
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


6.6: Observational Research
- Last updated
- Save as PDF
- Page ID 19655

- Rajiv S. Jhangiani, I-Chant A. Chiang, Carrie Cuttler, & Dana C. Leighton
- Kwantlen Polytechnic U., Washington State U., & Texas A&M U.—Texarkana
Learning Objectives
- List the various types of observational research methods and distinguish between each.
- Describe the strengths and weakness of each observational research method.
What Is Observational Research?
The term observational research is used to refer to several different types of non-experimental studies in which behavior is systematically observed and recorded. The goal of observational research is to describe a variable or set of variables. More generally, the goal is to obtain a snapshot of specific characteristics of an individual, group, or setting. As described previously, observational research is non-experimental because nothing is manipulated or controlled, and as such we cannot arrive at causal conclusions using this approach. The data that are collected in observational research studies are often qualitative in nature but they may also be quantitative or both (mixed-methods). There are several different types of observational methods that will be described below.
Naturalistic Observation
Naturalistic observation is an observational method that involves observing people’s behavior in the environment in which it typically occurs. Thus naturalistic observation is a type of field research (as opposed to a type of laboratory research). Jane Goodall’s famous research on chimpanzees is a classic example of naturalistic observation. Dr. Goodall spent three decades observing chimpanzees in their natural environment in East Africa. She examined such things as chimpanzee’s social structure, mating patterns, gender roles, family structure, and care of offspring by observing them in the wild. However, naturalistic observation could more simply involve observing shoppers in a grocery store, children on a school playground, or psychiatric inpatients in their wards. Researchers engaged in naturalistic observation usually make their observations as unobtrusively as possible so that participants are not aware that they are being studied. Such an approach is called disguised naturalistic observation. Ethically, this method is considered to be acceptable if the participants remain anonymous and the behavior occurs in a public setting where people would not normally have an expectation of privacy. Grocery shoppers putting items into their shopping carts, for example, are engaged in public behavior that is easily observable by store employees and other shoppers. For this reason, most researchers would consider it ethically acceptable to observe them for a study. On the other hand, one of the arguments against the ethicality of the naturalistic observation of “bathroom behavior” discussed earlier in the book is that people have a reasonable expectation of privacy even in a public restroom and that this expectation was violated.
In cases where it is not ethical or practical to conduct disguised naturalistic observation, researchers can conduct undisguised naturalistic observation where the participants are made aware of the researcher presence and monitoring of their behavior. However, one concern with undisguised naturalistic observation is reactivity. Reactivity refers to when a measure changes participants’ behavior. In the case of undisguised naturalistic observation, the concern with reactivity is that when people know they are being observed and studied, they may act differently than they normally would. This type of reactivity is known as the Hawthorne effect . For instance, you may act much differently in a bar if you know that someone is observing you and recording your behaviors and this would invalidate the study. So disguised observation is less reactive and therefore can have higher validity because people are not aware that their behaviors are being observed and recorded. However, we now know that people often become used to being observed and with time they begin to behave naturally in the researcher’s presence. In other words, over time people habituate to being observed. Think about reality shows like Big Brother or Survivor where people are constantly being observed and recorded. While they may be on their best behavior at first, in a fairly short amount of time they are flirting, having sex, wearing next to nothing, screaming at each other, and occasionally behaving in ways that are embarrassing.
Participant Observation
Another approach to data collection in observational research is participant observation. In participant observation , researchers become active participants in the group or situation they are studying. Participant observation is very similar to naturalistic observation in that it involves observing people’s behavior in the environment in which it typically occurs. As with naturalistic observation, the data that are collected can include interviews (usually unstructured), notes based on their observations and interactions, documents, photographs, and other artifacts. The only difference between naturalistic observation and participant observation is that researchers engaged in participant observation become active members of the group or situations they are studying. The basic rationale for participant observation is that there may be important information that is only accessible to, or can be interpreted only by, someone who is an active participant in the group or situation. Like naturalistic observation, participant observation can be either disguised or undisguised. In disguised participant observation, the researchers pretend to be members of the social group they are observing and conceal their true identity as researchers.
In a famous example of disguised participant observation, Leon Festinger and his colleagues infiltrated a doomsday cult known as the Seekers, whose members believed that the apocalypse would occur on December 21, 1954. Interested in studying how members of the group would cope psychologically when the prophecy inevitably failed, they carefully recorded the events and reactions of the cult members in the days before and after the supposed end of the world. Unsurprisingly, the cult members did not give up their belief but instead convinced themselves that it was their faith and efforts that saved the world from destruction. Festinger and his colleagues later published a book about this experience, which they used to illustrate the theory of cognitive dissonance (Festinger, Riecken, & Schachter, 1956) [1] .
In contrast with undisguised participant observation, the researchers become a part of the group they are studying and they disclose their true identity as researchers to the group under investigation. Once again there are important ethical issues to consider with disguised participant observation. First no informed consent can be obtained and second deception is being used. The researcher is deceiving the participants by intentionally withholding information about their motivations for being a part of the social group they are studying. But sometimes disguised participation is the only way to access a protective group (like a cult). Further, disguised participant observation is less prone to reactivity than undisguised participant observation.
Rosenhan’s study (1973) [2] of the experience of people in a psychiatric ward would be considered disguised participant observation because Rosenhan and his pseudopatients were admitted into psychiatric hospitals on the pretense of being patients so that they could observe the way that psychiatric patients are treated by staff. The staff and other patients were unaware of their true identities as researchers.
Another example of participant observation comes from a study by sociologist Amy Wilkins on a university-based religious organization that emphasized how happy its members were (Wilkins, 2008) [3] . Wilkins spent 12 months attending and participating in the group’s meetings and social events, and she interviewed several group members. In her study, Wilkins identified several ways in which the group “enforced” happiness—for example, by continually talking about happiness, discouraging the expression of negative emotions, and using happiness as a way to distinguish themselves from other groups.
One of the primary benefits of participant observation is that the researchers are in a much better position to understand the viewpoint and experiences of the people they are studying when they are a part of the social group. The primary limitation with this approach is that the mere presence of the observer could affect the behavior of the people being observed. While this is also a concern with naturalistic observation, additional concerns arise when researchers become active members of the social group they are studying because that they may change the social dynamics and/or influence the behavior of the people they are studying. Similarly, if the researcher acts as a participant observer there can be concerns with biases resulting from developing relationships with the participants. Concretely, the researcher may become less objective resulting in more experimenter bias.
Structured Observation
Another observational method is structured observation . Here the investigator makes careful observations of one or more specific behaviors in a particular setting that is more structured than the settings used in naturalistic or participant observation. Often the setting in which the observations are made is not the natural setting. Instead, the researcher may observe people in the laboratory environment. Alternatively, the researcher may observe people in a natural setting (like a classroom setting) that they have structured some way, for instance by introducing some specific task participants are to engage in or by introducing a specific social situation or manipulation.
Structured observation is very similar to naturalistic observation and participant observation in that in all three cases researchers are observing naturally occurring behavior; however, the emphasis in structured observation is on gathering quantitative rather than qualitative data. Researchers using this approach are interested in a limited set of behaviors. This allows them to quantify the behaviors they are observing. In other words, structured observation is less global than naturalistic or participant observation because the researcher engaged in structured observations is interested in a small number of specific behaviors. Therefore, rather than recording everything that happens, the researcher only focuses on very specific behaviors of interest.
Researchers Robert Levine and Ara Norenzayan used structured observation to study differences in the “pace of life” across countries (Levine & Norenzayan, 1999) [4] . One of their measures involved observing pedestrians in a large city to see how long it took them to walk 60 feet. They found that people in some countries walked reliably faster than people in other countries. For example, people in Canada and Sweden covered 60 feet in just under 13 seconds on average, while people in Brazil and Romania took close to 17 seconds. When structured observation takes place in the complex and even chaotic “real world,” the questions of when, where, and under what conditions the observations will be made, and who exactly will be observed are important to consider. Levine and Norenzayan described their sampling process as follows:
“Male and female walking speed over a distance of 60 feet was measured in at least two locations in main downtown areas in each city. Measurements were taken during main business hours on clear summer days. All locations were flat, unobstructed, had broad sidewalks, and were sufficiently uncrowded to allow pedestrians to move at potentially maximum speeds. To control for the effects of socializing, only pedestrians walking alone were used. Children, individuals with obvious physical handicaps, and window-shoppers were not timed. Thirty-five men and 35 women were timed in most cities.” (p. 186).
Precise specification of the sampling process in this way makes data collection manageable for the observers, and it also provides some control over important extraneous variables. For example, by making their observations on clear summer days in all countries, Levine and Norenzayan controlled for effects of the weather on people’s walking speeds. In Levine and Norenzayan’s study, measurement was relatively straightforward. They simply measured out a 60-foot distance along a city sidewalk and then used a stopwatch to time participants as they walked over that distance.
As another example, researchers Robert Kraut and Robert Johnston wanted to study bowlers’ reactions to their shots, both when they were facing the pins and then when they turned toward their companions (Kraut & Johnston, 1979) [5] . But what “reactions” should they observe? Based on previous research and their own pilot testing, Kraut and Johnston created a list of reactions that included “closed smile,” “open smile,” “laugh,” “neutral face,” “look down,” “look away,” and “face cover” (covering one’s face with one’s hands). The observers committed this list to memory and then practiced by coding the reactions of bowlers who had been videotaped. During the actual study, the observers spoke into an audio recorder, describing the reactions they observed. Among the most interesting results of this study was that bowlers rarely smiled while they still faced the pins. They were much more likely to smile after they turned toward their companions, suggesting that smiling is not purely an expression of happiness but also a form of social communication.
In yet another example (this one in a laboratory environment), Dov Cohen and his colleagues had observers rate the emotional reactions of participants who had just been deliberately bumped and insulted by a confederate after they dropped off a completed questionnaire at the end of a hallway. The confederate was posing as someone who worked in the same building and who was frustrated by having to close a file drawer twice in order to permit the participants to walk past them (first to drop off the questionnaire at the end of the hallway and once again on their way back to the room where they believed the study they signed up for was taking place). The two observers were positioned at different ends of the hallway so that they could read the participants’ body language and hear anything they might say. Interestingly, the researchers hypothesized that participants from the southern United States, which is one of several places in the world that has a “culture of honor,” would react with more aggression than participants from the northern United States, a prediction that was in fact supported by the observational data (Cohen, Nisbett, Bowdle, & Schwarz, 1996) [6] .
When the observations require a judgment on the part of the observers—as in the studies by Kraut and Johnston and Cohen and his colleagues—a process referred to as coding is typically required . Coding generally requires clearly defining a set of target behaviors. The observers then categorize participants individually in terms of which behavior they have engaged in and the number of times they engaged in each behavior. The observers might even record the duration of each behavior. The target behaviors must be defined in such a way that guides different observers to code them in the same way. This difficulty with coding illustrates the issue of interrater reliability, as mentioned in Chapter 4. Researchers are expected to demonstrate the interrater reliability of their coding procedure by having multiple raters code the same behaviors independently and then showing that the different observers are in close agreement. Kraut and Johnston, for example, video recorded a subset of their participants’ reactions and had two observers independently code them. The two observers showed that they agreed on the reactions that were exhibited 97% of the time, indicating good interrater reliability.
One of the primary benefits of structured observation is that it is far more efficient than naturalistic and participant observation. Since the researchers are focused on specific behaviors this reduces time and expense. Also, often times the environment is structured to encourage the behaviors of interest which again means that researchers do not have to invest as much time in waiting for the behaviors of interest to naturally occur. Finally, researchers using this approach can clearly exert greater control over the environment. However, when researchers exert more control over the environment it may make the environment less natural which decreases external validity. It is less clear for instance whether structured observations made in a laboratory environment will generalize to a real world environment. Furthermore, since researchers engaged in structured observation are often not disguised there may be more concerns with reactivity.
Case Studies
A case study is an in-depth examination of an individual. Sometimes case studies are also completed on social units (e.g., a cult) and events (e.g., a natural disaster). Most commonly in psychology, however, case studies provide a detailed description and analysis of an individual. Often the individual has a rare or unusual condition or disorder or has damage to a specific region of the brain.
Like many observational research methods, case studies tend to be more qualitative in nature. Case study methods involve an in-depth, and often a longitudinal examination of an individual. Depending on the focus of the case study, individuals may or may not be observed in their natural setting. If the natural setting is not what is of interest, then the individual may be brought into a therapist’s office or a researcher’s lab for study. Also, the bulk of the case study report will focus on in-depth descriptions of the person rather than on statistical analyses. With that said some quantitative data may also be included in the write-up of a case study. For instance, an individual’s depression score may be compared to normative scores or their score before and after treatment may be compared. As with other qualitative methods, a variety of different methods and tools can be used to collect information on the case. For instance, interviews, naturalistic observation, structured observation, psychological testing (e.g., IQ test), and/or physiological measurements (e.g., brain scans) may be used to collect information on the individual.
