- Applicant login

Open Faculty Positions
Associate director for clinical research.
Apply now Work type: University Medical Line Location: Stanford University Categories: School of Medicine
The Stanford Cancer Institute (SCI) seeks applicants for an Associate Director for Clinical Research (AD for CR) to join the School of Medicine at Stanford University as Associate Professor or Professor in the University Medical Line (UML). We are particularly interested in candidates who have outstanding record of innovation and accomplishment in research, translation, and education. Exceptional physician/physician-scientists with a strong clinical/translational emphasis in clinical trials development and implementation are encouraged to apply.
The major criteria for appointment for faculty in the University Medical Line shall be excellence in the overall mix of clinical care, clinical teaching, scholarly activity that advances clinical medicine, and institutional service appropriate to the programmatic need the individual is expected to fulfill.
Faculty rank and the department will be determined by the qualifications and experience of the successful candidate.
The successful applicant should have a MD or MD/PhD and be board certified in an oncological subspecialty of Medicine, Surgery, or in Radiation Oncology. Successful candidates will be expected to be clinically active, maintain a robust clinical research portfolio, and demonstrate a deep interest in cancer clinical research within a cancer center which has achieved comprehensive designation from the National Cancer Institute (NCI).
The Associate Director (AD) will provide overall leadership of the Stanford Cancer Institute’s cancer clinical research enterprise in alignment with the expectations established by the NCI, and help train the next generation of clinical and translational investigators. The incumbent will work closely with faculty and administrative leaders in the SCI and within the Stanford Health Care (SHC) Cancer Destination Service Line and the Stanford Children’s Health (SCH) oncology program. The SCI’s AD for Clinical Research will also represent and advocate for the interests and needs of cancer clinical research at the highest levels leadership within Stanford Medicine.
The mission of the Stanford Cancer Institute (SCI) is to leverage the broad and unique strengths of Stanford University in the sciences, in clinical care, and in translation to improve the diagnosis, treatment, and outcomes of cancer patients; to understand cancer etiologies among diverse populations; and to decrease cancer incidence in the SCI catchment area and beyond. The SCI offers unique opportunities for the successful candidate to be integral to its mission and to join a faculty highly engaged in improving cancer research and patient care.
Applications will be reviewed until the position is filled.
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law. Stanford also welcomes applications from others who would bring additional dimensions to the University’s research, teaching and clinical missions.
The Stanford Cancer Institute, School of Medicine, and Stanford University value faculty who are committed to advancing diversity, equity, and inclusion. Candidates may optionally include as part of their research or teaching statement a brief discussion of how their work will further these ideals.
Submit CV and a brief letter outlining your interest to:
https://facultypositions.stanford.edu/en-us/job/493542/

The expected base pay range for this position:
Associate Professor: $282,000 – $338,000
Professor: $356,000 – $375,000
Associate Professor: $355,000-$393,000
Professor: $424,000-$462,000
Radiation Oncology:
Associate Professor: $392,000 – $416,000
Professor: $466,000 – $484,000
This pay range reflects base pay, which is based on faculty rank and years in rank. It may not include all components of faculty compensation or pay from participation in departmental incentive compensation programs. For more information about compensation and our wide-range of benefits , including housing assistance , please contact the hiring department.
Stanford University has provided a pay range representing its good faith estimate of what the university reasonably expects to pay for the position. The pay offered to the selected candidate will be determined based on factors including (but not limited to) the experience and qualifications of the selected candidate including equivalent years in rank, training, and field or discipline; internal equity; and external market pay for comparable jobs.
For questions regarding this position, please contact Mihaela Bozdog at [email protected]
Advertised: 06 Feb 2023 5:00 PM Pacific Standard Time Applications close:
Back to search results Apply now Refer a friend
We will email you new jobs that match this search.
Great, we can send you jobs like this, if this is your first time signing up, please check your inbox to confirm your subscription.
The email address was invalid, please check for errors.
You must agree to the privacy statement
Search results
Current opportunities.
Powered by PageUp
Refine search
- University Medical Line 1
- Stanford University 1
- School of Medicine 1

CRA Careers at IQVIA
Help tackle healthcare’s greatest challenges to improve patient health.
Not ready to apply? Join our Global Talent Network .
Put your passion to work
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. Here, you'll find the autonomy and flexibility you need to take your CRA career to the next level.
Choose your path
Sponsor-dedicated.
When you join as a sponsor-dedicated CRA, you’ll embrace the stability of a global contract research organization and harness the power of industry-leading technology. As part of this alignment, you'll gain valuable experience, working side by side one of our pharmaceutical customers, and expand your knowledge in a wide variety of therapeutic areas.
Our sponsor-dedicated model brings together the right people, experience and ideas to deliver high quality solutions specific to the customer’s needs.

Full-service
When you become a CRA as part of our full-service alignment, you'll embrace your passion for a specific therapeutic area and support a variety of sponsors. With access to world-class training and cutting-edge technology, developed specifically for IQVIA CRAs, you'll have the resources you need to create the career you want.
Our full-service model delivers cutting-edge clinical research through state-of-the-art innovation.

IQVIA Biotech
When you pursue a CRA career with IQVIA Biotech, you'll work directly with customers in a collaborative environment to help change the face of biotech. Using your extensive therapeutic knowledge, you’ll oversee uniquely-focused clinical studies, where you’ll be exposed to pioneering protocols and experience a dedicated partnership with your team like none other.
IQVIA Biotech is at the forefront of clinical trials in a variety of therapeutic areas through the delivery of flexible solutions within the biotech sector.

IQVIA empowers you to drive your own path within the organization. As no person's route is the same and the clinical research landscape is forever changing, you are encouraged to continually seek career growth opportunities and align your interests with your career goals.

Senior Clinical Research Associate 2
At IQVIA, we are committed to supplying our CRAs with the right tools, training and development support to allow for success and career growth. In addition to the rewarding work, collaboration and engaged line management, there is a constant focus on development as your career growth supports also the growth of the company.

