

Understanding Clinical Data Analysis
Learning Statistical Principles from Published Clinical Research
- © 2017
- Ton J. Cleophas 0 ,
- Aeilko H. Zwinderman 1
Albert Schweitzer Hospital, Department Medicine Albert Schweitzer Hospital, Sliedrecht, The Netherlands
You can also search for this author in PubMed Google Scholar
Dept. Epidemiology and Biostatistics, Academic Medical Center Dept. Epidemiology and Biostatistics, Amsterdam, The Netherlands
- The book uses the best-help-there-is for making the difficult issues understandable by using real data examples rather than hypothetical examples
- Complementarily to real data examples, the book continually gives a philosophical treatise of the basics of the scientific method.
- The book explains all of the novel issues of clinical data analysis from the past few years.
20k Accesses
4 Citations
1 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This textbook consists of ten chapters, and is a must-read to all medical and health professionals, who already have basic knowledge of how to analyze their clinical data, but still, wonder, after having done so, why procedures were performed the way they were. The book is also a must-read to those who tend to submerge in the flood of novel statistical methodologies, as communicated in current clinical reports, and scientific meetings.
In the past few years, the HOW-SO of current statistical tests has been made much more simple than it was in the past, thanks to the abundance of statistical software programs of an excellent quality. However, the WHY-SO may have been somewhat under-emphasized. For example, why do statistical tests constantly use unfamiliar terms, like probability distributions, hypothesis testing, randomness, normality, scientific rigor, and why are Gaussian curves so hard, and do they make non-mathematicians getting lost all the time? Thebook will cover the WHY-SOs.
Similar content being viewed by others

Biostatistics

Essential Statistical Tests

- Statistical Reasoning
- Hypothesis Testing
- Clinical data analysis
- Statistical methodologies
- Medical Statistics
Table of contents (10 chapters)
Front matter.
- Ton J. Cleophas, Aeilko H. Zwinderman
Randomized and Observational Research
Randomized clinical trials, history, designs, randomized clinical trials, analysis sets, statistical analysis, reporting issues, discrete data analysis, failure time data analysis, quantitative data analysis, subgroup analysis, interim analysis, multiplicity analysis, medical statistics: a discipline at the interface of biology and mathematics, back matter, authors and affiliations.
Ton J. Cleophas
Aeilko H. Zwinderman
About the authors
The authors are well-qualified in their field. Professor Zwinderman is past-president of the International Society of Biostatistics (2012-2015), and Professor Cleophas is past-president of the American College of Angiology (2000-2002). From their expertise they should be able to choose the best-help-there-is for making difficult issues understandable, that is real data examples from the global literature rather than hypothetical examples.
The authors have been working and publishing together for 18 years, and their research can be characterized as a continued effort to demonstrate that clinical data analysis is not mathematics but rather a discipline at the interface of philosophy, biology, and mathematics.
The authors, as professors and teachers in statistics at universities in The Netherlands and France for the most part of their lives, are convinced, that the scientific method of statistical reasoning and hypothesis testing is little used by physicians and otherhealth workers, and they hope, that the current production will help them find the appropriate ways for answering their scientific questions.
Bibliographic Information
Book Title : Understanding Clinical Data Analysis
Book Subtitle : Learning Statistical Principles from Published Clinical Research
Authors : Ton J. Cleophas, Aeilko H. Zwinderman
DOI : https://doi.org/10.1007/978-3-319-39586-9
Publisher : Springer Cham
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer International Publishing Switzerland 2017
Hardcover ISBN : 978-3-319-39585-2 Published: 31 August 2016
Softcover ISBN : 978-3-319-81917-4 Published: 14 June 2018
eBook ISBN : 978-3-319-39586-9 Published: 23 August 2016
Edition Number : 1
Number of Pages : X, 234
Number of Illustrations : 119 b/w illustrations, 92 illustrations in colour
Topics : Medicine/Public Health, general
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Introduction
- Conclusions
- Article Information
eTable 1. Identification of Existing Guidance on the Content of Statistical Analysis Plans.
eTable 2. Consensus Criteria.
eTable 3. Consensus Meeting Contributors and the Areas of Representation.
eTable 4. Items That While Important When Implementing a SAP Do Not Necessarily Need to be Included.
eAppendix 1. Survey of UK Clinical Research Collaborative Registered Clinical Trials Units.
eAppendix 2. Explanation and Elaboration of Essential Items.
eReferences.
- Guidelines for Statistical Analysis Plans JAMA Editorial December 19, 2017 David L. DeMets, PhD; Thomas D. Cook, PhD; Kevin A. Buhr, PhD
- Statistical Analysis Plans for Clinical Trials JAMA Comment & Response May 8, 2018 Bruno Mario Cesana, MD
- Statistical Analysis Plans for Clinical Trials—Reply JAMA Comment & Response May 8, 2018 Carrol Gamble, PhD; Steff Lewis, PhD; Stephen Senn, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Gamble C , Krishan A , Stocken D, et al. Guidelines for the Content of Statistical Analysis Plans in Clinical Trials. JAMA. 2017;318(23):2337–2343. doi:10.1001/jama.2017.18556
Manage citations:
© 2024
- Permissions
Guidelines for the Content of Statistical Analysis Plans in Clinical Trials
- 1 Biostatistics Department, University of Liverpool, Liverpool, England
- 2 Clinical Trials Research Centre, University of Liverpool, Liverpool, England
- 3 Newcastle University, Newcastle, England
- 4 Currently with Leeds Institute of Clinical Trials Research, University of Leeds, Leeds, England
- 5 Edinburgh University, Edinburgh, Scotland
- 6 University of Oxford, Oxford, England
- 7 UCL Comprehensive Clinical Trials Unit, London, England
- 8 Centre for Statistics in Medicine, University of Oxford, Oxford, England
- 9 University of Nottingham, Nottingham, England
- 10 Janssen Research & Development LLC, Raritan, New Jersey
- 11 Johnson & Johnson, Titusville, New Jersey
- 12 Luxembourg Institute of Health, Strassen, Luxembourg
- 13 Clinical Trials Consulting & Training Limited, Buckingham, England
- 14 Medicines and Healthcare Products Regulatory Agency, London, England
- 15 BMJ , London, England
- Editorial Guidelines for Statistical Analysis Plans David L. DeMets, PhD; Thomas D. Cook, PhD; Kevin A. Buhr, PhD JAMA
- Comment & Response Statistical Analysis Plans for Clinical Trials Bruno Mario Cesana, MD JAMA
- Comment & Response Statistical Analysis Plans for Clinical Trials—Reply Carrol Gamble, PhD; Steff Lewis, PhD; Stephen Senn, PhD JAMA
Importance While guidance on statistical principles for clinical trials exists, there is an absence of guidance covering the required content of statistical analysis plans (SAPs) to support transparency and reproducibility.
Objective To develop recommendations for a minimum set of items that should be addressed in SAPs for clinical trials, developed with input from statisticians, previous guideline authors, journal editors, regulators, and funders.
Design Funders and regulators (n = 39) of randomized trials were contacted and the literature was searched to identify existing guidance; a survey of current practice was conducted across the network of UK Clinical Research Collaboration–registered trial units (n = 46, 1 unit had 2 responders) and a Delphi survey (n = 73 invited participants) was conducted to establish consensus on SAPs. The Delphi survey was sent to statisticians in trial units who completed the survey of current practice (n = 46), CONSORT (Consolidated Standards of Reporting Trials) and SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guideline authors (n = 16), pharmaceutical industry statisticians (n = 3), journal editors (n = 9), and regulators (n = 2) (3 participants were included in 2 groups each), culminating in a consensus meeting attended by experts (N = 12) with representatives from each group. The guidance subsequently underwent critical review by statisticians from the surveyed trial units and members of the expert panel of the consensus meeting (N = 51), followed by piloting of the guidance document in the SAPs of 5 trials.
Findings No existing guidance was identified. The registered trials unit survey (46 responses) highlighted diversity in current practice and confirmed support for developing guidance. The Delphi survey (54 of 73, 74% participants completing both rounds) reached consensus on 42% (n = 46) of 110 items. The expert panel (N = 12) agreed that 63 items should be included in the guidance, with an additional 17 items identified as important but may be referenced elsewhere. Following critical review and piloting, some overlapping items were combined, leaving 55 items.
Conclusions and Relevance Recommendations are provided for a minimum set of items that should be addressed and included in SAPs for clinical trials. Trial registration, protocols, and statistical analysis plans are critically important in ensuring appropriate reporting of clinical trials.
Transparency has been described as a fundamental value of society and initiatives to increase transparency in relation to clinical trial data have been launched. 1 Given the influence of statistical decisions on trial conclusions, well-documented and transparent statistical conduct is essential. This is relevant given concerns regarding research reproducibility. 2
Quiz Ref ID The contribution of the statistician to the design and analysis of clinical trials is acknowledged to be essential. 3 Guidance on statistical principles for clinical trials (International Conference for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [ICH] E9) 4 state that “the principal features of the eventual statistical analysis of the data should be described in the statistical section of the protocol.” However, ICH E9 4 and SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 5 guidelines refer to a separate statistical analysis plan (SAP). The level of detail appropriate for a SAP exceeds that of a protocol. According to ICH E9, 4 a SAP “contains a more technical and detailed elaboration of the principal features of the analysis described in the protocol, and includes detailed procedures for executing the statistical analysis of the primary and secondary variables and other data.” While guidance exists on the content of clinical trial protocols 5 and reporting standards for clinical trials, 6 both of which require a summary of the statistical analyses, there is no guidance on SAP content. Consequently, there is marked variation in practice.
This Special Communication provides recommendations for a minimum set of items that should be addressed and describes the methods used to develop this list. The recommendations are intended to aid the drafting of SAPs for clinical trials and improve their completeness.
The need to develop guidance on SAPs was raised during discussion by statisticians attending a UK Clinical Research Collaboration (UKCRC) Registered CTU (Clinical Trials Unit) Statisticians’ Operational Group meeting in November 2012. This group included 46 senior statisticians, each representing their CTU within the network. This wider group was engaged throughout the development process as well as user-testing and piloting. The members of the CTU network, based in the United Kingdom, conduct clinical trials funded by governmental agencies, foundations, and pharmaceutical companies under the remit of the European Medicines Agency, the UK Medicines and Healthcare Products Regulatory Agency (MHRA), and the US Food and Drug Administration. An application for funding was developed and submitted to the Medical Research Council Network of Hubs for Trials Methodology Research in December 2013 and the project started in May 2014. The SAP guidance document was developed with the primary intention of being applicable to the final analyses of later-phase randomized clinical trials addressing the minimum recommended content of a SAP within the context of the following assumptions:
The SAP is not a standalone document and should be read in conjunction with the clinical trial protocol;
The clinical trial protocol should be consistent with the principles of the SPIRIT 2013 Statement 5 ; and
The SAP is to be applied to a clean or validated data set for analysis.
This guidance document summarizes the findings of a comprehensive search to identify existing SAP guidance; a survey of current practice of statisticians within UKCRC-registered CTUs; and a Delphi survey to establish consensus. Consistent with advice received from the Central Office of Research Ethics, the UK Health Research Authority Decision Tool 7 indicated ethical approval was not required for the surveys and consent to take part was indicated by survey participation.
Major randomized clinical trial funding bodies and regulators were identified from responses to a previous survey, 8 which had generated a list of funders actively supporting clinical trials across at least 2 CTUs within the last 5 years. The full list is contained in eTable 1 in the Supplement and includes the European and Developing Countries Clinical Trials Partnership, FP7 Health Research, Medical Council of Canada, National Cancer Institute of Canada Clinical Trials Group, European Organisation for Research and Treatment for Cancer, National Institutes of Health, and the National Institute for Health Research. Quiz Ref ID The list, which was reviewed by the project team (May 2014), was extended to include regulators (US Food and Drug Administration, European Medicines Agency, and MHRA).
All funders and regulators were contacted by email (June 2014). If a response was not received, up to 2 further reminder emails were sent. If no response was received, the organization was contacted by telephone and the study team discussed whether alternative contacts within the organization could be approached to participate.
Journals were contacted in parallel to funders and regulators, and included JAMA , BMJ , the New England Journal of Medicine , and the Lancet as the leading medical journals publishing clinical trials. Journals identified via a PubMed search (June 2014) publishing SAPs as standalone publications were also contacted ( Trials , Critical Care and Resuscitation , and International Journal of Stroke ). The goal was to identify whether the journals had any internal guidance or recommendations on SAPs, if they followed any externally available guidance on SAPs, whether and how they used SAPs within the peer-review process, and any policies on the publications of SAPs. Each journal website was searched for information relating to SAPs within their support for authors and reviewers prior to contacting a journal editor.
The aim of the survey was to identify current practice and opinions about SAPs. A list of the 45 registered CTUs was accessed from the UKCRC website (June 2014). One CTU reported being split across 2 sites, with each using separate standard operating procedures, and requested that each site complete the survey separately. The survey was developed by A.K., C.G., and D.S. and adapted in response to comments from the project team. To reduce the number of survey questions, copies of standard operational procedures for SAPs and templates or examples of SAPs were also requested. In addition, the survey was piloted during July 2014 by statisticians from the CTUs of the study project team prior to distribution.
A senior statistician at each CTU, identified as the network’s nominated statistics contact, was asked to complete the survey to reflect practices and majority opinion within the statistician’s CTU (August 2014). For networks in which there was no nominated statistics contact, the survey was sent to the CTU director who was asked to delegate completion on behalf of the unit. Two reminder emails were sent to encourage responses. Survey completion was highlighted at network events at which nonresponders were approached to discuss completion. A copy of the survey and the participating CTUs is provided in eAppendix 1 in the Supplement .
The aim of the Delphi survey was to establish consensus among a broad range of stakeholders. The initial list of participants was sent to the project team for review and amendment (January 2015). The UKCRC-registered CTU participants were identified from the survey of current practice (n = 46). CONSORT and SPIRIT guideline authors were identified from relevant publications and websites (n = 16). Pharmaceutical industry contributors were selected from recommendations from the project team and aimed to have both industry and academic experience (n = 5). The journal editors contacted to identify existing guidance were also contacted to participate in the Delphi survey (n = 7). Regulators from the European Medicines Agency and the MHRA were included (n = 2). Contacts with the US Food and Drug Administration were unsuccessful in identifying a participant for the Delphi survey.
A comprehensive list of items that should or could be included within a SAP was derived after reviewing suggested guidance identified from contacting funders and regulators, considering the responses to the survey of current practice, and reviewing copies of standard operational procedures for SAPs and examples of SAPs provided with the survey responses or identified in the literature search. Items were listed individually but grouped under relevant domains.
The list was reviewed by the project team for completeness, comprehension, and suitability of the domains (January 2015). The Delphi survey was completed during February 2015, with each round lasting 2 weeks. During round 1, Delphi participants could suggest additional items for inclusion in round 2. Round 2 included all items from round 1 as well as the additional items suggested by participants. Suggestions were reviewed by the project team and checked for duplication prior to inclusion in round 2.
Participants were asked to score the importance of each item when writing, following, or reviewing a SAP. The scale was presented with 1 to 3 labeled “not important,” 4 to 6 labeled “important but not critical,” and 7 to 9 labeled “critical.” 9
All individual participants who completed round 1 were emailed and asked to complete round 2. In round 2, for each item, participants were presented with the number and percentage of participants who chose each score. Participants were shown their score from round 1 and provided with an option to revise their score for each of the items or keep it the same as their score in round 1.
The definition of consensus was predefined and is presented in eTable 2 in the Supplement . Items were determined to be in (consensus-in) if 70% or more of participants scored the item as critical and less than 15% of participants scored the item as not important. Items were deleted (consensus-out) if 70% or more of participants scored it as not important and less than 15% of participants scored it as critical.
Following round 2 of the Delphi process, a consensus meeting was held (March 2015) with expert representation from each group: CTU senior statisticians, regulators (MHRA), statisticians in the pharmaceutical industry, and journal editors. The 12 expert panel members are listed in eTable 3 in the Supplement .
All items included in the Delphi survey were reviewed at the consensus meeting. Items on which consensus had been reached were highlighted but not discussed further. The expert panel members were asked to discuss each item for which consensus had not been reached and, following discussion, to make a recommendation regarding its inclusion with consensus-in items within the minimum set of items that should be addressed and included in SAPs for clinical trials.
The aim of the critical review and piloting was to ensure the guidance produced was fit for purpose, appropriate to the needs of statisticians authoring and implementing SAPs, and to identify any items requiring clarification. The first draft of the guidance underwent critical review by attendees at the UKCRC Registered CTU Statisticians’ Operational Group meeting in April 2015. Meeting attendees were able to provide additional comments based on further discussions with the statistics team within their CTU until September 2015. Following incorporation of comments, the guidance was sent to the expert panel involved in the Delphi consensus meeting prior to being piloted by senior statisticians across 5 trials in January 2016.
Of the 39 funding bodies or regulators that were contacted and asked about their requirements or guidance for SAPs, 28 responded (72%). Four responders referred to ICH E9, 4 3 to the UK Medical Research Council website or ICH Good Clinical Practice guidance, 3 and 21 indicated an absence of guidance or recommendations relevant to SAPs. A comprehensive search of the literature and references of published SAPs did not identify any publications relevant to the content of SAPs.
The survey to establish current practice was distributed by email to each of the 45 UKCRC-registered CTUs (46 respondents), with a 100% response rate. Responses demonstrated variability in current practice around the processes of producing SAPs and their content. The production of guidance on SAP content was supported by 85% (n = 39) of responders.
Of the 73 invited participants in the Delphi process, 56 (77%) completed round 1 and 54 (73%), round 2. Those completing round 2 included CTU statisticians (40/46; 87%), editors (3/7; 43%), guideline authors (8/16; 50%), industry (5/5; 100%), and a regulator (1/2; 50%) (3 responders contributed to 2 groups each). Thirty percent of the responders were from outside the United Kingdom and included Canada, Germany, Ireland, Denmark, Australia, and the United States.
Round 1 contained 89 items, consensus for items to remain in was reached on 28 items, and an additional 21 items were suggested by responders. Round 2 contained 110 items (89 prepopulated items from round 1 and the 21 suggested items) and at the end of round 2, consensus was reached that 46 items should remain in with 1 item deleted (consensus-out).
At the end of the consensus meeting, there were 63 items in (consensus-in), 30 items deleted (consensus-out), and 17 items that the expert panel felt are important but do not necessarily need to be included (eTable 4 in the Supplement ). These 17 items may be found in other trial documents but the SAP should incorporate references to where details of these items can be found.
The critical review meeting, held in London, was attended by 51 statisticians from 37 CTUs (April 2015). Participants were asked to consider the ordering and clarity of the descriptions of each of the 63 items and to highlight any concerns. To ensure discussion and complete coverage of the items within the meeting, attendees were split into groups, with each group allocated 1 of the 6 sections to review and provide feedback on as a priority. Meeting attendees were also encouraged to discuss the draft guidance with other statisticians within their CTUs and return any additional collective responses. Additional responses were received from 8 CTUs.
Two issues were raised: the first was whether the sample size calculation should be replicated from the protocol in full or referenced and the second was concerning the use of a 2-stage analysis in which the assumptions of the analysis approach are tested and then the analysis determined by whether the assumptions are met or not. The sample size statement was amended to support an individual statistician’s preference to replicate or reference the protocol. The issue surrounding the 2-stage analysis was more controversial and in response to discussions, the guidance was amended to ensure that this was highlighted in the discussion of that item. During critical review of the 63 items, some items were found to overlap and were combined, leaving 55. The Table displays the essential items and their subitems. There are 6 sections: Title and Trial Registration (11 items/subitems); Introduction (2 items); Study Methods (9 items/subitems); Statistical Principles (8 items/subitems); Trial Population (8 items/subitems); and Analysis (17 items/subitems).
An open request for 5 volunteers to undertake piloting of the recommendations in the guidance document was made at the critical review meeting. Twelve statisticians expressed an interest and were invited to participate; 5 were selected to cover CTUs with varying experience in Wales, England, and Scotland, each of whom applied the guidance document to trials in adults and children, and included pharmaceutical and nonpharmaceutical interventions including devices and physiotherapy. The piloting feedback did not require any changes to the guidance and the comments received supported its content and usability.
An elaboration and explanation of each item is included within eAppendix 2 in the Supplement . Examples are provided to illustrate each item, along with an explanation of the rationale and detailed description of the issues to be addressed. Examples for each item are based on real SAPs either published in journals, provided by responders to the CTU survey, or contained within National Institute for Health Research’s Health Technology Assessment monographs.
Quiz Ref ID It is important that every clinical trial has a clear and comprehensive SAP to support reproducibility. Leading organizations and funding bodies openly support data sharing as best practice for clinical trials. 11 Such support will undoubtedly increase the availability of data from original research, resulting in an increase of attempts to replicate results. To support the reproducibility of research and allay concerns of misconduct and fraud in clinical research, a clear comprehensive and transparent account of preplanned statistical analyses must be available. 12 The aim of this guidance is to establish the minimum set of essential items required for a SAP for a clinical trial. It is intended to lead to improvements in the integrity of trial conduct and reporting by facilitating critical appraisal, execution, replication, and identification of any deviations from the prespecified methods.
This SAP guidance was developed following established transparent methods and involving a diverse range of stakeholders involved in the design, funding, conduct, review, and publication of clinical trials. Although the guidance was developed with a focus on the regulatory requirements of trials of medicinal products, and in particular later-phase trials, many aspects are transferable to studies of other types of interventions, phases, and designs.
Quiz Ref ID This guidance document does not cover when a SAP should be written, but early authoring of SAPs—before any data have been collected or analyzed—is the best approach. The final opportunity to amend the SAP should be in response to blind review, defined as the checking and assessment of data during the period between trial completion and the breaking of the blind, the act of unveiling each participant’s random allocation. 4 Following this point, deviations from the SAP and additional analyses should be clearly indicated as such within all reports and publications. 4 In the United Kingdom, the Health Research Authority has developed a protocol template 13 to improve consistency in the way that the items covered by SPIRIT are included within a protocol and a similar template may be beneficial for SAPs.
This guidance assumes that the SAP is not a standalone document, and therefore, it is not necessary to replicate large portions of the protocol, which should instead be clearly referenced. The SAP should contain a statement that it is consistent with the principal features of the statistical methods described in the protocol or a section detailing which analyses are different to those planned in the protocol and why. Any abbreviations used should be spelled out in full.
SAPs should be made publicly available. 14 A major step toward public availability of SAPs is the requirements of the US National Institutes of Health Final Rule for Clinical Trials Registration and Results Information Submission, 15 which in addition to posting of results within ClinicalTrials.gov also requires posting of the SAP if not contained within the protocol. In the discussion of public comments relating to the Final Rule, 15 it was noted that many of the benefits of the protocol that were cited by commenters were derived from the information regarding the statistical analyses. Quiz Ref ID This represents acknowledgment that SAPs have an important role in reducing the occurrence of, and facilitating the detection of, bias particularly in relation to selective analysis and reporting. 16 , 17 Some journals, including JAMA , require the SAP to be submitted alongside the report of a clinical trial for use within the peer-review process. The SAP may be made available as supplementary material or published as a standalone article. While this is encouraging, and increases public availability of SAPs, there is no guidance on how the SAP should be used or evaluated. Similar to protocols, the ability of a SAP to provide transparency is dependent on its content.
Any guidance needs to be responsive to relevant information from future projects and initiatives, as well as changes in legislation. Key initiatives that may influence SAP content include the addendum to ICH E9 on estimands and sensitivity analyses, 18 data-sharing initiatives, 19 and mandatory requirements to post clinical trial results in the European Clinical Trials Database and ClinicalTrials.gov. 15 , 20 , 21 Future revisions of this document will be made available periodically and extensions to other study designs, including observational studies 22 and studies with adaptive designs and Bayesian analyses, should be considered.
Recommendations are provided for a minimum set of items that should be addressed and included in SAPs for clinical trials. Trial registration, protocols, and statistical analysis plans are critically important in ensuring appropriate reporting of clinical trials.
Accepted for Publication: November 7, 2017.
Corresponding Author: Carrol Gamble, PhD, Biostatistics Department, Block F Waterhouse Building, 1-5 Brownlow St, University of Liverpool, Liverpool L69 3GL, England ( [email protected] ).
Author Contributions: Dr Gamble and Ms Krishan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Gamble, Stocken, Lewis, Dore, Williamson, Montgomery, Lim, Berlin, Senn.
Acquisition, analysis, or interpretation of data: Gamble, Krishan, Stocken, Juszczak, Dore, Williamson, Altman, Montgomery, Lim, Day, Barbachano, Loder.
Drafting of the manuscript: Gamble, Krishan, Stocken, Dore, Altman, Lim.
Critical revision of the manuscript for important intellectual content: Gamble, Stocken, Lewis, Juszczak, Dore, Williamson, Montgomery, Lim, Berlin, Senn, Day, Barbachano, Loder.
Statistical analysis: Gamble, Krishan, Stocken, Williamson, Senn.
Obtained funding: Gamble, Stocken, Lewis, Juszczak, Dore, Williamson, Montgomery.
Administrative, technical, or material support: Williamson, Lim.
Supervision: Gamble, Stocken.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Berlin is a full-time employee of Johnson & Johnson. Dr Loder is head of research for BMJ . No other disclosures were reported.
Funding/Support: This work was funded by grant MR/L004933/1-R44 from the UK Medical Research Council Network of Hubs for Trials Methodology Research and supported and endorsed by the UK Clinical Research Collaboration Registered Clinical Trials Unit Network.
Role of the Funder/Sponsor: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation review, or approval of the manuscript; and decision to submit the manuscript for publication.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 February 2021
Essentials of data management: an overview
- Miren B. Dhudasia 1 , 2 ,
- Robert W. Grundmeier 2 , 3 , 4 &
- Sagori Mukhopadhyay 1 , 2 , 3
Pediatric Research volume 93 , pages 2–3 ( 2023 ) Cite this article
4213 Accesses
6 Citations
5 Altmetric
Metrics details
What is data management?
Data management is a multistep process that involves obtaining, cleaning, and storing data to allow accurate analysis and produce meaningful results. While data management has broad applications (and meaning) across many fields and industries, in clinical research the term data management is frequently used in the context of clinical trials. 1 This editorial is written to introduce early career researchers to practices of data management more generally, as applied to all types of clinical research studies.
Outlining a data management strategy prior to initiation of a research study plays an essential role in ensuring that both scientific integrity (i.e., data generated can accurately test the hypotheses proposed) and regulatory requirements are met. Data management can be divided into three steps—data collection, data cleaning and transformation, and data storage. These steps are not necessarily chronological and often occur simultaneously. Different aspects of the process may require the expertise of different people necessitating a team effort for the effective completion of all steps.
Data collection
Data source.
Data collection is a critical first step in the data management process and may be broadly classified as “primary data collection” (collection of data directly from the subjects specifically for the study) and “secondary use of data” (repurposing data that were collected for some other reason—either for clinical care in the subject’s medical record or for a different research study). While the terms retrospective and prospective data collection are occasionally used, 2 these terms are more applicable to how the data are utilized rather than how they are collected . Data used in a retrospective study are almost always secondary data; data collected as part of a prospective study typically involves primary data collection, but may also involve secondary use of data collected as part of ongoing routine clinical care for study subjects. Primary data collected for a specific study may be categorized as secondary data when used to investigate a new hypothesis, different from the question for which the data were originally collected. Primary data collection has the advantage of being specific to the study question, minimize missingness in key information, and provide an opportunity for data correction in real time. As a result, this type of data is considered more accurate but increases the time and cost of study procedures. Secondary use of data includes data abstracted from medical records, administrative data such as from the hospital’s data warehouse or insurance claims, and secondary use of primary data collected for a different research study. Secondary use of data offers access to large amounts of data that are already collected but often requires further cleaning and codification to align the data with the study question.
A case report form (CRF) is a powerful tool for effective data collection. A CRF is a paper or electronic questionnaire designed to record pertinent information from study subjects as outlined in the study protocol. 3 CRFs are always required in primary data collection but can also be useful in secondary use of data to preemptively identify, define, and, if necessary, derive critical variables for the study question. For instance, medical records provide a wide array of information that may not be required or be useful for the study question. A CRF with well-defined variables and parameters helps the chart reviewer focus only on the relevant data, and makes data collection more objective and unbiased, and, in addition, optimize patient confidentiality by minimizing the amount of patient information abstracted. Tools like REDCap (Research Electronic Data Capture) provide electronic CRFs and offer some advanced features like setting validation rules to minimize errors during data collection. 4 Designing an effective CRF upfront during the study planning phase helps to streamline the data collection process, and make it more efficient. 3
Data cleaning and transformation
Quality checks.
Data collected may have errors that arise from multiple sources—data manually entered in a CRF may have typographical errors, whereas data obtained from data warehouses or administrative databases may have missing data, implausible values, and nonrandom misclassification errors. Having a systematic approach to identify and rectify these errors, while maintaining a log of the steps performed in the process, can prevent many roadblocks during analysis.
First, it is important to check for missing data. Missing data are defined as values that are not available and that would be meaningful for analysis if they were observed. 5 Missing data can bias the results of the study depending on how much data is missing and what is the pattern of distribution of missing data in the study cohort. Many methods for handling missing data have been published. Kang 6 provide a practical review of methods for handling missing data. If missing data cannot be retrieved and is limited to only a small number of subjects, one approach is to exclude these subjects from the study. Missing data in different variables across many subjects often require more sophisticated approaches to account for the “missingness.” These may include creating a category of “missing” (for categorical variables), simple imputation (e.g., substituting missing values in a variable with an average of non-missing values in the variable), or multiple imputations (substituting missing values with the most probable value derived from other variables in the dataset). 7
Second, errors in the data can be identified by running a series of data validation checks. Some examples of data validation rules for identifying implausible values are shown in Table 1 . Automated algorithms for detection and correction of implausible values may be available for cleaning specific variables in large datasets (e.g., growth measurements). 8 After identification, data errors can either be corrected, if possible, or can be marked for deletion. Other approaches, similar to those for dealing with missing data, can also be used for managing data errors.
Data transformation
The data collected may not be in the form required for analysis. The process of data transformation includes recategorization and recodification of the data, which has been collected along with derivation of new variables, to align with the study analytic plan. Examples include categorizing body mass index collected as a continuous variable into under- and overweight categories, recoding free-text values such as “growth of an organism” or “no growth,” and into a binary “positive” or “negative,” or deriving new variables such as average weight per year from multiple weight values over time available in the dataset. Maintaining a code-book of definitions for all variables, predefined and derived, can help a data analyst better understand the data.
Data storage
Securely storing data is especially important in clinical research as the data may contain protected health information of the study subjects. 9 Most institutes that support clinical research have guidelines for safeguards to prevent accidental data breaches.
Data are collected in paper or electronic formats. Paper data should be stored in secure file cabinets inside a locked office at the site approved by the institutional review board. Electronic data should be stored on a secure approved institutional server, and should never be transported using unencrypted portable media devices (e.g., “thumb drives”). If all study team members do not require access to study data, then selective access should be granted to the study team members based on their roles.
