Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Research Highlight
- Published: 16 February 2021

Elevated risk of COVID-19 in people with dementia
- Heather Wood 1
Nature Reviews Neurology volume 17 , page 194 ( 2021 ) Cite this article
2231 Accesses
4 Citations
12 Altmetric
Metrics details
- Alzheimer's disease
Individuals with dementia have an increased risk of contracting and experiencing a poor outcome from COVID-19, according to new research published in Alzheimer’s & Dementia . The findings underline the need to develop strategies to protect patients with dementia from SARS-CoV-2 infection while avoiding the potentially detrimental effects of social isolation.
“Previous studies by others showed that the altered blood–brain barrier in people with Alzheimer disease (AD) predisposes them to viral and bacterial infections,” explains corresponding author Rong Xu. “In addition, the memory impairment associated with dementia might interfere with the patient’s ability to adhere to preventive measures for COVID-19, such as social distancing, mask wearing and hand sanitizing.”
Xu and colleagues analysed de-identified, population-level electronic health record data from 61,916,260 individuals in the USA aged ≥ 18 years, 1,064,960 of whom were recorded as having dementia. The researchers used these data to examine the impact of dementia on the risk of developing COVID-19, and also on the probability of hospitalization and death as a result of the condition.
The analysis showed that people with dementia had a twofold increased risk of contracting COVID-19. The odds were highest in people with vascular dementia (adjusted OR (AOR) 3.17), followed by presenile dementia (AOR 2.62) and AD (AOR 1.86). In addition, dementia was associated with a greater likelihood of hospitalization and death as a consequence of COVID-19. The strong link between COVID-19 and vascular dementia indicates a possible role for pre-existing cerebrovascular pathology in SARS-CoV-2 infection.
The researchers also found that, among people with dementia, Black individuals had a higher risk of COVID-19 than white individuals, and were more likely to be hospitalized or die from the condition. These findings are consistent with COVID-19-related racial disparities that have been observed in the wider population.
Xu and colleagues noted that people with dementia often have comorbidities that are risk factors for COVID-19, such as hypertension, cardiovascular disease, obesity or type 2 diabetes. Moreover, many patients with dementia reside in nursing homes, which tend to be hotspots for SARS-CoV-2 infection. However, the correlations between COVID-19 and dementia remained statistically significant after the researchers had controlled for these potential confounding factors.
The analysis showed that people with dementia had a twofold increased risk of contracting COVID-19
“Prior evidence indicates a bidirectional relationship between viral infections and dementia, whereby people with dementia have an increased risk of viral infection, and a poor immune response to infection places individuals at an increased risk of dementia,” comments Xu. “Further research is warranted to understand the underlying mechanisms — both biological and socioeconomic — of the increased risk of COVID-19 in patients with dementia, and to determine whether SARS-CoV-2 infection accelerates cognitive decline or triggers dementia.”
Original article
Wang, Q. et al. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. https://doi.org/10.1002/alz.12296 (2021)
Article PubMed Google Scholar
Related articles
Numbers, K. & Brodaty, H. The effects of the COVID-19 pandemic on people with dementia. Nat. Rev. Neurol. 17 , 69–70 (2021)
Article CAS Google Scholar
Nolen, L. & Mejia, N. I. Inequities in neurology amplified by the COVID-19 pandemic. Nat. Rev. Neurol. 17 , 67–68 (2021)
Download references
Author information
Authors and affiliations.
Nature Reviews Neurology http://www.nature.com/nrneurol/
Heather Wood
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Heather Wood .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Wood, H. Elevated risk of COVID-19 in people with dementia. Nat Rev Neurol 17 , 194 (2021). https://doi.org/10.1038/s41582-021-00473-0
Download citation
Published : 16 February 2021
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41582-021-00473-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Health impact of the first and second wave of covid-19 and related restrictive measures among nursing home residents: a scoping review.
- Marjolein E. A. Verbiest
- Annerieke Stoop
- Katrien G. Luijkx
BMC Health Services Research (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Hospital Medicine Virtual Journal Club
Clinical Presentation of COVID19 in Dementia Patients.
Link to article at PubMed
J Nutr Health Aging. 2020 May 15;:1-3
Authors: Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, Bianchetti L, Trabucchi M
Abstract Objective: No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection. Design: Retrospective study. Setting: COVID wards in Acute Hospital in Brescia province, Northern Italy. Participants: We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia. Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results: Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09-3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status. Conclusion: The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
PMID: 32425646 [PubMed - as supplied by publisher]
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Mastodon (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
Leave a Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of new posts by email.
Dementia Clinical Care in Relation to COVID-19
Affiliations.
- 1 Medicine and Rehabilitation Department, Istituto Clinico S.Anna Hospital, Gruppo San Donato, Brescia, Italy.
- 2 Italian Association of Psychogeriatric (Associazione Italiana di Psicogeriatria -AIP), Brescia, Italy.
- 3 Geriatric Department, Fondazione Poliambulanza Istituto Ospedaliero Hospital, Brescia, Italy.
- 4 Geriatric Rehabilitation Unit, Anni Azzurri, Rezzato, Brescia, Italy.
- 5 Geriatric Research Group, Brescia, Italy.
- PMID: 35221646
- PMCID: PMC8863507
- DOI: 10.1007/s11940-022-00706-7
Purpose of review: This review discusses the complex relationship between COVID-19 and dementia and how the pandemic has affected the management of patients with dementia. This population resulted particularly susceptible to SARS-CoV-2 infection and its effects and also to the negative effects of the measures taken worldwide to control the spread of the virus.
Recent findings: Patients with dementia were at increased risk for COVID-19 compared to patients without dementia, and diagnosis of dementia represents an independent risk factor for hospitalization in COVID-19 patients. Mortality due to SARS-CoV2 infection in subjects with dementia is 2-5 times higher than in the general population. Cognitive impairment and delirium have been described in COVID-19 survivors. SARS-COV2 pandemic exacerbates the vulnerability of dementia patients and their caregivers, due to the morbidity and mortality from COVID-19, the indirect effects of the pandemic on the social supports, and the effects on healthcare system on which they depend.
Summary: The COVID-19 pandemic requires people with dementia to move from traditional models of health care to innovative models for home care, to support caregivers' burden, and to improve long term care.
Keywords: Alzheimer’s disease; COVID-19; Dementia; Frailty; Long-term care.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature 2022.
Publication types
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,954,836 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
Clinical Presentation of COVID19 in Dementia Patients.
Author information, affiliations.
- Bianchetti A 1
ORCIDs linked to this article
- BIANCHETTI A | 0000-0002-2914-0627
The Journal of Nutrition, Health & Aging , 01 Jan 2020 , 24(6): 560-562 https://doi.org/10.1007/s12603-020-1389-1 PMID: 32510106 PMCID: PMC7227170
Abstract
Participants, measurements, free full text .

Clinical Presentation of COVID19 in Dementia Patients
Angelo bianchetti.
1 Department Medicine and Rehabilitation, Istituto Clinico S.Anna Hospital, via del Franzone 31, 25122 Brescia, Italy
4 Italian Association of Psychogeriatrics, Rome, Italy
2 Geriatric Department, Fondazione Poliambulanza Istituto Ospedaliero Hospital, Brescia, Italy
S. Boffelli
L. bianchetti.
3 Geriatric Reahabilitation Unit, Anni Azzurri, Rezzato, Brescia, Italy
M. Trabucchi
No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection.
Retrospective study.
COVID wards in Acute Hospital in Brescia province, Northern Italy.
We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia.
Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status.
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
Introduction
In Italy, SARS-CoV-2 outbreak was catastrophic with 135,586 confirmed cases and 17,127 deaths at April, 8th ( 1 ). In clinical series of patients who died of COVID-19 comorbidities (especially hypertension, cardiac ischemic disease, diabetes and obesity) were identified as significant risk factors for mortality, while dementia was described as a comorbid condition in only 6.8% of COVID-19 patients ( 2 ).
Although dementia is known to be an important mortality risk factor among older people, so far there are no studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19 ( 3 , 4 ).
In the Province of Brescia, an administrative district in eastern Lombardy home to 1.2 million people, between February 22nd and April 8th, 9,900 cases of Covid-19 have been diagnosed and 1,800 deaths have been reported. About 53% (2265 out of 4200) of hospital beds have been dedicated to treat patients affected by Covid-19 pneumonia. Specific units were created to cater to these patients: acute medical units, named COVID Wards, and intensive care units, with the last accounting fort the 8.5% of all the beds dedicated to COVID-19 patients.
Methods and study population
During this period, 627 patients diagnosed with COVID-19 pneumonia were admitted to our hospitals. All patients admitted to COVID Wards were positive to RT-PCR for SARS-Cov-2 conducted on a nasopharyngeal specimen and presented respiratory failure. Each patient underwent a thorough medical evaluation and, if over 65, a geriatric multi-dimensional assessment, comprehensive of evaluation of cognitive and functional status and presence of delirium.
Dementia was diagosed according to clinical history and results of the cognitive assessment. The modalities of onset of the COVID-19 infection, the symptoms of presentation at the hospital emergency department and the outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mean age of patients diagnosed with dementia was 82.6 (SD 5.3; IQR 80–86), versus 68.9 (SD 12.7; IQR 60–68) in patients not affected by dementia (p<0.001; Student’s t test). Females were 47 (57.3%) among patients with dementia and 288 (52.8%) among patients not diagnosed with dementia, respectively.
The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). (Table (Table1 1 )
Characteristics of 627 patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals according to the diagnosis of dementia
* Pearson’s chi-squared test; ** Student’s t-test
The Clinical Dementia Rating Scale (CDR) ( 5 ) was used to determine the severity of dementia: 36 patients (43.4%) were classified in stage 1, 15 (18.3%) in stage 2 and 31 (37.8%) in stage 3. The Mortality rates were, respectively, 41.7%, 66.7%, and 83.9% (p<0.001, one-way ANOVA). (table (table2 2 )
Characteristics of 627 patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals according to CDR classification
* one-way ANOVA
To assess if the diagnosis of dementia was associated with a worse outcome regardless of age and sex, we built a logistic regression model. According to this model age, and the diagnosis of dementia resulted independently associated with a higher mortality. For every increased year of age, the Odds Ratio (OR) for mortality was 1.09 (95% CI: 1.07–1.12, p<0.001), and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). According to this model sex was not associated with a change in mortality risk. (Table (Table3 3 )
Binary Logistic Regression Model for mortality by Age, Sex and Dementia
* Wald Test for Analysis of Variance
As shown in table table4, 4 , among patients diagnosed with dementia the most frequent symptoms of onset were delirium (67%, especially in the hypoactive form, 50%) and worsening of the functional status. The classic symptoms of COVID-19 infection were less frequent: only 47% of patients had fever, 44% dyspnea and 14% cough.
Symptoms at ER admission among 82 dementia patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals
Conclusions
Caring for patients with dementia during the current pandemic is a complex task, involving the management of patients in different settings. Some patients need to be treated at home, often with caregivers burdened by isolation due to lockdown measures and by limitation of home services. Other patients are cared in nursing homes, which often lack adequate and trained staffs and access to personal protective equipment. Hospital patient’s management has been difficult due to the scarce collaboration offered by the patient and difficulties in communication, immobility, and limited availability of trained staff members ( 6 ). There are also ethical concerns regarding hospitalization of patients with dementia due to resource constraints during the current pandemic ( 7 ).
To our knowledge, the proportion of subjects with dementia among patients admitted to an acute hospital for COVID-19 has never been evaluated. The prevalence of demented patient found in the present study (13.1%) is lower than the previous estimates of the prevalence of dementia in hospital, which vary from 15% to 42% ( 7 ). According to our data, the diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. We suggest that the onset of hypoactive delirium and worsening functional status in people with dementia may be considered a sign of possible COVID-19 infection during this epidemic. Early recognition of COVID-19 in demented people can help provide timely treatment and adequate isolation. Hospitals should develop integrated care models, create Special Care Geriatric COVID units and promote guidelines to ensure the better possible treatment for frail older persons.
Acknowledgement
Anita Chizzoli, MD; Marzia Cristo, MD; Silvia Comini, MD; Assunta Di Stasio, MD and Antonella Ricci, MD for the support in clinical evaluation of patients.
Funding: No funding.
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: This is a review study; the protocol was approved by the institutional committee.
All the authors contributed equally to the drafting of this manuscript.
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12603-020-1389-1
Citations & impact
Impact metrics, citations of article over time, smart citations by scite.ai smart citations by scite.ai include citation statements extracted from the full text of the citing article. the number of the statements may be higher than the number of citations provided by europepmc if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1007/s12603-020-1389-1, article citations, a longitudinal cohort study on the use of health and care services by older adults living at home with/without dementia before and during the covid-19 pandemic: the hunt study..
Ibsen TL , Strand BH , Bergh S , Livingston G , Lurås H , Mamelund SE , Voshaar RO , Rokstad AMM , Thingstad P , Gerritsen D , Selbæk G
BMC Health Serv Res , 24(1):485, 19 Apr 2024
Cited by: 0 articles | PMID: 38641570
COVID-19 and Alzheimer's disease: Impact of lockdown and other restrictive measures during the COVID-19 pandemic.
Nawaz AD , Haider MZ , Akhtar S
Biomol Biomed , 24(2):219-229, 11 Mar 2024
Cited by: 0 articles | PMID: 38078809 | PMCID: PMC10950341
New score to predict COVID-19 progression in vaccine and early treatment era: the COVID-19 Sardinian Progression Score (CSPS).
De Vito A , Saderi L , Colpani A , Puci MV , Zauli B , Fiore V , Fois M , Meloni MC , Bitti A , Moi G , Maida I , Babudieri S , Sotgiu G , Madeddu G
Eur J Med Res , 29(1):123, 15 Feb 2024
Cited by: 0 articles | PMID: 38360688 | PMCID: PMC10868088
Investigating the Potential Shared Molecular Mechanisms between COVID-19 and Alzheimer's Disease via Transcriptomic Analysis.
Fan Y , Liu X , Guan F , Hang X , He X , Jin J
Viruses , 16(1):100, 09 Jan 2024
Cited by: 0 articles | PMID: 38257800 | PMCID: PMC10821526
COVID-19 and Alzheimer's Disease Share Common Neurological and Ophthalmological Manifestations: A Bidirectional Risk in the Post-Pandemic Future.
Amadoro G , Latina V , Stigliano E , Micera A
Cells , 12(22):2601, 10 Nov 2023
Cited by: 0 articles | PMID: 37998336 | PMCID: PMC10670749
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
- http://www.ebi.ac.uk/biostudies/studies/S-EPMC7227170?xr=true
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital.
Ticinesi A , Cerundolo N , Parise A , Nouvenne A , Prati B , Guerra A , Lauretani F , Maggio M , Meschi T
Aging Clin Exp Res , 32(10):2159-2166, 18 Sep 2020
Cited by: 56 articles | PMID: 32946031 | PMCID: PMC7498987
Effectiveness of Streptococcus Pneumoniae Urinary Antigen Testing in Decreasing Mortality of COVID-19 Co-Infected Patients: A Clinical Investigation.
Desai A , Santonocito OG , Caltagirone G , Kogan M , Ghetti F , Donadoni I , Porro F , Savevski V , Poretti D , Ciccarelli M , Martinelli Boneschi F , Voza A
Medicina (Kaunas) , 56(11):E572, 29 Oct 2020
Cited by: 6 articles | PMID: 33138045 | PMCID: PMC7693839
Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy.
Inciardi RM , Adamo M , Lupi L , Cani DS , Di Pasquale M , Tomasoni D , Italia L , Zaccone G , Tedino C , Fabbricatore D , Curnis A , Faggiano P , Gorga E , Lombardi CM , Milesi G , Vizzardi E , Volpini M , Nodari S , Specchia C , [...] Metra M
Eur Heart J , 41(19):1821-1829, 01 May 2020
Cited by: 296 articles | PMID: 32383763 | PMCID: PMC7239204
Delirium Assessment in Critically Ill Older Adults: Considerations During the COVID-19 Pandemic.
Duggan MC , Van J , Ely EW
Crit Care Clin , 37(1):175-190, 14 Aug 2020
Cited by: 12 articles | PMID: 33190768 | PMCID: PMC7427547
Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy.
Toniati P , Piva S , Cattalini M , Garrafa E , Regola F , Castelli F , Franceschini F , Airò P , Bazzani C , Beindorf EA , Berlendis M , Bezzi M , Bossini N , Castellano M , Cattaneo S , Cavazzana I , Contessi GB , Crippa M , Delbarba A , [...] Latronico N
Autoimmun Rev , 19(7):102568, 03 May 2020
Cited by: 473 articles | PMID: 32376398 | PMCID: PMC7252115
Europe PMC is part of the ELIXIR infrastructure
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Covid 19: a fork in...
Dementia patients: a vulnerable population during the COVID-19 Pandemic
Rapid response to:
Covid 19: a fork in the road for general practice
Read our latest coverage of the coronavirus outbreak.
- Related content
- Article metrics
- Rapid responses
Rapid Response:
Dear editor, We are facing extraordinarily challenging times with a profound impact on the core competencies of primary care.(4) The novel coronavirus SARS-CoV-2 (COVID-19) has infected nearly 42.512.186 million people and has caused over 1.147.301 deaths worldwide.(1) The high risk of infection in the workplace catapulted healthcare professionals into a new reality; from the classic outpatient visit they were obliged to move to telemedicine.(2)
The WHO reports that dementia is perhaps the 21st century's most serious health challenge. Worldwide around 50 million people live with dementia, and by 2050 this number is expected to reach 152 million.(11) Since February 2020, there has been a steady decline in dementia diagnosis rates in England, dropping from 67.6% in February 2020 to 63.2% in July. Misdiagnosis of dementia was always a concern; however, we now face a risk of not diagnosing at all (8).
Patients with an official dementia diagnosis require systematic follow-up visits and close management of their condition. In the UK, 27.5% of all deaths involving COVID-19 (from March to June 2020), Alzheimer’s disease and other types of dementia were the most common underlying conditions.(10) Furthermore, among 10 576 people with confirmed COVID-19 in US nursing homes, residents living with dementia were 52% of COVID-19 cases; yet, accounted for 72% of all deaths (an increased risk of 1.7). (11)
People with dementia are particularly vulnerable to COVID-19 infection because of their age, multimorbidity, and difficulties in maintaining physical distancing11. Appropriate hand hygiene can be challenging, especially in physically debilitated patients. People with dementia might not remember or comprehend required changes to their behavior (9), they might not understand why people are wearing masks, recognize who is behind it, or understand speech when lips are covered.(4)
Dementia already isolates patients to a certain extent and to varying degrees. But now patients are physically confined, which can place a significant strain on their well-being, and may lead to a marked decline in their cognitive abilities or a setback in their progress. (4-6)
Daily routines of dementia patients shouldn’t be disrupted as this may lead to further distress and disorientation.(4) The current situation is already a huge burden for patients and their families.(4) During lockdown in the UK, people with dementia felt lonelier, 56% of them live alone and 23% live with others. They reported difficulties in concentration (48%), memory loss (47%), and agitation or restlessness (45%).(7)
We urgently need to be prepared for the dementia sufferers future problems, and try to best answer their medical needs. Primary care and its patient-centered model is the key to providing comprehensive assessment for diagnosis and follow up (12); and must reaffirm its crucial role in being aware in the detection of any underlying cognitive issues.
We should contemplate building a new approach to treat dementia with more creative methods of communication, to diagnose dementia remotely, or consider a solution to receive these patients in a safe way.(3) It’s time to face the opportunity to improve our model of care and learn from all the struggles that the COVID-19 pandemic has brought.(12)
1. World Health Organization. (25 october 2020). Coronavirus disease (COVID-19). available at https://covid19.who.int/?gclid=Cj0KCQjwxNT8BRD9ARIsAJ8S5xZ-dTRtE-YeXMadD... 2. Klein, BC and Busis, NA. (2020) COVID-19 is catalyzing the adoption of teleneurology. Neurology. 94, 903-904. 3. NHS. (June 2020) “COVID-19 and dementia: The interface between primary care and memory assessment services”. Available at: https://www.southeastclinicalnetworks.nhs.uk/covid-dementia-mas/ 4. Migliaccioa R, and Bouziguesa A. “Dementia and COVID-19 Lockdown: More Than a Double Blow for Patients and Caregivers.” Journal of Alzheimer’s Disease. Reports 4 , 2020, pp. 231–235. 5. Giebel C, Cannon J, et al. (2020) “Impact of COVID-19 related social support service closures on people with dementia and unpaid carers: a qualitative study”, Aging & Mental Health, Available at DOI: 10.1080/13607863.2020.1822292 6. Alzheimers’s Society. (2020) “Worst hit Dementia during coronavirus report.” Available at https://www.alzheimers.org.uk/sites/default/files/2020-09/Worst-hit-Deme... . 7. Alzheimer’s Society. (2020) “Alzheimer’s Society online survey: The impact of COVID-19 on People Affected by Dementia.” Available at https://www.alzheimers.org.uk/news/2020-07-30/lockdownisolation-causes-s... 8. NHS (2020) Digital. “Recorded Dementia Diagnoses.” Available https://digital.nhs.uk/data-andinformation/publications/statistical/reco... . 9. Wang H, Li T, et al. (2020) “Dementia care during COVID-19”. Lancet. 395(10231):1190-1191. Available at doi: 10.1016/S0140-6736(20)30755-8. 10. Office for National Statistics. (2020) “Deaths involving COVID-19, England and Wales: deaths occurring in June 2020”. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarri... deathsinvolvingcovid19englandandwales/deathsoccurringinjune2020. 11. Livingston G, Huntley J, et al. (2020) “Dementia prevention, intervention, and care: 2020 report of the Lancet Commission.” Lancet. 396:413-46. Available at doi:10.1016/S0140-6736(20)30367-6 12. Benaque A, Gurruchaga MJ, et al. Research Center and Memory Clinic, Fundació ACE (2020). “Dementia Care in Times of COVID-19: Experience at Fundació ACE in Barcelona, Spain.” Journal of Alzheimer's disease: JAD, 76(1), 33–40. Available at https://doi.org/10.3233/JAD-200547
Competing interests: No competing interests
- Advanced search
- Peer review
- Record : found
- Abstract : found
- Article : not found
Clinical Presentation of COVID19 in Dementia Patients
Read this article at.
- open (via crossref license)
- oa repository (via OAI-PMH doi match)
- oa repository (via pmcid lookup)

- Review article
- Invite someone to review
No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection.
Retrospective study.
COVID wards in Acute Hospital in Brescia province, Northern Italy.
Participants
We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia.
Measurements
Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status.
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
Author and article information
Contributors, affiliations.
This article is made available via the PMC Open Access Subset for unrestricted research re-use and secondary analysis in any form or by any means with acknowledgement of the original source. These permissions are granted for the duration of the World Health Organization (WHO) declaration of COVID-19 as a global pandemic.
Comment on this article
REVIEW article
Management of cognitive impairment associated with post-covid-19 syndrome: recommendations for primary care.

- 1 Rudolfinerhaus private clinic GmbH, Rudolfinerhaus, Vienna, Austria
- 2 Internal Medicine Family Practice, Bad Camberg, Germany
- 3 Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 4 Center for Brain Research, Department of Molecular Neuroscience, Medical University of Vienna, Vienna, Austria
Introduction: Although post-COVID-19 syndrome (PCS) with cognitive impairment is increasingly encountered in primary care, evidence-based recommendations for its appropriate management are lacking.
Methods: A systematic literature search evaluating the diagnosis and treatment of cognitive impairment associated with PCS was conducted. Practical recommendations for the management of PCS-associated cognitive impairment in primary care are summarized, based on an evaluation of pharmacological plausibility and clinical applications.
Results: Currently, the pathology of cognitive impairment associated with PCS remains unclear with no high-quality data to support targeted interventions. Existing treatment approaches are directed towards symptom relief where counseling on the chronicity of the disease and regular reassessments at 4- to 8-week intervals is considered reasonable. Patients should be informed and encouraged to adopt a healthy lifestyle that centers around balanced nutrition and appropriate physical activities. They may also benefit from the intake of vitamins, micronutrients, and probiotics. The administration of Ginkgo biloba extract could offer a safe and potentially beneficial treatment option. Other non-pharmacological measures include physiotherapy, digitally supported cognitive training, and, if indicated, ergotherapy or speech therapy. In most patients, symptoms improve within 8 weeks. If serious, ambiguous, or when new symptoms occur, specialized diagnostic measures such as comprehensive neurocognitive testing or neuroimaging should be initiated. Very few patients would require inpatient rehabilitation.
Conclusion: PCS with cognitive impairment is a debilitating condition that could affect daily functioning and reduce work productivity. Management in primary care should adopt a multidisciplinary approach, centering around physical, cognitive, and pharmacological therapies.
