An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Recent Advances and Clinical Outcomes of Kidney Transplantation
Charat thongprayoon.
1 Division of Nephrology, Department of Medicine, Mayo Clinic, Rochester, MN 55905, USA; [email protected]
Panupong Hansrivijit
2 Department of Internal Medicine, University of Pittsburgh Medical Center Pinnacle, Harrisburg, PA 17105, USA; ude.cmpu@ptijivirsnah
Napat Leeaphorn
3 Department of Nephrology, Department of Medicine, Saint Luke’s Health System, Kansas City, MO 64111, USA; [email protected]
Prakrati Acharya
4 Division of Nephrology, Department of Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA; [email protected]
Aldo Torres-Ortiz
5 Department of Medicine, Ochsner Medical Center, New Orleans, LA 70121, USA; moc.liamtoh@68t_odlA
Wisit Kaewput
6 Department of Military and Community Medicine, Phramongkutklao College of Medicine, Bangkok 10400, Thailand; moc.liamg@orhpentisiw
Karthik Kovvuru
7 Division of Nephrology, Department of Medicine, University of Mississippi Medical Center, Jackson, MS 39216, USA; ude.cmu@uruvvokk (K.K.); ude.cmu@irudnaks (S.R.K.)
Swetha R. Kanduri
Tarun bathini.
8 Department of Internal Medicine, University of Arizona, Tucson, AZ 85724, USA; moc.liamg@bbocajnurat
Wisit Cheungpasitporn
Recent advances in surgical, immunosuppressive and monitoring protocols have led to the significant improvement of overall one-year kidney allograft outcomes. Nonetheless, there has not been a significant change in long-term kidney allograft outcomes. In fact, chronic and acute antibody-mediated rejection (ABMR) and non-immunological complications following kidney transplantation, including multiple incidences of primary kidney disease, as well as complications such as cardiovascular diseases, infections, and malignancy are the major factors that have contributed to the failure of kidney allografts. The use of molecular techniques to enhance histological diagnostics and noninvasive surveillance are what the latest studies in the field of clinical kidney transplant seem to mainly focus upon. Increasingly innovative approaches are being used to discover immunosuppressive methods to overcome critical sensitization, prevent the development of anti-human leukocyte antigen (HLA) antibodies, treat chronic active ABMR, and reduce non-immunological complications following kidney transplantation, such as the recurrence of primary kidney disease and other complications, such as cardiovascular diseases, infections, and malignancy. In the present era of utilizing electronic health records (EHRs), it is strongly believed that big data and artificial intelligence will reshape the research done on kidney transplantation in the near future. In addition, the utilization of telemedicine is increasing, providing benefits such as reaching out to kidney transplant patients in remote areas and helping to make scarce healthcare resources more accessible for kidney transplantation. In this article, we discuss the recent research developments in kidney transplants that may affect long-term allografts, as well as the survival of the patient. The latest developments in living kidney donation are also explored.
1. Introduction
Kidney transplantation is the optimal treatment for improving survival and quality of life for patients with end-stage kidney disease (ESKD) [ 1 ]. Advances in surgical, immunosuppressive and monitoring protocols have led to a significant improvement in overall one-year kidney allograft survival of >95% [ 2 ]. Nonetheless, there has not been a significant change in long-term kidney allograft outcomes. In fact, chronic and acute antibody-mediated rejection (ABMR) has continued to cause kidney allograft failures [ 3 ]. In addition, non-immunological complications following kidney transplantation, such as the recurrence of primary kidney disease and other complications, such as cardiovascular diseases, infections, and malignancy also play important roles in poor long-term allografts and patient survival [ 4 , 5 , 6 ].
In their research into immunologic monitoring and diagnostics in kidney transplants [ 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ], a number of groups have made attempts in the recent past towards determining the peripheral molecular fingerprints of ongoing rejection [ 7 , 8 ] and predicting acute rejection [ 7 ]. Contemporary researchers have measured the levels of donor-derived cell-free DNA (dd-cfDNA) and showed higher predictive abilities for acute rejection [ 9 , 10 , 11 , 12 ], especially antibody-mediated rejection (ABMR) diagnostics in cases with a combination of donor specific antibodies (DSA) and dd-cfDNA [ 13 , 14 ]. In addition, a molecular microscope diagnostic system for the evaluation of allograft biopsies has been recently introduced within transplant practice, particularly in complex cases. This has mainly been introduced for the purpose of enhancing histological diagnostics [ 15 ].
Recent studies have been conducted aimed at preventing or treating ABMR [ 16 , 17 ]. In 2017, imlifidase (IdeS), an endopeptidase derived from Streptococcus pyogenes, was utilized in a desensitization regimen in an open-label phase 1–2 trial [ 16 ]. An instant impact was observed by a significant decline in plasma IgG levels. Another single-center phase 2 study that focused mainly on the pharmacokinetics, effectiveness and safety of IdeS treatment was conducted and proved a reduction in anti-human leukocyte antigen (HLA) antibodies using a complement-dependent cytotoxicity test [ 17 ].
In recent years, there has been significant progress in research into kidney transplantation and kidney donation [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 ], including articles [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ] published in our current Special Issue "Recent Advances and Clinical Outcomes of Kidney Transplantation" ( https://www.mdpi.com/journal/jcm/special_issues/outcomes_kidney_transplantation ).
In this article, we discuss the recent research developments in kidney transplantation that may impact long-term allografts and patient survival, as well as the latest developments in living kidney donation.
2. Non-HLA Antibodies in Transplantation
When it comes to solid organ transplantation, one major immunological obstacle is the detection the non-self structures that exist in the donor cells. Human leukocyte antigens (HLA) are considered the most important non-self allo-antigens in organ transplantation. In addition, patients can form antibodies against targets other than HLA [ 85 ]. Multiple targets for these non-HLA antibodies have been studied in kidney transplantation over the last decade ( Figure 1 ). Recent studies have provided findings that suggest the an importance of non-HLA mismatches between donors and recipients in the development of acute rejection and long-term kidney allograft outcomes [ 68 , 78 , 86 , 87 , 88 , 89 , 90 , 91 , 92 ].

Post-transplant antibodies against human leukocyte antigen (HLA) and non-HLA antigens [ 68 , 78 , 86 , 87 , 88 , 89 , 90 , 91 , 92 ]. Abbreviations: human leukocyte antigen (HLA), major histocompatibility complex class I related chain A antigen (MICA); angiotensin type 1 receptor (AT1R); endothelin-1 type A receptor (Anti-ETAR); FMS-like tyrosine kinase 3 (FLT3); Epidermal growth factor-like repeats and discoidin I-like domain 3 (EDIL3); Intercellular adhesion molecule 4 (ICAM4).
3. Active AMR
Chronic active ABMR is one of the major causes of long-term allograft loss [ 93 , 94 , 95 ]. Tocilizumab, a humanized monoclonal antibody targeting the interleukin (IL)-6 receptor, has been assessed in patients with acute and chronic active ABMR [ 96 , 97 , 98 ], given that IL-6 mediates various inflammatory and immunomodulatory pathways, including the expansion and activation of T cells and B cells [ 98 ]. Furthermore, there is a genetically engineered humanized Immunoglobulin (Ig)G1 monoclonal antibody that binds to IL-6, inhibiting its interaction with IL-6R. Direct inactivation of IL-6 may limit a rebound induced by the accumulation of IL-6 [ 99 , 100 ]. Preliminary investigations from phase 1–2 trials demonstrated the efficacy of the C1q inhibitor for the prevention of a delayed graft function (DGF) and to lessen the occurrence of chronic active ABMR [ 101 , 102 ]. Although the inhibition of the first step in both the classical and lectin pathways of complement activation may serve as another tool to overcome critical sensitization, such data need to be validated in larger cohorts. Several trials are currently being conducted, and new developments will conceivably provide us with practical ways to counteract the deleterious consequences of ABMR [ 103 ].
4. Cardiovascular Diseases in Kidney Transplant Recipients
The burden of cardiovascular diseases on ESKD is improved after kidney transplantation [ 104 ]. However, it remains the leading cause of reduced early renal graft loss and mortality, as it is associated with significant morbidity and healthcare costs [ 104 ]. Major phenotypes of cardiovascular diseases among kidney transplant recipients include ischemic heart disease, congestive heart failure, valvular heart disease, arrhythmias and pulmonary hypertension ( Figure 2 ).

Incidence (%) of cardiovascular disease in kidney transplant recipients.
Reported risk factors for cardiovascular disease in kidney transplant recipients include inflammatory and immunosuppressive agents, episodes of allograft rejection, as well as traditional cardiovascular risk factors, such as hypertension, hyperlipidemia, smoking, obesity, chronic kidney disease, proteinuria, and diabetes mellitus, all of which add to a transplant recipient’s cardiovascular risk profile [ 104 ]. Hypertension is common among kidney transplant recipients and uncontrolled hypertension in kidney transplant recipients is associated with increased cardiovascular mortality and morbidity, and reduced allograft survival [ 105 ]. Furthermore, weight gain is also a significant problem in post-kidney transplant patients. Weight gain after transplantation can unfavorably affect patient outcomes [ 106 ]. Identifying these risk factors and adopting strategies to abolish these risk factors may potentially prevent, and help manage, post-transplant obesity. The underlying mechanisms for the increased occurrence of dyslipidemia post-transplant are due to immunosuppressive medications, proteinuria, and post-transplant diabetes [ 107 , 108 ].
The medical management of risk factors includes strategies employed in the chronic kidney disease (CKD) population, with credence given to approaches specific for kidney transplant recipients, such as the choice of maintenance immunosuppression, steroid tapering or withdrawal, and particular anti-hypertensive regimens ( Table 1 ). Overall, cardiovascular morbidity and mortality in kidney transplant recipients has decreased over the last few decades, likely due to improved detection and the timely management of risk factors. Recognition of these complications is important in assessing cardiovascular disease risk in kidney transplant recipients, and optimizing screening and therapeutic approaches. These include lifestyle and immunosuppressive regimen modification, as well as the best feasible regimen for glycemic and lipid controls according to an individual’s metabolic profile and medical history.
Cardiovascular risk factors among kidney transplant recipients and suggested management.
American College of Cardiology (ACC); angiotensin-converting enzyme inhibitor (ACEI); American Heart Association (AHA); angiotensin-II receptor blocker (ARB); aspirin (ASA); body mass index (BMI); blood pressure (BP); complete blood count (CBC); calcium-channel blockers (CCB); chronic kidney disease (CKD); cardiovascular disease (CVD); diabetes mellitus (DM); electrocardiography (ECG); estimated glomerular filtration rate (eGFR); Kidney Diseases Improving Global Outcomes (KDIGO); kidney transplant (KTx).
5. Preexisting Diabetes and Post-Transplantation Diabetes
Preexisting diabetes and post-transplantation diabetes confer reduced patient and graft survival in kidney transplant recipients [ 71 , 73 , 125 ]. Hyperglycemia is present in nearly 90% of kidney transplant recipients in the immediate postoperative period, but it is not sustained in the majority [ 126 ]. In addition to the general risk factors for diabetes, there are also certain transplantation-related factors (e.g., specific immunosuppressive agents, surgical stress and inflammation, nutritional interventions) placing kidney transplant recipients at elevated risk of hyperglycemia [ 126 ]. Some transplant immunosuppressive medications, including corticosteroids, calcineurin Inhibitors (CNIs), and mammalian target of rapamycin (mTOR) inhibitors, are associated with a higher incidence of metabolic complications such as post-transplantation diabetes. CNIs impair insulin secretion and sensitivity and directly damage pancreatic islet cells [ 127 ].
A robust evidence base guiding precise glycemic goals is currently lacking in kidney transplant recipients. Management is largely guided by evidence from the general diabetes population [ 71 , 73 , 125 ]. Hospital management of hyperglycemia is primarily achieved through an insulin regimen that takes into account rapid changes in glucocorticoid doses, nutritional modalities and renal function during the immediate post-transplantation period. There is an opportunity to use oral or non-insulin injectable agents in a considerable number of patients by the time they are discharged from the hospital, or in the long run. The use of specific oral or non-insulin injectable agents is guided by patient specifics and the pharmacologic properties of medications. Although several studies have suggested the safe use of sodium glucose transport 2 (SGLT2) inhibitors in kidney transplant recipients [ 128 ], future studies assessing their efficacy and safety are needed, since SGLT2 inhibitor treatment also carries an increased risk of genital tract infections and, possibly, of urinary tract infections [ 129 ]; kidney transplant recipients are particularly susceptible to infections due to immunosuppressive regimens.
6. Posttransplant Malignancy
Cancer is one of the three major causes of death after kidney transplantation [ 130 , 131 ]. Posttransplant malignancy occurrence is widely recognized ( Table 2 ). The effect of viral infections, induction and immunosuppressive maintenance regimens have been proposed as important risk factors for posttransplant malignancy. The increased risk of cancer may be due to viral reactivation induced by immunosuppressive agents or impaired immune surveillance leading to faster tumor growth [ 132 ]. A higher degree of immunosuppression is associated with an increased risk of malignancy, and calcineurin inhibitors can promote carcinogenesis [ 132 ].
Standardized incidence ratio of cancers in kidney transplant recipients [ 133 ].
Confidence Interval (CI).
7. Infection
Solid organ transplant recipients are at greater risk of infection than the non-immunosuppressed population ( Table 3 ) [ 134 ]. Infections are the most common non-cardiovascular causes of mortality following kidney transplantation, accounting for 15%–20% of mortality [ 131 , 135 ]. The first six months post-transplant is the time of greatest infection risk. There are also times when patients encounter adverse reactions to immunosuppressive agents [ 136 , 137 ]. Among all infectious complications, viruses are considered to be the most common agents [ 138 ]. Herpes simplex virus, varicella zoster virus, BK polyomavirus, cytomegalovirus, Epstein–Barr virus, hepatitis B virus, and adenovirus are well-known etiologic agents of viral infections in kidney transplant patients worldwide [ 138 ]. In order to prevent opportunistic infections in kidney transplant recipients, antimicrobial prophylaxis is recommended after kidney transplantation. The recommended prophylactic method after transplant differs based on the organism, as well as individual patient characteristics.
Infection post kidney transplantation.
* Center-dependent multidrug resistant bacteria like Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), extended-spectrum beta-lactamases (ESBLs); ** With prophylaxis – with Bactrim and Gancyclovir/Valganciclovir; Abbreviations: cytomegalovirus (CMV), lymphocytic choriomeningitis virus (LCM), Epstein-Barr Virus (EBV), hepatitis B virus (HBV), hepatitis C virus (HCV), Trypanosoma cruzi (T. cruzi), Varicella Zoster virus (VZV), human herpes virus 8 (HHV-8).
8. Latest Developments in Living Kidney Donation
Living donor kidney transplants are the best option for many patients with ESKD for several reasons, including (1) better long-term graft survival, (2) no need to wait on the transplant waiting list for a kidney from a deceased donor, (3) transplant surgery can be planned and (4) lower risks of rejection and DGF [ 139 ]. Living donor kidney transplantation is the optimal treatment for patients with ESKD [ 139 ]. The expansion of living donor programs was made possible by new modes of living donation and by the extension of the living donor pool [ 139 ].
To expand the donor pool, a well-developed paired kidney donation program and the adequate reimbursement of costs associated with donation are fundamental elements [ 140 ]. Paired kidney donation provides living kidney donation for noncompatible donor/recipient pairs that otherwise would not be feasible or need desensitization [ 141 ]. Other possible approaches for increasing the donor pool include ABO-incompatible transplantation [ 142 ], the utilization of higher risk donors, advanced donation with a voucher system, and providing donors with financial incentives [ 141 , 143 , 144 ].
Over the past decade, the long-term risks of kidney donation have been described. Living donors seem to have a higher risk of ESKD, particularly in obese donors and also for African American donors with an apolipoprotein L1 (APOL1) high-risk genotype. In African American living kidney donors, those with the APOL1 high-risk genotype (prevalent in about 13% of African Americans in the United States) had an almost three times more accelerated decline in estimated glomerular filtration rate (eGFR) after adjusting for pre-donation eGFR than those with a low-risk genotype [ 145 ].
9. Post-Transplant Hyperparathyroidism and Bone Disease
Successful renal transplantation results in a reduction in parathyroid hormone (PTH), especially during the first 3 months after transplantation [ 146 ]. However, elevated PTH levels can still be found in 30% to 60% of patients 1 year after transplantation. Persistent hyperparathyroidism following kidney transplantation can result in notable complications, such as fracture/bone diseases, cardiovascular disease, vascular calcification, and allograft dysfunction ( Figure 3 ). Associated factors for persistent hyperparathyroidism are long dialysis duration, high PTH levels prior to transplantation, lower eGFR post-transplant, post-transplant hypercalcemia, and post-transplant high alkaline phosphatase.

