An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Prev Med

How to Write a Systematic Review: A Narrative Review
Ali hasanpour dehkordi.
Social Determinants of Health Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran
Elaheh Mazaheri
1 Health Information Technology Research Center, Student Research Committee, Department of Medical Library and Information Sciences, School of Management and Medical Information Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
Hanan A. Ibrahim
2 Department of International Relations, College of Law, Bayan University, Erbil, Kurdistan, Iraq
Sahar Dalvand
3 MSc in Biostatistics, Health Promotion Research Center, Iran University of Medical Sciences, Tehran, Iran
Reza Ghanei Gheshlagh
4 Spiritual Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
In recent years, published systematic reviews in the world and in Iran have been increasing. These studies are an important resource to answer evidence-based clinical questions and assist health policy-makers and students who want to identify evidence gaps in published research. Systematic review studies, with or without meta-analysis, synthesize all available evidence from studies focused on the same research question. In this study, the steps for a systematic review such as research question design and identification, the search for qualified published studies, the extraction and synthesis of information that pertain to the research question, and interpretation of the results are presented in details. This will be helpful to all interested researchers.
A systematic review, as its name suggests, is a systematic way of collecting, evaluating, integrating, and presenting findings from several studies on a specific question or topic.[ 1 ] A systematic review is a research that, by identifying and combining evidence, is tailored to and answers the research question, based on an assessment of all relevant studies.[ 2 , 3 ] To identify assess and interpret available research, identify effective and ineffective health-care interventions, provide integrated documentation to help decision-making, and identify the gap between studies is one of the most important reasons for conducting systematic review studies.[ 4 ]
In the review studies, the latest scientific information about a particular topic is criticized. In these studies, the terms of review, systematic review, and meta-analysis are used instead. A systematic review is done in one of two methods, quantitative (meta-analysis) and qualitative. In a meta-analysis, the results of two or more studies for the evaluation of say health interventions are combined to measure the effect of treatment, while in the qualitative method, the findings of other studies are combined without using statistical methods.[ 5 ]
Since 1999, various guidelines, including the QUORUM, the MOOSE, the STROBE, the CONSORT, and the QUADAS, have been introduced for reporting meta-analyses. But recently the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement has gained widespread popularity.[ 6 , 7 , 8 , 9 ] The systematic review process based on the PRISMA statement includes four steps of how to formulate research questions, define the eligibility criteria, identify all relevant studies, extract and synthesize data, and deduce and present results (answers to research questions).[ 2 ]
Systematic Review Protocol
Systematic reviews start with a protocol. The protocol is a researcher road map that outlines the goals, methodology, and outcomes of the research. Many journals advise writers to use the PRISMA statement to write the protocol.[ 10 ] The PRISMA checklist includes 27 items related to the content of a systematic review and meta-analysis and includes abstracts, methods, results, discussions, and financial resources.[ 11 ] PRISMA helps writers improve their systematic review and meta-analysis report. Reviewers and editors of medical journals acknowledge that while PRISMA may not be used as a tool to assess the methodological quality, it does help them to publish a better study article [ Figure 1 ].[ 12 ]

Screening process and articles selection according to the PRISMA guidelines
The main step in designing the protocol is to define the main objectives of the study and provide some background information. Before starting a systematic review, it is important to assess that your study is not a duplicate; therefore, in search of published research, it is necessary to review PREOSPERO and the Cochrane Database of Systematic. Sometimes it is better to search, in four databases, related systematic reviews that have already been published (PubMed, Web of Sciences, Scopus, Cochrane), published systematic review protocols (PubMed, Web of Sciences, Scopus, Cochrane), systematic review protocols that have already been registered but have not been published (PROSPERO, Cochrane), and finally related published articles (PubMed, Web of Sciences, Scopus, Cochrane). The goal is to reduce duplicate research and keep up-to-date systematic reviews.[ 13 ]
Research questions
Writing a research question is the first step in systematic review that summarizes the main goal of the study.[ 14 ] The research question determines which types of studies should be included in the analysis (quantitative, qualitative, methodic mix, review overviews, or other studies). Sometimes a research question may be broken down into several more detailed questions.[ 15 ] The vague questions (such as: is walking helpful?) makes the researcher fail to be well focused on the collected studies or analyze them appropriately.[ 16 ] On the other hand, if the research question is rigid and restrictive (e.g., walking for 43 min and 3 times a week is better than walking for 38 min and 4 times a week?), there may not be enough studies in this area to answer this question and hence the generalizability of the findings to other populations will be reduced.[ 16 , 17 ] A good question in systematic review should include components that are PICOS style which include population (P), intervention (I), comparison (C), outcome (O), and setting (S).[ 18 ] Regarding the purpose of the study, control in clinical trials or pre-poststudies can replace C.[ 19 ]
Search and identify eligible texts
After clarifying the research question and before searching the databases, it is necessary to specify searching methods, articles screening, studies eligibility check, check of the references in eligible studies, data extraction, and data analysis. This helps researchers ensure that potential biases in the selection of potential studies are minimized.[ 14 , 17 ] It should also look at details such as which published and unpublished literature have been searched, how they were searched, by which mechanism they were searched, and what are the inclusion and exclusion criteria.[ 4 ] First, all studies are searched and collected according to predefined keywords; then the title, abstract, and the entire text are screened for relevance by the authors.[ 13 ] By screening articles based on their titles, researchers can quickly decide on whether to retain or remove an article. If more information is needed, the abstracts of the articles will also be reviewed. In the next step, the full text of the articles will be reviewed to identify the relevant articles, and the reason for the removal of excluded articles is reported.[ 20 ] Finally, it is recommended that the process of searching, selecting, and screening articles be reported as a flowchart.[ 21 ] By increasing research, finding up-to-date and relevant information has become more difficult.[ 22 ]
Currently, there is no specific guideline as to which databases should be searched, which database is the best, and how many should be searched; but overall, it is advisable to search broadly. Because no database covers all health topics, it is recommended to use several databases to search.[ 23 ] According to the A MeaSurement Tool to Assess Systematic Reviews scale (AMSTAR) at least two databases should be searched in systematic and meta-analysis, although more comprehensive and accurate results can be obtained by increasing the number of searched databases.[ 24 ] The type of database to be searched depends on the systematic review question. For example, in a clinical trial study, it is recommended that Cochrane, multi-regional clinical trial (mRCTs), and International Clinical Trials Registry Platform be searched.[ 25 ]
For example, MEDLINE, a product of the National Library of Medicine in the United States of America, focuses on peer-reviewed articles in biomedical and health issues, while Embase covers the broad field of pharmacology and summaries of conferences. CINAHL is a great resource for nursing and health research and PsycINFO is a great database for psychology, psychiatry, counseling, addiction, and behavioral problems. Also, national and regional databases can be used to search related articles.[ 26 , 27 ] In addition, the search for conferences and gray literature helps to resolve the file-drawn problem (negative studies that may not be published yet).[ 26 ] If a systematic review is carried out on articles in a particular country or region, the databases in that region or country should also be investigated. For example, Iranian researchers can use national databases such as Scientific Information Database and MagIran. Comprehensive search to identify the maximum number of existing studies leads to a minimization of the selection bias. In the search process, the available databases should be used as much as possible, since many databases are overlapping.[ 17 ] Searching 12 databases (PubMed, Scopus, Web of Science, EMBASE, GHL, VHL, Cochrane, Google Scholar, Clinical trials.gov, mRCTs, POPLINE, and SIGLE) covers all articles published in the field of medicine and health.[ 25 ] Some have suggested that references management software be used to search for more easy identification and removal of duplicate articles from several different databases.[ 20 ] At least one search strategy is presented in the article.[ 21 ]
Quality assessment
The methodological quality assessment of articles is a key step in systematic review that helps identify systemic errors (bias) in results and interpretations. In systematic review studies, unlike other review studies, qualitative assessment or risk of bias is required. There are currently several tools available to review the quality of the articles. The overall score of these tools may not provide sufficient information on the strengths and weaknesses of the studies.[ 28 ] At least two reviewers should independently evaluate the quality of the articles, and if there is any objection, the third author should be asked to examine the article or the two researchers agree on the discussion. Some believe that the study of the quality of studies should be done by removing the name of the journal, title, authors, and institutions in a Blinded fashion.[ 29 ]
There are several ways for quality assessment, such as Sack's quality assessment (1988),[ 30 ] overview quality assessment questionnaire (1991),[ 31 ] CASP (Critical Appraisal Skills Program),[ 32 ] and AMSTAR (2007),[ 33 ] Besides, CASP,[ 34 ] the National Institute for Health and Care Excellence,[ 35 ] and the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information checklists.[ 30 , 36 ] However, it is worth mentioning that there is no single tool for assessing the quality of all types of reviews, but each is more applicable to some types of reviews. Often, the STROBE tool is used to check the quality of articles. It reviews the title and abstract (item 1), introduction (items 2 and 3), implementation method (items 4–12), findings (items 13–17), discussion (Items 18–21), and funding (item 22). Eighteen items are used to review all articles, but four items (6, 12, 14, and 15) apply in certain situations.[ 9 ] The quality of interventional articles is often evaluated by the JADAD tool, which consists of three sections of randomization (2 scores), blinding (2 scores), and patient count (1 scores).[ 29 ]
Data extraction
At this stage, the researchers extract the necessary information in the selected articles. Elamin believes that reviewing the titles and abstracts and data extraction is a key step in the review process, which is often carried out by two of the research team independently, and ultimately, the results are compared.[ 37 ] This step aimed to prevent selection bias and it is recommended that the chance of agreement between the two researchers (Kappa coefficient) be reported at the end.[ 26 ] Although data collection forms may differ in systematic reviews, they all have information such as first author, year of publication, sample size, target community, region, and outcome. The purpose of data synthesis is to collect the findings of eligible studies, evaluate the strengths of the findings of the studies, and summarize the results. In data synthesis, we can use different analysis frameworks such as meta-ethnography, meta-analysis, or thematic synthesis.[ 38 ] Finally, after quality assessment, data analysis is conducted. The first step in this section is to provide a descriptive evaluation of each study and present the findings in a tabular form. Reviewing this table can determine how to combine and analyze various studies.[ 28 ] The data synthesis approach depends on the nature of the research question and the nature of the initial research studies.[ 39 ] After reviewing the bias and the abstract of the data, it is decided that the synthesis is carried out quantitatively or qualitatively. In case of conceptual heterogeneity (systematic differences in the study design, population, and interventions), the generalizability of the findings will be reduced and the study will not be meta-analysis. The meta-analysis study allows the estimation of the effect size, which is reported as the odds ratio, relative risk, hazard ratio, prevalence, correlation, sensitivity, specificity, and incidence with a confidence interval.[ 26 ]
Estimation of the effect size in systematic review and meta-analysis studies varies according to the type of studies entered into the analysis. Unlike the mean, prevalence, or incidence index, in odds ratio, relative risk, and hazard ratio, it is necessary to combine logarithm and logarithmic standard error of these statistics [ Table 1 ].
