
- 2022 Best Tech
- Creating a Lasting Impact on the Future of Sight
- Opt-Out Screening for STIs
- RSV Roundtable
- Food Insecurity and the Dangers of Infant Formula Dilution
- Opt-Out Chlamydia Screening in Adolescent Care
- Pediatric ADHD: Zeroing in on an Untenable Burden
- The Role of the Healthcare Provider Community in Increasing Public Awareness of RSV in All Infants
- Update in Pediatric COVID-19 Vaccines

- Publications
- Conferences
- Partnerships

Case Studies
If you are interested in submitting a Puzzler of your own, please see Puzzler guidelines here .

Quiz: Can you diagnose these muscle spasms in a 19-year-old-male?
Take this Contemporary Pediatrics quiz, and see if you can correctly diagnose the patient in this case study. Submit your answer to see if you were correct.

11-year-old boy with testicular pain and rash
An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. Read the full case to see if you can correctly diagnose the patient.
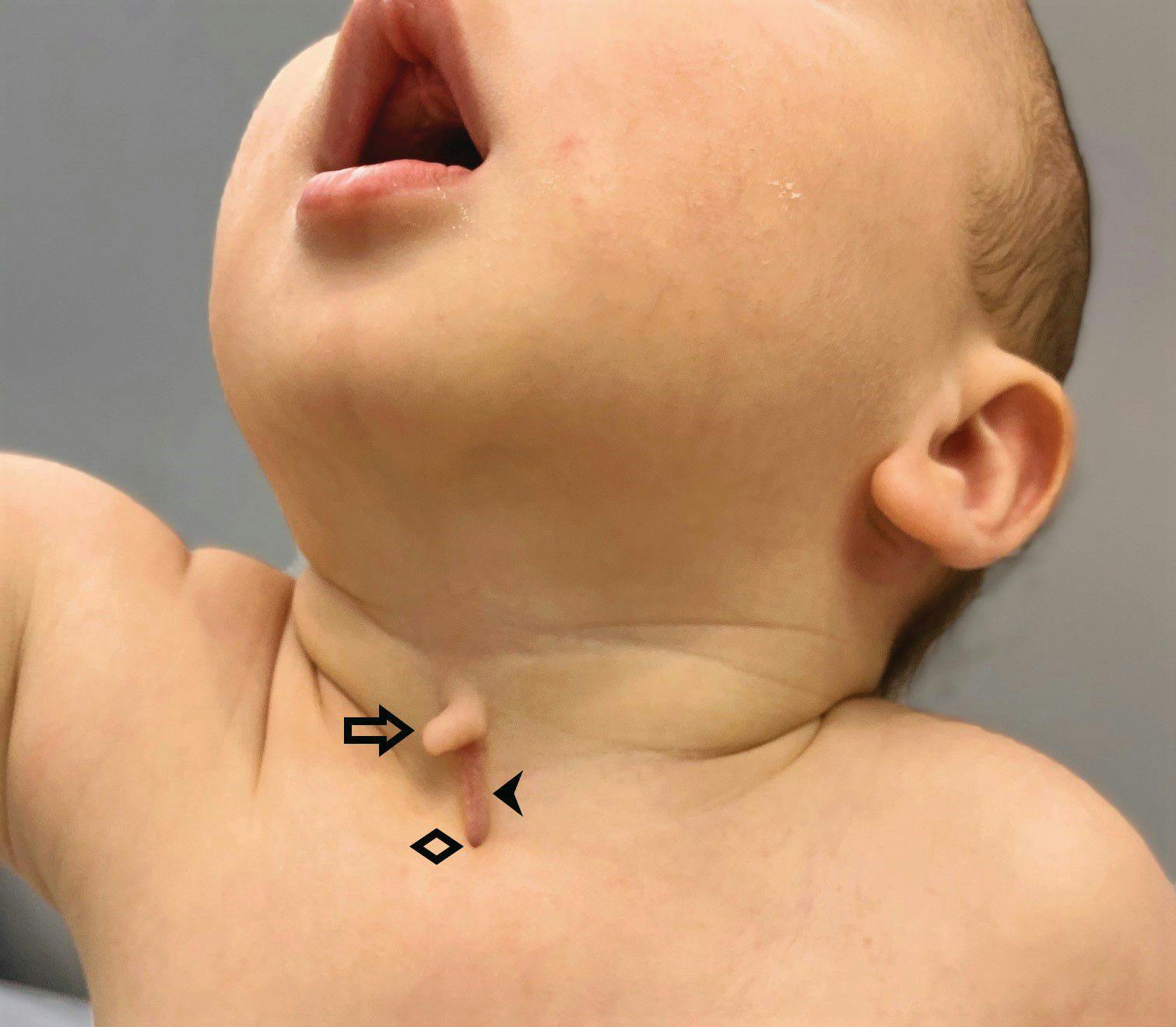
Newborn with midline neck lesion
A 4-day-old boy with a midline neck lesion was born at term by normal vaginal delivery. After birth, mid line lesion had the configuration of a linear cleft with a cephalocaudal orientation, extending from the level below the hyoid bone to the suprasternal notch with a length of 2.5 cm and a width of 0.5 cm. What's the diagnosis?
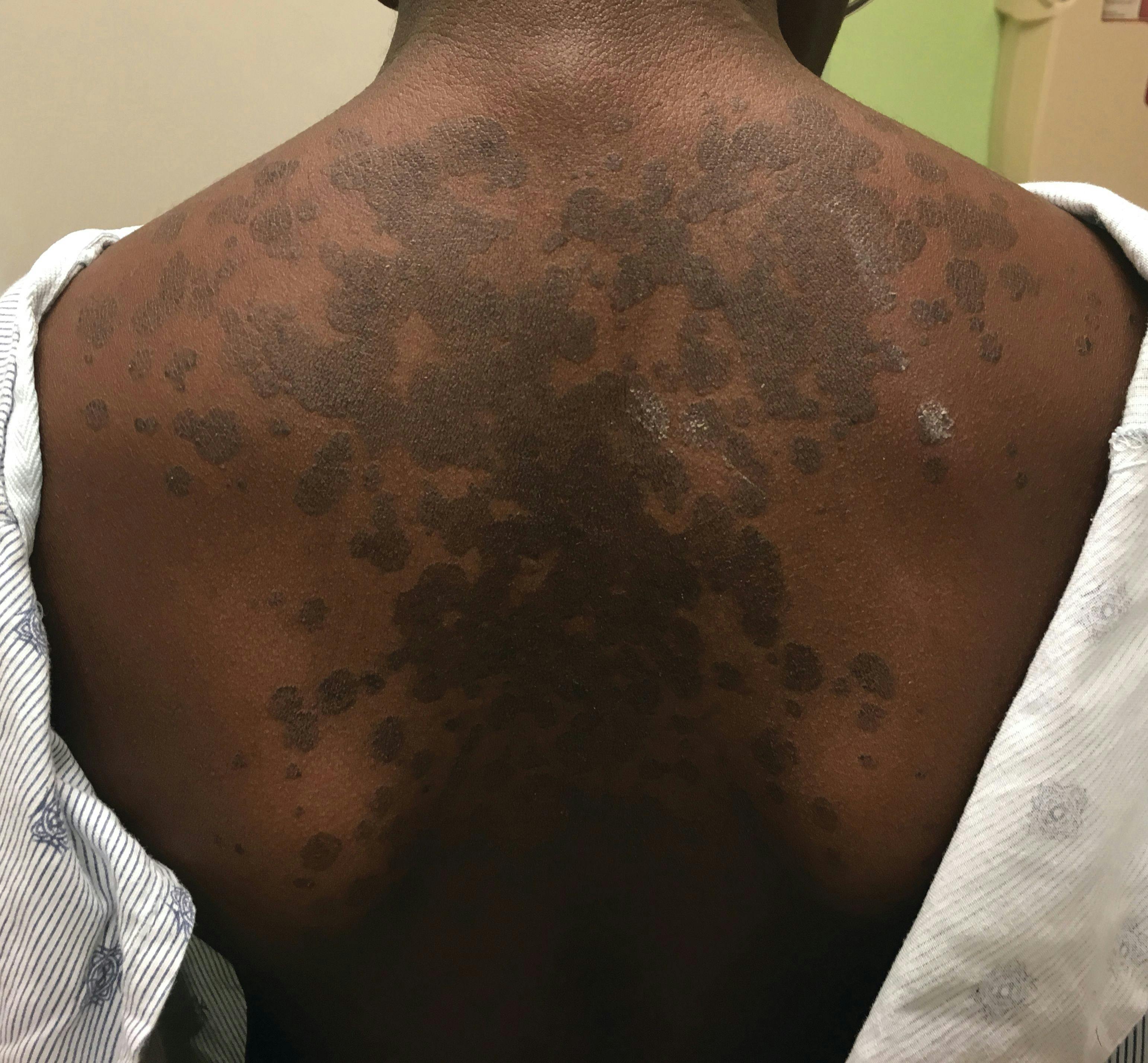
A 13-year-old girl with well-demarcated rash on back and chest
A healthy 13-year-old girl presented with a 1-month history of an asymptomatic, well-demarcated rash on her back and upper chest. The eruption consisted of discrete, dark brown papules that coalesced into large, flat-topped plaques with mild superficial scale and accentuation of skin markings. What's the diagnosis?

Suspicious facial swelling in a 22-month-old girl
A 22-month-old female patient with sickle cell disease on folic acid and penicillin prophylaxis with a 3-day history of nasal congestion, rhinorrhea, fever and decreased oral intake presents to the emergency department (ED) for acute facial swelling noted when she woke up from a nap. What's the diagnosis?
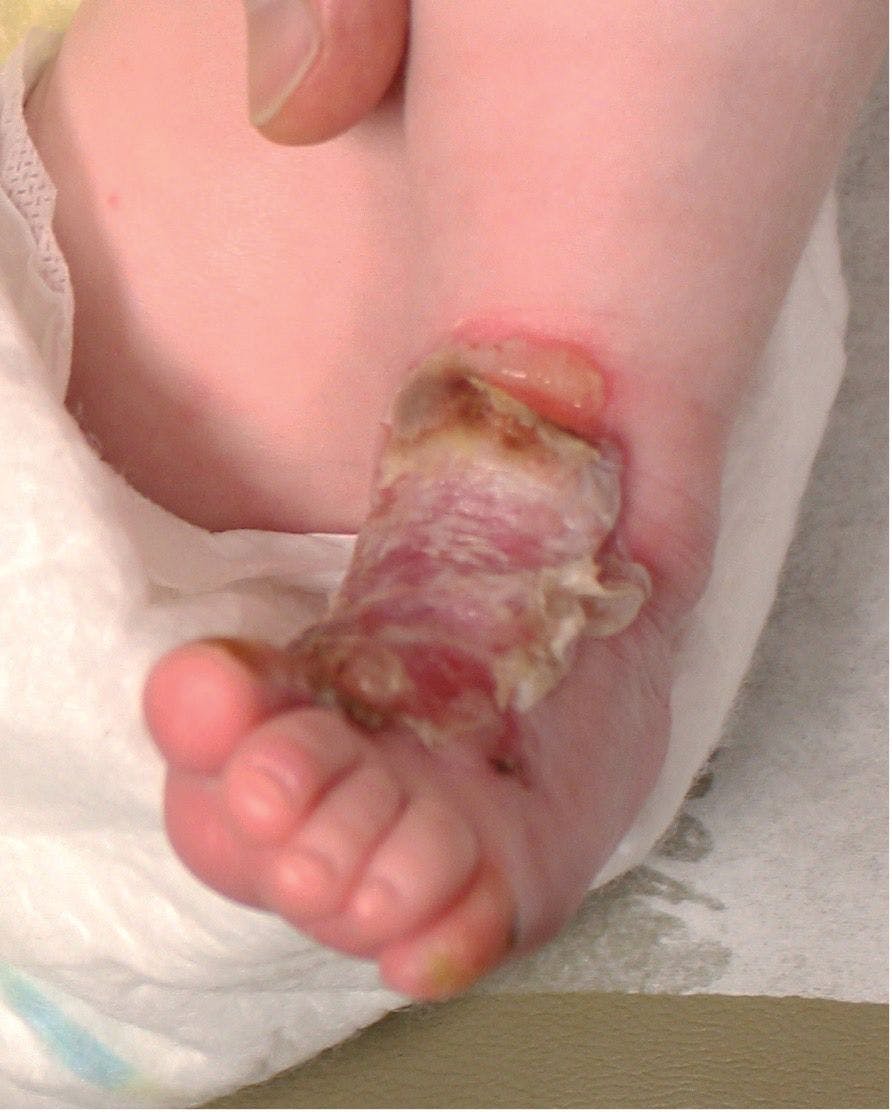
Friction-induced blistering on a child’s feet
You are called to the hospital nursery to evaluate a healthy full-term newborn boy who developed painful flaccid blisters and erosions on the tops of his feet and ankles shortly after birth. His mother had a history of similar recurrent skin lesions that healed with scarring. She also had oral and gastrointestinal tract involvement. What's the diagnosis?

Hypothermia and abnormal eye movements in a 5-week-old infant
A 5-week-old female infant born at 38 weeks presents to her pediatrician with abnormal eye movements. What’s the diagnosis?

Neonate experiences coffee ground emesis
The infant did not show signs of illness; her mother experienced a routine pregnancy and prenatal lab test results were normal. What is the diagnosis?
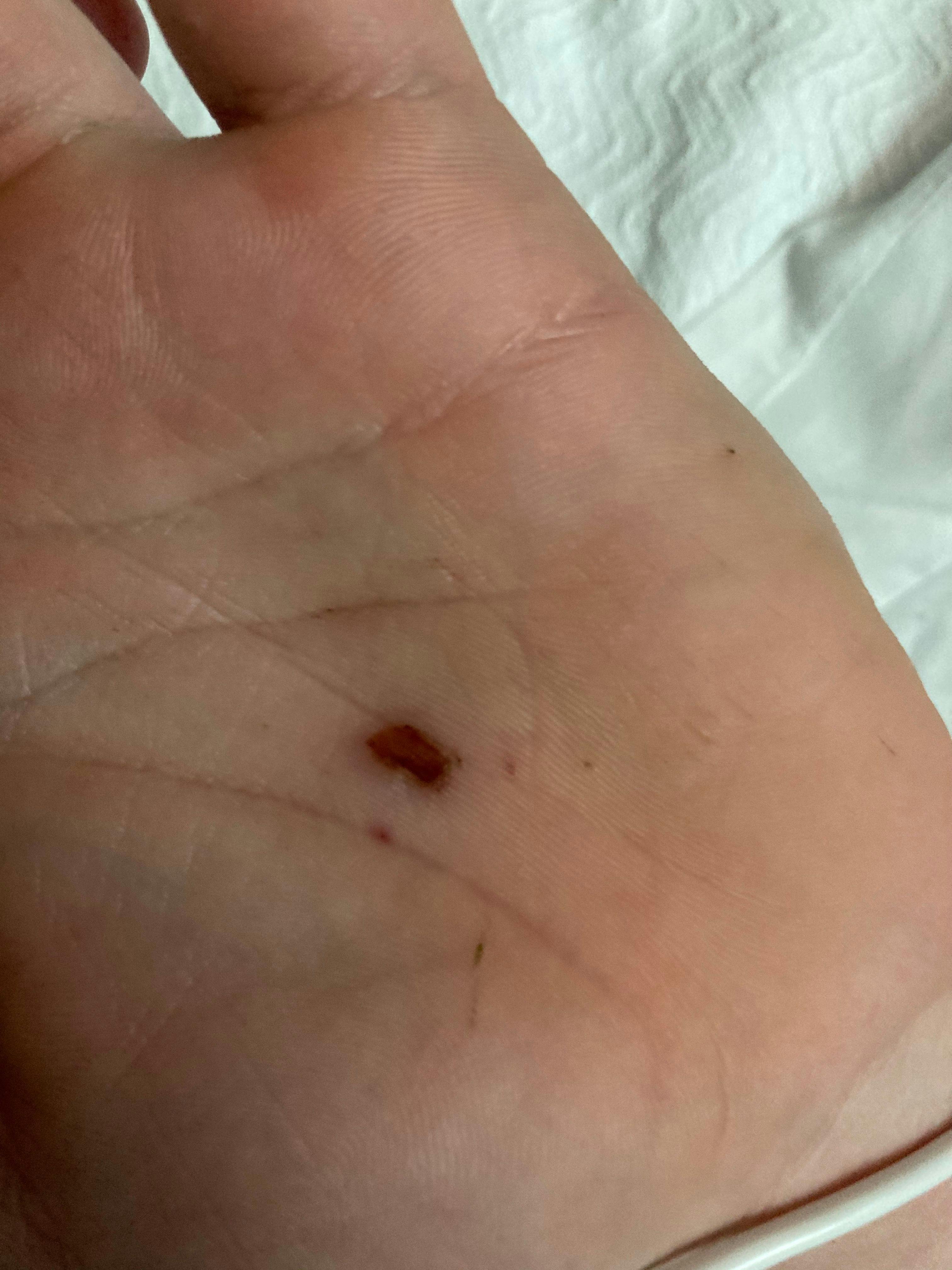
Muscle spasms in a 19-year-old male
A 19-year-old male presents to the emergency department (ED) with headache and fever of 4 days’ duration. Six days earlier, his left palm had been punctured by a rusty nail. What's the diagnosis?

Treating an 18-month-old who tested positive for cannabis exposure
As more and more states legalize recreational marijuana, caregivers need to be vigilant about keeping products out of reach of children.

Scalp thickening and folding in a pubertal boy
A 16-year-old boy with developmental delay and intellectual disability developed dramatic chronic wrinkling of his scalp over a year ago. The lesions were persistent but not symptomatic. What's the diagnosis?
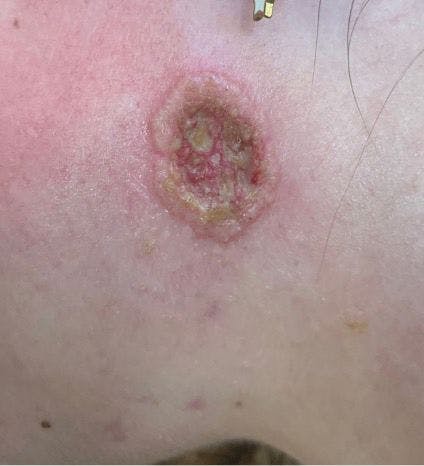
Worsening acne after isotretinoin treatment in an adolescent girl
A healthy 17-year-old girl with inflammatory acne had failed to respond to topical tretinoin, benzoyl peroxide, and oral minocycline. What's the diagnosis?

Psychosis in an 18-year-old male
Alex, an 18-year-old male, presented to the emergency department with a 4-day history of paranoia, agitation, and disorganized behavior. He had no psychiatric history or prior mental health contact and no known medical conditions.
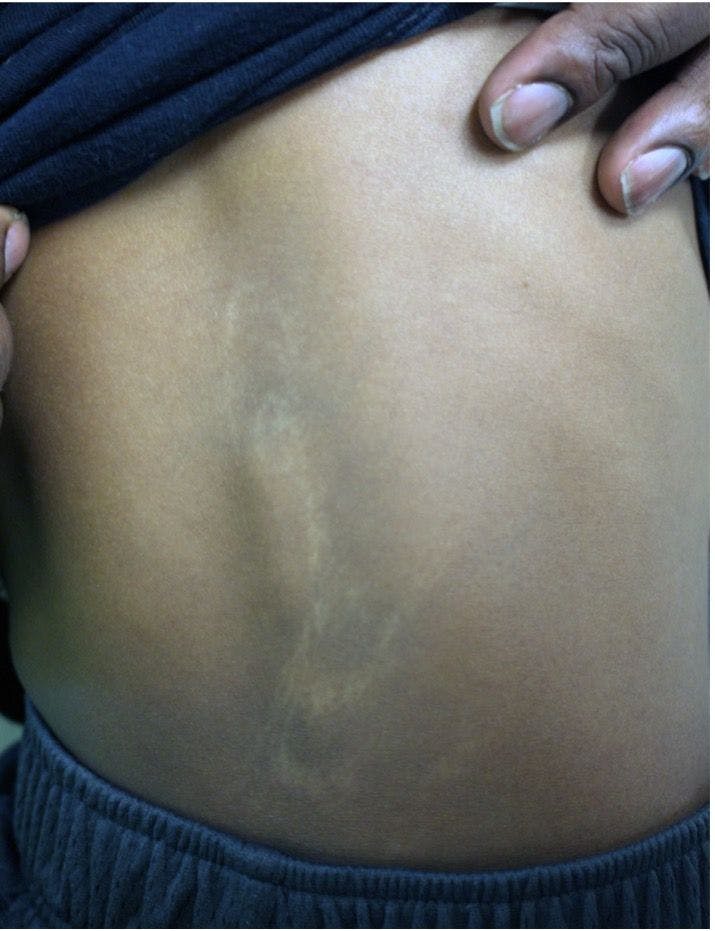
Congenital hypopigmented macules on a healthy child
You are asked to evaluate an African American boy aged 4 years with a birthmark on his back and right arm. He is healthy with normal growth and development. What's the diagnosis?
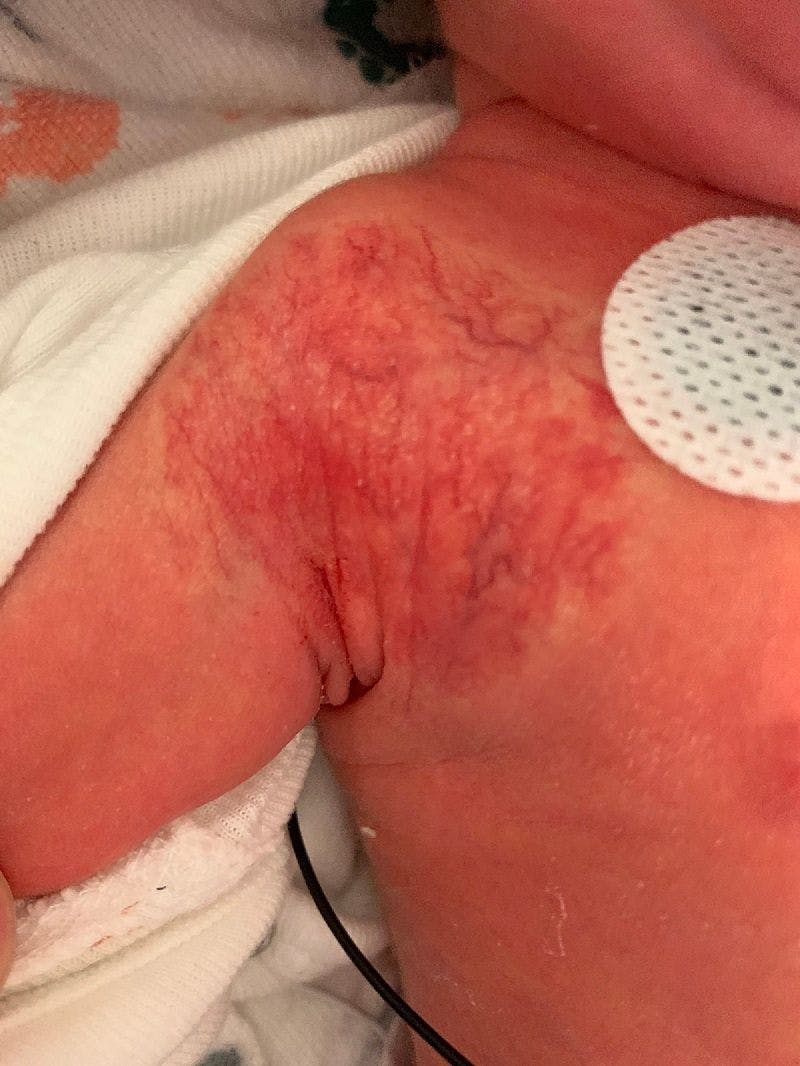
Just a little birthmark?
You are asked to evaluate a healthy 1.5-day-old girl who has a congenital red patch with coarse telangiectasias and a surrounding ring of pallor on the right shoulder. What's your diagnosis?

