Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 19 November 2022
- Epidemiology

UK Biobank: a globally important resource for cancer research
- Megan C. Conroy ORCID: orcid.org/0000-0002-3847-6202 1 ,
- Ben Lacey 1 ,
- Jelena Bešević 1 ,
- Wemimo Omiyale 1 ,
- Qi Feng 1 ,
- Mark Effingham 2 ,
- Jonathan Sellers 2 ,
- Simon Sheard 2 ,
- Mahesh Pancholi 2 ,
- Gareth Gregory 2 ,
- John Busby 2 ,
- Rory Collins 1 , 2 &
- Naomi E. Allen 1
British Journal of Cancer volume 128 , pages 519–527 ( 2023 ) Cite this article
10k Accesses
27 Citations
19 Altmetric
Metrics details
- Cancer epidemiology
- Genetics research
- Research data
UK Biobank is a large-scale prospective study with deep phenotyping and genomic data. Its open-access policy allows researchers worldwide, from academia or industry, to perform health research in the public interest. Between 2006 and 2010, the study recruited 502,000 adults aged 40–69 years from the general population of the United Kingdom. At enrolment, participants provided information on a wide range of factors, physical measurements were taken, and biological samples (blood, urine and saliva) were collected for long-term storage. Participants have now been followed up for over a decade with more than 52,000 incident cancer cases recorded. The study continues to be enhanced with repeat assessments, web-based questionnaires, multi-modal imaging, and conversion of the stored biological samples to genomic and other ‘–omic’ data. The study has already demonstrated its value in enabling research into the determinants of cancer, and future planned enhancements will make the resource even more valuable to cancer researchers. Over 26,000 researchers worldwide are currently using the data, performing a wide range of cancer research. UK Biobank is uniquely placed to transform our understanding of the causes of cancer development and progression, and drive improvements in cancer treatment and prevention over the coming decades.
Similar content being viewed by others
The Personal Genome Project-UK, an open access resource of human multi-omics data
The Project Baseline Health Study: a step towards a broader mission to map human health
dbTMM: an integrated database of large-scale cohort, genome and clinical data for the Tohoku Medical Megabank Project
Cancer is now the most common cause of death in many parts of the world, including North America, Europe, Australia, and China [ 1 , 2 , 3 ]. However, the major determinants of many cancers remain unclear, despite decades of biological and epidemiological research [ 4 ]. UK Biobank is a large-scale biomedical database and research resource, containing in-depth information on genetic, physiological, lifestyle, and environmental factors on half a million UK participants, with their health followed up through linkage to electronic health records. The resource is available to all bona fide researchers to perform health-related research, and its unique combination of scale, depth, maturity and accessibility has led to it becoming the world’s most important biomedical resource, offering enormous potential to improve understanding of the determinants of a wide range of cancers.
The risk of developing cancer reflects the combined effect of genetic and environmental factors, each of which may have only a modest effect on cancer risk [ 4 , 5 , 6 ]. As such, research on the effects of these factors requires epidemiological studies that collect detailed information on a very large number of people. However, previous epidemiological studies have typically involved collection of either a large amount of data on a small number of participants, or a small amount of data on a large number of participants. By contrast, UK Biobank contains extensive questionnaire data, physical measures and biological samples for a very large number of participants (i.e., both depth and breadth have been achieved). This was made possible by the establishment of highly efficient, purpose-designed centralised processes with detailed input from UK Biobank’s extensive academic collaborative network [ 7 ].
UK Biobank was established by the Medical Research Council (UK) (MRC), Wellcome, the UK Department of Health, and the Scottish Government in response to the challenge of understanding the determinants of common complex disease [ 8 ]. Participants have been followed up for over a decade, and there are now ~50,000 incident cancer cases. From inception, the study data was intended to be made available to academic and commercial researchers worldwide and the resource is now uniquely placed to enable major scientific discoveries into the causes, treatment and prevention of cancer and other diseases. UK Biobank now receive core funding from the MRC, Wellcome, Cancer Research UK, British Heart Foundation and the National Institute of Health and Care Research [ 9 ].
UK Biobank: data collection and enhancements
Recruitment and data collection.
Between 2006 and 2010, about 9.2 million people aged 40–69 years, who were registered with the NHS and living within reasonable travelling distance (up to 25 miles) of one of 22 assessment centres across the UK, were invited to join UK Biobank. Overall, 502,000 adults (5.5% of those invited) were recruited [ 10 , 11 ]. Participants underwent an extensive range of baseline assessments, including touchscreen questionnaires on sociodemographic factors, family history, lifestyle, medical history, cognitive function tests and environmental exposures. Physical measurements were taken, including blood pressure, bone mineral density, hand grip strength, eye and lung function, and cardiorespiratory fitness; and blood, urine and saliva samples were collected for long-term storage (Table 1 ) [ 7 , 12 ]. A proportion of the cohort also underwent an eye examination (including refractive index, intraocular pressure, retinal photograph, and optical coherence tomography), a hearing test, a cardiorespiratory fitness test with 4 lead ECG test, calcaneal ultrasound for bone density, and pulse wave velocity of arterial stiffness.
The original sample size was selected to maximise the number of incident cases of a wide range of important diseases to support the reliable investigation of their potential determinants. Power calculations prior to study recruitment indicated that for an exposure in 10% of the cohort, 5000 cases of a health outcome (i.e., 1% of the cohort) would be required to identify a minimum detectable odds ratio of 1.26 at a critical P value of 10 −4 [ 8 ]. For gene-by-environment analyses, assuming a 10% prevalence of both the genotype and environmental exposure, 5000 cases would enable the identification of a minimum odds ratio of 1.98. As a result, very large numbers of participants are needed to identify large numbers of cases of particular diseases during a reasonable follow-up period. However, despite the large sample size of UK Biobank, some gene-by-environment analyses will not be possible for rare exposures or outcomes, and pooling data across other cohort studies is necessary to ensure adequate sample sizes for reliable investigation.
Due to the volunteer nature of the cohort, the UK Biobank cohort is not representative of the current general UK population in a number of ways [ 11 ]. However, the extent to which this actually matters depends on the aims of the specific research question. To ensure associations are generalisable to a wider population (or future populations), what may be more important is to have sufficiently large numbers of participants with different levels of exposures and incident disease [ 13 ]. For example, although the UK Biobank cohort contains a lower proportion of participants who live in more deprived areas compared with the UK population (16% [82,000] vs. 33% in the UK population), it still includes sufficiently large numbers to allow associations of socio-economic deprivation with disease risk to be investigated with high internal validity.
As a consequence of the healthy volunteer effect, cancer incidence rates are generally lower in UK Biobank in comparison to the general UK population but this varies by cancer site, as previously reported [ 11 ]. As such, UK Biobank should not be used to estimate cancer prevalence or incidence rates, but can be used to assess reliably the aetiological associations between exposures and cancer outcomes.
Outcome ascertainment
Participants provided consent for UK Biobank to follow their health over time through linkage to electronic medical and other health-related records. To date, linkage has been achieved to national death and cancer registries and hospital inpatient admissions (including critical care), with linkage to primary care available for ~45% of the cohort (Table 2 and Fig. 1 ). Cancer registry data provides curated data on the histological tumour type and date of diagnosis, both prior to recruitment (with data from the mid-1950s onwards) and during follow-up. Cancer registry data are considered the gold standard method for ascertaining cancer outcomes in the UK, owing to mandatory reporting of cancer outcomes within the NHS [ 14 ]. However, due to data being curated from multiple sources, there is a time lag to completeness, with data from cancer registries usually complete within 2 years of diagnosis. Primary care records include data on rapid referral under the 2-week pathway, cancer-relevant biomarkers (e.g., prostate-specific antigen testing and CA-125 measurements) and other information on the route to diagnosis, co-morbidities and medication use. Linked health data are updated approximately annually within UK Biobank (except GP data). UK Biobank also periodically contacts participants directly to obtain information on health-related conditions that are not well-captured in healthcare records (e.g., cognitive function, mental health, pain, etc.) through a series of web-based questionnaires (Table 1 ). These data are potentially important for cancer research as they can be used, for example, to assess pain among cancer patients, as well as enabling research into cognition and mental health of cancer survivors. Further details on data linkages, cleaning, validation and data availability (including summary statistics for all data fields) can be found on the UK Biobank data showcase webpage ( https://biobank.ctsu.ox.ac.uk/crystal/ ).
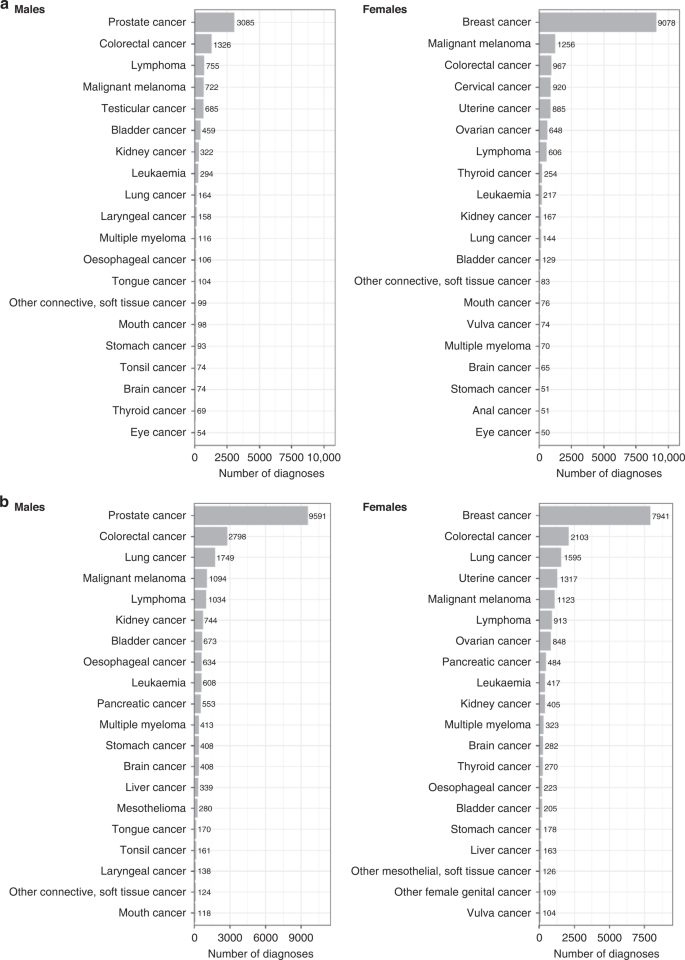
a Prevalent cancers by sex; b incident cancers by sex. Cancer registry data available until February 29, 2020 for England and Wales and January 31, 2021 for Scotland. Graphs reproduced from UK Biobank cancer summary report ( https://biobank.ndph.ox.ac.uk/~bbdatan/CancerSummaryReport.html accessed 27/9/2022).
Enhancements to the resource
Following the baseline assessment between 2006 and 2010, additional data have continued to be collected to enhance the value of the resource for health-related research. During 2013, a reasonably representative sample of about 20,000 participants was invited back for a repeat of the baseline assessment visit (including sample collection and storage) in order that researchers can make essential allowance for regression dilution bias due to measurement error and within-person fluctuations in exposure levels in their disease association analyses [ 15 ].
UK Biobank has also collected data on physical activity using wrist-worn accelerometers in 100,000 participants between 2013 and 2015, which was repeated on a seasonal basis in a subset of 2500 participants a few years later to assess changes in activity over time [ 16 ]. National guidelines on physical activity are based mainly on epidemiological studies that have used self-reported data, and the accelerometer data [ 16 ] in UK Biobank is now enabling robust research into the associations of objectively measured physical activity and sleep patterns with health outcomes (Table 1 ).
In 2014, UK Biobank initiated the world’s largest imaging sub-study, which aims to recruit up to 100,000 participants to undergo magnetic resonance imaging (MRI) of the brain, heart, and body, whole-body dual-energy X-ray absorptiometry (DXA), carotid ultrasound, together with a repeat of the baseline assessment, including questionnaires, physical measures and biological sampling (blood and urine) [ 17 ]. By the end of 2021, 50,000 participants had been scanned at one of four bespoke UK Biobank imaging centres, with a subset also invited to wear a cardiac monitor for 2 weeks. Furthermore, repeat imaging of up to 60,000 participants has also started, allowing research into the relationship between changes in internal physiology (such as muscle and fat distribution), and risk of disease onset and progression, which is likely to be of particular value for research into identifying early detection biomarkers and for refining risk prediction models.
Plans are underway to incorporate further information on cancer phenotyping by expanding its linkage to national datasets with information on tumour aggressiveness (i.e., stage, grade), morphology, and treatment (including radiotherapy, chemotherapy, immunotherapy and hormone treatments). These data will allow for more detailed research into risk factors for different cancer subtypes, as well as identifying suitable prognostic markers for survival and provide data for pharmacogenomics research [ 18 ]. However, researchers should be aware that data completeness varies by cancer site, as these data are not compulsory to provide to the National Cancer Registration and Analysis service [ 19 ]. For tumour grade, the amount of missing data varies from 0% to about 70%, with breast, colorectal, pancreatic and oesophageal cancers having the most complete data, and brain and uterine having the least. Tumour stage data are available for between 55 and 90% of cancers, with brain, hepatobiliary and pancreatic being most complete and colorectal, endometrial and ovarian the least. Pilot studies are also currently underway to assess the feasibility of incorporating digitised histopathology slides into UK Biobank to enable researchers to ascertain different morphological subtypes of cancer. It may also be possible to link to datasets that contain information on the molecular characterisation of cancer subtypes (e.g., biomarkers or genetic changes in cancer tissue), which will accelerate research into their aetiological pathways and how best to treat and manage them. For example, it is now well-established that colorectal cancer evolves through multiple pathways, which can be classified according to their molecular features (e.g., DNA microsatellite instability and methylation) [ 20 ]. Detailed phenotyping of cancers, together with better characterisation of key exposures (such as imaging-derived body composition and genomic data), will support powerful research into the determinants of different cancer subtypes.
UK Biobank’s policy has been, wherever possible, to perform cohort-wide assays on the biological samples, thereby allowing the limited biological samples to be used for the widest possible range of research [ 21 ]. This unique approach facilitates good quality control and effective management of the limited and depletable sample volume [ 21 ]. The availability of a wide range of biomarkers in all 500,000 participants increases the resource’s utility, as it allows research between biomarkers and a wide range of outcomes (which is simply not possible if using a case–control design). To date, cohort-wide data have been made available on: haematological and biochemistry assays [ 22 ] (including several biomarkers of relevance to cancer research, such as sex hormones and insulin-like growth factor-I); leukocyte telomere length; [ 23 ] and genome-wide genotyping using an Affymetrix array of ~850,000 variants, with imputation on >90 million variants (Table 3 ) [ 24 ]. In addition, industry consortia have performed whole-exome [ 25 ] and whole-genome sequencing for all 500,000 participants, making this biomedical database the world’s largest resource for scientists to gain valuable insights into the genetic determinants of disease. Of course, the availability of genetic data - coupled with lifestyle information and clinical outcomes on such a large-scale - will also accelerate the identification of potential drug targets.
Arising from previous consortia to fund genetic sequencing, a pharmaceutical consortium is investing in proteomic measurements for 3000 proteins in 57,000 participants using the O-LINK platform. These samples were selected randomly (~45,000) or enhanced for diseases of interest by the consortium members (~8000). These data are expected to be released in 2023 (Table 3 ), and there is significant interest in extending these measures to the full cohort to accelerate the development of drug targets and identifying early detection biomarkers [ 26 ].
Metabolomics assays using nuclear magnetic resonance (NMR) spectroscopy (funded by Nightingale Health) are underway for all 500,000 participants, with the first tranche of data released in 2021 for >200 circulating metabolites for 120,000 participants at baseline and 3000 participants at resurvey [ 27 ]. Data on serological markers of infectious agents, including a number of known or potential oncogenic pathogens, are also available for a subset of participants [ 28 ], with recent funding (from Open Philanthropy) to extend these data to an additional 50,000 participants (Table 3 ).
Dates for the future release of data, such as the enhanced cancer data and proteomics, are available on our website ( https://www.ukbiobank.ac.uk/enable-your-research/about-our-data/future-data-release-timelines?src=future_timelines ).
UK Biobank works with, and is guided by, the research community to ensure the resource is continually enhanced, and welcomes proposals from researchers to improve its utility. In addition to samples being available for assay, proposals for exposure and outcome measurement to develop the resource are considered. Researchers that wish to discuss potential enhancements (such as further linkages) that would be beneficial to the cancer research community are encouraged to contact UK Biobank’s access team ( https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/contact-us ).
UK Biobank and cancer research
UK Biobank is an important resource for population-based cancer research. There are already over 43,000 incident cancer cases recorded to date in the national cancer registry among UK Biobank participants (in addition to the 26,000 prevalent cases at baseline, including 9000 prevalent breast cancers, 3000 prevalent prostate cancers and 2200 prevalent colorectal cancers (Fig. 1 )). This includes 9500 incident prostate cancers, 7900 incident breast cancers, 4900 incident colorectal cancers and 3300 incident lung cancers (Fig. 1 ). Even for some relatively rare cancers, such as renal cell carcinoma and endometrial cancer, there are already over 1100 incident cases. As the cohort ages (the average age is now 70 years), the number of cancer cases will increase substantially, with incident prostate, breast, colorectal and lung cancers predicted to increase to 16,000, 14,000, 8000 and 6000 cases, respectively, by 2027 (these estimated numbers are adjusted for age, sex, and the healthy volunteer effect seen in UK Biobank). The full cancer reports and methodology can be accessed on the UK Biobank data website [ 29 , 30 ].