Effect size in systematic review and meta-analysis
OR=Odds ratio; RR=Relative risk; RCT= Randomized controlled trial; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio; DOR: diagnostic odds ratio
Interpreting and presenting results (answers to research questions)
A systematic review ends with the interpretation of results. At this stage, the results of the study are summarized and the conclusions are presented to improve clinical and therapeutic decision-making. A systematic review with or without meta-analysis provides the best evidence available in the hierarchy of evidence-based practice.[ 14 ] Using meta-analysis can provide explicit conclusions. Conceptually, meta-analysis is used to combine the results of two or more studies that are similar to the specific intervention and the similar outcomes. In meta-analysis, instead of the simple average of the results of various studies, the weighted average of studies is reported, meaning studies with larger sample sizes account for more weight. To combine the results of various studies, we can use two models of fixed and random effects. In the fixed-effect model, it is assumed that the parameters studied are constant in all studies, and in the random-effect model, the measured parameter is assumed to be distributed between the studies and each study has measured some of it. This model offers a more conservative estimate.[ 40 ]
Three types of homogeneity tests can be used: (1) forest plot, (2) Cochrane's Q test (Chi-squared), and (3) Higgins I 2 statistics. In the forest plot, more overlap between confidence intervals indicates more homogeneity. In the Q statistic, when the P value is less than 0.1, it indicates heterogeneity exists and a random-effect model should be used.[ 41 ] Various tests such as the I 2 index are used to determine heterogeneity, values between 0 and 100; the values below 25%, between 25% and 50%, and above 75% indicate low, moderate, and high levels of heterogeneity, respectively.[ 26 , 42 ] The results of the meta-analyzing study are presented graphically using the forest plot, which shows the statistical weight of each study with a 95% confidence interval and a standard error of the mean.[ 40 ]
The importance of meta-analyses and systematic reviews in providing evidence useful in making clinical and policy decisions is ever-increasing. Nevertheless, they are prone to publication bias that occurs when positive or significant results are preferred for publication.[ 43 ] Song maintains that studies reporting a certain direction of results or powerful correlations may be more likely to be published than the studies which do not.[ 44 ] In addition, when searching for meta-analyses, gray literature (e.g., dissertations, conference abstracts, or book chapters) and unpublished studies may be missed. Moreover, meta-analyses only based on published studies may exaggerate the estimates of effect sizes; as a result, patients may be exposed to harmful or ineffective treatment methods.[ 44 , 45 ] However, there are some tests that can help in detecting negative expected results that are not included in a review due to publication bias.[ 46 ] In addition, publication bias can be reduced through searching for data that are not published.
Systematic reviews and meta-analyses have certain advantages; some of the most important ones are as follows: examining differences in the findings of different studies, summarizing results from various studies, increased accuracy of estimating effects, increased statistical power, overcoming problems related to small sample sizes, resolving controversies from disagreeing studies, increased generalizability of results, determining the possible need for new studies, overcoming the limitations of narrative reviews, and making new hypotheses for further research.[ 47 , 48 ]
Despite the importance of systematic reviews, the author may face numerous problems in searching, screening, and synthesizing data during this process. A systematic review requires extensive access to databases and journals that can be costly for nonacademic researchers.[ 13 ] Also, in reviewing the inclusion and exclusion criteria, the inevitable mindsets of browsers may be involved and the criteria are interpreted differently from each other.[ 49 ] Lee refers to some disadvantages of these studies, the most significant ones are as follows: a research field cannot be summarized by one number, publication bias, heterogeneity, combining unrelated things, being vulnerable to subjectivity, failing to account for all confounders, comparing variables that are not comparable, just focusing on main effects, and possible inconsistency with results of randomized trials.[ 47 ] Different types of programs are available to perform meta-analysis. Some of the most commonly used statistical programs are general statistical packages, including SAS, SPSS, R, and Stata. Using flexible commands in these programs, meta-analyses can be easily run and the results can be readily plotted out. However, these statistical programs are often expensive. An alternative to using statistical packages is to use programs designed for meta-analysis, including Metawin, RevMan, and Comprehensive Meta-analysis. However, these programs may have limitations, including that they can accept few data formats and do not provide much opportunity to set the graphical display of findings. Another alternative is to use Microsoft Excel. Although it is not a free software, it is usually found in many computers.[ 20 , 50 ]
A systematic review study is a powerful and valuable tool for answering research questions, generating new hypotheses, and identifying areas where there is a lack of tangible knowledge. A systematic review study provides an excellent opportunity for researchers to improve critical assessment and evidence synthesis skills.
Authors' contributions
All authors contributed equally to this work.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Open access
- Published: 19 April 2021
How to properly use the PRISMA Statement
- Rafael Sarkis-Onofre 1 ,
- Ferrán Catalá-López 2 , 3 ,
- Edoardo Aromataris 4 &
- Craig Lockwood 4
Systematic Reviews volume 10 , Article number: 117 ( 2021 ) Cite this article
73k Accesses
187 Citations
103 Altmetric
Metrics details
A Research to this article was published on 29 March 2021
It has been more than a decade since the original publication of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [ 1 ], and it has become one of the most cited reporting guidelines in biomedical literature [ 2 , 3 ]. Since its publication, multiple extensions of the PRISMA Statement have been published concomitant with the advancement of knowledge synthesis methods [ 4 , 5 , 6 , 7 ]. The PRISMA2020 statement, an updated version has recently been published [ 8 ], and other extensions are currently in development [ 9 ].
The number of systematic reviews (SRs) has increased substantially over the past 20 years [ 10 , 11 , 12 ]. However, many SRs continue to be poorly conducted and reported [ 10 , 11 ], and it is still common to see articles that use the PRISMA Statement and other reporting guidelines inappropriately, as was highlighted recently [ 13 ].
The PRISMA Statement and its extensions are an evidence-based, minimum set of recommendations designed primarily to encourage transparent and complete reporting of SRs. This growing set of guidelines have been developed to aid authors with appropriate reporting of different knowledge synthesis methods (such as SRs, scoping reviews, and review protocols) and to ensure that all aspects of this type of research are accurately and transparently reported. In other words, the PRISMA Statement is a road map to help authors best describe what was done, what was found, and in the case of a review protocol, what are they are planning to do.
Despite this clear and well-articulated intention [ 2 , 3 , 4 , 5 ], it is common for Systematic Reviews to receive manuscripts detailing the inappropriate use of the PRISMA Statement and its extensions. Most frequently, improper use appears with authors attempting to use the PRISMA statement as a methodological guideline for the design and conduct reviews, or identifying the PRISMA statement as a tool to assess the methodological quality of reviews, as seen in the following examples:
“This scoping review will be conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Statement.”
“This protocol was designed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
“The methodological quality of the included systematic reviews will be assessed with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement.”
Some organizations (such as Cochrane and JBI) have developed methodological guidelines that can help authors to design or conduct diverse types of knowledge synthesis rigorously [ 14 , 15 ]. While the PRISMA statement is presented to predominantly guide reporting of a systematic review of interventions with meta-analyses, its detailed criteria can readily be applied to the majority of review types [ 13 ]. Differences between the role of the PRISMA Statement to guide reporting versus guidelines detailing methodological conduct is readily illustrated with the following example: the PRISMA Statement recommends that authors report their complete search strategies for all databases, registers, and websites (including any filters and limits used), but it does not include recommendations for designing and conducting literature searches [ 8 ]. If authors are interested in understanding how to create search strategies or which databases to include, they should refer to the methodological guidelines [ 12 , 13 ]. Thus, the following examples can illustrate the appropriate use of the PRISMA Statement in research reporting:
“The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.”
