StudyMonkey
Your personal ai chemistry tutor.

Learn Smarter, Not Harder with Chemistry AI
Introducing StudyMonkey, your AI-powered Chemistry tutor .
StudyMonkey AI can tutor complex Chemistry homework questions, enhance your essay writing and assess your work—all in seconds.
No more long all-nighters
24/7 solutions to Chemistry questions you're stumped on and essays you procrastinated on.
No more stress and anxiety
Get all your Chemistry assignments done with helpful answers in 10 seconds or less.
No more asking friends for Chemistry help
StudyMonkey is your new smart bestie that will never ghost you.
No more staying after school
AI Chemistry tutoring is available 24/7, on-demand when you need it most.
Chemistry is the branch of science that studies the properties and behavior of matter, the changes it undergoes during chemical reactions, and the energy that accompanies those processes.
AI Tutor for any subject
American college testing (act), anthropology, advanced placement exams (ap exams), arabic language, archaeology, biochemistry, chartered financial analyst (cfa) exam, communications, computer science, certified public accountant (cpa) exam, cultural studies, cyber security, dental admission test (dat), discrete mathematics, earth science, elementary school, entrepreneurship, environmental science, farsi (persian) language, fundamentals of engineering (fe) exam, gender studies, graduate management admission test (gmat), graduate record examination (gre), greek language, hebrew language, high school entrance exam, high school, human geography, human resources, international english language testing system (ielts), information technology, international relations, independent school entrance exam (isee), linear algebra, linguistics, law school admission test (lsat), machine learning, master's degree, medical college admission test (mcat), meteorology, microbiology, middle school, national council licensure examination (nclex), national merit scholarship qualifying test (nmsqt), number theory, organic chemistry, project management professional (pmp), political science, portuguese language, probability, project management, preliminary sat (psat), public policy, public relations, russian language, scholastic assessment test (sat), social sciences, secondary school admission test (ssat), sustainability, swahili language, test of english as a foreign language (toefl), trigonometry, turkish language, united states medical licensing examination (usmle), web development, step-by-step guidance 24/7.
Receive step-by-step guidance & homework help for any homework problem & any subject 24/7
Ask any Chemistry question
StudyMonkey supports every subject and every level of education from 1st grade to masters level.
Get an answer
StudyMonkey will give you an answer in seconds—multiple choice questions, short answers, and even an essays are supported!
Review your history
See your past questions and answers so you can review for tests and improve your grades.
It's not cheating...
You're just learning smarter than everyone else
How Can StudyMonkey Help You?
Hear from our happy students.
"The AI tutor is available 24/7, making it a convenient and accessible resource for students who need help with their homework at any time."
"Overall, StudyMonkey is an excellent tool for students looking to improve their understanding of homework topics and boost their academic success."
Upgrade to StudyMonkey Premium!
Why not upgrade to StudyMonkey Premium and get access to all features?

Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
In-cite ai reference generator, legal text refiner, job search ai assistant.
Chemistry AI Homework Solver
Omni is the most accurate AI homework solver on the market!
See Omni in Action
Introducing Chemistry AI Homework Solver: The Ultimate Solution for Your Chemistry Assignments!
Are you struggling with your chemistry homework? Are you tired of spending hours trying to solve complex equations and formulas? Look no further! Smodin Chemistry Homework Solver is here to help you ace your assignments with ease.
How does it work?
Our AI-powered tool uses advanced algorithms and machine learning to provide accurate and efficient solutions to your chemistry problems. Simply input your assignment or question, and Smodin Chemistry AI homework solver will generate step-by-step solutions in seconds.
Why choose Chemistry AI Homework Solver?
- Save Time: With our solver, you can complete your assignments in a fraction of the time it would take you to solve them manually.
- Accuracy: Smodin’s Chemistry homework solver provides accurate solutions every time, eliminating the risk of errors and mistakes.
- Boost your Grades: You can enhance your school success by guaranteeing the precision and correctness of your responses using Smodin’s AI Chemistry solver.
- Convenience: 24/7 availability guarantees that you can access it at any time for your chemistry homework needs.
Experience the Best Chemistry AI Homework Solver
Smodin’s Chemistry AI Homework Solver is the most advanced and reliable solution for all your chemistry assignments. Whether you're a high school or college student, Smodin can help you tackle even the most challenging problems. Give it a try today and experience the convenience and accuracy of Smodin Chemistry solver. Say goodbye to the frustration of chemistry homework and hello to success!
Get Instant Help with Chemistry Questions Using Smodin’s AI-powered Chemistry Solver!
Are you stuck on a chemistry question and don't know where to turn for help? Smodin’s Chemistry AI Solver is here to assist you. With our advanced algorithms, we can provide accurate and efficient solutions to your chemistry problems in no time. Say goodbye to frustration and confusion and hello to quick and easy answers to your chemistry questions.
Ace Your Exams with the Help of Smodin’s Chemistry Homework Assistant!
Preparing for a chemistry exam can be stressful and time-consuming. Let Smodin’s Chemistry Homework Assistant take some of the weight off your shoulders. With Smodin you can practice solving problems and reviewing concepts to ensure that you're fully prepared for your exam. Smodin’s assistant can help you achieve your academic goals and excel in your chemistry studies.
The Benefits of Using a Chemistry AI Solver for Your Homework
Using a Chemistry AI Solver for your homework has many benefits. Not only can it save you time and provide accurate solutions, but it can also improve your understanding of chemistry concepts. Smodin Chemistry AI solver generates step-by-step solutions, allowing you to learn from the process and apply it to future assignments. Try Smodin homework solver today and see the difference it can make in your academic performance.
Say Goodbye to Late-Night Study Sessions with Smodin’s Chemistry Question Solver
Late-night study sessions can be exhausting, especially when you're struggling with chemistry problems. Let Smodin Chemistry Question Solver lighten the load. Smodin can provide quick and accurate answers to your questions, allowing you to complete your assignments faster and more efficiently. Get the rest you need and the academic success you deserve with the help of Smodin’s solver.
Unlock Your Potential with Smodin Chemistry AI Homework Solver
Don't let challenging chemistry assignments hold you back from achieving your academic goals. Smodin’s Chemistry AI Homework Solver is the ultimate solution to your chemistry struggles. With Smodinl, you can unlock your potential and excel in your chemistry studies. Smodin Solver is easy to use and provides accurate solutions every time. Experience the convenience and efficiency of Smodin Chemistry AI Homework solver and say hello to academic success!
© 2024 Smodin LLC

