Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here

NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
NECO Chemistry Questions and Answers 2023. I will be showing you past Chemistry objectives and theory repeated questions for free in this post. You will also understand how NECO Chemistry questions are set and how to answer them.
The National Examinations Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and December/January respectively.
Table of Contents
NECO Chemistry Objectives and Essay Answers 2023 (Expo)
The 2023 NECO Chemistry expo will be posted here today 24th July during the NECO Chemistry examination. Keep checking and reloading this page for the answers.
NECO 2023 Chemistry Answers Loading.
OBJ Answers:
1-10: DEADADECAD
11-20: BAEDDBDBAE
21-30: CCDCABDDCD
31-40: EBEECEBCEE
41-50: BCCECDDADD
51-60: DABBDEAECA
————————————————————————————————————-
NECO Chemistry Questions and Answers For Practice
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
See: NECO Timetable
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
Recommended: How to check NECO Result
NECO Chemistry Questions And Answers 2023 (Paper 2)
Don’t worry about these NECO Chemistry Questions And Answers 2023. All you need to do is to keep on refreshing this page for the 2023 NECO Chemistry Questions And Answers for this year. It will be posted here in few minutes.
Tips on How to Pass 2023 NECO Chemistry Examinations
The following guidelines will help you pass the 2023 NECO Chemistry examination with flying colours.
Have a Target and Work Towards Actualizing it
You have decided to pass NECO Chemistry 2023 and I am sure of that. Now, the next thing you should do is set targets.
You have told yourself, “I will score A in NECO Chemistry 2023”, that’s not all. You need to plan on how to make it happen. Create a timetable and master plan to achieve your goals.
Get the Recommended Textbook on Chemistry for 2023 NECO Examination
Normally, NECO recommends books for the examination. But apart from NECO Literature in English where certain novels are compulsory, you are free to use any good Chemistry textbook to prepare for NECO 2023 exam.
Some textbooks are more difficult to understand. If you have any topic you are finding difficult to understand, then get a textbook that will simplify the topics and make life better for you.
Do not Skip Chemistry Examples and Exercise you Will Come Across While Reading:
Many candidates are fond of skipping exercises and even examples while studying textbooks. In fact, we like notebooks so much that we could ask, “can I read my notebook and pass NECO Chemistry 2023?” Don’t be scared of attempting exercises in Biology. Face the challenges.
If you have any questions about the NECO Chemistry Questions and Answers 2023 , kindly drop your question in the comment box.
Last Updated on July 25, 2023 by Admin
Related posts:

122 thoughts on “NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
Please when will the answer come up
I just need questions for the two
I am really greatfull for the coperation you showed to us most especially me if not for you guys thisneco would have been trouble so sincear thank you
WHERE IS THE ANSWER FOR THE OBJ QUESTIONS THAT IS UP THERE
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

NECO Chemistry Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Get free Verified NECO Chemistry Questions and Answers 2023. NECO June/July Free Chemistry EXPO answers. National Examination Council Chemistry Theory and Objective Answers for you to have good NECO result. You will also understand how NECO Chemistry questions are set and how to answer them. The National Examination Council is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
Click Here Now To Chat Us on WhatsApp For Subscription Details

Click Here To See All NECO Chemistry Question Paper 2023
Please Note that the NECO 2023 Chemistry Questions and Answers and any other NECO expo is provided by us for free. We understand that a lot of website charge of collect money from student to provide NECO expo Chemistry Answers to them. NECO questions and answers are provided for free. We will do same during Other Exam like NECO GCE.
NECO 2023 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once the verification process complete, we will go ahead to upload it here.
Be at alert! Keep refreshing this page for NECO Chemistry Theory Questions and Answers
2023 Chemistry Essay Questions Loading 93.8%
Also Read: How To Check Your JAMB Result
Click Here To Join The 2023 NECO Direct Link answers Group
NECO Chemistry Questions and Answers 2023 OBJECTIVES (OBJ) ANSWERS Loading
2023 VERIFIED Chemistry OBJ:…Loading
CHEMISTRY-OBJ
Please Click Here To See The NECO Chemistry OBJ Answers
CHEMISTRY-Obj! 1-10 DEADADECAD 11-20 BAEDDBDBAE 21-30 CCDCABDDCD 31-40 EBEECEBCEE 41-50 BCCECDDADD 51-60 DABBDEAECA Solved By beafans.com Completed!!
NECO Chemistry Questions and Answers 2023 loading…
Please do not panic and fall into the right hand. 2023 Chemistry Answers will be posted for free here once ready.
We are right now getting things ready for you. The NECO 2023 Chemistry theory questions and answers will be posted any moment from now. All you need do is to keep refreshing this page until you see the answers.
NECO Chemistry -ESSAY-ANSWERS-joberplanet.com
Answers Loading….
CHEMISTRY-ANSWERS
(1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides
(1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent
(1aiii) Soaps are made from natural products while detergents are made from synthetic products.
(1aiv) Detergents is for household cleaning and laundry purposes
(1bi) Number of neutrons = Mass number (A) – Atomic number (Z)
I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number)
Number of neutrons = 23 – 11 = 12
II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number)
Number of neutrons = 39 – 19 = 20
(1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol
Now, let’s calculate the mass of oxygen needed:
First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol
Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol
Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g
Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium.
(1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l)
From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂).
Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol
Now, let’s calculate the number of moles of CaCO₃ in 50g:
Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol
Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol.
Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium.
(1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation.
(I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃.
The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol
Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol
Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol.
(II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O.
The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O)
Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol)
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol
If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box.
We are happy you are here for the 2023 Chemistry answers. The complete solution will be made free in some minutes before the Chemistry examination.
NECO Chemistry questions 2023, NECO 2023 Chemistry questions and answers, Chemistry NECO 2023, NECO questions 2023/2023, Chemistry 2023,
Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C
Substituting the values to calculate the mass of silver deposited:
Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C
Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C
Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)
(2bi) Coming
(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol
(3aii) (i) Fermentation (ii) Ethylene hydration
(3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72
(3bi) 1s², 2s², 2p⁴
(3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless
(3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically
(3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O
(3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system.
(3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g)
(3ciii) Redox reaction.
(3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
(4aii) 15/345 = Solubility *25/1000
Solubility =1000*15/25*345=15000/8625
Solubility = 1.79mol/dm³
(4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆
(4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures
(4bii) (i) Noble gases (ii) Carbon (iv) oxide
(4biii) H₂SO₄ —-> 2H+ + SO₄²⁻
1 mole of H₂SO₄ = 2 mole of H⁺
0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant
No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions
(4biv) (i) Dative bonding (ii) Hydrogen bonding
(4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures.
(ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Now Loading…94.5%
We want to hear from you concerning the NECO Chemistry Questions and Answers 2023 , kindly use the comment section below to get to us.
Related Posts

LASUSTECH School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Caritas University, Enugu School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Bingham University, New Karu School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Emmanuel Alayande University of Education Oyo State School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Covenant University Ota School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Caleb University, Lagos School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
itsmyschoollibrary
Your lesson notes in a blink.
NECO ANSWERS , Past Questions & Answer
Protected: 2023 NECO: Chemistry Essay and Objectives Questions with Solutions

This content is password protected. To view it please enter your password below:
Discover more from itsmyschoollibrary
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

How to Pass NECO Chemistry: Objective and Essay Questions and Answers Explained (With Examples)