“This scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).”
“The protocol is being reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement.”
Systematic Reviews supports the complete and transparent reporting of research. The Editors require the submission of a populated checklist from the relevant reporting guidelines, including the PRISMA checklist or the most appropriate PRISMA extension. Using the PRISMA statement and its extensions to write protocols or the completed review report, and completing the PRISMA checklists are likely to let reviewers and readers know what authors did and found, but also to optimize the quality of reporting and make the peer review process more efficient.
Transparent and complete reporting is an essential component of “good research”; it allows readers to judge key issues regarding the conduct of research and its trustworthiness and is also critical to establish a study’s replicability.
With the release of a major update to PRISMA in 2021, the appropriate use of the updated PRISMA Statement (and its extensions as those updates progress) will be an essential requirement for review based submissions, and we encourage authors, peer reviewers, and readers of Systematic Reviews to use and disseminate that initiative.
Availability of data and materials
We do not have any additional data or materials to share.
Abbreviations
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
Systematic reviews
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005 .
Article PubMed Google Scholar
Caulley L, Cheng W, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, et al. Citation impact was highly variable for reporting guidelines of health research: a citation analysis. J Clin Epidemiol. 2020;127:96–104. https://doi.org/10.1016/j.jclinepi.2020.07.013 .
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. https://doi.org/10.1186/s13643-017-0663-8 .
Article PubMed PubMed Central Google Scholar
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. https://doi.org/10.1186/s13643-020-01542-z .
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850 .
Article Google Scholar
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi/10.1186/s13643-021-01626-4.
EQUATOR Network: Reporting guidelines under development for systematic reviews. https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/ . Accessed 11 Feb 2021.
Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. Plos Med. 2016;13(5):e1002028. https://doi.org/10.1371/journal.pmed.1002028 .
Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016;94(3):485–514. https://doi.org/10.1111/1468-0009.12210 .
Niforatos JD, Weaver M, Johansen ME. Assessment of Publication Trends of Systematic Reviews and Randomized Clinical Trials, 1995 to 2017. JAMA Intern Med. 2019;179(11):1593–4. https://doi.org/10.1001/jamainternmed.2019.3013.
Caulley L, Catala-Lopez F, Whelan J, Khoury M, Ferraro J, Cheng W, et al. Reporting guidelines of health research studies are frequently used inappropriately. J Clin Epidemiol. 2020;122:87–94. https://doi.org/10.1016/j.jclinepi.2020.03.006 .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition ed. Chichester: Wiley; 2019.
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. ed. Adelaide: JBI; 2020.
Download references
Acknowledgements
RSO is funded in part by Meridional Foundation. FCL is funded in part by the Institute of Health Carlos III/CIBERSAM.
Author information
Authors and affiliations.
Graduate Program in Dentistry, Meridional Faculty, IMED, Passo Fundo, Brazil
Rafael Sarkis-Onofre
Department of Health Planning and Economics, National School of Public Health, Institute of Health Carlos III, Madrid, Spain
Ferrán Catalá-López
Department of Medicine, University of Valencia/INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain
JBI, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, Australia
Edoardo Aromataris & Craig Lockwood
You can also search for this author in PubMed Google Scholar
Contributions
RSO drafted the initial version. FCL, EA, and CL made substantial additions to the first and subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rafael Sarkis-Onofre .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
CL is Editor-in-Chief of Systematic Reviews, FCL is Protocol Editor of Systematic Reviews, and RSO is Associate Editor of Systematic Reviews.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sarkis-Onofre, R., Catalá-López, F., Aromataris, E. et al. How to properly use the PRISMA Statement. Syst Rev 10 , 117 (2021). https://doi.org/10.1186/s13643-021-01671-z
Download citation
Published : 19 April 2021
DOI : https://doi.org/10.1186/s13643-021-01671-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Doing a systematic review
Creating a protocol, pico framework for structuring your research, alternative frameworks, registering your protocol.
- Step 2: choosing where to search
- Step 3: developing a search strategy
- Step 4: running & recording your search
- Step 5: managing your search results
- Step 6: screening & evaluating
- Step 7: synthesis and writing it up (including PRISMA flow diagrams)
Finding protocol examples

Use the PROSPERO database to find examples of protocols and details of reviews in progress. If you are planning to publish your review check PROSPERO to make sure someone isn't already investigating that area. It is also a good idea to register your own review here once you've established its uniqueness.
- PROSPERO This international database covers prospectively registered systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome.
Software for managing your review
- RevMan Review Manager (RevMan) is Cochrane's software for preparing and maintaining Cochrane reviews, although it can be used for non-Cochrane reviews too. If you are doing a professional systematic review leading to publication you may wish to consider using it to help manage the process. RevMan facilitates preparation of protocols and full reviews, including text, characteristics of studies, comparison tables, and study data. It can perform meta-analysis of the data entered, and present results graphically. Note that a subscription is required to use this software.

This may be revised as you work through the next step which is to develop a protocol which outlines the study methodology including:
- research question and aims
- criteria for inclusion and exclusion - these specify attributes which selected studies must have, or attributes which will disqualify them. They must be sufficiently clear and detailed to enable you to accurately assess the relevance of each study.
- search strategy
- quality assessment
- data extraction & analysis
- synthesis of results
- dissemination
- time frame.
Whilst you are in the early stages of choosing and defining your research question try doing some scoping searches , using simple search terms, on a couple of relevant databases. These will help you identify key papers, boost your understanding of the topic, and give you a feel for how many studies have been done. If there are too few results how can your broaden your search? If too many, can you focus in on a specific aspect or population, or apply limits such as study type or publication date?
- Systematic review protocol template Use this Word document to help you plan your review and develop your protocol
- Systematic review protocol example An example of using the protocol template to plan a systematic review.
- The review protocol Produced by the Centre for Reviews and Dissemination this detailed guidance covers elements to consider when creating a review protocol.
- Watch our video introductions to doing a systematic review Watch video 2 in our series of videos on doing a systematic review for brief guidance on creating your protocol.
There are several different frameworks you can use to help structure your research and ensure you have clear parameters for your search. The most commonly used one used for health-related reviews is the PICO framework:
- Population This could be the general population, or a specific group defined by: age (e.g. infants, children, adolescents, elderly); socioeconomic status (e.g. low-income, homeless); risk status; location (rural or urban)
- Intervention Refers to the therapy, test, strategy to be investigated (e.g. drug, behavioural change, environmental factors, counselling)
- Comparator A measure you will use to compare results against (e.g. no treatment, alternative treatment/exposure, standard/routine interventions)
- Outcome What outcome is significant to your population or issue? This may be different from the outcome measures used in the studies.
PICO example
This example is extracted from: PROSPERO 2018 CRD42018100888 .
Further information
- Developing an efficient search strategy using PICO A tool created by Health Evidence which can be used to: develop a clear, answerable question that can be used to generate a search strategy; identify key search terms to facilitate a more efficient search; and to document search strategies/terms for future search updates, either in the same or similar topics.
Another framework may be more suitable depending on your review topic. Here are some other options:
- PECO – Population | Environment | Comparison | Outcome Very similar to PICO but looking at the effect of exposure to something e.g. smoky atmosphere
- SPICE - Setting | Population | Intervention | Comparison | Evaluation Another variant of PICO but this time including the setting (where? in what context?)
- CIMO - Context | Intervention | Mechanisms | Outcome A variant of PICO suitable for management and organisation studies
- ECLIPSE - Expectation | Client group | Location | Impact | Professionals | SErvice Recommended for health policy/management searches
- SPIDER – Sample | Phenomenon of Interest | Design | Evaluation | Research Type Developed to create effective search strategies of qualitative and mixed-methods research - more specific than PICO/PECO
- Developing a research question This guide from the University of Maryland includes a comprehensive list of other frameworks.
- How CLIP became ECLIPSE: a mnemonic to assist in searching for health policy/management information Article about the development of the ECLIPSE framework.
- Beyond PICO: the SPIDER tool for qualitative evidence synthesis. A comparison of the PICO and SPIDER frameworks for creating search strategies.
If you are doing a systematic review for potential publication then it is a good idea to register it with one of the following sites. This helps ensure you are not duplicating an existing study. Also by registering the protocol prior to starting your research there is a reduced risk of reframing the study to fit the results.
Sites only accepting reviews
- PROSPERO (International Prospective Register of Systematic Reviews) Accepts systematic review outlines in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome. The most established of all the registers - starting in 2011. Free to register your own review but might be rejected if it does not meet their criteria (they accept systematic reviews, rapid reviews, umbrella reviews, and meta-analyses - they don’t accept scoping, mapping, or literature reviews). You also have to register your review before you start your searches.
- Research Registry (Registry of Systematic Reviews/Meta-Analyses in Research Registry) Accepts outlines of systematic reviews and meta-analyses in any field. Established in 2015 but still much smaller than PROSPERO. Charges a fee to register each review.
- INPLASY (International Platform of Registered Systematic Review and Meta-analysis Protocols) Accepts systematic and scoping review outlines in any field. Much smaller than PROSPERO as it started in 2020. Minimal registration costs.
Sites accepting any type of study
Both these sites accepts all study designs in any field. Both are free to register. Unlike the previously mentioned sites there is no checking of records.
- Open Science Framework
- protocols.io
The following resources give more information about why it is important to register your review protocol and the sites which are currently available.
