- Career Blog

Quality Manager Resume: Examples and Writing Guide

In this article, we will explore the world of Quality Manager Resumes. A Quality Manager Resume is a document that showcases the skills, experiences, and achievements of a Quality Manager. Employers need Quality Managers to ensure that their products or services meet a high standard of quality. Crafting an effective Quality Manager Resume is essential for standing out in a competitive job market. In this article, we will discuss the elements that make a great Quality Manager Resume and provide examples and tips to help you write your own. So, let’s dive in!
Understanding the Role of a Quality Manager
A Quality Manager is a professional who is responsible for ensuring that products, services, and processes of an organization meet the required standards and specifications. They oversee quality control measures, testing, and inspection, and are responsible for making sure that products meet regulatory and industry requirements.
What does a Quality Manager do?
A Quality Manager plays a critical role in ensuring the quality and consistency of products, services, and processes within an organization. Their duties may include:
- Establishing and implementing quality control procedures
- Analyzing and interpreting data
- Identifying areas for improvement and implementing solutions
- Developing quality standards and specifications
- Conducting quality audits and inspections
- Training, coaching, and mentoring team members on quality processes and procedures
What skills are required to be a Quality Manager?
To be a successful Quality Manager, you will need to possess a combination of technical and soft skills. These may include:
- Strong analytical and problem-solving skills
- Excellent communication and interpersonal abilities
- Knowledge of quality control procedures and methodologies
- Attention to detail and accuracy
- Proficiency in data analysis and interpretation
- Experience with production processes and industry-specific regulations
- Strong leadership and organizational skills
- Ability to work effectively in a team and independently
- Continuous improvement mindset
What qualities are employers looking for in a Quality Manager?
Employers are typically looking for Quality Managers who have a strong track record of success in creating and implementing quality control procedures and methodologies. Additionally, they often look for candidates who possess:
- Professional certifications in quality management or a related field
- Experience in their specific industry or sector
- Strong leadership and management skills
- A customer-oriented approach
- The ability to manage multiple projects simultaneously
- A commitment to continuous improvement and learning
- A positive attitude and strong work ethic
A successful Quality Manager will possess a combination of technical and soft skills, as well as a commitment to continuous improvement and a customer-oriented approach. By demonstrating these qualities and competencies, they stand to become an invaluable asset to any organization.

Tips for Writing an Attention-Grabbing Quality Manager Resume
When crafting your Quality Manager resume, it’s important to keep in mind that your goal is to make an impactful first impression on your potential employer. This can be achieved by following these tips:
Understanding the importance of a targeted and relevant resume
The first step in writing an attention-grabbing resume is to understand the importance of tailoring your resume to the job you’re applying for. Your resume should reflect the requirements of the job posting, and highlight how your specific skill set and experience can meet those requirements.
Highlighting core skills and achievements
Be sure to include a section in your resume that highlights your core skills and achievements. This is your opportunity to showcase your unique strengths and what sets you apart from other applicants.
Using a professional format and structure
A hiring manager will appreciate a well-organized and easy-to-read resume. Ensure that your resume is structured in a professional manner, and that all sections are clearly labeled. Be consistent with font size and formatting throughout the document.
Making sure your resume is easy to read and visually appealing
In addition to a professional structure, it’s also important to make sure your resume is visually appealing. Avoid using too many graphics or fancy fonts, as this can be distracting. Instead, use white space to break up sections and make the resume easy to read.
By following these tips, you can create an attention-grabbing Quality Manager resume. Remember, your goal is to stand out from the competition and land an interview. A well-crafted, targeted resume can help you achieve that goal.
Key Components of a Quality Manager Resume
When it comes to crafting a quality manager resume, there are several essential components that should be included in order to impress potential employers and stand out from the competition.
The Necessary Sections to Include
The following sections are a must-have in any quality manager resume:
Contact Information: Your full name, phone number, email address, and LinkedIn profile URL should be clearly visible at the top of your resume.
Professional Summary/Objective Statement: This section should be a brief, three to five sentence summary of your qualifications and career goals.
Skills: When drafting your Skills section, try to focus on relevant quality management skills such as ISO certification, Six Sigma, root cause analysis, and statistical process control.
Work Experience: This section should list your work experience in reverse chronological order, along with your job title, employer, dates of employment, and key accomplishments.

Education: List your highest degree first, including the name of the institution, your major, and the date of graduation.
How to Showcase Relevant Education and Experience
When it comes to featuring your education and experience in a quality manager resume, it’s essential to highlight the aspects that are most relevant to the job you’re applying for. You can do this by using specific keywords and phrases from the job posting in your resume.
Be sure to include any relevant certifications or training programs as well. Quality managers with certifications in ISO or Six Sigma are particularly valuable to employers.
Writing a Compelling Summary or Objective Statement
Your professional summary or objective statement should be concise but impactful. Focus on your most significant accomplishments and unique skills, and demonstrate how these factors make you the ideal candidate for the job.
For example:
“Results-driven quality manager with over 10 years of experience in improving product quality and reducing defects. Skilled in leading cross-functional teams, implementing process improvements, and developing robust quality management systems. Seeking a challenging role in a fast-paced manufacturing environment that values quality and continuous improvement.”
By following these guidelines, you will be well on your way to crafting a standout quality manager resume that will land you your dream job.
Example Quality Manager Resumes
Finding the right job as a quality manager can be tough, especially if you’re not sure where to start. That’s why we’ve created a collection of example resumes to help you showcase your skills and experience effectively. Whether you’re a seasoned pro or just starting out, we’ve got you covered.
Examples and Templates for Different Levels of Experience
On our website, you can find quality manager resume examples at different levels of experience, so you can choose the one that best suits your needs. For example, if you’re a recent graduate or just starting out, we have entry-level resumes that showcase your education and skills. And for those with more experience, we have resumes that highlight your accomplishments and leadership skills.
In addition, we also offer different templates that will help you create a professional and well-organized resume in no time.
Quality Manager Resume (Entry-Level)
Emily Johnson
Quality Manager
Dedicated and detail-oriented Quality Manager with a strong academic background and a passion for ensuring product quality. Seeking an entry-level role to apply my knowledge of quality management principles and gain hands-on experience in implementing quality control processes. Strong analytical and problem-solving skills, with a focus on continuous improvement and customer satisfaction.
- Bachelor’s Degree in Industrial Engineering, University of XYZ
Academic Quality Improvement Project
- Led a team project to analyze and improve the quality of a manufacturing process in a simulated environment.
- Conducted data analysis to identify process variations and implemented corrective actions to improve product quality.
- Developed quality control charts to monitor process performance and ensure adherence to specifications.
Internship – Quality Assurance, ABC Manufacturing Company
- Assisted in implementing quality control measures to ensure compliance with industry standards.
- Conducted inspections and tests to verify product quality and identify non-conformities.
- Participated in root cause analysis and corrective action implementation to address quality issues.
- Quality Management Principles
- Data Analysis
- Root Cause Analysis
- Quality Control
- Continuous Improvement
- Problem-solving
- Communication
Certifications
- Six Sigma Yellow Belt (In Progress)
Quality Manager Resume (Experienced)
Robert Thompson
Quality Manager | Continuous Improvement Expert
Results-driven Quality Manager with over 8 years of experience in implementing and managing quality control processes. Proven track record in leading cross-functional teams, driving process improvements, and ensuring product quality. Skilled in quality management systems, root cause analysis, and implementing corrective actions. Strong leadership, problem-solving, and communication skills.
Professional Experience
Quality Manager, XYZ Manufacturing Company
- Led a team of quality professionals to implement and maintain a quality management system based on ISO 9001 standards.
- Developed and implemented quality control processes and procedures to ensure product conformity and customer satisfaction.
- Conducted root cause analysis and implemented corrective and preventive actions to address quality issues and improve process performance.
- Led continuous improvement initiatives, resulting in a reduction in non-conformities and improved process efficiency.
Senior Quality Engineer, ABC Automotive Company
- Implemented statistical process control (SPC) techniques to monitor and control manufacturing processes.
- Led cross-functional teams in problem-solving and root cause analysis to address quality issues and improve product quality.
- Developed and delivered training programs on quality control and process improvement methodologies to enhance employee knowledge and skills.
- Quality Management Systems (ISO 9001)
- Corrective and Preventive Actions
- Statistical Process Control (SPC)
- Leadership and Team Management
- Problem-solving and Decision-making
- Six Sigma Black Belt
- Certified Quality Manager (CQM)
How to Personalize Each Resume to Meet Specific Job Requirements
At the heart of an effective quality manager resume is the ability to tailor it to the specific job requirements. This means that each resume should be personalized to fit the company and position you’re applying for.
We recommend that you read the job description carefully and identify the key skills and experience that the employer is looking for. Then, you can highlight and emphasize those skills in your resume. For instance, if the job requires experience in ISO 9001:2015 or Lean Six Sigma methodologies, make sure to include your related experience in your resume.
It’s also important to include examples of your accomplishments and how they relate to the job you’re applying for. This will give the employer a better understanding of your capabilities and how you can help their organization.
Using our quality manager resume examples and templates, you can create an effective resume that showcases your skills and helps you stand out from other candidates. And by personalizing each resume to fit the specific job requirements, you can increase your chances of landing your dream job.
Tailoring Your Quality Manager Resume to Specific Industries
Quality Managers work across many different industries, and each industry has its own unique quality standards and requirements. Therefore, it is important to tailor your Quality Manager resume to the specific industry in which you are seeking employment. This can increase your chances of being noticed by potential employers and ultimately securing the job you desire.
Understanding the different industries in which Quality Managers work
Quality Managers can be found in a variety of industries such as manufacturing, healthcare, construction, food and beverage, and pharmaceuticals, to name a few. Each of these industries has their own quality standards and compliance requirements that must be met to ensure the delivery of safe and high-quality products or services to customers. For instance, a Quality Manager in the pharmaceutical industry is responsible for ensuring that drugs are manufactured in compliance with regulations set by the FDA, whereas a Quality Manager in healthcare is responsible for maintaining patient safety and regulatory compliance.
Adapting your resume to highlight relevant experiences and skills based on industry
When tailoring your resume, it is important to highlight relevant experiences and skills that are specific to the industry in which you are applying. For instance, if you are applying for a Quality Manager position in the food and beverage industry, highlight your experience with HACCP (Hazard Analysis and Critical Control Points) as well as your knowledge of food safety regulations.
Similarly, if you are applying for a Quality Manager position in the construction industry, highlight your experience with ISO 9001 (Quality Management System) and your knowledge of OSHA (Occupational Safety and Health Administration) regulations.
Tailoring your Quality Manager resume to specific industries can help you stand out in a competitive job market. Be sure to research the industry and the company you are applying to, and customize your resume to highlight your relevant experiences and skills that will make you an ideal candidate for the position.
Writing a Cover Letter for a Quality Manager Position
In the job application process, a cover letter serves as an introduction of yourself to the hiring manager. It is your chance to grab their attention and make a great first impression. As a Quality Manager, your cover letter should not only showcase your qualifications but also demonstrate your ability to communicate clearly and effectively.
Here are some tips for writing a targeted and engaging cover letter:
Understanding the role of a cover letter in the job application process
A cover letter is your chance to set yourself apart from other applicants. It should be tailored to the specific company and position you are applying for. It is an opportunity to highlight your most relevant skills and experiences, and explain how they make you the best candidate for the position. A well-written cover letter can also demonstrate your attention to detail and professional writing skills.
Tips for writing a targeted and engaging cover letter
Research the company: Before writing your cover letter, research the company you are applying to. Learn about their mission, values, and culture. Understanding the company’s goals and needs can help you tailor your cover letter to the position.
Highlight your qualifications: Use your cover letter to highlight your relevant experience and qualifications. Explain how your skills and experiences match the job requirements. Be specific and use examples to support your claims.
Use a professional tone: Your cover letter should be professional and polished. Avoid using slang, abbreviations, or casual language. Use a formal tone and make sure to proofread for errors.
Personalize your letter: Address your cover letter to the hiring manager by name if possible. If you are unable to find their name, use a professional greeting such as “Dear Hiring Manager.”
Keep it concise: Your cover letter should be no longer than one page. Keep your writing clear and to the point. Use bullet points or short paragraphs to make it easy to read.
Example cover letters for Quality Manager positions
Dear Hiring Manager,
I am excited to apply for the Quality Manager position at XYZ Company. With six years of experience in the industry, I have developed a deep understanding of quality control processes and the ability to lead a team to success.
At my past company, I reduced product defects by 10% through process improvement initiatives. My experience in Lean and Six Sigma methodologies has also been valuable in identifying areas of improvement and implementing changes that have resulted in cost savings.
I am confident that my skills and experiences make me an ideal candidate for the Quality Manager position at XYZ Company. Thank you for considering my application.
Sincerely, [Your name]
I am writing to express my interest in the Quality Manager position at ABC Corporation. As a certified Quality Engineer with over eight years of experience in the field, I have a proven track record of success in managing quality control processes.
My experience leading cross-functional teams has been instrumental in driving process improvements and increasing product quality.
Best Practices for Optimizing Your Quality Manager Resume for Applicant Tracking Systems (ATS)
When applying for a quality manager position, it is essential to understand how applicant tracking systems (ATS) screen resumes. ATS is software used by recruiters to manage and sort resumes based on specific keywords, phrases, and job-related criteria.
To optimize your resume for ATS, follow the tips below:
Use relevant keywords: Read the job posting carefully and pick out relevant keywords and phrases. Include them in your resume to increase the chances of being selected by the ATS.
Keep it simple: Use a simple and clear resume format. Avoid using fancy fonts, graphics, and formatting that can confuse the ATS.
Use relevant headings: Use headings such as “work experience,” “skills,” and “education” that are easy for ATS to recognize.
Use bullet points: Use bullet points to highlight your relevant experience, skills, and achievements. This makes it easier for the ATS to scan and identify the information.
Always use a text-based document: Use a simple and common file format such as .docx or .pdf (text-based) to ensure that the ATS can easily scan your resume.
Common mistakes when preparing your resume for ATS include:
Adding unnecessary information and graphics that can confuse the ATS.
Using headers, footers, and tables that the ATS may not recognize.
Using abbreviations, slang, or technical jargon that the ATS may not identify.
When preparing your quality manager resume for ATS, focus on using relevant keywords, a clear format, relevant headings, bullet points and avoid using unnecessary information and graphics. This will help increase your chances of getting shortlisted and landing a quality manager job.
Proofreading and Editing your Quality Manager Resume
One of the most crucial stages in creating a quality manager resume is the proofreading and editing process. It’s important to ensure that your resume is free from errors and inconsistencies so that it presents you in the best light possible.
The importance of proofreading and editing your resume
Proofreading and editing your quality manager resume is essential because it can make the difference between getting an interview for your dream job or being dismissed by potential employers. Even the tiniest error or inconsistency can reflect poorly on you and your attention to detail. In a world where employers receive hundreds of resumes for a single role, a resume that contains mistakes is unlikely to stand out.
Tips for spotting errors and inconsistencies
To ensure the quality of your manager resume, here are some tips for spotting errors and inconsistencies:
- Read your resume out loud – this helps you to identify awkward phrasing and grammatical errors.
- Take a break before proofreading – give yourself some space before you proofread, as this can help you approach your resume with fresh eyes.
- Use proofreading software – programs like Grammarly and Hemingway can help you identify errors and suggest corrections.
- Enlist a friend or family member to help – sometimes you need outside help to catch mistakes that you might have missed.
Tools and resources for editing and proofreading
Several tools and resources can help you in your editing and proofreading process. Here are some of the most popular:
- Grammarly – A widely used grammar-checking tool that helps detect grammar, spelling, and punctuation errors.
- Hemingway – A writing app that helps you simplify your writing, making it clear and concise.
- Microsoft Word – Microsoft Word has an in-built grammar-checking feature that can help you detect errors and offer suggestions.
- Online proofreading services – Services like Scribendi and ProWritingAid offer professional proofreading services that can help you take your resume to the next level.
Proofreading and editing your quality manager resume is an essential part of the job-seeking process. By following our tips and using the tools and resources provided, you can ensure that your resume is free from errors and inconsistencies and presents you as the best candidate for the job.
X. Frequently Asked Questions about Quality Manager Resumes
Quality Manager positions require a certain level of expertise and specialized knowledge. When crafting a resume for such a role, it’s important to highlight the specific skills and experiences that make you a qualified candidate. Here are some commonly asked questions about writing resumes for Quality Manager positions, along with answers and advice to help job seekers stand out from the competition.
1. What skills should I include on my Quality Manager resume?
Skills are a vital component of any Quality Manager resume. A few key skills to consider including are quality control, process improvement, data analysis, problem-solving, and leadership. Additionally, soft skills such as communication, collaboration, and attention to detail are important to emphasize. Review the job description and industry standards to tailor your skills to the position specifically.
2. How can I showcase my accomplishments on my resume?
It’s important to quantify your accomplishments on your resume, such as improving product quality, increasing efficiency, or reducing costs. Consider using bullet points to highlight specific achievements and numbers that indicate your impact on the organization. Use strong action verbs and avoid generic descriptions that do not convey the scope of your responsibilities.
3. Should I include certifications on my Quality Manager resume?
Adding certifications that reflect your expertise to your Quality Manager resume is beneficial, especially if the certification is relevant to the position you are applying for. Examples of certifications that would be valuable for Quality Managers are ISO 9001 Quality Management System certifications, Six Sigma certifications or industry-specific certifications.
4. How should I format my Quality Manager resume?
When searching for Quality Manager positions, recruiters and hiring managers look for resumes that are easy to read and visually pleasing. Use a clean, organized format that is easy to follow, with consistent formatting and clear section headings. Include a summary statement at the beginning of your resume that highlights your key achievements, job experience, and relevant skills.
5. What common mistakes should I avoid on my Quality Manager resume?
Mistakes on a Quality Manager resume can be costly for candidates, as it portrays a lack of attention to detail, a crucial trait for Quality Manager roles. Avoid grammatical errors, typos or inconsistency in formatting, education details, or job descriptions. Do not oversell or use empty phrases, as this may backfire during the hiring process.
By answering these frequently asked questions and heeding these recommendations, job seekers can make a strong impression on potential employers, increasing their chances of landing a Quality Manager position. The key is to maintain a balance between highlighting your skills and experiences while keeping the focus on how the organization benefits from your expertise.
Related Articles
- QA Team Lead Job Description: Complete Guide for 2023
- 25 Fast Tips for Finding a New Job in 2023
- Acing Interviews with the STAR Method: Best Examples
- 25 Restaurant Manager Resume Examples with Helpful Tips
- 30 Common Situational Interview Questions and Expert Answers
Rate this article
0 / 5. Reviews: 0

More from ResumeHead

Quality Manager resume examples for 2024
Quality managers need to have an eye for detail and a knack for problem-solving. According to Dr. Cindy O'Reilly, Director of Quality, "You have to be able to analyze data, to understand what it's telling you, and to do something with that information. That's a very important quality for a quality manager." quality managers also need strong interpersonal skills to effectively communicate with their team, customers, and suppliers. They must be able to "work closely and advised production supervisors and production staff to ensure all quality procedures were followed and the disposition of material." They must also be able to "investigate customer complaints associated with product quality and coordinate the development of root cause analyses and the development of corrective actions."

Quality Manager resume example
How to format your quality manager resume:.
- Use the same job title on your resume as the job you're applying for: Quality Manager
- Highlight achievements rather than responsibilities in your work experience section
- Aim to fit your resume on one page, focusing on relevant information for the Quality Manager role
Choose from 10+ customizable quality manager resume templates
Choose from a variety of easy-to-use quality manager resume templates and get expert advice from Zippia’s AI resume writer along the way. Using pre-approved templates, you can rest assured that the structure and format of your quality manager resume is top notch. Choose a template with the colors, fonts & text sizes that are appropriate for your industry.

Quality Manager resume format and sections
1. add contact information to your quality manager resume.
Quality Manager Resume Contact Information Example # 1
Dhruv Johnson
[email protected] | 333-111-2222 | www.linkedin.com/in/dhruv-johnson
2. Add relevant education to your quality manager resume
Your resume's education section should include:
- The name of your school
- The date you graduated ( Month, Year or Year are both appropriate)
- The name of your degree
If you graduated more than 15 years ago, you should consider dropping your graduation date to avoid age discrimination.
Optional subsections for your education section include:
- Academic awards (Dean's List, Latin honors, etc. )
- GPA (if you're a recent graduate and your GPA was 3.5+)
- Extra certifications
- Academic projects (thesis, dissertation, etc. )
Other tips to consider when writing your education section include:
- If you're a recent graduate, you might opt to place your education section above your experience section
- The more work experience you get, the shorter your education section should be
- List your education in reverse chronological order, with your most recent and high-ranking degrees first
- If you haven't graduated yet, you can include "Expected graduation date" to the entry for that school
Check More About Quality Manager Education
Quality Manager Resume Relevant Education Example # 1
Bachelor's Degree In Business 1996 - 1999
Arizona State University Phoenix, AZ
Quality Manager Resume Relevant Education Example # 2
Bachelor's Degree In Business 2010 - 2013
Strayer University Washington, DC
3. Next, create a quality manager skills section on your resume
Your resume's skills section should include the most important keywords from the job description, as long as you actually have those skills. If you haven't started your job search yet, you can look over resumes to get an idea of what skills are the most important.
Here are some tips to keep in mind when writing your resume's skills section:
- Include 6-12 skills, in bullet point form
- List mostly hard skills ; soft skills are hard to test
- Emphasize the skills that are most important for the job
Hard skills are generally more important to hiring managers because they relate to on-the-job knowledge and specific experience with a certain technology or process.
Soft skills are also valuable, as they're highly transferable and make you a great person to work alongside, but they're impossible to prove on a resume.
Example of skills to include on an quality manager resume
Continuous improvement is an ongoing process of improvement of products, services, and processes with the help of innovative ideas. It is an organized approach that helps an organization to find its weaknesses and improve them.
Quality standards are a specific level of standards of products that are set by the companies for the customers that have to be met and maintained throughout the process until the time of delivery. Quality standards are information that includes the customer's requirements, guidelines, and characteristics for the needed final product or service.
Product quality is the basic element of a business. It means to add features in a product or service in such a way that it meets the needs and wants of the customers. Enhancing the product quality also means improve the goods from any existing defects to ensure customer satisfaction.
In Greek alphabets, sigma is the 18th letter that means "to sum up". In statistics, the lower case symbol of sigma is the unit of measurement for standard deviation which is used to assess the variability in a given set of data. While the upper case symbol is for summation notation means to add up all the given numbers in the data set.
A quality management system is a set of techniques that deals with the procedure, process, and duties to achieve a quality goal and purpose. This particular skill focuses on the coordination and management of the company's policy. With this system, they can determine what needs improvement and understand the level of awareness and efficiency.
Customer service is the process of offering assistance to all the current and potential customers -- answering questions, fixing problems, and providing excellent service. The main goal of customer service is to build a strong relationship with the customers so that they keep coming back for more business.
Quality control is a set of instructions or procedures to ensure a manufactured product or a service is up to the highest quality standards. This set of quality control criteria are either defined by the clients or the company itself.
Top Skills for a Quality Manager
- Continuous Improvement , 7.7%
- Corrective Action , 5.5%
- Quality Standards , 5.1%
- Product Quality , 4.7%
- Other Skills , 77.0%
4. List your quality manager experience
The most important part of any resume for a quality manager is the experience section. Recruiters and hiring managers expect to see your experience listed in reverse chronological order, meaning that you should begin with your most recent experience and then work backwards.
Don't just list your job duties below each job entry. Instead, make sure most of your bullet points discuss impressive achievements from your past positions. Whenever you can, use numbers to contextualize your accomplishments for the hiring manager reading your resume.
It's okay if you can't include exact percentages or dollar figures. There's a big difference even between saying "Managed a team of quality managers" and "Managed a team of 6 quality managers over a 9-month project. "
Most importantly, make sure that the experience you include is relevant to the job you're applying for. Use the job description to ensure that each bullet point on your resume is appropriate and helpful.
- Rated as Top Performer for SMB Sales FORMULATED IT SVCS 2001 - 2002 Sr.
- Worked with CAD engineers on PCB layout.
- Coordinated all Pro/Engineering hardware, software, and training requirements for the Commercial Engineering group and Presented team status to management.
- Designed high speed data cable assemblies and developed cable routing workplans for high-performance computational systems.
- Implemented virtual NIC driver on Linux Platform utilizing the APIs exported by the NTB PCI driver.
- Performed data validations on XML Service calls between the Clients and Vendors.
- Extracted data from flat files using UNIX shell scripts.
- Provided support to the Business QA team during the Business Acceptance Testing (BAT) phase of the project.
- Utilized tools and methodologies to improve individual effectiveness and increases efficiency in the QA process.
- Coordinated the test efforts of on-site and off-shore teams and reported directly to the QA Manager.
- Trained on the latest HDP-CVD process technology, documenting new process and maintenance procedures for the Applied Materials 300mm Ultima Systems.
- Supported DUT windows OS host systems, setup, operation, updates and hardware repair.
- Implemented a Perl based web page for ETEST critical success indicators saving $500K daily and reduced factory downtime.
- Completed static analysis of McAfee Central windows 8 application using sharplinter command line tool and python script.
- Programmed motion controls systems and the associated interfacing with PLCs and HMI, simulation, testing and start-up.
- Executed the LEAN-Sigma optimization and downtime avoidance of 77% ETO sterilization process.
- Coordinated management reviews and third party audits (FDA, ISO, etc.)
- Reviewed and audited installation (IQ), operational (OQ), and procedural qualifications (PQ).
- Led Six Sigma projects to improve business processes.
- Performed first articles and implemented inspection procedures for new products/transfer components to MPROC, equipments and tool calibrations.
5. Highlight quality manager certifications on your resume
Specific quality manager certifications can be a powerful tool to show employers you've developed the appropriate skills.
If you have any of these certifications, make sure to put them on your quality manager resume:
- Certified Quality Engineer (CQE)
- Six Sigma Green Belt
- Certified Quality Auditor (CQA)
- Master Quality Manager (MQM)
- Lead Auditor
- Certified Manager of Quality/Organizational Excellence (CMQ/OE)
- NSF-IFSEA HACCP Manager (HACCP)
- Certified Manager Certification (CM)
- Certified Quality Technician (CQT)
- Certified Welding Inspector (CWI)
6. Finally, add an quality manager resume summary or objective statement
A resume summary statement consists of 1-3 sentences at the top of your quality manager resume that quickly summarizes who you are and what you have to offer. The summary statement should include your job title, years of experience (if it's 3+), and an impressive accomplishment, if you have space for it.
Remember to emphasize skills and experiences that feature in the job description.
Common quality manager resume skills
- Continuous Improvement
- Corrective Action
- Quality Standards
- Product Quality
- Quality Management System
- Customer Service
- Quality Issues
- Lean Six Sigma
- Process Improvement
- Quality Procedures
- Customer Complaints
- Supplier Quality
- Process Control
- Customer Satisfaction
- Lean Manufacturing
- Quality Audits
- Quality Metrics
- Internal Audit
- Data Analysis
- External Audits
- Quality Program
- Excellent Interpersonal
- Regulatory Compliance
- Direct Reports
- Preventive Actions
- Pharmaceutical Industry
- Customer Audits
- Product Specifications
- Various Training
Quality Manager Jobs
Links to help optimize your quality manager resume.
- How To Write A Resume
- List Of Skills For Your Resume
- How To Write A Resume Summary Statement
- Action Words For Your Resume
- How To List References On Your Resume
Quality Manager resume FAQs
How do you write a quality manager resume, what is the role of a qa manager, what should be on a manager's resume, search for quality manager jobs.
Updated April 25, 2024
Editorial Staff
The Zippia Research Team has spent countless hours reviewing resumes, job postings, and government data to determine what goes into getting a job in each phase of life. Professional writers and data scientists comprise the Zippia Research Team.
Quality Manager Related Resumes
- Auditor/Quality Resume
- Director Of Quality Resume
- Laboratory Manager Resume
- Manager, Quality Engineer Resume
- Quality Assurance Manager Resume
- Quality Assurance Supervisor Resume
- Quality Consultant Resume
- Quality Control Engineer Resume
- Quality Control Manager Resume
- Quality Coordinator Resume
- Quality Engineer Resume
- Quality Lead Resume
- Quality Specialist Resume
- Quality Supervisor Resume
- Quality Technician Resume
Quality Manager Related Careers
- Auditor/Quality
- Director Of Quality
- Laboratory Manager
- Lead Quality Control
- Manager, Quality Engineer
- Quality Assurance Director
- Quality Assurance Manager
- Quality Assurance Supervisor
- Quality Consultant
- Quality Control Engineer
- Quality Control Manager
- Quality Control Supervisor
- Quality Coordinator
- Quality Engineer
- Quality Lead
Quality Manager Related Jobs
Quality manager jobs by location.
- Quality Manager Aliso Viejo
- Quality Manager Chicago
- Quality Manager Colorado Springs
- Quality Manager Cumberland
- Quality Manager Elizabethtown
- Quality Manager Fairfield
- Quality Manager Glendale
- Quality Manager Kettering
- Quality Manager Milwaukee
- Quality Manager Norwalk
- Quality Manager Philadelphia
- Quality Manager San Gabriel
- Quality Manager Spanish Lake
- Quality Manager Sunrise
- Quality Manager Upper Chichester
- Zippia Careers
- Executive Management Industry
- Quality Manager
- Quality Manager Resume
Browse executive management jobs
Quality Manager Resume Samples
The role of a quality manager is concerned with monitoring and supervising the performance of the quality management systems , measuring against set standards and producing data on performance. A well-crafted Quality Manager Resume includes the following tasks – devising and establishing quality procedures, reviewing customer requirements, establishing quality requirements, setting standards for quality, defining quality procedures , monitoring performance, supervising employees use relevant quality tools and making suggestions for improvement.
To successfully execute these tasks, the quality managers are supposed to exhibit a keen eye for details, have outstanding communication skills and be an excellent leader. These experts should have a deep understanding of quality control procedures and be aware of the latest legal standards. While a degree in the field of science may be considered sufficient to enter into this profession, holding a QC certification would prove to be advantageous.

