Organization Menu
Additional organization links, search and explore, primary menu.
Vaccine FAQ

Top 20 Questions about Vaccination
Last updated 22 April 2022
Our most frequently asked questions. Expand for detailed answers from experts.
Vaccines work to prime your immune system against future “attacks” by a particular disease. There are vaccines against both viral and bacterial pathogens, or disease-causing agents.
When a pathogen enters your body, your immune system generates antibodies to try to fight it off. Depending on the strength of your immune response and how effectively the antibodies fight off the pathogen, you may or may not get sick.
If you do fall ill, however, some of the antibodies that are created will remain in your body playing watchdog after you’re no longer sick. If you’re exposed to the same pathogen in the future, the antibodies will “recognize” it and fight it off.
Vaccines work because of this function of the immune system. They’re made from a killed, weakened, or partial version of a pathogen. When you get a vaccine, whatever version of the pathogen it contains isn’t strong or plentiful enough to make you sick, but it’s enough for your immune system to generate antibodies against it. As a result, you gain future immunity against the disease without having gotten sick: if you’re exposed to the pathogen again, your immune system will recognize it and be able to fight it off.
Some vaccines against bacteria are made with a form of the bacteria itself. In other cases, they may be made with a modified form of a toxin generated by the bacteria. Tetanus, for example, is not directly caused by the Clostridium tetani bacteria. Instead, its symptoms are primarily caused by tetanospasmin, a toxin generated by that bacterium. Some bacterial vaccines are therefore made with a weakened or inactivated version of the toxin that actually produces symptoms of illness. This weakened or inactivated toxin is called a toxoid. A tetanus immunization, for example, is made with tetanospasmin toxoid.
Vaccines are designed to generate an immune response that will protect the vaccinated individual during future exposures to the disease. Individual immune systems, however, are different enough that in some cases, a person’s immune system will not generate an adequate response. Therefore, he or she will not be effectively protected after immunization.
That said, the effectiveness of most vaccines is high. After receiving the second dose of the MMR vaccine (measles, mumps and rubella) or the standalone measles vaccine, 99.7% of vaccinated individuals are immune to measles. The inactivated polio vaccine offers 99% effectiveness after three doses. The varicella (chickenpox) vaccine is between 85% and 90% effective in preventing all varicella infections, but 100% effective in preventing moderate and severe chicken pox.
Currently, the U.S. childhood vaccination schedule for children between birth and six years of age recommends immunizations for 14 different diseases. Some parents worry this number seems high, particularly since some vaccine-preventable diseases are now extremely rare in the United States.
Each disease for which vaccinations are recommended can cause serious illness or death in unvaccinated populations, and could quickly begin to appear again if vaccination rates dropped. The United States has seen mumps outbreaks in recent years, since vaccination rates have dropped, with severe complications and hospitalizations required for some patients. And before the introduction of the Hib (Haemophilus Influenzae Type b) vaccine, Hib meningitis affected more than 12,000 American children annually, killing 600 and leaving many others with seizures, deafness, and developmental disabilities. After the vaccine was introduced, the number of deaths from Hib dropped to fewer than 10 per year.
Each vaccine on the schedule continues to be recommended because of the risks posed by wild infection.
In some cases, natural immunity is longer-lasting than the immunity gained from vaccination. The risks of natural infection, however, outweigh the risks of immunization for every recommended vaccine. For example, wild measles infection causes encephalitis (inflammation of the brain) for one in 1,000 infected individuals. Overall, measles infection kills two of every 1,000 infected individuals. In contrast, the combination MMR (measles, mumps and rubella) vaccine results in a severe allergic reaction only once in every million vaccinated individuals, while preventing measles infection. The benefits of vaccine-acquired immunity extraordinarily outweigh the serious risks of natural infection. (For more on this topic, see our .)
Additionally, the Hib ( Haemophilus influenzae type b) and tetanus vaccines actually provide more effective immunity than natural infection.
It is unclear why the length of acquired immunity varies with different vaccines. Some offer lifelong immunity with only one dose, while others require boosters to maintain immunity. Recent research has suggested that the persistence of immunity against a particular disease may depend on the speed with which that disease typically progresses through the body. If a disease progresses rapidly, the immune system’s memory response (that is, the “watchdog antibodies” generated after a previous infection or vaccination) may not respond quickly enough to prevent infection—unless they’ve been “reminded” about the disease fairly recently and are already watching for it. Boosters serve as a “reminder” to your immune system.
Research continues on the persistence of immunity generated by vaccines.
The idea of “pox parties” is generally tied to the perception of chickenpox as a harmless illness. Before the varicella vaccine became available, however, chickenpox infections required 10,000 hospitalizations and caused more than 100 deaths each year in the United States. Exposing a child to wild chickenpox puts him at risk for a severe case of the disease.
Even uncomplicated cases of chickenpox cause children to miss a week or more of school, with a caregiver missing work to care for the sick child. Natural infection also means a risk of infecting others: while successful vaccination protects a child against chickenpox without this risk, children infected with chickenpox naturally are contagious. They can spread the disease to other people—not just other children, but also adults, who have a higher risk of complications from the disease.
Meanwhile, vaccination for chickenpox typically prevents future infection with the disease. In rare cases where individuals do not develop adequate protection from vaccination to prevent future infection, chickenpox infection is typically mild, results in fewer symptoms, and ends more quickly than natural infection. (People with this mild form are contagious, however, and should take care not to expose others to the virus.)
Vaccines made with killed versions of pathogens—or with only a part of the pathogen—are not able to cause illness. When a person receives these vaccines, it is impossible for him or her to become ill with the disease.
Live, attenuated (or weakened) vaccines are theoretically capable of causing illness: because they can still replicate (though not well), mutation is possible, which can lead to a virulent form of the pathogen. However, they are designed with this in mind, and attenuated to minimize this possibility. Reversion to virulent form is a problem with some forms of the oral polio vaccine (OPV), which is why only the inactivated form (IPV) is now used in the United States.
It is important to note that attenuated vaccines can cause serious problems for individuals with weakened immune systems, such as cancer patients. These individuals may receive a killed form of the vaccine if one is available. If not, their doctors may recommend against vaccination. In such cases, individuals rely on herd immunity for protection.
Why some vaccines contain live pathogens and others contain killed pathogens, the reasons vary by illness. However, live, attenuated vaccines generally generate longer-lasting immunity than killed vaccines. Thus, killed vaccines are more likely to require boosters to maintain immunity. Killed vaccines, however, tend to be more stable for storage purposes, and can’t cause illness. The medical community must weigh these trade-offs in deciding which approach to use against a particular disease.
Yes. Studies demonstrate that infants’ immune systems can handle receiving many vaccines simultaneously—more than the number currently recommended. The immunization schedule is based on infants’ ability to generate immune responses, as well as when they are at risk of certain illnesses. For example, the immunity passed from mother to child at birth is only temporary, and typically does not include immunity against polio, hepatitis B, Haemophilus influenzae type b, and other diseases that can be prevented by vaccination.
Unlike most vaccines, which contain the most common strains of a given pathogen (if more than one exists) and are rarely changed, the seasonal flu vaccine changes frequently, though one or more flu strains in the vaccine may be retained from one year to the next. This is because the strains of influenza viruses that circulate are constantly changing. Each year, researchers choose viruses for the vaccine based on which ones are likely to circulate over the coming flu season, providing protection against the most prevalent strains. So when you get a seasonal flu vaccine, you’re usually not getting another “dose” of the same flu vaccine you were given before. Instead, you’re usually getting protection against a whole new batch of flu viruses.
Herd immunity, also known as community immunity, refers to the protection offered to everyone in a community by high vaccination rates. With enough people immunized against a given disease, it’s difficult for the disease to gain a foothold in the community. This offers some protection to those who are unable to receive vaccinations—including newborns and individuals with chronic illnesses—by reducing the likelihood of an outbreak that could expose them to the disease.
Some vaccines, including most vaccines against influenza, are cultured in chicken eggs. During the process of creating the vaccine, most egg protein is removed, but there is some concern that these vaccines could generate an allergic reaction in individuals with an egg allergy.
A recent report found that most children with egg allergies who were given a flu shot had no adverse reactions. About 5% of children in the studied group developed relatively minor reactions, such as hives, which resolved without treatment. Additional research is underway to study this issue further.
In most cases, only people with a severe (life-threatening) allergy to eggs are recommended against receiving egg-based vaccines. Your doctor can provide specific information.
No. Vaccines do not cause autism. This possibility was publicized after a 1998 paper by a British physician who claimed to have evidence that the MMR (measles, mumps and rubella) vaccine was linked to autism. The potential link has been thoroughly explored; study after study has found no such link, and , which had originally published it. Studies were also done regarding the possibility of a link between the preservative thimerosal, which is used in some vaccines, and autism; again, no such link was found.
It’s likely that this misconception persists because of the coincidence of timing between early childhood vaccinations and the first appearance of symptoms of autism.
All vaccines have possible side effects. Most, however, are mild and temporary. Adverse effects from vaccines are thoroughly monitored via multiple reporting systems, and there is no evidence from these systems to support these claims.
Every vaccine has potential side effects. Typically they are mild: soreness at the injection site (for a vaccine delivered via a shot), headaches, and low-grade fevers are examples of common vaccine side effects. Serious side effects are possible, including severe allergic reactions. However, these side effects are rare. (Your doctor can explain the risks for individual vaccines in detail; .)
When considering possible side effects from vaccination, it’s important to do so in context. While some possible side effects are serious, they are rare. It’s important to remember that choosing not to vaccinate also has serious risks. Vaccines protect against potentially fatal infectious diseases. Avoiding vaccination raises the risk of contracting those diseases and spreading them to others.
Vaccines are tested repeatedly before being approved, and continue to be monitored for adverse reactions after their release. See our article on vaccine testing and safety for more information and details about this topic.
No. The rubella vaccine virus included in the MMR (measles, mumps and rubella) shot is cultured using human cell lines. The vaccine material is carefully separated from the cells in which it was grown before being used.
Some of these cell lines were generated from fetal tissue obtained in the 1960s from legal abortions. No new fetal tissue is required to generate the rubella vaccine.
Improved hygiene and nutrition, among other factors, can certainly lower the incidence of some diseases. Data documenting the number of cases of a disease before and after the introduction of a vaccine, however, demonstrate that vaccines are responsible for the largest drops in disease rates. Measles cases, for example, numbered anywhere from 300,000 to 800,000 a year in the United States between 1950 and 1963, when a newly licensed measles vaccine went into widespread use. By 1965, U.S. measles cases were beginning a dramatic drop. In 1968, about 22,000 cases were reported (a drop of 97.25% from the height of 800,000 cases in just three years). By 1998, the number of cases averaged about 100 per year or less. A similar post-vaccination drop occurred with most diseases for which vaccines are available.
Perhaps the best evidence that vaccines, not hygiene and nutrition, are responsible for the sharp drop in disease and death rates is chickenpox. If hygiene and nutrition alone were enough to prevent infectious diseases, chickenpox rates would have dropped long before the introduction of the varicella vaccine, which was not available until the mid-1990s. Instead, the number of chickenpox cases in the United States in the early 1990s, before the vaccine was introduced in 1995, was about four million a year. By 2004, the disease incidence had dropped by about 85%.
In theory, nearly any infectious disease for which an effective vaccine exists should be eradicable. With sufficient vaccination levels and coordination between public health organizations, a disease can be prevented from gaining a foothold anywhere. Without anyone to infect, it must die off. (A notable exception is tetanus, which is infectious but not contagious: it’s caused by a bacterium commonly found in animal feces, among other places. Thus, tetanus could not be eradicated without completely removing the Clostridium tetani bacterium from the planet.)
Smallpox is unusual, however, in the characteristics that made it susceptible to eradication. Unlike many other infectious diseases, smallpox has no animal reservoir. That is, it can’t “hide” in an animal population and re-emerge to infect humans, while some diseases can do exactly that (yellow fever, for example, can infect some primates; if a mosquito bites an infected primate, it can transmit the virus back to humans).
Another obstacle to eradication for many infectious diseases is visibility. People with smallpox were highly visible: the smallpox rash was easily recognizable, so that new cases could be detected quickly. Vaccination efforts could be focused on the location of the cases and potential exposure to other individuals. Polio, by contrast, causes no visible symptoms in about 90% of the people it infects. As a result, tracking the spread of the polio virus is extremely difficult, making it a difficult eradication target.
Perhaps most importantly, smallpox patients generally did not reach their highest level of infectivity (that is, their ability to infect others) until after the appearance of the smallpox rash. As a result, quick action to quarantine infected individuals upon the eruption of the rash usually left enough time to vaccinate anyone already exposed, and prevent additional exposures. Many infectious diseases do not allow for this type of reaction time. Measles patients, for example, can become infectious up to four days before the appearance of the measles rash. As a result, they can pass the virus on to many, many other people before anyone even knows they are infected.
Many people still think eradication is possible for certain diseases. Efforts are ongoing to eradicate polio and Guinea worm disease (Dracunculiasis), with both eliminated in many regions, but remaining endemic in several countries. Meanwhile, the Carter Center International Task Force for Disease Eradication has declared additional diseases potentially eradicable: lymphatic filariasis (Elephantiasis), mumps, pork tapeworm, and yaws.
[For more about this topic, see our article on .]
The polio vaccines developed by Jonas Salk and Albert Sabin in the mid-20th century were made with monkey cells. Years later, microbiologist Maurice Hilleman found a monkey virus in both vaccines—the 40th monkey virus to be discovered, which he called Simian Virus 40 (SV40). (Salk’s killed vaccine, which was treated with formaldehyde, had very small amounts of the virus; Sabin’s live vaccine was heavily contaminated.) Worried about the potential effects the virus could have on humans, Hilleman injected it into hamsters, finding that nearly all of them developed massive cancerous tumors. But the initial panic this caused gave way in the face of future studies.