- Registering protocols of systematic reviews (blog post on the Cochrane Community)
- Where to prospectively register a systematic review (article)
- << Previous: Home
- Next: Step 2: choosing where to search >>
- Last Updated: Jun 3, 2024 5:03 PM
- URL: https://libguides.reading.ac.uk/systematic-review
A Guide to Writing a Qualitative Systematic Review Protocol to Enhance Evidence-Based Practice in Nursing and Health Care
Affiliations.
- 1 PhD candidate, School of Nursing and Midwifey, Monash University, and Clinical Nurse Specialist, Adult and Pediatric Intensive Care Unit, Monash Health, Melbourne, Victoria, Australia.
- 2 Lecturer, School of Nursing and Midwifery, Monash University, Melbourne, Victoria, Australia.
- 3 Senior Lecturer, School of Nursing and Midwifery, Monash University, Melbourne, Victoria, Australia.
- PMID: 26790142
- DOI: 10.1111/wvn.12134
Background: The qualitative systematic review is a rapidly developing area of nursing research. In order to present trustworthy, high-quality recommendations, such reviews should be based on a review protocol to minimize bias and enhance transparency and reproducibility. Although there are a number of resources available to guide researchers in developing a quantitative review protocol, very few resources exist for qualitative reviews.
Aims: To guide researchers through the process of developing a qualitative systematic review protocol, using an example review question.
Methodology: The key elements required in a systematic review protocol are discussed, with a focus on application to qualitative reviews: Development of a research question; formulation of key search terms and strategies; designing a multistage review process; critical appraisal of qualitative literature; development of data extraction techniques; and data synthesis. The paper highlights important considerations during the protocol development process, and uses a previously developed review question as a working example.
Implications for research: This paper will assist novice researchers in developing a qualitative systematic review protocol. By providing a worked example of a protocol, the paper encourages the development of review protocols, enhancing the trustworthiness and value of the completed qualitative systematic review findings.
Linking evidence to action: Qualitative systematic reviews should be based on well planned, peer reviewed protocols to enhance the trustworthiness of results and thus their usefulness in clinical practice. Protocols should outline, in detail, the processes which will be used to undertake the review, including key search terms, inclusion and exclusion criteria, and the methods used for critical appraisal, data extraction and data analysis to facilitate transparency of the review process. Additionally, journals should encourage and support the publication of review protocols, and should require reference to a protocol prior to publication of the review results.
Keywords: guidelines; meta synthesis; qualitative; systematic review protocol.
© 2016 Sigma Theta Tau International.
- Evidence-Based Practice / standards*
- Information Seeking Behavior
- Nursing / methods
- Qualitative Research*
- Research Design / standards*
- Systematic Reviews as Topic*
- Writing / standards*
Research Guides
Systematic reviews and related evidence syntheses: proposal.
- Standards & Guidelines
Getting started with proposal of review
The proposal stage is the most important step of a review project as it determines the feasibility of the review and its rationale.
The steps are:
1. Determining review question and review type.
- Right Review : free tool to assist in selecting best review type for a given question
- Trying to choose between a scoping or a systematic review? try this article- Munn, Z., Peters, M.D.J., Stern, C. et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach . BMC Med Res Methodol 18 , 143 (2018). https://doi.org/10.1186/s12874-018-0611-x
- This article provides 10 different types of questions that systematic reviews can answer- Munn, Z., Stern, C., Aromataris, E. et al. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 18, 5 (2018).
- For scoping reviews, the framework is: Population, Concept, Context (see JBI Scoping Review guide )
2. Search for related reviews to proposed question. Places to search include:
- Prospero : database of protocols for systematic reviews, umbrella reviews, and rapid reviews with human health outcomes
- Open Science Framework : open access registry for any type of research, including scoping reviews and more
- Cochrane Collaboration Handbook : systematic reviews of clinical interventions
- Campbell Collaboration : accepts many types reviews across many disciplines: Business and Management, Crime and Justice, Disability, Education, International Development, Knowledge Translation and Implementation, Methods, Nutrition, and Social Welfare
Collaboration for Environmental Evidence : reviews in environmental research
Systematic Reviews for Animals & Food (SYREAF) : protocols and reviews on animals and food science related to animals
Also, consider searching subject related databases, adding a search concept "review"
3. Evaluate previous reviews for quality, as well as comparing their scope to the proposed review. The following tools can be used to
- ROBIS (Risk of Bias in Systematic reviews)
AMSTAR : Assessing the Methodological Quality of Systematic Reviews, for meta-analysis
- CASP Checklist : Critical Appraisal Skills Programme
4. Further refine question by defining the eligibility criteria
- Eligibility criteria are the characteristics of the studies/research to be collected. Inclusion criteria are those characteristics a study must have to be include. Exclusion criteria are exceptions to the inclusion criteria.
5. Develop a preliminary search and find a few studies that match the eligibility criteria
- Consider working with a librarian to develop a search. The purpose is to estimate the number of citations to be sorted (giving some idea of the amount time it will take complete the review) and to find at least a few studies that match the criteria.
6. Summarize proposal : A written proposal helps in framing the project and getting feedback. It should include:
- A descriptive title of project, which includes the type of review
- A brief introduction
- A description of previous reviews and the rationale for the proposed review
- An appropriate framed question for the review
- The eligibility criteria
- << Previous: About
- Next: Standards & Guidelines >>
- Last Updated: May 3, 2024 4:51 PM
- URL: https://tamu.libguides.com/systematic_reviews
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Research process
- How to Write a Research Proposal | Examples & Templates
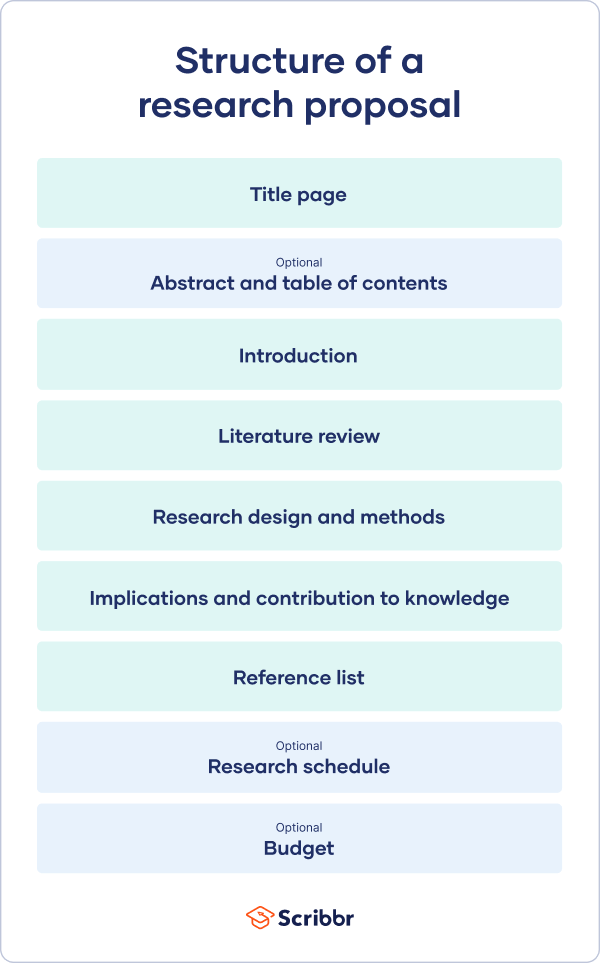
How to Write a Research Proposal | Examples & Templates
Published on 30 October 2022 by Shona McCombes and Tegan George. Revised on 13 June 2023.
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:

Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organised and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, frequently asked questions.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Prevent plagiarism, run a free check.
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: ‘A Conceptual Framework for Scheduling Constraint Management’
- Example research proposal #2: ‘ Medical Students as Mediators of Change in Tobacco Use’
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesise prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasise again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement.
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
McCombes, S. & George, T. (2023, June 13). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved 3 June 2024, from https://www.scribbr.co.uk/the-research-process/research-proposal-explained/
Is this article helpful?
Shona McCombes
Other students also liked, what is a research methodology | steps & tips, what is a literature review | guide, template, & examples, how to write a results section | tips & examples.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Research Proposal for a Systematic Review of the Effectiveness of Community Interventions in

The documented health effects of smoking are perhaps the most fundamental motivators of the efforts to curtail tobacco use. The efforts can be the work of governments or independent bodies that are concerned about the public health due to for instance the economic implications of smoking-related health diseases particularly on low-income earners. By illustration, governments may adopt statutory policies to curtail smoking by designating smoke zones and instituting legal penalties against individuals that smoke beyond such areas. Nevertheless, such measures, however stringent are undermined by their coercive nature that restricts smoking in public arenas but provides no incentive to curtail smoking at the household level. As such, the efficacy of affirmative action depends on the motivation that such provides to the person to quit tobacco use. For example, community efforts might be more impactful when they are frequently held to sensitize the population on the actual effects of tobacco use on health and the economic status of the family. The challenges to the mitigation strategies stem in principal from the business community thus detracting from the full impact of the efforts. In the light of the varying efficiency of tobacco use mitigation efforts, this paper presents a protocol for a systematic review of community interventions to prevent smoking among adults in the United Kingdom.
