- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
About The BMJ
- Resources for authors
- Forms, policies, and ethics
- Authorship & contributorship
The International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals ( ICMJE Recommendations 2018 ) recommend that authorship be based on the following four criteria:
• Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND • Drafting the work or revising it critically for important intellectual content; AND • Final approval of the version to be published; AND • Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
In addition to being accountable for the parts of the work he or she has done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors.
We include only one corresponding author per article. Any further contribution details (eg, equal contribution) must be included in the contributors or acknowledgement sections at the end of the article.
The BMJ requires that all those designated as authors should meet all four ICMJE criteria for authorship, and all who meet the four criteria should be identified as authors. We recognise only natural persons over 18 years of age as authors. These authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work. The criteria are not intended for use as a means to disqualify colleagues from authorship who otherwise meet authorship criteria by denying them the opportunity to meet criterion #s 2 or 3. Therefore, all individuals who meet the first criterion should have the opportunity to participate in the review, drafting, and final approval of the manuscript.
The individuals who conduct the work are responsible for identifying who meets these criteria and ideally should do so when planning the work, making modifications as appropriate as the work progresses. The corresponding author takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and typically ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements, are properly completed, although these duties may be delegated to one or more coauthors.
When a large multi-author group has conducted the work, the group ideally should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship, including approval of the final manuscript, and they should be able to take public responsibility for the work and should have full confidence in the accuracy and integrity of the work of other group authors. They will also be expected as individuals to complete conflict-of-interest disclosure forms.
The byline of the article identifies who is directly responsible for the manuscript, and Medline lists as authors whichever names appear on the byline. If the byline includes a group name, Medline will list the names of individual group members who are authors or who are collaborators, sometimes called non-author contributors, if there is a note associated with the byline clearly stating that the individual names are elsewhere in the paper and whether those names are authors or collaborators.
At The BMJ we want authors to assure us that all authors included on a paper fulfil the criteria of authorship. In addition we want assurance that there is no one else who fulfils the criteria but has not been included as an author.
When we encounter disagreements among authors we follow guidance from the Committee on Publication Ethics (COPE)—see here and here .
AI technologies will not be accepted as an author(s) of any content submitted to BMJ for publication. BMJ only recognises humans as being capable of authorship since they must be accountable for the work.
Contributorship
The BMJ lists contributors in two ways. Firstly, we publish a list of authors' names at the beginning of the paper and, secondly, we list contributors (some of whom may not be included as authors) at the end of the paper, giving details of who did what in planning, conducting, and reporting the work. This is a good place to include contributions by patients or members of the public who have assisted as research volunteers, giving their names and specific roles. We encourage authors to fully acknowledge the contribution of patients and the public to their research where appropriate.
One or more of these contributors are listed as guarantors of the paper. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. See Maintaining the integrity of the scientific record.
Contributorship and guarantorship are concepts that were applied first to original research papers, and are sometimes hard to define for other articles. Each contributorship statement should make clear who has contributed what to the planning, conduct, and reporting of the work described in the article, and should identify one, or occasionally more, contributor(s) as being responsible for the overall content as guarantor(s). For articles in The BMJ that do not report original research - such as editorials, clinical reviews, and education and debate - please state who had the idea for the article, who performed the literature search, who wrote the article, and who is the guarantor (the contributor who accepts full responsibility for the finished article, had access to any data, and controlled the decision to publish). For non-research articles that include case reports such as lessons of the week, drug points, and interactive case reports, please also state who identified and/or managed the case(s).
Researchers must determine among themselves the precise nature of each person's contribution, and we encourage open discussion among all participants. See Authorship is dying; long live contributorship.
Alteration to authorship or contributorship
Any change in authors and/or contributors after initial submission must be approved by all authors. This applies to additions, deletions, change of order to the authors, or contributions being attributed differently. Any alterations must be explained to the editor. The editor may contact any of the authors and/or contributors to ascertain whether they have agreed to any alteration.
Group authorship
If there is a very large number of authors we may ask for confirmation that everyone listed met the ICMJE criteria for authorship. If they did, we may then require that the authors form a group whose name will appear in the article byline.
We appreciate that authors may be concerned that their work will not be properly recognised if they form a group, but this is unfounded. Medline guidance can handle group authorship and still give each individual due credit:
"When a group name for a specific consortium, committee, study group, or the like appears in an article byline, the personal names of the members of that group may be published in the article text. Such names are entered as collaborator names for the Medline citation."
Key points:
• A Medline citation may contain an array of personal author names, group (or corporate) author names, and collaborator names. • Personal author names are included in Medline when the author names appear in the article byline, or are explicitly identified anywhere else in the text of the article as the authors or as the members of the writing group or writing committee for the article. • Group author names (also known as corporate, organization or collective names) are included in Medline when such names appear in the article byline. • When a group name for a specific consortium, committee, study group, or the like appears in an article byline, the personal names of the members of that group may be published in the article text. Such names are entered as collaborator names for the Medline citation. • Collaborator names are entered for a Medline citation only when a group (corporate) author name is present for the citation. • More than one group name may appear for a citation, and a group name may appear along with personal author names. • For articles that represent a formal guideline or practice guideline, the name of the guideline-issuing body is entered as a group name for the Medline citation, even if that name does not appear in the article byline.
What this means for The BMJ 's authors
a) if authors form a group for the article's main byline they will also be listed individually:
• As collaborators in the article's Medline/PubMed record; • As authors in a group authorship statement at the end of the article on thebmj.com; and • As contributors in the contributorship statement at the end of the article on thebmj.com.
b) however, for The BMJ 's research articles with many authors, where those authors do not opt to form a group, we will not be able to publish a BMJ pico in the print issue of The BMJ . Such research articles will be for online only (thebmj.com) publication only.
Here's a research article in The BMJ with group authorship as it appeared on Medline, with all collaborators clearly listed as individuals:
http://www.ncbi.nlm.nih.gov/pubmed/20123835
And here's how the individual authors for that article were listed on thebmj.com:
1. What appeared at the top of the article and was dowloadable to citation manager:
Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries. Wei-Hong Zhang, Catherine Deneux-Tharaux, Peter Brocklehurst, Edmund Juszczak, Matthew Joslin, Sophie Alexander, on behalf of the EUPHRATES Group. BMJ 2010;340:c293, doi: 10.1136/bmj.c293 (Published 1 February 2010)
2. What appeared at the end of the article in an authorship statement:
The following are members of EUPHRATES (EUropean Project on obstetric Haemorrhage, Reduction, Attitudes, Trial and Early warning System): Sophie Alexander (project leader, Belgium), Diogo Ayres-de-Campos (Portugal), Istvan Berbik (Hungary), Marie-Hélène Bouvier-Colle (France), Gérard Bréart (France), Peter Brocklehurst (UK), Vicenç Cararach (Spain), Anna Maria Marconi (Italy), Catherine Deneux-Tharaux (France), Risto Erkkola (Finland), Mathias Klein (Austria), Jens Langhoff-Roos (Denmark), Alison Macfarlane (UK), Walter Prendiville (Republic of Ireland), Jos van Roosmalen (Netherlands), Babill Stray-Pedersen (Norway), Carolyn Troeger (Switzerland), Clare Winter (UK), and Wei-Hong Zhang (Belgium). Also see web extra for a list of people who helped in each country.
3. What appeared at the end of the article in the contributorship statement:
Contributors: W-HZ designed data collection tools, monitored data collection for the whole trial, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. She is guarantor. CD-T implemented the trial in France, analysed the data, and drafted and revised the paper. PB analysed the data and drafted and revised the paper. EJ wrote the statistical analysis plan, monitored data collection for the whole trial, and revised the draft paper. MJ designed data collection tools,, monitored data collection for the whole trial, and revised the draft paper. SA initiated the collaborative project, designed data collection tools, implemented the trial for the all countries, monitored data collection for the whole trial, analysed the data, and drafted and revised the paper. All members of EUPHRATES designed the trial. Diogo Ayres-de-Campos, Istvan Berbik, Marie-Hélène Bouvier-Colle, Vicenç Cararach, Risto Erkkola, Mathias Klein, Walter Prendiville, Jos van Roosmalen, Babill Stray-Pedersen, and Carolyn Troeger implemented the trial in, respectively, Portugal, Hungary, France, Spain, Finland, Austria, Republic of Ireland, Netherlands, Norway, and Switzerland, and revised the draft paper. Gérard Bréart analysed the data and revised the draft paper. Alison Macfarlane and Clare Winter revised the draft paper.
- Publishing model
- Editorial staff
- Advisory panels
- Explore The BMJ
- BMJ Student
- How green is The BMJ?
- Sources of revenue
- Article types and preparation
- Article submission
- Competing interest policy
- Copyright, open access, and permission to reuse
- Patient consent and confidentiality
- Research Ethics
- BMJ papers audit
- Guidance for new authors
- BMJ Christmas issue
- Resources for advertisers and sponsors
- Resources for BMA members
- Resources for media
- Resources for subscribers
- Resources for readers
- Resources for reviewers
- About The BMJ app
- Poll archive
- International jobs
This week's poll
Read related article
See previous polls
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 26 September 2012
Authorship: Who's on first?
- Amber Dance 1
Nature volume 489 , pages 591–593 ( 2012 ) Cite this article
43k Accesses
68 Citations
214 Altmetric
Metrics details
When scientists collaborate on an experiment and a paper, it can be hard to decide who gets the credit and how much.
Stephen Kosslyn first started to consider how author lists come together when he found himself mediating a dispute. A postdoc and a graduate student each wanted to be listed as the first author on a study. “They both had a case,” recalls Kosslyn. “It got heated.”
Disagreements often happen when contributors put in similar amounts of effort on different aspects of a project, says Kosslyn, a psychologist at Stanford University in California. For example, one person might have developed the idea for the project and the other performed most of the data analysis. “The force of the dispute usually revolves around the feeling that whatever they did was more important than what the other person did,” says Kosslyn.

Such disputes are common. “As authorship is our academic currency, it tends to be a hot-button topic,” says Karen Peterson, scientific ombudsman at the Fred Hutchinson Cancer Research Center in Seattle, Washington. She says that one-fifth of the disputes she adjudicates concern authorship. Similar conflicts are among the most common issues mediated by the Committee on Publication Ethics (COPE), says Virginia Barbour, the organization's chairwoman and chief editor of PLoS Medicine in Cambridge, UK.
Authorship disagreements can be mitigated with careful discussions, explicit lab guidelines and a good understanding of authorship practices in one's field. There is no perfect approach, but deciding on who gets an authorship credit, and how they are ranked, is a crucial part of doing science responsibly.
Precise statistics on authorship disputes are hard to come by, says Mario Biagioli, a science historian at the University of California, Davis, who has studied authorship. Scientists may be reluctant to admit that they have demanded undeserved authorship or otherwise subverted the system, and the US Office of Research Integrity does not track such disagreements because they are not considered scientific misconduct, says Biagioli, who co-edited the book Scientific Authorship: Credit and Intellectual Property in Science (Routledge, 2002). However, in a 2005 survey 1 of researchers who had received a grant from the US National Institutes of Health (NIH), 10% of respondents admitted to assigning authorship “inappropriately”.
Credit confusion
Questions of who deserves credit for a paper are a fairly recent phenomenon, says Biagioli. Once upon a time, a paper had one author, maybe two. But with modern big science and large collaborations, a study might have hundreds or even thousands of authors — as in the case of the ATLAS experiment 2 at the Large Hadron Collider at CERN, Europe's particle-physics laboratory near Geneva, Switzerland.
And what authorship means varies by scientific discipline. For example, in particle physics, hundreds of researchers may contribute to the development and maintenance of a single piece of equipment, such as an accelerator. At big physics labs such as CERN, everyone who was working at the lab when the discovery was made gets a slot on the author list — even if they haven't seen the paper, says Biagioli. The authors are usually listed alphabetically, regardless of how much they contributed.
In the biological sciences, by contrast, the author list is often strictly ranked. The top spot is at the end of the list, where the principal investigator gets credit for running the lab. The student or postdoc who actually did the work goes first. As for the authors in the middle, it is hard to tell whether they participated a lot or a little, says Biagioli.
The International Committee of Medical Journal Editors (ICMJE), headquartered in Philadelphia, Pennsylvania, has developed authorship guidelines that are used by many journals and institutions. These rules state that to be listed as an author, each researcher must meet three key criteria: they must have been involved in designing the project, collecting data or analysing the results; they must have participated in drafting or revising the manuscript; and they must have approved the final, published paper. Many universities that have their own guidelines base them on the ICMJE's wording, says Biagioli.

Kosslyn has his own definition: the crucial element, he says, is creativity. For example, a researcher could work with study participants in the lab, but just be following a protocol. “Anybody could have run the subjects, so running the subjects is not enough,” says Kosslyn. To earn authorship, the researcher would be intellectually engaged: they might point out a feature of the data that leads the team to reshape the experiment. The paper wouldn't look the same without them.
The author in question
COPE recommends that researchers decide who will be an author and what order they will be listed in before they even conduct experiments, and that the group revisits the author list as a project evolves. A handshake isn't enough to seal the deal — researchers should keep author agreements in writing.
Whenever they occur, authorship discussions need not be confrontational (see 'Aggravation-free authorship' ). Mark Groudine, deputy director of the Hutchinson Center, says that the parties in a dispute should sit down and try to talk the matter over. “People get so locked into their positions that they don't make the effort to understand the other person's point of view,” he says, “and therefore they don't understand why it's a dispute.”
If talking doesn't work, Groudine suggests asking the opinion of an unbiased third party. For example, on one project he collaborated with another principal investigator. When it came to writing up the paper, both wanted to be senior author. They invited two trusted colleagues to mediate.
The jury awarded the senior slot to Groudine, but he felt uneasy about it. He suggested that the other investigator be the corresponding author, who communicates with the journal and any scientists who enquire about the work. “I consider corresponding author as equivalent, almost, to senior author,” says Groudine. Co-senior authorship is also an option, he adds.
But sharing credit too broadly can be risky. Sometimes authors are listed more as a courtesy than because they made a key contribution, says Chris Sneden, an astronomer at the University of Texas at Austin, who will step down from his post as editor of The Astrophysical Journal Letters at the end of this year. Accepting courtesy authorship is a “double-edged sword”, he says. If the paper becomes famous, “every author gets to claim credit”. But if it becomes infamous, everyone gets a share of the blame. Researchers need to be aware of the potential risks of adding their names to manuscripts that they know little about (see 'Ghosts and guests' ).
Gerald Schatten, a stem-cell researcher at the University of Pittsburgh in Pennsylvania, learned that lesson when he lent his good name to a high-profile but eventually discredited stem-cell paper by Woo Suk Hwang, then at Seoul National University. Schatten was investigated by his university, which cleared him of misconduct, but chastised him for 'research misbehaviour' because he failed to check the quality of the science 3 .
The decision to accept courtesy authorship is a matter of preference, says Sneden. “Personally, if I haven't actually contributed something to the specific paper, I just won't have my name on it,” he says. In that case, he politely tells his colleagues that he shouldn't be on the list. “I make sure they understand that it's not a negative reflection on the paper,” he says.
Taken in vain
Sometimes, the recipient of this courtesy may not get the chance to bow out. A researcher who has been added to the author list without their permission might be surprised to see their name when the paper comes out, says Sneden, or even angry if they don't agree with the conclusions. Those who find themselves unexpectedly an author on a paper that they would prefer not to be associated with should contact the editor of the journal, he recommends. The editor will get in touch with the study's corresponding author, and decide whether a corrigendum is necessary to explain that the author in question was not involved with the work.
These kinds of conflicts shouldn't occur. Corresponding authors are expected to have the approval of their co-authors — but some don't realize it. “People, do you read the publication agreement that you sign?” Sneden asks his colleagues. (Often, the answer is no.)
Increasingly, journals are attempting to keep authors in line by asking for details on who did what. In cases of fraud, those descriptions should lay the blame at the right person's door.
Biagioli agrees that delineating each person's contribution should help, but he says that the descriptions are frequently too brief. As an example, he cites the study published this month in Nature by the ENCODE Project Consortium 4 . It ascribes generic tasks such as “data analysis”, “writing” or “scientific management” to large sets of authors, making it impossible to tell, for example, who analysed which data. When scientists sit down to plan a project — and ideally draft the author list — they should also decide how to describe everyone's contributions, says Biagioli. The relevant details will probably vary by discipline, he adds.
In his own lab, Kosslyn has instituted a scheme to make authorship requirements explicit from the outset. As he listened to his student and postdoc arguing their cases several years ago, he started to develop what eventually became a 1,000-point system. The researchers who come up with the idea get 250 points, split between them according to their contribution; writing the paper is worth the same. A further 500 points are available for designing and running the experiment and analysing the data. Researchers who score at least 100 points make the author list, with each person's point total determining their rank.
Disagreements still occur; in those cases, Kosslyn decides how the points are allocated. When the balance of contributions is unclear, he does his best. However, it rarely comes to tallying points. “Usually it's very obvious what the order's going to be,” he says.
In recent years, no disputes have ever risen to the level of the argument that led to the point system. “That,” says Kosslyn, “was the last heated dispute we had in the lab.”
Box 1: Aggravation-free authorship
When many scientists work together, determining authorship isn't always easy. Here are some tips for settling the line-up.
Make sure that you choose collaborators with whom you can work well.
Discuss authorship early, and keep doing so often as a project evolves. Put it in writing.
When there are disputes, first try to talk it out amicably and understand the other person's point of view. For example, try to work out how the idea first came about.
If you must approach your supervisor about an authorship decision that you don't like, keep the tone inquisitive, not accusatory. Explain that you want to understand how authorship was decided.
If a contributor's authorship is in question, it can help to consider what the paper would have looked like without their efforts, and whether someone else could have made the same contribution.
Familiarize yourself with your institution's or journal's authorship guidelines, or those of the International Committee of Medical Journal Editors. Use them to back up your case.
Be prepared to compromise or share credit.
If you can't agree among yourselves, engage a supervisor, trusted colleagues or an ombudsman to investigate the matter and make a recommendation. A.D.
Box 2: Ghosts and guests
Authorship can be misused when there is money to be made. Medical journals contain a mixture of original scientific findings and veiled advertisements for drugs, and scientists and physicians must read papers critically to understand a medicine's true merits, says Alastair Matheson, a biomedical-research consultant in Toronto, Canada.
Some pharmaceutical companies make drugs and run clinical trials, then engage medical writers to draft manuscripts. These contributors are often ghostwriters not listed as authors on the paper. Instead, the company's marketing team finds a big academic name to headline the project — even if this guest author makes no contribution to the paper apart from scanning the final version. Companies sometimes use the same technique to produce reviews promoting their latest medicines, says Joseph Ross, a physician who studies health policy at Yale University in New Haven, Connecticut. One survey 5 found that guests and ghosts haunted 21% of papers published in six leading medical journals in 2008.
“This vast production line of information about drugs is passed off as the work of academics rather than the work of industry,” says Matheson. The companies get to advertise their products; the ghostwriters receive a pay cheque; and the academics get another line on their CVs. But the patients and the integrity of science all lose out, says Matheson.
For example, Merck, a pharmaceutical company based in Whitehouse Station, New Jersey, minimized reporting of the risks observed for its painkiller Vioxx (rofecoxib) until the drug was taken off the market in 2004. Ross was a consultant to people who had taken Vioxx and developed heart problems, or their families, in two court cases against Merck, and he saw some of the company's internal documents 6 . “We were sort of shocked to find pretty rampant evidence that a lot of the trials were ghostwritten,” says Ross. “We would stumble across a full draft of a manuscript that just said, 'external author?'.”
There are ways to identify traces of guests and ghosts in a manuscript: “Check the small print,” says Matheson. That is where a medical writer or communications company may be acknowledged. Funding from a drug-maker is another tell-tale sign. “These are pointers to the likelihood that this is something originated and planned by industry prior to the involvement of the headline authors,” says Matheson. Author disclosures are less helpful, he adds, because academic authors may list several affiliations and it is difficult to tell which commercial relationship is relevant.
With commerce and medicine intimately intertwined, it would be impractical for academics to cut ties with companies, says Matheson. But, he adds, when academics are offered guest authorship, “I would advise them, for the sake of their reputation, to do two things”. First, he says, be more than a guest: make sure that your contribution is author-level. Second, insist that company employees involved in the study are also listed as authors.
Matheson says it is the responsibility of journals to make participation by drug-makers more apparent. He would like to see papers marked right at the top with 'commercial article'. He also suggests that journals use labels to indicate who funded the study, and what drug it supports. A.D.
Martinson, B. C., Anderson, M. S. & de Vries, R. Nature 435 , 737–738 (2005).
Article ADS CAS Google Scholar
The ATLAS Collaboration J. Instrum. 3 , S08003 (2008).
Maris, E. & Check, E. Nature 439 , 768–769 (2006).
Article ADS Google Scholar
The ENCODE Project Consortium Nature 489 , 57–74 (2012).
Wislar, J. S., Flanagin, A., Fontanarosa, P. B. & DeAngelis, C. D. Br. Med. J. 343 , D6128 (2011).
Article Google Scholar
Ross, J. S., Hill, K. P., Egilman, D. S. & Krumholz, H. M. J. Am. Med. Assoc. 299 , 1800–1812 (2008).
Article CAS Google Scholar
Download references
Author information
Authors and affiliations.
Amber Dance is a freelance science writer in Los Angeles, California.,
Amber Dance
You can also search for this author in PubMed Google Scholar
Additional information
See World View p.475
Related links
Related links in nature research.
Authorship policies
Credit where credit's due
Merck accused of disguising its role in research
Taking the industry road
Nature authorship policy
Related external links
ICJME authorship guidelines
Stephen Kosslyn's authorship criteria
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Dance, A. Authorship: Who's on first?. Nature 489 , 591–593 (2012). https://doi.org/10.1038/nj7417-591a
Download citation
Published : 26 September 2012
Issue Date : 27 September 2012
DOI : https://doi.org/10.1038/nj7417-591a
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Honorary authorship in health sciences: a protocol for a systematic review of survey research.
- Reint Meursinge Reynders
- Gerben ter Riet
- Mario Malički
Systematic Reviews (2022)
Gender disparities in pediatric research: a descriptive bibliometric study on scientific authorships
- Katja Böhme
- Doris Klingelhöfer
- Michael H. K. Bendels
Pediatric Research (2022)
Can authors’ position in the ascription be a measure of dominance?
Scientometrics (2019)
Text Recycling in Scientific Writing
- Cary Moskovitz
Science and Engineering Ethics (2019)
The Perception of Scientific Authorship Across Domains
- David Johann
- Sabrina Jasmin Mayer
Minerva (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Publication Recognition
What is a Corresponding Author?
- 3 minute read
- 411.1K views
Table of Contents
Are you familiar with the terms “corresponding author” and “first author,” but you don’t know what they really mean? This is a common doubt, especially at the beginning of a researcher’s career, but easy to explain: fundamentally, a corresponding author takes the lead in the manuscript submission for publication process, whereas the first author is actually the one who did the research and wrote the manuscript.
The order of the authors can be arranged in whatever order suits the research group best, but submissions must be made by the corresponding author. It can also be the case that you don’t belong in a research group, and you want to publish your own paper independently, so you will probably be the corresponding author and first author at the same time.
Corresponding author meaning:
The corresponding author is the one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process. Normally, he or she also ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements, are properly completed, although these duties may be delegated to one or more co-authors.
Generally, corresponding authors are senior researchers or group leaders with some – or a lot of experience – in the submission and publishing process of scientific research. They are someone who has not only contributed to the paper significantly but also has the ability to ensure that it goes through the publication process smoothly and successfully.
What is a corresponding author supposed to do?
A corresponding author is responsible for several critical aspects at each stage of a study’s dissemination – before and after publication.
If you are a corresponding author for the first time, take a look at these 6 simple tips that will help you succeed in this important task:
- Ensure that major deadlines are met
- Prepare a submission-ready manuscript
- Put together a submission package
- Get all author details correct
- Ensure ethical practices are followed
- Take the lead on open access
In short, the corresponding author is the one responsible for bringing research (and researchers) to the eyes of the public. To be successful, and because the researchers’ reputation is also at stake, corresponding authors always need to remember that a fine quality text is the first step to impress a team of peers or even a more refined audience. Elsevier’s team of language and translation professionals is always ready to perform text editing services that will provide the best possible material to go forward with a submission or/and a publication process confidently.
Who is the first author of a scientific paper?
The first author is usually the person who made the most significant intellectual contribution to the work. That includes designing the study, acquiring and analyzing data from experiments and writing the actual manuscript. As a first author, you will have to impress a vast group of players in the submission and publication processes. But, first of all, if you are in a research group, you will have to catch the corresponding author’s eye. The best way to give your work the attention it deserves, and the confidence you expect from your corresponding author, is to deliver a flawless manuscript, both in terms of scientific accuracy and grammar.
If you are not sure about the written quality of your manuscript, and you feel your career might depend on it, take full advantage of Elsevier’s professional text editing services. They can make a real difference in your work’s acceptance at each stage, before it comes out to the public.
Language Editing Services by Elsevier Author Services:
Through our Language Editing Services , we correct proofreading errors, and check for grammar and syntax to make sure your paper sounds natural and professional. We also make sure that editors and reviewers can understand the science behind your manuscript.
With more than a hundred years of experience in publishing, Elsevier is trusted by millions of authors around the world.
Check our video Elsevier Author Services – Language Editing to learn more about Author Services.
Find more about What is a corresponding author on Pinterest:

- Research Process
What is Journal Impact Factor?

What is a Good H-index?
You may also like.

How to Make a PowerPoint Presentation of Your Research Paper

How to Submit a Paper for Publication in a Journal
Input your search keywords and press Enter.

In this section:
- Advertising and sponsorship
- Authorship and contributorship
- Competing interests
- Copyright and authors’ rights
- Correction and retraction policies
- Data protection policy
- Data sharing
- Editor Roles & Responsibilities
- Gender identity and race & ethnicity data in ScholarOne
- Patient and public partnership
- Patient consent and confidentiality
- Peer Review Terms and Conditions
- Publication embargo
- Reproducing third party illustrative materials
- Research Ethics
- Rapid responses
- Scientific misconduct
- Trial registration
This policy ensures that contributors who have made substantive intellectual contributions to an article are given credit and that contributors understand their role in taking responsibility and being accountable for what is published. Contributors are either author contributors (meaning that they meet all four authorship criteria – see below) or non-author contributors.
BMJ credits and lists contributors in two ways:
- Authorship – we publish a list of authors’ names at the beginning of the paper in the byline
- Contributorship – we publish a contributorship statement at the end of the paper, giving details of who did what in planning, conducting, and reporting the work. This should include all author contributors and may include non-author contributors.
We also publish an acknowledgements statement at the end of the paper, detailing those who helped in carrying out the research but that have not been recognised as contributors, and for personal expressions of gratitude.
Submitting author
Corresponding author, joint first authorship, collaborators (group authorship), deceased authors, alteration to authorship, acknowledgements.
The International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals ( ICMJE Recommendations 2019 ) recommend that authorship be based on the following four criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
BMJ requires that all those designated as authors should meet all four ICMJE criteria for authorship, and all who meet the four criteria should be identified as author contributors. We recognise only natural persons (an individual human being, as opposed to a private or public organisation) as authors. These authorship criteria are intended to reserve the status of authorship for those who deserve credit and can take responsibility for the work. The criteria should not be used to disqualify colleagues from authorship who otherwise meet authorship criteria by denying them the opportunity to meet criterion number 2 or 3. Therefore, all individuals who meet the first criterion should have the opportunity to participate in the review, drafting and final approval of the manuscript.
Contributors who have contributed materially to the paper but whose contributions do not justify authorship should be described clearly in the contributorship statement .
In addition to being accountable for the parts of the work they have done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors.
Submitting authors should provide assurance that all authors included on a paper fulfil the criteria of authorship. We also ask for assurance that there is no one else who fulfils the criteria that has been excluded as an author.
When we encounter disagreements among authors we follow guidance from the Committee on Publication Ethics (COPE).
The submitting author takes primary responsibility for submitting the article to the journal using our manuscript submission system ScholarOne and for communicating with the journal during the article submission, peer review and revision process. They ensure that all of the journal’s administrative requirements are properly completed. These include, but are not limited to, providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements. These tasks may be delegated to one or more co-authors, but the submitting author remains responsible for them.
When you submit your article through our submission system you will be asked to provide a name, email address and institutional affiliation for all author contributors. In the final published article author names, institutions and addresses will be taken from these completed fields and not from the submitted Word document.
Affiliations listed should be those where the work was carried out at the time the research/article was written. If institution details appear incorrectly these can be directly amended under ‘Actions’ by selecting the ‘Edit’ drop down next to each author.
All author contributors receive a confirmation email when an article has been submitted and when a final decision is made.
The submitting author should assign the corresponding author when providing author details (see below for more information about the corresponding author role). The submitting author and corresponding author can be the same person.
The corresponding author, as listed on ScholarOne, takes primary responsibility for completing all necessary actions after acceptance of the manuscript and communicating with the journal and with readers after publication. All email communication from BMJ will be sent to the corresponding author including:
- The timeline for your article proof with a link to Publishing at Work where you can track your article’s status
- If your article will be published open access or in colour in the print edition of the journal, you will receive an email from Rightslink with payment options and instructions. If you are not making the payment yourself, you may forward the email to the person or organisation that will be paying on your behalf
- A link to review and approve the proof when available
- Confirmation that your article has been published online
- Notifications when a response has been posted to your article
Find out more about what to expect when your article has been accepted .
Although we include only one corresponding author on ScholarOne for email communication, multiple authors can be listed with correspondence information in the author byline of the final published article. This information can be included at the article proof stage, after acceptance.
Note, the policy for The BMJ differs and can be found here
Joint first authors can be indicated by the inclusion of the statement ‘X and X contributed equally to this paper’ in the contributorship statement.
Collaborators are a large group of multi-author contributors (e.g. a specific consortium, committee, study group or the like). Collaborators should decide who will be an author before the work is started and confirm who is an author before submitting the manuscript for publication. All members of the group named as authors should meet all four criteria for authorship as detailed above. They will also be expected as individuals to complete conflict-of-interest disclosure forms and provide a summary in the relevant section.
The collaborator group name(s) should be included in the main author list on ScholarOne. The collaborator group name(s) followed by the individual names should also be listed in the ‘Collaborator’ field on ScholarOne. BMJ will list the author group name(s) in the author byline, with the full list of individual names included in a collaborator statement at the end of the article. Details of the group’s contributions should also be listed in the ‘Contributorship statement’ field on ScholarOne.
If the journal is indexed in PubMed (MEDLINE and/or PubMed Central), the group name will be listed in the author byline and the names of individual group members entered as collaborators on the PubMed record to ensure individual due credit.
The BMJ Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 European countries
PubMed record >>
BMJ Open Establishing a core outcome set for treatment of uncomplicated appendicitis in children: study protocol for an international Delphi survey
AI technologies will not be accepted as an author(s) of any content submitted to BMJ for publication. BMJ only recognises humans as being capable of authorship since they must be accountable for the work.
Deceased persons deemed appropriate as authors should be highlighted to the Editorial Assistant when submitting your article and should also be included in your contributorship statement.
If an author’s affiliation has changed during the course of the work, the author may either list the affiliation at the time that the research (or most significant portion of the research) was conducted, or their current affiliation, or both. The change of affiliation can be explained in an acknowledgements section.
Any change in authors after initial submission and before publication must be approved by all authors. This applies to additions, deletions, a change of order to the authors’ names or a change to the attribution of contributions. Any alterations must be explained to the Editor. The Editor may contact any of the authors and/or contributors to ascertain whether they have agreed to any alteration.
Contributorship statement
A contributorship statement is required for every article submitted and should outline who has contributed what to the planning, conduct and reporting of the work described in the article. A contributorship statement should include author contributors, non author contributors and group author contributors (collaborators). Contributors who have contributed materially to the paper but whose contributions do not justify authorship should be described clearly in the contributorship statement; for example, “served as scientific advisors”, “critically reviewed the study proposal”, “collected data” or “provided and cared for study patients”.
Researchers must determine among themselves the precise nature of each person’s contribution, and we encourage open discussion among all participants to reach a consensus.
This is also the appropriate place to include contributions by patients or members of the public who have assisted as research volunteers, giving their names and specific roles. We encourage authors to fully acknowledge the contribution of patients and the public to their research where appropriate.
All individuals named in the contributorship statement must give permission to be included, as readers may infer their endorsement of the data and conclusions of the paper. It is the responsibility of the submitting author to ensure that permission is obtained and to be able to provide evidence of this if required.
Each contributorship statement must make clear who is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
- To ensure transparent declaration of AI, authors should:
2. Transparent declaration includes a description of:
- What AI technology was used (the name of the technology)
- Why this AI technology was used (the reason for its use)
- How the AI technology was used (what the task of the technology was)
- Consider including a summary of the input, output, and the way in which the AI output was reviewed on the part of the authors as supplementary files or additional information for the editor to review. The editor may ask for more information and/or for information to be added to the content for internal use and/or for publication.
An acknowledgements statement may be included at the end of the paper, detailing those who helped in carrying out the research but who have not been recognised as contributors, as well as for personal expressions of gratitude.
Because acknowledgment may imply endorsement by acknowledged individuals of a study’s data and conclusions, authors are strongly advised to obtain permission to be acknowledged from all acknowledged individuals before submitting to any BMJ journal.
A uthor name change requests
As an inclusive publisher, BMJ wishes to ensure a smooth process and experience to facilitate author name changes after publication. For more information on how to request an author name change in an existing publication see our corrections policies.
Last updated: March 2023
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Reumatologia
- v.58(6); 2020

