
- May 20, 2024 | When Cells Turn Against Us: The Ferroptosis Link in COVID-19 Lung Destruction
- May 20, 2024 | Ancient Egypt’s Engineering Marvel: Archaeologists Uncover Hidden River Beneath the Desert
- May 20, 2024 | From Ancient Egyptian Myths to Astrophysics: A Jewel in the Queen’s Hair
- May 19, 2024 | Trustnet Unveils a New Era in Decentralizing Online Fact-Checking
- May 19, 2024 | Quantum Coherence: Harvard Scientists Uncover Hidden Order in Chemical Chaos

Redefining Dementia Treatment: Berkeley Scientists Unveil Promising New Breakthrough
By University of California - Berkeley February 28, 2024
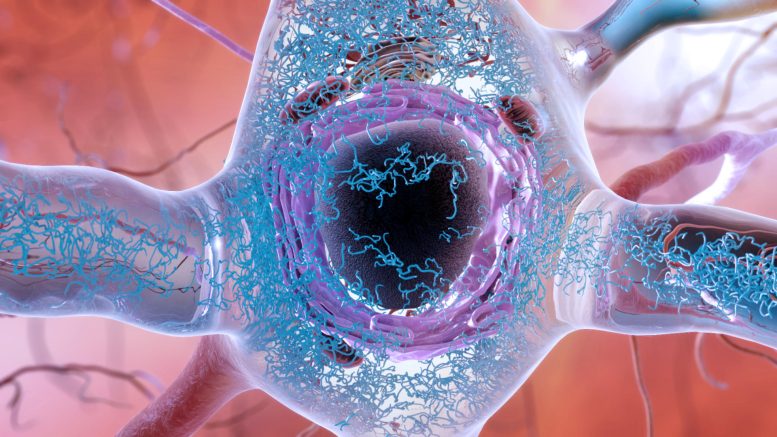
An illustration of a brain cell in a person with Alzheimer’s disease, showing the accumulation and clumping of tau proteins (blue squiggles) in the cytoplasm of a brain cell. Protein clumps, also known as aggregates, are thought to lead to cell death and dementia. New research suggests that such clumps may not cause brain cell death directly, but rather throw the cell’s response to stress off balance so that it never gets switched off. Credit: National Institute on Aging, National Institutes of Health
Research from UC Berkeley indicates that ongoing stress caused by protein aggregation is leading to the death of brain cells.
Numerous neurodegenerative conditions, including Alzheimer’s and Parkinson’s, involve the buildup of protein clusters, known as aggregates, within the brain. This phenomenon has prompted researchers to hypothesize that these protein masses are responsible for the death of brain cells. Despite this, efforts to develop treatments that break up and remove these tangled proteins have had little success.
But a new discovery by University of California, Berkeley , researchers suggests that the accumulation of aggregated proteins isn’t what kills brain cells. Rather, it’s the body’s failure to turn off these cells’ stress response.
In a study recently published in the journal Nature , the researchers reported that delivering a drug that forces the stress response to shut down saves cells that mimic a type of neurodegenerative disease known as early-onset dementia.
The Role of the SIFI Complex in Neurodegeneration
According to lead researcher Michael Rapé, the finding could offer clinicians another option for treatment for some neurodegenerative diseases, at least for those caused by mutations in the protein that switches off the cellular stress response. These include inherited diseases that lead to ataxia, or loss of muscle control, and early-onset dementia.
In addition, Rapé noted that other neurodegenerative diseases, including Mohr–Tranebjærg syndrome, childhood ataxia, and Leigh syndrome, are also characterized by stress responses in overdrive and have symptoms similar to those of the early onset dementia mimicked in the new study.
“We always thought that protein clumps directly kill neurons, for example by puncturing membrane structures within these cells. Yet, we now found that aggregates prevent the silencing of a stress response that cells originally mount to cope with bad proteins. The stress response is always on, and that’s what kills the cells,” said Rapé, head of the new division of molecular therapeutics in UC Berkeley’s Department of Molecular and Cell Biology and a Howard Hughes Medical Institute investigator. “We think that the same mechanisms may underlie more common pathologies that also show widespread aggregation, such as Alzheimer’s disease or frontotemporal dementia, but more work is needed to investigate the role of stress signaling in these diseases.”
Key to the discoveries by Rapé’s lab was the researchers’ finding that stress responses need to be turned off once a brain cell has successfully addressed a difficult situation. Rapé explained this finding to his son in simple terms: You not only need to clean up your room, but also turn out the light before going to bed. If you don’t turn off the light, you can’t fall asleep, but if you turn it off before you cleaned up your room, you would stumble if you had to get up in the dark.
Similarly, a cell has to clean up protein aggregates before turning off the stress response. If it doesn’t turn off the stress response, the cell will ultimately die.
“Aggregates don’t kill cells directly. They kill cells because they keep the light on,” he said. “But that means that you can treat these diseases, or at least the dozen or so neurodegenerative diseases that we found have kept their stress responses on. You treat them with an inhibitor that turns off the light. You don’t have to worry about completely getting rid of large aggregates, which changes how we think about treating neurodegenerative diseases. And most importantly, it makes this really doable.”
In their paper, Rapé and his colleagues describe a very large protein complex they discovered called SIFI (SIlencing Factor of the Integrated stress response). This machine serves two purposes: It cleans up aggregates and, afterward, turns off the stress response triggered by the aggregated proteins. The stress response controlled by SIFI is switched on to deal with specific intracellular problems — the abnormal accumulation of proteins that end up at the wrong location in the cell. If components of SIFI are mutated, the cell will accumulate protein clumps and experience an active stress response. But it is the stress response signaling that kills the cells.
“The SIFI complex would normally clear out the aggregating proteins. When there are aggregates around, SIFI is diverted from the stress response, and the signaling continues. When aggregates have been cleared — the room has been cleaned up before bedtime — then the SIFI is not diverted away anymore, and it can turn off the stress response,” he said. “Aggregates kind of hijack that natural stress response-silencing mechanism, interfere with it, stall it. And so that’s why silencing never happens when you have aggregates, and that’s why cells die.”
A future treatment, Rapé said, would likely involve the administration of a drug to turn off the stress response and a drug to keep SIFI turned on to clean up the aggregate mess.
Rapé, who is also the Dr. K. Peter Hirth Chair of Cancer Biology, studies the role of ubiquitin — a ubiquitous protein in the body that targets proteins for degradation — in regulating normal and disease processes in humans. In 2017, he discovered that a protein called UBR4 assembles a specific ubiquitin signal that was required for the elimination of proteins that tend to aggregate inside cells.
Only later did other researchers find that mutations in UBR4 are found in some inherited types of neurodegeneration. This discovery led Rapé to team up with colleagues at Stanford University to find out how UBR4 causes these diseases.
“This was a unique opportunity: We had an enzyme that makes an anti-aggregation signal, and when it’s mutated, it causes aggregation disease,” he said. “You put these two things together and you can say, ‘If you figure out how this UBR4 allows sustained cell survival, that probably tells you how aggregates kill cells.'”
They found that UBR4 is actually part of a much larger protein complex, which Rapé dubbed SIFI, and they found that this SIFI machinery was needed when a cell couldn’t sort proteins into its mitochondria. Such proteins that end up at the wrong location in cells tend to clump and, in turn, cause neurodegeneration.
“Surprisingly, though, we found that the core substrates of the SIFI complex were two proteins, one of which senses when proteins don’t make it into mitochondria. That protein detects that something is wrong, and it then activates a kinase that shuts down most of new protein synthesis as part of a stress response, giving the cell time to correct its problem with bringing proteins to the right location,” he said.
This kinase is also degraded through SIFI. A kinase is an enzyme that adds a phosphate group to another molecule, in this case, a protein, to regulate important activities in the cell. By helping degrade these two proteins, the SIFI complex turns off the stress response that is caused by clumpy proteins accumulating at the wrong location.
“That’s the very first time that we’ve seen a stress response turned off in an active manner by an enzyme — SIFI — that happens to be mutated in neurodegeneration,” Rapé said.
While investigating how SIFI can turn off the stress response at the right time — only after the room had been cleaned up — the researchers found that SIFI recognizes a short protein segment that acts as a kind of ZIP code that allows proteins or protein precursors to get into the mitochondria, where they are processed. When they are prevented from getting in, they accumulate in the cytoplasm, but SIFI homes in on that ZIP code to eliminate them. The ZIP code looks just like the light switch.
“When you have aggregates accumulating in the cytoplasm, now the ZIP code is still in the cytoplasm, and there’s a lot of it there,” he said. “And it’s the same signal as you would have in the proteins that you want to turn off. So it basically diverts the SIFI complex from the light switch back to the mess. SIFI tries to clean up the mess first, and it cannot turn off the light. And so when you have an aggregate in the cell, the light is always on. And if the light is always on, if stress signaling is always on, the cell will die. And that’s a problem.”
Implications for Treatment and Future Research
Rapé suspects that many intracellular protein aggregates characteristic of neurodegenerative diseases have similar consequences and may prevent the cell from switching off the stress response. If so, the fact that a drug can turn off the response and rescue brain cells bodes well for the development of treatments for potentially many neurodegenerative diseases.
Already, another stress response inhibitor, a drug called ISRIB discovered at UCSF in 2013, has been shown to improve memory in mice and reduce age-related cognitive decline.
“That means there is the prospect that by manipulating stress silencing, by turning off the light with chemicals, you might target other neurodegenerative diseases, as well,” he said. “At the very least, it’s another way we could help patients with these diseases. In the best possible way, I think it will change how we treat neurodegenerative diseases. That’s why this is a really important story, why I think it’s very exciting.”
Rapé, already a co-founder of two startups, Nurix Therapeutics Inc., and Lyterian Therapeutics, is now looking to develop therapies to silence the stress response while maintaining the cell’s cleanup of protein aggregates.
Reference: “Stress response silencing by an E3 ligase mutated in neurodegeneration” by Diane L. Haakonsen, Michael Heider, Andrew J. Ingersoll, Kayla Vodehnal, Samuel R. Witus, Takeshi Uenaka, Marius Wernig and Michael Rapé, 31 January 2024, Nature . DOI: 10.1038/s41586-023-06985-7
Co-authors with Rapé are postdoctoral fellows Diane Haakonsen, Michael Heider, and Samuel Witus and graduate student Andrew Ingersoll, all of UC Berkeley, and Kayla Vodehnal, Takeshi Uenaka, and Marius Wernig of Stanford. The work was supported primarily by the Stinehart–Reed Foundation and the National Institutes of Health (RF1 AG048131, T32MH020016-25).
More on SciTechDaily

Precision and Peril: Stunning New Image of Intuitive Machines Odysseus Landing on Moon

Challenging “Rule Breakers” – Children Will Confront Their Peers, but How They Do So Varies Across Cultures
Climate change and extreme weather will have complex effects on infectious disease transmission.

