- Search Menu
- Advance articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Open Access
- Why Submit?
- About Journal of Language Evolution
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
1. introduction, 2. materials and methods, 3. analysis, 4. evaluation and discussion, 5. conclusion, acknowledgements, data availability, references–textbooks, the representation of animal communication and language evolution in introductory linguistics textbooks.
- Article contents
- Figures & tables
- Supplementary Data
Sławomir Wacewicz, Michael Pleyer, Aleksandra Szczepańska, Aleksandra Ewa Poniewierska, Przemysław Żywiczyński, The representation of animal communication and language evolution in introductory linguistics textbooks, Journal of Language Evolution , Volume 7, Issue 2, July 2022, Pages 147–165, https://doi.org/10.1093/jole/lzac010
- Permissions Icon Permissions
The last three decades have brought a wealth of new empirical data and methods that have transformed investigations of language evolution into a fast-growing field of scientific research. In this paper, we investigate how the results of this research are represented in the content of the most popular introductory linguistic textbooks. We carried out a comprehensive computer-assisted qualitative study, in which we inspected eighteen English-language textbooks for all content related to the evolutionary emergence of language and its uniqueness in nature, in order to evaluate its thematic scope, selection of topics, theories covered, researchers cited, structural soundness, currency, and factual accuracy. Overall, we found that the content of interest lacks a defined canonical representation across the textbooks. The coverage of animal communication was relatively broad, with some recurring classic examples, such as vervet monkeys or honeybees; this content was mostly structured around the ‘design features’ approach. In contrast, the coverage of topics related to language origins and evolution was much less extensive and systematic, and tended to include a relatively large the proportion of content of historical value (i.e. creation myths, ‘bow-wow’ theories). We conclude by making recommendations for future editions of textbooks, in particular, a better representation of important frameworks such as signalling theory, and of current research results in this fast-paced field.
Research on the origins and evolution of language origins has a troubled history of being viewed as unscientific (see e.g. Whitney 1873[1872 ], Doerfer 1973 , Fisiak 1985 ; for a discussion, see Kaplan 2021 ). However, as also stressed in a number of recent introductions to this field (e.g. Fitch 2010 , 2017 ; Gong et al. 2014 ; Dediu and de Boer 2016 ; Progovac 2019 ; Boeckx 2021 ), the field has transformed significantly. It no longer can be seen as armchair philosophizing producing just-so-stories ( Gould and Lewontin 1979 ; cf. Lewontin 1998 ), or a kind of ‘intellectual game’ ( Kendon 1991 : 202). Instead, it has progressed to regular research centred around the day-to-day addressing of solvable Kuhnian puzzles, including both empirical research and carefully constructed theoretical models. In particular, the perception of language evolution as a scientific field has been academically consolidated in terms of institutional indicators, such as prestigious flagship publications, research centres, funded projects, conferences, and journals (see esp. Dediu and de Boer 2016 ), to yield a program that is institutionally as well as theoretically progressive ( Lakatos 1978 ; for a discussion, see Żywiczyński 2018 : 200–202). In sum, it is fair to say that ‘[r]esearch on language evolution is undoubtedly among the fastest-growing topics in linguistics’ ( Nölle et al. 2020 ).
Has all of this progress also percolated to the teaching of linguistics, as reflected in the content of linguistics textbooks? Introductory textbooks are generally seen as places where Kuhnian ‘“normal science” is defined and acknowledged fact is represented’ ( Hyland 2004 : 105). They thus serve an important function of ‘canonizing discourse’ within a discipline ( Brown 1993 ; cf. Hyland 2004 ; Love 2006 ). That is, they provide an orthodox, coherent ‘epistemological map’ of the landscape of the discipline and what it is about ( Hyland 2004 : 105). In fact, many students view introductory textbooks as representing ‘concrete embodiments of the knowledge of their disciplines’ ( Hyland 2004 : 105). Textbooks are, therefore, an important source for students to develop academic literacy in a discipline and become acculturated into it ( Johns 1997 ; Love 2006 ). As Myers (1992 : 3) puts it, ‘writing and reading [textbooks] reproduces knowledge and reproduces academics’. As outlined above, language evolution research has reached the stage of Kuhnian ‘normal science’ and is playing an expanding and important role in the language sciences. As such, it should be well represented in introductory linguistic textbooks, given that these texts strive to offer a ‘balanced and uniformly excellent coverage of the full range of modern linguistics’ ( Fasold & Connor-Linton ), address ‘all the topics that a student will need in their study of language’ ( McGregor ), and ‘up-to-date coverage of all the important areas of linguistics’ ( O’Grady et al. ).
In 2016, in a conference abstract, we reported on an exploratory investigation of topics related to language evolution research in fourteen introductory-level linguistic textbooks, finding that language evolution was not given the same status as, for example, language acquisition, language change, language and the brain, language and culture, or language and society, which are most extensively covered. We also judged that ‘the teaching of language evolution to students of general linguistics rests on out-dated and largely inadequate conceptual frameworks, and fails to communicate major theoretical breakthroughs and empirical results’ ( Wacewicz et al. 2016 ). Here, we revisit this issue with more rigorous tools and an updated and extended scope. Specifically, we investigate what introductory textbooks say about two closely intertwined (see, in particular, the now-classic work by Hockett 1960 and Hauser et al. 2002 ) topics: the questions on how language emerged and how it differs from the communication of non-human animals (henceforth: animals). In this paper, we present a first-of-its-kind, large computer-assisted qualitative study, in which we inspected eighteen textbooks for all contents related to the evolutionary emergence of language and its uniqueness in nature, in order to evaluate its thematic scope, selection of topics, theories covered, researchers cited, structural soundness, currency, and factual accuracy. Our primary goal is to guide better-quality teaching to the new generations of linguists, but our study may also serve as a blueprint for similar projects in other areas relevant to linguistics, as well as in other disciplines.
2.1 Materials and design
To evaluate the popularity and influence of linguistic textbooks, we consulted Open Syllabus (OS), a non-profit service with a corpus of over nine million English-language syllabi from 140 countries ( https://opensyllabus.org ). 1 Based on the OS popularity metrics, OS appearances , and OS score (as of 20 January 2021—see Table 1 ), we selected sixteen general introductory textbooks to linguistics, which we complemented with two very recent textbooks. In our analyses, we worked with the most recent edition of each textbook available as of 20 January 2021.
The introductory linguistics textbooks analysed. Edition: most recent edition available as of 20 January 2021. Year: year of publication of the most recent edition; OS App, OS Score - appearances (App) and popularity score on a 1-100 scale (Score) indicators at Open Syllabus; Chapter (Animal Communication, Language Evolution): page count of the chapter dedicated to this category (if present), this number is inclusive of any sections in the chapter; Section: LTA - page count of the section dedicated to the subcategory Language Trained Animals (if present). Section: other - page count of the section dedicated to subcategories Animal Communication Behaviors and Animal vs Human Communication (if present).
2.1.1 Scope of the analysis
The content of interest for our analyses can be described as any content related to the origins of language and its status among animal communication systems, or more generally, a species-comparative or evolutionary perspective on language. This thematic range closely matches the scope of the Evolang , http://evolang.org/ , conference series, which is commonly regarded as the main conference in this field and often assumed as a reference point in language evolution research ( Bergmann and Dale 2016 ; Wacewicz and Żywiczyński 2017 ). Hence, in order to help operationalize the exact thematic profile of the content to be coded, we stipulated it as ‘content with a good fit to the Evolang conference’. Following up on Wacewicz et al. (2016 ; see also Bergmann and Dale 2016 , on the main Evolang topic clusters), we divided the content top-down into two main thematic categories of interest as Animal Communication and Language Evolution (comprising both explanations of how language originated and interdisciplinary research relevant to language evolution, but excluding purely historical language change). Including animal communication in addition to language evolution was motivated by the fact that the comparison of human and non-human communication systems represents one of the most central methods for the investigation of the evolution of language. Finding similarities and differences between aspects of human language and the communication systems of other animals can be informative as to the evolutionary foundations of language and evolutionary pressures shaping it. As such, the comparative methods are well represented in work on language evolution (e.g. Fitch 2010 ; Tallerman and Gibson 2012 ; Macmahon and McMahon 2013 ). For example, at the recent Joint Conference on Language Evolution ( https://sites.google.com/view/joint-conf-language-evolution/home ) in Kanazawa, Japan, in August 2022, out of the 196 plenaries, invited talks, talks, and posters, 43 (22%) were on animal communication and cognition. We coded passages appearing anywhere in the content of each book, including boxes, footnotes, captions, and exercises (but not indexes or references). Section 2.2 explains the details of the coding procedure, and Section 3.1 provides a more detailed breakdown and discussion of the final set of codes and categories.
2.2 Procedure
The material was coded by two expert coders (AP, AS) with the computer-assisted qualitative research software NVivo 1.3 ( QSR International Pty Ltd., released August 2020 ), in collaboration with two language evolution experts (SW, MP).
The cyclical coding/re-coding process was completed in five steps (cf. Saldaña 2015 ):
1) Training: Two coders were trained with a training set consisting of excerpts from two textbooks. The coders were instructed to identify and mark all passages related to language origins, language evolution, or animal communication as described above, assuming one sentence as a minimal passage and one paragraph as a maximum passage. Each of the identified passages was assigned a short label, that is, a code. The coders were instructed to adopt a bottom-up approach and use the topics discussed at the Evolang conferences as the reference point for coding. Two language evolution experts provided feedback.
2) Individual open coding: each textbook was open coded by the two expert coders, working independently of each other. The coders identified the relevant passages through a three-step procedure:
(a) The coder analysed the table of contents and the indexes of each textbook to pre-select the potentially most relevant chapters, sections, and pages for close reading;
(b) The coder then manually skimmed the full content of each textbook;
(c) Finally, the coder completed a series of targeted keyword searches for a broad range of general keywords (e.g. evolution, emergence, origins, etc.) and specific keywords including names of animal species, names of disciplines (e.g. archaeology), and key concepts (e.g. FOXP2).
The coders coded all identified passages in the bottom-up approach described above.
3) Consensus I: in the first consensus phase, the output files from both expert coders were merged into a single NVivo file, containing over 1000 codes. The coders discussed each coded passage to arrive at a consensual coding and synthesized the material into a hierarchical structure of codes and categories (cf. Saldaña 2015 ). The coding scheme was progressively updated in the process, resulting in a roughly twofold reduction in the number of codes.
4) Verification: in this step, two language evolution experts (MP, SW), working together, reviewed the correctness of the coding scheme. The two language evolution experts discussed each coded passage and recommended changes to be considered in the next consensus phase as discussion points. They also did an additional manual skimming of the full text of each book. Additionally, they evaluated the coded passages for the factual accuracy of the information contained therein. This resulted in an additional set of codes and their annotations (see below, Section 4.1), which were then incorporated into the NVivo codebook.
5) Consensus II: all passages marked during the verification phase as discussion points were discussed together by all four experts to determine consensual coding and consensual classification into categories. This led to a final total of 462 codes and coding categories, available in Supplementary Material .
3.1 Topics overview
The main categories of investigation are presented here in a top-down manner. First, we will deal with the question of how many textbooks mention our two main coding categories: Animal Communication and Language Evolution.
First, only the Language Files , O’Grady et al . 2 and Yule have chapters on Animal Communication, and only Yule has a chapter on Language Evolution. Five other textbooks have sections dedicated to Animal Communication. Four other textbooks have sections dedicated to Language Evolution (see Table 1 ). However, it has to be noted that these sections also differ in length, ranging from less than one page to several pages. Out of eighteen textbooks, three do not make any references to either Animal Communication or Language Evolution. For the remaining fifteen, references to Animal Communication and Language Evolution can be further sub-categorized (see Figs. 1 and 2 ). For Animal Communication, there were three emergent broad topics (subcategories), and for Language Evolution, there were two subcategories.

Numbers of references in the five main coding subcategories in each of the linguistics textbooks analysed.

Proportion of the number of references in the two main categories (Animal Communication vs. Language Evolution) and their main subcategories.
Main category : Animal Communication
Subcategories:
Animal communication behaviours : References to specific animal communicative behaviours or general characterizations of aspects of animal communication,
Animal versus human communication : Comparisons of animal versus human communication,
Language-trained animals : References to research with language-trained animals. 3
Main category: Language Evolution
Language origin theories : References to explanations of how language originated. This category includes references to larger theoretical frameworks as well as more specific scenarios of language origins, most of them historical rather than contemporary. Examples include creation myths like the story of Babel, historical onomatopoeic hypotheses of language emergence, or modern-day scientific accounts such as Dunbar’s gossip theory of language origins ( Dunbar 1996 ).
Language evolution research : References to interdisciplinary research directly connected to the field of language evolution (see Section 2). This category includes passages that do not describe a particular account or scenario of language origins, but instead present results and data, mostly empirical and relatively recent, that provide an evidential basis for inferences about the evolutionary history of language, and empirical and theoretical building blocks for scenarios and theories. Examples include references to the relevant fossil record (e.g. hominin braincases or speech organs), or relevant genetic research (e.g. the FOXP2 gene in ancient humans). Importantly, other bodies of research that are often drawn on to inform evolutionary scenarios (such as on language acquisition, sign language, neurolinguistics, or historical linguistics 4 ) were not included as a default—we only did so when they were presented in a context relating them to the questions of the evolutionary emergence and development of language in human phylogeny. This decision is based on the fact that these areas of research on their own are not only independent subjects of linguistic study, but also because they are most frequently not discussed in an evolutionary framework or with respect to their potential implications for language evolution.
3.1.1 Results
There are two main general findings: firstly, references to Animal Communication (80.4% of coded references) are overall much more frequent than to Language Evolution (19.6%), and secondly, there is significant variation between textbooks in the extent of coverage and selection of topics.
Regarding the subcategories of Animal Communication, these are roughly distributed evenly within that category, with 35.5% of references to specific animal communicative behaviours, 32.7% of references comparing animal and human communication, and 32.8% of references discussing language-trained animals. Within the category Language Evolution, results are also evenly split, with 50.2% of references to language evolution research, and 49.8% of references to specific language origin theories.
Secondly, the number of references to Animal Communication and Language Evolution differs significantly from textbook to textbook. As already mentioned, three textbooks do not include references to these topics at all, and a number of them also only make very little reference to these topics. The bulk of references come from ten textbooks, which, however, still vary quite extensively in their coverage, from 32 ( Fasold & Connor-Linton ) to 318 references ( O’Grady et al ). An overview of the frequencies of topics by textbooks can be seen in Fig. 1 .
Moreover, as can be seen in Fig. 2 , textbooks also differ in how much they discuss a particular topic, for example, the degree to which they discuss animal versus human communication, language-trained animals, and the other categories.
3.2 Animal communication
3.2.1 animal communication behaviours.
Thirteen out of fifteen textbooks (87%, with the exception of Bauer and Hayes ) make references to specific animal communicative behaviours or general characterizations of aspects of animal communication. Among those, references to primate communication are most frequent (12 textbooks, 125 references), followed by birds (7 textbooks, 108 references), arthropods, especially bees (11 textbooks, 82 references), and mammals other than primates (12 textbooks, 56 references). Overall, textbooks differ quite strongly in which specific animal communicative behaviours they refer to. This also holds for references to species. Textbooks reference a total of sixty-three different species. However, forty-four (69.8%) of these species are only mentioned in one textbook, and only four species/clades (6.3%) are mentioned in five textbooks or more. These are vervet monkeys (five textbooks), chimpanzees (six textbooks), dogs (seven textbooks) and honeybees (eleven textbooks).
In addition, sometimes textbooks also make more general statements, for example, about monkeys or primates, fish, or songbirds. Some textbooks also mention more general properties of animal communication, for example, discussions of non-vocal communication and whether animal signals are innate or learnt. Furthermore, some textbooks also discuss the general properties of animal signs, such as animal signs as expressions of emotional states, graded instead of discrete signals, and their limited range of meanings.
3.2.2 Animal versus human communication
Fourteen out of fifteen textbooks (93.33%, with the exception of Dirven & Verspoor ) include an explicit comparison of human language with the communication systems of non-human animals. As outlined in Section 3.1, three of them have chapters dedicated to animal communication or chapters on animal versus human communication.
As shown in Fig. 3 , the two topics that are mentioned by most textbooks are human language as a species-specific trait (11 textbooks, 24 references), as well as discussions of animal versus human communication in the context of Hockett’s design features (11 textbooks, 183 references).

Topics in the subcategory ‘animal vs. human communication’ by the number of textbooks and references.
Another prominent topic is discussions of the biological differences between humans and animals. A number of textbooks also discuss animals’ ability to learn and understand human language and differences in the cognitive abilities of animals and humans. Also frequent are specific comparisons between humans and chimpanzees and the claimed distinction that human communication is stimulus-free whereas animal communication is stimulus bound.
The last group of topics is differences between the features of human language and animal communication that are not captured by Hockett’s design features. These include the degree of iconicity in human language versus animal communication, the pragmatic function of human language, differences in the mode of communication, the property of redundancy in communication systems, and turn-taking as a characteristic property of human communication.
In terms of the number of references to concepts, references to Hockettian design features of language far outweigh all other references.
For this reason, we are now going to turn to discussions of Hockettian design features of the language. Out of the eleven textbooks that mention such features, only four (36.4%) explicitly mention Hockett by name and six (55.55%) reference the characteristic properties of human language as ‘design features’. All eleven, however, mention properties that were popularized by Hockett, either directly using Hockett’s terminology or expressing the same concept (see Fig. 4 ).

Topics in the sub-subcategory ‘Hockettian design features’ by the number of textbooks and references.
3.2.3 Language-trained animals
Discussions of ‘language-trained animals’, that is, animals exposed to some kind of sign system, can also be found frequently in textbooks. They can be found in ten out of fifteen textbooks (66.67%, with the exception of Bauer , Dirven and Verspoor , Hayes , Hazen and Meyer ). Textbooks focus mostly on research with great apes, which are mentioned in ten textbooks, whereas studies with parrots and dogs are both mentioned in four textbooks (40%). Only one textbook also reports on research with dolphins.
Regarding the great apes, all ten textbooks mention language-trained chimpanzees, eight mention bonobos; seven mention gorillas, and two textbooks also mention language-trained orangutans.
A sceptical perspective on language-trained animals is prevalent with eight textbooks discussing scepticism towards these experiments, and seven textbooks discussing these experiments in the context of stimulus-response conditioning. Three textbooks explicitly discuss the question of to what degree language-trained animals can be said to have acquired symbols, and three textbooks discuss the question if chimpanzees have acquired syntactic knowledge. Five textbooks explicitly compare humans’ and primates’ abilities to learn a language.
3.3 Language evolution
References in the category Language Evolution can be found in fifteen textbooks. However, as already shown in Table 1 and Fig. 1 (cf. Section 3.1), textbooks differ quite significantly in how much space they give to this topic and also in which research and language origin scenarios they discuss. As already mentioned, only Yule has a chapter dedicated to language evolution, and only Denham & Lobeck , Finegan , Hayes , and McGregor have dedicated sections on language evolution. But even here the amount of space devoted to language evolution differs, ranging from less than one page to six pages. The other textbooks sometimes only discuss language evolution in passing.
3.3.1 Language evolution research
Regarding the topics discussed, twelve textbooks make reference to biological aspects of human evolution (fifty-three references). This includes references to the evolution and structure of the human brain (eight textbooks, thirteen references), the FOXP2 gene (seven textbooks, sixteen references), and the evolution and structure of the human speech organs (five textbooks, eight references). Other topics in this area that are also mentioned are the evolution of bipedalism (three textbooks, four references), changes of the digestive and respiratory system (one textbook, two references), and handedness (one textbook, one reference). One other frequent feature is that many textbooks (eight textbooks, thirteen references) discuss that there is still much scepticism towards language evolution research and that many proposals are speculative. Explicitly positive attitudes towards the field of language evolution and its future are only expressed in two textbooks ( Finegan and McGregor ). Other topics discussed in a number of textbooks are extinct hominins (six textbooks, fifteen references), archaeological evidence (five textbooks, six references), and that language evolution research is an interdisciplinary endeavour. An overview of the main topics discussed and their frequencies can be found in Fig. 5 .

Main topics in the subcategory ‘language evolution research’ by the number of textbooks and references.
As illustrated by Fig. 6 , textbooks also differ considerably in the potential timeframes of language emergence, with estimates in eight textbooks ranging from 50 000 years ago to as much as 500 000 years ago, and even older estimates for related capacities such as the evolution of ‘speech areas’ of the brain (2 million years ago) or the ‘capacity for language-type communication’ (2.6 million years ago).

Different timeframe estimations for the evolution of language by the numbers of textbooks and references. Some textbooks mention more than one estimate.
3.3.2 Language origin theories
Twelve textbooks mention language origin scenarios (with the exception of Dirven & Verspoor , Hayes and Hazen ). As Fig. 7 shows, the most frequently mentioned factor is language as an evolutionary adaptation (seven textbooks, eleven references) and discussions of the evolution of the properties of the faculty of language (six textbooks, fourteen references). Notably, cultural evolution is only mentioned explicitly in one textbook ( McGregor , three references), which we consider a major oversight (see Section 4.3.2). Another frequently discussed topic is divine origins myths, which can be found in five textbooks (thirty-two references). Equally frequent are discussions of social factors involved in the emergence of language (five textbooks, thirteen references) and the question in which modality language began, that is, if it was primarily vocal, gestural, or multimodal at first (five textbooks, nine references). Other aspects of language origins scenarios are discussed infrequently. This includes references to onomatopoeic scenarios of language evolution (three textbooks, seven references), the role of tool use in language evolution (three textbooks, seven references), the relationship of music and language in language evolution (two textbooks, three references), and catastrophic origin theories (four textbooks, nine references). In contrast, explicit references to gradualistic theories of language evolution can only be found in one textbook ( Fromkin et al.). The same holds for the important concept of protolanguage as an intermediary stage before fully modern language (e.g. Tallerman 2012), which is only mentioned in one textbook ( Fasold & Connor-Linton ).