Chemistry is one of the core subjects in the Senior Secondary Certificate Examination (SSCE) administered by the National Examinations Council (NECO). It is a required subject for students who wish to study science-related courses at a higher institution. As a result, it is important to properly prepare for the NECO chemistry exam and score high marks.
In this article, we will provide you with some helpful information on how to prepare for the NECO chemistry exam, as well as some sample questions and answers for both the objective and essay sections. We will also give you some helpful tips on how to answer the questions correctly and avoid typical mistakes.
What is NECO Chemistry Exam?
The NECO chemistry exam is a test of your knowledge and comprehension of the fundamental ideas and principles of chemistry. It covers subjects like organic chemistry, physical chemistry, analytical chemistry, chemical reactions, the periodic table, and environmental chemistry. It also covers topics like atomic structure and chemical bonding.
The NECO chemistry exam is divided into two sections: Paper 1 consists of 60 multiple-choice questions that must be answered in one hour. Paper 2 contains two sections: Section A (Theory) and Section B (Practical). Section A contains six essay questions, four of which must be answered in two hours. Section B contains three practical questions, one of which you must answer in one hour.
The NECO chemistry exam has a total of 200 marks . Paper 1 carries 60 marks , Paper 2 Section A carries 80 marks , and Paper 2 Section B carries 60 marks . To pass the exam, you must score at least 50% on each paper.
Related Article: Lester B. Pearson Scholarship in Canada 2023-2024 [ Fully Funded]
How to Prepare for NECO Chemistry Exam?
You must do the following to properly prepare for the NECO chemistry exam:
- Thoroughly study the NECO chemistry syllabus and understand the objectives and outcomes of each topic.
- Review your class notes and textbooks on a regular basis to ensure you understand the key concepts, formulas, definitions, laws, and equations.
- Practice answering past questions and answers from previous years’ exams and mock tests. You can get them online or in bookstores.
- Revise your weak areas and clarify any uncertainties with your teachers or classmates.
- Attend revision classes or tutorials, if possible, and learn from your instructors’ feedback and corrections.
- Prepare your practical materials and equipment in advance, and become familiar with the procedures and safety rules.
- Before the exam, eat well, sleep well, and stay healthy. Avoid stressful situations and distractions that can affect your concentration and performance.
Related post: How to Apply for Student Loan in Nigeria
NECO Chemistry Objective Questions and Answers 2023
The objective section of the NECO chemistry exam tests your ability to recall facts, recognize concepts, apply principles, analyze data, and solve problems. For each question, you must select the correct option from four alternatives (A, B, C, or D).
Here are some sample objective questions and answers for the NECO chemistry exam 2023:
- Which of the following elements has the highest electronegativity?
A) Fluorine B) Chlorine C) Oxygen D) Nitrogen Answer: A) Fluorine
2. What is the name of the compound with the formula CH₃COOH?
A) Methanoic acid B) Ethanoic acid C) Propanoic acid D) Butanoic acid Answer: B) Ethanoic acid
3. What is the oxidation number of sulfur in SO₄²⁻?
A) +2 B) +4 C) +6 D) +8 Answer: C) +6
4. What type of reaction occurs when zinc reacts with hydrochloric acid?
A) Combination reaction B) Decomposition reaction C) Displacement reaction D) Neutralization reaction Answer: C) Displacement reaction
5. What is the empirical formula of benzene?
A) CH B) CH₂ C) C₂H₂ D) C₂H₄ Answer: B) CH₂
See Also: How to Borrow Money from Opay
Tips for Answering Objective Questions
To answer the objective questions correctly, you need to follow these tips:
- Carefully read the question and fully understand what it is asking.
- Remove any options that are obviously incorrect or irrelevant.
- Choose the best option that fits the question based on your knowledge of chemistry and logic.
- If you are not sure of the answer, make an educated guess based on clues from the question or the options.
- Don’t spend too much time on one question. If you get stuck, move on to the next one and come back later if you have time.
- On the answer sheet, write your answers clearly and neatly. Do not make any stray marks or erase any marks.
NECO Chemistry Essay Questions and Answers 2023
The NECO chemistry exam essay section tests your ability to explain concepts, describe processes, compare and contrast phenomena, evaluate arguments, and communicate effectively. For each question, you must write detailed and coherent answers.
Here are some sample essay questions and answers for the NECO chemistry exam 2023:
- (a) Define the following terms:
(i) Atomic number (ii) Mass number (iii) Isotopes (b) Write the electronic configuration of the following elements: (i) Sodium (ii) Magnesium (iii) Chlorine (c) Draw the dot-and-cross diagram of sodium chloride.
(a) (i) The atomic number is the number of protons in the nucleus of an element’s atom. It determines the element’s identity as well as its chemical properties.
(ii) Mass number is the sum of the number of protons and neutrons in the nucleus of an atom of an element. It determines the mass and stability of the atom.
(iii) Isotopes are atoms of the same element with different numbers of neutrons in their nuclei. They have the same atomic number but different mass numbers.
(b) (i) Sodium has an atomic number of 11 and a mass number of 23. Its electronic configuration is 2,8,1 or 1s²2s²2p⁶3s¹.
(ii) Magnesium has an atomic number of 12 and a mass number of 24. Its electronic configuration is 2,8,2 or 1s²2s²2p⁶3s².
(iii) Chlorine has an atomic number of 17 and a mass number of 35. Its electronic configuration is 2,8,7 or 1s²2s²2p⁶3s²3p⁵.
(c) Sodium chloride is formed when one electron is transferred from sodium to chlorine, resulting in the formation of sodium ion (Na+) and chloride ion (Cl).
2. (a) State two differences between metals and non-metals based on their physical properties. (b) Give two examples of metals and two examples of non-metals. (c) Explain why metals are good conductors of electricity.
Read Also: How to Apply as a Teacher in the UK from Nigeria
(a) The following are two physical property distinctions between metals and nonmetals:
- Nonmetals have low melting and boiling points, whereas metals have high melting and boiling points.
- Metals are malleable and ductile, whereas nonmetals are brittle and easily broken.
(b) Two examples of metals are iron and copper . Two examples of non-metals are oxygen and carbon .
(c) Metals are good conductors of electricity because their outermost shells contain free electrons that can easily move through the metal lattice when a potential difference is applied across it. These free electrons transfer electric charge from one end to the other, resulting in an electric current.
3. (a) Define the term acid-base titration. (b) List two types of indicators used in acid-base titrations and state their colour changes. (c) Write a balanced equation for the reaction between hydrochloric acid and sodium hydroxide.
(a) Acid-base titration is a laboratory technique for determining the concentration of an acid or a base by neutralizing it with a known-concentration standard solution of the opposite type.
(b) Two types of indicators used in acid-base titrations are:
- Methyl orange: In acidic solutions, it turns red; in alkaline solutions, it turns yellow.
- Phenolphthalein: In acidic solutions, it is colorless; in alkaline solutions, it is pink.
(c) The balanced equation for the reaction between hydrochloric acid and sodium hydroxide is:
HCl + NaOH → NaCl + H₂O
Read Also: How to Apply for an International Passport in Nigeria 2023 and How Much it Costs
In this article, we have provided you with some useful information on how to prepare for the NECO chemistry exam in 2023, as well as some sample questions and answers for both the objective and essay sections. We hope that this article will help you pass the exam and achieve your academic goals. Goodluck in your exams!
You can also check other of our related articles here
Hi Guys! I have been using DStv, GOtv, and Startimes for more than a decade now and I will be sharing some information about them here.
That's not all, I also love On-Demand streaming platforms such as Netflix and ShowMax equally.
Read more about Ibiok Samuel
Similar Posts
Lester b. pearson scholarship in canada 2023-2024 [ fully funded].
The Lester B. Pearson Scholarship in Canada is a remarkable opportunity for international students who wish to pursue their undergraduate studies at the University of Toronto. This scholarship is named after Lester Bowles Pearson, a former Canadian Prime Minister and Nobel Peace Prize laureate. The scholarship program seeks to recognize and support individuals who have…
The Chevening Africa Media Freedom Fellowship Application
Introduction Welcome to our comprehensive guide to the Chevening Africa Media Freedom Fellowship application process. As an aspiring fellow, you have embarked on an extraordinary adventure to empower African journalists and promote media freedom in Africa. In this post, we will delve into the complexities of the fellowship, emphasizing its significance, qualifying criteria, application process,…

PREMIUM EXAM RUNZ WEBSITE
Vibrant exam expo website for jamb runz, waec runz, neco runz, nabteb expo, bece expo, ijmb runz, jupeb runz, nabteb expo, joint exam runz and all school updates, for easy & fast contact 07086897910 || 07086897910, free neco gce 2023 chemistry expo essay & objectives answers.
December 5, 2023 Examgenius GCE 0

