people coming back for their second or third or fourth transplant,” he said.
A kidney and stem cells from his sister
Alex Hernandez was 27 when he received a kidney transplant and a dose of stem cells from his sister as one of Akkina’s patients and a trial participant.
Hernandez, of Milwaukee, was born with vesicoureteral reflux, a condition that causes urine to flow backward in the urinary tract, leaving his kidneys scarred and ultimately causing permanent kidney damage. In college, his kidneys started to fail, and he had to go on dialysis.

In order to prepare his body for the stem cells, Hernandez had radiation therapy just days after his surgery. That temporarily suppressed his immune system so the stem cells would be accepted. Eleven days after surgery, the new cells were transferred, mixing with Hernandez’s immune system to become a hybrid of both donor and recipient, a phenomenon called “mixed chimerism.”
This prevents the body from attacking the new kidney and allows the patient to eventually stop all those immunosuppressant drugs.
“The stem cells basically reprogram your immune system , telling them to recognize that this donor kidney coming in is actually going to be part of your own,” Akkina said.
For Hernandez, now 30 and about to start his second year of dental school at Marquette University, the results have been life-changing. His surgery was in 2021 and he was fully off of all of the anti-rejection drugs that same year.
“I had to carry this pill container labeled Monday, Tuesday, Wednesday, Thursday, Friday,” he said. “It’s empty now.”
What’s next?
The results are an “important next step” in the field of organ transplantation, but Segev stressed that there is a lot more work to be done in the field of tolerance.
The results presented Monday were for those who had received organs from closely matched siblings who are genetically similar, he said — that’s not the majority of transplant recipients.
“Ultimately we need to figure out a way for this to apply to many more patients who undergo transplants, which is why we’re still very early in this field,” Segev said.
Veale said the goal is to get everyone to come off their anti-rejection medications, not just those who get an organ from a sibling.
The approach used in the clinical trial also requires both the transplant and stem cell donation to occur within a two-week period, which can be a significant burden on patients, donors and health care systems.
Veale’s own research focuses on extending the amount of time that can elapse between the organ transplant and the stem cell transplant. He’s had success weaning patients off anti-rejection drugs as many as five years after their transplant.
“They may already be starting to feel the toxic effects of those medications, so I wanted to open up tolerance to that pool of patients,” he said.
Doctors are also hopeful this could one day apply to other types of organ transplants beyond the kidney.
“I think as we understand how chimerism occurs and what we can do to successfully achieve chimerism, we would be able to apply that to any solid organ transplant,” Segev said.
Akshay Syal, M.D., is a medical fellow with the NBC News Health and Medical Unit.
Jessica Herzberg is a producer in the NBC News Medical Unit.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 31 May 2024
Efficacy and safety of stem cell transplantation for multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials
- Asmaa Ahmed Nawar 1 ,
- Aml Mostafa Farid 1 ,
- Rim Wally 2 ,
- Engy K. Tharwat 3 ,
- Ahmed Sameh 4 ,
- Yomna Elkaramany 4 ,
- Moamen Mostafa Asla 1 &
- Walaa A. Kamel 5
Scientific Reports volume 14 , Article number: 12545 ( 2024 ) Cite this article
806 Accesses
3 Altmetric
Metrics details
Multiple sclerosis
- Stem-cell research
Multiple sclerosis (MS) is a common autoimmune neurological disease affecting patients’ motor, sensory, and visual performance. Stem Cell Transplantation (SCT) is a medical intervention where a patient is infused with healthy stem cells with the purpose of resetting their immune system. SCT shows remyelinating and immunomodulatory functions in MS patients, representing a potential therapeutic option. We conducted this systematic review and meta-analysis that included randomized control trials (RCTs) of SCT in MS patients to investigate its clinical efficacy and safety, excluding observational and non-English studies. After systematically searching PubMed, Web of Science, Scopus, and Cochrane Library until January 7, 2024, nine RCTs, including 422 patients, were eligible. We assessed the risk of bias (ROB) in these RCTs using Cochrane ROB Tool 1. Data were synthesized using Review Manager version 5.4 and OpenMeta Analyst software. We also conducted subgroup and sensitivity analyses. SCT significantly improved patients expanded disability status scale after 2 months (N = 39, MD = − 0.57, 95% CI [− 1.08, − 0.06], p = 0.03). SCT also reduced brain lesion volume (N = 136, MD = − 7.05, 95% CI [− 10.69, − 3.4], p = 0.0002). The effect on EDSS at 6 and 12 months, timed 25-foot walk (T25-FW), and brain lesions number was nonsignificant. Significant adverse events (AEs) included local reactions at MSCs infusion site (N = 25, RR = 2.55, 95% CI [1.08, 6.03], p = 0.034) and hematological disorders in patients received immunosuppression and autologous hematopoietic SCT (AHSCT) (N = 16, RR = 2.33, 95% CI [1.23, 4.39], p = 0.009). SCT can improve the disability of MS patients and reduce their brain lesion volume. The transplantation was generally safe and tolerated, with no mortality or significant serious AEs, except for infusion site reactions after mesenchymal SCT and hematological AEs after AHSCT. However, generalizing our results is limited by the sparse number of RCTs conducted on AHSCT. Our protocol was registered on PROSPERO with a registration number: CRD42022324141.
Similar content being viewed by others

Looking backward to move forward: a meta-analysis of stem cell therapy in amyotrophic lateral sclerosis

Effect of autologous hematopoietic stem cell transplantation on multiple sclerosis and neuromyelitis optica spectrum disorder: a PRISMA-compliant meta-analysis
Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study
Introduction.
Multiple Sclerosis (MS) is a common autoimmune demyelinating neurological disease affecting more than 2.8 million patients worldwide 1 . It presents with different manifestations such as visual loss, weakness, sensory, and even sphincteric disturbances 2 . MS includes five main clinical courses: Primary Progressive Multiple Sclerosis (PPMS), Secondary Progressive Multiple Sclerosis (SPMS), Relapsing–Remitting Multiple Sclerosis (RRMS), Clinically Isolated Syndrome (CIS), and Radiologically Isolated Syndrome (RIS). These clinical phenotypes are assessed according to two descriptors: disease activity and progression. Disease activity is evidenced by clinical relapses or lesions activity on MRI, while disability progression is associated with increasing neurological dysfunction 3 .
Currently, approved medications for MS only aim to alleviate the symptoms or slow disease progression and reduce relapses through disease-modifying therapies (DMTs). Of these drugs, interferon beta (INF-β) and glatiramer acetate can reduce the relapse rate by one-third in relapsing MS (RMS; includes RRMS, CIS, and SPMS with relapses) through their immunomodulatory effect. Oral immunomodulators, including dimethyl-fumarate and teriflunomide, are also effective in reducing the disease activity in RMS 4 , 5 . Other DMTs can inhibit the migration of immune cells to the CNS, such as fingolimod which decreases the disease activity with considerable safety concerns, including bradycardia, liver injury, and infections. Siponimod is another novel cell migration inhibitor that decreases the relapse rate in SPMS by about 20% with the same adverse events as fingolimod 5 . For SPMS and worsening RMS, mitoxantrone induces immunosuppression but carries a high risk of cardiotoxicity and hematological malignancies 4 . Cladribine is another agent used for the relapsing forms of MS. Novel B-cell depleting drugs, including natalizumab, ocrelizumab, alemtuzumab, and ofatumumab can be used for RRMS and active SPMS 6 , 7 . However, patients with SPMS and PPMS have fewer options of medications with limited efficacy and safety issues. To date, finding an ultimate cure for MS is still an unmet need 8 , 9 .
