Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED PATHWAYS
Related topics.
INTRODUCTION
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately.
● (See "Screening for type 2 diabetes mellitus" .)
● (See "Prevention of type 2 diabetes mellitus" and "Type 1 diabetes mellitus: Prevention and disease-modifying therapy" .)
● (See "Classification of diabetes mellitus and genetic diabetic syndromes" .)
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
- What Is Diabetes?
Type 2 Diabetes
- Español
On this page:
What is type 2 diabetes?
Who is more likely to develop type 2 diabetes, what are the symptoms of diabetes, what causes type 2 diabetes, how do health care professionals diagnose type 2 diabetes, how can i manage my type 2 diabetes, what medicines do i need to treat my type 2 diabetes, what health problems can people with diabetes develop, how can i lower my chances of developing type 2 diabetes.
Type 2 diabetes, the most common type of diabetes, is a disease that occurs when your blood glucose, also called blood sugar, is too high. Blood glucose is your main source of energy and comes mainly from the food you eat. Insulin , a hormone made by the pancreas , helps glucose get into your cells to be used for energy. In type 2 diabetes, your body doesn’t make enough insulin or doesn’t use insulin well. Too much glucose then stays in your blood, and not enough reaches your cells.
The good news is that you can take steps to prevent or delay the development of type 2 diabetes.
You can develop type 2 diabetes at any age, even during childhood. However, type 2 diabetes occurs most often in middle-aged and older people. You are more likely to develop type 2 diabetes if you are age 45 or older, have a family history of diabetes, or are overweight or have obesity . Diabetes is more common in people who are African American, Hispanic/Latino, American Indian, Asian American, or Pacific Islander.
Physical inactivity and certain health problems such as high blood pressure affect your chances of developing type 2 diabetes. You are also more likely to develop type 2 diabetes if you have prediabetes or had gestational diabetes when you were pregnant. Learn more about risk factors for type 2 diabetes .

Symptoms of diabetes include
- increased thirst and urination
- increased hunger
- feeling tired
- blurred vision
- numbness or tingling in the feet or hands
- sores that do not heal
- unexplained weight loss
Symptoms of type 2 diabetes often develop slowly—over the course of several years—and can be so mild that you might not even notice them. Many people have no symptoms. Some people do not find out they have the disease until they have diabetes-related health problems, such as blurred vision or heart disease .
Type 2 diabetes is caused by several factors, including
- overweight and obesity
- not being physically active
- insulin resistance
Learn more about the causes of type 2 diabetes .
Your health care professional can diagnose type 2 diabetes based on blood tests. Learn more about blood tests for diabetes and what the results mean.
Managing your blood glucose, blood pressure , and cholesterol , and quitting smoking if you smoke, are important ways to manage your type 2 diabetes . Lifestyle changes that include planning healthy meals, limiting calories if you are overweight, and being physically active are also part of managing your diabetes. So is taking any prescribed medicines. Work with your health care team to create a diabetes care plan that works for you.

Along with following your diabetes care plan, you may need diabetes medicines, which may include pills or medicines you inject under your skin, such as insulin. Over time, you may need more than one diabetes medicine to manage your blood glucose. Even if you don’t take insulin, you may need it at special times, such as during pregnancy or if you are in the hospital. You also may need medicines for high blood pressure, high cholesterol, or other conditions.
Learn more about medicines, insulin, and other diabetes treatments .
Following a good diabetes care plan can help protect against many diabetes-related health problems. However, if not managed, diabetes can lead to problems such as
- heart disease and stroke
- nerve damage
- kidney disease
- foot problems
- eye disease
- gum disease and other dental problems
- sexual and bladder problems
Many people with type 2 diabetes also have nonalcoholic fatty liver disease (NAFLD) . Losing weight if you are overweight or have obesity can improve NAFLD. Diabetes is also linked to other health problems such as sleep apnea , depression, some types of cancer, and dementia .
You can take steps to lower your chances of developing these diabetes-related health problems .
Research such as the Diabetes Prevention Program , sponsored by the National Institutes of Health, has shown that you can take steps to reduce your chances of developing type 2 diabetes if you have risk factors for the disease. Here are some things you can do to lower your risk:
- Lose weight if you are overweight, and keep it off. You may be able to prevent or delay diabetes by losing 5 to 7 percent of your current weight. 1 For instance, if you weigh 200 pounds, your goal would be to lose about 10 to 14 pounds.
- Move more. Get at least 30 minutes of physical activity, such as walking, at least 5 days a week. If you have not been active, talk with your health care professional about which activities are best. Start slowly and build up to your goal.
- Eat healthy foods. Eat smaller portions to reduce the amount of calories you eat each day and help you lose weight. Choosing foods with less fat is another way to reduce calories. Drink water instead of sweetened beverages.
Ask your health care team what other changes you can make to prevent or delay type 2 diabetes.
Most often, your best chance for preventing type 2 diabetes is to make lifestyle changes that work for you long term. Get started with Your Game Plan to Prevent Type 2 Diabetes .
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Rita Basu, M.D., Mayo Clinic
- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is usually diagnosed using the glycated hemoglobin (A1C) test. This blood test indicates your average blood sugar level for the past two to three months. Results are interpreted as follows:
- Below 5.7% is normal.
- 5.7% to 6.4% is diagnosed as prediabetes.
- 6.5% or higher on two separate tests indicates diabetes.
If the A1C test isn't available, or if you have certain conditions that interfere with an A1C test, your health care provider may use the following tests to diagnose diabetes:
Random blood sugar test. Blood sugar values are expressed in milligrams of sugar per deciliter ( mg/dL ) or millimoles of sugar per liter ( mmol/L ) of blood. Regardless of when you last ate, a level of 200 mg/dL (11.1 mmol/L ) or higher suggests diabetes, especially if you also have symptoms of diabetes, such as frequent urination and extreme thirst.
Fasting blood sugar test. A blood sample is taken after you haven't eaten overnight. Results are interpreted as follows:
- Less than 100 mg/dL (5.6 mmol/L ) is considered healthy.
- 100 to 125 mg/dL (5.6 to 6.9 mmol/L ) is diagnosed as prediabetes.
- 126 mg/dL (7 mmol/L ) or higher on two separate tests is diagnosed as diabetes.
Oral glucose tolerance test. This test is less commonly used than the others, except during pregnancy. You'll need to not eat for a certain amount of time and then drink a sugary liquid at your health care provider's office. Blood sugar levels then are tested periodically for two hours. Results are interpreted as follows:
- Less than 140 mg/dL (7.8 mmol/L ) after two hours is considered healthy.
- 140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L ) is diagnosed as prediabetes.
- 200 mg/dL (11.1 mmol/L ) or higher after two hours suggests diabetes.
Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
- People younger than 35 who are overweight or obese and have one or more risk factors associated with diabetes.
- Women who have had gestational diabetes.
- People who have been diagnosed with prediabetes.
- Children who are overweight or obese and who have a family history of type 2 diabetes or other risk factors.
After a diagnosis
If you're diagnosed with diabetes, your health care provider may do other tests to distinguish between type 1 and type 2 diabetes because the two conditions often require different treatments.
Your health care provider will test A1C levels at least two times a year and when there are any changes in treatment. Target A1C goals vary depending on age and other factors. For most people, the American Diabetes Association recommends an A1C level below 7%.
You also receive tests to screen for complications of diabetes and other medical conditions.
More Information
- Glucose tolerance test
Management of type 2 diabetes includes:
- Healthy eating.
- Regular exercise.
- Weight loss.
- Possibly, diabetes medication or insulin therapy.
- Blood sugar monitoring.
These steps make it more likely that blood sugar will stay in a healthy range. And they may help to delay or prevent complications.
Healthy eating
There's no specific diabetes diet. However, it's important to center your diet around:
- A regular schedule for meals and healthy snacks.
- Smaller portion sizes.
- More high-fiber foods, such as fruits, nonstarchy vegetables and whole grains.
- Fewer refined grains, starchy vegetables and sweets.
- Modest servings of low-fat dairy, low-fat meats and fish.
- Healthy cooking oils, such as olive oil or canola oil.
- Fewer calories.
Your health care provider may recommend seeing a registered dietitian, who can help you:
- Identify healthy food choices.
- Plan well-balanced, nutritional meals.
- Develop new habits and address barriers to changing habits.
- Monitor carbohydrate intake to keep your blood sugar levels more stable.
Physical activity
Exercise is important for losing weight or maintaining a healthy weight. It also helps with managing blood sugar. Talk to your health care provider before starting or changing your exercise program to ensure that activities are safe for you.
- Aerobic exercise. Choose an aerobic exercise that you enjoy, such as walking, swimming, biking or running. Adults should aim for 30 minutes or more of moderate aerobic exercise on most days of the week, or at least 150 minutes a week.
- Resistance exercise. Resistance exercise increases your strength, balance and ability to perform activities of daily living more easily. Resistance training includes weightlifting, yoga and calisthenics. Adults living with type 2 diabetes should aim for 2 to 3 sessions of resistance exercise each week.
- Limit inactivity. Breaking up long periods of inactivity, such as sitting at the computer, can help control blood sugar levels. Take a few minutes to stand, walk around or do some light activity every 30 minutes.
Weight loss
Weight loss results in better control of blood sugar levels, cholesterol, triglycerides and blood pressure. If you're overweight, you may begin to see improvements in these factors after losing as little as 5% of your body weight. However, the more weight you lose, the greater the benefit to your health. In some cases, losing up to 15% of body weight may be recommended.
Your health care provider or dietitian can help you set appropriate weight-loss goals and encourage lifestyle changes to help you achieve them.
Monitoring your blood sugar
Your health care provider will advise you on how often to check your blood sugar level to make sure you remain within your target range. You may, for example, need to check it once a day and before or after exercise. If you take insulin, you may need to check your blood sugar multiple times a day.
Monitoring is usually done with a small, at-home device called a blood glucose meter, which measures the amount of sugar in a drop of blood. Keep a record of your measurements to share with your health care team.
Continuous glucose monitoring is an electronic system that records glucose levels every few minutes from a sensor placed under the skin. Information can be transmitted to a mobile device such as a phone, and the system can send alerts when levels are too high or too low.
Diabetes medications
If you can't maintain your target blood sugar level with diet and exercise, your health care provider may prescribe diabetes medications that help lower glucose levels, or your provider may suggest insulin therapy. Medicines for type 2 diabetes include the following.
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively.
Some people experience B-12 deficiency and may need to take supplements. Other possible side effects, which may improve over time, include:
- Abdominal pain.
Sulfonylureas help the body secrete more insulin. Examples include glyburide (DiaBeta, Glynase), glipizide (Glucotrol XL) and glimepiride (Amaryl). Possible side effects include:
- Low blood sugar.
- Weight gain.
Glinides stimulate the pancreas to secrete more insulin. They're faster acting than sulfonylureas. But their effect in the body is shorter. Examples include repaglinide and nateglinide. Possible side effects include:
Thiazolidinediones make the body's tissues more sensitive to insulin. An example of this medicine is pioglitazone (Actos). Possible side effects include:
- Risk of congestive heart failure.
- Risk of bladder cancer (pioglitazone).
- Risk of bone fractures.
DPP-4 inhibitors help reduce blood sugar levels but tend to have a very modest effect. Examples include sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta). Possible side effects include:
- Risk of pancreatitis.
- Joint pain.
GLP-1 receptor agonists are injectable medications that slow digestion and help lower blood sugar levels. Their use is often associated with weight loss, and some may reduce the risk of heart attack and stroke. Examples include exenatide (Byetta, Bydureon Bcise), liraglutide (Saxenda, Victoza) and semaglutide (Rybelsus, Ozempic, Wegovy). Possible side effects include:
SGLT2 inhibitors affect the blood-filtering functions in the kidneys by blocking the return of glucose to the bloodstream. As a result, glucose is removed in the urine. These medicines may reduce the risk of heart attack and stroke in people with a high risk of those conditions. Examples include canagliflozin (Invokana), dapagliflozin (Farxiga) and empagliflozin (Jardiance). Possible side effects include:
- Vaginal yeast infections.
- Urinary tract infections.
- Low blood pressure.
- High cholesterol.
- Risk of gangrene.
- Risk of bone fractures (canagliflozin).
- Risk of amputation (canagliflozin).
Other medicines your health care provider might prescribe in addition to diabetes medications include blood pressure and cholesterol-lowering medicines, as well as low-dose aspirin, to help prevent heart and blood vessel disease.
Insulin therapy
Some people who have type 2 diabetes need insulin therapy. In the past, insulin therapy was used as a last resort, but today it may be prescribed sooner if blood sugar targets aren't met with lifestyle changes and other medicines.
Different types of insulin vary on how quickly they begin to work and how long they have an effect. Long-acting insulin, for example, is designed to work overnight or throughout the day to keep blood sugar levels stable. Short-acting insulin generally is used at mealtime.
Your health care provider will determine what type of insulin is right for you and when you should take it. Your insulin type, dosage and schedule may change depending on how stable your blood sugar levels are. Most types of insulin are taken by injection.
Side effects of insulin include the risk of low blood sugar — a condition called hypoglycemia — diabetic ketoacidosis and high triglycerides.
Weight-loss surgery
Weight-loss surgery changes the shape and function of the digestive system. This surgery may help you lose weight and manage type 2 diabetes and other conditions related to obesity. There are several surgical procedures. All of them help people lose weight by limiting how much food they can eat. Some procedures also limit the amount of nutrients the body can absorb.
Weight-loss surgery is only one part of an overall treatment plan. Treatment also includes diet and nutritional supplement guidelines, exercise and mental health care.
Generally, weight-loss surgery may be an option for adults living with type 2 diabetes who have a body mass index (BMI) of 35 or higher. BMI is a formula that uses weight and height to estimate body fat. Depending on the severity of diabetes or the presence of other medical conditions, surgery may be an option for someone with a BMI lower than 35.
Weight-loss surgery requires a lifelong commitment to lifestyle changes. Long-term side effects may include nutritional deficiencies and osteoporosis.
People living with type 2 diabetes often need to change their treatment plan during pregnancy and follow a diet that controls carbohydrates. Many people need insulin therapy during pregnancy. They also may need to stop other treatments, such as blood pressure medicines.
There is an increased risk during pregnancy of developing a condition that affects the eyes called diabetic retinopathy. In some cases, this condition may get worse during pregnancy. If you are pregnant, visit an ophthalmologist during each trimester of your pregnancy and one year after you give birth. Or as often as your health care provider suggests.
Signs of trouble
Regularly monitoring your blood sugar levels is important to avoid severe complications. Also, be aware of symptoms that may suggest irregular blood sugar levels and the need for immediate care:
High blood sugar. This condition also is called hyperglycemia. Eating certain foods or too much food, being sick, or not taking medications at the right time can cause high blood sugar. Symptoms include:
- Frequent urination.
- Increased thirst.
- Blurred vision.
Hyperglycemic hyperosmolar nonketotic syndrome (HHNS). This life-threatening condition includes a blood sugar reading higher than 600 mg/dL (33.3 mmol/L ). HHNS may be more likely if you have an infection, are not taking medicines as prescribed, or take certain steroids or drugs that cause frequent urination. Symptoms include:
- Extreme thirst.
- Drowsiness.
- Dark urine.
Diabetic ketoacidosis. Diabetic ketoacidosis occurs when a lack of insulin results in the body breaking down fat for fuel rather than sugar. This results in a buildup of acids called ketones in the bloodstream. Triggers of diabetic ketoacidosis include certain illnesses, pregnancy, trauma and medicines — including the diabetes medicines called SGLT2 inhibitors.
The toxicity of the acids made by diabetic ketoacidosis can be life-threatening. In addition to the symptoms of hyperglycemia, such as frequent urination and increased thirst, ketoacidosis may cause:
- Shortness of breath.
- Fruity-smelling breath.
Low blood sugar. If your blood sugar level drops below your target range, it's known as low blood sugar. This condition also is called hypoglycemia. Your blood sugar level can drop for many reasons, including skipping a meal, unintentionally taking more medication than usual or being more physically active than usual. Symptoms include:
- Irritability.
- Heart palpitations.
- Slurred speech.
If you have symptoms of low blood sugar, drink or eat something that will quickly raise your blood sugar level. Examples include fruit juice, glucose tablets, hard candy or another source of sugar. Retest your blood in 15 minutes. If levels are not at your target, eat or drink another source of sugar. Eat a meal after your blood sugar level returns to normal.
If you lose consciousness, you need to be given an emergency injection of glucagon, a hormone that stimulates the release of sugar into the blood.
- Medications for type 2 diabetes
- GLP-1 agonists: Diabetes drugs and weight loss
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 2 diabetes can reduce the risk of serious — even life-threatening — complications. Consider these tips:
- Commit to managing your diabetes. Learn all you can about type 2 diabetes. Make healthy eating and physical activity part of your daily routine.
- Work with your team. Establish a relationship with a certified diabetes education specialist, and ask your diabetes treatment team for help when you need it.
- Identify yourself. Wear a necklace or bracelet that says you are living with diabetes, especially if you take insulin or other blood sugar-lowering medicine.
- Schedule a yearly physical exam and regular eye exams. Your diabetes checkups aren't meant to replace regular physicals or routine eye exams.
- Keep your vaccinations up to date. High blood sugar can weaken your immune system. Get a flu shot every year. Your health care provider also may recommend the pneumonia vaccine. The Centers for Disease Control and Prevention (CDC) also recommends the hepatitis B vaccination if you haven't previously received this vaccine and you're 19 to 59 years old. Talk to your health care provider about other vaccinations you may need.
- Take care of your teeth. Diabetes may leave you prone to more-serious gum infections. Brush and floss your teeth regularly and schedule recommended dental exams. Contact your dentist right away if your gums bleed or look red or swollen.
- Pay attention to your feet. Wash your feet daily in lukewarm water, dry them gently, especially between the toes, and moisturize them with lotion. Check your feet every day for blisters, cuts, sores, redness and swelling. Contact your health care provider if you have a sore or other foot problem that isn't healing.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can go a long way toward controlling high blood pressure and cholesterol. Take medication as prescribed.
- If you smoke or use other types of tobacco, ask your health care provider to help you quit. Smoking increases your risk of diabetes complications. Talk to your health care provider about ways to stop using tobacco.
- Use alcohol sparingly. Depending on the type of drink, alcohol may lower or raise blood sugar levels. If you choose to drink alcohol, only do so with a meal. The recommendation is no more than one drink daily for women and no more than two drinks daily for men. Check your blood sugar frequently after drinking alcohol.
- Make healthy sleep a priority. Many people with type 2 diabetes have sleep problems. And not getting enough sleep may make it harder to keep blood sugar levels in a healthy range. If you have trouble sleeping, talk to your health care provider about treatment options.
- Caffeine: Does it affect blood sugar?
Alternative medicine
Many alternative medicine treatments claim to help people living with diabetes. According to the National Center for Complementary and Integrative Health, studies haven't provided enough evidence to recommend any alternative therapies for blood sugar management. Research has shown the following results about popular supplements for type 2 diabetes:
- Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions.
- Magnesium supplements have shown benefits for blood sugar control in some but not all studies. Side effects include diarrhea and cramping. Very large doses — more than 5,000 mg a day — can be fatal.
- Cinnamon, in some studies, has lowered fasting glucose levels but not A1C levels. Therefore, there's no evidence of overall improved glucose management.
Talk to your health care provider before starting a dietary supplement or natural remedy. Do not replace your prescribed diabetes medicines with alternative medicines.
Coping and support
Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network.
Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the lifestyle changes and stress that come with a type 2 diabetes diagnosis.
Support groups can be good sources of diabetes education, emotional support and helpful information, such as how to find local resources or where to find carbohydrate counts for a favorite restaurant. If you're interested, your health care provider may be able to recommend a group in your area.
You can visit the American Diabetes Association website to check out local activities and support groups for people living with type 2 diabetes. The American Diabetes Association also offers online information and online forums where you can chat with others who are living with diabetes. You also can call the organization at 800-DIABETES ( 800-342-2383 ).
Preparing for your appointment
At your annual wellness visit, your health care provider can screen for diabetes and monitor and treat conditions that increase your risk of diabetes, such as high blood pressure, high cholesterol or a high BMI .
If you are seeing your health care provider because of symptoms that may be related to diabetes, you can prepare for your appointment by being ready to answer the following questions:
- When did your symptoms begin?
- Does anything improve the symptoms or worsen the symptoms?
- What medicines do you take regularly, including dietary supplements and herbal remedies?
- What are your typical daily meals? Do you eat between meals or before bedtime?
- How much alcohol do you drink?
- How much daily exercise do you get?
- Is there a history of diabetes in your family?
If you are diagnosed with diabetes, your health care provider may begin a treatment plan. Or you may be referred to a doctor who specializes in hormonal disorders, called an endocrinologist. Your care team also may include the following specialists:
- Certified diabetes education specialist.
- Foot doctor, also called a podiatrist.
- Doctor who specializes in eye care, called an ophthalmologist.
Talk to your health care provider about referrals to other specialists who may be providing care.
Questions for ongoing appointments
Before any appointment with a member of your treatment team, make sure you know whether there are any restrictions, such as not eating or drinking before taking a test. Questions that you should regularly talk about with your health care provider or other members of the team include:
- How often do I need to monitor my blood sugar, and what is my target range?
- What changes in my diet would help me better manage my blood sugar?
- What is the right dosage for prescribed medications?
- When do I take the medications? Do I take them with food?
- How does management of diabetes affect treatment for other conditions? How can I better coordinate treatments or care?
- When do I need to make a follow-up appointment?
- Under what conditions should I call you or seek emergency care?
- Are there brochures or online sources you recommend?
- Are there resources available if I'm having trouble paying for diabetes supplies?
What to expect from your doctor
Your health care provider is likely to ask you questions at your appointments. Those questions may include:
- Do you understand your treatment plan and feel confident you can follow it?
- How are you coping with diabetes?
- Have you had any low blood sugar?
- Do you know what to do if your blood sugar is too low or too high?
- What's a typical day's diet like?
- Are you exercising? If so, what type of exercise? How often?
- Do you sit for long periods of time?
- What challenges are you experiencing in managing your diabetes?
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Diabetes prevention: 5 tips for taking control
- Hyperinsulinemia: Is it diabetes?
Associated Procedures
News from mayo clinic.
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
- Mayo Clinic Q and A: Diabetes risk in Hispanic people Oct. 20, 2022, 12:15 p.m. CDT
- The importance of diagnosing, treating diabetes in the Hispanic population in the US Sept. 28, 2022, 04:00 p.m. CDT
- Mayo Clinic Minute: Managing Type 2 diabetes Sept. 28, 2022, 02:30 p.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Are You Eligible to Join the National DPP Lifestyle Change Program?
- Information for Employers and Insurers
- Testimonials From Participants
- About the National Diabetes Prevention Program
- Conversation Tips
- How Pharmacists Can Participate
- Resources for Referring Patients to the Lifestyle Change Program
- Becoming a Lifestyle Change Program Provider
- Lifestyle Change Program Provider
About Prediabetes and Type 2 Diabetes
- Certain factors increase your risk for prediabetes and type 2 diabetes.
- Type 2 diabetes is common, is serious, and with lifestyle changes can be prevented or delayed.
- The National Diabetes Prevention Program (National DPP) lifestyle change program can help.

What are prediabetes and diabetes?
Having prediabetes means your blood glucose (sugar) levels are higher than normal, but not high enough to be diagnosed as diabetes. Prediabetes can lead to heart disease, stroke, and type 2 diabetes, the most common form of diabetes. Prediabetes can often be reversed.
Take the test
In the United States, about 1 in 3 adults has prediabetes, and more than 8 in 10 of them don't know they have it. Without taking action, many people with prediabetes could develop type 2 diabetes within 5 years.
With type 2 diabetes, your body can't effectively use insulin (a hormone that helps glucose get into the cells of the body). You can get type 2 diabetes at any age, but certain factors increase your risk.
If you have prediabetes, the National DPP lifestyle change program is one of the most effective ways to prevent type 2 diabetes. In the program, you'll learn skills to help you lose weight, become more active, and improve your overall health.
Type 2 diabetes has serious consequences
Diabetes is serious .
Diabetes increases the risk of serious health problems, including:
- Heart attack
- Kidney failure
- Loss of toes, feet, or legs
Diabetes is costly
In 2022, the total cost of care for people with diagnosed diabetes was $413 billion. About 1 in 4 health care dollars is spent on people with diagnosed diabetes. Most expenses are related to hospitalizations and medicines used to treat diabetes complications.
- Albright A, Gregg EW. Preventing type 2 diabetes in communities across the US: the National Diabetes Prevention Program. Am J Prev Med 2013;44(4):S346-S351. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539613/
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. (2010). Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr . 2010;8:29. http://www.pophealthmetrics.com/content/8/1/29 .
- Knowler WC, Barrett-Conner E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. https://www.nejm.org/doi/full/10.1056/NEJMoa012512#t=articleTop .
National Diabetes Prevention Program
The National Diabetes Prevention Program is building a nationwide network for its lifestyle change program, which is proven to cut type 2 diabetes risk in half.
For Everyone
Health care providers, public health.