Can intranasal corticosteroids improve obstructive sleep apnea syndrome in children?
A study featured at CHEST 2022 investigated INCS in children with OSAS to determine if the therapy improved their symptoms, polysomnography findings, behavior, and quality of life.
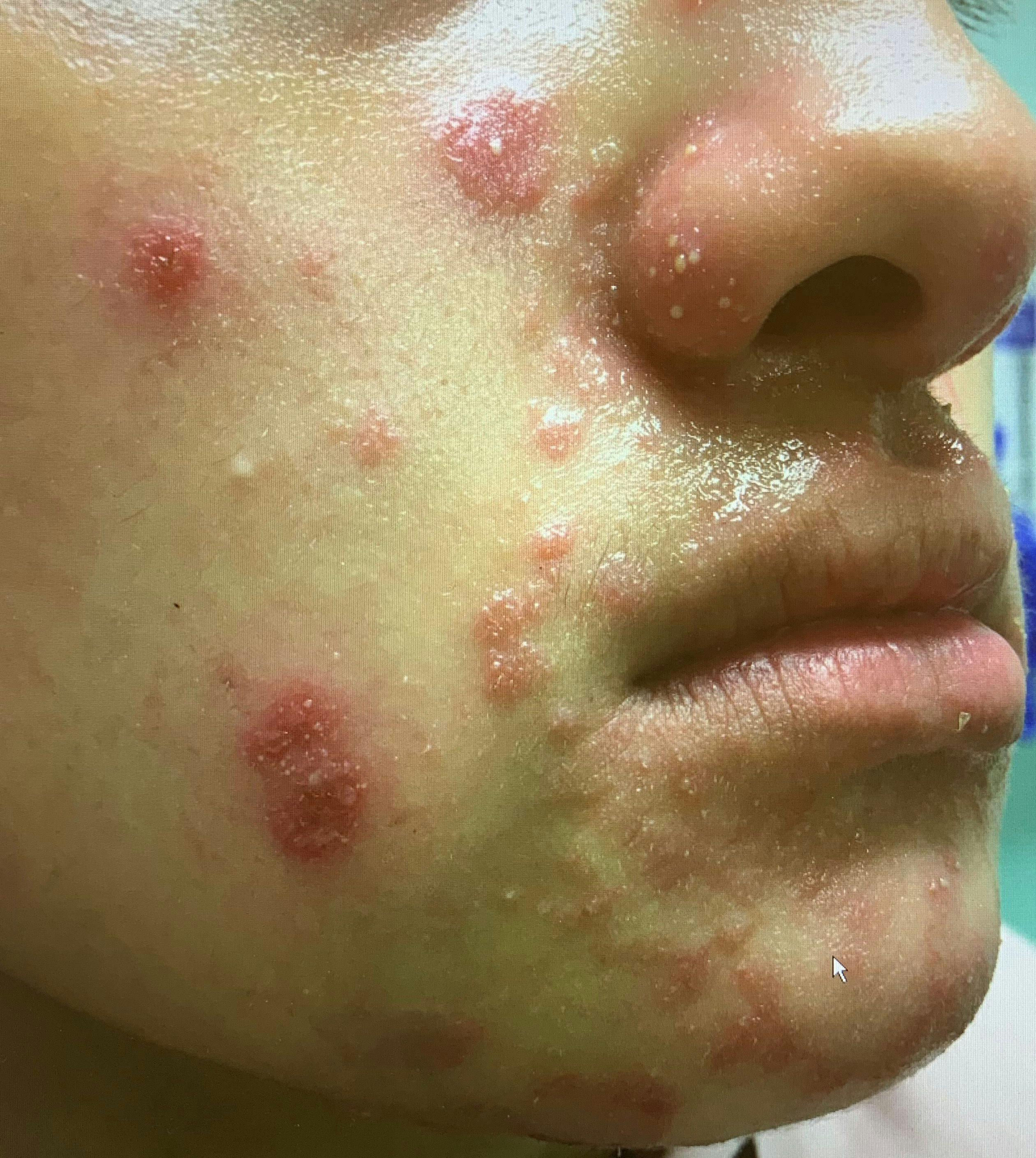
Case of inflammatory acne or something else?
This case study analyzes variants of majocchi granuloma and potential treatment.

A case of late-onset group B Streptococcus infection in fraternal twins
A 29-year-old White woman presented to the labor and delivery unit due to preterm premature rupture of membranes and delivered twins. The twins were transferred to the neonatal intensive care unit following delivery.
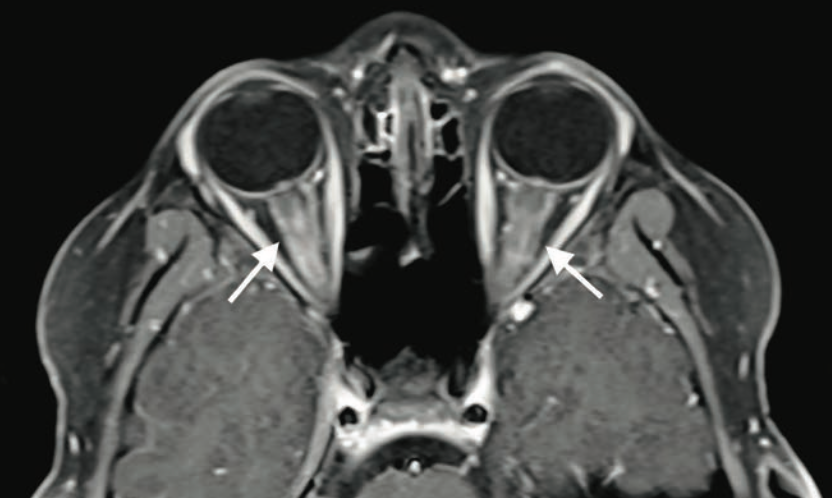
Bilateral blurry vision in a 9-year-old boy
A 9-year-old boy with no significant medical history presented to the emergency department with 2 days of painless blurry vision. What's the diagnosis?
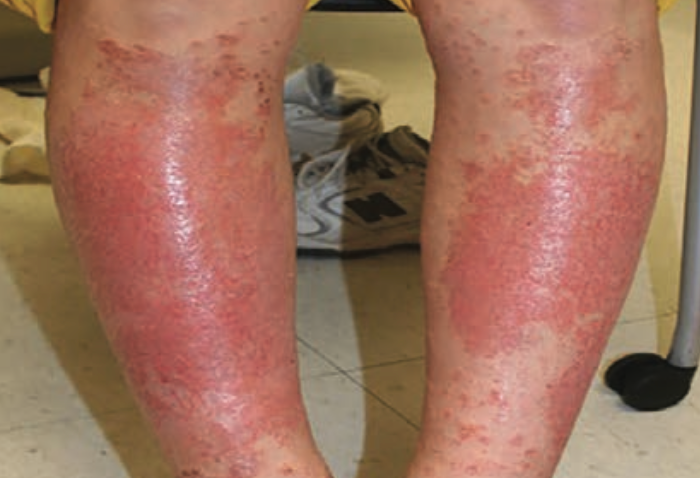
A case of shin guard dermatitis?
A healthy 14-year old boy was evaluated for an intensely pruritic shin rash that developed 2 weeks prior and had been treated with oral antibiotics for 10 days.

A renal anomaly? Look for a Müllerian anomaly as well
A retrospective study found that postmenarchal women with a renal anomaly were also at risk of having a Müllerian anomaly.

A case of progressive joint pain and rash in a 5-year-old
A 5-year-old nonverbal boy with autism spectrum disorder and global developmental delay presented to the emergency department with bilateral lower-extremity bruising and progressive difficulty ambulating. What's the diagnosis?
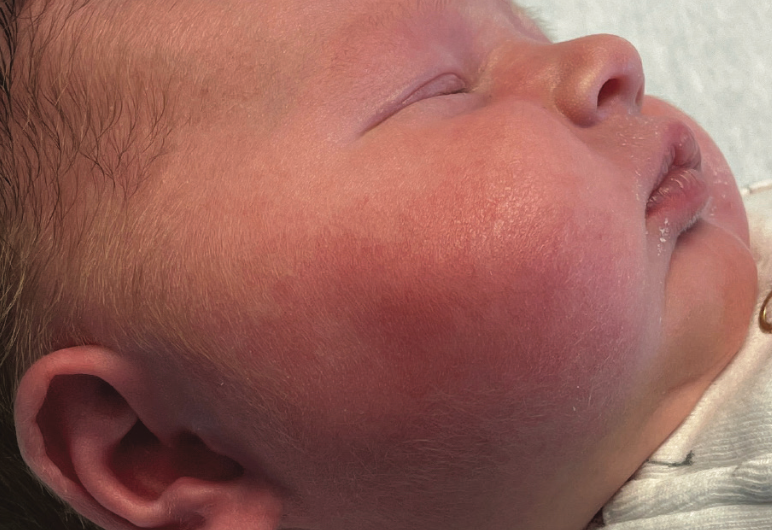
Fever and facial swelling in a neonate
An 18-day-old girl whose right cheek had become increasingly red and warm over 24 hours was directly admitted to an inpatient unit. She had firmness and pain to the affected area, fussiness, increased sleeping, and poor feeding, preferring the bottle to breastfeeding. What's the diagnosis?

Altered mental state in a 2-year-old boy
A 26-month-old boy presents for mild altered mental status and balance issues following a fall the day before. There was no loss of consciousness or vomiting but he subsequently complained of left-sided head pain. What's the diagnosis?
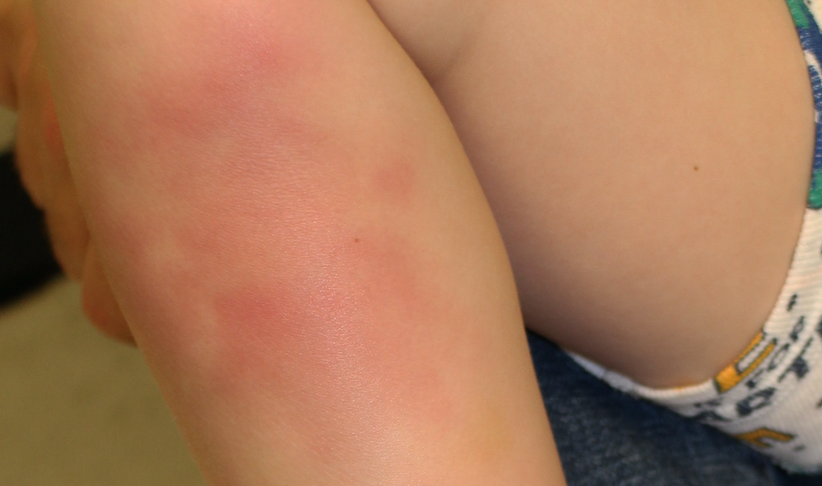
Painful red lumpy leg
A healthy 4-year-old boy presents with painful deep-seated bumps on the front of his right leg. He also complains of a sore throat for the last 4 days. What's the diagnosis?
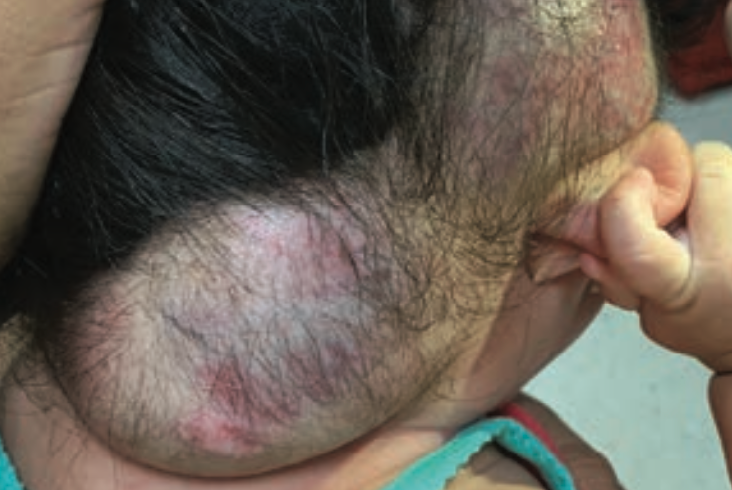
Severe hemorrhage from infantile hemangioma
A 5-month-old girl with a large scalp infantile hemangioma (IH), present since 6 weeks of age, is evaluated in the emergency department for lethargy and pallor.
Generalized, eruptive lichen planus in a pediatric patient
A healthy 14-year-old boy presented at our dermatology practice with acute onset of an intensely itchy rash that first appeared 2 months prior.
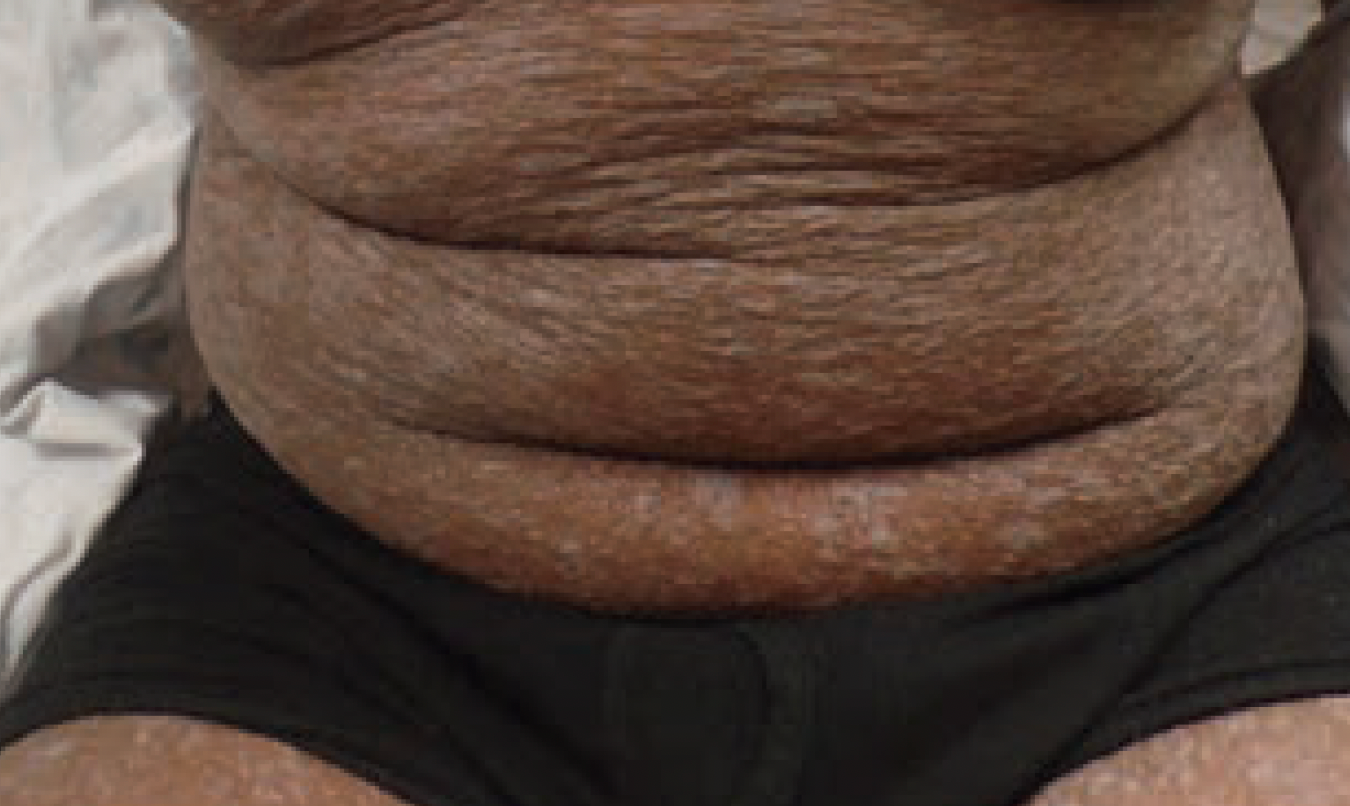
Annular scars with hyperpigmentation
An 18-month-old girl presents with ringed scars with hyperpigmentation on the right side of her buttocks and back. What's the diagnosis?
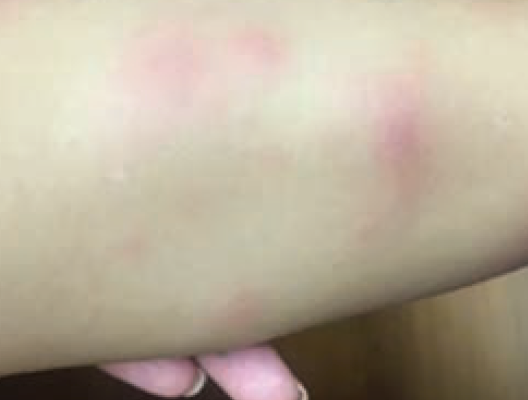
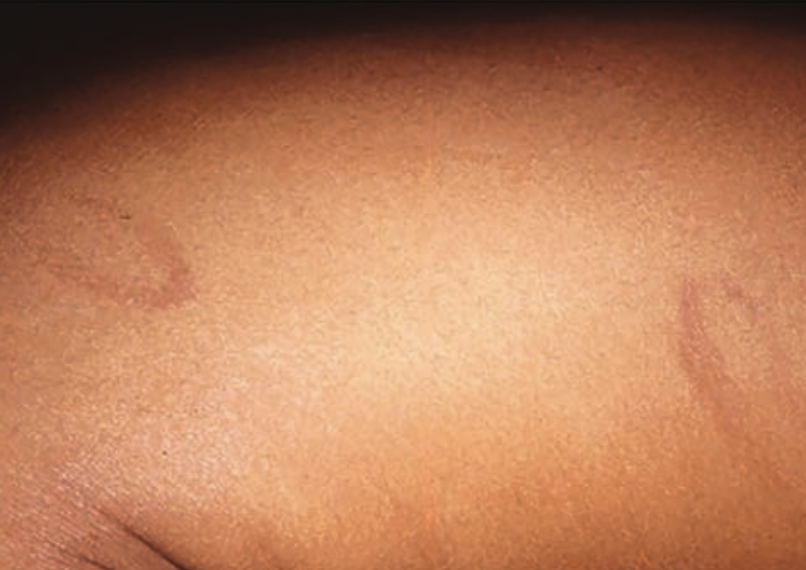
Lower-extremity nodules in a 2-year-old girl
A 2-year-old girl presents with an itchy, bilateral leg rash. Additionally, the child had several bruises that felt like "hard welts" and were warm to the touch. What's the diagnosis?
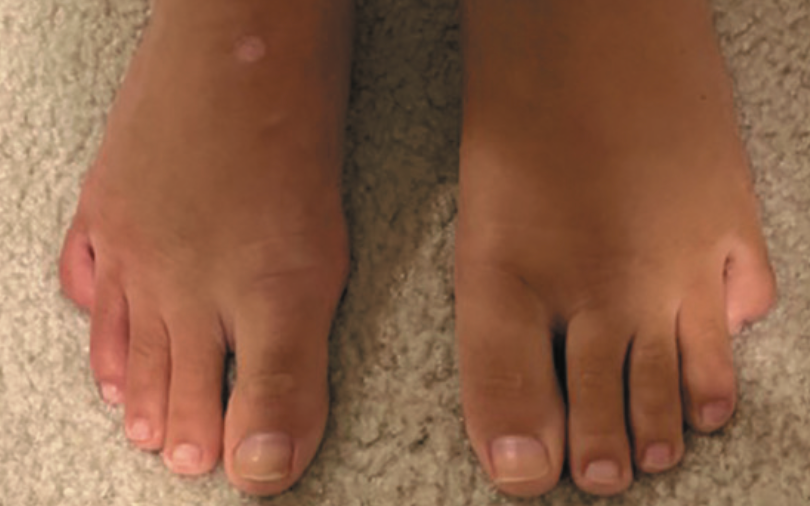
Persistent foot and leg swelling in a 17-year-old female
A 17-year-old girl presents with a 2-year history of unilateral swelling of the left lower extremity as well as a poorly healed ankle sprain of the affected extremity 3 years prior that slowly resolved but left persistent swelling. What's the diagnosis?
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Paediatrics
Showing results 1 - 10 of 100
Sorted by most recent
10 May 2024
16 April 2024
11 April 2024
8 April 2024
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs
- Case Files®: Pediatrics
- Pediatrics Examination and Board Review
- Neonatal-Perinatal Medicine: Specialty Board Review
- Symptom-Based Diagnosis in Pediatrics

Case Files: Pediatrics, 6e
Author(s): Eugene C. Toy; Mark D. Hormann; Robert J. Yetman; Margaret C. McNeese; Sheela L. Lahoti; Emma A. Omoruyi; Abby M. Geltemeyer
- 2 Infant of a Diabetic Mother
- 3 Neonatal Hyperbilirubinemia
- 4 Sepsis and Group B Streptococcal Infections
- 6 Neonatal Herpes Simplex Virus Infection
- 8 Transient Tachypnea of the Newborn
- 10 Failure to Thrive
- 38 Child Abuse
- 14 Pneumonia
- 17 Pelvic Inflammatory Disease
- 27 Bacterial Meningitis
- 28 Bacterial Enteritis
- 40 Immunodeficiency
- 54 Appendicitis
- 55 Acute Epstein-Barr Virus (Infectious Mononucleosis)
- 29 Subdural Hematoma
- 30 Complex Febrile Seizure
- 48 Migraine Without Aura
- 56 Myasthenia Gravis
- 60 Concussion
- 22 Patent Ductus Arteriosus With Heart Failure
- 23 Truncus Arteriosus
- 18 Cystic Fibrosis
- 20 Asthma Exacerbation
- 61 Electronic Cigarette or Vaping Induced Acute Lung Injury (EVALI) Online Only
- 7 Esophageal Atresia
- 15 Rectal Bleeding
- 34 Intestinal Malrotation
- 53 Inflammatory Bowel Disease
- 35 Posterior Urethral Valves
- 52 Acute Postinfectious Glomerulonephritis
- 11 Anemia in the Pediatric Patient
- 13 Sickle Cell Disease With Vaso-Occlusive Crisis
- 19 Acute Lymphoblastic Leukemia
- 33 Suspected Neuroblastoma
- 42 Diabetic Ketoacidosis
- 44 Growth Hormone Deficiency
- 45 Precocious Puberty
- 41 Trisomy 21
- 50 Turner Syndrome
- 37 Immune Thrombocytopenic Purpura
- 39 Kawasaki Disease
- 51 Systemic Lupus Erythematosus
- 57 Oligoarticular Juvenile Idiopathic Arthritis
- 58 Anaphylactoid Purpura (Henoch-Schönlein Purpura)
- 49 Adolescent Substance Use Disorder
- 62 Alopecia Areata Online Only
- 5 Accidental Ingestion of Opioids
- 25 Lead Ingestion (Microcytic Anemia)
- 59 Attention Deficit Hyperactivity Disorder
- 31 Duchenne Muscular Dystrophy
- 36 Nursemaid’s Elbow (Radial Head Subluxation)
- 47 Slipped Capital Femoral Epiphysis
- 1 Infant Rashes
- 26 Stevens-Johnson Syndrome
- 32 Atopic Dermatitis
- 9 Congenital Cataracts
- 16 Acute Otitis Media
- 43 Obstructive Sleep Apnea Syndrome
- 46 Retropharyngeal Abscess
- 21 Sudden Infant Death Syndrome
- Accidental Ingestion of Opioids
- Acute Epstein-Barr Virus (Infectious Mononucleosis)
- Acute Lymphoblastic Leukemia
- Acute Otitis Media
- Acute Postinfectious Glomerulonephritis
- Adolescent Substance Use Disorder
- Alopecia Areata Online Only
- Anaphylactoid Purpura (Henoch-Schönlein Purpura)
- Anemia in the Pediatric Patient
- Appendicitis
- Asthma Exacerbation
- Atopic Dermatitis
- Attention Deficit Hyperactivity Disorder
- Bacterial Enteritis
- Bacterial Meningitis
- Child Abuse
- Complex Febrile Seizure
- Congenital Cataracts
- Cystic Fibrosis
- Diabetic Ketoacidosis
- Duchenne Muscular Dystrophy
- Electronic Cigarette or Vaping Induced Acute Lung Injury (EVALI) Online Only
- Esophageal Atresia
- Failure to Thrive
- Growth Hormone Deficiency
- Immune Thrombocytopenic Purpura
- Immunodeficiency
- Infant of a Diabetic Mother
- Infant Rashes
- Inflammatory Bowel Disease
- Intestinal Malrotation
- Kawasaki Disease
- Lead Ingestion (Microcytic Anemia)
- Migraine Without Aura
- Myasthenia Gravis
- Neonatal Herpes Simplex Virus Infection
- Neonatal Hyperbilirubinemia
- Nursemaid’s Elbow (Radial Head Subluxation)
- Obstructive Sleep Apnea Syndrome
- Oligoarticular Juvenile Idiopathic Arthritis
- Patent Ductus Arteriosus With Heart Failure
- Pelvic Inflammatory Disease
- Posterior Urethral Valves
- Precocious Puberty
- Rectal Bleeding
- Retropharyngeal Abscess
- Sepsis and Group B Streptococcal Infections
- Sickle Cell Disease With Vaso-Occlusive Crisis
- Slipped Capital Femoral Epiphysis
- Stevens-Johnson Syndrome
- Subdural Hematoma
- Sudden Infant Death Syndrome
- Suspected Neuroblastoma
- Systemic Lupus Erythematosus
- Transient Tachypnea of the Newborn
- Truncus Arteriosus
- Turner Syndrome
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Center for Bloodless Medicine and Surgery
Case study: pediatrics, bloodless surgical treatment of a medulloblastoma in a 9-year old boy.
A 9-year old boy presented with loss of balance, headache, vomiting, double vision and was found to have a large left cerebellar brain tumor (5.6x4.8x4.8cm) extending to the margin of the fourth ventricle. An MRI showed no evidence of metastasis. As soon as the surgery was posted, the patient was placed on oral iron supplements (325 mg Ferrous Sulfate, twice a day). A discussion with the parents about Bloodless Medicine revealed their wish to avoid transfusion of the major blood fractions (red blood cells, plasma, or platelets) but they agreed to minor fractions (albumin, cryoprecipitate), as well as autologous blood salvage (Cell Saver) if necessary. We explained the risks and benefits of using Cell Saver blood in Oncology cases, including the use of special filters (leukoreduction filters), which have been shown to remove cancer cells. The clinical and legal aspects of parents requesting to avoid transfusion for their children who are under 18 years of age were discussed. Our team informed the parents that we would do everything to avoid transfusion, however, if it came to a life or death situation, we could not honor the parents' wishes. The parents were comforted to know that all blood conserving measures would be implemented.
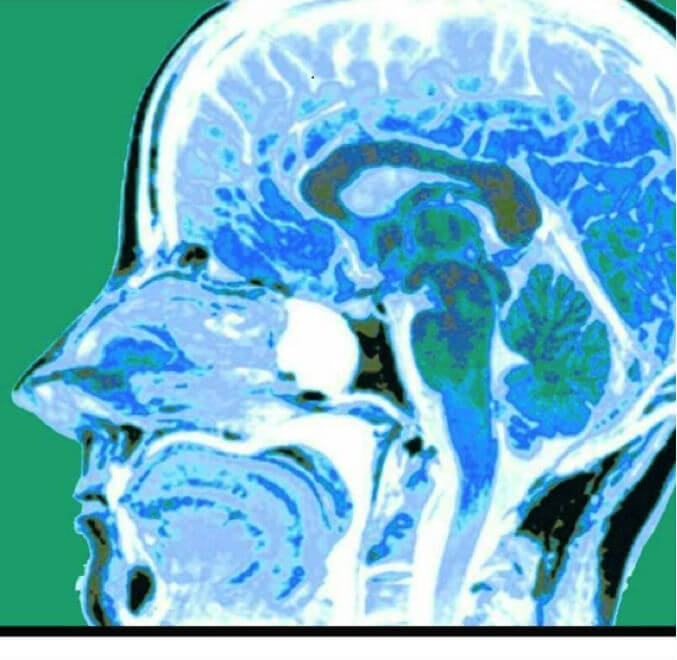
The surgical procedure was a suboccipital craniotomy for cerebellar tumor, which was performed under general anesthesia provided by the pediatric anesthesia team. Blood loss was 600 mLs, which represented 25% of his total blood volume. This calculation is based on 80 mLs/kg total blood volume in a 30 Kg child or 2,400 mLs. With a 25% blood loss we expected a 25% decrease in hemoglobin level, which is exactly what occurred. The preoperative hemoglobin level was 12.5 g/dL, and the postoperative level was 9.4. Because the tumor was adherent to the ventricles, the plan was to bring him back for a second surgery 4-6 weeks later. At that time a complete resection after chemo and radiation therapy with a regimen prescribed by the National Cancer Institute under protocol ACNS0331 for standard risk medulloblastoma was planned. This involved radiotherapy in combination with chemotherapy comprising vincristine, cisplatin, lomustine, and cyclophosphamide.
Resection of the residual tumor was accomplished 6 weeks later with only 200 mLs of blood loss. At the end of the procedure an intraventricular drain was placed to prevent hydrocephalus from adhesions at the cervicomedullary junction. The intraventricular drain was removed two weeks later and a ventriculoperitoneal shunt was placed. Follow up care was provided at the Kennedy Kreiger Institute with audiology testing and physical therapy rehabilitation. Brain and spine MRIs as well as CSF from a lumbar puncture were negative for signs of tumor. His current hemoglobin level is 11.1 and he has not required a blood transfusion through all this treatment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(10); 2023 Oct
- PMC10680406