- Resume Samples
- Quality Manager
Quality Manager Resume
Summary : Tested and proven Assistant Quality Manager, with multiple certifications and vast knowledge of process improvement possibilities, ranging from Lean, Continuous Improvement, and Six Sigma. Well versed in Quality systems and auditing from ISO to AS9100. To obtain employment with a growth-oriented business, which will engage my past and present capabilities and for the development of additional skills leading to further advancement opportunities.
Skills : Coaching, Quality Management, Quality Control, Organizational Development, Leadership Training, Auditing

Description :
- Responsible for planning, directing, and coordinating quality activities for all inspection personnel.
- Ensuring that the repair and overhaul of all articles are being conducted in accordance with all regulatory and customer requirements and that the work is inspected and returned to service by a qualified inspector.
- Ensuring that current versions of the required technical data are always available and used for the overhaul or repair of every article where maintenance has been performed by maintenance personnel.
- Administering the precision tool and test equipment calibration program which requires the performance of periodic checks and calibrations on all new and existing precision tools and equipment as well as the maintenance of current records detailing these checks/calibrations.
- Evaluating and maintaining the annual internal and external evaluation and surveillance programs and the subsequent evaluation plan and checklists necessary for assessing the implemented policies.
- Maintaining a current list of approved suppliers through which the repair station and PMA manufacturing departments may purchase aeronautical products and/or direct use material.
- Managing a list of maintenance providers (both faa certified and noncertified), for the performance of repairs, maintenance, modification, and/or overhaul of aviation articles.
- Managing existing and ongoing employee training while maintaining current records that verify individual participation.
Software Quality Manager Resume
Objective : Motivated Software Quality Manager, seeking an opportunity to utilize my skills and experience to benefit the right organization. Proven leader with the ability to motivate people to meet objectives, exceed expectations and deliver results. To obtain a continuous improvement management position focused on quality and continuous improvement initiatives that will utilize my engineering education, supervisory experience, Six Sigma knowledge, and diverse technical background.
Skills : Word, Access, Excel, PowerPoint, Outlook, Instructor, Trainer, Microsoft Office, QI Macros And Minitab

- Supporting customer and end item users with technical issues and concerns and promptly performing preventative and corrective action.
- Designing, administering and auditing conformance of the QA system to AS9100/AS9102FAI/AS9110 and Nadcap requirements.
- Leading the implementation of proactive quality management practices in all functional areas, spanning marketing and product development, manufacturing, and test.
- Teaching and facilitating the implementation of the tools of quality to the broader organization and supply chain.
- Possessing working knowledge of 8d and root cause problem-solving methodology.
- Providing reports depicting the quality levels, quality problems, and cost of quality.
- Driving quality improvement with through more effective launch of new programs.
- Providing quality reports to customers as required, and ensures frequent, effective communication with customers on all corrective action plans.
Data And Quality Manager Resume
Summary : Analytical, business-minded Data And Quality Manager with extensive experience planning and improving manufacturing operations within fast-paced automotive and aerospace industries. Innovative strategist, skilled in developing and implementing continuous improvement initiatives aimed at cutting costs, bolstering productivity and supporting the goal of operational excellence. Highly-skilled quality assurance supervisor with a proven track record of consistently improving manufacturing processes.
Skills : Microsoft Office Applications, Mini-tab, Auto-cad, Uni Graphics, Lean Manufacturing, Automotive Quality Experience, Automotive OEM, And Tier 1 Experience.

- Created a corrective action policy to reduce the number of technical support cases resulting from internal issues.
- Led teams through the process of root-cause analysis and corrective action methods and reduced the number of tech support cases by 20% in a 3 month period.
- Developed a system to address the alarming number of parts replaced under warranty, many of which were never returned and the cause of the failures was not investigated.
- The system included failure analysis reports that enabled a feedback loop to all relevant internal and external stakeholders.
- As a result, returns were reduced by greater than 30% in less than 4 months which, in turn, significantly reduced man-hours and warranty costs.
- Focused on a modular design in software and hardware for strategic reuse and scalability.
- Also, led continuous improvement efforts which improved test accuracy by 20% and reduced test time by 80%.
- Created and executed schedule, cost, capacity, scalability, risk analysis, and product qualification plan to successfully transfer more 10 + new products into full-scale production, on schedule, complete with documentation, test hardware/software.
Operations Manager/Quality Manager Resume
Summary : Motivated, personable Operations Manager/ Quality Manager - with extensive experience in a number of different high-tech manufacturing environments (machining, EDM, metal stamping, welding, and assembly). Talent for being able to think out-of-the-box to develop solutions to complex problems. Diplomatic and tactful with professionals and manufacturing personnel at all levels of the organization. Flexible and versatile - able to maintain control and focus under pressure. Poised and competent, with demonstrated ability to easily transcend cultural differences.
Skills : MS Office, Leadership, Problem Solving, Lean Manufacturing, Quality Circle Leader, Six Sigma Black Belt

- Driving, leading and troubleshooting quality investigations to ensure issues are thoroughly investigated with appropriate caps.
- Effectively interacting with production and development teams to maintain product supply and help introduce new products.
- Working with purchasing staff to establish quality requirements from external suppliers.
- Bringing together the staff of different disciplines and driving the group to plan, formulate and agree on comprehensive quality procedures.
- Persuading reluctant staff to change their way of working to incorporate quality methods.
- Managing raw material testing, in-process testing and finished producing and release procedures.
- Responsible for the creation, issuance, tracking, review, approval and/or control of labeling materials as per 21 CFR 101 regulations.
- Responsible for the overall health of the quality department through technical guidance, employee development, and actively collaborating with production.
- Upgrading and/creation of quality plans for legacy and new products.
Regional Quality Manager Resume
Summary : Accomplished Regional Quality Manager with extensive experience leading results-driven training, project management, product testing, and quality assurance. Proven ability to enhance workflow and standardize effective project management tools and systems. To bring to an established corporation: enthusiasm, dedication, responsibility, and excellent work ethic, combined with a desire to utilize the skills obtained through my experience and education.
Skills : Microsoft Office, Six Sigma Green Belt, AS9100 Internal Auditor, SAP User, Lean Improvements, Cycle Time Reduction

- Managing and maintaining the quality management system in relation to AS9100C and ISO 9000/2008.
- Working one-on-one with AS9100C lead certification auditors to supply any quality documentation needed during recertification and surveillance audits.
- Writing non-conformance documents, corrective action reports, and preventive action report to continually improve companywide quality.
- Utilizing lean six sigma principles to investigate non-conformances/ identify root causes.
- Revising and maintaining process maps, procedures, and any other quality documents to reflect processes as they are carried out, as well as in relation to AS9100C's standard for quality management systems and any other customer specific requirements.
- Writing a new company employee safety manual and forklift safety manual create a FOD training program and an AS9100C training program for familiarizing and training employees with these standards and regulations.
- Tracking shipment summary and jobs returned data to create reports to detect potential problem areas and better improve delivery times.
- Responsible for forklift safety training and ensuring all facility safety regulations are observed.
Corporate Quality Manager Resume
Headline : Corporate Quality Manager with 5 years of progressive experience in a broad range of technical precision, manufacturing, and developmental growth system environments. Proven ability to combine vision creativity and strong business acumen in order to develop lean, well-documented quality management leadership programs compliant with Sigma Six Lean, ISO 9001 and API Q1.
Skills : HACCP, FDA Food Defense, ISO, As400, Microsoft Office, Forklift Operator, SQF Implementation

- Brought on board to develop and implement a corporate-wide API spec Q1, spec 11AX & 5B program.
- Transitioned all existing recordkeeping systems from manual tracking to a digital record keeping information system.
- Represented management during all QSI preliminary setup reviews and compliance audits.
- Streamlined operational processes management including ordering, inventory, manufacturing, calibration tracking, and quality assurance methodologies reducing loss and NCP production to cycling target guidelines.
- Integrated automated digital barcode system for our calibrated precision instrument distribution.
- By connecting tooling to the employee and the workstation, shrinkage tracking and timely calibration processes were implemented.
- Developed innovative product line management processes that expedited barrel honing and quality staging prior to coating.
- Performed corporate acquisition conversion to concurrent API practices including machine maintenance scheduling, precision tooling calibration, product line sorting, critical designator print review and floor plan layout.
- Reviewed and oversaw internship program to correct print layout including the addition of API spec callouts, critical designators, non-compliant tolerances and inserting proper logo, revision creation, review, and approval formatting to meet Q1 requirement.
Industrial Quality Manager Resume
Summary : Proactive and innovative Industrial Quality Manager and problem-solver with over 10 years of experience in manufacturing, operations and quality management. Accomplished communicator with proven experience in dispute resolution and maintaining peaceful, productive and dynamic business environments.
Skills : leadership And Development, Creative Problem Solving / Decision-making, Process Improvement / Organizational Development, Resource Allocation And Management, Client Education / Training And Presentation Efficiency

- Ensured an effective ISO 9001:2008 Quality Management System (QMS) process was established and implemented, to recommend corrective and preventative action where necessary to ensure its effective implementation and improvement.
- Effectively managed and coordinated all quality management activities and staff to deliver the scope of work in line with all project expectations.
- Liaised with clients on all quality related issues during design, procurement, fabrication, construction and commissioning matters.
- Monitored and controlled project quality performance against quality requirements using appropriate control tools and report same to management, clients and project management team.
- Prepared, issued and revised the contract quality plan, inspection/verification strategy, and compliance strategies.
- Established a team of competent and experienced Quality Management (QM) discipline engineers to meet the scope of work and specify contract quality requirements.
- Ensured that contract personnel was provided with the tools and knowledge required to execute the contract/project with legislation, procedures, and compliance.
- Convened and attended meetings concerning project quality management matters as appropriate.
Operations And Quality Manager Resume
Objective : High-energy Operations And Quality Manager successful in building and motivating dynamic teams. Cultivates a company culture in which staff members feel comfortable voicing questions and concerns, as well as contributing new ideas that drive company growth. A full-time position, using my vast knowledge of Quality and Technical systems gained over many years of work in the automotive and technical industries.
Skills : Microsoft Office, Develop Quality Systems, Start-Ups, Document Control, Continuous Improvement, Customer Service, Statistical Process Control, Root Cause Analysis

- Responsible for developing, maintaining, and reporting departmental goals for quality and continual improvement.
- Participate in group, team, and departmental meetings to collect, analyze, and act on issues to improve facility performance.
- Planned, coordinated, and directed the quality management system designed to ensure continuous production consistent with established standards.
- Built, maintained, and promoted a quality driven culture within the QMS and through regularly scheduled meetings held with plant employees.
- Developed, initiated, and performed corrective actions ranging from external complaints, rejects from customers.
- Using tools such as 6 panel, fishbone, deep dive root cause analysis, 8d corrective actions, as well as executing and training on these areas.
- Performed and managed process audit schedule for the facility utilizing the layered audit process.
- Maintained MSA data and calibration system of all inspection tools used by the facility.
Maintenance Quality Manager Resume
Summary : Senior Quality Engineer position supporting a manufacturing organization through process analysis and system implementation to solve problems resulting in significant cost savings. Energetic and hands-on Quality Management and Operations leader with over ten years of experience in manufacturing, quality, software programs, and supplier management. Proven ability to lead, mentor and motivate team members in order to maximize performance.
Skills : Quality Engineering, Team Problem Solving, Training & Facilitation, Reliability Engineering, Customer Service, Advanced Quality Planning, Quality Auditing, Continuous Improvement, Lead Time Reduction, System Development, Supplier Development, New Product Launches

- Responsible for implementing and maintaining the Alcoa business system (modeled after the Toyota business system) continuous improvement process for this location of alcoa's building and construction division.
- Training and coaching all levels of employees in problem-solving and corrective action reporting.
- Facilitating quality Kaizen events quarterly to identify opportunities for improving current processes and soliciting all levels of team member involvement in brainstorming, analysis and improvement execution.
- Facilitating "take time out for problem-solving" events weekly using 5-why and 8-discipline methods to assist team members in determining root cause, developing corrective & preventive action plans and ensuring execution/follow-through.
- Initiated level 3 "flash" communication meetings combining verbal & visual tracking daily management where all departments come together & report out the key measurable status, identify daily constraints & present problem-solving results all in 15 minutes total.
- Managing quality control department with 4 direct salaried reports and 8 hourly technicians.
- Resolving customer claim issues, vendor claim issues, manage change control process and oversee operational excellence process auditing to ensure quality system compliance.
- Developing & executing annual departmental operational plans in concert with business plan & track performance objectives.
- Coordinatiing development of process FMEAs & control plans with cross-functional teams for existing processes without and proposed new processes.
Safety Manager-Quality Manager Resume
Summary : A proven Safety Manager-Quality Manager who is rich with diverse experience. Enjoys challenges, teamwork, and continuous improvement. Results-oriented Quality Assurance Manager with expertise in root cause analysis and implementation of targeted process and personnel improvements to reduce the overall cost of quality while maintaining or improving production efficiency. Seeking a challenging and rewarding position in quality assurance, engineering, or process improvement utilizing proven technical expertise, quality process knowledge, and procedure analysis.
Skills : Quality Management, Project Management, CAD Engineering Tools, Electrical System Design, Continual Improvements, Metrics Reporting, Process Engineering

- Developing, implementing and managing quality management system to ISO 9001:2008 certification.
- Responsible for continuous improvements, value mapping, inspection & testing, preventative and corrective actions, root cause analysis, solutions implementation & follow up.
- Providing excellent customer service handling customer concerns and quality action plans.
- Conducting management reviews, internal audits, process audits, and product audits.
- Authoring and standardizing checklists, process documents, and leading continuous improvement activities for engineering and manufacturing.
- Managing subcontractor quality and work processes and quality via audits, data analysis, communications, & metrics reporting.
- Leading diverse workgroups and teams through process and product improvement initiatives.
- Designing technical project overviews, system designs, and control charts.
- Constructing an intranet website providing accessibility to reports, tools, processes, and reference documents.
- Programming PLC equipment, application design verification testing, and control panel testing.
Table of Contents
Recent posts, download this pdf template., creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option., unlock the power of over 10,000 resume samples., take your job search to the next level with our extensive collection of 10,000+ resume samples. find inspiration for your own resume and gain a competitive edge in your job search., get hired faster with resume assistant., make your resume shine with our resume assistant. you'll receive a real-time score as you edit, helping you to optimize your skills, experience, and achievements for the role you want., get noticed with resume templates that beat the ats., get past the resume screeners with ease using our optimized templates. our professional designs are tailored to beat the ats and help you land your dream job..
Quality Manager Resume Examples
Writing a great quality manager resume is important because it is one of the first things a potential employer will see when they are considering you for a position. It is your opportunity to make a good first impression and sell yourself as the best candidate for the job.
Create your resume Select from 7 professional resume templates
If you're looking for inspiration when it comes to drafting your own quality manager resume, look no further than the samples below. These resumes will help you highlight your experience and qualifications in the most effective way possible, giving you the best chance of landing the quality manager job you're after.

or download as PDF
Essential Components of an Effective Quality Manager Resume
An impactful Quality Manager resume is a critical tool for showcasing your expertise in quality assurance and control. It should highlight your proficiency in developing, implementing, and maintaining quality management systems within an organization.
Your resume must demonstrate your ability to identify issues, conduct audits, and implement improvements to ensure exceptional product or service quality. In the following sections, we will delve into the various elements of a Quality Manager's resume, discussing the significance of each and offering guidance on how to optimize them.
1. Contact Information
The first section of your resume should list your contact information , which is essential for employers to connect with you. This includes your name, phone number, professional email address, and your location (city and state).

Ensure your contact details are accurate and up-to-date. A professional email address that incorporates your name or initials is recommended. Refrain from using casual or unprofessional email addresses.
Indicating your location is helpful, but a full address is unnecessary; city and state suffice. If relevant, include your LinkedIn profile or personal website, ensuring they are current and professionally presented.
Double-check this section for accuracy to facilitate smooth communication with potential employers.
- Phone Number
- Professional Email Address
- City and State
- LinkedIn Profile (optional)
- Personal Website (optional)
2. Objective Statement
The objective statement is a concise and compelling introduction at the top of your resume. It should succinctly convey your career goals and your suitability for the Quality Manager position.
An effective objective statement for a Quality Manager might read: "Experienced Quality Manager seeking a challenging role to leverage my five-year track record in establishing quality control systems and improving operational efficiency. Keen to contribute my strong leadership skills and commitment to product excellence to XYZ Company."
Customize your objective statement for each application to reflect your genuine interest in the role and alignment with the company's goals.
- Highlight your relevant skills, experiences, and aspirations in quality management.
- Be concise yet impactful to capture the attention of hiring managers.
- Illustrate how your qualifications align with the company's needs and your potential impact on their operations.
- Personalize your objective statement for each job application.
- Ensure your career objectives resonate with the company's vision.
Related : Top Quality Manager Resume Objective Examples
3. Work Experience
The work experience section is where you chronicle your professional journey, emphasizing your responsibilities and achievements in previous quality management roles. This section can significantly enhance your chances of securing an interview.
Focus on positions relevant to the Quality Manager role, starting with your most recent job. Include the company name, location, dates of employment, and your job title.
- Use bullet points to detail your responsibilities and accomplishments, employing action verbs and quantifiable results to demonstrate your impact. For example, instead of "Managed product quality," you could write, " Implemented a new quality control system, reducing product defects by 20% ."
Highlight skills such as quality assurance , process improvement , regulatory compliance , team leadership , and project management in relation to your past roles.
Showcase the diversity of your experience if you've worked across various sectors or managed quality for different products or services, as this can indicate adaptability.
Include significant projects or initiatives where you played a key role, especially if they led to cost savings, efficiency improvements, or increased customer satisfaction.
Your work experience section should not only summarize your career but also clearly demonstrate your application of essential Quality Manager skills in various contexts.
4. Skills and Competencies
The "Skills and Competencies" section is a snapshot of your professional capabilities that qualify you for the Quality Manager position. It allows employers to quickly assess your potential contributions to their organization.
- Technical Skills: Proficiency in quality assurance systems, statistical analysis, process improvement methodologies, and industry-specific software is essential. Knowledge of quality standards such as ISO 9001 is also crucial.
- Analytical Skills: The ability to identify issues in workflows and develop effective solutions is key. Quality Managers should be adept at interpreting complex data and quality reports.
- Leadership Skills: Effective team management, decision-making, conflict resolution, delegation, and motivational skills are necessary for leading quality initiatives.
- Communication Skills: Clear verbal and written communication is vital for articulating quality-related information to both technical and non-technical stakeholders.
- Attention to Detail: Precision is paramount in quality management, as minor oversights can lead to significant issues.
- Problem-Solving Skills: The role demands quick and critical thinking to identify and rectify process or product deficiencies.
- Project Management Skills: Familiarity with project management principles is beneficial for overseeing specific quality-related projects within an organization's strategy.
- Knowledge of Regulatory Standards: Understanding regulatory requirements ensures compliance with product safety and efficacy laws.
- Customer Service Orientation: A customer-centric approach aids in understanding consumer needs and enhancing product quality to meet expectations.
Provide examples in your resume's experience section to illustrate how you've applied these skills in practice.
Related : Quality Manager Skills: Definition and Examples
5. Educational Background
Your educational background is a critical element of your Quality Manager resume, offering insight into your academic qualifications. A bachelor's degree in Business Administration, Industrial Engineering, Quality Assurance, or a related field is typically required.
Advanced degrees such as an MBA or MQM may be preferred for certain positions, indicating a deep commitment to the field and advanced business acumen.
Certifications from recognized organizations like the American Society for Quality (ASQ) can distinguish you from other candidates. Credentials such as the Certified Manager of Quality/Organizational Excellence (CMQ/OE) or Certified Quality Engineer (CQE) are highly valued.
Include relevant coursework or projects, such as Total Quality Management (TQM), Lean Manufacturing, Six Sigma, ISO standards, and Risk Management, which are attractive to employers.
- Total Quality Management (TQM)
- Lean Manufacturing
- ISO standards
- Risk Management
List your educational achievements in reverse chronological order, providing the institution's name, degree obtained, and years attended. This section should be clear and informative, reflecting your academic suitability for a Quality Manager role.
6. Certifications and Training in Quality Management
Including Quality Management certifications and training on your resume can set you apart from other candidates by demonstrating your commitment to continuous professional development.
These credentials validate your expertise in various quality management areas, showing that you have undergone rigorous training and passed examinations. Prominent certifications for Quality Managers include the Certified Manager of Quality/Organizational Excellence (CMQ/OE), Certified Quality Auditor (CQA), and Certified Six Sigma Black Belt.
- The CMQ/OE certification denotes comprehensive knowledge in leadership and strategic planning.
- The CQA certification reflects proficiency in auditing practices.
- A Six Sigma Black Belt certification indicates expertise in business process improvement through statistical analysis.
Relevant training courses also enhance your profile, from specific quality control software training to broader topics like Lean Manufacturing or TQM. List any pertinent courses you've completed, along with the competencies acquired.
Adding certifications and training to your resume underscores your qualifications and your dedication to keeping abreast of industry standards and best practices. Include the name of the certification or course, the issuing organization, and the completion date.
Emphasizing your certifications and training in quality management can significantly elevate your credibility as a candidate, showcasing your skill set, commitment to lifelong learning, and preparedness to contribute to an organization's quality assurance objectives.
Related : Quality Manager Certifications
7. Achievements and Awards in Quality Management
Including notable achievements and awards in quality management on your resume can be a powerful testament to your skills and expertise. These distinctions provide tangible proof of your accomplishments and can instill confidence in potential employers.
Achievements may encompass successful projects you've led, process improvements you've initiated, cost savings from efficiency measures, or recognition from industry organizations. For instance, implementing a quality control system that reduced product defects by 20% is a noteworthy accomplishment.
Awards may be internal company recognitions or honors from industry associations or independent bodies. If you've received accolades such as 'Quality Manager of the Year' or 'Best Innovation' for a project that enhanced production quality, be sure to highlight them.
Describe each award or achievement succinctly, detailing the reason for the recognition and its impact on the company. Quantify your achievements where possible to strengthen your case.
Ensure that all achievements and awards listed are relevant to quality management roles, helping employers envision how your past successes could translate to their organization.
Keep this section updated with recent awards and achievements to accurately reflect your current capabilities and successes.
Listing awards and achievements is not about boasting; it's about demonstrating your potential effectiveness as a Quality Manager and giving employers a preview of the value you can bring to their team.
Related Resume Examples
- Supplier Quality Manager
- Quality Assurance Manager
- Quality Control Manager
- Quality Systems Manager
- Quality Assurance Analyst
- Quality Assurance Assistant
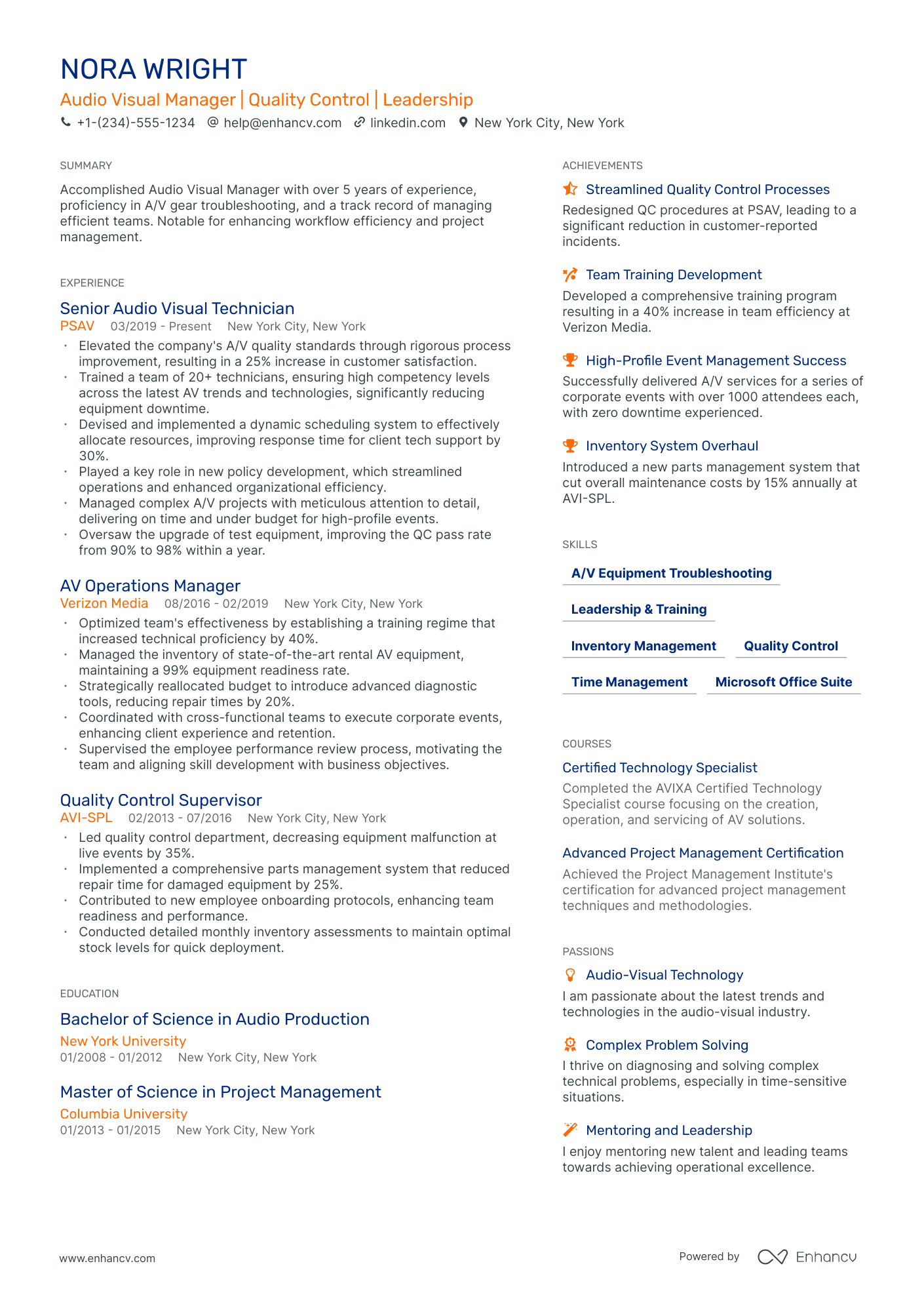
- • Elevated the company's A/V quality standards through rigorous process improvement, resulting in a 25% increase in customer satisfaction.
- • Trained a team of 20+ technicians, ensuring high competency levels across the latest AV trends and technologies, significantly reducing equipment downtime.
- • Devised and implemented a dynamic scheduling system to effectively allocate resources, improving response time for client tech support by 30%.
- • Played a key role in new policy development, which streamlined operations and enhanced organizational efficiency.
- • Managed complex A/V projects with meticulous attention to detail, delivering on time and under budget for high-profile events.
- • Oversaw the upgrade of test equipment, improving the QC pass rate from 90% to 98% within a year.
- • Optimized team's effectiveness by establishing a training regime that increased technical proficiency by 40%.
- • Managed the inventory of state-of-the-art rental AV equipment, maintaining a 99% equipment readiness rate.
- • Strategically reallocated budget to introduce advanced diagnostic tools, reducing repair times by 20%.
- • Coordinated with cross-functional teams to execute corporate events, enhancing client experience and retention.
- • Supervised the employee performance review process, motivating the team and aligning skill development with business objectives.
- • Led quality control department, decreasing equipment malfunction at live events by 35%.
- • Implemented a comprehensive parts management system that reduced repair time for damaged equipment by 25%.
- • Contributed to new employee onboarding protocols, enhancing team readiness and performance.
- • Conducted detailed monthly inventory assessments to maintain optimal stock levels for quick deployment.
5 Quality Control Manager Resume Examples & Guide for 2024
Your quality control manager resume must highlight your meticulous attention to detail. Demonstrate a proven track record in identifying and rectifying process deviations. Additionally, showcase your strong analytical skills. They are crucial in interpreting data to make informed decisions that uphold quality standards.
All resume examples in this guide