Introduction
The post-COVID-19 syndrome (PCS, also referred to as long-COVID) is defined by the absence of complete recovery after an acute episode of SARS-CoV-2 infection. According to the clinical case definition published by the World Health Organization (WHO), the post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection usually 3 months from the onset of COVID-19, with symptoms lasting at least 2 months, and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, and cognitive dysfunction, which could impact on daily functioning ( World Health Organization, 2021 ). However, there is no widely accepted definition of this condition ( Sykes et al., 2021 ) to date. The prevalence of PCS is estimated at approximately 10% ( Siso-Almirall et al., 2021 ; Davis et al., 2023 ) of patients infected with SARS-CoV-2, and especially higher among hospitalized patients with COVID-19 pneumonia in excess of 80% ( Carfi et al., 2020 ). Although PCS can develop in patients of all ages, the highest percentage of diagnoses is observed in patients between 36 and 50 years of age, which could impact on work productivity through an increased absence from work (absenteeism) ( Davis et al., 2023 ). A 2021 retrospective cross-sectional study with 1,378 employees undergoing their annual medical check-up in Italy found that PCS was associated with reduced ability to work ( Magnavita et al., 2023 ).
In addition to fatigue, both cognitive impairment and memory complaints in previously cognitively healthy individuals are among the most prominent components in the neurological presentation of PCS. In patients who required hospitalization during SARS- CoV-2 infection, the prevalence of persistent cognitive impairment in the post-acute phase was reported to be 50%–65% ( Frontera et al., 2023 ). According to a meta-analysis of 81 studies, more than one-fifth of patients reported cognitive impairment ( Ceban et al., 2022 ). It is important to note that subjective cognitive dysfunction and objective findings of cognitive impairment post-COVID-19 may be incongruous.
Most patients with persistent symptoms following SARS-CoV-2 infection are managed within the primary care setting. A retrospective data analysis of 63 patients in the COVID-REHA outpatient clinic of the Medical University Hannover in Germany found that only 8% of patients underwent immediate inpatient rehabilitation after first diagnosis ( Teixido et al., 2023 ). An online survey of 11 general practitioners (GPs) in Germany conducted between May and July 2021 revealed that each general practice treated an average of 12 patients with PCS at that time ( Schrimpf et al., 2022 ). In a retrospective cohort analysis of data from health insurance claims for ambulatory care in Bavaria, Germany, between January 2020 and March 2022, 14.2% of patients with confirmed COVID-19 were diagnosed with PCS, and 6.7% received the diagnosis in at least two quarterly periods during a 2-year follow-up ( Donnachie et al., 2022 ). Although the management of PCS-associated cognitive impairment is of utmost importance in primary care, there are no evidence-based guidelines for diagnosis and therapy to date.
The aim of this scoping review was to provide an overview of literature references on PCS-associated cognitive impairment and to reflect the current research perspective. From the outcome combined with personal experience, we aimed to develop pragmatic recommendations with special emphasis on diagnosis and therapy in primary clinical care.
Materials and methods
A systematic literature search was conducted in PubMed ( https://pubmed.ncbi.nlm.nih.gov ), covering references published between 1 January 2020 and 7 March 2024 and reporting the management of cognitive impairment associated with PCS (search terms: post-COVID-19 syndrome OR post-acute COVID-19 syndrome OR post-COVID OR long-COVID OR post-COVID-19 condition OR post-acute sequelae of COVID-19 infection OR long-haul COVID OR PSC AND cognitive impairment). By means of additional filters implemented in the PubMed database for the research field COVID-19, the records were further selected electronically for diagnosis (broad scope) and therapy (broad scope). The results were further restricted to English and German language. In addition, a manual search was carried out in the literature known to the authors. The information derived from the publications was summarized and analyzed descriptively.
Key topics of this scoping review include the diagnosis of cognitive impairment associated with PCS and the follow-up of therapeutic outcomes. We thus reviewed publications on cognitive tests, including their sensitivity and specificity for PCS based on published clinical studies, as well as their suitability for the primary care setting.
As illustrated in the flow diagram ( Figure 1 ), our literature search resulted in 867 hits. Of these, a total of nine scientific publications were considered as relevant for therapy and 44 for diagnosis of PCS-associated cognitive impairment. Two other publications retrieved by hand search, i.e., the German S1 guideline on long/post-COVID ( Rabady et al., 2023 ) and recommendations for an interdisciplinary and multimodal practical approach for PCS ( Teixido et al., 2023 ), were also included.
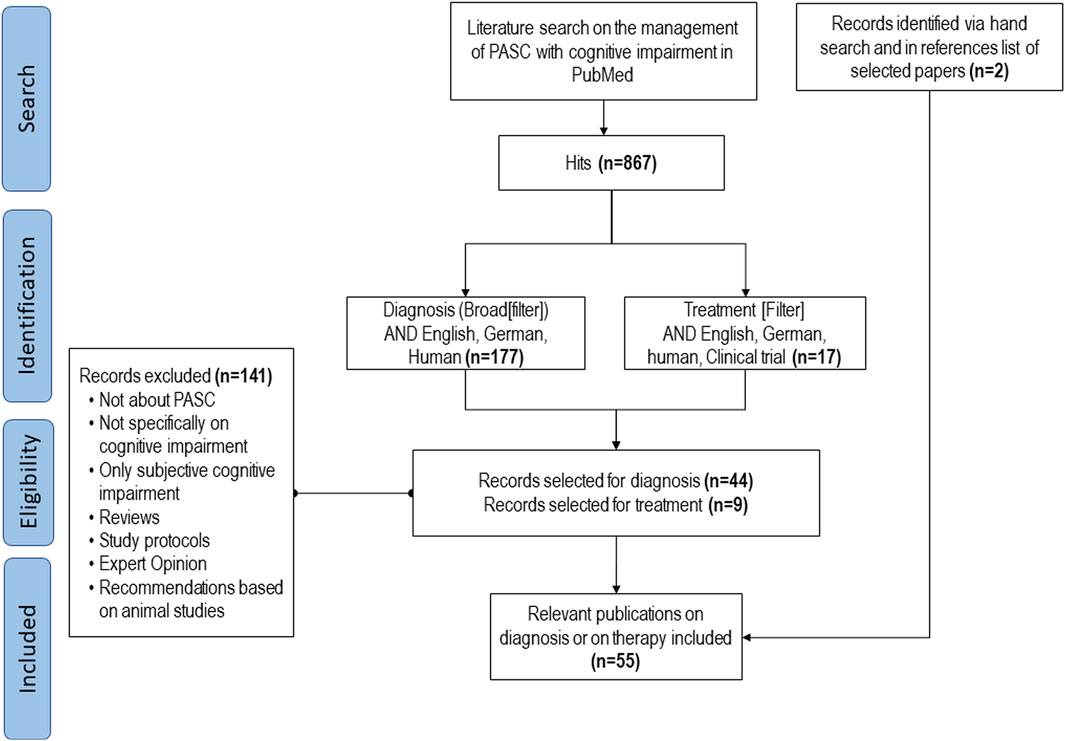
Figure 1 . Flow-chart of systematic literature search.
Pathogenesis of cognitive impairment associated with PCS
The pathophysiology underlying PCS remains unclear. Hypotheses on pathogenic mechanisms implicate both systemic effects and sequelae from acute SARS-CoV-2 organ injury. Hyperinflammation ( Proal and VanElzakker, 2021 ), abnormal immunological response and autoimmunity ( Leng et al., 2023 ), viral persistence ( Sherif et al., 2023 ), reactivation of latent Epstein-Barr virus ( Su et al., 2022 ), microvascular dysfunction ( Navis, 2023 ) , as well as coagulopathies and endotheliopathy ( Leng et al., 2023 ) have been suggested in the etiopathogenesis of PCS.
Specific pathogenic mechanisms involved in COVID-19-associated cognitive impairment are also not fully understood. The three main factors identified by recent literature reviews ( Yang et al., 2021 ; Monje and Iwasaki, 2022 ), i.e., neuroinflammation, neurovascular dysfunction, and disruption of cellular energy metabolism, are summarized below. Several other mechanisms are currently being researched, e.g., microbial dysbiosis ( Gang et al., 2022 ), adverse effects of the viral spike protein on the angiotensin-converting enzyme two receptor, or inhibition of the gamma-aminobutyric acid (GABA) receptors ( Manganotti et al., 2023 ).
Neuroinflammation
As shown by viral detection in the cerebrospinal fluid in some patients, SARS-CoV-2 is neuroinvasive and may spread through various pathways into the central nervous system ( Song et al., 2021 ). This has been suggested to trigger neuroinflammation ( Castanares-Zapatero et al., 2022 ), which may account for neurotoxicity and neuronal damage. Microglia, the resident mononuclear immune cells of the central nervous system (CNS), play an essential role in the response to neuroinflammation. Activated microglia cells in the white matter of the brain further amplify neuroinflammation and can be associated with brain tissue damage ( Fernandez-Castaneda et al., 2022 ). Furthermore, the generation of autoantibodies has a negative impact on neurogenesis and neuronal repair ( Elizalde-Diaz et al., 2022 ).
Neurovascular dysfunction
Microvascular injury and endothelial damage can trigger excessive thrombin production and inhibit fibrinolysis that leads to formation of microthrombi. These pathogenetic factors may cause hypoperfusion and oxidative stress ( Ostergaard, 2021 ). In a prospective observational cohort study, prolonged endothelial dysfunction with impairments of the microcirculation was observed and may explain ongoing symptoms in PCS ( Kuchler et al., 2023 ).
Disruption of cellular energy metabolism
Mitochondrial dysfunction, possibly caused by oxidative stress, leads to systemic reduction of metabolic activity and cellular bioenergetics within the CNS structures, which adversely affects neuronal function ( Astin et al., 2023 ; Davis et al., 2023 ).
Diagnostic recommendations
To date, no clear diagnostic criteria or biological markers for PCS have been established. Unless there are warning signs, diagnostics should be handled within the primary care setting. The steps described below have been recommended in a guideline ( Koczulla et al., 2022 ) and are based on clinical experience. An algorithm for pragmatic management of individuals with cognitive impairment associated with post-COVID syndrome in primary care is also depicted in Figure 2 .
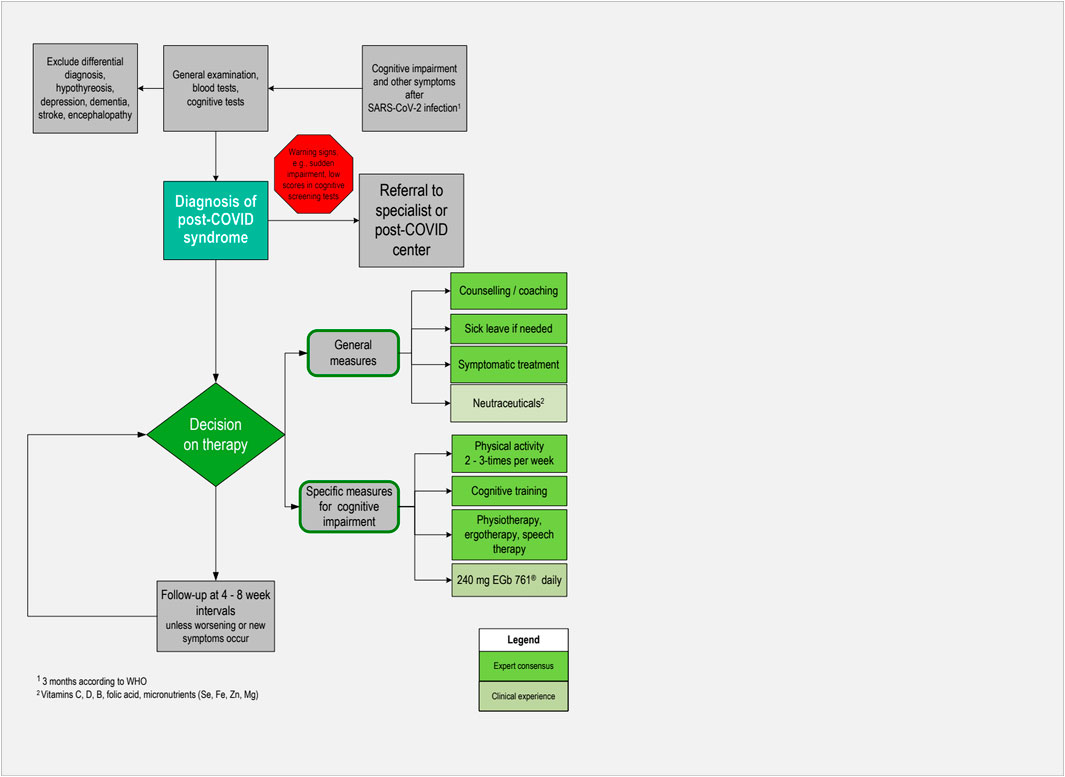
Figure 2 . Algorithm for pragmatic management of individuals with cognitive impairment associated with post-COVID syndrome in primary care.
History of COVID-19 infection
Firstly, the preceding SARS-CoV-2 infection should be confirmed in patients’ medical history, ideally by polymerase chain reaction (PCR) or by a positive antigen test, even if asymptomatic. In primary care, this should be documented in the relevant patient files. The severity of the preceding infection has relevance for the individual prognosis and can be classified by means of WHO criteria. If patients report cognitive complaints, they should be asked about the onset of symptoms and whether complaints are related to PCS or may have another cause.
Physical examination
Physical examinations should be tailored to the specific concerns of patients, but should also include assessment of the cardiovascular system and a neurological examination. To assess functional status, the 1-min sit-to-stand test can be used, with the patient sitting down and standing up as often as possible within 1 minute. Depending on their age group, women can perform an average of 27–40 repeats per minute and men 30–45 repeats per minute. Lower values may indicate a reduced physical performance, which then should be examined further. Handgrip strength was shown to be a good indicator for fatigue. It requires a dynamometer and an accurate and standardized conduct.
Laboratory tests
Blood tests should be performed routinely and include heart, liver, and kidney function parameters. Furthermore, inflammatory markers, i.e., primarily C-reactive protein, immunological parameters, and blood coagulation parameters, should be determined. Testing for COVID-infection markers must be decided case by case, e.g., to exclude an acute infection. In PCS, tests for SARS-CoV-2 have often turned negative when the patient presents.
Tests of cognitive functioning
Most of the objective cognitive tests applied in clinical studies of PCS were developed in the context of dementia research. As can be seen from the literature found, the domains of cognition that are most frequently impaired in the case of PCS are executive functioning ( Calabria et al., 2022 ; Garcia-Sanchez et al., 2022 ; Hadad et al., 2022 ; Serrano-Castro et al., 2022 ; Shanley et al., 2022 ; Diez-Cirarda et al., 2023 ; Ferrucci et al., 2023 ; Ludwig et al., 2023 ; Manganotti et al., 2023 ; Matias-Guiu et al., 2023 ; Taruffi et al., 2023 ), attention ( Calabria et al., 2022 ; Garcia-Sanchez et al., 2022 ; Hadad et al., 2022 ; Serrano-Castro et al., 2022 ; Diez-Cirarda et al., 2023 ; Ferrucci et al., 2023 ; Ludwig et al., 2023 ; Matias-Guiu et al., 2023 ), and delayed recall ( Calabria et al., 2022 ; Crivelli et al., 2022 ; Garcia-Sanchez et al., 2022 ; Serrano-Castro et al., 2022 ; Shanley et al., 2022 ). Notably, up to two-thirds of patients with subjective cognitive impairment due to PCS still pass these tests successfully, either in the total scores or in single domains ( Lauria et al., 2022 ), which shows the limitations of using these cognitive tests in this patient group. Nevertheless, reliable, short, and easy-to-perform cognitive tests such as the Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), or the Demenz-Detektion (DemTect) are useful in primary care, especially if their conduct can be delegated to the nursing staff.
Since there is no specific test for PCS-associated cognitive impairment, the MoCA or the MMSE are recommended as global screening tools ( Frontera et al., 2023 ). In 19 of the 44 included studies, the MoCA was applied as an outcome criterion ( Nasreddine et al., 2005 ). Twelve of the studies applied the MMSE. In studies where both tests were applied, the MMSE tended to be less sensitive than the MoCA ( Aiello et al., 2022 ; Schild et al., 2023 ). Overall, although there are concerns that it may not be sensitive enough to reliably detect mild impairment or impairment in single domains of cognition, MoCA appears to be the most used tool ( Lynch et al., 2022 ). Noteworthy, a positive screening result may indicate a severe course or a comorbid condition requiring special attention while a negative result does not exclude mild cognitive deficits.
Patient self-assessment tools can be used both to identify cognitive impairment and for follow-up ( Koczulla et al., 2022 ). The German S1 guideline on long/post-COVID ( Rabady et al., 2023 ) recommends the abbreviated Measurement of Everyday Cognition (ECog12), which is an assessment tool for self-reported cognitive decline. Another self-assessment tool is the Clinical Global Impression Scale which offers an alternative in terms of a robust measure assessing the global improvement of a patient’s condition. It can be applied to cognition and memory by asking the patient to score from 1 = ‘very much improved’ to 7 = ‘very much worse’. Finally, the five-item version of the Perceived Deficits Questionnaire may be used ( Walker et al., 2023 ), which contains five simple cognition-related questions related to the previous week that are answered by the patients by means of a scale ranging from ‘never’ to ‘almost always’.
Imaging procedures
In eight studies relating to diagnosis of PCS, imaging procedures such as magnetic resonance imaging (MRI) or computed tomography (CT) were most frequently reported. In a retrospective analysis of case files from 243 patients, 37% were referred for neuroradiological examinations, 31% for electroencephalogram (EEG), and 28% for color Doppler of blood vessels of the head and neck. The results of the tests were not stated ( Hegna et al., 2023 ).
In five studies with patient numbers ranging from 12 to 156, MRI scans of the brain ( Franke et al., 2023 ; Ludwig et al., 2023 ; Mina et al., 2023 ; Taruffi et al., 2023 ), or MRI or CT ( Hadad et al., 2022 ) did not reveal specific pathological findings correlating with cognitive impairment.
From another study including 86 patients with subjective PCS and 36 healthy controls, persistent structural and functional brain abnormalities following MRI examination 11 months after the acute infection were reported ( Diez-Cirarda et al., 2023 ). Bilateral hypometabolism of regions of cerebrum and cerebellum associated with cognitive impairment was detected by positron emission tomography (PET) scan ( Guedj et al., 2021 ) even 1 year after COVID-19 infection ( Ferrucci et al., 2023 ).
Differential diagnosis
Differential diagnosis is of utmost importance to ensure that cognitive impairment has not been present before the acute phase of COVID-19. The patient, or a caregiver, should be asked since when cognitive problems have existed. Primary care physicians usually have an advantage of knowing the patient’s medical history and thus do not have to rely solely on the patient’s own assessment.
Results from a qualitative study conducted in Germany in primary care provided some insights into how GPs managed patients with PCS ( Bachmeier et al., 2023 ). The exclusion of other underlying conditions, such as hypothyroidism, and other neurologic-psychiatric conditions such as depression, dementia, stroke, or encephalopathy was the most common diagnostic approach.
Therapeutic recommendations
Since most patients present with moderate or mild symptoms in primary care, a conservative approach including non-pharmacological and pharmacological measures is suitable ( Figure 2 ). According to a practice-based recommendation based on experiences at the COVID-rehabilitation ambulance ( Teixido et al., 2023 ), this includes counseling or coaching of patients and addressing their individual concerns. Besides the restitution of health and capabilities to carry out daily activities, maintaining or regaining the ability to work is of major importance for many patients. They should therefore be informed that complaints due to PCS are mostly reversible within several weeks or months, and usually disappear without any sequelae. It is important for patients to accept that they have to give themselves time for recovery ( Bachmeier et al., 2023 ). Granting sufficiently long sick leave is therefore reasonable in most cases. In a recent study, guided qualitative interviews were conducted with 25 people with PCS ( Schmachtenberg et al., 2023 ). Results showed that many interviewees reached their stress limit during routine household activities or childcare. Of the 25 participants, 19 experienced limitations in pursuing leisure activities, and 10 of those 23 interviewees with jobs reported being on sick leave for several months. Returning to work is possible if daily activities are manageable and 500 m can be walked symptom-free. Work intensity should be discussed with the employer and other restrictions may apply, e.g., avoiding night shifts ( Magnavita et al., 2023 ). Moreover, physicians should discuss with their patients if their cognitive impairment may be a safety issue for professional or leisure activities, particularly for driving or operating machinery.
Follow-up visits at 4- to 8-week intervals are considered appropriate. If patients present with serious, unclear, suddenly evolving symptoms, poor general condition, or other warning signs such as disorientation and confusion, referrals to specialized care are indicated. However, sometimes there are waiting times, particularly for psychotherapy or inpatient rehabilitation.
Non-pharmacological interventions
General recommendations for patients with cognitive impairment associated with PCS include counseling on lifestyle factors such as improving sleep, reducing stress, adopting a healthy diet, and stopping smoking. A healthy diet consists of ample portions of fruits and salad, prioritizing freshly cooked meals, and reducing meat consumption ( Bachmeier et al., 2023 ). Physical activity plays a crucial role in reducing the impact of PCS and engagement in exercise two to three times a week is recommended ( Bachmeier et al., 2023 ). However, setting realistic and achievable goals and avoiding overexertion is of great importance.
Excessive body weight has been identified as a prognostic factor for poor outcomes of COVID-19. Obesity is likely to impair immune response to viral infections, leading to the development of a chronic low-grade inflammatory state and an elevated level of oxidative stress. Hence, body weight reduction may potentially have a positive effect in obese patients with PCS. In this context, there is ongoing discussion regarding the use of ketogenic diets, which are high-fat diets characterized by a marked carbohydrate restriction. It is important to note that particularly very low-calorie ketogenic diets should be supervised by professionals ( Barrea et al., 2022 ). Implementation of weight reduction training aligns with the goal of strengthening muscles by exercise to improve PCS symptoms and has been found beneficial ( Bachmeier et al., 2023 ; Jimeno-Almazan et al., 2023 ).
Behavioral interventions mentioned by Müller and Di Benedetto ( Muller and Di Benedetto, 2023 ) comprise mind-body interventions, musical therapy, and meditation. These interventions, which are of low to moderate cost, can be conducted as self-practice at home and can alleviate symptoms of PCS. On a biochemical level, meditation is associated with a release of anti-inflammatory cytokines, modulation of neuroimmune responses, and decrease in C-reactive protein levels.
A practical guideline on PCS including cognitive impairment recommends various non-pharmacological therapeutic measures including digital solutions for cognition or memory training ( Teixido et al., 2023 ). Some of them are freely available on the internet (e.g., for German-speaking patients: https://www.mental-aktiv.de/uebungen-klassisch/ ) or as YouTube videos with brain training exercises. Others can be prescribed by physicians, e.g., the App NeuroNation MED as digital health application (Digitale Gesundheitsanwendung, DiGA) in Germany. A currently ongoing study is evaluating the efficacy of computer-aided cognitive training in adult patients with PCS ( ClinicalTrials.gov ID NCT05338749). Despite the pending results, this study presents an interesting approach to use game-based computer-delivered cognitive training to address mental symptoms such as attention, memory, or deficits in executive functions.
Further elements of outpatient rehabilitation may be physiotherapy, physical rehabilitation, or special fitness measures ( Teixido et al., 2023 ). The RECOVE trial evaluated the effectiveness of exercise and respiratory muscle training administered in outpatient settings for patients with PCS. The authors found that exercises based on concurrent training (including supervised resistance and endurance exercises of low-to-moderate intensity) and concurrent training combined with inspiratory muscle training significantly alleviated dyspnea and fatigue, as well as enhanced overall health status ( Jimeno-Almazan et al., 2023 ).
In specific circumstances, ergotherapy and speech therapy may be recommended. The latter may be necessary if given cognitive communication problems lead to the impairment of language fluency.
Nutraceuticals
The authors of a review article concluded that high-dose intravenous vitamin C (3.5 g–75 g daily) might be a reasonable treatment option for PCS, due to its antioxidant, anti-inflammatory, endothelial-restoring, and immunomodulatory effects ( Vollbracht and Kraft, 2021 ).
Although almost all PCS patients experience vitamin D deficiency, no correlation was found between vitamin D levels and severity of PCS symptoms ( Mohamed Hussein et al., 2022 ). Nevertheless, supplementation of 2,000 IU daily is recommended for patients with vitamin D blood levels below 30 ng/mL ( Vieth, 2022 ).
Vitamins of the B complex are beneficial for the nervous and the immune system. Anecdotal evidence suggests that low-dose vitamin B supplementation (10 mg thiamine, 4 mg riboflavin, 40 mg nicotinamide, 6 mg dexpanthenol daily) improved COVID-19 mortality ( Majidi et al., 2022 ). Dosage recommendations for folic acid supplementation specifically for PSC were not found in the literature. As a general recommendation, vitamins of the B complex should be supplemented unless the blood levels are at the upper limit of the normal range.
Micronutrients
Micronutrients, including selenium, iron, zinc, and magnesium, are also critical for proper functioning of the immune system. Although there is no evidence for a general benefit in PCS patients, daily supplementation with 35–40 µg selenium, 15 mg iron, 15 mg zinc, or 350 mg magnesium daily can be considered in cases where a deficiency of these nutrients is present ( Tosato et al., 2022 ; Pavlidou et al., 2024 ).
Pre- or probiotics
A persisting reduction in the richness of normal composition of gut microbiota can be found even 6 months after recovering from COVID-19 infection. Although controlled clinical trials focusing on patients with PCS are currently lacking, the use of probiotics and prebiotics may be considered as a supportive measure ( Catalano et al., 2022 ). Additionally, immunomodulatory effects of probiotics may help in restoring the gut microbiome altered during viral infections ( Muller and Di Benedetto, 2023 ).