Topics in the subcategory ‘language origin theories’ by the number of textbooks and references.
4.1. Suggestions and resources for improvement
In this section, we offer some short notes on suggestions for improvements for introductory textbooks, along with suggestions for good resources regarding language evolution research (see Box 1 ).
Fitch, W. T. (2010). The Evolution of Language. Cambridge University Press.
The best book-length introduction to the subject with a special emphasis on biological evolution and the evolution of speech.
McMahon, A., & McMahon, R. (2013). Evolutionary Linguistics . Cambridge University Press.
Part of the ‘Cambridge Textbooks in Linguistics’ series, the only introductory textbook on the subject so far.
Progovac, Liljana (2019). A Critical Introduction to Language Evolution: Current Controversies and Future Prospects . Springer.
Part of the ‘SpringerBriefs in Linguistics’ series, explicitly aimed at acquainting ‘scholars with recent developments outside their own research areas’. Contrasts sudden (saltationist) and gradualist approaches to language evolution and calls for an empirical research paradigm to study the co-evolutionary loop between language, brains, and genes.
Tallerman, M. & Gibson, K. R., eds. (2012). The Oxford Handbook of Language Evolution . Oxford University Press.
A comprehensive handbook on the subject, featuring sixty-five chapters written by international experts on all aspects of language evolution. It covers ‘insights from comparative animal behaviour’, ‘the biology of language evolution’, ‘the prehistory of language’, theories on the initial emergence of language and ‘language change, creation, and transmission in modern humans’.
Christiansen, M. H. & Chater, N. (2022). The Language Game: How Improvisation Created Language and Changed the World . Basic Books.
A recent monograph that focusses on the cultural evolution of language. It adopts a view of linguistic behaviour as a game of communicative charades played over multiple generations. This process in turn explains the emergence of language and its structure.
Planer, R.J. & Sterelny, K. (2021). From Signal to Symbol: The Evolution of Language . MIT Press.
A novel, integrative account of language evolution, treating language as a result of successively developing protolanguages over the last two million year. The book focusses on archaeological data and the social and sociocognitive infrastructure enabling the emergence of language.
Hurford, J. R. (2014). Origins of language: A slim guide . Oxford University Press.
A condensed introductory version of Hurford’s two-part opus magnum on ‘Language in the Light of Evolution’ (2007, 2012) that focusses on the origins of meaning and the origins of grammar from an interdisciplinary perspective.
Fitch, W. T. 2017 (eds.) Special Issue on the Biology and Evolution of Language. Psychonomic Bulletin & Review 24 .
A collection of thirty-six Articles by international experts focussing on different empirical perspectives on language evolution from evolutionary biology, neuroscience, palaeoanthropology, comparative psychology, cultural evolution, linguistics and cognitive science.
Engesser, S. & Townsend, S. W. (2019). Combinatoriality in the vocal systems of nonhuman animals. WIREs Cognitive Science 10(4), e1493.
An article that reviews the current evidence for combinatorial systems in the vocal communication of different animals.
Tamariz, M. (2017). Experimental studies on the cultural evolution of language. Annual Review of Linguistics , 3, 389-407.
Gives an overview of experiments on cultural language evolution, emphasizing the role interaction and transmission play in the emergence of linguistic structure.
Krebs, J. R. & Dawkins, R. (1984). “Animal Signals: Mind-Reading and Manipulation”, in Behavioral Ecology: An Evolutionary Approach , eds. J. R. Krebs and R. Dawkins (Oxford: Blackwell), 380–402.
This groundbreaking work laid the foundation for signalling theory. It argues that the cooperative design of human language that we naturally take for granted is an exception rather than the rule in animal communication, and requires special conditions to emerge.
Overall, we found that the number of problematic passages about language evolution and animal communication was relatively low, with a total of sixty problematic passages of different dimensions found in all textbooks (thirty-eight for animal communication and twenty-two for language evolution). 5 The most frequent inaccurate statement concerns non-human primate vocal anatomy, with the claim that the vocal tracts of non-human primates are anatomically incapable of producing speech sounds. This has been demonstrated by more recent research to be inaccurate (e.g. Fitch 2000 ; Fitch et al. 2016 ). However, given that this is a relatively recent research development, it is understandable why it hasn’t permeated introductory textbooks yet.
One aspect we judged to be more problematic is that some textbooks ( Akmajian et al., Fasold & Connor-Linton , Rowe & Levine) make extensive reference to popular press publications, which in addition sometimes are also quite outdated. For example, in the 2017 edition of their textbook, Akmajian et al. cite a 1989 National Geographic Magazine article on the speech capacities of Neanderthals.
In addition, some textbooks, including Yule , cite and draw conclusions from research on the famous alarm call system of vervet monkeys alone (e.g. Cheney and Seyfarth 1990 ). Here, we suggest that introductory textbooks could profit from including more recent work on more complex signalling systems, such as those found in putty-nosed monkeys ( Arnold and Zuberbühler 2006 ) and Campbell’s monkeys ( Zuberbühler 2002 ; Ouattara et al. 2009 ), among others (see e.g. Townsend and Manser 2013 ; Engesser and Townsend 2019 ; Suzuki and Zuberbühler 2019 , for recent overviews).
In Box 1 , we list some useful recent resources that introductory textbooks could use to improve their representation of language evolution research (see also Box 2 ). In addition, Johansson (2020) offers a comprehensive bibliography of language evolution research.
There are a number of important alternatives to the design features framework that do not receive enough attention in textbooks. In particular, the distinction into the Faculty of Language in the broad versus narrow sense (FLB vs. FLN) , influentially put forward 20 years ago by Chomsky and collaborators ( Hauser et al. 2002 ; Fitch et al. 2005), is only briefly discussed in one textbook. Notwithstanding its serious limitations (see e.g. Wacewicz et al. 2020 ), the FLN/FLB distinction is now well established in the study of language.
Equally important are the approaches to both the evolution and uniqueness of language that highlight its social grounding and cognitive-interactional aspects (esp. Tomasello 1999 , 2008 ; Levinson 2006 ; Levinson and Holler 2014 ). Such approaches underscore the centrality of the cooperative nature of language as well as the cognitive infrastructure that enables language; the former completely eludes the Hockett-type classifications, and the latter is only indirectly or marginally present in features such as displacement.
Even if no particular theoretical formulation has yet reached textbook status, a forming consensus in the language evolution community is that the evolutionary history of cooperation in the hominin line plays a central role in the problem of language emergence ( Zlatev 2014 ). Cooperation is understood here widely and inclusive not only of the Gricean notion of cooperation but also signalling theory with its focus on cooperation as information donation (discussed in Section 4.2.2).
With regard to the cognitive infrastructure necessary for language , current research in language evolution focuses on prerequisites for communication based on the attribution of intentions ( Levinson 2006 ), Theory of Mind and social motivations ( Tomasello 1999 , 2008 ; Botha 2020 ), ostensive signals ( Scott-Phillips 2014 ), bodily mimesis ( Zlatev 2014 ) as well as turn-taking ( Levinson 2006 ; Levinson and Holler 2014 ).
4.2. Animal communication
4.2.1 hockett’s design features.
Section 3.2.2 reveals that the received way of comparing language with animal communication is through the list of ‘design features’ based more or less closely on those proposed by Hockett (esp. 1960 , but see also Hockett 1958 , 1959 ; 1966 ). This is understandable, given that the Hockettian system is still the classic, best known, and widely accepted system of comparing animal communication to language (e.g. Beecher 2021 ) and has been highly influential both in and beyond linguistics, in fields such as ethology (e.g. Hinde 1982 ), biosemiotics ( Noth 1990 ), and also language evolution (e.g. Fitch 2010 ; Cuskley 2020 ). However, more than 60 years have passed since Hockett’s first proposals ( 1958 , 1959 ), meaning that some of the deeper theoretical foundations of Hockett’s system rest on an outdated understanding of both biology and linguistics.
Biologically, Hockett’s approach is implicitly informed by once-popular misconceptions including that of evolution proceeding exclusively through incremental progressions, or anthropocentric views of animal communication systems as ‘incomplete languages’. We suggest that while Hockett’s design features might prove a good entry point to comparisons of human language and animal communication systems, textbooks would profit from extending their discussions by including insights from signalling theory (see Section 4.2.2). In contrast, Hockett’s approach rests implicitly on the ‘classic ethological’ model of communication, which is no longer supported in behavioural ecology (see Searcy and Nowicki 2005 : 7–9). From the point of view of linguistic theory, the Hockettian system inevitably reflects the mid-20 th -century understanding of what language is, with a heavy focus on its formal and structural properties; even if not incorrect, this understanding is at least incomplete, through its neglect of more recent cognitive, functional and interactionist perspectives (see esp. Wacewicz and Zywiczynski 2015 ).
In sum, the Hockettian system of design features has long been a cornerstone of understanding the status and origins of language, and its classic status still translates into its considerable descriptive and educational utility. However, textbooks would benefit from an overhaul of this system to the inclusion of more recent perspectives. Small steps in this direction are already evident in some textbooks. For example, O’Grady et al . feature a text box on ‘Updating the Design Features of Language’ with reference to a recent paper by Hauser et al. (2014) . However, these attempts are unsystematic and not yet standardized. In Box 2 , we briefly describe several points that we see as deserving inclusion.
4.2.2 Signalling theory
In biology, the received approach to studying the form, function and in particular evolution of animal communication is signalling theory: it is ‘the main body of theory applied to animal communication’ ( Power 2014 : 50), and it underlies contemporary textbooks on this topic (e.g. Maynard Smith and Harper 2003 ; Searcy and Nowicki 2005 ; Bradbury and Vehrencamp 2011 ). Signalling theory is a theoretical framework that applies neo-Darwinian principles to the study of communication. From that perspective, it construes communicators as agents designed to maximise their evolutionary fitness, and communication, like all behaviour, is seen as a tool for such fitness maximization. This perspective is interested in the underlying economics of communication, that is, what the animal stands to lose versus what it stands to gain from the communicative interaction. These costs and benefits ultimately affect the animal’s evolutionary fitness, and in this way, they translate into selection pressures shaping the evolution of communication systems. In particular, a central tenet of signalling theory is that the ‘goal’ of communication, understood from that perspective as its evolved function, is not to provide information to others—since making investments into the fitness of unrelated individuals instead of one’s own would be biologically inexplicable. Rather, the ‘goal’ of communication is to pursue one’s own fitness-enhancing goals, such as, for example, advertising one’s biological quality to potential mates, which can be trivialized into saying that communication is ‘self-interested’.
Signalling theory thus elucidates the deeper design principles of all animal communication. It is, therefore, fundamental from the species-comparative perspective, as it provides the only established unified framework to study all animal communication systems that do not presuppose language as a special case or a reference point. Signalling theory makes it possible to understand the design reasons for the limited range of expression in non-human animals (esp. Heintz and Scott-Phillips 2022 ), or explain certain unexpected patterns in the relation between communicative and cognitive capacities, such as the advanced communication system in honeybees (e.g. Wacewicz and Żywiczyński 2018 ). Most importantly, this perspective also clearly and independently identifies important ways in which human language stands out in nature (cf. esp. Knight 2016 ; Dessalles 2020 ). This is mostly related to the through-and-through cooperativeness of language, which is taken for granted in linguistics, but not normally expected in animal communication systems. This is as important for the species-comparative perspective as it is for research on the evolutionary origins of language, where the emergence of cooperative signalling as well as its continued evolutionary stability is considered a ‘central problem’ (e.g. Maynard Smith and Harper 2003 ; Fitch 2010 ; Heintz and Scott-Phillips 2022 ). Adding the perspective of signalling theory to textbooks would improve the theoretical context for discussing communication systems, and the differences and similarities between human language and non-human animal communication systems.
4.3 Language origins
Sections 3.3.1 and 3.3.2 reveal a relative paucity of information on those topics, especially when compared to much more extensive and detailed coverage of animal communication (see Fig. 2 ). While a majority of textbooks include mentions of language origins and language evolution research, only one textbook has a chapter on this topic and two more have sections extending beyond a single page. Further, we see a disproportionate representation of content of purely historical value, in particular, divine or mythical accounts of language origins (such as the story of Babel), or accounts developed in the 19th century (e.g. the ‘bow-wow’ or ‘pooh-pooh’ theories of language emerging from mimicking animals or from emotional cries). The inclusion of such themes may be substantiated on historical grounds as classic points of reference in the debates of language origins; however, their over-representation relative to the current state of the art may be felt as painting an unfavourable picture of contemporary language evolution research.
A particularly interesting example is Yule , the only textbook with a whole chapter dedicated to the topic of language origins. It is divided into sub-chapters representing several ‘sources’ of language: the divine source, the social interaction source, the natural sound source, the tool-making source, the physical adaptation source, and the genetic source. This presentation is problematic on a number of grounds. First, such a structuring juxtaposes explanations on incomparable levels ( Tinbergen 1963 ): implementation, versus adaptive function, versus phylogeny—that is the underlying physiological implementation of a feature, versus the selection pressures shaping it, versus its development in the species. For example, the level of physical implementation, here discussed in terms of the speech-adapted anatomy of the vocal tract, is listed in parallel to the level of genetic specification, even though the former is clearly causally dependent on the latter rather than being a ‘different source’. Furthermore, a juxtaposition of natural and supernatural ‘sources’ poses the danger of casting unscientific approaches as equal-status contenders—not only through the structure of subchapters already mentioned, but occasionally through unfortunate phrasing, for example, ‘If human language did emanate from a divine source, we have no way of reconstructing that original language, especially given the events in a place called Babel, <because the Lord did there confound the language of all the earth,> as described in Genesis’ (11: 9).
Another notable example is McGregor . This textbook presents an explicitly favourable evaluation of the field of language evolution research (pages 265, 267—the only such textbook except for Finegan ), and it offers a relatively brief but accurate and up-to-date coverage of this field, with a good selection of the most influential theories and relevant bodies of evidence. On the other hand, McGregor inexplicably extends Max Müller’s derogatory ‘bow-wow’ terminology to these approaches, using the labels ‘noddy’ (the gestural approach), ‘yackety-yack’ (Dunbar’s grooming hypothesis), and the ‘just genes’ theory, further subdivided into the ‘oops!’ and ‘chatting-up’ theories, which stand for the views of Chomsky and Pinker, respectively. As noted, for example, by Sprinker (1980 : 117), ‘it is hard to suppress one’s natural amusement when discussing them under such labels. […] [I]t is sometimes difficult for us to [take such ideas seriously], particularly with the persistent ring of Muller’s appellatives in our ears’.
4.4 Language evolution research
4.4.1 genetic, fossil, and material-culture data sources for modern language evolution research.
As shown in Section 3, textbooks do not rely extensively on the bodies of data that go beyond comparative evidence from animals. Of these, genetic evidence, and specifically the FOXP2 gene, is referenced relatively frequently (seven books, sixteen references), but information provided on this particular topic is often problematic (see Appendix A.1 in Supplementary Material). Palaeoanthropological and archaeological information is typically absent from textbooks or only mentioned as isolated facts ( fossil record : three books, four references; extinct hominins : six books, fifteen references; archaeology : five books, six references).
Still, the recently available genetic, fossil, and material-culture record of extinct hominins does allow us to shed considerable light on their linguistic abilities. Arguably the most interesting example, also for its significance to the science of language, is the strong interdisciplinary evidence pointing to the presence of recognizably human-like forms of communication in Neanderthals (see esp. Botha 2020 , for a critical overview). First cases for Neanderthal language backed up by comprehensive multidisciplinary evidence became available already a decade ago (e.g. Dediu and Levinson 2013 ), and additional evidence has accrued since then, such as on systematic neanderthalensis—sapiens interbreeding, the presence of the same derived mutations of the FOXP2 gene, very similar speech production and hearing apparatuses, and behavioural modernity of Neanderthals (see e.g. Johansson 2015 , Dediu and Levinson 2018 , for reviews).
Of course, we cannot say with absolute certainty whether Neanderthals did or did not have language ( Berwick et al. 2013 ; Botha 2020 ). Nevertheless, the perception that arguments regarding language in the prehistoric past must solely rely on speculation is no longer true. The last two to three decades have dramatically expanded the evidential basis for inferences about the language capacities of ancient hominins, making such inferences more robust through grounding them in multiple lines of converging evidence. Further, as shown above, it currently appears that the extent and weight of this converging evidence make it reasonable to change the null hypothesis: the bulk of evidence makes assuming the presence of language in Neanderthals more parsimonious than assuming its absence. Finally, this case is arguably of global importance to linguistics at large, as it is one of the very few potential ‘windows’ on dating the beginnings of language. Linguistic literature, including introductory textbooks (see Section 3.3.1) will keep on making estimates of the anciency of language, speculative as they are. We believe that basing these estimates on state-of-the-art information is no less important than hedging them for speculativeness.
4.4.2 Cultural evolution
The second cluster of empirical results relevant to language evolution comes from modelling and experimental studies focussing on the cultural evolution of language (e.g. Kirby 2012 ; Tamariz and Kirby 2016 ; Tamariz 2017 ). 6 This research is typically less concerned with the questions of the biological origin of the language faculty, but instead elucidates the emergence of meaning and structure in the linguistic code itself (see Section 4.3.2). This type of research now constitutes the core of empirical studies presented at Evolang conferences, but is virtually absent from linguistics textbooks.
Cultural evolution as a framework is particularly valuable, since it provides the most direct data on language evolution, and in particular on the evolutionary pressures shaping language structure. While comparative evidence from fields such as primatology or palaeoanthropological evidence from the hominin fossil record is necessarily indirect, the cultural evolution of communication can be studied in the laboratory, through experiments with human participants. As such, it represents a quintessentially hypothesis-testing approach, and the adoption of such methods to study the evolution of language was in a large part responsible for the change of profile of language evolution as a research field, from speculative to evidence and experiment based.
In stark contrast to its central role in current language evolution research, barring a single exception ( McGregor , three references), the topic of the cultural evolution of language is absent from the introductory linguistic textbooks that we examined. This can be partly explained by the relative recency of cultural evolution as a research area (e.g. Richerson and Boyd 2005 ; Mesoudi et al. 2006 ), both in a general sense and with regard to its role in language origins and language change. However, from today’s perspective, the almost complete neglect of this topic in introductory linguistic textbooks should be seen as a major oversight. Cultural evolution deserves a better representation in textbooks, as it addresses many of the concerns that language evolution research lacks an experimental, predictive framework. Furthermore, it is not only relevant for the study of the evolutionary origins of language, but provides a set of experimental methods that can be used to study the emergence of semiotic systems (e.g. Galantucci et al. 2012 , see Section 4.3.1). In addition, it can also be used to study the cultural change of languages more generally from an interdisciplinary perspective, in essence representing an analogue to the biologist’s Drosophila (cf. Roberts 2017 ). Integrating these research frameworks into discussions of language evolution in introductory textbooks is therefore likely to prove highly profitable.
In sum, in our examination of the most popular ‘introduction to linguistics’ textbooks, we found that the topic of language evolution and language origins is not prominently represented. Among the research areas that were most frequently mentioned were biological aspects of human evolution, references to extinct hominins, and discussions of the scepticism towards language evolution research. While animal communication is discussed much more frequently, textbooks also show wide variation in how much space they devote to the topic. Some of the most extensively covered topics include animal communicative behaviours, comparisons of animal versus human communication, and references to research with language-trained animals.
We found the factual accuracy to be good overall, with the most frequent mistake relating to the long-held belief that the vocal tract of non-human primates is incapable of producing speech sounds, which is understandable given that this is a relatively recent research development. However, the topics covered in our analysis are treated as a peripheral rather than core linguistic topic. We found the content on animal communication and in particular language origins and evolution to vary considerably between the textbooks, and this missing homogeneity is indicative of a lack of consensus on a canonical body of knowledge that students of linguistics should be expected to internalize. They also appear to be considered marginal, ‘luxury’ topics that are sometimes not covered at all or just in passing, especially in the shorter textbooks. An indicative example of this can be seen in O’Grady et al ., where animal communication is relegated to an online-only chapter only accessible via subscription (cf. Section 3.1).
The most important conclusion of our study is that the representation of the topics covered in our analysis, and in particular the origins and evolution of language, does not reflect the state of the art. Furthermore, this problem appears to disproportionately concern the most influential textbooks as measured by their OS score (esp. Fromkin , Yule— see Section 1 and Table 1 ), whose consecutive editions to a large degree inherit their content and organization from previous editions, which favours only local instead of global changes.
We recommended amendments on several dimensions. First, the relatively extensive representation of non-scientific content such as myths, religious stories, and 19th-century ‘bow-wow’-type accounts to the detriment of presentations of modern language evolution research, which was perhaps merited several decades ago, is no longer tenable in the face of the wealth of currently available scientific content that informs language origins questions. Non-scientific motifs should be reduced to symbolic proportions that can be justified on the grounds of their historical importance as cultural codes, in favour of a better representation of contemporary accounts, debates, and evidence (see esp. Section 4.3.1). Second, several specific approaches or perspectives that are at the heart of current language evolution research are absent from linguistics textbooks, in particular signalling theory (Section 4.2.2) and cultural evolution (Section 4.3.2). Their inclusion would be highly beneficial to young adepts of linguistics, for whom it would be an early (and in many cases perhaps the only) opportunity to become aware of dynamically developing approaches to the study of language-related phenomena. Third, although abandoning the reliance on Hockett-type lists of design features would be neither feasible nor fully desirable, such lists should be redefined to accommodate the recent findings such as on the status of arbitrariness in language or the importance of concepts such as cooperativeness, domain generality and turn-taking (Sections 4.2 and 4.2.1).
One limitation of this study is that we only examined English-language textbooks published which, with the exception of Dirven and Verspoor (2004) , were published in the USA and UK. For future studies, it would be interesting to see if this pattern is also apparent in introductory linguistics textbooks in other languages, which might come from different disciplinary and intellectual traditions.
We are grateful to Arkadiusz Jasiński, who helped develop an early version of this study. We would also like to thank the two anonymous reviewers as well as Dan Dediu for their insightful comments on the manuscript.
This research was supported by the Polish National Science Centre under grant agreement UMO-2019/34/E/HS2/00248. MP was supported by a postdoctoral fellowship from the University Centre of Excellence IMSErt: Interacting Minds, Societies, Environments, Nicolaus Copernicus University in Toruń.
Conflict of interest statement : The authors declare that there is no conflict of interest regarding the publication of this article.
The data underlying this article are available in OSF, at https://osf.io/5ta7x/
Akmajian , A. et al. ( 2017 ) Linguistics: An Introduction to Language and Communication . Cambridge, MA : MIT Press .
Google Scholar
Google Preview
Bauer , L. ( 2007 ) Linguistics Student’s Handbook . Edinburgh : Edinburgh University Press .
Dawson , H. , and M. Phelan ( 2016 ) Language Files: Materials for an Introduction to Language and Linguistics . Columbus, OH : The Ohio State University Press .
Denham , K. , and A. Lobeck ( 2013 ) Linguistics for Everyone: An Introduction . Boston, MA : Cengage Learning .
DeVito , J. A. ( 2017 ) Human Communication: The Basic Course . Hoboken, NJ : Prentice Hall .
Dirven , R. , and M. Verspoor , eds. ( 2004 ) Cognitive Exploration of Language and Linguistics . Amsterdam : John Benjamins Publishing .
Fasold , R. W. , and J. Connor-Linton , eds. ( 2014 ) An Introduction to Language and Linguistics . Cambridge : Cambridge University Press .
Finegan , E. ( 2015 ) Language: Its Structure and Use . Boston, MA : Cengage Learning .
Fromkin , V. , R. Rodman, and N. Hyams ( 2017 ) An Introduction to Language . Boston, MA : Cengage Learning .
Hayes , B. P. ( 2015 ) Introductory Linguistics . https://linguistics.ucla.edu/people/hayes/20/Text/HayesIntroductoryLinguistics2015.pdf
Hazen , K. ( 2015 ) An Introduction to Language . Hoboken, NJ : John Wiley & Sons .
McGregor , W. B. ( 2015 ) Linguistics: An Introduction . London : Bloomsbury Publishing .
Meyer , C. F. ( 2009 ) Introducing English Linguistics . Cambridge : Cambridge University Press .
O’Grady W. , J. Archibald , and M. Aronoff ( 2017 ) Contemporary Linguistics: An Introduction . New York : Bedford Books .