“Unusual” Findings Overturn Current Battery Wisdom

600 Million-Year-Old Time Capsule – New Discoveries From the Himalayas Shed Light on Earth’s Past

Billion Year Old Surface Water Found in Oceanic Plates
Beware the Splice of Life: This Killer Protein Causes Pancreatic Cancer

Reviving Ancient Skills to Solve Prehistoric Puzzles
Be the first to comment on "redefining dementia treatment: berkeley scientists unveil promising new breakthrough", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
MIT Technology Review
- Newsletters
New breakthroughs on Alzheimer’s
MIT scientists have pinpointed the first brain cells to show signs of neurodegeneration in the disorder and identified a peptide that holds potential as a treatment.
- Anne Trafton archive page
Neuronal hyperactivity and the gradual loss of neuron function are key features of Alzheimer’s disease. Now researchers led by Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory, have identified the cells most susceptible to this damage, suggesting a good target for treatment. Even more exciting, Tsai and her colleagues have found a way to reverse neurodegeneration and other symptoms by interfering with an enzyme that is typically overactive in the brains of Alzheimer’s patients.
In one study , the researchers used single-cell RNA sequencing to distinguish two populations of neurons in the mammillary bodies, a pair of structures in the hypothalamus that play a role in memory and are affected early in the disease. Previous work by Tsai’s lab found that they had the highest density of amyloid beta plaques, abnormal clumps of protein that are thought to cause many Alzheimer’s symptoms.
The researchers found that neurons in the lateral mammillary body showed much more hyperactivity and degeneration than those in the larger medial mamillary body. They also found that this damage led to memory impairments in mice and that they could reverse those impairments with a drug used to treat epilepsy.
In the other study , the researchers treated mice with a peptide that blocks a hyperactive version of an enzyme called CDK5, which plays an important role in development of the central nervous system. They found dramatic reductions in neurodegeneration and DNA damage in the brain, and the mice got better at tasks such as learning to navigate a water maze.
CDK5 is activated by a smaller protein known as P35, allowing it to add a phosphate molecule to its targets. However, in Alzheimer’s and other neurodegenerative diseases, P35 breaks down into a smaller protein called P25, which allows CDK5 to phosphorylate other molecules—including the Tau protein, leading to the Tau tangles that are another characteristic of Alzheimer’s.
Pharmaceutical companies have tried to target P25 with small-molecule drugs, but these drugs also interfere with other essential enzymes. The MIT team instead used a peptide—a string of amino acids, in this case a sequence matching that of a CDK5 segment that is critical to binding P25.
In tests on neurons in a lab dish, the researchers found that treatment with the peptide moderately reduced CDK5 activity. But in a mouse model that has hyperactive CDK5, they saw myriad beneficial effects, including reductions in DNA damage, neural inflammation, and neuron loss.
The treatment also produced dramatic improvements in a different mouse model of Alzheimer’s, which has a mutant form of the Tau protein. Tsai hypothesizes that the peptide might confer resilience to cognitive impairment in the brains of people with Tau tangles.
“We found that the effect of this peptide is just remarkable,” she says. “We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
The researchers hope the peptide could eventually be used as a treatment not only for Alzheimer’s but for frontotemporal dementia, HIV-induced dementia, diabetes-linked cognitive impairment, and other conditions.
Keep Reading
Most popular, how scientists traced a mysterious covid case back to six toilets.
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
- Cassandra Willyard archive page
It’s time to retire the term “user”
The proliferation of AI means we need a new word.
- Taylor Majewski archive page
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
- Casey Crownhart archive page
Sam Altman says helpful agents are poised to become AI’s killer function
Open AI’s CEO says we won’t need new hardware or lots more training data to get there.
- James O'Donnell archive page
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How do you read organization’s silence over rise of Nazism?

Got milk? Does it give you problems?

Cancer risk, wine preference, and your genes
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.
“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this
Newly identified genetic variant protects against Alzheimer’s
Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.
Medical historians look to cultural context, work of peer publications in wrestling with case of New England Journal of Medicine

Biomolecular archaeologist looks at why most of world’s population has trouble digesting beverage that helped shape civilization

Biologist separates reality of science from the claims of profiling firms
Epic science inside a cubic millimeter of brain
Researchers publish largest-ever dataset of neural connections
Finding right mix on campus speech policies
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.
Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
In 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (Leqembi) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
A phase 3 clinical trial found that the medicine slowed cognitive decline in people with early Alzheimer's disease. The medicine prevents amyloid plaques in the brain from clumping. The phase 3 trial was the largest so far to study whether clearing clumps of amyloid plaques from the brain can slow the disease.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Also, people taking lecanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. It also recommends being monitored with brain MRI s during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends being tested for this gene before starting treatment with lecanemab.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your health care professional before taking lecanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab. Other research is looking at how effective lecanemab may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
Another medicine being studied is donanemab. It targets and reduces amyloid plaques and tau proteins. It was found to slow declines in thinking and functioning in people with early Alzheimer's disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Latest Latest
- The West The West
- Sports Sports
- Opinion Opinion
- Magazine Magazine
Utahn may be on verge of a significant breakthrough in treating Alzheimer’s
University of utah research professor donna j. cross has helped mice overcome brain disorders. are humans next.
By Lee Benson
Is a research professor at the University of Utah on the verge of a significant breakthrough in the treatment of Alzheimer’s disease and other brain disorders?
Not only would a bunch of lab mice vote yes, they’d remember to do so.
Donna J. Cross, who has a doctoral degree in neuroscience, has spent the past 25 years shepherding research that favorably suggests a small, specialized dose of a chemotherapy drug called Paclitaxel might be capable of repairing injuries, whether caused by pathology or by trauma, to the human brain.
The quest began at the University of Michigan, where Cross earned her doctorate, then to the University of Washington when she joined that faculty, and finally to the University of Utah, when Cross’ mentor and the man who began the research, Dr. Satoshi Minoshima, came to the U. as chair of the department of radiology and imaging sciences.
In a nutshell, when the scientists have administered their version of the cancer drug to mice that have been bred to develop Alzheimer’s, the mice have experienced “a complete reversal of their cognitive deficit.” Same thing happened when mice that had suffered concussions were given the medication.
Suddenly, mice that couldn’t remember things and/or had traumatic brain injury were acting cognitively normal again.
“Whether that would happen in humans, we have a lot of work still to do,” says Cross. But if it did? She doesn’t equivocate. “It would be huge.”
This is personal for Donna Cross. Like so many of us, she knows what it’s like to watch loved ones suffer from brain diseases. Her grandmother’s Alzheimer’s diagnosis was her chief motivation for joining Minoshima’s team when she started graduate school. Recently, a father-in-law with severe dementia has only heightened her sense of urgency.
“Even though it’s too late for my grandmother and likely too late for my father-in-law, it’s not too late for vast, vast numbers of people in the world,” she says, “that’s why we have to keep moving forward.”
Still, scientific research takes time and money. With dwindling amounts of both when she came to the University of Utah (Alzheimer’s research isn’t her only iron in the fire), Cross confesses she was “almost ready to give up.”
She desperately needed help in repurposing Paclitaxel into a form that could be applied to the human brain and didn’t know where to turn.
Once she’d settled into her lab at the U. she did a Google search to see if any pharmaceutical scientists might be in the area.
That’s when she discovered she’d been given an office, quite randomly, in the Biomedical Polymers Research Building, where the world-renowned Czech-born pharmaceutical chemist Jindrich (Henry) Kopecek has set up his headquarters.
The drug makers she needed were literally right next door.
Kopecek and Dr. Jiyuan (Jane) Yang have been integrally involved ever since, lending their expertise in figuring out how to develop and safely deliver to the brain a potential game-changing drug.
“I’m just very, very lucky to be placed in their building for no other reason than they had space,” says Cross. “These guys are rock stars. I came to them as a brain person who was interested in treating neurological conditions and they are the drug developer/drug delivery people. It’s a collaboration that is very strong because of our different areas of expertise.”
The goal now is to get the drug developed and ready for clinical trials. It will be costly — Cross estimates they need to raise at least $2 million — but the potential upside could be priceless.
“We would treat not just Alzheimer’s,” she says, “but also any kind of dementia: ALS, Parkinson’s, multiple sclerosis, spinal cord injury, any kind of condition where nerve cells are dying.”
In a best case scenario, not only would the neurological damage be halted, but the brain would conceivably be healed.
How long will it take to find out? As long as it takes, says Cross. “This is my passion. It started out personal to me; it still is, extremely so.”
This week Cross will be giving a presentation about her work at the free Alzheimer’s & Caregiving Education Conference scheduled to be held Wednesday, May 15, from 10 a.m. to 1 p.m. at the Embassy Suites Hotel in West Valley City. The conference, hosted by the Alzheimer’s Foundation of America, is open to the public.
“My motivation for doing these public lectures is twofold: one, to draw attention to the work we’re doing, and two, to give hope,” says Cross. “I think it’s extremely important that people have hope.”
To register in advance for the conference, go to www.alzfdn.org/tour . You can stay connected with Cross’ research by following @UofURadiology.

Research Offers New Ideas for Treating Alzheimer’s
“out-of-the-clump,” mitochondrial, and other theories offer hope on alzheimer’s..
Posted May 17, 2024 | Reviewed by Tyler Woods
- What Is Dementia?
- Find counselling to help with dementia
- The need for a new "out-of-the-clump" way of thinking about AD is emerging as a top priority in brain science.
- In Alzheimer’s, the brain’s immune system fails to differentiate between bacteria and brain cells.
- Probiotics may not only support a healthier gut, but a healthier brain as well.
- For the elderly, in particular those with cognitive impairment, good oral hygiene is essential.
Dementia refers to an array of symptoms characterized by failing short-term memory , confused thinking, and a decline in language skills. Of all the dementias, Alzheimer’s disease (AD) constitutes approximately 60 to 80 percent of cases.
Two drugs, Lecanemab and Donanemab, have been hailed as part of a new class of monoclonal antibody (MOA) drugs that could mark a turning point for Alzheimer’s (AZ) drug research. These drugs are incredibly expensive and carry risks of brain microbleeds and swelling. More importantly, they do not cure or even halt the disease, they delay it by about six months on average. At least 98 unique compounds tested in Phase 2 or 3 trials that pursued the various MOA classes have failed over the years. Howard Chertow, of McGill University, commented, “They’re not a home run.”
Personally, I think they’re more like a strike-out, in view of the fact that most neuroscientists and the drug companies employed by them may be looking in the wrong places in the wrong way.
In 2006, a research paper published in the highly regarded journal Nature asserted that the development of Alzheimer’s is caused by the formation in the brain of abnormally high levels of the naturally occurring protein beta-amyloid that clumps together to form plaques and the intracellular accumulations of neurofibrillary tangles of tau protein that disrupt cell function.
In 2023, a critical review in the journal Brain , collaboratively written by scientists from Denmark, the U.S., Italy, and Australia, stated that “Despite the importance of amyloid in the definition of Alzheimer's disease, we argue that the data point to Aβ playing a minor aetiological role.” They further asserted that the search for more effective ways to treat Alzheimer’s should involve more than amyloid as the single causative agent.
I propose to discuss the currently leading "out-of-the-clump" research, a term coined by Donald Weaver of the University of Toronto, that may eventually usher in new and better ways of dealing with Alzheimer’s.
One of the most auspicious of these novel directions comes from the above-mentioned Weaver, who found that significant resemblances between bacterial membranes and brain cell membranes exist. Beta-amyloid erroneously mistakes the brain cells for invading bacteria and attacks them. These brain cells gradually decay, ultimately leading to dementia. According to Weaver, Alzheimer’s is an autoimmune disease.
If this theory gains traction in the scientific world, treatments that are effective in autoimmune diseases such as celiac disease, Crohn’s disease, diabetes type 1, eczema, etc. may prove successful in the treatment of Alzheimer’s.
In addition to this autoimmune theory of Alzheimer’s, many other new and varied theories are appearing. John Mamo of Curtin University in Australia, demonstrated already in 2021 that the liver also makes amyloid protein.
It follows that finding ways to either prevent the liver from manufacturing the amyloid protein or destroying it before it enters the circulation ought to be explored.
A recent study from Portugal suggests that Alzheimer’s is a disease of the mitochondria . Mitochondria are tiny organelles (similar to organs like the heart or liver but much smaller inside cells) that generate most of the chemical energy required to power the cell's functions. The authors of this study reported positive outcomes in Alzheimer’s with animals fed a diet rich in antioxidants.
This is good news because we are in familiar territory here. We have known for a long time that antioxidants scavenge free radicals from the body cells and prevent or reduce the damage caused by oxidation. Of course, further research is necessary before it is proven that antioxidants in humans can lessen the risk of developing Alzheimer’s or benefit people in the early stages of Alzheimer’s. However, the consumption of antioxidants like vitamins A, C, and E, the minerals copper, zinc, and selenium, as well as nuts, fruits and vegetables, pecans, blueberries, and dark chocolate, seems well-proven to benefit the health of everyone, at any stage of life.
Scientists from the University of Bern, Switzerland, contend that Alzheimer’s is the end result of a brain infection, particularly with bacteria from the mouth. Since our hands and fingers swarm with viruses and bacteria, a recent paper that advanced the hypothesis that nose picking could play a role in increasing the risk of developing Alzheimer’s makes much sense. Digging around our noses is encouraging all those little critters to hop on the olfactory nerve train and take a vacation in our brains.
Recent research has focussed on probiotics as potentially beneficial in preventing the development or slowing the progression of Alzheimer’s. Probiotics are foods or supplements that contain live microorganisms that help to maintain or improve a diverse microflora in the gut. A systematic review of the literature on the effect of probiotics on Alzheimer’s by scientists from Malaysia in conjunction with researchers from Baghdad in 2022 write, “Probiotics are known to be one of the best preventative measures against cognitive decline in AD. Numerous in vivo trials and recent clinical trials have proven the effectiveness of selected bacterial strains in slowing down the progression of AD. It is proven that probiotics modulate the inflammatory process, counteract [with] oxidative stress , and modify gut microbiota.”