Pharmacological treatments
To date, the pathologic mechanisms of cognitive impairment associated with PCS are unclear and no evidence-based treatments are available ( Bonilla et al., 2023 ). Thus, management is currently focused on symptomatic treatment including anti-inflammatory drugs such as corticosteroids, anticoagulants, or analgesics, if indicated. The following treatment option specific for cognitive impairment was selected from the literature search based on empirical pharmacological plausibility. Due to the lack of rigorously controlled clinical trials, a favorable safety profile is of high importance.
Ginkgo biloba extract
Most published nonclinical and clinical studies investigating Ginkgo biloba extract were done using the proprietary Ginkgo biloba leaves extract EGb 761 ® . EGb 761 ® was shown to display multimodal effects on a variety of pathogenetic processes which may be involved in PCS ( Mueller and Muller, 2024 ). Flavonoids, terpenoids, and anthocyanidins exhibit neuroprotective effects by modulating signaling pathways known to be impacted by COVID-19 ( Zaa et al., 2023 ). They have been shown to inhibit neuroinflammation by reducing inflammatory activation in microglia cells ( Gargouri et al., 2018 ). Importantly, it protects the function of endothelial cells ( Pierre et al., 2008 ; Zhang et al., 2017 ) and improves brain and sensory organ perfusion by reducing blood viscosity ( Erdinҫler et al., 1996 ). Randomized controlled trials demonstrated the efficacy of Ginkgo extract at the dose of 240 mg daily in mild cognitive impairment ( Grass-Kapanke et al., 2011 ; Gavrilova et al., 2014 ). A meta-analysis of seven randomized, placebo-controlled trials in patients with dementia showed that treatment-associated risks (relative risk of adverse events, rates of premature withdrawal) in patients taking EGb 761 ® did not differ noticeably compared to the placebo group and confirmed the safety and tolerability of this extract ( Gauthier and Schlaefke, 2014 ). However, even if these pharmacological and clinical results are promising, the available data are still preliminary and require additional proof by further studies ( Mueller and Muller, 2024 ).
In patients with cognitive impairment, treatment with Ginkgo extract can be started immediately at first consultation. Follow-up is recommended after 8 weeks of treatment. In a small case series with five patients aged 26–59 years and suffering from concentration and attention deficits, cognitive deficiencies, and/or fatigue, cognitive deficits and other symptoms, such as fatigue and hyposmia, were substantially improved or completely restored by treatment with EGb 761 ® within 6 months ( Zifko et al., 2022 ). The authors therefore recommended randomized controlled clinical trials to be conducted in order to confirm efficacy in this indication.
Research perspectives for the management of cognitive impairment associated with PCS
Our literature search revealed nine publications on experimental studies investigating therapeutic approaches. Table 1 presents an overview of study methodologies and main outcomes Table 2 . Out of the studies found, only three were small scale randomized controlled studies. Each one was a controlled study on the efficacy of the H2 antagonist famotidine ( Momtazmanesh et al., 2023 ) or donezepil hydrochloride ( Pooladgar et al., 2023 ), and one investigated the effectiveness of a neuro-meditation program ( Hausswirth et al., 2023 ). A case control study evaluated the efficacy of cognitive remediation therapy ( Palladini et al., 2023 ), a feasibility pilot study was carried out on a personalized computerized cognitive training ( Dunabeitia et al., 2023 ), and one observational pilot study evaluated a multimodal therapy concept with behavioral therapy-oriented, disorder-specific psychotherapy and exercise therapy ( Kupferschmitt et al., 2023 ). Moreover, a retrospective analysis was performed with data from 64 patients suffering from PCS who were treated with a day-by-day individualized psychological intervention of cognitive stimulation in addition to a standard in-hospital rehabilitation program ( Rabaiotti et al., 2023 ). A case series with 23 outpatients investigated the effect of transcranial magnetic stimulation ( Noda et al., 2023 ) and another case series reported on five patients treated with EGb 761 ® following presentation with concentration and attention deficits, cognitive deficiencies, and/or fatigue 9–35 weeks after COVID-19 infection ( Zifko et al., 2022 ).
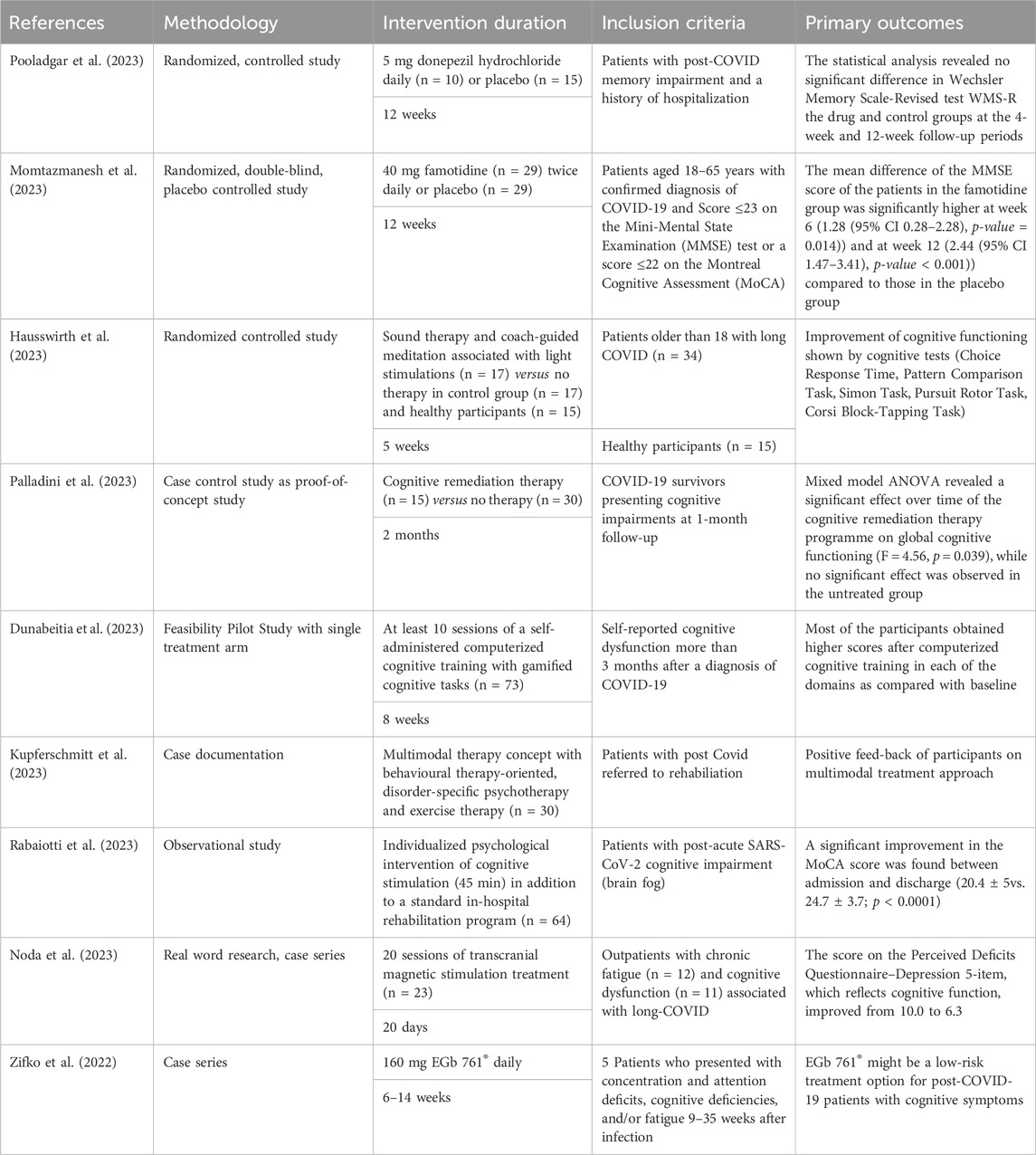
Table 1 . Data collections on therapy of cognitive impairment associated with PCS.
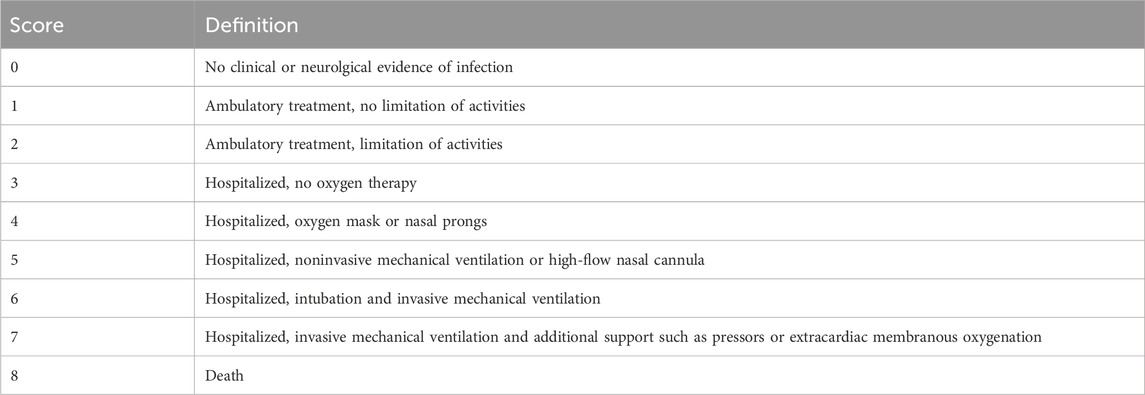
Table 2 . nine-point WHO ordinal clinical severity scale for COVID-19.
In August 2023, the US National Library of Medicine of the National Institutes of Health (NIH) clinical research registry ( www.clinicaltrials.gov ) reported 28 clinical trials investigating treatments of cognitive impairment associated with PCS. Of these, 18 clinical trials investigated various non-pharmacological interventions (ranging from Tai Chi, psychoeducation to computer-based cognitive trainings) and 10 investigated pharmacological treatments (atorvastatin, the NMDA receptor antagonist DAOIB, the antidepressant vortioxetine, an amniotic fluid product VIX001 for intravenous injection, ketamine (CI-581a and b) as glutamatergic modulator, as well as medium chain triglycerides, safflower oil, or nicotinamide riboside as dietary supplements).
PCS-associated cognitive impairment as complication after acute SARS-CoV-2 infection has a negative impact on daily functioning and quality of life and leads to loss of working days or reduced productivity at work and to increased use of healthcare resources. Most patients are managed in a primary care setting requiring a multifactorial and/or multidisciplinary approach with longitudinal follow-up ( Siso-Almirall et al., 2021 ). For cognitive impairment associated with PCS, there are neither clinical guidelines nor well designed randomized controlled trials providing an evidence base for diagnostics and therapy so far. When considering the body of evidence, GPs may feel that they lack sufficient knowledge on this topic. Nevertheless, it is crucial for the healthcare system that only patients requiring secondary or tertiary care are referred to specialists, especially as waiting times amount to several weeks or even months in some countries.
This scoping review provides an overview of literature references on PCS-associated cognitive impairment and develops pragmatic recommendations with special emphasis on diagnosis and therapy in primary clinical care. A similar review of PCS by Nicotra et al. was recently published ( Nicotra et al., 2023 ), which did, however, not focus on primary care. The systematic search resulted in 947 unique records available until May 2023, from which 180 studies were retrieved. The authors stated that only a minority of studies included patients according to stringent temporal criteria for syndrome onset (34%), while most studies reported a required minimum duration of symptoms (77%). In our search, we applied the filters of the literature database for diagnosis and thereby identified 44 clinical studies that applied cognitive tests or imaging procedures (mostly MRI). Nicotra et al. found 36 studies which employed cognitive measures: screening tests alone (n = 19), full neuropsychological batteries (n = 25), or both (n = 29), while 30 studies performed psychiatric testing ( Nicotra et al., 2023 ). Although the numbers vary, the conclusions of the systematic searches are similar. Nicotra et al. reported that cognitive deficits were documented in 39% of subjects, the most frequently affected domains being attention/executive functions (90%) and memory (67%). In our review, only a few individual tests reached positive results in more than 50% of participants. In patients reporting subjective cognitive complaints, measurement based on objective criteria is challenging due to the fact that only half of the patients respond to cognitive tests. In research settings, the conduct of cognitive test batteries is recommended for diagnosis of PCS-associated cognitive impairment. This is time-consuming and not practicable under the conditions of primary care. Since many patients suffer from fatigue, a complete test battery may be too strenuous and is therefore not feasible. Screening tools developed for dementia such as MoCA or MMSE are not sensitive enough for this patient group but are nevertheless useful to identify patients with severe cognitive impairment for whom specialist care is necessary.
Currently, there are many ongoing research activities and clinical studies evaluating PCS. Since there is no uniform definition of the disease, the results are sometimes difficult to interpret. So far, no clearly defined anatomical equivalents or biomarkers for the condition have been found. It seems that most research teams did not find characteristic features in imaging procedures, although this is still an important step in excluding other causes. Moreover, tools for the measurement of cognitive impairment applied in clinical studies are heterogenous and results are therefore not transferrable. The diagnosis or inclusion of patients in almost all studies published relies on self-assessment of patients. All these factors may explain why the results of different studies are equivocal. However, due to the great burden of PCS on the healthcare system, research on the pathogenesis, diagnosis, and therapy is needed. Harmonized methodological approaches are required for future research.
Factors influencing cognitive disturbances in PCS are currently being researched, but the pathogenesis is still not elucidated. Therefore, it is not possible to develop causative drugs that target the condition. Our literature search retrieved only very few publications on clinical studies investigating non-pharmacological or individual pharmacological therapeutic approaches. This is not surprising, given that PCS is a relatively new disease and many studies are therefore still ongoing, as shown by our search in the NIH clinical research registry. While some studies specific to the treatment of PSC-associated cognitive impairment have been published, no confirmatory clinical trials on the efficacy of treatment options or proven therapeutic strategies are available ( Frontera et al., 2023 ). This is due to the relatively new disease and to the fact that there still is a lack of data on the underlying pathophysiological mechanisms. Further research in both fields is therefore needed.
A conservative approach is recommended in primary care unless warning signs such as poor general condition or sudden onset of severe symptoms appear. Pragmatic management strategies consist of a multidisciplinary approach tailored for the individual patient involving counseling on the nature of the disease, optimal lifestyle, digital cognitive training, and non-pharmacological as well as pharmacological therapies.
If indicated, physiotherapy, ergotherapy, or speech therapy can be implemented. Neurocognitive rehabilitation should only be initiated in serious cases, with support from social services ( Aiyegbusi et al., 2021 ). These recommendations may help to allocate resources more efficiently.
Due to its potent anti-inflammatory properties, enhancement of neuroplasticity, and well-proven clinical efficacy, EGb 761 ® may be beneficial for use in patients with cognitive impairment associated with PCS. In a case series involving patients who experienced persistent cognitive symptoms following SARS-CoV-2 infection, treatment with EGb 761 ® improved or eliminated cognitive deficits ( Zifko et al., 2022 ). The favorable safety and tolerability profile of EGb 761 ® supports its use additionally ( Schulz et al., 2018 ). Thus, EGb 761 ® might be a low-risk treatment option for cognitive impairment associated with PCS.
Symptomatic treatment may include the supplementation of vitamin B complex, vitamin D and micronutrients. Vitamin C may be beneficial due to its antioxidant effect and probiotics have been shown to improve dysbiosis and thereby support the immune system.
Our scoping review may be limited by the fact that no systematic data extraction was carried out and the selection of reports was rather based on subjective assessment of their relevance. Since personal experiences can vary and may not be representative of the broader clinical landscape, the conclusions drawn in our work could be subject to a certain bias. Like all expert recommendations, our findings therefore represent the lowest level of evidence. Nevertheless, we provide a comprehensive overview and analysis of the huge amount of published literature, which might be helpful and time-saving in clinical practice. To our knowledge, this is the first review focusing on primary care as well as on cognitive sequels. Our work also shows that further research is urgently needed to develop evidence-based treatments.
Author contributions
UZ: Conceptualization, Data curation, Visualization, Writing–review and editing. KG: Conceptualization, Data curation, Visualization, Writing–review and editing. RS: Conceptualization, Data curation, Visualization, Writing–review and editing. SK: Conceptualization, Data curation, Visualization, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Małgorzata Biernikiewicz and Patricia Buchholz of Valid Insight, Macclesfield, United Kingdom for providing medical writing support. These Medical writing services were funded by Dr. Willmar Schwabe.
Conflict of interest
This research received funding from Dr. Willmar Schwabe GmbH & Co. KG. The funder was involved in the study design and data collection. The final decision to submit the article for publication remained with the authors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aiello, E. N., Fiabane, E., Manera, M. R., Radici, A., Grossi, F., Ottonello, M., et al. (2022). Screening for cognitive sequelae of SARS-CoV-2 infection: a comparison between the mini-mental state examination (MMSE) and the Montreal cognitive assessment (MoCA). Neurol. Sci. 43 (1), 81–84. doi:10.1007/s10072-021-05630-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Aiyegbusi, O. L., Hughes, S. E., Turner, G., Rivera, S. C., McMullan, C., Chandan, J. S., et al. (2021). Symptoms, complications and management of long COVID: a review. J. R. Soc. Med. 114 (9), 428–442. doi:10.1177/01410768211032850
Astin, R., Banerjee, A., Baker, M. R., Dani, M., Ford, E., Hull, J. H., et al. (2023). Long COVID: mechanisms, risk factors and recovery. Exp. Physiol. 108 (1), 12–27. doi:10.1113/EP090802
Bachmeier, B. E., Holzle, S., Gasser, M., and van den Akker, M. (2023). How do German general practitioners manage long-/post-COVID? A qualitative study in primary care. Viruses 15 (4), 1016. doi:10.3390/v15041016
Barrea, L., Vetrani, C., Caprio, M., Cataldi, M., Ghoch, M. E., Elce, A., et al. (2022). From the ketogenic diet to the mediterranean diet: the potential dietary therapy in patients with obesity after CoVID-19 infection (post CoVID syndrome). Curr. Obes. Rep. 11 (3), 144–165. doi:10.1007/s13679-022-00475-z
Bonilla, H., Peluso, M. J., Rodgers, K., Aberg, J. A., Patterson, T. F., Tamburro, R., et al. (2023). Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 14, 1129459. doi:10.3389/fimmu.2023.1129459
Calabria, M., Garcia-Sanchez, C., Grunden, N., Pons, C., Arroyo, J. A., Gomez-Anson, B., et al. (2022). Post-COVID-19 fatigue: the contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 269 (8), 3990–3999. doi:10.1007/s00415-022-11141-8
Carfi, A., Bernabei, R., Landi, F., and Gemelli Against, C.-P.-A. C. S. G. (2020). Persistent symptoms in patients after acute COVID-19. JAMA 324 (6), 603–605. doi:10.1001/jama.2020.12603
Castanares-Zapatero, D., Chalon, P., Kohn, L., Dauvrin, M., Detollenaere, J., Maertens de Noordhout, C., et al. (2022). Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 54 (1), 1473–1487. doi:10.1080/07853890.2022.2076901
Catalano, A., Iacopetta, D., Ceramella, J., Maio, A. C., Basile, G., Giuzio, F., et al. (2022). Are nutraceuticals effective in COVID-19 and post-COVID prevention and treatment? Foods 11 (18), 2884. doi:10.3390/foods11182884
Ceban, F., Ling, S., Lui, L. M. W., Lee, Y., Gill, H., Teopiz, K. M., et al. (2022). Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135. doi:10.1016/j.bbi.2021.12.020
Crivelli, L., Calandri, I., Corvalan, N., Carello, M. A., Keller, G., Martinez, C., et al. (2022). Cognitive consequences of COVID-19: results of a cohort study from South America. Arq. Neuropsiquiatr. 80 (3), 240–247. doi:10.1590/0004-282X-ANP-2021-0320
Davis, H. E., McCorkell, L., Vogel, J. M., and Topol, E. J. (2023). Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21 (3), 133–146. doi:10.1038/s41579-022-00846-2
Diez-Cirarda, M., Yus, M., Gomez-Ruiz, N., Polidura, C., Gil-Martinez, L., Delgado-Alonso, C., et al. (2023). Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 146 (5), 2142–2152. doi:10.1093/brain/awac384
Donnachie, E., Hapfelmeier, A., Linde, K., Tauscher, M., Gerlach, R., Greissel, A., et al. (2022). Incidence of post-COVID syndrome and associated symptoms in outpatient care in Bavaria, Germany: a retrospective cohort study using routinely collected claims data. BMJ Open 12 (9), e064979. doi:10.1136/bmjopen-2022-064979
Dunabeitia, J. A., Mera, F., Baro, O., Jadad-Garcia, T., and Jadad, A. R. (2023). Personalized computerized training for cognitive dysfunction after COVID-19: a before-and-after feasibility pilot study. Int. J. Environ. Res. Public Health 20 (4), 3100. doi:10.3390/ijerph20043100
Elizalde-Diaz, J. P., Miranda-Narvaez, C. L., Martinez-Lazcano, J. C., and Martinez-Martinez, E. (2022). The relationship between chronic immune response and neurodegenerative damage in long COVID-19. Front. Immunol. 13, 1039427. doi:10.3389/fimmu.2022.1039427
Erdinҫler, D. S., Karakoҫ, Y., Toplan, S., Önen, S., Sukyasyan, A., Beğer, T., et al. (1996). The effect of ginkgo biloba glycoside on the blood viscosity and erythrocyte deformability. Clin. Hemorheol. 16 (3), 271–276. doi:10.3233/ch-1996-16306
CrossRef Full Text | Google Scholar
Fernandez-Castaneda, A., Lu, P., Geraghty, A. C., Song, E., Lee, M. H., Wood, J., et al. (2022). Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185 (14), 2452–2468.e16. doi:10.1016/j.cell.2022.06.008
Ferrucci, R., Cuffaro, L., Capozza, A., Rosci, C., Maiorana, N., Groppo, E., et al. (2023). Brain positron emission tomography (PET) and cognitive abnormalities one year after COVID-19. J. Neurol. 270 (4), 1823–1834. doi:10.1007/s00415-022-11543-8
Franke, C., Boesl, F., Goereci, Y., Gerhard, A., Schweitzer, F., Schroeder, M., et al. (2023). Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav. Immun. 109, 139–143. doi:10.1016/j.bbi.2023.01.006
Frontera, J. A., Guekht, A., Allegri, R. F., Ashraf, M., Baykan, B., Crivelli, L., et al. (2023). Evaluation and treatment approaches for neurological post-acute sequelae of COVID-19: a consensus statement and scoping review from the global COVID-19 neuro research coalition. J. Neurol. Sci. 454, 120827. doi:10.1016/j.jns.2023.120827
Gang, J., Wang, H., Xue, X., and Zhang, S. (2022). Microbiota and COVID-19: long-term and complex influencing factors. Front. Microbiol. 13, 963488. doi:10.3389/fmicb.2022.963488
Garcia-Sanchez, C., Calabria, M., Grunden, N., Pons, C., Arroyo, J. A., Gomez-Anson, B., et al. (2022). Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 12 (3), e2508. doi:10.1002/brb3.2508
Gargouri, B., Carstensen, J., Bhatia, H. S., Huell, M., Dietz, G. P. H., and Fiebich, B. L. (2018). Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 44, 45–55. doi:10.1016/j.phymed.2018.04.009
Gauthier, S., and Schlaefke, S. (2014). Efficacy and tolerability of Ginkgo biloba extract EGb 761 ® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Interv. Aging 9, 2065–2077. doi:10.2147/CIA.S72728
Gavrilova, S. I., Preuss, U. W., Wong, J. W., Hoerr, R., Kaschel, R., Bachinskaya, N., et al. (2014). Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int. J. Geriatr. Psychiatry 29 (10), 1087–1095. doi:10.1002/gps.4103
Grass-Kapanke, B., Busmane, A., Lasmanis, A., Hoerr, R., and Kaschel, R. (2011). Effects of ginkgo biloba special extract EGb 761&#174; in very mild cognitive impairment (vMCI). Neurosci. Med. 2 (01), 48–56. doi:10.4236/nm.2011.21007
Guedj, E., Campion, J. Y., Dudouet, P., Kaphan, E., Bregeon, F., Tissot-Dupont, H., et al. (2021). (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 48 (9), 2823–2833. doi:10.1007/s00259-021-05215-4
Hadad, R., Khoury, J., Stanger, C., Fisher, T., Schneer, S., Ben-Hayun, R., et al. (2022). Cognitive dysfunction following COVID-19 infection. J. Neurovirol 28 (3), 430–437. doi:10.1007/s13365-022-01079-y
Hausswirth, C., Schmit, C., Rougier, Y., and Coste, A. (2023). Positive impacts of a four-week neuro-meditation program on cognitive function in post-acute sequelae of COVID-19 patients: a randomized controlled trial. Int. J. Environ. Res. Public Health 20 (2), 1361. doi:10.3390/ijerph20021361
Hegna, E., Racki, V., Hero, M., Papic, E., Rozmaric, G., Radovic, K., et al. (2023). Post-COVID-19 syndrome in neurology patients: a single center experience. Pathogens 12 (6), 796. doi:10.3390/pathogens12060796
Jimeno-Almazan, A., Buendia-Romero, A., Martinez-Cava, A., Franco-Lopez, F., Sanchez-Alcaraz, B. J., Courel-Ibanez, J., et al. (2023). Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: the RECOVE trial. J. Appl. Physiol. 134 (1), 95–104. doi:10.1152/japplphysiol.00489.2022
Koczulla, A. R., Ankermann, T., Behrends, U., Berlit, P., Berner, R., Böing, S., et al. (2022). AWMF S1-leitlinie long/post-COVID (S1 guideline long/post-COVID). Available at: https://register.awmf.org/de/leitlinien/detail/020-027 (Accessed August, 2023).