- Previous Article
- Next Article
Classification
Diagnostic tests for diabetes, type 1 diabetes, prediabetes and type 2 diabetes, cystic fibrosis–related diabetes, posttransplantation diabetes mellitus, monogenic diabetes syndromes, pancreatic diabetes or diabetes in the context of disease of the exocrine pancreas, gestational diabetes mellitus, 2. classification and diagnosis of diabetes: standards of medical care in diabetes—2021.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
American Diabetes Association; 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 1 January 2021; 44 (Supplement_1): S15–S33. https://doi.org/10.2337/dc21-S002
Download citation file:
- Ris (Zotero)
- Reference Manager
The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee ( https://doi.org/10.2337/dc21-SPPC ), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations, please refer to the Standards of Care Introduction ( https://doi.org/10.2337/dc21-SINT ). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC .
Diabetes can be classified into the following general categories:
Type 1 diabetes (due to autoimmune β-cell destruction, usually leading to absolute insulin deficiency, including latent autoimmune diabetes of adulthood)
Type 2 diabetes (due to a progressive loss of adequate β-cell insulin secretion frequently on the background of insulin resistance)
Specific types of diabetes due to other causes, e.g., monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young), diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis), and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS, or after organ transplantation)
Gestational diabetes mellitus (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation)
This section reviews most common forms of diabetes but is not comprehensive. For additional information, see the American Diabetes Association (ADA) position statement “Diagnosis and Classification of Diabetes Mellitus” ( 1 ).
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis. The traditional paradigms of type 2 diabetes occurring only in adults and type 1 diabetes only in children are no longer accurate, as both diseases occur in both age-groups. Children with type 1 diabetes typically present with the hallmark symptoms of polyuria/polydipsia, and approximately one-third present with diabetic ketoacidosis (DKA) ( 2 ). The onset of type 1 diabetes may be more variable in adults; they may not present with the classic symptoms seen in children and may experience temporary remission from the need for insulin ( 3 – 5 ). Occasionally, patients with type 2 diabetes may present with DKA ( 6 ), particularly ethnic and racial minorities ( 7 ). It is important for the provider to realize that classification of diabetes type is not always straightforward at presentation and that misdiagnosis is common (e.g., adults with type 1 diabetes misdiagnosed as having type 2 diabetes; individuals with maturity-onset diabetes of the young [MODY] misdiagnosed as having type 1 diabetes, etc.). Although difficulties in distinguishing diabetes type may occur in all age-groups at onset, the diagnosis becomes more obvious over time in people with β-cell deficiency.
In both type 1 and type 2 diabetes, various genetic and environmental factors can result in the progressive loss of β-cell mass and/or function that manifests clinically as hyperglycemia. Once hyperglycemia occurs, patients with all forms of diabetes are at risk for developing the same chronic complications, although rates of progression may differ. The identification of individualized therapies for diabetes in the future will require better characterization of the many paths to β-cell demise or dysfunction ( 8 ). Across the globe many groups are working on combining clinical, pathophysiological, and genetic characteristics to more precisely define the subsets of diabetes currently clustered into the type 1 diabetes versus type 2 diabetes nomenclature with the goal of optimizing treatment approaches. Many of these studies show great promise and may soon be incorporated into the diabetes classification system ( 9 ).
Characterization of the underlying pathophysiology is more precisely developed in type 1 diabetes than in type 2 diabetes. It is now clear from studies of first-degree relatives of patients with type 1 diabetes that the persistent presence of two or more islet autoantibodies is a near certain predictor of clinical hyperglycemia and diabetes. The rate of progression is dependent on the age at first detection of autoantibody, number of autoantibodies, autoantibody specificity, and autoantibody titer. Glucose and A1C levels rise well before the clinical onset of diabetes, making diagnosis feasible well before the onset of DKA. Three distinct stages of type 1 diabetes can be identified ( Table 2.1 ) and serve as a framework for future research and regulatory decision-making ( 8 , 10 ). There is debate as to whether slowly progressive autoimmune diabetes with an adult onset should be termed latent autoimmune diabetes in adults (LADA) or type 1 diabetes. The clinical priority is awareness that slow autoimmune β-cell destruction can occur in adults leading to a long duration of marginal insulin secretory capacity. For the purpose of this classification, all forms of diabetes mediated by autoimmune β-cell destruction are included under the rubric of type 1 diabetes. Use of the term LADA is common and acceptable in clinical practice and has the practical impact of heightening awareness of a population of adults likely to develop overt autoimmune β-cell destruction ( 11 ), thus accelerating insulin initiation prior to deterioration of glucose control or development of DKA ( 4 , 12 ).
Staging of type 1 diabetes ( 8 , 10 )
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; 2-h PG, 2-h plasma glucose.
The paths to β-cell demise and dysfunction are less well defined in type 2 diabetes, but deficient β-cell insulin secretion, frequently in the setting of insulin resistance, appears to be the common denominator. Type 2 diabetes is associated with insulin secretory defects related to inflammation and metabolic stress among other contributors, including genetic factors. Future classification schemes for diabetes will likely focus on the pathophysiology of the underlying β-cell dysfunction ( 8 , 9 , 13 – 15 ).
Diabetes may be diagnosed based on plasma glucose criteria, either the fasting plasma glucose (FPG) value or the 2-h plasma glucose (2-h PG) value during a 75-g oral glucose tolerance test (OGTT), or A1C criteria ( 16 ) ( Table 2.2 ).
Criteria for the diagnosis of diabetes
DCCT, Diabetes Control and Complications Trial; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; WHO, World Health Organization; 2-h PG, 2-h plasma glucose.
In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples.
Generally, FPG, 2-h PG during 75-g OGTT, and A1C are equally appropriate for diagnostic screening. It should be noted that the tests do not necessarily detect diabetes in the same individuals. The efficacy of interventions for primary prevention of type 2 diabetes ( 17 , 18 ) has mainly been demonstrated among individuals who have impaired glucose tolerance (IGT) with or without elevated fasting glucose, not for individuals with isolated impaired fasting glucose (IFG) or for those with prediabetes defined by A1C criteria.
The same tests may be used to screen for and diagnose diabetes and to detect individuals with prediabetes ( Table 2.2 and Table 2.5 ) ( 19 ). Diabetes may be identified anywhere along the spectrum of clinical scenarios—in seemingly low-risk individuals who happen to have glucose testing, in individuals tested based on diabetes risk assessment, and in symptomatic patients.
Fasting and 2-Hour Plasma Glucose
The FPG and 2-h PG may be used to diagnose diabetes ( Table 2.2 ). The concordance between the FPG and 2-h PG tests is imperfect, as is the concordance between A1C and either glucose-based test. Compared with FPG and A1C cut points, the 2-h PG value diagnoses more people with prediabetes and diabetes ( 20 ). In people in whom there is discordance between A1C values and glucose values, FPG and 2-h PG are more accurate ( 21 ).
Recommendations
2.1 To avoid misdiagnosis or missed diagnosis, the A1C test should be performed using a method that is certified by the NGSP and standardized to the Diabetes Control and Complications Trial (DCCT) assay. B
2.2 Marked discordance between measured A1C and plasma glucose levels should raise the possibility of A1C assay interference and consideration of using an assay without interference or plasma blood glucose criteria to diagnose diabetes. B
2.3 In conditions associated with an altered relationship between A1C and glycemia, such as hemoglobinopathies including sickle cell disease, pregnancy (second and third trimesters and the postpartum period), glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes. (See other conditions altering the relationship of a1c and glycemia below for more information.) B
The A1C test should be performed using a method that is certified by the NGSP ( www.ngsp.org ) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Although point-of-care A1C assays may be NGSP certified and cleared by the U.S. Food and Drug Administration (FDA) for use in monitoring glycemic control in people with diabetes in both Clinical Laboratory Improvement Amendments (CLIA)-regulated and CLIA-waived settings, only those point-of-care A1C assays that are also cleared by the FDA for use in the diagnosis of diabetes should be used for this purpose, and only in the clinical settings for which they are cleared. As discussed in Section 6 “Glycemic Targets” ( https://doi.org/10.2337/dc21-S006 ), point-of-care A1C assays may be more generally applied for assessment of glycemic control in the clinic.
A1C has several advantages compared with FPG and OGTT, including greater convenience (fasting not required), greater preanalytical stability, and less day-to-day perturbations during stress, changes in diet, or illness. However, these advantages may be offset by the lower sensitivity of A1C at the designated cut point, greater cost, limited availability of A1C testing in certain regions of the developing world, and the imperfect correlation between A1C and average glucose in certain individuals. The A1C test, with a diagnostic threshold of ≥6.5% (48 mmol/mol), diagnoses only 30% of the diabetes cases identified collectively using A1C, FPG, or 2-h PG, according to National Health and Nutrition Examination Survey (NHANES) data ( 22 ).
When using A1C to diagnose diabetes, it is important to recognize that A1C is an indirect measure of average blood glucose levels and to take other factors into consideration that may impact hemoglobin glycation independently of glycemia, such as hemodialysis, pregnancy, HIV treatment ( 23 , 24 ), age, race/ethnicity, pregnancy status, genetic background, and anemia/hemoglobinopathies. (See other conditions altering the relationship of a1c and glycemia below for more information.)
The epidemiologic studies that formed the basis for recommending A1C to diagnose diabetes included only adult populations ( 22 ). However, recent ADA clinical guidance concluded that A1C, FPG, or 2-h PG can be used to test for prediabetes or type 2 diabetes in children and adolescents (see screening and testing for prediabetes and type 2 diabetes in children and adolescents below for additional information) ( 25 ).
Race/Ethnicity/Hemoglobinopathies
Hemoglobin variants can interfere with the measurement of A1C, although most assays in use in the U.S. are unaffected by the most common variants. Marked discrepancies between measured A1C and plasma glucose levels should prompt consideration that the A1C assay may not be reliable for that individual. For patients with a hemoglobin variant but normal red blood cell turnover, such as those with the sickle cell trait, an A1C assay without interference from hemoglobin variants should be used. An updated list of A1C assays with interferences is available at www.ngsp.org/interf.asp .
African Americans heterozygous for the common hemoglobin variant HbS may have, for any given level of mean glycemia, lower A1C by about 0.3% compared with those without the trait ( 26 ). Another genetic variant, X-linked glucose-6-phosphate dehydrogenase G202A, carried by 11% of African Americans, was associated with a decrease in A1C of about 0.8% in homozygous men and 0.7% in homozygous women compared with those without the variant ( 27 ).
Even in the absence of hemoglobin variants, A1C levels may vary with race/ethnicity independently of glycemia ( 28 – 30 ). For example, African Americans may have higher A1C levels than non-Hispanic Whites with similar fasting and postglucose load glucose levels ( 31 ). Though conflicting data exists, African Americans may also have higher levels of fructosamine and glycated albumin and lower levels of 1,5-anhydroglucitol, suggesting that their glycemic burden (particularly postprandially) may be higher ( 32 , 33 ). Similarly, A1C levels may be higher for a given mean glucose concentration when measured with continuous glucose monitoring ( 34 ). Despite these and other reported differences, the association of A1C with risk for complications appears to be similar in African Americans and non-Hispanic Whites ( 35 , 36 ).
Other Conditions Altering the Relationship of A1C and Glycemia
In conditions associated with increased red blood cell turnover, such as sickle cell disease, pregnancy (second and third trimesters), glucose-6-phosphate dehydrogenase deficiency ( 37 , 38 ), hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes ( 39 ). A1C is less reliable than blood glucose measurement in other conditions such as the postpartum state ( 40 – 42 ), HIV treated with certain protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs) ( 23 ), and iron-deficient anemia ( 43 ).
Confirming the Diagnosis
Unless there is a clear clinical diagnosis (e.g., patient in a hyperglycemic crisis or with classic symptoms of hyperglycemia and a random plasma glucose ≥200 mg/dL [11.1 mmol/L]), diagnosis requires two abnormal test results, either from the same sample ( 44 ) or in two separate test samples. If using two separate test samples, it is recommended that the second test, which may either be a repeat of the initial test or a different test, be performed without delay. For example, if the A1C is 7.0% (53 mmol/mol) and a repeat result is 6.8% (51 mmol/mol), the diagnosis of diabetes is confirmed. If two different tests (such as A1C and FPG) are both above the diagnostic threshold when analyzed from the same sample or in two different test samples, this also confirms the diagnosis. On the other hand, if a patient has discordant results from two different tests, then the test result that is above the diagnostic cut point should be repeated, with careful consideration of the possibility of A1C assay interference. The diagnosis is made on the basis of the confirmed test. For example, if a patient meets the diabetes criterion of the A1C (two results ≥6.5% [48 mmol/mol]) but not FPG (<126 mg/dL [7.0 mmol/L]), that person should nevertheless be considered to have diabetes.
Each of the tests has preanalytic and analytic variability, so it is possible that a test yielding an abnormal result (i.e., above the diagnostic threshold), when repeated, will produce a value below the diagnostic cut point. This scenario is likely for FPG and 2-h PG if the glucose samples remain at room temperature and are not centrifuged promptly. Because of the potential for preanalytic variability, it is critical that samples for plasma glucose be spun and separated immediately after they are drawn. If patients have test results near the margins of the diagnostic threshold, the health care professional should discuss signs and symptoms with the patient and repeat the test in 3 – 6 months.
In a patient with classic symptoms, measurement of plasma glucose is sufficient to diagnose diabetes (symptoms of hyperglycemia or hyperglycemic crisis plus a random plasma glucose ≥200 mg/dL [11.1 mmol/L]). In these cases, knowing the plasma glucose level is critical because, in addition to confirming that symptoms are due to diabetes, it will inform management decisions. Some providers may also want to know the A1C to determine the chronicity of the hyperglycemia. The criteria to diagnose diabetes are listed in Table 2.2 .
2.4 Screening for type 1 diabetes risk with a panel of islet autoantibodies is currently recommended in the setting of a research trial or can be offered as an option for first-degree family members of a proband with type 1 diabetes. B
2.5 Persistence of autoantibodies is a risk factor for clinical diabetes and may serve as an indication for intervention in the setting of a clinical trial. B
Immune-Mediated Diabetes
This form, previously called “insulin-dependent diabetes” or “juvenile-onset diabetes,” accounts for 5 – 10% of diabetes and is due to cellular-mediated autoimmune destruction of the pancreatic β-cells. Autoimmune markers include islet cell autoantibodies and autoantibodies to GAD (GAD65), insulin, the tyrosine phosphatases IA-2 and IA-2β, and zinc transporter 8 (ZnT8). Numerous clinical studies are being conducted to test various methods of preventing type 1 diabetes in those with evidence of islet autoimmunity ( www.clinicaltrials.gov and www.trialnet.org/our-research/prevention-studies ) ( 12 , 45 – 49 ). Stage 1 of type 1 diabetes is defined by the presence of two or more of these autoimmune markers. The disease has strong HLA associations, with linkage to the DQA and DQB genes. These HLA-DR/DQ alleles can be either predisposing or protective ( Table 2.1 ). There are important genetic considerations, as most of the mutations that cause diabetes are dominantly inherited. The importance of genetic testing is in the genetic counseling that follows. Some mutations are associated with other conditions, which then may prompt additional screenings.
The rate of β-cell destruction is quite variable, being rapid in some individuals (mainly infants and children) and slow in others (mainly adults) ( 50 ). Children and adolescents may present with DKA as the first manifestation of the disease. Others have modest fasting hyperglycemia that can rapidly change to severe hyperglycemia and/or DKA with infection or other stress. Adults may retain sufficient β-cell function to prevent DKA for many years; such individuals may have remission or decreased insulin needs for months or years and eventually become dependent on insulin for survival and are at risk for DKA ( 3 – 5 , 51 , 52 ). At this latter stage of the disease, there is little or no insulin secretion, as manifested by low or undetectable levels of plasma C-peptide. Immune-mediated diabetes is the most common form of diabetes in childhood and adolescence, but it can occur at any age, even in the 8th and 9th decades of life.
Autoimmune destruction of β-cells has multiple genetic predispositions and is also related to environmental factors that are still poorly defined. Although patients are not typically obese when they present with type 1 diabetes, obesity is increasingly common in the general population, and there is evidence that it may also be a risk factor for type 1 diabetes. As such, obesity should not preclude the diagnosis. People with type 1 diabetes are also prone to other autoimmune disorders such as Hashimoto thyroiditis, Graves disease, celiac disease, Addison disease, vitiligo, autoimmune hepatitis, myasthenia gravis, and pernicious anemia (see Section 4 “Comprehensive Medical Evaluation and Assessment of Comorbidities,” https://doi.org/10.2337/dc21-S004 ).
Idiopathic Type 1 Diabetes
Some forms of type 1 diabetes have no known etiologies. These patients have permanent insulinopenia and are prone to DKA but have no evidence of β-cell autoimmunity. However, only a minority of patients with type 1 diabetes fall into this category. Individuals with autoantibody-negative type 1 diabetes of African or Asian ancestry may suffer from episodic DKA and exhibit varying degrees of insulin deficiency between episodes (possibly ketosis-prone diabetes). This form of diabetes is strongly inherited and is not HLA associated. An absolute requirement for insulin replacement therapy in affected patients may be intermittent. Future research is needed to determine the cause of β-cell destruction in this rare clinical scenario.
Screening for Type 1 Diabetes Risk
The incidence and prevalence of type 1 diabetes is increasing ( 53 ). Patients with type 1 diabetes often present with acute symptoms of diabetes and markedly elevated blood glucose levels, and approximately one-third are diagnosed with life-threatening DKA ( 2 ). Multiple studies indicate that measuring islet autoantibodies in individuals genetically at risk for type 1 diabetes (e.g., relatives of those with type 1 diabetes or individuals from the general population with type 1 diabetes–associated genetic factors) identifies individuals who may develop type 1 diabetes ( 10 ). Such testing, coupled with education about diabetes symptoms and close follow-up, may enable earlier identification of type 1 diabetes onset. A study reported the risk of progression to type 1 diabetes from the time of seroconversion to autoantibody positivity in three pediatric cohorts from Finland, Germany, and the U.S. Of the 585 children who developed more than two autoantibodies, nearly 70% developed type 1 diabetes within 10 years and 84% within 15 years ( 45 ). These findings are highly significant because while the German group was recruited from offspring of parents with type 1 diabetes, the Finnish and American groups were recruited from the general population. Remarkably, the findings in all three groups were the same, suggesting that the same sequence of events led to clinical disease in both “sporadic” and familial cases of type 1 diabetes. Indeed, the risk of type 1 diabetes increases as the number of relevant autoantibodies detected increases ( 48 , 54 , 55 ). In The Environmental Determinants of Diabetes in the Young (TEDDY) study, type 1 diabetes developed in 21% of 363 subjects with at least one autoantibody at 3 years of age ( 56 ).
There is currently a lack of accepted and clinically validated screening programs outside of the research setting; thus, widespread clinical testing of asymptomatic low-risk individuals is not currently recommended due to lack of approved therapeutic interventions. However, one should consider referring relatives of those with type 1 diabetes for islet autoantibody testing for risk assessment in the setting of a clinical research study (see www.trialnet.org ). Individuals who test positive should be counseled about the risk of developing diabetes, diabetes symptoms, and DKA prevention. Numerous clinical studies are being conducted to test various methods of preventing and treating stage 2 type 1 diabetes in those with evidence of autoimmunity with promising results (see www.clinicaltrials.gov and www.trialnet.org ).
2.6 Screening for prediabetes and type 2 diabetes with an informal assessment of risk factors or validated tools should be considered in asymptomatic adults. B
2.7 Testing for prediabetes and/or type 2 diabetes in asymptomatic people should be considered in adults of any age with overweight or obesity (BMI ≥25 kg/m 2 or ≥23 kg/m 2 in Asian Americans) and who have one or more additional risk factors for diabetes ( Table 2.3 ). B
2.8 Testing for prediabetes and/or type 2 diabetes should be considered in women with overweight or obesity planning pregnancy and/or who have one or more additional risk factor for diabetes ( Table 2.3 ). C
2.9 For all people, testing should begin at age 45 years. B
2.10 If tests are normal, repeat testing carried out at a minimum of 3-year intervals is reasonable, sooner with symptoms. C
2.11 To test for prediabetes and type 2 diabetes, fasting plasma glucose, 2-h plasma glucose during 75-g oral glucose tolerance test, and A1C are equally appropriate ( Table 2.2 and Table 2.5 ). B
2.12 In patients with prediabetes and type 2 diabetes, identify and treat other cardiovascular disease risk factors. A
2.13 Risk-based screening for prediabetes and/or type 2 diabetes should be considered after the onset of puberty or after 10 years of age, whichever occurs earlier, in children and adolescents with overweight (BMI ≥85th percentile) or obesity (BMI ≥95th percentile) and who have one or more risk factor for diabetes. (See Table 2.4 for evidence grading of risk factors.) B
2.14 Patients with HIV should be screened for diabetes and prediabetes with a fasting glucose test before starting antiretroviral therapy, at the time of switching antiretroviral therapy, and 3−6 months after starting or switching antiretroviral therapy. If initial screening results are normal, fasting glucose should be checked annually. E
Criteria for testing for diabetes or prediabetes in asymptomatic adults
CVD, cardiovascular disease; GDM, gestational diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
Risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 202 )
GDM, gestational diabetes mellitus.
After the onset of puberty or after 10 years of age, whichever occurs earlier. If tests are normal, repeat testing at a minimum of 3-year intervals (or more frequently if BMI is increasing or risk factor profile deteriorating) is recommended. Reports of type 2 diabetes before age 10 years exist, and this can be considered with numerous risk factors.
Prediabetes
“Prediabetes” is the term used for individuals whose glucose levels do not meet the criteria for diabetes but are too high to be considered normal ( 35 , 36 ). Patients with prediabetes are defined by the presence of IFG and/or IGT and/or A1C 5.7 – 6.4% (39 – 47 mmol/mol) ( Table 2.5 ). Prediabetes should not be viewed as a clinical entity in its own right but rather as an increased risk for diabetes and cardiovascular disease (CVD). Criteria for testing for diabetes or prediabetes in asymptomatic adults is outlined in Table 2.3 . Prediabetes is associated with obesity (especially abdominal or visceral obesity), dyslipidemia with high triglycerides and/or low HDL cholesterol, and hypertension.
Criteria defining prediabetes *
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; 2-h PG, 2-h plasma glucose.
For all three tests, risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at the higher end of the range.
IFG is defined as FPG levels from 100 to 125 mg/dL (from 5.6 to 6.9 mmol/L) ( 57 , 58 ) and IGT as 2-h PG during 75-g OGTT levels from 140 to 199 mg/dL (from 7.8 to 11.0 mmol/L) ( 59 ). It should be noted that the World Health Organization (WHO) and numerous other diabetes organizations define the IFG cutoff at 110 mg/dL (6.1 mmol/L).
As with the glucose measures, several prospective studies that used A1C to predict the progression to diabetes as defined by A1C criteria demonstrated a strong, continuous association between A1C and subsequent diabetes. In a systematic review of 44,203 individuals from 16 cohort studies with a follow-up interval averaging 5.6 years (range 2.8 – 12 years), those with A1C between 5.5% and 6.0% (between 37 and 42 mmol/mol) had a substantially increased risk of diabetes (5-year incidence from 9% to 25%). Those with an A1C range of 6.0–6.5% (42 – 48 mmol/mol) had a 5-year risk of developing diabetes between 25% and 50% and a relative risk 20 times higher compared with A1C of 5.0% (31 mmol/mol) ( 60 ). In a community-based study of African American and non-Hispanic White adults without diabetes, baseline A1C was a stronger predictor of subsequent diabetes and cardiovascular events than fasting glucose ( 61 ). Other analyses suggest that A1C of 5.7% (39 mmol/mol) or higher is associated with a diabetes risk similar to that of the high-risk participants in the Diabetes Prevention Program (DPP) ( 62 ), and A1C at baseline was a strong predictor of the development of glucose-defined diabetes during the DPP and its follow-up ( 63 ). Hence, it is reasonable to consider an A1C range of 5.7 – 6.4% (39 – 47 mmol/mol) as identifying individuals with prediabetes. Similar to those with IFG and/or IGT, individuals with A1C of 5.7 – 6.4% (39 – 47 mmol/mol) should be informed of their increased risk for diabetes and CVD and counseled about effective strategies to lower their risks (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ). Similar to glucose measurements, the continuum of risk is curvilinear, so as A1C rises, the diabetes risk rises disproportionately ( 60 ). Aggressive interventions and vigilant follow-up should be pursued for those considered at very high risk (e.g., those with A1C >6.0% [42 mmol/mol]).
Table 2.5 summarizes the categories of prediabetes and Table 2.3 the criteria for prediabetes testing. The ADA diabetes risk test is an additional option for assessment to determine the appropriateness of testing for diabetes or prediabetes in asymptomatic adults ( Fig. 2.1 ) ( diabetes.org/socrisktest ). For additional background regarding risk factors and screening for prediabetes, see screening and testing for prediabetes and type 2 diabetes in asymptomatic adults and also screening and testing for prediabetes and type 2 diabetes in children and adolescents below.