Traditional
Resume Guide
Resume Format Tips
Resume Experience
Skills on Resume
Education & Certifications
Resume Summary Tips
Additional Resume Sections
Key Takeaways

As a quality control manager, you might find it challenging to succinctly showcase your extensive experience in process improvement and regulatory compliance on your resume. Our guide will provide you with targeted advice to effectively highlight your expertise and achievements, ensuring your resume stands out to potential employers.
- Which sections do you need to include in your resume to meet recruiters' requirements;
- How to write your quality control manager resume experience section - even if you have don't have little to no work experience;
- Real-life professional examples to guide you how to write the most important quality control manager resume sections;
- Adding even more sections so your quality control manager resume stands out with professionalism and your personality.
We've also selected some of the best (and most relevant) resume guides for the quality control manager role you're applying for:
- Quality Assurance Engineer Resume Example
- Quality Assurance Officer Resume Example
- Quality Consultant Resume Example
- Release Manager Resume Example
- Quality Technician Resume Example
- Quality Assurance Analyst Resume Example
- Quality Specialist Resume Example
- Quality Assurance Associate Resume Example
- Data Quality Analyst Resume Example
- Quality Manager Resume Example
The importance of format and layout in your quality control manager resume
Achieve this balance by:
- Listing your experience, beginning with the most recent and relevant , in reverse chronological order;
- Ensuring your header contains essential information, such as contact details , a headline, and a portfolio link. Include a professional photo in the quality control manager resume header if you have one;
- Including only the most important and relevant resume sections to showcase your expertise and stand out from other candidates;
- Editing your quality control manager resume to be no longer than two pages if you have extensive relevant experience. Use your limited resume space judiciously.
Also, remember that your quality control manager resume might initially be scanned by an Applicant Tracker System (ATS).
When it comes to ATS:
- Opt for simple and legible fonts like Raleway, Rubik, Lato, etc., making your experience easy for the ATS to scan;
- Use serif and sans-serif fonts, both of which are ATS-friendly;
- Avoid overused options like Arial and Times New Roman, which, while suitable, may lack personality.
Contrary to a common myth, our recent study shows that the ATS can effectively process both one-column and two-column resumes. Learn more about this in the ATS myths guide .
Finally, when submitting your quality control manager resume, always export it as a PDF to ensure all information remains intact, making the document easier to print, read, and scan.
Upload & Check Your Resume
Drop your resume here or choose a file . PDF & DOCX only. Max 2MB file size.
If you failed to obtain one of the certificates, as listed in the requirements, but decide to include it on your resume, make sure to include a note somewhere that you have the "relevant training, but are planning to re-take the exams". Support this statement with the actual date you're planning to be re-examined. Always be honest on your resume.
Recruiters' preferred quality control manager resume sections:
- A header with relevant contact information and headline, listing your current job title
- A resume summary or objective pinpointing what is most impressive about your expertise (that aligns with the role)
- An experience section highlighting the specifics of your responsibilities and achievements
- A skills sidebar to intertwine job advert keywords with your unique talents
- An education and certifications sections to serve as further accreditation to your professional experience
What recruiters want to see on your resume:
- Proven experience in implementing and monitoring quality control systems and standards in a manufacturing or production environment.
- In-depth knowledge of relevant quality control tools and methodologies such as Six Sigma, lean manufacturing, or total quality management (TQM).
- Strong analytical skills with a detailed-oriented mindset, able to analyze data, understand trends, and make data-driven decisions to improve product quality.
- Experience with regulatory compliance and certifications, showing understanding of industry standards like ISO 9001, FDA, etc., relevant to the sector.
- Leadership experience demonstrating the ability to manage and develop a quality control team, including training employees on quality standards and processes.
Defining your professional expertise in your quality control manager resume work experience section
The work experience section, often the most detailed part of your resume , is where you discuss your past roles and achievements. To effectively list your experience, consider these four key tips:
- Align your expertise with the job requirements. It's vital to integrate keywords matching the job criteria to pass initial assessments;
- Show, don’t just tell. Quantify your responsibilities by stating your actual achievements in previous roles;
- Include measurable metrics. For instance, how did your performance impact the annual ROI?
- Highlight crucial industry skills. Mention both technological knowledge and interpersonal skills in this section.
These guidelines will help you craft an impressive quality control manager resume work experience section that is bound to catch recruiters' attention.
- Developed and executed strategic plans for quality control in alignment with company objectives, leading to a 20% increase in overall product quality year-over-year.
- Managed a team of 15 quality control analysts, providing continuous training and development that resulted in a 30% reduction in error rate.
- Pioneered the use of advanced statistical analysis tools for monitoring product quality, which significantly improved detection of defects and non-conformance issues.
- Orchestrated the revision and enhancement of standard operating procedures, which contributed to a 25% improvement in compliance with regulatory standards.
- Led the successful certification process for ISO 9001:2015, resulting in an improved market reputation and trust from clients.
- Spearheaded a successful cross-functional team initiative to standardize quality control processes across multiple manufacturing sites.
- Implemented a company-wide quality management system, cutting costs by 15% through the reduction of waste and non-value-added activities.
- Directed and improved the supplier quality management program, which led to higher quality raw materials and components with a 20% decrease in supplier defects.
- Initiated and supervised a product quality improvement project that enhanced customer satisfaction ratings by 35%.
- Introduced a real-time quality monitoring system that reduced production downtime by 40% due to quicker response to quality deviations.
- Managed and optimized product testing protocols, improving testing efficiency by 50%, while maintaining rigorous quality standards.
- Collaborated with R&D to incorporate quality by design principles in new product development, ensuring quality objectives were met prior to launch.
- Cultivated a culture of continuous improvement that led to a sustained reduction in customer complaints by an average of 20% annually.
- Implemented lean manufacturing techniques that resulted in a 15% faster quality check process without compromising the precision of inspections.
- Negotiated and established lasting relationships with external quality assurance consultants to enhance the in-house team's expertise.
- Led the quality control team through a significant regulatory audit, with zero major findings for the first time in company history.
- Drove the automation of quality control processes, reducing manual processing time by 60% and improving data integrity for regulatory reporting.
- Championed a collaborative approach with the product development teams to infuse quality perspectives early in the design phase, leading to a reduction in redesign cycles.
- Carried out comprehensive risk assessments that identified critical control points, decreasing the likelihood of quality incidents by 25%.
- Coordinated with international manufacturing partners to align quality standards, facilitating a smoother global supply chain operation.
- Advanced employee quality awareness programs that increased staff competency in quality control measures and standards.
- Expertly managed the quality control lifecycle of over 300 products, ensuring all items met or exceeded stringent quality benchmarks.
- Implemented a scalable quality management system to support a 50% increase in production output while maintaining defect rates below 0.5%.
- Conducted targeted training programs that led to a highly skilled quality control workforce capable of adapting to evolving industry standards.
Quantifying impact on your resume
- Quantify the number of quality control checks completed per month, demonstrating efficiency and thoroughness.
- Include the percentage decrease of defective products under your management to show your effectiveness in reducing errors.
- State the amount of cost savings achieved through quality control improvements to highlight your financial impact.
- List the number of quality assurance policies you've developed or revised, indicating your proactive approach to quality management.
- Mention any increase in customer satisfaction scores related to product quality to reflect customer-centric outcomes.
- Specify the number of team members you've managed or trained in quality control practices to show leadership and mentorship.
- Detail the number of audits conducted and the subsequent compliance rates to underscore your commitment to standards.
- Present the percentage improvement of process cycle times as a result of your quality control initiatives to demonstrate efficiency gains.
Action verbs for your quality control manager resume
What if my quality control manager experience doesn't match the requirements?
You've just graduated from college and may have no real world job experience . What should you include within your resume then?
Instead of making up information or adding irrelevant past jobs (e.g. your on-campus work during freshman year), you can:
- Shift the focus from your professional experience to your community impact with your volunteer work. This would showcase numerous soft skills you've built over time (e.g. interpersonal communication);
- Highlight the projects you've completed, as part of your coursework, or, on your own. Thus, you will align your technical background with recruiters' requirements;
- Consider spotlighting your transferrable skills. Or, what lessons and talents your current professional and personal experience has taught you and how they could benefit your potential employers;
- Even if you've had a few months of internship experience, that is relevant for the role, make sure to include this. Recruiters do care about the years of experience you happen to have, but, at the end of the day, your profile would also be assessed based on role alignment.
Recommended reads:
- Should I Put In An Incomplete Degree On A Resume?
- How To List Certifications On A Resume (Examples Included)
In-demand hard skills and soft skills for your quality control manager resume
A vital element for any quality control manager resume is the presentation of your skill set.
Recruiters always take the time to assess your:
- Technological proficiency or hard skills - which software and technologies can you use and at what level?
- People/personal or soft skills - how apt are you at communicating your ideas across effectively? Are you resilient to change?
The ideal candidate presents the perfect balance of hard skills and soft skills all through the resume, but more particular within a dedicated skills section.
Building your quality control manager skills section, you should:
- List up to six skills that answer the requirements and are unique to your expertise.
- Include a soft skill (or two) that defines you as a person and professional - perhaps looking back on feedback you've received from previous managers, etc.
- Create up to two skills sections that are organized based on the types of skills you list (e.g. "technical skills", "soft skills", "quality control manager skills", etc.).
- If you happen to have technical certifications that are vital to the industry and really impressive, include their names within your skills section.
At times, it really is frustrating to think back on all the skills you possess and discover the best way to communicate them across.
We understand this challenge - that's why we've prepared two lists (of hard skills and soft skills) to help you build your next resume, quicker and more efficiently:
Top skills for your quality control manager resume:
Quality Assurance Methodologies
Statistical Process Control
ISO Standards Knowledge
Audit Planning and Execution
Regulatory Compliance
Data Analysis
Process Improvement Techniques
Quality Management Systems
Root Cause Analysis
Product Testing
Attention to Detail
Communication
Problem Solving
Decision Making
Time Management
Adaptability
Critical Thinking
Conflict Resolution
If you're in the process of obtaining your certificate or degree, list the expected date you're supposed to graduate or be certified.
How to include your education and certifications on your resume
We're taking you back to your college days with this part of our guide, but including your relevant higher education is quite important for your resume.
Your degree shows recruiters your dedication to the industry, your recent and relevant know-how, and some form of experience in the field.
Your quality control manager resume education should:
- Include your applicable degrees, college (-s) you've graduated from, as well as start and end dates of your higher education;
- Skip your high school diploma. If you still haven't graduated with your degree, list that your higher education is ongoing ;
- Feature any postgraduate diplomas in your resume header or summary - this is the perfect space to spotlight your relevant MBA degree ;
- Showcase any relevant coursework , if you happen to have less professional experience and think this would support your case in being the best candidate for the role.
As far as your job-specific certificates are concerned - choose up to several of the most recent ones that match the job profile, and include them in a dedicated section.
We've saved you some time by selecting the most prominent industry certificates below.
The top 5 certifications for your quality control manager resume:
- Certified Quality Manager/Organizational Excellence (CQM/OE) - American Society for Quality (ASQ)
- Certified Six Sigma Black Belt (CSSBB) - American Society for Quality (ASQ)
- Certified Quality Auditor (CQA) - American Society for Quality (ASQ)
- ISO 9001:2015 Certified Lead Auditor - Exemplar Global
- Lean Six Sigma Green Belt Certification (LSSGB) - International Association for Six Sigma Certification (IASSC)
If you happen to have plenty of certificates, select the ones that are most applicable and sought-after across the industry. Organize them by relevance to the role you're applying for.
- Perfecting the Education Section on Your Resume
- When Should You Include Your High School on Your Resume?
Your quality control manager resume top one third: choosing between a resume summary or an objective
The top third of your resume is crucial, as recruiters might focus only on this section rather than reading the entire document. Therefore, it's important to carefully decide whether to include a resume summary or an objective.
- The resume summary encapsulates your most significant experiences, key achievements, and skills in the field. Ideal for candidates with substantial relevant experience, the summary previews what recruiters will find in the rest of your resume.
- The resume objective outlines your professional aspirations. It describes your career goals for the coming years and how you envision your role evolving in the prospective company. The resume objective is suitable if you have less professional experience and wish to emphasize various soft skills such as motivation, vision, and planning.
Explore some of the best examples of resume summaries and objectives from real-life professional resumes in the industry.
Resume summaries for a quality control manager job
- With over 15 years of experience mastering the intricacies of quality control in the pharmaceutical sector, I have effectively implemented robust quality monitoring systems. Spearheaded an initiative that slashed defect rates by 30% within a year, my expertise in GMP regulations, risk assessment, and process optimization sets a high bar for excellence in product integrity and safety.
- As a software developer transitioning into quality control management, I bring acute analytical skills and a honed understanding of agile methodology. With 10 years under my belt coding and resolving complex system bugs, my career pivot is fueled by a drive to elevate product quality standards and bridge the gap between technical development and end-user satisfaction.
- Leading a team in the competitive aerospace sector, I leveraged my 12-year tenure to consistently surpass quality benchmarks and propel our company to achieve ISO 9001 certification. My ability to troubleshoot process bottlenecks and integrate cutting-edge inspection technologies led to a record-low 2% product return rate due to quality issues.
- A seasoned professional in automotive engineering, I am now channeling a decade's worth of project management and technical acumen into a quality control management role. My successes in launching innovative vehicle safety features and optimizing supply chain protocols underscore my commitment to uphold and amplify product excellence standards.
- Keen to transition into quality control management, my background in consumer electronics retail management has endowed me with a unique perspective on consumer expectations and product standards. With a relentless focus on customer satisfaction and a track record of managing a top-performing store, I'm set to apply my insights to enhance quality processes and outcomes.
- As a recent graduate with a degree in Biochemistry and a passion for pharmaceutical excellence, I am eager to embark on a career path in quality control management. Armed with internship experience and knowledge of FDA regulatory compliance, I am committed to contributing a fresh perspective and unwavering dedication to ensuring that products meet and exceed quality and safety standards.
Taking your quality control manager resume to the next level with these four additional resume sections
Your quality control manager resume can feature a variety of skills (both hard and soft) in diverse sections . Choose those that align best with the job requirements and reflect your suitability for the company culture.
Consider these four additional resume sections recommended by our experts:
- Languages - State any languages you are proficient in and your level of proficiency. This demonstrates your commitment to communication and potential for international growth.
- Projects - Highlight up to three significant projects you've completed outside of work, showcasing skill development. Include a link to your project portfolio in the quality control manager resume header, if applicable.
- My Time - How you allocate your time outside work can indicate your organizational skills and cultural fit within the company.
- Volunteering - Detail causes you're passionate about, roles you've held, and achievements in volunteering. Such experiences likely have honed a range of soft skills crucial for your dream job.
Key takeaways
At the end of our guide, we'd like to remind you to:
- Invest in a simple, modern resume design that is ATS friendly and keeps your experience organized and legible;
- Avoid just listing your responsibilities in your experience section, but rather focus on quantifiable achievements;
- Always select resume sections that are relevant to the role and can answer job requirements. Sometimes your volunteering experience could bring more value than irrelevant work experience;
- Balance your technical background with your personality traits across various sections of your resume to hint at how much time employers would have to invest in training you and if your profile would be a good cultural fit to the organization;
- Include your academic background (in the form of your relevant higher education degrees and certifications) to show recruiters that you have the technical basics of the industry covered.

Looking to build your own Quality Control Manager resume?

- Resume Examples
Alice's resume for a masters scholarship
How do i build a resume, do you put your birthday on a resume, how to answer the “are you a team player” interview question, how to answer the "what do you least like about your job" interview question, how to include a hackathon on a resume in 2024.
- Create Resume
- Terms of Service
- Privacy Policy
- Cookie Preferences
- Resume Templates
- AI Resume Builder
- Resume Summary Generator
- Resume Formats
- Resume Checker
- Resume Skills
- How to Write a Resume
- Modern Resume Templates
- Simple Resume Templates
- Cover Letter Builder
- Cover Letter Examples
- Cover Letter Templates
- Cover Letter Formats
- How to Write a Cover Letter
- Resume Guides
- Cover Letter Guides
- Job Interview Guides
- Job Interview Questions
- Career Resources
- Meet our customers
- Career resources
- English (UK)
- French (FR)
- German (DE)
- Spanish (ES)
- Swedish (SE)
© 2024 . All rights reserved.
Made with love by people who care.
Resume Worded | Proven Resume Examples
- Resume Examples
- Engineering Resumes
16 Quality Assurance Resume Examples - Here's What Works In 2024
If the tech industry were the publishing industry, quality assurance professionals would be the proofreaders. so much of our world runs on extensive lines of code these days, and it’s mathematically impossible for it all to be perfect. this article will show you how qa professionals are integral to the tech industry and give you resume examples to target these roles..

Quality Assurance professionals design automated code tests, identify software bugs, and help to fix the problems identified (or send them to the department that does). They also collaborate with other designers to create effective tests for new software and web applications. The user data collected in these tests is what allows the organization to make user-friendly, effective applications. As our world becomes ever more connected, even in the most mundane tasks like doing the laundry or turning on a light, the potential to collect this data and utilize it is everywhere. What this means is that the best QA professionals are both reactive and proactive. Entry-level jobs will likely deal mostly with legacy code, finding and fixing bugs. Growth from that position then has engineers creating automated tools to complete these jobs. However, the intermediate and higher skill-level QA professionals are both reactive and proactive. Knowing what questions to ask to avoid problems -- that is, effectively designing tests -- means working with multiple tech-industry teams, including UX/UI designers, software and web application designers, and, of course, the IT department. The path to this career involves the combination of critical thinking and good coding skills. Most professionals in this industry have a BA, and often they’ve completed specialized courses from either a university, a respected industry leader, or their corporations. Keep in mind that new technologies like machine learning will rapidly change this industry in the next few years, so that penchant for life-long learning and problem solving that led you to QA will serve you well in QA career longevity.
Quality Assurance Resume Templates
Jump to a template:
- Quality Assurance Tester
- QA (Quality Assurance) Automation Engineer
- QA (Quality Assurance) Engineer
- QA (Quality Assurance) Manager
- QA (Quality Assurance) Software Tester
- Entry Level QA (Quality Assurance) Tester
- QA (Quality Assurance) Analyst/Specialist
- Medical Quality Assurance Inspector
Jump to a resource:
- Keywords for Quality Assurance Resumes
Quality Assurance Resume Tips
- Action Verbs to Use
- Related Engineering Resumes
Get advice on each section of your resume:
Template 1 of 16: Quality Assurance Tester Resume Example
Many companies use quality assurance (QA) testing to ensure the products they create meet specific standards. Quality assurance testing allows companies to avoid sending out products to customers that are faulty or low-quality. Quality assurance testers can be found in almost every industry, from clothing to software. The quality assurance tester is responsible for performing a variety of tests on each product, constantly monitoring product performance, flagging issues, and troubleshooting them. When looking for a quality assurance tester, hiring managers will want a candidate with the right mix of experience and education. Typically, ideal candidates for the role will have a bachelor's degree in a related field such as computer science or analytics. They will want a candidate with related experience, such as prior roles as a quality analyst or test engineer. Additionally, it’s important that candidates for these roles demonstrate specific skills, such as knowledge of testing methodology, problem-solving abilities, and critical thinking skills.
We're just getting the template ready for you, just a second left.
Tips to help you write your Quality Assurance Tester resume in 2024
detail your experience utilizing different testing methodologies.
There are lots of testing methodologies that quality assurance professionals can use. Black box, user acceptance, and agile testing are just a few. Hiring managers will like to see an applicant who has experience with as many testing methods as possible. Showcasing what testing methods you are fluent in on your resume will show hiring managers that you are a dynamic and knowledgeable candidate.


Showcase any certifications you have in quality assurance testing
You can earn certifications specific to quality assurance testing to showcase your knowledge in a concrete way on your resume. You can earn the ISTQB tester or analytics professional certificate to strengthen your resume.

Skills you can include on your Quality Assurance Tester resume
Template 2 of 16: quality assurance tester resume example.
Quality Assurance testers are the individuals and teams that run every test you can think of trying to see if a web application or software program will break. They have strong problem-solving skills, attention to detail, and excellent written communication skills. They’re the best at documentation and take the minute information and put it together in a system that the entire QA chain can understand.
Related job experience is applied to current QA career path
Even if you choose to diversify your skill set by taking a position in a parallel career path (as this person did with the electrical project engineer role), it’s important that you highlight aspects of the role that apply to the QA job you’re seeking. If you’re applying for multiple roles in related industries, make sure you have a resume for each one with different highlighted achievements. Note how in this case we highlight achievements which are relevant to the quality assurance industry.

Demonstrate impact with hard data in the QA field
As a QA tester, you’re going to need to demonstrate communication skills. The best way to do this is to measure the results or impact of actions you’ve taken, which this resume does by measuring the efficiency of tests when working with a team of software engineers. As a QA tester, you’re in an excellent position to measure the impact of your actions -- you are the one who tracks how many bugs are found after all!

Template 3 of 16: QA (Quality Assurance) Automation Engineer Resume Example
The quality assurance (QA) automation engineer plays an integral role in optimizing a company’s quality assurance procedures. These professionals plan and create automated tests to assess the function of software. Being able to automate the QA process allows companies to test their technology more quickly and efficiently, leading to increased revenue. To become a QA automation engineer, you will need the right education and skills. Hiring managers will look for someone with a degree in computer science or IT. It’s important that QA automation engineers are talented coders with a breadth of technical knowledge. Hiring managers will look for someone with experience in QA, such as previous roles as a manual tester or QA tester. Strong candidates for this role will have knowledge of different testing methods and coding, superb problem-solving skills, and organizational abilities.

Tips to help you write your QA (Quality Assurance) Automation Engineer resume in 2024
highlight your knowledge of qa testing tools and frameworks.
To become a QA automation engineer, you will need to understand the variety of testing tools and frameworks you can use to solve QA testing issues. For this reason, you should highlight the testing methods and frameworks you have used in the past on your resume.

Show your ability to creatively solve problems
QA automation engineers must be savvy problem solvers in order to create testing automations that are effective. As such, one important soft skill to highlight on your resume is your problem-solving skills. Be sure to highlight any complicated QA issues you have successfully solved in the past on your resume.

Skills you can include on your QA (Quality Assurance) Automation Engineer resume
Template 4 of 16: qa automation engineer resume example.
A QA automation engineer develops automated tests that are used to test the functionality of applications. They are tasked with designing the tests, creating the code for them, activating the tests, and reporting on how they perform. To succeed in this role, you will need to have very strong programming skills, as well as a wealth of experience as a QA automation engineer. Recruiters will be expecting at least a bachelor’s degree in computer science, software engineering, or a similar field.
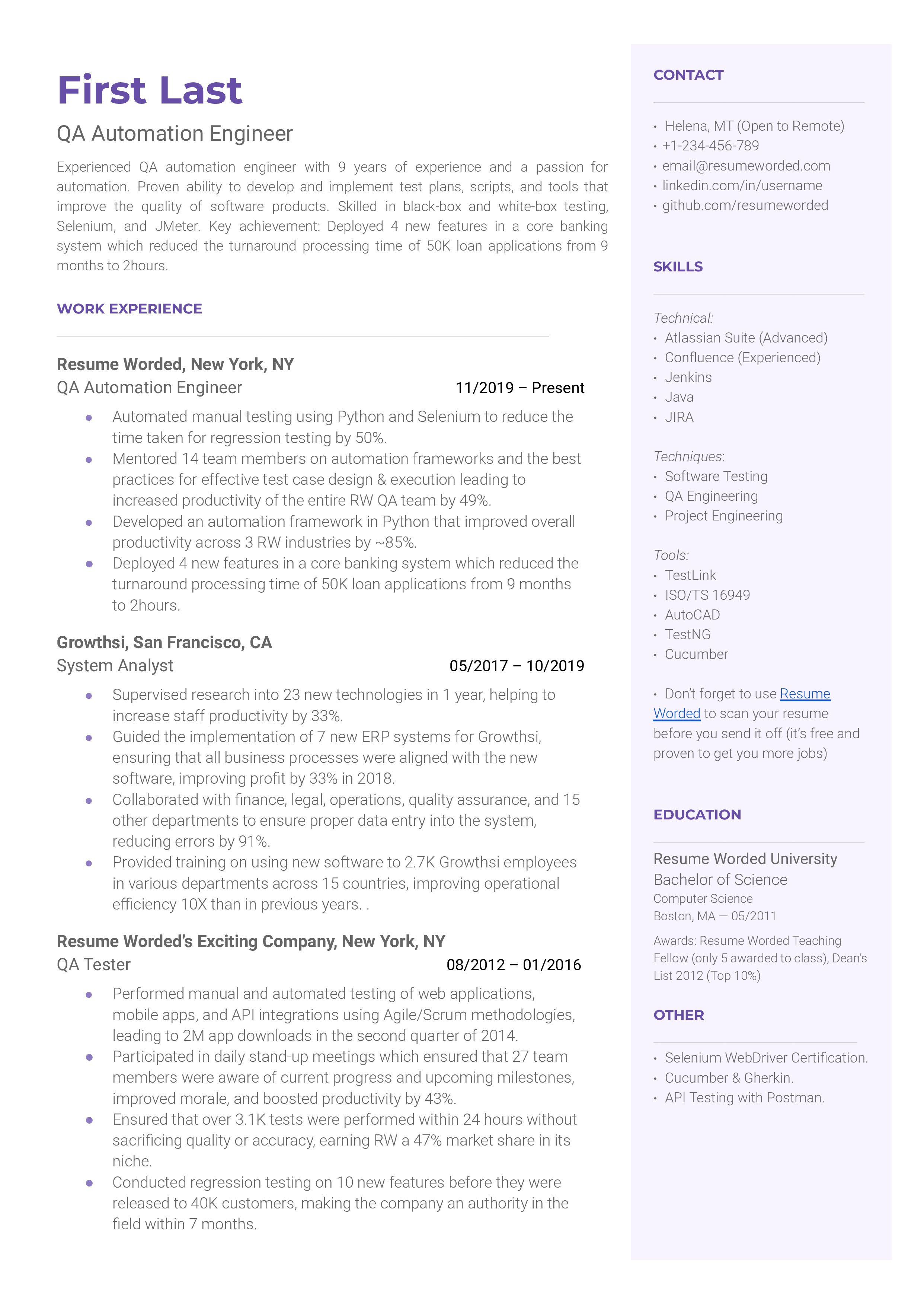
Tips to help you write your QA Automation Engineer resume in 2024
stay up to date with the latest development software and languages..
This is a very technical position that needs you to stay up to date with the latest software and software languages to stay relevant and useful. So make sure you stay up to date on the same. When any new relevant tech and software is developed, ensure you are quick to acquaint yourself with it. Do a course to learn the new tech or get as much experience with it as possible.

Quantify your QA testing success.
Recruiters want to know how successful and efficient the tests you create and run are. So make sure you include actual numbers in your experience section. How many tests have you run in a short period of time? How many errors and bugs did your tests identify?

Skills you can include on your QA Automation Engineer resume
Template 5 of 16: qa (quality assurance) automation engineer resume example.
Quality Assurance Automation Engineers are the bigger-picture thinkers of the QA world. They excel at writing tests that can be iterated over large-scale and enterprise technologies, including databases. They know every aspect of the design of the program and how it should function. They often work on mobile and web applications. As with all QA professionals, they work with other engineers and developers, but they are also likely to have a team of QA testers who work with their code to identify particular bugs.

Shows leadership amongst in-house and remote teams.
The larger-scale you get in technology, the more likely it is that you’re going to be working with distributed teams, even internationally. Your ability to communicate well with these teams on a technological and professional level is absolutely essential to the QA team working well.