Google Scholar
Kuchler, T., Gunthner, R., Ribeiro, A., Hausinger, R., Streese, L., Wohnl, A., et al. (2023). Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis 26, 547–563. doi:10.1007/s10456-023-09885-6
Kupferschmitt, A., Etzrodt, F., Kleinschmidt, J., and Kollner, V. (2023). Not only multimodal, but also interdisciplinary: a concept for interdisciplinary cooperation in the rehabilitation of post-COVID syndrome. Psychother. Psychosom. Med. Psychol. 73 (1), 34–41. doi:10.1055/a-1838-3055
Lauria, A., Carfi, A., Benvenuto, F., Bramato, G., Ciciarello, F., Rocchi, S., et al. (2022). Neuropsychological measures of long COVID-19 fog in older subjects. Clin. Geriatr. Med. 38 (3), 593–603. doi:10.1016/j.cger.2022.05.003
Leng, A., Shah, M., Ahmad, S. A., Premraj, L., Wildi, K., Li Bassi, G., et al. (2023). Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells 12 (5), 816. doi:10.3390/cells12050816
Ludwig, B., Deckert, M., Krajnc, N., Keritam, O., Macher, S., Bsteh, G., et al. (2023). Reported neurological symptoms after severe acute respiratory syndrome coronavirus type 2 infection: a systematic diagnostic approach. Eur. J. Neurol. 30 (9), 2713–2725. doi:10.1111/ene.15923
Lynch, S., Ferrando, S. J., Dornbush, R., Shahar, S., Smiley, A., and Klepacz, L. (2022). Screening for brain fog: is the montreal cognitive assessment an effective screening tool for neurocognitive complaints post-COVID-19? Gen. Hosp. Psychiatry 78, 80–86. doi:10.1016/j.genhosppsych.2022.07.013
Magnavita, N., Arnesano, G., Di Prinzio, R. R., Gasbarri, M., Meraglia, I., Merella, M., et al. (2023). Post-COVID symptoms in occupational cohorts: effects on health and work ability. Int. J. Environ. Res. Public Health 20 (9), 5638. doi:10.3390/ijerph20095638
Majidi, N., Bahadori, E., Shekari, S., Gholamalizadeh, M., Tajadod, S., Ajami, M., et al. (2022). Effects of supplementation with low-dose group B vitamins on clinical and biochemical parameters in critically ill patients with COVID-19: a randomized clinical trial. Expert Rev. Anti Infect. Ther. , 1–7. doi:10.1080/14787210.2022.2125867
Manganotti, P., Michelutti, M., Furlanis, G., Deodato, M., and Buoite Stella, A. (2023). Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin. Neurophysiol. 151, 83–91. doi:10.1016/j.clinph.2023.04.010
Matias-Guiu, J. A., Herrera, E., Gonzalez-Nosti, M., Krishnan, K., Delgado-Alonso, C., Diez-Cirarda, M., et al. (2023). Development of criteria for cognitive dysfunction in post-COVID syndrome: the IC-CoDi-COVID approach. Psychiatry Res. 319, 115006. doi:10.1016/j.psychres.2022.115006
Mina, Y., Enose-Akahata, Y., Hammoud, D. A., Videckis, A. J., Narpala, S. R., O'Connell, S. E., et al. (2023). Deep phenotyping of neurologic postacute sequelae of SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm 10 (4), e200097. doi:10.1212/NXI.0000000000200097
Mohamed Hussein, A. A. R., Galal, I., Amin, M. T., Moshnib, A. A., Makhlouf, N. A., Makhlouf, H. A., et al. (2022). Prevalence of vitamin D deficiency among patients attending Post COVID-19 follow-up clinic: a cross-sectional study. Eur. Rev. Med. Pharmacol. Sci. 26 (8), 3038–3045. doi:10.26355/eurrev_202204_28635
Momtazmanesh, S., Ansari, S., Izadi, Z., Shobeiri, P., Vatankhah, V., Seifi, A., et al. (2023). Effect of famotidine on cognitive and behavioral dysfunctions induced in post-COVID-19 infection: a randomized, double-blind, and placebo-controlled study. J. Psychosom. Res. 172, 111389. doi:10.1016/j.jpsychores.2023.111389
Monje, M., and Iwasaki, A. (2022). The neurobiology of long COVID. Neuron 110 (21), 3484–3496. doi:10.1016/j.neuron.2022.10.006
Mueller, J. K., and Muller, W. E. (2024). Multi-target drugs for the treatment of cognitive impairment and fatigue in post-COVID syndrome: focus on Ginkgo biloba and Rhodiola rosea. J. Neural Transm. (Vienna) 131 (3), 203–212. doi:10.1007/s00702-024-02749-3
Muller, L., and Di Benedetto, S. (2023). Aged brain and neuroimmune responses to COVID-19: post-acute sequelae and modulatory effects of behavioral and nutritional interventions. Immun. Ageing 20 (1), 17. doi:10.1186/s12979-023-00341-z
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53 (4), 695–699. doi:10.1111/j.1532-5415.2005.53221.x
Navis, A. (2023). A review of neurological symptoms in long COVID and clinical management. Semin. Neurol. 43 (2), 286–296. doi:10.1055/s-0043-1767781
Nicotra, A., Masserini, F., Calcaterra, F., Di Vito, C., Doneddu, P. E., Pomati, S., et al. (2023). What do we mean by long-COVID? A scoping review of the cognitive sequelae of SARS-CoV-2 infection. Eur. J. Neurol. 30, 3968–3978. doi:10.1111/ene.16027
Noda, Y., Sato, A., Shichi, M., Sato, A., Fujii, K., Iwasa, M., et al. (2023). Real world research on transcranial magnetic stimulation treatment strategies for neuropsychiatric symptoms with long-COVID in Japan. Asian J. Psychiatr. 81, 103438. doi:10.1016/j.ajp.2022.103438
Ostergaard, L. (2021). SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 9 (3), e14726. doi:10.14814/phy2.14726
Palladini, M., Bravi, B., Colombo, F., Caselani, E., Di Pasquasio, C., D'Orsi, G., et al. (2023). Cognitive remediation therapy for post-acute persistent cognitive deficits in COVID-19 survivors: a proof-of-concept study. Neuropsychol. Rehabil. 33 (7), 1207–1224. doi:10.1080/09602011.2022.2075016
Pavlidou, E., Poulios, E., Papadopoulou, S. K., Fasoulas, A., Dakanalis, A., and Giaginis, C. (2024). Clinical evidence on the potential beneficial effects of diet and dietary supplements against COVID-19 infection risk and symptoms' severity. Med. Sci. (Basel) 12 (1), 11. doi:10.3390/medsci12010011
Pierre, S. V., Lesnik, P., Moreau, M., Bonello, L., Droy-Lefaix, M. T., Sennoune, S., et al. (2008). The standardized Ginkgo biloba extract Egb-761 protects vascular endothelium exposed to oxidized low density lipoproteins. Cell Mol. Biol. (Noisy-le-grand) 54, OL1032–1042.
PubMed Abstract | Google Scholar
Pooladgar, P., Sakhabakhsh, M., Soleiman-Meigooni, S., Taghva, A., Nasiri, M., and Darazam, I. A. (2023). The effect of donepezil hydrochloride on post-COVID memory impairment: a randomized controlled trial. J. Clin. Neurosci. 118, 168–174. doi:10.1016/j.jocn.2023.09.005
Proal, A. D., and VanElzakker, M. B. (2021). Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 12, 698169. doi:10.3389/fmicb.2021.698169
Rabady, S., Hoffmann, K., Aigner, M., Altenberger, J., Brose, M., Costa, U., et al. (2023). S1 guidelines for the management of postviral conditions using the example of post-COVID-19. Wien Klin. Wochenschr 135 (4), 525–598. doi:10.1007/s00508-023-02242-z
Rabaiotti, P., Ciraci, C., Donelli, D., Oggioni, C., Rizzi, B., Savi, F., et al. (2023). Effects of multidisciplinary rehabilitation enhanced with neuropsychological treatment on post-acute SARS-CoV-2 cognitive impairment (brain fog): an observational study. Brain Sci. 13 (5), 791. doi:10.3390/brainsci13050791
Schild, A. K., Goereci, Y., Scharfenberg, D., Klein, K., Lulling, J., Meiberth, D., et al. (2023). Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: results from a prospective monocentric cohort. J. Neurol. 270 (3), 1215–1223. doi:10.1007/s00415-022-11444-w
Schmachtenberg, T., Muller, F., Kranz, J., Dragaqina, A., Wegener, G., Konigs, G., et al. (2023). How do long COVID patients perceive their current life situation and occupational perspective? Results of a qualitative interview study in Germany. Front. Public Health 11, 1155193. doi:10.3389/fpubh.2023.1155193
Schrimpf, A., Braesigk, A., Lippmann, S., and Bleckwenn, M. (2022). Management and treatment of long COVID symptoms in general practices: an online-based survey. Front. Public Health 10, 937100. doi:10.3389/fpubh.2022.937100
Schulz, M., Hoerr, R., and Mueller, H. (2018). 46th ESCP symposium on clinical pharmacy "Science meets practice: towards evidence-based clinical pharmacy services", Heidelberg, Germany, October 9th-11th, 2017. Int. J. Clin. Pharm. 40 (1), 203–317. doi:10.1007/s11096-017-0565-9
Serrano-Castro, P. J., Garzon-Maldonado, F. J., Casado-Naranjo, I., Ollero-Ortiz, A., Minguez-Castellanos, A., Iglesias-Espinosa, M., et al. (2022). The cognitive and psychiatric subacute impairment in severe Covid-19. Sci. Rep. 12 (1), 3563. doi:10.1038/s41598-022-07559-9
Shanley, J. E., Valenciano, A. F., Timmons, G., Miner, A. E., Kakarla, V., Rempe, T., et al. (2022). Longitudinal evaluation of neurologic-post acute sequelae SARS-CoV-2 infection symptoms. Ann. Clin. Transl. Neurol. 9 (7), 995–1010. doi:10.1002/acn3.51578
Sherif, Z. A., Gomez, C. R., Connors, T. J., Henrich, T. J., Reeves, W. B., and Force, R. M. P. T. (2023). Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 12, e86002. doi:10.7554/eLife.86002
Siso-Almirall, A., Brito-Zeron, P., Conangla Ferrin, L., Kostov, B., Moragas Moreno, A., Mestres, J., et al. (2021). Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int. J. Environ. Res. Public Health 18 (8), 4350. doi:10.3390/ijerph18084350
Song, E., Zhang, C., Israelow, B., Lu-Culligan, A., Prado, A. V., Skriabine, S., et al. (2021). Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 218 (3), e20202135. doi:10.1084/jem.20202135
Su, Y., Yuan, D., Chen, D. G., Ng, R. H., Wang, K., Choi, J., et al. (2022). Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185 (5), 881–895.e20. doi:10.1016/j.cell.2022.01.014
Sykes, D. L., Holdsworth, L., Jawad, N., Gunasekera, P., Morice, A. H., and Crooks, M. G. (2021). Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung 199 (2), 113–119. doi:10.1007/s00408-021-00423-z
Taruffi, L., Muccioli, L., Mitolo, M., Ferri, L., Descovich, C., Mazzoni, S., et al. (2023). Neurological manifestations of long COVID: a single-center one-year experience. Neuropsychiatr. Dis. Treat. 19, 311–319. doi:10.2147/NDT.S387501
Teixido, L., Andreeva, E., Gartmann, J., Lemhofer, C., Sturm, C., and Gutenbrunner, C. (2023). Outpatient rehabilitative care for patients with Long-COVID - a guideline-based clinical practice guideline. Laryngorhinootologie 102 (7), 521–532. doi:10.1055/a-1985-0450
Tosato, M., Ciciarello, F., Zazzara, M. B., Pais, C., Savera, G., Picca, A., et al. (2022). Nutraceuticals and dietary supplements for older adults with long COVID-19. Clin. Geriatr. Med. 38 (3), 565–591. doi:10.1016/j.cger.2022.04.004
Vieth, R. (2022). Critique of public health guidance for vitamin D and sun exposure in the context of cancer and COVID-19. Anticancer Res. 42 (10), 5027–5034. doi:10.21873/anticanres.16011
Vollbracht, C., and Kraft, K. (2021). Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue. Nutrients 13 (4), 1154. doi:10.3390/nu13041154
Walker, S., Goodfellow, H., Pookarnjanamorakot, P., Murray, E., Bindman, J., Blandford, A., et al. (2023). Impact of fatigue as the primary determinant of functional limitations among patients with post-COVID-19 syndrome: a cross-sectional observational study. BMJ Open 13 (6), e069217. doi:10.1136/bmjopen-2022-069217
World Health Organization (2021). A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021 . Geneva: World Health Organization .
Yang, F., Zhao, H., Liu, H., Wu, X., and Li, Y. (2021). Manifestations and mechanisms of central nervous system damage caused by SARS-CoV-2. Brain Res. Bull. 177, 155–163. doi:10.1016/j.brainresbull.2021.09.015
Zaa, C. A., Espitia, C., Reyes-Barrera, K. L., An, Z., and Velasco-Velazquez, M. A. (2023). Neuroprotective agents with therapeutic potential for COVID-19. Biomolecules 13 (11), 1585. doi:10.3390/biom13111585
Zhang, C., Wang, D. F., Zhang, Z., Han, D., and Yang, K. (2017). EGb 761 protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury and exerts inhibitory effect on the ATM pathway. J. Microbiol. Biotechnol. 27 (3), 584–590. doi:10.4014/jmb.1611.11024
Zifko, U. A., Yacob, M., Braun, B. J., and Dietz, G. P. H. (2022). Alleviation of post-COVID-19 cognitive deficits by treatment with EGb 761 ® : a case series. Am. J. Case Rep. 23, e937094. doi:10.12659/AJCR.937094
Keywords: post-acute covid, long-COVID syndrome, cognitive impairment, management, Ginkgo biloba extract, EGb 761 ®
Citation: Zifko U, Guendling K, Seet R and Kasper S (2024) Management of cognitive impairment associated with post-COVID-19 syndrome: recommendations for primary care. Front. Pharmacol. 15:1338235. doi: 10.3389/fphar.2024.1338235
Received: 14 November 2023; Accepted: 22 March 2024; Published: 22 April 2024.
Reviewed by:
Copyright © 2024 Zifko, Guendling, Seet and Kasper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Udo Zifko, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 19 April 2024
A longitudinal cohort study on the use of health and care services by older adults living at home with/without dementia before and during the COVID-19 pandemic: the HUNT study
- Tanja Louise Ibsen 1 ,
- Bjørn Heine Strand 1 , 2 , 3 ,
- Sverre Bergh 1 , 4 ,
- Gill Livingston 5 , 6 ,
- Hilde Lurås 7 , 8 ,
- Svenn-Erik Mamelund 9 ,
- Richard Oude Voshaar 10 ,
- Anne Marie Mork Rokstad 1 , 11 ,
- Pernille Thingstad 12 , 13 ,
- Debby Gerritsen 14 &
- Geir Selbæk 1 , 15 , 16
BMC Health Services Research volume 24 , Article number: 485 ( 2024 ) Cite this article
92 Accesses
Metrics details
Older adults and people with dementia were anticipated to be particularly unable to use health and care services during the lockdown period following the COVID-19 pandemic. To better prepare for future pandemics, we aimed to investigate whether the use of health and care services changed during the pandemic and whether those at older ages and/or dementia experienced a higher degree of change than that observed by their counterparts.
Data from the Norwegian Trøndelag Health Study (HUNT4 70 + , 2017–2019) were linked to two national health registries that have individual-level data on the use of primary and specialist health and care services. A multilevel mixed-effects linear regression model was used to calculate changes in the use of services from 18 months before the lockdown, (12 March 2020) to 18 months after the lockdown.
The study sample included 10,607 participants, 54% were women and 11% had dementia. The mean age was 76 years (SD: 5.7, range: 68–102 years). A decrease in primary health and care service use, except for contact with general practitioners (GPs), was observed during the lockdown period for people with dementia ( p < 0.001) and those aged ≥ 80 years without dementia ( p = 0.006), compared to the 6-month period before the lockdown. The use of specialist health services decreased during the lockdown period for all groups ( p ≤ 0.011), except for those aged < 80 years with dementia. Service use reached levels comparable to pre-pandemic data within one year after the lockdown.
Older adults experienced an immediate reduction in the use of health and care services, other than GP contacts, during the first wave of the COVID-19 pandemic. Within primary care services, people with dementia demonstrated a more pronounced reduction than that observed in people without dementia; otherwise, the variations related to age and dementia status were small. Both groups returned to services levels similar to those during the pre-pandemic period within one year after the lockdown. The increase in GP contacts may indicate a need to reallocate resources to primary health services during future pandemics.

Trial registration
The study is registered at ClinicalTrials.gov, with the identification number NCT 04792086.
Peer Review reports
In Norway, similar to most European countries [ 1 , 2 , 3 ], the first wave of the COVID-19 pandemic lasted from 12 March to 15 June 2020 [ 4 ]. During this period, strict infection control measures were introduced to minimise the number of infected people. Health and care services were reduced or locked down, because health professionals were transferred to COVID-19-related services, or hospital wards were reserved for COVID-19 patients. Facilities such as day care services were closed to prevent the spread of infection through social contact, and some services were employed with digital technology. People were urged to stay at home to maintain social distancing and prevent the spread of the virus [ 4 ].
The strict infection control measures aimed mainly to prevent people from hospitalisation and/or death by COVID-19. By 13 November 2022 (last published data), Norway recorded 4,399 cumulative COVID-19-related deaths, of which approximately two-thirds occurred in 2022 (in people of an average age of 85.6 years in 2022) [ 5 ]. From March 2020 to March 2021, compared to the mean all-cause mortality from 2016 to 2019 as a reference, Norway recorded significantly lower all-cause mortality than those recorded by other European Union countries [ 6 ], indicating that Norway had a successful public health strategy. The topic being raised in the present paper, is how infection control measures affected the use of health and care services by the older population, to better prepare ourselves for future health crisis like a pandemic.
Older adults are particularly vulnerable to COVID-19 and at a higher risk of hospitalisation and death [ 7 ]. People with dementia are anticipated to have an even higher risk of mortality than that of people without dementia, because of an impaired immune system [ 8 ]. Fearing the virus, some older adults personally imposed strict infection control measures and cancelled scheduled healthcare appointments. A German study, including participants aged ≥ 73 years, has reported that approximately 30% of the participants reduced or cancelled their medical consultations during the first wave of the pandemic [ 1 ]. A qualitative study including participants aged 65–79 years from Portugal, Brazil, and the United Kingdom has reported that the majority refrained from face-to-face contact with their family doctors in the first wave of the pandemic, as it implied using public transport making social distancing difficult [ 2 ]. Some health and care services have been replaced with online or telephone consultations, which have been beneficial for some parts of the population and challenging for others, especially older adults [ 2 , 3 , 9 ].
People with dementia often need health and care services and practical assistance in their homes to manage their everyday lives [ 10 ]. A Norwegian study including 105 caregivers of people with dementia has reported that 60% experienced a reduction or full cessation of formal care during the first wave of the pandemic as the services were cancelled by the service provider [ 11 ]. This is in line with studies from Sweden and the USA, which reported a significant drop in the use of health and care services during this period [ 12 , 13 ]. However, how the use of primary and specialist healthcare services affected older adults, including people with dementia, as society began a cautious reopening after the first wave of the pandemic remains unclear. A study from the USA conducted a predictive analysis for the post-lockdown period (June 2020–October 2021) on inpatient, outpatient, and emergency services. They found that people with mild cognitive impairment (MCI), Alzheimer’s disease, and related dementia experienced greater and more sustained disruptions in primary and specialist health and care service use than those experienced by people without MCI or dementia [ 13 ].
In the present study, we used a large population-based dataset from the Norwegian Trøndelag Health Study (HUNT) [ 14 ], linked to national registry data on primary and specialist health and care services, to investigate whether the use of health and care services changed during the pandemic, and those with older ages and/or dementia had a higher degree of change than that observed in their counterparts.
Study design and setting
We used a longitudinal cohort design, linking participant data on sex, year of birth, and cognitive status from the HUNT4 70 + survey with later registry data on the use of health and care services from 12 September 2018 to 11 September 2021. This time period equals 18 months before- and 18 months after the Norwegian lockdown on 12 March 2020. This 36-month period was grouped into six periods of six months each, including three pre-lockdown periods (pre1, pre2, and pre3), one lockdown period, and two post-lockdown periods (post1 and post2) (Fig. 1 ). We included a longer lockdown period than the generally denoted period from March to June 2020, as the reopening started slowly, and many older adults imposed strict social distancing on themselves. The next period, 12 September 2020 to 11 March 2021 also included periods with restrictions on social life and activity, such as a maximum of five people gathering and recommendations for wearing a face mask where maintaining distance is difficult. In the last period from 12 March to 11 September 2021, all infection control measures were gradually lifted until Norway was completely reopened on 25 September 2021 [ 4 ]. Trøndelag, the county where the study was conducted, followed national infection control regulations.
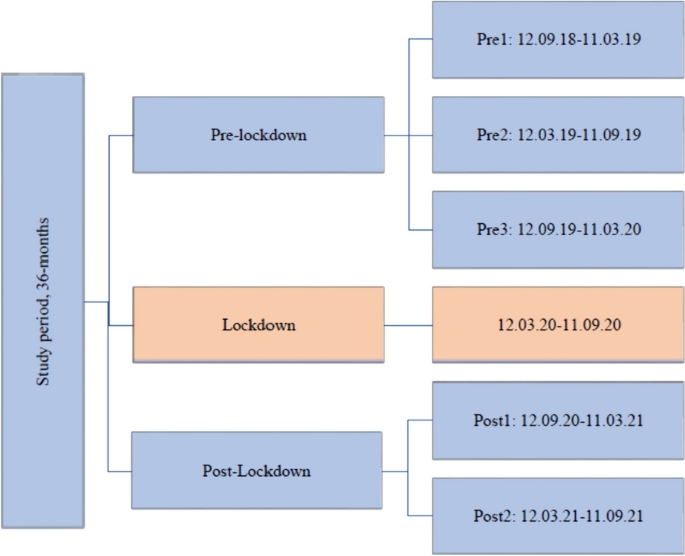
Flow-chart of the study periods
Participants
The study included participants aged > 70 years in the fourth wave of the HUNT Study (HUNT4 70 +), which took place between September 2017 and March 2019. The HUNT is a population-based study that has invited the entire adult population from the same geographic area, North-Trøndelag, in four waves, first time in 1984 [ 14 ]. As North-Trøndelag does not comprise any large cities, a random sample of people aged > 70 years from a city in Trondheim (212,000 inhabitants) was also invited. In total, 11,675 participants were included, with 9,930 from North-Trøndelag (response rate 51%) and 1,745 from Trondheim (response rate 34%). We do not judge that there is likely to be any systematic bias introduced by the difference in response rates in different municipalities as the people living at home are similar populations.”. The participants answered a questionnaire that included socio-demographic and clinical data, and they attended a comprehensive clinical evaluation by health professionals [ 15 ]. Participants without sufficient information on their cognitive status ( n = 202) and nursing home residents ( n = 866) were excluded (Fig. 2 ). The mean age (76 years, SD 5.7 years) of those included was lower than that of those excluded (82 years, SD 7.9) ( p < 0.001). The study population included 10,607 participants with complete data on cognitive status. We do not have information on dementia status on the population not included in HUNT4 70 + .