Stem cell transplantation (SCT) has emerged as another treatment option for multiple sclerosis in addition to different autoimmune neurological diseases such as myasthenia gravis, Neuromyelitis optica, and systemic inflammatory diseases including rheumatoid arthritis and systemic lupus erythematosus 10 , 11 . SCT involves ablation of the patient's aberrant immune system and reconstitution of a new immune system derived after the infusion of healthy stem cells 12 . The European Group for Blood and Marrow Transplantation has recommended autologous hematopoietic SCT (AHSCT) for MS patients showing inflammatory disease activity, including RRMS patients not responding to the approved DMTs and SPMS patients with worsening disability 13 . Young and ambulatory MS patients are considered the optimal candidates for AHSCT 14 .
In multiple sclerosis, stem cells migrate into the brain lesions, contribute to regenerating the impaired myelin and induce tissue repair. This regenerative process is attributed to the ability of stem cells to differentiate into both neuronal and myelin-producing cells 15 . Stem cells also show immunomodulatory functions by inhibiting the autoimmune lymphocytes that attack the white matter of the brain, providing a neuroprotective potential as observed in preclinical and clinical studies 15 , 16 .
Clinical trials have detected promising clinical recovery and improvement of the quality of life of MS patients after SCT with minimal safety concerns 17 , 18 , 19 . However, these clinical trials show variations in the transplantation procedure, including the dose, the origin of the cells, and their route of administration. Stem cells can be found in embryonic tissue or in adult tissue, including hematopoietic stem cells (in the bone marrow or peripheral blood), mesenchymal stem cells (in bone marrow or adipose tissue), and neural stem cells in the brain 18 , 20 . Furthermore, autogenic stem cells are isolated from the patient who gets the transplantation, and allogenic stem cells are derived from a donor. Stem cells are usually infused via the intravenous route; however, the intrathecal or the intraventricular routes are expected to be more effective in MS 21 . These variations limited finding the transplantation approach that produces the optimal benefits for MS patients. We conducted this systematic review and meta-analysis of randomized control trials to assess the efficacy and safety of various SCT approaches in MS.
Applying the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement 2020 guidelines 22 , we proceeded as follows :
Protocol and registration
Our protocol was registered on PROSPERO with a registration number: CRD42022324141.
Eligibility criteria
We included studies satisfying the following PICOS (Population, Intervention, Comparator, Outcomes, and Study design) criteria in our meta-analysis: Population: patients with multiple sclerosis; Intervention: stem cells transplantation without restrictions on the dose or the source of stem cells; Comparator: control group received placebo or active treatment; Outcome: efficacy and/or safety of stem cell transplantation; Study design: randomized controlled trials (RCTs).
Exclusion criteria
We excluded studies that didn’t meet the PICOS criteria, observational studies, animal or experimental trials, reviews, book chapters, conference abstracts, trial registries, and protocols.
Information sources
We searched four electronic databases: PubMed, Scopus, Web of Science (WOS), and Cochrane Central Register of Controlled Trials from inception to January 7, 2024. Figure 1 presents the flow diagram of studies selection.
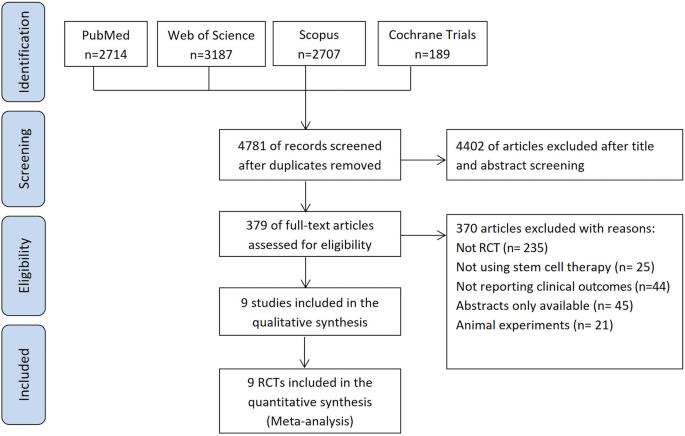
PRISMA Flow chart of the study selection process.
Search strategy
We used the following search terms: multiple sclerosis, disseminated sclerosis, stem cells, mesenchymal cells, and hematopoietic cells. The PubMed advanced search strategy was as follows: (((Multiple[Title/Abstract] OR Disseminated[Title/Abstract]) AND Sclerosis[Title/Abstract])) AND ((Stem[Title/Abstract] OR Hematopoietic[Title/Abstract] OR Progenitor[Title/Abstract] OR Mesenchymal[Title/Abstract]) AND (Cells[Title/Abstract] OR cell[Title/Abstract])). The search strategy was modified for each database individually.
Selection process
Two authors independently screened the titles and abstracts of the records retrieved from the literature search according to our prespecified inclusion criteria. Full texts of the eligible records were obtained for further screening by another two authors. Any discrepancy between the authors in these two screening steps was resolved by a third reviewer.
Data extraction
Data were extracted to structured excel sheets by two independent authors and disagreements between them were resolved through discussion with a third author.
The data were (1) characteristics of the included studies, including setting, design, intervention details, and summary of main findings; (2) demographic and baseline characteristics of the studies population; (3) quality assessment information (4) safety and efficacy endpoints.
Risk of bias assessment
Two authors evaluated the quality of the included RCTs independently using the domains of Cochrane Collaboration’s tool for assessing risk of bias 1. We assessed the presence of bias in selection, performance, detection, attrition, and reporting, then classified the risk of these biases as high, low, or unclear 23 . The overall quality of each study was categorized into good, fair, and poor quality. Disagreements between the two authors were discussed and resolved with a third author.
Effect measures
We included the following outcomes in our meta-analysis:
Clinical outcomes
The primary outcome :
Expanded Disability Status Scale (EDSS) changes from baseline to 2, 6, and 12 months. An increased score indicates worsening of patient disability 25 .
The secondary outcomes :
− Timed 25 Foot Walk (T25-FW) change at 6 months to assess the effect on motor performance. Better walking ability is associated with lower scores 26
− Change in the Nine-hole peg test (9-HPT) at 6 months that measures finger dexterity with lower scores indicating improvement 27
− Paced auditory serial addition test (PASAT-3) at the last follow-up to assess the effect on cognitive functions indicating improvement with increased scores 28
− Number of relapses evaluated in 6 months of follow-up.
Radiological outcomes
− Change in the volume of MRI T2 weighted lesions at the end of treatment.
− Change in the number of MRI T2-weighted lesions at 12 months.
− Change in the number of gadolinium-enhancing lesions (GELs) at the end of the treatment period.
Safety endpoints
We analyzed the reported incidence of headache, anemia, gastrointestinal disturbance, blood and lymphatic system disorders, total infections, administration site adverse events (AEs), and serious AEs.
Clinical and radiological outcomes were continuous data that were pooled as mean change from baseline (MC) and standard deviation (SD) of mean change. On the other hand, safety endpoints were dichotomous variables and were extracted as frequency of events in the total patients’ number in the group.
Calculating the missing data
When the mean change from baseline to the time point of measurement of the outcome wasn’t reported, it was calculated by subtracting the pre-intervention values from the post-intervention values of the outcomes 29 .
When the standard deviations (SD) of mean change weren’t provided in the included studies, we calculated these SD values using Cochrane Handbook for Systematic Reviews of Interventions methods 29 . We also followed Wan et al. 30 method to calculate mean and SD from median and (range or interquartile range), if provided.
Statistical analysis
Outcomes reported by two or more studies were included in our analysis. We conducted the meta-analysis using Cochrane systematic review software Review Manager Version 5.4 for windows for continuous data; and OpenMeta Analyst software for windows for dichotomous data. We presented Continuous variables as mean differences (MDs) with 95% confidence intervals (CIs) and dichotomous variables as relative risks (RRs) with 95% CIs. We considered data statistically significant when p value is ≤0.05.