Related Papers
Central European Journal of Public Health
Egemen Ünal
Janet Ferguson , Amanda Amos
Tobacco Control
Christine Godfrey
Preventive Medicine
Lynne Parkinson
Public Health
Richard Edwards
Linda Bauld
Martin White , Amanda Amos
Drug and alcohol review
Prabhat Jha
Cigarette smoking and other tobacco use imposes a huge and growing public health burden globally. Currently, approximately 5 million people are killed annually by tobacco use; by 2030, estimates based on current trends indicate that this number will increase to 10 million, with 70% of deaths occurring in low- and middle-income countries. Numerous studies from high-income countries, and a growing number from low- and middle-income countries, provide strong evidence that tobacco tax increases, dissemination of information about health risks from smoking, restrictions on smoking in public places and in work-places, comprehensive bans on advertising and promotion and increased access to cessation therapies are all effective in reducing tobacco use and its consequences. Despite this evidence, tobacco control policies have been unevenly applied—due partly to political constraints. This paper provides a summary of these issues, beginning with an overview of trends in global tobacco use and its consequences and followed by a review of the evidence on the effectiveness of tobacco control policies in reducing tobacco use. A description of the types and comprehensiveness of policies currently in place and a discussion of some of the factors correlated with the strength and comprehensive of these policies follows.
American Journal of Preventive Medicine
Sajal Chattopadhyay
Afaf Girgis
RELATED PAPERS
Journal of Neurology, Neurosurgery & Psychiatry
Matthew Walker
Birat Journal of Health Sciences
Roshan Yadav
Fertilizer Research
julian zuluaga henao
Intercultural Communication Studies
Planta Medica
Muhammad Bello
Digest of the 9th International Conference on Optical Internet (COIN 2010)
Lian Kuan Chen
saksham sharma
Green Energy and Technology
Giulia De Aloysio
Journal of Interdisciplinary History
riccardo rosolino
Cultura del cuidado
Natalia Betancur
Journal of Geoscience and Environment Protection
Ejembi Emmanuel
Actas de la I Jornada Didáctica para Profesores de ELE Enseñar español en el sur de Brasil: realidades, prácticas y retos de los estados de frontera. Instituto Cervantes de Curitiba. 28 de septiembre de 2019.
Javier Badiola González
Electrochimica Acta
Feridun Hamdullahpur
World Journal of Microbiology and Biotechnology
Mônica Rossi
IEEE Journal of Photovoltaics
Ahmed Saïdi
Proceedings of the Nigerian Academy of Science
Aliyu Sani Ado
Farnoush Faridbod
Journal for the Study of the Old Testament
Cristiana Conti-Easton
International Journal of Engineering Research in Africa
Gideon Bamigboye
Research in Computing Science
ADRIAN MACIAS ESTRADA
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Systematic Review
- Open access
- Published: 30 May 2024
Patient experiences: a qualitative systematic review of chemotherapy adherence
- Amineh Rashidi 1 ,
- Susma Thapa 1 ,
- Wasana Sandamali Kahawaththa Palliya Guruge 1 &
- Shubhpreet Kaur 1
BMC Cancer volume 24 , Article number: 658 ( 2024 ) Cite this article
177 Accesses
Metrics details
Adherence to chemotherapy treatment is recognized as a crucial health concern, especially in managing cancer patients. Chemotherapy presents challenges for patients, as it can lead to potential side effects that may adversely affect their mobility and overall function. Patients may sometimes neglect to communicate these side effects to health professionals, which can impact treatment management and leave their unresolved needs unaddressed. However, there is limited understanding of how patients’ experiences contribute to improving adherence to chemotherapy treatment and the provision of appropriate support. Therefore, gaining insights into patients’ experiences is crucial for enhancing the accompaniment and support provided during chemotherapy.
This review synthesizes qualitative literature on chemotherapy adherence within the context of patients’ experiences. Data were collected from Medline, Web of Science, CINAHL, PsychINFO, Embase, Scopus, and the Cochrane Library, systematically searched from 2006 to 2023. Keywords and MeSH terms were utilized to identify relevant research published in English. Thirteen articles were included in this review. Five key themes were synthesized from the findings, including positive outlook, receiving support, side effects, concerns about efficacy, and unmet information needs. The review underscores the importance for healthcare providers, particularly nurses, to focus on providing comprehensive information about chemotherapy treatment to patients. Adopting recommended strategies may assist patients in clinical practice settings in enhancing adherence to chemotherapy treatment and improving health outcomes for individuals living with cancer.
Peer Review reports
Introduction
Cancer can affect anyone and is recognized as a chronic disease characterized by abnormal cell multiplication in the body [ 1 ]. While cancer is prevalent worldwide, approximately 70% of cancer-related deaths occur in low- to middle-income nations [ 1 ]. Disparities in cancer outcomes are primarily attributed to variations in the accessibility of comprehensive diagnosis and treatment among countries [ 1 , 2 ]. Cancer treatment comes in various forms; however, chemotherapy is the most widely used approach [ 3 ]. Patients undergoing chemotherapy experience both disease-related and treatment-related adverse effects, significantly impacting their quality of life [ 4 ]. Despite these challenges, many cancer patients adhere to treatment in the hope of survival [ 5 ]. However, some studies have shown that concerns about treatment efficacy may hinder treatment adherence [ 6 ]. Adherence is defined as “the extent to which a person’s behaviour aligns with the recommendations of healthcare providers“ [ 7 ]. Additionally, treatment adherence is influenced by the information provided by healthcare professionals following a cancer diagnosis [ 8 ]. Patient experiences suggest that the decision to adhere to treatment is often influenced by personal factors, with family support playing a crucial role [ 8 ]. Furthermore, providing adequate information about chemotherapy, including its benefits and consequences, can help individuals living with cancer gain a better understanding of the advantages associated with adhering to chemotherapy treatment [ 9 ].
Recognizing the importance of adhering to chemotherapy treatment and understanding the impact of individual experiences of chemotherapy adherence would aid in identifying determinants of adherence and non-adherence that are modifiable through effective interventions [ 10 ]. Recently, systematic reviews have focused on experiences and adherence in breast cancer [ 11 ], self-management of chemotherapy in cancer patients [ 12 ], and the influence of medication side effects on adherence [ 13 ]. However, these reviews were narrow in scope, and to date, no review has integrated the findings of qualitative studies designed to explore both positive and negative experiences regarding chemotherapy treatment adherence. This review aims to synthesize the qualitative literature on chemotherapy adherence within the context of patients’ experiences.
This review was conducted in accordance with the Joanna Briggs Institute [ 14 ] guidelines for systemic review involving meta-aggregation. This review was registered in PROSPERO (CRD42021270459).
Search methods
The searches for peer reviewed publications in English from January 2006-September 2023 were conducted by using keywords, medical subject headings (MeSH) terms and Boolean operators ‘AND’ and ‘OR’, which are presented in the table in Appendix 1 . The searches were performed in a systematic manner in core databases such including Embase, Medline, PsycINFO, CINAHL, Web of Science, Cochrane Library, Scopus and the Joanna Briggs Institute (JBI). The search strategy was developed from keywords and medical subject headings (MeSH) terms. Librarian’s support and advice were sought in forming of the search strategies.
Study selection and inclusion criteria
The systematic search was conducted on each database and all articles were exported to Endnote and duplicates records were removed. Then, title and abstract of the full text was screened by two independent reviewers against the inclusion criteria. For this review, populations were patients aged 18 and over with cancer, the phenomenon of interest was experiences on chemotherapy adherence and context was considered as hospitals, communities, rehabilitation centres, outpatient clinics, and residential aged care. All peer-reviewed qualitative study design were also considered for inclusion. Studies included in this review were classified as primary research, published in English since 2006, some intervention implemented to improve adherence to treatment. This review excluded any studies that related to with cancer and mental health condition, animal studies and grey literature.
Quality appraisal and data extraction
The JBI Qualitative Assessment and Review Instrument for qualitative studies was used to assess the methodological quality of the included studies, which was conducted by the primary and second reviewers independently. There was no disagreement between the reviews. The qualitative data on objectives, study population, context, study methods, and the phenomena of interest and findings form the included studies were extracted.
Data synthesis
The meta-aggregation approach was used to combine the results with similar meaning. The primary and secondary reviewers created categories based on the meanings and concept. These categories were supported by direct quotations from participants. The findings were assess based on three levels of evidence, including unequivocal, credible, and unsupported [ 15 , 16 ]. Findings with no quotation were not considered for synthesis in this review. The categories and findings were also discussed by the third and fourth reviewers until a consensus was reached. The review was approved by the Edith Cowan University Human Research Ethics Committee (2021–02896).
Study inclusion
A total of 4145 records were identified through a systematic search. Duplicates ( n = 647) were excluded. Two independent reviewers conducted screening process. The remaining articles ( n = 3498) were examined for title and abstract screening. Then, the full text screening conducted, yielded 13 articles to be included in the final synthesis see Appendix 2 .
Methodological quality of included studies
All included qualitative studies scored between 7 and 9, which is displayed in Appendix 3 . The congruity between the research methodology and the research question or objectives, followed by applying appropriate data collection and data analysis were observed in all included studies. Only one study [ 17 ] indicated the researcher’s statement regarding cultural or theoretical perspectives. Three studies [ 18 , 19 , 20 ] identified the influence of the researcher on the research and vice-versa.