UK Biobank is particularly suited to enable studies on the determinants of disease; identifying risk factors that make people more or less likely to develop a particular disease, and quantifying the strength of the associations. This can often be challenging using small-scale studies, due to the limited power from low numbers of disease events. UK Biobank’s size, together with its deep phenotyping, allow associations to be quantified with greater precision, and across levels of other risk factors. Furthermore, variation in the strength of the associations across a broad range of demographic, socio-economic, and lifestyle characteristics can be used to assess the generalisability of the associations to important population subgroups [ 11 , 31 ].
Since the release of genome-wide genotyping data for all UK Biobank participants in 2017, the study has played a central role in accelerating the identification of genetic variants associated with cancer risk. Recent studies using UK Biobank data have identified new susceptibility loci for specific cancers, including endometrial cancer [ 32 ], colon cancer [ 33 ] and cervical cancer [ 34 ]. Such studies are particularly valuable in understanding the biological mechanisms underlying the development of cancer. For example, many genetic variants associated with cervical cancer risk are in the region of PAX8, CLPTM1L and HLA genes, suggesting a disruption in apoptotic and immune function pathways [ 34 ]. Research has also identified genes that affect the risk of more than one type of cancer (many of which appear to be regulatory elements and/or influence cross-tissue gene expression), offering further insight into the complex genetic architecture of cross-cancer susceptibility [ 35 ]. Further, research has identified shared genetics between known cancer risk factors and cancer development (such as alcohol consumption and oral cancer [ 36 ] and obesity and progression of a number of cancers [ 37 ]) which will help to disentangle the causal pathways of known associations.
Genotyping data have also facilitated causal inference through the use of Mendelian randomisation, a technique whereby genetic variants that are associated with a given exposure are used to investigate the causality of associations between an exposure and outcome of interest [ 38 ]. Mendelian randomisation takes advantage of the random assortment of genes from parents to offspring that occurs during gamete formation and conception to mimic the effect of a randomised controlled trial for a particular exposure. Analyses using Mendelian randomisation have supported the causality of the associations of circulating insulin-like growth factor-1 concentration with colorectal, breast and prostate cancer risk [ 39 , 40 , 41 , 42 ], obesity with endometrial cancer [ 43 ], and height with overall cancer risk [ 44 ]; but refute previous observational evidence for an inverse association between vitamin D concentration and colorectal cancer risk [ 45 ].
In addition to research on the causes of disease, GWAS data can also be used to construct polygenic risk scores, which combine the effects of genetic variants (each of which may have only a small effect on cancer risk) to improve risk prediction [ 46 , 47 , 48 , 49 , 50 , 51 ]. These scores could then be used to stratify the population according to their genetic risk, or used to enhance existing risk prediction algorithms (such as QCancer for cancer risk [ 52 , 53 ]) that use information on sociodemographic, lifestyle or clinical factors. Polygenic risk scores have been developed for a wide range of cancers using UK Biobank data. For example, an academic consortia (Breast Cancer Association Consortium) have developed a polygenic risk score for breast cancer composed of 313 genetic variants, with those in the highest group having a lifetime risk of about 30% for developing ER + breast cancer, compared to 2% in the lowest, with a range of 0.55–4% for ER− disease [ 54 ]. Many of those in the high PRS category do not have a strong family history of breast cancer, so would not be identified by standard risk screening tools. The clinical utility of polygenic risk scores is being assessed, but such scores may well be used to inform clinical decision-making or to inform screening programmes (e.g., to target individuals with a high polygenic risk score for certain cancers to undergo earlier or higher frequency screening programmes [ 54 , 55 ]).
The release of whole-exome and whole-genome sequencing for 500,000 participants will be extremely valuable to research into new cancer therapies. In particular, variants in the exome region of the genome (which encode for proteins) can be used to identify genetic variants of particular relevance for drug discovery. For example, a study using UK Biobank data has already found that a genetic variant in the gene that encodes the GPR75 receptor is associated with a significantly lower rate of obesity in homozygous carriers [ 56 ]. This has subsequently been confirmed in animal models [ 56 ], and paves the way for pharmaceutical trials to develop new treatments for obesity. Whole-genome sequencing makes it possible for scientists to investigate the impact of coding and non-coding DNA and of repeated, missing or extra sequences of DNA, on disease risk. These data offer an opportunity to understand the potential impact of inhibiting or agonising the product of a gene, with relevance to drug development [ 57 ]. It also allows the detection of rare, non-coding variants that will help us understand gene regulation and disease mechanisms, as well as the identification of structural variations, such as short tandem repeats, which can be used to further understand the aetiologies of complex diseases.
In addition to understanding the genetic determinants of disease, the rich characterisation of participants in UK Biobank has been used to assess the behavioural and environmental determinants of cancer, such as from dietary factors [ 58 , 59 ] and physical activity [ 60 , 61 , 62 ] to shift work [ 63 ] and exogenous hormone use [ 64 , 65 ], with some results directly impacting public health policy. For example, researchers using dietary data collected at baseline and at resurvey (supplemented by the dietary web-based questionnaire) found higher consumption of red and processed meat was positively related to risk of colorectal rectal, even within the current UK guidelines that recommend no more than 90 g of red and processed meat per day [ 59 ]. Researchers have also used accelerometer-based measures of physical activity to improve understanding of the associations of physical activity and risk of breast cancer [ 66 ]. The study found that greater physical activity was associated with a reduction in breast cancer risk in both pre- and post-menopausal women, independent of any association it may have on risk through its effects on adiposity.
A wide range of anthropometric measures were collected as part of the baseline survey in UK Biobank, and these have been used to assess the impact of adiposity on cancer risk. A recent study assessed the association of six adiposity-related markers (including body-mass index, body fat percentage, waist-hip ratio, waist-height ratio, and waist and hip circumference), with risk of 24 different cancers [ 67 ]. The study found strong associations with a number of cancers, including cancers of the stomach cardia, gallbladder, liver and kidney. The availability of imaging data on large numbers of participants will substantially enhance research into the effect of adiposity (and other endogenous markers of body size and structure and composition) with disease risk, allowing more precise analyses of the risks associated with specific measures of body composition. Imaging-derived adiposity measures from DXA and MRI images have already been used to assess the relation between the distribution of body fat and risk of several cancers. For example, a recent study found that for a given level of total body fat, increased central adiposity was associated with an increased risk of colorectal cancer, but increased hip fat was associated with a reduced colorectal cancer risk [ 68 ].
The cohort-wide assays performed on the blood samples from all 500,000 participants are already enabling robust research into the role of sex hormones and risk of cancer onset and progression. For example, analyses using UK Biobank have shown that the risk of endometrial cancer is positively related to circulating levels of total and free testosterone but inversely related to levels of sex hormone-binding globulin [ 69 ] with Mendelian randomisation analyses supporting the causality of these association [ 69 ]. In men, higher free testosterone, but not total testosterone, has been found to be associated with risk of prostate cancer [ 41 ]. Conversely, biomarkers of inflammation do not appear to be related to risk of glioma [ 70 ] and circulating lipid levels are not strongly associated with ovarian cancer risk [ 71 ]. Biomarkers can also be used to investigate the pathways between known risk factors and cancer diagnosis, with research showing that the increased risk of colorectal cancer associated with obesity is unlikely to be driven by adiposity-induced chronic inflammation, insulin resistance or sex-steroid hormone levels [ 72 ]. Proteomic data, in particular, may help identify individuals at high risk of specific cancers or may aid in their diagnosis, with small-scale analyses in other cohorts indicating its utility [ 73 ]. Proteomics—particularly when combined with genetics and metabolomics data in a single cohort—will enhance the opportunities to investigate the biological pathways by which genes affect cancer risk, with the potential to identify novel drug targets and treatments [ 73 ].
With such a complex dataset, researchers have employed artificial intelligence tools to identify risk factors for cancer incidence and to improve risk prediction models for cancer onset and survival [ 74 , 75 ]. For example, machine-learning algorithms have been used to predict overall survival in breast cancer patients from whole-exome sequencing data in UK Biobank [ 76 ]. Researchers have also used machine learning to derive phenotypes from complex data, such as sleep phenotypes from the accelerometer data or imaging phenotypes, such as organ segmentation, from the MRI data [ 77 , 78 , 79 ]. Machine-learning methods allow the relationships among different variables and types of data to be learnt from the data itself, and this may have advantages over classical statistical methods, where the relationships among variables need to be pre-specified, and only a limited number of factors can be modelled at a time [ 80 ].
Accessing UK Biobank data
What makes UK Biobank so unique is the easy accessibility of a vast range of data on 500,000 participants to all bona fide academic or commercial researchers, anywhere in the world [ 81 ]. Researchers must register prior to submitting an application, and the application must be for health-related research that is in the public interest. UK Biobank has a policy of no preferential access, ensuring all applicants (whether academic, governmental, charitable or commercial) are treated in the same way [ 82 ], and has seen an exponential rise in registered researchers, with over 25,000 registered researchers and 2800 applications by the end of 2021 (Fig. 2 ). This has been borne out with over 1600 publications arising from UKB data in 2021 alone (Fig. 2 ).
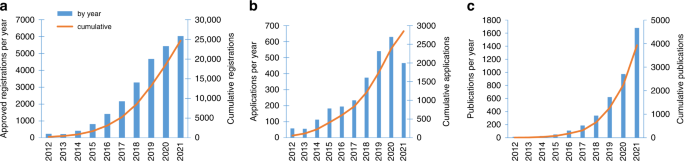
a Researchers registrations; b Project applications; c Publications, by year.
UK Biobank is a registered charity, and manages access fees on a cost recovery basis, which are reviewed on a periodic basis and are subsidised for student projects and research groups based in low and low-to-middle-income countries [ 83 ]. Applications to access biological samples are reviewed more stringently, due to the limited and depletable nature of the samples. Information on how to access the dataset can be found on the UK Biobank website ( https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access ).
The data included in the UK Biobank resource is expected to grow to 50 petabytes by 2027. In the past, UK Biobank data have been provided to approved researchers for download, which requires a non-trivial level of local computing power and storage. The continuing expansion of data requires a more democratic approach to ensure the data are available to all researchers. Consequently, UK Biobank has made available a new cloud-based Research Analysis Platform, developed by DNAnexus (Mountain View, CA) and hosted by Amazon Web Services (London, UK). This ensures that access to UK Biobank data will remain open to all, and not just those with the information technology infrastructure to store and analyse such large data. Further, research credits to subsidise the cost of running analyses on the Research Analysis Platform have been provided to support early career researchers and those from low-and middle-income countries ( https://www.ukbiobank.ac.uk/enable-your-research/research-analysis-platform/the-uk-biobank-platform-credits-programme ).
UK Biobank is a large-scale prospective study with deep phenotyping and genomic data. Easy accessibility to this vast biomedical resource allows researchers from around the world to make scientific discoveries to improve population health. The sheer depth and breadth of data mean that UK Biobank is now arguably the world’s most important health resource for understanding the determinants major diseases in middle and old age; it is now being used by over 25,000 researchers internationally and generating thousands of peer-reviewed publications. The resource has already demonstrated it value in enabling novel and robust research into the determinants of cancer, and will only grow in value as more incident cancer cases occur over time. In particular, the combination of whole-genome sequencing, imaging, proteomics, and metabolomic data, will enable the world’s best minds to transform our understanding of the causes of cancer development and progression and drive improvements in cancer treatment and prevention.
Data availability
UK Biobank is an open-access resource. Applications to access the data from bone fide researchers can be made at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access .
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Article PubMed Google Scholar
Ahmad AS, Ormiston-Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer. 2015;112:943–7.
Article CAS PubMed PubMed Central Google Scholar
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–89.
Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–41.
Article PubMed PubMed Central Google Scholar
Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17.
Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505–17.
Article CAS PubMed Google Scholar
Downey P, Peakman TC. Design and implementation of a high-throughput biological sample processing facility using modern manufacturing principles. Int J Epidemiol. 2008;37:i46–50.
UK Biobank. UK Biobank: Protocol for a large-scale prospective epidemiological resource (AMENDMENT ONE FINAL) [Internet]. 2007 [cited 2018 Nov 15]. http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf?phpMyAdmin=trmKQlYdjjnQIgJ%2CfAzikMhEnx6
Our funding [Internet]. [cited 2022 May 6]. https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/our-funding
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with the general population. Am J Epidemiol. 2017;186:1026–34.
Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44.
Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368. https://www.bmj.com/content/368/bmj.m131
National Cancer Registration and Analysis Service. CancerData. [cited 2022 Sep 22]. https://www.cancerdata.nhs.uk/
Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol [Internet]. 1999;150:341–53.
Doherty A, Jackson D, Hammerla N, Plö tz T, Olivier P, Granat MH, et al. Large scale population assessment of physical activity using wrist worn accelerometers: The UK Biobank Study. PLoS ONE. 2017;12:e0169649.
Littlejohns TJ, Holliday J, Gibson LM, Garratt S, Oesingmann N, Alfaro-Almagro F, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. 2020;11:2624.
Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001;1:99–108.
National Cancer Registration and Analysis Service. CancerData. https://www.cancerdata.nhs.uk/ [accessed 22/09/2022].
Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30.
UK Biobank. Sample release policy and procedure. 2017. https://www.ukbiobank.ac.uk/media/0nhcwiff/sample-release-policy-and-procedures.pdf
Allen NE, Arnold M, Parish S, Hill M, Sheard S, Callen H, et al. Approaches to minimising the epidemiological impact of sources of systematic and random variation that may affect biochemistry assay data in UK Biobank. Wellcome Open Res. 2021;5:222.
Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, et al. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging. 2022;2:170–9.
Article Google Scholar
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Szustakowski JD, Balasubramanian S, Kvikstad E, Khalid S, Bronson PG, Sasson A, et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat Genet. 2021;53: 942–8.
Suhre K, McCarthy MI, Schwenk JM. Genetics meets proteomics: perspectives for large population-based studies. Nat Rev Genet. 2020;22:19–37.
Julkunen, H, Cichońska, A, Slagboom, P, Würtz, P, Nightingale Health UK Biobank Initiative. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. eLife. 2021;10:e63033. https://doi.org/10.7554/eLife.63033 (2021).
Mentzer AJ, Brenner N, Allen N, Littlejohns TJ, Chong AY, Cortes A, et al. Identification of host–pathogen-disease relationships using a scalable multiplex serology platform in UK Biobank. Nat Commun. 2022;13:1–12.
UK Biobank. UK Biobank Malignant Cancer Summary Report. 2022 [cited 2022 Sep 27]. https://biobank.ndph.ox.ac.uk/~bbdatan/CancerSummaryReport.html
UK Biobank. UK Biobank Cancer Numbers Summary Report. 2022 [cited 2022 Sep 27]. https://biobank.ndph.ox.ac.uk/~bbdatan/CancerNumbersReport.html
Richiardi L, Pizzi C, Pearce N. Commentary: representativeness is usually not necessary and often should be avoided. Int J Epidemiol. 2013;42:1018–22.
O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018;9:3166.
Law PJ, Timofeeva M, Fernandez-Rozadilla C, Broderick P, Studd J, Fernandez-Tajes J, et al. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun. 2019;10:1–15.
Takeuchi F, Kukimoto I, Li Z, Li S, Li N, Hu Z, et al. Genome-wide association study of cervical cancer suggests a role for ARRDC3 gene in human papillomavirus infection. Hum Mol Genet. 2019;28:341–8.
Rashkin SR, Graff RE, Kachuri L, Thai KK, Alexeeff SE, Blatchins MA, et al. Pan-cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nat Commun. 2020;11:1–14.
Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol Psychiatry. 2017;22:1376–84.
Christakoudi S, Evangelou E, Riboli E, Tsilidis KK. GWAS of allometric body-shape indices in UK Biobank identifies loci suggesting associations with morphogenesis, organogenesis, adrenal cell renewal and cancer. Sci Rep. 2021;11:10688.
Smith GD, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98.
Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin‐like growth factor‐1 and site‐specific cancers: a Mendelian randomization study. Cancer Med. 2020;9:6836–42.