Plag , I. et al. . ( 2015 ) Introduction to English Linguistics . Boston, MA : De Gruyter Mouton .
Radford , A. et al. . ( 2009 ) Linguistics: An Introduction . Cambridge : Cambridge University Press .
Rowe , B. M. , and D. P. Levine ( 2018 ) A Concise Introduction to Linguistics . London : Routledge .
Yule , G. ( 2020 ) The Study of Language . Cambridge : Cambridge University Press .
Arnold , K. , and K. Zuberbühler ( 2006 ) ‘Semantic Combinations in Primate Calls’ , Nature , 441 : 303 .
Beecher , M. D. ( 2021 ) ‘ Why Are No Animal Communication Systems Simple Languages?’ Frontiers in Psychology 12 , 701 . doi: 10.3389/fpsyg.2021.722685 .
Bergmann , T. , and R. Dale ( 2016 ) ‘ A Scientometric Analysis of Evolang: Intersections and Authorships’. In: Roberts , S. et al. (eds.) The Evolution of Language: Proceedings of the 11th Conference (EVOLANGXI) . http://evolang.org/neworleans/papers/182.html .
Berwick , R. C. , M. D. Hauser , and I. Tattersall . ( 2013 ) ‘Neanderthal Language? Just-So Stories Take Center Stage’ , Frontiers in Psychology , 4 : 671 . doi: 10.3389/fpsyg.2013.00671 .
Boeckx , C. ( 2021 ) Reflections on Language Evolution . Berlin : Language Science Press . doi: 10.5281/zenodo.5524633 .
Botha , R. ( 2020 ) Neanderthal Language: Demystifying the Linguistic Powers of Our Extinct Cousins . Cambridge : Cambridge University Press .
Bradbury , J. W. , and S. L. Vehrencamp ( 2011 ) Principles of Animal Communication , 2nd edn. Sunderland, MA : Sinauer Associates .
Brown , V. ( 1993 ) ‘ Decanonizing Discourses: Textual Analysis and the History of Economic Thought’. In: Henderson , Willie , Tony Dudley-Evans , and Roger Backhouse , (eds.) Economics and Language , pp. 44 – 84 . London : Routledge .
Campbell , L. ( 2013 ) Historical Linguistics: An Introduction , 3rd edn. Edinburgh : Edinburgh University Press .
Cheney , D. L. , and R. M. Seyfarth ( 1990 ) How Monkeys See the World: Inside the Mind of Another Species . Chicago, IL : University of Chicago Press .
Cuskley , C. ( 2020 ) ‘Language Evolution: A Brief Overview’ , PsyArXiv . doi: 10.31234/osf.io/3y98j .
Dediu , D. , and B. De Boer . ( 2016 ) ‘Language Evolution Needs its Own Journal’ , Journal of Language Evolution , 1 : 1 – 6 .
———, and S. C. Levinson . ( 2013 ) ‘On the Antiquity of Language: The Reinterpretation of Neandertal Linguistic Capacities and its Consequences’ , Frontiers in Psychology , 4 : 397 . doi: 10.3389/fpsyg.2013.00397 .
———. ( 2018 ) ‘Neanderthal Language Revisited: Not Only Us’ , Current Opinion in Behavioral Sciences , 21 : 49 – 55 .
Dessalles , J. -L. ( 2020 ) ‘Language: The Missing Selection Pressure’ , Theoria et Historia Scientiarum , 17 : 7 – 57 .
Doerfer , G. ( 1973 ) ‘Lautgesetz und Zufall. Betrachtungen zum Omnicomparativismus [Sound Law and Chance. Notes on Omnicomparativism]’ . Innsbrucker Beiträge zur Sprachwissenschaft , Vol. 10 . Innsbruck : Universität .
Dunbar , R. ( 1996 ) Grooming, Gossip and the Evolution of Language . London : Faber and Faber .
Engesser , S. , and S. W. Townsend ( 2019 ) ‘Combinatoriality in the Vocal Systems of Nonhuman Animals’ , WIREs Cognitive Science , 10 : e1493 .
Fisiak , J. ( 1985 ) Wstęp do współczesnych teorii lingwistycznych . Warszawa : WSiP .
Fitch , W. T. ( 2000 ) ‘The Phonetic Potential of Nonhuman Vocal Tracts: Comparative Cineradiographic Observations of Vocalizing Animals’ , Phonetica , 57 : 205 – 18 .
——— et al. . ( 2016 ) ‘Monkey Vocal Tracts Are Speech-Ready’ , Science Advances , 2 : e1600723 .
——— ( 2017 ) ‘Empirical Approaches to the Study of Language Evolution’ , Psychonomic Bulletin & Review , 24 : 3 – 33 .
Fitch , W. T. ( 2010 ) The Evolution of Language . Cambridge : Cambridge University Press .
Fitch , W. T. , eds. ( 2017 ) ‘Special Issue on the Biology and Evolution of Language’ , Psychonomic Bulletin & Review , 24 .
Galantucci , B. , S. Garrod , and G. Roberts ( 2012 ) ‘Experimental Semiotics’ , Language and Linguistics Compass , 6 : 477 – 93 .
Gong , T. , L. Shuai , and B. Comrie ( 2014 ) ‘Evolutionary Linguistics: Theory of Language in an Interdisciplinary Space’ , Language Sciences , 41 : 243 – 53 .
Gould , S. J. , and R. C. Lewontin ( 1979 ) ‘The Spandrels of San Marco and the Panglossian Paradigm: A Critique of The Adaptationist Programme’ , Proceedings of the Royal Society of London B: Biological Sciences , 205 : 581 – 98 .
Hartmann , S. ( 2020 ) ‘Language Change and Language Evolution: Cousins, Siblings, Twins?’ , Glottotheory , 11 : 15 – 39 .
Hauser , M. , N. Chomsky , and W. T. Fitch ( 2002 ) ‘The Faculty of Language: What Is It, Who Has It, and How Did It Evolve?’ Science 298 . 1569 – 1579 .
Hauser , M. D. et al. . ( 2014 ) ‘The Mystery of Language Evolution’ , Frontiers in Psychology , 5 : 401 . doi: 10.3389/fpsyg.2014.00401 .
Heintz , C. , and T. Scott-Phillips ( 2022 ) ‘Expression Unleashed: The Evolutionary & Cognitive Foundations of Human Communication’ , Behavioral and Brain Sciences , 46 : 1 – 53 . doi: 10.1017/S0140525X22000012 .
Hinde , R. A. ( 1982 ) Ethology. Its Nature and Relations with Other Sciences . Oxford : Oxford University Press .
Hockett , C. F. ( 1959 ) ‘Animal “languages” and human language’ , Human Biology , 31 : 32 – 9 .
——— ( 1960 ) ‘The Origin of Speech’ , Scientific American , 203 : 88 – 96 .
Hockett , C. F. ( 1958 ) A Course in Modern Linguistics . New York : Macmillan .
——— ( 1966 ) ‘ The problem of universals in language’. In: Greenberg , Joseph H. (ed.) Universals of Language , pp. 1 – 29 . Cambridge : The MIT Press .
Hyland , K. ( 2004 ) Disciplinary Discourses: Social Interactions in Academic Writing . Ann Arbor : University of Michigan Press .
Johansson , S. ( 2015 ) ‘Language Abilities in Neanderthals’ , Annual Review of Linguistics , 1 : 311 – 32 .
——— ( 2020 ) ‘Evolution of Language’ , Oxford Bibliographies: Evolutionary Biology . doi: 10.1093/OBO/9780199941728-0079 .
Johns , A. M. ( 1997 ) Text, Role and Context: Developing Academic Literacies . Cambridge : Cambridge University Press .
Kaplan , J. ( 2021 ) ‘Unravelling Babel’: Mary LeCron Foster on the Origins of Language’ , BJHS Themes , 6 : 1 – 17 . doi: 10.1017/bjt.2021.4 .
Kendon , A. ( 1991 ) ‘Some Considerations for a Theory of Language Origins’ , Man , 26 : 199 – 221 .
Kirby , S. ( 2012 ) ‘ Language Is an Adaptive System: The Role of Cultural Evolution in the Origins of Structure’. In: Tallerman , M. , and K. R. Gibson (eds.) The Oxford Handbook of Language Evolution , pp. 589 – 604 . Oxford : Oxford University Press .
Knight , C. ( 2016 ) ‘Puzzles and Mysteries in the Origins of Language’ , Language & Communication , 50 : 12 – 21 .
Krebs , J. R. , and R. Dawkins ( 1984 ) ‘ Animal Signals: Mind-Reading and Manipulation’. In: Krebs , J. R. and R. Dawkins (eds.) Behavioral Ecology: An Evolutionary Approach , pp. 380 – 402 . Oxford : Blackwell .
Lakatos , I. ( 1978 ) The Methodology of Scientific Research Programmes: Volume I . Cambridge : Cambridge University Press .
Levinson , S. C. , and J. Holler ( 2014 ) ‘The Origin of Human Multi-modal Communication’ , Philosophical Transactions of the Royal Society B: Biological Sciences , 369 : 20130302 .
Levinson , S. C. ( 2006 ) ‘ On the Human “Interaction Engine”’. In: Enfield , Nick J. , and Stephen C. Levinson (eds.) Roots of Human Sociality , pp. 39 – 69 . Oxford : Berg .
Lewontin , R. C. ( 1998 ) ‘ The Evolution of Cognition: Questions We Will Never Answer’. In: Scarborough , Don , and Saul Sternberg (eds.) An Invitation to Cognitive Science. Volume 4: Methods, Models, and Conceptual Issues , pp. 106 – 32 . Cambridge, MA : MIT Press .
Love , A. ( 2006 ) ‘ Introductory Textbooks and Disciplinary Acculturation: A Case Study from Social Anthropology. In: Martin Hewings (ed.) Academic Writing in Contexts: Implications and Applications , pp. 122 – 139 . Birmingham : The University of Birmingham Press .
Lyn , H. ( 2012 ) ‘Apes and the Evolution of Language: Taking Stock of 40 Years of Research’. In Shackelford , Todd K. and Jennifer Vonk (eds.) The Oxford Handbook of Comparative Evolutionary Psychology , pp. 356 – 78 . Oxford : Oxford University Press .
Maynard Smith , J. , and D. Harper ( 2003 ) Animal Signals . Oxford : Oxford University Press .
McMahon , A. , and R. McMahon ( 2013 ). Evolutionary Linguistics . Cambridge : Cambridge University Press .
Mesoudi , A. , A. Whiten , and K. N. Laland . ( 2006 ) ‘Towards a Unified Science of Cultural Evolution’ , Behavioral and Brain Sciences , 29 : 329 – 47 .
Myers , G. ( 1992 ) ‘Textbooks and the Sociology of Scientific Knowledge’ , English for Specific Purposes , 11 : 3 – 17 .
Nölle , J. et al. . ( 2020 ) ‘Language as Shaped by the Environment: Linguistic Construal in a Collaborative Spatial Task’ , Palgrave Communications , 6 : 1 – 10 .
Noth , W. ( 1990 ) Handbook of Semiotics . Bloomington, IN : Indiana University Press .
Ouattara , K. , A. Lemasson , and K. Zuberbühler ( 2009 ) ‘Campbell’s Monkeys Concatenate Vocalizations into Context-Specific Call Sequences’ , Proceedings of the National Academy of Sciences , 106 : 22026 – 31 .
Power , C. ( 2014 ) ‘Signal Evolution and the Social Brain’. In: Daniel Dor , Chris Knight , and Jerome Lewis (eds.) The Social Origins of Language , pp. 47 – 55 . Oxford : Oxford University Press .
Progovac , L. ( 2019 ) A Critical Introduction to Language Evolution: Current Controversies and Future Prospects . Berlin : Springer .
QSR International Pty Ltd. ( 2020 ) NVivo (ver. 1.3 released in August 2020. https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
Richerson , P. J. , and R. Boyd ( 2005 ) Not by Genes Alone: How Culture Transformed Human Evolution . Chicago, IL : University of Chicago Press .
Roberts , G. ( 2017 ) ‘The linguist’s Drosophila: Experiments in Language Change’ , Linguistics Vanguard , 3 : 20160086 .
Saldaña , J. ( 2015 ) The Coding Manual for Qualitative Researchers . New York : SAGE Publications Ltd .
Scott-Phillips , T. C. ( 2014 ) Speaking Our Minds: Why Human Communication Is Different, and How Language Evolved to Make It Special . London : Macmillan International Higher Education .
Searcy , W. A. ; and S. Nowicki ( 2005 ) The Evolution of Animal Communication . Princeton, NJ : Princeton University Press .
Sprinker , M. ( 1980 ) ‘Gerard Manley Hopkins on the Origin of Language’ , Journal of the History of Ideas , 41 : 113 – 28 .
Suzuki , T. N. , and K. Zuberbühler . ( 2019 ) Animal syntax . Current Biology , 29 : R669 – 71 .
Tamariz , M. ( 2017 ) ‘Experimental Studies on the Cultural Evolution of Language’ , Annual Review of Linguistics , 3 : 389 – 407 .
Tallerman , M. , and K. R. Gibson , eds. ( 2012 ) The Oxford Handbook of Language Evolution . Oxford : Oxford University Press .
Tamariz , M. , and S. Kirby . ( 2016 ) ‘The Cultural Evolution of Language’ , Current Opinion in Psychology , 8 : 37 – 43 .
Tinbergen , N. ( 1963 ) ‘On Aims and Methods of Ethology’ , Zeitschrift für Tierpsychologie , 20 : 410 – 33 .
Tomasello , M. ( 1999 ) The Cultural Origins of Human Cognition . Cambridge, MA : Harvard University Press .
——— ( 2008 ) Origins of Human Communication . Cambridge, MA : MIT Press .
Townsend , S. W. , and M. B. Manser . ( 2013 ). Functionally referential communication in mammals: the past, present and the future . Ethology , 119 : 1 – 11 .
Wacewicz , S. et al. . ( 2020 ) ‘Language in Language Evolution Research: In Defense of a Pluralistic View’ , Biolinguistics , 14 : 59 – 101 .
———, and P. Żywiczyński ( 2015 ) ‘Language Evolution: Why Hockett’s Design Features Are a Non-starter’ , Biosemiotics , 8 : 29 – 46 .
——— ( 2017 ) ‘The Multimodal Origins of Linguistic Communication’ , Language & Communication , 54 : 1 – 8 .
——— ( 2018 ) ‘Language Origins: Fitness Consequences, Platform of Trust, Cooperation, and Turn-Taking’ , Interaction Studies , 19 : 167 – 82 .
Wacewicz , S. , P. Żywiczyński , and A. Jasiński ( 2016 ) ‘ Language Evolution and Language Origins in Teaching Linguistics at the University Level’. In: Roberts , Sean G. , et al. . (eds.) The Evolution of Language: Proceedings of the 11th International Conference (EVOLANG11) . http://evolang.org/neworleans/papers/62.html
Whitney , W. D ( 1873 [1872] ) Oriental and Linguistic Studies . New York : Charles Scribner’s Sons .
Zlatev , J. ( 2014 ) ‘Human Uniqueness, Bodily Mimesis and the Evolution of Language’ , Humana. Mente Journal of Philosophical Studies , 7 : 197 – 219 .
Zuberbühler , K. ( 2002 ) ‘A Syntactic Rule in Forest Monkey Communication’ , Animal Behaviour , 63 : 293 – 9 .
Żywiczyński , P. ( 2018 ) Language Origins: From Mythology to Science . New York : Peter Lang .
It is of course important to note that this selection of textbooks is biased in that it is limited to textbooks in English, which come with their own biases in terms of a predominant focus on English as the language discussed most frequently. This bias in the Open Syllabus list is very likely due to the fact that although it covers 140 countries in total, it is a list of English-language syllabi and the majority of syllabi are taken from schools in English-speaking countries. In a future study, it would be highly interesting if the patterns found in this paper regarding English-language linguistics textbooks also hold for textbooks in other languages.
However, in O’Grady et al. , the chapter on animal communication is an ‘online only’ chapter that is only accessible with a subscription to the Macmillan Launchpad Solo service.
Language-trained animals were chosen as a subcategory on the basis of their frequent discussion both in linguistics textbooks and language evolution textbooks with relation to language evolution, and because the capacities of language- and symbol-trained animals have been of central interest to researchers in language evolution for a very long time (e.g. Lyn 2012 ).
While it could be argued that the history of languages is directly relevant to language evolution, most of the textbooks did not make explicit references to language evolution when discussing historical language change. This is in line with the widely held view in traditional historical linguistics that a ‘topic not generally considered to be properly part of historical linguistics is the ultimate origin of human language and how it may have evolved from non-human primate call systems, gestures, or whatever, to have the properties we now associate with human languages in general’ ( Campbell 2013 : 2; see Hartmann 2020 , for discussion).
For a comprehension description of problematic passages, see the Appendix A.1 in Supplementary Data.
For a short description of research on the cultural evolution of language, see the Appendix A.2 in Supplementary Data.
Supplementary data
Email alerts, citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 2058-458X
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
This page has been archived and is no longer updated
An Introduction to Animal Communication
The ability to communicate effectively with other individuals plays a critical role in the lives of all animals. Whether we are examining how moths attract a mate, ground squirrels convey information about nearby predators, or chimpanzees maintain positions in a dominance hierarchy, communication systems are involved. Here, I provide a primer about the types of communication signals used by animals and the variety of functions they serve. Animal communication is classically defined as occurring when “...the action of or cue given by one organism [the sender] is perceived by and thus alters the probability pattern of behavior in another organism [the receiver] in a fashion adaptive to either one both of the participants” (Wilson 1975). While both a sender and receiver must be involved for communication to occur (Figure 1), in some cases only one player benefits from the interaction. For example, female Photuris fireflies manipulate smaller, male Photinus fireflies by mimicking the flash signals produced by Photinus females. When males investigate the signal, they are voraciously consumed by the larger firefly (Lloyd 1975; Figure 2). This is clearly a case where the sender benefits and the receiver does not. Alternatively, in the case of fringe-lipped bats, Trachops cirrhosus , and tungara frogs, Physalaemus pustulosus , the receiver is the only player that benefits from the interaction. Male tungara frogs produce advertisement calls to attract females to their location; while the signal is designed to be received by females, eavesdropping fringe-lipped bats also detect the calls, and use that information to locate and capture frogs (Ryan et al . 1982). Despite these examples, there are many cases in which both the sender and receiver benefit from exchanging information. Greater sage grouse nicely illustrate such “true communication”; during the mating season, males produce strutting displays that are energetically expensive, and females use this honest information about male quality to choose which individuals to mate with (Vehrencamp et al . 1989).
Figure 1 A model of animal communication.
Figure 2: Photinus fireflies. Courtesy of Tom Eisner.
Signal Modalities
Animals use a variety of sensory channels, or signal modalities, for communication. Visual signals are very effective for animals that are active during the day. Some visual signals are permanent advertisements; for example, the bright red epaulets of male red-winged blackbirds, Agelaius phoeniceus, which are always displayed, are important for territory defense. When researchers experimentally blackened epaulets, males were subject to much higher rates of intrusion by other males (Smith 1972). Alternatively, some visual signals are actively produced by an individual only under appropriate conditions. Male green anoles, Anolis carolinensis, bob their head and extend a brightly colored throat fan (dewlap) when signaling territory ownership. Acoustic communication is also exceedingly abundant in nature, likely because sound can be adapted to a wide variety of environmental conditions and behavioral situations. Sounds can vary substantially in amplitude, duration, and frequency structure, all of which impact how far the sound will travel in the environment and how easily the receiver can localize the position of the sender. For example, many passerine birds emit pure-tone alarm calls that make localization difficult, while the same species produce more complex, broadband mate attraction songs that allow conspecifics to easily find the sender (Marler 1955). A particularly specialized form of acoustic communication is seen in microchiropteran bats and cetaceans that use high-frequency sounds to detect and localize prey. After sound emission, the returning echo is detected and processed, ultimately allowing the animal to build a picture of their surrounding environment and make very accurate assessments of prey location. Compared to visual and acoustic modalities, chemical signals travel much more slowly through the environment since they must diffuse from the point source of production. Yet, these signals can be transmitted over long distances and fade slowly once produced. In many moth species, females produce chemical cues and males follow the trail to the female’s location. Researchers attempted to tease apart the role of visual and chemical signaling in silkmoths, Bombyx mori , by giving males the choice between a female in a transparent airtight box and a piece of filter paper soaked in chemicals produced by a sexually receptive female. Invariably, males were drawn to the source of the chemical signal and did not respond to the sight of the isolated female (Schneider 1974; Figure 3). Chemical communication also plays a critical role in the lives of other animals, some of which have a specialized vomeronasal organ that is used exclusively to detect chemical cues. For example, male Asian elephants, Elaphus maximus , use the vomeronasal organ to process chemical cues in female’s urine and detect if she is sexually receptive (Rasmussen et al . 1982).
Figure 3 Male silkmoths are more strongly attracted to the pheromones produced by females (chemical signal) than the sight of a female in an airtight box (visual signal). Tactile signals, in which physical contact occurs between the sender and the receiver, can only be transmitted over very short distances. Tactile communication is often very important in building and maintaining relationship among social animals. For example, chimpanzees that regularly groom other individuals are rewarded with greater levels of cooperation and food sharing (de Waal 1989). For aquatic animals living in murky waters, electrical signaling is an ideal mode of communication. Several species of mormyrid fish produce species-specific electrical pulses, which are primarily used for locating prey via electrolocation, but also allow individuals searching for mates to distinguish conspecifics from heterospecifics. Foraging sharks have the ability to detect electrical signals using specialized electroreceptor cells in the head region, which are used for eavesdropping on the weak bioelectric fields of prey (von der Emde 1998).
Signal Functions
Some of the most extravagant communication signals play important roles in sexual advertisement and mate attraction. Successful reproduction requires identifying a mate of the appropriate species and sex, as well as assessing indicators of mate quality. Male satin bowerbirds, Ptilonorhynchus violaceus , use visual signals to attract females by building elaborate bowers decorated with brightly colored objects. When a female approaches the bower, the male produces an elaborate dance, which may or may not end with the female allowing the male to copulate with her (Borgia 1985). Males that do not produce such visual signals have little chance of securing a mate. While females are generally the choosy sex due to greater reproductive investment, there are species in which sexual roles are reversed and females produce signals to attract males. For example, in the deep-snouted pipefish, Syngnathus typhle , females that produce a temporary striped pattern during the mating period are more attractive to males than unornamented females (Berglund et al . 1997). Communication signals also play an important role in conflict resolution, including territory defense. When males are competing for access to females, the costs of engaging in physical combat can be very high; hence natural selection has favored the evolution of communication systems that allow males to honestly assess the fighting ability of their opponents without engaging in combat. Red deer, Cervus elaphus , exhibit such a complex signaling system. During the mating season, males strongly defend a group of females, yet fighting among males is relatively uncommon. Instead, males exchange signals indicative of fighting ability, including roaring and parallel walks. An altercation between two males most often escalates to a physical fight when individuals are closely matched in size, and the exchange of visual and acoustic signals is insufficient for determining which animal is most likely to win a fight (Clutton-Brock et al . 1979). Communication signals are often critical for allowing animals to relocate and accurately identify their own young. In species that produce altricial young, adults regularly leave their offspring at refugia, such as a nest, to forage and gather resources. Upon returning, adults must identify their own offspring, which can be especially difficult in highly colonial species. Brazilian free-tailed bats, Tadarida brasiliensis , form cave colonies containing millions of bats; when females leave the cave each night to forage, they place their pup in a crèche that contains thousands of other young. When females return to the roost, they face the challenge of locating their own pups among thousands of others. Researchers originally thought that such a discriminatory task was impossible, and that females simply fed any pups that approached them, yet further work revealed that females find and nurse their own pup 83% of the time (McCracken 1984, Balcombe 1990). Females are able to make such fantastic discriminations using a combination of spatial memory, acoustic signaling, and chemical signaling. Specifically, pups produce individually-distinct “isolation calls”, which the mother can recognize and detect from a moderate distance. Upon closer inspection of a pup, females use scent to further confirm the pup’s identity. Many animals rely heavily on communication systems to convey information about the environment to conspecifics, especially close relatives. A fantastic illustration comes from vervet monkeys, Chlorocebus pygerythrus , in which adults give alarm calls to warn colony members about the presence of a specific type of predator. This is especially valuable as it conveys the information needed to take appropriate actions given the characteristics of the predator (Figure 4). For example, emitting a “cough” call indicates the presence of an aerial predator, such as an eagle; colony members respond by seeking cover amongst vegetation on the ground (Seyfarth & Cheney 1980). Such an evasive reaction would not be appropriate if a terrestrial predator, such as a leopard, were approaching.
Figure 4 Vervet monkeys. Many animals have sophisticated communication signals for facilitating integration of individuals into a group and maintaining group cohesion. In group-living species that form dominance hierarchies, communication is critical for maintaining ameliorative relationships between dominants and subordinates. In chimpanzees, lower-ranking individuals produce submissive displays toward higher-ranking individuals, such as crouching and emitting “pant-grunt” vocalizations. In turn, dominants produce reconciliatory signals that are indicative of low aggression. Communication systems also are important for coordinating group movements. Contact calls, which inform individuals about the location of groupmates that are not in visual range, are used by a wide variety of birds and mammals. Overall, studying communication not only gives us insight into the inner worlds of animals, but also allows us to better answer important evolutionary questions. As an example, when two isolated populations exhibit divergence over time in the structure of signals use to attract mates, reproductive isolation can occur. This means that even if the populations converge again in the future, the distinct differences in critical communication signals may cause individuals to only select mates from their own population. For example, three species of lacewings that are closely related and look identical are actually reproductively isolated due to differences in the low-frequency songs produced by males; females respond much more readily to songs from their own species compared to songs from other species (Martinez, Wells & Henry 1992). A thorough understanding of animal communication systems can also be critical for making effective decisions about conservation of threatened and endangered species. As an example, recent research has focused on understanding how human-generated noise (from cars, trains, etc) can impact communication in a variety of animals (Rabin et al . 2003). As the field of animal communication continues to expand, we will learn more about information exchange in a wide variety of species and better understand the fantastic variety of signals we see animals produce in nature.
Vomeronasal organ – auxiliary olfactory organ that detects chemosensory cues
Altricial – the state of being born in an immature state and relying exclusively on parental care for survival during early development
Refugia – areas that provide concealment from predators and/or protection from harsh environmental conditions
Conspecifics – organisms of the same species
References and Recommended Reading
Balcombe, J.P. Vocal recognition of pups by mother Mexican free-tailed bats, Tadarida brasiliensis mexicana . Animal Behaviour 39 , 960-966 (1990). Berglund, J., Rosenqvist G. and Bernet P. Ornamentation predicts reproductive success in female pipefish. Behavioral Ecology and Sociobiology 40 , 145-150 (1997). Clutton-Brock, T., Albon S., Gibson S. & Guinness F. The logical stag: Adaptive aspects of fighing in the red deer. Animal Behaviour 27 , 211-225 (1979). de Waal F.B.M. Food sharing and reciprocal obligations among chimpanzees. Journal of Human Evolution 18 , 433–459 (1989).
Hauser, M. 1997. The Evolution of Communication . Cambridge, MA: MIT Press. Lloyd, J.E. Aggressive mimicry in Photuris: signal repertoires by femmes fatales. Science 197 , 452-453 (1975).
Marler, P. Characteristics of some animal calls. Nature 176 , 6-8 (1955). Martinez Well, M. & Henry C.S. The role of courtship songs in reproductive isolation among populations of green lacewings of the genus Chrysoperla . Evolution 46 , 31-43 (1992). McCracken, G.F. Communal nursing in Mexican free-tailed bat maternity colonies. Science 223 , 1090-1091(1984).
Rabin, L.A., McCowan B., Hooper S.L & Owings D.H. Anthropogenic noise and its effect on animal communication: an interface between comparative psychology and conservation biology. International Journal of Comparative Psychology 16 , 172-192 (2003). Ryan M.J., Tuttle M.D., & Rand A.S. Sexual advertisement and bat predation in a neotropical frog. American Naturalist 119 , 136–139 (1982). Schneider, D. The sex attractant receptors of moths. Scientific American 231 , 28-35 (1974). Seyfarth, R.M., Cheney D.L. & Marler P. Monkey responses to three different alarm calls: Evidence for predator classification and semantic communication. Science 210 , 801-803 (1980). Smith, D. The role of the epaulets in the red-winged blackbird, ( Agelaius phoeniceus ) social system. Behaviour 41 , 251-268 (1972).
Vehrencamp, S.L., Bradbury J.W., & Gibson R.M. The energetic cost of display in male sage grouse. Animal Behaviour 38 , 885-896 (1989). von der Emde, G. Electroreception. In D. H. Evans (ed.). The Physiology of Fishes , pp. 313-343. Boca Raton, FL: CRC Press (1998). Wilson, E.O. Sociobiology: The New Synthesis . Cambridge, MA: Harvard University Press (1975).
Article History
Flag inappropriate.