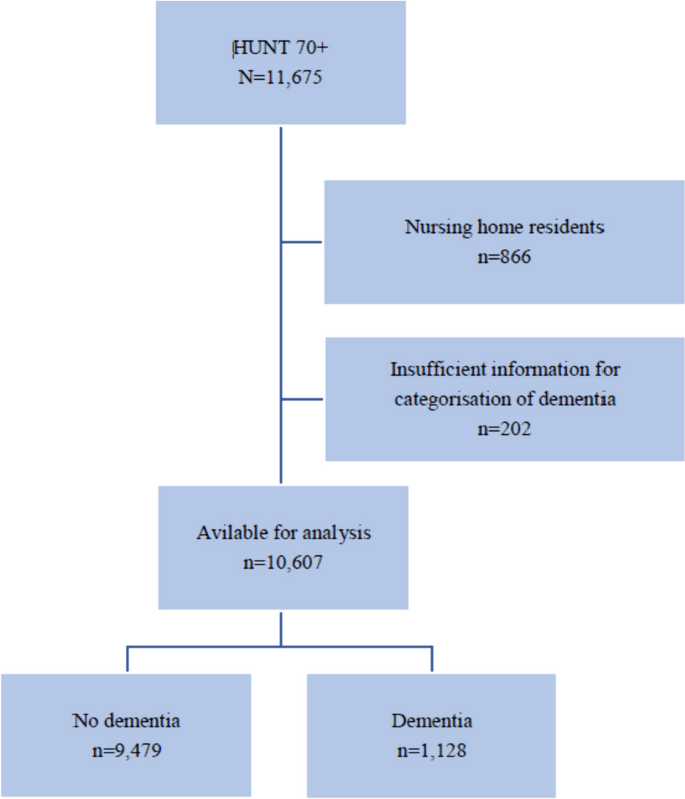
Flow-chart of included participants. HUNT4 70 + : The fourth wave of the Trøndelag health study, 70 year and older cohort
Dementia diagnosis
Two specialists from a diagnostic workgroup of nine medical doctors with comprehensive scientific and clinical expertise (geriatrics, old-age psychiatry, or neurology) independently diagnosed each patient with dementia using the Diagnostic and Statistical Manual of Mental Disorders-5 [ 16 ]. Discrepancies were resolved and consensuses were obtained by the involvement of a third expert. During the diagnostic process, the experts had access to all relevant information from the HUNT4 70 + dataset, such as education, function in activities of daily living, neuropsychiatric symptoms, onset and course of cognitive symptoms, cognitive tests (the Montreal Cognitive Assessment (MoCA) scale [ 17 ] and the Word List Memory Task (WLMT) [ 18 ], and structured interviews with the closest family proxy. A more comprehensive description of the diagnostic process has been published [ 15 ].
Health and care services
Data from two national registries were collected for the entire study period, from September 2018 to September 2021. Health and care services used in primary health care were obtained from The Norwegian Registry of Primary Health Care [ 19 ]. This registry includes individual-level data on municipal health services (contacts with general practitioners (GPs), emergency rooms, and physiotherapists) and care services (care, such as home nurses, and practical assistance in the recipient’s home, day care, respite services and short-term nursing home stays, municipal housing, and nursing home admission) [ 20 ]. Information on the use of specialist health services was based on data from the Norwegian Patient Registry (NPR) [ 21 ]. The NPR holds individual-level data on patients’ use of specialist health services (contacts with somatic hospitals, mental health care, and rehabilitation institutions). The NPR also registers whether the contact was an outpatient consultation, hospitalisation, or day-treatment [ 20 ].
Data were analysed using the STATA 16 software [ 22 ]. Participant characteristics are reported as means with SD, frequencies, or percentages, as appropriate. Those who were admitted to a nursing home ( n = 364) or died ( n = 821) during the study period were censored and participated in only half of the person-time during the study period. Duplicates were removed (3,293 observations). The mean number of health and care services per person in each period (with 95% confidence interval [CI]) was predicted from a multilevel mixed-effects linear regression model with random intercept, where random effects varied across the individuals. In the regression model, the number of services per person was the outcome variable and sex, age, cognitive status (no dementia/dementia), and period were covariates.Age and cognitive status are relevant confounders to address the aim of the present study, and sex is included as a key sociodemographic measure in epidemiological research. [ 23 , 24 ]. To allow for different time trends by cognitive status group, the interaction term period by cognitive status was included in the regression model. In the predictions, the adjusted variables were fixed at their mean values. The significance level was set at p < 0.05. To investigate the use of health and care services before and during the pandemic, the number of care services implemented within each period and the number of contacts within each period for primary and specialist health services were aggregated. Hence, for care services, we used the date on which the service was implemented, for example the date on which practical assistance at home was implemented. For health services, we used the date when the service occurred, for example, the date a person had contact with a GP or the date a person had contact with a hospital, either for outpatient consultation, hospitalisation, or day-treatment.
In the Results section, we report significant differences between the lockdown period and all the pre- and post-lockdown periods, and between pre2 and post2, as these periods comprise the same seasonal months, one year before and one year after the lockdown, respectively.
The study included 10,607 participants, of whom 54% were women, and 11% had dementia (Table 1 ). The mean age of the participants on 1 January 2017 was 76 years (SD 5.7, range: 68–102 years), and 7,769 participants (73%) were < 80 years old. During the 36-month follow-up period, the study sample was reduced by 10% (from 10,607 to 9,568) due to censoring for death and/or nursing home admission (Table 2 ). The dropout rate was higher in those with dementia than in those without dementia (37% vs. 7%, p < 0.001). During these 36-months, the total number of contacts with primary health services was 554,061, which corresponded to 9.2 contacts per person per 6-month period (Table 3 ). People with dementia had more contact with health services in the municipality than the contact made by those without dementia (11.3 vs. 8.8 contacts per person per 6-month period, p < 0.001). The total number of care services implemented for our study population was 20,411, which corresponded to 0.3 care services per person per 6-month period. People with dementia received more care services than those received by people without dementia (1.2 vs. 0.2 care services per person per 6-month period, p < 0.001). The total number of contacts with specialist health services was 141,994, which corresponded to 2.3 contacts per person per 6-month period. People with dementia had less contact with specialist health services than the contact made by those without dementia (2.2 vs. 2.6 contacts per person per 6-month period, p < 0.001).
Primary health and care services
Health services.
During the 36-month study period, contact with GPs was the most used health service (66%), followed by physiotherapy services (30%), and contact with GPs in emergency rooms (4%).
The following model only presents contact with GPs, including GPs in emergency rooms, as contact with GPs was the most frequently used primary health service.
The age- and sex-adjusted model (Fig. 3 ) shows that for those aged < 80 years with dementia, the mean number of GP contacts during the lockdown period was higher than that during pre1 (1.27, p < 0.001) and pre3 (0.82, p = 0.002) and lower than that during post1 (1.67, p < 0.001) and post2 (0.84, p < 0.002). The mean number of GP contacts during post2 was higher than that during pre2 (0.32, p < 0.001).
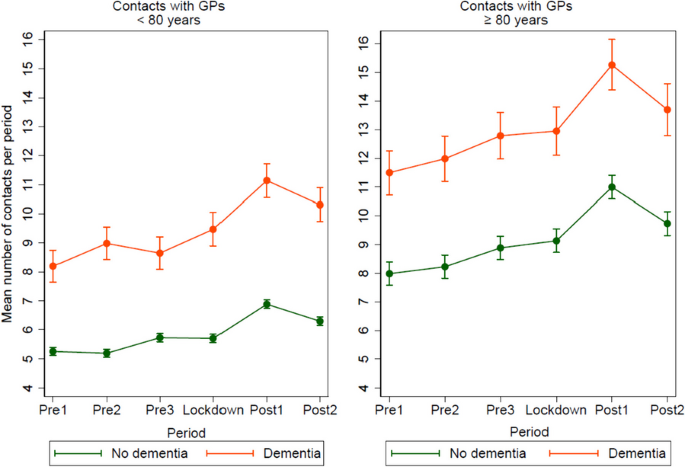
Mean number of registered contacts with general practitioners (GPs) per period, pre-lockdown, during lockdown and post-lockdown, including GPs at emergency rooms, for participants < 80 versus ≥ 80 years, divided in people with- or without dementia. Mean number of contacts was predicted in a mixed-effects linear regression model adjusted by period, cognitive status, sex, age, and the interaction period*cognitive status. In the predictions, the adjustment variables age and sex were fixed at the mean values
For those without dementia, the mean number of GP contacts during the lockdown was higher than that during pre1 (0.45, p < 0.001) and pre2 (0.51, p < 0.001) and lower than that during post1 (1.18, p < 0.001) and post2 (0.59, p < 0.001). The mean number of GP contacts during post2 was higher than that during pre2 (1.11, p < 0.001).
For those aged ≥ 80 years with dementia, the mean number of GP contacts during the lockdown was higher than that during pre1 (1.45, p < 0.001) and pre2 (0.96, p = 0.015) and lower than that during post1 (2.31, p < 0.001). The mean number of GP contacts during post2 was higher than that during pre2 (1.72, p < 0.001).
For those without dementia, the mean number of GP contacts during the lockdown was higher than that during pre1 (1.15, p < 0.001) and pre2 (0.91, p < 0.001) and lower than that during post1 (1.86, p < 0.001) and post2 (0.60, p < 0.002). The mean number of GP contacts during post2 was higher than that during pre2 (1.51, p < 0.001).
Care services
During the 36-month study period, care and practical assistance at home represented the largest service group (69%), followed by short-term nursing home stays and respite services (21%), nursing home admissions (4%), municipal housing (3%), and day care services (4%). The following model presents all combined care services.
The age- and sex-adjusted model (Fig. 4 ) shows that for those aged < 80 years with dementia, the mean number of care services implemented during the lockdown was lower than that during pre3 (0.37, p < 0.001) and post1 (0.43, p < 0.001). The mean number of care services implemented in post2 was higher than that during pre2 (0.13, p = 0.039).
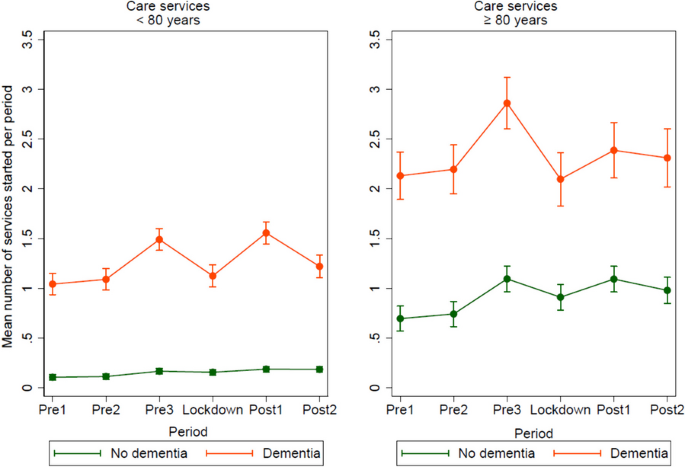
Mean number of care services implemented per period, pre-lockdown, during lockdown and post-lockdown, as health care and practical assistance in the home, day- and respite services, short-term institutional stay, and nursing home admission, for participants < 80 versus ≥ 80 years, divided in people with- and without dementia. Mean number of care services implemented was predicted in a mixed-effects linear regression model adjusted by period, cognitive status, sex, age, and the interaction period*cognitive status. In the predictions, the adjustment variables age and sex were fixed at the mean values
For those without dementia, the mean number of care services implemented during the lockdown was higher than that during pre1 (0.5, p = 0.001) and pre2 (0.04, p = 0.005) and lower than that during post1 (0.03, p = 0.044). The mean number of care services implemented during post2 was higher than that during pre2 (0.07, p < 0.001).
For those aged ≥ 80 years with dementia, the mean number of care services implemented during the lockdown was lower than that during pre3 (0.76, p < 0.001).
For those without dementia, the mean number of care services implemented during the lockdown was higher than that during pre1 (0.22, p = 0.001) and pre2 (0.17, p = 0.011) and lower than that during pre3 (0.18, p = 0.006) and post1 (0.18, p = 0.007). The mean number of care services implemented during post2 was higher than that during pre2 (0.24, p < 0.001).
Specialist health services
During the study period, service provision from somatic hospitals was the most used service (96%), followed by mental health care (3%), and treatment at a rehabilitation institution (1%). Somatic hospital services included outpatient consultations (88%), hospitalisation (9%), and daily treatment (3%). The following model only presents contacts with somatic hospital services, as this is the most frequently used specialist health service.
The age- and sex-adjusted models (Fig. 5 ) show that for those aged < 80 years with dementia, the mean number of contacts with somatic hospital services during the lockdown was lower than that during post1 (0.67, p = 0.002) and post2 (0.48, p = 0.025). The mean number of contacts with somatic hospital services in post2 was higher than that during pre2 (0.61, p = 0.004).
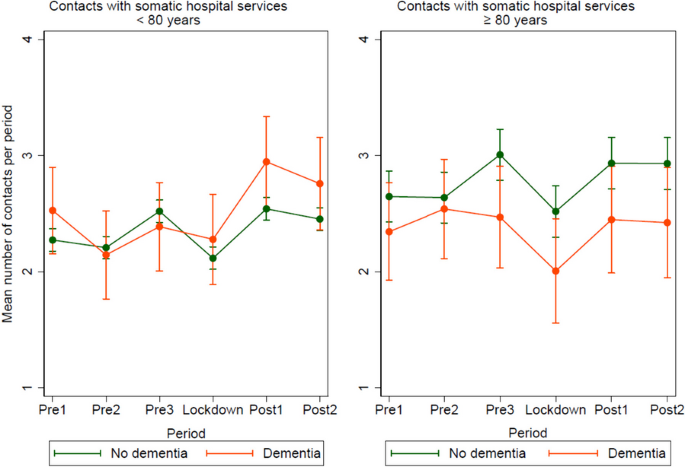
Mean number of registered contacts with somatic hospital services per period, pre-lockdown, during lockdown and post-lockdown, for participants < 80 versus ≥ 80 years, divided in people with- or without dementia. Mean number of contacts was predicted in a mixed-effects linear regression model adjusted by period, cognitive status, sex, age, and the interaction period*cognitive status. In the predictions, the adjustment variables age and sex were fixed at the mean values
For those without dementia, the mean number of contacts with somatic hospital services during the lockdown was lower than that during pre1 (0.16, p = 0.002), pre3 (0.40, p < 0.001), post1 (0.43, p < 0.001), and post2 (0.34, p < 0.001). The mean number of contacts with somatic hospital services in post2 was higher than that during pre2 (0.25, p < 0.001).
For those aged ≥ 80 years with dementia, the mean number of contacts with somatic hospital services during the lockdown was lower than that during pre2 (0.54, p = 0.003), pre3 (0.46, p = 0.011), post1 (0.44, p = 0.022), and post2 (0.42, p = 0.040).
For those without dementia, the mean number of contacts with somatic hospital services during the lockdown was lower than that during pre3 (0.49, p < 0.001), post1 (0.41, p < 0.001), and post2 (0.41, p < 0.001). The mean number of contacts with somatic hospital services in post2 was higher than that during pre2 (0.29, p = 0.001).
This population-based study revealed that people with dementia experienced a larger decrease in the use of primary care services implemented during the lockdown than that experienced by people without dementia. Contact with GPs was maintained at a normal level or increased in both groups during the lockdown. The use of specialist health services decreased in both groups during the lockdown period except for those aged < 80 years with dementia. The use of primary health and care services, and specialist health services was at the same or higher-level post-lockdown (post2) as pre-lockdown (pre2). Collectively, these results indicate an increased burden on primary health services during the lockdown.
Both cognitive groups had a similar number of GP contacts during lockdown as pre-lockdown. Those aged < 80 years with dementia experienced an increased number of GP contacts during the lockdown compared to the numbers during the 6-month period before the lockdown (pre3). Furthermore, all the groups had an increased number of GP contacts in the first 6-months period post-lockdown (post1). Unfortunately, we were unable to identify whether the consultations were digital in our material; however, digital consultations may have contributed to maintaining contact with GPs during the pandemic. This corresponds with the results of a previous study which has reported that the Norwegian population experienced an increased use of telephone and video consultations during the pandemic [ 3 ]. However, a survey during the pandemic in the same study population as that of the present study (HUNT4 70 +) revealed that only 8% reported contact with healthcare professionals via screen-based media or telephone at least once a month during the pandemic [ 9 ]. In addition, a survey of video consultations among Norwegian GPs during the pandemic revealed that video consultations were unsuitable for the oldest population [ 25 ].
The results of the present study may indicate that GPs managed to serve older adults in Norway during the pandemic and that the cancellations of medical consultations described among older adults in other countries [ 1 , 2 ] have been less extensive in Norway. Meanwhile, contact with GPs may have shifted towards more severe cases, where patients in need of specialist health services who postponed contact because of COVID-19 used the primary care service. In addition, the increase in GP contact post-lockdown may imply an increased stress level among older adults and an increase in health problems during the lockdown, which will be discussed in more detail in a later section.
Our finding that people with dementia experienced a larger decrease in the number of care services implemented during the lockdown than that experienced by people without dementia is in line with those of earlier studies [ 11 , 13 ]. This is most likely a consequence of the fact that people with dementia use care services more often and thus, are more affected when such services are reduced or locked down. Interestingly, those with dementia in both age groups experienced a significant increase in new services implemented in the 6-month period before the lockdown (pre3). However, the possible cause for the increase in care services implemented, such as a reduction in other services or societal changes during this period, remains unconfirmed. The most likely explanation is an increase in service needs related to dementia progression, although some random fluctuations cannot be ruled out.
Care service providers have reported a deterioration in older adults’ health during the pandemic related to the absence of social support, which, in turn, has led to less support with meals, practical help, and physical activity [ 26 ]. Next of kin reported that people with dementia had a reduction in cognitive- and functional abilities because of the limited possibility of meaningful activities and mental stimulation when they had to stay at home [ 27 , 28 ]. Furthermore, a lack of social connections [ 29 ] and perceived social support [ 30 ] are associated with cognitive decline and depression. Based on these findings, it can be assumed that the need for care services may be the same or higher post-lockdown than that in the 6-month period before the pandemic (pre3). However, the number of care services implemented post-lockdown (post2) was at the same level as that at pre-lockdown (pre2).
This study revealed that somatic hospital services for those aged ≥ 80 years were the only services with a lower level of contact during the lockdown period than during the comparable pre-lockdown period (pre2). Both those with and without dementia had a decrease in somatic hospital services during the lockdown period, compared to the 6-months period before the lockdown. This corresponds with findings from an Italian study conducted in the autumn of 2020, reporting that hospitalisations and outpatient visits among older adults aged ≥ 65 years were reduced by 18.3% during the pandemic [ 31 ].
The decrease in the use of somatic hospital services during the lockdown observed in the present study was most likely related to strict infection control measures that prevented a widespread COVID-19 outbreak. Furthermore, it may be interpreted as a precautionary measure taken to minimize the risk of exposing older adults to hospitals, where a considerable number were affected by COVID-19. Hospital services experienced the greatest decline in activity during the lockdown due to preparedness for COVID-19 patients [ 32 ]. In the present study, all the groups returned to the same or a higher level of contact with somatic hospital services post-lockdown (post2), than they had pre-lockdown (pre2). Conversely, a study from the USA has suggested that people with dementia or MCI would experience more sustained disruption in primary and specialist health services than that experienced by people without such diagnoses [ 13 ]. Another study from the USA has revealed that those with comorbidities, often present among people with dementia, were at a higher risk of delayed or missed care during the pandemic [ 33 ]. The contrast in the findings may be related to differences in the healthcare system. In addition, the World Health Organization has reported disruptions in both primary and specialist health services worldwide two years into the pandemic. High-income countries reported fewer service disruptions than those reported by low-income ones [ 34 ]. The increase in GP contact post-lockdown in the present study may indicate that primary health services have been able to relieve specialist health services in Norway, so that people with dementia and others in need of specialist health services may be prioritised.
The variation in the frequency of contact with both somatic hospital services and GPs may be observed in the context of normal seasonal variations, where contact might be higher in the autumn and winter months (pre1, pre3, and post2) than in the spring and summer months (pre2, lockdown, and post2). However, the Norwegian Institute of Public Health has reported that the seasonal flu outbreak from December 2019 to March 2020, which corresponds with the 6-month period before the lockdown (pre3), was limited compared to those in previous years [ 35 ]. Thus, normal variations due to seasonal flu cannot provide a full explanation for more contact with GPs and somatic hospital services in the 6-month period before lockdown (pre3). The next seasonal flu, expected from December 2020 to March 2021 (post1), did not appear as expected, most likely because of the infection control measures in connection with the COVID-19 outbreak [ 36 , 37 ]. The increase in the frequency of contact with GPs and somatic hospital services detected in the 6-month period after the lockdown (post1) may be explained by the fact that people had less contact with these services for diseases other than COVID-19 during the first wave of the pandemic [ 32 ], and that these consultations accumulated when society started reopening. Furthermore, the increase in contact with GPs and somatic hospital services after the lockdown may be explained by the increased contact between people, which may have caused an increased spread of infections [ 37 ].
Finally, the increase in mental health problems during the pandemic [ 27 , 28 , 30 ], may have required additional medical supervision. Studies have reported an increase in depression among older adults during the pandemic, a related increase in the prescription of antidepressant medication [ 30 , 38 ], and the need for primary health services, such as GPs, and specialist services, such as hospital services [ 38 ].
Strength and limitations
The main strength of the present study is its large population-based survey sample merged with unique national registry data on primary and specialist health care services. This provided objective data regarding the participants’ service use. Despite the large study sample, all the participants were from the middle region of Norway, which may differ from the population in other parts of the country and outside Norway. Furthermore, the study sample was a homogenous group of participants mainly born in Norway, and the results cannot be generalised to other ethnic groups. Although the diagnostic process for dementia was thorough, the diagnosis was based on collected research data without access to imaging or biomarker data which may have caused misclassification. As our goal was to estimate the actual change in service use based on dementia status among younger and older adults, the analysis does not include health-related covariates such as comorbidity and functional level. Finally, the information on dementia status was collected from 2017 to 2019 and may have changed during the study period from September 2018 to September 2021.
The use of primary care and specialist health services was immediately reduced during the COVID-19 lockdown period. Within primary care services, people with dementia experienced a more pronounced reduction than that experienced by people without dementia; however, age and dementia status only demonstrated small variations. One year after the lockdown, service provisions returned to a level similar to or higher than that of one year before the lockdown for all groups. Our findings indicate that infection control and management limited the scope of action within care services and specialist health services during the lockdown, leaving GPs on the front line to manage medical problems and psychological stress in the population. In any future pandemic, the reallocation of resources for primary health services could make us better equipped to meet the needs of the population.
Availability of data and materials
The data that support the findings of this study are available from the HUNT database and the Norwegian registry database, Helsedata, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the HUNT database and the Norwegian registry database Helsedata.
Brandl C, Zimmermann ME, Günther F, Dietl A, Küchenhoff H, Loss J, Stark KJ, Heid IM. Changes in healthcare seeking and lifestyle in old aged individuals during COVID-19 lockdown in Germany: the population-based AugUR study. BMC Geriatr. 2022;22(1):34.
Article CAS PubMed PubMed Central Google Scholar
von Humboldt S, Low G, Leal I. Health Service Accessibility, Mental Health, and Changes in Behavior during the COVID-19 Pandemic: A Qualitative Study of Older Adults. Int J Environ Res Public Health. 2022;19(7):4277.
Article Google Scholar
Tu K, Sarkadi Kristiansson R, Gronsbell J, de Lusignan S, Flottorp S, Goh LH, Hallinan CM, Hoang U, Kang SY, Kim YS, et al. Changes in primary care visits arising from the COVID-19 pandemic: an international comparative study by the International Consortium of Primary Care Big Data Researchers (INTRePID). BMJ Open. 2022;12(5):e059130.
Article PubMed Google Scholar
Governmentof Norway: Timeline: News from Norwegian Ministries about the Coronavirus disease Covid-19 [ https://www.regjeringen.no/no/tema/Koronasituasjonen/tidslinje-koronaviruset/id2692402/ ]. Accessed 12 May 2023.
Norwegian Institute of Public Health: Dødelighet i Norge under koronapandemien 2020 til høsten 2022 [Mortality in Norway during the corona pandemic 2020 to autumn 2022] [ https://www.fhi.no/publ/2022/Dodelighet-under-pandemien/ ]. Accessed 22 June 2023.
Statistics Norway: Hvordan gikk det? Korona i Norge og EU [How did it go? Corona in Norway and EU] [ https://www.ssb.no/helse/faktaside/konsekvenser-av-korona ]. Accessed 22 June 2023.
Ho FK, Petermann-Rocha F, Gray SR, Jani BD, Katikireddi SV, Niedzwiedz CL, Foster H, Hastie CE, Mackay DF, Gill JMR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE. 2020;15(11):e0241824.
Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, Bianchetti L, Trabucchi M. Clinical Presentation of COVID19 in Dementia Patients. J Nutr Health Aging. 2020;24(6):560–2.
Eriksen SRA, Selbæk G, Bjørkløf G, Tveito M, Bergh S, Langhammer A, Næss M, Ibsen T. Bruk av skjermbaserte medier blant eldre under covid-19-pandemien En HUNT-studie [Use of screen-based media among older people during the COVID-19 pandemic A HUNT study]. Sykepleien Forskning. 2022;17(88131):e-88131.
Google Scholar
Bradbury KM, Moody E, Aubrecht K, Sim M, Rothfus M. Equity in Changes to Dementia Care in the Community during the First Wave of the COVID-19 Pandemic in High Income Countries: A Scoping Review. Societies. 2022;12(2):30.
Vislapuu M, Angeles RC, Berge LI, Kjerstad E, Gedde MH, Husebo BS. The consequences of COVID-19 lockdown for formal and informal resource utilization among home-dwelling people with dementia: results from the prospective PAN.DEM study. BMC Health Serv Res. 2021;21(1):1003.
Article PubMed PubMed Central Google Scholar
Ekman B, Arvidsson E, Thulesius H, Wilkens J, Cronberg O. Impact of the Covid-19 pandemic on primary care utilization: evidence from Sweden using national register data. BMC Res Notes. 2021;14(1):424.
