MRC Dyspnoea Scale
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea.
It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life scales (particularly in COPD) the scores are only variably associated with patients' perceptions of respiratory symptom burden. It is used as a component of the BODE Index, which predicts adverse outcomes, including mortality and risk of hospitalisation. The scale is easy and efficient to use.
The mMRC breathlessness scale ranges from grade 0 to 4. It is very similar to the original version and is now widely used in studies. It should be noted that the MRC clearly states on its website that it is unable to give permission for use of any modified version of the scale (including therefore, the mMRC scale). Use of the MRC questionnaire is free but should be acknowledged.
The modified MRC was developed by D A Mahler, see https://pubmed.ncbi.nlm.nih.gov/3342669/

Diagnostic testing
Your essential guide to respiratory diagnostic testing from FeNO and spirometry to CRP Point of Care Testing.
Clinical resources
Step by step guides, expert opinion, the latest insights and case studies - our resources cover a range of respiratory topics and a vital resource for any practitioner working in the delivery of respiratory healthcare
PCRS Respiratory Conference
The UK's leading respiratory conference for clinicians working primary, community and integrated care comes to Telford in September.
You may also be interested in...
Step by step guides, podcasts and webinars cover prevention, diagnosis, testing and management. They will help you to support your patients and improve their outcomes.
Inhaler devices
Inhaler devices may seem simple to use but they are often used incorrectly by patients and healthcare professionals alike.
Chronic Obstructive Pulmonary Disease (COPD) is the fifth leading cause of death in the UK. It's a serious condition which calls for a patient centric approach.
Join PCRS Today
Become part of the UK's largest network of dedicated respiratory professionals working in primary, community and integrated care settings.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Measuring Shortness of Breath (Dyspnea) in COPD
How the Perception of Disability Directs Treatment
Dyspnea is the medical term used to describe shortness of breath, a symptom considered central to all forms of chronic obstructive pulmonary disease (COPD) including emphysema and chronic bronchitis.
As COPD is both a progressive and non-reversible, the severity of dyspnea plays a key role in determining both the stage of the disease and the appropriate medical treatment.
Challenges in Diagnosis
From a clinical standpoint, the challenge of diagnosing dyspnea is that it is very subjective. While spirometry tests (which measures lung capacity) and pulse oximetry (which measures oxygen levels in the blood) may show that two people have the same level of breathing impairment, one may feel completely winded after activity while the other may be just fine.
Ultimately, a person's perception of dyspnea is important as it helps ensure the person is neither undertreated nor overtreated and that the prescribed therapy, when needed, will improve the person's quality of life rather than take from it.
To this end, pulmonologists will use a tool called the modified Medical Research Council (mMRC) dyspnea scale to establish how much an individual's shortness of breath causes real-world disability.
How the Assessment Is Performed
The process of measuring dyspnea is similar to tests used to measure pain perception in persons with chronic pain. Rather than defining dyspnea in terms of lung capacity, the mMRC scale will rate the sensation of dyspnea as the person perceives it.
The severity of dyspnea is rated on a scale of 0 to 4, the value of which will direct both the diagnosis and treatment plan.
Role of the MMRC Dyspnea Scale
The mMRC dyspnea scale has proven valuable in the field of pulmonology as it affords doctors and researchers the mean to:
- Assess the effectiveness of treatment on an individual basis
- Compare the effectiveness of a treatment within a population
- Predict survival times and rates
From a clinical viewpoint, the mMRC scale correlates fairly well to such objective measures as pulmonary function tests and walk tests . Moreover, the values tend to be stable over time, meaning that they are far less prone to subjective variability that one might assume.
Using the BODE Index to Predict Survival
The mMRC dyspnea scale is used to calculate the BODE index , a tool which helps estimate the survival times of people living with COPD.
The BODE Index is comprised of a person's body mass index ("B"), airway obstruction ("O"), dyspnea ("D"), and exercise tolerance ("E"). Each of these components is graded on a scale of either 0 to 1 or 0 to 3, the numbers of which are then tabulated for a final value.
The final value—ranging from as low as 0 to as high as 10—provides doctors a percentage of how likely a person is to survive for four years. The final BODE tabulation is described as follows:
- 0 to 2 points: 80 percent likelihood of survival
- 3 to 4 points: 67 percent likelihood of survival
- 5 of 6 points: 57 percent likelihood of survival
- 7 to 10 points: 18 percent likelihood of survival
The BODE values, whether large or small, are not set in stone. Changes to lifestyle and improved treatment adherence can improve long-term outcomes, sometimes dramatically. These include things like quitting smoking , improving your diet and engaging in appropriate exercise to improve your respiratory capacity.
In the end, the numbers are simply a snapshot of current health, not a prediction of your mortality. Ultimately, the lifestyle choices you make can play a significant role in determining whether the odds are against you or in your favor.
Janssens T, De peuter S, Stans L, et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program . Chest. 2011;140(3):618-625. doi:10.1378/chest.10-3257
Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study . BMC Pulm Med . 2010;10:32. doi:10.1186/1471-2466-10-32
Esteban C, Quintana JM, Moraza J, et al. BODE-Index vs HADO-score in chronic obstructive pulmonary disease: Which one to use in general practice? . BMC Med . 2010;8:28. doi:10.1186/1741-7015-8-28
Chhabra, S., Gupta, A., and Khuma, M. " Evaluation of Three Scales of Dyspnea in Chronic Obstructive Pulmonary Disease. " Annals of Thoracic Medicine. 2009; 4(3):128-32. DOI: 10.4103/1817-1737.53351 .
Perez, T.; Burgel, P.; Paillasseur, J.; et al. " Modified Medical Research Council scale vs Baseline Dyspnea Index to Evaluate Dyspnea in Chronic Obstructive Pulmonary Disease. " International Journal of Chronic Obstructive Pulmonary Disease . 2015; 10:1663-72. DOI: 10.2147/COPD.S82408 .
By Deborah Leader, RN Deborah Leader RN, PHN, is a registered nurse and medical writer who focuses on COPD.

MedicalCRITERIA.com
Unifying concepts, modified medical research council (mmrc) dyspnea scale.
The modified Medical Research Council (mMRC) scale is recommended for conducting assessments of dyspnea and disability and functions as an indicator of exacerbation.
The modified Medical Research Council (mMRC) scale
An mMRC scale grade of 3 have a significantly poorer prognosis and that the mMRC scale can be used to predict hospitalization and exacerbation.
References:
- Natori H, Kawayama T, Suetomo M, Kinoshita T, Matsuoka M, Matsunaga K, Okamoto M, Hoshino T. Evaluation of the Modified Medical Research Council Dyspnea Scale for Predicting Hospitalization and Exacerbation in Japanese Patients with Chronic Obstructive Pulmonary Disease. Intern Med. 2016;55(1):15-24. [Medline]
- Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, Lebargy F, Deslee G. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012 Oct 1;12:61. [Medline]
Created Feb 10, 2021.
Related Posts
The muscle scale grades muscle power on a scale of 0 to 5 in relation…
The Hachinski ischemic score (HIS) is known to be a simple clinical tool, currently used…
The Karnofsky Performance Status Scale (KPS) was designed to measure the level of patient activity…
Instructions: This scale is intended to record your own assessment of any sleep difficulty you…
The FAST scale is a functional scale designed to evaluate patients at the more moderate-severe…

Users Online
Medical disclaimer, recent posts.
- Psoriasis Area Severity Index (PASI) Score
- Diagnostic Criteria for Catatonia
- Diagnostic Criteria for Dengue Hemorrhagic Fever (DHS) and Dengue Shock Syndrome (DSS)
- Diagnosis of Posterior Reversible Encephalopathy Syndrome (PRES)
- Manifestations of Right-Sided Heart Failure (RHF)
- About Us (1)
- Allergy (3)
- Anesthesiology (5)
- Cardiology (54)
- Critical Care (17)
- Dermatology (7)
- Diabetes (14)
- Endocrinology and Metabolism (20)
- Epidemiology (6)
- Family Practice (7)
- Gastroenterology (63)
- Hematology (40)
- Immumology (6)
- Infectious Disease (44)
- Internal Medicine (1)
- Nephrology (17)
- Neurology (79)
- Nutrition (29)
- Obstetrics & Gynecology (20)
- Oncology (13)
- Ophthalmology (4)
- Orthopedic (5)
- Otolaryngology (5)
- Pathologic (1)
- Pediatrics (14)
- Pharmacology (3)
- Physical Therapists (7)
- Psychiatry (50)
- Pulmonary (23)
- Radiology (5)
- Rheumatology (52)
- Surgery (8)
- Urology (5)
Popular of the Last Month
- DSM-5 Diagnostic Criteria for Schizophrenia 750 vistas
- Diagnostic Criteria for Anorexia Nervosa (DSM-V) 673 vistas
- DSM-5 Diagnostic Criteria for Panic Disorder 606 vistas
- Diagnostic Criteria for Bulimia Nervosa (DSM-5) 575 vistas
- DSM-5 Diagnostic Criteria for Major Depressive Disorder 415 vistas
- GLIM Criteria for the Diagnosis of Malnutrition 365 vistas
- Edmonton Obesity Staging System (EOSS) 337 vistas
- DSM-5 Diagnostic Criteria for Dyspraxia/Developmental Coordination Disorder 284 vistas
- Medical Research Council (MRC) Scale for Muscle Strength 269 vistas
- Stage Classification of Gastric Ulcer by Sakita-Miwa 261 vistas
Copyright by MedicalCriteria.com
Last Updated on 21 June, 2021 by Guillermo Firman
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
- Research article
- Open access
- Published: 01 October 2012
The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study
- Claire Launois 1 ,
- Coralie Barbe 2 ,
- Eric Bertin 3 ,
- Julie Nardi 1 ,
- Jeanne-Marie Perotin 1 ,
- Sandra Dury 1 ,
- François Lebargy 1 &
- Gaëtan Deslee 1
BMC Pulmonary Medicine volume 12 , Article number: 61 ( 2012 ) Cite this article
61k Accesses
74 Citations
2 Altmetric
Metrics details
Dyspnea is very frequent in obese subjects. However, its assessment is complex in clinical practice. The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute walk test (6MWT), lung function and biological parameters.
Forty-five obese subjects (17 M/28 F, BMI: 43 ± 9 kg/m 2 ) were included in this pilot study. Dyspnea in daily living was evaluated by the mMRC scale and exertional dyspnea was evaluated by the Borg scale after 6MWT. Pulmonary function tests included spirometry, plethysmography, diffusing capacity of carbon monoxide and arterial blood gases. Fasting blood glucose, total cholesterol, triglyceride, N-terminal pro brain natriuretic peptide, C-reactive protein and hemoglobin levels were analyzed.
Eighty-four percent of patients had a mMRC ≥ 1 and 40% a mMRC ≥ 2. Compared to subjects with no dyspnea (mMRC = 0), a mMRC ≥ 1 was associated with a higher BMI (44 ± 9 vs 36 ± 5 kg/m 2 , p = 0.01), and a lower expiratory reserve volume (ERV) (50 ± 31 vs 91 ± 32%, p = 0.004), forced expiratory volume in one second (FEV 1 ) (86 ± 17 vs 101 ± 16%, p = 0.04) and distance covered in 6MWT (401 ± 107 vs 524 ± 72 m, p = 0.007). A mMRC ≥ 2 was associated with a higher Borg score after the 6MWT (4.7 ± 2.5 vs 6.5 ± 1.5, p < 0.05).
This study confirms that dyspnea is very frequent in obese subjects. The differences between the “dyspneic” and the “non dyspneic” groups assessed by the mMRC scale for BMI, ERV, FEV 1 and distance covered in 6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects.
Peer Review reports
Obesity, defined as a Body Mass Index (BMI) greater than or equal to 30 kg/m 2 , is a significant public health concern. According to the World Health Organization, worldwide obesity has more than doubled since 1980 and in 2008 there were about 1.5 billion overweight adults (25 ≤ BMI < 30 kg/m 2 ). Of these, over 200 million men and nearly 300 million women were obese [ 1 ].
Dyspnea is very frequent in obese subjects. In a large epidemiological study, 80% of obese patients reported dyspnea after climbing two flights of stairs [ 2 ]. In a series of patients with morbid obesity, Collet et al. found that patients with a BMI > 49 kg/m 2 had more severe dyspnea assessed with BDI (Baseline Dyspnea Index) than obese patients with a BMI ≤ 49 kg/m 2 [ 3 ]. The most frequent pulmonary function abnormalities associated with obesity [ 4 , 5 ] are a decrease in expiratory reserve volume (ERV) [ 6 – 8 ], functional residual capacity (FRC) [ 6 – 8 ], and an increase in oxygen consumption [ 9 ]. Although the mechanisms of dyspnea in obesity remain unclear, it is moderately correlated with lung function [ 3 , 10 – 16 ]. Of note, type 2 diabetes [ 17 ], insulin resistance [ 18 ] and metabolic syndrome [ 19 ] have been shown to be associated with reduced lung function in obesity. It must be pointed out that dyspnea is a complex subjective sensation which is difficult to assess in clinical practice. However, there is no specific scale to assess dyspnea in daily living in obesity. The modified Medical Research Council (mMRC) scale is the most commonly used validated scale to assess dyspnea in daily living in chronic respiratory diseases [ 20 – 22 ] but has never been assessed in the context of obesity without a coexisting pulmonary disease.
The objectives of this pilot study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute walk distance (6MWD), lung function and biological parameters.
Adult obese patients from the Department of Nutrition of the University Hospital of Reims (France) were consecutively referred for a systematic respiratory evaluation without specific reason and considered for inclusion in this study. Inclusion criteria were a BMI ≥ 30 kg/m 2 and an age > 18 year-old. Exclusion criteria were a known coexisting pulmonary or neuromuscular disease or an inability to perform a 6MWT or pulmonary function testing. The study was approved by the Institutional Review Board (IRB) of the University Hospital of Reims, and patient consent was waived.
Clinical characteristics and mMRC scale
Demographic data (age, sex), BMI, comorbidities, treatments and smoking status were systematically recorded. Dyspnea in daily living was evaluated by the mMRC scale which consists in five statements that describe almost the entire range of dyspnea from none (Grade 0) to almost complete incapacity (Grade 4) (Table 1 ).
- Six-minute walk test
The 6MWT was performed using the methodology specified by the American Thoracic Society (ATS-2002) [ 23 ]. The patients were instructed that the objective was to walk as far as possible during 6 minutes. The 6MWT was performed in a flat, long, covered corridor which was 30 meters long, meter-by-meter marked. Heart rate, oxygen saturation and modified Borg scale assessing subjectively the degree of dyspnea graded from 0 to 10, were collected at the beginning and at the end of the 6MWT. When the test was finished, the distance covered was calculated.
Pulmonary function tests
Pulmonary function tests (PFTs) included forced expiratory volume in one second (FEV 1 ), vital capacity (VC), forced vital capacity (FCV), FEV 1 /VC, functional residual capacity (FRC), expiratory reserve volume (ERV), residual volume (RV), total lung capacity (TLC) and carbon monoxide diffusing capacity of the lung (DLCO) (BodyBox 5500 Medisoft Sorinnes, Belgium). Results were expressed as the percentage of predicted values [ 24 ]. Arterial blood gases were measured in the morning in a sitting position.
Biological parameters
After 12 hours of fasting, blood glucose, glycated hemoglobin (HbAIc), total cholesterol, triglyceride, N-terminal pro brain natriuretic peptide (NT-pro BNP), C-reactive protein (CRP) and hemoglobin levels were measured.
Statistical analysis
Quantitative variables are described as mean ± standard deviation (SD) and qualitative variables as number and percentage. Patients were separated in two groups according to their dyspnea: mMRC = 0 (no dyspnea in daily living) and mMRC ≥ 1 (dyspnea in daily living, ie at least short of breath when hurrying on level ground or walking up a slight hill).
Factors associated with mMRC scale were studied using Wilcoxon, Chi-square or Fisher exact tests. Factors associated with Borg scale were studied using Wilcoxon tests or Pearson’s correlation coefficients. A p value < 0.05 was considered statistically significant. All analysis were performed using SAS version 9.0 (SAS Inc, Cary, NC, USA).
Results and discussion
Demographic characteristics.
Fifty four consecutive patients with a BMI ≥ 30 kg/m 2 were considered for inclusion. Of these, 9 patients were excluded because of an inability to perform the 6MWT related to an osteoarticular disorder (n = 2) or because of a diagnosed respiratory disease (n = 7; 5 asthma, 1 hypersensitivity pneumonia and 1 right pleural effusion).
Results of 45 patients were considered in the final analysis. Demographic characteristics of the patients are presented in Table 2 . Mean BMI was 43 ± 9 kg/m 2 , with 55% of the patients presenting an extreme obesity (BMI ≥ 40 kg/m 2 , grade 3). Regarding smoking status, 56% of patients were never smokers and 11% were current smokers. The main comorbidities were hypertension (53%), dyslipidemia (40%) and diabetes (36%). Severe obstructive sleep apnea syndrome was present in 16 patients (43%).
Dyspnea assessment by the mMRC scale and 6MWT
Results of dyspnea assessment are presented in Table 3 . Dyspnea symptom assessed by the mMRC scale was very frequent in obese subjects with 84% (n = 38) of patients with a mMRC scale ≥ 1 and 40% (n = 18) of patients with a mMRC scale ≥ 2 (29% mMRC = 2, 9% mMRC = 3 and 2% mMRC = 4).
The mean distance covered in 6MWT was 420 ± 112 m. Sixteen percent of patients had a decrease > 4% of SpO2 during the 6MWT and one patient had a SpO2 < 90% at the end of the 6MWT (Table 4 ). The dyspnea sensation at rest was very slight (Borg = 1 ± 1.5) but severe after exertion (Borg = 5.4 ± 2.4). Fifty-three percent of patients exhibited a Borg scale ≥ 5 after the 6MWT which is considered as severe exertional dyspnea. No complication occurred during the 6MWT. Subjects with a mMRC score ≥ 2 had a higher Borg score after the 6MWT than subjects with a mMRC score < 2 (6.5 ± 1.5 vs 4.7 ± 2.5, p < 0.05).
Lung function tests
Results of spirometry, plethysmography and arterial blood gases are shown in Table 4 . Overall, the PFTs results remained in the normal range for most of the patients, except for ERV predicted values which were lower (ERV = 56 ± 34%). There were an obstructive ventilatory disorder defined by a FEV 1 /VC < 0.7 in 5 patients (11%) with 5 patients (13%) exhibiting a mMRC ≥ 1, a restrictive ventilatory disorder defined by a TLC < 80% in 5 patients (13%) with 5 patients (16%) exhibiting a mMRC ≥ 1, and a decrease in alveolar diffusion defined by DLCO < 70% in 10 patients (26%) with 9 patients (28%) exhibiting a mMRC ≥ 1. Arterial blood gases at rest were in the normal range with no hypoxemia < 70 mmHg and no significant hypercapnia > 45 mmHg.
Fifteen percent (n = 7) of patients presented anemia. All patients had a hemoglobin level ≥ 11 g/dL. Mean NT pro-BNP was 117 ± 285 pg/mL. Four patients (10%) had a pro-BNP > 300 pg/mL.Forty-five percent of patients had a fasting glucose level > 7 mmol/L, 51% a Hba1c > 6%, 29% a triglyceride level ≥ 1.7 mmol/L, 35% a total cholesterol level > 5.2 mmol/L and 31% a CRP level > 10 mg/L.
Relationships between the mMRC scale and clinical characteristics, PFTs and biological parameters
The comparisons between the mMRC scale and demographic, lung functional and biological parameters are shown in Table 5 . Subjects in the mMRC ≥ 1 group had a higher BMI (p = 0.01) (Figure 1 A), lower ERV (p < 0.005) (Figure 1 B), FEV 1 (p < 0.05), covered distance in 6MWT (p < 0.01) (Figure 1 C) and Hb level (p < 0.05) than subjects in the mMRC = 0 group. Of note, there was no association between the mMRC scale and age, sex, smoking history, arterial blood gases, metabolic parameters and the apnea/hypopnea index.
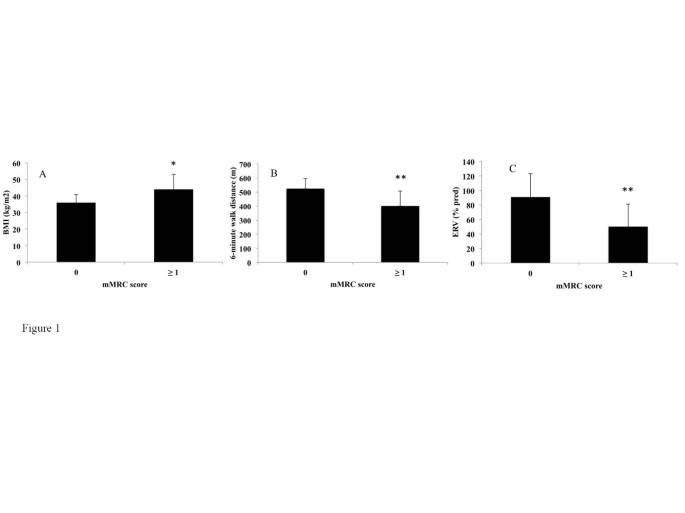
Differences in Body Mass Index (BMI) (A), Expiratory reserve volume (ERV) (B) and 6-minute walk distance (C) between non-dyspneic (modified Medical Research Council score = 0) and dyspneic (mMRC score ≥ 1) subjects. *p < 0.05, **p < 0.01. A Wilcoxon test was used.
The relationships between the Borg scale after 6MWT and demographic, lung functional and biological parameters were also analysed. The Borg score after 6MWT was correlated with a higher BMI (correlation coefficient = +0.44, p < 0.005) and a lower FEV 1 (correlation coefficient = -0.33, p < 0.05). No relationship was found between the Borg score after 6MWT and ERV or hemoglobin level. The Borg score after 6MWT was correlated with a higher fasting glucose (correlation coefficient = +0.46, p < 0.005) whereas this parameter was not associated with the mMRC scale (data not shown). We found no statistically different change in Borg scale ratings of dyspnea from rest to the end of the 6MWT between the two groups (p = 0.39).
In this study, 45 consecutive obese subjects were specifically assessed for dyspnea in daily living using the mMRC scale. Our study confirms the high prevalence of dyspnea in daily living in obese subjects [ 2 ] with 84% of patients exhibiting a mMRC scale ≥ 1 and 40% a mMRC scale ≥ 2. Interestingly, the presence of dyspnea in daily living (mMRC ≥ 1) was associated with a higher BMI and a lower ERV, FEV 1 , distance covered in 6MWT and hemoglobin level. Furthermore, a mMRC score ≥ 2 in obese subjects was associated with a higher Borg score after the 6MWT (data not shown).