Knuppel A, Fensom GK, Watts EL, Gunter MJ, Murphy N, Papier K, et al. Circulating insulin-like growth factor-I (IGF-I) concentrations and incidence of 30 cancers: prospective analyses in UK Biobank. Cancer Res. 2020;80:4014–21.
Watts EL, Fensom GK, Smith Byrne K, Perez‐Cornago A, Allen NE, Knuppel A, et al. Circulating insulin‐like growth factor‐I, total and free testosterone concentrations and prostate cancer risk in 200,000 men in UK Biobank. Int J Cancer. 2020;ijc.33416. https://onlinelibrary.wiley.com/
Murphy N, Knuppel A, Papadimitriou N, Martin RM, Tsilidis KK, Smith-Byrne K, et al. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: observational and Mendelian randomization analyses with ∼ 430 000 women. Ann Oncol. 2020;31:641–9
Ahmed M, Mulugeta A, Lee SH, Mäkinen V-P, Boyle T, Hyppönen E. Adiposity and cancer: a Mendelian randomization analysis in the UK biobank. Int J Obes. 2021;45:2657–65.
Ong J-S, An J, Law MH, Whiteman DC, Neale RE, Gharahkhani P, et al. Height and overall cancer risk and mortality: evidence from a Mendelian randomisation study on 310,000 UK Biobank participants. Br J Cancer. 2018;118:1262–7.
He Y, Zhang X, Timofeeva M, Farrington SM, Li X, Xu W, et al. Bidirectional Mendelian randomisation analysis of the relationship between circulating vitamin D concentration and colorectal cancer risk. Int J Cancer. 2021; https://onlinelibrary.wiley.com/
Salvatore M, Beesley LJ, Fritsche LG, Hanauer D, Shi X, Mondul AM, et al. Phenotype risk scores (PheRS) for pancreatic cancer using time-stamped electronic health record data: discovery and validation in two large biobanks. J Biomed Inform. 2020;113:103652.
Smith T, Gunter MJ, Tzoulaki I, Muller DC. The added value of genetic information in colorectal cancer risk prediction models: development and evaluation in the UK Biobank prospective cohort study. Br J Cancer. 2018;119:1036–9.
McCarthy CE, Bonnet LJ, Marcus MW, Field JK. Development and validation of a multivariable risk prediction model for head and neck cancer using the UK Biobank. Int J Oncol. 2020;57:1192–202.
CAS PubMed Google Scholar
Usher-Smith JA, Harshfield A, Saunders CL, Sharp SJ, Emery J, Walter FM, et al. External validation of risk prediction models for incident colorectal cancer using UK Biobank. Br J Cancer. 2018;118:750–9.
Hunter RF, Murray JM, Coleman HG. The association between recreational screen time and cancer risk: findings from the UK Biobank, a large prospective cohort study. Int J Behav Nutr Phys Act. 2020;17:1–25.
Kachuri L, Graff RE, Smith-Byrne K, Meyers TJ, Rashkin SR, Ziv E, et al. Pan-cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat Commun. 2020;11:1–11.
Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pr. 2013;63:e1–10.
Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pr. 2013;63:e11–21.
Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2018;104:21–34.
Hung RJ, Warkentin MT, Brhane Y, Chatterjee N, Christiani DC, Landi MT, et al. Assessing lung cancer absolute risk trajectory based on a polygenic risk model. Cancer Res. 2021;81:1607–15.
Akbari P, Gilani A, Sosina O, Kosmicki JA, Khrimian L, Fang YY, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373:eabf8683.
King EA, Davis JW, Degner JF. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019;15:e1008489.
Knuppel A, Papier K, Fensom GK, Appleby PN, Schmidt JA, Tong TYN, et al. Meat intake and cancer risk: prospective analyses in UK Biobank. Int J Epidemiol. 2020;49:1540–52.
Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2019;49:246–58.
Morris JS, Bradbury KE, Cross AJ, Gunter MJ, Murphy N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118:920–9.
Murray JM, Coleman HG, Hunter RF. Physical activity and cancer risk: findings from the UK Biobank, a large prospective cohort study. Cancer Epidemiol. 2020;68:101780.
Celis-Morales CA, Lyall DM, Welsh P, Anderson J, Steell L, Guo Y, et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ. 2017;357:j1456.
Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang X-S, et al. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst. 2016;108:djw169.
Yaghjyan L, Rich S, Mao L, Mai V, Egan KM. Interactions of coffee consumption and postmenopausal hormone use in relation to breast cancer risk in UK Biobank. Cancer Causes Control. 2018;29:519–25.
Petrick JL, McMenamin ÚC, Zhang X, Zeleniuch-Jacquotte A, Wactawski-Wende J, Simon TG, et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br J Cancer. 2020;123:316–24.
Guo W, Fensom GK, Reeves GK, Key TJ. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br J Cancer. 2020;122:726–32.
Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. A body shape index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. Cancer Med. 2021;10:5614–28.
Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. Association of body-shape phenotypes with imaging measures of body composition in the UK Biobank cohort: relevance to colon cancer risk. BMC Cancer. 2021;21:1–15.
Mullee A, Dimou N, Allen N, O’Mara T, Gunter MJ, Murphy N. Testosterone, sex hormone-binding globulin, insulin-like growth factor-1 and endometrial cancer risk: observational and Mendelian randomization analyses. Br J Cancer. 2021;125:1308–17.
Cote DJ, Smith-Warner SA, Creed JH, Furtado J, Gerke T, Wang M, et al. Circulating lipids and glioma risk: results from the UK Biobank, Nurses’ Health Study, and Health Professionals Follow-Up Study. Cancer Causes Control. 2021;4:347–55.
Trabert B, Hathaway CA, Rice MS, Rimm EB, Sluss PM, Terry KL, et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol Biomark Prev. 2021;30:2044–51.
Article CAS Google Scholar
Dashti SG, Viallon V, Simpson JA, Karahalios A, Moreno-Betancur M, English DR, et al. Explaining the link between adiposity and colorectal cancer risk in men and postmenopausal women in the UK Biobank: a sequential causal mediation analysis. Int J Cancer. 2020;147:1881–94.
Kwon YW, Jo HS, Bae S, Seo Y, Song P, Song M, et al. Application of proteomics in cancer: recent trends and approaches for biomarkers discovery. Front Med. 2021;8:1644.
Widen E, Raben TG, Lello L, Hsu SDH. Machine learning prediction of biomarkers from SNPs and of disease risk from biomarkers in the UK Biobank. Genes. 2021;12:991.
Capobianco E. High-dimensional role of AI and machine learning in cancer research. Br J Cancer. 2022;126:523–32.
Jang BS, Kim IA. Machine-learning algorithms predict breast cancer patient survival from UK Biobank whole-exome sequencing data. Biomark Med. 2021;15:1529–39.
Cetin I, Raisi-Estabragh Z, Petersen SE, Napel S, Piechnik SK, Neubauer S, et al. Radiomics signatures of cardiovascular risk factors in cardiac MRI: results from the UK Biobank. Front Cardiovasc Med. 2020;7:591368.
Kart T, Fischer M, Küstner T, Hepp T, Bamberg F, Winzeck S, et al. Deep learning‐based automated abdominal organ segmentation in the UK Biobank and German National Cohort Magnetic Resonance Imaging Studies. Invest Radio. 2021;56:401–8.
Willetts M, Hollowell S, Aslett L, Holmes C, Doherty A. Statistical machine learning of sleep and physical activity phenotypes from sensor data in 96,220 UK Biobank participants. Sci Rep. 2018;8:7961.
Weng SF, Vaz L, Qureshi N, Kai J. Prediction of premature all-cause mortality: a prospective general population cohort study comparing machine-learning and standard epidemiological approaches. PLoS ONE. 2019;14:e0214365.
UK Biobank. AMS—Log in [Internet]. [cited 2019 Jan 29]. https://bbams.ndph.ox.ac.uk/ams/
Conroy M, Sellors J, Effingham M, Littlejohns TJ, Boultwood C, Gillions L, et al. The advantages of UK Biobank’s open-access strategy for health research. J Intern Med. 2019;286:389–97.
UK Biobank. New costs for 2021. [cited 2021 Mar 24]. https://www.ukbiobank.ac.uk/enable-your-research/new-costs-for-2021
Download references
Acknowledgements
We would like to thank all the participants of the UK Biobank for their vital contribution to the resource.
UK Biobank is funded by the Medical Research Council, Wellcome, Department of Health, Scottish Government, Welsh Assembly Government, British Heart Foundation, Cancer Research UK, Diabetes UK, National Institute for Health and Care Research (NIHR), and the Northwest Regional Development Agency.
Author information
Authors and affiliations.
Nuffield Department of Population Health (NDPH), University of Oxford, Oxford, UK
Megan C. Conroy, Ben Lacey, Jelena Bešević, Wemimo Omiyale, Qi Feng, Rory Collins & Naomi E. Allen
UK Biobank, Stockport, Greater Manchester, UK
Mark Effingham, Jonathan Sellers, Simon Sheard, Mahesh Pancholi, Gareth Gregory, John Busby & Rory Collins
You can also search for this author in PubMed Google Scholar
Contributions
The review was conceived and written by MCC, BL and NEA. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Megan C. Conroy .
Ethics declarations
Competing interests.
All authors are current members of UK Biobank scientific team and/or executive management team. The authors declare no other competing interests.
Ethics approval and consent to participate
UK Biobank received ethical approval from the National Information Governance Board for Health and Social Care and the National Health Service North West Centre for Research Ethics Committee (Ref: 21/NW/0157). All participants provided informed consent at recruitment to the study for their data to be used for health-related research that was in the public interest.
Consent to publish
No participant data were used in the review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Conroy, M.C., Lacey, B., Bešević, J. et al. UK Biobank: a globally important resource for cancer research. Br J Cancer 128 , 519–527 (2023). https://doi.org/10.1038/s41416-022-02053-5
Download citation
Received : 21 June 2022
Revised : 26 October 2022
Accepted : 27 October 2022
Published : 19 November 2022
Issue Date : 16 February 2023
DOI : https://doi.org/10.1038/s41416-022-02053-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Identification of phenomic data in the pathogenesis of cancers of the gastrointestinal (gi) tract in the uk biobank.
- Shirin Hui Tan
- Catherina Anak Guan
- Edmund Ui Hang Sim
Scientific Reports (2024)
Exploring potential causal associations between autoimmune diseases and colorectal cancer using bidirectional Mendelian randomization
- Feifan Wang
- Baoshan Cao
The Relationship Between Systemic Lupus Erythematosus and Osteoporosis Based on Different Ethnic Groups: a Two-Sample Mendelian Randomization Analysis
Calcified Tissue International (2024)
Multi-Trait Body Shape Phenotypes and Breast Cancer Risk in Postmenopausal Women: A Causal Mediation Analysis in the UK Biobank Cohort
- Amina Amadou
- Heinz Freisling
- Béatrice Fervers
Journal of Epidemiology and Global Health (2024)
Multi-morbidity and its association with common cancer diagnoses: a UK Biobank prospective study
- Megan C. Conroy
- Gillian K. Reeves
- Naomi E. Allen
BMC Public Health (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 05 June 2020
Evidencing the impact of cancer trials: insights from the 2014 UK Research Excellence Framework
- Catherine R. Hanna ORCID: orcid.org/0000-0002-0907-7747 1 ,
- Lauren P. Gatting 2 ,
- Kathleen Anne Boyd ORCID: orcid.org/0000-0002-9764-0113 2 ,
- Kathryn A. Robb ORCID: orcid.org/0000-0002-1672-0411 3 &
- Rob J. Jones 1
Trials volume 21 , Article number: 486 ( 2020 ) Cite this article
1821 Accesses
8 Citations
3 Altmetric
Metrics details
Introduction
An impactful clinical trial will have real-life benefits for patients and society beyond the academic environment. This study analyses case studies of cancer trials to understand how impact is evidenced for cancer trials and how impact evaluation can be more routinely adopted and improved.
The United Kingdom (UK) Government allocates research funding to higher-education institutions based on an assessment of the institutions’ previous research efforts, in an exercise known as the Research Excellence Framework (REF). In addition to each institution’s journal publications and research environment, for the first time in 2014, allocation of funding was also dependent on an evaluation of the wider, societal impact of research conducted. In the REF2014, impact assessment was performed by evaluation of impact case studies. In this study, case studies ( n = 6637) submitted by institutions for the REF2014 were accessed and those focussing on cancer trials were identified. Manual content analysis was then used to assess the characteristics of the cancer trials discussed in the case studies, the impact described and the methods used by institutions to demonstrate impact.
Forty-six case studies describing 106 individual cancer trials were identified. The majority were phase III randomised controlled trials and those recruiting patients with breast cancer. A list of indicators of cancer trial impact was generated using the previous literature and developed inductively using these case studies. The most common impact from a cancer trial identified in the case studies was on policy, in particular citation of trial findings in clinical guidelines. Impact on health outcomes and the economy were less frequent and health outcomes were often predicted rather than evidenced. There were few descriptions identified of trialists making efforts to maximise trial impact.
Cancer trial impact narratives for the next REF assessment exercise in 2021 can be improved by evidencing actual rather than predicted Impact, with a clearer identification of the beneficiaries of cancer trials and the processes through which trial results are used. Clarification of the individuals responsible for performing impact evaluations of cancer trials and the provision of resources to do so needs to be addressed if impact evaluation is to be sustainable.
Peer Review reports
The success of a modern cancer trial should not be determined solely by the trial results or the impact factor of the journal of publication. In addition, this success should be based on the real-life benefits that the trial makes to patients and society. Several institutions that fund or perform cancer trials, including Cancer Research UK, the Institute of Cancer Research and the Dana-Farber Cancer Institute, have formally endorsed the San Francisco Declaration on Research Assessment [ 1 ]. This declaration states that the evaluation of scholarly output should focus on meaningful benefits arising from research rather than narrow, quantitative metrics.
Cancer trials attract substantial investment from public and private funding. In 2019, the National Cancer Institute received over US$6 billion from Congress to fund cancer research, with over US$800 million spent on clinical trials [ 2 , 3 ]. Cancer Research UK, which is the single largest funder of cancer research in the United Kingdom (UK), spent £546 million on cancer research in 2018/2019 [ 4 ], has pledged £45 million specifically to its eight clinical trials units and [ 5 ] recruits over 25,000 patients to its clinical trials per annum [ 6 ].
In order to show accountability for these investments and to demonstrate to the public that money is invested wisely, it is crucial to show that academic outputs from cancer trials are leading to broader changes and benefits to society. These benefits are commonly referred to as the impact of research. The UK Higher Education Funding Council for England states that impact is ‘ an effect on, change or benefit to the economy, society, culture, public policy or services, health, the environment or quality of life, beyond academia’ [ 7 ].
Demonstrating the real-life impact of cancer trials can illustrate to patients and the public the value of participating in clinical trials. Outlining to healthcare managers the benefits that cancer trials bring to the health system may increase the time allocated to clinicians for trial recruitment. Demonstrating to funders that trials are impactful and identifying which types of trial have most impact means that funders can prioritise clinical trial investment. This is important because there is an opportunity cost that accompanies the decision to develop and perform one trial rather than another, due to the limited pool of patient volunteers and administrative support available. For example, Carlisle et al. [ 8 ] have demonstrated that clinical trials of cancer monotherapy conducted in the post-regulatory approval setting contribute less to subsequent drug approval and clinical guidelines than trials conducted for approval purposes. This is despite an at least equivalent burden for patients in terms of numbers needed for recruitment and the proportion who experience serious adverse events related to trial treatment. Only by understanding the impact of previous trials can funders, policy-makers and clinicians design, prioritise and invest in increasingly impactful trials in future.
Although the evaluation of research quality is not new, the assessment of research impact is a more recent phenomenon. The UK Government allocates research funding to higher-education institutions based on an assessment of the institutions’ previous research efforts. This allocation has traditionally focussed on an assessment of institutions’ journal publications and the research environment and prior to 2014 (1986–2008), was known as the Research Assessment Exercise. For the first time in 2014, allocation of funding was also dependent on an evaluation of the wider, societal impact from research. The name of the assessment was changed to the Research Excellence Framework (REF), and, in the exercise conducted in 2014 (REF2014), assessment of research impact was performed by evaluation of case studies. Impact case studies are narratives written by the institutions to describe the downstream effects that the institution perceive to represent the wider, societal impact related to their research, that is external to academia. The REF was piloted in the UK in 2010, formally employed in 2014, and the next assessment is due in 2021. Through this exercise the government allocates over £2 billion per annum to higher-education institutions and in 2021, impact case studies will attract an even greater proportion of funds (25%) compared to 2014 (20%). Partly because of the REF, the ability of UK universities to demonstrate that their research has led to real-life, tangible benefits to society, has become a major determinant of core income and status for these institutions. Other countries, such as Australia and Canada, are now (re-) investigating the use of impact assessment as part of their national evaluation frameworks [ 9 , 10 ].
Several authors have reflected on how universities evidenced the impact of their research in the REF2014. Greenhalgh and Fahy [ 11 ] outlined 14 types of impact evidenced by higher-education institutions in 162 impact case studies submitted to the REF2014 community-based disciplines’ panel. They found that an influence on guidelines was most commonly described, followed by impact on informing policy change and changes in clinical or public health practice. Chowdhury, Koya and Philipson [ 12 ] reviewed 363 case studies in six disciplines from either top-ranking or bottom-performing institutions in the REF2014 and identified variables that predicted the average REF scores received by the institutions. For 92 case studies submitted under the discipline of Clinical Medicine, the number of publications in highly cited journals was the variable most consistently associated with higher REF scores. These authors also used automated word frequency analysis to identify themes of research submitted under different disciplines. For clinical medicine, these included oncology, paediatrics, genetics, diabetes and heart disease research. Terämä et al. [ 13 ] used computational text-mining of the REF2014 case studies to understand how higher-education institutions interpreted impact. By analysing 6637 case studies, six classes of impact were identified (1 – Education, 2 – Public engagement, 3 – Environment and energy solutions, 4 – Enterprise, 5 – Policy; 6 – Clinical uses) and the class of impact described differed according to discipline. Similarly, a review of the REF2014, commissioned by the Higher Education Funding Council for England, discovered that frameworks and taxonomies of impact were often context specific [ 14 ].