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Chemistry Worksheets and Handouts (PDF for Printing)

This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Here is a list of worksheets. This site also has articles explaining these topics in detail.
- Label Parts of the Atom [ Google Apps worksheet ][ worksheet PDF ][ worksheet PNG ][ answers PNG ]
- Acid formulas [ PDF ][ Answers ]
- Balancing equations Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ] Worksheet #3 [ PDF ][ Answers ] Worksheet #4 [ PDF ][ Answers ]
- Chemical and Physical Changes [ PDF ][ Answers ]
- Chemistry scavenger hunt [ PDF clues ][ Answers ]
- Element names crossword [ PDF ][ Answers ]
- Element symbols – Symbols that make words [ PDF worksheet ][ Answers ]
- Element symbols – Countries of the world [ PDF ][ Answers ]
- More element symbol worksheets
- Homogeneous or Heterogeneous Mixtures [ PDF ][ Answers ]
- Intensive and Extensive Properties [ Worksheet ][ Answer Key ]
- Intrinsic and Extrinsic Properties [ PDF ][ Answers ]
- Ionic and Covalent Compounds (Names and Identification) [ PDF Worksheet ][ Answer Key ]
- Ionic Compound Names and Formulas [ PDF Worksheet ][ Answer Key ]
- Metric to English Unit Conversions [ PDF Worksheet ][ Answer Key ]
- Mixtures [ PDF ][ Answers ]
- Periodic table scavenger hunt [ PDF clues ][ Answers ]
- Reading a meniscus [ PDF ][ Answers ]
- Reading periodic table element information Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ]
- Scientific Notation [ PDF ][ Answers ]
- Significant digits Rules [ PDF ][ Answers ] Addition and subtraction [ PDF ][ Answers ] Multiplication and division [ PDF ][ Answers ]
- Types of Chemical Reactions [ Worksheet ][ Answers ]
In addition to these chemistry worksheets, there is a collection of word search puzzles .
Chemistry Handouts
These chemistry handouts illustrate chemistry concepts and offer examples.
- Amino acid side chains [ PDF ]
- Antimatter examples [ PNG ]
- Atom facts [ PNG ]
- Chemical properties [ JPG ]
- Colligative properties [ JPG ]
- Electron configurations [ PDF ]
- Element electronegativities [ PDF ]
- 118 Element Flash Cards [ PDF ]
- Element list [ PDF ]
- Endothermic reactions [ PNG ]
- Error calculations [ JPG ]
- Exothermic reactions [ JPG ]
- Heterogeneous mixtures [ JPG ]
- Hydrocarbon prefixes [ JPG ]
- Ionic compound properties [ PNG ]
- Genetic codons [ PDF ]
- Lewis structures [ JPG ]
- Litmus test [ PNG ]
- Magnetic vs non-magnetic metals [ JPG ]
- Mole ratio [ JPG ]
- Organic vs inorganic [ JPG ]
- Oxidation numbers [ JPG ]
- Periodic table Bingo game [ PDF ]
- pH indicators [ PNG ]
- Physical change [ JPG ]
- Physical properties [JPG ]
- Noble metals [ JPG ]
- Reactants and products [ JPG ]
- RNA vs DNA [ JPG ]
- States of matter [ JPG ]
- Visible spectrum [ JPG ]
Periodic Tables
There’s a printable periodic table for just about any purpose, but some of the most popular are listed here.

- 118 element vibrant periodic table [ PNG ]
- Actinides [ JPG ]
- Blank periodic table [ PDF ]
- Element charges [ JPG ]
- Element density [ PDF ]
- Element electrical conductivity [ PDF ]
- Element state of matter [ PDF ]
- Muted color 118 element periodic table [ PDF ]
- Native elements [ JPG ]
- Valence [ JPG ]