The assessment of dyspnea in clinical practice is difficult. Regarding the mMRC scale, two versions of this scale have been used, one with 5 grades [ 20 ] as used in this study and an other with 6 grades [ 25 ] leading to some confusion. Other scales have been also used to assess dyspnea [ 26 ]. Collet at al. [ 3 ], Ofir et al. [ 11 ] and El-Gamal [ 27 ] et al provided some evidence to support the use of the BDI, Oxygen cost diaphragm (OCD) and Chronic Respiratory Disease Questionnaire (CRQ) to evaluate dyspnea in obesity. El-Gamal et al [ 27 ] demonstated the responsiveness of the CRQ in obesity as they did measurements before and after gastroplaty-induced weight loss within the same subjects. The Baseline Dyspnea Index (BDI) uses five grades (0 to 4) for 3 categories, functional impairment, magnitude of task and magnitude of effort with a total score from 0 to 12 [ 28 ]. The University of California San Diego Shortness of Breath Questionnaire comprises 24 items assessing dyspnea over the previous week [ 29 ]. It must be pointed out that these scores are much more time consuming than the mMRC scale and are difficult to apply in clinical practice.
To our knowledge, the mMRC scale has not been investigated in the assessment of dyspnea in daily living in obese subjects without a coexisting pulmonary disease. The mMRC scale is an unidimensional scale related to activities of daily living which is widely used and well correlated with quality of life in chronic respiratory diseases [ 20 ] such as chronic obstructive pulmonary disease (COPD) [ 21 ] or idiopathic pulmonary fibrosis [ 22 ]. The mMRC scale is easy-to-use and not time consuming, based on five statements describing almost the entire range of dyspnea in daily living. Our study provides evidence for the use of the mMRC scale in the assessment of dyspnea in daily living in obese subjects. Firstly, as expected, our results demonstrate an association between the mMRC scale and the BMI in the comparison between “dyspneic” and “non dyspneic” groups. Secondly, in our between-group comparisons, the mMRC scale was associated with pulmonary functional parameters (lower ERV, FEV 1 and distance walked in 6MWT) which might be involved in dyspnea in obesity. The reduction in ERV is the most frequent functional respiratory abnormality reported in obesity [ 6 – 8 ]. This decrease is correlated exponentially with BMI and is mainly due to the effect of the abdominal contents on diaphragm position [ 30 ]. While the FEV 1 might be slightly reduced in patients with severe obesity, the FEV 1 /VC is preserved as seen in our study [ 31 ]. The determination of the walking distance and the Borg scale using the 6MWT is known to be a simple method to assess the limitations of exercise capacity in chronic respiratory diseases [ 23 ]. Two studies have shown a good reproducibility of this test [ 32 , 33 ] but did not investigate the relationships between the 6MWD and dyspnea in daily living. Our study confirms the feasibility of the 6MWD in clinical practice in obesity and demonstrates an association between covered distance in 6MWT and the presence or the absence of dyspnea in daily living assessed by the mMRC scale. It must be pointed out that the 6MWT is not a standardized exercise stimulus. Exercise testing using cycloergometer or the shuttle walking test could be of interest to determine the relationships between the mMRC scale and a standardize exercise stimulus. In our between-group comparisons, BMI and FEV 1 were associated with the mMRC scale and correlated with the Borg scale after 6MWT. Surprisingly, the ERV was associated with the mMRC scale but not with the Borg scale. Moreover, the fasting glucose was correlated with the Borg scale after 6MWT but not associated with the mMRC scale. Whether these differences are due to a differential involvement of these parameters in dyspnea in daily living and at exercise, or simply related to a low sample size remains to be evaluated.
As type 2 diabetes, insulin resistance, metabolic syndrome [ 17 – 19 ], anemia and cardiac insufficiency have been shown to be associated with lung function and/or dyspnea, we also investigated the relationships between dyspnea in daily living and biological parameters. A mMRC scale ≥ 1 was associated with a lower hemoglobin level. However, all patients had a hemoglobin level > 11 g/dL and the clinical significance of the association between dyspnea in daily living and a mildly lower hemoglobin level has to be interpreted cautiously and remains to be evaluated. Of note, we did not find any associations between the mMRC scale and triglyceride, total cholesterol, fasting glucose, HbA1C, CRP or NT pro-BNP.
The strength of this study includes the assessment of the relationships between the mMRC scale and multidimensional parameters including exertional dyspnea assessed by the Borg score after 6MWT, PFTs and biological parameters. The limitations of this pilot study are as follows. Firstly, the number of patients included is relatively low. This study was monocentric and did not include control groups of overweight and normal weight subjects. Due to the limited number of patients, our study did not allow the analysis sex differences in the perception of dyspnea. Secondly, we did not investigate the relationships between the mMRC scale and other dyspnea scales like the BDI which has been evaluated in obese subjects and demonstrated some correlations with lung function [ 3 ]. Thirdly, it would have been interesting to assess the relationships between the mMRC scale and cardio-vascular, neuromuscular and psycho-emotional parameters which might be involved in dyspnea. Assessing the relationships between health related quality of life and dyspnea would also be useful. Finally, fat distribution (eg Waist circumferences or waist/hip ratios) has not been specifically assessed in our study but might be assessed at contributing factor to dyspnea. Despite these limitations, this pilot study suggests that the mMRC scale might be of value in the assessment of dyspnea in obesity and might be used as a dyspnea scale in further larger multicentric studies. It remains to be seen whether it is sensitive to changes with intervention.
Conclusions
This pilot study investigated the potential use of the mMRC scale in obesity. The differences observed between the “dyspneic” and the “non dyspneic” groups as defined by the mMRC scale with respect to BMI, ERV, FEV 1 and distance covered in 6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects.
Abbreviations
Body Mass Index
- Modified Medical Research Council scale
Expiratory volume in one second
Vital capacity
Forced vital capacity
Functional residual capacity
Expiratory reserve volume
Residual volume
Total lung capacity
Carbon monoxide diffusing capacity of the lung
Glycated hemoglobin
N-terminal pro brain natriuretic peptide
Serum C reactive protein.
WHO: Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ ,
Sjöström L, Larsson B, Backman L, Bengtsson C, Bouchard C, Dahlgren S, Hallgren P, Jonsson E, Karlsson J, Lapidus L: Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992, 16: 465-479.
PubMed Google Scholar
Collet F, Mallart A, Bervar JF, Bautin N, Matran R, Pattou F, Romon M, Perez T: Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes (Lond). 2007, 31: 700-706.
CAS Google Scholar
Salome CM, King GG, Berend N: Physiology of obesity and effects on lung function. J Appl Physiol. 2010, 108: 206-211. 10.1152/japplphysiol.00694.2009.
Article PubMed Google Scholar
Gibson GJ: Obesity, respiratory function and breathlessness. Thorax. 2000, 55 (Suppl 1): S41-S44.
Article PubMed PubMed Central Google Scholar
Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K: Effects of obesity on respiratory function. Am Rev Respir Dis. 1983, 128: 501-506.
Article CAS PubMed Google Scholar
Jones RL, Nzekwu M-MU: The effects of body mass index on lung volumes. Chest. 2006, 130: 827-833. 10.1378/chest.130.3.827.
Biring MS, Lewis MI, Liu JT, Mohsenifar Z: Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999, 318: 293-297. 10.1097/00000441-199911000-00002.
Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B: Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008, 178: 116-123. 10.1164/rccm.200706-875OC.
Sahebjami H: Dyspnea in obese healthy men. Chest. 1998, 114: 1373-1377. 10.1378/chest.114.5.1373.
Ofir D, Laveneziana P, Webb KA, O’Donnell DE: Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007, 102: 2217-2226. 10.1152/japplphysiol.00898.2006.
Romagnoli I, Laveneziana P, Clini EM, Palange P, Valli G, de Blasio F, Gigliotti F, Scano G: Role of hyperinflation vs. deflation on dyspnoea in severely to extremely obese subjects. Acta Physiol (Oxf). 2008, 193: 393-402. 10.1111/j.1748-1716.2008.01852.x.
Article CAS Google Scholar
Jensen D, Webb KA, Wolfe LA, O’Donnell DE: Effects of human pregnancy and advancing gestation on respiratory discomfort during exercise. Respir Physiol Neurobiol. 2007, 156: 85-93. 10.1016/j.resp.2006.08.004.
Scano G, Stendardi L, Bruni GI: The respiratory muscles in eucapnic obesity: their role in dyspnea. Respir Med. 2009, 103: 1276-1285. 10.1016/j.rmed.2009.03.023.
Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE: Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009, 180: 964-971.
Sava F, Laviolette L, Bernard S, Breton M-J, Bourbeau J, Maltais F: The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010, 10: 55-10.1186/1471-2466-10-55.
Lecube A, Sampol G, Muñoz X, Hernández C, Mesa J, Simó R: Type 2 diabetes impairs pulmonary function in morbidly obese women: a case-control study. Diabetologia. 2010, 53: 1210-1216. 10.1007/s00125-010-1700-5.
Lecube A, Sampol G, Muñoz X, Lloberes P, Hernández C, Simó R: Insulin resistance is related to impaired lung function in morbidly obese women: a case-control study. Diabetes Metab Res Rev. 2010, 26: 639-645. 10.1002/dmrr.1131.
Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M: Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009, 179: 509-516. 10.1164/rccm.200807-1195OC.
Mahler DA, Wells CK: Evaluation of clinical methods for rating dyspnea. Chest. 1988, 93: 580-586. 10.1378/chest.93.3.580.
Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T: Analysis of Clinical Methods Used to Evaluate Dyspnea in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1998, 158: 1185-1189.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, Watanabe F, Arizono S: A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J. 2010, 36: 1067-1072. 10.1183/09031936.00152609.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002, 166: 111-117.
Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993, 16: 1-100.
Eltayara L, Becklake MR, Volta CA, Milic-Emili J: Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996, 154: 1726-1734.
Gerlach Y, Williams MT, Coates AM: Weighing up the evidence-a systematic review of measures used for the sensation of breathlessness in obesity. Int J Obes. 2012
Google Scholar
El-Gamal H, Khayat A, Shikora S, Unterborn JN: Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest. 2005, 128: 3870-3874. 10.1378/chest.128.6.3870.
Mahler DA, Weinberg DH, Wells CK, Feinstein AR: The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984, 85: 751-758. 10.1378/chest.85.6.751.
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM: Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998, 113: 619-624. 10.1378/chest.113.3.619.
Parameswaran K, Todd DC, Soth M: Altered respiratory physiology in obesity. Can Respir J. 2006, 13: 203-210.
Sin DD, Jones RL, Man SFP: Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002, 162: 1477-1481. 10.1001/archinte.162.13.1477.
Beriault K, Carpentier AC, Gagnon C, Ménard J, Baillargeon J-P, Ardilouze J-L, Langlois M-F: Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009, 30: 725-727. 10.1055/s-0029-1231043.
Larsson UE, Reynisdottir S: The six-minute walk test in outpatients with obesity: reproducibility and known group validity. Physiother Res Int. 2008, 13: 84-93. 10.1002/pri.398.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2466/12/61/prepub
Download references
Acknowledgements
We thank the personnel of the Department of Nutrition and Pulmonary Medicine of the University Hospital of Reims for the selection and clinical/functional assessment of the patients.
Author information
Authors and affiliations.
Service des Maladies Respiratoires, INSERM UMRS 903, Hôpital Maison Blanche, CHU de Reims, 45 rue Cognacq Jay 51092, Reims, Cedex, France
Claire Launois, Julie Nardi, Jeanne-Marie Perotin, Sandra Dury, François Lebargy & Gaëtan Deslee
Unité d'Aide Méthodologique, Pôle Recherche et Innovations, Hôpital Robert Debré, CHU de Reims, Reims, France
Coralie Barbe
Service d’Endocrinologie-Diabétologie-Nutrition, Hôpital Robert Debré, CHU de Reims, Reims, France
Eric Bertin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Claire Launois .
Additional information
Competing interests.
None of the authors of the present manuscript have a commercial or other association that might pose a conflict of interest.
Authors’ contributions
CL, CB, EB, JN, JMP, SD, FL and GD conceived the study. CL acquired data. CB performed the statistical analysis. CL and GD drafted the manuscript. All authors read and approved the manuscript prior to submission.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Launois, C., Barbe, C., Bertin, E. et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med 12 , 61 (2012). https://doi.org/10.1186/1471-2466-12-61
Download citation
Received : 06 April 2012
Accepted : 22 September 2012
Published : 01 October 2012
DOI : https://doi.org/10.1186/1471-2466-12-61
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung function
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Impact of pulmonary rehabilitation on patients with different chronic respiratory diseases during hospitalization
Affiliation.
- 1 Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
- PMID: 38608115
- DOI: 10.1097/MD.0000000000037778
The impact of pulmonary rehabilitation (PR) on patients with different chronic respiratory diseases (CRDs) during hospitalization has not been thoroughly evaluated before. The objectives of the current research were to assess the effect of comprehensive PR management on inpatients' self-management skills, exercise capacity, nutrition assessment and mental health issues and explore whether impacts of PR vary in different CRDs. This retrospective study analyzed the clinical data from 272 inpatients with CRDs receiving PR management during hospitalization between October 2020 and March 2022 in Beijing Chao-Yang Hospital. Significant improvements were found in the patients' ability of daily living (ADL), dyspnea (assessed by modified medical research council dyspnea scale (MMRC)), handgrip strength, maximal inspiratory and expiratory pressure, anxiety (using the 7-item generalized anxiety disorder scale (GAD-7)) and depression (the 9-item patient health questionnaire score (PHQ-9)). There was no significant change in nutrition assessment pre-post PR management during hospitalization. The subgroup analyses were conducted on hospitalized patients with chronic obstructive pulmonary disease (COPD), bronchiectasis, asthma, interstitial lung diseases (ILDs) and other CRDs (e.g., lung cancer, diaphragm hemiparesis, obesity, etc.). The results showed that ADL, MMRC score, MIP, MEP, PHQ-9 score improved in all subgroups with CRDs. Handgrip strength of left hand was increased in COPD inpatients and anxiety was improved in all subgroups except for ILDs. Comprehensive PR management was necessary and beneficial for patients with different CRDs during hospitalization.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

Study of Longidaze in the Prevention & Treatment of Pulmonary Fibrosis, Interstitial Lung Diseases Caused by COVID-19
- Study Details
- Tabular View
- No Results Posted

Change in the degree of dyspnea on the MMRC scale from baseline after 2.5 months and 6 months in patients of the Longidaze® group compared with the dynamic observation group.
MMRC scale (Modified Medical Research Council scale) 0 - no - Dyspnea does not bother, except for very intense exercise
- - mild - Shortness of breath bothers with brisk walking or climbing a small elevation
- - moderate to severe - Shortness of breath results in slower walking compared to other people of the same age, or need to stop while walking at normal pace on a level surface
- - Severe - Shortness of breath makes you stop when walking about 100 m or after a few minutes of walking on a flat surface
- - very severe - Shortness of breath makes it impossible to leave the house or appears when dressing and undressing
Inclusion Criteria:
- Patients with residual lung changes after complicated COVID-19
- Residual changes were detected no later than 2 months after the discharge after disease
- Treatment of COVID-19 was in accordance with the standard of the then current temporary guidelines for the treatment of COVID-19
- Age of patients over 18 years old
- Negative polymerase chain reaction (PCR) test COVID-19 at least 2 times in respiratory samples or based on serology results in blood samples
- Patients in the framework of routine clinical practice, in accordance with the instructions for use before inclusion in the study, were prescribed intramuscular treatment with Longidaze® at a dose of 3000 IU, 1 injection every 5 days for a total course of 15 injections or dynamic observation without the use of active therapy
- The patient did not participate in other drug clinical trials within 1 month prior to Visit 1.
- The patient or patient's caregiver agrees to participate in the trial and sign an informed consent form
- Patient understands and agrees to follow the planned procedures.
- Women with fertile potential must agree to use at least one method of contraception before completing participation in the study.
Exclusion Criteria:
- Women during pregnancy and lactation and women planning to become pregnant during the study period
- Severe background diseases, such as severe heart failure (class IV heart function), severe liver and kidney disease, severe bronchial asthma, severe chronic obstructive pulmonary disease, bronchiectasis, bullous emphysema and previously identified interstitial lung diseases, neurological diseases, tumors.
- Long-term bed rest, regardless of its cause
- Increased individual sensitivity to the components of the studied drug
- Pathological conditions that determine the impossibility of patient participation in the study (by the decision of the investigator)
- Medical history that, according to the investigator, does not allow the patient to be included in the study
- A burdened allergic anamnesis, which, according to the investigator, does not allow the patient to be included in the study
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, risk factors for anxiety and its impacts on acute exacerbation in older patients with chronic obstructive pulmonary disease.

- Department of Pulmonary and Critical Care Medicine, Huadong Hospital, Fudan University, Shanghai, China
Background: Anxiety is common in patients with chronic obstructive pulmonary disease (COPD), especially in older patients with the definition of age over 60 years old. Few studies have focused on anxiety in older COPD patients. This study aimed to analyze the risk factors of anxiety in older COPD patients and the impacts of anxiety on future acute exacerbation.
Methods: The general information, questionnaire data, previous acute exacerbation and pulmonary function were collected. Hamilton Anxiety Rating Scale (HAMA) was used to evaluate the anxiety of older COPD patients. The patients were followed up for one year, the number and the degrees of acute exacerbations of COPD were recorded.
Results: A total of 424 older COPD patients were included in the analysis. 19.81% ( N = 84) had anxiety symptoms, and 80.19% ( N = 340) had no anxiety symptoms. There were increased pack-years, more comorbidities, and more previous acute exacerbations in older COPD patients with anxiety compared to those without anxiety ( P < 0.05). Meanwhile, a higher modified Medical Research Council (mMRC), a higher COPD assessment test (CAT) score and a shorter six-minute walking distance (6MWD) were found in older COPD patients with anxiety ( P < 0.05). The BODE index, mMRC, CAT score, comorbidities and acute exacerbations were associated with anxiety. Eventually, anxiety will increase the risk of future acute exacerbation in older COPD patients (OR = 4.250, 95% CI: 2.369–7.626).
Conclusion: Older COPD patients with anxiety had worsening symptoms, more comorbidities and frequent acute exacerbation. Meanwhile, anxiety may increase the risk of acute exacerbation in the future. Therefore, interventions should be provided to reduce the risk of anxiety in older COPD patients at an early stage.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most frequent respiratory diseases among middle-aged and old individuals, contributing to significant global morbidity and mortality ( 1 ). The annual death toll associated with COPD reaches approximately 3 million, and it is predicted to rise to over 4.5 million by 2030 worldwide ( 2 ). There are currently 99.9 million people with COPD in China, and the prevalence of COPD in people over 40 years old and over 60 years old are 13.7 and 27%, respectively ( 3 ). With the increasing levels of air pollution and aging population, COPD is expected to become the primary economic burden of chronic diseases in the future ( 4 ). Therefore, it is crucial for the society to display special concern on COPD.
Recently, there has been growing attention toward comorbidities in individuals with COPD ( 5 ). Comorbidity prevalence is quite high among COPD patients: more than half have one or two comorbidities; while around 15.8% have three or four comorbidities; additionally, about 6.8% suffer from five or more comorbidities ( 6 ). Anxiety is a common comorbidity observed in individuals with COPD. In the general adult population of China, anxiety was found to have a prevalence of 5.3% according to the Hospital Anxiety and Depression Scale (HADS) and 5.6% based on the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) scale ( 7 , 8 ). The prevalence of anxiety ranged from 10 to 55% for inpatients and 13–46% for outpatients among patients with COPD ( 9 ).
Patients with COPD often experience poor mental health and older COPD patients are more likely to develop mental health especially anxiety ( 10 ). There are many risk factors for anxiety in COPD patients, such as continued smoking, poor knowledge, loneliness, and low social status ( 11 , 12 ). As COPD progressing and age increasing, patients experience increased dyspnea, decreased physical function, and limited physical and social activities which leads to more severe anxiety symptoms ( 13 ). They often faced accelerated health deterioration, increased risk of adverse events, reduced quality of life, and experienced frequent acute exacerbation ( 14 ). However, there have been limited clinical trials conducted in this age group. In this study, we attempted to identify the risk factors of anxiety in older COPD patients and the impacts of anxiety on future acute exacerbation.
2 Materials and methods
2.1 study design and participants.
This study involved 424 older COPD patients who visited pulmonary outpatient clinic at fifteen hospitals in Shanghai from June 2017 to December 2020 (ChiCTR2000030911). All the patients were in a stable condition and randomly admitted. Written informed consent was obtained. The study was approved by the Ethics Committee of Huadong Hospital.
Inclusion criteria were as follows: (1) primary diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria ( 1 ). The forced expiratory volume in the first second of forced vital capacity (FEV1/FVC) < 0.7 after inhaling bronchodilators (BD) confirmed persistent airflow limitation ( 15 ); (2) participants with age ≥ 60 years; (3) signed written informed consent in the study. Exclusion criteria were as follows: (1) history of COPD acute exacerbation within one month prior to enrollment; (2) history of respiratory infection within one month prior to enrollment; (3) mental disorders (e g, schizophrenia, cognitive disorder, senile dementia, or Alzheimer) impairing capacity for informed consent; (4) missing follow-up information.
2.2 Demographic data
All COPD patients were required to complete a structured questionnaire and were given a thorough physical examination. All data were collected by physicians. The frequency and severity of acute exacerbation in the previous year were recorded at the first visit. Patients were followed up for one year. Demographic characteristics and clinical features were recorded. Comorbidity included diseases of respiratory system (asthma, allergic rhinitis, lung cancer, pulmonary embolism and bronchiectasis), cardiovascular system (angina, arrhythmia, hypertension and heart failure), metabolism system (diabetes, osteoporosis and metabolism syndrome), nervous system (stroke, subarachnoid and dementia), digestive system (peptic ulcer, digestive tumor and liver disease) and other diseases like connective tissue disease, peripheral vascular disease, lymphoma, leukemia, and anxiety.
2.3 Assessment of anxiety
The Hamilton Anxiety Rating Scale (HAMA) was widely used to screen anxiety in the general hospital. All participants were assessed by the same physician. All items of the HAMA were scored on a scale of 0–4 points. The HAM-A included 14 items covering two types of symptom factors which were psychic anxiety factors and somatic anxiety factors. The psychic anxiety factors were as follows: anxiety mood, tension, fears, insomnia, difficulties in concentration and memory, depression mood and behaviors during the interview. The somatic anxiety factors included somatic symptoms concerning seven symptoms: muscle, sensory, cardiovascular, respiratory, gastrointestinal, genito-urinary and other autonomic nervous system symptoms. HAMA ≥ 14 was defined as COPD with anxiety ( 16 ).