The aim of this paper was to use the REF2014 case studies to understand how higher-education institutions evidenced the impact of their cancer trials. Such an understanding will allow reflection on if, and how, impact assessment for cancer trials can be performed outside the context of the REF, and how impact evaluation can improve, both for REF2021 and beyond.
The objectives were:
To identify cancer trials included by higher-education institutions in the REF2014 case studies
To quantify and explore the characteristics of these trials and the types of impacts they were claimed to have had
To identify the types of evidence used by higher-education institutions to substantiate those claims of impact
To identify any examples of researchers or research users making active attempts to maximise impact
Data collection
The REF2014 impact case studies are stored online and are publicly available via the Research Excellence Framework 2014 website [ 15 ]. A search of the non-confidential case studies was performed by combining the terms ‘cancer’ and ‘trial’ in the website search function [ 15 ]. This search function identified case studies that included these words in any part of the submission (title, main text or references). The case studies identified were read in full and the application of inclusion and exclusion criteria at this stage allowed the selection for final analysis. Inclusion criteria required that the case study focussed on the impact of adult (aged 16 years or over) clinical trials that prospectively recruited patients with a diagnosis of malignancy, or individuals without a known diagnosis but where the aim of the trial was to investigate the development of, diagnosis or screening of cancer. All stages of cancer and clinical trials of all phases were included. Impact case studies were excluded if they described paediatric cancer trials (age < 16 years) and/or if clinical trials were mentioned but were not the focus of the case study.
Data analysis
Manual content analysis of the case studies meeting these criteria was performed [ 16 ]. The initial coding manual was based on previous literature [ 11 , 17 , 18 , 19 ], collected descriptive information about the case studies and cancer trials, and contained pre-defined categories of impact that were identified from a systematic review (unpublished). Supplementary material 2 explains in more detail how these categories of impact were identified. The manual was developed iteratively through three stages by two researchers (CH and LG) to better reflect the specific context of cancer trial impacts. For a detailed outline of the coding process, see Fig. 1 b. This iteration included the inclusion of specific examples, often referred to as indicators [ 12 ], of how higher-education institutions evidenced impact within each categories. The second reviewer (LG) coded a randomly selected sub-sample of the case studies to assess coding validity. The final inter-coder reliability estimate for this was 80.2%.
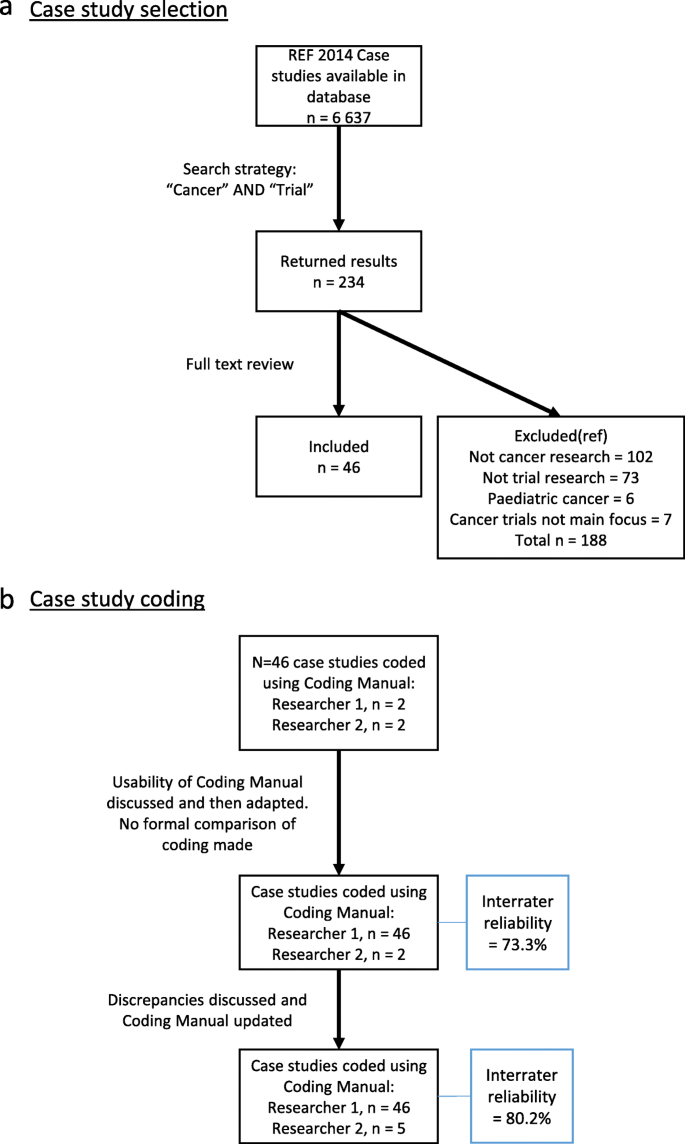
a Case study selection. b Case study coding
In Part 1 of the coding manual (Supplementary material 1 ) the following information was recorded: (1) the institution responsible for the submission; (2) the Unit of Assessment and (3) the Summary Impact Type. The Units of Assessment are 36 subject areas, each with its own REF expert review panel. The Summary Impact Types are eight categories of impact, assigned to each case study by text analysis after submission to the REF. These categories are technological, economic, health, political, legal, cultural, societal and environmental [ 13 ]. For the clinical trials identified, the following key characteristics were extracted: (1) name; (2) phase of the trial; (3) type of cancer investigated; (4) focus of the trial (screening, diagnosis and treatment, other); (5) journal of publication cited in the case study; (6) category of funder; (7) primary endpoint and (8) whether the primary endpoint was met. For the purposes of the final characteristic, trials were marked as positive if they met their pre-specified primary endpoint with statistical significance. For non-inferiority trials, if the experimental arm of the trial was deemed to be statistically non-inferior than the control arm at the level of significance pre-defined by the trialists, this was considered a positive result. For earlier-phase trials such as phase I trials focussing on safety, if, for example, the authors set out to find a recommended phase II dose of a novel drug, and this was achieved and reporting in the trial findings, this was considered as having a positive result.
Part 2 of the coding manual (Supplementary material 1 ) captured the following information for each impact case study: (1) all categories of impact described; (2) examples of dissemination and knowledge transfer of trial information and results; (3) methods used by institutions to evidence impact; (4) clinical guidelines cited and (5) examples of when researchers or research users acted to enhance trial impact [ 20 ]. Dissemination and knowledge transfer describe the communication of trial information or results to stakeholders. This information was collected by reading and manually coding the ‘Details of Impact’ section of each case study using Nvivo version 12.1 (2018). The pre-defined categories of cancer trial impact were (i) ‘New knowledge and immediate research outputs’, (ii)‘Capacity building for future research ’, (iii) ‘Policy and guidelines’, (iv)‘Health sector services and clinical practice’, (v) ‘Improved health for patients and public’, (vi)‘Economic’ and (vii) ‘Social and cultural’ impact. A distinction was made between those case studies in which institutions’ described potential health impacts versus those in which the institution evidenced health improvements that had actually occurred; for example, through the use of audit data or epidemiological studies.
Impact case studies
Out of 6637 publicly available REF2014 impact case studies, 234 were returned as potentially relevant based on the combined word search of “Cancer” AND “Trial”. On reading the full submissions of these 234 case studies, 46 met the pre-defined inclusion criteria. Figure 1 a presents the search results in a PRISMA style diagram [ 21 ] and details the reasons for exclusion. The REF Unit of Assessment, Summary Impact Type and name of institutions responsible for the submission for each case study are shown in Table 1 .
Characteristics of the cancer trials identified
The number of trials specifically cited in each case study ranged from 1 to 7. Overall, 106 individual trials were referenced 110 times. The majority of trials identified (68%) were phase III randomised clinical trials and most trials focussed on the treatment of cancer (88%); trials investigating screening and diagnosis were much less common at 5% and 4%, respectively. A large proportion recruited patients with a diagnosis of breast cancer (35%) (Table 1 ). The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial [ 22 ] was discussed in five separate case studies by four universities [ 23 , 24 , 25 , 26 , 27 ]. The ATAC trial investigated the efficacy of an orally administered aromatase inhibitor compared to an orally administered anti-oestrogen for the adjuvant endocrine treatment for postmenopausal women with hormone-receptor-positive, localised breast cancer. When used by the same university, one case study focussed on the impact on clinical practice change worldwide and the sales for the drug company responsible for the production of the aromatase inhibitor [ 25 ]. The second focussed on subsequent research by the same university in response to knowledge generated from the ATAC trial around drug-associated bone loss [ 26 ]. Impacts described in the other three ATAC trial case studies included the provision of tumour specimens for translational research and investigation of novel biomarkers [ 27 ], citation of the trial results in guidelines with subsequent impact on clinical practice and breast cancer relapse [ 24 , 27 ]. The Prostate Testing for Cancer and Treatment (ProtecT) trial [ 28 ], which was still recruiting at the time of REF2014 submissions, was described by two universities as an example of their work [ 29 , 30 ]. Both institutions outlined the collaborative approach to designing and performing this trial and the impact that the background work for the trial contributed to the concept of active monitoring for men with prostate cancer and on providing evidence to support a government decision not to introduce prostate cancer screening.
As shown in Table 1 , there were often collaborative funding streams for these clinical trials from industry, the charity sector and government-led research councils. Figure 2 a shows that the journals of publication included both cancer-specific journals and those aimed at a more generic clinical readership. The most common primary outcomes evaluated were overall or cancer-specific survival (18%; 20/110) or a measure of disease recurrence or progression (18%; 20/110). Several trials used a co-primary endpoint (16%; 18/110). Although most trials (78%; 86/110) met their primary endpoint, one fifth of trials (20%; 22/110) did not and, for a minority of the trials (2%; 2/110) this was unclear.
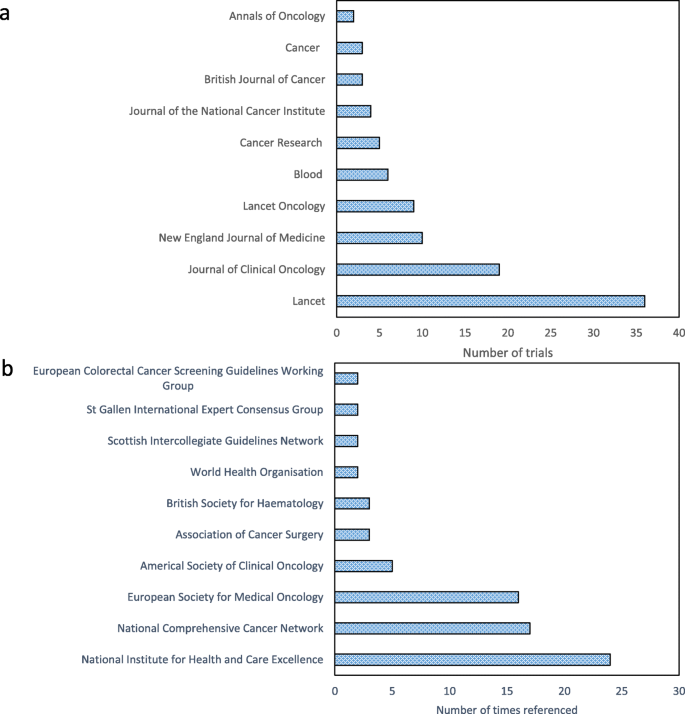
a Ten most common journals of trial publication. b Ten most frequently referenced national or international clinical guidelines
Categories of cancer trial impact
The frequency with which different categories of impact were identified in the case studies are shown in Table 2 . Most case studies (93%) described the impact of cancer trials on policy, and in particular, the citation of trial results in national or international clinical guidelines. A list of the ten clinical guidelines in which these trials are most cited is in Fig. 2 b. None of the case studies referred to social or cultural impacts of clinical trials. One case study did explain that a clinical trial had changed ‘culture and behaviour’, but on reading the narrative this was coded as a change in the prescribing practice of clinicians [ 31 ]. Another case study [ 32 ] discussed differences in cancer screening uptake between different socioeconomic groups which was partly identified by a clinical trial and has led to funding for a future trial to investigate and tackle this problem. There is potential for this subsequent trial to have substantial social impact if it successfully identifies ways to address this screening uptake imbalance.
Dissemination and knowledge transfer
Overall, half (50%, 23/46) of case studies mentioned at least one type of dissemination or knowledge transfer. These examples were divided into a description of the publication of trial results in an academic journal (20% of case studies; 9/46), citation of the results publication in other academic articles (7%; 3/46) or other methods of communication (35%; 16/46) such as reports in the lay or social media, patient-facing websites and conference presentations.
Methods of evidencing cancer trial impact
Common methods used by higher-education institutions to evidence the cancer trial impacts that were identified included: (1) identification of citations of trial publications in policy documents (78%; 36/46); (2) interrogation of real-life patient- or population-level data on clinical practice or health-service use (52%; 24/46); (3) the use of expert or user testimony (30%; 13/46) and (4) surveys (both quantitative and qualitative) (15%; 7/46). Interestingly, testimonies were only from researchers and funders, with none from policy-makers or patients. Although many (70%; 32/46) case studies described the impact that cancer trials had on changing health outcomes (Section 5 of the coding manual, Supplementary material ), only seven (15%) described an actual, rather than predicted or estimated, change in health of patients (Section 5.1 of the coding manual) (Table 2 ). Several (39%; 18/46) case studies specifically quoted the monetary value of the funding linked to the research described in their case studies, totalling approximately £90 million. None incorporated this monetary value in an estimation of the economic return on research investment.
Researchers and research users enhancing cancer trial impact
A minority (15%; 7/46) of case studies mentioned that researchers actively enhanced the impact of a clinical trial. Examples included researchers interacting with policy-makers to give advice on how to pilot implementation of clinical trial findings [ 33 ] and researchers making efforts to ensure that trial findings are presented in the lay media, health blogs and charity websites [ 31 ]. There was also an example of researchers training clinicians in the selection of patients who would benefit from radiotherapy treatment that had been developed in the context of a clinical trial [ 34 ]. The submitting institution explained that these actions help to ensure implementation of trial findings and improved uptake of this radiotherapy treatment in the UK. There was one example of when a research user enhanced the impact of a cancer trial. This occurred when a patient used the results from a cancer trial to lobby the UK government to fund a novel drug to treat breast cancer for treatment of patients within the UK [ 35 ]. Overall, the fact that there is a limited number of these examples does not imply that researchers or research users did not play an important role in the promotion, implementation and wider impact of cancer trial findings, but if this did occur, it was not identified by universities as an important part of their impact narrative within these case studies.
There have been prior reviews of the REF2014 case studies [ 19 , 36 , 37 , 38 , 39 ], but this is the first analysis that focuses specifically on cancer research or clinical trials. This study shows that UK universities recognise cancer trials as impactful research undertaken at their institutions. Nineteen (12%) out of 154 institutions participating in the REF2014 submitted 46 case studies that specifically focussed on cancer trials. Most of the higher-education institutions were Russell Group Universities (89%; 16/19) [ 40 ], a self-selected association of 24 leading public research universities in the UK, whose member institutions submitted 68% of the highest-ranked (4* outstanding) case studies in the REF2014 [ 41 ]. The relatively small number of universities submitting cancer trial case studies implies that this type of research is concentrated at specific locations. Over half (54%) of the case studies described the impact of more than one trial, raising the question of whether it is feasible to expect a single trial, rather than a combination of trials or a programme of trials’ research, to lead to tangible impacts on patients and society. Lastly, several universities described the impact of the same trial, illustrating the collaborative approach adopted at those institutions.
Trials recruiting patients with breast cancer constituted over a third of the included trials; a much greater proportion than those recruiting patients with, for example, lung cancer (7%). Although breast cancer is the most common cancer (15% incidence) in men and women combined in the UK [ 42 ], lung cancer has the highest mortality rate and accounts for over one fifth of all cancer deaths (2017) [ 43 ]. Skin cancer, including melanoma, germ-cell cancer and sarcoma were in the coding manual but no trials were identified that solely included patients with these diagnoses. It is likely that, rather than accurately reflect the relative burden of these cancers in the UK [ 44 ], these case studies reflect the landmark trials that reported results within the assessment REF2014 eligible period (1993–2014). There were no trials reporting the benefits of immunotherapy, widely regarded as a major recent advance in cancer treatment. Again, it is likely that this reflects the publication dates of key trials investigating the novel immunotherapies and it will be interesting to analyse whether these trials are in the case studies submitted to the REF2021. The ten journals in which the clinical trials described in these case studies were most frequently published all have a Journal Impact Factor over 5 and the top three have an Journal Impact Factor above 25 [ 45 ]. This supports the findings from Chowdhury, Koya and Philipson [ 38 ] that, although not an article-level metric and not a measure of impact, the research outputs underlying REF2014 impact case studies were often published in journals with a high average citation count.
Higher-education institutions did not exclusively use clinical trials that met their pre-specified primary endpoints in these case studies as examples of impactful research. For example, the LIBERATE trial [ 46 ] closed early because an increase in breast cancer recurrence was found to occur in patients being managed with hormone replacement therapy to treat symptoms following cancer treatment. The submitting university argued that the impact of this trial was a change in guidelines to prevent subsequent use of hormone replacement therapy for this group of patients. Another example was the FOCUS2 trial [ 47 ], which tested the optimal treatment for elderly and frail patients with metastatic colorectal cancer. Although the trial did not meet its primary endpoint, it demonstrated the feasibility of recruiting patients from an often under-researched patient cohort. It also provided important information around toxicity and quality of life that has subsequently been cited in clinical guidelines and changed clinical practice. This demonstrates that the pathway to impact is not solely dictated by practice-changing trials, but that practice-affirming trials may be impactful by preventing harmful variation in practice [ 48 , 49 ].
The fact that some institutions used early phase trials as standalone examples of impactful research shows that robust examples of real-life impact do not only emerge from large, later-phase trials. As an example, a portfolio of trials which demonstrated the safety, optimal dosing and blood-brain-barrier penetration of a drug for patients with brain tumours, led to both direct (licensing of the drug) and indirect impacts (a phase III trial performed at another institution, subsequent introduction of the drug into routine practice and increased revenue for the pharmaceutical company) [ 50 ]. Another case study described the impact of early phase trials investigating the use a targeted treatment for patients with BRCA -associated breast and ovarian cancer. The significant improvement in outcomes for this sub-group of patients meant that these trials directly influenced international guidelines for genetic testing and led to further research investment and collaboration with industry for that institution. Submission guidelines indicate that examples of indirect impact will be welcomed in the REF2021 [ 51 , 52 ].