Cites specific automation tools in the QA space
As a QA automation engineer, you’re going to be approaching complex web and mobile applications as well as enterprise-level software. As a result, you want a wide coding toolbox to draw from. Demonstrating how your solutions work -- and that you have a diversity of hows at your disposal -- makes you an asset to your company.

Template 6 of 16: QA Engineer Resume Example
If you’re a technical whiz with great attention to detail, a position as a QA (quality assurance) engineer might be right for you. Before going to a customer, every product must be vetted and tested for quality. The QA engineer is responsible for giving each product the final check before it is shipped out. In order to ensure each product is flawless, QA engineers conduct continuous tests throughout the manufacturing cycle. The QA engineer must understand the larger picture, and work in accordance with the business needs and budget. To become a QA engineer, you will need a specific set of technical skills. You’ll need a bachelor’s degree in software engineering or another related field. Hiring managers will also look for a candidate who is proficient in a variety of testing methods. Previous experience as a quality inspector or tester can help you land this role. In addition to technical skills, it’s important that candidates for this role are detail-oriented with strong project management skills.
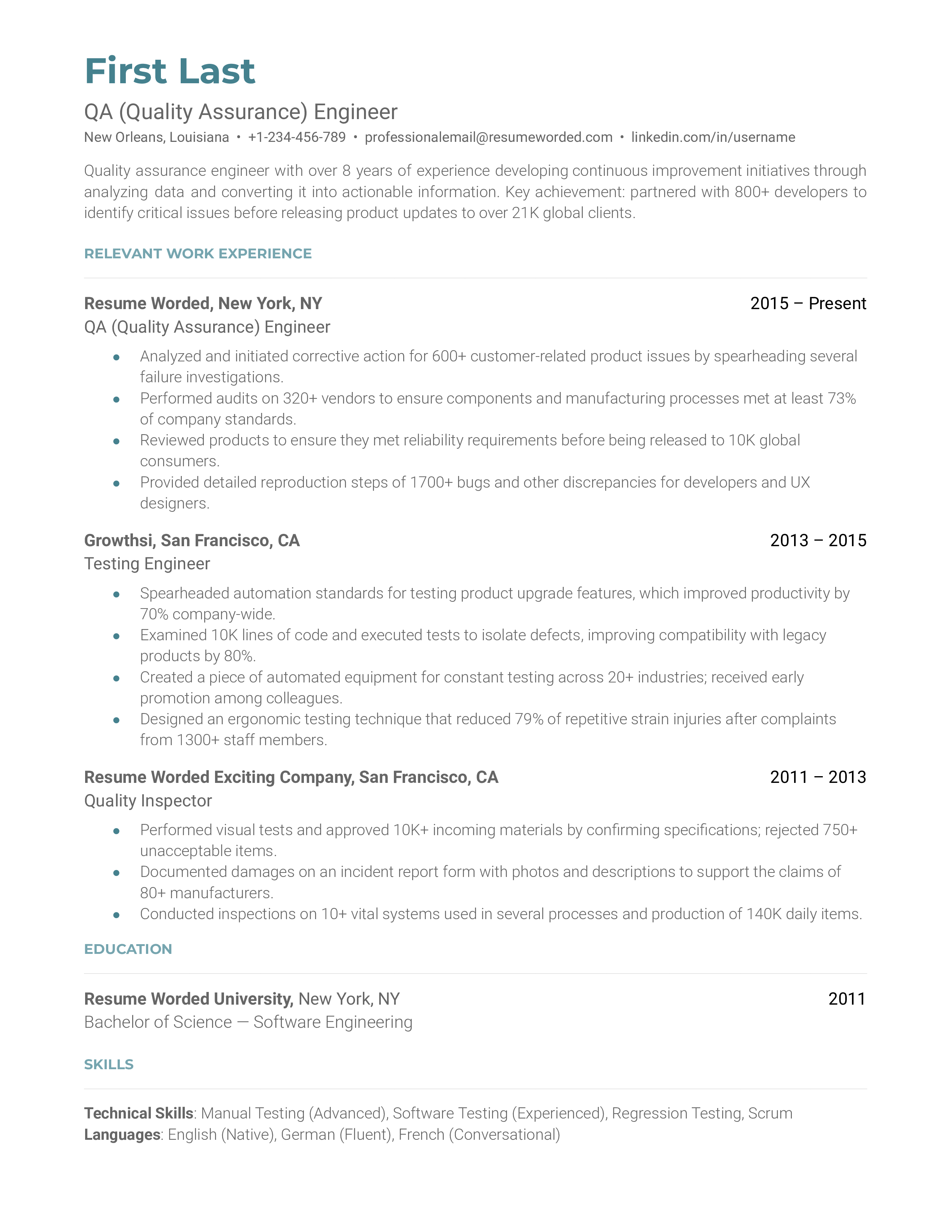
Tips to help you write your QA Engineer resume in 2024
showcase your knowledge of testing methodologies.
QA engineers have to understand different quality assurance testing methods. Showcasing the testing methods you are knowledgeable in on your resume can help you land this role. You should put certain testing methodologies, like regression testing, in your skills section on your resume.

Demonstrate your Scrum knowledge
Scrum is an agile project management framework that allows teams to manage projects through a set of specific principles. Having scrum knowledge as a QA engineer can help set you apart from the crowd and show hiring managers you understand how to organize projects in an effective manner.
Skills you can include on your QA Engineer resume
Template 7 of 16: qa (quality assurance) engineer resume example.
Quality Assurance Engineers are some of the generalists in the QA world. They’re often divided into manual and automated career paths, but in such an in-demand industry, the truth is that there’s more demand for QA engineers than there is supply. They work with software that already exists in the product market and with new and releasing products. They may also focus on internal processes of the company, not just customer-facing software.

Tips to help you write your QA (Quality Assurance) Engineer resume in 2024
skills section reflects new trends in the qa industry and qa software.
As a QA engineer (or really any kind of engineer or developer), you’re going to be constantly on the forefront of new tools changing your industry. It’s important to note that you have the drive to continually learn these techniques when they become relevant and necessary to your job.
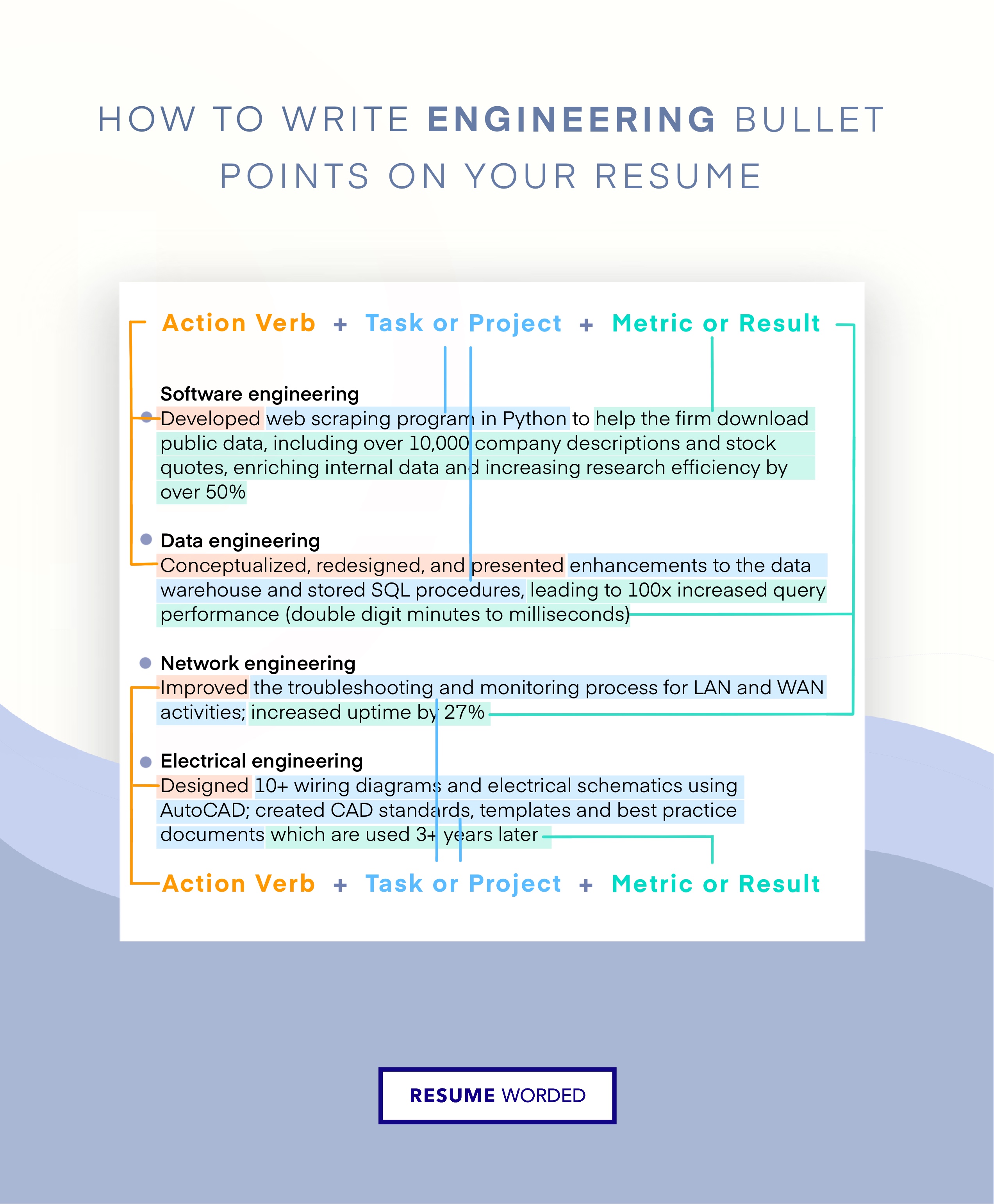
Inclusion of the QA engineer title combats ATS (applicant tracking system) filters.
Anytime you’re applying for a specific role, you’ll get a higher ranking from ATS filters if you include the job title at the top of your resume as well as in specific job titles.

Skills you can include on your QA (Quality Assurance) Engineer resume
Template 8 of 16: qa (quality assurance) manager resume example.
As a QA (Quality Assurance) Manager, you'll be responsible for ensuring the quality and reliability of software or product releases. This role requires strong communication, analytical skills and attention to detail. With the recent shift towards automation and continuous integration and delivery, the value of a skilled QA manager has become more critical in today's industries. When writing your resume, be sure to highlight your technical expertise, adaptability, and strategic approach to problem-solving, as these are key aspects that companies are looking for in a QA manager candidate. In the ever-evolving tech landscape, it's essential to keep up with new tools and methodologies. For QA managers, this means staying current with industry standards and best practices in areas like test automation, performance testing, and security. Your resume should demonstrate your ability to learn and adapt to new technologies and processes, as well as showcase your track record of successful QA projects and process improvements.

Tips to help you write your QA (Quality Assurance) Manager resume in 2024
highlight relevant certifications.
As a QA manager, having certifications such as ISTQB, CSTE, or CSQA, can be a significant advantage to set you apart from other candidates. Include any relevant certifications in your resume to showcase your dedication to the field and your expertise in various quality assurance methodologies.

Demonstrate leadership and teamwork skills
As a QA manager, you'll need to work closely with cross-functional teams and lead the quality assurance team towards success. Your resume should showcase your leadership and teamwork abilities, including examples of how you've managed and motivated teams to achieve their goals and drive process improvements.

Skills you can include on your QA (Quality Assurance) Manager resume
Template 9 of 16: qa (quality assurance) manager resume example.
A Quality Assurance manager is a high-level employee who has experience as a QA engineer and tester. Their job is to understand how to best employ QA techniques and teams across the entire system. In other words, they create the strategy for effective testing so that no product hits the market without being thoroughly vetted.

Emphasizes educational achievement combined with work experience in QA.
If you’re an advanced degree holder, you’re doing yourself a disservice if you simply list it without fanfare. You certainly should include any awards received as well as their rarity, particularly if they’re in a technical field (e.g. QA). If possible, you should include work achievements that you were commended that show your leadership experience, which is critical to being a manager. It shows that you can handle balancing your priorities and still excel.

Highlights adaptability in industry by citing different project management/workflow strategies.
As a manager, you’re expected to be flexible enough to adapt to different teams but experienced enough to have definite opinions about how to help that team succeed. This resume shows that the application worked in Agile, waterfall, and iterative methods of software development, which covers the essentials of how any software team would work in the industry.

Template 10 of 16: QA (Quality Assurance) Software Tester Resume Example
Quality assurance is an important factor in the production process in many industries, particularly in software. QA (quality assurance) software testers help companies ensure their software is functioning correctly and effectively, before putting it in the hands of customers. QA software testers are responsible for assessing software, running various tests, identifying and solving bugs, generating result reports, and more. To become a QA software tester, you will need a degree in computer science or IT. Hiring managers will look for someone with previous technical and/or software experience, such as previous roles as a functional tester or application engineer. The ideal candidate for this role will have technical expertise, in addition to strong problem-solving and critical-thinking skills.
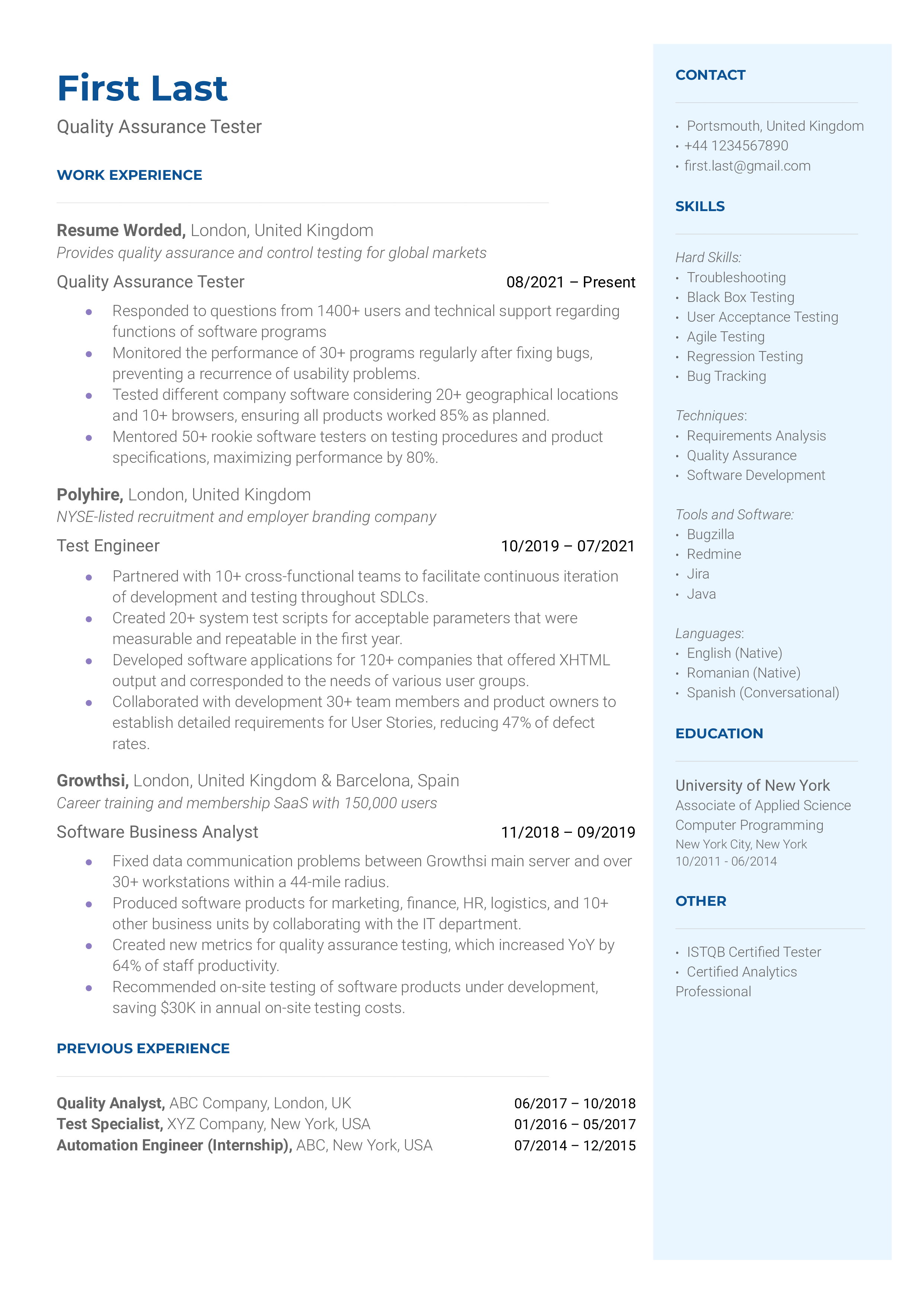
Tips to help you write your QA (Quality Assurance) Software Tester resume in 2024
detail your experience identifying and solving bugs.
A big piece of the QA software tester’s job is to find and solve for bugs or issues found within the software. For this reason, you should highlight any experience you have identifying and/or solving for bugs in software. Even if your experience was in a different position, the ability to troubleshoot and problem solve is crucial to landing this role.

Showcase your ability to make suggestions to stakeholders
As a QA software tester, in addition to testing for issues, you will have the opportunity to create ideas about how to improve or add integrations to existing software. If you can bring valuable insight to the role, companies will be more likely to hire you. For this reason, you should highlight any experience you have making improvement recommendations to stakeholders and leadership teams.
Skills you can include on your QA (Quality Assurance) Software Tester resume
Template 11 of 16: qa (quality assurance) software tester resume example.
Traditionally, Quality Assurance Testers come in at the end of the development cycle of a new product. The title QA software tester is often used to emphasize that the QA team is a part of the development of the product from start to finish. This means that the team works in an iterative and integrative way, and that QA software testers will pay attention to all things that will affect the function of the program, from bugs, to slow load times, to poorly written customer copy.

Clear skills section to highlight QA testing skills to help with ATS (applicant tracking system) filters.
Especially in any tech field, your skills section tells a story. It shows the tools you have used and those you can bring to bear on the problems at hand. This is also an easy way to customize your resume to better fit a company’s needs -- if you have the tech skills they’re asking for, change them to match what’s in the job description and you’ll read as a better fit to ATS filters.

Promotions within an organization show strong leadership skills and an employee that exceeds expectations.
Whenever you’re a person in a senior position with management responsibilities, one of the best things you can do is show when you were rewarded ahead of schedule or exceeded expectations. Any position, especially as a QA tester, is looking for strong soft skills and ability to lead — being promoted within 18 months of receiving a new position is a great example.

Template 12 of 16: Entry Level QA (Quality Assurance) Tester Resume Example
As an entry-level QA tester, you'll be starting your journey in the fascinating world of software quality assurance, ensuring that products meet the necessary standards for functionality and user experience. In this position, staying informed on industry practices and tools is crucial to staying relevant and developing your skills. As you write your resume, showcase your understanding of key testing methodologies and emphasize your passion for learning and improvement. In recent years, there's been a shift towards automation and data-driven testing processes. Companies are increasingly looking for candidates who can leverage these technologies to improve testing efficiency. Therefore, highlighting any experience or knowledge you have in these areas will make your resume stand out.
Tips to help you write your Entry Level QA (Quality Assurance) Tester resume in 2024
show proficiency in testing methodologies.
On your resume, be sure to demonstrate your understanding of various testing methodologies, such as functional, integration, and performance testing. This shows employers that you're well-versed in the core responsibilities of a QA tester.
Highlight automation and technical skills
Given the increasing role of automation in the QA field, it's essential to showcase any experience or knowledge you have with automated testing tools, scripting languages, or frameworks. Additionally, mention any relevant programming languages and technical skills that can demonstrate your ability to handle the technical aspects of QA testing.
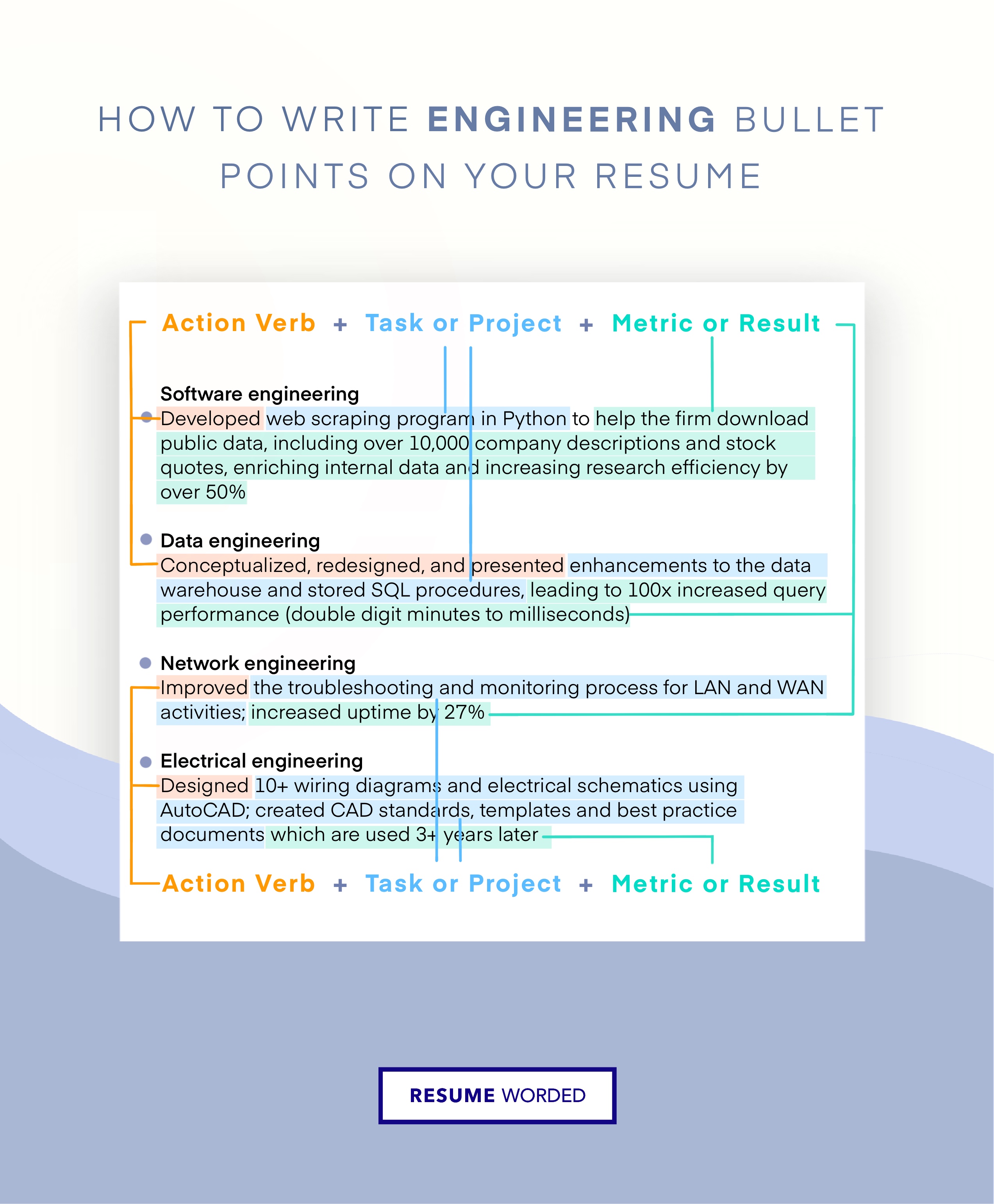
Skills you can include on your Entry Level QA (Quality Assurance) Tester resume
Template 13 of 16: entry level qa (quality assurance) tester resume example.
An entry-level Quality Assurance tester can expect to be doing a lot of work that seems tedious or repetitive to most. They should have an excellent attention to detail and be thorough as they will spend most of their time identifying and documenting bugs. They should also be great at teamwork. The power of QA lies in the orchestration of a team to identify all the bugs in a software or application release, and it certainly is not a job that can be completed alone.

Supplements limited work experience with related volunteer or school projects.
If you’re applying for an entry-level job, it’s a great idea to expand your work experience in this way. These can be school projects or volunteer projects you seek out, but make sure you clearly explain your successes with data. Try to highlight projects that use relevant skills in a QA role, whether that’s programming, software testing, bug fixing or website development.

Highlights industry interest in extracurricular activities.
Another good way to show your involvement in the industry is to include your activity and leadership in clubs or sports. As above, you should point out what skills you think translate to your chosen career path and why. Soft skills like leadership, communication and teamwork are always transferable and are relevant to QA roles — so highlight them through your experiences.
Template 14 of 16: QA (Quality Assurance) Analyst/Specialist Resume Example
As a Quality Assurance (QA) Analyst or Specialist, you're a vital player in product development, helping make sure that all the bugs are identified and resolved before software gets to the customer. Lately, there's a shift towards automation in QA, so having experience with automated testing tools can be a real bonus. When crafting your resume, remember that hiring managers want to see a mix of hard skills (like knowledge of specific testing tools) and soft skills (like attention to detail). One more thing - in this field, employers love seeing concrete examples of your problem-solving prowess on your resume. In your QA resume, don't overlook the significance of showcasing your collaborative skills. More companies are adopting Agile methodologies, making it crucial for QA specialists to work effectively within cross-functional teams. Make sure your resume highlights experience with Agile or Scrum, if you have it. One last advice - continuously updating your skills can make the difference between a resume that gets set aside and one that earns an interview, as the QA field is ever-evolving.

Tips to help you write your QA (Quality Assurance) Analyst/Specialist resume in 2024
include specific testing tools knowledge.
As a QA Analyst/Specialist, having a grasp of both manual and automated testing tools is essential. List the specific testing tools you've used, like JUnit or Selenium, as they are the ones that will grab a hiring manager's attention.

Showcase project management skills
In the QA realm, you often need to manage multiple test plans simultaneously. Including past experiences where you successfully juggled multiple projects or led a testing team can showcase your ability to handle these scenarios in your future role.

Skills you can include on your QA (Quality Assurance) Analyst/Specialist resume
Template 15 of 16: qa (quality assurance) analyst/specialist resume example.
The roles of a Quality Assurance Specialist and a Quality Assurance Tester are quite similar. The biggest difference is that a QA Specialist is more likely to choose a more general or managerial path, and so those skill sets should be highlighted on your resume.

Summary targets the intent of the resume, specializing in QA
Especially earlier in your career, it helps hiring managers to understand how you see your current position in relation to your desired career path. Consider it something of an elevator pitch. You have 30 seconds to influence how hiring managers are going to read the rest of your resume, so make it count.

Demonstrate conversion from university-led program to workplace.
If you’re lucky enough to be singled out for placement programs early in your career, that’s a great thing for employers to know! It shows that you excelled enough to be taken note of, but also that you made the most of that time and entered the workplace with some solid (and accelerated) experience under your belt. Even better, highlight QA or software development experience to show recruiters that you’re technically minded.

Template 16 of 16: Medical Quality Assurance Inspector Resume Example
A medical quality assurance inspector is someone who ensures the products being manufactured at a facility meet specific standards. When it comes to medical equipment, this is particularly important. It’s the medical quality assurance inspectors' responsibility to inspect each piece of equipment post-production, using tools given by their employer, and meticulously record their findings after each inspection. If you want to go right into a job in medical quality assurance, an educational background in science or medicine will make your resume attractive to hiring managers. Hiring managers may also want to see certifications in quality assurance. When hiring for this role, managers will be looking for someone who demonstrates an eye for detail and superb communication skills.
Tips to help you write your Medical Quality Assurance Inspector resume in 2024
start with a lower stakes quality assurance inspector role.
Most companies that produce a physical product have a quality assurance department. Medical devices are one of the most high-stakes products on the market, given that a faulty medical device can have serious consequences. Gaining a couple of years of experience in a “lower-stakes” industry allows you to learn the ropes in a less stressful environment, and shows hiring managers you have foundational experience inspecting products.
Obtain quality assurance inspector certifications
Certifications show hiring managers you have the knowledge needed to competently inspect products and create reports. There are several certifications you can earn, such as the CQAP or CQI. You can also get a medical device quality assurance certification, which shows hiring managers you have specialized knowledge in inspecting medical devices.