ADA risk test ( diabetes.org/socrisktest ).
Type 2 Diabetes
Type 2 diabetes, previously referred to as “noninsulin-dependent diabetes” or “adult-onset diabetes,” accounts for 90 – 95% of all diabetes. This form encompasses individuals who have relative (rather than absolute) insulin deficiency and have peripheral insulin resistance. At least initially, and often throughout their lifetime, these individuals may not need insulin treatment to survive.
There are various causes of type 2 diabetes. Although the specific etiologies are not known, autoimmune destruction of β-cells does not occur, and patients do not have any of the other known causes of diabetes. Most, but not all, patients with type 2 diabetes have overweight or obesity. Excess weight itself causes some degree of insulin resistance. Patients who do not have obesity or overweight by traditional weight criteria may have an increased percentage of body fat distributed predominantly in the abdominal region.
DKA seldom occurs spontaneously in type 2 diabetes; when seen, it usually arises in association with the stress of another illness such as infection, myocardial infarction, or with the use of certain drugs (e.g., corticosteroids, atypical antipsychotics, and sodium–glucose cotransporter 2 inhibitors) ( 64 , 65 ). Type 2 diabetes frequently goes undiagnosed for many years because hyperglycemia develops gradually and, at earlier stages, is often not severe enough for the patient to notice the classic diabetes symptoms caused by hyperglycemia. Nevertheless, even undiagnosed patients are at increased risk of developing macrovascular and microvascular complications.
Patients with type 2 diabetes may have insulin levels that appear normal or elevated, yet the failure to normalize blood glucose reflects a relative defect in glucose-stimulated insulin secretion. Thus, insulin secretion is defective in these patients and insufficient to compensate for insulin resistance. Insulin resistance may improve with weight reduction, exercise, and/or pharmacologic treatment of hyperglycemia but is seldom restored to normal. Recent interventions with intensive diet and exercise or surgical weight loss have led to diabetes remission ( 66 – 72 ) (see Section 8 “Obesity Management for the Treatment of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S008 ).
The risk of developing type 2 diabetes increases with age, obesity, and lack of physical activity. It occurs more frequently in women with prior gestational diabetes mellitus (GDM), with hypertension or dyslipidemia, with polycystic ovary syndrome, and in certain racial/ethnic subgroups (African American, American Indian, Hispanic/Latino, and Asian American). It is often associated with a strong genetic predisposition or family history in first-degree relatives (more so than type 1 diabetes). However, the genetics of type 2 diabetes is poorly understood and under intense investigation in this era of precision medicine ( 13 ). In adults without traditional risk factors for type 2 diabetes and/or younger age, consider islet autoantibody testing (e.g., GAD65 autoantibodies) to exclude the diagnosis of type 1 diabetes.
Screening and Testing for Prediabetes and Type 2 Diabetes in Asymptomatic Adults
Screening for prediabetes and type 2 diabetes risk through an informal assessment of risk factors ( Table 2.3 ) or with an assessment tool, such as the ADA risk test ( Fig. 2.1 ) (online at diabetes.org/socrisktest ), is recommended to guide providers on whether performing a diagnostic test ( Table 2.2 ) is appropriate. Prediabetes and type 2 diabetes meet criteria for conditions in which early detection via screening is appropriate. Both conditions are common and impose significant clinical and public health burdens. There is often a long presymptomatic phase before the diagnosis of type 2 diabetes. Simple tests to detect preclinical disease are readily available. The duration of glycemic burden is a strong predictor of adverse outcomes. There are effective interventions that prevent progression from prediabetes to diabetes (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ) and reduce the risk of diabetes complications ( 73 ) (see Section 10 “Cardiovascular Disease and Risk Management,” https://doi.org/10.2337/dc21-S010 , and Section 11 “Microvascular Complications and Foot Care,” https://doi.org/10.2337/dc21-S011 ). In the most recent National Institutes of Health (NIH) Diabetes Prevention Program Outcomes Study (DPPOS) report, prevention of progression from prediabetes to diabetes ( 74 ) resulted in lower rates of developing retinopathy and nephropathy ( 75 ). Similar impact on diabetes complications was reported with screening, diagnosis, and comprehensive risk factor management in the U.K. Clinical Practice Research Datalink database ( 73 ). In that report, progression from prediabetes to diabetes augmented risk of complications.
Approximately one-quarter of people with diabetes in the U.S. and nearly half of Asian and Hispanic Americans with diabetes are undiagnosed ( 57 , 58 ). Although screening of asymptomatic individuals to identify those with prediabetes or diabetes might seem reasonable, rigorous clinical trials to prove the effectiveness of such screening have not been conducted and are unlikely to occur. Based on a population estimate, diabetes in women of childbearing age is underdiagnosed ( 76 ). Employing a probabilistic model, Peterson et al. ( 77 ) demonstrated cost and health benefits of preconception screening.
A large European randomized controlled trial compared the impact of screening for diabetes and intensive multifactorial intervention with that of screening and routine care ( 78 ). General practice patients between the ages of 40 and 69 years were screened for diabetes and randomly assigned by practice to intensive treatment of multiple risk factors or routine diabetes care. After 5.3 years of follow-up, CVD risk factors were modestly but significantly improved with intensive treatment compared with routine care, but the incidence of first CVD events or mortality was not significantly different between the groups ( 59 ). The excellent care provided to patients in the routine care group and the lack of an unscreened control arm limited the authors' ability to determine whether screening and early treatment improved outcomes compared with no screening and later treatment after clinical diagnoses. Computer simulation modeling studies suggest that major benefits are likely to accrue from the early diagnosis and treatment of hyperglycemia and cardiovascular risk factors in type 2 diabetes ( 79 ); moreover, screening, beginning at age 30 or 45 years and independent of risk factors, may be cost-effective (<$11,000 per quality-adjusted life year gained—2010 modeling data) ( 80 ). Cost-effectiveness of screening has been reinforced in cohort studies ( 81 , 82 ).
Additional considerations regarding testing for type 2 diabetes and prediabetes in asymptomatic patients include the following.
Age is a major risk factor for diabetes. Testing should begin at no later than age 45 years for all patients. Screening should be considered in adults of any age with overweight or obesity and one or more risk factors for diabetes.
BMI and Ethnicity
In general, BMI ≥25 kg/m 2 is a risk factor for diabetes. However, data suggest that the BMI cut point should be lower for the Asian American population ( 83 , 84 ). The BMI cut points fall consistently between 23 and 24 kg/m 2 (sensitivity of 80%) for nearly all Asian American subgroups (with levels slightly lower for Japanese Americans). This makes a rounded cut point of 23 kg/m 2 practical. An argument can be made to push the BMI cut point to lower than 23 kg/m 2 in favor of increased sensitivity; however, this would lead to an unacceptably low specificity (13.1%). Data from WHO also suggests that a BMI of ≥23 kg/m 2 should be used to define increased risk in Asian Americans ( 85 ). The finding that one-third to one-half of diabetes in Asian Americans is undiagnosed suggests that testing is not occurring at lower BMI thresholds ( 86 , 87 ).
Evidence also suggests that other populations may benefit from lower BMI cut points. For example, in a large multiethnic cohort study, for an equivalent incidence rate of diabetes, a BMI of 30 kg/m 2 in non-Hispanic Whites was equivalent to a BMI of 26 kg/m 2 in African Americans ( 88 ).
Medications
Certain medications, such as glucocorticoids, thiazide diuretics, some HIV medications ( 23 ), and atypical antipsychotics ( 66 ), are known to increase the risk of diabetes and should be considered when deciding whether to screen.
Individuals with HIV are at higher risk for developing prediabetes and diabetes on antiretroviral (ARV) therapies, so a screening protocol is recommended ( 89 ). The A1C test may underestimate glycemia in people with HIV; it is not recommended for diagnosis and may present challenges for monitoring ( 24 ). In those with prediabetes, weight loss through healthy nutrition and physical activity may reduce the progression toward diabetes. Among patients with HIV and diabetes, preventive health care using an approach used in patients without HIV is critical to reduce the risks of microvascular and macrovascular complications. Diabetes risk is increased with certain PIs and NRTIs. New-onset diabetes is estimated to occur in more than 5% of patients infected with HIV on PIs, whereas more than 15% may have prediabetes ( 90 ). PIs are associated with insulin resistance and may also lead to apoptosis of pancreatic β-cells. NRTIs also affect fat distribution (both lipohypertrophy and lipoatrophy), which is associated with insulin resistance. For patients with HIV and ARV-associated hyperglycemia, it may be appropriate to consider discontinuing the problematic ARV agents if safe and effective alternatives are available ( 91 ). Before making ARV substitutions, carefully consider the possible effect on HIV virological control and the potential adverse effects of new ARV agents. In some cases, antihyperglycemic agents may still be necessary.
Testing Interval
The appropriate interval between screening tests is not known ( 92 ). The rationale for the 3-year interval is that with this interval, the number of false-positive tests that require confirmatory testing will be reduced and individuals with false-negative tests will be retested before substantial time elapses and complications develop ( 92 ). In especially high-risk individuals, particularly with weight gain, shorter intervals between screening may be useful.
Community Screening
Ideally, testing should be carried out within a health care setting because of the need for follow-up and treatment. Community screening outside a health care setting is generally not recommended because people with positive tests may not seek, or have access to, appropriate follow-up testing and care. However, in specific situations where an adequate referral system is established beforehand for positive tests, community screening may be considered. Community testing may also be poorly targeted; i.e., it may fail to reach the groups most at risk and inappropriately test those at very low risk or even those who have already been diagnosed ( 93 ).
Screening in Dental Practices
Because periodontal disease is associated with diabetes, the utility of screening in a dental setting and referral to primary care as a means to improve the diagnosis of prediabetes and diabetes has been explored ( 94 – 96 ), with one study estimating that 30% of patients ≥30 years of age seen in general dental practices had dysglycemia ( 96 , 97 ). A similar study in 1,150 dental patients >40 years old in India reported 20.69% and 14.60% meeting criteria for prediabetes and diabetes using random blood glucose. Further research is needed to demonstrate the feasibility, effectiveness, and cost-effectiveness of screening in this setting.
Screening and Testing for Prediabetes and Type 2 Diabetes in Children and Adolescents
In the last decade, the incidence and prevalence of type 2 diabetes in children and adolescents has increased dramatically, especially in racial and ethnic minority populations ( 53 ). See Table 2.4 for recommendations on risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 25 ). See Table 2.2 and Table 2.5 for the criteria for the diagnosis of diabetes and prediabetes, respectively, which apply to children, adolescents, and adults. See Section 13 “Children and Adolescents” ( https://doi.org/10.2337/dc21-S013 ) for additional information on type 2 diabetes in children and adolescents.
Some studies question the validity of A1C in the pediatric population, especially among certain ethnicities, and suggest OGTT or FPG as more suitable diagnostic tests ( 98 ). However, many of these studies do not recognize that diabetes diagnostic criteria are based on long-term health outcomes, and validations are not currently available in the pediatric population ( 99 ). The ADA acknowledges the limited data supporting A1C for diagnosing type 2 diabetes in children and adolescents. Although A1C is not recommended for diagnosis of diabetes in children with cystic fibrosis or symptoms suggestive of acute onset of type 1 diabetes and only A1C assays without interference are appropriate for children with hemoglobinopathies, the ADA continues to recommend A1C for diagnosis of type 2 diabetes in this cohort to decrease barriers to screening ( 100 , 101 ).
2.15 Annual screening for cystic fibrosis–related diabetes (CFRD) with an oral glucose tolerance test should begin by age 10 years in all patients with cystic fibrosis not previously diagnosed with CFRD. B
2.16 A1C is not recommended as a screening test for cystic fibrosis–related diabetes. B
2.17 Patients with cystic fibrosis–related diabetes should be treated with insulin to attain individualized glycemic goals. A
2.18 Beginning 5 years after the diagnosis of cystic fibrosis–related diabetes, annual monitoring for complications of diabetes is recommended. E
Cystic fibrosis–related diabetes (CFRD) is the most common comorbidity in people with cystic fibrosis, occurring in about 20% of adolescents and 40 – 50% of adults ( 102 ). Diabetes in this population, compared with individuals with type 1 or type 2 diabetes, is associated with worse nutritional status, more severe inflammatory lung disease, and greater mortality. Insulin insufficiency is the primary defect in CFRD. Genetically determined β-cell function and insulin resistance associated with infection and inflammation may also contribute to the development of CFRD. Milder abnormalities of glucose tolerance are even more common and occur at earlier ages than CFRD. Whether individuals with IGT should be treated with insulin replacement has not currently been determined. Although screening for diabetes before the age of 10 years can identify risk for progression to CFRD in those with abnormal glucose tolerance, no benefit has been established with respect to weight, height, BMI, or lung function. OGTT is the recommended screening test; however, recent publications suggest that an A1C cut point threshold of 5.5% (5.8% in a second study) would detect more than 90% of cases and reduce patient screening burden ( 103 , 104 ). Ongoing studies are underway to validate this approach. Regardless of age, weight loss or failure of expected weight gain is a risk for CFRD and should prompt screening ( 103 , 104 ). The Cystic Fibrosis Foundation Patient Registry ( 105 ) evaluated 3,553 cystic fibrosis patients and diagnosed 445 (13%) with CFRD. Early diagnosis and treatment of CFRD was associated with preservation of lung function. The European Cystic Fibrosis Society Patient Registry reported an increase in CFRD with age (increased 10% per decade), genotype, decreased lung function, and female sex ( 106 , 107 ). Continuous glucose monitoring or HOMA of β-cell function ( 108 ) may be more sensitive than OGTT to detect risk for progression to CFRD; however, evidence linking these results to long-term outcomes is lacking, and these tests are not recommended for screening outside of the research setting ( 109 ).
CFRD mortality has significantly decreased over time, and the gap in mortality between cystic fibrosis patients with and without diabetes has considerably narrowed ( 110 ). There are limited clinical trial data on therapy for CFRD. The largest study compared three regimens: premeal insulin aspart, repaglinide, or oral placebo in cystic fibrosis patients with diabetes or abnormal glucose tolerance. Participants all had weight loss in the year preceding treatment; however, in the insulin-treated group, this pattern was reversed, and patients gained 0.39 (± 0.21) BMI units (P = 0.02). The repaglinide-treated group had initial weight gain, but this was not sustained by 6 months. The placebo group continued to lose weight ( 110 ). Insulin remains the most widely used therapy for CFRD ( 111 ). The primary rationale for the use of insulin in patients with CFRD is to induce an anabolic state while promoting macronutrient retention and weight gain.
Additional resources for the clinical management of CFRD can be found in the position statement “Clinical Care Guidelines for Cystic Fibrosis–Related Diabetes: A Position Statement of the American Diabetes Association and a Clinical Practice Guideline of the Cystic Fibrosis Foundation, Endorsed by the Pediatric Endocrine Society” ( 112 ) and in the International Society for Pediatric and Adolescent Diabetes's 2014 clinical practice consensus guidelines ( 102 ).
2.19 Patients should be screened after organ transplantation for hyperglycemia, with a formal diagnosis of posttransplantation diabetes mellitus being best made once a patient is stable on an immunosuppressive regimen and in the absence of an acute infection. B
2.20 The oral glucose tolerance test is the preferred test to make a diagnosis of posttransplantation diabetes mellitus. B
2.21 Immunosuppressive regimens shown to provide the best outcomes for patient and graft survival should be used, irrespective of posttransplantation diabetes mellitus risk. E
Several terms are used in the literature to describe the presence of diabetes following organ transplantation ( 113 ). “New-onset diabetes after transplantation” (NODAT) is one such designation that describes individuals who develop new-onset diabetes following transplant. NODAT excludes patients with pretransplant diabetes that was undiagnosed as well as posttransplant hyperglycemia that resolves by the time of discharge ( 114 ). Another term, “posttransplantation diabetes mellitus” (PTDM) ( 114 , 115 ), describes the presence of diabetes in the posttransplant setting irrespective of the timing of diabetes onset.
Hyperglycemia is very common during the early posttransplant period, with ∼90% of kidney allograft recipients exhibiting hyperglycemia in the first few weeks following transplant ( 114 – 117 ). In most cases, such stress- or steroid-induced hyperglycemia resolves by the time of discharge ( 117 , 118 ). Although the use of immunosuppressive therapies is a major contributor to the development of PTDM, the risks of transplant rejection outweigh the risks of PTDM and the role of the diabetes care provider is to treat hyperglycemia appropriately regardless of the type of immunosuppression ( 114 ). Risk factors for PTDM include both general diabetes risks (such as age, family history of diabetes, etc.) as well as transplant-specific factors, such as use of immunosuppressant agents ( 119 ). Whereas posttransplantation hyperglycemia is an important risk factor for subsequent PTDM, a formal diagnosis of PTDM is optimally made once the patient is stable on maintenance immunosuppression and in the absence of acute infection ( 117 – 120 ). In a recent study of 152 heart transplant recipients, 38% had PTDM at 1 year. Risk factors for PTDM included elevated BMI, discharge from the hospital on insulin, and glucose values in the 24 h prior to hospital discharge ( 121 ). In an Iranian cohort, 19% had PTDM after heart and lung transplant ( 122 ). The OGTT is considered the gold standard test for the diagnosis of PTDM (1 year posttransplant) ( 114 , 115 , 123 , 124 ). However, screening patients using fasting glucose and/or A1C can identify high-risk patients requiring further assessment and may reduce the number of overall OGTTs required.
Few randomized controlled studies have reported on the short- and long-term use of antihyperglycemic agents in the setting of PTDM ( 119 , 125 , 126 ). Most studies have reported that transplant patients with hyperglycemia and PTDM after transplantation have higher rates of rejection, infection, and rehospitalization ( 117 , 119 , 127 ). Insulin therapy is the agent of choice for the management of hyperglycemia, PTDM, and preexisting diabetes and diabetes in the hospital setting. After discharge, patients with preexisting diabetes could go back on their pretransplant regimen if they were in good control before transplantation. Those with previously poor control or with persistent hyperglycemia should continue insulin with frequent home self-monitoring of blood glucose to determine when insulin dose reductions may be needed and when it may be appropriate to switch to noninsulin agents.
No studies to date have established which noninsulin agents are safest or most efficacious in PTDM. The choice of agent is usually made based on the side effect profile of the medication and possible interactions with the patient's immunosuppression regimen ( 119 ). Drug dose adjustments may be required because of decreases in the glomerular filtration rate, a relatively common complication in transplant patients. A small short-term pilot study reported that metformin was safe to use in renal transplant recipients ( 128 ), but its safety has not been determined in other types of organ transplant. Thiazolidinediones have been used successfully in patients with liver and kidney transplants, but side effects include fluid retention, heart failure, and osteopenia ( 129 , 130 ). Dipeptidyl peptidase 4 inhibitors do not interact with immunosuppressant drugs and have demonstrated safety in small clinical trials ( 131 , 132 ). Well-designed intervention trials examining the efficacy and safety of these and other antihyperglycemic agents in patients with PTDM are needed.
2.22 All children diagnosed with diabetes in the first 6 months of life should have immediate genetic testing for neonatal diabetes. A
2.23 Children and those diagnosed in early adulthood who have diabetes not characteristic of type 1 or type 2 diabetes that occurs in successive generations (suggestive of an autosomal dominant pattern of inheritance) should have genetic testing for maturity-onset diabetes of the young. A
2.24 In both instances, consultation with a center specializing in diabetes genetics is recommended to understand the significance of these mutations and how best to approach further evaluation, treatment, and genetic counseling. E
Monogenic defects that cause β-cell dysfunction, such as neonatal diabetes and MODY, represent a small fraction of patients with diabetes (<5%). Table 2.6 describes the most common causes of monogenic diabetes. For a comprehensive list of causes, see Genetic Diagnosis of Endocrine Disorders ( 133 ).
Most common causes of monogenic diabetes ( 133 )
AD, autosomal dominant; AR, autosomal recessive; IUGR, intrauterine growth restriction; OGTT, oral glucose tolerance test; UPD6, uniparental disomy of chromosome 6; 2-h PG, 2-h plasma glucose.
Neonatal Diabetes
Diabetes occurring under 6 months of age is termed “neonatal” or “congenital” diabetes, and about 80 – 85% of cases can be found to have an underlying monogenic cause ( 134 – 137 ). Neonatal diabetes occurs much less often after 6 months of age, whereas autoimmune type 1 diabetes rarely occurs before 6 months of age. Neonatal diabetes can either be transient or permanent. Transient diabetes is most often due to overexpression of genes on chromosome 6q24, is recurrent in about half of cases, and may be treatable with medications other than insulin. Permanent neonatal diabetes is most commonly due to autosomal dominant mutations in the genes encoding the Kir6.2 subunit ( KCNJ11 ) and SUR1 subunit ( ABCC8 ) of the β-cell K ATP channel. A recent report details a de novo mutation in EIF2B1 affecting eIF2 signaling associated with permanent neonatal diabetes and hepatic dysfunction, similar to Wolcott-Rallison syndrome but with few severe comorbidities ( 138 ). Correct diagnosis has critical implications because most patients with K ATP -related neonatal diabetes will exhibit improved glycemic control when treated with high-dose oral sulfonylureas instead of insulin. Insulin gene ( INS ) mutations are the second most common cause of permanent neonatal diabetes, and, while intensive insulin management is currently the preferred treatment strategy, there are important genetic counseling considerations, as most of the mutations that cause diabetes are dominantly inherited.
Maturity-Onset Diabetes of the Young
MODY is frequently characterized by onset of hyperglycemia at an early age (classically before age 25 years, although diagnosis may occur at older ages). MODY is characterized by impaired insulin secretion with minimal or no defects in insulin action (in the absence of coexistent obesity). It is inherited in an autosomal dominant pattern with abnormalities in at least 13 genes on different chromosomes identified to date. The most commonly reported forms are GCK-MODY (MODY2), HNF1A-MODY (MODY3), and HNF4A-MODY (MODY1).
For individuals with MODY, the treatment implications are considerable and warrant genetic testing ( 139 , 140 ). Clinically, patients with GCK-MODY exhibit mild, stable fasting hyperglycemia and do not require antihyperglycemic therapy except sometimes during pregnancy. Patients with HNF1A- or HNF4A-MODY usually respond well to low doses of sulfonylureas, which are considered first-line therapy. Mutations or deletions in HNF1B are associated with renal cysts and uterine malformations (renal cysts and diabetes [RCAD] syndrome). Other extremely rare forms of MODY have been reported to involve other transcription factor genes including PDX1 ( IPF1 ) and NEUROD1 .
Diagnosis of Monogenic Diabetes
A diagnosis of one of the three most common forms of MODY, including GCK-MODY, HNF1A-MODY, and HNF4A-MODY, allows for more cost-effective therapy (no therapy for GCK-MODY; sulfonylureas as first-line therapy for HNF1A-MODY and HNF4A-MODY). Additionally, diagnosis can lead to identification of other affected family members. Genetic screening is increasingly available and cost-effective ( 138 , 140 ).
A diagnosis of MODY should be considered in individuals who have atypical diabetes and multiple family members with diabetes not characteristic of type 1 or type 2 diabetes, although admittedly “atypical diabetes” is becoming increasingly difficult to precisely define in the absence of a definitive set of tests for either type of diabetes ( 135 – 137 , 139 – 145 ). In most cases, the presence of autoantibodies for type 1 diabetes precludes further testing for monogenic diabetes, but the presence of autoantibodies in patients with monogenic diabetes has been reported ( 146 ). Individuals in whom monogenic diabetes is suspected should be referred to a specialist for further evaluation if available, and consultation is available from several centers. Readily available commercial genetic testing following the criteria listed below now enables a cost-effective ( 147 ), often cost-saving, genetic diagnosis that is increasingly supported by health insurance. A biomarker screening pathway such as the combination of urinary C-peptide/creatinine ratio and antibody screening may aid in determining who should get genetic testing for MODY ( 148 ). It is critical to correctly diagnose one of the monogenic forms of diabetes because these patients may be incorrectly diagnosed with type 1 or type 2 diabetes, leading to suboptimal, even potentially harmful, treatment regimens and delays in diagnosing other family members ( 149 ). The correct diagnosis is especially critical for those with GCK-MODY mutations where multiple studies have shown that no complications ensue in the absence of glucose-lowering therapy ( 150 ). Genetic counseling is recommended to ensure that affected individuals understand the patterns of inheritance and the importance of a correct diagnosis.
The diagnosis of monogenic diabetes should be considered in children and adults diagnosed with diabetes in early adulthood with the following findings:
Diabetes diagnosed within the first 6 months of life (with occasional cases presenting later, mostly INS and ABCC8 mutations) ( 134 , 151 )
Diabetes without typical features of type 1 or type 2 diabetes (negative diabetes-associated autoantibodies, nonobese, lacking other metabolic features, especially with strong family history of diabetes)
Stable, mild fasting hyperglycemia (100 – 150 mg/dL [5.5 – 8.5 mmol/L]), stable A1C between 5.6% and 7.6% (between 38 and 60 mmol/mol), especially if nonobese
Pancreatic diabetes includes both structural and functional loss of glucose-normalizing insulin secretion in the context of exocrine pancreatic dysfunction and is commonly misdiagnosed as type 2 diabetes. Hyperglycemia due to general pancreatic dysfunction has been called “type 3c diabetes” and, more recently, diabetes in the context of disease of the exocrine pancreas has been termed pancreoprivic diabetes ( 1 ). The diverse set of etiologies includes pancreatitis (acute and chronic), trauma or pancreatectomy, neoplasia, cystic fibrosis (addressed elsewhere in this chapter), hemochromatosis, fibrocalculous pancreatopathy, rare genetic disorders ( 152 ), and idiopathic forms ( 1 ), which is the preferred terminology. A distinguishing feature is concurrent pancreatic exocrine insufficiency (according to the monoclonal fecal elastase 1 test or direct function tests), pathological pancreatic imaging (endoscopic ultrasound, MRI, computed tomography), and absence of type 1 diabetes–associated autoimmunity ( 153 – 157 ). There is loss of both insulin and glucagon secretion and often higher-than-expected insulin requirements. Risk for microvascular complications is similar to other forms of diabetes. In the context of pancreatectomy, islet autotransplantation can be done to retain insulin secretion ( 158 , 159 ). In some cases, autotransplant can lead to insulin independence. In others, it may decrease insulin requirements ( 160 ).
2.25 Test for undiagnosed prediabetes and diabetes at the first prenatal visit in those with risk factors using standard diagnostic criteria. B
2.26 Test for gestational diabetes mellitus at 24 – 28 weeks of gestation in pregnant women not previously found to have diabetes. A
2.27 Test women with gestational diabetes mellitus for prediabetes or diabetes at 4 – 12 weeks postpartum, using the 75-g oral glucose tolerance test and clinically appropriate nonpregnancy diagnostic criteria. B
2.28 Women with a history of gestational diabetes mellitus should have lifelong screening for the development of diabetes or prediabetes at least every 3 years. B
2.29 Women with a history of gestational diabetes mellitus found to have prediabetes should receive intensive lifestyle interventions and/or metformin to prevent diabetes. A
For many years, GDM was defined as any degree of glucose intolerance that was first recognized during pregnancy ( 60 ), regardless of the degree of hyperglycemia. This definition facilitated a uniform strategy for detection and classification of GDM, but this definition has serious limitations ( 161 ). First, the best available evidence reveals that many, perhaps most, cases of GDM represent preexisting hyperglycemia that is detected by routine screening in pregnancy, as routine screening is not widely performed in nonpregnant women of reproductive age. It is the severity of hyperglycemia that is clinically important with regard to both short- and long-term maternal and fetal risks. Universal preconception and/or first trimester screening is hampered by lack of data and consensus regarding appropriate diagnostic thresholds and outcomes and cost-effectiveness ( 162 , 163 ). A compelling argument for further work in this area is the fact that hyperglycemia that would be diagnostic of diabetes outside of pregnancy and is present at the time of conception is associated with an increased risk of congenital malformations that is not seen with lower glucose levels ( 164 , 165 ).
The ongoing epidemic of obesity and diabetes has led to more type 2 diabetes in women of reproductive age, with an increase in the number of pregnant women with undiagnosed type 2 diabetes in early pregnancy ( 166 – 169 ). Because of the number of pregnant women with undiagnosed type 2 diabetes, it is reasonable to test women with risk factors for type 2 diabetes ( 170 ) ( Table 2.3 ) at their initial prenatal visit, using standard diagnostic criteria ( Table 2.2 ). Women found to have diabetes by the standard diagnostic criteria used outside of pregnancy should be classified as having diabetes complicating pregnancy (most often type 2 diabetes, rarely type 1 diabetes or monogenic diabetes) and managed accordingly. Women who meet the lower glycemic criteria for GDM should be diagnosed with that condition and managed accordingly. Other women should be rescreened for GDM between 24 and 28 weeks of gestation (see Section 14 “Management of Diabetes in Pregnancy,” https://doi.org/10.2337/dc21-S014 ). The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) GDM diagnostic criteria for the 75-g OGTT as well as the GDM screening and diagnostic criteria used in the two-step approach were not derived from data in the first half of pregnancy, so the diagnosis of GDM in early pregnancy by either FPG or OGTT values is not evidence based ( 171 ) and further work is needed.
GDM is often indicative of underlying β-cell dysfunction ( 172 ), which confers marked increased risk for later development of diabetes, generally but not always type 2 diabetes, in the mother after delivery ( 173 , 174 ). As effective prevention interventions are available ( 175 , 176 ), women diagnosed with GDM should receive lifelong screening for prediabetes to allow interventions to reduce diabetes risk and for type 2 diabetes to allow treatment at the earliest possible time ( 177 ).
GDM carries risks for the mother, fetus, and neonate. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study ( 178 ), a large-scale multinational cohort study completed by more than 23,000 pregnant women, demonstrated that risk of adverse maternal, fetal, and neonatal outcomes continuously increased as a function of maternal glycemia at 24 – 28 weeks of gestation, even within ranges previously considered normal for pregnancy. For most complications, there was no threshold for risk. These results have led to careful reconsideration of the diagnostic criteria for GDM.
GDM diagnosis ( Table 2.7 ) can be accomplished with either of two strategies:
The “one-step” 75-g OGTT derived from the IADPSG criteria, or
The older “two-step” approach with a 50-g (nonfasting) screen followed by a 100-g OGTT for those who screen positive, based on the work of Carpenter and Coustan's interpretation of the older OʼSullivan ( 179 ) criteria.
Screening for and diagnosis of GDM
GDM, gestational diabetes mellitus; GLT, glucose load test; OGTT, oral glucose tolerance test.
American College of Obstetricians and Gynecologists notes that one elevated value can be used for diagnosis ( 189 ).
Different diagnostic criteria will identify different degrees of maternal hyperglycemia and maternal/fetal risk, leading some experts to debate, and disagree on, optimal strategies for the diagnosis of GDM.
One-Step Strategy
The IADPSG defined diagnostic cut points for GDM as the average fasting, 1-h, and 2-h PG values during a 75-g OGTT in women at 24 – 28 weeks of gestation who participated in the HAPO study at which odds for adverse outcomes reached 1.75 times the estimated odds of these outcomes at the mean fasting, 1-h, and 2-h PG levels of the study population. This one-step strategy was anticipated to significantly increase the incidence of GDM (from 5 – 6% to 15–20%), primarily because only one abnormal value, not two, became sufficient to make the diagnosis ( 180 ). Many regional studies have investigated the impact of adopting the IADPSG criteria on prevalence and have seen a roughly one- to threefold increase ( 181 ). The anticipated increase in the incidence of GDM could have a substantial impact on costs and medical infrastructure needs and has the potential to “medicalize” pregnancies previously categorized as normal. A recent follow-up study of women participating in a blinded study of pregnancy OGTTs found that 11 years after their pregnancies, women who would have been diagnosed with GDM by the one-step approach, as compared with those without, were at 3.4-fold higher risk of developing prediabetes and type 2 diabetes and had children with a higher risk of obesity and increased body fat, suggesting that the larger group of women identified by the one-step approach would benefit from increased screening for diabetes and prediabetes that would accompany a history of GDM ( 182 , 183 ). The ADA recommends the IADPSG diagnostic criteria with the intent of optimizing gestational outcomes because these criteria are the only ones based on pregnancy outcomes rather than end points such as prediction of subsequent maternal diabetes.
The expected benefits of using IADPSG to the offspring are inferred from intervention trials that focused on women with lower levels of hyperglycemia than identified using older GDM diagnostic criteria. Those trials found modest benefits including reduced rates of large-for-gestational-age births and preeclampsia ( 184 , 185 ). It is important to note that 80 – 90% of women being treated for mild GDM in these two randomized controlled trials could be managed with lifestyle therapy alone. The OGTT glucose cutoffs in these two trials overlapped with the thresholds recommended by the IADPSG, and in one trial ( 185 ), the 2-h PG threshold (140 mg/dL [7.8 mmol/L]) was lower than the cutoff recommended by the IADPSG (153 mg/dL [8.5 mmol/L]). No randomized controlled trials of treating versus not treating GDM diagnosed by the IADPSG criteria but not the Carpenter-Coustan criteria have been published to date. Data are also lacking on how the treatment of lower levels of hyperglycemia affects a mother's future risk for the development of type 2 diabetes and her offspring's risk for obesity, diabetes, and other metabolic disorders. Additional well-designed clinical studies are needed to determine the optimal intensity of monitoring and treatment of women with GDM diagnosed by the one-step strategy ( 186 , 187 ).
Two-Step Strategy
In 2013, the NIH convened a consensus development conference to consider diagnostic criteria for diagnosing GDM ( 188 ). The 15-member panel had representatives from obstetrics and gynecology, maternal-fetal medicine, pediatrics, diabetes research, biostatistics, and other related fields. The panel recommended a two-step approach to screening that used a 1-h 50-g glucose load test (GLT) followed by a 3-h 100-g OGTT for those who screened positive. The American College of Obstetricians and Gynecologists (ACOG) recommends any of the commonly used thresholds of 130, 135, or 140 mg/dL for the 1-h 50-g GLT ( 189 ). A systematic review for the U.S. Preventive Services Task Force compared GLT cutoffs of 130 mg/dL (7.2 mmol/L) and 140 mg/dL (7.8 mmol/L) ( 190 ). The higher cutoff yielded sensitivity of 70–88% and specificity of 69 – 89%, while the lower cutoff was 88 – 99% sensitive and 66 – 77% specific. Data regarding a cutoff of 135 mg/dL are limited. As for other screening tests, choice of a cutoff is based upon the trade-off between sensitivity and specificity. The use of A1C at 24–28 weeks of gestation as a screening test for GDM does not function as well as the GLT ( 191 ).
Key factors cited by the NIH panel in their decision-making process were the lack of clinical trial data demonstrating the benefits of the one-step strategy and the potential negative consequences of identifying a large group of women with GDM, including medicalization of pregnancy with increased health care utilization and costs. Moreover, screening with a 50-g GLT does not require fasting and is therefore easier to accomplish for many women. Treatment of higher-threshold maternal hyperglycemia, as identified by the two-step approach, reduces rates of neonatal macrosomia, large-for-gestational-age births ( 192 ), and shoulder dystocia, without increasing small-for-gestational-age births. ACOG currently supports the two-step approach but notes that one elevated value, as opposed to two, may be used for the diagnosis of GDM ( 189 ). If this approach is implemented, the incidence of GDM by the two-step strategy will likely increase markedly. ACOG recommends either of two sets of diagnostic thresholds for the 3-h 100-g OGTT—Carpenter-Coustan or National Diabetes Data Group ( 193 , 194 ). Each is based on different mathematical conversions of the original recommended thresholds by O'Sullivan ( 179 ), which used whole blood and nonenzymatic methods for glucose determination. A secondary analysis of data from a randomized clinical trial of identification and treatment of mild GDM ( 195 ) demonstrated that treatment was similarly beneficial in patients meeting only the lower thresholds per Carpenter-Coustan ( 193 ) and in those meeting only the higher thresholds per National Diabetes Data Group ( 194 ). If the two-step approach is used, it would appear advantageous to use the Carpenter-Coustan lower diagnostic thresholds as shown in step 2 in Table 2.7 .
Future Considerations
The conflicting recommendations from expert groups underscore the fact that there are data to support each strategy. A cost-benefit estimation comparing the two strategies concluded that the one-step approach is cost-effective only if patients with GDM receive postdelivery counseling and care to prevent type 2 diabetes ( 196 ). The decision of which strategy to implement must therefore be made based on the relative values placed on factors that have yet to be measured (e.g., willingness to change practice based on correlation studies rather than intervention trial results, available infrastructure, and importance of cost considerations).
As the IADPSG criteria (“one-step strategy”) have been adopted internationally, further evidence has emerged to support improved pregnancy outcomes with cost savings ( 197 ), and IADPSG may be the preferred approach. Data comparing population-wide outcomes with one-step versus two-step approaches have been inconsistent to date ( 198 , 199 ). In addition, pregnancies complicated by GDM per the IADPSG criteria, but not recognized as such, have outcomes comparable to pregnancies with diagnosed GDM by the more stringent two-step criteria ( 200 , 201 ). There remains strong consensus that establishing a uniform approach to diagnosing GDM will benefit patients, caregivers, and policy makers. Longer-term outcome studies are currently underway.
Suggested citation: American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15−S33
Email alerts
- Addendum. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15–S33
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides constitutes appropriate attribution).
Permission is required from the Association for any commercial use or for reproduction in any print materials (contact [email protected] ).
Using the links to the slide deck will download the deck. You may need to check the folder for downloaded documents on your device to see/open the file.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Pathophysiology of Type 2 Diabetes Mellitus
Unai galicia-garcia.
1 Fundación Biofisika Bizkaia, Barrio Sarriena s/n., 48940 Leioa (Bizkaia), Spain; [email protected] (U.G.-G.); se.oohay@aerralreisa (A.L.-S.)
2 Biofisika Institute (UPV/EHU, CSIC), Barrio Sarriena s/n., 48940 Leioa (Bizkaia), Spain; [email protected] (A.B.-V.); sue.uhe.elsaki@100irabejs (S.J.); se.uhe@clpambfo (H.O.)
Asier Benito-Vicente
3 Department of Biochemistry and Molecular Biology, Universidad del País Vasco UPV/EHU, Apdo. 644, 48080 Bilbao, Spain
Shifa Jebari
Asier larrea-sebal, haziq siddiqi.
4 Havard Medical School, 25 Shattuck St, Boston, MA 02115, USA; [email protected]
Kepa B. Uribe
5 Center for Cooperative Research in Biomaterials (CIC biomaGUNE), Basque Research and Technology Alliance (BRTA), Paseo de Miramon 182, 20014 Donostia San Sebastián, Spain; se.enugamoibcic@osollebk
Helena Ostolaza
César martín.
Type 2 Diabetes Mellitus (T2DM), one of the most common metabolic disorders, is caused by a combination of two primary factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond appropriately to insulin. Because insulin release and activity are essential processes for glucose homeostasis, the molecular mechanisms involved in the synthesis and release of insulin, as well as in its detection are tightly regulated. Defects in any of the mechanisms involved in these processes can lead to a metabolic imbalance responsible for the development of the disease. This review analyzes the key aspects of T2DM, as well as the molecular mechanisms and pathways implicated in insulin metabolism leading to T2DM and insulin resistance. For that purpose, we summarize the data gathered up until now, focusing especially on insulin synthesis, insulin release, insulin sensing and on the downstream effects on individual insulin-sensitive organs. The review also covers the pathological conditions perpetuating T2DM such as nutritional factors, physical activity, gut dysbiosis and metabolic memory. Additionally, because T2DM is associated with accelerated atherosclerosis development, we review here some of the molecular mechanisms that link T2DM and insulin resistance (IR) as well as cardiovascular risk as one of the most important complications in T2DM.
1. Introduction
Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic disorders worldwide and its development is primarily caused by a combination of two main factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin [ 1 ]. Insulin release and action have to precisely meet the metabolic demand; hence, the molecular mechanisms involved in the synthesis and release of insulin, as well as the insulin response in tissues must be tightly regulated. Therefore, defects in any of the mechanisms involved can lead to a metabolic imbalance that leads to the pathogenesis of T2DM.
This review analyses the key aspects of T2DM, as well as the molecular mechanisms and pathways implicated in insulin metabolism and associations between T2DM and cardiovascular pathophysiology. In this review, we describe the global trends of T2DM, the roles of major risk factors, in particular, obesity, lifestyle factors, genetic predispositions, gut dysbiosis, epigenetics and mitochondrial deregulation. We highlight physiological and molecular mechanisms leading to T2DM and its complications.
2. Type 2 Diabetes Mellitus: Background and Epidemiology
According to the World Health Organization (WHO) diabetes mellitus is a chronic, metabolic disease characterized by elevated levels of blood glucose, which leads over time to damage to the heart, vasculature, eyes, kidneys and nerves. Over 90% of diabetes mellitus cases are T2DM, a condition marked by deficient insulin secretion by pancreatic islet β-cells, tissue insulin resistance (IR) and an inadequate compensatory insulin secretory response [ 2 , 3 ]. Progression of the disease makes insulin secretion unable to maintain glucose homeostasis, producing hyperglycaemia. Patients with T2DM are mostly characterized by being obese or having a higher body fat percentage, distributed predominantly in the abdominal region. In this condition, adipose tissue promotes IR through various inflammatory mechanisms, including increased free fatty acid (FFA) release and adipokine deregulation. The main drivers of the T2DM epidemic are the global rise in obesity, sedentary lifestyles, high caloric diets and population aging, which have quadrupled the incidence and prevalence of T2DM [ 4 , 5 ].
The organs involved in T2DM development include the pancreas (β-cells and α-cells), liver, skeletal muscle, kidneys, brain, small intestine, and adipose tissue [ 6 ]. Evolving data suggest a role for adipokine dysregulation, inflammation, and abnormalities in gut microbiota, immune dysregulation, and inflammation have emerged as important pathophysiological factors [ 7 ].
Epidemiological data show alarming values that predict a worrisome projected future for T2DM. According to the International Diabetes Federation (IDF), in 2019, diabetes caused 4.2 million deaths; and 463 million adults aged between 20 and 79 years old were living with diabetes, a number that will likely rise up to 700 million by 2045. Diabetes was the underlying cause of at least 720 billion USD in health expenditure in 2019. Additionally, the true disease burden of T2DM is likely an underrepresentation as 1 in 3 diabetic people were underdiagnosed, equivalent to 232 million people. The greatest number of people suffering from diabetes are aged between 40 and 59 years old. Incidence and prevalence of T2DM vary according to geographical region, with more than 80% of patients living in low-to-middle-income countries, which poses additional challenges in effective treatment. Patients with T2DM have a 15% increased risk of all-cause mortality compared with people without diabetes with cardiovascular disease (CVD) as the greatest cause of morbidity and mortality associated with T2DM [ 8 ]. The association of diabetes with increased risk of coronary heart disease (hazard ratio [HR] 2.00; 95% CI 1.83–2.19), ischaemic stroke (HR 2.27; 1.95–2.65), and other vascular disease-related deaths (HR 1.73; 1.51–1.98) has been shown in a meta-analysis [ 9 ].
Epidemiology of T2DM is affected both by genetics and the environment. Genetic factors exert their effect following exposure to an environment characterized by sedentary behavior and high-calorie intake. Common glycaemic genetic variants for T2DM have been identified by genome-wide association studies, but these only account for 10% of total trait variance, suggesting that rare variants are important [ 10 ]. People of different ethnic origins may have different specific phenotypes that increase predisposition to clusters of CVD risk factors, including hypertension, insulin resistance, and dyslipidemia [ 11 ].