2.4 Definition of acute exacerbation
An acute exacerbation of chronic obstructive pulmonary disease (AECOPD) defines as an acute worsening of respiratory symptoms that result in additional therapy ( 1 ). Exacerbation events are classified as mild [treated with short acting bronchodilators (SABDs) only], moderate (relieved by SABDs plus antibiotics, with or without oral corticosteroids), or severe (refer to acute exacerbation requiring hospitalization, emergency admission or ICU transferring) ( 17 ). The number of total exacerbations, mild, moderate, or severe exacerbations in the previous year and in the following-up one year were documented.
2.5 Assessment of pulmonary function
Spirometry was obtained from a Jaeger Toennies spirometer (Höchberg, Germany) according to the American Thoracic Society (ATS) guidelines ( 1 ). Each patient completed the spirometry test and bronchodilator reversibility test (BDR). The parameters including FEV1/predicted post BD, FEV1/FVC post BD and residual volume/total lung capacity (RV/TLC) % were recorded. The spirometry tests were performed by professional technicians and the results were interpreted by two physicians. COPD severity was evaluated according to the severity of airflow obstruction. GOLD1: FEV1 ≥ 80% predicted means mild; GOLD2: 50% ≤ FEV1 < 80% predicted means moderate; GOLD3: 30% ≤ FEV1 < 50% predicted means severe; GOLD4: FEV1 ≤ 30% predicted means very severe.
2.6 Assessment of COPD symptoms and health-related quality of life
The BODE index, a multidimensional grading system, is based on the body-mass index (B), the degree of airflow obstruction (O) evaluated by FEV1, the grade of dyspnea (D) assessed by the modified Medical Research Council (mMRC) dyspnea score, and the exercise capacity (E) assessed by the six-minute walking distance test (6MWD). The total scores of the BODE index ranged from 0 to 10 points (higher scores indicated more severity). The BODE index predicted death and other poor outcomes in COPD ( 18 ). mMRC dyspnea score was used to estimate the impact of dyspnea in everyday activities. The COPD assessment test (CAT) and St. George’s respiratory questionnaire (SGRQ) were used to evaluate health-related quality of life (HRQL) ( 19 ). 6MWD was carried out to evaluate exercise capacity of COPD patients ( 20 ). The evaluation was done by professional physicians.
2.7 Statistical analyses
All statistical analyses were performed by a commercially software program (SPSS 22.0 for Windows; SPSS, Chicago, IL, USA). Continuous variates were presented as mean ± standard deviation for the normally distributed data or median (25th and 75th percentile) for the non-normally distributed data, while categorical variates were presented as n or n (%). Student’s t -test was used for normally distributed data, while the Mann Whitney u test was used for non-normally distributed data. The categorical variates were analyzed by chi-square test. We used Logistic regression to evaluate risk factors of anxiety in COPD patients. We used Logistic regression and Poisson regression to predict the effect of anxiety on future exacerbation. P < 0.05 was considered statistically significant.
3.1 General characteristics of older COPD patients
A total of 424 older COPD patients were included to analyze the relation between anxiety and its associated factors in older COPD patients. There were 380 (89.60%) males and 44 females (10.40%) with a median age of 70 years. The median pack-years were 30 (15–50). The median FEV1/predict post BD was 31.30 (28.00–52.40) %. The median score on SGRQ was 36 (31.00–51.75), while the median 6MWD (m) was 310 (295.50–340.00). Among the 424 patients, 86.79% had one or more comorbidities, and 56.13% had at least one exacerbation in the previous one year ( Table 1 ).
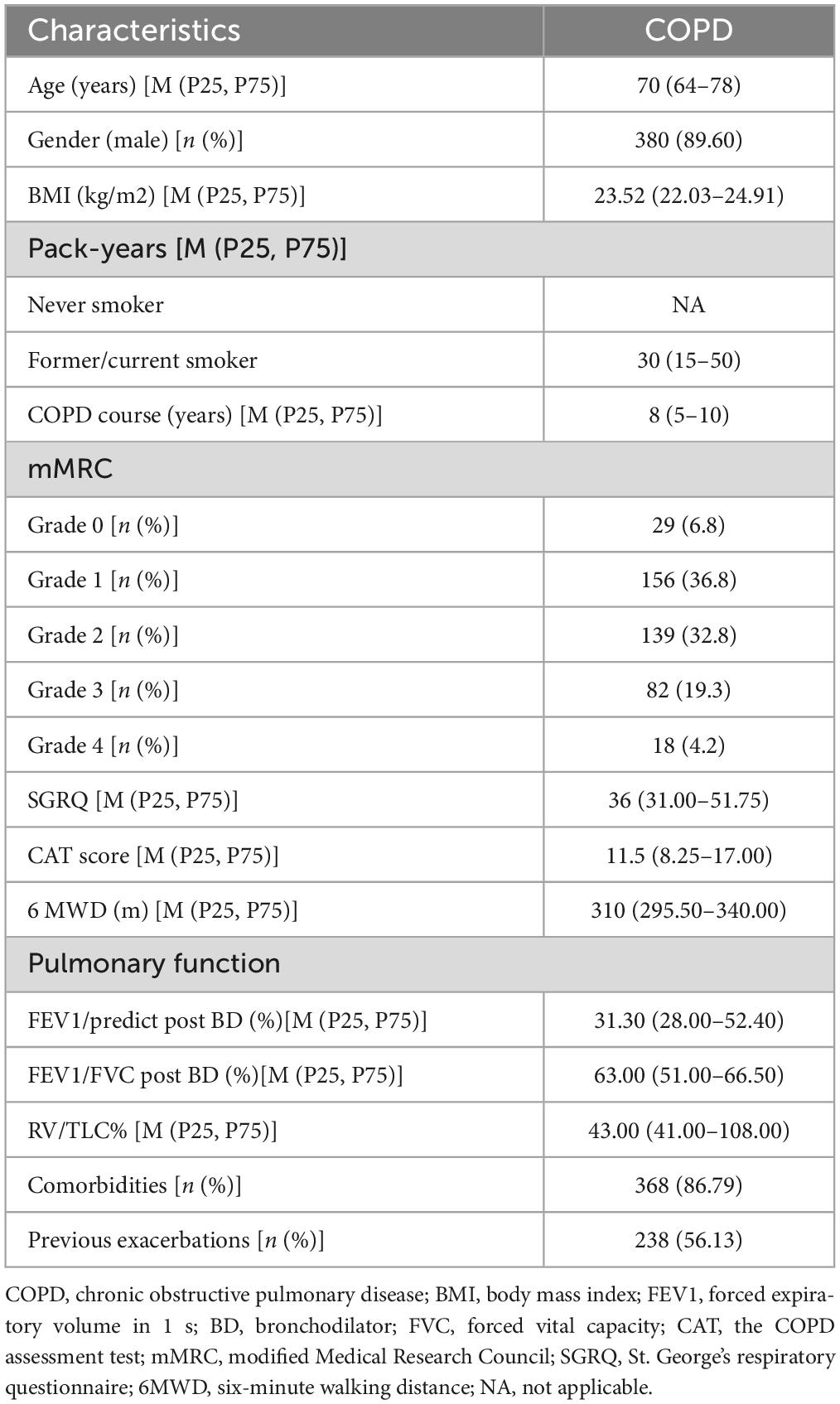
Table 1. Baseline characteristics of the 424 subjects.
3.2 Comparison of baseline data between older COPD patients with anxiety and older COPD patients without anxiety
Older COPD patients in our analysis were divided into two groups ( Table 2 ). Group 1, Older COPD patients with anxiety; Group 2, Older COPD patients without anxiety. Table 2 showed the baseline data and comparisons between the two groups. Group 1 included 84 older COPD patients with anxiety: 76 (90.48%) males and 8 females (9.52%) were included, while the average age was 68 (63–77) years. Group 2 consisted of 340 older COPD patients without anxiety: 304 (89.41%) males and 36 females (10.59%) were included, while the average age was 70 (65–78) years. There were increased pack-years, more comorbidities, and more acute exacerbations in older COPD patients with anxiety. They were statistically different. There were statistically differences in mMRC, CAT score and 6MWD between the two groups. However, there were no statistically differences in gender, BMI, COPD courses, SGRQ score between the two groups.
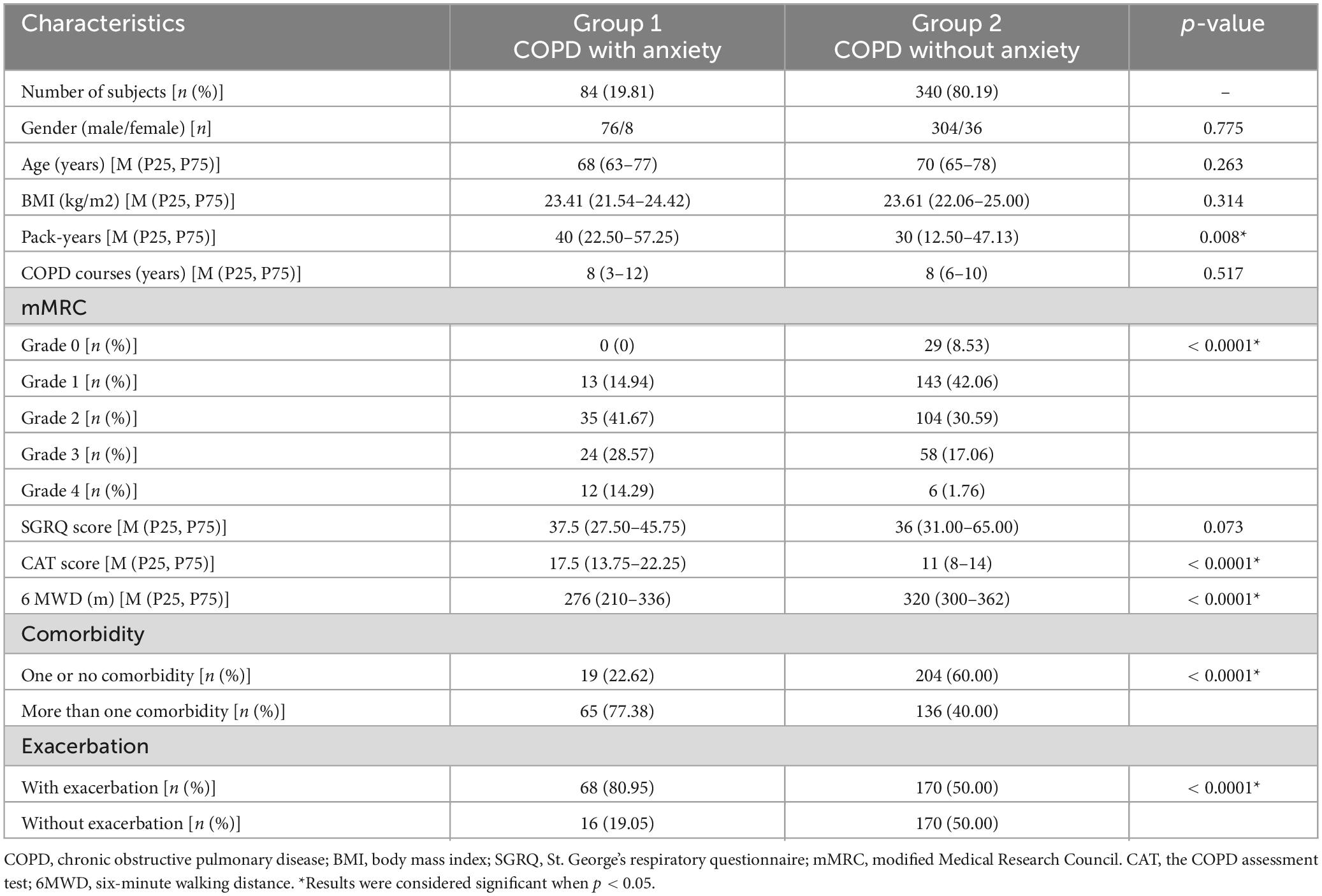
Table 2. Comparison of baseline data between COPD with anxiety and COPD without anxiety.
3.3 Possible factors of anxiety in terms of symptoms, disease severity, and exercise capacity
Higher COPD severity evaluated by BODE index was associated with a higher risk of anxiety in older COPD patients. Degree of dyspnea, evaluated by mMRC had association with the risk of anxiety. The higher the CAT score, the higher the risk of anxiety. CAT score in severe and very severe COPD was 3.547 times of that in mild and moderate COPD. However, 6MWD were not related to anxiety ( Table 3 ).
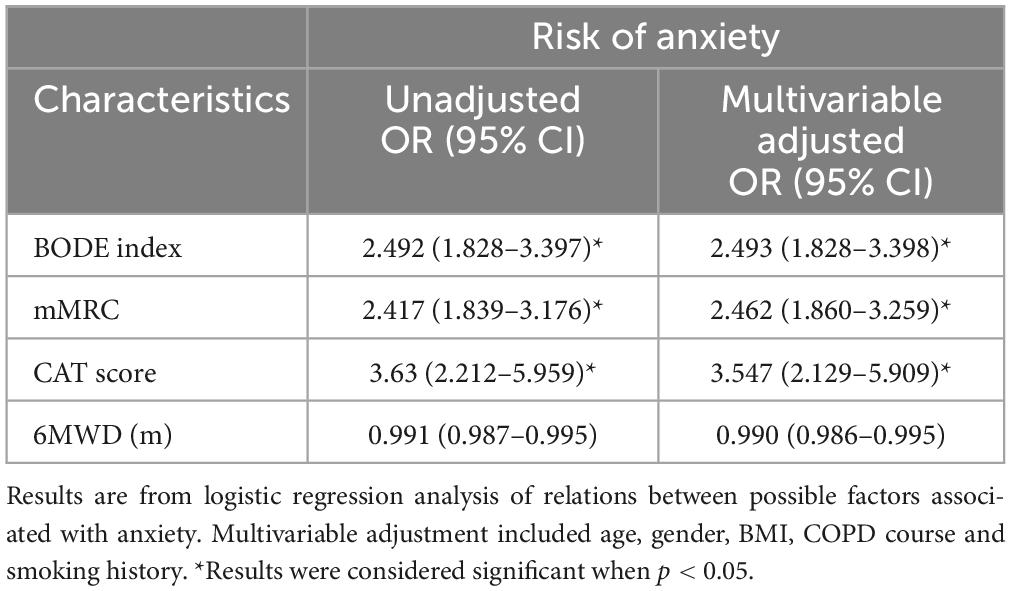
Table 3. Possible factors associated with anxiety in older COPD patients.
3.4 Related factors of anxiety in terms of comorbidities and acute exacerbations
Older COPD patients with more than one comorbidity had greater risk of anxiety than those with none or one comorbidity with the odds ratio of 5.671 (95% CI: 3.193–10.07). Compared with older COPD patients without acute exacerbation in the previous year, the odds ratio of anxiety in older COPD patients with acute exacerbation was 4.004 (95% CI: 2.204–2.273) ( Table 4 ).
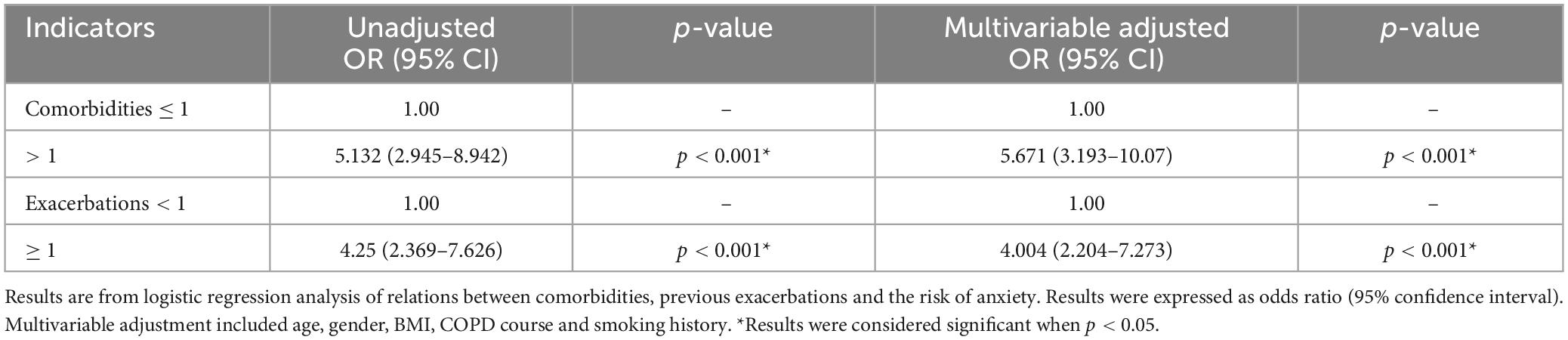
Table 4. Relations between comorbidities and exacerbations and anxiety in older COPD patients.
3.5 Anxiety associated with the increased risk of future acute exacerbation
Anxiety was associated with increased risk of various degrees of future exacerbation from the aspect of both incidence and frequency. Unadjusted odds ratio (95% CI) of future exacerbation for older COPD patients with anxiety was 4.250 (2.369–7.626) compared to those without anxiety. Corresponding unadjusted odds ratios (95% CI) were 2.653 (1.526–4.611) and 2.006 (1.221–3.297) for moderate and severe exacerbations in one year ( Table 5 ). Meanwhile, Unadjusted incidence-rate ratio (95% CI) for total acute exacerbation was 2.000 (1.572–2.545) in older COPD patients with anxiety compared to those without anxiety. Corresponding incidence-rate ratios (95% CI) were 2.285 (1.422–3.669) and 2.080 (1.350–3.203) for moderate and severe acute exacerbations within one year ( Table 6 ).
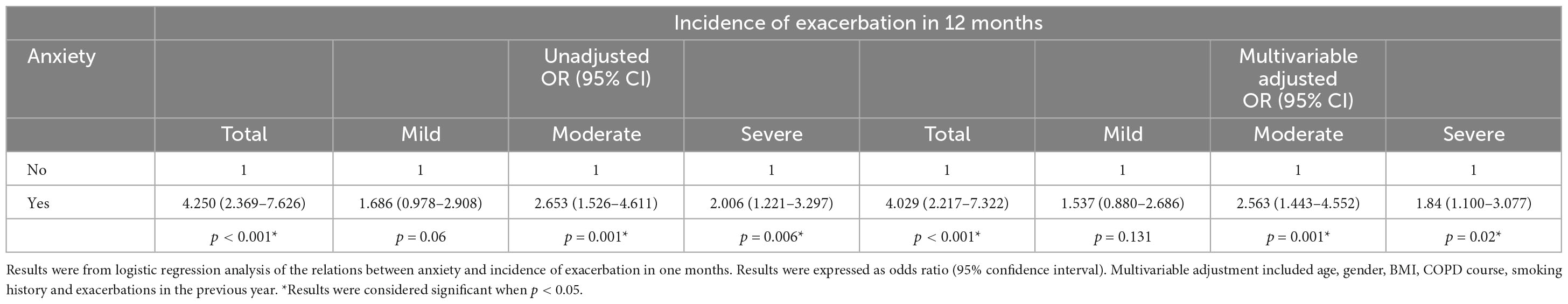
Table 5. Anxiety in relation to incidence of different levels of acute exacerbations in COPD.
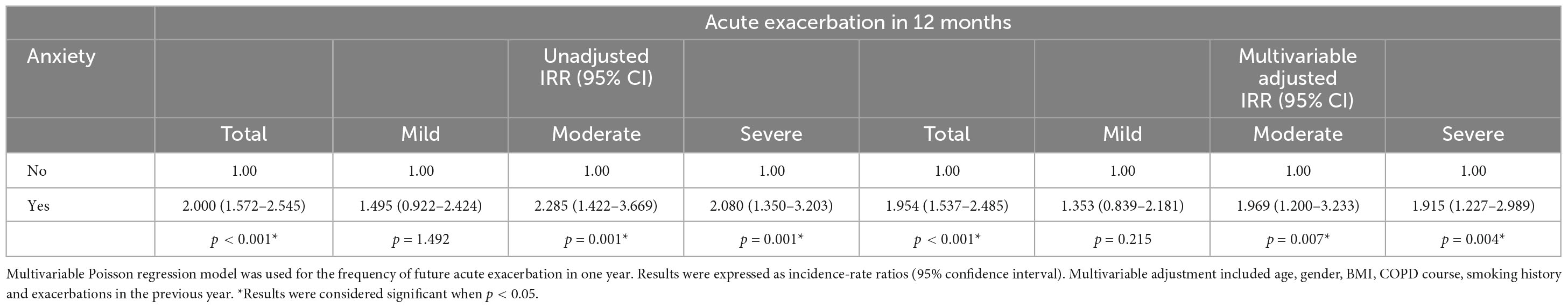
Table 6. Anxiety in relation to frequency of different levels of acute exacerbations in COPD.
Multivariable adjusted odds ratio (95% CI) of future acute exacerbation in older COPD patients with anxiety was 4.029 (2.217–7.322) compared to those without anxiety. Multivariable adjusted odds ratios (95% CI) were 2.563 (1.443–4.552) and 1.84 (1.100–3.077) for moderate and severe acute exacerbations in one year ( Table 5 ). Meanwhile, Multivariable adjusted incidence-rate ratio (95% CI) for total acute exacerbation was 1.954 (1.537–2.485) in older COPD patients with anxiety compared to those without anxiety. Corresponding incidence-rate ratios (95% CI) were 1.969 (1.200–3.233) and 1.915 (1.227–2.989) for moderate and severe acute exacerbations within one year after additional adjustment for potential confounders ( Table 6 ).
4 Discussion
Chronic obstructive pulmonary disease (COPD) usually coexists with various comorbidities. Anxiety, an important comorbidity of COPD, is frequently under-diagnosed and significantly impacts the prognosis of COPD patients, especially in older COPD patients.
In our analysis, the prevalence of anxiety in older COPD patients was 19.81%. However, different studies had reported various prevalence rates. For instance, a cross-sectional study conducted in Shanghai included 275 mild COPD patients from urban communities and found that 7.6% had anxiety ( 21 ). Another study evaluated 491 Chinese COPD patients by Hospital Anxiety and Depression Scale (HADS) and reported an anxiety prevalence rate of 10% ( 22 ). The China Pulmonary Health Study (CPH) revealed that anxiety affected approximately 10.79% COPD patients ( 23 ). This discrepancy could be attributed to differences sample size, methodological design, participant sources, screening instruments, and severity levels of COPD ( 24 ).
Chronic obstructive pulmonary disease (COPD) primarily affected older populations and exhibited male predominance; this trend was also evident in our cohort where there were more male participants. However, no gender differences were observed between the two groups. Our findings indicated that COPD patients with anxiety tended to have higher pack-years, have greater comorbidities, and experience more frequent exacerbations. Additionally, COPD patients with anxiety exhibited higher levels of dyspnea (mMRC), worse health status (CAT score), and less exercise capacity (6MWD). Our analysis indicated that the BODE index, mMRC score and CAT score were associated with anxiety ( P < 0.05).