The REF2021 expects that institutions will describe the process through which impact occurs, including, where possible, evidence of dissemination leading to impact. Ensuring transparency by informing patients and the public of the results of research, in particular clinical trials, is one of the UK’s Health Research Authority’s major priorities for ethical research practice [ 53 ]. It was, therefore, encouraging that some institutions in REF2014 described methods of knowledge transfer other than journal publication. In contrast, although there were examples of researchers or research users enhancing trial impact, these were identified in only a selection of case studies. Improved and more frequent descriptions of how trialists engage with end users of clinical trials to maximise timely trial impact could help submitting institutions to better demonstrate the process through which impact occurs in the REF2021. Finally, there was a small number of case studies evidencing actual impact that has occurred using methods such as the analysis of national audit data [ 34 ] or quantification of drug sales to indicate practice change [ 27 , 54 ], or referencing epidemiological studies to show improved health outcomes [ 31 ]. Describing actual impact presents significant challenges in terms of timelines and planning, but gives a much stronger indication of the real-life benefits from cancer trials compared to estimations of potential impacts and it is likely that the former will be viewed favourably in the REF2021 [ 51 ].
Reflecting on the findings of this study provides optimism towards the more routine adoption of cancer trial impact evaluation, but also highlights challenges going forward. It is reassuring to see that cancer trials, a type of applied scientific research, are having real-life benefits for patients. Looking at the narratives submitted by higher-education institutions it is clear that impact evaluation is a useful way to scrutinize and reflect on the merits of the vast amount of work and investment required to perform these trials and that institutions have been able to evidence this real-life impact. In addition, by paying careful attention to trial impact, it is likely that this will contribute to better research prioritisation in the future. What is less clear from this study is who should be responsible for performing these evaluations, and if there is an expectation on primary researchers, such as clinicians, statisticians and health economists, to adopt this role, or if a new breed of researchers will emerge to answer this call. Impact assessment requires the utilisation of methods such as surveys, interviews and the analysis of large datasets, which are skills that may not be routinely utilised by cancer trialists. In addition, preparation of submissions to the REF2014 costs UK higher-education institutions £246 million, with £55 million spent on impact evaluation. This was a 133% increase from the Research Assessment Exercise in 2008 [ 55 ]. If the assessment of real-life cancer trial impact is to become a priority for the government and funders, provision of resources to perform such evaluations will need to be addressed either through core funding or specifically within clinical trial research grants.
Table 3 offers some suggestions of how to better evaluate, communicate and maximise cancer trial impact in the future. Whether trialists will hold responsibility for impact assessment or not, articulating the expected impact of a trial during the design phase, in collaboration with patients, will make subsequent impact evaluation easier and may focus trial design to address unmet needs. Tracking the impact of clinical practice on a national level will require access to routinely collected healthcare data, with sufficient granularity to make meaningful claims regarding the evidencing of impact and the identification of barriers to impact. Although the REF2014 website offers a list of impact case studies from many disciplines, it would be more useful if future impact narratives relating to cancer trials were to be publicised on more clinician- and patient-friendly platforms. Opening dialogue about research impact in a way that resonates with funders and trialists may encourage trial design with a focus on longer-term outcomes, such as changes in health or clinical practice, in a way that actually makes trials more impactful. The coding manual used in this study (Supplementary material 1 ) may offer a starting point for trialists to consider how they could embed impact evaluation into the routine review of their clinical trial outputs.
There are several limitations to our analysis. Firstly, as with any review of the REF2014 impact case studies, these case studies were not specifically intended for this type of secondary analysis. Secondly, although having content validity for this study, the list of indicators of impact used to code these case studies (Supplementary material 1 ) will not be an exhaustive list of cancer trial impacts. In addition, for the purposes of this study we focussed on evaluating impact. Going forward, it would also be useful to make an assessment of the investment, both economic and non-monetary, into cancer trials. This would allow the impact of trials to be contextualised in terms of the investment provided from funders, and burden for patients from participating in these trials [ 8 ]. Finally, we used a binary assessment to indicate whether the primary endpoint was met for each trial. In future, this could be evaluated in greater detail by also looking at secondary endpoints or widening the evaluation to explore whether a trial met its objective to recruit sufficient patients to answer a clinical question.
Further research is required to understand which types of impact are important to patients and other stakeholders and the processes through which cancer trial impact occurs. It will be useful to repeat this exercise using the REF2021 case studies to identify which cancer trials conducted during 2000–2020 are regarded as most impactful by higher-education institutions, and to understand whether the methods of impact assessment have changed. Although not coded for the purposes of this study, a comparison of the dates of both the clinical trial and the impact evidenced would be useful. This would improve understanding of the time taken to achieve impact from UK cancer trials, which has previously been estimated to be in the order of 15 years for cancer research [ 56 ]. A better understanding of time lags specifically for cancer trials would provide insight into when an analysis of the return in cancer trial investment should ideally be performed and may identify opportunities to speed up impact in some scenarios [ 57 ].
This study should be helpful to institutions in any country who conduct cancer trials, and, in particular, in the UK as they currently prepare for their REF2021 submissions. It will also allow cancer trial funders to contextualise responses received when trialists describe the actual or potential impact of their work. The results should help conscientious cancer trialists and cancer trial units to consider how they can demonstrate the wider impact of their work to funders and patients. Ultimately, a better understanding and more routine adoption of impact assessment will provide the knowledge and vision required to ensure that we are conducting meaningful cancer trials research for patients.
Availability of data and materials
REF2014 impact case studies freely available online.
San Francisco Declaration on Research Assessment (DORA). DORA signers Organisations and Individuals 2019. Available from: https://sfdora.org/signers/ . Accessed 8 Aug 2019.
National Cancer Institute. NCI Budget and Appropriations 2018. Accessed on 8 August 2019. Available from: https://www.cancer.gov/about-nci/budget .
National Cancer Institute. Funding for Research Areas 2018. Accessed on 8 August 2019. Available from: https://www.cancer.gov/about-nci/budget/fact-book/data/research-funding .
Cancer Research UK. How we spend your money 2019. Accessed on 8 August 2019. Available from: https://www.cancerresearchuk.org/how-we-spend-your-money .
Cancer Research UK. Cancer Research UK to invest £45 million in clinical trials 2018. Available from: https://www.cancerresearchuk.org/about-us/cancer-news/press-release/2018-02-15-cancer-research-uk-to-invest-ps45-million-in-clinical-trials .
Cancer Research UK. Current clinical trial research 2019. Accessed on 8 August 2019. Available from: https://www.cancerresearchuk.org/our-research-by-cancer-topic/our-clinical-trial-research/current-clinical-trial-research .
REF2021. Consultation on the draft panel criteria and working methods. Nicholson House, Lime Kiln Close, Stoke Gifford, Bristol, BS34 8SR: 2018 July 2018. Report No.
Carlisle BG, Doussau A, Kimmelman J. Patient burden and clinical advances associated with postapproval monotherapy cancer drug trials: a retrospective cohort study. BMJ Open. 2020;10(2):e034306.
Article Google Scholar
Williams K, Grant J. A comparative review of how the Policy and Procedures to Assess Research Impact evolved in Australia and the UK. Res Evaluat. 2018;27(2):93–105 13.
Langton J. LSE Impact Blog [Internet]: The London School of Economics and Political Science. 2018. Accessed on 8 August 2019. Available from: https://blogs.lse.ac.uk/impactofsocialsciences/2018/10/19/developing-approaches-to-research-impact-assessment-and-evaluation-lessons-from-a-canadian-health-research-funder/ .
Guthrie S, Krapels J, Lichten CA, Wooding S. 100 metrics to assess and communicate the value of biomedical research: an ideas book. Cambridge: RAND Europe; 2016..
Parks S, Ioppolo B, Stepanek M, Gunashekar S. Guidance for standardising quantiative indicators of impact within REF case studies. Santa Monica, and Cambridge: RAND Europe; 2018.
Higher Education Funding Council for England. REF2014 Impact Case Studies 2014. Accessed on 5 October 2019. Available from: https://impact.ref.ac.uk/casestudies/FAQ.aspx .
King’s College London and Digital Science. The nature, scale and beneficiaries of research impact: An initial analysis of Research Excellence Framework (REF) 2014 impact case studies. HEFCE: Policy Institute at King's College London; 2015.
REF2014. Search REF Impact case studies. Accessed on 8 August 2019. Available from: https://impact.ref.ac.uk/casestudies/ .
Bryman A. Social research methods. 4th ed. Oxford: Oxford University Press; 2012. p. 809.
Google Scholar
Buxton M, Hanney S, Jones T. Estimating the economic value to societies of the impact of health research: a critical review. Bull World Health Organ. 2004;82(10):733–9.
PubMed PubMed Central Google Scholar
Kuruvilla S, Mays N, Pleasant A, Walt G. Describing the impact of health research: a Research Impact Framework. BMC Health Serv Res. 2006;6:134.
Greenhalgh T, Fahy N. Research impact in the community-based health sciences: an analysis of 162 case studies from the 2014 UK Research Excellence Framework. BMC Med. 2015:13.
Lavis J, Ross S, McLeod C, Gildiner A. Measuring the impact of health research. J Health Serv Res Policy. 2003;8(3):165–70.
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–30.
A Howell 1, J Cuzick, M Baum, A Buzdar, M Dowsett, J F Forbes, G Hoctin-Boes, J Houghton, G Y Locker, J S Tobias, ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2.
University College London. Introduction of aromatase inhibitors for the treatment of breast cancer. 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=34811 . Accessed 8th August 2019 (not cited).
Queen Mary University of London. Anastrozole for oestogen receptor positive breast cancer. 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=18352 . Accessed 8 Aug 2019.
University of Manchester. Improving outcomes of women diagnosed with and at increased risk of breast cancer: the results of translational research and national and international clinical trials: Higher Education Funding Council; 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=28102 . Accessed 8 Aug 2019.
University of Manchester. Preventing bone loss in patients treated for breast cancer: Higher Education and Funding Coucil; 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=28032 . Accessed 8 Aug 2019.
Institute of Cancer Research. Aromatase inhibitors in breast cancer treatment: Higher Education and Funding Council; 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=41981 . Accessed 8 Aug 2019.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24.
University of Bristol. Avoiding harm and evaluating benefit: establishing and implementing an evidence-based policy for prostate cancer screening in the UK: Higher Education Funding Council for England; 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=40165 . Accessed 8 Aug 2019.
University of Cambridge. The ProtecT Trial and Associated Translational Research – Management of Localised Prostate Cancer – Neal 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=29986 . Accessed 8 Aug 2019.
University of Oxford. UOA02–05: Hormone replacement therapy and cancer risk: the Million Women Study 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=9539 . Accessed 8 Aug 2019.
University College London. Inclusion of flexible sigmoidoscopy in the UK Bowel Cancer Screening Programme 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=23145 . Accessed 8 Aug 2019.
University of Nottingham. Saving lives through faecal occult blood screening for bowel cancer 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=40998 . Accessed 8 Aug 2019.
University of Leeds. Case study 4. Improving chemotherapy, radiotherapy and patient outcomes for colorectal cancer through patient-focused integrated clinical trials 2014. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=27417 .
University of Edinburgh. C: Detailed analysis of trial of lapatinib in combination with capecitabine in advanced, HER2+ breast cancer leads to marketing authorisation worldwide. 2014.
Higher Education Funding Council. Decisions on assessing research impact. Northavon House, Coldharbour Lane, Bristol, BS16 1QD: March 2011. Report No. REF 01.2011.
Hinrichs S, Grant J. A new resource for identifying and assessing the impacts of research. BMC Med. 2015;13(1):148.
Chowdhury G, Koya K, Philipson P. Measuring the impact of research: lessons from the UK's Research Excellence Framework 2014. PLoS One. 2016;11(6):e0156978.
Terämä E, Smallman M, Lock SJ, Johnson C, Austwick MZ. Beyond academia – interrogating research impact in the Research Excellence Framework. PLoS One. 2016;11(12):e0168533.
Russell Group. About 2019. Accessed on 8 August 2019. Available from: https://www.russellgroup.ac.uk/ .
Russell Group. Research Excellence Framework 2014. Accessed on 8 August 2019. Available from: https://russellgroup.ac.uk/news/research-excellence-framework/ .
Cancer Research UK. Cancer incidence for common cancers 2020. Accessed on 2020 19 April 2020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Zero .
Cancer Research UK. Cancer mortality for common cancers 2020. Accessed on April 2020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality/common-cancers-compared#heading-Zero . Accessed 19 Apr.
World Health Organisation. Health Statistics and Information systems Disease burden and mortality estimates 2019. Accessed on 8 August 2019.
Clarivate Analytics. InCites Journal Citation Reports 2019. Accessed on November 2019. Available from: https://jcr.clarivate.com/JCRJournalHomeAction.action . Accessed 20 Nov.
Bundred NJ, Kenemans P, Yip CH, Beckmann MW, Foidart J-M, Sismondi P, et al. Tibolone increases bone mineral density but also relapse in breast cancer survivors: LIBERATE trial bone substudy. Breast Cancer Res. 2012;14(1):R13.
Article CAS Google Scholar
Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–59.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2018;20(2):273–86.
Strzebonska K, Waligora M. Umbrella and basket trials in oncology: ethical challenges. BMC Med Ethics. 2019;20(1):58.
Imperial College London. Clinical development of temozolomide: an anticancer drug that improves survival of patients with brain cancer (glioma). 2014.
Department for the Economy, Higher Education Funding Council for Wales, Research England, Scottish Funding Council. REF 2018/02 Consultation on the draft panel criteria and working methods 2018.
Department for the Economy, Higher Education Funding Council for Wales, Research England, Scottish funding Council. REF 2019/01 Guidance on Submissions 2019.
Health Research Authority. Our transparency agenda 2019. Accessed on 8 October 2019. Available from: https://www.hra.nhs.uk/about-us/what-we-do/our-transparency-agenda/ .
Queen Mary University of London. Improved sensitivity of breast cancer screening with two-view mammography 2014. Accessed on 2019. Available from: https://impact.ref.ac.uk/casestudies/CaseStudy.aspx?Id=18319 . Accessed 8 Aug.
Stern N. Research Excellence Framework (REF) review: building on success and learning from experience; 2016.
Glover M, Buxton M, Guthrie S, Hanney S, Pollitt A, Grant J. Estimating the returns to UK publicly funded cancer-related research in terms of the net value of improved health outcomes. BMC Med. 2014;12:99.
Guthrie S, Pollitt A, Hanney S, Grant J. Investigating time lags and attribution in the translation of cancer research: a case study approach. RAND Health Q. 2014;4(2):16.
Download references
Acknowledgements
Dr. Catherine Hanna holds a Clinical Trials Fellowship Grant from CRUK and the University of Glasgow (Grant ID: 174279–01).
Author information
Authors and affiliations.
CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, 1053 Great Western Road, Glasgow, G12 OYN, UK
Catherine R. Hanna & Rob J. Jones
Institute of Health and Wellbeing, University Of Glasgow Gartnavel Royal Hospital, Admin Building, 1st Floor, 1055 Great Western Road, Glasgow,, G12 0XH, UK
Lauren P. Gatting & Kathleen Anne Boyd
Institute of Health and Wellbeing, University Of Glasgow, Health Economics and Health Technology Assessment, 1 Lilybank Gardens, Glasgow, G12 8RZ, UK
Kathryn A. Robb
You can also search for this author in PubMed Google Scholar
Contributions
CH designed the study and carried out the analysis and wrote/edited the manuscript and approved the final version. LG helped with coding manual development and acted as the secondary coder for manual content analysis. LG also edited the manuscript and approved the final version. KAR helped design the study, edited the manuscript and approved the final version. KAB helped interpret the study results, edited the manuscript and approved the final version. RJJ helped interpret the study results, edited the manuscript and approved the final version.
Corresponding author
Correspondence to Catherine R. Hanna .
Ethics declarations
Ethics approval and consent to participate.
No specific ethical approval was sought for this study.
Consent for publication
No individual personal data included.
Competing interests
The authors declare that they have no conflicts of interest.

Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: supplementary material 1:.
Excel spreadsheet.
Additional file 2: Supplementary material 2:
Diagram and Table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hanna, C.R., Gatting, L.P., Boyd, K.A. et al. Evidencing the impact of cancer trials: insights from the 2014 UK Research Excellence Framework. Trials 21 , 486 (2020). https://doi.org/10.1186/s13063-020-04425-9
Download citation
Received : 20 December 2019
Accepted : 16 May 2020
Published : 05 June 2020
DOI : https://doi.org/10.1186/s13063-020-04425-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Evidencing the impact of cancer trials: insights from the 2014 UK Research Excellence Framework
Affiliations.
- 1 CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, 1053 Great Western Road, Glasgow, G12 OYN, UK. [email protected].
- 2 Institute of Health and Wellbeing, University Of Glasgow Gartnavel Royal Hospital, Admin Building, 1st Floor, 1055 Great Western Road, Glasgow,, G12 0XH, UK.
- 3 Institute of Health and Wellbeing, University Of Glasgow, Health Economics and Health Technology Assessment, 1 Lilybank Gardens, Glasgow, G12 8RZ, UK.
- 4 CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, 1053 Great Western Road, Glasgow, G12 OYN, UK.
- PMID: 32503612
- PMCID: PMC7275320
- DOI: 10.1186/s13063-020-04425-9
Introduction: An impactful clinical trial will have real-life benefits for patients and society beyond the academic environment. This study analyses case studies of cancer trials to understand how impact is evidenced for cancer trials and how impact evaluation can be more routinely adopted and improved.
Methods: The United Kingdom (UK) Government allocates research funding to higher-education institutions based on an assessment of the institutions' previous research efforts, in an exercise known as the Research Excellence Framework (REF). In addition to each institution's journal publications and research environment, for the first time in 2014, allocation of funding was also dependent on an evaluation of the wider, societal impact of research conducted. In the REF2014, impact assessment was performed by evaluation of impact case studies. In this study, case studies (n = 6637) submitted by institutions for the REF2014 were accessed and those focussing on cancer trials were identified. Manual content analysis was then used to assess the characteristics of the cancer trials discussed in the case studies, the impact described and the methods used by institutions to demonstrate impact.
Results: Forty-six case studies describing 106 individual cancer trials were identified. The majority were phase III randomised controlled trials and those recruiting patients with breast cancer. A list of indicators of cancer trial impact was generated using the previous literature and developed inductively using these case studies. The most common impact from a cancer trial identified in the case studies was on policy, in particular citation of trial findings in clinical guidelines. Impact on health outcomes and the economy were less frequent and health outcomes were often predicted rather than evidenced. There were few descriptions identified of trialists making efforts to maximise trial impact.
Discussion: Cancer trial impact narratives for the next REF assessment exercise in 2021 can be improved by evidencing actual rather than predicted Impact, with a clearer identification of the beneficiaries of cancer trials and the processes through which trial results are used. Clarification of the individuals responsible for performing impact evaluations of cancer trials and the provision of resources to do so needs to be addressed if impact evaluation is to be sustainable.
- Clinical Trials as Topic / economics
- Clinical Trials as Topic / standards*
- Cost-Benefit Analysis
- Financing, Government / classification
- Outcome Assessment, Health Care*
- Quality Indicators, Health Care*
- Research / economics
- Research / standards*
- United Kingdom
- Universities
Grants and funding
- 15960/CRUK_/Cancer Research UK/United Kingdom
- 174279-01/CRUK_/Cancer Research UK/United Kingdom
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
Cancer Research UK
Donate to help us beat cancer.
So every month, these moments can keep happening
Support our life-saving work
Fundraise. Pledge. Volunteer. Donate.