Scientific authorship: a primer for researchers
Olena zimba.
1 Department of Internal Medicine No. 2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
Armen Yuri Gasparyan
2 Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, West Midlands, United Kingdom
The International Committee of Medical Journal Editors (ICMJE) proposed the authorship criteria which can be employed by medics and allied specialists. Scholars who substantively contribute to research and writing, revise, approve final drafts for target journal submissions, and take responsibility for all aspects of the work deserve authorship. Increasing awareness of the ICMJE criteria, incorporating related points in journal instructions, and enforcing them in daily practice may have positive impact for healthcare. Instances of inappropriate authorship are ethical transgressions which can be avoided by editors employing strategies of author profile evaluations. There are several platforms for recording author accomplishments which may improve the discoverability of scholarly works and prevent unethical conduct.
Most publishers advise authors to submit their Open Researcher and Contributor IDs (ORCID) at the manuscript submission. Other identifiers, such as Twitter handles, are also emerging as tools to stimulate post-publication communication and increase authors’ accountability for published articles.
Introduction
Scientific authorship is based on principles of contribution, responsibility, and credit [ 1 ]. Some experts additionally advocate for equity, diversity, and inclusion to avoid gender and country-based biases and endorse the concept of equal opportunities for all able contributors [ 2 ]. Improving awareness of authorship norms may add to the quality of research and prevent research and publication misconduct [ 3 ].
The issue of authorship is becoming more complex in the time of the COVID-19 pandemic and global crisis [ 4 , 5 ], affecting established research infrastructure and career opportunities and mandating switch to online collaboration. Early-career researchers are now facing challenges with motivation and involvement in influential studies [ 6 ]. Renewed initiatives are particularly warranted in research-intensive fields, such as rheumatology and immunology, to adapt to the new realities of cross-country and cross-disciplinary collaborations to re-evaluate anti-inflammatory drugs and antiviral vaccines. More attention to the issue of authorship in these fields is also required to ensure the crediting of those who meet certain authorship criteria and contribute to the science growth.
Authorship criteria
Defining authorship and employing strategies for verifying author qualifications distinguish ethical journals from substandard, or “predatory”, journals [ 7 ]. Responsible journal editors and publishers alike regularly update their instructions for employing mechanisms of reporting author roles and preventing instances of inappropriate authorship [ 8 ].
The absolute majority of medical, biomedical, and allied journals currently declare the adherence to the International Committee of Medical Journal Editors (ICMJE) authorship criteria, mandating substantive contribution (1), participation in the manuscript revision (2), its approval for submission to a target journal (3), and responsibility for all aspects of the work (4) [ 9 ]. The main requirements are to fulfil all four criteria to be credited as authors and acknowledge non-author contributions, such as language editing and other services offered by commercial editing agencies [ 10 ]. The agreement to take responsibility for all aspects of the scholarly work is perhaps the most critical and not entirely clear to some authors [ 11 ]. This criterion was introduced by the ICMJE in 2013 to curb the problems with inappropriate crediting for technical assistance, funding, language editing, statistical analyses, and other types of support in the absence of responsibility for all aspects of the work and (un)intentional wrongdoings [ 12 ]. Notably, technical (non-intellectual) contributions and financial support cannot justify an addition to the author bylines [ 13 ].
Biomedical journal editors, particularly those who process submissions from non-Anglophone countries and emerging professional societies, should implement mechanisms to increase the awareness of the ICMJE criteria and avoid instances of unethical authorship [ 14 ]. In this regard, an analysis of 296 names listed as authors of a top Iranian journal demonstrated that 37% of them did not meet the ICMJE criteria (“guest” authors) [ 15 ]. A large survey of physical medicine and rehabilitation specialists who published their research in the top specialist journals (n = 246) revealed that 45% of the respondents were unaware of the ICMJE authorship guidelines and 30% were unaware of what constitutes honorary authorship [ 16 ].
Ideally, author contributions, order, and responsibilities throughout the publishing and post-publication communication should be defined at the start of research [ 17 ]. Such an approach may prevent human errors and misleading ethics notes in published articles.
Non-medical journal editors may adapt and enforce the adherence to the ICMJE authorship criteria, crediting those with substantive contributions [ 18 ]. Alternatively, they may refer to the Council of Science Editors (CSE) definitions and procedures that aim to assign authorship to persons with “sufficient” scholarly contributions. The CSE also recommends to identify roles of all co-authors and inform readers of the same [ 19 ].
Finally, all stakeholders in science communication are advised to familiarize with the updated ethics statements of the Committee on Publication Ethics (COPE) that prioritize strategies to identify authors and their contributions and manage related disputes [ 20 ].
Creative ideas and authorship order
Although the current taxonomy of author contributions, which is employed by some publishers and journals, is sufficiently detailed and quantifiable [ 21 ], journal editors should not overlook the importance of immeasurable creative ideas. Traditionally, such ideas and overall intellectual input bring about the main credit in multi-authored research and review articles [ 22 ]. Ideas formulated as scientific hypotheses often result in solo-authored articles which are increasingly published in the time of the COVID-19 pandemic [ 23 , 24 ].
The sequence of author names on article bylines is often determined by established global and local crediting norms, level of contribution, and academic discipline [ 25 , 26 ]. Traditionally, the first authorship is reserved for senior research fellows, those who secure funds, or administrators in post-Socialist countries. The same actors are listed last in most developed and Western countries. The first and last places on the bylines are often viewed as the most important in the context of technical and conceptual contributions. The first and last author entries are often processed by grant funding agencies and bibliographic databases. One of the rational and globally acceptable approaches to the authorship order is based on listing author names in declining order, i.e. those with the greatest contributions are listed first and followed by those with declining contributions [ 25 , 27 , 28 ].
Journal editors often process manuscripts with co-first authors who may jointly benefit from their privileged place and claim their status during the academic promotion. Editorial policies of designating co-first authorship vary across journals since there are no universally acceptable instructions [ 29 ]. By properly denoting co-first authorship, editors may facilitate searches through online databases which should adjust their options to the growing trend in authorship [ 30 ].
Instances of inappropriate authorship
Inappropriate (honorary) authorship is a frequently reported publication ethics violation ( Table I ). In obvious cases of misconduct, individuals with minimal or no scholarly contributions are credited as authors. No any journal is immune to this transgression which is confounded by the unawareness of acceptable norms and inadequate editorial strategies. Numerous reports have pointed to honorary authorship even in articles of flagship journals due to corresponding authors’ irresponsible conduct [ 31 ].
Common instances of inappropriate authorship
A landmark survey involving 630 corresponding authors of high-impact general medical journals revealed the following descending prevalence of honorary authorship: 25% in original research reports, 15% in reviews, and 11.2% in editorials [ 32 ]. A recent large survey of 3859 scholars demonstrated that 74% of respondents had been involved in a study where someone was added as a co-author without substantive contribution (honorary authorship) and 34% had witnessed the opposite – not listing a co-author despite substantive contribution (ghost writing) [ 33 ]. And when asked about the widely publicized ICMJE authorship criteria, only 74% confirmed their awareness.
Honorary authorship may present in different forms. An extreme case of the violation involves non-professionals who lack any knowledge in the subject like kids of influential administrators who abuse their power and fabricate profiles of their family members [ 34 ]. Members of research groups and collaborating centers may be involved in the so-called gift authorship by unethically assigning authorship to colleagues and expecting a reciprocal attitude from them [ 1 ]. Senior researchers and administrators with impressive profiles can be listed as “guest” authors in an attempt to increase the chances of the manuscript acceptance and publication [ 1 ].
Finally, “paid” authorship is offered by some predatory journals that sell authorship to individuals who wish to pay and unethically boost their publication records [ 35 ]. Such a predatory practice has surfaced in the times of mass proliferation of open-access journals and commercial editing agencies that offer their services in violation of established ethical norms. Importantly, most articles with paid authorship turned to be fabricated and published with “fake” reviews [ 36 ].
Sophisticated forms of misconduct include instances of guest authorship and ghost writing. In such cases, eminent scientists appear as lead authors in articles drafted by industry-sponsored medical “ghost” writers who hide their identities. Related articles often violate authorship and conflicts of interest disclosure norms and contain misleading drug information that may have dire consequences for healthcare [ 37 , 38 ].
Finally, contributors with substantive input to research and writing may encounter the denial to be credited as authors [ 39 ]. The denial often affects junior research fellows and students who satisfy acceptable authorship criteria but judged unsuitable of scholarly credits [ 40 ]. Journal editors processing manuscripts from research environments where denial of authorship is possible due to subjective reasons, such as interpersonal conflicts and internal policies, should ask all listed authors to disclose their contributions and reassure that no-one deserving authorship credit has been denied such a privilege.
Author identifiers
Author identifiers and online profiling platforms play several important roles. One of the basic functions of such e-links is to distinguish scholars with identical names and help journal editors to increase visibility of scholarly contributions [ 41 ]. Assigning identifiers to non-Anglophone authors with variably spelled, misplaced, and missing names may prevent technical mistakes and avoid losing their article records in bibliographic databases [ 42 ]. In the era of digitization and open access, author identifiers are essential for making scholarly accomplishments more discoverable by cross-linking individual records with digital platforms, libraries, and editorial management systems [ 43 , 44 ].
Since its launch in 2012, Open Researcher and Contributor ID (ORCID; https://orcid.org/ ) has become a universal and multifunctional tool for increasing visibility of author profiles and their scholarly contributions. As of November 23, 2020, the number of the registered users is 10,091,759. Any scholar may freely register with the ORCID platform and maintain a persistent online profile for life. The main advantage of the platform is its ease of registration, safety and accuracy of data recording, and availability of options for listing author, reviewer, and editor accomplishments ( Table II ). ORCID IDs are now endorsed and integrated with numerous funding agencies, bibliographic databases and search platforms, reviewer crediting platform (Publons; http://publons.com/ ), and social media, enabling comprehensive evaluation of the ID holders’ background and current scientific interests. Journal editors employ these IDs to pick peer reviewers and display editorial board member activities [ 45 ]. Importantly, non-Anglophone scholars may benefit a lot from the ORCID initiative by increasing transparency and validity of their academic activities [ 46 ].
Some advantages and limitations of Open Researcher and Contributor ID (ORCID)
Perhaps the main limitation of the ORCID initiative is the unavailability of scholars’ photographs, video clips, interaction tools, and full-text repository, which are available on digital repositories and platforms for generating academic curriculum vitae such as ResearchGate ( https://www.researchgate.net/ ). Also, some registrants may avoid publicly sharing their information, limiting the use of their IDs for scholarly evaluation purposes.
Listing several IDs with publicly available information may partly overcome limitations of ORCID IDs. Relevant examples are Scopus Author ID, PubMed list of articles, and Publons profile with merged Web of Science entries, which are now widely employed by journal editors for displaying academic profiles of their board members.
There are also successful precedents of listing author Twitter handles alongside emails and ORCID IDs in their articles for post-publication interactions with readers and followers. One of the relevant examples is Academic Medicine where corresponding author Twitter handles are increasingly listed in the published articles. Given the global use of Twitter for scholarly communication and dissemination of journal updates [ 47 ], it is expected that author Twitter handles will soon appear in most peer-reviewed journals. Listing author Twitter handles may increase the responsibility for post-publication communication and overall accountability for published articles.
Conclusions
Improving awareness of ethical authorship and updating related journal instructions may help fairly credit individuals who deserve authorship. Perceptions of scholarly contributions and authorship criteria may vary across academic disciplines. However, adapting and adhering to the authorship recommendations of the ICMJE, CSE and COPE may curb numerous ethical problems.
Journal editors should employ strategies for evaluating author profiles and integrating their digital identifiers with published works. Authors, in turn, should familiarize with acceptable authorship criteria and fulfil their functions at pre- and post-publication stages. By adhering to authorship norms, editors and authors may prevent other forms of misconduct and ensure the integrity of published contents.
All are invited to watch the meeting on Facebook channel of the Reumatologia/Rheumatology journal, the official edition of the National Institute of Geriatrics, Rheumatology and Rehabilitation and Polish Society for Rheumatology at @ReumatologiaJ
https://www.facebook.com/ReumatologiaJ/
This article overviews authorship criteria, author identifiers and online profiling platforms, common instances of inappropriate authorship, and suggests options to solve related problems by authors, reviewers, and editors of scholarly journals. This is the first review in a series of articles devoted to scientific writing and editing. Related topics were discussed during the two-day online workshop titled “Good practices in the writing of scientific articles” organized under the patronage of the journal Reumatologia on 18 and 19 November 2020.
Editorial Team of Reumatologia
The authors declare no conflict of interest.
AFP Authors' Guide
American family physician ( afp ) is a monthly, editorially independent, peer-reviewed journal of the american academy of family physicians. afp ’s mission is to empower family physicians to improve the health of patients and communities as the leading source of medical information while advancing science and health equity. the circulation of afp ’s print journal is more than 175,000 and afp is consistently rated the number one journal among primary care physicians. afp editors seek original articles from experienced clinicians who write concise, evidence-based, authoritative clinical reviews to aid family physicians in patient care. submitted manuscripts must be original, not previously published, and not under consideration for publication by any other publication. articles demonstrating a family medicine perspective and an approach to common clinical conditions are particularly desirable., authors' guide - cme conflict of interest, article proposals.
Before beginning work on a manuscript, authors should submit an article proposal to [email protected] with the words “Article Proposal for AFP Editor” in the subject line. This allows tailoring of the topic to AFP ’s needs and prevents overlap with recently published content or articles in preparation.
Authors must demonstrate expertise in their area of interest or manuscript topic. The most experienced author should take the lead in evaluating the available evidence and writing the manuscript. Article bylines may include a maximum of three contributing authors. In our experience, allowing more than three authors leads to an uneven-quality paper.
Resident physicians must work with an experienced attending physician who serves as the first and corresponding author. Students can be coauthors for only the following departments: Diary of a Family Physician, FPIN’s Clinical Inquiries, FPIN’s Help Desk Answers, Letters to the Editor, Photo Quiz, Practice Guidelines, video submissions , and the AFP Community Blog. Clinical review articles, editorials, and other submissions with student authors are not considered for publication.
When submitting an article proposal, the lead author must complete an Author Credentialing form , and all authors must log into their AAFP account and complete a Conflict of Interest form . If authors do not have an AAFP account, they need to create one to access the Conflict of Interest form .
Note: To avoid bias or the perception of bias, our strict conflict of interest policy precludes us from considering manuscripts sponsored directly or indirectly by a pharmaceutical company, medical education company, or other commercial entity or ineligible company, or those written by an author who has a financial relationship with or interest in any commercial entity or ineligible company that may have an interest in the subject matter of the article within the previous 36 months or in the foreseeable future. This policy also includes serving on a commercial speaker's bureau or advisory board, or receiving commercial research support related to the subject matter of the article, among other relationships outlined in our conflict of interest policy .
Articles and Departments
Clinical review articles.
Submit articles to Sumi M. Sexton, MD, Editor-in-Chief, using AFP ’s Editorial Manager submission system . For instructions, see Submitting the Manuscript . Updated information about AFP ’s acceptance rates and timelines to publication is also available.
AFP articles are evidence-based clinical reviews. AFP focuses on clinical conditions and diseases that are encountered frequently by practicing family physicians, with an emphasis on diagnosis and treatment. Clinical reviews should be 1,500 to 1,800 words in length.
AFP does not publish original research articles. Although case reports are not featured as articles, brief case summaries may be submitted in the form of a Letter to the Editor (see also Curbside Consultation and Diary of a Family Physician ).
Authors are expected to reply to accepted Letters to the Editor about their article, especially any that question the science involved. Failure to do so disqualifies an author from future publication in AFP .
Curbside Consultation
Curbside Consultation is a feature that addresses legal, psychological, and ethical issues that physicians may encounter in their day-to-day practice. Each piece contains a case scenario with a clinical question, a commentary section, and a resolution of the scenario. Examples are available in the Curbside Consultation department collection .
Authors may submit a topic proposal to Caroline Wellbery, MD, Associate Deputy Editor at [email protected] . If the topic is approved, the author should prepare a brief scenario that describes the challenge to resolve, ending with a clinical question. Although the case is hypothetical, it should be presented as a query to a consultant. The commentary then addresses the pertinent issues presented in the case and should be limited to approximately 1,000 words. The piece should conclude with a resolution of the case scenario, which can include actionable advice for the family physician. One to two small tables (if warranted) can be added, and up to 15 references can be used.
Diary of a Family Physician
The goal of Diary of a Family Physician is to share experiences and foster a sense of camaraderie among readers. We are looking for stories that provide a real-life flavor of day-to-day practice. These may reflect the broad range of experience in family medicine and diverse clinical settings (e.g., solo or group practice, rural, urban, hospitals, nursing homes, emergency department/urgent care, house calls, telehealth, residency programs, community health centers, universities). Submissions should be sent to [email protected] . Examples are available in the Diary of a Family Physician department collection .
Content may focus on the joys and challenges of everyday family practice, clinical treatment tips, personal experiences with patients and their families, or diagnostic puzzles that can be addressed adequately in the space provided.
Diary submissions should be 150 to 300 words. Six to seven entries should be provided in a submission (representing different hours or patient encounters on a busy day in family medicine with the inclusion of an evening hour if appropriate to describe a longer day or interesting personal or family event). Avoid providing information that might allow anyone to identify a patient. Avoid stories that promote a specific commercial product or political opinions. Diverse perspectives are welcome, but the focus should be on the clinical experience. First person is preferred when referring to yourself, but third person may be acceptable in certain cases, such as when referring to the patient.
Most editorials in AFP are solicited by the editors; however, freestanding editorials are occasionally accepted. Editorials should range from 500 to 800 words in length and may include six to 12 references. Submit editorials to [email protected] with the subject heading "Editorial."
Letters to the Editor
Letters to the editor are published in most issues of AFP in print or online only. Online only letters are listed in the print table of contents. Authors may comment on a previously published article or submit a freestanding letter on an important clinical topic. Letters must be submitted in a Word document and be less than 400 words, with a limit of one table or figure, six or fewer references, and no more than three authors. If the letter is in response to a previously published article, that article should be listed as one of the references. Letters must not be submitted to any other publication. Possible conflicts of interest must be disclosed at the time of submission. All authors must sign an Author Statement form and log into their AAFP account to complete a Conflict of Interest form . If authors do not have an AAFP account, they need to create one to access the Conflict of Interest form . These forms should be completed and submitted with the Letter to the Editor submission. If an author of an accepted letter has a conflict of interest that is not deemed to be disqualifying, the editor will add a disclosure statement at the end of the letter. Submission of a letter grants the AAFP permission to publish the letter in any of its publications in any form. Accepted letters are edited to meet style and space requirements. Send letters to Kenny Lin, MD, Deputy Editor of AFP at [email protected] .
Photo Quiz presents readers with a clinical challenge based on a photograph or other figure. Submissions should conform to AFP guidelines. Send submissions to [email protected] . Please note that we receive many submissions each month and it can take six to eight weeks for a decision about acceptance.
Photo Quizzes should be original and not published or under consideration for publication elsewhere. Articles that demonstrate a family medicine perspective on and approach to a common clinical condition are particularly desirable.
Authorship:
- The first/corresponding author must be an experienced physician and preferably the same author. Residents and medical students may be coauthors.
- All authors must sign and submit an author statement form .
- All authors must log into their AAFP account and complete a Conflict of Interest form , with any financial relationship or interest disclosed in a separate email to [email protected] . If authors do not have an AAFP account, they need to create one to access the Conflict of Interest form . The AAFP requires all authors to disclose any commercial association that might pose a conflict of interest in connection with the submitted manuscript. View more information about what constitutes a conflict of interest .
- Authors should submit original color photographs, slides, radiographs, or digital images that conform to the illustration guidelines outlined in "Figures" under Preparation of the Manuscript .
- Figures should be original images. Do not obtain images from textbooks, journals, the Internet, etc. Acceptance of your Photo Quiz constitutes transfer of copyright.
- Images must be in focus and clearly show the feature you describe for readers.
- If you add wording, arrows, etc., also provide a clean image for our production department to work with.
- Each figure should be submitted as a separate digital file, not embedded in a Word document.
- Photographs in which a patient is identifiable (i.e., the patient would be able to identify themselves) must be accompanied by a signed Consent for Publication form granting AFP permission to publish the photo. Please note that obscuring the eyes does not provide adequate anonymity.
The text of your Photo Quiz should include the following elements: 1) title, 2) introduction paragraph presenting the clinical scenario, 3) a question with one correct and three or four incorrect answers, 4) discussion of correct answer, 5) brief explanation of incorrect answers, and 6) a summary table. The following are specific instructions for each element ( view PQ template ):
- The Photo Quiz department intends to help our readers improve their clinical skills through learning about common clinical conditions with visual components. The primary avenues for this are images (e.g., a skin rash), radiographs, sonograms, and ECGs.
- Photo Quiz is not a case report. It uses case-based teaching to illustrate and educate on a common clinical topic. Two primary criteria for publishing a Photo Quiz are 1) the problem is commonly seen by practicing family physicians and 2) the image shows a typical example. Thus, we prefer images that show typical pathology or common variants rather than “once-in-a-lifetime” cases.
- The title should hint at the diagnosis without giving it away.
- The introduction paragraph presents the scenario that goes with the image. Include clinical information that would logically be included for the presenting complaint.
- Reader chooses the correct diagnosis: “Based on the patient’s history and physical examination findings, which one of the following is the most likely diagnosis?"
- Your answer choices can include other physical findings, laboratory values, typical pathology, appropriate treatment, typical clinical course, appropriate treatment setting, etc.: “Based on the patient’s history and physical examination findings, which one of the following (physical finding, laboratory value, etc.) is most likely?”
- Reader chooses the appropriate treatment for the condition: “Based on the patient’s history and physical examination findings, which one of the following treatment options is most appropriate?”
- Please provide one correct and three or four incorrect answer choices . Answers should come from an appropriate differential diagnosis for the condition you present.
- The discussion of the correct diagnosis should cover important key features of the diagnosis, including defining features, epidemiology, and clinical findings. Begin with a short explanation of why the photo make the diagnosis correct. Please limit your discussion to 300–500 words.
- Follow your discussion with brief (one to two sentences) explanations of each incorrect answer, describing why they are incorrect or not typical of the photo.
- Create a Summary Table listing your answer choices and key characteristics of each (see PQ template ). Make sure the information in the table is consistent with what you present in the main text.
Preparation of the Manuscript
Please review this checklist carefully before submitting your manuscript to ensure it includes all required components and conforms with AFP style. This checklist is required and will be uploaded with your manuscript.
- Literature Search and Data Sources
In a short paragraph, please succinctly describe your search strategy, the key word(s) used, the date(s) of the search, and the data sources you accessed in identifying the highest-quality evidence on your topic. By "data sources," we mean sources such as Cochrane reviews and Agency for Healthcare Research and Quality, in addition to a PubMed search using the Clinical Query function ( https://www.ncbi.nlm.nih.gov ). Do not list the actual references you found in each source; simply include them in your bibliography. We strongly recommend that you search the following freely accessible evidence-based sources of information and also consult our EBM Toolkit .
As part of your paragraph, please also include a statement about the use of individual characteristic classification variables (e.g., race, gender, sexual orientation, etc.), if applicable. Please see the sample paragraph below.
If studies used individual characteristic classification variables in analyses or models and you choose to include this information in your manuscript, your manuscript should clearly state the following: (1) what the variables are truly measuring (i.e., is self-identified race acting as an indicator of systemic racism?); (2) what hypothesis or research question justifies their inclusion; and (3) how the variables were defined/identified in the included studies.
If you believe that there are studies that have important information even though there were questions about how individual characteristic classification variables were identified, please include a statement to the effect in your manuscript. As an example of the limitations inherent in this process, we acknowledge that many studies more than a few years old offered participants only the binary male/female option for gender identify. A brief statement that acknowledges this issue when that study is utilized is acceptable.
The AFP Diversity, Equity, and Inclusion Committee is happy to help with issues like this. Please contact the editor assigned to your manuscript with questions about how to review and evaluate this information in a particular study.
Some examples of how two individual characteristic classification variables, in this case race and gender, are not clearly identified or are identified inappropriately include, but are not limited to, the following:
- Race and gender are key demographics leading to specific outcome recommendations, yet there is no mention in the study about how race and gender were defined.
- The study investigators assigned race/ethnicity or gender based on the patient's appearance, the patient's first or last name, or other subjective assessment.
- Authors make overarching statements about entire ethnicities or races in their conclusions based on country location of the study.
- Authors offered only male/female as binary options for gender and made overarching assumptions about other demographic groups that are nonbinary.
Sample Data Sources paragraph: Data Sources: A PubMed search was completed in Clinical Queries using the key terms gout and hyperuricemia. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Agency for Healthcare Research and Quality Effective Healthcare Reports, the Cochrane database, DynaMed, and Essential Evidence Plus were also searched. We critically reviewed studies that used patient categories such as race and/or gender but did not define how these categories were assigned, stating their limitations in the text. Search date: November 18, 2022.
Websites for Sources of Evidence-Based Clinical Information:
Free Access Sites
McMaster University’s compendium of pre-appraised evidence to support clinical decisions. Content is presented in a hierarchical way, with the highest level of available evidence listed first.
Agency for Healthcare Research and Quality In particular, see AHRQ’s Effective Healthcare Reports on various clinical topics. Note: Many of these reports are published in AFP under the Implementing AHRQ Effective Health Care Reviews department collection.
Cochrane Database of Systematic Reviews Free for abstracts only, which in most cases provide the key findings of interest. The complete review requires a subscription. The Cochrane database contains systematic reviews of narrowly focused clinical questions (e.g., “Colchicine for treating acute gout attacks”) as opposed to broad, general reviews of topics (e.g., “Management of an acute gout attack”). Note: AFP publishes summaries of Cochrane abstracts in Cochrane for Clinicians .
ECRI Guidelines Trust
Repository of evidence-based clinical practice guidelines, appraised using the National Academy of Medicine’s Standards for Trustworthy Clinical Practice Guidelines.
National Center for Complementary and Integrative Health Although NCCIH has been criticized for political interference and questionable science, we include it in this list because a few comprehensive sources of information in this field are freely available.
Trip (Turning Research Into Practice) Contains links to a wide range of journal articles, medical organization clinical guidelines, online medical references, and other sources. A limited version is freely available; additional content requires an annual subscription.
U.S. Preventive Services Task Force Premier source of evidence-based, graded recommendations for clinical preventive services. Note: AFP publishes Recommendations and Reports from the USPSTF as well as CME case studies in the Putting Prevention Into Practice series.
Subscription Required Sites Most of these are point-of-care clinical information and decision support tools. These sites provide important background information, but authors should review the primary source to use as a citation for the article.
Essential Evidence Plus Includes POEMS (collections of patient-oriented evidence that matters).
Natural Medicines Comprehensive Database Reviews of the use of natural medicines in the treatment of various diseases.
Natural Medicines Database of dietary supplements, natural medicines, and complementary, alternative, and integrative therapies.
UpToDate To ensure adequate searching on your topic, we strongly recommend that the above sources be reviewed, in addition to a PubMed search using the Clinical Query function . This is done by using the provided link, or by going to the PubMed home page, and selecting “Clinical queries” from the lower center of the screen.
Manuscript Format
Manuscripts formatted to conform to the “ Uniform requirements for manuscripts submitted to biomedical journals ” 1 are acceptable for submission. AFP endorses these guidelines. Format the manuscript with margins of 1 inch on all sides. Double-space the entire manuscript, including components, and arrange the manuscript in the following sequence, with each section beginning on a new page:
- Tables, including an Evidence Table (each table begins on a new page)
Acknowledgments
- Biographic sketch
The title page, abstract, text, references, and tables should be contained in a single word processor document, ideally in Microsoft Word (DOC) or Rich Text Format (RTF). The acknowledgments and the biographic sketch should be in a separate file in order to accommodate blinded peer review. Each figure should be submitted as a separate computer file as described in this document under Figures.
Number pages consecutively in the upper right-hand corner, beginning with the title page. To accommodate blinded peer review, place the names of authors only in the biographical sketch for each author. See the section on Submitting the Manuscript for details on how to submit your manuscript to AFP .
Please carefully review the detailed instructions for each section that follows:
This page should contain only the title of the manuscript and the word counts (word count for the entire manuscript and word count for text only--excluding abstract, references, tables, figures). This is to facilitate blinded peer review of the manuscript in the Editorial Manager system. Pertinent information such as names of authors, institutional affiliation, and contact information is entered into Editorial Manager by the authors and will be placed in a pre-formatted cover sheet for editorial use.
Sources of support in the form of grants, equipment, or drugs should be mentioned in the Conflict of Interest Form ; this information will be included as a footnote to the article. For details, see the Conflict of Interest section .
Include an abstract of 150 to 250 words, depending on the length of the text. The abstract should provide factual and specific (rather than general and nonspecific) information summarizing the main points of the manuscript. For example, instead of saying, “This article will describe the differential diagnosis of chest pain in adolescents,” say, “The most common causes of chest pain in adolescents include musculoskeletal strain, hyperventilation syndrome, and anxiety.” For clinical reviews, highlight key points in the diagnosis and treatment of the condition discussed.
Article length should be 1,500 to 1,800 words (maximum 2,000 words, about six to eight manuscript pages of text), not including the title page, abstract, tables, reference list, etc. Manuscripts of more than 2,000 words of text are rarely accepted.
Do not include a summary or conclusion section in your manuscript; anything that you would ordinarily put in such a section should go into the abstract.
Provide appropriate reference citations to support key clinical recommendations, statistical information, reports of previous studies, controversial statements, etc. Use the following guidelines in choosing references:
- Avoid citing other clinical review articles—you should emphasize original research articles, systematic reviews, Cochrane Library reviews, citations from BMJ’s Clinical Evidence , validated clinical decision rules, randomized trials, and evidence-based practice guidelines where possible. Clinical review articles may be cited as sources for tables, figures, or general background information.
- Emphasize recent references (within the past 10 years); in general, avoid letters to the editor, editorials, and references that are older than 10 years or of historic interest only.
- Avoid references from obscure or non–English-language journals.
- Do not cite abstracts, unpublished observations, manuscripts in preparation or submitted for publication, or personal communications.
- To avoid plagiarism, do not to use the language, content, or concepts of another source without an appropriate reference. Do not use extensive verbatim or near-verbatim portions of text from another source, even with appropriate citation.
Begin the writing process by identifying key clinical questions and controversies related to your topic, and then answer them with the best available evidence. Do not write the article and then find selected references to support your opinions!
Please number references in the text in the order of citation. Use double-line spacing in your reference list; arrange references numerically, not alphabetically. Do NOT use "Endnotes" or any other automated reference function in any word processor.
Titles of journals should be abbreviated according to the style used in PubMed . If there are six or fewer authors, list them all; if there are more than six, list the first three followed by "et al." Please note that no periods are used after the authors’ initials. Include beginning and ending page numbers for journal and book references. The average number of references for a full-length article ranges from 30 to 40. Most articles will not exceed 50 references.
References first cited in tables or figure legends must be numbered to remain in sequence with references cited in the text. Note the following examples of reference style:
Standard Journal Article
- Weiss BD. Nonpharmacologic treatment of urinary incontinence. Am Fam Physician . 1991;44(2):579-586.
- Gold D, Bowden R, Sixbey J, et al. Chronic fatigue. A prospective clinical and virologic study. JAMA. 1990;264:48-53.
Chapter in a Book
- Murray JL. Care of the elderly. In: Taylor RB, ed. Family Medicine: Principles and Practice . 3rd ed. New York, NY: Springer-Verlag, 1988:521-532.
- BMJ Publishing Group. Clinical evidence on tinnitus. Accessed November 12, 2013. http://www.clinicalevidence.com
Authors should maximize the educational value of tables. Give complete reference data for each item in a table. For all tables that are borrowed or adapted from other sources, include scanned photocopies of the tables as they appeared in the original source, making sure that complete reference data are included for the original source.
Do NOT attempt to obtain reprint permission from the original publisher. AFP will seek permission from the copyright owner to publish the material in print and other formats. However, it is possible that the rightsholder will not grant permission for use of copyrighted materials, and AFP reserves the right to withhold tables or figures from print and other formats based on the rightsholder’s terms. Due to the increasing difficulty with obtaining permission to adapt previously published material, we strongly encourage authors to create original tables and figures. See Tips for Creating Original Tables and Algorithms and How to Create an Algorithm in Word . Note that tables and figures that were previously published, and for which we obtain reprint or adaptation permission, must be removed from AFP articles sent to content aggregators, such as EBSCO and MDConsult.
Tables should be interpretable without reference to the text. Each table should have a title and be numbered sequentially with Arabic numerals. Put each table with double-line spacing on a separate page. Use the “Tables” function of your word processor to create the table rather than just using spaces and tabs (which quickly get out of alignment as the manuscript is transferred into other computer formats). In general, make tables part of your main document rather than creating a separate file for each table.
For tables that include drug pricing, please use information from GoodRx to find representative undiscounted prices, not using GoodRx coupons.
Clinical Decision Tools: Please mention and include links to relevant clinical decision tools and calculators, especially those published in AFP ’s Point-of-Care Guides and on the medical calculator website MDCalc .
Costs of laboratory tests, imaging studies, and clinical procedures: Where relevant, please include approximate, representative costs for tests or procedures, especially in tables where comparative cost is discussed. We use Healthcare Bluebook as the source for pricing when possible: https://www.healthcarebluebook.com . If you use another source, please provide a complete citation for it.
SORT: Evidence Table of Key Clinical Recommendations
We would like each article to include an Evidence Table (also called “SORT” or “Strength of Recommendations Table”). This table will help readers understand the main points of your article, and the strength of evidence that supports its recommendations. The table should contain the key clinical recommendations and strength of recommendation ratings for your article as shown in the sample below:
The SORT table is intended to highlight the most important three to seven recommendations from your article for clinicians. Each recommendation must be accompanied by a SORT rating of A, B, or C . Your recommendations should emphasize interventions and approaches that improve patient-oriented outcomes (e.g. morbidity, mortality, quality of life) over disease-oriented evidence (e.g. biomarkers, surrogate endpoints).
- You should have three to seven recommendations. Try to identify a range of recommendations, for example, one each about screening, prevention, diagnosis, and two about treatment.
- Each statement should be in the form of a recommendation and should not just present a fact or piece of medical trivia. For example, “Use the Wells score to determine the risk of DVT in patients with leg pain” is a recommendation, while “Of patients presenting with leg pain, 16% have a DVT” is not.
- An “A” recommendation should be based on consistent evidence of improved patient-oriented outcomes from well-designed studies. Use clear, directive language as this is a recommendation that should be applied to most patients, such as “Patients age 50 to 74 years should receive screening for colorectal cancer."
- A “B” recommendation is based on lower quality evidence of improved patient-oriented outcomes or inconsistent evidence. These statements should use language such as “Consider…” or “…is a practice option” or “…may be effective.”
- A “C” recommendation is often something that is standard of care, but for which there have been no clinical trials or trials have only reported disease-oriented outcomes. In this case, the recommendation statement should reflect the strength of recommendation, and the “Comment” column should clarify that this is a recommendation “based on expert opinion in the absence of clinical trials” or “based on evidence from clinical trials with blood pressure reduction as the outcome.”
If you are not comfortable assigning the strength of recommendation (below), our medical editors will do that for you.
To rate the strength of evidence supporting key clinical recommendations, please use the following guidelines:
Use the table below to determine whether a study measuring patient-oriented outcomes is of good or limited quality, and whether the results are consistent or inconsistent between studies:
* High quality diagnostic cohort study: cohort design, adequate size, adequate spectrum of patients, blinding, and a consistent, well-defined reference standard. + High quality RCT: allocation concealed, blinding if possible, intention-to-treat analysis, adequate statistical power, adequate follow-up (> 80%). ++ An all-or-none study is one where the treatment causes a dramatic change in outcomes, such as antibiotics for meningitis or surgery for appendicitis, which precludes study in a controlled trial.
Please use the following algorithm for determining the strength of a recommendation based on a body of evidence (applies to clinical recommendations regarding diagnosis, treatment, prevention, or screening). Although this provides a general guideline, authors and editors should adjust the strength of recommendation based on the benefits, harms, and costs of the intervention being recommended. Again, if you are unsure how to apply these ratings, the medical editors will do this for you. At a minimum, you should create a summary table with recommendations and references for each recommendation.
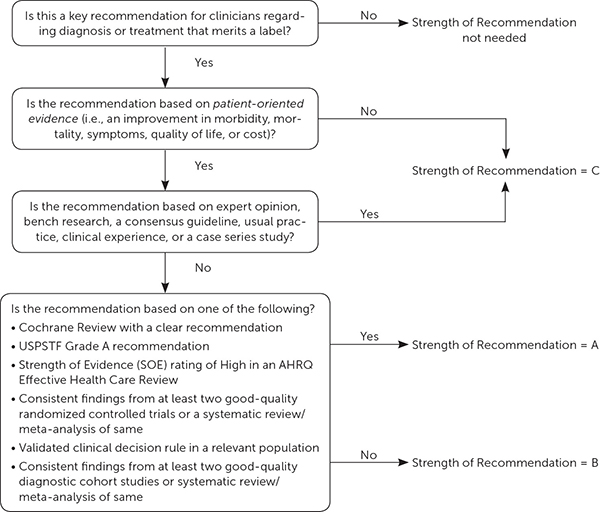
For more information on how to apply these ratings, please see the explanatory article published in the February 1, 2004, issue of American Family Physician .
AFP encourages the submission of original figures that clarify the text. The term "figures" refers to illustrations, photographs, radiographs, scans, sonograms, diagrams, graphs, flow charts, algorithms, etc. AFP requires authors to transfer copyright ownership of original figures to the AAFP. For all figures that are borrowed or adapted from other sources, include scanned photocopies of the figures as they appeared in the original source, making sure that complete reference data for the original source are included. Clearly identify figures that have not been previously published and are supplied by a person other than the author and include complete contact information for the owner of the material. For figures supplied by your institution or a colleague, clearly indicate whether that institution/person is retaining copyright (in which case we will need to contact them) or whether copyright is being transferred to AFP with the article.
Do NOT attempt to obtain reprint permission from the original publisher. AFP will seek permission from the copyright owner to publish the material in print and other formats. However, it is possible that the rightsholder will not grant permission for use of copyrighted materials, and AFP reserves the right to withhold tables or figures from print and other formats based on the rightsholder’s terms. Due to the increasing difficulty with obtaining permission to adapt previously published material, we strongly encourage authors to create original tables and figures. See Tips for Creating Original Tables and Algorithms and How to Create an Algorithm in Word . Note that tables and figures that were previously published, and for which we obtain reprint or adaptation permission, must be removed from AFP articles sent to content aggregators, such as EBSCO and MDConsult.
Each figure should be submitted as a separate digital file and numbered sequentially as it appears in the text. Diagnostic images (e.g., ECGs, sonograms, radiographs), artwork, line drawings, and nondigital photographs should be scanned at a resolution of at least 600 DPI before submission and saved as TIFF files. Only the following file formats are acceptable; others will be returned to the author for reformatting and resubmission.
- TIFF (Tagged Image File Format)
- PNG (Portable Network Graphic)
- JPG (only high-resolution images of at least 300 pixels or dots per inch [ppi or dpi])
- Word (acceptable ONLY for tables or algorithms; NOT acceptable for imported images)
- PowerPoint (acceptable ONLY for tables or algorithms; NOT acceptable for imported images)
Image resolution is typically measured in pixels per inch, or ppi (some use the term "dots per inch," or dpi). The image’s resolution and its dimensions determine the overall file size of the image, as well as the quality of the output. Images with a resolution of 72 ppi (28.35 pixels per cm) are adequate for materials posted on the Web; however, this resolution is inadequate for print media. If your file size is less than 200 kb, it is almost certainly of too low a resolution for a print journal. For color and grayscale images of 3 to 5 inches, we recommend a resolution of 300 ppi (118.11 pixels per cm). Line drawings in black and white require a higher resolution of 600 ppi (236.22 pixels per cm). An image generated by a digital camera as a 72 ppi JPEG file may still be acceptable if it measures at least 14 inches wide or high.
We strongly prefer original photographs/images because images downloaded from websites or taken from other publications rarely reproduce well, even if we are able to obtain permission to reprint them.
Because the quality of original illustration varies, it may be necessary to have the art you supply redrawn to meet AFP ’s artistic standards. AFP ’s art department is available to assist authors in the creation of original, high-quality artwork to illustrate manuscripts accepted for publication. This service is provided at no charge to authors but is subject to editorial judgment.
Other guidelines for artwork include the following:
- Symbols, lettering, and arrows in figures should be clearly marked and large enough to remain legible if the size of the illustration is reduced for publication.
- Photographs in which a patient is identifiable MUST be accompanied by the patient’s written permission for publication . "Identifiable" means that the person in the photo (or a parent of a child subject) could reasonably be expected to recognize himself/herself. A bar obscuring the eyes does not provide adequate anonymity and is not acceptable [see NEJM , August 24, 1989, p. 550].
- Because of the poor quality inherent in reproducing previously published images, photographs and radiographic images from textbooks and journals cannot be reproduced in AFP , regardless of whether permission has been obtained from the publisher.
- Do not save images within a Microsoft Word or PowerPoint document or use the “Drawing” features of your word processor.
- The legends for each figure should be typed with double-line spacing and combined on a separate page at the end of the manuscript.
If you are submitting figures in digital format, save each figure as a separate file. Each file should be saved with a name that includes the AFP manuscript number and figure number as referenced in the manuscript. Files should be uploaded at the time of manuscript submission in AFP's Editorial Manager site and clearly labeled.
Acceptance of a manuscript for publication is contingent on provision of artwork that meets the above specifications. If you have any questions about the preparation of art or digital images for your manuscript, contact Dave Klemm, AFP Medical Art Coordinator, at [email protected] . After your manuscript has been accepted for publication, address questions about art to the medical editor.
You may acknowledge professional help in the preparation or review of your manuscript. Written permission is required to publish the names of persons acknowledged. See Author Statements Form .
Biographic Sketch
Please complete a brief Biographic Sketch form for each author, which includes information on medical training, current position, and academic appointments.
Author's Guide References
- International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. http://www.icmje.org/index.html . Accessed June 17, 2010.
AFP Style Guidelines
Headings. Use ALL CAPITALS to indicate major sections of the manuscript , and Initial Capitals for subsections.
SI Units. Include SI units in parentheses after conventional units (https://academic.oup.com/amamanualofstyle/si-conversion-calculator).
Measurements. Do not include periods after metric measurements.
Numbers. Spell out numbers one through nine. Use numerals for 10 and greater. Exceptions: always use numerals in dosages, percentages, degrees of temperature, and metric measurements.
Drug names. Use the generic name for all drugs. Include the trade name in parentheses after the first mention of a drug in the text. Trade names used in AFP are typically the first brand that was approved. If a drug is not available in the United States, indicate so in parentheses after the drug name.
Abbreviations. Except for units of measurement, abbreviations are discouraged. If used, an abbreviation should be preceded by the words for which it stands. For example, computed tomography (CT). This need only be done on the first use, not throughout the manuscript.
Percentages. Use the percent sign (%) rather than the word “percent.”
Style questions. For questions about medical writing style, consult the American Medical Association Manual of Style . 1
Formatting text. Do not justify the right margin; do not use bold print or italics; use a single, standard 12-point typeface (e.g., Times New Roman, Arial); and double-space the entire manuscript.
- American Medical Association. AMA Manual of Style: A Guide for Authors and Editors . 11th ed. Oxford University Press, 2020.
Submitting the Manuscript
Please submit the manuscript as a Microsoft Word document (DOC or DOCX) via AFP ’s Editorial Manager system .
View instructions for submitting the article in Editorial Manager . These instructions show how to create a login and password for first-time users and explain each stage of the submission process in detail.
Please see the Figures section for guidelines on file formats for artwork and photographs. Do not save images within the Microsoft Word or PowerPoint document or use the “Drawing” features of Word.
Author Statements
Submitted manuscripts must be accompanied by an Author Statements form signed by all authors. This form includes an authorship statement, a copyright transfer statement or statement of federal employment, and an acknowledgment statement. Upload your signed form to AFP ’s Editorial Manager system at the time of manuscript submission.
Copyright Transfer
Each author who was not an employee of the U.S. federal government when this manuscript was prepared must complete the copyright assignment statement in the Author Statements Form, which assigns, transfers, and conveys all rights, title, and interest in the work and its accompanying original tables and figures, including copyright ownership, to the AAFP in the event that this work is published by the AAFP. All accepted manuscripts become the permanent property of the AAFP and may not be published elsewhere without written permission from the AAFP.
Federal Employment
Each author who was an employee of the U.S. federal government when this manuscript was prepared must sign the statement of federal employment in the Author Statements Form , which indicates that the work is not protected by the Copyright Act and there is no copyright of which ownership can be transferred.
Acknowledgment
The corresponding author must sign the acknowledgment statement in the Author Statements Form , which affirms that all persons who have made substantial contributions to the work reported in the manuscript but who are not authors are named in the Acknowledgment section of the manuscript and have given their written permission to be named.
Artificial Intelligence Technologies
Artificial intelligence technologies (AI), machine learning, and similar technologies, including chatbots like ChatGPT (Chat Generative Pretrained Transformer), have been used in the drafting of scientific manuscripts. Authors who choose to use this technology must disclose at the time of manuscript submission their use of AI, the type of AI utilized, and how they used AI. In addition, when incorporating AI-generated statements, authors must provide correct references in the established literature for all AI-generated items to ensure accuracy and appropriate attribution. AI technology does not qualify for authorship credit, since it cannot guarantee the veracity of the language generated, but its use must be acknowledged in the manuscript.
Conflict of Interest Policy
The AAFP requires all authors to disclose any commercial association that might pose a conflict of interest in connection with the submitted manuscript. All authors must log into their AAFP account and complete a Conflict of Interest form , with any financial relationship or interest disclosed in a separate email to [email protected] . If authors do not have an AAFP account, they need to create one to access the Conflict of Interest form .
To avoid bias or the perception of bias, AFP will not consider manuscripts sponsored directly or indirectly by a pharmaceutical company, medical education company, or other commercial entity or ineligible company* ( as defined by the Accreditation Council for Continuing Medical Education [ACCME] ) or those written by an author who has or whose spouse/partner has a financial relationship with or interest in any commercial entity or ineligible company that may have an interest in the subject matter of the article within the previous 36 months or in the foreseeable future. This policy includes, but is not limited to, the following relationships/interests:
- Consultant or Advisory Board
- Manuscript preparation assistance
- Partnership
- Receipt of equipment or supplies
- Research grants or support
- Speakers’ Bureaus
- Stock/Bond holdings (excluding mutual funds)
- Other financial support
- Other personal or professional relationships
To avoid writing an article that we will not be able to consider, please contact us first with any questions about this policy. Manuscripts without signed Conflict of Interest forms from all authors will not be considered for peer review.
All funding sources supporting a manuscript should be acknowledged on the title page. The editorial staff may inquire further about financial disclosure after submission. If accepted for publication, any nondisqualifying financial disclosure or potential conflict of interest will be acknowledged at the end of the manuscript text. More information on our editorial policies can be found in this editorial .
Note: If you develop, or your spouse/partner develops, new financial relationships with or interests in a relevant commercial entity or ineligible company after initially completing the Conflict of Interest form but before publication, please let us know and complete an updated form immediately. Any such changes may preclude your article from publication.
*“Commercial entities” include pharmaceutical companies, medical education companies, or other entities producing, marketing, re-selling, or distributing health care goods or services to patients or health care professionals. The ACCME uses a similar definition for "ineligible company" with additional examples.
Advocacy Bias Policy
Certain medical topics are more controversial than others, and therefore run the risk of attracting authors who have strong advocacy positions on the topic, leading to what could be called “advocacy bias.” In this context, “advocacy bias” refers to a strong preference for a position borne out of something other than scientific controversy (i.e., the science is conflicting, and one may reasonably argue for one approach over another), but primarily based on religious, political, social, or other non-medical considerations. In general, we prefer relatively neutral authors who can cover topics in a way that is less subject to this type of bias.
For topics at risk of advocacy bias, authors will be carefully vetted to ensure a relatively neutral presentation, and we may exclude those who we deem to have an undue degree of advocacy bias. However, there are instances in which it is acceptable and even desirable to have “advocacy” authors write for AFP . These include presentations where we specifically address various advocacy viewpoints, such as our series of pro/con editorials, “Controversies in Family Medicine,” or Curbside Consultation. Letters to the Editor are another venue where advocacy authors can be given a platform. All instances are subject to editorial review and approval, as is the case with all our content. More information regarding controversial topics in family medicine and AFP’s approach to such topics can be found in these editorials:
Editorials: Ethics, Education, and American Family Physician
Editorials: Controversial Topics in Family Medicine and Our Duty to Engage
Manuscript Processing and Review
Acknowledgment and manuscript number.
When the manuscript is initially submitted, the corresponding author will receive an automated email from the Editorial Manager system with a notification that the manuscript has been received. After it has been processed by editorial staff, the corresponding author will receive an automated email from Editorial Manager that contains the manuscript number. Please refer to this number in any communication about the manuscript. Details about timelines of the various stages of review can be found within an updated report of AFP 's acceptance rates .
Peer Review
Manuscripts are initially reviewed by the editors for suitability and adherence to the guidelines. Acceptable manuscripts are reviewed by at least one family physician and one expert in the subject discussed. The latter may be a family physician or a subspecialist in another discipline.
Editorial Decision
A decision about acceptance, revision, or rejection is sent to the corresponding author, generally within eight to 12 weeks of receipt of the manuscript. All acceptable manuscripts require some revision based on reviewers' comments and medical editor guidance. Instructions, showing how to handle each comment, and a sample response letter are provided in the Authors' Responses to Reviews .
Return of Manuscripts and Illustrations
If submitted, hard copies of original artwork, photographs, slides, and other illustrations will automatically be returned if the manuscript is not accepted for publication. For manuscripts accepted for publication, any hard copies of illustrations submitted will be returned after the article is published.
Manuscript Editing
When a manuscript is accepted, it will be edited to conform to AFP ’s style as well as to improve its educational value. An edited manuscript will be sent to the corresponding author for review before publication. Authors are responsible for all statements made in their work, including any changes made by the editors and authorized by the corresponding author.
Professionalism
We want to emphasize the importance of professionalism when interacting with AFP staff members and medical editors. This includes, but is not limited to, considerate and respectful communication, as well as being responsive to queries from our editors and replying to these queries in a timely manner. We take many factors into consideration when deciding whether to invite authors to write for AFP again in the future. Although our top priority is producing high-quality content for our readers, the failure of any author to comply with these requests of professionalism may result in not being invited to write for AFP again.
Videos help expand the knowledge and expertise of AFP ’s readers. Videos can be freestanding or accompany clinical review articles. All videos are peer-reviewed by at least two medical editors and, if accepted, are published on the AFP YouTube channel . Videos are promoted on the AFP 's Video Collection Page , which receives 2.5 million unique visitors each month and 31 million unique visitors each year, including from AAFP members.
We are seeking original, high-quality, concise, clinically impactful videos that teach office procedures, physical examination skills, and clinical findings. We consider existing videos and welcome proposals for videos on new topics. The first author of the video must be an experienced physician. Residents and medical students may be coauthors. We cannot provide financial support or equipment.
Seek approval for your submission by sending a completed video proposal form to Chris Bunt, MD, at [email protected] .
Once approved, submit your video to [email protected] using a common, cloud-based service (e.g., Google Drive, OneDrive). Include a Word document containing the video title, a plain language description of the video, and a copyright transfer form . Completed consent forms are required from anyone appearing in the video (e.g., physicians, patients).
Technical Requirements
Delete or obscure any identifying information about the patient, hospital, or health care system, including the date. Our goal is to avoid commercialization of any product, hospital system, or institution. When referring to drugs or devices, use generic names or descriptions.
The video file should be complete, in final format, and at as high a resolution as possible. Any editing is the authors’ responsibility, and AFP staff may request additional edits following peer review. AFP staff may abridge the video for content, length, or quality.
Submit your video in a mpeg or QuickTime (.mov) format. If possible, include a still image from the video for use in print or on a nonanimated website page.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Instructions for Authors
Contact Monica Mungle for help if edits are needed to the top section.
Original Investigation
Caring for the critically ill patient, brief report, research letter, systematic review (without meta-analysis), narrative review, special communication, clinical challenge, diagnostic test interpretation, a piece of my mind, letter to the editor, letter in reply.
- Randomized Clinical Trial
- Parallel-Design Double-blind Trial
- Crossover Trial
- Equivalence and Noninferiority Trial
- Cluster Trial
- Nonrandomized Controlled Trial
Meta-analysis
- Cohort Study
- Case-Control Study
- Cross-sectional Study
- Case Series
- Economic Evaluation
- Decision Analytical Model
- Comparative Effectiveness Research
- Genetic Association Study
- Diagnostic/Prognostic Study
- Quality Improvement Study
- Survey Study
- Qualitative Study
Manuscript Submission
Copies of previous editorial and reviewer comments, cover letter, manuscript style, manuscript components, recommended file sizes, manuscript file formats, abbreviations, units of measure, names of drugs, devices, and other products, gene names, symbols, and accession numbers, reproduced and re-created material, online-only supplements and multimedia.
What to Expect
Editorial and Peer Review
The jama network advantage.
- JAMA-Express
Authorship Form and Publishing Agreement
Publication.
- Postpublication Online Commenting
Reprints/e-Prints
Corrections, previous publication, related manuscripts and reports, and preprints, previous or planned meeting presentation or release of information, embargo policy, research article public access, depositing in repositories, and discoverability.
Editorial Policies for Authors
Authorship and Disclosures
Authorship criteria and contributions, role of the corresponding author, changes in authorship, name change policy, group authorship, conflicts of interest and financial disclosures, funding/support and role of funder/sponsor, data access, responsibility, and analysis, acknowledgment section, equator reporting guidelines, use of causal language, timeliness of data, statistical methods and data presentation, reporting demographic information for study participants, ethical approval of studies and informed consent, patient identification, use of ai in publication and research, personal communications and unpublished data, manuscripts that pose security risks.
Journal Policies, Forms, Resources
Decisions and Management of Editorial Conflicts of Interest
Publishing agreement, unauthorized use.
- Patient Permission Form
- AMA Manual of Style
- EQUATOR Network
- About This Journal
Contact Information
JAMA , Kirsten Bibbins-Domingo, PhD, MD, MAS, Editor in Chief, 330 N Wabash Ave, Chicago, IL 60611-5885; telephone: (312) 464-4444; fax: (312) 464-5824; email: [email protected] . Manuscripts should be submitted online at http://manuscripts.jama.com .
Determine My Article Type
Categories of articles.
Original Investigation full info
Clinical trial Meta-analysis Intervention study Cohort study Case-control study Epidemiologic assessment Survey with high response rate Cost-effectiveness analysis Decision analysis Study of screening and diagnostic tests Other observational study
- ≤5 tables and/or figures
- Structured abstract
Data Sharing Statement
Follow EQUATOR Reporting Guidelines
Caring for the Critically Ill Patient full info
Original research reports, preferably clinical trials or systematic reviews that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care.
- See also requirements for Clinical Trial , Meta-analysis , and Systematic Review
Brief Report full info
Short reports of original studies or evaluations or unique, first-time reports of clinical case series.
It is very rare for this journal to publish case reports.
- 15 references
- ≤3 tables and/or figures
Research Letter full info
Concise, focused reports of original research. Can include any of the study types listed under Original Investigation.
- No more than 7 authors
- ≤6 references
- ≤2 small tables and/or figures
- No Abstract or Key Points
Back to top
Clinical Review and Education
Systematic Review (without meta-analysis) full info
This article type requires a presubmission inquiry. See the "full info" below for requirements and contact information.
Critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ).
- 50-75 references
- A PRISMA-style flow diagram should be included as an online supplement
- Include a table with ratings of the quality of the studies/evidence
- Subtitle should be "A Systematic Review"
Narrative Review full info
Up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines.
The focus should be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment.
These reviews should address a specific question or issue that is relevant for clinical practice.
- 2000-3500 words
- 3-part structured abstract
- No Key Points
- Subtitle should be "A Review"
Special Communication full info
This journal publishes very few of these types of articles.
These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
- 50 references
- ≤4 tables and/or figures
- Requires a presubmission inquiry
Clinical Challenge full info
Presents an actual patient case with a specific disease or condition with an accompanying clinical image.
- "What Would You Do Next?" with 4 single-phrase plausible treatment options describing possible courses of action with 1 being preferred
- Case presentation: 250 words
- Discussion: 500-600 words
- ≤10 references
- 1-2 small figures
- Patient permission required
Diagnostic Test Interpretation full info
This article requires a presubmission inquiry.
Presentation of the results of a diagnostic test from a single patient with exploration of the clinical application of the test result; intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
- How Do You Interpret These Test Results? (or What Would You Do Next?) with 4 plausible responses
- Case presentation: 200 words
- Discussion: 650 words
Viewpoint full info
May address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article.
- 1200 words (or 1000 words with 1 small table or figure)
- ≤7 references at submission
- ≤3 authors, with no more than 2 affiliations per author
A Piece of My Mind full info
Personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession.
- ≤1600 words
- Patient permission may be needed
Poetry full info
Original poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer.
- No longer than 44 lines
Correspondence
Letter to the Editor full info
Letters discussing a recent article in this journal should be submitted within 4 weeks of the article's publication in print.
- ≤5 references (1 of which should be to the recent article)
Letter in Reply full info
Replies by authors of original articles to letters from readers.
Determine My Study Type
Randomized Clinical Trial full info
A trial that prospectively assigns participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like.
- ≤5 tables and/or figures, including CONSORT flow diagram
- Subtitle should be "A Randomized Clinical Trial"
- Trial registration and ID
- Trial protocol
- CONSORT checklist
- Follow CONSORT Reporting Guidelines
Parallel-Design Double-blind Trial full info
A randomized trial that prospectively assigns participants to 2 or more groups to receive different interventions. Participants and those administering the interventions are unaware of which intervention individual participants are receiving.
Crossover Trial full info
A trial in which participants receive more than 1 of the treatments under investigation, usually in a randomly determined sequence, and with a prespecified amount of time (washout period) between sequential treatments.
Equivalence and Noninferiority Trial full info
A trial designed to assess whether the treatment or intervention under study (eg, a new intervention) is no worse than an existing alternative (eg, an active control). In these trials, authors must prespecify a margin of noninferiority that is consistent with all relevant studies and within which the new intervention can be assumed to be no worse than the active control.
Cluster Trial full info
A trial that includes random assignment of groups rather than individuals to intervention and control groups.
Nonrandomized Controlled Trial full info
A trial that prospectively assigns groups or populations to study the efficacy or effectiveness of an intervention but in which the assignment to the intervention occurs through self-selection or administrator selection rather than through randomization. Control groups can be historic, concurrent, or both. This design is sometimes called a quasi-experimental design.
- ≤5 tables and/or figures, including a trial flow diagram
- Subtitle should be "A Nonrandomized Controlled Trial"
- TREND checklist
Meta-analysis full info
A systematic review that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate.
- Subtitle should include "A Meta-analysis"
- Follow PRISMA Reporting Guidelines or MOOSE Reporting Guidelines
Cohort Study full info
An observational study that follows a group (cohort) of individuals who are initially free of the outcome of interest. Individuals in the cohort may share some underlying characteristic, such as age, sex, diagnosis, exposure to a risk factor, or treatment.
- Follow STROBE Reporting Guidelines
Case-Control Study full info
An observational study designed to determine the association between an exposure and outcome in which study participants are selected by outcome. Those with the outcome (cases) are compared with those without the outcome (controls) with respect to an exposure or event. Cases and controls may be matched according to specific characteristics (eg, age, sex, or duration of disease).
Cross-sectional Study full info
An observational study of a defined population at a single point in time or during a specific interval, in which exposure and outcome are ascertained simultaneously.
Case Series full info
An observational study that describes a selected group of participants with similar exposure or treatment and without a control group. A case series may also involve observation of larger units such as groups of hospitals or municipalities, as well as smaller units such as laboratory samples.
- Follow Reporting Guidelines
Economic Evaluation full info
A study using formal, quantitative methods to compare 2 or more treatments, programs, or strategies with respect to their resource use and expected outcomes. This includes cost-effectiveness, cost-benefit, and cost-minimization analyses.
- Follow CHEERS Reporting Guidelines
Decision Analytical Model full info
A mathematical modeling study that compares consequences of decision options by synthesizing information from multiple sources and applying mathematical simulation techniques, usually with specific software. Reporting should address the relevant non-cost aspects of the CHEERS guideline.
Comparative Effectiveness Research full info
A study that compares different interventions or strategies to prevent, diagnose, treat, and monitor health conditions to determine which work best for which patients, under what circumstances, and are associated with the greatest benefits and harms.
- Follow ISPOR Reporting Guidelines
Genetic Association Study full info
A study that attempts to identify and characterize genomic variants that may be associated with susceptibility to multifactorial disease.
- Follow STREGA Reporting Guidelines
Diagnostic/Prognostic Study full info
A prospective study designed to develop, validate, or update the diagnostic or prognostic accuracy of a test or model.
- Follow STARD Reporting Guidelines or TRIPOD Reporting Guidelines
Quality Improvement Study full info
A study that uses data to define, measure, and evaluate a health care practice or service to maintain or improve the appropriateness, quality, safety, or value of that practice or service.
- Follow SQUIRE Reporting Guidelines
Survey Study full info
A survey study includes a representative sample of individuals who are asked to describe their opinions, attitudes, or behaviors. Survey studies should have sufficient response rates (generally ≥60%) and appropriate characterization of nonresponders to ensure that nonresponse bias does not threaten the validity of the findings.
- Follow AAPOR Best Practices for Survey Research
- Optional: Survey instrument as supplemental file
Qualitative Study full info
A study based on observation and interview with individuals that uses inductive reasoning and a theoretical sampling model and that focuses on social and interpreted, rather than quantifiable, phenomena and aims to discover, interpret, and describe rather than to test and evaluate. This includes mixed-methods studies that combine quantitative and qualitative designs in a sequential or concurrent manner.
- Follow SRQR Reporting Guidelines or COREQ Reporting Guidelines
These reports typically include randomized trials (see Clinical Trial ), intervention studies, cohort studies, case-control studies, epidemiologic assessments, other observational studies, surveys with high response rates (see Reports of Survey Research ), cost-effectiveness analyses and decision analyses (see Reports of Cost-effectiveness Analyses and Decision Analyses ), and studies of screening and diagnostic tests (see also Reports of Diagnostic Tests ). Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are original research reports, preferably clinical trials, or systematic reviews (see above classifications for manuscript submission requirements by category of article) that address virtually any aspect of critical illness, from prevention and triage, through resuscitation and acute treatment, to rehabilitation and palliative care. Manuscripts that provide new insights into the diagnosis, prognosis, and treatment of critically ill patients, as well as those that explore pathophysiological, technological, ethical, or other related aspects of critical care medicine, are welcome. Follow EQUATOR Reporting Guidelines . For reports of original data and systematic reviews, a structured abstract is required; see instructions for preparing Abstracts for Reports of Original Data or Abstracts for Reviews . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 5 tables and/or figures.
These manuscripts are short reports of original studies or evaluations or unique, first-time reports of clinical case series. Follow EQUATOR Reporting Guidelines . A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Recommended length: 1200 words (not including abstract, tables, figures, acknowledgments, references, and online-only material) with no more than a total of 3 tables and/or figures and no more than 15 references. Note: It is very rare for this journal to publish case reports.
Research Letters are concise, focused reports of original research. These should not exceed 600 words of text and 6 references and may include up to 2 tables or figures. Online supplementary material is only allowed for brief additional and absolutely necessary methods but not for any additional results or discussion. Research Letters may have no more than 7 authors. The text should include the full name, academic degrees, and a single institutional affiliation for each author and the email address for the corresponding author. Other persons who have contributed to the study may be indicated in an Acknowledgment, with their permission, including their academic degrees, affiliation, contribution to the study, and an indication if compensation was received for their role. Letters must not duplicate other material published or submitted for publication. In general, Research Letters should be divided into the following sections: Introduction, Methods, Results, and Discussion. They should not include an abstract or key points, but otherwise should follow all of the guidelines in Manuscript Preparation and Submission Requirements . Letters not meeting these specifications are generally not considered.
This article type requires a presubmission inquiry to [email protected] .
The journal will consider 2 types of review articles:
Systematic Reviews
These types of Review articles differ by the scope and level of analysis of the literature searches and the titles used. Systematic Reviews require a complete systematic search of the literature using multiple databases, covering many years, and grading of the quality of the cited evidence. Narrative Reviews do not require a rigorous literature search but should rely on evidence and should be written by established experts in the field. See below for more detail on each type of Review.
Titles for these Reviews should include a concise description of the main topic. Use specific and not overly broad wording for the title; the type of review should be indicated in the subtitle. For example:
Behavioral Treatment of Obesity: A Systematic Review
Behavioral Treatment of Obesity: A Review (note: the word "narrative" is not included in the subtitle)
Systematic Reviews are critical assessments of the literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention. Systematic Reviews without meta-analysis are published as Reviews; those with meta-analysis are published as Original Investigations (see Meta-analysis ). Systematic Reviews should address a specific question or issue that is relevant for clinical practice and provide an evidence-based, balanced, patient-oriented review on a focused topic. Follow EQUATOR Reporting Guidelines .
The basic structure of manuscripts reporting Systematic Reviews should include the following: Abstract (structured abstract of no more than 350 words); Introduction (150-250 words); Methods (150-250 words); Results (1000-1250 words, with the following subsections, if appropriate, depending on the specific question or issue addressed: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); Discussion (1000 words); and Conclusions (2-3 sentences).
Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. For an example of a published Systematic Review, see JAMA . 2014;312(6):631-640 and below for the general structure of a Systematic Review article.
Prospective authors interested in submitting a review manuscript should prepare a detailed outline of the proposed article. There should also be a brief summary of the extent and quality of the literature supporting the proposed review. Alternatively, if a draft of the manuscript has been completed, this can be sent. Prospective authors should also summarize their publication record in the field. Send this information to the editorial office via email to Mary McDermott, MD, at [email protected] .
Specific Components of a Systematic Review
Key Points (75-100 words)
This feature provides a quick structured synopsis of the Review, following 3 key points: Question, Findings, and Meaning. Limit to no more than 100 words. This is different from the Abstract.
Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Abstract (350 words)
A structured abstract is required; Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Introduction (150-250 words)
The first 2 to 3 sentences of the Introduction should draw in readers such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). The epidemiology of the disease or condition should be briefly summarized and generally should include disease prevalence and incidence. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments reported in trials with a minimum follow-up of 2 years including 80% of the original cohort).
Methods/Literature Search (150-250 words)
The literature search should be as current as possible, ideally with end dates within a month or two before manuscript submission. A search of the primary literature should be conducted, including multiple bibliographic databases (eg, PubMed/MEDLINE, Embase, CINAHL, PsycINFO). This can be facilitated by collaborating with a medical librarian to help with the search.
Briefly describe characteristics of the literature searched and included in the review, following the PRISMA reporting guidelines , including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, screening process, language limitations, and inclusion and exclusion criteria. The rating system used to evaluate the quality of the evidence should be specified (see table below) and the methods used to evaluate quality should be described, including number of quality raters, how agreement on quality ratings was assessed, and how disagreements on quality ratings were resolved.
The highest-quality evidence (eg, randomized clinical trials, meta-analyses, systematic reviews, and high-quality prospective cohort studies) should receive the greatest emphasis. Clinical practice guidelines ordinarily should not be used as a primary component of the evidence base for the systematic review, although relevant guidelines should be addressed in the Discussion section of the article.
The search methods should be described in sufficient detail so the search can be reproduced based on the information provided in the manuscript. A summary of the methods of the literature search including this information should be included in the main article; details can be included in an online-only supplement. A PRISMA-style flow diagram showing this information should also be included as an online-only supplement. In addition, a completed PRISMA checklist should be submitted for the items completed that apply to systematic reviews (the checklist items that apply to meta-analyses do not need to be completed for systematic reviews without meta-analysis). The checklist will be used during review but will not be published.
Results (1000-1250 words)
First, briefly report the results of the literature search, including the number of articles reviewed and included, numbers of various types of studies (eg, clinical trials, cohort studies) included, and the aggregate numbers of patients included in the reviewed studies. Also provide a brief summary of the quality of the evidence. Details of this information can be included in a PRISMA-style flow diagram and table(s).
Next, the subsections listed below should generally appear in the Results sections of most Reviews although all of these subsections may not be necessary for some topics, depending on the specific question or issue addressed. The word counts following each subsection are suggested to assist with keeping the overall Results section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a clinician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review of the literature, either performed by the author of the Review or published in the form of a high-quality review or guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included. Discussion (Approximately 1000 words)
Key findings should be summarized in the first paragraph of the Discussion section. All statements made should be supported by evidence. It is very important to not simply list findings from the studies reviewed. This information is best presented in tables. The Discussion should provide a critical synthesis of data and information based on the results of the review, an assessment of the quality of studies summarized, and a description of how studies can be interpreted and used to guide clinical practice. The limitations of the evidence and of the review should be discussed, and gaps in evidence should be addressed. A discussion of controversial or unresolved issues and topics in need of future research also should be included.
Clinical Practice Guidelines: In the Discussion section, describe current clinical practice guidelines, relevant to the topic of the review, if available, and whether the conclusions of this review agree with, or disagree with, the current clinical practice guidelines. If this is done and there is more than 1 guideline, a table should be prepared comparing the major features that differ between the guidelines. Guideline quality should be discussed using the standards outlined for the JAMA Clinical Guidelines Synopsis .
Conclusions
Include a 2- to 3-sentence summary of the major conclusions of the review.
Construct tables that summarize the search results. Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns should include the name of the treatment, strength of evidence supporting the treatment, the treatment's effect (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Ratings of the quality of the evidence. Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
There are several other preferred systems for rating the quality of evidence in Review articles. For Reviews that synthesize findings from numerous studies into a single summary recommendation, use the rating scale shown above or the Oxford Centre for Evidence-based Medicine's Levels of Evidence and Grades of Recommendation or the recommendations in the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . For reviews that include diagnostic studies, use The Rational Clinical Examination Levels of Evidence table .
Follow additional instructions for preparation and submission of Tables .
A PRISMA-style flow diagram should be included as an online supplement that summarizes the results of the literature search and the numbers of articles/records/studies and patients/participants represented in the studies identified, screened, eligible, and included in the final review.
Additional figures that illustrate pathophysiology or clinical presentation may be considered. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Follow additional instructions for preparation and submission of Figures and Video .
Narrative Reviews on clinical topics provide an up-to-date review for clinicians on a topic of general common interest from the perspective of internationally recognized experts in these disciplines. The focus of Narrative Reviews will be an update on current understanding of the physiology of the disease or condition, diagnostic consideration, and treatment. These reviews should address a specific question or issue that is relevant for clinical practice. Narrative Reviews do not require (but may include) a systematic review of the literature search. Recommendations should be supported with evidence and should rely on recent systematic reviews and guidelines, if available, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention.
The basic structure of manuscripts reporting Narrative Reviews should include the following: Abstract (structured abstract of no more than 300 words); Introduction (150-250 words); Methods, if included (150-250 words); Discussion/Observations (1000-1250 words, with the following subsections, if appropriate: Pathophysiology, Clinical Presentation, Assessment and Diagnosis, Treatment, and Prognosis); and Conclusions (2-3 sentences).
Typical length: 2000-3500 words (maximum), with no more than a total of 5 tables and/or figures, and no more than 50-75 references. For an example of this type of article, see JAMA . 2015;314(23):2544-2554 .
Specific Components of a Narrative Review
Abstract (300 words)
Narrative Review articles should include a 3-part structured abstract of no more than 300 words using the headings listed below:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
The first 2 to 3 sentences of the Introduction should draw in readers in such that they want to continue reading the article and should establish the importance of the Review. Reviews should include the clinical question or issue and its importance for general medical practice, specialty practice, or public health. The first paragraph should provide a general summary of the clinical problem (eg, obesity). The next paragraph should focus on the specific aspect of the clinical problem the article will explore (eg, treatments for obesity). Briefly summarize the epidemiology of the disease. This information should include disease prevalence and incidence and perhaps discussion of the presence and frequency of any relevant subpopulations and any geographic or seasonal variations of the disease if these are relevant. The third paragraph should discuss exactly what material will be covered in the Review (eg, obesity treatments).
Methods (150-250 words)
A Methods section is not required for Narrative Reviews, but may be included to summarize a literature search that was conducted for this Review. If included, briefly describe the characteristics of the literature searched and included in the review, including the bibliographic databases and other sources searched, search terms used, dates included in the search, date the literature search was conducted, and any process used to evaluate the literature.
Discussion/Observations (1000-1250 words)
The principal observations of the Narrative Review generally should include the subsections listed below, although each section may not be necessary for some topics. The word counts following each subsection are suggested to assist with keeping the overall Observations section limited to 1000-1250 words.
Pathophysiology (150-250 words). Provide a brief overview of the pathophysiology of the disease. The intent is to provide readers with sufficient background information about the underpinnings of a disease to provide context for the rest of the article. Clinical Presentation (150-250 words). Briefly describe the clinical characteristics that result in a patient seeking medical care for the condition or what features of the disease should lead a physician to evaluate or treat it. Assessment and Diagnosis (250-300 words). Describe the clinical examination for evaluation of the disease and explain the most salient physical examination findings. If laboratory or imaging studies are necessary, provide the sensitivity and specificity and diagnostic accuracy of these tests and consider providing positive and negative likelihood ratios. Sequences of diagnostic tests are best presented as algorithms or in tables. Treatment (250-500 words). Treatments should be based on the most recently available and highest level of evidence. Treatment options should be summarized in the text and presented in detail in tables along with an indication of the strength of evidence supporting the individual treatments. In general, treatment recommendations should be supported by a systematic review or a high-quality guideline. If possible, the costs for various treatments should be provided. Prognosis (100-150 words). A section outlining the overall prognosis for the condition, once treated, should be included.
For most Narrative Reviews, tables should be included that summarize the epidemiology, diagnostic tools, and therapies available for the disease. In some cases, these 3 topics may not all be relevant to the review topic and tables may be appropriately modified to fit the review. Include a fourth table that compares the findings of the review and current clinical practice recommendations or diagnostic and therapeutic uncertainty or controversies.
Table 1: Major epidemiologic and burden of disease facts Table 2: Major diagnostic tools available Table 3: Major therapies available Table 4: Current clinical practice recommendations and/or diagnostic and therapeutic uncertainty, and controversies
Tables summarizing treatments should have information organized by category of treatment and then by individual treatments. Columns may include the treatment, strength of evidence supporting the treatment, the effect of the treatment (preferably shown as the treatment's effect as compared to control on the measured outcome together with 95% confidence intervals), adverse effects, and very brief explanatory comments, if necessary. Lengthy text-based tables should be avoided. Additional or lengthy tables may be published online only, if justified.
Figures that illustrate pathophysiology or clinical presentation may be included. Note: All figures will be re-created. For each proposed illustration, the authors should provide a list of the elements to be included in the illustration; 3-4 relevant recent references; example illustrations, if available; a working figure title and legend; and an explanation of how this new illustration would add to the published literature. We encourage videos, if appropriate, to illustrate a point made or process described in the Review.
Note: This journal publishes very few of these types of articles. These manuscripts describe an important issue in clinical medicine, public health, health policy, or medical research in a scholarly, thorough, well-referenced, systematic, and evidence-based manner.
A structured abstract is required. Maximum length: 3000 words of text (not including tables, figures, or references) with no more than a total of 4 tables and/or figures and no more than 50 references. For a recently published example, see JAMA . 2019;322(20):1996-2016 .
Clinical Challenge presents an actual patient scenario about a specific disease or condition with an accompanying clinical image.
Authors should provide 4 single-phrase plausible treatment options describing possible courses of action with one of these being the most correct response for the question "What Would You Do Next?" Manuscripts should include a brief discussion of the relevant clinical issues and provide well-supported (evidence-based) explanations discussing the 4 potential courses of action. For a recently published example, see JAMA . 2022;327(24):2448-2449. doi:10.1001/jama.2022.8384 .
All diagnostic and treatment recommendations should be supported by referencing recent authoritative texts or journal articles. Preferably, these recommendations should be supported by governmental or multisociety guidelines, clinical trials, meta-analyses, or systematic reviews. The text should have a maximum length of 850 words, consisting of no more than 250 words for the case presentation, question, and 4 one-sentence answers, followed by no more than 600 words that include the diagnosis and a brief discussion. There should be no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. There should be no more than 10 references, and no more than 2 small figures totaling 3 image components (Figure 1, with no more than 2 components, for the case presentation; and Figure 2, with no more than 1 component, for the diagnosis and discussion).
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or the first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
In addition, the JAMA Network Patient Permission form must be completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. Please read Patient Identification before submitting your manuscript.
The image and case presentation should be from the same patient and must not have been published previously. In some cases, additional figures may be included to accompany the answer explanations (see description of additional figure(s) above). All images submitted should be high-quality .jpg or .tif files. Submit the original version of all image files at the highest resolution possible without labels. In general, the original image file should have a minimum resolution of 350 dpi at a width of about 5 inches. Do not increase the original resolution, resize, or crop the image; where applicable, we will crop to maintain patient confidentiality. If any labels, arrowheads, or A/B panel indicators are desired, provide a separate labeled version of the figure(s) for reference. All labels will be reformatted to journal style.
For more information on how to submit figures, see Figures.
We would like to receive common problems presenting uncommonly, rather than unusual or rare conditions (ie, "zebras"). These cases should be of interest to clinicians; they should be problems that clinicians are likely to encounter and have an outstanding image that illustrates the disorder and contributes to the diagnostic challenge.
Manuscripts not meeting these guidelines will not be considered.
Diagnostic Test Interpretation presents the results of a diagnostic test from a single patient and explores the clinical application of the test result. The Diagnostic Test Interpretation is intended to help clinicians understand the underlying rationale in ordering tests, interpreting test results, and acting on the diagnostic test findings.
The diagnostic test result must be obtained from the care of an actual patient and must include that patient's written permission. The JAMA Network Patient Permission form should be read and completed and signed by the patient (or a family member if the patient has died, is a minor, or is an adult without decisional capacity) and included at the time of manuscript submission. The results of laboratory, pathologic, or radiographic tests are appropriate but clinical images are not. Results of the diagnostic test of interest (and related tests) and the range of reference values should be included after the case. Authors of manuscripts based on clinical images should consult the instructions for Clinical Challenge .
Provide a short title that briefly describes the disease entity or case presentation and does not include the diagnosis. Do not include the patient's race, ethnicity, or country of origin in the title or first line of the article. If this information is clinically relevant and necessary, it can be included in the case description.
Manuscripts for Diagnostic Test Interpretation should have the following sections:
Case presentation. The case presentation should be brief and focus on the diagnostic test in question. At the end of the case presentation the pertinent diagnostic test results and reference ranges should be provided (200 words). Include: JAMA Exclude: Specialty Journals, JNO Comments: How do you interpret these test results? How do you interpret these test results? (or What would you do next?) Four plausible responses should be provided. While most Diagnostic Test Interpretation articles will pose the question "How do you interpret these results?" a subset may more appropriately focus on the next best step regarding workup of the abnormal test result. In these cases, the question "How do you interpret these test results?" can be replaced with "What would you do next?" Either question should be presented in the format of a multiple choice question with a single correct (or best) answer. The answers may be brief phrases or short sentences, should be similar in length, and should be arranged alphabetically by first word in the answer. Response options should not describe treatments (about 50 words). Include: CAR,ONC Exclude: JAMA, DER, IMD, NEU, OPH, PED, OTO, PSY, SUR, JNO Comments: How do you interpret these test results? Test characteristics. A brief review of the diagnostic test should be provided (approximately 200 words). For biomarkers, this should include a brief description of the related physiology. Test accuracy should be reported using sensitivity and specificity or likelihood ratios, and predictive values should be provided for common clinical scenarios. Please use likelihood ratios whenever possible, since they do not depend on disease prevalence. The prevalence of the disease should be stated so that the pretest probability may be estimated. For example, "For patients with a typical disease prevalence of 10%, the predictive values of positive and negative test results are approximately 50% and 1%, respectively." Discussion of the application and utility of the diagnostic test should be based on a high-quality systematic review or authoritative practice guideline. If a more recent, original study supersedes or adds meaningfully to the prior synthesis of research, that article also should be cited. The approximate fee for the test should be provided. For example, some fees for laboratory tests can be obtained from the Medicare fee schedules . Radiology procedure fees can be found at the Medicare Physician Fee Schedule website . Application of test result to this patient. A brief discussion of how the diagnostic test result will facilitate the next steps in a patient's management should be presented. Please also address the correct answer to the question about test interpretation in this section (200 words). What Are Alternative Diagnostic Testing Approaches? If there are different testing strategies that can be used to evaluate patients to establish a diagnosis, please discuss them (100 words). Patient Outcome. Long-term follow-up (most recent as possible) regarding the patient's condition and outcome of treatment is necessary (100 words). Clinical Bottom Line. Please provide a bulleted list of 3-5 items that reflect the most important message readers should obtain from this article.
The overall text of the manuscript should have a maximum of 850 words, no more than 10 references, and no more than 3 authors. At least 1 of the authors, ideally the corresponding author, should have sufficient expertise and experience with the topic. The case presentation must not have been previously published.
For an example of this article type, see JAMA . 2022;327(13):1284-1285. doi:10.1001/jama.2022.2037 .
If there are questions about patient identifiability, please contact the editorial office. Authors interested in submitting a manuscript for Diagnostic Test Interpretation should contact the editorial office prior to manuscript preparation and submission by sending an email to Kristin Walter at [email protected] .
Viewpoints may address virtually any important topic in medicine, public health, research, discovery, prevention, ethics, health policy, or health law and generally are not linked to a specific article. Viewpoints should be well focused, scholarly, and clearly presented but should not include the findings of new research or data that have not been previously published.
Viewpoints must have no more than 3 authors. Editors encourage diversity of gender, race, ethnicity, geographic location, and discipline for Viewpoint authors, and the first author should have sufficient expertise and experience with the topic to provide an authoritative opinion. The text should include the full name, academic degrees, and no more than 2 institutional affiliations for each author. Maximum length: up to 1200 words of text—or 1000 words of text with 1 small table or figure—and no more than 7 references, which should be as current as possible. Viewpoints not meeting these guidelines will not be considered.
Most essays published in A Piece of My Mind are personal vignettes (eg, exploring the dynamics of the patient-physician relationship) taken from wide-ranging experiences in medicine; occasional pieces express views and opinions on the myriad issues that affect the profession. If the patient(s) described in these manuscripts is identifiable, a Patient Permission form , which provides consent for publication, must be completed and signed by the patient(s) or family member(s) and submitted with the manuscript. Manuscripts that describe identifiable patients that do not have a signed form will not be reviewed. Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Fictional or composite accounts are not permitted.
Manuscripts are not published anonymously or pseudonymously and must have no more than 3 authors. All manuscripts must be submitted formally via the journal's manuscript submission system; we do not review drafts or unfinished manuscripts prior to submission. Length limit: 1600 words.
Poems related to the medical experience, whether from the point of view of a health care worker or patient, or simply an observer, will be considered. Poems should be original, not previously published or under consideration elsewhere, no longer than 44 lines, and with individual lines no longer than 55 characters (including spaces). Authors should submit each poem separately (ie, one poem per submission record, and only one author per poem). Submissions containing multiple poems will be returned with instructions to split into individual files. Do not submit artwork, music/audio, or other accompanying materials, which are not considered. All poems must be submitted online via the online manuscript submission and review system . Authors of poems that are accepted for publication are required to complete Authorship Forms and transfer copyright to the publisher as part of a publishing agreement. An email with links to the Authorship Form will be sent to authors for completion before final acceptance. Author requests to republish poems are generally granted by our permissions department following a formal request.
Questions about submitting poems (but not submissions) may be sent to [email protected] .
Letters discussing a recent article in this journal should be submitted within 4 weeks of publication of the article in print. 3 Letters received after 4 weeks will rarely be considered. Letters should not exceed 400 words of text and 5 references, 1 of which should be to the recent article. Letters may have no more than 3 authors. The text should include the full name, academic degrees, and a single institutional affiliation for each author and the email address for the corresponding author. Letters must not duplicate other material published or submitted for publication and should not include unpublished data. Letters not meeting these specifications are generally not considered. Letters being considered for publication ordinarily will be sent to the authors of the original article, who will be given the opportunity to reply. Letters will be published at the discretion of the editors and are subject to abridgement and editing for style and content. To read more about Letters, see the AMA Manual of Style .
Replies by authors should not exceed 500 words of text and 6 references. They should have no more than 3 authors.
Clinical Trial
These manuscripts include reports of Randomized Clinical Trials, Parallel-Design Double-blind Trials, Crossover Trials, Equivalence and Noninferiority Trials, Cluster Trials, and Nonrandomized Controlled Trials.
The ICMJE defines a clinical trial as any research project that prospectively assigns human participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. 4 Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, process-of-care changes, and the like. All manuscripts reporting clinical trials, including those limited to secondary exploratory or post hoc analysis of trial outcomes, must include the following:
- Copy of the original trial protocol, including the complete statistical analysis plan and any amendments. The journal recommends using the SPIRIT reporting guidelines when preparing original protocols (see Protocols ).
- CONSORT flow diagram (see Figure ).
- Completed trial checklist (see Checklist ).
- Registry at an appropriate online public clinical trial registry (see Trial Registration requirements).
- A Data Sharing Statement to indicate if data will be shared or not. Specific questions regarding the sharing of data are included in the manuscript submission system.
For additional guidance on reporting Randomized Clinical Trial, Parallel-Design Double-blind Trial, Crossover Trial, Equivalence and Noninferiority Trial, Cluster Trial, and Nonrandomized Controlled Trial, see Study Types .
Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria, or data sources, and how these were selected for the study); the essential features of any interventions; the primary and secondary outcome measures (consistent with those reported in the trial protocol); the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions.
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Randomized Clinical Trial" or, for Nonrandomized Controlled Trials, "A Nonrandomized Controlled Trial." To read more about clinical trials, see the AMA Manual of Style .
Trial Registration:
In concert with the ICMJE, JAMA Network requires, as a condition of consideration for publication, registration of all trials in a public trials registry that is acceptable to the ICMJE (ie, the registry must be owned by a not-for-profit entity, be publicly accessible, and require the minimum registration data set as described by ICMJE). 4 , 8 , 9
Acceptable trial registries include the following and others listed at http://www.icmje.org :
- anzctr.org.au
- clinicaltrials.gov
- trialregister.nl
- umin.ac.jp/ctr
All clinical trials, regardless of when they were completed, and secondary analyses of original clinical trials must be registered before submission of a manuscript based on the trial. Secondary data analyses of primary (parent) clinical trials should not be registered as separate clinical trials, but instead should reference the trial registration number of the primary trial. Please note: for clinical trials starting patient enrollment after July 2005, trials must have been registered before onset of patient enrollment. For trials that began before July 2005 but that were not registered before September 13, 2005, trials must have been registered before journal submission. Trial registry name, registration identification number, and the URL for the registry should be included at the end of the abstract and also in the space provided on the online manuscript submission form.
Authors of manuscripts reporting clinical trials must submit trial protocols (including the complete statistical analysis plan) along with their manuscripts. Protocols in non-English languages should be translated into English. This should include the original approved protocol and statistical analysis plan, and all subsequent amendments to either document. Do not submit a summary version that was published as an article in another journal. If the manuscript is accepted, the protocol and statistical analysis plan will be published as a supplement.
CONSORT Flow Diagram and Checklist:
Manuscripts reporting the results of randomized trials must include the CONSORT flow diagram showing the progress of patients throughout the trial. The CONSORT checklist also should be completed and submitted with the manuscript. 10
Figure. Profile of a Randomized Clinical Trial