3. Risk Factors and Pathophysiology
T2DM risk factors include a complex combination of genetic, metabolic and environmental factors that interact with one another contributing to its prevalence. Although individual predisposition to T2DM due to non-modifiable risk factors (ethnicity and family history/genetic predisposition) has a strong genetic basis, evidence from epidemiological studies suggests that many cases of T2DM can be prevented by improving the main modifiable risk factors (obesity, low physical activity and an unhealthy diet) [ 12 , 13 ].
3.1. Ethnicity and Family History/Genetic Predisposition
Globally, the incidence and prevalence of T2DM are found to vary widely depending on ethnicity and geographical region with Japanese, Hispanics and Native Americans having the highest risks [ 14 , 15 , 16 ]. It has been shown higher incidence rates in Asians compared with a White American population [ 17 , 18 ], and white population in the UK, [ 19 ], where the highest risk is among the black population [ 20 ]. Whilst no clear reasons have been found, contributing factors such as modern lifestyle factors (which promote obesity), socioeconomic and direct genetic propensity or gene environmental interactions have been postulated.
Genetic predisposition plays an important part in the risk of developing T2DM. Over the past decade, several T2DM genome-wide association studies have shown the complex polygenic nature of T2DM in which most of these loci increase T2DM risk through primary effects on insulin secretion, and a minority act through reducing insulin action [ 21 , 22 ]. Dimas et al. grouped these variants on the basis of their potential intermediate mechanisms in T2DM pathophysiology, with four variants fitting a clear IR pattern; two reducing insulin secretion with fasting hyperglycemia; nine lowering insulin secretion with normal fasting glycemia; and one altering insulin processing [ 23 ]. According to these data, the genetic architecture of T2DM is highly polygenic, and additional association studies are needed to identify most T2DM loci [ 24 ]. Interactions between susceptibility loci and environmental factors could underlie the missing heritability of T2DM thus the impact of a given genetic variant can be modulated by the environmental factors (and vice versa) as evidenced by both observational studies and clinical trials [ 25 ].
3.2. Obesity, Low Physical Activity and Unhealthy Diet
Obesity (body-mass index [BMI]≥30 kg/m 2 ) is the strongest risk factor for T2DM [ 26 , 27 ] and is associated with metabolic abnormalities resulting in IR [ 28 ]. There exist an inverse linear relationship between BMI and the age at diagnosis of T2DM [ 29 ]. The exact mechanisms by which obesity induces T2DM and IR remain to be elucidated; however, numerous factors have shown a significant role in the development of this pathological process, which involves both cell-autonomous mechanisms and inter-organ communications.
A sedentary lifestyle is another risk factor for T2DM as shown by the Women’s Health Study and in the Kuipio Ischemic Heart Disease Risk Factor Study, which showed a reduction of 34% and 56% reduction of developing T2DM in participants walking 2–3 h a week or at least 40 min a week, respectively [ 30 , 31 ]. There are three primary benefits of physical activity on the delay of T2DM onset. First, the contraction of skeletal muscle cells induces an increase in blood flow into the muscle, enhancing glucose uptake from plasma [ 32 ]. Second, physical activity reduces the notorious intra-abdominal fat, which is a known risk factor that promotes IR [ 33 ]. Finally, moderate-intensity exercise has been shown to improve glucose uptake by 40% [ 34 ]. Physical activity improves glucose uptake and insulin sensitivity but it can also improve or even reverse inflammation and oxidative stress, which are T2DM predisposing factors [ 32 ].
3.3. Pathophysiology
Regarding the pathophysiology of the disease, a malfunctioning of the feedback loops between insulin action and insulin secretion results in abnormally high glucose levels in blood [ 2 ]. In the case of β-cell dysfunction, insulin secretion is reduced, limiting the body’s capacity to maintain physiological glucose levels. On the other hand, IR contributes to increased glucose production in the liver and decreased glucose uptake both in the muscle, liver and adipose tissue. Even if both processes take place early in the pathogenesis and contribute to the development of the disease, β-cell dysfunction is usually more severe than IR. However, when both β-cell dysfunction and IR are present, hyperglycaemia is amplified leading to the progression of T2DM [ 35 , 36 ].
4. Mechanisms Leading to T2DM and Pathophysiology
4.1. insulin secretion: physiological and dysfunctional mechanisms leading to t2dm, 4.1.1. β-cell physiology.
To safeguard proper β-cell function, cellular integrity must be ensured and the mechanisms and pathways implicated in the physiology of β-cell must be tightly regulated [ 35 ].
β-cells are responsible for insulin production, which is synthesized as pre-proinsulin. In the maturation process, pre-proinsulin undergoes a conformational modification carried out with the help of several proteins in the endoplasmic reticulum (ER) to yield proinsulin [ 37 ]. Afterwards, proinsulin is translocated from the ER to the Golgi apparatus (GA), entering into immature secretory vesicles and being cleaved into C-peptide and insulin [ 38 , 39 ].
Once matured, insulin is stored in granules until insulin release is triggered. Insulin release is primarily triggered by a response to high glucose concentrations. It is worth noting that some other factors can also induce insulin release such as amino acids, fatty acids and hormones [ 40 ]. When circulating glucose levels increase, β-cells take in glucose mainly through the glucose transporter 2 (GLUT2), a solute carrier protein that also works as a glucose sensor for β-cells. Once glucose enters, glucose catabolism is activated, increasing the intracellular ATP/ADP ratio, which induces the closing of ATP-dependant potassium channels in the plasma membrane. This leads to membrane depolarization and opening of the voltage dependant Ca 2+ channels, enabling Ca 2+ to enter the cell. The rise in the intracellular Ca 2+ concentration triggers the priming and fusion of the secretory insulin-containing granules to the plasma membrane, resulting in insulin exocytosis [ 38 , 40 , 41 , 42 ] ( Figure 1 A).

