- Representative Publications
- Grant Writing
- YCCI Mentor Training
- Foundations of Community-Engaged Research Course
- 2022 Scholars
- 2021 Scholars
- Scholars: 2020-2010
- Scholars: 2009-2006
- Masters of Health Science Research Program
- Research in Progress Online Lectures
- YCCI Scholars RFA
- Fund to Retain Clinical Scientists at Yale
- COVID-19 Fund to Retain Clinical Scientists
- Past Awardees
- 2023 Summer Interns
- 2022 Summer Interns
- 2021 Summer Interns
- 2023 YSERE Interns
- 2022 YSERE Interns
- Additional Training Programs

Past and Current YCCI Interns
- Faculty Dinner Series
- GCP Training
- Introduction to Clinical Research
- Coffee and Conversation
- Lunch and Learn
- SOCRA Training and Certification Exam
- Research Practitioner
- 2020 Training
- 2019 Training
- 2018 Training
- 2017 Training
- 2016 Training
- 2015 Training
- 2014 Training
- 2013 Training
- 2012 Training
- 2011 Training
INFORMATION FOR
- Residents & Fellows
- Researchers
Yale Center for Clinical Investigation Internship Program
- Yale Center for Clinical Investigation (YCCI)
- Center for Musculoskeletal Disorders
- Child Study Center
- Department of Psychiatry
- Program of Applied Translational Research
- Yale Cancer Center
- Yale Children’s Diabetes Program
- Yale Diabetes Center
- Yale Equity Research and Innovation Center
- Yale Stress Center
- … and many others
- Working with and shadowing senior clinical research faculty as they conduct clinical research
- Human subject protection and regulatory training
- Meeting regularly with YCCI and faculty leadership to provide updates and receive mentoring
- Working with our regulatory and subject recruitment units
- Rotations in our inpatient and outpatient research clinics
Interested in joining our clinical research team?
Meet the Past and Current YCCI Interns!
The long conversations and mentoring I received from Dr. Sherwin inspired me to become a physician-scientist. Ania Jastreboff, MD, PhD, Assistant Professor of Medicine (Endocrinology) and of Pediatrics (Pediatric Endocrinology)
Skip to content
Meet the Team
Welcome message.
Read a message from the Chair of the Department of Emergency Medicine.
Fellowships
Research questions.
Get Answers for frequently asked questions about research in the Department of Emergency Medicine.
Patient Care
- In Memory of Dr. Breen
Clinical Research Internship Program
The Clinical Research Internship is a unique opportunity for students to directly participate in the performance of clinical research, such as enrolling patients in study programs, while also observing overall emergency department (ED) operations and interacting with physicians, nurses and other health care personnel. The ED Research Internship offers a broad diversity of research from basic observational studies to complex randomized controlled trials thereby exposing students to the full spectrum of research applications in a single clinical setting. Students will gain research experience working in the ED that will strengthen their skills in developing a career in public health and/or medicine. Students will be exposed to the practical application of research to provide critical context and texture to research theory they may have received in the classroom.
Our Mission and Goal:
The program's mission is to conduct innovative and practice-changing science while supporting and training a diverse group of clinician-scientists dedicated to improving patient care. Our program goals are to provide a clinical research educational experience for pre-health professionals, hands-on training to gain research-related skills, and exposure to an acute clinical setting.
Clinical Research Intern Position Roles:
- Attend study-related training sessions held by Clinical Research Coordinators (CRC) throughout the course of the internship.
- Master the processes of properly obtaining informed consent and practicing good clinical practice (GCP) in an acute care setting.
- Develop proficiency in clinical research activities, such as screening, pre-consenting, and enrolling eligible patients with a CRC mentor.
- Increase understanding of the principals of compliance, patient confidentiality, and other ethical obligations involved with conducting clinical research.
- Interact with Principal Investigators (PIs) and other departments by attending program-exclusive lectures, health fairs, trainings, workshops, etc.
- Interact and observe patient care with clinical staff.
Pediatric & Adult ED Differences:
Pediatric Emergency Medicine (PEM)
- Peds ED treats 0-20 y/o patients
- Larger RI Cohort (~20 total)
- PEM Supervising RC Team
General Emergency Medicine (GEM)
- Adult ED treats 18-100+ y/o patients
- Smaller RI Cohort (~10 total)
- GEM Supervising RC Team
Research Intern Program Requirements:
- Must be a current pre-medical or pre-health student who’s academic program requires they gain experience in a medical setting
- Commit to ONE FULL YEAR (12 months) of two 4-hour shifts or one 8-hour shift per week
- A mandatory 4-hour program orientation,
- A protocol training session for each active research study
- At least one (of the many) Principal Investigator (PI) Lecture Series seminars held throughout the course of the year.
For more information
Please contact us at [email protected](link sends e-mail)
Vanderbilt Summer Science Academy
Undergraduate clinical research internship program.
The Vanderbilt Undergraduate Clinical Research Internship Program (UCRIP) gives college students earning a four-year degree the opportunity to participate in both research and clinical patient care at an academic medical center. This program is designed for students who are interested in a career in medicine. Participants will complete a research project under the directorship of a research mentor and also directly observe clinical patient care while spending time with residents and attending physicians.
- The 2024 session will run from the beginning of June through the first week in August, with the Sunday before and Saturday after allotted for moving.
- Program participants will be required to attend on-site orientation and training prior to beginning their research and clinical experiences.
- Students will round with a hospital-based general medicine physician team each week.
- Students will be assigned a research mentor and project by the Program Director. The director will try to place each participant with a mentor in an area of his/her interest as is available. The project may be either clinical or basic science research.
- Students will attend weekly seminars to discuss various topics, including medical school admissions, medical student life, medical education, and other issues related to healthcare.
- Program participants will be required to present their research at the conclusion of the program.
- The program provides each student housing on the Vanderbilt campus for the summer. Information on Vanderbilt summer housing can be found here: https://www.vanderbilt.edu/meetatvanderbilt/academic-intern-housing/
- Participants will receive a stipend of $1,500. The program does not provide support for travel or meals.
- Students will have the opportunity to interact socially and academically with students participating in other Vanderbilt Summer Science Academy programs.
- Disadvantaged students accepted into the program may be eligible for financial assistance.
Eligibility
- The Program is limited to U.S. citizens (or persons having U.S. Permanent Resident status) earning a four-year undergraduate degree at an accredited US college or university.
- Applying students must be a college undergraduate during the summer of the program year.
- Applying students must have a cumulative GPA of 3.5 or greater on a 4.0 scale.
- Preference is given to candidates with prior research experience and who have taken advanced science courses.
- Applying students should have a strong desire to pursue an M.D. degree or a combined M.D./Ph.D. degree.
Application Instructions
The application will include:
- A brief personal statement which addresses your interests, your qualifications for the program, and how you believe this program will help you achieve your professional goals.
- Two letters of recommendation
- Uploaded unofficial transcript
The application opens on October 1. The application deadline is February 1, 2024. Click here to start the application through the Vanderbilt Summer Science Academy
Questions can be emailed to:
- Fernando Murphy Senior Administrative Assistant Office for Diversity Affairs Email: [email protected]
- About the School
- Quick Facts
- Administration
- Basic Sciences
- A-Z Directory
- Contact Information
- Campus Maps & Parking
- Current Students
- Basic Sciences Faculty Affairs
- Clinical Faculty Affairs
- Eskind Biomedical Library
- People Finder