First, hamsters that ingested SV40 instead of being injected with it didn’t get cancer. Sabin’s live vaccine (which contained more SV40 than Salk’s) was given orally. Additional studies showed that children given Sabin’s vaccine did not develop antibodies to SV40; it simply passed through their digestive system, never causing infection.
That left only Salk’s vaccine, which contained very little SV40, but was given by injection. Studies performed eight years, fifteen years, and thirty years after SV40-contaminated vaccines were given to children found they had the same cancer incidence as unvaccinated groups. No credible evidence suggests SV40 has ever caused cancer in humans.
For a discussion on why the polio vaccine is not associated with HIV, read our article discussing this proposed association:
The mRNA vaccines developed in response to the COVID-19 pandemic caused concern among many people who claimed the mRNA technology was “too new” to be considered safe, or that it would be a while before we would know all the risks.
First, the clinical trials to show the vaccine safety and efficacy were carried out in the same manner as other clinical trials for vaccines. They had the same number of participants, same steps, and same oversight. The only aspect of those trials that was different was the timeframe in which they were done. Because of the need for new vaccines to counter to the COVID-19 pandemic, the studies and vaccine development/manufacturing were done simultaneously, not subsequently. Those clinical trials showed that the mRNA vaccines were safe and effective in preventing severe disease.
Second, mRNA technology has been around since the 1990s. So why have they not been used in vaccines? Two reasons: Lack of funding to use them as vaccines against infectious agents, and lack of interest, since the existing licensed vaccines worked well. In 2020, the Trump Administration authorized the use of funds for “Operation Warp Speed” to fund the rapid development of vaccines, taking care of the funding part. Once the mRNA vaccines were shown to be safe and effective against COVID-19, other infectious diseases were targeted for mRNA vaccine development.
- Gever, J. . MedPage Today. (2010) Accessed 01/25/2018.
- Carroll-Pankhurst, C., Engels, E.A., Strickler, H.D., Goedert, J.J., Wagner, J., Mortimer Jr, E.A. . British Journal of Cancer . 2001 Nov;85(9):1295. Accessed 01/25/2018.
- The Carter Center. . (20 KB PDF) Accessed 01/25/2018.
- Centers for Disease Control and Prevention. . Accessed 01/25/2018.
- CDC. . Accessed 01/25/2018.
- Sharp, P.M., Hahn, B.H. . Philosophical Transactions of the Royal Society of London. Biological Sciences . 2010 Aug 27;365(1552):2487-94. Accessed 01/25/2018.
- Worobey, M., Santiago, M.L., Keele, B.F., Ndjango, J.B., Joy, J.B., Labama, B.L., Dhed'a, B.D., Rambaut, A., Sharp, P.M., Shaw, G.M., Hahn, B.H. Origin of AIDS: contaminated polio vaccine theory refuted. Nature. 2004 Apr 22;428(6985):820-.
- Departments
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
More Questions and Answers About COVID-19 Vaccines
Interview by Stephanie Desmond
How is it possible that COVID-19 vaccines prevent serious illness and death but may not prevent mild infection? How effective are vaccines at preventing long-haul COVID? How soon might we see flu mRNA vaccines and would those have to go through clinical trials?
Josh Sharfstein answers a list of important questions about COVID-19 vaccines.
Most COVID vaccine information is focused on how effective they are at preventing serious disease, hospitalization, and death. How is it possible that the vaccine is more effective at preventing serious illness and death than it is preventing a mild infection?
It’s actually very common for vaccines to be much better at preventing serious illness and death than preventing infection or mild infection. For example, with the flu vaccine, people can still often get the flu, but they are much less likely to get seriously ill or die if they get the flu vaccine.
The question is why. It partly depends on how the immune system responds to vaccines. Any infection whatsoever is a certain type of immune response, and very few vaccines give what people call a “sterilizing immune response.”
What vaccines do cause is an immune response that is strong and multifaceted inside your body. So, even if you knew that the virus can replicate a bit for a mild infection, it can’t cause that huge overwhelming infection that really puts people at risk.
Early on in the pandemic, before we even had vaccines, some vaccine experts were saying the most important thing is going to be [preventing] serious illness and death, and [vaccines will] probably will be much better for that than for mild illness, just like almost every other vaccine out there. Sure enough, that proved to be the case.
How effective are vaccines at preventing long-haul COVID?
We don’t know. It’s a good question, because people can get these long-term symptoms from relatively mild infection.
There are some studies being set up to assess this, but we don’t know for sure. The safe bet would be that the chance of getting a long-haul infection is going to be much lower [for] someone who’s vaccinated compared to someone who’s not, just because that person is much less likely to get infected at all.
There’s also this related question of whether people with long-term symptoms from COVID actually might benefit from getting vaccinated. Somebody who had an infection and has been suffering some of those symptoms like fatigue and brain fog—does it get better if you get vaccinated? There’s no answer to that; however, at multiple clinical sites, some of the doctors are hearing from their patients that they’re feeling somewhat better. I think that the real answer to that, though, is going to depend on studies that will be completed, to see whether it makes a difference.
If I have no symptoms at all after receiving the Pfizer or Moderna vaccines, does this indicate that if I had gotten COVID, I would have been asymptomatic or had mild symptoms?
I do not think it means that.
What determines how sick you are from COVID-19 is a complex set of things that include how much virus your body actually took in. That’s one reason why people who get exposed to lower levels of virus are more likely to have an infection without symptoms, for example.
It also relates to different aspects of people’s immune system and probably some other factors we haven’t figured out, so I would not assume that the response to the vaccine is the same as the response to the actual virus.
Is this the first time mRNA technology has been used in a vaccine?
It is not, actually. There are several vaccines that are in development with mRNA technology. They’ve completed safety studies for them, and that includes influenza—so there could be an mRNA flu vaccine in the future—cytomegalovirus, Zika virus, and the rabies virus.
[These vaccine trials] haven’t made it all the way to the end [because] those were going through the regular vaccine process where you go one step at a time. Those companies aren’t going to invest in a big, next trial until they’ve really analyzed the data from the previous study.
In the case of [COVID-19 vaccines], we had a lot of urgency and all the money was put up, up front. The companies didn’t have to find the money for each stage—they were just able to just proceed from the safety study to the effectiveness study very quickly. This let the coronavirus vaccines go to the front of the line because of the urgency.
This is a technology that’s been well studied, not just for vaccines, but also for therapeutics.
Do you think that having successful mRNA COVID vaccines will pave the way for these other vaccines?
It’s going to be great for people’s comfort level with the vaccine, both at a level of understanding—like, “Wow, that’s going to be like the coronavirus vaccine, and it was so successful!”—and also scientifically, I think there’ll be a greater understanding of mRNA vaccines, and that will help with the development and the review of other mRNA vaccines for different different viruses.
Having said that, just because an mRNA vaccine works for coronavirus doesn’t mean it’s necessarily going to work for a rabies or influenza virus. They’re going to have to do studies to find out.
Do we know yet how soon flu vaccines may be made as mRNA vaccines, and will they have to go through clinical trials as a new vaccine?
I would expect that they would go to clinical trials … but I do know that some studies have already been done, and hopefully this will proceed and we’ll get another great vaccine.
One of the long-held goals for flu vaccination is a vaccine that lasts more than one year, and maybe a vaccine that doesn’t require a strain change every year. The mRNA vaccines may be a way to get to that goal, but there obviously has to be a lot more research.
Why are mRNA vaccines so encouraging for the future?
This is a platform that has certain advantages, among them, that you can stand it up so quickly. It doesn’t require a lot of different ingredients—it’s a very, very small number of things that go into the vaccine—and it can be updated, very quickly, so if you need to change the strain, it’s very possible to do that.
I think we’ll look back and think that mRNA kind of had its coming out party with coronavirus, but [was] around beforehand, and it will hopefully lead to some other important advances in medicine.
How are side effects from COVID-19 vaccines being monitored?
They’re being monitored in multiple ways. One thing that people who have gotten vaccinated know is that you have an opportunity to get texted about the potential side effects you’re experiencing. The Centers for Disease Control is looking at that from millions of people who are getting vaccinated to understand the profile of side effects. People also submit reports to the manufacturers and to the FDA about potential side effects, and there are studies that are done in large insurance databases or clinical databases where you can look at the people who got the vaccine compared to people who didn’t get the vaccine to see whether there’s any difference in case there’s a question about whether or not a particular side effect might be caused by the vaccine.
On a regular basis, there is a big group that comes together and looks at data from all these different sources to see what the safety profile is and, so far, it’s been very, very strong.
I was just looking at a 60-page document that’s posted on the CDC website where they went through all these different sources and they have a huge analysis of allergic reactions. I think the Pfizer vaccine had five serious allergic reactions per million doses given, and per 2.8 million for the Moderna vaccine. Almost always, those allergic reactions are in the first dose. Not always, but almost always.
It also talks about the evidence of the mild side effects people get. Seventy percent of the people get a sore arm; I think about a third got a headache, a third got fatigue, but then of course they feel better in just a couple of days.
They’ve been even doing studies in these insurance databases to compare people who are vaccinated and people who aren’t vaccinated just for things that people think “Well, maybe, could it possibly relate to this [vaccine]?” and they have not found any serious red flags coming up.
So, there’s a lot of analysis of safety data and there will continue to be. It’s a very important part of vaccination and the vaccination program to look at safety and not just in one way, but in multiple ways.
Does someone who recovered from COVID and then gets vaccinated have a higher immunity than someone who hasn’t had COVID and also gets vaccinated?
In general, people who have had COVID have some immune reaction to COVID when they recover. But it’s variable—some people may have a pretty mild immune reaction, and some people may have a very protective immune reaction—and right now, we don’t have an easy way to tell the difference between them.
That’s why vaccination is recommended for everyone, even if you’ve had COVID before. There will be studies of different types of people, their vaccination status and when they got vaccinated, and hopefully we’ll get a picture and some markers like a blood test that you could take to find out how protected you are. We have that for certain infectious diseases. You can, for example, for hepatitis B, see whether you have antibodies.
One of the things we’ll learn from some of these studies is, is there a way to test people for their ability to withstand a coronavirus infection? When we have that, I think that might be more important than these general questions because probably it will depend on the individual and having some way to test to figure that out over time is what will be helpful to people.
If I’ve had COVID, how long should I wait to get vaccinated? Is it okay to get my first dose if I no longer have symptoms?
The basic standard requirements are that if you are in that period where you’re sick and could be spreading COVID to stay home until you get better, which I think is around 10 days and no symptoms—then it’s fine to get vaccinated.
[Some] people have said you’re probably relatively protected from another infection for a couple months after that infection and, if you want to wait a couple of months to get vaccinated, you can do that. But there’s no requirement to do that. It’s perfectly fine to get vaccinated.
There are people who may get COVID right after their first shot, before there’s any protection, and they could get vaccinated for their second shot on time if they want, with one exception: If they’ve been treated for that COVID infection with antibody treatment, then there’s a recommendation to wait 90 days so that that antibody treatment doesn’t interfere with the vaccination.
What will happen if everyone gets vaccinated? Won’t the variants get tougher as their source of food gets eliminated?
The virus is constantly mutating and every time that it replicates, there’s a chance that you could develop a variant. If the virus can’t replicate, the virus can’t develop a variant. If the virus is replicating a lot, then you’re more likely to get variants.
The goal of a vaccination campaign now is to reduce the spread of the virus, which reduces the replication of the virus, which will reduce the chance that there will be more variants.
With less virus, fewer people are dying. And with less virus, fewer variants.
The CDC recently released guidance for what vaccinated people can do safely. What do you think of this?
One important principle is that vaccination is important to people both directly and indirectly.
Directly, it’s important if you’re protected, and there may be some things that are different, like you can meet up in small groups with people who are vaccinated.
There’s also the indirect benefit, which is the more people get vaccinated, the less coronavirus is spreading out there. The less coronavirus spreading out there, the easier it is to open things up again. That’s the indirect benefit, and that may not happen the day you get vaccinated or the day you’re protected from your vaccine. But, the more people in your community get vaccinated, the more likely the benefit is going to come help you.
This is exciting because we can see what the end of the pandemic might look like, but we just have to get there. We can’t trip on our way running too fast to the end of the pandemic.
Meanwhile, states like Texas and Mississippi have both rescinded their mask mandates. Is this getting a little too far ahead?
We have to push COVID as far into the end zone as it can go through good mask wearing, social distancing, and vaccination until we really are able to open things with competence. The risk of doing it too soon is that the virus keeps spreading, you get mutations, you get potential variants spreading, and we wind up taking a step back. That takes longer, in the end, to get to the place that we all want to go.
I’m also concerned about the mixed messaging. Mask wearing really does reduce infection, and we still have a lot of infections in the United States, even though it has come down. Just to hear from one level of government “Do this,” and another level of government “Do that,” it just stirs the pot again and makes it harder for people just to stick with the program long enough to put coronavirus back in a box, which I think is within reach.
Now, will what the governors do really upend that? We don’t know. But will it increase the risk of a problem? It might, and I think that’s why you hear so many people saying, “We’re headed toward the end zone, don’t blow it.”
Joshua Sharfstein, MD , is the vice dean for Public Health Practice and Community Engagement and a professor in Health Policy and Management . He is also the director of the Bloomberg American Health Initiative and a host of the Public Health On Call podcast.
Stephanie Desmon is the co-host of the Public Health On Call podcast. She is the director of public relations and marketing for the Johns Hopkins Center for Communication Programs , the largest center at the Bloomberg School of Public Health.
RELATED CONTENT
- Monica Gandhi and Vaccine Optimism (Podcast)
- Vaccine Week Finale: Q&A with Dr. Josh Sharfstein (podcast)
- For mRNA Vaccines, COVID-19 Is Just the Beginning
Facebook Live Q&A
This conversation is excerpted from a March 5 Facebook Live.
See More Like This
Related Content