This and many other academic papers present robust evidence on the role of probiotics in alleviating the progression of Alzheimer’s.
As opposed to drugs, probiotics are readily available in foods such as yogurt, buttermilk, sauerkraut, pickles, and many others.
If we are going to make significant advances in the prevention and treatment of Alzheimer’s, we urgently require new approaches outside the old amyloid plaque box. Here I reviewed a number of such studies that promise to make a difference in the near future.
Understanding the condition, its origins, and effective strategies for prevention should be a top priority of our healthcare system.
Lee, Y. R., Ong, L., Gold, M., Kalali, A., & Sarkar, J. (2022). Alzheimer’s disease: key insights from two decades of clinical trial failures. Journal of Alzheimer's Disease, 87(1), 83-100.
Van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., ... & Iwatsubo, T. (2023). Lecanemab in early Alzheimer’s disease. New England Journal of Medicine, 388(1), 9-21.
Romanenko, M., Kholin, V., Koliada, A., & Vaiserman, A. (2021). Nutrition, gut microbiota, and Alzheimer's disease. Frontiers in psychiatry, 12, 712673
Prater, K. E., Green, K. J., Smith, C. L., ... & Jayadev, S. (2023). Human microglia show unique transcriptional changes in Alzheimer’s disease. Nature Aging, 3(7), 894-907.

Thomas R. Verny, M.D. , the author of eight books, including The Embodied Mind , has taught at Harvard University, University of Toronto, York University, and St. Mary’s University of Minnesota. His podcast, Pushing Boundaries , may be viewed on Youtube or listened to on Spotify and many other platforms.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
As populations age, Alzheimer’s and dementia are becoming more prevalent. A new drug could offer hope

As populations age, the number of cases of dementia rises. Image: Unsplash/centelm
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Charlotte Edmond

.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Global Health is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, global health.
Listen to the article
- A new drug, lecanemab, has been shown to reduce the decline in memory and thinking associated with Alzheimer's.
- As populations age, dementia cases are on the rise, with 10 million new people diagnosed each year.
- Dementia is a collective term for a group of diseases or brain injuries that can lead to a change in cognitive functioning as well as other symptoms like lack of emotional control.
It is one of the biggest diseases of our time: 10 million new cases of dementia are diagnosed every year, according to the World Health Organization (WHO). More than 55 million people worldwide live with a form of dementia and it is the seventh leading cause of death among all diseases.
Now a new drug is offering a glimmer of hope after years of searching for a treatment. In clinical trials, lecanemab has been shown to slow the cognitive decline associated with the disease. The drug attacks the protein clumps in the brain that many think are the cause of the disease.
Although dementia patients are currently offered drugs, none of them affect the progression of the disease which is why scientists in the field are so excited about this latest development. Alzheimer's Research UK called the findings "a major step forwards" .
But while this is undoubtedly positive news, the body also points out that the benefits of the drug were small and came with significant side effects. In addition, lecanemab has been proven to work in the early stages of the disease, so would rely on doctors spotting it before it had progressed too far.
With the number of dementia cases expected to rise to 78 million by 2030 and 139 million in 2050, according to the WHO, the race is on for scientific developments and research that will help us understand, treat and possibly prevent the disease.
A global impact
As populations age, the number of cases of dementia rises. While the deterioration of cognitive functioning is not caused by age itself, it does primarily affect the older generation. For many elderly people it also results in disability and loss of independence - which can have psychological, social and economic implications for them and their families, carers and society more broadly.
The estimated global cost of dementia to society was placed at $1.3 trillion in 2019, and is expected to rise to $2.8 trillion by 2030, WHO says.
Alzheimer’s Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
To catalyse the fight against Alzheimer's, the World Economic Forum is partnering with the Global CEO Initiative (CEOi) to form a coalition of public and private stakeholders – including pharmaceutical manufacturers, biotech companies, governments, international organizations, foundations and research agencies.
The initiative aims to advance pre-clinical research to advance the understanding of the disease, attract more capital by lowering the risks to investment in biomarkers, develop standing clinical trial platforms, and advance healthcare system readiness in the fields of detection, diagnosis, infrastructure and access.
What is dementia?
Dementia is a collective term for a group of diseases or injuries which primarily or secondarily affect the brain. Alzheimer’s is the most common of these and accounts for around 60-70% of cases. Other types include vascular dementia , dementia with Lewy bodies (abnormal protein clumps) and a group of diseases that contribute to frontotemporal dementia. It can also be triggered by strokes, excessive use of alcohol, repetitive head injuries, nutritional deficiencies, or follow some infections like HIV, the Alzheimer’s Society explains.
The different forms of dementia can often be indistinct and can co-exist.
Different people are affected in different ways, depending on the underlying cause. But the syndrome is usually progressive and can affect a range of functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language and judgement.
Changes in mood and ability to control emotions often accompany these cognitive variations.

Can it be treated?
There is no cure for dementia, although there are numerous treatments being worked on and at clinical trial phase. Dementia care currently focuses on early diagnosis, optimizing health and wellbeing and providing long-term support to carers.
Besides age, there are a number of other risk factors, which if avoided, can decrease the chances of dementia and slow its progression. Preventative steps include being physically active, not smoking, avoiding the harmful use of alcohol, as well as maintaining a healthy diet, weight, blood pressure, cholesterol and blood sugar levels.
Other risk factors associated with dementia include depression, social isolation, low educational attainment, cognitive inactivity and even air pollution .

What is the impact?
People with dementia rely heavily on informal care - i.e., friends and family. These carers spent on average five hours a day looking after people living with dementia in 2019, according to WHO figures. Informal care is thought to cover half of the overall financial burden of dementia.
There is also a disproportionate impact on women. They account for 65% of all dementia-related deaths, and also have a greater number of years affected by the disease. Women also typically provide the majority of informal care - covering over two-thirds of the carer hours for people living with dementia.

What are the latest developments?
The fact that dementia is only diagnosed once symptoms appear means that by the time people take part in clinical trials the disease is often quite well advanced. This can hamper the development of drugs. However, research analyzing data from the UK Biobank has indicated there are a collection of signals that could indicate a problem years before dementia is currently being diagnosed .
Other scientists postulate that, rather than being a disease of the brain, Alzheimer’s is in fact a disorder of the immune system within the brain . They believe research should instead focus on drugs targeting auto-immune pathways .
On a less positive note, researchers found that people who have recently received a dementia diagnosis, or diagnosed with the condition at a younger age, are at an increased risk of suicide . This underlines the importance of a strong support network, particularly among those newly diagnosed.
Have you read?
Here’s how exercise alters our brain chemistry – and could prevent dementia, dementia cases are expected to double in many countries over the next 30 years, these 7 simple habits could halve your risk of dementia, don't miss any update on this topic.
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} weekly.
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Wellbeing and Mental Health .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

The fascinating link between biodiversity and mental wellbeing
Andrea Mechelli
May 15, 2024

How philanthropy is empowering India's mental health sector
Kiran Mazumdar-Shaw
May 2, 2024

From 'Quit-Tok' to proximity bias, here are 11 buzzwords from the world of hybrid work
Kate Whiting
April 17, 2024

Young people are becoming unhappier, a new report finds

Promoting healthy habit formation is key to improving public health. Here's why
Adrian Gore
April 15, 2024

What's 'biophilic design' and how can it benefit neurodivergent people?
Fatemeh Aminpour, Ilan Katz and Jennifer Skattebol
- International edition
- Australia edition
- Europe edition