A Brief Overview of Recent Pediatric Physical Therapy Practices and Their Importance
Chavan srushti sudhir.
1 Department of Paedatric Physiotherapy, Ravi Nair Physiotherapy College, Datta Meghe Institute of Higher Education & Research (Deemed to be University), Wardha, IND
H V Sharath
2 Department of Paediatric Physiotherapy, Ravi Nair Physiotherapy College, Datta Meghe Institute of Higher Education & Research (Deemed to be University), Wardha, IND
Recent years have seen a substantial increase in interest in pediatric physical therapy, which is a reflection of improvements in its methods and the rising understanding of its significance in child development and rehabilitation. This review provides a concise overview of the latest trends and techniques in pediatric physical therapy, emphasizing the integration of innovative technologies, evidence-based interventions, and holistic approaches. For children with varied developmental, congenital, and acquired disorders, the significance of early intervention and individualized treatment programs is emphasized, underlining the important influence of prompt interventions on long-term functional results and quality of life. To guarantee comprehensive and coordinated care, the study also examines the interdisciplinary character of pediatric physical therapy placing special emphasis on collaboration with families, caregivers, educators, and healthcare professionals. It also emphasizes the importance of continuing research, instruction, and lobbying to improve the effectiveness and availability of pediatric physical therapy services, eventually promoting the overall well-being of kids and their families.
Introduction and background
Pediatric physical therapy is a branch of physiotherapy that helps treat or develop movement issues in infants and young children and enhances a child's developmental stages. Orthopedics, congenital malformations, neurology, neuropsychiatry, respiration, and preterm are among the systems that affect children [ 1 ]. Pediatric physical therapists address developmental delay and neuromotor disorders. They treat children of all ages, from infants to teenagers, and they have particular knowledge and training in growth and development, syndromes, and diagnosis (Table (Table1) 1 ) [ 2 ].
The pediatric physical therapist may collaborate with a wide range of specialists due to the complicated requirements of the child and the family, including teams from medicine, nursing, social work, education, and psychiatry, as well as speech and occupational therapists [ 3 , 4 ]. Even though India is still in its infancy, the breadth has significantly expanded in the last several years to decades. Despite this point of view, there is controversy around using therapeutic touch in pediatric physical therapy, particularly for children with cerebral palsy [ 5 , 6 ]. Physiotherapists who work with children are experts in evaluating, identifying, diagnosing, and treating movement abnormalities and physiological conditions (Table (Table2). 2 ). They treat children (infants up to 19 years) in the areas of orthopedics, congenital anomalies, neurology, neuropsychiatry, breathing, and preterm [ 7 - 9 ].
ASD: autism spectrum disorder; ADHD: attention-deficit hyperactivity disorder; ARDS: acute respiratory distress syndrome.
Study selection
We included all research articles like randomized and non-randomized clinical studies, systematic reviews, and experimental studies of recent pediatric physical practices over the past five years; the English-language literature was searched on Google Scholar, Medline, Cochrane Library, and PubMed. Between 2019 and March 2023, 346 articles were searched using the terms paediatric physiotherapy, modern treatment practices, and advanced physiotherapy. Twenty-three papers were included in the 50 articles eligible for full-text review out of the 346 articles (Figure 1 ), which displays a database search, and data extraction demonstrates the outcome of the article selection process. A list of the examined papers is shown in Table Table3, 3 , which provides a quick summary of current pediatric physical procedures.
GMA: General Movement Assessment; AIMS: Alberta Infant Motor Scale; NDD: Neurodevelopmental Delay; IH: Intermittent Hypoxia; LRT: Lower Respiratory Tract; NDT-B: Neurodevelopmental Therapy Method-Bobath; CMT: Congenital Muscular Torticollis; NTDs: Neural Tube Defects; SB: Spina Bifida; SSC: Stretch Shortening Cycle; DS: Down Syndrome; ADHD: Attention-Deficit Hyperactivity Disorder; HCs: Healthy Asymptomatic Controls; MOOSE: Meta-analysis of Observational Studies in Epidemiology; NBPP: Newborn Brachial Plexus Palsy.

PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Pediatric physical therapists give outstanding care to everyone, from infants in the neonatal intensive care unit to young adults with childhood illnesses. Worldwide, prematurity is the leading cause of neonatal deaths, and pneumonia is the second-leading cause of death among children under five. According to the World Health Organization (WHO), India has the world's highest rate of preterm deliveries (Table 4 ). Studies have shown that early intervention therapy and chest physiotherapy for neonatal intensive care unit secretion clearance that comprises manual hyperinflation and vibrations significantly reduce the preterm death ratio. Even children who are malnourished can benefit from physical therapy to build muscle while they are recovering. For children with cerebral palsy, the most cutting-edge treatment, Pedi suit/Rehab suit therapy, is required. The benefits of multimodal stimulation for preterm infants include promoting eating and psychomotor growth. The multimodal stimulus has also been shown to enhance visual function. The General Movement Assessment approach is utilized for baby neurodevelopment. It is a method that aids in the early diagnosis of cerebral palsy. The Albert Infant Motor Scale and Optimality Score are two components of the General Movement Assessment. The handling techniques are especially useful in respiratory disorders like the incidence of intermittent hypoxemia, where it is stated that touch features are basic trigger mechanisms for intermittent hypoxemia [ 31 - 33 ].
Refs. [ 1 - 35 ].
Deformities involving muscles, tissue, or joints are included in musculoskeletal conditions. Exercises for stretching, handling placement, and strengthening are used to treat this issue [ 34 ]. Even adjustments to the surroundings help treat the illnesses. Higher-level data are emerging in favor of microcurrent therapy for torticollis. As the cesarean section appears to be a precaution, Erb's palsy, also known as neonatal brachial plexus palsy, is decreasing. Physiotherapy therapies are useful in assisting and enhancing the range of motion, strength, and functional recovery after brachial plexus and polytrauma injuries. One of the cardiorespiratory illnesses is acute respiratory disorder syndrome (ARDS). In premature neonates, chest physical therapy can treat lung issues. The Vojta approach is useful for treating lung problems in chest physical therapy. Both prone and supine positions were used while measuring oxygen saturation, partial pressure of arterial oxygen, oxygenation index, and thoracoabdominal synchronization [ 35 ].
Children with cerebral palsy are offered stretches and progressive resistance exercises to maintain and build strength. The core stability activities also improve children's balance and coordination. Patients with cerebral palsy and feeding issues might use the Bobath techniques for neurodevelopmental therapy to help with feeding and swallowing tasks. The spastic diplegic condition that children experience in cerebral palsy may be supported by simultaneous proprioceptive and visual training, but it has little or no impact on kinetic gait characteristics. Stretch-shortening cycle exercises based on the trampoline are used to address children's postural control issues as well as to improve strength in children with Down syndrome. Pediatric physiotherapists have a bright future in the rehabilitation of young athletes. Depending on the child's needs, a therapist will undertake sports conditioning, which may involve balance, proprioception, agility, coordination training, postural and gait retraining, dynamic core strengthening, and flexibility training. This is done to reduce the likelihood of sports-related injuries [ 12 ].
Limitations
The limitation of the topic presents a difficulty in fully encompassing the broad range of current pediatric physical therapy approaches and their significance within the context of this review. The overview may not adequately cover all the specific methods, case studies, or clinical applications needed for a complete grasp of pediatric physical therapy practices because of being very condensed.
Furthermore, given that these factors can have a substantial impact on the execution and efficacy of the interventions, the brief summary might not fully address regional or cultural variations in pediatric physical therapy practices. The review does not take into consideration the variations in access to pediatric physical therapy treatments and different socioeconomic backgrounds, which could restrict the generalizability of the findings to all populations and communities.
Additionally, the review might not go into great detail on the difficulties and barriers that pediatric physical therapists encounter in actual clinical settings, such as resource limitations, staffing problems, and changing healthcare regulations. These difficulties, which are so important in determining how pediatric physical therapy services are actually provided, might not be adequately covered in a succinct description. The succinct summary can also miss some important trends and future developments in pediatric physical therapy, such as the potential effects of new interdisciplinary collaborations, technological innovations, and scientific breakthroughs. These factors are essential for comprehending the changing environment of pediatric physical therapy and assuring its ongoing expansion and efficacy. As a result, while providing an insightful introduction, the succinct overview might not fully capture the breadth and depth of the topic of pediatric physical therapy, leaving room for further exploration and analysis.
Conclusions
This study focuses on the current situation of physiotherapy techniques for resolving and enhancing the issues that are developing in pediatric patients. In conclusion, pediatric diseases like spina bifida, congenital torticollis, and cerebral palsy can be managed and improved with physiotherapy. The in-depth investigation reveals the widespread usage of strengthening and stretching workouts for condition control. Beyond correcting errors, it encourages children's physical, cognitive, emotional, and social development, enabling them to realize their full potential. Additionally, the use of cutting-edge methods and technology has improved pediatric physical therapy's effectiveness and engagement levels, improving the experience for patients as a whole. A child's sense of independence and self-confidence are also fostered by therapists in addition to physical disabilities. The importance of pediatric physical therapy must be acknowledged and supported by healthcare professionals, carers, and legislators as the discipline develops. By doing this, we can work together to give physically challenged children a better future, so that they can have healthier, more contented lives. Pediatric physical therapy is a glimmer of hope and advancement for the most impressionable individuals of our society in this constantly changing environment.
The authors have declared that no competing interests exist.
- Open access
- Published: 09 May 2024
Evaluation of integrated community case management of the common childhood illness program in Gondar city, northwest Ethiopia: a case study evaluation design
- Mekides Geta 1 ,
- Geta Asrade Alemayehu 2 ,
- Wubshet Debebe Negash 2 ,
- Tadele Biresaw Belachew 2 ,
- Chalie Tadie Tsehay 2 &
- Getachew Teshale 2
BMC Pediatrics volume 24 , Article number: 310 ( 2024 ) Cite this article
89 Accesses
Metrics details
Integrated Community Case Management (ICCM) of common childhood illness is one of the global initiatives to reduce mortality among under-five children by two-thirds. It is also implemented in Ethiopia to improve community access and coverage of health services. However, as per our best knowledge the implementation status of integrated community case management in the study area is not well evaluated. Therefore, this study aimed to evaluate the implementation status of the integrated community case management program in Gondar City, Northwest Ethiopia.
A single case study design with mixed methods was employed to evaluate the process of integrated community case management for common childhood illness in Gondar town from March 17 to April 17, 2022. The availability, compliance, and acceptability dimensions of the program implementation were evaluated using 49 indicators. In this evaluation, 484 mothers or caregivers participated in exit interviews; 230 records were reviewed, 21 key informants were interviewed; and 42 observations were included. To identify the predictor variables associated with acceptability, we used a multivariable logistic regression analysis. Statistically significant variables were identified based on the adjusted odds ratio (AOR) with a 95% confidence interval (CI) and p-value. The qualitative data was recorded, transcribed, and translated into English, and thematic analysis was carried out.
The overall implementation of integrated community case management was 81.5%, of which availability (84.2%), compliance (83.1%), and acceptability (75.3%) contributed. Some drugs and medical equipment, like Cotrimoxazole, vitamin K, a timer, and a resuscitation bag, were stocked out. Health care providers complained that lack of refreshment training and continuous supportive supervision was the common challenges that led to a skill gap for effective program delivery. Educational status (primary AOR = 0.27, 95% CI:0.11–0.52), secondary AOR = 0.16, 95% CI:0.07–0.39), and college and above AOR = 0.08, 95% CI:0.07–0.39), prescribed drug availability (AOR = 2.17, 95% CI:1.14–4.10), travel time to the to the ICCM site (AOR = 3.8, 95% CI:1.99–7.35), and waiting time (AOR = 2.80, 95% CI:1.16–6.79) were factors associated with the acceptability of the program by caregivers.
Conclusion and recommendation
The overall implementation status of the integrated community case management program was judged as good. However, there were gaps observed in the assessment, classification, and treatment of diseases. Educational status, availability of the prescribed drugs, waiting time and travel time to integrated community case management sites were factors associated with the program acceptability. Continuous supportive supervision for health facilities, refreshment training for HEW’s to maximize compliance, construction clean water sources for HPs, and conducting longitudinal studies for the future are the forwarded recommendation.
Peer Review reports
Integrated Community Case Management (ICCM) is a critical public health strategy for expanding the coverage of quality child care services [ 1 , 2 ]. It mainly concentrated on curative care and also on the diagnosis, treatment, and referral of children who are ill with infectious diseases [ 3 , 4 ].
Based on the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) recommendations, Ethiopia adopted and implemented a national policy supporting community-based treatment of common childhood illnesses like pneumonia, Diarrhea, uncomplicated malnutrition, malaria and other febrile illness and Amhara region was one the piloted regions in late 2010 [ 5 ]. The Ethiopian primary healthcare units, established at district levels include primary hospitals, health centers (HCs), and health posts (HPs). The HPs are run by Health Extension Workers (HEWs), and they have function of monitoring health programs and disease occurrence, providing health education, essential primary care services, and timely referrals to HCs [ 6 , 7 ]. The Health Extension Program (HEP) uses task shifting and community ownership to provide essential health services at the first level using the health development army and a network of woman volunteers. These groups are organized to promote health and prevent diseases through community participation and empowerment by identifying the salient local bottlenecks which hinder vital maternal, neonatal, and child health service utilization [ 8 , 9 ].
One of the key steps to enhance the clinical case of health extension staff is to encourage better growth and development among under-five children by health extension. Healthy family and neighborhood practices are also encouraged [ 10 , 11 ]. The program also combines immunization, community-based feeding, vitamin A and de-worming with multiple preventive measures [ 12 , 13 ]. Now a days rapidly scaling up of ICCM approach to efficiently manage the most common causes of morbidity and mortality of children under the age of five in an integrated manner at the community level is required [ 14 , 15 ].
Over 5.3 million children are died at a global level in 2018 and most causes (75%) are preventable or treatable diseases such as pneumonia, malaria and diarrhea [ 16 ]. About 99% of the global burden of mortality and morbidity of under-five children which exists in developing countries are due to common childhood diseases such as pneumonia, diarrhea, malaria and malnutrition [ 17 ].
In 2013, the mortality rate of under-five children in Sub-Saharan Africa decreased to 86 deaths per 1000 live birth and estimated to be 25 per 1000live births by 2030. However, it is a huge figure and the trends are not sufficient to reach the target [ 18 ]. About half of global under-five deaths occurred in sub-Saharan Africa. And from the top 26 nations burdened with 80% of the world’s under-five deaths, 19 are in sub-Saharan Africa [ 19 ].
To alleviate the burden, the Ethiopian government tries to deliver basic child care services at the community level by trained health extension workers. The program improves the health of the children not only in Ethiopia but also in some African nations. Despite its proven benefits, the program implementation had several challenges, in particular, non-adherence to the national guidelines among health care workers [ 20 ]. Addressing those challenges could further improve the program performance. Present treatment levels in sub-Saharan Africa are unacceptably poor; only 39% of children receive proper diarrhea treatment, 13% of children with suspected pneumonia receive antibiotics, 13% of children with fever receive a finger/heel stick to screen for malaria [ 21 ].
To improve the program performance, program gaps should be identified through scientific evaluations and stakeholder involvement. This evaluation not only identify gaps but also forward recommendations for the observed gaps. Furthermore, the implementation status of ICCM of common childhood illnesses has not been evaluated in the study area yet. Therefore, this work aimed to evaluate the implementation status of integrated community case management program implementation in Gondar town, northwest Ethiopia. The findings may be used by policy makers, healthcare providers, funders and researchers.
Method and material
Evaluation design and settings.
A single-case study design with concurrent mixed-methods evaluation was conducted in Gondar city, northwest Ethiopia, from March 17 to April 17, 2022. The evaluability assessment was done from December 15–30, 2021. Both qualitative and quantitative data were collected concurrently, analyzed separately, and integrated at the result interpretation phase.
The evaluation area, Gondar City, is located in northwest Ethiopia, 740 km from Addis Ababa, the capital city of the country. It has six sub-cities and thirty-six kebeles (25 urban and 11 rural). In 2019, the estimated total population of the town was 338,646, and 58,519 (17.3%) were under-five children. In the town there are eight public health centers and 14 health posts serving the population. All health posts provide ICCM service for more than 70,852 populations.
Evaluation approach and dimensions
Program stakeholders.
The evaluation followed a formative participatory approach by engaging the potential stakeholders in the program. Prior to the development of the proposal, an extensive discussion was held with the Gondar City Health Department to identify other key stakeholders in the program. Service providers at each health facility (HCs and HPs), caretakers of sick children, the Gondar City Health Office (GCHO), the Amhara Regional Health Bureau (ARHB), the Minister of Health (MoH), and NGOs (IFHP and Save the Children) were considered key stakeholders. During the Evaluability Assessment (EA), the stakeholders were involved in the development of evaluation questions, objectives, indicators, and judgment criteria of the evaluation.
Evaluation dimensions
The availability and acceptability dimensions from the access framework [ 22 ] and compliance dimension from the fidelity framework [ 23 ] were used to evaluate the implementation of ICCM.
Population and samplings
All under-five children and their caregivers attended at the HPs; program implementers (health extension workers, healthcare providers, healthcare managers, PHCU focal persons, MCH coordinators, and other stakeholders); and ICCM records and registries in the health posts of Gondar city administration were included in the evaluation. For quantitative data, the required sample size was proportionally allocated for each health post based on the number of cases served in the recent one month. But the qualitative sample size was determined by data saturation, and the samples were selected purposefully.
The data sources and sample size for the compliance dimension were all administrative records/reports and ICCM registration books (230 documents) in all health posts registered from December 1, 2021, to February 30, 2022 (three months retrospectively) included in the evaluation. The registries were assessed starting from the most recent registration number until the required sample size was obtained for each health post.
The sample size to measure the mothers’/caregivers’ acceptability towards ICCM was calculated by taking prevalence of caregivers’ satisfaction on ICCM program p = 74% from previously similar study [ 24 ] and considering standard error 4% at 95% CI and 10% non- responses, which gave 508. Except those who were seriously ill, all caregivers attending the ICCM sites during data collection were selected and interviewed consecutively.
The availability of required supplies, materials and human resources for the program were assessed in all 14HPs. The data collectors observed the health posts and collected required data by using a resources inventory checklist.
A total of 70 non-participatory patient-provider interactions were also observed. The observations were conducted per each health post and for health posts which have more than one health extension workers one of them were selected randomly. The observation findings were used to triangulate the findings obtained through other data collection techniques. Since people may act accordingly to the standards when they know they are observed for their activities, we discarded the first two observations from analysis. It is one of the strategies to minimize the Hawthorne effect of the study. Finally a total of 42 (3 in each HPs) observations were included in the analysis.
Twenty one key informants (14 HEWs, 3 PHCU focal person, 3 health center heads and one MCH coordinator) were interviewed. These key informants were selected since they are assumed to be best teachers in the program. Besides originally developed key informant interview questions, the data collectors probed them to get more detail and clear information.
Variables and measurement
The availability of resources, including trained healthcare workers, was examined using 17 indicators, with weighted score of 35%. Compliance was used to assess HEWs’ adherence to the ICCM treatment guidelines by observing patient-provider interactions and conducting document reviews. We used 18 indicators and a weighted value of 40%.
Mothers’ /caregivers’/ acceptance of ICCM service was examined using 14 indicators and had a weighted score of 25%. The indicators were developed with a five-point Likert scale (1: strongly disagree, 2: disagree, 3: neutral, 4: agree and 5: strongly agree). The cut off point for this categorization was calculated using the demarcation threshold formula: ( \(\frac{\text{t}\text{o}\text{t}\text{a}\text{l}\, \text{h}\text{i}\text{g}\text{h}\text{e}\text{s}\text{t}\, \text{s}\text{c}\text{o}\text{r}\text{e}-\,\text{t}\text{o}\text{t}\text{a}\text{l}\, \text{l}\text{o}\text{w}\text{e}\text{s}\text{t} \,\text{s}\text{c}\text{o}\text{r}\text{e}}{2}) +total lowest score\) ( 25 – 27 ). Those mothers/caregivers/ who scored above cut point (42) were considered as “satisfied”, otherwise “dissatisfied”. The indicators were adapted from the national ICCM and IMNCI implementation guideline and other related evaluations with the participation of stakeholders. Indicator weight was given by the stakeholders during EA. Indicators score was calculated using the formula \(\left(achieved \,in \%=\frac{indicator \,score \,x \,100}{indicator\, weight} \right)\) [ 26 , 28 ].
The independent variables for the acceptability dimension were socio-demographic and economic variables (age, educational status, marital status, occupation of caregiver, family size, income level, and mode of transport), availability of prescribed drugs, waiting time, travel time to ICCM site, home to home visit, consultation time, appointment, and source of information.
The overall implementation of ICCM was measured by using 49 indicators over the three dimensions: availability (17 indicators), compliance (18 indicators) and acceptability (14 indicators).
Program logic model
Based on the constructed program logic model and trained health care providers, mothers/caregivers received health information and counseling on child feeding; children were assessed, classified, and treated for disease, received follow-up; they were checked for vitamin A; and deworming and immunization status were the expected outputs of the program activities. Improved knowledge of HEWs on ICCM, increased health-seeking behavior, improved quality of health services, increased utilization of services, improved data quality and information use, and improved child health conditions are considered outcomes of the program. Reduction of under-five morbidity and mortality and improving quality of life in the society are the distant outcomes or impacts of the program (Fig. 1 ).
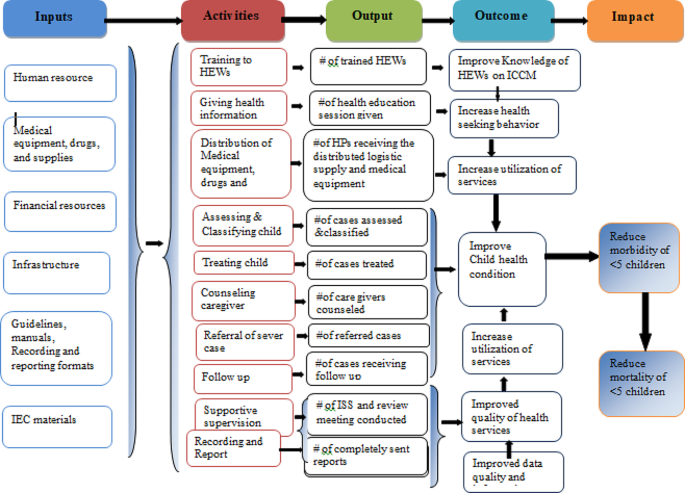
Integrated community case management of childhood illness program logic model in Gondar City in 2022
Data collection tools and procedure
Resource inventory and data extraction checklists were adapted from standard ICCM tool and check lists [ 29 ]. A structured interviewer administered questionnaire was adapted by referring different literatures [ 30 , 31 ] to measure the acceptability of ICCM. The key informant interview (KII) guide was also developed to explore the views of KIs. The interview questionnaire and guide were initially developed in English and translated into the local language (Amharic) and finally back to English to ensure consistency. All the interviews were done in the local language, Amharic.
Five trained clinical nurses and one BSC nurse were recruited from Gondar zuria and Wegera district as data collectors and supervisors, respectively. Two days training on the overall purpose of the evaluation and basic data collection procedures were provided prior to data collection. Then, both quantitative and qualitative data were gathered at the same time. The quantitative data were gathered from program documentation, charts of ICCM program visitors and, exit interview. Interviews with 21 KIIs and non-participatory observations of patient-provider interactions were used to acquire qualitative data. Key informant interviews were conducted to investigate the gaps and best practices in the implementation of the ICCM program.
A pretest was conducted to 26 mothers/caregivers/ at Maksegnit health post and appropriate modifications were made based on the pretest results. The data collectors were supervised and principal evaluator examined the completeness and consistency of the data on a daily basis.
Data management and analysis
For analysis, quantitative data were entered into epi-data version 4.6 and exported to Stata 14 software for analysis. Narration and tabular statistics were used to present descriptive statistics. Based on established judgment criteria, the total program implementation was examined and interpreted as a mix of the availability, compliance, and acceptability dimensions. To investigate the factors associated with ICCM acceptance, a binary logistic regression analysis was performed. During bivariable analysis, variables with p-values less than 0.25 were included in multivariable analysis. Finally, variables having a p-value less than 0.05 and an adjusted odds ratio (AOR) with a 95% confidence interval (CI) were judged statistically significant. Qualitative data were collected recorded, transcribed into Amharic, then translated into English and finally coded and thematically analyzed.
Judgment matrix analysis
The weighted values of availability, compliance, and acceptability dimensions were 35, 40, and 25 based on the stakeholder and investigator agreement on each indicator, respectively. The judgment parameters for each dimension and the overall implementation of the program were categorized as poor (< 60%), fair (60–74.9%), good (75-84.9%), and very good (85–100%).
Availability of resources
A total of 26 HEWs were assigned within the fourteen health posts, and 72.7% of them were trained on ICCM to manage common childhood illnesses in under-five children. However, the training was given before four years, and they didn’t get even refreshment training about ICCM. The KII responses also supported that the shortage of HEWs at the HPs was the problem in implementing the program properly.
I am the only HEW in this health post and I have not been trained on ICCM program. So, this may compromise the quality of service and client satisfaction.(25 years old HEW with two years’ experience)
All observed health posts had ICCM registration books, monthly report and referral formats, functional thermometer, weighting scale and MUAC tape meter. However, timer and resuscitation bag was not available in all HPs. Most of the key informant finding showed that, in all HPs there was no shortage of guideline, registration book and recording tool; however, there was no OTP card in some health posts.
“Guideline, ICCM registration book for 2–59 months of age, and other different recording and reporting formats and booklet charts are available since September/2016. However, OTP card is not available in most HPs.”. (A 30 years male health center director)
Only one-fifth (21%) of HPs had a clean water source for drinking and washing of equipment. Most of Key-informant interview findings showed that the availability of infrastructures like water was not available in most HPs. Poor linkage between HPs, HCs, town health department, and local Kebele administer were the reason for unavailability.
Since there is no water for hand washing, or drinking, we obligated to bring water from our home for daily consumptions. This increases the burden for us in our daily activity. (35 years old HEW)
Most medicines, such as anti-malaria drugs with RDT, Quartem, Albendazole, Amoxicillin, vitamin A capsules, ORS, and gloves, were available in all the health posts. Drugs like zinc, paracetamol, TTC eye ointment, and folic acid were available in some HPs. However, cotrimoxazole and vitamin K capsules were stocked-out in all health posts for the last six months. The key informant also revealed that: “Vitamin K was not available starting from the beginning of this program and Cotrimoxazole was not available for the past one year and they told us they would avail it soon but still not availed. Some essential ICCM drugs like anti malaria drugs, De-worming, Amoxicillin, vitamin A capsules, ORS and medical supplies were also not available in HCs regularly.”(28 years’ Female PHCU focal)
The overall availability of resources for ICCM implementation was 84.2% which was good based on our presetting judgment parameter (Table 1 ).
Health extension worker’s compliance
From the 42 patient-provider interactions, we found that 85.7%, 71.4%, 76.2%, and 95.2% of the children were checked for body temperature, weight, general danger signs, and immunization status respectively. Out of total (42) observation, 33(78.6%) of sick children were classified for their nutritional status. During observation time 29 (69.1%) of caregivers were counseled by HEWs on food, fluid and when to return back and 35 (83.3%) of children were appointed for next follow-up visit. Key informant interviews also affirmed that;
“Most of our health extension workers were trained on ICCM program guidelines but still there are problems on assessment classification and treatment of disease based on guidelines and standards this is mainly due to lack refreshment training on the program and lack of continuous supportive supervision from the respective body.” (27years’ Male health center head)
From 10 clients classified as having severe pneumonia cases, all of them were referred to a health center (with pre-referral treatment), and from those 57 pneumonia cases, 50 (87.7%) were treated at the HP with amoxicillin or cotrimoxazole. All children with severe diarrhea, very severe disease, and severe complicated malnutrition cases were referred to health centers with a pre-referral treatment for severe dehydration, very severe febrile disease, and severe complicated malnutrition, respectively. From those with some dehydration and no dehydration cases, (82.4%) and (86.8%) were treated at the HPs for some dehydration (ORS; plan B) and for no dehydration (ORS; plan A), respectively. Moreover, zinc sulfate was prescribed for 63 (90%) of under-five children with some dehydration or no dehydration. From 26 malaria cases and 32 severe uncomplicated malnutrition and moderate acute malnutrition cases, 20 (76.9%) and 25 (78.1%) were treated at the HPs, respectively. Of the total reviewed documents, 56 (93.3%), 66 (94.3%), 38 (84.4%), and 25 (78.1%) of them were given a follow-up date for pneumonia, diarrhea, malaria, and malnutrition, respectively.
Supportive supervision and performance review meetings were conducted only in 10 (71.4%) HPs, but all (100%) HPs sent timely reports to the next supervisory body.
Most of the key informants’ interview findings showed that supportive supervision was not conducted regularly and for all HPs.
I had mentored and supervised by supportive supervision teams who came to our health post at different times from health center, town health office and zonal health department. I received this integrated supervision from town health office irregularly, but every month from catchment health center and last integrated supportive supervision from HC was on January. The problem is the supervision was conducted for all programs.(32 years’ old and nine years experienced female HEW)
Moreover, the result showed that there was poor compliance of HEWs for the program mainly due to weak supportive supervision system of managerial and technical health workers. It was also supported by key informants as:
We conducted supportive supervision and performance review meeting at different time, but still there was not regular and not addressed all HPs. In addition to this the supervision and review meeting was conducted as integration of ICCM program with other services. The other problem is that most of the time we didn’t used checklist during supportive supervision. (Mid 30 years old male HC director)
Based on our observation and ICCM document review, 83.1% of the HEWs were complied with the ICCM guidelines and judged as fair (Table 2 ).
Acceptability of ICCM program
Sociodemographic and obstetric characteristics of participants.
A total of 484 study participants responded to the interviewer-administered questionnaire with a response rate of 95.3%. The mean age of study participants was 30.7 (SD ± 5.5) years. Of the total caregivers, the majority (38.6%) were categorized under the age group of 26–30 years. Among the total respondents, 89.3% were married, and regarding religion, the majorities (84.5%) were Orthodox Christian followers. Regarding educational status, over half of caregivers (52.1%) were illiterate (unable to read or write). Nearly two-thirds of the caregivers (62.6%) were housewives (Table 3 ).
All the caregivers came to the health post on foot, and most of them 418 (86.4%) arrived within one hour. The majority of 452 (93.4%) caregivers responded that the waiting time to get the service was less than 30 min. Caregivers who got the prescribed drugs at the health post were 409 (84.5%). Most of the respondents, 429 (88.6%) and 438 (90.5%), received counseling services on providing extra fluid and feeding for their sick child and were given a follow-up date.
Most 298 (61.6%) of the caregivers were satisfied with the convenience of the working hours of HPs, and more than three-fourths (80.8%) were satisfied with the counseling services they received. Most of the respondents, 366 (75.6%), were satisfied with the appropriateness of waiting time and 431 (89%) with the appropriateness of consultation time. The majority (448 (92.6%) of caregivers were satisfied with the way of communicating with HEWs, and 269 (55.6%) were satisfied with the knowledge and competence of HEWs. Nearly half of the caregivers (240, or 49.6%) were satisfied with the availability of drugs at health posts.
The overall acceptability of the ICCM program was 75.3%, which was judged as good. A low proportion of acceptability was measured on the cleanliness of the health posts, the appropriateness of the waiting area, and the competence and knowledge of the HEWs. On the other hand, high proportion of acceptability was measured on appropriateness of waiting time, way of communication with HEWs, and the availability of drugs (Table 4 ).
Factors associated with acceptability of ICCM program
In the final multivariable logistic regression analysis, educational status of caregivers, availability of prescribed drugs, time to arrive, and waiting time were factors significantly associated with the satisfaction of caregivers with the ICCM program.
Accordingly, the odds of caregivers with primary education, secondary education, and college and above were 73% (AOR = 0.27, 95% CI: 0.11–0.52), 84% (AOR = 0.16, 95% CI: 0.07–0.39), and 92% (AOR = 0.08, 95% CI: 0.07–0.40) less likely to accept the program as compared to mothers or caregivers who were not able to read and write, respectively. The odds of caregivers or mothers who received prescribed drugs were 2.17 times more likely to accept the program as compared to their counters (AOR = 2.17, 95% CI: 1.14–4.10). The odds of caregivers or mothers who waited for services for less than 30 min were 2.8 times more likely to accept the program as compared to those who waited for more than 30 min (AOR = 2.80, 95% CI: 1.16–6.79). Moreover, the odds of caregivers/mothers who traveled an hour or less for service were 3.8 times more likely to accept the ICCM program as compared to their counters (AOR = 3.82, 95% CI:1.99–7.35) (Table 5 ).
Overall ICCM program implementation and judgment
The implementation of the ICCM program in Gondar city administration was measured in terms of availability (84.2%), compliance (83.1%), and acceptability (75.3%) dimensions. In the availability dimension, amoxicillin, antimalarial drugs, albendazole, Vit. A, and ORS were available in all health posts, but only six HPs had Ready-to-Use Therapeutic Feedings, three HPs had ORT Corners, and none of the HPs had functional timers. In all health posts, the health extension workers asked the chief to complain, correctly assessed for pneumonia, diarrhea, malaria, and malnutrition, and sent reports based on the national schedule. However, only 70% of caretakers counseled about food, fluids, and when to return, 66% and 76% of the sick children were checked for anemia and other danger signs, respectively. The acceptability level of the program by caretakers and caretakers’/mothers’ educational status, waiting time to get the service and travel time ICCM sites were the factors affecting its acceptability. The overall ICCM program in Gondar city administration was 81.5% and judged as good (Fig. 2 ).
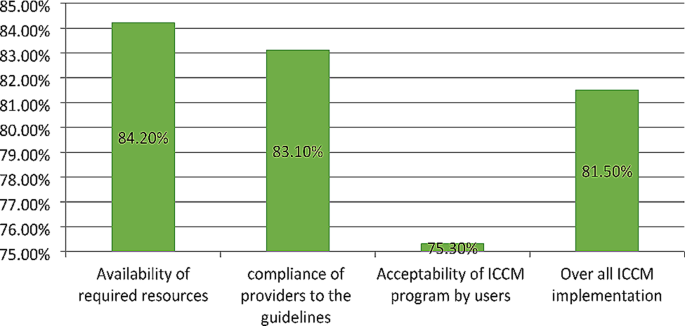
Overall ICCM program implementation and the evaluation dimensions in Gondar city administration, 2022
The implementation status of ICCM was judged by using three dimensions including availability, compliance and acceptability of the program. The judgment cut of points was determined during evaluability assessment (EA) along with the stakeholders. As a result, we found that the overall implementation status of ICCM program was good as per the presetting judgment parameter. Availability of resources for the program implementation, compliance of HEWs to the treatment guideline and acceptability of the program services by users were also judged as good as per the judgment parameter.
This evaluation showed that most medications, equipment and recording and reporting materials available. This finding was comparable with the standard ICCM treatment guide line [ 10 ]. On the other hand trained health care providers, some medications like Zink, Paracetamol and TTC eye ointment, folic acid and syringes were not found in some HPs. However the finding was higher than the study conducted in SNNPR on selected health posts [ 33 ] and a study conducted in Soro district, southern Ethiopia [ 24 ]. The possible reason might be due to low interruption of drugs at town health office or regional health department stores, regular supplies of essential drugs and good supply management and distribution of drug from health centers to health post.
The result of this evaluation showed that only one fourth of health posts had functional ORT Corner which was lower compared to the study conducted in SNNPR [ 34 ]. This might be due poor coverage of functional pipe water in the kebeles and the installation was not set at the beginning of health post construction as reported from one of ICCM program coordinator.
Compliance of HEWs to the treatment guidelines in this evaluation was higher than the study done in southern Ethiopia (65.6%) [ 24 ]. This might be due to availability of essential drugs educational level of HEWs and good utilization of ICCM guideline and chart booklet by HEWs. The observations showed most of the sick children were assessed for danger sign, weight, and temperature respectively. This finding is lower than the study conducted in Rwanda [ 35 ]. This difference might be due to lack of refreshment training and regular supportive supervision for HEWs. This also higher compared to the study done in three regions of Ethiopia indicates that 88%, 92% and 93% of children classified as per standard for Pneumonia, diarrhea and malaria respectively [ 36 ]. The reason for this difference may be due to the presence of medical equipment and supplies including RDT kit for malaria, and good educational level of HEWs.
Moreover most HPs received supportive supervision and performance review meeting was conducted and all of them send reports timely to next level. The finding of this evaluation was lower than the study conducted on implementation evaluation of ICCM program southern Ethiopia [ 24 ] and study done in three regions of Ethiopia (Amhara, Tigray and SNNPR) [ 37 ]. This difference might be due sample size variation.
The overall acceptability of the ICCM program was less than the presetting judgment parameter but slightly higher compared to the study in southern Ethiopia [ 24 ]. This might be due to presence of essential drugs for treating children, reasonable waiting and counseling time provided by HEWs, and smooth communication between HEWs and caregivers. In contrast, this was lower than similar studies conducted in Wakiso district, Uganda [ 38 ]. The reason for this might be due to contextual difference between the two countries, inappropriate waiting area to receive the service and poor cleanness of the HPs in our study area. Low acceptability of caregivers to ICCM service was observed in the appropriateness of waiting area, availability of drugs, cleanness of health post, and competence of HEWs while high level of caregiver’s acceptability was consultation time, counseling service they received, communication with HEWs, treatment given for their sick children and interest to return back for ICCM service.
Caregivers who achieved primary, secondary, and college and above were more likely accept the program services than those who were illiterate. This may more educated mothers know about their child health condition and expect quality service from healthcare providers which is more likely reduce the acceptability of the service. The finding is congruent with a study done on implementation evaluation of ICCM program in southern Ethiopia [ 24 ]. However, inconsistent with a study conducted in wakiso district in Uganda [ 38 ]. The possible reason for this might be due to contextual differences between the two countries. The ICCM program acceptability was high in caregivers who received all prescribed drugs than those did not. Caregivers those waited less than 30 min for service were more accepted ICCM services compared to those more than 30 minutes’ waiting time. This finding is similar compared with the study conducted on implementation evaluation of ICCM program in southern Ethiopia [ 24 ]. In contrary, the result was incongruent with a survey result conducted by Ethiopian public health institute in all regions and two administrative cities of Ethiopia [ 39 ]. This variation might be due to smaller sample size in our study the previous one. Moreover, caregivers who traveled to HPs less than 60 min were more likely accepted the program than who traveled more and the finding was similar with the study finding in Jimma zone [ 40 ].
Strengths and limitations
This evaluation used three evaluation dimensions, mixed method and different data sources that would enhance the reliability and credibility of the findings. However, the study might have limitations like social desirability bias, recall bias and Hawthorne effect.
The implementation of the ICCM program in Gondar city administration was measured in terms of availability (84.2%), compliance (83.1%), and acceptability (75.3%) dimensions. In the availability dimension, amoxicillin, antimalarial drugs, albendazole, Vit. A, and ORS were available in all health posts, but only six HPs had Ready-to-Use Therapeutic Feedings, three HPs had ORT Corners, and none of the HPs had functional timers.
This evaluation assessed the implementation status of the ICCM program, focusing mainly on availability, compliance, and acceptability dimensions. The overall implementation status of the program was judged as good. The availability dimension is compromised due to stock-outs of chloroquine syrup, cotrimoxazole, and vitamin K and the inaccessibility of clean water supply in some health posts. Educational statuses of caregivers, availability of prescribed drugs at the HPs, time to arrive to HPs, and waiting time to receive the service were the factors associated with the acceptability of the ICCM program.
Therefore, continuous supportive supervision for health facilities, and refreshment training for HEW’s to maximize compliance are recommended. Materials and supplies shall be delivered directly to the health centers or health posts to solve the transportation problem. HEWs shall document the assessment findings and the services provided using the registration format to identify their gaps, limitations, and better performances. The health facilities and local administrations should construct clean water sources for health facilities. Furthermore, we recommend for future researchers and program evaluators to conduct longitudinal studies to know the causal relationship of the program interventions and the outcomes.
Data availability
Data will be available upon reasonable request from the corresponding author.
Abbreviations
Ethiopian Demographic and Health Survey
Health Center/Health Facility
Health Extension Program
Health Extension Workers
Health Post
Health Sector Development Plan
Integrated Community Case Management of Common Childhood Illnesses
Information Communication and Education
Integrated Family Health Program
Integrated Management of Neonatal and Childhood Illness
Integrated Supportive Supervision
Maternal and Child Health
Mid Upper Arm Circumference
Non-Government Organization
Oral Rehydration Salts
Outpatient Therapeutic program
Primary health care unit
Rapid Diagnostics Test
Ready to Use Therapeutic Foods
Sever Acute Malnutrition
South Nation Nationalities People Region
United Nations International Child Emergency Fund
World Health Organization
Brenner JL, Barigye C, Maling S, Kabakyenga J, Nettel-Aguirre A, Buchner D, et al. Where there is no doctor: can volunteer community health workers in rural Uganda provide integrated community case management? Afr Health Sci. 2017;17(1):237–46.
Article PubMed PubMed Central Google Scholar
Mubiru D, Byabasheija R, Bwanika JB, Meier JE, Magumba G, Kaggwa FM, et al. Evaluation of integrated community case management in eight districts of Central Uganda. PLoS ONE. 2015;10(8):e0134767.
Samuel S, Arba A. Utilization of integrated community case management service and associated factors among mothers/caregivers who have sick eligible children in southern Ethiopia. Risk Manage Healthc Policy. 2021;14:431.
Article Google Scholar
Kavle JA, Pacqué M, Dalglish S, Mbombeshayi E, Anzolo J, Mirindi J, et al. Strengthening nutrition services within integrated community case management (iCCM) of childhood illnesses in the Democratic Republic of Congo: evidence to guide implementation. Matern Child Nutr. 2019;15:e12725.
Miller NP, Amouzou A, Tafesse M, Hazel E, Legesse H, Degefie T, et al. Integrated community case management of childhood illness in Ethiopia: implementation strength and quality of care. Am J Trop Med Hyg. 2014;91(2):424.
WHO. Annual report 2016: Partnership and policy engagement. World Health Organization, 2017.
Banteyerga H. Ethiopia’s health extension program: improving health through community involvement. MEDICC Rev. 2011;13:46–9.
Article PubMed Google Scholar
Wang H, Tesfaye R, Ramana NV, Chekagn G. CT. Ethiopia health extension program: an institutionalized community approach for universal health coverage. The World Bank; 2016.
Donnelly J. Ethiopia gears up for more major health reforms. Lancet. 2011;377(9781):1907–8.
Legesse H, Degefie T, Hiluf M, Sime K, Tesfaye C, Abebe H, et al. National scale-up of integrated community case management in rural Ethiopia: implementation and early lessons learned. Ethiop Med J. 2014;52(Suppl 3):15–26.
Google Scholar
Miller NP, Amouzou A, Hazel E, Legesse H, Degefie T, Tafesse M et al. Assessment of the impact of quality improvement interventions on the quality of sick child care provided by Health Extension workers in Ethiopia. J Global Health. 2016;6(2).
Oliver K, Young M, Oliphant N, Diaz T, Kim JJNYU. Review of systematic challenges to the scale-up of integrated community case management. Emerging lessons & recommendations from the catalytic initiative (CI/IHSS); 2012.
FMoH E. Health Sector Transformation Plan 2015: https://www.slideshare.net . Accessed 12 Jan 2022.
McGorman L, Marsh DR, Guenther T, Gilroy K, Barat LM, Hammamy D, et al. A health systems approach to integrated community case management of childhood illness: methods and tools. The American Journal of Tropical Medicine and Hygiene. 2012;87(5 Suppl):69.
Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. The American journal of tropical medicine and hygiene. 2012;87(5 Suppl):6.
Ezbakhe F, Pérez-Foguet A. Child mortality levels and trends. Demographic Research.2020;43:1263-96.
UNICEF, Ending child deaths from pneumonia and diarrhoea. 2016 report: Available at https://data.unicef.org. accessed 13 Jan 2022.
UNITED NATIONS, The Millinium Development Goals Report 2015: Available at https://www.un.org.Accessed 12 Jan 2022
Bent W, Beyene W, Adamu A. Factors Affecting Implementation of Integrated Community Case Management Of Childhood Illness In South West Shoa Zone, Central Ethiopia 2015.
Abdosh B. The quality of hospital services in eastern Ethiopia: Patient’s perspective.The Ethiopian Journal of Health Development. 2006;20(3).
Young M, Wolfheim C, Marsh DR, Hammamy DJTAjotm, hygiene. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children.2012;87(5_Suppl):6–10.
Obrist B, Iteba N, Lengeler C, Makemba A, Mshana C, Nathan R, et al. Access to health care in contexts of livelihood insecurity: a framework for analysis and action.PLoS medicine. 2007;4(10):e308.
Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implementation science. 2007;2(1):1–9.
Dunalo S, Tadesse B, Abraham G. Implementation Evaluation of Integrated Community Case Management of Common Childhood Illness (ICCM) Program in Soro Woreda, Hadiya Zone Southern Ethiopia 2017 2017.
Asefa G, Atnafu A, Dellie E, Gebremedhin T, Aschalew AY, Tsehay CT. Health System Responsiveness for HIV/AIDS Treatment and Care Services in Shewarobit, North Shewa Zone, Ethiopia. Patient preference and adherence. 2021;15:581.
Gebremedhin T, Daka DW, Alemayehu YK, Yitbarek K, Debie A. Process evaluation of the community-based newborn care program implementation in Geze Gofa district,south Ethiopia: a case study evaluation design. BMC pregnancy and childbirth. 2019;19(1):1–13.
Pitaloka DS, Rizal A. Patient’s satisfaction in antenatal clinic hospital Universiti Kebangsaan Malaysia. Jurnal Kesihatan Masyarakat (Malaysia). 2006;12(1):1–10.
Teshale G, Debie A, Dellie E, Gebremedhin T. Evaluation of the outpatient therapeutic program for severe acute malnourished children aged 6–59 months implementation in Dehana District, Northern Ethiopia: a mixed-methods evaluation. BMC pediatrics. 2022;22(1):1–13.
Mason E. WHO’s strategy on Integrated Management of Childhood Illness. Bulletin of the World Health Organization. 2006;84(8):595.
Shaw B, Amouzou A, Miller NP, Tafesse M, Bryce J, Surkan PJ. Access to integrated community case management of childhood illnesses services in rural Ethiopia: a qualitative study of the perspectives and experiences of caregivers. Health policy and planning.2016;31(5):656 – 66.
Organization WH. Annual report 2016: Partnership and policy engagement. World Health Organization, 2017.
Berhanu D, Avan B. Community Based Newborn Care Baseline Survey Report Ethiopia,October 2014.
Save the children, Enhancing Ethiopia’s Health Extension Package in the Southern Nations and Nationalities People’s Region (SNNPR) Shebedino and Lanfero Woredas report.Hawassa;. 2012: Avalable at https://ethiopia.savethechildren.net
Kolbe AR, Muggah R, Hutson RA, James L, Puccio M, Trzcinski E, et al. Assessing Needs After the Quake: Preliminary Findings from a Randomized Survey of Port-au-Prince Households. University of Michigan/Small Arms Survey: Available at https://deepbluelibumichedu PDF. 2010.
Teferi E, Teno D, Ali I, Alemu H, Bulto T. Quality and use of IMNCI services at health center under-five clinics after introduction of integrated community-based case management (ICCM) in three regions of Ethiopia. Ethiopian Medical Journal. 2014;52(Suppl 3):91 – 8.
Last 10 Km project, Integrated Community Case Management (iCCM) Survey report in Amhara, SNNP, and Tigray Regions, 2017: Avaialable at https://l10k.jsi.com
Tumuhamye N, Rutebemberwa E, Kwesiga D, Bagonza J, Mukose A. Client satisfaction with integrated community case management program in Wakiso District, Uganda, October 2012: A cross sectional survey. Health scrip org. 2013;2013.
EPHI. Ethiopia service provision assessment plus survey 2014 report: available at http://repository.iifphc.org
Gintamo B. EY, Assefa Y. Implementation Evaluation of IMNCI Program at Public Health Centers of Soro District, Hadiya Zone, Southern Ethiopia,. 2017: Available at https://repository.ju.edu.et
Download references
Acknowledgements
We are very grateful to University of Gondar and Gondar town health office for its welcoming approaches. We would also like to thank all of the study participants of this evaluation for their information and commitment. Our appreciation also goes to the data collectors and supervisors for their unreserved contribution.
No funding is secured for this evaluation study.
Author information
Authors and affiliations.
Metema District Health office, Gondar, Ethiopia
Mekides Geta
Department of Health Systems and Policy, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, P.O. Box 196, Gondar, Ethiopia
Geta Asrade Alemayehu, Wubshet Debebe Negash, Tadele Biresaw Belachew, Chalie Tadie Tsehay & Getachew Teshale
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the preparation of the manuscript. M.G. conceived and designed the evaluation and performed the analysis then T.B.B., W.D.N., G.A.A., C.T.T. and G.T. revised the analysis. G.T. prepared the manuscript and all the authors revised and approved the final manuscript.
Corresponding author
Correspondence to Getachew Teshale .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from Institutional Review Board (IRB) of Institute of Public Health, College of Medicine and Health sciences, University of Gondar (Ref No/IPH/1482/2013). Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
All authors declared that they have no competing interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Geta, M., Alemayehu, G.A., Negash, W.D. et al. Evaluation of integrated community case management of the common childhood illness program in Gondar city, northwest Ethiopia: a case study evaluation design. BMC Pediatr 24 , 310 (2024). https://doi.org/10.1186/s12887-024-04785-0
Download citation
Received : 20 February 2024
Accepted : 22 April 2024
Published : 09 May 2024
DOI : https://doi.org/10.1186/s12887-024-04785-0