Effects and risk factors of post-transplant hyperparathyroidism.
10. Potential Directions and Future Scope
Researchers need to instantly shift their focus on the unaddressed concerns with respect to kidney transplants. Because of the limited supply of organs, numerous potential recipients still have to spend more time in dialysis, waiting for a transplant. Sensitization to HLA antigens inhibits the recipients’ access to transplants, compromising the survival of the graft due to chronic and acute AMR. The publication of complete data from a multi-center second-phase test that explores how IdeS is useful in desensitization is underway ( {"type":"clinical-trial","attrs":{"text":"NCT02790437","term_id":"NCT02790437"}} NCT02790437 ). The phase 3 trial, uncovering the impact of clazakizumab following transplantation, was launched recently, with the outcomes of the phase 2 trial to be released soon.
Moreover, the lack of experienced and skilled professionals could hinder the diagnostic correctness of complications following transplantation. Furthermore, medication non-adherence among patients could increase the alloimune reaction. Notably, medical research on the costimulation blockade during kidney transplantation is underway. A randomized sixty-month multi-center study (CIRRUS, {"type":"clinical-trial","attrs":{"text":"NCT03663335","term_id":"NCT03663335"}} NCT03663335 ) in kidney transplant is also underway, with the aim of defining the range of dosage and assessing the tolerability, safety, and effectiveness of some newly developed anti-CD40 monoclonal antibodies in two distinct cohorts in comparison to a tacrolimus-based regimen. Recently, a phase 2a clinical trial, with the purpose of assessing how effective the dual costimulation blockade with anti-CD40 (VIB4920) is when combined with belatacept in kidney transplantation patients ( {"type":"clinical-trial","attrs":{"text":"NCT04046549","term_id":"NCT04046549"}} NCT04046549 ), was registered.
Big data is increasingly being utilized, with the establishment of a large collection of cohorts and the usage of electronic health records (EHRs) in kidney transplantation and artificial intelligence, which might be useful in solving problems related to the survival analysis of patients who have gone through kidney transplantation [ 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 ]. In the present era, it is strongly believed that big data and artificial intelligence will greatly reshape the research done on kidney disease and, consequently, improve the general clinical practice of nephrology [ 156 ].
The benefits of telemedicine include reaching out to patients in remote areas and helping to make scarce healthcare resources more accessible. As telemedicine applications continue to proliferate, studies have demonstrated that telehealth for transplant care may be associated with a reduction in cost and time, and may also improve access to transplantation for ESKD patients [ 157 , 158 ].
11. Conclusions
The most recent endeavors in kidney transplantation tend to mainly focus on noninvasive monitoring, as well as the improvement of histological diagnostics with the aid of molecular techniques. Such studies offer creative means that can be used to find immunosuppressive agents, which can effectively overcome critical sensitization, prevent the creation of anti-HLA antibodies, treat chronic active ABMR, and reduce non-immunological complications following kidney transplantation, such as the recurrence of primary kidney disease and other complications, such as cardiovascular diseases, infections, and malignancy. In the present era of utilizing EHRs, it is strongly believed that big data and artificial intelligence will reshape the research done on kidney transplantation in the near future. In addition, the utilization of telemedicine is increasing, providing benefits such as reaching out to kidney transplant patients in remote areas and helping to make scarce healthcare resources more accessible for kidney transplantation.
This research received no external funding.
Conflicts of Interest
We do not have any potential financial or non-financial conflicts of interest.
- Search Menu
- Advance Articles
- Editor's Choice
- Cover Archive
- Author videos
- Supplements
- Cover Images
- Author Guidelines
- Submission Site
- Open Access Options
- Why publish with NDT?
- About the ERA
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Terms and Conditions
- Editorial Fellowship
- The ERA Journals
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Conflict of interest statement, 2020 landmark papers in transplantation published in ndt : clinical research highlights in the area of kidney transplantation.
- Article contents
- Figures & tables
- Supplementary Data
Rainer Oberbauer, 2020 landmark papers in transplantation published in NDT : clinical research highlights in the area of kidney transplantation, Nephrology Dialysis Transplantation , Volume 36, Issue 4, April 2021, Pages 569–571, https://doi.org/10.1093/ndt/gfab011
- Permissions Icon Permissions
NDT published 32 original research papers in the field of kidney transplantation in the year 2020. My selection of only eight featured transplant papers is owed to the restricted word count of editorials and is clearly subjective with a focus on findings with potential clinical impact. All other transplant papers in Volume 35 of NDT are also highly recommended and acknowledged.
In the January 2020 issue of NDT , the research group from the Westmead Hospital and University of Sydney investigated a potential obesity and gender bias in access to deceased donor kidney transplantation [ 1 ]. This is an important clinical issue and all colleagues involved in selection of transplant candidates should be reminded that we are prone to unconscious biases based on maybe not so objective mental priors. The investigators used the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) to determine a potential association of obesity and waitlisting and effect modification by gender. Of the 12 000 patients included, 4400 (38%) were obese. A total of 80% (3515) of the obese patients were waitlisted and 1662 (47% of obese waitlisted subjects) subsequently transplanted between 2007 and 2014. The main finding was that obesity was associated not only with a reduced chance of waitlisting for a deceased donor kidney [adjusted hazard ratio (aHR) 0.66, 95% confidence interval (CI) 0.58–0.76] but also with a lower chance of receiving a transplant while waitlisted (aHR 1.10, 95% CI 0.97–1.24). The effect was modified by gender. Obese women were 34% less likely to be waitlisted than non-obese women. This rate was only 14% in males. The authors’ conclusion of this important paper is that we need to be aware of this inequality and sensitive enough to overcome these biases.
Once the candidate has been waitlisted for a deceased donor kidney, thoughts on potential acceptance of older or marginal donor organs are required and discussed with the prospective recipient. The trade-off between waiting time on dialysis and acceptance of older/marginal quality organs especially for younger candidates with longer life expectancy needs support from scientific data. Researchers from the ERA-EDTA Registry specifically studied that enigma by merging the ERA-EDTA Registry with nine national databases [ 2 ]. The investigators selected almost 6500 kidneys of deceased donors aged between 20 and 70 years that were transplanted in recipients between 20 and 50 years of age. Donor age was dichotomized at 50 years and recipient age at 35 years. Not unexpectedly, the main finding was that the 10-year cumulative incidence of graft loss was lowest in recipients of donor kidneys below 50 years of age (roughly 20%) compared with 30% and 37% in recipients below and above 35 years of age, respectively, for donor organs above 50 years of age. Based on these findings, the authors concluded that future studies will determine whether this observation will remain valid in recently transplanted patients.
At the other end of the age spectrum, i.e. waitlisted candidates older than 75 years offered deceased donor kidneys >75 years old, the trade-off is similar. A decision has to be made on whether to accept a marginal/old organ or to remain on dialysis. This intrinsic question was addressed by colleagues from Madrid [ 3 ]. The authors identified 138 such transplants performed between 2002 and 2015 in their centre. Univariable analysis showed 5-year survival rates of 60%, with infection as the leading cause of death. Death censored graft survival was 93% at 5 years. Given the reported primary non-function of 8%, this may imply that many of these patients died subsequently. The main conclusion was that patients above 75 years of age can be transplanted with older organs. Even if a likely selection bias is taken into consideration, the unfortunate lack of a dialysis control group weakens the conclusion. Nevertheless, the paper is important because the highest relative increase in age categories waitlisted for transplantation is above 70 years in most European centres.
Once the transplant is successfully performed, it is time to refocus on cardiovascular comorbidities as main reason for death with a functioning graft in the majority of patients. In the February 2020 issue of NDT , Sotomayor and colleagues from Groningen studied the association between nutrition, specifically fruit and vegetable consumption, and overall and cardiovascular mortality [ 4 ]. The difficulty in performing observational studies on food constituents and clinical outcomes is the high risk of unaccounted confounding. Furthermore, food consists of a large number of ingredients, which makes it even more complex to dissociate. The modified Alternative Healthy Eating Index (mAHEI) was created by McCullough as a standard tool to assess such associations unambiguously [ 5 ]. Sotomayor and colleagues focused on fruits and vegetable consumption in 400 renal allograft recipients and found beneficial associations with cardiovascular mortality (about 50 events in this study) for both items. This is very encouraging and supports our healthy lifestyle recommendations, but at the same time it is difficult to draw any causal inference from such findings. Especially in nutritional sciences in patients of industrialized countries, often the main finding is that ‘less is more’ of benefit.
Exercise and cardiovascular events are major players in all-cause mortality [ 6 ]. As with nutrition, randomization is not an option to address this question and thus this post hoc study of a randomized controlled trial on folic acid supplementation is a well-thought-out design. The investigators categorized about 3000 participants of the FAVORIT (Folic Acid for Vascular Outcome Reduction In Transplantation) study (NIH Clinical Trial Number U01DK061700) into three tertiles according to their self-reported physical activity over a median of 3.7 years. The top tertile with most exercise rigor exhibited a lower risk for cardiovascular events and mortality as well as overall mortality. As acknowledged by the authors, the remaining risk of unmeasured confounding and reverse causation is an intrinsic problem in observational studies [ 7 ].
By presenting the interesting but somehow sobering findings from the study of Deak and colleagues we remain in the area of cardiovascular risk assessment. Deak and colleagues from Austria investigated whether the many existing and continuously updated guidelines of different prominent bodies on cardiovascular risk assessment [ 8 ] and potentially therapy led to a better risk assessment and reduced events post-transplant in the clinic [ 9 ]. Although the updated guidelines on cardiovascular risk stratification between the years 2003 and 2015 (split into three eras) were followed and more workup was done pretransplant, the incidence of cardiovascular events remained unchanged over time. This somehow fits to the findings from the ISCHEMIA-CKD trial, which showed that a percutaneous intervention in stable patients does not lead to prolonged survival compared with conservative therapy [ 10 ]. The certainly correct conclusion of the authors states that after one and a half decades of unchanged policy it may be time to reconsider our screening strategy for waitlisting.
Accordingly, a meta-analysis on comparative effectiveness of antihypertensive drugs in kidney transplant patients was published in the May issue by epidemiologists from Reggio Calabria in collaboration with colleagues from other ERA-EDTA member countries [ 11 ]. The authors searched the four main life-sciences publication databases for studies on antihypertensive drug classes in renal allograft recipients. Without going too much into detail, calcium channel blocker was found to be associated with a reduced risk of graft loss by almost 40% in the 26 identified (old) trials. Accordingly, the 13 trials on angiotensin-converting enzyme inhibitors showed a similar ‘protection’. Interestingly, angiotensin receptor blocker did not modify the association with graft loss or death. Therefore, the authors concluded that calcium channel blocker could be the first drug class of choice in these patients. One caveat, however, is that the studies on calcium channels blockers were older and of lesser quality than the more recent studies on renin–angiotensin blocker. This fact was also specifically addressed by the authors.
Finally, a selection of 2020 papers would not be complete without a coronavirus disease 2019 (COVID-19) review. The DESCARTES (Developing Education Science and Care for Renal Transplantation in European States) study group of the ERA-EDTA nicely summarized the evidence available until May 2020 in the prognosis, management and treatment of kidney transplant recipients with COVID-19 [ 12 ]. Given the fact that no established therapy was available until fall of 2020 when the first monoclonal antibody combinations against the early corona viremia in patients with respiratory symptoms were applied to prominent patients such as the president of the USA (REGEN-COV2). The availability of the first doses of mRNA vaccines at the end of 2020 must be considered as a strong statement of the strength of biomedical academic research in cooperation with big pharma. The authorization of the mRNA vaccines from Pfizer/Biontech and Moderna was the fastest approval of drugs in the history of the European Medicines Agency and the US Food and Drug Administration. This is a very good sign, and shows that a pandemic brings out the best in each field, and that cooperation can be successfully accomplished even under these harsh conditions. This example and the global availability of a cheap vaccine will save millions of lives, and may serve as bolster for all those who are skeptical of fundamental discoveries in the live sciences field.
This work was in part supported by the Vienna Science and Technology Fund (WWTF grant #LS18-031 to R.O.).
None declared.
Ladhani M , Craig JC , Wong G. Obesity and gender-biased access to deceased donor kidney transplantation . Nephrol Dial Transplant 2020 ; 35 : 184 – 189
Google Scholar
Pippias M , Jager KJ , Asberg A et al. Young deceased donor kidneys show a survival benefit over older donor kidneys in transplant recipients aged 20–50 years: a study by the ERA-EDTA Registry . Nephrol Dial Transplant 2020 ; 35 : 534 – 543
Cabrera J , Fernandez-Ruiz M , Trujillo H et al. Kidney transplantation in the extremely elderly from extremely aged deceased donors: a kidney for each age . Nephrol Dial Transplant 2020 ; 35 : 687 – 696
Sotomayor CG , Gomes-Neto AW , Eisenga MF et al. Consumption of fruits and vegetables and cardiovascular mortality in renal transplant recipients: a prospective cohort study . Nephrol Dial Transplant 2020 ; 35 : 357 – 365
McCullough ML , Feskanich D , Stampfer MJ et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance . Am J Clin Nutr 2002 ; 76 : 1261 – 1271
Kang AW , Bostom AG , Kim H et al. Physical activity and risk of cardiovascular events and all-cause mortality among kidney transplant recipients . Nephrol Dial Transplant 2020 ; 35 : 1436 – 1443
Dunkler D , Kohl M , Heinze G et al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus . Kidney Int 2015 ; 87 : 784 – 791
Rangaswami J , Mathew RO , Parasuraman R et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies . Nephrol Dial Transplant 2019 ; 34 : 760 – 773
Deak AT , Ionita F , Kirsch AH et al. Impact of cardiovascular risk stratification strategies in kidney transplantation over time . Nephrol Dial Transplant 2020 ; 35 : 1810 – 1818
Spertus JA , Jones PG , Maron DJ et al. Health status after invasive or conservative care in coronary and advanced kidney disease . N Engl J Med 2020 ; 382 : 1619 – 1628
Pisano A , Bolignano D , Mallamaci F et al. Comparative effectiveness of different antihypertensive agents in kidney transplantation: a systematic review and meta-analysis . Nephrol Dial Transplant 2020 ; 35 : 878 – 887
Maggiore U , Abramowicz D , Crespo M et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion . Nephrol Dial Transplant 2020 ; 35 : 899 – 904
- renal transplantation
- transplantation
- clinical research
Email alerts
Citing articles via.
- ndt Twitter
- ERA Twitter
- ERA Facebook
- ERA Instagram
- ERA LinkedIn
- Recommend to Your Librarian
Affiliations

- Online ISSN 1460-2385
- Print ISSN 0931-0509
- Copyright © 2024 European Renal Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 30 July 2020
The current and future landscape of dialysis
- Jonathan Himmelfarb ORCID: orcid.org/0000-0002-3319-1224 1 , 2 ,
- Raymond Vanholder ORCID: orcid.org/0000-0003-2633-1636 3 ,
- Rajnish Mehrotra ORCID: orcid.org/0000-0003-2833-067X 1 , 2 &
- Marcello Tonelli ORCID: orcid.org/0000-0002-0846-3187 4
Nature Reviews Nephrology volume 16 , pages 573–585 ( 2020 ) Cite this article
86k Accesses
259 Citations
130 Altmetric
Metrics details
- Haemodialysis
- Health care economics
- Health services
- Medical ethics
The development of dialysis by early pioneers such as Willem Kolff and Belding Scribner set in motion several dramatic changes in the epidemiology, economics and ethical frameworks for the treatment of kidney failure. However, despite a rapid expansion in the provision of dialysis — particularly haemodialysis and most notably in high-income countries (HICs) — the rate of true patient-centred innovation has slowed. Current trends are particularly concerning from a global perspective: current costs are not sustainable, even for HICs, and globally, most people who develop kidney failure forego treatment, resulting in millions of deaths every year. Thus, there is an urgent need to develop new approaches and dialysis modalities that are cost-effective, accessible and offer improved patient outcomes. Nephrology researchers are increasingly engaging with patients to determine their priorities for meaningful outcomes that should be used to measure progress. The overarching message from this engagement is that while patients value longevity, reducing symptom burden and achieving maximal functional and social rehabilitation are prioritized more highly. In response, patients, payors, regulators and health-care systems are increasingly demanding improved value, which can only come about through true patient-centred innovation that supports high-quality, high-value care. Substantial efforts are now underway to support requisite transformative changes. These efforts need to be catalysed, promoted and fostered through international collaboration and harmonization.
The global dialysis population is growing rapidly, especially in low-income and middle-income countries; however, worldwide, a substantial number of people lack access to kidney replacement therapy, and millions of people die of kidney failure each year, often without supportive care.
The costs of dialysis care are high and will likely continue to rise as a result of increased life expectancy and improved therapies for causes of kidney failure such as diabetes mellitus and cardiovascular disease.
Patients on dialysis continue to bear a high burden of disease, shortened life expectancy and report a high symptom burden and a low health-related quality of life.
Patient-focused research has identified fatigue, insomnia, cramps, depression, anxiety and frustration as key symptoms contributing to unsatisfactory outcomes for patients on dialysis.
Initiatives to transform dialysis outcomes for patients require both top-down efforts (that is, efforts that promote incentives based on systems level policy, regulations, macroeconomic and organizational changes) and bottom-up efforts (that is, patient-led and patient-centred advocacy efforts as well as efforts led by individual teams of innovators).
Patients, payors, regulators and health-care systems increasingly demand improved value in dialysis care, which can only come about through true patient-centred innovation that supports high-quality, high-value care.
Similar content being viewed by others
Epidemiology of haemodialysis outcomes