HM is one of the most notorious case studies in psychology. HM suffered from intractable and very severe epilepsy. A surgeon localized HM’s epilepsy to his medial temporal lobe and in 1953 he removed large sections of his hippocampus in an attempt to stop the seizures. The treatment was a success, in that it resolved his epilepsy and his IQ and personality were unaffected. However, the doctors soon realized that HM exhibited a strange form of amnesia, called anterograde amnesia. HM was able to carry out a conversation and he could remember short strings of letters, digits, and words. Basically, his short term memory was preserved. However, HM could not commit new events to memory. He lost the ability to transfer information from his short-term memory to his long term memory, something memory researchers call consolidation. So while he could carry on a conversation with someone, he would completely forget the conversation after it ended. This was an extremely important case study for memory researchers because it suggested that there’s a dissociation between short-term memory and long-term memory, it suggested that these were two different abilities sub-served by different areas of the brain. It also suggested that the temporal lobes are particularly important for consolidating new information (i.e., for transferring information from short-term memory to long-term memory),
The history of psychology is filled with influential cases studies, such as Sigmund Freud’s description of “Anna O.” (see Note 6.1 “The Case of “Anna O.””) and John Watson and Rosalie Rayner’s description of Little Albert (Watson & Rayner, 1920) [7] , who allegedly learned to fear a white rat—along with other furry objects—when the researchers repeatedly made a loud noise every time the rat approached him.
The Case of “Anna O.”
Sigmund Freud used the case of a young woman he called “Anna O.” to illustrate many principles of his theory of psychoanalysis (Freud, 1961) [8] . (Her real name was Bertha Pappenheim, and she was an early feminist who went on to make important contributions to the field of social work.) Anna had come to Freud’s colleague Josef Breuer around 1880 with a variety of odd physical and psychological symptoms. One of them was that for several weeks she was unable to drink any fluids. According to Freud,
She would take up the glass of water that she longed for, but as soon as it touched her lips she would push it away like someone suffering from hydrophobia.…She lived only on fruit, such as melons, etc., so as to lessen her tormenting thirst. (p. 9)
But according to Freud, a breakthrough came one day while Anna was under hypnosis.
[S]he grumbled about her English “lady-companion,” whom she did not care for, and went on to describe, with every sign of disgust, how she had once gone into this lady’s room and how her little dog—horrid creature!—had drunk out of a glass there. The patient had said nothing, as she had wanted to be polite. After giving further energetic expression to the anger she had held back, she asked for something to drink, drank a large quantity of water without any difficulty, and awoke from her hypnosis with the glass at her lips; and thereupon the disturbance vanished, never to return. (p.9)
Freud’s interpretation was that Anna had repressed the memory of this incident along with the emotion that it triggered and that this was what had caused her inability to drink. Furthermore, he believed that her recollection of the incident, along with her expression of the emotion she had repressed, caused the symptom to go away.
As an illustration of Freud’s theory, the case study of Anna O. is quite effective. As evidence for the theory, however, it is essentially worthless. The description provides no way of knowing whether Anna had really repressed the memory of the dog drinking from the glass, whether this repression had caused her inability to drink, or whether recalling this “trauma” relieved the symptom. It is also unclear from this case study how typical or atypical Anna’s experience was.

Case studies are useful because they provide a level of detailed analysis not found in many other research methods and greater insights may be gained from this more detailed analysis. As a result of the case study, the researcher may gain a sharpened understanding of what might become important to look at more extensively in future more controlled research. Case studies are also often the only way to study rare conditions because it may be impossible to find a large enough sample of individuals with the condition to use quantitative methods. Although at first glance a case study of a rare individual might seem to tell us little about ourselves, they often do provide insights into normal behavior. The case of HM provided important insights into the role of the hippocampus in memory consolidation.
However, it is important to note that while case studies can provide insights into certain areas and variables to study, and can be useful in helping develop theories, they should never be used as evidence for theories. In other words, case studies can be used as inspiration to formulate theories and hypotheses, but those hypotheses and theories then need to be formally tested using more rigorous quantitative methods. The reason case studies shouldn’t be used to provide support for theories is that they suffer from problems with both internal and external validity. Case studies lack the proper controls that true experiments contain. As such, they suffer from problems with internal validity, so they cannot be used to determine causation. For instance, during HM’s surgery, the surgeon may have accidentally lesioned another area of HM’s brain (a possibility suggested by the dissection of HM’s brain following his death) and that lesion may have contributed to his inability to consolidate new information. The fact is, with case studies we cannot rule out these sorts of alternative explanations. So, as with all observational methods, case studies do not permit determination of causation. In addition, because case studies are often of a single individual, and typically an abnormal individual, researchers cannot generalize their conclusions to other individuals. Recall that with most research designs there is a trade-off between internal and external validity. With case studies, however, there are problems with both internal validity and external validity. So there are limits both to the ability to determine causation and to generalize the results. A final limitation of case studies is that ample opportunity exists for the theoretical biases of the researcher to color or bias the case description. Indeed, there have been accusations that the woman who studied HM destroyed a lot of her data that were not published and she has been called into question for destroying contradictory data that didn’t support her theory about how memories are consolidated. There is a fascinating New York Times article that describes some of the controversies that ensued after HM’s death and analysis of his brain that can be found at: https://www.nytimes.com/2016/08/07/magazine/the-brain-that-couldnt-remember.html?_r=0
Archival Research
Another approach that is often considered observational research involves analyzing archival data that have already been collected for some other purpose. An example is a study by Brett Pelham and his colleagues on “implicit egotism”—the tendency for people to prefer people, places, and things that are similar to themselves (Pelham, Carvallo, & Jones, 2005) [9] . In one study, they examined Social Security records to show that women with the names Virginia, Georgia, Louise, and Florence were especially likely to have moved to the states of Virginia, Georgia, Louisiana, and Florida, respectively.
As with naturalistic observation, measurement can be more or less straightforward when working with archival data. For example, counting the number of people named Virginia who live in various states based on Social Security records is relatively straightforward. But consider a study by Christopher Peterson and his colleagues on the relationship between optimism and health using data that had been collected many years before for a study on adult development (Peterson, Seligman, & Vaillant, 1988) [10] . In the 1940s, healthy male college students had completed an open-ended questionnaire about difficult wartime experiences. In the late 1980s, Peterson and his colleagues reviewed the men’s questionnaire responses to obtain a measure of explanatory style—their habitual ways of explaining bad events that happen to them. More pessimistic people tend to blame themselves and expect long-term negative consequences that affect many aspects of their lives, while more optimistic people tend to blame outside forces and expect limited negative consequences. To obtain a measure of explanatory style for each participant, the researchers used a procedure in which all negative events mentioned in the questionnaire responses, and any causal explanations for them were identified and written on index cards. These were given to a separate group of raters who rated each explanation in terms of three separate dimensions of optimism-pessimism. These ratings were then averaged to produce an explanatory style score for each participant. The researchers then assessed the statistical relationship between the men’s explanatory style as undergraduate students and archival measures of their health at approximately 60 years of age. The primary result was that the more optimistic the men were as undergraduate students, the healthier they were as older men. Pearson’s r was +.25.
This method is an example of content analysis —a family of systematic approaches to measurement using complex archival data. Just as structured observation requires specifying the behaviors of interest and then noting them as they occur, content analysis requires specifying keywords, phrases, or ideas and then finding all occurrences of them in the data. These occurrences can then be counted, timed (e.g., the amount of time devoted to entertainment topics on the nightly news show), or analyzed in a variety of other ways.
- Festinger, L., Riecken, H., & Schachter, S. (1956). When prophecy fails: A social and psychological study of a modern group that predicted the destruction of the world. University of Minnesota Press. ↵
- Rosenhan, D. L. (1973). On being sane in insane places. Science, 179 , 250–258. ↵
- Wilkins, A. (2008). “Happier than Non-Christians”: Collective emotions and symbolic boundaries among evangelical Christians. Social Psychology Quarterly, 71 , 281–301. ↵
- Levine, R. V., & Norenzayan, A. (1999). The pace of life in 31 countries. Journal of Cross-Cultural Psychology, 30 , 178–205. ↵
- Kraut, R. E., & Johnston, R. E. (1979). Social and emotional messages of smiling: An ethological approach. Journal of Personality and Social Psychology, 37 , 1539–1553. ↵
- Cohen, D., Nisbett, R. E., Bowdle, B. F., & Schwarz, N. (1996). Insult, aggression, and the southern culture of honor: An "experimental ethnography." Journal of Personality and Social Psychology, 70 (5), 945-960. ↵
- Watson, J. B., & Rayner, R. (1920). Conditioned emotional reactions. Journal of Experimental Psychology, 3 , 1–14. ↵
- Freud, S. (1961). Five lectures on psycho-analysis . New York, NY: Norton. ↵
- Pelham, B. W., Carvallo, M., & Jones, J. T. (2005). Implicit egotism. Current Directions in Psychological Science, 14 , 106–110. ↵
- Peterson, C., Seligman, M. E. P., & Vaillant, G. E. (1988). Pessimistic explanatory style is a risk factor for physical illness: A thirty-five year longitudinal study. Journal of Personality and Social Psychology, 55 , 23–27. ↵

- Research Process
What is Observational Study Design and Types
- 4 minute read
- 109.1K views
Table of Contents
Most people think of a traditional experimental design when they consider research and published research papers. There is, however, a type of research that is more observational in nature, and it is appropriately referred to as “observational studies.”
There are many valuable reasons to utilize an observational study design. But, just as in research experimental design, different methods can be used when you’re considering this type of study. In this article, we’ll look at the advantages and disadvantages of an observational study design, as well as the 3 types of observational studies.
What is Observational Study Design?
An observational study is when researchers are looking at the effect of some type of intervention, risk, a diagnostic test or treatment, without trying to manipulate who is, or who isn’t, exposed to it.
This differs from an experimental study, where the scientists are manipulating who is exposed to the treatment, intervention, etc., by having a control group, or those who are not exposed, and an experimental group, or those who are exposed to the intervention, treatment, etc. In the best studies, the groups are randomized, or chosen by chance.
Any evidence derived from systematic reviews is considered the best in the hierarchy of evidence, which considers which studies are deemed the most reliable. Next would be any evidence that comes from randomized controlled trials. Cohort studies and case studies follow, in that order.
Cohort studies and case studies are considered observational in design, whereas the randomized controlled trial would be an experimental study.
Let’s take a closer look at the different types of observational study design.
The 3 types of Observational Studies
The different types of observational studies are used for different reasons. Selecting the best type for your research is critical to a successful outcome. One of the main reasons observational studies are used is when a randomized experiment would be considered unethical. For example, a life-saving medication used in a public health emergency. They are also used when looking at aetiology, or the cause of a condition or disease, as well as the treatment of rare conditions.
Case Control Observational Study
Researchers in case control studies identify individuals with an existing health issue or condition, or “cases,” along with a similar group without the condition, or “controls.” These two groups are then compared to identify predictors and outcomes. This type of study is helpful to generate a hypothesis that can then be researched.
Cohort Observational Study
This type of observational study is often used to help understand cause and effect. A cohort observational study looks at causes, incidence and prognosis, for example. A cohort is a group of people who are linked in a particular way, for example, a birth cohort would include people who were born within a specific period of time. Scientists might compare what happens to the members of the cohort who have been exposed to some variable to what occurs with members of the cohort who haven’t been exposed.
Cross Sectional Observational Study
Unlike a cohort observational study, a cross sectional observational study does not explore cause and effect, but instead looks at prevalence. Here you would look at data from a particular group at one very specific period of time. Researchers would simply observe and record information about something present in the population, without manipulating any variables or interventions. These types of studies are commonly used in psychology, education and social science.
Advantages and Disadvantages of Observational Study Design
Observational study designs have the distinct advantage of allowing researchers to explore answers to questions where a randomized controlled trial, or RCT, would be unethical. Additionally, if the study is focused on a rare condition, studying existing cases as compared to non-affected individuals might be the most effective way to identify possible causes of the condition. Likewise, if very little is known about a condition or circumstance, a cohort study would be a good study design choice.
A primary advantage to the observational study design is that they can generally be completed quickly and inexpensively. A RCT can take years before the data is compiled and available. RCTs are more complex and involved, requiring many more logistics and details to iron out, whereas an observational study can be more easily designed and completed.