Trial Protocol
These manuscripts are documents that describe the organization and plan for a randomized clinical trial, including the trial's objective(s), design, methodology, all outcomes to be measured, and statistical analysis plan. All trial protocol manuscripts must include a copy of the trial protocol including the complete statistical analysis plan (see Protocols ). All clinical trials that have begun randomization must be registered at an appropriate online public registry (see Trial Registration requirements). Follow SPIRIT Reporting Guidelines .
A structured abstract is required, and trial registration information (registry name, trial ID, and URL) must be listed at the end of the abstract; for more information, see instructions for preparing Abstracts for Trial Protocols . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Trial Protocol."
These manuscripts are systematic, critical assessments of literature and data sources pertaining to clinical topics, emphasizing factors such as cause, diagnosis, prognosis, therapy, or prevention, and that includes a statistical technique for quantitatively combining the results of multiple studies that measure the same outcome into a single pooled or summary estimate. All articles or data sources should be searched for and selected systematically for inclusion and critically evaluated, and the search and selection process should be described in the manuscript. The specific type of study or analysis, population, intervention, exposure, and tests or outcomes should be described for each article or data source. The data sources should be as current as possible, ideally with the search having been conducted within several months of manuscript submission. Authors of reports of meta-analyses of clinical trials should submit the PRISMA flow diagram and checklist . Authors of meta-analyses of observational studies should submit the MOOSE checklist . Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Meta-analysis . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and online-only material), with no more than a total of 5 tables and/or figures and no more than 50-75 references. The subtitle should include the phrase "A Meta-analysis." To read more about meta-analyses, see the AMA Manual of Style .
Other Observational Studies
These manuscripts include Cohort Study, Case-Control Study, Cross-sectional Study, Case Series, Economic Evaluation, Decision Analytical Model, Comparative Effectiveness Research, Genetic Association Study, Diagnostic/Prognostic Study, Quality Improvement Study, Survey Study, and Qualitative Study. Each manuscript should clearly state an objective or hypothesis; the design and methods (including the study setting and dates, patients or participants with inclusion and exclusion criteria and/or participation or response rates, or data sources, and how these were selected for the study); the essential features of any interventions or exposures; the main outcome measures; the main results of the study; a discussion section placing the results in context with the published literature and addressing study limitations; and the conclusions and relevant implications for clinical practice or health policy. Data included in research reports must be original and should be as timely and current as possible (see Timeliness of Data ). Follow EQUATOR Reporting Guidelines .
A structured abstract is required; for more information, see instructions for preparing Abstracts for Reports of Original Data . A list of 3 Key Points is required (see guidance on preparing Key Points ). Maximum length: 3000 words of text (not including abstract, tables, figures, acknowledgments, references, and supplemental material) with no more than a total of 5 tables and/or figures and no more than 50-75 references.
Format My Manuscript
Manuscript preparation and submission requirements.
All manuscripts must be submitted online via the online manuscript submission and review system .
At the time of submission, complete contact information (affiliation, postal/mail address, email address, and telephone numbers) for the corresponding author is required. First and last names, email addresses, and institutional affiliations of all coauthors are also required. After the manuscript is submitted, the corresponding author will receive an acknowledgment confirming receipt and a manuscript number. Authors will be able to track the status of their manuscripts via the online system. After manuscript submission, all authors of papers under consideration for publication will be sent a link to the Authorship Form to complete and submit. See other details in these instructions for additional requirements. 2 , 4
As recommended by the ICMJE, "if the manuscript has been submitted previously to another journal, it is helpful to include the previous editors' and reviewers' comments with the submitted manuscript, along with the authors' responses to those comments." 4 It is not uncommon for manuscripts to have been submitted to and peer reviewed by other journals and sharing this information will not bias an editor's decision for this journal. Thus, authors are encouraged to submit these previous comments in their entirety and indicate how they have revised the manuscript in response to these comments, which may expedite the review process. In the submission system, there is a file type for Previous Peer Review and Editorial Comments.
Include a cover letter and complete contact information for the corresponding author (affiliation, postal/mail address, email address, and telephone number) and whether the authors have published, posted, or submitted any related papers from the same study (see Previous Publication, Related Manuscripts and Reports, and Preprints ).
Manuscripts should be prepared in accordance with the AMA Manual of Style , 11th edition, 2 and/or the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals . 4
Include in the manuscript file a title page, abstract, text, references, and as appropriate, figure legends and tables. Start each of these sections on a new page, numbered consecutively, beginning with the title page. Figures should be submitted as separate files (1 file per figure) and not included in the manuscript text.
We recommend individual file sizes of no more than 500 kB and not exceeding 1 MB, with the total size for all files not exceeding 5 MB (not including any video files).
For submission and review, please submit the manuscript as a Word document. Do not submit your manuscript in PDF format.
Use 10-, 11-, or 12-point font size, double-space text, and leave right margins unjustified (ragged).
The title page should be the first page of your manuscript file. It should include a manuscript title; the full names, highest academic degrees, and affiliations of all authors (if an author's affiliation has changed since the work was done, the new affiliation also should be listed); name and complete contact information for corresponding author; and manuscript word count (not including title, abstract, acknowledgment, references, tables, and figure legends).
Titles should be concise, specific, and informative. 2(p8) Please limit the length of titles to 100 characters (including spaces) for reports of research and other major articles and 60 characters for shorter article types such as opinion articles and Letters as well as for subtitles to major articles. For scientific manuscripts, do not use overly general titles, declarative titles, titles that include the direction of study results, or questions as titles. For reports of clinical trials, meta-analyses, and systematic reviews, include the type of study as a subtitle (eg, A Randomized Clinical Trial, A Meta-analysis, A Systematic Review). For reports of other types of research, do not include study type or design in the title or subtitle. Depending on the context, avoid inclusion of specific locations (eg, state, province, or country) and specific years. To read more about titles, see the AMA Manual of Style .
In the manuscript, include a separate section called "Key Points" before the Abstract.
This feature provides a quick structured synopsis of the findings of your manuscript (required only for research and review manuscripts), following 3 key points: Question, Findings, and Meaning. Limit this section to 75-100 words or less.
Question: Focused question based on the study hypothesis or goal/purpose. Limit to 1 sentence. Findings: Results of the study/review. Include the design (eg, clinical trial, cohort study, case-control study, meta-analysis). Focus on primary outcome(s) and finding(s). Do not emphasize secondary outcomes. Report basic numbers only but state if results are statistically significant or not significant; do not include results of statistical tests or measures of variance (see example below). Can include 1 to 2 sentences. Meaning: Key conclusion and implication based on the primary finding(s). Limit to 1 sentence. Example of Research Article Question: What is the immunogenicity of an inactivated influenza A vaccine with and without adjuvant? Findings: In this randomized clinical trial that included 980 adults, the proportion achieving an effective antibody response was 84% with adjuvant vs 2% without adjuvant, a significant difference. Meaning: In an influenza pandemic the use of an adjuvant with inactivated influenza A vaccine may be warranted. Include: All Journals except JNO and JHF Exclude: JNO and JHF Comments: Example of Review Article Example of Review Article Question: What are the most effective medical treatments for adult chronic sinusitis? Findings: In this systematic review, symptoms of chronic sinusitis were improved with saline irrigation and topical corticosteroid therapy compared to no therapy. Compared with placebo, 3-week courses of systemic corticosteroids or oral doxycycline were associated with reduced polyp size, and a 3-month course of macrolide antibiotic was associated with improved symptoms in patients without polyps. Meaning: First-line therapy for chronic sinusitis should begin with daily topical intranasal corticosteroid in conjunction with saline irrigation; subsequent therapies should be based on the patient's polyp status and severity of symptoms.
Include a structured abstract for reports of original data, meta-analyses, and systematic reviews. Abstracts should be prepared in JAMA Network style—see instructions for preparing abstracts below. Abstracts are not required for Editorials, Viewpoints, and special features. No information should be reported in the abstract that does not appear in the text of the manuscript. To read more about abstracts, see the AMA Manual of Style .