Characteristics of included studies
Most of studies conducted semi-structured and in-depth interviews, one study used narrative stories [ 19 ], one study used focus group discussion [ 21 ], and one study combined focus group and interview [ 22 ] to collect data. All studies conducted outpatient’s clinic, community, or hospital settings [ 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. The study characteristics presented in Appendix 4 .
Review findings
Eighteen findings were extracted and synthesised into five categories: positive outlook, support, side effects, concern about efficacy and unmet information needs.
Positive outlook
Five studies discussed the link between positivity and hope and chemotherapy adherence [ 19 , 20 , 23 , 27 , 28 ]. Five studies commented that feeling positive and avoid the negativity and worry could encourage people to adhere in their mindset chemotherapy: “ I think the main thing for me was just keeping a positive attitude and not worrying, not letting myself worry about it ” [ 20 ]. Participants also considered the positive thoughts as a coping mechanism, that would help them to adhere and complete chemotherapy: “ I’m just real positive on how everything is going. I’m confident in the chemo, and I’m hoping to get out of her soon ” [ 23 ]. Viewing chemotherapy as part of their treatment regimen and having awareness of negative consequences of non-adherence to chemotherapy encouraged them to adhere chemotherapy: “ If I do not take medicine, I do not think I will be able to live ” [ 28 ]. Adhering chemotherapy was described as a survivor tool which helped people to control cancer-related symptoms: “ it is what is going to restore me. If it wasn’t this treatment, maybe I wasn’t here talking to you. So, I have to focus in what he is going to give me, life !” [ 27 ]. Similarly, people accepted the medical facts and prevent their life from worsening; “ without the treatment, it goes the wrong way. It is hard, but I have accepted it from the beginning, yes. This is how it is. I cannot do anything about it. Just have to accept it ” [ 19 ].
Finding from six studies contributed to this category [ 20 , 21 , 23 , 24 , 25 , 29 ]. Providing support from families and friends most important to the people. Receiving support from family members enhanced a sense responsibility towards their families, as they believed to survive for their family even if suffered: “ yes, I just thought that if something comes back again and I say no, then I have to look my family and friends in the eye and say I could have prevented it, perhaps. Now, if something comes back again, I can say I did everything I could. Cancer is bad enough without someone saying: It’s your own fault!!” [ 29 ]. Also, emotional support from family was described as important in helping and meeting their needs, and through facilitation helped people to adhere chemotherapy: “ people who genuinely mean the support that they’re giving […] just the pure joy on my daughter’s face for helping me. she was there day and night for me if I needed it, and that I think is the main thing not to have someone begrudgingly looking after you ” [ 20 ]. Another study discussed the role family, friends and social media as the best source of support during their treatment to adhere and continue “ I have tons of friends on Facebook, believe it or not, and it’s amazing how many people are supportive in that way, you know, just sending get-well wishes. I can’t imagine going through this like 10 years ago whenever stuff like that wasn’t around ” [ 23 ]. Receiving support from social workers was particularly helpful during chemotherapy in encouraging adherence to the chemotherapy: “ the social worker told me that love is courage. That was a huge encouragement, and I began to encourage myself ” [ 25 ].
Side effects
Findings from five studies informed this category [ 17 , 21 , 22 , 25 , 26 ]. Physical side effects were described by some as the most unpleasure experience: “ the side effects were very uncomfortable. I felt pain, fatigue, nausea, and dizziness that limited my daily activities. Sometimes, I was thinking about not keeping to my chemotherapy schedule due to those side effect ” [ 17 ]. The impact of side effects affected peoples’ ability to maintain their independence and self-care: “ I couldn’t walk because I didn’t have the energy, but I wouldn’t have dared to go out because the diarrhoea was so bad. Sometimes I couldn’t even get to the toilet; that’s very embarrassing because you feel like you’re a baby ” [ 26 ]. Some perceived that this resulted in being unable to perform independently: “ I was incredibly weak and then you still have to do things and you can’t manage it ” [ 22 ]. These side effect also decreased their quality of life “ I felt nauseated whenever I smelled food. I simply had no appetite when food was placed in front of me. I lost my sense of taste. Food had no taste anymore ” [ 25 ]. Although, the side effects impacted on patients´ leisure and free-time activities, they continued to undertake treatment: “ I had to give up doing the things I liked the most, such as going for walks or going to the beach. Routines, daily life in general were affected ” [ 21 ].
Concern about efficacy
Findings form four studies informed this category [ 17 , 18 , 24 , 28 ]. Although being concerned about the efficacy of the chemotherapy and whether or not chemotherapy treatment would be successful, one participant who undertook treatment described: “the efficacy is not so great. It is said to expect about 10% improvement, but I assume that it declines over time ” [ 28 ]. People were worried that such treatment could not cure their cancer and that their body suffered more due to the disease: “ I was really worried about my treatment effectiveness, and I will die shortly ” [ 17 ]. There were doubts expressed about remaining the cancer in the body after chemotherapy: “ there’s always sort of hidden worries in there that whilst they’re not actually taking the tumour away, then you’re wondering whether it’s getting bigger or what’s happening to it, whether it’s spreading or whatever, you know ” [ 24 ]. Uncertainty around the outcome of such treatment, or whether recovering from cancer or not was described as: “it makes you feel confused. You don’t know whether you are going to get better or else whether the illness is going to drag along further” [ 18 ].
Unmet information needs
Five studies contributed to this category [ 17 , 21 , 22 , 23 , 26 ]. The need for adequate information to assimilate information and provide more clarity when discussing complex information were described. Providing information from clinicians was described as minimal: “they explain everything to you and show you the statistics, then you’re supposed to take it all on-board. You could probably go a little bit slower with the different kinds of chemo and grappling with these statistics” [ 26 ]. People also used the internet search to gain information about their cancer or treatments, “I’ve done it (consult google), but I stopped right away because there’s so much information and you don’t know whether it’s true or not ” [ 21 ]. The need to receive from their clinicians to obtain clearer information was described as” I look a lot of stuff up online because it is not explained to me by the team here at the hospital ” [ 23 ]. Feeling overwhelmed with the volume of information could inhibit people to gain a better understanding of chemotherapy treatment and its relevant information: “ you don’t absorb everything that’s being said and an awful lot of information is given to you ” [ 22 ]. People stated that the need to know more information about their cancer, as they were never dared to ask from their clinicians: “ I am a low educated person and come from a rural area; I just follow the doctor’s advice for my health, and I do not dare to ask anything” [ 17 ].
The purpose of this review was to explore patient’s experiences about the chemotherapy adherence. After finalizing the searches, thirteen papers were included in this review that met the inclusion criteria.
The findings of the present review suggest that social support is a crucial element in people’s positive experiences of adhering to chemotherapy. Such support can lead to positive outcomes by providing consistent and timely assistance from family members or healthcare professionals, who play vital roles in maintaining chemotherapy adherence [ 30 ]. Consistent with our study, previous research has highlighted the significant role of family members in offering emotional and physical support, which helps individuals cope better with chemotherapy treatment [ 31 , 32 ]. However, while receiving support from family members reinforces individuals’ sense of responsibility in managing their treatment and their family, it also instils a desire to survive cancer and undergo chemotherapy. One study found that assuming self-responsibility empowers patients undergoing chemotherapy, as they feel a sense of control over their therapy and are less dependent on family members or healthcare professionals [ 33 ]. A qualitative systematic review reported that support from family members enables patients to become more proactive and effective in adhering to their treatment plan [ 34 ]. This review highlights the importance of maintaining a positive outlook and rational beliefs as essential components of chemotherapy adherence. Positive thinking helps individuals recognize their role in chemotherapy treatment and cope more effectively with their illness by accepting it as part of their treatment regimen and viewing it as a tool for survival. This finding is supported by previous studies indicating that positivity and positive affirmations play critical roles in helping individuals adapt to their reality and construct attitudes conducive to chemotherapy adherence [ 35 , 36 ]. Similarly, maintaining a positive mindset can foster more favourable thoughts regarding chemotherapy adherence, ultimately enhancing adherence and overall well-being [ 37 ].
This review identified side effects as a significant negative aspect of the chemotherapy experience, with individuals expressing concerns about how these side effects affected their ability to perform personal self-care tasks and maintain independent living in their daily lives. Previous studies have shown that participants with a history of chemotherapy drug side effects were less likely to adhere to their treatment regimen due to worsening symptoms, which increased the burden of medication side effects [ 38 , 39 ]. For instance, cancer patients who experienced minimal side effects from chemotherapy were at least 3.5 times more likely to adhere to their treatment plan compared to those who experienced side effects [ 40 ]. Despite experiencing side effects, patients were generally willing to accept and adhere to their treatment program, although one study in this review indicated that side effects made some patients unable to maintain treatment adherence. Side effects also decreased quality of life and imposed restrictions on lifestyle, as seen in another study where adverse effects limited individuals in fulfilling daily commitments and returning to normal levels of functioning [ 41 ]. Additionally, unmet needs regarding information on patients’ needs and expectations were common. Healthcare professionals were considered the most important source of information, followed by consultation with the internet. Providing information from healthcare professionals, particularly nurses, can support patients effectively and reinforce treatment adherence [ 42 , 43 ]. Chemotherapy patients often preferred to base their decisions on the recommendations of their care providers and required adequate information retention. Related studies have highlighted that unmet needs among cancer patients are known factors associated with chemotherapy adherence, emphasizing the importance of providing precise information and delivering it by healthcare professionals to improve adherence [ 44 , 45 ]. Doubts about the efficacy of chemotherapy treatment, as the disease may remain latent, were considered negative experiences. Despite these doubts, patients continued their treatment, echoing findings from a study where doubts regarding efficacy were identified as a main concern for chemotherapy adherence. Further research is needed to understand how doubts about treatment efficacy can still encourage patients to adhere to chemotherapy treatment.