Signaling pathways involved in insulin secretion in β-cells in physiological conditions ( A ) and mechanisms leading to dysfunction ( B ). ( A ) Insulin release is primarily triggered by a response to high glucose concentrations and glucose in mainly internalized mainly through GLUT2 transporter. Glucose catabolism increases ATP/ADP ratio, ATP-dependant potassium channels are closed leading to membrane depolarization and opening of the voltage dependant Ca 2+ channels. The latter enables Ca 2+ influx triggering insulin exocytosis. Additional Ca 2+ channels as P2X, P2Y, SERCA and RYR contribute to Ca 2+ mobilization and insulin secretion. ( B ) hyperglycemia and hyperlipidemia promote oxidative stress leading to ROS generation that inhibits Ca 2+ mobilization and activates proapoptotic signals. Additionally, an excess of FFAs and hyperglycemia lead to the activation of the apoptotic unfolded protein response (UPR) pathways and generation of ER stress. Sustained high glucose levels increase proinsulin and IAAP biosynthesis, which generate ROS. GLUT2: glucose transporter 2, P2X: purinergic receptor X; P2Y: purinergic receptor Y; IP2: inositol 1,3-bisphosphate; IP3: inositol 1,4,5-trisphosphate; RYR: ryanodine receptor channel; SERCA: sarco-endoplasmic reticulum Ca 2+ -ATPase; FFA: free fatty acid, ROS: reactive oxygen species; UPR: unfolded protein response.
Additionally, Ca 2+ signals can be amplified by the RY receptors (RYR) and may play important roles in stimulus- insulin secretion coupling by virtue of their strategic locations within the cell and their ability to mediate Ca 2+ induced Ca 2+ release (CICR). RYR amplifies Ca 2+ signals when the channel is sensitized by messenger molecules generated from the nutrient metabolism or ligand-binding and are involved in the amplification of insulin secretion [ 43 ] ( Figure 1 A).
Nevertheless, other cell signals can also assist or enhance insulin release from β-cells. Among them, cAMP might be the most important messenger potentiating insulin release. Accumulated evidence suggests that cAMP induces insulin-containing secretory vesicle mobilization by depleting intracellular Ca 2+ reservoirs, thereby increasing intracellular Ca 2+ concentrations [ 44 ]. There is also compelling evidence that extracellular ATP is another important regulator of β-cell function. It is well-documented that β-cells release ATP through exocytosis of insulin granules upon glucose stimulation. Purinergic signaling via P2Y and P2X purinergic receptors stimulates Ca 2+ mobilization and regulates insulin exocytosis also independently of glucose. P2Y purinoreceptors have been reported to be coupled to G-proteins [ 45 , 46 ] whereas P2X-type receptors are ATP-activated ligand-gated ion channels non-selective for cations [ 47 ]. In the case of P2Y receptors, it has been proposed that insulin release could be mediated by intracellular Ca 2+ mobilization in response to inositol-1,4,5-trisphosphate (IP3) formation that triggers the release of Ca 2+ from ER stores, which amplifies the exocytosis-triggering Ca 2+ signal [ 48 , 49 ] ( Figure 1 A).
4.1.2. Mechanisms Leading to β-Cell Dysfunction
β-cell dysfunction has been traditionally associated with β-cell death [ 50 ]. However, recent evidence suggests that the dysfunction of β-cells in T2DM might be due to a more complex network of interactions between the environment and different molecular pathways implicated in cell biology [ 51 ]. In an excessive nutritional state, similar to that found in obesity, hyperglycemia and hyperlipidemia are often present, favoring IR and chronic inflammation. Under these circumstances, β-cells, due to differences in their genetic susceptibility, are subject to toxic pressures including inflammation, inflammatory stress, ER stress, metabolic/oxidative stress, amyloid stress, with the potential of ultimately leading to a loss of islet integrity [ 50 ].
An excess of FFAs and hyperglycemia lead to β-cell dysfunction by inducing ER stress through the activation of the apoptotic unfolded protein response (UPR) pathways [ 52 ]. In fact, lipotoxicity, glucotoxicity and glucolipotoxicity occurring in obesity, induce metabolic and oxidative stress that leads to β-cell damage [ 51 ]. Stress derived from high levels of saturated FFAs can activate the UPR pathway by several mechanisms including inhibition of the sarco/endoplasmic reticulum Ca 2+ ATPase (SERCA) responsible for ER Ca 2+ mobilization; activation of IP3 receptors or direct impairment of ER homeostasis. In addition, sustained high glucose levels increase proinsulin biosynthesis and islet amyloid polypeptides (IAAP) in β-cells, leading to the accumulation of misfolded insulin and IAAP and increasing the production of oxidative protein folding-mediated reactive oxygen species (ROS) [ 52 ]. These effects alter physiological ER Ca 2+ mobilization and favor proapoptotic signals, proinsulin mRNA degradation and induce interleukin (IL)-1 β release that recruits macrophages and enhances local islet inflammation [ 51 ] ( Figure 1 B).
As previously mentioned, insulin secretion has to be finely regulated to precisely meet metabolic demand. For that reason, proper islet integrity must be conserved in order to allow β-cells to respond to metabolic needs. Under pathogenic conditions, the mechanism described above can ultimately lead to disruption of islet integrity/organization, impairing optimal cell-to-cell communication within pancreatic islets, contributing to poor regulation of insulin and glucagon release and ultimately exacerbating the hyperglycemia. Defects in the synthesis of any insulin precursors, or insulin itself, as well as disruption of the secretion mechanism, can lead to insulin secretory dysfunction, the primary driver of β-cell failure, and a foundation of T2DM. For instance, reduced expression in the GLUT2 glucose transporter would affect the downstream signaling pathway [ 53 ], while failure in the folding of proinsulin is another finding commonly linked to deficient insulin production and diabetes [ 54 ].
4.1.3. Pathological Conditions Perpetuating T2DM
Nutritional factors.
High-caloric Western diet contains large amounts of fats and carbohydrates that elevate blood glucose and circulating very-low-density lipoproteins (VLDLs), chylomicrons (CMs) and their remnants (CMRs) that are rich in triglycerides (TG). This induces a spike in reactive oxygen species (ROS) concentrations, which in turn leads to an abnormal generation of inflammatory molecules. Given that inflammation is a recognized inducer of oxidative stress, a synergistic interaction occurs between the two processes after a heavy meal, with consequent amplification of harmful postprandial effects. The sustained and marked increase in steady-state levels of ROS contributes significantly to the pathogenesis of T2DM and IR. Therefore, a pro-oxidant environment leads to mitochondrial dysfunction, ER stress, activation of NADPH oxidase (NOX) and superoxide (O 2 − ) production. The increase in O 2 − production activates the five major pathways involved in the pathogenesis of diabetes complications: enhancement of the polyol pathway, increased formation of advanced glycation end products (AGEs), increased expression of AGEs receptor and its activating ligands, activation of protein kinase C (PKC) isoforms, and overactivity of the hexosamine pathway [ 55 , 56 , 57 ]. Through these pathways, increased intracellular ROS causes defective angiogenesis in response to ischemia, activates a number of proinflammatory pathways, and cause long-lasting epigenetic changes which drive persistent expression of proinflammatory genes even after glycemia is normalized [ 58 ]. Additionally, increased blood levels of FFAs also lead to mitochondrial dysfunction through two different mechanisms: (1) FFA metabolism by-products disturb the electron flow throughout the mitochondrial respiratory chain and (2) through the incorporation of FFAs into the mitochondrial membranes, thus likely favoring electron leakage [ 59 ].
Physical Activity
Reduced physical activity and exercise training, and increased sedentary behaviors constitute a link between obesity and T2DM and are associated with increased markers of chronic low-grade systemic inflammation [ 60 , 61 ]. In this condition, proinflammatory molecules are released into the bloodstream and within specific tissues such as interleukin 6 (IL-6), C-Reactive Protein (CRP), tumor necrosis factor-alpha (TNF-α) or IL-1 induces an inflammatory state known as metabolic inflammation [ 37 ]. Indeed, IL-1 is involved in the autoimmune response to β-cells in the pancreas, inhibition of β-cell function and activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor, thus inhibiting β-cell function and promoting apoptosis [ 32 ]. Preclinical data suggest that inflammation resolution could prevent the development of T2DM in obesity and prediabetes, which was substantiated by preclinical animal data showing that deletion of the macromolecular complex NLRP3 inflammasome, responsible for the production of IL-1β and IL-18, resulted in improved insulin sensitivity [ 62 ].
Intentional weight loss remains the cornerstone therapy to improve insulin sensitivity and in some circumstances to prevent the incidence of T2DM in individuals with obesity and prediabetes [ 63 ]. Regular exercise and increased physical activity enhance the production of anti-inflammatory cytokines such as IL-1 Receptor antagonist (IL-1Ra) and soluble TNF receptor (s-TNF-R) that are antagonists of IL-1 and TNF-α, respectively. Individuals with increased physical activity also show reduced circulating levels of IL-6, IL-18 and CRP, together with lower levels of leptin, a molecule associated with CRP [ 64 ]. Physical exercise can improve T2DM-inducing oxidative stress by inducing the synthesis of antioxidants such as glutathione (GSH), a major non-enzymatic antioxidant [ 65 ] and other antioxidant enzymes which lead to a long-term reduction in free radical levels [ 32 ].
Finally, irisin is an exercise-regulated myokine, which improves glucose tolerance [ 66 ] secreted by skeletal muscle [ 67 ] and adipose tissue [ 57 ] in response to exercise [ 68 ]. T2DM patients have been found to have lower circulating levels of irisin compared to control subjects. Additionally, diabetic patients with CVD had significantly lower serum irisin than non-CVD patients [ 69 ]. Low levels of serum irisin have been associated with 1.6 times increased risk of CVD incidence in T2DM patients [ 70 ].
Gut Dysbiosis
Gut microbiota is composed of many microbial species that impact human physiology and participate in different biological processes [ 71 ]. They can modulate the immune system and inflammatory response, regulate gut barrier integrity and human metabolism, take part in the synthesis of metabolites. Gut resident microorganisms produce many metabolites that contribute to physiology in healthy individuals. However, changes due to both inherited and acquired factors such as age, nutrition, lifestyle, genetic predisposition, or underlying diseases can affect the gut microbiota produced metabolite proportion leading to metabolic disturbances that can culminate in disease [ 72 , 73 , 74 ]. The better understating of gut microbiota has evidenced its important role in the development of diabetes and recent studies indicate that changes in dysbiosis can promote IR and T2DM [ 75 ]. A high-fat diet can induce up to threefold lipopolysaccharide (from Gram-negative bacteria) production in mice models, thereby contributing to low-grade inflammation and insulin resistance [ 76 , 77 ]. Furthermore, intestinal dysbiosis can reduce short-chain fatty acid synthesis that promotes gut barrier integrity, pancreatic β-cell proliferation and insulin biosynthesis [ 78 , 79 ]. Dysbiosis can also compromise the production of other metabolites such as branched aminoacids and trimethylamine thus disrupting glucose homeostasis and triggering T2DM development [ 80 , 81 ]. Understanding the clinical implications of the gut microbiome is a relatively new field, and requires further research to better elucidate the connection between gut microbiota and T2DM.
Metabolic Memory
Metabolic memory refers to the persistence of diabetic complications even after maintained glycemic control. This concept arose from the results of multiple large-scale clinical trials, which showed that after diabetes onset, diabetes complications persist and progress even when glycemic control is restored through pharmaceutical intervention [ 82 , 83 , 84 ]. Among them, the UKPDS post-trial study and Steno-2 trial showed that specifically early glycemic interventions prevent diabetic complication and has a marked decrease in CVD endpoints in patients that received either standard or intensive treatment following their diagnosis [ 84 ]. Later on, animal models of diabetes and in vitro cell cultures demonstrated that the initial hyperglycemic period results in permanent abnormalities (including aberrant gene expression) of target organs/cells [ 85 , 86 , 87 , 88 ]. Metabolic memory involves four mechanisms: epigenetics, oxidative stress, non-enzymatic glycation of proteins and chronic inflammation.
Epigenetics involves genetic modulation by factors other than individuals’ DNA sequence, and can regulate gene expression and determine which proteins are transcribed [ 89 ]. There are different epigenetic regulation mechanisms: direct methylation of cytosine or adenine residues, covalent modifications of histone proteins, higher-order chromatin structure and non-coding RNAs. Disruptions or imbalances in epigenetic mechanisms can lead to the development of diabetic pathophysiology [ 90 ].
MicroRNAs (miRNAs) are small non-coding RNA sequences synthesized as non-mature molecules that undergo several processing steps both in the nucleus and in the cytoplasm to become fully matured miRNAs. Once matured, miRNAs bind to their target gene’s mRNA, leading to mRNA silencing or degradation [ 91 ]. Increasing evidence highlights the importance of miRNA mediated post-transcriptional regulation in different aspects of β-cell biology such as cell differentiation, cytokine and growth factor-mediated signaling, glucose metabolism and insulin synthesis and secretion [ 92 ]. Deregulation of miRNA expression can directly impair β-cell function leading to the development of T2DM [ 93 ]. To date, more than 2600 miRNAs have been described within the human genome (miRBase, v.22.1), and multiple miRNAs have been shown to be involved in the pathogenesis of T2DM, including miR-200, miR-7, miR-184, miR-212/miR132 and miR-130a/b/miR-152 [ 94 ]. For instance, overexpression of miR-7 results in reduced insulin secretion via inhibition of genes involved in vesicle fusion and SNARE activity such as Snca, Cspa and Cplx1 [ 95 ]. In the case of miR-375, over-expression results in impaired exocytosis and thereby reduced insulin secretion. Conversely, it is the downregulation of miR-375 expression that causes a reduction in β-cell mass [ 93 , 96 , 97 ].
Several studies have evidenced that deregulation of the microRNA (miRNA) profile (post-translational histone methylation and non-canonical histone variant inclusion in octomers) may persist even after normoglycemia restoration [ 98 , 99 , 100 , 101 ]. MiRNAs participate in metabolic memory by targeting the mRNA of genes encoding enzymes involved in DNA methylation and those tightly regulated at the level of promoter methylation, transcription, and processing [ 102 ]. It has been shown that high glucose levels can alter post-translational histone modifications (PTHMs) and the activity of DNA methyltransferases generating irreversible changes that explain the long-term harmful effects of metabolic memory [ 103 , 104 , 105 , 106 ].
Hyperglycaemia induces an excess of ROS generation by mitochondria, which gives rise to diabetes complications [ 107 ] that may persist even when hyperglycemia is controlled. The damage following hyperglycemia-induced oxidative stress can be prevented when good glycemic control is initiated very early, but is not easily reversed if poor control is maintained for a longer duration [ 108 , 109 ]. At the early stages of T2DM, there is a relationship between hyperglycemia, increased oxidative stress, and excessive AGE formation. As the disease progresses, there is persistent protein glycation of the components of the respiratory chain that together with mitochondrial DNA damage can generate a hyperglycemia-independent concatenation of events leading to a synergy between oxidative stress and AGEs [ 86 ]. The effects of this metabolic imbalance activate inflammatory processes through receptor binding of AGEs or ROS which can modify the composition and structure of the extracellular matrix [ 98 ]. These structural changes may cause endothelial dysfunction and then atherosclerosis [ 98 ].
Finally, low-grade inflammation, which is involved in T2DM development and its vascular complications, has been shown to mediate metabolic memory. Many environmental factors (age, obesity, sedentarism and diet) that promote T2DM development trigger an inflammatory response leading to IR and endothelial dysfunction [ 105 , 110 , 111 ]. Obesity leads to NF-κB activation, which mediates the expression of inflammatory genes, which enhances monocyte binding to endothelial and vascular smooth muscle cells, subsequently promoting monocyte-to-macrophage differentiation [ 105 ]. In addition, NF-κB activation induces expression of inflammatory cytokines that are involved in vascular inflammation, with subsequent generation of endothelial adhesion molecules, proteases, and other mediators [ 111 ]. Another important factor that links inflammation and oxidative stress in obesity condition is the Toll-like receptor, which contributes to hypertension, insulin resistance, and obesity [ 105 ].
In summary, T2DM is a heterogeneous and progressive disorder that represents a series of metabolic conditions associated with hyperglycemia and caused by defects in insulin secretion and/or insulin action due to a complex network of pathological conditions. There are many different paths, driven by various genetic and environmental factors, that interact and mutually reinforce each other leading to an increased risk of other diseases including heart, peripheral arterial and cerebrovascular disease, obesity and nonalcoholic fatty liver disease, among others. The complex network of pathological conditions leading to T2DM development are summarized in Figure 2 .

Type 2 Diabetes Mellitus (T2DM) risk factors and the pathological changes leading to the perpetuation of insulin dysfunction. Complex combinations of genetic, metabolic and environmental factors that interact with one another constitute both non-modifiable (ethnicity and family history/genetic predisposition) and modifiable risk factors (obesity, low physical activity and an unhealthy diet). These states affect cell function resulting in a complex network of pathological changes that influence mutually and lead to the perpetuation of insulin dysfunction. ROS: reactive oxygen species; ER: endoplasmic reticulum; AGEs: advanced glycation end products; PKC: protein kinase C; LPS: lipopolysaccharide; miRNA: microRNA.
Mitochondrial Dysfunction
There is increasing evidence associating mitochondrial dysfunction with T2DM development, age-related IR and T2DM complications [ 112 ]. Indeed, oxidative stress, defective mitochondrial biogenesis, genetic mutations affecting mitochondrial integrity and aging promote mitochondrial dysfunction and are closely associated with T2DM development ( Figure 3 ) [ 113 , 114 ].

Mitochondrial dysfunction and contribution to T2DM development. Oxidative stress, defective mitochondrial biogenesis and impaired mitophagy promote mitochondrial dysfunction. Generation of ROS links mitochondrial dysfunction and IR. As a consequence of nutrient overload, electron supply to the mitochondrial ETC increases and the electron excess is transferred to oxygen generating O 2 − and H 2 O 2 . ROS oxidize proteins, damage DNA and membrane lipids. Mitofusin-2 and PGC 1α are downregulated leading to reduced mitochondrial biogenesis. Cellular stress and ROS production contribute to higher mitochondrial fission and impaired mitophagy. PCG 1α: Peroxisome proliferator-activated receptor-gamma coactivator-1.
The main function of mitochondria is ATP synthesis through oxidative phosphorylation in response to metabolic demand [ 115 ]. Mitochondria also participate in the production of different metabolites used as precursors of several macromolecules (lipids, proteins, and DNA). In addition, mitochondria play an important role in maintaining ion homeostasis, ROS clearance, the stress response, and serve to integrate multiple signaling pathways [ 116 , 117 ]. An imbalance between energy intake and expenditure in the mitochondria generates mitochondrial dysfunction, a state characterized by a reduced ratio of energy production to respiration [ 112 ]. Under these circumstances, nutrient oxidation efficiency is reduced leading to a decreased ratio of ATP synthesis/oxygen consumption, which increases O 2 − production [ 118 ]. In fact, the accumulation of ROS in the mitochondria is one proposed mechanism linking mitochondrial dysfunction to IR [ 119 ]. This relationship was corroborated in studies showing decreased mitochondria oxidative capacity in skeletal muscle and impaired lipid metabolism in obese and insulin-resistant individuals compared to healthy controls [ 120 , 121 , 122 ]. In addition, patients with T2DM have been found to have downregulation of genes involved in oxidative metabolism that are regulated by the peroxisome proliferator-activated receptor γ co-activator 1α (PGC 1α) [ 123 , 124 ] and a diminished phosphocreatine re-synthesis rate, both indicative of impaired mitochondrial function [ 125 ] ( Figure 3 ). Moreover, some relatives of T2DM patients have been found to have decreased mitochondrial respiration suggesting that mitochondrial dysfunction may precede T2DM development. It has also been proposed that T2DM development may be a direct consequence of defects in the oxidative phosphorylation system and the electron transport chain (ETC) rather than a decrease in mitochondrial content [ 126 ].
The generation of ROS is highly implicated in the relationship between mitochondrial dysfunction and insulin resistance. ROS production takes place mainly at complex I and complex III of the ETC and increases when ETC is not able to handle excessive electron input. In these circumstances, as a consequence of nutrient overload, electron supply to the mitochondrial ETC increases and the electron excess is transferred to oxygen generating O 2 − and subsequent hydrogen peroxide [ 127 ]. ROS generated in mitochondria oxidize the Cys and Met residues in proteins, damaging protein structure, impairing their function and eventually causing cell death. ROS species also damage DNA and membrane lipids, thus promoting mitochondrial dysfunction [ 128 ]. In addition, ROS overproduction activates the polyol pathway, the formation of AGEs, and the expression of the AGEs receptor and its activating ligands. It also activates PKC isoforms and upregulates the hexosamine pathway contributing to T2DM worsening [ 129 , 130 ]. In sum, excessive ROS generation by mitochondria contributes to accelerated T2DM progression ( Figure 3 ).
Mitochondrial dysfunction includes a reduction in mitochondrial biogenesis, along with a decrease in the expression of mitochondrial oxidative proteins, such as ETC complexes, which leads to decreased substrate oxidation. The damage produced by high oxidative stress in the mitochondria activates mitophagic processes in order to eliminate dysfunctional mitochondria or in case of excessive cellular stress to apoptosis [ 131 ]. These two processes reduce substrate utilization and enhance the accumulation of lipid intermediates such as diacylglycerols (DAG) and ceramide (CER) that disrupt the insulin signaling pathway [ 132 ]. DAG induces an increment of the serine/threonine phosphorylation of IRS-1, reducing its insulin-stimulated tyrosine phosphorylation and downstream propagation of the insulin signaling pathway [ 133 ] while CER inhibits protein kinase AKT [ 133 ]. The accumulation of DAG and CER contributes to the mitochondrial dysfunction seen in IR ( Figure 3 ).
Defects in mitochondrial biogenesis may be mediated by the downregulation of PGC 1α that has also been detected in T2DM patients [ 123 , 124 ]. PGC 1α is a transcription coactivator that regulates the expression of key genes involved in mitochondrial biogenesis, adaptive thermogenesis and metabolic substrate metabolism [ 134 ]. Furthermore, some of the genes involved in oxidative metabolism that are downregulated in individuals with T2DM are under the control of PGC 1α [ 124 ]. Mitofusin-2 , a key driver in mitochondria biogenesis is also downregulated in humans with T2DM [ 135 ]. Interestingly, mitofusin-2 levels increase upon weight loss indicating that nutrient and energy oversupply leads to mitochondrial dynamics defects [ 135 ].
Mitochondrial homeostasis is maintained via mitochondrial biogenesis and the selective clearance of damaged organelles. Mitochondrial dynamics are crucial to maintaining healthy mitochondria and control their quantity. Mitochondria fission promotes the removal of damaged mitochondria in a process known as mitophagy, which has to be efficiently and tightly regulated in order to preserve cell homeostasis [ 136 ]. Thus, mitophagy is considered to be one of the core mechanisms controlling the mitochondrial quantity and quality [ 137 ]. The process of removing damaged mitochondria consists of two steps: the induction of general autophagy and the priming of damaged mitochondria for selective autophagy recognition [ 138 ]. Once the degradation process is completed, the products are released back into the cytosol where macromolecular constituents are recycled. This process generates energy to maintain cell viability under unfavorable conditions and protects the cell during stress conditions [ 136 , 139 ]. When mitophagy is impaired, cellular stress and ROS production increase contributing to reduced hepatic insulin sensitivity and glucose homeostasis, two of the major pathological branches of T2DM development [ 112 , 140 ]. Deregulation of mitochondria dynamics with a shift towards fission promotes metabolic dysfunction as demonstrated by the onset of obesity and IR following the ablation of fusion protein in mice [ 141 , 142 ]. Furthermore, increased mitochondrial fission and mitochondrial fragmentation have been associated with mitochondrial depolarization, impaired ATP production and decreased insulin-dependent glucose uptake as well as increased mitochondrial ROS and impaired insulin signalling in C2C12 murine cell line and cybrids, respectively [ 143 , 144 ]. These studies highlight that the deleterious effect of unbalanced mitochondrial dynamics on metabolic health. Enhanced mitochondria fission also negatively impacts fatty acid β-oxidation, which is a pivotal metabolic defect in obesity and IR [ 120 , 121 ] contributing to the accumulation of lipotoxic lipid species. Fusion-shifted mitochondria dynamics has been also associated with an increase in fatty acid utilization putatively preventing lipotoxicity [ 145 ].
The role of mitochondrial genetics in the risk of T2DM has been clearly established. Indeed, several mtDNA variants (homoplasmic or heteroplasmic) have been associated with T2DM development. To date, the group of heteroplasmic variants associated with a higher risk of T2DM development includes A3243G, T14577C and A5178C [ 146 , 147 , 148 , 149 ]. The group of homoplasmic variants associated with T2DM risk includes C1310T, G1438A, A12026G, T16189C and A14693G [ 150 , 151 , 152 ]. It is important to note that additional studies are necessary to determine whether more metabolically active tissues that generate more mitochondrial ROS have increased rates of mtDNA heteroplasmy in T2DM.
To summarize, there is a highly nuanced and bi-directional relationship between mitochondrial dysfunction and T2DM. On one hand, aspects of T2DM such as insulin resistance can lead to mitochondrial dysfunction, such as through nutrient overload leading to ROS accumulation. On the other hand, mitochondrial dysfunction may predispose patients to subsequently developing T2DM, as evidenced by the presence of mtDNA variants associated with T2DM. Additional research is needed to better characterize the relationship between mitochondrial health and diabetes.
5. Insulin Resistance
IR refers to a decrease in the metabolic response of insulin-responsive cells to insulin or, at a systemic level, an impaired/lower response to circulating insulin by blood glucose levels [ 153 ]. There are three broad categories of IR or insulin-deficient conditions: (1) diminished insulin secretion by β-cells; (2) insulin antagonists in the plasma, due either to counter-regulatory hormones or non-hormonal bodies that impair insulin receptors or signaling; and (3) impaired insulin response in target tissues [ 154 ]. The action of insulin is influenced by the interplay of additional molecules including growth hormone and IGF-1 in the fed state. While fasting, the insulin response is mitigated by glucagon, glucocorticoids and catecholamines in order to prevent insulin-induced hypoglycemia. The ratio of insulin/glucagon plays a major role in this regulation, since it determines the relative degree of phosphorylation of downstream enzymes in the regulatory signaling pathways. While catecholamines promote lipolysis and glycogenolysis, glucocorticoids promote muscle catabolism, gluconeogenesis and lipolysis. Hence, excessive secretion of these hormones may be responsible for inducing IR [ 155 , 156 ]. Regarding the last category, there are three main extra-pancreatic insulin-sensitive organs that play major roles in the aforementioned processes: skeletal muscle, adipose tissue and liver. A defective action of insulin in these tissues often precedes the development of systemic IR, thus progressively leading T2DM.
5.1. Skeletal Muscle
Skeletal muscle IR is considered to be the most important extra-pancreatic factor in the development of T2DM [ 157 ]. Under physiological conditions, insulin stimulates muscle glycogen synthesis by enhancing glucose uptake from plasma. There are three primary rate-limiting factors implicated in glucose uptake and glycogen synthesis: glycogen synthase, hexokinase and the glucose transporter GLUT4 [ 158 ]. Upon insulin binding to insulin receptor (INSR) in muscle cells, GLUT4 translocates from intracellular compartments (early endosomes (EE), endosomal recycling compartment (ERC) and trans-Golgi network (TGN)) to the plasma membrane. This process allows glucose uptake and reduces circulating glucose levels [ 159 ].
Mutations that reduce the expression of insulin receptor or GLUT4, as well as any defect in either upstream or downstream signaling pathway would reduce glucose intake into the muscle resulting in a hyperglycaemic state [ 153 , 160 ]. The activation of INSR tyrosine kinase activity is essential for the action of insulin on glucose metabolism. Insulin binding to the α-subunit of the INSR causes phosphorylation of the β-subunit on multiple tyrosine residues and allows insulin-mediated signaling. Thus, mutations in any of the main phosphorylation sites can impair INSR tyrosine kinase activity, thereby impairing insulin action on skeletal muscle [ 161 ]. As mentioned above, mutations in key proteins of the downstream signaling pathway such as IRS-1 and IRS-2 or phosphoinositide 3-kinase (PI3K) also impair insulin action on the muscle. Apart from mutations or defective epigenetic regulation, environmental factors can also play an important role in glucose uptake by muscle. Physical activity increases blood flow into skeletal muscle cells and thereby enhances glucose utilization [ 32 ]. Obesity, which is associated with chronic inflammation, contributes to IR and T2DM. Increasing evidence suggests that as a consequence of obesity, increased immune cell infiltration and secretion of proinflammatory molecules in intermyocellular and perimuscular adipose tissue leads to skeletal muscle inflammation. This ultimately leads to myocyte inflammation, impaired myocyte metabolism, and contributes to IR via paracrine effects [ 162 ].
5.2. Adipose Tissue
Adipose tissue is a metabolically dynamic tissue capable of synthesizing a wide range of biologically active compounds that regulate metabolic homeostasis at a systemic level [ 163 ]. Indeed, adipose tissue participates in a broad range of biological processes involving, among others, immunity, coagulation, angiogenesis, fibrinolysis, reproduction, vascular tone control, appetite regulation, body weight homeostasis and glucose and lipid metabolism [ 164 ].
Insulin acts on adipose tissue in two different ways: (1) stimulating glucose uptake and triglyceride synthesis; and (2) suppressing triglyceride hydrolysis and inducing the uptake of FFA and glycerol from circulation [ 165 ]. In the fed state, GLUT4 allows uptake of glucose from the bloodstream into adipocytes, activating glycolysis in which glycerol-3-phospate (glycerol-3-P) is produced and incorporated into lipogenic pathways. Glycerol-3-P, along with the fatty acids coming from VLDLs, are esterified, forming triacylglycerol (TGA) that is stored in lipid droplets. During metabolic stress, TGA droplets the adipocyte are depleted, in order to provide FFA to be used as an energy source in other tissues.
An impaired response to insulin stimulation by adipose tissue is known as adipose IR (Adipose-IR). Adipose-IR can lead to impaired suppression of lipolysis, impaired glucose uptake, and enhanced FFA release into plasma even in the presence of high insulin levels [ 166 ]. Among the signaling elements affected by adipose-IR, we found that defective AKT activation impairs GLUT4 translocation to the membrane and promotes the activation of lipolytic enzymes that aggravate hyperglycemia [ 153 ]. Adipose-IR, as mentioned before, is associated with glucose intolerance and elevated release of FFA into a plasma that accumulates in other tissues such as muscle or liver. In the case of the liver, FFA accumulation results in impaired insulin signaling that promotes hepatic gluconeogenesis and impairs the glucose-stimulated insulin response, inducing T2DM development.
It has been shown that abnormally increased adipose tissue mass and adipocyte size correlate with pathologic vascularisation, hypoxia, fibrosis and macrophage-mediated inflammation [ 167 ]. A high-fat diet and obesity can activate saturated FFA-stimulated adenine nucleotide translocase 2 (ANT2), an inner mitochondrial protein that results in adipocyte hypoxia and triggers the transcription factor hypoxia-inducible factor-1α (HIF-1α). This culminates in adipose tissue dysfunction and inflammation [ 1 ]. Hypertrophied adipocytes as well as adipose tissue-resident immune cells contribute to increased circulating levels of proinflammatory cytokines. This increase in circulating proinflammatory molecules, together with an increase in local cytokine releases such as TNF and IL-1β and IL-6 facilitates the emergence of a chronic state of low-grade systemic inflammation, also known as metabolic inflammation [ 1 ]. This chronic inflammatory state is considered to be a key part in the pathogenesis of IR and T2DM [ 168 ]. The insulin stimulation effects on healthy and hypertrophic adipose tissue are shown in Figure 4 .