Welcome to the Duke University School of Medicine Clinical Research Internship Portal (CRISP)!
Our goal is to assist students earning a degree with finding an opportunity to participate in research at an academic medical center. Students will work in a mentored team environment that supports learning in clinical research.
At this time, only students from institutions that have existing internship agreements with Duke University are eligible to participate in internships at Duke. These schools include:
Campbell University
Durham Technical Community College
Elon University
Greensboro College
Meredith College
North Carolina Central University
Northern Illinois University
University of North Carolina, Chapel Hill
University of North Carolina, Greensboro
University of North Carolina, Wilmington
University of North Florida
Wake Forest University
If you are not from one of the above schools, review our additional opportunities page here .
This program is supported by the Duke Clinical & Translational Science Institute (CTSI) and the Duke Office of Clinical Research .

- Career Opportunities
- Working Here
- Returning Applicants
- Current Employees
- Mission, Vision, Values
- Corporate Responsibility
- Patient Care Technician
- Travel Careers
- Clinical Managers
- Allied Health
- Quality & Compliance
- Research and Development
- Manufacturing, Distribution and Drivers
- Student Programs
- Nursing Overview
- Acute Nursing
- Clinic Nursing
- Home Therapies Nursing
- Travel Nursing
- Azura Vascular Nursing
- Patient Care Technician Overview
- Travel Careers Overview
- Allied Health Overview
- Biomedical Technician
- Medical (MD, PA/NP, Surg Tech)
- Social Work
- Corporate Overview
- Customer Experience
- Human Resources
- Information Technology
- Marketing & Communications
- Quality & Compliance Overview
- Post Market Surveillance
- Research and Development Overview
- Pharmaceutical
- Research & Development
- Regulatory Affairs
- Manufacturing, Distribution and Drivers Overview
- Distribution
- Manufacturing
- Market Leadership
- Training & Development
- University Relations
Radius Miles 5 miles 15 miles 25 miles 35 miles 50 miles
Im prove Healthcare with You r care er
Clinical research intern, new york, new york.
As an intern with Fresenius Medical Care, a student will apply classroom based knowledge to workplace experience and will benefit from learning experiences in their major area of study.
Supports FMCNA’s mission, vision, core values and customer service philosophy. Adheres to the FMCNA Compliance Program, including following all regulatory and division/company policy requirements.
PURPOSE AND SCOPE:
Under supervision, the Clinical Research Intern will assist in carrying out research activities related to clinical research. In addition, they will be given the opportunity to enhance their research skills by working with our diverse team of researchers. The Clinical Research Intern will become more familiar with renal (patho)physiology, renal replacement therapies, and research being done to improve patient outcomes.
PRINCIPAL DUTIES AND RESPONSIBILITIES:
- Under close supervision, utilizes established procedures to perform routine assigned research tasks
- Learns to use good clinical practice and research integrity concepts
- Applies company policies and procedures to resolve routine issues
- Works on problems of limited scope. Follows standard practices and procedures in analyzing situations or data from which answers can be readily obtained
- Conducts literature reviews
- Assists in the preparation of study-related materials, including screening forms, case report forms and questionnaires
- Assists in medical record review
- Under supervision, assists in the recruitment of study subjects
- Assists in the collection and analysis of data
- Organizes, maintains, and updates project-related files and databases
- Attends routine project meetings
- Attends seminars and other meetings as necessary
- Builds stable working relationships internally
- Escalates issues to supervisor/manager for resolution, as deemed necessary
- Assists with various projects as assigned by direct supervisor
- Other duties as assigned
PHYSICAL DEMANDS AND WORKING CONDITIONS :
- The physical demands and work environment characteristics described here are representative of those an employee encounters while performing the essential functions of this job. Reasonable accommodations may be made to enable individuals with disabilities to perform the essential functions.
- Day to day work includes desk and personal computer work as well as interaction with study subjects and clinic personnel. The position requires travel to participating research sites in New York City.