NECO GCE 2023 CHEMISTRY EXPO | NECO GCE CHEMISTRY THEORY & OBJECTIVES QUESTIONS AND ANSWERS NOV/DEC EXPO – 6TH DEC., 2023
Table of Contents
Are you Searching for How to Get NECO GCE 2023 CHEMISTRY Expo, 2023 CHEMISTRY NECO GCE EXPO ESSAY & OBJ. QUESTIONS AND ANSWERS | 2023 NECO GCE CHEMISTRY Expo midnight before exam. Welcome to Examgenius.com.ng Solution portal where you will get correct and accurate CHEMISTRY Questions And Answers for 2023 Neco Gce Exam We have gotten the Confirmed/Verified 2023 NECO GCE CHEMISTRY Questions and Answers Expo
Please Note that you must Keep Refreshing this page To get this year NECO GCE CHEMISTRY QUESTIONS AND ANSWERS
In this post you will get your Get NECO GCE 2023 CHEMISTRY Expo 8 hours Before exam time, NECO GCE 2023 MIDNIGHT CHEMISTRY QUESTIONS AND ANSWERS | 2023 CHEMISTRY NECO GCE Questions and Answers before exam
NECO GCE 2023 CHEMISTRY QUESTIONS AND ANSWERS 2023 VERIFIED ESSAY AND OBJECTIVE ANSWERS (Expo) when we have Solve and release the Answers…
STEPS TO GET NECO GCE 2023 CHEMISTRY EXPO (ESSAY &OBJECTIVES) ANSWERS
BEAR IN MIND: – If you want us to Help you send you Real NECO GCE 2023 CHEMISTRY Questions and Answers before exam you have to Pay IT’S NOT FREE.
i.WHATSAPP PACKAGE :-
On this Packages the Question & Answers Will be Sent to your WhatsApp Inbox Directly >>> Send Your N800 MTN CARD to 09064489646 on whatsApp and your will receive the exam questions and answers after your Subscription is confirmed.
ii. PASSWORD PAYMENT
On this package of subscription, We will send The PASSWORD/PIN which will be Used to access the Answers Online on our Answerpage Examgenius.com.ng/answerpage Send Your N700 MTN CARD to 09064489646 on WhatsApp or SMS and wait patiently for your Pin Code after confirming your Payment
CLICK HERE TO VIEW THE ANSWERS
=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=
EXAMGENIUS.COM.NG TEAM ASSURES YOU NOTHING LESS THAN B2 IN THIS SUBJECT… ALL YOU HAVE TO DO IS TO SUBSCRIBE FOR OUR HELP.
ALL THE QUESTIONS AND ANSWERS ARE LOCKED WITH PIN 🔐🔐🔐🔐

ENTER THE PIN SENT TO YOU BE OUR ADMIN ON THE ANSWERPAGE TO VIEW YOUR ANSWERS
CHEMISTRY PIN IS 91**
ONLY THOSE WHO SUBSCRIBE WILL GET THE FULL PIN TO VIEW THE ANSWER ON THE PORTAL ONLINE
3. SMS PACKAGE
On this Package the Questions & Answers Will be Sent to your Phone SMS Inbox Directly >>> Send Your N1,000 MTN CARD to 09064489646 on SMS Text and receive your Answers after validating your subscription. Note: This method is only advisable to those who do not have internet connection or using small phone.
Things you Should Know in This Year NECO GCE 2023 CHEMISTRY EXPO – QUESTIONS AND ANSWERS 2023 VERIFIED ESSAY AND OBJECTIVE ANSWERS Questions and Answers Expo
www.examgenius.com.ng portal is the only reliable source that is capable of accessing the NECO GCE 2023 CHEMISTRY EXPO OBJECTIVES & THEORY QUESTIONS AND ANSWERS before exam day/ time. On a daily basis, majority of candidates fall victim to scams related to November/December External NECO (GCE) Questions and Answers. Examgenius Team stands out as the first trustworthy Team, evidenced by the early delivery and response to all exams. T here are so Many Website posting Questions and Answers on their different websites telling you that the Answers to the Questions is Out; the Solutions you are seeing is not for this year NECO GCE Questions, Kindly Take note of that.
How TO GET VERIFIED NECO GCE 2023 CHEMISTRY EXPO Questions and Answers From Examgenius.com.ng
NOTE – getting your NECO GCE 2023 CHEMISTRY QUESTIONS AND ANSWERS VERIFIED ESSAY AND OBJECTIVE ANSWERS expo – questions and answers before exam time or day is not for free. We charge a little token for the services, but trust me” you won’t regret spending your money to get the leak papers.
NECO GCE CHEMISTRY 2023
Ready to subscribe Now? Send The Following details:-
- MTN CARD Pin(s) or Ask for Bank Details on WhatsApp/SMS
- Phone Number to ===> 09064489646 via sms or WhatsApp
Before You Chat Me on whatsapp make sure you are ready to pay either by bank transfer or pos deposit or Mtn Card.
ITS NOT A FREE STUFF
Most Of the NECO GCE EXAM ANSWERS/Solutions you are seeing from others Exam Dealers Are gotten From www.examgenius.com.ng, and The Funniest part is those people you paid to join their NECO GCE vip group, also paid to us to join my Vip Group in other to Get NECO GCE Questions and Answers and send to their vip members that paid to them
Others Maybe Dropping 10mins before exam, But here at Examgenius.com.ng we Drop 10-8hours before the exam
Just as our name implies, Examgenius.com.ng is the number one, the champion when it comes to assisting students in their external exams. It’s not by pride! Examgenius.com.ng is the best and most reliable exam runs website you can ever rely on. We give answers before others and the amazing thing here is that, we don’t charge much for our services.
NOTE: QUESTIONS AND ANSWERS WILL NOT BE POSTED ON THIS PAGE. THE VERIFIED EXAM QUESTIONS AND ANSWERS WILL BE SENT TO OUR SUBCRIBERS ONLY. HURRY UP AND PAY FOR IT NOW.

Interested in getting your NECO GCE CHEMISTRY QUESTIONS AND ANSWERS 2023 VERIFIED ESSAY AND OBJECTIVE ANSWERS questions and answers 10hrs or midnight before exam, kindly join us on WhatsApp. That’s where you can easily chat the admin and get the correct answers before exam time. Use the below details to join or reach out to us;
CLICK HERE TO JOIN EXAMGENIUS EXPO GROUP
How To Pay/ Subscribe And Get 2023 NECO GCE CHEMISTRY Questions And Answers.
There are Two Different Acceptable Payment Methods, which are listed below
- Payment Through Bank Transfer or POS: To Pay Through Bank Transfer or Pos Deposit; Text “I Need Bank Details” To 09064489646. Please Specify The Subject(s) you’re paying for E.g Neco Gce CHEMISTRY ESSAY AND OBJ.
- Payment through Airtime (MTN CARD ONLY): All MTN Card should be forwarded to 09064489646 on WhatsApp or Sms.
NOTE: ALWAYS MENTION THE MEDIUM THROUGH WHICH YOU PREFER RECEIVING YOUR QUESTIONS AND ANSWERS (Whatsapp, Online Answer Pin or SMS)
EMPHASIS – getting your NECO GCE CHEMISTRY QUESTIONS AND ANSWERS 2023 VERIFIED ESSAY AND OBJECTIVE ANSWERS expo – questions and answers before exam time is NOT FOR FREE . We charge a little token for the services, but trust me” you won’t regret spending your money to get the leak papers.
Do not allow any motivational speaker to deceive you with” you can do it by yourself. NECO GCE examination is not like your random class exams. The exam is set by external bodies and the questions are very difficult. Well, the choice is yours though.
Keep refreshing to get answers for the NECO GCE Questions….
Answers coming in form of pictures….
Answers Loading……… Soon……
- NECO GCE 2023 CHEMISTRY EXPO
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2024 | Designed by Examgenius.Com.Ng
JOIN EXAMGENIUS WhatsApp Group For Exam Expo and Latest Exam Updates

Home » NECO » NECO Chemistry Questions & Answers 2023 (OBJ-Essay) Released
Home → NECO
Neco chemistry questions & answers 2023 (obj-essay) released.
See 2023 NECO Chemistry Answers & Questions Here.
The Neco chemistry answers for 2023 questions can now be seen here. The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm.
This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write.
Here, we will be posting the neco chemistry questions for candidates that will participate in the examination. Note that below is the questions from past questions and answers that we feel are likely questions for SSCE preparation.