Insulin stimulation effects on healthy and hypertrophic adipose tissue. In healthy adipose tissue insulin stimulates glucose uptake and TG synthesis, induces FFA uptake and diminishes macrophage-mediated inflammation. Hypertrophic adipose tissue leads to diminished glucose uptake, TG synthesis and enhances FFA release, hypoxia and macrophage-mediated inflammation. FFA: free fatty acid.
In the liver, insulin does not only regulate glucose production/utilization but also affects lipid metabolism more broadly. When circulating glucose levels increase and insulin is secreted by pancreatic β-cells, insulin binding to liver INSR induces autophosphorylation of the receptor. Consequently, insulin receptor substrates (IRSs) are recruited and phosphorylated. In turn, IRSs activate PI3K, which phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2), generating phosphatidylinositol (3,4,5)-triphosphate (PIP3). PIP3 then activates PDK1, which phosphorylates AKT. In addition, AKT is phosphorylated by mTORC2. Once AKT is fully activated, it participates in several downstream pathways that regulate multiple metabolic processes including glycogen synthesis, gluconeogenesis, glycolysis and lipid synthesis [ 169 ].
In physiological states, the combined action of glucagon and insulin allows the precise regulation of hepatic glucose output. While glucagon induces hepatic glucose production, insulin acts as a potent inhibitor of glucose production when its concentration in the blood is elevated [ 170 ]. The effect of insulin on hepatic glucose production is due to both direct and indirect mechanisms. However, the relative importance of each of these mechanisms remains unclear [ 171 ].
In addition to inducing glycogen synthesis, insulin also inhibits hepatic glucose production by activating FOXO1, resulting in a reduction of hepatic glucose release. FOXO1 is a transcription factor that belongs to a subclass of the forkhead family of transcription factors that possess a forkhead box-type DNA binding domain. FOXO1 recognizes a specific regulatory element termed the insulin response element (IRE) on the promoters of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) genes, both of which play important roles in maintaining glucose level in states of starvation [ 172 , 173 , 174 ]. Thus, through inhibition of FOXO1, insulin promotes glucose storage as glycogen and inhibits glucose synthesis and hepatic glucose output [ 175 ] ( Figure 5 ).

Signaling pathways involved in insulin signaling in hepatocytes. Binding of insulin to INSR induces IRSs recruitment and phosphorylation. Phosphorylated IRSs activate PI3K, generating PIP3 which activates PDK resulting in AKT phosphorylation. AKT is fully activated by further mTORC2 phosphorylation and participates in several downstream pathways that regulate multiple metabolic processes including glycogen synthesis, gluconeogenesis, glycolysis and lipid synthesis. INSR: insulin receptor; PIP2: inositol 1,3-bisphosphate; PIP3: inositol 1,4,5-trisphosphate; IRS1: insulin receptor substrate 1; PI3K: phosphoinositide 3 kinase; mTORC2: mammalian target of rapamycin complex 2; PDK1: Phosphoinositide-dependent kinase-1; AKT: protein kinase B; AS160: Akt substrate of 160 kDa; GLUT4: glucose transporter 4; GSk3β: Glycogen Synthase Kinase 3 Beta; GS: Glycogen synthase; FOXO1: Forkhead box protein O1; G6pc:Glucose 6 phosphate; Pck1: Phosphoenolpyruvate Carboxykinase 1.
Similar to the case in insulin-sensitive tissues, in states of IR, physiologic levels of circulating insulin are insufficient to elicit the appropriate insulin response in hepatic cells [ 176 ]. In the liver, IR impairs glycogen synthesis, fails to suppress glucose production, enhances lipogenesis, and increases the synthesis of proteins such as the proinflammatory CRP. In fact, the abnormal production of proinflammatory proteins such as adipocytokines and cytokines, combined with conditions such as oxidative stress, can lead to an inflammatory state responsible for altered insulin response by the liver [ 175 ].
6. T2DM Outcomes/Complications: Cardiovascular Risk
As described in the previous sections, T2DM is a multisystem disease with a strong correlation with CVD development [ 177 ]. T2DM leads to a two- to four-fold increase in the mortality rate of adults from heart disease and stroke and is associated with both micro- and macro-vascular complications, the latter consisting of accelerated atherosclerosis leading to severe peripheral vascular disease, premature coronary artery disease (CAD) and increased risk of cerebrovascular diseases [ 178 , 179 , 180 ]. These factors lead to T2DM being considered a significant risk factor for CVD [ 181 ], likely through the involvement of several molecular mechanisms and pathological pathways. These include the role of IR in atherosclerosis, vascular function, oxidative stress, hypertension, macrophage accumulation and inflammation [ 182 , 183 , 184 , 185 ]. The following sections describe in detail the main factors implicated in cardiovascular risk outcomes from T2DM and the interactions between them ( Figure 6 ).

Factors implicated in cardiovascular risk outcomes from T2DM and the interactions between them. T2DM derived hyperglycemia, hyperinsulinemia and IR causes endothelial dysfunction, diabetic dyslipidemia and inflammation leading to CVD. The flowchart illustrates the multiple interactions among the implicated factors.
6.1. Diabetic Dyslipidaemia and Atherosclerosis Development
Dyslipidaemia is a common feature of T2DM, and increases the incidence of atherosclerosis and mortality of diabetic patients [ 186 ]. The hallmark of diabetic dyslipidemia is a characteristic dyslipidaemic profile consisting of elevated TG, TG-rich lipoproteins (TRLs), small dense LDLs (sdLDL), and reduced HDL levels [ 187 , 188 , 189 ]. Although the pathophysiology of dyslipidemia in T2DM is not completely characterized, several factors such as hyperglycemia, insulin resistance, hyperinsulinemia, abnormalities in adipokines and adipocytokines have been implicated [ 190 ]. Epidemiological studies indicate that TG-rich lipoproteins and their remnants contribute to atherogenesis and CVD risk [ 191 , 192 , 193 , 194 , 195 ] and experimental studies indicated a connection between cholesterol deposition and inflammation as a result of TRLs entry into the artery wall [ 196 , 197 ]. TRLs consist of a great variety of nascent and metabolically modified lipoprotein particles including intestine-derived apoB48 (chylomicrons and chylomicron remnants) and liver-derived apoB100 (VLDL and its remnants). TRLs are highly heterogeneous in size, density, and apolipoprotein composition [ 198 ].
Under physiological conditions, chylomicrons deliver dietary lipids and lipid-biliary sources to the liver, which upon entering central circulation acquire apoE, apo-CI, apo-CII and apo-CIII from circulating HDL ( Figure 7 ). Apo-CII, an activator of lipoprotein lipase (LPL), hydrolyzes TG within the chylomicron core, thereby releasing free fatty acids (FFAs). The progressive removal of TGs leads to the formation of chylomicron remnants (CR), which upon apoE incorporation, are cleared by hepatocytes ( Figure 7 ). This, together with the uptake of FFA generated by lipolysis in adipose tissue provides the major source of hepatic VLDL assembly and secretion. Once in the circulation, VLDL particles incorporate apo-CII and apoE from HDL allowing VLDL to be progressively lipolyzed leading to the generation of smaller VLDL particles (VLDL1, VLDL2 and VLDL3), IDL, and finally LDL ( Figure 7 ). Lipoprotein production, metabolism, and clearance are efficient processes. However, T2DM and IR are among the most important metabolic derangements in these process and they give rise to impaired metabolism and clearance of chylomicrons and VLDLs [ 199 , 200 ].

Diabetic dyslipidemia: mechanisms leading to T2DM dyslipidemia and lipoprotein clearance in physiological an IR conditions. ( A ) IR leads to an impaired adipose tissue fat storage, resulting in constitutive FFA release from the intracellular TG stores of adipocytes. The released FFAs are taken up by hepatocytes, where they can be directed to the mitochondria and undergo β-oxidation; be re-assimilated into TG to assemble new VLDL particles; shifted to gluconeogenesis resulting in a worsening of hyperglycemia; or stored as TG leading to hepatic steatosis. ( B ) Under physiological conditions, VLDL particles incorporate apo-CII and apoE from HDL allowing VLDL to be progressively lipolyzed leading to the generation of smaller VLDL particles (upper panel). T2DM and IR impair metabolism and clearance of chylomicrons and VLDLs. Activation of CETP promotes an exchange of TG out of RLPs and incorporates CE from HDL and LDL particles leading to reduced levels of circulating HDL-C and an increase in the more atherogenic sdLDL particles (lower panel). TG: triglyceride; FFA: free fatty acid, LPL: lipoprotein Lipase; CR: chylomicron remnants; HL: hepatic lipase; CETP: Cholesteryl Ester Transfer Protein; ApoE: apolopoprotein E; ApoC-II: apolipoprotein CII; apoC-III: apolipoprotein CIII; VLDL: very low-density lipoprotein; sdLDL: small dense lipoprotein.
Mechanisms Leading to T2DM Dyslipidemia and Atherosclerosis
Increased hepatic TG content present in T2DM patients leads to elevated hepatic production of VLDL and normal or slightly elevated LDL-C levels, most commonly sdLDLs enriched in TG [ 189 , 201 ]. One of the primary abnormalities in IR is impaired adipose tissue fat storage, resulting from insulin’s inability to inhibit hormone-sensitive lipase (HSL). This results in constitutive FFA release from the intracellular TG stores of adipocytes. The released FFAs are taken up by hepatocytes, where they can be directed to the mitochondria and undergo β-oxidation; be re-assimilated into TG to assemble new VLDL particles; shifted to gluconeogenesis resulting in a worsening of hyperglycemia; or stored as TG leading to hepatic steatosis.
The dominant feature of diabetic dyslipidemia is the increased production rate of VLDL-apoB100 by the liver, mainly VLDL1, which is related to insulin sensitivity indices [ 202 ]. This highlights the role of insulin on VLDL assembly and secretion by hepatocytes [ 203 ]. Insulin plays a role in almost all the steps of VLDL assembly and secretion. It is known that insulin inhibits the transcription of Mttp, the gene coding for the microsomal transfer protein (MTP), the protein responsible for assembling TG with apoB100 [ 204 ]. MTP facilitates concerted lipid transfer and apoB100 folding as it enters the ER lumen and lipidation determines the amount of the active pool of apoB100 [ 205 ]. Lipidation of apoB100 is a co-translational event and a rate-limiting step of apoB100 mRNA stability thus low availability of TG leads to apoB100 degradation. The addition of TG to apoB100 generates nascent VLDL particles that are transported to the GA by Sar2/COPII-containing vesicles. Within the GA, VLDL maturation occurs in a process promoted by the phospholipase D1 (PLD1) [ 203 ]. Therefore, in insulin-resistant condition, MTP expression and activity is increased thus contributing to raising apoB100 lipidation and to its rescue from degradation. Indeed, IR leads to a loss of the acute insulin-mediated inhibition of apoB100 secretion [ 205 ].
Availability of TGs within hepatocytes is important for VLDL synthesis [ 206 ] and the liver uses both de novo synthesized FAs and extra-hepatic FFAs as a substrate for TG synthesis [ 207 ]. De novo lipogenesis occurs primarily in the fed state in which the expression of lipogenic genes is regulated by the sterol regulatory element-binding protein (SREBP). The SREBP-1c isoform up-regulates almost all the enzymes involved in FA synthesis as well as enzymes that supply acetyl-CoA units and reducing equivalents to the pathway [ 208 ]. Insulin regulates SREBP-1c, which explains the lipogenic effect of chronic hyperinsulinemia [ 208 ]. FFAs derived from adipose tissue is also a major source of liver TGs and VLDL production. As mentioned above, T2DM is characterized by increased production of FFAs by adipose tissue [ 207 ]. Therefore, in IR, an increase in TG lipolysis in adipose tissue and FFA influx serves as another source of lipid to the liver [ 207 ].
As mentioned above, in the IR milieu, insulin has reduced capacity to inhibit VLDL secretion in the fed state, the availability of apo-CII is lower and apo-CIII production is increased [ 209 ]. These events result in the accumulation of VLDL remnants and IDL due to diminished clearance of TRLs by hepatocytes [ 210 ]. Additionally, hepatic IR also impairs LRP1 translocation from intracellular vesicles to the hepatocyte plasma membrane, which contributes to impaired clearance of TRLs [ 211 , 212 ] ( Figure 7 ).
In an effort to offload TG from remnant lipoproteins (VLDL 2+3 and IDL; RLPs), CETP is activated and promotes an exchange of TG out of RLPs and incorporates CE from HDL and LDL particles [ 213 ]. The TG-enriched HDL and LDL particles are better substrates for lipolysis by hepatic lipase leading to reduced levels of circulating HDL-C and an increase in sdLDL particles, which are more atherogenic [ 214 ]. The increased movement of CE into circulating TRLs mediated by enhanced activity of CETP [ 215 ] plays a key role in generating small dense HDL and LDL particles, the former being less atheroprotective and the latter more atherogenic [ 216 ]. TG enrichment of HDL enhances circulating HDL clearance [ 217 ].
The lower HDL concentration and modified composition of HDL have an important impact in diminishing the particle capacity of inducing cholesterol efflux from the cells, which is the first step in reverse cholesterol transport (RCT) [ 218 ]. The impaired RCT activity has been associated with increased risk of CAD [ 219 ] and with flow-mediated vasodilation in T2DM patients [ 220 ].
Atherosclerosis is accelerated by the increased permeability of sdLDL into the subendothelial space ( Figure 7 ) [ 221 , 222 ]. SdLDL particles are characterized by a lower affinity for LDLR due to conformational rearrangements occurring in apoB100 as the particle decreases in volume and size [ 223 ]. Furthermore, sdLDL particles are more susceptible to oxidation and thus avidly scavenged by activated macrophages in the subendothelial space, giving rise to foam cells [ 224 ]. In addition, sdLDL particles show increased proteoglycan binding and facilitated entry into the arterial wall, increased arterial retention, and a longer half-life [ 225 ]. sdLDL particles are also more likely to be glycated, more resistant to breakdown, and more susceptible to oxidation by free radicals [ 226 ].
There is an insulin response element in the gene for apoA-I, the primary apolipoprotein constituent of HDL particles [ 227 , 228 ]. As the liver becomes more insulin resistant, less apoA-I is produced and there is less HDL biogenesis. Adipocytes express the ATP-binding membrane cassette transport protein A1 (ABCA1). IR downregulates the expression of ABCA1 on the surface of adipocytes and reduces HDL formation by these cells [ 229 , 230 , 231 ]. Chylomicrons are enriched with apoA-I. IR reduces the release of this apoA-I into the serum by inhibiting LPL. In addition, within the milieu of IR of diabetes, HDL particle concentrations are not only quantitatively reduced, but also tend to be dysfunctional and unable to perform their primary functions, including reversal of cholesterol transport and inhibition of oxidative and inflammatory phenomena [ 232 ].
This highly atherogenic lipid profile is a pivotal contributor to atherogenic dyslipidemia, which is causally linked to the development and progression of atherosclerotic CV disease (ASCVD) [ 233 , 234 ]. The relationship between atherogenic dyslipidemia and ASCVD is supported by prospective longitudinal cohorts, clinical evidence and genetic linkage studies. As an example, the best predictor of risk of myocardial infarction at the population level in the INTERHEART study was the apolipoprotein (apo) B100/apoA-I ratio, reflecting the correlation between all apoB (atherogenic lipoproteins) and HDL (representing classically anti-atherogenic particles) [ 235 ]. The relationship between atherogenic dyslipidemia and ASCVD has also been demonstrated in prospective randomized clinical trials using statins. Even when treated with statins, patients with the atherogenic dyslipidemia phenotype have a higher risk of CV events than those without AD [ 236 , 237 ].
Diabetic dyslipidemia acts in concert with other metabolic and vascular abnormalities to further compound vascular risk. Chronic hyperglycemia induces endothelial dysfunction through a variety of mechanisms such as by reducing vasodilation, increasing vasoconstriction, increasing exposure to free radicals and impairing endothelial cell function, with a net effect of facilitating pro-atherogenic conditions [ 238 ]. Increased activity of the renin-angiotensin axis has also been found to further increase oxidative stress [ 239 ].
6.2. Impaired Endothelial Function and Atherosclerosis Development
Endothelium plays an important role in the regulation of vascular tone and structure through a balanced release of endothelial-derived relaxing and contracting factors. This balance is altered in T2DM leading to alteration of the physicochemical properties of the vascular wall via endothelial dysfunction, oxidative stress, platelet hyperreactivity, and inflammation [ 240 , 241 ]. These abnormalities lead to enhanced vasoconstriction, development of atherosclerosis, and favored thrombus formation [ 179 , 180 ].
6.2.1. Mechanisms Leading to Endothelial Dysfunction in T2DM
Vascular endothelial cells are particularly susceptible to developing intracellular hyperglycemia because glucose diffuses passively through their plasma membrane. In T2DM, the excess of glucose can be metabolized in the sorbitol pathway to sorbitol and fructose by aldose reductase, which activates the aldose reductase secondary metabolic pathway, with concomitant oxidation of NADPH to NADP + and reduction of NAD + to NADH. NADPH depletion and an increased NADH/NAD + cytosolic ratio leads to a change in redox potential that accelerates glycolysis and increases de novo synthesis of DAG [ 242 ]. As a result, protein kinase C (PKC) is activated, nitric oxide (NO) is reduced. These effects cause vascular permeability and increase contractility. Simultaneously, the increased NADH/NAD + ratio also results in higher production of O 2 − , LDL oxidation, cytotoxic effects on endothelial cells and reduced NO availability, leading to endothelial dysfunction [ 241 , 242 ].
The overproduction of aldoses by the sorbitol pathway promotes protein glycosylation that yields the formation of the stable Amadori products (such as glycosylated hemoglobin) and AGEs. AGEs are associated with several molecules that augment oxidant activity and consequently the production of ROS, which increases oxidative stress and prevents the release of NO, resulting in vascular lesions. AGEs may also reduce endothelium-derived NO bioavailability and activity, further compromising vascular activity [ 243 ]. In addition, AGEs can trigger an inflammatory and pro-coagulant state and can cause endothelial activation through the induction of receptor-mediated gene transcription. AGE binding to the RAGE-receptor, nuclear transcription factor NF-κB [ 242 , 243 ] is activated leading to transcription of endothelin-1, VCAM-1, ICAM-1, E-selectin, thrombomodulin, TF, vascular endothelial growth factor (VEGF), IL-1, IL-6, and TNF-α [ 242 , 244 ]. Increased expression of inflammatory and adhesion molecules amplifies the inflammatory response and aggravates diabetic vascular complications. These pro-inflammatory cytokines stimulate the expression and release of pro-coagulant molecules and inhibit the expression of anti-coagulant molecules by endothelial cells [ 245 ]. This leads to a pro-coagulant state in the surface of the endothelium and increases growth factor production resulting in a thickening of the basement membrane, thus favoring protein and lipid deposition and impairing vasodilation [ 242 , 246 ].
6.2.2. Endothelial Dysfunction in T2DM and Atherosclerosis Development
Hyperglycemia-associated vascular injury, oxidative stress, inflammation and altered hemodynamic balance may initiate atherosclerosis development and formation of arterial thrombus [ 247 ]. At early stages of atherosclerosis, circulating LDL binds to matrix proteoglycans where their oxidation is favored, giving rise to highly pro-inflammatory particles that stimulate the expression of several adhesion molecules by endothelial cells [ 242 , 248 ]. This promotes selective binding of leukocytes and their transmigration into the vascular wall along with recruitment and activation of circulating monocytes that differentiate into macrophages. The excess of oxidized LDL is removed by macrophages by a non-regulated mechanism that leads to the formation of foam cells and the onset of fatty streaks. Mononuclear cells release inflammatory cytokines, including IL-1 and IL-6, promoting the recruitment of additional inflammatory cells. As a result, smooth muscle cells proliferate and migrate into the intima where they synthesize and secrete extracellular matrix facilitating fibroatheroma formation [ 242 ]. As the process progresses, if a fissure or ulceration of the plaque occurs, highly thrombogenic substances are exposed leading to adhesion and aggregation of platelets, which promotes thrombus formation [ 249 ]. In addition, platelets can also release pro-inflammatory cytokines and growth factors promoting monocyte recruitment to atherosclerotic plaques, which stimulates fibroblasts and smooth muscle cell proliferation thus accelerating the atherosclerotic process.
6.3. Diabetes-Associated Chronic Inflammation and Atherosclerosis Progression
A critical component of T2DM is a chronic low-grade inflammatory state, referred to as “metaflammation” [ 250 ]. This chronic condition involves the same cellular and molecular players of acute inflammatory responses and has been suggested as an underlying cause of the progression of atherosclerosis in T2DM. Hyperglycaemia can increase circulating cytokines that can lead to chronic inflammation in T2DM [ 250 ]. Among them, patients with T2DM have higher levels of IL-1b, IL-6, IL-8, MCP-1, and other major cytokines in both monocytes and macrophages [ 130 ]. The underlying mechanisms involved in this process are ROS-mediated activation of p38 and other proinflammatory kinases, upregulation of NF-kB induction, oxidative stress, and activation of the AGE-RAGE pathway [ 129 , 130 ]. In addition, exposure to high glucose levels impairs the phagocytic activity of macrophages, which partially explains the increased incidence of chronic infection among T2DM patients [ 251 ]. Indeed, T2DM is associated with increased activity of the inflammasome, upregulation of the nucleotide-binding oligomerization domain-like receptor 3 (NLRP3), increased levels of IL-1β and IL-18 [ 252 , 253 , 254 ]. These events trigger neutrophil extracellular trap activation, or NETosis, a characteristic cell death of macrophages causing chronic inflammation [ 255 ]. High levels of these markers have been found in T2DM patients [ 256 ], which are enhanced in hyperglycaemic conditions [ 257 ].
6.4. Adipokine Balance and CVD Risk
Adipose tissue dysfunction as a result of T2DM can result in an imbalance between pro-inflammatory and anti-inflammatory adipokines, and is one of the mechanisms of T2DM complications. Several studies indicate that adipokines are related to IR, and can result in endothelial dysfunction, and pro-inflammatory and pro-atherogenic states [ 258 , 259 ].
Adiponectin is a well-described insulin-sensitizing hormone and its expression and circulating levels are inversely proportional to the extent of adiposity. Adiponectin has insulin-sensitizing properties [ 260 , 261 ]. Adiponectin acts through ADIPOR1 and ADIPOR2 receptors [ 262 ] and the peroxisome proliferator-activated receptor α (PPARα) pathway, leading to decreased hepatic gluconeogenesis, increased liver and skeletal muscle fatty acid oxidation, increased glucose uptake in skeletal muscle and white adipose tissue, and decreased white adipose tissue inflammation [ 263 ]. In addition, adiponectin ameliorates β-cell death by neutralizing inflammatory and lipotoxic ceramides and DAGs [ 264 ] and shows strong anti-inflammatory effects on other cell types such as macrophages and fibrogenic cells [ 263 , 265 , 266 ]. Low concentrations of adiponectin have been found in T2DM patients and are correlated with increased risk of developing premature arteriosclerosis, and are thus considered an additional CVD risk factor [ 267 ]. Notably, adiponectin deficiency is associated with coronary artery disease, hypertension, endothelial dysfunction and greater carotid intima-media thickness [ 268 , 269 , 270 , 271 ]. Low concentrations of adiponectin lead to an increased expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, promotes differentiation of macrophages into foam cells and enhances the proliferation and migration of smooth muscle cells [ 272 ].
Omentin is an adipokine secreted from white adipose tissue and is involved in glucose homeostasis [ 273 , 274 ]. Omentin circulates in the blood [ 275 , 276 ], and is associated with reduced levels in T2DM patients [ 277 , 278 ]. In vitro studies have shown that omentin enhances insulin-stimulated glucose uptake in human adipocytes by AKT signaling pathway activation [ 273 ]. In humans, an inverse correlation between omentin levels and IR is seen, both at the protein and mRNA levels [ 277 , 279 , 280 ]. Additional studies show that omentin has anti-inflammatory properties, diminishes cytokine expression [ 281 , 282 ], and is negatively associated with systemic inflammatory markers such as TNF and IL-6 [ 283 ].
Vaspin (visceral adipose tissue-derived serine protease inhibitor) is an adipokine that inhibits proteases responsible for IR and protects against atherosclerosis and plaque development [ 284 , 285 ]. It has been shown that T2DM patients have higher serum vaspin levels than healthy controls. Higher vaspin levels are associated with a 1.7-fold increased risk of CVD [ 70 ]. High vaspin is also associated with increased severity of coronary artery disease [ 286 ].
7. Conclusions
The importance of research in the fields of glucose homeostasis, insulin and diabetes has not faded. In fact, due to rapid globalization and the normalization of a sedentary lifestyle, along with increased obesity, diabetes and their consequent co-morbidities, research in this topic must continue to grow. Understanding the mechanisms implicated in every step in the development and complications of T2DM is crucial in order to prevent, control, treat or revert the pathophysiology of T2DM its complications. Although quality outcomes for patients are optimized by early detection of T2DM through screening and intensive patient-centered management, research efforts are needed to define causative factors accounting for correlations among different demographic subsets and the corresponding variable risks for T2DM as well as the drivers of increased risk in individuals of low socioeconomic status. Being the pathophysiology and underlying mechanisms of T2DM increasingly understood, precision medicine should be implemented and treatments individualized and targeted appropriately with the help of molecular genetic tools by identifying specific variants contributing to disease development as well as by searching biomarkers to assess progression and response to therapeutic interventions. Additional research is needed to determine a direct causal role of the intestinal microbiota in pathogenesis of T2DM and response to therapies needs to be determined.
Taking everything in this review into consideration, it is clear that there is still a long way until we fully understand each of the many stakeholders in glucose homeostasis.
Author Contributions
U.G.-C., A.B.-V., S.J., A.L.-S., H.S., K.B.U., H.O. and C.M. have contributed to the writing of the manuscript. U.G.-G. and C.M. conceptualized the review. A.B.-V. and C.M. drawn the images. H.S. edited language. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Basque Government (Grupos Consolidados IT-1264-19). U.G-G. was supported by Fundación Biofísica Bizkaia. A.B.-V. was supported by Programa de especializaci.n de Personal Investigador Doctor en la UPV/EHU (2019) 2019–2020. S.J. and A.L-S. were supported by a grant PIF (2017–2018) and (2019–2020), Gobierno Vasco, respectively. A.L.-S. was partially supported by Fundación Biofísica Bizkaia.
Conflicts of Interest
The authors declare no conflict of interest.
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