Skills you can include on your Medical Quality Assurance Inspector resume
As a hiring manager who has recruited Quality Assurance professionals at companies like Microsoft, Apple, and Google, I have reviewed hundreds of resumes. I know what makes a strong resume that will get you noticed by top employers. Here are some tips to help your Quality Assurance resume stand out:
Highlight your technical skills and tools
Employers want to see the specific technical skills and tools you have used in your Quality Assurance roles. Instead of simply listing the skills, provide examples of how you used them to deliver results.
- Performed manual and automated testing using Selenium, Cypress, and Postman to ensure high-quality software releases
- Developed and executed test plans and test cases using JIRA and TestRail, resulting in a 25% reduction in bug leakage
- Collaborated with development teams to identify and troubleshoot defects using debugging tools like Chrome DevTools and Xcode

Quantify your impact with metrics
Use numbers and metrics to show the impact of your work. This helps employers understand the value you can bring to their organization.
- Tested software applications to ensure quality
- Identified and reported bugs and defects
Instead, quantify your accomplishments like this:
- Executed 500+ test cases per release, ensuring 99% test coverage and reducing post-release defects by 40%
- Identified and reported 150+ high-priority bugs, collaborating with developers to resolve them before release deadlines
Show your experience with different testing types
Quality Assurance involves various types of testing, such as functional, performance, usability, and security testing. Showcase your experience with different testing types to demonstrate your versatility.
- Conducted functional testing on web and mobile applications, ensuring features worked as expected across different devices and browsers
- Performed load and stress testing using JMeter to identify performance bottlenecks and optimize application speed
- Collaborated with UX designers to conduct usability testing, gathering user feedback to improve application design and user experience
Demonstrate your problem-solving skills
Quality Assurance professionals need strong problem-solving skills to identify and troubleshoot issues. Use examples to show how you have used your problem-solving skills to deliver results.
Investigated and identified root cause of a critical production issue impacting 50,000+ users. Collaborated with development and operations teams to implement a hotfix within 24 hours, minimizing downtime and user impact.
This example shows how you used your problem-solving skills to quickly identify and resolve a critical issue, demonstrating your value to the organization.
Tailor your resume to the job description
Customize your resume for each job application to highlight the skills and experience most relevant to that specific role. This shows employers that you have taken the time to understand their needs and how you can contribute to their team.
For example, if the job description emphasizes experience with agile methodologies, make sure to highlight your experience working in agile teams and participating in agile ceremonies like daily stand-ups and retrospectives.
- Collaborated with cross-functional agile teams to plan and execute testing activities in 2-week sprints
- Participated in daily stand-ups, sprint planning, and retrospectives to continuously improve quality and delivery speed
Include relevant certifications and training
Quality Assurance professionals often pursue certifications and training to stay up-to-date with the latest tools and best practices. Including relevant certifications and training on your resume can help you stand out from other candidates.
- Certified Software Quality Analyst (CSQA) - International Software Testing Qualifications Board (ISTQB)
- Agile Testing Certification - Scrum Alliance
- Completed training on Selenium WebDriver and JMeter
These certifications and training demonstrate your commitment to your profession and your ability to apply industry-standard practices to your work.
Writing Your Quality Assurance Resume: Section By Section
header, 1. put your name first and make it stand out.
Your name should be the most prominent element in your resume header. Use a larger font size than the rest of your contact details to make it pop.
Compare these two examples:
- John Smith, 123 Oak St, New York, NY 10001 | (555) 123-4567 | [email protected]
Instead, format your header like this:
- John Smith New York, NY | (555) 123-4567 | [email protected]
By putting your name on its own line in a larger font, it becomes the focal point and makes it easier for hiring managers to remember you.
2. Include essential contact details only
In your resume header, stick to the key details hiring managers need to contact you:
- Phone number
- Professional email address
- LinkedIn profile URL
- City and state of residence
Avoid listing your full mailing address, as it's no longer expected and takes up valuable space. And never include personal details like your date of birth, marital status, or headshot. Keep your header concise and professional.
John Smith Seattle, WA | (555) 123-4567 | [email protected] | linkedin.com/in/johnsmith
3. Tailor your header to the QA role
If you're applying for a specific QA position, consider tailoring your resume header to better align with the role. For example:
- John Smith Senior QA Engineer San Francisco, CA | (555) 123-4567 | [email protected]
By adding your current or desired job title that matches the role you're applying for, you quickly convey to employers that you're a relevant candidate. However, avoid listing multiple job titles or stuffing your header with keywords, as it can appear cluttered and spammy.
Keep your header tailored but concise, so hiring managers can easily see your fit for the QA job at hand.
Summary
A resume summary for a Quality Assurance (QA) role is optional, but it can be a valuable addition if you want to provide context or highlight key skills and experiences that may not be immediately evident from the rest of your resume. As a QA professional, your summary should focus on your ability to ensure product quality, identify and report issues, and collaborate with cross-functional teams. Avoid using an objective statement, as it is outdated and does not provide value to the employer.

To learn how to write an effective resume summary for your Quality Assurance resume, or figure out if you need one, please read Quality Assurance Resume Summary Examples , or Quality Assurance Resume Objective Examples .
1. Highlight your QA expertise
In your summary, showcase your QA expertise by mentioning your experience with specific testing methodologies, tools, and processes. This helps employers quickly understand your background and how you can contribute to their organization.
For example:
- Experienced Quality Assurance professional with 5+ years of expertise in manual and automated testing, ensuring high-quality software releases.
- Detail-oriented QA specialist skilled in developing and executing test plans, identifying and reporting defects, and collaborating with development teams to deliver exceptional products.
2. Quantify your achievements
Whenever possible, use metrics to quantify your achievements and demonstrate your impact in previous roles. This helps employers understand the scale and significance of your contributions.
Avoid vague statements like:
- Improved software quality and reduced bugs.
Instead, provide specific numbers:
- Implemented a comprehensive testing strategy that reduced production defects by 35% and increased customer satisfaction ratings by 20%.
3. Tailor your summary to the job
While it's essential to highlight your overall QA experience, make sure to tailor your summary to the specific job you're applying for. Review the job description and identify the key skills and requirements the employer is looking for, then incorporate those into your summary.
Quality Assurance professional with 7+ years of experience in the e-commerce industry. Skilled in developing and executing comprehensive test plans, collaborating with cross-functional teams, and ensuring the delivery of high-quality, user-friendly web and mobile applications. Proven track record of reducing defect leakage by 30% and improving time-to-market by 20% through the implementation of efficient testing processes and automation frameworks.
Experience
The work experience section is the heart of your resume. It's where you show the impact you've made at each company. Hiring managers and recruiters look to this section to see how your career has progressed and to find evidence that you're the best candidate for the job.
In this section, we'll break down exactly what to include in your work experience section, and share tips to make your experience stand out to employers.
1. Focus on your impact and results
It's not enough to simply list your day-to-day responsibilities in your work experience section. Employers want to see the impact you made and the results you achieved.
For each role, think about how you contributed to the success of projects, initiatives, or the company as a whole. Then, use metrics and data to quantify your impact wherever possible.
- Implemented new testing procedures which reduced defect rate by 25% and improved customer satisfaction scores by 15%
- Developed and executed test plans for new software releases, resulting in a 98% pass rate and on-time delivery for all major milestones
- Led a team of 5 QA analysts to thoroughly test new features, catching and documenting 150+ bugs before release
2. Highlight your QA expertise and technical skills
Quality assurance roles require a mix of technical know-how, problem-solving skills, and attention to detail. Make sure your work experience section showcases your expertise in these areas.
Mention the specific QA methodologies, tools, and technologies you've used. This shows employers you have the technical chops to excel in the role.
- Tested software for bugs and issues
- Worked with developers to improve quality
Instead, get specific about your QA expertise:
- Expertise in Agile testing methodologies, including Scrum and Kanban
- Proficient with test automation tools such as Selenium, Appium, and JMeter
- Skilled in writing detailed bug reports and test cases using Jira and TestRail
3. Show your career progression
Employers like to see candidates who have grown and taken on more responsibility throughout their career. If you've been promoted or taken on leadership roles, make sure to highlight that in your work experience section.
One way to show progression is by listing your roles in reverse-chronological order, with your most recent position first. For each company, start with your most recent title and then list any previous roles below.
QA Manager, Acme Inc. (2019-Present) - Manage a team of 8 QA analysts and oversee all testing activities for web and mobile apps - Collaborate with cross-functional teams to define and implement QA strategy - Improved test coverage by 30% and reduced escaped defects by 20% QA Analyst, Acme Inc. (2016-2019) - Developed and executed manual and automated test cases for new features and releases - Reported and tracked bugs using Jira and collaborated with dev team on fixes - Contributed to a 15% reduction in customer-reported issues
Education
The education section of your resume is where you showcase your academic background and training. When applying for quality assurance roles, it's important to highlight relevant coursework, certifications, and degrees that demonstrate your knowledge and skills in this field. Here are some tips to help you craft an effective education section on your resume.
1. List your degrees in reverse chronological order
Start with your most recent degree and work backwards. Include the name of the institution, the degree earned, and the year of graduation.
If you have a degree in a field related to quality assurance, such as computer science, engineering, or business, make sure to highlight it. You can also include relevant coursework, such as software testing, quality management, or statistics.
Bachelor of Science in Computer Science University of California, Los Angeles Graduated: 2020 Relevant Coursework: Software Testing and Quality Assurance Database Management Systems Data Structures and Algorithms
If you have multiple degrees, consider listing only the most relevant one for the quality assurance role you're applying for. This helps keep your resume concise and focused.
2. Include relevant certifications
Certifications demonstrate your expertise and commitment to the field of quality assurance. If you have earned any certifications, such as Certified Software Quality Analyst (CSQA) or Certified Quality Engineer (CQE), include them in your education section.
List the name of the certification, the issuing organization, and the date of completion. If the certification required passing an exam or completing a certain number of hours of training, you can mention that as well.
Certified Software Quality Analyst (CSQA) American Society for Quality (ASQ) Completed: 2022 Passed comprehensive exam covering software testing, quality assurance, and quality management principles Completed 40 hours of training and hands-on projects
Only include certifications that are relevant to quality assurance and recognized in the industry. Avoid listing generic or outdated certifications that may not add value to your resume.
3. Highlight your technical skills
Quality assurance professionals often need to have a strong technical background, especially when working with software or technology products. Use your education section to showcase your technical skills and knowledge.
If you have taken courses or earned certifications in specific programming languages, tools, or methodologies, list them. This can include things like Java, Python, Selenium, Agile, or Six Sigma.
Associate's Degree in Information Technology Community College of Denver Graduated: 2018 Technical Skills: Programming Languages: Java, Python, C++ Testing Tools: Selenium, JUnit, TestNG Methodologies: Agile, Scrum, Waterfall
Be specific about your technical skills and how you acquired them. This helps employers understand the depth of your knowledge and how it can be applied to the quality assurance role.
4. Keep it brief if you're a senior professional
If you have several years of experience in quality assurance, your education section can be brief. List your degree, institution, and graduation year, but avoid going into detail about coursework or projects.
Master of Science in Engineering Stanford University Bachelor of Science in Computer Science University of Michigan
However, if your education is not directly related to quality assurance or is outdated, consider leaving it off your resume entirely. Your work experience and accomplishments will carry more weight.
- Associate's Degree in Liberal Arts Community College of Philadelphia Graduated: 1985
As a senior quality assurance professional, your education becomes less important than your work experience and accomplishments. Focus on highlighting your most impressive and relevant degrees or certifications.
Action Verbs For Quality Assurance Resumes
A big part of your career as a Quality Assurance professional is going to be in generating reports that identify vulnerabilities in software. Those reports should be data-driven, persuasive, and clear so that the myriad of stakeholders involved in the design of the application or software can act on it. In some ways, it’s very similar to how you write a resume. You choose a word that suggests the course of action in a descriptive way, include the supporting data, and don’t use seven words when you can use five. Clarity and impact are key!
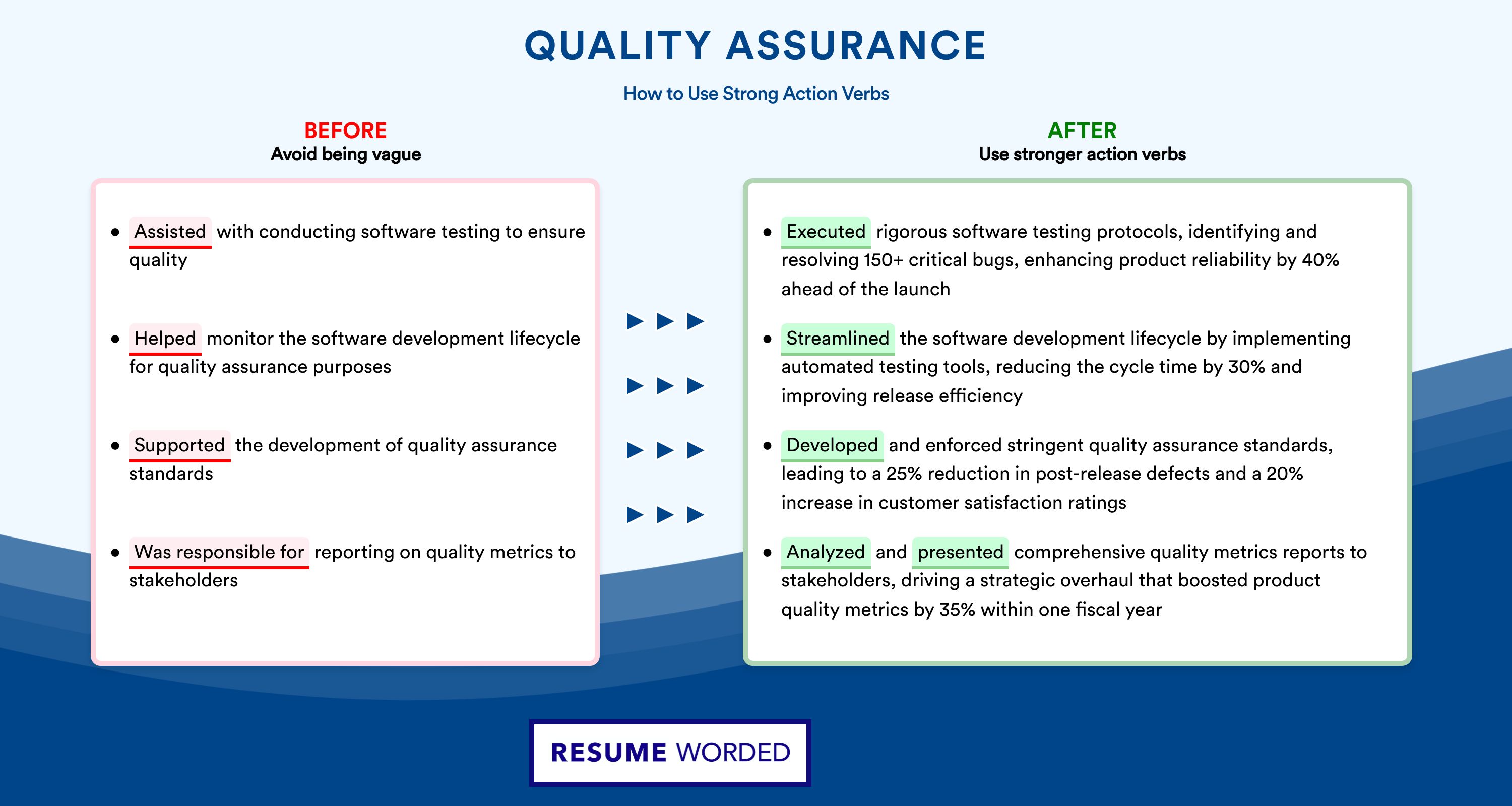
- Systematized
- Demonstrated
- Facilitated
- Implemented
For a full list of effective resume action verbs, visit Resume Action Verbs .
Action Verbs for Quality Assurance Resumes
Skills for quality assurance resumes.
As a Quality Assurance professional in the tech industry, the skill box is your best friend. You can quickly and easily tell employers which skills you can bring to bear and you can even point towards your forward thinking in a growing field (such as including machine learning tools). It’s also a simple and easy way to target your resume to the job listing. This puts you in a better position with ATS (applicant tracking system) filters. Remember that a skill in and of itself doesn’t create a robust picture of your abilities. As a Quality Assurance professional, you’re going to be expected to use data to make your argument. What better way to show you’re expert at this than having data driven bullet points on your resume?
- Quality Assurance
- Manual Testing
- Regression Testing
- Test Planning
- Test Automation
- Software Quality Assurance
- Software Testing
- Agile Methodologies
- Functional Testing
Quality Control
- Engineering
- Manufacturing
- Statistical Process Control (SPC)
- Failure Mode and Effects Analysis (FMEA)
- QA Engineering
- Good Manufacturing Practice (GMP)
- Quality Management
How To Write Your Skills Section On a Quality Assurance Resumes
You can include the above skills in a dedicated Skills section on your resume, or weave them in your experience. Here's how you might create your dedicated skills section:
Skills Word Cloud For Quality Assurance Resumes
This word cloud highlights the important keywords that appear on Quality Assurance job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more 'important' it is.

How to use these skills?
Other engineering resumes.

Manufacturing Engineer

- Software Engineer Resume Guide
- Software Developer Resume Guide
- Web Developer Resume Guide
- Programmer Resume Guide
- Front End Developer Resume Guide
- DevOps Resume Guide
- Full Stack Developer Resume Guide
- Java Developer Resume Guide
- Python Developer Resume Guide
- IT Manager Resume Guide
- Cyber Security Resume Guide
- Salesforce Resume Guide
Quality Assurance Resume Guide
- Quality Engineer Resume Guide
- Electrical Engineer Resume Guide
- System Administrator Resume Guide
- Scrum Master Resume Guide
- Civil Engineer Resume Guide
- Network Administrator Resume Guide
- Mechanical Engineer Resume Guide
- Manufacturing Engineer Resume Guide
- Network Engineer Resume Guide
- Node.js Resume Guide
- SQL Developer Resume Guide
- Integration Architect Resume Guide
- Engineering Manager Resume Guide
- Software Tester Resume Guide
- Service Technician Resume Guide
- Platform Engineer Resume Guide
- Automation Engineer Resume Guide
- C, C++, and C# Developer Resume Guide
- Technical Support Resume Guide
- Project Engineer Resume Guide
- Security Manager Resume Guide
- Electronic Technician Resume Guide
- System Engineer Resume Guide
- IT Specialist Resume Guide
- Packaging Engineer Resume Guide
- Oracle Resume Guide
- Planning Engineer Resume Guide
- Blockchain Resume Guide
- Cloud Developer Resume Guide
- ETL Developer Resume Guide
- SharePoint Developer Resume Guide
- Kafka Resume Guide
- Audio Engineer Resume Guide
- HVAC Resume Guide
- Industrial Engineer Resume Guide
- Maintenance Technician Resume Guide
- Solutions Architect Resume Guide
- Implementation Specialist Resume Guide
- Software Architect Resume Guide
- PHP Developer Resume Guide
- Biomedical Engineer Resume Guide
- Robotics Resume Guide
- Chief Digital Officer Resume Guide
- Innovation Resume Guide
- Security Analyst Resume Guide
- IT Auditor Resume Guide
- Director of Software Engineering Resume Guide
- Environmental Engineer Resume Guide
- Technology Director Resume Guide
- Director of Information Technology Resume Guide
- AWS Resume Guide
- Director of Engineering Resume Guide
- Materials Engineer Resume Guide
- UAT Tester Resume Guide
- Quality Assurance Tester Resume Example
- QA (Quality Assurance) Automation Engineer Resume Example
- QA (Quality Assurance) Engineer Resume Example
- QA (Quality Assurance) Manager Resume Example
- QA (Quality Assurance) Software Tester Resume Example
- Entry Level QA (Quality Assurance) Tester Resume Example
- QA (Quality Assurance) Analyst/Specialist Resume Example
- Medical Quality Assurance Inspector Resume Example
- Tips for Quality Assurance Resumes
- Skills and Keywords to Add
- All Resume Examples
- Quality Assurance CV Examples
- Quality Assurance Cover Letter
- Quality Assurance Interview Guide
- Explore Alternative and Similar Careers
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 15 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 15 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

- Quality Control Manager Resume Example
Resume Examples
- Common Tasks & Responsibilities
- Top Hard & Soft Skills
- Action Verbs & Keywords
- Resume FAQs
- Similar Resumes
Common Responsibilities Listed on Quality Control Manager Resumes:
- Develop and implement quality control systems and procedures
- Monitor and inspect production processes and final output
- Ensure that products meet quality standards
- Investigate customer complaints and non-conformance issues
- Analyze data to identify areas for improvement in the quality system
- Develop and implement testing procedures and standards
- Monitor all aspects of product performance
- Ensure compliance with applicable laws and regulations
- Train and supervise quality control technicians
- Prepare reports to communicate outcomes of quality activities
- Monitor performance by gathering relevant data and producing statistical reports
- Investigate root causes of failures to identify areas of improvement
Speed up your resume creation process with the AI-Powered Resume Builder . Generate tailored achievements in seconds for every role you apply to.
Quality Control Manager Resume Example:
- Developed and implemented a comprehensive quality control system, resulting in a 20% reduction in product defects and a 10% increase in customer satisfaction.
- Conducted thorough inspections and analysis of production processes, leading to a 15% improvement in overall product quality and a 5% decrease in non-conformance issues.
- Implemented data-driven decision-making processes, utilizing statistical analysis to identify areas for improvement and achieving a 10% increase in production efficiency.
- Investigated and resolved customer complaints and non-conformance issues, resulting in a 25% decrease in customer complaints and a 20% improvement in product quality.
- Developed and implemented testing procedures and standards, leading to a 15% reduction in product failures and a 10% increase in compliance with quality standards.
- Trained and supervised a team of quality control technicians, resulting in a 20% improvement in their performance and a 10% increase in overall team productivity.
- Prepared and presented reports on quality activities, highlighting areas for improvement and driving a 15% increase in management awareness and decision-making.
- Monitored performance by gathering relevant data and producing statistical reports, leading to a 10% reduction in defects and a 5% increase in production efficiency.
- Investigated root causes of failures and implemented corrective actions, resulting in a 20% decrease in failures and a 15% improvement in overall product quality.
- Quality control system development and implementation
- Production process inspection and analysis
- Data-driven decision making
- Statistical analysis
- Customer complaint resolution
- Non-conformance issue resolution
- Testing procedures and standards development
- Quality control team training and supervision
- Quality activity reporting
- Performance monitoring and data gathering
- Root cause analysis
- Corrective action implementation
- Compliance with quality standards
- Quality control technician performance improvement
- Management of quality control technicians
- Quality improvement strategies
- Process improvement
- Risk management
- Quality assurance
- Leadership and team management
- Communication skills
- Problem-solving skills
- Attention to detail
- Time management skills
- Project management skills
- Knowledge of quality control standards and regulations
- Proficiency in quality control software.
Top Skills & Keywords for Quality Control Manager Resumes:
Hard skills.
- Quality Assurance and Control
- Statistical Process Control (SPC)
- Six Sigma Methodology
- Root Cause Analysis
- ISO 9001 Standards
- Lean Manufacturing Principles
- Process Improvement
- Risk Assessment and Management
- Supplier Quality Management
- Regulatory Compliance
- Auditing and Inspection
- Documentation and Reporting
Soft Skills
- Attention to Detail
- Analytical Thinking
- Problem Solving
- Communication Skills
- Time Management
- Decision Making
- Adaptability
- Conflict Resolution
- Critical Thinking
- Organizational Skills
Resume Action Verbs for Quality Control Managers:
- Implemented
- Streamlined
- Collaborated
- Standardized
- Investigated
- Communicated
Generate Your Resume Summary
Resume FAQs for Quality Control Managers:
How long should i make my quality control manager resume, what is the best way to format a quality control manager resume, which keywords are important to highlight in a quality control manager resume, how should i write my resume if i have no experience as a quality control manager, compare your quality control manager resume to a job description:.
- Identify opportunities to further tailor your resume to the Quality Control Manager job
- Improve your keyword usage to align your experience and skills with the position
- Uncover and address potential gaps in your resume that may be important to the hiring manager
Complete the steps below to generate your free resume analysis.
Related Resumes for Quality Control Managers:
Quality control analyst, quality control auditor, quality control inspector, quality control specialist, quality control technician, operations manager, production manager, procurement manager.
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
- Resume Samples
- Quality Assurance
Senior Quality Manager Resume Samples
The guide to resume tailoring.
Guide the recruiter to the conclusion that you are the best candidate for the senior quality manager job. It’s actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get hired.
Craft your perfect resume by picking job responsibilities written by professional recruiters
Pick from the thousands of curated job responsibilities used by the leading companies, tailor your resume & cover letter with wording that best fits for each job you apply.
Create a Resume in Minutes with Professional Resume Templates
- Provide leadership and develop and implement methods to improve teams’ effectiveness and performance versus established quantitative measures
- Train and develop QC managers and SQE; make department training plan
- Establish and manage departments performance metrics and goals
- Facilitate resolution of issues by working with Project Quality Managers and Project Managers
- Develop and improve relationships with key Suppliers through projects and improvement strategies
- Manage a team of quality program managers building and improvement the data transformation quality processes
- Utilize quality system performance KPI to drive performance improvement
- Provide Quality Systems leadership using recognized standards as the basis for process development and optimization, procedure development, data collection, and Quality Systems training
- Develops and maintains the Program Quality policy, processes, procedures, and related documents. Reviews Quality Manuals, documents and implementation procedures to verify compliance with industry codes and standards, and regulations
- Participates in Client Quality planning and preparation meetings
- Work closely with the Program Management to develop, implement, and manage the Quality Program for the Client, including design and construction phases of each element, as well as program-wide activities
- Manage the Program Quality Management team for each element during mobilization, implementation, and closeout phases
- Develop and manage quality training program
- Manage the Quality team in the oversight of developers/contractors' quality programs for each element via the following
- Assist in establishing robust and consistent evaluation methodology ensuring reporting of adverse events are consistenly applied across all Dx sites
- Provide quality support for the Dx service organization and partner with local sites for improvement activities
- Drive continuous improvement and LEAN activities with the sites to improve efficiencies in post market activities
- Manage regional quality managers in Mexico and APAC to suppor the Dx business
- Ownership responsibility for the Dx business level QMR, establishing and disseminating metrics applied across all sites
- Function as the Subject Matter Expert on post market surveillance matters
- Function as the Dx champion for IT projects impacting the Quality function and/or systems
- You will have a strong solutions focus and be comfortable working in an environment which demands strong deliverables
- Ability to learn and apply knowledge of applicable local, state/province, and federal/national statutes and guidelines
- Ability to work with mathematical concepts such as probability and statistical inference, and fundamentals of plane and solid geometry and trigonometry
- Strong experience in using HP Quality Center tool. Quality Center Admin activity as an added advantage
- Strong working knowledge of ISO Quality Management Systems Standards
- Strong quality management credentials, with at least 5 years of experience managing teams, ideally with hands-on quality improvement experience
- Good knowledge of audit methods and quality improvement tools (Statistics)
- Strong technical skills in the manufacturing of solid oral dose, semi-solids, devices, and biologics
- Good and proven ability to analyze and evaluate Good Manufacturing Practice compliance
- Ability to attend to detail and work in a time-conscious and time-effective manner
15 Senior Quality Manager resume templates

