
Research Topics & Ideas: Healthcare
100+ Healthcare Research Topic Ideas To Fast-Track Your Project

Finding and choosing a strong research topic is the critical first step when it comes to crafting a high-quality dissertation, thesis or research project. If you’ve landed on this post, chances are you’re looking for a healthcare-related research topic , but aren’t sure where to start. Here, we’ll explore a variety of healthcare-related research ideas and topic thought-starters across a range of healthcare fields, including allopathic and alternative medicine, dentistry, physical therapy, optometry, pharmacology and public health.
NB – This is just the start…
The topic ideation and evaluation process has multiple steps . In this post, we’ll kickstart the process by sharing some research topic ideas within the healthcare domain. This is the starting point, but to develop a well-defined research topic, you’ll need to identify a clear and convincing research gap , along with a well-justified plan of action to fill that gap.
If you’re new to the oftentimes perplexing world of research, or if this is your first time undertaking a formal academic research project, be sure to check out our free dissertation mini-course. In it, we cover the process of writing a dissertation or thesis from start to end. Be sure to also sign up for our free webinar that explores how to find a high-quality research topic.
Overview: Healthcare Research Topics
- Allopathic medicine
- Alternative /complementary medicine
- Veterinary medicine
- Physical therapy/ rehab
- Optometry and ophthalmology
- Pharmacy and pharmacology
- Public health
- Examples of healthcare-related dissertations
Allopathic (Conventional) Medicine
- The effectiveness of telemedicine in remote elderly patient care
- The impact of stress on the immune system of cancer patients
- The effects of a plant-based diet on chronic diseases such as diabetes
- The use of AI in early cancer diagnosis and treatment
- The role of the gut microbiome in mental health conditions such as depression and anxiety
- The efficacy of mindfulness meditation in reducing chronic pain: A systematic review
- The benefits and drawbacks of electronic health records in a developing country
- The effects of environmental pollution on breast milk quality
- The use of personalized medicine in treating genetic disorders
- The impact of social determinants of health on chronic diseases in Asia
- The role of high-intensity interval training in improving cardiovascular health
- The efficacy of using probiotics for gut health in pregnant women
- The impact of poor sleep on the treatment of chronic illnesses
- The role of inflammation in the development of chronic diseases such as lupus
- The effectiveness of physiotherapy in pain control post-surgery

Topics & Ideas: Alternative Medicine
- The benefits of herbal medicine in treating young asthma patients
- The use of acupuncture in treating infertility in women over 40 years of age
- The effectiveness of homoeopathy in treating mental health disorders: A systematic review
- The role of aromatherapy in reducing stress and anxiety post-surgery
- The impact of mindfulness meditation on reducing high blood pressure
- The use of chiropractic therapy in treating back pain of pregnant women
- The efficacy of traditional Chinese medicine such as Shun-Qi-Tong-Xie (SQTX) in treating digestive disorders in China
- The impact of yoga on physical and mental health in adolescents
- The benefits of hydrotherapy in treating musculoskeletal disorders such as tendinitis
- The role of Reiki in promoting healing and relaxation post birth
- The effectiveness of naturopathy in treating skin conditions such as eczema
- The use of deep tissue massage therapy in reducing chronic pain in amputees
- The impact of tai chi on the treatment of anxiety and depression
- The benefits of reflexology in treating stress, anxiety and chronic fatigue
- The role of acupuncture in the prophylactic management of headaches and migraines
Topics & Ideas: Dentistry
- The impact of sugar consumption on the oral health of infants
- The use of digital dentistry in improving patient care: A systematic review
- The efficacy of orthodontic treatments in correcting bite problems in adults
- The role of dental hygiene in preventing gum disease in patients with dental bridges
- The impact of smoking on oral health and tobacco cessation support from UK dentists
- The benefits of dental implants in restoring missing teeth in adolescents
- The use of lasers in dental procedures such as root canals
- The efficacy of root canal treatment using high-frequency electric pulses in saving infected teeth
- The role of fluoride in promoting remineralization and slowing down demineralization
- The impact of stress-induced reflux on oral health
- The benefits of dental crowns in restoring damaged teeth in elderly patients
- The use of sedation dentistry in managing dental anxiety in children
- The efficacy of teeth whitening treatments in improving dental aesthetics in patients with braces
- The role of orthodontic appliances in improving well-being
- The impact of periodontal disease on overall health and chronic illnesses

Tops & Ideas: Veterinary Medicine
- The impact of nutrition on broiler chicken production
- The role of vaccines in disease prevention in horses
- The importance of parasite control in animal health in piggeries
- The impact of animal behaviour on welfare in the dairy industry
- The effects of environmental pollution on the health of cattle
- The role of veterinary technology such as MRI in animal care
- The importance of pain management in post-surgery health outcomes
- The impact of genetics on animal health and disease in layer chickens
- The effectiveness of alternative therapies in veterinary medicine: A systematic review
- The role of veterinary medicine in public health: A case study of the COVID-19 pandemic
- The impact of climate change on animal health and infectious diseases in animals
- The importance of animal welfare in veterinary medicine and sustainable agriculture
- The effects of the human-animal bond on canine health
- The role of veterinary medicine in conservation efforts: A case study of Rhinoceros poaching in Africa
- The impact of veterinary research of new vaccines on animal health
Topics & Ideas: Physical Therapy/Rehab
- The efficacy of aquatic therapy in improving joint mobility and strength in polio patients
- The impact of telerehabilitation on patient outcomes in Germany
- The effect of kinesiotaping on reducing knee pain and improving function in individuals with chronic pain
- A comparison of manual therapy and yoga exercise therapy in the management of low back pain
- The use of wearable technology in physical rehabilitation and the impact on patient adherence to a rehabilitation plan
- The impact of mindfulness-based interventions in physical therapy in adolescents
- The effects of resistance training on individuals with Parkinson’s disease
- The role of hydrotherapy in the management of fibromyalgia
- The impact of cognitive-behavioural therapy in physical rehabilitation for individuals with chronic pain
- The use of virtual reality in physical rehabilitation of sports injuries
- The effects of electrical stimulation on muscle function and strength in athletes
- The role of physical therapy in the management of stroke recovery: A systematic review
- The impact of pilates on mental health in individuals with depression
- The use of thermal modalities in physical therapy and its effectiveness in reducing pain and inflammation
- The effect of strength training on balance and gait in elderly patients
Topics & Ideas: Optometry & Opthalmology
- The impact of screen time on the vision and ocular health of children under the age of 5
- The effects of blue light exposure from digital devices on ocular health
- The role of dietary interventions, such as the intake of whole grains, in the management of age-related macular degeneration
- The use of telemedicine in optometry and ophthalmology in the UK
- The impact of myopia control interventions on African American children’s vision
- The use of contact lenses in the management of dry eye syndrome: different treatment options
- The effects of visual rehabilitation in individuals with traumatic brain injury
- The role of low vision rehabilitation in individuals with age-related vision loss: challenges and solutions
- The impact of environmental air pollution on ocular health
- The effectiveness of orthokeratology in myopia control compared to contact lenses
- The role of dietary supplements, such as omega-3 fatty acids, in ocular health
- The effects of ultraviolet radiation exposure from tanning beds on ocular health
- The impact of computer vision syndrome on long-term visual function
- The use of novel diagnostic tools in optometry and ophthalmology in developing countries
- The effects of virtual reality on visual perception and ocular health: an examination of dry eye syndrome and neurologic symptoms
Topics & Ideas: Pharmacy & Pharmacology
- The impact of medication adherence on patient outcomes in cystic fibrosis
- The use of personalized medicine in the management of chronic diseases such as Alzheimer’s disease
- The effects of pharmacogenomics on drug response and toxicity in cancer patients
- The role of pharmacists in the management of chronic pain in primary care
- The impact of drug-drug interactions on patient mental health outcomes
- The use of telepharmacy in healthcare: Present status and future potential
- The effects of herbal and dietary supplements on drug efficacy and toxicity
- The role of pharmacists in the management of type 1 diabetes
- The impact of medication errors on patient outcomes and satisfaction
- The use of technology in medication management in the USA
- The effects of smoking on drug metabolism and pharmacokinetics: A case study of clozapine
- Leveraging the role of pharmacists in preventing and managing opioid use disorder
- The impact of the opioid epidemic on public health in a developing country
- The use of biosimilars in the management of the skin condition psoriasis
- The effects of the Affordable Care Act on medication utilization and patient outcomes in African Americans
Topics & Ideas: Public Health
- The impact of the built environment and urbanisation on physical activity and obesity
- The effects of food insecurity on health outcomes in Zimbabwe
- The role of community-based participatory research in addressing health disparities
- The impact of social determinants of health, such as racism, on population health
- The effects of heat waves on public health
- The role of telehealth in addressing healthcare access and equity in South America
- The impact of gun violence on public health in South Africa
- The effects of chlorofluorocarbons air pollution on respiratory health
- The role of public health interventions in reducing health disparities in the USA
- The impact of the United States Affordable Care Act on access to healthcare and health outcomes
- The effects of water insecurity on health outcomes in the Middle East
- The role of community health workers in addressing healthcare access and equity in low-income countries
- The impact of mass incarceration on public health and behavioural health of a community
- The effects of floods on public health and healthcare systems
- The role of social media in public health communication and behaviour change in adolescents
Examples: Healthcare Dissertation & Theses
While the ideas we’ve presented above are a decent starting point for finding a healthcare-related research topic, they are fairly generic and non-specific. So, it helps to look at actual dissertations and theses to see how this all comes together.
Below, we’ve included a selection of research projects from various healthcare-related degree programs to help refine your thinking. These are actual dissertations and theses, written as part of Master’s and PhD-level programs, so they can provide some useful insight as to what a research topic looks like in practice.
- Improving Follow-Up Care for Homeless Populations in North County San Diego (Sanchez, 2021)
- On the Incentives of Medicare’s Hospital Reimbursement and an Examination of Exchangeability (Elzinga, 2016)
- Managing the healthcare crisis: the career narratives of nurses (Krueger, 2021)
- Methods for preventing central line-associated bloodstream infection in pediatric haematology-oncology patients: A systematic literature review (Balkan, 2020)
- Farms in Healthcare: Enhancing Knowledge, Sharing, and Collaboration (Garramone, 2019)
- When machine learning meets healthcare: towards knowledge incorporation in multimodal healthcare analytics (Yuan, 2020)
- Integrated behavioural healthcare: The future of rural mental health (Fox, 2019)
- Healthcare service use patterns among autistic adults: A systematic review with narrative synthesis (Gilmore, 2021)
- Mindfulness-Based Interventions: Combatting Burnout and Compassionate Fatigue among Mental Health Caregivers (Lundquist, 2022)
- Transgender and gender-diverse people’s perceptions of gender-inclusive healthcare access and associated hope for the future (Wille, 2021)
- Efficient Neural Network Synthesis and Its Application in Smart Healthcare (Hassantabar, 2022)
- The Experience of Female Veterans and Health-Seeking Behaviors (Switzer, 2022)
- Machine learning applications towards risk prediction and cost forecasting in healthcare (Singh, 2022)
- Does Variation in the Nursing Home Inspection Process Explain Disparity in Regulatory Outcomes? (Fox, 2020)
Looking at these titles, you can probably pick up that the research topics here are quite specific and narrowly-focused , compared to the generic ones presented earlier. This is an important thing to keep in mind as you develop your own research topic. That is to say, to create a top-notch research topic, you must be precise and target a specific context with specific variables of interest . In other words, you need to identify a clear, well-justified research gap.
Need more help?
If you’re still feeling a bit unsure about how to find a research topic for your healthcare dissertation or thesis, check out Topic Kickstarter service below.

You Might Also Like:

15 Comments
I need topics that will match the Msc program am running in healthcare research please
Hello Mabel,
I can help you with a good topic, kindly provide your email let’s have a good discussion on this.
Can you provide some research topics and ideas on Immunology?
Thank you to create new knowledge on research problem verse research topic
Help on problem statement on teen pregnancy
This post might be useful: https://gradcoach.com/research-problem-statement/
can you provide me with a research topic on healthcare related topics to a qqi level 5 student
Please can someone help me with research topics in public health ?
Hello I have requirement of Health related latest research issue/topics for my social media speeches. If possible pls share health issues , diagnosis, treatment.
I would like a topic thought around first-line support for Gender-Based Violence for survivors or one related to prevention of Gender-Based Violence
Please can I be helped with a master’s research topic in either chemical pathology or hematology or immunology? thanks
Can u please provide me with a research topic on occupational health and safety at the health sector
Good day kindly help provide me with Ph.D. Public health topics on Reproductive and Maternal Health, interventional studies on Health Education
may you assist me with a good easy healthcare administration study topic
May you assist me in finding a research topic on nutrition,physical activity and obesity. On the impact on children
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
December 22, 2020
2020 Research Highlights — Promising Medical Findings
Results with potential for enhancing human health.
With NIH support, scientists across the United States and around the world conduct wide-ranging research to discover ways to enhance health, lengthen life, and reduce illness and disability. Groundbreaking NIH-funded research often receives top scientific honors. In 2020, these honors included one of NIH’s own scientists and another NIH-supported scientist who received Nobel Prizes . Here’s just a small sample of the NIH-supported research accomplishments in 2020.
Full 2020 NIH Research Highlights List
20200929-covid.jpg
New approaches to COVID-19
As the global pandemic unfolded, researchers worked at unprecedented speed to develop new treatments and vaccines. Scientists studied antibodies from the blood of people who recovered from COVID-19 and identified potent, diverse ones that neutralize SARS-CoV-2 . Some antibody treatments have now been given emergency use authorization by the FDA, with many others in development . However, such antibodies—called monoclonal antibodies—are difficult to produce and must be given intravenously. NIH-researchers have been pursuing other approaches, including using antibodies from llamas , which are only about a quarter of the size of a typical human antibody and could be delivered directly to the lungs using an inhaler. Computer-designed “miniproteins” and other antiviral compounds are also under investigation.
20200622-mosquito.jpg

Universal mosquito vaccine tested
Most mosquito bites are harmless. But some mosquitoes carry pathogens, like bacteria and viruses, that can be deadly. A small trial showed that a vaccine against mosquito saliva—designed to provide broad protection against mosquito-borne diseases—is safe and causes a strong immune response in healthy volunteers. More studies are needed to test its effectiveness against specific diseases.
20201006-knee-stock.jpg

Machine learning detects early signs of osteoarthritis
Osteoarthritis is the most common type of arthritis. It results when cartilage, the tissue that cushions the ends of the bones, breaks down. People with osteoarthritis can have joint pain, stiffness, and swelling. Some develop serious pain and disability from the disease. Using artificial intelligence and MRI scans, scientists identified signs of osteoarthritis three years before diagnosis. The results suggest a way to identify people who may benefit from early interventions.
20201103-eye.jpg

Advances in restoring vision
Several common eye diseases, such as age-related macular degeneration and retinitis pigmentosa, damage the retina, the light-sensitive tissue in the eye. They can eventually lead to vision loss. Two studies looked at ways to restore vision in mouse models. Researchers reprogrammed skin cells into light-sensing eye cells that restored sight in mice. The technique may lead to new approaches for modeling and treating eye diseases. Other scientists restored vision in blind mice by using gene therapy to add a novel light-sensing protein to cells in the retina. The therapy will soon be tested in people.
20200107-aging.jpg

Blood protein signatures change across lifespan
The bloodstream touches all the tissues of the body. Because of the constant flow of proteins through the body, some blood tests measure specific proteins to help diagnose diseases. Researchers determined that the levels of nearly 400 proteins in the blood can be used to determine people’s age and relative health. More research is needed to understand if these protein signatures could help identify people at greater risk of age-related diseases.
20201027-hiv-thumb.jpg

Understanding HIV’s molecular mechanisms
More than a million people nationwide are living with HIV, the virus that causes AIDS. HIV attacks the immune system by destroying immune cells vital for fighting infection. Researchers uncovered key steps in HIV replication by reconstituting and watching events unfold outside the cell. The system may be useful for future studies of these early stages in the HIV life cycle. In other work, experimental treatments in animal models of HIV led to the viruses emerging from their hiding places inside certain cells—a first step needed to make HIV vulnerable to the immune system.
20200225-parkinsons.jpg
Test distinguishes Parkinson’s disease from related condition
A protein called alpha-synuclein plays a major role in Parkinson’s disease as well as other brain disorders. Early symptoms of Parkinson’s disease and another disease involving alpha-synuclein, multiple system atrophy, can be similar. Researchers created a test using cerebrospinal fluid that can distinguish between these two diseases with 95% accuracy. The results have implications for the early diagnosis and treatment of these conditions and may help in the development of new targeted therapies.
20200114-cream.jpg

Understanding allergic reactions to skin care products
Personal care products like makeup, skin cream, and fragrances commonly cause rashes called allergic contact dermatitis. It’s not well understood how chemical compounds in personal care products trigger such allergic reactions. Scientists gained new insight into how personal care products may cause immune responses that lead to allergic responses in some people. Understanding how compounds in these products trigger immune reactions could lead to new ways to prevent or treat allergic contact dermatitis.
Connect with Us
- More Social Media from NIH
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Health sciences articles from across Nature Portfolio
The health sciences study all aspects of health, disease and healthcare. This field of study aims to develop knowledge, interventions and technology for use in healthcare to improve the treatment of patients.
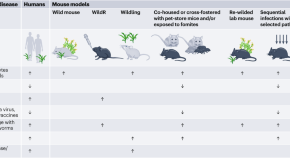
Leveraging dirty mice that have microbial exposure to improve preclinical models of human immune status and disease
The National Institute of Allergy and Infectious Diseases (NIAID) hosted a two-day virtual workshop on leveraging microbial exposure to improve mouse models of human immune status and disease. The workshop’s objective was to evaluate the current state of knowledge in the field and to identify gaps, challenges and future directions.
- Thames Pickett
- Barbara Rehermann
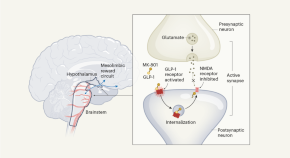
Dual-action obesity drug rewires brain circuits for appetite
A two-in-one drug that modulates neural pathways involved in appetite and reward might prove to be more effective and longer lasting than current weight-loss drugs on the market.
- Tyler M. Cook
- Darleen Sandoval
Patient-centered development of clinical outcome assessments in early Parkinson disease: key priorities and advances
Novel therapies with the ability to delay disease progression are a gap in the care of people living with Parkinson disease (PD) today. Clinical outcomes assessments (COAs) that are sensitive to the earliest clinical changes in PD are deemed essential for a successful therapeutic development. To understand the current landscape of COAs use in clinical trials in PD and define priorities for future research in the field, a stakeholder roundtable meeting was held in November 2022. The current paper 1) proposes the collaborative development of patient-centric COAs that can adequately document the effectiveness of disease modification therapies in PD based on key priorities identified during this initial meeting, 2) summarizes the progress made in the subsequent 12 months, and 3) presents the deliverables expected in the near future. Key priorities include 1) the development of a consensus conceptual model of early PD experiences, 2) the adaptation of existing patient-reported outcomes (PROs), 3) the investigation of the role of observer-reported outcomes in addition to 4) enabling diversity in PD research and advocacy, 5) fostering data sharing, and 6) reaching consensus on a biological staging system for PD to drive the development of appropriate PROs for biologically defined populations.
- Tiago A. Mestre
- Glenn T. Stebbins
- Tanya Simuni
Related Subjects
- Endocrinology
- Gastroenterology
- Health care
- Health occupations
- Medical research
- Molecular medicine
- Pathogenesis
- Rheumatology
- Risk factors
- Signs and symptoms
Latest Research and Reviews
Postoperative liver injury after sevoflurane or propofol anesthesia in patients undergoing non-cardiac surgery: a retrospective cohort study
- Dae Kyun Ryu
- Jeong-Jin Min
Inulin supplementation exhibits increased muscle mass via gut-muscle axis in children with obesity: double evidence from clinical and in vitro studies
- Chonnikant Visuthranukul
- Asada Leelahavanichkul
- Sirinuch Chomtho
A ResNet mini architecture for brain age prediction
- Si-Yuan Duan
- Xiao-Lei Zhang

