- Open access
- Published: 02 September 2017

A systematic literature review of health state utility values in head and neck cancer
- Michela Meregaglia ORCID: orcid.org/0000-0003-0092-5970 1 , 2 &
- John Cairns 1 , 3
Health and Quality of Life Outcomes volume 15 , Article number: 174 ( 2017 ) Cite this article
5881 Accesses
32 Citations
1 Altmetric
Metrics details
Health state utility values (HSUVs) are essential parameters in model-based economic evaluations. This study systematically identifies HSUVs in head and neck cancer and provides guidance for selecting them from a growing body of health-related quality of life studies.
We systematically reviewed the published literature by searching PubMed, EMBASE and The Cochrane Library using a pre-defined combination of keywords. The Tufts Cost-Effectiveness Analysis Registry and the School of Health and Related Research Health Utilities Database (ScHARRHUD) specifically containing health utilities were also queried, in addition to the Health Economics Research Centre database of mapping studies. Studies were considered for inclusion if reporting original HSUVs assessed using established techniques. The characteristics of each study including country, design, sample size, cancer subsite addressed and demographics of responders were summarized narratively using a data extraction form. Quality scoring and critical appraisal of the included studies were performed based on published recommendations.
Of a total 1048 records identified by the search, 28 studies qualified for data extraction and 346 unique HSUVs were retrieved from them. HSUVs were estimated using direct methods (e.g. standard gamble; n = 10 studies), multi-attribute utility instruments (MAUIs; n = 13) and mapping techniques ( n = 3); two studies adopted both direct and indirect approaches. Within the MAUIs, the EuroQol 5-dimension questionnaire (EQ-5D) was the most frequently used ( n = 11), followed by the Health Utility Index Mark 3 (HUI3; n = 2), the 15D ( n = 2) and the Short Form-Six Dimension (SF-6D; n = 1). Different methods and types of responders (i.e. patients, healthy subjects, clinical experts) influenced the magnitude of HSUVs for comparable health states. Only one mapping study developed an original algorithm using head and neck cancer data. The identified studies were considered of intermediate quality.
This review provides a dataset of HSUVs systematically retrieved from published studies in head and neck cancer. There is currently a lack of research for some disease phases including recurrent and metastatic cancer, and treatment-related complications. In selecting HSUVs for cost-effectiveness modeling purposes, preference should be given to EQ-5D utility values; however, mapping to EQ-5D is a potentially valuable technique that should be further developed in this cancer population.
Cost-utility models are increasingly used to establish whether the cost of a new treatment is justified in terms of health gains. This approach usually adopts the quality-adjusted life year (QALY) as a measure of health effectiveness. According to Neumann et al., the QALY corresponds to the time spent in a series of quality-weighted health states , where the weights represent the desirability of living in that state [ 1 ]. The basic idea is that individuals move through health states over time and that each health state has a preference weight attached to it [ 2 ], also known as a health state utility value (HSUV). Thus, the HSUV can be interpreted as the strength of preference for a given health state on a cardinal scale anchored at 0 (‘death’) and 1 (‘full health’), with some instruments also allowing for negative values representing states worse than death [ 3 ]. Therefore, QALYs are obtained by summing-up the products of the time spent in each health state and its corresponding preference-based value [ 4 ].
HSUVs can be estimated in a variety of ways including direct methods, multi-attribute utility instruments (MAUIs), mapping functions and expert opinion. The most common ways of eliciting HSUVs directly are gambling with respect to a hypothetical treatment that may result in perfect health or death (standard gamble, SG) or trading-off part of future life for a shorter time in perfect health (time trade-off, TTO) [ 5 ]. A further, simpler option is to use a visual analog scale (VAS), also known as rating scale, which provides an immediate valuation of the current (or a hypothetical) health state on a graduated scale, usually ranging between 0 and 100. This technique is generally considered to be methodologically inferior to choice tasks such as SG and TTO, which incorporate some extra information about the individual risk attitude [ 4 ]; VAS scores, indeed, are elicited in a choice-less context, and thus do not required respondents to make trade-offs within their utility function [ 6 ]. Moreover, the rating scales are well-known to present measurement biases such as context bias, spacing-out bias, and end-aversion bias [ 4 , 7 ]. Additionally, there is now consensus that health-related quality of life (HRQoL) is a multi-dimensional concept, which includes domains related to physical, mental, emotional, and social functioning that are difficult to measure on a single scale [ 8 ].
Direct measurement of health utility through SG or TTO can be complicated and time-consuming and lead to incomparable results across the studies due to arbitrary health state descriptions (also called ‘vignettes’) [ 9 , 10 ]. Consequently, in recent years, HSUVs have been increasingly estimated indirectly using multi-attribute utility instruments (MAUIs). These tools are formed of a generic HRQoL questionnaire and an accompanying formula or set of weights (or “tariffs”) elicited from a sample of the general population for converting responses into HSUVs; thus, the utility measure can be considered as a preference-based evaluation of a given health state described by the dimensions of the tool [ 11 , 12 ]. The National Institute for Health and Care Excellence (NICE) and the European Network for Health Technology Assessment (EUnetHTA) recommend the EuroQol 5-dimension questionnaire (EQ-5D) ( https://euroqol.org ) [ 13 , 14 ]. Accordingly, the TTO with a 10-year time horizon is the most frequently used approach among the direct techniques, because of greater comparability with the method used to develop the EQ-5D scoring algorithm [ 15 ]. The other generic MAUIs mostly adopted in the literature [ 11 ] are the Health Utility Index (HUI mark 2, HUI2 or mark 3, HUI3) [ 16 ], the Short Form-6-dimension (SF-6D) questionnaire derived from the 36-item Short Form Survey (SF-36) ( https://www.sheffield.ac.uk/scharr/sections/heds/mvh/sf-6d ), the 15D ( www.15d-instrument.net ), the Quality of Wellbeing (QWB) index [ 17 ] and the Assessment of Quality of Life (AQoL) instruments [ 18 ].
In many situations, clinical studies neither administer preference-based MAUIs nor elicit HSUVs directly, but collect instead disease-specific HRQoL data or other clinical measures that are not associated with a preference-based scoring system; thus, QALY calculation from these studies is not possible. As a second-best solution, “mapping” or “cross-walking” has been developed to predict HSUVs from non-preference-based scores, provided that a statistical relationship can be established between the two instruments and, sometimes, allowing for the mediating effect of demographic and clinical characteristics [ 19 ].
This study focuses on HSUVs in head and neck cancer (HNC). Patients with HNC often undergo several rounds of treatment during which they experience acute toxicity and other side effects, such as loss of verbal abilities, difficulties in swallowing, and considerable pain [ 20 ]. This HRQoL impairment may continue long after treatment through persistent functional deficits, physical disfigurement, psychological distress, and recurrent disease. There is an extensive HRQoL literature in HNC, although mainly comprised of disease-specific, non-preference based data unsuitable for cost-utility comparisons. Due to the paucity of HSUVs for some health states in HNC, some previous cost-effectiveness analyses [ 21 , 22 ] relied on values calculated for other cancers (such as breast or lung) to populate their models with utility parameters. A systematic review published in 2006 [ 23 ] identified eight studies providing utility values in HNC elicited through VAS, TTO or SG. Our study extends the collection of utility values related to this medical condition by systematically reviewing the studies published to date. This paper considers for inclusion studies of any design in which utility values in HNC were:
directly elicited using standard techniques such as TTO or SG either in patient-based studies or in the general population;
calculated indirectly from patient’s responses to generic MAUIs (e.g. EQ-5D) through a set of tool- and country-specific preference weights;
predicted from non-preference based HRQoL instruments using mapping algorithms.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 24 ] is not entirely applicable to systematic reviews of HSUVs [ 25 ], since the standard Population, Intervention, Comparator, and Outcome (PICO) elements do not provide a useful framework for identifying utility values for health states that are not necessarily attached to a given intervention [ 26 ]. Thus, in this study we follow the recommendations provided by Papaioannou et al. [ 26 ]. The ultimate objective is to generate a database of HSUVs that might be useful to populate future cost-utility studies of interventions in HNC. In addition, we critically appraise the included studies by highlighting a few elements that should be considered when selecting utility parameters for modeling.
A systematic literature search was carried out of the PubMed, EMBASE and Cochrane Library databases for studies published from 2000 until the end of 2016 using a range of free-text terms in title/abstract (Fig. 1 ). Since Medical Subject Headings (MeSH) terms provide little coverage of HSUVs [ 25 , 26 ], we identified a few relevant free-text terms by referring to the published recommendations [ 26 ] and recent analogous systematic reviews [ 25 , 27 , 28 ]. Tool- (e.g. EQ-5D) and method-specific (e.g. SG) terms were combined with vocabulary related to HNC including the most frequent cancer sites; in using free-text terms, we considered that some instruments may be referred to or spelled in different ways. We did not explicitly included VAS among the keywords, due to the above-mentioned limitations in using this tool for measuring utility. Other search strings were used to identify cost-effectiveness and cost-utility studies using HSUVs to calculate QALYs. We searched directly utility weights in the Tufts Cost-Effectiveness Analysis (CEA) Registry [ 29 ] and the University Sheffield School of Health and Related Research Health Utilities Database (ScHARRHUD) [ 30 ]. An additional search was carried out of the Health Economics Research Centre (HERC) database [ 31 ] to retrieve mapping studies deriving utility values from non-preference based instruments in HNC. We selected the relevant databases based on previous recommendations [ 26 ] and systematic reviews on the topic [ 32 ]. Web searches of grey literature were not performed to avoid obtaining contents which are frequently subject to changes and cannot be identified in a systematic manner.
Free-text terms for electronic database searching
All search results were extracted in an Excel spreadsheet and duplicates removed. Titles and abstracts were screened by two independent reviewers and records excluded if not meeting the inclusion criteria; full-text papers were retrieved in case of doubtful results. Articles estimating HNC utility values using established methods were included; studies using the VAS instrument were not considered for inclusion, unless alongside other valuation techniques. This choice is consistent with the suggestion that VAS should be used as an introductory task but not as a definitive method to elicit utility values alone [ 33 ]. The included studies had to be published as full-text with no time or language restrictions; conference abstracts, editorials, and reviews were not suitable for inclusion. Studies were excluded if they did not report original utility values in HNC; however, the bibliography of studies referring to secondary sources for HSUVs was checked to avoid missing any relevant publications. The authors resolved any disagreements by discussion until consensus was reached.
The characteristics of the included studies were extracted by the first reviewer (MM) using a form developed following previous studies [ 25 , 26 , 27 ], and subsequently crosschecked by the other author (JC). Information collected included: study country, study design, sample size, valuation technique, administration method, cancer subsite addressed, and clinical and demographic characteristics of respondents. For each HSUV, we recorded the number of respondents, the point estimate (i.e. mean or median) and its measure of variance (e.g. standard deviation); the same information was collected for each study subgroup (or time point) whenever applicable.
Although there are no agreed reporting standards for HSUVs studies, the methodological quality of each included study was evaluated through a set of generic criteria as reported by the guidelines from Papaioannou et al. [ 26 ]. Thereafter, one point was awarded to each of the following criteria: (1) sample size ≥100; (2) description of respondent selection and recruitment; (3) description of inclusion/exclusion criteria; (4) response rate ≥ 60% [ 34 ]; (5) reporting of the amount and reasons of loss to follow-up (only for longitudinal studies); (6) reporting of missing data pattern and methods to deal with it; (7) appropriateness of measure (based on the authors’ judgment). Lastly, the scores were summed for each article to yield an overall quality score, ranging from 0 to 7 where higher scores indicated higher quality [ 35 ]. Any other problems arising from the studies (criterion 8) were narratively discussed. Additionally, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) recently published a set of recommendations for mapping studies [ 19 ] that were used to evaluate the quality of mapping studies retrieved by the systematic search.
Study selection
The PRISMA diagram [ 24 ] for this literature search is presented in Fig. 2 . In total, the search strategy identified 1048 articles: 1046 were retrieved by searching the online databases (PubMed; EMBASE; The Cochrane Library; CEA Registry; HERC database), and two by manually searching the bibliography of model-based economic evaluations retrieved from the online search. No articles were obtained from the ScHARRHUD database. After removing 743 duplicates, 305 records were scanned for title/abstract and 221 were excluded in this first phase for a variety of reasons reported in the chart. Subsequently, 84 full-text articles were retrieved and a further 56 records were excluded for not complying with the inclusion/exclusion criteria. Accordingly, 28 studies were definitively included in the review.
PRISMA flow chart
Study characteristics
The 28 journal articles included in the review are categorized into three groups: studies using direct elicitation methods ( n = 10), studies administering MAUIs ( n = 13) and studies deriving HSUVs using mapping ( n = 3); two studies [ 36 , 37 ] adopted both direct methods and MAUIs. The characteristics of the 25 studies using direct and indirect techniques (i.e. MAUIs) are listed in Table 1 , while the three mapping studies are separately described in Table 2 .
Studies using direct or indirect methods
Among the studies using direct elicitation techniques, SG alone was adopted in two cases [ 20 , 38 ] and TTO alone in five [ 39 , 40 , 41 , 42 , 43 ]. In four studies [ 37 , 44 , 45 , 46 ], more than one direct methodology (i.e. SG, TTO, VAS) was adopted to derive utility values. The study by Noel et al. [ 37 ] compared these direct techniques with MAUIs (i.e. EQ-5D, HUI3), while a further study [ 36 ] used both TTO and EQ-5D instruments.
In studies administering MAUIs, EQ-5D was the most common ( n = 11); five of these studies [ 36 , 37 , 47 , 48 , 49 ] did not report which scoring algorithm was used, two studies [ 50 , 51 ] explicitly adopted the UK algorithm, another two [ 52 , 53 ] adopted the US one, one study [ 54 ] used the Dutch tariff and another one [ 55 ] the Belgian one. Moreover, nine of the studies using EQ-5D [ 36 , 47 , 48 , 50 , 51 , 52 , 53 , 54 , 55 ] explicitly referred to the 3-level version (EQ-5D-3 L) and one [ 37 ] to the newer 5-level one (EQ-5D-5 L); one study [ 49 ] did not specify the instrument’s version adopted. Additional generic, preference-based HRQoL tools retrieved by our search were 15D ( n = 2), HUI3 ( n = 2) and SF-6D ( n = 1); no studies used the QWB scale or the AQoL-8D utility instrument.
The 25 articles reported on HNC utility-related studies conducted in several European (Belgium, n = 1; Finland, n = 2; Italy, n = 2; Netherlands, n = 3 Spain; n = 1 United Kingdom, n = 2) and non-European countries (Australia, n = 1; Canada, n = 5 Morocco, n = 1; United States, n = 7). The great majority of the HSUVs came from cross-sectional surveys ( n = 18); the remaining articles ( n = 7) adopted a longitudinal design, including five prospective cohort studies [ 45 , 49 , 55 , 56 , 57 ] and two clinical trials [ 47 , 53 ].
Sample sizes varied widely from 3 [ 43 ] to 818 [ 53 ], with a mean of 152 respondents per study. The response rate was between 49% [ 42 ] and 95% [ 20 ]. In most of the studies ( n = 18), the participants were HNC patients at various stages of disease and treatment pathway; in two studies [ 20 , 38 ] healthy individuals from the general population were surveyed through the SG techniques, while in one case [ 43 ] the utility assessment was based on a consultation with a panel of experienced physicians. The remaining four studies [ 39 , 41 , 44 , 46 ] retrieved utility measures from multiple subjects (i.e. healthy people, clinical experts, HNC patients and patients with other medical conditions) and reported HSUVs from each group separately.
In studies recruiting HNC patients, most were male and the mean age was always above 55. Conversely, responders were generally younger and with a higher proportion of females in studies surveying individuals from the general population or clinical experts. The range of cancer subsites addressed by each study was quite broad: ten studies [ 36 , 37 , 40 , 41 , 47 , 48 , 51 , 52 , 55 , 56 ] generally investigated utility in HNC without specifying any cancer site, six [ 39 , 42 , 45 , 46 , 49 , 58 ] were related to laryngeal cancer, two [ 38 , 44 ] addressed cancer in the oropharynx, one [ 54 ] recruited patients affected by cancer in the oral cavity and the remaining six [ 20 , 43 , 50 , 53 , 57 , 59 ] focused on selected multiple sites (e.g. oropharynx, hypopharynx, and larynx).
The most common way ( n = 12 [ 41 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ]) of collecting utility data was by self-completion of a written survey (administered on site or by post/e-mail), followed by face-to-face interviews ( n = 6 [ 37 , 39 , 42 , 44 , 45 , 46 ]); four studies adopted different administration options including group session ( n = 1 [ 38 ]), telephone or mail interview ( n = 1 [ 59 ]), interview using a script/prop ( n = 1 [ 20 ]), and computer-guided data collection ( n = 1 [ 36 ]). The administration method was not specified in three cases [ 40 , 43 , 47 ]. When HSUVs were obtained from the patients, the survey (or the interview) was usually scheduled during a clinical appointment or a hospital admission; in longitudinal studies [ 55 , 57 ], surveys after the first were frequently delivered by post to the patient’s home address.
Mapping studies
The three studies deriving HSUVs in HNC using a mapping technique are described in Table 2 . Among them, only one [ 60 ] developed an original mapping algorithm using responses from HNC patients and was retrieved from the HERC database. Ordinary Least Square (OLS) regression was applied to establish a statistical relationship between the University of Washington Quality of Life questionnaire version 4 (UW QOL v4) and the EQ-5D-3 L using a dataset of 89 patients treated for HNC. Thereafter, the responses of an additional 48 patients enrolled in the study were used as a validation database.
The other two studies [ 61 , 62 ] were model-based economic evaluations reporting HSUVs for several HNC-related health states by applying previously published algorithms [ 63 , 64 ] to HRQoL data retrieved from the literature [ 65 , 66 ].
Overview of HSUVs
A total of 346 original HSUVs were retrieved from 27 studies included in the review (Additional file 1 : Table S1), since one study [ 41 ] reported results only graphically in the article. The studies [ 37 , 44 , 46 ] providing the highest number of HSUVs (i.e. over 40) either adopted multiple techniques or interviewed several groups of respondents that yielded different values for each health state. In other cases [ 45 , 49 , 53 , 55 , 56 , 57 , 62 ], different HSUVs have been collected by the same participants over the study time points. HSUVs were reported as means in the great majority of studies ( n = 25), of which four [ 20 , 38 , 51 , 53 ] also reported the median; the remaining two studies [ 47 , 55 ] calculated a median value only. Among the measures of variance, standard deviation was the most frequently adopted ( n = 12), followed by the min-max range ( n = 7), and the interquartile range ( n = 5); several studies reported more than one measure type. In some cases [ 39 , 43 , 46 , 58 , 62 ], no measures of variability were reported, thus limiting the usefulness of the utility data.
Study quality assessment
The quality assessment of the 25 studies using direct or indirect methods was based upon eight criteria, of which seven were given a score (Table 3 ). In all studies, the instrument adopted to estimate HSUVs was considered appropriate in relation with the participants enrolled. Additionally, most studies (84%) reported a description of the participants recruitment process, whilst only 56% of them clearly stated the inclusion/exclusion criteria. Information on missing data and techniques to deal with them were reported by a limited number of studies (24%). In half of the studies, the sample size was rather small (<100) and response rate was either low (<60%) or not reported. In reviewing these studies, we highlighted a few additional issues (criterion 8 [ 26 ]) that should be considered when selecting sources to populate health economic models with utility parameters. First, some of the included studies are quite dated (published before 2000), thus describing health states that might not be realistic nowadays because of emerging treatment modalities, improvements in treatment-related morbidity and organ preservation techniques. Second, there might be potential sources of bias in reporting HRQoL results in clinical studies investigating one or more interventions, although the number of comparative trials retrieved by our search was very limited. Third, in some studies [ 36 , 48 , 59 , 60 ], and especially those analyzing HRQoL in multiple cancers including head and neck [ 36 , 59 ], patient’s characteristics (e.g. cancer stage/site, treatment phase) are poorly reported, thus making it difficult to match the study’s HSUVs with the health states described in a cost-effectiveness model. Lastly, the great majority of studies are cross-sectional surveys, representing the quickest and cheapest method for gathering HRQoL data; however, longitudinal data collections are often more valuable since they facilitate capture of changes in utility values as cancer progresses through different phases.
With reference to mapping, we retrieved only one original algorithm [ 60 ] through the search, thus preventing a comparative evaluation of studies. This study presented a four-variable model to predict EQ-5D-3 L utilities using OLS regression; coefficient values and error terms were clearly reported and box-plot distributions of actual and predicted utilities provided. However, the authors did not justify the model choice in relation to the observed EQ-5D distribution, nor any additional tests or judgments made. The goodness-of-fit was presented as R, 2 mean absolute error (MAE) and root mean squared error (RMSE), which are considered of limited value in the mapping field. No demographic or clinical variables were included as covariates, which was recognized as a study limitation by the same authors. Moreover, when the sample size is small (as it was in this study), the most recent guidelines do not recommend splitting it for empirical validation [ 19 ].
This study reviews systematically published studies reporting HSUVs in HNC. Compared to a previous review [ 23 ], many more studies have been identified, most of which use the EQ-5D and were published from 2011 onwards. Overall, this review shows that HNC patients suffer from substantial HRQoL impairment over the different disease phases. However, there is a lack of research into the HRQoL in the recurrent and/or metastatic health states, with only one study [ 47 ] reporting a median EQ-5D utility value (i.e. 0.7) from the patients, which is less useful for the purposes of economic evaluation that focuses on mean costs and effects. Another study [ 44 ] elicits values for a range of recurrent disease states from healthy subjects and clinical experts using SG and VAS and obtains extremely heterogeneous results across the types of participants and methods. The same paucity of HSUVs was observed for treatment-related complications, which are addressed by three studies [ 20 , 44 , 45 ] only, possibly because of the infrequency of some of these events that restricts the data from patients in that health state.