Abstracts for Reports of Original Data:
Reports of original data should include an abstract of no more than 350 words using the headings listed below. For brevity, parts of the abstract may be written as phrases rather than complete sentences. Each section should include the following content:
Importance: The abstract should begin with a sentence or 2 explaining the clinical (or other) importance of the study question. Objective: State the precise objective or study question addressed in the report (eg, "To determine whether..."). If more than 1 objective is addressed, the main objective should be indicated and only key secondary objectives stated. If an a priori hypothesis was tested, it should be stated. Design: Describe the basic design of the study and include the specific study type (eg, randomized clinical trial, cohort, cross-sectional, case-control, case series, survey, meta-analysis, bibliometric analysis). State the years of the study and the duration of follow-up. For older studies (eg, those completed >3 years ago), add the date of the analysis being reported. If applicable, include the name of the study (eg, the Framingham Heart Study). As relevant, indicate whether observers were blinded to patient groupings, particularly for subjective measurements. Setting: Describe the study setting to assist readers to determine the applicability of the report to other circumstances, for example, multicenter, population-based, primary care or referral center(s), etc. Participants: State the clinical disorders, important eligibility criteria, and key sociodemographic features of patients (or other study participants). The numbers of eligible participants and how they were selected should be provided, including the number approached but who refused or were excluded. For selection procedures, these terms should be used, if appropriate: random sample (where random refers to a formal, randomized selection in which all eligible individuals have a fixed and usually equal chance of selection); population-based sample; referred sample; consecutive sample; volunteer sample; convenience sample. If matching is used for comparison groups, characteristics that are matched should be specified. In follow-up studies, the proportion of participants who completed the study must be indicated.
Note: The preceding 3 sections are usually combined for accepted papers during the editing process as "Design, Setting, and Participants," but for manuscript submission these sections should be kept separate.
Intervention(s) (for clinical trials) or Exposure(s) (for observational studies): The essential features of any interventions, or exposures, should be described, including their method and duration. The intervention, or exposure, should be named by its most common clinical name, and nonproprietary drug names should be used. Main Outcome(s) and Measure(s): Indicate the primary study outcome measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurements unfamiliar to a general medical readership. Results: Summary demographic information (eg, characteristics such as sex and age) and the number of study participants should be reported in the first sentence of the Results paragraph. The main outcomes of the study should be reported and quantified, including final included/analyzed sample. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P =.13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Measures of relative risk also may be reported (eg, relative risk, hazard ratios) and should include confidence intervals. Studies of screening and diagnostic tests should report sensitivity, specificity, and likelihood ratio. If predictive value or accuracy is reported, prevalence or pretest likelihood should be given as well. All randomized clinical trials should include the results of intention-to-treat analysis as well. In intervention studies, the number of patients withdrawn because of adverse effects should be given. Approaches such as number needed to treat to achieve a unit of benefit may be included when appropriate. All surveys should include response/participation rates. Conclusions and Relevance: Provide only conclusions of the study that are directly supported by the results. Give equal emphasis to positive and negative findings of equal scientific merit. Also, provide a statement of relevance indicating implications for clinical practice or health policy, avoiding speculation and overgeneralization. The relevance statement may also indicate whether additional study is required before the information should be used in clinical settings. Trial Registration: For clinical trials only (not nontrial observational studies), the name of the trial registry, registration number, and URL of the registry must be included. See Trial Registration .
Abstracts for Meta-analysis:
Manuscripts reporting the results of meta-analyses should include an abstract of no more than 350 words using the headings listed below. The text of the manuscript should also include a section describing the methods used for data sources, study selection, data extraction, and data synthesis. Each heading should be followed by a brief description:
Importance: A sentence or 2 explaining the importance of the systematic review question that is used to justify the meta-analysis. Objective: State the precise primary objective of the meta-analysis. Indicate whether the systematic review for the meta-analysis emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being analyzed. Data Sources: Succinctly summarize data sources, including years searched. The search should include the most current information possible, ideally with the search being conducted within several months before the date of manuscript submission. Potential sources include computerized databases and published indexes, registries, meeting abstracts, conference proceedings, references identified from bibliographies of pertinent articles and books, experts or research institutions active in the field, and companies or manufacturers of tests or agents being reviewed. If a bibliographic database is used, state the exact indexing terms used for article retrieval, including any constraints (for example, English language or human study participants). If abstract space does not permit this level of detail, summarize sources in the abstract including databases and years searched, and place the remainder of the information in the Methods section. Study Selection: Describe inclusion and exclusion criteria used to select studies for detailed review from among studies identified as relevant to the topic. Details of selection should include particular populations, interventions, outcomes, or methodological designs. The method used to apply these criteria should be specified (for example, blinded review, consensus, multiple reviewers). State the proportion of initially identified studies that met selection criteria. Data Extraction and Synthesis: Describe guidelines (eg, PRISMA , MOOSE ) used for abstracting data and assessing data quality and validity. The method by which the guidelines were applied should be stated (for example, independent extraction by multiple observers). Indicate whether data were pooled using a fixed-effect or random-effects model. Main Outcome(s) and Measure(s): Indicate the primary study outcome(s) and measurement(s) as planned before data collection began. If the manuscript does not report the main planned outcomes of a study, this fact should be stated and the reason indicated. State clearly if the hypothesis being tested was formulated during or after data collection. Explain outcomes or measurement unfamiliar to a general medical readership. Results: Provide the number of studies and patients/participants in the analysis and state the main quantitative results of the review. When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. See also Reporting Standards and Data Presentation . Meta-analyses should state the major outcomes that were pooled and include odds ratios or effect sizes and, if possible, sensitivity analyses. Evaluations of screening and diagnostic tests should include sensitivity, specificity, likelihood ratios, receiver operating characteristic curves, and predictive values. Assessments of prognosis should summarize survival characteristics and related variables. Major identified sources of variation between studies should be stated, including differences in treatment protocols, co-interventions, confounders, outcome measures, length of follow-up, and dropout rates. Conclusions and Relevance: The conclusions and their applications (clinical or otherwise) should be clearly stated, limiting interpretation to the domain of the review.
Abstracts for Systematic Reviews or Special Communications:
Systematic Review articles should include a structured abstract of no more than 350 words using the headings listed below.
Importance: Include 1 or 2 sentences describing the clinical question or issue and its importance in clinical practice or public health. Objective: State the precise primary objective of the review. Indicate whether the review emphasizes factors such as cause, diagnosis, prognosis, therapy, or prevention and include information about the specific population, intervention, exposure, and tests or outcomes that are being reviewed. Evidence Review: Describe the information sources used, including the search strategies, years searched, and other sources of material, such as subsequent reference searches of retrieved articles. Methods used for inclusion of identified articles and quality assessment should be explained. Findings: Include a brief summary of the number of articles included, numbers of various types of studies (eg, clinical trials, cohort studies), and numbers of patients/participants represented by these studies. Summarize the major findings of the review of the clinical issue or topic in an evidence-based, objective, and balanced fashion, with the highest-quality evidence available receiving the greatest emphasis. Provide quantitative data. Conclusions and Relevance: The conclusions should clearly answer the questions posed if applicable, be based on available evidence, and emphasize how clinicians should apply current knowledge. Conclusions should be based only on results described in the abstract Findings subsection.
Abstracts for Narrative Reviews or Special Communications:
Importance: An overview of the topic and discussion of the main objective or reason for this review. Observations: The principal observations and findings of the review. Conclusions and Relevance: The conclusions of the review that are supported by the information, along with clinical applications. How the findings are clinically relevant should be specifically stated.
Ratings of the quality of the evidence
Tables summarizing evidence should include ratings of the quality of the evidence. Use the rating scheme listed below with ratings of 1-5 for Reviews that include individual studies (modified from the Oxford Centre for Evidence-based Medicine for ratings of individual studies).
Do not use abbreviations in the title or abstract and limit their use in the text. Expand all abbreviations at first mention in the text. To read more about abbreviation use, see the AMA Manual of Style .
Laboratory values are expressed using conventional units of measure, with relevant Système International (SI) conversion factors expressed secondarily (in parentheses) only at first mention. Articles that contain numerous conversion factors may list them together in a paragraph at the end of the Methods section. In tables and figures, a conversion factor to SI should be presented in the footnote or legend. The metric system is preferred for the expression of length, area, mass, and volume. For more details, see the Units of Measure conversion table on the website for the AMA Manual of Style . 2
To read more about units of measure, click here .
Use nonproprietary names of drugs, devices, and other products and services, unless the specific trade name of a drug is essential to the discussion. 2(pp567-569) In such cases, use the trade name once and the generic or descriptive name thereafter. Do not include trademark symbols. To read more about names of drugs, see the AMA Manual of Style .
Authors describing genes or related structures in a manuscript should include the names and official symbols provided by the US National Center for Biotechnology Information (NCBI) or the HUGO Gene Nomenclature Committee . Before submission of a research manuscript reporting on large genomic data sets (eg, protein or DNA sequences), the data sets should be deposited in a publicly available database, such as NCBI's GenBank , and a complete accession number (and version number if appropriate) must be provided in the Methods section or Acknowledgment of the manuscript. To read more about gene nomenclature, see the AMA Manual of Style .
JAMA does not republish text, tables, figures, or other material from other publishers, except under rare circumstances. Please delete any such material and replace with originals.
The submission and publication of content created by artificial intelligence, language models, machine learning, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
Authors are responsible for the accuracy and completeness of their references and for correct text citation. Number references in the order they appear in the text; do not alphabetize. In text, tables, and legends, identify references with superscript arabic numerals. When listing references, follow AMA style and abbreviate names of journals according to the journals list in PubMed . List all authors and/or editors up to 6; if more than 6, list the first 3 followed by "et al." Note: Journal references should include the issue number in parentheses after the volume number.
Examples of reference style:
Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficileinfection. JAMA . 2014;312(17):1772-1778. Murray CJL. Maximizing antiretroviral therapy in developing countries: the dual challenge of efficiency and quality [published online December 1, 2014]. JAMA . doi:10.1001/jama.2014.16376 Centers for Medicare & Medicaid Services. CMS proposals to implement certain disclosure provisions of the Affordable Care Act. http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4221 . Accessed January 30, 2012. McPhee SJ, Winker MA, Rabow MW, Pantilat SZ, Markowitz AJ, eds. Care at the Close of Life: Evidence and Experience . New York, NY: McGraw Hill Medical; 2011.
For more examples of electronic references, click here .
Tables and Figures
Restrict tables and figures to those needed to explain and support the argument of the article and to report all outcomes identified in the Methods section. Number each table and figure and provide a descriptive title for each. Every table and figure should have an in-text citation. Verify that data are consistently reported across text, tables, figures, and supplementary material.
See also Tables and Figures .
Frequency data should be reported as "No. (%)," not as percentages alone (exception, sample sizes exceeding ~10,000). Whenever possible, proportions and percentages should be accompanied by the actual numerator and denominator from which they were derived. This is particularly important when the sample size is less than 100. Do not use decimal places (ie, xx%, not xx.xx%) if the sample size is less than 100. Tables that include results from multivariable regression models should focus on the primary results. Provide the unadjusted and adjusted results for the primary exposure(s) or comparison(s) of interest. If a more detailed description of the model is required, consider providing the additional unadjusted and adjusted results in supplementary tables.
Tables have a minimum of 2 columns. Comparisons must read across the table columns.
Do not duplicate data in figures and tables. For all primary outcomes noted in the Methods section, exact values with measures of uncertainty should be reported in the text or in a table and in the Abstract, and not only represented graphically in figures.
Pie charts and 3-D graphs should not be used and should be revised to alternative graph types.
Bar graphs should be used to present frequency data only (ie, numbers and rates). Avoid stacked bar charts and consider alternative formats (eg, tables or splitting bar segments into side-by-side bars) except for comparisons of distributions of ordinal data.
Summary data (eg, means, odds ratios) should be reported using data markers for point estimates, not bars, and should include error bars indicating measures of uncertainty (eg, SDs, 95% CIs). Actual values (not log-transformed values) of relative data (for example, odds ratios, hazard ratios) should be plotted on log scales.
For survival plots, include the number at risk for each group included in the analysis at intervals along the x-axis scale. For any figures in which color is used, be sure that colors are distinguishable.
All symbols, indicators, line styles, and colors in statistical graphs should be defined in a key or in the figure legend. Axes in statistical graphs must have labels. Units of measure must be provided for continuous data.
Note: All figures are re-created by journal graphics experts according to reporting standards using the JAMA Network style guide and color palette.
- Number all tables in the order of their citation in the text.
- Include a brief title for each table (a descriptive phrase, preferably no longer than 10 to 15 words).
- Include all tables at the end of the manuscript file.
- Refer to Categories of Articles for limits on the number of tables.
- NOTE: Do not embed tables as images in the manuscript file or upload tables in image formats, and do not upload tables as separate files.
Table Creation
Use the table menu in the software program used to prepare the text. Tables can be built de novo using Insert→Table or copied into the text file from another document (eg, Word, Excel, or a statistical spreadsheet).
Avoid using tabs, spaces, and hard returns to set up the table; such tables will have to be retyped, creating delays and opportunities for error.
Tables should be single-spaced and in a 10- or 12-point font (do not shrink the point size to fit the table onto the page). Do not draw extra lines or rules—the table grid will display the outlines of each cell.
Missing data and blank space in the table field (ie, an empty cell) may create ambiguity and should be avoided; use abbreviations such as NA for not applicable or not available. Each piece of data needs to be contained in its own cell. Do not try to align cells with hard returns or tabs; alignment will be imposed in the production system if the manuscript is accepted. To show an indent, add 2 spaces.
When presenting percentages, include numbers (numerator and denominator).
Include statistical variability where applicable (eg, mean [SD], median [IQR]). For additional detail on requirements for data presentation in tables, see Statistical Methods and Data Presentation .
Place each row of data in a separate row of cells, and note that No. (%) and measures of variability are presented in the same cell as in the example Table 1 below:
Table 1. Baseline Values in the Editors' Health Study

SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259.
Note that JAMA Network journals report laboratory values in conventional units. In a table, provide a footnote with the conversion factor to SI units. For a calculator of SI and conventional units, see the AMA Manual of Style . 2
To present data that span more than 1 row, merge the cells vertically. For example, in Table 2 the final column presents the P value for overall age comparisons.
Table 2. Blood Pressure Values Stratified by Age

The table should be constructed such that the primary comparison reads horizontally. For example, see Table 3 (incorrect) and Table 4 (correct).
Table 3. Patient Data by Study Group

Table 4. Patient Data by Study Group

If a table must be continued, repeat the title and column headings on the second page, followed by "(continued)."
Table Footnotes
Footnotes to tables may apply to the entire table, portions (eg, a column), or an individual entry.
The order of the footnotes is determined by the placement in the table of the item to which the footnote refers.
When both a footnote letter and reference number follow data in a table, set the superscript reference number first followed by a comma and the superscript letter.
Use superscript letters (a, b, c) to mark each footnote and be sure each footnote in the table has a corresponding note (and vice versa).
List abbreviations in the footnote section and explain any empty cells.
If relevant, add a footnote to explain why numbers may not sum to group totals or percentages do not add to 100%.
For more detail on the components and recommended structure of tables, see the AMA Manual of Style . 2
Number all figures (graphs, charts, photographs, and illustrations) in the order of their citation in the text. The number of figures should be limited. Avoid complex composite or multipart figures unless justified. See Categories of Articles for limits on the number of figures and/or tables according to article type.
For initial manuscript submissions, figures must be of sufficient quality and may be embedded at the end of the file for editorial assessment and peer review. If a revision is requested and before a manuscript is accepted, authors will be asked to provide figures that meet the requirements described in Figure File Requirements for Publication .
Graphs, charts, some illustrations, titles, legends, keys, and other elements related to figures in accepted manuscripts will be re-created and edited according to JAMA Network style and standards prior to publication. Online-only figures will not be edited or re-created (see Online-Only Supplements and Multimedia ).
Image Integrity
Preparation of scientific images (clinical images, radiographic images, micrographs, gels, etc) for publication must preserve the integrity of the image data. Digital adjustments of brightness, contrast, or color applied uniformly to an entire image are permissible as long as these adjustments do not selectively highlight, misrepresent, obscure, or eliminate specific elements in the original figure, including the background. Selective adjustments applied to individual elements in an image are not permissible. Individual elements may not be moved within an image field, deleted, or inserted from another image. Cropping may be used for efficient image display or to deidentify patients but must not misrepresent or alter interpretation of the image by selectively eliminating relevant visual information. Juxtaposition of elements from different parts of a single image or from different images, as in a composite, must be clearly indicated by the addition of dividing lines, borders, and/or panel labels.
The submission and publication of images created by artificial intelligence, machine learning tools, or similar technologies is discouraged, unless part of formal research design or methods, and is not permitted without clear description of the content that was created and the name of the model or tool, version and extension numbers, and manufacturer. Authors must take responsibility for the integrity of the content generated by these models and tools. See also Use of AI in Publication and Research .
When inappropriate images or image adjustments are detected by the journal staff, authors will be asked for an explanation and will be requested to submit the image as originally captured prior to any adjustment, cropping, or labeling. Authors may be asked to resubmit the image prepared in accordance with the above standards.
Acceptable Figure Files for Initial Submission and Review
Each figure for the main article may be uploaded as a separate file or appended to the end of the manuscript with the figure titles and legends. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ). Note: If a revision is requested and before acceptance, authors must upload each figure for the main article as a separate file and follow the instructions in Figure File Requirements for Publication .
See the Table of Figure Requirements for additional guidance for specific types of figures for suggested resolution and file formats. In general each figure should be no larger than 1 MB.
Figure File Requirements for Publication
Each figure for the main article must be uploaded as a separate file. Online-only figures must be combined into the PDF of the online-only supplement (see Online-Only Supplements and Multimedia ).
See the Table of Figure Requirements for additional guidance and file formats for specific types of figures.
Files created by vector programs are best for accurately plotting and maintaining data points. JAMA Network journals are unable to use file formats native to statistical software applications to prepare figures for publication; most statistical software programs allow users to save or export files in digital vector formats.
Images created digitally (by digital camera or electronically created illustrations) must meet the minimum resolution requirements at the time of creation. Electronically increasing the resolution of an image after creation causes a breakdown of detail and will result in an unacceptable poor-quality image. Each component of a composite image must be uploaded separately at submission and individually meet the minimum resolution requirement.
Color photographs should be submitted in RGB mode using profiles such as Adobe RGB or sRGB. Digital cameras capture images in RGB. Do not change any color settings once the file is on the computer. Black-and-white photographs (eg, radiographs, ultrasound images, CT and MRI scans, and electron micrographs) can be submitted in either RGB or grayscale modes.
Figure Titles and Legends (Captions)
At the end of the manuscript, include a title for each figure. The figure title should be a brief descriptive phrase, preferably no longer than 10 to 15 words. A figure legend (caption) can be used for a brief explanation of the figure or markers if needed and expansion of abbreviations. For photomicrographs, include the type of specimen, original magnification or a scale bar, and stain in the legend. For gross pathology specimens, label any rulers with unit of measure. Digitally enhanced images must be clearly identified in the figure legends as enhanced or manipulated, eg, computed tomographic scans, magnetic resonance images, photographs, photomicrographs, x-ray films.
Figures With Labels, Arrows, or Other Markers
Photographs, clinical images, photomicrographs, gel electrophoresis, and other types that include labels, arrows, or other markers must be submitted in 2 versions: one version with the markers and one without. Provide an explanation for all labels, arrows, or other markers in the figure legend. The Figure field in the File Description tab of the manuscript submission system allows for uploading of 2 versions of the same figure.
Number of Figures
Refer to Categories of Articles because there may be a limit on the number of figures by article type.
General Figure Guidelines
- Primary outcome data should not be presented in figures alone. Exact values with measure of variability should be reported in the text or table as well as in the abstract.
- All symbols, indicators (including error bars), line styles, colors, and abbreviations should be defined in a legend.
- Each axis on a statistical graph must have a label and units of measure should be labeled.
- Do not use pie charts, 3-D graphs, and stacked bar charts as these are not appropriate for accurate statistical presentation of data and should be revised to another figure type or converted to a table.
- Error bars should be included in both directions, unless only 1-sided variability was calculated.
- Values for ratio data—odds ratios, relative risks, hazard ratios—should be plotted on a log scale. Values for ratio data should not be log transformed.
- For footnotes, use letters (a, b, c, etc) not symbols.
- Do not submit figures with more than 4 panels unless otherwise justified.
- See the AMA Manual of Style for more guidance on figure types and components.
For images featuring patients or other identifiable persons, it is not acceptable to use black bars across the eyes in an attempt to deidentify. Cropping may be acceptable as long as the condition under discussion is clearly visible and necessary anatomic landmarks display. If the person in the image is possibly identifiable (not only by others but also by her/himself), permission for publication is required (see Patient Identification ).
Table of Figure Requirements

To present frequency data (numbers or percentages). Each bar represents a category.
Bar graphs are typically vertical but when categories have long titles or there are many of them, they may run horizontally.
The scale on the frequency axis should begin at 0, and the axis should not be broken.
If the data plotted are a percentage or rate, error bars may be used to show statistical variability.
Acceptable File Formats for Initial Submission: .ai, .bmp, .docx, .emf, .eps, .jpg, .pdf, .ppt, .psd, .tif, .wmf, .xls
Acceptable File Formats for Revision and Publication: .ai, .emf, .eps, .pdf, .wmf, .xls

To demonstrate the relationship between 2 or more quantitative variables, such as changes over time.
The dependent variable appears on the vertical axis (y) and the independent variable on the horizontal axis (x); the axes should be continuous, not broken.
Flow diagram

To show participant recruitment and follow-up or inclusions and exclusions (such as in a systematic review).
Acceptable File Formats for Initial Submission: .ai, .docx, .emf, .eps, .jpg, .pdf, .ppt
Acceptable File Formats for Revision and Publication: .ai, .docx, .emf, .eps, .pdf
Survival plot

To display the proportion or percentage of individuals (represented on the y-axis) remaining free of or experiencing a specific outcome over time (represented on the x-axis).
The curve should be drawn as a step function (not smoothed).
The number of individuals followed up for each time interval (number at risk) should be shown underneath the x-axis.
Box-and-whisker plot (box plot)

To show data distribution from 1 or more groups, particularly aggregate/summary data.
Each element should be described (the ends of the boxes, the middle line, and the whiskers). Data points that fall beyond the whiskers are typically shown as circles.
Forest plot

To illustrate summary data, particularly in meta-analyses and systematic reviews.
The data are presented both tabularly and graphically.
The sources (with years and citations, when relevant) should comprise the first column.
Provide indicators of both directions of results at the top of the plot on either side of the vertical line (eg, favors intervention).
Typically, proportionally sized boxes represent the weight of each study and a diamond shows the overall effect at the bottom of the plot.

To display quantitative data other than counts or frequencies on a single scaled axis according to categories on a baseline (horizontal or vertical). Point estimates are represented by discrete data markers, preferably with error bars (in both directions) to designate variability.
Scatterplot

To show individual data points plotted according to coordinate values with continuous, quantitative x- and y-axis scales.
A curve that is generated mathematically may be fitted to the data to summarize the relationship among the variables.
Illustration

To explain physiological mechanisms, describe clinical maneuvers and surgical techniques, or provide orientation to medical imaging.
Required minimum resolution for publication: ≥350 ppi
Acceptable File Formats for Initial Submission: .ai, .docx, .eps, .jpg, .pdf, .ppt, .psd., tif
Acceptable File Formats for Revision and Publication: .ai, .eps, .jpg, .pdf, .psd, .tif
Photographs and other clinical images

To display clinical findings, experimental results, or clinical procedures, including medical imaging, photomicrographs, clinical photographs, and photographs of biopsy specimens.
Legends for photomicrographs should include details about the type of stain used and magnification.
Acceptable File Formats for Initial Submission: .eps, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .eps, .jpg, .psd, .tif
Line drawings