Assessing the heterogeneity
The heterogeneity of the studies and subgroups was evaluated by visualizing the forest plot based on the Cochrane Q and I-square tests. We set a P value of P < 0.1 and I 2 ≥ 50% as the significance level for assessing heterogeneity 29 . We pooled data under a random-effects model due to the variation in the patients’ characteristics and procedural aspects among the included studies.
Subgroup analysis
We conducted the subgroup analyses for EDSS change from baseline based on (A) the time of assessment at 2, 6, and 12 months after the intervention; (B)baseline EDSS ≤ 6.5 or > 6.5 (EDSS of 6.5 correlates with walking ability of 20 m with two aids 25 ); (C) the administrated dose of stem cells in each study, either low doses (≤ 2 × 10 6 cells/kg) or higher doses (≥ 3 × 10 6 cells/kg); (D) the sources of the transplanted stem cells (adult or embryonic origin) and (E) whether the control was a placebo or active treatment.
Subgroups from B–D included EDSS change from baseline to the end of patients follow-up (last assessment).
Sensitivity analysis
We ran a sensitivity analysis using the leave-one-out procedure which includes conducting multiple meta-analyses for the outcomes and excluding a single study in each scenario to investigate the impact of these studies on the overall effect size. By this method, we ensure the statistical robustness of our results and that the results of our meta-analysis were not affected by any of the individual studies. OpenMeta Analyst software was used to perform the sensitivity analysis.
Publication bias assessment
We planned to explore the publication bias in the included studies using Egger’s method depending on funnel plot asymmetry 24 .
Study selection
From the initial literature search, we retrieved relevant 3948 records from PubMed, Web of Science, Scopus, and the Cochrane Library. After the title and abstract screening of them we screened the full text of 295 articles. Only nine studies met our criteria 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 . Figure 1 shows the PRISMA flow diagram of our search and selection process.
Study characteristics
The nine studies were RCTs and enrolled a total of 422 multiple sclerosis patients. All studies were parallel in design except 4 studies were cross-over RCTs 31 , 32 , 34 , 39 . These cross-over trials were reviewed up to the point of cross-over. All studies infused stem cells intravenously except Petrou et al. that included an additional intrathecal SCT subgroup 32 . This study showed that intrathecal SCT was more effective than intravenous SCT, but we pooled the data of both routes as single study data. Of the included studies, only two studies used autologous hematopoietic SCT (AHSCT) in addition to immune ablative regimen prior to the transplantation 37 , 38 . Burt et al. compared SCT to DMTs (natalizumab, fingolimod, and dimethyl fumarate) in RRMS patients 37 , and Mancardi et al., compared SCT to mitoxantrone in relapsing and progressive MS patients 38 .
Supplementary Table S1 summarizes the characteristics of the included trials, Table 1 shows the demographic and baseline characteristics of these studies’ population, and Supplementary Table S3 shows efficacy endpoints reported at 6 months.
Risk of bias within studies
We assessed seven domains in each study according to The Cochrane Collaboration’s tool for assessing risk of bias 1. The 9 studies were randomized but 4 studies 32 , 35 , 38 , 39 didn’t clarify the methods of random sequence generation. 6 RCTs confirmed concealment of patients’ allocation to the intervention 31 , 32 , 33 , 34 , 36 , 37 . Blinding of the outcome assessors was clearly stated in all studies except Nabavi et al. 39 but blinding of participants and personnel wasn’t fulfilled in three studies 35 , 37 , 38 . The reasons for incomplete outcome data are related to the treatment in Uccelli et al. 31 and the reasons weren’t clearly described in Burt et al 37 . Two studies reported the outcomes in an incomplete way that limited their inclusion in the meta-analysis inducing a reporting bias 33 , 38 . The overall quality of the studies was good for 2 studies 32 , 36 , fair for 3 studies 31 , 34 , 37 , and poor for 4 studies 33 , 35 , 38 , 39 . Figure 2 shows the risk of bias summary and graph.
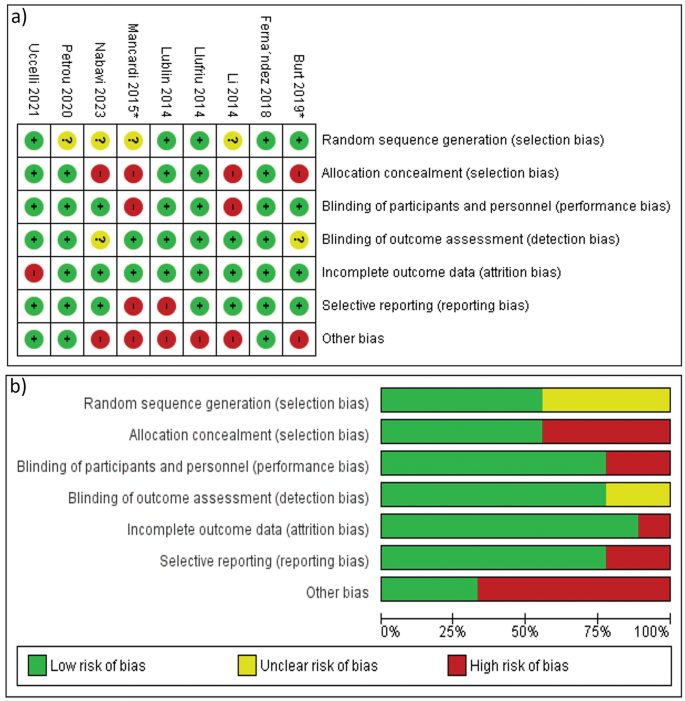
Risk of bias assessment: ( a ) Risk of bias summary, ( b ) Risk of bias graph.
Meta-analysis results
After analyzing the efficacy and safety outcomes for all studies collectively, we subdivided the results into studies that used immunosuppression before AHSCT 37 , 38 and studies that transplanted mesenchymal stem cells (MSCs) without immunosuppression 31 , 32 , 33 , 34 , 35 , 36 , 39 to minimize the procedural variations among the included trials.
Effect on patients disability
The majority of the studies 31 , 32 , 33 , 34 , 35 , 36 , 37 , 39 reported EDSS change for 211 patients in stem cell transplantation (SCT) arm and 176 controls. Because the time of reporting this outcome varied among the studies, we analyzed EDSS change at the last follow-up reported by each study. Our analysis showed nonsignificant difference between SCT group and the control group (MD = − 0.48, 95% CI [− 1.11, 0.14], p = 0.13). There was great heterogeneity between studies (χ2 = 116.74, df = 7, p < 0.00001, I 2 = 94%), so we pooled the data under the random-effects model (Table 2 and Supplementary Figure S1 ).
The subgroup analysis of the studies that used MSCs without immunosuppression also showed nonsignificant improvement (MD = − 0.3, 95% CI [− 0.87, 0.27], p = 0.3). However, Burt et al 37 . that used immunosuppression before AHSCT revealed significant EDSS reduction (Supplementary Figure S1 ).
The results remained nonsignificant after the leave-one-out sensitivity analysis (Supplementary Figure L1 ).
Subgroup analyses for EDSS
Edss at different timepoints, edss at 2 months.
The heterogeneity within the studies was not significant (χ2 = 1.61, df = 1, p = 0.2, I 2 = 38%), and we adopted a random effect model. The reduction of EDSS in SCT group was significantly greater than the control group (MD = − 0.57, 95% CI [− 1.08, − 0.06], p = 0.03) (Table 2 and Fig. 3 a).