Email your Friend

- | Lead Editor: Sara Tenney

Within this Subject (24)
- Basic (13)
- Intermediate (5)
- Advanced (6)
Other Topic Rooms
- Ecosystem Ecology
- Physiological Ecology
- Population Ecology
- Community Ecology
- Global and Regional Ecology
- Conservation and Restoration
- Animal Behavior
- Teach Ecology
- Earth's Climate: Past, Present, and Future
- Terrestrial Geosystems
- Marine Geosystems
- Scientific Underpinnings
- Paleontology and Primate Evolution
- Human Fossil Record
- The Living Primates
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse

REVIEW article
Why are no animal communication systems simple languages.
A commentary has been posted on this article:
Commentary: Why Are no Animal Communication Systems Simple Languages?
- Read general commentary
Commentary: Why Are No Animal Communication Systems Simple Languages?

- 1 Department of Psychology, University of Washington, Seattle, WA, United States
- 2 Department of Biology, University of Washington, Seattle, WA, United States
Individuals of some animal species have been taught simple versions of human language despite their natural communication systems failing to rise to the level of a simple language. How is it, then, that some animals can master a version of language, yet none of them deploy this capacity in their own communication system? I first examine the key design features that are often used to evaluate language-like properties of natural animal communication systems. I then consider one candidate animal system, bird song, because it has several of the key design features or their precursors, including social learning and cultural transmission of their vocal signals. I conclude that although bird song communication is nuanced and complex, and has the acoustic potential for productivity, it is not productive – it cannot be used to say many different things. Finally, I discuss the debate over whether animal communication should be viewed as a cooperative information transmission process, as we typically view human language, or as a competitive process where signaler and receiver vie for control. The debate points to a necessary condition for the evolution of a simple language that has generally been overlooked: the degree of to which the interests of the signaler and receiver align. While strong cognitive and signal production mechanisms are necessary pre-adaptations for a simple language, they are not sufficient. Also necessary is the existence of identical or near-identical interests of signaler and receiver and a socio-ecology that requires high-level cooperation across a range of contexts. In the case of our hominid ancestors, these contexts included hunting, gathering, child care and, perhaps, warfare. I argue that the key condition for the evolution of human language was the extreme interdependency that existed among unrelated individuals in the hunter-gatherer societies of our hominid ancestors. This extreme interdependency produced multiple prosocial adaptations for effective intragroup cooperation, which in partnership with advanced cognitive abilities, set the stage for the evolution of language.
Introduction
Research programs on animal communication systems in nature have proceeded essentially independently of research programs endeavoring to teach language to animals. This is surprising in light of the early, well-known efforts to relate these two research streams, especially by Hockett (1960) and Marler (1961) . These efforts spurred two questions. First, can animals be taught human language, even a simplified version? Second, do the natural communication systems of any animals rise to the level of simple language? Research since then has indicated that these two questions may have different answers: I would suggest a provisional yes to the first, and a provisional no to the second. If this view is correct, it raises a further question: why, then, if some animals can master a version of language, don’t they use this capacity in their natural communication system? In this paper I address this paradox, and make some suggestions toward its resolution.
My paper is divided into four parts. First I consider the main “design features” of language proposed by Hockett as a basis for evaluating language-like properties of animal communication systems. Hockett concluded that some animal communication systems have some of these design features, but none of them have all the design features, especially the key ones. I will designate an animal communication system as a ‘simple language’ system using a variation on the definition of Hewes (1973) : “language [is] any system of animal communication which exhibits most of the design features set forth by Hockett” ( Hewes, 1973 , p. 5). I narrow this definition by identifying four design features – semanticity, arbitrariness, learnability and cultural transmission, and productivity – as necessary for the system to be classified as a simple language. Second, I discuss bird song, a case where several but not all of the key design features are present. I will focus on one specific case of a song-based communication system that is clearly complex and nuanced, but nevertheless lacks three key design features, semanticity, arbitrariness and productivity. Third, I consider the debate, not yet fully concluded, over whether animal communication should be conceived of as a process of information transfer or as manipulation of receiver by the signaler. The debate is germane to our more specific question because it provides a clue as to why we find no simple languages among animals despite the apparent capacity for it in at least some of them. Finally, I suggest that although there appear to be at least some animals with the cognitive capacity for a language-like communication system, none of them have a social system with extreme interdependency among individuals on the scale of that which existed in the hominid hunter-gatherer system. I argue that this extreme interdependency was a necessary condition for the evolution of human language.
Design Features of Language
In this section I consider the extent to which the most important design features of human language are found in animal communication systems. I use Hockett’s (1960) design features as a basis for comparison of natural animal communication systems with human language. Although Hockett’s design features may have limited use as a theoretical framework for modern evolutionary linguistics ( Wacewicz and Żywiczyński, 2015 ), it is a useful starting point for the comparative analysis of this paper. I have winnowed Hockett’s original design features down to the few I consider the most fundamental ones that can be used to directly compare human language with animal communication systems.
Specialization: The Purpose of Linguistic Signals Is Communication and Not Some Other Biological Function
Specialization, in Hockett’s sense, is the first defining feature of a communication system, no matter how simple or complex it might be. Otte (1974) defines communication signals as traits “fashioned or maintained by natural selection because they convey information to other organisms”( Otte, 1974 , p. 385). I discuss the vigorous debate over the ‘information’ aspect of this definition in Section “Communication: Information or Influence? Mutual Benefit or Manipulation?”, but debaters on both sides would agree that this definition captures the key difference between true communication signals on the one hand, and tactical behaviors or inadvertent cues on the other. For example, while we might describe an individual delivering a blow to a potential opponent as ‘sending a message,’ we mean this only in a metaphorical sense. This behavior is primarily tactical, that is, the individual delivering the blow will directly benefit it if its opponent responds by backing down. If instead of delivering a blow the individual had said “I’m going to kill you,” or growled, or barked, or hissed, we would recognize these as true communication signals, having been shaped by natural selection for the purpose of (literally) sending a message, and requiring adaptations in the receiver as well.
Hockett listed prevarication – the ability to transmit misinformation, i.e., to lie or deceive – as one of his many design features, albeit a minor one, a corollary almost. In Section “Communication: Information or Influence? Mutual Benefit or Manipulation?”, I will argue that we should consider prevarication to be a fundamental, indeed foundational feature of animal communication systems: communication in animals is shaped by the tension between the sender’s and receiver’s interests, and truth in communication is not a given, but rather, when it occurs, hard won.
Semanticity: Specific Signals Are Directly Tied to Certain Meanings
To say that a communication system is semantic is to say that it uses signals to represent particular things or actions. A well-known example in animals are alarm signals given in response to different predators. We can say in such cases that each of these signals represents one of several different predators, or more precisely, the appearance on the scene of one of these predators. For example, vervet monkeys have three different alarm calls for three different classes of predators: raptors, terrestrial mammals and snakes, predators which depend on an element of surprise to capture the monkey. In response to an aerial predator, such as a martial eagle, a monkey emits ‘cough’ calls and sender and receivers take shelter in dense bushes or near the core of a tree. In response to leopards, a monkey emits a ‘bark’ call and the monkeys climb up to the tip of tree branches where leopards cannot safely go. Finally, if a monkey spots a dangerous snake, such as a python, it emits a ‘chutter’ call and the group gathers around the snake, standing upright and harassing it until it leaves the area. Although the vervets use these same signals in other contexts (e.g., intergroup fights) to represent different things, the modification of signal meaning in different contexts occurs in human language as well, and does not negatively impact the representational quality of these signals ( Seyfarth et al., 1990 ; Price et al., 2015 ). Indeed, it is not unusual for an animal to use a particular signal to mean different things in different contexts ( Smith, 1997 ), similar to some words meaning totally different things within different sentences.
Nevertheless, I will argue later in this paper that the semanticity of animal communication systems is limited: although some things are represented by animal signals, the number of things is generally small. Attempts to catalog the number of different things signaled in animal communication systems typically top out at 25 or so (vervet monkeys, Struhsaker, 1967 ; Japanese macaques, Green, 1975 ; review in Hauser, 2000 ). The limitation does not appear to be due to production constraints (the ability to produce enough distinct signals or to recombine enough of them to enlarge the signal set) or to perceptual-cognitive constraints.
Arbitrariness: Languages Are Made Up of Arbitrary Symbols Which Have No Intrinsic or Logical Connection to What They Represent
A distinctive feature of human language is that not only are words semantic, they are arbitrarily so. We could equally well call dogs ‘cats’ and cats ‘dogs,’ or any other two words, so long as sender and receiver knew the convention, a point illustrated by the existence of the many different languages of the world. These signals seem totally arbitrary with respect to what they signify, and in theory they could be interchanged without problems, so long as senders and receivers were both aware of the convention. How about animal signals? It appears that in theory we could interchange the vervet alarm signals without problems, provided of course that the receivers were aware of the ‘convention’ (i.e., were hard-wired appropriately). Identity signals – indicating species or individual identity, and occasionally group or kinship – are perhaps the most common animals signals that unequivocally have the arbitrariness feature.
But many, perhaps most, animal signals are not arbitrary. Signals used in agonistic and mate attraction contexts are typically “more of” signals, i.e., more effective signals are louder, longer, bigger, brighter, flashier, designed to impress or to shock and awe. I am unaware of any clear example where the reverse is true, where the more effective signal is the one that is less conspicuous, for example, a softer sound, a more subdued color, a less vigorous display. An apparent exception might be the ‘quiet song’ sung by many songbirds in intense conflict situations, but this typically happens only when the bird is close to its opponent so that the quiet song is audible to the receiver ( Searcy et al., 2014 ); ‘normal’ song is loud because it is a long-distance signal. Moreover, quiet song is typically different in other respects besides loudness, for example, having some elements seen only in quiet song, such as very high frequency elements.
Other animal signals are simple extensions or slight modifications of tactical behaviors, e.g., of attack behavior in agonistic situations. For example, a threat signal in many mammals is the open mouth display, where the teeth, the canines notably, are prominently displayed. Ethologists called this a ‘ritualized’ display ( Lorenz, 1966 ), i.e., one that has been modified by natural selection to be a display, since the mouth is held open, and attack withheld, rather than being the beginning of an actual attack. Another common threat signal is the raising of the hair or feathers, making the animal appear larger. Again, while these actions are plausibly considered ritualized displays, they are not arbitrary signals. If they were, you would also find cases where animals threaten by closing their mouths, or by making themselves appear small. In short, animal signals functioning to impress an opponent or potential mating partner are usually inherently impressive, not arbitrarily selected to represent threat or desirability. Any naïve observer viewing a ritualized dominance interaction between two wolves (or dogs) would have no difficulty determining which animal was dominant and which was subordinate. An upright animal, with its hair raised, its tail raised, and staring at its opponent inherently appears dominant, whereas one with a flattened, slinking body, hair down, tail down, and looking away from the opponent, inherently appears subordinate.
Many epigamic signals – signals designed to attract a mate and induce her to mate – are bright, striking ornaments, often ones that function like supernormal stimuli (e.g., the tail of the long-tailed widowbird, Andersson, 1982 ). Many epigamic signals are energetically expensive and highly skilled behaviors, such as the complex male courtship dances of wolf spiders and jumping spiders ( Hebets and Uetz, 1999 ; Elias et al., 2012 ). The motor performance revealed in these sorts of displays likely reflect whole-organism performance relating to survival, and thus should be good indicators of individual signaler quality. There is considerable evidence that females choose mates in nature based upon their evaluations of male motor performance (reviewed in Byers et al., 2010 ). The relevant point here is that these signals are not arbitrary, but inherently reflect the trait signaled: signaler quality.
Even in the example par excellence of communication of information about the external world – the honeybee dance language – the signals are not quite so arbitrary as generally assumed. For example, if the dance is done outside the hive, where the sun is visible, the bee dances with respect to the actual position of the sun, rather than with respect to the vertical ( Gould, 1975 ). That is, outside the hive, the symbology is not truly arbitrary. Moreover, the distance to the target is represented by the duration of the straight run – the further the distance, the longer the run – so this is at least partially non-arbitrary as well.
Although the words in human language are arbitrary – the existence of different languages is the clearest evidence on this point – they may be expressed in such a way to amplify or otherwise modify their meaning, as for example a loudly shouted “no” indicating stronger conviction. But what would be considered an extra-linguistic feature for humans is often the primary message in animals. For example, the initial stage of a battle between two male red deer consists of a roaring contest ( Clutton-Brock and Albon, 1979 ). This vocal signaling duel does far more than simply establish that each animal is a male conspecific ready to defend or fight for the harem – this undoubtedly was perceived by both parties before the contest began – rather, how loud and how long an individual roars establishes how motivated and formidable he is, and is used by the receiver to decide whether to continue the fight or depart. Similarly, the plumage ornaments and courtship dance of a male golden-collared manakin do far more than simply identify species and sex – that is simply the necessary first step – the brightness of the ornament and the skill of the dance determine whether the receiver, the female, will choose to mate with this particular male or continue her search for the best possible mate ( Stein and Uy, 2006 ; Barske et al., 2011 ).
In summary, although we have examples of animal signals that are totally arbitrary, many others – perhaps most? – are not. I would add that to date we have found nothing comparable to the many different human languages, which are a consequence of the arbitrariness feature. We do find geographical dialects in animals (e.g., Marler and Tamura, 1964 ; Wright and Dahlin, 2018 ), but as the name implies, these are relatively minor variations on the basic signal set, nothing like the wholesale variation seen in human languages.
Learnability and Cultural Transmission
Human language is both learned and taught. Most animal communication systems are neither. A well-known exception to this generalization are the learned vocal communication signals of several taxa, most notably the oscine passerines (songbirds), hummingbirds and parrots among birds, and cetaceans and at least some bat species among mammals (reviews in Janik, 2014 ; Knornschild, 2014 ; Nowicki and Searcy, 2014 ). Evidence for vocal learning and cultural transmission in some other birds and mammals as well ( Walcott et al., 2006 ; Kroodsma et al., 2013 ; Stoeger and Manger, 2014 ; Garland and McGregor, 2020 ; Barker et al., 2021 ) suggests that this ability may lie closer to the surface than is generally assumed, but at least at the present time, vocal learning is thought to be rare in animals. Later in this paper I return to the best-studied example of vocal learning, song learning in songbirds.
Where the communication signals are learned, we should expect to find dialects, geographical variation in the signals. The occurrence of dialects is one criterion for identifying the occurrence of learning and potentially evidence for the arbitrariness design feature. An example that may illustrate the arbitrary nature of dialects is the recently-discovered modification of the song in eastern white-throated sparrows to resemble the typical song of western white-throated sparrows. Investigators have traced this change to eastern birds learning the western version of the song on the migration grounds, where individuals of the two populations mix ( Otter et al., 2020 ). Most eastern birds now sing the ‘western’ version of the song on the breeding grounds, illustrating that the details of the song structure are not crucial for its function. Although Otter et al. (2020) suggest that this change might have been driven by a preference on the part of eastern females, they give no evidence for this hypothesis, nor plausible basis for it.
Perhaps even rarer in animal communication systems than learning is teaching. The commonly accepted criteria for demonstrating teaching in non-human animals are that (1) teachers should modify their behavior in the presence of the learner, (2) this change in behavior should result in no immediate benefit to the teacher, and (3) the learner should acquire a behavior quicker or better as a result ( Caro and Hauser, 1992 ). In song-learning studies the birds from whom the young bird learns its song are conventionally referred to as ‘tutors,’ and although live birds are invariably more effective song tutors than recorded song (review in Beecher, 2017 ), the term ‘tutor’ is used purely as matter of convenience. In fact, in the most common context for song learning in nature, young birds learn from older birds who are or will be their territorial rivals, a very different context from language learning in young humans, where ‘tutors’ are typically relatives or other interested parties who ultimately (but not immediately) benefit from tutoring. Nevertheless, even in the common songbird case where the young bird learns from territorial rivals, bird song tutoring would fit all three criteria for teaching if in fact the older bird reduces his usual aggression when a young bird appears on his territory, increases his counter-singing with the young bird in such a way as to facilitate learning, and benefits down the road from this tutoring (for example, the two cooperate in mutual defense of their territories, or against predators, or refrain from extra-pair mating with one another’s mates). We have indirect evidence for song learning/teaching in song sparrows: mutual survival is greater in young birds and their primary tutor-neighbor (the one from whom they learn most of their songs) the more songs the two of them ultimately share, i.e., the more songs the tutee learned from the tutor, or the tutor taught the tutee ( Beecher et al., 2020 ).
Productivity: By Combining a Small Number of Meaningless Units Into Larger Meaningful Signals, a Sender Is Capable of Producing Meaningful Statements About Virtually Anything
The sense in which I am using this term is captured by Hauser (2000 , p. 448): “the power of [human] language comes from our capacity to take meaningless syllables and combine them into an unbounded number of meaningful words, and then take these words and combine them into an unbounded number of meaningful expressions ( Chomsky, 1986 ; Studdert-Kennedy, 1998 ).” I will define productivity as recombining a smaller number of basic signal units to produce a larger number of signals, and thus, messages. Indeed, semanticity (representation) and productivity are probably the two central features of human language: by combining basic phonetic units into larger meaningful units, and combining these units further via syntactical rules, we can say almost anything.
Animal communication systems are not productive in this sense, and this is the primary reason we do not refer to them as languages. We would be impressed if a vervet could say something like “Grab your infant and run from the leopard coming from the west but watch out for the python who likes to hide in the bushes just to the east of you.” A human can say this kind of thing easily, combining a relatively small number of atomic units (phonemes) into very large number of basic signals (words) and combining these into a very large set of possible communications. I note that while there is some controversy in phonetics about exactly what are the units of productive combination, there is agreement that all natural languages (including sign language) are made up of meaningless atomic units that are combined into larger meaningful wholes ( Zuidema and de Boer, 2009 ).
Instead of productivity, we could describe the communication system in terms of information capacity. The information capacity of human language is essentially infinite, in the sense that, in theory, we can communicate virtually anything. Our motor, sensory and cognitive capacities obviously will reduce how much information actually gets transmitted and received. But still, the fact is that we can transmit an enormous amount of information with language. Attempts to measure information capacity or information transmission in animals, on the other hand, have given rather modest results. Two estimates of the information about distance and direction in the honeybee dance language have given a high value of 14.9 bits ( Gould, 1975 ) and a low value of 7.4 bits ( Schürch and Ratnieks, 2015 ). My group has estimated the information capacity of the call signature system that parents of the colonial cliff swallow use to find their offspring in their large breeding colonies ( Medvin et al., 1993 ). We estimated the capacity as 8.76 bits, and the estimate would be somewhat larger if we included information that can be derived from visual differences among cliff swallow chicks ( Stoddard and Beecher, 1983 ). The information capacity of human language of course is orders of magnitude larger than this.
We certainly find the potential for productivity in bird song. For example, most songbirds have multiple songs (song ‘repertoires’), and the different songs are made up of different syllables or notes in different orders, and these smaller units can be used in more than one song. Still, although the units are there, and although songbirds may possess the cognitive capacity to comprehend hierarchical structuring in vocal signals ( Gentner et al., 2006 ; but see van Heijningen et al., 2009 ), they do not use these capacities to form different songs representing different things. As Hauser (2000 , p. 450) puts it, “in contrast to the recombination of words into sentences by humans, the output of songbird recombination does not change its meaning.” A minor exception are some songbirds who use some song types in a territorial defense context and others in a mate attraction context (e.g., Byers, 1996 ). As discussed in the next section, theories on the function of song repertoires abound, but they all agree that the different songs function simply to provide diversity, rather than to represent different things.
Table 1 summarizes the conclusions of this section. The natural communication systems of animals fall short of human language on a number of the key design features of language. They come closest on semanticity, where signals sometimes represent things in the external world or within the signaler, and the signals are sometimes truly arbitrary. However, more commonly animal signals are not arbitrary but inherently meaningful, e.g., an animal making itself appear large is more frightening than an animal making itself appear small. Most animal communication signals and responses are neither learned nor culturally transmitted. And, so far as we know, no animal communication has the sine qua non of language: productivity.
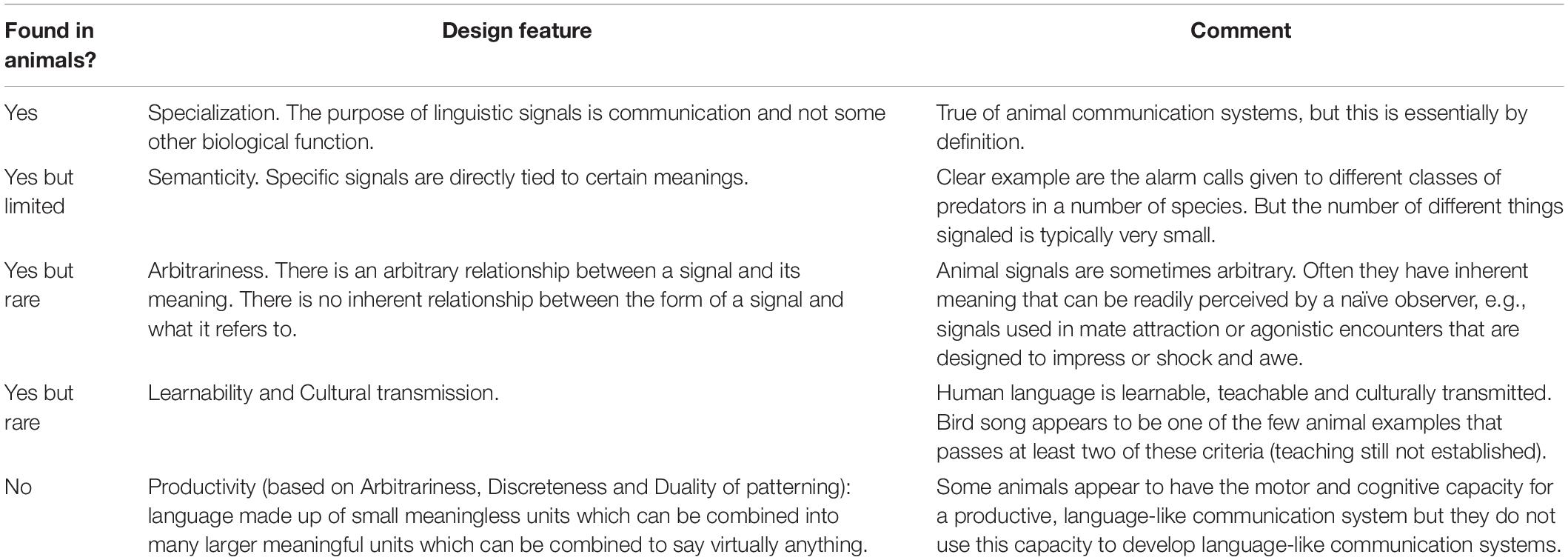
Table 1. Key design features of communication systems (after Hockett, 1960 , pruned and combined).
Bird Song: Complexity Without Productivity
The oscine passerines (songbirds) are one of the rare animal taxa in which individuals learn their vocal communication signals. In most animals, these vocal signals are ‘hard-wired,’ that is, they develop normally whether or not the animal is exposed to them early in life. It has long been noted that vocal learning in songbirds has many similarities to language learning in humans ( Marler, 1970 ; Doupe and Kuhl, 1999 ). These similarities include the following. (1) The young bird needs to be exposed to normal species vocal signals in order to produce them as an adult. (2) The sensory phase of song learning precedes the motor phase. (3) Auditory feedback (which can be abolished by deafening) is necessary for the translation of memorized sensory input into motor production. (4) Vocal learning is most efficient in (and sometimes restricted to) a sensitive period early in life. (5) There are specialized parts of the brain dedicated to the vocal control system. (6) Song is socially learned and culturally transmitted, and in at least some cases it may be actively taught (e.g., Carouso-Peck and Goldstein, 2019 ; Beecher et al., 2020 ). While notable differences exist among songbird species with regard to the normal progression of song learning ( Beecher and Brenowitz, 2005 ), these six features are essentially true for all of the many songbirds that have been studied to date.
Despite the notable parallels between bird song learning and human language learning, none of the many studies endeavoring to teach a version of human language to animals have focused on songbirds. This is all the more surprising given the language learning shown by Alex the African Gray Parrot, a member of another avian taxon with vocal learning, the psittacines ( Pepperberg, 1981 , 1987 ). Moreover, songbirds have strong cognitive capacities, a highly-developed vocal production mechanism, and a vocabulary of basic sound units in their song that rivals or exceeds the basic sound units of human language. There are even songbird species that can mimic human speech sounds (e.g., Hill Mynah birds). On the face of it, all the requisites would seem to be there to support a simple language in a songbird.
What Is the Function of a Song Repertoire?
In contrast to well-studied white-crowned sparrows and zebra finches, in most songbird species an individual bird will sing multiple songs (has a song ‘repertoire’). For example, song sparrows typically have nine (plus or minus two or so) very different songs. Each of these songs is made up of 5 or 6 distinct elements, and the order of these elements is important ( Horning et al., 1993 ). The songs do not have individual signatures and the nine or so songs in a song sparrow’s repertoire are as different among themselves as would be a collection of songs taken at random one from each of nine or so different birds ( Beecher et al., 1994 ). Song sparrows are somewhere on the middle of the song repertoire complexity scale: many species have larger and even more complex song repertoires. The key point for this discussion is that song repertoires provide clear potential for productivity, as song sparrows and many other songbirds have as many or more distinct units in their vocal communication systems (e.g., about 100 in indigo buntings, Thompson, 1970 ; and in swamp sparrows, Marler and Pickert, 1984 ) as there are in human language (a typical language has 40–45 phonemes).
The most popular hypothesis about song repertoires for north temperate zone songbirds – where only males sing – is that they are an epigamic signal produced by males to attract females and that larger repertoires are more attractive than smaller ones ( Catchpole, 1987 ; Searcy and Yasukawa, 1996 ; MacDougall-Shackleton, 1997 ; Collins, 2004 ). Focusing on just the well-studied song sparrow, the evidence for this hypothesis is mixed ( Searcy, 1984 ; Reid et al., 2004 ; Hill C. E. et al., 2011 ). The handicap principle, discussed in the next section, would suggest that if large song repertoires are preferred, it is because they are an indicator of some aspect of male quality. Reid et al. (2005) found support for this idea: song repertoire size in male song sparrows correlated with enhanced cell-mediated immune response (CMI) and relative heterozygosity. Anderson et al. (2017) hypothesized that female song sparrows might prefer large-repertoire males because this feature is an indicator the overall learning ability of the male. However, they found no correlations between repertoire size (or two other measures of song learning ability) with an overall measure of learning ability (based on five different learning tasks). I should note, however, that a correlation of vocal learning ability with both overall learning ability and mating success has been found in another songbird, the Satin Bowerbird, a vocal mimic: in this case the vocal learning ability is the ability of males to mimic the calls of other local bird species, both the number of species mimicked, and the accuracy of the mimicry ( Coleman et al., 2007 ; Keagy et al., 2009 ).
According to another hypothesis, song repertoires play a role in territorial competition, which in north temperate zone songbirds, where only males sing, is largely male-male competition, but outside the north temperate zone where both sexes sing, is pair-pair competition (e.g., Levin, 1996 ; Langmore, 1998 ; Logue and Gammon, 2004 ). There are several hypotheses as to how repertoires might work in the territorial competition context. Song is used by most territorial songbirds at least in part as a keep-out signal, to ‘post’ their territory. Kroodsma (1988) argues that the vocal diversity provided by a repertoire functions to hold the attention of territorial competitors by dishabituating them to the territory owner’s singing, i.e., by holding their attention. As one piece of evidence, he points to a positive correlation between repertoire size and population density in marsh wren populations, and also to the finding that birds in denser populations cycle through their songs faster, again a behavior that should reduce habituation ( Kroodsma, 1977 ). In contrast, song sparrows sing their much smaller repertoires with eventual variety, i.e., singing each one of their song types many times before switching to another type, and this would seem to argue against the dishabituation hypothesis. In western, resident populations of song sparrows, song repertoires may function primarily to provide a bird with songs matching all (or most) of his neighbors, and thus potential individualized replies to each one of them ( Beecher et al., 1997 ; and see next section).
Although as this brief discussion indicates, the theoretical debate has not yet concluded, the take-away point is that none of these hypotheses view song repertoires as a form of semantic communication. Rather they view repertoires as having a direct effect on the receiver (dishabituation), or as permitting individualized replies to multiple neighbors, or as quantitative signals with inherent rather than semantic meaning, that is, more songs (or more song syllables) are simply more effective.
I should add that most single-song species appear to have the potential to develop song repertoires yet do not tap into this potential. For example, when examined over an entire population, indigo buntings have a repertoire of over a 100 distinct song syllables, yet a given individual uses just 6–8 of these in the single song it develops ( Rice and Thompson, 1968 ; Thompson, 1969 ; Baker and Boylan, 1995 ).
An Example: Communication in a Negotiation Context
Although the different songs in a bird’s repertoire do not have different meanings, a bird having a song repertoire can still use the different songs to communicate in more subtle, nuanced ways than might at first be suspected. In this section I describe one such case: how song sparrows use the songs in their song repertoire to negotiate territorial disputes. The general point I will make is that their communication system is surprisingly complex and versatile, despite being neither semantic nor productive. Although I will not attempt to generalize to all songbirds given the incredible diversity of the song communication systems seen in this group ( Beecher and Brenowitz, 2005 ), I suspect that this conclusion – complexity without productivity – applies broadly to songbirds, and perhaps to all animals.
Song sparrows have a territorial system like that found in many animals and typical of many songbirds. An individual carves out a territory where the mated pair will nest and raise their young, doing most of their feeding on the territory. Suitable habitat is typically densely occupied by conspecifics, so territorial disputes can arise during both the establishment and maintenance stages. The relationship between territorial neighbors can become relatively non-hostile once established, however, on the principle that the enemy you know is better than the enemy you don’t know, generally referred to as the ‘Dear Enemy’ relationship ( Fisher, 1954 ; Akçay et al., 2009 , 2010 ; Beecher and Akçay, 2014 ). Because in territorial animals, neighbors have no fences, neighbors need to renegotiate territory boundaries from time to time. Negotiation can progress into fighting but avoiding fighting may benefit both parties and this common interest favors reliable signaling. Therefore, as I will discuss in Section “Communication: Information or Influence? Mutual Benefit or Manipulation?”, we should expect to find some degree of honest communication concerning not only fighting ability (resource-holding potential) but also motivation to fight (e.g., at a particular point in time, one party may have more to lose than the other).
Song sparrows in western, resident populations use their repertoires in a complex way to carry out territory negotiations. Although they will engage in serious fights, established neighbors use their signaling system to avoid fighting if possible. Before fighting they typically give their high-level threat signals, wing waves and soft song ( Searcy and Beecher, 2009 ; Searcy et al., 2014 ; Akçay et al., 2015a ). But before reaching this stage, they use the songs in their repertoires to escalate or de-escalate the dispute following a set of ‘conventions’ predicated on which songs the two birds happen to share ( Beecher et al., 1996 , 2000 ; Burt et al., 2001 , 2002 ; Beecher and Campbell, 2005 ; Akçay et al., 2011 ; Templeton et al., 2012 ; Akçay et al., 2013 , 2015b ). Because western song sparrows learn songs from their neighbors in the area to which they disperse after fledging, a bird typically shares some of his songs with each of his immediate neighbors. The set of songs he shares with one neighbor is typically different from the set he shares with another. A partial example is shown in Figure 1 . For example, if we represent the different songs of a bird with different capital letters, and the shared songs of neighbors with the same capital letter, then Bird 1 might share his song types A, B, and C with his neighbor Bird 2, his song types C, D, and E with another neighbor, his song types E and F with a third neighbor, and finally G, H, and I with no neighbors (e.g., the bird he learned these songs from may have died). A typical territorial negotiation might occur as follows. Suppose Bird 1’s mate finds an ideal place to build her nest just over the previously-established boundary with Bird 2. Bird 1, aiming to establish this new boundary, moves to that point and sings at his neighbor. Typically the two birds would still be a considerable distance apart at this point and out of sight of one another (territories are large and song is a long-distance signal). Although Bird 1 could sing any one of his 9 songs to Bird 2, in this circumstance he would typically ‘address’ Bird 2 by singing one of their shared types, A, B, or C. Let us say bird 1 sings B. Bird 2 can escalate by replying with his B’ (i.e., his most similar song to Bird 1’s B). This ‘type match’ is a low-level threat signal and would be the first step in escalation. Alternatively, he could ‘confirm’ without escalating by replying with A’ or C’ (‘repertoire matches’, Beecher et al., 1996 ). Note that this type of reply is only possible if Bird 2 knows Bird 1 well enough to know which songs they share and which songs they don’t. Finally, rather than type-matching or repertoire-matching, Bird 2 can de-escalate by singing one of his unshared types, e.g., D, E, F, G, H or I. Singing an unshared type is better than not singing at all because it signals that although the singer is not engaging, he is on territory and has heard his neighbor; it is a signal likely used for example when the bird is busy feeding recently-fledged young. If Bird 2 does type match bird 1 (sings B’), Bird 1 in turn can continue to sing that song type (‘stay on type’), or he can de-escalate by switching to another shared song (A or C, ‘repertoire match’), or de-escalate further by switching to an unshared type (e.g., D or E), or disengage totally by stopping singing.
Figure 1. Partial song repertoires of two neighboring birds. Shared songs are shown in the top three rows, and four of their unshared songs in the bottom two rows (they are arbitrarily paired). Frequency scale: 0–10 kHz. Songs are 2–3 s long.
Each ‘convention’ – type matching, repertoire matching, staying on type, switching to an unshared type – has a distinct signaling function in this graded signaling system, with both type matching and staying on type when type-matched signaling a readiness to escalate, repertoire matching signaling recognition of the sender and engagement but stopping short of escalation, and switching to an unshared type signaling de-escalation. The system while not in itself resolving anything, does give the neighbors time to defuse the situation or work out a compromise. Note, however, that the semantic content is limited. No particular song in the repertoire means a particular thing. A song’s meaning is defined entirely by the context of who the receiver is, and even then there are essentially only three meanings, roughly ‘back off,’ ‘I hear you and know who you are,’ and ‘I’m busy now.’
Songbirds check several of the design feature boxes and they would appear to have the potential to use their songs in a productive way, i.e., to use their signaling system to say many things. However, despite considerable debate concerning the function of song repertoires, the different repertoire hypotheses all agree on one point: that the function of the vocal diversity is diversity per se , not the transmission of different messages with different songs. Perhaps even more surprising, many single-song species have large song syllable repertoires an individual could tap into, but instead each individual uses just several of these syllables to develop its single song. No songbird rearranges its multiple song syllables into different songs that signal different things. I echo here the conclusion of Fitch and Jarvis (2013 , p. 502): although songbirds (and parrots) have vocal learning and a complex vocal repertoire, they do not “use their songs to communicate combinatorial propositional meanings, i.e., semantics.”. Songbirds may use their repertoires in subtle, nuanced ways, as with the song sparrow hierarchical signaling system I described above, but what the system achieves seems better described as the management of behavioral conflict than as an impressive transmission of information. That is, the system may function well, but it does not function like a language.
Communication: Information or Influence? Mutual Benefit or Manipulation?
In this section I discuss the debate within the field about the fundamental nature of animal communication. I believe this debate has provided us with a key to understanding why we find no examples of a simple language among the many communication systems of non-human animals, and true language only in the human animal.
We can trace the real beginning of the field of animal communication to the classical ethologists (e.g., Tinbergen, 1952 ). The ethologists provided detailed descriptions of animal signaling systems in nature, developed theories about the underlying proximate causes (e.g., sign stimuli, innate release mechanisms, and fixed action patterns) and evolutionary processes (e.g., ritualization), and most relevant here, established the view of animal communication as – like human language – an information transfer process. On the question of the function of animal signaling systems, they took a group-selectionist perspective: the benefit that a signaling system provided went not to signaler or receiver per se , but to the species (see Tinbergen, 1964 definition in Table 2 ).