To illustrate anatomy or procedures.
Line drawings are almost always black and white.
Required minimum resolution for publication: ≥600 ppi
Acceptable File Formats for Initial Submission: .docx, .jpg, .pdf, .ppt, .psd, .tif
Acceptable File Formats for Revision and Publication: .jpg, .psd, .tif
Authors may submit supporting material to accompany their article for online-only publication when there is insufficient space to include the material in the print article. This material should be important to the understanding and interpretation of the report and should not repeat material in the print article. The amount of online-only material should be limited and justified. Online-only material should be original and not previously published.
Online-only material will undergo editorial and peer review with the main manuscript. If the manuscript is accepted for publication and if the online-only material is deemed appropriate for publication by the editors, it will be posted online at the time of publication of the article as additional material provided by the authors. This material will not be edited or formatted; thus, authors are responsible for the accuracy and presentation of all such material.
Online-only material should be submitted in a single Word document with pages numbered consecutively. Each element included in the online-only material should be cited in the text of the main manuscript (eg, eTable in the Supplement) and numbered in order of citation in the text (eg, eTable 1, eTable 2, eFigure 1, eFigure 2, eMethods). The first page of the online-only document should list the number and title of each element included in the document.
Online-Only Text
Online-only text should be set in Times New Roman font, 10 point in size, and single-spaced. The main heading of the online-only text should be in 12 point and boldface; subheadings should be in 10 point and boldface.
Online-Only References
All references cited within the online-only document must be included in a separate reference section, including those that also were cited in the main manuscript. They should be formatted just as in the main manuscript and numbered and cited consecutively in the online-only material.
Online-Only Tables
Online-only tables should be inserted in the document and numbered consecutively according to the order of citation as eTable 1, eTable 2, etc. All online-only tables should be cited in the relevant text of the main manuscript. The text and data in online tables should be Arial font, 10 point in size, and single-spaced. The table title should be set in Arial font, 12 point, and bold. Headings within tables should be set in 10 point and bold. Table footnotes should be set in 8 point and single-spaced. See also instructions for Tables above. If a table runs on to subsequent pages, repeat the column headers at the top of each page. Wide tables may be presented using a landscape orientation.
If data are better displayed in a separate Excel file, this can be submitted, provided that the Excel file is cited as an eTable and is numbered in the order cited in the text. If multiple Excel files of data are submitted, these should be placed in a single Excel file, with multiple tabs (sheets) at the bottom of the file. The first tab (sheet) should include a table of contents with eTable numbers and titles, and the subsequent tabs (sheets) should be labeled as eTable 1, eTable 2, etc. Please note: the journal is not a data repository; large data sets should be deposited into publicly accessible data repositories, and a link should be provided in the Methods or Results section and the Data Sharing Statement .
Online-Only Figures
Online-only figures should be inserted in the document and numbered consecutively according to the order of citation as eFigure 1, eFigure 2, etc. All online-only figures should be cited in the relevant text of the main manuscript. Figure titles should be set in Arial font, 12 point, bold, and single-spaced. Text within figures should be set as Arial font, 10 point. Figure legends should be set in 8 point and single-spaced. Graphs and diagrams should be exported directly out of the software application used to create them in a vector file format, such as .wmf, and then inserted into the Word document. Image file formats such as .jpg, .tif, and .gif are generally not suitable for graphs. Photographs, including all radiological images, should be prepared as .jpg (highest option) or .tif (uncompressed) files at a resolution of 300 dpi and width of 3-5 inches, but the resolution of photographic files with an original resolution <300 dpi should not be increased digitally to achieve a 300-dpi resolution. Photographs should be inserted in the document with the "Link to File" button turned off. Wide figures may be presented using a landscape orientation.
For editorial and review of an initial submission, submit videos according to the following specifications:
- Acceptable file formats: .mov, .wmv, .mpg, .mpeg, .mp4, or .avi
- Maximum file size: ≤25 MB
- Preferred dimensions: 1920x1080 (HD) or greater (4k UHD footage is acceptable)
- Minimum dimensions: 640 pixels wide by 360 pixels deep
- Recommended frame rate: 24 fps (or 23.976 fps), 25 and 30 fps (or 29.97 fps)
- Maximum length: ≤5 minutes
- Desired aspect ratio: 4:3 (standard) or 16:9 (widescreen)
- If compression is required to reduce file size for uploading, please use a minimum bit rate of 10,000 kbit/s – 20,000 kbit/s
- When filming, please use a landscape orientation, not a portrait orientation. This is especially important when filming video or taking photographs with a smartphone or a mobile device.
Verify that the videos are viewable in QuickTime or Windows Media Player before uploading.
For each video, provide an in-text citation (eg, Video 1). At the end of the manuscript file, include a title (a brief phrase, preferably no longer than 10 to 15 words) and a caption that includes the file format and a brief explanation for each video. The same title and caption must be entered in the designated fields in the manuscript submission system when uploading each video. If multiple video files are submitted, number them in the order in which they should be viewed.
If patient(s) are identifiable in the video, authors must submit a Patient Permission form completed and signed by each patient. See also Patient Identification .
If the author does not hold copyright to the video, the author must obtain permission for the video to be published in the journal. This permission must be for unrestricted use in all print, online, and licensed versions of the journal.
NOTE: If your manuscript and accompanying videos are accepted for publication, the video files will be placed into a journal video frame and will be edited by JAMA Network video production staff according to journal style. In addition, a JAMA Network staff person may contact you to resubmit your videos to meet our production specifications. For example, a larger size may be needed, and if your videos were submitted with embedded text such as titles, annotations, labels, or captions, we will ask you to remove the text at this stage and resubmit the video without text, and JAMA Network video production will re-create all text using our house style.
Guidelines for Optimal Video Quality
- Use plenty of diffuse light; avoid shadows.
- Use the appropriate white-balance based on your lighting conditions. Different cameras have different settings, but most have presets for incandescent (yellow) light, fluorescent light, daylight, and tungsten light. Please make sure to select the correct one so that the color of your footage renders accurately.
- Do not overexpose the image; a bit underexposed is preferable.
- Use a tripod. This is especially important in close-ups.
- Avoid excessive zooming. Use the optical zoom only; do not use a digital zoom.
- Turn off all camera special effects.
- Avoid using autofocus. Manual focus is more accurate. Keep the camera at a fixed distance from the subject.
- Instruct people on camera to speak clearly and face the camera when speaking. Try to avoid large movements while speaking or immediately after speaking. Allow pauses before and after speaking for easier editing.
- If the situation permits, ensure that individuals being filmed are not wearing white clothing or clothing with busy patterns or stripes, especially shirts, jackets, and ties. Subdued medium blue, brown, tan, beige, and green colors all work well for shirt and clothing choices.
- Do not include an introduction by the physician as a "talking head" explaining a procedure. All footage should be of the procedure or relevant subject matter only.
- Record a few extra seconds before and after each cut or after changing the camera's position. This allows for easier editing.
Additional Considerations for Filming Surgical Procedures
- Coordinate with the surgical staff to establish a vantage point for the camera that has a clear view of the surgical field.
- Before the procedure, if the situation permits, identify the surgical staff's positions for access into and out of the surgical field to ensure there is no immediate obstruction of the camera.
- During the procedure, avoid typical obstructions of the camera's main view such as arms reaching across the field or soiled surgical sponges. Where possible, keep the heads, hands, and any instruments away from the immediate sightline of the camera. This will ensure that all moments of the procedure are captured in full view and focus.
- If the situation permits a choice of glove type, use brown or tan. White gloves reflect bright light; vividly colored surgical gloves can distract the viewer from the teaching point of the video.
- If the situation permits, avoid rapid movements for procedural steps that should be noticed and understood. To demonstrate a key moment or use of an instrument, movement that is deliberate and steady will allow a standard camera to focus properly.
For editorial and review of an initial submission, submit audio files according to the following minimum requirements:
- Acceptable file formats: .mp3, .wav, or .aiff
- Maximum file size: 25 MB
- To achieve the best quality, use a setting of 256 kbps or higher for stereo or 128 kbps or higher for mono.
- Sampling rate should be either 44.1 kHz or 48 kHz.
- Bit rate should be either 16 or 24 bit.
- To avoid audible clipping noise, please make sure that audio levels do not exceed 0 dBFS.
For each audio file, provide an in-text citation. At the end of the manuscript, include a title (a brief phrase, preferably no longer than 10-15 words) and a caption that includes the file format and a brief explanation for each audio.
NOTE: If your manuscript is accepted for publication, JAMA Network video production staff may contact you to request an original uncompressed audio file in .wav or .aiff format. There is no maximum file size requirement for publication at this stage.
After Submission
Authors will be sent notifications of the receipt of manuscripts and editorial decisions by email. During the review process, authors can check the status of their submitted manuscript via the online manuscript submission and review system . Authors should not disclose the fact that their manuscript has been submitted to anyone, except coauthors and contributors, without permission of the editor.
All submitted manuscripts are reviewed initially by one of the editors. Manuscripts are evaluated according to the following criteria: material is original and timely, writing is clear, study methods are appropriate, data are valid, conclusions are reasonable and supported by the data, information is important, and topic has general interest to readers of this journal. From these basic criteria, the editors assess a paper's eligibility for publication. Manuscripts with insufficient priority for publication are rejected promptly. Other manuscripts are sent to expert consultants for peer review. The journal uses a single-anonymized peer review process: peer reviewer identities are kept confidential (unless reviewers choose to reveal their names in their formal reviews); author identities are made known to reviewers. The existence of a manuscript under review is not revealed to anyone other than peer reviewers and editorial staff. Peer reviewers are required to maintain confidentiality about the manuscripts they review and must not divulge any information about a specific manuscript or its content to any third party without prior permission from the journal editors. Reviewers are instructed to not submit confidential manuscripts, abstracts, or other text into a chatbot, language model, or similar tool. At submission, authors may choose to have manuscripts that are not accepted by the journal referred to one of the JAMA Network specialty journals and/or JAMA Network Open along with reviewers' comments (if available). Information from submitted manuscripts may be systematically collected and analyzed as part of research to improve the quality of the editorial or peer review process. Identifying information remains confidential. Final decisions regarding manuscript publication are made by an editor who does not have any relevant conflicts of interest.
At the time of manuscript submission, authors may preselect the option to have their manuscript and reviewers' comments automatically referred to one of the JAMA Network specialty journals if the manuscript is not accepted by JAMA .
JAMA -EXPRESS
JAMA -EXPRESS provides rapid peer review and publication of major clinical trials and other original research studies that have immediate or public health importance. Authors who wish to have manuscripts considered for JAMA -EXPRESS should send the manuscript file and a request letter to [email protected] or call (312) 464-4444. Authors will be notified promptly whether the manuscript is approved for rapid peer review. Authors of those manuscripts determined not to qualify for rapid review may be invited to submit the manuscript for further consideration under the standard review process.
Authors may appeal decisions. All appeals are reviewed by the editor in chief, on a case-by-case basis, or a designated editor if the editor in chief is recused from the review.
After Revision/Acceptance
All authors are required to complete an Authorship Form and Publishing Agreement. See Authorship Criteria and Contributions .
Accepted manuscripts are edited in accordance with the AMA Manual of Style , 2 and returned to the corresponding author (or her/his designee) for approval. Authors are responsible for all statements made in their work, including changes made during editing and production that are authorized by the corresponding author.
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, until it is published without permission of the editor or as described in the guidance on Previous or Planned Meeting Presentaton or Release of Information and Embargo Policy .
If accepted for publication, all articles are published quickly in one of JAMA 's weekly print/online issues; selected articles are published Online First.
After Publication
Postpublication correspondence.
For accepted manuscripts, the corresponding author will be asked to respond to letters to the editor.
Reprints and e-prints may be ordered online when the edited manuscript is sent for approval to the corresponding author.
Requests to publish corrections should be sent to the editorial office. Errors and requests for corrections are reviewed by editors and authors, and, if warranted, a Correction notice summarizing the errors and corrections is published promptly and linked online to the original article, and the original article is corrected online with the date of correction. 15
First and last authors of peer-reviewed articles are eligible to receive CME credit. See CME From the JAMA Network .
About Previous Release of Information, Embargo, and Access
Manuscripts are considered with the understanding that they have not been published previously and are not under consideration by another publication.
Copies of all related or similar manuscripts and reports by the same authors (ie, those containing substantially similar content or using the same, similar, or a subset of data) that have been previously published or posted electronically or are under consideration elsewhere must be provided at the time of manuscript submission. All related previously published articles should be cited as references and described in the submitted manuscript along with explanation of how the submitted manuscript differs from the related previously published article(s).
Manuscripts that have been previously posted on a preprint server may be submitted for consideration for publication. When the manuscript is submitted, authors must provide information about the preprint, including a link to it and a description of whether the submitted manuscript has been revised or differs from the preprint.
See also Previous or Planned Meeting Presentation or Release of Information and Research Article Public Access, Depositing in Repositories, and Discoverability.
Meeting presentation: A complete manuscript submitted to the journal following or prior to presentation at a scientific meeting or publication of preliminary findings elsewhere (ie, as an abstract) is eligible for consideration for publication. Authors considering presenting or planning to present the work at an upcoming scientific meeting should indicate the name and date of the meeting on the manuscript submission form. For accepted papers, the editors may be able to coordinate publication with the meeting presentation. Authors of submitted papers, including those accepted but not yet published, should not disclose the status of such papers during such meeting presentations that occur before the work is published. Authors who present information contained in a manuscript that is under consideration by this journal during scientific or clinical meetings should not distribute complete reports (ie, copies of manuscripts) or full data presented as tables and figures to conference attendees or journalists. Publication of abstracts in print and online conference proceedings, as well as posting of slides or videos from the scientific presentation on the meeting website, is acceptable. However, for manuscripts under consideration by this journal, publication of full reports in meeting proceedings or online, issuing detailed news releases reporting the results of the study that go beyond the meeting abstract, or participation in formal news conferences will ordinarily jeopardize chances for publication of the submitted manuscript in this journal. 5 Media coverage of presentations at scientific meetings will not jeopardize consideration, but direct release of information through press releases or news media briefings may preclude consideration of the manuscript by this journal. 5 Rare instances of papers reporting public health emergencies should be discussed with the editor. Authors submitting manuscripts or letters to the editor regarding adverse drug or medical device reactions, reportable diseases, etc, should also report this information to the relevant government agency.
Authors should not release information about accepted manuscripts via social media until publication.
See also Previous Publication, Related Manuscripts and Reports, and Preprints . For more information, see the AMA Manual of Style .
Authors should not disclose the fact that their manuscript has been accepted to anyone, except coauthors and contributors, without permission of the editor until it is published. All information regarding the content and publication date of accepted manuscripts is strictly confidential. Unauthorized prepublication release of accepted manuscripts and information about planned publication date may result in rescinding the acceptance and rejecting the paper. This policy applies to all categories of articles, including research, review, opinion, correspondence, etc. Information contained in or about accepted articles cannot appear in print, audio, video, or digital form or be released by the news media until the specified embargo release date. 2 , 5 See also Previous or Planned Meeting Presentation or Release of Information .
The journal makes all JAMA research articles free public access 6 months after publication on the journal website.
Authors of research articles may deposit the accepted version (ie, the peer-reviewed manuscript that you submitted on which this decision is based) of the manuscript in a repository of your choice on or after the date of publication provided that it links to the final published version on the journal website. You may not deposit the published article (version of record), which is the final copyedited, formatted, and proofed version published by the journal. The journal will deposit a copy of the published research article into PubMed Central (PMC) at the time of publication, where it will be publicly available 6 months after publication. A few weeks after publication, you may obtain your PMCID on the PMC site at: https://www.ncbi.nlm.nih.gov/pmc/pmctopmid/ . These options apply only to research articles. Non-research articles may not be deposited into repositories.
In addition, the journal will add metadata to all articles to ensure web-based search engine discoverability and will provide publicly discoverable information about your article to PubMed/Medline and numerous other bibliographic databases on the day of publication.
Author Responsibilities
Most of the JAMA Network journals' editorial policies for authors are summarized in these instructions. Citations and links to the AMA Manual of Style: A Guide for Authors and Editors 2 and other publications with additional information are also provided.
Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. 2 One or more authors should take responsibility for the integrity of the work as a whole, from inception to published article. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE), 4 authorship credit should be based on the following 4 criteria:
- substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; and
- drafting of the work or reviewing it critically for important intellectual content; and
- final approval of the version to be published; and
- agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Each author should be accountable for the parts of the work he or she has done. In addition, each author should be able to identify which coauthors are responsible for specific other parts of the work and should have confidence in the integrity of the contributions of any coauthors.
All those designated as authors should meet all 4 criteria for authorship, and all who meet the 4 criteria should be identified as authors. Those who do not meet all 4 criteria should be acknowledged (see Acknowledgment Section ).
All authors (ie, the corresponding author and each coauthor) must read, complete, and submit an electronic Authorship Form with required statements on Authorship Responsibility, Criteria, and Contributions; Confirmation of Reporting Conflicts of Interest and Funding; and Publishing Agreement. 2(pp128-133) In addition, authors are required to identify their specific contributions to the work described in the manuscript. Requests by authors to designate equal contributions or shared authorship positions (eg, co-first authorship) may be considered if justified and within reason. 6 An email with links to the Authorship Form will be sent to authors for completion after manuscripts have been submitted.
For reports of original data, authors' specific contributions will be published in the Acknowledgment section (see Manuscript Preparation and Submission Requirements , Acknowledgment section ). 2 All other persons who have made substantial contributions to the work reported in this manuscript (eg, data collection, analysis, or writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions and affiliations in an Acknowledgment in the manuscript. Written permission to include the names of individuals in the Acknowledgment section must be obtained.
Nonhuman artificial intelligence, language models, machine learning, or similar technologies do not qualify for authorship. If these models or tools are used to create content or assist with writing or manuscript preparation, authors must take responsibility for the integrity of the content generated by these tools. Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or Methods section if this is part of formal research design or methods. See also Use of AI in Publication and Research , Reproduced and Re-created Material , and Image Integrity .
The authors also must certify that the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere (see also About Previous Release of Information, Embargo, and Access ). 2 Authors of manuscripts reporting original data or systematic reviews must provide an access to data statement from 1 or 2 named authors, often the corresponding author (see also Data Access, Responsibility, and Analysis ). If requested, authors should be prepared to provide the data and must cooperate fully in obtaining and providing the data on which the manuscript is based for examination by the editors or their assignees.
A single corresponding author (or coauthor designee in the event that the corresponding author is unavailable) will serve on behalf of all coauthors as the primary correspondent with the editorial office during the submission and review process. If the manuscript is accepted, the corresponding author will review an edited manuscript and proof, make decisions regarding release of information in the manuscript to the news media or federal agencies, handle all postpublication communications and inquiries, and will be identified as the corresponding author in the published article.
The corresponding author also is responsible for ensuring that the Acknowledgment section of the manuscript is complete (see Acknowledgment Section ) and that the conflict of interest disclosures reported in the Acknowledgment section of the manuscript are accurate, up-to-date, and consistent with the information provided in each author's potential conflicts of interest section in the Authorship Form (see Conflicts of Interest and Financial Disclosures ).
The corresponding author also must complete the Acknowledgment statement part of the Authorship Form confirming that all persons who have contributed substantially but who are not authors are identified in the Acknowledgment section and that written permission from each person acknowledged has been obtained (see Acknowledgment Section ).
Requests for co-corresponding authors will be considered on a very limited basis if justified, but no more than 2 co-corresponding authors will be permitted. In such cases, a primary corresponding author must be designated as the point of contact responsible for all communication about the manuscript and article, manage the tasks described above, and will be listed first in the corresponding author section. 6 To read more about the role and responsibilities of corresponding authors, see the AMA Manual of Style .
Authors should determine the order of authorship among themselves and should settle any disagreements before submitting their manuscript. Changes in authorship (ie, order, addition, and deletion of authors) should be discussed and approved by all authors. Any requests for such changes in authorship after initial manuscript submission and before publication should be explained in writing to the editor in a letter or email from all authors. 2(pp128-133)
The JAMA Network recognizes that authors may change their names for personal reasons, and the editors respect authors' rights to autonomy and privacy in this regard. Authors who request confidential name changes after publication because of changes in identity, marital status, religion, or other reasons may have their names changed in articles without indication of the reason for the change and without a formal correction notice. If an author prefers this change to be public, a formal Correction notice can be issued, with or without the reason per author preference. The journal will not request the approval of coauthors, but the requesting author may wish to notify coauthors if this change will affect subsequent citations to the article. The requester may be asked to notify the corresponding author about this change to the published article; alternatively, the journal may inform the corresponding author of this change (without explaining the reason for the change). The journal will make this change to the online and PDF versions of the published article and will notify postpublication indexes and databases as a standard process but cannot guarantee when or if the change will be reflected in these indexes and databases.
If authorship is attributed to a group (either solely or in addition to 1 or more individual authors), all members of the group must meet the full criteria and requirements for authorship as described above, and all group member authors must complete Authorship Forms. 6 If all members of a group do not meet all authorship criteria, a group must designate 1 or more individuals as authors or members of a writing group who meet full authorship criteria and requirements and who will take responsibility for the group. 2 , 6 Group names should appear at the end of the byline and should not be interspersed within the list of individually named authors. Group authors may not be included for article types with limited numbers of authors (eg, opinion articles).
For articles with a large number of authors (eg, >50), a long list of authors will not fit in the byline of a print/PDF version of the article. In such cases, a group byline will be recommended with the individual names of each author listed at the end of the article. All author names would still be individually indexed, displayed, and easily searchable in bibliographic records such as PubMed. 6
Nonauthor Collaborators: Other group members who do not meet the criteria for authorship (eg, investigators, advisors, assistants) may be identified. For group author manuscripts, a Nonauthor Collaborator Template (with names, academic degrees, institution, location, role/contribution, and subgroup) must be completed during revision. The template will be available to authors with the request for revision. The collaborators will be published in an online Supplement based on this template and will be deposited to PubMed.
To read more about authorship, click here .
A conflict of interest may exist when an author (or the author's institution or employer) has financial or personal relationships or affiliations that could influence (or bias) the author's decisions, work, or manuscript. All authors are required to report potential conflicts of interest including specific financial interests relevant to the subject of their manuscript in the Acknowledgment section of the manuscript 2 and in the Disclosure of Potential Conflicts of Interest section of the Authorship Form. Note: These forms will be requested after a manuscript has been submitted, but authors should also include conflict of interest disclosures in the Acknowledgment section of the submitted manuscript.
Definitions and Terms of Conflicts of Interest Disclosures:
Authors are expected to provide detailed information about all relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript) including, but not limited to, employment, affiliation, funding and grants received or pending, consultancies, honoraria or payment, speakers' bureaus, stock ownership or options, expert testimony, royalties, donation of medical equipment, or patents planned, pending, or issued.
Following the guidelines of the ICMJE, 4 the definitions and terms of such disclosures include
Any potential conflicts of interest "involving the work under consideration for publication" (during the time involving the work, from initial conception and planning to present), Any "relevant financial activities outside the submitted work" (over the 3 years prior to submission), and Any "other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing" what is written in the submitted work (based on all relationships that were present during the 3 years prior to submission).
Authors without conflicts of interest, including relevant financial interests, activities, relationships, and affiliations, should indicate such in their disclosures and include a statement of no such interests in the Acknowledgment section of the manuscript. Failure to include this information in the manuscript may delay evaluation and review of the manuscript. Authors should err on the side of full disclosure and should contact the editorial office if they have questions or concerns.
Although many universities and other institutions and organizations have established policies and thresholds for reporting financial interests and other conflicts of interest, the JAMA Network requires complete disclosure of all relevant financial relationships and potential financial conflicts of interest, regardless of amount or value. For example, authors of a manuscript about hypertension should report all financial relationships they have with all manufacturers and owners of products, devices, tests, and services used in the management of hypertension, not only those relationships with entities whose specific products, devices, tests, and services are mentioned in the manuscript. If authors are uncertain about what constitutes a relevant financial interest or relationship, they should contact the editorial office.
For all accepted manuscripts, the corresponding author will have been asked to confirm that each coauthor's disclosures of conflicts of interest and relevant financial interests, activities, relationships, and affiliations and declarations of no such interests are accurate, up-to-date, and consistent with the disclosures reported in the Acknowledgment section of the manuscript because this information will be published in the Acknowledgment section of the article. Decisions about whether such information provided by authors should be published, and thereby disclosed to readers, are usually straightforward. Although editors are willing to discuss disclosure of specific conflicts of interest with authors, JAMA Network policy is one of complete disclosure of all potential conflicts of interest, including relevant financial interests, activities, relationships, and affiliations (other than those affiliations listed in the title page of the manuscript). The policy requiring disclosure of conflicts of interest applies for all manuscript submissions, including letters to the editor. If an author's disclosure of potential conflicts of interest is determined to be inaccurate or incomplete after publication, a correction will be published to rectify the original published disclosure statement, and additional action may be taken as necessary.
All authors must also complete the Disclosure of Potential Conflicts of Interest section of the Authorship Form. 7
All financial and material support for the research and the work should be clearly and completely identified in an Acknowledgment section of the manuscript. At the time of submission, information on the funding source (including grant identification) must also be completed via the online manuscript submission and review system. The specific role of the funding organization or sponsor in each of the following should be specified: "design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication." 7 To read more about reporting funding and other support, see the AMA Manual of Style .
For all reports (regardless of funding source) containing original data, at least 1 named author (eg, the principal investigator), and no more than 2 authors, must indicate that she or he "had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis." 7 This exact statement should be included in the Acknowledgment section at the end of the manuscript. Modified statements or generic statements indicating that all authors had such access are not acceptable. In addition, for all reports containing original data, the names and affiliations of all authors (or other individuals) who conducted and are responsible for the data analysis must be indicated in the Acknowledgment section of the manuscript. If the individual who conducted the analysis is not named as an author, a detailed explanation of his/her contributions and reasons for his/her involvement with the data analysis should be included.
For all reports of research, authors are required to provide a Data Sharing Statement to indicate if data will or will not be shared. Specific questions regarding the sharing of data are included in the manuscript submission system. If authors choose to share or not share data, this information will be published in a Data Sharing Statement in an online supplement linked to the published article. Authors will be asked to identify the data, including individual patient data, a data dictionary that defines each field in the data set, and supporting documentation (eg, statistical/analytic code), that will be made available to others; when, where, and how the data will be available (eg, a link to a data repository); types of analyses that are permitted; and if there will be any restrictions on the use of the data. Authors also have the option to explain why data may not be shared. A list of generalist public repositories that authors may consider using is available from the National Library of Medicine .
The Acknowledgment section is the general term for the list of contributions, disclosures, credits, and other information included at the end of the text of a manuscript but before the references. The Acknowledgment section includes authors' contributions; information on author access to data; disclosure of potential conflicts of interest, including financial interests, activities, relationships, and affiliations; sources of funding and support; an explanation of the role of funder(s)/sponsor(s); names, degrees, and affiliations of participants in a large study or other group (ie, collaborators); any important disclaimers; information on previous presentation of the information reported in the manuscript; and the contributions, names, degrees, affiliations, and indication if compensation has been received for all persons who have made substantial contributions to the work but who are not authors. 2
All other persons who have made substantial contributions to the work reported in the manuscript (eg, data collection, analysis, and writing or editing assistance) but who do not fulfill the authorship criteria should be named with their specific contributions in an Acknowledgment in the manuscript.
Authors must obtain written permission to include the names of all individuals included in the Acknowledgment section, and the corresponding author must confirm that such permission has been obtained in the Authorship Form.
Authors should report the use of artificial intelligence, language models, machine learning, or similar technologies to create content or assist with writing or editing of manuscripts in the Acknowledgment section or the Methods section if this is part of formal research design or methods. This should include a description of the content that was created or edited and the name of the language model or tool, version and extension numbers, manufacturer, date(s) of use, and confirmation that the authors take responsibility for the integrity of the content generated. (Note: this does not include basic tools for checking grammar, spelling, references, etc.) See also Use of AI in Publication and Research and Statistical Analysis Subsection .
Requirements for Reporting
Authors of research articles should follow the EQUATOR Reporting Guidelines . See specific Study Types for detailed guidance on reporting.
Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording. To read more about use of causal language, see the AMA Manual of Style .
Research reports should be timely and current and should be based on data collected as recently as possible. Manuscripts based on data from randomized clinical trials should be reported as soon as possible after the trial has ended, ideally within 1 year after follow-up has been completed.
For cohort studies, the date of final follow-up should be no more than 5 years before manuscript submission. Likewise, data used in case-control or cross-sectional studies should have been collected as recently as possible, but no more than 5 years before manuscript submission. Manuscripts in which the most recent data have been collected more than 5 years ago ordinarily will receive lower priority for publication; thus, authors of such manuscripts should provide a detailed explanation of the relevance of the information in light of current knowledge and medical practice as well as the most recent date(s) of analysis of the study.
General Considerations
Authors are encouraged to consult "Reporting Statistical Information in Medical Journal Articles." 1 In the Methods section, describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to reproduce the reported results. Such description should include appropriate references to the original literature, particularly for uncommon statistical methods. For more advanced or novel methods, provide a brief explanation of the methods and appropriate use in the text and consider providing a detailed description in an online supplement.
In the reporting of results, when possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty, such as confidence intervals (see Reporting Standards and Data Presentation ). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important quantitative information. For observational studies, provide the numbers of observations. For randomized trials, provide the numbers randomized. Report losses to observation or follow up (see Missing Data ). For multivariable models, report all variables included in models, and report model diagnostics and overall fit of the model when available (see Statistical Procedures ).
Define statistical terms, abbreviations, and symbols, if included. Avoid nontechnical uses of technical terms in statistics, such as correlation, normal, predictor, random, sample, significant, trend. Do not use inappropriate hedge terms such as marginal significance or trend toward significance for results that are not statistically significant. Causal language (including use of terms such as effect and efficacy) should be used only for randomized clinical trials. For all other study designs (including meta-analyses of randomized clinical trials), methods and results should be described in terms of association or correlation and should avoid cause-and-effect wording.
Sample Size Calculations
For randomized trials, a statement of the power or sample size calculation is required (see the EQUATOR Network CONSORT Guidelines ). For observational studies that use an established population, a power calculation is not generally required when the sample size is fixed. However, if the sample size was determined by the researchers, through any type of sampling or matching, then there should be some justification for the number sampled. In any case, describe power and sample size calculations at the beginning of the Statistical Methods section, following the general description of the study population.
Descriptive Statistics
It is generally not necessary to provide a detailed description of the methods used to generate summary statistics, but the tests should be briefly noted in the Methods section (eg, ANOVA or Fisher exact test).
Statistical Procedures
Identify regression models with more than 1 independent variable as multivariable and regression models with more than 1 dependent variable as multivariate. Report all variables included in models, as well as any mathematical transformations of those variables. Provide the scientific rationale (clinical, statistical, or otherwise) for including variables in regression models.
For regression models fit to dependent data (eg, clustered or longitudinal data), the models should account for the correlations that arise from clustering and/or repeated measures. Failure to account for such correlation will result in incorrect estimates of uncertainty (eg, confidence intervals). Describe how the model accounted for correlation. For example, for an analysis based on generalized estimating equations, identify the assumed correlation structure and whether robust (or, sandwich) variance estimators were used. Or, for an analysis based on mixed-effects models, identify the assumed structure for the random effects, such as the level of random intercepts and whether any random slopes were included. Fixed-effects estimation should be described as conditional likelihood. Avoid the term fixed effects for describing covariates.
Missing Data
Report losses to observation, such as dropouts from a clinical trial or those lost to follow-up or unavailable in an observational study. If some participants are excluded from analyses because of missing or incomplete data, provide a supplementary table that compares the observed characteristics between participants with complete and incomplete data. Consider multiple imputation methods to impute missing data and include an assessment of whether data were missing at random. Approaches based on "last observation carried forward" should not be used.
Primary Outcomes, Multiple Comparisons, and Post Hoc Comparisons
Both randomized and observational studies should identify the primary outcome(s) before the study began, as well as any prespecified secondary, subgroup, and/or sensitivity analyses. Comparisons arrived at during the course of the analysis or after the study was completed should be identified as post hoc. For analyses of more than 1 primary outcome, corrections for multiple testing should generally be used. For secondary outcomes, address multiple comparisons or consider such analyses as exploratory and interpret them as hypothesis-generating. The reporting of all outcomes should match that included in study protocols. For randomized clinical trials, protocols with complete statistical analysis plans should be cited in the Methods section and submitted as online supplementary content. Randomized clinical trials should be primarily analyzed according to the intention-to-treat approach. Deviations from strict intention-to-treat analysis should be described as "modified intention-to-treat," with the modifications clearly described.
Statistical Analysis Subsection
At the end of the Methods section, briefly describe the statistical tests used for the analysis. State any a priori levels of significance and whether hypothesis tests were 1- or 2-sided. Also include the statistical software used to perform the analysis, including the version and manufacturer, along with any extension packages (eg, the svy suite of commands in Stata or the survival package in R). Do not describe software commands (eg, SAS proc mixed was used to fit a linear mixed-effects model). If analysis code is included, it should be placed in the online supplementary content.
Reporting Standards and Data Presentation
Analyses should follow EQUATOR Reporting Guidelines and be consistent with the protocol and statistical analysis plan, or described as post hoc.
When possible, present numerical results (eg, absolute numbers and/or rates) with appropriate indicators of uncertainty, such as confidence intervals. Include absolute numbers and/or rates with any ratio measures and avoid redundant reporting of relative data (eg, % increase or decrease). Use means and standard deviations (SDs) for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data that are not normally distributed. Avoid solely reporting the results of statistical hypothesis testing, such as P values, which fail to convey important quantitative information. For most studies, P values should follow the reporting of comparisons of absolute numbers or rates and measures of uncertainty (eg, 0.8%, 95% CI −0.2% to 1.8%; P = .13). P values should never be presented alone without the data that are being compared. If P values are reported, follow standard conventions for decimal places: for P values less than .001, report as " P <.001"; for P values between .001 and .01, report the value to the nearest thousandth; for P values greater than or equal to .01, report the value to the nearest hundredth; and for P values greater than .99, report as " P >.99." For studies with exponentially small P values (eg, genetic association studies), P values may be reported with exponents (eg, P = 1×10 −5 ). In general, there is no need to present the values of test statistics (eg, F statistics or χ² results) and degrees of freedom when reporting results.
For secondary and subgroup analyses, there should be a description of how the potential for type I error due to multiple comparisons was handled, for example, by adjustment of the significance threshold. In the absence of some approach, these analyses should generally be described and interpreted as exploratory, as should all post hoc analyses.
For randomized trials using parallel-group design, there is no validity in conducting hypothesis tests regarding the distribution of baseline covariates between groups; by definition, these differences are due to chance. Because of this, tables of baseline participant characteristics should not include P values or statements of statistical comparisons among randomized groups. Instead, report clinically meaningful imbalances between groups, along with potential adjustments for those imbalances in multivariable models. To read more about statistical tests and data presentation, see the AMA Manual of Style .
Researchers are encouraged to report studies that include diverse and representative participants and to indicate participant inclusion and exclusion criteria and how the findings generalize to the population(s) that are the focus of or are compatible with the research question. Aggregate, deidentified demographic information (eg, age, sex, race and ethnicity, and socioeconomic indicators) should be reported for all research reports along all prespecified outcomes. Demographic variables collected for a specific study should be reported in the Methods section. Demographic information assessed should be reported in the Results section, either in the main article or in an online supplement or both. If any demographic characteristics that were collected are not reported, the reason should be stated. Summary demographic information (eg, baseline characteristics of study participants) should be reported in the first line of the Results section of Abstracts.
Reporting Age
Study inclusion or exclusion criteria by age or age group should be defined in the Methods section. Stratification by age groups should be based on relevance to disease, condition, or population (eg, <5 or >65 years). The ages for study participants should be reported in aggregate (ie, mean and SD or median and IQR or range) in the Results section.
Reporting Sex and Gender
The term sex should be used when reporting biological factors and gender should be used when reporting gender identity or psychosocial/cultural factors. The methods used to obtain information on sex, gender, or both (eg, self-reported, investigator observed or classified, or laboratory test) should be explained in the Methods section. 12 The distribution of study participants or samples should be reported in the Results section, including for studies of humans, tissues, cells, or animals. All participants should be reported, not just the category that represents the majority of the sample. Studies that address pregnancy should follow these recommendations, and if the gender identity of participants was not assessed, use the terms "pregnant participants," "pregnant individuals," "pregnant patients," etc, as appropriate.
In research articles, sex or gender should be reported and defined, and how sex or gender was assessed should be described. Whenever possible, all main outcomes should be reported by sex (or gender if appropriate). In nonresearch reports, choose sex-neutral terms that avoid bias, suit the material under discussion, and do not intrude on the reader's attention.
Reporting Race and Ethnicity
The Methods section should include an explanation of who identified participant race and ethnicity and the source of the classifications used (eg, self-report or selection, investigator observed, database, electronic health record, survey instrument).
If race and ethnicity categories were collected for a study, the reasons that these were assessed also should be described in the Methods section. If collection of data on race and ethnicity was required by the funding agency, that should be noted.
Specific racial and ethnic categories are preferred over collective terms, when possible. Authors should report the specific categories used in their studies and recognize that these categories will differ based on the databases or surveys used, the requirements of funders, and the geographic location of data collection or study participants. Categories included in groups labeled as "other" should be defined.
Categories should be listed in alphabetical order in text and tables.
Race and ethnicity of the study population should be reported in the Results section.
For additional information, see " Updated Guidance on Reporting Race and Ethnicity in Medical and Science Journals " and the Summary Guide for Preferred Terms When Reporting Race and Ethnicity .
For all manuscripts reporting data from studies involving human participants or animals, formal review and approval, or formal review and waiver, by an appropriate institutional review board or ethics committee is required and should be described in the Methods section. 2(p226) For those investigators who do not have formal ethics review committees, the principles outlined in the Declaration of Helsinki should be followed. 13 For investigations of humans, state in the Methods section the manner in which informed consent was obtained from the study participants (ie, oral or written) and whether participants received a stipend. Authors of research studies involving humans should not make independent determinations of exemption or exclusion of IRB or ethical review; they should cite the institutional or regulatory policy for that determination and indicate if the data are deidentified and publicly available or protected by prior consent or privacy safeguards. Editors may request that authors provide documentation of the formal review and recommendation from the institutional review board or ethics committee responsible for oversight of the study.
A signed statement of informed consent to publish patient descriptions, photographs, video, and pedigrees should be obtained from all persons (parents or legal guardians for minors) who can be identified (including by the patients themselves) i/n such written descriptions, photographs, or pedigrees and should be submitted with the manuscript and indicated in the Acknowledgment section of the manuscript. Such persons should be offered the opportunity to see the manuscript before its submission. 2(pp229-232)
Omitting data or making data less specific to deidentify patients is acceptable, but changing any such data is not acceptable. Only those details essential for understanding and interpreting a specific case report or case series should be provided. Although the degree of specificity needed will depend on the context of what is being reported, specific ages, race/ethnicity, and other sociodemographic details should be presented only if clinically or scientifically relevant and important. 2 Cropping of photographs to remove identifiable personal features that are not essential to the clinical message may be permitted as long as the photographs are not otherwise altered. Please do not submit masked photographs of patients. Patients' initials or other personal identifiers must not appear in an image.
Patient Permission Form:
The Patient Permission form for publication of identifying material is available here . Translated versions in Arabic, Chinese, French, German, Hindi, Italian, Japanese, Portuguese, and Spanish are available on request.
AI Used in Manuscript Preparation
When traditional and generative AI technologies are used to create, review, revise, or edit any of the content in a manuscript, authors should report in the Acknowledgment section the following:
- Name of the AI software platform, program, or tool
- Version and extension numbers
- Manufacturer
- Date(s) of use
- A brief description of how the AI was used and on what portions of the manuscript or content
- Confirmation that the author(s) take responsibility for the integrity of the content generated
Note this guidance does not apply to basic tools for checking grammar, spelling, references, and similar.
AI Used in Research
When AI (eg, large language model [LLM] or natural language processing [NLP], supervised or unsupervised machine learning [ML] for predictive/prescriptive or clustering tasks, chatbots, or similar other technologies) is used as part of a scientific study, authors should:
- Follow relevant reporting guidelines for specific study designs when they exist and report each recommended guideline element with sufficient detail to enable reproducibility.
- Avoid inclusion of identifiable patient information in text, tables, and figures.
- Be aware of copyright and intellectual property concerns - if including content (text, images) generated by AI, and indicate rights or permissions to publish that content as determined by the AI service or owner.
Also address the following:
Methods Section
- Include the study design and, if a relevant reporting guideline exists, indicate how it was followed, with sufficient detail to enable reproducibility.
- Describe how AI was used for specific aspects of the study (eg, to generate or refine study hypotheses, assist in the generation of a list of adjustment variables, create graphs to show visual relationships).
- For studies using LLMs, provide the name of the platform or program, tool, version, and manufacturer; specify dates and prompt(s) used and their sequence and any revisions to prompts in response to initial outputs.
- For studies reporting ML and algorithm development, include details about data sets used for development, training, and validation. Clearly state if algorithms were trained and tested only on previously collected or existing data sets or if the study includes prospective deployment. Include the ML model and describe the variables and outcome(s) and selection of the fine-tuning parameters. Describe any assumptions involved (eg, log linearity, proportionality) and how these assumptions were tested.
- Indicate the metric used to evaluate the performance of the algorithms, including bias, discrimination, calibration, reclassification, and others as appropriate.
- Indicate the methods used to address missing data.
- Indicate institutional review board/ethics review, approval, waiver, or exemption.
- Describe methods or analyses included to address and manage AI-related methodologic bias and inaccuracy of AI-generated content.
- Indicate, when appropriate, if sensitivity analyses were performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
- Provide a data sharing statement, including if code will be shared.
Results Section
- When reporting comparisons, provide performance assessments (eg, against standard of care), include effect sizes and measures of uncertainty (eg, 95% CIs) and other measurements such as likelihood ratios, and include information about performance errors, inaccurate or missing data, and sufficient detail for others to reproduce the findings.
- Report the results of analyses to address methodologic bias and population representation.
- If examples of generated text or content are included in tables or figures, be sure to indicate the source and licensing information, as noted above.
Discussion Section
- Discuss the potential for AI-related bias and what was done to identify and mitigate such bias.
- Discuss the potential for inaccuracy of AI-generated content and what was done to identify and manage this.
- Discuss generalizability of findings across populations and results of analyses performed to explore the performance of the AI model in vulnerable or underrepresented subgroups.
A signed statement of permission should be included from each individual identified as a source of information in a personal communication or as a source for unpublished data, and the date of communication and whether the communication was written or oral should be specified. 2(p199) Personal communications should not be included in the list of references but added to the text parenthetically.
Authors and reviewers are expected to notify editors if a manuscript could be considered to report dual use research of concern (ie, research that could be misused by others to pose a threat to public health and safety, agriculture, plants, animals, the environment, or material). 14 The editor in chief will evaluate manuscripts that report potential dual use research of concern and, if necessary, consult additional reviewers.
Journal Policies
Final decisions regarding manuscript publication are made by the editor in chief or a designated editor who does not have any relevant conflicts of interest. The journal has a formal recusal process in place to help manage potential conflicts of interest of editors. In the event that an editor has a conflict of interest with a submitted manuscript or with the authors, the manuscript, review, and editorial decisions are managed by another designated editor without a conflict of interest related to the manuscript.
All authors are required to complete and submit a Publishing Agreement that is part of the journal's electronic Authorship Form. In this agreement, authors will transfer copyright or a publication license; or indicate that they are employed by a federal government; or indicate that they are an employee of an institution that considers the work in the manuscript a work for hire, in which case an authorized representative of that institution will assign copyright or a publication license on the author's behalf.
Published articles become the permanent property of the American Medical Association (AMA) and may not be published elsewhere without written permission. Unauthorized use of the journal's name, logo, or any content for commercial purposes or to promote commercial goods and services (in any format, including print, video, audio, and digital) is not permitted by the JAMA Network or the AMA.
1. Cummings P, Rivara FP. Reporting statistical information in medical journal articles. Arch Pediatr Adolesc Med . 2003;157(4):321-324. doi:10.1001/archpedi.157.4.321
2. Iverson C, Christiansen S, Flanagin A, et al. AMA Manual of Style: A Guide for Authors and Editors . 11th ed. Oxford University Press; 2020. http://www.amamanualofstyle.com
3. Golub RM. Correspondence course: tips for getting a letter published in JAMA . JAMA . 2008;300(1):98-99. doi:10.1001/jama.300.1.98
4. International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Updated May 2023. Accessed May 18, 2023. http://www.icmje.org/recommendations/
5. Fontanarosa PB, Flanagin A, DeAngelis CD. Update on JAMA 's policy on release of information to the public. JAMA . 2008;300(13):1585-1587. doi:10.1001/jama.300.13.1585
6. Fontanarosa P, Bauchner H, Flanagin A. Authorship and team science. JAMA . 2017;318(24):2433-2437. doi:10.1001/jama.2017.19341
7. Fontanarosa PB, Flanagin A, DeAngelis CD. Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies. JAMA . 2005;294(1):110-111. doi:10.1001/jama.294.1.110
8. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA . 2004;292(11):1363-1364. doi:10.1001/jamainternmed.2014.6933
9. DeAngelis CD, Drazen JM, Frizelle FA, et al; International Committee of Medical Journal Editors. Is this clinical trial fully registered? a statement from the International Committee of Medical Journal Editors. JAMA . 2005;293(23):2927-2929. doi:10.1001/jama.293.23.jed50037
10. The CONSORT Group. The CONSORT statement. Updated 2014. Accessed September 23, 2016. http://www.consort-statement.org/consort-2010
11. American Association for Public Opinion Research. Best practices for survey research. Accessed March 23, 2023. https://aapor.org/standards-and-ethics/best-practices/
12. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA . 2016;316(18):1863-1864. doi:10.1001/jama.2016.16405
13. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA . 2013;310(20):2191-2194. doi:10.1001/jama.2013.281053
14. Journal Editors and Authors Group. Statement on scientific publication and security. Science . 2003;299(5610):1149. doi:10.1126/science.299.5610.1149 . Published correction appears in Science . 2003;299(5614):1845.
15. Christiansen S, Flanagin A. Correcting the medical literature: "to err is human, to correct divine." JAMA . 2017;318(9):804-805. doi:10.1001/jama.2017.11833
Last Updated: April 25, 2024
Quick Links
- Why Publish in JAMA
- Submit and Track Your Manuscript
- Author Reprints
- Permissions Requests
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 15 May 2024
Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: a case control study
- Shivaani Mariapun 1 , 2 ,
- Weang-Kee Ho 1 , 2 ,
- Mikael Eriksson 3 , 9 ,
- Nur Aishah Mohd Taib 4 , 5 ,
- Cheng-Har Yip 5 , 6 ,
- Kartini Rahmat 4 , 7 ,
- Per Hall 3 , 8 &
- Soo-Hwang Teo 1 , 4
Breast Cancer Research volume 26 , Article number: 79 ( 2024 ) Cite this article
Metrics details
Mammographic density (MD) has been shown to be a strong and independent risk factor for breast cancer in women of European and Asian descent. However, the majority of Asian studies to date have used BI-RADS as the scoring method and none have evaluated area and volumetric densities in the same cohort of women. This study aims to compare the association of MD measured by two automated methods with the risk of breast cancer in Asian women, and to investigate if the association is different for premenopausal and postmenopausal women.
In this case–control study of 531 cases and 2297 controls, we evaluated the association of area-based MD measures and volumetric-based MD measures with breast cancer risk in Asian women using conditional logistic regression analysis, adjusting for relevant confounders. The corresponding association by menopausal status were assessed using unconditional logistic regression.
We found that both area and volume-based MD measures were associated with breast cancer risk. Strongest associations were observed for percent densities (OR (95% CI) was 2.06 (1.42–2.99) for percent dense area and 2.21 (1.44–3.39) for percent dense volume, comparing women in highest density quartile with those in the lowest quartile). The corresponding associations were significant in postmenopausal but not premenopausal women (premenopausal versus postmenopausal were 1.59 (0.95–2.67) and 1.89 (1.22–2.96) for percent dense area and 1.24 (0.70–2.22) and 1.96 (1.19–3.27) for percent dense volume). However, the odds ratios were not statistically different by menopausal status [ p difference = 0.782 for percent dense area and 0.486 for percent dense volume].
Conclusions
This study confirms the associations of mammographic density measured by both area and volumetric methods and breast cancer risk in Asian women. Stronger associations were observed for percent dense area and percent dense volume, and strongest effects were seen in postmenopausal individuals.
Mammographic density (MD) reflects the composition of fibro-glandular tissue of the breast, as visualised on a mammogram. MD is an independent predictor of breast cancer risk, although the strength of its association varies across studies, due in part to the different methods of MD assessment and different partitioning thresholds used to define high and low MD [ 1 , 2 , 3 ]. Efforts to make measuring MD less reader-dependent and more reproducible have resulted in the development of a number of fully-automated methods for measuring MD [ 4 , 5 , 6 ], including both volumetric and area-based assessments methods.
In women of European ancestry Volumetric assessments of density have been shown to be a stronger predictor of risk compared to area-based density [ 7 , 8 ]. Volumetric methods are less influenced by compression force and are more sensitive to breast thickness, and may more accurately estimate the amount of fibroglandular tissue for women with larger breasts [ 9 , 10 , 11 ]. However Asian women have smaller and denser breasts compared to women of European ancestry, and the performance of area and volume-based densities have hitherto not been compared in the same study.
In this study, we aim to determine and compare the effects of two automated MD measures, namely STRATUS measurements of area densities, and Volpara measurements of volumetric densities, on breast cancer risk in the Asian population, and to explore the potential variation by menopausal status.
Study participants, data collection and eligibility criteria
Cases comprised of patients who were recruited sequentially into the Malaysian Breast Cancer Genetics (MyBrCa) study from Subang Jaya Medical Centre (SJMC), between 2012 and 2020, and University Malaya Medical Centre (UMMC), between 2003 and 2020. Controls were women between 40 and 74 years old with no prior history of breast cancer that were recruited into the Malaysian Mammography Study (MyMammo) from the same participating hospitals as cases. The study details have been previously published [ 12 ]. All participants answered a detailed questionnaire which included information on lifestyle and reproductive risk factors, socio-demographic factors, and family history and provided blood sample for genetic testing.
Bilateral full-field digital mammograms (FFDMs) for cases were retrieved from the medical image storage servers retrospectively starting in June 2018 and for controls were collected at recruitment. The bilateral cranio-caudal (CC) and medio-lateral oblique (MLO) views for both raw and processed images, where possible, were retrieved. Cases were excluded from the research study if: (a) digital mammograms were conducted more than 12 months prior to cancer diagnosis, (b) only mammograms ipsilateral to the breast cancer were available. Controls were excluded from the research study if no mammograms were available for analysis. All participants included in the study were of self-declared Chinese, Malay or Indian ethnicity and had information on age at mammography, body mass index (BMI) and/or menopausal status. In total, 10% of cases and 69% of controls were available and eligible for matching.
For the case–control analysis of mammographic density and breast cancer risk, as raw and processed images were not available for all women, cases and controls in the full dataset were matched for age (within 5 years) and ethnicity (exact) separately for the analyses of STRATUS, which measures processed images, and Volpara, which measures raw images. Age-matching was performed in each ethnic group using a 1:4 case to control ratio nearest neighbour propensity score matching using the matchit package in R . For the STRATUS study, a total of 488 cases and 1796 controls were included in the matched case–control study, of which 82.2% of cases were matched to four controls, 9% to three controls, 3.5% to two controls and 5.3% to only one control. For the Volpara study, a total of 436 cases and 1623 controls were included, of which 81.4% were matched to four controls, 12.4% to three controls, 3.2% to two controls and 3% to only one control. In total, 531 cases and 2297 controls were included for analysis of which data was available for both STRATUS and Volpara in 393 cases and 1122 controls.
Mammographic density (MD) assessments
Mammography was performed using machines from three different manufacturers; Hologic [Models: Lorad Selenia, Selenia Dimensions and Tomo Selenia Dimensions], General Electric (GE) Senographe Essential, and Siemens Mammomat Novation. Area-based MD was determined using STRATUS, a fully automated machine-learning method for assessing MD based on image features assessed using thresholding methods, by the developers of STRATUS at the Karolinska Institute, Sweden [ 4 ]. Volumetric MD was computed using Volpara Data Manager version 1.1.109 [ 5 ]. Six MD phenotypes were considered in this study: absolute dense area (DA) and volume (DV), percent dense area (PDA, i.e., absolute dense area/total breast area) and volume (PDV, i.e., absolute dense volume/total breast volume), and non-dense area (NDA) and volume (NDV). We also categorised MD according to the computer-generated BI-RADS scores (cBIRADS) generated by STRATUS, and the clinical classification score (Volpara Density Grades (VDG)).
Image laterality
Pearson’s correlation coefficients and previous studies showed that there were strong correlations between CC and MLO measurements [ 13 , 14 ]. The Wilcoxon rank sum test was performed to compare the distribution of MD in the left and right mammograms in the control group. For the CC view mammograms, percent dense volume was higher in the right breast (Left median 9.1%; Right 9.5%, P = 0.035), whereas for the MLO view, three measures were higher in the left breast [dense volume (Left 57.6 cm 3 ; Right 56.5 cm 3 , P = 0.006), non-dense volume (Left 591.8 cm 3 ; Right 561.7 cm 3 , P = 0.011) and total breast volume (Left 653.4 cm 3 ; Right 628.0 cm 3 , P = 0.006)]. As there was less variation in MD measurements for the CC view, MD measurements from the CC view mammograms of unaffected breasts of cases were used in all analyses, and matched by laterality in the controls.
Statistical analyses
Box-Cox transformation was used to transform MD phenotypes into approximately normal distribution.
Confounder selection
Covariates that were assessed include socio-demographic factors, known lifestyle and reproductive risk factors of breast cancer, mammogram machine and compressed breast thickness. A covariate was considered confounding if: (a) it was significantly associated with MD in controls at P < 0.05, after accounting for other associated variables; (b) it was significantly associated with breast cancer risk at P < 0.05, after accounting for other associated variables; and (c) it had a magnitude of confounding that was greater than 5%.
Age at first full term pregnancy, total number of live births and breast feeding were only evaluated among parous women. Parous women were defined as those who have had at least one full-term pregnancy. The use of hormone replacement therapy (HRT) was only evaluated among postmenopausal women. Postmenopausal women were defined as women who have not had their periods for at least 12 months prior to their enrolment into the study or if they self-reported that they were postmenopausal at enrolment.
Association of mammographic density (MD) phenotypes and breast cancer risk
We assessed the association between mammographic density phenotypes (treated either as continuous or categorical variables) and breast cancer risk using conditional logistic regression, adjusting for selected confounders. When MD was treated as a continuous variable, odds ratios per-adjusted standard deviations (OPERA [ 15 ]) was calculated to allow comparison across MD phenotypes. When MD was treated as a categorical variable, MD phenotypes were categorised into four equal quartiles based on the MD distribution in controls, using the first quartile as the reference group. We also categorised MD according to the computer-generated BI-RADS scores, cBIRADS, generated by STRATUS, and Volpara Density Grades (VDG), which is the classification used to report density, measured by Volpara, in the clinic. Weighted kappa, using quadratic weighting, was calculated to assess the concordance between quantiles of STRATUS and Volpara measurements.
The association between MD phenotypes and breast cancer risk by menopausal status were conducted using unconditional regression. Z-tests were conducted to determine whether the odds ratios for mammographic densities and breast cancer risk were different for premenopausal and postmenopausal women.
All statistical analyses were performed with R version 3.6.1.
Characteristics of study participants
Participant selection and descriptive statistics of cases and controls are presented in Fig. 1 and Table 1 . The majority of controls within the STRATUS study (67.7%) were recruited from the private tertiary hospital (SJMC), while approximately half of the controls within the Volpara study were from the government-funded teaching hospital (UMMC). Most of the mammograms were obtained from the Hologic machine.
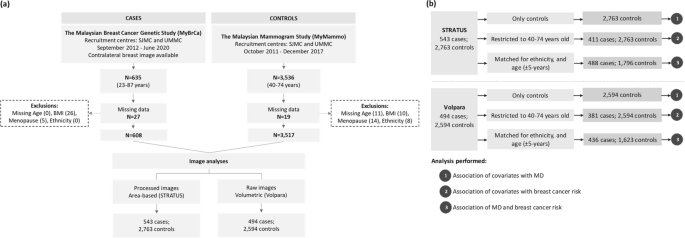
Flowchart illustrating a the selection of cases and controls for mammographic density (MD) assessment by STRATUS and Volpara, and b participants included in the different analyses performed including the analysis of (1) the association of covariates with MD, (2) the association of covariates with breast cancer risk, and (3) the association of MD and breast cancer risk
Confounders
We identified potential confounders as covariates with P value < 0.05 with both MD phenotypes and breast cancer risk in the multivariable models, and these were breastfeeding for absolute dense area and dense volume, alcohol intake for non-dense volume and breast thickness for all MD phenotypes except dense area (Additional file 1 : Table S1). Additionally, although not significant in our study, menopausal status and parity were included as potential confounders as these variables have consistently been reported to be associated with both MD and breast cancer risk in the literature. Of the list of potential confounders, only those resulting in > 5% change in the magnitude of MD association with risk were retained in the model for adjustment. The final list of variables included in the association analyses for adjustment can be found Additional file 1 : Table S2.
When treated as a continuous variable, both dense area and dense volume were significantly associated with breast cancer risk, the odds per adjusted standard deviation (OPERA) and the corresponding 95% CI were 1.19 (1.08–1.32) and 1.14 (1.02–1.28), respectively (Fig. 2 ). However, when categorised into quartiles, only the highest quartile of dense area was significantly associated with risk (odds ratio (95% CI) was 1.44 (1.03–1.21)). This association was no longer significant in analyses limited to overlapping samples between the STRATUS and Volpara studies (1.26, 95% CI: 0.83-1.91) (Fig. 3 ).
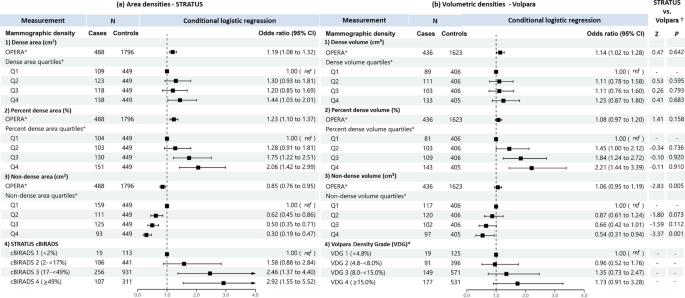
Associations of a STRATUS area mammographic densities and b Volpara volumetric mammographic densities, with breast cancer risk. *Adjusted for relevant confounding factors. † Z-tests comparing estimated regression coefficients between the STRATUS and Volpara studies
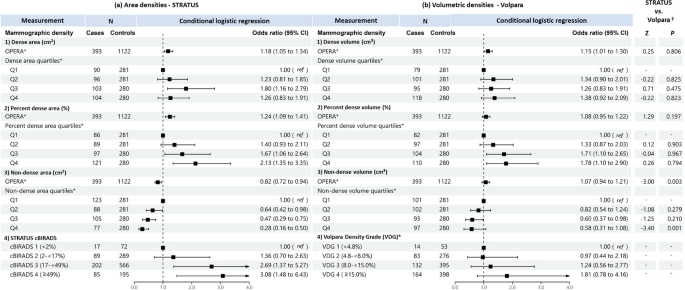
Associations of a STRATUS area mammographic densities, b Volpara volumetric mammographic densities, with breast cancer risk in the dataset of 393 cases and 1122 controls included in both STRATUS and Volpara studies. *Adjusted for relevant confounding factors. † Z-tests comparing estimated regression coefficients between the STRATUS and Volpara studies
For percent density, OPERA for percent dense area was significant (1.23, 95% CI 1.10–1.37) while the OPERA for percent dense volume was not significant (1.08, 95% CI 0.97–1.20). However, quartiles analyses of percent density showed significant association for both MD measurement methods, with risk estimates increased consistently across quartiles. The OR of highest versus lowest quartile was 2.06 (95% CI 1.42–2.99) for percent dense area and 2.21 (95% CI 1.44–3.39) for percent dense volume (Fig. 2 ). There was no significant difference between the ORs of percent dense area and percent dense volume (p-value of Z-test < 0.05). Similar results were observed for analyses limited to overlapping samples between the STRATUS and Volpara studies (Fig. 3 ).
Non-dense area was significantly associated with a lower breast cancer risk (OPERA 0.85, 95% CI 0.76–0.95). Risk estimates decreased consistently, from OR 0.62 (95% CI: 0.45-0.86) to 0.50 (95% CI: 0.35-0.71) and 0.30 (95% CI: 0.19-0.47), comparing the first quartile of non-dense area with the second, third and fourth quartile, respectively. By contrast, the OPERA estimate for non-dense volume was not significant (1.06, 95% CI: 0.95-1.19), although the pattern of association for quartiles was similar to that observed for non-dense area i.e., OR 0.87 (95% CI: 0.61-1.24), OR 0.66 (95% CI: 0.42-1.01) and OR 0.54 (95% CI: 0.31-0.94) for the second, third and fourth quartiles respectively, the corresponding strengths of association were weaker (Figs. 2 and 3 ).
Where densities were categorised according to area-based cBIRADS and volume-based VDG, women in cBIRADS 3 and cBIRADS 4 were associated with a 2.5-fold ( P = 0.003) and 2.9-fold ( P < 0.001) greater odds of disease, respectively (Fig. 2 a). By contrast, VDG was not associated with breast cancer risk (Fig. 2 b). The same pattern was observed for analyses limited to overlapping samples between the STRATUS and Volpara studies (Fig. 3 ).
There are no appreciable differences between the results generated using the CC and MLO view measurements (Additional file 1 : Table S2).
The agreement between the STRATUS and Volpara measurements for classifying women into mammographic density quartiles was fair for absolute density (Weighted Kappa, κw = 0.28) and percent density (0.35), and moderate for non-dense area and volume (0.50). Figure 4 illustrates the magnitude of concordance for the classification of area and volumetric MD quartiles. Although there is some agreement between STRATUS and Volpara, there are instances of discordance where individuals shift to adjacent quartiles or even skip one quartile altogether.
Concordance between the classification of a absolute dense area/volume, b percent dense area/volume and c non-dense area/volume into quartiles using STRATUS and Volpara measurements. Note: Agreement between the STRATUS and Volpara measurements for classifying women into mammographic density quartiles was calculated using Cohen’s weighted kappa. Weighted Kappa, κw values for dense area/volume, percent dense area/volume, and non-dense area/volume were 0.28, 0.35 and 0.50, respectively
Analyses by menopausal status
Figures 5 and 6 show the association of MD with breast cancer risk for premenopausal and postmenopausal women, respectively. For dense area and dense volume, both OPERAs and quantile analyses were not significantly associated with breast cancer risk in premenopausal women. By contrast, consistent with the all-women analysis, OPERA for both dense area (OR 1.23, 95% CI: 1.07–1.41) and dense volume (OR 1.30, 95% CI: 1.10–1.54) were significant in postmenopausal women, but the corresponding quartile analyses did not show significant associations for dense area and was only significant for the association of the highest dense volume quartile and risk when compared to the lowest dense volume quartile among postmenopausal women (OR 1.65, 95% CI: 1.01-2.71).
Associations of a STRATUS area mammographic densities and b Volpara volumetric mammographic densities, with breast cancer risk in premenopausal women. *Adjusted for relevant confounding factors. † Reference category is cBIRADS 1 (< 2%) + cBIRADS 2 (2− < 17%). ‡ Reference category is VDG 1 (< 4.8%) + VDG 2 (4.8—< 8.0%). § Z-tests comparing estimated regression coefficients between the STRATUS and Volpara studies
Associations of a STRATUS area mammographic densities, b Volpara volumetric mammographic densities, with breast cancer risk in postmenopausal women and, c comparison of regression coefficients for premenopausal and postmenopausal women. *Adjusted for relevant confounding factors. † Z-tests comparing estimated regression coefficients between the STRATUS and Volpara studies. ‡ Z-tests comparing estimated regression coefficients between premenopausal and postmenopausal women
For percent density, OPERAs for percent dense area and percent dense volume were significant in postmenopausal women but not premenopausal women. The OPERAs were 1.23 (95% CI 1.07–1.41) and 1.16 (95% CI 1.01–1.34) for precent dense area and percent dense volume, respectively, in postmenopausal women. The corresponding estimates in premenopausal women were 1.16 (95% CI 0.99–1.35) and 1.01 (95% CI 0.86–1.19). The observed significant association of highest versus lowest quartile in all women analysis was replicated in postmenopausal women (OR 1.89, 95% CI: 1.22-2.96 for percent dense area; OR 1.96, 95% CI: 1.19-3.27 for percent dense volume), but not premenopausal women (OR 1.59, 95% CI: 0.95-2.67 for percent dense area; OR 1.24, 95% CI: 0.70-2.22 for percent dense volume).
For non-dense MD phenotype in both premenopausal and postmenopausal women, OPERAs were not significant in both non-dense area (0.88, 95% CI: 0.75-1.02 and 0.87, 95% CI: 0.75-1.01 for premenopausal and postmenopausal women, respectively) and non-dense volume (1.11, 95% CI: 0.95-1.31 and 1.00, 95% CI: 0.85-1.18, respectively) measurements. However, the quartile analyses for both non-dense area and non-dense volume were significant in premenopausal women, comparing the highest and lowest non-dense area quartiles (OR 0.37, 95% CI: 0.20-0.66) and non-dense volume quartiles (OR 0.31, 95% CI: 0.14-0.67), but not postmenopausal women (OR 0.60, 95% CI: 0.36-1.01 for non-dense area; OR 0.88, 95% CI: 0.51-1.54 for non-dense volume).
In this study of women of Asian-ancestry, we found that percent mammographic density is a strong breast cancer risk factor, with similar magnitudes of association for both area and volumetric mammographic density measures. Comparing women in the lowest quartiles, women with percent density in the highest quartiles had approximately two-fold higher odds of breast cancer. The observed association was however significant only in postmenopausal women but not in premenopausal women.
The two-fold risk estimates reported in this study are consistent with those found in a meta-analysis of Japanese, Korean and Singaporean women comprising of one cohort study and five case–control studies, which reported a summary effect size of 2.2 (95% CI 1.5–3.2) [ 16 ], as well as with a large meta-analysis of European women using the BI-RADS density four-category classification [ 3 ]. The corresponding odds ratio per adjusted standard deviation (OPERA) was similar to a Korean study of 213 cases and 630 controls [ 17 ], but lower than those previously reported in women of European ancestry. A study of Australian women reported OPERA of 1.52 (95% CI 1.34–1.73) for percent dense area, compared to 1.23 (95% CI 1.10–1.37) this study, suggesting potential ethnic differences in MD-risk associations [ 18 ].
Our findings of lack of MD-risk association in premenopausal women align with similar-sized studies in other Asian populations [ 19 , 20 , 21 , 22 ]. For instance, a multicentre Japanese study (530 cases, 1043 controls) found a near three-fold increase in breast cancer odds (OR 2.9, 95% CI 1.1–7.2) among postmenopausal women with extremely dense breast (>75% glandular tissue), while no significant association was observed in premenopausal women [ 19 ]. Similarly, another study in Japanese women (146 cases, 659 controls) revealed a four-fold higher odds of breast cancer among postmenopausal women with > 75% percent densities, with no significant association in premenopausal women [ 21 ]. However, it is important to note that a recent large prospective Korean study comprising of ~ 65,000 breast cancer cases reported that breast density is associated with breast cancer risk in both premenopausal (OR 2.4, 95% CI 2.2–2.5) and postmenopausal (OR 2.9, 95% CI 2.8–3.0) women, suggesting that larger sample sizes in premenopausal women are required to detect a significant association with breast cancer risk [ 20 ].
Our study did not yield conclusive evidence regarding the association of absolute MD measures with breast cancer risk. While the odds ratios for continuous dense area and dense volume were significant at a nominal level (1.19, 95% CI 1.08–1.32 and 1.14, 95% CI 1.02–1.28, respectively), the results from quartile analysis did not support the significant associations. We also observed a stronger inverse association with non-dense area compared to non-dense volume that was significant in our analyses of all women and premenopausal women, but not that of postmenopausal women. This inverse association is consistent with previous studies in women of European ancestry reporting a protective effect of having greater amounts of fat or non-dense tissue in the breast [ 23 ].
In summary, our study confirms the significance of MD as a robust breast cancer risk factor in Asian-ancestry women, with percent density showing consistent associations across area and volumetric-based measures. However, the lack of MD-risk association in premenopausal women underscores the need for further investigation in larger datasets. While our findings contribute to the understanding of MD and breast cancer risk, the inconclusive evidence regarding absolute MD measures prompts a critical evaluation of their utility in risk prediction models for this population.
This study had several limitations. First, more than 90% of the cases were recruited from one recruitment centre, making it impossible to match cases and controls based on centre. However, we adjusted our analyses for this factor. Second, some covariates have missingness rates greater than 10%, which may explain some of the unexpected results (e.g. the protective effect observed for HRT among postmenopausal women and alcohol consumption). Third, the healthy controls were women attending an opportunistic screening mammography programme and may be enriched for a family history of breast cancer. This is likely to be the reason family history of breast cancer is not associated with breast cancer risk in this study. Finally, only the mammograms performed at the time of cancer detection (or close to cancer detection) were available for the cases. Given that densities measured from the unaffected contralateral breasts have been shown to be similarly associated with risk of disease [ 8 ], densities of the contralateral breasts were used as surrogate measurements.
In conclusion, our study underscores the significance of mammographic density (MD) as a strong predictor of breast cancer risk in women of Asian-ancestry, particularly in postmenopausal individuals. While percent density, for both area- and volume-based measures, consistently demonstrated significant association, absolute MD measures yielded inconclusive results. Future research should aim to elucidate ethnic-specific MD-risk associations and refine risk prediction models to incorporate the most predictive MD measures, thus enabling more targeted preventive strategies for women of Asian ancestry.
Availability of data and materials
Datasets described and analysed in this manuscript are available from the corresponding author on reasonable request.
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36.
Article CAS PubMed Google Scholar
McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–69.
Article Google Scholar
Bodewes FTH, van Asselt AA, Dorrius MD, Greuter MJW, de Bock GH. Mammographic breast density and the risk of breast cancer: a systematic review and meta-analysis. Breast. 2022;66:62–8.
Article CAS PubMed PubMed Central Google Scholar
Eriksson M, Li J, Leifland K, Czene K, Hall P. A comprehensive tool for measuring mammographic density changes over time. Breast Cancer Res Treat. 2018;169(2):371–9.
Article PubMed PubMed Central Google Scholar
Highnam R, et al., editors. Robust breast composition measurement – Volpara. In: Proceedings of the 10th international workshop on Digital Mammography IWDM’2010; 2010; Berlin, Heidelberg: Springer
Destounis S, Arieno A, Morgan R, Roberts C, Chan A. Qualitative versus quantitative mammographic breast density assessment: Applications for the US and Abroad. Diagnostics (Basel). 2017;7(2):30.
Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, et al. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomark Prev. 2011;20(7):1473–82.
Astley SM, Harkness EF, Sergeant JC, Warwick J, Stavrinos P, Warren R, et al. A comparison of five methods of measuring mammographic density: a case-control study. Breast Cancer Res. 2018;20(1):10.
Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. 2017;161(2):353–62.
Article PubMed Google Scholar
Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159(2):140–7.
Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30(5):959–65.
Tan MM, Ho WK, Yoon SY, Mariapun S, Hasan SN, Lee DS, et al. A case-control study of breast cancer risk factors in 7663 women in Malaysia. PLoS ONE. 2018;13(9):e0203469.
van Engeland S, Snoeren PR, Huisman H, Boetes C, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms. IEEE Trans Med Imaging. 2006;25(3):273–82.
Winkel RR, von Euler-Chelpin M, Nielsen M, Diao P, Nielsen MB, Uldall WY, et al. Inter-observer agreement according to three methods of evaluating mammographic density and parenchymal pattern in a case control study: impact on relative risk of breast cancer. BMC Cancer. 2015;15:274.
Hopper JL. Odds per adjusted standard deviation: comparing strengths of associations for risk factors measured on different scales and across diseases and populations. Am J Epidemiol. 2015;182(10):863–7.
Bae JM, Kim EH. Breast density and risk of breast cancer in asian women: a meta-analysis of observational studies. J Prev Med Public Health. 2016;49(6):367–75.
Kim BK, Choi YH, Nguyen TL, Nam SJ, Lee JE, Hopper JL, et al. Mammographic density and risk of breast cancer in Korean women. Eur J Cancer Prev. 2015;24(5):422–9.
Nguyen TL, Aung YK, Evans CF, Dite GS, Stone J, MacInnis RJ, et al. Mammographic density defined by higher than conventional brightness thresholds better predicts breast cancer risk. Int J Epidemiol. 2017;46(2):652–61.
PubMed Google Scholar
Nishiyama K, Taira N, Mizoo T, Kochi M, Ikeda H, Iwamoto T, et al. Influence of breast density on breast cancer risk: a case control study in Japanese women. Breast Cancer. 2020;27(2):277–83.
Tran TXM, Moon SG, Kim S, Park B. Association of the interaction between mammographic breast density, body mass index, and menopausal status with breast cancer risk among Korean women. JAMA Netw Open. 2021;4(12):e2139161.
Nagata C, Matsubara T, Fujita H, Nagao Y, Shibuya C, Kashiki Y, et al. Mammographic density and the risk of breast cancer in Japanese women. Br J Cancer. 2005;92(12):2102–6.
Park IH, Ko K, Joo J, Park B, Jung SY, Lee S, et al. High volumetric breast density predicts risk for breast cancer in postmenopausal, but not premenopausal. Korean Women Ann Surg Oncol. 2014;21(13):4124–32.
Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011;13(5):R100.
Download references
Acknowledgements
We want to thank all the study participants, clinicians, and support staff at Subang Jaya Medical Centre and University Malaya Medical Centre for their generous contributions to the Malaysian Mammography Study (MyMammo) and the Malaysian Breast Cancer Genetic Study (MyBrCa) studies. We thank all the research associates, scientists and staff at Cancer Research Malaysia and University of Malaya who were involved in the MyMammo and MyBrCa studies – this work would not have been possible without their support and contributions.
The Malaysian Ministry of Science and the Malaysian Ministry of Higher Education High Impact Research Grant (grant number: UM.C/HIR/MOHE/06) was used to fund the Malaysian Breast Cancer Genetic Study (MyBrCa). Funds raised through the Sime Darby LPGA tournament and from the High Impact Research Grant were used to support the Malaysian Mammography Study (MyMammo). Additional funds were received from Yayasan Sime Darby, PETRONAS, Estee Lauder Group of Companies, and other donors of Cancer Research Malaysia. WKH and SM are recipients of the L’Oreal-UNESCO For Women in Science National Fellowship.
Author information
Authors and affiliations.
Cancer Research Malaysia, Subang Jaya, Selangor, Malaysia
Shivaani Mariapun, Weang-Kee Ho & Soo-Hwang Teo
School of Mathematical Sciences, Faculty of Science and Engineering, University of Nottingham Malaysia, Semenyih, Selangor, Malaysia
Shivaani Mariapun & Weang-Kee Ho
Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Mikael Eriksson & Per Hall
Faculty of Medicine, University Malaya Cancer Research Institute, University Malaya, Kuala Lumpur, Malaysia
Nur Aishah Mohd Taib, Kartini Rahmat & Soo-Hwang Teo
Department of Surgery, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Nur Aishah Mohd Taib & Cheng-Har Yip
Subang Jaya Medical Centre, Subang Jaya, Malaysia
Cheng-Har Yip
Biomedical Imaging Department, Faculty of Medicine, Universiti Malaya Research Imaging Centre, University of Malaya, Kuala Lumpur, Malaysia
Kartini Rahmat
Department of Oncology, Södersjukhuset, Stockholm, Sweden
Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
Mikael Eriksson
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization and design: SM, WKH and ST; Data collection: SM, NAMT, CHY, and KR; Data analysis and interpretation: SM, WKH, ME, PH and ST; Manuscript writing: SM, WKH and ST; Reviewed manuscript: SM, WKH, ME, NAMT, CHY, KR, PH and ST.
Corresponding author
Correspondence to Soo-Hwang Teo .
Ethics declarations
Ethics approval and consent to participate.
The Malaysian Mammography Study (MyMammo) and the Malaysian Breast Cancer Genetics (MyBrCa) studies were approved by the Independent Ethics Committee, Ramsay Sime Darby Health Care (reference numbers 201109.4 and 201208.1) and the Medical Ethics Committee, University Malaya Medical Centre (reference numbers 1030.8 and 842.9). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no nonfinancial conflicts of interest but the following financial conflict interests: Mikael Eriksson and Per Hall report research grants and a patent on system and method for assessing breast cancer risk using imagery with a license to iCAD, Inc.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: .
Results for confounder selection analyses and sensitivity analysis using MLO view images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mariapun, S., Ho, WK., Eriksson, M. et al. Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: a case control study. Breast Cancer Res 26 , 79 (2024). https://doi.org/10.1186/s13058-024-01829-2
Download citation
Received : 13 December 2023
Accepted : 19 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s13058-024-01829-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mammographic density
- Breast cancer
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
Understanding coordinated development through spatial structure and network robustness: A case study of the Beijing-Tianjin-Hebei region
- Research Articles
- Published: 10 May 2024
- Volume 34 , pages 1007–1036, ( 2024 )
Cite this article