NECO Chemistry Answers 2023.
1. a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca.
2. a) (i) Define nuclear fission. (ii) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1. Explain briefly the effect of each of the following changes on the equilibrium composition:
- increase in the concentration of B;
- decrease in pressure of the system;
- addition of catalyst.
b) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
c) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2.
3. a) (i) Define saturated solution. (ii) Distinguish between dative bond and covalent bond. (iii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in: hydrogen fluoride; argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give reason for your answer.
(b) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p. [ standard vapour pressure of water at 18°C = 15 mmHg]
4. a) (i) Define ionic bond. (ii) What type of bond(s) exist(s) in: magnesium oxide; ammonium ion?
b) (i) Determine the oxidation number of sulphur in Na2S2O3. (ii) State Faraday’s first law. (iii) Give one example each of: acid salt; base salt.
c) (i) Name the type of energy change that occurs in each of the following processes; I2(s) ———> I2(g); C1(g) + e- ——> C1-(g). (ii) State the effect of each of the following aqueous solutions on litmus paper: Na2SO4(aq); AlC13(aq) (iii) Define the term efflorescence. (iv) Give two uses of activated charcoal.
d) (i) State one use of each of the following processes in the chemical industry: hydrogenation of vegetable oil; cracking; esterification. (ii) Calculate the amount of silver deposited in moles when 10920 coulombs of electricity is passed through a solution of a silver salt. [Faraday constant = 96500 C mol-1]
5. a) (i) Define in terms of electron transfer I. oxidizing agent; II. reducing agent. (ii) Write a balanced equation to show that carbon is a reducing agent. (iii) State the change in oxidation number of the specie that reacted with carbon in 5 (a)(ii).
b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H2S to form sulphur. Using the information provided: identify gas X; state two properties exhibited by X; give two uses of X.
c) Consider the following substances: (1) sodium; (2) lead (II) iodide; (3) hydrogen; (4) magnesium; (5) oxygen. Which of the substances (i) conducts electricity? (ii) is produced at the cathode during electrolysis of H2SO4(aq)? (iii) corresponds to the molecular formula AB2 ? (iv) is an alkaline earth metal?
d) (i) Define the term salt. (ii) Mention two types of salt. (iii) Give an example of each of the salts mentioned in 5(d)(ii) above.
e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution. (i) Write a balanced equation for the reaction. (ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
Note: There is nothing like Neco chemistry Expo online. Neco Ssce candidates are to desist from patronizing online fraudsters / vendors who says they can provide such services as they are not real.
Neco Chemistry Objective Questions 2023.
NOTE: There is nothing like Neco chemistry expo online. Do not be deceived by fraudsters posing fake NECO chemistry answers on the internet.
13. At a particular temperature and pressure, 15.0 g of CO2 occupy 7.16 liters. What is the volume of 12.0 g of CH4 at the same temperature and pressure? A.
Keep following this page. If you have any questions, endeavour to use the comment box below…
Industrial Action: ASUU, FG Schedule Another Meeting for November 20
Neco animal husbandry answers 2023 [essay-obj-practical] is out, other recommended posts for you, neco bece 2023 registration form & time table is now out, neco data processing answers 2023 practicals is out, comments (33).
Are the questions legitimate
It was very useful Please add me up
Can i get 2022 neco chemistry theory…
This is very good
You are in point but not at all will people read there is expo
Can I get chemistry practical question of 2023?
We should all read our books & not rely on expo
Why u con dey this site
Today chemistry answers
Yes I agree wholeheartedly agree with you.
So what are u doing here U are cross checking ur work nonsense
I hope the questions are not fake
I need to join this group so that I can excel in my exam. So lovely. Thanks.
I want to join this group
I really need to join this group
I need the Neco 2021 questions and answers
How about the answers
Obj and theory answers chemistry pls
Objective pls
How can I join please
I loved to join this group,this website is great, thanks so much
These chemistry questions above, the correct ones for neco 2021?
I wish to joined dis group
For chemistry obj n essay
please i need neco 2022 answers
I just love unn-edu.info. I wrote Agric exams neco today main paper and can you imagine that some of the questions they posted here came out today . So I advise anything that is posted here should be given utmost attention.
Oyebode precious read your books, stop looking for expo
Where are the solution for those questions above.
The answer for this
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Privacy Policy
- ADMISSION UPDATES
- SCHOOL GISTS

NECO Chemistry 2023 Questions and Answers Expo Obj & Essay Free

What You'll Learn in this Post
NECO Chemistry 2023 Answers Objective and Essay
01-10: DEADADECAD 11-20: BAE… for more click HERE now

3a(ii) Two methods to prepare ethanol commercially: – Fermentation: Ethanol can be produced by the fermentation of sugars using yeast. This is commonly used to produce alcoholic beverages and biofuels. – Hydration of ethene: Ethanol can be produced by the hydration of ethene (ethylene) in the presence of a catalyst, such as phosphoric acid.

(3bi) The elec…
For more NECO 2023 Chemistry Objective and Essay Answers, click HERE now.
https://wa.link/mz5r
Loading….
- Recommended: Neco Chemistry Questions 2023 | Check Now
NECO 2023 Chemistry Questions and Answers
(3civ) Three uses of hydrogen: (i) Hydrogen is used as a fuel for combustion engines and fuel cells. (ii) It is used in the production of ammonia for fertilizer and other chemicals. (iii) Hydrogen is used in the hydrogenation of oils and fats in the food industry.
For more / assistance click Here now
https://paystack.com/pay/necopayment
Thanks for reading.