Differences in utilities were found across studies even in the pre-treatment state. The choice of baseline utility is particularly relevant because it affects the incremental gain achievable by different therapeutic options [ 13 ], thus potentially biasing the estimated cost-effectiveness. The two Finnish studies [ 56 , 57 ] using the 15D yielded higher utility values in patients shortly after diagnosis than those using the EQ-5D [ 53 , 54 ]. This phenomenon has previously been observed in studies addressing other medical conditions [ 32 , 67 , 68 ]. There are many possible explanations for these discrepancies: different number of dimensions, the EQ-5D has generally been valued using TTO rather than VAS [ 69 ], the preference weights have come from different populations (a Finnish value set is usually adopted for 15D) [ 67 ], and the EQ-5D, unlike the 15D, can take negative values [ 69 , 70 ]. The participants’ characteristics might have also affected study results. For example, a study [ 55 ] addressing HRQoL in patients aged ≥65 years with HNC consistently provided lower HSUVs than other studies in either the pre-treatment, treatment, and follow-up phases, probably because of comorbidities and functional impairments usually affecting elderly people independently from cancer. Moreover, the use of different scoring algorithms may have contributed to variation in HSUVs in studies administering the EQ-5D.
Heterogeneity in utility values was particularly evident in the studies applying more than one technique to evaluate the same health state. Among them, in a study reporting HSUVs for different treatments, treatment-related complications, and remission/recurrence states in oropharyngeal cancer [ 44 ], the values obtained using a VAS scale were consistently lower that for the SG. In the study by Marcellusi et al. [ 36 ], patients in follow-up after treatment for HNC reported lower utility values when performing the TTO task than when responding to the EQ-5D questionnaire. Another study [ 37 ] compared five different (direct and indirect) methods to retrieve HSUVs from patients experiencing a similar health state (i.e. three months after completion of treatments and no evidence of recurrent disease). Unlike Marcellusi et al. [ 36 ], the method yielding the highest utility value in the overall sample ( n = 100) was TTO (0.94), followed by SG (0.91), EQ-5D (0.82), VAS (0.76) and HUI3 (0.75). That VAS scores are consistently lower than SG scores is well-known in the literature; in 2001, Torrance et al. [ 33 ], after reviewing several studies, concluded that the relationship between the two instruments can be represented by a concave curve passing through 0 and 1. Moreover, the indirect methods involving MAUIs have been shown to yield systematically lower utility values than the direct ones in a wide range of diseases [ 71 ] for a variety of reasons. First, in MAUIs participants are not asked to consider their health status relative to death and thus, there is no disincentive in reporting more severe health problems [ 72 ]. Second, respondents are forced to describe their complex medical conditions through a limited number of attributes, thus ignoring any positive feelings that would boost utility values. Third, it is likely that the general population used to obtain tariffs for MAUIs make a different trade-off between a given health state and death because they tend to be younger and healthier. Finally, the vignettes described in direct valuation tasks are usually more detailed than the MAUI health states [ 71 ]. In studies comparing alternative MAUIs, EQ-5D has been shown to provide higher utilities values compared to HUI2 and HUI3, which in turn yield higher values than SF-6D. As for the differences between EQ-5D and 15D, potential explanations are likely to be found in descriptive systems, preference measurement, source of community preferences, and scoring methods [ 73 ].
In addition, studies can be classified by the type of responders who valued the health states, either patients or healthy subjects. In the literature, some argue that patients are best placed to value the relevant health states, while others advocate valuation by healthy people who will not directly benefit from a new treatment but, in tax-based systems, will bear its cost. The latter claim that this will provide an unbiased estimate of the hypothetical health states [ 7 , 74 ] and more consistency across appraisal of very different interventions. The review by Komatsuzaki et al. [ 23 ] showed that patients usually reported lower utilities than physicians and healthy people for health states associated to HNC. In this review, only a few studies recruited participants from the general population, thus limiting the number of utilities comparisons across different types of responders. One study [ 46 ] confirms the conclusions reached by the previous review [ 23 ], whilst others [ 41 , 44 ] found healthy subjects consistently providing lower utility estimates compared to patients and healthcare professionals.
This study facilitates the identification of HSUVs for use in future HNC economic evaluations. The number of retrieved studies was quite large, with almost 350 distinct HSUVs collected from them. Most of the utility values were collected during the treatment phase or shortly after the completion of treatment, whilst limited evidence is available for the health-related utility assessment in HNC recurrent and end-of-life states. Due to the variety of health state definitions and valuation techniques across the studies, we were not able to perform a quantitative synthesis of the results [ 3 ]. Moreover, unlike cost-effectiveness studies where structured guidelines exist to support authors and reviewers in assessing their quality [ 75 , 76 ], recommendations for valuation studies specifically aimed at measuring HSUVs are more fragmented or method-specific [ 10 ]. In this review, the assessment of study quality was based on a set of generic recommendations elaborated by a previous study [ 26 ] and arbitrarily modified to allow a quantitative scoring of the studies adopting direct and indirect techniques to estimate HSUVs; for mapping studies, we relied instead on recent ISPOR guidelines [ 19 ].
Although there is no universally accepted theoretical basis for choosing direct or indirect methods [ 71 ], the use of the EQ-5D, is favored by several agencies including NICE, the Canadian Agency for Drugs and Technologies in Health and the French National Authority for Health [ 3 ]. In a recent position statement [ 77 ], NICE recommends the use of EQ-5D-3 L for base-case analyses, or mapping EQ-5D-5 L responses onto the 3 L valuation set, to derive HSUVs, since further research is needed to explore the impact of adopting the EQ-5D-5 L valuation set on technology appraisal. In model-based cost-effectiveness studies, where there is a choice of HSUVs, those using the value set of the jurisdiction for which a decision is being made are usually preferred. Moreover, HSUVs should be collected from studies enrolling patients with demographic and clinical characteristics that mostly resemble those of potential recipients of the intervention under investigation in the model. Until now, studies relying on direct techniques represent the only available source to retrieve HSUVs for recurrent disease, palliative states, or treatment-related complications in HNC. Although considered as qualitatively inferior to MAUIs [ 3 ], these methods can provide values for cost-effectiveness analyses where the ‘vignettes’ presented in the choice task fit with the health states addressed in the model. Finally, in the absence of preference-based data, mapping from disease-specific instruments to generic MAUIs may represent a valuable alternative [ 74 ]; however, the only algorithm published to date in HNC [ 60 ] does not map from one of the HRQoL tools most frequently adopted in cancer studies, such as the European Organization for Research and Treatment of Cancer 30-item Quality of Life Core Questionnaire (EORTC QLQ-C30 [ 78 ]) and the Functional Assessment of Cancer Therapy - General (FACT-G [ 79 ]). Greater availability of mapping functions would facilitate the comparison of treatments using HRQoL data from many randomized controlled trials that only collected disease-specific health status information. Overall, the use of different techniques for utility elicitation might have substantial implications in cost-utility analyses; for example, it has been shown [ 71 ] that MAUIs, compared to direct valuation, tend to favor non-lifesaving treatments over interventions preventing or delaying death. Thus, regulatory bodies should avoid a mixture of methods in their decision processes to avoid a biased allocation of healthcare resources. Moreover, health economic modelers are always recommended to extensively test the uncertainty around the utility parameters in sensitivity analyses [ 71 ].
Conclusions
This study improves understanding of preference-based HRQoL measurement in HNC by systematically reviewing and critically evaluating studies that estimated HSUVs in this cancer setting. Utility values are an essential parameter but also a major source of uncertainty in model-based economic evaluations, where it is common to select them from a single study based on clinical considerations [ 3 , 28 ]. Further studies on the health-related utility assessment from HNC patients using MAUIs in recurrent and terminal states are encouraged. Additional research on mapping algorithms to convert disease-specific HRQoL results onto preference-based HSUVs would be of value in this cancer population. Overall, the methods used to identify utility values within a growing body of HRQoL literature should be increasingly systematic and justified in future studies.
Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611.
Article CAS PubMed Google Scholar
Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(Suppl 1):S5–9.
Article PubMed Google Scholar
Wolowacz SE, Briggs A, Belozeroff V, Clarke P, Doward L, Goeree R, et al. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19(6):704–19.
Rashidi AA, Anis AH, Marra CA. Do visual analogue scale (VAS) derived standard gamble (SG) utilities agree with health utilities index utilities? A comparison of patient and community preferences for health status in rheumatoid arthritis patients. Health Qual Life Outcomes. 2006;4:25.
Article PubMed PubMed Central Google Scholar
Witney AG, Treharne GJ, Tavakoli M, Lyons AC, Vincent K, Scott DL, et al. The relationship of medical, demographic and psychosocial factors to direct and indirect health utility instruments in rheumatoid arthritis. Rheumatology. 2006;45(8):975–81.
Dolan P, Sutton M. Mapping visual analogue scale health state valuations onto standard gamble and time trade-off values. Soc Sci Med. 1997;44(10):1519–30.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21.
Post MWM. Definitions of quality of life: what has happened and how to move on. Top Spinal Cord Inj Rehabil. 2014;20(3):167–80.
Wiedermann W, Frick U. Using surveys to calculate disability-adjusted life-year. Alcohol Res. 2013;35(2):128–33.
PubMed Google Scholar
Attema AE, Edelaar-Peeters Y, Versteegh MM, Stolk EA. Time trade-off: one methodology, different methods. Eur J Health Econ. 2013 Jul;14(Suppl 1):S53–64.
Richardson J, Khan MA, Iezzi A, Maxwell A. Comparing and explaining differences in the magnitude, content, and sensitivity of utilities predicted by the EQ-5D, SF-6D, HUI3, 15D, QWB, and AQoL-8D multi-attribute utility instruments. Med Decis Mak. 2015;35:276–91.
Article Google Scholar
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
EUnetHTA. Methods for health economic evaluations – a guideline based on current practices in Europe. Copyright © EUnetHTA 2014.
Hao Y, Wolfram V, Cook J. A structured review of health utility measures and elicitation in advanced/metastatic breast cancer. Clinicoecon Outcomes Res. 2016;8:293–303.
PubMed PubMed Central Google Scholar
Boye KS, Matza LS, Feeny DH, Johnston JA, Bowman L, Jordan JB. Challenges to time trade-off utility assessment methods: when should you consider alternative approaches? Expert Rev Pharmacoecon Outcomes Res. 2014;14(3):437–50.
Horsman J, Furlong W, Feeny D, Torrance G. The health utilities index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
Button B. Quality of well-being (QWB) scale. Encyclopedia of quality of life and well-being research; 2014. p. 5314–20. https://doi.org/10.1007/978-94-007-0753-5_654 .
Book Google Scholar
Hawthorne G, Richardson J, Osborne R. The assessment of quality of life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res. 1999;8(3):209–24.
Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, et al. Mapping to estimate health-state utility from non-preference-based outcome measures: an ISPOR good practices for outcomes research task force report. Value Health. 2017;20(1):18–27.
Szabo SM, Dobson RL, Donato BMK, L’Italien G, Hotte SJ, Levy AR. The quality-of-life impact of head and neck cancer: preference values from the Canadian general public. Health Outcomes Res Med. 2012;3(1):e11–23.
Hannouf MB, Sehgal C, Cao JQ, Mocanu JD, Winquist E, Zaric GS. Cost-effectiveness of adding cetuximab to platinum-based chemotherapy for first line treatment of recurrent or metastatic head and neck cancer. PLoS One. 2012;7(6):e38557. https://doi.org/10.1371/journal.pone.0038557 .
Article CAS PubMed PubMed Central Google Scholar
Liberato NL, Rognoni C, Rubrichi S, Quaglini S, Marchetti M, Gorlia T, et al. Adding docetaxel to cisplatin and fluorouracil in patients with unresectable head and neck cancer: a cost-utility analysis. Ann Oncol. 2012;23(7):1825–32.
Komatsuzaki Y, Gramegna P, Stephens JM, Botteman MF, Pashos CL, Redaelli A. Preferences and utilities of health outcomes and treatments associated with head and neck cancer: a systematic review. Am J Cancer. 2006;5(1):27–34.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, PRISMA-P Group, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. https://doi.org/10.1186/2046-4053-4-1 .
Sampson CJ, Tosh JC, Cheyne CP, Broadbent D, James M. Health state utility values for diabetic retinopathy: protocol for a systematic review and meta-analysis. Systematic review. 2015;4:15. https://doi.org/10.1186/s13643-015-0006-6 .
Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health. 2013;16:686–95.
Jeong K, Cairns J. Systematic review of health state utility values for economic evaluation of colorectal cancer. Health Econ Rev. 2016;6(1):36.
Gheorghe A, Moran G, Duffy H, Roberts T, Pinkney T, Calvert M. Health utility values associated with surgical site infection: a systematic review. Value Health. 2015;18(8):1126–37.
CEA Registry. Available at: http://healtheconomics.tuftsmedicalcenter.org/cear4/Home.aspx . Accessed 18 Nov 2016.
ScHARRHUD Health Utilities Database. Available at: http://www.scharrhud.org /. [Accessed 22 Nov 2016].
Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes. 2013;11:151. https://doi.org/10.1186/1477-7525-11-151 .
Smith-Palmer J, Bae JP, Boye KS, Norrbacka K, Hunt B, Valentine WJ. Evaluating health-related quality of life in type 1 diabetes: a systematic literature review of utilities for adults with type 1 diabetes. Clinicoecon Outcomes Res. 2016;8:559–71.
Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Mak. 2001;21(4):329–34.
Article CAS Google Scholar
Fincham JE. Response rates and responsiveness for surveys, standards, and the journal. Am J Pharm Educ. 2008;72(2):43.
Linendoll N, Saunders T, Burns R, Nyce JD, Wendell KB, Evens AM, et al. Health-related quality of life in Hodgkin lymphoma: a systematic review. Health Qual Life Outcomes. 2016;14(1):114.
Marcellusi A, Capone A, Favato G, Mennini FS, Baio G, Haeussler K, HPV Italian Collaborative Study Group, et al. Health utilities lost and risk factors associated with HPV-induced diseases in men and women: the HPV Italian collaborative study group. Clin Ther. 2015;37(1):156–167.e4.
Noel CW, Lee DJ, Kong Q, Xu W, Simpson C, Brown D, et al. Comparison of health state utility measures in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(8):696–703.
Conway EL, Farmer KC, Lynch WJ, Rees GL, Wain G, Adams J. Quality of life valuations of HPV-associated cancer health states by the general population. Sex Transm Infect. 2012;88(7):517–21.
Hamilton DW, Bins JE, McMeekin P, Pedersen A, Steen N, De Soyza A, et al. Quality compared to quantity of life in laryngeal cancer: a time trade-off study. Head Neck. 2016;38(Suppl 1):E631–7.
Hollenbeak CS, Lowe VJ, Stack BC Jr. The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer. 2001;92(9):2341–8.
Jalukar V, Funk GF, Christensen AJ, Karnell LH, Moran PJ. Health states following head and neck cancer treatment: patient, health-care professional, and public perspectives. Head Neck. 1998;20(7):600–8.
Ringash J, Redelmeier DA, O'Sullivan B, Bezjak A. Quality of life and utility in irradiated laryngeal cancer patients. Int J Radiat Oncol Biol Phys. 2000;47(4):875–81.
Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120(7):699–702.
de Almeida JR, Villanueva NL, Moskowitz AJ, Miles BA, Teng MS, Sikora A, et al. Preferences and utilities for health states after treatment for oropharyngeal cancer: transoral robotic surgery versus definitive (chemo)radiotherapy. Head Neck. 2014;36(7):923–33.
Llewellyn-Thomas HA, Sutherland HJ, Thiel EC. Do patients' evaluations of a future health state change when they actually enter that state? Med Care. 1993;31(11):1002–12.
van der Donk J, Levendag PC, Kuijpers AJ, Roest FH, Habbema JD, Meeuwis CA, et al. Patient participation in clinical decision-making for treatment of T3 laryngeal cancer: a comparison of state and process utilities. J Clin Oncol. 1995;13(9):2369–78.
del Barco ME, Mesía R, Adansa Klain JC, Vázquez Fernández S, Martínez-Galán J, Pastor Borgoñon M. Et al; Spanish head and neck cancer cooperative group (TTCC). Phase II study of panitumumab and paclitaxel as first-line treatment in recurrent or metastatic head and neck cancer. TTCC-2009-03/VECTITAX study. Oral Oncol. 2016;62:54–9.
Ouattassi N, Benmansour N, El Fakir S, Nejjari C, Alami MN. Translation and validation of EORTC QLQ-H&N 35 into Moroccan Arabic for ENT head and neck cancer patients in Morocco. Eur Arch Otorhinolaryngol. 2016;273(9):2727–34.
Parrilla C, Minni A, Bogaardt H, Macri GF, Battista M, Roukos R, et al. Pulmonary rehabilitation after Total Laryngectomy: a multicenter time-series clinical trial evaluating the Provox XtraHME in HME-Naïve patients. Ann Otol Rhinol Laryngol. 2015;124(9):706–13.
Rogers SN, Miller RD, Ali K, Minhas AB, Williams HF, Lowe D. Patients' perceived health status following primary surgery for oral and oropharyngeal cancer. Int J Oral Maxillofac Surg. 2006;35(10):913–9.
Ramaekers BL, Joore MA, Grutters JP, van den Ende P. Jong Jd, Houben R, et al. the impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768–74.
Pickard AS, Jiang R, Lin HW, Rosenbloom S, Cella D. Using patient-reported outcomes to compare relative burden of cancer: EQ-5D and functional assessment of cancer therapy-general in eleven types of cancer. Clin Ther. 2016;38(4):769–77.
Truong MT, Zhang Q, Rosenthal DI, List M, Axelrod R, Sherman E, et al. Quality of life and performance status from a sub-study conducted within a prospective phase 3 randomized trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for locally advanced head and neck carcinoma: NRG oncology radiation therapy oncology group 0522. Int J Radiat Oncol Biol Phys. 2017;97(4):687–99.
Govers TM, Schreuder WH, Klop WM, Grutters JP, Rovers MM, Merkx MA, et al. Quality of life after different procedures for regional control in oral cancer patients: cross-sectional survey. Clin Otolaryngol. 2016;41(3):228–33.
Pottel L, Lycke M, Boterberg T, Pottel H, Goethals L, Duprez F, et al. G-8 indicates overall and quality-adjusted survival in older head and neck cancer patients treated with curative radio-chemotherapy. BMC Cancer. 2015;15:875.
Aro K, Bäck L, Loimu V, Saarilahti K, Rogers S, Sintonen H, et al. Trends in the 15D health-related quality of life over the first year following diagnosis of head and neck cancer. Eur Arch Otorhinolaryngol. 2016;273(8):2141–50.
Loimu V, Mäkitie AA, Bäck LJ, Sintonen H, Räsänen P, Roine R, et al. Health-related quality of life of head and neck cancer patients with successful oncological treatment. Eur Arch Otorhinolaryngol. 2015;272(9):2415–23.
Higgins KM. What treatment for early-stage glottic carcinoma among adult patients: CO2 endolaryngeal laser excision versus standard fractionated external beam radiation is superior in terms of cost utility? Laryngoscope. 2011;121(1):116–34.
Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–65.
Chan KK, Willan AR, Gupta M, Pullenayegum E. Underestimation of uncertainties in health utilities derived from mapping algorithms involving health-related quality-of-life measures: statistical explanations and potential remedies. Med Decis Mak. 2014;34(7):863–72.
Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2009;31(10):1255–62.
Yong JH, Beca J, O'Sullivan B, Huang SH, McGowan T, Warde P, et al. Cost-effectiveness of intensity-modulated radiotherapy in oropharyngeal cancer. Clin Oncol (R Coll Radiol). 2012;24(7):532–8.
Krabbe PF, Peerenboom L, Langenhoff BS, Ruers TJ. Responsiveness of the generic EQ-5D summary measure compared to the disease-specific EORTC QLQ C-30. Qual Life Res. 2004;13(7):1247–53.
Nichol MB, Sengupta N, Globe DR. Evaluating quality-adjusted life years: estimation of the health utility index (HUI2) from the SF-36. Med Decis Mak. 2001;21(2):105–12.
Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–91.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M; EORTC 24971/TAX 323 Study Group, .et al, Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704.
Heiskanen J, Tolppanen AM, Roine RP, Hartikainen J, Hippeläinen M, Miettinen H, et al. Comparison of EQ-5D and 15D instruments for assessing the health-related quality of life in cardiac surgery patients. Eur Heart J Qual Care Clin Outcomes. 2016;2(3):193–200.
Vainiola T, Pettilä V, Roine RP, Räsänen P, Rissanen AM, Sintonen H. Comparison of two utility instruments, the EQ-5D and the 15D, in the critical care setting. Intensive Care Med. 2010;36(12):2090–3.
Lunde L. Can EQ-5D and 15D be used interchangeably in economic evaluations? Assessing quality of life in post-stroke patients. Eur J Health Econ. 2013;14:539–50.
Vartiainen P, Mäntyselkä P, Heiskanen T, Hagelberg N, Mustola S, Forssell H, et al. Validation of EQ-5D and 15D in the assessment of health-related quality of life in chronic pain. Pain. 2017;158(8):1577–85.
Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:b2688.
Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health. 2008;11(3):478–86.
McDonough CM, Tosteson AN. Measuring preferences for cost-utility analysis: how choice of method may influence decision-making. Pharmaco Economics. 2007;25(2):93–106.
Brazier J, Rowen D. NICE DSU Technical Support Document 11: Alternatives to EQ-5D for Generating Health State Utility Values [Internet]. In: NICE Decision Support Unit Technical Support Documents. London: National Institute for Health and Care Excellence (NICE); 2011.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16:231–50.