11 templates

20 templates

holy spirit
36 templates

9 templates

25 templates

memorial day
12 templates
Diabetes Presentation templates
Here at slidesgo, we would like to help you raise awareness about diabetes, a metabolic disorder related to insulin and the levels of glucose in our blood. with these google slides & powerpoint templates, you can make sure that the audience will pay attention to your speech, as these slides will keep them focused on the screen and your explanations..
It seems that you like this template!
Diabetes infographics.
Information on health issues requires pedagogical resources to make it understandable to others. At Slidesgo we have created this diabetes infographic template so that you can easily and entertainingly report on this disease. It contains numerous icons and illustrations to make the data more visual and easier to remember. With...

World Diabetes Day
Diabetes is a serious illness that affects millions of people all around the world, and making proper treatment, care and information accessible to everyone who needs it is an unresolved issue that needs more awareness. For reasons like these, the United Nations decided to establish November 14th as the World...

Type 2 Diabetes Breakthrough
Type 2 diabetes is a metabolic disorder with a high prevalence in the world. Hundreds of millions of people suffer from it every year. Basically, a person cannot produce enough insulin, which can cause very severe consequences. Are there news on the treatment of type 2 diabetes? Waste no time...

Diabetes Breakthrough: Insulin Increase
This is a modern template full of illustrations and resources that gives medical presentations a visual and creative touch. This design focuses on diabetes: a medical issue that affects the lives of millions of people. If you have investigated on this and have come up with a new treatment, these...

World Diabetes Day Infographics
Slidesgo is aware of how healthcare professionals love to have extra help when sharing information about diseases, treatments or breakthroughs. Today, we're releasing a series of infographic designs for presentations about diabetes, a serious illness that affects millions of people. Check them out! The main color used is blue, a...

Gestational Diabetes Breakthrough
Download the Gestational Diabetes Breakthrough presentation for PowerPoint or Google Slides.Treating diseases involves a lot of prior research and clinical trials. But whenever there’s a new discovery, a revolutionary finding that opens the door to new treatments, vaccines or ways to prevent illnesses, it’s great news. Should there be a...

Premium template
Unlock this template and gain unlimited access
Gestational Diabetes
Download the "Gestational Diabetes" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking medical attention....

Gestational diabetes is diabetes that first appears during pregnancy. It usually occurs in the middle of the gestation period and it is necessary to take care of it so that it does not cause problems. For this, information is key, so we have created this beautiful pink template, with creative...

Treatment of Hypoglycemia Breakthrough
When the glucose levels in our blood drop below normal, our body can experience symptoms such as shakiness, confusion, sweating, and fatigue. A more correct name for this condition is hypoglycemia, but there's treatment (usually, ingesting sugar and carbohydrates), but perhaps there's more to it. Any new breakthrough in this...

Type 2 Diabetes Disease
Type 2 diabetes is a chronic condition that affects the way the body processes glucose. With this disease, the patient's body does not produce enough insulin or is resistant to it. Share your research on this important disease using this modern and minimalist template designed in pastel colors, with which...

Nutrition for People with Diabetes Workshop
Download the Nutrition for People with Diabetes Workshop presentation for PowerPoint or Google Slides. If you are planning your next workshop and looking for ways to make it memorable for your audience, don’t go anywhere. Because this creative template is just what you need! With its visually stunning design, you...

Diabetes Education Center
Running a medical center specialized on diabetes involves lots of organization and management. What is the admission procedure? How are patients going to be allocated? What fun/free time activities do you offer? That’s an important question to think about, because offering your patients activities to disconnect can improve their mental...

Happy World Diabetes Day!
Celebrate World Diabetes Day in style with our fully editable Google Slides and PowerPoint template. Complete with illuminating light green design and vivid photographs, it provides a perfect platform to give an informative presentation about diabetes, its varied types, and summarize significant research. It also offers a fantastic opportunity to...

Diabetes Devices and Technology Breakthrough
This modern and cool template is the perfect way to showcase the amazing breakthroughs in diabetes technologies and devices. With this template, you can easily present the latest developments to help treat and control diabetes. It features modern visuals and graphics that provide insight into the technology, as well as...

Diabetes Mellitus Disease
Diabetes mellitus refers to a group of diseases that affects the way the body uses blood glucose. Glucose is vital for health, as it is an important source of energy for the cells that make up muscles and tissues. Understanding a disease thoroughly is the first step to being able...

Diabetes Mellitus Breakthrough
Diabetes mellitus refers to a group of diseases that affects the way the body uses blood glucose. Many people in the world suffer from this disease, and we are sure that your breakthrough will give a new perspective to its diagnosis and treatment. Present it with this complete and impressive...

Kidney Diseases: Diabetic Nephropathy
Download the Kidney Diseases: Diabetic Nephropathy presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking...

Atypical Diabetic Ketoacidosis Case Report
Are you tired of scribbling notes on scratch paper, or worse - forgetting vital details about your patients' cases? Well, don’t worry about it anymore! We've got a template that will make organizing your atypical diabetic ketoacidosis case reports a breeze! This watercolor beauty features a gentle pastel palette with...
- Page 1 of 2
Great presentations, faster
Slidesgo for Google Slides :
The easy way to wow
Questionnaire-Based Polyexposure Assessment Outperforms Polygenic Scores for Classification of Type 2 Diabetes in a Multiancestry Cohort
Add to collection, downloadable content.
- Other Affiliation: Biostatistics & Computational Biology Branch, National Institute of Environmental Health Sciences, Durham, NC, United States
- Other Affiliation: National Institute of Environmental Health Sciences, Durham, NC, United States
- Affiliation: School of Medicine, Department of Medicine
- Other Affiliation: Clinical Research Branch, National Institute of Environmental Health Sciences, Durham, NC, United States
- Other Affiliation: National Institute of Environmental Health Science, Durham, NC, United States
- OBJECTIVE Environmental exposures may have greater predictive power for type 2 diabetes than polygenic scores (PGS). Studies examining environmental risk factors, however, have included only individuals with European ancestry, limiting the applicability of results. We conducted an exposome-wide association study in the multiancestry Personalized Environment and Genes Study to assess the effects of environmental factors on type 2 diabetes. RESEARCH DESIGN AND METHODS Using logistic regression for single-exposure analysis, we identified exposures associated with type 2 diabetes, adjusting for age, BMI, household income, and self-reported sex and race. To compare cumulative genetic and environmental effects, we computed an overall clinical score (OCS) as a weighted sum of BMI and prediabetes, hyperten-sion, and high cholesterol status and a polyexposure score (PXS) as a weighted sum of 13 environmental variables. Using UK Biobank data, we developed a multiancestry PGS and calculated it for participants. RESULTS We found 76 significant associations with type 2 diabetes, including novel associations of asbestos and coal dust exposure. OCS, PXS, and PGS were significantly associated with type 2 diabetes. PXS had moderate power to determine associations, with larger effect size and greater power and reclassification improvement than PGS. For all scores, the results differed by race. CONCLUSIONS Our findings in a multiancestry cohort elucidate how type 2 diabetes odds can be at-tributed to clinical, genetic, and environmental factors and emphasize the need for exposome data in disease-risk association studies. Race-based differences in predictive scores highlight the need for genetic and exposome-wide studies in diverse populations. EmR2TaFJ.
- Risk Factors
- Diabetes Mellitus, Type 2
- questionnaire
- disease classification
- structured questionnaire
- Hypertension
- Environmental Exposure
- genetic risk score
- cholesterol level
- patient participation
- household income
- cohort analysis
- self report
- environmental exposure
- Genome-Wide Association Study
- controlled study
- genome-wide association study
- clinical assessment
- Surveys and Questionnaires
- environmental factor
- non insulin dependent diabetes mellitus
- Multifactorial Inheritance
- middle aged
- groups by age
- complication
- major clinical study
- United Kingdom
- effect size
- population structure
- impaired glucose tolerance
- risk factor
- hypertension
- multifactorial inheritance
- https://doi.org/10.17615/xqbp-5w10
- https://doi.org/10.2337/dc22-0295
- In Copyright
- Diabetes Care
- National Institute of Environmental Health Sciences, NIEHS
- National Institutes of Health, NIH
- American Diabetes Association Inc.
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Research Day Recap: Hunter Lecture and Fellow Presentations
Keeley Higley May 16, 2024

Hunter Lecture
Fellow thesis presentations , “early pregnancy associations with gestational diabetes: the hoosier moms cohort” .
Hani Faysal, MD, PREGMED Postdoctoral Research Fellow Faculty Advisor: David Haas, MD, MS Summary: Gestational Diabetes (GDM) raises the risk of adverse perinatal outcomes and long-term risk of type 2 diabetes. There is a lack of comprehensive GDM prediction models based on more than simple clinical features. The objective of this study was to collect a comprehensive set of clinical, sociodemographic, biobehavioral, and genomic features in a prospective high-risk cohort for GDM, to discover novel predictive and therapeutic targets for GDM during early pregnancy. The Hoosier Moms Cohort identified that participants with a previous GDM diagnosis, hypertension, elevated BMI, and insomnia have significantly increased odds of developing GDM in a diverse cohort of participants. These factors will be integrated in a machine learning model with multi-omics data to develop a comprehensive predictor for GDM.
“Sterilization Rates Following the Overturn of Roe v. Wade”
Deena Elsahy, MD, MIGS Fellow Faculty Advisors: Kelly Kasper, MD Summary: On June 24, 2022, the Supreme Court of the United States overturned the 1973 Roe v. Wade and 1992 Planned Parenthood v Casey decisions. In doing so, the right to abortion was no longer protected by the Constitution and was instead left to each state to decide. This decision has caused significant uncertainty regarding the future of reproductive health care. We conducted a retrospective cohort study with data collected over the span of 20 months to compare rates of sterilization pre- and post- the overturn of Roe v. Wade. Our study found that at our institution, there was a significant increase in the weekly number of sterilizations performed following the overturn of Roe v. Wade. Further studies are needed to assess motivation for sterilization and whether fear of being unable to access contraception and/or abortion care influenced patient’s decisions.
“Maternal Weight Gain Among Individuals with Type 2 Diabetes and Associated Perinatal Outcomes”
Stacey Stivers, MD, MFM Fellow Faculty Advisors: Christina Scifres, MD Summary: The prevalence of Type 2 Diabetes Mellitus (T2DM) in pregnancy is increasing, and adverse perinatal outcomes are common. We sought to assess whether weight gain below the recommendations per National Academy of Medicine is associated with adverse perinatal outcomes in T2DM. Our study found that weight gain <5kg in women with obesity and T2DM is associated with a reduced risk for certain outcomes. The increased risk for stillbirth deserves further study.
“The Presacral Space Re-Visited: Correlation Between Presacral Depth and Body Mass Index (BMI)”
Keeley Higley
- Reference Manager
- Simple TEXT file
People also looked at
Mini review article, acute complicated jejunum diverticulitis: a case report with a short literature review.

