- Search Menu
- Advance Articles

Editor's Choice
- Information for authors
- Submission Site
- Open Access Options
- Why publish with the journal
- About DNA Research
- About the Kazusa DNA Research Institute
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Editor-in-Chief
Satoshi Tabata
About the journal
DNA Research is an internationally peer-reviewed journal which aims at publishing papers of highest quality in broad aspects of DNA and genome-related research …
Latest Articles

High-Impact Research Collection
Explore a collection of freely available high-impact research from 2020 and 2021 published in DNA Research .
Browse the collection

DNA Research is the official journal of Kazusa DNA Research Institute, published by Oxford University Press and supported by funding from Chiba Prefecture, Japan.

Why publish in DNA Research?
Growing Impact Factor, fully open access journal, low open access charges, and more.

Volume 26, Issue 6: TASUKE+: a web-based platform for exploring GWAS results and large-scale resequencing data
Read the Executive Editor’s commentary
Resource Articles: Genomes Explored

Email alerts
Register to receive table of contents email alerts as soon as new issues of DNA Research are published online.

Recommend to your library
Fill out our simple online form to recommend DNA Research to your library.
Recommend now

Committee on Publication Ethics (COPE)
This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE)
publicationethics.org

PubMed Central
This journal enables compliance with the NIH Public Access Policy Read more

- Open access
Open access options for authors.

Accepting high quality papers on broad aspects of DNA and genome-related research.
Related Titles

- Author Guidelines
Affiliations
- Online ISSN 1756-1663
- Copyright © 2024 Kazusa DNA Research Institute
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
DNA structure and function
Affiliations.
- 1 MRC Laboratory of Molecular Biology, Cambridge, UK.
- 2 Department of Biochemistry, University of Cambridge, UK.
- 3 Jacobs University Bremen, Germany.
- PMID: 25903461
- DOI: 10.1111/febs.13307
The proposal of a double-helical structure for DNA over 60 years ago provided an eminently satisfying explanation for the heritability of genetic information. But why is DNA, and not RNA, now the dominant biological information store? We argue that, in addition to its coding function, the ability of DNA, unlike RNA, to adopt a B-DNA structure confers advantages both for information accessibility and for packaging. The information encoded by DNA is both digital - the precise base specifying, for example, amino acid sequences - and analogue. The latter determines the sequence-dependent physicochemical properties of DNA, for example, its stiffness and susceptibility to strand separation. Most importantly, DNA chirality enables the formation of supercoiling under torsional stress. We review recent evidence suggesting that DNA supercoiling, particularly that generated by DNA translocases, is a major driver of gene regulation and patterns of chromosomal gene organization, and in its guise as a promoter of DNA packaging enables DNA to act as an energy store to facilitate the passage of translocating enzymes such as RNA polymerase.
Keywords: A-DNA; B-DNA; DNA as an energy store; DNA backbone conformation; DNA elasticity; DNA information; DNA structure; DNA topology; alternative DNA structures; genome organisation.
© 2015 FEBS.
Publication types
- Historical Article
- Chromatin Assembly and Disassembly
- DNA / chemistry*
- DNA / metabolism
- DNA, Superhelical / chemistry
- DNA, Superhelical / metabolism
- Energy Metabolism
- Genetic Phenomena*
- Genetics / history*
- History, 20th Century
- History, 21st Century
- Nucleic Acid Conformation
- DNA, Superhelical
Grants and funding
- MC_U105178783/MRC_/Medical Research Council/United Kingdom
Help | Advanced Search
Computer Science > Information Theory
Title: representing information on dna using patterns induced by enzymatic labeling.
Abstract: Enzymatic DNA labeling is a powerful tool with applications in biochemistry, molecular biology, biotechnology, medical science, and genomic research. This paper contributes to the evolving field of DNA-based data storage by presenting a formal framework for modeling DNA labeling in strings, specifically tailored for data storage purposes. Our approach involves a known DNA molecule as a template for labeling, employing patterns induced by a set of designed labels to represent information. One hypothetical implementation can use CRISPR-Cas9 and gRNA reagents for labeling. Various aspects of the general labeling channel, including fixed-length labels, are explored, and upper bounds on the maximal size of the corresponding codes are given. The study includes the development of an efficient encoder-decoder pair that is proven optimal in terms of maximum code size under specific conditions.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
May 14, 2024
15 min read
Revolutionary Genetics Research Shows RNA May Rule Our Genome
Scientists have recently discovered thousands of active RNA molecules that can control the human body
By Philip Ball
T homas Gingeras did not intend to upend basic ideas about how the human body works. In 2012 the geneticist, now at Cold Spring Harbor Laboratory in New York State, was one of a few hundred colleagues who were simply trying to put together a compendium of human DNA functions. Their project was called ENCODE, for the Encyclopedia of DNA Elements. About a decade earlier almost all of the three billion DNA building blocks that make up the human genome had been identified. Gingeras and the other ENCODE scientists were trying to figure out what all that DNA did.
The assumption made by most biologists at that time was that most of it didn’t do much. The early genome mappers estimated that perhaps 1 to 2 percent of our DNA consisted of genes as classically defined: stretches of the genome that coded for proteins, the workhorses of the human body that carry oxygen to different organs, build heart muscles and brain cells, and do just about everything else people need to stay alive. Making proteins was thought to be the genome’s primary job. Genes do this by putting manufacturing instructions into messenger molecules called mRNAs, which in turn travel to a cell’s protein-making machinery. As for the rest of the genome’s DNA? The “protein-coding regions,” Gingeras says, were supposedly “surrounded by oceans of biologically functionless sequences.” In other words, it was mostly junk DNA.
So it came as rather a shock when, in several 2012 papers in Nature , he and the rest of the ENCODE team reported that at one time or another, at least 75 percent of the genome gets transcribed into RNAs. The ENCODE work, using techniques that could map RNA activity happening along genome sections, had begun in 2003 and came up with preliminary results in 2007. But not until five years later did the extent of all this transcription become clear. If only 1 to 2 percent of this RNA was encoding proteins, what was the rest for? Some of it, scientists knew, carried out crucial tasks such as turning genes on or off; a lot of the other functions had yet to be pinned down. Still, no one had imagined that three quarters of our DNA turns into RNA, let alone that so much of it could do anything useful.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Some biologists greeted this announcement with skepticism bordering on outrage . The ENCODE team was accused of hyping its findings; some critics argued that most of this RNA was made accidentally because the RNA-making enzyme that travels along the genome is rather indiscriminate about which bits of DNA it reads.