Meregaglia M, Cairns J. Economic evaluations of follow-up strategies for cancer survivors: a systematic review and quality appraisal of the literature. Expert Rev Pharmacoecon Outcomes Res. 2015;15(6):913–29.
National Institute for Health and Care Excellence. Position Statement on use of the EQ-5D-5L valuation set. Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf [Accessed 10 Aug 2017].
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group: The EORTC QLQ-C30 scoring manual, 3 rd edition. European Organization for Research and Treatment of Cancer (EORTC), Brussels (2001).
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–61.
Download references
Acknowledgments
Not applicable.
The authors declare that this study received no financial support.
Availability of data and materials
The datasets supporting the conclusions of this article are included within its tables and additional files.
Author information
Authors and affiliations.
Department of Health Services Research and Policy, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH, UK
Michela Meregaglia & John Cairns
CeRGAS (Research Centre on Health and Social Care Management), Bocconi University, Via Roentgen 1, 20136, Milan, Italy
Michela Meregaglia
CCBIO (Centre for Cancer Biomarkers), University of Bergen, Postboks 7804, N-5020, Bergen, Norway
John Cairns
You can also search for this author in PubMed Google Scholar
Contributions
MM conducted the systematic literature search, extracted, analyzed, and interpreted the data, and drafted the work. JC conceived the work, interpreted the results, and contributed to the manuscript preparation. Both authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Michela Meregaglia .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: table s1..
Details of HSUVs reported by the studies ( n = 27). (DOCX 107 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Meregaglia, M., Cairns, J. A systematic literature review of health state utility values in head and neck cancer. Health Qual Life Outcomes 15 , 174 (2017). https://doi.org/10.1186/s12955-017-0748-z
Download citation
Received : 20 February 2017
Accepted : 24 August 2017
Published : 02 September 2017
DOI : https://doi.org/10.1186/s12955-017-0748-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health state utility values (HSUVs)
- Health-related quality of life (HRQoL)
- Head and neck cancer (HNC)
- Multi-attribute utility instruments (MAUIs)
- Standard gamble (SG)
- Time trade-off (TTO)
- Systematic literature review
Health and Quality of Life Outcomes
ISSN: 1477-7525
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Health State Utilities of Patients with Heart Failure: A Systematic Literature Review
Gian luca di tanna.
1 Statistics Division, The George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney, NSW Australia
7 The George Institute for Global Health, Level 5, 1 King St, Newtown, NSW 2042 Australia
Michael Urbich
2 Amgen (Europe) GmbH, Global Value & Access, Modeling Center of Excellence, Rotkreuz, Switzerland
Heidi S. Wirtz
3 Amgen Inc, Global Health Economics, Thousand Oaks, CA USA
Barbara Potrata
4 Pharmerit - an OPEN Health company, Rotterdam, The Netherlands
Marieke Heisen
Craig bennison.
5 Pharmerit - an OPEN Health company, York, UK
John Brazier
6 Health Economics and Decision Science, University of Sheffield, Sheffield, UK
Associated Data
Background and objectives.
New treatments and interventions are in development to address clinical needs in heart failure. To support decision making on reimbursement, cost-effectiveness analyses are frequently required. A systematic literature review was conducted to identify and summarize heart failure utility values for use in economic evaluations.
Databases were searched for articles published until June 2019 that reported health utility values for patients with heart failure. Publications were reviewed with specific attention to study design; reported values were categorized according to the health states, ‘chronic heart failure’, ‘hospitalized’, and ‘other acute heart failure’. Interquartile limits (25th percentile ‘Q1’, 75th percentile ‘Q3’) were calculated for health states and heart failure subgroups where there were sufficient data.
The systematic literature review identified 161 publications based on data from 142 studies. Utility values for chronic heart failure were reported by 128 publications; 39 publications published values for hospitalized and three for other acute heart failure. There was substantial heterogeneity in the specifics of the study populations, methods of elicitation, and summary statistics, which is reflected in the wide range of utility values reported. EQ-5D was the most used instrument; the interquartile limit for mean EQ-5D values for chronic heart failure was 0.64–0.72.
Conclusions
There is a wealth of published utility values for heart failure to support economic evaluations. Data are heterogenous owing to specificities of the study population and methodology of utility value elicitation and analysis. Choice of value(s) to support economic models must be carefully justified to ensure a robust economic analysis.
Electronic supplementary material
The online version of this article (10.1007/s40273-020-00984-6) contains supplementary material, which is available to authorized users.
Introduction
Heart failure (HF) is the inability of the heart to pump sufficient blood to meet the body’s needs, causing symptoms such as dyspnea, fatigue, and edema. It affects about 64 million patients worldwide and carries a heavy morbidity and mortality burden [ 1 , 2 ]. Approximately 50% of patients die within 5 years of diagnosis and HF is the leading cause of hospitalization in patients aged over 65 years [ 2 , 3 ]. Heart failure therefore presents a large public health burden, which is anticipated to grow with an aging global population.
Heart failure may be chronic or acute. Chronic HF is a relatively stable condition. However, periods of stable heart function are punctuated by acute events (also referred to as decompensations). Chronic HF is commonly classified by the New York Heart Association (NYHA) classification, which describes the severity of symptoms and their impact on the patient’s physical activity and daily functioning [ 4 – 6 ]. Acute heart failure (AHF) is the rapid onset of new or worsening of symptoms and signs of HF, requiring urgent medical attention [ 7 ]. The majority of AHF cases occur in patients with worsening chronic HF, i.e., as a decompensation of existing disease rather than new-onset (‘de novo’) presentation of HF [ 4 ].
New treatments and models of care are under exploration for both chronic HF with reduced left ventricular ejection fraction (HFrEF, LVEF < 40%) and chronic HF with preserved left ventricular ejection fraction (HFpEF, LVEF ≥ 50%). As HF has a substantial impact on health-related quality of life (HRQoL) [ 6 ], it is important to understand the effect of treatment on HRQoL and consequently assess cost effectiveness (CE) through a cost-utility analysis, where effectiveness is measured in terms of quality-adjusted life-years (QALYs). Health state utility values can be obtained via generic measures, such as the EQ-5D, or condition-specific measures. It is essential that a choice-based evaluation is applied, for instance, in the shape of a time trade-off, to derive a utility scoring algorithm, also called a value set [ 8 ]. Utility values are therefore key components that inform health technology assessment decisions. In methodological reviews of HF cost-effectiveness models, utility is one of the key drivers, and sources of heterogeneity, of incremental cost-effectiveness ratios, making it an important parameter for consideration [ 9 , 10 ]. It is therefore essential that the values used in economic evaluations can be justified. The latest reporting standards from the International Society for Pharmacoeconomics and Outcomes Research recommend values are obtained systematically, are reviewed for quality, and are consistent in the methods used to derive the values [ 11 ].
To the best of our knowledge, there have been no systematic literature reviews (SLR) of HF utility values. The aim of this SLR is to identify, summarize, and appraise HF utility values to support and inform economic evaluations.
Search Strategy
Sources from the National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, and Canadian Agency for Drugs and Technologies in Health were used to develop the search strategy (Electronic Supplementary Material [ESM] 1), which adhered to a prespecified protocol and methods recommended by the Cochrane Collaboration and the Centre for Reviews and Dissemination. MEDLINE, EMBASE, EconLit, and Centre for Reviews Dissemination York database (which included the National Health Service Economics and Evaluation Database and Health Technology Assessment Database) were searched for relevant articles published from the beginning of database records until June 2019. Databases were searched for primary studies that published utility values for adult patients (aged ≥ 18 years) with HF, regardless of treatment or intervention. The search strategy allowed for the inclusion of studies conducted in broader patient populations if utility values were reported for a HF sub-population, and studies that reported HF utility values as valued or perceived by the general population and caregivers. No minimum sample size was set for inclusion.
Conference abstracts (ESM 1) published between 2016 and June 2019 were searched to identify data from the gray literature. In addition, websites for the Canadian Agency for Drugs and Technologies in Health, European Medicines Agency, National Institute for Health and Care Excellence, Scottish Medicines Consortium, US Food and Drug Administration, and School of Health and Related Research Health Utilities Database were also reviewed. The search was also supplemented with relevant publications identified in a parallel SLR on CE models and economic evaluations in HF.
Only reports, abstracts and manuscripts published in English were selected for further review. References cited in retrieved articles were reviewed for additional publications that had not been already identified (citation snowballing).
As only primary studies publishing new utility values were of interest, CE studies, health technology assessment submissions, SLRs, and meta-analyses were excluded from the review, unless they used or published de-novo data; however, references for utility inputs cited by these publications were assessed for inclusion as part of the citation snowballing exercise. The review was registered (Registration Number CRD42019134288) with the International Prospective Register of Systematic Reviews (PROSPERO) and reported according to the following guidelines: Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and International Society for Pharmacoeconomics and Outcomes Research good practices on identification, review, and use of health state utilities in CE models (SpRUCE) [ 11 , 12 ].
Selection, Extraction, and Quality Assessment
Two reviewers independently screened database records and identified relevant studies for review (Fig. 1 ) in accordance with pre-defined search strategy. Data were extracted and primary studies with a full publication were assessed for quality, based on relevant criteria from the Papaioannou et al. [ 13 ] checklist. Data extraction and quality assessment were performed by one reviewer and quality checked by a second reviewer. Any discrepancies between the two reviewers during selection, extraction, and quality assessment were adjudicated by a third reviewer.

Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow chart displaying the number of publications included as well as the number of publications that were excluded, with reasons. CE cost effectiveness, HF , heart failure, SLR systematic literature review
Data Review
Publications were reviewed with specific attention to the design of the study that generated the utility data, the method of utility elicitation, the value set used to generate utility values, and the utility value summary statistic and data. There are two versions of the EQ-5D instrument, the original three-level (− 3L) version and the five-level (− 5L) version that was introduced in 2009. All papers that were published in or prior to 2009 were assumed to use the EQ-5D-3L version, regardless of whether this was specified.
Publications were categorized according to the health state(s) for which utility data were reported: ‘chronic HF’, ‘hospitalized’, and ‘other AHF’. Other AHF captured those publications that presented data on acute events but did not specify restriction to hospitalization events. Categorization was based on the study population for which the utility values were elicited. In instances where the utility value publication did not report sufficient detail around the study population, where feasible, further information on the study was sourced from either clinicaltrials.gov or the study publication. Publications were assigned the category ‘HF’ where there was insufficient information to assign it to a more specific health state.
The interquartile limits (IQLs) of study means (25th percentile ‘Q1’, 75th percentile ‘Q3’) were calculated for HF subgroups where there were sufficient data. For inclusion in the IQLs, calculation studies had to have a sample size ≥ 100. Weighted averages were calculated where publications reported utility values for two or more study arms and baseline data used where utility values were reported for multiple timepoints. Baseline data were used so as to not confound the analysis with the effect of different therapies, which adds further heterogeneity. Publications that reported utility values for individual NYHA classes were omitted from the calculation if it was not possible to calculate an overall utility value as a weighted average of the classes’ values, as there is often an unequal distribution of patients across the NYHA classes, only a weighted average would avoid bias [ 14 , 15 ]. Follow-up studies were also omitted. In occasional cases where publications reported utility values for multiple value sets, values based on the most frequently reported value set for the UK were used to calculate Q1 and Q3.
Study Identification and Description
The SLR identified 161 primary publications that reported utility value data (including disutilities) for HF, based on elicitation from 142 studies (Fig. 1 , PRISMA flow diagram, Table 1 , ESM 2). There was considerable heterogeneity in the design of studies; 78 were observational studies, 43 randomized controlled trials, and 13 cost-utility studies. Studies differed in terms of sample size (range 6–28,500) and treatment arms. Furthermore, substantial diversity was seen within study populations. Some studies (28.5%) recruited a broad population of patients with HF (e.g., patients with chronic HF), others (7%) recruited hypothetical patients with HF or the general population, while the majority (64.5%) had much more specific inclusion criteria based on HF type, severity, or comorbidity (e.g., patients in specific NYHA classes and below pre-specified LVEF thresholds). There was also heterogeneity in how utility values were reported (Table 1 ). The mean was the most reported statistic ( n = 87), eight publications reported both mean and median, and five reported the median. Other reported data included variability statistics, coefficients, estimates, weights, and base-case/model inputs; 35 studies did not state the statistic for the reported data.
Table 1
Overview of studies ( n = 142) and publications ( n = 161) identified in the systematic literature review
AHF acute heart failure, CHF chronic heart failure, HF heart failure, HRQoL health-related quality of life, HUI Health Utilities Index, NR not reported, NS not specified, RCT randomized controlled trial, SF Short Form
a Exceeds 142, some studies (16) used multiple elicitation instruments, from which utility values were calculated
b Includes HUI, HUI-2, HUI-3, SF-6D, SF-12, SF-36, and Quality of Well-Being Index
c Exceeds 161, as some studies published data on multiple HF populations/value sets/parameters
Publications were categorized according to the health state for which utility data were reported. Although new-onset cases of ‘de novo’ acute HF do occur, the disease course of HF is typically that of a chronic condition, with hospitalization episodes due to a worsening of the previously stable condition [ 4 ]. This is reflected in the literature with 128 publications focusing on chronic HF, 39 on hospitalization, and only 3 on other AHF (Table 1 ). The health state could not be defined for ten publications. Of note, some publications reported utility values for several HF health states. EQ-5D (3L and 5L) was the most common elicitation instrument, being used in 104 studies. Value sets used also varied between studies. The UK value set was the most frequently reported value set ( n = 33); however, the majority of publications ( n = 89) did not report the value set used.
Most studies ( n = 130) recruited patients with HF, of which 113 elicited data using patient-reported outcomes. One study used proxy report by caregivers (both informal and healthcare providers) [ 16 ]; six studies recruited a general population and one study used healthcare providers, and elicited utility values for vignettes describing specific health states [ 17 – 23 ]; and five cost-utility models defined hypothetical HF populations deriving utility values from the literature [ 24 – 28 ].
Full manuscripts ( n = 91) included in the review were assessed for quality according to relevant criteria from the Papaioannou et al. checklist (ESM 3). In general, publications were of good quality in reporting response rates, using population characteristics that matched those modeled (e.g., chronic HF, hospitalization), using generic preference-based instruments, and assessing utility values elicited directly from patients. However, loss to follow-up and missing data were not reported or addressed by many of the papers.
This high level of heterogeneity between publications limits the ability to compare and synthesize studies. Consequently, focus was predominantly placed on those papers that used the EQ-5D instrument and published mean health utility values as the summary statistic.
Utility Values in Patients with Chronic HF
The SLR identified 52 studies that published mean utility values for patients with chronic HF, using the EQ-5D (− 3L or − 5L) instrument, of which 35 (Table 2 ) met inclusion criteria for calculating Q1 and Q3 limits of 0.64–0.72. In a subgroup analysis of those publications that used the EQ-5D-3L instrument ( n = 22), the IQL did not substantially change (0.64–0.71). Only two publications that met criteria for inclusion in the IQL calculation used the EQ-5D-5L instrument. Squire et al. reported a mean utility value of 0.60 for patients with HF with NYHA II–IV who had been diagnosed for at least 12 months [ 29 ]. Zhu et al. reported a mean utility value of 0.73 for a broad population of patients with chronic HF [ 30 ]. Eleven of the 35 papers were excluded from − 3L and 5L subgroup analyses because the EQ-5D version could not be determined.
Table 2
Mean EQ-5D (3L or 5L) utility values for patients with chronic heart failure (CHF)
CI confidence interval, CRT - D cardiac resynchronization therapy defibrillator, EQ - 5D EuroQol 5-dimensional questionnaire, EQ - 5D - 3L EQ-5D 3 levels, EQ - 5D - 5L EQ-5D 5 levels, HF heart failure, HRQoL health-related quality of life, HTx Heart transplantation, ICD implantable cardiac device, LVEF left ventricular ejection fraction, NR not reported, NYHA New York Heart Association, RCT randomized controlled trial, SD standard deviation
a Baseline data presented for studies that published utility values at multiple timepoints
b Based on information given in clinicaltrials.gov or primary study publication
The chronic HF populations of studies included in the IQL calculations were varied ranging, for example, from advanced HF populations (with or waiting for heart transplant), to more general chronic HF populations who were stable for at least 3 months [ 31 , 32 ]. A review of data by NYHA class clearly illustrates the impact of the severity of chronic HF on utility values. The SLR identified 11 publications that provided data on mean EQ-5D utility values according to NYHA class, of which nine were included in the IQL calculation; one study was omitted from the IQL calculation as it grouped NYHA classes (I/II and III/IV), and a second study was omitted because it published the mean difference in utility values between classes [ 33 , 34 ]. Aside from Zhu et al. [ 30 ], which reported lower utility values for NYHA I vs NYHA class II, increasing NYHA class was associated with lower utility values (Fig. 2 ). Interquartile limits were 0.79–0.86 for NYHA class I, 0.75–0.81 for class II, 0.61–0.69 for class III, and 0.51–0.66 for class IV.

Mean utility scores for chronic heart failure, based on EQ-5D health-related quality-of-life data, according to New York Heart Association (NYHA) class [ 30 , 57 – 64 ]. Black circle: Comin-Colet 2013, white circle: Delgardo 2014, black square: Gohler 2009, white square: Grustam 2018, white up-pointing trianlge: Kularatna 2017, white up-pointing trianlge: Marti 2010, black diamond: Marti 2011, white diamond: Yao 2007, grey diamond: Zhu 2017
The impact of a value set on the utility value is illustrated in two publications. Berg et al. calculated mean utility values for a large population of Swedish patients with chronic HF, using Swedish and UK value sets [ 35 ]. Baseline mean utility score calculated using the UK value set was 0.696; whereas the utility value was nearly 20% higher when calculated with the Swedish value set (0.828) [ 35 ]. Eurich et al. calculated mean utility values for patients with HFrEF managed in an outpatient setting using US and UK value sets that gave mean scores of 0.74 and 0.66, respectively [ 36 ].
Of those papers included in the IQL calculation, nine publications published utility values for patients with HFrEF, giving an IQL of 0.67–0.74. None of the publications eligible for inclusion in the IQL calculation focused on patients with HFpEF; however, two publications that were not eligible compared utility values in HFrEF and HFpEF. Berg et al., which used the EQ-5D-3L instrument but did not specify the summary statistic, reported a lower utility in HFpEF (0.65) than in HFrEF (0.72–0.73) [ 37 ]. Nafees et al., which used a vignette elicitation method, reported similar values between HFrEF and HFpEF populations [ 21 ].
In addition to understanding utility values for HF populations, understanding disutility due to chronic HF in the general population (or other patient populations) may be of value for modeling studies. The SLR identified ten papers, regardless of the elicitation instrument or statistic reported, that provided data on the disutility of chronic HF (ESM 4). The large degree of heterogeneity between study designs, in particular, the background population, instrument used, and statistic reported, prevents a detailed collective review of these studies. However, in most cases, the presence of HF was associated with a reduction in the utility value (although statistical significance was not assessed or demonstrated for all disutility values).
Utility Values in Hospitalized Patients with HF
Acute HF events, whether new-onset (‘de novo’) events or, more commonly, acute decompensations of chronic HF, usually lead to urgent hospital admission [ 7 ]. The SLR identified 31 publications that reported EQ-5D utility values for hospitalization, of which two were based on the ACEND-HF trial and four on the WHICH study. Patients with HF are at risk of all-cause hospitalization as well as hospitalization for HF [ 2 ]. Twenty of the 31 EQ-5D publications focused on hospitalization for HF, three on all-cause hospitalization, and eight failed to clearly report the cause of hospitalization.
Many of the studies did not report, or poorly defined, the timing of HRQoL elicitation during the hospitalization event. Understanding when HRQoL questionnaires are administered is important as two studies suggest that utility values change rapidly during a hospitalization event. Ambrosy et al. published EQ-5D-3L utility values for the ASCEND-HF trial, which investigated the effect of nesiritide in patients hospitalized with AHF. Mean utility value, for the total study population (regardless of treatment arm) increased rapidly from 0.56 at baseline (assumed near to time of admission) to 0.67 at 24 h of hospitalization; by the time the patient was discharged (day 10), the utility value had further increased to 0.79 [ 38 ]. A second smaller study by Swinburn et al. reported mean utility values of patients with HF, as perceived by caregivers and healthcare professionals, following admission to hospital. EQ-5D-3L values, as perceived by experienced cardiac nurses ( n = 50), increased from 0.199 on day 1 post-hospital admission to 0.563 on day 3 [ 16 ]. By day 7, utility values had increased to 0.817.
For the IQL calculation, papers that published mean EQ-5D utility values (for patients with HF) were included regardless of the cause of hospitalization, if they reported inpatient or discharge utility values. Four papers were identified that provided utility values collected during hospital admission, and six at discharge (Table 3 ), yielding IQLs of 0.54–0.63 and 0.64–0.73, respectively. Differences in study design, including whether the study focused on all-cause hospitalization or hospitalization for HF, are likely to have contributed to the variability in estimates. In addition, when the EQ-5D questionnaire was administered during hospital stay is also likely to have contributed to the variation in utility values.
Table 3
Mean EQ-5D (3-level or 5-level) utility values during hospitalization and at discharge
AHF acute heart failure, CBI clinic-based intervention program, CHF chronic heart failure, CI confidence interval, HRQoL health-related quality of life, HBI home-based intervention program, HF heart failure, NR not reported, NYHA New York Heart Association, RCT randomized controlled trial, SD standard deviation, SE standard error
a Based on information given in clinicaltrials.gov or primary study publication
b Baseline (admission): study also reports 24 h
Four papers provided longer term, follow-up, mean EQ-5D utility values on patients hospitalized for HF (ESM 5) [ 38 – 41 ]. Temporal changes in utility values following discharge vary between studies and follow-up care. In most studies, utility values maintain or increase following discharge.