Table 2. Definitions.
Following the revolution of the 1960’s and 1970’s first known as sociobiology ( Wilson, 1975 ) and subsequently as behavioral ecology ( Krebs and Davies, 1978 ), natural selection came to be viewed as acting on individuals, rather than species or groups ( Williams, 1966 ). For some researchers, the shift from naïve group selection to individual selection did not entail a significant change in view: it was simply assumed that signaler and receiver both benefited from the transmission of information, and so this basic parallel with human language was maintained (see Table 2 definitions of Marler, 1968 ; Otte, 1974 ). The assumption of mutual benefit seemed natural in cases where sender and receiver have a strong common interest, e.g., the honeybee ‘dance language’ where scout and recruit are both working toward the same end, to provide food for their relatives in the hive. But as investigators began considering the many cases where signaler and receiver have conflicting interests, such as in agonistic encounters over an indivisible resource, they began to question the mutual-benefit, information transmission view. They asked two questions about such cases. First, do both parties have to benefit? Second, do we need to even talk about ‘information transmission’? Isn’t the signaler simply selected to manipulate (or influence) the behavior of the receiver to its advantage? The manipulation viewpoint was famously developed by Dawkins and Krebs (1978) who argued that rather than expecting signalers to signal honestly, we should expect them to manipulate the receiver to their own advantage, e.g., to convince opponents to retreat, or potential partners to mate with them.
Since the Dawkins and Krebs (1978) paper, the debate has continued as to whether it is justified or productive to conceptualize animal signaling as an information transmission process in which both parties benefit. Simplifying somewhat, I will distinguish between the Information Transmission and Manipulation approaches to animal communication. Strong arguments on the manipulation side since Dawkins and Krebs (1978) include Krebs and Dawkins (1984) , Owings and Morton (1998) , Scott-Phillips (2008) , Rendall et al. (2009) , and Owren et al. (2010) . Strong arguments on the information side over this same period include Green and Marler (1979) , Smith (1997) , Bradbury and Vehrencamp (1998) , Searcy and Nowicki (2005) , Carazo and Font (2010) , Seyfarth et al. (2010) , and Wiley (2013) . Definitions from some of these sources are included in Table 2 .
In conceiving of signaling as manipulation, Dawkins and Krebs (1978) essentially treated the communication interaction like a zero-sum game. This seems reasonable in cases like disputes over an indivisible resource (a food item, a territory, and a mate), and also in epigamic selection, where a male tries to persuade a female to mate with him now rather than to continue searching for a possibly better male. Although the manipulation view was enlightening in many respects, as originally presented it had a serious weakness: it gave no agency to the receiver. While it was sensible to expect signalers to signal for their own benefit, why should we expect receivers to be passive in these evolutionary scenarios, especially if being manipulated by the signaler is costly? Rather, we should expect receivers to show ‘sales resistance’ to signals that carry misinformation or are pure propaganda (“I am the best,” “I will fight you to death”). Indeed, receivers can do more than simply ignore signals that do not benefit them: they can require signals that do benefit them, even if those signals are costly to the sender. For example, in many species males must sing or call to attract a female for mating. If the male does not vocalize, potential female receivers will simply not engage. Moreover, these vocal signals may attract predators, a cost borne by the signaler but not the receiver. Indeed, the most effective or most-preferred signals may be the most costly, e.g., most conspicuous not just to the intended receiver but to predators as well. This is the case for a male túngara frog ( Ryan and Rand, 1990 ). Males attract females to mate with a ‘whine’ call or a ‘whine-chuck’ call. When a male adds chucks to his calls, he not only attracts more females, but also predators: frog-eating bats that home in specifically on the chucks. Similarly, a calling male field cricket attracts more females than does a silent male, but he also attracts more parasitoid flies, and louder calls attract both more females and more parasitoid flies ( Cade, 1975 ). In some populations the rate of fly parasitism is so high that males have lost the ability to sing ( Zuk et al., 2006 ). As another example, territorial animals often vocalize as a “keep-out” signal. When a territorial songbird is deprived of its voice, however, potential rivals show up and proceed to take over its territory (e.g., McDonald, 1989 ).
If we reframe our view of the communication system as beginning with the implicit requirement that the receiver imposes on the signaler—to signal—rather than with the signal itself, it is apparent that receivers can be conceived of as manipulating signalers, and in the ‘receiver manipulation’ view, the potential costs to the sender are secondary to the potential benefits to the receiver. A possible benefit for the female túngara frog – the receiver in our example – might be a shorter search time in navigating to the male who adds the more localizable chucks to his calls, perhaps lessening her vulnerability to predation.
The receiver manipulation view prompts us to consider how the receiver might demand a more honest signal. There are two related possibilities. First, the receiver can selectively attend to signals that are inherently honest due to physical constraints. For example, in many frogs and toads, size is the most important weapon in male battles over mating opportunities and size is reliably predicted by the pitch of the animal’s vocalization: larger animals give lower-pitched calls. Davies and Halliday (1978) showed that playback of low-pitched calls was sufficient to discourage smaller males from entering into battle with an apparently larger male. A second way to require a more reliable signal has generally been discussed under the rubric of the ‘handicap’ principle. This principle was first proposed by Zahavi (1975) , modified and formalized by Grafen (1990) , given the intuitively pleasing graphical formulation by Johnstone (1997) shown in Figure 2 , and is still being subjected to further modification and clarification (e.g., Penn and Számadó, 2018 ). But the basic principle is straight-forward, and can be verbalized as follows: signals whose degree of expression is dependent on the health, general condition or vigor of the signaler are inherently honest expressions of that individual’s quality. For a high-quality signaler, a ‘bigger’ signal is a smaller handicap (less costly, or more affordable) than it is for a low-quality signaler, thus ‘big’ signals are reliable signals of signaler quality. One of the clearest demonstrations of honesty in an epigamic signal was carried out by Petrie and her colleagues on that poster animal for epigamic signaling, the peacock. Petrie and colleagues demonstrated that in their peacock population, females preferred a mate with more eyespots in his feather train (whether the difference was natural, or produced by experimental manipulation), and that females mated with males with more eyespots had more young surviving to a year of age than females mated to males with fewer eyespots ( Petrie et al., 1991 ; Petrie, 1994 ; Petrie and Halliday, 1994 ). Although the generality of these results has been questioned by studies on other populations ( Takahashi et al., 2008 ; Dakin and Montgomerie, 2011 ), the example provides a clear illustration of the predictions generated by the handicap principle, and how they should be tested.
Figure 2. Johnstone’s graphical model of the Handicap principle. The basic assumption is that it costs a high-quality signaler less to signal at its optimum level than it costs a low-quality signaler to signal at that level. The optimum or equilibrium level (where the difference between the costs and benefits of signaling are greatest) for the low quality signaler is lower (opt low) than that for the high-quality signaler (opt high). Thus the signaling level is a reliable indicator of signaler quality.
The handicap principle should maintain some degree of honesty in any signaling system where signaler and receiver have non-identical interests, such as virtually all mating and agonistic contexts. A low-quality individual can only ‘lie’ by diverting energy into signal development and expression that it needs for maintenance, and so as Searcy and Nowicki (2005) succinctly put it, lying becomes more costly than signaling honestly. Searcy and Nowicki suggest that ‘reliable’ is a better word here than ‘honest,’ for several reasons. First, as with reliability testing in science and elsewhere, we understand that although perfect reliability is unattainable, partial reliability may be good enough. In contrast, ‘honesty’ is generally taken to mean absolute honesty. Second, reliability of a signal is empirically measurable. Thus instead of debating whether an animal signal is informative or not, we can measure if it predicts something important about the present state of affairs or future events. Thus for example, in an agonistic situation a ‘threat signal’ should predict subsequent escalation, and the strongest ‘threat’ signal should predict attack ( Searcy and Beecher, 2009 ).
Summing Up: Two Perspectives
Historically, the Information Transmission and Manipulation views of animal communication systems have been presented as in opposition. I suggest that in fact they are simply different perspectives on the same process. Once we give the receiver agency, and accept that manipulation is a two-way or reciprocal process in animal communication, we see that the two views have more in common than was at first thought. This rapprochement is nicely captured in the evolution of Dawkins and Krebs’s papers on the topic. In their original paper, Dawkins and Krebs (1978) focused on signalers and argued that “natural selection favors [signalers] who successfully manipulate [receivers] whether or not this is to the advantage of the manipulated individuals.” However, 6 years later in a follow-up paper ( Krebs and Dawkins, 1984 ) they expanded their view to include receiver interests, noting that receivers would be favored to resist manipulation and to attempt to “read the minds” of signalers. Finally, Krebs (1991) , discussing Zahavi’s handicap principle, concluded that the manipulation and honest signaling views are probably not incompatible: “ Dawkins and Krebs (1978) discussed a coevolutionary process without specifying an end point, whereas Zahavi was concerned mainly with the end-point itself, so it is possible to imagine an evolutionary arms race of manipulation and sales resistance which end up with honest signaling” ( Krebs, 1991 , p. 67).
Figure 3 is a schematic representation of what I will call the Reciprocal Manipulation view. It shows communication taking place on a battleground in which signaler and receiver are each selected to manipulate the other, the battle being settled in the long run with the compromise of mostly-honest (reliable) signals. The “management-assessment” theory of Owings and Morton (1997 , 1998) is quite similar to the Reciprocal Manipulation view. Their theory captures the dynamics of signalers attempting to manage receivers and receivers assessing signalers. In their words “the process of assessment is more active than has been generally recognized, and is responsible for the ‘informational’ couplings between individuals” (1997, p. 359). However, receivers do more than just assess signalers, they manipulate them as well, requiring them to signal in the first place, and requiring a relatively honest signal as a prerequisite for responding to the signal. The Reliable Signaling view of Searcy and Nowicki (2005) is essentially identical to the Reciprocal Manipulation view, with the superficial difference that the former focuses on the information transmission aspect (reliable signaling) while the latter focuses on the manipulation aspect (the conflicting motivations of signaler and receiver).
Figure 3. Schematic suggesting the opposing pressures favoring signaler over receiver or vice-versa. Where interests of signaler and receiver are coincident or nearly so (light gray to white) reliable communication will occur. At the extremes of the space (darker), where interests of one or the other of the two parties predominates, signaling will be disfavored. In the intermediate (gray) region, one party may benefit more than the other, but signaling may still be ‘reliable enough.’
The Reciprocal Manipulation and Information Transmission views each seem most helpful in different circumstances ( Table 3 ). Where the interests and thus motivations of the two parties differ, the Reciprocal Manipulation highlights the clash. In contrast, where the interests and motivations of the two parties are more in line, the Information Transmission viewpoint focuses on the essence of the interaction. Indeed, where the overlap of sender and receiver interests is considerable, as for example between related individuals, or mates caring for offspring, or individuals in a social group where individuals are strongly interdependent, reliable, mutually beneficial signals will be favored. But even where the interests of sender and receiver are partially opposed, selection acting on both parties will move them to the region where both parties benefit on average, and signals will still be reliable, if less so. This game theory dynamic has been clearly laid out elsewhere ( Maynard Smith and Harper, 2003 ; Godfrey-Smith, 2013 ).