- Hao Wang 1 ,
- Xiaoyuan Zhang 2 ,
- Xiaoyu Zhang 3 ,
- Ruowen Liu 1 &
- Xiaogang Ning 1
60 Accesses
Explore all metrics
In the context of accelerated globalization, intercity factor flows are becoming increasingly dependent on a reasonable and orderly spatial structure. Therefore, an in-depth study of the optimization and adjustment of spatial structure is essential for coordinated development. This study quantitatively evaluated urban development levels and introduced network analysis methods to analyse the spatial structure and robustness of development. The results indicated the following: (1) The urban development level in the Beijing-Tianjin-Hebei (BTH) region increased in all dimensions, and the transmission efficiency significantly improved. (2) The spatial structure of the BTH region has been relatively stable, as illustrated by the main pattern of the spatial distribution of central cities, with a trend towards contiguous development. (3) The ranking of network robustness is environment>society>economy, and the core network and key nodes are primarily located within the radiation of the three central cities of Beijing, Tianjin, and Shijiazhuang. (4) The coordinated development of the BTH region is effective but still needs to be optimized and adjusted, and the strategic significance of edge cities has not been completely exploited. This study aims to provide an emerging analytical perspective for optimizing regional spatial structure and promoting regional coordinated development.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The spatial correlation network structure and its formation mechanism of urban high-quality economic development: a comparative analysis from the Yangtze River Economic Belt and the Yellow River Basin in China