Ten publications reported EQ-5D disutility values for a hospitalization event, with four using data from the SHIFT study (Table 4 ). While a large degree of study heterogeneity (in particular, the summary statistic reported) prevents IQL from being calculated, it is evident that a hospitalization event reduces utility (Table 4 ). The publications based on the SHIFT study provide several interesting insights. Two publications by Griffiths et al., both of which use EQ-5D data from the SHIFT study, indicate that disutility because of hospitalization increases with NYHA class; differences in the reported values between the publications suggest sensitivity to differences in the analyses applied in the different publications (both papers report the results of a mixed model using NYHA classes as a time-varying covariate but the model building strategies appear to be different with an automatic backward elimination used to retain covariates in the latter paper) [ 42 , 43 ]. Kansal et al. provide disutilities specifically for HF hospitalizations based on SHIFT data; disutilities for one or two HF hospitalizations are similar (parameter estimates [standard error] − 0.076 [0.007] and − 0.074 [0.013]), but increases for patients with three or more HF hospitalizations (− 0.133 [0.016]) [ 44 ]. Apart from McMurray et al. [ 45 ], none of the papers that publish hospitalization disutility values provide time boundaries around the data, i.e., when the decrement is applied and how long the effect lasts. According to McMurray et al., disutility is − 0.105 for patients hospitalized in the previous 30 days and reduces to − 0.054 for patients hospitalized in the previous 30–90 days (UK value set) [ 45 ]. This suggests that disutility because of hospitalization reduces over time, which is consistent with other studies publishing trends in utility values following discharge (ESM 5).
Table 4
Disutility because of a hospitalization event, EQ-5D (3-level or 5-level) values
CHF chronic heart failure, CI confidence interval, HF heart failure, HFrEF heart failure reduced ejection fraction, MI myocardial infarction, NR not reported, NYHA New York Heart Association, RCT randomized controlled trial, SD standard deviation, SE standard error
a Based on information given in clinicaltrials.gov
Utility Values in Patients with Other AHF Events
Acute HF events do not always result in hospitalization. Three studies reported utility values for a broader group of patients with AHF, none of which used EQ-5D. Collins et al. is a modeling study [ 46 ], while Davies et al. and Matza et al. use vignette methodology and surveyed general populations [ 17 , 20 ].
This SLR identified a wealth of HF utility data, with 161 publications reporting data from 142 studies. This large evidence base provides opportunities for the relevant utility values to be identified and used in cost-utility analyses. However, opportunities to compare and synthesize the studies were limited, as heterogeneity between the studies was considerable. This degree of heterogeneity is not unique to HF, a review of CE analyses in cardiovascular disease by Ara et al. found utilities values varied hugely in terms of the patient population and the methods (in particular, the instruments and value set) used to obtain them, resulting in considerable heterogeneity in the data [ 47 ].
From the quality assessment, reporting of loss to follow-up and missing data in HF utility publications needs to be improved to enable the reader to establish whether bias might have been introduced [ 48 ]. However, as this review focused on baseline data, lack of reporting loss to follow-up did not pose a risk of bias in this instance. Furthermore, the specifics of the instrument (e.g., EQ-5D-3L vs EQ-5D-5L) and country value set applied should also be reported routinely.
In this SLR, EQ-5D was the dominant instrument accounting for 73% of utility studies. While other instruments may be relevant for specific uses, for the purpose of a comparative synthesis, heterogeneity was reduced by focusing the detailed review on those studies that used the EQ-5D instrument. Furthermore, as utility values are sensitive to study specificities, such as study population and value set used (as well as instrument for elicitation), IQLs were calculated for the comparative synthesis, as utilities for a broad population cannot be accurately represented by a single value.
The IQL for chronic HF was 0.64–0.72, with a trend of decreasing utility with increasing disease severity observed (IQLs 0.79–0.86 for NYHA class I, 0.75–0.81 for class II, 0.61–0.69 for class III and 0.51–0.66 for class IV). As expected, utilities were lower for hospitalized patients with HF (compared with chronic HF), with an IQL of 0.54–0.63. However, IQLs at discharge (0.64–0.73) were near identical to those reported for the general chronic HF population.
Hospitalization of patients with HF is an area of focus for this review as a treatment goal of HF is to prevent hospital admission. Consequently, ‘hospitalization for heart failure’ is a key outcome in many HF trials [ 7 , 49 ]. Understanding the impact of hospitalization on utility is likely to be central to economic evaluations of new treatments. While 39 publications reported utility data following hospitalization of patients with HF, there were limitations in the data. In particular, the timing of administration of the EQ-5D questionnaire was poorly defined and some publications failed to report the cause of hospitalization, e.g., HF specific, cardiovascular, or all-cause. Longitudinal studies of HF utility were rare; only four studies reported utilities during hospitalization (admission or discharge) as well as at follow-up timepoints, none provided pre-admission data. Furthermore, studies reporting disutility because of hospitalization did not, in general, specify when disutility was assessed during hospitalization or time-bound the effect of a hospitalization event. Consequently, despite the large number of publications, there are important limitations to the hospitalization data that need careful consideration when applying these values in economic models.
Acute HF events may not always result in hospitalization but may require urgent medical attention and treatment; a recent HF trial included ‘urgent HF visit’ alongside hospitalization for HF and cardiovascular death in the primary composite endpoint [ 50 ]. However only three studies reported utility values for a broader group of patients with acute HF, none of which used EQ-5D. Consequently, utility data of acute heart failure, not restricted to hospitalization, are limited, highlighting it as an area for further investigation.
To the best of our knowledge, this is the first dedicated SLR of utility in HF. Dyer et al. reviewed EQ-5D utility values in a broad group of cardiovascular diseases [ 51 ]. They identified 150 studies that published EQ-5D values for chronic HF, with mean values ranging from 0.31 to 0.78 [ 51 ]. The IQLs for mean EQ-5D values for chronic HF calculated in this review fall within the range published by Dyer et al. This comprehensive SLR expands on Dyer et al. While mainly focusing on EQ-5D (because of its high usage), this review is not restricted to this instrument and captures an additional 9 years of the latest data. Furthermore, utility values for specific health states (specifically chronic HF and hospitalization) are analyzed.
Rankin et al. reviewed trial-based economic evaluations of HF interventions that derive QALYs as an outcome measure, to identify approaches used to measure and value change in HRQoL [ 52 ]. They identified 20 studies reporting economic evaluations based on 18 individual trials, with most studies ( n = 17) using generic preference-based measures to describe HRQoL and derive QALYs, commonly the EQ-5D-3L. Rankin et al. did not provide the utility values reported per study but rather they examined whether the evaluations undertaken alongside trials identified significant changes in QALYs. Our review expands on Rankin et al. to identify, summarize, and appraise primary studies publishing HF utility values, regardless of the treatment or intervention and study design provided the studies report on de-novo utility data.
Limitations
Whilst we were inclusive in our approach to selecting studies, this review might be affected by publication bias which, although beyond the control of a systematic review, could have distorted our summaries with an over-representation of studies with larger and/or statistically significant results. Further, language bias is also possible, as only publications in English were included in the review.
Because of the fact that HF is asymptomatic in its first stages, early assessment of the severity of HF is a crucial task [ 53 ]. The most commonly employed classifications for HF severity are NYHA and American College of Cardiology/American Heart Association stages of HF. The NYHA classification system has been criticized because of the fact that it is based on a subjective evaluation and thus intra-observer variability can be introduced [ 54 ]. Although we do acknowledge the criticism on the NHYA classification system, we did investigate how NYHA class impacted utility as this was the most frequently reported classification for HF severity; while none of the identified studies provided utilities stratified per American College of Cardiology/American Heart Association stages of HF.
Acute HF events, whether ‘de novo’ events or, more commonly, acute decompensations of chronic HF, usually lead to urgent hospital admission. In this review, acute HF was driven by hospitalization except from three studies (listed under ‘other AHF events’). The differentiation between ‘de novo’ and ‘acute-on-chronic’ HF cases would have some merit because the initial hospitalization with diagnosis of HF is generally considered more costly. However, we did not differentiate among the two types of acute events because of studies that either do not clearly define the population with HF hospitalization or they include both patients with chronic HF and newly diagnosed HF in the sample of patients admitted to hospital [ 40 , 41 , 55 ]. To avoid grouping the studies based on the reviewers’ interpretation of the data, we did present the findings according to the health state for which utility data were reported: ‘chronic HF’, ‘hospitalized’, and ‘other AHF’. Consequently, all studies reporting utilities for HF hospitalization were grouped together regardless of ‘de novo’ or ‘acute-on-chronic’ events. It is possible that many chronic HF studies may have investigated patients with an exacerbation of chronic HF (‘acute-on-chronic’) and therefore the study population may not be per se stable chronic patients. The manner in which we approached the studies of patients with chronic HF is that when the study reported utility for an acute event (e.g., hospitalization) clearly defined by the authors, we grouped the study under ‘hospitalized HF’ or ‘other AHF’.
Although age of respondents was reported in most of the studies, direct comparison of outcomes based on age between studies was not possible owing to the large differences in the set-up of the studies. Looking at within-study reporting of the role of age, some insights have been offered only by the studies by Calvert et al. and McMurray et al. [ 45 , 56 ]. The former reported utilities for patients with HF stratified by age, and revealed that the impact of HF on quality of life appears to be independent of age with no specific trend identified (25–34 years: 0.55; 35–44 years: 0.65; 45–54 years: 0.60; 55–64 years: 0.60; 65–74 years: 0.60; 75 + years: 0.60). McMurray et al., in the supplemental results, reported the results of multivariable mixed models for utilities, but for both cohorts (UK and Colombian, Danish analysis) the centered-on-the-mean coefficient for age was very small (− 0.001) and borderline statistically significant (95% confidence interval − 0.001, 0.000), indicating a weak disutility effect by older ages.
There is a wealth of published utility values providing a useful source for health economic modelers. In line with latest International Society for Pharmacoeconomics and Outcomes Research recommendations, utility values should be obtained systematically, reviewed for quality, and derived using consistent methods [ 11 ]. This SLR provides evidence on suitable values to support future economic evaluations in HF and, where feasible, summarizes the data; utility value IQLs for chronic HF were 0.64–0.72. We advocate the use of systematic reviews to inform the parameters of the models used for cost-effectiveness analyses because utilities are among the key drivers of the models used in HF [ 10 ]. This study is an exhaustive repository of data from which utility values can be selected, justified (relevant to specific modeling scenarios), and used. Meanwhile, for those modelers using de novo utility values, data identified in this SLR provide a useful resource for benchmarking.
Below is the link to the electronic supplementary material.

Acknowledgements
Dr. Aruna Jeans and Krystallia Pantiri provided medical writing support.
Declarations
This work was funded by Amgen Inc. Pharmerit - an OPEN Health company commissioned by Amgen Inc. to carry out the systematic review
Michael Urbich, Gary Globe, and Heidi S. Wirtz are employees of Amgen and hold corporate stock in Amgen. Heidi S. Wirtz also holds corporate stock in Teva Pharmaceutical Industries Ltd. Barvara Potrata, Marieke Heisen, and Craig Bennison report funding from Amgen Inc. to Pharmerit - an OPEN Health company, during the conduct of the study, and employment from Pharmerit - an OPEN Health company, outside the submitted work. Gian Luca Di Tanna was an employee of Amgen until February 2019. He received an honorarium from Amgen during the conduct of the study for providing methodological support. John Brazier has no conflicts of interest that are directly relevant to the content of this article.
Not applicable.
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
All co-authors contributed to the design, data collection, analysis, and interpretation.
Advertisement
Evaluating the conduct and application of health utility studies: a review of critical appraisal tools and reporting checklists
- Original Paper
- Published: 11 April 2021
- Volume 22 , pages 723–733, ( 2021 )
Cite this article

- Michael J. Zoratti 1 ,
- A. Simon Pickard 2 ,
- Peep F. M. Stalmeier 3 ,
- Daniel Ollendorf ORCID: orcid.org/0000-0001-6016-8129 4 ,
- Andrew Lloyd 5 ,
- Kelvin K W Chan 6 , 7 ,
- Don Husereau 8 ,
- John E. Brazier 9 ,
- Murray Krahn 10 ,
- Mitchell Levine 1 , 11 ,
- Lehana Thabane 1 , 12 ,
- Feng Xie ORCID: orcid.org/0000-0003-3454-6266 1 on behalf of
Health Utility Book (HUB) Working Group
1228 Accesses
9 Citations
4 Altmetric
Explore all metrics
Published health utility studies are increasingly cited in cost–utility analyses to inform reimbursement decision-making. However, there is limited guidance for investigators looking to systematically evaluate the methodological quality of health utility studies or their applicability to decision contexts.
To describe how health utility concepts are reflected in tools intended for use with the health economic literature, particularly with respect to the evaluation of methodological quality and context applicability.
We reviewed the critical appraisal and reporting tools described in a 2012 report published by the Agency for Healthcare Research and Quality (AHRQ), supplemented with a keyword search of MEDLINE and EMBASE, to identify existing tools which include health utility constructs. From these tools, a list of relevant items was compiled and grouped into domain categories based on the methodological or applicability aspect they were directed toward.
Of the 24 tools we identified, 12 contained items relevant to the evaluation of health utilities. Sixty-five items were considered relevant to the evaluation of quality, while 44 were relevant to the evaluation of applicability. Items were arranged into four domains: health state descriptions; selection and description of respondents; elicitation and measurement methods; and other considerations.
As key inputs to cost–utility analyses, health utilities have the potential to significantly impact estimates of cost-effectiveness. Existing tools contain only general items related to the conduct or use of health utility studies. There is a need to develop tools that systematically evaluate the methodological quality and applicability of health utility studies.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
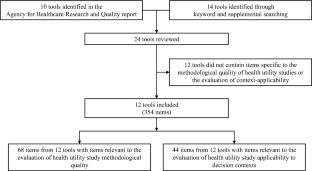
Similar content being viewed by others

Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach

The ABC of systematic literature review: the basic methodological guidance for beginners

A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research
Klarman, H.E., Francis, J.O.S., Rosenthal, G.D.: Cost Effectiveness Analysis Applied to the Treatment of Chronic Renal Disease. Med. Care 6 (1), 48–54 (1968)
Article Google Scholar
Bremner, K.E., Chong, C.A., Tomlinson, G., Alibhai, S.M., Krahn, M.D.: A review and meta-analysis of prostate cancer utilities. Med. Decis. Making 27 (3), 288–298 (2007). https://doi.org/10.1177/0272989x07300604
Article PubMed Google Scholar
Sturza, J.: A review and meta-analysis of utility values for lung cancer. Med. Decis. Making 30 (6), 685–693 (2010). https://doi.org/10.1177/0272989x10369004
Paracha, N., Thuresson, P.O., Moreno, S.G., MacGilchrist, K.S.: Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 16 (5), 549–559 (2016). https://doi.org/10.1080/14737167.2016.1222907
Peasgood, T., Ward, S.E., Brazier, J.: Health-state utility values in breast cancer. Expert Rev. Pharmacoecon. Outcomes Res. 10 (5), 553–566 (2010). https://doi.org/10.1586/erp.10.65
Hao, Y., Wolfram, V., Cook, J.: A structured review of health utility measures and elicitation in advanced/metastatic breast cancer. Clin. Econ. Outcomes Res. CEOR 8 , 293–303 (2016). https://doi.org/10.2147/ceor.s100448
Schiller-Fruhwirth, I.C., Jahn, B., Arvandi, M., Siebert, U.: Cost-effectiveness models in breast cancer screening in the general population: a systematic review. Appl. Health Econ. Health Policy 15 (3), 333–351 (2017). https://doi.org/10.1007/s40258-017-0312-3
Carter, G.C., King, D.T., Hess, L.M., Mitchell, S.A., Taipale, K.L., Kiiskinen, U., Rajan, N., Novick, D., Liepa, A.M.: Health state utility values associated with advanced gastric, oesophageal, or gastro-oesophageal junction adenocarcinoma: a systematic review. J. Med. Econ. 18 (11), 954–966 (2015). https://doi.org/10.3111/13696998.2015.1066380
Djalalov, S., Rabeneck, L., Tomlinson, G., Bremner, K.E., Hilsden, R., Hoch, J.S.: A review and meta-analysis of colorectal cancer utilities. Med. Decis. Making 34 (6), 809–818 (2014). https://doi.org/10.1177/0272989x14536779
Jeong, K., Cairns, J.: Systematic review of health state utility values for economic evaluation of colorectal cancer. Heal. Econ. Rev. 6 (1), 36 (2016). https://doi.org/10.1186/s13561-016-0115-5
Richardson, J., Khan, M.A., Iezzi, A., Maxwell, A.: Comparing and explaining differences in the magnitude, content, and sensitivity of utilities predicted by the EQ-5D, SF-6D, HUI 3, 15D, QWB, and AQoL-8D multiattribute utility instruments. Med. Decis. Making 35 (3), 276–291 (2015). https://doi.org/10.1177/0272989x14543107
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35 (11), 1095–1108 (1997). https://doi.org/10.1097/00005650-199711000-00002
Article CAS PubMed Google Scholar
Mulhern, B.J., Bansback, N., Norman, R., Brazier, J.: Valuing the SF-6Dv2 classification system in the United Kingdom using a discrete-choice experiment with duration. Med. Care 58 (6), 566–573 (2020). https://doi.org/10.1097/mlr.0000000000001324
Galante, J., Augustovski, F., Colantonio, L., Bardach, A., Caporale, J., Marti, S.G., Kind, P.: Estimation and comparison of EQ-5D health states' utility weights for pneumoccocal and human papillomavirus diseases in Argentina, Chile, and the United Kingdom. Value Health 14 (5, Supplement), S60–S64 (2011). https://doi.org/10.1016/j.jval.2011.05.007
Takemoto, M.L., Lopes da Silva, N., Ribeiro-Pereira, A.C., Schilithz, A.O., Suzuki, C.: Differences in utility scores obtained through Brazilian and UK value sets: a cross-sectional study. Health Qual. Life Outcomes 13 , 119 (2015). https://doi.org/10.1186/s12955-015-0318-1
Article PubMed PubMed Central Google Scholar
Pollard, C., Hartz, S., Leage, S.L., Paget, M.A., Cook, J., Enstone, A.: Elicitation of health state utilities associated with varying severities of flares in systemic lupus erythematosus. Health Qual. Life Outcomes 13 , 66 (2015). https://doi.org/10.1186/s12955-015-0262-0
Article CAS PubMed PubMed Central Google Scholar
Brazier, J., Ara, R., Azzabi, I., Busschbach, J., Chevrou-Severac, H., Crawford, B., Cruz, L., Karnon, J., Lloyd, A., Paisley, S., Pickard, A.S.: Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR Good practices for outcomes research task force report. Value Health 22 (3), 267–275 (2019). https://doi.org/10.1016/j.jval.2019.01.004
Xie, F., Zoratti, M., Chan, K., Husereau, D., Krahn, M., Levine, O., Clifford, T., Schunemann, H., Guyatt, G.: Toward a centralized, systematic approach to the identification, appraisal, and use of health state utility values for reimbursement decision making: introducing the health utility book (HUB). Med. Decis. Making 39 (4), 370–378 (2019). https://doi.org/10.1177/0272989x19837969
Walker, D.G., Wilson, R.F., Sharma, R., Bridges, J., Niessen, L., Bass, E.B., Frick, K.: Best practices for conducting economic evaluations in health care: a systematic review of quality assessment tools. AHRQ Publication No. 12(13)-EHC132-EF. Agency for Healthcare Research and Quality (2012)
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., Augustovski, F., Briggs, A.H., Mauskopf, J., Loder, E.: Consolidated health economic evaluation reporting standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 16 (2), 231–250 (2013). https://doi.org/10.1016/j.jval.2013.02.002
Evers, S., Goossens, M., de Vet, H., van Tulder, M., Ament, A.: Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int. J. Technol. Assess. Health Care 21 (2), 240–245 (2005)
Nerich, V., Saing, S., Gamper, E.M., Holzner, B., Pivot, X., Viney, R., Kemmler, G.: Critical appraisal of health-state utility values used in breast cancer-related cost-utility analyses. Breast Cancer Res. Treat. (2017). https://doi.org/10.1007/s10549-017-4283-8
Chiou, C.F., Hay, J.W., Wallace, J.F., Bloom, B.S., Neumann, P.J., Sullivan, S.D., Yu, H.T., Keeler, E.B., Henning, J.M., Ofman, J.J.: Development and validation of a grading system for the quality of cost-effectiveness studies. Med. Care 41 (1), 32–44 (2003). https://doi.org/10.1097/01.mlr.0000039824.73620.e5
Simoens, S.: Assessment of methodological quality of economic evaluations in belgian drug reimbursement applications. PLoS One 8 (12), e85411 (2013). https://doi.org/10.1371/journal.pone.0085411
Stalmeier, P.F., Goldstein, M.K., Holmes, A.M., Lenert, L., Miyamoto, J., Stiggelbout, A.M., Torrance, G.W., Tsevat, J.: What should be reported in a methods section on utility assessment? Med. Decis. Making 21 (3), 200–207 (2001)
Article CAS Google Scholar
Petrou, S., Rivero-Arias, O., Dakin, H., Longworth, L., Oppe, M., Froud, R., Gray, A.: Preferred reporting items for studies mapping onto preference-based outcome measures: the MAPS statement. Pharmacoeconomics 33 (10), 985–991 (2015). https://doi.org/10.1007/s40273-015-0319-2
Xie, F., Pickard, A.S., Krabbe, P.F., Revicki, D., Viney, R., Devlin, N., Feeny, D.: A checklist for reporting valuation studies of multi-attribute utility-based instruments (CREATE). Pharmacoeconomics 33 (8), 867–877 (2015). https://doi.org/10.1007/s40273-015-0292-9
Brazier, J., Deverill, M., Green, C.: A review of the use of health status measures in economic evaluation. J. Health Serv. Res. Policy 4 (3), 174–184 (1999). https://doi.org/10.1177/135581969900400310
Drummond, M.F., Jefferson, T.O.: Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 313 (7052), 275–283 (1996)
Ungar, W.J., Santos, M.T.: The pediatric quality appraisal questionnaire: an instrument for evaluation of the pediatric health economics literature. Value Health 6 (5), 584–594 (2003). https://doi.org/10.1046/j.1524-4733.2003.65253.x
Clemens, K., Townsend, R., Luscombe, F., Mauskopf, J., Osterhaus, J., Bobula, J.: Methodological and conduct principles for pharmacoeconomic research. Pharmaceutical Research and Manufacturers of America. PharmacoEconomics 8 (2), 169–174 (1995)
Adams, M.E., McCall, N.T., Gray, D.T., Orza, M.J., Chalmers, T.C.: Economic analysis in randomized control trials. Med. Care 30 (3), 231–243 (1992)
Gerard, K.: Cost-utility in practice: a policy maker’s guide to the state of the art. Health Policy 21 (3), 249–279 (1992)
Sacristan, J.A., Soto, J., Galende, I.: Evaluation of pharmacoeconomic studies: utilization of a checklist. Ann. Pharmacother. 27 (9), 1126–1133 (1993). https://doi.org/10.1177/106002809302700919
Drummond, M., Manca, A., Sculpher, M.: Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int. J. Technol. Assess. Health Care 21 (2), 165–171 (2005)
Ramsey, S., Willke, R., Briggs, A., Brown, R., Buxton, M., Chawla, A., Cook, J., Glick, H., Liljas, B., Petitti, D., Reed, S.: Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 8 (5), 521–533 (2005). https://doi.org/10.1111/j.1524-4733.2005.00045.x
Goetghebeur, M.M., Wagner, M., Khoury, H., Levitt, R.J., Erickson, L.J., Rindress, D.: Evidence and value: Impact on decisionMaking–the EVIDEM framework and potential applications. BMC Health Serv. Res. 8 , 270 (2008). https://doi.org/10.1186/1472-6963-8-270
Davis, J.C., Robertson, M.C., Comans, T., Scuffham, P.A.: Guidelines for conducting and reporting economic evaluation of fall prevention strategies. Osteoporos Int. 22 (9), 2449–2459 (2011). https://doi.org/10.1007/s00198-010-1482-0
Vintzileos, A.M., Beazoglou, T.: Design, execution, interpretation, and reporting of economic evaluation studies in obstetrics. Am. J. Obstet. Gynecol. 191 (4), 1070–1076 (2004). https://doi.org/10.1016/j.ajog.2004.05.021
Grutters, J.P., Seferina, S.C., Tjan-Heijnen, V.C., van Kampen, R.J., Goettsch, W.G., Joore, M.A.: Bridging trial and decision: a checklist to frame health technology assessments for resource allocation decisions. Value Health 14 (5), 777–784 (2011). https://doi.org/10.1016/j.jval.2011.01.005
Russell, L.B., Gold, M.R., Siegel, J.E., Daniels, N., Weinstein, M.C.: The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. Jama 276 (14), 1172–1177 (1996)
Siegel, J.E., Weinstein, M.C., Russell, L.B., Gold, M.R.: Recommendations for reporting cost-effectiveness analyses. Panel on cost-effectiveness in health and medicine. Jama 276 (16), 1339–1341 (1996)
Weinstein, M.C., Siegel, J.E., Gold, M.R., Kamlet, M.S., Russell, L.B.: Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 276 (15), 1253–1258 (1996)
Sanders, G.D., Neumann, P.J., Basu, A., Brock, D.W., Feeny, D., Krahn, M., Kuntz, K.M., Meltzer, D.O., Owens, D.K., Prosser, L.A., Salomon, J.A., Sculpher, M.J., Trikalinos, T.A., Russell, L.B., Siegel, J.E., Ganiats, T.G.: Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 316 (10), 1093–1103 (2016). https://doi.org/10.1001/jama.2016.12195
Higgins, J.P.T., Altman, D.G., Gøtzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., Savović, J., Schulz, K.F., Weeks, L., Sterne, J.A.C.: The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343 (2011). https://doi.org/10.1136/bmj.d5928
Mokkink, L.B., Terwee, C.B., Patrick, D.L., Alonso, J., Stratford, P.W., Knol, D.L., Bouter, L.M., de Vet, H.C.: The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Quality Life Res. 19 (4), 539–549 (2010). https://doi.org/10.1007/s11136-010-9606-8
Download references
No funding was received for this research.