New Alzheimer’s drug slows cognitive decline by 35%, trial results show
Donanemab is second drug in a year to succeed in trials in what could be ‘beginning of the end’ of disease
A new Alzheimer’s drug slowed cognitive decline by 35%, according to late-stage trial results, raising the prospect of a second effective treatment for the disease.
Donanemab met all goals of the trial and slowed progression of the condition by 35% to 36% compared with a placebo in 1,182 people with early-stage Alzheimer’s, the drugmaker Lilly said.
It comes after trial results published last year showed that lecanemab , made by Eisai and Biogen, reduced the rate of cognitive decline by 27% in patients with early Alzheimer’s.
“This could be the beginning of the end of Alzheimer’s disease,” said Dr Richard Oakley, the associate director of research at the Alzheimer’s Society in the UK. “After 20 years with no new Alzheimer’s drugs, we now have two potential new drugs in just 12 months – and for the first time, drugs that seem to slow the progression of disease.”
Maria Carrillo, the chief science officer of the Alzheimer’s Association in the US, also hailed donanemab’s trial results. “These are the strongest phase 3 data for an Alzheimer’s treatment to date,” she said.
Alzheimer’s is the most common cause of dementia, one of the world’s biggest health threats. The number of people living with dementia globally is forecast to nearly triple to 153 million by 2050, and experts have said it presents a rapidly growing threat to future health and social care systems in every community, country and continent.
In patients on donanemab, 47% showed no signs of the disease progressing after a year, according to a statement issued by Lilly . That compared to 29% on a placebo.
The drug resulted in 40% less decline in the ability to perform activities of daily living, the company said. Patients on donanemab also experienced a 39% lower risk of progressing to the next stage of disease compared to those on a placebo.
However, the company also reported side-effects.
Brain swelling occurred in 24% of those on donanemab, with 6.1% experiencing symptoms, Lilly said. Brain bleeding occurred in 31.4% of the donanemab group and 13.6% of the placebo group.
Lilly also said the incidence of serious brain swelling in the donanemab study was 1.6%, including two deaths attributed to the condition and a third death after an incident of serious brain swelling.
“The treatment effect is modest, as is the case for many first-generation drugs, and there are risks of serious side-effects that need to be fully scrutinised before donenemab can be marketed and used,” said Dr Susan Kohlhaas, the executive director of research and partnerships at Alzheimer’s Research UK.
But she said the results were still “incredibly encouraging” and represented “another hugely significant moment for dementia research”.
“We’re now on the cusp of a first generation of treatments for Alzheimer’s disease, something that many thought impossible only a decade ago,” she added. “People should be really encouraged by this news, which is yet more proof that research can take us ever closer towards a cure.”
Lilly said it planned to apply for approval from the US Food and Drug Administration next month, and with regulators in other countries shortly thereafter.
“At face value, these data look positive, but we need to see the full dataset,” said Dr Liz Coulthard, an associate professor in dementia neurology at the University of Bristol.
“Donanemab seems to help people with early Alzheimer’s retain cognitive function for longer – and this effect looks to be clinically meaningful. Donanemab might help people live well with Alzheimer’s for longer. If approved alongside lecanemab, this potentially brings a choice of treatments for patients.”
- Alzheimer's
- Pharmaceuticals industry
Most viewed
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 5, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Scientists discover new cause of Alzheimer's, vascular dementia
by Erik Robinson, Oregon Health & Science University
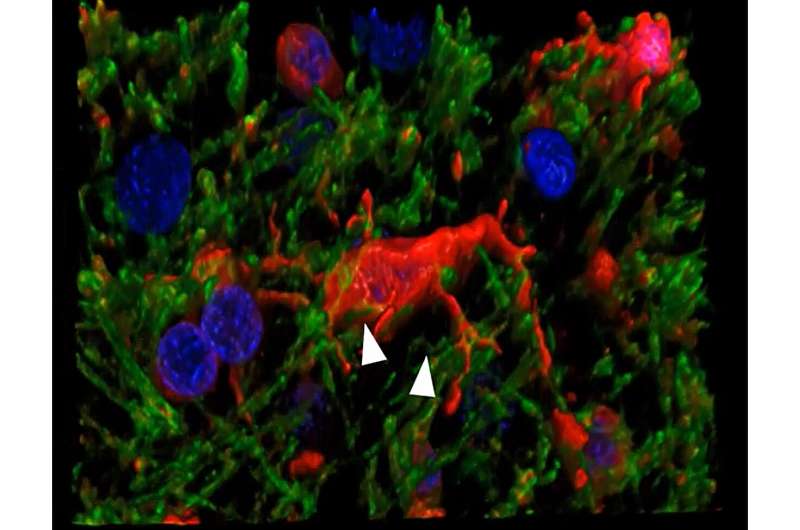
Researchers have discovered a new avenue of cell death in Alzheimer's disease and vascular dementia.
A new study, led by scientists at Oregon Health & Science University and published in the journal Annals of Neurology , reveals for the first time that a form of cell death known as ferroptosis—caused by a buildup of iron in cells—destroys microglia cells, a type of cell involved in the brain 's immune response, in cases of Alzheimer's and vascular dementia .
The researchers conducted the study examining post-mortem human brain tissue of patients with dementia.
"This is a major finding," said senior author Stephen Back, M.D., Ph.D., a neuroscientist and professor of pediatrics in the OHSU School of Medicine.
Back has long studied myelin, the insulation-like protective sheath covering nerve fibers in the brain, including delays in forming myelin in premature infants. The new research extends that line of work by uncovering a cascading form of neurodegeneration triggered by deterioration of myelin. They made the discovery using a novel technique developed by the study's lead author Philip Adeniyi, Ph.D., a postdoctoral researcher in Back's laboratory.
The researchers discovered that microglia degenerates in the white matter of the brain of patients with Alzheimer's and vascular dementia.
Microglia are resident cells in the brain normally involved in clearing cellular debris as part of the body's immune system. When myelin is damaged, microglia swarm in to clear the debris. In the new study, researchers found that microglia themselves are destroyed by the act of clearing iron-rich myelin—a form of cell death known as ferroptosis.
Given the intense scientific focus on the underlying cause of dementia in older adults , Back called it amazing that researchers hadn't made the connection to ferroptosis until now.
"We've missed a major form of cell death in Alzheimer's disease and vascular dementia," Back said. "We hadn't been giving much attention to microglia as vulnerable cells, and white matter injury in the brain has received relatively little attention."
Co-author Kiera Degener-O'Brien, M.D., initially discovered the degeneration of microglia in tissue samples, Back said. Adeniyi subsequently developed a novel immunofluorescence technique to determine that iron toxicity was causing microglial degeneration in the brain. This was likely a result of the fact that the fragments of myelin are themselves rich in iron, Back said.
In effect, the immune cells were dying in the line of duty.
"Everyone knows that microglia are activated to mediate inflammation," Back said. "But no one knew that they were dying in such large numbers. It's just amazing that we missed this until now."
The study finds that the cascading effect of degenerating microglia appears to be a mechanism in advancing cognitive decline in Alzheimer's disease and vascular dementia , Back said. He expects pharmaceutical companies will use this new finding to develop compounds focused on reducing microglial degeneration in the brain.
"That's where the field will go next," he said. "A discovery like ours will stimulate a lot of excitement in the pharmaceutical industry to develop therapeutically important compounds."
He said the underlying cause initiating the cycle of decline likely relates to repeated episodes of low blood flow and oxygen delivery to the brain over time due to acute stroke or chronic conditions such as hypertension and diabetes.
"Dementia is a process that goes on for years and years," Back said. "We have to tackle this from the early days to have an impact so that it doesn't spin out of control."
Explore further
Feedback to editors

New CRISPR screening method could reveal what drives brain diseases
11 minutes ago

Happy or angry: Researchers discover brain network that recognizes emotions
40 minutes ago
Study shows exercise spurs neuron growth and rewires the brain, helping mice forget traumatic and addictive memories
41 minutes ago
Brain 'assembloids': Researchers develop first human mini-brain with fully functional blood-brain barrier
52 minutes ago
Study finds fat cells influence heart health in Chagas disease

New AI algorithm may improve autoimmune disease prediction and therapies

Genetically engineered pig hearts transplanted in two brain-dead patients reveal more about immune response
2 hours ago

Researcher finds mothers live longer as child mortality declines
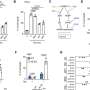
Scientists use new approach to disable brain cancer cells' ability to survive

Low-dose iron supplementation has no benefit for breastfed infants, shows study
Related stories.
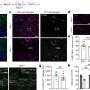
Immune cells help protect brain health and cognition, finds study
Dec 14, 2022
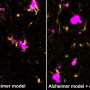
Poorly insulated nerve cells shown to promote Alzheimer's disease in old age
Jun 2, 2023

Research reveals clues to why immune cells seem to cause brain damage
May 30, 2023
Study shows how iron dysregulation might contribute to neurodegenerative diseases
Jan 18, 2023

Researchers find a backup mechanism that removes cellular debris from the brain
Nov 9, 2020
Reprogramming the brain's cleaning crew to mop up Alzheimer's disease
Aug 11, 2022
Recommended for you

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

New blood test for stroke detection combines blood-based biomarkers with a clinical score
May 17, 2024