Strengths and limitation
The strength of this review lies in its comprehensive search strategy across databases to select appropriate articles. Additionally, the use of JBI guidelines provided a comprehensive and rigorous methodological approach in conducting this review. However, the exclusion of non-English studies, quantitative studies, and studies involving adolescents and children may limit the generalizability of the findings. Furthermore, this review focuses solely on chemotherapy treatment and does not encompass other types of cancer treatment.
Conclusion and practical implications
Based on the discussion of the findings, it is evident that maintaining a positive mentality and receiving social support can enhance chemotherapy adherence. Conversely, experiencing treatment side effects, concerns about efficacy, and unmet information needs may lead to lower adherence. These findings present an opportunity for healthcare professionals, particularly nurses, to develop standardized approaches aimed at facilitating chemotherapy treatment adherence, with a focus on providing comprehensive information. By assessing patients’ needs, healthcare professionals can tailor approaches to promote chemotherapy adherence and improve the survival rates of people living with cancer. Raising awareness and providing education about cancer and chemotherapy treatment can enhance patients’ understanding of the disease and its treatment options. Utilizing videos and reading materials in outpatient clinics and pharmacy settings can broaden the reach of educational efforts. Policy makers and healthcare providers can collaborate to develop sustainable patient education models to optimize patient outcomes in the context of cancer care. A deeper understanding of individual processes related to chemotherapy adherence is necessary to plan the implementation of interventions effectively. Further research examining the experiences of both adherent and non-adherent patients is essential to gain a comprehensive understanding of this topic.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. on our submission system as well.
World Health Organization. Cancer 2021 [ https://www.who.int/news-room/fact-sheets/detail/cancer .
Klapheke A, Yap SA, Pan K, Cress RDDHSDCA. Sociodemographic disparities in chemotherapy treatment and impact on survival among patients with metastatic bladder cancer. Urologic Oncology: Seminars Original Investigations. 2018;36(6):19–308.
Article Google Scholar
Moth EB, Kiely BE, Naganathan V, Martin A, Blinman P. How do oncologists make decisions about chemotherapy for their older patients with cancer? A survey of Australian oncologists. Support Care Cancer. 2018;26(2):451–60.
Article CAS PubMed Google Scholar
Khamboon T, Pakanta I. Intervention for symptom cluster management of fatigue, loss of appetite, and anxiety among patients with lung cancer undergoing chemotherapy. Asia-Pacific J Oncol Nurs. 2021;8(3):267–75.
Garcia ACM, Camargos Junior JB, Sarto KK, Silva Marcelo CAd, Paiva EMC, Nogueira DA, Mills J. Quality of life, self-compassion and mindfulness in cancer patients undergoing chemotherapy: a cross-sectional study. Eur J Oncol Nurs. 2021;51:N.PAG-N.PAG.
Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about Medicines prescribed for long-term conditions: a Meta-Analytic Review of the necessity-concerns Framework. PLoS ONE. 2013;8(12):e80633.
Article PubMed PubMed Central Google Scholar
WHO. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organisation; 2003.
Google Scholar
Warby A, Dhillon HM, Kao S, Vardy JL. A survey of patient and caregiver experience with malignant pleural mesothelioma. Support Care Cancer. 2019;27(12):4675–86.
Article PubMed Google Scholar
Arunachalam SS, Shetty AP, Panniyadi N, Meena C, Kumari J, Rani B, et al. Study on knowledge of chemotherapy’s adverse effects and their self-care ability to manage - the cancer survivors impact. Clin Epidemiol Global Health. 2021;11:100765.
Article CAS Google Scholar
Nizet P, Touchefeu Y, Pecout S, Cauchin E, Beaudouin E, Mayol S, et al. Exploring the factors influencing adherence to oral anticancer drugs in patients with digestive cancer: a qualitative study. Support Care Cancer. 2022;30(3):2591–604.
Clancy C, Lynch J, Oconnor P, Dowling M. Breast cancer patients’ experiences of adherence and persistence to oral endocrine therapy: a qualitative evidence synthesis. Eur J Oncol Nurs. 2020;44.
Magalhães B, Fernandes C, Lima L, Martinez-Galiano JM, Santos C. Cancer patients’ experiences on self-management of chemotherapy treatment-related symptoms: A systematic review and thematic synthesis. Eur J Oncol Nurs. 2020;49.
Peddie N, Agnew S, Crawford M, Dixon D, MacPherson I, Fleming L. The impact of medication side effects on adherence and persistence to hormone therapy in breast cancer survivors: a qualitative systematic review and thematic synthesis. Breast. 2021;58:147–59.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ: Br Med J. 2009;339(7716):332–6.
Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews. Checklist for qualitative research. 2017.
Zachary M, Kylie P, Craig L, Edoardo A, Alan P. Establishing confidence in the output of qualitative research synthesis: the ConQual approach. BMC Med Res Methodol [Internet]. 2014;14(1):108.
Iskandarsyah A, de Klerk C, Suardi DR, Soemitro MP, Sadarjoen SS, Passchier J. Psychosocial and cultural reasons for Delay in seeking help and Nonadherence to treatment in Indonesian women with breast Cancer: a qualitative study. Health Psychol. 2014;33(3):214–21.
Chircop D, Scerri J. The lived experience of patients with Non-hodgkin’s lymphoma undergoing chemotherapy. Eur J Oncol Nurs. 2018;35:117–21.
Kvåle K, Synnes O. Living with life-prolonging chemotherapy—control and meaning‐making in the tension between life and death. Eur J Cancer Care. 2018;27(1):1.
Staneva AA, Beesley VL, Niranjan N, Gibson AF, Rowlands I, Webb PM. I wasn’t gonna let it stop me: exploring women’s experiences of getting through chemotherapy for ovarian cancer. Cancer Nurs. 2019;42(2):E31–8.
Talens A, Guilabert M, Lumbreras B, Aznar MT, López-Pintor E. Medication Experience and Adherence to Oral Chemotherapy: A Qualitative Study of Patients’ and Health Professionals’ Perspectives. Int J Environ Res Public Health. 2021;18(8).
Dumas L, Lidington E, Appadu L, Jupp P, Husson O, Banerjee S, et al. Exploring older women’s attitudes to and experience of treatment for advanced ovarian cancer: a qualitative phenomenological study. Cancers. 2021;13(6):1207.
Albrecht TA, Keim-Malpass J, Boyiadzis M, Rosenzweig M. Psychosocial experiences of young adults diagnosed with acute leukemia during hospitalization for induction chemotherapy treatment. J Hospice Palliat Nurs. 2019;21(2):167–73.
Beaver K, Williamson S, Briggs J. Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur J Oncol Nurs. 2016;20:77–86.
Chou J-F, Lu YY. Intraperitoneal chemotherapy: the lived experiences of Taiwanese patients with ovarian cancer. Clin J Oncol Nurs. 2019;23(6):E100–6.
Farrell C, Heaven C. Understanding the impact of chemotherapy on dignity for older people and their partners. Eur J Oncol Nurs. 2018;36:82–8.
Wakiuchi J, Silva Marcon S, de Oliveira DC, Aparecida Sales C. Rebuilding subjectivity from the experience of cancer and its treatment. Revista Brasileira De Enfermagem. 2019;72(1):125–33.
Yagasaki K, Komatsu H, Takahashi T. Inner conflict in patients receiving oral anticancer agents: a qualitative study. BMJ Open [Internet]. 2015; 5(4).
Gassmann C, Kolbe N, Brenner A. Experiences and coping strategies of oncology patients undergoing oral chemotherapy: first steps of a grounded theory study. Eur J Oncol Nurs. 2016;23:106–14.
Tang GX, Yan PP, Yan CL, Fu B, Zhu SJ, Zhou LQ, et al. Determinants of suicidal ideation in gynecological cancer patients. Psycho-oncology. 2016;25(1):97–103.
Oven Ustaalioglu B, Acar E, Caliskan M. The predictive factors for perceived social support among cancer patients and caregiver burden of their family caregivers in Turkish population. Int J Psychiatry Clin Pract. 2018;22(1):63–9.
Levkovich I, Cohen M, Karkabi K. The experience of fatigue in breast Cancer patients 1–12 Month Post-chemotherapy: a qualitative study. Behav Med. 2019;45(1):7–18.
Simchowitz B, Shiman L, Spencer J, Brouillard D, Gross A, Connor M, Weingart SN. Perceptions and experiences of patients receiving oral chemotherapy. Clin J Oncol Nurs. 2010;14(4):447–53.
Rashidi A, Kaistha P, Whitehead L, Robinson S. Factors that influence adherence to treatment plans amongst people living with cardiovascular disease: a review of published qualitative research studies. Int J Nurs Stud 2020;110(103727).
Aydogan U, Doganer YC, Komurcu S, Ozturk B, Ozet A, Saglam K. Coping attitudes of cancer patients and their caregivers and quality of life of caregivers. Indian J Palliat Care. 2016;22(2):150–6.