Biology Worksheets and Handouts
Is biology more your thing? We’ve got similar resources for the life sciences, including biology, biochemistry, cell biology, and anatomy.
Chemistry Worksheets Terms of Use
You are welcome to print these resources for personal or classroom use. They may be used as handouts or posters. They may not be posted elsewhere online, sold, or used on products for sale.
This page doesn’t include all of the assets on the Science Notes site. If there’s a table or worksheet you need but don’t see, just let us know!
Related Posts
The starting materials consist of one green sphere and two purple spheres. The products consist of two green spheres and two purple spheres. This violates Dalton’s postulate that that atoms are not created during a chemical change, but are merely redistributed.
This statement violates Dalton’s fourth postulate: In a given compound, the numbers of atoms of each type (and thus also the percentage) always have the same ratio.
Dalton originally thought that all atoms of a particular element had identical properties, including mass. Thus, the concept of isotopes, in which an element has different masses, was a violation of the original idea. To account for the existence of isotopes, the second postulate of his atomic theory was modified to state that atoms of the same element must have identical chemical properties.
Both are subatomic particles that reside in an atom’s nucleus. Both have approximately the same mass. Protons are positively charged, whereas neutrons are uncharged.
(a) The Rutherford atom has a small, positively charged nucleus, so most α particles will pass through empty space far from the nucleus and be undeflected. Those α particles that pass near the nucleus will be deflected from their paths due to positive-positive repulsion. The more directly toward the nucleus the α particles are headed, the larger the deflection angle will be. (b) Higher-energy α particles that pass near the nucleus will still undergo deflection, but the faster they travel, the less the expected angle of deflection. (c) If the nucleus is smaller, the positive charge is smaller and the expected deflections are smaller—both in terms of how closely the α particles pass by the nucleus undeflected and the angle of deflection. If the nucleus is larger, the positive charge is larger and the expected deflections are larger—more α particles will be deflected, and the deflection angles will be larger. (d) The paths followed by the α particles match the predictions from (a), (b), and (c).
(a) 133 Cs + ; (b) 127 I − ; (c) 31 P 3− ; (d) 57 Co 3+
(a) Carbon-12, 12 C; (b) This atom contains six protons and six neutrons. There are six electrons in a neutral 12 C atom. The net charge of such a neutral atom is zero, and the mass number is 12. (c) The preceding answers are correct. (d) The atom will be stable since C-12 is a stable isotope of carbon. (e) The preceding answer is correct. Other answers for this exercise are possible if a different element of isotope is chosen.
(a) Lithium-6 contains three protons, three neutrons, and three electrons. The isotope symbol is 6 Li or 3 6 Li . 3 6 Li . (b) 6 Li + or 3 6 Li + 3 6 Li +
(a) Iron, 26 protons, 24 electrons, and 32 neutrons; (b) iodine, 53 protons, 54 electrons, and 74 neutrons
(a) 3 protons, 3 electrons, 4 neutrons; (b) 52 protons, 52 electrons, 73 neutrons; (c) 47 protons, 47 electrons, 62 neutrons; (d) 7 protons, 7 electrons, 8 neutrons; (e) 15 protons, 15 electrons, 16 neutrons
Let us use neon as an example. Since there are three isotopes, there is no way to be sure to accurately predict the abundances to make the total of 20.18 amu average atomic mass. Let us guess that the abundances are 9% Ne-22, 91% Ne-20, and only a trace of Ne-21. The average mass would be 20.18 amu. Checking the nature’s mix of isotopes shows that the abundances are 90.48% Ne-20, 9.25% Ne-22, and 0.27% Ne-21, so our guessed amounts have to be slightly adjusted.
Turkey source: 20.3% (of 10.0129 amu isotope); US source: 19.1% (of 10.0129 amu isotope)
The symbol for the element oxygen, O, represents both the element and one atom of oxygen. A molecule of oxygen, O 2 , contains two oxygen atoms; the subscript 2 in the formula must be used to distinguish the diatomic molecule from two single oxygen atoms.
(a) molecular CO 2 , empirical CO 2 ; (b) molecular C 2 H 2 , empirical CH; (c) molecular C 2 H 4 , empirical CH 2 ; (d) molecular H 2 SO 4 , empirical H 2 SO 4
(a) C 4 H 5 N 2 O; (b) C 12 H 22 O 11 ; (c) HO; (d) CH 2 O; (e) C 3 H 4 O 3
(a) CH 2 O; (b) C 2 H 4 O
(a) ethanol
(b) methoxymethane, more commonly known as dimethyl ether
(c) These molecules have the same chemical composition (types and number of atoms) but different chemical structures. They are structural isomers.
Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams.
Formic acid. Its formula has twice as many oxygen atoms as the other two compounds (one each). Therefore, 0.60 mol of formic acid would be equivalent to 1.20 mol of a compound containing a single oxygen atom.
The two masses have the same numerical value, but the units are different: The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × × 10 23 molecules.