RELATED ARTICLES MORE FROM AUTHOR
Waec mathematics 2024 questions and answers essay & obj, nigeria national anthem nigeria we hail thee lyrics, waec animal husbandry practical 2024 questions and answers free expo.
I need answer
Aslm yakake
LEAVE A REPLY Cancel reply
Save my name, email, and website in this browser for the next time I comment.
NECO Chemistry Past Questions and Answers | Theory and Objectives –Download PDF.
NECO Chemistry Past Questions and Answers | Theory and Objectives –Download PDF. The National Examination Council of Nigeria (NECO) Examination is around the corner. This article provides the readers with the information on how to download NECO Chemistry Past Questions and Answers. NECO Chemistry Questions are essential for you to know if you want to pass the NECO SSCE Chemistry Exams 2023.
The NECO Chemistry past questions and answers is a compiled material in PDF that contains past questions from NECO Chemistry Exams of previous years. The material comes with answers and is easily downloadable.
- NECO Commerce Past Questions and Answers | Theory and Objectives –Download PDF.
- NECO English Past Questions and Answers –Download PDF.
You can download the materials here. The instructions on how to download the NECO Chemistry can be found below. You are expected to follow the instructions in order for you to succeed. The material does not only contain information on NECO Chemistry Questions and answers but how to pass exams as well. You can see this article on the 21 indispensable steps to pass any exams with high grade .
Download also: WAEC past Questions and Answers .
NECO Chemistry Sample Questions and Answers
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
Related Posts:
- NECO CRS Past Questions and Answers | Theory, and…
- NECO Physics Past Questions and Answers | Theory and…
- NECO Biology Past Questions and Answers | Theory and…
- NECO Government Past Questions and Answers | Theory…
- NECO Economics Past Questions and Answers | Theory…
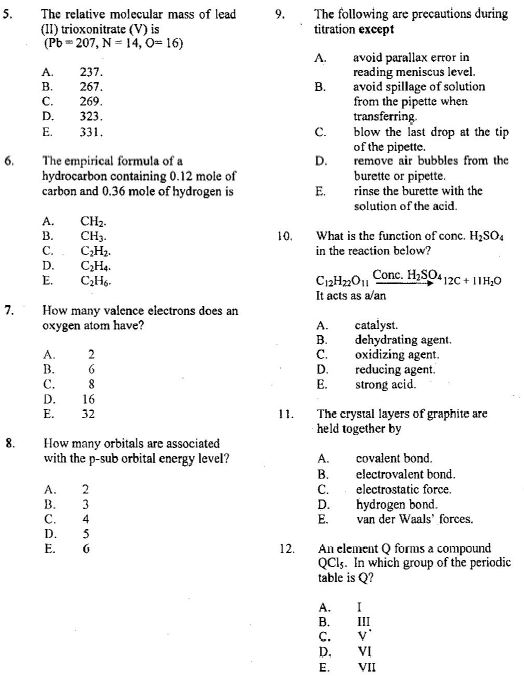
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
How to Download NECO Chemistry past Questions and Answers
Here are the instructions to be followed in order to download the NECO Chemistry Questions and answers without any issue. Please follow our instructions and guide very well.
The cost of the newly updated NECO Past Questions and Answers is 2,000.00 naira only for One Subject e.g
- Use of English 1000 naira
- Biology 1000 naira
- Chemistry 1000 naira
- Physics 1000 naira
See The Payment Detail Below.
To get NECO Past Questions and Answers follow the steps below.
Pay into the account below.
Account Details Account Name: ETUK, OTO-OBONG EMMANUEL
Account Number : 0318379097 Bank : GTBANK .
After Payment , send the following
(1) Depositors name,
(2) Teller number,
(3) Amount paid to 07063986527 on whatsapp or [email protected]
The purchased NECO Past Questions and Answer will be uploaded immediately into your email address within the next 24 hours.
If you have purchased yours, kindly drop a testimony in the comment box below.
CALL or WhatsApp EXAMSTUTS: 07063986527. Send all your mails to [email protected]
Tips on How to Pass NECO Examination 2023
If you want to Past the NECO Examination this year, you will need to follow the instructions below.
- Practice daily with NECO Past Questions and Answers
- Write out your subjects, the dates and time.
- Engage in general revision few days to the examination and dwell more on the subjects you find difficult.
- Focus on only your first paper a week to the exam. This would make it sweet and you will have energy to read for other papers.
- Don’t read any other subject when it is two days to a particular subject.
- When you have two papers that are separated by only one day, read the second one first before going to the subject you have first.
- Ensure to be in the NECO examination venue at least two hours to the examination so that you would be balanced.
- Try to be up to date in cases of changes in the timetable.
- Have a personal copy of the NECO timetable and syllabus.
- Go through your timetable daily so that you don’t miss any paper.
- Don’t go to the hall when you are not having paper that day.
Disclaimer: Please note these are not the actual questions and answers that you will find in the NECO Examination this year, but they are the likely questions. to get the actual NECO Past Questions and answers, please call 07063986527.
Download Actual NECO Past Questions and Answers.
If this article on NECO Chemistry Questions and Answers has been helpful Please share and Like us on Facebook@Examstuts
If You Want More Information, please subscribe to our email list and leave a comment with your number below, also Follow us on Facebook, Twitter, and Linkedin
For Inquiries on all Past Questions Call 07063986527 or Chat on WhatsApp.
Disclaimer: Please note that we are not in any way affiliated with any of the organizations or institutions here. All articles here are for the sole purpose of providing information. All Past questions are gotten from previous years’ examinations and likely questions from the Internet and related exams. We are not in any way promising that what you find in the past questions is what you will find in your examination. We are not in any way responsible for how the reader uses the information here. Please do consult an expert or professional in your field should the need arise. Copyrights Infringement: No article from this website should be copied without a proper reference and link to the page picked from. Anything otherwise will lead to legal action of copyright infringement. All articles on this website are products of painstaking research from our writers and journalist. Should you find any material bearing semblance here to any material on your page, please quickly notify us by sending a mail to [email protected] and we will immediately commence the process of taking it down.
NECO Data Science Past Questions and Answers | Theory, and Objectives –Download PDF.
Neco past questions and answers for all subjects | free pdf download, related articles, neco further mathematics past questions and answers | theory and objectives –download pdf., neco physical education past questions and answers | theory and objectives –download pdf., neco french past questions and answers | theory and objectives –download pdf., neco syllabus pdf download (ssce) 2023 for all subjects- download here., neco agric practical questions and answers | theory and objectives –download pdf., neco computer studies past questions and answers | theory and objectives –download pdf..
- NECO Timetable 2023 PDF Download (June/July)- Download Here.

Home » PAST QUESTIONS » 2023 NECO Chemistry Essay Questions and Answers For all Candidates
2023 NECO Chemistry Essay Questions and Answers For all Candidates

2023 Neco Chemistry Questions: If you are a candidate who is in search of Neco Agricultural Science Questions then you are on the right website because we try to make sure all our audience studies hard to pass with proper information.
If you need us to help you with more updated information at the right time about the 2023 Neco Chemistry Questions, kindly subscribe or bookmark our website for us to provide you with it.
Nkedugists understand how students feel whenever it comes to closing time for an external exam such as Waec, Neco , Jamb, and Post Utme, that is why we make sure any question we are releasing/publishing is always the best option questions you are to see in your exam day because we always follow scholars and examiners. below are what we taught about before publishing it, which are;
- NECO GCE Chemistry Questions Questions
- NECO Chemistry Questions Questions
- 2020/2021 NECO Chemistry Questions
- NECO Chemistry Questions
- GCE Chemistry Questions
- NECO Chemistry Questions
- Chemistry Questions for GCE
- 2020 NECO Chemistry Questions
- 2022 Neco Chemistry Questions
- Neco Chemistry Questions 2022/2023
CHEMISTRY PAPER II Paper 2 will last for 1 hour 30 min Attempt two questions from this section
(1a) Ammonium chloride —> sublimation Ink —> chromatography Iron filling —> magnetic separation
(1aii) -Example Of physical Change (i)change of matter (ii)evaporation of sodium chloride
-Example of chemical change (i)burning of firewood (ii)rusting of iron
(1bi) (i)they are good reducing agent (ii)they form basic oxide (iii)they formed electro valent bonding
(1bii) Aluminum tetraoxosulphate(vi) is prepared by the action of hot concentrated tetraoxosulphate(vi)acid on aluminum oxide Al2O3(s) + 3H2SO4(aq)—->Al2(SO4)3(aq)+3H2O(i) Aluminum tetraoxosulphate(vi) is moderately soluble in water
(1ci) Hydrogen
(1cii) (i)it is denser than air (ii)it is very poisonous
(1ciii) Pb(NO3)2+H2S—->Pbs+2HNO3
(1civ) It turn black due to the formation of black lead (II) sulphide
(1di) Water gas contains flammable gases whereas the producer gas contains both flammable and non-flammable gases
(1dii) (I) Diamond —> Octahedral in shape (II) Graphite —> Hexagonal in shape
(1e) (i) For manufacturing of ammonia and fertilizer (ii) It is used as a refrigerant and also used to shrink metal parts (iii) It is used in grinding substances that are too tough to grind at normal temperature
(2aii) Avogadro’s law states that equal volumes of all gases under the same temperature and pressure contain the same number of molecules
(2aii) Given : 2SO2(aq) + O2(g) –> 2SO3(aq)
The volume of oxygen, O2 required = 1/2*7.50dm³ =3.75dm³
No of moles required = 3.75/22.4moles =0.1674moles
No of oxygen molecules required = 0.1674×6.02×10^23 =1.008×10^23molecules
(2bi) (I) NaCl —–> Valency of chlorine is 1 (II) NaO2 —–> Valency of oxygen is 2 (III) Na3N ——> Valency of Nitrogen is 3
(2bii) (i)Formation of black soap (ii)Formation of glycerol (iii)It helps in the precipitation of large biomolecules such as protein
(2ci) (i)Na2O (ii)MgO (iii)CaO
(2cii) Because they react with water to form acids
(2ciii) – Reaction with sulphur S(s) + O2(g) —> SO2(g)
– Reaction with metals 4Na(s) + O2(g) —-> 2NaO(s)
(2di) Ion is any atom or group of atoms which possess an electric charge
(2dii) TABULATE PLS ION : H^+, F^-, N^3+
NUMBER OF PROTONS: 1,9,13
NUMBER OF NEUTRONS : 0, 10, 14
NUMBER OF ELECTRONS : 0, 10, 10
(2ei) Monosaccharides are simple six-carbon sugar having the formula C6H12O6 eg glucose and its isomers are fructose and galactose, both obtainable from fruits and honey
(2eii) (i)fructose (ii)galactose
What’s your take on this? drop your comment box below Nkedugists urge you to use this same opportunity to share this information across to others using our Facebook, Twitter, or Google+ share button below.
You may also like

Download KANO State Compendium Past Questions and...
Kaduna state free nda past questions and answers for....