Langford DJ, Morgan S, Cooper B, Paul S, Kober K, Wright F, et al. Association of personality profiles with coping and adjustment to cancer among patients undergoing chemotherapy. Psycho-oncology. 2020;29(6):1060–7.
Jamie MJ, Pensak NA, Sporn NJ, MacDonald JJ, Lennes IT, Safren SA et al. Treatment satisfaction and adherence to oral chemotherapy in patients with Cancer. J Oncol Pract. 2017;13(2).
Tsai Y-F, Huang W-C, Cho S-F, Hsiao H-H, Liu Y-C, Lin S-F, et al. Side effects and medication adherence of tyrosine kinase inhibitors for patients with chronic myeloid leukemia in Taiwan. Medicine. 2018;97(26):415.
D S, M P, G R, S H. Importance of medication adherence and factors affecting it. IP Int J Compr Adv Pharmacolog. 2020;3(2):69–77.
Bekalu YE, Wudu MA, Gashu AW. Adherence to Chemotherapy and Associated factors among patients with Cancer in Amhara Region, Northeastern Ethiopia, 2022. A cross-sectional study. Cancer Control. 2023;30.
Hsu H-C, Liou W-S, Chiang A-J, Tsai S-Y, Jeang S-R, Wu S-L, et al. Longitudinal perceptions of the side effects of chemotherapy in patients with gynecological cancer. Support Care Cancer. 2017;25(11):3457–64.
Gow K, Rashidi A, Whithead L. Factors influencing medication adherence among adults living with diabetes and comorbidities: a qualitative systematic review. Curr Diab Rep. 2023:1–7.
Rashidi A, Whitehead L, Kaistha P. Nurses’ perceptions of factors influencing treatment engagement among patients with cardiovascular diseases: a systematic review. BMC Nurs. 2021;20(1):251.
Zebrack BJ, Block R, Hayes-Lattin B, Embry L, Aguilar C, Meeske KA, et al. Psychosocial service use and unmet need among recently diagnosed adolescent and young adult cancer patients. Cancer. 2013;119(1):201–14.
Timmers L, Boons CCLM, van den Verbrugghe M, Van Hecke A, Hugtenburg JG. Supporting adherence to oral anticancer agents: clinical practice and clues to improve care provided by physicians, nurse practitioners, nurses and pharmacists. BMC Cancer. 2017;17(1).
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
School of Nursing and Midwifery, Edith Cowan University, 270 Joondalup Drive, Joondalup, Perth, WA, 6027, Australia
Amineh Rashidi, Susma Thapa, Wasana Sandamali Kahawaththa Palliya Guruge & Shubhpreet Kaur
You can also search for this author in PubMed Google Scholar
Contributions
First author (AR) and second author (ST) conceived the review and the second author oversight for all stages of the review provided by the second author. All authors (AR), (ST), (WG) and (SK) undertook the literature search. Data extraction, screening the included papers and quality appraisal were undertaken by all authors (AR), (ST), (WG) and (SK). First and second authors (AR) and (ST) analysed the data and wrote the first draft of the manuscript and revised the manuscript and all authors (AR), (ST), (WG) and (SK) approved the final version of the manuscript.
Corresponding author
Correspondence to Amineh Rashidi .
Ethics declarations
Ethics approval and consent to participate.
The review was approved by the Edith Cowan University Human Research Ethics Committee (2021–02896). A proposal for the systematic review was assessed by the Edith Cowan University Human Research Ethics Committee and deemed not appropriate for full ethical review. However, a Data Management Plan (2021-02896-RASHIDI) was approved and monitored as part of this procedure. Raw data was extracted from the published manuscripts and authors could not identify individual participants during or after this process.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, supplementary material 5, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rashidi, A., Thapa, S., Kahawaththa Palliya Guruge, W. et al. Patient experiences: a qualitative systematic review of chemotherapy adherence. BMC Cancer 24 , 658 (2024). https://doi.org/10.1186/s12885-024-12353-z
Download citation
Received : 17 November 2023
Accepted : 07 May 2024
Published : 30 May 2024
DOI : https://doi.org/10.1186/s12885-024-12353-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chemotherapy treatment
- Medication adherence
- Qualitative research
- Patients experiences
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
RESEARCH PROPOSAL Written in line with PRISMA-P 2015 statement Title Thoracic dysfunction in whiplash associated disorders: a systematic review and meta-synthesis Registration To be registered in PROSPERO. A protocol following method guidelines Cochrane handbook (Higgins ... sample characteristics, sample size, outcomes, and timescales to ...
Research by Vincent R et. al assessed the uptake of the HOME COS in treatment trials, the results showed a lack of universal implementation(5), it follows that the primary outcome of the proposed systematic review is to assess the uptake of the COS domains and instruments in systematic reviews of AD intervention. The four core outcome domains
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer. Example: Systematic review. In 2008, Dr. Robert Boyle and his colleagues published a systematic review in ...
Background. A systematic review, as its name suggests, is a systematic way of collecting, evaluating, integrating, and presenting findings from several studies on a specific question or topic.[] A systematic review is a research that, by identifying and combining evidence, is tailored to and answers the research question, based on an assessment of all relevant studies.[2,3] To identify assess ...
A systematic review is a type of review that uses repeatable methods to find, select, and synthesise all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer. Example: Systematic review. In 2008, Dr Robert Boyle and his colleagues published a systematic review in ...
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology. To minimise bias, systematic reviews utilise a ...
A systematic review aims to bring evidence together to answer a pre-defined research question. This involves the identification of all primary research relevant to the defined review question, the critical appraisal of this research, and the synthesis of the findings.13 Systematic reviews may combine data from different.
Systematic reviews are characterized by a methodical and replicable methodology and presentation. They involve a comprehensive search to locate all relevant published and unpublished work on a subject; a systematic integration of search results; and a critique of the extent, nature, and quality of evidence in relation to a particular research question.
guiding reviewers on conducting a systematic review, using examples from published systematic reviews and different types of studies. To illustrate the approach, we use example research questions and elaborate the stepwise proposed methodology for conducting a systematic review. Some of the potential research questions are: 1.
This article is organized as follows: The next section presents the methodology adopted by this research, followed by a section that discusses the typology of literature reviews and provides empirical examples; the subsequent section summarizes the process of literature review; and the last section concludes the paper with suggestions on how to improve the quality and rigor of literature ...
Thus, the following examples can illustrate the appropriate use of the PRISMA Statement in research reporting: "The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement.". "This scoping review was reported according to the Preferred ...
for Systematic reviews and meta Analyses).3These guidelines facilitate the reporting of appropriate information (Figure 1). Conducting and Reviewing the Search Once a justified study question and detailed study protocol are in place, the systematic review process can proceed. First, accounts must be created with each database (medline,
Your first step is to devise a focused, clear and answerable question which your review will address.. This may be revised as you work through the next step which is to develop a protocol which outlines the study methodology including:. background; research question and aims; criteria for inclusion and exclusion - these specify attributes which selected studies must have, or attributes which ...
The paper highlights important considerations during the protocol development process, and uses a previously developed review question as a working example. Implications for research: This paper will assist novice researchers in developing a qualitative systematic review protocol. By providing a worked example of a protocol, the paper ...
Getting started with proposal of review. The proposal stage is the most important step of a review project as it determines the feasibility of the review and its rationale. The steps are: 1. Determining review question and review type. Right Review: free tool to assist in selecting best review type for a given question.
A systematic review is a means of identifying, evaluating and interpreting all available research relevant to a particular research question, or topic area, or phenomenon of interest. Individual studies contributing to a systematic review are called primary studies; a systematic review is a form of secondary study.
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management".
Research proposal aims. Relevance. Show your reader why your project is interesting, original, and important. Context. Demonstrate your comfort and familiarity with your field. Show that you understand the current state of research on your topic. Approach. Make a case for your methodology. Demonstrate that you have carefully thought about the ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
extraction tool has been developed specifically for quantitative research data extraction based on the work of the Cochrane Collaboration and the Centre for Reviews and Dissemination (Appendix 3). Qualitative research data and expert opinion will be extracted using the data extraction tools developed for the Systematic Review Protocol Example 175
For example, community efforts might be more impactful when they are frequently held to sensitize the population on the actual effects of tobacco use on health and the economic status of the family. ... Research Proposal for a Systematic Review of the Effectiveness of Community Interventions in Decreasing Smoking Prevalence among Adults in the ...
Studies included in this review were classified as primary research, published in English since 2006, some intervention implemented to improve adherence to treatment. ... (2021-02896). A proposal for the systematic review was assessed by the Edith Cowan University Human Research Ethics Committee and deemed not appropriate for full ethical ...
Work on the "double empathy problem" (DEP) is rapidly growing in academic and applied settings (e.g., clinical practice). It is most popular in research on conditions, like autism, which are characterized by social cognitive difficulties. Drawing from this literature, we propose that, while research on the DEP has the potential to improve understanding of both typical and atypical social ...
Background: The ubiquity of short videos has demonstrated vast potential for health communication. An expansion of research has examined the persuasive effect of health communication in short videos, yet a synthesis of the research is lacking. Objective: This paper aims to provide an overview of the literature by examining the persuasive effect of health communication in short videos, offering ...