Tannous J, Pan A, Bako A, Potter T, Jones SL, Janjan N, Smith ML, Seshadri S, Ory MG, Vahidy FS. COVID-19 associated disruptions in routine health care of people with mild cognitive impairment or dementia. Alzheimers Dement (Amst). 2022;14(1):e12323.
Åsvold BO, Langhammer A, Rehn TA, Kjelvik G, Grøntvedt TV, Sørgjerd EP, Fenstad JS, Heggland J, Holmen O, Stuifbergen MC, Vikjord SAA, Brumpton BM, Skjellegrind HK, Thingstad P, Sund ER, Selbæk G, Mork PJ, Rangul V, Hveem K, Næss M, Krokstad S. Cohort Profile Update: The HUNT Study. Norway Int J Epidemiol. 2023;52(1):80–91.
Gjøra L, Strand BH, Bergh S, Borza T, Brækhus A, Engedal K, Johannessen A, Kvello-Alme M, Krokstad S, Livingston G, et al. Current and Future Prevalence Estimates of Mild Cognitive Impairment, Dementia, and Its Subtypes in a Population-Based Sample of People 70 Years and Older in Norway: The HUNT Study. J Alzheimers Dis. 2021;79(3):1213–26.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Warshington, DC: American Psychiatric Association; 2013.
Book Google Scholar
Nasreddine Z, Phillips N, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings J, Chertkow H. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. JAGS. 2005;53:695–9.
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65.
Article CAS PubMed Google Scholar
Norwegian Registry for Primary Health Care [ https://helsedata.no/en/forvaltere/norwegian-directorate-of-health/norwegian-registry-for-primary-health-care-kpr/ ]. Accessed 12 May 2023.
Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE. The Norwegian Patient Registry and the Norwegian Registry for Primary Health Care: Research potential of two nationwide health-care registries. Scand J Public Health. 2020;48(1):49–55.
Norwegian Patient Registry [ https://helsedata.no/en/forvaltere/norwegian-directorate-of-health/norwegian-patient-registry-npr/ ]. Accessed 12 May 2023.
StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp LLC; 2019.
Zhang X, Dupre ME, Qiu L, Zhou W, Zhao Y, Gu D. Age and sex differences in the association between access to medical care and health outcomes among older Chinese. BMC Health Serv Res. 2018;18(1):1004.
Bale TL, Epperson CN. Sex as a Biological Variable: Who, What, When, Why, and How. Neuropsychopharmacology. 2017;42(2):386–96.
Johnsen TM, Norberg BL, Kristiansen E, Zanaboni P, Austad B, Krogh FH, Getz L. Suitability of Video Consultations During the COVID-19 Pandemic Lockdown: Cross-sectional Survey Among Norwegian General Practitioners. J Med Internet Res. 2021;23(2):e26433.
Bell SA, Krienke L, Brown A, Inloes J, Rettell Z, Wyte-Lake T. Barriers and facilitators to providing home-based care in a pandemic: policy and practice implications. BMC Geriatr. 2022;22(1):234.
Rokstad AMM, Røsvik J, Fossberg M, Eriksen S. The COVID-19 pandemic as experienced by the spouses of home-dwelling people with dementia - a qualitative study. BMC Geriatr. 2021;21(1):583.
Tuijt R, Frost R, Wilcock J, Robinson L, Manthorpe J, Rait G, Walters K. Life under lockdown and social restrictions - the experiences of people living with dementia and their carers during the COVID-19 pandemic in England. BMC Geriatr. 2021;21(1):301.
Morina N, Kip A, Hoppen TH, Priebe S, Meyer T. Potential impact of physical distancing on physical and mental health: a rapid narrative umbrella review of meta-analyses on the link between social connection and health. BMJ Open. 2021;11(3):e042335.
Greenblatt-Kimron L, Shinan-Altman S, Alperin M, Levkovich I. Depression and Medicine Use among Older Adults during the COVID-19 Pandemic: The Role of Psychosocial Resources and COVID-19 Perceived Susceptibility. Int J Environ Res Public Health. 2023;20(4):3398.
Vigezzi GP, Bertuccio P, Amerio A, Bosetti C, Gori D, Cavalieri d’Oro L, Iacoviello L, Stuckler D, Zucchi A, Gallus S, et al. Older Adults’ Access to Care during the COVID-19 Pandemic: Results from the LOckdown and LifeSTyles (LOST) in Lombardia Project. Int J Environ Res Public Health. 2022;19(18):11271.
Helgeland J, Telle KE, Grøsland M, Huseby BM, Håberg S, Lindman ASE. Admissions to Norwegian Hospitals during the COVID-19 Pandemic. Scand J Public Health. 2021;49(7):681–8.
Smith M, Vaughan Sarrazin M, Wang X, Nordby P, Yu M, DeLonay AJ, Jaffery J. Risk from delayed or missed care and non-COVID-19 outcomes for older patients with chronic conditions during the pandemic. J Am Geriatr Soc. 2022;70(5):1314–24.
World Health Organization. Third round of the global pulse survey on continuity of essential health services during the COVID-19 pandemic: November–December 2021. In. Geneva: World Health Organization; 2022.
Norwegian Institute of Public Health: Influensasesongen i Norge 2019–2020 [Influenza season in Norway 2019–2020] [ https://www.fhi.no/publ/2020/influensasesongen-i-norge-2019-2020/ ]. Accessed 22 June 2023.
Norwegian Institute of Public Health: Influensasesongen i Norge 2021–2022 [Influenza season in Norway 2021–2022] [ https://www.fhi.no/publ/2022/influensasesongen-i-norge-2021-2022/ ]. Accessed 22 June 2023.
Oh KB, Doherty TM, Vetter V, Bonanni P. Lifting non-pharmaceutical interventions following the COVID-19 pandemic - the quiet before the storm? Expert Rev Vaccines. 2022;21(11):1541–53.
Greig F, Perera G, Tsamakis K, Stewart R, Velayudhan L, Mueller C. Loneliness in older adult mental health services during the COVID-19 pandemic and before: Associations with disability, functioning and pharmacotherapy. Int J Geriatr Psychiatry. 2021;37(1):10.
PubMed Central Google Scholar
Download references
Acknowledgements
HUNT is a collaborative project between the HUNT Research Centre at the Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, the Trøndelag County Council, the Central Norway Regional Health Authority and the Norwegian Institute of Public Health. We would like to thank everyone who participated in HUNT 70+ for their valuable contributions to this research.
This study was supported by the Norwegian Health Association (grant no. 22687).
Author information
Authors and affiliations.
The Norwegian National Centre for Ageing and Health (Ageing and Health), Vestfold Hospital Trust, Tønsberg, Norway
Tanja Louise Ibsen, Bjørn Heine Strand, Sverre Bergh, Anne Marie Mork Rokstad & Geir Selbæk
Department of Geriatric Medicine, Oslo University Hospital, Oslo, Norway
Bjørn Heine Strand
Department of Physical Health and Ageing, Norwegian Institute of Public Health, Oslo, Norway
Research Centre for Age-Related Functional Decline and Disease (AFS), Innlandet Hospital Trust, Ottestad, Norway
Sverre Bergh
Division of Psychiatry, University College London, London, UK
Gill Livingston
Camden and Islington NHS Foundation Trust, London, UK
Health Services Research Unit, Akershus University Hospital, Oslo, Norway
Hilde Lurås
Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Centre for Research On Pandemics & Society (PANSOC), at Oslo Metropolitan University, Oslo, Norway
Svenn-Erik Mamelund
Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands
Richard Oude Voshaar
Faculty of Health Sciences and Social Care, Molde University College, Molde, Norway
Anne Marie Mork Rokstad
Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Science, Norwegian University of Science and Technology, Trondheim, Norway
Pernille Thingstad
Department of Health and Social Services, Trondheim Municipality, Trondheim, Norway
Department of Primary and Community Care, Research Institute for Medical Innovation, Radboudumc Alzheimer Center, Radboud University Medical Center, Nijmegen, Netherlands
Debby Gerritsen
Geir Selbæk
Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
You can also search for this author in PubMed Google Scholar
Contributions
GS led the study project and is responsible for the concept and design of the study, together with BHS, SB and TLI. BHS was a major contributor in the analysis prosses together with TLI. TLI, BHS, SB, GL, HL, SEM, ROV, AMMR, PT og GS contributed to interpreting the data. TLI drafted the paper, with substantially contributions from all the authors in revising the drafted work. DG made significant contributions on the revised version after peer review. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tanja Louise Ibsen .
Ethics declarations
Ethics approval and consent to particpate.
This study was approved by the Regional Committee for Medical and Health Research Ethics of Norway (REK Southeast B 182575). All methods were carried out in accordance with REK’s guidelines which correspond to the Declaration of Helsinki. The present study is part of a larger project registered at ClinicalTrials.gov (identification number: NCT 04792086). Informed written consent was obtained from all participants in the HUNT4 70 + study. Participants with reduced capacity to consent were included if they had a next of kin who consented on their behalf. In the consent form, it was thoroughly described that collected data can be linked to other registers in order to carry out approved research projects, as has been done in the present project.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ibsen, T.L., Strand, B.H., Bergh, S. et al. A longitudinal cohort study on the use of health and care services by older adults living at home with/without dementia before and during the COVID-19 pandemic: the HUNT study. BMC Health Serv Res 24 , 485 (2024). https://doi.org/10.1186/s12913-024-10846-y
Download citation
Received : 07 July 2023
Accepted : 11 March 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12913-024-10846-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health care services
- Older adults
- Longitudinal cohort study
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Aging Neurosci
Dementia as Risk Factor for Severe Coronavirus Disease 2019: A Case-Control Study
Mariantonietta pisaturo.
1 Infectious Diseases Unit, Department of Mental Health and Public Medicine, University of Campania Luigi Vanvitelli, Naples, Italy
Federica Calò
Antonio russo, clarissa camaioni, agnese giaccone.
2 Infectious Diseases Unit, Federico II University, Naples, Italy
Biagio Pinchera
Ivan gentile, filomena simeone.
3 Infectious Disease Unit, AORN Caserta, Caserta, Italy
Angelo Iodice
Paolo maggi, nicola coppola, associated data.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The aim of the present study was to investigate the outcome of patients with SARS-CoV-2 infection and dementia.
Patients and Methods
In a multicenter, observational, 1:2 matched case-control study all 23 patients with a history of dementia, hospitalized with a diagnosis of SARS-CoV-2 infection from February 28th 2020 to January 31st 2021 were enrolled. For each Case, 2 patients without dementia observed in the same period study, pair matched for gender, age (±5 years), PaO 2 /FiO 2 (P/F) ratio at admission (<200, or >200), number of comorbidities (±1; excluding dementia) were chosen (Control group).
The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: 75.5–85) in the Cases and 80 (IQR: 75.5–83.75) in the Controls]. The prevalence of co-pathologies was very high: all the Cases and 43 (93.5%) Controls showed a Charlson comorbidity index of at least 2. During hospitalization the patients in the Case group less frequently had a moderate disease of COVID-19 (35 vs. 67.4%, p = 0.02), more frequently a severe disease (48 vs. 22%, p = 0.03) and more frequently died (48 vs. 22%, p = 0.03). Moreover, during coronavirus disease 2019 (COVID-19), 14 (60.8%) patients in the Case group and 1 (2.1%; p < 0.000) in the Control group showed signs and symptoms of delirium.
Patients with dementia are vulnerable and have an increased risk of a severe disease and death when infected with COVID-19.
Introduction
The novel coronavirus SARS-CoV-2, first identified in China on 31 December 2019, has rapidly spread around the world causing a global pandemic with over two million deaths.
The clinical presentation of the coronavirus disease 2019 (COVID-19) is variable, ranging from an asymptomatic infection to mild and more severe progressive respiratory failure ( Macera et al., 2020 ; Cascella et al., 2021 ). Several risk factors for poor outcomes and mortality have been identified, such as age, hypertension, obesity, diabetes, and cancer ( Gautret et al., 2020 ; Marfella et al., 2020 ; Onder et al., 2020 ; Zhou et al., 2020 ; Fedeli et al., 2021 ; Monari et al., 2021 ).
Older adults are particularly susceptible to COVID-19 infection due to the presence of multiple comorbidities and chronic diseases ( Wynants et al., 2020 ). Moreover, the cognitive decline due to dementia, such as Alzheimer’s disease, exposes elderly subjects to a greater risk of becoming infected with COVID-19 ( Korczyn, 2020 ); in fact, the poor adherence to infection control measures (e.g., hand washing, social distancing, and wearing masks) and their close physical contact with caregivers are risk factors for SARS-CoV-2 infection ( Canevelli et al., 2020a ). Furthermore, they often show an atypical clinical presentation ( Bianchetti et al., 2020 ; Isaia et al., 2020 ; Ward et al., 2020 ) that may delay diagnosis and appropriate treatment and consequently impact their prognosis and survival ( Alonso-Lana et al., 2020 ). Moreover, in the case of respiratory failure, the compliance with oxygen (O 2 ) treatment with non-invasive or invasive ventilation is very low, with a possible poor prognosis.
Few data have been published on the impact of SARS-CoV-2 infection in patients with dementia ( Canevelli et al., 2020b ; Caratozzolo et al., 2020 ; Burns et al., 2021 ; Tsapanou et al., 2021 ; Wang et al., 2021 ; West et al., 2021 ). Although results are controversial, a worse outcome has been described among these patients ( Hariyanto et al., 2020 ; Liu et al., 2020 ; McMichael et al., 2020 ). However, being older the patients with dementia had multiple comorbidities, so the nature of the association between dementia and poor prognosis of COVID-19 without the evaluation of age and co-pathologies associated has not yet been clearly evaluated.
The aim of the present pair-matched case-control study was to investigate the outcome of patients with SARS-CoV-2 infection and dementia, compared with patients without dementia but of the same age, presence of co-morbidities and clinical presentation at hospitalization, in order to assess its impact on the mortality and severity of the disease.
Study Design and Setting
We performed a multicenter, observational, 1:2 matched case-control study involving three COVID-19 Units in two cities in the Campania region in southern Italy, Naples and Caserta.
The patients enrolled were adults (≥18 years), hospitalized with a diagnosis of SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) on a naso-oropharyngeal swab. Viral RNA was extracted by naso-oropharyngeal swab with QIAamp Viral RNA Kits (Qiagen GmbH, Hilden, Germany); the detection of SARS-CoV-2 was performed by RT-PCR test using Bosphore ® Novel Coronavirus (Anatolia Diagnostics and Biotechnology Products Inc., İstanbul, Turkey) Detection Kit V3, by primers designed on three viral regions: E, ORF1ab, and N regions.
The study period was from February 28th 2020 to January 31st 2021. All the patients with a diagnosis of dementia observed in the study period in one of the three centers participating were enrolled as Cases (Case group). For each Case, two patients without dementia observed by the same centers in the same study period, pair matched for gender, age (±5 years), PaO 2/ FiO 2 (P/F) ratio at admission (<200, or >200), number of comorbidities (±1; excluding dementia) were chosen (Control group).
All demographic, clinical and laboratory data of both Cases and Controls were collected in a database. From this database we extrapolated the data.
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples ( n °10877/2020). All procedures performed in this study were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards. Informed consent was obtained from all participants included in the study.
Variables and Definitions
The microbiological diagnosis of SARS-CoV-2 infection was defined as a positive RT-PCR test on a naso-oropharyngeal swab.
Dementia was diagnosed according to the clinical history of the patients.
The P/F ratio was considered as the arterial partial pressure of oxygen (aPP O 2 ) investigated through hemogas analysis divided by the fraction of inspired oxygen concentration (FiO 2 ) at the time of hospital admission.
The presence of underlying chronic diseases were defined according to the Charlson age-comorbidity index ( Charlson et al., 1987 ), while medical conditions at risk of clinical deterioration were defined through a modified Early Warning Score (MEWS) ( Subbe et al., 2001 ).
We defined patients with a mild, moderate or severe disease according to the clinical presentation of COVID-19. Precisely, patients with a mild infection did not need O 2 therapy and/or had a MEWS score below 3 points. Patients with a moderate infection required low flow O 2 therapy or non-invasive O 2 therapy and/or had a MEWS score equal to or above 3 points (≥3). Lastly, patients with a severe infection needed management in an intensive care unit (ICU) and/or mechanical ventilation; in this definition we also included patients who died because of respiratory failure, multi-organ failure or septic shock. Patients were followed until SARS-CoV-2-RNA negativity at naso-oropharyngeal swab or discharge from hospital.
Statistical Analysis
For the descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies. Continuous variables were summarized as mean and standard deviation if normally distributed or as median and interquartile range (IQR) if not normally distributed. We performed a comparison of patients with dementia and without dementia using Pearson chi-square or Fisher exact test for categorical variables and Student’s t - or Mann-Whitney tests for continuous variables.
A p -value below 0.05 was considered statistically significant. Analyses were performed by STATA.
During the study period, overall 672 patients with SARS-CoV-2 infection were observed in the three centers participating in the study. Of these 672 patients enrolled, 23 had a pre-existing diagnosis of dementia before the development of COVID-19 and were included in the Case group. Among the 649 patients observed without dementia, 46 were chosen as the Control group.
Of the 23 patients in the Case group, nine had a history of senile dementia, six of vascular dementia, five of Alzheimer’s disease, one of frontotemporal dementia, one of Parkinson dementia, and one of human immunodeficiency virus (HIV)-related dementia. Twelve patients in the Case group were in chronic treatment ( Table 1 ): memantine in two, dopaminergic drug in two, benzodiazepine in one, selective serotonin reuptake inhibitors in one, antiepileptic drug in one, antipsychotic drug and benzodiazepine in one, antipsychotic drug and NMDA receptor antagonist in one, antipsychotic drug and gabapentin in one, antipsychotic drug and lithium in one; antipsychotic drug and acetylcholinesterase inhibitors in one. Eight patients had a history of delirium before COVID-19 that required pharmacological treatment.
The therapies of the patients in case group.
Table 2 shows the epidemiological and clinical characteristics of the Cases and Controls. There were no statistically significant differences in age, gender, and co-morbidities among COVID-19 patients with and without dementia. The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: 75.5–85) in the Cases and 80 (IQR: 75.5–83.75) in the Controls] ( Table 2 ). The prevalence of co-pathologies was very high: all the Cases and 43 (93.5%) Controls showed a Charlson comorbidity index of at least 2; moreover, the median Charlson comorbidity index was similar in the two groups of patients [median 6 (IQR: 5–7) in Case group vs. 6 (IQR: 4–6) in the Control group] ( Table 2 ). However, the patients in the Control group more frequently showed as underlying chronic diseases arterial hypertension (86.9 vs. 56.8%, p = 0.004).
Demographic and clinical characteristics of the patients according to the presence or absence of dementia.
Table 3 shows the data on the clinical presentation of COVID-19 in the Cases and Controls. No statistically significant differences were found at admission between P0 2 [median 69.5 mmHg (IQR: 56.3–79) in the Cases and 65 (IQR: 59.5–74. 5) in the Controls] and P/F [median 244.5 (IQR: 169.7–320.5) in the Cases and 245 (IQR: 205–290) in the Controls] ( Table 3 ).
Clinical presentation of coronavirus disease 2019 (COVID-19) in case and control groups.
As regards the most serious respiratory support needed during hospitalization, a similar prevalence was found in high flow nasal cannulas (HFNC) [9 (39.1%) vs. 9 (19.6%; p = 0.08] in continuous positive airway pressure (CPAP) [1 (4.3%) vs. 8 (17.4%); p = 0.25] in non-invasive ventilation (NIV) [4 (17.4%) vs. 6 (13%); p = 0.72]. In the Case group, the prevalence of patients needing invasive ventilation was higher [10 (43.5) vs. 10 (21.7); p = 0.06], but with a difference not significant to the statistical analysis ( Table 2 ). However, during hospitalization, with respect to the patients in the Control group, those in the Case group less frequently had a moderate disease of COVID-19 (35 vs. 67.4%, p = 0.02), more frequently a severe disease (48 vs. 22%, p = 0.03) and more frequently died (48 vs. 22%, p = 0.03) ( Table 2 ). Moreover, although no difference between the two group of patients was observed in time from admission to discharge, the patients with dementia had a shorter period between admission and death [median and IQR of 12 (9–21) days vs. 19 (12.5–30) days], a difference without statistical significance ( Table 3 ).
During COVID-19, 14 (60.8%) patients in the Case group and 1 (2.1%; p < 0.000) in the Control group showed signs and symptoms of delirium and required the addition of drugs to control these ( Table 1 ): antipsychotic drug in three, benzodiazepine in seven and both in three patients in the Case group; the only patient in the Control group showing signs of delirium required the addition of an antipsychotic drug and benzodiazepine.
In the Case group, no difference in mortality was observed between the 14 patients with signs and symptoms of delirium during COVID-19 and the nine without (35.7 vs. 66.6%, p = 0.21).
In the present 1:2 case-control study performed in three COVID-19 Units in southern Italy we found that the patients with pre-existing dementia showed a worse prognosis of COVID-19. They more frequently showed a severe clinical outcome and more frequently died than those without dementia, but showed a similar age, number of pre-existing co-pathologies and respiratory failure at admission.
We know that globally more than 50 million people have dementia that has emerged as a pandemic in an aging society ( Fox and Petersen, 2013 ; Alzheimer’s Disease International, 2019 ). Thus, the double hit of dementia and COVID-19 pandemics has raised great concern.
A meta-analysis on 24 studies with 46,391 dementia patients showed that dementia was associated with severe COVID-19 [RR 2.63 (95% CI 1.41–4.90), p = 0.002] and mortality from COVID-19 infection [RR 2.62 (95% CI 2.04–3.36), p < 0.00001] ( Hariyanto et al., 2020 ). However, the data available in the literature on this topic cannot be considered conclusive. In fact, since the patients with dementia were very elderly, they had a lot of co-pathologies. Thus, the impact of age and the presence of co-pathologies in the clinical presentation of patients with dementia have not been clearly analyzed. For example, Bianchetti et al. (2020) showed that the mortality rate was higher (62.2%) among 82 patients suffering from dementia than that (26.2%) observed in 545 without. Instead, the 82 patients with dementia were older (mean age of 82.6 ± standard deviation 5.3) than the 545 without (68.9 ± 12.7), with no analysis of the presence of co-pathologies.
Interestingly, the majority (69.5%) of patients with dementia in the present study during COVID-19 showed symptoms that required the addition of antipsychotic or benzodiazepine drugs. Thus, as already suggested by other authors ( Kales et al., 2019 ), the SARS-CoV-2 patients with dementia who need hospital care represent a challenge for COVID-19 units and an increase in stress to manage non-compliant patients and with behavioral problems. In fact, delirium caused by hypoxia, a prominent clinical feature of COVID-19, can complicate the presentation of dementia ( Marcantonio, 2017 ) and increase the suffering of people with dementia hospitalized for COVID-19, as well as the cost of medical care and the need for dementia support.
Although without a difference in the statistical significance probably due to the small number of patients enrolled, it seems interesting that the patients with dementia had a shorter period between admission and death compared with those without. These data are in agreement with the observation of the Italian Institute of Health: considering the data on the 2,621 deaths due to COVID-19 in Italy, the patients with dementia showed a more rapid clinical worsening compared with individuals with intact cognition ( Canevelli et al., 2020a ).
The factors involved in the association between dementia and worse prognosis of COVID-19 could be many. Of course, the patient’s lack of cooperation in performing the main therapy for SARS-CoV-2 pneumonia could be one of the reasons for the negative outcome of the disease in these patients. Then, the neurotropism of the virus and the presence of angiotensin-converting enzyme 2 (ACE-2) receptor, the cellular receptor for the SARS-CoV-2, on the brain and glial tissue makes the central nervous system a potential target for the virus ( Yan et al., 2020 ; Barillari et al., 2021 ). The virus could infect the brain also through a disrupted blood-brain barrier that was often compromised in the aging brain and in neurodegenerative diseases, such as Alzheimer’s disease ( Hascup and Hascup, 2020 ). In view of this, it is likely that neurological manifestations caused by the virus worsen the already damaged neurological function of patients with dementia, making the prognosis worse. Furthermore, some studies had shown how the ACE receptor polymorphisms could influence the prognosis of patients with COVID 19 and are associated with Alzheimer’s disease ( Cao et al., 2020 ; Delanghe et al., 2020 ; Gómez et al., 2020 ).
Finally, severe COVID-19 outcomes are often associated with a “cytokine storm” ( Castelli et al., 2020 ); so elderly individuals affected by dementia could be at a higher risk due to a higher baseline of inflammation that steadily increases with age ( Rea et al., 2018 ; Naughton et al., 2020 ).
Our study shows several limits; first, the retrospective nature of the study; second, we evaluated only in-hospital mortality; third, the number of patients enrolled with dementia was low. The strengths of the study are the multicenter and case-control nature of the design, which makes it possible to look at multiple risk factors at the same time, especially age and the presence of co-pathologies.
In conclusion, patients with dementia are vulnerable and have an increased risk of serious morbidity, admission to ICUs, and death when infected with COVID-19. Thus, it is necessary to carry out an early diagnosis of SARS-CoV-2 infection in this population and to implement all measures to ensure proper management of the disease at home, with the use of telemedicine and digital technological devices, such as smart phones, which can be very useful in remote monitoring and care. Ideally, the use of monoclonal antibodies can be considered in these patients in an early phase to reduce the need of hospitalization and progression of the disease. In addition, it is necessary to establish a multidisciplinary team with an infectious disease specialist, a psychiatrist, a psychologist, social workers, nurses and volunteers to manage this difficult-to-treat-population. Finally, implementing the anti-COVID-19 vaccination in these patients is a priority.