What to Know About COVID FLiRT Variants

Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds

To Protect Human Health, We Must Protect the Earth’s Health

Outbreak Preparedness for All

What’s Happening With Dairy Cows and Bird Flu
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Year in Review
- Published: 14 December 2021
Eight unanswered questions about the COVID-19 pandemic
- Mike May 1
Nature Medicine volume 27 , pages 2058–2061 ( 2021 ) Cite this article
13k Accesses
2 Citations
70 Altmetric
Metrics details
We asked experts around the world what surprised them about SARS-CoV-2 and COVID-19, and what questions remain unanswered.
Linfa Wang: Bat immunology
Linfa Wang is a professor in the Emerging Infectious Diseases Program, Duke–National University of Singapore Medical School, Singapore.

From day one, when this mysterious pneumonia started happening in late 2019, I thought the causative agent could be another coronavirus — most likely another bat coronavirus. By the end of 2019, I had been studying bat coronaviruses for almost 17 years, and it’s no surprise anymore for people like me to see another animal coronavirus jumping into humans. From serological data from humans, I know that spillover of viruses from bats to humans happens often, but most of that spillover happens in remote areas, and it usually either is not very transmissible or doesn’t cause severe disease. But if any of these viruses get to a major city with lots of wildlife trading, the virus can adapt, via an animal intermediate host, allowing human-to-human transmission.
When spillover causes an outbreak of human infections, there are three types of viruses to consider: ancestral, progenitor, and outbreak. We know the ancestral virus in bats and the outbreak virus in humans (SARS-CoV-2), but not the progenitor virus — the virus that allowed the spillover. So, we need to focus on identifying the early transmission event.
Our lab developed a serological test for neutralizing antibodies to SARS-CoV-2. This can find antibodies that defend against the virus. With a large collection of pre-pandemic samples, serology could be used for origin studies. If we could randomly take a large number — for example, 10,000 — of pre-pandemic samples from different parts around the world, we could see when and where spillover occurred and determine which samples are the closest to the outbreak virus, SARS-CoV-2. This serosurveillance can be done only if the international community is willing to provide samples, but it’s going to be very difficult, if not impossible. Nobody wants to say COVID-19 started in their country.
Although the world is focused on bats as the source of this pandemic, I’d like to emphasize that we could learn so much from these animals. Bats are well known for studies of flight and echolocation, but they are also important reservoirs of viruses, although they are not susceptible to diseases caused by most viruses — with rabies as the main exception. Bats could be used as a model system for studying immunological defense in mammals, and these studies would surely benefit human health.
Bryan Grenfell, Caroline Wagner and Chadi Saad-Roy: New variants
Bryan Grenfell is the Kathryn Briger and Sarah Fenton Professor of Ecology and Evolutionary Biology and Public Affairs at the School of Public and International Affairs, Princeton University; Caroline Wagner is an assistant professor in the Department of Bioengineering, McGill University; Chadi Saad-Roy is a doctoral candidate at the Lewis-Sigler Institute for Integrative Genomics, Princeton University.

The population dynamics of SARS-CoV-2 are strongly influenced by a variety of parameters, including characteristics of immunity after natural infection or vaccination, availability of vaccines, non-pharmaceutical interventions, potential seasonal climatic or behavioral drivers, and — perhaps most importantly — emerging viral variants. In all likelihood, the virus will remain in society for a long time, and population dynamics may eventually equilibrate to some sort of endemic seasonal and/or longer-term epidemic cycle, given experience with previous acute pandemic and endemic diseases. However, we don’t know how long this shift from pandemic to endemic or epidemic will take, nor what the characteristics of the steady state will be — or how vaccination strategies will affect these patterns.
One surprise was how much non-pharmaceutical interventions for COVID-19 reduced the transmission of other respiratory infections, such as infection with influenza virus and respiratory syncytial virus, viruses that are now bouncing back.
Real-world studies have shown the considerable impact of vaccines, but the way vaccines are distributed strongly influences global population dynamics. Large pockets of unvaccinated individuals, particularly when they are spatially clustered, can drive large outbreaks. Our models, and a variety of other studies, illustrate that vaccine equity is crucial. Allowing transmission in unvaccinated populations presents an ideal scenario for pathogen evolution and new variants of concern, which ultimately threatens gains in immunity around the world, including in highly vaccinated populations.
There are several key unanswered questions about the evolution of SARS-CoV-2. In which populations are new variants occurring? What are the characteristics of breakthrough infections? In particular, what’s the susceptibility of vaccinees relative to that of unvaccinated people, and are breakthrough infections less transmissible? Are infections more likely to occur for different immune profiles, depending on prior natural infection, specific vaccination and specific viral strains? How long do natural and vaccinal immunity last — in terms of both clinical protection and infection?
We still don’t fully understand the dynamics and impact of new variants, but we are beginning to incorporate increasing amounts of real-world data related to immune parameters into our epidemic models, as well as continuing to refine how we incorporate human behavior.
Kathryn Edwards: Better data on boosters
Kathryn Edwards is a professor of pediatrics, the Sarah H. Sell and Cornelius Vanderbilt Chair, and the scientific director of the Vanderbilt Vaccine Research Program, Vanderbilt University Medical Center.

The really hard question is how we are going to live in the future with this pathogen, as SARS-CoV-2 is likely to be a permanent endemic virus. In the short term, everyone needs to get vaccinated. It is clear that areas with lower vaccination rates are having higher burdens of disease and higher death rates. What we don’t know is whether everybody needs to be boosted or how often everybody needs to be boosted. Understanding the need, or not, for boosters, will require that the research community and policymakers have an open mind, and let the data tell us what we need to do.
Worries from the public about vaccine safety are an ongoing concern. All the vaccine companies and the Centers for Disease Control and Prevention (CDC) are calling on people who have been studying vaccines all their life to make sure of safety, which has included my working on a CDC contract to look at vaccine safety, and on the safety monitoring committee for Pfizer. It is important that researchers continue to better understand when adverse events happen, how could they be prevented and what the reasons for the adverse events are.
But overall, vaccines for COVID-19 are incredibly, incredibly safe. There have been some safety signals, but they’ve been quite rare — just a few in a million. Those of us who work on vaccine safety need to better communicate how seriously we take assessing safety and making sure that the vaccines are as safe as they can be. If the public understands and appreciates this work, then this can reduce vaccine hesitancy.
Manoj Murhekar: Reasons for hesitancy
Manoj Murhekar is the director of the National Institute of Epidemiology, Indian Council of Medical Research, Chennai.

One unexpected challenge early in the pandemic was the stigma associated with COVID-19. Some people were reluctant to get tested, fearing isolation or quarantine. This even affected healthcare workers, who faced hardships and alienation because of their vulnerability to catch SARS-CoV-2.
India has a huge population, with about 940 million adults eligible to receive a vaccine against COVID-19. As of September 2021, nearly 70% of the eligible individuals had received one dose and 25% had received both doses. Sustaining the pace of vaccination to cover the remaining unvaccinated population is the greatest challenge facing India in 2021 and 2022.
Despite the availability of effective and safe vaccines, and even after seeing their near and dear ones dying, some sections of society are hesitant to receive a vaccine. More research is needed to understand the reasons for vaccine hesitancy and how to tackle it.
Malik Peiris: Mucosal immunity
Malik Peiris is a professor of virology at the School of Public Health, and a co-director of the World Health Organization H5 Reference Laboratory and SARS-CoV-2 Reference Laboratory, University of Hong Kong.

Although we have learned a lot about transmission of SARS-CoV-2, questions still remain. We still don’t know the relative contributions of small airborne aerosols versus droplets versus fomites in transmission. Clearly, the contribution of different modalities differs depending on context and the environmental setting. We also do not know how the more infectious variants, such as Delta, increase transmission. It could arise from an increased viral load shed from infected persons, increased duration of infectiousness or increased survival of infectious virus in the aerosols.
We also need to know more about the impact of various public-health measures, such as how much different types of masks affect transmission. Similarly, we don’t yet fully understand how much different vaccines reduce transmission and what is the durability of this impact, and if it differs with the vaccine type and viral variants.
It is clear that currently available vaccines alone do not entirely prevent transmission. But what happens when vaccinated individuals get — hopefully mild — breakthrough infections? Vaccine immunity followed by natural infection — even if asymptomatic — may elicit enhanced transmission-blocking immunity because the mucosal immune system is now engaged after infection via the nose and throat. If vaccination followed by a usually mild breakthrough infection provides broadened immunity to or protection against a range of variants, we may be seeing light at the end of the tunnel.
This raises questions about the best route of delivery of COVID-19 vaccines. Injected vaccines do provide good immunity against severe disease, but that is not the ideal route for eliciting immunity in the nasopharyngeal mucosa, which is needed for full protection from infection and transmission. An intranasal vaccine could be more successful in preventing transmission. Perhaps a combination of conventional injected vaccines and intranasal vaccines would elicit both systemic immunity and mucosal immunity that will provide better protection from infection and reduce transmission.
Ester Cerdeira Sabino: Political obstacles
Ester Sabino is a professor at the Institute of Tropical Medicine, University of São Paulo, Brazil.

In Brazil, the autochthonous transmission of the Delta variant started in Rio de Janeiro in June 2021 and then spread across the country. The Delta variant is now responsible for most of the cases, but cases and deaths are decreasing, and only Rio de Janeiro suffered a new wave due to this variant.
The lack of a new wave of infections across Brazil is clearly a positive development, but we need to understand why this is happening, as by the end of September 2021 only 42% of the population were fully vaccinated. It may be that the first vaccine dose, combined with prior immunity from natural infection, has helped to contain a third wave in Brazil. Approximately 72% of the Brazilian population had received at least one dose of the vaccine.
One unexpected development was that the federal government delayed the process of vaccine acquisition in Brazil. When the outbreak caused by the Gamma variant exploded in February 2021, there were vaccines only for healthcare workers and senior citizens. At that point, mainly the CoronaVac (Sinovac) and ChAdOx1 (Oxford–AstraZeneca) vaccines were used, because public vaccine companies in Brazil were able to have contracts to manufacture the vaccines, and they were manufactured in part locally. Unexpected delays in procurement meant that large-scale vaccination of individuals under the age of 60 only started in May 2021, a program that also included the BNT162b2 (BioNTech–Pfizer) vaccine.
Brazil has a long history of successful vaccine programs and a well-organized system for delivering vaccines across the country, including to remote regions in the Amazon basin. In contrast to some other countries, Brazil does not have a problem with vaccine hesitancy — only with a lack of vaccine. Now that we have the vaccine, immunization has started to move very fast.
One unanswered question concerns the need for boosters. Rollout of a test to measure the population level of immunity can inform the health authorities if and when a vaccine booster will be necessary. Boosters will be impacted by new variants of concern, which can be identified only by continued genomic surveillance. Brazil is contributing to this worldwide sequencing effort, with more than 40,000 sequences submitted to the GISAID database in 2021.
Tim Spector: Symptom tracking
Tim Spector is a professor of genetic epidemiology at Kings College London, and is lead investigator of the ZOE COVID Study.

In March 2020, we started the ZOE COVID Study to collect information on symptoms from people with suspected COVID-19. This was so that we could predict who had COVID-19 and who didn’t, early in the pandemic while testing capacity was low. Four and a half million people downloaded the app in the United Kingdom and United States. One of our biggest discoveries was how loss of smell and taste was predictive of PCR positivity, as well as the risk factors for developing long COVID . With nearly a million people regularly logging in, we’ve also seen a recent change in symptoms: as the variants and environment change, including weather, seasons and social factors, symptoms are becoming more respiratory and less systemic.
The ZOE team has now identified an incredible range of symptoms, with over 25 symptoms being recognized for the combination of COVID-19 and long COVID. A big question is how the same virus gives some people a skin rash on their toes, but others get gastrointestinal issues, and 40% get cold-like symptoms, and so on. Why do some symptoms develop three weeks or three months after being infected? SARS-CoV-2 is a mysterious virus, and we need to keep an open mind on why these symptoms are occurring as the science and our knowledge changes.
The marked variations in symptoms and in immune responses, whereby only 80% of people produce antibodies, could lead to personalized infection advice. We should probably have a personalized risk approach to vaccination, knowing when to get boosters, and selecting the best treatments when someone gets sick. If we start now and use the technology at our disposal, we should be in a much better place for future pandemics.
John Nkengasong: Rebuilding trust
John Nkengasong is the director of the Africa Centres for Disease Control and Prevention.