Another important aspect of data storage is data de-identification. Data de-identification is a process by which identifying characteristics of the study participants are removed from the data, in order to mitigate privacy risks to individuals. 10 Identifying characteristics of a study subject includes name, medical record number, date of birth/death, and so on. To de-identify data, these characteristics should either be removed from the data or modified (e.g., changing the medical record number to study IDs, changing dates to age/duration, etc.). If feasible, study data should be de-identified when storing. If you anticipate that reidentification of the study participants may be required in future, then the data can be separated into two files, one containing only the de-identified data of the study participants, and one containing all the identifying information, with both files containing a common linking variable (e.g., study ID), which is unique for every subject or record in the two files. The linking variable can be used to merge the two files when reidentification is required to carry out additional analyses or to get further data. The link key should be maintained in a secure institutional server accessible only to authorized individuals who need access to the identifiers.
To conclude, effective data management is important to the successful completion of research studies and to ensure the validity of the results. Outlining the steps of the data management process upfront will help streamline the process and reduce the time and effort subsequently required. Assigning team members responsible for specific steps and maintaining a log, with date/time stamp to document each action as it happens, whether you are collecting, cleaning, or storing data, can ensure all required steps are done correctly and identify any errors easily. Effective documentation is a regulatory requirement for many clinical trials and is helpful for ensuring all team members are on the same page. When interpreting results, it will serve as an important tool to assess if the interpretations are valid and unbiased. Last, it will ensure the reproducibility of the study findings.
Krishnankutty, B., Bellary, S., Kumar, N. B. & Moodahadu, L. S. Data management in clinical research: an overview. Indian J. Pharm. 44 , 168–172 (2012).
Article Google Scholar
Weinger, M. B. et al. Retrospective data collection and analytical techniques for patient safety studies. J. Biomed. Inf. 36 , 106–119 (2003).
Avey, M. in Clinical Data Management 2nd edn. (eds Rondel, R. K., Varley, S. A. & Webb, C. F.) 47–73 (Wiley, 1999).
Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42 , 377–381 (2009).
Little, R. J. et al. The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 367 , 1355–1360 (2012).
Article CAS Google Scholar
Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 64 , 402 (2013).
Rubin, D. B. Inference and missing data. Biometrika 63 , 581–592 (1976).
Daymont, C. et al. Automated identification of implausible values in growth data from pediatric electronic health records. J. Am. Med. Inform. Assoc. 24 , 1080–1087 (2017).
Office for Civil Rights, Department of Health and Human Services. Health insurance portability and accountability act (HIPAA) privacy rule and the national instant criminal background check system (NICS). Final rule. Fed. Regist. 81 , 382–396 (2016).
Google Scholar
Office for Civil Rights (OCR). Methods for de-identification of PHI. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html (2012).
Download references
Acknowledgements
This work was partially supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health grant (K23HD088753).
Author information
Authors and affiliations.
Division of Neonatology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Miren B. Dhudasia & Sagori Mukhopadhyay
Center for Pediatric Clinical Effectiveness, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Miren B. Dhudasia, Robert W. Grundmeier & Sagori Mukhopadhyay
Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA
Robert W. Grundmeier & Sagori Mukhopadhyay
Department of Biomedical and Health Informatics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Robert W. Grundmeier
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sagori Mukhopadhyay .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Dhudasia, M.B., Grundmeier, R.W. & Mukhopadhyay, S. Essentials of data management: an overview. Pediatr Res 93 , 2–3 (2023). https://doi.org/10.1038/s41390-021-01389-7
Download citation
Received : 11 December 2020
Revised : 27 December 2020
Accepted : 06 January 2021
Published : 18 February 2021
Issue Date : January 2023
DOI : https://doi.org/10.1038/s41390-021-01389-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Advancing clinical and translational research in germ cell tumours (gct): recommendations from the malignant germ cell international consortium.
- Adriana Fonseca
- Matthew J. Murray
British Journal of Cancer (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An overview of commonly used statistical methods in clinical research
Affiliations.
- 1 Center for Surgical Outcomes Research, The Research Institute at Nationwide Children's Hospital, Columbus, OH, USA.
- 2 Department of Surgery, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA. Electronic address: [email protected].
- PMID: 30473041
- DOI: 10.1053/j.sempedsurg.2018.10.008
Statistics plays an essential role in clinical research by providing a framework for making inferences about a population of interest. In order to interpret research datasets, clinicians involved in clinical research should have an understanding of statistical methodology. This article provides a brief overview of statistical methods that are frequently used in clinical research studies. Descriptive and inferential methods, including regression modeling and propensity scores, are discussed, with focus on the rationale, assumptions, strengths, and limitations to their application.
Keywords: Descriptive statistics; Inferential statistics; Propensity scores; Regression analysis; Survival analysis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Publication types
- Biomedical Research / methods*
- Clinical Trials as Topic / methods*
- Data Interpretation, Statistical*
- Propensity Score
- Regression Analysis
- Research Design*
- Survival Analysis
An easy guide to understanding healthcare data analytics
In this brave new world, virtually every person generates data. Whether it’s from accessing social media apps on their smartphone or wearing a fitness-tracking device, most of us create information trails that can be recorded, stored and used . This data can be extremely helpful to gain insight into the behaviour or composition of large groups of people, for example. In turn, this can help companies design products that meet certain needs most closely, and proactively fulfil certain customer expectations. Like many industries, the healthcare sector is increasingly moving towards data as the foundation of its decision making.
The field of healthcare data analytics stands at the intersection of technology and healthcare, promising to transform how we manage, deliver and access medical services. In practice, hospital data , medical data and clinical data are already being used to improve inventory management and provide personalized healthcare to patients. However, the potential of healthcare data extends much, much further.
This guide aims to unwrap the complex world of healthcare data analytics for those who work within the healthcare industry and those who don’t.
Table of contents
What is healthcare data analytics.
At its heart, healthcare data analytics is the uncovering of patterns and insights from raw healthcare data like patient histories, bloodwork and genetic trackers to help healthcare providers determine the best course of treatment.
This field leverages technologies like machine learning and data visualization to enhance medical practices, optimize resource allocation, and drive evidence-based decision making in the healthcare industry. In short, healthcare data analytics seeks to transform vast amounts of raw data into meaningful, actionable knowledge.
How to make healthcare data analytics fit for purpose
There are various subsets of healthcare analytics, each serving a distinct purpose:
- Medical data analytics is done on data from the electronic health records, medical imaging, laboratory tests and wearable health devices of individual patients. The practice aims to draw insights into patients’ health status and clinical outcomes to optimize healthcare delivery at the patient level, including diagnosis, treatment planning and monitoring.
- Clinical data analytics encompasses the analysis of data collected during clinical care processes, including patient interactions, medical procedures and healthcare interventions. The idea is to spot patterns that could improve clinical workflows.
- Hospital data analytics specifically refers to the analysis of data generated within hospital settings, including administrative data, operational metrics and financial performance indicators.
While medical data analytics, clinical data analytics and hospital data analytics all target specific facets of healthcare, they each empower healthcare professionals to make well-informed decisions that can lead to revolutionary improvements in patient care and healthcare management.
Four types of healthcare data analytics explained
When looking at the more technical side of healthcare data analytics, we can discern four fundamental types of analytic techniques:
- Descriptive analytics is the initial phase that creates a historical narrative of healthcare events.
- Diagnostic analytics goes a little deeper to identify trends and explain them.
- Predictive analytics uses past and current data to forecast future events. As such, predictive analytics in healthcare is medicine’s attempt at a crystal ball.
- Prescriptive analytics is the final stage. By suggesting actions in response to the predictions made, this analytics process seeks to find a strategy. When done well, it is key to driving informed and data-driven decision making.
Sign up for email updates
Register for additional resources and updates on health topics and related standards!
Almost done! You are only one step away from joining the ISO subscriber list. Please confirm your subscription by clicking on the email we've just sent to you. You will not be registered until you confirm your subscription. If you can't find the email, kindly check your spam folder and/or the promotions tab (if you use Gmail).
To learn how your data will be used, please see our privacy notice .
Big data in healthcare
By definition, the analysis of any kind of data requires, first and foremost, vast amounts of data. Enter big data, which refers to datasets too large for traditional analytics methods or tools. Big data is often used in the study of human behaviour or interactions, making it the perfect foundation for healthcare data analytics.
The sheer size of these datasets means leveraging big data in healthcare is dependent on advanced technologies like distributed computing, cloud infrastructure and specialized software. Machine learning and data visualization can supplement statistical discoveries and help human operators derive valuable insights.
Applications of big data in healthcare
The potential of big data in healthcare is unlimited, but there are six obvious applications in the healthcare sector:
- Early disease detection : By anticipating health deterioration through predictive analytics, medical professionals can intervene proactively.
- Faster and more accurate diagnostics : Analysing medical data can lead to quicker treatment decisions and better patient care.
- Personalized medicine : Medical data analytics personalizes treatment by considering an individual’s genetic makeup, lifestyle and environmental factors.
- Improved operational decisions : By analysing operational data, healthcare organizations can better optimize patient flow, staffing levels and resource allocation within hospitals.
- Faster drug development : Healthcare data analytics can help to predict drug interactions and streamline clinical trials.
- Oncology research : The benefits of data analytics in cancer research include the accelerated discovery of new treatments and a deeper understanding of cancer biology.
- Procurement and supply chain optimization : Simply put, the analysis of supply chain data enables providers to predict demand, optimize inventory levels and reduce waste.
Precision and personalized medicine
Healthcare data analytics plays a pivotal role in advancing precision medicine, a paradigm shift in healthcare that aims to tailor medical treatment and interventions to the individual characteristics of each patient. Running personal patient information like genomic information, clinical records and lifestyle factors through machine learning and other analytics tools can help design treatment strategies that meet the unique needs and makeup of individuals.
Data analytics for precision medicine holds the promise of maximizing efficacy while minimizing adverse effects, ultimately improving patient outcomes.
Challenges of big data in healthcare
None of the above is possible without big data, but it can be difficult to collect and use big data for a variety of reasons:
- Data privacy and security : The growing storage and use of health data has already made hospitals and practitioners the targets of cybercriminals and hackers. It is the responsibility of healthcare providers to safeguard this sensitive information by improving their cybersecurity protocols and de-identifying aggregated health data, for example.
- Data quality and accuracy : Healthcare data is collected from a number of systems and stored for different purposes. As such, it is diverse. This diversity can result in data silos and inconsistencies, making it difficult to integrate and analyse the datasets. It can also lead to inconsistencies in the accuracy and completeness of datasets.
- Unstructured data : This is especially relevant to organizations and providers undergoing the transition to digital records from traditional methods. Paper notes, charts and records can be referred to as unstructured data, and can be very difficult to include into datasets without sophisticated techniques for text mining, natural language processing and image recognition.
- Interoperability : Providers are likely to be at different stages of the data analytics adoption process, so collaboration can be tricky. Even if both partners are fully digital, the varying technical infrastructures, security concerns, legal complexities and differing priorities can hinder data exchanges.
Addressing these challenges is no small feat. It will require a commitment from healthcare providers to break down data silos, and the development of tech solutions that not only enhance interoperability but also guarantee the data’s integrity and security.
Promoting interoperability and security
In this context, the safe and reliable development of healthcare data analytics depends on the seamless exchange of data between patients, providers and third parties. International Standards for healthcare data analytics play a crucial role in achieving interoperability across global healthcare systems by providing a common language, shared objectives and monitoring tools.
For healthcare organizations, implementing standards like ISO/HL7 27931 is an effective approach to regulating, managing and handling sensitive data. These steps are crucial for healthcare providers aiming to align with international best practices in data management and patient information security. When implemented effectively, this standard ensures health practitioners have access to information that is accurate, valid, reliable, timely, relevant, legible and complete.
ISO/HL7 27931 Data Exchange Standards – An application protocol for electronic data exchange in healthcare environments
ISO/TS 24289 Health informatics – Hierarchical file structure specification for secondary storage of health-related information
The future of data-driven healthcare
The potential impact of healthcare data analytics on patient care is monumental, driving a healthcare revolution that is more proactive, personalized and efficient. Innovations such as predictive analytics in healthcare, precision medicine, enhanced disease research and improved drug development – which all stem from big data analytics – would ultimately contribute to a healthier world for all.
While it is already essential to the industry, the importance of healthcare data analytics is set to grow in years to come. Developing this field safely, responsibly and effectively is therefore crucial, but it must be a collective endeavour by all actors in the healthcare space. International Standards can provide the foundation for the seamless, safe and private exchange of data to ensure that this new era of healthcare does not compromise on the very essence of patient care – trust.
- An easy guide to understanding healthcare data …
Add to cart
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
Apologies; the page you are requesting is currently unavailable. The request resembles an abusive automated request. If you believe this an error, please contact us and we will assist in resolving the issue.
Thank you for visiting!
- Open access
- Published: 16 May 2024
Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey
- Mark McJury 1
BMC Health Services Research volume 24 , Article number: 635 ( 2024 ) Cite this article
533 Accesses
Metrics details
In healthcare, regulation of professions is an important tool to protect the public. With increasing regulation however, professions find themselves under increasing scrutiny. Recently there has also been considerable concern with regulator performance, with high profile reports pointing to cases of inefficiency and bias. Whilst reports have often focused on large staff groups, such as doctors, in the literature there is a dearth of data on the experiences of smaller professional groups such Clinical Scientists with their regulator, the Health and Care Professions Council.
This article reports the findings of a survey from Clinical Scientists (Physical Sciences modality) about their experiences with their regulator, and their perception of the quality and safety of that regulation.
Between July–October 2022, a survey was conducted via the Medical Physics and Engineering mail-base, open to all medical physicists & engineers. Questions covered typical topics of registration, communication, audit and fitness to practice. The questionnaire consisted of open and closed questions. Likert scoring, and thematic analysis were used to assess the quantitative and qualitative data.
Of 146 responses recorded, analysis was based on 143 respondents. Overall survey sentiment was significantly more negative than positive, in terms of regulator performance (negative responses 159; positive 106; significant at p < 0.001). Continuous Professional Development audit was rated median 4; other topics were rated as neutral (fitness to practice, policies & procedures); and some as poor (value).
Conclusions
The Clinical Scientist (Physical Sciences) professional registrants rated the performance of their regulator more negatively than other reported assessments (by the Professional Standards Authority). Survey respondents suggested a variety of performance aspects, such as communication and fitness to practice, would benefit from improvement. Indications from this small dataset, suggest a larger survey of HCPC registrants would be useful.
Peer Review reports
In Healthcare, protection of patients and the public is a core principle. Part the framework of protections, includes regulation of professions [ 1 ]. This aims to mitigate risks such as the risk from bogus practitioners – insufficiently trained people acting as fully-trained professional practitioners, see Fig. 1 .
Recent UK media report on a bogus healthcare practitioner [ 2 ]
Regulation of professions ensures that titles (e.g. Doctor, Dentist, Clinical Scientist) are protected in law. The protected title means someone may only use that title, if they are on the national register, managed by the regulator – the Health and Care Professions Council (HCPC). It is a criminal offence to use a protected title if you are not entitled to do so [ 3 ]. There are a large number of regulators in healthcare – see Table 1 . Most of the regulators manage a register for one profession, except the HCPC which regulates 15 professions.
To be included on the register, a candidate must meet the regulators criteria for knowledge and training, and a key element to remain, is to show evidence of continuous professional development (CPD). Being on the register ensures that a practitioner has met the appropriate level of competence and professional practice.
For many healthcare workers, being on the HCPC register is a compulsory requirement to be appointable to a post. They must pay the necessary annual fees, and abide by the policies drawn-up by the regulator, and generally professions have no choice of regulator – these are statutory bodies, setup by government.
Recently, there has been considerable public dissatisfaction with the activity & performance of some regulators, notably Ofwat [ 4 ], and Ofgem [ 5 ]. Healthcare workers should expect a high level of professionalism, efficiency, and integrity from a regulator, as the regulator’s performance directly affects staff and public safety.
In terms of the regulation of UK Clinical Scientists, there is a dearth of data regarding experiences with the HCPC and views on the quality of regulation provided.
Findings are reported here from a 2022 survey of Medical Physicists and Engineers (one of the 16 job roles or ‘modalities’ under the umbrella of Clinical Scientist). The research aim was to assess experiences, and the level of ‘satisfaction’ with the regulator. For the remainder of this report, the term Clinical Scientist will be taken to mean Clinical Scientist (Medical Physicist/Engineer). The survey was designed to gather & explore data about opinions and experiences regarding several key aspects of how the HCPC performs its role, and perception of the quality & safety of regulation delivered.
A short survey questionnaire was developed, with questions aimed to cover the main regulatory processes, including registration & renewal, CPD audit, and fitness-to-practice. There were also questions relating more generally to HCPC’s performance as an organisation, e.g. handling of personal data. Finally, participants were asked to rate the HCPC’s overall performance and what they felt was the ‘value’ of regulation. The survey questions are listed in the Supplementary file along with this article.
Questions were carefully worded and there was a balance of open and closed questions. A five-point Likert score was used to rate closed questions. The survey was anonymous, and the questions were not compulsory, allowing the responders to skip irrelevant or difficult questions. The survey also aimed to be as short & concise as possible, to be a minimal burden to busy clinical staff & hopefully maximise response rate. There were a small number of questions at the start of the survey, to collect basic demographics on the respondents (role, grade, UK nation etc.).
The survey was advertised on the online JISC-hosted UK Medical Physics and Engineering (UKMPE) mail-base. This offered convenient access for the majority of Clinical Scientists. The survey was advertised twice, to allow for potential work absence, holiday/illness etc. It was active from the end of July 2002 until October 2022, when responses appeared to saturate.
The data is a combination of quantitative rating scores, and qualitative text responses. This allows a mixed-methods approach to data analysis, combining quantitative assessment of the Likert scoring, and (recursive) thematic analysis of the free-text answers [ 6 ]. Thematic analysis is a standard tool, and has been reported as a useful & appropriate for assessing experiences, thoughts, or behaviours in a dataset [ 7 ]. The survey questions addressed the main themes, but further themes were identified using an inductive, data-driven approach. Qualitative data analysis (QDA) was performed using NVivo (QSR International).
Two survey questions attempted to obtain an overall perception of HCPC’s performance: the direct one (Q12), and a further question’Would you recommend HCPC as a regulator…?’. This latter question doesn’t perhaps add anything more, and in fact a few respondents suggested it was a slightly awkward question, given professions do not have a choice of regulator – so that has been excluded from the analysis.
Study conduct was performed in accordance with relevant guidelines and regulations [ 8 , 9 ]. Before conducting the survey of Clinical Scientists, the survey was sent to their professional body, the Institute of Physics and Engineering in Medicine (IPEM). The IPEM Professional Standards Committee reviewed the survey questions [ 10 ]. Written informed consent was obtained from participants.
Data analysis
Data was collected via an MS form, in a single excel sheet and stored on a secure network drive. The respondents were anonymised, and the data checked for errors. The data was then imported into NVivo v12.
Qualitative data was manually coded for themes, and auto-coded for sentiment. An inductive approach was used to develop themes.
The sample size of responses allowed the use of simple parametric tests to establish the level of statistical significance.
Survey demographics
A total of 146 responses were collected. Two respondents noted that they worked as an HCPC Partner (a paid role). They were excluded from the analysis due to potential conflict of interest. One respondent’s responses were all blank aside from the demographic data, so they were also excluded from further analysis.
Analysis is based on 143 responses, which represents ~ 6% of the UK profession [ 11 ]. It is arguable whether it is representative of the profession at this proportion of response – but these responses do offer the only sizeable pool of data currently available. The survey was aimed at those who are on the statutory register as they are most likely to have relevant interactions & experiences of the HCPC, but a small number of responses were also received from Clinical Technologists (Medical Technical Officers-MTOs) and Engineers (CEs) and these have been included in the analysis. Figure 2 shows the breakdown in respondents, by nation.
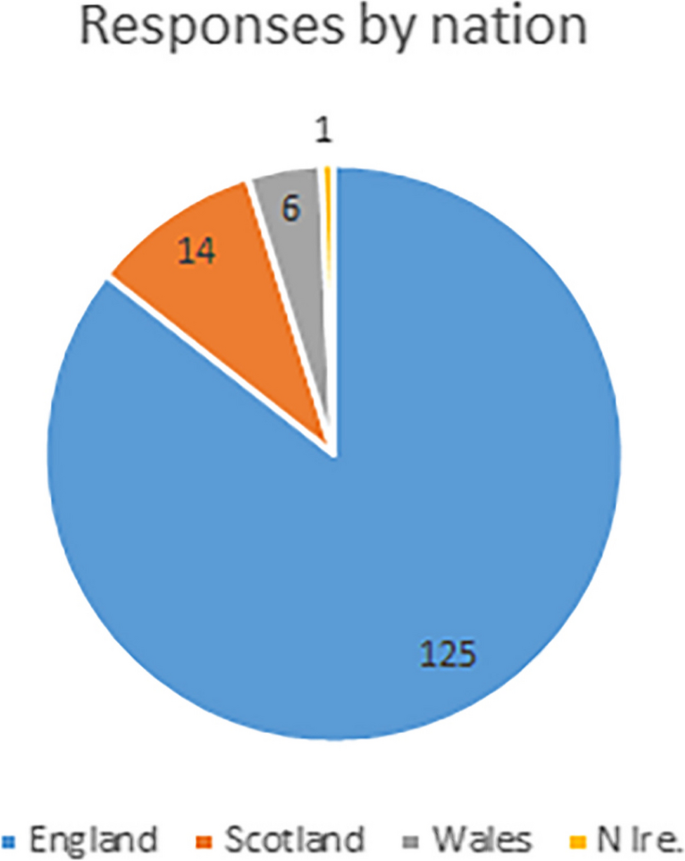
Proportion of respondents, by nation
Of the respondents, 91% are registered Clinical Scientists, and would therefore have a broad range of experience with HCPC and its processes. Mean time on the register was 12 yrs. Respondents show a large range in seniority, and their roles are shown in Fig. 3 (CS-Clinical Scientist; CE-Clinical Engineer; MTO-Medical Technical Officer/Technician; CS-P are those working in private healthcare settings, so not on Agenda for Change (AfC) pay bands).
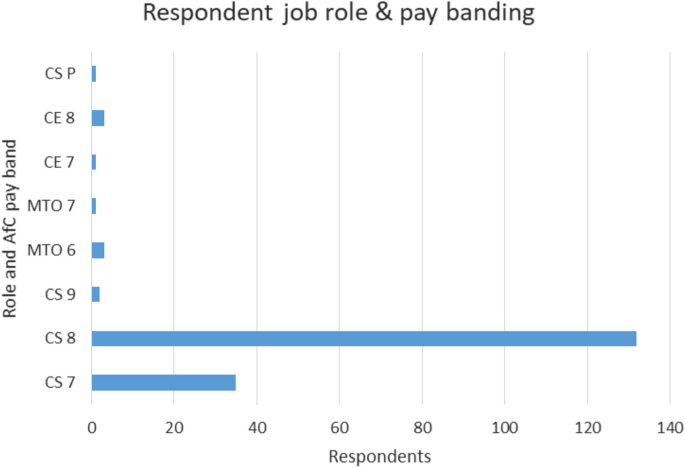
Breakdown in respondents, by role and pay banding
These data can be compared with the most recent HCPC ‘snapshot’ of the CS registrants (find here: Registrants by profession snapshot—1967 to 2019 | ( https://www.hcpc-uk.org/resources/data/2019/registrant-snapshot/ )).
The perception of overall regulator performance, can be assessed in two ways – one interview question directly asked for a rating score, and the overall survey sentiment also offers additional insight.
The score for overall performance was a median of 3 (mean 2.7; response rate 90%) which suggests neutral satisfaction.
Respondents were not asked directly to explain this overall performance rating – themes were extracted from the questionnaire as a whole.
The auto-coded sentiment scores generated in the NVivo software are shown in Table 2 . There is a significantly stronger negative sentiment than positive for HCPC performance – moderate, strong and total sentiment scores are all higher for negative sentiment. The normal test for a single proportion (109), shows the negative and positive sentiment differences have statistical significance with p < 0.001. Whilst the PSA assessment of HCPC performance in 2022–23 shows 100% performance for 4 out of 5 assessment areas, survey data here from regulated professionals suggests considerably less satisfaction with HCPC. This raises associated questions about the relevance and validity of PSA assessment.
A large number of respondents seem to question the value of regulation. Whilst many accepted the value for it in terms of protecting the safety of the public, many questioned its relevance & benefit to themselves. Many respondents also queried the payment model where although the main beneficiaries of regulation are the public & the employer, it is the registrants actually pay the fees for registration. There was very little mention in survey responses, of benefit in terms of protected-title. These issues were amalgamated into Theme 1— Value of regulation , with the two sub-themes Value in monetary terms (value-for-money) and Value in professional terms (benefit and relevance to the individual professional) (see Table 3 ).
In the survey, several aspects of HCPC organisational performance were scored – handling of personal data, registration and renewal, engagement with the profession, audit, and the quality and usefulness of HCPC policies. These formed Theme 2 and its sub-themes.
A third theme Registrant competence and vulnerability , was developed to focus on responses to questions related to the assessment of registrant competence and Fitness To Practice (FTP) processes.
Finally, the survey also directly asked respondents if they could suggest improvements which would have resulted in higher scoring for regulation quality and performance. These were grouped into Theme 4.
Theme 1 – Value of regulation
Value in monetary terms.
The Likert score for value-for-money was a median of 2 (mean 2.3; response rate 100%) which suggests dissatisfaction. This is one of the few survey questions to elicit a 100% response rate – a clear signal of its importance for registrants.
There was a high number of responses suggesting fees are too expensive (and a significantly smaller number suggesting good value). This ties in with some respondents explaining that the ‘benefit’ from registration is mainly for the employer (an assurance of high quality, well-trained staff). Several respondents point to little ‘tangible’ benefit for registrants and query whether the payment model is fair and if the employer should pay registrant fees.
“Expensive fees for what appears to be very little support.” Resp094
“It seems that I pay about £100 per year to have my name written on a list. It is unclear to me what the HCPC actually does in order to justify such a high fee.” Resp014
“I get, quite literally, nothing from it. It’s essentially a tax on work.” Resp008
Several respondents suggested that as registration was mandated by the employer, it was in essence an additional ‘tax’ on their employment, which was highlighted previously by Unison [ 12 ]. A comparator for payment model, are the checks preformed on potential staff who will be working with children and vulnerable adults. In general, these ‘disclosure’ checks are paid for by the employer, however the checks are not recurrent cost for each individual, but done once at recruitment.
Value in professional terms & relevance
This was not a direct question on the questionnaire, but emerged consistently in survey responses. Aside from value-for-money, the value of regulation can also refer to more general benefit and relevance for a professional, for example in protecting a professional title or emphasising the importance of a role. Many respondents commented, in relation to the ‘value’ of regulation, about the relevance of the HCPC to them and their job/role.
The largest number of responses highlighted the lack of clarity about HCPC’s role, and also to note its lack of relevance felt by a significant proportion of respondents.
“Not sure I have seen any value in my registration except that it is a requirement for my role” Resp017
“I really fail to understand what (sic) the benefits of registration.” Resp018
“They do not promote the profession. I see no evidence of supporting the profession. I pay to have the title and I am not aware of any other benefits.” Resp038
Theme 2 – HCPC performance
Communication & handling data.
The survey questionnaire did not have a specific question relating to communication, therefore no specific Likert scores are available. Rather, communication was a sub-theme which emerged in survey responses. The response numbers related to positive (1) and negative experiences (50) clearly suggest an overall experience of poor communication processes (and statistically significant at p < 0.001 for a normal proportion test).
One respondent noted they had ‘given up’ trying to communicate with HCPC electronically. Several respondents also noted issues with conventional communication—letters from HCPC going to old addresses, or being very slow to arrive.
“…I have given up on contacting by electronic means.” Resp134
When trying to renew their registration, communication with HCPC was so difficult that two respondents noted they raised a formal complaint.
A number of respondents noted that when they eventually got through to the HCPC, staff were helpful, so the main communication issue may relate to insufficiently resourced lines of communication (phones & email) or the need for a more focussed first point of contact e.g. some form of helpdesk or triaging system.
“Recently long wait to get through to speak to someone… Once through staff very helpful.” Resp126
This topic overlaps with the next (Processing Registration & renewals) in that both involve online logins, website use etc.
Security & data handling was rated as neutral (median 3, mean 3.4; response rate 91%). Although responses were balanced in terms of satisfaction, a significant number noted a lack of knowledge about HCPC processes. There are almost equal proportions of respondents reporting no issues, some problems with handling of personal data, or insufficient knowledge to express an opinion.
Registration and renewal
The score for processing registrations & renewals, was a median of 4 (mean 3.5; response rate 92%) which suggests modest satisfaction.
The overall rating also suggests that the issues may have been experienced by a comparative minority of registrants and that for most, renewal was straightforward.
“They expected people to call their phone number, which then wasn’t picked up. They didn’t reply to emails except after repeated attempts and finally having to resort to raising a complaint.” Resp023
“Difficult to get a timely response. Difficult to discuss my situation with a human being…” Resp044
Although the Likert score is positive, the themes in responses explaining the rating, are more mixed. Many respondents mentioned either having or knowing others who had issues with registration renewal, and its online processes including payments. A few respondents mentioned that the process was unforgiving of small errors. One respondent, for example, missed ticking a box on the renewal form, was removed from the register and experienced significant difficulties (poor communication with HCPC) getting the issue resolved.
Some respondents noted issues related to a long absence from work (e.g. maternity/illness etc.) causing them to miss registration deadlines – for some, this seems to have resulted in additional fees to renew registration. It seems rather easy for small errors (on either side) to result in registrants being removed from the register. For registrants, this can have very serious consequences and it can then be difficult and slow to resolve this, sometimes whilst on no pay. There have also been other reported instances of renewal payment collection errors [ 13 ].
“I had been off work… and had missed their renewal emails…I was told that there would be no allowances for this situation, and I would have to pay an additional fee to re-register…” Resp139.
Some respondents raised the issue of exclusion – certain staff groups not being included on the register—such as Clinical Technologists and Clinical Engineers. This desire for inclusion, also points to a perception of value in being on the register. One respondent raised an issue of very difficult and slow processing of registration for a candidate from outside the UK.
“Staff member who qualified as medical physicist abroad…has had a dreadful, drawn out and fruitless experience.” Resp135
Overall, many respondents noted difficulties in renewing registration and issues with HCPC’s online processes. Some of these issues (e.g. website renewal problems) may have been temporary and are now resolved, but others (e.g. available routes for registration) remain to be resolved.
Audit process & policies
In the survey, 12% respondents reported having been audited by HCPC regarding their CPD (response rate 97%). This is well above the level of 2.5% of each profession, which HCPC aims to review at each renewal [ 14 ], and similar values reported by some professional bodies [ 15 ]. The participants seem representative, although two respondents mentioned their perception of low audit rates. Data on CPD audit is available here: https://www.hcpc-uk.org/about-us/insights-and-data/cpd/cpd-audit-reports/
Respondents rated the process of being audited as a median of 4 (mean 3.7), which is the joint highest score on the survey, pointing to satisfaction with the process. From the responses, the overall perception could be summed up as straight-forward, but time-consuming. Without regular record-keeping, unfortunately most audits will be time-consuming – the HCPC more so, as it is not an annual audit, but covers the two preceding years.
Some respondents did find the process not only straight-forward, but also useful (related to feedback received). However, responses regarding feedback were mixed, with comments on both good, and poor feedback from HCPC.
“Not difficult but quite long-winded” Resp008
“Very stressful and time consuming” Resp081
“While it was a lot of work the process seemed very thorough and well explained.” Resp114
The HCPC’s policies & procedures were rated as a median of 3 (mean 3.2; response rate 98%). This neutral score could suggest a mixture of confidence in HCPC practise. This score may also reflect the fact that the majority of respondents had either not read, or felt they had no need to read the policies, and so are largely unfamiliar with them.