Epidemiology of peritoneal dialysis outcomes
Kidney disease trials for the 21st century: innovations in design and conduct
Introduction.
Haemodialysis as a treatment for irreversible kidney failure arose from the pioneering efforts of Willem Kolff and Belding Scribner, who together received the 2002 Albert Lasker Clinical Medical Research Award for this accomplishment. Kolff treated his first patient with an artificial kidney in 1943 — a young woman who was dialysed 12 times successfully but ultimately died because of vascular access failure. By 1945, Kolff had dialysed 15 more patients who did not survive, when Sofia Schafstadt — a 67-year-old woman who had developed acute kidney injury — recovered, becoming the first long-term survivor after receipt of dialysis. In 1960, Belding Scribner, Wayne Quinton and colleagues at the University of Washington, WA, USA, designed shunted cannulas, which prevented the destruction of blood vessels and enabled repeated haemodialysis sessions. The first patient who received long-term treatment (named Clyde Shields) lived a further 11 years on haemodialysis. In their writings, both Kolff and Scribner eloquently described being motivated by their perception of helplessness as physicians who had little to offer for the care of young patients who were dying of uraemia and stated that the goal of dialysis was to achieve full rehabilitation to an enjoyable life 1 .
The potential to scale the use of dialysis to treat large numbers of patients with kidney failure created great excitement. At the 1960 meeting of the American Society for Artificial Internal Organs (ASAIO), Scribner introduced Clyde Shields to physicians interested in dialysis, and Quinton demonstrated fabrication of the shunt. The following decade saw rapid gains in our understanding of kidney failure, including the discovery of uraemia-associated atherogenesis and metabolic bone disease, and in virtually every aspect of haemodialysis, including improvements in dialyser technology, dialysate composition, materials for haemocompatibility and water purification systems. The Scribner–Quinton shunt rapidly became an historical artefact once Brescia and colleagues developed the endogenous arteriovenous fistula in 1966 (ref. 2 ), and prosthetic subcutaneous interpositional ‘bridge’ grafts were developed shortly thereafter. Concomitant with these pioneering efforts, in 1959, peritoneal dialysis (PD) was first used successfully to sustain life for 6 months. Within 2 years a long-term PD programme was established in Seattle, WA, USA, and within 3 years the first automated PD cycler was developed 3 .
In 1964, Scribner’s presidential address to the ASAIO described emerging ethical issues related to dialysis, including considerations for patient selection, patient self-termination of treatment as a form of suicide, approaches to ensure death with dignity and selection criteria for transplantation 4 . Indeed, the process of selecting who would receive dialysis contributed to the emergence of the field of bioethics. The early success of dialysis paradoxically created social tensions, as access to this life-sustaining therapy was rationed by its availability and the ‘suitability’ of patients. In the early 1970s, haemodialysis remained a highly specialized therapy, available to ~10,000 individuals, almost exclusively in North America and Europe, with a high frequency of patients on home haemodialysis. In a portentous moment, Shep Glazer, an unemployed salesman, was dialysed in a live demonstration in front of the US Congress House Ways and Means Committee. Soon thereafter, in October 1972, an amendment to the Social Security Act creating Medicare entitlement for end-stage renal disease (now known as kidney failure), for both dialysis and kidney transplantation, was passed by Congress and signed into law by President Nixon.
The resulting expansion of dialysis, previously described as “from miracle to mainstream” 5 , set in motion dramatic changes 6 , including the development of a for-profit outpatient dialysis provider industry; relaxation of stringent patient selection for dialysis eligibility in most HICs; a move away from home towards in-centre dialysis; efforts on the part of single payors such as Medicare in the USA to restrain per-patient costs through the introduction of bundled payments and the setting of composite rates; the development of quality indicators — such as adequate urea clearance per treatment — that were readily achievable but are primarily process rather than outcome measures; consolidation of the dialysis industry, particularly in the USA owing to economies of scale, eventually resulting in a duopoly of dialysis providers; the development of joint ventures and other forms of partnerships between dialysis providers and nephrologists; the globalization of dialysis, which is now available, albeit not necessarily accessible or affordable in many low-income and middle-income countries (LMICs); and finally, a dramatic slowing in the rate of true patient-centred innovation, with incremental gains in dialysis safety and efficiency replacing the pioneering spirit of the early innovators.
The population of patients receiving dialysis continues to grow rapidly, especially in LMICs, as a result of an increase in the availability of dialysis, population ageing, increased prevalence of hypertension and diabetes mellitus, and toxic environmental exposures. However, despite the global expansion of dialysis, notable regional differences exist in the prevalence of different dialysis modalities and in its accessibility. Worldwide, a substantial number of people do not have access to kidney replacement therapy (KRT), resulting in millions of deaths from kidney failure each year. Among populations with access to dialysis, mortality remains high and outcomes suboptimal, with high rates of comorbidities and poor health-related quality of life. These shortcomings highlight the urgent need for innovations in the dialysis space to increase accessibility and improve outcomes, with a focus on those that are a priority to patients. This Review describes the current landscape of dialysis therapy from an epidemiological, economic, ethical and patient-centred framework, and provides examples of initiatives that are aimed at stimulating innovations in dialysis and transform the field to one that supports high-quality, high-value care.
Epidemiology of dialysis
Kidney failure is defined by a glomerular filtration rate <15 ml/min/1.73 m 2 (ref. 7 ) and may be treated using KRT (which refers to either dialysis or transplantation) or with supportive care 8 . The global prevalence of kidney failure is uncertain, but was estimated to be 0.07%, or approximately 5.3 million people in 2017 (ref. 9 ), with other estimates ranging as high as 9.7 million. Worldwide, millions of people die of kidney failure each year owing to a lack of access to KRT 10 , often without supportive care. Haemodialysis is costly, and current recommendations therefore suggest that haemodialysis should be the lowest priority for LMICs seeking to establish kidney care programmes. Rather, these programmes should prioritize other approaches, including treatments to prevent or delay kidney failure, conservative care, living donor kidney transplantation and PD 11 . Nonetheless, haemodialysis is the most commonly offered form of KRT in LMICs, as well as in high-income countries (HICs) 12 , and continued increases in the uptake of haemodialysis are expected worldwide in the coming decades. Here, we review the basic epidemiology of kidney failure treated with long-term dialysis and discuss some of the key epidemiological challenges of the future (Fig. 1a ).
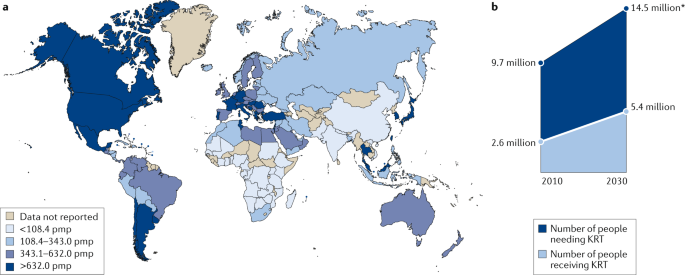
Growth is continuously outpacing the capacity of kidney replacement therapy (KRT), defined as maintenance dialysis or kidney transplant, especially in low-income and middle-income countries. a | Global prevalence of chronic dialysis. b | Estimated worldwide need and projected capacity for KRT by 2030. pmp, per million population. Adapted with permission from the ISN Global Kidney Health Atlas 2019.
Prevalence of dialysis use
Prevalence of haemodialysis.
Worldwide, approximately 89% of patients on dialysis receive haemodialysis; the majority (>90%) of patients on haemodialysis live in HICs or the so-called upper middle-income countries such as Brazil and South Africa 12 , 13 . The apparent prevalence of long-term dialysis varies widely by region but correlates strongly with national income 14 . This variation in prevalence in part reflects true differences in dialysis use 12 , 15 but also reflects the fact that wealthier countries are more likely than lower income countries to have comprehensive dialysis registries. Of note, the prevalence of haemodialysis is increasing more rapidly in Latin America (at a rate of ~4% per year) than in Europe or the USA (both ~2% per year), although considerable variation between territories exists in all three of these regions, which again correlates primarily (but not exclusively) with wealth 16 , 17 . The prevalence of haemodialysis varies widely across South Asia, with relatively high prevalence (and rapid growth) in India and lower prevalence in Afghanistan and Bangladesh 18 . Limited data are available on the prevalence of dialysis therapies in sub-Saharan Africa 19 . A 2017 report suggests that haemodialysis services were available in at least 34 African countries as of 2017, although haemodialysis was not affordable or accessible to the large majority of resident candidates 13 .
Prevalence of peritoneal dialysis
Worldwide, PD is less widely available than haemodialysis. In a 2017 survey of 125 countries, PD was reportedly available in 75% of countries whereas haemodialysis was available in 96% 20 . In 2018, an estimated 11% of patients receiving long-term dialysis worldwide were treated with PD; a little over half of these patients were living in China, Mexico, the USA and Thailand 21 .
Large variation exists between territories in the relative use of PD for treating kidney failure; in Hong Kong for example, >80% of patients on dialysis receive PD, whereas in Japan this proportion is <5% 22 . This variation is, in part, determined by governmental policies and the density of haemodialysis facilities 23 . In some countries such as the USA, rates of PD utilization also vary by ethnicity with African Americans and Hispanics being much less likely than white Americans to receive PD 24 . Disparate secular trends in PD use are also evident, with rapid growth in the use of PD in some regions such as the USA, China and Thailand and declining or unchanging levels of PD use in other regions, for example, within Western Europe 22 . As for haemodialysis, access to PD is poor in many LMICs for a variety of reasons, as comprehensively discussed elsewhere 25 .
Incidence of dialysis use
Following a rapid increase in dialysis use over a period of approximately two decades, the incidence of dialysis initiation in most HICs reached a peak in the early 2000s and has remained stable or slightly decreased since then 22 , 26 , 27 . Extrapolation of prevalence data from LMICs suggests that the incidence of dialysis initiation seems to be steadily increasing in LMICs 10 , 28 , 29 , 30 , with further increases expected over the coming decades. However, incidence data in LMICs are less robust than prevalence data, although neither reflect the true demand for KRT given the lack of reporting.
Of note, the incidence of dialysis initiation in HICs is consistently 1.2-fold to 1.4-fold higher for men than for women, despite an apparently higher risk of chronic kidney disease (CKD) in women 31 . Whether this finding reflects physician or health system bias, different preferences with regard to KRT, disparities in the competing risk of death, variation in rates of kidney function loss in women versus men, or other reasons is unknown and requires further study. Few data describe the incidence of haemodialysis by sex in LMICs.
Dialysis outcomes
Mortality is very high among patients on dialysis, especially in the first 3 months following initiation of haemodialysis treatment. Approximately one-quarter of patients on haemodialysis die within a year of initiating therapy in HICs, and this proportion is even higher in LMICs 32 , 33 , 34 . Over the past two decades, reductions in the relative and absolute risk of mortality have seemingly been achieved for patients on haemodialysis. Data suggest that relative gains in survival may be greater for younger than for older individuals; however, absolute gains seem to be similar across age groups 35 . Although controversial, improvements in mortality risk seem to have been more rapid among patients on dialysis than for the general population 36 , suggesting that better care of patients receiving dialysis treatments rather than overall health gains might be at least partially responsible for these secular trends. The factors responsible for these apparent trends have not been confirmed, but could include better management of comorbidities, improvements in the prevention or treatment of dialysis-related complications such as infection, and/or better care prior to the initiation of dialysis (which may translate into better health following dialysis initiation). Historically, although short-term mortality was lower for patients treated with PD than for those treated with haemodialysis, the long-term mortality risk was higher with PD 37 , 38 . In the past two decades, the reduction in mortality risk has been greater for patients treated with PD than with haemodialysis, such that in most regions the long-term survival of patients treated with PD and haemodialysis are now similar 39 , 40 , 41 .
Despite these improvements, mortality remains unacceptably high among patients on dialysis and is driven by cardiovascular events and infection. For example, a 2019 study showed that cardiovascular mortality among young adults aged 22–29 years with incident kidney failure was 143–500-fold higher than that of otherwise comparable individuals without kidney failure, owing to a very high burden of cardiovascular risk factors 42 . The risk of infection is also markedly greater among patients on dialysis than in the general population, in part driven by access-related infections in patients on haemodialysis with central venous catheters and peritonitis-related infections in patients on PD 43 , 44 , 45 , 46 , 47 . Hence, strategies to reduce the risk of infection associated with dialysis access should continue to be a high clinical priority.
The risk of mortality among patients on dialysis seems to be influenced by race. In the USA, adjusted mortality is lower for African American patients than for white patients on dialysis, although there is a significant interaction with age such that this observation held only among older adults, and the converse is actually true among younger African American patients aged 18 to 30 years 48 . A similar survival advantage is observed among Black patients compared with white patients or patients of Asian heritage on haemodialysis in the Netherlands 49 . In Canada, dialysis patients of indigenous descent have higher adjusted mortality, and patients of South Asian or East Asian ethnicity have lower adjusted mortality than that of white patients. In addition, between-region comparisons indicate that mortality among incident dialysis patients is substantially lower for Japan than for other HICs. Whether this difference is due to ethnic origin, differences in health system practices, a combination of these factors or other, unrelated factors is unknown 30 . No consistent evidence exists to suggest that mortality among incident adult dialysis patients varies significantly by sex 50 , 51 , 52 .
Other outcomes
Hospitalization, inability to work and loss of independent living are all markedly more common among patients on dialysis than in the general population 53 , 54 , 55 . In contrast to the modest secular improvements in mortality achieved for patients on dialysis, health-related quality of life has remained unchanged for the past two decades and is substantially lower than that of the general population, due in part to high symptom burden 56 , 57 , 58 , 59 . Depression is also frequent among patients on dialysis 60 , and factors such as high pill burden 61 , the need to travel to dialysis sessions and pain associated with vascular access puncture all affect quality of life 62 .
Future epidemiological challenges
The changing epidemiology of kidney failure is likely to present several challenges for the optimal management of these patients. For example, the ageing global population together with continuing increases in the prevalence of key risk factors for the development of kidney disease, such as diabetes mellitus and hypertension, mean that the incidence, prevalence and costs of kidney failure will continue to rise for the foreseeable future. This increased demand for KRT will undoubtedly lead to an increase in the uptake of haemodialysis, which will pose substantial economic challenges for health systems worldwide. Moreover, as growth in demand seems to be outpacing increases in KRT capacity, the number of deaths as a result of kidney failure is expected to rise dramatically (Fig. 1b ).
The same risk factors that drive the development of kidney disease will also increase the prevalence of multimorbidities within the dialysis population. These comorbidities will in turn require effective management in addition to the management of kidney failure per se 63 and will require technical innovations of dialysis procedures, as well as better evidence to guide the management of comorbidities in the dialysis population.
Finally, the particularly rapid increases in the incidence and prevalence of kidney failure among populations in LMICs will place considerable strain on the health systems of these countries. The associated increases in mortality resulting from a lack of access to KRT will create difficult choices for decision makers. Although LMIC should prioritize forms of KRT other than haemodialysis, some haemodialysis capacity will be required 11 , for example, to manage patients with hypercatabolic acute kidney injury or refractory PD-associated peritonitis, which, once available, will inevitably increase the use of this modality.
Health economy-related considerations
The cost of dialysis (especially in-centre or in-hospital dialysis) is high 64 , and the cost per quality-adjusted life-year associated with haemodialysis treatment is often considered to be the threshold value that differentiates whether a particular medical intervention is cost-effective or not 65 . Total dialysis costs across the population will probably continue to rise, owing to increases in life expectancy of the general population and the availability of improved therapeutics for causes of kidney failure such as diabetes mellitus, which have increased the lifespan of these patients and probably will also increase their lifespan on dialysis. KRT absorbs up to 5–7% of total health-care budgets, despite the fact that kidney failure affects only 0.1–0.2% of the general population in most regions 66 . Although societal costs for out-of-centre dialysis (for example, home or self-care haemodialysis, or PD) are in general lower than that of in-centre haemodialysis in many HICs, these options are often underutilized 67 , adding to the rising costs of dialysis.
Reimbursement for haemodialysis correlates with the economic strength of each region 68 , but in part also reflects willingness to pay . In most regions, the correlation curve for PD or reimbursement with respect to gross domestic product projects below that of in-centre haemodialysis, which in part reflects the lower labour costs associated with PD 68 . Unfortunately, little clarity exists with regard to the aggregated cost of single items that are required to produce dialysis equipment for both PD and haemodialysis and the labour costs involved in delivering haemodialysis 69 , which makes it difficult for governments to reimburse the real costs of haemodialysis.
Although increasing reimbursement of home dialysis strategies would seem to be an appropriate strategy to stimulate uptake of these modalities, evidence from regions that offer high reimbursement rates for PD suggests that the success of this strategy is variable 23 , 68 . However, financial incentives may work. In the USA, reimbursement for in-centre and home dialysis (PD or home haemodialysis) has for a long time been identical. The introduction of the expanded prospective payment system in 2011 further enhanced the financial incentives for PD for dialysis providers, which led to a doubling in both the absolute number of patients and the proportion of patients with kidney failure treated with PD 70 , 71 , 72 , 73 .
Although in countries with a low gross domestic product, dialysis consumes less in absolute amounts, it absorbs a higher fraction of the global health budget 68 , likely at the expense of other, potentially more cost-effective interventions, such as prevention or transplantation. Although society carries most of the costs associated with KRT in most HICs, some costs such as co-payment for drugs or consultations are borne by the individual, and these often increase as CKD progresses. In other regions, costs are covered largely or entirely by the patient’s family, leading to premature death when resources are exhausted 74 . In addition, costs are not limited to KRT but also include the costs of medication, hospitalizations and interventions linked to kidney disease or its complications (that is, indirect costs), as well as non-health-care-related costs such as those linked to transportation or loss of productivity.
Dialysis also has an intrinsic economic impact. Patients on dialysis are often unemployed. In the USA, >75% of patients are unemployed at the start of dialysis, compared with <20% in the general population 53 . Unemployment affects purchasing power but also lifestyle, self-image and mental health. Moreover, loss of productivity owing to unemployment and/or the premature death of workers with kidney failure also has economic consequences for society 75 . Therefore, continued efforts to prevent kidney failure and develop KRT strategies that are less time consuming for the patient and allow more flexibility should be an urgent priority. Concomitantly, employers must also provide the resources needed to support employees with kidney failure.
Hence, a pressing need exists to rethink the current economic model of dialysis and the policies that direct the choice of different treatment options. The cost of dialysis (especially that of in-centre haemodialysis) is considerable and will continue to rise as the dialysis population increases. Maintaining the status quo will prevent timely access to optimal treatment for many patients, especially for those living in extreme poverty and with a low level of education and for patients living in LMICs.
Ethical aspects
A 2020 review by a panel of nephrologists and ethicists appointed by three large nephrology societies outlined the main ethical concerns associated with kidney care 76 . With regard to management of kidney failure (Box 1 ), equitable access to appropriate treatment is probably the most important ethical issue and is relevant not only in the context of haemodialysis but also for the other modalities of kidney care (including transplantation, PD and comprehensive conservative care) 76 . Of note, conservative care is not equivalent to the withdrawal of treatment, but rather implies active management excluding KRT.
As mentioned previously, access to such care is limited in many countries 10 , 77 . Inequities in access to dialysis at the individual level are largely dependent on factors such as health literacy, education and socio-economic status, but also on the wealth and organization of the region in which the individual lives. Even when dialysis itself is reimbursed, a lack of individual financial resources can limit access to care. Moreover, elements such as gender, race or ethnicity and citizenship status 78 , 79 can influence an individual’s ability to access dialysis 80 . These factors impose a risk that patients who are most vulnerable are subject to further discrimination. In addition, without necessarily being perceived as such, dialysis delivery may be biased by the financial interests of dialysis providers or nephrologists, for example, by influencing whether a patient receives in-centre versus home dialysis, or resulting in the non-referral of patients on dialysis for transplantation or conservative care 81 , 82 .
A potential reason for the high utilization of in-centre haemodialysis worldwide is a lack of patient awareness regarding the alternatives. When surveyed, a considerable proportion of patients with kidney failure reported that information about options for KRT was inadequate 83 , 84 . Patient education and decision support could be strengthened and its quality benchmarked, with specific attention to low health literacy, which is frequent among patients on dialysis 85 . Inadequate patient education might result from a lack of familiarity with home dialysis (including PD) and candidacy bias among treating physicians and nurses. Appropriate education and training of medical professionals could help to solve this problem. However, the first step to increase uptake of home dialysis modalities is likely policy action undertaken by administrations, but stimulated by advocacy by patients and the nephrology community, as suggested by the higher prevalence of PD at a lower societal cost of regions that already have a PD-first policy in place 68 .
Although the provision of appropriate dialysis at the lowest possible cost to the individual is essential if access is to be improved 86 , approaches that unduly compromise the quality of care should be minimized or avoided. General frameworks to deal with this challenge can be provided by the nephrology community, but trade-offs between cost and quality may be necessary and will require consultation between authorities, medical professionals and patient representatives. Consideration must also be given to whether the societal and individual impact of providing dialysis would be greater than managing other societal health priorities (for example, malaria or tuberculosis) or investing in other sectors to improve health (for example, access to clean drinking water or improving road safety).
The most favourable approach in deciding the most appropriate course of action for an individual is shared decision-making 87 , which provides evidence-based information to patients and families about all available therapeutic options in the context of the local situation. Providing accurate and unbiased information to support such decision-making is especially relevant for conservative care, to avoid the perception that this approach is being recommended to save resources rather than to pursue optimal patient comfort. Properly done, shared decision-making should avoid coercion, manipulation, conflicts of interest and the provision of ‘futile dialysis’ to a patient for whom the harm outweighs the benefits, life expectancy is low or the financial burden is high 88 . However, the views of care providers do not always necessarily align with those of patients and their families, especially in multicultural environments 89 . Medical professionals are often not well prepared for shared decision-making, and thus proper training is essential 90 . Policy action is also required to create the proper ethical consensus and evidence-based frameworks at institutional and government levels 91 to guide decision-making in the context of dialysis care that can be adapted to meet local needs.
Box 1 Main ethical issues in dialysis
Equity in access to long-term dialysis
Inequities in the ability to access kidney replacement therapy exist worldwide; however, if dialysis is available, the ability to transition between different dialysis modalities should be facilitated as much as possible. Specific attention should be paid to the factors that most prominently influence access to dialysis, such as gender, ethnicity, citizenship status and socio-economic status
Impact of financial interests on dialysis delivery
Financial interests of dialysis providers or nephrologists should in no way influence the choice of dialysis modality and/or result in the non-referral of patients for transplantation or conservative care
Cost considerations
Local adaptations are needed to ensure that the costs of dialysis provision are as low as possible without compromising quality of care
The high cost of dialysis means that consideration must be given to whether the benefits obtained by dialysis outweigh those obtained by addressing other health-care priorities, such as malaria or tuberculosis
Shared decision-making
Shared decision-making, involving the patient and their family, is recommended as an approach to allow an informed choice of the most appropriate course to follow
Approaches to shared decision-making must be evidence based and adapted to local circumstances
Futile dialysis should be avoided
Proper training is required to prepare physicians for shared decision-making
Clinical outcomes to measure progress
Over the past six decades, the availability of long-term dialysis has prolonged the lives of millions of people worldwide, often by serving as a bridge to kidney transplantation. Yet, patients on dialysis continue to bear a high burden of disease, both from multimorbidity and owing to the fact that current dialysis modalities only partially replace the function of the native kidney, resulting in continued uraemia and its consequences. Thus, although dialysis prevents death from kidney failure, life expectancy is often poor, hospitalizations (particularly for cardiovascular events and infection) are frequent, symptom burden is high and health-related quality of life is low 22 , 92 , 93 .
Given the multitude of health challenges faced by patients on dialysis, it is necessary to develop a priority list of issues. For much of the past three decades, most of this prioritization was performed by nephrology researchers with the most effort to date focusing on approaches to reducing all-cause mortality and the risk of fatal and non-fatal cardiovascular events. However, despite the many interventions that have been tested, including increasing the dose of dialysis (in the HEMO and ADEMEX trials 94 , 95 ), increasing dialyser flux (in the HEMO trial and MPO trial 94 , 96 ), increasing haemodialysis frequency (for example, the FHN Daily and FHN Nocturnal trials 97 , 98 ), use of haemodiafiltration (the CONTRAST 99 , ESHOL 100 and TURKISH-OL-HDF trials 101 ), increasing the haemoglobin target (for example, the Normal Haematocrit Trial 102 ), use of non-calcium-based phosphate binders (for example, the DCOR trial 103 ), or lowering of the serum cholesterol level (for example, the 4D, AURORA and SHARP trials 104 , 105 , 106 ), none of these or other interventions has clearly reduced all-cause or cardiovascular mortality for patients on dialysis. These disappointments notwithstanding, it is important that the nephrology community perseveres in finding ways to improve patient outcomes.
In the past 5 years, nephrology researchers have increasingly engaged with patients to understand their priorities for meaningful outcomes that should be used to measure progress. The overarching message from this engagement is that although longevity is valued, many patients would prefer to reduce symptom burden and achieve maximal functional and social rehabilitation. This insight highlights the high symptom burden experienced by patients receiving long-term dialysis 92 , 93 , 96 , 107 . These symptoms arise as a consequence of the uraemic syndrome. Some of these symptoms, such as anorexia, nausea, vomiting, shortness of breath and confusion or encephalopathy, improve with dialysis initiation 108 , 109 , 110 , but many other symptoms, such as depression, anxiety and insomnia do not. Moreover, other symptoms, such as post-dialysis fatigue, appear after initiation of haemodialysis.
Of note, many symptoms of uraemic syndrome might relate to the persistence of protein-bound uraemic toxins and small peptides (so-called middle molecules) that are not effectively removed by the current dialysis modalities. The development of methods to improve the removal of those compounds is one promising approach to improving outcomes and quality of life for patients on dialysis, as discussed by other articles in this issue.
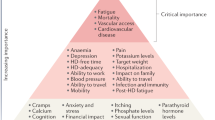
Patients on dialysis report an average of 9–12 symptoms at any given time 92 , 93 , 107 . To determine which of these should be prioritized for intervention, the Kidney Health Initiative used a two-step patient-focused process involving focus groups and an online survey to identify six symptoms that should be prioritized by the research community for intervention. These include three physical symptoms (fatigue, insomnia and cramps) and three mood symptoms (depression, anxiety and frustration) 111 . Parallel to these efforts, the Standardizing Outcomes in Nephrology Group (SONG) workgroup for haemodialysis ( SONG-HD ) has identified several tiers of outcomes that are important to patients, caregivers and health-care providers. Fatigue was identified as one of the four core outcomes, whereas depression, pain and feeling washed out after haemodialysis were identified as middle-tier outcomes 112 , 113 , 114 . Along these same lines, the SONG workgroup for PD ( SONG-PD ) identified the symptoms of fatigue, PD pain and sleep as important middle-tier outcomes 115 , 116 . Despite the importance of these symptoms to patients on dialysis, only a few studies have assessed the efficacy of behavioural and pharmacological treatments on depression 117 , 118 , 119 , 120 , 121 . Even more sobering is the observation that very few, if any, published studies have rigorously tested interventions for fatigue or any of the other symptoms. The nephrology community must now develop standardized and psychometrically robust measures that accurately capture symptoms and outcomes that are important to patients and ensure that these are captured in future clinical trials 122 , 123 .
Approaches to maximizing functional and social rehabilitation are also important to patients with kidney failure. In addition to the above-mentioned symptoms, SONG-HD identified ability to travel, ability to work, dialysis-free time, impact of dialysis on family and/or friends and mobility as important middle-tier outcomes 112 , 113 , 114 . SONG-PD identified life participation as one of five core outcomes, and impact on family and/or friends and mobility as other outcomes that are important to patients 115 , 116 . Given the importance of these outcomes to stakeholders, including patients, it is imperative that nephrology researchers develop tools to enable valid and consistent measurement of these outcomes and identify interventions that favourably modify these outcomes.
Fostering innovation
As described above, the status quo of dialysis care is suboptimal. Residual symptom burden, morbidity and mortality, and economic cost are all unacceptable, which begs the question of what steps are needed to change the established patterns of care. Patients are currently unable to live full and productive lives owing to the emotional and physical toll of dialysis, its intermittent treatment schedule, the dietary and fluid limitations, and their highly restricted mobility during treatment. Current technology requires most patients to travel to a dialysis centre, and current modalities are non-physiological, resulting in ‘washout’, which is defined as extensive fatigue, nausea and other adverse effects, caused by the build-up of uraemic toxins between treatments and the rapid removal of these solutes and fluids over 4-h sessions in the context of haemodialysis. LMICs face additional difficulties in the provision of dialysis owing to infrastructural requirements, the high cost of this treatment, the need for a constant power supply and the requirement for high volumes of purified water. For LMICs, innovations that focus on home-based, low-cost therapies that promote rehabilitation would be especially beneficial.
We contend that initiatives to transform dialysis outcomes for patients require both top-down efforts (for example, those that involve systems changes at the policy, regulatory, macroeconomic and organizational levels) and bottom-up efforts (for example, patient-led and patient-centred advocacy and individual teams of innovators). Top-down efforts are required to support, facilitate and de-risk the work of innovators. Conversely, patient-led advocacy is essential for influencing governmental and organizational policy change. Here, by considering how selected programmes are attempting to transform dialysis outcomes through innovation in support of high-value, high-quality care, we describe how top-down and bottom-up efforts can work synergistically to change the existing ecosystem of dialysis care (Fig. 2 ). The efforts described below are not an exhaustive list; rather, this discussion is intended to provide a representative overview of how the dialysis landscape is changing. Additional articles in this issue describe in more detail some of the bottom-up efforts of innovators to create wearable 124 , portable 125 , more environmentally friendly 126 and more physiological dialysis systems 127 , 128 , priorities from the patients’ perspective 129 , and the role of regulators in supporting innovation in the dialysis space 130 .
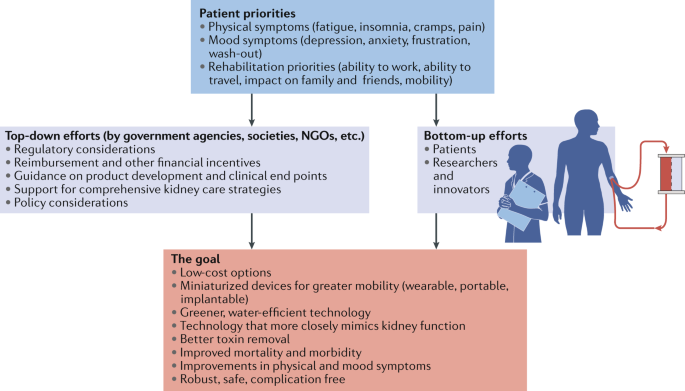
Initiatives to transform dialysis outcomes for patients require both top-down efforts (for example, those that involve systems-level changes at the policy, regulatory, macroeconomic and organizational level) and bottom-up efforts (for example, patient-led and patient-centred advocacy efforts and efforts from individual teams of innovators). Both of these efforts need to be guided by priorities identified by patients. Such an approach, focused on patient-centred innovation, has the potential to result in meaningful innovations that support high-quality, high-value care. NGOs, non-governmental organizations.
The Kidney Health Initiative
In 2012, the American Society of Nephrology (ASN) and the FDA established the KHI as an umbrella organization through which the kidney community can work collaboratively to remove barriers to the development of innovative drugs, devices, biologics and food products, in order to improve outcomes for people living with kidney diseases. To advance its mission, KHI has initiated a number of projects composed of multidisciplinary workgroups. A major accomplishment for the KHI was the establishment of a precompetitive environment to promote innovation while ensuring patient safety.
The KHI is the largest consortium in the kidney community, with over 100 member organizations including patient groups, health professional organizations, dialysis organizations, pharmaceutical and device companies, and government agencies. During the first 7 years of its existence, the KHI has launched and in many cases completed projects that have facilitated the development of new therapeutic options for dialysis patients (Box 2 ), as well as published position papers on topics relevant to innovation in haemodialysis care, including innovations in fluid management 131 and symptom management 132 in patients on haemodialysis, recommendations for clinical trial end points for vascular access 133 , perspectives on pragmatic trials in the haemodialysis population 134 and regulatory considerations for the use of haemodiafiltration 135 .
Box 2 Kidney Heath Initiative Projects that Support Dialysis Innovation
Patient and Family Partnership Council
Since 2015, the Kidney Health Initiative (KHI) Patient and Family Partnership Council (PFPC) has helped KHI stakeholders to engage and network with patients and patient organizations. The PFPC also advises industry and research partners of patient needs and preferences as new products are planned and developed. The PFPC continually emphasizes that innovation will only be successful if built around the needs of people with kidney disease and focused on improving their quality of life.
ESRD Data Standard Project
The aim of this project is to create a harmonized common data standard for kidney failure. The availability of a uniform data standard could accelerate the pace of scientific discovery, facilitate the creation of scientific registries for epidemiological surveillance and allow the development of common metrics for value-based health care.
Building Capacity to Incorporate Patient Preferences into the Development of Innovative Alternatives to kidney replacement therapy (KRT)
This project, which is supported by a 3-year contract with the FDA, is based on the premise that access to scientifically valid patient preference information could positively inform the decisions of industry and regulators as they design and review new devices for individuals with kidney failure. This project will collect patients’ preference information and also address a stated goal of the Advancing American Kidney Health (AAKH) initiative, which instructs the FDA to “develop a new survey to gain insight into patient preferences for new kidney failure treatments” 137 .
Clinical Trial Design to Support Innovative Approaches to KRT
This project is intended to facilitate coordinated efforts between regulators and the nephrology community to streamline the clinical development pathway. The primary objectives of the project are to define terminology for future KRT products (for example, wearable, portable, implantable and artificial kidney) and identify the most appropriate trial designs and end points for a variety of KRT products.
Advancing American Kidney Health
In July 2019, President Donald Trump signed an Executive Order on Advancing American Kidney Health (AAKH) 136 , which promises to fundamentally change the clinical care of kidney disease in general and kidney failure in particular. Components of the AAKH that are relevant to dialysis care include a directive for education and support programmes to promote awareness of kidney disease; a shift in the focus of reimbursement initiatives from in-centre haemodialysis to home therapies, transplantation and upstream CKD care; a system that rewards clinicians and dialysis facilities for providing a range of treatments for kidney failure, with the aim of increasing uptake of home dialysis and transplantation; and incentives for nephrology care teams to focus on reducing costs and improving outcomes by providing longitudinal care of patients with kidney disease.