(a) 256.48 g/mol; (b) 72.150 g mol −1 ; (c) 378.103 g mol −1 ; (d) 58.080 g mol −1 ; (e) 180.158 g mol −1
(a) 197.382 g mol −1 ; (b) 257.163 g mol −1 ; (c) 194.193 g mol −1 ; (d) 60.056 g mol −1 ; (e) 306.464 g mol −1
(a) 0.819 g; (b) 307 g; (c) 0.23 g; (d) 1.235 × × 10 6 g (1235 kg); (e) 765 g
(a) 99.41 g; (b) 2.27 g; (c) 3.5 g; (d) 222 kg; (e) 160.1 g
(a) 9.60 g; (b) 19.2 g; (c) 28.8 g
zirconium: 2.038 × × 10 23 atoms; 30.87 g; silicon: 2.038 × × 10 23 atoms; 9.504 g; oxygen: 8.151 × × 10 23 atoms; 21.66 g
AlPO 4 : 1.000 mol or 26.98 g Al Al 2 Cl 6 : 1.994 mol or 53.74 g Al Al 2 S 3 : 3.00 mol or 80.94 g Al The Al 2 S 3 sample thus contains the greatest mass of Al.
3.113 × × 10 25 C atoms
0.865 servings, or about 1 serving.
20.0 g H 2 O represents the least number of molecules since it has the least number of moles.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-atoms-first-2e/pages/1-introduction
- Authors: Paul Flowers, Edward J. Neth, William R. Robinson, PhD, Klaus Theopold, Richard Langley
- Publisher/website: OpenStax
- Book title: Chemistry: Atoms First 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-atoms-first-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-atoms-first-2e/pages/chapter-2
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.15: Assignment—Atoms, Molecules, and Ions
- Last updated
- Save as PDF
- Page ID 232991
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
To download a copy of the assignment, please click on the link Sample Questions .
As you work these matter and measurement problems, consider and explain:
- What type of question is it?
- How do you know what type of question it is?
- What information are you looking for?
- What information do they give?
- How will you go about solving this?
- Show how to solve the problem.
- Be able to answer for a different reaction, number, set of conditions, etc.
Sample Questions
- NH 4 and NH 4 Cl
- ZnO 2 and ZnCl 2
- H 2 O and HCl
- NO and NO 2
- CH 4 and CO 2
- A sample of chemical X is found to contain 5.0 grams of oxygen, 10.0 grams of carbon, and 20.0 grams of nitrogen. The law of definite proportion would predict that a 70 gram sample of chemical X should contain how many grams of carbon?
- All atoms of the same element are identical.
- Compounds are combinations of different atoms.
- A chemical reaction changes the way atoms are grouped together.
- Atoms are indestructible.
- Who was the first scientist to show that atoms emit any negative particles?
- The Rutherford experiment proved the Thomson “plum-pudding” model of the atom to be essentially correct.
- The Rutherford experiment was useful in determining the nuclear charge on the atom.
- Millikan’s oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.
- The electric discharge tube proved that electrons have a negative charge.
- All of the above experiments gave the results described.
- An atom is mostly empty space.
- Almost all of the mass of the atom is concentrated in the nucleus.
- The protons and neutrons in the nucleus are very tightly packed.
- The number of protons and neutrons is always the same in the neutral atom.
- All of the above statements (A–D) are true.
- The element rhenium (Re) exists as two stable isotopes and 18 unstable isotopes. What does Rhenium-185 have in its nucleus?
- 20 protons and 20 neutrons
- 21 protons and 19 neutrons
- 22 neutrons and 18 protons
- 20 protons and 22 neutrons
- 21 protons and 20 neutrons
- There is twice as much mass of hydrogen as oxygen in each molecule.
- There are two hydrogen atoms and one oxygen atom per water molecule.
- There is twice as much mass of oxygen as hydrogen in each molecule.
- There are two oxygen atoms and one hydrogen atom per water molecule.
- None of these.
- K, alkali metal
- Ba, alkaline earth metal
- Ne, noble gas
- Ni, transition metal
- Calcium, Ca
- good conductors of heat
- often lustrous
- tend to gain electrons in chemical reactions
- cobalt(II) chloride
- magnesium oxide
- aluminum(III) oxide
- diphosphorus pentoxide
- All of the above names are correct
- iodine trichloride, ICl 3
- phosophorus pentoxide, P 2 O 5
- ammonia, NH 3
- sulfur hexafluoride, SF 6
- All of the above pairs are correct.
- How many oxygen atoms are there in one formula unit of Ca 2+ ?
- What is the correct name for FeO?
- What is the correct name for Ca 2+ ?
- What is the correct name for V 3+ ?
- What is the subscript of barium in the formula of barium sulfate?
- What is the formula for calcium bisulfate?
- Pb(NO 3 ) 2 , lead(II) nitrate
- NH 4 ClO 4 , ammonium perchlorate
- PO 4 3− , phosphate ion
- Mg(OH) 2 , magnesium hydroxide
- NO 3− , nitrite ion
[reveal-answer q=”279540″]Show Sample Answers[/reveal-answer] [hidden-answer a=”279540″]
- J. J. Thomson
- 75 protons, 110 neutrons
- I, IV and II, V
- 20 protons, 20 neutrons, and 18 electrons
- 19 p, 20 n, 18 e
- iron(II) oxide
- calcium ion
- vanadium(III) ion
- Ca(HSO 4 ) 2
[/hidden-answer]
- Authored by : Jessica Garber. Provided by : Tidewater Community College. License : CC BY: Attribution
Faculty Resources
Assignments.