The most urgent and important question for COVID-19 in Africa is how to gain access to vaccines. Without a deliberate commitment globally to scale up vaccination in Africa, its people will be threatened by endemic COVID-19 forever. Africa has vaccinated about 5% of its population in a continent of 1.2 billion people. Africa is dealing with a vaccine famine. Everybody in Africa now knows somebody who has died of COVID-19, so it has become very personal. The solution is producing the vaccines in Africa, but it will take time to build factories and run them appropriately.
Once African nations have access to vaccines, we need to sign a social contract that says ‘I will vaccinate myself, because I want to protect myself, and by protecting myself, I protect my loved ones and I protect my community and I protect my country’. Without that, we are very quickly moving toward endemicity, and who wants to live with another endemic disease like AIDS, tuberculosis or malaria?
I’m very encouraged that people across Africa are moving in the right direction, but there are clearly some issues with trust of vaccines. There is a rupture in the relationship between science and the community that to me is very surprising. What we don’t know is why anyone might not want to get vaccines and how we can have a proper dialogue on how to change that. It should be part of preparing for the next pandemic.
Editorial note
These interviews have been edited for length and clarity.
Author information
Authors and affiliations.
Freelance writer and editor, Seattle, WA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mike May .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
May, M. Eight unanswered questions about the COVID-19 pandemic. Nat Med 27 , 2058–2061 (2021). https://doi.org/10.1038/s41591-021-01598-x
Download citation
Published : 14 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41591-021-01598-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
How Americans View the Coronavirus, COVID-19 Vaccines Amid Declining Levels of Concern
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
Americans’ Largely Positive Views of Childhood Vaccines Hold Steady
About nine-in-ten (88%) Americans say, overall, the benefits of childhood vaccines for measles, mumps and rubella outweigh the risks, identical to the share who said this before the coronavirus outbreak. U.S. adults are less confident in COVID-19 vaccines: Fewer than half rate them as having high health benefits and a low risk of side effects.
About half of recent online daters in U.S. say it’s important to see COVID-19 vaccination status on profiles
Online dating users who are Democrats are far more likely their Republican counterparts to say someone’s vaccination status is important for them to see.
Sign up for our Internet, Science, and Tech newsletter
New findings, delivered monthly
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
Lack of Preparedness Among Top Reactions Americans Have to Public Health Officials’ COVID-19 Response
Overall, 46% of Americans say the statement “public health officials were unprepared for the outbreak” describes their views extremely or very well, including similar shares of Republicans and Democrats.
Gay or bisexual men express concern about monkeypox, are critical of government’s response
Men who describe themselves as gay or bisexual are more likely to say they have received or intend to get a monkeypox vaccine.
Partisan differences are common in the lessons Americans take away from COVID-19
Here’s what Americans said they learned about the development of vaccines and medical treatments and their advice for handling a future outbreak.
Americans Reflect on Nation’s COVID-19 Response
Americans offer a lackluster evaluation of how the country has balanced priorities during the coronavirus outbreak. Fewer than half say the country has given the right amount of priority to the needs of K-12 students, public health or quality of life.
Americans skeptical about religious objections to COVID-19 vaccines, but oppose employer mandates
Most U.S. adults do not believe that requests for religious exemptions from the COVID-19 vaccine are sincere.
Two Years Into the Pandemic, Americans Inch Closer to a New Normal
Americans in 2022 find themselves in an environment that is at once greatly improved and frustratingly familiar.
COVID-19 Pandemic Continues To Reshape Work in America
Nearly two years into the COVID-19 pandemic, roughly six-in-ten U.S. workers who say their jobs can mainly be done from home (59%) are working from home all or most of the time.
REFINE YOUR SELECTION
Research teams.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
© 2024 Pew Research Center
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8; 2021 Aug
Willingness to vaccinate against Covid-19: A qualitative study involving older adults from Southern Switzerland
The Covid-19 pandemic is causing unprecedented disruption and suffering to people across the globe, with a disproportionate toll on the elderly. The development and equitable distribution of a vaccine seems to be the most promising and sustainable route ahead. The goal of this study was to explore older adults’ attitudes towards and beliefs regarding the Covid-19 vaccination in Southern Switzerland.
We conducted a qualitative study employing telephone interviews to understand older adults’ attitudes towards and beliefs about the Covid-19 vaccine. No Covid-19 vaccine had yet been approved at the moment of data collection. A convenience and snowball sample of 19 participants was recruited. Participants had to be at least 65 years old, without any hearing impairments, and be resident in the Canton of Ticino.
Most participants were women (n = 12), Swiss nationals (n = 14), retired (n = 18), resident in urban areas (n = 14), and had obtained a secondary school degree (n = 14). The average age was 75 (SD = 6.04; range = 64–85). We found that the majority of participants were in favor of the vaccination and highlighted its positive consequences, such as the abandonment of current freedom-limiting protective measures. Those participants who were against or unsure about the vaccination had concerns regarding the novelty of the vaccine and its impact on its safety and efficacy, stated they would prefer other protective measures rather than the vaccination, and identified contextual and individual drivers of their concerns.
Conclusions
Independently from the outbreak’s trajectory, efforts to foster vaccination acceptance should focus on the benefit of relapsing freedom-limiting protective measures. Vaccination strategies should be grounded in an evidence-based, participatory approach, ongoing community engagement, trust-building activities, and communication about vaccine developments and how the vaccine will be combined with other outbreak response measures.
Introduction
With almost 200 million confirmed cases and almost 4 million deaths as of June 21, 2021 [1] , the Covid-19 pandemic is causing unprecedented disruption and suffering to people across the globe, with a disproportionate toll on the elderly [2] . The risk for severe illness from Covid-19 increases with age, with older adults being more likely to require hospitalization, intensive care, or artificial ventilation [3] . Eight out of 10 Covid-19-related deaths reported in the United States have been among adults aged 65 years and older [4] , and case fatality rate for those over 80 years of age is five times the global average [3] . Less evident but no less worrying are the psychological, social, and economic implications of the current pandemic [5] , [6] . Compared to the general population, the impact on well-being and mental health is more dramatic among those who were already vulnerable before the onset of the outbreak, and stigma and discrimination are significantly higher among the older adult population [2] .
With no known therapeutic interventions and considering the necessity of sustained preventive behaviors, including physical distancing, the development and equitable distribution of a vaccine seems to be the most promising and sustainable route ahead to mitigate and possibly suppress the pandemic. The introduction of a SARS-CoV-2 vaccine is considered essential not only to reduce morbidity and mortality if the virus establishes itself in the population, but also to lessen the impact of the pandemic on the global economy [7] , [8] . Research groups around the world have been using different next-generation vaccine technologies and platforms, and innovative approaches to develop a vaccine that could stop the outbreak [9] , [10] , [11] , while a mechanism called COVAX has been created to guarantee rapid, fair and equitable access to covid-19 vaccines worldwide [8] . Over 280 vaccine candidates are either in preclinical or clinical development, 15 of which have entered Phase 3 clinical trials at the time of writing [12] . On December 11, 2020, the U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA) for a vaccine for the prevention of covid-19 in individuals 16 years of age and older [13] . The Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, coordinated by the Foundation for the National Institutes of Health (FNIH) and involving government, academic, and industry partners (including FDA) [14] , [15] , was created to prioritize vaccine and therapeutic candidates, maximise clinical trial capacity and effectiveness, and rapidly expand the clinical research resources focused on developing therapies for the covid-19 pandemic [16] .
In Switzerland, the first covid-19 vaccine was approved on December 19, 2020, and its administration started in January, free of charge and without obligation [17] , [18] . As in most countries in Europe, vulnerable individuals and frontline health-care and social workers in targeted and supervised settings were given priority [18] , [19] . However, research shows that the sole availability of a vaccine does not equal its acceptance [20] . The vaccination decision may be driven by emotional, cultural, social, spiritual, logistical, political and cognitive factors. Furthermore, the current pandemic entails unique challenges for public confidence in a future, licensed covid-19 vaccine [21] . These include the speed of development and approval of this vaccine, limited information on the specific SARS-CoV-2 antigen(s) used in vaccine development, the fact that most active vaccine candidates have been developed or are being developed by private/industry developers, and the lack of a long-term safety and efficacy record [22] . A final, considerable challenge is the spread of falsehoods as mis-informed people, anti-vaccine groups and other anti-establishment groups (e.g., lockdown opponents) use powerful tools to amplify vaccine scares and spread mis-information, thus potentially threatening the success of future vaccination campaigns against covid-19 and other public health efforts to contain the pandemic [23] , [24] , [25] .
Understanding the concerns, beliefs, and needs for information of potential recipients of currently approved and/or future covid-19 vaccines is crucial for effective public health communication. A global survey conducted in 19 countries to determine potential acceptance rates and factors influencing acceptance of a covid-19 vaccine found that, on average, 71.5% of respondents would be very or somewhat likely to take a covid-19 vaccine, and 48.1% reported that they would accept their employer’s recommendation to do so [26] . However, the study found marked differences in acceptance rates across countries, which ranged from almost 90% in China to less than 55% in Russia [26] . Studies conducted in the US found that between 50% and 69% of people intended to receive a (future) covid-19 vaccine, with another quarter either unsure about or against it [27] , [28] , [29] . Other studies found that 20% of Canada residents would refuse a vaccine or were unsure [30] , compared to 15% in Australia [31] . In line with findings from other studies conducted in Europe [32] , [33] , [34] , willingness to get vaccinated against covid-19 was found to be 73.9% in a European study (including Denmark, France, Germany, Italy, Portugal, the Netherlands, and the UK), while more than a quarter of respondents stated that they were not sure or did not want to get vaccinated [35] .In another study, French residents showed the lowest levels of willingness in Europe [36] . Lower levels of education and working in health care were associated with lower intent, while liberal political views, altruism, believing in the natural origin of the virus, and perceived threat to physical health were associated with higher intent [29] , [33] , [37] . This expanding body of evidence is difficult to reconcile because quantitative studies vary in scope and methods. Despite the importance of collecting qualitative data when planning to introduce a new vaccine [38] , no qualitative investigation has been conducted to explore the specific beliefs that the elderly may have regarding a future covid-19 vaccine, at the time when no covid-19 vaccine had been approved yet. The goal of this study was to explore older adults’ attitudes towards and beliefs regarding a future covid-19 vaccination in the Italian-speaking Canton of Ticino in Southern Switzerland.
Study design and participants
We conducted a qualitative study employing telephone interviews to capture and understand older adults’ attitudes towards and beliefs about a future covid-19 vaccine. It was conducted in the Canton of Ticino, Switzerland, a region severely affected by the covid-19 epidemic. We conducted all interviews over the telephone to meet physical distancing measures in force at the time of data collection. A convenience sample of 16 participants was recruited from a readily available database of older adults who previously took part in other qualitative studies focusing on ageing carried out by the research team. The database comprised 22 older adults who had previously accepted to be contacted for other studies. The interviewer invited them by either e-mail or phone to participate in the present study and asked those who accepted to participate to invite additional participants among their acquaintances. To be eligible for the study, participants had to be at least 65 years old at the time of the interview, without any hearing impairments, and be resident in the Canton of Ticino. Three additional participants were recruited through participant’s referral. We combined convenience and snowball sampling strategies to maximize the variance of our participants’ attitudes and beliefs towards a future covid-19 vaccine and to ensure we reached a target sample of approximately 20 participants quickly. The Ethics Committee of the Canton of Ticino approved the study (ID REQ-2020-00291).
The objectives and voluntary nature of the study were explained to participants when we first contacted them to schedule the interview (either by e-mail or over the phone) and when starting the interview over the phone. Oral informed consent was obtained before each interview began. Confidentiality was assured by replacing names with numbers and removing any identifying information from the interview transcripts. All audio recordings, transcripts, and personal data were saved on a password-protected computer. We adhere with the Standards for Reporting Qualitative Research guidelines [39] .
Data collection
Semi-structured, in-depth telephone interviews were done at a time convenient for participants between April 2 and May 15, 2020. We conducted all interviews in the local language, i.e., Italian, the native language of both the interviewers and the interviewees After explicit consent from participants, all interviews were audio-recorded using a free call recorder smartphone application. When not already available, participant’s gender, age, education, relational status, occupation, place of residence, and type of dwelling were obtained at the end of the interview. The duration of the interviews spanned from 16 to 120 min. Based on a semi-structured guideline, participants were asked open-ended questions to elicit their attitudes towards a future covid-19 vaccine (“What is your attitude towards a future covid-19 vaccine?”) and related beliefs (“Why would you say you are in favor/unsure/against a future covid-19 vaccine?”), information on its status (“What do you know about the development of a covid-19 vaccine?”) and related sources (“Do you remember where you read this/heard this from?”), and expected social reactions (“To what extent do you think people will accept the vaccine once it is licensed?”). Furthermore, participants were also asked questions regarding their representation of the virus and of the disease and the strategies they used to manage the risk of covid-19 in their day-to-day life. Questions were developed based on previous qualitative studies conducted by the authors [40] , [41] . The interviewer was a purposely trained female research assistant who, at the time of data collection, was undertaking her postgraduate training in cognitive psychology, and had substantial, previous experience in qualitative research. Debriefing between the interviewer and an experienced member of the research team (MF) took place shortly after each interview.
Data analysis
Audio-recordings were transcribed verbatim within one week after each interview. The experienced member of the research team (MF) independently conducted an inductive thematic analysis of the transcripts in the original language (Italian) following the six-stage comprehensive thematic analysis approach developed by Braun and Clarke [42] . The analysis included reading the transcripts multiple times to familiarize with the content, identifying meaningful quotes regardless of their length, labelling them under broader concepts, organizing the generated labels around more general themes, and creating relationships between them. To validate the results, discussion between the coder and the other members of the research team (SS and EA) took place at the end of the analysis. Disagreements in the interpretation of the findings were resolved through discussion and by making reference to the transcripts. Participant’s answers to the question regarding their attitudes towards a future covid-19 vaccine were used to categorize them as either in favor, against or unsure about this vaccination.
Nineteen older adults participated in an interview. Most participants were women (n = 12), Swiss nationals (n = 14), retired (n = 18), resident in urban areas (n = 14), and had obtained a secondary school degree (n = 14). The average age was 75 (SD = 6.04; range = 64–85). Most participants reported to be living alone (n = 12) and in a flat (n = 14). See Table 1 for participants’ characteristics. Most were in favor of a future covid-19 vaccination, while a minority were either against or unsure. In three cases, it was impossible to infer participant’s attitude towards the vaccination from their reports. The thematic analysis resulted in three main themes in relation to our research question. The first theme refers to participants’ understanding of the vaccination as a means to loosen current freedom-limiting protective measures; the second theme refers to participants’ belief that protective measures will always be safer than the vaccine; the third theme refers to participant’s reported contextual and individual drivers of vaccine hesitancy, namely the pandemic’s trajectory and one’s personal health status, respectively. These three themes are presented in detail in the next paragraphs.
Socio-demographic characteristics of participants (N = 19). *
Vaccination as freedom
More than half of the participants reported to be in favor of a future covid-19 vaccination and explained that the main reason they would accept to be vaccinated is that immunization will accelerate the easing of some of the covid-19 restrictive measures, such as those limiting people’s movement and demanding physical distance.
I think that, at that moment, everyone will be willing to get vaccinated because it means freedom of movement, so basically everything. If everyone is vaccinated, everyone will be alright (Participant #7, pro-vaccination, female, 79, secondary school) They must find this vaccine! If they do not find this vaccine, we need to use all these precautions! (Participant #13, pro-vaccination, female, 85, primary school)
For some participants, the vaccination will allow to regain normality in everyday life. As one participant pointed out when asked what her hopes for the future were:
I absolutely want to get out of this situation, to find a vaccine and a cure, so that we can return to normal life with the hope that everything will make us better than we are, and that we will understand the importance of life and friendship (Participant #4, pro-vaccination, female, 71, secondary school)
According to a few participants, a future covid-19 vaccination will also mean people will have less anxiety, thanks to the easing of some of the current non-pharmaceutical public health interventions including social and physical distancing, and hygiene measures.
When a vaccine that protects [from covid-19] is available, there will be a race to the vaccine, because it gives mental serenity and removes that anxiety that someone may have… Avoiding people, extreme hand and face hygiene… It is fine that everything is disinfected, including the chairs where you sit, but now they seem to be a bit exaggerated (Participant #11, pro-vaccination, male, 81, secondary school).
Despite agreeing on the positive consequences of a future covid-19 vaccination, unsure participants stressed the importance of being recommended to get vaccinated by a trustworthy source such as their family doctor:
If they say it is recommended, I listen to those who know more about it than me. Clearly, I do not get advice from anyone. It has to be the trusted doctor or someone who tells me that it is better that you do it because … I mean it can be the urologist or the family doctor. If it is recommended to get it, then I say “why not to listen?” (Participant #6, pro-vaccination, male, 73, secondary school). So, yes, the vaccine… Someone will refuse it. For example, I always get the flu vaccination, but my wife never does. It’s a matter of trust (Participant #11, pro-vaccination, male, 81, secondary school).
Other participants stressed the need to ensure a fair distribution of the vaccine that takes into account people’s age, with younger people having the right to be vaccinated first:
People will line up to receive the first ones. However, we will have to wait a little once these products are available… I mean, I hope there is a strategy for distribution… We will need to be less selfish and help those people who really need it. […] People up to 65 should have the right to have this vaccine. […] They still have all their lives ahead; we have lived our own. We had nothing, but we built something, and now we try to move on. Young people have to rebuild everything now, and they must have the possibility to do it, and that comes with the chance of being vaccinated (Participant 5, pro-vaccination, female, 79, secondary school).
Protective measures will be safer than the vaccine
One third of participants was either against or unsure about the vaccination, and reported that, even when a covid-19 vaccine will be available, protective measures such as physical distancing, increased hand hygiene and mask usage will be safer than the vaccine. This participant reported that will surely not get vaccinated, arguing that the vaccine can have fatal consequences:
I certainly will not [get vaccinated], I really will not! Look, when I had the flu vaccination, the nurse who treated my husband for 14 years - now, unfortunately, he’s dead - has always recommended me not to get the vaccine. Because, some told me, they got the vaccine and they died as a result. I don’t want to be against the vaccine, but I know that they died after getting the shot. When they gave my husband the shot without telling me, he died after 2–3 days. I… I'm really allergic to vaccines. I will never get it, even if I am obliged to. (Participant #15, anti-vaccination, female, 85, secondary school).
The same participant added that the vaccination will give the illusion to be protected and people will stop using other precautions, putting others in danger:
I think that most of them, out of fear, will get the vaccine and then go around without precautions. So I prefer to go around with a mask but avoid the vaccine, personally.
Another participant stressed that the future covid-19 vaccine will be similar to the flu vaccine, and that vaccination weakens the immune system, exposing people to other diseases:
As with the flu, I have never done it [the vaccination]. They say that you get a milder disease. But it’s been 6 or 7 years now that I haven’t got a cold. I don't take anything if I don't really need it. I am not against medications, but I am against mandatory vaccinations. Maybe, if you get it [the vaccine]… it kills you. I have been years now without taking anything. I either go to the supermarket and get the virus, or I stay here and get the shot. (Participant #10, anti-vaccination, male, 71, secondary school).
Some of those participants who reported to be in favor of a future covid-19 vaccine stated that they will likely wait for some time before accepting the vaccination, so that more people can be vaccinated before them and the vaccine safety can be further tested.