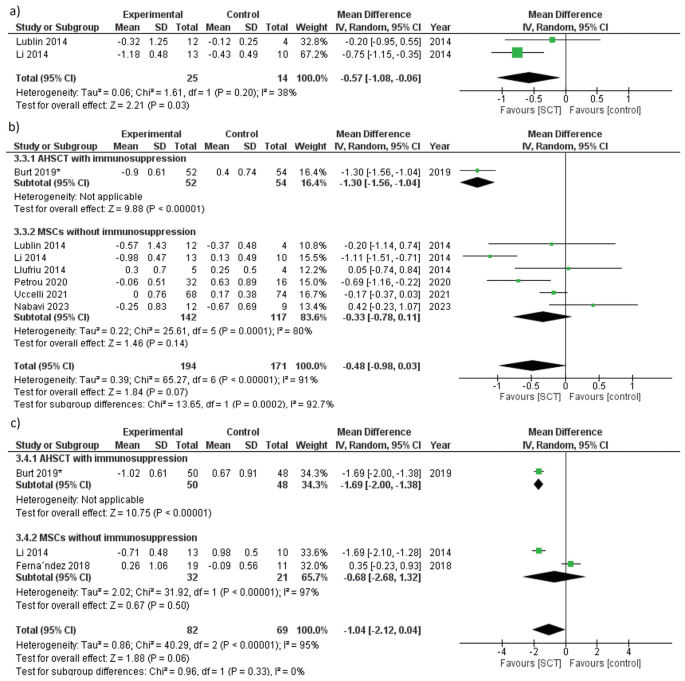
Forest plot of EDSS change from baseline at ( a ) 2 months, ( b ) 6 months, ( c ) 12 months.
EDSS at 6 months
Adopting the random-effects model, the heterogeneity between the studies was significant (χ2 = 65.27, df = 6, p < 0.00001, I 2 = 91%), and SCT showed nonsignificant improvement of EDSS compared to the control (MD = − 0.48, 95% CI [− 0.98, 0.03], p = 0.07) (Table 2 and Fig. 3 b). MSCs without immunosuppression also resulted in nonsignificant EDSS reduction at 6 months (MD = − 0.33, 95% CI [− 0.78, 0.11], p = 0.14) (Fig. 3 b).
The effect estimate changed to (MD = − 0.62,95% CI [− 1.14, − 0.09], p = 0.02) favoring SCT over the control after excluding Nabavi et al. 39 from the analysis (Supplementary Figure L2 and Table L1 ).
EDSS at 12 months
We adopted the random-effects model because heterogeneity was significant, and the difference between the SCT group and the control was not significant at 12 months for both collective studies analysis and studies used MSCs without immunosuppression ( p = 0.06 and p = 0.5, respectively). However, the study that used AHSCT plus immunosuppression 37 showed significant improvement in patients disability ( p < 0.00001) (Table 2 and Fig. 3 c).
After performing a sensitivity analysis by excluding Ferna´ndez et al. 36 , the results changed from nonsignificant to significant improvement in SCT arm (MD = − 1.69, 95% CI [− 1.94, − 1.44], p < 0.00001) (Supplementary Figure L3 and Table L1 ).
EDSS improvement according to baseline EDSS
We compared the effect of SCT on patients’ disability depending on baseline EDSS. Six studies 31 , 32 , 33 , 34 , 37 , 39 included 334 MS patients with baseline EDSS ≤ 6.5, while two studies 35 , 36 included 53 patients with baseline EDSS > 6.5. Using a random effects model, both subgroups showed significant heterogeneity ( p < 0.00001 and p < 0.00001). Both subgroups revealed nonsignificant effect of SCT on EDSS, (MD = − 0.41, 95% CI [− 1.11, 0.29], p = 0.25) for baseline EDSS ≤ 6.5 subgroup and (MD = − 0.68, 95%CI [− 2.68, 1.32], p = 0.5) for baseline EDSS > 6.5 subgroup (Table 2 and Supplementary Figure S2 ).
EDSS according to the doses of stem cells
We pooled data of EDSS change from baseline to the last assessment time under a random-effects model, and the differences were nonsignificant for both low and high doses subgroups, (MD = − 0.31, 95% CI [− 1, 0.38], p = 0.37) and (MD = − 0.57, 95% CI [− 1.94, 0.8], p = 0.41), respectively. The studies of both subgroups showed significant heterogeneity (I 2 = 95%, p < 0.00001) for the low doses subgroup, and (I 2 = 89%, p = 0.0001) for the high doses subgroup (Table 2 and Supplementary Figure S3 ).
EDSS analysis with stem cells of adult and embryonic origin
Adopting a random-effects model, stem cells from embryonic as well as adult origin showed nonsignificant effect on EDSS ( p = 0.17, and p = 0.37, respectively), With significant heterogeneity among the studies (I 2 = 88%, p = 0.004), and (I 2 = 94%, p < 0.00001), respectively (Table 2 and Supplementary Figure S4 ).
EDSS analysis according to the control group
We pooled data using a random-effects model. Five studies 31 , 32 , 33 , 36 , 39 , in which placebo was the control, showed substantial heterogeneity (I 2 = 63%, p = 0.03) and the difference between SCT and placebo was not significant (MD = − 0.09, 95% CI [− 0.46, 0.28], p = 0.62). Three studies 34 , 35 , 37 , in which the control was active treatment, showed significant reduction of EDSS with SCT compared to the active drugs (MD = − 1.21, 95% CI [− 1.98, − 0.43], p = 0.002) and the heterogeneity was significant (I 2 = 88%, p = 0.0002) (Table 2 and Supplementary Figure S5 ).
Number of relapses during 6 months of follow-up
Only two studies 32 , 34 reported the number of relapses in the 6 months following the intervention. Under a random-effects model, the heterogeneity was moderate ( p = 0.14, I 2 = 53%), and the decrease in relapses number was nonsignificant ( p = 0.23) (Supplementary Figure S6 ).
Timed-25 foot walk (T25-FW) change at 6 months
Four studies 31 , 32 , 34 , 37 assessed T25-FW in 154 and 136 patients in the SCT and control groups, respectively. We pooled data under a random-effect model, and heterogeneity was moderate (χ2 = 5.99, df = 3, p = 0.11, I 2 = 50%). SCT resulted in a nonsignificant improvement in patients’ T25-FW scores compared to the control group (MD = − 0.69, 95% CI [− 1.93, 0.56], p = 0.28), as shown in Fig. 4 .
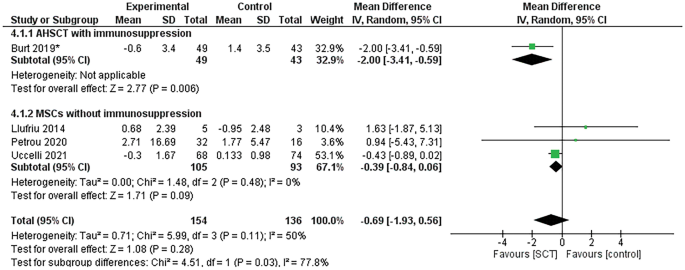
Forest plot of T25-FW change from baseline.
In the studies that included mesenchymal SCT without immunosuppression, the improvement in patients’ T25-FW scores after SCT was not significant (MD = − 0.39, 95% CI [− 0.84, 0.06], p = 0.09), but T25-FW significantly improved in the study that used AHSCT and immunosuppression 37 ( p = 0.006). Figure 4 demonstrates these analyses. The p value of the results didn’t change after the one-study-removed sensitivity analysis (Supplementary Figure L4 ).