Now it looks like ENCODE was basically right. Dozens of other research groups, scoping out activity along the human genome, also have found that much of our DNA is churning out “noncoding” RNA. It doesn’t encode proteins, as mRNA does, but engages with other molecules to conduct some biochemical task. By 2020 the ENCODE project said it had identified around 37,600 noncoding genes—that is, DNA stretches with instructions for RNA molecules that do not code for proteins. That is almost twice as many as there are protein-coding genes. Other tallies vary widely, from around 18,000 to close to 96,000. There are still doubters, but there are also enthusiastic biologists such as Jeanne Lawrence and Lisa Hall of the University of Massachusetts Chan Medical School. In a 2024 commentary for the journal Science , the duo described these findings as part of an “RNA revolution.”
What makes these discoveries revolutionary is what all this noncoding RNA—abbreviated as ncRNA—does. Much of it indeed seems involved in gene regulation: not simply turning them off or on but also fine-tuning their activity. So although some genes hold the blueprint for proteins, ncRNA can control the activity of those genes and thus ultimately determine whether their proteins are made. This is a far cry from the basic narrative of biology that has held sway since the discovery of the DNA double helix some 70 years ago, which was all about DNA leading to proteins. “It appears that we may have fundamentally misunderstood the nature of genetic programming,” wrote molecular biologists Kevin Morris of Queensland University of Technology and John Mattick of the University of New South Wales in Australia in a 2014 article.
Another important discovery is that some ncRNAs appear to play a role in disease, for example, by regulating the cell processes involved in some forms of cancer. So researchers are investigating whether it is possible to develop drugs that target such ncRNAs or, conversely, to use ncRNAs themselves as drugs. If a gene codes for a protein that helps a cancer cell grow, for example, an ncRNA that shuts down the gene might help treat the cancer.
A few noncoding RNAs had been known for many decades, but those seemed to have some role in protein manufacture. For instance, only a few years after Francis Crick, James Watson and several of their colleagues deduced the structure of DNA, researchers found that some RNA, called transfer RNA, grabs onto amino acids that eventually get strung together into proteins.
In the 1990s, however, scientists realized ncRNA could do things quite unrelated to protein construction. These new roles came to light from efforts to understand the process of X-inactivation, wherein one of the two X chromosomes carried by females is silenced, all 1,000 or so of its genes (in humans) being turned off. This process seemed to be controlled by a gene called XIST . But attempts to find the corresponding XIST protein consistently failed.
The reason, it turned out, was that the gene did not work through a protein but instead did so by producing a long noncoding (lnc) RNA molecule. Such RNAs are typically longer than about 200 nucleotides, which are the chemical building blocks of DNA and RNA. Using a microscopy technique called fluorescence in situ hybridization, Lawrence and her colleagues showed that this RNA wraps itself around one X chromosome (selected at random in each cell) to induce persistent changes that silence the genes. “This was the first evidence of a lncRNA that does something,” Lawrence says, “and it was totally surprising.”
If noncoding RNAs power the way a cell processes genetic information, it is possible they can be used in medicine.
XIST isn’t that unusual in generating an ncRNA, though. In the early 2000s it became clear that transcription of noncoding DNA sequences is widespread. For example, in 2002 a team at biotech company Affymetrix in Santa Clara, Calif., led by Gingeras, who was working there at the time, reported that much more of human chromosomes 21 and 22 gets transcribed than just the protein-coding regions.
It was only after ENCODE published its results in 2012, however, that ncRNA became impossible to ignore. Part of the antipathy toward those findings, says Peter Stadler, a bioinformatics expert at Leipzig University in Germany, is that they seemed like an unwanted and unneeded complication. “The biological community figured we already knew how the cell works, and so the discovery of [ncRNAs] was more of an annoyance,” he says. What’s more, it showed that simpler organisms were not always a reliable guide to human biology: there is far less ncRNA in bacteria, studies of which had long shaped thinking about how genes are regulated.
But now there is no turning back the tide: many thousands of human lncRNAs have been reported, and Mattick suspects the real number is greater than 500,000. Yet only a few of these have been shown to have specific functions, and how many of them really do remains an open question. “I personally don’t think all of those RNAs have an individual role,” Lawrence says. Some, though, may act in groups to regulate other molecules.
How lncRNAs perform such regulation is also still a matter of debate. One idea is that they help to form so-called condensates: dense fluid blobs containing a range of different regulatory molecules. Condensates are thought to hold all the relevant players in one place long enough for them to do their job collectively. Another idea is that lncRNAs affect the structure of chromatin—the combination of DNA and proteins that makes up chromosome fibers in the cell nucleus. How chromatin is structured determines which of its genes are accessible and can be transcribed; if parts of chromatin are too tightly packed, the enzyme machinery of transcription can’t reach it. “Some lncRNAs appear to be involved with chromatin-modifying complexes,” says Marcel Dinger, a genomics researcher at the University of Sydney.
If only 1 to 2 percent of the RNA from our genome was encoding proteins, what was the rest for? Some, scientists knew, carried out crucial tasks such as turning genes on or off.
Lawrence and Hall suspect that lncRNAs could supply scaffolds for organizing other molecules, for example, by holding some of the many hundreds of RNA-binding proteins in functional assemblies. One lncRNA called NEAT1, which is involved in the formation of small compartments in the nucleus called paraspeckles, has been shown capable of binding up to 60 of these proteins. Or such RNA scaffolding could arrange chromatin itself into particular structures and thereby affect gene regulation. Such RNA scaffolding could have regularly repeating modules and thus repetitive sequences—a feature that has long been regarded as a hallmark of junk DNA but lately is appearing to be not so junky after all. This view of lncRNA as scaffolding is supported by a 2024 report of repeat-rich ncRNAs in mouse brain cells that persist for at least two years. The research, by Sara Zocher of the German Center for Neurodegenerative Diseases in Dresden and her co-workers. found these ncRNAs seem to be needed to keep parts of chromatin in a compact and silent state.