Compositional and functional differences of the vaginal microbiota of women with and without cervical dysplasia
- Johanna Norenhag
- Gabriella Edfeldt
- Matts Olovsson
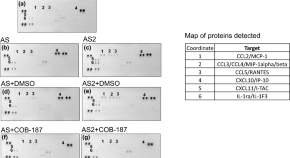
Mechanistic insights into SARS-CoV-2 spike protein induction of the chemokine CXCL10
- Davoud Ghazanfari
- Maria Cecilia Courreges
- Douglas J. Goetz
Effectiveness of Bariatric Metabolic Surgery versus Glucagon-Like Peptide-1 Receptor Agonists for prevention of Congestive Heart Failure
- Yael Wolff Sagy
- Dror Dicker
News and Comment
Prosthetic embodiment or what makes a limb part of your body.
Prosthetic embodiment, or the incorporation of a prosthesis into one’s sensory and functional body schema, may be achieved by engineering bionic limbs that leverage a closed-loop mechanoneural–machine interface. However, the subjective experience of embodiment remains difficult to define and assess.
Development of MKK4 inhibitors for liver regeneration
- Jordan Hindson

AUA24 — pioneering shared decision-making and patient engagement strategies
- Maria Chiara Masone
Organoids merge to model the blood–brain barrier
Combining a brain organoid with a blood-vessel organoid yields a system similar to a protective mesh in the brain.
Spatial and molecular exploration of glioblastoma heterogeneity
- Michael Fletcher
Telemedicine for hepatitis C virus treatment in opioid treatment programmes
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
151+ Public Health Research Topics [Updated 2024]

The important area of public health research is essential to forming laws, influencing medical procedures, and eventually enhancing community well-being. As we delve into the vast landscape of public health research topics, it’s essential to understand the profound impact they have on society.
This blog aims to provide a comprehensive guide to selecting and understanding the diverse array of public health research topics.
Overview of Public Health Research Topics
Table of Contents
Public health research encompasses a wide range of subjects, reflecting the interdisciplinary nature of the field. From epidemiology and health policy to environmental health and infectious diseases, researchers navigate through various dimensions to address complex health challenges.
Each category holds its own significance, contributing to the overall understanding of public health dynamics.
Key Considerations in Selecting Public Health Research Topics
- Current Relevance: Assess the timeliness of potential topics by considering recent health trends, emerging issues, and societal concerns.
- Impact on Public Health: Evaluate the potential impact of the research on improving health outcomes, addressing disparities, or influencing policy and interventions.
- Feasibility and Resources: Gauge the practicality of conducting research on a particular topic, considering available resources, data accessibility, and research infrastructure.
- Ethical Considerations: Scrutinize the ethical implications of the research, ensuring it aligns with ethical standards and guidelines, especially when dealing with vulnerable populations or sensitive topics.
Top 151+ Public Health Research Topics
Epidemiology.
- The Impact of Social Determinants on Disease Outcomes
- Patterns and Trends in Emerging Infectious Diseases
- Investigating Health Disparities among Different Ethnic Groups
- Childhood Obesity and its Long-Term Health Consequences
- Assessing the Effectiveness of Contact Tracing in Disease Control
Health Policy
- Universal Healthcare: Comparative Analysis of Global Models
- The Role of Telemedicine in Improving Healthcare Access
- Evaluating Mental Health Policies and Their Impact on Communities
- Assessing the Impact of Affordable Care Act on Public Health
- Vaccine Policies and Public Perception: A Comprehensive Study
Environmental Health
- Climate Change and Health: Adapting to the Challenges
- Air Quality and Respiratory Health in Urban Environments
- Waterborne Diseases and Strategies for Safe Water Supply
- Occupational Health Hazards: A Comprehensive Workplace Analysis
- The Impact of Green Spaces on Mental Health in Urban Areas
Infectious Diseases
- Antimicrobial Resistance: Strategies for Mitigation
- Vaccination Strategies and Herd Immunity
- Global Health Security: Preparedness for Pandemics
- The Impact of Vector-Borne Diseases on Public Health
- Emerging Trends in Antibiotic-Resistant Infections
Chronic Diseases
- Lifestyle Interventions for Preventing Cardiovascular Diseases
- Genetic Factors in the Development of Cancer: A Comprehensive Study
- Aging and Health: Addressing the Healthcare Needs of the Elderly
- Diabetes Prevention Programs: Efficacy and Implementation
- Mental Health in Chronic Disease Patients: Bridging the Gap
Maternal and Child Health
- Maternal Mortality: Understanding Causes and Prevention
- The Impact of Breastfeeding on Infant Health and Development
- Childhood Immunization: Barriers and Strategies for Improvement
- Teenage Pregnancy and Its Long-Term Health Consequences
- Mental Health Support for Postpartum Women: Current Gaps and Solutions
Health Behavior and Promotion
- Smoking Cessation Programs: Effectiveness and Challenges
- Physical Activity Promotion in Schools: Strategies for Success
- Nutrition Education and Its Impact on Healthy Eating Habits
- Mental Health Awareness Campaigns: Assessing Public Perceptions
- The Role of Social Media in Health Promotion
Global Health
- Assessing the Impact of International Aid on Global Health
- Water, Sanitation, and Hygiene (WASH) Programs in Developing Countries
- The Role of Non-Governmental Organizations in Global Health
- Communicable Disease Control in Refugee Populations
- Global Access to Essential Medicines: Challenges and Solutions
Community Health
- Community-Based Participatory Research: Best Practices and Challenges
- The Impact of Community Health Workers on Health Outcomes
- Health Literacy and its Relationship to Health Disparities
- Assessing the Effectiveness of Mobile Health (mHealth) Interventions
- Community Resilience in the Face of Public Health Crises
Healthcare Quality and Patient Safety
- Hospital-Acquired Infections: Strategies for Prevention
- Patient Safety Culture in Healthcare Organizations
- Quality Improvement Initiatives in Primary Care Settings
- Healthcare Accreditation: Impact on Patient Outcomes
- Implementing Electronic Health Records: Challenges and Benefits
Mental Health
- Stigma Reduction Programs for Mental Health Disorders
- Integrating Mental Health into Primary Care Settings
- The Impact of COVID-19 on Mental Health: Long-Term Implications
- Mental Health in the Workplace: Strategies for Employee Well-being
- Suicide Prevention Programs: Effectiveness and Outreach
Health Disparities
- Racial Disparities in Healthcare: Addressing Systemic Inequities
- LGBTQ+ Health Disparities and Inclusive Healthcare Practices
- Socioeconomic Status and Access to Healthcare Services
- Geographical Disparities in Health: Rural vs. Urban
- The Impact of Gender on Health Outcomes and Access to Care
Public Health Education
- Evaluation of Public Health Education Programs
- Innovative Approaches to Teaching Public Health Concepts
- Online Health Education Platforms: Opportunities and Challenges
- Interdisciplinary Training in Public Health: Bridging Gaps
- Continuing Education for Public Health Professionals: Current Landscape
Digital Health
- The Role of Wearable Devices in Health Monitoring
- Telehealth Adoption: Barriers and Opportunities
- Health Apps for Chronic Disease Management: User Perspectives
- Blockchain Technology in Healthcare: Privacy and Security Implications
- Artificial Intelligence in Disease Diagnosis and Prediction
Health Economics
- Cost-Effectiveness of Preventive Health Interventions
- The Impact of Healthcare Financing Models on Access to Care
- Pharmaceutical Pricing and Access to Essential Medicines
- Economic Evaluation of Health Promotion Programs
- Health Insurance Coverage and Health Outcomes: A Global Perspective
Innovations in Public Health
- 3D Printing in Healthcare: Applications and Future Prospects
- Gene Editing Technologies and their Ethical Implications
- Smart Cities and Public Health: Integrating Technology for Well-being
- Nanotechnology in Medicine: Potential for Disease Treatment
- The Role of Drones in Public Health: Surveillance and Intervention
Food Safety and Nutrition
- Foodborne Illness Outbreaks: Investigating Causes and Prevention
- Sustainable Food Systems: Implications for Public Health
- Nutritional Interventions for Malnutrition in Developing Countries
- Food Labeling and Consumer Understanding: A Critical Review
- The Impact of Fast Food Consumption on Public Health
Substance Abuse
- Opioid Epidemic: Strategies for Prevention and Treatment
- Harm Reduction Approaches in Substance Abuse Programs
- Alcohol Consumption Patterns and Public Health Outcomes
- Smoking and Mental Health: Exploring the Connection
- Novel Psychoactive Substances: Emerging Threats and Strategies
Occupational Health
- Workplace Stress and Mental Health: Intervention Strategies
- Occupational Hazards in Healthcare Professions: A Comparative Analysis
- Ergonomics in the Workplace: Improving Worker Health and Productivity
- Night Shift Work and Health Consequences: Addressing Challenges
- Occupational Health and Safety Regulations: A Global Overview
Disaster Preparedness and Response
- Pandemic Preparedness and Lessons from COVID-19
- Natural Disasters and Mental Health: Post-Traumatic Stress
- Emergency Response Systems: Improving Timeliness and Efficiency
- Communicating Health Risks During Emergencies: Public Perception
- Collaborative Approaches to Disaster Response in Global Health
Cancer Research
- Precision Medicine in Cancer Treatment: Current Advancements
- Cancer Screening Programs: Efficacy and Challenges
- Environmental Factors and Cancer Risk: Exploring Connections
- Survivorship Care Plans: Enhancing Quality of Life after Cancer
- Integrative Therapies in Cancer Care: Complementary Approaches
Sexual and Reproductive Health
- Access to Contraception in Developing Countries: Challenges and Solutions
- Comprehensive Sex Education Programs: Impact on Teen Pregnancy
- Reproductive Health Rights: Global Perspectives and Challenges
- Infertility Treatment: Ethical Considerations and Societal Impact
- Maternal and Child Health in Conflict Zones: Addressing Challenges
Cardiovascular Health
- Hypertension Prevention Programs: Strategies and Effectiveness
- Cardiovascular Disease in Women: Gender-Specific Risk Factors
- Innovations in Cardiac Rehabilitation Programs
- Artificial Heart Technology: Advancements and Ethical Implications
- Impact of Air Pollution on Cardiovascular Health: A Global Concern
Social Determinants of Health
- Educational Attainment and Health Outcomes: Exploring Links
- Income Inequality and its Impact on Population Health
- Social Support Networks and Mental Health: A Comprehensive Study
- Neighborhood Environments and Health Disparities
- Employment and Health: The Interplay of Work and Well-being
Genomics and Public Health
- Population Genomics and its Implications for Public Health
- Genetic Counseling and Education: Empowering Individuals and Families
- Ethical Issues in Genetic Research: Privacy and Informed Consent
- Pharmacogenomics: Tailoring Drug Therapies to Individual Genotypes
- Gene-Environment Interactions in Disease Risk: Unraveling Complexities
Public Health Ethics
- Informed Consent in Public Health Research: Current Practices
- Ethical Challenges in Global Health Research: Balancing Priorities
- Confidentiality in Public Health Reporting: Striking the Right Balance
- Research with Vulnerable Populations: Ethical Considerations
- Ethical Implications of Emerging Technologies in Healthcare
Health Communication
- The Role of Media in Shaping Public Health Perceptions
- Health Literacy Interventions: Improving Understanding of Health Information
- Social Media Campaigns for Public Health Promotion: Best Practices
- Tailoring Health Messages for Diverse Audiences: Cultural Competency
- Risk Communication in Public Health Emergencies: Lessons Learned
Nutrigenomics
- Personalized Nutrition Plans based on Genetic Makeup
- Impact of Nutrigenomics on Chronic Disease Prevention
- Ethical Considerations in Nutrigenomics Research
- Public Perceptions of Nutrigenomic Testing: A Qualitative Study
- Integrating Nutrigenomics into Public Health Policies
Public Health and Artificial Intelligence
- Predictive Analytics in Disease Surveillance: Harnessing AI for Early Detection
- Ethical Considerations in AI-Driven Health Decision Support Systems
- Machine Learning in Epidemiology: Predicting Disease Outbreaks
- Natural Language Processing in Public Health: Text Mining for Insights
- Bias in AI Algorithms: Implications for Health Equity
Health Disparities in Aging
- Geriatric Health Disparities: Bridging the Gap in Elderly Care
- Ageism in Healthcare: Addressing Stereotypes and Discrimination
- Social Isolation and Health Consequences in Aging Populations
- Access to Palliative Care for Older Adults: A Global Perspective
- Alzheimer’s Disease and Ethnic Disparities in Diagnosis and Treatment
- Loneliness and Mental Health in the Elderly: Interventions and Support
Research Methodologies in Public Health
Public health research employs various methodologies, including quantitative, qualitative, and mixed-methods approaches. Each method brings its own strengths to the research process, allowing researchers to gain a comprehensive understanding of the complex issues they investigate.
Community-based participatory research is another valuable approach, emphasizing collaboration with communities to address their specific health concerns.
Challenges and Opportunities in Public Health Research
While public health research is immensely rewarding, it comes with its own set of challenges. Funding constraints, ethical dilemmas, the need for interdisciplinary collaboration, and the integration of technology pose both obstacles and opportunities.
Researchers must navigate these challenges to ensure their work has a meaningful impact on public health.
In conclusion, public health research topics are diverse and dynamic, reflecting the complex nature of the field. As researchers embark on their journeys, they must carefully consider the relevance, impact, and ethical implications of their chosen topics.
The collaborative and interdisciplinary nature of public health research positions it as a powerful tool in addressing the health challenges of our time. By exploring the depths of these topics, researchers contribute to the collective effort to build healthier and more equitable communities.
As we move forward, a continued exploration of relevant public health research topics is essential for shaping the future of healthcare and improving the well-being of populations worldwide.
Related Posts

Step by Step Guide on The Best Way to Finance Car

The Best Way on How to Get Fund For Business to Grow it Efficiently
Healthcare Research Paper Topics