Director, Clinical Operations
For me, it’s the people and knowing I’m making a difference in creating a healthier world that inspire me to come to work every day. I can go to anyone with a challenge, and they are willing to help. Together, we’re thinking outside of the box to navigate each sites’ unique needs and challenges.

Senior Clinical Research Associate
Explore unlimited opportunities
Clinical Research Associates can leverage their experience in a variety of ways that enable them to pursue their unique passions. Whether you choose to advance within a specific therapeutic area or want to explore something new, you can create the career you want. Explore the various career opportunities when you join IQVIA as a CRA.
What you can expect
All CRAs at IQVIA, regardless of alignment, thrive within our dynamic culture and experience:
Professional Development

Work-Life Balance
Supportive Leadership

Best-in-Class Training

Collaboration
Explore clinical monitoring jobs.
- R1407289 未経験CRA (臨床開発モニター)/ IQVIA Services Learn more Multiple Locations
- R1423040 Sr CRA, Sponsor Dedicated IVDx, Hybrid In-House Learn more Multiple Locations
- R1388789 Senior Clinical Research Associate - Sponsor Dedicated Learn more Multiple Locations
Join our Global Talent Network
Let’s stay connected. Sign up to receive alerts when new opportunities become available that match your career ambitions.
Job Category Select a Job Category Administrative Support Advanced Analytics Business Systems Analysis Client Services Clinical Data Management Clinical Operations Clinical Project Management/Leadership Clinical Trial Supply & Logistics Communications Connected Devices Consulting Contract Management Database Management Systems Finance General Management Human Resources Information Security Internships IT Design & Development IT Infrastructure IT Support Lab Science Lab Science Advisors Lab Services Laboratory Laboratory Projects Legal and Regulatory Lifecycle Safety Marketing Medical Medical Affairs Medical Communications Medical Sales & Services Monitoring Patient Centric Services (PCS) Phase 1/Clinic Operations Product Support (Tier 3) Production Project and Program Management QA & Testing Quality Assurance Sales Sales Support Software Development Engineering Statistician Strategic Supplier Services Strategy and Corporate Development Technical Writing
Remote Select... Yes
Areas of Interest
Confirm Email
Search for a location and select one from the list of suggestions. Select a job category from the list of options. Finally, click “Add” to create your job alert. Multiple job alerts may be created by repeating these steps.
Explore life at IQVIA
Explore more Johnson & Johnson sites:
- Saved jobs 0
- English Deutsch English Español Français Indonesian Italiano 日本語 한국어 Nederlands Portuguese ไทย Türkçe 中文(简体) 中文(繁體)
Discovery & Pre-Clinical/Clinical Development
Associate Director, Clinical Sciences - Ophthalmology
- Job Title Associate Director, Clinical Sciences - Ophthalmology
- Function Discovery & Pre-Clinical/Clinical Development
- Sub Function Clinical Development & Research & Non-MD
- Category Senior Principal Scientist, Clinical Development & Research - Non-MD (ST8)
- Location Raritan, New Jersey, United States
- Date Posted May 30 2024
- Requisition Number 2406190657W
Description
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at https://www.jnj.com/.
Johnson & Johnson is recruiting for an Associate Director, Clinical Project Scientist - Ophthalmology to be located in Raritan, NJ, Spring House, PA, Cambridge, MA, or La Jolla, CA . Remote work options may be considered on a case-by-case basis and if approved by the Company.
The Associate Director, Clinical Project Scientist is a responsible member of a clinical team dedicated to the development and execution of clinical strategies and the clinical and operational implementation of a complex compound program. The Associate Director, Clinical Project Scientist provides active scientific contribution to a cross-functional clinical team developing a molecular entity. The Associate Director, Clinical Project Scientist provides input to the clinical development plan, works on the development of the clinical trial protocol, clinical trial materials, and takes responsibility for coordinating completion of clinical study reports and supports preparation of relevant documents for regulatory filings. This role involves extensive team matrix interactions with colleagues from several different disciplines. The Associate Director, Clinical Project Scientist may be asked to contribute to the evaluation of scientific opportunities in the therapeutic area.
ESSENTIAL FUNCTIONS:
- Leads/Supports preparation of clinical development plans, trial protocols, and supports the operations group with trial set up and monitoring and authoring of clinical study reports.
- Assists Physicians in evaluation of adverse events (pre and post-marketing) for relationship to treatment and supports the interpretation and reporting of results.
- Assists Regulatory Affairs in determining requirements for any corrective actions or health authority reporting.
- Interprets, reports, and prepares oral and written results of product research, in concert with senior clinical personnel, in preparation for health authority submissions.
- Responsible for clinical data review, including medical data review, coding, in conjunction with other clinical team members.
OTHER RESPONSIBILITIES / DETAILED DUTIES:
- Assists Regulatory Affairs in the development of drug regulatory strategies.
- Participate on and may lead cross-functional teams for evaluation of new product ideas, implement franchise business strategies, etc.
- Reviews medical literature and related new technologies
- May be asked to lead the execution of contracts.
- May be asked to assess medical publications emerging from the team and its affiliates.
- May be responsible, with appropriate colleagues, for review of Company advertising and promotion.
Qualifications
Education and Experience: A minimum of a Bachelor's degree with required therapeutic area experience in ophthalmology. An advanced degree (e.g., MS, RN, RD, PhD or PharmD) is preferred. A minimum of at least 6 years of clinical research and development experience within the pharmaceutical industry is required.
Required Technical Knowledge and Skills:
o Experience in ophthalmology product development and cross company alliances is preferred.
o Significant experience with managing/ supervising clinical trials, clinical research programs is required.
o Medical Data Review experience strongly preferred.
o Strong interpersonal and communication skills are essential.
The anticipated base pay range for this position is $135,000 to $232,300 USD.