Read our complete resume writing guides
How to tailor your resume, how to make a resume, how to mention achievements, work experience in resume, 50+ skills to put on a resume, how and why put hobbies, top 22 fonts for your resume, 50 best resume tips, 200+ action words to use, internship resume, killer resume summary, write a resume objective, what to put on a resume, how long should a resume be, the best resume format, how to list education, cv vs. resume: the difference, include contact information, resume format pdf vs word, how to write a student resume, senior quality manager resume examples & samples.
- Manager China QA/QC team;organize effective working process in SE departments among offices in different places
- ITS setting up for the audit and inspections in Vietnam and Taiwan
- Weekly / Monthly report to HQ management team
- Build up strong SE department team; team manpower and schedule monitor, coordination and application
- Business travel within China, VC meeting with HQ PM periodically for working communication and solving quality problems
- QC manual building up, translation, compile working and monitoring
- Make team KPI and appraisal review; set up QC working policy, monitor and evaluate achievements
- Factory audit conduct before SQE team setting up
- PPS control before the production season
- Establish Quality Operating Systems with adherence to all the global standards as required in marketplace
- Ensure compliance to certification agencies
- Conduct internal audits and ensure corrective actions
- Provide reports and documentation of key measurables as required, and maintain a system for communicating ongoing performance
- Report on the performance of the quality system to the management team
- Represent the needs of the customer in internal functions in addressing quality requirements
- Represent Quality function in dealings with external parties like customers or suppliers
- Provide conduit to Magna International Corporate Quality initiatives for continuous improvements
- Manage departmental and program budgets for Quality Activities
- Report on Cost of Quality metrics
- Support standardization of processes and systems between corporate and manufacturing plants
- Support Failure mode and effects management in design and manufacturing phases (DFMEA and PFMEA)
- Provide sign-off on design, drawings, validations to seek sign-off from customer
- Ensure effective change management process and tools for internal and external changes
- Manage direct reports, dotted line personnel, and other employees to accomplish company / program goals
- Motivate all company personnel to focus on Total Quality
- B.S. in Science or Engineering or related technical field
- Minimum 5 years in leadership roles in manufacturing organizations
- Hands on knowledge of automotive business, manufacturing processes
- Excellent interpersonal and communication skills both written and verbal
- Experience in training, coaching and moderating meetings
- Strong PC skills in excel, visio, powerpoint and internet applications
- Superior organization, multi-tasking skills
- Although this position is based in a corporate office environment, frequent visits and stays at manufacturing plants environments may be required
- Although travel is not an inherent part of this position, it may be frequently required as needed between corporate office, manufacturing plants, suppliers etc. with little or no notice
- International travel may be required. Valid passport and entry document for US are required
- As a management representative of TS16949/ISO14000, and ensuring establish, implement and continuously improve the quality system and policy required by the development of plant and customer
- To ensure that the company's products and service to meet the quality requirements of the customers, including the establishment and reach of quality objectives of the organization, the formulation and implementation of corrective and preventive measures and the continuous improvement of quality
- Be responsible for establishing new method and balancing standard of quality management; organizing a variety of continuous improvement activities within the plant
- Be responsible for operation supervising and internal audit activity of management system, and examining and put forward disposals of the implementation of management system in all department
- Implement product quality management process and guarantee the product quality
- Be responsible for solving problems of quality
- Leading the quality management of suppliers
- Leading the cost management of quality
- The quality department; finishing operating and financial budget
- Minimum 5 years quality control experience in manufacturing industry, auto parts industry is preferred
- Be familiar with 5 tools(APQP, MAS,FMEA,QFD and 6 sigma)
- Be familiar with quality management system(i.e. VDA6.1, TS16949, ISO14000)
- Be familiar with quality management skill and have experience in compiling QSD
- Bachelor degree in automotive manufacturing, technician or related subject or above
- Be skilled in using office software
- CET4, be fluent in oral and written English
- Work diligently, think clearly
- Good communication skill and team work spirit, good sense of coordination, good project management skill and problem solving
- Define quality measures for onsite product listing to influence conversion rate
- Gain a deep understanding of all processes used by cross functional teams to add and update products onsite
- Define quality metrics for all processes at various stages and work with each team to measure, monitor and tracks these results
- Identify core issues via root cause analysis, do not settle for mediocrity
- Propose improvements to current workflows to increase product quality metrics. The improvements will cover optimizations needed across many different processes, tools, vendor communication and more
- Drive the implementation of the proposed improvements across teams and monitor progress
- Gain alignment across teams and with senior leadership using data driven approach and a customer centric view
- You have solid knowledge of all areas of quality and/or compliance based upon practical experience, including root cause problem solving (e.g., 8D, CAPA), quality gates, Six Sigma, cGMP regulation, and/or remediation
- You have professional experience in introducing and implementing quality and compliance concepts
- You have an excellent university degree, with a technical or business specialization
- You have outstanding analytical and conceptual skills
- You show great initiative and creativity, working well both on your own and in a team
- You are able to convince others through your written and spoken communication
- Experience in Consulting or Service industry or e-commerce with quality and process improvement a plus
- Implement and drive a process of continuous improvement and cost reduction at the site by embracing ACE/Lean principles and tools including Kaizen, Value Stream Mapping & other related methodologies to achieve highest quality, lowest cost & customer focused standards
- Lead and develop talent within the quality organization
- Lead the site in complying with the Quality Management System (QMS)
- Responsible for the preparation and maintenance of the plant Quality Assurance Program, FAA Repair Station Manual, and the North Carolina State Radiation Safety Manual
- Responsible for the preparation and maintenance of Quality Procedures to support the Quality Assurance Program
- Lead and delegate third party inspection services and direct the activities thereof to support plant quality and manufacturing objectives
- Responsible for the preparation and maintenance of the nonconforming materials program
- Responsible for external and internal quality audits, source inspection, including the reporting of findings, recommendations, nonconformance and suitable corrective actions
- Responsible for the review of customer proposals prior to contract resulting in exception, clarification and/or comment
- Provide first point contact with customers, vendors, and regulatory representatives on all communications regarding Quality Assurance
- Responsible for the monitoring and analysis of scrap and rework costs; serve as a primary member of the Material Review Board
- Actively support the Company policies, procedures, and workplace rules including environmental, health, and safety objectives to ensure compliance, keep our people safe and minimize risk
- Responsible for compliance to all UTC, UTAS and outside regulatory policies and procedures
- Quality culture program: as role model to promote TQM culture in whole business unit
- Quality system/ legal compliance: ensure Mars Quality Management System fully implement, maintain and continuously improve in end to end process. Ensure full legal compliance
- Continuous improvement: leading TPM QM pillar to drive quality continuous improvement
- Quality capability building: talent expert in quality team, quality ownership with capable quality knowledge in operation team
- Quality Team building: high engaged team with strong quality capability
- Education & Professional Qualification
- Develops and manages the implementation of the strategic quality plan and ensures alignment / priority setting with strategic business plan and the alignment of appropriate resources. Guides business in making appropriate risk / reward decisions associated with product and service quality
- Oversees the development, implementation, and maintenance of effective, fact-based processes for establishing final product specifications and manufacturing specifications, and maintaining consistency between them
- Oversees implementation of global Quality initiatives. Develops project plans, allocates resources and manages project team members across locations in achieving project goals. Determines priorities and manages budgets and other resources
- Ensures ongoing compliance with ISO and Quality systems requirements and ensure total quality excellence in all activities. Ensures that manuals are updated and lab procedures are standardized at all plants in the region. Follows up on regional and other audits and oversees corrections and improvements
- Coordinates business complaints database, providing customer complaint resolution both at the plant and business levels. Works with cross-functional groups as needed to ensure solutions are found that meet both the customer and Cabot’s standards
- Ensures that the business or region has appropriate technical strengths in the areas of analytical chemistry, statistical methods and laboratory management
- The Senior Quality Manager will coordinate with the Director of Quality Operations and Facility General Manager and assure that PharMEDium’s core values are continually emphasized and kept in the forefront of facility operations
- Will be responsible for all daily quality operations within the facility. Ensures compliance to procedures, governing regulations and addresses non-compliance issues
- As the leader of Quality in the Center the Senior Quality Manager will set the tone for quality processes and services to deliver safe compounded preparations to hospital customers
- Working with Facility General Manger will assure timely closure of audit items
- Will initiate SOP revisions as needed to assure compliance, continuous improvement of overall operation
- Conduct root cause analysis for compounding errors and in-process errors generated at the facility during all phases of the Center processes
- Coordinate and schedule testing of all compounded services for the on-going stability and on-going sterility monitoring programs with the appropriate contract labs (ensure product is produced and shipped to the appropriate lab in a timely manner)
- Perform review of quality related documents generated at the Sugar Land facility
- Coordinate efforts with the Director of Quality Operations in the management of the Facility Testing Laboratory. Will ensure the day to day operation of the lab is meeting its testing goals in a precise, accurate and efficient manner
- Bachelor’s Degree in Science or related field
- Aseptic processing experience desired
- Minimum of 10-15 years’ experience in Quality, or related field in the healthcare or pharmaceutical industry
- 5-7 years Management experience
- Demonstrated understanding of quality assurance in a regulated (FDA, DEA, ISO) environment
- Effective problem solving, supervisory, strong communication (written and verbal) and organizational skills
- Develops and maintains a quality plan for one or more service center operations and programs, including service quality standards, quality scorecards, monitoring, coaching, performance metric assessment, and improvement actions
- Manages a team of Quality Supervisors, Monitors and/or Specialists, establishing roles, work standards, performance objectives, and enforcement through regular oversight
- Monitors and reports program performance against service level agreements and performance standards, initiating improvement actions when opportunities are identified
- Represents the quality function to clients, serving as a point of contact for quality inquiries
- Conducts process and product audits to confirm compliance with company policies
- Effective supervisory skills
- Proficiency with MS Office Products: Word, Excel, PowerPoint, Project
Senior Quality Manager Training & Compliance Resume Examples & Samples
- Collaborate closely with NBS training functions, NBS QA functions and Group Quality Training to create alignment across the business, optimize cross-functional GxP training strategies, cross-organizational synergies, and improve transparency of cross-functional GxP training standards and requirements. Provide periodic scheduled reports on progress against process improvement objectives
- Drive the design and implementation of robust quality-related training programs (e.g. Novartis quality requirements, GxP, SOPs, etc.) to facilitate continued growth and development of NBS personnel
- Define and implement the strategy for new quality processes, systems, and standards related to QMS training activities
- Drive structure, tools and processes to ensure highest quality standards for both internally and externally developed learning solutions and operational technologies, while maximizing the effectiveness of learning and training com-pliance
- Act as SPOC for audit and inspection queries from a NBS training and compli-ance perspective, as required. Oversee the implementation of corrective and preventive action plans resulting from Health Authority inspections or internal Novartis quality audits related to NBS training processes
- Ensure timely resolution of Customer quality issues through leadership within the ADD Quality Management system
- Responsibility for GSCQA Operations at ADD Manufacturing sites; TPMs and approved Supplier base worldwide
- Position requires domestic and international travel as well as outstanding relationship and interpersonal skills
- Support the continuous improvement of the GSCQA Organization
- Go / No-Go decisions related to Supplier selection
- Lead efforts to harmonize and simplify GSCQA processes
- Monitor quality performance (Supplier / Customer Complaints) and elevate through the appropriate channels
- Performs Drug Product batch disposition activities upon review of executed batch records, analytical data, and certificate of analysis for commercial drug products (oral dose)
- Provides quality oversight for root-cause analysis and product impact assessment for investigations as a result of deviations, OOS, and OOT investigations ensuring appropriate CAPA actions are identified
- Develops relationships with strategic external partners in support of Vertex’s expanding commercial portfolio and manages ongoing relationships
- Represents Vertex Commercial Quality Assurance on cross functional working teams and is recognized for strong communication and influencing skills
- Models Vertex Core Values and leadership behaviors and fosters their demonstration in others
- Experience with drug product (oral solid dosage forms) development with proficient knowledge of the following in a pharmaceutical setting
- Serves as a CQA Compound Lead for assigned clinical programs providing GCP compliance interpretation, consultation, training, and other supportive services necessary to maintain and/or improve the quality of Vertex research to ensure the protection of subjects’ safety, rights, and well-being as well as the integrity and credibility of data generated
- Maintains a contemporary knowledge of current industry trends, standards and methodologies as it relates to Good Clinical Practice (GCP)
- Fosters Vertex Core Values when collaborating with cross functional teams to advance quality activities while remaining independent and autonomous
- Responsible for developing strategic audit plans assigned programs, ensures audits are conducted in accordance with the plan, evaluates CAPA plans and drives CAPAs to closure in accordance with company standards and policies
- Manages and/or leads domestic and international audits for Clinical Investigators, Trial Master Files (TMF) and/or GCP document audits to ensure compliance to ICH GCP, applicable regulations, and company standards
- Assures reporting of potential or confirmed violations, as appropriate, in accordance with company standards
- As assigned, reviews and approves audit reports for content, format, regulatory risks, and compliance with applicable standards
- Identifies and escalates critical quality issues appropriately to CQA Associate Director for Quality Oversight of Clinical Programs
- Contributes to the development and implementation of continuous quality improvement initiatives
- Provides inspection support as necessary for Regulatory Agency inspections held at Vertex facilities or Clinical Investigator sites
- Nine years of relevant GCP QA work experience, or relevant comparable background and 5 years of relevant GCP QA experience
- In depth knowledge of FDA regulations, EU Regulations and Directives, MHRA Statutory Instruments and ICH GCP guidelines
- Experience with clinical trials involving drugs, biologics, devices and drug/ device combinations
- Communicates professionally, clearly, concisely and consistently both verbally and in writing to internal and external customers
- Possess the necessary scientific education and knowledge to manage quality oversight for clinical trials, understands medical terminology, standards of care and disease states to assure the ethical treatment of subjects
- Demonstrates proficiency in managing complex projects, delivering all expected deliverables in a timely manner, and proactively communicating changes in pre-established goals and deadlines
- Proficient in using Microsoft Office applications is an asset (MS Word, MS Excel, MS PowerPoint)
- This position may require up to 30% travel
- Bachelors or Master’s Degree in Engineering, Business or College Diploma
- 5 years Aerospace Quality experience
- 5 years Management/Supervisory experience
- 10+ years of experience dealing with quality management systems, regulations, guidelines and associated standards preferably in an aerospace manufacturing environment and experience with Canadian Aviation Regulations related to Manufacturing and Maintenance of aeronautical products
- Leading Lean and/or Six Sigma initiatives
- Broad knowledge of safety in aviation operations and the aviation regulatory framework (TCCA, FAA, EASA or other NAA’s); ability to provide guidance to Contract Manufacturer to ensure compliance in the Honeywell Manufacturing Policy Manual and Maintenance Policy Manual
- AS9100 and/or ISO 9001 knowledge and experience on regulations, guidelines and associated standards
- Experience in successfully taking companies through required quality management certification processes and audits (AS9100, Regulatory, Customer etc...)
- Experience in Management (training and development) of staff in various disciplines of quality (Customer Quality Engineers, Quality Engineers, Auditors, Inspectors)
- Proficient with Microsoft Office tools such as MS Word, Excel, and PowerPoint
- Practices and maintains integrity while following company requirements
- Creates a positive work environment by demonstrating and sharing functional/technical knowledge
- Ability to interface easily with customers and suppliers as the quality leader in the Integrated Supply Chain. Proven track record in building and maintain lasting relationships with other departments, key business partners, and government agencies
- Excellent communication skills for liaising and communicating with Contract Manufacturers, external suppliers, customers, and internal departments
- A well-defined sense of diplomacy, including solid negotiation, conflict resolution, and people management skills. Must maintain a sense of urgency to resolve quality issues
Senior Quality Manager for SAP Custom Development Resume Examples & Samples
- Managing the quality assurance activities for the full lifecycle of the development projects Supporting different project methodologies like waterfall and scrum
- Being a decisive member of large project teams advocating the quality of the solution
- Ensuring together with the project team that the project meets customer expectations
- Alignment with other departments like Solution Validation department or production unit concerning quality activities
- Coaching and consulting of development projects regarding Custom Development methodology and processes
- Project assessments and audits
- Improvement of QM processes and tools within SAP Custom Development methodology
- Advising customers regarding quality related topics throughout the whole project lifecycle
- SAP Custom Development takes care for its new team members. Therefore we will provide different kind of support to get you started for instance trainings and mentoring
- Test execution in the assigned projects
- Strong quality and customer focus
- Flexible and quickly adopting to new tasks
- Good project management skills is a plus
- Knowledge of I2M tools and I2M processes for Standard Development is a plus
- Knowledge of agile development processes
- At least 3 years of experience in Quality Management or Project Management
- Experience in working with cross location and multi-cultural teams
- Experience with new technologies like UI5 or hybris is a plus
- Drive a Zero Defect culture by managing controllable root-cause defects and ensure implementation of controls and minimize losses
- Own the development of robust quality feedback loops into training, analytics, and process to drive continuous improvement
- Interact with stakeholders at multiple levels and dynamically lead business and product teams to define and deliver solutions to establish quality standards and processes
- Identify and analyze data to isolate issues, identify trends, develop solutions and prioritize opportunities for overall process improvements
- Create, maintain and disseminate project information to stakeholders and senior management
- 5+ Years project/program/operations management within a quality environment
- Experience with Six Sigma tools and Lean techniques
- Maintain a positive and professional demeanor always portraying the company in a positive light and effectively managing sensitive issues
- For us, the culture fit is non-negotiable – you will have high energy, fantastic communication skills and huge amounts of drive
- Quality management in a contact center environment
- Advanced analytics background
- Additional European languages
- Experience with Lean and/or 6 sigma methodologies
- Leads and manages customer audits and regulatory/compliance agency audits
- Reviews weekly quality performance with CFT/BU management team and customer to drive for problem resolution and continuous improvement
- Works as a partner with suppliers to ensure that the material spec. is followed, the process is under control to ensure the material quality and on time delivery
- Identifies the potential risk to product long term reliability in the end to end manufacturing process and drive for proactive solution
- Typically requires 8 years’ experience in PCBA manufacturing, assembly and integration process, and at least 4 years works as Quality Manager
- Demonstrates maturity of people/process management and communication skills
- Good command of English and Chinese
- Lean Green Belt and Six Sigma Black Belt certification are preferred
- Characters of Intense Collaboration, Passionate Customer Focus, Thoughtful Fast Discipline Execution and Tenacious Commitment to Continuous Improvement
Senior Quality Manager Apparel Resume Examples & Samples
- You lead a team of 4 and you play a central role in the strategic quality management
- You are the lead for quality management projects and you follow them through until the end
- You set new strategies for the quality management in collaboration with other quality control@source pillars and the quality control inbound
- Your set KPIs for the evaluation of your team members, 3rd party providers and the quality control abroad
- You manage data analysis in order to identify weaknesses and strengths in structures
- You develop SOPs, guidelines, manuals and requirements for 3rd party provider and quality control abroad
- You support the team lead in budget planning and hiring process management
- Your take care of efficient utilization of resources as well as train and develop team members
- Your steer all activities in the quality control@source team; enhance, standardize, monitor and develop quality management processes internally
- You provide support in the existing product lifecycle management systems
- You are the interface for our Stakeholders
- You report autonomous to the team lead quality management
- You drive seasonal reviews for our supplier performances
- Boredom doesn't get a look in here! Zalando is a working environment where something new happens every day A corporate culture where your skills are judged only by your results - our motto: Every Day is a Casual Friday
- A dynamic and highly motivated international team of experts to inspire and support you
- Also a feel-good team: We don't just work well together, we also like to get together after work
- Training and further development opportunities to expand your skills and help you on your way
- The security of an expanding company with more than 10,000 employees and a company pension scheme
- Everything your retail-loving heart could desire thanks to our attractive employee discounts in our online shop
- Global Test Machine Product Line Quality Leadership for Ultrasound testing machines (UTTM)
- Site Quality Leader and responsible for sites in Huerth (Germany), Nove Mesto (SK) and product quality produced by other IT manufacturing sites
- Drive continuous cross functional efforts to improve key site metrics: Productivity, Manufacturing Defects, Rework, Cost and Customer Satisfaction
- As Site Quality Leader responsibility to manage (ensure, maintain and improve) site Quality Management System (QMS)
- Establish, monitor, and drive improvements for Site and product line quality goals including: audit program, corrective action (CA) system, customer issue resolution (CIR) cases, Net Promoter Score (NPS), Cost of Quality (CoQ), on-time delivery (OTD), continuous improvement (CI), Technical Regulations and Standards (TRS) compliance, and other relevant metrics for the location
- Work closely with the site leadership to drive quality and LEAN programs and raise quality culture and awareness at site and other related functions like Services etc
- Be voice and advocate of our customers
- Lead Quality team
- Relevant professional experience in Quality
- Experience implementing and influencing quality metrics
- Strong personality and Change management process
- Fluent German/English speaking skills
- Lean Six Sigma GB/BB
- Ability to document, plan, market, and execute programs
- Bachelors or Master’s Degree in Engineering, Business or equivalent related education
- 10 years Quality Management experience
- Broad knowledge of safety in aviation operations and the aviation regulatory framework (TCCA, FAA, EASA or other NAA’s)
- AS9100 and/or ISO 9001 knowledge and experience on regulations , guidelines and associated standards
- Sound knowledge to be able to provide expertise in the airworthiness standards and processes described in the Honeywell Manufacturing Policy Manual and Maintenance Policy Manual in compliance with TCCA requirements
- Experience in training and developing personnel at all levels in quality management
- Experience in Management of staff in various disciplines of quality (Customer Quality Engineers, Quality Engineers, Auditors, Inspectors)
- Experience with Microsoft Office tools such as MS Word, Excel, and PowerPoint
- Displays leadership qualities and practices
- Strong work ethic and positive team attitude
- Able to interface directly with cross-functional teams
- Able to build and maintain lasting relationships with other departments, key business partners, and government agencies
- Promotes and seeks excellence in all aspects of quality and compliance
- Good presentation skills including presentation to Senior Management
- 4-year degree in Electrical Engineering, Quality discipline or related field experience as an inspector (or equivalent work experience)
- A minimum of 15 years of related work experience in the field of Quality, including 7 years experience in a management capacity
- Lead Auditor certification (ISO 9001 or equal) or ASQ certification (CMQ/CQE.)
- Active membership in a nationally recognized Quality organization
- Materials, methods, regulations and requirements relative to the construction of electrical and related facilities associated with building, radio antenna structures and related projects
- Defects and faults in electrical construction installations
- Tools, surveying instruments and equipment used in construction projects
- Basic mathematics including algebra, geometry and trigonometry
- Working knowledge of "Codes and Standards" listed below
- Electrical installation best practices
- National Fire Protection Association (NFPA)
- Ability to communicate clearly and concisely, orally and in writing with a diverse group of professionals
- Requires management ability and extensive knowledge of Quality Management practices, processes and current technology
- Requires thorough experience in directing and implementing quality management systems and standards specified by ISO 9000, ASME, ANSI, ASTM and similar organizations
- Requires the ability to communicate clearly, conduct presentations to management and interface effectively with a diverse group of professionals
- Proficiency in analyzing situations in business terms, i.e. ROI, cost per unit, etc
- Strong interpersonal skills to work with other departments in a tactful and effective manner
- Strong leadership and management skills, excellent written and verbal communication, strong analytical, problem solving, and risk assessment skills. Ability to make public presentations
- Computer proficiency with advanced knowledge of Microsoft Office Products, including Word, Excel, Power Point, Access and Visio. Project Planning skills are essential
- Ability to direct and manage team members with day to day activities as well as special projects to assure timely completion
- Lead in the development, improvement or maintenance of compliance requirements supporting corrective activities, PAI readiness, and government agencies (FDA) inspection management
- Participate and lead in internal/external audit and inspection preparation and management. Manage remediation projects, leading cross-functional teams. Manage various source observations (audit, inspection, risk assessment) to assure corrective actions are implemented
- Ensure that the internal audit processes are conducted and that the corrective actions are implemented
- Develop government agency communications, including written correspondence, responses to inspection observations, presentations and verbal communication including but not limited to Escalations and Field Alert Reports
- Support the implementation and tracking of Key Quality Indicators
- Lead the creation and maintenance of the annual Quality Plan
- Facilitate with close interaction with the local, region and global QA functions, product quality investigations processes related to Field Alerts, Product Recall, Counterfeit and/or Withdrawals
- Facilitates the Deviation and Change Control Governance process at the site
- Actively manages the Compliance team and sets up their development and objective targets
- 1) Thorough knowledge of applicable procedures, specifications, regulations and standards. 2) Ability to manage/supervise a large team of employees 3) Strong analytical and problem solving skills. 4) Strong communication and leadership skills. 5) Strong interpersonal/communication/influencing/negotiation skills 6) Strong project management skills. 7) BS in science or engineering; advanced degree helpful. 8) 7 years in Quality, Manufacturing, 9) Engineering or related field. 5 years of management experience
- Promote and maintain effective communications and relations with district management, project management teams, authorized inspectors and engineer or client as required
- Recruit, hire, train, mentor and supervise district and project quality control inspectors
- Assist project teams in setting up the quality department and preparing for project kick-off and pre-job planning meetings. Assist in identifying the proper Inspection and Test Plans (ITP’s), checklists, and other documentation for the project
- Update the American Society of Mechanical Engineers (ASME) Quality Control Manual at least annually as required by code revisions, lessons learned, best practices, corporate initiatives, or changes in the work practices in the field
- Support project teams in the development and implementation of project quality plans and supplemental procedures as needed
- Oversee code compliance for work which falls under the ASME Quality Control Manual
- Bachelor’s or Master’s degree in mechanical, electrical, welding, civil engineering or construction management/engineering is preferred
- Certifications from AWS, CWB, API, ACI, ICBO, ASNT, NACE, etc. are advantageous
- Exceptional math, computer, verbal and written communication skills; proficient with Microsoft Office Suite
- Ability to freely access all points of a construction site in wide-ranging climates and environments
- Serve as People Manager with strong leadership skills
- Project Management / Continuous Improvement
- Experience providing QA support and oversight of GMP manufacturing operation including batch release
- Experience with drug product (oral solid dosage forms preferred) development and manufacturing with proficient knowledge of the following in a pharmaceutical setting
- O o cGMP’s and associated CMC regulatory considerations
- Lead the Quality Engineering group primarily responsible with providing quality oversight and support to the Annex Drug Product Facility operation in the following key areas/activities; engineering, validation/qualification and facilities and utilities support
- Provides change control assessments and approval relative to product quality. Chair the weekly change control review board meeting. Support investigations and CAPA for deviations within areas of responsibility. Collaborate with customer groups (Manufacturing Operations, Formulation Development, Analytical Development, and Facility Operations) to ensure quality systems are monitored and established metrics are met
- Provide quality oversight of site validation activities including qualification of manufacturing equipment and control systems, facilities and utilities system, and analytical instrument,
- Provide quality oversight of various site wide programs including the equipment and facilities maintenance, instrument calibration, periodic assessment, environmental and utilities monitoring, stability chambers trending, and SCADA alarm management
- Provide support of facilities and engineering projects, including capital projects, annual plant shutdown, and various improvement projects as assigned
- Responsible for tracking QA-Eng performance metrics; including site metrics for change control
- Participate in inspection readiness activities and provide support during regulatory site inspections. Lead compliance walkthroughs of the site and help drive the closure of any observations
- Lead/participate in continuous improvements of department processes
- May be required to assist the QA GMP Operations group with commercial and clinical batch disposition activities
- Strong leadership skills with the ability to thrive in a high throughput environment
- Ability to lead and manage complex projects/teams within corporate objectives and project timelines
- Ability to independently lead cross-functional teams, represent the Quality unit
- Ability to evaluate quality matters and make decisions utilizing risk based approach
- Expert knowledge of global GMP requirements governing oral drug products and knowledge of oral drug product manufacturing practices. Strong validation background
- May serve as People Manager
- M.S (or equivalent degree) and 4-5 years of relevant work experience, or B.S in scientific or allied health field (or equivalent degree) and 9+years of relevant work experience
- Experience providing QA support and oversight of GMP manufacturing operation
- Quality KPI (Scorecard / Satisfaction) reporting globally to stakeholders in China, other regions and HQ
- Support the Risk assessments and decision competence with Business Division
- Installation and definition of effective process flow for complaint management in China
- Manage and control spending within budget
- Use effective verbal and written communication skills. Add, subtract, multiply and divide whole numbers and fractions rapidly and accurately
- Read and understand plans and specifications. Visualize two-dimensional drawings in three dimensions
- Examine/inspect field conditions and identify problems, inaccuracies, and cost saving measures that arise or that may be encountered. Take corrective actions as needed
- Inspect subordinates’ work for compliance with the contract plans and specifications, point out deficiencies and explain and take any corrective action needed
- Listen to other employee's and supervisor's suggestions, complaints, problems, safety concerns and recommendations, evaluate each and then devise and implement a plan of action based on that evaluation
- Observe functioning of installed equipment or systems to determine hazards and need for adjustments, relocation or replacement
- Identify safety hazards you or other employees may be subject to and take all necessary corrective actions to eliminate or minimize hazards
- Understand and respond appropriately to all safety hazards and warning devices (i.e. back-up alarms, smell of smoke, different colored warning tags, warning sirens.)
- Understand and implement lockout/tagout procedures in safe manner
- Be motivated and work productively
- Have a thorough knowledge of the District’s Quality Management System, Work Instructions and Procedures
- Develop the Project Quality Plan, goals and objectives. Identify project level risks and implement measures to limit liabilities
- Develop a complete understanding of contract, specifications, applicable codes and jurisdictional requirements and client quality expectations
- Promote and maintain effective communications and relations (verbally and in writing and through periodic management meetings) with District management, project management teams, and the engineer/client as required. Conduct and coordinate client interviews and surveys
- Develop future leaders by mentoring and coaching quality personnel. Hire, train, mentor and supervise project quality personnel and provide input on compensation
- Coordinate Quality staffing needs on the project with the District Quality Manager and assign qualified personnel
- Manage implementation and effectiveness of the Project Quality Plan through periodic reviews, document findings and implement corrective/preventive actions
- Facilitate quality walk-arounds (tours) and quality plan implementation reviews (surveys/self-assessments) to status the program effectiveness
- Ensure that tests are performed, received and interpreted timely so that impacts due to failure are minimized. Review/approve (initial/date) all third party test results for compliance with project requirements
- Provide training to supervision and craft/trades regarding the project specific quality process and requirements
- When required, develop written plans and procedures for project specific activities to supplement task planning, i.e. pipe line cleaning
- When required, assist in the development and implementation of the Turnover Package Procedure and assist in assembling turnover packages
- Manage Non-Conformance reporting. Ensure the cost of rework is tracked and reported
- Be familiar with District’s ASME Quality Control Manual; be capable of managing the ASME Quality Control Inspector’s work on the assigned project including Pre and Post Weld Heat Treat (PWHT) activities
- Research and answer questions of a technical nature. Assist in the interpretation of construction codes and standards
- Coordinate with the District for needed codes, standards and procedures, including Welding Procedure Specifications
- Ensure that welder certifications and status logs are maintained current and submitted as required
- Provide input on continuous improvement of District’s quality program
- If assigned, oversee QA/QC on multiple projects
- Perform all other job-related duties as requested by supervisor
- You are responsible for leading, planning, and implementing quality management support projects to ensure regulatory compliance and improve competitiveness of Siemens Healthineers businesses worldwide (Business Areas, Business Lines, Key functions and Countries)
- You will provide solution oriented and practical support of the Siemens Healthineers units in the implementation of national and international requirements (standards, laws, regulations) in their QM systems and processes
- You will identify trends and focus topics to use as an input for knowledge sharing sessions to ensure dissemination of best practices and lessons learned throughout Siemens Healthineers
- You will lead, plan, and execute healthcare regulatory compliance audits on a global scale
- You are responsible for the definition and maintenance of process descriptions, quality regulations, and requirements
- You will create content and perform training sessions on quality relevant topics
- You hold a master‘s degree in science or a comparable subject (e.g. Chemistry, Biomedical, Hardware or Software engineering) – alternatively you have a comparable professional experience
- You have 7-10 years of relevant experience in Medical Device industry (in-Vitro and/or In-Vivo) in the field of Quality Management
- You have expert knowledge of standards and requirements in the area of software development in medical engineering in accordance with IEC 62304 and FDA Software Validation Guidance
- You have several years of hands-on quality auditing experience (ISO 19011 or similar certification desired)
- You have advanced knowledge in continuous improvement methods and processes (e.g. CAPA) as well as knowledge in risk management in accordance with ISO 14971
- You have fluent written and verbal communication skills in German and English as well as strong communication and presentation skills
- On a personal level you exhibit customer oriented behavior, high motivation and initiative, you are creative, proactive and assertive with strong leadership skills
- Provides necessary definition, development, and deployment of product/package quality assurance strategy by addressing all phases of product development from water quality and injection molding to the finished package
- Aid in the development, publishing, and/or maintenance of all Niagara Bottling Quality Assurance procedures, policies, and standards in use at each designated production location
- Coordinate the development and/or implementation of Niagara Quality Assurance SOPs, policies, and standards through the appropriate corporate personnel within Niagara Bottling
- Ensure that all in-house Quality Assurance standard operating procedures (SOPs), policies, and standards are written and/or published with the intent of assuring that all finished products meet and/or exceed the product/packaging standards as set forth by Niagara Bottling, FDA, local state requirements, and the IBWA (bottled water only)
- Ensure all designated production locations maintain all required regulatory information and certifications. (i.e. kosher certificates, SQF certificates, 3rd party audits, etc)
- Ensure all in-house bottled water, beverage analyses, laboratory tests, and procedures are properly understood and carried out. When required by a customer, ensure that relative/routine quality and production documents are forwarded to the customer in a timely manner
- Review chemical usage, storage, and safety practices with each designated production locations
- Work with each designated production locations to ensure water processing, batching, thermal processing, bottling, packaging and logistics quality compliance. Ensure compliance and improvement through GMP auditing and implementation of corrective actions
- Maintain a strong knowledge of food processing and plant sanitation principles and techniques
- When necessary, monitor the product/package quality as well as GMPs at regional 3PL warehouses and or re-packs
- Develop a strong quality team to improve customer satisfaction and quality objectives. Acts as quality interface for all customers, implementing customer specific quality requirements and assuring conformance
- Responsible for planning, leading, educating and assisting the site staff in the execution of the established continuous improvement strategy through recommendation and strategic implementation of metrics, tools and training
- Instills a disciplined approach to seek out and eliminate quality problems at all levels of the organization, achieved through a sustained, results-oriented process to instill this culture changing mindset into all levels of the organization through leadership, tools, metrics and training
- Defines a road map of continuous improvement for the organization by establishing priorities, methods and strategies for implementation using data to both guide the process as well as monitor the result
- Analyzes failures, determines cause, determines responsibility, and initiates corrective action
- Implements Advanced Quality Planning in new product development process, participates in program and design reviews
- Promotes quality achievement and performance improvement across the organisation
- Ensuring compliance with national and international standards
- Other duties as assigned – it is understood that this list of major duties and responsibilities is not an inclusive list and that other duties and responsibilities, which may include helping others in the same or different departments, may be assigned by supervision
- Have an absolute commitment to and passion for achieving Quality improvements in a made to order technical product focused environment
- Have a high energy level and drive required to lead critical functional areas within a dynamic business unit
- Have strong persuasive skills and the ability to exercise influence beyond direct authority
- Have a hands-on style and a willingness to perform a range of detail-level work, i.e. a doer rather than an administrator
- Ability to teach and train others
- Demonstrated leadership and facilitation skills in a matrix environment and ability to motivate all levels of the organization. Excellent oral and written communication skills, to include presentation skills and facilitation of groups toward deliverables
- Troubleshooting and Complex Problem Solving with ability to evaluate options and implement solutions
- Ability to link customer needs and business processes; Deductive Reasoning (the ability to apply general rules to specific problems to produce answers that make sense); Inductive Reasoning (the ability to combine pieces of information to form general rules or conclusions, includes finding a relationship among seemingly unrelated events
- Extensive conceptual and analytical ability in order to conceive quality requirements in designs, production operations and processes
- Ability to manage multiple projects and adapt to changing priorities
- Ability to establish and maintain effective working relations with a wide variety of individuals
- Ability to represent the company in a positive and professional manner
- Minimum of 3 years experience as Senior Quality Manager
- Certification is an advantage including Quality Improvement Associate, Six Sigma
- Knowledge of tools, concepts and methodologies of QA and relevant regulatory requirements
Senior Quality Manager Development Projects Resume Examples & Samples
- Manages all quality aspects at external manufacturers for Drug Product and Medical Devices of Biopharmaceuticals, particularly for late stage development phases (product transfer, process validation, dossier submission)
- Ensures the operational business complies with international standards of cGMP, regulatory as well as internal requirements
- Single point of contact for all quality related activities at the contract manufacturer and within the Novartis Contract Manufacturing Relationship Team
- Ensures pre-approval inspection (PAI) readiness and drives remediation efforts to ensure sustained quality performance and reliable supply of products
- Interact with SSPA stakeholders at multiple levels to define and deliver solutions to establish quality standards and processes
- Remain flexible to changing priorities, open to new ideas and have relevance and moderation quality as the top focus
- Can think big and develop new ideas, simultaneously well at nurturing new ideas and applying high standards to weed out those that are least valuable
- Is comfortable collaborating and influencing team members, working with engineering teams and other business groups, and presenting projects to senior leadership on a regular basis
- Has the technical aptitude to quickly grasp complex technical issues and communicate directly with technical teams
- 5-7 years project/program management /Quality Assurance or Lean 6-Sigma is required
- Bachelor’s degree in business discipline
- Experience developing operational process and technologies to drive quality and continuous improvement
- Experience in customer experience and quality initiatives
- Map business requirements, understand business process, study and analyze workflows, design solutions and communicating results throughout the organization
- Proficient with MS Excel / MS office
- MBA is preferred
- Good oral and written communication skills are crucial
- Experience in online retail is a plus
- Ability to think and act both strategically and tactically
- Proven track record of taking ownership and driving results in an fast paced business environment
- Demonstrated ability to build quantitative reporting and dashboards for defect analysis
- Curiosity to dig several layers deep into metrics, have an innate desire to understand key drivers and whether they are the correct or best metrics to measure/manage a business or process
- Experience in managing people
- Innovative and self-directed
- Participate in weekly communications meeting with the safety team (regional and global safety leads) including reporting out on active issues and new issue tracking through the PPIRP system
- Participates in the Structures Weekly Quality call to ensure understanding and escalation of any potential Safety Compliance issues
- Leads all identified Structures Safety and Compliance concerns through the verification and documentation phases
- Leads and confirms all identified Structures Safety Compliance related read across and lessons learned activity through the verification, documentation and implementation phases
- Assist in the development and review of white paper summaries for each identified issue and distributes them as determined by the concern escalation and notification protocols
- Assist in timely issue closure through the PPIRP system as defined and agreed upon in the problem solving process
- Review and sign-off that all necessary Engineering Specifications and Testing/Inspection Instructions to prove compliance to identified Safety and Compliance Standards are included in the DV, PV, and IP mandated sampling frequencies. 100% compliance is required
- Complete documented Safety and Compliance check sheet to confirm that all applicable program standards and associated controls have been developed for the product. Document shall be signed by all Advance program functions and provided to the Structures APQP Manager for attachment to APQP profile record. 100% compliance is required
- Minimum of 7 years of Experience in Automotive Product, Advance Manufacturing Engineering or Automotive Quality Manager position
- Strong Systems or validation background in Seat Systems Structures highly preferred
- Strong Welding background and testing knowledge for Automotive Structures highly preferred
- Implement and supervise technical quality (i.e., adherence to client, regulatory agency, and company requirements), perform administrative functions (i.e., budgeting, forecasting, staffing, etc.), and help to maintain harmonious client relations
- Serve as project Quality Manager on medium to large projects, if necessary
- Serve as the office Quality Manager, if necessary
- Facilitate the implementation of the Operating System Requirements (OSR) in offices for which responsibility is allocated
- Ability to communicate effectively with audiences that include but are not limited to management, coworkers, clients, vendors, contractors, and other stakeholders
- Minimum of 7 years of experience in Automotive Product, Advance Manufacturing Engineering or Automotive Manager position
- Strong Systems or validation background in Automotive components highly preferred
- Participates in the JIT Weekly Quality call to ensure understanding and escalation of any potential Safety Compliance issues
- Leads all identified JIT Safety and Compliance concerns through the verification and documentation phases
- Leads and confirms all identified JIT Safety Compliance related read across and lessons learned activity through the verification, documentation and implementation phases