I will accept it, but I'll probably wait a month or two to see the consequences. Let’s say I won’t get it on the first day. (Participant #12, pro-vaccination, female, 79, secondary school). I would wait at least a month or two to see what effects it has, this is my idea. […] Some will be afraid and hope that this vaccine will not harm us, and that it will not impose an additional burden on us. Some think that it’s a bad thing because they inject us with a product that is still unknown. I don’t know if everyone will agree to get vaccinated. I need to see if healthy people can tolerate this vaccine. Only then we can be sure that we can give it to other people as well. (Participant #5, pro-vaccination, female, 79, secondary school). I think I will get vaccinated, but always according to what will be defined, what will be said. I don't want to be a guinea pig. I will get it when there is enough information on its effectiveness. (Participant #11, pro-vaccination, male, 81, secondary school).
Contextual and individual drivers of vaccine hesitancy
Unsure and favorable participants stressed that two main factors will be crucial for acceptance. The first one is a contextual factor, i.e., if the pandemic fades and cases significantly decrease, people’s perception of the risk of getting infected will also decrease and they will be more reluctant to accept the vaccination.
This will be another big problem, because it depends on whether the virus disappears. If the vaccine becomes available, the question “what is this vaccine for?” may be raised. If the vaccine was developed for a specific strain of virus, it might no longer be needed at some point. If, on the other hand, this vaccine will work like a broad-spectrum antibiotic, then yes. Even when vaccines were offered against the seasonal flu, I never got them and never got the flu. And there are people who got the vaccine and also got the flu. So, you are right, it will be a big question when the vaccine is available: what will we do? My impression is that, if this [pandemic] completely fades and there is no more risk, I will most likely not get the vaccine. (Participant #9, unsure, male, 80, university degree).
The second factor is an individual one and has to do with people’s perceived health and past experiences. Participants argued that, if older adults will perceive themselves to be in good health, they will not perceive the likely consequences of covid-19 to be serious enough to require the vaccination.
A group of older adults whom I know would say that they are well and that they would not do it. But just as I get the shot for the flu, I would get it [covid-19 vaccine] without any problems. Because I am convinced that if they recommend doing it, then I should do it. I really noticed that every year that I get it [flue vaccine], I get nothing. Nothing, neither a cold nor a flu… only when I got the shot for the first time did I get something, but then nothing again, and now I’m fine (Participant #17, pro-vaccination, male, 71, secondary school).
Other participants stated that their parents’ or family members’ experiences with past infectious diseases increased the perception of the risk of covid-19 and made them in favor of the vaccination, even if they were not used to vaccinating against the flu.
I never got the flu shot, I have to say, but if a vaccine against coronavirus becomes available now, I would accept it. I would be the first to call and ask to be vaccinated. I come from a family of hairdressers and both were infected with tuberculosis by their customers. My mom had to stay in the sanatorium for a year and she used to tell me to take care of my lungs, because you never know… here is just to tell you that I've always been on that side [in favor]… It's not that I lived with the fear of getting sick with tuberculosis, because it was eradicated at some point. But my mother told me about this period of [her life]… At that time they didn't have penicillin and you could only recover at the sanatorium. I am very cautious, in a few words. Even if I don't wear a mask, I am very sensitive to this pandemic. (Participant #8, pro-vaccination, male, 76, university degree).
The goal of this study was to qualitatively explore older adults’ willingness to vaccinate against covid-19 and, in particular, their attitudes towards and beliefs regarding a future covid-19 vaccination. We found that a majority of participants were in favor of the vaccination and highlighted its positive consequences, such as the removal of other protective measures. Those participants who were against or unsure about the vaccination had concerns regarding its safety and efficacy and identified contextual and individual drivers of such concerns.
The current, global response to the covid-19 pandemic involves the aggressive implementation of containment, mitigation, or suppression strategies, and the enforcement of a variety and combination of non-pharmacological (i.e. public health) measures including case reporting, contact tracing, isolation of sick individuals, quarantine, voluntary sheltering, national and international travel impositions, and a variety of community restrictions including physical distancing, and partial to complete lockdowns of schools, universities, workplaces, businesses, and shops. The finding that participants support a future covid-19 vaccination for its liberating effect (possibility to return to a normal life, have freedom of movement and no longer live with anxiety) is consistent with evidence on both the psycho-social benefits of vaccination [43] , and the disruptive impact of public health measures such as isolation and lockdown on individuals’ mental health and well-being [44] , [45] , [46] . The literature on vaccine hesitancy has repeatedly found a number of variables related to the health and psycho-social benefits of the vaccination (e.g., anticipated regret for not vaccinating, avoidance of the anxiety related to a possible infection, desire to protect the health of family members or the community) to be important predictors of the vaccination decision, particularly among parents [47] , [48] , [49] . The novelty of our findings lies in participants’ awareness of the unprecedented effects of the pandemic on multiple levels (mental and physical well-being, relationships) and the resulting importance of the vaccination for returning to a normal, pre-pandemic life, and having freedom of movement again. Despite being observed in the context of other vaccinations, for example smallpox and polio vaccinations, such perceived benefits of the vaccination may constitute a much stronger potential driver of the immunization decision than in the past. Our findings also suggest that participants were aware of their vulnerability and recognized the need to be sufficiently informed and recommended to get vaccinated by trustworthy sources, such as their family doctor. Participants cited the scarce evidence on vaccine safety and efficacy and the key role of their family members’ experiences with past epidemics as the main two reasons for this. This is confirmed by previous evidence that social interactions with family members, friends, politicians, bloggers, religious leaders, or anti-vaccination campaigners may play a significant role in the vaccination decision, with healthcare provider advice consistently found to be the most important predictor of vaccine uptake [48] . Furthermore, participants were concerned about vaccine manufacture and deployment, both issues that are gaining increasing attention [50] , [51] , [52] . The literature has repeatedly shown that practical issues such as vaccine availability, logistic or financial inconvenience in accessing vaccines, service quality and satisfaction, personal requirements, and incentives may further delay vaccination or lead to its complete refusal [53] . Participants showed sensitivity to the need to ensure a fair distribution of any future covid-19 vaccine, adding that younger individuals should be given priority despite recognizing their at-risk status. This latter finding contributes to the debate on who should be vaccinated first, as long as vaccine availability is limited. While the elderly and other vulnerable groups might seem the most obvious candidates to receive priority, some scholars have argued that prioritizing the vaccination of children would maximize the benefits of indirect immunity for older and vulnerable individuals. [54] . Our participants’ preference to let children have first access to the vaccine seems to be rooted in the altruistic belief that they will benefit most, rather than in selfish motives, indicating that the population’s view of what an acceptable vaccination strategy should be may differ from that of the experts. However, there are unresolved issues related to the indirectness of evidence of vaccines’ safety and efficacy in children due to their systematic, by-design exclusion from pivotal randomized controlled trials.
Our findings that protective measures are perceived as safer and potentially more effective than the vaccine resonate with previous evidence that individuals may believe vaccines can cause harm or even death, particularly when vaccines are novel and do not have a long track record of safety tests [49] , [55] . Even pro-vaccination participants would likely wait several months before getting vaccinated after a covid-19 vaccine is licensed and officially recommended, and may prefer to continue to use protective measures rather than getting vaccinated even in the long term [56] . This can be explained by the stronger influence of safety over efficacy concerns in relation to the vaccine. Even if individuals believe that the vaccine is more effective in protecting from covid-19 than other measures (such as wearing a mask), however intrusive and disruptive they may be, they may opt for active refusal and give preference to what they believe to be the safer measure. Knowledge and understanding of the disease, of the vaccine manufacture process (including information on its safety and efficacy), as well as the reputation of vaccines developers has been shown to contribute to instil trust [48] . Finally, our results that individual’s hesitancy may be altered by the pandemic’s trajectory is in line with previous studies showing that individual’s risk perception in terms of susceptibility depends on the communicated diffusion of a certain disease within a population [48] .
The findings also have a number of implications for the success of future covid-19 vaccination strategies targeting older adults and the general population. As it is never too early to understand the nature and extent of vaccine hesitancy in order to address it with suitable measures, such strategies should be grounded in an evidence-based, participatory approach and include ongoing community engagement, trust-building activities, and communication about vaccine developments and how the vaccine will be combined with other outbreak response measures. Information must be understandable and channelled through both traditional and new media [57] . In addition, communication training of healthcare providers and other key stakeholders in the immunisation landscape should also be provided [58] . Importantly, research on hesitancy and communication strategies should inform each other, through an iterative process, and because education and socio-economic status influence hesitancy in an extremely complex manner [48] , an effort should be made to include the public as a whole [49] . Furthermore, the findings obtained from this qualitative study could be used to guide future or further research related to Covid-19 vaccination acceptance among those 65 years old and older. A way to do this could be to include additional items to already validated vaccine hesitancy scales to measure to what extent the novelty of this vaccine impacts its perceived safety and efficacy, what type of trade-off people make between the use of protective measures or the acceptance of the vaccine, or in which measure older adults are willing to let younger people have priority access to the vaccine as a way to protect themselves.
Some limitations of the present study are to be mentioned. We used telephone interviews for data collection to allow participation during the Covid-19 pandemic, while physical distancing was in place, and face to face interactions with older adults impracticable. Despite studies suggesting that data quality is comparable between face-to-face and telephone interviews, the latter have known limitations. The lack of an in-person contact may have limited the formation of trust between interviewer and participant. Social desirability bias might have contributed to express possible frustrations connected to the lockdown less freely. To mitigate these challenges, we introduced long pauses during the interviews and adopted a familiar, warm, and understanding approach to the interview to allow participants to express their thoughts at their own pace, reduce inhibitions, and increase their confidence that their honest responses were welcomed. A second limitation has to do with the study sampling. Most participants were part of previous studies conducted by our research team. We cannot exclude that some similarities exist between participants in both their characteristics and their previous experience of research participation. To reduce this potential bias and increase socio-demographic variance, we approached participants in a non-systematic manner and combined the initial sampling strategy with a snowball sampling. Previous interactions between participants and the interviewers may, however, have positively influenced mutual trust and bonding, and contributed to the elicitation of themes during the interviews. Finally, the nature and small sample size of qualitative studies limit the generalizability of results to similar contexts, populations, and circumstances. However, the internal validity of the study is supported by the robustness of our methods, and is confirmed by the saturation of the themes after 15 interviews.
We found that pro-vaccination older adults see the vaccination as the single, best route to ending current restrictive measures, while anti-vaccination participants have safety and efficacy concerns that may be amplified by the trajectory of the pandemic. To integrate research efforts and communication actions into covid-19 vaccination strategies and promote public confidence in the vaccine and its acceptance, a dialogue between governments, the scientific community, vaccine developers, regulators, healthcare providers, and the public is essential [59] . Through a coordinated action individuals can be placed in the position to understand the value of vaccination, in general, and of the covid-19 vaccination, in particular, and demand it as both a right and a responsibility once the vaccines are approved and become available [60] . Following a social marketing approach [61] , researchers from both the public and private sectors are urged to employ or adapt existing, validated tools to collect evidence on individuals’ knowledge about, and attitudes towards a future covid-19 vaccine, informative needs and information sources, trust in (public health) institutions, social influences or other factors that are known to drive confidence and uptake [62] , [63] . In this regard, the value of our qualitative study stretches far beyond the geographic area where our data were collected, by providing useful insights and novel themes worth further investigation to others interested in conducting research on Covid-19 vaccine hesitancy among older adults.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [The authors declare that they have no conflict of interest. LSS served on the MSD European Vaccines Advisory Board in 2019].
Acknowledgements
We are grateful to all study participants for their valuable input.
The study was conducted with internal funding of the Institute of Public Health.
- Frontiers in Immunology
- Vaccines and Molecular Therapeutics
- Research Topics
A Global Perspective on Vaccines: Priorities, Challenges and Online Information
Total Downloads
Total Views and Downloads
About this Research Topic
Vaccines have been one of the most important medical discoveries of the last three centuries. Thanks to their development, millions of deaths have been avoided. Together with improved hygiene and antibiotics, vaccines have significantly contributed to the prolongation of life expectancy in high-income ...
Keywords : Vaccine, Vaccination
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines.
Submission closed.
Participating Journals
Total views.
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Development of next-generation covid-19 vaccines: barda supported phase 2b study designs.
- Article contents
- Figures & tables
- Supplementary Data
Daniel N Wolfe, Elizabeth Arangies, Gloria L David, Brian Armstrong, Theresa Z Scocca, Janel Fedler, Ramya Natarajan, James Zhou, Lakshmi Jayashankar, Ruben Donis, Mirjana Nesin, H Cody Meissner, Laurence Lemiale, Gerald R Kovacs, Shyam Rele, Robin Mason, Huyen Cao, Development of Next-Generation COVID-19 Vaccines: BARDA Supported Phase 2b Study Designs, Clinical Infectious Diseases , 2024;, ciae286, https://doi.org/10.1093/cid/ciae286
- Permissions Icon Permissions
In response to the COVID-19 pandemic, vaccines were quickly and successfully developed and deployed, saving millions of lives globally. While first generation vaccines are safe and effective in preventing disease caused by SARSCoV-2, next-generation vaccines have the potential to improve efficacy and safety. Vaccines delivered by a mucosal route may elicit greater protective immunity at respiratory surfaces thereby reducing transmission. Inclusion of viral antigens in addition to the spike protein may enhance protection against emerging variants of concern. Next-generation vaccine platforms with a new mechanism of action may necessitate efficacy trials to fulfill regulatory requirements. The Biomedical Advanced Research and Development Authority (BARDA) will be supporting Phase 2b clinical trials of candidate next-generation vaccines. The primary endpoint will be improved efficacy in terms of symptomatic disease relative to a currently approved COVID-19 vaccine. In this paper, we discuss the planned endpoints and potential challenges to this complex program.
- antigens, viral
- drug administration routes
- mucous membrane
- pharmacokinetics
- surrogate endpoints
- covid-19 vaccines
- coronavirus pandemic
- spike protein, human
Email alerts
More on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants
FDA News Release
Today, the U.S. Food and Drug Administration took action approving and authorizing for emergency use updated COVID-19 vaccines formulated to more closely target currently circulating variants and to provide better protection against serious consequences of COVID-19, including hospitalization and death. Today’s actions relate to updated mRNA vaccines for 2023-2024 manufactured by ModernaTX Inc. and Pfizer Inc. Consistent with the totality of the evidence and input from the FDA’s expert advisors, these vaccines have been updated to include a monovalent (single) component that corresponds to the Omicron variant XBB.1.5.
What You Need to Know
- Individuals 5 years of age and older regardless of previous vaccination are eligible to receive a single dose of an updated mRNA COVID-19 vaccine at least 2 months since the last dose of any COVID-19 vaccine.
- Individuals 6 months through 4 years of age who have previously been vaccinated against COVID-19 are eligible to receive one or two doses of an updated mRNA COVID-19 vaccine (timing and number of doses to administer depends on the previous COVID-19 vaccine received).
- Unvaccinated individuals 6 months through 4 years of age are eligible to receive three doses of the updated authorized Pfizer-BioNTech COVID-19 Vaccine or two doses of the updated authorized Moderna COVID-19 Vaccine.
- The FDA is confident in the safety and effectiveness of these updated vaccines and the agency’s benefit-risk assessment demonstrates that the benefits of these vaccines for individuals 6 months of age and older outweigh their risks.
- Individuals who receive an updated mRNA COVID-19 vaccine may experience similar side effects as those reported by individuals who previously received mRNA COVID-19 vaccines as described in the respective prescribing information or fact sheets.
- The updated vaccines are expected to provide good protection against COVID-19 from the currently circulating variants. Barring the emergence of a markedly more virulent variant, the FDA anticipates that the composition of COVID-19 vaccines may need to be updated annually, as is done for the seasonal influenza vaccine.
- The U.S. Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet tomorrow (Sept. 12), to discuss clinical recommendations on who should receive an updated vaccine, as well as further considerations for specific populations such as immunocompromised and older individuals.
- Manufacturers have publicly announced that the updated vaccines would be ready this fall, and the FDA anticipates that the updated vaccines will be available in the near future.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
The updated mRNA vaccines are each approved for individuals 12 years of age and older and are authorized under emergency use for individuals 6 months through 11 years of age. As part of today’s actions, the bivalent Moderna and Pfizer-BioNTech COVID-19 vaccines are no longer authorized for use in the United States.
Data Supporting the Updated mRNA COVID-19 Vaccines (2023-2024 Formula)
The mRNA COVID-19 vaccines approved and authorized today are supported by the FDA’s evaluation of manufacturing data to support the change to the 2023-2024 formula and non-clinical immune response data on the updated formulations including the XBB.1.5 component.
- The updated mRNA vaccines are manufactured using a similar process as previous formulations. In studies that have been recently conducted, the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5 and BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with prior versions of the vaccines against corresponding prior variants against which they had been developed to provide protection. This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants.
- The benefit-risk profile of previously authorized and approved mRNA COVID-19 vaccines is well understood as these vaccines have been administered to hundreds of millions of people in the United States.
Based on an evaluation of the totality of the evidence, the benefit-risk profile is favorable for individuals 6 months of age and older to receive an updated COVID-19 mRNA vaccine. Although serious outcomes from COVID-19 are less common in younger individuals, they do occur, and it has been demonstrated that recently receiving a COVID-19 vaccine reduces the risk of such serious outcomes.
Additional Details on Today’s Actions
Specifically, today’s actions include:
- Approval of Comirnaty (COVID-19 Vaccine, mRNA) to include the 2023-2024 formula, and a change to a single dose for individuals 12 years of age and older. Comirnaty was previously approved as a two-dose series for individuals 12 years of age and older.
- Approval of Spikevax (COVID-19 Vaccine, mRNA) to include the 2023-2024 formula, a change to a single dose for individuals 18 years of age and older, and approval of a single dose for individuals 12 through 17 years of age. Spikevax was previously approved as a two-dose series for individuals 18 years of age and older.
- Authorization of Moderna COVID-19 Vaccine for emergency use in individuals 6 months through 11 years of age to include the 2023-2024 formula and lower the age eligibility for receipt of a single dose from 6 years to 5 years of age. Additional doses are also authorized for certain immunocompromised individuals ages 6 months through 11 years, as described in the fact sheets.
- Authorization of Pfizer-BioNTech COVID-19 Vaccine for emergency use in individuals 6 months through 11 years of age to include the 2023-2024 formula. Additional doses are also authorized for certain immunocompromised individuals ages 6 months through 11 years, as described in the fact sheets.
The approval of Comirnaty (COVID-19 Vaccine, mRNA) (2023-2024 Formula) was granted to BioNTech Manufacturing GmbH. The EUA amendment for the Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) was issued to Pfizer Inc.
The approval of Spikevax (COVID-19 Vaccine, mRNA) (2023-2024 Formula) was granted to ModernaTX Inc. and the EUA amendment for the Moderna COVID-19 Vaccine (2023-2024 Formula) was issued to ModernaTX Inc.
Related Information
- Comirnaty (COVID-19 Vaccine, mRNA) (2023-2024 Formula)
- Spikevax (COVID-19 Vaccine, mRNA) (2023-2024 Formula)
- Moderna COVID-19 Vaccine (2023-2024 Formula)
- FDA Resources for the Fall Respiratory Illness Season
- Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula)
- Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2023
- June 15, 2023, Meeting of the Vaccines and Related Biological Products Advisory Committee
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
- hot-topics Extras
- Newsletters
- Reading room
Tell us what you think. Take part in our reader survey
Celebrating twenty years
- Back to parent navigation item
- Collections
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- Energy storage and batteries
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus
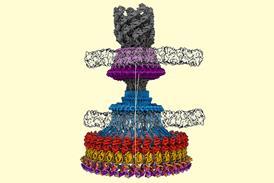
- More from navigation items
Genetic engineering feat coaxes yeast to produce valuable vaccine compound
By James Urquhart 2024-05-30T08:30:00+01:00
- No comments
Yeast has been engineered to perform the complete biosynthesis of QS-21, a potent and highly sought-after saponin-based adjuvant that boosts the immune response to certain vaccines.