The compensation and benefits information set forth in this posting applies to candidates hired in the United States. Candidates hired outside the United States will be eligible for compensation and benefits in accordance with their local market.
Employees may be eligible to participate in Company employee benefit programs such as health insurance, savings plan, pension plan, disability plan, long term incentives, vacation pay, sick time, holiday pay, and work, personal and family time off in accordance with the terms of the applicable plans. Additional information can be found through the link below. For additional general information on Company benefits, please go to: - https://www.careers.jnj.com/employee-benefits
At Johnson & Johnson, we’re on a mission to change the trajectory of health for humanity. That starts by creating the world’s healthiest workforce. Through cutting-edge programs and policies, we empower the physical, mental, emotional, and financial health of our employees and the ones they love. As such, depending on location and subject to local legislation, candidates offered employment may be required to show proof of COVID-19 vaccination or, in certain countries, secure an approved accommodation prior to the commencement of employment to support the well-being of our employees, their families and the communities in which we live and work. If you are invited to interview for the position, your recruiter will advise on the vaccine requirement status in your geographic location.
Johnson & Johnson Family of Companies are equal opportunity employers, and all qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, genetic information, national origin, protected veteran status, disability status, or any other characteristic protected by law.
For more information on how we support the whole health of our employees throughout their wellness, career, and life journey, please visit www.careers.jnj.com.
Related Jobs
You might be interested
Director, Project Responsible Physician - Neuroscience
- Discovery & Pre-Clinical/Clinical Development - Clinical Development & Research & MD
- Titusville, United States; La Jolla, United States; Spring House, United States; Cambridge, United States
Associate Director, Translational Clinical Medical Lead Translational Science & Medicine- Immunology
- Spring House, United States
Medical Director/ Sr. Medical Director, Clinical Development
- Raritan, United States
Your next move could mean our next breakthrough.
We invite you to join our Global Talent Hub, where we keep in touch with people around the world who share our passions for bold innovations and are inspired by our mission of changing the trajectory of human health.
应聘者知情同意函 Job Applicant Consent Letter
鉴于强生全球集团业务的全球化经营性质,当您向强生中国 [1] 应聘职位时,强生中国会按照《应聘者隐私政策》所述情形,向境外接收方及其经授权的第三方提供您的个人信息,包括但不限于:
Due to the global nature of the operation of Johnson & Johnson Family of Companies, in the course of processing your application with J&J China, J&J China may provide your personal information to the overseas recipient and authorized third parties in accordance with J&J’s Careers Privacy Policy, including but not limited to:
- 基本信息:姓名、生日、民族、性别、国籍、照片; Basic Information: Name, Birthday, Ethnicity, Gender, Nationality, Headshot;
- 联系信息:住址、电话号码、电子邮件地址; Contact Information: Address, Phone Number, Email;
- 教育工作信息:职业、职位、工作单位、工作经验、教育背景、学历、学位、培训记录。 Academic & Professional Information: Occupation, Position, Employer, Work Experience, Educational Background, Degree, Training Records.
除强生中国或法律特别要求外,请您避免在您的申请中提交可能会被视为敏感个人信息的信息,即生物识别、宗教信仰、特定身份、医疗健康、金融账户、行踪轨迹,以及不满十四周岁未成年人的信息等一旦遭遇泄露或非法使用,容易导致人格尊严受到侵害或者人身、财产安全受到危害的个人信息。
Except as specifically requested by J&J China or legally required, please avoid submitting information in your application which may be deemed as sensitive personal information, which includes biometrics, religious belief, specific identity, medical health, financial accounts, whereabouts, as well as information of minors under the age of fourteen (14), and other information that the breach or illegal use of which may easily lead to the infringement of an individual’s personal dignity or harm to personal or property safety.
前述境外接收方为:美国强生公司(Johnson & Johnson Services, Inc.),其联系方式为:美国新泽西州新不伦瑞克市强生广场一号。
The overseas recipient is Johnson & Johnson Services, Inc., which can be contacted at One Johnson & Johnson Plaza, New Brunswick, New Jersey 08933, U.S.
境外接收方将按照如下目的和方式处理您的个人信息。
The overseas recipient will process your personal information in accordance with the following purpose and method:
- 处理目的:根据强生全球集团“统一人力资源(One HR)”原则,对人才招募进行全球统一管理,具体请参见《应聘者隐私政策》中“我们收集此类个人信息的原因”部分。
Processing Purpose: Conduct talent acquisition management globally following the One HR principle of the Johnson & Johnson Family of Companies, as described in the “Reasons We Collect This Information” section of J&J’s Careers Privacy Policy.
- 处理方式:通过统一流程和全球互联应用实现对人才招募的统一管理,涉及存储、使用、加工、传输、删除已出境个人信息。境外接收方将采取适当的管理和技术措施保障出境个人信息的机密性、完整性和可用性,并将在完成上述目的最小必要范围内保存出境个人信息。
Processing Method: Conduct talent acquisition management through the unified process and globally connected applications, including storage, use, handling, transmission, and deletion of provided personal information. Overseas recipients will apply appropriate managerial and technical measures to ensure the confidentiality, integrity, and availability of the provided personal information and shall store such personal information to the minimum extent necessary to complete the above purposes.
您可以通过[email protected]联系强生中国公司的个人信息保护官以向境外接收方行使您享有的相关权利,包括要求访问、更正、复制或删除您的个人信息。
You may contact the Personal Information Protection Officer of the J&J China at [email protected] to exercise your relevant rights to the overseas recipient, including access, correct, copy, or delete your personal information.
鉴于强生全球集团业务的全球化经营性质,当您向强生中国 应聘职位时,强生中国会向境外接收方及其经授权的第三方提供您的个人信息。具体而言:
除强生中国或法律特别要求外,请您避免在您的申请中提交可能会被视为敏感个人信息的信息,即物识别、宗教信仰、特定身份、医疗健康、金融账户、行踪轨迹,以及不满十四周岁未成年人的信息等一旦遭遇泄露或非法使用,容易导致人格尊严受到侵害或者人身、财产安全受到危害的个人信息。
Due to the global nature of the operation of the Johnson & Johnson Family of Companies, in the course of processing your application with J&J China, J&J China may provide your personal information to the overseas recipient and authorized third parties. Specifically:
[1] 在本个人信息出境同意函中,“强生中国”是指与您申请的职位所属的强生全球集团内具体公司。强生全球集团指美国强生公司(Johnson & Johnson Services, Inc.)以及其在世界范围内的关联公司,包括强生中国。
In this Employee Consent Letter, “J&J China” means the company in the Johnson & Johnson Family of Companies which you applied for, and “Johnson & Johnson Family of Companies” means Johnson & Johnson Services, Inc. and its worldwide affiliates, including J&J China.
We care about your privacy
Thank you, your preferences have been updated.
By clicking "Accept All", you agree to the storing of cookies on your device to give you the most optimal experience using our website. We may also use cookies to enhance performance, analyse site usage and to personalise your experience.