Author information
Authors and affiliations.
Department of Health Research Methodology, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Michael J. Zoratti, Mitchell Levine, Lehana Thabane & Feng Xie
Department of Pharmacy Systems, Outcomes and Policy, College of Pharmacy, The University of Illinois at Chicago, Chicago, IL, USA
A. Simon Pickard
Health Evidence, Radboud University Medical Centre, Nijmegen, The Netherlands
Peep F. M. Stalmeier
Center for the Evaluation of Value and Risk in Health, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA, USA
Daniel Ollendorf
Acaster Lloyd Consulting Ltd, London, UK
Andrew Lloyd
Sunnybrook Odette Cancer Centre, Toronto, ON, Canada
Kelvin K W Chan
Canadian Centre for Applied Research in Cancer Control, Toronto, Canada
School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada
Don Husereau
School of Health and Related Research, University of Sheffield, Sheffield, UK
John E. Brazier
Toronto General Hospital Research Institute, University Health Network, Toronto, ON, Canada
Murray Krahn
Department of Medicine, Division of Clinical Pharmacology and Toxicology, McMaster University, Hamilton, ON, Canada
Mitchell Levine
St Joseph’s Healthcare, Hamilton, ON, Canada
Lehana Thabane
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Feng Xie .
Ethics declarations
Conflicts of interest.
None to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 59 KB)
Rights and permissions.
Reprints and permissions
About this article
Zoratti, M.J., Pickard, A.S., Stalmeier, P.F.M. et al. Evaluating the conduct and application of health utility studies: a review of critical appraisal tools and reporting checklists. Eur J Health Econ 22 , 723–733 (2021). https://doi.org/10.1007/s10198-021-01286-0
Download citation
Received : 22 July 2020
Accepted : 12 March 2021
Published : 11 April 2021
Issue Date : July 2021
DOI : https://doi.org/10.1007/s10198-021-01286-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health utility
- Cost utility analysis
- Methodology
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 14 May 2024
Effect of cytoplasmic fragmentation on embryo development, quality, and pregnancy outcome: a systematic review of the literature
- Ariella Yazdani 1 , 3 ,
- Iman Halvaei 2 ,
- Catherine Boniface 1 &
- Navid Esfandiari ORCID: orcid.org/0000-0003-0979-5236 1 , 4
Reproductive Biology and Endocrinology volume 22 , Article number: 55 ( 2024 ) Cite this article
241 Accesses
Metrics details
The role of cytoplasmic fragmentation in human embryo development and reproductive potential is widely recognized, albeit without standard definition nor agreed upon implication. While fragmentation is best understood to be a natural process across species, the origin of fragmentation remains incompletely understood and likely multifactorial. Several factors including embryo culture condition, gamete quality, aneuploidy, and abnormal cytokinesis seem to have important role in the etiology of cytoplasmic fragmentation. Fragmentation reduces the volume of cytoplasm and depletes embryo of essential organelles and regulatory proteins, compromising the developmental potential of the embryo. While it has been shown that degree of fragmentation and embryo implantation potential are inversely proportional, the degree, pattern, and distribution of fragmentation as it relates to pregnancy outcome is debated in the literature. This review highlights some of the challenges in analysis of fragmentation, while revealing trends in our evolving knowledge of how fragmentation may relate to functional development of the human embryos, implantation, and pregnancy outcome.
Introduction
Human preimplantation embryo scoring systems have been widely used to predict blastocyst development and implantation rate after in-vitro fertilization (IVF). The grading of embryos on day-2 and -3 after fertilization is largely subjective and interpretation varies across IVF laboratories, as it is commonly based on morphological appearance. Characteristics in early embryo grading schema include the amount of cytoplasmic fragmentation (CF) during early cleavage, speed of cellular division, number, size, and symmetry of cells (blastomeres). As defined by the Istanbul consensus workshop on embryo assessment, a fragment is “an extracellular membrane-bound cytoplasmic structure that is < 45 µm diameter in a day-2 embryo and < 40 µm diameter in a day-3 embryo” [ 1 ]. There are several different systems to evaluate embryo morphology including Hill’s scoring system [ 2 ] Cummins' grading system [ 3 ] ASEBIR grading system [ 1 ], the UK/ACE grading scheme [ 4 ]; each system has its own classification for degree of fragmentation as well as embryo grade. This heterogeneity further complicates analysis of fragmentation in relation to outcomes.
CF has been shown to occur early in embryonic division and is a common phenomenon seen in embryos cultured in vitro. CF has traditionally been used as a metric of embryo implantation potential [ 3 , 5 , 6 , 7 ]. The amount and pattern of fragments are analyzed in early development, incorporated into the embryo grade depending on grading system, and used to help select the most developmentally competent embryo to be transferred during an IVF cycle. This classification system is important as a proportion of embryos within a single cohort will not successfully develop to the blastocyst stage in vitro. Although there are various contributing factors to an embryo’s developmental capacity and viability, it is largely agreed upon that fragmentation plays an important role. It seems that the etiology of embryo fragmentation is not fully understood but it may be related to several factors like gamete quality, culture condition, and genetic abnormalities in the embryo [ 8 ]. It is difficult to directly compare and quantify relative degrees of fragmentation across studies. However, it has been repeatedly shown that the extent of fragmentation and implantation potential are inversely proportional [ 5 , 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ]. While a low degree of fragmentation does not seem to significantly impact embryo viability, severe fragmentation does [ 7 , 22 , 23 ]. Alongside the cell to cytoplasmic ratio, the pattern and distribution of fragmentation influence the developmental quality of the embryo [ 7 , 24 ]. There are two main patterns of embryo cytoplasmic fragments: scattered and concentrated. The former is characterized by fragment contact within several blastomeres and is related to aneuploidy [ 25 ]. Time-lapse studies have shown that fragmentation is thought to be a dynamic process, where some fragments can be expelled or reintroduced into the cells as the embryo continues to divide [ 25 , 26 ]. Fragments can also easily move or rotate around the associated blastomere and change their position in the embryo [ 27 ].
Current grading systems used to evaluate cleavage-stage embryos are largely based on day-2 or -3 morphology. This can be problematic, as developmental growth of an embryo is variable and the grade of a developing embryo at one point in time is not guaranteed to persist. For example, studies have suggested that embryo selection on day-2 or -3 based on morphological grade can be unreliable and lead to negative pregnancy outcomes [ 28 , 29 , 30 ]. Accordingly, new parameters for predicting implantation success have been proposed including extended embryo culture to the blastocyst stage to day-5, -6 or -7 [ 31 ]. Delaying embryo transfer to the blastocyst stage is advantageous as it can limit the number of unsuccessful embryo transfers and biochemical pregnancies or clinical pregnancy losses in IVF. While there are multiple reports on the impact of cleavage-stage embryo quality on blastocyst formation and blastocyst quality [ 32 , 33 ], few have specifically looked at the degree of fragmentation as a predictive variable.
In this systematic review, we comprehensively reviewed the available literature on the origin and characteristics of CF, factors affecting CF, and the effect of CF and fragment removal on embryo development and pregnancy rate.
Materials and methods
A search was conducted on October 10, 2023, using PubMed and Google Scholar databases in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [ 34 ]. In PubMed, the search terms “embryo*[tw] OR cleavage stage [tw] OR "Embryonic Structures"[Mesh] OR "Embryonic Development"[Mesh] OR "Embryo, Mammalian"[Mesh] OR "Cleavage Stage, Ovum"[Mesh]” AND “cytoplasm*[tw] AND fragment*[tw] AND “(Blastocyst*[tw] OR "Blastocyst"[Mesh]) AND (form* OR develop* OR quality*)” were used. A title search in Google Scholar using search terms as above and “embryo cytoplasm fragmentation”, “blastocyst quality”, “blastocyst development” was performed. Only full-text publications in English were included. Full-text articles which did not have any mention of cytoplasmic or embryo fragmentation were excluded, however articles which mentioned both DNA fragmentation and CF were included. Since most of the studies discussing CF also discussed other morphologic features of the embryo, studies that mention embryo morphology, grade or quality were also included. Articles that looked at non-human embryo fragmentation, case reports, case series, book chapters and review papers were excluded. Titles and abstracts were screened, and study quality and bias were assessed. The primary outcomes of interest were embryo quality, blastocyst formation, and pregnancy outcome.
Figure 1 provides details of study screening and inclusion. There were 206 studies screened between the two search engines PubMed ( n =106) and Google Scholar ( n =100). There were 18 duplicates giving a total of 188 articles. Due to the small number of studies from the search criteria, no filter of time was placed. After removal of non-full text articles, articles that used non-human embryos, and articles not relevant to the topic, 20 articles were eligible for inclusion. Forty relevant references from the articles were also extracted, reviewed, and included in this review. These additional articles were reviewed with the same inclusion and exclusion criteria as mentioned above. A total of 60 articles were included in the qualitative synthesis of this review.
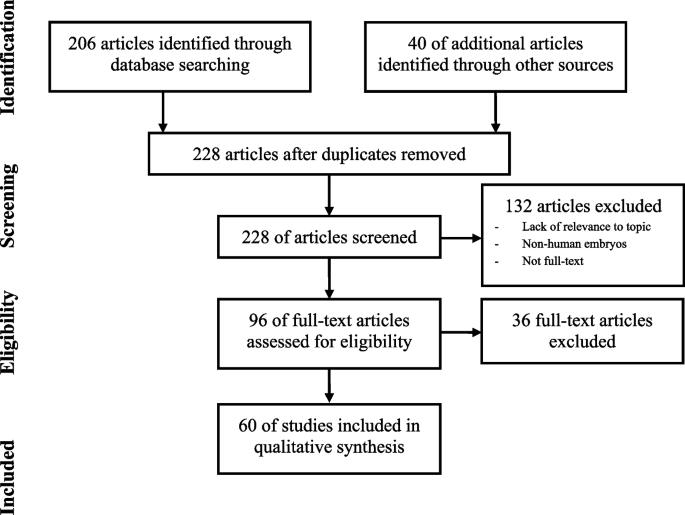
Article Identification and Screening
Origin and etiology of CF
The etiology of CF is not completely understood. There are several proposed theories as to why embryos display variable degrees of fragmentation. Fragmentation has been shown to be a natural, unpredictable process both in vitro and in vivo and is documented in various species [ 35 , 36 ]. This suggests that embryo fragmentation is neither species-specific nor solely a byproduct of in vitro culture. Assisted reproductive technology (ART) and IVF techniques, such as time-lapse microscopy (TLM) and transmission electron microscopic (TEM) analyses, have recently allowed for further understanding of embryo developmental potential and fragmentation (Figs. 2 and 3 ). Seven of the included studies in this review propose potential hypotheses as to the origin of CF (Table 1 ). Three of the articles evaluated gamete quality as related to fragmentation in a developing embryo [ 37 , 38 , 39 ].
Human cleavage stage embryos a) Day-2 embryo at 4-cell stage with no fragmentation, b) fragmented Day-2 embryo, c) Day-3 embryo at 8-cell stage with no fragmentation, d) fragmented Day-3 embryo, e) Day-5 cavitating Morula with no fragmentation, f) fragmented Day-5 cavitating Morula
Ultrastructure and organelle microtopography of an embryo fragment by transmission electron microscopy. Ly: primary lysosome, M: mitochondrion, rM: remnant of regressing mitochondrion, MV: mitochondria-vesicle complex, V: vesicle; scale bar: 1 µM
An early study showed that sperm DNA oxidation has been associated with embryo development and quality, and therefore linked to CF [ 37 ]. Nucleolar asynchrony in the zygote from sperm DNA fragmentation has previously been shown to predict future low-quality blastocyst development. A positive correlation has also been found between the percentage of sperm OxiDNA-stained cells with embryo fragmentation on day-2 and -3 of development. Sperm DNA oxidation may therefore be associated with fragmented, nonviable, poor-quality embryos [ 37 ] . A recent study also showed the negative correlation between sperm DNA fragmentation and blastomere DNA fragmentation and blastulation rate [ 40 ]. Further studies are needed to confirm the impact of sperm DNA oxidation on embryo fragmentation.
An observational study documented the degree of fragmentation of human embryos as they progressed through mitotic cell cycles [ 38 ]. In this study, the authors analyzed nearly 2,000 oocytes and 372 embryos, and found that increased embryo fragmentation (>50%) was associated with a specific pattern of development: delayed first division (oocyte spindle detected at 36.2 hours after hCG injection vs. 35.5 hours in low fragmentation), a significantly earlier start of the second mitosis (8.9 hours vs. 10.8 hours after the first mitosis), and a significant delay of the third mitosis after the second mitosis (2.2. hours vs. 0.6 hours). The authors did not comment on whether fragmentation could be a result of the cell dividing before proper chromosome alignment, or if existing aneuploidy resulted in erroneous cleavage patterns [ 38 ].
Polar body (PB) fragmentation has also been investigated in relation to cytoplasmic fragmentation. Ebner et al., in a prospective study analyzed the relationship between a fragmented first PB and embryo quality in patients undergoing ICSI. Two groups of oocytes were analyzed according to PB fragmentation: intact first PBs and those with fragmented PBs. Forty-two hours after ICSI, embryo morphology (i.e., number of blastomeres and degree of fragmentation) was recorded. Overall, a significantly higher percentage of cytoplasmic fragmentation was seen in day-2 embryos that originated from oocytes with fragmented first PBs than those with intact PBs ( P < 0.05). This study further supports the concept that oocyte quality contributes to overall embryo fragmentation and provides evidence that preselection of oocytes may contribute to the prognosis of embryo quality and blastocyst development [ 39 ]. The role of PB fragmentation on embryo quality was confirmed in other studies [ 41 , 42 ], however, a recent study has not recommended considering PB status as a tool for embryo selection [ 43 ].
Beyond analysis of gamete quality, other studies have shown a biochemical relationship between embryo competence and fragmentation. One study showed that disturbances in E-cadherin, a cell adhesion protein that plays a critical role in morphogenesis, occur in embryos with cleavage abnormalities and extensive cytoplasmic fragmentation, suggesting a possible mechanism to the loss of embryonic viability [ 44 ]. Further, by using mitochondrial fluorescence techniques, Van Blerkom et al., found that mitochondrial distribution at the pronuclear stage may be an epigenetic factor related to the organization of the embryo and further embryonic development [ 45 ]. Blastomeres that were deficient in mitochondria and thus ATP at the first or second cell division remained undivided and often died during subsequent culture. Although this study examined morphologically normal (unfragmented) cleavage-stage embryos, it may support the idea that perinuclear mitochondrial distribution and microtubular organization influence developmental capacity of early cleavage-stage embryos [ 45 ]. Higher numbers of mitochondria reported in fragmented compared to the normal blastomeres show the rapid depletion of ATP in the fragmented embryos [ 21 ]. There have also been reports of increased gene transcription of mitochondrial factors like OXPHOS complexes, ATP synthase, and mtDNA content in highly fragmented embryos compared to controls [ 46 ]. Mitochondrial activity is lower and more centralized in fragmented embryos compared to good quality embryos on day-3 [ 47 ]. Mitochondria are the main source of ATP for embryo mitosis, and their proper function is essential for embryo development. More research is needed to elucidate the morphology and role of mitochondria in embryo development, especially in relation to fragmentation.
A subsequent study by Van Blerkom et al., analyzed the temporal and spatial aspects of fragmentation through TLM and TEM analyses from the pronuclear to the 10-12-cell stage. Through TLM, the authors visualized the non-discrete, dynamic nature of fragments and noted that many were “bleb-elaborations” of the plasma membrane and cytoplasm. They characterized two patterns of fragmentation: definitive and pseudo-fragmentation. Definitive fragmentation was described as fragments detached from a blastomere, and pseudo-fragmentation was assigned when the fragments were no longer detectable during subsequent development. Often one developing embryo would show both fragmentation patterns at different stages of development, suggesting that these patterns may have different etiologies and effects on embryo development competence [ 47 ]. Hardarson et al., similarly used TLM to document that fragments are dynamic and can be internalized throughout cleavage during culture periods. The contents of the fragments were noted to be internalized and released into the cytoplasm of the blastomere and seen on multiple time-lapse photographs as a cytoplasmic turbulence. This is the first reported evidence that cellular fragments can “disappear” during the culture period in human IVF [ 26 ]. It seems that in mild to moderate CF, the timing of embryo evaluation and grading can affect the reported percent of fragmentation.
Lastly, we have included a preliminary study performed by Sermondade et al., that suggests a specific subgroup of patients who have had repeated IVF failures (presumably due to a recurring high rate of fragmented embryos) may benefit from early intrauterine embryo transfer at the zygote stage (2PN) [ 48 ]. Data showed a delivery rate per oocyte retrieval of 18.9%, which was significantly higher than the delivery rate of 7.5% in the matched control group. The results were encouraging and suggestive of a safe, non-invasive rescue strategy for patients who experience recurrent highly fragmented embryos and failed IVF attempts. The data further suggests that fertilized oocytes of this subgroup may have deficiencies in certain maternal factors (i.e., stress-response factors) that do not allow normal embryo development in culture environments [ 48 ]. Another study was also confirmed application of zygote transfer in patients with history of low-quality embryos [ 49 ]. However, further studies are required to verify the impact of this technique for patients with history of fragmented embryos.