Change in Nine-hole peg test (9-HPT) at 6 months
9-HPT was evaluated in four RCTs 31 , 32 , 34 , 37 . We used a random-effects model because heterogeneity was significant ( p = 0.0003, I 2 = 84%). 9-HPT showed nonsignificant improvement in the collective analysis and the sub-analysis of MSCs without immunosuppression. However, Burt et al. 37 . revealed a significant improvement ( p < 0.00001) (Supplementary Figure S7 ). The results remained nonsignificant after sensitivity analysis (Supplementary Figure L5 ).
Change of Paced auditory serial addition test (PASAT-3) score
We pooled PASAT-3 scores assessed at the end of treatment in four trials under a random-effects model 31 , 34 , 36 , 37 . Heterogeneity was minimal ( p = 0.35, I 2 = 9%), and the differences were nonsignificant in the collective analysis and the sub-analysis of autologous and mesenchymal SCT ( p = 0.35, p = 0.96, and p = 0.31, respectively) (Supplementary Figure S8 ). Effect estimate remained nonsignificant after one-study-removed sensitivity analysis (Supplementary Figure L6 ).
Change in the volume of MRI T2-weighted lesions
We analyzed the change in brain lesion volume from baseline to the end of the follow-up. Data were pooled under a random-effects model, heterogeneity was absent ( p = 0.38, I 2 = 0%). Our analysis revealed a significant reduction in T2 lesions volume (MD = − 7.05, 95% CI [− 10.69, − 3.4], p = 0.0002). In the studies that used MSCs without immunosuppression, the reduction of brain lesions volume was nonsignificant ( p = 0.1) (Fig. 5 a).
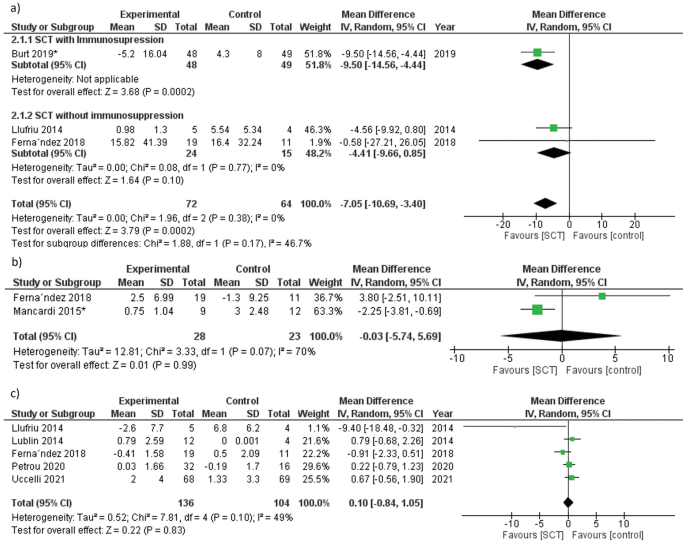
Forest plot of radiological outcomes change from baseline ( a ) MRI T2-weighted lesions volume at the end of treatment, ( b ) MRI T2-weighted lesions number at 12 months, ( c ) number of GELs at the end of treatment. *the study used immunosuppression before AHSCT.
The results became nonsignificant and changed to (MD = − 4.41, 95% CI [− 9.66, 0.85], p = 0.1) after a sensitivity analysis performed by excluding Burt et al. 37 (Supplementary Figure L7 and Table L1 ).
Change in the number of MRI T2-weighted lesions
Adopting a random-effects model, the studies showed substantial heterogeneity ( p = 0.07, I 2 = 70%). And the differences between SCT and the control after 12 months were nonsignificant ( p = 0.99) (Fig. 5 b).
Change in the number of gadolinium-enhancing lesions (GELs) at the end of the treatment
Five studies 31 , 32 , 33 , 34 , 36 assessed this outcome. Four studies reported the change of GELs number from baseline at 6 months except Ferna´ndez et al. 36 at 12 months. We pooled data under a random-effects model and heterogeneity was not significant (χ2 = 7.81, df = 4, p = 0.1, I 2 = 49%). Our analysis revealed nonsignificant differences in GELs number change ( p = 0.83) (Fig. 5 c). The results didn’t change after sensitivity analysis (Supplementary Figure L8 ).
Incidence of adverse events (AEs)
Seven studies 31 , 32 , 33 , 34 , 36 , 37 , 38 reported adverse events that occurred during the follow-up period. Two studies 35 , 39 didn’t provide data about AEs. Nabavi et al. mentioned only pain at the site of bone marrow aspiration 39 . Our analysis revealed that the difference was nonsignificant between SCT and the control group regarding the incidence of most AEs. Administration-related AEs, including infusion site swelling, hematoma, and pain, were significantly more common in the SCT group compared to the control (N = 25, RR = 2.55, 95% CI [1.08, 6.03], p = 0.034). On the other hand, the SCT group had a lower incidence of total infections (any infection during the follow-up period, including viral infections, respiratory, urinary infections, scabies, and other infestations) than the control group (N = 60, RR = 0.58, 95% CI [0.37, 0.9], p = 0.02). Regarding the use of immunosuppression, AHSCT combined with immunosuppression was significantly associated with a higher incidence of blood and lymphatic system disorders (N = 16, RR = 2.33, 95% CI [1.23, 4.39], p = 0.009). The analyses of the adverse events are shown in Table 3 and Supplementary Figures S9 – S14 . No transplant-related mortality was noted in all trials during the follow-up period, except for two unrelated deaths compacted by Ferna´ndez et al. in the placebo arm (one due to choking while feeding and the other due to respiratory infection) 36 .
Publication bias
We examined the publication bias among the studies that reported the effect of SCT on patients’ disability using the funnel plot test. Although there was funnel plot asymmetry, the test isn’t reliable because the included studies were less than ten studies 24 (Supplementary Figure S15 ).
Most of multiple sclerosis (MS) patients suffer from sensory, motor, and cognitive impairment. However, no curative treatment for this disease is available up till now. The approved disease modifying therapies (DMTs) for relapsing remittent MS showed effective short-term results, but the benefit for progressive subtypes is limited with fewer treatment options 40 . On the other hand, autologous hematopoietic stem cell transplantation (AHSCT) could achieve complete suppression of MS disease activity in 70–80% of patients for 4–5 years, which is superior to other MS therapeutic options 14 . AHSCT also showed promising results with aggressive MS and patients refractory to DMTs 41 . Also, AHSCT is more frequently used to treat aggressive MS than allogeneic hematopoietic SCT, because allogenic hematopoietic SCT is associated with a risk of graft-versus-host disease that increases patients morbidity and mortality rates 42 . On the other hand, various preclinical studies using animal models revealed that mesenchymal stem cells (MSCs) could ameliorate MS symptoms and delay disease progression 43 . In recent human clinical trials, MSCs improved MS symptoms and showed immunoregulatory and anti-inflammatory functions without the need for intense immunosuppression 18 .
To assess the effect of SCT on MS, we included nine RCTs in our meta-analysis with an overall population of 422 patients. Our results revealed that SCT was significantly superior to the control in improving patients EDSS at 2 months and reducing MRI-T2 weighted lesions volume. However, improvement in EDSS in other clinical outcomes was not significant. Regarding SCT safety, adverse events showed nonsignificant differences except for site reactions. However, patients who received SCT significantly experienced a lower incidence of total infections.