It is worth noting that anxiety negatively impacts COPD. On one hand, the symptoms of COPD, such as gradually increasing dyspnea, cough, and expectoration, may be the main cause of anxiety in COPD patients ( 25 ). On the other hand, other comorbidities such as lung cancer, cardiovascular disease, and gastroesophageal reflux disease contribute to the occurrence of anxiety in COPD patients. Our study revealed that an increased comorbidities was associated with a higher risk of anxiety (OR 5.671; 95% CI: 3.193–10.07). Acute exacerbation of COPD is associated with increased mortality rate ( 26 , 27 ). 25% of patients experiencing acute exacerbation was required for ICU admission, further increasing the economic burden of COPD ( 28 ). Additionally, frequent acute exacerbation severely worsened patients’ quality of life. A previous study conducted by our group identified that anxiety, angina, and hypertension were independent risk factors for acute exacerbation within a year ( 29 , 30 ). In our study, we found that acute exacerbation in the previous year were related to anxiety and increased the risk of anxiety (OR 4.004; 95% CI: 2.204–7.273). We also discovered that older COPD patients with anxiety increased the risk of future exacerbation in one year, especially moderate and severe acute exacerbation compared to those without anxiety.
Older COPD patients with anxiety have worse dyspnea symptoms, more comorbidities, and experience more frequent acute exacerbations. Therefore, early diagnosis of COPD with anxiety is very important. However, the current scales for the diagnosis and assessment of anxiety are professional and complex. Fortunately, respiratory physicians are sensitive to clinical indicators. If there was a possibility that respiratory physicians can evaluate COPD patients with anxiety through clinical indicators, they would transfer them to psychologists as soon as possible for further treatment including psychotherapy, medications, and exercise. That would improve treatment compliance, improve symptoms, and reduce acute exacerbations of older COPD with anxiety.
There are some limitations to consider regarding our study findings. Firstly, the data on acute exacerbations were obtained from the medical records of COPD patients. Considering that some patients may have sought treatment from other hospitals during acute exacerbation episodes, there was a possibility of underreporting the frequency of acute exacerbations. Additionally, it should be noted that different assessment tools for evaluating anxiety may yield different results. In our study, HAMA was used to assess anxiety in older COPD patients.
5 Conclusion
In summary, our study found that older COPD patients with anxiety had worse symptoms, more comorbidities and more frequent. In addition, our study also found anxiety can increase the risk of future acute exacerbation in older COPD patients. In COPD management, routine screening for psychiatric symptoms should be an integral part of clinical work to reduce the risk of anxiety in older COPD patients at an early stage.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethic Committee of Huadong Hospital (protocol code 20180064). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YM: Methodology, Project administration, Writing – original draft, Writing – review and editing. LS: Methodology, Investigation, Writing – review and editing. YL: Software, Validation, Writing – original draft. YW: Data curation, Resources, Writing – review and editing. ZH: Methodology, Software, Writing – review and editing. XL: Validation, Writing – review and editing. HZ: Visualization, Writing – review and editing. HG: Project administration, Supervision, Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (2020YFC2009001), Scientific Research Project of Shanghai Science and Technology Commission (2022XD030, 22Y11901200, and 21140902500), Scientific Research Project of Shanghai Municipal Health Commission (202140036), Shanghai Municipal Key Clinical Specialty (shslczdzk02801), Bethune Research and Development Fund Project (BJ-RW2020002J), Investigator-initiated clinical trials Foundation of Huadong Hospital (HDLC2022018, ZDXK2216, ZDZB2226, and JYRC202209), and Shanghai Health System Young Talent Fund Project Hengjie-Special Support Program (2022-020).

Acknowledgments
We thank the chronic obstructive pulmonary disease (COPD) patients who participated in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor ZC declared a shared parent affiliation with the authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Global Initiative for Chronic Obstructive Lung Disease. GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD: 2024 Report. (2024). Available online at: https://goldcopd.org/2024-gold-report/ (accessed November 17, 2023).
Google Scholar
2. Chuchalin A, Avdeev S, Aysanov Z, Belevskiy A, Leshchenko I, Meshcheryakova N, et al. RUSSIAN RESPIRATORY SOCIETY. FEDERAL GUIDELINES ON DIAGNOSIS AND TREATMENT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE. Pulmonologiâ (Mosk). (2020) 3:15–54. doi: 10.2147/COPD.S153770
PubMed Abstract | Crossref Full Text | Google Scholar
3. Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): A national cross-sectional study. Lancet. (2018) 391:1706–17.
4. Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: A systematic review. Int J Chron Obstruct Pulmon Dis. (2018) 13:1353–64. doi: 10.2147/COPD.S161555
5. Barnes P. Senescence in COPD and its comorbidities. Annu Rev Physiol. (2017) 79:517–39.
6. Cavaillès A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, et al. Comorbidities of COPD. Eur Respir Rev. (2013) 22:454–75.
7. Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Prevalence and correlations with depression, anxiety, and other features in outpatients with chronic obstructive pulmonary disease in China: A cross-sectional case control study. BMC Pulm Med. (2012) 12:53. doi: 10.1186/1471-2466-12-53
8. Phillips M, Zhang J, Shi Q, Song Z, Ding Z, Pang S, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: An epidemiological survey. Lancet. (2009) 373:2041–53. doi: 10.1016/S0140-6736(09)60660-7
9. Willgoss T, Yohannes A. Anxiety disorders in patients with COPD: A systematic review. Respir Care. (2013) 58:858–66.
10. Kunik M, Roundy K, Veazey C, Souchek J, Richardson P, Wray N, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. (2005) 127:1205–11.
11. Zhao X, Liu G, Liu D, Zou L, Huang Q, Chen M, et al. Clinical and economic burden of anxiety/depression among older adult COPD patients: Evidence from the COPD-AD China registry study. Front Psychiatry. (2024) 14:1221767. doi: 10.3389/fpsyt.2023.1221767
12. Zhang Q, Liao J, Liao X, Wu X, Wan M, Wang C, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: A cross-sectional study. BMC Pulm Med. (2014) 14:92.
13. Cleland J, Lee A, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. (2007) 24:217–23.
14. Gudmundsson G, Nagorni-Obradovic L, Vukovic D. The prevalence of COPD co-morbidities in Serbia: Results of a national survey. NPJ Prim Care Respir Med. (2014) 24:14008. doi: 10.1038/npjpcrm.2014.8
15. Long J, Ouyang Y, Duan H, Xiang Z, Ma H, Ju M, et al. Multiple factor analysis of depression and/or anxiety in patients with acute exacerbation chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:1449–64.
16. Labaki W, Rosenberg S. Chronic obstructive pulmonary disease. Ann Intern Med. (2020) 173:Itc17–32.
17. Celli B, Fabbri L, Aaron S, Agusti A, Brook R, Criner GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: The rome proposal. Am J Respir Crit Care. (2021) 204:1251–8.
18. Celli B, Cote C, Marin J, Casanova C, Montes de Oca M, Mendez R, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. New Engl J Med. (2004) 350:1005–12.
19. Jones P, Harding G, Berry P, Wiklund I, Chen W, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. (2009) 34:648–54.
20. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care. (2002) 166:111–7.
21. Xiao T, Qiu H, Chen Y, Zhou X, Wu K, Ruan X, et al. Prevalence of anxiety and depression symptoms and their associated factors in mild COPD patients from community settings, Shanghai, China: A cross-sectional study. BMC Psychiatry. (2018) 18:89. doi: 10.1186/s12888-018-1671-5
22. Xu W, Collet J, Shapiro S, Lin Y, Yang T, Platt R, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care. (2008) 178:913–20.
23. Huang K, Huang K, Xu J, Yang L, Zhao J, Zhang X, et al. Anxiety and depression in patients with chronic obstructive pulmonary disease in China: Results from the China pulmonary health [CPH] study. Int J Chron Obstruct Pulmon Dis. (2021) 16:3387–96.
24. Zareifopoulos N, Bellou A, Spiropoulou A, Spiropoulos K. Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: A narrative review. COPD. (2019) 16:406–17. doi: 10.1080/15412555.2019.1679102
25. Hill K, Geist R, Goldstein R, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. (2008) 31:667–77.
26. Hu G, Zhou Y, Wu Y, Yu Y, Liang W, Ran P. The pneumonia severity index as a predictor of in-hospital mortality in acute exacerbation of chronic obstructive pulmonary disease. PLoS One. (2015) 10:e0133160. doi: 10.1371/journal.pone.0133160
27. Ho T, Tsai Y, Ruan S, Huang C, Lai F, Yu C. In-hospital and one-year mortality and their predictors in patients hospitalized for first-ever chronic obstructive pulmonary disease exacerbations: A nationwide population-based study. PLoS One. (2014) 9:e114866. doi: 10.1371/journal.pone.0114866
28. Ai-Ping C, Lee K, Lim T. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: A retrospective study. Respir Med. (2006) 1:104.
29. Ge H, Liu X, Gu W, Feng X, Zhang F, Han F, et al. Distribution of COPD comorbidities and creation of acute exacerbation risk score: Results from SCICP. J Inflamm Res. (2021) 14:3335–48. doi: 10.2147/JIR.S315600
30. Graham B, Steenbruggen I, Miller M, Barjaktarevic I, Cooper B, Hall G, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
Keywords : COPD, anxiety, Hamilton Anxiety Rating Scale (HAMA), comorbidity, acute exacerbation
Citation: Mou Y, Shan L, Liu Y, Wang Y, He Z, Li X, Zhu H and Ge H (2024) Risk factors for anxiety and its impacts on acute exacerbation in older patients with chronic obstructive pulmonary disease. Front. Med. 11:1340182. doi: 10.3389/fmed.2024.1340182
Received: 17 November 2023; Accepted: 20 March 2024; Published: 05 April 2024.
Reviewed by:
Copyright © 2024 Mou, Shan, Liu, Wang, He, Li, Zhu and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Ge, [email protected]
† These authors have contributed equally to this work
This article is part of the Research Topic
Women in Science - Pulmonary Medicine 2023
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Prognostic indicators and outcomes of hospitalised COVID-19 patients with neurological disease: An individual patient data meta-analysis
Contributed equally to this work with: Bhagteshwar Singh, Suzannah Lant
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom, Department of Infectious Diseases, Christian Medical College, Vellore, India
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom
Affiliation Department of Health Data Science, University of Liverpool, Liverpool, United Kingdom
Roles Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing
Roles Investigation, Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Queen Square Institute of Neurology, University College London, London, United Kingdom
Roles Writing – review & editing
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Department of Neurology, Wayne State University, Detroit, Michigan, United States of America
Affiliation Instituto de Medicina Tropical, Universidade de São Paulo, São Paulo, Brazil
Affiliation Department of Medicine, King Saud University, Riyadh, Saudi Arabia
Affiliation Department of Clinical and Experimental Sciences, Neurology Unit, University of Brescia, Brescia, Italy
Affiliation Bangur Institute of Neurosciences, Institute of Post-Graduate Medical Education and Research, Kolkata, India
Roles Project administration, Writing – review & editing
Roles Data curation, Investigation, Writing – review & editing
Affiliation Homerton University Hospital NHS Foundation Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Neurovirology, National Institute of Mental Health and Neurosciences, Bangalore, India
Affiliation Department of Infection, Manchester University NHS Foundation Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Neurology Unit, Neuromotor & Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Affiliation Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Virology, UK Health Security Agency, Manchester University NHS Foundation Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Barts Health NHS Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Department of Infectious Diseases & Tropical Medicine, North Manchester General Hospital, Manchester University Foundation NHS Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Warrington Hospital, Warrington and Halton Teaching Hospitals NHS Foundation Trust, Warrington, United Kingdom of Great Britain and Northern Ireland
Affiliation Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi
Affiliation Kingston Hospital NHS Foundation Trust, Kingston upon Thames, United Kingdom of Great Britain and Northern Ireland
Affiliation Institute of Clinical Neurosciences, University of Bristol, Bristol, United Kingdom of Great Britain and Northern Ireland
Affiliation North Manchester General Hospital, Manchester University Foundation NHS Trust, Manchester, United Kingdom of Great Britain and Northern Ireland
Affiliation Epsom and St Helier University Hospitals NHS Foundation Trust, United Kingdom of Great Britain and Northern Ireland
Affiliation King’s College Hospital NHS Foundation Trust, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Regional Infectious Diseases Unit, NHS Lothian, Edinburgh, United Kingdom of Great Britain and Northern Ireland
Affiliation Yerevan State Medical University named after Mkhitar Heratsi, Neuroscience Laboratory, Cobrain Center, Yerevan, Armenia
Affiliation St Vincent’s Hospital, Sydney, Australia
Affiliation Saint-Luc University Hospital, Brussels, Belgium
Affiliation Université de Mons, Mons, Belgium
Affiliation AZ Glorieux, Ronse, Belgium
Affiliation Hospital da Restauração, Recife, Brazil
Affiliation Hospital Federal dos Servidores do Estado, Rio de Janeiro, Brazil
Affiliation Hospital Dr. Sótero del Río, Santiago, Chile
Affiliation Universidad de Chile - Hospital Barros Luco Trudeau, Santiago, Chile
Affiliation The 940th Hospital of Joint Logistic Support Force of the People’s Liberation Army, Lanzhou, China
Affiliation Cairo University Hospital, Cairo, Egypt
Affiliation Kasr Alainy Teaching Hospital, Cairo, Egypt
Affiliation Mataria Teaching Hospital, Cairo, Egypt
Affiliation Fondation Rothschild, Paris, France
Affiliation Pitié Salpetriere Hospital, Paris, France
Affiliation Rennes University Hospital, Rennes, France
Affiliation Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Affiliation Children’s Hospital, Dresden Municipal Hospital Teaching Hospital TUD, Dresden, Germany
Affiliation Medical Center University of Freiburg, Freiburg, Germany
Affiliation Department of Neurology, Technical University of Munich, Munich, Germany
Affiliation Mazandaran University of Medical Science, Sari, Islamic Republic of Iran
Affiliation Institute for Research in Fundamental Sciences (IPM), Tehran, Islamic Republic of Iran
Affiliation Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences, Tehran, Iran
Affiliation Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy
Affiliation University of Brescia, Brescia, Italy
Affiliation San Gerardo Hospital ASST Monza, University of Milano Bicocca, Monza, Italy
Affiliation Fondazione Mondino IRCCS, Pavia, Italy
Affiliation Santa Maria delle Croci Hospital, AUSL Romagna, Ravenna, Italy
Affiliation Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Affiliation University Hospital of Rome Tor Vergata, Rome, Italy
Affiliation Healthcare Trust of the Autonomous Region of Trento, Rovereto, Italy
Affiliation Città della Salute e della Scienza di Torino, Regina Margherita Children’s Hospital, Turin, Italy
Affiliation University of Verona, Verona, Italy
Affiliation Halcyon Healthcare Limited, Nairobi, Kenya
Affiliation Hôpitaux Robert Schuman, Luxembourg, Luxembourg
Affiliation Leiden University Medical Center, Leiden, Netherlands
Affiliation Hospital Regional Docente de Trujillo, Trujillo, Peru
Affiliation Centro Hospitalar São João, Porto, Portugal
Affiliation Centro Hospitalar Universitário do Porto, Porto, Portugal
Affiliation Buyanov Moscow City Hospital, Moscow, Russian Federation
Affiliation Moscow Research and Clinical Center for Neuropsychiatry and Buyanov Moscow City Hospital, Moscow, Russian Federation
Affiliation National Neuroscience Institute, Singapore, Singapore
Affiliation Complejo Hospitalario Universitario de Albacete, Albacete, Spain
Affiliation Hospital Universitario Virgen de las Nieves, Granada, Spain
Affiliation University Hospital Sanchinarro, Madrid, Spain
Affiliation University Hospital Ramón y Cajal, Madrid, Spain
Affiliation Hospital Virgen de la Salud, Toledo, Spain
Affiliation Hospital del Río Hortega, Valladolid, Spain
Affiliation Hopitaux Universitaires de Genève, Geneva, Switzerland
Affiliation Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
Affiliation Acibadem Mehmet Ali Aydinlar University Medical School, Istanbul, Turkey
Affiliation Ulster Hospital, Belfast, United Kingdom of Great Britain and Northern Ireland
Affiliation University of Bristol and North Bristol NHS Trust, Bristol, United Kingdom of Great Britain and Northern Ireland
Affiliation Gloucestershire Royal Hospital, Gloucester, United Kingdom of Great Britain and Northern Ireland
Affiliation Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
Affiliation The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
Affiliation Great Ormond Street Hospital for Children, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Imperial College London, London, United Kingdom of Great Britain and Northern Ireland
Affiliation The National Hospital for Neurology & Neurosurgery, London, United Kingdom of Great Britain and Northern Ireland
Affiliation University College London, London, United Kingdom of Great Britain and Northern Ireland
Affiliation University College London Queen Square Institute of Neurology, London, United Kingdom of Great Britain and Northern Ireland
Affiliation Eastern Pathology Alliance Department of Microbiology, Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, United Kingdom of Great Britain and Northern Ireland
Affiliation Sheffield Teaching Hospitals Trust, Sheffield, United Kingdom of Great Britain and Northern Ireland
Affiliation Wessex Neurological Centre, Southampton, United Kingdom of Great Britain and Northern Ireland
Affiliation Emory University School of Medicine, Atlanta, Georgia, United States of America
Affiliation Massachusetts General Hospital / Harvard Medical School, Boston, Massachusetts, United States of America
Affiliation Yale New Haven Health Bridgeport Hospital, Bridgeport, Connecticut, United States of America
Affiliation Rush University Medical Center, Chicago, Illinois, United States of America
Affiliation University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States of America
Affiliation Children’s Hospital Los Angeles and Keck School of Medicine at the University of Southern California, Los Angeles, California, United States of America
Affiliation Ochsner Medical Center, New Orleans, Los Angeles, United States of America
Affiliation Columbia University Irving Medical Center, New York, New York, United States of America
Affiliation New York University Grossman School of Medicine, New York, New York, United States of America
Affiliation Department of Neurology, National Neuroscience Institute, Singapore, Singapore
Affiliation Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom
Affiliation Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, United States of America
Roles Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
¶ Membership of The Brain Infections Global COVID-Neuro Network Study Group is provided in S1 Appendix .
¶ ‡ CTS and TS also contributed equally to this work.
- [ ... ],
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom, Tropical and Infectious Diseases Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
- [ view all ]
- [ view less ]
- Bhagteshwar Singh,
- Suzannah Lant,
- Sofia Cividini,
- Jonathan W. S. Cattrall,
- Lynsey C. Goodwin,
- Laura Benjamin,
- Benedict D. Michael,
- Ayaz Khawaja,
- Aline de Moura Brasil Matos,

- Published: June 2, 2022
- https://doi.org/10.1371/journal.pone.0263595
- Reader Comments
Neurological COVID-19 disease has been reported widely, but published studies often lack information on neurological outcomes and prognostic risk factors. We aimed to describe the spectrum of neurological disease in hospitalised COVID-19 patients; characterise clinical outcomes; and investigate factors associated with a poor outcome.
We conducted an individual patient data (IPD) meta-analysis of hospitalised patients with neurological COVID-19 disease, using standard case definitions. We invited authors of studies from the first pandemic wave, plus clinicians in the Global COVID-Neuro Network with unpublished data, to contribute. We analysed features associated with poor outcome (moderate to severe disability or death, 3 to 6 on the modified Rankin Scale) using multivariable models.
We included 83 studies (31 unpublished) providing IPD for 1979 patients with COVID-19 and acute new-onset neurological disease. Encephalopathy (978 [49%] patients) and cerebrovascular events (506 [26%]) were the most common diagnoses. Respiratory and systemic symptoms preceded neurological features in 93% of patients; one third developed neurological disease after hospital admission. A poor outcome was more common in patients with cerebrovascular events (76% [95% CI 67–82]), than encephalopathy (54% [42–65]). Intensive care use was high (38% [35–41]) overall, and also greater in the cerebrovascular patients. In the cerebrovascular, but not encephalopathic patients, risk factors for poor outcome included breathlessness on admission and elevated D-dimer. Overall, 30-day mortality was 30% [27–32]. The hazard of death was comparatively lower for patients in the WHO European region.
Interpretation
Neurological COVID-19 disease poses a considerable burden in terms of disease outcomes and use of hospital resources from prolonged intensive care and inpatient admission; preliminary data suggest these may differ according to WHO regions and country income levels. The different risk factors for encephalopathy and stroke suggest different disease mechanisms which may be amenable to intervention, especially in those who develop neurological symptoms after hospital admission.
Citation: Singh B, Lant S, Cividini S, Cattrall JWS, Goodwin LC, Benjamin L, et al. (2022) Prognostic indicators and outcomes of hospitalised COVID-19 patients with neurological disease: An individual patient data meta-analysis. PLoS ONE 17(6): e0263595. https://doi.org/10.1371/journal.pone.0263595
Editor: Patricia T. Bozza, Fundacao Oswaldo Cruz, BRAZIL
Received: October 14, 2021; Accepted: January 21, 2022; Published: June 2, 2022
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The study was funded by the UK Medical Research Council’s Global Effort on COVID-19 Programme (MR/V033441/1) ( https://mrc.ukri.org/ ); UK National Institute for Health Research (NIHR)- funded Global Health Research Group on Acute Brain Infections (17/63/110) ( https://www.nihr.ac.uk/ ); and the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections (NIHR200907), at University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford (Grant Nos. IS-HPU-1112-10117 and NIHR200907). These grants were awarded to TS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: TS is part of the Data Safety Monitoring Committee of a study to evaluate the safety and immunogenicity of a candidate Ebola Vaccine in children - the GSK3390107A (ChAd3 EBO-Z) vaccine; he is a panel member of Covid-19 Vaccine Benefit Risk Expert Working Group for the Medicines and Healthcare Regulatory Agency (UK); he is a member of COVID-19 Therapeutics Advisory Panel for the UK Department of Health & Social Care; he is the Chair/Co-Chair of the COVID-19 Rapid Response and Rolling Funding Initiatives, which supported the development of the Oxford-AstraZeneca Covid-19 vaccine. In addition, Dr. Solomon has a diagnostic test for bacterial meningitis, based on a blood test, filed for patent pending.