The assignments in this course are openly licensed, and are available as-is, or can be modified to suit your students’ needs. Available answer keys will be provided to faculty who adopt Waymaker or OHM courses with paid support from Lumen Learning. This approach helps us protect the academic integrity of these materials by ensuring they are shared only with authorized and institution-affiliated faculty and staff.
If you import this course into your learning management system (Blackboard, Canvas, etc.), the assignments will automatically be loaded into the assignment tool.
You can view these faculty-contributed assignments below or throughout the course.
Discussions
The following discussion assignments will also be preloaded (into the discussion-board tool) in your learning management system if you import the course. They can be used as is, modified, or removed. You can preview them below:
Optional Reading-Based Discussions
If you wish, you can include the following prompt to encourage students to complete their reading and discuss class topics with their peers.
Discussion Board Standards
It is expected that students will complete the assigned textbook readings prior to posting responses to the discussion board. Student interaction is encouraged. The goal of these online discussions is to simulate the sort of group discussion that can occur in traditional classroom settings. Therefore, students must post comments to at least two classmates. In addition, the instructor may post their reaction to student comments.
The instructor will assess the quality of student contributions towards group discussion and determine a grade for each unit/chapter.
- Student discussion must be relevant to the specific question being discussed.
- Students should demonstrate their understanding of the issues, theories, and problems from their textbook readings and homework. Good student commentary will make reference to specific textbook readings and make use of the terminology introduced in the chapter.
- Students should respond to the discussion questions with an attitude of proper objectivity and a willingness to discuss matters with others who do not share their viewpoint. Criticism of theories or ideas is appropriate; however, the tone of this criticism should remain scholarly rather than personal.
- Students are encouraged to make use of examples and counter-examples, compare and contrast theories, make reference to past learning, indicate problems or difficulties they have with the theories, and draw out the relevant implications of the discussion.
- Students may also raise questions they have about the readings and discuss possible answers provided these questions are relevant to the topic of the discussion.
Consider the discussions as opportunities to share ideas about this exciting material with your classmates—enjoy this!
- Assignments. Provided by : Lumen Learning. License : CC BY: Attribution
- Reading-Based Discussions. Authored by : Shawn Shields. Provided by : Germanna Community College. License : CC BY: Attribution
- Pencil Cup. Authored by : IconfactoryTeam. Provided by : Noun Project. Located at : https://thenounproject.com/term/pencil-cup/628840/ . License : CC BY: Attribution
Chemistry 10th Edition Whitten, Kenneth W.; Davis, Raymond E.; Peck, Larry; Stanley, George G.
Chemistry (12th edition) wilbraham, chemistry 12th edition chang, raymond; goldsby, kenneth, chemistry (4th edition) burdge, julia, chemistry (7th edition) mcmurry, john e.; fay, robert c.; robinson, jill kirsten, chemistry 9th edition zumdahl, steven s.; zumdahl, susan a., chemistry: a molecular approach (3rd edition) tro, nivaldo j., chemistry: an atoms-focused approach gilbert, thomas r.; kirss, rein v.; foster, natalie, chemistry and chemical reactivity (9th edition) kotz, john c.; treichel, paul m.; townsend, john r.; treichel, david a., chemistry: an introduction to general, organic, and biological chemistry (12th edition) timberlake, karen c., chemistry: atoms first (2nd edition) zumdahl, steven s.; zumdahl, susan a., chemistry: molecular approach (4th edition) tro, nivaldo j., chemistry: the molecular science (5th edition) moore, john w.; stanitski, conrad l., chemistry: principles and practice (3rd edition) reger, daniel l.; goode, scott r.; ball, david w., chemistry: the central science (13th edition) brown, theodore e.; lemay, h. eugene; bursten, bruce e.; murphy, catherine; woodward, patrick; stoltzfus, matthew e., chemistry: the molecular nature of matter and change 7th edition silberberg, martin; amateis, patricia, chemistry: the science in context (4th edition) gilbert, thomas r.; kirss, rein v., general chemistry (4th edition) mcquarrie, donald a., general chemistry 10th edition ebbing, darrell; gammon, steven d., general chemistry: principles and modern applications (10th edition) petrucci, ralph h.; herring, f. geoffrey; madura, jeffry d.; bissonnette, carey, general, organic, and biological chemistry: structures of life (5th edition) timberlake, karen c., general, organic, & biological chemistry 3rd edition smith, janice, introductory chemistry (5th edition) tro, nivaldo j., organic chemistry, 5th edition smith, janice, organic chemistry (8th edition) wade jr., l. g., organic chemistry 9th edition mcmurry, john e., organic chemistry as a second language, 3e: first semester topics klein, david, physical chemistry: thermodynamics, structure, and change atkins, peter; de paula, julio.
Please ensure that your password is at least 8 characters and contains each of the following:
- a special character: @$#!%*?&
- Book A Demo