In this page, we provide a comprehensive list of healthcare research paper topics , expert advice on selecting compelling topics, guidance on writing an impactful research paper, and information about iResearchNet’s writing services. By exploring these resources, students in the health sciences field can choose relevant and significant healthcare research paper topics, develop their papers effectively, and access professional writing assistance to excel in their academic endeavors.
100 Healthcare Research Paper Topics
The field of healthcare research encompasses a vast array of topics that are crucial for understanding, improving, and transforming healthcare practices. As students in the health sciences, you have the opportunity to explore these diverse areas and contribute to the knowledge base of healthcare research. This comprehensive list aims to inspire and guide you in selecting healthcare research paper topics that align with your interests and academic goals. The topics are divided into ten distinct categories, each containing ten thought-provoking and relevant research ideas. Let this list serve as a springboard for your exploration and a catalyst for impactful research in the dynamic field of healthcare.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code.
1. Healthcare Policy and Management
- The Impact of Health Policies on Access to Care
- Assessing the Effectiveness of Health Insurance Programs
- Analyzing the Role of Healthcare Leadership in Quality Improvement
- Exploring Strategies for Healthcare Cost Containment
- Investigating the Relationship Between Healthcare Regulations and Patient Outcomes
- Evaluating the Impact of Electronic Health Records on Healthcare Delivery
- Examining the Role of Public-Private Partnerships in Healthcare
- Analyzing the Influence of Political Factors on Healthcare Decision-Making
- Assessing the Ethical Implications of Resource Allocation in Healthcare
- Investigating the Effectiveness of Health Promotion Programs in Primary Care Settings
2. Healthcare Ethics and Legal Issues
- Analyzing the Ethical Challenges of Healthcare Research Involving Human Subjects
- Exploring the Impact of Cultural and Religious Beliefs on Healthcare Decision-Making
- Examining Legal Issues in End-of-Life Care and Advance Directives
- Investigating the Ethical Implications of Genetic Testing and Personalized Medicine
- Assessing the Ethical Dilemmas in Access to Experimental Treatments
- Exploring the Role of Ethics Committees in Healthcare Organizations
- Analyzing the Intersection of Healthcare Ethics and Artificial Intelligence
- Evaluating the Legal and Ethical Implications of Telemedicine
- Investigating the Ethics of Healthcare Resource Allocation during Public Health Emergencies
- Examining the Legal and Ethical Issues of Patient Privacy in the Digital Age
3. Healthcare Technology and Innovation
- Assessing the Impact of Artificial Intelligence in Healthcare Diagnostics
- Exploring the Potential of Wearable Devices for Remote Patient Monitoring
- Investigating the Role of Big Data Analytics in Healthcare Decision-Making
- Analyzing the Use of Robotics in Surgery and Patient Care
- Examining the Impact of Telehealth on Healthcare Access and Delivery
- Evaluating the Benefits and Challenges of Electronic Health Records Implementation
- Exploring the Applications of Virtual Reality in Healthcare Education and Training
- Investigating the Role of Mobile Health Applications in Health Behavior Change
- Assessing the Potential of Blockchain Technology in Healthcare Data Security
- Analyzing the Ethical and Social Implications of Genetic Engineering in Healthcare
4. Healthcare Quality and Patient Safety
- Evaluating the Impact of Patient-Centered Care on Health Outcomes
- Analyzing the Role of Quality Improvement Initiatives in Reducing Medical Errors
- Assessing the Effectiveness of Medication Safety Practices in Healthcare Settings
- Exploring Strategies to Improve Healthcare Communication and Interprofessional Collaboration
- Investigating the Relationship Between Nursing Workforce and Patient Safety
- Examining the Impact of Clinical Practice Guidelines on Healthcare Quality
- Analyzing the Role of Patient Engagement in Enhancing Healthcare Quality
- Evaluating the Effectiveness of Lean Six Sigma in Healthcare Process Improvement
- Exploring the Role of Health Information Technology in Enhancing Patient Safety
- Investigating the Influence of Organizational Culture on Healthcare Quality and Safety
5. Mental Health and Psychological Well-being
- Analyzing the Impact of Stigma on Mental Health Help-Seeking Behavior
- Exploring the Effectiveness of Psychotherapy Approaches in Treating Mental Health Disorders
- Assessing the Role of Early Intervention in Preventing Mental Health Disorders
- Investigating the Relationship Between Adverse Childhood Experiences and Mental Health Outcomes
- Examining the Intersection of Mental Health and Substance Abuse Disorders
- Evaluating the Impact of Mindfulness-Based Interventions on Psychological Well-being
- Exploring the Role of Social Support in Mental Health Recovery
- Analyzing the Effectiveness of Mental Health Awareness Campaigns
- Investigating the Influence of Cultural Factors on Mental Health Help-Seeking Behavior
- Examining the Mental Health Needs and Challenges among Specific Populations (e.g., LGBTQ+, Veterans, Refugees)
6. Chronic Diseases and their Management
- Assessing the Impact of Lifestyle Factors on Chronic Disease Prevention and Management
- Exploring the Role of Community-Based Interventions in Chronic Disease Control
- Investigating the Relationship Between Social Determinants of Health and Chronic Disease Burden
- Analyzing the Use of Digital Health Technologies in Chronic Disease Management
- Examining the Impact of Health Literacy on Chronic Disease Outcomes
- Evaluating the Effectiveness of Self-Management Programs for Chronic Conditions
- Exploring the Role of Healthcare Providers in Chronic Disease Prevention and Management
- Analyzing the Impact of Health Policies on Chronic Disease Prevention Efforts
- Investigating the Relationship Between Mental Health and Chronic Disease Management
- Examining the Disparities in Access to Chronic Disease Care and Treatment
7. Healthcare Disparities and Access to Care
- Analyzing Racial and Ethnic Disparities in Healthcare Access and Quality
- Exploring the Role of Socioeconomic Factors in Healthcare Disparities
- Assessing the Impact of Geographic Location on Healthcare Access and Health Outcomes
- Investigating Gender Disparities in Healthcare Utilization and Treatment
- Examining the Influence of Health Insurance Status on Healthcare Disparities
- Evaluating the Effectiveness of Culturally Competent Care in Reducing Disparities
- Exploring the Relationship Between Language Barriers and Healthcare Access
- Analyzing the Impact of Implicit Bias on Healthcare Disparities
- Investigating the Role of Health Literacy in Healthcare Disparities
- Examining the Disparities in Mental Health Services and Access to Mental Healthcare
8. Healthcare Education and Training
- Assessing the Effectiveness of Simulation-Based Training in Healthcare Education
- Exploring the Role of Interprofessional Education in Improving Collaborative Practice
- Investigating the Impact of Technology-Enhanced Learning in Healthcare Education
- Analyzing the Use of Gamification in Healthcare Training and Skill Development
- Examining the Role of Continuing Education in Enhancing Healthcare Providers’ Competence
- Evaluating the Effectiveness of Mentorship Programs in Healthcare Education
- Exploring Strategies to Address Cultural Competence in Healthcare Education
- Analyzing the Role of Reflective Practice in Healthcare Professional Development
- Investigating the Use of Team-Based Learning in Healthcare Education
- Examining the Impact of Experiential Learning in Healthcare Training Programs
9. Public Health and Preventive Medicine
- Assessing the Impact of Vaccination Programs on Public Health Outcomes
- Exploring the Role of Health Promotion Campaigns in Preventing Non-communicable Diseases
- Investigating the Effectiveness of Community-Based Interventions in Disease Prevention
- Analyzing the Impact of Environmental Factors on Public Health
- Examining the Role of Social Determinants of Health in Health Disparities
- Evaluating the Effectiveness of Public Health Policies in Tobacco Control
- Exploring Strategies for Preventing and Managing Infectious Diseases
- Analyzing the Role of Health Education in Promoting Healthy Lifestyles
- Investigating the Influence of Media on Public Health Perceptions and Behaviors
- Examining the Challenges and Opportunities in Global Health Initiatives
10. Emerging Topics in Healthcare Research
- Assessing the Implications of Artificial Intelligence in Healthcare
- Exploring the Role of Precision Medicine in Personalized Healthcare
- Investigating the Impact of Genomic Research on Healthcare Delivery
- Analyzing the Use of Telemedicine in Rural and Underserved Areas
- Examining the Integration of Traditional and Complementary Medicine in Healthcare
- Evaluating the Potential of Digital Therapeutics in Disease Management
- Exploring the Ethical Considerations of Gene Editing Technologies in Healthcare
- Analyzing the Influence of Social Media on Healthcare Decision-Making
- Investigating the Role of Health Information Exchange in Coordinated Care
- Examining the Implications of Health Equity in Healthcare Research and Practice
This comprehensive list of healthcare research paper topics encompasses a wide range of areas within the healthcare field. Each category offers diverse research ideas that can inspire students in the health sciences to explore pressing issues, propose innovative solutions, and contribute to the advancement of healthcare knowledge. Whether you are interested in healthcare policy, ethics, technology, mental health, chronic diseases, healthcare disparities, education, public health, or emerging healthcare research paper topics, this list serves as a valuable resource to kickstart your research journey. Choose a topic that resonates with you, aligns with your academic goals, and enables you to make a meaningful impact in the field of healthcare research. Remember, the pursuit of knowledge and the drive to improve healthcare practices are at the heart of your journey as a student in the health sciences.
Choosing Healthcare Research Paper Topics
Choosing the right healthcare research paper topic is a crucial step in conducting a successful and impactful study. With the vast array of healthcare issues and areas to explore, it can be challenging to narrow down your focus. To help you navigate this process effectively, we have compiled expert advice and ten essential tips for selecting compelling healthcare research paper topics. Consider these insights as you embark on your research journey in the dynamic field of healthcare:
- Follow Your Passion : Choose a topic that genuinely interests you. Passion and enthusiasm will drive your motivation, ensuring that you remain engaged throughout the research process.
- Stay Informed : Keep up with the latest healthcare trends, emerging issues, and ongoing debates. Stay informed through reputable sources, academic journals, conferences, and professional networks to identify current and relevant research gaps.
- Identify a Research Gap : Conduct a thorough literature review to identify areas where there is a need for further research. Look for unanswered questions, controversies, or gaps in knowledge that you can address in your study.
- Consider Relevance and Significance : Choose a topic that is relevant to current healthcare challenges or contributes to improving healthcare practices, policies, or patient outcomes. Aim for a topic that has real-world implications and societal impact.
- Delve into Specific Areas : Narrow down your focus by selecting a specific aspect or subtopic within the broad field of healthcare. This allows for a more focused and in-depth analysis of the chosen area.
- Consult with Your Advisor or Faculty : Seek guidance from your research advisor or faculty members who specialize in healthcare research. They can provide valuable insights, help you refine your topic, and direct you to relevant literature and resources.
- Brainstorm with Peers : Engage in discussions with your peers and classmates to explore different perspectives and gain inspiration. Collaborative brainstorming sessions can generate new ideas and offer fresh insights.
- Consider Ethical Considerations : Take ethical considerations into account when selecting a healthcare research topic. Ensure that your research adheres to ethical guidelines and respects the rights and privacy of participants, especially in studies involving human subjects.
- Think Interdisciplinary : Consider interdisciplinary approaches to healthcare research. Explore how other disciplines, such as sociology, psychology, economics, or technology, intersect with healthcare, providing a broader perspective and enhancing the depth of your research.
- Feasibility and Available Resources : Assess the feasibility of your chosen topic, considering the resources, time, and data availability required for your research. Ensure that you have access to relevant data sources, research tools, and necessary support to carry out your study effectively.
By following these expert tips, you will be equipped to choose a healthcare research paper topic that aligns with your interests, is relevant to current healthcare challenges, and has the potential to make a meaningful impact in the field. Remember, selecting the right topic sets the foundation for a successful research endeavor, allowing you to contribute to the advancement of healthcare knowledge and practices.
How to Write a Healthcare Research Paper
Writing a healthcare research paper requires careful planning, organization, and attention to detail. To help you navigate the intricacies of the writing process, we have compiled ten essential tips to guide you towards crafting a well-written and impactful healthcare research paper. Follow these expert recommendations to enhance the quality and effectiveness of your research paper:
- Develop a Clear Research Question : Start by formulating a clear and concise research question that will serve as the central focus of your paper. Ensure that your question is specific, measurable, achievable, relevant, and time-bound (SMART).
- Conduct a Thorough Literature Review : Before diving into your research, conduct a comprehensive literature review to familiarize yourself with existing knowledge on the topic. Identify key theories, concepts, methodologies, and gaps in the literature that your research aims to address.
- Create a Solid Research Design : Design a robust research plan that aligns with your research question. Define your study population, sampling strategy, data collection methods, and statistical analyses. A well-designed research plan enhances the validity and reliability of your findings.
- Collect and Analyze Data : Implement your data collection methods, ensuring ethical considerations and adherence to research protocols. Once collected, analyze the data using appropriate statistical techniques and tools. Provide a clear description of your analytical methods.
- Structure your Paper Effectively : Organize your research paper into logical sections, including an introduction, literature review, methodology, results, discussion, and conclusion. Use headings and subheadings to enhance readability and guide the reader through your paper.
- Write a Compelling Introduction : Start your paper with a strong introduction that captures the reader’s attention and provides a concise overview of the research topic, objectives, and significance. Clearly state your research question and the rationale for your study.
- Present Clear and Concise Results : Present your research findings in a clear and concise manner. Use tables, graphs, and figures where appropriate to enhance the readability of your results. Provide a comprehensive interpretation of the results, highlighting key findings and their implications.
- Engage in Critical Analysis and Discussion : Analyze and interpret your findings in the context of existing literature. Discuss the strengths and limitations of your study, addressing potential biases or confounders. Consider alternative explanations and provide a thoughtful discussion of the implications of your findings.
- Follow Proper Citation and Referencing Guidelines : Adhere to the appropriate citation style (such as APA, MLA, or Chicago) consistently throughout your paper. Cite all sources accurately and include a comprehensive list of references at the end of your paper.
- Revise and Edit : Before finalizing your research paper, revise and edit it thoroughly. Pay attention to clarity, coherence, grammar, spelling, and punctuation. Ensure that your arguments flow logically and that your paper is well-structured and cohesive.
By following these tips, you will be well-equipped to write a high-quality healthcare research paper that effectively communicates your findings, contributes to the existing knowledge in the field, and engages readers with your insights and conclusions. Remember to seek feedback from your peers, professors, or research advisors to further refine your paper and ensure its overall excellence.
iResearchNet’s Custom Writing Services
At iResearchNet, we understand the challenges students face when it comes to writing healthcare research papers. To support you in your academic journey and ensure the highest quality of your work, we offer a comprehensive range of writing services. With a team of expert degree-holding writers and a commitment to excellence, we are dedicated to providing customized solutions tailored to your specific needs. Here are the features that set our writing services apart:
- Expert Degree-Holding Writers : Our team consists of highly qualified writers with advanced degrees in healthcare and related fields. They possess in-depth knowledge and expertise in various areas of healthcare, ensuring that your research paper is handled by professionals with subject matter expertise.
- Custom Written Works : We understand the importance of originality and uniqueness in academic writing. Our writers craft each research paper from scratch, tailoring it to your specific requirements and ensuring that it is entirely original and plagiarism-free.
- In-Depth Research : Our writers are skilled in conducting extensive research using reputable sources. They delve deep into the literature to gather the most relevant and up-to-date information, providing a solid foundation for your research paper.
- Custom Formatting : We offer custom formatting options to meet the specific guidelines of your institution and chosen citation style. Whether it’s APA, MLA, Chicago/Turabian, Harvard, or any other formatting style, our writers are well-versed in the intricacies of each.
- Top Quality : We are committed to delivering research papers of the highest quality. Our writers follow strict quality control measures to ensure that your paper meets the academic standards, including proper structure, clarity of writing, and logical flow of ideas.
- Customized Solutions : We recognize that every research paper is unique. Our writers work closely with you to understand your research objectives, guidelines, and preferences. They tailor their approach to ensure that your research paper reflects your vision and academic goals.
- Flexible Pricing : We offer flexible pricing options to accommodate students’ budgets. We understand the financial constraints students often face, and we strive to provide competitive and affordable pricing for our writing services.
- Short Deadlines : We understand that time is often a critical factor. We offer short turnaround times, allowing you to meet tight deadlines without compromising the quality of your research paper. With our dedicated team, we can handle urgent requests efficiently.
- Timely Delivery : We prioritize timely delivery to ensure that you receive your research paper well before your deadline. We understand the importance of submitting your work on time and offer our commitment to punctuality.
- 24/7 Support : Our customer support team is available 24/7 to assist you with any inquiries or concerns you may have. We are here to provide prompt and helpful assistance at any stage of the writing process.
- Absolute Privacy : We value your privacy and confidentiality. We have strict measures in place to protect your personal information and ensure that your identity remains anonymous throughout the process.
- Easy Order Tracking : We provide a user-friendly platform that allows you to track the progress of your order. You can stay updated on the status of your research paper and communicate directly with your assigned writer.
- Money-Back Guarantee : We are confident in the quality of our writing services. In the rare event that you are not satisfied with the final product, we offer a money-back guarantee, ensuring your peace of mind and commitment to your satisfaction.
At iResearchNet, we are dedicated to your success. We strive to exceed your expectations and provide you with a seamless and exceptional experience. Trust us with your healthcare research paper and let our expert writers bring your ideas to life with professionalism, accuracy, and academic excellence.
Customized Solutions for Your Research Needs
Are you a health sciences student in search of professional assistance for your healthcare research paper? Look no further than iResearchNet. We are here to empower your academic journey and help you excel in your research endeavors. With our comprehensive writing services and commitment to excellence, we provide the necessary tools and expert guidance to ensure your success.
At iResearchNet, we understand the unique challenges that come with writing healthcare research papers. Our team of expert degree-holding writers specializes in the health sciences field, allowing us to deliver customized solutions tailored to your specific research needs. Whether you need assistance in selecting a research topic, conducting in-depth literature reviews, analyzing data, or crafting a well-structured paper, we have the expertise to guide you every step of the way.
Take the next step in your healthcare research journey and unlock your academic potential with iResearchNet. Order your custom healthcare research paper today and let our expert writers bring your ideas to life with professionalism, accuracy, and academic excellence. Trust us to provide you with the guidance and support you need to achieve your research goals and make a meaningful impact in the field of healthcare.
ORDER HIGH QUALITY CUSTOM PAPER

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Global Health Research Topics
Subscribe to Fogarty's Global Health Matters newsletter , and weekly funding news for global health researchers .

The Fogarty International Center and its NIH partners invest in research on a variety of topics vital to global health. For each of these global health research topics, find an in-depth collection of news, resources and funding from Fogarty, the NIH, other U.S. government agencies, nongovernmental organizations and others.
- Chronic noncommunicable diseases (NCDs)
- Climate change
- Deafness and other communication disorders
- Diversity, equity, inclusion, and accessibility
- Eye disease, vision health and blindness
- Global health security
- Household air pollution
- Implementation science
- Infectious diseases
- Coronaviruses
- Ebola virus disease
- Tuberculosis (TB)
- Maternal and child health
- Mentoring and mentorship training
- Mobile health (mHealth)
- Neurological and mental disorders and diseases
- Oral and dental health
- Trauma and injury
- Tobacco control
- Women’s leadership in global health research

Health Topic Information
- NIH Health Topics
- MedlinePlus Health Topics
- Diseases and Conditions from the U.S. Centers for Disease Control and Prevention (CDC)
Updated January 3, 2024
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
Health Policy
Pursuing policies that improve the public health, including health economics, health services, advocacy, and preparedness
Health Policy Headlines
Explore the latest public health research and insights about health policy.

OB-GYN Training and Practice in Dobbs’ Shadow
Abortion bans are changing where prospective doctors study and work—and stand to exacerbate health care shortages and disparities.

Research Gaps Around Type 1 Diabetes
Recent research has highlighted disparities in research between Type 1 and Type 2 diabetes, with Type 1 long—and mistakenly—associated only with childhood onset. Elizabeth Selvin and Michael Fang challenge previously held assumptions about Type 1 diabetes.

Summer Institute in Data to Policy
Registration now open for courses in strategic leadership, youth engagement, policy analysis, resource management, and evaluation of programs and/or research.

The Omnipresence of PFAS—and What We Can Do About Them
PFAS—also known as “forever chemicals”—are everywhere, but we don’t yet know the extent of their damage to the environment, or our health.

Student Spotlight: Glendedora Dolce
For graduate students who are passionate about putting research and policy recommendations into action, the Johns Hopkins Health Policy Institute (HPI) is an invaluable experience. Among the Spring 2024 HPI Fellows is Glendedora Dolce, now in her second year as a Health and Public Policy doctoral student. Glendora's research aims to prevent injuries, illnesses, and health disparities as it pertains to child passenger safety.

HOPE Challenge: Heart of the Matter Recap
In observance of American Heart Month, HPM’s Hopkins Center for Health Disparities Solutions (HCHDS) hosted discussions with leading policy and medical experts about equity in cardiovascular health and how to advance policies that can positively change the health destinies of marginalized communities in the U.S. today and for generations to come.
- View more Headlines
Health Policy Faculty Experts
Looking for prominent public health experts in the field of health policy? They’re here at the Bloomberg School of Public Health.

Joshua M. Sharfstein
Joshua Sharfstein, MD, works to develop and promote public health strategies, healthcare payment approaches, and regulatory policies that advance health and equity.

Rajiv N. Rimal
Rajiv N. Rimal, PhD, MA, works globally on social and behavior change interventions with a focus on women and children’s nutrition and well-being. He adopts a social norms-based approach to address health inequities in low- and middle-income countries.

Gerard Anderson
Gerard Anderson, PhD, works with policymakers to control health care spending.

Albert Wu, MD, MPH, studies the impact of safety problems on patients and health care workers.
Departments
Biostatistics
Health Policy and Management
Health, Behavior and Society
Centers & Institutes
Center of Excellence in Maternal and Child Health
Child and Adolescent Health Measurement Initiative (CAHMI)
Early Childhood Services Research Program
Institute for Health and Social Policy (IHSP)
Women, Infants and Children Program (WIC)
Upcoming Events
Hope challenge hands that rock the cradle: expanding policies to protect black maternal and infant health.
77 interesting medical research topics for 2024
Last updated
25 November 2023
Reviewed by
Brittany Ferri, PhD, OTR/L
Medical research is the gateway to improved patient care and expanding our available treatment options. However, finding a relevant and compelling research topic can be challenging.
Use this article as a jumping-off point to select an interesting medical research topic for your next paper or clinical study.
- How to choose a medical research topic
When choosing a research topic , it’s essential to consider a couple of things. What topics interest you? What unanswered questions do you want to address?
During the decision-making and brainstorming process, here are a few helpful tips to help you pick the right medical research topic:
Focus on a particular field of study
The best medical research is specific to a particular area. Generalized studies are often too broad to produce meaningful results, so we advise picking a specific niche early in the process.
Maybe a certain topic interests you, or your industry knowledge reveals areas of need.
Look into commonly researched topics
Once you’ve chosen your research field, do some preliminary research. What have other academics done in their papers and projects?
From this list, you can focus on specific topics that interest you without accidentally creating a copycat project. This groundwork will also help you uncover any literature gaps—those may be beneficial areas for research.
Get curious and ask questions
Now you can get curious. Ask questions that start with why, how, or what. These questions are the starting point of your project design and will act as your guiding light throughout the process.
For example:
What impact does pollution have on children’s lung function in inner-city neighborhoods?
Why is pollution-based asthma on the rise?
How can we address pollution-induced asthma in young children?
- 77 medical research topics worth exploring in 2023
Need some research inspiration for your upcoming paper or clinical study? We’ve compiled a list of 77 topical and in-demand medical research ideas. Let’s take a look.
- Exciting new medical research topics
If you want to study cutting-edge topics, here are some exciting options:
COVID-19 and long COVID symptoms
Since 2020, COVID-19 has been a hot-button topic in medicine, along with the long-term symptoms in those with a history of COVID-19.
Examples of COVID-19-related research topics worth exploring include:
The long-term impact of COVID-19 on cardiac and respiratory health
COVID-19 vaccination rates
The evolution of COVID-19 symptoms over time
New variants and strains of the COVID-19 virus
Changes in social behavior and public health regulations amid COVID-19
Vaccinations
Finding ways to cure or reduce the disease burden of chronic infectious diseases is a crucial research area. Vaccination is a powerful option and a great topic to research.
Examples of vaccination-related research topics include:
mRNA vaccines for viral infections
Biomaterial vaccination capabilities
Vaccination rates based on location, ethnicity, or age
Public opinion about vaccination safety
Artificial tissues fabrication
With the need for donor organs increasing, finding ways to fabricate artificial bioactive tissues (and possibly organs) is a popular research area.
Examples of artificial tissue-related research topics you can study include:
The viability of artificially printed tissues
Tissue substrate and building block material studies
The ethics and efficacy of artificial tissue creation
- Medical research topics for medical students
For many medical students, research is a big driver for entering healthcare. If you’re a medical student looking for a research topic, here are some great ideas to work from:
Sleep disorders
Poor sleep quality is a growing problem, and it can significantly impact a person’s overall health.
Examples of sleep disorder-related research topics include:
How stress affects sleep quality
The prevalence and impact of insomnia on patients with mental health conditions
Possible triggers for sleep disorder development
The impact of poor sleep quality on psychological and physical health
How melatonin supplements impact sleep quality
Alzheimer’s and dementia
Cognitive conditions like dementia and Alzheimer’s disease are on the rise worldwide. They currently have no cure. As a result, research about these topics is in high demand.
Examples of dementia-related research topics you could explore include:
The prevalence of Alzheimer’s disease in a chosen population
Early onset symptoms of dementia
Possible triggers or causes of cognitive decline with age
Treatment options for dementia-like conditions
The mental and physical burden of caregiving for patients with dementia
- Lifestyle habits and public health
Modern lifestyles have profoundly impacted the average person’s daily habits, and plenty of interesting topics explore its effects.
Examples of lifestyle and public health-related research topics include:
The nutritional intake of college students
The impact of chronic work stress on overall health
The rise of upper back and neck pain from laptop use
Prevalence and cause of repetitive strain injuries (RSI)
- Controversial medical research paper topics
Medical research is a hotbed of controversial topics, content, and areas of study.
If you want to explore a more niche (and attention-grabbing) concept, here are some controversial medical research topics worth looking into:
The benefits and risks of medical cannabis
Depending on where you live, the legalization and use of cannabis for medical conditions is controversial for the general public and healthcare providers.
Examples of medical cannabis-related research topics that might grab your attention include:
The legalization process of medical cannabis
The impact of cannabis use on developmental milestones in youth users
Cannabis and mental health diagnoses
CBD’s impact on chronic pain
Prevalence of cannabis use in young people
The impact of maternal cannabis use on fetal development
Understanding how THC impacts cognitive function
Human genetics
The Human Genome Project identified, mapped, and sequenced all human DNA genes. Its completion in 2003 opened up a world of exciting and controversial studies in human genetics.
Examples of human genetics-related research topics worth delving into include:
Medical genetics and the incidence of genetic-based health disorders
Behavioral genetics differences between identical twins
Genetic risk factors for neurodegenerative disorders
Machine learning technologies for genetic research
Sexual health studies
Human sexuality and sexual health are important (yet often stigmatized) medical topics that need new research and analysis.
As a diverse field ranging from sexual orientation studies to sexual pathophysiology, examples of sexual health-related research topics include:
The incidence of sexually transmitted infections within a chosen population
Mental health conditions within the LGBTQIA+ community
The impact of untreated sexually transmitted infections
Access to safe sex resources (condoms, dental dams, etc.) in rural areas
- Health and wellness research topics
Human wellness and health are trendy topics in modern medicine as more people are interested in finding natural ways to live healthier lifestyles.
If this field of study interests you, here are some big topics in the wellness space:
Gluten sensitivity
Gluten allergies and intolerances have risen over the past few decades. If you’re interested in exploring this topic, your options range in severity from mild gastrointestinal symptoms to full-blown anaphylaxis.
Some examples of gluten sensitivity-related research topics include:
The pathophysiology and incidence of Celiac disease
Early onset symptoms of gluten intolerance
The prevalence of gluten allergies within a set population
Gluten allergies and the incidence of other gastrointestinal health conditions
Pollution and lung health
Living in large urban cities means regular exposure to high levels of pollutants.
As more people become interested in protecting their lung health, examples of impactful lung health and pollution-related research topics include:
The extent of pollution in densely packed urban areas
The prevalence of pollution-based asthma in a set population
Lung capacity and function in young people
The benefits and risks of steroid therapy for asthma
Pollution risks based on geographical location
Plant-based diets
Plant-based diets like vegan and paleo diets are emerging trends in healthcare due to their limited supporting research.
If you’re interested in learning more about the potential benefits or risks of holistic, diet-based medicine, examples of plant-based diet research topics to explore include:
Vegan and plant-based diets as part of disease management
Potential risks and benefits of specific plant-based diets
Plant-based diets and their impact on body mass index
The effect of diet and lifestyle on chronic disease management
Health supplements
Supplements are a multi-billion dollar industry. Many health-conscious people take supplements, including vitamins, minerals, herbal medicine, and more.
Examples of health supplement-related research topics worth investigating include:
Omega-3 fish oil safety and efficacy for cardiac patients
The benefits and risks of regular vitamin D supplementation
Health supplementation regulation and product quality
The impact of social influencer marketing on consumer supplement practices
Analyzing added ingredients in protein powders
- Healthcare research topics
Working within the healthcare industry means you have insider knowledge and opportunity. Maybe you’d like to research the overall system, administration, and inherent biases that disrupt access to quality care.
While these topics are essential to explore, it is important to note that these studies usually require approval and oversight from an Institutional Review Board (IRB). This ensures the study is ethical and does not harm any subjects.
For this reason, the IRB sets protocols that require additional planning, so consider this when mapping out your study’s timeline.
Here are some examples of trending healthcare research areas worth pursuing:
The pros and cons of electronic health records
The rise of electronic healthcare charting and records has forever changed how medical professionals and patients interact with their health data.
Examples of electronic health record-related research topics include:
The number of medication errors reported during a software switch
Nurse sentiment analysis of electronic charting practices
Ethical and legal studies into encrypting and storing personal health data
Inequities within healthcare access
Many barriers inhibit people from accessing the quality medical care they need. These issues result in health disparities and injustices.
Examples of research topics about health inequities include:
The impact of social determinants of health in a set population
Early and late-stage cancer stage diagnosis in urban vs. rural populations
Affordability of life-saving medications
Health insurance limitations and their impact on overall health
Diagnostic and treatment rates across ethnicities
People who belong to an ethnic minority are more likely to experience barriers and restrictions when trying to receive quality medical care. This is due to systemic healthcare racism and bias.
As a result, diagnostic and treatment rates in minority populations are a hot-button field of research. Examples of ethnicity-based research topics include:
Cancer biopsy rates in BIPOC women
The prevalence of diabetes in Indigenous communities
Access inequalities in women’s health preventative screenings
The prevalence of undiagnosed hypertension in Black populations
- Pharmaceutical research topics
Large pharmaceutical companies are incredibly interested in investing in research to learn more about potential cures and treatments for diseases.
If you’re interested in building a career in pharmaceutical research, here are a few examples of in-demand research topics:
Cancer treatment options
Clinical research is in high demand as pharmaceutical companies explore novel cancer treatment options outside of chemotherapy and radiation.
Examples of cancer treatment-related research topics include:
Stem cell therapy for cancer
Oncogenic gene dysregulation and its impact on disease
Cancer-causing viral agents and their risks
Treatment efficacy based on early vs. late-stage cancer diagnosis
Cancer vaccines and targeted therapies
Immunotherapy for cancer
Pain medication alternatives
Historically, opioid medications were the primary treatment for short- and long-term pain. But, with the opioid epidemic getting worse, the need for alternative pain medications has never been more urgent.
Examples of pain medication-related research topics include:
Opioid withdrawal symptoms and risks
Early signs of pain medication misuse
Anti-inflammatory medications for pain control
- Identify trends in your medical research with Dovetail
Are you interested in contributing life-changing research? Today’s medical research is part of the future of clinical patient care.
As your go-to resource for speedy and accurate data analysis , we are proud to partner with healthcare researchers to innovate and improve the future of healthcare.
Should you be using a customer insights hub?
Do you want to discover previous research faster?
Do you share your research findings with others?
Do you analyze research data?
Start for free today, add your research, and get to key insights faster
Editor’s picks
Last updated: 11 January 2024
Last updated: 15 January 2024
Last updated: 17 January 2024
Last updated: 12 May 2023
Last updated: 30 April 2024
Last updated: 18 May 2023
Last updated: 25 November 2023
Last updated: 13 May 2024
Latest articles
Related topics, .css-je19u9{-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-flex-direction:row;-ms-flex-direction:row;flex-direction:row;-webkit-box-flex-wrap:wrap;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;-webkit-box-pack:center;-ms-flex-pack:center;-webkit-justify-content:center;justify-content:center;row-gap:0;text-align:center;max-width:671px;}@media (max-width: 1079px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}}@media (max-width: 799px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}} decide what to .css-1kiodld{max-height:56px;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;}@media (max-width: 1079px){.css-1kiodld{display:none;}} build next, decide what to build next.