Introduction
Since the first reported patients in December 2019, the COVID-19 pandemic has spread globally to cause more than 225 million cases, with over 4.5 million deaths [ 1 ]. SARS-CoV-2 virus principally causes respiratory disease, although neurological manifestations were also reported from early in the pandemic, including acute cerebrovascular events, other central and peripheral nervous system disease [ 2 ]. There have now been many such reports, but their use of standardised case definitions, detailed clinical and diagnostic evaluation has varied, making comparisons difficult; clinical outcomes and prognostic factors are often not well characterised. Several meta-analyses have also been published [ 3 – 8 ], but given they are based on these original reports, drawing firm conclusions is challenging. In July 2020 we published standardised case definitions for neurological COVID-19 disease [ 2 ], including an assessment of the strength of evidence for their association with SARS-CoV-2 infection, which are being used increasingly [ 2 , 9 – 11 ]. Using this framework and related data tools, we have now conducted an individual patient data (IPD) meta-analysis of patients with neurological COVID-19 disease from the global first wave. We aimed to firstly describe the spectrum of neurological disease in hospitalised COVID-19 patients using a uniform approach with standardised case definitions; secondly, characterise clinical outcomes; thirdly, investigate factors associated with a poor outcome; and finally, define how frequently acute neurological disease was observed as a proportion of all hospitalised COVID-19 patients. The protocol was registered prospectively on the PROSPERO registry (CRD42020196542).
Search strategy and selection criteria
We searched the following sources for articles published between 1st January 2020 and 3rd July 2020, without language restrictions: PubMed and Scopus; the preprint servers medRxiv and SSRN (Social Science Research Network); and the Brain Infections Global COVID-Neuro Resource and the Journal of Neurology, Neurosurgery and Psychiatry “Neurology and Neuropsychiatry of COVID-19” Blog. We used prespecified search terms modified as needed for each database (S1 Table in S1 Appendix ). We applied the following inclusion criteria to studies and then to individual patients: 1) hospitalised patients of any age; 2) diagnosed with COVID-19; and 3) acute onset of neurological symptoms, not explained fully by a pre-existing condition (e.g. progression of chronic neurological disease), with neurological illnesses classified according to our pre-defined syndromes [ 2 ], or a defined other neurological or neuropsychiatric diagnosis. Onset of neurological symptoms could have been before or after hospitalisation. We excluded studies that did not report original data, reported patients that were not hospitalised, or gave insufficient information. We selected abstracts and obtained full texts of potentially eligible studies. To compare the results of our IPD meta-analysis with other systematic reviews, meta-analyses and primary studies, including evidence from after the global second wave, we used the same search strategy to obtain articles published up to 30 th September 2021.
Data extraction and processing
We invited authors of published studies, and members of the COVID-Neuro Network of the Brain Infections Global Programme, to participate by providing IPD. Contributors ensured local ethical, regulatory and data sharing agreements were in place. We designed and piloted a standard data collection tool early in the pandemic (S2 Appendix, Section 1 to 3). Details included demographics, comorbidities and pre-admission medications; COVID-19 clinical features, including “typical” COVID-19 symptoms of cough, fever and breathlessness (patient-reported or clinician-assessed), the latter of which was taken as a proxy of COVID-19 severity (oxygen usage and ventilation were not chosen as proxies because access to these varied early in the pandemic, although these data were also collected); investigation results, including PCR (with cycle threshold if positive) and antibody testing for SARS-CoV-2 in blood and cerebrospinal fluid (CSF), with evidence of intrathecal production; COVID-19 disease severity as defined by the World Health Organization (WHO) [ 12 ]: neurological features and diagnosis; evidence for association between COVID-19 and neurological disease using pre-defined criteria (S2 Appendix, Section 3.3) [ 2 ]; dates of onset of typical and neurological COVID-19 symptoms (including symptoms that were part of the neurological diagnosis), hospital admission and discharge; treatment for COVID-19 (including maximum oxygen or respiratory support) and for neurological disease; admission to critical care, need for invasive ventilation, death, and modified Rankin Scale (mRS) score at discharge. We did not collect data for patients with no neurological disease.
Submitted datasets were cleaned and processed by at least two investigators from a core team of clinical reviewers. This was to harmonise data recording across studies in accordance with pre-defined variable types, descriptions and definitions; complete missing fields where details were available elsewhere in the dataset; and clarify outlying, unexpected or residual missing data with contributors where necessary. If a contributor was unable to harmonise their data with our format, we allowed original study data to be shared with a corresponding data code dictionary; these data were extracted by one reviewer and then checked fully by a second reviewer using an approach standardised through piloting and frequent team discussions.
Quality assessment
We designed and piloted a bespoke tool to classify study design (S1 Fig in S1 Appendix ) and assessed the quality of studies using an appropriate established assessment tool: for case reports and case series we used the Joanna Briggs Institute (JBI) critical appraisal tools [ 13 , 14 ]; for case-control, cohort and cross-sectional studies, we used the Newcastle-Ottawa Scale (NOS) [ 15 , 16 ]. Two independent reviewers appraised and assessed the quality of IPD studies, with disagreements resolved by consensus or involvement of a third reviewer.
Spectrum of neurological disease
Neurological syndromic diagnoses were made by contributors and checked by reviewers using standardised case definitions with levels of diagnostic certainty (S2 Appendix, Section 3.2) [ 2 ]. Pre-defined syndromic diagnoses included encephalopathy, encephalitis, meningitis, myelitis, acute disseminated encephalomyelitis (ADEM), and cerebrovascular events (including stroke, vasculitis, and cerebral venous sinus thrombosis). The definitions for encephalopathy (including delirium, coma, subsyndromal delirium and other encephalopathy not classified as delirium or coma, each defined as per the Ten Societies’ recommendations), and for encephalitis were combined for the purpose of the primary subgroup analysis [ 17 ]. A secondary analysis was performed for the encephalopathy subgroup excluding patients with encephalitis, who potentially have a different pathophysiological mechanism and so maybe different outcomes. We also included patients with Guillain-Barré syndrome (GBS) and variants, radiculopathy, cranial neuropathy, peripheral neuropathy, myopathy and myositis. Patients with a diagnosis outside our pre-defined criteria were categorised as ‘other neurological presentation’.
Clinical outcomes
Primary outcome..
We used the mRS to characterise outcome at hospital discharge, with a mRS score of 3 to 6 (moderate to severe disability or death) defined as a poor outcome.
Secondary outcomes.
- Mortality and days from hospital admission to death from any cause.
- Admission to critical care or receipt of invasive ventilation, referred to hereafter as “need for intensive care”.
- Length of stay in intensive care.
- Length of stay in hospital.
Statistical analysis
We used an ordinal logistic regression model with random effects to account for clustering within studies, and cumulative link function to estimate log cumulative odds of being at or above each mRS category, for all studies providing mRS for patients systematically. We fitted models for patients with any neurological syndrome, and then for the largest subgroups: cerebrovascular events, and encephalopathy. To identify factors associated with a ‘poor outcome’, an mRS of 3 to 6, we first fitted univariate models using a list of covariates. We then adjusted for a predefined subset of these covariates, which we considered important potential confounders, in multivariable logistic regression models (S2 Table in S1 Appendix ).
Mortality was analysed using Kaplan–Meier survival curves and marginal Cox regression model using the robust sandwich covariance estimates to account for the clustering of individuals within each study. For outcomes with competing risks (need for intensive care, length of stay in critical care and length of stay in hospital), the cumulative incidence curve for the event of interest in the presence of competing events (death) was estimated, and the subdistribution hazards for clustered data were modelled using the approach described by Zhou et al. [ 18 ]. For mortality and need for intensive care (i.e. admission to critical care or receipt of invasive ventilation), a pre-defined set of risk factors were explored in univariate regression models as well as in multivariable models to adjust for confounding factors (S2 Table in S1 Appendix ). We used a 5% significance level throughout. In a post-hoc analysis, we compared mortality and need for intensive care estimates between the two largest subgroups, encephalopathy, and cerebrovascular events, using the log-rank test (mortality) and Gray’s test (intensive care). In a post-hoc sensitivity analysis we compared the whole encephalopathy subgroup with a smaller subgroup of patients with encephalopathy that excluded those with a diagnosis of encephalitis.
Finally, we used data from cohort and cross-sectional studies providing verified totals of all patients hospitalised with COVID-19 in their respective centres, to estimate a pooled proportion of COVID-19 patients with neurological disease. Through inspection of study protocols, reports and other information provided by contributors, we ensured that the approaches used to screen and include participants, and to define denominators were similar across studies selected for meta-analysis.
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Results and discussion
Study selection and ipd obtained.
We identified 4092 records by database searches. After screening these and adding a further 64 records from preprint servers and reference lists, 413 published studies were included ( Fig 1 ). We contacted all study authors, received responses from 128 and received 85 IPD study datasets (2505 patients), comprising 54 published studies, and 31 unpublished studies contributed by Global COVID-Neuro Network collaborators, five of which have now been published. Two studies (143 patients) were excluded as they did not meet inclusion criteria. When inclusion criteria were applied at individual patient-level to 83 studies, 383 patients were excluded, leaving 1979 patients for analyses ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
IPD = individual patient data.
https://doi.org/10.1371/journal.pone.0263595.g001
Characteristics of included studies
The make-up of the 83 studies is summarised in S3 Table in S1 Appendix . Case series accounted for the majority of studies (61 [73%] studies, 1049 patients); 26 [31%] studies collected data prospectively; patients were hospitalised across 101 sites; 75 studies included adult patients only (1844 [93%] patients); 1179 [60%] patients were male; and most were aged 60 years and above ( Fig 2A ). Nineteen (23%) studies reported from low- or middle-income countries (LMICs); 64 (77%) were from high-income countries (HICs). Most studies (53 [64%]) reported from the WHO European region; 16 (19%) from the Americas region; eight (10%) Eastern Mediterranean; three (4%) Western Pacific region; two (2%) Southeast Asian region; and one (1%) African region (S4 Table in S1 Appendix ). Fig 2B shows the distribution of age classes by WHO region and World Bank income group. The locations of the included studies are displayed in Fig 3 . For 11 of the 83 studies, all patients were on ICU; 17 studies had no patients on ICU; and 55 studies included some patients on ICU. Quality assessments were performed as described above for all studies. Most case reports and case series were of high methodological quality in most domains assessed: 11 of the 12 case reports had an answer of ‘Yes’ for the mandatory domains 1 to 6; and the majority of case series had positive responses for domains 1, 3 and 6–9 of their respective JBI assessment scales. The cohort and cross-sectional studies had lower quality in several domains (for complete assessments see S5 Table in S1 Appendix ).
https://doi.org/10.1371/journal.pone.0263595.g002
WHO regions are depicted in different colours. Countries from which we received IPD are depicted in a darker shade. Country names and numbers of patients for which we had IPD are displayed in boxes, grouped according to region.
https://doi.org/10.1371/journal.pone.0263595.g003
Spectrum of neurological disease in patients with COVID-19
First, we looked at the spectrum of neurological disease observed in patients with COVID-19 ( Table 1 ). From 83 studies, a total of 1979 patients had a syndromic or specific neurological diagnosis. The most commonly reported syndromes were encephalopathy (978 [49%]), and cerebrovascular events (506 [26%]); other important syndromes included smell or taste disturbance (13%), peripheral neuropathy (6%), GBS (3%) and neuropsychiatric disorders (2.5%). Less than 1% were reported to have each of meningitis, ADEM, myelitis, radiculitis, and myositis. For 1027 patients with both dates available, the median time from the onset of typical COVID-19 symptoms to the onset of neurological symptoms was 5 days (IQR 0–12). For patients with encephalopathy, this was 5 days (IQR 1–10); for cerebrovascular events, 7 days (IQR 0–15); peripheral neuropathy, 13 days (IQR 1–24); and GBS, 12 days (IQR 7–22). Of 807 patients for whom the dates of neurological symptom onset and admission were available, 532 (66%) had neurological symptom onset before the admission to hospital, and 275 (34%) after. This varied by neurological diagnosis: while a similar proportion of patients with encephalopathy (66%) and cerebrovascular events (68%) had neurological features at or before admission, the corresponding proportion was 77% for GBS and 38% for other peripheral neuropathy. The majority of patients (93% [1849/1979] of all patients; 95% [932/978] of the encephalopathy subgroup; 89% [450/506] of the cerebrovascular subgroup) had confirmation of COVID-19 by PCR of a respiratory sample for SARS-CoV-2. Two with myelitis had virus detected in the CSF by PCR; no patient had antibody detected in the CSF. The remaining 7% were either cases confirmed by antibody testing, or clinically probable or suspected cases, based on our prescribed definitions (S3.1 Table in S2 Appendix).
https://doi.org/10.1371/journal.pone.0263595.t001
Overall, 887 (45%) of the 1979 patients had severe or critical COVID-19, as per WHO definitions; this proportion was similar in the encephalopathy (47% [457/978]) and cerebrovascular event (51% [260/506]) subgroups ( Fig 2C and S6 Table in S1 Appendix ). Typical COVID-19 symptoms were present before admission in 747 (93%) of 807 patients.
The 978 (49%) encephalopathy cases were reported across 61 studies, of which 161 (16%) of 978 patients had delirium, 37 (4%) had coma, and 92 (9%) had possible or confirmed encephalitis; 688 (70%) of 978 had features of encephalopathy but did not meet criteria for the aforementioned subtypes and so were described as ‘encephalopathy other’, being not otherwise defined. Of the 506 (26%) patients with a cerebrovascular event, 308 (61%) had an ischaemic stroke, 90 (18%) of 506 haemorrhagic stroke, 2 (0.4%) vasculitis, and 106 (21%) another cerebrovascular event ( Table 1 ). Of these 506 patients, 90% (454 of 506) had neuroimaging that informed diagnosis.
According to our definitions for strength of evidence for an association between infection with SARS-CoV-2 and the development of neurological disease (S2 Appendix, Section 3.3), only two patients met criteria for confirmed association—both had myelitis with a positive CSF PCR test for SARS-CoV-2. Most patients were defined as having a probable association between neurological disease and COVID-19: this applied to 792 (96%) of 826 patients with encephalopathy or encephalitis. The majority of patients with cerebrovascular events for whom this assessment was available were classified as having a possible rather than probable association (362 of 454 [80%]) due to the presence of other pre-defined vascular risk factors. More complete details of the strength of association between neurological disease and infection in patients are provided in S8 Table in S1 Appendix . Four (5%) of the 83 studies included all consecutive patients with neurological COVID-19 disease in a given hospital or region (S10 Table in S1 Appendix ). For two of them encephalopathy was the most common presentation, accounting for 50% and 76% of patients, for two cerebrovascular disease predominated (both 64%).
A poor outcome (moderate to severe disability or death, mRS 3–6) was recorded for 50% (95% CI 41–59) of the 1052 patients in 73 studies reporting mRS systematically, after adjusting for clustering within studies ( Table 2 ). The predicted probability of having no symptoms at discharge (mRS 0) was estimated as 7%. Table 2 shows the probability of each mRS score for 413 patients with encephalopathy and 326 patients with cerebrovascular events, for whom an mRS score was available. There was a higher probability of a poor outcome for cerebrovascular patients (76% [95% CI 67–82]), than encephalopathy patients (54% [95% CI 42–65]). The crude probability of death at 30 days ( Fig 4A ) was estimated from a Kaplan-Meier analysis as 30% (95% CI 27–32) for all 1745 patients for whom the outcome was available and did not differ significantly for the encephalopathy and cerebrovascular subgroups. For the 1428 patients with adequate data, the crude cumulative incidence of need for intensive care by 30 days was 38% (95% CI 35–41; Fig 4B ); this was significantly higher for cerebrovascular patients (47% [95% CI 41–53]; 368 patients) than encephalopathy patients (38% [95% CI 34–42]; 617 patients; Gray’s test p = 0.03). The cumulative incidence of discharge from hospital by 30 days was 55% (95% CI 53–58; Fig 4D ) and did not differ significantly between subgroups. Outcomes for the encephalopathy subgroup excluding patients with a diagnosis of encephalitis were all similar to the outcomes of the whole encephalopathy subgroup (S9 Table in S1 Appendix ).
1. These figures show results of analyses for the whole IPD database (i.e., patients with any neurological disease diagnosis), and other than for A, the analyses use death as a competing risk. 2. A total of 1745 patients were included in this analysis. Of the 1979, 115 had no dates; 14 patients had no hospital admission date; 9 dead patients had no date of death; 88 alive patients had no discharge date; it was unknown if 8 patients were dead or alive. For time to death, individuals that were alive at discharge or last follow-up were censored. 3. This analysis uses date of hospital admission as day 0. A total of 1428 patients were included in this analysis: 404 patients had no dates; 17 had no hospital admission date; 123 (23 dead; 100 alive) patients had neither the date of admission to critical care or the date of commencement of invasive ventilation; 7 patients only had a hospital admission date, but it was unknown if they were dead or alive. For time to critical care admission, individuals who were alive at discharge or last follow-up and had not been admitted to intensive care were censored. Individuals who died without receiving critical care or invasive ventilation were treated as competing events in a competing risks analysis. 4. This analysis uses date of critical care admission as day 0. A total of 486 patients who were admitted critical care were included in this analysis: 1482 patients had no date of admission to critical care; 5 dead patients had no death date; 5 alive patients had no hospital discharge date; there were no dates for 1 patient. 5. For discharge from critical care, individuals that were alive and not yet discharged at last follow-up were censored. Individuals that died after admission to intensive care were treated as competing events in a competing risks analysis. 10. For length of hospital stay, individuals that were alive and not yet discharged at last follow-up were censored. Individuals that died were treated as competing events in a competing risks analysis.
https://doi.org/10.1371/journal.pone.0263595.g004
https://doi.org/10.1371/journal.pone.0263595.t002
Factors associated with clinical outcomes
On multivariable analysis, after adjusting for potential confounders (S3 Table in S1 Appendix ), we identified several factors associated with a poorer outcome (i.e. higher mRS score at hospital discharge) ( Table 3 ). For patients with any neurological diagnosis, these were: age (with the odds ratio [OR] up to 15.3 [95% CI 7.7–30.5] with increasing age); pre-existing dementia (OR 2.6 [1.2–5.7]); breathlessness on admission (OR 1.7 [1.1–2.4]); severely elevated initial blood D-dimer concentration (OR 2.5 [1.4–4.6] for >3000ng/mL vs. <500ng/mL); and corticosteroid use during admission (OR 2.8 [1.8–4.3]). For the encephalopathy subgroup, significant factors on multivariable analysis associated with a poor outcome were: age (OR 5.4 [95% CI 1.4–20.7] for 70–79 years; OR 12.2 [2.8–53.0] for ≥80 years); corticosteroid treatment in hospital (OR 3.6 [1.5–8.9]); anticoagulation in hospital (OR 3.1 [1.3–7.4]); and low initial lymphocyte count (OR 0.4 [0.2–0.9] for normal or high lymphocyte count). For patients with cerebrovascular events, the following were significant: age (OR 3.7 [1.2–11.2] for 60–69 years; OR 4.53 [1.59–12.9] for 70–79 years; OR 6.7 [2.2–20.7] for ≥80 years); elevated D-dimer (OR 2.8 [1.3–6.2] for 500-3000ng/mL; OR 3.5 [1.3–9.7] for ≥3000ng/mL); breathlessness on admission (OR 2.8 [1.4–5.5]); and corticosteroid use during admission (OR 4.8 [1.9–11.9]). For patients with cerebrovascular events, being in the WHO African/Eastern Mediterranean region was associated with a poor outcome relative to the WHO European region (OR 4.4 [1.4–14.4]), whereas being in the Southeast Asia/Western Pacific region was associated with a better outcome relative to the European region (OR 0.2 [0.1–0.9]).
https://doi.org/10.1371/journal.pone.0263595.t003
Hazard of death among patients with any neurological disease was found be associated with age, dementia, breathlessness at presentation, corticosteroid use in hospital, WHO Region (higher for all regions compared with Europe) and World Bank income group (higher for low- and lower-middle income countries), following adjustment for confounders in multivariable models ( Table 4 ). For patients with encephalopathy, age, dementia, corticosteroid use in hospital, WHO region and World Bank income group were statistically significant after adjustment for confounders. Adjusted multivariable models for the cerebrovascular patients found a significant association with increased hazard of death and low lymphocyte count, corticosteroid treatment, and WHO region, whereas anticoagulant use in hospital was protective.
https://doi.org/10.1371/journal.pone.0263595.t004
Multivariable regression analysis found a statistically significant association with requiring intensive care for male sex, breathlessness at presentation, pre-existing dementia or diabetes, increased CRP, elevated D-dimer, anticoagulant use, corticosteroid use, WHO region and World Bank income group ( Table 5 ). After fitting models for the encephalopathy subgroup, a statistically significant increased hazard of requiring intensive care was found for age (≥80 years), obesity, dementia, breathlessness, elevated CRP and D-dimer, corticosteroid use, WHO region, and World Bank income group. In the cerebrovascular event subgroup, pre-existing cardiac disease or dementia, corticosteroid treatment in hospital, income group and WHO region were significant after adjustment for confounders.
https://doi.org/10.1371/journal.pone.0263595.t005
Proportion of patients with neurological COVID-19 disease
Eight of the 83 studies included the total number of neurological and other hospitalised COVID-19 patients, admitted over a specified time period, in a comparable way which could be analysed. Five were case series. Fig 5 illustrates that, overall, 7.8% (95% CI 1.6–31.2) of hospitalised COVID-19 patients had neurological disease. The I 2 statistic showed a high degree of statistical heterogeneity among studies (100%). The studies contributing data are summarised in S11 Table in S1 Appendix . When one study, which included patients admitted to community isolation facilities as well as to hospitals, was excluded in a sensitivity analysis (S2 Fig in S1 Appendix ), the pooled percentage of hospitalised COVID-19 patients who had neurological disease was 14.7% (95% CI 4.7–37.8; I 2 98%).
Neurological disease = number of patients with neurological COVID-19 disease. All COVID-19 = number of patients with all COVID-19 disease hospitalised in the same centre over the same time period.
https://doi.org/10.1371/journal.pone.0263595.g005
Since the onset of the COVID-19 pandemic, there has been a plethora of studies reporting associated neurological disease, initially without the use of standardised case definitions, and often still without detailed clinical and diagnostic evaluation, investigation of prognostic markers or clinical outcomes. To some extent, this reflected the difficulties of studying a new highly infectious disease that was swamping health services, plus the desire to publish important information quickly [ 2 ]. Several meta-analyses have now also been published based on these original reports [ 3 – 8 ], but they may not accurately capture the true clinical picture, given the limitations of the original data.