Apoptosis is another proposed etiology of fragmentation. Apoptosis may occur in blastomeres with defective cytoplasm or abnormal chromosomes, leading to embryo fragmentation [ 50 ]. There are several studies reporting apoptosis in both fragments and neighboring blastomeres in a fragmented embryo [ 24 , 50 ]. Chi et al., showed that fragments are associated with both apoptosis and necrosis [ 21 ]. One of the factors that appears to induce apoptosis in blastomeres is suboptimal culture conditions such as hypoxia [ 51 ]. In addition, there are controversial reports on the role of reactive oxygen species (ROS) in embryo fragmentation [ 52 , 53 ]. It has been shown that ROS are present at high levels in the culture media of fragmented embryos [ 52 , 54 ]. Chen et al., recently showed that embryo culture in 5% oxygen, from days 1 to 3, is associated with higher embryo quality and live birth rate compared to 20% oxygen [ 55 ]. The effects of culture condition modifications, such as hypoxia and ROS, on embryo fragmentation need to be clarified to understand the importance of culture condition in this process.
Membrane compartmentalization of DNA, abnormal cytokinesis, and extra vesicular formation are other proposed theories for embryo fragmentation [ 8 ]. Defects or damages in mitochondria are associated with low ATP and high ROS production leading to a compromised cell division and cytokinesis [ 27 ]. In addition, there is a correlation between embryo fragmentation and ploidy status. Chavez et al., showed that CF was seen in a high proportion of aneuploid embryos, and that meiotic and mitotic errors may cause fragmentation in different cell development stages. Meiotic errors were associated with fragmentation at one-cell stage while mitotic errors were associated with fragmentation at interphase or after first cytokinesis [ 56 ]. Chromosomally abnormal embryos often have severe fragmentation, which may be another cause of CF [ 55 , 57 ].
Overall, the precise cause of CF has yet to be clearly defined. The above investigations have elucidated potential sources and associations of what is likely a complex and multifactorial process and represent our current understanding of CF origin.
What is contained in CF?
Four of the included studies used various technological advances to study the contents of CF in human embryos (Table 2 ). Two studies used TEM methods to evaluate fragment ultrastructure (Fig. 3 ) [ 21 , 58 ]. Fragments were extracted from embryos with 10-50% fragmentation and the ultrastructure evaluated by TEM. Micrographs showed that the fragments had a distinct membrane containing cytoplasmic organelles including mitochondria, mitochondria-vesicle complexes, Golgi apparatus, primary lysosomes, and vacuoles. Mitochondria were the most abundant structure.
In an additional evaluation of CF contents, Johansson et al., analyzed DNA content of fragments to define a cutoff diameter for an anucleate fragment or blastomere. Findings showed that 98% of fragments <45 µm on day-2 and 97% of those <40 µm on day-3 contained no DNA and, if not reabsorbed into a blastomere, showed a loss of cytoplasm. Presence of essential blastomere organelles such as mitochondria, mRNA, and proteins within cytoplasmic fragments were related to embryo development arrest [ 59 ]. Lastly, Chi et al., also used TEM to examine ultrastructure of the human fragmented embryos and found that blastomeres with anucleate fragments contained fewer mitochondria in their cytoplasm compared to normal blastomeres [ 21 ].
Cell death and CF
Eight of the included studies analyzed the relationship between cell death and embryo fragmentation (Table 3 ). Five studies analyzed the status of chromatin in arrested fragmented embryos through a combined technique for simultaneous nuclear and terminal transferase-mediated DNA end labelling (TUNEL) [ 24 , 60 , 61 , 62 , 63 ]. Two studies used a comet assay to analyze DNA fragmentation [ 21 , 63 ]. Four of the eight studies used Annexin V staining [ 21 , 61 , 62 , 63 ] with three including the presence of propidium iodide (PI) to compare apoptosis to necrosis [ 21 , 61 , 63 ].
Jurisicova et al., used a combined nuclear and fragmented DNA labeling approach which allowed distinction between chromatin status and DNA fragmentation, which serve as markers of apoptosis versus necrosis respectively [ 60 ]. After fertilization, embryos were stained with 4,6-diamidino-2-phenylindole (DAPI). In cases of compromised cell membrane integrity, DAPI stain was observed in the cytoplasm as a sign of necrosis. Concomitant use of TUNEL labeling reflected the integrity of the DNA and allowed distinction between necrotic and apoptotic cells. Through combined techniques of DAPI/TUNEL, TEM, scanning electron microscopy (SEM) and stereomicroscopic observations, 153 of 203 (75.4%) fragmented early cleavage-stage embryos displayed signs of apoptosis (i.e., chromatin condensation, cellular shrinkage, DNA fragmentation, presence of cell corpses) with or without normal nuclei [ 60 ].
Similarly, Levy et al., analyzed early arrested or fragmented preimplantation embryos and the pattern of DNA fragmentation using TUNEL assay and the presence of phosphatidylserine through Fluorescein isothiocyanate (FITC)-labelled Annexin V, a phosphatidylserine binding protein. The authors observed TUNEL staining in one or more nuclei of 15 out of 50 (30%) arrested embryos from the 2-cell stage to uncompacted morulae, all of which had high degrees of CF. Furthermore, embryos with regular-sized blastomeres without fragmentation were all TUNEL negative [ 50 ].
A separate prospective study by Antczak et al., explored the possible association between fragmentation and apoptosis using PI and Annexin V staining of plasma membrane phosphatidylserine and TUNEL analysis of blastomere DNA [ 24 ]. In contradistinction to prior studies, these authors found no direct correlation between fragmentation and apoptosis. Virtually all blastomeres that were PI negative, intact or fragmented, showed no TUNEL or annexin V fluorescence, suggesting no signs of apoptosis [ 24 ].
Liu et al., used a similar methodology of TUNEL labeling and Annexin V staining to detect markers of apoptosis in fragmented human embryos derived from IVF [ 61 ]. Overall, highly fragmented embryos had apoptotic features including bright fluorescence (positive TUNEL labeling signifying DNA fragmentation) on the cell corpses and in intact blastomeres [ 61 ]. By staining cells with both annexin V and PI, this study was able to demonstrate that apoptosis occurs frequently in fragmented human embryos and the coexistence of apoptotic, necrotic and viable sibling blastomeres can occur. Sibling blastomeres within an embryo often showed apoptotic features that led to secondary necrosis while others did not initiate apoptosis. The authors did not find a significant difference in the expression frequency of apoptotic genes between viable and nonviable or arrested embryos [ 61 ].
Chi et al., stained human embryos ( n =10) with annexin V and PI and found that human fragmented embryos exhibited characteristics of both necrosis and apoptosis [ 20 ]. Rather than TUNEL assay, these authors used a modified sperm comet assay to investigate DNA fragmentation of human fragmented embryos. They found that 6/7 human fragmented embryos (85.1%) stained positively for PI with the intensity of staining increasing with the degree of fragmentation. Of note, DNA fragmentation was observed in fragmented human embryos but not in the normal embryo [ 21 ].
Metcalfe et al., analyzed the expression of 11 BCL-2 family genes in normally developing embryos and in severely fragmented embryos [ 64 ]. They found that the expression of BCL-2 family genes was highest in the pronuclear stage and eight-cell stages, and lowest at the two-cell, four-cell, and blastocyst stages in developmentally intact embryos. Furthermore, the expression did not change in fragmented embryos, suggesting that embryo fragmentation does not likely compromise mRNA integrity and gene detection [ 64 ]. However, like Liu et al., [ 61 ] these authors did detect far fewer pro-apoptotic BCL-2 genes in fragmented embryos at the eight-cell stage. The authors noted that these findings do not distinguish between iatrogenic apoptosis from suboptimal in-vitro culture conditions [ 64 ]. A separate study by Jurisicova et al. similarly analyzed gene expression at the 2-, 4- and 8-cell stage of fragmented embryos. Embryos that had 30-50% fragmentation showed a significant increase in Hrk mRNA levels, a BCL-2 protein encoding gene ( P = 0.016). Further, these authors found an increase in Caspase-3 mRNA in fragmented embryos, as well as induction of Caspase-3-like enzyme activity in nucleated fragments, although this finding was not statistically significant [ 65 ].
Van Blerkom et al., also used TUNEL assay in conjunction with the comet assay as a method of identifying the specific pattern of cell death (necrosis, lysis or apoptosis) and the extent of DNA damage in developing embryos [ 47 ]. They analyzed the integrity of the plasma membrane through annexin V staining with PI. They examined both transient and persistent fragment clusters at day-3 and 3.5 embryos for evidence of programed cell death using time-lapse video and TEM. In contrast to previous studies, they found no indication of nuclear DNA damage or loss of membrane integrity. These results, led the authors to hypothesize that the fragmentation observed was not characteristic of programed cell death, but rather resembled features of oncosis. The culture in this study was not severely oxygen-deprived and thus the authors concluded that this oncosis-like process was potentially a result of disproportionate mitochondrial segregation during the first cleavage division. Without sufficient mitochondria, the early blastomeres did not maintain adequate ATP for normal cell function which may have precipitated an ATP-driven oncosis-like process [ 47 ].
Lastly, a study by Bencomo et al., found correlations between the degree of apoptosis in human granulosa-lutein (GL) cells, the outcome of IVF-ET cycle, the percentage of embryo fragmentation, and patient’s age [ 66 ]. Human GL cells were collected from follicular fluid, cultured for 48 hours, and marked with caspACE FITC-VAD-FMK, a fluorescent marker for activated caspases. Results showed that GL cells of older women (>38 years old) were significantly more susceptible to apoptosis at 43.2 ± 18.0% compared to the younger group (<38 years old) with a mean percentage of apoptotic cells 33 ± 17.2%. Women who had a positive pregnancy had a lower level of apoptosis in GL cultures than those who did not get pregnant (30.2 ± 14% vs. 40.4 ± 19.5%). There was a positive correlation between embryo fragmentation and GL cell apoptosis ( r = 0.214). Overall, the level of apoptosis of cultured GL cells was correlated with IVF outcome [ 66 ].
These studies demonstrate the diversity among techniques to evaluate cell death in the developing embryo. TUNEL labeling, sperm comet assay, annexin V staining or some combination of these techniques have been described. Furthermore, there are discrepancies between the stage at which apoptosis might occur, with majority of studies cited here suggesting that cell death occurs in early stages of development before blastocyst formation. While some studies suggest that fragmented embryos display signs of apoptosis, these findings are still disputed and the distinction between apoptosis and necrosis is not clearly defined in the literature.
Patient age and CF
There are inconsistencies within the literature regarding the relationship between maternal age and CF. A total of six studies in this review focused on this relationship (Table 4 ). Three of the studies found a positive correlation between patient age and degree of embryo fragmentation [ 67 , 68 , 69 ]. The other three studies found no age-related correlation between embryo fragmentation or quality [ 7 , 70 , 71 ].
A retrospective study by Ziebe et al., compared the relationship between age of women undergoing IVF and the proportion of anucleate fragmentation in cleavage-stage embryos. Using a logistic regression analysis, the authors compared the percentage of transfers using fragmented embryos with age; the odds of fragmentation increased by 3% per year (OR 1.033 [95% CI 0.996, 1.071]). There was a linear relationship between age and embryo fragmentation rate, with an increase in fragmentation of 0.76% per year (95% CI -0.09%, 1.61%) [ 68 ].
Keltz et al., assessed various predictors of embryo fragmentation in IVF and found that increased maternal age and lower number of oocytes and embryos were associated with increased embryo fragmentation. There was a significant difference between cycles with fragmented embryos ( n =74) at a mean age of 36.9 ± 4.24 years as compared to cycles with no fragmented embryos ( n =234) at a mean age of 35.4 ± 4.74 years. Overall, this retrospective analysis of fresh IVF cycles found that embryo fragmentation is indeed associated with older age and ultimately poor cycle outcome [ 67 ].
Contrary to these findings, an early study by Alikani et al., showed no relationship between maternal age and CF [ 7 ]. In a retrospective analysis of degree and pattern of embryo fragmentation on days 2 and 3, they defined five patterns of fragmentation. Both the degree and pattern of fragmentation impacted pregnancy and implantation rate, but the authors found no correlation between appearance of any CF pattern and maternal age. The average maternal age in their population was 35.7 ± 4.25 years [ 7 ]. Another study by Stensen et al., analyzed the effect of chronological age on oocyte quality (assessed by maturity) and embryo quality (assessed by cleavage-stage, blastomere size and embryo fragmentation). Women were divided into five age groups: ≤25, 26–30, 31–35, 36–40 and ≥41 years. The embryo morphological score was based on fragmentation and blastomere size with score of 0-4 where score of 4 being equally sized blastomeres and no fragmentation and score of 0 being cleavage arrest or morphologically abnormal embryo. The mean oocyte score and embryo morphology score were not found to be significantly different across the age groups [ 70 ]. Wu et al., also showed that age does not influence embryo fragmentation. Patient ages ranged from 20 to 44 years with a mean age of 30.6 ± 4.6 years and were divided into age groups of ≤29, 30–34, 35–37, 38–40, and ≥41 years of age. Analysis of embryos with similar degrees of fragmentation was used to assess whether maternal age was associated with embryo fragmentation and blastocyst development. There was no correlation between age and embryo fragmentation as a continuous variable ( r = 0.02; P = 0.25) nor was there a correlation when age was divided into the groups ( P = 0.2). They also found that neither age ( r = -0.08; P =0.16) nor degree of fragmentation ( r = -0.01; P = 0.81) had a significant impact on blastocyst development [ 71 ].
Recently, a retrospective time-lapse study evaluated the implantation rate of 379 fragmented embryos. The results showed that there was an association between advanced maternal age and fragmentation. Fragmentation rate was higher in patients ˃35 compared to patients ≤35 years old. It seems that the lower quality of oocytes in older patients results in increasing fragmentation [ 69 ]. Overall, the included studies have differing conclusions on the effect of maternal age and CF; varying definitions and analysis of CF remain a limitation.
IVF vs ICSI procedures and CF
Five of the included studies compared embryo quality between conventional IVF and intracytoplasmic sperm injection (ICSI) procedures (Table 5 ). Two of these studies found that ICSI was associated with impaired embryo morphology compared to IVF [ 72 , 73 ], while the other three showed no difference in embryo quality between the two fertilization modalities [ 74 , 75 , 76 ]. There were no studies within our search that identified embryos created by ICSI having greater morphology grade, or less embryo fragmentation, than IVF.
Frattarelli et al., directly examined the effect of ICSI on embryo fragmentation and implantation rate compared to IVF. There was a significant difference in mean embryo grade between IVF and ICSI. IVF patients had significantly more grade I, or non-fragmented, embryos compared to the ICSI group ( P < 0.01). However, there was no significant difference in mean number of embryos per embryo grade II – IV [ 72 ].
Similarly, Hsu et al., compared embryo quality, morphology, and cleavage after ICSI with standard IVF patients. They defined the grading system from 1 – 5, ranging from no fragments (grade 1) to severe or complete fragmentation (grade 5). They found that for the overall population, when comparing ICSI and IVF patients after matching for age and number of embryos transferred, the number of embryos with good morphology was significantly greater in the IVF group compared to ICSI ( P < 0.006). The average morphology scores, similar to the results of Frattarelli et al., were significantly different between the ICSI group and the IVF group. They also found IVF patients’ embryos to have significantly better cleavage rate than those from ICSI patients ( P < 0.001) [ 73 ].
Garello et al., evaluated if fertilization via ICSI influences pronuclear orientation, PB placement, and embryo quality when compared to IVF. Embryos were assessed using morphology, and grouped as good (grades 1-2), average (grades 3-4), or poor (grades 5-6). Embryos were also assessed for cleavage regularity and proportion of fragmentation (0, <20%, 20–50%, >50%). There was no statistically significant difference in mean morphology (good, average, poor) between the groups, although they did note an apparent increase in grade 4 versus grade 3 embryos after ICSI procedure. The two groups had similar proportions of fragmentation [ 74 ].
Two other studies took a unique approach in comparing embryo quality in ICSI and IVF patients by using randomized sibling oocytes [ 75 , 76 ]. Yoeli et al., studied oocytes retrieved from patients with a less than 40% fertilization rate in a previous standard IVF cycle and divided these oocytes into a conventional insemination group and an ICSI group. Each group had over 1400 oocytes. Overall, there was no significant difference between the IVF and ICSI groups in terms of cleavage rate or rate of high-quality embryos (both Grade A embryos with ≤10% fragmentation and embryos with ≤20% fragmentation) [ 75 ]. Ruiz et al., also analyzed sibling oocytes in patients who had failed intrauterine insemination attempts. The authors similarly found no significant difference in fertilization rates and degree of fragmentation between ICSI and standard IVF groups [ 76 ]. Most studies included in the search criteria showed that ART techniques such as ICSI do not significantly impact fragmentation rate in developing embryos, suggesting that ICSI is not a significant contributor to poorer outcomes by way of embryo fragmentation. Of note, the timing of cumulus cell denudation after conventional IVF is a matter of debate; none of the included studies in this review performed short-time insemination. In a meta-analysis reviewing denudation times, the number of good quality embryos produced after retaining cumulus cells was similar to those produced after early removal of these cells, suggesting that brief insemination has no impact on CF [ 77 ]. Liu et al. also showed that short insemination time is not associated with different outcomes in terms of embryo development [ 78 ].
Effect of CF on embryo development
It is commonly believed that CF has detrimental effects on embryo development. Thirteen of the included studies found a negative effect of CF on embryo development (Table 6 ). Various approaches have been used to propose a hypothesis as to how increased fragmentation impedes embryo development.
Van Blerkom et al., showed through time-lapse video and TEM that fragments physically impede cell-cell interactions, interfering with compaction, cavitation, and blastocyst formation [ 63 ]. In an ultrastructural observational study by Sathananthan et al., 15 embryos were cultured with human ampullary cell lines and TEM used to evaluate embryo development. They noted degeneration of blastomeres, including incomplete incorporation of chromatin into nuclei and formation of micronuclei, which was possibly a consequence of being adjacent to blastomere fragments [ 79 ]. A much larger prospective study by Antczak and Van Blerkom analyzed 2293 fertilized eggs from 257 IVF cycles to examine the effect of fragmentation on the distribution of eight regulatory proteins. Fragmentation reduced the volume of cytoplasm and depleted embryos of essential organelles or regulatory proteins, compromising the embryo developmental potential. They also found that specific fragmentation patterns during various stages of embryo development, i.e., 2- and 4-cell stages, were associated with embryo viability and therefore could have clinical application in the selection of embryos for transfer [ 24 ]. As previously mentioned, fragmentation may affect compacted/morula and blastocyst quality [ 80 ]. Cell exclusion at this stage is due to failure or abnormal expression of proteins involved in compaction [ 44 , 81 ]. Blastomeres may also irregularly divide, resulting in fragmentation and exclusion from compaction [ 82 ], and excluded cells have a high rate of aneuploidy [ 83 ]. Blastocyst quality from fully compacted embryos has been reported to be higher than blastocysts with partial compaction [ 84 ].
The hypothesis that fragmentation reflects inherent embryogenetic abnormalities, such as aneuploidy, increased mosaicism, or polyploidy, is supported by multiple studies in this review [ 55 , 57 , 85 ]. Morphologically poor-quality embryos, defined by amount of fragmentation, were often found to have concomitant chromosomal abnormalities [ 57 , 85 ]. Culture environment has also been implicated in presence and degree of fragmentation. For example, Morgan et al., using video-cinematography found that embryos cultured on a monolayer of feeder cells had fewer fragments than did embryos cultured alone [ 86 ]. In addition to aneuploidy and external environment, degree of fragmentation also appears to be related to embryo quality. Both Alikani et al., and Hardy et al., have shown that a small degree of fragmentation (<15%) on day-2 embryos did not affect blastocyst formation but increased (> 15%) fragmentation was associated with significantly reduced blastocyst development [ 23 , 87 ]. Similarly, a prospective study of over 4000 embryos by Guerif et al., showed that the rate of blastocyst formation increased significantly with decreased fragmentation (<20%) on day-2 embryos [ 32 ].
A separate study by Ivec et al., graded day-4 and -5 morulae based on the degree of fragmentation (<5%, 5%–20%, or >20%) and compared their blastocyst development rate. They found a negative correlation between degree of fragmentation and clinically usable blastocysts, optimal blastocysts, and those with a hatching zona pellucida. Through logistic regression analysis, they found that with each increase in percentage of fragmentation in morulae, there was a 4% decrease in the odds of hatching (OR: 0.96, 95% CI: 0.95–0.98; P < 0.001) and optimal blastocyst formation (OR: 0.96, 95% CI: 0.94–0.97; P < 0.001) [ 88 ]. It is important to point out that the degree of embryo fragmentation, no matter at what stage of development, is measured subjectively without standardized methods. One study from Hnida et al., included here recognized this limitation and used a computer-controlled system for multilevel embryo morphology analysis [ 89 ]. The degree of fragmentation was evaluated based on digital image sequences and correlated to the blastomere size. Fragments were defined to be anucleate with an average diameter of <40 µm. Not surprisingly, the mean blastomere volume decreased significantly with increasing degree of fragmentation ( P < 0.001). In addition, average blastomere size was significantly affected by the degree of fragmentation and multinuclearity which may function as a biomarker for embryo quality [ 89 ]. Furthermore, Sjöblom et al., analyzed the relationship of morphological characteristics to the developmental potential of embryos [ 90 ]. These authors, similar to Hnida et al., found that a large cytoplasmic deficit, i.e., blastomeres not filling the space under the zona, was detrimental to blastocyst development (P < 0.044). However, this is the only study in which the extent of CF observed was not significantly associated with blastocyst development [ 90 ]. Another study using time-lapse imaging showed an association between cytoplasmic fragments at the two-cell stage and perivitelline threads. Perivitelline threads can be observed as the cytoplasmic membrane withdraws from the zona pellucida during embryo cleavage. Ultimately, the presence of these threads, despite the level of fragmentation, did not affect embryo development [ 91 ]. As demonstrated by the studies described here, the degree of CF has a largely negative effect on embryo development.