Table 3. Differences between reciprocal manipulation and information transmission perspectives.
I believe that the clash between these views of animal communication has ultimately led us to a clearer view of animal communication systems than the original human-oriented information transmission view. Most animal communication systems are somewhere on the continuum from pure manipulation to pure communication, from arms race (where sender and receiver have different interests, each selected to behave so as to benefit themselves) to pure information transmission (where sender and receiver have identical interests, and where signals benefit or cost both parties in the same way or to the same degree). A fuller development of these ideas can be found in Beecher (2020) .
In conclusion, I have argued that we should expect that natural communication systems will generally be reliable, even if not perfectly honest, with signaler and receiver both benefiting on average. However, returning to the main theme of this paper, there is no reason to expect such systems to blossom into simple languages unless signalers and receivers have identical or near-identical interests, and if the ecological selective context requires strong cooperation. There are cognitive prerequisites as well – otherwise one might predict that honeybees should have a simple language – but the brake on the evolution to language-like signaling systems in species with the requisite cognitive capacity is provided by the generally divergent interests of signaler and receiver. Otherwise, bonobos, dolphins and some other vertebrates who seem to have the necessary cognitive prerequisites would have a more language-like natural communication systems than they do.
Why Are There No Natural Language Systems in Animals?
Research on teaching animals simple human language indicate that at least some animals appear to have the cognitive capacity to decode language or language-like expressions. Herman’s dolphins could comprehend a sign language command such as “take the ball to the hoop” and to distinguish it from a similar but syntactically different command like “take the hoop to the ball” ( Herman, 2010 ). Kanzi the bonobo could respond correctly to novel verbal commands such as “Can you put the pine needles in the refrigerator?” ( Savage-Rumbaugh et al., 1993 ). Pepperberg (1981 , 1987) and Pailian et al. (2020) have shown that African gray parrots can follow verbal directions to solve difficult problems, including some that challenge humans. Yet despite having the apparent capacities, at least to some extent, no non-human animal uses even a rudimentary language in its day-to-day existence. This includes groups like the songbirds that seem to have a crucial design feature, the learning and cultural transmission of a complex set of vocal signals. Some animals appear to be smart enough, or capable enough to handle a simple language, but we have yet to discover an animal communication system – in nature – that rises to this level. Thus it appears that some missing element other than cognitive or motor limitations has blocked language evolution in non-human animals. Although it is possible that yet some other cognitive limitation has not been clearly identified ( Hauser et al., 2002 ; Pinker and Jackendoff, 2005 ), I focus in this final section on a candidate for the missing element that is not purely a cognitive mechanism.
A clue as to the missing element comes from the honeybee ‘dance language.’ Despite a relatively simple nervous system, honeybees are able not only to transmit precise information about events in the external world, but also to use this system in two very different contexts (when talking about the location of desirable food sources or about the location of suitable hive sites). The key ingredient for the evolution of this system, I would argue, is zero conflict of interest between sender and receiver. Both scout and recruit are sister sterile workers and they are both working to feed sisters and brothers slated to be future reproductives. Humans also evolved in a social system featuring extraordinary levels of cooperation, but significantly this cooperation was not restricted to close relatives, as it is in the honeybees and other social insects, ruling out kin selection as a sufficient explanation (but see Fitch, 2004 ).
I will reframe the question from “why not them?” to the question of “why us” (phrasing suggested by Hrdy, 2009 )? How did the human animal become the one species to evolve language? As I argued in the previous section, the field has arrived at a consensus concerning the factors that shape animal communication systems: the pressure for sender and receiver each to shape the interaction to its benefit inevitably both stimulates and constrains the evolution of the communication system. Very unusual circumstances are required for a true language system to evolve. Three essential conditions have to be met. First, the species must have the underlying cognitive capacity. Honeybees may lack this, but some other animals may have it. Second, and this is the clue provided by honeybees, sender and receiver must have identical or near identical interests. Third, individuals must have a compelling need to transmit information across multiple contexts. These are precisely the conditions that existed in pre-human and early human hunter-gatherer societies, the context in which humans and our hominid precursors spent some 95% of our evolutionary history. The description of the prototypical hunter-gatherer society that follows is based on information from a number of sources (including Boehm, 1999 ; Bowles, 2006 ; Hrdy, 2009 ; Hill K. et al., 2011 ; Knight and Power, 2011 ; Lee, 2018 ).
Our hunter-gather ancestors lived in small social groups where individuals were strongly interdependent, and cooperation across multiple contexts was essential for survival. Most highly cooperative animal societies such as the eusocial insects are typically just very large families, but the human hunter-gatherer societies we know – and which we assume to be typical of the ancestral type – consisted of members of several kin lines. Thus human societies then – and now as well – required extensive cooperation among unrelated individuals. Humans are the supreme cooperators in the animal world, but because this cooperation is not supported by high kin relatedness, it has to withstand a strong undercurrent of individual competition. We sometimes lose sight of the human affinity for within-group cooperation because of its paradoxical coexistence with intense between-group competition and tribalism. Irreconcilable conflicts within ancestral hunter-gatherer groups surely occurred, but were often resolved by individuals leaving one group for another (hunter-gatherer societies being classic examples of fission-fusion societies).
Students of human evolution, while differing as to what were the key selective contexts, or the key adaptations, all agree that human evolution has been characterized by remarkable levels of within-group cooperation among unrelated individuals, on a scale not seen in any non-human animal. Several contexts stand out as crucial for the high level of cooperation found in hunter-gather societies. They begin, of course, with hunting and gathering. Effective group hunting (usually done by men) requires sharing of information about distant prey and discussion of strategies for capturing prey. In essentially the same way, gathering of plants and fruits (usually done by women) requires the ability to track the growing schedules and locations of many plants and fruits in the area and the ability to discuss and coordinate foraging activities efficiently. Furthermore, hunter-gatherer societies periodically have to pick up and move to a new, more abundant locale. These moves require discussion and group consensus, with input from all parties, especially older, more experienced men and women.
A second, equally important axis of cooperation is child-raising. Humans are unique among primates in the time and cost required to raise an offspring. Humans solved this problem by involving the whole group in the process. Hrdy (2009) has pointed out that this pattern of cooperative breeding sets humans apart from the exclusive mother-centered parenting of our closest relatives, the great apes. In these early human societies, many individuals played a role in the cooperative care. For starters, the whole group participated in that food brought back to the camp was typically shared among all individuals, without reference to their role in procuring the food. Then unlike most mammals, the father participated in child care alongside the mother. Other relatives were routinely involved in direct child care, especially older siblings and grandparents, often aunts and uncles too, and sometimes non-relatives as well.
Finally, within-group cooperation is essential for success in between-group competition, warfare in particular. This aspect of our hunter-gather heritage is strongly debated in anthropology. Using the terms of Lee (2018) , the Peaceful school views significant inter-group competition as not beginning until the Agricultural era, when property gave humans something to fight over. The Bellicose school (e.g., Kelly, 2000 ; Gat, 2015 ) believes inter-group competition dates further back in our evolutionary past. But whenever it started, warfare would certainly promote adaptations for within-group cooperation.
In recent years various investigators have proposed key adaptations that may have allowed human societies to achieve this high level of cooperation in the absence of the glue of a very high level of kinship. Although there is not complete agreement as to which of these adaptations were most crucial, taken together they coalesce into a suite of psychological adaptations that promote prosocial within-group interactions within a context of near-complete interdependence. Indeed, Tomasello et al. (2012) have dubbed this the Interdependence hypothesis. The specific adaptations include: shared intentionality ( Tomasello et al., 2005 ), egalitarianism ( Boehm, 1999 ), social learning and communication ( Herrmann et al., 2007 ), intersubjectivity and empathy ( Hrdy, 2009 ), moral intuitions ( Haidt, 2012 ), adaptations for teaching and receiving teaching, and thus cultural transmission ( Sterelny, 2012 ; Henrich, 2016 ; Whiten, 2017 ), proactive aggression ( Wrangham, 2018 ) and self-domestication ( Wrangham, 2019 ). These adaptations of our social mind appear to be what set us apart from the other great apes, who it has been argued are otherwise just as cognitively advanced ( Herrmann et al., 2007 ). This suite of adaptations has enabled us to live in complex, cooperative societies. Despite our equally extraordinary proactive (deliberate and planned) aggressive tendencies, directed typically at out-groups, as in wars, pogroms, crusades and the like ( Wrangham, 2018 ), no other social animal has achieved the level of within-group docility and cooperation without high within-group relatedness that is found in the human species. I note that Knight (2018) has an advanced an argument similar to the one I have presented here.
Language unquestionably represents the pinnacle of evolved animal communication systems, and as noted at the beginning of this section, attempts to teach language to animals have not significantly changed this view. Language is often given pride of place in human evolution. In this view the other adaptations mentioned above came only after some form of language was in place. I favor the view of Hrdy (2009) , that this may well reverse cause and effect. The evolution of language may have only become possible when the posited unique suite of prosocial, communicative and mind-reading adaptations were in place. The crucial importance of communication in the strongly interdependent social system of early humans would have created this prosocial suite of adaptations, and would have laid the groundwork for evolving a true language.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many thanks to editor IP, three reviewers, John Byers, Doug Mock, Trish Schwagmeyer, and Bill Searcy for their very thoughtful reviews of the manuscript.
Akçay, Ç, Anderson, R. C., Nowicki, S., Beecher, M. D., and Searcy, W. A. (2015a). Quiet threats: soft song as an aggressive signal in birds. Anim. Behav. 105, 267–274. doi: 10.1016/j.anbehav.2015.03.009
CrossRef Full Text | Google Scholar
Akçay, Ç, Campbell, S. E., and Beecher, M. D. (2015b). The fitness consequences of honesty: under-signalers have a survival advantage in song sparrows. Evolution 69, 3186–3193. doi: 10.1111/evo.12818
PubMed Abstract | CrossRef Full Text | Google Scholar
Akçay, Ç, Reed, V. A., Campbell, S. E., Templeton, C. N., and Beecher, M. D. (2010). Indirect reciprocity: song sparrows distrust aggressive neighbors based on eavesdropping. Anim. Behav. 80, 1041–1047. doi: 10.1016/j.anbehav.2010.09.009
Akçay, Ç, Tom, M. E., Campbell, S. E., and Beecher, M. D. (2013). Song type matching is an honest early threat signal in a hierarchical animal communication system. Proc. R. Soc. Lond. B Biol. Sci. 280:20122517. doi: 10.1098/rspb.2012.2517
Akçay, Ç, Tom, M. E., Holmes, D., Campbell, S. E., and Beecher, M. D. (2011). Sing softly and carry a big stick: signals of aggressive intent in song sparrows. Anim. Behav. 82, 377–382. doi: 10.1016/j.anbehav.2011.05.016
Akçay, Ç, Wood, W. E., Searcy, W. A., Templeton, C. N., Campbell, S. E., and Beecher, M. D. (2009). Good neighbour, bad neighbour: song sparrows retaliate against aggressive rivals. Anim. Behav. 78, 97–102. doi: 10.1016/j.anbehav.2009.03.023
Anderson, R. C., Searcy, W. A., Peters, S., Hughes, M., DuBois, A. L., and Nowicki, S. (2017). Song learning and cognitive ability are not consistently related in a songbird. Anim. Cogn. 20, 309–320. doi: 10.1007/s10071-016-1053-7
Andersson, M. (1982). Female choice selects for extreme tail length in a widowbird. Nature 299, 818–820. doi: 10.1038/299818a0
Baker, M. C., and Boylan, J. T. (1995). A catalog of song syllables of indigo and lazuli buntings. Condor 97, 1028–1040. doi: 10.2307/1369541
Barker, A. J., Veviurko, G., Bennett, N. C., Hart, D. W., Mograby, L., and Lewin, G. R. (2021). Cultural transmission of vocal dialect in the naked mole-rat. Science 371, 503–507. doi: 10.1126/science.abc6588
Barske, J., Schlinger, B. A., Wikelski, M., and Fusani, L. (2011). Female choice for male motor skills. Proc. R. Soc. Lond., B Biol. Sci. 278, 3523–3528. doi: 10.1098/rspb.2011.0382
Beecher, M. D. (2017). Birdsong learning as a social process. Anim. Behav. 124, 233–246. doi: 10.1016/j.anbehav.2016.09.001
Beecher, M. D. (2020). “Animal communication,” in Oxford Encyclopedia of the History of Psychology , ed. W. Pickren (Oxford, UK: Oxford University Press).
Google Scholar
Beecher, M. D., and Akçay, Ç (2014). “Friends and enemies: how social dynamics shape communication and song learning in song sparrows,” in Animal Behavior , ed. K. Yakusawa (Santa Barbara, CA: Praeger), 33–61.
Beecher, M. D., and Akçay, Ç, and Campbell, S. E. (2020). Birdsong learning is mutually beneficial for tutee and tutor in song sparrows. Anim. Behav. 166, 281–288. doi: 10.1016/j.anbehav.2020.05.015
Beecher, M. D., and Brenowitz, E. A. (2005). Functional aspects of song learning in songbirds. Trends Ecol. Evol. 20, 143–149. doi: 10.1016/j.tree.2005.01.004
Beecher, M. D., and Campbell, S. E. (2005). The role of unshared songs in singing interactions between neighbouring song sparrows. Anim. Behav. 70, 1297–1304. doi: 10.1016/j.anbehav.2005.03.008
Beecher, M. D., Campbell, S. E., and Burt, J. M. (1994). Song perception in the song sparrow: birds classify by song type but not by singer. Anim. Behav. 47, 1343–1351. doi: 10.1006/anbe.1994.1182
Beecher, M. D., Campbell, S. E., Burt, J. M., Hill, C. E., and Nordby, J. C. (2000). Song type matching between neighboring song sparrows. Anim. Behav. 59, 21–27. doi: 10.1006/anbe.1999.1276
Beecher, M. D., Nordby, J. C., Campbell, S. E., Burt, J. M., Hill, C. E., and O’Loghlen, A. L. (1997). “What is the function of song learning in songbirds?,” in Communication. Perspectives in Ethology , Vol. 12, eds D. H. Owings, M. D. Beecher, and N. S. Thompson (New York, NY: Plenum Press), 77–97. doi: 10.1007/978-1-4899-1745-4_4
Beecher, M. D., Stoddard, P. K., Campbell, S. E., and Horning, C. L. (1996). Repertoire matching between neighbouring song sparrows. Anim. Behav. 51, 917–923. doi: 10.1006/anbe.1996.0095
Boehm, C. (1999). Hierarchy in the Forest: The Evolution of Egalitarian Behavior. Cambridge, MA: Harvard University Press.
Bowles, S. (2006). Group competition, reproductive leveling, and the evolution of human altruism. Science 314, 1569–1572. doi: 10.1126/science.1134829
Bradbury, J. W., and Vehrencamp, S. L. (1998). The Principles of Animal Communication. Sunderland, MA: Sinauer Associates.
Burt, J. M., Bard, S. C., Campbell, S. E., and Beecher, M. D. (2002). Alternative forms of song matching in song sparrows. Anim. Behav. 63, 1143–1151. doi: 10.1006/anbe.2002.3011
Burt, J. M., Campbell, S. E., and Beecher, M. D. (2001). Song type matching as threat: a test using interactive playback. Anim. Behav. 62, 1163–1170. doi: 10.1006/anbe.2001.1847
Byers, B. E. (1996). Messages encoded in the songs of chestnut-sided warblers. Anim. Behav. 52, 691–705. doi: 10.1006/anbe.1996.0214
Byers, J., Hebets, E., and Podos, J. (2010). Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778. doi: 10.1016/j.anbehav.2010.01.009
Cade, W. (1975). Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. doi: 10.1126/science.190.4221.1312
Carazo, P., and Font, E. (2010). Putting information back into biological communication. J. Evol. Biol. 23, 661–669. doi: 10.1111/j.1420-9101.2010.01944.x
Caro, T. M., and Hauser, M. D. (1992). Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174.
Carouso-Peck, S., and Goldstein, M. H. (2019). Female social feedback reveals non-imitative mechanisms of vocal learning in zebra finches. Curr. Biol. 29, 631–636.e633. doi: 10.1016/j.cub.2018.12.026
Catchpole, C. K. (1987). Bird song, sexual selection and female choice. Trends Ecol. Evol. 2, 94–97. doi: 10.1016/0169-5347(87)90165-0
Chomsky, N. (1986). Knowledge of Language: Its Nature, Origin, and Use. New York, NY: Praeger.
Clutton-Brock, T. H., and Albon, S. D. (1979). The roaring of red deer and the evolution of honest advertisement. Behaviour 69, 145–170. doi: 10.1163/156853979X00449
Coleman, S. W., Patricelli, G. L., Coyle, B., Siani, J., and Borgia, G. (2007). Female preferences drive the evolution of mimetic accuracy in male sexual displays. Biol. Lett. 3, 463–466. doi: 10.1098/rsbl.2007.0234
Collins, S. A. (2004). “Vocal fighting and flirting: the functions of birdsong,” in Nature’s Music: The Science of Birdsong , ed. P. M. H. Slabbekoorn (New York, NY: Academic Press), 39–79. doi: 10.1016/b978-012473070-0/50005-0
Dakin, R., and Montgomerie, R. (2011). Peahens prefer peacocks displaying more eyespots, but rarely. Anim. Behav. 82, 21–28. doi: 10.1016/j.anbehav.2011.03.016
Davies, N. B., and Halliday, T. R. (1978). Deep croaks and fighting assessment in toads Bufo bufo. Nature 274, 683–685. doi: 10.1038/274683a0
Dawkins, R., and Krebs, J. R. (1978). “Animal Signals: information or manipulation?,” in Behavioural Ecology: An Evolutionary Approach , eds J. R. Krebs and N. B. Davies (Oxford: Blackwell), 282–309.
Doupe, A. J., and Kuhl, P. K. (1999). Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631. doi: 10.1146/annurev.neuro.22.1.567
Elias, D. O., Maddison, W. P., Peckmezian, C., Girard, M. B., and Mason, A. C. (2012). Orchestrating the score: complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Salticidae). Biol. J. Linn. Soc. 105, 522–547. doi: 10.1111/j.1095-8312.2011.01817.x
Fisher, J. B. (1954). “Evolution and bird sociality,” in Evolution as Process , eds J. Huxley, A. C. Hardy, and E. B. Ford (London: Allen & Unwin), 71–83.
Fitch, W. T. (2004). “Evolving honest communication systems: kin delection and ‘mother tongues’,” in Evolution of Communication Systems: A Comparative Approach , eds D. K. Oller and U. Griebel (Cambridge, MA: MIT Press), 275–296.
Fitch, W. T., and Jarvis, E. (2013). “Birdsong and other animal models for human speech, song, and vocal learning,” in Language, Music, and the Brain: A Mysterious Relationship , ed. M. A. Arbib (Cambridge, MA: MIT Press).
Garland, E. C., and McGregor, P. K. (2020). Cultural transmission, evolution, and revolution in vocal displays: insights from bird and whale song. Front. Psychol. 11:544929. doi: 10.3389/fpsyg.2020.544929
Gat, A. (2015). Proving communal warfare among hunter-gatherers: the quasi-rousseauan error. Evol. Anthropol. 24, 111–126. doi: 10.1002/evan.21446
Gentner, T. Q., Fenn, K. M., Margoliash, D., and Nusbaum, H. C. (2006). Recursive syntactic pattern learning by songbirds. Nature 440, 1204–1207. doi: 10.1038/nature04675
Godfrey-Smith, P. (2013). “Information and influence in sender-receiver models, with applications to animal behavior,” in Animal Communication Theory: Information and Influence , ed. U. E. Stegmann (Cambridge: Cambridge University Press).
Gould, J. L. (1975). Honey bee recruitment: the dance-language controversy. Science 189, 685–693. doi: 10.1126/science.1154023
Grafen, A. (1990). Biological signals as handicaps. J. Theor. Biol. 144, 517–546. doi: 10.1016/s0022-5193(05)80088-8
Green, S. (1975). “Variation of vocal pattern with social situation in the Japanese macaque ( Macaca fuscata ): a field study,” in Primate Behavior , ed. L. A. Rosenblum (New York, NY: Academic Press), 1–102. doi: 10.1016/b978-0-12-534004-5.50006-3
Green, S., and Marler, P. (1979). “The analysis of animal communication,” in Social Behavior and Communication , eds P. Marler and G. Vandenbergh (New York, NY: Plenum), 73–158. doi: 10.1007/978-1-4615-9116-0_3
Haidt, J. (2012). The Righteous Mind: Why Good People are Divided by Politics and Religion. New York, NY: Pantheon.
Hauser, M. D. (2000). A primate dictionary? Decoding the function and meaning of another species’ vocalizations. Cogn. Sci. 24, 445–475. doi: 10.1207/s15516709cog2403_5
Hauser, M. D., Chomsky, N., and Fitch, W. T. (2002). The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579. doi: 10.1126/science.298.5598.1569
Hebets, E. A., and Uetz, G. W. (1999). Female responses to isolated signals from multimodal male courtship displays in the wolf spider genus Schizocosa (Araneae: Lycosidae). Anim. Behav. 57, 865–872. doi: 10.1006/anbe.1998.1048
Henrich, J. (2016). The Secret of our Success: How Culture is Driving Human Evolution, Domesticating our Species, and Making us Smarter. Princeton, NJ: Princeton University Press.
Herman, L. M. (2010). What laboratory research has told us about dolphin cognition. Int. J. Comp. Psychol. 23, 310–330.
Herrmann, E., Call, J., Hernandez-Lloreda, M. V., Hare, B., and Tomasello, M. (2007). Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. doi: 10.1126/science.1146282
Hewes, G. W. (1973). Primate communication and the gestural origin of language. Curr. Anthrop. 14, 5–24.
Hill, C. E., Akçay, Ç, Campbell, S. E., and Beecher, M. D. (2011). Extrapair paternity, song and genetic quality in song sparrows. Behav. Ecol. 22, 73–81. doi: 10.1093/beheco/arq171
Hill, K., Walker, R. S., Božičević, M., Eder, J., Headland, T., Hewlett, B., et al. (2011). Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–1289. doi: 10.1126/science.1199071
Hockett, C. F. (1960). The origin of speech. Sci. Am. 203, 88–111.
Horning, C. L., Beecher, M. D., Stoddard, P. K., and Campbell, S. E. (1993). Song perception in the song sparrow: importance of different parts of the song in song type classification. Ethology 94, 46–58. doi: 10.1111/j.1439-0310.1993.tb00546.x
Hrdy, S. B. (2009). Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Harvard University Press.
Janik, V. M. (2014). Cetacean vocal learning and communication. Curr. Opin. Neurobiol. 28, 60–65. doi: 10.1016/j.conb.2014.06.010
Johnstone, R. A. (1997). “The evolution of animal signals,” in Behavioural Ecology: An Evolutionary Approach , eds J. Krebs and R. Davies (Oxford: Blackwell), 155–178.
Keagy, J., Savard, J.-F., and Borgia, G. (2009). Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817. doi: 10.1016/j.anbehav.2009.07.011
Kelly, R. (2000). Warless Societies and the Origin of War. Ann Arbor: Uinversity of Michigan Press.
Knight, C. (2018). “Pressure for trust-based efficiency shaped the evolution of language,” in The Evolution of Language: Proceedings of the 12th International Conference (EVOLANGXII) , eds C. Christine, F. Molly, L. Hannah, M. Luke, R. Andrea, and V. Tessa.
Knight, C., and Power, C. (2011). “Social conditions for the evolutionary emergence of language,” in Handbook of Language Evolution , ed. K. G. M. Tallerman (Oxford: Oxford University Press), 346–349.
Knornschild, M. (2014). Vocal production learning in bats. Curr. Opin. Neurobiol. 28, 80–85. doi: 10.1016/j.conb.2014.06.014
Krebs, J. R. (1991). “Animal communication: ideas derived from Tinbergen’s activities,” in The Tinbergen Legacy , eds M. D. Dawkins, T. R. Halliday, and R. Dawkins (London: Chapman & Hall), 60–74. doi: 10.1007/978-0-585-35156-8_5
Krebs, J. R., and Davies, R. (1978). Behavioural Ecology: An Evolutionary Approach. Oxford: Blackwell.
Krebs, J. R., and Dawkins, R. (1984). “Animal signals: mind-reading and manipulation,” in Behavioural Ecology: An Evolutionary Approach , 2nd Edn, eds J. R. Krebs and R. Davies (Oxford: Blackwell), 380–402.
Kroodsma, D., Hamilton, D., Sánchez, J. E., Byers, B. E., Fandiño-Mariño, H., Stemple, D. W., et al. (2013). Behavioral evidence for song learning in the suboscine bellbirds (Procnias spp.; Cotingidae). Wilson J. Ornithol. 125, 1–14. doi: 10.1676/12-033.1
Kroodsma, D. E. (1977). Correlates of song organization among North American wrens. Am. Nat. 111, 995–1008. doi: 10.1086/283228
Kroodsma, D. E. (1988). “Contrasting styles of song development and their consequences among passerine birds,” in Evolution and Learning , eds R. C. Bolles and M. D. Beecher (Hillsdale, NJ: Lawrence Erlbaum Associates), 157–184.
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. doi: 10.1016/s0169-5347(97)01241-x
Lee, R. L. (2018). Hunter-gatherers and human evolution: new light on old debates. Annu. Rev. Anthrop. 47, 513–531. doi: 10.1146/annurev-anthro-102116-041448
Levin, R. N. (1996). Song behaviour and reproductive strategies in a duetting wren, Thryothorus nigricapillus : II. Playback experiments. Anim. Behav. 52, 1107–1117. doi: 10.1006/anbe.1996.0258
Logue, D. M., and Gammon, D. E. (2004). Duet songs and sex roles during territory defence in a tropical bird, the black-bellied wren. Anim. Behav. 68, 721–731. doi: 10.1016/j.anbehav.2003.10.026
Lorenz, K. Z. (1966). The psychobiological approach: methods and results–Evolution of ritualization in the biological and cultural spheres. Philos. Trans. R. Soc. Lond. B Biol. Sci 251, 273–284. doi: 10.1098/rstb.1966.0011
MacDougall-Shackleton, S. A. (1997). Sexual selection and the evolution of song repertoires. Curr. Ornithol. 14, 81–124. doi: 10.1007/978-1-4757-9915-6_3
Marler, P. (1961). The logical analysis of animal communication. J. Theor. Biol. 1, 295–317. doi: 10.1016/0022-5193(61)90032-7
Marler, P. (1968). “Visual systems,” in Animal Communication , ed. T. A. Sebeok (Bloomington, IN: Indiana University Press), 103–126.
Marler, P. (1970). Birdsong and speech development: could there be parallels? Am. Sci. 58, 669–673.
Marler, P., and Pickert, R. (1984). Species-universal microstructure in the learned song of the swamp sparrow ( Melospiza georgiana ). Anim. Behav. 32, 673–689. doi: 10.1016/s0003-3472(84)80143-8
Marler, P., and Tamura, M. (1964). Culturally transmitted patterns of vocal behavior in sparrows. Science 146, 1483–1486. doi: 10.1126/science.146.3650.1483
Maynard Smith, J., and Harper, D. (2003). Animal Signals. Oxford: Oxford University Press.
McDonald, M. V. (1989). Function of song in Scott’s seaside sparrow. Anim. Behav. 38, 468–485. doi: 10.1016/s0003-3472(89)80040-5
Medvin, M. B., Stoddard, P. K., and Beecher, M. D. (1993). Signals for parent-offspring recognition: a comparative analsysis of the begging calls of cliff swallows and barn swallows. Anim. Behav. 45, 841–850. doi: 10.1006/anbe.1993.1105
Nowicki, S., and Searcy, W. A. (2014). The evolution of vocal learning. Curr. Opin. Neurobiol. 28, 48–53. doi: 10.1016/j.conb.2014.06.007
Otte, D. (1974). Effects and functions in the evolution of signaling systems. Annu. Rev. Ecol. Syst. 5, 385–417. doi: 10.1146/annurev.es.05.110174.002125
Otter, K. A., Mckenna, A., LaZerte, S. E., and Ramsay, S. M. (2020). Continent-wide shifts in song dialects of white-throated sparrows. Curr. Biol. 30, 1–5. doi: 10.1016/j.cub.2020.05.084
Owings, D. H., and Morton, E. S. (1997). “The role of information in communication: an assessment/management approach,” in Communication , eds D. H. Owings, M. D. Beecher, and N. S. Thompson (New York, NY: Plenum Press), 359–390. doi: 10.1007/978-1-4899-1745-4_12
Owings, D. H., and Morton, E. S. (1998). Animal Vocal Communication: A New Approach. Cambridge: Cambridge University Press.
Owren, M. J., Rendall, D., and Ryan, M. J. (2010). Redefining animal signaling: influence versus information in communication. Biol. Philos. 25, 755–780. doi: 10.1007/s10539-010-9224-4 Corpus ID: 13443399,
Pailian, H., Carey, S. E., Halberda, J., and Pepperberg, I. M. (2020). Age and species comparisons of visual mental manipulation ability as evidence for its development and evolution. Sci. Rep. 10:7689. doi: 10.1038/s41598-020-64666-1
Penn, D. J., and Számadó, S. (2018). The handicap principle: how an erroneous hypothesis became a scientific principle. Biol. Rev. 95, 267–290. doi: 10.1111/brv.12563
Pepperberg, I. M. (1981). Functional vocalizations by an African grey parrot ( Psittacus erithacus ). Z. Tierpsychol. 55, 139–160. doi: 10.1111/j.1439-0310.1981.tb01265.x
Pepperberg, I. M. (1987). Acquisition of the same/different concept by an African grey parrot ( Psittacus erithacus ): learning with respect to categories of color, shape, and material. Anim. Learn. Behav. 15, 423–432. doi: 10.3758/bf03205051
Petrie, M. (1994). Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371, 598–599. doi: 10.1038/371598a0
Petrie, M., Halliday, T., and Sanders, C. (1991). Peahens prefer peacocks with elaborate trains. Anim. Behav. 41, 323–331. doi: 10.1016/s0003-3472(05)80484-1
Petrie, M., and Halliday, T. R. (1994). Experimental and natural changes in the peacock’s ( Pavo cristatus ) train can affect mating success. Behav. Ecol. Sociobiol. 35, 213–217. doi: 10.1007/bf00167962
Pinker, S., and Jackendoff, R. (2005). The faculty of language: what’s special about it? Cognition 95, 201–236. doi: 10.1016/j.cognition.2004.08.004
Price, T., Wadewitz, P., Cheney, D., Seyfarth, R., Hammerschmidt, K., and Fischer, J. (2015). Vervets revisited: a quantitative analysis of alarm call structure and context specificity. Sci. Rep. 5:13220. doi: 10.1038/srep13220
Reid, J. M., Arcese, P., Cassidy, A. L. E. V., Hiebert, S. M., Smith, J. N. M., Stoddard, P. K., et al. (2004). Song repertoire size predicts initial mating success in male song sparrows, Melospiza melodia . Anim. Behav. 68, 1055–1063. doi: 10.1016/j.anbehav.2004.07.003
Reid, J. M., Arcese, P., Cassidy, A. L. E. V., Hiebert, S. M., Smith, J. N. M., Stoddard, P. K., et al. (2005). Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia) . Am. Nat. 165, 299–310. doi: 10.2307/3473407
Rendall, D., Owren, M. J., and Ryan, M. J. (2009). What do animal signals mean? Anim. Behav. 78, 233–240. doi: 10.1016/j.anbehav.2009.06.007
Rice, J. O., and Thompson, W. L. (1968). Song development in the indigo bunting. Anim. Behav. 16, 462–469. doi: 10.1016/0003-3472(68)90041-9
Ryan, M. J., and Rand, A. S. (1990). The sensory basis of sexual selection for complex calls in the Túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 44, 305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x
Savage-Rumbaugh, E. S., Murphy, J., Sevcik, R. A., Brakke, K. E., Williams, S. L., and Rumbaugh, D. M. (1993). Language comprehension in ape and child. Monogr. Soc. Res. Child Dev. 58, 1–222.
Schürch, R., and Ratnieks, F. W. (2015). The spatial information content of the honey bee waggle dance. Front. Ecol. Evol. 3:22. doi: 10.3389/fevo.2015.00022
Scott-Phillips, T. C. (2008). Defining biological communication. J. Evol. Biol. 21, 387–395. doi: 10.1111/j.1420-9101.2007.01497.x
Searcy, W. A. (1984). Song repertoire size and female preferences in song sparrows. Behav. Ecol. Sociobiol. 14, 281–286. doi: 10.1007/bf00299499
Searcy, W. A., Akçay, Ç, Nowicki, S., and Beecher, M. D. (2014). Aggressive signaling in song sparrows and other songbirds. Adv. Study Behav. 46, 89–125. doi: 10.1016/b978-0-12-800286-5.00003-1
Searcy, W. A., and Beecher, M. D. (2009). Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292. doi: 10.1016/j.anbehav.2009.08.011
Searcy, W. A., and Nowicki, S. (2005). The Evolution of Animal Communication. Princeton, NJ: Princeton University Press.
Searcy, W. A., and Yasukawa, K. (1996). “Song and female choice,” in Ecology and Evolution of Acoustic Communication in Birds , eds D. E. M. Kroodsma and H. Edward (London: Cornell University Press), 454–473.
Seyfarth, R. M., Cheney, D. L., Bergman, T., Fischer, J., Zuberbühler, K., and Hammerschmidt, K. (2010). The central importance of information in studies of animal communication. Anim. Behav. 80, 3–8. doi: 10.1016/j.anbehav.2010.04.012
Seyfarth, R. M., Cheney, D. L., and Marler, P. (1990). Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science 210, 801–803. doi: 10.1126/science.7433999
Smith, W. J. (1997). “The behavior of communicating, after twenty years,” in Communication , eds D. H. Owings, M. D. Beecher, and N. S. Thompson (New York, NY: Plenum), 7–53. doi: 10.1007/978-1-4899-1745-4_2
Stein, A. C., and Uy, J. A. C. (2006). Plumage brightness predicts male mating success in the lekking golden-collared manakin, Manacus vitellinus . Behav. Ecol. 17, 41–47. doi: 10.1093/beheco/ari095
Sterelny, K. (2012). The Evolved Apprentice: How Evolution Made Humans Unique. Cambridge, MA: The MIT Press.
Stoddard, P. K., and Beecher, M. D. (1983). Parental recognition of offspring in the cliff swallow. Auk 100, 795–799. doi: 10.1093/auk/100.4.795
Stoeger, A., and Manger, P. (2014). Vocal learning in elephants: neural bases and adaptive context. Curr. Opin. Neurobiol. 28, 101–107. doi: 10.1016/j.conb.2014.07.001
Struhsaker, T. T. (1967). “auditory communication among vervet monkeys (Cercopithecus aethiops) ,” in Social Communication Among Primates , ed. S. A. Altmann (Chicago, IL: Chicago University Press), 281–324.
Studdert-Kennedy, M. (1998). “The particulate origins of language generativity: from syllable to gesture,” in Approaches to the Evolution of Language: Social and Cognitive Bases , eds J. Hurford, M. Studdert-Kennedy, and C. Knight (Cambridge: Cambridge University Press), 202–221.
Takahashi, M., Arita, H., Hiraiwa-Hasegawa, M., and Hasegawa, T. (2008). Peahens do not prefer peacocks with more elaborate trains. Anim. Behav. 75, 1209–1219. doi: 10.1016/j.anbehav.2007.10.004
Templeton, C. N., Akçay, Ç, Campbell, S. E., and Beecher, M. D. (2012). Soft song is a reliable signal of aggressive intent in song sparrows. Behav. Ecol. Sociobiol. 66, 1503–1509. doi: 10.1007/s00265-012-1405-5
Thompson, W. L. (1969). Song recognition by territorial male buntings (Passerin A). Anim. Behav. 17, 658–663. doi: 10.1016/S0003-3472(69)80008-4
Thompson, W. L. (1970). Song variation in a population of indigo buntings. Auk 87, 58–71. doi: 10.2307/4083658
Tinbergen, N. (1952). Derived activities: their causation, biological significance, origin and emancipation during evolution. Q. Rev. Biol. 27, 1–32. doi: 10.1086/398642
Tinbergen, N. (1964). “The evolution of signaling devices,” in Social Behavior and Evolution Among Vertebrates , ed. W. Etkin (Chicago, IL: University of Chicago Press), 206–230.
Tomasello, M., Carpenter, M., Call, J., Behne, T., and Moll, H. (2005). Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–691. doi: 10.1017/s0140525x05000129
Tomasello, M., Melis, A. P., Tennie, C., Wyman, E., and Herrmann, E. (2012). Two key steps in the evolution of human cooperation: the interdependence hypothesis. Curr. Anthropol. 53, 673–692. doi: 10.1086/668207
van Heijningen, C. A. A., de Visser, J., Zuidema, W., and ten Cate, C. (2009). Simple rules can explain discrimination of putative recursive syntactic structures by a songbird species. Proc. Natl. Acad. Sci. U.S.A. 106, 20538. doi: 10.1073/pnas.0908113106
Wacewicz, S., and Żywiczyński, P. (2015). Language evolution: why Hockett’s design features are a non-starter. Biosemiotics 8, 29–46. doi: 10.1007/s12304-014-9203-2
Walcott, C., Mager, J. N., and Piper, W. (2006). Changing territories, changing tunes: male loons, Gavia immer , change their vocalizations when they change territories. r Anim. Behav. 71, 673–683. doi: 10.1016/j.anbehav.2005.07.011
Whiten, A. (2017). Social learning and culture in child and chimpanzee. Annu. Rev. Psychol. 68, 129–154. doi: 10.1146/annurev-psych-010416-044108
Wiley, R. H. (2013). “Communication as a transfer of information: measurement, mechanism, and meaning,” in Animal Communication Theory: Information and Influence , ed. U. Stegmann (Cambridge: Cambridge University Press), 113–129. doi: 10.1017/cbo9781139003551.007
Williams, G. C. (1966). Adaptation and Natural Selection. Princeton, NJ: Princeton University Press.
Wilson, E. O. (1975). Sociobiology. Cambridge, MA: Harvard University Press.
Wrangham, R. W. (2018). Two types of aggression in human evolution. Proc. Natl. Acad. Sci. U.S.A. 115, 245–253. doi: 10.1073/pnas.1713611115
Wrangham, R. W. (2019). Hypotheses for the evolution of reduced reactive aggression in the context of human self-domestication. Front. Psychol. 10:1914. doi: 10.3389/fpsyg.2019.01914
Wright, T. F., and Dahlin, C. R. (2018). Vocal dialects in parrots: patterns and processes of cultural evolution. Emu 118, 50–66. doi: 10.1080/01584197.2017.1379356
Zahavi, A. (1975). Mate selection- a selection for handicap. J. Theor. Biol. 53, 205–214. doi: 10.1016/0022-5193(75)90111-3
Zuidema, W., and de Boer, B. (2009). The evolution of combinatorial phonology. J. Phon. 37, 125–144. doi: 10.1016/j.wocn.2008.10.003
Zuk, M., Rotenberry, J. T., and Tinghitella, R. M. (2006). Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524. doi: 10.1098/rsbl.2006.0539
Keywords : animal communication, language evolution, animal cognition, animal language studies, information
Citation: Beecher MD (2021) Why Are No Animal Communication Systems Simple Languages? Front. Psychol. 12:602635. doi: 10.3389/fpsyg.2021.602635
Received: 03 September 2020; Accepted: 18 February 2021; Published: 19 March 2021.
Reviewed by:
Copyright © 2021 Beecher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael D. Beecher, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Philos Trans R Soc Lond B Biol Sci
- v.375(1789); 2020 Jan 6
Animal cognition and the evolution of human language: why we cannot focus solely on communication
Associated data.
This article has no additional data.
Studies of animal communication are often assumed to provide the ‘royal road’ to understanding the evolution of human language. After all, language is the pre-eminent system of human communication: doesn't it make sense to search for its precursors in animal communication systems? From this viewpoint, if some characteristic feature of human language is lacking in systems of animal communication, it represents a crucial gap in evolution, and evidence for an evolutionary discontinuity. Here I argue that we should reverse this logic: because a defining feature of human language is its ability to flexibly represent and recombine concepts, precursors for many important components of language should be sought in animal cognition rather than animal communication. Animal communication systems typically only permit expression of a small subset of the concepts that can be represented and manipulated by that species. Thus, if a particular concept is not expressed in a species' communication system this is not evidence that it lacks that concept. I conclude that if we focus exclusively on communicative signals, we sell the comparative analysis of language evolution short. Therefore, animal cognition provides a crucial (and often neglected) source of evidence regarding the biology and evolution of human language.
This article is part of the theme issue ‘What can animal communication teach us about human language?’
1. Introduction
I have not, to my knowledge, spoken the word ‘octopus’ today or indeed in the past week, but no one would therefore conclude that I lack the concept OCTOPUS (here I follow the philosopher's convention, when necessary, of denoting conceptual representations in capital letters). Indeed, I have spent many hours observing these creatures and read books about them but, like most of my mental concepts, OCTOPUS goes unexpressed in my speech most of the time. This is not only true of concepts captured by single words (like ‘octopus’, ‘chartreuse’, ‘quasar’ or ‘exponent’) but for more complex cognitive constructs that I possess (like how to walk from the Jardin de Luxembourg to the Place Stravinsky in Paris, via Notre Dame) but have never spoken at all. Humans possess many concepts, within individual minds, that go unexpressed via their language output for long periods of time (and some may never be expressed verbally). However, my assumption in what follows is that pretty much any human concept could be expressed in language, with perhaps hours or days of effort, and with varying degrees of accuracy, difficulty and concision. This capability to express any concept goes far beyond what any other species can do.
In what follows, I will take the basic observation that most concepts go unexpressed as axiomatic and argue that the same is true regarding animal communication, only more so (using ‘animal’ as shorthand for ‘non-human animal’ hereafter). For at least in principle, I might, under some circumstances, exclaim ‘Octopus!’ (e.g. when seeing one unexpectedly) or tell you the way to the Place Stravinsky (if you asked me), providing evidence that I indeed possess these concepts. By contrast, it is the nature of all known animal communication systems that they allow their bearers to express only a small subset of the concepts they can remember, represent and manipulate productively (cf. [ 1 ]). For example, honeybees have excellent colour vision and can remember the colours of the flowers they visit, but the honeybee dance ‘language’ allows a forager to communicate only the spatial location of the flower and has no provision for expressing colour information. I will provide evidence for this below and review similar evidence for other species, including non-human primates. I conclude that animal communication systems appear to be intrinsically limited to a smallish set of fitness-relevant messages that relate to such factors as food, danger, aggression, appeasement or personal prowess. But a substantial literature in animal cognition reveals that they know much more than this, even if they have no way of saying it [ 2 ].
The core argument is that, just as a person's utterances reveal only a subset of what they know, animal communication signals express an intrinsically limited subset of that species' conceptual storehouse. The argument that most thoughts are not expressed is by no means new: it follows Jackendoff's (2002) model of linguistic semantics closely and is also consonant with Chomsky's model [ 3 , 4 ]. Both Hurford [ 2 ] and Bickerton [ 5 ] have explored its implications for language evolution at book length [ 2 , 5 ], as have I more briefly [ 1 ]. My aim here is simply to argue this crucial point sharply and concisely, for although these ideas should not be controversial, they are rejected by some prominent philosophers, and even when accepted, their implications are ignored in many recent discussions of language evolution (e.g. [ 6 , 7 ]).
The central implication of my thesis is that the field of animal cognition has a very important role to play in our understanding of human language evolution because the fact that animals have concepts (whether expressible via signalling or not) erases a potentially gaping evolutionary chasm that would exist if they did not. Apparent discontinuities between humans and animal cognition that ‘pose a severe challenge for evolutionary explanation’ ([ 6 ], p.3), may in fact be based on discontinuities between language and other species' communication systems. This elision between two different things—cognition and communication—is at best misleading and often pernicious. The study of animal communication is indeed important for comparative analysis of language evolution, most obviously relevant for factors involved in externalization, such as vocal learning, speech perception and gestural communication. But to get the full comparative picture, we need to embrace animal cognition as a central and in some cases the central source of information relevant to the biology and evolution of language (and human cognition more generally).
2. Words ≠ concepts
Before discussing animals, it is important to first clarify some basic issues about the nature of human concepts, and to at least dip our toes into the philosophical quagmire surrounding the term ‘concept’ (for a concise introduction see [ 8 ]). My take on concepts in this essay will be essentially that of mainstream cognitive (neuro)science today, where a concept is simply ‘a nonlinguistic psychological representation of a class of entities in the world’ (Murphy [ 9 ], p. 335).
More specifically, my perspective is mentalistic and representationalist. I assume that concepts are mind-internal entities—‘representations’—that often, but not necessarily, correspond to some entities ‘out there’ in the world. It is physicalist: conceptual representations ultimately consist of neural activity in brains (they have no platonic existence, independent of minds). Finally, it is pluralistic, meaning that it allows for different types of concepts, some best captured by definitions, others by prototypes and still others as abilities to discriminate or act. Although much ink has been shed regarding the virtues and flaws of these different interpretations, both in cognitive science [ 9 ] and philosophy [ 10 – 13 ], precisely where one stands on these philosophical issues will have little relevance to my comparative argument here.
However, one central issue, illustrated in figure 1 , cannot be ignored, concerning a long-running philosophical debate between ‘mentalists’ (virtually all modern cognitive scientists) and ‘referentialist’ philosophers like Quine or Putnam [ 12 , 13 ]. Referentialists posit a direct referential linkage between utterances and their real-world referents. The referentialist doctrine was dominant in behaviourist psychology of language, which privileged observable behaviours (such as speaking words and pointing) over invisible mental constructs. But it has fallen by the wayside in modern cognitive science—at least regarding human language [ 3 , 14 ]. The alternative mentalist perspective (also termed the ‘internalist’ or ‘conceptualist’ perspective, [ 3 , 4 ]) holds that words do not refer directly to things in the world, but rather express our (mind-internal) concepts. To paraphrase Strawson ‘words don't refer, people refer’ [ 15 ]. The concepts we express linguistically may correspond to real entities in the world, but in many cases (e.g. ‘Sherlock Holmes’, ‘the unicorn in my dream’), they do not.