- Enrolled in a bachelor’s degree program, with a minimum of 1 year of undergraduate program completed, preferably with a focus in healthcare, research, biology, chemistry, mathematics, or engineering
EXPERIENCE AND REQUIRED SKILLS :
- 0 - 2 years’ related experience preferred
EO/AA Employer: Minorities/Females/Veterans/Disability/Sexual Orientation/Gender Identity
Fresenius Medical Care North America maintains a drug-free workplace in accordance with applicable federal and state laws.
You do not have any recently viewed jobs
You do not have any saved jobs
- Louisville.edu
- Health Sciences Center
- PeopleSoft HR
- PeopleSoft Campus Solutions
- PeopleSoft Financials
- Business Ops
- Cardinal Careers
Division of Infectious Diseases
- Departments /
- Department of Medicine /
- Divisions /
- Division of Infectious Diseases /
- Education /
- Clinical Research Internship
Clinical Research Internship Program
Overview program structure application.
The Center of Excellence for Research in Infectious Diseases organizes the only comprehensive Clinical Research Internship to provide participants with immersive learning opportunities in clinical research. This university approved internship offers a combination of in-class, hands-on, and project-based learning in all aspects of the clinical research process.
Forest Arnold, DO Program Director
Program Structure
The program curriculum is designed to achieve multiple learning goals that equip participants with the necessary skills to develop and implement a clinical research program in the United States and internationally.
- In-Class Clinical Research Learning: Members of CERID provide the didactic portion of the program lectures. The curriculum describes the theoretical elements of planning and performing clinical studies, analyzing study results, and dissemination of new knowledge generated through clinical research.
- Hands-On Clinical Research Learning: Interns move beyond clinical research theory into the practice of clinical research. They acquire experiential learning working with members of different CERID research units, including Data Management, Research Implementation, Laboratory, Biorepository, Quality Assurance, Biostatistics, Medical Writing, Medical Informatics, Community Outreach, Research Administration, and Research Finances. First-hand experience with patients participating in clinical studies occurs at any of the ten CERID participating hospitals. These hospitals serve the entire population of the city of Louisville.
- Project-Based Clinical Research Learning: Under the guidance and monitoring of a CERID mentor, interns will perform a series of projects related to research dissemination. These projects may include oral presentations, literature reviews, abstracts, posters, or manuscripts. The mentor will offer advice to the intern regarding their research topic and the type of research dissemination activity. The project work may be performed by a single intern, or by a group of interns according to the project scope and difficulty.
These three learning approaches prepare interns by bridging the gap between clinical research theory and clinical research practice.
Application
The application for the internship program will be open from tuesday 04/09/2024 10:30 am edt to tuesday 04/23/2024 10:30 am edt. , how to apply.
The Clinical Research Internship program with the University of Louisville Division of Infectious Diseases hosts two cohorts each year with 20-30 participants per cohort. Applications from qualified candidates are accepted twice per year. To be considered, applicants must submit all required materials during the open application process.
Minimum Qualifications
- Demonstrate English language proficiency
- Provide documentation of personal financial support in the amount of $1,200 USD per month
- Provide proof of Repatriation and Medical Insurances
- Available for a 1-year dedicated schedule, Monday through Friday from 8:00a - 4:30p
Application Process
The table below outlines the application process and the required documents for each step. Please note that if you have previously applied to the program you will need to complete the full application and resubmit all requested documents.
Back to Top
Monday-Friday 8AM-4:30PM
501 East Broadway Suite 100 Louisville, Kentucky 40202
Phone and Fax
Office: 502-852-7844
550 Clinic: 502-561-8844
Vaccine Clinic/ Travel : 502-852-6464
Civil Surgeon : 502-852-1867
Office Fax: 502-852-1961
Clinic Fax: 502-561-8843
Infectious Diseases : [email protected]
Journal of Respiratory Infections: [email protected]
Med School Admissions: [email protected]
Twitter @UofLDID
LinkedIn UL Division of Infectious Diseases
Exploring the Art, Science & Joy of Medicine
- See us on facebook
- See us on twitter
- See us on instagram