Finally, and perhaps most radically, the AAKH calls on the US Department of Health and Human Services to support premarket approval of wearable and implantable artificial kidneys and welcomes other strategies to facilitate transformative innovation in dialysis devices. The AAKH directive specifically identifies the KidneyX programme (described below) as the vehicle with which to drive this innovation. The AAKH is the most ambitious US policy initiative ever undertaken to transform the care of patients with advanced kidney disease. Its agenda is still being shaped by the federal governmental agencies, with input from professional societies and other kidney community stakeholders, but this initiative provides a framework and support for transformative innovation in dialysis care.
The KHI Technology Roadmap and KidneyX
The KHI Technology Roadmap for Innovative Approaches to KRT, published in 2019 (ref. 137 ), is aimed at supporting the development of innovative dialysis devices by providing guidance on technical criteria, patient preferences, assessment of patient risk tolerances and regulatory, reimbursement and marketing considerations. Key strengths of the Roadmap include its patient-centred focus and the description of multiple solution pathways for different technologies (for example, portable, wearable and implantable devices that may be purely mechanical, cell-based or hybrid systems), each with appropriate timeline projections.
The KRT Roadmap was designed to be complementary to the Kidney Innovation Accelerator (also known as KidneyX). KidneyX is a public–private partnership between the Department of Health and Human Services and the ASN, and is aimed at accelerating the development of drugs, devices, biologics and other therapies across the spectrum of kidney care. The current major focus of KidneyX is to catalyse the fundamental redesign of dialysis, supported by a series of competitions. Phase I prizes focused on innovations in biomaterials, novel biosensors and safety monitors used for haemodialysis, as well as approaches for improved vascular access and the development of novel technologies that replicate kidney function more precisely than conventional dialysis. Phase II focuses on strategies to build and test prototype solutions or components of solutions that can replicate normal kidney function or improve haemodialysis access. KidneyX has also awarded a series of Patient Innovator Challenge prizes to patients who have proposed innovative solutions to problems emanating from their everyday experiences with kidney disease, including approaches to monitoring blood electrolyte levels and increasing the accessibility of patient education resources.
Dutch Kidney Foundation and Neokidney
The Dutch Kidney Foundation (DKF; or Nierstichting Nederland ) was founded in 1968. It supports research into the causes, prevention and treatment of kidney failure. Furthermore, it works to improve the quality of dialysis treatment and increase the number of kidney transplants. All projects are planned and organized with considerable patient involvement. The DKF also offers financial support to kidney research projects by large Dutch multi-centric consortia. These projects not only promote innovation in the Netherlands but also support trans-national European Union (EU)-supported projects with Dutch participation or leadership, such as Horizon 2020 and Horizon Europe.
Neokidney is a partnership between the DKF and several companies that specialize in miniaturization of dialysis equipment (including dialysis pumps) and sorbent technology for dialysate regeneration. This partnership is aimed at developing a small, portable haemodialysis device that will enable more frequent dialysis sessions, permit more flexibility for patients and improve patient quality of life, as well as reduce health-care costs. The first prototype is currently undergoing preclinical testing and is expected to be tested in humans soon, with the aim of demonstrating proof-of-concept for the first portable haemodialysis machine for daily use, requiring only a limited volume of dialysate. In addition to the development of miniaturization technologies, the partnership is also investigating the use of polymer membranes that permit combined filtration and absorption to achieve more effective haemodialysis 138 .
Nephrologists Transforming Hemodialysis Safety
Nephrologists Transforming Hemodialysis Safety (NTDS) is a collaborative initiative of the ASN and Centers for Disease Control and Prevention (CDC) that is aimed at addressing a specific complication inherent to contemporary dialysis — infection. In 2016, the CDC observed that 10% of dialysis patients in the USA died each year as the result of infections — most of which were preventable. The aim of NTDS is to develop and deploy innovations to achieve zero preventable infections in dialysis facilities across the USA. To reach this goal, NTDS uses a multi-pronged approach. For example, education strategies via publications 139 , 140 , 141 , 142 , 143 and webinars that address various aspects of infection prevention and standards of care, use of social media, development of an interactive chapter for trainees and clinicians, and invited lectures are aimed at ensuring that nephrologists, nurses, dialysis administrators and other professionals understand the risk of dialysis-related infections and evidence-based best working practices.
NTDS also interacts with experts in infection detection, prevention and treatment within federal, state and local health departments who can provide advice and assistance that is independent of the regulatory and potentially punitive arms of health departments. NTDS promotes the appropriate use of these experts in settings where expert advice is needed.
To promote leadership among physicians and nurses, NTDS is sponsoring a leadership academy to ensure that knowledge pertaining to evidence-based best working practices is applied to improve workflows in clinical practice. Effective leadership is a requirement, particularly in complex settings, to ensure that patient safety is prioritized and to motivate staff to use best practices.
NTDS are also collaborating with human factors engineers to study the workflows used in haemodialysis facilities and help to define ways of avoiding errors that lead to infection. As a first step in this process, NTDS and human factors engineers have spent time in various haemodialysis facilities to obtain information about the complex processes of care within those facilities, particularly with regard to the use of haemodialysis catheters and approaches to hand hygiene, injection safety and disinfection. Better understanding of current processes may lead to better workflow design.
Finally, based on lessons learned during the Ebola Crisis of 2014, an NTDS work group has designed processes to anticipate and respond to unexpected health-care crises. At the time of writing this Review, the NTDS team is working with CDC and haemodialysis organizations to anticipate and respond to the COVID-19 epidemic and its effect on dialysis care.
The Affordable Dialysis Prize
As discussed earlier, kidney failure remains a death sentence for many residents of LMICs owing to a lack of access to dialysis. In response to the pressing need for cost-effective dialysis options, the International Society of Nephrology in collaboration with the George Institute for Global Health and the Asian Pacific Society of Nephrology launched the Affordable Dialysis Prize in 2017 with the objective of facilitating the design of a dialysis system that would cost less than US $1,000, and provide treatment for less than $5 a day, yet be as safe and effective as existing dialysis systems. The prize was awarded to an engineer for a system that runs off solar power and includes a miniature distiller for producing pure water from any source via steam distillation. The purified water can then be mixed with electrolytes in empty PD bags to produce cheap, homemade dialysis solutions. This strategy identifies the lack of cheap, high-quality water as a major impediment to dialysis in LMICs and LICs. The system will ideally fit into a small suitcase 144 . This device remains under development with the goal of initiating clinical trials and ultimately commercializing the technology.
Empowered in-centre haemodialysis
For some patients with kidney failure, maintenance in-centre haemodialysis will always be the preferred treatment, and despite incentivizing policy levers, they will not be interested in pursuing home dialysis or kidney transplantation. In-centre self-dialysis (also referred to as empowered haemodialysis) originated in Sweden, when a young engineer named Christian Farman returned to haemodialysis in 2010 after a failed transplant. Farman began negotiating with his nurses to perform his own dialysis treatments with staff supervision and caught the attention of other patients 145 . Eventually, the process of self-dialysis within this centre — whereby coaches in the dialysis unit train people to take over control of their own treatments and health — grew so popular that a new unit was built at the hospital for self-dialysis patients only, with patient input into the design of the unit. Since then, self-care units were installed in several haemodialysis units in Europe and the USA, offering patients the autonomy and flexibility of home haemodialysis within the safety of a controlled environment. This approach to empowering patients has not been widely used to date, but deserves rigorous study and evaluation 146 .
Remote monitoring to support self-care
Telemedicine is defined as the electronic exchange of medical information between sites with the aim of improving a patient’s health. Telehealth encompasses a broader set of services such as the provision of educational content. New technologies have broadened the scope of telemedicine and telehealth applications and services, making these tools more accessible and useful in the care of patients who live remotely or have difficulty visiting a clinic. The range of services that can be delivered by telehealth now includes two-way interactive video, device data programming, asynchronous messaging , sensors for remote monitoring and portals to enable patients to access electronic health records. Although relatively understudied in haemodialysis patients to date, telehealth has the potential to increase the acceptance of home dialysis and improve patient satisfaction, while potentially decreasing costs and improving outcomes.
Telehealth and the remote monitoring of dialysis patients has become more commonplace in the past decade, particularly in Australia, where telehealth is used widely for patients receiving home dialysis. Telemedicine is also considered a support tool for kidney care in disaster situations such as earthquakes where many individuals in remote locations can be affected. Telemedicine has also been used for distance monitoring of patients receiving PD 147 , 148 . In the USA, the Bipartisan Budget Act of 2018 included provisions to expand telehealth coverage to include patients on home dialysis. This legislation allows patients on home dialysis to choose to have their monthly care-provider visits take place via telehealth, without geographic restrictions. The ongoing COVID-19 pandemic has also resulted in an unprecedented and rapid expansion in the use of telemedicine for providing health care in many regions worldwide, including for the care of patients undergoing in-centre haemodialysis. The experience gained during this pandemic has the potential to permanently embed telemedicine in health-care delivery in many health-care systems.
Although telehealth has considerable promise for the care of dialysis patients, the implementation of telehealth in clinical practice can be challenging 149 . Telehealth-guided digital interactions have the potential to improve outcomes through the provision of activities such as individualized patient-centred education, remote communication and data exchange, in-home clinical guidance and monitoring, assessment of prescription and/or treatment efficacy and adherence, real-time modification of treatments and early alerts for problems that require intervention, although all of these interventions need to be rigorously tested 150 .
The European Kidney Health Alliance
The European Kidney Health Alliance (EKHA) is a non-governmental organization based in Brussels, Belgium, which advocates for kidney patients and the nephrology community at relevant bodies of the EU and also at European national organizations. The EKHA represents all of the major stakeholders in kidney care, including physicians, patients, nurses and foundations. The actions of the EKHA are supported by a dedicated group of Members of European Parliament. Of note, according to the treaty of Lisbon 151 , health-care systems are the responsibility of the national authorities of EU countries, which limits the role of the European Commission to one of complementing national policies and fostering cooperation. The EKHA has undertaken several initiatives in the area of kidney care, mainly focusing on measures to decrease the costs of kidney care while maintaining quality of care and access for all appropriate candidates, and to reduce demand for dialysis by promoting efforts to prevent the progression of kidney disease, and encouraging kidney transplantation as the KRT of choice 66 , 152 . In 2021, the EKHA will focus on reimbursement strategies and access to KRT, especially home haemodialysis.
The Nephrology and Public Policy Committee is a similar initiative created by the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA). This committee aims to translate important kidney-related clinical topics into public policy, including the search for novel biomarkers of CKD, improving transition between paediatric and adult nephrology, and improving collaboration between the ERA-EDTA Registry and the guidance body of the ERA-EDTA, European Renal Best Practice 153 .
Beating Kidney Disease
Together with the Dutch Federation for Nephrology and the Dutch Kidney Patients Association, the DKF has initiated a strategic agenda for research and innovation in the Netherlands. This initiative, called Beating Kidney Disease (Nierziekte de Baas) will promote four specific research areas 154 : prevention of kidney failure, including root causes such as other chronic diseases; personalized medicine including genome and big data analyses, and studies of rare diseases; patient-centred outcomes and quality of life, transplantation and home haemodialysis; and regenerative medicine including bio-artificial kidneys. In collaboration with the EKHA, the Beating Kidney Disease initiative will be proposed as a framework for future initiatives at the Directorate General for Health and Food Safety of the European Commission, and the European Commissioner of Health. Similar to European initiatives that have promoted transplantation 152 , 155 , 156 , these efforts will emphasize shifts in policy action to strengthen institutional frameworks, improve education, training and information, optimize registries, and ensure appropriate benchmarking in nephrology.
Conclusions
The past 50 years have seen rapid changes in how and to whom dialysis is provided. From a global perspective, the escalating numbers of patients who require dialysis mean that even current costs are not sustainable, and yet most people who develop kidney failure forego treatment owing to a lack of access, with millions of lives lost every year as a consequence. Also important, the limitations of current dialysis treatment in alleviating patient suffering, morbidity and mortality are now viewed as unacceptable. Consequently, patients, payors, regulators and health-care systems are increasingly demanding improved value, which can only come about through true patient-centred innovation that supports high-quality, high-value care. Substantial efforts are now underway to support requisite transformative changes. These efforts need to be catalysed, promoted and fostered through international collaboration and harmonization to ensure that in the future, people living with kidney failure have more and better treatment options than exist today.
Peitzman, S. J. Chronic dialysis and dialysis doctors in the United States: a nephrologist-historian’s perspective. Semin. Dial. 14 , 200–208 (2001).
Article CAS PubMed Google Scholar
Brescia, M. J., Cimino, J. E., Appel, K. & Hurwich, B. J. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N. Engl. J. Med. 275 , 1089–1092 (1966).
Blagg, C. R. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am. J. Kidney Dis. 49 , 482–496 (2007).
Article PubMed Google Scholar
Scribner, B. H. Ethical problems of using artificial organs to sustain human life. Trans. Am. Soc. Artif. Intern. Organs 10 , 209–212 (1964).
CAS PubMed Google Scholar
Blagg, D. C. R. From Miracle to Mainstream: Creating the World’s First Dialysis Organization (Northwest Kidney Centers, 2017).
Himmelfarb, J., Berns, A., Szczech, L. & Wesson, D. Cost, quality, and value: the changing political economy of dialysis care. J. Am. Soc. Nephrol. 18 , 2021–2027 (2007).
KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3 , 163 (2013).
Google Scholar
Hole, B. et al. Supportive care for end-stage kidney disease: an integral part of kidney services across a range of income settings around the world. Kidney Int. Suppl. 10 , e86–e94 (2020).
Article Google Scholar
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395 , 709–733 (2020).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385 , 1975–1982 (2015).
Tonelli, M. et al. Framework for establishing integrated kidney care programs in low- and middle-income countries. Kidney Int. Suppl. 10 , e19–e23 (2020).
Pecoits-Filho, R. et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int. Suppl. 10 , e3–e9 (2020).
Bello A. K. L. et al. Global Kidney Health Atlas: a report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe. https://www.kidneycareuk.org/documents/52/ISN_Global_kidney_health_atlas.pdf (2017).
White, S. et al. How can we achieve global equity in provision of renal replacement therapy? Bull. World Health Organ. 86 , 229–237 (2008).
Article PubMed PubMed Central Google Scholar
Bello, A. K. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 367 , l5873 (2019).
Luxardo, R. et al. The epidemiology of renal replacement therapy in two different parts of the world: the Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev. Panam. Salud Publica 42 , e87 (2018).
United States Renal Data System. Volume 2: ESRD in the United States https://www.usrds.org/2018/download/2018_Volume_2_ESRD_in_the_US.pdf (2018).
Jha, V. et al. The state of nephrology in South Asia. Kidney Int. 95 , 31–37 (2019).
Barsoum, R. S., Khalil, S. S. & Arogundade, F. A. Fifty years of dialysis in Africa: challenges and progress. Am. J. Kidney Dis. 65 , 502–512 (2015).
Bello, A. K. et al. Assessment of Global Kidney Health Care Status. JAMA 317 , 1864–1881 (2017).
Fresenius Medical Care. Annual Report 2018: Care and Live. https://www.freseniusmedicalcare.com/fileadmin/data/com/pdf/Media_Center/Publications/Annual_Reports/FME_Annual-Report_2018.pdf (2018).
United States Renal Data System. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. https://www.usrds.org/2019/view/USRDS_2019_ES_final.pdf (2019).
Liu, F. X. et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit. Dial. Int. 35 , 406–420 (2015).
Article CAS PubMed PubMed Central Google Scholar
Mehrotra, R. et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. J. Am. Soc. Nephrol. 27 , 2123–2134 (2016).
Li, P. K.-T. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13 , 90–103 (2017).
ANZDATA Registry. ANZDATA 42nd Annual Report 2019. https://www.anzdata.org.au/report/anzdata-42nd-annual-report-2019/ (2019).
Kramer, A. et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry annual report 2015: a summary. Clin. Kidney J. 11 , 108–122 (2018).
Anand, S., Bitton, A. & Gaziano, T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS ONE 8 , e72860 (2013).
Modi, G. K. & Jha, V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int. 70 , 2131–2133 (2006).
Robinson, B. M. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388 , 294–306 (2016).
Antlanger, M. et al. Sex differences in kidney replacement therapy initiation and maintenance. Clin. J. Am. Soc. Nephrol. 14 , 1616 (2019).
Chan, K. E. et al. Early outcomes among those initiating chronic dialysis in the United States. Clin. J. Am. Soc. Nephro 6 , 2642–2649 (2011).
Article CAS Google Scholar
Thamer, M. et al. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am. J. Kidney Dis. 66 , 1024–1032 (2015).
Garcia-Garcia, G. et al. Survival among patients with kidney failure in Jalisco, Mexico. J. Am. Soc. Nephrol. 18 , 1922–1927 (2007).
Foster, B. J., Mitsnefes, M. M., Dahhou, M., Zhang, X. & Laskin, B. L. Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin. J. Am. Soc. Nephrol. 13 , 91–99 (2018).
Storey, B. C. et al. Declining comorbidity-adjusted mortality rates in English patients receiving maintenance renal replacement therapy. Kidney Int. 93 , 1165–1174 (2018).
Fenton, S. S. et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am. J. Kidney Dis. 30 , 334–342 (1997).
Vonesh, E. F., Snyder, J. J., Foley, R. N. & Collins, A. J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 66 , 2389–2401 (2004).
Mehrotra, R., Chiu, Y. W., Kalantar-Zadeh, K., Bargman, J. & Vonesh, E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch. Intern. Med. 171 , 110–118 (2011).
Mehrotra, R., Devuyst, O., Davies, S. J. & Johnson, D. W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 27 , 3238–3252 (2016).
Mehrotra, R. et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J. Am. Soc. Nephrol. 18 , 2781–2788 (2007).
Modi, Z. J. et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: an analysis of the us renal data system. JAMA Cardiol. 4 , 353–362 (2019).
Wetmore, J. B. et al. Insights from the 2016 peer kidney care initiative report: still a ways to go to improve care for dialysis patients. Am. J. Kidney Dis. 71 , 123–132 (2018).
Skov Dalgaard, L. et al. Risk and prognosis of bloodstream infections among patients on chronic hemodialysis: a population-based cohort study. PLoS One 10 , e0124547 (2015).
Article PubMed PubMed Central CAS Google Scholar
Nelveg-Kristensen, K. E., Laier, G. H. & Heaf, J. G. Risk of death after first-time blood stream infection in incident dialysis patients with specific consideration on vascular access and comorbidity. BMC Infect. Dis. 18 , 688 (2018).
Chaudry, M. S. et al. Risk of infective endocarditis in patients with end stage renal disease. Clin. J. Am. Soc. Nephrol. 12 , 1814–1822 (2017).
Pruthi, R., Steenkamp, R. & Feest, T. UK renal registry 16th annual report: chapter 8 survival and cause of death of UK adult patients on renal replacement therapy in 2012: national and centre-specific analyses. Nephron. Clin. Pract. 125 , 139–169 (2013).
Kucirka, L. M. et al. Association of race and age with survival among patients undergoing dialysis. JAMA 306 , 620–626 (2011).
CAS PubMed PubMed Central Google Scholar
van den Beukel, T. O. et al. The role of psychosocial factors in ethnic differences in survival on dialysis in The Netherlands. Nephrol. Dial. Transpl. 27 , 2472–2479 (2012).
Depner, T. et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO study. Kidney Ing. 65 , 1386–1394 (2004).
Villar, E., Remontet, L., Labeeuw, M. & Ecochard, R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J. Am. Soc. Nephrol. 18 , 2125–2134 (2007).
Hecking, M. et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS). PLoS Med. 11 , e1001750 (2014).
Erickson, K. F., Zhao, B., Ho, V. & Winkelmayer, W. C. Employment among patients starting dialysis in the United States. Clin. J. Am. Soc. Nephrol. 13 , 265–273 (2018).
Kurella Tamura, M. et al. Functional status of elderly adults before and after initiation of dialysis. N. Eng. J. Med. 361 , 1539–1547 (2009).
Daratha, K. B. et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin. J. Am. Soc. Nephrol. 7 , 409–416 (2012).
Davison, S. N. & Jhangri, G. S. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J. Pain. Symptom Manage. 39 , 477–485 (2010).
Eneanya, N. D. et al. Longitudinal patterns of health-related quality of life and dialysis modality: a national cohort study. BMC Nephrol. 20 , 7 (2019).
Ju, A. et al. Patient-reported outcome measures for fatigue in patients on hemodialysis: a systematic review. Am. J. Kidney Dis. 71 , 327–343 (2018).
Rhee, E. P. et al. Prevalence and persistence of uremic symptoms in incident dialysis patients. Kidney360 1 , 86–92 (2020).
Kimmel, P. L. & Peterson, R. A. Depression in patients with end-stage renal disease treated with dialysis: has the time to treat arrived? Clin. J. Am. Soc. Nephrol. 1 , 349–352 (2006).
Burnier, M., Pruijm, M., Wuerzner, G. & Santschi, V. Drug adherence in chronic kidney diseases and dialysis. Nephrol. Dial. Transpl. 30 , 39–44 (2015).
Viecelli, A. K. et al. Identifying critically important vascular access outcomes for trials in haemodialysis: an international survey with patients, caregivers and health professionals. Nephrol. Dial. Transpl. 35 , 657–668 (2020).
Ceretta, M. L. et al. Changes in co-morbidity pattern in patients starting renal replacement therapy in Europe-data from the ERA-EDTA registry. Nephrol. Dial. Transpl. 33 , 1794–1804 (2018).
Vanholder, R. et al. Reimbursement of dialysis: a comparison of seven countries. J. Am. Soc. Nephrol. 23 , 1291–1298 (2012).
Winkelmayer, W. C., Weinstein, M. C., Mittleman, M. A., Glynn, R. J. & Pliskin, J. S. Health economic evaluations: the special case of end-stage renal disease treatment. Med. Decis. Mak. 22 , 417–430 (2002).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13 , 393–409 (2017).
Klarenbach, S. & Manns, B. Economic evaluation of dialysis therapies. Semin. Nephrol. 29 , 524–532 (2009).
van der Tol, A., Lameire, N., Morton, R. L., Van Biesen, W. & Vanholder, R. An international analysis of dialysis services reimbursement. Clin. J. Am. Soc. Nephrol. 14 , 84–93 (2019).
Beaudry, A. et al. Cost of dialysis therapy by modality in Manitoba. Clin. J. Am. Soc. Nephrol. 13 , 1197–1203 (2018).
Golper, T. A. The possible impact of the US prospective payment system (“bundle”) on the growth of peritoneal dialysis. Perit. Dial. Int. 33 , 596–599 (2013).
Lin, E. et al. Home dialysis in the prospective payment system era. J. Am. Soc. Nephrol. 28 , 2993–3004 (2017).
Shen, J. I. et al. Expanded prospective payment system and use of and outcomes with home dialysis by race and ethnicity in the United States. Clin. J. Am. Soc. Nephrol. 14 , 1200–1212 (2019).
Wang, V. et al. Medicare’s new prospective payment system on facility provision of peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 13 , 1833–1841 (2018).
Swanepoel, C. R., Wearne, N. & Okpechi, I. G. Nephrology in Africa–not yet uhuru. Nat. Rev. Nephrol. 9 , 610–622 (2013).
Wang, V., Vilme, H., Maciejewski, M. L. & Boulware, L. E. The economic burden of chronic kidney disease and end-stage renal disease. Semin. Nephrol. 36 , 319–330 (2016).
Martin, D. E. et al. A call for professional collaboration to address ethical challenges in nephrology. Nat Rev Nephrol. https://doi.org/10.1038/s41581-020-0295-4 (2020).
Harris, D. C. H. et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 95 , S1–S33 (2019).
Rodriguez, R. A. Dialysis for undocumented immigrants in the United States. Adv. Chronic Kidney Dis. 22 , 60–65 (2015).
Saunders, M. R., Lee, H., Maene, C., Schuble, T. & Cagney, K. A. Proximity does not equal access: racial disparities in access to high quality dialysis facilities. J. Racial Ethn. Health Disparities 1 , 291–299 (2014).
Shaikh, M. et al. Utilization, costs, and outcomes for patients receiving publicly funded hemodialysis in India. Kidney Int. 94 , 440–445 (2018).
Ladin, K. & Smith, A. K. Active medical management for patients with advanced kidney disease. JAMA Intern. Med. 179 , 313–315 (2019).
Boulware, L. E., Wang, V. & Powe, N. R. Improving access to kidney transplantation: business as usual or new ways of doing business? JAMA 322 , 931–933 (2019).
Van Biesen, W., van der Veer, S. N., Murphey, M., Loblova, O. & Davies, S. Patients’ perceptions of information and education for renal replacement therapy: an independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS ONE 9 , e103914 (2014).
Mehrotra, R., Marsh, D., Vonesh, E., Peters, V. & Nissenson, A. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int. 68 , 378–390 (2005).
Taylor, D. M. et al. A systematic review of the prevalence and associations of limited health literacy in CKD. Clin. J. Am. Soc. Nephrol. 12 , 1070–1084 (2017).
Vanholder, R., Van Biesen, W. & Lameire, N. Renal replacement therapy: how can we contain the costs? Lancet 383 , 1783–1785 (2014).
Moss, A. H. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin. J. Am. Soc. Nephrol. 5 , 2380–2383 (2010).
Williams, A. W. et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin. J. Am. Soc. Nephrol. 7 , 1664–1672 (2012).
Rinehart, A. Beyond the futility argument: the fair process approach and time-limited trials for managing dialysis conflict. Clin. J. Am. Soc. Nephrol. 8 , 2000–2006 (2013).
Ladin, K. et al. Characterizing approaches to dialysis decision making with older adults: a qualitative study of nephrologists. Clin. J. Am. Soc. Nephrol. 13 , 1188–1196 (2018).
Luyckx, V. A. et al. Developing the ethical framework of end-stage kidney disease care: from practice to policy. Kidney Int. Suppl. 10 , e72–e77 (2020).
Davison, S. N., Jhangri, G. S. & Johnson, J. A. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 69 , 1621–1625 (2006).
Weisbord, S. D. et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2 , 960–967 (2007).
Eknoyan, G. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 347 , 2010–2019 (2002).
Paniagua, R. et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J. Am. Soc. Nephrol. 13 , 1307–1320 (2002).
Locatelli, F. et al. Effect of membrane permeability on survival of hemodialysis patients. J. Am. Soc. Nephrol. 20 , 645–654 (2009).
Group, F. H. N. T. et al. In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 363 , 2287–2300 (2010).
Rocco, M. V. et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int. 80 , 1080–1091 (2011).
Grooteman, M. P. C. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J. Am. Soc. Nephrol. 23 , 1087 (2012).
Farrington, K. & Davenport, A. The ESHOL study: hemodiafiltration improves survival - but how? Kidney Int. 83 , 979–981 (2013).
Maduell, F. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 24 , 487 (2013).
Besarab, A. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 339 , 584–590 (1998).
Suki, W. N. et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 72 , 1130–1137 (2007).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353 , 238–248 (2005).
Fellstrom, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360 , 1395–1407 (2009).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377 , 2181–2192 (2011).
Abdel-Kader, K., Unruh, M. L. & Weisbord, S. D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin. J. Am. Soc. Nephrol. 4 , 1057–1064 (2009).
Mehrotra, R., Berman, N., Alistwani, A. & Kopple, J. D. Improvement of nutritional status after initiation of maintenance hemodialysis. Am. J. Kidney Dis. 40 , 133–142 (2002).
Pupim, L. B. et al. Improvement in nutritional parameters after initiation of chronic hemodialysis. Am. J. Kidney Dis. 40 , 143–151 (2002).
Rivara, M. B. et al. Changes in symptom burden and physical performance with initiation of dialysis in patients with chronic kidney disease. Hemodial. Int. 19 , 147–150 (2015).
Flythe, J. E. et al. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin. J. Am. Soc. Nephrol. 13 , 735–745 (2018).
Tong, A. et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop. Am. J. Kidney Dis. 69 , 97–107 (2017).
Evangelidis, N. et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am. J. Kidney Dis. 70 , 464–475 (2017).
Urquhart-Secord, R. et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am. J. Kidney Dis. 68 , 444–454 (2016).
Manera, K. E. et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clin. J. Am. Soc. Nephrol. 14 , 74–83 (2019).
Manera, K. E. et al. An international Delphi survey helped develop consensus-based core outcome domains for trials in peritoneal dialysis. Kidney Int. 96 , 699–710 (2019).
Duarte, P. S., Miyazaki, M. C., Blay, S. L. & Sesso, R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 76 , 414–421 (2009).
Taraz, M. et al. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: results of a randomized double-blind, placebo-controlled clinical trial. Int. Immunopharmacol. 17 , 917–923 (2013).
Cukor, D. et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J. Am. Soc. Nephrol. 25 , 196–206 (2014).
Friedli, K. et al. Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis: a randomized, controlled feasibility trial. Clin. J. Am. Soc. Nephrol. 12 , 280–286 (2017).
Mehrotra, R. et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Ann. Intern. Med. 170 , 369–379 (2019).
Mendu, M. L. et al. Measuring quality in kidney care: an evaluation of existing quality metrics and approach to facilitating improvements in care delivery. J. Am. Soc. Nephrol. 31 , 602–614 (2020).
Nair, D. & Wilson, F. P. Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am. J. Kidney Dis. 74 , 791–802 (2019).
Himmelfarb, J. & Ratner, B. Wearable artificial kidney: problems, progress and prospects. Nat. Rev. Nephrol. in press [doi to be supplied]
Foo, M. W. Y. & Htay, H. Innovations in peritoneal dialysis. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0283-8 (2020).
Agar, J. W. M. & Barraclough, K. A. Water use in dialysis: environmental considerations. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0296-3 (2020).
Masereeuw, R. & Verhaar, M. C. Innovations in approaches to remove uraemic toxins. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0299-0 (2020).
Geremia, I. & Stamatialis, D. Innovations in dialysis membranes for improved kidney replacement therapy. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0293-6 (2020).
Gedney, N., Sipma, W. & Søndergaard, H. Innovations in dialysis: the user’s perspective. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0292-7 (2020).
Wieringa, F. P., Sheldon, M. I. & Hidalgo-Simon, A. Regulatory approaches to stimulate innovative renal replacement therapies. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0275-8 (2020).
Kidney Health Initiatve. Fostering Innovation in Fluid Management. https://khi.asn-online.org/uploads/KHI_InnovationsInFluidManagement.pdf (2019).
Flythe, J. E. et al. Fostering innovation in symptom management among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 14 , 150 (2019).
Shenoy, S. et al. Clinical trial end points for hemodialysis vascular access. Clin. J. Am. Soc. Nephrol. 13 , 490 (2018).
Dember, L. M. et al. Pragmatic trials in maintenance dialysis: perspectives from the kidney health initiative. J. Am. Soc. Nephrol. 27 , 2955 (2016).
Canaud, B., Vienken, J., Ash, S. & Ward, R. A. Hemodiafiltration to address unmet medical needs ESKD patients. Clin. J. Am. Soc. Nephrol. 13 , 1435 (2018).
Trump, D. J. Executive Order on Advancing American Kidney Health. The White House https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/ (2019).
Kidney Health Initiative. Technology Roadmap for Innovative Approaches to Renal Replacement Therapy. https://www.asn-online.org/g/blast/files/KHI_RRT_Roadmap1.0_FINAL_102318_web.pdf (2018).
Tijink, M. S. L. et al. Mixed matrix membranes: a new asset for blood purification therapies. Blood Purif. 37 , 1–3 (2014).
Vijayan, A. & Boyce, J. M. 100% use of infection control procedures in hemodialysis facilities: call to action. Clin. J. Am. Soc. Nephrol. 13 , 671–673 (2018).
Wong, L. P. Achieving dialysis safety: the critical role of higher-functioning teams. Semin. Dial. 32 , 266–273 (2019).
Kliger, A. S. & Collins, A. J. Long overdue need to reduce infections with hemodialysis. Clin. J. Am. Soc. Nephrol. 12 , 1728–1729 (2017).
Kliger, A. S. Targeting zero infections in dialysis: new devices, yes, but also guidelines, checklists, and a culture of safety. J. Am. Soc. Nephrol. 29 , 1083–1084 (2018).
Collins, A. J. & Kliger, A. S. Urgent: stop preventable infections now. Clin. J. Am. Soc. Nephrol. 13 , 663–665 (2018).
The George Institute. World’s first affordable dialysis machine a finalist in 2017 Eureka Awards. https://www.georgeinstitute.org.au/media-releases/worlds-first-affordable-dialysis-machine-a-finalist-in-2017-eureka-awards (2017).
Institute for Healthcare Improvement. A patient directs his own care. http://www.ihi.org/resources/Pages/ImprovementStories/APatientDirectsHisOwnCareFarmanSelfDialysis.aspx (2020).
Shinkman, R. Is “empowered dialysis” the key to better outcomes? NEJM Catalyst Carryover https://doi.org/10.1056/CAT.18.0232 (2018).
Nayak, K. S., Ronco, C., Karopadi, A. N. & Rosner, M. H. Telemedicine and remote monitoring: supporting the patient on peritoneal dialysis. Perit. Dial. Int. 36 , 362–366 (2016).
Rohatgi, R., Ross, M. J. & Majoni, S. W. Telenephrology: current perspectives and future directions. Kidney Int. 92 , 1328–1333 (2017).
Lew, S. Q. & Sikka, N. Operationalizing telehealth for home dialysis patients in the United States. Am. J. Kidney Dis. 74 , 95–100 (2019).
Bieber, S. D. & Gadegbeku, C. A. A call to action for the kidney community: nephrologists’ perspective on advancing American kidney health. Clin. J. Am. Soc. Nephrol. 14 , 1799–1801 (2019).
Foundation for EU democracy. Consolidated Reader-Friendly Edition of the Treaty on European Union (TEU) and the Treaty on the Functioning of the European Union (TFEU) as amended by the Treaty of Lisbon (2007) Third edition. http://en.euabc.com/upload/books/lisbon-treaty-3edition.pdf (2009).
European Kidney Health Alliance. Thematic Network on Improving Organ Donation and Transplantation in the EU 2019. http://ekha.eu/wp-content/uploads/FINAL_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (2019).
Massy, Z. A. et al. Nephrology and public policy committee propositions to stimulate research collaboration in adults and children in Europe. Nephrol. Dial. Transpl. 34 , 1469–1480 (2019).
Beating kidney disease. A joint agenda for research and innovation. https://www.nierstichting.nl/media/filer_public/4d/6d/4d6d6b4e-ce56-4a4b-8ba2-f5ac957d0df8/beating_kidney_disease_-_joint_agenda_for_ri_june_2018.pdf (2018).
Matesanz, R., Marazuela, R., Coll, E., Mahillo, B. & Dominguez-Gil, B. About the Opt-Out system, live transplantation, and information to the public on organ donation in Spain… Y ole! Am. J. Transpl. 17 , 1695–1696 (2017).
Zivcic-Cosic, S. et al. Development of the Croatian model of organ donation and transplantation. Croat. Med. J. 54 , 65–70 (2013).
Download references
Author information
Authors and affiliations.
Kidney Research Institute, Seattle, WA, USA
Jonathan Himmelfarb & Rajnish Mehrotra
Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA, USA
Nephrology Section, Department of Internal Medicine and Pediatrics, University Hospital, Ghent, Belgium and European Kidney Health Alliance (EKHA), Brussels, Belgium
Raymond Vanholder
Division of Nephrology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada
Marcello Tonelli
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Jonathan Himmelfarb .
Ethics declarations
Competing interests.
J.H. declares that The Kidney Research Institute and the Center for Dialysis Innovation at the University of Washington, which he directs, has received gift and grant support from the Northwest Kidney Centers, a not-for-profit dialysis provider. The Center for Dialysis Innovation has also received a Phase I prize from KidneyX, and a grant from the Veterans Administration. J.H. is also a founder and holds equity in AKTIV-X Technologies, Inc. R.V. has consulted for Baxter Healthcare, B. Braun and Neokidney. R.M. has received an honorarium from Baxter Healthcare and serves as a member of the Board of Trustees of the Northwest Kidney Centers. M.T. has received a lecture fee from B. Braun, which was donated to charity.
Additional information
Peer review information.
Nature Reviews Nephrology thanks M. Verhaar, who co-reviewed with M. van Gelder, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Affordable Dialysis Prize: https://www.dialysisprize.org/
Dutch Kidney Foundation: https://www.narcis.nl/organisation/RecordID/ORG1238896/Language/en
ESRD Data Standard Project: https://khi.asn-online.org/projects/project.aspx?ID=78
European Kidney Health Alliance: http://ekha.eu/
Kidney Health Initiative: https://khi.asn-online.org/
KidneyX: https://www.kidneyx.org/
Neokidney: https://www.nextkidney.com/
Nephrologists Transforming Hemodialysis Safety: https://www.asn-online.org/ntds/
Nephrology and Public Policy Committee: https://www.era-edta.org/en/nppc/
Nierstichting Nederland: https://nierstichting.nl/
Patient and Family Partnership Council: https://khi.asn-online.org/pages/?ID=1
SONG-HD: https://songinitiative.org/projects/song-hd/
SONG-PD : https://songinitiative.org/projects/song-pd/
Standardizing Outcomes in Nephrology Group (SONG): https://songinitiative.org/
The total ‘real’ price of a given treatment, including the amount paid by the individual and the amount paid by society.
The ratio of the cost of the intervention compared with a relevant measure of its effect.
The share of treatment cost paid by society; that is, by government or insurers.
Money paid by governments or insurers to health-care providers for money spent on treatment.
The value of everything produced in that country at the prices prevailing in that country, usually expressed as gross domestic product.
The maximum price at or below which a society is prepared to buy a product.
The monetary value of all finished goods and services made within a country during a specific period.
Total amount of money spent by government on health care.
Engineers who design systems, devices, software and tools to fit human capabilities and limitations.
A communication method where the message is placed in a queue, and can be processed at a later time point.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Himmelfarb, J., Vanholder, R., Mehrotra, R. et al. The current and future landscape of dialysis. Nat Rev Nephrol 16 , 573–585 (2020). https://doi.org/10.1038/s41581-020-0315-4
Download citation
Accepted : 19 June 2020
Published : 30 July 2020
Issue Date : October 2020
DOI : https://doi.org/10.1038/s41581-020-0315-4