Researchers reveal molecular mechanisms of different donor arteries for coronary artery bypass grafting
Study investigates cardiac cell regeneration in search of novel therapeutics
May 16, 2024
Researchers identify immunosuppressive pathway that helps newborn hearts regenerate in mouse models
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Alzheimer's disease articles from across Nature Portfolio
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the most common cause of dementia.
APOE4 homozygosity is a new genetic form of Alzheimer’s disease
New data confirm that APOE4 homozygosity is a major genetic cause of Alzheimer’s disease, warranting the development of specialized research strategies, treatment approaches and clinical trials.
- Zherui Liang
- Yadong Huang
Latest Research and Reviews
Detecting the effect of genetic diversity on brain composition in an Alzheimer’s disease mouse model
QUINT workflow analysis of cell and amyloid density in AD-BXD mice demonstrates age-dependent and strain-specific increases in gliosis and amyloid accumulation. These changes in brain composition influence age-related variations in gene expression.
- Brianna Gurdon
- Sharon C. Yates
- Catherine C. Kaczorowski
p75 neurotrophin receptor modulation in mild to moderate Alzheimer disease: a randomized, placebo-controlled phase 2a trial
A phase 2a trial of LM11A-31 in mild to moderate Alzheimer disease suggests that p75 NTR modulation is safe and attenuates measures of degeneration.
- Hayley R. C. Shanks
- Taylor W. Schmitz
G-protein coupled estrogen receptor 1, amyloid-β, and tau tangles in older adults
GPER1 RNA expression is linked to tau tangles and modifies the relation of amyloidβ with tau tangles.
- Shahram Oveisgharan
- David A. Bennett
Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities
- Linda Söderberg
- Malin Johannesson
- Christer Möller
Pirh2 modulates the mitochondrial function and cytochrome c-mediated neuronal death during Alzheimer’s disease
- Abhishek Singh
- Shubhangini Tiwari
- Sarika Singh
Effects of SPI1 -mediated transcriptome remodeling on Alzheimer’s disease-related phenotypes in mouse models of Aβ amyloidosis
Although SPI1 gene was identified as a risk factor for Alzheimer’s disease, its role in the disease remains unclear. Here, the authors show that decreasing SPI1 level exacerbates disease symptoms, whereas increasing its level ameliorates phenotypes.
- Byungwook Kim
- Luke Child Dabin
News and Comment
Genetic protection against alzheimer disease, including tau inclusions.
- Rebecca Wright
Neuronal activity drives glymphatic waste clearance
Two new studies show that clearance of waste, including pathogenic amyloid, through the glymphatic system is driven by synchronized neuronal activity.
Five biomarkers from one cerebrospinal fluid sample to stage Alzheimer’s disease
Staging Alzheimer’s disease on the basis of the disease’s biological underpinnings might help with stratification and prognostication, both in the clinical setting and in clinical trials. We propose a staging model based on only five biomarkers, which are related to amyloid-β and tau pathologies in different ways and can be measured with a single sample of cerebrospinal fluid.
A test for Alzheimer’s-disease stage predicts dementia risk
Levels of a host of molecules in the cerebrospinal fluid reliably assess development of the disease.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
A gene long thought to just raise the risk for Alzheimer’s may cause some cases
FILE - A section of a human brain with Alzheimer’s disease is displayed at the Museum of Neuroanatomy at the University at Buffalo, in Buffalo, N.Y., Oct. 7, 2003. A long-feared gene appears to do more than raise people’s risk of Alzheimer’s: Inheriting two copies can cause the mind-robbing disease, according to research published in the journal Nature Medicine on Monday, May 6, 2024. (AP Photo/David Duprey, File)
- Copy Link copied
WASHINGTON (AP) — For the first time, researchers have identified a genetic form of late-in-life Alzheimer’s disease — in people who inherit two copies of a worrisome gene.
Scientists have long known a gene called APOE4 is one of many things that can increase people’s risk for Alzheimer’s, including simply getting older. The vast majority of Alzheimer’s cases occur after age 65. But research published Monday suggests that for people who carry not one but two copies of the gene, it’s more than a risk factor, it’s an underlying cause of the mind-robbing disease.
The findings mark a distinction with “profound implications,” said Dr. Juan Fortea, who led the study the Sant Pau Research Institute in Barcelona, Spain.
Among them: Symptoms can begin seven to 10 years sooner than in other older adults who develop Alzheimer’s.
An estimated 15% of Alzheimer’s patients carry two copies of APOE4, meaning those cases “can be tracked back to a cause and the cause is in the genes,” Fortea said. Until now, genetic forms of Alzheimer’s were thought to be only types that strike at much younger ages and account for less than 1% of all cases.
Scientists say the research makes it critical to develop treatments that target the APOE4 gene. Some doctors won’t offer the only drug that has been shown to modestly slow the disease, Leqembi, to people with the gene pair because they’re especially prone to a dangerous side effect, said Dr. Reisa Sperling, a study coauthor at Harvard-affiliated Brigham and Women’s Hospital in Boston.
Sperling hunts ways to prevent or at least delay Alzheimer’s and “this data for me says wow, what an important group to be able to go after before they become symptomatic.”
But the news doesn’t mean people should race for a gene test. “It’s important not to scare everyone who has a family history” of Alzheimer’s because this gene duo isn’t behind most cases, she told The Associated Press.
HOW DO GENETICS AFFECT ALZHEIMER’S?
More than 6 million Americans, and millions more worldwide, have Alzheimer’s. A handful of genes are known to cause rare “early-onset” forms, mutations passed through families that trigger symptoms unusually young, by age 50. Some cases also are linked to Down syndrome.
But Alzheimer’s most commonly strikes after 65, especially in the late 70s to 80s, and the APOE gene – which also affects how the body handles fats -- was long known to play some role. There are three main varieties. Most people carry the APOE3 variant that appears to neither increase nor decrease Alzheimer’s risk. Some carry APOE2, which provides some protection against Alzheimer’s.
APOE4 has long been labeled the biggest genetic risk factor for late-in-life Alzheimer’s, with two copies risker than one. About 2% of the global population is estimated to have inherited a copy from each parent.
RESEARCH POINTS TO A CAUSE FOR A SUBSET OF ALZHEIMER’S
To better understand the gene’s role, Fortea’s team used data from 3,297 brains donated for research and from over 10,000 people in U.S. and European Alzheimer’s studies. They examined symptoms and early hallmarks of Alzheimer’s such as sticky amyloid in the brain.
People with two APOE4 copies were accumulating more amyloid at age 55 than those with just one copy or the “neutral” APOE3 gene variety, they reported in the journal Nature Medicine. By age 65, brain scans showed significant plaque buildup in nearly three-quarters of those double carriers – who also were more likely to have initial Alzheimer’s symptoms around that age rather than in the 70s or 80s.
Fortea said the disease’s underlying biology was remarkably similar to young inherited types.
It appears more like “a familial form of Alzheimer’s,” said Dr. Eliezer Masliah of the National Institute on Aging. “It is not just a risk factor.”
Importantly, not everyone with two APOE4 genes develops Alzheimer’s symptoms and researchers need to learn why, Sperling cautioned.
“It’s not quite destiny,” she said.
HOW THE NEW FINDINGS MAY AFFECT ALZHEIMER’S RESEARCH AND TREATMENT
The drug Leqembi works by clearing away some sticky amyloid but Sperling said it’s not clear if carriers of two APOE4 genes benefit because they have such a high risk of a side effect from the drug – dangerous brain swelling and bleeding. One research question is whether they’d do better starting such drugs sooner than other people.
Masliah said other research aims to develop gene therapy or drugs to specifically target APOE4. He said it’s also crucial to understand APOE4’s effects in diverse populations since it’s been studied mostly in white people of European ancestry.
As for gene tests, for now they’re typically used only to evaluate if someone’s a candidate for Leqembi or for people enrolling in Alzheimer’s research – especially studies of possible ways to prevent the disease. Sperling said the people most likely to carry two APOE4 genes had parents who both got Alzheimer’s relatively early, in their 60s rather than 80s.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
New group training tool for the prevention of dementia
Solving a quiz as a group while moving around the room at the same time -- this combination is the basis of a new tool designed to prevent dementia. Researchers developed and evaluated it in the "go4cognition" project with industry partners and brought it to market maturity. Vanessa Lissek and Professor Boris Suchan from Ruhr University Bochum, Germany, and Stefan Orth from the company Ontaris describe how effective the training with the system is in the Journal of Alzheimer's Disease . The article was published online on April 30, 2024.
"In the project, we've developed a completely new system that can be used for dementia prevention training," says Boris Suchan. "We were specifically interested in designing a tool for group training, as it produces very good results and increases acceptance. Also, all participants benefit and learn from each other."
Passing the baton from quiz station to quiz station
The new system is designed for groups of seven to ten people. It consists of six stations arranged around the room, each equipped with a tablet. The tablets display different tasks, such as listing German chancellors, memorizing series of numbers or planning a trip around the world to specific locations. In order to complete the tasks, a participant has to move to the relevant station and insert a baton fitted with a microchip into a specific device. "We can then digitally record how long it took to complete the task and whether the answer was correct," explains Boris Suchan. As everyone has their own baton, their individual performance can be evaluated.
Significant reduction of cognitive impairments through training
The researchers tested the system with 30 volunteers aged between 60 and 89 who had been diagnosed with mild cognitive impairment, i.e. who showed moderate cognitive impairment in neuropsychological tests. "Our participants are not restricted in their everyday activities, but have an increased risk of developing dementia at a later stage," says Boris Suchan. Mild cognitive impairment occurs in 15 to 20 percent of people above the age of 60.
The participants trained with the go4cognition system two days a week for six weeks. After completing the training, 70 percent of them were no longer diagnosed with mild cognitive impairment.
System already in use in retirement homes
The group training tool is marketed by the company Ontaris. "It's already used in retirement homes, for example in Oberhausen -- also for residents who have not yet been diagnosed with mild cognitive impairment," says Boris Suchan, and offers an outlook: "In future, we're also considering testing the system for people with acquired brain damage."
For people who don't have access to the go4cognition system but still wish to take steps to prevent dementia, the Bochum-based researcher recommends physical and mental activities and a healthy diet. "All of this helps to prevent dementia or at least delay it for as long as possible," says Suchan. As part of the go4cognition project, he and his colleagues published a review article on this topic.
About go4cognition
The go4cognition project ran from 2018 to 2023, supported by Leitmarkt Agentur.NRW, the European Union and the state of NRW (funding number ERDF -0801287). Specialists from various institutions developed new tools to prevent cognitive impairment in old age.
- Alzheimer's Research
- Healthy Aging
- Medical Education and Training
- Intelligence
- Alzheimer's
- Therapy dog
- Stem cell treatments
- Neurobiology
- Neuroscience
- Scientific method
- Collaboration
- Milgram experiment
Story Source:
Materials provided by Ruhr-University Bochum . Original written by Julia Weiler. Note: Content may be edited for style and length.
Journal Reference :
- Vanessa J. Lissek, Stefan Orth, Boris Suchan. go4cognition: Evaluation of a Newly Developed Multicomponent Intervention in Mild Cognitive Impairment . Journal of Alzheimer's Disease , 2024; 99 (1): 377 DOI: 10.3233/JAD-230802
Cite This Page :
Explore More
- First Glimpse of an Exoplanet's Interior
- High-Efficiency Photonic Integrated Circuit
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
- Toward Human Brain Gene Therapy
- Whale Families Learn Each Other's Vocal Style
- AI Can Answer Complex Physics Questions
- Otters Use Tools to Survive a Changing World
- Monogamy in Mice: Newly Evolved Type of Cell
Trending Topics
Strange & offbeat.
![ARUK-FOR-A-CURE logo Core-colours-RGB[1] alzheimer's research uk for a cure logo](https://www.alzheimersresearchuk.org/wp-content/uploads/2023/05/ARUK-FOR-A-CURE-logo-Core-colours-RGB1-1024x192.png)
If you have a press enquiry please visit our Press Office page .

“Why wouldn’t we want to do that?” Volunteer perspectives on taking part in dementia research
When Alison Littleford’s husband Frank was diagnosed with Alzheimer’s in February 2020, the couple told no one about his diagnosis other than family and close friends. But they soon began to question this, and Alison said they “began a process of complete change. We realised that, by speaking publicly about dementia and by taking part…

Eleanor: My dad’s diagnosis took 10 years – we must do better
Eleanor’s dad, Mike, is living with young onset Alzheimer’s disease. Eleanor shares how a drawn-out diagnosis journey impacted her family and explains why she supports Alzheimer’s Research UK’s efforts to revolutionise dementia diagnosis.

Cadbury release Memory Bar Boxes as part of partnership with Alzheimer’s Research UK
Cadbury has launched special Memory Bar Boxes, as part of their partnership with Alzheimer’s Research UK.

Hike For A Cure: what’s your why?
We speak to our supporter, Tom Manning, about how dementia has impacted his life, and why he decided to Hike For A Cure.

Blood pressure and brain health: what’s the link?
Let’s dig into the research to explore what we know about the way our blood pressure affects the brain’s ability to function.

Candice Brown shares three healthy recipes for your brain health
Challenge your brain with a healthy recipe to chew over, created by Alzheimer’s Research UK supporter and Great British Bake Off winner, Candice Brown.

Inheriting two copies of APOE4 linked to risk of Alzheimer’s at a younger age, study suggests
A new study confirms that people who carry two copies of an Alzheimer’s risk gene, APOE4, have a very high chance of developing Alzheimer’s.

“You want your loved ones to have the best life possible. We want that too.”
Drug discovery researcher Dr Emma Mead shares how her team are focused on the next generation of dementia treatments

340,000 people took our Brain Health Check-in – here’s what we’ve learnt
It’s now over a year since we launched the Think Brain Health Check-in. We look back at five of the biggest lessons we’ve learnt.

“I’ve got three degrees, but I can’t tell the time” – new film reveals reality of dementia with Lewy bodies
Alzheimer’s Research UK launches a new film to shine a spotlight on dementia with Lewy bodies (DLB), featuring supporters Des and Valli.

Antipsychotics for dementia linked to more harms than previously acknowledged
A large, UK-based study published in the BMJ has suggested that antipsychotic drugs carry greater risks of serious health conditions than previously thought.

Three Slimming World members share their motivations for running the London Marathon for a cure
This month, a team of nine members and colleagues from our Corporate Partner, Slimming World, are heading to the start line in aid of Alzheimer’s Research UK, to raise £18,000 for a cure.

‘I can’t rest until we find a way to stop dementia’ – Iceland Foods chief to run London Marathon for Alzheimer’s Research UK
Iceland Foods Executive Chairman, Richard Walker OBE, will be joining thousands of others running the TCS London Marathon on Sunday 21 April.

My reflections on the UK’s largest gathering of dementia researchers
Our new Director of Research looks back on our Research Conference 2024 in Liverpool

Brothers Jordan and Cian receive Prime Minister’s ‘Points of Light’ award in recognition of fundraising achievements
Brothers Jordan and Cian Adams receive Point of Light award in recognition of their support for dementia research.

Our highlights from this year’s AD/PD conference
Our new Head of Research shares her three take homes from the 2024 AD/PD conference.

Study reveals combination of earlier menopause and higher cardiovascular risk may be linked to memory and thinking problems
A large Canadian research study, has reported that women with an earlier onset of menopause and higher risk of cardiovascular disease show signs of reduced cognitive function.

Two revolutionary studies set to bring blood tests for dementia a step closer to NHS
The Blood Biomarker Challenge will see two teams of researchers conduct UK-wide clinical trials to identify quick and accurate blood tests for diagnosing dementia.

Melissa: Putting Alzheimer’s on the agenda at No. 10
Alzheimer’s Research UK supporter, Melissa Williams, shares a moving account of how dementia has and continues to impact her family.

Canadian study in mice offers new insight into ‘transmissible’ Alzheimer’s disease
New study finds transplanting stem cells from mice with an Alzheimer’s gene can trigger Alzheimer’s symptoms in mice without the gene.

Walk for a cure – and for your brain health
Our supporter Hat Hewitt explains why it was important for her to walk for a cure – and how walking is part of her toolkit for looking after her brain health.

What’s it like to get a blood pressure test?
We spoke to our supporter, Andy Watts, about what it’s like to get your blood pressure tested – and why he recommends it as a way to help give back to your brain.

Alzheimer’s Research UK supporter, Frank Rothwell, awarded ‘Point of Light’ by Downing Street
Oldham businessman Frank Rothwell has been recognised as a ‘Point of Light’ by Prime Minister Rishi Sunak for his outstanding fundraising efforts for Alzheimer’s Research UK.