Mission: Stanford CSI brings together curious learners from differing backgrounds to actively engage in the exploration of the art and science behind world-class medicine. Discover, contribute and make meaningful connections and friendships while working alongside dedicated and dynamic Stanford medical students, residents and faculty, who are all eager to share the joy they have found in medicine.
2024 stanford clinical summer internship, session 1 (in person): july 8 - 19, 2024, session 2 (virtual): july 22 - aug 2, 2024, fall intensive (virtual): sept 5 - nov 7, 2024 , a transformative experience for rising high school juniors, seniors, and undergraduate pre-medical students.
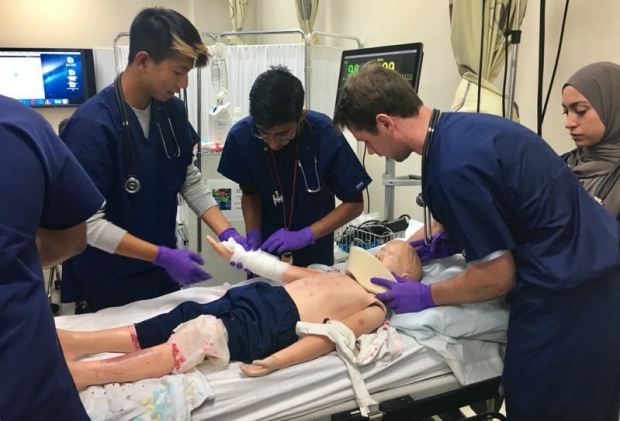
Simulations of real medical cases

Sessions with current medical students, residents, and medical school admissions officers

Visits to the emergency "Life Flight" station & Stanford clinics

Guided heart, brain and kidney dissections lead by Stanford faculty and doctors
Csi keynotes.

Dean Winslow, MD: Professor of Medicine (Hospital Medicine) and Senior Fellow, by courtesy, at the Freeman Spogli Institue for International Studies - CSI 2023 Graduation

Tamara Dunn, MD: Associate Chair, Diversity & Inclusion (DoM), Program Director, Hematology Felowship, Co-Chair Diversity & Inclusion Council - CSI 2022 and 2021 Graduation

Abraham Verghese, MD: Lane Provostial Professor, and Vice Chair for the Theory and Practice of medicine at the School of Medicine at Stanford University and best-selling author - 2022 and 2019 CSI Graduation

Megan Mahoney, MD: Chief of Staff, Stanford Health Care - CSI 2020 Graduation

Neera Ahuja, MD: Division Chief, Hospital Medicine, Stanford University School of Medicine, Medical Director, General Medicine Inpatient Wards, Stanford, Medical Director, Pharmacy, Stanford
"This is the best time to be in Medicine."

Sang-ick Chang, MD: Division Chief, Primary Care and Population Health, Department of Medicine, Associate Dean for Primary Care & Population Health, Stanford School of Medicine
"Medicine continues to be the most rewarding profession anyone could choose. A balance of head and heart, and the ability to make a real difference, every day."
Research and Training Opportunities
New section.
Looking for ways to enrich your medical school experience? Check out our directories of clinical, research, and public health opportunities.

Looking for ways to enrich your medical school experience? Search for fellowships, internships, summer programs, scholarships, and grants currently available in the United States and abroad.

Earn two degrees in four to five years to improve the health of the individuals and communities you serve.
The National Institutes of Health (NIH) Medical Research Scholars Program (MRSP) is a comprehensive, year-long research enrichment program designed to attract the most creative, research-oriented medical, dental, and veterinary students to the intramural campus of the NIH in Bethesda, MD.
Summer programs at the National Institutes of Health (NIH) provide an opportunity to spend a summer working at the NIH side-by-side with some of the leading scientists in the world, in an environment devoted exclusively to biomedical research.

- Areas of Research
- Benefits of Research
- Research Studies
- Physician-Initiated Research
- Patient Stories
- Grand Rounds
- Clinical Research Internship
- International Scholar Program
- Ways to Give
- Memorial & Tribute Giving
- Make a Planned Gift
- Become a Monthly Donor
- Articles & Newsletters
- Board of Directors

Clinical Research Internship Program
Developing and empowering the next generation of cardiovascular health professionals through a cutting-edge research internship program.
- Internship Application
- Meet The Interns
Educating the Next Generation
The Minneapolis Heart Institute Foundation® (MHIF) offers one of the most outstanding and unique internship opportunities available to undergraduates who are pre-med or planning a career in medicine. The goal of the research internship program is to develop and empower the next generation of cardiovascular health professionals by offering a robust research experience in the field of cardiology.
During this unique educational experience, the research intern is paired with a physician mentor who is actively engaged as a clinician and researcher at a nationally recognized hospital. At the conclusion of the program, the interns will showcase their research work at an open house and poster session. Many projects result in the intern having the opportunity to be an author on an article published in a national scientific journal. Some interns also have the opportunity to present their work at a national cardiovascular conference.
This internship is made possible thanks to philanthropic support from generous donors.