The reasons for this lack of familiarity are also explained by some respondents – four commented that the policies & procedures are rather too generic/vague. Three respondents noted that they felt the policies were not sufficiently relevant to their clinical roles to be useful. This may be due to the policies being written at a level to be applicable to registrants from all 16 modalities – and perhaps a limitation of the nature of HCPC as a very large regulator. Familiarity seemed mainly to be restricted to policies around registration, and CPD. There were slightly lower response levels for positive sentiment (6), than negative sentiment (9).
“I’ve never had cause to read them.” Resp115
“Detached from the real clinical interface for our professions…” Resp083
HCPC split their policies into ‘corporate’- which relate to organisational issues (e.g. equality & diversity; find them here: Our policies and procedures | ( https://www.hcpc-uk.org/about-us/corporate-governance/freedom-of-information/policies/#:~:text=Our%20main%20policies%20and%20procedures%201%20Customer%20feedback,scheme%20...%207%20Freedom%20of%20Information%20Policy%20 )) and those more relevant to professions (e.g. relating to the register; find them here: Resources | ( https://www.hcpc-uk.org/resources/?Query=&Categories=76 )).
One respondent noted not only that the policies were ‘as you might expect’, but felt the policies were less demanding than those from other similar bodies such as the CQC ( https://www.cqc.org.uk/publications ).
“…Other regulatory bodies (such as the CQC for example) have policies and procedures that are a lot more challenging to comply with.” Resp022
Theme 3 – Registrant competence and vulnerability
In this survey, 3.5% (5/143) of respondents noted some involvement with the HCPC’s Fitness to Practice service. These interactions were rated at a median of 3 (mean 2.8) suggesting neutral sentiment.
Firstly, we can immediately see the level of interaction with the FTP team is very small. CS registrants represent approx. 2% of HCPC registrants, and the level of CS referrals to FTP in 2020–21 was 0.2% [ 16 ].
The data is a very small sample, but responses vary strongly, so it is worth digging a little further into the granularity of individual responses. Response scores were 1, 1, 2, 5, 5 – which are mainly at the extremes of the rating spectrum. The majority of respondents described poor experiences with the FTP team: errors, a process which was ‘extremely prolonged’, involved slow/poor communication, and processes which were ‘entirely opaque’.
“It is slow, the process was badly managed… and the system was entirely opaque,” Resp37
“They were hard to contact and I didn't feel they listened…no explanation, apology or assurance it would not happen again. It left my colleague disillusioned and me very angry on their behalf…” Resp044
Some respondents commented that the team were not only difficult to contact, but also didn’t seem to listen. At the end of a process which involved errors from HCPC, one respondent noted were ‘no explanation, apologies or assurance that it would not happen again’, leaving the registrant ‘disillusioned’. These experiences do not fit with the HCPC’s stated goal to be a compassionate regulator, see Fig. 4 . Arguably it is more difficult to change a culture of behaviour and beliefs, than to publish a corporate goal or statement of vision.
HCPC’s vision statement & purpose [ 17 ]
Some survey respondents have noted the necessity of regulation for our profession.
“Ultimately I am very grateful that I can register as a professional.” Resp024
Theme 4 – Suggestions for improved regulation
Following the question relating to overall performance, respondents were invited to suggest things which might improve their rating for HCPC’s performance and value. These suggestions were also combined with those which appeared in earlier survey responses.
Although we are in a current cost-of-living crisis, responses did not query simply high absolute cost of fees, but also queried the value/benefit of HCPC regulation for registrants. Many responses expressed doubt as to the added value & relevance of HCPC registration for them. They seem to point to a desire for more tangible benefit from their fees. Perhaps, given the costs and levels of scrutiny, registrants want some definite benefit to balance the scales .
“Cost less and do more for the people who are on the register.” Resp089
“Vastly reduced cost. Employer paying registrant fees.” Resp074
A significant number of responses pointed out that the main benefits of registration are for the public, and for employers – but that it is the registrants who pay for registration. Many queries why this should be, and whether there should be a different payment model, where for example employers pay.
Similarly, some respondents felt that the HCPC’s unusual position of regulating a large swathe of healthcare professions was not necessarily helpful for their profession or others.
Communication and response times are obviously an issue of concern for registrants, and improvements are needed based on the low satisfaction levels reported here. This is also linked to a wish for increased engagement with the CS profession.
“Engagement with the workforce, specialism specific development, reduced fees” Resp025
Some responses suggested they would be comforted by increased accountability / governance of HCPC including improved FTP efficiency.
“More accountability to registrants” Resp130
Finally, improvement in terms of additional registration routes for Engineers & Technical staff were also suggested. It may be damaging to work-place moral, if two professionals doing roles of a similar nature are not being governanced is the same way and if there is not parity of their gross salary due to mandatory professional fees & reductions.
Value-for-money : This will vary between individuals depending on many variables, such as upbringing & environment, salary, lifestyle priorities, political persuasion, and so on. However, many of these factors should balance in a large sample. In general, it can be suggestive of satisfaction (or lack of) with a service. The score here suggesting dissatisfaction, echoes with other reports on HCPC’s spending, and financial irregularities [ 18 , 19 ].
In the survey findings, respondents have voiced dissatisfaction with registration value for money. In fact, HCPC’s registration fees are not high when compared to the other healthcare professions regulators. Table 1 shows data from 2021–22 for regulator annual registration fees. However, the HCPC has risen from having the lowest regulator fees in 2014–5, to its current position (9 th of 13) slightly higher in the table. Perhaps more concerning than the absolute level of fees, are when large increases are proposed [ 12 , 20 , 21 , 22 ].
However, fees have regularly increased to current figure of £196.48 for a two-year cycle. During a consultation process in 2018, the Academy for Healthcare Clinical Scientists (AHCS) wrote an open letter to the HCPC, disputing what they felt was a disproportionate fee increase [ 23 ]. Further fee rises have also been well above the level of inflation at the time.
HCPC expenditure (which is linked to registration fees) has arguably been even more controversial than fee increases – noted by several respondents. A freedom of information (FOI) request in 2016 showed HCPC’s spending of £17,000 for their Christmas party [ 18 ] – which amounts to just over £76 per person. This cost was close to the annual registration fee (at that time) for registrants.
In 2019, regulation of social workers in England moved from HCPC, to Social Work England. This resulted in a loss of over 100,000 registrants, and a loss in registration fee income. HCPC raised fees to compensate, but a freedom of information (FoI) request in 2020 [ 18 ] showed that even though there was an associated lowering in workload associated with the loss of 100 k registrants, the HCPC had no redundancies, suggesting the loss of income was compensated mainly by the fees increase.
Inherent value & relevance
One of HCPC’s aims is to promote ‘the value of regulation’ [ 24 ]. However, not only is there dissatisfaction with value-for-money, the second highest response suggests a lack of inherent value (or benefit) from regulation to the individual registrant. In some ways, there is a lack of balance – registrants are under increasing scrutiny, but feel there is little direct benefit, to provide balance.
This also suggests that HCPC’s aim or message is not getting through to the CS profession. It’s not clear what the HCPC 2021–22 achieved milestone – ‘Embedded our registrant experiences research into employee learning and development and inductions’ has actually achieved.
A large number of responses pointed to the lack of clarity about HCPC’s role, and also to note its lack of relevance for respondents. Some of this is understandable – until recently, many CS registrants will have little interaction with HCPC. They would typically get one email reminder each year to renew their registration and pay those fees, and hear little else from the HCPC. That is beginning to change, and HCPC have recently begun to send more regular, direct emails/updates to registrants.
However, for many registrants, the HCPC appears not to be clearly communicating its role, or the relevance/importance of regulation. As mentioned above, this also links in to previous mentions of the lack of any tangible benefit for registrants. Some note little more relevance other than the mandatory aspects of regulation.
Finally, relevance is also queried in relation to the limited access for some professional groups to a professional register. The current situation of gaps in registration for some groups, results in two situations – firstly, for Clinical Scientists and Clinical Engineers/Technologists, one group has to compulsorily pay a fee to be allowed/approved to do their job and the other does not; also, the public are routinely helped and assisted by Clinical Scientists and Clinical Engineers/Technologists – but only one group is regulated to ensure public safety.
HCPC Communication
This was highlighted by respondents as often poor. Recently in the media, there has been a concern raised by The College of Paramedics (CoP) about communications issues with HCPC—changes to the HCPC policy on the use of social media [ 25 ]. They raised particular concerns about the use of social media content and ‘historical content’ in the context of investigations of fitness-to practice.
There have previously been some concerns raised on the UKMPE mail-base regarding handling of personal data, and lack of efficiency in addressing the issue [ 26 ]. Several messages detailed HCPC communicating unencrypted registrant passwords in emails and sending personal data to the incorrect registrant. Some on the forum noted that they had reported this problem over a period of several years to HCPC, suggesting HCPC’s response to these serious issues was extremely slow. Several responses noted these previous issues.
Registration processes
Although responses here show some satisfaction, there have been reports in the media of significant issues with registration (such as removing registrants from the register in error) with associated impact for patients and the public [ 27 , 28 ]. Similarly, there were reports on the UKMPE mail-base of significant issues with registration renewals being problematic [ 26 ]. In Scotland, NHS.net email accounts ceased to be supported in July-Sept 2020 and the associated lack of access to email accounts and messages used for HCPC communication and registration, caused a major issue in registration renewal. This coincided with COVID lockdowns and a period of unusually difficult communication with HCPC. If NHS staff lose registration (irrespective of the reason), respondents noted that some Human Resources (HR) departments were quick to suspend staff from work, and in some cases withhold pay. That spike in difficulties is likely the cause of the most common responses suggesting issues with a complicated process.
In safe-guarding public safety, a key task for a healthcare regulator is assessing the competence of registrants. This is done via a small set of related activities. Registrants must return regular evidence of CPD, and these are audited for 2.5% registrants. This process is simple and routine, and as seen in Theme 2 responses here suggest registrants are reasonably satisfied with this process.
More formal and in-depth competence assessment happens when a complaint is raised against a registrant, either by a work colleague/management, a member of the public or occasionally by the HCPC itself. The process is complex, lengthy and can end in a registrant attending a court hearing [ 29 ].
It is usual for registrants to continue in their normal job during FTP investigations – effectively the public remains at risk from a registrant if their competence is eventually proven to be below the regulators standards, so there is a need for investigations to be efficient both in timeliness, and outcome.
Obviously, being under investigation can be highly stressful, and has the potential for the registrant to be ‘struck off’ the register, and lose their job if registration is mandated (e.g. NHS posts). There are many reports of the process & experience either provoking or increasing underlying mental health challenges [ 30 , 31 , 32 ]. Along with efficiency, a regulator needs to behave compassionately. Investigations of highly-skilled professionals engaging in complex work activities, is also necessarily complex and requires a high degree of knowledge and experience from the regulator’s investigational panel.
The Professional Standards Authority (PSA) regulate the HCPC, and publish annual reviews of their performance ( https://www.professionalstandards.org.uk/publications/performance-reviews ) (see Table 4 ). HCPC performance as reported by PSA, seems to be generally higher than noted by survey respondents here. For 2022–23, aside from one area, the HCPC has scored 100% for performance, which seems at odds with these survey responses [ 33 ]. The FTP team is notable in repeatedly performing very poorly compared to most other sections of the HCPC (even though the majority of the HCPC budget goes to FTP activity, see Fig. 4 ). The HCPC Annual Report 2018–9 [ 34 ] highlighted the completion of the first phase of the Fitness-To-Practice Improvement Plan. This delivered “A root and branch review of this regulatory function… a restructure, tightened roles and processes and the introduction of a new Threshold Policy”, but this seems to have no impact on the performance reported by the PSA for the next few years shown in Table 4 . However, the most recent data does suggest improvement, and HCPC continues to develop FTP team practice [ 17 ].
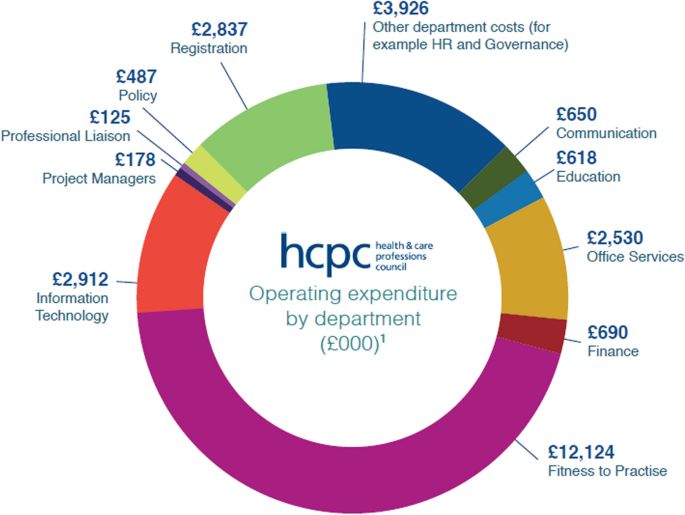
HCPC expenditure for the year 2020–21 [ 17 ]
There are other reports of poor experiences with this team [ 35 , 36 ], and in one report the FTP team’s processes have been noted as being rather inhumane [ 35 ].
Regulation is an important part of public protection, but how effectively it is managed & enforced is also a concern, given it involves increased scrutiny of registrants. A topical comparator is the current dissatisfaction by a large section of the public about several other government regulators allowing seemingly poor performance to go unchecked [ 4 , 5 ].
It is arguable, that registrants remain on the register as long as the HCPC allows them. Several respondents in this survey noted being removed from the register through HCPC administrative error. Removal could also happen through poor judgement/decision-making – the FTP team handle large numbers of very complex investigational cases – 1603 concluded cases for the year 2021–22 and 1024 hearings [ 16 ]. Every justice system is subject to a level of error – guilty parties can be erroneously ‘cleared’, and vice-versa. It is essential therefore, that policies & procedures relating to FTP are fit for purpose—that the FTP team work effectively and humanely, and that there is genuine & effective governance of HCPC to ensure accountability. In this survey, some respondents seem to be saying that currently this seems not to be the case.
It might have been anticipated that the greatest concern is costs, especially in the current cost-of-living crisis. The recent HCPC consultation to increase fees [ 37 ] seems particularly tone-deaf and has caused concern across the professions [ 21 , 22 ].
Above findings show respondents are interested in lower fees, but also increased benefit for their fees. Some respondents pointed out that whilst registrants pay for registration, benefit is mainly for the public and employers. The HCPC is a statutory body, its funding model will have been designed/decided upon by government, and may be unlikely to change. However, there are a variety of potential regulation models [ 38 ], and so change is possible. A review of the financial model for regulation may be welcome.
Regulator size
Some aspects of HCPC performance, policies, and distribution of spending, is related to the nature of it being the largest and only multi-professional regulator in the healthcare sector. Data from the HCPC suggests (see Fig. 5 ) that the majority of spending relates to FTP activity. Data also points to Clinical Scientists having very low levels of FTP investigation compared to others in HCPC [ 16 ]. This suggests that a significant proportion of CS registrant fees are used to investigate other professions. It’s possible (perhaps simplistically) that if, like many other healthcare professions such as doctors & dentists who’s regulator is concerned only with that single profession, if CSs were regulated separately, their registrant fees may be reduced. This model of single-profession regulation may also mitigate against other disadvantages of the HCPC’s practice, such as the ‘generic’ policies aiming to apply to a pool of 15 professions.
Although there is a very low level of data for this topic, the concerned raised by registrants are serious in nature. There also seems to be issues in handling of complaints related to this service and advocacy for registrants. Certainly, there is a clear governance path via PSA, to the Health Secretary. However, this does not offer a route for individual complaints to be raised and addressed. Unlike complaints from the public in other areas, there is no recourse to an ombudsman for registrants. The only option for individual registrants, is the submission of a formal complaint to the HCPC itself, which is dealt with internally. Comments from survey respondents suggest this process does not guarantee satisfaction. Indeed, one of the respondents who mentioned submitting a complaint, made it clear they remained unhappy with HCPC’s response. Overall, there seems to be a lack of clear & effective advocacy for registrants.
“…the HCPC’s stance appeared to be guilty until proven innocent… At no point did I feel the HCPC cared that their (sic) was an individual involved....” Resp044.
FTP processes affect a comparatively small number of CS registrants, compared to other professions. However, it seems clear that the majority of those who have interacted with the FTP team have had poor experiences, and respondents have suggested improvements are needed. The reason for FTP investigations, is protection of staff and the public. If processes are slow, and investigations prolonged, or decisions flawed, the public may be exposed to increased levels of risk, as healthcare practitioners who may be lacking in competence continue to practice. The data in Table 4 shows concerning but improving trends in FTP performance levels.
Limitations
There are two main limitations to this work. Firstly, due to time constraints, there was no pilot work done when designing the survey questionnaire. This may have helped, as noted earlier, a few responses pointed to some awkwardness with one survey question. Although no pilot work was done, the questionnaire was reviewed by the IPEM Professional Standards Committee, as noted in the Acknowledgements section.
The other obvious limitation is the low response rate (~ 6% of UK Medical Physicists). Circulation of the survey was performed via the only online forum for the profession currently available. The survey was advertised multiple times to ensure visibility to staff who may have missed it initially due to leave etc. However, the forum does reach 100% of the profession, and some addressees may have filters set to send specific posts to junk folders etc. The professional body IPEM declined to offer support in circulating the survey (believing the issues involved would affect/be of interest only to a small minority of members.)
The low response rate also has a particular impact on the pool of responses relating to FTP issues, which inherently affect low numbers of registrants.
However, the importance of some of the findings here (e.g. expressed dissatisfaction with regulation in terms of value; the poor experience of some members with the Registration, Communication and FTP teams) and the low sample surveyed, both justify the need for a larger follow-on survey, across all of Clinical Science.
In Healthcare, regulation of professions is a key aspect of protecting the public. However, to be effective, regulation must be performed professionally, impartially, and associated concerns or complaints investigated efficiently and respectfully.
This report presents findings from a survey aimed at collecting a snap-shot of the experiences of Clinical Scientists with their regulator, and their perception of the quality and safety of that regulation performance.
Overall survey sentiment scores showed a significantly more negative responses than positive. Survey comments relate not only to current issues, but to previous problems and controversial issues [ 18 , 26 ]. It seems that some respondents have at some point lost confidence and trust in the HCPC, and survey responses suggest there has not been enough engagement and work done by HCPC to repair and rebuild this trust.
In the midst of a cost of living crisis, costs are a large concern for many. The HCPC fees are neither the highest not lowest amongst the healthcare regulators. Spending is transparent, and details can be found in any of the HCPC’s annual reports.
A repeating sub-theme in responses, was a lack of tangible value for the registrant, and that the employer should pay the costs of registration, where registration is mandated by the job.
Many respondents have suggested that they feel there should be more proactive engagement from HCPC with the profession. Most respondents were not familiar with or felt the HCPC policies are relevant/important to them.
Survey data showed moderate satisfaction with registration processes for the majority of respondents. Some respondents also noted a lack of registration route for engineering & technical healthcare staff. CPD processes also achieved a score indicating registrant satisfaction. This generated the highest ratings in the survey. Communication scored poorly and many respondents suggests there needs to be improved levels of communication in terms of response times and access to support.
The CS profession experiences low levels of interaction with the FTP service. However, those interactions which were recorded in the survey, show some poor experiences for registrants. There also seems to be a lack of advocacy/route for complaints about HCPC from individual registrants. There may need to be more engagement between registrants and their professional body regarding HCPC performance, and more proactivity from the stake-holder, IPEM.
Some of the findings reported here relate to important issues, but the survey data are based on a low response rate. A larger survey across all of Clinical Science is being planned.
Availability of data and materials
To protect confidentiality of survey respondents, the source data is not available publicly, but are available from the author on reasonable request.
Abbreviations
Agenda for Change
Academy for Healthcare Clinical Scientists
Continuous professional development
Clinical Engineer
Clinical Scientist
College of Paramedics
Clinical Technologist
Freedom of Information
Fitness-to-practice
Health and Care Professions Council
Human resources
Institute of Physics and Engineering in Medicine
Joint Information Systems Committee
Medical Technical Officer
Professional Standards Authority
Professional Standards Committee
Qualitative data analysis
UK Medical Physics and Engineering
Professional Standards Authority. Professional healthcare regulation in the UK. https://www.professionalstandards.org.uk/news-and-blog/blog/detail/blog/2018/04/10/professional-healthcare-regulation-explained#:~:text=Regulation%20is%20simply%20a%20way,may%20face%20when%20receiving%20treatment . Accessed 26 Jul 2023
Evening Standard. Bogus surgeon treated hundreds. https://www.standard.co.uk/hp/front/bogus-surgeon-treated-hundreds-6326549.html . Accessed 26 Jul 2023.
HCPC . About registration: protected titles. http://www.hcpc-uk.org/aboutregistration/protectedtitles/ . Accessed 27 Jul 23.
The Guardian. Public patience is wearing thin. Ofwat must wield the big stick | Nils Pratley | https://www.theguardian.com/business/nils-pratley-on-finance/2022/dec/08/public-patience-is-wearing-thin-ofwat-must-wield-the-big-stick . Accessed 19 Jul 2023.
TrustPilot. Reviews of Ofgem. Ofgem Reviews | Read Customer Service Reviews of ofgem.com (trustpilot.com). Accessed 19 Jul 2023.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Article Google Scholar
Kiger ME, Varpio L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach. 2020;42(8):846–54.
Article PubMed Google Scholar
Declaration of Helsinki. 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 12 Sept 2023.
UK Data Protection Act. 2018. https://www.gov.uk/data-protection . Accessed 15 Sept 2023.
Rowbottom C. Private communication on behalf of the IPEM Professional Standards Committee; 2022.
IPEM Workforce Team. Clinical scientist & engineer workforce data. Personal communication. 2022.
Unison. HCPC fee increase is an unjustified ‘tax on practising.’ https://www.unison.org.uk/news/press-release/2019/02/hcpc-fee-increase-unjustified-tax-practising/ . Accessed 27 Jul 2023.
HCPC. Direct debit collection errors. https://www.hcpc-uk.org/news-and-events/news/2020/early-direct-debit-collections/?dm_i=2NJF,141CO,7C0ZNI,4A8IE,1 . Accessed 27 Jul 23.
HCPC. CPD audit rates. https://www.hcpc-uk.org/cpd/cpd-audits/ . Accessed 21 Jul 2023.
IPEM. CPD audit rates. https://www.ipem.ac.uk/your-career/cpd-career-development/cpd-audit/ . Accessed 21 Jul 2023.
HCPC. Fitness to practice annual report 2020–21. https://www.hcpc-uk.org/about-us/insights-and-data/ftp/fitness-to-practise-annual-report-2020-21/ . Accessed 23 Jul 2023.
HCPC. Annual report and accounts, 2020–21. https://www.hcpc-uk.org/resources/reports/2022/annual-report-and-accounts-2020-21/ . Accessed 19 Jul 2023.
Wikipedia. The health and care professions council. https://en.wikipedia.org/wiki/Health_and_Care_Professions_Council . Accessed 2 Jul 23.
HCPC. Annual report 2005–06. https://www.hcpc-uk.org/resources/reports/2006/annual-report-2005-06/ . Accessed 19 Jul 2023.
British Dental Association. BDA very disappointed by HCPC decision to raise registration fees by 18%. https://www.bda.uk.com/resource/bda-very-disappointed-by-hcpc-decision-to-raise-registration-fees-by-18.html . Accessed 27 Jul 2023.
British Psychological Society. HCPC fees consultation – share your views. https://www.bps.org.uk/news/hcpc-fee-consultation-share-your-views . Accessed 27 Jul 23.
IBMS. IBMS response to the HCPC registration fees consultation. https://www.ibms.org/resources/news/ibms-response-to-hcpc-registration-fees-consultation/ . Accessed 17 Jul 23.
Association of HealthCare Scientists. Open letter to HCPC. https://www.ahcs.ac.uk/wp-content/uploads/2018/11/HCPC-Open-Letter.pdf . Accessed 27 Jul 23.
HCPC. Corporate plan 2022–23. https://www.hcpc-uk.org/resources/reports/2022/hcpc-corporate-plan-2022-23/ . Accessed 23 Jul 2023.
College of Paramedics. Our formal response to the HCPC consultation. https://collegeofparamedics.co.uk/COP/News/2023/Our%20formal%20response%20to%20the%20HCPC%20consultation.aspx . Accessed 27 Jul 23.
JISC Mail - MPE mailbase. JISCMail - Medical-physics-engineering list at www.jiscmail.ac.uk . Accessed 19 July 2023.
The Guardian. Thousands miss out on treatment as physiotherapists are taken off UK register. https://www.theguardian.com/society/2022/may/14/thousands-miss-out-on-treatment-as-physiotherapists-are-struck-off-uk-register . Accessed 27 Jul 2023.
HSJJobs.com. https://www.hsjjobs.com/article/thousands-of-clinicians-unable-to-work-after-registration-blunder . Accessed 27 Jul 2023.
HCPC. How we investigate. https://www.hcpc-uk.org/concerns/how-we-investigate/ . Accessed 21 Nov 2023.
Sirriyeh R, Lawton R, Gardner P, Armitage G. Coping with medical error: a systematic review of papers to assess the effects of involvement in medical errors on healthcare professionals’ psychological well-being. Br Med J Qual Saf. 2010;19:6.
Google Scholar
Bourne T, Wynants L, Peters M, van Audenhove C, Timmerman D, van Calster B, et al. The impact of complaints procedures on the welfare, health and clinical practise of 7926 doctors in the UK: a cross-sectional survey. BMJ Open. 2015;5:e006687.
Article PubMed PubMed Central Google Scholar
Jones-Berry S. Suicide risk for nurses during fitness to practice process. Ment Health Pract. 2016;19:8.
Professional Standards Authority. HCPC performance review 2022–23. https://www.professionalstandards.org.uk/publications/performance-review-detail/periodic-review-hcpc-2022-23 . Accessed 25 Jul 2023
HCPC. Annual report and accounts, 2018–19. https://www.hcpc-uk.org/resources/reports/2019/hcpc-annual-report-and-accounts-2018-19/ . Accessed 19 Jul 2023.
Maben J, Hoinville L, Querstret D, Taylor C, Zasada M, Abrams R. Living life in limbo: experiences of healthcare professionals during the HCPC fitness to practice investigation process in the UK. BMC Health Serv Res. 2021;21:839–54.
Leigh J, Worsley A, Richard C, McLaughlin K. An analysis of HCPC fitness to practise hearings: fit to practise or fit for purpose? Ethics Soc Welfare. 2017;11(4):382–96.
HCPC. Consultation changes to fees. https://www.hcpc-uk.org/news-and-events/consultations/2022/consultation-on-changes-to-fees/ . Accessed 27 Jul 23
Department of Health. Review of the regulation of public health professions. London: DoH; 2010.
Download references
Acknowledgements
The author wishes to kindly acknowledge the input of Dr Carl Rowbottom (IPEM Professional Standards Committee), in reviewing the survey questions. Thanks also to Dr Nina Cockton for helpful advice on ethics and recruitment issues.
There were no sources of funding required for this work.
Author information
Authors and affiliations.
University of Glasgow, Level 2, ICE Building, Queen Elizabeth University Hospital Campus, 1345 Govan Road, Glasgow, G51 4TF, UK
Mark McJury
You can also search for this author in PubMed Google Scholar
Contributions
All work to collect, analyse & publish this survey, are the work of the author Dr Mark McJury.
Corresponding author
Correspondence to Mark McJury .
Ethics declarations
Ethics approval and consent to participate.
As this study relates to low risk, survey data, formal ethics committee approval is not required (exemption obtained from NHSGGC REC04 REC Officer Dr Judith Godden [email protected]). As the survey responses were from members of a professional body (The Institute of Medical Physics and Engineering in Medicine (IPEM) it was consulted. Its Professional Standards Committee (PSC) reviewed the survey and raised no objections. The survey questions were assessed for bias and approved unchanged (acknowledged in the manuscript). Written informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
The survey questionnaire has been provided as a supplementary file.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
McJury, M. Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey. BMC Health Serv Res 24 , 635 (2024). https://doi.org/10.1186/s12913-024-10956-7
Download citation
Received : 06 September 2023
Accepted : 05 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12913-024-10956-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Regulation of professions
- Clinical scientists
- Medical physicists
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Open access
- Published: 14 May 2024
Correlating Reiff scores with clinical, functional, and prognostic factors: characterizing noncystic fibrosis bronchiectasis severity: validation from a nationwide multicenter study in Taiwan
- Wen-Chien Cheng 1 , 2 , 3 ,
- Chia-Ling Chang 4 , 5 ,
- Chau-Chyun Sheu 6 , 7 ,
- Ping-Huai Wang 8 ,
- Meng-heng Hsieh 10 , 9 ,
- Ming-Tsung Chen 11 ,
- Wei-Fan Ou 12 ,
- Yu-Feng Wei 13 , 14 ,
- Tsung-Ming Yang 15 ,
- Chou-Chin Lan 16 ,
- Cheng-Yi Wang 17 ,
- Chih-Bin Lin 18 , 19 ,
- Ming-Shian Lin 20 ,
- Yao-Tung Wang 21 , 22 ,
- Ching-Hsiung Lin 23 , 24 , 25 , 26 ,
- Shih-Feng Liu 27 , 28 , 29 ,
- Meng-Hsuan Cheng 30 , 4 ,
- Yen-Fu Chen 31 , 32 ,
- Chung-Kan Peng 11 , 33 ,
- Ming-Cheng Chan 34 , 35 ,
- Ching-Yi Chen 13 ,
- Lun-Yu Jao 36 ,
- Ya-Hui Wang 37 ,
- Chi-Jui Chen 18 ,
- Shih-Pin Chen 21 , 22 ,
- Yi-Hsuan Tsai 27 , 38 ,
- Shih-Lung Cheng 8 ,
- Horng-Chyuan Lin 10 , 39 , 9 ,
- Jung-Yien Chien 40 ,
- Hao-Chien Wang 40 , 41 ,
- Wu-Huei Hsu 1 , 2 , 3 &
Taiwan Bronchiectasis Research Collaboration (TBARC)
European Journal of Medical Research volume 29 , Article number: 286 ( 2024 ) Cite this article
147 Accesses
1 Altmetric
Metrics details
Our study aimed to confirm a simplified radiological scoring system, derived from a modified Reiff score, to evaluate its relationship with clinical symptoms and predictive outcomes in Taiwanese patients with noncystic fibrosis bronchiectasis (NCFB).
This extensive multicenter retrospective study, performed in Taiwan, concentrated on patients diagnosed with NCFB verified through high-resolution computed tomography (HRCT) scans. We not only compared the clinical features of various types of bronchiectasis (cylindrical, varicose, and cystic). Furthermore, we established relationships between the severity of clinical factors, including symptom scores, pulmonary function, pseudomonas aeruginosa colonization, exacerbation and admission rates, and HRCT parameters using modified Reiff scores.
Data from 2,753 patients were classified based on HRCT patterns (cylindrical, varicose, and cystic) and severity, assessed by modified Reiff scores (mild, moderate, and severe). With increasing HRCT severity, a significant correlation was found with decreased forced expiratory volume in the first second (FEV1) ( p < 0.001), heightened clinical symptoms ( p < 0.001), elevated pathogen colonization (pseudomonas aeruginosa) ( p < 0.001), and an increased annual hospitalization rate ( p < 0.001). In the following multivariate analysis, elderly age, pseudomonas aeruginosa pneumonia, and hospitalizations per year emerged as the only independent predictors of mortality.