The main disadvantage of observational study designs is that they’re more open to dispute than an RCT. Of particular concern would be confounding biases. This is when a cohort might share other characteristics that affect the outcome versus the outcome stated in the study. An example would be that people who practice good sleeping habits have less heart disease. But, maybe those who practice effective sleeping habits also, in general, eat better and exercise more.
Language Editing Plus Service
Need help with your research writing? With our Language Editing Plus service , we’ll help you improve the flow and writing of your paper, including UNLIMITED editing support. Use the simulator below to check the price for your manuscript, using the total number of words of the document.

Clinical Questions: PICO and PEO Research

Paper Retraction: Meaning and Main Reasons
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.

Design Science Methodology for Information Systems and Software Engineering pp 225–245 Cite as
Observational Case Studies
- Roel J. Wieringa 2
- First Online: 01 January 2014
9648 Accesses
1 Citations
An observational case study is a study of a real-world case without performing an intervention. Measurement may influence the measured phenomena, but as in all forms of research, the researcher tries to restrict this to a minimum.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
D. Damian, J. Chisan, An empirical study of the complex relationships between requirements engineering processes and other processes that lead to payoffs in productivity, quality and risk management. IEEE Trans. Softw. Eng. 32 (7), 433–453 (2006)
Article Google Scholar
M. Denscombe, The Good Research Guide For Small-Scale Social Research Projects , 4th edn. (Open University Press, Maidenhead, 2010)
Google Scholar
K.M. Eisenhardt, Building theories from case study research. Acad. Manag. Rev. 14 (4), 532–550 (1989)
B. Flyvberg, Five misunderstandings about case-study research. Qual. Inq. 12 (2), 219–245 (2006)
R.L. Glass, Pilot studies: What, why, and how. J. Syst. Softw. 36 , 85–97 (1997)
M.M. Kennedy, Generalizing from single case studies. Eval. Q. 3 (4), 661–678 (1979)
B. Kitchenham, L. Pickard, S.L. Pfleeger, Case studies for method and tool evaluation. IEEE Softw. 12 (4), 52–62 (1995)
C. Robson, Real World Research , 2nd edn. (Blackwell, Oxford, 2002)
P. Runeson, M. Höst, A. Rainer, B. Regnell, Case Study Research in Software Engineering: Guidelines and Examples (Wiley, Hoboken, 2012)
Book Google Scholar
J.M. Verner, J. Sampson, V. Tosic, N.A.A. Bakar, B.A. Kitchenham, Guidelines for industrially-based multiple case studies in software engineering, in Research Challenges in Information Science, 2009. RCIS 2009. Third International Conference on , 2009, pp. 313–324
L. Warne, D. Hart, The impact of organizational politics on information systems project failure-a case study, in Proceedings of the Twenty-Ninth Hawaii International Conference on System Sciences , vol. 4, 1996, pp. 191–201
R.J. Wieringa, Towards a unified checklist for empirical research in software engineering: first proposal, in 16th International Conference on Evaluation and Assessment in Software Engineering (EASE 2012) , ed. by T. Baldaresse, M. Genero, E. Mendes, M. Piattini (IET, Ciudad Real, 2012), pp. 161–165
R.J. Wieringa, A unified checklist for observational and experimental research in software engineering (version 1). Technical Report TR-CTIT-12-07, Centre for Telematics and Information Technology University of Twente (2012)
R.K. Yin, Case Study research: Design and Methods (Sage, Thousand Oaks, 1984)
R.K. Yin, Case Study research: Design and Methods , 3rd edn. (Sage, Thousand Oaks, 2003)
Download references
Author information
Authors and affiliations.
University of Twente, Enschede, The Netherlands
Roel J. Wieringa
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter.
Wieringa, R.J. (2014). Observational Case Studies. In: Design Science Methodology for Information Systems and Software Engineering. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43839-8_17
Download citation
DOI : https://doi.org/10.1007/978-3-662-43839-8_17
Published : 20 August 2014
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-662-43838-1
Online ISBN : 978-3-662-43839-8
eBook Packages : Computer Science Computer Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Case Study? | Definition, Examples & Methods
What Is a Case Study? | Definition, Examples & Methods
Published on May 8, 2019 by Shona McCombes . Revised on November 20, 2023.
A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research.
A case study research design usually involves qualitative methods , but quantitative methods are sometimes also used. Case studies are good for describing , comparing, evaluating and understanding different aspects of a research problem .
Table of contents
When to do a case study, step 1: select a case, step 2: build a theoretical framework, step 3: collect your data, step 4: describe and analyze the case, other interesting articles.
A case study is an appropriate research design when you want to gain concrete, contextual, in-depth knowledge about a specific real-world subject. It allows you to explore the key characteristics, meanings, and implications of the case.
Case studies are often a good choice in a thesis or dissertation . They keep your project focused and manageable when you don’t have the time or resources to do large-scale research.
You might use just one complex case study where you explore a single subject in depth, or conduct multiple case studies to compare and illuminate different aspects of your research problem.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Once you have developed your problem statement and research questions , you should be ready to choose the specific case that you want to focus on. A good case study should have the potential to:
- Provide new or unexpected insights into the subject
- Challenge or complicate existing assumptions and theories
- Propose practical courses of action to resolve a problem
- Open up new directions for future research
TipIf your research is more practical in nature and aims to simultaneously investigate an issue as you solve it, consider conducting action research instead.
Unlike quantitative or experimental research , a strong case study does not require a random or representative sample. In fact, case studies often deliberately focus on unusual, neglected, or outlying cases which may shed new light on the research problem.
Example of an outlying case studyIn the 1960s the town of Roseto, Pennsylvania was discovered to have extremely low rates of heart disease compared to the US average. It became an important case study for understanding previously neglected causes of heart disease.
However, you can also choose a more common or representative case to exemplify a particular category, experience or phenomenon.
Example of a representative case studyIn the 1920s, two sociologists used Muncie, Indiana as a case study of a typical American city that supposedly exemplified the changing culture of the US at the time.
While case studies focus more on concrete details than general theories, they should usually have some connection with theory in the field. This way the case study is not just an isolated description, but is integrated into existing knowledge about the topic. It might aim to:
- Exemplify a theory by showing how it explains the case under investigation
- Expand on a theory by uncovering new concepts and ideas that need to be incorporated
- Challenge a theory by exploring an outlier case that doesn’t fit with established assumptions
To ensure that your analysis of the case has a solid academic grounding, you should conduct a literature review of sources related to the topic and develop a theoretical framework . This means identifying key concepts and theories to guide your analysis and interpretation.
There are many different research methods you can use to collect data on your subject. Case studies tend to focus on qualitative data using methods such as interviews , observations , and analysis of primary and secondary sources (e.g., newspaper articles, photographs, official records). Sometimes a case study will also collect quantitative data.
Example of a mixed methods case studyFor a case study of a wind farm development in a rural area, you could collect quantitative data on employment rates and business revenue, collect qualitative data on local people’s perceptions and experiences, and analyze local and national media coverage of the development.
The aim is to gain as thorough an understanding as possible of the case and its context.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

In writing up the case study, you need to bring together all the relevant aspects to give as complete a picture as possible of the subject.
How you report your findings depends on the type of research you are doing. Some case studies are structured like a standard scientific paper or thesis , with separate sections or chapters for the methods , results and discussion .
Others are written in a more narrative style, aiming to explore the case from various angles and analyze its meanings and implications (for example, by using textual analysis or discourse analysis ).
In all cases, though, make sure to give contextual details about the case, connect it back to the literature and theory, and discuss how it fits into wider patterns or debates.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Degrees of freedom
- Null hypothesis
- Discourse analysis
- Control groups
- Mixed methods research
- Non-probability sampling
- Quantitative research
- Ecological validity
Research bias
- Rosenthal effect
- Implicit bias
- Cognitive bias
- Selection bias
- Negativity bias
- Status quo bias
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). What Is a Case Study? | Definition, Examples & Methods. Scribbr. Retrieved April 12, 2024, from https://www.scribbr.com/methodology/case-study/
Is this article helpful?
Shona McCombes
Other students also liked, primary vs. secondary sources | difference & examples, what is a theoretical framework | guide to organizing, what is action research | definition & examples, what is your plagiarism score.
- En español – ExME
- Em português – EME
An introduction to different types of study design
Posted on 6th April 2021 by Hadi Abbas

Study designs are the set of methods and procedures used to collect and analyze data in a study.
Broadly speaking, there are 2 types of study designs: descriptive studies and analytical studies.
Descriptive studies
- Describes specific characteristics in a population of interest
- The most common forms are case reports and case series
- In a case report, we discuss our experience with the patient’s symptoms, signs, diagnosis, and treatment
- In a case series, several patients with similar experiences are grouped.
Analytical Studies
Analytical studies are of 2 types: observational and experimental.
Observational studies are studies that we conduct without any intervention or experiment. In those studies, we purely observe the outcomes. On the other hand, in experimental studies, we conduct experiments and interventions.
Observational studies
Observational studies include many subtypes. Below, I will discuss the most common designs.
Cross-sectional study:
- This design is transverse where we take a specific sample at a specific time without any follow-up
- It allows us to calculate the frequency of disease ( p revalence ) or the frequency of a risk factor
- This design is easy to conduct
- For example – if we want to know the prevalence of migraine in a population, we can conduct a cross-sectional study whereby we take a sample from the population and calculate the number of patients with migraine headaches.
Cohort study:
- We conduct this study by comparing two samples from the population: one sample with a risk factor while the other lacks this risk factor
- It shows us the risk of developing the disease in individuals with the risk factor compared to those without the risk factor ( RR = relative risk )
- Prospective : we follow the individuals in the future to know who will develop the disease
- Retrospective : we look to the past to know who developed the disease (e.g. using medical records)
- This design is the strongest among the observational studies
- For example – to find out the relative risk of developing chronic obstructive pulmonary disease (COPD) among smokers, we take a sample including smokers and non-smokers. Then, we calculate the number of individuals with COPD among both.
Case-Control Study:
- We conduct this study by comparing 2 groups: one group with the disease (cases) and another group without the disease (controls)
- This design is always retrospective
- We aim to find out the odds of having a risk factor or an exposure if an individual has a specific disease (Odds ratio)
- Relatively easy to conduct
- For example – we want to study the odds of being a smoker among hypertensive patients compared to normotensive ones. To do so, we choose a group of patients diagnosed with hypertension and another group that serves as the control (normal blood pressure). Then we study their smoking history to find out if there is a correlation.
Experimental Studies
- Also known as interventional studies
- Can involve animals and humans
- Pre-clinical trials involve animals
- Clinical trials are experimental studies involving humans
- In clinical trials, we study the effect of an intervention compared to another intervention or placebo. As an example, I have listed the four phases of a drug trial:
I: We aim to assess the safety of the drug ( is it safe ? )
II: We aim to assess the efficacy of the drug ( does it work ? )
III: We want to know if this drug is better than the old treatment ( is it better ? )
IV: We follow-up to detect long-term side effects ( can it stay in the market ? )
- In randomized controlled trials, one group of participants receives the control, while the other receives the tested drug/intervention. Those studies are the best way to evaluate the efficacy of a treatment.
Finally, the figure below will help you with your understanding of different types of study designs.

References (pdf)
You may also be interested in the following blogs for further reading:
An introduction to randomized controlled trials
Case-control and cohort studies: a brief overview
Cohort studies: prospective and retrospective designs
Prevalence vs Incidence: what is the difference?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on An introduction to different types of study design
you are amazing one!! if I get you I’m working with you! I’m student from Ethiopian higher education. health sciences student
Very informative and easy understandable
You are my kind of doctor. Do not lose sight of your objective.
Wow very erll explained and easy to understand
I’m Khamisu Habibu community health officer student from Abubakar Tafawa Balewa university teaching hospital Bauchi, Nigeria, I really appreciate your write up and you have make it clear for the learner. thank you
well understood,thank you so much
Well understood…thanks
Simply explained. Thank You.
Thanks a lot for this nice informative article which help me to understand different study designs that I felt difficult before
That’s lovely to hear, Mona, thank you for letting the author know how useful this was. If there are any other particular topics you think would be useful to you, and are not already on the website, please do let us know.
it is very informative and useful.
thank you statistician
Fabulous to hear, thank you John.
Thanks for this information
Thanks so much for this information….I have clearly known the types of study design Thanks
That’s so good to hear, Mirembe, thank you for letting the author know.
Very helpful article!! U have simplified everything for easy understanding
I’m a health science major currently taking statistics for health care workers…this is a challenging class…thanks for the simified feedback.
That’s good to hear this has helped you. Hopefully you will find some of the other blogs useful too. If you see any topics that are missing from the website, please do let us know!