In July 2020, we published standardised case definitions for neurological COVID-19 disease [ 2 ], which included assessment of the strength of evidence for an association, and have been modified and are being adopted by the Global Covid-19 Neuro Research Coalition and the WHO [ 19 ]. We therefore decided to perform an IPD meta-analysis of published and unpublished data from patients admitted to hospital during the first wave of the pandemic, using these case definitions and a standardised data collection tool. To date, no other published meta-analysis has included IPD for multiple pre-defined neurological diagnoses, though one large analysis combined data from two cohorts of patients with neurological COVID-19 and a third with or without neurological disease [ 20 ]. We received data on 1979 patients supplied from 83 studies (including 31 that were originally unpublished). Most previous systematic reviews described symptoms and diagnoses, with some estimating the proportion of COVID-19 patients that develop neurological disease. Here, we concentrated on detailed descriptions of the neurological diseases, their outcomes, and risk factors for a poor prognosis. This latter is especially important for neurologists and other hospital specialists who care for such patients. We also compared WHO regions and World Bank income groups, to initiate thinking about differences in outcomes across the global community.
The neurological syndromes seen most commonly were encephalopathy (49%), including encephalitis, coma, and delirium, and cerebrovascular events (26%), principally ischaemic stroke ( Table 1 ). There were also many patients with smell or taste disturbance (19%), and some with peripheral neuropathy (6%), GBS (3%) and neuropsychiatric disorders (2.5%). The cerebrovascular case definitions worked relatively well in terms of classifying patients. The encephalopathy definitions worked less well with 35% of patients being classed as “encephalopathy other” because they did not fit into the main categories of delirium, coma and encephalitis; although there has been considerable debate on encephalopathy case definitions among the neurology, geriatric, and psychiatric community [ 10 , 21 , 22 ] these results suggest clinicians may be unfamiliar with the definitions, or they may need further revision. Encephalopathy may be precipitated by many different factors, in the context of different diseases, and the spectrum of clinical features of this syndrome can make succinct classification a challenge. Assessment of patients with suspected delirium in our study was performed by clinicians, guided by the variables included in our data collection form; existing tools such as CAM-ICU, 4AT or AMT-4 could also be used to quantify neurocognitive features in more detail. In anticipation of other factors that can impact on conscious level, cognition, and behaviour, we also collected data on brain imaging and use of hypnotic and anxiolytic agents during hospital admission. For most encephalitis patients the aetiological link to SARS-CoV-2 was classed as “probable” or “possible”, because no virus was detected in their CSF. This is in contrast to herpes simplex virus encephalitis where virus is frequently detected, and there is marked inflammatory change on brain imaging or autopsy. Over a year into the pandemic, we now know that virus detection in the CSF is extremely rare, and the case definitions should probably be refined to reflect this, perhaps following the approach for enterovirus 71, which also causes severe brain disease with inflammatory changes despite virus rarely being detected in the CSF [ 23 , 24 ]. Of note, two myelitis patients had virus detected in CSF; we did not have the PCR cycle threshold values from this testing, but given the implications of true confirmed viral myelitis on management, this finding should be rigorously confirmed by by performing PCR for SARS-CoV-2 on CSF of myelitis patients who have concurrent or recent COVID-19, or who present during a pandemic wave.
In previous systematic reviews encephalopathy and cerebrovascular disease were the most commonly reported neurological presentations, though which of these was most important varied [ 25 – 29 ]. This likely reflects differences in study populations and case definitions. Even for the four studies in our analysis that recruited consecutive neurological patients, and where we could apply strict case definitions to the individual patient data, two studies had a predominance of patients with cerebrovascular events and the other two had a majority of patients with encephalopathy (S10 Table in S1 Appendix ). These differences may stem from varying approaches to screening for neurological symptoms and inclusion of hospitalised COVID-19 patients. In our database overall, we found encephalopathy was reported for about half the patients, and stroke for about a quarter. This is similar to one of the larger prospective series of 606 unselected neurological patients in New York, which found encephalopathy in 50% and stroke in 14% [ 30 ]. Another recent study combining COVID-19 and neurological disease patient registries reported encephalopathy in 49%, and stroke in 6% [ 20 ].
Approximately half of the 1052 patients with neurological COVID-19 disease and a mRS score available had a poor outcome on discharge from hospital, as determined by a mRS score of 3–6 (moderate to severe disability or death; Table 2 ); the proportion was higher in those with cerebrovascular events (76%) than encephalopathy (54%), and this was largely accounted for by those that died (33% versus 17%). Our findings highlight the degree of disability experienced by patients with COVID-19 and neurological disease; a recent report of hospitalised UK patients in the UK ISARIC-4C study found that functional outcomes are worse in those with neurological complications compared to those with other severe but non-neurological complications of COVID-19 [ 31 ]. In another study, the adjusted odds ratio of in-hospital death was 5.99 (95%CI: 4.33–8.28) for those with any neurological signs and/or syndromes compared to those without, though the odds ratio was greater for encephalopathy than stroke [ 20 ].
The mRS was devised for stroke and although it is not particularly reliable for brain injuries that result in cognitive disability [ 32 ], it is still widely used in this group. Future studies of neurological disability should use a more generic outcome score such as the Glasgow Outcome Scale which is equally simple to administer and may better capture the impact of neuropsychiatric manifestations [ 33 ]. Clinicians were not blinded to the patients neurological condition at the time of mRS assessment, but the outcome measure was clearly defined. Variable time to discharge (at which point mRS was calculated) may have affected our results, as we did not account for this in our multivariable models.
Overall, 30% of the 1745 neurological patients with outcome information available had died by 30 days, which is higher than the mortality of around 25% reported by meta-analyses of all hospitalised COVID-19 patients from North America, Europe, and China [ 34 , 35 ]. Our higher mortality rate is in keeping with the report of the ISARIC-4C study, which found patients with neurological COVID-19 complications (specifically meningitis, encephalitis, seizure, or stroke) had an increased hazard of mortality [ 31 ]. A previous systematic review of patients with neurological disease reported a lower mortality of 10%, but this study may have included non-hospitalised patients with neurological symptoms such as headache [ 3 ]. Nearly 40% of our patients needed intensive care (higher for those with a cerebrovascular event than for the encephalopathic patients). No previous systematic reviews of neurological COVID-19 patients have meta-analysed for these outcomes, though in the ISARIC-4C study, 22% of those with neurological complications were admitted to critical care compared with 14% of patients overall [ 31 ]. Approximately half of our neurological COVID-19 patients who required intensive care support still needed this at 30 days. Whilst we did not have a control group, this is considerably longer than what has been reported in published studies for all COVID-19 patients in intensive care: 12 days in one large UK cohort study [ 36 ], and 8 days in a meta-analysis [ 37 ]. 45% of our patients were still in hospital at 30 days. While previous systematic reviews of neurological COVID-19 do not report this, our estimate appears longer than studies reporting on all hospitalised COVID-19 patients: median length of stay was 12 days for one study of 1321 patients in France [ 38 ], and 8 days for 2005 patients in Germany [ 39 ] Collectively our results on the need for and duration of intensive care, length of hospital stay, and patient outcomes underscore the significant burden of neurological COVID-19 disease on health care resources, compared with COVID-19 disease as a whole. Post-acute COVID-19 neurological symptoms and outcomes are also an emerging and important issue, though longer-term data were not available for us to investigate this [ 40 ].
We found age, and markers of disease severity including breathlessness and elevated D-dimer were associated with a poor outcome among all patients with neurological disease ( Table 3 ). These same factors were also important for the subgroup with cerebrovascular events, but not those with encephalopathy. This is in keeping with an earlier report from the ISARIC-4C study indicating that patients presenting with encephalopathy, with or without typical COVID-19 symptoms, had a higher mortality [ 41 ]. Low initial lymphocyte count was associated with poor outcome in our encephalopathy patients, as has been shown in a meta-analysis of over 10,000 patients with COVID-19 [ 42 ]. Other biomarkers shown to be important in COVID-19 generally, such as neutrophil and platelet counts, were not available consistently for our patients [ 43 ].
Corticosteroid use in hospital was associated with a worse outcome in all neurological patients, as well as the cerebrovascular and encephalopathic subgroups ( Table 3 ). This is likely to be because clinicians were more inclined to use corticosteroids in these patients with severe disease. Anticoagulation use in hospital was also associated with a worse outcome in the encephalopathic patients, but intriguingly it was associated with a lower hazard of death in those with cerebrovascular events ( Table 4 ), suggesting it may be beneficial in these patients. Further work is needed to understand the role of anticoagulation in COVID-19 patients with stroke. While these variables might have been susceptible to immortal time bias, this is unlikely to have influenced the results significantly, as these drugs are usually started at admission.
Although international comparison was not the primary aim of our study, we could begin to explore differences in outcomes between different WHO regions, and World Bank income groups. We found that compared with neurological COVID-19 patients in the WHO European region, those in other regions had a higher hazard ratio for death ( Table 4 ); the hazard ratio was also higher for patients from low- and lower-middle-income countries compared to high-income countries (HICs), though with wide confidence intervals, reflecting fewer patients in the lower-income category. Differences in mRS scores were also seen across WHO regions ( Table 3 ), but only in patients with cerebrovascular disease and again with wide confidence intervals. Although these are only preliminary data, these differences may reflect broader public health approaches and capacities in different countries [ 44 ]. Further larger-scale studies including LMICs are needed to investigate these potential findings.
Although we applied standard case definitions (S2 Appendix, Section 3) and eligibility criteria to our IPD database, the original studies or case series had been conducted using different protocols, and many were small and did not capture all patients with neurological disease, potentially leading to selection bias. However, 47% of our patients were from cross-sectional and cohort studies, and the case series scored highly across several quality assessment domains. Our novel approach of capturing unpublished data through the Global Covid-Neuro Network meant we included patients, especially from LMICs, that would not otherwise have ever been included in a publication, thus improving accessibility and equity (S4 Table in S1 Appendix ). Despite this, just 6 (7%) of 83 studies providing 42 (2%) of the total 1979 patients were from low- or lower-middle income countries. Only 2% of our patients were children. This may reflect some degree of residual selection bias, despite not relying upon published literature. Finally, despite using multivariable analyses with pre-defined exposures and confounders, associations do not equate to causation; determining these would require further research.
Conclusions
We have shown that encephalopathy and stroke are the most commonly reported neurological manifestations of COVID-19, with the latter group having a worse outcome, as judged by the mRS. Nearly 40% of patients needed intensive care, and the burden in terms of prolonged intensive care and hospital stay was higher than for other hospitalised COVID-19 patients. Markers of disease severity such as breathlessness and elevated D-dimer were associated with poor outcome in the cerebrovascular event, but not the encephalopathic, patients, suggesting different disease mechanisms. For one third of the patients, the neurological symptoms started after hospital admission, providing a potential window for intervention if risk factors and neurological disease mechanisms were better understood. Prospective case-control studies across multiple WHO regions are needed to better understand the factors leading to neurological COVID-19 and point to potential interventions.
Supporting information
S1 checklist. prisma-ipd checklist..
https://doi.org/10.1371/journal.pone.0263595.s001
S1 Appendix. Author list, supplementary figures and tables.
https://doi.org/10.1371/journal.pone.0263595.s002
S2 Appendix. Data extraction tool and case definitions.
https://doi.org/10.1371/journal.pone.0263595.s003
Acknowledgments
Please see the S1 Appendix for details of the Group Authors, members of The Brain Infections Global COVID-Neuro Network. We acknowledge invaluable administrative support from Ms Clare Fotheringham in the Institute of Infection, Veterinary & Ecological Sciences and the Research Contracts Team at the University of Liverpool.
- View Article
- PubMed/NCBI
- Google Scholar
- 12. WHO. Coronavirus disease 2019 (COVID-19) Situation Report– 76. Geneva: World Health Organization; 2020.
- 13. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. Aromataris E, Z M, editors: Joanna Briggs Institute; 2017.
- 15. Wells G, Shea B, O’Connell D, Peterson j, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 31. Drake TM RA, Fairfield CJ, et al, on behalf of the ISARIC4C investigators. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: A prospective, multicentre cohort study2021 April 5, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/962826/s1081-in-hospital-complications-isaric.pdf
- 43. Wijeratne T, Sales C, Karimi L, Jakovljevic M. Elevated Neutrophil to Lymphocyte Ratio Predicts In-hospital Mortality Among Stroke Patients in a Metropolitan hospital in Australia, Universal Value-added measure in Stroke Care. medRxiv [Preprint]. 2021 [posted 2021 Mar 3]. https://www.medrxiv.org/content/10.1101/2021.03.01.21252317v1
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- ERJ Open Res
- v.3(4); 2017 Oct

mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis
Kaisa rajala.
1 Helsinki University Hospital, Comprehensive Cancer Center, Dept of Palliative Care, Helsinki, Finland
2 Faculty of Medicine, University of Helsinki, Helsinki, Finland
Juho T. Lehto
3 Dept of Oncology, Palliative Care Unit, Tampere University Hospital, Tampere, Finland
4 Faculty of Medicine and Life Sciences, University of Tampere, Tampere, Finland
Eva Sutinen
Hannu kautiainen.
5 Primary Health Care Unit, Kuopio University Hospital, Kuopio, Finland
6 Folkhälsan Research Center, Helsinki, Finland
Marjukka Myllärniemi
7 University of Helsinki and Helsinki University Hospital, Heart and Lung Center, Dept of Pulmonary Medicine, Helsinki, Finland
Tiina Saarto
Associated data.
J.T. Lehto 00084-2017_Lehto
M. Myllärniemi 00084-2017_Myllarniemi
K. Rajala 00084-2017_Rajala
E. Sutinen 00084-2017_Sutinen
This study was undertaken to investigate idiopathic pulmonary fibrosis (IPF) patients' health-related quality of life (HRQoL) and symptoms in a real-life cross-sectional study. Our secondary aim was to create a simple identification method for patients with increased need for palliative care by studying the relationship between modified Medical Research Council (mMRC) dyspnoea scale, HRQoL and symptoms.
We sent a self-rating HRQoL questionnaire (RAND-36) and modified Edmonton Symptom Assessment Scale (ESAS) to 300 IPF patients; 84% of the patients responded to these questionnaires.
The most prevalent (>80%) symptoms were tiredness, breathlessness, cough and pain in movement. An increasing mMRC score showed a linear relationship (p<0.001) to impaired HRQoL in all dimensions of RAND-36 and the severity of all symptoms in ESAS. Dimensions of RAND-36 fell below general population reference values in patients with mMRC score ≥2. The intensity of pain in movement (p<0.001) and at rest (p=0.041), and the prevalence of chest pain (p<0.001) had a positive linear relationship to increased mMRC score.
An increasing mMRC score reflects impaired HRQoL and a high symptom burden. In clinical practice, the mMRC scale could be used for screening and identification of IPF patients with increased need for palliative care.
Short abstract
mMRC indicates impaired HRQoL and pain in IPF http://ow.ly/oRB430gIW7U
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive and severe disease of unknown cause, seen primarily in older adults [ 1 ]. Even with recent advances in pharmacological treatment, IPF is still a disease with a high morbidity and poor survival [ 2 – 4 ]. As the disease trajectory in IPF is comparable to many advanced malignant disorders, guidelines recommend early-integrated palliative care in addition to pharmacological treatment and referral for lung transplantation [ 5 , 6 ].
Patients with IPF suffer from difficult symptoms, of which breathlessness and cough are the most common ones [ 7 , 8 ]. In addition, there is some evidence that IPF patients frequently experience pain, although the location and mechanism of the pain have not been reported [ 7 ]. Comorbidities are frequently reported in IPF patients, as shown in a recent study, where 88% of the patients had at least one and 30% more than four other diagnoses [ 9 ]. The total number of comorbidities and especially the occurrence of cardiovascular disease are associated with increased mortality [ 9 – 12 ].
There exist a limited number of studies on the heath-related quality of life (HRQoL) of IPF patients in a real-life setting [ 13 ]. Most recent studies have either concentrated on pharmaceutical treatment or have included a very limited number of patients [ 7 , 13 ]. However, there are clear indications of a decreased HRQoL in IPF patients [ 13 , 14 ].
The primary aim of this cross-sectional study was to describe the HRQoL and symptom burden among IPF patients derived from a national IPF registry (FinnishIPF). The secondary aim was the identification of patients with increased need for palliative care by investigating the relationship between dyspnoea score and HRQoL.
Materials and methods
Study population.
The FinnishIPF study is a prospective national clinical registry study of IPF patients initiated in 2012. IPF diagnosis is made according to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society 2011/2015 criteria [ 1 , 6 ]. In Finland, practically all IPF patients are initially evaluated in public hospitals (university and central hospitals). The FinnishIPF registry consists of all IPF patients from specialist centres who have given their informed consent to participate to the study. K aunisto et al . [ 2 ] have published a detailed description of the FinnishIPF study. Overall, 76% of confirmed IPF patients have given consent to participate to the study [ 2 ].
This study was initiated in April 2015, when all 300 patients registered to FinnishIPF study at that time were contacted and asked for a written informed consent to participate in this substudy. The questionnaires were sent to the patients with the consent form. The patients who did not respond within 2 weeks were contacted by telephone and reminded to answer to the questionnaire.
Data collection and questionnaires
Sociodemographic and disease characteristics were collected from patient records and by a separate questionnaire. Collected data included age, sex, date of birth, marital status, living conditions, education, physical activity, the need for help in daily activities, the date of IPF diagnosis, comorbidities and smoking status. The participants' exercise habits during the preceding 6 months (≥30 min at least moderate-intensity leisure time physical exercise, i.e. causing breathless and sweating) were asked.
The specific questionnaires of symptoms and HRQoL were modified Edmonton Symptom Assessment Scale (ESAS), modified Medical Research Council (mMRC) dyspnoea scale and the RAND 36-Item Health Survey (RAND-36).
The ESAS is a self-rated, numeric-rating, symptom-based scale developed for assessing the symptoms of cancer patients [ 15 ]. ESAS measures different symptoms on a scale from 0 (no symptoms) to 10 (the worst possible symptoms) [ 16 , 17 ]. In this study, we used a modified version, including 12 questions on symptoms, one question on general wellbeing and a standardised body diagram on which patients could mark the areas of pain.
The mMRC scale is a self-rating tool to measure the degree of disability that breathlessness poses on day-to-day activities on a scale from 0 to 4: 0, no breathlessness except on strenuous exercise; 1, shortness of breath when hurrying on the level or walking up a slight hill; 2, walks slower than people of same age on the level because of breathlessness or has to stop to catch breath when walking at their own pace on the level; 3, stops for breath after walking ∼100 m or after few minutes on the level; and 4, too breathless to leave the house, or breathless when dressing or undressing [ 18 , 19 ].
The RAND-36 [ 20 ] is a general HRQoL measurement tool, for which Finnish general population reference values exist [ 21 ]. The Short Form-36, which is commonly used in IPF patients, is similar to RAND-36 [ 21 ]. It is divided into eight health concepts, as explained by H ays et al. [ 20 ] and A alto [ 21 ], with scale from 0 to 100 (lower score meaning worse HRQoL). The concepts are: “physical functioning” (10 questions from ability to move and exercise to the ability to take care of personal hygiene), “role physical” (four questions on role limitations due to physical health), “bodily pain” (two questions), “general health” (five questions), “vitality” (four questions on energy level and tiredness), “social functioning” (two questions), “role emotional” (three questions on role limitations due to emotional problems) and “mental health” (five questions on anxiety, depression and mood) during the past 4 weeks [ 20 , 21 ].
Statistics and ethical aspects
The data are presented as mean± sd or n (%). The statistical significance for the hypothesis for linearity across groups in RAND-36 domains and symptoms were determined by ANCOVA and logistic regression analysis with an appropriate contrast (orthogonal polynomial). In the case of violation of the assumptions ( e.g. non-normality), a bootstrap-type test was used. The normality of the variables was tested by using the Shapiro–Wilk W-test. Stata 14.1 (StataCorp LP, College Station, TX, USA) was used for the analysis.
The ethical committee of Helsinki University Central Hospital (Helsinki, Finland) approved this study (381/13/03/01/2014). Permission to screen hospital registries for patients with IPF was approved by the Finnish National Institute for Health and Welfare (Dnro THL/1161/5.05.01/2012). All patients who participated to this study gave a written informed consent to participate this substudy.
Of 300 registered patients, 47 were excluded: 42 did not want to participate or did not answer our questionnaire; one received lung transplantation and one was found not to be IPF patient, so these two also were excluded; three patients died before they answered.
Patient characteristics
The patient characteristics are shown in table 1 . The mean duration of IPF at the time of the study entry was 3.9 years. At least one comorbidity was reported in 79% (n=200) and more than two comorbidities in 30% (n=77) of the patients, respectively. 37% of the patients had performed at least moderate-intensity leisure time physical exercise for ≥30 min a week during the last 6 months, whereas 21% had not been engaged in any physical exercise. A majority (65%) of the patients did not need help in everyday life, whereas 26% had received assistance in their daily routines. The remaining patients ( 9% ) did not receive help but considered themselves to be in need of it.
TABLE 1
Data are presented as mean± sd unless otherwise stated. IPF: idiopathic pulmonary fibrosis; FVC: forced vital capacity; COPD: chronic obstructive pulmonary disease. # : smoking status and FVC were recorded at the time of diagnosis, and other factors at the time of questionnaire; ¶ : including three patients with lung cancer.
mMRC for breathlessness
The severity of breathlessness on exertion reported by mMRC score was 0 (no breathlessness) in 33 (13%), 1 (breathless when hurrying or walking up a hill) in 88 (35%), 2 (breathless when walking slower than people of same age or has to stop when walking) in 75 (30%), 3 (breathlessness stops walking after ∼100 m or a few minutes) in 34 (13%) and 4 (breathless when dressing or not able to leave the house) in 23 (9%) of the patients.
RAND-36 for HRQoL
The different dimensions of HRQoL measured by RAND-36 are presented in table 2 . There was a linear relationship between impaired HRQoL and all RAND-36 dimensions and a higher mMRC score (linearity p<0.001) ( figure 1 ). All HRQoL dimensions of RAND-36 were significantly impaired in patients with mMRC 2–4 as compared to the general population except “bodily pain”, which was significantly below the general population level only in patients with mMRC score 4 ( figure 1 ). Physical dimensions (“physical functioning” and “role physical”) were the most impaired ones. “Role physical” derives from four questions in the questionnaire and reflects limitations in everyday life due physical health problems [ 20 , 21 ].
TABLE 2
Symptoms by Edmonton Symptom Assessment Scale (ESAS) questionnaire and health-related quality of life by RAND 36-Item Health Survey (RAND-36)
Data are presented as mean± sd unless otherwise stated. # : numeric rating scale, 0–10.

Health-related quality of life measured by the RAND 36-Item Health Survey according to modified Medical Research Council (mMRC) dyspnoea scale groups. Data are presented as mean values with 95% confidence intervals. Values adjusted for age, sex, comorbidities, education and living status. Dashed lines mark Finnish general population levels.
ESAS for symptoms
The prevalence and mean intensity of symptoms as measured by ESAS are shown in table 2 . There was positive linear relationship between the intensity of all symptoms in ESAS questionnaire and increasing mMRC breathlessness score ( figure 2 ).