Based on our large cohort study, the simplified CT scoring system (Reiff score) can serve as a useful adjunct to clinical factors in predicting disease severity and prognosis among Taiwanese patients with NCFB.
Introduction
Bronchiectasis is identified by persistent abnormal enlargement of an airway [ 1 ]. Precise diagnosis of bronchiectasis is achieved using high-resolution computed tomography (HRCT), which allows for quantitative assessment of morphological changes related to the condition [ 2 ]. There are two main types of bronchiectasis: noncystic fibrosis bronchiectasis (NCFB) and cystic fibrosis bronchiectasis (CFB). CFB, a genetic condition, results in the accumulation of thick mucus buildup in the lungs and other organs, causing respiratory challenges and lung damage [ 3 ]. NCFB is known for its heterogeneity, with symptoms and pulmonary damage varying widely in severity [ 3 , 4 ]. Over the years, several radiological scoring systems have been proposed to evaluate the severity of the disease.
Bhalla et al. created a comprehensive scoring system to measure structural lung abnormalities in 14 patients with CFB using thin-section CT scans [ 5 ]. Reiff et al. presented a scoring system encompassing both CFB and NCFB, outlining the site, type, and extent of bronchiectasis [ 6 ]. Bedi et al. introduced the Bronchiectasis Radiologically Indexed CT Score (BRICS), which combines bronchial dilation and emphysema-affected segments on CT scans. BRICS correlated significantly with clinic prognostic markers, providing a useful tool for evaluating NCFB in clinical practice [ 7 ]. There were some limitations to these scoring systems. The Bhalla score, initially developed for patients with CFB, has been expanded to include all bronchiectasis types, but its complexity limits its clinical utility [ 8 ]. The Reiff score, based on subjective evaluation of bronchiectasis features, fails to capture the diversity of the disease. It has the potential to assign high scores to patients with localized but severe abnormalities while neglecting those with widespread yet less pronounced structural problems [ 9 ]. While BRICS considers clinic prognostic factors, it lacks a relationship between specific CT features and disease activity degree, a vital factor in treatment decisions [ 7 ]. These scoring systems lack integration with clinical parameters, which is a significant drawback. Moreover, all these scoring systems are based on western populations to evaluate the severity of bronchiectasis.
Among these score systems, modified Reiff scores have been extensively used in studies [ 6 ]. However, a few studies have comprehensively correlated modified Reiff scores with clinical parameters in East Asian patients with NCFB. As a result, we performed a multicenter study in Taiwan, including patients from northern, central, southern, and eastern regions, without limiting to specific causes of NCFB. Our aim was not only to confirm the association between the modified Reiff score and clinical prognostic factors but also to analyze the clinical characteristics of patients with NCFB based on bronchiectasis patterns (cylindrical, varicose, and cystic).
Study setting and participants
The Taiwan Bronchiectasis Registry is a multicenter, retrospective, observational cohort study. We performed a retrospective review of medical records from 16 healthcare sites across Taiwan, involving patients diagnosed with NCFB based on the 2017 European Respiratory Society guidelines[ 10 ] between January 2017 and June 2020. Excluding individuals aged less than 20 years, and those with less than one year of follow-up, the study cohort consisted of 2753 patients. The study protocol was approved by the Institutional Review Board of each site and followed the amended Declaration of Helsinki. Patient information was anonymized and deidentified before analysis, eliminating the requirement for informed consent due to the retrospective nature of the study.
Collection of clinical and demographics data
Upon enrollment, detailed medical records were gathered, including demographics, clinical manifestations, comorbidities, lung function, exacerbations, microbiology, and radiology. A 1-year follow-up involved tracking acute exacerbation frequency and monitoring survival status after enrollment. CT scans were assessed collaboratively by a pulmonologist and a radiologist, with the date of CT serving as the index date. Discrepancies were addressed through discussion, resulting in a consensus. Patients were then divided into three distinct groups: cylindrical, varicose, and cystic. If a patient exhibits multiple different types of bronchiectasis on HRCT, they will be classified on the basis of the most severe type, such as varicose or cystic type. The severity of bronchiectasis was assessed using the modified Reiff score [ 6 ], which considered the involvement and degree of dilation within each lobe. With each lung containing three lobes (including the lingular segment as a separate lobe of the left lung), the scoring for bronchial dilation was as follows: cylindrical = 1, varicose = 2, and cystic = 3. The sum of the scores from all six lobes constituted the modified Reiff score (ranging from 1 to 18). The severity of bronchiectasis was also categorized into three groups based on Reiff scores: Group 1 (mild: 1–6 points), Group 2 (moderate: 7–12 points), and Group 3 (severe: 13–18 points).
Clinical symptoms and outcome assessment
We attempted to analyze the variations in fundamental demographic data, lung function, clinical symptoms, incidence of pneumonia, and hospitalization among the three groups of patients (cylindrical, varicose, and cystic). Furthermore, we examined the relationship between modified Reiff scores and clinical symptoms, lung function, hospitalization, and pseudomonas pneumonia. Hospitalization and pneumonia data were collected from medical records within one year of the index date. Pneumonia was defined on the basis of alterations in respiratory symptoms, changes in sputum characteristics, and chest radiological findings requiring additional antibiotic treatment. Pseudomonas aeruginosa pneumonia was confirmed through sputum culture results positive for P. aeruginosa in patients treated with antibiotics for pneumonia. Surgical intervention in this study refers to the treatment of hemoptysis resulting from bronchiectasis. Macrolide use in this study encompasses treatment with erythromycin, clarithromycin, or azithromycin for a minimum duration of three months. Hospitalization specifically concentrated on cases with a primary admission diagnosis of pneumonia or bronchiectasis with exacerbation. Mortality data were monitored through medical records until June 2022. Symptom scores were computed, assigning 1 point each for cough, sputum, hemoptysis, and dyspnea, leading to a total score ranging from 1 to 4 points.
Statistical analysis
All data are expressed as the mean ± standard deviation for continuous variables and numbers (percentage) for categorical variables. Statistical analysis was performed using MedCalc for Windows version 18.10 (MedCalc Software, Ostend, Belgium). Continuous variables were compared using one-way ANOVA tests, whereas categorical variables were assessed using Chi-squared tests. Variables with p-values below 0.1 in the univariate analysis were included in the multivariate analysis. Statistical significance was set at p < 0.05. The strength of association is expressed as HR and the associated 95% confidence interval (CI).
Baseline characteristics
This study recruited 2753 subjects across 16 sites in Taiwan. The distribution included 1282 patients (47%) from northern Taiwan, 398 patients (14%) from central Taiwan, 1015 patients (37%) from southern Taiwan, and 58 patients (2%) from eastern Taiwan. Among these patients, 1527 patients (55%) were classified as cylindrical type, 625 patients (23%) as varicose type, and 601 patients (22%) as cystic type (Fig. 1 ).
A Enrolled patients from North, Central, South, and East Taiwan. B Enrolled patients were categorized into cylindrical, varicose, and cystic types of NCFB. NCFB Noncystic Fibrosis Bronchiectasis
The baseline characteristics of 2753 patients with NCFB are depicted in Table 1 . Cylindrical-type patients showed a higher percentage of males (45.3%) and a smoking history (29.6%) than other types ( p < 0.001). Cystic patients had the lowest body mass index (BMI) ( p < 0.001). Cystic-type patients revealed higher clinical symptoms and symptoms scores (2.5, from 1 to 4; p < 0.001), including cough (88.2%), sputum (81.5%), hemoptysis (29.8%), and dyspnea (53.7%) ( p < 0.001). Patients with the cystic type also demonstrated the highest prevalence of comorbidities, including chronic obstructive pulmonary disease (COPD) (40.3%, p < 0.001), asthma (21.6%, p = 0.043), allergic bronchopulmonary aspergillosis (ABPA) (1.2%, p = 0.003), and end-stage renal disease (ESRD) (29.8%, p < 0.001), compared with the overall study population. There was no significant relationship between autoimmune disease and bronchiectasis type. However, patients with cystic bronchiectasis showed a higher incidence of prior pneumonia (44.3%) and tuberculosis infections (22.0%) ( p < 0.001). In addition, they exhibited an increased risk of bacterial pneumonia (45.3%), with notable occurrences of pseudomonas aeruginosa (14.3%) and nontuberculous mycobacterial pneumonia (8.7%) in the whole cohort ( p < 0.001). In terms of lung function, patients with the cystic type had the lowest FEV1 (1.31 L) and forced vital capacity (FVC) (1.81 L) compared with the other types ( p < 0.001). Patients with cystic bronchiectasis have higher rates of surgical intervention ( p = 0.037) and macrolide use ( p < 0.001). Cystic bronchiectasis patients faced a high risk of hospitalization (0.49) because of bronchiectasis exacerbations ( p < 0.001). Cystic bronchiectasis patients had a higher severity score according to the modified Reiff score (9.01) ( p < 0.001).
Correlation between clinical parameters and outcomes with the modified Reiff score
Figure 2 shows the correlation between clinical symptoms and the modified Reiff score. Patients experiencing clinical symptoms such as cough, sputum production, hemoptysis, and wheezing showed higher Reiff scores than those without these symptoms. Moreover, the Reiff score was classified into three groups based on severity, and as the severity increased, the symptom scores also increased ( p < 0.001). As depicted in Fig. 3 , there was a considerable decline in forced expiratory volume in the first second (FEV1) ( p < 0.001), FVC ( p < 0.001), and FEV1/FVC% ( p = 0.015) with an increase in the modified Reiff score. Furthermore, as the severity of Reiff scores increased, the hospitalization frequency for bronchiectasis due to acute exacerbations or pneumonia also elevated last year. The incidence of Pseudomonas aeruginosa pneumonia also increased with the severity of Reiff scores (Fig. 4 ).
A The Reiff scores were compared between patients with and without clinical symptoms (cough, hemoptysis, dyspnea, and sputum). B The symptom scores of patients with different severity levels of Reiff scores. Group 1: mild; Group 2: moderate; Group 3: severe
Relationship between the modified Reiff score and FEV1, FVC, and FEV1/FVC. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s
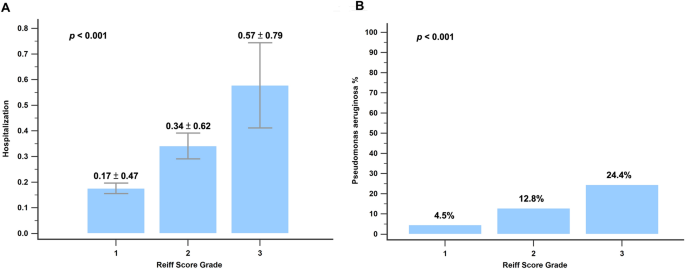
A The hospitalization frequency of patients across various severity levels of Reiff scores. B The occurrence of Pseudomonas aeruginosa pneumonia among patients at different severity levels of Reiff scores. Group 1: mild; Group 2: moderate; Group 3: severe
Independent predictors of hospitalization and mortality
We conducted both univariate and multivariate analyses to detect clinical predictors of hospitalization and mortality, as illustrated in Tables 2 and 3 . Lower FEV1 (Hazard Ratio (HR) = 0.51, 95% CI 0.37–0.68; p < 0.001), pseudomonas aeruginosa pneumonia (HR = 2.24, 95% CI 1.35–3.73; p = 0.002), HR scores (HR = 1.34, 95% CI 1.02–1.76; p = 0.035), and surgical intervention (HR = 2.52, 95% CI 1.02–6.22; p = 0.044) emerged as independent predictors for hospitalization. Furthermore, elderly age (HR = 1.05, 95% CI 1.02–1.09; p = 0.005), pseudomonas aeruginosa pneumonia (HR = 3.48, 95% CI 1.46–8.31; p = 0.005), and hospitalization due to acute exacerbations (HR = 3.79, 95% CI 2.26–6.37; p < 0.001) were identified as independent predictors of mortality.
To the best of our knowledge, this is the first research to comprehensively evaluate the clinical features, symptoms, and disease severity based on the HRCT pattern using the modified Reiff score in Taiwanese patients with NCFB. The present study demonstrated that individuals with cystic-type NCFB showed a greater symptom burden and more comorbidities, such as COPD, asthma, and ABPA. Furthermore, they faced a heightened risk of pseudomonas bacterial pneumonia, hospitalization, and lung function impairment. Cystic-type NCFB patients showed HR scores, which were closely related to poorer lung function, increased symptom severity, higher risk of pseudomonas aeruginosa pneumonia, and higher rates of hospitalization. Our results show that the simplified modified Reiff score aligns with clinical prognostic factors, offering ease of use for clinicians.
The Bronchiectasis Severity Index (BSI) [ 11 ] and the FACED score [ 12 ] were created based on different clinical parameters to predict the severity and prognosis of bronchiectasis. However, the BSI and FACED scores did not show significant associations with the percent predicted FEV1, sputum purulence, and hospital admissions for bronchiectasis exacerbations. Moreover, these two score systems are relatively complex and need the gathering of more information, making them less user-friendly. As a result, we try to use a simple radiology score to associate with all clinical parameters.
A Korean study of 506 bronchiectasis patients found that obstructive disorders led to worse dyspnea, disease severity, and radiologic findings, with significant reductions in FVC%, FEV1/FVC%, and FEV1% correlating with higher modified Reiff scores ( p < 0.001) [ 13 ]. In a study of 114 bronchiectasis patients, 83.3% exhibited obstructive lung patterns and 68.7% had air trapping, with severe dyspnea correlating with obstructive spirometry findings [ 14 ]. The current study not only showed a significant decrease in FEV1 ( p < 0.001), FVC ( p < 0.001), and FEV1/FVC% ( p = 0.015) but also noted an increased symptom burden with higher modified Reiff scores. Our research also indicates that among patients with the same Reiff score but different HRCT patterns, the cystic type of NCFB shows the lowest pulmonary function (FEV1 and FVC) and higher symptoms burden compared with other HRCT patterns (cylindrical or varicose). Previous studies have established a connection between sputum purulence and bronchiectasis severity [ 15 ]. Airflow limitation is commonly found in advanced stages of bronchiectasis [ 16 ]. Studies show a connection between airflow obstruction in bronchiectasis and structural abnormalities detected on CT scans [ 12 , 17 ].
Chalmers et al. studied bronchiectasis patients from 10 centers across Europe and Israel over 5 years, finding that exacerbations, associated with more severe disease, reduced quality of life, and higher mortality, were predicted by pseudomonas aeruginosa infections, lower FEV1, radiologic severity, and concurrent COPD [ 18 ]. The current study found that lower FEV1, the presence of pseudomonas aeruginosa pneumonia, and HR scores emerged as independent predictors for hospitalization due to acute exacerbation of bronchiectasis. The incidence of pseudomonas aeruginosa pneumonia also increased with the severity of modified Reiff scores. Our research also discovered that advanced age, pseudomonas aeruginosa pneumonia, and hospitalization due to acute exacerbations of bronchiectasis were independent predictors of mortality. This study included NCFB patients from 16 sites in Taiwan, with a follow-up period of up to 3 years. In these two studies concentrating on bronchiectasis across two distinct ethnic groups, a consistent pattern emerged: as the modified Reiff score increased, it was associated positively with clinical symptoms burden, decreasing lung function, increased risk of pseudomonas pneumonia, higher rates of hospitalization due to acute exacerbations, and ultimately mortality. Hence, using a simplified Reiff score can efficiently stratify the severity of NCFB across several ethnic populations.
This study also demonstrated a high incidence of COPD among patients with cystic NCFB. Recent studies show that bronchiectasis and COPD coexist in 20–60% of cases [ 19 , 20 , 21 ]. The coexistence of these diseases can lead to an increased symptom burden and a poorer prognosis compared with COPD or bronchiectasis alone [ 22 ]. Patients with cystic-type NCFB not only revealed a high risk of prior pneumonia and a history of tuberculosis infections but also had a higher incidence of ABPA. Yang et al. found that COPD, previous pulmonary tuberculosis, and nontuberculous mycobacterial disease raised aspergillosis risk in bronchiectasis patients [ 23 ]. Ma et al. also reported that asthmatic patients with bronchiectasis had more frequent asthma exacerbations [ 24 ]. The current study observed that individuals with cystic NCFB had a higher incidence of comorbid asthma. This may also explain why cystic NCFB is more prone to acute exacerbations. Furthermore, our study highlighted the increased risk of pseudomonas aeruginosa infection in patients with cystic NCFB. Wang et al. discovered that pseudomonas aeruginosa colonization associates with greater lung involvement and a higher risk of exacerbations requiring hospitalization [ 25 ]. Lee et al. reported that being underweight is linked with heightened mortality among individuals with bronchiectasis [ 26 ]. In this study, it was also observed that cystic-type NCFB was associated with the lowest BMI and poor clinical prognosis. To the best of our knowledge, our study is the first to emphasize that cystic-type NCFB is connected with high comorbidities and a high risk of pseudomonas aeruginosa pneumonia, beyond the scope of modified Reiff scores alone.
This study had several limitations that should be mentioned. First, its retrospective design may introduce selection bias. Although bronchiectasis can present differently across geographical regions, our results align with those of previous studies in Europe and Israel [ 18 ]. Second, due to constraints in healthcare data, identifying the etiology of bronchiectasis was limited. Third, patients were recruited in June 2020, and mortality data were obtained by June 2022. This timeframe may have resulted in some subjects being censored before the 3-year mark, which could impact the accuracy of the estimated 3-year mortality rate. Finally, due to the retrospective nature of the study, the microbiological survey and timing were not strictly regulated, potentially resulting in an underestimation of the incidence of pseudomonas aeruginosa pneumonia. To confirm these findings, a prospective registry is essential.
The current study represents the first large cohort study of Taiwanese NCFB patients, aiming to confirm the modified Reiff score’s relationship with clinical symptoms, pulmonary function, hospitalization due to acute exacerbations of bronchiectasis, and the incidence of pseudomonas aeruginosa pneumonia. Our results suggest that the modified Reiff score serves as a simplified radiological tool for clinical decision-making. In addition, cystic-type NCFB is associated with more comorbidities and poorer clinical prognosis.
Availability of data and materials
The corresponding author is willing to provide the datasets used and/or analyzed in the current study upon reasonable request.
Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8:691–702.
CAS PubMed Google Scholar
Grenier P, Maurice F, Musset D, Menu Y, Nahum H. Bronchiectasis: assessment by thin-section CT. Radiology. 1986;161:95–9.
Article CAS PubMed Google Scholar
Schafer J, Griese M, Chandrasekaran R, Chotirmall SH, Hartl D. Pathogenesis, imaging and clinical characteristics of CF and non-CF bronchiectasis. BMC Pulm Med. 2018;18:79.
Article PubMed PubMed Central Google Scholar
Edwards EA, Narang I, Li A, Hansell DM, Rosenthal M, Bush A. HRCT lung abnormalities are not a surrogate for exercise limitation in bronchiectasis. Eur Respir J. 2004;24:538–44.
Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, Naidich DP. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179:783–8.
Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–7.
Bedi P, Chalmers JD, Goeminne PC, Mai C, Saravanamuthu P, Velu PP, Cartlidge MK, Loebinger MR, Jacob J, Kamal F, et al. The BRICS (Bronchiectasis Radiologically Indexed CT Score): a multicenter study score for use in idiopathic and postinfective bronchiectasis. Chest. 2018;153:1177–86.
Article PubMed Google Scholar
Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, Bandla H, Farrell PM. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21:14–21.
Ledda RE, Balbi M, Milone F, Ciuni A, Silva M, Sverzellati N, Milanese G. Imaging in non-cystic fibrosis bronchiectasis and current limitations. BJR Open. 2021;3:20210026.
PubMed PubMed Central Google Scholar
Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, Murris M, Canton R, Torres A, Dimakou K, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50:1700629.
Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–85.
Martinez-Garcia MA, de Gracia J, Vendrell Relat M, Giron RM, Maiz Carro L, de la Rosa CD, Olveira C. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43:1357–67.
Kim SH, Yang B, Yoo JY, Cho JY, Kang H, Shin YM, Kim EG, Lee KM, Choe KH. Clinical characteristics, radiological features, and disease severity of bronchiectasis according to the spirometric pattern. Sci Rep. 2022;12:13167.
Article CAS PubMed PubMed Central Google Scholar
Nucci M, Fernandes FLA, Salge JM, Stelmach R, Cukier A, Athanazio R. Characterization of the severity of dyspnea in patients with bronchiectasis: correlation with clinical, functional, and tomographic aspects. J Bras Pneumol. 2020;46: e20190162.
Murray MP, Pentland JL, Turnbull K, MacQuarrie S, Hill AT. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34:361–4.
Cherniack NS, Carton RW. Factors associated with respiratory insufficiency in bronchiectasis. Am J Med. 1966;41:562–71.
Roberts HR, Wells AU, Milne DG, Rubens MB, Kolbe J, Cole PJ, Hansell DM. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198–204.
Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, Fardon TC, Obradovic D, Gerlinger C, Sotgiu G, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med. 2018;197:1410–20.
O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42.
Martinez-Garcia MA, Soler-Cataluna JJ, Donat Sanz Y, Catalan Serra P, Agramunt Lerma M, Ballestin Vicente J, Perpina-Tordera M. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–7.
Bafadhel M, Umar I, Gupta S, Raj JV, Vara DD, Entwisle JJ, Pavord ID, Brightling CE, Siddiqui S. The role of CT scanning in multidimensional phenotyping of COPD. Chest. 2011;140:634–42.
Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–11.
Yang B, Kim T, Ryu J, Park HY, Hwangbo B, Kong SY, Kwon YS, Lee SJ, Ra SW, Oh YM, et al. Increased incidence and associated risk factors of aspergillosis in patients with bronchiectasis. J Pers Med. 2021;11:422.
Ma D, Cruz MJ, Ojanguren I, Romero-Mesones C, Varona-Porres D, Munoz X. Risk factors for the development of bronchiectasis in patients with asthma. Sci Rep. 2021;11:22820.
Kwok WC, Ho JCM, Tam TCC, Ip MSM, Lam DCL. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir Res. 2021;22:132.
Lee JM, Lee SA, Han CH, Lee SM, Kim CJ, Lee SC, Park SC. Body mass index as a predictor of mortality in bronchiectasis: a nationwide population-based study. Respir Med. 2021;180: 106370.
Download references
Acknowledgements
The authors thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for technical support during this study.This study was also funded by China Medical University Hospital under Grant/Award Number: DMR-112-027. We extend our gratitude to China Medical University Hospital for their support of this research.
This study was supported by funding from the Taiwan Society of Pulmonary and Critical Care Medicine, Ministry of Science and Technology, Taiwan (MOST 111-2314-B-002 -201 -MY3 and NSTC 112-2314-B-002-310) and National Taiwan University Hospital (112-S0108).
Author information
Authors and affiliations.
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
Wen-Chien Cheng & Wu-Huei Hsu
Critical Medical Center, China Medical University Hospital, Taichung, Taiwan
School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
Department of Internal Medicine, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan
Chia-Ling Chang & Meng-Hsuan Cheng
Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan
Chia-Ling Chang
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Chau-Chyun Sheu
Department of Internal Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Division of Thoracic Medicine, Far Eastern Memorial Hospital, Taipei, Taiwan
Ping-Huai Wang & Shih-Lung Cheng
Department of Thoracic Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Meng-heng Hsieh & Horng-Chyuan Lin
College of Medicine, Chang Gung University, Taoyuan, Taiwan
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
Ming-Tsung Chen & Chung-Kan Peng
Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
Department of Internal Medicine, E-Da Cancer Hospital, I-Shou University, Kaohsiung, Taiwan
Yu-Feng Wei & Ching-Yi Chen
School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan
Yu-Feng Wei
Division of Pulmonary and Critical Care Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan
Tsung-Ming Yang
Division of Pulmonary Medicine, Department of Internal Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan, Republic of China
Chou-Chin Lan
Department of Internal Medicine, Cardinal Tien Hospital and School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
Cheng-Yi Wang
Division of Pulmonary Medicine, Department of Internal Medicine, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan
Chih-Bin Lin & Chi-Jui Chen
School of Medicine, Tzu-Chi University, Hualien, Taiwan
Chih-Bin Lin
Department of Respiratory Care, Chang Gung University of Science and Technology, Chiayi, 613016, Taiwan
Ming-Shian Lin
Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
Yao-Tung Wang & Shih-Pin Chen
School of Medicine, Chung Shan Medical University, Taichung, Taiwan
Department of Internal Medicine, Division of Chest Medicine, Changhua Christian Hospital, Changhua, Taiwan
Ching-Hsiung Lin
Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung, Taiwan
Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan
Department of Recreation and Holistic Wellness, MingDao University, Changhua, Taiwan
Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Shih-Feng Liu & Yi-Hsuan Tsai
Department of Respiratory Therapy, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Shih-Feng Liu
Chang Gung University College of Medicine, Taoyuan, Taiwan
Department of Respiratory Therapy, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Meng-Hsuan Cheng
Department of Internal Medicine, National Taiwan University Hospital Yun-Lin Branch, Yun-LIn, Taiwan
Yen-Fu Chen
Thoracic Medicine Center, Department of Medicine and Surgery, National Taiwan University, Taipei, Taiwan
Department of Medical Planning, Medical Affairs Bureau Ministry of National Defense, Taipei, Taiwan
Chung-Kan Peng
Department of Critical Care Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
Ming-Cheng Chan
School of Post Baccalaureate Medicine, College of Medicine National Chung Hsing University, Taichung, Taiwan
School of Medicine, Tzu-Chi University, Hualien, Taiwan, Republic of China
Medical Research Center, Cardinal Tien Hospital and School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
Ya-Hui Wang
Department of Pulmonary Medicine, Lee’s Clinic, Pingtung, Taiwan
Yi-Hsuan Tsai
Department of Respiratory Therapy, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Horng-Chyuan Lin
Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
Jung-Yien Chien & Hao-Chien Wang
Department of Medicine, National Taiwan University Cancer Center, National Taiwan University College of Medicine, Taipei, Taiwan
Hao-Chien Wang
You can also search for this author in PubMed Google Scholar
Contributions
HCW, WHH, JYC, and WCC did the study design. All authors contributed to patient enrollment and data collection. WHH and WCC analyzed the data. WHH and WCC drafted the manuscript.
Corresponding authors
Correspondence to Wen-Chien Cheng , Hao-Chien Wang or Wu-Huei Hsu .
Ethics declarations
Ethics approval and consent to participate.
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by Institutional Review Board of China Medical University Hospital—approval: (CMUH112-REC2-046). Adult participant consent was not required because the need for individual patient consent was waived by the Institutional Review Board (IRB) of China Medical University Hospital (CMUH) due to the retrospective design.
Consent for publication
Not applicable.
Competing interests
No conflicts exist for the specified authors.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cheng, WC., Chang, CL., Sheu, CC. et al. Correlating Reiff scores with clinical, functional, and prognostic factors: characterizing noncystic fibrosis bronchiectasis severity: validation from a nationwide multicenter study in Taiwan. Eur J Med Res 29 , 286 (2024). https://doi.org/10.1186/s40001-024-01870-z
Download citation
Received : 14 March 2024
Accepted : 25 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s40001-024-01870-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Handbook home
- Search the Handbook
- Undergraduate courses
- Graduate courses
- Research courses
- Undergraduate subjects
- Graduate subjects
- Research subjects
- Breadth Tracks
- CAPS Login - Staff only
- Data Analysis in Clinical Research
Data Analysis in Clinical Research (CLRS90010)
Graduate coursework Points: 12.5 On Campus (Parkville)
View full page
About this subject
- Eligibility and requirements
- Dates and times
- Further information
- Timetable (login required) (opens in new window)
Contact information
Email: [email protected]
Phone: + 61 3 8344 0149
Contact hours : https://unimelb.edu.au/professional-development/contact-us
Data analysis methods are an integral part of modern clinical research. They are powerful techniques that enable researchers to draw meaningful conclusions from data collected through observation, survey, or experimentation.
However, data analysis is a huge discipline with different paradigms, schools of thought and alternative methodologies. Therefore consideration of the appropriate methods used must be undertaken when designing a study and selecting variables and groups.
This subject introduces students to the basic principles of qualitative and quantitative data analysis techniques. It will provide a functional grounding in the theoretical concepts behind each type of analysis, as well as exploration of the interpretation of data and the difference, where applicable, between clinical vs statistical significance.
Intended learning outcomes
On completion of this subject students should be able to:
- describe the theoretical concepts behind a range of qualitative and quantitative data analysis techniques
- compare and contrast the strengths and weaknesses of different qualitative and quantitative data analysis techniques
- describe a strategy for selecting an appropriate data analysis technique based on the study design selected and/or research data collected
- competently perform a range of basic data analysis techniques using appropriate analysis software and interpret analysis output/s
- provide a rationale for the importance of statistical power and perform power calculations
- identify and discuss the key elements associated with ensuring data integrity including storage, management, collation and coding
- critically compare and contrast statistical vs clinical significance and its relevance to clinical practice
- demonstrate confidence in discussing the validity of data analysis outcomes reported in the scientific literature.
Generic skills
- to engage with unfamiliar problems and identify relevant data analysis strategies
- to construct and express logical arguments and to work in abstract or general terms to increase the clarity and efficiency of data analysis
- communicate advanced data analysis concepts in written and oral form;
- the ability to comprehend complex data analysis information
- exercise responsibility for their own learning;
- manage their time effectively.
Last updated: 31 January 2024
- Open access
- Published: 13 May 2024
Comparison of clinical characteristics and disease burden of febrile seizures in children with and without COVID-19
- Zhongli Jiang 1 ,
- Cuiyun Fang 2 ,
- Fengyimei Peng 1 &
- Wei Fan 1
BMC Pediatrics volume 24 , Article number: 329 ( 2024 ) Cite this article
171 Accesses
1 Altmetric
Metrics details
Febrile seizures (FS) are the most common seizure disorder in children and a common neurologic complication in children with coronavirus disease 2019 (COVID-19). This study aimed to identify differences in clinical characteristics and disease burden between FS with and without COVID-19.
Materials and methods
We conducted a retrospective analysis of medical data at our hospital from December 2019 to July 2023, focusing on hospitalized patients under the age of 14 diagnosed with FS who underwent COVID-19 polymerase chain reaction (PCR) testing. Descriptive statistics and analysis of variance were employed to compare the COVID-19 and non-COVID-19 groups in terms of clinical characteristics and disease burden.
A total of 514 patients were included, with 106 testing positive for COVID-19 and 408 testing negative. Patients with COVID-19 were older (34.87 ± 6.16 vs. 28.61 ± 11.35 months, P < 0.001) and had a higher proportion of males (79.2% vs. 62.3%, P = 0.001). The COVID-19 group had longer seizure durations (4.57 ± 4.38 vs. 3.22 ± 2.91 min, P = 0.006) and more complex FS (25.5% vs. 15.9%, P = 0.022). Laboratory tests showed lower lymphocyte counts in the COVID-19 group (1.87 ± 1.48 vs. 2.75 ± 1.51 × 103/µL, P < 0.001) and higher creatine kinase levels (158.49 ± 82.89 vs. 110.89 ± 56.11 U/L, P < 0.001). No significant differences were found in hospital costs, length of hospitalization, and intensive care unit admissions.