- 1 Department of Surgery, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 2 Department of Surgery, Emergency Clinical County Hospital, Cluj-Napoca, Romania
- 3 Internal Medicine Department, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 4 Department of Internal Medicine, Emergency Clinical County Hospital, Cluj-Napoca, Romania
- 5 Department of Hematology, Oncology Institute "Prof. Dr. Ion Chiricuta ", Cluj-Napoca, Romania
Introduction: Jejunal diverticulosis is a rare condition. Most of the time, it is asymptomatic; but it can cause severe complications such as intestinal perforation, mechanical occlusion, and hemorrhage.
Case presentation: A patient aged 78 years, with a history of biological aortic valve prosthesis, atrial fibrillation, type 2 diabetes mellitus, and chronic obstructive pulmonary disease, presented in the emergency department for acute abdominal pain in the lower abdominal floor, nausea, and inappetence. Abdominal computed tomography revealed an inflammatory block in the hypogastrium, agglutinated small intestinal loops, fecal stasis, and air inclusions. Pulled mesentery and associated internal hernia are suspected. Exploratory laparotomy was performed, revealing an inflammatory block in the hypogastrium, whose dissection revealed inner purulent collection and the appearance of jejunal diverticulitis, a diagnosis confirmed by histopathological examination. Segmental resection of the jejunum with double-layer terminal–terminal enteroenteric anastomosis, lavage, and drainage was performed. The evolution was favorable.
Conclusion: Based on our brief review, the diagnosis of complicated jejunal diverticulosis is difficult and sometimes not accurately established, even by high-resolution imaging techniques, with diagnostic laparotomy being necessary for these situations. Surgical treatment should be considered before severe complications develop.
1 Introduction
Jejunal diverticulosis is a rare pathology that occurs in 0.3–1.3% of patients ( 1 ). Most often, it is asymptomatic; but sometimes it can give serious complications such as diverticulitis, perforation, mechanical occlusion, and hemorrhage. Due to its position, most of the time, high-evolution imaging techniques cannot establish the diagnosis. Therefore, this diagnosis should be considered in patients with intense abdominal pain localized periumbilically or in the hypogastrium. Performing an emergency laparoscopy is preferable to a conservative medical attitude in these situations. We present the case of a 78-year-old woman who presented with a complication of small intestine diverticulosis – diverticulitis and intestinal perforation requiring emergency surgery.
This paper aims to describe a case of small intestine diverticulitis and intestinal perforation and review the cases of small intestine diverticulitis published in the last 10 years to determine the best method of diagnosis and appropriate conservative or surgical treatment for these cases.
2 Case presentation
A 78-year-old female patient was referred to our emergency department with complaints of lower abdominal pain, slowed gastrointestinal transit, nausea, loss of appetite, and fatigue.
Her medical history was significant for hypertension, biological aortic valve, mitral valve regurgitation, atrial fibrillation, heart failure NYHA II/III, chronic obstructive pulmonary disease Gold II, type 2 diabetes mellitus, and dyslipidemia. There was no relevant family history. Her medication history consisted of Perindopril 10 mg/Indapamide 2.5 mg, Acenocoumarol 2 mg/day, Digoxin 0.25 mg/day, 5/7 days, Budesonide 160 microg/Formoterol 4.5 micron 2× two puffs/day, and Metformin 1,000 mg/day.
The pain was moderate without irradiation. There were no aggravating or relieving factors. The symptoms were worsening in the last 4 days before admission.
On admission, the patient had a body temperature of 37.7°C, a pulse rate of 80 beats/min, a blood pressure of 140/80 mmHg, and a saturation of 96% in ambient air. Physical examination revealed a new periumbilical mass associated with lower abdominal tenderness but no rigidity or rebound tenderness.
Blood tests showed leukocytosis (22 × 109/L), neutrophilia (20.09 × 109/L), C-reactive protein >30 mg/dL, procalcitonin 9.5 ng/mL, creatinine = 3.42 mg/dL, urea = 127 mg/dL, and INR > 9.
An abdominal ultrasound was performed, which revealed intestinal loops with peristalsis present at the level of the descending colon – a slightly dilated intestinal loop with a slightly thickened intestinal wall. We completed with abdominal and pelvic computer tomography scan (CT), which showed an inflammatory block at the level of the hypogastrium – thin intestinal loops, agglutinated, forming a lesional block of 90/69/60 mm, with fecal stasis and air inclusions. Adjacent fat infiltrated, with multiple fluid fuses present. Pulled mesentery and mesenteric vessels – an associated internal hernia is suspected ( Figure 1 ).
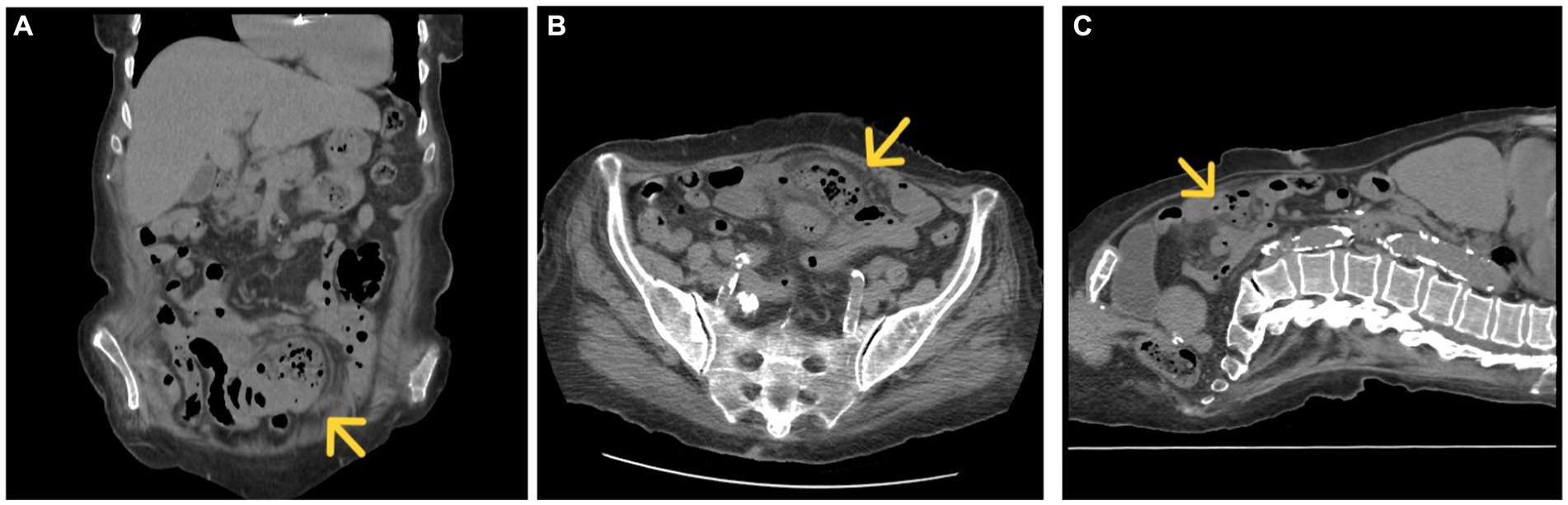
Figure 1 . CT, coronal (A) , axial (B) , and sagittal section (C) inflammatory block marked with a yellow arrow.
An echocardiogram reported neurofunctional biological aortic valve, concentric left ventricular hypertrophy, and mitral valve regurgitation. There were no vegetations of valves observed.
Due to high suspicion of intestinal subocclusion, the patient underwent exploratory laparotomy via a median incision. The abdominal cavity was explored, detecting an epigastric inflammatory block that includes several loops of the small intestine (jejunum and ileum) and omentum, the dissection of which reveals the minimal interileal purulent collection and a tumor at about 50 cm from the duodenojejunal angle, with the appearance of diverticulitis. At 40 cm from the duodenojejunal angle, another uncomplicated intestinal diverticulum of about 2 cm diameter is identified ( Figure 2 ).
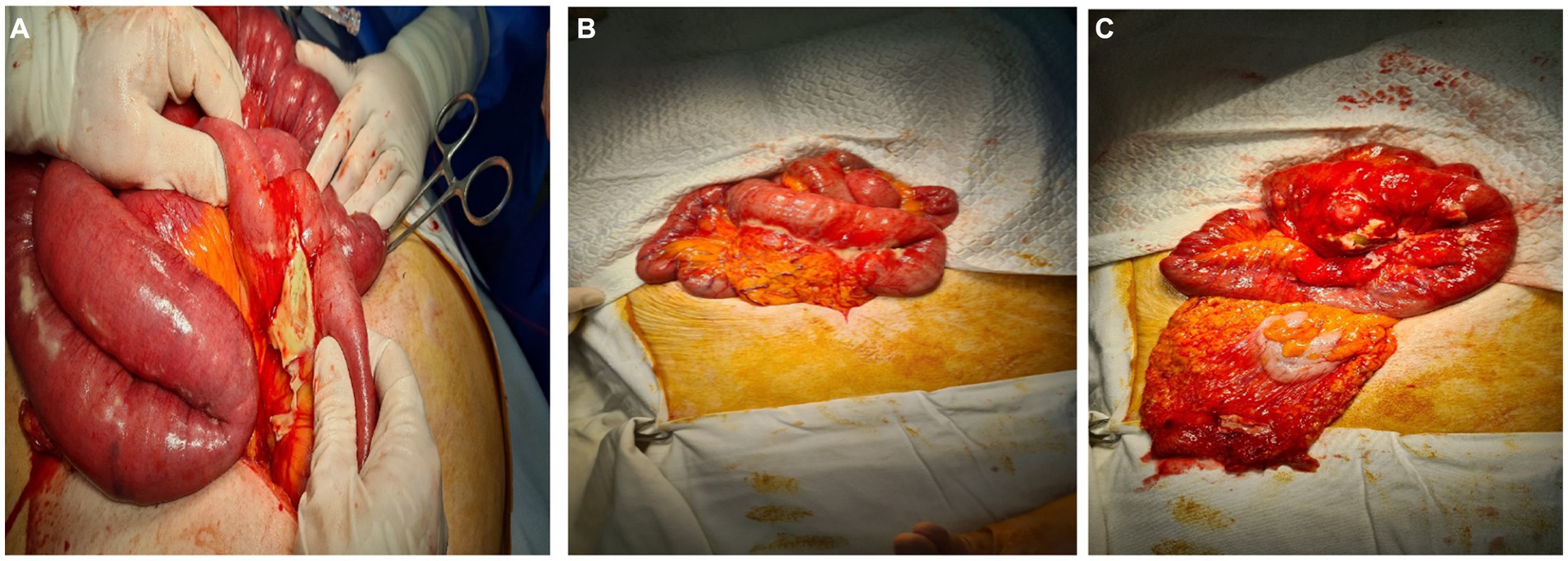
Figure 2 . (A–C) Intraoperative appearance. Intestinal inflammatory block, the dissection of which reveals an abscessed jejunal diverticulum.
We performed segmental resection of the jejunum with terminal–terminal enteroenteric anastomosis.
in a double layer, lavage, and drainage. IV fluids, Ceftriaxone 2 g/day once daily, Metronidazole 500 mg every 8 h, probiotics, and Famotidine 20 mg were administered. The treatment of associated diseases continued.
On Day 4, the patient had normal gastrointestinal transit. During hospitalization, the patient presented one episode of upper gastrointestinal bleeding. The patient underwent an esogastroduodenal endoscopy, which revealed a Forrest III antral gastric ulcer and congestive corporeal gastritis without an active source of bleeding. Under treatment with proton pump inhibitors, erythrocyte mass transfusion evolution was favorable. The patient was discharged home on day 21.
Histopathological examination confirmed the diagnosis of jejunal diverticulitis. Stasis, hemorrhagic areas, and lymphoid follicles were observed at the resection margins. The intestinal wall shows areas of necrosis spread throughout its thickness, hemorrhagic areas, abscesses, and a marked transparietal predominantly neutrophilic inflammatory infiltrate. At the level of the diverticulum, fibrin–hematic exudate is observed at the level of the serosa, vascular stasis, hemorrhagic areas, and mixed inflammatory infiltrate, with the presence of lymphoid follicles and erosions at the level of the mucosa ( Figures 1 , 2 ).
3 Discussion
Diverticula are hernias of the mucosa and submucosa through the muscular layer of the intestinal wall. It is usually located in the sigmoid and descending colon ( 1 ).
Localization in the small intestine is rare; the incidence varies between 0.5 and 2.3%. It is usually identified at the level of the proximal jejunum (75%), with the ileum being identified only in 5% of cases ( 1 ). Jejunal diverticula are usually multiple and occur more frequently in men in the sixth or seventh decade ( 2 , 3 ).
Diverticula form in the intestinal wall, in areas of low resistance, due to increased intraluminal pressure ( 4 ). They may have a genetic determinism that should be suspected, especially in the case of diffuse forms ( 5 , 6 ). It is located more frequently in the jejunum than the ileum because the penetrating jejunal arteries have a larger diameter. Other favorable factors for the appearance of diverticles are dysmotility and abnormalities in the mesenteric plex ( 1 , 4 ). They differ from Merkel diverticula, because they appear on the mesenteric margin ( 1 ).
Clinically, jejunal diverticulosis is usually asymptomatic (80%) of cases. When it is symptomatic, it is manifested by nonspecific abdominal pain, transit disorders (diarrhea/constipation), and flatulence ( 1 , 7 ). Complications of jejunal diverticulosis occur in about 10% of cases, most commonly consisting of acute diverticulitis, mechanical obstruction, volvulus, perforation, peritonitis, and hemorrhage ( 1 , 4 ). Perforation with peritonitis can be caused by an inflammatory diverticulum or a ruptured diverticular abscess, as was the case with our patient.
Because of its nonspecific symptoms and because it is rare, jejunal diverticulitis is often misinterpreted as appendicitis, peptic ulcer, cholecystitis, Crohn’s disease, or colonic diverticulitis ( 8 ). To avoid misdiagnosis, which inevitably leads to delayed treatment, clinicians should be aware of this entity.
Abdominal ultrasonography is used to establish the diagnosis at the first stage. This can sometimes indicate a thickened intestinal wall, irregular-looking formations related to the intestine, hypoechogenic having a hyperechogenic center – characteristic aspect for diverticula, and hyperechogenic tissue around these formations, indicating infiltration of surrounding fat or air bubbles ( 9 ).
Computed tomography (CT) is more sensitive in the diagnosis of acute diverticulitis and its complications compared to abdominal ultrasound and is therefore preferred ( 1 , 10 ).
CT scans identify diverticular inflammation characterized by peridiverticular edema and thickening of the diverticular wall ( 8 ). The presence of pneumoperitoneum is not a definite sign of peritonitis, because the thin wall of the diverticulum can allow air to pass through ( 9 ).
CT diagnosis is difficult and remains uncertain in advanced local forms, in which diverticula can no longer be identified due to extensive local inflammation that causes fluid and gaseous infiltration. Highlighting other diverticula on the mesenteric edge of the loop of the small intestine helps clarify the diagnosis ( 9 ).
Selective mesenteric angiography or CT angiography may be used to locate active bleeding in cases of jejunal diverticular hemorrhage ( 11 ).
Treatment of diverticulitis can be conservative and medical (antibiotic therapy, according to table) ( 10 ).
The most common bacterial etiology of diverticulitis are:
1. Enterobacteriaceae: Escherichia coli , Klebsiella sp., aerobic high gram-negative bacillus.
2. Bacteroides species.
3. Enterococcus species: Enterococcus faecalis most common, Enterococcus faecium.
4. Pseudomonas aeruginosa : 3–15% ( 12 ) (see Table 1 ).
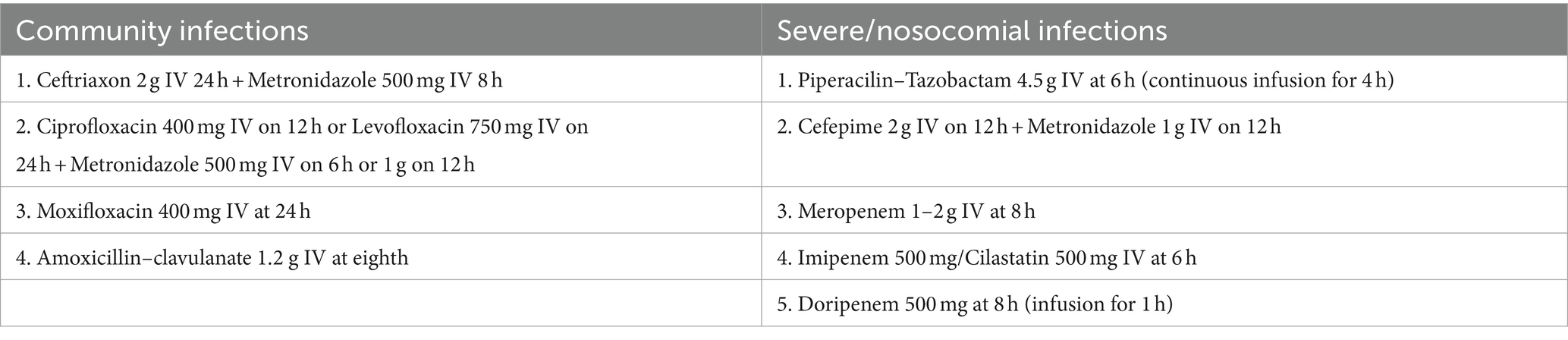
Table 1 . Antibiotic treatment in case of acute diverticulitis ( 12 ).
The average duration of antibiotic treatment is 5–10 days. The criteria for discontinuing treatment are clinical improvement, normalization of leukocytes, and resumption of intestinal transit ( 12 ).
In the case of localized limited perforation, conservative management treatment may be indicated in hemodynamically stable patients ( 4 , 7 ), with the caveat that surgery should be performed if clinical improvement is not achieved within 48–72 h. In patients with peridiverticular abscess, antibiotic treatment and image-guided drainage (CT) may theoretically be sufficient, depending on the size of the collection and the possibility of a percutaneous approach ( 8 ).
For perforated jejunal diverticula, with peritonitis, or in the case of abuse or significant bleeding, literature data recommend emergency laparotomy, segmental intestinal resection, and primary anastomosis to avoid complications ( 13 ). Resection should be limited to the intestinal loop with complicated diverticulum (local abscess, peritonitis, or bleeding) to prevent short bowel syndrome ( 4 , 11 ). An exception is pan-jejunoileal diverticulosis, for which conservative treatment may be preferred. This is because surgery can lead to severe malnutrition ( 3 ).
Risk factors for unfavorable evolution are old age, comorbidities, delay in diagnosis, and duration interval between perforation and surgery ( 9 ).
No consensus exists on the therapeutic strategy and management of jejunales diverticulitis ( 8 ).
To determine the best diagnostic method for complicated jejunal diverticulitis and the most appropriate treatment, we searched PubMed, MedNar, and Cochrane Library electronic databases for literature reviews on cases of jejunal diverticulitis published between 1.01.2014 and 31.12.2023. We considered the following terms in the studies’ title or abstract: “jejunal diverticulitis.” We excluded studies in languages other than English and French and excluded articles that did not cover several cases. The results are summarized in Table 2 .
Table 2 . Management of patients with acute diverticulitis according to specialized reviews from 2014–2023.
As Table 2 shows, a CT scan is more sensitive in diagnosing acute diverticulitis, but angiography is used in cases of gastrointestinal bleeding. Sometimes, exploratory laparoscopy is needed for diagnosis.
Thus, surgical treatment remains the management of choice in patients with jejunal diverticulitis, possibly due to late diagnosis in the complication phase ( 8 ).
In our case, due to advanced local inflammation, the diagnosis could not be established correctly by CT, and the abscessed and perforated intestinal diverticulum was misinterpreted as an internal hernia with intestinal occlusion. It was necessary to perform a laparotomy for diagnostic purposes and surgical treatment. The postoperative evolution was favorable, although the patient had an increased surgical risk of presenting multiple comorbidities.
4 Conclusion
Diagnosing complicated jejunal diverticulosis is complex and sometimes not accurately established, even by high-resolution imaging, such as a CT scan, the more sensitive diagnostic technique. Diagnostic laparotomy is necessary in these situations. Surgical treatment should be considered in complicated jejunal diverticulitis before severe complications develop.
Author contributions
SC: Writing – original draft, Writing – review & editing, Investigation, Methodology. MM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. MS: Investigation, Writing – original draft, Writing – review & editing. FM: Investigation, Methodology, Writing – original draft, Writing – review & editing. RC: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for this article’s research, authorship, and publication. The publication fees were partially reimbursed by the University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pajtak, R, Ramadan, A, and Strauss, P. Strangulated diverticulum: a new acute complication of small bowel diverticulosis. J Surg Case Rep . (2023) 5:rjad253. doi: 10.1093/jscr/rjad253
Crossref Full Text | Google Scholar
2. Karas, L, Asif, M, Chun, V, and Khan, FA. Complicated small bowel diverticular disease: a case series. BMJ Case Rep . (2017) 2017:bcr2017219699. doi: 10.1136/bcr-2017-219699
3. Prough, H, Jaffe, S, and Jones, B. Jejunal Diverticulitis. J Surg Case Rep . (2019) 1:rjz005. doi: 10.1093/jscr/rjz005
4. Khan, M, Arshad, R, Malik, I, Kamran, A, Gul, F, and Lee, KY. Jejunal diverticulosis presenting as intestinal obstruction - a case report of a rare association. Clin Case Rep . (2023) 11:e7033. doi: 10.1002/ccr3.7033
5. Scheese, D, Alwatari, Y, Khan, J, and Slaughter, A. Complicated jejunal diverticulitis: a case report and review of literature. Clin Case Reports . (2022) 10:e657011. doi: 10.1002/ccr3.6570
6. Barbaro, MR, Cremon, C, Fuschi, D, Marasco, G, Palombo, M, Stanghellini, V, et al. Pathophysiology of diverticular disease: from diverticula formation to symptom generation. Int J Mol Sci . (2022) 23:6698. doi: 10.3390/ijms23126698
7. Khsiba, A, Bradai, S, Mahmoudi, M, Mohamed, AB, Bradai, J, Bouzaidi, K, et al. Jejunal diverticulitis as a rare cause of abdominal pain: a case report. Pan Afr Med J . (2022) 17:222. doi: 10.11604/pamj.2022.41.222.29095
8. Zafouri, EB, Ben Ismail, I, Sghaier, M, Rebii, S, and Zoghlami, A. Jejunal diverticulitis: a new case report and a literature review. Int J Surg Case Rep . (2022) 97:107395. doi: 10.1016/j.ijscr.2022.107395
9. Lebert, P, Ernst, O, and Zins, M. Acquired diverticular disease of the jejunum and ileum: imaging features and pitfalls. Abdom. Radiol. (2019) 44:1734–43. doi: 10.1007/s00261-019-01928-1
PubMed Abstract | Crossref Full Text | Google Scholar
10. Rangan, V, and Lamont, JT. Small bowel diverticulosis: pathogenesis, clinical management, and new concepts. Curr Gastroenterol Rep . (2022) 22:4. doi: 10.1007/s11894-019-0741-2
11. Lin, CH, Hsieh, HF, Yu, CY, Yu, JC, Chan, DC, Chen, TW, et al. Diverticulosis of the jejunum with intestinal 5416Obstruction: a case report. World J Gastroenterol . (2005) 11:5416. doi: 10.3748/wjg.v11.i34.5416
12. CDC. Antibiotic Use in the United States, 2020 Update: Progress and Opportunities. Atlanta, GA: US Department of Health and Human Services, CDC , (2021)
Google Scholar
13. Ng, ZQ, Theophilus, M, Navadgi, S, Menon, T, and Wijesuriya, R. Jejunal diverticulitis: a single-Center experience and proposed management algorithm. Surg Infect . (2019) 20:499–503. doi: 10.1089/sur.2019.070
14. Johnson, KN, Fankhauser, GT, Chapital, AB, Merritt, MV, and Johnson, DJ. Emergency management of complicated jejunal diverticulosis. Am Surg . (2014) 80:600–3. doi: 10.1177/000313481408000625
15. Horesh, N, Klang, E, Gravetz, A, Nevo, Y, Amiel, I, Amitai, MM, et al. Jejunal Diverticulitis. J Laparoendosc Adv Surg Tech A . (2016) 26:596–9. doi: 10.1089/lap.2016.0066
16. Kumar, D . Complicated jejunal diverticulitis with unusual presentation. Radiol Case Rep . (2018) 13:58–64. doi: 10.1016/j.radcr.2017.10.002
17. De Simone, B, Alberici, L, Ansaloni, L, Sartelli, M, Coccolini, F, and Catena, F. Not all diverticulites are colonic: small bowel diverticulitis - a systematic review. Minerva Chir . (2019) 74:137–45. doi: 10.23736/S0026-4733.18.07745-3
18. López Marcano, AJ, Ramia, JM, De la Plaza, LR, Alonso, S, Gonzales Aguilar, JD, and Kühnhardt Barrantes, AW. Complicated jejunoileal diverticular disease: a 12 cases' serie and literature review. Rev Gastroenterol Peru . (2017) 37:240–5.
PubMed Abstract | Google Scholar
19. Mazahreh, TS, Aleshawi, AJ, Alorjani, MS, Elayyan, R, and Al-Zoubi, NA. Arteriovenous malformations within jejunal diverticulosis: cb ase report and literature review. BMC Surg . (2019) 19:70. doi: 10.1186/s12893-019-0538-0
Keywords: intestinal perforation, jejunal diverticulosis, diverticulitis, segmental resection, surgical treatment
Citation: Chiorescu S, Mocan M, Santa ME, Mihăileanu F and Chiorescu RM (2024) Acute complicated jejunum diverticulitis: a case report with a short literature review. Front. Med . 11:1413254. doi: 10.3389/fmed.2024.1413254
Received: 06 April 2024; Accepted: 23 April 2024; Published: 15 May 2024.
Reviewed by:
Copyright © 2024 Chiorescu, Mocan, Santa, Mihăileanu and Chiorescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mihaela Mocan, [email protected]
This article is part of the Research Topic
Diverticulitis - A Neglected Disease Despite its Clinical Burden

IMAGES
VIDEO
COMMENTS
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
DM is broadly classified into three types by etiology and clinical presentation, type 1 diabetes, type 2 diabetes, and gestational diabetes (GDM). Some other less common types of diabetes include monogenic diabetes and secondary diabetes. Type 1 Diabetes Mellitus (T1DM) Type 1 diabetes mellitus (T1DM) accounts for 5% to 10% of DM and is ...
Next: Physical Examination. Type 2 diabetes mellitus consists of an array of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. Poorly controlled type 2 diabetes is associated with an array of ...
Causes. Type 2 diabetes is mainly the result of two problems: Cells in muscle, fat and the liver become resistant to insulin As a result, the cells don't take in enough sugar. The pancreas can't make enough insulin to keep blood sugar levels within a healthy range. Exactly why this happens is not known.
Type 2 diabetes (T2D) is a chronic condition that happens when you have persistently high blood sugar levels (hyperglycemia). Healthy blood sugar (glucose) levels are 70 to 99 milligrams per deciliter (mg/dL). If you have undiagnosed Type 2 diabetes, your levels are typically 126 mg/dL or higher.
Type 2 Diabetes in Children and Teens. Childhood obesity rates are rising, and so are the rates of type 2 diabetes in youth. More than 75% of children with type 2 diabetes have a close relative who has it, too. But it's not always because family members are related; it can also be because they share certain habits that can increase their risk.
Type 2 diabetes, the most common type of diabetes, is a disease that occurs when your blood glucose, also called blood sugar, is too high. Blood glucose is your main source of energy and comes mainly from the food you eat. Insulin, a hormone made by the pancreas, helps glucose get into your cells to be used for energy.
Type 2 diabetes is a metabolic disorder that causes your blood sugar levels to increase. The severity of diabetes can vary quite a bit: Some people get the disease well under control, and in others it leads to more health problems over time. There are two main types of diabetes: Type 1 diabetes usually develops in childhood or the teenage years.
140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L) is diagnosed as prediabetes. 200 mg/dL (11.1 mmol/L) or higher after two hours suggests diabetes. Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
Diabetes follows a progressive pattern with complex pathogenesis and varied presentation.[1,2] Hyperglycemia and its associated carbohydrate, fat, and protein metabolic dysfunctions affect multiple organs of the body and disrupt their normal functioning. ... Type 2 diabetes mellitus. T2DM, also known as non-insulin-dependent diabetes mellitus ...
PDF-1.4 %âãÏÓ 368 0 obj ¿
6 /25. Some health habits and medical conditions related to your lifestyle can raise your odds of having type 2 diabetes, including: Being overweight, especially at the waist. A couch potato ...
Diabetes Basics. Diabetes is a chronic (long-lasting) disease that affects how your body turns food into energy. There are three main types of diabetes: type 1, type 2, and gestational diabetes (diabetes while pregnant). More than 133 million Americans are living with diabetes (37.3 million) or prediabetes (96 million).
In the United States, about 1 in 3 adults has prediabetes, and more than 8 in 10 of them don't know they have it. Without taking action, many people with prediabetes could develop type 2 diabetes within 5 years. With type 2 diabetes, your body can't effectively use insulin (a hormone that helps glucose get into the cells of the body).
Type 2 Diabetes Warning Signs. Warning Signs and Symptoms - Can occur slowly over time. Blurred vision. Tingling or numbness in legs, feet or fingers. Recurring skin, gum or urinary tract infections. Drowsiness. Slow healing of cuts and bruises. Any symptoms that occur with Type 1 diabetes.
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis.
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides ...
1. Introduction. Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic disorders worldwide and its development is primarily caused by a combination of two main factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin [].Insulin release and action have to precisely meet the metabolic demand; hence, the ...
2. INTRODUCTION Type 2 diabetes is sometimes called a "life style" disease as it more common in people who don't do enough exercise, have an unhealthy diet and obese. Type 2 Diabetes was previously seen mainly in older adults, however it is becoming more common in young children due to obesity and overweight children. 3.
Oct 3, 2009 • Download as PPT, PDF •. 21 likes • 10,279 views. D. dommilewis. Education Health & Medicine. 1 of 10. Download now. Type 2 Diabetes. Type 2 Diabetes PPT - Download as a PDF or view online for free.
Free Google Slides theme, PowerPoint template, and Canva presentation template. Type 2 diabetes is a chronic condition that affects the way the body processes glucose. With this disease, the patient's body does not produce enough insulin or is resistant to it. Share your research on this important disease using this modern and minimalist ...
Diabetes Presentation templates Here at Slidesgo, we would like to help you raise awareness about diabetes, a metabolic disorder related to insulin and the levels of glucose in our blood. ... Type 2 diabetes is a metabolic disorder with a high prevalence in the world. Hundreds of millions of people suffer from it every year. Basically, a person ...
Questionnaire-Based Polyexposure Assessment Outperforms Polygenic Scores for Classification of Type 2 Diabetes in a Multiancestry Cohort ... If you would like to deposit a poster, presentation, conference paper or white paper, use the "Scholarly Works" deposit form. Undergraduate Honors Theses.
Correlation of dietary inflammation index and dietary pattern with mild cognitive impairment in patients with type 2 diabetes. Shengdan Pu, Yuxin Xu, Xuewei Tong, Yitong Zhang, ... Xinyuan Gao ... An unusual presentation in a patient with Prader-Willi syndrome. Laura Costa, Emma Garcia-Grau, Laura Toledo, Nuria Burgaya, ... Assumpta Caixas ...
"The fellow presentations also show that the different divisions in our department do a really good job of preparing their fellows to utilize their research time effectively for their investigations into important topics." Read a summary of each fellow's presentation below. ... Summary: The prevalence of Type 2 Diabetes Mellitus (T2DM) in ...
Case presentation: A patient aged 78 years, with a history of biological aortic valve prosthesis, atrial fibrillation, type 2 diabetes mellitus, and chronic obstructive pulmonary disease, presented in the emergency department for acute abdominal pain in the lower abdominal floor, nausea, and inappetence. Abdominal computed tomography revealed ...