Get involved and make a difference
Cancer is relentless. But so are we. Whether you fundraise, pledge to leave a gift in your will or donate. Every part supports life-saving research. Play your part and together we will beat cancer.

About Cancer
If you've been diagnosed with cancer, or know someone who has, we provide practical information on everything from symptoms and screening, to coping after treatment.

Cancer Chat
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.

Race for Life 2024
Raise money for life-saving cancer research and join our biggest series of fundraising events this summer. No matter how cancer affects is, life is worth Racing for.

King Charles III announced as our new patron
We’re delighted to announce His Majesty King Charles III’s patronage and are excited by the opportunities this brings to help us further progress our mission to ensure more people live longer, better lives, free from the fear of cancer.

Summer Superdraw 2024
Enter today for just £1 to be in with a chance of winning our top prize of £15,000! Play now and help fund life-saving research. The Summer Superdraw closes on 17 July 2024.
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Health & Medicine
Contraception and cancer – a look behind the headlines

21 March 2023
You may have heard about a new study into contraception and cancer recently. New information about cancer risks can be hard to apply to everyday life, and headlines about this paper are highlighting some alarming-looking numbers. I f you or someone you’re close to uses hormonal contraception, there’s a chance you’re asking what the latest findings actually mean. Let’s break this research down and see.
First, we should say a bit about what hormonal contraception is. Unlike barrier contraception, such as condoms, hormonal contraception releases hormones into a woman’s body to prevent her becoming pregnant. There are two main types:
- Combined contraception, which releases two hormones called oestrogen and progestogen.
- Progestogen-only contraception, which, as the name suggests, only releases progestogen.
Combined contraception usually comes as a pill or a patch. Progestogen-only contraception can be a pill, implant, injection, or intrauterine system (IUS).
What did we already know about contraception and cancer?
Previous research has already established that the combined pill increases the risk of breast cancer. This increased risk is small and only lasts while someone is taking the pill. After they stop taking it, the increase in risk slowly disappears.
The combined pill also decreases the risk of womb and ovarian cancer . So, for most women, the benefits of using the combined pill outweigh the risks. Still, health guidelines encourage health professionals to inform their patients about the small difference it can make to their risk of breast cancer.
Up till now, the evidence on other types of contraception, such as the progestogen-only pill, and cancer has not been as strong as the evidence on the combined pill. This is partly because there weren’t as many people using other forms of contraception, which meant researchers couldn’t put together big enough studies.
However, since 2010 the number of people using the combined pill has gone down, and the number of people using the progestogen-only pill has gone up. By 2020, they were being used by almost equal numbers of people. Now that there are more people using progestogen-only contraceptives, researchers can more accurately study the population to see how they affect cancer risk.
What did this new study show?
There are two parts to this new study into contraception and breast cancer, which we helped fund. First, the authors compared the health records of nearly 10,000 people who had breast cancer to the records of about 18,000 people who weren’t affected by the disease. They looked at how many people in each group used contraception, as well as what types of contraception they used, to see how it impacted their risk.
After accounting for BMI and alcohol – two factors that we already know increase the risk of breast cancer – the researchers found that the progestogen-only pill, injection, and IUS have a similar effect on the risk of breast cancer as the combined pill. The results for the progestogen-releasing implant were too inconsistent to draw conclusions from, and the study didn’t investigate the effects of the combined patch.
However, the authors also realised that many women who have used hormonal contraception may have tried more than one type. It’s not unusual for women to use a few different types while they find the one that works best for them. This means the first analysis doesn’t provide enough evidence for us to be sure which type of contraception was having an effect on breast cancer, or whether the risk was actually linked to people using a combination of different types.
So, to round out part one, the researchers re-did their analysis. This time they limited it to women who had only ever taken one type of hormonal contraception. This meant the sample size was smaller, so the results are less reliable. Even so, it gives us a clearer idea of how specific types of contraception might affect people’s risk levels.
These results still show an increased risk of breast cancer from using the combined pill, progestogen-only pill and IUS. However, as with the implant in the first analysis, the second set of results is too inconsistent for us to draw conclusions about the injection.
Importantly, this follow-up analysis also showed that, similarly to the combined pill, the increase in breast cancer risk from using the progestogen-only pill goes away once someone stops using it.
The second part of the study was a meta-analysis. This means the researchers combined a range of smaller past studies that weren’t reliable enough by themselves into one larger, more reliable study. That gave more evidence that progestogen-only contraceptives increased the risk of breast cancer.
What does this mean for you?
We already knew that the combined pill has a small, temporary effect on the risk of breast cancer – and that this risk is outweighed by its benefits. This new research suggests other forms of contraception have a similar risk that also goes away after people stop using them.
It’s also important to remember that women who use contraception are usually below the age of 50. This means their risk of breast cancer is low to begin with, so any increase will have a relatively small effect.
As such, the researchers estimated that for every 100,000 women who use oral contraception from the age of 16 to 20, there will be an extra 8 cases of breast cancer (this is an increase in incidence from 0.084% to 0.093%). And for every 100,000 women who use it between the ages of 35 and 39, they estimate there will be around 265 extra cases (an increase in incidence from 2.0% to 2.2%).
So, the take-home message around cancer and contraception has not changed – for most women, the benefits of using contraception still outweigh the risks. The information from this new study means most women can continue using their contraception with a new level of understanding about their choices.
To put this into context, it’s estimated that in the UK, around 400 breast cancer cases are caused by the pill every year. Around 4,400 are caused by alcohol. This difference is partly because drinking alcohol is much more common than taking the pill.
If you’re worried about using contraception, speak to your doctor. The best way to lower your risk of cancer is to stop smoking, eat a healthy, balanced diet, be physically active and keep a healthy weight.
Maxine Lenza is a health information officer at Cancer Research UK
Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case–control study and meta-analysis Fitzpatrick D, Pirie K, Reeves G, Green J, Beral V (2023) Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case–control study and meta-analysis. PLOS Medicine 20(3): e1004188. https://doi.org/10.1371/journal.pmed.1004188
This advice completely misses the point about breast cancer. Hormonal contraceptives give a small increase in the risk of breast cancer, as does alcohol, being overweight and taking HRT. At the end of all these “small” risks breast cancer has the highest incidence of cancer in the UK.
Thank you for reading the article and taking the time to leave a comment.
People who use the pill are generally below the age of 50. During this time, a person’s risk of breast cancer is low. 91% of female breast cancers in the UK develop in people aged 45 and over.
When someone stops taking the pill, the increased risk of breast cancer goes down. Ten years after stopping the pill, a person’s risk is no longer increased – as if the pill was never used.
The pill is an effective and convenient form of birth control that helps many women avoid pregnancy. If someone is worried about breast cancer, they can lower their risk by drinking less alcohol, eating a healthy balanced diet, and keeping a healthy weight.
I hope that helps Max, Cancer Research UK
I got triple negative breast cancer at the age of 38. I was healthy, have a healthy BMI. Don’t smoke and not a big drinker (it always made me sick) yet I still got breast cancer!
Highlighted content
General election 2024: what does this mean for the tobacco and vapes bill, skin cancer cases reach all-time high, an animal's guide to staying safe in the sun, hpv vaccine slashes cervical cancer rates across society, one to one with penny: volunteering special 1 - that cancer conversation podcast, the ‘mystery’ culprit causing kidney cancer worldwide, proteins in blood could give cancer warning seven years earlier, related topics.
- Oral contraceptives
- Cancer in the news
The SOFII One Hundred want to help other fundraisers be their best. Find out how you can join them, here .
Ideas, information, initiatives to help you change the world
Cancer research uk : the race for life.
A great idea that puts the oft-repeated ‘it’ll never work here’ firmly in its place. It crossed from the USA to the UK and has continued to evolve and inspire women for many years. Helped by constant innovation they have raised nearly £500 million to beat cancer.
Creator / originator
Imperial Cancer Research Fund/Cancer Research UK.
Summary / objectives
Race for life is a nationwide fundraising event for women only. They walk, jog, or run five kilometres in return for sponsorship to raise funds for Cancer Research UK.
The original ideal came from the American charity Susan G Komen. They held the first ‘race for the cure’ (as it’s known there), in Dallas in 1983.
In the early 1990s, race for the cure inspired an events manager at Imperial Cancer Research Fund (one of two charities that would later merge to form Cancer Research UK) to try something similar. The first ‘race for life’ took place in Battersea Park in 1994; 650 women took part and it raised £35,000. But more importantly it proved that this concept from across the Atlantic could work in the UK. Over the 19 years since, the event has grown massively.
Special characteristics
Race for life has been so successful for so long because innovation is at its heart – it doesn’t stand still. This innovation seeks to make the event as inspiring and supportive for all the women who take part by giving them the opportunity to take their stand against cancer. Examples include:
- Since 1998 particpants wear signs on their backs which are a simple way for them to celebrate the survival of a cancer patient, or commemorate the life of someone they’ve lost to cancer
- In 2001 they introduced the minute’s silence before every event, which creates an emotional and inspiring moment of reflection
Influence / impact
Race for life has created two very big innovations the whole sector has benefited from.
- Online donors raise and return more money than offline donors, so it’s important to get more people to create an online fundraising page when they register. JustGiving and Cancer Research UK have worked hard over years to make this as simple as possible. It’s now seamless. Many charities are also now raising more money as a result of this technology.
- In 2004 there was a small telemarketing test to see if race for life participants would also support with a monthly direct debit donation. They did and at impressive rates. After re-testing the concept it was rolled out. The team formed as a result has been responsible for recruiting over 100,000 new regular givers. Most charities now do something similar – but it was Cancer Research UK who paved the way.
Since the first race for life in Battersea in 1994:
- Six million women have taken part (that’s 19 per cent of the UK female population).
- Over £493 million pounds has been raised.
At its peak in 2009, there were nearly 750,000 participants and still over half a million women take part today.
Race for life is a true inspiration: six million women have come together to achieve a common goal. Every one of them has a personal story to tell. They are taking part because of their personal connection to the cause, to honour the memory of a loved one they have lost to cancer or to celebrate the life of someone who has survived.
Race for life creates the moment for these women to take action against cancer. Helped by constant innovation they have raised nearly £500 million to beat cancer.
Paul De Gregorio presents Cancer Research UK at SOFII’s IWITOT 2013
Other relevant information

Related case studies or articles
- The London marathon: a human race
- Cancer Research UK: the letter from cancer
Also in Categories
Make sofii better still….
We love receiving new exhibits from around the world, so please share your fundraising stories with us. Add a case-study or article to SOFII .
SOFII speaks your language
Sofii en français, sofii in italiano, sofii em português, sofii en español, sofii auf deutsch.