Homework & Practice
Aktiv Chemistry is a comprehensive online homework platform that helps students build mastery of chemistry. Take advantage of over 15,000 built-in chemistry problems, scaffolded practice, a powerful instructor dashboard, LMS grade syncing, and much more.

All-in-one platform
Comprehensive learning & assessment.
From in-class active learning, homework assignments, and now secure online quizzes and exams, Aktiv Chemistry’s all-in-one platform provides a comprehensive set of features for formative and summative assessments.
In-class or Synchronous Online
Take attendance, post polls & quizzes, enhance traditional worksheets, or promote think-pair-share activities. Learn More
Assign interactive problem sets with instant feedback and automatic grading that sync with institutional LMS gradebooks.

Timed Online Quizzes & Exams
Create summative assessments with question pools, algorithmic problems, and optional integrated proctoring. Learn More
15,000 PROBLEMS OR WRITE YOUR OWN
Extensive content library.
Building engaging classroom activities has never been easier. Aktiv Chemistry provides an extensive library of questions for chemistry courses such as General Chemistry, Introductory Chemistry, GOB Chemistry, Organic Chemistry. The content library includes discipline-specific problem types such as molecule drawing, chemical equations and naming, dimensional analysis, numerical entry, and more.
Easy and flexible authoring also allows instructors to add their own questions.
Learn More About
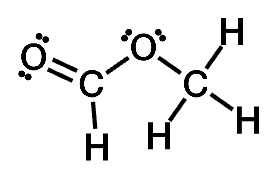
Draw the Lewis structure of (CHO)OCH₃ and then choose the appropriate set of molecular geometries of the three central atoms. Your answer choice is independent of the orientation of your drawn structure.
The solubility of He in water at 25.0 °C is 7.0 × 10⁻⁵ M when the partial pressure of He is 0.20 atm. What is the value of the Henry's law constant for He ?
- monosaccharide
- oligosaccharide
- monopolysaccharide
- heteropolysaccharide
- homopolysaccharide
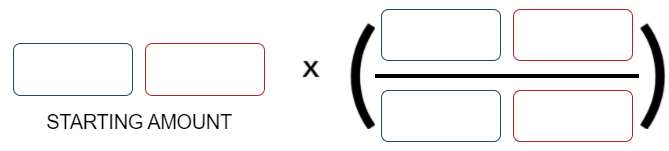
According to the balanced reaction below, calculate the moles of NH₃ that form when 4.2 mol of N₂H₄ completely reacts 3 N₂H₄(l) → 4 NH₃(g) + N₂(g)
AFFORDABLE & FLEXIBLE
Built to work with any textbook.
Aktiv Chemistry ’s textbook independent approach means instructors have more flexibility for their courses. Build assignments that align to any major publisher textbook or increasingly popular OER options.
For General Chemistry courses, Aktiv Chemistry also offers seamless integration with the OpenStax Chemistry textbooks.
Student Success
More chemistry, less technology.
Drawing chemical structures, entering subscripts and superscripts, and working with significant figures just shouldn’t be hard. That’s why Aktiv Chemistry was designed from the ground up with the student experience in mind. With intuitive user interfaces and scaffolded and visual modules, our goal is to use technology to lower the barrier to chemistry.
Bring Your Own Device. Any Device.
Students and instructors can access the Aktiv Learning app from any iPhone, iPad, or Android Device. Additionally, the platform is fully accessible on Web with any Mac, PC, or Chromebook.
Aktiv Chemistry ’s mobile-first design ensures that students receive the same experience no matter where they are. Students take advantage of the Aktiv Learning mobile app to work and study on-the-go in places like riding the bus or train, or even on campus when they have downtime.
RANDOMIZATION WITH
Algorithmic problems & question pools.
Aktiv Chemistry offers additional security with both Question Pools and Algorithmic Problems. These features randomize the content that is delivered to each student during homeworks, quizzes, or exams. Instructors can also randomize the order of questions on any assignment.
Built-in variables create thousands of question variants to be delivered that randomize numbers, words, or compounds within the problem statement.
Group together a set of similar questions and deliver a subset of them at random to students.
Problem Pool 1
14 problems in this pool
- Problems to Assign
What is the correct IUPAC name for HBrO(aq)?
What is the correct IUPAC name for Ca(BrO₂)₂?
What is the correct IUPAC name for Ti(ClO₄)₄?
Success Story
Professors from over 700 colleges and universities use Aktiv Chemistry to engage students inside and outside of the classroom. Learn how some of them have transformed their courses.

Helping Students Learn, Interact, and Visualize Chemistry in New Ways

¹ This quote was provided at a time where Aktiv Chemistry was named Chem101. We have replaced the use of Chem101 in any direct quotes with Aktiv Chemistry to minimize confusion.
DESIGNED TO
Support your course, flexible grading policies.
Every Aktiv Chemistry activity has a multitude of grading policies that can be customized depending on the assignment type. Settings include points per problem, participation credit, late submissions with penalties, variable attempts per problem, penalties for incorrect attempts, and more. The Aktiv Chemistry gradebook can be set up to display summary columns or individual columns depending on the assignment type as well.
Industry-leading Support
Any course using Aktiv Chemistry comes with our industry-leading support featuring average response times of 14 minutes and extended hours that include late nights and weekends. Additionally, every Aktiv Chemistry instructor works with an individual member of our Success team that helps with initial course set up, gradebook/LMS integrations, and recommendations for best practices.
LMS Integrated
Aktiv Chemistry’s gradebook can be synced with any popular campus LMS such as Canvas, Blackboard, Moodle, or D2L. The gradebook and associated columns can be customized to separate the various assignment types, to present individual assignments, or display summary columns for each type.
Speak to a Specialist
One of our Learning Specialists will give you a tour of the Aktiv Chemistry or Aktiv Mathematics learning platforms and provide a free instructor playground account with access to the content library.
I Want to Learn More About:
Chemistry 30
Assignments & unit exams, other links.
This page provides links to answer keys for all assignments in this resource, as well as a unit exam for each unit with answer key.
Collapse All | Expand All
Switch content script available from Dynamic Drive
- How it works
- Homework answers