Users report unexpectedly high data usage, especially during streaming sessions.

Users find it hard to navigate from the home page to relevant playlists in the app.

It would be great to have a sleep timer feature, especially for bedtime listening.

I need better filters to find the songs or artists I’m looking for.
Log in or sign up
Get started for free
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Research Topics
General research, cancer research, genetics research, genome biology.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009.

Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research.
- Hardcopy Version at National Academies Press
3 The Value, Importance, and Oversight of Health Research
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2 , the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
In addition to defining health research and delineating its value to individuals and society, this chapter provides an overview and historical perspective of federal research regulations that were in place long before the Privacy Rule was implemented. Because a great deal of medical research falls under the purview of multiple federal regulations, it is important to understand how the various rules overlap or diverge. The chapter also explains how the definition of research has become quite complex under the various federal regulations, which make a distinction between research and some closely related health practice activities that also use health data, such as quality improvement initiatives.
The chapter also reviews the available survey data regarding public perceptions of health research and describes the importance of effective communication about health research with patients and the public.
- CONCEPTS AND VALUE OF HEALTH RESEARCH
Definitions
Under both the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and the Common Rule , “research” is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” This is a broad definition that may include biomedical research, epidemiological studies, 1 and health services research, 2 as well as studies of behavioral, social, and economic factors that affect health.
Perhaps the most familiar form of health research is the clinical trial, in which patients volunteer to participate in studies to test the efficacy and safety of new medical interventions. But an increasingly large portion of health research is now information based. A great deal of research entails the analysis of data and biological samples that were initially collected for diagnostic, treatment, or billing purposes, or that were collected as part of other research projects, and are now being used for new research purposes. This secondary 3 use of data is a common research approach in fields such as epidemiology, health services research, and public health research, and includes analysis of patterns of occurrences, determinants, and natural history of disease; evaluation of health care interventions and services; drug safety surveillance; and some genetic and social studies ( Lowrance, 2002 ; Lowrance and Collins, 2007 ).
The Importance of Health Research
Like privacy, health research has high value to society. It can provide important information about disease trends and risk factors, outcomes of treatment or public health interventions, functional abilities, patterns of care, and health care costs and use. The different approaches to research provide complementary insights. Clinical trials can provide important information about the efficacy and adverse effects of medical interventions by controlling the variables that could impact the results of the study, but feedback from real-world clinical experience is also crucial for comparing and improving the use of drugs, vaccines, medical devices, and diagnostics. For example, Food and Drug Administration (FDA) approval of a drug for a particular indication is based on a series of controlled clinical trials, often with a few hundred to a few thousand patients, but after approval it may be used by millions of people in many different contexts. Therefore, tracking clinical experience with the drug is important for identifying relatively rare adverse effects and for determining the effectiveness in different populations or in various circumstances. It is also vital to record and assess experience in clinical practice in order to develop guidelines for best practices and to ensure high-quality patient care.
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the population contributes greatly to the national economy ( Hatfield et al., 2001 ; Murphy and Topel, 1999 ) in addition to the individual benefits of improved health. If the research enterprise is impeded, or if it is less robust, important societal interests are affected.
The development of Herceptin as a treatment for breast cancer is a prime example of the benefits of research using biological samples and patient records ( Box 3-1 ) ( Slamon et al., 1987 ). Many other examples of findings from medical records research have changed the practice of medicine as well. Such research underlies the estimate that tens of thousands of Americans die each year from medical errors in the hospital, and research has provided valuable information for reducing these medical errors by implementing health information technology, such as e-prescribing ( Bates et al., 1998 ; IOM, 2000b ). This type of research also has documented that disparities in health care and lack of access to care in inner cities and rural areas result in poorer health outcomes ( Mick et al., 1994 ). Furthermore, medical records research has demonstrated that preventive services (e.g., mammography) substantially reduce mortality and morbidity at reasonable costs ( Mandelblatt et al., 2003 ), and has established a causal link between the nursing shortage and patient health outcomes by documenting that patients in hospitals with fewer registered nurses are hospitalized longer and are more likely to suffer complications, such as urinary tract infections and upper gastrointestinal bleeding ( Needleman et al., 2002 ). These findings have all informed and influenced policy decisions at the national level. As the use of electronic medical records increases, the pace of this form of research is accelerating, and the opportunities to generate new knowledge about what works in health care are expanding ( CHSR, 2008 ).
Examples of Important Findings from Medical Database Research. Herceptin and breast cancer: Data were collected from a cohort of more than 9,000 breast cancer patients whose tumor specimens were consecutively received at the University (more...)
Advances in health information technology are enabling a transformation in health research that could facilitate studies that were not feasible in the past, and thus lead to new insights regarding health and disease. As noted by the National Committee on Vital and Health Statistics, “Clinically rich information is now more readily available, in a more structured format, and able to be electronically exchanged throughout the health and health care continuum. As a result, the information can be better used for quality improvement, public health, and research, and can significantly contribute to improvements in health and health care for individuals and populations” ( NCVHS, 2007a ). The informatics grid recently developed with support from the National Cancer Institute (Cancer Biomedical Informatics Grid, or caBIG) is an example of a how information technologies can facilitate health research by enabling broader sharing of health data while still ensuring regulatory compliance and protecting patient privacy ( Box 3-2 ).
caBIG (Cancer Biomedical Informatics Grid). The National Cancer Institute’s caBIG Data Sharing and Intellectual Capital Workspace’s mission is to enable all constituencies in the cancer community—including researchers, physicians, (more...)
Science today is also changing rapidly and becoming more complex, so no single researcher or single site can bring all the expertise to develop and validate medical innovations or to ensure their safety. Thus, efficient sharing of information between institutions has become even more important than in previous eras, when there were fewer new therapies introduced. The expansion of treatment options, as well as the escalating expense of new therapies, mandates greater scrutiny of true effectiveness, 5 once efficacy has been demonstrated. This requires registries of patient characteristics, outcomes, and adverse events. Large populations are required to facilitate comparison of patient populations and to calculate risk/benefit estimates. For example, INTERMACS 6 (Interagency Registry for Mechanically Assisted Circulatory Support) is a national registry for patients who are receiving mechanical circulatory support device therapy to treat advanced heart failure. This registry was devised as a joint effort of the National Heart, Lung and Blood Institute, Centers for Medicare & Medicaid Services, FDA, clinicians, scientists and industry representatives. Analysis of the data collected is expected to facilitate improved patient evaluation and management while aiding in better device development. Registry results are also expected to influence future research and facilitate appropriate regulation and reimbursement of such devices. Similarly, the Extracorporeal Life Support Organization (ELSO), 7 an international consortium of health care professionals and scientists who focus on the development and evaluation of novel therapies for support of failing organ systems, maintains a registry of extracorporeal membrane oxygenation and other novel forms of organ system support. Registry data are used to support clinical practice and research, as well as regulatory agencies. Another example is the database developed by the United Network for Organ Sharing (UNOS) for the collection, storage, analysis and publication of data pertaining to the patient waiting list, organ matching, and transplants. 8 Launched in 1999, this secure Internet-based system contains data regarding every organ donation and transplant event occurring in the United States since 1986.
Information-based research, such as research using health information databases has many advantages (reviewed by Lowrance, 2002 ). It is often faster and less expensive than experimental studies; it can analyze very large sets of data and may detect unexpected phenomena or differences among subpopulations that might not be included in a controlled experimental study; it can often be undertaken when controlled trials are simply not possible for ethical, technical, or other reasons, and it can be used to study effectiveness of a specific test or intervention in clinical practice, rather than just the efficacy as determined by a controlled experimental study. It can also reexamine data accrued in other research studies, such as clinical trials, to answer new questions quickly and inexpensively. However, information-based research does have limitations. Often it has less statistical rigor than controlled clinical studies because it lacks scientific control over the original data collection, quality, and format that prospective experimental research can dictate from the start. In addition to these scientific limitations, because of its relational and often distant physical separation from the data subjects, and the sheer volume of the records involved, obtaining individual consent for the research can be difficult or impossible.
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. The goal of personalized medicine is to tailor prevention strategies and treatments to each individual based on his/her genetic composition and health history. In spite of the strides made in improving health through new treatments, it is widely known that most drugs are effective in only a fraction of patients who have the condition for which the drug is indicated. Moreover, a small percentage of patients are likely to have adverse reactions to drugs that are found to be safe for the majority of the population at the recommended dose. Both of these phenomena are due to variability in the patient population. Revolutionary advances in the study of genetics and other markers of health and disease are now making it possible to identify and study these variations, and are leading to more personalized approaches to health care—that is, the ability to give “the appropriate drug, at the appropriate dose, to the appropriate patient, at the appropriate time.” Achieving the goals of personalized medicine will lead to improvements in both the effectiveness and the safety of medical therapies.
Public Perceptions of Health Research
A number of studies have been undertaken to gauge the public’s attitude toward research and the factors that influence individuals’ willingness to participate in research. The surveys reviewed in this chapter focus on interventional clinical trials. A review of survey questions to gauge the public willingness to allow their medical records to be used in research can be found in Chapter 2 .
The Public Values Health Research
A number of studies suggest that most Americans have a positive view of medical research and believe that research is beneficial to society. A recent Harris poll found that nearly 80 percent of respondents were interested in health research findings, consistent with previous survey results ( Westin, 2007 ). A study in 2005 compiled data from 70 state surveys and 18 national surveys and found that the majority of Americans believe maintaining world leadership in health-related research is important. Seventy-eight percent of respondents said that it is very important, and 17 percent said that it is somewhat important. Only 4 percent of Americans reported that maintaining world leadership in health-related research is not impor tant ( Woolley and Propst, 2005 ). Similar results were found in a 2007 survey—76 percent of respondents reported that science plays a very important role in our health, and 78 percent reported that science plays a very important role in our competitiveness ( Research!America, 2007 ).
The Virginia Commonwealth University 2004 Life Sciences Survey also found that most Americans have a positive view of research. In this study, 90 percent of respondents agreed that developments in science have made society better; 92 percent reported that “scientific research is essential for improving the quality of human lives”; and 84 percent agreed that “the benefits of scientific research outweigh the harmful results” ( NSF, 2006 ).
Overall Experience When Participating in Research
Little is known about the attitudes of individuals who have actually participated in medical research. However, the available evidence suggests that most research participants have positive experiences. A recent Harris Poll found that 13 percent of respondents had participated in some form of health research, and 87 percent of those felt comfortable about their experience ( Westin, 2007 ). In a study focused on cancer, 93 percent of respondents who participated in research reported it as a very positive experience; 76 percent said they would recommend participation in a clinical trial to someone with cancer. Most physicians surveyed in this study stated that they believe clinical trial participants receive the best possible care, and have outcomes at least as good as patients receiving standard cancer treatment ( Comis et al., 2000 ). Another study found that 55 percent of individuals who participated in a research study would be willing to participate again in a future research study ( Trauth et al., 2000 ).
Willingness to Participate in Research
Public opinion surveys indicate that a majority of Americans are willing to participate in clinical research studies. In 2001, a compilation of studies commissioned by Research !America found that 63 percent of Americans would be willing to participate in a clinical research study ( Woolley and Propst, 2005 ). This percentage has remained stable over time. A 2007 Research!America survey also found that 63 percent of Americans would be very likely to participate in a clinical research study if asked ( Research!America, 2007 ); 68 percent of respondents reported that their desire to improve their own health or the health of others was a major factor in deciding whether to participate in a clinical research project ( Research!America, 2007 ).
Other surveys also suggest that willingness to participate in research focused on specific diseases is quite high. In one survey, the percentage of respondents indicating a willingness to participate in a medical research study was 88 percent for cancer, 86 percent for heart disease, 83 percent for a noncurable fatal disease, 79 percent for addiction, 78 percent for depression, and 76 percent for schizophrenia ( Trauth et al., 2000 ). Respondents with greater knowledge of how research is conducted were more willing to participate ( Trauth et al., 2000 ). Another study found that 8 of 10 Americans would consider participating in a clinical trial if faced with cancer. More than two-thirds of respondents said they would be willing to participate in a clinical trial designed to prevent cancer ( Comis et al., 2000 ).
Americans also seem to be very supportive of medical research that relies on genetic data. A 2007 survey found that 93 percent of Americans supported the use of genetic testing if the information collected is used by researchers to find new ways to diagnose, prevent, or treat disease ( Genetics & Public Policy Center, 2007 ). Two separate surveys found that 66 percent of Americans would be willing to donate their genetic material for medical research ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ). However, despite this apparent positive view of genetic research, 92 percent of Americans reported they were concerned about their genetic information being used in a “harmful way” ( Genetics & Public Policy Center, 2007 ).
Many factors, in addition to concerns about privacy and confidentiality ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ), may influence an individual’s willingness to participate in a medical research study. The Trauth survey found that individuals with higher income levels, with a college or graduate degree, or with children were more likely to participate in research. Age affected willingness to participate: 57 percent of respondents ages 18–34 were willing to participate in research, but only 31 percent of respondents ages 65 or older were willing ( Trauth et al., 2000 ).
Other factors that potentially influence an individual’s willingness to participate in research are race and ethnicity. It is well documented that minorities participate in health research at a much lower percentage than white Americans. Many cultural, linguistic, and socioeconomic barriers could be responsible for this difference ( Giuliano et al., 2000 ), and study results have been variable on this issue. Several studies suggest that the low participation rates by racial and ethnic minority groups are due to their strong distrust of the medical research community compared to the general population ( Braunstein et al., 2008 ; Corbie-Smith et al., 1999 ; Farmer et al., 2007 ; Grady et al., 2006 ; Shavers et al., 2002 ).
However, other evidence suggests that the low percentage of minorities participating in research is related to minority groups’ lack of access to the research community ( Brown et al., 2000 ; Wendler et al., 2006 ; Williams and Corbie-Smith, 2006 ). Thus, it is likely that the low number of minority individuals participating in medical research is at least partly due to recruitment techniques that are ineffective for minority populations.
The survey that focused on cancer research suggests that one of the main reasons why individuals do not participate in research is lack of knowledge about the availability of clinical trials. In a survey of nearly 6,000 cancer patients, 85 percent said they were unaware of the opportunity to participate in a clinical trial. Respondents who did participate said they did so because of one of the following beliefs: (1) trials provide access to the best quality of care (76 percent), (2) their participation would benefit future cancer patients (72 percent), (3) they would receive newer and better treatment (63 percent), and (4) participation would get them more care and attention (40 percent) ( Comis et al., 2000 ).
A recommendation from a physician can also impact participation. In the United States, 48 percent of respondents to one survey reported that a physicians’ recommendation would be a major factor in deciding whether to take part in a research study. Nearly three-fourths of respondents also cited an institution’s reputation as a key factor to consider when deciding whether to participate in a study ( Research!America, 2007 ). Twenty percent of respondents in an Italian public survey indicated that the presence of a physician as a reference during a research study influenced their willingness to participate ( Mosconi et al., 2005 ).
In sum, surveys indicate that the vast majority of Americans have a positive view of medical research, believe that research is beneficial to society, and are interested in health research findings. Although little is known about the attitudes of individuals who have actually participated in medical research, the available evidence suggests that most research participants have positive experiences. Surveys also suggest that a majority of Americans are willing to participate in clinical research studies. Similar to the findings in Chapter 2 , surveys indicate that many factors, in addition to concerns about privacy and confidentiality, can potentially influence an individual’s willingness to participate in medical research, including the type of research and personal characteristics such as health status, age, education, and race. Notably, respondents with greater knowledge of how research is conducted were more willing to participate in research.
- OVERSIGHT OF HEALTH RESEARCH
Historical Development of Federal Protections of Health Information in Research
The development of international codes, federal legislation, and federal regulation of human subjects often occurred in response to past abuses in biomedical experiments (reviewed by Pritts, 2008 ) ( Box 3-3 ). The most well-known examples included (1) reported abuses of concentration camp prisoners in Nazi experiments during World War II, and (2) the Tuskegee syphilis study begun in 1932, in which researchers withheld effective treatment from affected African American men long after a cure for syphilis was found. Most of the current principles and standards for conducting human subjects research were developed primarily to protect against the physical and mental harms that can result from these types of biomedical experiments. Therefore, they focus on the principles of autonomy and consent. Although the standards apply to research that uses identifiable health information, research based solely on information is not their primary focus.
The Basis for Human Subjects Protections in Biomedical Research. Nuremberg Code The Nuremberg Code, created by the international community after the Nazi War Crimes Trials, is generally seen as the first codification (more...)
In the United States, perhaps the most influential inquiry into the protection of human subjects in research was the Belmont Report. The Belmont principles have been elaborated on in many settings, and served as the basis for formal regulation of human subjects research in the United States. In general, states do not directly regulate the activity of most researchers ( Burris et al., 2003 ). However, the Belmont Commission’s recommendations were reflected in the Department of Health and Human Services’ (HHS’s) Policy for Protection of Human Subjects Research , Subpart A of 45 C.F.R. 46 (“Subpart A”) in 1979. 9 These protections were considered a benchmark policy for federal agencies, and in December 1981, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research recommended 10 that all federal departments and agencies adopt the HHS regulations. 11
In 1982, the President’s Office of Science and Technology Policy appointed a Committee for the Protection of Human Research Subjects to respond to the recommendations of the President’s commission. The committee agreed that uniformity of federal regulations on human subjects protection is desirable to eliminate unnecessary regulations and to promote increased understanding by institutions that conduct federally supported or regulated research. As a result, in 1991, other federal departments and agencies joined HHS in adopting a uniform set of rules for the protection of human subjects of research, identical to Subpart A of 45 C.F.R. 46, which is now informally known as the “ Common Rule .” Eighteen federal agencies have now adopted the Common Rule as their own respective regulations.
Overview of the Common Rule
The Common Rule governs most federally funded research conducted on human beings and aims to ensure that the rights of human subjects are protected during the course of a research project. The Common Rule stresses the importance of individual autonomy and consent; requires independent review of research by an Institutional Review Board (IRB); and seeks to minimize physical and mental harm. Privacy and confidentiality protections, although not defined in a detailed and prescriptive manner, are included as important components of risk in research.
The framework for achieving the goal of protecting human subjects is based on two foundational requirements: the informed consent of the research participant and the review of proposed research by an IRB. This section describes some of the basic parameters of the Common Rule (reviewed by Pritts, 2008 ). Particular provisions that interact with the HIPAA Privacy Rule are described in more detail in Chapter 4 .
Scope of the Common Rule
In general, the Common Rule applies only to research on human subjects that is supported by the federal government. 12 As noted previously, research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” 13
Under the Common Rule , a “human subject” is defined as “a living individual about whom an investigator … conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information.” Private information is considered to be personally identifiable if the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
The Common Rule applies to most human subjects research conducted using federal funds, but its influence is broader because most institutions that accept federal funds sign an agreement (a Federalwide Assurance or FWA) with HHS to abide by the Common Rule requirements in all research, regardless of funding source. Nonetheless, some privately funded human subjects research is conducted outside the purview of federal regulation ( Goldman and Choy, 2001 ; Williams, 2005 ). Companies and other organizations may voluntarily choose to apply the Common Rule to their research projects, and many do. However, research projects in which compliance is voluntary are not subject to oversight or disciplinary action by HHS ( Goldman and Choy, 2001 ; Williams, 2005 ).
Informed Consent 14
The Common Rule requires that a researcher obtain informed consent (usually in writing) from a person before he/she can be admitted to a study ( Williams, 2005 ). Informed consent is sought through a process in which a person learns key facts about a research study, including the potential risks and benefits, so that he/she can then agree voluntarily to take part or decide against it.
The Common Rule informed consent regulations focus primarily on the elements and documentation of informed consent rather than on the process used to obtain it. As to the process, the regulations require that informed consent be sought only under circumstances that provide the prospective subject with adequate opportunity to consider whether to participate. The Common Rule requires that information pertaining to informed consent be given in language understandable to the subject, and that the consent does not imply that the subject is giving up his/her legal rights or that the investigator is released from liability for negligence during the conduct of the study. 15
The Common Rule also specifies a number of elements that must be provided when informed consent is sought. These elements include:
- an explanation of the purposes of the research,
- the expected duration of the subject’s participation,
- the potential risks and benefits of the research,
- how confidentiality will be maintained,
- the fact that participation is strictly voluntary, and
- who the subject can contact to answer questions about the study or about his/her rights as a research participant.
In certain limited circumstances, the Common Rule allows an informed consent to be for unspecified future research. For example, under the Common Rule an informed consent can be used to obtain a person’s permission to study personally identifiable information maintained in a repository for future, unspecified research purposes ( HHS, 2003 ).
For the most part, the required elements of an informed consent address all types of research, although some are more relevant to biomedical research (e.g., the consent must include a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject). One required element of informed consent is particularly relevant to research involving personally identifiable health information. The Common Rule requires an informed consent to include a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. 16
Institutional Review Boards
Adopting the principles of the Belmont Report, the Common Rule requires that protocols for human subjects research be reviewed by an IRB ( Box 3-4 ) before research may begin. 17 The IRB must meet certain membership requirements, including having members with different expertise and at least one member who is not affiliated with the investigator’s institution. The Common Rule specifies which level of IRB review is needed for various types of research and provides criteria for the IRB to consider during the review. Although the Common Rule does not specify the procedures an IRB must follow in its review of protocols, it does require the IRB to have written procedures for how it will review protocols and document IRB decisions.
Institutional Review Boards. According to the Department of Health and Human Services (HHS) Institutional Review Board (IRB) guidebook, “the IRB is an administrative body established to protect the rights and welfare of human research subjects (more...)
The Common Rule requires that an IRB determine the following factors are satisfied to approve proposed research:
- Risks to subjects are minimized;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result;
- The selection of subjects is equitable;
- Informed consent will be sought in accordance with the rules and will be documented;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects; and
- When appropriate, adequate provisions are in place to protect the privacy of subjects and to maintain the confidentiality of data. 18
An IRB may waive the requirement to obtain informed consent or approve an alteration of the consent form for some minimal risk research. The IRB may also waive the requirement for signed consent in certain circumstances. 19
Anonymized Data
As noted above, the Common Rule considers use of “private identifiable information” to be human subjects research. Data are considered personally identifiable if the identity of the subject is or may be readily ascertained by the investigator or associated with the information accessed by the researcher. 20 However, the Common Rule exempts from its requirements research that involves:
[T]he collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. 21
Otherwise identifiable data may be deidentified or “anonymized” for purposes of the Common Rule if it is coded and certain other conditions are met ( HHS, 2004 ). Under Guidance issued by the Office for Human Research Protection, information is “coded” if identifying information (such as name or Social Security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (the code), and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimen.
Research involving only coded private information or specimens is not considered to involve human subjects under the Common Rule if the following conditions are met:
- The private information or specimens were not collected specifically for the currently proposed research project through an interaction or intervention with living individuals; and
- —The key to decipher the code is destroyed before the research begins;
- —The investigators and the holder of the key enter into an agreement prohibiting the release of the key to the investigators under any circumstances, until the individuals are deceased;
- —IRB-approved written policies and operating procedures for a repository or data management center prohibit the release of the key to investigators under any circumstances, until the individuals are deceased; or
- —Other legal requirements prohibit the release of the key to the investigators, until the individuals are deceased.
Under this standard, when a researcher accesses or receives data that have been coded and does not have access to the identifying key, the research is not considered human subjects research and is not subject to the Common Rule ’s requirements of informed consent or IRB review and approval of protocol.
Enforcement of the Common Rule
The Common Rule requirements for informed consent do not preempt any applicable federal, state, or local laws that require additional information to be disclosed to a subject in order for informed consent to be legally effective. 22
Federal funding can be suspended or withdrawn from an institution when it is found to be in material violation of the Common Rule . 23 There is no authority to impose penalties directly on individual researchers for violations. Neither does the Common Rule expressly provide a research participant with a private right of action. It should be noted, however, that recent cases indicate that courts may be willing to hold an institution liable under common law negligence theories where the approved informed consent form is determined to be less than adequate ( Shaul et al., 2005 ). 24
FDA Protection of Human Research Subjects
Some health research is also subject to FDA regulations. The FDA is charged by statute with ensuring the protection of the rights, safety, and welfare of human subjects who participate in clinical investigations 25 involving articles subject to the Federal Food, Drug, and Cosmetic Act 26 (the Act), as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA, including drugs, medical devices, and biological products for human use ( Box 3-5 ).
FDA Protection of Human Subjects Regulations. The Food and Drug Administration (FDA) Protection of Human Subjects Regulations aim to protect the rights of human subjects enrolled in research involving products that the FDA regulates (i.e., drugs, medical (more...)
In January 1981, the FDA adopted regulations governing informed consent of human subjects 27 and regulations establishing standards for the composition, operation, and responsibilities of IRBs that review clinical investigations involving human subjects. 28 At the same time, HHS adopted the Common Rule regulations on the protection of human research subjects. 29 The FDA’s regulations were harmonized with the Common Rule in 1991 to the extent permitted by statute. Key differences between FDA and HHS regulations include that the FDA does not allow for waiver or alteration of informed consent and requires that subjects be informed that the FDA may inspect their medical records. In addition, studies of efficacy based solely on medical records research are not permitted to support registration. Remaining differences in the rules are due to differences in the statutory scope or requirements ( Lee, 2000 ).
- DISTINGUISHING HEALTH RESEARCH FROM PRACTICE
The Common Rule and Privacy Rule make a somewhat artificial distinction between health research and some closely related health care practices, such as public health practice, quality improvement activities, program evaluations, 30 and utilization reviews, 31 all of which may involve collection and analysis of personally identifiable health information. However, determining which activities meet the definition of “research” is a major challenge for IRBs, Privacy Boards , 32 investigators, and health care practitioners because neither the regulations nor their interpretations by HHS provide clear guidance on how to distinguish research from activities that use similar techniques to analyze health information ( IOM, 2000a ).
It is important for IRBs and Privacy Boards to correctly distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule . Only research requires formal IRB or Privacy Board review and informed consent. 33 Inappropriate classification of an activity as research can make it difficult or impossible for important health care activities, such as public health practice and quality improvement, to be undertaken. On the other hand, failure to correctly identify an activity as research could potentially allow improper disclosure of personally identifiable health information without sufficient oversight.
Thus, standard criteria are urgently needed for IRBs and Privacy Boards to use when making distinctions between health research and related activities, and the committee recommends that HHS consult with relevant stake holders to develop such standard criteria. HHS is aware of this need, and created a working document titled “What Is Research ?” However, the work on this project apparently has been delayed for unknown reasons ( NCURA, 2007 ). 34 As described below, a number of other models have already been proposed to help determine whether activities should be classified as research in the fields of public health and quality improvement, and these could be instructive for developing HHS guidance. Any criteria adopted by HHS should be regularly evaluated to ensure that they are helpful and producing the desired outcomes.
The following sections describe some ongoing efforts to develop such criteria in the fields of public health and quality improvement. The intent of the committee is not to endorse these particular models, but rather to illustrate the challenges associated with making these distinctions and establishing standard criteria.
Public Health Practice Versus Public Health Research
The Belmont Report defined health practice as “interventions designed solely to enhance the well-being of the person, patient or client, and which have reasonable expectation of success” ( CDC, 1999 ). To apply this definition to “public” health practice, the targeted beneficiary of the intervention must be expanded to include benefit to the community, rather than just a particular person. Neither the Common Rule nor the Privacy Rule provides a specific definition for public health research; rather public health research is included in the general definition of research. However, the Privacy Rule regulates public health practice differently from public health research (see Chapter 4 ).
An early model for distinguishing public health research from public health practice focused on the intent for which the activity was designed, noting that the intent of public health research is to “contribute to or generate generalizable knowledge,” while the intent of public health practice is to “conduct programs to prevent disease and injury and improve the health of communities” ( Snider and Stroup, 1997 ). The Centers for Disease Control and Prevention developed a similar method with an expanded assessment of intent. For example, the model posits that in public health research, the intended benefits of the project extend beyond the study participants, and the data collected exceed the requirements for the care of the study participants. But for public health practice, the intended benefits of the project are primarily for the participants in the activity, or for the participants’ community, and the only data collected are those needed to assess or improve a public health program or service, or the health of the participants and their community. The model also assumes that public health practice is based on well-established medical interventions and is nonexperimental ( CDC, 1999 ). However, these models both have been criticized as too subjective and too dependent on the opinion of the person conducting the activity ( Gostin, 2008 ; Hodge, 2005 ).
A new, more comprehensive model incorporating much of the previous two was recently proposed as a more objective checklist to be used by IRBs, Privacy Boards , and interested parties ( Hodge, 2005 ; Hodge and Gostin, 2004 ). The foundations for this model are specific definitions of public health research: “the collection and analysis of identifiable health data by a public health authority for the purpose of generating knowledge that will benefit those beyond the participating community who bear the risks of participation,” and public health practice: “the collection and analysis of identifiable health data by a public health authority for the purpose of protecting the health of a particular community, where the benefits and risks are primarily designed to accrue to the participating community.”
The model is based on two primary assumptions. First, the actor performing the activity in question is a governmental public health official, agent, agency, or entity at the federal, tribal, state, or local level. Second, the activity in question involves the acquisition, use, or disclosure of personally identifiable health data. The model is then divided into two stages. Stage 1 is applied to all activities, and can be used to distinguish practice from research in the easiest cases. Stage 2 is only applied to those cases that are hard to distinguish, and where Stage 1 failed to lead to a definitive IRB/ Privacy Board decision ( Box 3-6 ).
A Model for Distinguishing Public Health Practice from Research. Stage 1 Public health practice:
Quality Improvement Versus Health Research
Quality improvement has been defined as “systematic, data-guided activities designed to bring about immediate, positive change in the delivery of health care in a particular setting” ( Baily, 2008 ). Quality improvement activities do not require IRB or Privacy Board approval under the Common Rule or the Privacy Rule, which classify quality improvement as a component of health care operations. 35
However, in many cases, it is difficult for health care providers, IRBs, and Privacy Boards to determine whether a particular activity is purely for quality improvement, or whether it also entails research. One survey 36 exploring opinions in the health care community about the need for IRBs to review various quality-related activities found that physicians conducting quality improvement were less likely than IRB chairs to believe that IRB review was required for a given hypothetical activity, or that informed consent was necessary ( Lindenauer et al., 2002 ). Recently, a highly publicized case has again brought the issue to the forefront for all the stakeholders ( Box 3-7 ).
A Case Study of Quality Improvement and Research. Peter Pronovost of Johns Hopkins University (JHU) led a quality improvement effort at 103 intensive care units (ICUs) in Michigan hospitals to reduce the number of catheter-related bloodstream infections. (more...)
Some members of the health care community have proposed requiring that all prospective quality improvement activities go through external review ( Bellin and Dubler, 2001 ), while others have outlined specific criteria to differentiate quality improvement activities from research.
For example, Casarett and colleagues developed a two-part test to identify quality improvement activities. The first test is whether the majority of patients are expected to benefit directly from “the knowledge to be gained” from the initiative. This means that the patients must actually benefit from the knowledge learned during the evaluation, not just from being a recipient of the protocol itself. If the patients are generally expected to directly benefit from the knowledge gained during the activity, then the activity is quality improvement. If not, the activity is research. The second test is whether the participants would be subjected to additional risks or burdens, including the risk of privacy breach, beyond the usual clinical practice in order to make the results of the initiative generalizable. If yes, then the initiative should be reviewed as research ( Casarett et al., 2000 ).
More recently, the Hastings Center published a report exploring the similarities and differences between research and quality improvement. The report emphasized three fundamental characteristics of quality improvement and three fundamental characteristics of research. The authors argue that individuals have a responsibility to participate in the quality improvement activities because all patients have an interest in receiving high-quality medical care, and the success of a quality improvement activity depends on the cooperation of all patients. In addition, the report notes that quality improvement activities are a low risk to the patient, so there is little justification for not participating. The report also assumes that quality improvement activities are based on existing knowledge about human health and should lead to immediate local improvements in the provision of medical care.
In contrast, the report notes that participation in research should be voluntary, and decisions to participate should be based on researchers’ full disclosure of all the potential risks and benefits. In addition, the authors assert that research is designed to create new knowledge about human health, rather than relying solely on existing knowledge, and that most research does not result in any direct benefit to the institution where the research is being conducted.