Source: © Getty Images
Currently, the important vaccine adjuvant QS-21 is isolated from the soapbark tree that can only be found in Chile
The work represents one of the longest biosynthetic pathways ever transplanted into an organism, introducing 38 enzyme-encoding genes from six different species into yeast. The approach promises to enable a scalable, sustainable and cheaper way to make QS-21, as well as aid the design of new adjuvants.
Researchers have focused on the soap-like compound QS-21 as a vaccine adjuvant since the late 1990s for its ability to activate the immune system. Currently, it’s the only saponin-based vaccine adjuvant approved for clinical use in commercial vaccines, including ones for shingles, malaria and Covid-19, something which prompted concerns about QS-21 availability during the pandemic.
’From a world health perspective, there’s a lot of need for an alternative source of this adjuvant,’ says Jay Keasling at the University of California, Berkeley, US, who spearheaded the work with an international team of collaborators.
QS-21 is usually isolated from the bark of the soapbark tree, Quillaja saponaria , which is only found in Chile. Demand for QS-21 is high but supplies are limited because harvesting the bark requires mature trees and is tightly regulated. What’s more the isolation and purification of QS-21 from the mixture of compounds in bark extract is laborious, costly, uses toxic chemicals and has low yields.
Total synthesis of QS-21 has been previously achieved. However, it required the synthesis of an intermediate chemical first, takes 76 steps owing to the molecule’s complex structure – a glycosylated triterpene scaffold core coupled to a complex glycosylated 18-carbon acyl chain – and yields are poor.
One company, Botanical Solution in California, claims to have solved the supply and cost issues by devising and commercialising a plant tissue culture method to extract QS-21 using soapbark seedlings grown in the lab. Meanwhile, industry-led research published in March this year involving a number of biotech companies, presented another viable production route via cultured plant cells.
Keasling, however, thinks yeast offers the ideal alternative. ‘I want to make everything from a single sugar,’ he says. ‘I’d like to start with glucose, so when the production is performed in large tanks, they’re able to produce QS-21 as easily and inexpensively as possible.’
Pathway to success
The bioengineering feat of making QS-21 in yeast was possible following work published in January this year by some of the same team, including Keasling and Anne Osbourne at the John Innes Centre in Norwich, UK. They identified the complete 20-step biosynthetic pathway of QS-21, reproducing it in tobacco.
To reconstitute this pathway in yeast and make QS-21 from just glucose and galactose, the team first upregulated and fine tuned the passage of metabolites in the yeast strain’s native mevalonate pathway to produce quillaic acid, a key component of QS-21 synthesis. Meanwhile, using Crispr genome editing, enzyme-encoding genes from six other organisms, including plants that produce structurally similar saponins, fungi and bacteria were inserted. In total 38 enzymes spanning seven enzyme families were introduced, while ensuring critical metabolic pathways were unaffected for the yeast’s growth and survival.
‘This is a masterpiece of metabolic engineering, which shows how recent advances in synthetic biology like Crispr–Cas9 can be used to accelerate the discovery and engineering of pathways for the production of highly valuable molecules,’ says synthetic biologist Rodrigo Ledesma Amaro at Imperial College London, UK. ‘This is also a fantastic example of how microbial bioproduction can be an alternative to unsustainable plant extraction practices.’
‘The scale of the work undertaken in the study is quite extraordinary. It’s certainly up there as one of the most complex feats of metabolic engineering,’ says Paul Race , who researches natural product biosynthesis at Newcastle University, UK. ‘Work of this type is fraught with complications and I am in no doubt that a Herculean effort has been needed to get the system working as well as it does.’
However, both Amaro and Race note that low yields are an issue. Currently, it takes three days for the engineered yeast to produce around a third of the amount of QS-21 that the cells of the soapbark tree produces. Nevertheless, Keasling points out that yeast is around 1000 times faster than trees because only mature trees produce QS-21. ‘Even at the levels we’re producing it, it’s cheaper than producing it from the plant.’
‘Significant optimisation of the yeast platform is still required to achieve the yields needed to make this a viable route for QS-21 production at scale,’ Race comments. ‘This study represents the start of a journey that will hopefully result in access to this important molecule via a route that is uncoupled from the well-documented issues of isolation from the soapbark tree.’
Y Liu et al , Nature , 2024, DOI: 10.1038/s41586-024-07345-9
- biosynthesis
- Biotechnology
- Synthetic biology
Related articles
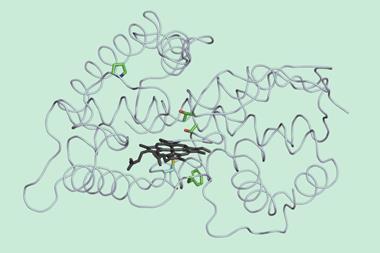
Carbene chemistry built into microbe’s metabolism in first for biosynthesis
2023-05-22T13:30:00Z
By James Urquhart