T hese lncRNAs are just one branch of the noncoding RNA family, and biologists keep discovering others that appear to have different functions and different ways of affecting what happens to a cell—and thus the entire human body.
Some of these RNAs are not long at all but surprisingly short. Their story began in the 1980s, when Victor Ambros, working as a postdoctoral researcher in the laboratory of biologist Robert Horvitz at the Massachusetts Institute of Technology, was studying a gene denoted lin-4 in the worm Caenorhabditis elegans . Mutations of lin-4 caused developmental defects in which “the cells repeated whole developmental programs that they should have transitioned beyond,” says Ambros, now at the University of Massachusetts Medical School. It seemed that lin-4 might be a kind of “master regulator” controlling the timing of different stages of development.

Jen Christiansen; Source: John Mattick, UNSW Sydney (consultant)
“We thought lin-4 would be a protein-coding gene,” Ambros says. To figure out what role this putative protein plays, Ambros and his colleagues cloned the C. elegans gene and looked at its product—and found that the effects of the gene may not be mediated by any protein but by the gene’s RNA product alone. This molecule looked ridiculously short: just 22 nucleotides long, a mere scrap of a molecule for such big developmental effects.
This was the first known microRNA (miRNA). At first “we thought this might be a peculiar characteristic of C. elegans ,” Ambros says. But in 2000 Gary Ruvkun, another former postdoc in the Horvitz lab, and his co-workers found that another of these miRNA genes in C. elegans , called let-7 , appears in essentially identical form in many other organisms, including vertebrates, mollusks and insects. This implies that it is a very ancient gene and “must have been around for 600 million to 700 million years” before these diverse lineages went their separate ways, Ambros says. If miRNAs are so ancient, “there had to be others out there.”
Indeed, there are. Today more than 2,000 miRNAs have been identified in the human genome, generally with regulatory roles. One of the main ways miRNAs work is by interfering with the translation of a gene’s mRNA transcript into its corresponding protein. Typically the miRNA comes from a longer molecule, perhaps around 70 nucleotides long, known as pre-miRNA. This molecule is seized by an enzyme called Dicer, which chops it into smaller fragments. These pieces, now miRNAs, move to a class of proteins called Argonautes, components of a protein assembly called the RNA-induced silencing complex (RISC). The miRNAs guide the RISC to an mRNA, and this either stops the mRNA from being translated into a protein or leads to its degradation, which has the same effect. This regulatory action of miRNAs guides processes ranging from the determination of cell “fate” (the specialized cell types they become) to cell death and management of the cell cycle.
Key insights into how such small RNAs can regulate other RNA emerged from studies in C. elegans in 1998 by molecular biologists Andrew Fire, Craig Mello and their co-workers, for which Fire and Mello were awarded the 2006 Nobel Prize in Physiology or Medicine. They learned that RISC is guided by slightly different RNA strands named small interfering (si) RNA. The process ends with the mRNA being snipped in half, a process called RNA interference.
MiRNAs do pose a puzzle, however. A given miRNA typically has a sequence that matches up with lots of mRNAs. How, then, is there any selectivity about which genes they silence? One possibility is that miRNAs work in gangs, with several miRNAs joining forces to regulate a given gene. The different combinations, rather than individual snippets, are what match specific genes and their miRNAs.
Why would miRNA gene regulation work in this complicated way? Ambros suspects it might allow for “evolutionary fluidity”: the many ways in which different miRNAs can work together, and the number of possible targets each of them can have, offer a lot of flexibility in how genes are regulated and thus in what traits might result. That gives an organism many evolutionary options, so that it is more able to adapt to changing circumstances.
One class of small RNAs regulates gene expression by directly interfering with transcription in the cell nucleus, triggering mRNA degradation. These PIWI-interacting (pi) RNAs work in conjunction with a class of proteins called PIWI Argonautes. PiRNAs operate in germline cells (gametes), where they combat “selfish” DNA sequences called transposons or “jumping genes”: sequences that can insert copies of themselves throughout the genome in a disruptive way. Thus, piRNAs are “a part of the genome’s immune system,” says Julius Brennecke of the Institute of Molecular Biotechnology of the Austrian Academy of Sciences. If the piRNA system is artificially shut down, “the gametes’ genomes are completely shredded, and the organism is completely sterile,” he says.
Still other types of ncRNAs, called small nucleolar RNAs, work within cell compartments called nucleoli to help modify the RNA in ribosomes—a cell’s protein-making factories—as well as transfer RNA and mRNA. These are all ways to regulate gene expression. Then there are circular RNAs: mRNA molecules (particularly in neurons) that get stitched into a circular form before they are moved beyond the nucleus into the cytoplasm. It’s not clear how many circular RNAs are important—some might just be transcriptional “noise”—but there is some evidence that at least some of them have regulatory functions.
In addition, there are vault RNAs that help to transport other molecules within and between cells, “small Cajal-body-specific RNAs” that modify other ncRNAs involved in RNA processing, and more. The proliferation of ncRNA varieties lends strength to Mattick’s claim that RNA, not DNA, is “the computational engine of the cell.”
I f ncRNAs indeed power the way a cell processes genetic information, it is possible they can be used in medicine. Disease is often the result of a cell doing the wrong thing because it gets the wrong regulatory instructions: cells that lose proper control of their cycle of growth and division can become tumors, for example. Currently medical efforts to target ncRNAs and alter their regulatory effects often use RNA strings called antisense oligonucleotides (ASOs). These strands of nucleic acid have sequences that are complementary to the target RNA, so they will pair up with and disable it. ASOs have been around since the late 1970s. But it has been hard to make them clinically useful because they get degraded quickly in cells and have a tendency to bind to the wrong targets, with potentially drastic consequences.
Some ASOs, however, are being developed to disable lncRNAs that are associated with cancers such as lung cancer and acute myeloid leukemia. Other lncRNAs might act as drugs themselves. One known as MEG3 has been found, preliminarily, to act as a tumor suppressor. Small synthetic molecules, which are easier than ASOs to fine-tune and deliver into the body as pharmaceuticals, are also being explored for binding to lncRNAs or otherwise inhibiting their interactions with proteins. Getting these approaches to work, however, has not been easy. “As far as I am aware, no lncRNA target or therapeutic has entered clinical development,” Gingeras says.