Research Internship Details
Research Internship Overview
Be a part of the Minneapolis Heart Institute Foundation’s exceptional 12-week cardiovascular clinical research internship designed for undergraduate students who are pre-med or planning a career in medicine. During this unique educational experience you will be paired with a physician mentor who is actively engaged as a clinician and researcher at a nationally recognized hospital. You will have the opportunity to:
- Participate in groundbreaking cardiovascular research
- Shadow a physician mentor in hospital rounds, watch procedures and listen as care teams discuss cases.
- Observe an open-heart procedure.
- Attend case conferences where cross-functional care teams discuss difficult cases.
- Shadow cardiovascular nurses in the ICU and post-surgical care area.
- Observe leading-edge cardiovascular diagnostic services.
- Reflect on your career options while hearing physicians’ candid stories of career journeys and passions during lunch and learns.
- Participate in interactive medical device industry tours and a hands-on tour of cardiovascular specimens at a nationally renowned heart registry.
At the conclusion of the internship, you will showcase your research work at an open house and poster session. Many projects result in the intern having the opportunity to be an author on an article published in a national scientific journal. Some interns will also have the opportunity to present their work at a national cardiovascular conference.
Location: On the campus of the flagship hospital of Allina Health, Abbott Northwestern Hospital, in Minneapolis, Minnesota.
Program dates: May 22 - August 9, 2024
Compensation: A $550 per week stipend paid bi-weekly to assist with expenses for housing and living expenses (no direct housing provided). Scholarship opportunities may be available for accepted applicants who need further support for travel or living expenses.
Application Opens: November 10, 2023
Deadline: January 3, 2024
Selection Notification: March 4, 2024
Program Dates: May 22 - August 9, 2024
Eligibility
- Undergraduate or recent graduate from a four-year college and not yet accepted to medical school. Preference will be given to those who have completed their junior year by the start of the research internship. Strong applicants who have completed their sophomore year or just graduated from college may also be considered.
- Must be enrolled in or a very recent graduate from an accredited degree program and not yet accepted to medical school.
- GPA of 3.60 or above.
- Applicants must be US citizens or have Permanent Resident status (be able to provide proof of employability through completion of the I-9 Form).
- The program welcomes applicants from all backgrounds, including but not limited to BIPOC (Black, Indigenous, People of Color) individuals, individuals who are historically underrepresented in health-related sciences and medical fields, individuals who face greater obstacles to educational achievements and individuals who can contribute to diversity, equity and inclusion efforts in the health-related sciences and medical fields.
- Must be able to complete 8-12 hours of independent, online CITI training (basic training required to participate in human subjects/clinical research) by April 30.
- Available to participate full-time (at least 40 hours per week) in the internship May 22 through August 9, 2024.
Steps Before You Apply
- Review the sample application PDF and prepare responses to the short answer and essay questions. Please note: this is an example and the application for the next cohort may change.
- Write a personal motivation statement (3,500 characters including spaces).
- Prepare a one-page resume (save as PDF or Word doc with file name First Name_Last Name).
- Note that you will have the opportunity to make changes to your application before submitting.
Program Components
In preparing for a career in medicine, this experience will position you well through a unique opportunity to participate in cardiovascular clinical research, shadow clinicians in action and witness everything from a routine day to extraordinary life-saving procedures.
- Orientation: Three days of orientation will prepare you for your research project, ground you in cardiovascular essentials and introduce you to the colleagues you will be working with, including your research physician, staff mentor and fellow interns.
- Research project: You will be paired with a physician who is the Principal Investigator on a research project and a staff mentor. In addition to capturing data from the electronic health record, organizing and analyzing the data and preparing conclusions, you may also write case reports and support other research projects.
- Observations: To provide you with exposure to what a career is really like in the field of medicine, you will observe various cardiovascular procedures, shadow healthcare professionals and visit cardiovascular related departments at Abbott Northwestern Hospital. Additionally, interns shadow healthcare professionals in the ICU and post-procedural care areas.
- Field Trips: Minneapolis is a hub for headquarters of major medical device companies and start-ups. You will have custom tours of some major medical device companies, the renowned Visible Heart Lab at the University of Minnesota and the Jesse E. Edwards Registry of Cardiovascular Disease. This registry is a repository for heart specimens of almost every known type of heart disease, including rare congenital and adult heart diseases.
- Poster Session: The culminating event of the research internship program, you will display your outcomes on a research poster and present to mentors, other medical professionals, MHIF board members, family and friends.
- Cohort Activities: The internship draws talent from across the country. Many interns gain friends for life through their summer experience in Minneapolis. In addition to MHIF-organized activities, interns plan activities for the weekends. Past interns have enjoyed camping, hiking, canoeing, BBQs and exploring the Twin Cities of Minneapolis and St. Paul.
Frequently Asked Questions
How many applications do you typically receive? 150-250 each year.
How many research interns are accepted? 7-9 new interns plus one lead intern from the previous cohort.
Should I apply if my GPA is below 3.6? A 3.6 GPA or above is highly preferred. The application has a space to explain extenuating circumstances.
Is a social security number (SSN) required to participate in this research internship? Yes, a SSN is required by the US Internal Revenue Service of all employers who report wages (stipend). Further information regarding SSNs can be found on the Social Security fact page .
Can I submit a letter of recommendation with my application? No. The online application with an attached resume are the accepted items.
The academic calendar for my school ends after Memorial Day or starts before the research internship program ends. Can I still apply? Interns are to be available to participate full-time in the internship from May 22 through August 9, 2024 with the first three days of orientation being key to a successful internship. Explain your circumstances in the eligibility section for consideration.
I anticipate attending summer classes. Can I still apply? This research internship is a full-time endeavor. It is not advised to apply.
What are the typical days/hours for this internship? What if I have a conflict? The internship program begins May 22 and ends August 9. MHIF is closed on May 27 for Memorial Day, June 19 and July 4. If interns have an important conflict, it should be pre-approved and should be limited to no more than 1-2 business days during the internship.
Core intern work days/hours are Monday to Friday from 7:30 am to 4:00 pm. Projects may require additional hours as deadlines approach. There are also some evening events or early morning observations.
I will be applying to medical school or doing medical school interviews during the summer. Can I still apply? Completing medical school applications is acceptable and needs to be completed outside of the research internship work hours. Attending medical school interviews are possible as long as the intern fulfills the expectations for the internship, which can require additional hours over and above scheduled work hours.
What statistical and software skills are needed? Interns will be using statistical software (including R), Excel, Word, and PowerPoint to build and maintain data dictionaries, do data cleaning, data validation, data verification, and work with demographic data. While some training will be provided, a working knowledge of these tools will be helpful.
Do you provide housing support? Generally we are not able to offer housing support. However, we share intern contact information for roommate collaborations and recommend researching the University of Minnesota campus (approximately two miles away), Facebook and Craigslist for short-term rental or sublet options. Scholarship opportunities may be available for accepted applicants who need housing/transportation support.
Can I apply again, if I am not selected this time? Yes, as long as you still meet the eligibility requirements.
Where can send a question if my question is not answered here? Email [email protected]
2024 Internship Application
Applications for the 2024 internship are closed. Applications for the 2025 cohort will open in November - keep watch for more details.
Cheers to 20 Years of Interns!
During the past 20 years, 235 clinical research interns have contributed to 223 posters and presentations at national scientific sessions, 219 publications in peer-reviewed journals, and 59% of former interns are now practicing physicians !
In celebration of this 20-year landmark, we were excited to reconnect with Dr. Aaron Tande, MD , who was part of our first internship program in 2002 . Hear what Aaron has to say about his internship experience 20 years later:
Intern Program Highlights
Since 2002, 235 research interns have contributed to….
223 posters and presentations at national scientific sessions
219 publications in peer-reviewed journals
Where are they now.
59% are physicians
24% are in medical school
12% are in healthcare related fields
5% are in non-healthcare fields