Symptoms measured by Edmonton Symptom Assessment Scale according to modified Medical Research Council (mMRC) dyspnoea scale groups. Data are presented as mean numeric rating scale (NRS) values with 95% confidence intervals. Values adjusted for age, sex, comorbidities, education and living status.
A striking increase in pain intensity in movement (p<0.001) and, to lesser extent, at rest (p=0.041) was found with an increased mMRC score ( figure 2 ). The prevalence of pain in different locations of body diagram according to mMRC groups is shown in table 3 . The prevalence of chest pain and increasing mMRC score showed a positive linear relationship (linearity p<0.001).
TABLE 3
Localisation of pain
mMRC: modified Medical Research Council dyspnoea scale. # : for linearity, adjusted for age, sex, comorbidities, education and living status; ¶ : four or more of the seven pain areas marked by the patient.
This was a cross-sectional, real-life study of the quality of life and symptoms of IPF patients. Our results show that increased breathlessness as measured by the mMRC questionnaire is related to impaired HRQoL and symptom burden. In addition to breathlessness and cough, pain in movement was detected in a majority of the patients. However, only chest pain had a linear relationship with increased mMRC breathlessness score. We suggest that pain and, more importantly, chest pain, may be an underdiagnosed symptom of IPF.
In our study, the HRQoL of IPF patients with at least moderate shortness of breath (mMRC ≥2), was impaired in all areas of HRQoL, especially physical function, when compared to the Finnish general population [ 21 ]. Our findings are in line with an American Internet survey of 220 IPF patients in which HRQoL was measured with PROMIS-29 [ 22 ]. A correlation between mMRC scores and all domains except sleep disturbance was found [ 22 ]. In a small, cross-sectional, longitudinal study of 32 Japanese IPF patients, lower scores were reported in all eight domains (HRQoL questionnaire SF-36) when compared to the general population [ 13 ]. Similarly, in another small observational validation study of 34 IPF patients, a decline was seen in seven of the eight measured domains of SF-36 compared to sex- and age-matched controls [ 14 ]. That particular study also showed correlation between baseline dyspnoea index and five SF-36 components: physical functioning, general health perceptions, vitality, social functioning and mental health [ 14 ]. Even though there was a significant correlation between baseline dyspnoea index and pulmonary function parameters, dyspnoea index seemed to predict HRQoL more sensitively than pulmonary function parameters [ 14 ]. Dyspnoea in daily living, measured by mMRC, is also stronger prognostic parameter than most physiological markers in the diagnostic phase of IPF [ 23 ]. N ishiyama et al. [ 23 ] showed that low arterial oxygen saturation in a 6-min walk test and mMRC score were the strongest predictors of IPF patient's survival.
In line with the American Internet survey, increasing mMRC score was related to the symptom burden of IPF patients in our study [ 22 ]. The three most common symptoms in our study were tiredness, shortness of breath and cough, which are in line with earlier findings [ 7 ]. Interestingly, however, pain in movement was the next most common symptom reported by the majority of our patients, and pain in rest was the sixth most common symptom, present in two thirds of the patients. In a Swedish register study of oxygen-dependent interstitial lung disease patients, pain was reported in 51% of the patients [ 7 ]. Similarly to our findings, Y ount et al . [ 22 ] demonstrated an association between dyspnoea severity in mMRC score and intensity of pain. In another small observational study, no correlation between baseline dyspnoea index and pain index was found [ 14 ]. These differences could be related to different stage of the disease in different study populations.
In our study, every third patient reported chest pain, which also had linear relationship to the intensity of breathlessness measured by mMRC. Unspecified thoracic pain has been reported in pulmonary sarcoidosis and chronic obstructive pulmonary disease but, to our knowledge, this is the first study to report chest pain in IPF [ 24 , 25 ]. The exact aetiology of chest pain in IPF falls beyond the scope of our study, and should be an aim of further studies. However, as the relationship between chest pain and breathlessness was maintained after adjusting for comorbidities and age, the results suggest that chest pain may be a symptom related to IPF itself. This finding should be taken into account when considering diagnostic tests and treatment strategies for patients with advanced IPF.
Study limitations
The cross-sectional nature of the study limits our results to a single time-point and does not allow us to describe the changes in symptoms or HRQoL over time. Our cohort may be subjected to some selection bias, as some patients at a very advanced stage of the disease or close to death are likely to be lost from the cohort. Another limitation is that although the diagnosis of IPF was made by pulmonologists according to international guidelines, there was no central confirmation of the diagnoses. The strength of our study is a relatively large population of IPF patients in different phases of disease trajectory, evaluated by several assessment tools in real-life setting, and a high response rate.
Conclusions
Pain is a relatively common symptom in IPF. In particular, chest pain is related to increasing mMRC score. This could indicate a causal relationship between chest pain and progressive IPF, but further studies are necessary to confirm and explain these findings. Our results show that mMRC not only reflects breathlessness in patients with IPF but indicates HRQoL and overall symptom burden. The HRQoL was significantly deteriorated and symptom burden rose in patients with mMRC score ≥2. Thus, mMRC could be used as a simple screening tool for palliative care needs of IPF patients.
Disclosures
Acknowledgements.
We are grateful for the patients that consented to participate in this study. The authors express gratitude to the participants of the FinnishIPF consortium: R. Kaarteenaho (University of Oulu, Oulu, Finland), S. Saarelainen (Tampere University Hospital, Tampere, Finland), H. Kankaanranta (Seinäjoki Central Hospital, Seinäjoki, Finland), A. Böök (Satakunta Central Hospital, Pori, Finland), E.R. Salomaa (University of Turku, Turku, Finland), J. Kaunisto (University of Turku, Turku, Finland), U. Hodgson (Helsinki University Central Hospital, Helsinki, Finland) and M. Purokivi (Kuopio University Hospital, Kuopio, Finland). The authors are also immensely grateful to the numerous pulmonary physicians who have contributed to the study by including patients and seeking informed consent: J. Vaden (Hämeenlinna Hospital, Hämeenlinna, Finland), M. Pekonen (Kanta-Häme Central Hospital, Hämeenlinna, Finland), H. Tapanainen (Hyvinkää Hospital, Hyvinkää, Finland), H. Lajunen (Jämsä Hospital, Jämsä, Finland), A. Saarinen (Seinäjoki Central Hospital, Seinäjoki, Finland), U. Suuronen (Etelä-Karjala Central Hospital, Lappeenranta, Finland), L. Lammi (Päijät-Häme Central Hospital, Lahti, Finland), K. Lehtonen (Pohjois-Kyme Hospital, Kouvola, Finland), J. Männistö (Kymeenlaakso Central Hospital, Kotka, Finland), I. Salmi (Pohjois-Karjala Central Hospital, Joensuu, Finland), M. Torkko (Etelä-Savo Central Hospital, Mikkeli, Finland), P. Torkko (Etelä-Savo Central Hospital, Mikkeli, Finland), M. Erkkilä (Savonlinna Central Hospital, Savonlinna, Finland), H. Andersen (Vaasa Central Hospital, Vaasa, Finland), J. Jaakkola (Pietarsaari Hospital, Pietarsaari, Finland), H. Rinne (Keski-Pohjanmaa Central Hospital, Kokkola, Finland), M-L. Alho (Rauma Hospital, Rauma Finland), M. Pietiläinen (Satkunta Central Hospital, Pori, Finland), T. Toljamo (Lapland Central Hospital, Rovaniemi, Finland), M. Palomäki (Kainuu Central Hospital, Kajaani, Finland), E. Nylund (Kainuu Central Hospital, Kajaani, Finland), E. Ahonen (Kainuu Central Hospital, Kajaani, Finland), P. Impola (Oulaskangas Hospital, Oulainen, Finland), S. Saviaro (Länsi-Pohja Central Hospital, Kemi, Finlan), L. Pusa (Raasepori Hospital, Raasepori, Finland), S. Vilkman (Porvoo Hospital, Porvoo, Finland), H. Ekroos (Porvoo Hospital, Porvoo, Finland), P. Vuori (Lohja Hospital, Lohja, Finland), J. Hedman (Etelä-Karjala Central Hospital, Lappeenranta, Finland), M. Lahti (Jokilaakso Hospital, Jämsä, Finland) and A. Mursu (City Hospital of Oulu, Oulu, Finland).
K. Rajala, J.T. Lehto, E. Sutinen, T. Saarto and M. Myllärniemi designed this study. K. Rajala, E. Sutinen and M. Myllärniemi were responsible for data collection. All authors analysed the data, drafted the manuscript, and read and approved the final manuscript. K. Rajala takes responsibility for the whole work.
A part of the results presented in this article have been presented as an abstract and poster at the 15th world Congress of the European Association for Palliative Care, May 18–20, 2017, Madrid, Spain.
Support statement: The Academy of Finland, the Sigrid Jusélius Foundation, the Foundation of the Finnish Anti-Tuberculosis Association and a governmental subsidy for health sciences research have supported Lung Factor research group. The sponsors had no role in the design of the study, the collection and the analysis of the data, or the preparation of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry .
Conflict of interest: Disclosures can be found alongside this article at openres.ersjournals.com
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Supplementary data.
- < Previous
Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection
D. M., N. A. N., P. B., D. B., and P. G. contributed equally.
- Article contents
- Figures & tables
Daniel Munblit, Nikita A Nekliudov, Polina Bugaeva, Oleg Blyuss, Maria Kislova, Ekaterina Listovskaya, Aysylu Gamirova, Anastasia Shikhaleva, Vladimir Belyaev, Peter Timashev, John O Warner, Pasquale Comberiati, Christian Apfelbacher, Evgenii Bezrukov, Mikhail E Politov, Andrey Yavorovskiy, Ekaterina Bulanova, Natalya Tsareva, Sergey Avdeev, Valentina A Kapustina, Yuri I Pigolkin, Emmanuelle A Dankwa, Christiana Kartsonaki, Mark G Pritchard, Victor Fomin, Andrey A Svistunov, Denis Butnaru, Petr Glybochko, Sechenov StopCOVID Research Team , Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection, Clinical Infectious Diseases , Volume 73, Issue 1, 1 July 2021, Pages 1–11, https://doi.org/10.1093/cid/ciaa1535
- Permissions Icon Permissions
The epidemiology, clinical course, and outcomes of patients with coronavirus disease 2019 (COVID-19) in the Russian population are unknown. Information on the differences between laboratory-confirmed and clinically diagnosed COVID-19 in real-life settings is lacking.
We extracted data from the medical records of adult patients who were consecutively admitted for suspected COVID-19 infection in Moscow between 8 April and 28 May 2020.
Of the 4261 patients hospitalized for suspected COVID-19, outcomes were available for 3480 patients (median age, 56 years; interquartile range, 45–66). The most common comorbidities were hypertension, obesity, chronic cardiovascular disease, and diabetes. Half of the patients (n = 1728) had a positive reverse transcriptase–polymerase chain reaction (RT-PCR), while 1748 had a negative RT-PCR but had clinical symptoms and characteristic computed tomography signs suggestive of COVID-19. No significant differences in frequency of symptoms, laboratory test results, and risk factors for in-hospital mortality were found between those exclusively clinically diagnosed or with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR. In a multivariable logistic regression model the following were associated with in-hospital mortality: older age (per 1-year increase; odds ratio, 1.05; 95% confidence interval, 1.03–1.06), male sex (1.71; 1.24–2.37), chronic kidney disease (2.99; 1.89–4.64), diabetes (2.1; 1.46–2.99), chronic cardiovascular disease (1.78; 1.24–2.57), and dementia (2.73; 1.34–5.47).
Age, male sex, and chronic comorbidities were risk factors for in-hospital mortality. The combination of clinical features was sufficient to diagnose COVID-19 infection, indicating that laboratory testing is not critical in real-life clinical practice.
In Russia, the first confirmed cases of coronavirus disease 2019 (COVID-19) were reported by the state authorities in early March 2020 [ 1 ]. Since then, the Russian Federation climbed into the top 3 nations in the world affected by COVID-19, surpassing 400 000 cases by the end of May 2020.
The rate of infections in Moscow and the Moscow metropolitan area, with its high population density and number of inhabitants (20 million), has exceeded 180 000 confirmed cases, accounting for half of all the COVID-19 cases in Russia [ 2 ].
The clinical characteristics of COVID-19 have been described in studies from China [ 3 ], Italy [ 4 ], the United States [ 5–7 ], and the United Kingdom [ 8 ]. At present, no information on the clinical epidemiology, including clinical course, and outcomes of patients with COVID-19 in the Russian population is available. A recent editorial in The Lancet highlighted a surprisingly low mortality rate (~1%) in Russia [ 9 ]. With no academic data, perspectives on the COVID-19 pandemic in Russia are mainly based on media reports and briefs from Russian officials.
This study aimed to present demographic characteristics, symptoms, comorbidities, clinical test results, outcomes, and risk factors associated with mortality in a cohort of consecutively admitted patients with COVID-19 at the Sechenov University Hospital Network in Moscow. Secondarily, we aimed to test whether patients presenting with symptoms and radiological findings consistent with COVID-19 but without laboratory confirmation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have outcomes similar to those with positive reverse transcriptase–polymerase chain reaction (RT-PCR).
Study Design and Ethics
StopCOVID is an observational cohort study that took place at 4 large adult tertiary university hospitals in Moscow, Russia. All persons aged 18 years or olrder admitted to any of 4 Sechenov University Hospital Network hospitals between 8 April and 28 May 2020 with suspected COVID-19 infection were included in the study. RT-PCR to SARS-CoV-2 was the recommended mode of testing by the Russian Ministry of Health and was used throughout the study period in all the hospitals ( Supplementary Box 1 ). We enrolled all patients with confirmed or suspected COVID-19 infection, due to concerns of a high false-negative rate from RT-PCR results [ 10 ].
This study was approved by the Sechenov University Institutional Review Board on 22 April 2020 (protocol number 08–20).
Data Collection Process
The data were collected between 22 April and 6 June 2020. We reviewed electronic medical records for signs and symptoms on admission, baseline comorbidities, computed tomography (CT) imaging, and laboratory results for all admitted patients. Weight and height were self-reported by the patients to the clinical staff.
The data extraction was performed by a group of 40 medical students and resident doctors who went through personal protocol explanation webinars and data entry training prior to the beginning of the study. The team was supervised by senior academic staff members. The baseline characteristics were collected using the case report form (CRF) that was developed by the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and the World Health Organization (WHO) for use in outbreak investigations [ 11 ]. REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA, hosted at Sechenov University) was used for data collection, storage, and management [ 12 , 13 ].
Study Definitions
Patients were defined as having confirmed COVID-19 if the diagnosis was confirmed by laboratory testing (at least 1 SARS-CoV-2 RT-PCR positive result).
Patients were defined as having “clinically diagnosed COVID-19” if laboratory confirmation was inconclusive or not available. Details of COVID-19 case definitions, criteria for hospitalization, grading of severity, and recommended treatment approaches are presented in Supplementary Box 1 .
We reviewed radiology reports of chest CT imaging during hospitalization. The data on the presence/absence of ground-glass opacities, consolidation, and severity of radiologic changes were retrieved. Incomplete reports containing no information on severity were excluded from the analysis. The severity of changes was graded by radiologists as per national COVID-19 guidelines using the modified visual assessment scale by Inui et al [ 14 ] ( Supplementary Table 1 ). The primary outcome in this study was in-hospital mortality.
Statistical Analysis
Descriptive statistics were calculated for baseline characteristics. Continuous variables were summarized as medians (interquartile range) and categorical variables as frequencies (percentage). The chi-square test or Fisher’s exact test was used for testing differences in proportions between individuals. The Wilcoxon rank-sum test was used to test for differences in laboratory test results between the groups.
We first ran univariate analysis to investigate associations between demographic characteristics and comorbidities with mortality. Then, we performed a multivariable logistic regression model, which included all statistically significant (at P = .001) potential predictors from the univariate analysis.
A Bonferroni correction was used to adjust for multiple comparisons, such that P values less than or equal to .001 were considered statistically significant for the analysis of symptoms and comorbidities and P values less than .001 were considered statistically significant for laboratory markers. All routine clinical laboratory measurements were used in the analysis, except the ones which were available for less than 10 deceased patients. Statistical analysis was performed using R version 3.5.1 (R Core Team).
A total of 4261 adults with suspected COVID-19 infection were admitted to the hospitals. Primary outcome data were available for 3535 patients who were discharged, died, or transferred to another hospital. The study primary endpoint was available for all but 55 individuals transferred to other hospitals; thus, 3480 (82%) individuals were included in the statistical analysis.
Half of the patients (n = 1728) had positive RT-PCR results, while the second half (n = 1748) were negative on RT-PCR but had clinical symptoms and CT signs suggestive of COVID-19. No differences were noted in the baseline demographic and clinical characteristics and laboratory and radiologic findings of those with RT-PCR–confirmed versus clinically diagnosed COVID-19 ( Table 1 , Supplementary Tables 2, 4, 5, 7 ).
Laboratory Test Results (Median [IQR]) in Patients With Clinically Diagnosed COVID-19 Infection (RT-PCR Negative) and Patients With RT-PCR–Confirmed COVID-19 Infection
Statistically significant results at P values <.001 are presented in bold. The number of patients is presented for each parameter.
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; RT-PCR, reverse transcriptase–polymerase chain reaction.
Baseline Characteristics
Table 2 and Supplementary Table 2 present an overview of baseline characteristics, stratified by the primary outcome and the RT-PCT result, respectively. The median age of all patients at admission was 56 years (interquartile range, 45–66; range, 18–100 years). Similar numbers of men (50.5%, n = 1758) and women (49.5%, n = 1722) were admitted to the hospitals ( P = .55). The median age of patients who died in the hospital was higher, 72 (61.5–81) years compared with 55 (44–65) years in survivors. Time from hospitalization to discharge/death was 14.5 (11.8–17.7) days, with shorter hospital stay in patients who died. Severity at admission was recorded as mild in 632 (18.2%), moderate in 2634 (75.7%), severe in 204 (5.9%), and critical in 7 (0.2%) patients, respectively.
Baseline Characteristics of Patients Admitted to Sechenov University Hospitals, Stratified by Outcome
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; RT-PCR, reverse transcriptase–polymerase chain reaction; PT, Prothrombin.
a The proportion of patients in each subgroup is calculated from the total number of patients receiving a particular type of care (ICU, noninvasive ventilation, and invasive mechanical ventilation). Calculations were performed for each type of care, regardless of whether patients were discharged/died within the ICU facilities or were transferred to the ward and were discharged/died there.
Only 218 (6.3%) patients required admission and/or transfer to the intensive care unit (ICU), with some patients requiring noninvasive ventilation and/or invasive mechanical ventilation: 80 (2.3%) and 171 (5.0%), respectively. Although the proportion discharged alive from the ICU facilities was 42.5%, among all patients who received care in the ICU during the hospital stay, 57 (26.1%) were discharged from the hospital alive. Eight (4.7%) patients who received invasive mechanical ventilation during the hospital stay were discharged alive.
Data on symptoms and comorbidities at the time of hospital admission were available in 3382 (97%) patients. The most common symptoms in the medical records were fever (3157, 93.3%), fatigue/malaise (2684, 79.4%), cough (2476, 73.2%), and shortness of breath (2013, 59.5%). We also found a significant overlap between the top 3 most common symptoms, with 1912 (56.5%) patients having all 3 symptoms ( Figure 1 ). Shortness of breath, altered consciousness, and inability to walk were present significantly more often in patients who died, while anosmia, sore throat, fever, and muscle pain were found more frequently in those discharged alive ( Supplementary Table 3 ). Symptoms at admission did not differ significantly between the patients with laboratory-confirmed and clinically diagnosed COVID-19 ( Supplementary Table 4 ).

Stacked bar charts presenting the ( A ) top 10 most common symptoms and ( B ) most common comorbidities. Venn diagrams showing the coexistence of the ( C ) top 3 symptoms and ( D ) top 3 comorbidities at the time of hospital admission.
Detailed information on comorbidities in our cohort is presented in Table 3 , Supplementary Table 5 , and Figure 1 . The most common comorbidities were hypertension (1539, 45.5%), obesity (1129, 33.4%), chronic cardiovascular disease (621, 18.4%), and diabetes (predominantly type 2; 459, 13.6%). One in 10 patients reported current (139, 4.1%) or former (235, 6.9%) smoking. There was little overlap between the top 3 most common comorbidities, with only 145 (4%) patients having all 3, while 965 (28.5%) did not report any comorbidities.
Patient-reported Comorbidities at the Time of Hospital Admission and Chest Computed Tomography Imaging Stratified by Outcome
Statistically significant results at P values ≤.001 are presented in bold.
Abbreviations: ART, antiretroviral therapy; CT, computed tomography; HIV, human immunodeficiency virus.
aExcluding asthma.
b Obesity defined as body mass index based on electronic medical records data, and if data on height and weight were missing, records were screened for obesity definition by clinical staff.
Clinical Investigations
Most patients (71.6%) had significant changes on chest CT, equivalent to CT-2–CT-3 severity grade. Ground-glass opacity was found in over 95% of the patients and 77.95% had lung consolidation in accordance with the radiologist’s reports.
We reviewed routine clinical test measurements at admission and found abnormal changes to the coagulation profile, greater median levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), aspartate aminotransferase (AST), and lactate dehydrogenase and decreased iron levels. Those patients who died in the hospital had more abnormal changes to their coagulation profile (D-dimer, international normalized ratio, prothrombin time, ferritin, fibrinogen), lymphocytopenia, and neutrophilia, and much higher levels of CRP and ESR, high blood urea nitrogen, AST, and γ-glutamyltransferase when compared with survivors ( Table 4 ). Platelet to lymphocyte ratio was associated with a higher in-hospital mortality odds ratio (1.003; 95% confidence interval, 1.002–1.004) adjusted for age and sex.
Laboratory Test Results (Median [IQR]), Stratified by Outcome
Statistically significant results at P values <.001 and parameters with levels higher/lower than the reference range are presented in bold. The number of patients is presented for each variable.
Abbreviations: IQR, interquartile range; PT, Prothrombin.
Results of the laboratory tests routinely performed in the clinical setting did not differ significantly between patients with confirmed and clinically diagnosed COVID-19 for 48 out of 51 parameters ( Table 1 ). Platelets, leukocytes, and neutrophil count were significantly lower in patients with confirmed COVID-19, but the differences were unlikely to be relevant, being within the normal reference ranges for both groups.
Patient Outcomes and Risk Factors
Among the 3480 patients who were discharged or died during hospitalization, the overall mortality was 5.5%, with a total number of 191 people who died.