Scott Mitchell named People’s Champion of the Dame Barbara Windsor Dementia Mission
Husband of the late Dame Barbara Windsor and Alzheimer’s Research UK Ambassador, Scott Mitchell, to play a key role in a national dementia initiative that honours her.

Five things Alzheimer’s Research UK is doing to change the ending
Find out how Alzheimer’s Research UK is changing the ending for everyone affected by dementia.
Privacy overview
- Introduction
- Conclusions
- Article Information
Models were stratified by age, cohort (sex), and calendar time, and adjusted for Southern European/Mediterranean ancestry (yes/no), married (yes/no), living alone (yes/no), smoking status (never, former, current smoker 1-14 cigarettes/d, 15-24 cigarettes/d, or ≥25 cigarettes/d), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent of task–h/wk), multivitamin use (yes/no), history of hypertension (yes/no), history of hypercholesterolemia (yes/no), history of diabetes (yes/no), in women postmenopausal status and menopausal hormone use (premenopausal, postmenopausal [no, past, or current hormone use]), total energy intake (kcal/d), family history of dementia (yes/no), history of depression (yes/no), census socioeconomic status (9-variable score, in quintiles), and body mass index calculated as weight in kilograms divided by height in meters squared (<23, 23-25, 25-30, 30-35, ≥35). Pooled results were obtained by pooling the datasets of the cohorts. AMED score is without monounsaturated:saturated fats intake ratio component. AHEI score is without polyunsaturated fats intake component. HR indicates hazard ratio.
a Reference value.
b P < .05.
Substitution analysis of 5 g/d intake of olive oil for the equivalent amount of butter, other vegetable oils, mayonnaise, and margarine. All Cox proportional hazards models were stratified by age and calendar time. Models were adjusted for Southern European/Mediterranean ancestry (yes/no), married (yes/no), living alone (yes/no), smoking status (never, former, current smoker 1-14 cigarettes/d, 15-24 cigarettes/d, or ≥25 cigarettes/d), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10.0-14.9, and ≥15.0 g/d), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent of task–h/wk), multivitamin use (yes/no), history of hypertension (yes/no), history of hypercholesterolemia (yes/no), in women postmenopausal status and menopausal hormone use (premenopausal, postmenopausal [no, past, or current hormone use]), total energy intake (kcal/d), family history of dementia (yes/no), history of depression (yes/no), census socioeconomic status (9-variable score, in quintiles), body mass index calculated as weight in kilograms divided by height in meters squared (<23, 23-25, 25-30, 30-35, ≥35), red meat, fruits and vegetables, nuts, soda, whole grains intake (in quintiles), and trans-fat. Pooled results were obtained by pooling the data sets of the cohorts and Cox proportional hazards model 3 was further stratified by cohort (sex). HR indicates hazard ratio.
eTable 1. Odds Ratios for Dementia-Related Mortality by APOE4 Allelic Dosage
eTable 2. Risk of Death With Dementia (Composite Outcome) According to Categories of Total Olive Oil
eTable 3. Joint Associations of Olive Oil Intake and AMED (A), and AHEI (B) With Dementia-Related Mortality Risk
eTable 4. Risk of Dementia-Related Mortality According to Categories of Total Olive Oil in the Genomic DNA Subsample
eFigure. Subgroup Analyses for 5g/d Increase in Olive Oil Intake With Dementia-Related Mortality Risk
eTable 5. Risk of Dementia-Related Mortality According to Categories of Total Olive Oil Without Stopping Diet Update Upon Report of Intermediate Non-Fatal Events
eTable 6. Risk of Dementia Mortality According to Categories of Total Olive Oil Applying a 4-Year Lag Period Between Dietary Intake and Dementia Mortality
eTable 7. Risk of Dementia-Related Mortality According to Categories of Total Olive Oil Adjusting for Other Covariates
eTable 8. Risk of Mortality From Dementia and Other Causes of Death According to Categories of Total Olive Oil Applying a Competing Risk Model
eReferences
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Tessier A , Cortese M , Yuan C, et al. Consumption of Olive Oil and Diet Quality and Risk of Dementia-Related Death. JAMA Netw Open. 2024;7(5):e2410021. doi:10.1001/jamanetworkopen.2024.10021
Manage citations:
© 2024
- Permissions
Consumption of Olive Oil and Diet Quality and Risk of Dementia-Related Death
- 1 Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts
- 2 School of Public Health, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3 Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts
- 4 Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts
- 5 Department of Public Health and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Question Is the long-term consumption of olive oil associated with dementia-related death risk?
Findings In a prospective cohort study of 92 383 adults observed over 28 years, the consumption of more than 7 g/d of olive oil was associated with a 28% lower risk of dementia-related death compared with never or rarely consuming olive oil, irrespective of diet quality.
Meaning These results suggest that olive oil intake represents a potential strategy to reduce dementia mortality risk.
Importance Age-standardized dementia mortality rates are on the rise. Whether long-term consumption of olive oil and diet quality are associated with dementia-related death is unknown.
Objective To examine the association of olive oil intake with the subsequent risk of dementia-related death and assess the joint association with diet quality and substitution for other fats.
Design, Setting, and Participants This prospective cohort study examined data from the Nurses’ Health Study (NHS; 1990-2018) and Health Professionals Follow-Up Study (HPFS; 1990-2018). The population included women from the NHS and men from the HPFS who were free of cardiovascular disease and cancer at baseline. Data were analyzed from May 2022 to July 2023.
Exposures Olive oil intake was assessed every 4 years using a food frequency questionnaire and categorized as (1) never or less than once per month, (2) greater than 0 to less than or equal to 4.5 g/d, (3) greater than 4.5 g/d to less than or equal to 7 g/d, and (4) greater than 7 g/d. Diet quality was based on the Alternative Healthy Eating Index and Mediterranean Diet score.
Main Outcome and Measure Dementia death was ascertained from death records. Multivariable Cox proportional hazards regressions were used to estimate hazard ratios (HRs) and 95% CIs adjusted for confounders including genetic, sociodemographic, and lifestyle factors.
Results Of 92 383 participants, 60 582 (65.6%) were women and the mean (SD) age was 56.4 (8.0) years. During 28 years of follow-up (2 183 095 person-years), 4751 dementia-related deaths occurred. Individuals who were homozygous for the apolipoprotein ε4 ( APOE ε4 ) allele were 5 to 9 times more likely to die with dementia. Consuming at least 7 g/d of olive oil was associated with a 28% lower risk of dementia-related death (adjusted pooled HR, 0.72 [95% CI, 0.64-0.81]) compared with never or rarely consuming olive oil ( P for trend < .001); results were consistent after further adjustment for APOE ε4 . No interaction by diet quality scores was found. In modeled substitution analyses, replacing 5 g/d of margarine and mayonnaise with the equivalent amount of olive oil was associated with an 8% (95% CI, 4%-12%) to 14% (95% CI, 7%-20%) lower risk of dementia mortality. Substitutions for other vegetable oils or butter were not significant.
Conclusions and Relevance In US adults, higher olive oil intake was associated with a lower risk of dementia-related mortality, irrespective of diet quality. Beyond heart health, the findings extend the current dietary recommendations of choosing olive oil and other vegetable oils for cognitive-related health.
One-third of older adults die with Alzheimer disease or another dementia. 1 While deaths from diseases such as stroke and heart disease have been decreasing over the past 20 years, age-standardized dementia mortality rates have been on the rise. 2 The Mediterranean diet has gained in popularity owing to its recognized, multifaceted health benefits, particularly on cardiovascular outcomes. 3 Accruing evidence suggests this dietary pattern also has a beneficial effect on cognitive health. 4 As part of the Mediterranean diet, olive oil may exert anti-inflammatory and neuroprotective effects due to its high content of monounsaturated fatty acids and other compounds with antioxidant properties such as vitamin E and polyphenols. 5 A substudy conducted as part of the Prevencion con Dieta Mediterranea (PREDIMED) randomized trial provided evidence that higher intake of olive oil for 6.5 years combined with adherence to a Mediterranean diet was protective of cognitive decline when compared with a low-fat control diet. 6 - 8
Given that most previous studies on olive oil consumption and cognition were conducted in Mediterranean countries, 7 - 10 studying the US population, where olive oil consumption is generally lower, could offer unique insights. Recently, we showed that olive oil consumption was associated with a lower risk of total and cause-specific mortality in large US prospective cohort studies, including a 29% (95% CI, 22%-36%) lower risk for neurodegenerative disease mortality in participants who consumed more than 7 g/d of olive oil compared with little or none. 11 However, this previous analysis was not designed to examine the association of olive oil and diet quality with dementia-related mortality, and therefore the latter remains unclear.
In this study, we examined the association between total olive oil consumption and the subsequent risk of dementia-related mortality in 2 large prospective studies of US women and men. Additionally, we evaluated the joint associations of diet quality (adherence to the Mediterranean diet and Alternative Healthy Eating Index [AHEI] score) and olive oil consumption with the risk of dementia-related mortality. We also estimated the difference in the risk of dementia-related mortality when other dietary fats were substituted with an equivalent amount of olive oil.
Analyses were performed in 2 large US prospective cohorts: the Nurses’ Health Study I (NHS) and the Health Professionals Follow-Up Study (HPFS). The NHS was initiated in 1976 and recruited 121 700 US female registered nurses aged 30 to 55 years. 12 The HPFS was established in 1986 and included 51 525 male health professionals aged 40 to 75 years. 13 The cohorts have been described elsewhere. 12 , 13 Lifestyle factors and medical history were assessed biennially through mailed questionnaires, with a follow-up rate greater than 90%. Baseline for this analysis was 1990, which is when the food frequency questionnaires (FFQs) first included information on olive oil consumption.
Participants with a history of cardiovascular disease (CVD) or cancer at baseline, with missing data on olive oil consumption, or who reported implausible total energy intakes (<500 or >3500 kcal/d for women and <800 or >4200 kcal/d for men) were excluded. The completion of the questionnaire self-selected cognitively highly functioning women and men. In total, 60 582 women and 31 801 men were included. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, which deemed the participants’ completion of the questionnaire to be considered as implied consent. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Dietary intake was measured using a validated greater than 130-item FFQ administered in 1990 and every 4 years thereafter. The validity and reliability of the FFQ have been described previously. 14 Participants were asked how frequently they consumed specific foods, including types of fats and oils used for cooking or added to meals in the past 12 months. Total olive oil intake was determined by summing up answers to 3 questions related to olive oil consumption (ie, olive oil used for salad dressings, olive oil added to food or bread, and olive oil used for baking and frying at home). The equivalent of 1 tablespoon of olive oil was considered to be 13.5 g. Intakes of other fats and nutrients were calculated using the United States Department of Agriculture and Harvard University Food Composition Database, 15 and biochemical analyses. The nutritional composition of olive oil and other types of fat, as well as trends of types of fat intake in the NHS and HPFS, have been reported previously. 11
Adherence to the Mediterranean diet was assessed using a modified version of the 9-point Alternative Mediterranean index (AMED) score. 16 Adherence to the AHEI (0-110), previously associated with lower risk of chronic disease, was also computed. 17 Higher scores indicated better overall diet quality.
The apolipoprotein E ε4 ( APOE ε4 ) allele is known to interfere with lipid and glucose metabolism such that it increases the risk of dementia. 18 APOE genotyping was conducted in a subset of 27 296 participants. Blood samples were collected between 1989 and 1990 in the NHS and between 1993 and 1995 in the HPFS. NHS participants who had not provided blood samples were invited to contribute buccal samples from 2002 to 2004. DNA was extracted with the ReturPureGene DNA Isolation Kit (Gentra Systems). The APOE genotype was determined using a Taqman Assay (Applied Biosystems) 19 in 5069 participants, and through imputation from multiple genome-wide association studies, 20 which has shown high accuracy, 20 in the remaining subset.