The authors concluded that IRBs are not the appropriate body for the ethical oversight of quality improvement activities. They argue that IRBs unnecessarily impose high transaction costs on these activities because of the difference in the way they are conducted compared to research. For example, in research, any changes in methodology require further IRB approval. In contrast, quality improvement activities involve frequent adjustments in the intervention, measurement, and goals of the activity based on the experience of the investigators. Requiring the investigator to revisit an IRB every time a small adjustment is needed in such an activity significantly increases the amount of time and effort required to conduct the initiative and to produce meaningful data. Also, the investigators involved in quality improvement activities ordinarily are already involved in the clinical care of participants and bear responsibility for the quality and safety of an intervention. Thus, the authors argue that there is no need for the additional oversight by an IRB to protect participant safety.
Rather, the report recommended integrating the ethical oversight of quality improvement activities into the ongoing management of an institution’s health care delivery system, suggesting that oversight of quality improvement could be left with the managers of clinical care organizations, and that consent to receive treatment should include consent to participate in any quality improvement project that is minimal risk. However, the report stated that if a project has the characteristics of both quality improvement and research, the project should be reviewed as both human subjects research and quality improvement ( Baily et al., 2006 ; Lynn et al., 2007 ).
In response to the ongoing confusion over when quality improvement rises to the level of research and requires IRB review, the IOM jointly hosted a meeting with the American Board of Internal Medicine in May 2008 to discuss this issue. Key members of the quality improvement community attended, and short- and long-term solutions to this problem were proposed. However, no written report from this meeting was produced and no general consensus was reached.
- THE IMPORTANCE OF EFFECTIVE COMMUNICATION WITH THE PUBLIC
As noted previously in this chapter, surveys indicate that the vast majority of Americans believe that health research is important and are interested in the findings of research studies. The majority of patients also appear to be willing to participate in health research, either by volunteering for a study to test a medical intervention or by allowing access to their medical records or stored biospecimens, under certain conditions. Their willingness to participate depends on trust in researchers to safeguard the rights and well-being of patients, including assurance of privacy and confidentiality, and the belief that it is a worthwhile endeavor that warrants their involvement. Yet patients often lack information about how research is conducted, and are rarely informed about research results that may have a direct impact on their health. The committee’s recommendations in this section are intended to address both the public’s desire for more information about health research and to help fulfill two of the committees overarching goals of the report: (1) improving the privacy and security of health information, and (2) improving the effectiveness of health research.
Disseminating Health Research Results
Ethicists have long suggested greater community involvement in health research studies, including more communication about research results (reviewed by Shalowitz and Miller, 2008a , b ). In addition, the IOM committee identified transparency—the responsibility to disclose clearly how and why personally identifiable information is being collected—as an important component of comprehensive privacy protections. A previous IOM report also recommended improved communication with the public and research participants to ensure that the protection process is open and accessible to all interested parties ( IOM, 2002 ). Effective communication would build the public’s trust of the research community and is consistent with the principles of fair information practices.
When patients consent to the use of their medical records in a particular study, health researchers should make greater efforts at the conclusion of the study to inform study participants about the results, and the relevance and importance of those results. Learning about clinically relevant findings from a study in which a patient has participated could make patients feel more integrated into the process and could encourage more to participate in future studies. A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community ( AMS, 2006 ). Moreover, direct feedback with study participants could lead to improved health care for the individuals if the results indicate that an altered course of care is warranted.
Nonetheless, there are multiple impediments, beyond cost, to providing meaningful feedback to participants. A summary of the results alone, while necessary and reasonable, can be seen as a token, and also raises questions about issues such as how best to write summaries, the stage at which results should be disseminated, and how to present research with uninformative outcomes. For example, one recent study found that sharing results directly with study participants was met with overwhelmingly favorable reactions from patients, but the study also revealed some obstacles ( Partridge et al., 2008 ). In a survey of women who had participated in a randomized trial of breast cancer therapy and had received a summary of the study results by mail, 95 percent reported that they were glad they received the results. Most respondents interpreted the results correctly, although incorrect interpretation of the results was associated with increased anxiety, as was dissatisfaction with treatment.
Although some guidelines for providing and explaining study results to research participants have been proposed, they differ in details because limited data are available on this subject, and thus standards are lacking ( Partridge and Winer, 2002 ; Partridge et al., 2008 ; Shalowitz and Miller, 2008b ; Zarin and Tse, 2008 ). Because transparency is best achieved by providing graded levels of information and guidance to interested parties ( IOM, 2002 ), it will be important to develop effective and efficient ways to communicate with various sectors of the population. A commitment to the principles of “plain language” 37 will be important. Broader adoption of electronic medical records may also be helpful in accomplishing this goal.
Research Registries
One way to make information about research studies more broadly available to the public is through registration of trials and other studies in public databases. HHS should encourage such registration of trials and other studies, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization (see Chapter 4 ). Numerous clinical trial registries already exist, and registration has increased in recent years (reviewed by Zarin and Tse, 2008 ). In 2000, the National Library of Medicine established a clinical trials registry ( ClinicalTrials.gov ), which has expanded to include information from several other trial registries and to serve as the FDA-required site for submissions about clinical trials subject to the FDA databank requirement. The FDA Amendments Act of 2007 38 expanded the scope of required registrations at ClinicalTrials.gov and provided the first federally funded trials results database. It mandates registrations of controlled clinical investigations, except for Phase I trials, of drugs, biologics, and devices subject to FDA regulation.
A policy of the International Committee of Medical Journal Editors (ICMJE), adopted in fall 2005, also requires prospective trial registration as a precondition for publication ( DeAngelis et al., 2004 ). This policy led to a 73 percent increase in trial registrations of all intervention types from around the world ( Zarin et al., 2005 ). Nearly 45,000 trials had been registered by fall 2007.
However, although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, no centralized system currently exists to disseminate information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. The current statutory requirements for registration and data reporting in the United States are not as broad as the transnational policies of the ICMJE or the World Health Organization, which call for the registration of all interventional studies in human beings regardless of intervention type ( Laine et al., 2007 ; Sim et al., 2006 ). Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/ Privacy Board approved waiver of consent or authorization, including those studies in a registry could be an important method for increasing public knowledge of such studies.
Informing the Public About the Methods and Value of Research
As noted previously, clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study subjects will be necessary to derive meaningful results.
However, many patients probably are not aware that their medical records are being used in information-based research. For example, the recent study that used focus groups to examine the views of veterans toward the use of medical records in research found that the majority of participants (75 percent) were not aware that “under some circumstances, [their] medical records could be used in some research studies without [their] permission,” despite the fact that a notice of privacy practices, which included a statement that such research could occur, had been mailed to all participants less than a year prior to the study ( Damschroder et al., 2007 ).
Moreover, surveys show that many patients desire not only notice, but also the opportunity to decide whether to consent to such research with medical records. Those surveys further indicate that patients who wish to be asked for consent for each study are most concerned about the potentially detrimental affects of inappropriate disclosure of their personally identifiable health information, including discrimination in obtaining health or life insurance or employment.
As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported on could also help to ease patient concerns about privacy and increase patients’ trust in the research community, which as noted above is important for the public’s continued participation in health research. For example, datasets are most often provided to researchers without direct identifiers such as name and Social Security number. Furthermore, identifiers are not included in publications about research results. Also, under both the Privacy Rule and the Common Rule , a waiver of consent and authorization is possible only under the supervision of an IRB or Privacy Board , and a waiver is granted only when the research entails minimal risk and when obtaining individual consent and authorization is impracticable (see the previous section and also Chapter 4 ). Finally, professional ethics dictate that researchers safeguard data and respect privacy.
Conveying the value of medical records research to patients will be important. Surveys show that people are more supportive of research that is relevant to them and their loved ones. At the same time, educational efforts should stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research ( Box 3-8 ), but datasets will not represent the entire population if some people withhold access to their health information.
Selection Bias in Health Research. When researchers are required to obtain consent or authorization to access each individual’s medical record for a research study, it is likely that individuals’ willingness to grant access will not be (more...)
In addition, an educated public could also decrease the potential for biased research samples. A universal requirement for consent or authorization in medical records research leads to incomplete datasets, and thus to biased results and inaccurate conclusions. Some large medical institutions with a strong research history and reputation (e.g., Mayo Clinic) can obtain authorization and consent rates as high as 80 percent, but the 20 percent who refuse have distinct demographic and health characteristics. In fact, even a refusal rate of less than 5 percent can create selection bias in the data ( Jacobsen et al., 1999 ; see Chapter 5 for more detail). Conveying to the public the importance of health care improvements derived from medical records research and stressing the negative impact of incomplete datasets on research findings may increase the public’s participation in research and their willingness to support information-based research that is conducted with IRB or Privacy Board oversight, under a waiver of patient consent or authorization.
Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required ( Box 3-1 ). For example, analysis of medical records showed that infants exposed to diethylstilbesterol (DES) during the first trimester of pregnancy had an increased risk of breast, vaginal, and cervical cancer as well as reproductive anomalies as adults. Similarly, studies of medical records led to the discovery that folic acid supplementation during pregnancy can prevent neural tube defects.
Thus, HHS and the health research community should work to edu cate the public about how research is done and the value it provides. All stakeholders, including professional organizations, nonprofit funders, and patient organizations, have different interests and responsibilities to make sure that their constituencies are well informed. For example, the American Society of Clinical Oncology and the American Heart Association already have some online resources to help patients gather information about research that may be relevant to their conditions. But coordination and identification of best practices by HHS would be helpful, and research is needed to identify which segments of the population would be receptive to and benefit from various types of information about how research is done and its value in order to create and implement an effective plan.
Greater use of community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained, 39 could help achieve this goal. These groups help researchers to recruit research participants by using the knowledge of the community to understand health problems and to design activities that the community is likely to value. They also inform community members about how the research is done and what comes out of it, with the goal of providing immediate community benefits from the results when possible.
- CONCLUSIONS AND RECOMMENDATIONS
Based on its review of the information described in this chapter, the committee agreed on a second overarching principle to guide the formation of recommendations. The committee affirms the importance of maintaining and improving health research effectiveness. Research discoveries are central to achieving the goal of extending the quality of healthy lives. Research into causes of disease, methods for prevention, techniques for diagnosis, and new approaches to treatment has increased life expectancy, reduced infant mortality, limited the toll of infectious diseases, and improved outcomes for patients with heart disease, cancer, diabetes, and other chronic diseases. Patient-oriented clinical research that tests new ideas makes rapid medical progress possible. Today, the rate of discovery is accelerating, and we are at the precipice of a remarkable period of investigative promise made possible by new knowledge about the genetic underpinnings of disease. Genomic research is opening new possibilities for preventing illness and for developing safer, more effective medical care that may eventually be tailored for specific individuals. Further advances in relating genetic information to predispositions to disease and responses to treatments will require the use of large amounts of existing health-related information and stored tissue specimens. The increasing use of electronic medical records will further facilitate the generation of new knowledge through research and accelerate the pace of discovery. These efforts will require broad participation of patients in research and broad data sharing to ensure that the results are valid and applicable to different segments of the population. Collaborative partnerships among communities of patients, their physicians, and teams of researchers to gain new scientific knowledge will bring tangible benefits for people in this country and around the world.
Surveys indicate that the majority of Americans believe that health research is important, are interested in the findings of research studies, and are willing to participate in health research. But patients often lack information about how research is conducted and are rarely informed about research results that may have a direct impact on their health. Effective communication could build the public’s trust of the research community, which is important because trust is necessary for the public’s continued participation in research. Moreover, direct feedback could lead to improved health care for study participants if the results indicate that an altered course of care is warranted.
Thus, the committee recommends that when patients consent to the use of their medical records in a particular study, health researchers should make greater efforts when the study ends to inform study participants about the results, and the relevance and importance of those results. Broader adoption of electronic health records may be helpful in accomplishing this goal, but standards and guidelines for providing and explaining study results to research participants or various sectors of the public are needed.
HHS should also encourage registration of trials and other studies in public databases, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization, as a way to make information about research studies more broadly available to the public. Numerous clinical trial registries already exist, and registration has increased in recent years, but no centralized system currently exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including such studies in a registry could be an important method for increasing public knowledge of those studies.
Interventional clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic health records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study participants will be necessary to derive meaningful results.
However, many patients are likely not aware that their medical records are being used in information-based research, and surveys show that many patients desire not only notice, but also the opportunity to decide about whether to consent to such research with medical records. As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported could also increase patients’ trust in the research community. Thus, HHS and the health research community should work to educate the public about how research is done.
It will also be important for HHS and researchers to convey the value of health care improvements derived from medical records research, and to stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research, but datasets will not be representative of the entire population if some people withhold access to their health information. A universal requirement for consent or authorization in information-based research may lead to incomplete datasets, and thus to biased results and inaccurate conclusions. Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required.
To ensure that beneficial health research and related activities continue to be undertaken with appropriate oversight under federal regulations, it will be important for HHS to also provide more guidance on how to distinguish the various activities. The Privacy Rule makes a distinction between health research and some closely related endeavors, such as public health and quality improvement activities, which also may involve collection and analysis of personally identifiable health information. Under the Privacy Rule (as well as the Common Rule ), these activities, which aim to protect the public’s health and improve the quality of patient care, are considered health care “practice” rather than health research. Therefore, they can be undertaken without consent or authorization, or an IRB/ Privacy Board waiver of consent or authorization. However, it can be a challenge for IRBs and Privacy Boards to distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule, and inappropriate decisions may prevent important activities from being undertaken or could potentially allow improper disclosure of personally identifiable health information.
To address these difficulties, a number of models have been proposed that outline the criteria IRBs and Privacy Boards should use to distinguish practice and research. For example, one recent model provides a detailed checklist for IRBs and Privacy Boards to use in determining whether an activity is public health research and required to comply with the research provisions of the Privacy Rule, or public health practice that does not need IRB/Privacy Board review. The committee believes that standardizing the criteria is essential to support the conduct of these important health care activities.
Thus, HHS should convene the relevant stakeholders to develop standard criteria for IRBs and Privacy Boards to use when making decisions about whether protocols entail research or practice. There should be flexibility in the regulation to allow important activities to go forward with appropriate levels of oversight. Also, it will be important to evaluate whether these criteria are effective in aiding IRB/Privacy Board reviews of proposed protocols, and whether they lead to appropriate IRB/Privacy Board decisions.
These changes suggested above could be accomplished without any changes to HIPAA by making them a condition of funding from HHS and other research sponsors and by providing some additional funds to cover the cost.
- AMS (Academy of Medical Sciences). Personal data for public good: Using health information in medical research. 2006. [accessed August 28, 2008]. http://www .acmedsci.ac .uk/images/project/Personal.pdf .
- Baily MA. Harming through protection? New England Journal of Medicine. 2008; 258 (8):768–769. [ PubMed : 18287599 ]
- Baily MA, Bottrell M, Lynn J, Jennings B. The ethics of using QI methods to improve health care quality and safety. A Hastings Center Special Report. 2006; 36 (4):S1–S40. [ PubMed : 16898359 ]
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander VM, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998; 280 (15):1311–1316. [ PubMed : 9794308 ]
- Bellin E, Dubler NN. The quality improvement-research divide and the need for external oversight. American Journal of Public Health. 2001; 91 (9):1512–1517. [ PMC free article : PMC1446813 ] [ PubMed : 11527790 ]
- Big Health. Big health consortium. 2008. [accessed October 29, 2008]. http://www .bighealthconsortium .org/about/
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87 (1):1–9. [ PubMed : 18204365 ]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention and treatment trials. Annals of Epidemiology. 2000; 10 :S13–S21. [ PubMed : 11189088 ]
- Burris S, Gable L, Stone L, Lazzarini Z. The role of state law in protecting human subjects of public health research and practice. Journal of Law, Medicine & Ethics. 2003; 31 :654. [ PubMed : 14968667 ]
- Casarett D, Karlawish J, Sugarman J. Determining when quality improvement initiatives should be considered research: Proposed criteria and potential implications. JAMA. 2000; 284 (7):2275–2280. [ PubMed : 10807388 ]
- Casarett D, Karlawish J, Andrews E, Caplan A. Bioethical issues in pharmacoepidemiological research. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 417–432.
- CDC (Centers for Disease Control and Prevention). Guidelines for defining public health research and public health non-research. 1999. [accessed March 4, 2008]. http://www .cdc.gov/od /science/regs/hrpp/researchdefinition .htm .
- CHSR (Coalition for Health Services Research). Framework for health services research policy for 2008. 2008. [accessed August 21, 2008]. http://www .chsr.org/Policy_Priorities .pdf .
- Comis RL, Aldige CR, Stovall EL, Krebs LU, Risher PJ, Taylor HJ. A quantitative survey of public attitudes towards cancer clinical trials. Philadelphia, PA: Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council, and Oncology Nursing Society; 2000.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans towards participation in medical research. Journal of General Internal Medicine. 1999; 14 :537–546. [ PMC free article : PMC1496744 ] [ PubMed : 10491242 ]
- Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Social Science & Medicine. 2007; 64 (1):223–235. [ PubMed : 17045717 ]
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA. 2004; 292 (11):1363–1364. [ PubMed : 15355936 ]
- Farmer D, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. Journal of Health Care for the Poor and Underserved. 2007; 18 :85–99. [ PubMed : 17337800 ]
- FDA (Food and Drug Administration). Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation. Federal Register. 1971; 36 (217):21537–21538.
- FDA. FDA public health advisory, deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/advisory/antipsychotics.htm .
- FDA. FDA alert [6/16/2008]: Information for healthcare professionals. Antipsychotics. 2008. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/infosheets/hcp /antipsychotics_conventional.htm .
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who’s paying? Health Affairs Web Exclusive. 2003. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w3.219v1 . [ PubMed : 14527256 ]
- Furrow BR, Greaney TL, Johnson SH, Jost TS, Schwartz RL. Bioethics: Health care law and ethics. St. Paul, MN: Thomson/West; 2004.
- GAO (Government Accountability Office). Scientific research: Continued vigilance critical to protecting human subjects. Washington, DC: GAO; 1996.
- Genetics & Public Policy Center. U.S. public opinion on uses of genetic information and genetic discrimination. 2007. [accessed August 21, 2008]. http://www .dnapolicy .org/resources/GINAPublic _Opinion_Genetic _Information_Discrimination.pdf .
- Gill S, Bronskill S, Normand S, Anderson G, Sykora K, Lam K, Bell C, Lee P, Fischer H, Herrmann N, Gurwitz J, Rochon P. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine. 2007; 146 :775–786. [ PubMed : 17548409 ]
- Giuliano AR, Mokuau N, Hughes C, Tortelero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000; 10 :S22–S34. [ PubMed : 11189089 ]
- Goldman J, Choy A. Ethical and policy issues in research involving human participants. Bethesda, MD: National Bioethics Advisory Commission; 2001. Privacy and confidentiality in health research; pp. C1–C34.
- Gostin LO. Public health law: Power, duty, restraint. Berkeley, CA: University of California Press; 2008. Surveillance and public health research: Personal privacy and the “right to know.”
- Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American Journal of Public Health. 2006; 96 (11):1996–2001. [ PMC free article : PMC1751807 ] [ PubMed : 17018826 ]
- Hatfield M, Sonnenschein HF, Rosenberg LE. Exceptional returns: The economic value of America’s investment in medical research. 2001. [accessed August 21, 2008]. http://www .laskerfoundation .org/advocacy/pdf/exceptional.pdf .
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971; 284 (15):878–881. [ PubMed : 5549830 ]
- HEW (Department of Health, Education and Welfare). The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. 1979. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/belmont.html . [ PubMed : 25951677 ]
- HHS (Department of Health and Human Services). Institutional review boards and the HIPAA Privacy Rule. 2003. [accessed August 21, 2008]. http: //privacyruleandresearch .nih.gov/pdf/IRB_Factsheet.pdf .
- HHS. Guidance on research involving coded private information or biological specimens. 2004. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /humansubjects/guidance/cdebiol.pdf .
- Hodge JG Jr. An enhanced approach to distinguishing public health practice and human subjects research. Journal of Law, Medicine & Ethics. 2005; 33 (1):125–141. [ PubMed : 15934670 ]
- Hodge JG, Gostin LO. Public health practice vs. research: A report for public health practitioners including cases and guidance for making distinctions. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004.
- IOM (Institute of Medicine). Health services research: Work force and educational issues. Washington, DC: National Academy Press; 1995.
- IOM. Protecting data privacy in health services research. Washington, DC: National Academy Press; 2000a. [ PubMed : 25057723 ]
- IOM. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000b. [ PubMed : 25077248 ]
- IOM. Responsible research: A systems approach to protecting research participants. Washington, DC: The National Academies Press; 2002. [ PubMed : 20669487 ]
- Jacobsen S, Xia Z, Campion M, Darby C, Plevak M, Seltman K, Melton L. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999; 74 :330–338. [ PubMed : 10221460 ]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van Der Weyden MB, Verheugt FWA. Clinical trial registration—looking back and moving ahead. New England Journal of Medicine. 2007; 356 (26):2734–2736. [ PubMed : 17548427 ]
- Lee B. Comparison of FDA and HHS human subject protection regulations. 2000. [accessed August 21, 2008]. http://www .fda.gov/oc/gcp/comparison .html .
- Lindenauer PK, Benjamin EM, Naglieri-Prescod D, Fitzgerald J, Pekow P. The role of the institutional review board in quality improvement: A survey of quality officers, institutional review board chairs, and journal editors. The American Journal of Medicine. 2002; 113 :575–579. [ PubMed : 12459404 ]
- Lowrance WW. Learning from experience, privacy and the secondary use of data in health research. London: The Nuffield Trust; 2002.
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007; 317 :600–602. [ PubMed : 17673640 ]
- Lynn J, Baily MA, Bottrell M, Jennings B, Levine RJ, Davidoff F, Casarett D, Corrigan J, Fox E, Wynia MK, Agich GJ, Speroff T, Schyve P, Batalden P, Tunis S, Berlinger N, Cronenwett L, Fitzmaurice M, Dubler NN, James B. The ethics of using quality improvement methods in health care. Annals of Internal Medicine. 2007; 146 (6):666–674. [ PubMed : 17438310 ]
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, Klein J, Helfand M. The cost-effectiveness of screening mammography beyond age 65: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003; 139 :835–842. [ PubMed : 14623621 ]
- Mick S, Morlock LL, Salkever D, de Lissovoy G, Malitz F, Wise CG, Jones A. Strategic activity and financial performance of U.S. rural hospitals: A national study, 1983 to 1988. Journal of Rural Health. 1994; 10 (3):150–167. [ PubMed : 10138031 ]
- Mosconi P, Poli P, Giolo A, Apolone G. How Italian health consumers feel about clinical research: A questionnaire survey. European Journal of Public Health. 2005; 15 (4):372–379. [ PubMed : 16014662 ]
- Murphy K, Topel R. The economic value of medical research. Chicago, IL: University of Chicago Press; 1999.
- NCI (National Cancer Institute). Getting connected with caBIG: Data sharing and security framework. 2008. [accessed October 27, 2008]. https://cabig .nci.nih .gov/working_groups /DSIC_SLWG/data_sharing_policy/
- NCURA (National Council of University Research Administrators). Report on research compliance. 2007. [accessed March 4, 2008]. http://www .reportonresearchcompliance .com .
- NCVHS (National Committee on Vital and Health Statistics). Enhanced protections for uses of health data: A stewardship framework for “secondary uses” of electronically collected and transmitted health data. 2007a. [accessed December 19, 2007]. http://ncvhs .hhs.gov/071221lt.pdf .
- NCVHS, Ad Hoc Work Group on Secondary Uses of Health Data. Testimony of the cancer Biomedical Informatics Grid (caBIG) Data Sharing and Intellectual Capital (DSIC) workspace. 2007b August 1, 2007
- Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002; 346 (22):1715–1722. [ PubMed : 12037152 ]
- NSF (National Science Foundation). Science and Engineering Indicators 2006. Arlington, VA: National Science Foundation; 2006. Science and technology: Public attitudes and understanding. Chapter 7.
- OHRP (Office for Human Research Protections). IRB guidebook, part I.A. 2008a. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /irb/irb_guidebook.htm .
- OHRP. OHRP concludes case regarding Johns Hopkins University research on hospital infections. 2008b. [accessed August 21, 2008]. http://www .hhs.gov/ohrp/news/recentnews .html#20080215 .
- Partridge A, Winer E. Informing clinical trial participants about study results. JAMA. 2002; 288 :363–365. [ PubMed : 12117402 ]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Research and Treatment. 2008 June 10 [ PubMed : 18543100 ]
- Pitkin R. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007; 85 (1):285S–288S. [ PubMed : 17209211 ]
- Pritts J. The importance and value of protecting the privacy of health information: Roles of HIPAA Privacy Rule and the Common Rule in health research. 2008. [accessed March 15, 2008]. http://www .iom.edu/CMS/3740/43729/53160 .aspx .
- Research!America. America speaks: Poll summary. Vol. 7. Alexandria, VA: United Health Foundation; 2007.
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang P. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal. 2007; 176 :672–632. [ PMC free article : PMC1800321 ] [ PubMed : 17325327 ]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. PLoS Medicine. 2008a May 13; 5 (5):e91. [ PMC free article : PMC2375946 ] [ PubMed : 18479180 ]
- Shalowitz DI, Miller FG. The search for clarity in communicating research results to study participants. Journal of Medical Ethics. 2008b September; 34 (9):e17. [ PubMed : 18757617 ]
- Shaul RZ, Birenbaum S, Evans M. Legal liability in research: Early lessons from North America. BMC Medical Ethics. 2005; 6 (4):1–4. [ PMC free article : PMC1182131 ] [ PubMed : 15953387 ]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002; 12 :248–256. [ PubMed : 11988413 ]
- Sim I, Chan A-W, Gülmezoglu AM, Evans T, Pang T. Clinical trial registration: Transparency is the watchword. The Lancet. 2006; 367 (9523):1631–1633. [ PubMed : 16714166 ]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987; 235 (4785):177–182. [ PubMed : 3798106 ]
- Snider DE, Stroup DF. Defining research when it comes to public health. Public Health Reports. 1997; 112 :29–32. [ PMC free article : PMC1381834 ] [ PubMed : 9018284 ]
- Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity in rising medical spending. Health Affairs Web Exclusive. 2004. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w4.480v1 . [ PubMed : 15496437 ]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health & Social Policy. 2000; 12 (2):23–43. [ PubMed : 11184441 ]
- Veurink M, Koster M, Berg L. The history of DES, lessons to be learned. Pharmacy World & Science. 2005; 27 (3):139–143. [ PubMed : 16096877 ]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3 (2):201–210. [ PMC free article : PMC1298944 ] [ PubMed : 16318411 ]
- Westin A. How the public views privacy and health research. 2007. [accessed November 11, 2008]. http://www .iom.edu/Object .File/Master/48 /528/%20Westin%20IOM %20Srvy%20Rept%2011-1107.pdf .
- Williams ED. Federal protection for human research subjects: An analysis of the Common Rule and its interactions with FDA regulations and the HIPAA Privacy Rule, CRS report for Congress. Washington, DC: Congressional Research Service; 2005.
- Williams IC, Corbie-Smith G. Investigator beliefs and reported success in recruiting minority participants. Contemporary Clinical Trials. 2006; 27 :580–586. [ PubMed : 16839822 ]
- Winston FK, Durbin DR, Kallan MJ, Moll EK. The danger of premature graduation to seat belts for young children. Pediatrics. 2000; 105 (6):1179–1183. [ PubMed : 10835054 ]
- WMA (World Medical Association). Declaration of Helsinki: Ethical principles for medical research involving human subjects. 1964. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/helsinki.html . [ PubMed : 16010903 ]
- Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294 (11):1380–1384. [ PubMed : 16174697 ]
- Zarin DA, Tse T. Moving toward transparency of clinical trials. Science. 2008; 319 :1340–1342. [ PMC free article : PMC2396952 ] [ PubMed : 18323436 ]
- Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine. 2005; 353 (26):2779–2787. [ PMC free article : PMC1568386 ] [ PubMed : 16382064 ]
Epidemiology is the study of the occurrence, distribution, and control of diseases in populations.
Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understanding of the structure, processes, and effects of health services for individuals and populations ( IOM, 1995 ).
The National Committee on Vital and Health Statistics has noted that “secondary uses” of health data is an ill-defined term, and urges abandoning it in favor of precise description of each use ( NCVHS, 2007a ). Thus, the committee chose to minimize use of the term in this report.
See Standards for Privacy of Individually Identifiable Health Information , 64 Fed. Reg. 59918, 59967 (preamble to rule proposed November 3, 1999) for a discussion on the benefits of health records research.
Effectiveness can be defined as the extent to which a specific test or intervention, when used under ordinary circumstances, does what it is intended to do. Efficacy refers to the extent to which a specific test or intervention produces a beneficial result under ideal conditions (e.g., in a clinical trial).
See http://www .intermacs.org .
See http://www .elso.med.umich.edu .
See http://www .unos.org/Data .
The Department of Health, Education and Welfare (now HHS) had previously issued policy and guidance on the protection of human subjects. See Williams (2005) .
In its report “First Biennial Report on the Adequacy and Uniformity of Federal Rules and Policies, and their Implementation, for the Protection of Human Subjects in Biomedical and Behavioral Research , Protecting Human Subjects.”
45 C.F.R. part 46 (2005).
See 45 C.F.R. § 46.101 (2005).
See 45 C.F.R. § 46.102(d) (2005).
This section on informed consent is based largely on a Congressional Research Service report ( Williams, 2005 ), as adapted by Pritts (2008) .
See 45 C.F.R. § 46.116 (2005).
See 45 C.F.R. § 46.116(b) (2005).
See 45 C.F.R. § 46.103 (2005).
See 45 C.F.R. § 46.111 (2005). There are additional factors if the study includes subjects who are likely to be vulnerable to coercion or undue influence.
See 45 C.F.R. § 46.116(d); 46.117(c) (2005).
See 45 C.F.R. § 46.102(f) (2005).
See 45 C.F.R. § 46.101(b)(4) (2005).
See 45 C.F.R. § 46.116(e) (2005).
See 45 C.F.R. § 46.123 (2005).
See also Grimes v. Kennedy Krieger Institute , 782 A. 2d 807 (Md. Ct. App. 2001); Gelsinger v. University of Pennsylvania (Philadelphia County Court of Common Pleas filed September 18, 2000), available at http://www .sskrplaw.com /links/healthcare2.html .
The FDA has defined “clinical investigation” to be synonymous with “research.”
The Food, Drug, and Cosmetic Act Section 505(i), 507(d), or 520(g) of 21 U.S.C. 355(i), 357(d), or 360j(g) (1972).
See 21 C.F.R. part 50 (2008); 46 Fed. Reg. 8942 (1981).
See 21 C.F.R. part 56 (2008); 46 Fed. Reg. 8958 (1981).
See 45 C.F.R. part 46 (2005); 46 Fed. Reg. 8366 (1981).
The Centers for Disease Control and Prevention defines program evaluation as the “systematic investigation of the merit, worth, or significance of organized public health action,” noting that such evaluations are “systematic ways to improve and account for public health actions by involving procedures that are useful, feasible, ethical, and accurate.” They can be based on goals, processes, outcomes, or value ( http://www .cdc.gov/mmwr /preview/mmwrhtml/rr4811a1.htm ).
The Utilization Review Accreditation Commission defines utilization review as “the evaluation of the medical necessity, appropriateness, and efficiency of the use of health care services, procedures, and facilities under the provisions of the applicable health benefits plans” ( http://www .urac.org/about/ ).
Another type of oversight board defined by the Privacy Rule. See Chapter 4 .
Under the Privacy Rule, consent is referred to as authorization. See Chapter 4 .
Personal communication, C. Heide, Office for Civil Rights, HHS, May 29, 2008.
The Privacy Rule defines the term “health care operations” by listing a number of specific activities that qualify as health care operations. These include “conducting quality assessment and improvement activities, population-based activities relating to improving or reducing health care costs, and case management and care coordination.” See 45 C.F.R. § 164.501 (2006).
A total of 444 surveys were mailed to the medical directors of quality improvement and IRB chairs at hospitals with 400 or more beds that belong to the Council of Teaching Hospitals of the Association of American Medical Colleges, and to the editors of all U.S.-based medical journals that publish original research and appear in the Abridged Index Medicus. 236 surveys were returned, for a 53 percent response rate. The survey consisted of six brief scenarios that asked respondents to determine whether the described project needed IRB review and informed consent.
See http: //plainlanguage.gov/index.cfm .
FDA, Public Law 110–85 § 801 (2007).
See http://www .ahrq.gov/research/cbprrole .htm .
- Cite this Page Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009. 3, The Value, Importance, and Oversight of Health Research.
- PDF version of this title (1.6M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Priva... The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Frontiers in Global Women's Health
- Maternal Health
- Research Topics
High-risk Pregnancy: Women's Experiences and New Approaches to Care
Total Downloads
Total Views and Downloads
About this Research Topic
Pregnancy involves physical, psychological, and social changes and when deemed high-risk, these experiences are amplified. High-risk pregnancy is characterized as a pregnancy in which the mother and/or fetus are at greater risk than normal of morbidity and mortality. Women and their families face significant physical, emotional, and psychological challenges due to uncertainty regarding the pregnancy outcome. The emotional and psychological experiences of women in high-risk pregnancies are profoundly influenced by the care they receive and their relationships with healthcare professionals are particularly important in this context. Person- and family-centered care models have the potential to significantly improve the quality of care and pregnancy outcomes, as they can address patient needs on an individual level. Understanding women’s experiences is essential to supporting them and their families throughout the course of the pregnancy and enabling the bonding process with their baby after the delivery. More than anything, the goal of individualized care should be to contribute to women’s well-being as well a positive pregnancy experience. This Research Topic welcomes papers concerning the experiences of women and their families with high-risk pregnancies and how their experience may be influenced by different models or approaches to care, acknowledging the complexity of factors involved. The goal is to highlight new interdisciplinary care models that are personalized, humanizing, and center women’s experience and ability to cope with the prolonged period of uncertainty that typically accompanies high-risk pregnancies. This collection will promote better-informed healthcare practices and policies that prioritize the well-being of women and their families facing high-risk pregnancies across different clinical care settings. This Research Topic, titled “High-risk Pregnancy: Women's Experiences and New Approaches to Care” welcomes papers from academics, clinicians, and other healthcare professionals in the field. Topics of interest include: - The psychosocial and emotional impact of a high-risk pregnancy diagnosis - Education needs arising from the diagnosis of a high-risk pregnancy - Aspects related to quality of care and how it influences outcomes such as access to personalized, culturally competent care and the importance of patients’ relationships with their healthcare providers - Factors related to women’s and their families’ ability to cope with prolonged uncertainty such as social support, the role of spirituality and religiousness and the perception of hope - Women’s autonomy and ethical considerations related to the decision-making during the care process - Opportunities presented by the implementation of innovative technologies in the care process Authors are welcome to submit quantitative and qualitative original research, review articles (systematic, integrative, scoping, mini, policy and practice), clinical trial, case report and brief research reports. Submissions should adhere to the guidelines and standards of Frontiers in Global Women’s Health.
Keywords : Pregnancy, High-Risk, Life Change Events, Pregnancy Complications, Prenatal Diagnosis, Mental Health, Psychological Well-Being, Hope, Spirituality, Family Relations, Life Course Perspective, Culture
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, submission deadlines, participating journals.
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
📕 Studying HQ
Exploring community health nursing research topics: a comprehensive guide for nursing students, carla johnson.
- August 25, 2023
- Essay Topics and Ideas
Community health nursing is a dynamic and vital field within the nursing profession, focused on providing holistic care to populations within a defined community. This specialized branch of nursing goes beyond individual patient care, extending its reach to families, groups, and entire communities. This article will delve into community health nursing, explore its significance, and provide valuable resources for nursing students to engage in research, evidence-based practice (EBP) projects, capstone projects, research paper topics, research questions, and essay ideas.
What You'll Learn
Understanding Community Health Nursing: A Holistic Approach
Community health nursing emphasizes preventive care , health promotion, and disease prevention within a specific community. This holistic approach involves understanding the community’s unique needs, cultures, and challenges to provide targeted interventions that improve health outcomes. As nursing students, you will find this field to be a gateway to understanding the broader healthcare landscape and the interconnectedness of various factors that influence health.