The primary outcome of our meta-analysis is EDSS, the most commonly used standardized and validated tool for disability progression 44 . Initially, we pooled EDSS changes from baseline to the last follow-up reported by the studies. Because it was reported that follow-up period variation represents a potential confounder of disability outcomes, we conducted a subgroup analysis based on the follow-up period 45 . Our results of these subgroups significantly confirmed the effect of SCT on delaying patients’ disability progression at 2 months and at 6 months (after the sensitivity analysis), agreeing with a previous meta-analysis on AHSCT 46 . Although EDSS change was not significant at 6 and 12 months, the sensitivity analysis showed a significant change in the effect size. EDSS at 6 months significantly favored SCT after excluding Nabavi et al., that revealed a non-remarkable difference between both arms 39 . Additionally, EDSS at 12 months significantly changed by excluding Fernández et al. 36 , that included only SPMS patients with longer disease duration and deteriorating patients disability affecting the analysis results. Previous literature found that SCT is more effective with RRMS than progressive MS and linked this to the lower baseline EDSS and early disease stage 10 , 46 , 47 . However, we performed a subgroup analysis based on baseline EDSS scores and detected nonsignificant effects on disability progression. The results of the other subgroup analyses regarding the source and the dose of stem cells supported the findings of a previous meta-analysis of preclinical trials of nonsignificant effects of these procedural aspects on disease progression 43 .
We assessed the effect of SCT on patients walking ability by the T25-FW test that is a reliable tool for the short and long-terms 26 . The analysis of T25-FW showed nonsignificant improvement in the collective analysis and in patients who received MSCs without immunosuppression. However, patients received AHSCT preceded by immunosuppression showed significant improvement. The results of T25-FW scores analysis is consistent with the EDSS analysis because both endpoints are affected by patients’ lower extremity disability. Regarding the radiological outcomes, the remission in MRI lesion volumes reflects the suppression of the inflammatory process in the brain and prevention of further disease progression, as explained by Genovese et al. 48 Also, the reduction of lesion volume is consistent with EDSS improvement according to a study that proved a positive correlation between MRI lesion volume and patients’ disability 49 . But after performing a sensitivity analysis by excluding Burt et al., the reduction in MRI-T2 lesion volume became nonsignificant indicating that Burt et al. may account for the significant improvement 37 .
The overall improvement in patients upper extremity function was nonsignificant, and none of the trials assessed this outcome showed a significant change in 9-HPT; however, Burt et al. 37 revealed a significant improvement in 9-HPT that can be justified by including only RRMS patients with low baseline EDSS compared to other trials. The presence of ceiling and floor effects in 9-HPT is a factor that may have affected this outcome. As reported, 9-HPT isn’t sensitive enough for detecting hand dexterity improvement in low or high disability patients 50 .
In terms of SCT safety, nonsignificant adverse events observed in the studies included headache and gastrointestinal disturbances. MS patients are known to suffer from a high incidence of infections, especially respiratory infections that may be complicated by death 51 . Surprisingly, in this meta-analysis SCT patients significantly experienced a lower incidence of total infections compared to their control, showing a protective effect against those infections. This can be explained by releasing antimicrobial substances from stem cells (particularly MSCs) such as beta-defensins, cathelicidin LL-37, and other peptides. Other functions of stem cells including, immunomodulatory, anti-inflammatory, and regenerative effects also contribute to fighting pathogens and combacting infections-related tissue damage 52 . Regarding transplant-related mortality, our results of no deaths in the follow-up period are similar to that reported by a previous meta-analysis that included 133 patients 53 . Treatment-related mortality of AHSCT in MS has dropped from 3.6% (in studies before 2005) to 0.3% (in studies since 2005), and this reduction in mortality was more evident in the younger population 14 . Additionally, allogeneic stem cells transplantation was associated with improved mortality rates in recent years in treating different autoimmune diseases 54 . Contrary to this evidence, a recent meta-analysis of AHSCT detected a higher transplant-related mortality of 4% in a 5 year follow-up duration 55 . However, other studies estimated the mortality rates during shorter post-transplant period, which indicates a need for standardizing the duration of assessing such an important endpoint. Another meta-analysis of AHSCT 56 revealed a significant association between patients’ mortality and both MS clinical subtypes and baseline EDSS. Lower mortality rates were observed in RRMS patients, and higher baseline EDSS were linked to higher mortality rates 56 .
Regarding the studies that included an immune ablative regimen before AHSCT, the reported results showed a significant improvement in EDSS, T25-FW, 9HPT, and lesions volume in Burt et al. 37 and a significant reduction in lesions number in Mancardi et al. 38 . Immunoablation followed by SCT has been considered in several autoimmune diseases to induce sustained remission. High-dose chemotherapy eradicates the autoreactive immunologic memory, and SCT following it would regain the immunologic self-tolerant state causing long-term remission of autoimmune diseases 57 . Future trials are needed to assess the long-term effects of immunoablation before SCT.
Finally, our meta-analysis provides an up-to-date valid conclusion on SCT efficacy and safety based on RCTs. We depended on a well-defined search strategy and criteria to include all eligible studies. We prepared this study following the PRISMA checklist and performed all the steps according to the Cochrane Handbook for Systematic Reviews of Interventions. We included cross-over trials to increase the sample size of the analysis to get credible results, and these studies were included until the cross-over point to avoid the carry-over effect in such trials. We analyzed all possible efficacy and safety outcomes to provide a comprehensive view of the role of SCT in MS.
Limitations
Our meta-analysis faced some limitations. First of all, we couldn’t provide a quantitative comparison between SCT and the approved DMTs for MS because few studies included DMTs as the control. And the number of studies was inadequate for conducting a comprehensive quantitative analysis of the efficacy of immunosuppression before SCT. Our study was also limited by the short follow-up periods (maximally 12 months) in the included trials, so the long-term effect of SCT is still questionable. Moreover, pooling the results of the cross-over studies up to the point of cross-over resulted in a further limitation in detecting long-term results.
Furthermore, heterogeneity was evident among the studies in most outcomes. This heterogeneity is attributed to the non-uniform patients’ characteristics and procedural parameters. Patients in the trials had variable disease duration, disease course, baseline clinical measures, and previous use of DMTs. Regarding the disease course, most studies included progressive and relapsing MS patients without reporting the outcomes of each subtype separately. This hindered us from performing a subgroup analysis depending on this variable, particularly in determining the effect of SCT on progressive MS that needs to be explored. Also, the studies showed variations in the transplantation procedure that could have contributed to this heterogeneity, including immunity suppression before the transplantation, the doses and sources of stem cells, and the routes of stem cell infusion. Particularly, the lack of RCTs investigating AHSCT combined with immunosuppression in MS limited comparing it to MSCs and generalizing our results. The previous use of DMTs varied among the studies’ population, also the washout periods of these DMTs before patients inclusion weren’t stated in some trials and were inadequate in other trials which may have impacted the results. Finally, assessing publication bias wasn’t reliable because the pooled studies were less than ten.
Recommendations
We recommend conducting future RCTs comparing SCT with the approved DMTs for more accurate and direct evidence. Also, comparing the transplantation of different sources of stem cells with and without immunosuppression is needed. Longer follow-up of RCTs will help to detect the long-term effect on disease progression and determine long-term safety concerns, particularly transplantation-related mortality. We also encourage RCTs to compare different routes of SCT, especially intrathecally, to determine the administration route that yields optimal results. Finally, detecting the effect of SCT on each MS clinical subtype separately is required to provide individualized treatment approaches.