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Lipid parameters, adipose tissue distribution and prognosis prediction in chronic kidney disease patients.
- Hui-fen Chen
- Bing-jie Xiao
Lipids in Health and Disease (2024)
Renal rehabilitation learning in Japanese physical therapy schools: a fact-finding study
- Toshiki Kutsuna
- Yuhei Otobe
- Ryota Matsuzawa
Renal Replacement Therapy (2024)
The disruptor of telomeric silencing 1-like (DOT1L) promotes peritoneal fibrosis through the upregulation and activation of protein tyrosine kinases
- Yingfeng Shi
Molecular Biomedicine (2024)
The Intersectoral Coordination Unit for the Sustainable Intensification of Peritoneal Dialysis in Schleswig–Holstein (SKIP-SH) cohort study
- Hauke S. Wülfrath
- Thorben Schrumpf
- Benedikt Kolbrink
BMC Nephrology (2024)
Quality of life and nutritional status in peritoneal dialysis patients: a cross-sectional study from Palestine
- Dania Haddad
- Inad Nawajah
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Transplant Research Center
Kidney and pancreas transplant research program.
The Kidney and Pancreas Transplant Research Program within the Mayo Clinic Transplant Research Center focuses its research efforts on overcoming the challenges that limit the success of kidney and pancreas transplantation.
Research focus areas
Research focus areas in the Kidney and Pancreas Transplant Research Program include:
Overcoming antibody barriers
Reducing disease recurrence, improving long-term success, expanding living-donor transplants, pre-emptive transplantation, immunosuppression characteristics and new treatments, managing cardiovascular complications.
Here's a closer look at the focus areas.
Researchers in the Kidney and Pancreas Transplant Research Program have led the way in developing treatment strategies that reduce antibody levels, which allowed for successful living-donor kidney transplants even with incompatible blood types.
Antibodies are proteins produced by the body to protect it against substances, cells or organs that are foreign. These antibodies are essential for health. However, at times these antibodies can prevent a patient from receiving a transplant, because they can quickly destroy the transplanted kidney or pancreas. Antibodies can be directed against blood groups or against donor cells (called a positive crossmatch).
Researchers have developed several strategies to allow transplantation even in the presence of antibodies.
- At Mayo Clinic, investigators apply sophisticated techniques to differentiate which antibodies pose a problem for transplantation and which don't.
- Researchers also apply techniques to remove these antibodies from blood before transplant and to control negative effects of these antibodies on the kidney with medications.
- Patients with kidney failure and high levels of antibodies can receive kidneys from a larger pool of living donors and may be able to participate in Mayo Clinic's paired donation program . As a result, qualified patients with kidney failure are able to avoid lengthy or indefinite waiting periods for deceased-donor transplants.
Ongoing research in the area of antibody resistance includes:
- The effect of antibody development on the transplanted kidney and pancreas
- Laboratory investigations of the cells responsible for producing damaging antibodies
- Clinical testing of new treatments for reducing or preventing the production of donor-specific antibodies
Researchers in the Kidney and Pancreas Transplant Research Program are studying ways to identify treatments that reduce the risk of recurrent disease after a kidney transplant.
Several of the diseases that can cause kidney failure can come back and attack a newly transplanted kidney. In fact, approximately 20% to 25% of kidney transplants are lost because of disease recurrence.
To address this problem, Mayo Clinic researchers have taken several steps.
- First, researchers identified diseases that are most likely to recur in the transplanted kidney.
- Second, with the use of protocol biopsies, researchers identified the earliest signs of disease recurrence in the transplanted kidney, with the intent of improving outcomes by treating patients when disease recurrence is detected.
- Third, researchers developed new treatments that can be given to the patient to prevent disease recurrence or to treat it when it occurs.
Researchers have made significant advances in the prevention and treatment of some of the most dangerous diseases for the transplanted kidney, including focal segmental glomerulosclerosis , membranous nephropathy and membranoproliferative glomerulonephritis.
Research in this area continues to further refine disease classification and identify optimal treatment options to prevent disease recurrence because each of these diseases seems to require specific management strategies.
The premature loss of transplanted organs traditionally has been attributed to poorly characterized diseases that are frequently lumped together under the term "chronic rejection."
Researchers in the Kidney and Pancreas Transplant Research Program have taken a different approach to this problem by first questioning what precisely causes the loss of kidney and pancreas transplants.
Using kidney biopsies and careful examination of laboratory and clinical parameters, the Mayo Clinic researchers determined that kidney transplants can be lost for multiple reasons. In most cases, the cause of the kidney loss was because of known diseases that could potentially be prevented and treated.
These investigations also make it abundantly clear that a comprehensive approach addressing all the threats that can harm the transplanted organ is needed for transplant success.
Researchers developed a series of tools that allow them to monitor outcomes-particular management strategies.
These tools include:
- A continually updated database to gather information about a multitude of clinical, laboratory and biopsy parameters in thousands of patients over time
- Protocol biopsies for pathology review of kidney transplants at specific time points during the first 10 years after transplant
- Microarray studies examining the expression of thousands of genes within kidney transplant tissues, helping researchers understand how the kidney reacts in response to injury
- Screening tests for potentially damaging viral infections and immune responses in kidney transplant recipients
- Monitoring of the production of antibodies against the transplanted kidney or pancreas, thus allowing researchers to determine whether immunosuppressive medications that the patient receives after the transplant are effective
Mayo Clinic has performed living-donor kidney transplants since 1963 and has the largest single-living-donor program in the United States.
In order for the practice of living-donor transplant to remain successful and safe for both donor and recipient, researchers in the Kidney and Pancreas Transplant Research Program have placed a high priority on research into the short- and long-term outcomes for kidney donation.
Ongoing clinical investigations include:
- Effect of kidney donation on blood pressure
- Impact of obesity on kidney donation
- Long-term outcomes of kidney donation
- Impact of donor age on transplant success
Researchers in the Kidney and Pancreas Transplant Research Program also have a strong commitment to expand pre-emptive transplantation — transplantation of a kidney in patients who have never received dialysis.
Investigators have shown in the past that dialysis prior to transplantation limits the life span of the recipient. Avoiding dialysis, particularly for more than one year, has beneficial consequences for the patient and perhaps the transplanted organ. In addition, pre-emptive transplantation reduces medical costs.
At times, patients are told that a period of dialysis is required before receiving a transplant. Researchers at Mayo Clinic believe that patients should be encouraged to consider transplantation as their kidneys start to fail.
The success of this program is closely tied to the success of living-donor kidney transplantation in the Kidney and Pancreas Transplant Research Program. However, it's possible to achieve pre-emptive transplantation with deceased-donor kidneys if patients are placed on the waiting list soon enough.
Ongoing investigations include:
- Evaluation of medical, psychological, quality of life and financial implications of pre-emptive transplantation
- Evaluation on how collaborative efforts with referring physicians and even insurance companies can facilitate pre-emptive transplantation
The Kidney and Pancreas Transplant Research Program has conducted and participated in many clinical studies designed to examine the benefits associated with new immunosuppressive medications and combinations of medications compared with established regimens.
Because most organ transplants require the use of two or more medications combined over time, the design of optimal immunosuppression regimens for individual organs and patient groups has grown more complex.
Mayo Clinic researchers have examined changes in the structure of transplanted kidneys using protocol biopsies and determined that prolongation of kidney survival likely will require re-examination of the approach to immunosuppression after the first year post-transplant.
By understanding what is happening inside the transplanted organ, researchers can design new strategies to achieve long-term success. Protocol biopsies are essential tools to achieve this goal.
Mayo Clinic researchers and physicians are conducting research to identify the causes of cardiovascular disease in kidney transplant recipients and the benefits of different treatment and prevention strategies.
Certain kidney transplant recipients are at higher risk of developing cardiovascular complications, such as heart attack, stroke and loss of blood supply to the limbs, typically from underlying medical diseases and medication-related toxicities.
Research studies using database information and measuring heart biomarkers in thousands of kidney transplant recipients at Mayo Clinic have improved the ability to identify patients who are at risk of heart problems after transplant
Investigators have found that the biomarker troponin levels can provide a more accurate measure of cardiac risk than can traditional cardiac measurements, such as a cardiac stress test, and can indicate whether surgical intervention is needed prior to kidney transplant.
The goals of this research are to achieve lower risk for patients and to conduct cardiac studies for patients who can benefit the most.
Ongoing studies include:
- Determining whether troponin can also be used to assess heart status after kidney or pancreas transplant
- Assessing how modifications in the treatment of patients before the transplant can reduce levels of troponin and perhaps reduce risk
Program faculty
Here's a list of faculty in the Kidney and Pancreas Transplant Research Program by campus location.
- Chakkera, Harini, M.D.
- Heilman, Raymond L., M.D.
- Huskey, Janna L. M.D.
- Kaplan, Bruce, M.D.
- Khamash, Hasan A, M.D.
- Mathur, Amit K., M.D.
- Morales, Alejandro, M.D.
- Moss, Adyr A., M.D.
- Petrides, Savas, M.D.
- Reddy, Kunam S., M.B.B.S.
- Singer, Andrew L., M.D., Ph.D.
- Sukumaran, Sumi Nair, M.B.B.S.
- Croome, Kristopher (Kris) P., M.D.
- Gonwa, Thomas A., M.D.
- Lee, David D., M.D.
- Mai, Martin L., M.D.
- Oshel, Katherine, M.D.
- Perry, Dana K., M.D.
- Prendergast, Mary B., M.D.
- Taner, Burcin C., M.D.
- Wadei, Hani M., M.D.
- Amer, Hatem, M.D.
- Cosio, Fernando G., M.D.
- Cramer, Carl H. II., M.D.
- Dean, Patrick G., M.D.
- Heimbach, Julie K., M.D.
- Hickson, LaTonya J., M.D.
- Issa, Naim S., M.D.
- Kudva, Yogish C., M.B.B.S.
- Kukla, Aleksandra M.D.
- Larsen, Brandon T., M.D., Ph.D.
- Lorenz, Elizabeth C., M.D.
- Norby, Suzanne M., M.D.
- Nyberg, Scott M.D., Ph.D.
- Prieto, Mikel, M.D.
- Schinstock, Carrie A., M.D.
- Stegall, Mark D., M.D.
- Taler, Sandra J., M.D.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