Chemistry Answers
Questions: 44 357
Answers by our Experts: 42 759
Ask Your question
Need a fast expert's response?
and get a quick answer at the best price
for any assignment or question with DETAILED EXPLANATIONS !
Find what you need to succeed.
- Our Mission
- Our Leadership
- Learning Science
- Macmillan Learning AI
- Sustainability
- Diversity, Equity, and Inclusion
- Accessibility
- Astronomy Biochemistry Biology Chemistry College Success Communication Economics Electrical Engineering English Environmental Science Geography Geology History Mathematics Music & Theater Nutrition and Health Philosophy & Religion Physics Psychology Sociology Statistics Value
- Digital Offerings
- Inclusive Access
- Lab Solutions
- LMS Integration
- Curriculum Solutions
- Training and Demos
- First Day of Class
- Administrators
- Affordable Solutions
- Badging & Certification
- iClicker and Your Content
- Student Store
- News & Media
- Contact Us & FAQs
- Find Your Rep
- Booksellers
- Macmillan International Support
- International Translation Rights
- Request Permissions
- Report Piracy
Achieve for Chemistry
NEW Pilot Feature: Achieve’s AI-powered Tutor provides next-level support for students. Learn more by contacting your rep.
Meeting students where they are, with content and a learning platform designed for the way they learn. Achieve is a comprehensive set of interconnected teaching and assessment tools. It incorporates the most effective elements from Macmillan’s market leading solutions—in a single, easy-to-use platform. Our resources were co-designed with instructors and students, using a foundation of learning research and rigorous testing. The resulting tools for before, during, and after class are proven to be engaging to students of all levels of preparedness, leading to better outcomes.
Sign into Achieve Schedule an Achieve Demo
Request Access to Achieve | See All Chemistry Titles
Introducing a New Pilot Feature: AI-powered Tutor!
Contact your local rep to learn how to use the new AI-powered Tutor this fall and transform study sessions into engaging deep-learning experiences. Explore how Macmillan Learning is using AI to enhance education.
Contact Your Rep
Achieve is a fully mobile, accessible, flexible, modular system to help you deploy and manage all your pre-class, in-class, and post-class curriculum in one place (and integrate it with your LMS if preferred) while gathering insights from it all in a simple and powerful interface.
Watch: What is Achieve?

Diagnostics with Personalized Study Plan
Personalized study plans provides instructional resources that target each student's identified growth areas.

Insightful Reports
Instructor reports provide insights into performance by full class, individual students and learning objectives.

Worked Example Videos
Worked example videos created exclusively for IGC from popular YouTube educator, Tyler DeWitt, make chemistry concepts clear and understandable through the effective use of whiteboard videos.

Interactives
In-line interactives simulations provide students with a way to interact with the material in a hands-on way, promoting the idea of learning by doing. Acclaimed PhET Simulations from the University of Colorado, Boulder further encourage quantitative exploration, while addressing specific problem-solving needs.

Homework Problems
Homework problems serve as a front door to learning. Students can either answer a question immediately or study further before answering by clicking on the Resources tab to go directly to the appropriate section in the e-book. Each problem provides help in the form of hints, targeted feedback and detailed solutions to ensure every problem provides learning through practice.

Insight Cards
Insight cards provide quick views of student work in a just-in-time way keeping what is important and current right at the surface! You can drill down for more detailed views to see individual student or whole class performance.

Instructor Activity Guides
The IGC instructor activity guides were built around the Interactive General Chemistry text and interactives. Each activity guide provides at-a-glance metadata to make it easier to prepare and implement. Instructors can pair "preflection" assignments with follow-up reflection supported by pre-built clicker slide decks and/or reinforce student learning with post-activity homework assignments to follow up on the in-class activity. These flexible resources support a variety of teaching styles all emphasizing student engagement.

Interactives & Worksheets
IGC worksheets were designed to work seamlessly with a set of Interactive General Chemistry interactives. The worksheets can be used for in-class or virtual group work or individual work outside of class.

iClicker Integration
Prebuilt iClicker slide desks assist with instruction and help to easily facilitate reflection on activities, allowing instructors to check understanding and get feedback in real time. In addition, iClicker seamlessly integrates into the Achieve gradebook.
Using Achieve & iClicker
Testimonial from Stephanie Katz
Transitioning from Sapling to Achieve
Stephanie Katz, Chemistry Professor from Villanova University shares her experience moving from Sapling to Achieve for a summer session of general Chemistry using Interactive General Chemistry in Achieve.
See How Achieve Works
Have one of our experts show you how Achieve works—and how it can work for you, your class, and your students.
- Introductory Chemistry
- General Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Analytical Chemistry
- General, Organic, and Biochemistry
See Available Chemistry Titles
Already using Achieve or Achieve Read & Practice?
Don't have an account? Create Account
Get Achieve maintenance updates. Check System Status
Instructors
Schedule a Grand Tour
See Achieve Titles
Get Achieve Support
Achieve Read & Practice
Schedule a Read & Practice Tour
See Read & Practice Titles
Get Read & Practice Support
Achieve Essentials
Schedule an Essentials Tour
See Essentials Titles
Get Essentials Support
Access Your Achieve Course
Purchase Read & Practice
Access Your Essentials Course