This meta-analysis showed that SCT improves multiple sclerosis patients’ disability at 2 months and reduces their brain lesions volume. SCT was tolerable and safe, with no mortality during the follow-up period. Patients who received MSCs significantly experienced local adverse events at the site of infusion. And in the studies that used AHSCT and immunosuppression, SCT patients significantly suffered from blood and lymphatic system disorders. However, we cannot generalize our results due to the sparse number of RCTs assessing AHSCT combined with immunosuppression for MS. We recommend conducting further RCTs of longer durations without cross-over, on specific subtypes of MS, using immunosuppression before the transplantation, and comparing SCT with approved DMTs to support evidence-based management. Including Naïve patients not previously treated with other DMTs will guarantee pure assessment of SCT safety and efficacy.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Abbreviations
Stem cell transplantation
Mesenchymal stem cells
Disease modifying therapies
Randomized controlled trial
Relapsing-remitting multiple sclerosis
Secondary-progressive multiple sclerosis
Primary-progressive multiple sclerosis
Expanded disability status scale
Timed 25 Foot Walk
Nine-hole peg test
Paced auditory serial addition test
Magnetic resonance imaging
Gadolinium-enhancing lesions
Adverse events
Browne, P. et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 83 , 1022–1024 (2014).
Article PubMed PubMed Central Google Scholar
Garg, N. & Smith, T. W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 5 , e00362 (2015).
Lublin, F. D. et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 83 , 278–286 (2014).
Wingerchuk, D. M. & Carter, J. L. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 89 , 225–240 (2014).
Article PubMed Google Scholar
Piehl, F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J. Intern. Med. 289 , 771–791 (2021).
Article CAS PubMed Google Scholar
Gavriilaki, M., Sakellari, I., Gavriilaki, E., Kimiskidis, V. K. & Anagnostopoulos, A. Autologous hematopoietic cell transplantation in multiple sclerosis: Changing paradigms in the era of novel agents. Stem. Cells Int. 2019 , 1–9 (2019).
Article Google Scholar
Yang, J. H., Rempe, T., Whitmire, N., Dunn-Pirio, A. & Graves, J. S. Therapeutic advances in multiple sclerosis. Front. Neurol. 13 , 824926 (2022).
Gholamzad, M. et al. A comprehensive review on the treatment approaches of multiple sclerosis: Currently and in the future. Inflammation Res. 68 , 25–38 (2019).
Article CAS Google Scholar
Liu, Z., Liao, Q., Wen, H. & Zhang, Y. Disease modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis. Autoimmun. Rev. 20 , 102826 (2021).
Sharrack, B. et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: Updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Acc. Bone Marrow Transplant 55 , 283–306 (2020).
Zeng, L. et al. Efficacy and safety of mesenchymal stem cell transplantation in the treatment of autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, multiple sclerosis, and ankylosing spondylitis): A systematic review and meta-analysis of randomized controlled trial. Stem Cells Int. 2022 , 1–20 (2022).
Fassas, A. et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: First results of a pilot study. Bone Marrow Transplant 20 , 631–638 (1997).
Snowden, J. A. et al. Haematopoietic SCT in severe autoimmune diseases: Updated guidelines of the European group for blood and marrow transplantation. Bone Marrow Transplant 47 , 770–790 (2012).
Muraro, P. A. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 13 , 391–405 (2017).
Karussis, D. & Kassis, I. The potential use of stem cells in multiple sclerosis: An overview of the preclinical experience. Clin. Neurol. Neurosurg. 110 , 889–896 (2008).
Muraro, P. A. et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201 , 805–816 (2005).
Article CAS PubMed PubMed Central Google Scholar
Markov, A. et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 12 , 192 (2021).
Rahim, F. & Arjmand, B. Stem cell clinical trials for multiple sclerosis: The past, present and future. Neurol. Regen. https://doi.org/10.1007/978-3-319-33720-3_9 (2017).
Oliveira, A. G., Gonçalves, M., Ferreira, H. & Neves, N. Growing evidence supporting the use of mesenchymal stem cell therapies in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 38 , 101860 (2020).
Genc, B., Bozan, H. R., Genc, S. & Genc, K. Stem cell therapy for multiple sclerosis. Tissue Eng. Regen. Med. https://doi.org/10.1007/5584_2018_247 (2018).
Yousefi, F., Lavi Arab, F., Saeidi, K., Amiri, H. & Mahmoudi, M. Various strategies to improve efficacy of stem cell transplantation in multiple sclerosis: Focus on mesenchymal stem cells and neuroprotection. J. Neuroimmunol. 328 , 20–34 (2019).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ 372 , n71 (2021).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) 343 , d5928–d5928 (2011).
PubMed Google Scholar
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315 , 629–634 (1997).
Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33 , 1444–1452 (1983).
Motl, R. W. et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Multiple Sclerosis 23 , 704–710 (2017).
Feys, P. et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Multiple Sclerosis 23 , 711–720 (2017).
Tombaugh, T. N. A comprehensive review of the paced auditory serial addition test (PASAT). Archiv. Clin. Neuropsychol. 21 , 53–76 (2006).
Higgins, J. P. T. et al. Cochrane handbook for systematic reviews of interventions (Wiley, Hoboken, 2019). https://doi.org/10.1002/9781119536604 .
Book Google Scholar
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 , 135 (2014).
Uccelli, A. et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): a phase 2, randomised, double-blind crossover trial. Lancet Neurol. 20 , 917–929 (2021).
Petrou, P. et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 143 , 3574–3588 (2020).
Lublin, F. D. et al. Human placenta-derived cells(pda-001) for the treatment of adults with multiple sclerosis: Arandomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 3 , 696–704 (2014).
Llufriu, S. et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One 9 , 1–15 (2014).
Li, J.-F. et al. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant 23 , 113–122 (2014).
Fernández, O. et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One 13 , 1–14 (2018).
Burt, R. K. et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: A randomized clinical trial. JAMA J. Am. Med. Ass. 321 , 165–174 (2019).
Mancardi, G. L. et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurology 84 , 981–988 (2015).
Nabavi, S. M. et al. Intravenous transplantation of bone marrow-derived mesenchymal stromal cells in patients with multiple sclerosis, a phase I/IIa, double blind, randomized controlled study. Mult. Scler. Relat .Disord. 78 , 104895 (2023).
Freedman, M. S. et al. Treatment optimization in multiple sclerosis: canadian ms working group recommendations. Can. J. Neurol. Sci. 47 , 437–455 (2020).
Rush, C. A., Maclean, H. J. & Freedman, M. S. Aggressive multiple sclerosis: Proposed definition and treatment algorithm. Nat. Rev. Neurol. 11 , 379–389 (2015).
Rush, C. A., Atkins, H. L. & Freedman, M. S. Autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Cold Spring Harb. Perspect. Med. 9 , a029082 (2019).
Yanwu, Y., Meiling, G., Yunxia, Z., Qiukui, H. & Birong, D. Mesenchymal stem cells in experimental autoimmune encephalomyelitis model of multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 44 , 102200 (2020).
Meyer-Moock, S., Feng, Y. S., Maeurer, M., Dippel, F. W. & Kohlmann, T. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 14 , 58 (2014).
Kalincik, T. et al. Defining reliable disability outcomes in multiple sclerosis. Brain 138 , 3287–3298 (2015).
Ge, F., Lin, H., Li, Z. & Chang, T. Efficacy and safety of autologous hematopoietic stem-cell transplantation in multiple sclerosis: A systematic review and meta-analysis. Neurol. Sci. 40 , 479–487 (2019).
Casanova, B. et al. Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: Comparison with secondary progressive multiple sclerosis. Neurol. Sci. 38 , 1213–1221 (2017).
Genovese, A. V. et al. Atrophied brain T2 lesion volume at MRI is associated with disability progression and conversion to secondary progressive multiple sclerosis. Radiology 293 , 424–433 (2019).
Kearney, H. et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Multiple Sclerosis 20 , 72–80 (2014).
Solaro, C. et al. Clinical correlates of 9-hole peg test in a large population of people with multiple sclerosis. Mult. Scler. Relat. Disord. 30 , 1–8 (2019).