Hello. I liked your presentation, the fact that you ranked them clearly is very helpful to understand for people like me who is a novelist researcher. However, I was expecting to read much more about the Experimental studies. So please direct me if you already have or will one day. Thank you
Dear Ay. My sincere apologies for not responding to your comment sooner. You may find it useful to filter the blogs by the topic of ‘Study design and research methods’ – here is a link to that filter: https://s4be.cochrane.org/blog/topic/study-design/ This will cover more detail about experimental studies. Or have a look on our library page for further resources there – you’ll find that on the ‘Resources’ drop down from the home page.
However, if there are specific things you feel you would like to learn about experimental studies, that are missing from the website, it would be great if you could let me know too. Thank you, and best of luck. Emma
Great job Mr Hadi. I advise you to prepare and study for the Australian Medical Board Exams as soon as you finish your undergrad study in Lebanon. Good luck and hope we can meet sometime in the future. Regards ;)
You have give a good explaination of what am looking for. However, references am not sure of where to get them from.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

A well-designed cohort study can provide powerful results. This blog introduces prospective and retrospective cohort studies, discussing the advantages, disadvantages and use of these type of study designs.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 12. Observational Study Designs
Bradley G. Hammill
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
- Introduction
- Analytic Study Designs
- Descriptive Study Designs
- Conclusions
- Full Chapter
- Supplementary Content
Observational studies in clinical research can be classified as either analytic or descriptive ( Table 12–1 ). Analytic observational studies are similar to randomized, controlled clinical trials in that the goal is to estimate the causal effect of an exposure on an outcome. Also similar to trials, analytic observational studies always include some type of comparison group, against which the experience of the exposed group is compared. Well-designed analytic studies can generate strong evidence for or against a stated hypothesis. Descriptive studies, on the other hand, aim to describe the characteristics or experiences of a particular patient group. Even well-designed descriptive studies cannot be used to draw strong conclusions about the effect of an exposure on an outcome. Instead, these studies are often used to generate study questions that can then be tested by more rigorous methods.
Although many observational study designs are available to researchers ( 1 ), a few are most widely used and will be described below. The analytic study designs presented are the case-control study and the cohort study. The descriptive study designs presented are the ecologic study, the cross-sectional prevalence survey, and case reports or case series.
Case-Control Studies
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.

Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Research Methods (Case Studies & Observation Studies) 0 Pages | Leaving School | 29/03/2024
- Case Studies & Observation Studies

——————————————————
Observation Studies

Categories of behaviour: If the researcher is undertaking a natural observation , he may need to divide the behaviour he sees into categories so that a quick record can be made. If the researcher wants to understand how the public respond to a woman collapsing in the street, for example, his categories might include 1.) Ignores and walks on. 2.) Hesitates and walks on. 3.) Checks to see if the woman is ok. 4.) Calls 999.
Inter-observer reliability: In order to test the reliability of an observer’s records, it might be sensible to have two observers who are working to exactly the same category and score sheet, so that they can compare their results at the end of the observation period. If these observations closely match each other then it can be assumed their observations have been accurate. If there is a significant difference it may be necessary to start the observation over again.
Advantages of natural observation Natural observations are high in ecological validity . A string of natural actions can be observed. In a laboratory situation people are often asked to complete unnatural tasks.
Disadvantages of natural observation In the absence ofcontrolled variables it is difficult to establish why someone behaved in a certain way. This type of study is reliant on the accuracy of the observation. There are ethical issues involved in an observation of this kind i.e. the people being observed may not know that this is the case. Should they be told? And if they are told, would their behaviour still be natural? Natural observations can be awkward to plan as well as time consuming.
- Research Methods
- Hypotheses and Experimental Designs
- Standardised Procedures & Instructions
- Ecological Validity & Sampling Methods
- Making Sense of Data & Anomalous Results
- Survey Methods & Ethical Considerations
- Remember it, Test it!
ADVERTISEMENTS
Observational vs. Experimental Study: A Comprehensive Guide
Explore the fundamental disparities between experimental and observational studies in this comprehensive guide by Santos Research Center, Corp. Uncover concepts such as control group, random sample, cohort studies, response variable, and explanatory variable that shape the foundation of these methodologies. Discover the significance of randomized controlled trials and case control studies, examining causal relationships and the role of dependent variables and independent variables in research designs.
This enlightening exploration also delves into the meticulous scientific study process, involving survey members, systematic reviews, and statistical analyses. Investigate the careful balance of control group and treatment group dynamics, highlighting how researchers meticulously assign variables and analyze statistical patterns to discern meaningful insights. From dissecting issues like lung cancer to understanding sleep patterns, this guide emphasizes the precision of controlled experiments and controlled trials, where variables are isolated and scrutinized, paving the way for a deeper comprehension of the world through empirical research.
Introduction to Observational and Experimental Studies
These two studies are the cornerstones of scientific inquiry, each offering a distinct approach to unraveling the mysteries of the natural world.
Observational studies allow us to observe, document, and gather data without direct intervention. They provide a means to explore real-world scenarios and trends, making them valuable when manipulating variables is not feasible or ethical. From surveys to meticulous observations, these studies shed light on existing conditions and relationships.
Experimental studies , in contrast, put researchers in the driver's seat. They involve the deliberate manipulation of variables to understand their impact on specific outcomes. By controlling the conditions, experimental studies establish causal relationships, answering questions of causality with precision. This approach is pivotal for hypothesis testing and informed decision-making.
At Santos Research Center, Corp., we recognize the importance of both observational and experimental studies. We employ these methodologies in our diverse research projects to ensure the highest quality of scientific investigation and to answer a wide range of research questions.
Observational Studies: A Closer Look
In our exploration of research methodologies, let's zoom in on observational research studies—an essential facet of scientific inquiry that we at Santos Research Center, Corp., expertly employ in our diverse research projects.
What is an Observational Study?
Observational research studies involve the passive observation of subjects without any intervention or manipulation by researchers. These studies are designed to scrutinize the relationships between variables and test subjects, uncover patterns, and draw conclusions grounded in real-world data.
Researchers refrain from interfering with the natural course of events in controlled experiment. Instead, they meticulously gather data by keenly observing and documenting information about the test subjects and their surroundings. This approach permits the examination of variables that cannot be ethically or feasibly manipulated, making it particularly valuable in certain research scenarios.
Types of Observational Studies
Now, let's delve into the various forms that observational studies can take, each with its distinct characteristics and applications.
Cohort Studies: A cohort study is a type of observational study that entails tracking one group of individuals over an extended period. Its primary goal is to identify potential causes or risk factors for specific outcomes or treatment group. Cohort studies provide valuable insights into the development of conditions or diseases and the factors that influence them.
Case-Control Studies: Case-control studies, on the other hand, involve the comparison of individuals with a particular condition or outcome to those without it (the control group). These studies aim to discern potential causal factors or associations that may have contributed to the development of the condition under investigation.
Cross-Sectional Studies: Cross-sectional studies take a snapshot of a diverse group of individuals at a single point in time. By collecting data from this snapshot, researchers gain insights into the prevalence of a specific condition or the relationships between variables at that precise moment. Cross-sectional studies are often used to assess the health status of the different groups within a population or explore the interplay between various factors.
Advantages and Limitations of Observational Studies
Observational studies, as we've explored, are a vital pillar of scientific research, offering unique insights into real-world phenomena. In this section, we will dissect the advantages and limitations that characterize these studies, shedding light on the intricacies that researchers grapple with when employing this methodology.
Advantages: One of the paramount advantages of observational studies lies in their utilization of real-world data. Unlike controlled experiments that operate in artificial settings, observational studies embrace the complexities of the natural world. This approach enables researchers to capture genuine behaviors, patterns, and occurrences as they unfold. As a result, the data collected reflects the intricacies of real-life scenarios, making it highly relevant and applicable to diverse settings and populations.
Moreover, in a randomized controlled trial, researchers looked to randomly assign participants to a group. Observational studies excel in their capacity to examine long-term trends. By observing one group of subjects over extended periods, research scientists gain the ability to track developments, trends, and shifts in behavior or outcomes. This longitudinal perspective is invaluable when studying phenomena that evolve gradually, such as chronic diseases, societal changes, or environmental shifts. It allows for the detection of subtle nuances that may be missed in shorter-term investigations.
Limitations: However, like any research methodology, observational studies are not without their limitations. One significant challenge of statistical study lies in the potential for biases. Since researchers do not intervene in the subjects' experiences, various biases can creep into the data collection process. These biases may arise from participant self-reporting, observer bias, or selection bias in random sample, among others. Careful design and rigorous data analysis are crucial for mitigating these biases.
Another limitation is the presence of confounding variables. In observational studies, it can be challenging to isolate the effect of a specific variable from the myriad of other factors at play. These confounding variables can obscure the true relationship between the variables of interest, making it difficult to establish causation definitively. Research scientists must employ statistical techniques to control for or adjust these confounding variables.
Additionally, observational studies face constraints in their ability to establish causation. While they can identify associations and correlations between variables, they cannot prove causality or causal relationship. Establishing causation typically requires controlled experiments where researchers can manipulate independent variables systematically. In observational studies, researchers can only infer potential causation based on the observed associations.
Experimental Studies: Delving Deeper
In the intricate landscape of scientific research, we now turn our gaze toward experimental studies—a dynamic and powerful method that Santos Research Center, Corp. skillfully employs in our pursuit of knowledge.
What is an Experimental Study?
While some studies observe and gather data passively, experimental studies take a more proactive approach. Here, researchers actively introduce an intervention or treatment to an experiment group study its effects on one or more variables. This methodology empowers researchers to manipulate independent variables deliberately and examine their direct impact on dependent variables.
Experimental research are distinguished by their exceptional ability to establish cause-and-effect relationships. This invaluable characteristic allows researchers to unlock the mysteries of how one variable influences another, offering profound insights into the scientific questions at hand. Within the controlled environment of an experimental study, researchers can systematically test hypotheses, shedding light on complex phenomena.
Key Features of Experimental Studies
Central to statistical analysis, the rigor and reliability of experimental studies are several key features that ensure the validity of their findings.
Randomized Controlled Trials: Randomization is a critical element in experimental studies, as it ensures that subjects are assigned to groups in a random assignment. This randomly assigned allocation minimizes the risk of unintentional biases and confounding variables, strengthening the credibility of the study's outcomes.
Control Groups: Control groups play a pivotal role in experimental studies by serving as a baseline for comparison. They enable researchers to assess the true impact of the intervention being studied. By comparing the outcomes of the intervention group to those of survey members of the control group, researchers can discern whether the intervention caused the observed changes.
Blinding: Both single-blind and double-blind techniques are employed in experimental studies to prevent biases from influencing the study or controlled trial's outcomes. Single-blind studies keep either the subjects or the researchers unaware of certain aspects of the study, while double-blind studies extend this blindness to both parties, enhancing the objectivity of the study.
These key features work in concert to uphold the integrity and trustworthiness of the results generated through experimental studies.
Advantages and Limitations of Experimental Studies
As with any research methodology, this one comes with its unique set of advantages and limitations.
Advantages: These studies offer the distinct advantage of establishing causal relationships between two or more variables together. The controlled environment allows researchers to exert authority over variables, ensuring that changes in the dependent variable can be attributed to the independent variable. This meticulous control results in high-quality, reliable data that can significantly contribute to scientific knowledge.
Limitations: However, experimental ones are not without their challenges. They may raise ethical concerns, particularly when the interventions involve potential risks to subjects. Additionally, their controlled nature can limit their real-world applicability, as the conditions in experiments may not accurately mirror those in the natural world. Moreover, executing an experimental study in randomized controlled, often demands substantial resources, with other variables including time, funding, and personnel.
Observational vs Experimental: A Side-by-Side Comparison
Having previously examined observational and experimental studies individually, we now embark on a side-by-side comparison to illuminate the key distinctions and commonalities between these foundational research approaches.
Key Differences and Notable Similarities
Methodologies
- Observational Studies : Characterized by passive observation, where researchers collect data without direct intervention, allowing the natural course of events to unfold.
- Experimental Studies : Involve active intervention, where researchers deliberately manipulate variables to discern their impact on specific outcomes, ensuring control over the experimental conditions.
- Observational Studies : Designed to identify patterns, correlations, and associations within existing data, shedding light on relationships within real-world settings.
- Experimental Studies : Geared toward establishing causality by determining the cause-and-effect relationships between variables, often in controlled laboratory environments.
- Observational Studies : Yield real-world data, reflecting the complexities and nuances of natural phenomena.
- Experimental Studies : Generate controlled data, allowing for precise analysis and the establishment of clear causal connections.