Soapbark tree’s biosynthetic pathway for vaccine adjuvant saponins
2023-03-31T11:37:00Z
By Anthony King

Yeast engineered to ferment sugars into chemotherapy drug precursors
2022-09-12T09:10:00Z

Nature-inspired crop protection
2022-09-07T14:44:00Z

Renewable rocket fuel made by genetically engineered soil bacteria
2022-07-11T13:46:00Z

Reprogrammed bacterium turns carbon dioxide into chemicals on industrial scale
2022-03-02T14:30:00Z
No comments yet
Only registered users can comment on this article., more from news.
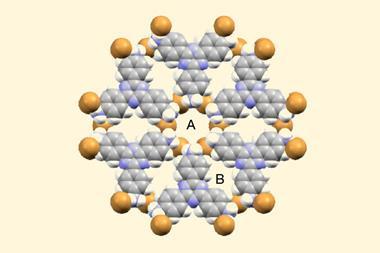
New metal-free MOFs show early promise for nuclear chemistry
2024-06-03T13:30:00Z
By Julia Robinson

‘Fire and devastation’: 50 years on from the Flixborough disaster what’s changed?
2024-06-03T08:30:00Z

Recycled construction waste could cut cement and steel’s carbon footprint
2024-05-31T11:42:00Z
Conjugate vaccine curbs xylazine effects in mice
2024-05-31T08:51:00Z
By Laura Cooper

Calls to kill off the European Institute of Innovation and Technology mount
2024-05-30T13:30:00Z
By Angeli Mehta

Graduate visa route stays, as UK government proposes ‘crackdown’ on abuses and migration
2024-05-29T13:30:00Z
By Jamie Durrani
- Contributors
- Terms of use
- Accessibility
- Permissions
- This website collects cookies to deliver a better user experience. See how this site uses cookies .
- This website collects cookies to deliver a better user experience. Do not sell my personal data .
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies .
Site powered by Webvision Cloud
Bird flu: Diverse range of vaccines platforms 'crucial' for enhancing human pandemic preparedness
New study launches following the discovery of a second case of avian influenza spreading from cows to humans.
Vaccination remains the most effective strategy for avian influenza prevention and control in humans, despite varying vaccine efficacy across strains.
That's according to the authors of a new review which delves into existing research into bird flu vaccines for humans.
Published in the peer-reviewed journal Human Vaccines & Immunotherapeutics , the results of the paper are particularly timely following news last week (Wednesday 22 nd May) that the bird flu strain H5N1 had once again, for a second time, jumped from cattle in America to a human -- prompting fears of subsequent human-to-human infection, with possible critical consequences.
Instances of the avian influenza were first recognized in US cattle in March. Since then, this strain has mainly spread from cow-to-cow and scientists have discovered very high levels of virus in raw milk (pasteurized milk is safe, having shown viral RNA but not infectious virus). To-date two people, however, are known to have contracted the bird flu virus. Both patients -- US farmers -- only reported eye symptoms and with treatment they made a full recovery.
Following tests on the first human instance, it was seen that the strain had mutated to be better adapted to mammalian cells, but as long as that human didn't pass it onto another person it likely stopped the spread at that point. With the second case, the CDC has released a statement to say it has been monitoring influenza surveillance systems intently, especially in impacted states. "There has been no sign of unusual influenza activity in people, including in syndromic surveillance," they report.
The concern now, though, is that if H5N1 continues to be given the environment in which to mutate (such as in close quarter cattle farms) -- and this continues long enough -- it has the potential to find a combination that will easily spread to humans.
The results of this new research, carried out by a team at the University of Georgia, USA, suggests vaccines still remain our "primary defense" against potential spread of avian influenzas such as the H5N1 and others assessed.
"The H5N1, H7N9, and H9N2 subtypes of avian influenza virus pose a dual threat, not only causing significant economic losses to the global poultry industry but also presenting a pressing public health concern due to documented spillover events and human cases," explains lead author Flavio Cargnin Faccin, who alongside his mentor Dr. Daniel Perez of the University of Georgia, USA, analyzed the current landscape of research into human vaccines for these bird flus.
"This deep delve into the landscape of avian influenza vaccines for humans shows vaccination remains the primary defense against the spread of these viruses."
The team examined studies of vaccines tested in mice, ferrets, non-human primates, and clinical trials of bird flu vaccines in humans, and assessed both established platforms and promising new directions.
The review carried out suggests inactivated vaccines are a safe and affordable option that primarily activate humoral immunity -- the part of our immune system that produces antibodies.
Live attenuated influenza vaccines (LAIVs) are known to induce a wider immune response than inactivated vaccines, activating not only antibody production but also mucosal and cellular defenses. In this review, the authors suggest this broader response may offer greater protection, though, the authors suggest further research is needed to fully understand and harness its potential benefits for both human and agricultural applications.
The review also examined alternatives, such as virus-like particle (VLP) vaccines and messenger RNA (mRNA) vaccines, that have emerged more recently. Although VLP vaccines for bird flu have limited clinical trial data in humans, results from studies in mice and ferrets showed promise, the authors found. mRNA vaccines against H5N1 and H7N9 bird flu subtypes also generated a rapid and strong immune response in mice and ferrets, and, while data in humans is scarce, results from a phase 1 study of an H7N9 mRNA vaccine in healthy humans were "encouraging."
Overall, the team suggests "exploring and employing a diverse range of vaccine platforms," will be "crucial for enhancing pandemic preparedness and mitigating the threat of avian influenza viruses."
- Cold and Flu
- Bird Flu Research
- Flu vaccine
- Influenza pandemic
- Global spread of H5N1
- MMR vaccine
Story Source:
Materials provided by Taylor & Francis Group . Note: Content may be edited for style and length.
Journal Reference :
- Flavio Cargnin Faccin, Daniel R. Perez. Pandemic preparedness through vaccine development for avian influenza viruses . Human Vaccines & Immunotherapeutics , 2024; 20 (1) DOI: 10.1080/21645515.2024.2347019
Cite This Page :
Explore More
- Kinship and Ancestry of the Celts
- How Statin Therapy May Prevent Cancer
- Origins of 'Welsh Dragons' Exposed
- Resting Brain: Neurons Rehearse for Future
- Observing Single Molecules
- A Greener, More Effective Way to Kill Termites
- One Bright Spot Among Melting Glaciers
- Martian Meteorites Inform Red Planet's Structure
- Volcanic Events On Jupiter's Moon Io: High Res
- What Negative Adjectives Mean to Your Brain
Trending Topics
Strange & offbeat.

- June 2, 2024 | Scientists Race To Perfect New Oral COVID-19 Treatment Poised To Replace Paxlovid
- June 2, 2024 | Tricking the Brain’s Inner GPS: Virtual Reality Study Reveals New Brain Mechanisms
- June 2, 2024 | Microscopic Marvel: A Photonic Device that Could Change Physics and Lasers Forever
- June 2, 2024 | Mediterranean Diet Tied to 23% Lower Risk of Death in Landmark 25-Year Study
- June 2, 2024 | From Ice Age to Geological Wonderland: The Rapid Transformation of Adam’s Inlet
Scientists Decode Deadly Blood Clot Disorder Triggered by COVID Vaccines
By Flinders University May 26, 2024
Researchers from Flinders University, along with international experts, have discovered that the PF4 antibodies causing VITT after adenovirus vector-based COVID-19 vaccination share identical molecular signatures with those found in similar cases following natural adenovirus infection. This finding, using a new approach developed at Flinders, has significant implications for understanding the genetic risk factors and improving future vaccine development.
New research has shown that the dangerous PF4 antibodies involved in vaccine-induced thrombosis (VITT) and similar disorders from common cold infections share identical molecular structures, highlighting implications for future vaccine development and disease management.
New research conducted by Flinders University and global specialists is deepening our knowledge of vaccine-induced immune thrombocytopenia and thrombosis (VITT). During the peak of the COVID-19 pandemic in 2021, VITT was recognized as a new condition linked to adenovirus vector-based vaccines, particularly the Oxford-AstraZeneca vaccine.
VITT was found to be caused by an unusually dangerous blood autoantibody directed against a protein termed platelet factor 4 (or PF4). In separate research in 2023, researchers from Canada, North America, Germany, and Italy described a virtually identical disorder with the same PF4 antibody that was fatal in some cases after natural adenovirus (common cold) infection.
Flinders University researchers Dr. Jing Jing Wang and Flinders Professor Tom Gordon, Head of Immunology at SA Pathology in South Australia, led a previous study in 2022 that cracked the molecular code of the PF4 antibody and identified a genetic risk factor related to an antibody gene termed IGLV3.21*02.
Flinders University immunology researchers Dr. Jing Jing Wang and Professor Tom Gordon. Credit: Flinders Foundation
Collaborative Efforts and Future Implications
Now, the Flinders group has collaborated with this international group of researchers to find that the PF4 antibodies in both adenoviruses infection-associated VITT and classic adenoviral vectored VITT share identical molecular fingerprints or signatures.
The research will also have implications for improving vaccine development, says Flinders University researcher Dr. Wang, the first author on the new article that was published in the eminent New England Journal of Medicine .
“These findings, using a completely new approach for targeting blood antibodies developed at Flinders University, indicate a common triggering factor on virus and vaccine structures that initiates the pathological pF4 antibodies,” explains Professor Gordon.
“Indeed, the pathways of lethal antibody production in these disorders must be virtually identical and have similar genetic risk factors. Our findings have the important clinical implication that lessons learned from VITT are applicable to rare cases of blood clotting after adenovirus (a common cold) infections, as well as having implications for vaccine development,” he says.
Reference: “Antibody Fingerprints Linking Adenoviral Anti-PF4 Disorders” by Jing Jing Wang, Linda Schönborn, Theodore E. Warkentin, Tim Chataway, Leonie Grosse, Paolo Simioni, Stephan Moll, Andreas Greinacher and Tom P. Gordon, 15 May 2024, New England Journal of Medicine . DOI: 10.1056/NEJMc2402592
The research was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) and a European Medicines Agency service contract. Dr. Schönborn was supported by the ASH Global Research Award from the American Society of Hematology and by the Gerhard Domagk Research Program through the Medical University of Greifswald. Dr. Wang was supported by a Flinders Foundation Health Seed Grant.
More on SciTechDaily
Scientists Discover “Startling” Levels of Hidden Mental Health Symptoms Among Autoimmune Disease Patients

How Cold Temperatures Boost Appetite – New Discovery Could Lead to Improved Weight Loss Treatments
Painkiller Paradox: 60 Years of Research, But Do They Really Work for Back Pain?

Evolution May Be to Blame for High Risk of Advanced Cancers in Humans – “Gene Has Gone Rogue”
COVID Omicron Variant: How Did It Emerge and Is It More Contagious Than Delta? A Virus Evolution Expert Explains

First Evidence of Coronal Rain on a Cold Star?