Clinicians should be knowledgeable about the distinct clinical characteristics of FS in children with COVID-19. Despite distinct features, the prognosis remains favorable and does not require excessive intervention. Ongoing monitoring and research are needed to fully understand the impact of COVID-19 on FS and optimize management strategies.
Peer Review reports
Introduction
Febrile seizures (FS) are the most prevalent seizure disorder in children, ranging from 2 to 5% in the United States and Western Europe, and higher prevalence rates of 5-10% in India and 6-9% in Japan among Asian populations [ 1 ]. FS are characterized by a seizure accompanied by a fever (temperature ≥ 100.4 °F) and do not involve central nervous system infection [ 2 ]. They typically occur in children aged 6 months to 5 years and can be categorized as simple or complex FS. Simple FS are primary generalized seizures that last for less than 15 min and do not recur within 24 h [ 3 ]. They occur during fevers unrelated to acute neurologic illness. On the other hand, complex febrile convulsions are defined as focal or prolonged seizures lasting ≥ 15 min and/or recurring within 24 h. These seizures may be linked to postictal neurologic abnormalities, commonly known as Todd’s palsy, or may occur in children with pre-existing neurologic deficits. This category also includes children whose seizures stopped before the 15th minute due to the administration of anti-seizure medication [ 4 ]. The exact etiology of FS is not fully understood, but it is believed to be multifactorial, involving genetics, viral infections, certain vaccinations, and incomplete neurological development in children [ 5 ]. Common viruses associated with FS include human herpesvirus 6, influenza virus, adenovirus, parainfluenza virus, varicella virus, respiratory syncytial virus, and rotavirus [ 6 ]. Despite having different seasonal distributions, the characteristics of seizures caused by different respiratory viruses do not show significant differences [ 7 ].
Coronavirus disease 2019 (COVID-19) is a severe infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with the epidemic starting in December 2019. In addition to respiratory abnormalities, COVID-19 also affects the nervous system and leads to a variety of complications [ 8 ]. Based on a multicenter prospective observational study, the most common neurological complications among children hospitalized for COVID-19 were fatigue, myalgia, altered consciousness, seizures, anosmia, and dysgeusia, in descending order of prevalence [ 9 ]. FS have become a common neurologic manifestation in children with COVID-19, particularly during the Omicron epidemic [ 10 ]. A multicenter cross-sectional study in the United States reported a 3.9% prevalence of FS with COVID-19 in hospitalized children [ 11 ]. While many studies have reported the clinical characteristics of FS in the context of COVID-19 at different time points, there is limited literature focusing on potential differences in clinical characteristics and disease burden between FS with and without COVID-19 [ 12 , 13 ]. Therefore, the aim of this study is to compare the demographic characteristics, clinical features, laboratory findings, and disease burden of febrile seizures in children with and without COVID-19, with the intention of contributing to this area of research.
Study design
We conducted a comprehensive search of the electronic medical record system at Liyang People’s Hospital, the sole tertiary hospital in the region, to retrieve the complete medical records of inpatients from December 2019 to July 2023. Two experienced pediatric specialists independently reviewed the medical records. Patients who met the diagnostic criteria for FS and underwent COVID-19 polymerase chain reaction (PCR) testing were included in the study. Exclusion criteria included central nervous system infections, prior history of seizures, metabolic disorders, head trauma, drug-induced seizures, incomplete medical records and those unable to obtain informed consent. In cases where a patient had multiple hospitalizations during the study period, only the information from the last medical record was collected.
We collected demographic data, which included age, sex, history of FS, and family history of FS. Clinical characteristics and laboratory test results were also gathered. Moreover, cerebrospinal fluid tests, electroencephalography, and brain computed tomography results were included in the data collection. Additionally, information regarding the disease burden, such as hospital costs, length of hospital stays, and admission to the intensive care unit, was documented. The calculation of hospital costs takes into consideration various factors such as the cost of medical supplies, equipment, staffing, overhead expenses, and administrative costs.
FS are characterized by a seizure accompanied by a fever (temperature ≥ 100.4 °F) and do not involve central nervous system infection [ 2 ]. Simple FS are primary generalized seizures that last for less than 15 min and do not recur within 24 h [ 3 ]. Complex febrile convulsions are defined as focal or prolonged seizures lasting ≥ 15 min and/or recurring within 24 h [ 3 ]. Status epilepticus was defined as a single sustained seizure lasting more than 5 min, or frequent clinical seizures without return to baseline clinical status in the interictal period [ 14 ]. COVID-19 PCR testing was conducted by a qualified laboratory that adheres to national standards [ 15 ]. The time lapse between febrile seizures and the COVID-19 PCR testing averaged around 0.5–3 h. Based on the results of the COVID-19 PCR tests, patients were divided into COVID-19 and non-COVID-19 groups. A case-control study unmatched was then conducted to analyze the differences between the two groups.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Liyang People’s Hospital (protocol code YJ2023012 and date of approval February 15, 2023). Informed consent was obtained from the participants’ legal guardian/next of kin.
Statistical analysis
Categorical variables were presented as n (%) and compared using appropriate statistical tests, such as chi-square tests, Fisher’s exact tests, or continuity corrections. Continuous variables were expressed as mean ± standard deviation. The significance of normally distributed data was analyzed using the t-test, while the significance of non-normally distributed data was analyzed using the Wilcoxon rank sum test. Statistical significance was defined as a two-sided P-value of less than 0.05. All statistical analyses in our study were performed using SPSS software (version 20.0; SPSS, Chicago, Illinois, United States of America).
General information and demographic data
During the study period, a total of 570 patients were diagnosed with FS and underwent COVID-19 PCR testing. A total of 56 patients were excluded from our study, including central nervous system infections (25 patients), epilepsy (13 patients), unable to provide informed consent (9 patients), incomplete medical data (7 patients), and metabolic disorders (2 patients). A total of 514 patients were included in this study. The flow of patient inclusion and exclusion was shown in Fig. 1 . Of these patients, 338 (65.8%) were male and 176 (34.2%) were female. The age of onset of FS ranged from 6 to 58 months, with a mean age of 29.9 ± 10.79 months. A history of FS was present in 166 (32.3%) patients, and 22 (4.3%) patients had a family history of FS.
The interval between febrile seizures and the COVID-19 PCR testing was approximately 0.5–3 h. COVID-19 PCR testing was positive in 106 (20.6%) patients and negative in 408 (79.4%) patients. Table 1 summarizes the demographic data of the two patient groups. Patients with COVID-19 were older compared to those without COVID-19 infection (34.87 ± 6.16 vs. 28.61 ± 11.35 months, P < 0.001). Additionally, there was a higher proportion of males in the COVID-19 group (79.2% vs. 62.3%, P = 0.001). There was no significant difference in the history of FS and family history of FS between the two groups.
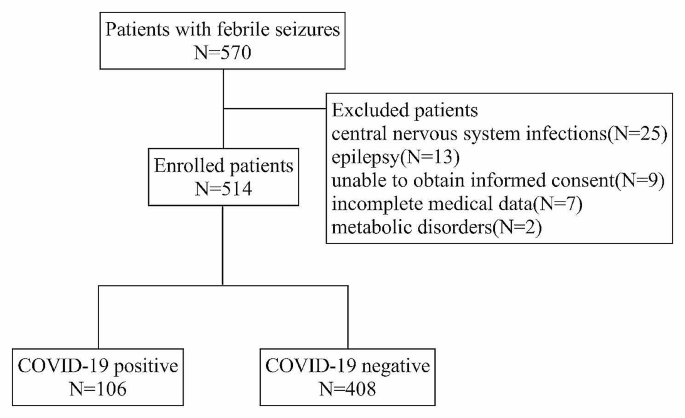
Flow chart for patient inclusion and exclusion
- Clinical characteristics
The highest recorded temperature during the course of the disease in all patients ranged from 38 °C to 41 °C, with a mean of 39.33 ± 0.50 °C. The duration of fever ranged from 1 to 5 days, with a mean duration of 2.37 ± 0.95 days. The most common type of seizure was generalized tonic-clonic seizure in 494 (96.1%) patients. Thirty-eight (7.4%) patients experienced seizure recurrences within 24 h. The mean duration of seizures was 3.49 ± 3.31 min. Fifty-four (10.6%) patients presented with status epilepticus and 92 patients were categorized as having complex FS. A total of 51 (10.0%) patients required antiseizure medications.
As shown in Table 1 , compared with FS without COVID-19, patients with COVID-19 had a significantly longer duration of seizures (4.57 ± 4.38 vs. 3.22 ± 2.91 min, P = 0.006) and a significantly higher proportion of complex FS (25.5% vs. 15.9%, P = 0.022). No significant differences were observed between the two groups in terms of fever peak, duration of fever, interval between seizures and fever, type of seizures, recurrence of seizures within 24 h, status epilepticus, and antiseizure medication needs.
Laboratory tests and brain tests
In laboratory tests, the COVID-19 group had significantly lower lymphocyte counts compared to the non-COVID-19 group (1.87 ± 1.48 vs. 2.75 ± 1.51 × 10 3 /µL, P < 0.001). Additionally, creatine kinase levels were significantly higher in the COVID-19 group compared to the control group (158.49 ± 82.89 vs. 110.89 ± 56.11 U/L, P < 0.001). There were no significant differences in terms of neutrophil count, hemoglobin, platelets, C-reactive protein, procalcitonin, serum sodium, serum potassium, aspartate aminotransferase, and alanine aminotransferase. The laboratory results are summarized in Table 2 .
A total of 49 patients underwent electroencephalography in the acute phase, with 17 in the COVID-19 group and 32 in the non-COVID-19 group. Among them, 9 patients in both groups showed background slow wave enhancement, which normalized during the outpatient review after 1 month. Sixteen patients underwent brain computed tomography, and no abnormalities were detected. Three patients underwent cerebrospinal fluid examination, and the results showed no abnormalities.
- Disease burden
The average hospital costs were 2041.34 ± 520.43 yuan, and the average length of hospitalization was 4.51 ± 1.48 days. Additionally, 17 (3.3%) patients received critical care services. No patients died or required chronic antiseizure medications. There were no significant differences between the COVID-19 and non-COVID-19 groups in terms of hospital costs, length of hospitalization, and proportion of intensive care unit admissions, as shown in Table 2 .
The objective of our study was to compare the clinical characteristics and disease burden of FS in children with and without COVID-19. Our study yielded several noteworthy findings. Firstly, FS associated with COVID-19 occurred at an older age and had a significantly higher prevalence among males. Secondly, these seizures had a longer duration and a higher incidence of complex FS. Lastly, FS with COVID-19 were associated with lower lymphocyte counts and higher creatine kinase levels. In terms of disease burden, there were no disparities in hospital costs, length of hospitalization, and proportion of intensive care unit admissions between FS with and without COVID-19.
Since the start of the pandemic in 2020, the neurological impact of COVID-19 has been a source of concern. Neurological complications in children with COVID-19 have been documented in recent international multicenter studies [ 9 , 16 ]. The most common neurological complication reported was malaise, followed by altered consciousness and myalgia [ 9 ]. It is important to note that these complications can occur not only in children with pre-existing underlying conditions but also in previously healthy children [ 16 ]. Additionally, although rare, there are serious and potentially life-threatening neurological complications associated with COVID-19. A large multi-center study conducted in the United States found that 2.5% of hospitalized children and adolescents with acute COVID-19 or multisystem inflammatory syndrome in children (MIS-C) developed various life-threatening neurological disorders associated with COVID-19 [ 17 ].
Our study uncovered a significant increase in the age of onset of FS in children with COVID-19 compared to those without COVID-19, aligning with previous studies [ 12 , 18 ]. This suggests that COVID-19 infection may exert distinct effects across different age groups. One plausible explanation for this pattern is the heightened severity of illness and higher fever observed in older children with COVID-19 [ 19 ]. FS are typically triggered by elevated body temperatures, and it is plausible that the more severe illness and higher fever in older children with COVID-19 may contribute to the delayed onset of FS. Another potential factor is the systemic inflammatory response and cytokine storm associated with COVID-19. Older children may possess a more mature immune system, which could result in a more robust inflammatory response to viral infections such as COVID-19 [ 20 ]. This heightened inflammatory response can impact the central nervous system and lower the seizure threshold, rendering older children more susceptible to FS. However, it is important to note that the effect of age on the severity of COVID-19 and the risk of death was significantly reduced after adjusting for important age-related risk factors such as immunocompromised conditions, prior respiratory diseases, and hypertension [ 21 ]. To comprehensively comprehend the mechanisms underpinning the age-specific effects of COVID-19 and FS, further research is imperative.
While both groups of children with FS were predominantly male, our study found a higher proportion of males in the COVID-19 group. The impact of COVID-19 on gender differences in FS has been observed in previous studies [ 18 ]. While both males and females are equally susceptible to COVID-19 infection, males tend to experience more severe complications and outcomes [ 22 ]. However, the reason for this gender difference is not well understood. One possible explanation for this finding is the potential differences in immune response between males and females. Studies have shown that females tend to mount stronger immune responses to viral infections than males, which may confer some degree of protection against FS [ 23 , 24 ]. Additionally, hormonal differences between males and females may also play a role in this gender discrepancy. For example, estrogen has been shown to have a neuroprotective effect, which may explain why females are less susceptible to FS [ 25 ].
In the COVID-19 group, patients had a significantly longer duration of seizures and a significantly higher proportion of complex FS. These imply that COVID-19 has stronger neurological effects. Various neurological manifestations have been associated with COVID-19, including altered consciousness, fatigue, seizures, and changes in smell and taste [ 9 ]. The exact mechanism of neurological complications caused by COVID-19 remains unclear, but some studies have suggested that COVID-19 may affect the central nervous system through the olfactory mucosa, blood-brain barrier, and axonal transport [ 26 , 27 ]. It is highly unlikely that the neurological complications in patients with COVID-19 were caused by direct infection of the central nervous system with SARS-CoV-2, as indicated by the negative PCR test results for the virus in the majority of cerebrospinal fluid tests [ 28 ]. Instead, it is believed that the inflammatory response theory, supported by blood-brain barrier dysfunction and elevated cytokine levels in patients with COVID-19, may play a role in these neurological complications [ 29 ].
The discrepancy observed in our research, where FS associated with COVID-19 exhibited significantly lower lymphocyte counts compared to FS without COVID-19, warrants further discussion. The immune response to viral infections, including COVID-19, is known to involve various immune cells, including lymphocytes. Lymphocytes play a crucial role in the adaptive immune response and are responsible for recognizing and eliminating viral pathogens [ 30 ]. The lower lymphocyte counts observed in FS with COVID-19 could be attributed to the direct impact of the virus on lymphocyte production or increased lymphocyte destruction. COVID-19 has been shown to cause lymphopenia in some patients, which may be due to the virus infecting and damaging lymphocytes or inducing their apoptosis [ 31 ]. Additionally, the systemic inflammatory response triggered by COVID-19 could lead to increased consumption and redistribution of lymphocytes to affected tissues [ 32 ]. It is important to note that our study was limited to laboratory tests and did not assess the functional capacity of lymphocytes or investigate the specific subsets of lymphocytes affected. Furthermore, our study revealed that patients with FS and COVID-19 had higher creatine kinase levels compared to those without COVID-19. Increased creatine kinase is frequently observed in COVID-19 patients and may be indicative of disease severity, serving as a predictor of poor prognosis [ 33 , 34 ]. It has also been reported that creatine kinase is associated with increased levels of inflammatory factors in COVID-19 patients [ 35 ]. However, the exact mechanism behind the elevated creatine kinase in the context of COVID-19 remains unclear. It is uncertain whether this elevation is a result of a viral-induced inflammatory response or direct muscle toxicity. Further investigation is needed to elucidate the underlying mechanisms involved in the association between COVID-19 and elevated creatine kinase levels in patients with FS.
The lumbar puncture rate observed in this study was remarkably low. Several factors may account for this phenomenon. Firstly, patients diagnosed with central nervous system infections post-lumbar puncture were intentionally excluded from the study. Secondly, the cautious approach required by physicians when considering lumbar puncture in China can be attributed to the general reluctance of Chinese parents towards invasive procedures. Our criteria for recommending lumbar puncture typically encompass patients presenting with unexplained lethargy, vomiting, or positive signs of meningeal irritation and/or pathology [ 36 , 37 ]; those lacking a history of influenza or pneumococcal vaccination, or with an unknown vaccination status between 6 and 12 months of age [ 3 ]; and individuals who have received antibiotic therapy, particularly those under 18 months old [ 2 ]. The decline in lumbar punctures among these patients may be associated with the increased vaccination rates and decreased antibiotic usage in China.
Despite presenting with more prolonged and complex FS, our study found that patients with FS and COVID-19 did not experience an increased burden of illness associated with FS, including length of hospitalization, hospital costs, and intensive care unit admissions. This finding suggests that while COVID-19 may contribute to the development of more severe and urgent seizures, it does not necessarily result in a greater need for extensive medical interventions or supportive measures. The majority of patients with FS and COVID-19 were able to recover without requiring additional medical interventions beyond standard febrile seizure management.
There are some limitations to our study. Firstly, it is a single-center retrospective study, which may be subject to selection bias and recall bias. Additionally, it is important to consider the timing and context of our study findings. Given that China’s anti-epidemic policy was fully liberalized during the Omicron period, caution should be exercised when extrapolating our results to all SARS-CoV-2 variants. Furthermore, our study only included patients who were admitted to the hospital, which may have excluded patients with milder symptoms who did not require hospitalization. As a result, our findings may not be applicable to children with FS who did not seek medical attention or were managed in an outpatient setting. Future studies with larger sample sizes and more diverse populations are needed to confirm our findings and provide a more comprehensive understanding of the impact of COVID-19 on FS in children.
In conclusion, patients with COVID-19 were older and had a higher proportion of males compared to those without the infection. Those with COVID-19 also experienced longer seizure durations and a higher rate of complex FS. Laboratory findings showed lower lymphocyte counts and higher creatine kinase levels in the COVID-19 group. In terms of disease burden, there were no disparities in hospital costs, length of hospitalization, and proportion of intensive care unit admissions between FS with and without COVID-19. As the pandemic continues to evolve, ongoing monitoring and research are necessary to understand the full impact of COVID-19 on FS and optimize management strategies for these patients.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- Febrile seizures
Coronavirus disease 2019
Polymerase chain reaction
Severe acute respiratory syndrome coronavirus 2
Generalized tonic-clonic
Intensive care unit
Leung AK, Hon KL, Leung TN. Febrile seizures: an overview. Drugs Context. 2018;7:212536. https://doi.org/10.7573/dic.212536 .
Article PubMed PubMed Central Google Scholar
Subcommittee on Febrile Seizures. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389–94. https://doi.org/10.1542/peds.2010-3318 .
Article Google Scholar
Capovilla G, Mastrangelo M, Romeo A, Vigevano F. Recommendations for the management of febrile seizures: ad Hoc Task Force of Lice Guidelines Commission. Epilepsia. 2009;50(Suppl 1):2–6. https://doi.org/10.1111/j.1528-1167.2008.01963.x .
Article PubMed Google Scholar
Mewasingh LD, Chin RFM, Scott RC. Current understanding of febrile seizures and their long-term outcomes. Dev Med Child Neurol. 2020;62(11):1245–9. https://doi.org/10.1111/dmcn.14642 .
Sawires R, Buttery J, Fahey MA, Review of Febrile Seizures. Recent advances in understanding of Febrile Seizure Pathophysiology and commonly implicated viral triggers. Front Pediatr. 2021;9:801321. https://doi.org/10.3389/fped.2021.801321 .
Pokorn M, Jevšnik M, Petrovec M, Steyer A, Mrvič T, Grosek Š, et al. Respiratory and enteric virus detection in children. J Child Neurol. 2017;32(1):84–93. https://doi.org/10.1177/0883073816670820 .
Han JY, Han SB. Febrile seizures and respiratory viruses determined by Multiplex polymerase chain reaction test and clinical diagnosis. Child (Basel). 2020;7(11):234. https://doi.org/10.3390/children7110234 .
Valderas C, Méndez G, Echeverría A, Suarez N, Julio K, Sandoval F. Covid-19 and neurologic manifestations: a synthesis from the child neurologist’s corner. World J Pediatr. 2022;18(6):373–82. https://doi.org/10.1007/s12519-022-00550-4 .
Article CAS PubMed PubMed Central Google Scholar
Cho SM, White N, Premraj L, Battaglini D, Fanning J, Suen J, et al. Neurological manifestations of Covid-19 in adults and children. Brain. 2023;146(4):1648–61. https://doi.org/10.1093/brain/awac332 .
Iijima H, Kubota M, Ogimi C. Change in seizure incidence in Febrile Children with Covid-19 in the era of Omicron variant of concern. J Pediatr Infect Dis Soc. 2022;11(11):514–7. https://doi.org/10.1093/jpids/piac085 .
Antoon JW, Hall M, Howard LM, Herndon A, Freundlich KL, Grijalva CG, et al. Covid-19 and Acute neurologic complications in children. Pediatrics. 2022;150(5):e2022058167. https://doi.org/10.1542/peds.2022-058167 .
Han MJ, Heo JH, Hwang JS, Jang YT, Lee M, Kim SJ. Incidence of febrile seizures in children with Covid-19. J Clin Med. 2023;12(3):1076. https://doi.org/10.3390/jcm12031076 .
Han MS, Kim KM, Oh KJ, Chang JY, Lee SY, Choi JE, et al. Distinct clinical and Laboratory features of Covid-19 in Children during the Pre-delta, Delta and Omicron Wave. Pediatr Infect Dis J. 2023;42(5):423–8. https://doi.org/10.1097/INF.0000000000003872 .
Becker LL, Gratopp A, Prager C, Elger CE, Kaindl AM. Treatment of Pediatric Convulsive Status Epilepticus. Front Neurol. 2023;14:1175370. https://doi.org/10.3389/fneur.2023.1175370 .
China Center for Disease Control and Prevention. Technical Guidelines for Laboratory Testing of Covid-19. https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202003/t20200309_214241.html . Accessed 01 March 2024.
Laçinel Gürlevik S, Günbey C, Ozsurekci Y, Oygar PD, Kesici S, Gocmen R, et al. Neurologic manifestations in children with Covid-19 from a Tertiary Center in Turkey and Literature Review. Eur J Paediatr Neurol. 2022;37:139–54. https://doi.org/10.1016/j.ejpn.2022.02.003 .
LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for Covid-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021;78(5):536–47. https://doi.org/10.1001/jamaneurol.2021.0504 .
Seo MJ, Yum MS, Park JS. Comparison of febrile seizures in children with or without Coronavirus Disease-2019: a single-Center Observational Study. Pediatr Int. 2023;65(1):e15461. https://doi.org/10.1111/ped.15461 .
Article CAS PubMed Google Scholar
Crespo FI, Mayora SJ, De Sanctis JB, Martínez WY, Zabaleta-Lanz ME, Toro FI, et al. Sars-Cov-2 infection in Venezuelan Pediatric Patients-a single center prospective observational study. Biomedicines. 2023;11(5):1409. https://doi.org/10.3390/biomedicines11051409 .
Ji SQ, Zhang M, Zhang Y, Xia K, Chen Y, Chu Q, et al. Characteristics of Immune and inflammatory responses among different age groups of Pediatric patients with Covid-19 in China. World J Pediatr. 2021;17(4):375–84. https://doi.org/10.1007/s12519-021-00440-1 .
Romero Starke K, Petereit-Haack G, Schubert M, Kämpf D, Schliebner A, Hegewald J, et al. The age-related risk of severe outcomes due to Covid-19 infection: a Rapid Review, Meta-Analysis, and Meta-Regression. Int J Environ Res Public Health. 2020;17(16):5974. https://doi.org/10.3390/ijerph17165974 .
Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with Covid-19: focus on severity and mortality. Front Public Health. 2020;8(152):152. https://doi.org/10.3389/fpubh.2020.00152 .
Kloc M, Ghobrial RM, Kubiak JZ. The role of genetic sex and Mitochondria in response to Covid-19 infection. Int Arch Allergy Immunol. 2020;181(8):629–34. https://doi.org/10.1159/000508560 .
Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of Sars-Cov-2 igg antibody between male and female Covid-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050–4. https://doi.org/10.1002/jmv.25989 .
Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of Circulating Sex Hormones with inflammation and disease severity in patients with Covid-19. JAMA Netw Open. 2021;4(5):e2111398. https://doi.org/10.1001/jamanetworkopen.2021.11398 .
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory Transmucosal Sars-Cov-2 Invasion as a Port of Central Nervous System Entry in individuals with Covid-19. Nat Neurosci. 2021;24(2):168–75. https://doi.org/10.1038/s41593-020-00758-5 .
Burks SM, Rosas-Hernandez H, Alejandro Ramirez-Lee M, Cuevas E, Talpos JC. Can Sars-Cov-2 infect the Central Nervous System Via the olfactory bulb or the blood-brain barrier? Brain Behav Immun. 2021;95:7–14. https://doi.org/10.1016/j.bbi.2020.12.031 .
Neumann B, Schmidbauer ML, Dimitriadis K, Otto S, Knier B, Niesen WD, et al. Cerebrospinal fluid findings in Covid-19 patients with neurological symptoms. J Neurol Sci. 2020;418:117090. https://doi.org/10.1016/j.jns.2020.117090 .
Jarius S, Pache F, Körtvelyessy P, Jelčić I, Stettner M, Franciotta D, et al. Cerebrospinal fluid findings in Covid-19: a Multicenter Study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19(1):19. https://doi.org/10.1186/s12974-021-02339-0 .
Jansen JM, Gerlach T, Elbahesh H, Rimmelzwaan GF, Saletti G. Influenza virus-specific Cd4 + and Cd8 + T cell-mediated Immunity Induced by infection and vaccination. J Clin Virol. 2019;119:44–52. https://doi.org/10.1016/j.jcv.2019.08.009 .
Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune Response to Sars-Cov-2 and mechanisms of Immunopathological Changes in Covid-19. Allergy. 2020;75(7):1564–81. https://doi.org/10.1111/all.14364 .
Delshad M, Tavakolinia N, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, Bagheri N, Bashash D. The contributory role of lymphocyte subsets, pathophysiology of Lymphopenia and its implication as prognostic and therapeutic opportunity in Covid-19. Int Immunopharmacol. 2021;95:107586. https://doi.org/10.1016/j.intimp.2021.107586 .
Orsucci D, Trezzi M, Anichini R, Blanc P, Barontini L, Biagini C, et al. Increased creatine kinase may predict a worse Covid-19 outcome. J Clin Med. 2021;10(8):1734. https://doi.org/10.3390/jcm10081734 .
Zhang T, Huang WS, Guan W, Hong Z, Gao J, Gao G, et al. Risk factors and predictors Associated with the severity of Covid-19 in China: a systematic review, Meta-analysis, and Meta-Regression. J Thorac Dis. 2020;12(12):7429–41. https://doi.org/10.21037/jtd-20-1743 .
Pitscheider L, Karolyi M, Burkert FR, Helbok R, Wanschitz JV, Horlings C, et al. Muscle involvement in Sars-Cov-2 infection. Eur J Neurol. 2021;28(10):3411–7. https://doi.org/10.1111/ene.14564 .
Son YY, Kim GH, Byeon JH, Eun SH, Eun BL. Need for lumbar puncture in children younger than 12 months presenting with simple febrile seizure. Pediatr Emerg Care. 2018;34(3):212–5. https://doi.org/10.1097/pec.0000000000000779 .
Guedj R, Chappuy H, Titomanlio L, De Pontual L, Biscardi S, Nissack-Obiketeki G, et al. Do all children who present with a Complex Febrile Seizure need a lumbar puncture? Ann Emerg Med. 2017;70(1):52–e626. https://doi.org/10.1016/j.annemergmed.2016.11.024 .
Download references
Acknowledgements
We thank Dr. Ali Cao for her advice on statistics.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Pediatrics, Liyang People’s Hospital, Liyang, China
Zhongli Jiang, Fengyimei Peng & Wei Fan
Department of Nursing, Liyang People’s Hospital, Liyang, China
Cuiyun Fang
You can also search for this author in PubMed Google Scholar
Contributions
WF, and FP conceived the study and reviewed the results; ZJ, FP, and CF collected data; ZJ, and CF analyzed the data and drafted the manuscript. All authors have read and approved the final version of manuscript for submission.
Corresponding author
Correspondence to Wei Fan .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jiang, Z., Fang, C., Peng, F. et al. Comparison of clinical characteristics and disease burden of febrile seizures in children with and without COVID-19. BMC Pediatr 24 , 329 (2024). https://doi.org/10.1186/s12887-024-04821-z
Download citation
Received : 06 November 2023
Accepted : 08 May 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12887-024-04821-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Pharmacol
- v.44(2); Mar-Apr 2012
Data management in clinical research: An overview
Binny krishnankutty.
Global Medical Affairs, Dr. Reddy's Laboratories Ltd., Ameerpet, Hyderabad, India
Shantala Bellary
Naveen b.r. kumar, latha s. moodahadu.
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right from inception to completion. They should have adequate process knowledge that helps maintain the quality standards of CDM processes. Various procedures in CDM including Case Report Form (CRF) designing, CRF annotation, database designing, data-entry, data validation, discrepancy management, medical coding, data extraction, and database locking are assessed for quality at regular intervals during a trial. In the present scenario, there is an increased demand to improve the CDM standards to meet the regulatory requirements and stay ahead of the competition by means of faster commercialization of product. With the implementation of regulatory compliant data management tools, CDM team can meet these demands. Additionally, it is becoming mandatory for companies to submit the data electronically. CDM professionals should meet appropriate expectations and set standards for data quality and also have a drive to adapt to the rapidly changing technology. This article highlights the processes involved and provides the reader an overview of the tools and standards adopted as well as the roles and responsibilities in CDM.
Introduction
Clinical trial is intended to find answers to the research question by means of generating data for proving or disproving a hypothesis. The quality of data generated plays an important role in the outcome of the study. Often research students ask the question, “what is Clinical Data Management (CDM) and what is its significance?” Clinical data management is a relevant and important part of a clinical trial. All researchers try their hands on CDM activities during their research work, knowingly or unknowingly. Without identifying the technical phases, we undertake some of the processes involved in CDM during our research work. This article highlights the processes involved in CDM and gives the reader an overview of how data is managed in clinical trials.
CDM is the process of collection, cleaning, and management of subject data in compliance with regulatory standards. The primary objective of CDM processes is to provide high-quality data by keeping the number of errors and missing data as low as possible and gather maximum data for analysis.[ 1 ] To meet this objective, best practices are adopted to ensure that data are complete, reliable, and processed correctly. This has been facilitated by the use of software applications that maintain an audit trail and provide easy identification and resolution of data discrepancies. Sophisticated innovations[ 2 ] have enabled CDM to handle large trials and ensure the data quality even in complex trials.
How do we define ‘high-quality’ data? High-quality data should be absolutely accurate and suitable for statistical analysis. These should meet the protocol-specified parameters and comply with the protocol requirements. This implies that in case of a deviation, not meeting the protocol-specifications, we may think of excluding the patient from the final database. It should be borne in mind that in some situations, regulatory authorities may be interested in looking at such data. Similarly, missing data is also a matter of concern for clinical researchers. High-quality data should have minimal or no misses. But most importantly, high-quality data should possess only an arbitrarily ‘acceptable level of variation’ that would not affect the conclusion of the study on statistical analysis. The data should also meet the applicable regulatory requirements specified for data quality.