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Stanford Center for Clinical Research (SCCR) is a growing academic research organization within the Stanford Department of Medicine. Our mission is to conduct and promote high-impact, innovative clinical research to improve human health.
At SCCR, we strive to find team members who are passionate about their work, flexible, fun, and want to deliver results. We place a high priority on equipping our staff to perform their job efficiently, helping them acquire new skills and grow within the organization. We encourage our team to have a healthy balance between work commitments and life outside of work and provide support to achieve this balance. If you are looking to make a large impact through global-reaching clinical research, we encourage you to apply!
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
DESIRED QUALIFICATIONS:
- Bachelor’s degree in a health/medical science or other related field.
- Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
EDUCATION & EXPERIENCE (REQUIRED):
- Two-year college degree and two years related work experience or a Bachelor's degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Working toward certification(s) to perform basic patient measurements and tests, such as phlebotomy and EKG.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
WORKING STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University’s Administrative Guide, http://adminguide.stanford.edu/ .
The expected pay range for this position is $31.73 to $36.54 per hour.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow . We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture . We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you . Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun . Stroll through historic sculptures, trails, and museums.
- Enviable resources . Enjoy free commuter programs, ridesharing incentives, discounts and more.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 103427
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: May 07, 2024
Post Date: Jan 29, 2024
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs

Search Results
Pursue progress.
Discover extraordinary.
Search Jobs
Job title, category, or keyword
City, region, or country
Sort by Relevancy Date Posted Job Title Location Distance
Here's what we found for you:
Sr. Associate - HEVA
Associate - heva, associate project specialist – medical communications, project specialist – medical communications, expert medical regulatory writer, manager – market access, country quality head chc korea, senior associate – heor modeling, eula manufacturing excellence lead, alternance 12 mois – communication interne et externe h/f, (senior)controller/(senior) financial analyst (german speaking) - budapest, hungary, estágio em produção farmacêutica / suzano - são paulo - sp (presencial), medical science liaison - hemophilia - mn, wi, mi, chc western europe public affairs lead, filter results.
- Administrative (23)
- Business Operations (80)
- Business Services (23)
- Clinical Development (17)
- CMC Development (25)
- Communication (10)
- Contingent Worker (40)
- Continuous Improvement and Performance (5)
- Corporate Affairs (3)
- Digital Data & Technology (155)
- Engineering (43)
- Ethics & Business Integrity (7)
- Facilities Management (5)
- Finance (74)
- Health, Safety and Environment (11)
- Human Resources (100)
- Laboratory (14)
- Leadership (11)
- Market Access (18)
- Marketing (81)
- Medical (103)
- Patient Support Services (3)
- Pharmacovigilance (5)
- Procurement (9)
- Production (96)
- Project Management (22)
- Public Affairs (4)
- Quality (103)
- Regulatory Affairs (31)
- Research & Development (3)
- Sales (258)
- Security (3)
- Strategy & Business Development (12)
- Supply Chain (46)
- Algeria (3)
- Argentina (12)
- Australia (2)
- Austria (5)
- Belgium (8)
- Brazil (16)
- Canada (26)
- China (216)
- Colombia (22)
- Czechia (7)
- Denmark (3)
- Ecuador (1)
- France (277)
- Germany (68)
- Hungary (107)
- India (233)
- Indonesia (2)
- Ireland (14)
- Malaysia (16)
- Morocco (3)
- Netherlands (8)
- Philippines (13)
- Poland (13)
- Portugal (1)
- Romania (6)
- Saudi Arabia (8)
- Singapore (13)
- Slovakia (3)
- South Africa (1)
- South Korea (5)
- Switzerland (2)
- Thailand (3)
- Tunisia (4)
- Turkey (22)
- Ukraine (10)
- United Arab Emirates (1)
- United Kingdom (12)
- United States (262)
- Vietnam (12)
- Abruzzo (2)
- Algiers Province (2)
- Auvergne-Rhône-Alpes (45)
- Beijing Municipality (24)
- Bogota D.C. (22)
- Borsod-Abaúj-Zemplén (2)
- Bratislava (1)
- Brittany Region (1)
- București (6)
- Budapest (102)
- Buenos Aires (4)
- Buenos Aires F.D. (1)
- Canton of Zug (2)
- Capital Region (3)
- Catalonia (24)
- Centre-Val de Loire (7)
- Corrientes (7)
- Emilia-Romagna (15)
- England (12)
- Flanders (7)
- Florida (1)
- Gouvernorat de Ben Arous (1)
- Gouvernorat de Tunis (3)
- Guangdong (25)
- Hauts-de-France (17)
- Hlavní město Praha (7)
- Ho Chi Minh (11)
- Île-de-France Region (172)
- Istanbul (13)
- Jakarta Special Capital Region (2)
- Jiangsu (1)
- Kırklareli (9)
- Kuala Lumpur (4)
- Kyiv City (10)
- Land Berlin (7)
- Leinster (1)
- Lisbon District (1)
- Lombardy (5)
- Maharashtra (3)
- Massachusetts (102)
- Mazovia (13)
- Mecca Region (3)
- Metro Manila (13)
- México (2)
- Mexico City (4)
- Munster (13)
- National Capital Territory of Delhi (1)
- New Jersey (158)
- Normandy (22)
- North Holland (8)
- North Rhine-Westphalia (5)
- Nouvelle-Aquitaine (6)
- Occitanie (11)
- Ontario (23)
- Pennsylvania (18)
- Pest megye (3)
- Provence-Alpes-Côte d'Azur Region (3)
- Provincia del Guayas (1)
- Queensland (2)
- Riyadh Region (6)
- São Paulo (16)
- Selangor (12)
- Shandong (17)
- Shanghai Municipality (54)
- Sichuan (33)
- Tamil Nadu (1)
- Telangana (109)
- Tennessee (32)
- Tianjin Municipality (17)
- Tipaza Province (1)
- Wallonia (1)
- Zhejiang (28)
- Alger Plage (2)
- Ambarès-et-Lagrave (3)
- Amsterdam (8)
- Barcelona (23)
- Beijing (24)
- Bogotá (22)
- Bridgewater (77)
- Bucharest (6)
- Buenos Aires (1)
- Cambridge (78)
- Campinas (2)
- Chattanooga (32)
- Chengdu (33)
- Chennai (1)
- City of Taguig (13)
- Cologne (5)
- Compiègne (17)
- Cuautitlán Izcalli (2)
- Framingham (15)
- Frankfurt am Main (57)
- Gentilly (67)
- Gobernador Juan E. Martínez (7)
- Guangzhou (24)
- Guarulhos (1)
- Guayaquil (1)
- Hangzhou (28)
- Ho Chi Minh City (11)
- Hyderabad (109)
- Jakarta (2)
- Le Trait (6)
- Lisieux (10)
- Lüleburgaz (9)
- Maagd van Gent (2)
- Maisons-Alfort (3)
- Marcy-l'Étoile (22)
- Mégrine (3)
- Mégrine (1)
- Milano Marittima (15)
- Miskolc (2)
- Mississauga (3)
- Montpellier (4)
- Morristown (1)
- Mourenx (3)
- Neuilly-sur-Seine (69)
- Neuville-sur-Saône (1)
- New Delhi (1)
- Northborough (1)
- Origgio (5)
- Orlando (1)
- Østerbro (3)
- Petaling Jaya (12)
- Petit Bruxelles (1)
- Ploërmel (1)
- Porto Salvo (1)
- Praha Klanovice (1)
- Reading (12)
- Ridgefield (6)
- Riells i Viabrea (1)
- Rotkreuz (2)
- São Paulo (5)
- Scoppito (2)
- Shanghai (54)
- Shenzhen (3)
- Sisteron (3)
- Swiftwater (18)
- Tianjin (17)
- Toronto (21)
- Val-de-Reuil (6)
- Veresegyház (3)
- Virginia (2)
- Vitry-sur-Seine (15)
- Waltham (8)
- Warsaw (13)
- Waterford (13)
- Zeralda (1)
- Business Operations (9)
- Corporate (104)
- Digital (78)
- Early Careers (355)
- Engineering (25)
- Manufacturing & Supply (78)
- Marketing & Sales (355)
- Medical (77)
- Quality Assurance (56)
- Regulatory Affairs (14)
- Research & Development (42)
- Apprentice (184)
- Fixed Term (263)
- International Internship Program (VIE) (19)
- Internship (152)
- Permanent (850)
- Temporary (3)
- Jobs you might like
- Jobs you've viewed
- Jobs you've saved
- View All of Our Available Opportunities

Hear from the ambitious, talented individuals who pull together to create better for patients.

Experience possibility

Your Saved Jobs
Privacy policy, terms and conditions.

Cambridge Crossing
We're bringing together 2,500 people from across our organization — R&D, Medical, Commercial and Global colleagues all working to realize the power of collaboration.

Innovation in Action
Our flexible lab of the future will transform how we conduct research, while our innovation center will be fully integrated with existing R&D locations.

Sustainable and Green
Our new facility was built to minimize the environmental impact — helping protect our planet and people. Using resources efficiently, we're providing greener, healthier workspaces.

Sanofi’s AI Centre of Excellence in Toronto
The Centre is focused on using leading technologies to develop world-class data and artificial intelligence (AI) products to create value for the health sector.

Ama puts her project management techniques and ServiceNow knowledge to use to help advance Sanofi’s Digital Data operating model. Learn how our team connects data and AI to do what’s never been done before.

Sanofi Canada's Philanthropic Efforts
By chasing the miracles of science to improve people’s lives, we surprise ourselves with what we can achieve. Our team is humbled by the impact our efforts make.

Emmanuel, Head of the Sanofi Digital Accelerator, shares how his team builds digital solutions that enable patients to receive new treatments to help improve their lives.

Dimitrije shares insights into the work carried out by the AI Centre of Excellence in Toronto.

Read Ziv's first-hand account describing the reasons he chose to join Sanofi – and many of the reasons why he now chooses to stay.

When you grow, we all grow
We strive to support your whole self with thoughtfully crafted rewards that benefit you physically, financially, mentally and socially. Whatever your role, you'll thrive in our inclusive teams.

Build a career with purpose
Bring your passion to your role and impact millions of people around the world. You're in the driver's seat – just set your goals, and we'll provide the training and support that will get you there.

Bolder, better futures
Change your life. And the lives of millions around the globe. How? By starting a career where you're supported to grow, while having a tangible impact and learning from the best.