Download Akwa Ibom State NDA Compendium Past Questions...
Adamawa state nda compendium past questions and....

Download Western Delta University Postgraduate Test...

Download Christopher University Postgraduate Exam Past...
About the author.
15 Comments
Thanks for the update
2023 NECO CHEMISTRY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE….
NECO 2023 CHEMISTRY SCIENCE QUESTION AND ANSWER NOW AVAILABLE!!!
IF YOU NEED THE ANSWERS ON WHATSAPP , IT COST 800
ADD US ON WHATSAPP WITH==>
09035742503
Please I need only the questions
Pls is this question real and correct
Please can I get the objectives and also the questions to the essay? Thank you.
Is it this year own
Is this 2023 neco question
2023 NECO CHEMISTRY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW………..
NO TIME TO WASTE………….
Number please
I want the objective and the essay answer
Leave a Comment X
Save my name, email, and website in this browser for the next time I comment.
You cannot copy content of this page

Neco 2023 Chemistry Obj & Essay Question And Answer Now Available
July 22, 2023 Wakagist Admin Neco 0

*NECO CHEMISTRY OBJECTIVE AND ESSAY ANSWERS/SOLUTIONS*
*CHEMISTRY OBJECTIVE* 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCABDDCD 31-40: EBEECEBCEE 41-50: BCCECDDADD 51-60: DABBDEAECA
=============================================
*INSTRUCTION*; ANSWER FOUR (4) QUESTIONS ONLY.
*(NUMBER 1)*
(1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides
(1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent
(1aiii) Soaps are made from natural products while detergents are made from synthetic products.
(1aiv) Detergents is for household cleaning and laundry purposes
(1bi) Number of neutrons = Mass number (A) – Atomic number (Z) I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number)
Number of neutrons = 23 – 11 = 12 II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number) Number of neutrons = 39 – 19 = 20
(1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol Now, let’s calculate the mass of oxygen needed:
First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium.
(1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l) From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂). Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol Now, let’s calculate the number of moles of CaCO₃ in 50g: Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol. Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium.
(1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation.
(1ciii) (I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃. The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol.
(II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O. The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O) Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol) Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol. =============================================
*(NUMBER 2)*
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C Substituting the values to calculate the mass of silver deposited: Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)
(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum) =============================================
*(NUMBER 3)*
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol
(3aii) (i) Fermentation (ii) Ethylene hydration
(3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72
(3bi) 1s², 2s², 2p⁴
(3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless
(3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically
(3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O
(3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system.
(3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g)
(3ciii) Redox reaction.
(3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer =============================================
*(NUMBER 4)*
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
(4aii) 15/345 = Solubility *25/1000 Solubility =1000*15/25*345=15000/8625 Solubility = 1.79mol/dm³
(4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆
(4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures
(4bii) (i) Noble gases (ii) Carbon (iv) oxide
(4biii) H₂SO₄ —-> 2H+ + SO₄²⁻ 1 mole of H₂SO₄ = 2 mole of H⁺ 0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions
(4biv) (i) Dative bonding (ii) Hydrogen bonding
(4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures.
(ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery. =============================================
*(NUMBER 5)*
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction
*(5c) DIAGRAM* [img]https://i.imgur.com/khwWNtS.jpg[/img]
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
ADVERTISE | CONTACT US | PRIVACY POLICY | ABOUT US | DISCLAIMER
- Neco answer
NECO CHEMISTRY PRACTICAL 2023 Verified Essay and Objective Answers
Posted by Hedowho on 12 July, 2023
Get Free Live 2023 NECO CHEMISTRY PRACTICAL Questions and Answers for Private Candidates Free of Charge | NECO June/July Free CHEMISTRY PRACTICAL Questions and Answers (Essay & OBJ) EXPO Room
NECO CHEMISTRY PRACTICAL Theory and Objective Answers (100%legit) CHEMISTRY PRACTICAL Essay verified Free (Expo) for National Examination Council. NECO CHEMISTRY PRACTICAL Questions For you to have good NECO result in CHEMISTRY PRACTICAL as well as repeated questions for free in this post.

NECO GCE June/July 2023 FREE CHEMISTRY PRACTICAL QUESTION AND ANSWER ROOM
(1a) [TABULATE]
Burette Reading (cm³) | Rough | Titration I | Titration II Final Reading (cm³) | 21.50 | 43.00 | 22.00 Initial Reading (cm³) | 0.00 | 21.50 | 00.00 Volume of A used (cm³) | 21.50 | 21.50 | 22.00
(1ai) Average Volume of acid used = 21.50 + 21.50/2 = 21.50cm³
(1aii) Solution B[Sodium trioxocarbonate (iv)] feels soapy
(1bi) Conc. B in mol/dm³ =? Ca= 0.04, Va= 21.50cm³, na= 1 Cb= ? , Vb= 25.00cm³ nb= 1
CaVa/CbVb = na/nb = 0.04×21.50/Cb×25.00 = 1/1 Cb= 0.04×21.50/25.00 = 0.86/25.00 Cb= 0.0344mol/dm³
(1bii) Conc. of B in g/dm³ = Conc. of B in mol/dm³ × molar mass of B
Molar mass of B(NaCO₃)= (23×2) + 12 + (16×3) = 106
:. Conc. of B in g/dm³ = 0.0344×106 = 3.6g/dm³
(1biii) 1 mole of Na₂Co₃ = 1mole of Na₂So₄
:. 0.0344 mole of Na₂Co₃ = 0.0344 mole of Na₂So₄ № of mole= mass/ molar mass
Molar mass of Na₂So₄= (23×2)+32+(16×4)=142 :. 0.0344 = mass/142 Mass=142×0.0344=4.9g
(1biv) 1 mole of Na₂Co₃= 1 mole of CO₂
0.0344 mole of Na₂Co₃ = 0.0344 mole of CO₂ :. № of mole = volume/ molar volume = 0.0344 = volume of CO₂/22400cm³ =771cm³ ========================================
(2ai) [TABULATE]
=TEST= C + heat
=OBSERVATION= Formation of yellow flame
=INFERENCE= Na⁺ present
(2aii) =TEST= C + distilled water + shake
=OBSERVATION= It readily dissolve in water
=INFERENCE= C is a soluble in water
(2aiii) =TEST= Solution C + litmus paper
=OBSERVATION= It changes moist red litmus to blue and no effect blue
=INFERENCE= Solution C is alkaline
(2bi) =TEST= 1st portion of solution C + barium chloride
=OBSERVATION= White precipitate formed
=INFERENCE= CO₃²⁻ , SO₄²⁻ , S²⁻ or SO₃²⁻
(2bii) =TEST= Add dilute HCl to product in (bi)
=OBSERVATION= White precipitate dissolved
=INFERENCE= CO₃ , SO₃²⁻ or S₃²⁻
(2biii) =TEST= To the second portion add phenolphthalein
=OBSERVATION= Purple colour observed
=INFERENCE= C is alkaline ========================================
(3ai) Sulphur (iv) oxide and hydrogen sulphide.
(3aii) Liberation of hydrogen gas with pop sound and formation of green iron (ii) chloride solution
(3aiii) Spatula, beaker, weighing balance, standard volumetric flask
(3bi) Solution turns red
(3bii) Turns from brown to green
(3biii) A colourless, odourless, acidic gas liberated which turns blue litmus paper red and turn lime water milky. ========================================
NECO CHEMISTRY PRACTICAL OBJ
Keep refreshing the page for CHEMISTRY PRACTICAL NECO GCE qu estions and answers
Answers coming inform of pictures ..
Answer loading………..Soon…..
Join this telegram group for updates
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
🎵 Want Latest Songs? Click HERE 1234 Views | No Responses
CLICK TO DROP YOUR COMMENT
Related posts.