Tools for CDM
Many software tools are available for data management, and these are called Clinical Data Management Systems (CDMS). In multicentric trials, a CDMS has become essential to handle the huge amount of data. Most of the CDMS used in pharmaceutical companies are commercial, but a few open source tools are available as well. Commonly used CDM tools are ORACLE CLINICAL, CLINTRIAL, MACRO, RAVE, and eClinical Suite. In terms of functionality, these software tools are more or less similar and there is no significant advantage of one system over the other. These software tools are expensive and need sophisticated Information Technology infrastructure to function. Additionally, some multinational pharmaceutical giants use custom-made CDMS tools to suit their operational needs and procedures. Among the open source tools, the most prominent ones are OpenClinica, openCDMS, TrialDB, and PhOSCo. These CDM software are available free of cost and are as good as their commercial counterparts in terms of functionality. These open source software can be downloaded from their respective websites.
In regulatory submission studies, maintaining an audit trail of data management activities is of paramount importance. These CDM tools ensure the audit trail and help in the management of discrepancies. According to the roles and responsibilities (explained later), multiple user IDs can be created with access limitation to data entry, medical coding, database designing, or quality check. This ensures that each user can access only the respective functionalities allotted to that user ID and cannot make any other change in the database. For responsibilities where changes are permitted to be made in the data, the software will record the change made, the user ID that made the change and the time and date of change, for audit purposes (audit trail). During a regulatory audit, the auditors can verify the discrepancy management process; the changes made and can confirm that no unauthorized or false changes were made.
Regulations, Guidelines, and Standards in CDM
Akin to other areas in clinical research, CDM has guidelines and standards that must be followed. Since the pharmaceutical industry relies on the electronically captured data for the evaluation of medicines, there is a need to follow good practices in CDM and maintain standards in electronic data capture. These electronic records have to comply with a Code of Federal Regulations (CFR), 21 CFR Part 11. This regulation is applicable to records in electronic format that are created, modified, maintained, archived, retrieved, or transmitted. This demands the use of validated systems to ensure accuracy, reliability, and consistency of data with the use of secure, computer-generated, time-stamped audit trails to independently record the date and time of operator entries and actions that create, modify, or delete electronic records.[ 3 ] Adequate procedures and controls should be put in place to ensure the integrity, authenticity, and confidentiality of data. If data have to be submitted to regulatory authorities, it should be entered and processed in 21 CFR part 11-compliant systems. Most of the CDM systems available are like this and pharmaceutical companies as well as contract research organizations ensure this compliance.
Society for Clinical Data Management (SCDM) publishes the Good Clinical Data Management Practices (GCDMP) guidelines, a document providing the standards of good practice within CDM. GCDMP was initially published in September 2000 and has undergone several revisions thereafter. The July 2009 version is the currently followed GCDMP document. GCDMP provides guidance on the accepted practices in CDM that are consistent with regulatory practices. Addressed in 20 chapters, it covers the CDM process by highlighting the minimum standards and best practices.
Clinical Data Interchange Standards Consortium (CDISC), a multidisciplinary non-profit organization, has developed standards to support acquisition, exchange, submission, and archival of clinical research data and metadata. Metadata is the data of the data entered. This includes data about the individual who made the entry or a change in the clinical data, the date and time of entry/change and details of the changes that have been made. Among the standards, two important ones are the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG) and the Clinical Data Acquisition Standards Harmonization (CDASH) standards, available free of cost from the CDISC website ( www.cdisc.org ). The SDTMIG standard[ 4 ] describes the details of model and standard terminologies for the data and serves as a guide to the organization. CDASH v 1.1[ 5 ] defines the basic standards for the collection of data in a clinical trial and enlists the basic data information needed from a clinical, regulatory, and scientific perspective.
The CDM Process
The CDM process, like a clinical trial, begins with the end in mind. This means that the whole process is designed keeping the deliverable in view. As a clinical trial is designed to answer the research question, the CDM process is designed to deliver an error-free, valid, and statistically sound database. To meet this objective, the CDM process starts early, even before the finalization of the study protocol.
Review and finalization of study documents
The protocol is reviewed from a database designing perspective, for clarity and consistency. During this review, the CDM personnel will identify the data items to be collected and the frequency of collection with respect to the visit schedule. A Case Report Form (CRF) is designed by the CDM team, as this is the first step in translating the protocol-specific activities into data being generated. The data fields should be clearly defined and be consistent throughout. The type of data to be entered should be evident from the CRF. For example, if weight has to be captured in two decimal places, the data entry field should have two data boxes placed after the decimal as shown in Figure 1 . Similarly, the units in which measurements have to be made should also be mentioned next to the data field. The CRF should be concise, self-explanatory, and user-friendly (unless you are the one entering data into the CRF). Along with the CRF, the filling instructions (called CRF Completion Guidelines) should also be provided to study investigators for error-free data acquisition. CRF annotation is done wherein the variable is named according to the SDTMIG or the conventions followed internally. Annotations are coded terms used in CDM tools to indicate the variables in the study. An example of an annotated CRF is provided in Figure 1 . In questions with discrete value options (like the variable gender having values male and female as responses), all possible options will be coded appropriately.

Annotated sample of a Case Report Form (CRF). Annotations are entered in coloured text in this figure to differentiate from the CRF questions. DCM = Data collection module, DVG = Discrete value group, YNNA [S1] = Yes, No = Not applicable [subset 1], C = Character, N = Numerical, DT = Date format. For xample, BRTHDTC [DT] indicates date of birth in the date format
Based on these, a Data Management Plan (DMP) is developed. DMP document is a road map to handle the data under foreseeable circumstances and describes the CDM activities to be followed in the trial. A list of CDM activities is provided in Table 1 . The DMP describes the database design, data entry and data tracking guidelines, quality control measures, SAE reconciliation guidelines, discrepancy management, data transfer/extraction, and database locking guidelines. Along with the DMP, a Data Validation Plan (DVP) containing all edit-checks to be performed and the calculations for derived variables are also prepared. The edit check programs in the DVP help in cleaning up the data by identifying the discrepancies.
List of clinical data management activities

Database designing
Databases are the clinical software applications, which are built to facilitate the CDM tasks to carry out multiple studies.[ 6 ] Generally, these tools have built-in compliance with regulatory requirements and are easy to use. “System validation” is conducted to ensure data security, during which system specifications,[ 7 ] user requirements, and regulatory compliance are evaluated before implementation. Study details like objectives, intervals, visits, investigators, sites, and patients are defined in the database and CRF layouts are designed for data entry. These entry screens are tested with dummy data before moving them to the real data capture.
Data collection
Data collection is done using the CRF that may exist in the form of a paper or an electronic version. The traditional method is to employ paper CRFs to collect the data responses, which are translated to the database by means of data entry done in-house. These paper CRFs are filled up by the investigator according to the completion guidelines. In the e-CRF-based CDM, the investigator or a designee will be logging into the CDM system and entering the data directly at the site. In e-CRF method, chances of errors are less, and the resolution of discrepancies happens faster. Since pharmaceutical companies try to reduce the time taken for drug development processes by enhancing the speed of processes involved, many pharmaceutical companies are opting for e-CRF options (also called remote data entry).
CRF tracking
The entries made in the CRF will be monitored by the Clinical Research Associate (CRA) for completeness and filled up CRFs are retrieved and handed over to the CDM team. The CDM team will track the retrieved CRFs and maintain their record. CRFs are tracked for missing pages and illegible data manually to assure that the data are not lost. In case of missing or illegible data, a clarification is obtained from the investigator and the issue is resolved.
Data entry takes place according to the guidelines prepared along with the DMP. This is applicable only in the case of paper CRF retrieved from the sites. Usually, double data entry is performed wherein the data is entered by two operators separately.[ 8 ] The second pass entry (entry made by the second person) helps in verification and reconciliation by identifying the transcription errors and discrepancies caused by illegible data. Moreover, double data entry helps in getting a cleaner database compared to a single data entry. Earlier studies have shown that double data entry ensures better consistency with paper CRF as denoted by a lesser error rate.[ 9 ]
Data validation
Data validation is the process of testing the validity of data in accordance with the protocol specifications. Edit check programs are written to identify the discrepancies in the entered data, which are embedded in the database, to ensure data validity. These programs are written according to the logic condition mentioned in the DVP. These edit check programs are initially tested with dummy data containing discrepancies. Discrepancy is defined as a data point that fails to pass a validation check. Discrepancy may be due to inconsistent data, missing data, range checks, and deviations from the protocol. In e-CRF based studies, data validation process will be run frequently for identifying discrepancies. These discrepancies will be resolved by investigators after logging into the system. Ongoing quality control of data processing is undertaken at regular intervals during the course of CDM. For example, if the inclusion criteria specify that the age of the patient should be between 18 and 65 years (both inclusive), an edit program will be written for two conditions viz . age <18 and >65. If for any patient, the condition becomes TRUE, a discrepancy will be generated. These discrepancies will be highlighted in the system and Data Clarification Forms (DCFs) can be generated. DCFs are documents containing queries pertaining to the discrepancies identified.
Discrepancy management
This is also called query resolution. Discrepancy management includes reviewing discrepancies, investigating the reason, and resolving them with documentary proof or declaring them as irresolvable. Discrepancy management helps in cleaning the data and gathers enough evidence for the deviations observed in data. Almost all CDMS have a discrepancy database where all discrepancies will be recorded and stored with audit trail.
Based on the types identified, discrepancies are either flagged to the investigator for clarification or closed in-house by Self-Evident Corrections (SEC) without sending DCF to the site. The most common SECs are obvious spelling errors. For discrepancies that require clarifications from the investigator, DCFs will be sent to the site. The CDM tools help in the creation and printing of DCFs. Investigators will write the resolution or explain the circumstances that led to the discrepancy in data. When a resolution is provided by the investigator, the same will be updated in the database. In case of e-CRFs, the investigator can access the discrepancies flagged to him and will be able to provide the resolutions online. Figure 2 illustrates the flow of discrepancy management.

Discrepancy management (DCF = Data clarification form, CRA = Clinical Research Associate, SDV = Source document verification, SEC = Self-evident correction)
The CDM team reviews all discrepancies at regular intervals to ensure that they have been resolved. The resolved data discrepancies are recorded as ‘closed’. This means that those validation failures are no longer considered to be active, and future data validation attempts on the same data will not create a discrepancy for same data point. But closure of discrepancies is not always possible. In some cases, the investigator will not be able to provide a resolution for the discrepancy. Such discrepancies will be considered as ‘irresolvable’ and will be updated in the discrepancy database.
Discrepancy management is the most critical activity in the CDM process. Being the vital activity in cleaning up the data, utmost attention must be observed while handling the discrepancies.
Medical coding
Medical coding helps in identifying and properly classifying the medical terminologies associated with the clinical trial. For classification of events, medical dictionaries available online are used. Technically, this activity needs the knowledge of medical terminology, understanding of disease entities, drugs used, and a basic knowledge of the pathological processes involved. Functionally, it also requires knowledge about the structure of electronic medical dictionaries and the hierarchy of classifications available in them. Adverse events occurring during the study, prior to and concomitantly administered medications and pre-or co-existing illnesses are coded using the available medical dictionaries. Commonly, Medical Dictionary for Regulatory Activities (MedDRA) is used for the coding of adverse events as well as other illnesses and World Health Organization–Drug Dictionary Enhanced (WHO-DDE) is used for coding the medications. These dictionaries contain the respective classifications of adverse events and drugs in proper classes. Other dictionaries are also available for use in data management (eg, WHO-ART is a dictionary that deals with adverse reactions terminology). Some pharmaceutical companies utilize customized dictionaries to suit their needs and meet their standard operating procedures.
Medical coding helps in classifying reported medical terms on the CRF to standard dictionary terms in order to achieve data consistency and avoid unnecessary duplication. For example, the investigators may use different terms for the same adverse event, but it is important to code all of them to a single standard code and maintain uniformity in the process. The right coding and classification of adverse events and medication is crucial as an incorrect coding may lead to masking of safety issues or highlight the wrong safety concerns related to the drug.
Database locking
After a proper quality check and assurance, the final data validation is run. If there are no discrepancies, the SAS datasets are finalized in consultation with the statistician. All data management activities should have been completed prior to database lock. To ensure this, a pre-lock checklist is used and completion of all activities is confirmed. This is done as the database cannot be changed in any manner after locking. Once the approval for locking is obtained from all stakeholders, the database is locked and clean data is extracted for statistical analysis. Generally, no modification in the database is possible. But in case of a critical issue or for other important operational reasons, privileged users can modify the data even after the database is locked. This, however, requires proper documentation and an audit trail has to be maintained with sufficient justification for updating the locked database. Data extraction is done from the final database after locking. This is followed by its archival.
Roles and Responsibilities in CDM
In a CDM team, different roles and responsibilities are attributed to the team members. The minimum educational requirement for a team member in CDM should be graduation in life science and knowledge of computer applications. Ideally, medical coders should be medical graduates. However, in the industry, paramedical graduates are also recruited as medical coders. Some key roles are essential to all CDM teams. The list of roles given below can be considered as minimum requirements for a CDM team:
- Data Manager
- Database Programmer/Designer
- Medical Coder
- Clinical Data Coordinator
- Quality Control Associate
- Data Entry Associate
The data manager is responsible for supervising the entire CDM process. The data manager prepares the DMP, approves the CDM procedures and all internal documents related to CDM activities. Controlling and allocating the database access to team members is also the responsibility of the data manager. The database programmer/designer performs the CRF annotation, creates the study database, and programs the edit checks for data validation. He/she is also responsible for designing of data entry screens in the database and validating the edit checks with dummy data. The medical coder will do the coding for adverse events, medical history, co-illnesses, and concomitant medication administered during the study. The clinical data coordinator designs the CRF, prepares the CRF filling instructions, and is responsible for developing the DVP and discrepancy management. All other CDM-related documents, checklists, and guideline documents are prepared by the clinical data coordinator. The quality control associate checks the accuracy of data entry and conducts data audits.[ 10 ] Sometimes, there is a separate quality assurance person to conduct the audit on the data entered. Additionally, the quality control associate verifies the documentation pertaining to the procedures being followed. The data entry personnel will be tracking the receipt of CRF pages and performs the data entry into the database.
CDM has evolved in response to the ever-increasing demand from pharmaceutical companies to fast-track the drug development process and from the regulatory authorities to put the quality systems in place to ensure generation of high-quality data for accurate drug evaluation. To meet the expectations, there is a gradual shift from the paper-based to the electronic systems of data management. Developments on the technological front have positively impacted the CDM process and systems, thereby leading to encouraging results on speed and quality of data being generated. At the same time, CDM professionals should ensure the standards for improving data quality.[ 11 ] CDM, being a speciality in itself, should be evaluated by means of the systems and processes being implemented and the standards being followed. The biggest challenge from the regulatory perspective would be the standardization of data management process across organizations, and development of regulations to define the procedures to be followed and the data standards. From the industry perspective, the biggest hurdle would be the planning and implementation of data management systems in a changing operational environment where the rapid pace of technology development outdates the existing infrastructure. In spite of these, CDM is evolving to become a standard-based clinical research entity, by striking a balance between the expectations from and constraints in the existing systems, driven by technological developments and business demands.
Source of Support: Nil.
Conflict of Interest: None declared.
- Open access
- Published: 14 May 2024
PSMD9 promotes the malignant progression of hepatocellular carcinoma by interacting with c-Cbl to activate EGFR signaling and recycling
- Yuting Su 1 na1 ,
- Lili Meng 2 na1 ,
- Chao Ge 1 ,
- Yuqi Liu 1 ,
- Chi Zhang 1 ,
- Yue Yang 1 ,
- Wei Tian 1 &
- Hua Tian 1 , 3 , 4
Journal of Experimental & Clinical Cancer Research volume 43 , Article number: 142 ( 2024 ) Cite this article
340 Accesses
Metrics details
Mounting evidences shows that the ubiquitin‒proteasome pathway plays a pivotal role in tumor progression. The expression of 26S proteasome non-ATPase regulatory subunit 9 (PSMD9) is correlated with recurrence and radiotherapy resistance in several tumor types. However, the role and mechanism of PSMD9 in hepatocellular carcinoma (HCC) progression remain largely unclear.
PSMD9 was identified as a prognosis-related biomarker for HCC based on analysis of clinical characteristics and RNA-seq data from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and the JP Project of the International Cancer Genome Consortium (ICGC-LIRI-JP). PSMD9 expression was analyzed in cancer tissues and adjacent noncancerous tissues via immunohistochemistry and Western blotting. Multiple in vivo and in vitro experimental techniques (such as CCK-8, colony formation, EdU, and Transwell assays; flow cytometry; Western blotting; quantitative RT-PCR; Coimmunoprecipitation assay and immunofluorescence confocal imaging) were used to assess the functions of PSMD9 in the pathogenesis of HCC.
We found that the expression of PSMD9 was upregulated and associated with a poor prognosis in HCC patients. PSMD9 promoted HCC cell proliferation, migration, invasion and metastasis. Knockdown of PSMD9 significantly inhibited HCC cell proliferation by inducing G1/S cell cycle arrest and apoptosis. Mechanistically, we demonstrated that PSMD9 promoted HCC cell proliferation and metastasis via direct interaction with the E3 ubiquitin ligase c-Cbl, suppresses EGFR ubiquitination, influenced EGFR endosomal trafficking and degradation and subsequently activated ERK1/2 and Akt signaling. In addition, we showed that PSMD9 knockdown sensitized HCC cells to the tyrosine kinase inhibitor erlotinib in vitro and in vivo.
Conclusions
Collectively, our results indicate that PSMD9 drives HCC progression and erlotinib resistance by suppressing c-Cbl mediated EGFR ubiquitination and therefore can be a potential therapeutic target for HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death and ranks sixth in incidence among cancers worldwide. Surgical resection is recognized as an effective treatment for early-stage HCC [ 1 ]. Unfortunately, the prognosis of HCC remains poor because of its propensity for metastatic progression and poor response to pharmacological treatment. Therefore, there is an unmet need for the identification of novel diagnostic and therapeutic targets to improve the prognosis of HCC.
The epidermal growth factor receptor (EGFR) is one of four members of the EGFR/ErbB subfamily of receptor tyrosine kinases (RTKs). EGFR plays a key role in the development of various cancers [ 2 ]. Small molecule inhibitors targeting EGFR have been applied to treat solid tumors, such as lung cancer [ 3 ]. However, EGFR antibodies and inhibitors have not achieved satisfactory clinical results in HCC [ 4 ]. Thus, a better understanding of the EGFR signaling cascade in HCC is needed.
The ubiquitin‒proteasome (Ub) system plays an important role in oncogenesis, cancer development and chemoresistance [ 5 ]. The 26S proteasome is composed of a 20S core proteasome (CP) and a 19S regulatory particle (RP). It is the 19S regulator that links the 20S CP to the Ub system. The 19S RP consists of at least 18 different subunits and orchestrates all the steps that lead to the degradation of ubiquitylated proteins [ 6 ]. The proteasome degrades most cellular proteins in a tightly controlled manner, and thereby, its dysregulation is involved in multiple diseases, including cancer. The proteasome has become an attractive target for therapy in many cancers. Several proteasome inhibitors have exhibited marked antitumor effects [ 7 , 8 ]. Our previous study showed that Rpn10/PSMD4 (a 19S regulator) promotes tumor progression by regulating hypoxia-inducible factor 1alpha through the PTEN/Akt signaling pathway in HCC [ 9 ]. Rpn10 may be a novel therapeutic target in HCC. PSMD9 (Rpn4) encodes a non-ATPase subunit of the 19S regulator. Many studies have shown that PSMD9 plays an important role in tumor progression. PSMD9 expression is correlated with recurrence after radiotherapy in patients with cervical cancer [ 10 ]. PSMD9 expression predicts the response to radiotherapy in breast cancer patients [ 11 ]. PSMD9 is associated with radiotherapy resistance and shorter survival in bone metastatic prostate cancer patients [ 12 ]. PSMD9 is implicated in ribosomal protein shuttling to the nucleolus and subsequent activation of p53 [ 13 ], which allows cells with PSMD9 to overcome the nucleolar stress induced by anticancer drugs and gain a survival advantage. However, the involvement of PSMD9 in HCC progression remains unknown. Thus, we explored the role of PSMD9 in HCC progression and the underlying molecular mechanism. In this study, we revealed that PSMD9 drives HCC progression and erlotinib resistance by decreasing c-Cbl-mediated EGFR ubiquitination.
Materials and methods
Cell lines and cell culture.
Huh7 and Hep3B cells were obtained from Riken Cell Bank (Tsukuba, Japan). HEK-293 T cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The MHCC-LM3 and MHCC-97H cell lines were obtained from the Liver Cancer Institute, Zhongshan Hospital of Fudan University (Shanghai, China). The HCC-LY10 cell line was established in our laboratory. The HCC cell lines used in this study were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) containing 10% heat-inactivated fetal bovine serum (Gibco) and incubated at 37°C in a humidified atmosphere with 5% CO 2 . All of the cell lines were authenticated and characterized by the suppliers. Cells were used within 6 months of resuscitation. These cell lines were mycoplasma-free and routinely authenticated by quality examinations of morphology and growth profile.
Lentivirus production and cell transduction
The PSMD9 lentiviral overexpression plasmid and shRNA plasmid were supplied by the CCSB-Broad Lentiviral Expression Library and Human TRC shRNA Library. The EGFR plasmid was maintained in our laboratory [ 14 ]. The plasmid was sequenced from the 5′ and 3′ ends to confirm its sequence. The target sequences are listed in Supplementary Table 1.
Viral packaging was performed in HEK-293 T cells after cotransfection of the PSMD9 overexpression or shRNA plasmid with the packaging plasmid psPAX2 and the envelope plasmid pMD2.G (Addgene) using Lipofectamine 2000 (Invitrogen). The viruses were harvested at 72 h after transfection, and the viral titers were determined. HCC cells were transduced with 1 × 10 6 recombinant lentivirus-transducing units in the presence of 6 μg/ml polybrene (Sigma).
Quantitative real-time RT‒PCR (qRT‒PCR)
Total RNA extraction, reverse transcription, and qRT‒PCR analyses were performed as previously described using an ABI Prism 7500 System (Applied Biosystems, Carlsbad, CA, USA) with SYBR® Premix Ex Taq (Takara, Dalian, China). The mRNA levels were normalized to those of the housekeeping gene GAPDH. Sequences of primers are listed in Supplementary Table 2.
Western blotting
Proteins in whole cell lysates were separated by SDS‒polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Millipore). The membranes were incubated overnight with primary antibodies at 4 °C and then with secondary antibodies conjugated to horseradish peroxidase (HRP) for 1 h at room temperature. The immunoreactive blots were visualized using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). β-Actin was used as a loading control. Information of the antibodies is listed in Supplementary Table 3.
Cell proliferation and colony formation assays
Cell proliferation was measured by the Cell Counting Kit-8 (CCK-8) (Bimake, USA) according to the manufacturer’s instructions. A cell proliferation EdU image Kit (Abbkine, Wuhan, China) was used for EdU staining following the manufacturer's protocols. The cells were observed and photographed with a fluorescence microscope after EdU staining. For colony formation assays, 1,000 cells were plated in each well of a 6-well plate and incubated at 37 °C for 2 weeks. Colonies were fixed with 4% phosphate-buffered formalin (pH 7.4) and subjected to Giemsa staining for 15 min. Three independent experiments were performed for each assay.
Migration and invasion assays
Cells were seeded in the upper chamber of a transwell (8-μm pore size) or in a Matrigel-coated transwell (BD Biosciences, NJ) in serum free media. The lower chamber contained DMEM supplemented with 10% fetal bovine serum as a chemoattractant. After incubating for 24 or 48 h, the nonmigrated or noninvaded cells were gently removed from the upper chamber using a cotton swab. The cells were fixed with formalin and stained with Giemsa solution. The number of cells in five randomly chosen fields of view was counted under a microscope.
Flow cytometry analysis
For cell cycle analysis, cells were washed twice with cold PBS, fixed in 70% cold ethanol and incubated overnight. Before analysis, the cells were stained with a solution containing 10 mg/ml RNAase and 400 mg/ml propidium iodide (PI) and incubated for 30 min at 37 °C. Finally, the cells were analyzed by flow cytometry.
For the apoptosis assay, the cells were harvested, washed, incubated with PE-conjugated Annexin V and 7-AAD, and incubated for 15 min at room temperature. The cells were analyzed by flow cytometry within 1 h. The results are the representative of 3 independent experiments with triplicate samples for each assay.
For the EGFR internalization assay, cells were trypsinized and collected after incubation with EGF at the indicated time points. After washing twice with cold PBS, nonpermeating cells were incubated with a PE-conjugated anti-EGFR antibody (BioLegend) for 30 min. After washing, the cells were immediately subjected to flow cytometry analysis (FlowJo 7.6.1).
In vivo growth and metastasis assays
To assess the in vivo growth and metastasis of HCC cells, four- to six- week-old male BALB/C nude mice used; HCC cells were orthotopically inoculated into the left hepatic lobes of the mice with a microsyringe through an 8-mm transverse incision in the upper abdomen under anesthesia. A total of 2 × 10 6 cells suspended in 40 μl of a mixture of serum-free DMEM/Matrigel (1:1 volume) (BD Biosciences, MA) were injected into each nude mouse. The mice were sacrificed at six weeks and the tissues were harvested and fixed with phosphate-buffered neutral formalin for at least 72 h. Metastases were identified by analyzing lung tissue sections followed by H&E staining.
For in vivo drug studies, six week old male BALB/C nude mice were injected subcutaneously with 2 × 10 6 cells. When the tumors reached a volume of approximately 100 mm 3 in size, the mice were randomized into 4 groups. The mice were treated with erlotinib (40 mg/kg, every 3 day) via oral gavage. The tumor dimensions were measured with Vernier calipers every 3 days, and tumor volume was calculated as follows: tumor volume = (length × width 2 )/2. On day 24, the mice were sacrificed, and the tumors were excised and fixed with 4% phosphate-buffered neutral formalin. All of the experiments were approved by the Renji Hospital Institutional Animal Care (RT2022-122u) and Use Committee and performed in accordance with the Institutional Guide for the Care and Use of Laboratory Animals.
Immunofluorescence analysis via confocal imaging
Briefly, cells were grown on Lab-Tek chamber slides (Nunc), fixed with 4% paraformaldehyde in PBS for 30 min, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. The slides were incubated with primary antibodies in blocking solution overnight at 4 °C in a humidified chamber. Subsequently, the glass slides were washed three times in PBS and incubated with Alexa Fluor 488-conjugated and Alexa Fluor 555-conjugated secondary antibodies and 4′, 6- diamidino-2-phenylindole (DAPI) in blocking solution for 30 min at 37 °C in a humidified chamber. Images were obtained with a Leica TCS SP8 confocal microscope (Leica, Microsystems). Information of the antibodies is listed in Supplementary Table S3.
Coimmunoprecipitation (Co-IP) assay
The cells were harvested in RIPA (Upstate, Biotechnology) lysis buffer containing protease inhibitors for 40 min on ice and centrifuged at 12,000 × g for 10 min. Protein A/G agarose beads were incubated with anti-PSMD9 or anti-CBL antibody or negative control IgG overnight on an orbital shaker at 4 °C. The immune complex was precipitated with protein-A/G agarose, washed five times and analyzed by western blotting.
Immunohistochemistry (IHC)
IHC assays were conducted as reported previously [ 15 ]. A total of 106 HCC tissues were obtained from Zhongshan Hospital of Fudan University. All samples were obtained with informed consent. The study was approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (KY2023-084-B). Briefly, the sections were deparaffinized with xylene and rehydrated before being heated to just below boiling temperature at a subboiling temperature in sodium citrate buffer (pH 6.0) for 20 min in a microwave oven for antigen retrieval. After being washed with PBS three times, the samples were incubated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. The sections were then incubated overnight with primary antibodies at 4 °C. After being rinsed with PBS, the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody at 37 °C for 30 min and then incubated with diaminobenzidine solution. Finally, the nuclei were counterstained with Mayer’s hematoxylin.
EGFR dimerization assay
Overnight serum-starved HCC cells were treated with EGF (25 ng/ml) at 4 °C for 1 h and then incubated with the 3 mM crosslinker BS3 (Thermo Fisher Scientific) at 4 °C for 20 min. The reaction was terminated by incubation with 250 mM glycine in PBS for 5 min. Samples were subjected to lysing and Western blotting.
Statistical analysis
All the data are presented as the means ± standard deviations (SDs). Unpaired t test was used to compare the means between two groups, and one-way ANOVA was used to compare the means among multiple groups. The Kaplan‒Meier method was used to plot survival curves, which were compared by the log-rank test. p < 0.05 was considered to indicate statistical significance.
Elevated PSMD9 expression in HCC is closely associated with increased tumor grade, metastasis and a poor prognosis
We first analyzed the expression of PSMD9 in numerous cancers using The Cancer Genome Atlas (TCGA) data. We found that PSMD9 was highly expressed in a variety of human tumors, including HCC (Supplementary Figure S1 A, Fig. 1 A). We further confirmed the difference in PSMD9 expression between adjacent cancer tissue and cancer tissue by analyses of the Gene Expression Omnibus (GEO) and the JP Project from the International Cancer Genome Consortium (ICGC-LIRI-JP) (Fig. 1 B-C). Moreover, PSMD9 protein levels were upregulated in HCC tissues compared with noncancerous tissues according to the Western blotting results (Fig. 1 D). Furthermore, PSMD9 expression was upregulated in patients with nodal metastasis patients compared with patients without nodal metastasis in the TCGA cohorts (Supplementary Figure S1 B).