Deaths were ascertained from state vital statistics records and the National Death Index or by reports from next of kin or the postal authorities. The follow-up for mortality exceeded 98% in these cohorts. Dementia deaths were determined by physician review of medical records, autopsy reports, or death certificates. Dementia deaths were those in which dementia was listed as the underlying cause of death, or as a contributing cause of death, or as reported by the family, in the absence of a more likely cause. The International Classification of Diseases, Eighth Revision (ICD-8) was used in the NHS and ICD-9 in the HPFS, which were the revisions used at the inception of those cohorts. Dementia deaths included codes 290.0 (senile dementia, simple type), 290.1 (presenile dementia), and 331.0 (Alzheimer disease). To test the validity of the dementia mortality outcome, we examined the likelihood of dementia mortality by APOE ε4 allelic dosage (eTable 1 in Supplement 1 ). 18 A composite outcome was also created including both participants who reported having dementia during follow-up and later died, with those who had dementia reported on their death certificate.
Participants completed biennial questionnaires reporting updates on body weight, smoking, physical activity, multivitamin use, menopausal status, and postmenopausal hormone use in women, family history of dementia, self-report of chronic diseases, and ancestry. History of depression was identified based on antidepressive medication use and self-report of depression. Socioeconomic status (SES) was established through a composite score derived from home address details and various factors such as income, education, and housing; the composite score methods are described in a previous report. 21 Body mass index (BMI) was obtained by dividing the weight in kilograms by the height in meters squared.
In each cohort, age-stratified Cox proportional hazard models were used to evaluate the association of olive oil intake with dementia-related mortality. Participant person-time was calculated from baseline until end of follow-up (June 30, 2018, in NHS; January 31, 2018, in HPFS), loss to follow-up, or death, whichever came first. The cumulative average (mean) of olive oil intake from all available FFQs, from baseline until 2014 (or loss to follow-up or death), was used as the exposure. Because potential diet modifications following cancer or CVD diagnosis may not represent long-term diet, we ceased updating dietary variables upon report of these conditions. For missing covariates, we carried forward nonmissing values from previous questionnaires and assigned median values for continuous variables.
Participants were categorized by olive oil intake frequency: never or less than once per month (reference group), greater than 0 to less than or equal to 4.5 g/d, greater than 4.5 g/d to less than or equal to 7 g/d, and greater than 7 g/d. P values for linear trends were obtained using the Wald test on a continuous variable represented by the median intake of each category. Multivariable Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% CIs for dementia mortality according to categories of olive oil intake, separately in each cohort. Participants were censored at death from causes other than dementia. Model 1 was stratified for age and calendar time. Multivariable model 2 was adjusted for Southern European/Mediterranean ancestry, married, living alone, smoking, alcohol intake, physical activity, multivitamin use, history of hypertension and hypercholesterolemia, in women postmenopausal status and menopausal hormone use, total energy intake, family history of dementia, history of depression, census SES, and BMI. Multivariable model 3 was further adjusted for intake of red meat, fruits and vegetables, nuts, soda, whole grains, and trans-fat, all indicative of diet quality.
In a secondary analysis we used the composite outcome for dementia-related deaths. We also repeated the main analysis in the genotyping subsample. We carried out mediation analyses to calculate the percentage of the association between olive oil intake and dementia-related mortality that is attributable to CVD, hypercholesterolemia, hypertension, and diabetes. We also performed stratified analyses by prespecified subgroups (eMethods in Supplement 1 ).
A joint analysis was performed to test whether olive oil intake (never or <1/mo, >0 to ≤7g/d, and >7g/d) and the AMED or the AHEI score (tertiles) combined as the exposure was associated with dementia mortality. In substitution analyses, we assessed the risk of dementia-related mortality by replacing 5 g/d of different fat sources, including margarine, mayonnaise, butter, and a combination of other vegetable oils (corn, safflower, soybean, and canola), with olive oil. Both continuous variables as 5-g/d increments were included in a multivariable model 3, mutually adjusted for other types of fat. The difference in the coefficients obtained for olive oil and the substituted fat provided the estimated HR and 95% CI for substituting 5 g/d of olive oil for an equivalent amount of the other fats.
Several exploratory sensitivity analyses were performed including a 4-year lagged analysis, analyses adjusting for other covariates, a cause-specific competing risk model and analyses excluding participants who self-reported having dementia at baseline (n = 12) (eMethods in Supplement 1 ). Analyses were performed from May 2022 to July 2023 using SAS version 9.4 (SAS Institute). All statistical tests were 2-sided with an α = .05.
Over 2 183 095 person-years of follow-up, this study documented a total of 4751 dementia deaths (3473 in NHS and 1278 in HPFS; 37 649 total deaths). Among 92 383 participants included at baseline in 1990, 60 582 (65.6%) were women, and the mean (SD) age was 56.4 (8.0) years. Mean (SD) olive oil intake was 1.3 (2.5) g/d in both NHS and HPFS; the mean (SD) adherence score for the Mediterranean diet was 4.5 (1.9) points in the NHS and 4.2 (1.9) points in the HPFS; and the mean (SD) AHEI diet quality score was 52.5 (11.1) points in the NHS and 53.4 (11.6) points in the HPFS.
Table 1 shows baseline characteristics of participants categorized by total olive oil intake. Participants with a higher olive oil intake (>7 g/d) at baseline had an overall higher caloric intake, but not a higher BMI, had better diet quality, had higher alcohol intake, were more physically active, and were less likely to smoke compared with those never consuming olive oil or less than once per month ( Table1 ). Individuals who were homozygous for the APOE ε4 allele were 5.5 to 9.4 times more likely to die with dementia compared with noncarriers for the APOE e4 allele (χ 2 P < .001) (eTable 1 in Supplement 1 ).
Olive oil intake was inversely associated with dementia-related mortality in age-stratified and multivariable-adjusted models ( Table 2 ). Compared with participants with the lowest olive oil intake, the pooled HR for dementia-related death among participants with the highest olive oil intake (>7 g/d) was 0.72 (95% CI, 0.64-0.81), after adjusting for sociodemographic and lifestyle factors. The association between each 5-g increment in olive oil consumption with dementia-related death was also inverse and significant in the pooled analysis. The multivariable-adjusted HR for dementia-related death for the highest compared with the lowest olive oil intake (>7 g/d) was 0.67 (95% CI, 0.59-0.77) for women and 0.87 (95% CI, 0.69-1.09) for men ( Table 2 ). Olive oil intake in 5-g increments was inversely associated with dementia-related mortality in women (HR, 0.88 [95% CI, 0.84-0.93]), but not in men (HR, 0.96 [95% CI, 0.88-1.04]). Analyses remained consistent when using the composite outcome for death with dementia (eTable 2 in Supplement 2 ). In the genotyping subsample, the results remained unchanged after further adjusting for the APOE ε4 allelic genotype (multivariable-adjusted pooled HR comparing high vs low olive oil intake, 0.66 [95% CI, 0.54-0.81]; P for trend < .001) (eTable 4 in Supplement 1 ). Pooled mediation analyses found that CVD, hypercholesterolemia, hypertension, and diabetes did not significantly attenuate the association (unchanged HRs with and without adjusting for the intermediate; data not shown).
In joint analyses, participants with the highest olive oil intake had a lower risk for dementia-related mortality, irrespective of their AMED score (28% to 34% lower risk compared with participants in the combined low olive oil and high AMED) ( Figure 1 A; eTable 3 in Supplement 1 ) and of their AHEI (27% to 38% lower risk compared with participants with low olive oil and high AHEI) ( Figure 1 B; eTable 3 in Supplement 1 ).
Replacing 5 g/d of mayonnaise with 5 g/d of olive oil was associated with a 14% (95% CI, 7%-20%) lower risk of dementia-related mortality in pooled multivariable-adjusted models ( Figure 2 ). As for the substitution of 5 g/d of margarine with the equivalent amount of olive oil, we estimated an 8% (95% CI, 4%-12%) lower risk. Substitutions of other vegetable oils or butter with olive oil were not statistically significant.
Exploratory subgroup analyses (eFigure in Supplement 1 ) showed associations between higher olive oil intake and lower risk of dementia-related mortality across most subgroups. No statistically significant associations were found in participants with a family history of dementia, living alone, using a multivitamin, and in non– APOE ε4 carriers. Results from exploratory sensitivity analyses (eTables 5-8 in Supplement 1 ) were comparable with the findings from the main analysis (eResults in Supplement 1 ).
In 2 large US prospective cohorts of men and women, we found that participants who consumed more than 7 g/d of olive oil had 28% lower risk of dying from dementia compared with participants who never or rarely consumed olive oil. This association remained significant after adjustment for diet quality scores including adherence to the Mediterranean diet. We estimated that substituting 5 g/d of margarine and mayonnaise with olive oil was associated with significantly lower dementia-related death risk, but not when substituting butter and other vegetable oils. These findings provide evidence to support dietary recommendations advocating for the use of olive oil and other vegetable oils as a potential strategy to maintain overall health and prevent dementia.
In the NHS and HPFS, a lower risk of neurodegenerative disease mortality, including dementia mortality, was observed with higher olive oil consumption (HR, 0.81 [95% CI, 0.78-0.84]). 11 Evidence that pertains to cognitive decline or incident dementia is more widely available than it is for dementia mortality. 6 , 22 In the French Three-City Study (n = 6947), participants with the highest olive oil intake were 17% (95% CI, 1%-29%) less likely to experience a 4-year cognitive decline related to visual memory, but no association was found for verbal fluency (odds ratio [OR], 0.85 [95% CI, 0.70-1.03]). 22 Furthermore, participants with a higher intake of olive oil (moderate or intensive vs never) had a lower risk of verbal fluency and visual memory cognitive impairment. Potential sex differences were not investigated. In the PREDIMED trial, which supplemented a Mediterranean-style diet with extra-virgin olive oil (1 L/wk/household) or nuts (30 g/d), 23 the authors investigated cognitive effects and status in 285 and 522 cognitively healthy participants using global and in-depth neuropsychological battery testing. Although the study was not originally designed for cognitive outcomes and the effect of olive oil cannot be isolated, after 6.5 years, the olive oil group exhibited improved cognitive performance in verbal fluency and memory tests compared with a low-fat diet (control), and they were less prone to develop mild cognitive impairment (OR, 0.34 [95% CI, 0.12-0.97]; n = 285). 6 Global cognitive performance was higher in both the olive oil and the nut groups compared with the control post trial (n = 522). 8 These studies were conducted in Europe, in populations with typically higher olive oil intake compared with US populations.
Observational studies and some trials have consistently found associations between following diets such as the Mediterranean, DASH, MIND, and AHEI, and prudent patterns to healthier brain structure, 24 reduced cognitive impairment and Alzheimer risk, and improved cognitive function. 4 In our study, those with the highest olive oil intake (>7 g/d) had the lowest dementia-related death risk compared with those with minimal intake (never or less than once per month), regardless of diet quality. This highlights a potentially specific role for olive oil. Still, the group with both high AHEI scores and high olive oil intake exhibited the lowest dementia mortality risk (HR, 0.68 [95% CI, 0.58-0.79]; reference: low AHEI score and low olive oil intake), suggesting that combining higher diet quality with higher olive oil intake may confer enhanced benefit.
Olive oil consumption may lower dementia mortality by improving vascular health. 18 Several clinical trials support the effect of olive oil in reducing CVD via improved endothelial function, coagulation, lipid metabolism, oxidative stress, platelet aggregation and decreased inflammation. 25 Nonetheless, the results of our study remained independent of hypertension and hypercholesterolemia. Mild cognitive impairment, Alzheimer disease, and related dementias were associated with abnormal blood brain barrier permeability, possibly allowing the crossing of neurotoxic molecules into the brain. 26 Mechanistical evidence from animal 27 - 29 and human studies 9 , 30 have shown that phenolic compounds in olive oil, particularly extra-virgin olive oil, may attenuate inflammation, oxidative stress and restore blood brain barrier function, thereby reducing brain amyloid-β and tau-related pathologies and improving cognitive function. However, incident CVD, hypercholesterolemia, hypertension, and diabetes were not significant mediators of the association between olive oil intake and dementia-related death in our study.