Targeting the smaller regulatory RNAs such as miRNAs might prove more clinically amenable. Because miRNAs typically hit many targets, they can do many things at once. For example, miRNAs in families denoted miR-15a and miR-16-1 act as tumor suppressors by targeting several genes that themselves suppress cell death (apoptosis, a defense against cancer) and are being explored for cancer therapies.
Yet a problem with using small RNAs as drugs is that they elicit an immune response. Precisely because the immune system aims to protect against viral RNA, it usually recognizes and attacks any “nonself” RNA. One strategy for protecting therapeutic RNA from immune assault and degradation is to chemically modify its backbone so that it forms a nonnatural “locked” ring structure that the degrading enzymes can’t easily recognize.
Some short ASOs that target RNAs are already approved for clinical use, such as the drugs inotersen to treat amyloidosis and golodirsen for Duchenne muscular dystrophy. Researchers are also exploring antisense RNAs fewer than 21 nucleotides long that target natural regulatory miRNAs because it is only beyond that length that an RNA tends to trigger an immune reaction.
These are early days for RNA-based medicine, precisely because the significance of ncRNA itself in human biology is still relatively new and imperfectly understood. The more we appreciate its pervasive nature, the more we can expect to see RNA being used to control and improve our well-being. Nils Walter of the Center for RNA Biomedicine at the University of Michigan wrote in an article early in 2024 that the burgeoning promise of RNA therapeutics “only makes the need for deciphering ncRNA function more urgent.” Succeeding in this goal, he adds, “would finally fulfill the promise of the Human Genome Project.”
Despite this potential of noncoding RNA in medicine, the debate continues about how much of it truly matters for our cells. Geneticists Chris Ponting of the University of Edinburgh and Wilfried Haerty of the Earlham Institute in Norwich, England, are among the skeptics. In a 2022 article they argued that most lncRNAs are just “transcriptional noise,” accidentally transcribed from random bits of DNA. “Relatively few human lncRNAs ... contribute centrally to human development, physiology, or behavior,” they wrote.
Brennecke advises caution about current high estimates of the number of noncoding genes. Although he agrees that such genes “have been underappreciated for a long time,” he says we should not leap to assuming that all lncRNAs have functions. Many of them are transcribed only at low levels, which is what one would expect if indeed they were just random noise. Geneticist Adrian Bird of the University of Edinburgh points out that the abundance of the vast majority of ncRNAs seems to be well below one molecule per cell. “It is difficult to see how essential functions can be exerted by an ncRNA if it is absent in most cells,” he says.
But Gingeras counters that this low expression rate might reflect the very tissue-specific roles of ncRNAs. Some, he says, are expressed more in one part of a tissue than in another, suggesting that expression levels in each cell are sensitive to signals coming from surrounding tissues. Lawrence points out that, despite the low expression levels, there are often shared patterns of expression across cells of a particular type, making it harder to argue that the transcription is simply random. And Hall doubts that cells are really so prone to “bad housekeeping” that they will habitually churn out lots of useless RNA. Lawrence and Hall’s suggestion that some lncRNAs have collective effects on chromatin structure would mean that no individual one of them is needed at high expression levels and that their precise sequence doesn’t matter too much.
That lack of specificity in sequence and binding targets, Dinger says, means that a mutation of a nucleotide in an ncRNA typically won’t have the same negative impact on its function as it tends to in a protein-coding DNA sequence. So it would not be surprising to see quite a lot of sequence variation. Dinger argues that it makes more sense to assume that “genetically encoded molecules are potentially functional until shown otherwise, rather than junk unless proven functional.” Some in the ENCODE team now agree that not all of the 75 percent or so of human genome transcription might be functionally significant. But many researchers make the point that surely many more of the noncoding molecules do meaningful things than was suspected before.
Demonstrating functional roles for lncRNAs is often tricky. In part, Gingeras says, this may be because lncRNA might not be the biochemically active molecule in a given process: it might be snipped up into short RNAs that actually do the work. But because long and short RNAs tend to be characterized via different techniques, researchers may end up searching for the wrong thing. What’s more, long RNAs are often cut up into fragments and then spliced back together again in various combinations, the exact order often depending on the condition of the host cell.
At its roots, the controversy over noncoding RNA is partly about what qualifies a molecule as “functional.” Should the criterion be based on whether the sequence is maintained between different species? Or whether deleting the molecule from an organism’s repertoire leads to some observable change in a trait? Or simply whether it can be shown to be involved in some biochemical process in the cell? If repetitive RNA acts collectively as a chromosome “scaffold” or if miRNAs act in a kind of regulatory swarm, can any individual one of them really be considered to have a “function”?
Gingeras says he is perplexed by ongoing claims that ncRNAs are merely noise or junk, as evidence is mounting that they do many things. “It is puzzling why there is such an effort to persuade colleagues to move from a sense of interest and curiosity in the ncRNA field to a more dubious and critical one,” he says.
Perhaps the arguments are so intense because they undercut the way we think our biology works. Ever since the epochal discovery about DNA’s double helix and how it encodes information, the bedrock idea of molecular biology has been that there are precisely encoded instructions that program specific molecules for particular tasks. But ncRNAs seem to point to a fuzzier, more collective, logic to life. It is a logic that is harder to discern and harder to understand. But if scientists can learn to live with the fuzziness, this view of life may turn out to be more complete.
Philip Ball is a science writer and former Nature editor based in London. His most recent book is How Life Works (University of Chicago Press, 2023).

Cellular activity hints that recycling is in our DNA
New research shows that 'spliceosomes' might reinsert problematic gene sequences after removing them.
Although you may not appreciate them, or have even heard of them, throughout your body, countless microscopic machines called spliceosomes are hard at work. As you sit and read, they are faithfully and rapidly putting back together the broken information in your genes by removing sequences called "introns" so that your messenger RNAs can make the correct proteins needed by your cells.
Introns are perhaps one of our genome's biggest mysteries. They are DNA sequences that interrupt the sensible protein-coding information in your genes, and need to be "spliced out." The human genome has hundreds of thousands of introns, about 7 or 8 per gene, and each is removed by a specialized RNA protein complex called the "spliceosome" that cuts out all the introns and splices together the remaining coding sequences, called exons. How this system of broken genes and the spliceosome evolved in our genomes is not known.