In a univariate analysis, chronic cardiovascular disease, hypertension, chronic pulmonary disease, chronic kidney disease, chronic neurological disorder, malignant neoplasm, diabetes, and dementia significantly differed between survivors and patients who died ( Table 3 ). In multivariable analysis, older age was a predictor of in-hospital mortality with an odds ratio (per 1-year increase) of 1.05 (95% confidence interval, 1.03–1.06). Other predictors associated with in-hospital mortality were male sex (1.71; 1.24–2.37), chronic kidney disease (2.99; 1.89–4.64), diabetes (2.1; 1.46–2.99), chronic cardiovascular disease (1.78; 1.24–2.57), and dementia (2.73; 1.34–5.47) ( Figure 2 ). The same risk factors were significantly associated with the admission/transfer to the ICU, with only dementia not reaching statistical significance ( Supplementary Figure 1 ).

Odds ratios and 95% CIs for in-hospital mortality from a multivariable logistic regression model. Abbreviation: CI, confidence interval.
When including COVID-19 laboratory-confirmed/suspected status as a covariate in the multivariable logistic regression model we found no evidence that it was associated with mortality (odds ratio, 1.22; 95% confidence interval, .89–1.69) and it did not have major impact on the effect size and significance of other predictors ( Supplementary Figure 2 ).
We did not find any statistically significant association of CT severity grade with in-hospital mortality, adjusting for age and sex ( Supplementary Table 6 ). With respect to CT imaging, no evidence of difference was found between the patients with confirmed and clinically diagnosed COVID-19 ( Supplementary Table 7 ).
Hydroxychloroquine was the most frequently used (84%) medication, followed by antibiotics (azithromycin [77.7%] and ceftriaxone [30.3%]), heparin (56.4%), paracetamol (34.4%), mucolytics (25.4%), lopinavir/ritonavir (16.2%), and systemic corticosteroids (10.4%), respectively ( Supplementary Table 8 ). There was a significant overlap between the top 3 most commonly used medications, with hydroxychloroquine, azithromycin, and heparin used in 1322 patients ( Supplementary Figure 3 ).
To our knowledge, StopCOVID cohort is the first large-scale study of consecutively hospitalized patients with COVID-19 in Russia assessing clinical characteristics and risk factors for in-hospital mortality. This is also the first large cohort, including both RT-PCR–confirmed COVID-19 cases and patients, diagnosed with COVID-19 based on clinical and radiological presentation in the absence of the SARS-CoV-2 RT-PCR confirmation. We found that older age and male sex as well as existing comorbidities were associated with in-hospital mortality. We found no significant difference between patients with clinical COVID-19 and laboratory-confirmed COVID-19, either in clinical presentation or in clinical measurements and risk factors for in-hospital mortality. We feel it is entirely appropriate to treat patients with clinical and radiological signs of COVID-19 who do not have an alternative diagnosis to explain their symptoms equivalently to PCR-confirmed cases. Sequential RT-PCR testing can identify patients with COVID-19 whose initial result was false-negative [ 15 ]. In settings where repeat testing is not performed, it can also be appropriate to include patients with clinical and radiological COVID-19 alongside those with laboratory-confirmed disease.
Patients in our study were of an age very similar to the New York cohort [ 6 ] and of a much lower median age than similar cohorts in Italy [ 4 ] and the United Kingdom [ 8 ]. This may be partly explained by a lack of a clear message from the authorities to the public with regard to whom should present to a hospital. Healthcare-seeking behavior may further explain a younger age at admission, which differs between the countries. Russian people are known for active specialist-seeking behavior [ 16 ], particularly in the presence of distrust of media sources [ 17 ] and easy access to free healthcare. It is, however, more likely to be a reflection of varying approaches from health services in different countries.
Patients in Moscow typically presented with fever, fatigue, cough, and shortness of breath, which is in agreement with the previously reported symptom patterns in other countries [ 5 , 8 , 18 ]. Among symptoms, anosmia was associated with a more favorable outcome, which is similar to the data from Hopkins et al [ 19 ], which showed rapid improvement in patients with COVID-19 presenting with a loss of smell.
Similar to other cohorts, cardiological conditions, hypertension, obesity, and diabetes were common problems in the hospitalized population. The lower median age of the patients in our cohort may explain the lower comorbidity rate when compared with some other studies [ 6 , 8 ]. We recorded a much lower number of patients with chronic pulmonary diseases, which is in agreement with data from Richardson et al [ 7 ] but in contrast to other US [ 6 ] and particularly UK [ 8 ] cohorts. We also found low rates of asthma in our cohort, which did not exceed the prevalence in the general population, which has been reported previously [ 20 ].
Patient age, male sex, and the presence of major comorbidities were all predictors of in-hospital mortality. These findings are in line with other international cohorts [ 6 , 21 ], including a UK ISARIC study using a similar data-collection protocol [ 8 ]. We also found common changes in the coagulation profile [ 6 ] and previously reported clinical patterns, such as lymphocytopenia, neutrophilia, and very high levels of CRP and ESR in patients who subsequently died from COVID-19. The platelet to lymphocyte ratio has been previously reported to be associated with higher severity and mortality in patients with COVID-19 [ 22 ]. Our findings agree with previous research but require further validation.
The proportion of patients admitted to the ICU in our cohort study was much lower than in the similar cohorts from the United Kingdom (17%) [ 8 ] and the United States (14.2%) [ 7 ], but similar to published data from China [ 18 ]. The decision for ICU admission within the Sechenov University Hospital Network is normally based on a joint opinion of a multidisciplinary team of respiratory physicians and intensivists. Due to good access to high-flow oxygen and noninvasive ventilation within the COVID-19 wards, only critical patients were transferred into the ICU, which may explain the lesser need for ICU admission in our cohort. Active use of noninvasive ventilation on the wards may explain the low in-hospital mortality in this group of patients. As only the most severely unwell patients were admitted for invasive mechanical ventilation, this may explain the high mortality recorded in ICU patients. The overall mortality rate in our cohort was similar to the average worldwide estimate [ 23 ] but much lower than in other international cohorts of hospitalized individuals, which may be a direct reflection of their much younger age and moderate state of disease at the time of admission in most of the patients.
Half of the patients admitted to the Sechenov University Hospital Network did not have positive RT-PCR test results, despite having clinical features of COVID-19 infection. Our findings are similar to the US data, with 42% [ 5 ] to 51.8% [ 6 ] of individuals having negative RT-PCR test results. The false-negative rate of the RT-PCR tests varies between 20% and 66% depending on the day since symptom onset [ 10 ], meaning that results must be cautiously interpreted [ 24 ], which represents a major concern related to control of the pandemic [ 25 ]. Previous research suggests that a negative RT-PCR test result does not exclude the possibility of COVID-19. Repeated testing and sampling were shown to improve the sensitivity of RT-PCR [ 15 ]. To our knowledge, previous studies of patients with COVID-19 excluded those with suspected COVID-19 infection in the absence of a positive test result [ 3–8 ]. However, this approach differs from pragmatic clinical practice, in which, in the absence of an alternative diagnosis, patients with a clinical diagnosis of COVID-19 are treated equally to laboratory-confirmed cases. When evaluating radiological findings in COVID-19, it must be born in mind that some patients may present with clinical symptoms or extrapulmonary manifestations, such as hepatic, cardiovascular, or kidney injury, but initially will have normal CT findings [ 26 ]. In our study we did not solely rely on CT findings for clinical diagnosis of COVID-19. However, new approaches to minimize the exclusion of patients with false-negative RT-PCR results should be sought, as highlighted in a recent report suggesting real-time lung ultrasound as an auxiliary method to rule-in COVID-19 during screening [ 27 ].
Limitations
This cohort study has some limitations. First, the study population only included patients within Moscow. Second, the data were collected retrospectively from the electronic medical records with no access to additional information that could be potentially retrieved from the medical notes. Third, half of the patients in our cohort did not have RT-PCR–confirmed COVID-19 infection, although this is unlikely to affect the outcomes as we failed to find any significant differences between clinically diagnosed and laboratory-confirmed cases. Fourth, endpoint outcome data were available for 83% of admitted patients. Patients admitted and/or transferred to the ICU and receiving invasive mechanical ventilation can spend a significant amount of time attached to the machine [ 7 , 8 ]. The absence of data on patients (18%) who remained in the hospital at the time of data analysis completion may lead to bias and may influence overall mortality calculations. Fifth, morbidity related to invasive procedures or sequelae in clinically suspected and/or laboratory-confirmed cases has not been recorded. Sixth, the definition of “clinically diagnosed COVID-19” implies changes on chest CT and nonspecific signs and symptoms, which may be present in other respiratory viral illnesses. The scoring system used for radiological signs is able to differentiate between symptomatic and asymptomatic cases of COVID-19 but is not fully able to differentiate between COVID-19 from other similar conditions.
Conclusions
The clinical features, chest CT, and blood test results did not differ between test-confirmed and clinically diagnosed patients. Furthermore, clinical outcomes were also identical. Our study results suggest that in order to assess the full impact of this pandemic on populations, all clinically diagnosed patients should be included. Comorbidities associated with death were similar to other published studies on COVID-19. Mortality in our cohort was low, which may have been due to the mean age of patients being lower than in some other published studies. Anosmia was associated with milder disease while asthma did not appear to pose an increased risk of adverse outcome. As with other studies, manifestations of nonrespiratory problems including coagulopathy, immune deficiency, hyperinflammation and renal deficits were associated with higher risks of death. The data collection within StopCOVID cohort is continuing and further analysis focused on predictive models of adverse outcomes for routine clinical practice is in progress.
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Sechenov StopCOVID Research Team . Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia:Anna Berbenyuk, Polina Bobkova, Semyon Bordyugov, Aleksandra Borisenko, Ekaterina Bugaiskaya, Olesya Druzhkova, Dmitry Eliseev, Yasmin El-Taravi, Natalia Gorbova, Elizaveta Gribaleva, Rina Grigoryan, Shabnam Ibragimova, Khadizhat Kabieva, Alena Khrapkova, Natalia Kogut, Karina Kovygina, Margaret Kvaratskheliya, Maria Lobova, Anna Lunicheva, Anastasia Maystrenko, Daria Nikolaeva, Anna Pavlenko, Olga Perekosova, Olga Romanova, Olga Sokova, Veronika Solovieva, Olga Spasskaya, Ekaterina Spiridonova, Olga Sukhodolskaya, Shakir Suleimanov, Nailya Urmantaeva, Olga Usalka, Margarita Zaikina, Anastasia Zorina; 1C First Bit, Moscow, Russia:Nadezhda Khitrina.
Author contributions. D. M.: Conceptualization, methodology, validation, formal analysis, resources, data curation, writing (original draft, review, and editing), supervision, project administration. N. A. N.: Conceptualization, methodology, formal analysis, investigation, writing (original draft, review, and editing), visualization, project administration. P. B.: Conceptualization, methodology, investigation, writing (original draft, review, editing), project administration. O. B.: Conceptualization, methodology, software, validation, formal analysis, data curation, writing (original draft, review, and editing), visualization. M. K.: Formal analysis, investigation, writing (original draft, review, and editing), visualization. E. L.: Investigation, writing (original draft, review, and editing), project administration. A. G.: Investigation, writing (original draft, review, and editing), project administration. A. S.: Investigation, project administration. V. B.: Resources, writing (review and editing). P. T.: Resources, project administration, writing (review and editing). J. O. W., P. C., and C. A.: Writing (original draft, review, and editing). E. Bezrukov: Funding acquisition, writing (review and editing). M. E. P., A. Y., E. Bulanova, and N. T.: Writing (review and editing). S. A.: Writing (review and editing), investigation. V. K. and Y. P.: Writing (review and editing). E. A. D., C. K., and M. P.: Methodology, writing (review and editing). V. F.: Writing (review and editing). A. A. S.: Funding acquisition, writing (review and editing). D. B.: Conceptualization, methodology, resources, writing (review and editing), project administration, funding acquisition. P. G.: Project administration, funding acquisition, writing (review and editing), supervision. StopCOVID Research Team: Investigation, writing (review and editing).
Acknowledgments. The authors are very grateful to the Sechenov University Hospital Network clinical staff and to the patients, carers, and families for their kindness and understanding during these difficult times of the COVID-19 pandemic. We thank Dr Inna Tulina, Dr Yuri Kitsenko, Mrs Ekaterina Rebrova, and Mr Maksim Kholopov for providing technical support in data collection and database administration. We are grateful to Ms Olga Burencheva, Dr Daria Levina, Ms Olga Sokova, Ms Natalia Chepelova, and Ms Elizaveta Mikhsin for assistance in data extraction. We highly appreciate the kind expert advice from Professor Gareth Tudor-Williams, Dr Jethro Herberg, Dr Nikita Sushentsev, and Dr Anna Pokshubina for assistance in data interpretation. Finally, we extend our gratitude to Laura Merson and the entire ISARIC team for their continuous support and expertise and for providing access to the REDCap CRF module.
Financial support. This work was supported by the Russian Academic Excellence Project “5–100” and Russian Foundation for Basic Research (RFBR) (grant number 20-04-60063).
Potential conflicts of interest. J. W. reports grants and personal fees from Danone/Nutricia and Airsonnet, nonfinancial support from Anaphylaxis Campaign, and lecture fees from Friesland Campina, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing . About confirmed case of the novel coronavirus infection COVID-2019 in Russia. Available at: https://www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=13870 . Accessed 9 June 2020 .
Government of Russian Federation . Stopcoronavirus.rf—Official information about Covid-19 in Russia. Available at: https://xn--80aesfpebagmfblc0a.xn--p1ai/ . Accessed 10 June 2020 .
Zhou F , Yu T , Du R , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study . Lancet 2020 ; 395 : 1054 – 62 .
Google Scholar
Grasselli G , Zangrillo A , Zanella A , et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy . JAM A 2020 ; 323 : 1574 – 81 . doi: 10.1001/jama.2020.5394 .
Argenziano MG , Bruce SL , Slater CL , et al. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series . medRxiv 2020 . doi: 10.1101/2020.04.20.20072116 .
Petrilli CM , Jones SA , Yang J , et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study . BMJ 2020 ; 369 : m1966 .
Richardson S , Hirsch JS , Narasimhan M , et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area . JAMA 2020 ; 323 : 2052 – 9 .
Docherty AB , Harrison EM , Green CA , et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study . BMJ 2020 ; 369 : m1985 .
Salient lessons from Russia’s COVID-19 outbreak . Lancet 2020 ; 395 : 1739 .
Kucirka LM , Lauer SA , Laeyendecker O , Boon D , Lessler J . Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure . Ann Intern Med 2020 ; 173 : 262 – 7 .
International Severe Acute Respiratory and Emerging Infection Consortium and World Health Organisation . Clinical data collection—the COVID-19 case report forms (CRFs). Available at: https://isaric.tghn.org/COVID-19-CRF/ . Accessed 22 June 2020 .
Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform 2009 ; 42 : 377 – 81 .
Harris PA , Taylor R , Minor BL , et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners . J Biomed Inform 2019 ; 95 : 103208 .
Inui S , Fujikawa A , Jitsu M , et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) . Radiol Cardiothoracic Imaging 2020 ; 2 : e200110 .
Zhang JJ , Cao YY , Dong X , et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2 . Allergy 2020 ; 75 : 1809 – 12 . doi: 10.1111/all.14316 .
Ipsos . Global views on healthcare in 2018. Available at: https://www.ipsos.com/sites/default/files/ct/news/documents/2018-07/Global%20Views%20on%20Healthcare%202018%20Graphic%20Report.pdf . Accessed 17 June 2020 .
Benisovich SV , King AC . Meaning and knowledge of health among older adult immigrants from Russia: a phenomenological study . Health Educ Res 2003 ; 18 : 135 – 44 .
Guan WJ , Ni ZY , Hu Y , et al. Clinical characteristics of coronavirus disease 2019 in China . N Engl J Med 2020 ; 382 : 1708 – 20 .
Hopkins C , Surda P , Whitehead E , Kumar BN . Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study . J Otolaryngol Head Neck Surg 2020 ; 49 : 26 .
Avdeev S , Moiseev S , Brovko M , et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19 . Allergy 2020 :1–3. doi: 10.1111/all.14420 .
Cummings MJ , Baldwin MR , Abrams D , et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study . Lancet 2020 ; 395 : 1763 – 70 .
Chan AS , Rout A . Use of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19 . J Clin Med Res 2020 ; 12 : 448 – 53 .
World Health Organization . Coronavirus disease 2019 (COVID-19) situation report—46. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4 . Accessed 22 June 2020 .
Tahamtan A , Ardebili A . Real-time RT-PCR in COVID-19 detection: issues affecting the results . Expert Rev Mol Diagn 2020 ; 20 : 453 – 4 .
Woloshin S , Patel N , Kesselheim AS . False negative tests for SARS-CoV-2 infection—challenges and implications . N Engl J Med 2020 ; 383 : e38 .
Harmon SA , Sanford TH , Xu S , et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets . Nat Commun 2020 ; 11 : 4080 .
Smallwood N , Walden A , Parulekar P , Dachsel M . Should point-of-care ultrasound become part of healthcare worker testing for COVID? Clin Med (Lond) 2020 ; 20 : 486 – 7 .
Author notes
- comorbidity
- hospital mortality
- hospitals, university
- laboratory techniques and procedures
- reverse transcriptase polymerase chain reaction
- signs and symptoms
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

COMMENTS
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea. It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life ...
The mMRC dyspnea scale is used to calculate the BODE index, a tool which helps estimate the survival times of people living with COPD. The BODE Index is comprised of a person's body mass index ("B"), airway obstruction ("O"), dyspnea ("D"), and exercise tolerance ("E"). Each of these components is graded on a scale of either 0 to 1 or 0 to 3 ...
The modified Medical Research Council (mMRC) scale is recommended for conducting assessments of dyspnea and disability and functions as an indicator of exacerbation. The modified Medical Research Council (mMRC) scale. An mMRC scale grade of 3 have a significantly poorer prognosis and that the mMRC scale can be used to predict hospitalization ...
The scale uses a simple and standardized method of categorizing disability in COPD (Cazzola M 2008). It quantifies disability related to dyspnea and has been widely used to describe co horts and stratify interventions including PR in COPD. It has been in use for over 50 years. Public domain. There is possible underestimation bias due to ...
The physical limitation or functional impact of breathlessness can be assessed using the Medical Research Council dyspnea scale (MRC; or modified MRC [mMRC] 39, 40 which is more widely used), 41 Dyspnea Exertion Scale (DES), 42 Oxygen Cost Diagram (OCD), 43 Baseline Dyspnea Index (BDI), 29 or Disability Related to COPD Tool (DIRECT). 44 The ...
The modified Medical Research Council (mMRC) dyspnea scale comprises five statements that describe a range of dyspnea effects in increasing order of severity. Use of this questionnaire is recommended in the GOLD 2020 report 5 ( Supplementary Table 1 ).
Assessment of dyspnea in COPD patients relies in clinical practice on the modified Medical Research Council (mMRC) scale, whereas the Baseline Dyspnea Index (BDI) is mainly used in clinical trials. Little is known on the correspondence between the two methods.
Calculator: Modified Medical Research Council (mMRC) scale for dyspnea - UpToDate.
Background Dyspnea is very frequent in obese subjects. However, its assessment is complex in clinical practice. The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese ...
The modified Medical Research Council (mMRC) dyspnoea scale is a measure of breathlessness severity recommended by guidelines and utilised as an inclusion criterion or endpoint for clinical trials. No studies have been conducted to validate the categorical descriptors against the dyspnoea severity grade.
Background: In multidimensional Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, the choice of the symptom assessment instrument (modified Medical Research Council dyspnea scale [mMRC] or COPD assessment test [CAT]) can lead to a different distribution of patients in each quadrant. Considering that physical activities of daily living (PADL) is an important ...
Introduction: Health-related quality of life (HRQoL) is an important patient-centred outcome in chronic obstructive pulmonary disease (COPD). The aim of the current study is to compare the discriminative capacity of the modified Medical Research Council (mMRC) dyspnoea scale and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric classification of COPD on HRQoL, as ...
Introduction. In the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the patients' symptoms can be assessed by either the modified Medical Research Council (mMRC) dyspnea score or the chronic obstructive pulmonary disease (COPD) assessment test (CAT) score. 1 MRC was developed to help physicians establish clinical grades of breathlessness for their patients with ...
UpToDate is a trusted source of evidence-based medical information for clinicians and patients. This image shows the modified Medical Research Council (mMRC) scale for dyspnea, a simple tool to assess the severity of breathlessness in patients with respiratory diseases.
Significant improvements were found in the patients' ability of daily living (ADL), dyspnea (assessed by modified medical research council dyspnea scale (MMRC)), handgrip strength, maximal inspiratory and expiratory pressure, anxiety (using the 7-item generalized anxiety disorder scale (GAD-7)) and depression (the 9-item patient health ...
Change in the degree of dyspnea on the MMRC scale from baseline after 2.5 months and 6 months in patients of the Longidaze® group compared with the dynamic observation group. MMRC scale (Modified Medical Research Council scale) 0 - no - Dyspnea does not bother, except for very intense exercise
We used the British Medical Research Council (MRC) dyspnoea scale, the EuroQoL five-dimension five-level (EQ-5D-5L) questionnaire, the EuroQoL Visual Analogue Scale (EQ-VAS) asking participants to score their QoL from 0 (worst imaginable health) to 100 (best imaginable health), UNICEF/Washington disability score and World Health Organisation ...
The BODE index, a multidimensional grading system, is based on the body-mass index (B), the degree of airflow obstruction (O) evaluated by FEV1, the grade of dyspnea (D) assessed by the modified Medical Research Council (mMRC) dyspnea score, and the exercise capacity (E) assessed by the six-minute walking distance test (6MWD).
Background Neurological COVID-19 disease has been reported widely, but published studies often lack information on neurological outcomes and prognostic risk factors. We aimed to describe the spectrum of neurological disease in hospitalised COVID-19 patients; characterise clinical outcomes; and investigate factors associated with a poor outcome. Methods We conducted an individual patient data ...
The modified Medical Research Council scale (mMRC scale) is largely used in the assessment of dyspnea in chronic respiratory diseases, but has not been validated in obesity. The objectives of this study were to evaluate the use of the mMRC scale in the assessment of dyspnea in obese subjects and to analyze its relationships with the 6-minute ...
The specific questionnaires of symptoms and HRQoL were modified Edmonton Symptom Assessment Scale (ESAS), modified Medical Research Council (mMRC) dyspnoea scale and the RAND 36-Item Health Survey (RAND-36). The ESAS is a self-rated, numeric-rating, symptom-based scale developed for assessing the symptoms of cancer patients .
Medical Research Council Population Health Research Unit, Nuffield Department of Population Health, University of Oxford ... The severity of changes was graded by radiologists as per national COVID-19 guidelines using the modified visual assessment scale by Inui et ... The most common symptoms in the medical records were fever (3157, 93.3% ...