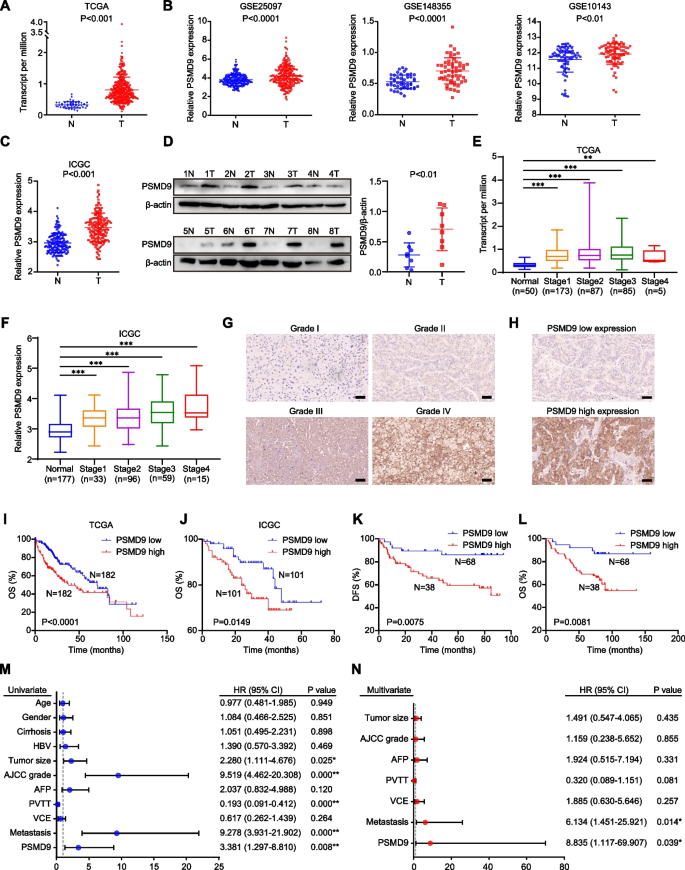
PSMD9 upregulation is associated with a poor prognosis in HCC patients. A - C The expression of PSMD9 in HCC tissues was compared with that in the corresponding noncancerous liver tissues in the TCGA datasets ( n = 50) ( A ), the GSE10143, GSE25097 and GSE148355 ( B ) datasets and the ICGC-LIRI-JP dataset ( C ). D The expression of PSMD9 in HCC tissues was compared with that in the corresponding noncancerous liver tissues by Western blotting. E – F The expression of PSMD9 in noncancerous liver tissues and HCC tissues of different grades was analyzed in the TCGA ( E ) and ICGC-LIRI-JP cohorts ( F ). G Immunohistochemical analysis of PSMD9 expression in HCC samples. Representative images are shown. H Representative images of samples with high and low PSMD9 expression. I - J Overall survival analysis of HCC patients in the TCGA cohort ( I ) and the ICGC-LIRI-JP cohort ( J ) stratified by the PSMD9 expression. K - L Overall and disease free survival analysis of 106 HCC patients stratified by the PSMD9 expression level. M – N Univariate and multivariate Cox proportional hazards analyses were conducted to evaluate the HR of PSMD9 in terms of the overall survival of patients with HCC. * p < 0.05; ** p < 0.01
We next investigated the clinical significance of PSMD9 in HCC patients. We found that the expression of PSMD9 was significantly correlated with the malignancy grade and metastasis in HCC patients in the TCGA and ICGC-LIRI-JP cohorts (Fig. 1 E-F). We next assessed the expression of PSMD9 in HCC patients using IHC. The clinicopathological features of the patients with HCC (106 patients) were shown in Supplementary Table S4.We found that high PSMD9 expression positively correlated with high tumor grade in HCC patients (Fig. 1 G). According to the IHC results, the patients were divided into two groups based on the expression of PSMD9 (Fig. 1 H). We found that PSMD9 expression was positively associated with tumor size, American Joint Committee on Cancer (AJCC) grade and metastasis (Table 1 ). However, there was no correlation between PSMD9 expression and other clinicopathological factors, including gender, age, cirrhosis status, serum alpha-fetoprotein (AFP) level, HBV positivity status, portal vein tumor thrombus (PVTT) and vessel carcinoma embolus (VCE) (Table 1 ). Kaplan–Meier survival analysis revealed that high PSMD9 expression was associated with significantly shorter overall survival (OS) time and disease-free survival (DFS) time than low PSMD9 expression according to TCGA, ICGC and GEO data (F i g. 1 I-J, Supplementary Figure S1 C). The results were also confirmed using our cohort (Fig. 1 K-L). Furthermore, univariate and multivariate Cox proportional hazard analyses suggested that high PSMD9 expression was associated with worse survival in HCC patients than low PSMD9 expression in HCC patients ( p < 0.05; Fig. 1 M-N). In addition, high expression of PSMD9 was associated with shorter OS times in patients with various types of tumors according to TCGA data (Supplementary Figure S2). Taken together, these findings indicate that high expression of PSMD9 is associated with a poor prognosis in HCC patients and that PSMD9 might play an important role in promoting the malignant progression of HCC.
PSMD9 promotes HCC cell proliferation
To verify the function of PSMD9 in HCC, we first examined the expression of PSMD9 in HCC cell lines. We found that PSMD9 expression was high in HCC-LY10 and MHCC-LM3 cells and that PSMD9 expression was low in Huh7 and Hep3B cells (Supplementary Figure S3). The HCC-LY10 and MHCC-LM3 cell lines were subjected to PSMD9 knockdown via shRNA in subsequent experiments (Fig. 2 A, supplementary Figure S4A). Our results showed that PSMD9 knockdown inhibited HCC cell proliferation and decreased the proportion of EdU-positive cells (Fig. 2 B-D, supplementary Figure S4B). Conversely, exogenous expression of PSMD9 markedly promoted cell growth and colony formation ability (Fig. 2 E-H, supplementary Figure S4C-D).
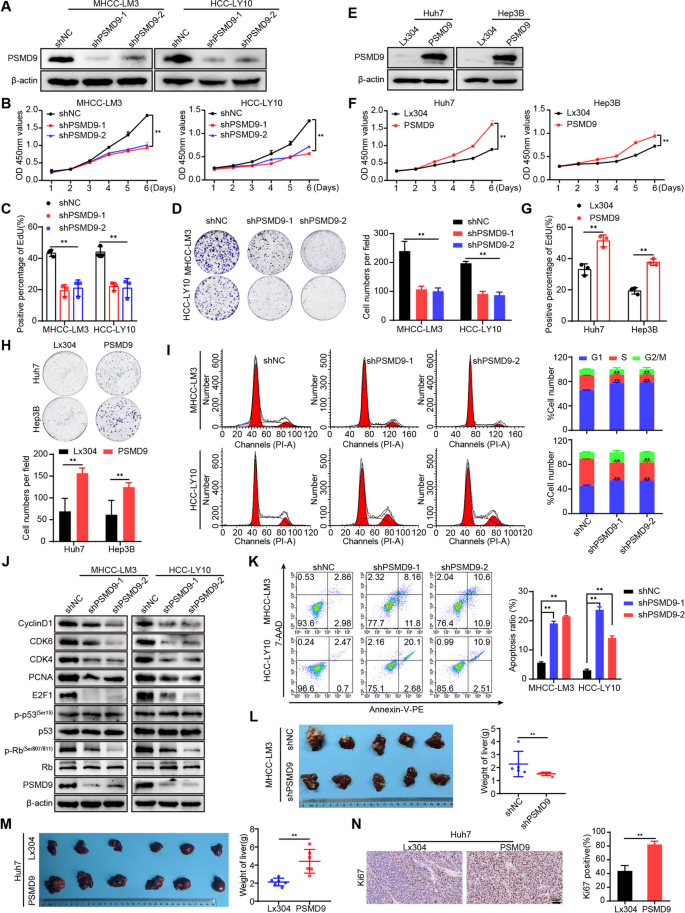
PSMD9 promotes HCC cell proliferation. A The expression levels of PSMD9 in PSMD9-knockdown HCC cells were determined by Western blotting. B - D The effect of PSMD9 knockdown on HCC cell proliferation was assessed by a CCK-8 assay ( B ), an EdU assay ( C ) and a colony formation assay ( D ). E The expression levels of PSMD9 in PSMD9-overexpressing HCC cells were determined by Western blotting. F – H The effect of PSMD9 overexpression on HCC cell proliferation was assessed by a CCK-8 assay ( F ), an EdU assay ( G ) and a colony formation assay ( H ). I The cell cycle distribution of cells was analyzed by flow cytometry. J The expression of cell cycle-related genes was detected by Western blotting. K Apoptosis was analyzed by flow cytometry. L Liver tissues from animals bearing xenografts from MHCC-LM3 cells with stable PSMD9 knockdown. The dot plots show the results of the quantitative analysis of liver weight. M Liver tissues from animals bearing xenografts from Huh7 cells with stable PSMD9 overexpression. The dot plots show the results of the quantitative analysis of liver weight. N Ki67 expression in xenograft tissues from Huh7 PSMD9-overexpressing cells was evaluated by IHC. Bar = 50 μm. * p < 0.05; ** p < 0.01
To further investigate the mechanism by which PSMD9 affects HCC proliferation, we determined the cell cycle distributions of HCC-LY10 and HCC-LM3 cells by flow cytometry. Our results showed that knockdown of PSMD9 increased the proportion of cells entering the G1 phase and decreased the proportion of cells entering the S phase, indicating that knockdown of PSMD9 induces cell cycle arrest at the G1 phase in HCC-LY10 and HCC-LM3 cells (F i g. 2 I). We next detected the expression of cell cycle regulators. The results showed that the levels of CDK4, CDK6, cyclin D1, p-Rb, E2F1 and PCNA were drastically lower in PSMD9-knockdown cells than in control cells (Fig. 2 J). Conversely, the expression of CDK4, CDK6, cyclin D1, p-Rb, E2F1 and PCNA was increased in PSMD9-overexpressing HCC cells(supplementary Figure S4E). However, expression of p53 was not affected in the PSMD9-knockdown and PSMD9-overexpressing HCC cells (Fig. 2 J, supplementary Figure S4E). Furthermore, we found that knockdown of PSMD9 induced apoptosis in HCC cells (Fig. 2 K). Conversely, overexpression of PSMD9 inhibited apoptosis in HCC cells (supplementary Figure S4F). Therefore, these results suggest that PSMD9 promotes the proliferation of HCC cells and inhibits their apoptosis.
Next, we assessed the effect of PSMD9 on the tumorigenicity of HCC cells in vivo by using an orthotopic liver tumor model in nude mice. As shown in Fig. 2 L, MHCC-LM3 tumors derived from shPSMD9 group cells weighed less than those derived from shNC group cells. In contrast, compared with the control cells, Huh7 cells overexpressing PSMD9 promoted tumor growth, as determined by the liver weight (Fig. 2 M). Furthermore, the expression of Ki-67 was significantly increased in HCC cells overexpressing PSMD9 (Fig. 2 N). Taken together, these results provide strong evidence that PSMD9 promotes the tumorigenesis.
PSMD9 promotes HCC cell migration, invasion and metastasis
Cancer cell migration and invasion have been identified as key events in cancer development. Therefore, we examined the effect of PSMD9 on HCC cell migration and invasion. The results showed that the overexpression of PSMD9 promoted HCC cell migration and invasion, whereas silencing PSMD9 resulted in decreased cell migration and invasion (Fig. 3 A-B).
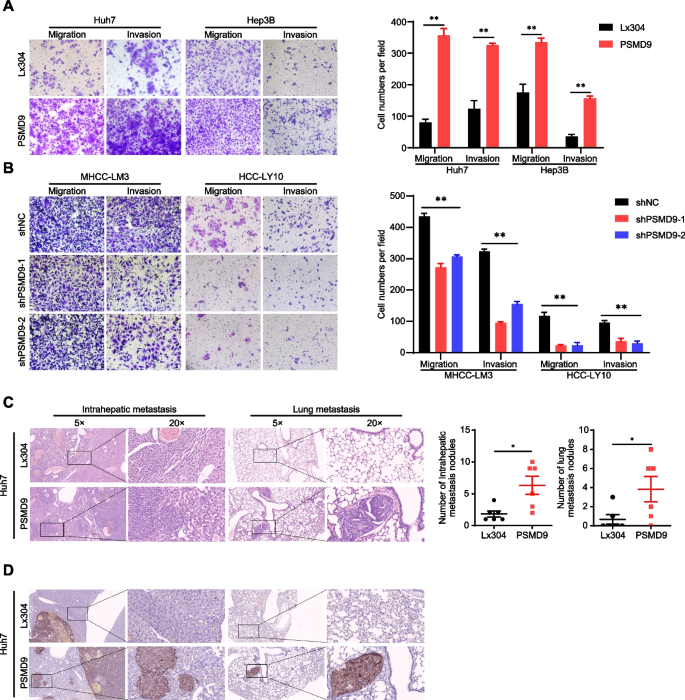
PSMD9 promotes HCC cell invasion and metastasis. A - B The effects of PSMD9 overexpression ( A ) and knockdown ( B ) on HCC cell migration and invasion were assessed by transwell assays. C Representative images of intrahepatic nodules and lung nodules formed by PSMD9-overexpressing Huh7 cells and control cells are shown. The numbers of intrahepatic metastatic nodules and lung metastatic nodules are shown in the right panel. Bar = 50 μm. D IHC analysis of metastasis using human-specific anti-mitochondria antibodies. * p < 0.05; ** p < 0.01
To further clarify the role of PSMD9 in HCC metastasis in vivo, PSMD9-overexpressing cells were orthotopically inoculated into the left hepatic lobe of mice via a microsyringe. Histological examination of lung and liver tissues indicated that the number of intrahepatic and lung metastasis nodules was significantly greater in the PSMD9 overexpression group than in the control group (Fig. 3 C). Metastasis was confirmed by anti-human mitochondria antibody staining, which is used to detect human cells in xenograft models (Fig. 3 D). Taken together, these findings suggest that PSMD9 promotes HCC metastasis.
PSMD9 promotes EGFR expression through inhibition of its ubiquitination
To explore the mechanism of PSMD9 in HCC, RNA sequencing was performed in PSMD9-overexpressing cells. Many differentially expressed genes (DEGs) were identified in cells based on RNA-seq, and DEGs were further subjected to Reactome enrichment analysis. The results of Reactome functional enrichment analysis showed that PSMD9 can regulate EGFR signaling in HCC cells (Fig. 4 A). Therefore, we next detected the expression of EGFR in PSMD9-overexpressing and PSMD9-knockdown HCC cells. Our results showed that the overexpression of PSMD9 increased the phosphorylation of EGFR in HCC cells (Fig. 4 B). In contrast, EGFR phosphorylation was inhibited in PSMD9-knockdown HCC cells (Fig. 4 C). EGFR triggers several signal transduction cascades, including those of Raf1-extracellular signal-regulated kinase (ERK) and PI3K-Akt. Therefore, we detected the expression of ERK1/2 and Akt in HCC cells. Our results showed that overexpression of PSMD9 promoted the phosphorylation of ERK1/2 and Akt in HCC cells (Fig. 4 B). Conversely, PSMD9 knockdown inhibited the phosphorylation of ERK1/2 and Akt in HCC cells (Fig. 4 C). Furthermore, phosphorylated EGFR and its downstream signaling molecule phosphorylated ERK1/2 were activated in murine xenografts from PSMD9-overexpressing Huh7 cells (Fig. 4 D). To determine whether PSMD9 affects EGF-induced EGFR degradation, we detected the expression of EGFR and its downstream signaling proteins in PSMD9-knockdown HCC cells treated with EGF. Our results showed that knocking down PSMD9 inhibited the phosphorylation of EGFR in HCC cells treated with EGF. Moreover, the activation of ERK1/2 and Akt was also altered in PSMD9-knockdown HCC cells treated with EGF (Fig. 4 E-F). Therefore, these results indicate that PSMD9 regulates EGFR signaling in HCC cells.
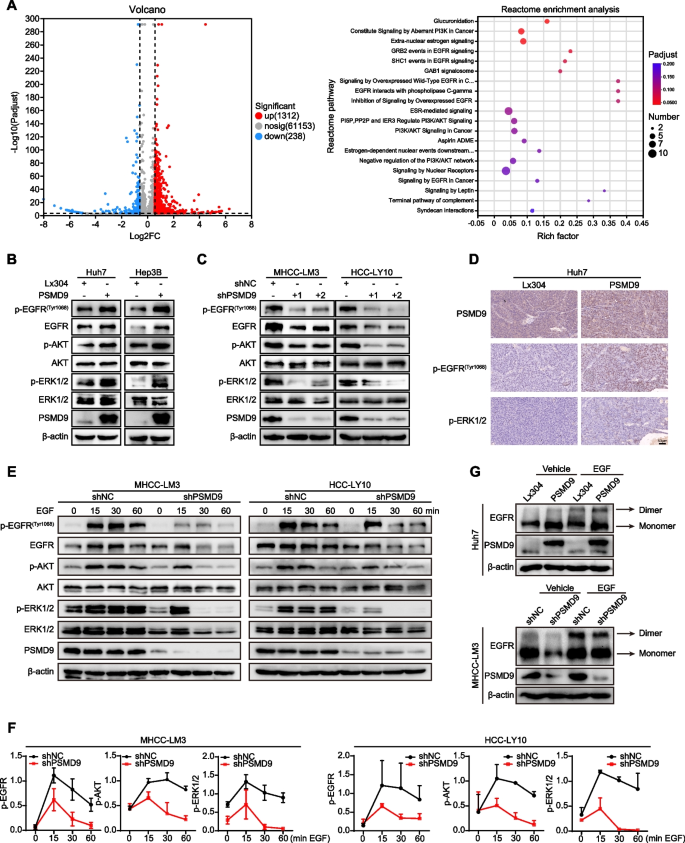
PSMD9 promotes EGFR, ERK1/2, and Akt phosphorylation. A Ectopic overexpression of PSMD9 activated the EGFR signaling pathway according to Reactome enrichment analysis. B - C The expression of p-EGFR, EGFR, p-ERK1/2, ERK1/2, p-Akt and Akt in PSMD9-overexpressing Huh7 and Hep3B cells ( B ) and PSMD9-knockdown HCC-LY10 and MHCC-LM3 cells ( C ) was detected by Western blotting. D The expression of p-EGFR and p-ERK1/2 in xenograft tumor tissues from Huh7-PSMD9 and control cells was detected by IHC. E Knocking down PSMD9 promoted p-EGFR, p-ERK1/2 and p-Akt degradation after EGF stimulation. F Densitometric analysis of the expression levels of p-EGFR, p-ERK1/2 and p-Akt. G The dimer and monomer forms of EGFR were evaluated in PSMD9-overexpressing and PSMD9-knockdown cells
EGFR is a cell surface protein that binds to epidermal growth factor. Binding of the protein to a ligand induces receptor dimerization and tyrosine autophosphorylation and leads to cell proliferation [ 16 ]. Therefore, crosslinking experiments were performed to examine the effects of PSMD9 on EGFR dimerization and EGF-induced dimerization of EGFR, and we found that forced expression of PSMD9 increased EGFR dimerization and EGF-induced EGFR dimerization. Conversely, PSMD9 knockdown inhibited EGFR dimerization and EGF-induced EGFR dimerization (Fig. 4 G).
In addition, we found that PSMD9 did not affect the expression of EGFR mRNA in HCC cells (Supplementary Figure S5A). These results indicate that PSMD9 promotes EGFR expression through posttranscriptional regulation. Next, PSMD9-overexpressing or control HCC cells were treated with cycloheximide (50 μg/ml), which blocks de novo protein synthesis. Our results showed that the rate of EGFR protein degradation was significantly lower in PSMD9-overexpressing cells than in control cells (Fig. 5 A). The half-life of the EGFR protein in the cells was extended (from 3 to 8 h in Huh7 cells and from 6 to 12 h in Hep3B cells) as a consequence of PSMD9 overexpression (Fig. 5 A). These results suggest that the overexpression of PSMD9 increases the stability of the EGFR protein in HCC cells.
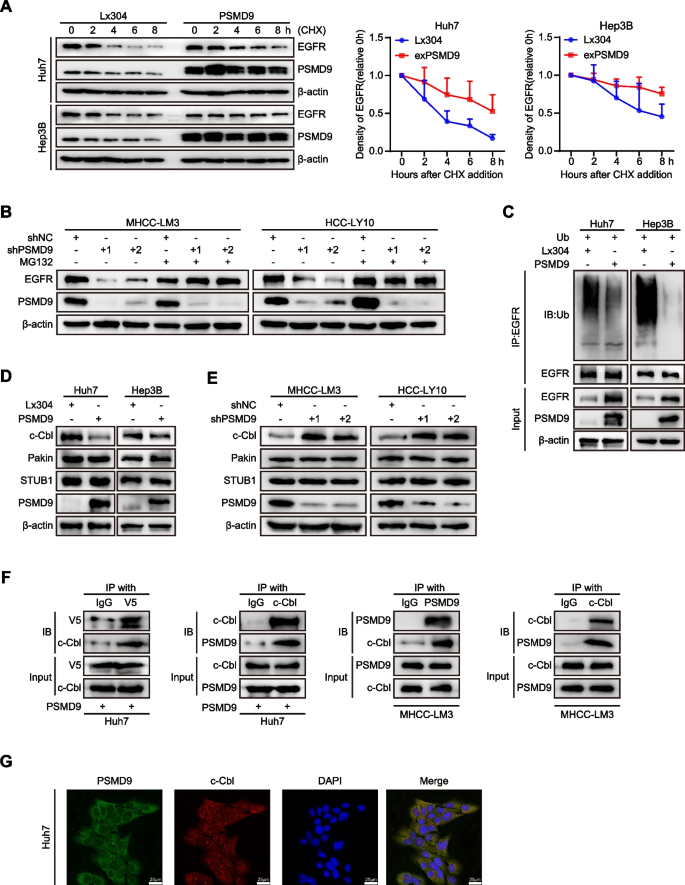
PSMD9 binds c-Cbl and is associated with EGFR ubiquitination. A Expression of EGFR in PSMD9-overexpressing HCC cells treated with cycloheximide (CHX) at the indicated time points. B Expression of EGFR in PSMD9-knockdown HCC cells treated with MG132 for 6 h. C Lysates of PSMD9-overexpressing and control Huh7 and Hep3B cells were immunoprecipitated with an anti-EGFR antibody, and the immunocomplexes were immunoblotted with antibodies against ubiquitinated proteins. D - E The expression of c-Cbl, Pakin and STUB1 in PSMD9-overexpressing ( D ) and PSMD9-knockdown ( E ) HCC cells was detected by Western blotting. F Co-IP and Western blotting showed that PSMD9 and c-Cbl bind to each other. G The protein expression of PSMD9 and c-Cbl in Huh7 cells was determined by immunofluorescence assays. ** p < 0.01
To investigate the involvement of the ubiquitin‒proteasome pathway in the proteolytic degradation of EGFR, we applied the proteasomal inhibitor MG132 to PSMD9-knockdown HCC cells. Our results showed that the downregulation of EGFR caused by PSMD9 knockdown was blocked by MG132 treatment (Fig. 5 B). Furthermore, overexpression of PSMD9 significantly reduced the ubiquitination of EGFR in an in vitro ubiquitination assay (Fig. 5 C). Therefore, these results indicated that PSMD9 increases the stability of EGFR by reducing its ubiquitination.
PSMD9 interacts with c-Cbl and stabilizes EGFR
To determine whether E3 ubiquitin ligases are involved in the regulation of EGFR ubiquitination by PSMD9, we next examined the expression of the ubiquitin ligases of EGFR (STUB1, Parkin and c-Cbl) in PSMD9-overexpressing and PSMD9-knockdown HCC cells [ 17 , 18 , 19 ]. Our results showed that overexpression of PSMD9 inhibited the expression of c-Cbl in HCC cells. Conversely, PSMD9 knockdown increased c-Cbl expression in HCC cells. However, the Pakin and STUB1 levels were not significantly influenced by PSMD9 in HCC cells (Fig. 5 D-E). Next, to investigate whether PSMD9 binds specifically to c-Cbl in HCC cells, we carried out co-IP and found that PSMD9 interacts with c-Cbl directly (Fig. 5 F, supplementary Figure S5B). Furthermore, immunofluorescence staining revealed that PSMD9 and c-Cbl were colocalized in HCC cells (Fig. 5 G). Taken together, these results indicate that PSMD9 interacts with c-Cbl and inhibits its expression thus decreasing the level of EGFR ubiquitination to increase EGFR stability in HCC cells.
PSMD9 influences EGFR endocytosis and degradation
EGFR endocytosis and degradation are regulated by the ubiquitination of EGFR [ 20 ]. Therefore, we first detected the cell surface expression of EGFR in the PSMD9-overexpressing HCC cells. We found that the overexpression of PSMD9 promoted the cell surface expression of EGFR (Fig. 6 A). Knockdown of PSMD9 inhibited the expression of EGFR in HCC cells as determined by immunofluorescence (Fig. 6 B). Next, we detected the cell surface expression of EGFR in the PSMD9-knockdown HCC cells treated with EGF. The results showed that knockdown of PSMD9 decreased the cell surface expression of EGFR compared with that in control cells (Fig. 6 C-D). Furthermore, PSMD9 knockdown promoted the colocalization of EGFR with the lysosomal degradation markers EEA1 (a marker of the early endosomal stage) and LAMP1 (a lysosomal marker) (Fig. 6 E-F). Taken together, these results indicate that PSMD9 inhibits c-Cbl expression and subsequently decreases EGFR ubiquitination and endocytosis from the cell membrane and suppresses ERK1/2 and Akt activation, which contributes to HCC progression.
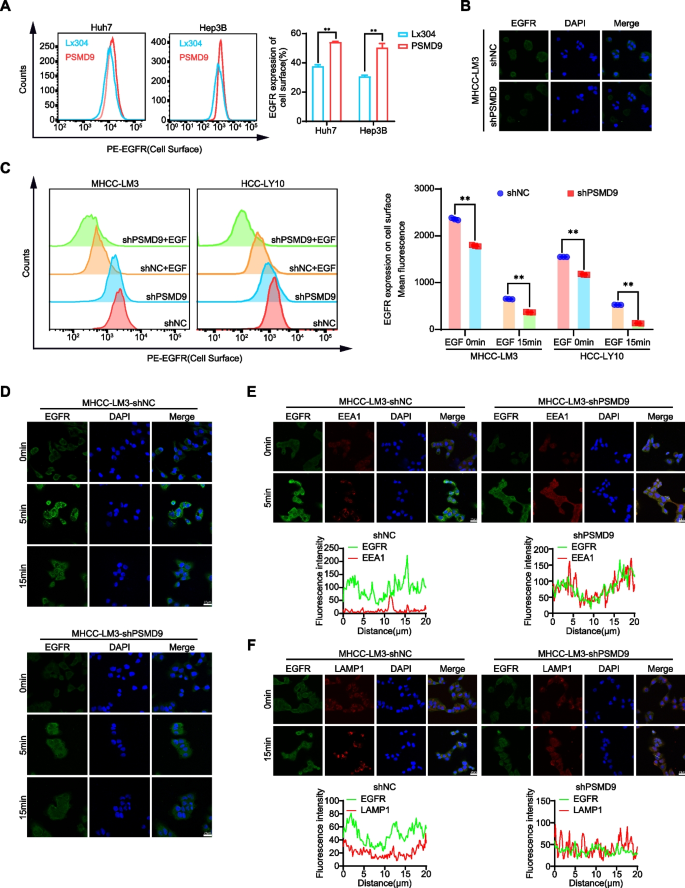
PSMD9 influences EGFR endosomal trafficking. A EGFR expression on the cell surface was assessed by flow cytometry. B The expression of EGFR in PSMD9-knockdown MHCC-LM3 cells was assessed by immunofluorescence. C EGFR expression on the cell surface of PSMD9-knockdown MHCC-LM3 cells was assessed by flow cytometry in the presence of EGF. D The expression of EGFR in the presence of EGF for the indicated time periods was assessed by immunofluorescence. E PSMD9-knockdown MHCC-LM3 cells incubated with EGF for the indicated time periods were subjected to an immunofluorescence assay. Antibodies against EGFR and EEA1 were used. F PSMD9-knockdown MHCC-LM3 cells incubated with EGF for the indicated time periods were subjected to an immunofluorescence assay. Antibodies against EGFR and LAMP1 were used. ** p < 0.01
PSMD9 promotes HCC cell proliferation, migration and invasion through the EGFR pathway
To confirm the role of EGFR in PSMD9-mediated HCC proliferation and invasion, we assessed the effect of EGFR expression on the cell proliferation and invasion of PSMD9-knockdown cells (Fig. 7 A). The results showed that the PSMD9 knockdown-induced suppression of cell proliferation, migration and invasion could be reversed by overexpressing EGFR in HCC cells (Fig. 7 B-G, supplementary Figure S6A-S6C). PSMD9 knockdown-induced apoptosis also was reversed by overexpressing EGFR in HCC cells (Fig. 7 E-F). In addition, the EGFR inhibitor erlotinib was used to treat PSMD9-overexpressing HCC cells. Our results showed that the PSMD9 overexpression-induced promotion of cell proliferation, migration and invasion could be attenuated by erlotinib in HCC cells (Fig. 7 H-K, supplementary Figure S6D-S6E). In addition, PSMD9 overexpression-induced cell proliferation, migration, and invasion were reversed by EGFR shRNAs (Supplementary Figure S7A-F).Therefore, these results suggested that PSMD9 promotes cell proliferation and invasion through the EGFR pathway in HCC cells.
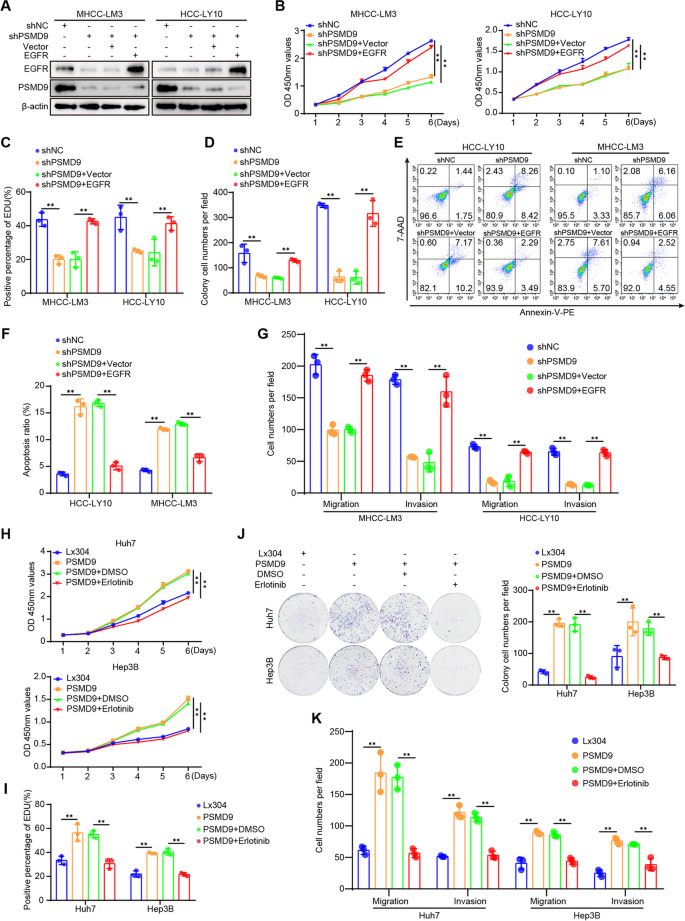
PSMD9 regulates HCC cell functions via the EGFR signaling. A PSMD9-knockdown HCC cells were transfected with EGFR, and the expression of EGFR and PSMD9 was detected by Western blotting. B - E PSMD9-knockdown HCC cells were transfected with EGFR as indicated, and cell proliferation, apoptosis, migration and invasion were evaluated by CCK-8 assay ( B ), EdU assay ( C ), colony formation ( D ), flow cytometry ( E – F ) and Transwell assays ( G ). H – K PSMD9-overexpressing HCC cells were treated with erlotinib or DMSO as indicated, and cell proliferation, migration and invasion were evaluated by CCK-8 assay ( H ), EdU assay ( I ),colony formation ( J ), and Transwell assays ( K ). * p < 0.05; ** p < 0.01
Knockdown of PSMD9 sensitizes HCC cells to erlotinib
According to the results mentioned above, we asked whether PSMD9 can be regarded as a potential target for HCC therapy. Erlotinib combined with sorafenib did not improve survival in patients with advanced HCC [ 4 ].To confirm whether the knockdown of PSMD9 enhances the potential effect of erlotinib on HCC cells, HCC cells with PSMD9 knockdown were incubated with erlotinib. As shown in Fig. 8 , PSMD9 knockdown sensitized HCC cells to erlotinib. Erlotinib treatment combined with PSMD9 knockdown had synergistic inhibitory effects on cell proliferation, migration and invasion (Fig. 8 A-E, supplementary Figure S8A-S8C). Knockdown of PSMD9 enhanced erlotinib-induced cell apoptosis (Fig. 8 D). Long-term colony formation assays revealed that compared with control cells, PSMD9-knockdown cells were sensitive to erlotinib treatment (Fig. 8 C, supplementary Figure S8B). In addition, erlotinib treatment combined with PSMD9 knockdown synergistically inhibited the expression of EGFR and the phosphorylation of ERK1/2 and Akt in HCC cells (Fig. 8 F). Therefore, these data indicate that the loss of PSMD9 sensitizes HCC cells to erlotinib. We next evaluated the synergistic effect of this combination treatment in vivo. We found that PSMD9 knockdown increased the sensitivity of HCC cells to erlotinib treatment. PSMD9 knockdown combined with erlotinib treatment reduced the overall tumor volume and mass (Fig. 8 G-J). In addition, the positive expression of Ki67 and EGFR was decreased significantly, as indicated by the immunohistochemistry results (Fig. 8 K).