Over his long career, Manny Ares, UC Santa Cruz distinguished professor of molecular, cellular, and developmental biology, has made it his mission to learn as much about RNA splicing as he can.
"I'm all about the spliceosome," Ares said. "I just want to know everything the spliceosome does -- even if I don't know why it is doing it."
In a new paper published in the journal Genes and Development , Ares reports on a surprising discovery about the spliceosome that could tell us more about the evolution of different species and the way cells have adapted to the strange problem of introns. The authors show that after the spliceosome is finished splicing the mRNA, it remains active and can engage in further reactions with the removed introns.
This discovery provides the strongest indication we have so far that spliceosomes could be able to reinsert an intron back into the genome in another location. This is an ability that spliceosomes were not previously believed to possess, but which is a common characteristic of "Group II introns," distant cousins of the spliceosome that exist primarily in bacteria.
The spliceosome and Group II introns are believed to share a common ancestor that was responsible for spreading introns throughout the genome, but while Group II introns can splice themselves out of RNA and then directly back into DNA, the "spliceosomal introns" that are found in most higher-level organisms require the spliceosome for splicing and were not believed to be reinserted back into DNA. However, Ares's lab's finding indicates that the spliceosome might still be reinserting introns into the genome today. This is an intriguing possibility to consider because introns that are reintroduced into DNA add complexity to the genome, and understanding more about where these introns come from could help us to better understand how organisms continue to evolve.
Building on an interesting discovery
An organism's genes are made of DNA, in which four bases, adenine (A), cytosine (C), guanine (G) and thymine (T) are ordered in sequences that code for biological instructions, like how to make specific proteins the body needs. Before these instructions can be read, the DNA gets copied into RNA by a process known as transcription, and then the introns in that RNA have to be removed before a ribosome can translate it into actual proteins.
The spliceosome removes introns using a two-step process that results in the intron RNA having one of its ends joined to its middle, forming a circle with a tail that looks like a cowboy's "lariat," or lasso. This appearance has led to them being named "lariat introns." Recently, researchers at Brown University who were studying the locations of the joining sites in these lariats made an odd observation -- some introns were actually circular instead of lariat shaped.
This observation immediately got Ares's attention. Something seemed to be interacting with the lariat introns after they were removed from the RNA sequence to change their shape, and the spliceosome was his main suspect.
"I thought that was interesting because of this old, old idea about where introns came from," Ares said. "There is a lot of evidence that the RNA parts of the spliceosome, the snRNAs, are closely related to Group II introns."
Because the chemical mechanism for splicing is very similar between the spliceosomes and their distant cousins, the Group II introns, many researchers have theorized that when the process of self-splicing became too inefficient for Group II introns to reliably complete on their own, parts of these introns evolved to become the spliceosome. While Group II introns were able to insert themselves directly back into DNA, however, spliceosomal introns that required the help of spliceosomes were not thought to be inserted back into DNA.
"One of the questions that was sort of missing from this story in my mind was, is it possible that the modern spliceosome is still able to take a lariat intron and insert it somewhere in the genome?" Ares said. "Is it still capable of doing what the ancestor complex did?"
To begin to answer this question, Ares decided to investigate whether it was indeed the spliceosome that was making changes to the lariat introns to remove their tails. His lab slowed the splicing process in yeast cells, and discovered that after the spliceosome released the mRNA that it had finished splicing introns from, it hung onto intron lariats and reshaped them into true circles. The Ares lab was able to reanalyze published RNA sequencing data from human cells and found that human spliceosomes also had this ability.
"We are excited about this because while we don't know what this circular RNA might do, the fact that the spliceosome is still active suggests it may be able to catalyze the insertion of the lariat intron back into the genome," Ares said.
If the spliceosome is able to reinsert the intron into DNA, this would also add significant weight to the theory that spliceosomes and Group II introns shared a common ancestor long ago.
Testing a theory
Now that Ares and his lab have shown that the spliceosome has the catalytic ability to hypothetically place introns back into DNA like their ancestors did, the next step is for the researchers to create an artificial situation in which they "feed" a DNA strand to a spliceosome that is still attached to a lariat intron and see if they can actually get it to insert the intron somewhere, which would present "proof of concept" for this theory.
If the spliceosome is able to reinsert introns into the genome, it is likely to be a very infrequent event in humans, because the human spliceosomes are in incredibly high demand and therefore do not have much time to spend with removed introns. In other organisms where the spliceosome isn't as busy, however, the reinsertion of introns may be more frequent. Ares is working closely with UCSC Biomolecular Engineering Professor Russ Corbett-Detig, who has recently led a systematic and exhaustive hunt for new introns in the available genomes of all intron-containing species that was published in the journal Proceedings of the National Academy of Sciences (PNAS) last year.
The paper in PNAS showed that intron "burst" events far back in evolutionary history likely introduced thousands of introns into a genome all at once. Ares and Corbett-Detig are now working to recreate a burst event artificially, which would give them insight into how genomes reacted when this happened.
Ares said that his cross-disciplinary partnership with Corbett-Detig has opened the doors for them to really dig into some of the biggest mysteries about introns that would probably be impossible for them to understand fully without their combined expertise.
"It is the best way to do things," Ares said. "When you find someone who has the same kind of questions in mind but a different set of methods, perspectives, biases, and weird ideas, that gets more exciting. That makes you feel like you can break out and solve a problem like this, which is very complex."
- Human Biology
- Back and Neck Pain
- Biochemistry Research
- New Species
- Evolutionary Biology
- Genetic code
- Human genome
- DNA microarray
Story Source:
Materials provided by University of California - Santa Cruz . Original written by Rose Miyatsu. Note: Content may be edited for style and length.
Journal Reference :
- Manuel Ares, Haller Igel, Sol Katzman, John P. Donohue. Intron lariat spliceosomes convert lariats to true circles: implications for intron transposition . Genes & Development , 2024; DOI: 10.1101/gad.351764.124
Cite This Page :
Explore More
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
- Toward Human Brain Gene Therapy
- Whale Families Learn Each Other's Vocal Style
- AI Can Answer Complex Physics Questions
- Otters Use Tools to Survive a Changing World
- Monogamy in Mice: Newly Evolved Type of Cell
- Sustainable Electronics, Doped With Air
- Male Vs Female Brain Structure
Trending Topics
Strange & offbeat.