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Integrated community case management
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Internet Explorer Alert
It appears you are using Internet Explorer as your web browser. Please note, Internet Explorer is no longer up-to-date and can cause problems in how this website functions This site functions best using the latest versions of any of the following browsers: Edge, Firefox, Chrome, Opera, or Safari . You can find the latest versions of these browsers at https://browsehappy.com
- Publications
- HealthyChildren.org
Shopping cart
Order Subtotal
Your cart is empty.
Looks like you haven't added anything to your cart.
- Career Resources
- Philanthropy
- About the AAP
- Confidentiality in the Care of Adolescents: Policy Statement
- Confidentiality in the Care of Adolescents: Technical Report
- AAP Policy Offers Recommendations to Safeguard Teens’ Health Information
- One-on-One Time with the Pediatrician
- American Academy of Pediatrics Releases Guidance on Maintaining Confidentiality in Care of Adolescents
- News Releases
- Policy Collections
- The State of Children in 2020
- Healthy Children
- Secure Families
- Strong Communities
- A Leading Nation for Youth
- Transition Plan: Advancing Child Health in the Biden-Harris Administration
- Health Care Access & Coverage
- Immigrant Child Health
- Gun Violence Prevention
- Tobacco & E-Cigarettes
- Child Nutrition
- Assault Weapons Bans
- Childhood Immunizations
- E-Cigarette and Tobacco Products
- Children’s Health Care Coverage Fact Sheets
- Opioid Fact Sheets
- Advocacy Training Modules
- Subspecialty Advocacy Report
- AAP Washington Office Internship
- Online Courses
- Live and Virtual Activities
- National Conference and Exhibition
- Prep®- Pediatric Review and Education Programs
- Journals and Publications
- NRP LMS Login
- Patient Care
- Practice Management
- AAP Committees
- AAP Councils
- AAP Sections
- Volunteer Network
- Join a Chapter
- Chapter Websites
- Chapter Executive Directors
- District Map
- Create Account

- About the National Coordinating Center for Epilepsy
Caring for Pediatric Patients with Epilepsy Utilizing Cultural Humility, Family-centered Care, and Shared Decision-making in the Medical Home Setting
The National Coordinating Center for Epilepsy has developed the Strengthen & Enhance Epilepsy Knowledge Training as a resource to help build capacity and start conversations amongst medical residents, school nurses, social workers, community health workers, and public health professionals regarding the care of children and youth with epilepsy. This training is intended to be self-guided or facilitated as a Lunch and Learn opportunity to encourage discussion in a short 30-minute presentation. This training consists of a pre-recorded didactic lecture presentation, presenter slides, and a case study for the facilitator.
Facilitated Lunch and Learn - Strengthen & Enhance Epilepsy Knowledge Training
Instructions: The story below presents an example of the complexities and nuances associated with various epileptic syndromes, from the perspective of a pediatric clinician/medical home. It is recommended that this story be presented and discussed during the Lunch and Learn series. Refer to the module slides to help guide the discussion.
Arianna is a 16-year-old Hispanic female who presents for a sports physical for soccer. She has a history of juvenile myoclonic epilepsy that has been well-controlled lately. She takes lamotrigine twice a day and her last seizure was 6 months ago. Her typical seizures are myoclonic jerks in the morning where she will drop her beverage and she has had 3 generalized tonic-clonic seizures in her life. Arianna also had infrequent absence seizures in middle school. In the past she has not needed rescue medications, however, she has a seizure action plan that recommends intranasal midazolam be administered if a seizure of more than 5 minutes occurs. She is asking about attending a sleep away soccer camp in a rural part of her state for a week this summer and is excited about the possibility because many of her high school teammates attended last year.
Arianna’s mother expresses apprehension about her attending camp given her epilepsy. However, Arianna is concerned that if she doesn’t attend, she may not be promoted to the Varsity team this year. She feels that her parents are overprotective of her compared to her siblings and expresses frustration that this is related to her epilepsy. She also expresses excitement about getting her learner’s permit and driving soon.
The clinic medical assistant mentions that when doing medication reconciliation her mother declined a refill for her intranasal midazolam rescue medication because it is “expensive and she has never needed it”.
Case study Questions & Answers
Use the questions below to guide conversation following the story. Possible answers are bulleted below each question. The responses are by no means exhaustive.
1) What might be the concerns regarding Arianna attending sleepaway camp? What would be the benefits? What activity limitations or precautions might be necessary? Specifically, her mother asks about swimming.
Suggested Answers:
a) Arianna’s mother likely has a number of concerns related to Arianna’s safety attending sleepaway camp, such concerns could include medication compliance, supervision by individuals not as familiar with Arianna and her seizure history as her family and friends, capability and comfort of staff responding to a seizure emergency, and possibility of situations that could lower the seizure threshold, such as sleep deprivation.
i) The potential benefits of attending camp are numerous. Attending may allow for opportunities for learning, team building, socialization, and physical activity to promote health. It is important to make sure that children and youth with epilepsy (CYE) are treated like the children and youth that they are. They are first and foremost individuals, who happen to have a chronic medical condition which does not define who they are.
b) It is feasible for CYE to safely participate in summer camp. There are some steps that can help to enhance participation in camp, such as, a discussion with their neurologist to ensure that the camper is on a stable dose of medication. Also, check to see if there are any recommended adjustments in the medication plan. Lastly, be sure to contact the neurologist if there are side effects during a medication change that could interfere with typical daily activities, and ultimately, with participation in camp.
c) Facilitate a conversation between the camper and camp staff regarding how seizures present, potential triggers, how to respond to different seizures, medication management, activity limitations and acceptable activities, to improve the comfort of all involved.
d) Encourage family/camper to review camp policies and discuss instructions well in advance of starting camp regarding expected routine and camp policies about medication administration/storage. Such preparation can help ease potential anxiety of receiving information at the last minute. Ensure that a seizure action plan is completed and submitted to the camp in advance of the start date. Make sure the camper knows the importance of taking medications as instructed and knows what to do if a dose is missed.
e) Patients with epilepsy are generally cautioned against activities where a fall could lead to significant injury, such as climbing. However, with proper safety equipment, precautions, and supervision, such activities could be considered for some campers. Generally, individuals with epilepsy should be seizure-free for over 1 year before considering higher-risk recreational activities like scuba diving, rock climbing, and motorsports.
f) When it comes to water safety, CYE should never swim alone. A seizure occurring in water can lead to drowning. A child with a history of seizures should be supervised by a person who is capable of swimming and can provide emergency assistance if needed. Showers are recommended instead of baths. Normal water safety precautions are advised (ie, personal floating devices (PFDs) for water sports, etc)
2) Does Arianna require seizure rescue medication? Are there any concerns about her having this at camp? (Consider: Is it a controlled substance? Refrigeration? Self-administration?)
a) A rescue medication is indicated for Arianna, who has a history of generalized tonic-clonic seizures. While her seizures have self-resolved within a few minutes in the past, epilepsy is unpredictable, and she is still at risk for having prolonged or frequent seizures.
b) Abortive seizure rescue medications are benzodiazepines, examples include diazepam, midazolam, clonazepam, and lorazepam.
c) Benzodiazepines are a schedule IV drug and a controlled substance. Like at schools, these medications should be kept at the camp medical office. These should not be carried by the CYE.
d) Since these medications are typically used for generalized tonic-clonic seizures over 5 minutes or clusters of seizures, the CYE would likely be unable to self-administer the medication.
e) It is important to remember that seizures can present in many different ways and seizure action plans can vary significantly among individuals. It is important to encourage CYE and caregivers to work with their neurologists to produce clearly documented seizure action plans that can be readily available by camp staff and school staff alike. The Epilepsy Foundation has a site with resources specifically for camp preparedness, including fillable seizure action plans (SAP), medical supplement form, seizure diary, and camp checklist: Resources and Seizure Action Plans for Summer Camp | Epilepsy Foundation.
3) Demonstrate how you might engage in shared decision making with Arianna and her mom, helping to address her mom’s concerns, while helping mom see how Arianna could safely be more independent.
a) As a teenager, Arianna is likely seeking more independence and her mother’s concerns over her safety could possibly lead to frustration in Arianna and tension in their relationship. Offering some ideas for Arianna to take steps towards achieving more independence and autonomy in her medical care may be helpful. Perhaps Arianna can begin to use an app on her phone to help her to track when she has taken her medication and provide reminders if she forgets. She can demonstrate maturity and responsibility that may lead to her family gaining more peace of mind and offering more independence. She may be able to use apps to track her seizures as well, maintaining some independence while providing her caregivers insight into her seizure frequency.
b) Primarily engage with Arianna and encourage her to answer questions/develop plans first with caregiver’s input as she becomes more autonomous.
i) The American Academy of Pediatrics outlines a 4-Step Framework for Shared Decision-making in Pediatrics .
4) Are there concerns regarding Arianna driving? What are some safety factors to discuss?
Suggested Answer:
a) If a driver has a seizure, their life, and the lives of others on the road can be put in jeopardy. The laws of each state differ in restrictions and limitations placed on drivers with epilepsy. It is important for patients and physicians to know the relevant laws in their state. In most circumstances, the Department of Motor Vehicles, not the physician, makes the decisions on driving. People with epilepsy are legally obligated to report seizures to the DMV, and to disclose any history of epilepsy when applying for or renewing a driver’s license.
5) Discuss the risks and benefits of having rescue medication. What resources might be available to help Arianna’s family with the cost associated with the medication?
a) The longer a seizure lasts, the more difficult it can become to stop. The intended benefit is to prevent longer or more frequent seizures from progressing to status epilepticus.
b) Potential risks include the side effect profile of benzodiazepines (sedation, respiratory depression, etc.), cost and ability to obtain, some routes of administration, and the potential for heightened stress and anxiety among CYE and/or caregivers about having the medication ready and when to administer.
c) There are several forms of benzodiazepines that can be utilized for seizure abortive medications. Rectal diazepam has been a common formulation; however, the route of administration can be burdensome and uncomfortable. Some medications, such as oral dissolvable clonazepam, can be administered inside the cheek, however, it is important to be cautious not to place a finger beyond the teeth, as an unexpected bite could occur. Furthermore, some patients may drool or have foaming of saliva during seizures, and the absorption of the medication could be compromised and potential for choking could be a concern. More recently, there are FDA approved nasal formulations of diazepam (6 years +) and midazolam (12 years +) for seizure clusters. Given that these formulations are newer, there is no available generic and cost may present a concern. However, both formulations have patients' savings programs.
d) Patients may also be eligible for state insurance programs based upon medical conditions that may also help cover additional medical costs.
If you have any questions regarding epilepsy or this facilitated mini training, please contact: The National Coordinating Center for Epilepsy
This project is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number U23MC26252, Awareness and Access to Care for Children and Youth with Epilepsy cooperative agreement. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS, or the U.S. Government.
Last Updated
American Academy of Pediatrics
Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis
- Original Research
- Open access
- Published: 11 May 2024
Cite this article
You have full access to this open access article