Search form
News: hhs announces new model to improve access to kidney transplants.
Last week, the United States Department of Health and Human Services (HHS) announced the proposed Increasing Organ Transplant Access (IOTA) Model .
This proposed model would aim to do the following:
- Increase access to kidney transplants for all people living with end-stage renal disease (ESRD)
- Improve the quality of care for people seeking kidney transplants
- Reduce disparities among individuals undergoing the process to receive a kidney transplant
- Increase the efficiency and capability of transplant hospitals selected to participate
In a press release published by CMS , HHS stated that people with ESRD who receive kidney transplants have better outcomes than people who receive dialysis, improvements in quality of life, and are freed from lengthy and burdensome dialysis treatments. They point to the scarcity of organs as a leading cause of increased patient mortality and a significant gap between demand and supply.
“Despite this scarcity, approximately 30% of donor kidneys go unused annually, highlighting gaps in procurement, distribution, and utilization,” they stated in the press release. “Prolonged waiting times, averaging three to five years or more, intensify patient suffering. With just over 28,000 kidney transplants performed in 2023 and more than 90,000 people on a waitlist during that same time, urgent measures are needed to improve the efficacy and efficiency of the system.”
The proposed IOTA model would measure participating transplant hospitals by increases in the number of transplants, increased organ acceptance rates, and post-transplant outcomes, and offer additional performance incentives to improve equity in the transplant process. IOTA would also hold kidney transplant hospitals accountable for the care they provide and require them to establish health equity plans that would identify and develop strategies to address gaps in access among populations in their communities.
Editor’s note : To read the CMS press release, click here . To access the proposed rule, click here .
More Like This
Conference corner: 2024 conference posters now available to acdis membership, news: upmc will pay $38 million to settle whistleblower lawsuit, news: antibiotic for treating sepsis linked to increased mortality, study shows.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 21, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New study uses health factors to predict kidney function recovery
by Cedric Ricks, University of Cincinnati
Researchers at the University of Cincinnati College of Medicine have created a scoring model that uses key health indicators to accurately predict recovery for patients who experience kidney failure due to acute kidney injury (AKI), which occurs when kidneys stop working properly, and can range from minor loss of kidney function to complete failure.
AKI is a major contributor to end-stage kidney disease (ESKD). About a third of patients with ESKD due to AKI recover kidney function.
Their findings, published in the Clinical Kidney Journal , predict kidney recovery within 90 days and 12 months after the start of dialysis for kidney failure patients due to AKI. The study looked at the health outcomes of 22,922 patients from the U.S. Renal Data System from 2005 to 2014 to offer predictions.
Lead author Silvi Shah, MD, associate professor in the Division of Nephrology at UC, explains that researchers examined several factors used in logistic regression models to analyze the effect of various covariates on a patient's health outcome and found that 24% and 34% of patients with kidney failure due to AKI recovered kidney function within 90 days and 12 months, respectively.
Patient factors such as age, race or ethnicity, body mass index, congestive heart failure, cancer, amputation, functional status, hemoglobin and prior nephrology care are used in the regression model.
"One of the significant comorbidities was a history of heart failure," Shah says. "If you had heart failure, you were at a lower chance of recovering as well. If you had a lower body mass index, you had a lower chance of recovery; if you had amputation or poor functional status, you had a low chance of recovery. These were some of the significant predictors of the score."
Shah notes that younger people got more points in her prediction model because older age was also associated with a likelihood of lower recovery for kidney failure patients due to AKI.
The scoring model was developed as a way to help staff providing clinical care in a dialysis unit quickly triage patients based on characteristics that would be easily available in their medical history, explains Charuhas Thakar, MD, a senior author in the study, professor at Queen's University Belfast in the United Kingdom and former division chief of nephrology at the University of Cincinnati.
"Patients who develop dialysis dependent AKI typically receive care in dialysis facilities among other patients with end stage kidney disease," says Thakar. "Our goal was to quickly triage and determine who has the higher, medium or low prospect of recovery based on clinical characteristics the patient had in their medical history ."
"That was our broad approach. If your score is high, you have the best chance of recovery," says Thakar. "Patients who are more likely to recover can be monitored closely for that prospect and we can focus our energies on preserving renal function. At the same time, those who are unlikely to recover should be allowed to undergo long term planning including transplantation. In summary, our study paves the way to individualize care as well as facilitate efficient use of treatment and resources."
Shah says it's important to understand that patients who have kidney failure due to AKI can recover.
"Around one-fourth of those patients will recover in 90 days, and around one-third of those patients will recover in 12 months," says Shah. "There are several factors which can predict the recovery rate. If you have lower body mass index, if you are Black, have congestive heart failure or a history of amputation, you have lower chances of recovery.
"It helps us to do a risk prediction score and at the same time helps us to tell patients and health care providers what is the percentage recovery that may be expected," adds Shah. "If you fall in the high score category, they have a 57% chance of recovery in 90 days. This is very encouraging for both patients and physicians."
She emphasizes that researchers included the largest data in the U.S.—the United States Renal Data System—and captures all patients on dialysis in the country, including information for women, men and different races and ethnicities.
"One of the biggest strengths of the paper was that it was inclusive, and the score was individualized," Shah says. "It can help in counseling and risk prediction and tailoring treatment specifically for patients that have dialysis-dependent AKI, and based on the score, we can tell patients what's the chance of their recovery ."
Other co-authors of the research at the University of Cincinnati are Department of Environmental and Public Health Sciences professors Anthony Leonard, Ph.D.; Karthikeyan Meganathan, Ph.D.; and Annette Christianson; along with Kathleen Harrison from the Division of Nephrology. Jia Ng, MD, assistant professor of medicine at Hofstra University, is also a co-author of the study.
Explore further
Feedback to editors