- Complete documented Safety and Compliance reviews to confirm that all applicable program standards and associated controls have been developed for the product. Should be confirmed in MGR reviews to complete the APQP requirements for each program phase. 100% compliance is required
- Leading the roll out of Global NGP quality standards and manage the ongoing performance and quality requirements of end markets in Geographies
- Leading delivery of NGPs on Geography level in scope of quality work streams. In this context, the role holder contributes to the way quality is integrated into each new product implementation project
- Manage and cooperate with Global, Regional and local stakeholders for the sign off of new product introductions and assessment of process capability in order to enable a smooth transition into local production and launches
- Deputy of NGP Quality Manager during TR/TA
- Responsibility for supporting the deployment of processes and systems needed to measure and report quality performance in the end markets. This includes the support to design, develop and embed end to end customer care processes aligned to global strategy while managing local uniqueness (i.e. call center, device & consumable retrieval process, device hyper care service)
- Manage the collation of region wide quality information such as quality data from NGP factories and consumer complaints from end markets, to ensure timely publication to the Global NGP team & senior management and other key stakeholders
- Ensure that information is reliable and up to date and escalate deviations from agreed reporting performance levels to the Geographic Quality Manager and appropriate business recipients
- Applies advanced quality planning tools (FMEA, DOE) for development and qualification of new materials and products and builds the quality plan for each innovation or NGP within the Geography
- Responsibility for maintaining external testing facilities within the region to support ongoing quality compliance measurement (where required) and root cause analysis of major quality incidents
- Meet internal and external guidelines regarding quality and safety (quality manuals, regulatory Current Good Practice guidelines, health authority guidance, Standard Operating Procedures, Health, Safety & Environment, etc). Promote and enforce compliance to guidelines. If managing associates, ensure same for them
- Act as counterpart for Technical Development Quality organization for new products entering the site
- Write/contribute to internal compliance policy and/or comment to regulations
- Lead and support direct reports in line with Novartis values and behavior including objectives setting, performance evaluations, development planning and participate in recruiting process
- At least 5 years of relevant experience in Quality Function
- At least 3 years in a commercial manufacturing site
- Ability to influence people, negotiate and communicate
- Sound scientific, technical and regulatory knowledge in a specific area
- Excellent knowledge of Current Good Practices, working knowledge of safety and environmental regulations and guidelines
- Good and proven ability to analyze and evaluate Current Good Practice compliance
- Establishing and maintaining metric capture programs, trending activities and implementing the QC plan
- Conducting and leading internal and external meetings and interfacing with customers
- Providing strategic support at a branch location level
- Performing corrective action investigations, determining root causes and defining corrective/preventive action measures
- Performing internal audits
- Performing training and supervising of the inspection workforce
- Evaluate and update current inspection and system procedures
- Perform Qualification tests on work benches
- Set up and participate in QAT''s and meetings
- Manage the Quality Information Systems (QIS)
- Conduct customer on-site visits
- Train new and current employees
- Initiate and manage new projects
- Provide strategic direction to take CTDI Quality to the next level
- Strong understanding and experience of TL9000 and/or ISO 9001 Quality standards
- Expertise involving electronic equipment, diagnostics, and/or electronics repair
- Working knowledge of IPC-610 and its application to process control
- Strong documentation keeping best practices
- Excellent analytical skills, organizational skills, and follow-up skills
- Ability to create and deliver presentations
- Must be able to communicate with all levels of management
- Must be detail oriented and a team player
- Ability to read electronic and electrical schematics and machine shop drawings
- Ability to motivate production/quality performances
- Collaborates with other functional managers in planning and implementing quality programs to ensure a customer perception of trust and reliability
- Establishes quality policies that ensure compliance at the lowest possible cost
- Reviews external complaints for the purpose of developing appropriate quality policies and standards
- Manages and executes quality programs that lead to improvements in quality, costs and overall efficiency
- Proactively advises management regarding the overall quality standard of products
- Effectively represent theNorth Carolinaplant to the customer through customer visits, visits to customer locations, during customer surveys and through problem resolution
- Monitors quality indicators and trends and makes this information available to the operating units
- Partners with engineering and marketing as required for effective product introduction and problem resolution
- Provide overall direction for the vendor quality program and incoming inspection of purchased material
- Monitor and stay abreast of customer requirements to assure they are integrated into the Fletcher Quality system
- Provide overall direction for warranty analysis and subsequent corrective action
- Prepare budgets and monitor performance to plan
- Identify capital requirements as they relate to items that could affect product quality
- Provide overall guidance for the gage control system
- Benchmark other organizations as well as other BorgWarner facilities for activities and systems that could assist in achieving world-class status
- Champion the Company Quality Award activities
- Manages TS 16949 activities and subsequent registration
- Provide guidance where required to assure successful application of Production Part Approval Process
- Participate in the deviation process to assure product quality is not adversely affected
- Participate in design reviews
- Acts as an integral member of the supplier performance improvement team along with the Materials Manager
- Represents the Fletcher plant and the Division at Corporate sponsored and mandated quality improvement initiatives
- Lead the management review process to evaluate the effectiveness of the Quality System
- B S Degree Engineering MUST HAVE AN ENGINEERING DEGREE
- Six Sigma Black Belt – strongly preferred
- 5+ years experience as a Quality Manager and Operation Management Experience in a fast paced manufacturing environment with full responsibilities of internal, external and customer quality (automotive strongly preferred)
- Operation Management Experience
- 5 years Experience
- Bachelor's Degree in Food Sciences
- Able to Relocate
- Knowledge of SAP systems
- Good written, verbal and computer skills
- Bachelor's Degree in Food Science or related field and/or training
- Food Engineer with 5 years' experience on industrial sites strongly preferred
- Knowledge of regulatory databases and applying to building and equipment for products in the USA (3A, USDA, FDA norms)
- Basic working knowledge of food microbiology
- Excellent knowledge of Current Good Manufacturing Practice working knowledge of safety and environmental regulations and guidelines
- Good knowledge of drug development. Novartis - Job details Page 1 of 2 https://sjobs.brassring.com/tgwebhost/jobdetails.aspx?partnerid=13617&si teid=5261... 09.03.2016 6. Good organizational skills
- Formulates and executes quality assurance objectives in alignment with Corporate, Business Unit, Plant goals and requirements. Coordinates objectives in collaboration with the plant manager to maximize product performance, reliability and minimize costs
- Performs Gap Analysis between established goals and objectives and evaluates current plant QA capability. Develops and communicates plans to close critical QA gaps
- Sets clear goals and performance expectations for all direct reports, continuously assesses the performance and capabilities of all QA direct reports (relative to goals and future business requirements) and provides them feedback and holds them accountable for excellent performance
- Develops, analyzes and reports statistical data relative to quality systems performance and implementation ensuring customer expectations are maintained
- Develops and maintains audit functions ensuring quality systems and products are consistent with internal documentation, standards, regulatory agencies and customer requirements
- Drives corrective and preventive action processes ensuring full identification of issues, root cause analysis and validation ensuring quality systems are effective
- Provides documentation systems that establish an accurate baseline of design specifications, component/product drawings, and manufacturing processes
- Develops and maintains failure analysis process; ensures feedback and corrective action communication and implementation is consistent with customer expectations
- Directs employees engaged in inspection and testing activities to ensure continual and effective control over materials, facilities, processes and products
- Acts as Plant Management representative and ensures full compliance to ISO 9000, ISO 13485, 21CFR 210-211, 21CFR820 and EQOS standards as applicable to the site
- Bachelor’s Degree in relevant field; minimum of 10 years quality management experience in a progressive quality environment
- Successful experience working within a Life Science progressive manufacturing environment
- Expertise implementing Six Sigma, Statistical Process Controls, FMEA, process audits and other proactive quality management practices
- Strong management and leadership skills with an ability to collaborate with manufacturing, R & D, Product management, Process Engineering and all other organizations
- Experience with SAP desired
- ASQ Certified Quality Manager or Auditor preferred
- Lean manufacturing certification/ experience highly desirable
- Experience leading a quality organization in the pharmaceutical or biopharmaceutical industry is desired
- Manages all personnel, activities, initiatives and site specific programs in the achievement of quality organizational goals, as well as Corporate driven initiatives related to the Quality System in support of the following services - incoming inspection, product release, material review, ship hold, field action, product complaints, RTM, SAP Quality Module, Quality Control inspection processes, process control & development and project management; Interfaces with other members of Management with respect to regulatory compliance initiatives, on-going compliance with all procedures and standards, as well as implementing continuous improvements initiatives; Sets policy and procedures for the Quality processes at the European Centre of Operations, in conjunction with the Director Quality Assurance, including systems which impact the European Distribution Centre from other BSC facilities. Responsible for interacting with internal BSC sites and external customers on Quality related issues; Provides vision and leadership for strategic projects with significant impact within the site and Corporation; Works collaboratively across functions, regions and business units to implement project strategies and tactical plans to deliver results; Contributes to organizational efficiencies through the recommendations regarding the creation, implementation and harmonization of new corporate standards and procedures; Acts as Subject Matter Expert in internal and external audits
- Quality Compliance: Ensures the Quality System is substantially compliant to GMP/QSR practices and standards as defined by the FDA, ISO and regulatory bodies in close coordination with the Quality Systems Manager. Works with Senior Management to identify areas of the quality system needing improvement. Takes a leadership role in developing and implementing new techniques and initiatives to enhance the quality management system on site; Maintains site compliance to external standards
- Quality Metrics & Communications: Develops and generates quality metrics reporting/tools as well as process improvements. Responsible for trending/analysis of any Quality data and develops/ maintains SOPs for recording, evaluation and reporting of Quality and reliability data. Shares periodic information across the Regional Quality Team on local issues, with the purpose of soliciting input on best practices and/or seeking consistent solutions to common problems; Provides the local management team with periodic reports on appropriate quality metrics; Establishes cross-functional team relationships; Where applicable, establishes and fosters relationships with local medical device industry groups to advance the interests of Boston Scientific in matters of quality and compliance
- B.S. Degree with minimum of 9 years’ experience in quality for medical devices and/or pharmaceuticals
- Demonstrated leadership & people management experience within a functional management role
- Comprehensive understanding of the QSR, cGMP and ISO/MDD standards
- Excellent communication skills with the ability to influence others
- High level of initiative & autonomy through continuous questioning of current practices
- Strong command of the English language (verbal & written)
- Effective team member, motivated to achieve and demonstrate best practices in line with the department & global objectives
- Develop and drive initiatives to improve and sustain manufacturing quality across all manufacturing locations, including third party locations
- Drive continuous improvement workstreams in support of product quality, customer experience, delivery and cost initiatives
- Directly partner with the other functions to improve customer satisfaction and drive customer loyalty
- Develop effective Quality Systems and deploy across all manufacturing sites. Establish sound process to ensure robustness of system
- Partner with business leadership to deploy customer scorecards across all manufacturing sites driving continuous improvement, connectivity to customers and enabling certifications
- Establish effective root cause corrective action and failure mode analysis processes and tools to drive effective mistake proofing and failure mode elimination
- Establish cross functional quality continuous improvement teams to drive complex problem resolution and set proactive activities to address problem statements
- Drive RMA and CA cycle time down and improve on response time
- Responsible for all site quality certifications and product validation plans
- Sets effective process and product audit programs to ensure exposure is limited and that quality aspect of product is in control
- Create and implement short and long term vision for manufacturing quality, centered on the customer and the built in quality strategy
- Creates development plans for all quality leadership employees setting up sound and robust succession plans and career paths
- Provide leadership, guidance and coaching to site leads and site quality teams in performing effective customer response and communication on quality and manufacturing related topics
- Directly responsible for all business quality metrics as well as all customer scorecards and certifications
- Partners with Technology to effectively launch products in line with launch protocols and processes and that meet customer specifications
- Establish COA’s which effectively represent requirements that are specified and measured for the end use application and/or the end-use customer
- Develop and deploy effective processes to ensure incoming raw materials meet proper specifications based on the end use application or customer
- Bachelors of Science Degree in Engineering or Technical discipline
- Minimum of10 years in field or an advanced degree and comparable experience
- Minimum 10 years quality, service or manufacturing experience
- Master’s degree in business is preferred
- Process oriented and customer focused
- Results driven with a positive can-do attitude
- Communicate effectively across multiple layers of the organization
- Excellent collaboration and negotiation skills
- Strong strategic and business Acumen
- Ability to foster teamwork
- Leadership skills including communication skills, mentoring and constructive feedback
- Ability to work collaboratively with cross functional
- Six Sigma / DFSS background, strong analytical skills and problem solving skills
- Must be capable of supervising direct reports in multiple locations
- Strong computer skills, particularly Excel or Access for data-mining and report generation, including Pivot charts & tables
- Ability to get Green Belt certified (After Hire)
- Trained/certified in Six Sigma and/or Lean Manufacturing techniques. Green Belt or Black Belt Certification highly desired
- Certified Quality Engineer by American Society for Quality (ASQ)
- Expert in quality management tools such as APQP, PPAP, FMEA, MSA, SPC
- Familiarity with Quality Systems (e.g., AS9100, ISO 9001, etc.), Lean Manufacturing, Manufacturing Process Control, Six Sigma
Senior Quality Manager / Engineering Resume Examples & Samples
- Plans and executes required audit activities (schedules, reports, finding resolutions). Facilitates all internal and external Quality audits and briefs management with results
- Manage the Quality team in the oversight of developers/contractors' quality programs for each element via the following
- Proficient with CMAR, design build, and traditional project delivery methods; in depth knowledge of the contracting relationships and typical contracting terms between the general contractor and all other parties
- Thorough knowledge of engineering principles and disciplines, construction technologies and job site construction management and OSHA
- Knowledge of scheduling and familiarity with project and document management software (such as Primavera, PROLOG, Expedition, etc.)
- Demonstrated ability to read and interpret civil, structural, and site utilities plans and detailed shop drawings
- Bachelors in engineering, construction or related field
- Provides oversight of inspection activity for product throughout production cycle. Directs workers engaged in inspection and testing activities to ensure continuous control over materials, facilities, and products in order to maintain compliance with all applicable codes, standards and/or specifications as well as all corporate and quality policies
- Responsible for all QA/QC functions and reporting including the following processes: Non-conformance (NCR); Corrective/Preventive action (CPAR); Field quality reports and customer complaints/returns; supplier monitoring, auditing and evaluation; gage calibration; quality inspection plans and contract packets( quality records); Material review board; Warranty analysis and investigations; Cost of Poor Quality reduction; first article qualification and production approval; and auditing ( internal and external). Plan and Conduct monthly Quality Management Reviews
- Oversee the process for design, release, and revision control to manufacturing and make suggestions to Engineering on necessary changes to reduce cost and assure consistent quality
- Direct the efforts and activities of Quality Assurance Team to ensure support to the shop floor and related suppliers, and contribute to the ongoing improvement of productivity and quality while reducing costs of operations for assigned processes
- Lead the quality program including establishment of required procedures, work instructions, and forms, internal audits, corrective action, and outside audits in meeting certification to recognized standards. Responsible for establishing and maintenance of 3rd party certification such ASME U-Stamp, China Code and other international standards
- Assure that compliance with established quality standards by all internal operating functions and external suppliers is attained
- Assist in the pre-quotation review of customer quality specifications to assure that any special requirements can be met and that adequate cycle time and cost is reflected
- Establish and maintain sound procedures to ensure the definition of proper quality standards and the means of measuring conformance to those standards
- Review internal and external failure costs and develops effective corrective/preventive action programs to reduce defect generation and associated costs
- Supervise the training, development and effectiveness of the quality group, while maintaining a safe operation and positive employee relations
- The Manager, Quality has an overall responsibility for developing, communicating, measuring and enforcing the Quality Policy through the establishment and monitoring of consistent work processes that fully meet internal and external quality requirements
- The incumbent is responsible for determining the frequency and detail of in-process quality audits so as to maintain cost effective processes and products that meet customer expectations and specifications. Establishes quality processes and procedures and publishes required internal and external quality documentation
- This position is responsible, in coordination with Sourcing group, for establishing vendor qualifications, and interfacing with such vendors to assure a clear understanding of and full compliance with required quality standards,
- Responsible for assuring that contract quality procedures and documentation requirements are met. This includes responsibility for assisting the Product Managers in reviewing customer quality specifications and attending customer meetings to achieve agreement on specific quality requirements. In this effort, consideration shall include the Division's design and production capabilities as well as schedule and cost impacts
- Responsibility for quality shall include units installed in the field. Maintain communication regarding problems and solutions between Manufacturing, Engineering, and the Service groups. In the event of repetitive or major field problems, provide leadership in warranty analysis and corrective action and visit customer sites as required for fact gathering
- The manager is responsible for managing the budget and all quality resources in meeting division goals
- 7 + years’ experience in a quality function within a manufacturing environment with progressing levels of responsibility
- Two plus years in quality assurance with working knowledge of quality systems, such as ISO
- Quality Management System Development
- Recognize industry standards and codes such as ASME Section VIII
- Experience managing a quality organization with multiple sites
- Ability to manage in a matrixed organization
- Experience with Oracle ERP/ QMS
- Lean/Six Sigma
- Demonstrated Leadership Ability
- Leading a team of 5 - 10 + direct reports
- Team Oriented, ability to motivate others
- Ability to make data based decisions in a timely manner
- Customer Focused mindset
- Strong interpersonal skills, with the ability to interact with all levels of the organization
- Strong Technical/ Analytical Skills
- Data-driven problem solving capacity (DMAIC, RCCA, etc.)
- Ability to analyze and manage operating expense to business expectations
- Ability to build and manage annual budgets (headcount, capital, and all related operating expenses)
- Demonstrated Communication Skills
- Bachelor's Degree in Science, Engineering or Technical field
- Pharmaceutical, biologics, and/or medical device experience
- Knowledgeable in FDA/QSR requirements and quality system development/management
- Previous experience mentoring and managing a team of professionals
- Experience with managing external agency audits
- Experience with product and process validations in the pharmaceutical/medical device industry
- Demonstrated project management and leadership skills
- Training and experience in statistics (DOE, SPC)
- Lead auditor certification
- Training in problem solving techniques or Lean Six Sigma Green belt
- Training in coaching and management skills
- Develop and execute against a quality strategy for delivering the highest quality data products
- Understand how all elements of the data transformation system work together and develop data drive quality approaches that fit the overall strategy
- Be responsible for development of improvement strategies with defined measurable metrics to gauge success
- Provide clear and concise quality assessments of data products from full data sets of test results
- Drive the team to build a Zero Defect culture by managing controllable root-cause defects and ensure implementation of controls and minimize losses
- Interact with stakeholders at multiple levels and dynamically quality teams to define and deliver solutions to establish quality standards and processes
- Disseminate project information to stakeholders and senior management
- Work with Customer and Operations teams to understand data and quality needs
- Set directions/goals for a team of Quality program managers
- Verify Quality Metrics in place and meet goals, trigger containment actions and corrective actions as appropriate
- Lead and support problem solving activities for Quality Alert as well as any other quality issues
- As a Sr. Manager Quality Engineering, on a day-to-day basis, you will lead a global organization of close-knit Quality Program Managers (multiple locations) that are incredibly data driven, quality obsessed, and highly collaborative
- You'll drive exciting Quality Improvement programs that set the standard for Quality across operations teams, drive automation efforts across various programs that become industry best practices
- You will also find yourself partnering across organizations with world-class technical leaders, Engineers, Research Scientists, Best in class operations leaders, and Data Analysts who have expertise in a wide range of disciplines
- As Quality Managers on our team we’d love to break things to see how they work, improve on what works, discard what doesn't, and learn from both. We do all of this using data driven approaches that measure quality of our services, processes and tools that enforce automation
- Establish quality standards by role, region and vertical. Engage data engineering and analytics to deliver automated solutions, where possible
- Work closely with Workforce, Analytics, Data Engineering, and Software Engineering teams to drive initiatives on Operational efficiency
- Bachelors or Masters in ME/IE/EE/CS, Systems engineering, or related Engineering degree
- At least three years of managing teams
- Hands-on experience with Quality engineering efforts focused on operations execution, planning, execution and designing, implementing and maintaining high quality processes
- Experience building customer facing quality services
- Ideal candidates must be innovative, creative, flexible and self-motivated, with the ability to design and implement high-performance, reliable, and measurable quality programs
- Strong understanding of process improvement techniques in a production environment
- Strong understanding and demonstrated success with practical applications of Lean / Six Sigma tools such as Failure Mode & Effects Analysis, Design of Experiments, Measurement System Analysis, Analysis of Variance, Hypothesis Testing, Value Stream Mapping, Pareto Charts, Gage Repeatability & Reproducibility, Cpk
- Must be able to multi-task setting priorities in-line with business needs
- Team work spirit
- Preferred 7+ years of working experience in engineering and/or quality role with 5+ years in a quality environment
- Leverages the efforts of the quality and manufacturing teams thru process improvement tools to reduce variation in operation. The incumbent will be a recognized expert for Structured Problem Solving, Process Controls tools required to drive the continuous improvement culture into the organization
- Reviews test procedures (functional, acceptance/qualification) for completeness with regard to technical content, test equipment and test data; provide assistance to inspection test stations. The Senior Quality Manager will resolve quality problems with Crane Aerospace suppliers by leading and managing the Supplier Quality Engineering staff
- Implement Advanced Quality Planning in new product development process, participates in program and design reviews, implement PPAP where required and assure that product quality objectives are met
- Maintain a Quality management System in compliance with FAA, EASA, AS9100 and government regulatory compliance
- Reviews charts and analyzes clinical record documentation
- Conducts ongoing activities which monitor established quality of care standards in the participating provider network and for other clinical staff
- Collects, analyzes and prepares clinical record information for projects related to assessing the efficiency, effectiveness and quality of the delivery of managed care services. Prepares monthly performance reports
- Assists in the planning and implementation of activities to improve delivery of services and quality of care including the development and coordination of in-service education programs for providers and other clinical staff
- Provides training, interpretation and support for QI Clinical Reviewer staff
- Responsible for auditing as well as validating internal audit results and/or corrective action plans
- Driving creation of quality initiatives, process change initiatives, and other Change/Six Sigma initiatives to enable the network to meet and exceed business plans
- Managing and developing direct reports
- Developing and following network quality roadmap
- Reviewing analysis of customer defect data with development of corresponding action plans to reduce defects
- Developing and executing daily, weekly & monthly action plans that improve quality and delivery
- Establishing and maintaining defect free handling procedures for all sites in the network
- Planning for and identifying root causes of quality and delivery deviation through cause and effect analysis
- Teaching and educating managers and associates on the importance of quality execution
- Gaining consensus to push change forward while keeping variation between sites to a minimum
- Traveling to sites to observe, investigate and create quality initiatives
- 5+ years Operations Management experience in manufacturing, production or distribution environment
- Experience performing root cause analysis and driving quality improvements in an operations environment
- Ability to handle changing priorities and use good judgment in stressful situations Six Sigma Black Belt preferred MBA preferred
Senior Quality Manager / Quality Manager Resume Examples & Samples
- Serve as the Diagnostics expert on post market surveillance activities, ensuring sites are supported through the decision making activity, closure, and termination of issues
- Oversee the Akron Quality team ensuring that all feedback mechanisms are captured and addressed in a timely maner
- Oversee Waltham quality activities related to R&D as well as CCMS
- Extensive knowledge of ISO 13485, FDA CFR 820, and emerging standards and regulations
- Excellent team building and leadership skills that motivate team members to drive projects to a successful conclusion; must be able to manage and lead without direct authority
- Excellent judgment skills in order to provide strong guidance and counsel to the site and line staff
- Time management skills for oneself and of others on a team, ability to drive projects to scheduled completion
- Superior interpersonal, verbal and written communication skills
- Exceptional organizational, negotiation, and problem solving skills
- Ability to work in a matrixed organizational structure, developing strong relationships with all levels of management
- Member of site leadership team, contributes and participates on issues and interactions related to site quality
- Responsible for working with Site Leadership Team to deliver on site goals and objectives
- Collaborate with the various functions to ensure the quality management system is comprehensive, incorporates best practices, and is consistent with the strategic plan
- Support and provide expertise to the Quality Management System (QMS) and Quality department to successfully meet or exceed goals and targets
- Educate and mentors employees on the site QMS and other quality/operational programs and processes
- Provide the necessary leadership and strategic planning to develop, implement and maintain a quality assurance and quality control program to support the business mission and ongoing continuous improvement effort
- Formulates and oversees maintenance of quality control objectives and coordination with production procedures to maximize product quality
- Instill and maintain a quality culture
- Provide input and direction to resolve quality related issues through proper inspections, root cause analysis and corrective actions plans
- Ensure continuous improvement through the Practical Process Improvement (PPI)
- Serves as key member of product teams, with focus on quality planning for new products/processes
- Monitors quality performance for the Millersburg site, reports progress, and keeps division leadership informed of progress, issues, corrective action, and customer complaints
- Provide direction to ensure compliance with all applicable customer requirements and government regulations involving product quality
- Proven communication and customer management skills
- Hands on knowledge of products and applications
- Understands the BioProduction customer and required compliance regulations for our products and processes
- Experience working and providing customer support, investigations, and relationship management
- Must model the highest degree of moral and ethical behavior
- Must maintain confidential information
- Experience working successfully in a fast paced environment
- Experience supporting change management efforts
- Proactive and takes ownership for producing positive results
- Contributes as a valued team member and colleague with leaders at all levels
- Demonstrates personal awareness and desire for continual learning and personal development
- Keeps commitments; exhibits candor and courage - is not afraid to establish a visible presence and point of view, to engage in spirited and constructive debate, to hold others accountable
- Normally receives little instruction on day-to-day work, general instructions on new assignments
- Must possess the presentation skills and professionalism to project a professional image, both internally and externally
- Strong interpersonal, verbal and written communications skills are essential
- Bachelor’s degree from an accredited higher learning institution
- 8+ years Quality experience
- Experience working in a manufacturing and/or production environment
- Good understanding of customers, products, and regulatory compliance
- Providing Packaging Quality Assurance support in the manufacture of new and existing products
- Ensuring the use of DanoneWave's innovation process methodology throughout the project
- Overall Risk Management (eliminating risks through strong problem solving or by use of a specific control)
- Coordinate package design activities with all other functional Quality managers involved in the Product Life Cycle (including Plant Quality Managers and the Sales & Supply Chain Quality Director)
- Bachelor degree minimum, Master degree in Sciences or Engineering ideal ( Packaging, Mechanical, Chemical or related engineering science)
- 3- 5 years' experience in a packaging quality field (preferably in the food or beverage industry)
- Experience in thermoforming, injection molding and extrusion blow molding
- Experience with polyolefins (PE,PP,HDPE,PS), their properties and use as food containers
- Knowledge in sachets, pouches and related flexible packaging
- Experience in barriers properties of packaging materials
- Experience in the use of statistical tools for problem solving
- Prior experience within Manufacturing, R&D or Supply Chain is preferable, preferably within a FMCG (CPG) food manufacturing environment
- Prior experience in packaging development
- Knowledge of commercialization process, quality and food safety systems
- Standing along/courage
- Leads the quality program and associated initiatives for the organization
- Serves as the liaison with customers/consumers on items of product quality or reliability
- Manages the continuous review of quality programs including auditing and the formulation of new or revised policies and procedures to effect improvements, reduce costs, and enhance efficiencies
- Assists technical and procurement teams in formulating new products and establishing standards for these products prior to initial production
- Manages objectives of Quality Initiatives such as the analysis of returned/defective products; prepares reports of findings
- Develops, formulates, and guides quality control programs that involve advanced inspection methods, devices, and control techniques
- Provides leadership and personally conducts the use of Pure Fishing’s 8 step problem solving process
- Conducts and/or oversees training as necessary to ensure organizational understanding of procedures, methods, uses of equipment and other quality control techniques
- Performs standard management responsibilities including but not limited to planning, organizing, delegating, evaluating, budgeting, scheduling, etc
- Develops and implements Lean training programs for the organization
- Deliver training, coaching and sponsor learning projects on CI concepts, tools and techniques
- Lead, facilitate and support Kaizens and other CI events and seeks corrective action from coworkers if results are not sustained
- Develops and implements structure for Kaizen events
- Leads and manages the value stream mapping process
- Co-Leads the 3P New Product and Process Development Process
- Assists and coaches in the development and implementation of MDI metrics, QCDSP Boards and the 5S Program
- Develops a program to measure and evaluate the success of Lean programs
- Establishes and controls budgets for appropriate cost centers
- Ensures the consistent application of organization policies
- Develops or approves recommendations to change systems, policies and procedures; ensures timely and accurate implementation
- Minimum of eight (8) years of progressive experience and training in quality assurance and control techniques within the Consumer Packaged Goods (CPG) industry required
- Minimum of four (4) years of experience in test laboratory procedures required
- Strong background in high volume manufacturing of consumer products required
- Strong computational and analytical ability as it relates to calculations and analysis of quality assessment in a manufacturing environment required
- Experience with International Standards Organization (ISO) standards required
- Experience implementing quality management systems and procedures required
- Minimum of intermediate level proficiency with Microsoft Office Suite (Excel, Word, PowerPoint, and Outlook) required
- Must be eligible to work in the United States for any employer
- Prior supervisory and managerial experience preferred
- Special training in Industrial Management and / or applied engineering services preferred
- Advanced proficiency with Microsoft Office Suite (Excel, Word, PowerPoint, and Outlook) preferred
- Experience functioning within a strong matrix environment of a global enterprise highly preferred
- Four year college or equivalent
- Five years job related experience
- Comprehensive knowledge of regulatory information regarding the use of human/animal participation in research and teaching
- Demonstrated understanding of the principles of Human/Animal research protection standards
- Strong knowledge of grants and contracts function
- In-depth understanding of operational requirements pertaining to management and implementation of protocol process
- Strong communication and presentation skills (verbal and written)
- Direct IRB or IACUC experience, preferably with an AAHRPP or AAALAC accredited institution. Advanced knowledge of human subjects/animal protection regulations and best practices, as evidenced by certification (e.g., ability to obtain or retain IRB or IACUC professional )
- Leads a program or multiple projects within a domain in a fast-paced, dynamic environment, which may include directing global sourcing resources as well as virtual teams. Projects are of a scope and complexity that usually impact multiple Lines of Business as well as functional areas, may have executive sponsorship and in some cases a steering or advisory committee and may span several years
- Defines and manages testing in these areas: scope, scope changes, objectives, requirements, deliverables, and value proposition that support the diverse business goals and interests of multiple stakeholders
- Drafts and submits testing budget proposals and recommends subsequent budget changes where necessary; conducts cost forecasting and actual tracking as well as variance reporting
- Reviewing all the test deliverables, ownership of the quality assurance process for testing, responsibility for the integration of testing into release management and change management within the ITIL/ ITSM process framework,
- Communicating accurate status/health information to delivery Project managers and Delivery managers including escalation along with risks & mitigations in summary level
- Ensuring quality milestones/reviews are done on timely manner and making sure the that Key performance indicators and service level agreements are being met and enforced
- Excellent Technical, Communication, Interpersonal and problem solving skills
- Thinks, plans and executes on multiple levels
- Can clearly articulate vision for future state; provide big-picture view/ enterprise view; decisive and directive when necessary
- Fosters and encourages frequent, open and honest feedback; learns from setbacks and mistakes to drive improvements; independently seeks out learning opportunities
- Establish operating policies and procedures defining requirements for responsible areas
- Maintain team performance to ensure objectives for key metrics are met, including timely regulatory filings, complaint closure, and patient data entry
- Provide leadership and develop and implement methods to improve teams’ effectiveness and performance versus established quantitative measures
- Frequently interact with senior-level management to inform, influence and achieve objectives
- Ensure completeness, thoroughness and compliance of all HVT complaint files
- Provide routine and ad hoc complaints reporting for management and external agencies
- Provide liaison between Quality, Manufacturing and R&D to ensure timely and value-adding analysis of customer complaints
- Support internal audits and external audits from various regulatory agencies
- Ensure compliance with regulations including 21CFR820 and 803, ISO13485, Canadian MDR, JPAL, and MDD 93/42/EEC, ISO14971
- Manage personnel ensuring growth and professional development
- A Bachelor’s degree in a scientific or related discipline is required; advanced degree is desirable
- A minimum of ten years’ experience in the medical device or similar regulated industry, with a demonstrated track record in management
- Complaint handling experience in the medical device industry required
- Experience working in a clinical setting is desirable
- People management including roles definition, team optimization, and conflict resolution
- Strong management and leadership skills to ensure growth, and development of personnel
- Ability to resolve issues and answer questions independently, with judgment to escalate issues when appropriate
- Strong communication skills, including written, verbal, and presentation skills
- Ability to identify appropriate means, style and depth of communication depending on audience
- Strong understanding of US FDA and international regulatory standards for medical devices
- Highly developed time management skills, including an ability to manage multiple and frequently changing projects and priorities
- Drug screen / background check
- U.S Citizenship (NO sponsorship)
- Participates in the interview and selection process (including hiring recommendations), and makes staffing assignments in accordance with schedule requirements. Conducts performance evaluations and makes appropriate recommendations for required actions, including recommendations for advancement, changes in status, or disciplinary action. Manages the salary administration process, including preparing the annual salary plan and recommending salary actions for merit increases and/or promotions
- Provides Client Quality Systems leadership using the ISO 9001 Standard as the basis for process development and optimization, procedure development, data collection and reporting, and Quality Systems training. Directs and coordinates efforts to initiate (and maintain) Regional/ Project ISO 9001 QMS certification
- Implements the defined Client? Corrective Action/ Preventive Action and Non-conformance
- Tracking program development including defining requirements and future administration and support of a unified, system to include tracking of NCRs, CARs, Audit Findings, etc
- Collaborates with the Vice President, OSS Quality or Vice President, PGS Quality and Compliance, in facilitating Quality Leadership programs, achieving present Quality objectives, setting future Quality objectives and providing input and coordinating output from the Quality Management Reviews
- Provides leadership, guidance and training to Quality personnel; monitors performance, and identifies skills, training, and capabilities needed for Quality activities. Develops and provides consistent quality training programs to key Resource Center personnel including direct reports. Provides Quality support in the Client Guidance Document Development Program initiative. Supports staffing requirements in the Regional Units, and assisting in the training needs of related Offices, Projects and Facilities
- Provides continued support and improvement of the Client? Quality Management System
- Directs preparation, issue and maintain Regional and/or project Quality program manual(s) and
- Procedures in compliance with Client and customer quality requirements, and ensure that these are implemented effectively
- Ensures that Client and customer’s quality requirements are met for products and services provided, by verifying compliance with quality standards and quality program requirements. Acts as a liaison with customers, contractors and/or internal departments on quality related matters. Interfaces with other Regional Quality Managers as member of Corporate Quality Working Group to maintain the PACE Quality Guide and advance the overall performance of quality at Client
- Identifies and reports to executive management, quality issues or trends based on data from a variety of sources. Provides input to management on organization performance metrics, and process or procedural system improvements
- Lead and develop team members through training, communication, and accountability
- Demonstrate knowledge of product and processes, and their impact on overall quality
- Primary technical liaison with customers, vendors, and regulatory agencies
- Support co-manufacturing sites for additional sites in the US
- Bachelor’s degree from an accredited institution in an applicable field of study such as Food Science, Chemistry, Biochemistry, Microbiology, or similar Science/Engineering
- Demonstrated success with relevant GFSI standards including SQF, BRC, IFS, or FS 22000
- Proven knowledge and understanding of Food Safety regulations, FSMA, and industry and market trends
- ASQ Quality Engineer certification (CQE) or equivalent. Candidates may pursue this certification concurrently, but must be able to demonstrate competency with statistical analysis
- Six sigma and/or lean manufacturing experience is desirable. Ability to drive continuous improvement and change management initiatives
- Proven success in communicating with customers and responding effectively to quality concerns
- Must have strong critical thinking ability along with analytical and problem solving skills
- Achievement in assessing, developing and retaining top talent
- Proven ability to lead with the following key competencies: Technical Expertise, Strategic Thinking & Influence, Process Optimization & Product Knowledge, and Leading & Managing Change
Customer Service Senior Quality Manager Resume Examples & Samples
- Ensure all internal,and external non-conformances are tracked with measurable progress of corrective actions in a timely fashion
- Produce, and report, Quality metrics and Key Process Indicators (KPI’s) as required by the QMS
- Manage internal audit programs to establish consistent adherence to all QMS and Regulatory requirements
- Administration of Corrective and Preventative Action (CAPA) system
- Lead and develop a quality team with skills and abilities to become a true service organization to support all customer service teams in training and technical troubleshooting to achieve company goals and objectives
- Develop and provide training to CS employees and other stakeholders as required
- May work on special projects/assignments upon request
- Be able to lead / sponsor / manage all aspects of Continuous Improvement programs to meet defined objectives and goals
- Job Requirements
- Bachelor’s or Master’s degree, or equivalent
- Fluent in English. Additional languages is a plus
- More than 5 years experience in Customer Service Quality departments minimum. Desired more than 10 years experience
- Coach, mentor and lead quality team
- Design, implement, maintain, and evaluate quality systems for aquatics, liquid filling and packaging operations
- General problem solving for aquatics and liquid packaging including root cause analysis and corrective action implementation
- Provides advice and counsel to Purchasing in connection with the selection of vendors and purchase of materials; requests the R&D department for required or changed material specifications; arrange where necessary for inspection of vendor’s plants to insure conformity to specifications and adherence to stated manufacturing process
- Manage and maintain network SPC data collection system (Gainseeker SPC)
- Analyzes, interprets, and prepares reports concerning Quality, effectiveness, costs and cost reductions, laboratory problems, status of equipment and initiates appropriate action
- Develop, analyze, and present Key Performance Indicators as they relate to plant quality
- Take ownership of the quality system and continued improvements to the plan
- Resolve quality issues related to product components
- Responsible for the selection and training of employees; planning, assigning, ad directing work; appraising employee performance; rewarding and disciplining employees; addressing complaints and resolving problems and disputes
- Comply with all Company policies and procedures
- Bachelor’s degree in Engineering or related science
- Seven to ten years of experience in Quality Management of consumer products (or equivalent combination of education and/or experience)
- Experience managing exempt and non-exempt employees
- Excellent Leadership Skills
- Ability to initiate resolutions for major quality issues
- Solid background in quality engineering; experience in experimental design is preferred
- Minimum of 10 years experience in Quality ideally in the Medical Device industry
- Supervisory and/or Managerial experience within a Quality department
- Extensive exp./knowledge in Quality Management Systems (ideally Agile)
- Very Hands-on role (80% hands-on & 20% managerial)
- Exp. working in a ISO 9000 and/or 1345 environment
- Microbiology background
- Consumable manufacturing background
- Cleanroom background
- Lean/Six sigma experience is a plus
- MRP/ERP experience
- Main duty will be oversight of the Quality Management System
- Will have a few direct reports
- Very hands-on role
- Lead, manage and develop team per WWT Integrated Management and Leadership Concepts
- Collaborate with onsite Quality teams to ensure standardization across QMS and new/changed requirements are being met
- Ensure overall effectiveness of the QMS across the organization
- Oversee the implementation of new and revised QMS requirements and standards
- Ensure Quality tools and concepts are being utilized properly within the organization, as needed (Risk Analysis, Root Cause Analysis, Change Management, Continuous Improvement)
- Collaborate with onsite quality teams to ensure KPIs are being monitored, measured and analyzed and corrective actions are assigned, as needed
- Minimum 10 years general business experience; quality, supply chain or logistics preferred
- Minimum 5 years of experience in the support with Quality Management Systems; ISO 9001 and/or TL 9000 certification preferred
- Bachelor’s Degree or Associate Degree with 5 years of experience supporting a mature QMS or equivalent experience
- Experience in the management and leadership of a team required
- Minimum 5-7 years’ experience in the management and leadership of a team required
- Proven track record in Closed-Loop Corrective Action resulting in sustainable process improvements
- Experience in leading others, establishing priorities and accomplishing objectives with minimal supervision
- Excellent oral and written communication and interpersonal skills
- Independent, professional, and self-motivated
- Potential to travel 10 percent
- Support co-manufacturing sites for additional sites in the US –Melrose Park IL Evansville IN, Sturtevant WI, Calhoun GA, Brampton Ontario
- Travel 50-75%
- Bachelor’s degree from an accredited institution in an applicable field of study such as Food Science, Chemistry, Biochemistry, Microbiology, or similar Science/Engineering and 7+ years of relevant progressive quality and leadership experience
- Experience in coatings, batters and seasonings is preferred. USDA experience is a plus
Related Job Titles