The association was significant in both sexes but did not remain in men after full adjustment of the model. Some previous research has reported cognitive-related sex differences. Evidence from trials also showed sex- and/or gender-specific responses to lifestyle interventions for preventing cognitive decline, possibly due to differences in brain structure, hormones (sex) and social factors (gender). 31 Olive oil intake may be protective of dementia and related mortality, particularly in women. Nonetheless, we did not observe significant heterogeneity or interaction of cohort by olive oil intake on the risk of fatal dementia. Sex and gender differences should be carefully considered in future studies examining the association or effect of olive oil on cognitive-related outcomes to improve our understanding.
We found that using olive oil instead of margarine and mayonnaise, but not butter and other vegetable oils, was associated with a lower risk of dementia-related death. At the time of the study, margarine and mayonnaise contained considerable levels of hydrogenated trans-fats. The latter were strongly associated with all-cause mortality, CVD, type 2 diabetes, and dementia, 32 , 33 which may explain the lower dementia-related death risk observed when replacing it with olive oil. The US Food and Drug Administration banned manufacturers from adding partially hydrogenated oils to foods in 2020. 34 Future studies examining intake of trans-fat–free margarine will be informative. Although the substitution of butter with olive oil was found to be associated with a lower risk of type 2 diabetes, CVD, and total mortality, 11 we did not find an association with the risk of dementia mortality. Although these previous studies did not examine the associations for butter per se, intake of regular fat dairy products, including cheese, yogurt, and milk, was reported to be either not associated or inversely associated with lower cognitive function, cognitive decline, and dementia. 35 - 37
Our cohort analyses include several strengths, namely the long follow-up period and large sample size with a high number of dementia death cases. Also, we included genotyping of the APOE ε4 allele in a large subsample of participants to reduce potential confounding attributed to this well-known risk factor for Alzheimer disease. Additionally, our repeated diet measurements, weight, and lifestyle variables permitted us to account for long-term olive oil intake and confounding factors. Furthermore, the use of dietary cumulative average updates reduced random measurement error by considering within-person variations in intake.
This study has limitations. The possibility of reverse causation cannot be excluded due to the observational nature of our study. However, the 4-year lagged analysis results, consistent with the primary analysis, suggest that olive oil intake is predictive of dementia mortality rather than a consequence of premorbid dementia. While it is plausible that higher olive oil intake could be indicative of a healthier diet and higher SES, our results remained consistent after accounting for the latter. Despite adjusting for key covariates, residual confounding may remain due to unmeasured factors. Also, our study was conducted among health professionals. While this minimizes the potential confounding effects of socioeconomic factors and likely increases reporting due to a high level of education, this may also limit generalizability. Our population was predominantly of non-Hispanic White participants, limiting generalizability to more diverse populations. Additionally, we could not differentiate among various types of olive oil that differ in their polyphenols and other nonlipid bioactive compounds content.
This study found that in US adults, particularly women, consuming more olive oil was associated with lower risk of dementia-related mortality, regardless of diet quality. Substituting olive oil intake for margarine and mayonnaise was associated with lower risk of dementia mortality and may be a potential strategy to improve longevity free of dementia. These findings extend the current dietary recommendations of choosing olive oil and other vegetable oils to the context of cognitive health and related mortality.
Accepted for Publication: March 6, 2024.
Published: May 6, 2024. doi:10.1001/jamanetworkopen.2024.10021
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Tessier AJ et al. JAMA Network Open .
Corresponding Authors: Anne-Julie Tessier, RD, PhD ( [email protected] ), and Marta Guasch-Ferré, PhD ( [email protected] ), Department of Nutrition, Harvard T.H. Chan School of Public Health, 655 Huntington Ave, Bldg 2, Boston, MA 02115.
Author Contributions: Drs Tessier and Guasch-Ferré had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Tessier, Chavarro, Hu, Willett, Guasch-Ferré.
Acquisition, analysis, or interpretation of data: Tessier, Cortese, Yuan, Bjornevik, Ascherio, Wang, Chavarro, Stampfer, Willett, Guasch-Ferré.
Drafting of the manuscript: Tessier.
Critical review of the manuscript for important intellectual content: Tessier, Cortese, Yuan, Bjornevik, Ascherio, Wang, Chavarro, Stampfer, Hu, Willett, Guasch-Ferré.
Statistical analysis: Tessier, Cortese, Wang, Willett, Guasch-Ferré.
Obtained funding: Chavarro, Stampfer, Hu, Guasch-Ferré.
Administrative, technical, or material support: Cortese, Yuan, Stampfer, Hu.
Supervision: Chavarro, Hu, Guasch-Ferré.
Conflict of Interest Disclosures: Dr Cortese reported a speaker honorarium from Roche outside the submitted work. Dr Ascherio reported receiving speaker honoraria from WebMD, Prada Foundation, Biogen, Moderna, Merck, Roche, and Glaxo-Smith-Kline. No other disclosures were reported.
Funding/Support: This study is supported by the research grant R21 AG070375 from the National Institutes of Health to Dr Guasch-Ferré. The NHS, NHSII and HPFS are supported by grants from the National Institutes of Health (UM1 CA186107, P01 CA87969, U01 CA167552, P30 DK046200, HL034594, HL088521, HL35464, HL60712). Dr Tessier is supported by the Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship Award. Dr Guasch-Ferré is supported the Novo Nordisk Foundation grant NNF23SA0084103.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
COMMENTS
Research from UC Berkeley indicates that ongoing stress caused by protein aggregation is leading to the death of brain cells. Numerous neurodegenerative conditions, including Alzheimer's and Parkinson's, involve the buildup of protein clusters, known as aggregates, within the brain. This phenomenon has prompted researchers to hypothesize that these protein masses are responsible for the ...
November 8, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
Full results about the Alzheimer's disease drug donanemab have been released today, supporting earlier trial results that suggested the breakthrough drug may slow down the progression of the disease.. Dr Richard Oakley, Associate Director of Research and Innovation at Alzheimer's Society, said: "Dementia is the biggest killer in the UK and over 60% of people living with dementia are ...
Anne Trafton. June 27, 2023. A pair of structures in the hypothalamus called the mammillary bodies (highlighted in green) are among the first brain regions to show neurodegeneration in Alzheimer ...
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by ...
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug development, lifestyle interventions ...
A new NIH intramural dementia research center designed to further accelerate a broad range of scientific discovery; FY 24 bypass budget. This year's progress report was preceded by the Fiscal Year 2024 Professional Judgment Budget for Alzheimer's Disease and Related Dementias announced in late July.
Alzheimer's disease (AD) is the most common form of dementia, and accounts for over 60% of cases. Aging is the biggest risk factor for dementia. According to the World Health Organization (WHO ...
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Dementia is one of the greatest health challenges of our generation. "Although dementia is the 7th leading cause of death globally, dementia research accounts for less than 1.5% of total health research output" said Dr Soumya Swaminathan, WHO's Chief Scientist. "Sadly, we are falling behind implementing the Global action plan on the public health response to dementia 2017-25.
We also built a new genetic risk score associated with the risk of future AD/dementia or progression from mild cognitive impairment to AD/dementia. The improvement in prediction led to a 1.6- to 1 ...
According to an NIA-funded study, these brain scan signatures may be combined with demographic, clinical, cognitive, and genetic information to help researchers better understand the underlying factors that influence a person's dementia, as well as improve Alzheimer's research. Results of the study were published in Alzheimer's & Dementia.
We performed a two-site, randomized, controlled trial involving older adults without cognitive impairment but with a family history of dementia, a body-mass index (the weight in kilograms divided ...
They're also studying whether the medicines may reduce the risk of dementia. Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's. Lifestyle choices.
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled ...
University of Utah research professor Donna J. Cross has spent the past 25 years studying brain disorders ... chief motivation for joining Minoshima's team when she started graduate school. Recently, a father-in-law with severe dementia has only heightened her sense of urgency. ... 81 What the New York Times got right and wrong in a new ...
Dementia refers to an array of symptoms characterized by failing short-term memory, confused thinking, and a decline in language skills. Of all the dementias, Alzheimer's disease (AD ...
Dementia is a collective term for a group of diseases or injuries which primarily or secondarily affect the brain. Alzheimer's is the most common of these and accounts for around 60-70% of cases. Other types include vascular dementia, dementia with Lewy bodies (abnormal protein clumps) and a group of diseases that contribute to frontotemporal ...
A new Alzheimer's drug slowed cognitive decline by 35%, according to late-stage trial results, raising the prospect of a second effective treatment for the disease. Donanemab met all goals of ...
Researchers have discovered a new avenue of cell death in Alzheimer's disease and vascular dementia. A new study, led by scientists at Oregon Health & Science University and published online in the journal Annals of Neurology on Aug. 21, reveals for the first time that a form of cell death known as ferroptosis — caused by a buildup of iron in cells — destroys microglia cells, a type of ...
Researchers have discovered a new avenue of cell death in Alzheimer's disease and vascular dementia. A new study, led by scientists at Oregon Health & Science University and published in the ...
Read the latest medical research on dementia. Causes, symptoms, lowering the risks, care, medications and new treatments for dementia.
New data confirm that APOE4 homozygosity is a major genetic cause of Alzheimer's disease, ... Latest Research and Reviews. ... A test for Alzheimer's-disease stage predicts dementia risk.
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. Get information and resources for Alzheimer's and other dementias from the Alzheimer's Association. Call our 24 hours, seven days a week helpline at 800.272.3900.
A new €7.5 million research project involving a team of academics from Queen's University Belfast seeks to improve the care and quality of life for those with advanced dementia living in care homes. People with advanced dementia who live and die in nursing homes experience varying quality of ...
RESEARCH POINTS TO A CAUSE FOR A SUBSET OF ALZHEIMER'S. To better understand the gene's role, Fortea's team used data from 3,297 brains donated for research and from over 10,000 people in U.S. and European Alzheimer's studies. They examined symptoms and early hallmarks of Alzheimer's such as sticky amyloid in the brain.
Alzheimer's disease is a form of dementia, and stories here track the latest dementia research and dementia treatments. Articles include a look at the new Alzheimer's drug Leqembi, the link ...
Ruhr-University Bochum. (2024, May 15). New group training tool for the prevention of dementia. ScienceDaily. Retrieved May 16, 2024 from www.sciencedaily.com / releases / 2024 / 05 / 240515122815.htm
Inheriting two copies of APOE4 linked to risk of Alzheimer's at a younger age, study suggests. A new study confirms that people who carry two copies of an Alzheimer's risk gene, APOE4, have a very high chance of developing Alzheimer's. "You want your loved ones to have the best life possible. We want that too.".
In the NHS and HPFS, a lower risk of neurodegenerative disease mortality, including dementia mortality, was observed with higher olive oil consumption (HR, 0.81 [95% CI, 0.78-0.84]). 11 Evidence that pertains to cognitive decline or incident dementia is more widely available than it is for dementia mortality. 6,22 In the French Three-City Study ...