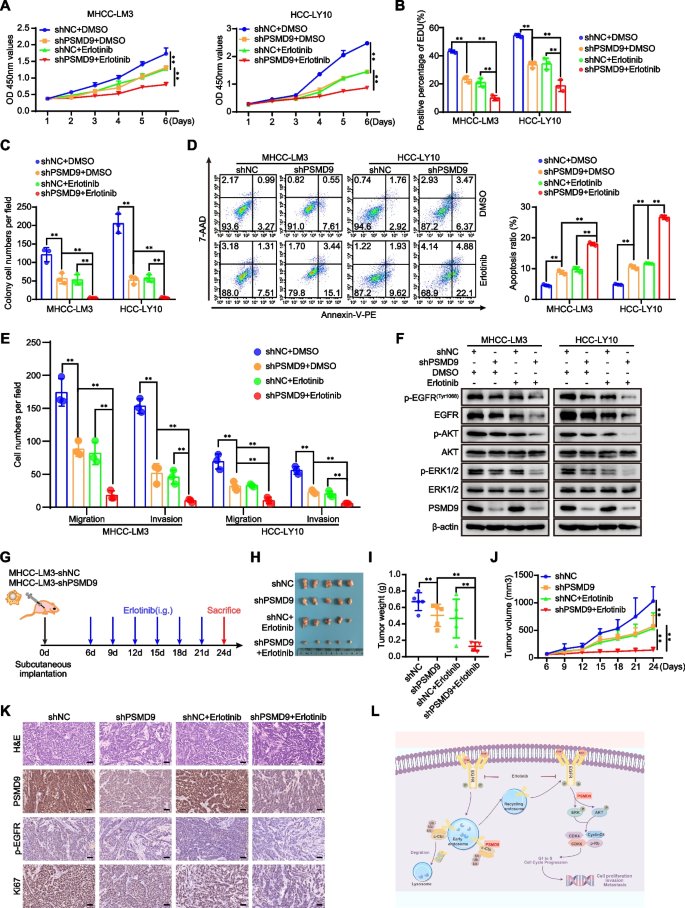
Knockdown of PSMD9 sensitizes HCC cells to erlotinib. A - E PSMD9 knockdown or control cells were incubated with erlotinib. Cell proliferation was detected by a CCK-8 assay ( A ), an EdU incorporation assay ( B ) and a colony formation assay ( C ). Apoptosis was detected by flow cytometry ( D ). Migration and invasion were detected by transwell assays ( E ). The expression of p-EGFR, EGFR, p-Akt, Akt, p-ERK1/2, ERK1/2 and PSMD9 was detected by western blotting ( F ). G - K In vivo xenograft tumorformation assays were performed using stably PSMD9 knockdown or control MHCC-LM3 cells (2 × 10 6 ) subcutaneously injected into the right posterior flanks of 6-week-old male BALB/C nude mice, followed by treatment with vehicle or erlotinib (40 mg/kg/d) when the tumors reached a volume of approximately 100 mm 3 in size. Schematic of the experimental design ( G ). On day 24, the mice were sacrificed, and the tumors were photographed ( H ). Tumor weights were measured and plotted ( I ). Tumor growth was measured every 3 days ( J ). The expression of Ki67, PSMD9 and p-EGFR in xenograft tissues was evaluated by IHC ( K ). L Model of the mechanisms of action of PSMD9 in HCC. PSMD9 interacts with c-Cbl, stabilizes EGFR, decreases EGFR ubiquitination, influences EGFR endosomal trafficking and degradation and subsequently activates downstream signaling and promotes HCC progression. Bar = 50 μm. * p < 0.05; ** p < 0.01
Several studies have reported that PSMD9 is correlated with the development of many tumor types. For example, low PSMD9 expression is associated with relative tumor radiosensitivity in breast cancer [ 11 ]. PSMD9 expression is correlated with recurrence after radiotherapy in patients with cervical cancer [ 10 ]. Although the role of PSMD9 in many tumor types has been studied, there are few reports about the role and molecular mechanism of PSMD9 in HCC. In the present work, we found that the expression of PSMD9 was significantly upregulated in HCC tissues. Furthermore, the expression of PSMD9 was significantly correlated with the malignancy grade, metastasis status and prognosis of HCC patient. We found that PSMD9 overexpression promoted cell growth and metastasis. Furthermore, PSMD9 knockdown sensitized HCC cells to erlotinib. Therefore, PSMD9 may be a therapeutic target or may be used to guide therapy. At present, proteasome inhibitors are an important class of drugs for the treatment of multiple myeloma [ 21 ]. Numerous preclinical experiments have demonstrated that combination treatment with proteasome inhibitors can markedly increase the therapeutic effects on cancer cells [ 22 ]. Highly selective specific proteasome inhibitors are beneficial for the treatment of solid tumors.
The main function of the proteasome is to regulate cell fate. Numerous studies have revealed that inhibition or knockdown of proteasomal subunits causes cell death in cancer cells. PSMD2 knockdown inhibits breast cancer cell proliferation and arrests the cell cycle [ 23 ]. Silencing PSMD4 regulates cell cycle arrest by modulating PTEN/Akt signaling in HCC [ 9 ]. PSMD10 inhibition suppresses autophagy and induces HCC cell sensitivity to drugs [ 24 ]. In this study, our results revealed that cell cycle arrest induced by PSMD9 knockdown is associated with decreased expression of G1 phase related proteins, such as cyclin D1 and CDK4/6. Apoptosis was also observed in PSMD9-knockdown HCC cells. Activated EGFR/ERK signaling has been tightly linked to the expression of cyclin D1 [ 25 ]. We found that PSMD9 promotes EGFR expression in HCC cells. Therefore, these data indicate that PSMD9 plays an important role in HCC cell proliferation.
The EGFR signaling pathway has been shown to be involved in the pathogenesis of several malignancies, including HCC [ 26 , 27 ]. EGFR is frequently mutated and/or overexpressed in different types of human cancers and the molecular target of multiple cancer therapies [ 28 ]. EGFR activation can accelerate intracellular signaling cascades, leading to the activation of downstream effectors, such as the PI3K/Akt, MAPK, Ras/Raf/Mek/Erk, JAK/STAT, and PLCγ1/PKC pathways [ 2 ]. ERK1/2 and Akt are thought to be downstream effectors of EGFR signaling. Activated ERK1/2 translocates to the nucleus to activate ternary complex factor (TCF) transcription factors, which bind to the cyclin D1 promoter to promote G1/S phase transition [ 29 ]. Activated Akt is associated with HCC progression [ 9 , 30 ]. Our results revealed that PSMD9 promotes EGFR expression and EGFR dimerization, leading to the activation of ERK1/2 and Akt in HCC cells. Knockdown of PSMD9 suppresses EGF-induced phosphorylation of EGFR and dimerization of EGFR and also suppresses ERK1/2 activation. Activated EGFR undergoes internalization, degradation or recycling in the absence of ligand [ 31 ]. Ubiquitin serves as a sorting signal during the endocytosis of EGFR [ 32 ]. Recruitment of the E3 ubiquitin ligase c-Cbl to activated EGFR is a key event leading to receptor ubiquitylation. C-Cbl directly binds to Py1045 or indirectly binds to the pY1068/pY1086 residues of EGFR via the GRB2 adaptor protein [ 33 ]. Our previous research also revealed that sorting nexin 5 interacts with EGFR and influences endosomal trafficking and degradation of EGFR in HCC [ 14 ]. In this study, we found that PSMD9 inhibits c-Cbl expression and subsequently suppresses EGFR ubiquitination and endocytosis from the cell membrane. Furthermore, we found that the effect of PSMD9 on HCC cell proliferation, migration and invasion was reversed by the EGFR inhibitor erlotinib and EGFR shRNA. Therefore, these data indicated that PSMD9 promotes HCC cell proliferation and metastasis through the EGFR-mediated signaling pathway.
First-generation drugs, including erlotinib and gefitinib, are reversible inhibitors. Approximately 60% of patients with acquired resistance to EGFR TKIs (erlotinib, gefitinib, and afatinib) develop a new mutation within the drug target. The EGFR T790M mutation is as the most common mechanism of acquired resistance [ 34 , 35 ]. The proteasome has become an attractive target for the treatment of many cancers. Several proteasome inhibitors have displayed remarkable antitumor effects. Many studies have shown that regulating the ubiquitin‒proteasome system has great potential as an approach for overcoming drug resistance [ 36 ]. Therefore, knockdown of PSMD9 or the discovery of PSMD9 inhibitors can promote the ubiquitination-mediated degradation of EGFR and Increase the sensitivity to EGFR TKIs.
In conclusion, our findings demonstrated that PSMD9 upregulation was associated with a poor prognosis in HCC patients. PSMD9 promotes HCC cell proliferation and metastasis via direct interaction with c-Cbl, subsequently decreasing EGFR ubiquitination and influencing EGFR endosomal trafficking and degradation (Fig. 8 K). Our findings highlight the molecular mechanism of PSMD9 in HCC progression and provide valuable information for cancer prognosis evaluation and treatment.
Abbreviations
- Hepatocellular carcinoma
The Cancer Genome Atlas
Gene Expression Omnibus
JP Project from International Cancer Genome Consortium
Epidermal growth factor receptor
Receptor tyrosine kinases
Ubiquitin-proteasome
Propidium iodide
Cycloheximide
Immunohistochemistry
Coimmunoprecipitation
Early endosome antigen 1
Lysosomal Associated Membrane Protein 1
Portal vein tumor thrombus
Vessel carcinoma embolus
Cbl proto-oncogene C
Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–62.
Article CAS PubMed Google Scholar
Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol. 2022;85:253–75.
Singh M, Jadhav HR. Targeting non-small cell lung cancer with small-molecule EGFR tyrosine kinase inhibitors. Drug Discov Today. 2018;23:745–53.
Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–66.
Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–89.
Collins GA, Goldberg AL. The Logic of the 26S Proteasome. Cell. 2017;169:792–806.
Article CAS PubMed PubMed Central Google Scholar
Majeed S, Aparnathi MK, Nixon KCJ, Venkatasubramanian V, Rahman F, Song L, et al. Targeting the Ubiquitin-Proteasome System Using the UBA1 Inhibitor TAK-243 is a Potential Therapeutic Strategy for Small-Cell Lung Cancer. Clin Cancer Res. 2022;28:1966–78.
Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14:417–33.
Jiang Z, Zhou Q, Ge C, Yang J, Li H, Chen T, et al. Rpn10 promotes tumor progression by regulating hypoxia-inducible factor 1 alpha through the PTEN/Akt signaling pathway in hepatocellular carcinoma. Cancer Lett. 2019;447:1–11.
Koster F, Sauer L, Hoellen F, Ribbat-Idel J, Brautigam K, Rody A, et al. PSMD9 expression correlates with recurrence after radiotherapy in patients with cervical cancer. Oncol Lett. 2020;20:581–8.
Article PubMed PubMed Central Google Scholar
Langlands FE, Dodwell D, Hanby AM, Horgan K, Millican-Slater RA, Speirs V, et al. PSMD9 expression predicts radiotherapy response in breast cancer. Mol Cancer. 2014;13:73.
Zhao Y, Wen S, Li H, Pan CW, Wei Y, Huang T, et al. Enhancer RNA promotes resistance to radiotherapy in bone-metastatic prostate cancer by m(6)A modification. Theranostics. 2023;13:596–610.
Ud Din Farooqee SB, Christie J, Venkatraman P. PSMD9 ribosomal protein network maintains nucleolar architecture and WT p53 levels. Biochem Biophys Res Commun. 2021;563:105–12
Zhou Q, Huang T, Jiang Z, Ge C, Chen X, Zhang L, et al. Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway. Oncogene. 2020;39:2140–55.
Zhou Q, Tian W, Jiang Z, Huang T, Ge C, Liu T, et al. A Positive Feedback Loop of AKR1C3-Mediated Activation of NF-kappaB and STAT3 Facilitates Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2021;81:1361–74.
Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem. 2020;20:815–34.
Hou J, Deng Q, Zhou J, Zou J, Zhang Y, Tan P, et al. CSN6 controls the proliferation and metastasis of glioblastoma by CHIP-mediated degradation of EGFR. Oncogene. 2017;36:1134–44.
Lin DC, Xu L, Chen Y, Yan H, Hazawa M, Doan N, et al. Genomic and Functional Analysis of the E3 Ligase PARK2 in Glioma. Cancer Res. 2015;75:1815–27.
Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–40.
Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–6.
Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–84.
Zhang L, Wu M, Su R, Zhang D, Yang G. The Efficacy and Mechanism of Proteasome Inhibitors in Solid Tumor Treatment. Recent Pat Anticancer Drug Discov. 2022;17:268–83.
Li Y, Huang J, Zeng B, Yang D, Sun J, Yin X, et al. PSMD2 regulates breast cancer cell proliferation and cell cycle progression by modulating p21 and p27 proteasomal degradation. Cancer Lett. 2018;430:109–22.
Luo T, Fu J, Xu A, Su B, Ren Y, Li N, et al. PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy. 2016;12:1355–71.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–72.
Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972–7.
Song S, Yu Z, You Y, Liu C, Xie X, Lv H, et al. EGFR/MET promotes hepatocellular carcinoma metastasis by stabilizing tumor cells and resisting to RTKs inhibitors in circulating tumor microemboli. Cell Death Dis. 2022;13:351.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Article PubMed Google Scholar
Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295–304.
CAS PubMed Google Scholar
Tian H, Ge C, Li H, Zhao F, Hou H, Chen T, et al. Ribonucleotide reductase M2B inhibits cell migration and spreading by early growth response protein 1-mediated phosphatase and tensin homolog/Akt1 pathway in hepatocellular carcinoma. Hepatology. 2014;59:1459–70.
Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34.
Berlin I, Sapmaz A, Stevenin V, Neefjes J. Ubiquitin and its relatives as wizards of the endolysosomal system. J Cell Sci. 2023;136(4):jcs260101.
Tang R, Langdon WY, Zhang J. Negative regulation of receptor tyrosine kinases by ubiquitination: Key roles of the Cbl family of E3 ubiquitin ligases. Front Endocrinol (Lausanne). 2022;13: 971162.
Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–501.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–7.
Narayanan S, Cai CY, Assaraf YG, Guo HQ, Cui Q, Wei L, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updat. 2020;48: 100663.
Download references
Acknowledgements
Not applicable.
This study was supported in part by grants from the National Natural Science Foundation of China (82273102, 81972581, 82103201, 82300165), Shanghai Sailing Program (21YF1445400), State Key Laboratory of Systems Medicine for Cancer Research Foundation (ZZ-94–2312), Guangxi Natural Science Foundation (2024GXNSFAA010061), Guangxi Training Program for Medical High-level Academic Leaders (No. Guiweikejiaofa [2020]-15), Bose Talent Highland (No. 2020–3-2), Building Projects of Guangxi Bagui Scholars (No. 2024), Building Projects from the Key Laboratory of Molecular Pathology (for Hepatobiliary Diseases) of Guangxi (No. Guiweikejiaofa [2020]-17) and the Key Laboratory of Tumor Molecular Pathology of Guangxi Higher Education Institutes (Guijiaokeyan [No. 2022]-10), and Clinical Key Specialty Building Project (For Pathology) of Guangxi (No. Guiweiyifa [2022]-21)..
Author information
Yuting Su and Lili Meng contributed equally to this work.
Authors and Affiliations
State Key Laboratory of Systems Medicine for Cancer, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao Tong University School of Medicine, 25/Ln 2200, Xietu Road, Shanghai, 200032, China
Yuting Su, Chao Ge, Yuqi Liu, Chi Zhang, Yue Yang, Wei Tian & Hua Tian
Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, 200032, China
Department of Pathology, The Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, 533000, China
The Key Laboratory of Molecular Pathology (Hepatobiliary Diseases) of Guangxi, Baise, 533000, China
You can also search for this author in PubMed Google Scholar
Contributions
SYT, TW, GC, LYQ and ZC conducted all experiments and analyzed the data. MLL collected the tumor samples and provided technical or material support. TH designed experiments, supervised the study and wrote the main manuscript text. All authors have read and approved the final manuscript.
Corresponding authors
Correspondence to Wei Tian or Hua Tian .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (KY2023-084-B). Informed consent was obtained and accepted by all of the patients before enrolment. All methods were performed according to relevant guidelines and regulations. All animal experiments were approved by the Laboratory Animal Ethics Committee of the Renji Hospital, Shanghai Jiao Tong University School of Medicine (approval number: RT2022-122u). All methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments.
Consent for publication
All authors have read and approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Su, Y., Meng, L., Ge, C. et al. PSMD9 promotes the malignant progression of hepatocellular carcinoma by interacting with c-Cbl to activate EGFR signaling and recycling. J Exp Clin Cancer Res 43 , 142 (2024). https://doi.org/10.1186/s13046-024-03062-3
Download citation
Received : 06 February 2024
Accepted : 06 May 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s13046-024-03062-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Progression
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Research Specialist
- Madison, Wisconsin
- SCHOOL OF MEDICINE AND PUBLIC HEALTH/DEPARTMENT OF MEDICINE
- Partially Remote
- Staff-Full Time
- Staff-Part Time
- Opening at: May 15 2024 at 16:50 CDT
- Closing at: May 29 2024 at 23:55 CDT
Job Summary:
This position will support the research program of Dr. Michael Lucey. Dr. Lucey's research focuses on chronic liver disease, particularly alcohol-associated liver disease (ALD). Dr. Lucey leads a multi-disciplinary and cross-departmental group, of whom key members are Dr. John Rice of the Division of Gastroenterology and Hepatology and Dr. Randy Brown of the Department of Family Medicine and Director of Addiction Services of UW Health. Our research encompasses electronic medical record data analysis, qualitative research techniques (focus groups and patient engagement), and clinical/health systems interventions using implementation science. Dr. Lucey is the principal investigator on a prospective 7-year study (starting date August 1, 2023), funded by the NIH, investigating the selection for and outcome of liver transplantation in patients with ALD who have been drinking in the 6 months prior to selection. The successful candidate for this position will assist and support Dr. Lucey and his team in all aspects of their research. Specific duties include (but not limited to): 1. Work with Dr. Lucey and his co-investigators to conduct high quality research; maintaining knowledge of specific study guidelines to assist Dr. Lucey in ensuring compliance with study mandates and federal regulations, managing project data, compliance, and assuring data consistency/security, and maintaining data documentation. 2. Coordinate Drs. Lucey and Rice's research projects within UW and with outside partners. This mandate includes coordinating patient and staff engagement groups and multidisciplinary research team meetings, maintaining working group meeting notes and project website data. 3. Working with the Division of Gastroenterology and Hepatology research office on identifying candidates for subject recruitment, helping with recruitment, maintaining study databases, helping with regulatory aspects and annual reports, 4. Specifically relating to the R01 study: a. maintaining coordination and communication with sub-sites to ensure that we are getting complete data, tracking invoices, and other duties as assigned to support the needs of the project. b. acting as liaison with the data managing site (University of Southern California) to ensure the accurate and timely transmission of data. 5. Assist Dr. Lucey and Dr. Rice with research-related tasks such as preparation of grants, manuscripts, institutional review board (IRB) applications/documentation, data presentations and research reports, and maintaining fiscal and granting agency records. 6. Under the direction of Dr. Lucey and his co-investigators, coordinate local data abstraction from EHR or clinical encounter notes and medical records, maintain project management databases and records, prepare protocols, training manuals, abstraction tools, and supervising other abstractors. 7. Interface with other departments and organizations within the University of Wisconsin School of Medicine & Public Health and the University of Wisconsin Hospitals and Clinics as necessary to identify, collect and disseminate research protocol information and results. 8. Conduct medical and scientific reference bibliographic searches, including abstracting data from published sources, summarizing articles, and maintaining a bibliographic database. Candidates must be well organized and have strong written communication skills, with the ability to present material concisely. Proficiency with Microsoft Word, Excel & PowerPoint is essential. Must be able to work well on a team. A background in research is helpful. Interest in and willingness to learn about alcohol-associated liver disease is a plus.
Responsibilities:
- 20% Conducts research experiments according to established research protocols with moderate impact to the project(s). Collects data and monitors test results
- 5% Operates, cleans, and maintains organization of research equipment and research area. Tracks inventory levels and places replenishment orders
- 20% Reviews, analyzes, and interprets data and/or documents results for presentations and/or reporting to internal and external audiences
- 10% Participates in the development, interpretation, and implementation of research methodology and materials
- 15% Provides operational guidance on day-to-day activities of unit or program staff and/or student workers
- 10% Performs literature reviews and writes reports
- 10% Assists in identification of candidates for subject recruitment and directly supports subject recruitment as needed
- 10% Coordinates patient and staff engagement groups and multidisciplinary research team meetings, maintaining working group meeting notes and project website data
Institutional Statement on Diversity:
Diversity is a source of strength, creativity, and innovation for UW-Madison. We value the contributions of each person and respect the profound ways their identity, culture, background, experience, status, abilities, and opinion enrich the university community. We commit ourselves to the pursuit of excellence in teaching, research, outreach, and diversity as inextricably linked goals. The University of Wisconsin-Madison fulfills its public mission by creating a welcoming and inclusive community for people from every background - people who as students, faculty, and staff serve Wisconsin and the world. For more information on diversity and inclusion on campus, please visit: Diversity and Inclusion
Preferred Bachelor's Degree Biological or social sciences or health-related field preferred
Qualifications:
At least 1 year of experience in clinical, health or social research environment preferred. Applicants with relevant experience in a clinical or community/public health role with direct participant are also encouraged to apply. REDCap experience preferred. Experience in providing project management or staff support to multidisciplinary teams preferred.
Full or Part Time: 50% - 100% This position may require some work to be performed in-person, onsite, at a designated campus work location. Some work may be performed remotely, at an offsite, non-campus work location.
Appointment Type, Duration:
Ongoing/Renewable
Minimum $45,000 ANNUAL (12 months) Depending on Qualifications The expected salary range for this position is $45,000 up to $65,000 for highly experienced candidates. Actual pay will depend on experience and qualifications. Employees in this position can expect to receive benefits such as generous vacation, holidays, and sick leave; competitive insurances and savings accounts; retirement benefits. Benefits information can be found at ( https://hr.wisc.edu/benefits/ ).
Additional Information:
It is anticipated that this will be filled as a 50% FTE position, with higher FTE possible in the future depending upon program needs and funding availability. University sponsorship is not available for this position, including transfers of sponsorship. The selected applicant will be responsible for ensuring their continuous eligibility to work in the United States (i.e. a citizen or national of the United States, a lawful permanent resident, a foreign national authorized to work in the United States without the need of an employer sponsorship) on or before the effective date of appointment. This position is an ongoing position that will require continuous work eligibility. UW-Madison is not an E-Verify employer, and therefore, is not eligible to employ F1-OPT STEM Extension participants. If you are selected for this position you must provide proof of work authorization and eligibility to work. This position has been identified as a position of trust with access to vulnerable populations. The selected candidate will be required to pass an initial caregiver check to be eligible for employment under the Wisconsin Caregiver Law and every four years.
How to Apply:
To apply for this position, please click on the "Apply Now" button. You will be asked to upload a current resume/CV and a cover letter briefly describing your qualifications and experience. You will also be asked to provide contact information for three (3) references, including your current/most recent supervisor during the application process. References will not be contacted without prior notice.
Jacqueline Giese [email protected] 608-263-1326 Relay Access (WTRS): 7-1-1. See RELAY_SERVICE for further information.
Official Title:
Research Specialist(RE047)
Department(s):
A53-MEDICAL SCHOOL/MEDICINE/GASTROENT
Employment Class:
Academic Staff-Renewable
Job Number:
The university of wisconsin-madison is an equal opportunity and affirmative action employer..
You will be redirected to the application to launch your career momentarily. Thank you!
Frequently Asked Questions
Applicant Tutorial
Disability Accommodations
Pay Transparency Policy Statement
Refer a Friend
You've sent this job to a friend!
Website feedback, questions or accessibility issues: [email protected] .
Learn more about accessibility at UW–Madison .
© 2016–2024 Board of Regents of the University of Wisconsin System • Privacy Statement

IMAGES
VIDEO
COMMENTS
This article is a practical guide to conducting data analysis in general literature reviews. The general literature review is a synthesis and analysis of published research on a relevant clinical issue, and is a common format for academic theses at the bachelor's and master's levels in nursing, physiotherapy, occupational therapy, public health and other related fields.
A necessary companion to well-designed clinical trial is its appropriate statistical analysis. Assuming that a clinical trial will produce data that could reveal differences in effects between two or more interventions, statistical analyses are used to determine whether such differences are real or are due to chance. Data analysis for small clinical trials in particular must be focused. In the ...
In this summary, we review standard statistical methodology used for data analysis in clinical research. We identify five common types of outcome data and provide an overview of the typical methods of analysis, effect estimates derived, and graphical presentation. We aim to provide a resource for the clinical researcher who is not a practicing ...
Statistical analysis is an essential technique that enables a medical research practitioner to draw meaningful inference from their data analysis. Improper application of study design and data analysis may render insufficient and improper results and conclusion. Converting a medical problem into a statistical hypothesis with appropriate ...
Four textbooks complementary to the current production and written by the same authors are Statistics applied to clinical studies 5th edition, 2012, Machine learning in medicine a complete overview, 2015, SPSS for starters and 2nd levelers 2nd edition, 2015, Clinical Data Analysis on a Pocket Calculator 2nd edition, 2016, all of them edited by ...
Understand and apply the principles of clinical trial design, such as using frameworks to create research questions and establish study objectives. Ensure diverse, representative study populations by studying participant selection criteria and recruitment and retention strategies. Minimize bias and ensure trial reliability and validity through ...
As a repercussion, the scientific process cycle is broken, leaving researchers who want to reuse prior results with three options: 1. Re-run the analysis if the code and original source data are ...
In order to interpret research datasets, clinicians involved in clinical research should have an understanding of statistical methodology. This article provides a brief overview of statistical methods that are frequently used in clinical research studies. Descriptive and inferential methods, including regression modeling and propensity scores ...
Data analysis methods are an integral part of modern clinical research. They are powerful techniques that enable researchers to draw meaningful conclusions from data collected through observation, survey, or experimentation. However, data analysis is a huge discipline with different paradigms, schools of thought and alternative methodologies.
Linear regression analysis. Linear regression is used to quantify a linear relationship or association between a continuous response/outcome variable or dependent variable with at least one ...
approaches in clinical trial data analysis. Adaptive designs, Bayesian methods, and machine learning SJIF Impact Factor 6.222 Review Article ISSN 2394-3211 EJPMR ... flexible clinical research.[3] Data management and analysis in clinical trials are closely intertwined with regulatory requirements and compliance. Regulatory agencies, such as the ...
Abstract. There are two types of data analyses of randomized clinical trials (RCTs). The primary analyses are pre-specified in the protocol and the findings form the basis for recommendations and clinical decisions. They typically adhere to the intention-to-treat principle. Secondary analyses are supplemental and of various sorts.
Transparency has been described as a fundamental value of society and initiatives to increase transparency in relation to clinical trial data have been launched. 1 Given the influence of statistical decisions on trial conclusions, well-documented and transparent statistical conduct is essential. This is relevant given concerns regarding research reproducibility. 2
While data management has broad applications (and meaning) across many fields and industries, in clinical research the term data management is frequently used in the context of clinical trials. 1 ...
Abstract. Statistical analysis is one of the foundations of evidence-based clinical practice, a key in conducting new clinical research and in evaluating and applying prior research. In this paper, we review the choice of statistical procedures, analyses of the associations among variables and techniques used when the clinical processes being ...
In order to interpret research datasets, clinicians involved in clinical research should have an understanding of statistical methodology. This article provides a brief overview of statistical methods that are frequently used in clinical research studies. Descriptive and inferential methods, including regression modeling and propensity scores ...
Integrative data analysis (IDA), a novel framework for conducting the simultaneous analysis of raw data pooled from multiple studies, offers many advantages including economy (i.e., reuse of extant data), power (i.e., large combined sample sizes), the potential to address new questions not answerable by a single contributing study (e.g., combining longitudinal studies to cover a broader swath ...
There are 6 modules in this course. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Understanding and implementing solid data management principles is critical for any scientific domain. Regardless of your current (or anticipated) role in the ...
Clinical Data Science Specialization. Launch your career in Clinical Data Science. A six-course introduction to using clinical data to improve the care of tomorrow's patients. Taught in English. 21 languages available. Some content may not be translated. Instructors: Laura K. Wiley, PhD. +1 more. Enroll for Free.
Clinical data analytics encompasses the analysis of data collected during clinical care processes, including patient interactions, medical procedures and healthcare interventions. The idea is to spot patterns that could improve clinical workflows. Hospital data analytics specifically refers to the analysis of data generated within hospital ...
U.S. Food and Drug Administration
Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design.
This allows a mixed-methods approach to data analysis, combining quantitative assessment of the Likert scoring, and (recursive) thematic analysis of the free-text answers . Thematic analysis is a standard tool, and has been reported as a useful & appropriate for assessing experiences, thoughts, or behaviours in a dataset . The survey questions ...
Background Our study aimed to confirm a simplified radiological scoring system, derived from a modified Reiff score, to evaluate its relationship with clinical symptoms and predictive outcomes in Taiwanese patients with noncystic fibrosis bronchiectasis (NCFB). Methods This extensive multicenter retrospective study, performed in Taiwan, concentrated on patients diagnosed with NCFB verified ...
Data analysis methods are an integral part of modern clinical research. They are powerful techniques that enable researchers to draw meaningful conclusions from data collected through observation, survey, or experimentation. However, data analysis is a huge discipline with different paradigms, schools of thought and alternative methodologies.
Febrile seizures (FS) are the most common seizure disorder in children and a common neurologic complication in children with coronavirus disease 2019 (COVID-19). This study aimed to identify differences in clinical characteristics and disease burden between FS with and without COVID-19. We conducted a retrospective analysis of medical data at our hospital from December 2019 to July 2023 ...
New analyses of the longest clinical trial yet of the weight-loss drug Wegovy are shedding light on how quickly it helps people lose weight, how long they sustain that weight loss and how safe the ...
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right ...
Background Mounting evidences shows that the ubiquitin‒proteasome pathway plays a pivotal role in tumor progression. The expression of 26S proteasome non-ATPase regulatory subunit 9 (PSMD9) is correlated with recurrence and radiotherapy resistance in several tumor types. However, the role and mechanism of PSMD9 in hepatocellular carcinoma (HCC) progression remain largely unclear. Methods ...
Our research encompasses electronic medical record data analysis, qualitative research techniques (focus groups and patient engagement), and clinical/health systems interventions using implementation science. Dr. Lucey is the principal investigator on a prospective 7-year...