Effect of CF on embryo implantation and pregnancy
In addition to evaluating the effect of CF on preimplantation embryo development, it is important to assess the effect of CF on implantation and pregnancy outcomes. Five of the included studies have shown a negative effect of CF on implantation or pregnancy outcome (Table 7 ). Assuming that increased fragmentation is detrimental to embryo development, implantation, and pregnancy outcome, it is important to understand the embryo scoring system that determines the best embryo for transfer. Giorgetti et al., used single embryo transfers to devise an embryo scoring pattern to best predict successful implantation. Not surprisingly, higher pregnancy rates were observed with embryos that displayed no fragmentation. The authors found that both pregnancy rate and live birth rate were significantly correlated with a 4-point score based on cleavage rate, fragmentation, irregularities displayed, and presence of a 4-cell embryo on day-2 [ 12 ].
Racowsky et al., assessed if multiple evaluations of an embryo improve selection quality and thus implantation and pregnancy success. They noted that an increased level of fragmentation on both day-2 and -3 was associated with a significant reduction in the number of fetuses that developed to 12 weeks. They also noted that severe fragmentation (>50%) impaired overall embryo viability and may be related to low pregnancy rates and high risk of congenital malformations. The authors ultimately concluded that single day morphological evaluation on day-2 or day-3 has the same predictive value to a multi-day scoring system [ 22 ].
Another retrospective analysis of 460 fresh embryo transfers by Ebner et al., sought to determine the impact of embryo fragmentation on not just pregnancy, but also obstetric and perinatal outcomes. There was a significant relationship between fragmentation and implantation and clinical pregnancy rate, but not with multiple pregnancy rate or ongoing pregnancy rate [ 10 ]. Alikani et al., also studied embryo fragmentation and its implications for implantation and pregnancy rate and included fragmentation pattern into their discussion. They too found a significant decrease in implantation and pregnancy rate as the degree of fragmentation increased. They identified an effect on pregnancy rate when the degree of fragmentation was greater than 35%. The authors went on to discuss that not all fragmentations are detrimental to the embryo development and that the pattern of fragmentation matters. They found that fragmentation pattern type IV, defined as having large fragments distributed randomly and associated with uneven cells, had significantly lower implantation and clinical pregnancy rates when compared to types I-III. They concluded that detaching blastomere cytoplasm as large fragments is most detrimental to embryo development and implantation rate. In contrast, small, scattered fragments (type III) did not seem to appreciably affect the cell number or pose a serious threat to further development [ 7 ].
Lastly, Paternot et al., used sequential imaging techniques and a computer-assisted scoring system to study blastocyst development and the effect of fragmentation on clinical pregnancy. The authors reviewed the volume reduction over time as a measure of embryo fragmentation. They analyzed volumes on day-1 to -3 and found a significant association between total embryo volume and pregnancy rate on both day-2 ( P = 0.003) and day-3 ( P = 0.0003), with the total volume measured on day-3 being the best predictor of pregnancy outcome [ 92 ]. In contrast, Lahav-Baratz recently showed that there was no association between fragmentation rate and abortion or live birth rate. It was concluded that fragmented embryos still have implantation potential and could be considered for transfer when applicable [ 69 ].
Effect of CF removal on embryo development
The effect of fragment removal on IVF outcomes has been controversial. Six of the studies included in this review discussed the impact of removing fragments on embryo development (Table 8 ) [ 7 , 67 , 93 , 94 , 95 , 96 ]. The literature is mixed, with some studies showing improvement in embryo development quality after fragmentation removal [ 7 , 93 ], and others showing no difference at all [ 70 , 94 , 95 ].
Alikani et al., were one of the first investigators to define various patterns of fragmentation and perform microsurgical fragment removal to improve implantation potential [ 7 ]. The authors found that the pattern and degree of fragmentation, and not merely the presence of fragmentation, was significant. When assisted hatching and microsurgical fragment removal was performed, there was an overall 4% increase in implantation rate. They concluded that the removal of the fragments possibly restored the spatial relationship of the cells and limited the interference of cell-cell contact. Further, their preliminary data showed that blastocysts formed after fragment removal were better organized than their unmanipulated counterparts [ 7 ].
Eftekhari-Yazdi et al., similarly studied the effect of fragment removal on blastocyst formation and quality of embryos [ 93 ]. They compared day-2 embryos without removal of fragments to those that fragments were microsurgically removed. There were significantly higher quality embryos in defragmented group compared to the control. Furthermore, fragment removal improved the blastocyst quality compared to the control group. There was also a reduction of apoptotic and necrotic cells in experimental group when compared with the control group [ 93 ].
Two separate studies by Keltz et al., assessed implantation, clinical pregnancy, and birth outcomes after defragmentation [ 67 ], as well as embryo development and fragmentation rate after day-3 embryo defragmentation [ 94 ]. The authors first compared cycle outcomes between low-grade embryos that underwent micromanipulation for fragment removal (>10% fragmentation) and high-grade embryos that did not undergo defragmentation but were hatched on day 3. When compared, the defragmented group showed no difference in rates of implantation, clinical pregnancy, live birth, spontaneous abortion, or fetal defects as compared to the cycles that included all top-grade embryos. Factors associated with poor IVF prognosis and formation of embryo fragments included advanced age, decreased number of oocytes and embryos, and embryo grade [ 67 ].
A separate prospective randomized study by Keltz et al., looked more specifically at day-5 fragmentation, compaction, morulation and blastulation rates after low grade day-3 embryo defragmentation [ 94 ]. Paired embryos from the same patient, not intended to be transferred, were randomly placed in either the experimental group, assisted hatching and embryo defragmentation, or control group (assisted hatching alone). Paired embryos had no difference in mean cell number, percent fragmentation, and grade before randomization. Results showed that on day-5, embryos in the defragmentation group had significantly diminished fragmentation when compared with controls; however, there was no difference in compaction rate, morula formation rate or blastocyst formation rate. Embryo grade generally improved in the treatment group, but this was not statistically significant. Overall, in both groups, improved embryo development was significantly associated with lower levels of fragmentation in the day-3 embryos, supporting the idea that defragmented embryos maintain their reduced fragmented state throughout preimplantation development. Of note, this study had 35 embryos in each group and was limited to lower grade embryos not intended for transfer [ 94 ].
Another, larger prospective randomized study by Halvaei et al., compared the effect of microsurgical removal of fragments on ART outcomes. The authors divided 150 embryos with 10-50% fragmentation into three groups, case ( n =50), sham ( n =50), and control ( n =50). They found no significant difference in rates of clinical pregnancy, miscarriage, live birth, multiple pregnancies, or congenital anomalies between these groups, ultimately showing that cosmetic microsurgery on preimplantation embryos to remove CFs had no beneficial effect [ 95 ].
Lastly, a pilot study by Yumoto et al., aimed to decrease CF in developing embryos by removing the zona pellucida of abnormally fertilized (3PN) donated oocytes [ 96 ]. Although they did not attempt to remove fragments themselves, this study is included as ZP-free oocytes are sometimes encountered in or because of ART procedures, i.e., ICSI. The results suggest that the rate of fragmentation is decreased after mechanical ZP removal. The authors concluded that ZP is not always necessary for normal embryo development since the ZP-free embryos developed normally, maintained their cell adhesions, and had a decreased rate of fragmentation [ 96 ]. It seems that defragmentation of an aneuploid or severely fragmented embryo, only improves the embryo morphology grade but the quality and fate of embryo is not changed [ 97 ].
CF and chromosomal abnormalities in embryo
Although the relationship between DNA fragmentation and chromosomal abnormalities has been more commonly explored in the literature, CF may also be related to intrinsic chromosomal abnormalities in developing embryos. Fourteen studies included in this review explored this relationship (Table 9 ) [ 55 , 56 , 85 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 ].
CF was rarely seen in embryos with normal chromosomal content. Findikli et al., studied DNA fragmentation and aneuploidy in poor quality embryos by TUNEL and fluorescent in situ hybridization (FISH) techniques. Within seven chromosomally abnormal embryos, each had variable degrees of CF [ 98 ]. This study suggests that DNA fragmentation, being a sign of chromosomal abnormalities, may exist together with CF.
An earlier study by Munne et al., examined 524 embryos using FISH analysis for three to five chromosomes. While controlling for age, they divided the embryos into three groups: arrested, slow and/or fragmented, or morphologically and developmentally normal. They found that polyploidy was the most common chromosomal abnormality in the arrested embryo group and decreased with increasing embryonic competence, with 44.5% polyploidy in arrested compared to 2.1% in morphologically normal embryos. Maternal age was not associated with polyploidy rates, but aneuploidy significantly increased with maternal age in morphologically normal human embryos [ 57 ]. Another early study by Almeida and Bolton also examined the relationship between chromosomal abnormalities and embryonic developmental potential. They found that cleavage-stage embryos with poor morphology, defined as irregular shaped blastomeres with severe fragmentation, showed a higher incidence of chromosomal abnormalities than those with good morphology [ 100 ]. Magli et al., found a more direct relationship between chromosomal abnormalities and embryo fragmentation in a larger retrospective study of nearly 1600 embryos. There was a strong association between percentage of fragmentation and chromosomal abnormalities (monosomies and trisomies), where 90% of chromosomal abnormalities were found in embryos with greater than 40% fragmentation [ 101 ].
Another retrospective study comparing maternal age to embryo morphology and chromosomal abnormalities was conducted by Moayeri et al., By examining nine chromosomes in day-3 embryos, they found that morphology predicted chromosomal status in the advanced maternal age group (≥38 years old), but not in younger patients. Fragmentation alone predicted euploidy in both the advanced maternal age and younger groups. This suggests that cellular fragmentation may be a predictor of chromosomal competence and thus embryo developmental potential [ 102 ].
In contrast, Baltaci et al., examined 1,000 embryos and concluded that embryo morphology was not predictive of euploidy and that a considerable number of chromosomally abnormal embryos with good development potential may be selected for embryo transfer. They used FISH for five chromosomes and found that a large proportion of both normal and aneuploid embryos were evaluated as top quality (grade I). For example, 66% of chromosomally abnormal embryos were of good quality (grade I and II). They found no significant difference among aneuploid embryos when distributed by age. However, a higher embryo quality found in normal compared to aneuploid embryos [ 103 ].
In addition, Pellestor et al., compared the relationship between morphology and chromosomal abnormalities in two separate studies. The first study found that aneuploidy was the most frequently observed abnormality after cytogenetic analysis of preimplantation embryos [ 55 ]. They defined the quality of embryos as good (grade I and II) and poor (grades III and IV). There was an increased chromosomal abnormality in poor quality embryos (84.3%) when compared to embryos with good quality (33.9%). Both aneuploidy and fragmentation were shown to be predominant in poor quality embryos, whereas mosaicism and polyploidy were the most frequent abnormalities in good quality embryos [ 55 ]. Pellestor et al., also performed cytogenetic analysis on 411 poor-quality embryos (grade IV) [ 85 ]. Ninety percent of the successfully analyzed cases showed abnormal chromosome complements, with aneuploidy being the most frequently observed. These results further support that a large majority of poor grade embryos are chromosomally abnormal and ultimately offer low chance of reproductive success for either embryo transfer or cryopreservation [ 85 ].
A separate study by Chavez et al., combined time-lapse imaging with karyotypic status of blastomeres in the 4-cell embryo to test whether blastomere behavior may reflect chromosomal abnormalities, using array comparative genomic hybridization (aCGH), during early cleavage [ 56 ]. In time-lapse observations, a large proportion of aneuploid and triploid, but not euploid embryos, exhibited cellular fragmentation. They showed that the probability of aneuploidy increased with higher fragmentation and only 65% of the fragmented embryo would be expected to form blastocyst. Furthermore, all the aneuploid embryos with additional unbalanced sub-chromosomal errors exhibited CF. The authors concluded that although fragmentation alone at a single point in time does not predict embryo developmental potential, time-lapse imaging with dynamic fragmentation screening may help detect embryonic aneuploidy [ 56 ].
Two more recent studies also used aCGH to evaluate the association between embryo ploidy and fragmentation. Vera-Rodriguez et al., in a retrospective study, compared the rate of embryo aneuploidy between two groups of high (≥25%) and low (˂25%) fragmentation. They found that the rate of aneuploidy in high and low fragmentation was 62.5 and 46.3%, respectively. However, the difference was not statistically significant concluding that using degree of fragmentation alone is not suggested to predict the embryo ploidy status [ 107 ]. Minasi et al., in a case series evaluated 1730 blastocyst ploidy with aCGH. They showed that there is no significant difference between day-3 embryo morphology and embryo ploidy. However, the quality of blastocyst (inner cell mass grade, trophectoderm grade, degree of expansion) was associated with embryo ploidy [ 106 ].
In a recent meta-analysis, it was shown there is trend between degree of fragmentation and rate of aneuploidy [ 109 ]. A major source of controversy in both early and recent studies on aneuploidy and fragmentation is the variation in the methods and criteria used to evaluate these factors. One of the aspects that differ across studies include the technique for detecting aneuploidy; FISH vs aCGH. Recent studies have used aCGH to detect aneuploidy and found no clear relationship in this regard. Also, the quality of the matching between groups, the design of the study (retrospective vs prospective), the timing of the fragmentation assessment, the use of time-lapse imaging to monitor the fate of fragments are the other reasons for this discrepancy. There is still the lack of a clear cut-off point for the percentage of fragmentation to predict aneuploidy. Further powerful studies using new methods like next gene sequencing and tile-lapse systems are recommended to shed light on the relationship between fragmentation and aneuploidy.
The literature highlights that poor quality embryos have a higher incidence of chromosomal abnormalities. Notably, CF is rarely observed in embryos with normal chromosomal content. Technological advancements, such as TLM, offer promising avenues to enhance our understanding and detection of embryonic aneuploidy. Overall, these studies underscore the complexity of the relationship between fragmentation and chromosomal abnormalities, emphasizing the need for continued research to refine embryo selection strategies and improve reproductive outcomes.
Discussion and conclusion
The role of fragmentation in human embryo development and reproductive potential is widely recognized, albeit without standard definition nor agreed upon implication. While it has been shown that degree of fragmentation and embryo implantation potential are inversely proportional [ 5 , 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ], the degree, pattern, and distribution of fragmentation as it relates to pregnancy outcome is debated in the literature. Our qualitative synthesis of 60 articles related to the study of embryo fragmentation and reproductive outcomes highlighted some of the challenges in analysis of fragmentation, while revealing trends in our evolving knowledge of how fragmentation may relate to functional development of the human embryo.
While fragmentation is best understood to be a natural process across species, the origin of fragmentation remains incompletely understood and likely multifactorial. Degree of fragmentation has been plausibly correlated to sperm DNA oxidation [ 37 ], errors in division [ 37 ], mitochondrial distribution [ 45 ], and overall embryo quality [ 39 ]. However, some causes of fragmentation are based on outdated studies and require validation in future research with higher quality and more advanced techniques. While cause of fragmentation remains a focus of investigation, advances in technology have allowed for more detailed analysis of its effect on embryo development and reproductive outcome. At the cellular level, increased fragmentation has been shown to be associated with higher rates of apoptosis, necrosis, and programmed cell death of cleavage-stage embryos [ 60 , 61 , 62 ]. Given the recognized significance of fragmentation on embryo development, it follows that many studies have been focused on IVF and ART impacts on fragmentation, as well as determining quantitative reproductive outcomes. In terms of other influences on degree of fragmentation, patient age was not universally found to be significantly associated with fragmentation [ 7 , 70 , 71 ] although age is certainly known to influence embryo quality. Most studies included in the search criteria showed that ART such as ICSI do not significantly impact fragmentation rate in developing embryos [ 74 , 75 , 76 ]. Those studies that found significant differences in embryo grading either between conventional fertilization and ICSI either did not find a difference in implantation or pregnancy rate or did not study it, suggesting that ICSI is not a significant contributor to poorer ART outcomes by way of embryo fragmentation.
In synthesizing the available data on ART and pregnancy outcomes with varying degrees of embryo fragmentation, most included studies did find a negative impact of increasing fragmentation on reproductive success while severe fragmentation does appear to be associated with poorer implantation rate and clinical pregnancy rate. This association may be related to the observation that increased fragmentation at the cleavage-stage embryo is related to chromosomal abnormalities incompatible with ongoing development or pregnancy.
The reviewed studies have several limitations. There are different grading systems in use that may impact detecting and reporting the degree of CF. Different criteria and terminology used in different studies may in turn make the comparison of outcome measures difficult. Another factor is the distribution pattern of CF. There are two types of scattered and concentrated fragments with different prognoses that is not considered in grading systems. Therefore, due to the lack of a standard cleavage-stage embryo grading system, comparing different studies should be done with caution. In addition, evaluation of embryo fragmentation is mostly based on individual observation which is subjective and has inter- and intra-observer subjectivity leading to high variable results even if performed by an experienced embryologist [ 110 ]. TLM is considered as a non-invasive tool and evaluates the embryo quality continuously and without the need to remove the embryo from the incubator [ 111 ]. The use of this technology allows for the analysis of embryo morphokinetics and has advanced knowledge of the developing embryo. Recently, artificial intelligence (AI) including machine learning and neural network has gained popularity in various fields of medicine including IVF and embryology. Accuracy of AI in prediction of fragmentation has been studied with encouraging results [ 112 ]. Further advances in technology will promote the use of AI as a tool in defining the effect of fragmentation on human embryo development and reproductive potential.
Although the precise origin and the importance of external or iatrogenic factors on fragmentation of cleavage-stage embryos varies in the literature, there is more consensus regarding severe fragmentation worsening reproductive outcomes. Given this important pattern, and the availability of increasingly sophisticated embryologic technology, further research is warranted to characterize more completely preventative or rescue techniques to improve reproductive outcomes.
Availability of data and materials
No datasets were generated or analysed during the current study.
Balaban B, Brison D, Calderon G, Catt J, Conaghan J, Cowan L, et al. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Article Google Scholar
Hill GA, Freeman M, Bastias MC, Jane Rogers B, Herbert CM, Osteen KG, et al. The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization-embryo transfer. Fertil Steril. 1989;52:801–6.
Article CAS PubMed Google Scholar
Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertil Embryo Transfer. 1986;3:284–95.
Article CAS Google Scholar
Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, on behalf of the BFS and ACE. Elective Single Embryo Transfer: Guidelines for Practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil. 2008;11:131–46.
Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilization for alleviating human infertility. J In Vitro Fert Embryo Transf. 1984;1:3–23.
Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–8.
Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42.
Cecchele A, Cermisoni GC, Giacomini E, Pinna M, Vigano P. Cellular and Molecular Nature of Fragmentation of Human Embryos. Int J Mol Sci. 2022;23:1349.
Article CAS PubMed PubMed Central Google Scholar
Claman P, Armant DR, Seibel MM, Wang TA, Oskowitz SP, Taymor ML. The impact of embryo quality and quantity on implantation and the establishment of viable pregnancies. J In Vitro Fert Embryo Transf. 1987;4:218–22.
Ebner T, Yaman C, Moser M, Sommergruber M, Pölz W, Tews G. Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril. 2001;76:281–5.
Erenus M, Zouves C, Rajamahendran P, Leung S, Fluker M, Gomel V. The effect of embryo quality on subsequent pregnancy rates after in vitro fertilization. Fertil Steril. 1991;56:707–10.
Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31.
Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22:548–57.
Roseboom TJ, Vermeiden JP, Schoute E, Lens JW, Schats R. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Hum Reprod. 1995;10:3035–41.
Shulman A, Ben-Nun I, Ghetler Y, Kaneti H, Shilon M, Beyth Y. Relationship between embryo morphology and implantation rate after in vitro fertilization treatment in conception cycles. Fertil Steril. 1993;60:123–6.
Staessen C, Janssenswillen C, Van den Abbeel E, Devroey P, Van Steirteghem AC. Avoidance of triplet pregnancies by elective transfer of two good quality embryos. Hum Reprod. 1993;8:1650–3.
Visser DS, Fourie FR. The applicability of the cumulative embryo score system for embryo selection and quality control in an in-vitro fertilization/embryo transfer programme. Hum Reprod. 1993;8:1719–22.
Volpes A, Sammartano F, Coffaro F, Mistretta V, Scaglione P, Allegra A. Number of good quality embryos on day 3 is predictive for both pregnancy and implantation rates in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82:1330–6.
Article PubMed Google Scholar
Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9.
Fujimoto VY, Browne RW, Bloom MS, Sakkas D, Alikani M. Pathogenesis, developmental consequences, and clinical correlations of human embryo fragmentation. Fertil Steril. 2011;95:1197–204.
Chi H-J, Koo J-J, Choi S-Y, Jeong H-J, Roh S-I. Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil Steril. 2011;96:187–92.
Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24:2104–13.
Article PubMed PubMed Central Google Scholar
Hardy K, Stark J, Winston RML. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol Reprod. 2003;68:1165–9.
Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14:429–47.
Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol. 2008;199(660):e1-5.
Google Scholar
Hardarson T, Löfman C, Coull G, Sjögren A, Hamberger L, Edwards RG. Internalization of cellular fragments in a human embryo: time-lapse recordings. Reprod Biomed Online. 2002;5:36–8.
Van Blerkom J. The Enigma of Fragmentation in Early Human Embryos: Possible Causes and Clinical Relevance. Essential IVF. Boston: Springer US; 2004. 377–421.
Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869–73.
Graham J, Han T, Porter R, Levy M, Stillman R, Tucker MJ. Day 3 morphology is a poor predictor of blastocyst quality in extended culture. Fertil Steril. 2000;74:495–7.
Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril. 2002;77:1191–5.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8.