- An Ta ORCID: orcid.org/0000-0003-0929-6365 1 ,
- Felicitas Kühne ORCID: orcid.org/0000-0001-9762-3842 2 ,
- Maren Laurenz 2 ,
- Christof von Eiff 2 ,
- Sophie Warren 3 &
- Johnna Perdrizet ORCID: orcid.org/0000-0002-4260-7607 4
61 Accesses
Explore all metrics
Introduction
Since 2009, a pneumococcal conjugate vaccine (PCV) covering 13 serotypes (PCV13) has been included by Germany’s Standing Committee on Vaccinations for infants, resulting in major reductions in pneumococcal disease (PD). Higher-valent vaccines may further reduce PD burden. This cost-effectiveness analysis compared 20-valent PCV (PCV20) under a 3+1 schedule with 15-valent PCV (PCV15) and PCV13, both under 2+1 schedule, in Germany’s pediatric population.
A Markov model with annual cycles over a 10-year time horizon was adapted to simulate the clinical and economic impact of pediatric vaccination with PCV20 versus lower-valent PCVs in Germany. The model used PCV13 clinical effectiveness and impact studies as well as PCV7 efficacy studies for vaccine direct and indirect effect estimates. Epidemiologic, utility, and medical cost inputs were obtained from published sources. Benefits and costs were discounted at 3% from a German societal perspective. Outcomes included PD cases, deaths, costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs).
In the base case, PCV20 provided greater health benefits than PCV13, averting more cases of invasive pneumococcal disease (IPD; 15,301), hospitalized and non-hospitalized pneumonia (460,197 and 472,365, respectively), otitis media (531,634), and 59,265 deaths over 10 years. This resulted in 904,854 additional QALYs and a total cost saving of €2,393,263,611, making PCV20 a dominant strategy compared with PCV13. Compared to PCV15, PCV20 was estimated to avert an additional 11,334 IPD, 704,948 pneumonia, and 441,643 otitis media cases, as well as 41,596 deaths. PCV20 was associated with a higher QALY gain and lower cost (i.e., dominance) compared with PCV15. The robustness of the results was confirmed through scenario analyses as well as deterministic and probabilistic sensitivity analyses.
PCV20 3+1 dominated both PCV13 2+1 and PCV15 2+1 over 10 years. Replacing lower-valent PCVs with PCV20 would result in greater clinical and economic benefits, given PCV20’s broader serotype coverage.
Plain Language Summary
Pneumococcal diseases (e.g., ear infections, pneumonia, bloodstream infections) are among the leading causes of illness and death in children worldwide. The pneumococcal conjugate vaccine protects against pneumococcal diseases and has significantly reduced the number of newly diagnosed cases. Higher-valent vaccines (which provide coverage for a greater number of disease-causing serotypes) have recently received European Commission approval for use in adults and children. This study examined costs and health benefits associated with the 20-valent pneumococcal conjugate vaccine (PCV20) under a 3+1 (i.e., three primary doses and one booster dose) schedule in Germany’s childhood vaccination program compared with 13-valent pneumococcal conjugate vaccine (PCV13) and the 15-valent pneumococcal conjugate vaccine (PCV15), both under a 2+1 (two primary doses, one booster) schedule. PCV20 was estimated to result in greater health benefits from avoiding more cases in pneumococcal diseases and lower costs compared with both PCV13 and PCV15. PCV20, therefore, is considered the best option among the three vaccines for children in Germany.
Avoid common mistakes on your manuscript.
Streptococcus pneumoniae is the leading cause of bacterial pneumonia and global mortality in children [ 2 , 3 , 4 , 5 ]. In 2016, S. pneumoniae has been estimated to account for approximately 197 million cases of pneumonia and 1.1 million deaths [ 6 ]. This encapsulated bacterium is the major cause of pneumococcal diseases ranging from otitis media (OM) and pneumonia to life-threatening invasive pneumococcal diseases (IPDs), including sepsis and meningitis.
Pneumococcal conjugate vaccines (PCV) elicit robust and durable immune responses in both pediatric and adult populations [ 4 ]. They have noticeably reduced IPD incidence across all age groups due to indirect effects (i.e., herd effects or the effect on the unvaccinated population) [ 7 , 8 ]. The 7-valent PCV (PCV7) was first approved in Europe in 2001 [ 9 ], and was recommended for high-risk children in July 2001 by Germany’s Standing Committee on Vaccinations [Ständige Impfkommission (STIKO)] with a schedule of three priming doses in infancy plus one booster (3+1) [ 10 ]. The recommendation was extended to the entire infant population (< 2 years of age) in July of 2006 [ 11 ].
The 13-valent PCV (PCV13) and 10-valent PCV (PCV10) replaced PCV7 and were introduced in 2009, and administered based on physician’s choice [ 12 ]. In 2015, STIKO changed its recommendations for full-term infants from a 3+1 schedule with the priming series administered at 2, 3, and 4 months and a booster at 11–14 months, to a 2+1 vaccination schedule with a priming series administered at 2 months and 4 months plus a booster at 11 months [ 3 , 13 ]. The 3+1 schedule remains in place for preterm infants [ 14 ].
Next-generation PCVs with increased serotype coverage [15-valent PCV (PCV15) and 20-valent PCV (PCV20)] were approved for adults aged 18 years and older by the European Commission in October 2021 and February 2022, respectively [ 15 , 16 ]. Since September 2023, PCV20 is recommended in Germany for all individuals aged 60 years and older and for all individuals aged 18‒59 years with underlying diseases. A cost-effectiveness analysis (CEA) in the German adult population concluded that a single dose of PCV20 for adults aged ≥ 60 years and adults aged 18‒59 years with moderate- and high-risk conditions would prevent pneumococcal disease cases, save lives, and would be cost-saving compared to the pneumococcal polysaccharide vaccine (PPSV23) alone, PCV13 followed by PPSV23, or PCV15 followed by PPSV23 [ 17 ].
Prior to the licensure of PCV15 in October of 2022, PCV13 was considered as the standard of care. With the inclusion of PCV15 in the STIKO recommendation for infants in Germany, the current clinical practice includes a market basket of PCV13 and PCV15. PCV20 covers all PCV15 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) and five additional serotypes (8, 10A, 11A, 12F, and 15B). On January 25, 2024, the Committee for Medicinal Products for Human Use adopted a positive opinion for the higher-valent option of PCV20 for the use in children [ 18 ] PCV20 was approved by the European Commission in a 3+1 schedule on March 12, 2024 [ 16 ]. The purpose of this CEA was to examine the health benefits and costs of implementing a PCV20 vaccination program under a 3+1 schedule in Germany’s pediatric population compared with PCV13 and PCV15, both administered in a 2+1 schedule.
Conceptual Framework and Model Structure
The CEA was structured in Microsoft Excel ® (Redmond, WA, US) using a decision-analytic Markov (state-transition) cohort model. The Markov model estimated pneumococcal disease-related events in both unvaccinated and vaccinated individuals (Fig. 1 ). The model captured an individual’s possible transition to several clinical events, including IPD (developing into either meningitis or sepsis/bacteremia), all-cause pneumonia (non-hospitalized or hospitalized), all-cause OM, no pneumococcal disease state, and death. Death captured both general mortality and case fatality, which could occur in any disease state and non-disease state. The transition occurred on an annual cycle and was age- and vaccination-specific. The non-mutually exclusive nature of pneumococcal disease was reflected through each 12-month interval during which persons could transition to one or more disease states or remain in a non-disease state. In the case of more than one pneumococcal disease, costs and quality-adjusted life year (QALY) decrements associated with all events were considered. At the beginning of each annual cycle, a new cohort of children (i.e., incoming birth cohort) entered the model and was eligible for vaccination.
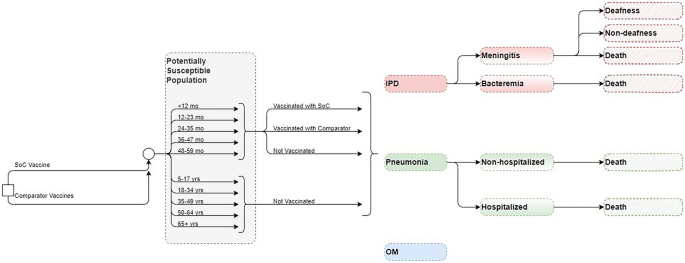
Model structure. IPD invasive pneumococcal disease, mo months, OM otitis media, SoC standard of care, yrs years
The full health benefit of vaccination was applied to the entire German population, of which the vaccinated cohort experienced the direct effects of vaccination immediately, while the rest of the unvaccinated population gradually received indirect effects over the model time horizon.
Target Population and Subgroups
The target population was composed of infants aged < 2 years (i.e., ,a vaccination cohort), while the model assumed that the groups aged 2–4 years, 5–17 years, and 18–49 years were not vaccinated with the higher-valent PCVs. In clinical practice, a proportion of the group ≥ 60 years is vaccinated under the adult immunization program [ 19 , 20 ]; hence, within this pediatric model, that proportion of adults was excluded from receiving indirect effects, while the remainder of the population ≥ 60 years remained unvaccinated and benefitted from indirect effects of pediatric vaccination.
Intervention and Comparator Strategies
STIKO currently recommends PCV10, PCV13, and PCV15 for infants and children in Germany [ 21 ]. However, PCV13 was shown to avoid more cases than PCV10 [ 22 ] and accounts for the majority of vaccination rate, at more than 90%, in children, remaining the most used PCV in the past decade in Germany [ 13 , 23 ]. Therefore, PCV10, although included in the STIKO recommendation, was not considered among comparators in this analysis. The analysis evaluated the clinical and economic outcomes of PCV20 in a 3+1 schedule as a potential vaccination strategy compared with PCV13 (i.e., standard of care) and PCV15, both in a 2+1 schedule.
Perspective, Time Horizon, Cycle Length, and Discount Rate
The base-case analysis was conducted from a German societal perspective using a 3% annual discount rate for both costs and benefits, according to the recommendations of the Institute for Quality and Efficiency in Health Care and STIKO [ 24 , 25 ]. The model used an annual cycle length over a 10-year time horizon to capture relevant costs and outcomes. The 10-year time horizon sufficiently captures the health benefits of the PCV vaccination program, based on the observation of the accrual and stabilization of indirect effects over a 5- to 10-year period following the introduction of PCV7 and PCV13 [ 26 , 27 ]. The life years and QALY loss is accumulated over the time loss between death occurrence and life expectancy (with the QALYs being age-dependent and discounted from the year the death occurs). Lifetime long-term costs related to a clinical event, such as sequalae following meningitis, were incorporated in the model as a one-time discounted cost in the cycle in which the event happens.
This study was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; as such, ethical approval was not required.
Population and Epidemiology Data
Population data were obtained from the German Federal Statistical Office to determine the size of the stratified population age groups [ 28 ] (Table S1 ; Supplementary Material) and the incoming birth cohort each year [ 29 ] (Table S2; Supplementary Material).
Age-specific disease incidence rates per 100,000 individuals were informed by German-specific published literature [ 30 , 31 , 32 , 33 ], adjusted for relevant age groups using population size from the German Federal Statistical Office [ 28 ] (Table 1 ).
Mortality in the analysis was considered as a combination of general mortality [ 34 ] and case fatality, which were applied to meningitis, sepsis/bacteremia, and all-cause hospitalized and non-hospitalized pneumonia (Table 1 ), while no mortality was assumed for OM [ 22 , 31 ].
The model considered sequela following meningitis (i.e., deafness and non-deafness) in the base case as sequela following meningitis are quite common among patients with IPD [ 22 ]. The data for the proportion of patients developing complications with IPD were sourced from published literature [ 22 ] (Table S3; Supplementary Material).
IPD serotype-specific distribution by each PCV stratified by age groups was obtained from [ 35 , 36 ] (Table 2 ). The serotype coverage for PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) was incorporated as one input, while the coverage for each additional serotype included in higher valent vaccines was input separately for each age group. The analysis did not consider cross-reactive serotypes. Non-invasive serotype distributions (i.e., for pneumonia and OM) were assumed to be the same as IPD serotype distribution.
Vaccine Effectiveness and Efficacy
The direct effect of PCVs against IPD for a complete vaccine schedule was assumed to be equivalent to the adjusted PCV13 effectiveness against PCV13-type IPD, of which 78.2% [95% confidence interval (CI) 56.0, 89.0] was applied for vaccines in a 2+1 schedule while 89.7% (95% CI 82.0, 94.0) was used for PCV20 in 3+1 schedule (Table 3 ) [ 37 ]. To estimate the direct effects of the higher-valent PCVs against all-cause pneumonia (non-hospitalized and hospitalized) and OM, the model adopted an approach commonly used in CEAs [ 38 , 39 , 40 , 41 , 42 ], in which the effectiveness of higher-valent PCVs against non-invasive disease was assumed to be the same as the reported trial-based efficacy data of PCV7, which was then adjusted based on study design, period, and country-specific factors. These results demonstrated an efficacy of 25.5% (95% CI 4.4, 34.0) [ 43 ], 6.0% (95% CI − 1.5, 11.0) [ 44 ], and 7.8% (95% CI 5.2, 10.5) [ 45 ] against radiographically confirmed non-invasive hospitalized pneumonia, non-hospitalized pneumonia, and OM, respectively (Table 3 ).
In addition, a < 12-month effect modifier was used to account for potential reduced effectiveness in the first year of life during which children have only received the priming series of the full vaccination schedule [i.e., at two-thirds (~ 67%) of the full effect for vaccination with a 2+1 schedule for PCV13 and PCV15, and at 75.6% for PCV20 3+1 based on the Advisory Committee on Immunization Practices’ assumption [ 46 ]]. Vaccine coverage was set at 89.9% for the priming series and at 76.8% for the booster dose [ 47 ].
Evidence has shown that direct effects of PCVs remain stable for a few years after the final dose. For example, the efficacy of PCV13 was steady for up to 4 years in infants after vaccination was completed [ 37 ] and for more than 5 years in people aged ≥ 65 years after a single dose [ 48 ]. Therefore, in the base case, a full direct effect for the first 5 years after the booster dose was assumed for all vaccines, followed by an annual waning of 10% from year 6 through year 10.
The analysis considered indirect effects in unvaccinated individuals since they are an important benefit from pediatric PCV national immunization programs. The indirect effect against serotypes covered represents the maximum protection the unvaccinated population could receive from a vaccine regimen. This was modeled as a percent reduction in the expected age-specific disease incidence. Indirect effect was not realized immediately and was only applied to newly covered serotypes in PCV15 and PCV20, as the indirect effect for PCV13 serotypes was assumed to have already reached a steady state. These benefits accrued gradually until a new steady state was reached for additional serotypes. Indirect effect for PCV15 and PCV20 was assumed to have no added effects on PCV13 steady-state serotypes. The model assumed that incidence trends for all newly covered serotypes would decrease consistently across ages. For IPD and non-hospitalized and hospitalized pneumonia, indirect effect was assumed for all age groups, while for OM, indirect effect was assumed only for the < 5 years age group. To estimate indirect effects, the model incorporated the reduction in incidence of the newly covered serotypes and the accrual of the indirect effects of higher-valent PCVs (see Supplementary Material, Appendix A).
Resource Use and Costs
Vaccine costs for PCV13, PCV20, and PCV15 were derived from retail pharmacy price per dose [ 49 ], and the administration cost were from [ 22 ], inflated to 2022 Euros (€). Additional vaccine cost and administration cost, which accounted for an additional visit, were applied to the extra dose under 3+1 schedule for PCV20. Medical costs per episode related to each disease state sourced from [ 22 ] were included for all relevant age groups in the model [ 22 ]. Lifetime medical costs per episode of sequela were assumed to be the same across all age groups for deafness and non-deafness [ 22 ]. Societal costs considered productivity loss per episode of meningitis, sepsis/bacteremia, inpatient and outpatient pneumonia and OM, as well copayment for adult patients. All costs were in Euros (€) and obtained from German published sources and the literature, then inflated to 2022 prices using the healthcare component of the consumer price index [ 50 ] where relevant. The summary of cost inputs is listed in Table 3 .
The model used baseline utility for the general population [ 51 ] minus disutilities related to disease states and acute events to assess quality of life related to each vaccination strategy (Table 3 ) [ 42 , 52 , 53 , 54 , 55 , 56 , 57 ].
Assessment of Uncertainty
Uncertainty around the analyses was evaluated using deterministic sensitivity analyses (DSA), probabilistic sensitivity analyses (PSA), and scenario analyses. DSA assessed uncertainty around the following variables: disease incidence, breakdown of IPD cases, case fatality rate (CFR), serotype distribution by age, vaccine effectiveness and utilities.
In the PSA, all parameters subject to any degree of uncertainty were assessed The incremental results for costs, QALYs, and incremental cost-effectiveness ratios (ICER) were recorded for each simulation of a total of 1000 simulations to examine the stability of the model findings.
Several scenarios were conducted, the description and results of which are reported in Table 6 . In addition to the scenario assessments, threshold analyses were conducted to assess the price per dose of PCV20 under a 3+1 schedule. These analyses aim to determine the price range at which PCV20 stays a cost-saving strategy compared to PCV13 2+1 and PCV15 2+1, assuming consistent input parameters for other variables (including the prices of PCV13 and PCV15).
Base-case Results
Over the 10-year time horizon, compared to both PCV13 2+1 and PCV15 2+1, PCV20 3+1 provided substantially greater health benefits and broader protection (Table 4 ). Compared to PCV13, PCV20 is estimated to result in greater health benefits due to a greater number of cases averted and total QALYs gained (Table 4 ). Compared with PCV13, PCV20 averted an additional 15,301 cases of IPD; 460,197 and 472,365 cases of hospitalized and non-hospitalized pneumonia, respectively; 531,634 cases of OM; and 59,265 deaths due to disease across all ages. Consequently, PCV20 was estimated with a higher QALY gain of 904,854. In comparison with PCV15, the number of additional cases averted by PCV20 were 11,334, 335,937, 369,012, and 441,643 in IPD, hospitalized pneumonia, non-hospitalized pneumonia, and OM, respectively. Additionally, an estimation of a reduction of 41,596 deaths due to disease was estimated for PCV20 versus PCV15, and PCV20 was associated with an incremental QALY of 646,235.
PCV20 was associated with higher direct vaccine costs, at €525,362,283 and €522,747,819 more than that of PCV13 and PCV15, respectively. This is the result of the higher price per dose considered for PCV20 and the additional dose included in PCV20 under the 3+1 schedule. However, it resulted in significant cost savings from lower direct costs of disease and lifetime costs of sequela compared to both comparators (Table 4 ) due to broader serotype coverage compared to PCV13 and PCV15. PCV20 was the dominant strategy in both comparisons, with a total cost saving of €2,393,263,611 versus PCV13 and of €1,628,000,506 versus PCV15.
The breakdown results by age groups were mostly consistent with the overall results where more cases were avoided related to PCV20 in all included age groups for IPD, hospitalized pneumonia, and OM than to PCV13, especially in the oldest group of 65+ year olds. Similarly, PCV20 is estimated to prevent more cases of non-hospitalized pneumonia than PCV13 in children less than 5 years old. The higher valent vaccines showed an increasing number of prevented non-invasive pneumonia. One could observe a shift from severe cases (i.e., hospitalized cases) in the lower valent vaccine strategies to less severe cases (i.e., non-hospitalized pneumonia) in the higher valent vaccine strategies. Furthermore, better health outcomes from PCV20 were shown by a reduction in deaths due to disease in all age groups, with the greatest reduction observed in those 65 years old and above (50,064 deaths averted) (Table S4; Supplementary Material). Similar results broken down by age groups were also observed when comparing PCV20 with PCV15 (Figs. 1 , 2 , 3 , 4 , 5 ; Table S4; Supplementary Material).
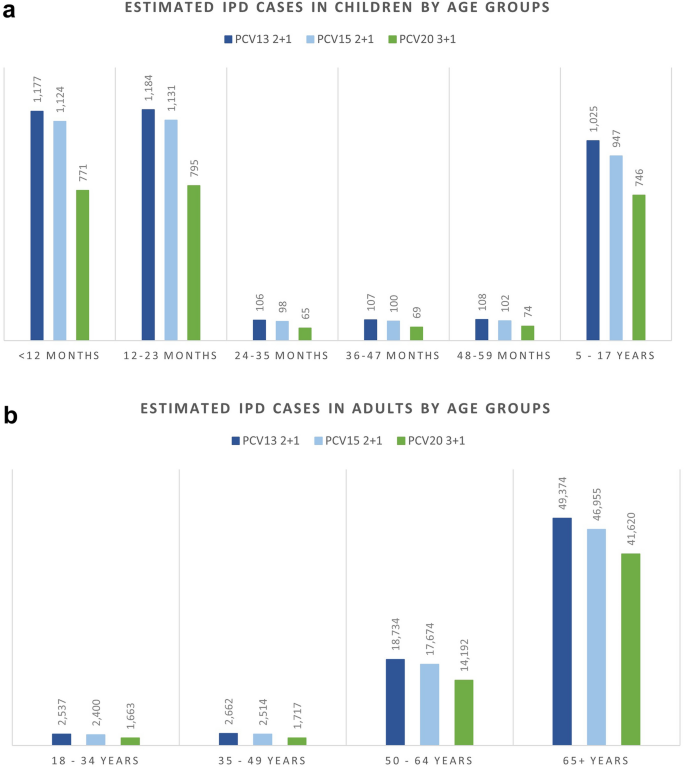
Estimated number of IPD cases stratified by age groups. IPD invasive pneumococcal disease, PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
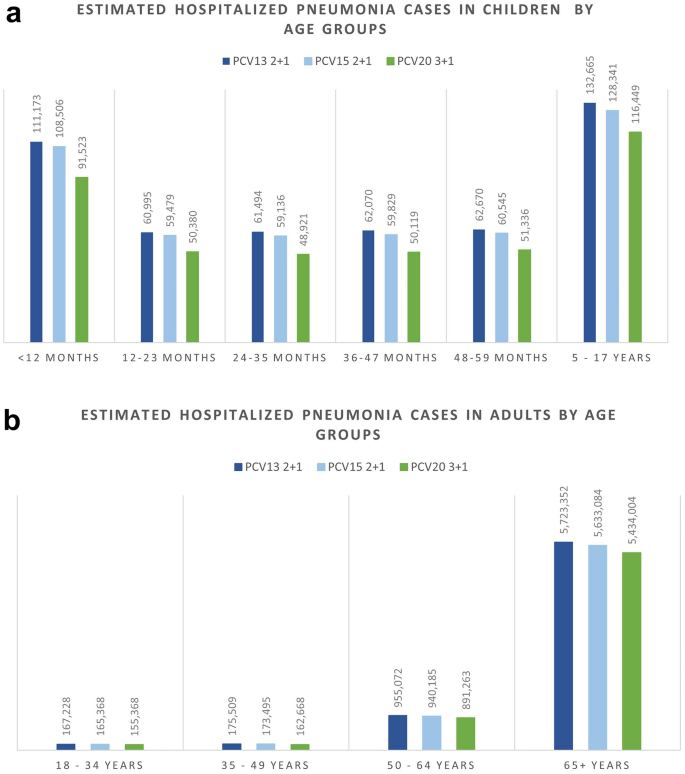
Estimated number of hospitalized pneumonia cases stratified by age groups. PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
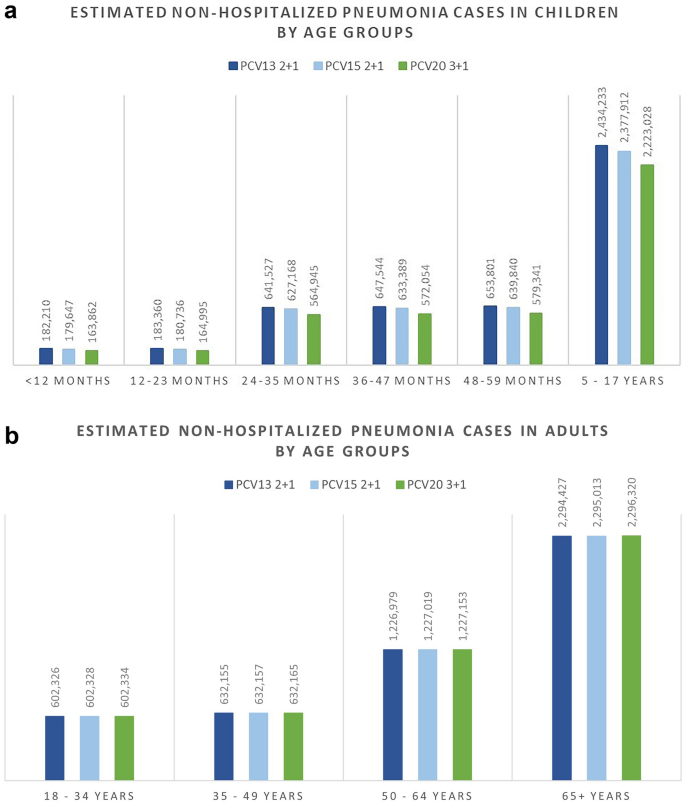
Estimated number of non-hospitalized pneumonia cases stratified by age groups. PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
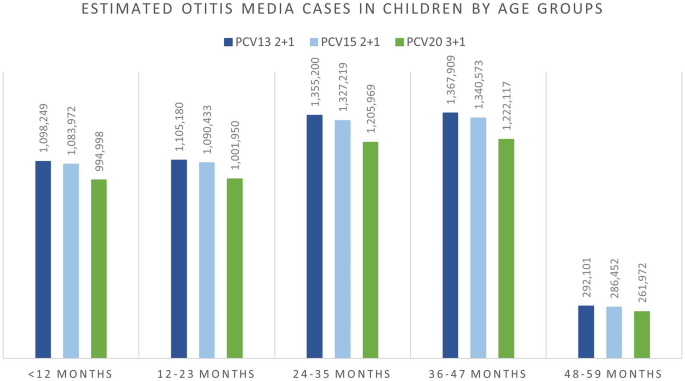
Estimated number of otitis media cases stratified by age groups. PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
Sensitivity Analyses
The results from DSA, where one parameter was varied in one direction while all other inputs were held constant, are reported in Fig. 6 for costs and Fig. 7 for QALYs. When compared to PCV13, the key drivers for costs included maximum indirect effect against hospitalized pneumonia (PCV20), serotype distribution by age, incidence of hospitalized pneumonia and medical costs of per episode of hospitalized pneumonia.
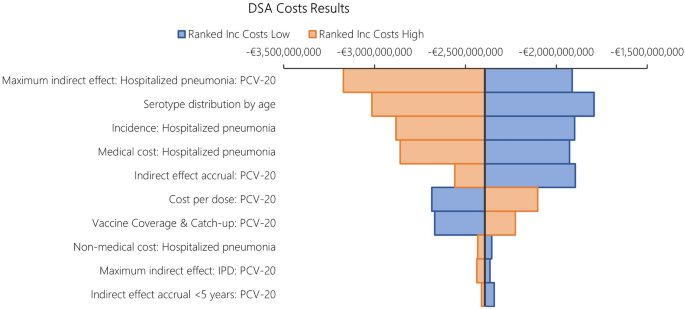
DSA results in costs: PCV20 versus PCV13. DSA deterministic sensitivity analysis, PCV13 13-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
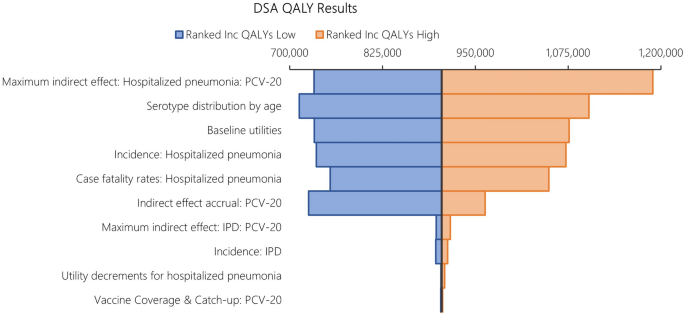
DSA results in QALYs: PCV20 versus PCV13. DSA, deterministic sensitivity analysis; PCV13 13-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, QALY quality-adjusted life years
The DSA of PCV20 versus PCV13 also illustrated that the top five most impactful parameters on QALYs were maximum indirect effects on hospitalized pneumonia (PCV20) and serotype distribution by age, baseline utilities, followed by hospitalized pneumonia incidence and CFR for hospitalized pneumonia. When comparing PCV20 and PCV15, the results were largely similar (Figs. 8 and 9 ).
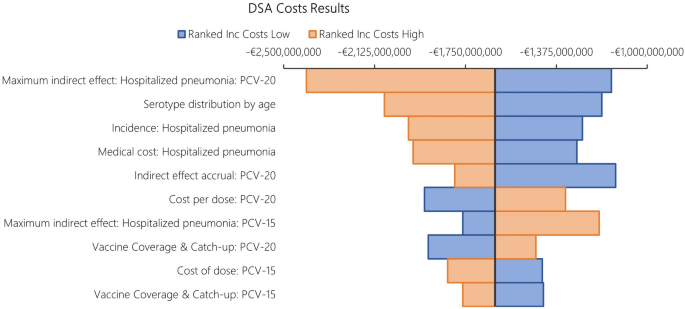
DSA results in costs: PCV20 versus PCV15. DSA deterministic sensitivity analysis; PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine
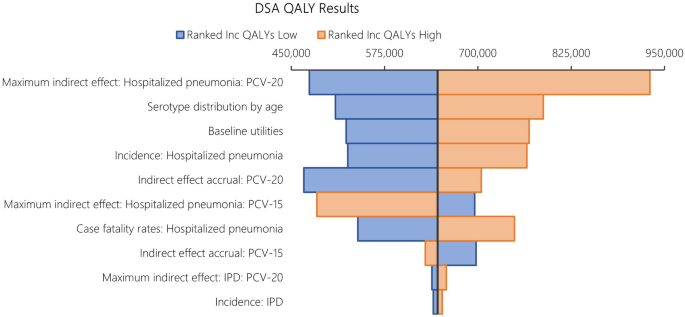
DSA results in QALYs: PCV20 versus PCV15. DSA deterministic sensitivity analysis, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, QALY quality-adjusted life years
Probabilistic results from the PSA based on 1,000 iterations aligned with the base case results, confirming robust findings with PCV20 being dominant in all simulations. Compared with PCV13, PCV20 was the dominant strategy in all iterations, while PCV20 dominated PCV15 in 98.40% of the total 1000 iterations (Table 5 ). The cost-effectiveness plane plots are reported in Figs. 10 and 11 .
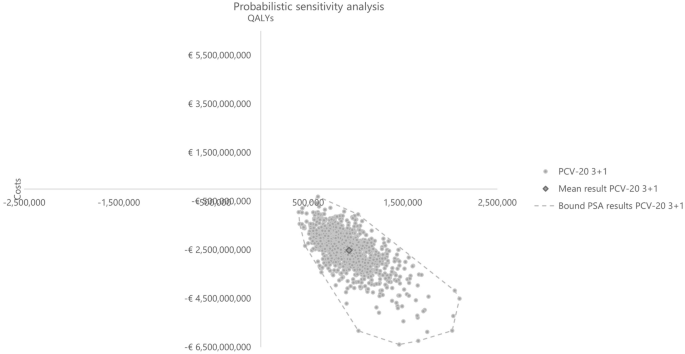
PSA cost-effectiveness plane versus baseline of PCV13. PCV13 13-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, PSA probabilistic sensitivity analysis
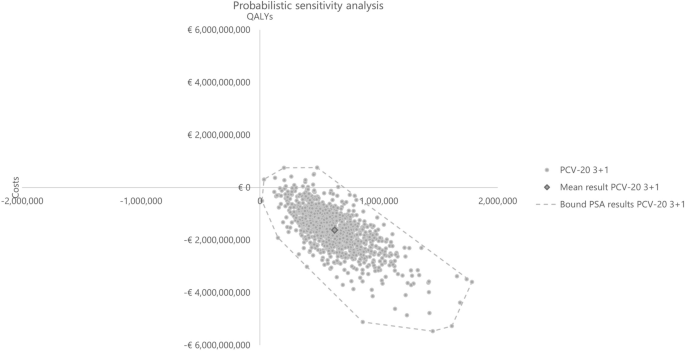
PSA cost-effectiveness plane versus baseline PCV15. PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, PSA probabilistic sensitivity analysis
Scenario Analyses
Scenario analysis results are summarized in Table 6 . In the three scenarios where different discount rates were applied for costs and benefits, the qualitative conclusion of PCV20 being the dominant strategy compared to both comparators remained robust. When reducing indirect effects (i.e., maximum reduction in disease incidence) by half, PCV20 was still estimated to have better health benefits and lower costs compared to both PCV13 and PCV15. Similarly, extending time to realize indirect effects (i.e., accrual data in the first 2 years at 0%) and increased vaccination uptake in adults 65+ years old aligned with the base case results of PCV20 being the dominant strategy versus both comparators. Using a payer perspective led to a decrease by 15% and 17% in ICER in the comparison between PCV20 versus PCV13 and PCV20 versus PCV15, respectively. However, the qualitative conclusion remained the same. Other scenarios testing a different waning assumption (i.e., reducing duration of full protection to 3 years) and serotype replacement were in line with the base case with minimal change in ICER. Overall, the results and conclusion were relatively robust. When assuming a high vaccine uptake in infant (90%), ICERs increased slightly, at less than 1%, for both PCV20 versus PCV13 and PCV20 versus PCV15. Considering disutility related to adverse events related to the administration of all vaccines (e.g., local reaction and systematic reaction or fever) resulted in the same conclusion of PCV20 3+1 being the dominant strategy among the three PCVs. In the threshold analyses, threshold prices per dose for PCV20 3+1 were €170 versus PCV13 2+1 and €135 versus PCV15 2+1. Given that all price ranges exceed double the current list price per dose of PCV20 for cost-saving analyses, it is reasonable to conclude that changes in the price per dose are unlikely to impact the study's conclusions.
This article is based on previously conducted studies or collected published data and does not contain any new studies with human participants or animals performed by any of the authors.
The introduction of next-generation PCVs with increased serotype coverage in Europe has provided options to be considered in national childhood immunization programs. This study examined the cost and health outcomes related to PCV20 under a 3+1 schedule compared with PCV13 and PCV15, both under a 2+1 schedule, in the pediatric population in Germany.
The base-case results demonstrated PCV20 as the dominant strategy over both lower-valent alternative vaccines. PCV20 was estimated to have greater health benefits than both PCV13 and PCV15 by averting more cases of pneumococcal diseases, including IPD, pneumonia, and OM, over a 10-year time horizon. This resulted in higher QALY gained and lower total costs related to PCV20, implying dominance of PCV20 compared with PCV13 and PCV15. Several scenarios assessed additional uncertainty, such as effects of different discount rates for costs and outcomes, several assumptions on vaccine effects (i.e., reduction in indirect effects and extension in accrual time of indirect effects), and waning duration. In addition, serotype replacement assumptions were examined to test how sensitive the results were to reduction in vaccine-type serotype coverage over time. Finally, payer perspective and an assumption of higher vaccine uptake in infant were explored. The results were robust across all sensitivity analyses including PSA and DSA.
Our findings are consistent with published studies comparing PCV20 to PCV13 and PCV15 in other settings. In Canada [ 58 ] and Greece [ 59 ], PCV20 was estimated to be cost-saving compared with PCV15 in a 2+1 schedule. In the United Kingdom, PCV20 2+1 was estimated to be cost-saving compared to PCV13 1+1 and cost-effective compared with PCV20 1+1 [ 60 ]. Moreover, a public health impact analysis in the Netherlands estimated that PCV20 could avert 45,127 pneumococcal cases compared to PCV10 over 5 years [ 61 ]. Our findings are also consistent with a historic economic evaluation in Germany comparing higher-valent versus lower-valent PCVs, in which PCV13 dominated both PCV10 and PCV7 [ 56 ]. The PCV13 infant immunization program in Germany was expected to have a substantial public health impact because of its broader serotype coverage compared with both PCV7 and PCV10, similar to our findings for PCV20 compared with both PCV13 and PCV15. Differences in disease incidence have been noted; for example, Strutton et al. 2012 [ 56 ] used an IPD incidence among those aged < 2 years of 43.35 per 100,000 individuals, whereas our analysis uses 15.8 per 100,000. The lower incidence values in our economic evaluation reflect the historic impact of PCV13 on disease incidence; however, substantial disease associated with PCV20-unique serotypes remains in Germany.
Our analyses have several limitations. Firstly, a Markov cohort model was considered appropriate for the decision problem based on previous cost-effectiveness assessments of PCVs [ 62 ]. Static models are commonly used for economic evaluations of PCVs in Germany [ 56 , 63 ] and globally [ 42 , 64 , 65 , 66 , 67 , 68 ]. While dynamic models are known to capture indirect effects, the decision-analytic Markov cohort model in this study utilizes the simplistic static Markov framework and incorporates components such as indirect effects. This notable improvement in the modeling approach helps quantify the far-reaching effects of vaccination at the population level while maintaining the clarity and transparency of the model.
The methodological framework adopted in this study was based on the examination of overall vaccine-type serotypes rather than individual serotypes, owing to the limited data suitable for modeling clinical outcomes at the individual serotype level, in particular non-invasive disease. While the analysis encompassed diverse distributions in serotype coverage pertaining to additional serotypes facilitated by higher-valent PCVs in contrast to PCV7, the capacity to scrutinize outcomes specific to distinct serotypes within this paradigm remained constrained. Future modeling endeavors could attempt to explore the nuanced dynamics inherent to pneumococcal serotypes, especially for IPD for which data are more readily available.
German data were prioritized to parameterize the model. When German data were not available, data were sourced from other high-income European countries with similar health care systems. Direct effects were estimated from different sources using PCV13 and PCV7 studies, given no studies have measured the effectiveness of PCV15 or PCV20 against pneumococcal disease outcomes. Differential herd effects were not modeled based on a PCV schedule but have been observed in countries that have implemented PCV13 in infant national immunization programs (i.e., increase in disease reduction under a 3+1 vs. 2+1) [ 26 , 69 ]. There are potential confounding factors, such as the rate of reduction and time to stabilization of IPD incidence across age groups and countries may be associated with multiple factors, including vaccine uptake, implementation of a catch-up program, duration of PCV use, availability of an adult pneumococcal vaccination program, serotypes in circulation, and general epidemiologic variability. To assess the uncertainty around the indirect effect estimations for IPD and non-invasive disease, extensive sensitivity analyses were conducted, such as PSA, DSA, and several scenarios.
The base-case analysis did not include serotype replacement. It is difficult to predict how the characteristics or composition of non-vaccine serotypes will change following higher-valent PCV introduction. Model simulations suggest that replacement may be less for high-valency PCVs [ 70 , 71 ]. To address uncertainty, we tested the impact of increasing non-vaccine serotypes over time, which all led to similar directional results as the base-case (i.e., PCV20 remained dominant).
We did not consider adverse events originating from vaccination by PCV20, PCV15, or PCV13 in our base-case analysis. Although adverse events after pneumococcal vaccination resulting in healthcare seeking are rare and event rates are similar for the different pneumococcal vaccines, PCV20, licensed in a 3+1 schedule, is likely to result in more adverse events than PCV15 and PCV13, both under a 2+1 schedule. We tested this scenario and found that, although PCV20 3+1 was estimated with slightly higher disutility related to the extra dose, the strategy still provides higher total QALY gain compared to lower-valent alternatives and remained dominant.
Despite a numerically higher immunological response reported against serotype 3 for PCV15, we did not model higher vaccine effectiveness against this serotype for PCV15, as data on clinical effectiveness of PCV15 against serotype 3 are unknown. In contrast, a meta-analysis of observational studies supports direct PCV13 protection against serotype 3 IPD in children. Without any real-world effectiveness data for PCV15, there is no way to assess the actual impact of PCV15 on serotype 3. For that reason, PCV15 and PCV20 are assumed to provide comparable protection to PCV13 against serotype 3 disease.
Recently, STIKO recommended PCV20 for adults aged 60 years and older and for adult patients with underlying diseases [ 19 ]. Vaccination rates among adults may increase in the future as PCV20 is only administered once in adults. However, given the recent recommendation and implementation (since January 2024), we were not able to draw conclusions regarding the full impact of adult PCV20 vaccination on epidemiology at this time. To account for the direct impact of adult vaccination, we assumed a proportion of adults received a PCV and therefore received no additional benefit of indirect effects from the pediatric program. We also tested the impact of less pronounced herd effects associated with higher-valent PCVs. Changes to the assumptions resulted in fewer cases avoided and smaller life expectancy gains under a PCV20 pediatric program. However, PCV20 under a 3+1 program remained the dominant strategy avoiding more cases, increasing life expectancy while costing less than PCV13 or PCV15 under a 2+1 program.
Indirect cost estimates were not based on a rigorous German database cost assessment. We applied estimates based on published assumptions. To avoid assumptions on the indirect costs, we carried out a scenario from the payer perspective, not accounting for any indirect costs. This is a very conservative approach given that parents stay at home or have another caregiver for their child. Even under conservative assumptions, PCV20 vaccination strategy was clearly dominating PCV15 and PCV13 strategies resulting in fewer cases and fewer costs.
The results of this CEA estimated that the implementation of PCV20 under a 3+1 schedule into the German immunization recommendation would be less costly and more effective than PCV13 and PCV15, both under a 2+1 schedule. PCV20 has the potential to substantially decrease the clinical and economic burden of pneumococcal diseases in Germany by providing substantially broader protection compared with lower-valent vaccines.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Ta A, et al. Cost-effectiveness of PCV20 to prevent pneumococcal disease in the pediatric population—a German societal perspective analysis. medRxiv. 2024.03.14.24304296; https://doi.org/10.1101/2024.03.14.24304296 .
Dadonaite, B. and M. Roser, Pneumonia. 2019. OurWorldInData.org.
European Centre for Disease Prevention and Control, Factsheet about pneumococcal disease. 2023.
Ganaie F, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020. https://doi.org/10.1128/mbio.00937-20 .
Article PubMed PubMed Central Google Scholar
World Health Organization. Pneumococcal Disease. [cited 2023 June]; Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease .
Troeger C, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Article Google Scholar
Ladhani SN, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51.
Article PubMed Google Scholar
Wahl B, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–57.
Tin Tin Htar M, Christopoulou D, Schmitt H-J. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15(1):419.
Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut. Begründung der STIKO-Empfehlungen zur Impfung gegen Pneumokokken und Meningokokken vom Juli 2006. Epidemiologisches Bulletin 2006;4(31).
Robert-Koch-Institut. Impfempfehlungen der Ständigen, I., Begründungen zur allgemeinen Empfehlung der Impfungen gegen Pneumokokken und Meningokokken im Säuglings- und Kindesalter, in Epidemiologisches Bulletin. Robert Koch-Institut. 2006.
van der Linden M, et al. Four years of universal pneumococcal conjugate infant vaccination in Germany: Impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine. 2012;30(40):5880–5.
Perniciaro S, et al. Regional variations in serotype distribution and vaccination status in children. PLoS One. 2019;14(1932-6203(electronic)):e0210278.
Article CAS PubMed PubMed Central Google Scholar
Robert Koch-Instituts (RKI). Epidemiologisches Bulletin. Robert Koch-Institut. 2015.
European Medicines Agency. European public assessment report. Vaxneuvance. 2021 [cited 2024 March]; Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxneuvance .
European Medicines Agency. European public assessment report. Prevenar 20 (previously Apexxnar). [cited 2024 March]; Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-20-previously-apexxnar .
Kühne Felicitas, et al. Cost-effectiveness of use of 20-valent pneumococcal conjugate vaccine among adults in Germany. Expert Rev Vacc. 2023;22(1):921–32. https://doi.org/10.1080/14760584.2023.2262575 .
Article CAS Google Scholar
European Medicines Agency. Apexxnar—opinion on variation to marketing authorisation. [cited 2024 February]; Available from: https://www.ema.europa.eu/en/medicines/human/variation/apexxnar .
Robert-Koch-Institut. STIKO: Aktualisierung der Empfehlungen zur Pneumokokken-Impfung in Epidemiologisches Bulletin. Robert-Koch-Institut. 2023.
Robert-Koch-Institut. Impfquoten bei Erwachsenen in Deutschland in Epidemiologisches Bulletin. Robert-Koch-Institut. 2022.
Robert-Koch-Institut. Impfempfehlungen der Ständigen, I., Stellungnahme der STIKO zum Einsatz von Pneumokokken-Konjugatimpfstoffen im Säuglings-, Kindes- und Jugendalter, in Epidemiologisches Bulletin. Robert Koch-Institut. 2023.
Kuhlmann A, von der Schulenburg JMG. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ. 2017;18(3):273–92.
Weiss S, et al. Impact of 10-and 13-valent pneumococcal conjugate vaccines on incidence of invasive pneumococcal disease in children aged under 16 years in Germany, 2009 to 2012. Eurosurveillance. 2015. https://doi.org/10.2807/1560-7917.ES2015.20.10.21057 .
Institut für Qualität und Wirtschaftlichkeit im, G., Allgemeine Methoden. 2022.
Ständige Impfkommission am Robert-Koch-Institut, Standardvorgehensweise (SOP) der Ständigen Impfkommission (STIKO) für die systematische Entwicklung von Impfempfehlungen Version 3.1 (Stand: 14.11.2018). Berlin. 2018.
Shiri T, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–9.
Centers for Disease Control and Prevention. 2019. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2019. [cited 2024 March]; Available from: https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf .
Statistisches Bundesamt (Destatis), Population: Germany Reporting date, age years. 2022.
Destatis Statistiches B. Current population of Germany. 2023 13 June 2023]; Available from: https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/_node.html .
Weinberger R, et al. Invasive pneumococcal disease in children under 16 years of age: Incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine. 2018;36(4):572–7.
Deb A, et al. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol Infect. 2022;150: e204.
Kuhlmann A, von der Schulenburg JMG. Authors’ reply to Gandjour: “Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany.” Eur J Health Econ. 2018;19(3):473–81.
Hu T, et al. Incidence of acute otitis media in children < 16 years old in Germany during 2014–2019. BMC Pediatr. 2022;22(1):204.
van der Linden M, Itzek A. Invasive pneumococcal disease among adults in Germany. Reporting period: 1 July 1992–30 June 2022, in Report for Pfizer Pharma GmbH. 2022, data on file.
van der Linden M, Itzek A. Invasive pneumococcal disease among adults in Germany. Reporting period: 1 July 1992–30 June 2022, in Report for Pfizer Pharma GmbH. 2022.