- Open access
- Published: 12 February 2019
Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method
- Nadia Aboul-Ftooh Aboul-Maaty 1 &
- Hanaa Abdel-Sadek Oraby ORCID: orcid.org/0000-0001-9779-2953 1
Bulletin of the National Research Centre volume 43 , Article number: 25 ( 2019 ) Cite this article
86k Accesses
119 Citations
3 Altmetric
Metrics details
Reliable measurement of DNA concentration and purity is important for almost all molecular genetics studies. Different plant species have varying levels of polysaccharides, polyphenols, and other secondary metabolites which combine with nucleic acids during DNA isolation and further affect the quality of the extracted DNA. The current extraction protocol is based upon the conventional cetyl trimethylammonium bromide (CTAB) method with further modifications for the extraction of DNA from variable plant seeds and crops belonging to seven different orders. The principle modifications currently employed for DNA extraction involved the use of higher CTAB concentration and higher levels of 2-β-mercaptoethanol. Additionally, higher concentrations of sodium chloride and potassium acetate were added simultaneously with absolute ice cold isopropanol for the precipitation of DNA free from polysaccharides.
Results and conclusion
The prescribed modifications in the present method establish a quick and efficient standardized protocol for DNA extraction from different plant orders. The current extraction protocol, therefore, can be of great value for molecular analysis involving large numbers of different plant samples from different orders. These modifications consistently produced pure and high-quality DNA suitable for further molecular analysis. Successful PCR amplification with random amplified polymorphic DNA primer, NPTII gene, and the complete digestion of the isolated DNA with the HindIII restriction enzyme validated the quality of the isolated DNA. Moreover, it reflects the efficiency of the protocol and proves its suitability for further applications for the assessment of food safety, detection of genetically modified (GM) crops, and conservation of biodiversity.
Isolation and purification of DNA are a crucial step in DNA molecular techniques used in plant studies for the identification of genotypes, economical traits associated with genes of interest, and genetic diversity. Reliable measurement of DNA concentration and purity is also important for the assessment of food safety, especially with the increase of the global cultivation area of genetically modified (GM) crops (Ateş Sönmezoğlu and Keskin 2015 ). To facilitate protection of biodiversity and to guarantee rational use of these GM crops, sufficient measurements for purity, quality, and amount of DNA present in these products must be determined to comply with labeling regulation requirements.
DNA molecular techniques are mainly based on polymerase chain reaction (PCR) assay that requires isolation of genomic DNA of suitable purity. Various extraction protocols have been established in order to isolate pure and intact whole genomic DNA from plant tissues (Saghai-Maroof et al. 1984 ; Doyle and Doyle 1990 ; Scott and Playford 1996 ; Sharma et al. 2000 ; Pirttilä et al. 2001 ; Shepherd et al. 2002 ; Mogg and Bond 2003 ; Haymes 1996 ).
However, many difficulties have been reported for isolating good-quality DNA from plants (Novaes et al. 2009 ; Silva 2010 ; Moreira and Oliveira 2011 ). These difficulties were attributed to the fact that different plant species have varying levels of polysaccharides, polyphenols, and other secondary metabolites. These components are usually hindering the process of DNA purification and its further use in molecular studies (Khanuja et al. 1999 ). These plant components have a similar structure of nucleic acids that allow secondary metabolites and polysaccharides to interfere with total DNA isolation (Shioda and Marakami-Muofushi 1987 ). They strongly combine with nucleic acids during DNA isolation and affect the quality of the extracted DNA from higher plants (Scott and Playford 1996 ). These metabolites also affect the quantity and purity of the isolated nucleic acids (Porebski et al. 1997 ). The removal of such contaminants needs complicated and time-consuming protocols. A single DNA isolation protocol is not likely to be applicable for all the plant tissues (Loomis 1974 ). Most of the cetyl trimethylammonium bromide (CTAB)-based protocols used for the extraction of DNA were tailored according to the internal components of each single plant species (Wang et al. 2012 ; Moreira and Oliveira 2011 ).
The present work describes an inexpensive CTAB-based method with modifications for the extraction of high-quality genomic DNA from 19 different plant seeds and crops belong to seven different plant orders. These plant samples are rich in proteins, polysaccharides, and polyphenols. In comparison, we used the classical protocol of Doyle and Doyle ( 1990 ) for isolation of DNA from the same samples. In order to validate the quality of the DNA extracted by the modified protocol, PCR amplification of genomic DNA extracted from different plant seeds applying the two utilized protocols was carried out using random amplified polymorphic DNA (RAPD). PCR amplification of neomycin phosphotransferase gene (nptII) was used to evaluate the efficacy of the present protocol to produce good-quality DNA suitable for detection of genetically modified crops.
Materials and methods
Plant materials.
Twenty-seven plant samples were purchased locally from plant seed suppliers in Egypt (Table 1 ). They were chosen to be enrolled in this study because they have varying amounts of polysaccharides, proteins, and polyphenols and they belong to seven different orders. These plant samples were mainly imported from different countries distributed in Europe, America, and Asia. Additionally, four animal diet samples (D1, D2, D3, and D4) were also purchased from different suppliers. These four diet samples contain mixtures of soybean and corn.
3× extraction buffer containing: 3% CTAB ( w / v ), 1.4 M NaCl, 0.8 M Tris-HCl pH 8.0, 0.5 M EDTA pH 8.0 (autoclaved)
0.3% 2-β-Mercaptoethanol.
Chloroform:isoamyl alcohol (24:1 v / v ).
3 M potassium acetate
Ice cold 100% isopropyl alcohol
70% ethanol
1× TE buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA, pH 8.0, autoclaved).
Agarose (molecular grade)
Modified DNA extraction protocol
Preheat the 3× extraction buffer in water bath at 65 °C. Add 0.3% 2-β-mercaptoethanol to the 3× CTAB extraction buffer immediately before use.
Grind 50 mg of plant samples into powder in liquid nitrogen using pre chilled mortar and pestle. While still in the mortar, add 800 μl of the preheated 3× CTAB extraction buffer to the grinded plant samples and swirl gently to mix using the pestle.
Transfer the sample mixture to a 2-ml microcentrifuge tube, incubate in water bath at 60–65 °C for 1 h, mix gently every 20 min by inverting the tube for 20 times each, then cool down to the room temperature.