NECO GCE Economic 2023 Verified Essay and Objective Answers
Neco financial accounting 2023 verified essay and objective answers, neco chemistry 2023 verified essay and objective answers, neco agricultural science 2023 verified essay and objective answers, neco literature 2023 verified essay and objective answers.

HOW KINGS REIGN (The Power Of Spoken Words) Apostle Joshua Selman

Best Friends in the World: Senior Year | Episode 13
Do you want latest songs click here, leave a reply.
NOTE:- Make your comment a bit long to get it approved .
© Copyright 2018 Flashgist | Privacy Policy | About Us | Contact Us | Advertise | Livescore
©2019, Developed by HEDOWHO ©2019, Developed HEDOWHO
NECO Past Questions and Answers For All Subjects PDF
- Post author: Study Admin
- Post published: October 26, 2023
- Post category: Past Questions
- Post comments: 0 Comments
NECO past questions and answers for all subjects are now available for download in PDF format. See how to access NECO past questions that will help boost your score and your overall performance in the 2023 NECO examination below.
NECO exam past questions and answers are provided here for download. All you have to do is to click on your favorite subjects and the downloading process will begin. Scroll down to download the free NECO past questions and answers below.
NECO Past Questions and Answers 2023
This web page was created for the purpose of helping all prospective undergraduate students who are searching for NECO past questions for both science and art subjects.
If you are one of them, then we wish you good luck as you embark on your journey to write this year’s examination. We have uploaded up-to-date NECO past questions to help you during your preparation.
These past questions will help you to know the nature and pattern of the National Examination Council (NECO). These materials will give you insight and prepares you for what to expect during the examination.
Our earnest desire is that you score higher in the upcoming Senior Secondary Certificate Examination (SSCE) for prospective undergraduates. That’s why we have gone to great lengths to upload the past questions for each subject to ease your preparation.
All you have to do is to set aside time each day for your preparation in order to have a distinction or at least a credit for each subject. Remember, preparation is key to success.
Click on each of the subjects to download the past questions in PDF format. Also, if there is any subject of your choice that we have not included, feel free to inform us using the comment box below this page. Below are NECO past questions PDF for both science and art subjects.
NECO Past Questions and Answers PDF
- NECO Agric past questions and answers
- NECO Animal Husbandry past questions and answers
- NECO Biology past questions and answers
- NECO Book Keeping past questions and answers
- NECO Chemistry past questions and answers
- NECO Civic Education past questions and answers
- NECO Commerce past questions and answers
- NECO CRK past questions and answers
- NECO Data Processing past questions and answers
- NECO Economics past questions and answers
- NECO English Language past questions and answers
- NECO French past questions and answers
- NECO Geography past questions and answers
- NECO Government past questions and answers
- NECO Hausa past questions and answers
- NECO History past questions and answers
- NECO Igbo past questions and answers
- NECO Literature in English past questions and answers
- NECO Marketing past questions and answers
- NECO Mathematics past questions and answers
- NECO Physics past questions and answers
- NECO Principle of Accounts past questions and answers
- NECO Yoruba past questions and answers
As we said before, if there is any subject we have not included, feel free to let us know using the comment box below and we will upload it immediately.
You Might Also Like
Udusok post utme past questions and answers pdf, mautech post utme past questions and answers pdf, eksuth school of nursing past questions and answers pdf, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

JOIN OUR WAEC & NECO WHATSAPP SAPP GROUP

Chemistry-Obj USE THIS OBJ FOR A1.. FEW CORRECTION MADE 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCADDECD 31-40: EBEEBEBCEE 41-50: BCCECEDADD 51-60: DABBDEAECA
CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent (1aiii) Soaps are made from natural products while detergents are made from synthetic products. (1aiv) Detergents is for household cleaning and laundry purposes (1bi) Number of neutrons = Mass number (A) – Atomic number (Z) I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number) Number of neutrons = 23 – 11 = 12 II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number) Number of neutrons = 39 – 19 = 20 (1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol Now, let’s calculate the mass of oxygen needed: First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium. (1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l) From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂). Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol Now, let’s calculate the number of moles of CaCO₃ in 50g: Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol. Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium. (1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation. (1ciii) (I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃. The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol. (II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O. The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O) Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol) Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C Substituting the values to calculate the mass of silver deposited: Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)

(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol (3aii) (i) Fermentation (ii) Ethylene hydration (3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72 (3bi) 1s², 2s², 2p⁴ (3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless (3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically (3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O (3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system. (3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g) (3ciii) Redox reaction. (3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. (4aii) 15/345 = Solubility *25/1000 Solubility =1000*15/25*345=15000/8625 Solubility = 1.79mol/dm³ (4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆ (4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures (4bii) (i) Noble gases (ii) Carbon (iv) oxide (4biii) H₂SO₄ —-> 2H+ + SO₄²⁻ 1 mole of H₂SO₄ = 2 mole of H⁺ 0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions (4biv) (i) Dative bonding (ii) Hydrogen bonding (4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures. (ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Completed!!

I’m a blogger living in Nigeria. I like to share education guides and information from various sources. I created Jambclass to serve as a platform to disseminate quality, credible and dependable information regarding various ways to excel academically. I strive to keep Nigerian youths and students informed. I started this Blog with the Vision To Inspire and Empower Young Persons; helping them Realize and Maximize their Potential.

Leave a Reply
Name (required)
Email (required, but never shared)
NOTE:- Your comment will appear after it has been approved by an admin .
Home | Recent Posts | Pages
About Jambclass
Copyright © 2022 Jambclass
NO.1 EDUCATIONAL BLOG FOR | WAEC | NECO | JAMB | NABTEB | GCE | IJMB | JUPEB | POST UTME & PDF BOOKS
NECO GCE Chemistry Practical 2023 Questions and Answers
November 21, 2023 Kazeem Waec Daily Updates 0
Table of Contents
The National Examination Council Has scheduled the NECO GCE Chemistry Practical 2023 paper to kick off by 22nd November, 2023.
This brings the attention of candidates writing the exam into searching for NECO gce Chemistry Practical 2023, neco gce chemistry practical questions and answers 2023 and etc.
NECO GCE Chemistry Practical 2023
In this article, I will provide you some simple steps to get accurate and verified solutions for NECO GCE Chemistry Practical 2023.