Our locations
We're in 60+ countries, all pulling together to define the future of healthcare. Wherever you work, you'll develop your career alongside experts, using technology to chase bigger breakthroughs.

Sanofi Stories
At Sanofi every voice matters. Get to know the talented Sanofians shaping our future and pushing us toward our ambitious goals.
Sanofi at Cambridge Crossing
Dubbed Sanofi at Cambridge Crossing, our new state-of-the-art facility will create an innovation hub promoting close collaboration and integration among business units. Join us and become part of a team dedicated to chasing the miracles of science that improve people’s lives.

Get access to the tools, training, and support to reach your goals. By fulfilling your potential, you’ll help us achieve our aim of halving the time from discovery to therapy.

Our people & culture
We're the first in Pharma to have a DE&I board. We also have Employee Resource Groups that create spaces for every Sanofian to be heard. Your voice matters – use it to shape our future.
Sanofi's Postdoctoral Program
Designed for high-caliber Ph.D. graduates, Sanofi's Postdoctoral Program helps you advance your scientific career in a state-of-the-art environment.
Physician Careers at Sanofi
At Sanofi, physicians like you have the opportunity to collaborate on new ideas and challenge established thinking. Learn about Physician careers here.
AI Centre of Excellence
The AI Centre of Excellence at Sanofi is a unique data-driven team based in Downtown Toronto. We pride ourselves on being data-obsessed and highly focused on using state-of-the-art technologies to drive global impact.
Your next role? We'll send it to you.
Job alerts help you find the right opportunity, so you can make the discoveries that change lives.
Job Category Select a Job Category Administrative Audit Business Operations Business Services Clinical Development CMC Development Communication Contingent Worker Continuous Improvement and Performance Corporate Affairs Digital Data & Technology Engineering Ethics & Business Integrity Facilities Management Finance Health, Safety and Environment Human Resources Laboratory Leadership Legal Market Access Marketing Medical Patient Support Services Pharmacovigilance Procurement Production Project Management Public Affairs Quality Regulatory Affairs Research & Development Sales Security Strategy & Business Development Supply Chain Trade
Location Select Location Algeria Argentina Australia Austria Belgium Brazil Canada China Colombia Czechia Denmark Ecuador Egypt France Germany Greece Hungary India Indonesia Ireland Italy Japan Malaysia Mexico Morocco Netherlands Philippines Poland Portugal Romania Saudi Arabia Singapore Slovakia South Africa South Korea Spain Switzerland Thailand Tunisia Turkey Ukraine United Arab Emirates United Kingdom United States Vietnam
Confirm Email
Sign up for job alerts
Get new jobs sent straight to your inbox.
An extraordinary career awaits. Make sure you never miss a thing.

- Prospective Students
- Current Students
- Faculty & Staff
- Parents & Family
- Alumni & Friends
- Campus Directory
- Request Info
- The Marist Experience
- Marist at a Glance
- History & Heritage
- President's Office
- Diversity, Equity, and Inclusion
- Marist 100 - Strategic Plan
- The Marist Gates
- Financial Statements
- Internships
- Academic Schools and Programs
- Academic Resources
- Academic Core
- Career Services
- Study Abroad
- Commencement
- Autumn Lecture Series
- Undergraduate
- Adult & Returning Students
- International
- Military & Veteran
- Summer Pre-College
- Student Financial Services
- Campus Living
- Student Services
- Student Involvement
- The Marist Community
- Safety & Security
- Diversity & Inclusion
Office of Human Resources
Pageup header, explore careers.
Thank you for your interest in a career with Marist College. For assistance and to follow up on an application, please contact the Office of Human Resources at [email protected] .
PageUptemplateMain
e.g. "Software Engineer, Poughkeepsie"
Filter results
Type of Position
- Full-time 24
- Part-time 44
- Poughkeepsie, NY 68
- Administration 20
- Part-Time Faculty 1
- School of Communication & the Arts 6
- School of Computer Science & Mathematics 2
- School of Liberal Arts 9
- School of Management 11
- School of Science 9
- School of Social & Behavioral Sciences 7
- Temporary 1
- Sorry, we can't provide additional information about this job right now.
We will email you new jobs that match this search.
Great, we can send you jobs like this, if this is your first time signing up, please check your inbox to confirm your subscription.
The email address was invalid, please check for errors.
You must agree to the privacy statement
Search results
More Jobs 48
Current Opportunities
Powered by PageUp
{{alert.message}} Close
- About company
- GENERAL CONTRACTOR