Add an equal volume of chloroform:isoamyl alcohol (24:1 v / v ) and mix by slight inversion.
Centrifuge at 13,000 rpm for 15 min at room temperature (RT).
Using a wide bore pipet, carefully transfer the upper aqueous phase, which contains the DNA, to a new 1.5-ml eppendorf tube.
Repeat the extraction steps (iv–vi), when necessary until the upper aqueous phase is clear.
Estimate the volume of the aqueous phase (approximately 700 μl) then add half this volume (350 μl) of 6 M NaCl and mix well. Successively, add 1/10 the volume (70 μl) 3 M potassium acetate and simultaneously mix with 500 μl ice cold 100% isopropyl alcohol (approximately two thirds the volume of the aqueous phase). Invert gently to precipitate DNA until the formation of DNA threads.
Incubate at − 20 °C for 30 min.
Centrifuge at 13,000 rpm for 5 min, discard supernatant.
Invert the tube containing the DNA pellet on tissue paper to complete draining off the supernatant.
Wash DNA pellet with 500 μl of 70% ethanol and invert once (to dissolve residual salts and to increase purity of the DNA).
Centrifuge at 13,000 rpm for 5 min.
Discard 70% alcohol from tubes. invert the on filter paper, and allow tubes containing pellet to air dry at room temperature for 15 min.
Re-suspend the DNA pellet in 50 μl 1× TE buffer. Incubate the DNA at 50 °C for 1 to 2 h to ensure complete re-suspension.
Store at − 20 °C till further use.
Quantitative and qualitative analysis of DNA extracted by established CTAB method and modified protocol
Dna concentration, purity, and quality.
DNA concentration was determined spectrophotometrically at 260 nm (A 260 ) absorption using NanoDrop1000 (Thermo Scientific). Purity of DNA from protein and polysaccharide contamination (Wilson and Walker 2005 ) was assessed by estimating the absorbance ratio at A 260 /A 280 and A 260 /A 230 respectively. The quality of the extracted DNA using both protocols was also evaluated by electrophoresis separation for all DNA samples on 0.8% agarose gel stained with ethidium bromide (1 μg/ml).
DNA digestion analysis
HindIII restriction enzyme was used to digest the DNA samples according to the procedure of Fang and colleagues ( 1992 ). Approximately 20 μg of genomic DNA was digested separately for 1 h at 37 °C with HindIII restriction enzyme (Amersham Pharmacia Biotech. UK Ltd). All stained electrophoresis separation matrices for PCR amplification and both extracted and digested DNA samples were resolved by SYNGENE Bio Imaging Gel Documentation System (UK).
Random amplified polymorphic DNA analysis
PCR amplification of genomic DNA extracted from different plant seeds applying the two protocols utilized was carried out using random amplified polymorphic DNA (RAPD) decamer primer (OPZ-09) that was synthesized by Operon Primer Kits (Operon, USA). The primer sequence is 5′-CAGCACTGAC-3′. PCR was performed for all samples according to the method described by Devi and colleagues ( 2013 ).
Detection of genetically modified (GM) crops
The efficacy of the present protocol to produce good-quality DNA suitable for detection of genetically modified crops was also assessed. The isolated genomic DNA from different plant samples by means of the present protocol and the conventional method was used as a template for PCR amplification of neomycin phosphotransferase gene (nptII), which is utilized as a selectable marker gene in the transformation processes. The existence of NPTII (173 bp target) was investigated in the plant seeds enrolled in the present work, using specific primers for this gene (F: 5′-GGATCTCCTGTCATCT-3′ and R: 5′-GGATCTCCTGTCATCT-3′). The PCR amplification was carried out in a 25-μl reaction mixture containing 12.75 μl of DNase free water, 100 ng template DNA (2 μl), 200 μM of each dNTP (2.5 μl), 2.5 pmol of each primer (2.5 μl), and 2.5 units of taq DNA polymerase (0.25 μl) in a reaction buffer (2.5 μl) containing 75 mM Tris-HCl, pH 8.0, 2 mM MgCl 2 , 50 mM KCl, 20 mM (NH 4 ) 2 SO 4 , and 0.001% BSA.
PCR amplifications were performed in a TM Thermal cycler (MJ Research PTC-100 thermocycler) programmed to perform an initial denaturation step of 98 °C for 2 min, followed by 40 cycles consisting of 30 s at 95 °C for denaturation, 45 s at annealing temperature (50 °C), and 30 s at 72 °C for extension. A final extension step of 7 min at 72 °C was performed. Following completion of the cycling reaction, 2 μl of a loading dye (bromophenol blue) was added to 10 μl of each reaction product and separated by 2% agarose gel electrophoresis stained with 1 μg/ml ethidium bromide. PCR products were analyzed, using SYNGENE Bio Imaging Gel Documentation System, for the presence of a fluorescent band of the expected base pair (bp) size (173 bp).
Applying the present standardized method, the extracted DNA concentrations varied with the different plant species used in the present work (Table 2 ). The yield of isolated DNA ranged from 2.238 ηg/mg of seeds in case of Cucurbitales maxima to 24.957 ηg/mg of seeds in the case of Lupinus lupinus. The other classical CTAB method employed (Doyle and Doyle 1990 ) also produced comparable range of DNA concentration (Table 2 ), yet with less purity in most cases. Most of DNA samples extracted by the original CTAB method had A 260 /A 280 ratio below 1.8, while the A 260 /A 280 ratios ranged from 2.08 to 2.23 in DNA samples extracted by our modified protocol.