Castelo-Branco, A. et al. Infections in patients with multiple sclerosis: A national cohort study in Sweden. Mult. Scler. Relat. Disord. 45 , 102420 (2020).
Ahmed Al-Anazi, K., K. Al-Anazi, W. & M. Al-Jasser, A. The Rising Role of Mesenchymal Stem Cells in the Treatment of Various Infectious Complications. Update on Mesenchymal and Induced Pluripotent Stem Cells (2020). https://doi.org/10.5772/intechopen.91475 .
Zhou, Y. et al. Autologous mesenchymal stem cell transplantation in multiple sclerosis: A meta-analysis. Stem. Cells Int. 2019 , 1–11 (2019).
Greco, R. et al. Allogeneic HSCT for autoimmune diseases: A retrospective study from the EBMT ADWP, IEWP, and PDWP working parties. Front. Immunol. 10 , 5170 (2019).
Nabizadeh, F. et al. Autologous hematopoietic stem-cell transplantation in multiple sclerosis: A systematic review and meta-analysis. Neurol. Ther. 11 , 1553–1569 (2022).
Sormani, M. P. et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurology 88 , 2115–2122 (2017).
Alexander, T., Arnold, R., Hiepe, F. & Radbruch, A. Resetting the immune system with immunoablation and autologous haematopoietic stem cell transplantation in autoimmune diseases. Clin. Exp. Rheumatol. 34 , 53–57 (2016).
Download references
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Faculty of Medicine, Zagazig University, Zagazig, Egypt
Asmaa Ahmed Nawar, Aml Mostafa Farid & Moamen Mostafa Asla
Faculty of Dentistry, Suez Canal University, Ismailia, Egypt
Bioinformatics Group, Centre for Informatics Science, School of Information Technology and Computer Science, Nile University, Giza, Egypt
Engy K. Tharwat
Biotechnology Department, Faculty of Science, Cairo University, Giza, Egypt
Ahmed Sameh & Yomna Elkaramany
Neurology Department, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
Walaa A. Kamel
You can also search for this author in PubMed Google Scholar
Contributions
A.A.N., W.A.K and M.M.A. participated in the conception of the study and critically revised the research article. A.A.N. and M.M.A. performed the statistical analyses. A.M.F, R.W., E.K.T., A.S., Y.E., and A.A.N. contributed to the data acquisition and manuscript writing. All the authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Asmaa Ahmed Nawar .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Nawar, A.A., Farid, A.M., Wally, R. et al. Efficacy and safety of stem cell transplantation for multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 14 , 12545 (2024). https://doi.org/10.1038/s41598-024-62726-4
Download citation
Received : 01 October 2023
Accepted : 21 May 2024
Published : 31 May 2024
DOI : https://doi.org/10.1038/s41598-024-62726-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
A peer-reviewed journal that publishes research articles on stem cell therapeutics and regenerative therapies, including clinical trials and ethical aspects. Find out the latest news, submission guidelines, thematic series and featured articles on the home page.
Stem cell therapy is a novel therapeutic approach that utilizes the unique properties of stem cells, including self-renewal and differentiation, to regenerate damaged cells and tissues in the ...
Stem cell-based therapies. Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies ().Stem cells offer the perfect solution when there is a need for tissue and organ ...
The term 'stem cells' encompasses various cells with self-renewal and differentiation properties, many of which can potentially be used therapeutically 1. Multipotent somatic stem cells, such ...
1 January 2024. Read latest issue. More opportunities to publish your research: Read the latest articles of Current Stem Cell Research & Therapy at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Interview with Dr. Helen Blau on stem cells in the treatment of disease. 9m 12s Download. The derivation of induced pluripotent stem cells (iPSCs) has revolutionized stem-cell research (see the ...
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
The Role of Various Factors in Neural Differentiation of Human Umbilical Cord Mesenchymal Stem Cells with a Special Focus on the Physical Stimulants, Current Stem Cell Research & Therapy, 19, 2 ...
Stem-cell therapies articles from across Nature Portfolio. Atom; RSS Feed; Definition. Stem cell therapies are a type of cell therapy in which the cells used are either stem cells (as in the case ...
As basic stem cell research has soared over the past few years, "translation research", a relatively new field of research, has recently greatly developed, making use of basic research results to develop new treatments. ... In autologous stem cell therapy, the stem cells are collected from the patient's own body. Culture-expanded ...
Current Stem Cell Research & Therapy. Impact Factor: 2.7. Indexed in: Scopus, SCI Expanded, MEDLINE/PubMed... View all. Volume 19 , Issues 11, 2024. Request Failed! Current Stem Cell Research & Therapy publishes reviews, research articles, drug clinical trial on all aspects of basic research on stem cells and their uses in clinical therapy.
Stem cell therapy is a novel therapeutic approach that utilizes the unique properties of stem cells, including self-renewal and differentiation, to regenerate damaged cells and tissues in the human body or replace these cells with new, healthy and fully functional cells by delivering exogenous cells into a patient. 7 Stem cells for cell-based ...
Scientists are researching how to use stem cells to regenerate or treat the human body. The list of conditions that stem cell therapy could help treat may be endless. Among other things, it could ...
Stem cell therapy is a type of regenerative medicine used to treat and study disease. It is used in cancer treatment and to reduce the risk of infection. Researchers are looking for other ways to use stem cells in medical therapies. This article will cover stem cells, which conditions they treat, and their safety.
Doug Melton and David Scadden, founding co-directors of the Harvard Stem Cell Institute. HSCI has been breaking down barriers to collaboration in stem cell science since 2004. We provide fertile ground for more than 350 research faculty and their labs, across the university's schools, centers, teaching hospitals, and partner companies, to ...
In 2023, the FDA approved a similar cell therapy treatment by a Chicago-based startup for type 1 diabetes. The Chinese researchers say more research is needed to confirm the long-term efficacy and ...
Stem cell therapy to treat such diseases often has disappointing results because the transplanted cells die off before they can take effect. In fact, more than 95% of neural progenitor cells (NPCs) transplanted into individuals with a spinal cord injury die following injection. ... Up until now, scientists have essentially put the stem cells ...
Stem cells have two important and unique characteristics: First, they are unspecialized and capable of renewing themselves through cell division. When a stem cell divides, each new cell has the potential either to remain a stem cell or to differentiate into other kinds of cells that form the body's tissues and organs.
Stem-cell research articles from across Nature Portfolio. Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells ...
Nara Institute of Science and Technology. "Tackling the hurdle of tumor formation in stem cell therapies." ScienceDaily. ScienceDaily, 31 May 2024. <www.sciencedaily.com / releases / 2024 / 05 ...
Stem Cell Research & Therapy aims to be the major forum for translational research into stem cell therapies. The journal has a special emphasis on basic, translational, and clinical research into stem cell therapeutics, including animal models, and clinical trials. Stem cell research for therapeutic purposes has largely used adult stem cell ...
The findings could help make blood stem cell transplants available to more people and improve the accessibility and safety of gene therapies that use these cells. UCLA scientists have identified a protein that plays a critical role in regulating human blood stem cell self-renewal by helping them sense and interpret signals from their environment.
DOI: 10.1038/s41467-024-47680-z. NDORMS researchers have identified a critical axis that controls the formation and behavior of cancer stem cells (CSCs), a subpopulation of cells that influence ...
The trial, funded by biotechnology company Medeor Therapeutics, is centered around MDR-101, a stem cell therapy that is derived from the person donating the organ. The findings have not yet been ...
Multiple sclerosis (MS) is a common autoimmune neurological disease affecting patients' motor, sensory, and visual performance. Stem Cell Transplantation (SCT) is a medical intervention where a ...