Intern Experience
During the summer research internship, interns will be exposed to a vast array of clinical research and educational experiences. They will be matched with a physician and staff mentor, who will guide them through a clinical research project throughout the summer. This valuable exposure takes place at a nationally ranked and award-winning hospital, Abbott Northwestern Hospital in Minneapolis, Minnesota.
In addition to many observations of surgery and procedures in the hospital, interns will embark on several field trips to destinations including the University of Minnesota Heart Lab, medical device companies and research organizations. Weekly Lunch and Learn presentations from the renowned cardiologists at the Minneapolis Heart Institute® and other guest speakers will offer exposure to the wide range of research happening at MHIF and unique career paths.
Meet Our Interns
Meet our 2023 Interns >
Meet our 2022 Interns >
Meet our 2021 Interns >
Meet our 2020 Interns >
Meet our 2019 Interns >

2024 Intern Program Donors
This cutting-edge educational experience is made possible by the generous contributions of individual donors, local foundations and industry organizations who share in the belief that hands-on experience is critical for tomorrow’s leaders. Financial support from donors means interns can work with both a staff and a physician mentor to learn about the research process and cardiology.
Thank you to the following generous donors for their contributions to the 2024 MHIF Internship Program:

Support the Intern Program
Help fund the next generation of cardiovascular experts!

The Minneapolis Heart Institute Foundation® (MHIF) strives to create a world without heart and vascular disease. To achieve this bold vision, we are dedicated to improving the cardiovascular health of individuals and communities through innovative research and education.
Thanks to the generosity of donors like you , we can continue this life-saving work. Please make a gift to support the area of greatest need.
All Gifts Matched! Educating the Next Generation of Heart Heroes

Since 2002, MHIF's Clinical Research Internship Program has hosted 235+ students for a unique physician-mentored cardiovascular research internship for undergraduate students. Interns not only gain exceptional experience that helps them prepare for medical school, they also help move MHIF research forward.
Community support is key to our ability to enlist the highest level of talent with annual internship costs exceeding $25,000 per intern. Build new opportunities for the next generation of world-class physician researchers by making a MATCHED gift by May 31st!
- Student/Faculty Portal
- Learning Hub (Brightspace)
- Continuous Professional Development