PICOT Questions on Community Health Nursing
- P: Adult population in psychiatric care ; I: Implementation of daily RS questionnaire; C: Units without the daily survey; O: Reduction in utilization of restraint and seclusion; T: 6 months. Can the implementation of a daily RS (Restraint and Seclusion) questionnaire for adults in psychiatric care lead to a significant decrease in the utilization of restraint and seclusion within a period of 6 months?
- P: Pediatric population in school settings; I: Introduction of daily exercise regimen; C: Schools without daily exercise; O: Improvement in BMI and overall fitness; T: 1 academic year. Does introducing a daily exercise regimen in school settings for pediatric populations result in a noticeable improvement in BMI and overall fitness over the course of 1 academic year?
- P: Elderly population in assisted living facilities; I: Implementation of fall prevention program; C: Facilities without fall prevention program; O: Reduction in fall-related injuries; T: 1 year. Is there a significant reduction in fall-related injuries among the elderly residing in assisted living facilities after the implementation of a comprehensive fall prevention program within 1 year?
- P: Low-income pregnant women; I: Provision of prenatal education classes; C: Those without access to prenatal education; O: Increase in prenatal knowledge and healthier pregnancy outcomes; T: Throughout gestation. Can providing prenatal education classes to low-income pregnant women lead to increased prenatal knowledge and improved pregnancy outcomes when compared to those without access to such education?
- P: Diabetic population within the community; I: Establishment of a mobile diabetic clinic; C: No mobile clinic available; O: Enhanced diabetic management and reduced hospitalizations; T: 2 years. Does the establishment of a mobile diabetic clinic within the community lead to better diabetic management and a decrease in hospitalizations over a span of 2 years?
- P: Adolescent population in schools; I: Implementation of comprehensive sexual education; C: Schools with standard sexual education; O: Reduction in teen pregnancies and sexually transmitted infections (STIs); T: 3 years. Over a period of 3 years, does the implementation of comprehensive sexual education in schools result in a significant decrease in the rates of teen pregnancies and STIs among adolescents compared to schools with standard sexual education?
- P: Homeless population; I: Launch of mobile healthcare unit; C: No access to regular healthcare; O: Improvement in overall health status and decrease in emergency room visits; T: 1 year. Can the introduction of a mobile healthcare unit for the homeless population improve their overall health status and a noticeable reduction in emergency room visits within a year?
- P: Rural elderly population; I: Initiation of telehealth services ; C: Lack of telehealth services; O: Enhanced access to healthcare and better management of chronic conditions; T: 18 months. Does the introduction of telehealth services for the rural elderly population lead to increased access to healthcare services and improved management of chronic conditions over the course of 18 months?
- P: New mothers; I: Implementation of postpartum support groups; C: No postpartum support groups available; O: Reduction in postpartum depression rates and improved maternal well-being; T: 1 year. Can the implementation of postpartum support groups for new mothers lead to a significant reduction in postpartum depression rates and an overall improvement in maternal well-being within a year?
- P: LGBTQ+ youth; I: Creating safe spaces in schools; C: Absence of designated safe spaces; O: Decreased mental health challenges and higher academic achievement; T: Ongoing. Does creating safe spaces within schools for LGBTQ+ youth lead to a noticeable decrease in mental health challenges and a rise in academic achievement over an ongoing period?
Evidence-Based Practice Projects Ideas
- Evaluating the effectiveness of community-wide vaccination drives in reducing vaccine-preventable diseases.
- Assessing the impact of a smoke-free policy in public spaces on community members’ respiratory health.
- Investigating the outcomes of a nutrition education program in improving dietary habits among low-income families.
- Analyzing the effectiveness of a community-based mental health awareness campaign in reducing stigma and increasing help-seeking behavior.
- Exploring the outcomes of a diabetes management intervention using mobile health apps in urban communities.
- Studying the effects of a community gardening initiative on physical activity levels and nutrition awareness.
- Investigating the utilization and impact of telemedicine services in remote rural areas.
- Assessing the benefits of a community fitness program on cardiovascular health and overall well-being.
- Evaluating the effectiveness of a school-based anti-bullying campaign on students’ mental health.
- Analyzing the outcomes of a community-driven initiative to increase access to clean drinking water in underserved areas.
Nursing Capstone Project Ideas
- Developing a comprehensive disaster preparedness plan for a local community.
- Designing and implementing a culturally sensitive prenatal care program for immigrant populations.
- Creating a curriculum for training community health workers in identifying and addressing social determinants of health.
- Establishing a support network for caregivers of elderly individuals living at home.
- Designing a mental health first aid training program for community leaders and volunteers.
- Implementing a community-based program to promote physical activity among children with obesity.
- Creating a resource guide for LGBTQ+ youth to access healthcare services without discrimination.
- Developing a community-wide initiative to combat opioid misuse and overdose.
- Establishing a telehealth platform for remote health consultations in underserved regions.
- Designing a comprehensive sexual education curriculum for high schools to address varying cultural norms.
Nursing Research Paper Topics
- The impact of community health nursing interventions on reducing health disparities .
- Exploring the role of community health nurses in disaster response and recovery.
- Analyzing the effectiveness of school-based health clinics in improving student health outcomes.
- Investigating the barriers and facilitators of healthcare access in underserved rural communities.
- The role of community health nursing in promoting healthy aging and elderly care.
- Addressing mental health stigma through community-based interventions led by nurses.
- Analyzing the outcomes of community health education programs on reducing tobacco use .
- Exploring the relationship between community engagement and positive maternal-child health outcomes.
- The effectiveness of telehealth services in bridging healthcare gaps in remote areas.
- Investigating the impact of community health nursing in preventing and managing chronic diseases.
Community Health Nursing Research Questions
- How does the presence of community health nurses influence health outcomes in underserved urban neighborhoods?
- What are the key components of successful school-based vaccination programs , and how do they impact disease prevention?
- How do cultural competence and sensitivity affect the effectiveness of community health nursing interventions?
- What are the main challenges community health nurses face in addressing social determinants of health ?
- How does community engagement contribute to the sustainability of community health initiatives led by nurses?
- What strategies effectively promote mental health awareness and reduce stigma within communities?
- How do telehealth services improve access to healthcare for individuals in geographically isolated regions?
- What role do community health nurses play in detecting and managing chronic diseases ?
- How do community health interventions impact healthcare utilization patterns and costs?
- What are the outcomes of community health nursing programs focused on improving maternal and child health?
Essay Topic Ideas & Examples
- The Role of Community Health Nursing in Promoting Population Health.
- Addressing Health Disparities: The Impact of Community Health Nursing.
- Community-Based Approaches to Preventing Teenage Pregnancy .
- Telehealth: Bridging Healthcare Gaps in Underserved Communities.
- Cultural Competence in Community Health Nursing: Challenges and Strategies.
- Disaster Preparedness and Response: The Critical Role of Community Health Nurses.
- The Influence of Social Determinants of Health on Community Health Nursing Interventions.
- Community Health Education: Strategies for Promoting Healthy Lifestyles.
- Exploring the Connection Between Mental Health and Community Well-being.
- Innovations in Community Health Nursing: Harnessing Technology for Better Outcomes.
As nursing students, you are poised to become the next generation of community health nurses, armed with the knowledge and skills to impact the health and well-being of diverse populations positively. Community health nursing offers numerous opportunities for research, practice, and advocacy. By delving into PICOT questions, evidence-based practice projects, capstone projects, research paper topics, and research questions, you can deepen your understanding of this vital field and contribute to its growth. Don’t hesitate to seek our writing services if you need assistance with your community health nursing assignments or essays. We understand the demands of nursing education and are here to support you in your academic journey. Your dedication to improving community health is commendable, and together, we can pave the way for healthier, more vibrant communities.
Frequently Asked Questions (FAQs) About Community Health Nursing
- Is community health nursing the same as nursing? Community health nursing is a specialized branch of nursing that focuses on providing holistic care to populations within specific communities. While nursing is a broader field encompassing various specialties, community health nursing is distinct in its emphasis on preventive care and health promotion within communities.
- What are the qualifications of a community health nursing? To practice community health nursing, one typically needs a registered nurse (RN) license. Many community health nurses also hold a Bachelor of Science in Nursing (BSN) degree, and advanced practice may require additional education such as a Master of Science in Nursing (MSN) with a specialization in community health.
- Do community health nurses work in hospitals? While community health nurses primarily work in community settings like public health departments, schools, and clinics, they can also collaborate with hospitals to provide education, preventive care, and continuity of care to patients transitioning from hospital to home.
- Can a community health nurse become a doctor? Community health nurses can certainly pursue further education and career advancement, but the path to becoming a doctor is different. Becoming a doctor requires completing medical school and earning a medical degree (MD) or a doctor of osteopathic medicine (DO) degree, whereas community health nursing involves nursing education and training.
Start by filling this short order form order.studyinghq.com
And then follow the progressive flow.
Having an issue, chat with us here
Cathy, CS.
New Concept ? Let a subject expert write your paper for You
Have a subject expert write for you now, have a subject expert finish your paper for you, edit my paper for me, have an expert write your dissertation's chapter, popular topics.
Business StudyingHq Essay Topics and Ideas How to Guides Samples
- Nursing Solutions
- Study Guides
- Free College Essay Examples
- Privacy Policy
- Writing Service
- Discounts / Offers
Study Hub:
- Studying Blog
- Topic Ideas
- How to Guides
- Business Studying
- Nursing Studying
- Literature and English Studying
Writing Tools
- Citation Generator
- Topic Generator
- Paraphrasing Tool
- Conclusion Maker
- Research Title Generator
- Thesis Statement Generator
- Summarizing Tool
- Terms and Conditions
- Confidentiality Policy
- Cookies Policy
- Refund and Revision Policy
Our samples and other types of content are meant for research and reference purposes only. We are strongly against plagiarism and academic dishonesty.
Contact Us:
📞 +15512677917
2012-2024 © studyinghq.com. All rights reserved
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Diabetes in Young People Is on the Rise
- Both type 1 and type 2 diabetes affect kids and teens.
- Diabetes in young people is projected to increase.
- New research shows what might be coming by the year 2060.