COMMENTS
A 24/7 free Chemistry homework AI tutor that instantly provides personalized step-by-step guidance, explanations, and examples for any Chemistry homework problem. Improve your grades with our AI homework helper! ... Get all your Chemistry assignments done with helpful answers in 10 seconds or less. No more asking friends for Chemistry help.
This AI tool helps answer chemistry questions and solve chemistry problems. HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.
HIX Tutor's AI chemistry homework helper is your reliable partner for assignment help and exam preparation. Try it now and experience a new standard in AI-powered education. Check out HIX Tutor's AI-powered chemistry problem solver. Effortlessly solve chemistry problems, understand complex reactions, and improve learning with step-by-step ...
Why choose Chemistry AI Homework Solver? Save Time: With our solver, you can complete your assignments in a fraction of the time it would take you to solve them manually. Accuracy: Smodin's Chemistry homework solver provides accurate solutions every time, eliminating the risk of errors and mistakes. Boost your Grades: You can enhance your school success by guaranteeing the precision and ...
Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Our resource for Pearson Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can take the guesswork out of studying and move forward with confidence. Find step-by-step solutions and answers to Pearson ...
13. (a) Carbon-12, 12 C; (b) This atom contains six protons and six neutrons. There are six electrons in a neutral 12 C atom. The net charge of such a neutral atom is zero, and the mass number is 12. (c) The preceding answers are correct. (d) The atom will be stable since C-12 is a stable isotope of carbon.
It's easier to figure out tough problems faster using Chegg Study. Unlike static PDF Mastering Chemistry for Chemistry 11th Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. No need to wait for office hours or assignments to be graded to find out where you took a wrong turn.
CH8. Problem. 1PE. Step-by-step solution. Step 1 of 2. The energy required to completely separate one mole of a solid ionic compound into gaseous ions is called lattice energy. The energy requires to separate the ions is given by Coulomb's law of electrostatic interaction. The equation of Coulomb's law is as follows:
ZnO 2 and ZnCl 2. H 2 O and HCl. NO and NO 2. CH 4 and CO 2. A sample of chemical X is found to contain 5.0 grams of oxygen, 10.0 grams of carbon, and 20.0. grams of nitrogen. The law of definite proportion would predict that a 70 gram sample of chemical.
Find step-by-step solutions and answers to Chemistry 11 - 9780176510381, as well as thousands of textbooks so you can move forward with confidence. ... Now, with expert-verified solutions from Chemistry 11 1st Edition, you'll learn how to solve your toughest homework problems. Our resource for Chemistry 11 includes answers to chapter ...
It's easier to figure out tough problems faster using Chegg Study. Unlike static PDF Pearson Chemistry 1st Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. No need to wait for office hours or assignments to be graded to find out where you took a wrong turn.
Exercise 3. Exercise 4. Exercise 5. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Chemistry: Matter and Change 1st Edition, you'll learn how to solve your toughest homework problems.
Module 19: Transition Metals and Coordination Chemistry: Assignment: Organic Chemistry Practice: Module 20: Organic Chemistry: Assignment: Nuclear Chemistry: ... Students may also raise questions they have about the readings and discuss possible answers provided these questions are relevant to the topic of the discussion.
Home Textbook Answers Science Chemistry Find Textbook Answers and Solutions. Browse ... Chemistry 10th Edition Whitten, Kenneth W.; Davis, Raymond E.; Peck, Larry; Stanley, George G. Publisher Brooks/Cole Publishing Co. ISBN 978-1-13361-066-3. Chemistry (12th Edition) Wilbraham Publisher
Free math problem solver answers your chemistry homework questions with step-by-step explanations. Mathway. Visit Mathway on the web. Start 7-day free trial on the app ... Download free on Amazon. Download free in Windows Store. get Go. Chemistry. Basic Math. Pre-Algebra. Algebra. Trigonometry. Precalculus. Calculus. Statistics. Finite Math ...
The Aktiv Chemistry gradebook can be set up to display summary columns or individual columns depending on the assignment type as well. Industry-leading Support Any course using Aktiv Chemistry comes with our industry-leading support featuring average response times of 14 minutes and extended hours that include late nights and weekends.
1 Intro to Energy Changes in Chemical Reactions. Word. RTF. PDF. 2 Energy Changes during Chemical Reactions. Word. RTF. PDF. 3 Calculating Heats of Reaction.
ALL Answered. Question #351043. Organic Chemistry. Draw a galvanic cell based on the net ionic equation for the reaction below and. complete the following: a) Label the anode and cathode. b) Write the oxidation and reduction half reactions for the cell. c) Indicate the electrode materials, and electrolyte components.
14th Edition • ISBN: 9780136873891 Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. 7,730 solutions. Get your Pearson Chemistry homework done with Quizlet! Browse through thousands of step-by-step solutions to end-of-chapter questions from the most popular Pearson Chemistry ...
Achieve for Chemistry. Meeting students where they are, with content and a learning platform designed for the way they learn. Achieve is a comprehensive set of interconnected teaching and assessment tools. It incorporates the most effective elements from Macmillan's market leading solutions—in a single, easy-to-use platform.
Chemistry 11 Stoichiometry Review Assignment Name: _____ Date:_____ Block: _____ Answer the following practice questions on a separate page Define the following terms: 1. Stoichiometry: quantitative relationships among substances as they participate in chemical reactions 2.
Select the Edition for Pearson Chemistry Below: Edition Name. HW Solutions. Pearson Chemistry 0th Edition by Dennis D Staley, Antony C Wilbraham, Edward L Waterman, Michael S Matta, Prentice Hall. 3285. Pearson Chemistry 0th Edition by Edward Waterman, Dennis D. Staley. 3280.