Discovery Alert: Water Vapor Detected on “Super Neptune” Exoplanet

Astronomers Discover Evidence for Substance on Liquid-Gas Boundary on Exoplanet WASP-31b
29 comments on "scientists decode deadly blood clot disorder triggered by covid vaccines".
all the people who forced vaccines on others need to be hanged
Ya, the Trump administration did this.
OK, Karen….
Seriously, how is it wrong? The vaccine mandates were during the Trump administration.
Presidents have no say over local policies, Cletus.
EVERY lockdown was by Demunists.
Hope you enjoyed that single term your bio weapon bought for you. It will be decades, if ever, before anyone trusts your party with power again.
Go back and look at a calendar, moron.
Your statement is clearly not true. I think you may be living on another planet…
WRONG, the mandates were all done under the Biden administration when he tried to mush it through OSHA in 2021 well after Trump left office.
So in conclusion the healthy appearing little girl dissected skinned and minced for science was made sick with adenovirus put in a bath of calf blood serum and sprinkled with fish semen. What could go wrong with this recipe other than the minced kidney could be a neuron and the calf semen could have been contaminated and the fish semen well I’ll leave that up to science? Anyway I think one of them said it wasn’t really for anything other than to see if it could be done. This cell line is a transformed cell line and as such is classified as a Genetically Modified Organism (GMO). The Human Embryonic Kidney cell lines have been transformed with Human Adenovirus 5.
You don’t have to be a biological researcher to know that the developers of mRNA vaccines don’t know WTF they are doing.
Or they knew/know exactly what they are doing. And just didn’t think they’d get CAUGHT.
The fruits of Malice and Ineptitude are virtually indistinguishable.
If you read the article you’ll see that this condition was not caused from the mRNA vaccines but rather from the vaccine that used an adenoviral vector.
Only those who were complicit or willfully blind could be surprised at this headline.
Rightly so. Most of us are sheeple to be driven easily.
Said it from the first. Being proven RIGHT every day since. The mRNA jabs do NOT create immunity against any actual pathogen, they induce AUTOIMMUNITY, turning your immune system against your own body’s cells.
Whatever you say Dr Fauci…
Seriously, READ the article. It says, “During the peak of the COVID-19 pandemic in 2021, VITT was recognized as a new condition linked to adenovirus vector-based vaccines, particularly the Oxford-AstraZeneca vaccine”.
@Pelosi’s Hammer – you really think they don’t know what they are doing? They know exactly what they are doing. It seems you don’t get it.
My long-time (unfortunately now retired) primary care physician said, (paraphrasing) “Whenever possible I never prescribe any medication which has not been on the market for 20 years. All medications have side effects, and we know what they are in those long-established meds.”
I also am old enough to remember thalidomide, and remember seeing many young college-age students in Germany as I traveled extensively there in 1980-81.
I and my family did not take the Covid jab. As my 34 year old grandson recently said, he knows many people his age who regret taking the jab, but he knows no one who did not take the jab who now is sorry.
Only you and your close loved ones care about you. One must think for themselves and carry out due diligence before buying into the common hype. Corporations are soulless, the government is not your friend, lying now is a badge of honor. A strain of evil lies in the hearts of mankind and as a society we have shed the restraints on that evil.
Want to know the breakdown of nearly any social question, simply overlay the question with the Bell curve to see how the way things fall out percent wise. That includes intelligence, awareness, curiosity, concern for others, financial planning, due diligence, independence, and on and on.
Also, the “Strauss–Howe generational theory” https://en.wikipedia.org/wiki/Strauss%E2%80%93Howe_generational_theory led to their 1997 book “The Fourth Turning,” which is well worth taking the time to read and contemplate.
I repeat, corporations are soulless and government is not your friend. Only you and your closest loved one really care about you. Become aware, think for yourself, carry out due diligence before jumping on a bandwagon.
Don’t be putting foreign stuff into your body if you are not clear on what may result from your action.
My wife and I had Covid at 79 years old. We were prepared with ivermectin and followed as best we could the FLCCC Alliance https://covid19criticalcare.com/ protocol and suffered only minimal effects. We recognized the red flags early on as Big Pharma, the government, and our corrupted medical system started flogging the deadly nature of Covid and pushing the vaccine. Luckily we had found a moral physician who provided advice and necessary prescription in preparation.
By the way, remember the push against chloroquine and hydroxychloroquine? Well, in 1979 I accepted a position working in the Indonesian part of New Guinea. My wife, pre-teen son, and I started taking chloroquine two weeks before departing the States and continued taking continuously it for more than two years. Over the years my wife and I spent considerable time traveling in Southeast Asia, where malaria is endemic, and each time took chloroquine as a preventive. We never caught malaria or suffered any side effects. We now are healthy at 82.
Don’t be a lemming, folks. Be aware, carry out some due diligence, and think for your self.
Congrats to you and your wife for not drinking the Kool-Aid.
Just a note. We know Democrats funded this bio weapon for over 20 years, developing it in secret with their CCP allies. All that is NOT known is EXACTLY how it got out.
Now, they are pushing the accident story hard – but it doesn’t wash. In over 5,000 years of recorded human history, there is little or no evidence there has ever been such a thing as a “political coincidence.” Absent proof it did not escape accidentally, the almost certain conclusion is that someone let it out on purpose. That leaves China or the DNC.
China risked global thermonuclear war had they released it. So, who does that leave?
Here’s an article about the hundreds, thousands, uncountable number of people who were in a hospital bed unable to breath, telling reporters they wish they had gotten the vaccine.
Remember: you can believe what you want if you stay in your echo chamber. But you will be bombarded by contradicting evidence when you scuttle out of it.
https://www.nbcnews.com/health/health-news/unvaccinated-hospitalized-patients-say-they-regret-not-getting-shot-n1273342
None of this is surprising to anyone in the field of virology. We knew early on this was a manufactured virus as it contained a furin cleavage site which was not naturally occurring in this family of viruses. All of the related and unusual side effects attributable to Coronavirae, namely autoimmune induced myocarditis, thrombosis, etc. were likely to occur with the vaccine and/or infection. That the political arms of medicine, namely big pharma, CDC, FDA, NIH, deliberately surpressed this likely outcome was and remains criminal.
This calls for Hague trials for every single decision maker who participated in making this experimental drug mandatory.
Wait, I was supposed to be a conspiracy theorist when I brought up this topic a couple years ago. Did I just get demoted?
The adenovirus used to carry the viral payload in the vaccine was not a human one. The vector is a chimpanzee adenovirus modified to avoid its replication. So it is not a “common cold virus”, though it may be a member of the same virus family. If a common cold adenovirus had been used, many humans would have mounted an immune response to the vector instead of the payload, rendering the vaccine ineffective. Still, with the benefit of hindsight, who’d have though “what could possibly go wrong with using a chimp virus?” OOPS! And AZ just withdrawn its vaccine worldwide.
Having said that, the consequences of infection with early variants of Covid-19 are almost certainly way worse on average than those of the vaccine. But I do think we were panicked into taking it, with the risks due to the vaccine being played down (or not yet known) and so informed consent was not properly obtained. In terms of lives saved it looks like a good job. Er, but what about this brain fog and these achey joints and muscles?
so someone figured out how they killed all those people … the govt is very crafty
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 3, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
US will make millions of bird flu vaccines this summer
by Robin Foster
As the H5N1 avian flu continues to spread among dairy cows in the United States, nearly 5 million doses of flu vaccine are now being prepared for possible use in humans.
Since the outbreak in livestock began this spring, bird flu has been confirmed in three humans who worked on dairy farms in Texas and Michigan and health experts are concerned the virus could mutate to the point where it could spread easily among humans.
In response, vaccine maker CSL Seqirus announced last week that it has been tasked with making the additional doses of flu vaccine at its North Carolina plant.
"It utilizes a highly scalable method of production and is currently positioned to deliver up to 150 million influenza vaccine doses to support an influenza pandemic response within six months of a pandemic declaration," the company noted in a news release .
"The CDC [U.S. Centers for Disease Control and Prevention] maintains the risk to public health as low. We are closely monitoring the situation because we are acutely aware of the threat that influenza virus strains like H5N1 can pose and take seriously our role in preparedness efforts alongside our government and public health partners," Marc Lacey, global executive director for pandemic at CSL Seqirus, said in the news release.
"This agreement... will help support the U.S. government's ability to respond swiftly in the event that the current avian flu situation changes."
The new vaccine doses combine an antigen that targets the H5 portion of the H5N1 virus with an ingredient designed to boost the immune response triggered by the vaccine.
While the production of these new flu vaccine doses is set to be completed "later this summer," they may not be ready for use right away.
The U.S. Food and Drug Administration has previously approved other vaccines for potential H5N1 pandemics, including a vaccine by Seqirus, but it isn't certain how soon the FDA might clear use of the new shots.
"If it is determined that the U.S. population needs to be vaccinated to prevent H5N1 influenza, then the FDA will use its regulatory pathways to take the appropriate steps to ensure vaccines are available in the timeliest manner possible," an FDA spokesperson told CBS News.
It is unclear who would be prioritized for the first shots if they are eventually rolled out for the public, CBS News reported.
A panel of the CDC's outside vaccine advisers is set to meet June 24 to discuss H5N1 alongside its routine votes on seasonal flu vaccines.
While the CDC says the risk to the general public remains low, the agency has warned that workers on dairy farms and in production facilities are at higher risk of infection and it has urged the industry to take safety precautions.
The latest human case of bird flu in Michigan involved respiratory symptoms for the first time, officials announced Thursday, which could make it easier for the virus to spread from person to person. The two previous patients only experienced eye symptoms.
"Simply put, someone who's coughing may be more likely to transmit the virus than someone who has an eye infection like conjunctivitis," the CDC's Nirav Shah said Thursday, CBS News reported.
The CDC is now investigating whether the strain that infected the latest Michigan patient might have developed key mutations that could raise its risk of spread among humans, CBS News reported.
"The mere fact that this individual displayed some respiratory symptoms, again we should be alert, but in and of itself is not a cause to change course or suggest that we're at an inflection point," Shah told reporters.
© 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Pollutants and climate change contribute to millions of deaths from cardiovascular disease each year, warn scientists
14 minutes ago
Chromatin openness sheds new light on prostate cancer plasticity
27 minutes ago

AI-driven tool can detect Lewy body dementia via changes in vocal emotional expression
56 minutes ago

Scientists develop AI tool to predict how cancer patients will respond to immunotherapy

Neoadjuvant immunotherapy for early stage melanoma shows positive results
Targeting leukemia's survival route: A novel approach to overcoming leukemia recurrence
Study finds timing of brain waves shapes the words we hear
2 hours ago

Study finds semaglutide associated with reduction in incidence and recurrence of alcohol-use disorder
3 hours ago

New analytical tool can improve understanding of heritable human traits and diseases
Q&A: Researchers determine that a subclass of stem cells replenish platelets more rapidly
Related stories.
Vaccines will be best defense against bird flu, experts say
May 29, 2024

CDC reports third dairy worker infected with bird flu, risk to public remains low
May 30, 2024

Another Michigan dairy worker has bird flu, the third US case this year

New study launches following the discovery of a second case of avian influenza spreading from cows to humans

US reports 2nd human case of bird flu tied to dairy cow outbreak
May 23, 2024
Researchers develop experimental mRNA avian flu vaccine
Recommended for you.

Antibody discovery promises new hope in influenza B battle, paves way for universal vaccine
Jun 1, 2024
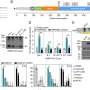
Study: The route into the cell influences the outcome of SARS-CoV-2 infection
May 31, 2024
AI model confirms vaccination is key to cutting COVID in prisons

Novel vaccine concept generates immune responses that could produce multiple types of HIV neutralizing antibodies
Ongoing clinical trials offer hope for people born with genetic mutation behind fibrodysplasia ossificans
New findings show risk of death from COVID-19 lessens, but infection still can cause issues three years later
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

COMMENTS
That said, the effectiveness of most vaccines is high. After receiving the second dose of the MMR vaccine (measles, mumps and rubella) or the standalone measles vaccine, 99.7% of vaccinated individuals are immune to measles. The inactivated polio vaccine offers 99% effectiveness after three doses. The varicella (chickenpox) vaccine is between ...
Josh Sharfstein answers a list of important questions about COVID-19 vaccines. Q&A. Most COVID vaccine information is focused on how effective they are at preventing serious disease, hospitalization, and death. How is it possible that the vaccine is more effective at preventing serious illness and death than it is preventing a mild infection?
Vaccination is a powerful method of disease prevention that is relevant to people of all ages and in all countries, as the Covid-19 pandemic illustrates. Vaccination can improve people's chances ...
Other research aims to develop new vaccines that might protect against current and future types of coronaviruses. The Journey of a Vaccine Learn about the four phases of clinical research, what questions researchers try to answer in each, and how a vaccine is developed, approved, and manufactured. View the Animation
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
In February 2020, we highlighted the top nine important research questions on SARS-CoV-2 and COVID-19 concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis. These and related questions are revisited at the end of 2021 to shed light on the ...
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
Answering Key Questions About COVID-19 Vaccines. The US government is investing in rapid development of vaccines against coronavirus disease 2019 (COVID-19), several relying on new technologies. 1 In the US, 4 vaccine candidates are in phase 3 studies with initial results expected soon. If studies succeed, 1 or more vaccines may become ...
Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines. ... Questions and answers about COVID-19 vaccine guidelines, development, and safety. EXPLORE VACCINES RESOURCES Featured Vaccines Resource.
The Journey of a Vaccine. Published: January 1, 2022. Learn about the four phases of clinical research, what questions researchers try to answer in each, and how a vaccine is developed, approved, and manufactured.
Vaccines are a clinical product that is composed of live or dead material from an infectious agent - bacterium, virus, fungus or parasite - that elicit protective immunity against the pathogen ...
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety. Vaccines go through various phases of development and testing - there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease ...
The most urgent and important question for COVID-19 in Africa is how to gain access to vaccines. Without a deliberate commitment globally to scale up vaccination in Africa, its people will be ...
About nine-in-ten (88%) Americans say, overall, the benefits of childhood vaccines for measles, mumps and rubella outweigh the risks, identical to the share who said this before the coronavirus outbreak. U.S. adults are less confident in COVID-19 vaccines: Fewer than half rate them as having high health benefits and a low risk of side effects.
The thematic analysis resulted in three main themes in relation to our research question. The first theme refers to participants' understanding of the vaccination as a means to loosen current freedom-limiting protective measures; the second theme refers to participants' belief that protective measures will always be safer than the vaccine ...
1. Listen with empathy. Start by listening with empathy to those who have questions around vaccination. Don't dismiss them, and acknowledge how they're feeling (without necessarily agreeing, for example "it's okay to have questions, or want more information before getting a vaccine"). 2. Ask open-ended questions.
As science advances and new information becomes available, this system will continue to improve. Vaccine safety research: Ensures the benefits of vaccines approved in the U.S. outweigh the risks. Defines which groups should not receive certain vaccines. Describes side effects and adverse events reported after vaccination.
In this Research Topic, we aim to gather a series of Review, Mini-Review and Perspective articles that discuss numerous questions and problems associated with vaccines. The future of vaccine development needs to take in account changing perspectives within our society and that the acceptance of preventative medicine may also change in the years ...
Research study visits are at the Vaccine Research Center (VRC) Clinic at the National Institutes of Health in Bethesda, Maryland. Free valet parking is available. You can also get there by Metro. Take the Red Line, and get off at the Medical Center Metro Station. Clinic hours are Monday through Friday from 8:00 a.m. to 4:30 p.m.
Yes. Vaccines are very safe. The United States' long-standing vaccine safety system ensures that vaccines are as safe as possible. Currently, the United States has the safest vaccine supply in its history. Millions of children safely receive vaccines each year. The most common side effects are very mild, such as pain or swelling at the ...
Any medicine, including vaccines, can cause side effects. Most of the time, these side effects are minor. Some examples are a low-grade fever, headache, fussiness or soreness at the injection site. Rarely, a child might experience a severe side effect, such as an allergic reaction or a seizure. These are rare side effects, and caregivers and ...
Q&A for Comirnaty (COVID-19 Vaccine mRNA) Information Pertaining to Measles, Mumps, Rubella and Varicella Virus Vaccine Live (Archived) Trumenba (Serogroup B Meningococcal Vaccine) Questions and ...
Vaccine hesitancy: 5 questions for Neetu Abad. ... Research has also demonstrated that recommendations from health care providers of all types can impact vaccination, so an endorsement from a psychologist can go a long way. There's so much room for research psychologists in this field. We need psychologists who understand behavioral theory ...
Because our research question focuses on vaccine refusal, we then subsetted to the 3711 URLs predicted to be hesitancy-inducing (see SM section S4.4 for an analysis of the full dataset of 13,206 URLs, which gives qualitatively similar results, and SM section S5.2 for discussion of potential impact of pro-vaccine content).
The Biomedical Advanced Research and Development Authority (BARDA) will be supporting Phase 2b clinical trials of candidate next-generation vaccines. The primary endpoint will be improved efficacy in terms of symptomatic disease relative to a currently approved COVID-19 vaccine.
Today's actions relate to updated mRNA vaccines for 2023-2024 manufactured by ModernaTX Inc. and Pfizer Inc. Consistent with the totality of the evidence and input from the FDA's expert ...
Researchers have focused on the soap-like compound QS-21 as a vaccine adjuvant since the late 1990s for its ability to activate the immune system. Currently, it's the only saponin-based vaccine ...
Review of research to-date suggests vaccination remains the most effective strategy for avian influenza prevention and control in humans, despite varying vaccine efficacy across strains.
New research conducted by Flinders University and global specialists is deepening our knowledge of vaccine-induced immune thrombocytopenia and thrombosis (VITT). During the peak of the COVID-19 pandemic in 2021, VITT was recognized as a new condition linked to adenovirus vector-based vaccines, particularly the Oxford-AstraZeneca vaccine.
As the H5N1 avian flu continues to spread among dairy cows in the United States, nearly 5 million doses of flu vaccine are now being prepared for possible use in humans. Topics Conditions