The Spatial Network of Beibu Gulf Urban Agglomeration and Its Challenges for Coordinated Integral Development

The spatial pattern and governance of Zhongyuan Urban-Rural System in its development trajectory
Adam Y O, Pretzsch J, Darr D, 2015. Land use conflicts in central Sudan: Perception and local coping mechanisms. Land Use Policy , 42: 1–6.
Article Google Scholar
Amin A, 2004. Regions unbound: Towards a new politics of place. Geografiska Annaler: Series B, Human Geography , 86(1): 33–44.
Anas A, Arnott R, Small K A, 1998. Urban spatial structure. Journal of Economic Literature , 36(3): 1426–1464.
Google Scholar
Antrop M, 2004. Landscape change and the urbanization process in Europe. Landscape and Urban Planning , 67(1–4): 9–26.
Batty M, 2013. The New Science of Cities. Cambridge, MA, USA: MIT Press.
Book Google Scholar
Bertaud A, 2004. The spatial organization of cities: Deliberate outcome or unforeseen consequence? Berkeley, CA: Institute of Urban and Regional Development, University of California. Working Paper Number 2004-01.
Burger M, Meijers E, 2012. Form follows function? Linking morphological and functional polycentricity. Urban Studies , 49(5): 1127–1149.
Cai J X, Huang B, Song Y M, 2017. Using multi-source geospatial big data to identify the structure of polycentric cities. Remote Sensing of Environment , 202: 210–221.
Camagni R, Diappi L, Stabilini S, 1994. City networks: an analysis of the Lombardy region in terms of communication flows. In: Cuadrado-Roura J, Nijkamp P, Salva P eds. Moving Frontiers: Economic Restructuring, Regional Development and Emerging Networks, 127–148. Aldershot: Avebury.
Cao Z, Derudder B, Peng Z W, 2018. Comparing the physical, functional and knowledge integration of the Yangtze River Delta city-region through the lens of inter-city networks. Cities , 82: 119–126.
Capello R, 2000. The city network paradigm: Measuring urban network externalities. Urban Studies , 37(11): 1925–1945.
Castells M, 1996. The Rise of the Network Society. The Information Age: Economy, Society, and Culture. New York, USA: Blackwell, 1:656.
Castells M, 2010. Globalisation, networking, urbanisation: Reflections on the spatial dynamics of the information age. Urban Studies , 47(13): 2737–2745.
Castells M, Cardoso G, 2006. The Network Society: From Knowledge to Policy. Washington, DC: Johns Hopkins Center for Transatlantic Relations, 1: 3–23.
Chong Z H, Pan S, 2020. Understanding the structure and determinants of city network through intra-firm service relationships: The case of Guangdong-Hong Kong-Macao Greater Bay Area. Cities , 103: 102738.
Cui X G, Fang C L, Liu H M et al. , 2019. Assessing sustainability of urbanization by a coordinated development index for an urbanization-resources-environment complex system: A case study of Jing-Jin-Ji region, China. Ecological Indicators , 96: 383–391.
Dong S J, Yu T B, Farahmand H et al. , 2020. Probabilistic modeling of cascading failure risk in interdependent channel and road networks in urban flooding. Sustainable Cities and Society , 62: 102398.
Dui H Y, Meng X Y, Xiao H et al. , 2020. Analysis of the cascading failure for scale-free networks based on a multi-strategy evolutionary game. Reliability Engineering & System Safety , 199: 106919.
Elvidge C D, Zhizhin M, Ghosh T et al. , 2021. Annual time series of global VIIRS nighttime lights derived from monthly averages: 2012 to 2019. Remote Sensing , 13(5): 922.
Fan Y P, Fang C L, Zhang Q, 2019. Coupling coordinated development between social economy and ecological environment in Chinese provincial capital cities: Assessment and policy implications. Journal of Cleaner Production , 229: 289–298.
Fang C L, 2017. Theoretical foundation and patterns of coordinated development of the Beijing-Tianjin-Hebei urban agglomeration. Progress in Geography , 36(1): 15–24. (in Chinese)
Fang C L, Yu X H, Zhang X L et al. , 2020. Big data analysis on the spatial networks of urban agglomeration. Cities , 102: 102735.
Fang X, Shi X Y, Phillips T K et al. , 2021. The coupling coordinated development of urban environment towards sustainable urbanization: An empirical study of Shandong Peninsula, China. Ecological Indicators , 129: 107864.
Feleki E, Vlachokostas C, Moussiopoulos N, 2018. Characterisation of sustainability in urban areas: An analysis of assessment tools with emphasis on European cities. Sustainable Cities and Society , 43: 563–577.
Feng Z J, Chen Z N, Cai H C et al. , 2022. Evaluation of the sustainable development of the social-economic-natural compound ecosystem in the Guangdong-Hong Kong-Macao Greater Bay Area urban agglomeration (China): Based on complex network analysis. Frontiers in Environmental Science , 10: 938450.
Frank W P, Hua C-I, 1981. An econometric procedure for estimation of a generalized systemic gravity model under incomplete information about the system. Regional Science and Urban Economics , 11(4): 585–606.
Gao W C, Zhao H T, Mao W J et al. , 2020. Construction research and application of fundamental geographic national condition monitoring quality control system. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences , XLIII-B3-2020: 1327–1331.
Garlaschelli D, Loffredo M I, 2004. Patterns of link reciprocity in directed networks. Physical Review Letters , 93(26): 268701.
Girvan M, Newman M E J, 2002. Community structure in social and biological networks. Proceedings of the National Academy of Sciences , 99(12): 7821–7826.
Article CAS Google Scholar
Gonzalez-Garcia S, Manteiga R, Moreira M T et al. , 2018. Assessing the sustainability of Spanish cities considering environmental and socio-economic indicators. Journal of Cleaner Production , 178: 599–610.
Han H, Guo L, Zhang J Q et al. , 2021. Spatiotemporal analysis of the coordination of economic development, resource utilization, and environmental quality in the Beijing-Tianjin-Hebei urban agglomeration. Ecological Indicators , 127: 107724.
Hao R, Wei Z, 2010. Fundamental causes of inland-coastal income inequality in post-reform China. The Annals of Regional Science , 45(1): 181–206.
He D, Chen Z X, Pei T et al. , 2023. Analysis of structural evolution and its influencing factors of the high-speed railway network in China’s three urban agglomerations. Cities , 132: 104063.
He J H, Li C, Yu Y et al. , 2017. Measuring urban spatial interaction in Wuhan urban agglomeration, central China: A spatially explicit approach. Sustainable Cities and Society , 32: 569–583.
Hirschi C, 2010. Strengthening regional cohesion: Collaborative networks and sustainable development in Swiss rural areas. Ecology and Society , 15(4): 299–305.
Hu Y N, Peng J, Liu Y X et al. , 2017. Mapping development pattern in Beijing-Tianjin-Hebei urban agglomeration using DMSP/OLS nighttime light data. Remote Sensing , 9(7): 760–773.
Huang H J, Xia T, Tian Q et al. , 2020. Transportation issues in developing China’s urban agglomerations. Transport Policy , 85: A1–A22.
Jing Z R, Wang J M, 2020. Sustainable development evaluation of the society–economy–environment in a resource-based city of China: A complex network approach. Journal of Cleaner Production , 263: 121510.
Kabir M, Salim R, Al-Mawali N, 2017. The gravity model and trade flows: Recent developments in econometric modeling and empirical evidence. Economic Analysis and Policy , 56: 60–71.
Lancichinetti A, Santo F, Radicchi F, 2008. Benchmark graphs for testing community detection algorithms. Phys Rev E Stat Nonlin Soft Matter Phys , 78(4): 046110.
Lao X, Zhang X L, Shen T Y et al. , 2016. Comparing China’s city transportation and economic networks. Cities , 53: 43–50.
Li J G, Li J W, Yuan Y Z et al. , 2019. Spatiotemporal distribution characteristics and mechanism analysis of urban population density: A case of Xi’an, Shaanxi, China. Cities , 86: 62–70.
Li L, Ma S J, Zheng Y L et al. , 2022. Integrated regional development: Comparison of urban agglomeration policies in China. Land Use Policy , 114: 105939.
Li T, Wang J E, Huang J et al. , 2020. Exploring temporal heterogeneity in an intercity travel network: A comparative study between weekdays and holidays in China. Journal of Geographical Sciences , 30(12): 1943–1962.
Li W W, Yi P T, 2020. Assessment of city sustainability: Coupling coordinated development among economy, society and environment. Journal of Cleaner Production , 256: 120453.
Li X Y, Lu Z H, 2021. Quantitative measurement on urbanization development level in urban agglomerations: A case of JJJ urban agglomeration. Ecological Indicators , 133: 108375.
Li Z F, Shi Y L, Xu M Q et al. , 2016. Position of the Asian container ports in global liner shipping network. Economic Geography , 36(3): 91–98. (in Chinese)
Liao F H F, Wei Y D, 2012. Dynamics, space, and regional inequality in provincial China: A case study of Guangdong province. Applied Geography , 35(1/2): 71–83.
Liu H, Ma L, Li G P, 2017. Pattern evolution and its contributory factor of cold spots and hot spots of economic development in Beijing-Tianjin-Hebei region. Geographical Research , 36(1): 97–108. (in Chinese)
CAS Google Scholar
Liu X B, Yan X D, Wang W et al. , 2021. Characterizing the polycentric spatial structure of Beijing metropolitan region using carpooling big data. Cities , 109: 103040.
Liu X J, Derudder B, Wu K, 2016. Measuring polycentric urban development in China: An intercity transportation network perspective. Regional Studies , 50(8): 1302–1315.
Liu Y L, Zhang X H, Pan X Y et al. , 2020. The spatial integration and coordinated industrial development of urban agglomerations in the Yangtze River Economic Belt, China. Cities , 104: 102801.
Luo D, Liang L W, Wang Z B et al. , 2021. Exploration of coupling effects in the Economy–Society–Environment system in urban areas: Case study of the Yangtze River Delta urban agglomeration. Ecological Indicators , 128: 107858.
Lv Y Q, Zhou L, Yao G B et al. , 2021. Detecting the true urban polycentric pattern of Chinese cities in morphological dimensions: A multiscale analysis based on geospatial big data. Cities , 116: 103298.
Ma H T, Wei Y D, Huang X D et al. , 2023. The innovation networks shaped by large innovative enterprises in urban China. Journal of Geographical Sciences , 33(3): 599–617.
Ma W Q, Jiang G H, Chen Y H et al. , 2020. How feasible is regional integration for reconciling land use conflicts across the urban–rural interface? Evidence from Beijing-Tianjin-Hebei metropolitan region in China. Land Use Policy , 92: 104433.
Mao W J, Zhao H T, Gao W H et al. , 2021. Research on quality control method of land cover classification data oriented to national geographic condition monitoring. ISPRS Annals of the Photogrammetry, Remote Sensing and Spatial Information Sciences , V-3-2021: 265–269.
Mcdonald R I, Green P, Balk D et al. , 2011. Urban growth, climate change, and freshwater availability. Proceedings of the National Academy of Sciences , 108(15): 6312–6317.
Meijers E, 2005. Polycentric urban regions and the quest for synergy: Is a network of cities more than the sum of the parts? Urban studies , 42(4): 765–781.
Meijers E, 2007. From central place to network model: Theory and evidence of a paradigm change. Tijdschrift voor economische en sociale geografie , 98(2): 245–259.
Meijers E, Hoekstra J, Aguado R, 2008. The Basque city network: An empirical analysis and policy recommendations. EUNIP International Conference , 10–12.
Meijers E, Hoogerbrugge M, Cardoso R, 2018. Beyond polycentricity: Does stronger integration between cities in polycentric urban regions improve performance? Tijdschrift voor Economische en Sociale Geografie , 109(1): 1–21.
Motter A E, Lai Y C, 2002. Cascade-based attacks on complex networks. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics , 66(6): 065102.
Mu X F, Fang C L, Yang Z Q, 2022. Spatio-temporal evolution and dynamic simulation of the urban resilience of Beijing-Tianjin-Hebei urban agglomeration. Journal of Geographical Sciences , 32(9): 1766–1790.
Neal Z, 2013. Does world city network research need eigenvectors? Urban Studies , 50(8): 1648–1659.
Parr J, 2004. The polycentric urban region: A closer inspection. Regional Studies , 38(3): 231–240.
Rosvall M, Axelsson D, Bergstrom C T, 2010. The map equation. The European Physical Journal Special Topics , 178(1): 13–23.
Rosvall M, Bergstrom C T, 2008. Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences , 105(4): 1118.
Shen J F, 2002. A study of the temporary population in Chinese cities. Habitat International , 26(3): 363–377.
Sigler T J, Martinus K, 2017. Extending beyond ‘world cities’ in World City Network (WCN) research: Urban positionality and economic linkages through the Australia-based corporate network. Environment and Planning A: Economy and Space , 49(12): 2916–2937.
Song J H, Abuduwayiti A, Gou Z H, 2023. The role of subway network in urban spatial structure optimization: Wuhan city as an example. Tunnelling and Underground Space Technology , 131: 104842.
Strogatz S H, 2001. Exploring complex networks. Nature , 410(6825): 268–276.
Sun X, Liu X S, Li F et al. , 2017. Comprehensive evaluation of different scale cities’ sustainable development for economy, society, and ecological infrastructure in China. Journal of Cleaner Production , 163: S329–S337.
Swyngedouw E, 2010. Globalisation or ‘glocalisation’? Networks, territories and rescaling. Cambridge Review of International Affairs , 17(1): 25–48.
Thompson C A, Saxberg K, Lega J et al. , 2019. A cumulative gravity model for inter-urban spatial interaction at different scales. Journal of Transport Geography , 79: 102461.
Ullman E L, 1957. American Commodity Flow. Seattle: University of Washington Press.
Van Nuffel N, Saey P, 2005. Commuting, hierarchy and networking: The case of Flanders. Tijdschrift voor Economische en Sociale Geografie , 96(3): 313–327.
Wall R S, Van Der Knaap G A, 2011. Sectoral differentiation and network structure within contemporary world-wide corporate networks. Economic Geography , 87(3): 267–308.
Wang H J, Wu Y, Deng Y et al. , 2022. Model construction of urban agglomeration expansion simulation considering urban flow and hierarchical characteristics. Journal of Geographical Sciences , 32(3): 499–516.
Wang J K, Han Q, Du Y H, 2021. Coordinated development of the economy, society and environment in urban China: A case study of 285 cities. Environment, Development and Sustainability , 24: 12917–129335.
Wang T Y, Yue W Z, Ye X Y et al. , 2020. Re-evaluating polycentric urban structure: A functional linkage perspective. Cities , 101: 102672.
Wang Y F, 2022. Population-land urbanization and comprehensive development evaluation of the Beijing-Tianjin-Hebei urban agglomeration. Environmental Science and Pollution Research , 29(39): 59862–59871.
Wang Z B, Liang L W, Sun Z et al. , 2019. Spatiotemporal differentiation and the factors influencing urbanization and ecological environment synergistic effects within the Beijing-Tianjin-Hebei urban agglomeration. Journal of Environmental Management , 243: 227–239.
Watts D J, Strogatz S H, 1998. Collective dynamics of ‘small-world’ networks. Nature , 393(6684): 440–442.
Wei J, Li Z Q, Lyapustin A et al. , 2021. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: Spatiotemporal variations and policy implications. Remote Sensing of Environment , 252: 112136.
Wei L, Luo Y, Wang M et al. , 2020. Multiscale identification of urban functional polycentricity for planning implications: An integrated approach using geo-big transport data and complex network modeling. Habitat International , 97: 102134.
Wei Y, Wang J E, Zhang S H et al. , 2022. Urban positionality in the regional urban network: Through the lens of alter-based centrality and national-local perspectives. Habitat International , 126: 102617.
Xia C, Zhang A Q, Wang H J et al. , 2019. Bidirectional urban flows in rapidly urbanizing metropolitan areas and their macro and micro impacts on urban growth: A case study of the Yangtze River middle reaches megalopolis, China. Land Use Policy , 82: 158–168.
Xie Y S, Wang C J, 2023. Spatial pattern of global submarine cable network and identification of strategic pivot and strategic channel. Journal of Geographical Sciences , 33(4): 719–740.
Xu H Z, Jiao M, 2021. City size, industrial structure and urbanization quality: A case study of the Yangtze River Delta urban agglomeration in China. Land Use Policy , 111: 105735.
Yang L J, Wang J, Yang Y C, 2022. Spatial evolution and growth mechanism of urban networks in western China: A multi-scale perspective. Journal of Geographical Sciences , 32(3): 517–536.
Ye C, Zhu J J, Li S M et al. , 2019. Assessment and analysis of regional economic collaborative development within an urban agglomeration: Yangtze River Delta as a case study. Habitat International , 83: 20–29.
Zhang D N, Chen Y, 2021. Evaluation on urban environmental sustainability and coupling coordination among its dimensions: A case study of Shandong Province, China. Sustainable Cities and Society , 75: 103351.
Zhang F, Ning Y M, Lou X Y, 2021. The evolutionary mechanism of China’s urban network from 1997 to 2015: An analysis of air passenger flows. Cities , 109: 103005.
Zhang L, Xu Y, Yeh C-H et al. , 2016. City sustainability evaluation using multi-criteria decision making with objective weights of interdependent criteria. Journal of Cleaner Production , 131: 491–499.
Zhang P F, Zhao Y Y, Zhu X H et al. , 2020. Spatial structure of urban agglomeration under the impact of high-speed railway construction: Based on the social network analysis. Sustainable Cities and Society , 62: 102404.
Zhang W J, Gong Z Y, Niu C C et al. , 2022a. Structural changes in intercity mobility networks of China during the COVID-19 outbreak: A weighted stochastic block modeling analysis. Computers, Environment and Urban Systems , 96: 101846.
Zhang X Y, Wang H, Ning X G et al. , 2022b. Identification of metropolitan area boundaries based on comprehensive spatial linkages of cities: A case study of the Beijing-Tianjin-Hebei region. ISPRS International Journal of Geo-Information , 11(7): 396.
Zhen F, Qin X, Ye X Y et al. , 2019. Analyzing urban development patterns based on the flow analysis method. Cities , 86: 178–197.
Zhong C, Arisona S M, Huang X F et al. , 2014. Detecting the dynamics of urban structure through spatial network analysis. International Journal of Geographical Information Science , 28(11): 2178–2199.
Zhu Y H, Yang S, Lin J P et al. , 2022. Spatial and temporal evolutionary characteristics and its influencing factors of economic spatial polarization in the Yangtze River Delta region. International Journal of Environmental Research and Public Health , 19(12): 6997.
Zou C, Ou X J, Tan J T, 2019. Temporal and spatial characteristics and early warning analysis of economic polarization evolution: A case study of Jiangsu province in China. Sustainability , 11(5): 1339.
Download references
Author information
Authors and affiliations.
Chinese Academy of Surveying and Mapping, Beijing, 100036, China
Hao Wang, Ruowen Liu & Xiaogang Ning
School of Information Engineering, China University of Geosciences, Beijing, 100083, China
Xiaoyuan Zhang
College of Land Science and Technology, China Agricultural University, Beijing, 100193, China
Xiaoyu Zhang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Xiaoyuan Zhang .
Additional information
Foundation: National Key Research and Development Program, No.2023YFC3804001; Natural Resources Planning and Management Project, No.A2417, No.A2418
Author: Wang Hao (1985–), PhD, specialized in territorial spatial planning and urban physical examination.
Supplementary material (Notations)
Supplementary material, approximately 165 kb., rights and permissions.
Reprints and permissions
About this article
Wang, H., Zhang, X., Zhang, X. et al. Understanding coordinated development through spatial structure and network robustness: A case study of the Beijing-Tianjin-Hebei region. J. Geogr. Sci. 34 , 1007–1036 (2024). https://doi.org/10.1007/s11442-024-2237-8
Download citation
Received : 29 July 2023
Accepted : 05 March 2024
Published : 10 May 2024
Issue Date : May 2024
DOI : https://doi.org/10.1007/s11442-024-2237-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- urban network
- spatial structure
- network robustness
- coordinated development
- Beijing-Tianjin-Hebei (BTH) region
- Find a journal
- Publish with us
- Track your research

Exploring variations in the implementation of a health system level policy intervention to improve maternal and child health outcomes in resource limited settings: A qualitative multiple case study from Uganda
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for David Roger Walugembe
- For correspondence: [email protected] [email protected]
- ORCID record for Katrina Plamondon
- Info/History
- Preview PDF
Background Despite growing literature, few studies have explored the implementation of policy interventions to reduce maternal and perinatal mortality in low- and middle-income countries (LMICs). Even fewer studies explicitly articulate the theoretical approaches used to understand contextual influences on policy implementation. This under-use of theory may account for the limited understanding of the variations in implementation processes and outcomes. We share findings from a study exploring how a health system-level policy intervention was implemented to improve maternal and child health outcomes in a resource limited LMIC.
Methods Our qualitative multiple case study was informed by the Normalization Process Theory (NPT). It was conducted across eight districts and among ten health facilities in Uganda, with 48 purposively selected participants. These included health care workers located at each of the case sites, policy makers from the Ministry of Health, and from agencies and professional associations. Data were collected using semi-structured, in-depth interviews to understand uptake and use of Uganda’s maternal and perinatal death surveillance and response (MPDSR) policy and were inductively and deductively analyzed using NPT constructs and subconstructs.
Results We identified six broad themes that may explain the observed variations in the implementation of the MPDSR policy. These include: 1) perception of the implementation of the policy, 2) leadership of the implementation process, 3) structural arrangements and coordination, 4) extent of management support and adequacy of resources, 5) variations in appraisal and reconfiguration efforts and 6) variations in barriers to implementation of the policy.
Conclusion and recommendations The variations in sense making and relational efforts, especially perceptions of the implementation process and leadership capacity, had ripple effects across operational and appraisal efforts. Adopting theoretically informed approaches to assessing the implementation of policy interventions is crucial, especially within resource limited settings.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study was undertaken with no funding.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Ethics approval for this study was sought from the Health Sciences Research Ethics Board (HSREB, IRB 00000940) Delegated Review of the University of Western Ontario. Additional ethical approval was sought from the School of Medicine Research and Ethics Committee, Makerere University College of Health Sciences (REC REF No. 2018-018), the Uganda National Council for Science and Technology (HS 2393) and the Ugandan Ministry of Health (ADM 130/313/05). Participation in the study was completely voluntary and written informed consent was sought at all times. Study participants were assured of privacy and confidentiality and approved the use of information for improving public health, clinical practices and policy implementation. The manuscript does not include details, images, or videos relating to individual participants.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available because it was a qualitative study but are available from the corresponding author on reasonable request.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Obstetrics and Gynecology
- Addiction Medicine (324)
- Allergy and Immunology (628)
- Anesthesia (165)
- Cardiovascular Medicine (2377)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (379)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (837)
- Epidemiology (11775)
- Forensic Medicine (10)
- Gastroenterology (703)
- Genetic and Genomic Medicine (3744)
- Geriatric Medicine (350)
- Health Economics (634)
- Health Informatics (2399)
- Health Policy (933)
- Health Systems and Quality Improvement (898)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13318)
- Intensive Care and Critical Care Medicine (767)
- Medical Education (365)
- Medical Ethics (105)
- Nephrology (398)
- Neurology (3507)
- Nursing (198)
- Nutrition (526)
- Obstetrics and Gynecology (674)
- Occupational and Environmental Health (664)
- Oncology (1824)
- Ophthalmology (538)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (233)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1035)
- Pharmacology and Therapeutics (426)
- Primary Care Research (420)
- Psychiatry and Clinical Psychology (3178)
- Public and Global Health (6145)
- Radiology and Imaging (1280)
- Rehabilitation Medicine and Physical Therapy (747)
- Respiratory Medicine (828)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (146)

IMAGES
VIDEO
COMMENTS
Because acknowledgment may imply endorsement by acknowledged individuals of a study's data and conclusions, editors are advised to require that the corresponding author obtain written permission to be acknowledged from all acknowledged individuals. Use of AI for writing assistance should be reported in the acknowledgment section. 4.
The corresponding author takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and typically ensures that all the journal's administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation ...
A corresponding author is someone who: Can assume responsibility for all communication with the co-authors and the journal and can ensure that this communication is transparent. Is willing to represent the team and take responsibility for the research output submitted on its behalf, especially in the event of a dispute.
3. Affiliation: The names of organizations for each author: 4. Corresponding author: Write the full name of the corresponding author and all contact details including email and mobile number: Abstract: 5. Background: what does this case report add to the medical literature? 6. Case summary: chief complaint, diagnosis, intervention, and outcome: 7.
The corresponding author, i.e., the author with whom the Editor communicates, is responsible for the integrity of the manuscript contents such as the veracity of the results, the lack of plagiarism, the contribution of each author, and other issues related to production of the manuscript. ... it seems intuitive that the corresponding should for ...
The corresponding author is usually the one who had the main idea and invited other collaborators or delegated tasks. While all authors are responsible for the work and review the final version of the manuscript with a fine-tooth comb, the corresponding author is ultimately in charge of submitting the paper and is responsible for the overall ...
A postdoc and a graduate student each wanted to be listed as the first author on a study. "They both had a case," recalls Kosslyn. "It got heated.". Disagreements often happen when ...
Corresponding author meaning: The corresponding author is the one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process. Normally, he or she also ensures that all the journal's administrative requirements, such as providing details of authorship ...
The submitting author and corresponding author can be the same person. Corresponding author. The corresponding author, as listed on ScholarOne, takes primary responsibility for completing all necessary actions after acceptance of the manuscript and communicating with the journal and with readers after publication. All email communication from ...
One of the relevant examples is Academic Medicine where corresponding author Twitter handles are increasingly listed in the ... Egilman DS, Krumholz HM. Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation. JAMA. 2008; 299:1800-1812. doi: 10.1001/jama.299.15.1800 ...
Never include a co-author or the name of a colleague in the Acknowledgments Section without his/her prior approval. 8.2. Corresponding author If there is more than one author on the report, you will need to have a corresponding author. The corresponding author is most often (but not always) the first author. The International Committee of
The corresponding author is also important or the most senior author in ... In this review paper we show - through personal real case studies and testimonials of writing CPC accumulated over the ...
As the corresponding author, you need to fill in this document and send it to the journal. Ensure ethical practices are followed: As corresponding author it is your job to ensure that the manuscript is prepared and submitted ethically. You (or your co-authors) should not be accused of any kind of malpractice such as salami slicing, plagiarism ...
The role of corresponding author means one thing in the eyes of editors, journals and publishers, but researchers often take it to mean something more.; There are two different roles for the corresponding author: pre-publication corresponding author and post-publication corresponding author. Journals should explicitly acknowledge these separate roles for corresponding authors in order to ...
The corresponding author is often the group leader or a senior researcher whose contact address is not likely to change in the near future. In cases where the main contributor of the study is also the group leader, he or she can be both the first and corresponding author for the study. Related reading: What corresponding authors are expected to ...
A case study is one of the most commonly used methodologies of social research. This article attempts to look into the various dimensions of a case study research strategy, the different epistemological strands which determine the particular case study type and approach adopted in the field, discusses the factors which can enhance the effectiveness of a case study research, and the debate ...
Resident physicians must work with an experienced attending physician who serves as the first and corresponding author. ... Case-control study: Case series: Level 3 Other evidence:
From the perspective of journals, high‐quality journals usually have higher proportions of co‐corresponding authorship. At the level of the article, our findings proved that, compared to articles with a single corresponding author, articles with multiple corresponding authors have a significant citation advantage.
Include a cover letter and complete contact information for the corresponding author (affiliation, postal/mail address, email address, and telephone number) and whether the authors have published, ... Include the design (eg, clinical trial, cohort study, case-control study, meta-analysis). Focus on primary outcome(s) and finding(s). Do not ...
This paper analyses the influence of a Brazilian institution delivering the corresponding author on its scientific citation impact, distinguishing between its collaborative papers with foreign institutions and ... "case studies" are used (Katz & Martin, 1997). Co-authorship reflects the formal result of the work of a group of researchers. The ...
This paper analyses the influence of a Brazilian institution delivering the corresponding author on its scientific citation impact, distinguishing between its collaborative papers with foreign institutions and those resulting from national collaboration. We retrieved from Scopus database a total of 607,454 Brazilian documents for all 443 Brazilian institutions with at least 100 documents ...
This would include the responsibilities of the corresponding author as well as whether multiple corresponding authors are allowed. In the current case the journal should make sure that all potential corresponding authors are aware of their responsibilities for the paper in its entirety. Year: 2024.
This case study aims to explore the role of pupils in educational design and what it means to have a say in primary school. Although many educational offers use the slogan 'pupil-centred' approach, examples are scarce in which children are seen as partners, let alone co-designers.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author. ... Michael, and Vladimir Frid. 2024. "A Case Study of the Integration of Ground-Based and Drone-Based Ground-Penetrating Radar (GPR) for an Archaeological Survey in Hulata (Israel): Advancements, ...
In this case-control study of 531 cases and 2297 controls, we evaluated the association of area-based MD measures and volumetric-based MD measures with breast cancer risk in Asian women using conditional logistic regression analysis, adjusting for relevant confounders. ... Corresponding author. Correspondence to Soo-Hwang Teo. Ethics ...
The authors claimed that the corresponding author did not have that much experience in article publication previously, and so did not pay attention to the list of authors. This is how the other authors' names were missed. It should be noted that the other six authors are included on their profile now.
In the context of accelerated globalization, intercity factor flows are becoming increasingly dependent on a reasonable and orderly spatial structure. Therefore, an in-depth study of the optimization and adjustment of spatial structure is essential for coordinated development. This study quantitatively evaluated urban development levels and introduced network analysis methods to analyse the ...
Senior Editor, Editage Insights. Researcher coach since 2015. Not all journals allow more than one corresponding author for a single study, but sometimes the research team needs a co-corresponding author. Authors need to keep this in mind and send pre-submission inquiries if they wish to have two corresponding authors for a paper.
Background Despite growing literature, few studies have explored the implementation of policy interventions to reduce maternal and perinatal mortality in low- and middle-income countries (LMICs). Even fewer studies explicitly articulate the theoretical approaches used to understand contextual influences on policy implementation. This under-use of theory may account for the limited ...
Community green spaces (CGSs) constitute a crucial element of urban land use, playing a pivotal role in maintaining the stability of urban ecosystems and enhancing the overall quality of the urban environment. Through the post-occupancy evaluation (POE) of green spaces, we can gain insights into residents' actual needs and usage habits, providing scientific evidence for the planning, design ...