Observational studies excel at exploring associations and uncovering patterns within the intricacies of real-world settings, while experimental studies shine as the gold standard for discerning cause-and-effect relationships through meticulous control and manipulation in controlled environments. Understanding these differences and similarities empowers researchers to choose the most appropriate method for their specific research objectives.
When to Use Which: Practical Applications
The decision to employ either observational or experimental studies hinges on the research objectives at hand and the available resources. Observational studies prove invaluable when variable manipulation is impractical or ethically challenging, making them ideal for delving into long-term trends and uncovering intricate associations between certain variables (response variable or explanatory variable). On the other hand, experimental studies emerge as indispensable tools when the aim is to definitively establish causation and methodically control variables.
At Santos Research Center, Corp., our approach to both scientific study and methodology is characterized by meticulous consideration of the specific research goals. We recognize that the quality of outcomes hinges on selecting the most appropriate method of research study. Our unwavering commitment to employing both observational and experimental research studies further underscores our dedication to advancing scientific knowledge across diverse domains.
Conclusion: The Synergy of Experimental and Observational Studies in Research
In conclusion, both observational and experimental studies are integral to scientific research, offering complementary approaches with unique strengths and limitations. At Santos Research Center, Corp., we leverage these methodologies to contribute meaningfully to the scientific community.
Explore our projects and initiatives at Santos Research Center, Corp. by visiting our website or contacting us at (813) 249-9100, where our unwavering commitment to rigorous research practices and advancing scientific knowledge awaits.
Recent Posts
Learn everything about Respiratory Syncytial Virus (RSV), from symptoms and diagnosis to treatment and prevention. Stay informed and protect your health with...
Discover key insights on Alzheimer's disease, including symptoms, stages, and care tips. Learn how to manage the condition and find out how you can...
Discover expert insights on migraines, from symptoms and causes to management strategies, and learn about our specialized support at Santos Research Center.
Explore our in-depth guide on UTIs, covering everything from symptoms and causes to effective treatments, and learn how to manage and prevent urinary tract infections.
Your definitive guide to COVID symptoms. Dive deep into the signs of COVID-19, understand the new variants, and get answers to your most pressing questions.
Unravel the differences between observational and experimental studies. Dive into the intricacies of each method and discover their unique applications in research.
Discover the different types of clinical trials and their significance in advancing medical science. Dive deep into the world of clinical research with Santos Research Center.
Santos Research Center, Corp. is a research facility conducting paid clinical trials, in partnership with major pharmaceutical companies & CROs. We work with patients from across the Tampa Bay area.
Contact Details
Navigation menu.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.10(2); Apr-Jun 2019
Study designs: Part 3 - Analytical observational studies
Priya ranganathan.
Department of Anaesthesiology, Tata Memorial Centre, Mumbai, Maharashtra, India
Rakesh Aggarwal
1 Director, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
In analytical observational studies, researchers try to establish an association between exposure(s) and outcome(s). Depending on the direction of enquiry, these studies can be directed forwards (cohort studies) or backwards (case–control studies). In this article, we examine the key features of these two types of studies.
INTRODUCTION
In a previous article[ 1 ] in this series, we looked at descriptive observational studies, namely case reports, case series, cross-sectional studies, and ecological studies. As compared to descriptive studies which merely describe one or more variables in a sample (or occasionally population), analytical studies attempt to quantify a relationship or association between two variables – an exposure and an outcome. As discussed previously, in observational analytical studies, the exposure is naturally determined as opposed to experimental studies where an investigator assigns each subject to receive or not receive a particular exposure.
COHORT STUDIES
A cohort is defined as a “group of people with a shared characteristic.” In cohort studies, different groups of people with varying levels of exposure are followed over time to evaluate the occurrence of an outcome. These participants have to be free of the outcome at baseline. The presence or absence of the risk factor (exposure) in each subject is recorded. The subjects are then followed up over time (longitudinally) to determine the occurrence of the outcome. Thus, cohort studies are forward-direction studies (moving from exposure to outcome) and are typically prospective studies (the outcome has not occurred at the start of the study).
An example of cohort study design is a study by Viljakainen et al ., which investigated the relation between maternal vitamin D levels during pregnancy and the bone health in their newborns.[ 2 ] Maternal blood vitamin D levels were estimated during pregnancy. Children born to these mothers were then followed up until 14 months of age, and bone parameters were evaluated. Based on the maternal serum 25-hydroxy vitamin D levels during pregnancy, children were divided into two groups – those born to mothers with normal blood vitamin D and those born to mothers with low blood vitamin D. The authors found that children born to mothers with low vitamin D levels had persistent bone abnormalities.
Advantages of cohort studies
- For an exposure to be causative, it must precede the outcome. In a cohort study, one starts with subjects who are known to have or not have the exposure and are free of the outcome at the start of the study, and the outcome develops later. Hence, one is certain that the exposure preceded the outcome, and temporality (and therefore probable causality) can be established. In the above example, one can be certain that the maternal vitamin D deficiency preceded the bone abnormalities.
- For a given exposure, more than one outcome can be studied. In the above example, the authors compared not only bone growth but also the age at which the babies born to low and high vitamin D mothers started walking independently.
- In cohort studies, often several exposures can be studied simultaneously. For this, the investigators begin by assessing several 'exposures', for example, age, sex, smoking status, diabetes, and obesity/overweight status in every member of a population. The entire population is then followed for the outcome of interest, for example, coronary artery disease. At the end of the follow-up, the data can then be analyzed for several contrasting cohorts defined by levels of each “exposure” – old/young, male/female, smoker/nonsmoker, diabetic/nondiabetic, and underweight/ideal body weight/overweight/obese, etc.
Limitations of cohort studies
- Cohort studies often require a long duration of follow-up to determine whether outcome will occur or not. This duration depends on the exposure-outcome pair. In the above example, a follow-up of at least 14 months was used. An even longer follow-up over several years or decades may be necessary – for instance, in the above example, if the investigators wanted to study whether maternal vitamin D levels influence the final height of a person, they would have needed to follow the babies till adolescence. During such follow-up, losses to follow-up, and logistic and cost issues pose major challenges.
- It is not uncommon for one or more unknown confounding factors to affect the occurrence of outcome. For example, in a cohort study looking at coffee drinking as a risk factor for pancreatic cancer, people who drink a large amount of coffee may also be consuming alcohol. In such cases, the finding that coffee drinkers have an increased occurrence of pancreatic cancer may lead the investigator to incorrectly conclude that drinking coffee increases the risk of pancreatic cancer, whereas it is the consumption of alcohol which is the true risk factor. Similarly, in the above study, the mothers with low and high vitamin D levels could have been different in another factor, e.g. overall nutrition or socioeconomic status, and that could be the real reason for the differences in the babies' bone health.
Uses of cohort studies
- Since cohort study design closely resembles the experimental design with the only difference being lack of random assignment to exposure, it is considered as having a greater validity compared to the other observational study designs.
- Since one starts with subjects known to have or not have exposure, one can determine the risk of outcome among exposed persons and unexposed persons, as also the relative risk.
- In situations where experimental studies are not feasible (e.g., when it is either unethical to randomize participants to a potentially harmful intervention, such as smoking, or impractical to create an exposure, such as diabetes or hypertension), cohort studies are a reasonable and arguably the best alternative.
Variations of cohort studies
Sometimes, a researcher may look back at data which have already been collected. For example, let us think of a hospital that records every patient's smoking status at the time of the first visit. A researcher may use these records from 10 years ago, and then contact the persons today to check if any of them have already been diagnosed or currently have features of lung cancer. This is still a forward-direction study (exposure traced forward among exposed and unexposed to outcome) but is retrospective (since the outcome may have already occurred). Such studies are known as 'retrospective cohort studies'.
Large cohort studies, such as the Framingham Heart Study or the Nurses' Health Study, have yielded extremely useful information about risk factors for several chronic diseases.
CASE-CONTROL STUDIES
In case-control studies, the researcher first enrolls cases (participants with the outcome) and controls (participants without the outcome) and then tries to elicit a history of exposure in each group. Thus, these are backward-direction studies (looking from outcome to exposure) and are always retrospective (the outcome must have occurred when the study starts). Typically, cases are identified from hospital records, death certificates or disease registries. This is followed by the identification and enrolment of controls.
Identification of appropriate controls is a key element of the case-control study design and can influence the estimate of association between exposure and outcome (selection bias). The controls should resemble cases in all respects, except for the absence of disease. Thus, they should be representative of the population from which the cases were drawn. For instance, if cases are drawn from a community clinic, an outpatient clinic or an inpatient setting, the controls should also ideally be from the same setting.
Sometimes, controls are individually matched with cases for factors (except for the one which is the exposure of interest) which are considered important to the development of the outcome. For example, in a study on relation of smoking with lung cancer, for each case of lung cancer enrolled, one control with similar age and sex is enrolled. This would reduce the risk of confounding by age and sex – the factors used for matching. Sometimes, the number of controls per case may be larger (e.g. two, three, or more).
Furthermore, to minimize assessment bias, it is important that the person assessing the history of exposure (e.g., smoking in this case) is unaware of (blinded to) whether the participant being interviewed is a case or a control.
For example, Anderson et al . conducted a case–control study to look at risk factors for childhood fractures.[ 3 ] They recruited cases from a hospital fracture clinic and individually matched controls (children without fractures) from a primary care research network. The cases and controls were matched on age, sex, height, and season. They found that the history of previous use of vitamin D supplements was significantly higher in the children without fractures, suggesting an inverse association between vitamin D supplementation and incidence of fractures.
Advantages of case–control studies
- Case-control studies are often cheap, and less time-consuming than cohort studies.
- Once cases and controls are identified and enrolled, it is often easy to study the relationship of outcome with not one but several exposures.
Limitations of case–control studies
- In case-control studies, temporality (whether the outcome or exposure occurred first) is often difficult to establish.
- There may be a bias in selecting cases or controls. For instance, if the cases studied differ from the entire pool of cases of a disease in an important characteristic, then the results of the study may apply only to the selected type of cases and not to the entire population of cases. In the above example,[ 3 ] the cases and controls were derived from different sources, and it is possible that the children that attended the hospital fracture clinic had different socioeconomic backgrounds to those attending the primary care facility from where controls were enrolled.
- Confounding factors, as discussed in cohort studies, also apply to case-control studies. For instance, the children with fractures and controls could have had different overall food intake, milk intake, and outdoor play time. These factors could influence both the likelihood of prior use of vitamin D supplements (exposure) and the risk of fracture (outcome), affecting the measurement of their association.
- The determination of exposure relies on existing records or history taking. Either can be problematic. The records may not contain information on exposure or contain erroneous data (e.g., those collected perfunctorily). This is particularly challenging if the missing or unreliable data are more likely to be present in one of the two groups being compared – cases or controls (misinformation bias). During history taking, cases may be more likely to recall exposure than controls (recall bias), for example, the mother of a child with a congenital anomaly is more likely to recall drugs ingested during pregnancy than a mother with a normal child. In the study by Anderson et al,[ 3 ] the mothers of children with fractures could have underestimated the amount of vitamin D their children have received, believing that this was the reason for the occurrence of fracture.
- Finally, since case–control studies are backward-directed, there is no “at risk” group at the start of the study; therefore, the determination of “risk” (and relative risk or risk ratio) is not possible, and one can only estimate “odds” (and odds ratio). For a detailed discussion on this, please refer to a previous article.[ 4 ]
Uses of case–control studies
- Case-control studies are ideal for rare diseases, where identifying cases is easier than following up large numbers of exposed persons to determine outcome.
- Case-control studies, because of their simplicity and need for fewer resources, are often the initial study design used to assess the relationship of a particular exposure and an outcome. If this study is positive, then a study with more complex and robust study design (cohort or interventional) can be undertaken.
A special variation of case–control study design
Nested case-control design is a special type of case-control study design which is built into a cohort study. From the main cohorts, participants who develop the outcome (irrespective of whether exposed or unexposed) are chosen as cases. From among the remaining study participants who have not developed the outcome, a subset of matched controls are selected. The cases and controls are then compared with respect to exposure. This is still a backward-direction (since the enquiry begins with outcome and then proceeds toward exposure) and retrospective study (since outcomes have already occurred when the study starts). The main advantage is that since one knows that the outcome had not occurred when the cohorts were established, temporal relation of exposure and outcome is ensured.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Open access
- Published: 12 April 2024
A prospective observational cohort study of covid-19 epidemiology and vaccine seroconversion in South Western Sydney, Australia, during the 2021–2022 pandemic period.