+7 (495) 526-30-40 +7 (49657) 0-30-99
THE HISTORY OF THE COMPANY CREATION
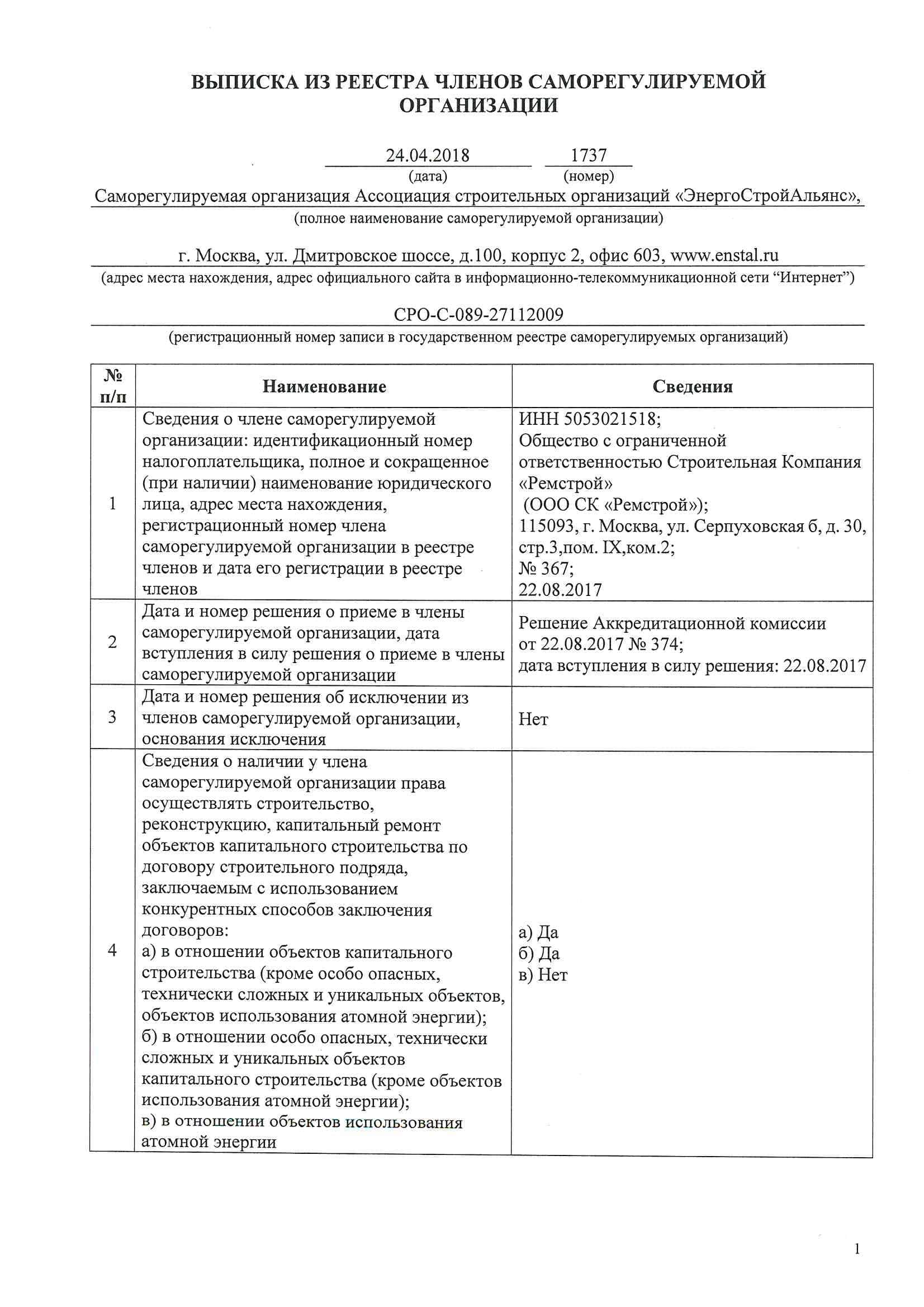
1993 how the construction company remstroy was created the year 1993 was a period when a lot of construction companies, which had been working successfully during the soviet times and had rich staff capacity, were forced to cease their activity for various reasons. a lot of capable specialists either had to look for another job or change their field. but there were also those who were willing to realise their potential in the field of construction in accordance with the received degree and the experience they had accumulated. thus, in 1993 in elektrostal (moscow oblast) a group of specialists and people sharing each other’s ideas, who had enormous educational background and the highest degree in architecture, organized and registered ooo firm erg which began its rapid development and successful work, offering its service both on the construction market and other areas. 2000 industrial construction is the main area seven years of successful work have shown that combining different types of activities in the same company is not always convenient. and in the year 2000 the founders of ooo firm erg decided to create and register a monoprofile construction company ooo remstroy construction company. industrial construction was chosen as the priority area. it was in this area that the directors of ooo sk remstroy began their working life and grew as specialists. in order to achieve the set goal, they selected a mobile team of professionals in the field of industrial construction, which allows us to cope with the tasks assigned to ooo sk remstroy throughout russia and the near abroad. 2010 manufacturing of metal structures we possess modern equipment that allows us to carry out the entire cycle of works on the manufacture of metal structures of any complexity without assistance. designing – production – installation of metal structures. a staff of professionals and well-coordinated interaction of the departments let us carry out the work as soon as possible and in accordance with all customer’s requirements.” extract from the list of members of self-regulatory organizations, construction.
LICENSE OF MINISTRY OF EMERGENCY SITUATIONS
Certificates, system of managing quality.

SYSTEM OF ECOLOGIAL MANAGEMENT

SYSTEM OF OCCUPATIONAL SAFETY AND HEALTH MANAGEMENT

LETTERS OF RECOMMENDATION

THE GEOGRAPHY OF CONSTRUCTION SITES
YOU CAN FIND MORE INFORMATION ON THE CONSTRUCTION SITES OF OOO REMSTROY ON THE PAGE OF THE SITE
OUR CLIENTS

http://remstroi.pro/yandex-promyshlennoe-stroitelstvo

Geographic coordinates of Elektrostal, Moscow Oblast, Russia
City coordinates
Coordinates of Elektrostal in decimal degrees
Coordinates of elektrostal in degrees and decimal minutes, utm coordinates of elektrostal, geographic coordinate systems.
WGS 84 coordinate reference system is the latest revision of the World Geodetic System, which is used in mapping and navigation, including GPS satellite navigation system (the Global Positioning System).
Geographic coordinates (latitude and longitude) define a position on the Earth’s surface. Coordinates are angular units. The canonical form of latitude and longitude representation uses degrees (°), minutes (′), and seconds (″). GPS systems widely use coordinates in degrees and decimal minutes, or in decimal degrees.
Latitude varies from −90° to 90°. The latitude of the Equator is 0°; the latitude of the South Pole is −90°; the latitude of the North Pole is 90°. Positive latitude values correspond to the geographic locations north of the Equator (abbrev. N). Negative latitude values correspond to the geographic locations south of the Equator (abbrev. S).
Longitude is counted from the prime meridian ( IERS Reference Meridian for WGS 84) and varies from −180° to 180°. Positive longitude values correspond to the geographic locations east of the prime meridian (abbrev. E). Negative longitude values correspond to the geographic locations west of the prime meridian (abbrev. W).
UTM or Universal Transverse Mercator coordinate system divides the Earth’s surface into 60 longitudinal zones. The coordinates of a location within each zone are defined as a planar coordinate pair related to the intersection of the equator and the zone’s central meridian, and measured in meters.
Elevation above sea level is a measure of a geographic location’s height. We are using the global digital elevation model GTOPO30 .
Elektrostal , Moscow Oblast, Russia