What did this study examine?
Researchers studied data from 2002 to 2017. They examined the number of young people (under 20) with type 1 or type 2 diabetes, and how cases have increased. They used mathematical models based on these past trends to predict how diabetes cases in young people will increase by 2060.
Terms to know
Type 1 diabetes is when the body does not produce enough insulin, the hormone that balances blood sugar levels. There is no known way to prevent type 1 diabetes at this time.
Type 2 diabetes is when the body loses the ability over time to use insulin. In some people, type 2 diabetes can be prevented or delayed through lifestyle changes.
Incidence refers to the number of people diagnosed with a condition over a certain timeframe. Incidence in this study is measured in two ways:
- Constant incidence is when the rate of new cases stays the same.
- Increasing incidence is when the rate of new cases increases based on past trends.
Study results
Researchers forecasted two scenarios of how many kids and teens will be diagnosed with diabetes by 2060:
Constant incidence: If the rate of new diagnoses stays the same, type 1 diabetes cases would remain about the same. Type 2 diabetes cases would increase about 70%.
Increasing incidence: If the rate of new diagnoses continues to increase, type 1 diabetes cases would increase about 65%. Type 2 diabetes cases would increase about 700%.
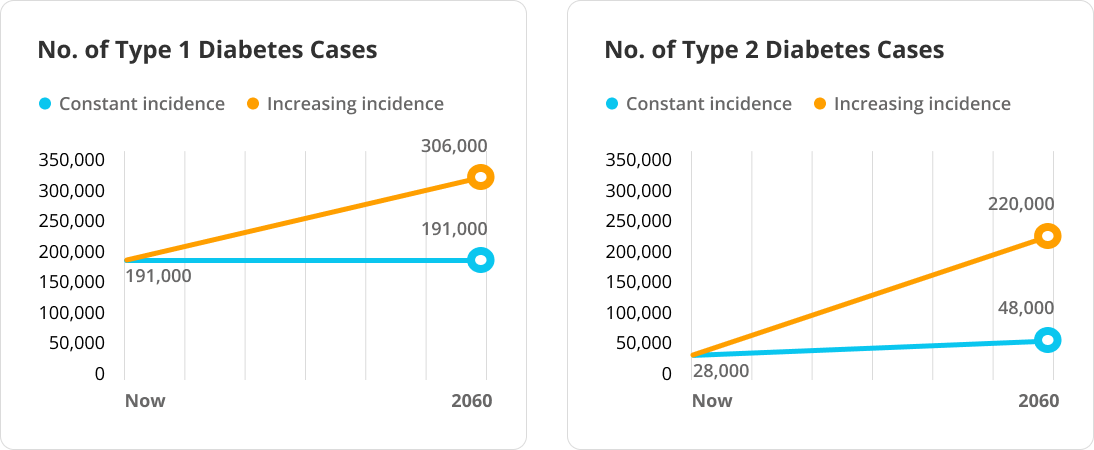
Researchers also examined data by race and ethnicity. They predicted higher diabetes increases in Black, Hispanic or Latino, Asian, Pacific Islander, and American Indian or Alaska Native young people. This finding reinforces CDC's actions to advance health equity .
Researchers created a hypothetical model that decreased the annual incidence of type 2 diabetes by 2% through prevention efforts. This model reduced the increase of diabetes among young people with to 294,000, compared to 526,000 without prevention efforts.
What's important about this study?
In both scenarios presented in this study, researchers expect diabetes in young people to increase over the next 40 years. This study highlights the importance of type 2 diabetes prevention efforts, where possible, for kids and teens. It also shows the increasing need for diabetes management for young people.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.
- Contributors
- Mission and Values
- Submissions
- The Regulatory Review In Depth