Data Availability Statement
Ethics statement.
The studies involving human participants were reviewed and approved by AOU Vanvitelli. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MP, FC, and NC were involved in study concept and design, and drafting of the manuscript. PM and IG were involved in critical revision of the manuscript for important intellectual content. CC, AR, AG, and BP were involved in acquisition of data, analysis and interpretation of data, and in critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. POR Campania FESR 2014-2020-Avviso per l’acquisizione di manifestazioni di interesse per la realizzazione di servizi di ricerca e sviluppo per la lotta contro il Covid-19 (DGR n. 140 del 17 marzo 2020), Project: “Identificazione dei Fattori Demografici, Clinici, Virologici, Genetici, Immunologici e Sierologici Associati ad Outcome Sfavorevole Nei Soggetti Con COVID-19”, Regione Campania, Italy.
- Alonso-Lana S., Marquié M., Ruiz A., Boada M. (2020). Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 12 : 588872 . 10.3389/fnagi.2020.588872 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alzheimer’s Disease International. (2019). World Alzheimer’s Report 2019: Attitudes to Dementia. Available online at: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf (accessed March 15, 2020). [ Google Scholar ]
- Barillari M. R., Bastiani L., Lechien J. R., Mannelli G., Molteni G., Cantarella G., et al. (2021). A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J. Med. Virol. 93 983–994. 10.1002/jmv.26354 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G., et al. (2020). Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 24 560–562. 10.1007/s12603-020-1389-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burns A., Lobo A., Olde Rikkert M., Robert P., Sartorius N., Semrau M., et al. (2021). COVID-19 and dementia: experience from six European countries. Int. J. Geriatr. Psychiatry. 36 943–949. 10.1002/gps.5497 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canevelli M., Palmieri L., Raparelli V., Lo Noce C., Colaizzo E., Tiple D., et al. (2020a). Prevalence and clinical correlates of dementia among COVID-19-related deaths in Italy. Alzheimers Dement (Amst). 12 : e12114 . 10.1002/dad2.12114 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canevelli M., Valletta M., Toccaceli Blasi M., Remoli G., Sarti G., Nuti F., et al. (2020b). Facing dementia during the COVID-19 outbreak. J. Am. Geriatr. Soc. 68 1673–1676. 10.1111/jgs.16644 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., et al. (2020). Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 6 : 11 . 10.1038/s41421-020-0147-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caratozzolo S., Zucchelli A., Turla M., Cotelli M. S., Fascendini S., Zanni M., et al. (2020). The impact of COVID-19 on health status of home-dwelling elderly patients with dementia in East Lombardy, Italy: results from COVIDEM network. Aging Clin. Exp. Res. 32 2133–2140. 10.1007/s40520-020-01676-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cascella M., Rajnik M., Aleem A., Dulebohn S. C., Di Napoli R. (2021). “ Features, evaluation, and treatment of coronavirus (COVID-19) ,” in StatPearls [Internet]. (Treasure Island: StatPearls Publishing; ). [ PubMed ] [ Google Scholar ]
- Castelli V., Cimini A., Ferri C. (2020). Cytokine storm in COVID-19: “When you come out of the storm, you won’t be the same person who walked in”. Front. Immunol. 11 : 2132 . 10.3389/fimmu.2020.02132 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40 373–383. 10.1016/0021-9681(87)90171-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delanghe J. R., Speeckaert M. M., De Buyzere M. L. (2020). COVID-19 infections are also affected by human ACE1 D/I polymorphism. Clin. Chem. Laboratory Med. (CCLM) 58 1125–1126. 10.1515/cclm-2020-0425 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fedeli U., Schievano E., Avossa F., Pitter G., Barbiellini Amidei C., Grande E., et al. (2021). Different approaches to the analysis of causes of death during the COVID-19 epidemic. Eur. Rev. Med. Pharmacol. Sci. 25 3610–3613. 10.26355/eurrev_202105_25844 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox N. C., Petersen R. C. (2013). The G8 dementia research summit–a starter for eight? Lancet 382 1968–1969. 10.1016/S0140-6736(13)62426-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gautret P., Million M., Jarrot P. A., Camoin-Jau L., Colson P., Fenollar F., et al. (2020). Natural history of COVID-19 and therapeutic options. Expert Rev. Clin. Immunol. 16 1159–1184. 10.1080/1744666X.2021.1847640 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gómez J., Albaiceta G. M., García-Clemente M., López-Larrea C., Amado-Rodríguez L., Lopez-Alonso I., et al. (2020). Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene 762 : 145102 . 10.1016/j.gene.2020.145102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hariyanto T. I., Putri C., Arisa J., Situmeang R. F. V., Kurniawan A. (2020). Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch. Gerontol Geriatr. 93 : 104299 . 10.1016/j.archger.2020.104299 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hascup E. R., Hascup K. N. (2020). Does SARS-CoV-2 infection cause chronic neurological complications? GeroScience 42 1083–1087. 10.1007/s11357-020-00207-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Isaia G., Marinello R., Tibaldi V., Tamone C., Bo M. (2020). Atypical presentation of Covid-19 in an older adult with severe Alzheimer disease. Am. J. Geriatr. Psychiatry 28 790–791. 10.1016/j.jagp.2020.04.018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kales H. C., Lyketsos C. G., Miller E. M., Ballard C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int. Psychogeriatr. 31 83–90. 10.1017/S1041610218000534 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korczyn A. D. (2020). Dementia in the COVID-19 period. J. Alzheimers Dis . 75 1071–1072. 10.3233/JAD-200609 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu N., Sun J., Wang X., Zhao M., Huang Q., Li H. (2020). The impact of dementia on the clinical outcome of COVID-19: a systematic review and meta-analysis. J. Alzheimers Dis. 78 1775–1782. 10.3233/JAD-201016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Macera M., De Angelis G., Sagnelli C., Coppola N. Vanvitelli Covid-Group. (2020). Clinical presentation of COVID-19: case series and review of the literature. Int. J. Environ. Res. Public Health 17 : 5062 . 10.3390/ijerph17145062 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marcantonio E. R. (2017). Delirium in hospitalized older adults. N. Engl. J. Med. 377 1456–1466. 10.1056/NEJMcp1605501 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marfella R., Paolisso P., Sardu C., Bergamaschi L., D’Angelo E. C., Barbieri M., et al. (2020). Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 46 403–405. 10.1016/j.diabet.2020.05.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McMichael T. M., Currie D. W., Clark S., Pogosjans S., Kay M., Schwartz N. G., et al. (2020). Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 382 2005–2011. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Monari M., Sagnelli C., Maggi P., Sangiovanni V., Numis F. G., Gentile I., et al. (2021). More severe covid-19 in patients with active cancer: the results of a multicenter cohort study. Front. Oncol. 11 : 662746 . 10.3389/fonc.2021.662746 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Naughton S. X., Raval U., Pasinetti G. M. (2020). Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J. Alzheimers Dis. 76 21–25. 10.3233/JAD-200537 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Onder G., Rezza G., Brusaferro S. (2020). Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323 1775–1776. 10.1001/jama.2020.4683 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rea I. M., Gibson D. S., McGilligan V., McNerlan S. E., Alexander H. D., Ross O. A., et al. (2018). Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 9 : 586 . 10.3389/fimmu.2018.00586 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Subbe C. P., Kruger M., Rutherford P., Gemmel L. (2001). Validation of a modified early warning score in medical admissions. QJM 94 521–526. 10.1093/qjmed/94.10.521 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tsapanou A., Papatriantafyllou J. D., Yiannopoulou K., Sali D., Kalligerou F., Ntanasi E., et al. (2021). The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int. J. Geriatr. Psychiatry. 36 583–587. 10.1002/gps.5457 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Q., Davis P. B., Gurney M. E., Xu R. (2021). COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement 10.1002/alz.12296 [Epub ahead of print]. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward C. F., Figiel G. S., Mcdonald W. M. (2020). Altered mental status as a novel initial clinical presentation for COVID-19 infection in the elderly. Am. J. Geriatr. Psychiatry 28 808–811. 10.1016/j.jagp.2020.05.013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West E., Nair P., Barrado-Martin Y., Walters K. R., Kupeli N., Sampson E. L., et al. (2021). Exploration of the impact of the COVID-19 pandemic on people with dementia and carers from black and minority ethnic groups. BMJ Open 11 : e050066 . 10.1136/bmjopen-2021-050066 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynants L., Van Calster B., Collins G. S., Riley R. D., Heinze G., Schuit E., et al. (2020). Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 369 : m1328 . 10.1101/2020.03.24.20041020 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. (2020). Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367 1444–1448. 10.1126/science.abb2762 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 1054–1062. 10.1016/S0140-6736(20)30566-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Open access
- Published: 20 April 2024
The role of colchicine in the management of COVID-19: a Meta-analysis
- Kholoud Elshiwy 1 ,
- Ghada Essam El-Din Amin 1 , 2 ,
- Mohamed Nazmy Farres 3 ,
- Rasha Samir 3 &
- Mohamed Farouk Allam 1 , 4
BMC Pulmonary Medicine volume 24 , Article number: 190 ( 2024 ) Cite this article
179 Accesses
Metrics details
The Coronavirus disease 2019 (COVID-19) pandemic has robustly affected the global healthcare and economic systems and it was caused by coronavirus-2 (SARS-CoV-2). The clinical presentation of the disease ranges from a flu-like illness to severe pneumonia and death. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million. Colchicine is an alkaloid drug that is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine inhibits the production of superoxide and the release of interleukins that stimulate the inflammatory cascade. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) that are related to the fibrosis. Moreover, colchicine has been used to traet viral myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. Additionally, colchicine is considered safe and affordable with wide availability.
The aim of the current study was to assess the evidence of colchicine effectiveness in COVID-19 treatment.
A comprehensive review of the literature was done till May 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only 8 clinical trials and cohort studies fulfilling the inclusion criteria were included for further steps of data collection, analysis, and reporting.
This meta-analysis involved 16,488 patients; 8146 patients in the treatment group and 8342 patients in the control group. The results showed that colchicine resulted in a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care (RR 0.35, 95%CI: 0.15–0.79). Colchicine resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95%CI 0.02–0.27, P = 0.000024). However, colchicine had no significant effect on the following outcomes among COVID-19 patients: the need for hospitalization, ICU admission, artificial ventilation, and hospital discharge rate. Among the PCR confirmed COVID-19 patients, colchicine decreased the hospitalization rate (RR 0.75, 95%CI 0.57–0.99, P = 0.042). However, colchicine had no effect on mortality and the need for mechanical ventilation among this subgroup.
Colchicine caused a significant clinical improvement among COVID-19 patients as compared with the standard care or placebo, in terms of the need for O2, and mortality. This beneficial effect could play a role in the management of COVID-19 especially severe cases to decrease need for oxygen and to decrease mortality among these patients.
Peer Review reports
Introduction
The Coronavirus disease 2019 (COVID-19) that was caused by coronavirus − 2 (SARS-CoV-2) has significantly impacted the healthcare and economic systems worldwide. The disease first began in Wuhan, China at the end of 2019. Then, it spread worldwide and became a pandemic. The clinical picture of the disease ranges from a flu-like illness to a massive inflammatory response and death [ 1 ]. In 2002 and 2003, there were outbreaks of severe respiratory distress syndrome in China. They occurred by SARS-CoV, another member of the coronavirus family. In 2012, another outbreak was documented in the Middle East and was caused by Middle East respiratory syndrome coronavirus (MERS-CoV) [ 2 ]. The current coronavirus is characterized by higher infectivity and geographical spread in comparison with both SARS and MERS. Therefore, COVID-19 was considered a significant global health threat that required robust efforts to minimize the burden of this pandemic [ 3 ].
The World Health Organization (WHO) announced that COVID-19 is a pandemic on 11 March 2020 [ 4 ]. Since then, the number of COVID-19 patients significantly increased. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million [ 5 ].
The clinical manifestations of COVID-19 encompass symptoms such as fever, cough, dyspnea, malaise, or anosmia or ageusia, which can aid in early detection of the disease [ 6 ]. The primary mode of COVID-19 transmission is predominantly through exposure to infectious respiratory droplets from close contact with either symptomatic patients or asymptomatic carriers, as well as through aerosol particles that can remain suspended in the air for extended periods [ 7 ]. Additionally, indirect transmission through contaminated fomites, fecal excretion, environmental contamination, and fluid pollution has been documented, with viral viability reaching up to 72 hours after infecting surfaces [ 7 , 8 ].
SARS-CoV-2 is a beta coronavirus that is a positive-stranded enveloped RNA virus. Similar to SARS-CoV and MERS-CoV, it is found in domestic and farm animals [ 9 , 10 ]. The SARS-CoV-2 is characterized by spike proteins called S proteins. These proteins facilitate the viral infection through binding the S proteins and the angiotensin-converting enzyme 2 receptors (ACE2). These receptors are found in many tissues such as pneumocytes, enterocytes, renal cells, and endothelial cells [ 11 ]. SARS-CoV-2 causes marked dysfunction of the epithelial barrier and the endothelial cells of the pulmonary capillaries which triggers the migration and accumulation of inflammatory cells. This initiates the inflammatory cascade by both innate and cell-mediated immunity which significantly influences the alveolar-capillary oxygen transmission and the oxygen diffusion capacity [ 12 ].
In severe cases of COVID-19, fulminant inflammation, stimulation of the coagulation pathways, and consumption of the clotting factors occur in the form of a “cytokine storm”. This happens under the effect of many inflammatory mediators including interleukins, tumor necrosis factor-α (TNF-α), and interferon (IFN-γ). In addition, vasodilators such as bradykinin increase vascular permeability and result in pulmonary edema [ 13 ].
These mechanisms of cell damage represent a target for already existing medications that modulate the immune response. Based on its anti-inflammatory effects, colchicine has gained attention to be utilized in the management of COVID-19 patients. Colchicine is an alkaloid drug that is formed from a plant called “ Colchicum autumnale ”, also named “autumn crocus”. Colchicine is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine has an anti-inflammatory effect that is mediated through its binding to the tubulins and inhibiting the polymerization of microtubules. Microtubules are a key component of the cytoskeleton and are composed of tubulin heterodimers. These structures are important in different cellular functions including intracellular trafficking, cell shape, cell migration, and division [ 14 ]..
Colchicine inhibits the production of superoxide and the release of interleukin 1β and IL-6. Colchicine also prevents the inflammatory cascade by decreasing the production of inflammasomes that stimulate caspase-1 activation and release of interleukins such as interlukin1β and interleukin IL18 [ 15 , 16 ]. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) [ 17 , 18 ]. Moreover, colchicine has been used in cardiac conditions caused by a viral infection like myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. These different mechanisms greatly decrease the inflammatory response that represents a cornerstone in the pathophysiologic process of COVID-19. Besides the aforementioned effects of colchicine, its usage is considered safe and affordable with wide availability [ 19 ].
The ongoing impact of COVID-19 on all life aspects, the scarcity of effective treatments and the emergence of new virus variants resulted in the urgent need to repurpose the already existing drugs and to invent new therapeutic agents. This raised concerns about the effectiveness of colchicine in COVID-19 treatment and the possibility of providing an improvement in the clinical course of the disease.
The aim of the current study was to evaluate the efficacy of colchicine on different clinical outcomes including mortality, duration of COVID-19 illness till recovery, need for hospitalization, need for O2 therapy, need for ICU admission, and need for artificial ventilation.
Methodology
Criteria for considering studies for this meta-analysis, types of studies.
The review was restricted to Clinical Trials and Cohort Studies, which investigated the Colchicine administration in COVID-19 patients, versus standard treatment/placebo.
Types of participants
Participants were adult patients with the diagnosis of COVID-19. Patients were considered to have a definite diagnosis of COVID-19 if they were laboratory-confirmed using reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. All healthcare settings (community/primary care, hospital outpatient, or long-stay institutional) were considered eligible.
Types of interventions
Clinical trials and Cohort Studies were included. Colchicine was administered in COVID-19 patients, versus standard treatment/placebo.
Types of outcome measures
At least one of these outcomes was considered; Mortality, Duration of COVID-19 illness till recovery, Need for hospitalization, Need for O2 therapy, Need for ICU admission, and Need for artificial ventilation.
Inclusion criteria
(i) Cohort studies. (ii) Randomized and non-randomized clinical trials. Studies conducted on adult human subjects. (iii) Studies conducted on patients diagnosed with COVID-19 confirmed with positive reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. (iv) Studies conducted in all healthcare settings (community/ primary care, hospital outpatient or long-stay institutional). Studies published in Arabic, English, French or Spanish languages.
Exclusion criteria
Review, opinion studies, Case series, Studies conducted on animals.
Search strategy for identification of studies
Published studies and abstracts on the role of colchicine in the management of COVID-19 were identified through a comprehensive search of electronic databases that included PubMed ( https://pubmed.ncbi.nlm.nih.gov/ ), ScienceDirect ( www.sciencedirect.com ), Scirus ( www.scirus.com/srsapp ), ISI Web of Knowledge ( http://www.isiwebofknowledge.com ), Google Scholar ( http://scholar.google.com ) and CENTRAL (Cochrane Central Register of Controlled Trials ( http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.htm ), using a combination of the following keywords: “Colchicine, COVID-19, Clinical Trail, Cohort Study”.
Methods of the meta-analysis
Locating and selecting studies.
Abstracts of articles identified using the search strategy above mentioned were viewed, and articles that appeared to fulfil the inclusion criteria were retrieved in full. Data on at least one of the outcome measures was included in the study. Each article identified was reviewed and categorized into one of the following groups: Included: Randomized and non-randomized clinical trials, and Cohort studies that met the described inclusion criteria and those where it was impossible to tell from the abstract, title or MESH headings. Excluded: review, opinion studies, case series, and studies conducted on animals. When there was a doubt, a second reviewer (MFA) assessed the article, and a consensus was reached. The literature was reviewed till May 31, 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only articles fulfilling the inclusion criteria were included (total 8 articles) for further steps of data collection, analysis, and reporting. The studies that met our inclusion criteria were Deftereos et al., Tardif et al., RECOVERY Collaborative Group, Lopes et al., Sandhu et al., Mareev et al., Brunetti et al. and Scarsi et al. [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ]. All were in English and there were no available studies published in Arabic, French or Spanish language.
Data extraction
A copy of each identified paper was obtained, and relevant data was abstracted by the first reviewer for a quantitative overview. We extracted the following study data from full-text articles: first author name, year of publication, study design, study location, eligibility criteria, sample size, age, sex, description of intervention and control groups, primary and secondary outcomes. In case of discrepancies or when the information presented in a study was unclear, abstraction by a second reviewer (MFA) was sought to resolve the discrepancy.
Statistical considerations
Data were abstracted from every study in the form of a risk estimate and its 95% confidence interval. When a risk estimate and its 95% confidence interval were not available from the article, we calculated unadjusted values from the published data of the article, using the Epi Info 6 computer program version 6.04d.
Pooled estimates of relative risks were obtained by weighing each study by the inverse variance of the effect measure on a logarithmic scale. This approach to pool the results assumed that the study populations being compared were similar and hence corresponded to a fixed effect analysis. The validity of pooling the relative risks was tested (test of homogeneity) using chi square test.
A violation of this test suggested that the studies being pooled differed from one another. In the presence of significant heterogeneity of the effect measure among studies being compared, we performed a random effect analysis that was based on the method described by DerSimonian and Laird. The random effect analysis accounted for the interstudy variation. Because the test of homogeneity had low power, we reported the figures of the random effect analysis even with the absence of significant heterogeneity.
All statistical analyses for pooling the studies were performed on the MetaXL Software.
In 6 databases, we identified 814 articles; 499 duplicates were removed. Out of the remaining 315 abstracts, we excluded 298 after screening. Thus, 17 full-text studies were assessed for eligibility and 9 were excluded. Finally, eight studies were included for further qualitative and quantitative analyses (Fig. 1 ).
PRISMA flow diagram showing selection of studies. PRISMA; Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics of the included studies
Two studies were cohort (Brunetti et al. and Scarsi et al.) while the other studies were four randomized controlled clinical trials (Deftereos et al., RECOVERY Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized controlled clinical trials (Mareev et al., and Sandhu et al.).
Two studies were multicentre clinical trials (RECOVERY Collaborative Group, and Tardif et al.) . The other six studies were conducted in Greece (Deftereos et al.), Brazil (Lopes et al.), the USA (Brunetti et al. and Sandhu et al.), Russia (Mareev et al.), and Italy (Scarsi et al.) [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ].
The studies included both hospitalized and non-hospitalized COVID-19 patients, who were diagnosed either clinically or by laboratory diagnosis with PCR–RT testing and CT chest imaging (Table 1 ).
Table 2 and Fig. 2 showed that the meta-analysis of all included studies showed a significant difference in mortality between the treatment group with colchicine and the control group (RR 0.35, 95% CI: 0.15–0.79). There is significant heterogeneity among the studies (Homogeneity Test X2: 42.219, P -value < 0.000).
Forest plot for the efficacy of colchicine on mortality in patients with COVID-19
The meta-analytical result of the six clinical trials was insignificant between the treatment and control groups (RR 0.48, 95% CI 0.22–1.07). There is significant heterogeneity among the studies (Homogeneity Test X2: 11.562, P -value: 0.000). The meta-analytical result of the two cohort studies was significant between the treatment and control groups (RR 0.17, 95%CI 0.08–0.35).
Duration of COVID-19 illness till recovery
Table 3 shows the efficacy of colchicine on the duration of COVID-19 illness till recovery. Lopes et al. reported that the median duration of COVID-19 illness in the treatment group with colchicine was 7 days vs 9 days in the control group ( P -value =0.003) [ 25 ]. While Sandhu et al., and Mareev et al., demonstrated that colchicine had no significant effect on the illness duration [ 26 , 27 ]. (Table 3 ).
Need for hospitalization
Tardif et al., reported that colchicine did not show a significant effect on the COVID-19 patients’ need for hospitalization RR 0.79, 95% CI 0.60–1.03, P-value =0.081) [ 23 ].
Need for O2 therapy
Lopes et al., demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95% CI 0.02–0.27, P = 0.000024) [ 25 ].
Need for ICU admission
Table 4 and Fig. 3 show the efficacy of colchicine on need for ICU admission in patients with COVID-19. The meta-analytical result did not show a significant effect (RR 0.29, 95% CI: 0.07–1.17).
Forest plot for the efficacy of colchicine on need for ICU admission in patients with COVID-19
Need for artificial ventilation
Table 5 and Fig. 4 show the efficacy of colchicine on need for artificial ventilation in patients with COVID-19. The meta-analysis of four studies demonstrated that colchicine has no significant effect on the need for artificial ventilation (RR 0.40, 95% CI 0.14–1.13). There is significant heterogeneity among the studies (Homogeneity Test X2: 18.417, P -value: 0.000).
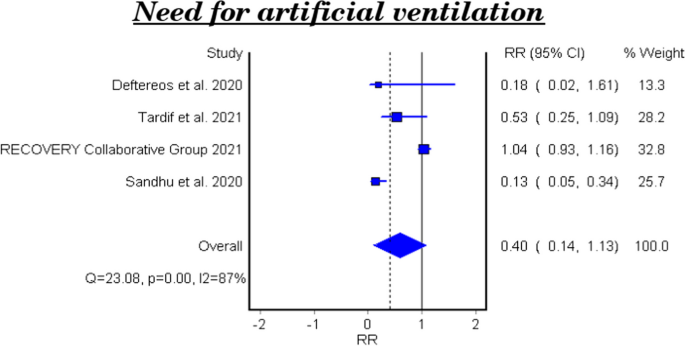
Forest plot for the efficacy of colchicine on need for artificial ventilation in patients with COVID-19
Hospital discharge rate
Table 6 and Fig. 5 show the efficacy of colchicine on hospital discharge rate in patients with COVID-19. The meta-analytical result of the three studies demonstrated that colchicine did not show a significant effect on the hospital discharge rate (RR 0.99, 95%CI 0.12–7.85).
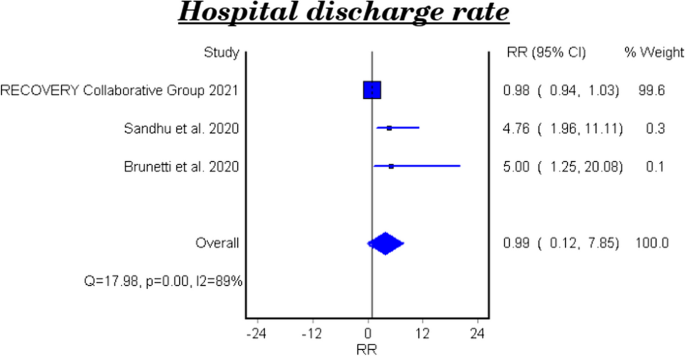
Forest plot for the efficacy of colchicine on hospital discharge rate in patients with COVID-19
The effect of colchicine on the hospital discharge rate in the clinical trials was not significant (RR 0.98, 95%CI 0.12–8.02), while a cohort study reported that colchicine showed a significant effect on the hospital discharge rate (RR 5.0, 95%CI 1.25–20.08, P-value 0.023) [ 28 ].