Clinical Research Internship Study Program (Florida)
Program overview.
Program length: 10 weeks Location : Jacksonville, Florida Stipend: $3,000
This internship is a paid 10-week summer program that gives college students an opportunity to participate in clinical research. This is a great way for you to test your inclination toward careers in medicine, health care, and science. You are placed with a faculty mentor to work on a clinical research project.
What is clinical research?
Clinical research represents a broad array of scientific studies and trials that help doctors and the healthcare team learn more about human disease, behavior, and treatments. It is typically differentiated from discovery science or "basic science research" in that there is not a wet lab or bench component. Varying methodologies and approaches may include, but are not limited to:
- Observational studies
- Epidemiological studies
- Retrospective studies
- Case series or reports
- Clinical trials
- Quality studies
Most CRISP students work with their faculty mentors in a largely computer-based or chart review manner to work on their research project.
A faculty mentor directs each student's Clinical Research Internship Study Program (CRISP) learning experience for the research project. The Mayo Clinic Institutional Review Board (IRB) approves all research projects.
To successfully complete the program, you must:
- Comply with all program and institutional policies
- Participate in an ongoing research investigation
- Develop skills in research methodology
- Participate in assigned educational activities
- Present a written summary and oral presentation of their research at the end of the program
CRISP students work on a variety of research topics. Some examples of previous projects:
- Injury in marathon runners
- Aortic aneurysm and smoking
- Liver transplant in patients older than 70
- Warfarin anticoagulation in acute intracranial hemorrhage
- Screw placement in the spine using 3-D images
You are expected to attend our lecture series aimed at helping to develop your professionalism.
You are expected to commit a minimum of 20 hours a week to be at Mayo Clinic to work on your research project.
Application process
Applications will open Nov. 1 and will close Jan. 15 at 11:59 p.m. EST.
Summer 2024 match
- The summer 2024 match is for May 28, 2024, through Aug. 2, 2024
- All students accepted into the program must be available on the first day to attend the mandatory orientation — no exceptions will be given
Prerequisites
To be eligible for the internship, you must:
- Recent graduates should typically have graduated no longer than 13 months prior to CRISP start date
- A limited number of spots may be awarded to exceptional applicants in professional degree-seeking programs
- Have completed your freshman year of college prior to the start of the program
- Have a GPA of at least 3.0 on a 4.0 scale
- Be seriously considering a career in health care or clinical research
Applicants are selected based on academic performance, work and volunteer experiences, leadership qualities, and representation of personal qualities and skills in a personal letter.
Non-U.S. citizen applicants
Admission to Mayo Clinic Office of Non-Clinical Programs is open to U.S. workers in the four categories below. Therefore, visa sponsorship is not available.
- U.S. citizens
- U.S. nationals
- Lawful permanent residents
- Asylees and refugees
Applicants whose primary language is not English must submit results from the Test of English as a Foreign Language Internet-based test (TOEFL iBT). Scores from the speaking portion of the exam are given particular consideration in admission decisions. Learn more about the exam and register online at the Educational Testing Service .
The Mayo Clinic Office of Non-Clinical Programs code for the TOEFL iBT is 5784.
Educational transcripts from schools outside the U.S. must be translated (if they are not already in English) and evaluated for U.S. equivalence by an accredited credential evaluation service company prior to submission. Please refer to the National Association of Credential Evaluation Services for a list of qualified companies. The applicant pays for the examination and credential-evaluation services.
Application instructions
Complete the following steps to apply:
- School of Study - Office of Non-Clinical Education Programs
- Area of Interest - Clinical Research Internship Study Program (CRISP)
- Once logged on, select "Create a New Application"
- Select "Start a Mayo Clinic College of Medicine and Science Experience Application"
- Complete each section of the application and submit
- Select the "View" button next to your application
- Upload your supplemental items
- Complete the Recommendation Request section
Additional required items
- In 500 words or less, write about your career goals and why you would be a good candidate for the program. To assist in the match, identify your specific research interests.
- Unofficial college transcripts may be uploaded in the Supplemental Items area.
- Recent CV/resume
- One letter of recommendation is required and must be completed in the Recommendation Request section. The recommender will receive an email with a link to upload a letter.
- Professional: References should be individuals who are/were an authority figure in your current/previous schooling or place of employment (i.e. supervisor, teacher)
Tuition and financial aid
Mayo Clinic Office of Non-Clinical Programs does not charge tuition or fees for the internship. A stipend of $3,000 will be provided for the experience.
You are responsible for your living accommodations and transportation.
You may hold outside employment during the program if it does not conflict with your program responsibilities.
Accreditation information
See accreditation information for Mayo Clinic College of Medicine and Science.
week program

Jacksonville, FL
Choosing mayo clinic.
Discover why people choose Mayo Clinic to complete their education
Mayo Clinic Office of Non-Clinical Education Programs Email: [email protected]