van der Linden M, Itzek A. Effects of the National Immunization Program for Pneumococcal Conjugate Vaccination (PCV7, PCV10, PCV13) on invasive pneumococcal disease among children in Germany. Reporting period: 1 July 1997–30 June 2022. Pfizer data on file. 2022.
Savulescu C, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022;40(29):3963–74.
Article CAS PubMed Google Scholar
Ansaldi F, et al. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to the 10-valent pneumococcal conjugate vaccine (PCV10) in Italy. Pathogens. 2020. https://doi.org/10.3390/pathogens9020076 .
Kim H-Y, et al. Cost-effectiveness of a national immunization program with the 13-valent pneumococcal conjugate vaccine compared with the 10-valent pneumococcal conjugate vaccine in South Korea. Hum Vaccin Immunother. 2021;17(3):909–18.
Klok RM, et al. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35(2):119–34.
Kulpeng W, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31(26):2839–47.
Rozenbaum MH, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340: c2509.
Hansen J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using world health organization standardized interpretation of chest radiographs. Pediat Infect Dis J. 2006;25(9):779–81.
Black SB, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5.
Black S, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19(3):187–95.
Stoecker C. Economic assessment of PCV15 &PCV20. In: Advisory Committee on Immunization Practices. Atlanta, GA. 2021.
Schley K, et al. Did the change of the vaccination schedule effect pneumococcal conjugate vaccination compliance and adherence of premature and mature born infants in Germany? Answers from a claims database analysis. Vaccine. 2023;41(28):4081–91. https://doi.org/10.1016/j.vaccine.2023.05.045 .
Patterson S, et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials Vaccinol. 2016;5:92–6.
Lauer-Fischer GmbH. LAUER-TAXE® Kompetenz online. 2023.
Statistisches Bundesamt (Destatis), Consumer Price Index: Germany Reporting date, application [Table 61111]. 2022. [cited 2024 March]. Available from: https://www.destatis.de/EN/Home/_node.html .
Grochtdreis T, et al. Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019;20(6):933–44.
Mangen M-JJ, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31):4203–14.
Rozenbaum MH, et al. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ Br Med J. 2012;345: e6879.
Stoecker C, et al. Cost-Effectiveness of Using 2 vs 3 Primary Doses of 13-Valent Pneumococcal Conjugate Vaccine. Pediatrics. 2013;132(2):e324–32.
Strutton DR, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J Infect. 2012;64(1):54–67.
Mangen M-JJ, Bonten MJM, de Wit GA. Rationale and design of the costs, health status and outcomes in community-acquired pneumonia (CHO-CAP) study in elderly persons hospitalized with CAP. BMC Infect Dis. 2013;13(1):597.
Lytle D, et al. Cost-effectiveness analysis of PCV20 to prevent pneumococcal disease in the Canadian pediatric population. Hum Vaccin Immunother. 2023;19(2):2257426.
Warren S, et al. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to higher-valent options in Greek infants. Vaccines. 2023;11(8):1369.
Wilson M, et al. Estimating the cost-effectiveness of switching to higher-valency pediatric pneumococcal conjugate vaccines in the United Kingdom. Vaccines. 2023;11(7):1168.
Wilson M, et al. Assessing public health impact of four pediatric pneumococcal conjugate vaccination strategies in the Netherlands. Infect Dis Ther. 2023;12(7):1809–21. https://doi.org/10.1007/s40121-023-00828-8 .
Løchen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infect. 2020;26(1):60–70.
Talbird SE, et al. Outcomes and costs associated with PHiD-CV, a new protein D conjugate pneumococcal vaccine, in four countries. Vaccine. 2010;28:G23–9.
Wilson M, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
Chuck AW, et al. Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine. 2010;28(33):5485–90.
Earnshaw SR, et al. Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect Dis. 2012;12(1):101.
Wals P, et al. Simulation model for comparing the costs and effectiveness of different pneumococcal conjugate vaccines. Proc Vaccinol. 2009;1:67–72.
Syeed MS, et al. Pneumococcal vaccination in children: a systematic review and meta-analysis of cost-effectiveness studies. Value Health. 2023;26(4):598–611.
Perdrizet J, et al. Historical population-level impact of infant 13-valent pneumococcal conjugate vaccine (PCV13) National immunization programs on invasive pneumococcal disease in Australia, Canada, England and Wales, Israel, and the United States. Infect Dis Ther. 2023;12(5):1351–64.
Croucher NJ, Løchen A, Bentley SD. Pneumococcal vaccines: host interactions, population dynamics, and design principles. Annu Rev Microbiol. 2018;72:521–49.
Masala GL, et al. Exploring the role of competition induced by non-vaccine serotypes for herd protection following pneumococcal vaccination. J R Soc Interface. 2017;14(136):20170620.
Robert Koch Institut; 2016. available from: RKI - Abgeschlossene Projekte - Evaluation unterschiedlicher Impfstrategien zur Prävention von Pneumokokken-Infektionen bei älteren Erwachsenen: Ergebnisse eines dynamischen Transmissionsmodells.
Levy C, et al. Impact of PCV13 on community-acquired pneumonia by C-reactive protein and procalcitonin levels in children. Vaccine. 2017;35(37):5058–64.
Rodrigo C, et al. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J. 2015;45(6):1632.
Lau WCY, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–9.
Claes C, Reinert RR, von der Schulenburg J-MG. Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur J Health Econ. 2009;10:25–38.
Statistisches Bundesamt (destatis.de). 40% der Mütter von Kindern unter drei Jahren sind erwerbstätig. 2023: destatis.de.
Mangen M-JJ, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208.
Erickson LJ, et al. Complications of meningococcal disease in college students. Clin Infect Dis. 2001;33(5):737–9.
Oostenbrink R, Henriëtte AM, Essink-Bot ML. The EQ-5D and the health utilities index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002;55(8):791–9.
Janoir C, et al. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. In: Open forum infectious diseases. Oxford: Oxford University Press; 2016.
Book Google Scholar
Download references
Medical Writing/Editorial Assistance
The original model was developed by Des Dillon-Murphy, PhD, and Ruth Chapman, PhD, of Evidera. Writing support for this manuscript was provided by Colleen Dumont and Sally Neath of Cytel Inc, Waltham, MA, US and by Allison Brackley, PhD, and Elizabeth Hubscher, PhD, who were employed by Cytel Inc, Waltham, MA, US at the time of the manuscript development. This support was funded by Pfizer. We thank Mark van der Linden for providing IPD data. An Ta is an employee of Cytel, which received funding from Pfizer in connection with the development of this manuscript.
Sponsorship for this study and Rapid Service Fee were funded by Pfizer Inc.
Author information
Authors and affiliations.
Cytel, London, United Kingdom
Pfizer Pharma GmbH, Berlin, Germany
Felicitas Kühne, Maren Laurenz & Christof von Eiff
Pfizer Inc, New York City, New York, USA
Sophie Warren
Pfizer Canada, Kirkland, Quebec, Canada
Johnna Perdrizet
You can also search for this author in PubMed Google Scholar
Contributions
Felicitas Kühne, Maren Laurenz, Christof von Eiff, Johnna Perdrizet and Sophie Warren contributed to the study conceptualization, investigation, and methodology. An Ta conducted the formal analysis and was responsible for project administration. Johnna Perdrizet was responsible for funding acquisition. Johnna Perdrizet and Felicitas Kühne provided supervision and validation. All authors contributed to writing the original draft, as well as reviewing and editing subsequent drafts, and approving the final version.
Corresponding author
Correspondence to Johnna Perdrizet .
Ethics declarations
Conflict of interest.
Felicitas Kühne, Maren Laurenz, Christof von Eiff, and Johnna Perdrizet are employees of Pfizer. Sophie Warren was employed by Pfizer at the time of the manuscript development (current affiliation: Adelphi Values, UK). An Ta is an employee of Cytel, which received consulting fees from Pfizer for the study and manuscript development.
Ethical Approval
This article was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; as such, ethical approval was not required.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: DGGÖ 2024 presentation and preprint (medRxiv) [ 1 ].
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 227 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Ta, A., Kühne, F., Laurenz, M. et al. Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00977-4
Download citation
Received : 27 February 2024
Accepted : 11 April 2024
Published : 11 May 2024
DOI : https://doi.org/10.1007/s40121-024-00977-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pneumococcal disease
- Cost-effectiveness
- Pneumococcal conjugate vaccine
- Pediatric, invasive pneumococcal disease, pneumonia, otitis media
- Find a journal
- Publish with us
- Track your research
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Background: Predictive Genetic Testing of Children for Adult-Onset-Only Conditions
Ethical challenges raised by the revised protocol, problems with the concept of family benefit, the controversy surrounding the disclosure of secondary findings, ethical issues in newborn sequencing research: the case study of babyseq.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Lainie Friedman Ross , Ellen Wright Clayton; Ethical Issues in Newborn Sequencing Research: The Case Study of BabySeq. Pediatrics December 2019; 144 (6): e20191031. 10.1542/peds.2019-1031
Download citation file:
- Ris (Zotero)
- Reference Manager
The BabySeq Project is a study funded by the National Institutes of Health and aimed at exploring the medical, behavioral, and economic impacts of integrating genomic sequencing into the care of both healthy newborns and newborns who are sick. Infants were randomly assigned to receive standard of care or standard of care plus sequencing. The protocol and consent specified that only childhood-onset conditions would be returned. When 1 child was found to carry a BRCA2 mutation despite a negative family history, the research team experienced moral distress about nondisclosure and sought institutional review board permission to disclose. The protocol was then modified to require participants to agree to receive results for adult-onset-only conditions as a precondition to study enrollment. The BabySeq team asserted that their new protocol was in the child’s best interest because having one’s parents alive and well provides both an individual child benefit and a “family benefit.” We begin with a short description of BabySeq and the controversy regarding predictive genetic testing of children for adult-onset conditions. We then examine the ethical problems with (1) the revised BabySeq protocol and (2) the concept of family benefit as a justification for the return of adult-onset-only conditions. We reject family benefit as a moral reason to expand genomic sequencing of children beyond conditions that present in childhood. We also argue that researchers should design their pediatric studies to avoid, when possible, identifying adult-onset-only genetic variants and that parents should not be offered the return of this information if discovered unless relevant for the child’s current or imminent health.
In 2014, the Eunice Kennedy Shriver National Institute of Children Health and Human Development and the National Human Genome Research Institute funded a consortium of 4 grantees to study newborn sequencing in genomic medicine and public health. 1 One of the awarded projects was entitled The BabySeq Project (Genome Sequence-Based Screening for Childhood Risk and Newborn Illness), a collaboration between Brigham and Women’s Hospital, Boston Children’s Hospital and the Broad Institute at Harvard University, and Baylor College of Medicine. Its aim was “to explore the medical, behavioral, and economic impacts of integrating genomic sequencing into the care of study families of both healthy and sick newborns” 2 , 3 via a randomized clinical trial in which half of participants were randomly assigned to receive standard of care (family history and standard newborn screening) and half were randomly assigned to have standard of care plus genomic sequencing. For those in the sequencing arm, a newborn genomic screening report was generated, “which lists pathogenic or likely pathogenic variants in genes that have been strongly linked to childhood-onset diseases or diseases for which intervention is possible during childhood.” 2 For newborns with a specific clinical presentation that potentially had a genetic etiology, an indication-based analysis was performed. 2
The study researchers sought to enroll 200 newborns and their parents into each cohort (healthy infants and infants admitted to the NICU), but despite 22 months of recruitment and approaching 3860 families, they were only able to recruit 268 infants (6.9%), 45 of 436 (10.3%) from the NICU and 223 of 3424 (6.5%) from the well-baby nursery. 3 Overall, 159 infants were sequenced, 127 healthy infants and 32 infants in the NICU. 4
In January 2019, Holm et al 5 reported that a male infant enrolled from the cardiac ICU at Boston Children’s Hospital was identified with a BRCA2 mutation despite a negative family history. The team wanted to return these results despite the facts that (1) the consent form clearly stated that only childhood-onset conditions would be returned,(2) the institutional review board (IRB) had approved the study knowing that only childhood-onset conditions would be returned, and (3) the study had obtained a US Food and Drug Administration nonsignificant risk determination on the basis of the plan to return only the results of conditions that could manifest in childhood. 5 The team went back to the IRB and obtained permission to recontact the infant’s parents to offer the return of adult-onset-only findings. The parents consented and were told the results. Although either parent could have been the BRCA2 carrier, when the findings were shared, the mother recalled some distant paternal relatives with breast and/or ovarian cancer, and she was referred to a familial cancer genetic risk clinic. The authors did not detail whether she went to the familial cancer genetic risk clinic or whether she was found to be the carrier, nor did the authors detail the perceived benefit from the discovery.
Because of their moral distress, the researchers proposed to modify their protocol to offer the optional return of adult-onset-only genetic variants if both parents agreed. In response to the IRB’s concern that some parents might still not receive results under the revised protocol, the researchers modified the amendment to require participants to receive results for adult-onset-only conditions as a precondition to participate in the study. 5 They explained that this “avoids the ethical dilemma of laboratory personnel knowing something that is widely considered to be actionable but cannot be returned.” 5 They asserted that their new protocol was in the child’s best interest because “the best interest of the child includes not only the child’s future autonomy to make a decision about what the child wants to know about him- or herself, but also having his or her parents alive and well,” 5 which they describe as “family benefit.” After a brief description of the debate regarding predictive genetic testing of children for adult-onset-only conditions in the clinical and research settings, we examine (1) the ethical challenges raised by the revised BabySeq protocol and (2) the ethical problems with the concept of family benefit.
There is a consensus within the pediatric, genetics, and ethics communities, in the Unites States and globally, that children should not be tested for adult-onset-only conditions. 6 – 14 The arguments to support this position are as follows: (1) the information is not clinically relevant to the child, and so testing is “not medically indicated” and could create anxiety without any potential for intervention; and (2) it preserves the child’s autonomy to decide as an adult whether to undergo testing.
Sequencing raises the possibility of discovering genetic information unrelated to the clinical question, known as “incidental” or “secondary” findings. Although the American College of Medical Genetics and Genomics (ACMG) issued a statement in 2012 that “patients should be given the option of not receiving certain or secondary findings,” 15 new recommendations in 2013 mandated that “laboratories performing clinical sequencing seek and report mutations…in all subjects, irrespective of age…,” 16 a form of opportunistic screening, despite acknowledging that there are “insufficient data on penetrance and clinical utility to fully support these recommendations.” 17 The original list included 57 genes, which was quickly revised to 56 genes, and has now been increased to 59 genes (ACMG 59). 17
Many ACMG members objected to these recommendations. 18 – 23 In response to the criticism that the new recommendations contradicted earlier policies about predictive genetic testing of children, the ACMG responded that the earlier policies were focused on children with “a known family history of risk, with the expectation that the child will be offered testing at an age when he or she can make an informed decision about testing,” 24 whereas opportunistic screening applied to unsuspecting families for whom the information may benefit the child and parents. However, in April 2014, in response to additional stakeholder feedback, 25 the ACMG modified its clinical recommendation to allow patients (and parents) to opt out of opportunistic screening and the return of the ACMG 59 results. 26
The ACMG guidelines were focused on the clinical setting, whereas BabySeq is a research protocol. Traditionally, research results were not returned to participants. In 2002, several researchers began to argue for informing clinical trial participants about aggregate results, 27 , 28 but many institutions had no policies on when, how, or what results should be returned. 29 The arguments in favor of returning results were both ethical (promoting trust and respecting participants as partners in research) and pragmatic (returning results could increase enrollment).
Within the decade, spurred on by public interest, the focus turned to returning individual participants’ research results. 30 , 31 A large body of scholarship addresses the return of these research results. 32 , 33 The National Heart, Lung, and Blood Institute published 2 conference reports focused on reporting genetic results in research studies in which they concluded that participants should not be forced to receive results. 34 , 35 Other reports were focused on the return of pediatric results. 36 – 39 Members of the Return of Results committees of the Clinical Sequencing Exploratory Research Consortium and the Electronic Medical Records Network Consent, Education, Regulation, and Consultation Working Group, 2 national multicenter projects actively engaged in returning research results, specifically argued that parents should have a right to refuse secondary results in research involving children “unless the return of results is of high health significance to the minor in childhood.” 40
The original BabySeq protocol was focused on the return of childhood-onset conditions, but the research sequencing methods identified a BRCA2 mutation, 1 of 3 genes classified as adult-onset-only in the ACMG 59 list.
What options did the team have to avoid this discovery? The BabySeq team could have devised an analytic pipeline to deliver only the information intended for the project, or it could have elected to sequence the whole genome or exome but not to interrogate the adult-onset-only regions unless there was substantial reason to suspect that they were pertinent to the child’s immediate clinical care rather than to other research goals. International guidelines support a more targeted approach, limiting search to genes relevant to the primary indication when possible. 41 , 42 Their failure to employ a more targeted approach created a situation that could have been avoided.
Although the parents in BabySeq were re-consented about whether to receive adult-only-onset information, the revised protocol going forward requires disclosure of all ACMG 59 results to all who undergo sequencing, even if the only person sequenced is a newborn and the results are adult-onset-only conditions. There is no opt-out policy, making the return of results in BabySeq more demanding than the ACMG recommendations, which were designed for the clinical setting, where providers have more stringent obligations to patients than researchers have to participants. 26 , 43
To be clear, participation in BabySeq is voluntary, and most parents (>90%) refuse. Given that sequencing is not standard of care, even for infants in the NICU, the 45 sets of parents who enrolled their NICU infants may have perceived the research protocol as a unique opportunity to learn about their child’s condition and to obtain information that could help providers best treat their child. Under the revised protocol, parents may elect to enroll their ill children, although it may mean getting unwanted information that is not relevant to their child’s current health status. That is, the mandatory return of results in BabySeq restricts parental freedom to choose what research results they want to know because it requires the reporting of AMCG 59 results as a precondition of participation even though the mandatory return of genetic research results is not consistent with many of the sequencing guidelines and consensus statements in the United States and globally. 38 – 42
In this case, however, a cancer risk variant was identified, and even if it were avoidable, the team was left in the position to decide what to do with the information. A major driver for the mandatory return of results by BabySeq was the moral distress of providers and laboratorians, a not-uncommon emotion when providers and families disagree about what is in a patient’s best interest or when patients refuse life-saving interventions. 44 In the clinical genetics context, providers may experience moral distress when patients refuse actionable genetic testing, fail to obtain appropriate surveillance, or refuse to share actionable genetic information with family. In contrast, in the research setting, providers are often more limited as to what they can offer, do, and say, given protocol restrictions. The providers’ distress does not (or at least should not) trump the participants’ interests or preferences, particularly if agreed to beforehand in the consent process.
In the case of children, providers and researchers do not get to impose on families their view of what is best. Clinicians could conceivably seek a court order to override parental refusals to obtain genetic testing of children for adult-onset-only disorders, asserting that the parents’ actions constituted medical neglect, but they would almost surely fail. Although providers may argue that a particular genetic test is in a child’s best interest, the courts will intervene only if the parents acts are abusive or neglectful. 45 , 46 Courts would not require clinical genetic testing of a young child for a condition that presents in adulthood when no intervention or surveillance is needed in childhood, even in a high-risk family, because the variant does not pose an imminent threat to the child.
The argument that there is an obligation to identify and then report genes associated with adult-onset-only conditions is even more misguided in families of children who are critically ill. In these cases, sequencing is performed in an effort to improve the particular infant’s care. Notably, other investigators who have explored the role of sequencing in these infants have specifically chosen not to look for secondary findings. 47 , 48 Saunders et al 47 published a methodology for rapid whole-genome sequencing in the NICU that masked many potential findings for expediency because the aim was to provide information that could inform emergent decision-making. Similarly, in 2016, Smith et al 48 stated that:
Only confirmed causative sequence changes that explain the observed phenotype are communicated to the parents by the treating physician and/or a certified genetic counselor. Because the primary analysis is directed toward the neonate with specific clinical findings, we do not evaluate for or report any of the 56 genes identified by the American College of Medical Genetics as reportable incidental findings, unless directly related to the underlying clinical presentation. 48
In the case that triggered the protocol change in BabySeq, the child died without leaving the hospital. Knowledge about late-onset conditions were a moot point for the child. If we want to know about parents’ risks, we should test them.
One objection to masking is that some genes have >1 function and excluding those associated with adult-onset-only conditions could hide the cause of the child’s health problems. Consider, for example, the case of an infant who carries 2 pathogenic variants of BRCA2 . BRCA causes adult-onset-only breast cancer in the heterozygous state but can cause Fanconi anemia in the homozygous state. 49 , 50 Masking the BRCA gene could lead to a failure to identify this health risk in a child. However, Alter et al 51 note the following:
The small group of patients with biallelic mutations in BRCA2 is distinctive in the severity of the phenotype, and early onset and high rates of leukemia and specific solid tumors, and may comprise an extreme variant of Fanconi anemia. Several of the alleles were not associated with cancer in presumed carriers, and thus counseling presents more uncertainties than usual. 51
That is, children with this form of Fanconi anemia will present with phenotypic findings that would justify BRCA genetic testing for diagnostic purposes. Even those who value the identification of both parents as BRCA carriers must pause, however, given that several of the variants were not associated with increased cancer risks and reporting back the results might lead to unnecessary screening and interventions. Harms from masking are rare and are outweighed by the benefits of focusing resources on the child’s immediate needs.
In its original recommendation to provide opportunistic screening, the ACMG stated that “after sequencing a child for a primary indication, it becomes relatively easy for a laboratory to report a limited number of variants for conditions that could be medically important to that child’s future or to the rest of the family.” 16 The ACMG argued that the risks to the child “were outweighed by the potential benefits to the future health of the child and the child’s parents of discovering an incidental finding for which intervention might be possible.” 16 The real focus was to provide risk information to the parents who might not otherwise be aware of their own risks, which could lead to preventive or therapeutic interventions that indirectly benefit the child. That is, opportunistic screening offers family benefit.
The ACMG has been arguing for the concept of family benefit at least since coauthoring, with the Health Resources and Services Administration, Newborn Screening: Toward a Uniform Screening Panel and System in 2006:
Historically, screening has focused on conditions for which the improvement in outcome for the infant has been substantial. However…the nature of genetic disease is such that knowledge of its presence can be of value to other family members. Previously, this factor has not been considered by newborn screening programs. 52
Natowicz 53 criticized the task force for including “a calculation of the benefits to the family and to society of early intervention—not just the benefit for the baby” because “it shifts the emphasis of newborn screening away from the medical interest of the child alone.” Others objected to the fact that family benefit was rated to be as important as clinical treatability when deciding what conditions to include in a uniform screening panel. 54
Wilfond et al 55 offered 2 arguments in support of family benefit. One benefit is derivative: by identifying an actionable adult-onset risk, the child benefits because prevention and treatment can promote the parent’s health, which enables the parent to care for the child. The other benefit is independent of a direct benefit to the child. It supports respecting the authority of parents to balance the competing interests of family members without state intervention unless their decision falls below a threshold that constitutes abuse and/or neglect.
Wilfond et al 55 examined the primary objections to testing children for adult-onset-only conditions: (1) the potential to cause adverse psychological impact and (2) depriving the child of a right to an open future. They argued that these objections failed because (1) the data fail to reveal evidence of significant harm 56 and (2) respect for parental authority to raise their children according to their own values means respecting parental decisions that foreclose options for their children and shape the direction of their children’s lives. 55
The arguments by Wilfond et al 55 are flawed, in part, because they are focused on the wrong people. Although families may consider the interests of other family members in their decision-making for a child, 57 , 58 the primary focus of health care providers must be the child’s well-being. 59 – 61 To focus otherwise is to treat the children as means for the ends of others (their parents). 62 If we want to reduce morbidity and mortality of adults, we can test adults. Interrogation of the areas of the genome that identify adult-onset - only conditions takes additional work and resources by the laboratorians and additional work on the clinicians and researchers in counseling families and providing follow-up evaluation and care. These results are not as easy to interpret and return as they are often made out to be, and they take large amounts of resources that may detract from the research aims. The parents in BabySeq were not demanding these results and had consented to participate without expecting to receive adult-onset-only results. Given the low participation rate in BabySeq, it will be important to see whether the policy change will decrease enrollment.
Second, even if the secondary findings are valid, parental benefits are not ensured unless participants understand their need for follow-up and can afford the necessary testing and treatments. BabySeq is not designed to support the mother beyond making a referral, which she would attend, presumably, at her own expense. Even if the mother were found to be a carrier, how much more of a risk she actually faces compared with other women who are premenopausal is unknown given that the high pathogenicity of the mutations in the ACMG 59 is based on studies in which high-risk families were enrolled. The meaning of these variants in a woman with a less compelling family history has not been adequately studied. Claims of high risk may be premature or even wrong, 57 , 58 , 63 – 65 particularly for minorities who are usually underrepresented in genetics research. 66 – 69 In any event, even if she is at high risk, she could not be required to pursue follow-up because it has been decades since the law has required women to undergo even life-saving medical treatment so that they can care for their children. 70
Third, both the ACMG and Wilfond et al 55 acknowledge that data about short- and long-term risks and harms (as well as benefits) of returning genetic information about adult-onset-only conditions are scant. They assume that identifying variants alone is a positive outcome before data are obtained on the psychological and health outcomes, and they ignore the risks, which include unnecessary testing due to false-positives or incomplete penetrance, as well as stigma, discrimination, and anxiety. At minimum, the returning of research results that have no immediate clinical indications for the child should be voluntary and not mandatory.
Fourth, although Wilfond et al 55 believe that the data are sufficiently equivocal that pediatricians should be neutral in counseling parents about requesting secondary findings, we argue that pediatricians should take a directive stance in opposition. A neutral position suggests not only that there is equipoise about the benefits and risks but also that the offer is morally neutral, which it is not. All pediatric professional statements argue against predictive genetic testing of children for adult-onset conditions to respect the child’s interests to decide for or against testing as an adult. 6 – 14 As such, the provider should counsel against such testing, even if the provider may accede to the parental demands.
Rather than requiring testing and reporting results of adult-onset genetic disorders in children on the basis of benefit to others in the family, providers and researchers should oppose testing and reporting of results even if parents can insist on getting them. Pediatric providers are moral agents and should counsel directively against predictive testing and reporting of results for adult-onset-only conditions in children in both the clinical and research settings, as Carl Elliott explains:
Finally, it is important to realize that the doctor is not a mere instrument of the patient’s wishes…[but] is also a moral agent who should be held accountable for his actions. If a patient undergoes a harmful procedure, the moral responsibility for that action does not belong to the patient alone; it is shared by the doctor who performs it. Thus a doctor is in the position of deciding not simply whether a subject’s choice is reasonable or morally justifiable, but whether he is morally justified in helping the subject accomplish it. 71
Requiring that all parents who authorize sequencing of their child be informed about adult-onset-only conditions included in the ACMG 59 assumes that the benefits of opportunistic screening outweigh the risks and harms, which has not been proven. 72 , 73 In fact, those sections of the genome associated with adult-onset-only conditions should not be analyzed at all unless pertinent to that child’s immediate medical care. Although we believe that there is much to be learned from BabySeq and the other newborn sequencing in genomic medicine and public health projects, the researchers should not intentionally search for adult-onset-only conditions.
Even if secondary findings are not intentionally sought, cases may occur in which known variants for later-onset conditions are identified: expect the unexpected. 74 We believe that studies should be designed to minimize the risk of identifying genes associated with adult-onset-only conditions. We believe that a policy of nondisclosure of adult-onset-only conditions is ideal, even when such genes are identified incidentally. This needs to be explained in the consent process so that parents do not expect otherwise. We support this position out of respect for the child’s future autonomy as an adult to decide what genetic information he or she wants and with whom he or she wants to share it.
We realize that there are those who disagree (who support disclosure of all ACMG high-risk alleles and other actionable secondary findings 55 , 75 , 76 ). At minimum, all participants and their surrogates should have the right to refuse learning about all research findings, even if researchers will feel distressed about knowing information that they believe is useful and actionable. The strongest case for mandatory disclosure is when researchers identify childhood-onset conditions for which early treatment would have health benefits for the child. If such information will be disclosed regardless of parental preferences, parents must understand this before consenting to sequencing so that they can make an informed decision about whether to enroll their child.
Parents may assert an interest in “knowing everything” and may experience significant distress and anger that some of their child’s results, particularly in the context of research, are withheld from them. 28 , 30 Clearly, researchers need to educate parents that everything is not and cannot be returned and that research teams do not interrogate or disclose every variant of unknown significance because of time, cost, and the sheer size of the genome (6 billion bp). Nor are researchers under any obligation to interrogate the genome fully, but, again, this must be made clear in the consent process. However, participants may be able to request and obtain their raw data under the Health Information Portability and Accountability Act to seek additional analyses not provided by the researchers. 32 , 77
How secondary research findings will be handled needs to be addressed upfront through a robust and transparent consent process. Additional psychosocial and clinical data about the benefits and harms that accrue from the return of results should be collected to inform future policy discussions.
Dr Ross conceptualized and designed the manuscript, drafted the initial manuscript, and revised the manuscript; Dr Clayton conceptualized and designed the manuscript and made critical revisions on multiple versions; and both authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: Dr Clayton received support from 5U01 HG008672-04 and 5RM 1HG009034-02. Funded by the National Institutes of Health (NIH).
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2019-3111 .
American College of Medical Genetics and Genomics
institutional review board
Competing Interests
Re: genetic screening in critically ill newborns.
In their Special Article, Ross and Clayton rightly asserted, "The argument that there is an obligation to identify and then report genes associated with adult-onset-only conditions is even more misguided in families of children who are critically ill. In these cases, sequencing is performed in an effort to improve the particular infant’s care." I agree that the proper goal of sequencing in the pediatric critical care setting is diagnosis, not screening of either the child or the parents for medically actionable genetic conditions.
Moreover, physicians must use sound clinical judgment when deciding whether or not to even offer to screen for secondary genetic findings when ordering genome scale sequencing. The physician-patient/parent relationship is necessarily fiduciary in nature. The potential harms of screening vary according to the clinical circumstances. In critically ill newborns with suspected genetic syndromes undergoing rapid genome-scale sequencing because it may be pivotal for medical or surgical management, including potential to support a palliative care option, the circumstances are far from ideal for parents to be confronted with decisions about screening their baby or themselves for adult genetic conditions of no immediate clinical relevance.
Is it wise and prudent to offer to screen baby and parents in the immediate postpartum period when a baby is critically ill, with survival in doubt and the potential to have to disclose some dire news, perhaps to mother, such as a hereditary breast and ovarian cancer susceptibility mutation? If physicians elect to offer secondary screening to a child-parent trio in such settings (and it would be ethical not to do so), then at least there should be discussion about the option to postpone disclosure of secondary findings that could otherwise come in days, right there in the intensive care unit. Perhaps parents can safely make the right choices for themselves even under the extreme psychic stress of such settings, but is it really ethical to put their ability to receive so much bad news all at once to the test? Patient autonomous choice is a pillar of medical ethics, but so is avoidance of harm, and especially when the choice to be screened or not can safely be deferred.
With the rapid pace of progress in our understanding of the risks and benefits of genetic screening as well as plummeting cost of screening oneself at will for gene mutations that may be "medically actionable," the argument for opportunistic screening incident to clinically indicated genomewide sequencing is weakening. It is especially weak in the case of a couple focused on diagnosis and treatment of their critically ill neonate. Parents can readily be screened at some more propitious time for receiving serious medical news, and any modest added cost associated with such screening does not create an imperative to screen under any and all clinical circumstances.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account