Guerif F, Le Gouge A, Giraudeau B, Poindron J, Bidault R, Gasnier O, et al. Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22:1973–81.
Rienzi L, Ubaldi F, Iacobelli M, Romano S, Minasi MG, Ferrero S, et al. Significance of morphological attributes of the early embryo. Reprod Biomed Online. 2005;10:669–81.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Killeen ID, Moore NW. The morphological appearance and development of sheep ova fertilized by surgical insemination. J Reprod Fertil. 1971;24:63–70.
Enders AC, Hendrickx AG, Binkerd PE. Abnormal development of blastocysts and blastomeres in the rhesus monkey. Biol Reprod. 1982;26:353–66.
Meseguer M, Martínez-Conejero JA, O’Connor JE, Pellicer A, Remohí J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89:1191–9.
Stensen MH, Tanbo TG, Storeng R, Åbyholm T, Fedorcsak P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil Steril. 2015;103:374-81.e4.
Ebner T. First polar body morphology and blastocyst formation rate in ICSI patients. Human Reproduction. 2002;17:2415–8.
Sedó CA, Bilinski M, Lorenzi D, Uriondo H, Noblía F, Longobucco V, et al. Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects. JBRA Assist Reprod. 2017;21:343–50.
PubMed Central Google Scholar
Rose BI, Laky D. Polar body fragmentation in IVM oocytes is associated with impaired fertilization and embryo development. J Assist Reprod Genet. 2013;30:679–82.
Zhou W, Fu L, Sha W, Chu D, Li Y. Relationship of polar bodies morphology to embryo quality and pregnancy outcome. Zygote. 2016;24:401–7.
Yang Y, Tan W, Chen C, Jin L, Huang B. Correlation of the position and status of the polar body from the fertilized oocyte to the euploid status of blastocysts. Front Genet. 2022;13:1006870. https://doi.org/10.3389/fgene.2022.1006870 .
Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod. 2005;20:3369–75.
Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization ATP content and competence. Hum Reprod. 2000;15:2621–33.
Otasevic V, Surlan L, Vucetic M, Tulic I, Buzadzic B, Stancic A, et al. Expression patterns of mitochondrial OXPHOS components, mitofusin 1 and dynamin-related protein 1 are associated with human embryo fragmentation. Reprod Fertil Dev. 2016;28:319–27.
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17.
Sermondade N, Delarouzière V, Ravel C, Berthaut I, Verstraete L, Mathieu E, et al. Characterization of a recurrent poor-quality embryo morphology phenotype and zygote transfer as a rescue strategy. Reprod Biomed Online. 2012;24:403–9.
Gat I, Levron J, Yerushalmi G, Dor J, Brengauz M, Orvieto R. Should zygote intrafallopian transfer be offered to all patients with unexplained repeated in-vitro fertilization cycle failures? J Ovarian Res. 2014;7:7.
Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002.
Chen EY, Fujinaga M, Giaccia AJ. Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology. 1999;60:215–25.
Lee T-H, Lee M-S, Liu C-H, Tsao H-M, Huang C-C, Yang Y-S. The association between microenvironmental reactive oxygen species and embryo development in assisted reproduction technology cycles. Reprod Sci. 2012;19:725–32.
Lan K-C, Lin Y-C, Chang Y-C, Lin H-J, Tsai Y-R, Kang H-Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J Assist Reprod Genet. 2019;36:325–34.
Bedaiwy MA, Falcone T, Mohamed MS, Aleem AAN, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil Steril. 2004;82:593–600.
Pellestor F, Girardet A, Andréo B, Arnal F, Humeau C. Relationship between morphology and chromosomal constitution in human preimplantation embryo. Mol Reprod Dev. 1994;39:141–6.
Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun. 2012;3:1251.
Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64(2):382–91. Corrected and republished in: Fertil Steril. 2019 Oct;112(4 Suppl1):e71–e80.
Halvaei I, Khalili MA, Nottola SA. A novel method for transmission electron microscopy study of cytoplasmic fragments from preimplantation human embryos. Microsc Res Tech. 2016;79:459–62.
Johansson M, Hardarson T, Lundin K. There is a cutoff limit in diameter between a blastomere and a small anucleate fragment. J Assist Reprod Genet. 2003;20:309–13.
Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–8.
Liu HC, He ZY, Mele CA, Veeck LL, Davis O, Rosenwaks Z. Expression of apoptosis-related genes in human oocytes and embryos. J Assist Reprod Genet. 2000;17:521–33.
Levy R, Benchaib M, Cordonier H, Souchier C, Guerin JF. Annexin V labelling and terminal transferasemediated DNA end labelling (TUNEL) assay in human arrested embryos. Mol Hum Reprod. 1998;4(8):775–83. https://doi.org/10.1093/molehr/4.8.775 .
Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod. 2001;16(4):719–29. https://doi.org/10.1093/humrep/16.4.719 .
Metcalfe AD, Hunter HR, Bloor DJ, Lieberman BA, Picton HM, Leese HJ, Kimber SJ, Brison DR. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev. 2004;68(1):35–50. https://doi.org/10.1002/mrd.20055 .
Jurisicova A, Antenos M, Varmuza S, Tilly J, Casper R. Expression of apoptosis-related genes during human preimplantation embryo development: potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol Hum Reprod. 2003;9:133–41.
Bencomo E, Pérez R, Arteaga M-F, Acosta E, Peña O, Lopez L, et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril. 2006;85:474–80.
Keltz MD, Skorupski JC, Bradley K, Stein D. Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil Steril. 2006;86:321–4.
Ziebe S, Loft A, Petersen JH, Andersen AG, Lindenberg S, Petersen K, et al. Embryo quality and developmental potential is compromised by age. Acta Obstet Gynecol Scand. 2001;80:169–74.
Lahav-Baratz S, Blais I, Koifman M, Dirnfeld M, Oron G. Evaluation of fragmented embryos implantation potential using time-lapse technology. J Obstet Gynaecol Res. 2023;49:1560–70.
Stensen MH, Tanbo T, Storeng R, Byholm T, Fèdorcsak P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21:118–25.
Wu DH, Reynolds K, Maxwell R, Lindheim SR, Aubuchon M, Thomas MA. Age does not influence the effect of embryo fragmentation on successful blastocyst development. Fertil Steril. 2011;95:2778–80.
Frattarelli JL, Leondires MP, Miller BT, Segars JH. Intracytoplasmic sperm injection increases embryo fragmentation without affecting clinical outcome. J Assist Reprod Genet. 2000;17:207–12.
Hsu MI, Mayer J, Aronshon M, Lanzendorf S, Muasher S, Kolm P, et al. Embryo implantation in in vitro fertilization and intracytoplasmic sperm injection: impact of cleavage status, morphology grade, and number of embryos transferred. Fertil Steril. 1999;72:679–85.
Garello C, Baker H, Rai J, Montgomery S, Wilson P, Kennedy CR, et al. Pronuclear orientation, polar body placement, and embryo quality after intracytoplasmic sperm injection and in-vitro fertilization: further evidence for polarity in human oocytes? Hum Reprod. 1999;14:2588–95.
Yoeli R, Orvieto R, Ashkenazi J, Shelef M, Ben-Rafael Z, Bar-Hava I. Comparison of embryo quality between intracytoplasmic sperm injection and in vitro fertilization in sibling oocytes. J Assist Reprod Genet. 2008;25:23–8.
Ruiz A, Remohí J, Minguez Y, Guanes PP, Simón C, Pellicer A. The role of in vitro fertilization and intracytoplasmic sperm injection in couples with unexplained infertility after failed intrauterine insemination. Fertil Steril. 1997;68:171–3.
Zhang XD, Liu JX, Liu WW, Gao Y, Han W, Xiong S, et al. Time of insemination culture and outcomes of in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2013;19:685–95.
Liu J, Zhang X, Yang Y, Zhao J, Hao D, Zhang J, et al. Long-time vs. short-time insemination of sibling eggs. Exp Ther Med. 2016;12:3756–60.
Sathananthan H, Bongso A, Ng SC, Ho J, Mok H, Ratnam S. Ultrastructure of preimplantation human embryos co-cultured with human ampullary cells. Hum Reprod. 1990;5:309–18.
Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, et al. Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update. 2021;27:848–65.
Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504.
Hur C, Nanavaty V, Yao M, Desai N. The presence of partial compaction patterns is associated with lower rates of blastocyst formation, sub-optimal morphokinetic parameters and poorer morphologic grade. Reprod Biol Endocrinol. 2023;21:12.
Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, et al. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod Biomed Online. 2017;34:137–46.
Ebner T, Moser M, Shebl O, Sommergruber M, Gaiswinkler U, Tews G. Morphological analysis at compacting stage is a valuable prognostic tool for ICSI patients. Reprod Biomed Online. 2009;18:61–6.
Pellestor F, Dufour MC, Arnal F, Humeau C. Direct assessment of the rate of chromosomal abnormalities in grade IV human embryos produced by in-vitro fertilization procedure. Hum Reprod. 1994;9(2):293–302. https://doi.org/10.1093/oxfordjournals.humrep.a138497 .
Morgan K, Wiemer K, Steuerwald N, Hoffman D, Maxson W, Godke R. Use of videocinematography to assess morphological qualities of conventionally cultured and cocultured embryos. Hum Reprod. 1995;10:2371–6.
Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15:2634–43.
Ivec M, Kovacic B, Vlaisavljevic V. Prediction of human blastocyst development from morulas with delayed and/or incomplete compaction. Fertil Steril. 2011;96:1473-1478.e2.
Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19:288–93.
Sjöblom P, Menezes J, Cummins L, Mathiyalagan B, Costello MF. Prediction of embryo developmental potential and pregnancy based on early stage morphological characteristics. Fertil Steril. 2006;86:848–61.
Kellam L, Pastorelli LM, Bastida AM, Senkbeil A, Montgomery S, Fishel S, et al. Perivitelline threads in cleavage-stage human embryos: observations using time-lapse imaging. Reprod Biomed Online. 2017;35:646–56.
Paternot G, Debrock S, De Neubourg D, D’Hooghe TM, Spiessens C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod. 2013;28:627–33.
Eftekhari-Yazdi P, Valojerdi MR, Ashtiani SK, Eslaminejad MB, Karimian L. Effect of fragment removal on blastocyst formation and quality of human embryos. Reprod Biomed Online. 2006;13:823–32.
Keltz M, Fritz R, Gonzales E, Ozensoy S, Skorupski J, Stein D. Defragmentation of low grade day 3 embryos resulted in sustained reduction in fragmentation, but did not improve compaction or blastulation rates. Fertil Steril. 2010;94:2406–8.
Halvaei I, Khalili MA, Esfandiari N, Safari S, Talebi AR, Miglietta S, et al. Ultrastructure of cytoplasmic fragments in human cleavage stage embryos. J Assist Reprod Genet. 2016;33:1677–84.
Yumoto K, Shimura T, Mio Y. Removing the zona pellucida can decrease cytoplasmic fragmentations in human embryos: a pilot study using 3PN embryos and time-lapse cinematography. J Assist Reprod Genet. 2020;37:1349–54.
Sordia-Hernandez LH, Morales-Martinez FA, Frazer-Moreira LM, Villarreal-Pineda L, Sordia-Piñeyro MO, Valdez-Martinez OH. Clinical Pregnancy After Elimination of Embryo Fragments Before Fresh Cleavage-stage Embryo Transfer. J Family Reprod Health. 2020;14(3):198–204. https://doi.org/10.18502/jfrh.v14i3.4674 .
Findikli N, Kahraman S, Kumtepe Y, Donmez E, Benkhalifa M, Biricik A, et al. Assessment of DNA fragmentation and aneuploidy on poor quality human embryos. Reprod Biomed Online. 2004;8:196–206.
Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–91.
Almeida PA, Bolton VN. The relationship between chromosomal abnormality in the human preimplantation embryo and development in vitro. Reprod Fertil Dev. 1996;8:235–41.
Magli MC, Gianaroli L, Ferraretti AP. Chromosomal abnormalities in embryos. Mol Cell Endocrinol. 2001;183(Suppl 1):S29-34.
Moayeri SE, Allen RB, Brewster WR, Kim MH, Porto M, Werlin LB. Day-3 embryo morphology predicts euploidy among older subjects. Fertil Steril. 2008;89:118–23.
Baltaci V, Satiroglu H, Kabukçu C, Ünsal E, Aydinuraz B, Üner Ö, et al. Relationship between embryo quality and aneuploidies. Reprod Biomed Online. 2006;12:77–82.
Ziebe S, Lundin K, Loft A, Bergh C, Nyboe Anderson A, Selleskog U. FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum Reprod. 2003;18:2575–81.
Delimitreva SM, Zhivkova RS, Vatev ITS, Toncheva DI. Chromosomal disorders and nuclear and cell destruction in cleaving human embryos. Int J Dev Biol. 2005;49:409–16.
Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–54. https://doi.org/10.1093/humrep/dew183 . Epub 2016 Sep 2.
Vera-Rodriguez M, Chavez SL, Rubio C, Reijo Pera RA, Simon C. Prediction model for aneuploidy in early human embryo development revealed by single-cell analysis. Nat Commun. 2015;6:7601. https://doi.org/10.1038/ncomms8601 .
Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87(3):534–41. https://doi.org/10.1016/j.fertnstert.2006.07.1512 . Epub 2006 Nov 21.
Bamford T, Barrie A, Montgomery S, Dhillon-Smith R, Campbell A, Easter C, et al. Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis. Hum Reprod Update. 2022;28:656–86.
Baxter Bendus AE, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril. 2006;86:1608–15.
Lundin K, Park H. Time-lapse technology for embryo culture and selection. Ups J Med Sci. 2020;125:77–84.
Leahy BD, Jang WD, Yang HY, Struyven R, Wei D, Sun Z, et al. Automated Measurements of Key Morphological Features of Human Embryos for IVF. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 2020.
Download references
We did not receive any funding to prepare this manuscript. We are grateful for receiving an editorial waiver for this manuscript.
Author information
Authors and affiliations.
Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Vermont Medical Center, The Robert Larner College of Medicine at the University of Vermont, Burlington, VT, 05405, USA
Ariella Yazdani, Catherine Boniface & Navid Esfandiari
Department of Anatomical Sciences, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Iman Halvaei
Present address: Obstetrics and Gynecology Institute, Cleveland Clinic Foundation, Cleveland, OH, 44195, USA
Ariella Yazdani
Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology and Reproductive Sciences, University of Vermont, 111 Colchester Avenue, Burlington, Vermont, 05401, USA
Navid Esfandiari
You can also search for this author in PubMed Google Scholar
Contributions
All authors have made substantial contributions to this manuscript. NE designed the work, critically reviewed and edited the manuscript. AY, IH and CB made substantial contribution in writing the manuscript. All authors have approved the paper for submission.
Corresponding author
Correspondence to Navid Esfandiari .
Ethics declarations
Ethics approval and consent to participate.
This is a review paper and does not involve human participants, human data or human tissue.
Consent for publication
This manuscript does not contain any individual person’s data in any form (including any individual details, images or videos).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yazdani, A., Halvaei, I., Boniface, C. et al. Effect of cytoplasmic fragmentation on embryo development, quality, and pregnancy outcome: a systematic review of the literature. Reprod Biol Endocrinol 22 , 55 (2024). https://doi.org/10.1186/s12958-024-01217-7
Download citation
Received : 20 November 2023
Accepted : 01 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12958-024-01217-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fragmentation
- Embryo development
- Implantation
- In vitro fertilization
- Pregnancy outcome
Reproductive Biology and Endocrinology
ISSN: 1477-7827
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The utilities for nonspecific hormone replacement therapy ranged from 0.52 to 0.93, whereas for tamoxifen, the utilities were on the higher end and ranged from 0.75 to 0.95. One study with a sample size of 152 patients 71 assessed the utility for goserelin therapy using SG and reported a mean utility value of 0.81.
Identify the most recent utility value estimates for cardiovascular disease (CVD) via systematic literature review (SLR) and explore trends in utility elicitation methods in the last 6 years. This SLR was updated on January 25, 2018, and identified studies reporting utilities for myocardial infarction (MI), stroke, angina, peripheral artery disease (PAD), and any-cause revascularization by ...
This review will facilitate such analyses by providing a comprehensive and up-to-date summary of the hepatitis C health utility literature. Methods This review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 13
This review aimed to identify and describe the utility values associated with MI/ACS reported in the available literature. PCV149 - A SYSTEMATIC LITERATURE REVIEW (SLR) OF HEALTH STATE UTILITIES ASSOCIATED WITH NON-FATAL MYOCARDIAL INFARCTION (MI) OR ACUTE CORONARY SYNDROME (ACS) - Value in Health
A systematic literature review (SLR) was performed to understand the HRQOL evidence for treatments in R/R LBCL and identify associated health utility values. ... Health utilities were assessed at baseline and months 1, 2, 3, 6, 9, 12, and 18 after infusion. At baseline, the mean [standard deviation (SD)] EQ-5D-5L health state score was 0.817 (0 ...
A systematic literature review was conducted to identify and summarize heart failure utility values for use in economic evaluations. ... Azzabi I, Busschbach J, Chevrou-Severac H, Crawford B, et al. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR Good Practices for Outcomes Research Task Force ...
A systematic literature search was carried out of the PubMed, EMBASE and Cochrane Library databases for studies published from 2000 until the end of 2016 using a range of free-text terms in title/abstract (Fig. 1).Since Medical Subject Headings (MeSH) terms provide little coverage of HSUVs [25, 26], we identified a few relevant free-text terms by referring to the published recommendations ...
Results. Eleven studies were included after reviewing 989 studies. Most hand conditions were associated with utilities less than 0.8. Utilities in the reviewed studies were measured using different methods and from different subjects. Three studies paradoxically mapped greater utilities for poorer heath states.
Utilities have a key role in the transition to a more economically and socially sustainable future. Driven by pressures from investors, regulators, government and society, companies across all sectors are setting bold ambitions for sustainability. ... This article aims to bridge this gap through a systematic literature review of 72 articles ...
A total of 25 752 articles were identified by the search strategy, with 383 articles meeting the inclusion criteria after the final review stage (see Fig. 1).Nine articles reported on health utilities, 131 on resource use, and 160 on costs associated with pneumococcal disease, with 95 articles reporting on both resource use and costs.
Assessment of health state utilities can be either direct or indirect, where direct utility assessment involves mapping preferences directly onto the utility scale ... Meregaglia, M., Cairns, J.: A systematic literature review of health state utility values in head and neck cancer. Health Qual. Life Outcomes. (2017).
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
This systematic literature review identifies and summarizes utility values for heart failure (HF) to support economic evaluations, derived from 161 publications reporting HF utility values from 142 studies. 79.5% of the publications provided utility values on chronic HF, 24% on hospitalization of patients with HF, and 2% on other acute events ...
A systematic review of utilities in hand surgery literature. J Hand Surg Am. 2015;40(5):997-1005. Article PubMed Google Scholar Magnus A, Isaranuwatchai W, Mihalopoulos C, Brown V, Carter R. A systematic review and meta-analysis of prostate cancer utility values of patients and partners between 2007 and 2016.
Laureani A, Antony J. Leadership and lean six Sigma: a systematic literature review. Total Qual Manag Bus Excel 2019; 30: 53-81. Google Scholar. 36. Ingelsson P, Bäckström I, Snyder K. Strengthening quality culture in private sector and health care. Leader Health Serv 2018; 31: 276-292.
Understanding the relationship between the intake of sugars and diet quality can inform public health recommendations. This systematic review synthesized recent literature on associations between sugar intake and diet quality in generally healthy populations aged 2 years or older. We searched databases from 2010 to 2022 for studies of any design examining associations between quantified sugar ...
Research in this systematic literature review has revealed the Third Space can be utilised successfully to support the development of children's identities in educational settings where multiple, sometimes competing discourses co-exist. The work can be extended by conducting similar reviews with children below the age of 4 or adolescents in ...
Med Decis Making. 2000;20 (2):186-193. Evaluating health-related quality of life in type 1 diabetes : a systematic literature review of utilities for adults with type 1 diabetes Jayne Smith-Palmer,1 Jay P Bae,2 Kristina S Boye,2 Kirsi Norrbacka,3 Barnaby Hunt,1 William J Valentine1 1Ossian Health Economics and Communications GmbH, Basel ...
et al., 2015). The systematic PRISMA review model, developed by Moher et al. (2009), was adopted for the systematic review process. Procedures for Systematic Literature Review A six-phase procedure was utilized to reflect logical thinking in the collection and screening of the literature in the scope of this study. The six phases are as follows:
tigated by scholars. This article aims to bridge this gap through a systematic literature review of 72 articles published from 1990 to 2023 in the accounting and management fields. After the analysis of bibliometric data and keywords used for science mapping, this study developed an in-depth review of the literature.
For instance, in a systematic review of health utilities in lung cancer, Sturza and colleagues reported that 16% of estimates (35/223, 7 studies) were derived using simple judgment, rather than established, valid preference elicitation techniques . Irrespective of the source of variation, these differences introduce additional uncertainty to ...
We conduct a systematic literature review to answer our RQs, and thus, assess systematically the fragmented knowledge base on success factors in growth-stage digital (health) companies. This review follows established guidelines proposed by Snyder and Tranfield et al. (21,22) , ensuring the generation of evidence-informed management knowledge.
The role of cytoplasmic fragmentation in human embryo development and reproductive potential is widely recognized, albeit without standard definition nor agreed upon implication. While fragmentation is best understood to be a natural process across species, the origin of fragmentation remains incompletely understood and likely multifactorial. Several factors including embryo culture condition ...
First, a qualitative analysis based on a systematic literature review of 29 relevant articles was conducted. Second, a meta analysis with harmonized methodological assumptions was applied to assess which of the proposed ex-situ carbonation routes in literature has the lowest environmental impacts (16 different impact categories assessed).