Mentalist model of concepts and meaning: contemporary cognitive scientists argue that words and sentences connection to their referents is indirect, and that reference requires the intervention of a (private) mental concept. Thus, an organism can have a concept (illustrated by the thought bubbles) independently of any words, sentences or other signals that express this concept. Referential links between real-world objects or events and non-verbal mental concepts (representations) can exist even if an organism has no means in its communication system to express those concepts.
The modern mentalist perspective in cognitive science sees acts of referring (e.g. by speaking) as being indirect. That is, reference involves two separable phenomena ( figure 1 ): first a mental representation of an entity is recognized, recalled or otherwise activated, and second some utterance is produced which may, if successful, elicit a similar though not identical mental representation in the listener. For example, observing a cat walk behind a tree, I may form a mental representation of CAT BEHIND TREE. This complex concept is the first step in reference: a correspondence between real-world events (e.g. visual patterns interpreted as cats and trees) and the resulting mental representation. Generating this particular non-verbal concept is accomplished by the visual system, is private, and (I argue below) essentially the same type of cognitive processing that occurs when a dog sees a cat go behind a tree (who perhaps indicates this knowledge by straining at its leash).
The second stage of reference—externalization—is the one with a public, perceivable component: under some circumstance, I may choose to say ‘there's a cat behind that tree’ or perhaps ‘hinter dem Baum ist eine Katze’ (in German). This second step in referring links my mental representation to some signal in English, German, American sign language, etc. Crucially, my mental representation is the same for either sentence (the very idea of translation—that different sentences in different languages can refer to the very same concept—assumes some language-independent conceptual world). Again the link between the concept CAT BEHIND TREE and either of these sentences is initially an internal matter, within the speaker's mind, and dependent on their personal conceptual and linguistic competences. However, if finally I utter one of these sentences, the utterance enters the public sphere and may cause an appropriately equipped listener to form their own mental representation CAT BEHIND TREE (probably different in detail from mine). Linguistic communication—concept sharing—has occurred.
This indirect model may sound overly complicated or obscure. We have a strong intuition that words themselves ‘mean things’ and sentences ‘refer’, regardless of whether anyone reads or understands them. This intuition about direct reference is hard to shake and still taken quite seriously by some philosophers. This may be because the intuition is biologically grounded, stemming from a ‘referential drive’ to interpret words as meaningful, part of the species-typical ‘instinct to learn’ that underpins child language acquisition [ 1 ]. For the child inferring word meanings, the simple notion that words mean things provides a useful shortcut to get the semantic system up and running. This intuition persists into adulthood, leading to superstitious beliefs (the magical powers of names or ritual chants). Despite providing a concise shorthand for denoting the more circuitous process detailed above, the referentialist intuition is completely inadequate as a full description of linguistic meaning [ 3 ]. Freeing ourselves from the shackles of this prescientific intuition is the first step to insightful scientific analysis.
Embracing this indirect, two-step nature of reference, I can now state my argument more clearly: the first stage of reference—building representations that tie sensory input to conceptual representations—is built upon a chassis of cognitive processes (sensory processing, recognition, categorization, combination and inference) that has fundamental shared components between humans and other animals. These components long predated language. The second stage of ‘externalization’—the capacity to form signals representing these non-verbal concepts—represents a crucial difference in humans and was one of the key innovations in human language evolution [ 16 ]. As Jackendoff puts it ‘phonology and syntax… evolved to express meaning, which has a far longer evolutionary pedigree’ ([ 3 ], p. 428).
It was once common to take a link between concepts and language as definitional, such that a ‘true’ concept must be linked to a word [ 17 , 18 ], but this traditional notion seems unsustainable in the face of infant research, where infants can clearly represent and reason about things they have no words for [ 19 – 22 ].
3. Do animals have concepts?
The considerations above lead most cognitive scientists to assume that the meanings of words and sentences are to be cashed out in non-linguistic mental representations: ‘concepts’ hereafter. However, the cognitive revolution remains incomplete: while few today deny the existence of internal mental representations (concepts) in humans, many remain suspicious when attributing them to animals. Animal cognition researchers are typically required to reject all possible associative explanations, regardless of their complexity, before attributing mental representations to animals [ 23 ] and the discipline spends considerable energy and ingenuity refuting so-called killjoy associative explanations [ 10 , 24 ]. Fortunately, the field has matured to the point where, for many phenomena, there can be little doubt that mental representations exist in animals, and can be recalled, manipulated and themselves represented [ 25 – 27 ].
Concepts should be, in some sense, general and flexible, and might initially be equated with mental ‘categories’. It is uncontested that birds and mammals learn and recall categories [ 28 , 29 ], but some have claimed that animal categories are little more than reflexes, reactively elicited in sensory cortices by sensory inputs and lacking the flexibility and generality of human concepts [ 18 , 30 ]. However, current data demonstrate that many species form cross-modal associations, showing that their categories are flexibly multi-modal [ 31 – 33 ]. Animals can summon categorical representations in the absence of relevant triggering stimuli, for instance seeking hidden food items at particular times, or re-hiding food items a potential thief saw them hide, in the absence of that thief [ 34 ]. They can compute abstract relationships like ‘same’ and ‘different’, for example, correctly choosing novel ‘same’ pairs when presented with two matched objects, and vice versa when given unmatched pairs [ 35 , 36 ]. Many species can compute transitive inferences: knowing that if A > B and B > C, then A must be greater than C as well [ 37 – 39 ]. These data fulfil the philosophers' desideratum that (animal) concepts should be more than unimodal, reflexive, stimulus-driven dispositions to react appropriately: they have an abstract categorical and relational structure.
A sceptical philosopher might still object that however impressive these cognitive abilities are, they do not ‘really’ constitute concepts. Concepts require not just categorization (first-order representations), but a second-order representation of that knowledge: knowing that (or doubting that, or being surprised that) some perceptual object belongs to the category. Animal concepts are limited, philosophers like Davidson argue, to first-order representations [ 40 ]. The most telling evidence against this ‘first-order’ view comes from studies on ‘metacognition’, where animals exhibit an understanding of their own conceptual representations (beliefs about beliefs). If uncertain about their own knowledge, they will choose a ‘don't know’ response, for lesser reward, rather than guessing [ 41 ]. Most research in this experimental paradigm been done on rhesus macaques but related work documents metacognition in dolphins, rats and pigeons (cf. [ 42 ]). Such experiments involve a response to some discrimination task, yielding a food reward, but an additional response is allowed for uncertain cases, often glossed as ‘I don't know’. The animal can choose the ‘don't know’ option when uncertain, receiving a smaller food reward than they would receive for a correct answer, but no punishment. Typically, in situations of high uncertainty (e.g. stimuli ambiguous from a human perspective), animals in these experiments choose the ‘uncertain’ button.
Although some critics have suggested that animals in such experiments simply form a new perceptual category (e.g. ‘unfamiliar’) and pushing the button for this, this possibility can be ruled out in most of the primate experiments (for the refutation of this and other ‘killjoy’ hypotheses, cf. [ 43 ]). Recent experiments are most compelling. Monkeys are first trained on one set of experimental stimuli, for example, based on colour discrimination, to learn the ‘don't know’ option. If this response was really tied to perceptual cues (e.g. colour) about the training stimuli, there should be no carry-over of this third option to novel stimulus sets. Instead, monkeys immediately transfer their appropriate use of the third option to novel situations (e.g. area discrimination) or even from past (retrospective) judgements to future (prospective) judgements [ 44 ]. This strongly suggests that the animals truly doubt their knowledge (represent their own uncertainty) and can transfer a response based on this meta-knowledge to novel situations. These and other data have convinced even previous sceptics that animals possess representations about representations, and therefore ‘concepts’ in this more demanding Davidsonian sense [ 45 ]. Of course, human metacognition is more sophisticated, involving thoughts about thoughts about thoughts… But that fact provides no empirical grounds to deny basic second-order metacognition to other animals. Given these modern data, denials that animals possess basic non-verbal concepts seem misinformed and anti-scientific (e.g. [ 30 ]).
I hasten to add that my claim here is not that animal concepts are of the same complexity or flexibility as those of humans. That would be absurdly anthropomorphic and would ignore the fact that language, as a multi-component system [ 16 ], also includes recursive compositional machinery that allows us to flexibly combine basic concepts into complex, hierarchically structured thoughts. This compositionality is a key component of linguistically structured thought, independent of externalization. Indeed, Chomsky terms it the ‘Basic Property’ of language and argues that it was selected in the human lineage precisely for its value in structuring internal thought, rather than externalizing these thoughts via speech [ 4 , 46 ]. There is at present little evidence of complex compositionality in animal communication or cognition (beyond things like transitivity, discussed below) [ 47 ]. But crucially, if we want to understand the evolution of this component, the appropriate starting point is animal conceptual abilities, and cannot be limited to the signals animal produce.
I now turn to the empirical data supporting my main contention that animals possess more concepts than their communication systems allow them to express. For reasons of space and concision, this is a very selective review—the data are so abundant that a full treatment requires an entire book (for this I recommend [ 2 , 29 ]). I will thus focus on a few examples from clever species, like primates and dolphins, plus honeybees, because these are well documented in easily accessible publications.
4. Animal signals ≠ animal concepts
To empirically demonstrate that a species can conceptualize more than they can express requires both an understanding of their communication system and independent data concerning their cognition. A nice example to start with is the honeybee Apis mellifera , in which communication and cognition are well-studied. The honeybee communication system allows a forager who has discovered flowers, upon returning to the hive, to inform other foragers of their location [ 48 , 49 ]. In the darkness of the hive, the bee performs a stereotyped (and apparently innate) ‘waggle dance’ whose direction, relative to gravity, signals the azimuth direction of the flowers (relative to the sun). The duration of the waggle portion correlates with the distance to the flowers, and by combining these cues, the dance provides a remarkably accurate indication of the location of these flowers. This system is also remarkable in ‘referring’ to an entity not currently present or visible to the communicators (thus sharing the property of ‘displacement’ with human language; [ 50 ]). Finally, the system is flexible, because a honeybee can ‘refer’ to the location of other objects than flowers when necessary, for instance, water or a new nest-site (I put ‘refer’ in quotes to avoid philosophical debate—I simply mean that a honeybee's dance reliably allows naive honeybees to locate the object in question).
Despite this impressive communication system, detailed studies of honeybee cognition reveal even more impressive cognitive abilities (reviewed in [ 51 ]). For example honeybees have excellent colour vision and can remember the colour of rewarding versus unrewarding nectar sources over days [ 52 , 53 ]. Nonetheless, their dance ‘language’ has no way to communicate colour information. Even more impressive, a honeybee can judge whether two stimuli are the same or different in colour or pattern [ 54 ] and generalize this behaviour to novel modalities (trained on colour, she immediately transfers the same/different decision to patterns or vice versa). Again, however, the honeybee dance language lacks signals for ‘same’ or ‘different’. Thus, even an insect whose brain occupies 1 mm 3 and contains less than a million neurons has cognitive abilities that significantly outstrip its ability to communicate them.
Turning now to a large-brained species, the bottlenose dolphin Tursiops truncatus is another species for which we have solid data about both cognition and communication. Dolphins have sophisticated cognitive abilities rivalling those of non-human primates [ 31 ]. They rapidly learn a ‘delayed match-to-sample’ task and generalize across hundreds of novel sounds [ 55 ]. Dolphins can remember lists of items (spatial locations, visual objects or sounds), correctly indicating whether a probe stimulus was or was not in the list, and show a classic recency effect, like humans [ 31 ]. Dolphins show cross-modal integration, matching visually and acoustically perceived (via echolocation) object shapes, and show mirror self-recognition, inspecting themselves in a mirror when marked in an otherwise invisible location (and not doing so when sham-marked). Dolphins readily learn to interpret human signals, whether gestural (e.g. pointing) or auditory [ 56 ] and can understand novel combinations of signals (‘sentences’ made up of multiple gestures or sounds) on the first try, based on a simple order-based grammar (e.g. responding correctly to ‘take the hoop to the ball’ versus ‘take the ball to the hoop’). Dolphins can understand the abstract command ‘create’ indicating ‘do something novel’ by performing some new action or ‘repeat’ to perform the act again (thus requiring the dolphin to keep track of what it itself had done). All of these data indicate that dolphins have a flexible, productive capacity to learn, can self-monitor and can retain and manipulate novel concepts across multiple modalities.
However, turning to bottlenose dolphins' well-studied communication system, we get a very different picture. Early studies indicated a quite complex vocal communication system, and the ability of dolphins to learn human words suggested that they might have a ‘language’ of their own [ 57 ]. These suggestions led to careful experiments attempting to understand dolphin communication via observation and playback experiments that, on the contrary, suggested an ordinary mammalian repertoire of vocal signals [ 58 ], with the exception that dolphins are vocal learners and readily learn to mimic both conspecific and human-generated sounds [ 31 , 59 , 60 ]. Vocal learning is put to use in a ‘signature whistle’ system: dolphins emit an individual-specific whistle pattern (for example, when captured) that can be imitated by other dolphins, leading to exchanges and reuniting of separated animals [ 61 ]. Young dolphins initially acquire their whistles, by imitation [ 62 , 63 ]. Although this is an interesting system, with a capacity to signal individuals (reminiscent of ‘names’), it appears to be the most productive aspect of their vocal system.
The evidence against greater expressive ability comes from experiments where two dolphins are allowed to communicate vocally while solving a joint task [ 64 – 66 ]. Individual dolphins readily learn to push on a right or left paddle depending on a visual signal. With more training, two dolphins who can see each other can learn a social version: a signal perceived by one dolphin must be responded to by the other dolphin first, and only afterwards by the second, to provide a food reward to both. The crucial experimental condition involves blocking visual contact between the two individuals. If dolphins possessed a flexible language-like communication system, it should be a simple matter to signal ‘push the left one’ and succeed. Although initial experiments suggested this [ 64 ], more careful follow-up studies showed that these initial successes did not reflect anything language-like. When the roles were reversed (so that the former responder had to become the signaller), the pair totally failed. Furthermore, when the contingency between signal and response was changed, the dolphins had to be retrained from scratch and were not able to simply switch vocal signals to indicate the other action. The researchers concluded that the initial success was a result of trial-and-error learning where incidental sounds made by one animal, or vocal sounds produced whether or not the other animal was present, were used to solve the task [ 58 , 65 ]. Bastian, who led this research project concisely concluded ‘No evidence was found to support the supposition that the social signalling of dolphins is capable of the transfer of arbitrary environmental information’ (p. iii, [ 65 ]). Summarizing, dolphins have very sophisticated cognitive and learning abilities, revealing complex internal concepts, but their capacity to communicate those concepts via their species-typical signals is quite limited.
5. Concepts and communication in primates
My final examples come from two non-human primate species—vervet monkeys and chimpanzees—but similar examples could be provided for many other well-studied primates.
Vervets Chlorocebus pygerythrus (previously Cercopithecus aethiops ) are small common African monkeys, possessing a suite of different alarm calls that are typically emitted in the presence of different predators [ 67 , 68 ]. The vervet monkey alarm call system is frequently cited as a potential precursor to language [ 10 , 69 ]. However, the three different alarm calls produced to leopards, eagles and snakes in no way exhaust the concepts that vervets can represent. In addition to ‘standard’ primate concepts like individuality and dominance [ 70 ], vervets maintain complex spatial representations of their environment [ 71 ] and can mentally track the locations of hidden group members [ 72 ]. They can socially learn how to access food and rapidly absorb new social preferences about what to eat based on colour [ 73 ]. None of this cognitive sophistication is in any way detectable in their vocal communication system.
Turning finally to our nearest living relatives, the chimpanzees and bonobos ( Pan troglodytes and Pan paniscus ), there is abundant evidence that chimpanzees have highly developed cognitive abilities and can represent basic concepts like colour and shape, as well as abstract concepts including sameness, location, and sequence [ 27 , 74 , 75 ]. Chimpanzees also have social representations including individual identity, dominance and relationships (e.g. ‘child of’) and are capable of transitive inference [ 76 ]. With extensive training, very abstract concepts like number are within their cognitive reach [ 77 , 78 ]. They show at least the beginnings of theory of mind, in that they can represent what competitors do or do not see [ 79 ]. Their tool-using abilities are sophisticated and incorporate future planning [ 80 ]. When trained intensively with human communication systems, they can understand multi-word sentences and indicate an impressive variety of objects and events [ 81 , 82 ] and exhibit flexible cross-modal transfer of information without further training [ 83 ]. In general then, chimpanzees exhibit some of the most sophisticated cognitive abilities known among animals—unsurprising given their close biological relationship to humans.
By contrast, chimpanzee vocal communication is comparable to that seen in many other primates or mammals, with a small repertoire of 30-odd innate vocalizations [ 84 ] including food calls that differ for different food quality [ 85 , 86 ], screams and threats, and complex display calls like pant-hoots [ 87 ]. Chimpanzees are not known to have predator-specific alarm calls like vervets. Their gestural communication system is considerably richer, and perhaps more intentionally informative than their vocal communication [ 88 – 90 ]. But both their vocal and gestural communication skills pale in comparison to their rich and sophisticated cognitive abilities. Cognitive studies demonstrate beyond a reasonable doubt that chimpanzees possess many concepts that their species-typical communication systems cannot express (nor indeed do the utterances of ‘language trained’ chimpanzees come close to expressing the complexity of concepts like number, transitivity or tool use [ 82 , 91 ]). Thus, chimpanzees clearly possess and manipulate concepts that they are unable to communicate. Even the most exhaustive analysis of chimpanzee communication would vastly underestimate the complexity of their non-verbal conceptual world.
It is crucial not to conflate these communicative limitations with the false but frequently repeated claim that primates (or animals more generally) have no voluntary control over their vocalizations. A sizeable body of data clearly demonstrates that they do (cf. [ 92 ]). For example, in the wild, many species (including chickens and monkeys) exhibit ‘audience effects,’ producing vocalizations only when appropriate listeners are around [ 70 , 93 , 94 ], and chimpanzee screams and alarm vocalizations are clearly modulated by the presence and composition of the audience [ 95 , 96 ]. Several bird species produce ‘false’ alarm calls when no predator is present, frightening away competitors and then taking remaining food [ 97 , 98 ]. In an operant setting in the laboratory, numerous studies have demonstrated voluntary production (or inhibition) of vocalizations on command [ 97 , 98 ] in chimpanzees, other primates [ 99 , 100 ] and various other mammals (e.g. cats and dogs, [ 101 ]). Thus, despite a common misconception, animal vocalizations are not reflexive actions, performed inevitably upon the appearance of some external stimulus; but this fact does not imply that their vocalizations provide exhaustive access to their conceptual world.
6. Conclusion: discontinuities in signalling do not indicate cognitive discontinuities
I end by clarifying the key implication of this essay: when considering the evolution of human cognition, we will be fundamentally misled if we attribute to animals only those concepts they can communicate. Externalization of concepts is just one component of language, and another is to help structure our private internal thought [ 4 ]. Thus, we cannot accurately limit our estimation of what humans know to what they say . The same is true of animals, only more so. The flexibility of human language means that we can use it to represent virtually anything we can think (perhaps with considerable effort, in the case of visual, musical or highly abstract concepts). The same flexibility and expressivity is simply not present in animal communication systems. This limitation, rather than any fundamental non-existence of animal concepts, was surpassed by humans during language evolution. Thus, our (linguistic) ability to refer, not our basic ability to conceptually represent, must be explained if we hope to understand the neural and ultimately genetic basis of human language.
This is not to deny that externalized language gives humans a huge conceptual advantage over other species. We acquire many concepts via language that we have no direct access by personal experience, vastly enlarging our potential store of knowledge (some readers may never have personally seen an octopus, but most will nonetheless have some concept OCTOPUS). Blind people, thanks to language, have surprisingly rich conceptions of colour terms [ 102 ], and many abstract or scientific terms such as ‘electron’ or ‘truth’ have no sensory manifestations at all. My argument is not that animals have precisely the same concepts as humans (that would be absurd, because even individual humans do not share precisely the ‘same’ concepts, figure 1 ). My argument concerns the neural and cognitive machinery underlying the formation of mental representations, along with many of the cognitive processes that allow concepts to be formed based on sensory experience and combined at a basic level. These capabilities are shared across species and were therefore present before language evolved and provided the precursors of more complex human concepts.
In many circumstances, the study of animal communication can provide crucial insights into what animals know and remains an important part of comparative investigation of language evolution. But accepting the fundamental fact that animals know much more than they can express implies that the evolution of human language built upon a pre-existing conceptual apparatus much richer than that observable in animal communicative capabilities. It is therefore critical that future scholarly explorations of human language evolution take results from animal cognition research as crucial data for understanding the evolutionary path to human language. Even more crucial is a dedicated research programme to explore in detail animals' abilities to combine concepts. To the extent that they can do so in a flexible, hierarchical manner [ 103 , 104 ], I think we can see the germs of the recursive symbolic system that underlies human linguistic concepts.
Acknowledgements
This essay is dedicated to the fundamental contributions to the study of both animal cognition and communication made by Dorothy Cheney (1950–2018). The author thanks Gesche Westphal-Fitch, Barry Smith and two anonymous reviewers for comments on previous drafts, and Nadja Kavcik for her help with the figure.
Data accessibility
Competing interests.
The author declares that he has no competing interests.
Preparation of this paper was supported by Austrian Science Fund (FWF) DK Grant ‘Cognition & Communication’ (grant no. W1262-B29).
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Animal communication
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- Animal Behavior Follow Following
- Animal Cognition Follow Following
- Bioacoustics Follow Following
- Acoustic Communication Follow Following
- Acoustic Ecology Follow Following
- Behavioral Ecology Follow Following
- Social behavior in animals Follow Following
- Cognitive Ethology Follow Following
- Animal Behaviour Follow Following
- Primatology Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Animal Behavior
Social networks and the complexity and beauty of animal life, lee dugatkin's new book is full of fascinating surprises..
Updated May 16, 2024 | Reviewed by Ray Parker
- Lee Dugatkin uncovers a world of cooperation, communication, and bonding in the animal kingdom.
- Numerous animals display flexible, intricate, and varied social networks that are used in their daily lives.
- "The Well-Connected Animal" is a stand-alone tribute to the complexity, depth, and beauty of animal societies.
- Female macaques with strong friendships with their grooming partners had higher survival than other females.