IMAGES
VIDEO
COMMENTS
A 13-year-old boy presents to his primary care provider with a 5-day history of abdominal pain and a 2-day history of diarrhea and vomiting. He describes the quality of the abdominal pain as sharp, originating in the epigastric region and radiating to his back, and exacerbated by movement. Additionally, he has had several episodes of nonbloody, nonbilious vomiting and watery diarrhea. His ...
Fifteen months before this admission, the patient was evaluated at the pediatric clinic of another hospital. On examination, the pulse was 95 beats per minute and the blood pressure 117/78 mm Hg.
The Diverse Spectrum of Invasive Meningococcal Disease in Pediatric and Adolescent Patients: Narrative Review of Cases and Case Series, Infectious Diseases and Therapy, 13, 2, (251-271), (2024 ...
A 13-year-old boy with a recent diagnosis of ulcerative colitis presented with fever and chest pain to the emergency department. The sharp pain had begun 2 days previous and radiated to his shoulders. It was constant, although it improved with sitting up and leaning forward. On the day before, he had developed a fever to 38.3°C. His past medical history was notable for a diagnosis of ...
Presentation Of Case. Dr. Caitlin E. Naureckas Li (Pediatrics): A 3-year-old boy was admitted to this hospital during the summer because of a seizure. The patient had been well until 3 days before ...
Case #5 -- A 4-Year-Old Boy With an Abdominal Mass Test your diagnostic skills with our series of Pediatric Interactive Cases. Clinical Case, May 12, 2003. Interactive Case #4 - A Child With ...
11-year-old boy with testicular pain and rash. William A. Frese, MD, MPH. January 19th 2024. An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. Read the full case to see if you can correctly diagnose the ...
9 May 2024. Persistent omphalomesenteric duct in an infant with trisomy 21. Arameh Aghababaie, Jennifer Ho, Arun Kelay, Penny Salt. 8 May 2024. Mandibuloacral dysplasia type A. Urmila Dahake, Akash Bang, Abhijit Choudhary, Shikha Jain. 6 May 2024. Large birth mark and unilateral swelling of the lower extremity in a young teenager.
View by: Case Topic Case Number. Accidental Ingestion of Opioids. Acute Epstein-Barr Virus (Infectious Mononucleosis) Acute Lymphoblastic Leukemia. Acute Otitis Media. Acute Postinfectious Glomerulonephritis. Adolescent Substance Use Disorder. Alopecia Areata Online Only. Anaphylactoid Purpura (Henoch-Schönlein Purpura)
18 Apr 2024. 03 Apr 2024. 28 Mar 2024. Case Reports in Pediatrics publishes case reports and case series related to pediatric subspecialities such as adolescent medicine, cardiology, critical care, dentistry, developmental and behavioral medicine, endocrinology, gastroenterology etc.
A 4-month-old girl presents with a 1-week history of a temperature to 102°F (38.9°C), congestion, rhinorrhea, and cough. She has had fatigue and diaphoresis with feedings over the last week, although this did not occur before this time. She was born at term following normal findings on prenatal ultrasonography. Initially, she had difficulty gaining weight, but she is now growing along the ...
Cases in Pediatric Acute Care presents over 100 real-world pediatric acute care cases, each including a brief patient history, a detailed history of present illness, presenting signs and symptoms, vital signs, and physical examination findings. Ideal for developing a systematic approach to diagnosis, evaluation, and treatment, this resource provides students and advanced practitioners with the ...
We present 3 scenarios that are commonly encountered in clinical practice: isolated elevation of low-density lipoprotein cholesterol (LDL-C), combined dyslipidemia, and severe hypertriglyceridemia. Treatment with statin is indicated when the LDL-C is ≥190 mg/dL (4.9 mmol/L) in children ≥10 years of age. For LDL-C levels between 130 and 189 ...
ia, fever, nuchal rigidity, myalgias, and fatigue. Character and significance of symptoms vary by patient age. Symptoms of infection may improve spontaneously or worsen, becoming potentially lethal. Early recognition and treatment of meningitis are crucial to prevent morbidity and mortality. The case reviewed in this article focuses on viral meningitis in a pediatric patient that may be ...
Results from published studies suggest that omalizumab should be continued for > 1 year [77, 78]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was 'excellent' in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years . After the ...
Case 1. Previously healthy 11-year-old male presents with respiratory distress and dehydration. On exam: Afebrile. Patient is ill appearing with sunken eyes. Appears breathless. Cannot complete full sentence without taking another breath. Taking deep and fast respirations, RR 27. Lungs clear to auscultation.
This calculation is based on 80 mLs/kg total blood volume in a 30 Kg child or 2,400 mLs. With a 25% blood loss we expected a 25% decrease in hemoglobin level, which is exactly what occurred. The preoperative hemoglobin level was 12.5 g/dL, and the postoperative level was 9.4. Because the tumor was adherent to the ventricles, the plan was to ...
Case 9-2013 — A 9-Year ... one study showed the incidence of uncomplicated infection to be essentially unaffected by vaccination in the group 5 to 17 years of age. 2 Primary or acquired ...
Abstract. Nephrotic syndrome (NS) is a glomerular disorder typically characterized by gross proteinuria, hypoalbuminemia, hyperlipidemia, and peripheral oedema. We report the case of a 2-year-old male toddler weighing 15 kg with a 1-week history of swelling around the eyes and both legs, and generalized body swelling.
The causes of constipation in children are poorly understood, and for the individual child the cause is often unknown. Organic constipation is due to an underlying pathology. It occurs in approximately 5%-10% of cases and is more likely to present in the first month of life. Causes in babies and toddlers include:
The pediatric physical therapist may collaborate with a wide range of specialists due to the complicated requirements of the child and the family, including teams from medicine, nursing, social work, education, and psychiatry, as well as speech and occupational therapists [3,4].]. Even though India is still in its infancy, the breadth has significantly expanded in the last several years to ...
J.S. is a former 32-weeks'-gestation infant who presents as a new patient for his 4-month health supervision visit. His birth history includes a 4-week neonatal intensive care unit stay caused by difficulty feeding, but ever since his discharge, he has been doing well on fortified human (breast) milk of 22 kcal/fl oz.
Case Study: Pediatrics Outpatient Setting Primary Concepts: Skin Integrity, Primary Healthcare, Rural Healthcare, Collaborative Care, Telehealth. Present Problem: You are entering an outpatient clinic room located in a small rural town of a patient that is 9 years old. The child, C., was referred from the local elementary school nurse because ...
Ovarian vein thrombosis is a rare type of venous thromboembolism that can be the cause of puerperal fever in approximately a third of women and Mortality related to OVT is nowadays low due to the combination treatment of parenteral broad-spectrum antibiotics and anticoagulation.
Integrated Community Case Management (ICCM) of common childhood illness is one of the global initiatives to reduce mortality among under-five children by two-thirds. It is also implemented in Ethiopia to improve community access and coverage of health services. However, as per our best knowledge the implementation status of integrated community case management in the study area is not well ...
Case study Questions & Answers. Use the questions below to guide conversation following the story. Possible answers are bulleted below each question. ... The American Academy of Pediatrics outlines a 4-Step Framework for Shared Decision-making in Pediatrics. 4) Are there concerns regarding Arianna driving? What are some safety factors to discuss?
The first of these Advocacy Case Studies, by Bronstein et al., (10.1542/peds.2018-1886) focuses on how legislation was created and then passed by the California State legislature in 2016 to require the state to develop processes to comply with any new federal recommendations for newborn screening within two years of that recommendation. In this ...
This study examined the cost and health outcomes related to PCV20 under a 3+1 schedule compared with PCV13 and PCV15, both under a 2+1 schedule, in the pediatric population in Germany. The base-case results demonstrated PCV20 as the dominant strategy over both lower-valent alternative vaccines.
The study researchers sought to enroll 200 newborns and their parents into each cohort (healthy infants and infants admitted to the NICU), but despite 22 months of recruitment and approaching 3860 families, they were only able to recruit 268 infants (6.9%), 45 of 436 (10.3%) from the NICU and 223 of 3424 (6.5%) from the well-baby nursery. 3 Overall, 159 infants were sequenced, 127 healthy ...
Due to low susceptibility of coronavirus disease of 2019 (COVID-19) in children, limited studies are available regarding COVID-19 in the pediatric population in Tunisia. The current study evaluated the incidence, clinical characteristics, and outcomes of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection among children hospitalized at Béchir Hamza Children's Hospital.