IMAGES
VIDEO
COMMENTS
Clinical Research Fellow Short Term Fellow/Intern. AdventHealth Orlando. Celebration, FL 34747. ( Celebration Place area) $26.17 - $39.26 an hour. Full-time + 1. Monday to Friday + 1. Established competency in research as evidenced by published (peer reviewed) research and/or prior work experience. Paid Days Off from Day One.
Clinical Research Fellow Short Term Fellow/Intern. AdventHealth Orlando. Celebration, FL 34747. ( Celebration Place area) $26.17 - $39.26 an hour. Full-time + 1. Monday to Friday + 1. Established competency in research as evidenced by published (peer reviewed) research and/or prior work experience.
Search similar titles. Today's top 84 Clinical Research Intern jobs in United States. Leverage your professional network, and get hired. New Clinical Research Intern jobs added daily.
Editorial Internship: Copy-editing, Writing, or Operations (6 Months) GoodRx. Hybrid work. $22.12 - $32.70 an hour. Full-time. Writing - working with assigning editors to conduct in-depth research and write articles for topics spanning all aspects of healthcare, with a primary focus on…. Posted 25 days ago ·.
Search similar titles. Today's top 232 Clinical Research Internship jobs in United States. Leverage your professional network, and get hired. New Clinical Research Internship jobs added daily.
The top companies hiring now for clinical research intern jobs in United States are Healthy Young Minds Inc, MEDIDATA, Clinical Trials of South Carolina, CEDARS-SINAI, Taiho Oncology, Inc., Olema Oncology, Children's National Hospital, RHSC, MannKind Corporation, Minnesota Urology P.A.
Internship Overview: Clinical Research Intern We are seeking a highly motivated and detail-oriented Clinical Research Intern to join our team. This internship will provide invaluable hands-on experience in clinical research and offer the opportunity to contribute to important projects aimed at helping people with serious medical conditions live ...
Yale School of Medicine has established a Clinical and Translational Research Internship program that offers unique and exciting opportunities to gain experience in research at a renowned academic institution. YCCI is sponsoring clinical and translational research internships in departments and programs across the School of Medicine.
Clinical Research Internship Program. The Clinical Research Internship is a unique opportunity for students to directly participate in the performance of clinical research, such as enrolling patients in study programs, while also observing overall emergency department (ED) operations and interacting with physicians, nurses and other health care ...
Through internship programs at the Biomedical Research (BR) division of Novartis, your contribution to the search for new disease therapies can begin early in your scientific education journey. Our internship programs are designed to introduce talented students and recent college graduates to drug discovery research together with rigorous ...
Clinical Research Fellow Short Term Fellow/Intern. AdventHealth Orlando. Celebration, FL 34747. ( Celebration Place area) $26.17 - $39.26 an hour. Full-time + 1. Monday to Friday + 1. Established competency in research as evidenced by published (peer reviewed) research and/or prior work experience. Paid Days Off from Day One.
The Vanderbilt Undergraduate Clinical Research Internship Program (UCRIP) gives college students earning a four-year degree the opportunity to participate in both research and clinical patient care at an academic medical center. This program is designed for students who are interested in a career in medicine. Participants will complete a research project under the directorship of...
Hartford HealthCare. Meriden, CT. Be an early applicant. 4 months ago. Today's top 9 Clinical Research Internship jobs in New York City Metropolitan Area. Leverage your professional network, and ...
Welcome to the Duke University School of Medicine Clinical Research Internship Portal (CRISP)! Our goal is to assist students earning a degree with finding an opportunity to participate in research at an academic medical center. Students will work in a mentored team environment that supports learning in clinical research. At this time, only students from institutions that have existing ...
Under supervision, the Clinical Research Intern will assist in carrying out research activities related to clinical research. In addition, they will be given the opportunity to enhance their research skills by working with our diverse team of researchers. The Clinical Research Intern will become more familiar with renal (patho)physiology, renal ...
The Clinical Research Internship program with the University of Louisville Division of Infectious Diseases hosts two cohorts each year with 20-30 participants per cohort. Applications from qualified candidates are accepted twice per year. To be considered, applicants must submit all required materials during the open application process.
What does a Clinical Research Intern do? Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure ...
University of Miami. Miami, FL. Be an early applicant. 6 days ago. Today's top 37 Clinical Research Associate Intern jobs in United States. Leverage your professional network, and get hired. New ...
Clinical Research Fellow Short Term Fellow/Intern. AdventHealth Orlando. Celebration, FL 34747. ( Celebration Place area) $26.17 - $39.26 an hour. Full-time + 1. Monday to Friday + 1. Established competency in research as evidenced by published (peer reviewed) research and/or prior work experience.
Discover, contribute and make meaningful connections and friendships while working alongside dedicated and dynamic Stanford medical students, residents and faculty, who are all eager to share the joy they have found in medicine. 2024 Stanford Clinical Summer Internship. Session 1 (In person): July 8 - 19, 2024.
Summer programs at the National Institutes of Health (NIH) provide an opportunity to spend a summer working at the NIH side-by-side with some of the leading scientists in the world, in an environment devoted exclusively to biomedical research. Find opportunities available for current medical students.
The Minneapolis Heart Institute Foundation® (MHIF) offers one of the most outstanding and unique internship opportunities available to undergraduates who are pre-med or planning a career in medicine. The goal of the research internship program is to develop and empower the next generation of cardiovascular health professionals by offering a ...
Research Intern. Children's National Hospital. Washington, DC 20010. ( Catholic University area) Pay information not provided. Part-time. Day shift. The Research Intern is an entry level clinical research position. Collects data in order to facilitate operational and clinical research activities.
This internship is a paid 10-week summer program that gives college students an opportunity to participate in clinical research. This is a great way for you to test your inclination toward careers in medicine, health care, and science. You are placed with a faculty mentor to work on a clinical research project.
Apply for Finance Intern, Clinical Research Group job with Thermo Fisher Scientific in Wilmington, North Carolina, United States of America. Students & Internships jobs at Thermo Fisher Scientific