IMAGES
VIDEO
COMMENTS
Your quality manager resume must clearly demonstrate your attention to detail. It should reflect your ability to meticulously uphold standards. Showcase your expertise in developing and implementing quality assurance protocols. Your resume needs to convey your track record of improving processes effectively. Use This Example.
Here's a list of steps you can follow to write a quality assurance manager resume: 1. Add your contact information. Begin your resume by adding a header with your contact information to the top right, left or center of the document. Include your full name, phone number, email address and the city and state where you live.
Quality Manager Resume Examples. Quality Managers coordinate teams of Quality Control Technicians and make sure clients are delivered functional and safe products. Common duties listed on a Quality Control Technician resume example are running tests, analyzing products, assigning tasks, organizing work schedules, documenting defects, and ...
Find tips and sample resumes for quality manager positions in various industries. Learn how to highlight your skills, experience, and achievements in quality management, assurance, and improvement.
Tips to help you write your QA (Quality Assurance) Manager resume in 2024. Highlight relevant certifications. As a QA manager, having certifications such as ISTQB, CSTE, or CSQA, can be a significant advantage to set you apart from other candidates. Include any relevant certifications in your resume to showcase your dedication to the field and ...
A QA manager resume example better than 9 out of 10 other resumes. How to write a QA manager resume that will land you more interviews. Tips and examples of how to put skills and achievements on a quality assurance manager resume. How to describe your work experience on a QA manager resume to get any job your heart desires.
Employers need Quality Managers to ensure that their products or services meet a high standard of quality. Crafting an effective Quality Manager Resume is essential for standing out in a competitive job market. In this article, we will discuss the elements that make a great Quality Manager Resume and provide examples and tips to help you write ...
Quality Manager Resume Examples & Samples. Directs, implements, and maintains operational-level strategies and objectives. Develops short to moderate-term goals and translates them into short-term plans in support of operational goals and objectives. Monitors performance against plan and ensures that goals are met.
Quality Management Manager Resume Examples & Samples. Pediatric health and wellbeing, Maternal and Child development, QM practices, service authorizations, utilization review and coordination of healthcare services. Health care administration related to children in Out-Of-Home care. AHCCCS/Medicaid rules and policies.
The top 5 certifications for your qa manager resume: Certified Manager of Quality/Organizational Excellence (CMQ/OE) - American Society for Quality (ASQ) Certified Software Quality Engineer (CSQE) - American Society for Quality (ASQ) Professional Scrum Master (PSM) - Scrum.org.
Welcome, aspiring Quality Manager! You're about to embark on a journey that transforms your resume into a gateway for your dream job. We'll guide you, step by step, on how to tailor your resume for a Quality Manager position, ensuring it shines under the scrutiny of hiring managers and passes through Applicant Tracking Systems (ATS) like a breeze.
Montgomery, AL | 334-555-0196 | [email protected]. Summary. Data-driven quality professional with four years of experience reducing costs and optimizing testing processes in the tech industry. Seeking a Quality Control Manager position to expand my leadership skills and precise approach to continuous improvement.
How to format your quality manager resume: Highlight achievements in your work experience, not just responsibilities. For example, 'Led team in achieving CMM level 3 implementation' or 'Coordinated company-wide Lean Six Sigma training initiative with local college'. Keep your resume concise and relevant.
Quality Manager Resume Samples. The role of a quality manager is concerned with monitoring and supervising the performance of the quality management systems, measuring against set standards and producing data on performance.A well-crafted Quality Manager Resume includes the following tasks - devising and establishing quality procedures, reviewing customer requirements, establishing quality ...
Resumes; Cover Letters; Skills; Interview Questions; Resume Examples; Quality Manager; Quality Manager. Resume Examples. Writing a great . quality manager resume is important because it is one of the first things a potential employer will see when they are considering you for a position.. It is your opportunity to make a good first impression and sell yourself as the best candidate for the job.
Quality Control Manager Resume Sample. A quality control manager is a senior-level position in which they create quality standards and manage the quality control team. They will provide directions to quality control technicians and ensure everyone follows quality benchmarks. They are involved in every phase of the manufacturing process to make ...
We've also selected some of the best (and most relevant) resume guides for the quality control manager role you're applying for: Quality Assurance Engineer Resume Example. Quality Assurance Officer Resume Example. Quality Consultant Resume Example. Release Manager Resume Example. Quality Technician Resume Example.
This is a resume example for an Operations Manager and Quality Manager transitioning from Operations positions in the Air Force. The resume is a useful document for anyone coming from federal or military positions. The resume begins by using a headline to target key areas of experience in production, operations and quality management.
Experience. Roscoe Technologies. 9/1/2009 - 10/1/2010. Company Name. City, State. Manage training Document Management System and Quality Management system. Planned and executed multiple tasks to ensure controlled documents were managed per procedures. Provided coaching to personnel on Quality systems (QS) to include document control and training.
Template 11 of 16: QA (Quality Assurance) Software Tester Resume Example. Traditionally, Quality Assurance Testers come in at the end of the development cycle of a new product. The title QA software tester is often used to emphasize that the QA team is a part of the development of the product from start to finish.
Coordination of problem solving in development process. Create and maintain project specific quality assurance plan. Participation in test plan reviews between test departments and development. Evaluate attainment of quality milestones in compliance with the agreed standards. Escalate in case of non-fulfillment of quality milestones.
Common Responsibilities Listed on Quality Control Manager Resumes: Develop and implement quality control systems and procedures. Monitor and inspect production processes and final output. Ensure that products meet quality standards. Investigate customer complaints and non-conformance issues. Analyze data to identify areas for improvement in the ...
Senior Quality Manager Resume Examples & Samples. Develops and manages the implementation of the strategic quality plan and ensures alignment / priority setting with strategic business plan and the alignment of appropriate resources. Guides business in making appropriate risk / reward decisions associated with product and service quality.
Quality Manager Cover Letter Example Here is an example of a cover letter that you use as a reference when applying for a quality manager position: Monika Paul Pune, Maharashtra (91) 92544-59888 [email protected] March 14, 2023 Mr. Rajiv Tyagi Wavewood Private Limited Pune, Maharashtra Dear Mr. Rajiv Tyagi, I am writing to express my interest in the quality manager position as advertised ...