The Future of Technology in Health Care
Alyson diaz , julia englebert , and carson turner.

Scholars discuss the need for federal regulations to combat risks associated with technology in health care.
Most U.S. adults use technology to improve their health—nearly 60 percent browse the Internet for medical information, and over 40 percent obtain care through telemedicine . Despite technology’s health care potential, however, six out of ten Americans are uncomfortable with their health care provider relying on AI to diagnose diseases and recommend treatments.
AI can enhance quality of care by helping physicians verify their diagnoses and detect diseases earlier. For example, researchers have found that AI technology can help predict a patient’s risk of breast cancer. Similarly, a combination of physician expertise and AI algorithms can increase the accuracy of diagnoses.
Yet, AI systems can fail, and if humans rely too much on software, an underlying problem in one algorithm can injure more patients than a single physician’s error. In addition, AI algorithms incorporate biases from available data. For example, Black patients receive , on average, less pain medication than white patients. An algorithm trained to recommend pain treatment from these health records could suggest lower doses of painkillers for Black patients, irrespective of biological needs.
At the same time, technology can help underserved communities gain access to health care. These communities often experience shortages of trained practitioners and standard health care facilities, resulting in higher risk of disease and misdiagnoses. Telehealth, as one example, increases access to quality care by allowing patients to meet with doctors online or have their vitals monitored remotely.
Currently, no federal law regulates the use of AI in health care. Although the U.S. Food and Drug Administration (FDA) reviews most products using technology or AI software on patients, it does not currently make determinations as to whether uses of AI in health care are safe for patients. Instead, FDA approves AI-enabled devices through a process known as 510(k) review . During a 510(k) review, a manufacturer must show that its technology is “substantially equivalent” to a product already available in the market. The process allows AI-enabled devices to be approved without clinical trials proving their safety or accuracy.
Last year, the Biden Administration pledged to oversee the responsible development of AI, including in health-related fields. President Joseph R. Biden’s executive order on the subject includes requirements for health care providers to inform users when the content they provide is AI-generated and not reviewed by a physician. In addition, providers are responsible for mitigating potential risks posed by the technology and ensuring that it expands access to care.
Health professionals have also expressed concern about adolescents self-diagnosing medical conditions discussed by influencers who promote telemedicine on social media. Currently, FDA does not require telemedicine companies to disclose information about potential risks of services, and companies receive free speech protections as “advertisers.”
Advocates for stricter regulation of technology in health care point out that telehealth providers escape regulation by classifying themselves as communication platforms that connect patients with doctors, and not as providers of medical services. Telehealth companies maintain their independence from medical providers, allowing them to avoid legal liability for those providers’ actions.
In this week’s Saturday Seminar, scholars offer varying suggestions on regulating the use of technology in health care.
- AI algorithms are inherently biased, yet no federal regulation addresses the risk of biased diagnostics when AI is used in health care, recent Seattle University School of Law graduate Natalie Shen argues in an article in the Seattle Journal of Technology, Environmental & Innovation Law . Shen explains that in the absence of federal action, states have taken the lead in passing laws to address automated decision systems such as AI in health care. By analyzing New Jersey’s and California’s approaches, Shen recommends improvements to future state legislation, including extending any future law’s coverage to the private health insurance sector, and imposing continuous assessment requirements as AI technology evolves.
- In an article for the Virginia Law Review , Berkeley Law Schools Khiara M. Bridges argues that educating patients about the risk of race-based algorithmic bias should be a prerequisite before using AI in health care. Bridges explains that people of color are more likely to distrust physicians and health care institutions and thus, are likely to be skeptical of medical AI. Furthermore, medical algorithms are developed based on a primarily white “general population,” reducing their predictive accuracy for communities of color, Bridges notes . She argues that disclosure of AI-related risks would foster patient-physician dialogue in communities of color, encouraging more patients of color to use the technology and ultimately remedying existing algorithmic biases.
- Regulation of AI-enabled health tools must include pre-market authorization and continued performance monitoring processes, urge Joana Gonçalves-Sá of Complexity Science Hub and Flávio Pinheiro of NOVA Information Management School in an chapter in Multidisciplinary Perspectives on Artificial Intelligence and the Law . Gonçalves-Sá and Pinheiro propose improvements to FDA’s pilot program, Total Product Lifecycle , which tracks the safety risks of AI. Under the program, an AI company can achieve “precertified status” if it can demonstrate that it develops high quality algorithms and continues to monitor their effectiveness after market entry, Gonçalves-Sá and Pinheiro explain . FDA should also investigate the reliability of datasets and engineers that train AI tools, Gonçalves-Sá and Pinheiro recommend .
- Regulators should lower legal barriers that prevent community organizations such as Black churches from helping poor and marginalized people to gain access to telehealth services, argues Meighan Parker of the University of Chicago Law School in a recent article in the Columbia Science and Technology Law Review . Parker notes that although community organizations such as Black churches could help some people to overcome mistrust of health care providers, involving them could cause conflicts between the churches’ beliefs and patients’ medical needs, or open the churches to malpractice liability. In response, Parker proposes softening or adjusting regulatory barriers to ensure that churches will not face ethical conflict or legal liability for connecting people with needed telehealth services.
- In a note in the Washington Journal of Law, Technology & Arts , Kaitlin Campanini , a student at Pace University Elisabeth Haub School of Law , argues that the U.S. Drug Enforcement Administration’s lax regulation of telehealth providers has worsened inadequate mental health treatment and increased excessive drug prescriptions. Although telehealth providers’ business models can render treatment more convenient and affordable, the expedited treatment model they offer “blurs the line between offering health care to patients and selling controlled substances to customers.” This is because such companies fall into a regulatory gray area. They disclaim providing medical services by maintaining that they are independent from providers. Yet they aggressively market stimulants to consumers and facilitate questionable prescriptions after short, virtual evaluations.
- In a recent note in the Belmont Law Review , J.D. candidate Nora Klein argues that regulators should close legal loopholes that allow direct-to-consumer (DTC) pharmaceutical companies to unfairly influence social media users. Klein notes that DTC pharmaceutical companies have avoided FDA advertising regulations in part by labeling themselves as entities over which FDA has no regulatory authority. Accordingly, these entities are only subject to FTC advertising regulations, which are difficult to enforce, Klein observes . She argues that the DTC model is harmful because it leads to misdiagnoses and patient complications more often than traditional health care services. To address the problem, Klein proposes that FDA require DTC pharmaceutical companies to disclose important drug information to consumers.
The Saturday Seminar is a weekly feature that aims to put into written form the kind of content that would be conveyed in a live seminar involving regulatory experts. Each week, The Regulatory Review publishes a brief overview of a selected regulatory topic and then distills recent research and scholarly writing on that topic.
Related Essays

The Troubled Teen Industry’s Troubling Lack of Oversight
The federal government must step in to regulate an industry fraught with youth abuse.

Reforming Accountable Care Organizations
Experts explore opportunities to reduce costs and promote health equity.

A Review of Health Care in the Court
Several U.S. Supreme Court cases from this past term have significant implications for the provision of medical care.

Browse all our research projects by topic We have funded more than 150 external research projects across a range of themes over the last five years
- Share on Twitter
- Share on LinkedIn
- Share on Facebook
- Share on WhatsApp
- Share by email
- Link Copy link

Please click below to see the research projects we've funded on the following topics.
- Analytics and data
- Children and young people
- Commissioning
- Community and voluntary
- Digital technology
- Efficiency and productivity
- Emergency medicine
- End of life care
- Funding and sustainability
- Improvement science
- Inequalities
- Integrated care
- Long-term conditions
- Mental health
- Older people
- Patient experience
- Patient safety
- Person-centred care
- Primary care
- Public health
- Quality improvement
- Quality of care
- Social care
- Social determinants of health
Share this page:
Health Foundation @HealthFdn
- Work with us
We look for talented and passionate individuals as everyone at the Health Foundation has an important role to play.
The Q community
Q is an initiative connecting people with improvement expertise across the UK.
Quick links
- News and media
- Events and webinars
Hear from us
Receive the latest news and updates from the Health Foundation
- 020 7257 8000
- [email protected]
- The Health Foundation Twitter account
- The Health Foundation LinkedIn account
- The Health Foundation Facebook account
Copyright The Health Foundation 2024. Registered charity number 286967.
- Accessibility
- Anti-slavery statement
- Terms and conditions
- Privacy policy


IMAGES
COMMENTS
Finding and choosing a strong research topic is the critical first step when it comes to crafting a high-quality dissertation, thesis or research project. If you've landed on this post, chances are you're looking for a healthcare-related research topic, but aren't sure where to start. Here, we'll explore a variety of healthcare-related research ideas and topic thought-starters across a ...
With NIH support, scientists across the United States and around the world conduct wide-ranging research to discover ways to enhance health, lengthen life, and reduce illness and disability. Groundbreaking NIH-funded research often receives top scientific honors. In 2021, these honors included Nobel Prizes to five NIH-supported scientists.
The findings suggest that people can learn to reduce the brain activity causing some types of chronic pain that occur in the absence of injury or persist after healing. 2021 Research Highlights — Basic Research Insights >>. NIH findings with potential for enhancing human health include new drugs and vaccines in development for COVID-19 ...
They also assess the future evolution of payment systems leading toward sustainable health, changes in provider roles, and the entrance of new nontraditional players. For more on this topic, watch Health Systems in 2030, a free NEJM Catalyst virtual event held on March 4, 2021.
Health research entails systematic collection or analysis of data with the intent to develop generalizable knowledge to understand health challenges and mount an improved response to them. The full spectrum of health research spans five generic areas of activity: measuring the health problem; understanding its cause(s); elaborating solutions; translating the solutions or evidence into policy ...
Health care is the discipline concerned with the provision and delivery of health services to a patient population by personnel in professional and allied health occupations ...
Change is an ongoing process in any organizations. Over years, healthcare organizations have been exposed to multiple external stimuli to change (eg, ageing population, increasing incidence of chronic diseases, ongoing Sars-Cov-2 pandemic) that pointed out the need to convert the current healthcare organizational model.
Here, the authors investigate the fluctuations of physiological indices along aging trajectories and observed a characteristic decrease in the organism state recovery rate. Timothy V. Pyrkov ...
NIH Research Matters. Bldg. 31, Rm. 5B52, MSC 2094. Bethesda, MD 20892-2094. Editor: Assistant Editors: NIH Research Matters Office of Communications and Public Liaison NIH Office of the Director. NIH findings with potential for enhancing human health include new approaches to COVID-19, a universal mosquito vaccine, and advances in restoring ...
Health sciences articles from across Nature Portfolio. Atom. RSS Feed. The health sciences study all aspects of health, disease and healthcare. This field of study aims to develop knowledge ...
151+ Public Health Research Topics [Updated 2024] The important area of public health research is essential to forming laws, influencing medical procedures, and eventually enhancing community well-being. As we delve into the vast landscape of public health research topics, it's essential to understand the profound impact they have on society.
The greatest strength of the qualitative research approach lies in the richness and depth of the healthcare exploration and description it makes. In health research, these methods are considered as the most humanistic and person-centered way of discovering and uncovering thoughts and actions of human beings. Table 1.
Precision medicine for lung cancer theragnostic is an advanced model combining prevention, diagnosis, and treatment for individual or specific population diseases to match individual patient differences. The scientific community has recently defined it as a medical care that takes advantage of large data sets of individuals such as their genome or their entire electronic health record to ...
Topic IDs 3 and 4 reveal challenges associated with homelessness, access to housing and treatment/care, and HIV-related care/health outcomes. While prior research raised concerns regarding HIV-infected older persons' acceptance into retirement homes and long-term care facilities, ref. identified barriers to subsidized housing based on their ...
100 Healthcare Research Paper Topics. The field of healthcare research encompasses a vast array of topics that are crucial for understanding, improving, and transforming healthcare practices. As students in the health sciences, you have the opportunity to explore these diverse areas and contribute to the knowledge base of healthcare research.
The Fogarty International Center and its NIH partners invest in research on a variety of topics vital to global health. For each of these global health research topics, find an in-depth collection of news, resources and funding from Fogarty, the NIH, other U.S. government agencies, nongovernmental organizations and others. Bioethics.
March 20, 2024. For graduate students who are passionate about putting research and policy recommendations into action, the Johns Hopkins Health Policy Institute (HPI) is an invaluable experience. Among the Spring 2024 HPI Fellows is Glendedora Dolce, now in her second year as a Health and Public Policy doctoral student.
Health Services Research and Primary Care Research. AHRQ convened an interactive roundtable meeting of leaders in health systems, public health preparedness, and resiliency, facilitated by the Department of Health and Human Services Office of Climate Change and Health Equity interim director. The roundtable discussion laid the groundwork for advancing work on health system resilience, along ...
Since 2020, COVID-19 has been a hot-button topic in medicine, along with the long-term symptoms in those with a history of COVID-19. Examples of COVID-19-related research topics worth exploring include: The long-term impact of COVID-19 on cardiac and respiratory health. COVID-19 vaccination rates.
Department of Anesthesiology and Critical Care Medicine. Arrhythmia, cardiomyopathy, heart failure, preventative cardiology and vascular topics research. Heart Institute. Biomechanics research, gait and mobility disorders, swallowing dysfunction research. Department of Physical Medicine and Rehabilitation.
Breaking Barriers in LGBT+ Health: Innovations and Insights. Michał Czapla. Raúl Juárez-Vela. Piotr Karniej. 310 views. The most cited cited journal in its field, which promotes discussion around inter-sectoral public health challenges spanning health promotion to climate change, transportation, environmental change...
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2, the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
CAR T-cell immunotherapy changes these collected T cells to produce chimeric antigen receptors (or CARs) that adhere to tumors to destroy them. A recent study published in the New England Journal of Medicine, suggests CAR T-cell therapy could become a highly effective treatment for SLE patients who do not respond to current lupus therapeutics ...
Findings show U.S. university student journalists emphasized efficacy rather than threats countering past content analysis research. We also found environmental and sustainability communities of practice did not predict threat and efficacy information, but topics did.
This Research Topic, titled "High-risk Pregnancy: Women's Experiences and New Approaches to Care" welcomes papers from academics, clinicians, and other healthcare professionals in the field. Topics of interest include: - The psychosocial and emotional impact of a high-risk pregnancy diagnosis - Education needs arising from the diagnosis of ...
Community health nursing is a dynamic and vital field within the nursing profession, focused on providing holistic care to populations within a defined community. This specialized branch of nursing goes beyond individual patient care, extending its reach to families, groups, and entire communities. This article will delve into community health ...
Diabetes projections for young people under 20 years old. Researchers also examined data by race and ethnicity. They predicted higher diabetes increases in Black, Hispanic or Latino, Asian, Pacific Islander, and American Indian or Alaska Native young people. This finding reinforces CDC's actions to advance health equity.
Laura Panozzo is the Assistant Director for DNP Executive, PhD, and DNP/PHD Recruitment at Johns Hopkins School of Nursing. She can help you take the next step in your nursing career, contact her at 443-287-7430 or [email protected]. Research is what drives nursing innovation forward, and is an important part of improving health care delivery.
Scholars discuss the need for federal regulations to combat risks associated with technology in health care. Most U.S. adults use technology to improve their health—nearly 60 percent browse the Internet for medical information, and over 40 percent obtain care through telemedicine. Despite technology's health care potential, however, six out ...
REAL Centre: making health and care services more sustainable; Improving national health and care policy; Funding and partnerships. Current opportunities; ... Browse all our research projects by topic We have funded more than 150 external research projects across a range of themes over the last five years Share on Twitter ...