IMAGES
VIDEO
COMMENTS
Associate Director, Clinical Research. Takeda Pharmaceutical. Lexington, MA 02421. $149,100 - $234,300 a year. Full-time. Minimum of 5 years' experience as a clinical project/program manager leading complex pharmaceutical clinical research deliverables in a multi-disciplinary,….
The top companies hiring now for associate director clinical research jobs in United States are MEDSTAR HEALTH, Midwestern Career College, Save the Chimps, Inc., Ascend Healthcare, South University, Malibu Recovery Center, Twinkle Autism Therapy, LLC, Family Service and Children's Aid Society of Venango County, Pediatric Therapies, Inc ...
Today's top 1,000+ Clinical Research Associate Director jobs in United States. Leverage your professional network, and get hired. New Clinical Research Associate Director jobs added daily.
Today's top 1,000+ Director Clinical Research Associate jobs in United States. Leverage your professional network, and get hired. New Director Clinical Research Associate jobs added daily.
Today's top 2,000+ Associate Clinical Research Director jobs in United States. Leverage your professional network, and get hired. New Associate Clinical Research Director jobs added daily.
The Associate Director, Clinical Research provides clinical project management and leadership to ensure successful operational execution of all US Medical Clinical Research including, Medical Affairs Company Sponsored Studies (MACS) collaborative studies and Investigator Initiated Research (IIR). Specific objectives include:
Clinical Research Site Director. Our clinical research company is looking to hire a qualified candidate for the full-time position of Clinical Research Site Director. The ideal candidate will possess certification such as RNB, LPN, phlebotomy, or similar, prior clinical research experience, and have a strong desire to work directly with patients.
The typical day of a clinical research associate includes planning and managing clinical research projects for pharmaceutical companies. They may recruit participants, coordinate schedules, input data, and oversee trials. In their career, clinical researchers may also be in charge of ensuring that researchers follow all local and federal ...
Associate Director for Clinical Research. Apply now Work type: University Medical Line ... to any employee with a disability who requires accommodation to perform the essential functions of his or her job. Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for ...
Apply for Associate Director-Clinical Research Operations job with University of Virginia in Charlottesville, Virginia, United States of America. Research at University of Virginia
Explore Clinical Monitoring Jobs. R1418422 Senior Clinical Research Associate- Sponsor Dedicated Team- Home-based Learn more Multiple Locations. R1415288 Experienced Clinical Research Associate (m/w/d), Multi-Sponsor Learn more Multiple Locations. R1419786 CRA 2 Learn more Multiple Locations.
The Associate Director, Clinical Project Scientist provides input to the clinical development plan, works on the development of the clinical trial protocol, clinical trial materials, and takes responsibility for coordinating completion of clinical study reports and supports preparation of relevant documents for regulatory filings.
Apply for Associate Director, Clinical Development Science job with Thermo Fisher Scientific in Bleiswijk, Netherlands. Clinical Research jobs at Thermo Fisher Scientific
Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms. Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents.
Janssen Research and Development, L.L.C., a member of Johnson & Johnson's Family of Companies, is recruiting for a Medical Director/Senior Medical Director-Clinical Research-Prostate. They will be a member of a matrix team dedicated to the implementation of clinical research studies that are part of a global compound development program.
Select a job category from the list of options. Search for a location and select one from the list of suggestions. You can also search and select 'ALL' categories or locations. Finally, click "+" to create your job alert. By signing up, you acknowledge that you have read our privacy notice, and consent to receive email communication from ...
Director, Sponsored Programs and Research Poughkeepsie, NY The Director is responsible for working with faculty and deans on program needs and assisting in the identification of potential funding sources, coordination of the preparation, submission, and review of selected grant proposals, and providing internal communication to the academic ...
Andover Animal Hospital is seeking an Associate Veterinarian to join our team at the Big Red Barn! Andover Animal Hospital is a full-service veterinary medical and surgical facility founded in 1958. We are an AAHA-accredited hospital dedicated to the care of small animals. We have a large, modern facility at the Big Red Barn.
Today's top 1,000+ Associate Director Clinical Research jobs in United States. Leverage your professional network, and get hired. New Associate Director Clinical Research jobs added daily.
Today's top 160 Director Clinical Research jobs in Chicago, Illinois, United States. Leverage your professional network, and get hired. New Director Clinical Research jobs added daily.
2000. Seven years of successful work have shown that combining different types of activities in the same company is not always convenient. And in the year 2000 the founders of OOO Firm ERG decided to create and register a monoprofile construction company OOO Remstroy Construction Company. Industrial construction was chosen as the priority area.
Geographic coordinates of Elektrostal, Moscow Oblast, Russia in WGS 84 coordinate system which is a standard in cartography, geodesy, and navigation, including Global Positioning System (GPS). Latitude of Elektrostal, longitude of Elektrostal, elevation above sea level of Elektrostal.
March 17, 2011. Pavel Oderov was appointed as Head of the International Business Department pursuant to a Gazprom order. Pavel Oderov was born in June 1979 in the town of Elektrostal, Moscow Oblast. He graduated from Gubkin Russian State University of Oil and Gas with an Economics degree in 2000 and a Management degree in 2002.
The Judge Group. New York City Metropolitan Area. Actively Hiring. 1 week ago. Today's top 7,000+ Director Clinical Research jobs in United States. Leverage your professional network, and get ...