Study: Newborns whose mother spoke in a mix of languages during pregnancy are more sensitive to a range of sound pitches
Study finds ultraviolet radiation may affect subcutaneous fat regulation, could lead to new obesity treatments

Regular fish oil supplement use might increase first-time heart disease and stroke risk
6 hours ago

Pedestrians may be twice as likely to be hit by electric/hybrid cars as petrol/diesel ones
Study finds jaboticaba peel reduces inflammation and controls blood sugar in people with metabolic syndrome
8 hours ago

Study reveals how extremely rare immune cells predict how well treatments work for recurrent hives
9 hours ago

Researchers find connection between PFAS exposure in men and the health of their offspring

Researchers develop new tool for better classification of inherited disease-causing variants

Specialized weight navigation program shows higher use of evidence-based treatments, more weight lost than usual care

Exercise bouts could improve efficacy of cancer drug
10 hours ago
Related Stories
Mortality rates higher following kidney injury, research finds
Jun 19, 2020

Comparing pregnancy rates in patients undergoing two types of dialysis
Mar 8, 2024

Study explores barriers to contraceptive use in females with kidney disease
Jan 31, 2024

Certain factors linked with kidney function recovery in children on dialysis
Sep 20, 2018
Research shows racial disparities in pregnant women on dialysis
Sep 26, 2019

Brief dialysis may be best for some kidney patients
Sep 28, 2023
Recommended for you
Drug-like inhibitor shows promise in preventing flu

Hope for a cure for visceral leishmaniasis, an often fatal infectious disease
14 hours ago
Matcha mouthwash shown to inhibit bacteria that cause periodontitis
16 hours ago

AI can help improve ER admission decisions, study finds

New AI algorithm may improve autoimmune disease prediction and therapies
May 20, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
UC study uses health factors to predict kidney function recovery
Age, race, heart health, body mass among key factors in kidney care with dialysis-dependent aki.