- Daniela Potter 1 , 2 , 3 ,
- Jason Diep 3 ,
- Colleen Munro 3 ,
- Noelle Lin 3 ,
- Ramon Xu 3 ,
- Jeffrey Wong 3 ,
- Robert Porritt 4 ,
- Michael Maley 4 ,
- Hong Foo 4 &
- Angela Makris 1 , 2 , 3
BMC Nephrology volume 25 , Article number: 131 ( 2024 ) Cite this article
Metrics details
It is known that COVID-19 disproportionally adversely affects the immunocompromised, including kidney transplant recipients (KTR), as compared to the general population. Risk factors for adverse outcomes and vaccine seroconversion patterns are not fully understood. Australia was uniquely positioned to reduce initial case numbers during the 2021–2022 pandemic period due to its relative isolation and several significant public health interventions. South-Western Sydney Local Heath District was one of the predominant regions affected.
A single centre, prospective cohort study of prevalent renal transplant recipients was conducted between 25th July 2021 and 1st May 2022. Baseline characteristics, COVID-19 vaccination status, COVID-19 diagnosis and outcomes were determined from the electronic medical record, Australian vaccination register and Australian and New Zealand Dialysis and Transplant Registry. Assessment of vaccine-induced seroconversion was assessed with ELISA in a subpopulation. Analysis was performed using SPSS v.28.
We identified 444 prevalent transplant recipients (60% male, 50% diabetic, median age 58 years (Interquartile range (IQR)21.0) and eGFR 56 ml/min/1.73m 2 (IQR 21.9). COVID-19 was identified in 32% ( n = 142) of patients, of which 38% ( n = 54) required hospitalisation and 7% ( n = 10) died. At least one COVID-19 vaccination was received by 95% ( n = 423) with 17 (4%) patients remaining unvaccinated throughout the study period. Seroconversion after 2 and 3 doses of vaccine was 22% and 48% respectively. Increased COVID-19 related deaths were associated with older age (aOR 1.1, 95% CI 1.004–1.192, p = 0.040), smoking exposure (aOR 8.2, 05% CI 1.020-65.649, p = 0.048) and respiratory disease (aOR 14.2, 95%CI:1.825–110.930, p = 0.011) on multi-variable regression analysis. Receipt of three doses of vaccination was protective against acquiring COVID-19 (aOR 0.48, 95% CI 0.287–0.796, p = 0.005) and death (aOR 0.6, 95% CI: 0.007–0.523, p = 0.011), but not against hospitalisation ( p = 0.32). Seroconversion was protective for acquiring COVID-19 on multi-variable regression independent of vaccination dose (aOR 0.1, 95%CI: 0.0025–0.523, p = 0.011).
Conclusions
COVID-19 was associated with a high mortality rate. Older age, respiratory disease and prior smoking exposure may be risk factors for increased mortality. Vaccination of 3 doses is protective against acquiring COVID-19 and death, however not hospitalisation. Antibody response is protective for acquiring COVID-19, however seroconversion rates are low.
Peer Review reports
Introduction
It is known that COVID-19 disproportionally adversely affects the immunocompromised, including kidney transplant recipients (KTR), as compared to the general population. The advent of specific COVID-19 therapies and novel vaccination improved outcomes, however mortality rates for organ transplant recipients from large cohort studies remained as high as 14% into 2021 [ 1 , 2 ]. Factors predicting mortality are not fully understood but age, cardiovascular disease, diabetes, and certain immunosuppression regimens have been suggested [ 1 , 3 , 4 , 5 , 6 , 7 ]. KTRs were prioritised for vaccine administration, however, were not included in original vaccination trials [ 8 , 9 ]. Subsequent data suggests conventional 2-dose regimens are insufficient for KTRs, with 3 doses potentially ineffective against later strains such as BA.1 (Omicron) [ 10 , 11 , 12 ]. The primary course of vaccination was extended, between March 2021 and July 2022, to 5 doses in Australia, however adequate ongoing vaccination strategies are unclear [ 13 ].
COVID-19 in Australia and South Western Sydney
Australia was protected from high case numbers during the early phases of the pandemic due to its geographical isolation, strict initial international border controls and aggressive case tracking. Although Australia comprises 6 states and two territories, each have a significant degree of independence and power in health policy making. Those states with low case numbers throughout 2020–2022, such as South and Western Australia, maintained strict international and interstate border controls, but relaxed internal restrictions with almost near normal, pre-COVID, living conditions. They enacted limited, “snap lockdowns” in response to small numbers of detected cases to keep COVID-19 suppressed, until the majority of the population could be vaccinated [ 14 ]. Within New South Wales, however, several significant outbreaks occurred in 2021–2022, prompting repeated modification of public orders and prolonged periods of community lockdown and restrictions [ 15 ]. South Western Sydney Local Health District (SWSLHD) was one of the first areas in New South Wales (NSW) to be affected by COVID-19, and experienced one of the higher reported case numbers and the highest reported deaths of any Local Health District in NSW [ 16 ]. In response to the high rates of infection, SWSLHD experienced the most restrictive lockdown regulations in NSW during the pandemic period. During the second NSW wave in 2021, several local government areas within SWSLHD were classed as “areas of concern” and had additional public orders imposed, including: a stay at home order, restrictions on entering or leaving a district except for specific work exemptions (which required a permit), not allowed to travel more than 5 km for exercise, mandatory mask wearing outside and, at one point, a 9pm to 5am curfew [ 17 ]. August and September 2021 was associated with peak B.1.617.2 (Delta) wave incidence, followed by peak BA.1 (Omicron) in January 2022 [ 16 ]. COVID-19 vaccination was available for immunosuppressed individuals in Australia from 22nd March 2021 [ 18 ]. There is also a large burden of chronic kidney disease (CKD), with SWSLHD accounting for approximately 3.3% of prevalent KTRs in Australia. SWSLHD is also diverse, multiethnic population with 54% of people speaking a language other than English, predominantly Arabic or Vietnamese, and 43% of the population were born overseas, in comparison to 29% to the rest of NSW [ 19 , 20 ].
This study was conducted in the 2nd to 3rd year of the pandemic, during two dominant strain outbreaks, B.1.617.2 (Delta) and BA.1.(Omicron), after vaccination was available for all recipients [ 15 ]. Our objective was to ascertain the impact of COVID-19 on KTRS, with a focus on acquisition, hospitalisation, and mortality from COVID-19, and to perform a serology assessment of vaccine seroconversion.
Study design
A single centre (SWSLHD) prospective cohort study of prevalent kidney transplant recipients was undertaken between 25th July 2021 and 1st May 2022.
The study was commenced prospectively, coinciding with the onset of rising COVID-19 transmission and initiation of community stay at home orders. After restrictions had ended, vaccination numbers had increased, and it was clear no further public health orders were likely to be initiated, the study was terminated. All KTRs were strongly encouraged to receive vaccination throughout the study period, via national public health messaging, family practitioner support, and nephrologist advice. The Renal department at SWSLHD undertook a program at this time to encourage immunisation by developing a multi-lingual (Arabic, Vietnamese) information letter in view of the multi-ethnic population (distributed, mailed or emailed) to all KTRs and dialysis patients. A dedicated contact nephrologist was available to answer vaccination specific queries to facilitate timely immunisation.
Participants
All prevalent KTRs, aged ≥ 18, were included in the initial observational component of the study (see ethics below). Patients were identified from an existing clinical database and cross-referenced by searching the entire health district electronic coding system for renal transplantation to reduce the risk of selection bias. After the final data collection point on 1st May 2022 the cohorts of COVID-19 positive and COVID-19 negative patients were identified.
Variables and data sources
Baseline clinical and transplant characteristics, including: age, sex, body mass index, place of birth, smoking status, primary renal disease, co-morbidities, baseline eGFR, use of any blood thinner, prior dialysis modality and modality change during study, requirement for an interpreter, number of transplants, donor type, number of mismatches, transplant vintage, baseline immunosuppression regiment, dosage and levels and administration of Anti-thymocyte globulin, were determined from the electronic medical record, the Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) records, and locally available Nephrologist letters. The date and brand of each COVID-19 vaccination is recorded into the Australian Immunisation Register and electronic health record prospectively. We obtained information on every dose of COVID-19 vaccination provided to patients. We assessed the impact of increasing vaccination dose, from 1 onwards. COVID-19 diagnosis, and outcomes were determined from the electronic medical record, including date of diagnosis, administration of sotrovimab or molpurinovir, hospitalisation and level of care for COVID-19, oxygen requirement, use of dexamethasone and other adjunctive agents including baricitinib, tocilizumab, remdesivir and sarilumab, length of stay and mortality from COVID-19.
The combination of both ANZDATA records, local electronic health record and Nephrologist letters was utilised to reduce missing data and increase accuracy of data imputation. The study period encompassed a period of mandatory reporting of all positive COVID-19 polymerase chain results and rapid antigen tests to the NSW Health Service. In SWSLHD each positive result was reviewed by a dedicated COVID-19 Community Health service and documented in the electronic health record, which we anticipated would reduce the impact of sampling bias and missing data.
Serology assessment
All patients were invited to participate in the post COVID-19 vaccination serology conversion assessment component of the study. At study commencement all patients received a multi-lingual text message (English, Arabic, or Vietnamese) offering participation in COVID-19 serologic conversion testing. Additional written informed consent for this component of the study was obtained from those willing to participate. Blood tests we requested to be performed at least 14 days after their 2nd and 3rd vaccine dose. These patients were planned to be analysed as a subgroup from the main cohort.
All patient serum underwent testing at NSW Health Pathology– Liverpool, using both the Roche Elecsys Anti-SARS-CoV-2 assay (“Elecsys”) and the EUROIMMUN Anti-SARS-CoV-2 QuantiVAC ELISA (“QuantiVAC”), which have different targets. The Elecsys assay is an electrochemiluminescence assay for the qualitative detection of antibodies to SARS-CoV-2 nucleocapsid protein in human serum, and is considered reflective of wild-type infection [ 21 ]. A result (cutoff index; signal to cutoff ratio) of ≥ 1.0 is considered reactive. The QuantiVAC ELISA is an enzyme immunoassay, providing quantitative in vitro determination of antibodies to the immunoglobulin class IgG against the S1 antigen and receptor binding domain of SARS-CoV-2 [ 22 ]. Detection of the anti-S1 (spike) antibody is considered to indicate either a wild-type infection or a response to vaccination. A result of < 8RU/ml was considered negative, ≥ 8-<11 RU/ml borderline and ≥ 11RU/ml positive. Utilising the results from these two assays, in conjunction with the patient’s vaccination status and any noted clinical COVID-19, it was possible to determine whether the patient’s antibody response was secondary to clinical infection or to vaccination (Supp Table 1 ).
To determine vaccine-induced seroconversion in patients who undertook serial testing, we reviewed the relationship of serial serum collections, vaccination and known COVID-19. The serum sample collected closest in time to the date of vaccination, with a minimum of 14 days post-vaccination was included in the analysis. If there was evidence of seroconversion from a reactive QuantiVac ELISA, subsequent reactive samples were not included. If there was evidence of seroconversion after a subsequent incrementing vaccine dose, with no known interval COVID-19, patients were considered to have vaccine-induced seroconversion at the incrementation. If there was no evidence of seroconversion, despite additional vaccine administration, the final sample was included to reflect this. Borderline results were considered as seroconverted in the context of immunocompromise.
This study was approved by the SWSLHD Human Research and Ethics Committee (Approval Reference: 2019/STE00860) with a waiver of consent for the initial cohort analysis of all KTRs in the district and individual informed consent for the serology component if a patient elected to participate.
Statistical methods
Data was analysed using parametric and non-parametric tests for normally distributed and non-normally distributed variables respectively. Univariate analysis was performed with chi-squared, or Fishers test as appropriate, on categorical variables, and either independent t-test or Mann-Whitney U for continuous variables. Missing data was left as null with no imputation. Any variable with > 10% missing data was not included in any model Multi-variable binary logistic regression (backward stepwise conditional) was undertaken. Probability of entry for any variable was 0.05, removal 0.1. A goodness-of-fit test was undertaken ((Hosmer-Lemeshow test) was utilised to assess the goodness of fit and stability of the model. Statistical analysis was performed using SPSS v.28. P < 0.05 (2-sided) was considered significant. Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were followed for the reporting of the results [ 26 ].