For many years, Lee Alan Dugatkin, a prolific author and leading researcher in animal behavior , has been one of my "go-to-guys" for learning about cutting -edge research in this and related fields. His book about power-brokering in the wild was one of the most novel books I've read in years, and his latest book, The Well-Connected Animal: Social Networks and the Wondrous Complexity of Animal Societies , is yet another one of his extremely thoughtful and readable works, this time outlining what we know about social networks in diverse species, how the research is conducted, and where future studies should best focus. In my own research, I often think of emotional social networks in which individual feelings serve as a "social glue" for others in their group.
I’m sure many people will be pleasantly surprised by the flexible, intricate, and varied social networks that many nonhumans form and use in their daily lives. Here's what Lee had to say about this fascinating discussion of social networks in widely diverse animals.
Marc Bekoff: Why did you write The Well-Connected Animal ?
Lee Dugatkin : I’ve been studying the complex, nuanced, strategic behavior of animals for 35 years, but even I was surprised when I discovered that non-humans are deeply embedded in their own social networks. When the first detailed work on social networks emerged in the 1980s and 1990s, there was skepticism in the animal behavior research community. The general sense was that nonhumans displayed a rich array of complex social behavior, but not that complex: not social network complex. When researchers began to publish more and more examples of social networks in animals in the early 2000s, the skepticism waned.
Since then, animal behavior researchers have been building models and testing hypotheses—most often in the field—about how social networks operate, why they work, who gets what, who matters most, and who does not so much, and more. Scientists have come to discover that being embedded in social networks plays a critical role in almost every aspect of animal life: what they eat, how they protect themselves, whom they mate with, the dynamics of parent-offspring relations, power struggles, navigation, communication, play, cooperation , and culture. Microbes—some good, some not so good—also hitchhike rides on the animals in these social networks. It's time to tell the story of all this and more. Hence, The Well-Connected Animal.
MB: What are some of the major topics you consider?
LD: This book walks the reader through findings from nonhuman social networks by taking a deep dive into animal behavior, evolution, computer science, psychology, anthropology, genetics , and neurobiology literature, and incorporating interviews and insights from researchers swimming with manta rays, avoiding pigeon poop, and stopping monkeys from stealing their iPads. The Well-Connected Animal tells of animal networks in Australia and Asia to Africa, Europe, and the Americas, taking readers into the midst of social networks in giraffes, elephants, kangaroos, chimpanzees, Tasmanian devils, crickets, honeybees, whales, bats, and more.

To give your readers a sense of what I mean, The Well-Connected Animal tells the tale of how Hurricane Maria upended the long-standing social networks in place among macaques on Cayo Santiago, Puerto Rico, and how the macaques rebuilt those networks, shedding light on how animal social systems respond to catastrophic natural disasters. Data that Laurent Brent and her team had collected between 2010 to 2017 found that female macaques who had strong friendships (tight connections) with their favored grooming partners in their social network had higher survival probabilities than other females. Yet another way a female increased her chances of survival was through weaker connections but many grooming partners. Friends of friends also mattered to female macaques. The more friends of friends a female had, the more offspring she produced.
Then Hurricane Maria struck, devastating everything in its path. About three months after Hurricane Maria, when the shock had partly worn off, Brent and her colleagues began thinking seriously again about the effects of Maria on the dynamics of macaque social networks. What they discovered was that post-Hurricane, macaques had more social partners, but the average intensity of their relationship with a partner in their social grooming network had not changed: they had formed more friendships in their network, not strengthened already existing ones. The hurricane had brought the macaques in a group closer together, with additional grooming partners buffering them from the devastating effects that Maria left in its trail. And again, friends of friends mattered: macaques took the path of least resistance in forming new grooming relationships in their social network. If Monkey 1 had been in a grooming relationship with Monkey 2 before Maria, it was more likely to initiate a grooming relationship with one of Monkey 2’s grooming partners after the storm. Disaster, in the form of Hurricane Maria, had brought the monkeys closer to one another, and social network analysis was the perfect means to show how.
MB: Who do you hope to reach in your interesting and important book?
LD: “Every enlightened reader” may sound glib, but it is not far from the truth. From a human-centric view, we are all embedded in many overlapping social networks, and so a realization that such networks have been in place in nature for eons may help us understand our own social networks better. That said, we don’t need to reference human networks to appreciate nonhuman social networks: in that sense, The Well-Connected Animal is a stand-alone tribute to the complexity, depth, and, dare I say, the beauty of life in animal societies. The book not only delves into the science, but also the behind-the-scenes, everyday stories that go on when scientists study social networks in nonhumans in the wild. Readers get a sense of the science, but just as important, what it is like to do work in this field, including the role of the ups and downs and twists and turns that are sometimes sanitized away in science books. This approach provides the narrative backdrop to complement the remarkable science readers will learn about social networks in nonhumans.
In conversation with Dr. Lee Alan Dugatkin , prolific author professor and University Scholar in the Department of Biology at the University of Louisville. His main areas of research are the evolution of social behavior and the history of science. Dr. Dugatkin is the author of over 175 articles on evolution and behavior and two textbooks— Principles of Animal Behavior (2020, 4th edition) and Evolution (co-authored with Carl Bergstrom, 2016, 2nd edition). Dr. Dugatkin is also the author of many trade books, including How to Tame a Fox (and Build a Dog): Visionary Scientists and a Siberian Tale of Jump-Started Evolution , which the New York Times called "Sparkling ... a story that is part science, part Russian fairy tale, and part spy thriller," Mr. Jefferson and the Giant Moose: Natural History in Early America , and, most recently, Behind the Crimson Curtain: The Rise and Fall of Peale's Museum . For another fascinating interview with Lee see The Subtleties of Power-Brokering Among Wild Animals .
T he Emotional Lives of Animals and Why They Matter ; The Swarm Intelligence of Piping Hot and Boisterous Honey Bees ; The Fascinating Complex Minds of Bees and Why They Matter ; "The Internet of Animals" and Earth's Collective Intelligence ,

Marc Bekoff, Ph.D. , is professor emeritus of ecology and evolutionary biology at the University of Colorado, Boulder.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
We can help you reset your password using the email address linked to your BioOne Complete account.

- BioOne Complete
- BioOne eBook Titles
- By Publisher
- About BioOne Digital Library
- How to Subscribe & Access
- Library Resources
- Publisher Resources
- Instructor Resources
- FIGURES & TABLES
- DOWNLOAD PAPER SAVE TO MY LIBRARY
Virtual fence (VF) is the use of a global positioning system (GPS) to dictate where on the landscape livestock can graze without relying on traditional physical fence such as barbed wire. The recent acceleration in the development and adoption of VF technology for grazing management has been characterized by the evolution of divergent terminology. Different research and commercial entities have adopted terms and definitions independently. Some terms and definitions are inherently problematic, while others are more aligned, and the simple fact that differences exist contributes to confusion in communication among scientists, producers, land managers, manufacturers, government agencies, and the public. In this paper, we propose a standard terminology determined during a 2-d in-service workshop at the annual meeting of the Society of Rangeland Management in February 2023. Standard terminology will aid in efficient and effective communication among all entities and interested parties.

KEYWORDS/PHRASES
Publication title:, collection:, publication years.

IMAGES
VIDEO
COMMENTS
1. Introduction. This theme issue is dedicated to the memory of Dorothy Cheney—an extraordinary and insightful primatologist who, with her husband Robert Seyfarth, studied vervet and baboon vocal communication and illuminated the importance of social cognition in primate evolution and language origins [1,2].For centuries, scientists have been interested in the biological origins of human ...
non-human animal communication have been doing extensive research over the past 25 years, on humans, apes, rodents, and birds, among others. Mainly, the purpose of these studies has been t o dete ...
The introductory linguistics textbooks analysed. Edition: most recent edition available as of 20 January 2021. Year: year of publication of the most recent edition; OS App, OS Score - appearances (App) and popularity score on a 1-100 scale (Score) indicators at Open Syllabus; Chapter (Animal Communication, Language Evolution): page count of the chapter dedicated to this category (if present ...
This theme issue is driven by a recognition of the value of comparative perspectives on human language and the powerful insights that can be gained from studies of animal communication and cognition. The inspiration for this special issue, and many of the contributed articles, arose from an international conference entitled 'New Perspectives ...
The past few years have seen a surge of interest in using machine learning (ML) methods for studying the behavior of nonhuman animals (hereafter "animals") ().A topic that has attracted particular attention is the decoding of animal communication systems using deep learning and other approaches ().Now is the time to tackle challenges concerning data availability, model validation, and ...
The present paper draws attention to this promising research avenue by providing an overview of the state of the art of turn-taking in four animal taxa—birds, mammals, insects and anurans. ... that comparative research into human-animal communication often tends to separate 'animal communication' from 'human language' - assuming the ...
Animals is an international peer-reviewed open access semimonthly journal published by MDPI. Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English.
As an example, recent research has focused on understanding how human-generated noise (from cars, trains, etc) can impact communication in a variety of animals (Rabin et al. 2003). As the field of ...
Detailed comparative studies have revealed many surface similarities between linguistic communication and the communication of nonhumans. How should we interpret these discoveries in linguistic and cognitive perspective? We review the literature with a specific focus on analogy (similar features and function but not shared ancestry) and homology (shared ancestry).
Motivated by Understanding Animal Communication Shafi Goldwasser ∗ UC Berkeley & Project CETI [email protected] David F. Gruber City University of New York & Project CETI [email protected] Adam Tauman Kalai ∗ Microsoft Research & Project CETI [email protected] Orr Paradise UC Berkeley & Project CETI [email protected] Abstract
Most animal communication signals and responses are neither learned nor culturally transmitted. And, so far as we know, no animal communication has the sine qua non of language: productivity. Table 1. Key design features of communication systems (after Hockett, 1960, pruned and combined).
The evolution of animal communication. Marc Naguib a,∗and J. Jordan Price b. a Wageningen University, Department of Animal Sciences, Behavioural Ecology Group, De Elst 1, 6708 WD Wageningen, The ...
Research into the semantics of animal signals began in 1980, with evidence that alarm calls of a non-human primate designated predators as external referents. These studies have challenged the historical assumption that such referential signaling is a unique feature of human language and produced a paradigm shift in animal communication research.
Advanced Research in Animal Communication. Special Issue Editors. Special Issue Information. Keywords. Published Papers. A special issue of Animals (ISSN 2076-2615). This special issue belongs to the section "Human-Animal Interactions, Animal Behaviour and Emotion". Deadline for manuscript submissions: closed (31 December 2021) | Viewed by 21334.
This Special Issue is interested in both reviews and research papers on all aspects of human-animal communication, from both human and animals (farmed and pet) point of view. It is also interested in understanding how empathy can affect the ability of humans to interpret communication cues. Papers can also present results on automatic systems ...
impact research involving animal communication. Every paper at PNAS is handled by a member of the National Academy of Sciences, a nonprofit organization comprising nearly 2,400 active members and 500 international members, of whom more than 200 are Nobel laureates. PNAS makes research on animal communication accessible
2. Words ≠ concepts. Before discussing animals, it is important to first clarify some basic issues about the nature of human concepts, and to at least dip our toes into the philosophical quagmire surrounding the term 'concept' (for a concise introduction see []).My take on concepts in this essay will be essentially that of mainstream cognitive (neuro)science today, where a concept is ...
of published papers on animal communication is also staggering. What this review can do is allude to main lines of argument and development and provide insights aboutalarmcallsasjustone,albeitpertinent,example of animal communication, note the present currents and in"uences on the !eld, and provide a few pointers
There should, then, be insights from pragmatics that can inform the study of animal communication. I will highlight three: (1) that pragmatics has developed an account of meaning that is inherently functional, and hence a consilient account of meaning can be developed; (2) that, consistent with Rendall et al.'s emphasis on influencing rather ...
An internal committee at Dolphin Research Center ethically approved this research. All aspects of animal care and habitat complied with the Standards and Guidelines of the Alliance of Marine Mammal Parks and Aquariums. Research was non-invasive and strictly adhered to the laws of the United States of America.
Combining key concepts from machine learning and linguistic theory could thus substantially advance the study of non-human communication and, more broadly, bring a data-centric paradigm shift to the study of animal communication. In this paper, we describe the current state of knowledge on sperm whale communication and outline the key ...
A topic that has attracted particular attention is the decoding of animal communication systems using deep learning and other approaches (2). Now is the time to tackle challenges concerning data availability, model validation, and research ethics, and to embrace opportunities for building collaborations across disciplines and initiatives.
The "Animal" in the Humanities Research Group was founded in 2017 with the support of the Humanities Center at Texas Tech in order to foster interdisciplinary, collaborative inquiry into the role played by both "the animal" and real animals in human intellectual landscapes, historical and contemporary.
Animal Behaviour is published for the Association for the Study of Animal Behaviour in collaboration with the Animal Behavior Society. First published in 1953, Animal Behaviour is a leading international publication and has wide appeal, containing critical reviews, original papers, and research articles on all aspects of animal behaviour. Book Reviews and Books Received sections are also included.
Researchers have developed a method to investigate sperm whale communication by determining their vocal style, finding that groups living in close proximity can develop similar styles to each other.
Animal Communication at PNAS. Every paper at PNAS is handled by a member of the National Academy of Sciences, a nonprofit organization comprising nearly 2,400 active members and 500 international members, of whom more than 200 are Nobel laureates. Recent and highly cited research in animal communication published in PNAS include:
Key points. Lee Dugatkin uncovers a world of cooperation, communication, and bonding in the animal kingdom. Numerous animals display flexible, intricate, and varied social networks that are used ...
Virtual fence (VF) is the use of a global positioning system (GPS) to dictate where on the landscape livestock can graze without relying on traditional physical fence such as barbed wire. The recent acceleration in the development and adoption of VF technology for grazing management has been characterized by the evolution of divergent terminology. Different research and commercial entities ...
Elephants are long-lived, large brained, and cognitive animals, but we still know very little about how learning (i.e., social learning) affects elephant behavior in general. Most research papers have focused on a single modality (e.g., sound or olfaction), but a more holistic approach is needed.