Researchers at the University of Cincinnati College of Medicine have created a scoring model that uses key health indicators to accurately predict recovery for patients who experience kidney failure due to acute kidney injury (AKI), which occurs when kidneys stop working properly and can range from minor loss of kidney function to complete failure.
AKI is a major contributor to end-stage kidney disease (ESKD). About a third of patients with ESKD due to AKI recover kidney function.
Their findings, published in the Clinical Kidney Journal , predict kidney recovery within 90 days and 12 months after the start of dialysis for kidney failure patients due to AKI. The study looked at the health outcomes of 22,922 patients from the U.S. Renal Data System from 2005 to 2014 to offer predictions.
Lead author Silvi Shah, MD, associate professor in the Division of Nephrology at UC, explains that researchers examined several factors used in logistic regression models to analyze the effect of various covariates on a patient’s health outcome and found that 24% and 34% of patients with kidney failure due to AKI recovered kidney function within 90 days and 12 months, respectively.
Patient factors such as age, race or ethnicity, body mass index, congestive heart failure, cancer, amputation, functional status, hemoglobin and prior nephrology care are used in the regression model.
“One of the significant comorbidities was a history of heart failure,” Shah says. “If you had heart failure, you were at a lower chance of recovering as well. If you had a lower body mass index, you had a lower chance of recovery; if you had amputation or poor functional status, you had a low chance of recovery. These were some of the significant predictors of the score.”
Dr. Charuhas Thakar and Dr. Silvi Shah are shown in the UC College of Medicine. Photo by Colleen Kelley/UC Marketing + Brand.
Shah notes that younger people got more points in her prediction model because older age was also associated with a likelihood of lower recovery for kidney failure patients due to AKI.
The scoring model was developed as a way to help staff providing clinical care in a dialysis unit quickly triage patients based on characteristics that would be easily available in their medical history, explains Charuhas Thakar, MD, a senior author in the study, professor at Queen’s University Belfast in the United Kingdom and former division chief of nephrology at the University of Cincinnati.
“Patients who develop dialysis dependent AKI typically receive care in dialysis facilities amongst other patients with end stage kidney disease,” says Thakar. “Our goal was to quickly triage and determine who has the higher, medium or low prospect of recovery based on clinical characteristics the patient had in their medical history.”
“That was our broad approach. If your score is high, you have the best chance of recovery,” says Thakar. “Patients who are more likely to recover can be monitored closely for that prospect and we can focus our energies on preserving renal function. At the same time, those who are unlikely to recover should be allowed to undergo long term planning including transplantation. In summary, our study paves the way to individualize care as well as facilitate efficient use of treatment and resources.”
Shah says it’s important to understand that patients who have kidney failure due to AKI can recover.
“Around one-fourth of those patients will recover in 90 days, and around one-third of those patients will recover in 12 months,” says Shah. “There are several factors which can predict the recovery rate. If you have lower body mass index, if you are Black, have congestive heart failure or a history of amputation, you have lower chances of recovery.
“It helps us to do a risk prediction score and at the same time helps us to tell patients and healthcare providers what is the percentage recovery that may be expected,” adds Shah. “If you fall in the high score category, they have a 57% chance of recovery in 90 days. This is very encouraging for both patients and physicians."
She emphasizes that researchers included the largest data in the U.S. — the United States Renal Data System — and captures all patients on dialysis in the country, including information for women, men and different races and ethnicities.
“One of the biggest strengths of the paper was that it was inclusive, and the score was individualized,” Shah says. “It can help in counseling and risk prediction and tailoring treatment specifically for patients that have dialysis-dependent AKI, and based on the score, we can tell patients what’s the chance of their recovery.”
Other co-authors of the research at the University of Cincinnati are Department of Environmental and Public Health Sciences professors Anthony Leonard, PhD; Karthikeyan Meganathan, PhD; and Annette Christianson; along with Kathleen Harrison from the Division of Nephrology. Jia Ng, MD, assistant professor of medicine at Hofstra University, is also a co-author of the study.
Both Silvi Shah and Jia Ng had career development awards from the National Institutes of Health. Ng also received support from the Breslin Family Foundation. Shah received intramural funds from the UC Division of Nephrology.
Disclosures: Jia Ng received consultancy fees from Vifor Pharmaceuticals. She is a founder of PublishedMD Consulting LLC.
Read the research study online .
Impact Lives Here
The University of Cincinnati is leading public urban universities into a new era of innovation and impact. Our faculty, staff and students are saving lives, changing outcomes and bending the future in our city's direction. Next Lives Here.
- College of Medicine
- Public Health
- Environmental Health
- Academic Health Center
Related Stories
May 21, 2024
Researchers at the University of Cincinnati College of Medicine have created a scoring model that uses key health indicators to accurately predict recovery for patients who suffer kidney failure due to acute kidney injury (AKI).
U.S. News & World Report: Metformin may help young patients with bipolar disorder avoid weight gain
October 31, 2023
U.S. News & World Report highlighted recent research led by the University of Cincinnati and Northwell Health that found the drug metformin can help prevent or reduce weight gain in youth taking medication to treat bipolar disorder.
UC research examines workplace stress in the aftermath of the COVID-19 pandemic
November 28, 2022
A new study from the University of Cincinnati examined the impact of workplace stress and provided insights for organizations to explore ways to reduce workplace stress for a better and healthier working environment. The study had two parts: a survey in which respondents described their experiences of workplace stress during the COVID-19 pandemic and a quantitative study using saliva cortisol as a biomarker of stress along with a stress diary to find out where and when workplace stress commonly occurred.
Studies reveal cell-by-cell changes caused when pig hearts and kidneys are transplanted into humans
Surgical teams at NYU Langone Health performed the world's first genetically modified pig kidney transplants into a human body in September and November 2021, and then transplanted two pig hearts in the summer of 2022. These procedures were done in patients declared dead based on neurologic criteria (decedents) and maintained on ventilators with the consent of their families. Demonstrating the field's progress, NYU Langone in April 2024 transplanted a pig kidney into a living patient.
Now two new analyses, one published online on May 17 in Nature Medicine and the other May 21 in Med , reveal changes at the single-cell level in the organs and recipient's bodies before, during, and just after the xenotransplantation surgeries in the decedents. Teams of scientists had worked alongside the surgeons, taking blood and tissue samples to analyze changes in tens of thousands of collected cells.
Led by researchers at NYU Grossman School of Medicine and the Broad Institute of MIT and Harvard, the Med paper tracked the genetic and cellular activity in the two pig kidneys transplanted into humans, and compared them against pig kidney samples that had not been translated. To do so, the research team used several techniques, including single-cell RNA sequencing, which determined the order (sequence) of the molecular letters making up the pig and human genes active in various cell types during the procedures.
The study showed that the transplanted pig kidneys, while not rejected outright by the recipients' bodies (no immediate kidney failure), caused a strong reaction in human peripheral blood mononuclear cells (PBMCs). This set of immune cells can attack transplanted (foreign) organs much like they attack foreign invaders (e.g. viruses). While immediate rejection was not seen, in part due to treatment with medications that suppressed it, the new study found evidence of subtler reactions that could cause xenotransplants to fail over time.
Specifically, the pig kidneys were seen to trigger "antibody-mediated rejection" at the molecular level. As the body develops immune proteins called antibodies specific to a transplanted organ, they recruit natural killer cells, macrophages, and T cells that can injure it. The team also saw an uptick in pig kidneys of tissue repair mechanisms, where certain cells multiply as part of the growth involved in healing. Normal cells that transform into cancer cells also grow aggressively, so the mechanism bears watching.
"We have detailed the cellular mechanisms that dictate how human immune cells react to a xenotransplant in the short term," said Jef Boeke, PhD, a co-senior author on both studies, and director of the Institute for System Genetics at NYU Grossman School of Medicine. "These results give us new insights into how we might further engineer pig organs for transplant, or tailor immunosuppression treatments to improve tolerance of a foreign organ."
By tracking the interplay between the kidneys and human system several times each day, the researchers found that pig kidney immune cells drove reactions right after the transplant, but that human immune cells infiltrated the pig organs by 48 hours to dominate signaling. Measuring the degree to which pig immune cells trigger the first wave of immune attack on xenotransplants will shape efforts to prevent irreversible cellular damage to them, say the study authors.
Transplanted Hearts
The other new paper, published in Nature Medicine , featured a "multiomics" analysis of pig hearts and surrounding human cells in decedents. This included analyses every six hours after transplant of gene activity (transcriptomics), as well of proteins (proteomics), lipids, and metabolites (intermediates in biological pathways) present in cells.
Rapid, massive increases in the number of certain cell types were also seen in decedents receiving pig hearts. In one of the decedents (D1) but not the other, activated T cell and natural killer (NK) cell populations within the PBMC group increased from about one percent 30 hours post-transplant to more than 20 percent of the entire PBMC population by 66 hours after the procedure. This dramatic immune reaction to the organ, a complication called perioperative cardiac xenograft dysfunction (PCXD), came with a damaging inrush of immune cells (inflammation), and misplaced healing attempts (tissue remodeling) that thicken tissue and can hinder function.
The worse outcomes experienced by the one decedent may be partly because this heart was smaller than anticipated for the recipient's size, and required an extra procedure to compensate for it, the researchers said. These factors may have cut off blood flow and the oxygen supply to the heart for longer, which is known to cause ischemia reperfusion injury when the supply is restored. The research team observed that PCXD-related immune reactions to the pig organ got worse in the presence of this recipient's reperfusion injury.
"This study demonstrated that multiomics can be used to reveal a broad picture of what is happening in the recipient of a xenograft," said Brendan Keating, PhD, a co-senior author on both studies and faculty in Department of Surgery at NYU Grossman School of Medicine. "The team that did the xenotransplant had several theories about why the first decedent was having more issues, but multiomics helped to define the complications, and may be used to counter them moving forward."
- Immune System
- Medical Topics
- Biotechnology and Bioengineering
- Biotechnology
- Molecular Biology
- Human physiology
- Human anatomy
- Development of the urinary and reproductive organs
- Developmental biology
- Somatic cell nuclear transfer
- Morphogenesis
- Trait (biology)
- Cell (biology)
Story Source:
Materials provided by NYU Langone Health / NYU Grossman School of Medicine . Note: Content may be edited for style and length.
Journal Reference :
- Wanqing Pan, Weimin Zhang, Binghan Zheng, Brendan R. Camellato, Jeffrey Stern, Ziyan Lin, Alireza Khodadadi-Jamayran, Jacqueline Kim, Philip Sommer, Karen Khalil, Elaina Weldon, Jiangshan Bai, Yinan Zhu, Peter Meyn, Adriana Heguy, Massimo Mangiola, Adam Griesemer, Brendan J. Keating, Robert A. Montgomery, Bo Xia, Jef D. Boeke. Cellular dynamics in pig-to-human kidney xenotransplantation . Med , 2024; DOI: 10.1016/j.medj.2024.05.003
Cite This Page :
Explore More
- Stopping Flu Before It Takes Hold
- Cosmic Rays Illuminate the Past
- Star Suddenly Vanish from the Night Sky
- Dinosaur Feather Evolution
- Warming Climate: Flash Droughts Worldwide
- Record Low Antarctic Sea Ice: Climate Change
- Brain 'Assembloids' Mimic Blood-Brain Barrier
- 'Doomsday' Glacier: Catastrophic Melting
- Blueprints of Self-Assembly
- Meerkat Chit-Chat
Trending Topics
Strange & offbeat.
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- National Health Service
- Patient safety
- SaBTO microbiological safety guidelines
- Department of Health & Social Care
Chapter 4: referral for donation
Updated 21 May 2024

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/guidance-on-the-microbiological-safety-of-human-organs-tissues-and-cells-used-in-transplantation/chapter-4-referral-for-donation
Deceased donors
The donor’s family and/or the most relevant life partner should be interviewed, and supporting information obtained from relevant health professionals such as the donor’s GP. Standard questionnaires are used to seek relevant information and should be kept as part of the donor record. Wherever possible any post-mortem findings will need to be available to ensure that an appropriate risk assessment is complete and that all information pertaining to the cause of death is taken into account.
Current microbiological results on the donor should be available from the donor’s clinician and must be included in the comprehensive patient assessment process conducted by the trained healthcare professional. Where circumstances dictate, the risk arising from the use of materials from potentially infected, or known to be infected, donors should be discussed with a consultant or specialist with relevant knowledge regarding and expertise regarding that infectious risk. Advice from a specialist centre may be required for defining the balance between risk and benefit. Such discussion is normally between medical staff in the transplant unit receiving the organ and the relevant local transplant unit consultant microbiologist or virologist.
The information gathered by the trained healthcare professional must include the relevant microbiological findings.
Assessment of donor risk does not end at the time of retrieval of tissues and organs. Important information relating to the risk of transmission of infection may become available after transplantation. This information must be made available by the organ donation team or tissue establishment to the recipient centres for appropriate management of the recipient.
Living donors
Some people wish to donate an organ to a relative, to another person they are close to or to an individual they do not know. The latter situation may occur in the setting of paired donation, pooled donation and non-directed altruistic donation. Others may agree to donate surplus tissue, such as heart valves from the replaced heart after heart transplantation, bone following hip-replacement surgery, or umbilical cells to a cord blood bank. The information needed to assess any risk should be gathered from the potential donor or, in the case of child donors too young to understand the issues, from the adult with parental responsibility for that child as specified by the Human Tissue Act 2004 [footnote 1] and the current Human Tissue Authority ( HTA ) code of practice on consent .
Autologous tissue donation is a special example of living donation where tissue or cells previously taken from an individual is transplanted to meet that same individual’s clinical needs. Where tissues and cells for autologous donation are removed and are to be stored and/or cultured, then microbiological screening of the donor prior to the procedure is a requirement of the Human Tissue (Quality and Safety for Human Application) Regulations 2007 . HTA has a statutory requirement that while awaiting microbiological testing results, tissues and cells are quarantined regardless of ultimate destination. An autologous donation from an infected donor must be stored securely in a way that does not pose an infection risk to other donations stored in the same facility.
Donors of gametes (spermatozoa or eggs) and embryos
The various uses of gametes and embryos for reproductive purposes have additional considerations to organ or tissue transplantation. Their use in the human body carries a small risk of infection transmission not only to recipients, but also to any child that might result from fertility procedures.
Partner donation is defined as a procedure that intends to produce a pregnancy in a woman who is in an intimate relationship with a man who is providing the spermatozoa. In relation to infection risk, it may be considered to be similar to autologous donation.
Donation of gametes or embryos to a non-partner recipient requires further assessments and testing. The definition of such donors is given in the Human Fertilisation and Embryology Authority ( HFEA ) code of practice . Where cryopreserved embryos are donated to a third party, there may be a period of several years between the embryo creation and donation.
Embryonic stem cell ( ESC ) lines for therapeutic use may be derived from embryos created for treatment of infertility that are surplus to clinical requirements. Similar criteria apply as for other stem cell lines taking account of the potential time interval between embryo creation and donation. If the embryos are created for the purpose of creation of ESCs rather than fertility, the same criteria will apply as for tissue and cell donation. Further guidance relating to the regulatory requirements for conducting human stem cell research is available on the EuroStemCell website.
SaBTO has previously published a review considering risks of infection from cell based advanced therapies: Donation of starting material for cell-based advanced therapies: a SaBTO review .
Table 1 gives examples of donations relevant to these guidelines.
Table 1: examples of donors and circumstances of donation
Note 1: donation for corneas should take place within 24 hours of death.
See a list of all chapters in this guidance
Donation in Scotland is undertaken according to the Human Tissue (Scotland) Act 2006 . ↩
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .

COMMENTS
Abstract. Recent advances in surgical, immunosuppressive and monitoring protocols have led to the significant improvement of overall one-year kidney allograft outcomes. Nonetheless, there has not been a significant change in long-term kidney allograft outcomes. In fact, chronic and acute antibody-mediated rejection (ABMR) and non-immunological ...
Kidney transplantation is limited by graft rejection, complications owing to long-term immunosuppression and a shortage of donor organs. ... Kidney Research Institute, New York City, NY, USA.
Over the past 65 years, kidney transplantation has evolved into the optimal treatment for patients with kidney failure, dramatically reducing suffering through improved survival and quality of life. However, access to transplant is still limited by organ supply, opportunities for transplant are inequitably distributed, and lifelong transplant survival remains elusive. To address these ...
The New England Journal of Medicine has published the most manuscripts on kidney transplantation (n = 26) and is the most cited journal (n = 15,642). The United States has the highest number of publications (n = 61). Kashika is the corresponding author with the most published papers (n = 5; 2892 citations).
R.A. Montgomery and OthersN Engl J Med 2022;386:1889-1898. Genetically modified pig kidney xenografts were transplanted into two brain-dead human recipients. The xenografts functioned immediately ...
The survival advantages of transplantation over long-term dialysis are generally well described, provided a given patient with end-stage kidney disease is deemed a candidate for a transplant. 1,2 ...
Research Topics. See all (4) Learn more about Research Topics. Transplant-related clinical and translational research, reflecting the dynamic field of kidney transplantation from immunobiology to noninvasive genomic biomarkers in the diagnosis of acute rejection.
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Background: In kidney ...
The demand for kidney transplants is far from met by human donors — a problem that may be solved by the clinical translation of porcine kidney xenotransplantation. A new paper describes the ...
Combined islet and kidney xenotransplantation for diabetic nephropathy: an update in ongoing research for a clinically relevant application of porcine islet transplantation, Frontiers in ...
All other transplant papers in Volume 35 of NDT are also highly recommended and acknowledged. In the January 2020 issue of NDT, the research group from the Westmead Hospital and University of Sydney investigated a potential obesity and gender bias in access to deceased donor kidney transplantation . This is an important clinical issue and all ...
Adult kidney transplant recipients (KTRs) fully vaccinated against COVID-19 have substantial morbidity and mortality related to SARS-CoV-2 infection compared with the general population. However, little is known regarding the safety and efficacy of the COVID-19 vaccination series in pediatric KTRs.
This review seeks to improve understanding of kidney disease, dialysis and transplant and identify future areas of research to improve kidney outcomes in end-stage renal disease population ...
Kidney failure is defined by a glomerular filtration rate <15 ml/min/1.73 m 2 (ref. 7) and may be treated using KRT (which refers to either dialysis or transplantation) or with supportive care 8 ...
The purpose of this trial is to evaluate the reduction in incidence and severity of delayed graft function when using QPI-1002 with kidney transplants from donors older than 45 years who have brain death. J&J Vaccine Booster in Transplant Recipients Scottsdale/Phoenix, AZ; Jacksonville, FL; Rochester, MN.
We aimed to assess the performance of a published genotype-informed tacrolimus dosing model in an independent set of adult AA kidney transplant (KTx) recipients. CYP3A5 genotypes were obtained for all AA KTx recipients (n = 232) from 2010 to 2019 who met inclusion criteria at a single transplant center in Philadelphia, Pennsylvania, USA.
Researchers in the Kidney and Pancreas Transplant Research Program have taken a different approach to this problem by first questioning what precisely causes the loss of kidney and pancreas transplants. Using kidney biopsies and careful examination of laboratory and clinical parameters, the Mayo Clinic researchers determined that kidney ...
Last week, the United States Department of Health and Human Services (HHS) announced the proposed Increasing Organ Transplant Access (IOTA) Model. This proposed model would aim to do the following: Increase access to kidney transplants for all people living with end-stage renal disease (ESRD) Improve the quality of care for people seeking ...
More information: Silvi Shah et al, A clinical score to predict recovery in end-stage kidney disease due to acute kidney injury, Clinical Kidney Journal (2024). DOI: 10.1093/ckj/sfae085
AKI is a major contributor to end-stage kidney disease (ESKD). About a third of patients with ESKD due to AKI recover kidney function. Their findings, published in the Clinical Kidney Journal, predict kidney recovery within 90 days and 12 months after the start of dialysis for kidney failure patients due to AKI. The study looked at the health ...
Jan. 20, 2022 — Researchers have announced the first peer-reviewed research outlining the successful transplant of genetically modified, clinical-grade pig kidneys into a brain-dead human ...
Kidney transplantation occurs within an overall organ donation and transplantation system (also known and referred to as the transplant ecosystem) that comprises a vast network of institutions dedicated to ensuring that patients are evaluated and, if appropriate, placed onto the organ transplant waitlist, and that those on the organ transplant ...
The donor's family and/or the most relevant life partner should be interviewed, and supporting information obtained from relevant health professionals such as the donor's GP. Standard ...