A total of 537 patients were initially identified. After excluding patients that were deceased ( n = 37), already on dialysis ( n = 26), moved out of area ( n = 23), or lost to follow up ( n = 7), a total of 444 patients remained for analysis (Fig. 1 .). A total of 84 patients elected to participate in testing for the seroconversion analysis, who were analysed as a subgroup. Baseline characteristics of the final 444 prevalent KTRs are shown in Table 1 . They were predominantly male (60%), with a median age 58 years (Interquartile range [IQR]21.0) and baseline mean estimated glomerular filtration rate (eGFR) of 57 ml/min/1.73m 2 (Standard Deviation [SD] 21.9). Patients were primarily deceased donor recipients (69%) due to glomerulonephritis (50%) or diabetes (15%), with a median transplant vintage of 69.0 months (IQR 111.0). The primary immunosuppression regimen consisted of prednisolone (93%), mycophenolate (80%), and tacrolimus (72%).
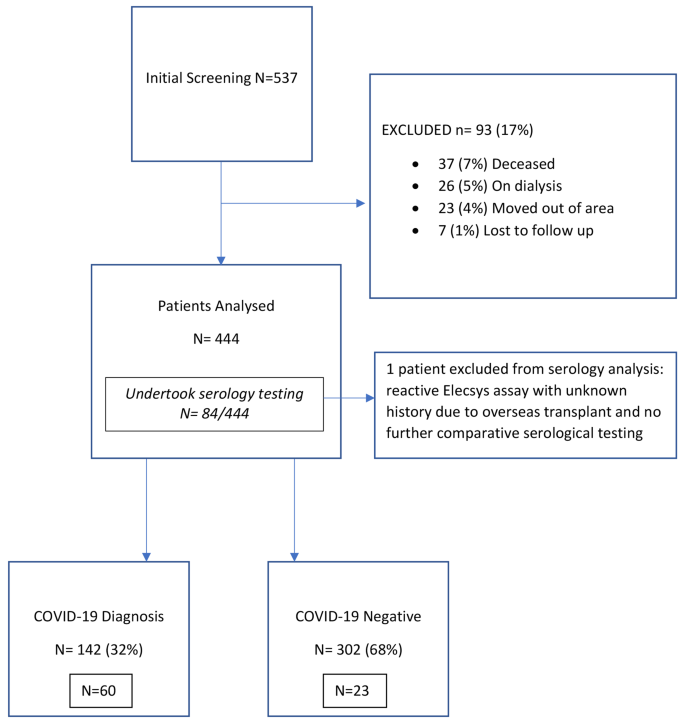
Flow chart of patient inclusion and COVID-19 diagnosis
Vaccination status
Vaccination status was acquired for 440 (99%) patients. By study end, 95% ( n = 423) of patients had received at least 1 vaccination. The number of patients that received 1,2,3, or 4 vaccine doses was 4 (1%), 75 (17%), 239 (54%) and 105 (24%) respectively. 17 (4%) patients remained unvaccinated throughout the study period. The vaccines administered included Pfizer BioNTech BNT162b2 (70%), AstraZeneca ChAdOx1 nCoV-19 (26%) and Moderna mRNA-1273 (3%) (Supp Table 2 ).
COVID-19 outcomes
COVID-19 was reported in 142 (32%) patients, and of these 54 (38%) required admission for COVID-19 with 10 (7%) deaths due to COVID-19. 17 (4%) patients died from any cause during the study period, with COVID-19 accounting for 59% of all deaths.
COVID-19 diagnosis
Univariate factors associated with acquiring COVID-19 are shown in Table 2 . On multivariable analysis, an increased risk of acquiring COVID-19 was associated with male sex (aOR 1.7, 1.093–2.701, p = 0.019), younger age (aOR, 0.98, 0.964–0.994, p = 0.006) and lower eGFR (aOR 0.99, 0.978–0.998, p = 0.020), after adjusting for significant univariate associations, body mass index (BMI) and diabetes. (Table 3 ). Receipt of 3 or more doses of vaccine was protective (aOR 0.48, 95% CI 0.287–0.796, p = 0.005).
COVID-19 mortality
Deaths from COVID-19 occurred throughout the study period, with 3 deaths in September 2021, 1 death in January 2022, 4 deaths in February 2022 and 1 death in both April and May 2022. Univariate analyses are shown in Table 2 . On multivariate analysis, increased mortality due to COVID-19 was associated with older age (aOR1.1, 95%CI 1.004–1.192, p = 0.04), respiratory disease (aOR 14.2, 95%CI 1.825–110.930, p = 0.011) and current or past smoking exposure (aOR 8.2, 95% CI 1.020-65.649, p = 0.048) after adjusting for significant univariate associations, sex, BMI, diabetes, and vaccination (3 + doses). Vaccination of 3 or more doses was protective (aOR 0.6, 95% CI 0.007–0.523, p = 0.011) (Table 3 ).
COVID-19 hospitalisation
Of those with reported COVID-19, 62 (44%) received sotrovimab and 11 (8%) received molnupiravir (Suppl Table 6.). 54 (38%) patients required hospitalisation for COVID-19, and 16 (11%) required intensive care unit (ICU) care. 33 (23%) patients required oxygen therapy. The maximum level of oxygen required was: low flow nasal prong oxygen in 13 (9%), high flow nasal prong oxygen in 4 (3%), non-invasive ventilation in 8 (6%) and invasive ventilation in 8 (6%) patients. Sotrovimab and molnupiravir were given in the community. When provided, neither were found to be protective for hospital admission ( p = 0.11, p = 021 respectively). Among hospitalised patients, those who received sotrovimab had evidence of protection for ICU admission (OR 0.2, 95%CI 0.035–0.886, p = 0.030). Median length of hospital stay was 8 days (IQR ± 13). There was an association between prior sotrovimab use and shorter length of stay (5 vs. 10 days, p = 0.027). Vaccination with 3 doses did not impact hospital admission ( p = 0.32), ICU admission ( p = 0.14) or length of stay (0.54).
Immunosuppression alteration occurred frequently in hospitalised patients (85%), as compared to those who were not hospitalised (10%). Hospitalisation with COVID-19 increased the odds of a reduction of immunosuppression (OR 50.5, 95% CI 18.211-139.883, p < 0.001), however it was not significant for those who required an ICU admission among hospitalised patients ( p = 0.41) or mortality ( p = 0.64). Univariate factors associated with hospitalisation for COVID-19 are shown in Table 2 .
On multivariable analysis, increased hospitalisation was associated with older age (aOR 1.0, 95% CI 1.007–1.0092, p = 0.021), lower eGFR (aOR 0.96, 95% CI 0.994 − 0.982, p < 0.001) and receipt of a deceased donor graft (aOR 4.1, 95% CI 1.128–14.747, p = 0.032), after adjusting for significant univariable associations, sex, BMI and vaccination (3 doses) (Table 3 ). Vaccination was not protective.
Seroconversion
84 patients underwent serological testing, including: 71 patients who had a single test, 12 who had 2 serial tests and 1 patient who had 3 serial tests. All but one patient, had a non-reactive Eleycs assay, indicating no prior exposure to COVID-19. The single patient with a reactive Eleycs assay was not known to have had prior COVID-19, however, was transplanted overseas with limited details prior to returning to Australia before the study period. This patient had no further serological evaluation and was excluded from the seroconversion analysis, resulting in 83 patients providing 97 serological tests assessed for vaccine-induced seroconversion.
All but 2/97 tests were collected prior to documented COVID-19. These two patients participated in serial testing. Prior to known COVID-19 they were Elecsys assay and QuantiVac ELISA negative. Post COVID-19 they remained Elecsys assay negative, however seroconverted on the QuantiVac ELISA. During this interval they received additional vaccinations, incrementing from 2 to 3 doses. As it is not possible to determine if these patients seroconverted due to wild type COVID-19 infection or vaccination, the serial samples prior to known COVID-19 were analysed. Of the remaining 95 tests, 5 were excluded based on QuantiVac ELISA results: 1 patient who did not have a QuanitVac ELISA processed on initial collection 1, but undertook repeat testing which was utilised, 3 patients with serial reactive tests performed after 2 and 3 doses of vaccine with no status change, therefore sampling after the 2nd dose was included, and 1 patient with 2 serial reactive tests, both after the 4th dose of vaccine and the earlier sample was included.
This resulted in 90 analysed samples: 1, 64, 21 and 4 samples after 1,2,3 and 4 doses of vaccine respectively (Suppl Table 3). Seroconversion rates after 1, 2, 3 and 4 doses were: 0, 22%, 48%, and 75% respectively (Suppl Table 4). Overall seroconversion rate at study end was 33% (27/83).
Univariate factors associated with COVID-19 diagnosis in this subgroup are shown in Supplementary Table 5. On multivariable analysis, after adjusting for univariate associations, in addition to age and diabetes, factors associated with an increased rate of acquiring COVID-19 included Asian place of birth (aOR 9.0, 95% CI 1.803–44.888, p = 0.007) and higher dose of prednisolone (aOR 1.5, 95% CI 1.125–1.949, p = 0.005). Seroconversion was protective (aOR 0.1, 95% CI 0.025–0.627, p = 0.011), independent of vaccination of 3 + doses ( p = 0.108) (Table 3 ).
The number of hospitalised patients in this subgroup was small ( n = 6). No hospitalised patients demonstrated evidence of seroconversion, however this did not reach statistical significance ( p = 0.539). No patient who died underwent serology assessment.
In this large, observational study of KTRs in Australia, during a period following community stay-at-home orders and two strain outbreaks, COVID-19 resulted in significant morbidity and mortality throughout the 2021–2022 pandemic period. Over 30% of the cohort developed breakthrough COVID-19, despite 78% receiving 3 or more doses of vaccine. Early monoclonal or antiviral treatment was provided to 51% of positive patients, however 38% of patients still required hospitalisation, with death occurring in 7% [ 15 , 16 ]. Overall seroconversion rates were low, with 3 doses of vaccine achieving a seroconversion rate of 48%.
Several risk factors for mortality amongst KTRs have been suggested, including older age, sex, cardiometabolic or respiratory co-morbidities and obesity [ 1 , 3 , 4 , 5 , 6 , 7 , 11 , 23 ].. This data supports older age, respiratory disease and smoking exposure may be independent factors for mortality for COVID-19 in KTRs. On systematic review and registry data analysis, no single co-morbidity had consistently been identified as a risk factor, other than age [ 3 , 11 ].
It has been suggested certain immunosuppression regimens are associated with increased COVID-19 mortality [ 1 , 6 , 7 ]. We did not find any effect of individual immunosuppressive agent on mortality, however there were high rates of baseline steroid (93%) and anti-metabolite (89%) use. A higher dose of prednisolone was associated with increased risk of acquiring COVID-19 in the serology subset.
Prior recommendations suggested temporarily altering immunosuppressive regimens during COVID-19 infections, and we noted high rates of alteration on hospitalisation in line with this trend [ 29 ]. There was no association with ICU admission or mortality among this group, and of patients who were not hospitalised, the majority did not have drug alteration (90%). Drug alteration, therefore, is likely reflective of a response to the severity of COVID-19. Current advice, with new strain evolution, suggests immunosuppression alteration is not required, particularly in the asymptomatic or those with a mild illness, with our data supportive of this [ 30 ]. Systematic review has not supported an association between immunosuppression and mortality, and there is limited comparative data to guide reduction of immunosuppression therefore decisions should be based on individualized assessment and the risk of rejection [ 11 , 23 ].
This data covered a period until May 2022, during a predominant Omicron outbreak from January 2022, whereby most patients had received 3 or more vaccine doses [ 15 , 16 ].. The Omicron era heralded decreased virulence, however the neutralising capability after 3 doses of vaccine was suggested to be diminished [ 12 ]. In this data, vaccination of 3 or more doses was protective for death and acquiring COVID-19, with no effect on hospitalisation, ICU admission or length of stay. In the serology subset, seroconversion, independent of dose of vaccination, was protective for acquiring COVID-19. Mortality rates during periods of Omicron predominance among solid organ transplant recipients have been reported to be 3 − 4%, however hospitalisation rates have remained 24–32%, with ICU admission rates of 28–36% [ 24 , 31 ]. Ongoing hospitalisation rates remain a concern for KTRs and further data regarding vaccine schedule optimisation and seroconversion assessment, independent of vaccination dose number, is needed.
This data demonstrated protection against death, reduced rates of ICU admission and length of stay with the use of sotrovimab, with no protective effects of molnupiravir. Our study reflects a period where sotrovimab was the primary agent of choice in early COVID-19 disease (approved August 2021) as opposed to molnupirovir (approved January 2022), likely influencing our results [ 27 , 28 ].
Current Australian recommendations do not recommend either sotrovimab or molnupirovir. Tixagevimab plus cilgavimab (Evusheld) has also lost its recommendation. Nirmatrelvir plus ritonavir (Paxlovid) retains its conditional recommendation, however, its use in KTRs is challenging due to effects on calcineurin inhibitor levels [ 25 ]. Remdesivir remains recommended only for patients requiring oxygen due to symptomatic COVID-19. There remains a paucity of agents effective at treating early COVID-19 in renal transplant recipients.