CLICK HERE TO CHAT ME UP NOW & GET IT
Neco gce chemistry practical expo 2023
==> Direct SMS: N1000 MTN CARD Direct SMS MEANS all answers(theory & obj) will come direct to ur phone as sMs.
==> Online PIN: N500 MTN CARD Online PIN MEANS The Pin to access our answers online via https://zamgist.com.ng/answer-page will be sent to u at least 4hours before the exam to access our answers.
==> WhatsApp: N500 MTN CARD Whatsapp MEANS The answer will be sent to you on WhatsApp after we confirm your subscription. Add Us In WhatsApp With 08023429251.
Send The Following details:-
(i) MTN CARD Pin(s) (ii) Your Name (iii) Subject (iv) Phone number ===> 08023429251 via sms
NOTE:- All SMS Sent To The Above Number Are Attended To, Our Phone Number Might Be Diverted To Avoid Distraction. Always Send Us SMS Of Your Complaint Even When Our Number Is Not Available, Your Message(s) Will Get To Us And We Will Reply You ASAP.
ALSO CHECK: Neco Gce Agric Practical Question And Answer 2023
Past Neco gce chemistry practical answers 2022
(1ai) Tabulate
Burette reading/cm3 | 1st titre | 2nd titre Final reading/cm3 | 22.50 | 22.50 Initial reading/cm3 | 0.00 | 0.00 Volume of A used/cm3 | 22.50 | 22.50
(1aii) This is used for the easy identification of end-point or neutrality point.
(1aiii) To avoid error in measurement which may occur through droplet of A into the burette affecting the reading
(1bi) Mass conc. of A = 4.015/dm3 Mass conc. of B = 5.55/dm3 Volume of A, Va = 22.5cm3 Volume of B, Vb = 25.0cm3 Molar mass of A, Hcl = 1+35.5 = 36.5g/mol
:- Conc. of A in mol/dm3 = ?? Conc. of A (mol/dm3) = conc. Of A (g/dm3) ÷ molar mass = 4.015/36.5 Conc. of A = 0.11mol/dm3
(1bii) Conc. of B in mol/dm3 = ?? Cb = ?? Ca = 0.11mol/dm3, Va = 22.5cm3, V6 = 25.0cm3, na = 1, nb = 1 CaVa/CbVb = na/nb Cb = CaVanb/naVa Cb = 0.11×22.5×1/1×25.0 Cb = 2.475/25.0 Cb = 0.099mol/dm3
(1biii) Molar mass of B = ?? ing Conc (mol/dm3) = conc. (g/dm3) ÷ molar mass Molar mass = conc. (g/dm3) ÷ conc. (mol/dm3) Molar mass = 5.55/0.099 Molar mass = 56.0g/mol
(1biv) XOH = 56 X + 16 + 1 = 56 X + 17 = 56 X = 56 – 17 X = 39
(1c) Potassium
(1cii) It is an analytical value
(1ciii) KOH
(1civ) Potassium hydroxide
=============================
(2ai) Is a soluble salt.
(2aii) Ca²+ suspected.
(2aiii) Ca2+ Present.
(2aiv) Ppt remains insoluble.
(2av) White Ppt formed which Remain insoluble
(2avi) Brick red flame observed.
(2bi) SO₂, H₂S Or CO₂ gas suspected
(2bii) Lead(II) Ethanoate paper turns black
(2biii) H₂s gas Evolved. S²- present
===========================
(3ai) I – KMnO4 II – Bromine water III – Litmus paper
(3aii) (i) A rotten egg odour is perceived (ii) A colourless gas is formed (iii) A green coloured compound is formed
(3bi) -I- – A precipitate is formed – Asoluble xompound is also formed
-II- – Filtration – Decantation
(3bii) AgNO3 (silver trioxonitrate(v))
Dear Neco gce 2023 Candidates always make sure that you subscribe to a legit platform such as ZAMGIST to Avoid stories that touches. We wish all of you a successful results

Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2024 | WordPress Theme by ZG Themes

IMAGES
VIDEO
COMMENTS
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
The NECO Chemistry Practical Syllabus is similar to the WAEC Syllabus, if not the same. This video, which is the detailed solution to past WAEC and NECO ques...
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol. Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol. If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box. We are happy you are here for the 2023 Chemistry answers.
Welcome to the NECO 2023 CHEMISTRY Objectives & Essay Answers to Questions for the year 2023. 2023 NECO Chemistry Objectives & Essay Questions and
The 2023 NECO chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 95% of the questions are repeated. You don't have to worry about the 2023 NECO chemistry questions and answers PDF (essay and objective).
The National Examination Council (NECO) has released the official time table for the November/December NECO GCE. Before proceeding to the time table, candidates concerned should take note of the instructions below Candidates should confirm the specific venues for Oral French, Arabic and Stenography I & IV from NECO State…. 2023 neco chemistry ...
NECO CHEMISTRY Theory and Objective Answers (100%legit) CHEMISTRY Essay verified Free (Expo) for National Examination Council. NECO CHEMISTRY Questions For you to have good NECO result in CHEMISTRY as well as repeated questions for free in this post.
Here are some sample essay questions and answers for the NECO chemistry exam 2023: (a) Define the following terms: (i) Atomic number (ii) Mass number (iii) Isotopes (b) Write the electronic configuration of the following elements: (i) Sodium (ii) Magnesium (iii) Chlorine (c) Draw the dot-and-cross diagram of sodium chloride. Answer:
2023 NECO Chemistry Answers: Get NECO 2023 Chemistry Questions and Answers for 2023 Paper 2 and 3 (Essay and Objectives). We have the 2023 NECO Chemistry Questions and Answers Expo, so count yourself lucky to be here on our website, where we provide accurate and verified answers.
B. 2023 NECO CHEMISTRY (ESSAY) ANSWERS: TO SUBSCRIBE FOR NECO CHEMISTRY OBJ & THEORY ANSWERS VIA LINK ONLY. JUST GO OUT AND BUY MTN CARDS OF N600 ( 200 + 200 + 200 = 600) GO TO YOUR MESSAGE, TYPE THE MTN CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL THE NUMBER, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU ...
GET Live Neco GCE Chemistry Questions and Answers 8hours before exam. PREMIUM EXAM RUNZ WEBSITE Vibrant Exam Expo Website for Jamb Runz, Waec Runz, Neco Runz, Nabteb Expo, Bece Expo, Ijmb Runz, Jupeb runz, Nabteb Expo, Joint Exam Runz And All School Updates ... FREE NECO GCE 2023 CHEMISTRY EXPO ESSAY & OBJECTIVES ANSWERS ...
The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm. This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write. Here, we will be posting the neco chemistry questions for candidates that will ...
NECO Chemistry Likely Questions And Answers With Detailed Explanations...00:00 - Intro01:13 - Glucose With Fehling's Solution02:43 - Oxidation and Reduction ...
NECO Chemistry 2023 Questions & Answers: Get NECO Chemistry 2023 answers Objective and Essay for Monday, 24th July 2023, Paper III & II: Objective & Essay - Chemistry - 10:00am- 1:00pm.
NECO Chemistry Sample Questions and Answers. The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. 2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is.
NECO GCE CHEMISTRY ESSAY (THEORY) ANSWERS 2023: TO SUBSCRIBE FOR NECO CHEMISTRY ANSWERS VIA LINK ONLY. JUST GO OUT AND BUY MTN CARDS OF N600 ( 200+ 200 + 200 = 600) GO TO YOUR MESSAGE, TYPE THE CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU CONFIRMING THAT YOU HAVE ...
Neco Chemistry Questions 2022/2023. CHEMISTRY PAPER II. Paper 2 will last for 1 hour 30 min. Attempt two questions from this section. (1a) Ammonium chloride —> sublimation. Ink —> chromatography. Iron filling —> magnetic separation. (1aii)
Neco 2023 Chemistry Obj & Essay Question And Answer Now Available. July 22, 2023 Wakagist Neco 0 *NECO CHEMISTRY OBJECTIVE AND ESSAY ANSWERS/SOLUTIONS* *CHEMISTRY OBJECTIVE* 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCABDDCD 31-40: EBEECEBCEE 41-50: BCCECDDADD 51-60: DABBDEAECA ===== *INSTRUCTION*; ANSWER FOUR (4) QUESTIONS ONLY. ...
NECO CHEMISTRY PRACTICAL Theory and Objective Answers (100%legit) CHEMISTRY PRACTICAL Essay verified Free (Expo) for National Examination Council. NECO CHEMISTRY PRACTICAL Questions For you to have good NECO result in CHEMISTRY PRACTICAL as well as repeated questions for free in this post.
NECO past questions and answers for all subjects are now available for download in PDF format. See how to access NECO past questions that will help boost your score and your overall performance in the 2023 NECO examination below. NECO exam past questions and answers are provided here for download. All you have to do is to click on your favorite ...
Get 2023 NECO Chemistry Practicals (CHEM PRAT) Questions and Answers were given out in a free live EXPO Room on the 13th July, 2023. You can get them for yourself: NECO June/July Chemistry (Practicals) Questions and Answers. CHEMISTRY PRACTICALS (CHEM) FREE QUESTION AND ANSWER ROOM AT NECO MAY/JUNE 2023
51-60: DABBDEAECA. CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid. (ii) Vulcanization of rubber. (iii) Formulation of Pesticides and fungicides. (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs. (ii) Hydrogen sulphide is soluble in water to some extent.
The National Examination Council Has scheduled the NECO GCE Chemistry Practical 2023 paper to kick off by 22nd November, 2023. This brings the attention of candidates writing the exam into searching for NECO gce Chemistry Practical 2023, neco gce chemistry practical questions and answers 2023 and etc.