Subgroup analysis among PCR confirmed COVID-19 patients
Mortality among pcr confirmed covid-19 patients.
Table 7 and Fig. 6 show the efficacy of colchicine on mortality among PCR confirmed COVID-19 Patients. Colchicine did not show a significant effect on mortality among PCR confirmed COVID-19 patients (RR 1.02, 95% CI 0.74–1.41).
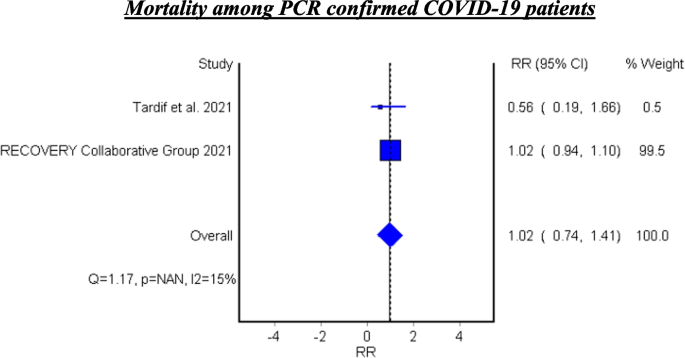
Forest plot for the efficacy of colchicine on mortality among PCR confirmed COVID-19 patients
See Fig. 6 .
Hospitalization among PCR confirmed COVID-19 patients
Tardif et al. assessed the efficacy of colchicine on hospitalization and reported that colchicine resulted in decreased hospitalization among the PCR confirmed COVID-19 patients (RR 0.75, 95%CI 0.57–0.99, P 0.042) [ 23 ].
Mechanical ventilation among PCR confirmed COVID-19 patients
Tardif et al. found that colchicine has no significant effect on mechanical ventilation among PCR confirmed COVID-19 Patients (RR 0.50, 95%CI 0.23–1.07, P 0.042) [ 23 ].
In this meta-analysis, the studies investigated the role of colchicine in the management of COVID-19 were reviewed.
After a comprehensive search, eight studies were identified. Two of them were cohort studies (Brunetti et al., and Scarsi et al.) while the other studies were four randomized control trials (Deftereos et al., Recovery Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized trials (Mareev et al., and Sandhu et al.). The current meta-analysis involved 16,488 patients; 8146 were in the treatment group who received colchicine and 8342 were in the control group who received a placebo or standard treatment [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ].
The efficacy of colchicine on mortality
The eight pooled studies evaluated the efficacy of colchicine on mortality among COVID-19 patients and showed a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care. This result coincides with the findings of a recent systematic review that reported a significant decrease in the all-cause mortality in three observational studies [ 28 ]. In addition, a recently published meta-analysis reported that colchicine resulted in decreased mortality among COVID-19 patients. This study pooled four randomized control trials and five observational studies and involved 5522 patients only [ 29 ].
On the other hand, Mehta, et al. and Toro-Huamanchumo, et al. documented that colchicine had no effect on the mortality rate among COVID-19 patients [ 30 , 31 ].
The heterogeneity test between the pooled studies showed a significant difference, which indicates interstudy variation. Pooling of these heterogeneous studies added more useful information.
According to our result, colchicine may have a beneficial effect to decrease mortality among COVID-19 patients. It was obvious that this effect occurred when colchicine was used within the early days of the disease. These findings can be explained by the anti-inflammatory role of colchicine that is mediated through the interaction between colchicine and microtubules which play an important role in cellular division, migration, and adhesion. This effect robustly influences the immune system response and reduces the inflammatory reaction. Also, colchicine decreases the release of cytokines and inflammatory mediators that stimulate the immune cells [ 32 ].
The subgroup analysis of the two cohort studies demonstrated a significant effect of colchicine on mortality among COVID-19 patients. However, the subgroup analysis for the six clinical trials showed that colchicine has no effect on mortality in the management of COVID-19. This result is consistent with the pooled analysis of a recent study where four clinical trials only were included [ 33 ]. This variation could be attributed to difference of the study design, variation in follow up duration and the colchicine regimen used in these studies.
The efficacy of colchicine on the duration of COVID-19 illness till recovery
The efficacy of colchicine on the duration of COVID-19 illness was assessed in three clinical trials. Lopes et al. found that hospitalized COVID-19 patients who received colchicine had a shorter duration of illness till recovery in comparison with the patients who received placebo [ 23 ]. This is similar to the result reported by a recent study [ 34 ]. This finding can be related to the anti-inflammatory and immune modulatory roles of colchicine in the management of COVID-19. On the other hand, two clinical trials reported that colchicine did not affect the duration of COVID-19 illness [ 23 , 25 ]. These findings agree with the results of a recently published study investigated the efficacy of colchicine on the duration of COVID-19 clinical course [ 31 ].
The efficacy of colchicine on need for hospitalization
Tardif et al., investigated the efficacy of colchicine among non-hospitalized COVID-19 patients vs placebo. They found that colchicine did not influence the need for hospitalization among the non-hospitalized patients [ 21 ]. A recent clinical trial was conducted to assess the effect of colchicine on the prognosis of non-hospitalized COVID-19 patients and the results showed no significant effect of colchicine on hospitalization rate of the patients [ 35 ].
The efficacy of colchicine on need for O2 therapy
Lopes et al., assessed the efficacy of colchicine on the need for O2 therapy and the results demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 [ 23 ]. This result can be understood based on the beneficial effect of colchicine on the inflammatory response.
The efficacy of colchicine on need for ICU admission
The pooled results of two clinical trials showed that colchicine did not improve the need of ICU admission compared to placebo or standard care. This finding is concomitant with a recent study that included six studies only [ 30 ].
The efficacy of colchicine on need for artificial ventilation
Four pooled studies evaluated the efficacy of colchicine on need for artificial ventilation and showed that colchicine did not decrease the need for artificial ventilation compared to placebo or standard care [ 20 , 21 , 22 , 24 ].
The heterogeneity test between the pooled studies regarding the need for artificial ventilation showed a significant difference, which indicates interstudy variation.
This can be attributed to the variation of duration and dose of colchicine regimens in these studies, and the severity of the disease. Tardif et al., included non-hospitalized COVID-19 patients while the other three studies involved hospitalized patients.
The efficacy of colchicine on hospital discharge rate
Three pooled studies evaluated the efficacy of colchicine on hospital discharge rate and showed that colchicine did not improve the hospital discharge rate in comparison with placebo or standard treatment [ 22 , 24 , 26 ].
Furthermore, the subgroup analysis of the pooled results included two clinical trials and showed that colchicine did not cause a significant improvement in the hospital discharge rate compared to placebo or standard treatment [ 22 , 24 ]. On the other hand, the cohort study demonstrated a beneficial effect of colchicine on the hospital discharge rate compared to standard care [ 26 ].
The variation of the results of the three studies could be attributed to the difference of study design, number of included patients, and the treatment regimens used.
Two pooled studies evaluated the efficacy of colchicine among PCR confirmed COVID-19 patients and showed that colchicine did not significantly decrease mortality among PCR confirmed patients [ 21 , 22 ].
In addition, Tardif et al. assessed the efficacy of colchicine on hospitalization rate among PCR confirmed COVID-19 patients and found that colchicine significantly decreased the hospitalization rate compared to placebo. Also, Tardif et al. evaluated the effectiveness of colchicine on mechanical ventilation rate among PCR confirmed COVID-19 patients and showed no beneficial effect of colchicine on mechanical ventilation in comparison with placebo [ 21 ].
The study demonstrates that colchicine administration leads to a notable reduction in mortality rates and a decrease in the necessity for oxygen therapy among individuals with COVID-19. Although its impact on broader outcomes like hospitalization rates, ICU admissions, and discharge rates remains minimal, there’s a significant finding regarding its efficacy in lowering hospitalizations specifically among PCR-confirmed COVID-19 patients. This detailed understanding highlights the potential of colchicine as a therapeutic intervention for COVID-19, particularly in mitigating mortality risks and oxygen therapy requirements. These results offer valuable insights for clinicians, highlighting the need to consider colchicine as a viable treatment option for COVID-19 patients, while also emphasizing the necessity for further exploration to optimize its clinical utility.
Availability of data and materials
Our study is a Systematic Review/Meta-analysis. The datasets analyzed during the current study are available in the published pooled study. Also, the datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Rahman MT, et al. Early prediction and HRCT evaluation of post covid-19 related lung fibrosis. Microbiol Insights. 2023;16:11786361231190334.
Article PubMed PubMed Central Google Scholar
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–97.
Article CAS PubMed PubMed Central Google Scholar
Han Q, Lin Q, Jin S, You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J Infect. 2020;80(4):373–7.
Hageman JR. The coronavirus disease 2019 (COVID-19). Pediatr Ann. 2020;49(3):e99–e100.
Article PubMed Google Scholar
WHO. World Health Organization. Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. 2022. Available from: https://covid19.who.int/info/ .
Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, Hooft L, Emperador D, Domen J, Tans A, Janssens S, Wickramasinghe D, Lannoy V, Horn SRA, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2022;5(5):CD013665. https://doi.org/10.1002/14651858.CD013665.pub3 .
Mehraeen E, Salehi MA, Behnezhad F, Moghaddam HR, SeyedAlinaghi S. Transmission modes of COVID-19: a systematic review. Infect Disord Drug Targets. 2021;21(6):e170721187995.
Article CAS PubMed Google Scholar
van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–7.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Pandit R, Matthews QL. A SARS-CoV-2: companion animal transmission and variants classification. Pathogens. 2023;12(6):775.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7.
Bhattacharyya B, Panda D, Gupta S, et al. Anti-mitotic activity of colchicine and the structural basis for its interaction withTubulin. Med Res Rev. 2007;28(1):155–83.
Article Google Scholar
Cronstein BN, Esserman PR, Sunkureddi P. Mechanistic aspects of inflammation and clinical Management of Inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29.
Korkmaz S, Erturan I, NazIroǧlu M, et al. Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of ca 2+ release and antioxidant system. J Membr Biol. 2011;244(3):113–20.
Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, Sen S, Duman S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(5):53-57.
Lho Y, Do JY, Heo JY, Kim AY, Kim SW, Kang SH. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. Int J Mol Sci. 2021;22(9):4739.
Schlesinger, N., Firestein, B. L., & Brunetti, L. Colchicine in COVID-19: an old drug, New Use In Current Pharmacology Reports 6(4): 137–145 (2020).
Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6)
Tardif JC, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924–32.
Group, R. C. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9(12):1419–26.
Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1):1–8.
Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection. Can J Infect Dis Med Microbiol. 2020;2020:1–9.
Mareev VY, Orlova YA, Plisyk AG, et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiya. 2021;61(2):15–27.
Brunetti L, Diawara O, Tsai A, et al. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J Clin Med. 2020;9(9):1–12.
Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-Centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–9.
Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med. 2022;54(1):775–89.
Elshafei MN, El-Bardissy A, Khalil A, et al. Colchicine use might be associated with lower mortality in COVID-19 patients: a meta-analysis. Eur J Clin Investig. 2021;51(9):1–5.
Mehta KG, Patel T, Chavda PD, et al. Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials. RMD Open. 2021;7(3):1–10.
Toro-Huamanchumo CJ, Benites-Meza JK, Mamani-García CS, et al. Efficacy of colchicine in the treatment of COVID-19 patients: a systematic review and Meta-analysis. J Clin Med. 2022;11(9)
Hariyanto TI, Halim DA, Jodhinata C, et al. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–30.
Zein AFMZ, Raffaello WM. Effect of colchicine on mortality in patients with COVID-19 – a systematic review and meta-analysis. Diabet Metabol Syndrome: Clin Res Rev. 2022;16(2):102395.
Article CAS Google Scholar
Kow CS, Lee LH, Ramachandram DS, et al. The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: a meta-analysis of randomized trials. Immun Inflamm Disease. 2022;10(2):255–64.
Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022;19(22):1–9.
Google Scholar
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Department of Family Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Kholoud Elshiwy, Ghada Essam El-Din Amin & Mohamed Farouk Allam
Department of Community, Environmental and Occupational Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Ghada Essam El-Din Amin
Department of Internal Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Mohamed Nazmy Farres & Rasha Samir
Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Cordoba, 14004, Cordoba, Spain
Mohamed Farouk Allam
You can also search for this author in PubMed Google Scholar
Contributions
Kholoud Elshiwy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Ghada Essam El-Din Amin: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Nazmy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Rasha Samir: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Farouk Allam: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft.
Corresponding author
Correspondence to Kholoud Elshiwy .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elshiwy, K., Amin, G.E.ED., Farres, M.N. et al. The role of colchicine in the management of COVID-19: a Meta-analysis. BMC Pulm Med 24 , 190 (2024). https://doi.org/10.1186/s12890-024-03001-0
Download citation
Received : 04 July 2023
Accepted : 08 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1186/s12890-024-03001-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Meta-analysis
- Ain Shams University
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Permissive, Overbearing Parenting Is Connected to Hikikomori
It doesn’t always cause hikikomori, but all hikikomori have that kind of parent..
Posted April 23, 2024 | Reviewed by Abigail Fagan
- A Parent's Role
- Find a family counsellor near me
- Parenting influences an individual’s ability to actualize and feel self-confident.
- Effective parenting has clear communication, boundaries, and consistent rules and reinforcements.
- Interventions should include behavioral approaches and changes to the home environment.
The media, and columnists in particular, often criticize the roles of teachers, healthcare professionals, and parents in some way. After all, what else are they going to do? Sure, all these areas play some part in our development, and while it may be helpful to debate the state of things, one would hope that the readers (including you) would add a pinch of salt and digest slowly what’s said. With that in mind, consider that parenting does play a role in an individual’s ability to self-actualize and transition to adulthood with self-confidence . It’s not the only role; a myriad of other factors — genetic predispositions, social circles, technology, access to services — count, too.
Nevertheless, a recent study in Clinical Child and Family Psychology Review identified the role of parenting as a key environmental element in the presentation of those with Extreme Social Withdrawal (ESW) and its less extreme variants of Not Employed in Education or Training, Adultolescence, etc. “All in all,” it reported, “it can be concluded that adverse family processes seem to play a significant role in the development and maintenance of ESW” (Muris & Ollendick, 2023, 468). These processes involved parents who had “some kind of psychopathology” and had parenting styles “characterized by less communication and cohesion,” which led to children being “subjected to detrimental rearing practices” (Muris & Ollendick, 2023, 468). Within this realm, individuals had parents who displayed some combination of being overbearing (helicoptering) and/or overly indulgent, which includes the sort of permissive parenting bereft of rules, structure, expectations, and consequences.
Interestingly, this shows up not only in the clinical research but in the Reddit feeds and anime watched by the self-identifying hikikomori. Not all permissive parenting leads to a delayed transition to adulthood, but all hikikomori have this sort of parent. The findings of this study came as no surprise to me. I have worked in residential treatment centers, both with adolescents and adults as well as in outpatient, and all of my patients presenting with extreme social withdrawal and anxiety around self-actualization come from parents like the ones I’ve just outlined. Not mentioned in the article, is the fact that most folks with this presentation come from wealthy families, and the pressure to maintain status-quo can lead to a big drop in an individual’s standard of living, hindering the motivation to step outside of a plush comfort zone.
Stop for a second to think about it: if someone hovers over you all your life and does not let you try things and fail and figure it out for yourself, would you sense that you’re capable of solving your problems or taking risks? Perhaps the parents are ambivalent about their child’s transition to independence because they’re not ready to let go, or the child is avoiding a drop in living standards. Add on top of that a technological landscape that favors social isolation via dopaminergic rewards, school and healthcare systems that err on the side of coddling lest they get canceled, and one gets the makings of a cycle. To break that cycle we have to address the parenting, for that’s where it lives, literally.
To wit, I have yet to treat a patient with this presentation who has parents who clearly communicated their expectations and rules and boundaries and reinforced that stuff with positivity, compassion and meaningful rewards and consequences. None of these patients had chores or summer jobs, nor was there an expectation of it. Please notice that I excluded other presentations here. Effective parenting isn’t a guarantee against mental illness; thought disorders, neurocognitive disorders, trauma , death anxieties and grief : there are a million ways things can go for us in this world. But parenting definitely plays into those with ESW and a delayed transition to adulthood.

The typical refrain from parents when this is broached is that they struggle to practice restraint because it’s too difficult to play that role — they don’t want to be the bad guy. But that’s their job. Parenting is very difficult for this reason, and all the more reason our society should provide better support for it. Until then, the best way for a parent to help a child with ESW or a delayed transition to adulthood is to take part in their own behavioral therapy as well as Parent Effectiveness Training. Parent Effectiveness Training is a program that takes 24 hours to complete and involves improving communication, setting clear boundaries, rules and expectations, coupled with meaningful reinforcers (aka, rewards and consequences). The behavioral therapy will help address the discomfort a parent has in implementing rules and structure. Plus, the child should be doing a behavioral approach, too, like Acceptance and Commitment Therapy, which will confront the experiential avoidance that keeps them from self-actualizing.
It can be said that the job of a parent is to raise an adult. For those parents who want nothing more than to be a friend to their child, consider patience; that type of relationship will blossom once the child is out of your house and into their own. For as Mark Twain noted: “When I was a boy of 14, my father was so ignorant I could hardly stand to have the old man around. But when I got to be 21, I was astonished at how much the old man had learned in seven years.”
California Evidence-Based Clearing House for Child Welfare. (n.d.). CEBC » Topic › Parent Training Programs Behavior Problems. The California Evidence-Based Clearinghouse for Child Welfare. Retrieved April 22, 2024, from https://www.cebc4cw.org/topic/parent-training-programs-behavior-problem…
Muris, P., & Ollendick, T. H. (2023, January 18). Contemporary Hermits: A Developmental Psychopathology Account of Extreme Social Withdrawal (Hikikomori) in Young People. Clinical Child and Family Psychology Review, 26, 459-481. https://doi.org/10.1007/s10567-023-00425-8

Reuben Brody, LCSWA, is a licensed clinical social worker associate. He works at Matone Counseling and Testing in Asheville, NC.
- Find Counselling
- Find Online Therapy
- South Africa
- Johannesburg
- Port Elizabeth
- Bloemfontein
- Vereeniging
- East London
- Pietermaritzburg
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience

IMAGES
COMMENTS
The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. COVID19 infection; dementia; mortality risk. The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients.
Severe COVID-19 affects elderly with chronic diseases, including cognitive decline, in high proportion compared to the general adult population [2, 3]. According to recent studies, dementia is a major risk factor for COVID-19 severity [4, 5]. Concomitantly, the risk of exposure to the infection is more important in patients with dementia ...
Dementia is a relevant risk for COVID-19 development and influences the clinical presentation, course, and management of affected individuals. ... COVID-19 patients with dementia may be hospitalized due to the characteristic symptoms of the infection (pneumonia, fever, respiratory failure).
Clinical characteristics of dementia patients who died from COVID between Feb‐Apr 2020. Dementia patients were less likely to present with cough, to have faster clinical deterioration, have reduced access to intensive care, antivirals, hydroxychloroquine and chloroquine. ... Eight studies reported that presentation of COVID‐19 in dementia ...
BMC Geriatrics (2023) The COVID-19 pandemic has posed unique risks to people with Alzheimer disease and dementia. Research from 2020 has shown that these people have a relatively high risk of ...
Delirium caused by hypoxia, a prominent clinical feature of COVID-19, could complicate the presentation of dementia, increasing the suffering of the people living with dementia, the cost of medical care, and the need for dementia support. During the COVID-19 outbreak in China, five organisations, including the Chinese Society of Geriatric ...
The strong link between COVID-19 and vascular dementia indicates a possible role for pre-existing cerebrovascular pathology in SARS-CoV-2 infection. The researchers also found that, among people ...
Not only that, but up to 5.7% of patients with severe presentation of COVID-19 have stroke, which can precipitate cognitive decline in people already living with progressive cognitive difficulties (4). ... Bianchetti A, Rozzini R, Guerini F., et al. Clinical presentation of COVID-19 in dementia patients. J Nutr Health Aging 24, 560-562 doi.org ...
Overall, the meta-analysis of the 10 studies showed that the incidence of dementia in COVID-19 patients was (R: 9%, [95% CI: 6% to 13%]). Moreover, the meta-analysis of 9 studies showed that the mortality rate of individuals with dementia after being infected with COVID-19 was higher than that of individuals with no dementia (OR: 5.17 [95% CI ...
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients, and the clinical presentation in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. Objective No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19.
Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results: Dementia was diagnosed in 82 patients (13.1%).
SARS-COV2 pandemic exacerbates the vulnerability of dementia patients and their caregivers, due to the morbidity and mortality from COVID-19, the indirect effects of the pandemic on the social supports, and the effects on healthcare system on which they depend. Summary: The COVID-19 pandemic requires people with dementia to move from ...
Europe PMC is an archive of life sciences journal literature. Search life-sciences literature (41,605,652 (41,605,652
In the UK, 27.5% of all deaths involving COVID-19 (from March to June 2020), Alzheimer's disease and other types of dementia were the most common underlying conditions.(10) Furthermore, among 10 576 people with confirmed COVID-19 in US nursing homes, residents living with dementia were 52% of COVID-19 cases; yet, accounted for 72% of all ...
This review provides an overview of the effects of COVID-19 on dementia patients, and the potential effects of COVID-19 on the development of neurodegenerative diseases including dementia. ... Guerini F,. et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging 2020; 24:560-562. [PMC free article] [Google Scholar] 20 ...
Of the 30 laboratory-diagnosed COVID-19 elderly patients with dementia, only three (10⋅0%) were detected with delirium ... Indeed, such a clinical presentation in elderly patients with dementia might represent a prodromal phase of SARS-CoV-2 infection. As also suggested by other authors, (10. Bianchetti A. Rozzini R. Guerini F. Boffelli S.
A year after contracting COVID-19, all of the patients with dementia had experienced a significant increase in fatigue and depression, as well as worsening attention, memory, speech, visuospatial capabilities, and executive functions. All the patients also had cerebral atrophy, which is the loss of neurons and connections between neurons, and ...
Approximately 40% - 60% of people with dementia in residential care facilities experience behavioral and psychological symptoms (BPSD), such as agitation, psychosis, or apathy [1]. During the COVID-19, older adults with dementia were likely to develop behavioral changes [2]. Among multiple factors contributing to the behavioral disturbances in unprecedented times, delirium was not well ...
Measurements Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results Dementia was diagnosed in 82 patients (13.1%).
People with dementia have a higher risk of getting COVID-19, are more likely to require hospitalization, and are more likely to have severe or fatal cases of this disease compared with people without dementia. This risk is even higher in Black patients with dementia, according to a new study funded in part by NIA and published in Alzheimer's ...
The aim of the study is to describe the clinical characteristics and outcomes of a series of older patients consecutively admitted into a non-ICU ward due to SARS-CoV-2 infection (14, males 11), developing delirium. Hypokinetic delirium with lethargy and confusion was observed in 43% of cases (6/14 patients). A total of eight patients exhibited ...
The investigators identified 17,290 patients who had been hospitalized for CAP and determined that 12.0% (2079 patients; median [interquartile range] age, 71.8 [60.1-82.8] years; 50.3% women) were inappropriately diagnosed. Of the group that was inappropriately diagnosed, 87.6% (1821/2079 patients) received full antibiotic courses.
This manuscript reviews the atypical neurological presentation of COVID-19 in dementia patients and methods of prevention, diagnosis and management. ... Boffelli S, Ranieri P, Minelli G, Bianchetti L, Trabucchi M. Clinical presentation of COVID-19 in dementia patients. J Nutr Health Aging. 2020; 24:560-562. doi: 10.1007/s12603-020-1389-1.
A case series with 23 outpatients investigated the effect of transcranial magnetic stimulation (Noda et al., 2023) and another case series reported on five patients treated with EGb 761 ® following presentation with concentration and attention deficits, cognitive deficiencies, and/or fatigue 9-35 weeks after COVID-19 infection (Zifko et al ...
Dementia diagnosis. Two specialists from a diagnostic workgroup of nine medical doctors with comprehensive scientific and clinical expertise (geriatrics, old-age psychiatry, or neurology) independently diagnosed each patient with dementia using the Diagnostic and Statistical Manual of Mental Disorders-5 [].Discrepancies were resolved and consensuses were obtained by the involvement of a third ...
Please use one of the following formats to cite this article in your essay, paper or report: APA. Bose, Priyom. (2024, April 18). Study shows antipsychotic drugs increase health risks in dementia ...
There were no statistically significant differences in age, gender, and co-morbidities among COVID-19 patients with and without dementia. The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: ... Clinical presentation of COVID-19: case series and review of the literature. Int. J. Environ.
The Coronavirus disease 2019 (COVID-19) pandemic has robustly affected the global healthcare and economic systems and it was caused by coronavirus-2 (SARS-CoV-2). The clinical presentation of the disease ranges from a flu-like illness to severe pneumonia and death. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million.
Key points. Parenting influences an individual's ability to actualize and feel self-confident. Effective parenting has clear communication, boundaries, and consistent rules and reinforcements ...