I Wish I’d Thought of That
- IWITOT 2021 : UK & Europe was epic — order your recording now!
- IWITOT : The Americas was a hit in January 2021
- I Wish I’d Thought of That ( IWITOT ): the supporter experience edition
- I Wish I’d Thought Of That Virtual 2020
- I Wish I’d Thought Of That, London 2019
See all Jobs in Fundraising
Get SOFII delivered to your inbox

- SOFII Contributors
- Featured Organisations
Carolina Herrera, project manager — [email protected] Joanna Culling — [email protected]
The SOFII Foundation – Registered charity no. 1124743 19 Chelsea Park Gardens, London SW3 6AF
Company limited by guarantee. Company registered number 06530274
© 2024 The SOFII Foundation
Policies: Cookies , Privacy , Terms of use , Copyright
Made by 1810 .
Sie verwenden einen veralteten Browser. Bitte aktualisieren Sie Ihren Browser damit die Seite verwendet werden kann.
Get free insights about optimizing your procurement!
Request info or schedule a demo.
A sales expert will contact you within the next 24 hours.
We use cookies to ensure that we give you the best experience on our website. This includes cookies from third party social media websites and advertising cookies that may analyze your use of this site. Learn more
- All agencies in USA
- Los Angeles
- San Francisco
- Philadelphia
- All services in USA
- AI Marketing
- Digital Marketing
- Social Media Marketing
- Email Marketing
- Content Marketing
- All industries in USA
- Travel & Tourism
- Real Estate
- Fashion & Retail
- Media & Entertainment
- Food & Beverage
- Agency of the Month

- All agencies in the UK
- Bournemouth
- All services in the UK
- All industries in the UK

- All agencies in Canada
- All services in Canada
- Influencer Marketing
- All industries in Canada
- Travel Tourism
- All agencies in Australia
- All services in Australia
- PPC Marketing
- All industries in Australia
- Beauty & Cosmetics
- Hospitality

- All agencies in Europe
- All services in Europe
- Web Development
- All industries in Europe
- IT & Technology

- All agencies in Asia
- All services in Asia
- B2B Marketing
- All industries in Asia

- Agency News
- Marketing Resources
- Industry News

- Digital Ad Campaigns
- Case Studies
- Social Media Campaigns

- Marketing Blog
- Advertising
- Ecommerce Marketing

- Industrial Blog
- Fashion Marketing
- Sports Marketing
- Luxury Marketing
- Legal Marketing
- Healthcare Marketing

- Digital Marketing Tools
- Marketing Reporting Tools
- Digital Marketing Analytics Tools
- Email Marketing Tools
- Other Tools
- Social Media Management Tools
- Social Media Marketing Tools
- Social Media Analytics Tools
- Social Media Monitoring Tools
- Influencer Marketing Platforms
- Web Design Tools
- Landing Page Builders
- UI / UX Design Tools
- Website Builder Software
- Front End Development Tools
- Team Management Softw...
- Project Management Tools
- Agency Management Software
- Productivity Management Software
- Time Tracking Tools
- Sales Tools
- Sales Automation Tools
- Product Feed Management Tools
- Sales Enablement Tools
- AI Design Tools
- AI Content Tools
- AI Analytics Tools
- AI Marketing Tools
- Performance & Software
- Website Optimization Tools
- Content Delivery Network Tools
- Cybersecurity Software
- Web Accessibility Tools
Market your SaaS Tools and reach digital agencies & marketing professionals worldwide.
- All Categories in USA
- Artificial Intelligence Events
- Design & Development Events
- Digital Marketing Conferences
- Social Media Events

UK Content Awards 2024

Ecommerce Forum 2024
Submit your exclusive marketing event today.
Submit your event to reach a wider audience! Whether it's digital marketing, AI, or any related theme, we would love to help spread the word out!
- All Categories in UK
- All Categories in Canada
- All Categories in Australia
- All Categories in Europe
- All Categories in Asia

Impression Has Collaborated with Cancer Research UK to Drive Awareness
Impression worked with all of the marketing teams at Cancer Research UK to meet the changing audience need, marketing the right message and the right product to the right audiences.
Client Intro
Cancer Research UK is the world’s largest independent funder of cancer research with the vision to bring forward the day when all cancers are cured.
They appointed Impression in early 2019 and Impression has been working together since to manage their paid search activity and to work collaboratively with their internal marketing teams and external agencies responsible for the management of other marketing channels.
The Challenge
There are multiple CRUK PPC accounts all with different aims and objectives. Each marketing team has varying KPIs that change year-on-year, from lead generation to donations and e-commerce purchasing.
Throughout the COVID period, Impression worked with all of the marketing teams at Cancer Research UK to meet the changing audience need – marketing the right message and the right product to the right audiences. Impression was tasked with ensuring CRUK audiences could find the health information that they needed as well as developing a contingency plan. This contingency plan allowed CRUK to reduce spend in lower-performing areas, focus on maximizing higher-performing products and align the strategy with the messaging and timing of ATL’s ‘appeal’ activity.
In-house marketing teams and various agencies work together to achieve wider business goals:
- Donation revenue
- Fundraising pack downloads
- Legacies referrals and Will Guide Downloads
- Weekly Lottery Revenue
- Stand Up to Cancer Revenue and Sign Ups
- Online Shop (eCommerce) Revenue
- Hero product sign-ups eg. Walk All Over Cancer
- Race for Life Sign Ups
- Sport Sign-Ups eg. Half Marathons
- Volunteer Sign-Ups
- Donate in Memory Tribute Pages
The Strategy
1. implementing new account structures and campaign consolidation.
Their first step was to restructure campaigns to be more data-dense and consolidated to allow for easier management when there are so many other things to consider as well as more efficient management through SA360 bidding strategies.
2. Integration with ATL Activity and Consideration of Budget
New account structures allow for more control over ad messaging and keywords being prioritized. This was crucial for their alignment with TV, radio, and social as it ensured consistent messaging for those interacting with “Above the Line” targeting, activity ran on TV, Radio, VOD, Digital Audio, and upper-funnel marketing on Social and Display.
Integrating with CRUK’s media agencies was also important when ensuring budgets and bidding are weighted when a strong brand message is conveyed through a burst of ATL activity, including weeks and months of TV, social, and radio bursts.
3. Reallocating Spend to Provide Income through Higher-Performing Channels
Working with the CRUK accounts team, a plan was drawn up to suggest which products had lower intent or soon to be canceled events and could therefore be cut or dropped, to allow an increase spend in top of mind, more urgent products.
The Results
Impression was pleased to have helped them reduce overall spend by 17.4% whilst maintaining year-on-year and month-on-month growth by focusing on, key priorities. Overall they were able to drive an increase in revenue of 10% despite the challenging context.
Impression launched the very first Microsoft Ads EMEA advertising grant account supporting charities with Cancer Research UK. This grant allowed them to drive higher awareness through the account, specifically:
- 68.8% increase in impressions
- 8.19% increase in clicks
- 9.58% increase in cost
Whilst Impression is pleased to have had a positive impact on CRUK’s performance, it’s not all about the numbers. Since 2019, their relationship has gone from strength to strength and their two-way feedback loop allows them to have honest conversations on a regular basis. These regular check-ins have led to changes in requirements for the number of meetings and honest communication about hours and priorities to ensure Impression always meets expectations… They are proud to work as an extension of Cancer Research UK’s team and integrating with their other agencies.
Working in true partnership, a member of the CRUK team has appeared as a guest speaker on an Impression podcast and Impression has also helped their internal marketing teams by delivering various virtual training sessions which are saved to their learning hub for new starters.
Their work has also been recognized and shortlisted for awards. In 2020, their campaigns were shortlisted for the ‘most effective use of paid search’ (DADI Awards, 2020), for ‘best use of search – not for profit’ (UK Search Awards, 2020), and for the ‘community response award’ (Microsoft Advertising Partner Awards, 2020).
About Impression
Impression is a multi-award-winning digital agency, specializing in the delivery of high-performance campaigns across SEO, PPC, analytics and digital PR.
Share this post

Agencies of the Month

Related Works

This website uses cookies. Continued use of this website indicates that you have read and agree to our Terms & Conditions and Privacy Policy .

IMAGES
COMMENTS
We fund a number of major research programmes and collaborations led by our world-leading senior cancer researchers. These case studies illustrate the kinds. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
In these case studies you can read advice on applying for funding from some of the leading researchers we fund, and explore the career path that they have taken. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee. Registered ...
These case studies feature researchers who. Read advice on applying for funding from some of our current grantees with a range of backgrounds and expertise. These case studies feature researchers who are developing their careers with our ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle ...
Successful applicant case studies; How we deliver research. Our research strategy; Our research infrastructure; Events and conferences; Our research partnerships; Facts & figures about our funding; ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A ...
When this study was done, COVID-19 was a new illness. Nobody knew how it would affect people with cancer. Or how well the COVID-19 vaccines would work for people with cancer. The research team wanted to find out more about the effect of the COVID-19 vaccines on the different cells of the immune system. Antibodies are proteins made by our immune ...
Case study 4 - Dr Rajesh Jena. Personalising radiotherapy - how patient data is helping reduce the side effects of treatment. Radiotherapy is the gold standard of treatment for many types of cancers. In fact, more than 130,000 patients benefit from radiotherapy every year in the UK.
Definition of cases and controls. As the main objective was to evaluate the effect of mammography screening on breast cancer mortality, cases were defined as women whose primary cause of death was ...
A Neoadjuvant Chemotherapy Trial for Early Breast Cancer is Impacted by COVID-19: Addressing Vaccination and Cancer Trials Through Education, Equity, and Outcomes, Clinical Cancer Research, 27, 16 ...
Abstract. UK Biobank is a large-scale prospective study with deep phenotyping and genomic data. Its open-access policy allows researchers worldwide, from academia or industry, to perform health ...
ICR scientists discovered and helped develop one of the very first in a new class of PI3 kinase inhibitors. Working with Piramed Pharma - an ICR spinout company ultimately acquired by Roche for $175m - and others, we discovered and helped to develop pictilisib, which is currently in clinical trials. Our PI3K research has also led to the ...
And that's just as important than ever, with new analysis showing that melanoma skin cancer rates have increased by almost a third over the past decade. In fact, researchers have projected a record high of 20,800 cases this year in the UK. This rise may sound alarming, but it's important to note that around 17,000 cases of melanoma each ...
An impactful clinical trial will have real-life benefits for patients and society beyond the academic environment. This study analyses case studies of cancer trials to understand how impact is evidenced for cancer trials and how impact evaluation can be more routinely adopted and improved. The United Kingdom (UK) Government allocates research funding to higher-education institutions based on ...
Soaring skin cancer cases hit a record high. Melanoma skin cancer cases in the UK have reached an all-time high. Our latest analysis shows there are 17,500 cases being diagnosed per year and projections reveal that these high numbers could continue to increase by around 50% over the next 20 years.
The research, led by Karsten Bach and Dr Sara Pensa at the University of Cambridge, set out to develop a method to detect the early changes occurring in BRCA1-affected breast cells, indicating that they are progressing towards breast cancer. "Over the last decades scientists have catalogued the mutations present in breast cancers.
This study analyses case studies of cancer trials to understand how impact is evidenced for cancer trials and how impact evaluation can be more routinely adopted and improved. Methods: The United Kingdom (UK) Government allocates research funding to higher-education institutions based on an assessment of the institutions' previous research ...
Successful applicant case studies; How we deliver research. Our research strategy; Our research infrastructure; Events and conferences; Our research partnerships; Facts & figures about our funding; ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A ...
Maxine Lenza is a health information officer at Cancer Research UK. References and further reading . Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case-control study and meta-analysis Fitzpatrick D, Pirie K, Reeves G, Green J, Beral V (2023) Combined and progestagen-only hormonal contraceptives and ...
Cancer Research UK's race for life is an event for women only, so it gives them the chance to take action against cancer. Through constant innovation over many years, nearly £500 million has been raised to beat cancer. ... Related case studies or articles. The London marathon: a human race Cancer Research UK: the letter from cancer Also in ...
2. Top performing organisations like Cancer Research UK strive for employee engagement excellence, as they know they'll be more productive, grow faster and innovate more. They develop thriving cultures and winning people practices that propel them into the upper quartile of engaging organisations.
Research; About us; Search Talk to a nurse: 020 7923 5475; Target Ovarian Cancer. ... Campaigning on ovarian cancer across the UK; Influence your local health systems. Ovarian cancer 'Red Flags' ... Sarah was diagnosed with stage 3c ovarian cancer in March 2023 after an elevated CA125 result of over 1,700. She's now taking the maintenance drug ...
The Challenge. Cancer Research UK, like most charities, was hit hard by the COVID-19 pandemic in 2020. The charity had to close its shops temporarily and cancel fundraising events including Race for Life. As a result of the pandemic, Cancer Research UK expected to see a decline in its income of £30m in 2020, and £300m over three years.
Cancer Research UK is the world's largest independent funder of cancer research with the vision to bring forward the day when all cancers are cured. They appointed Impression in early 2019 and Impression has been working together since to manage their paid search activity and to work collaboratively with their internal marketing teams and ...
CASE STUDY ___ Cancer Research UK. Challenge. To create a Premium Retail brand which resonates with a new growing Conscious Consumer base in order to support the long term objective of defeating Cancer. It's 2018 and the High Street and Retail at large are in decline. CRUK's Premium offering has not had a re-design in over 13 years during ...
News. Researchers at the Francis Crick Institute and King's College London have found that a new method of drug delivery using proline, an amino acid found in chicken feathers and skin tissue, could be used to limit the side effects of chemotherapy and repair important enzymes. The small cages, with an internal cavity 1-4 nanometres in length ...
The Queen's University Northern Ireland Cancer Registry (NICR) today (Tuesday 21 May) released the official statistics on cancer diagnosed in Northern Ireland during 1993-2021. This release provides details of the number of cancer cases diagnosed each year along with incidence rates over time and ...