This data supports concern surrounding ongoing mortality and hospitalisation risk for KTRs, in the context of low seroconversion rates despite increasing vaccination dose schedule. This reiterates vaccination of at least 3 doses, and potentially evidence of seroconversion, is protective, however, in the absence of effective early treatments, encouragement of protective behaviours, such as social distancing, mask compliance and hand hygiene should continue.
This study is limited as a single centre and results are not generalisable. As with all observational data our analyses are limited to associations. Those undertaking the serology assessment were a self-selected population, which is likely to result in unmeasured patient bias, especially with regards to protective behaviours. They were highly vaccinated, with more than 90% receiving 3 or more doses. In addition, our data spanned two predominant strain periods, Delta and Omicron, and we were not able to specify strains in individual patients. While it is likely we captured most noted infections due to mandatory government reporting, cases could have been omitted if patients did not note an infection, obtain testing, or report a positive test, resulting in potential underdiagnosis of mild and asymptomatic cases. In addition, seroconversion does not always reflect in-vivo activity of antibodies and we did not assess the effect of waning immunity over time.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Sahota A, Tien A, Yao J, Dong E, Herald J, Javaherifar S, et al. Incidence, risk factors, and outcomes of COVID-19 infection in a large cohort of solid organ transplant recipients. Transplantation. 2022;106(12):2426–34.
Article CAS PubMed PubMed Central Google Scholar
Hall VG, Solera JT, Al-Alahmadi G, Marinelli T, Cardinal H, Poirier C, et al. Severity of COVID-19 among solid organ transplant recipients in Canada, 2020–2021: a prospective, multicentre cohort study. CMAJ. 2022;194(33):E1155–63.
Article PubMed PubMed Central Google Scholar
Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transpl. 2020;35(11):1973–83.
Article CAS Google Scholar
Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transpl. 2021;21(3):1295–303.
Udomkarnjananun S, Kerr SJ, Townamchai N, Susantitaphong P, Tulvatana W, Praditpornsilpa K, et al. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep. 2021;11(1):20073.
Gerard AO, Barbosa S, Anglicheau D, Couzi L, Hazzan M, Thaunat O, et al. Association between Maintenance Immunosuppressive Regimens and COVID-19 mortality in kidney transplant recipients. Transplantation. 2022;106(10):2063–7.
Requião-Moura LR, Modelli de Andrade LG, de Sandes-Freitas TV, Cristelli MP, Viana LA, Nakamura MR, et al. The mycophenolate-based Immunosuppressive Regimen is Associated with increased mortality in kidney transplant patients with COVID-19. Transplantation. 2022;106(10):e441–51.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Article CAS PubMed Google Scholar
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16.
Sanders JF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, et al. The RECOVAC Immune-response study: the immunogenicity, tolerability, and Safety of COVID-19 vaccination in patients with chronic kidney Disease, on Dialysis, or living with a kidney transplant. Transplantation. 2022;106(4):821–34.
Opsomer R, Kuypers D. COVID-19 and solid organ transplantation: finding the right balance. Transpl Rev (Orlando). 2022;36(3):100710.
Article Google Scholar
Kumar D, Hu Q, Samson R, Ferreira VH, Hall VG, Ierullo M, et al. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am J Transpl. 2022;22(8):2089–93.
(ATAGI) ATAGoI. ATAGI updated recommendations for a winter dose of COVID-19 vaccine. In: Care DoHaA, editor. 2022.
Edwards BB, Rehill R, Zhong PEL, Killigrew F, Riquelme Gonazalez A, Sheard P, Zhi E. R., Philips, T. Variation in policy response to COVID-19 across Australian states and territories. Blavatinik School Of Government: University of Oxford; 2022.
Google Scholar
Australian Bureau of Statistic. COVID-19 Mortality by wave2022. https://www.abs.gov.au/articles/covid-19-mortality-wave .
South Western Sydney Local Health District Public Health Unit. Personal Communication: Epidemiology of COVID-19 in SWSLHD. 2023.
NSW Government. Public Health (COVID-19 Additional Restrictions for Delta Outbreak) Order (No 2) Amendment Order 2021. In: Research MfHaM, editor. 2021.
Australian Goverment. Priority groups for COVID-19 Vaccination Program Phase 1B. In: Health, editor. Online2021.
Australian and New Zealand Dialysis and Transplant Registry. ANZDATA 45th Annual Report 2022 (Data to 2021). 2022.
ID (Informed Decisions). [Data Extraction from Australian Bureau of Statistic and Census]. https://home.id.com.au/ .
United States Food and Drugs Administration. Elecsys (R) Anti-SARS-CoV-2 Intruction for Use. 2020.
United States Food and Drugs Administration. Anti-SARS-CoV2 ELISA (IgG) Instructions for Use. 2022.
Nimmo A, Gardiner D, Ushiro-Lumb I, Ravanan R, Forsythe JLR. The global impact of COVID-19 on solid organ transplantation: two years into a pandemic. Transplantation. 2022;106(7):1312–29.
Anjan S, Khatri A, Viotti JB, Cheung T, Garcia LAC, Simkins J, et al. Is the Omicron variant truly less virulent in solid organ transplant recipients? Transpl Infect Dis. 2022;24(6):e13923.
National Clinical Evidence Taskforce. Australian Guideline for the clinical care of people with COVID-19. 2022;v70.1.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296.
Australian Government. TGA approves new COVID-19 treatment for use in Australia. In: Health and Aged Care, editor. 2021.
Government A. TGA Provisionally approves Merck Sharp and Dohme (Australia) Pty Ltd’s oral COVID-19 treatment, LAGEVRIO (Molnupiravir). 2022.
Maggiore U, Abramowicz D, Crespo M, Mariat C, Mjoen G, Peruzzi L, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transpl. 2020;35(6):899–904.
Gandolfini I, Crespo M, Hellemans R, Maggiore U, Mariat C, Mjoen G, et al. Issues regarding COVID-19 in kidney transplantation in the ERA of the Omicron variant: a commentary by the ERA Descartes Working Group. Nephrol Dial Transpl. 2022;37(10):1824–9.
Solera JT, Arbol BG, Alshahrani A, Bahinskaya I, Marks N, Humar A, et al. Impact of vaccination and early monoclonal antibody therapy on Coronavirus Disease 2019 outcomes in Organ Transplant recipients during the Omicron Wave. Clin Infect Dis. 2022;75(12):2193–200.
Download references
Acknowledgements
SWSLHD Renal Unit and Research Staff. NSW Health Pathology Laboratories– Liverpool. All the patients who participated in the study.
Locally (non-peer reviewed) funded (renal department trust fund).
Author information
Authors and affiliations.
University of Western Sydney, Sydney, NSW, Australia
Daniela Potter & Angela Makris
University of New South Wales, Sydney, NSW, Australia
Department of Renal Medicine, Liverpool Hospital, Liverpool, Sydney, NSW, Australia
Daniela Potter, Jason Diep, Colleen Munro, Noelle Lin, Ramon Xu, Jeffrey Wong & Angela Makris
Department of Microbiology and Infectious Diseases, NSW Health Pathology, Liverpool, NSW, Australia
Robert Porritt, Michael Maley & Hong Foo
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the work, as well as reading and approving the final manuscript. DP is the primary corresponding author and was responsible for the design of study and the primary data interpretation and drafting of work. AM was responsible for study conception, study design, data analysis and contributed to draft revisions of the work. JP, CM, NL and RX were responsible for data acquisition and analysis, as well as draft revision. JW was responsible for data interpretation and draft revisions. RP was the primary technical laboratory advisor and analyst. MM and HF were both advisors on laboratory, infectious and epidemiological components of the study and contributed to draft revisions.
Corresponding author
Correspondence to Daniela Potter .
Ethics declarations
Ethics approval and consent.
Written informed consent for this component of the study was obtained from those willing to participate. This study was approved by the SWSLHD Human Research and Ethics Committee (Approval Reference: 2019/STE00860).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Potter, D., Diep, J., Munro, C. et al. A prospective observational cohort study of covid-19 epidemiology and vaccine seroconversion in South Western Sydney, Australia, during the 2021–2022 pandemic period.. BMC Nephrol 25 , 131 (2024). https://doi.org/10.1186/s12882-024-03560-8
Download citation
Received : 16 September 2023
Accepted : 26 March 2024
Published : 12 April 2024
DOI : https://doi.org/10.1186/s12882-024-03560-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Kidney Transplantation
- COVID-19 Epidemiology
- COVID-19 Vaccination
- COVID-19 Vaccination Seroconversion
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Case-control study. A case-control study is an observational analytic retrospective study design [].It starts with the outcome of interest (referred to as cases) and looks back in time for exposures that likely caused the outcome of interest [13, 20].This design compares two groups of participants - those with the outcome of interest and the matched control [].
An observational study is used to answer a research question based purely on what the researcher observes. There is no interference or manipulation of the research subjects, and no control and treatment groups. These studies are often qualitative in nature and can be used for both exploratory and explanatory research purposes.
A case study is an in-depth examination of an individual. Sometimes case studies are also completed on social units (e.g., a cult) and events (e.g., a natural disaster). ... Like many observational research methods, case studies tend to be more qualitative in nature. Case study methods involve an in-depth, and often a longitudinal examination ...
Case Control Observational Study. Researchers in case control studies identify individuals with an existing health issue or condition, or "cases," along with a similar group without the condition, or "controls." These two groups are then compared to identify predictors and outcomes. This type of study is helpful to generate a hypothesis ...
Observational studies play a significant role in healthcare, including the study of the use and effects of medicines in large populations known as 'pharmacoepidemiology', ... Case-control studies compare the proportion of cases with a specific exposure to the proportion of controls with the same exposure (the odds ratio). Case-control ...
An observational case study is a study of a real-world case without performing an intervention. Measurement may influence the measured phenomena, but as in all forms of research, the researcher tries to restrict this to a minimum. The researcher may study a sample of two or even more cases, but the goal of case study research is not to acquire ...
based studies, observation methods were incorporated from the outset in the design of the research. However, as conventional case study models, such as Yin (2014), do not distinguish observation data from other types of data collection in terms of their unique significance and poten-tial, we modified Yin's CSR method. This observation-
Revised on November 20, 2023. A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are ...
The most common forms are case reports and case series; In a case report, we discuss our experience with the patient's symptoms, signs, diagnosis, and treatment; In a case series, several patients with similar experiences are grouped. Analytical Studies. Analytical studies are of 2 types: observational and experimental.
Observational studies provide critical descriptive data and information on long-term efficacy and safety that clinical trials cannot provide, at generally much less expense. Observational studies include case reports and case series, ecological studies, cross-sectional studies, case-control studies and cohort studies.
An observational study would typically start with a group of symptomatic subjects and work backwards to find those who were given the medication and later developed the symptoms. Thus a subset of the treated group was determined based on the presence of symptoms, instead of by random assignment. ... Case-control study: study originally ...
There are seven types of observational studies. Researchers might choose to use one type of observational study or combine any of these multiple observational study approaches: 1. Cross-sectional studies. Cross-sectional studies happen when researchers observe their chosen subject at one particular point in time.
Observational studies in clinical research can be classified as either analytic or descriptive (Table 12-1). Analytic observational studies are similar to randomized, controlled clinical trials in that the goal is to estimate the causal effect of an exposure on an outcome. ... The analytic study designs presented are the case-control study ...
This article describes the distinctive characteristics of case study observational research, a modified form of Yin's 2014 model of case study research the authors used in a study exploring interprofessional collaboration in primary care. In this approach, observation data are positioned as the central component of the research design.
Case Studies & Observation Studies. A case study is a detailed study of a particular person, group or organisation. Focus is placed on the case study because the basic facts tend to help us understand a wider scientific truth. On the other hand they can also be used to challenge an accepted theory and so prompt scientists to change their thinking.
Observational research studies involve the passive observation of subjects without any intervention or manipulation by researchers. These studies are designed to scrutinize the relationships between variables and test subjects, uncover patterns, and draw conclusions grounded in real-world data. Researchers refrain from interfering with the ...
In analytical observational studies, researchers try to establish an association between exposure (s) and outcome (s). Depending on the direction of enquiry, these studies can be directed forwards (cohort studies) or backwards (case-control studies). In this article, we examine the key features of these two types of studies.
Australia was uniquely positioned to reduce initial case numbers during the 2021-2022 pandemic period due to its relative isolation and several significant public health interventions. South-Western Sydney Local Heath District was one of the predominant regions affected. ... In this large, observational study of KTRs in Australia, during a ...