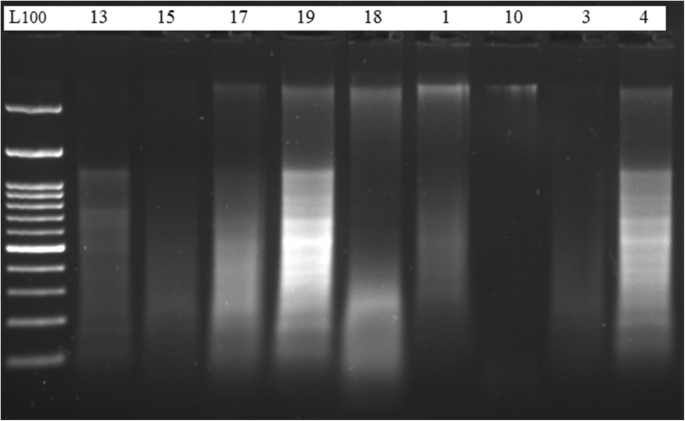
The quality of the total DNA extracted by the present protocol, from different plant species, was also evaluated by electrophoresis separation. Results showed intense bands very close to the gel wells (Fig. 1 , upper lane). Genomic DNA extracted by the CTAB method of Doyle and Doyle ( 1990 ) from the same samples did not produce distinct or intact bands (Fig. 1 , lower lane). The NanoDrop spectrophotometer measurement profile showed a single absorbance peak at 260 nm in DNA samples extracted by our standardized protocol. Figure 2 shows an example of a NanoDrop measurement profile of extracted genomic DNA from Glycine max sample using our protocol. DNA samples extracted by the present modified extraction protocol were efficiently digested with the HindIII restriction enzyme (Fig. 3 ).
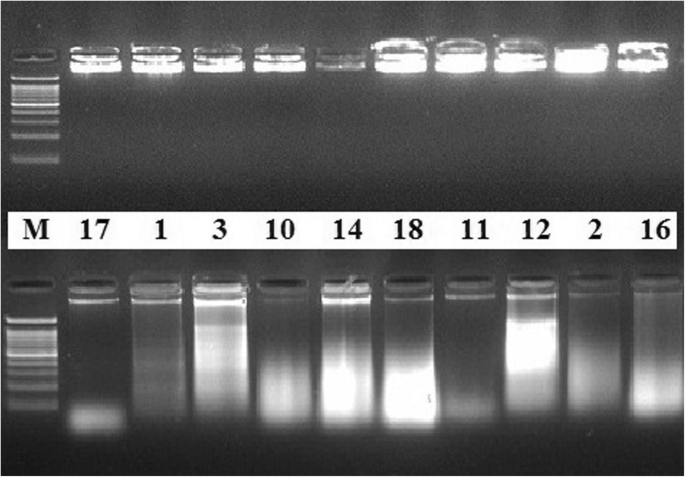
Quality of extracted DNA. Quality of DNA extracted from some of the plant samples using both DNA extraction methods. Electrophoresis separation was performed on 0.8% agarose gel matrix, stained with ethidium bromide. The upper lane is for samples extracted by the standardized method after modification; the lower lane is for the same samples extracted by the conventional method. M is a molecular marker (100 bp)
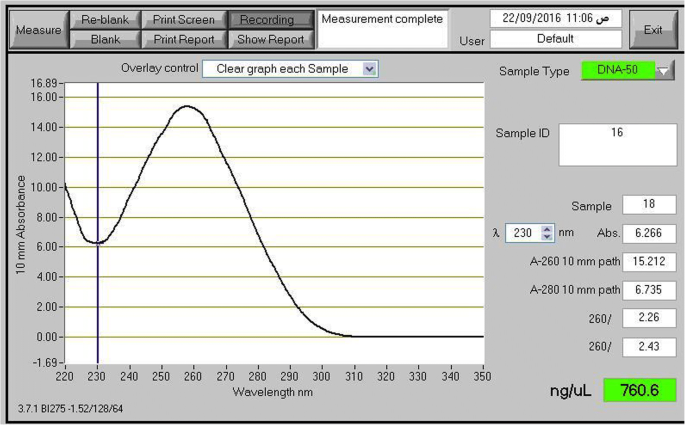
NanoDrop measurement profile. NanoDrop measurement profile of the extracted genomic DNA from the Glycine max sample using the modified protocol
DNA digested with HindIII enzyme. DNA samples extracted by the standardized method and digested with HindIII enzyme. Digested products were separated in 1.5% agarose gel stained with ethidium bromide, in 1 × TAE buffer. The digested products were visualized by UV fluorescence. M is a molecular marker (50 bp)
PCR amplification with RAPD primer (OPZ-09) showed clear and well-differentiated band patterns (Fig. 4 ) in case of DNA samples extracted by the present DNA extraction protocol, whereas genomic DNA extracted by the other method from the same plant seed samples was rather difficult to be amplified (Fig. 4 ). Figure 5 shows the differences in the quality of the PCR amplification products of nptII (173 bp target) in plant samples which were extracted by both the conventional method and by the modified protocol.
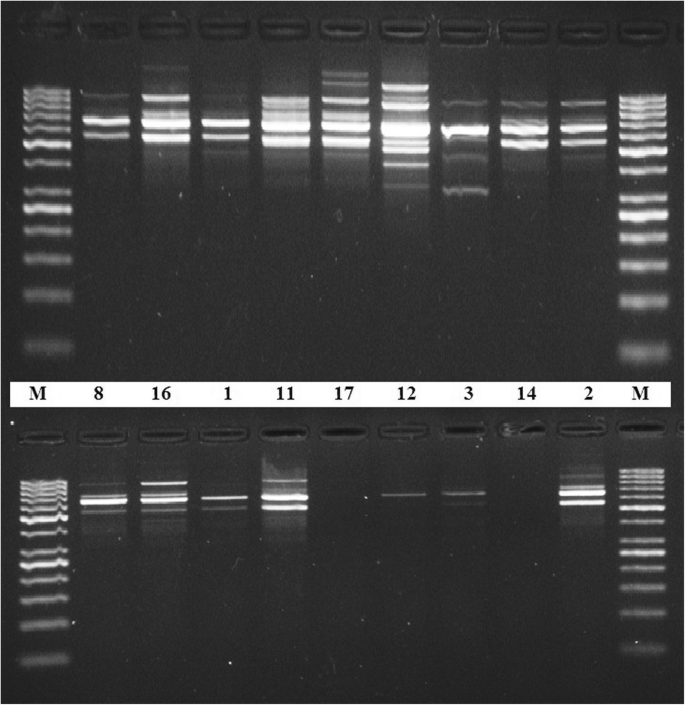
PCR amplification with RAPD primer (OPZ-09). PCR amplification of OPZ-09 primer and electrophoresis separation by 1.5% agarose gel of some DNA samples extracted by the standardized method (upper lane) and the classical method (lower lane). M is for molecular marker (50 bp)
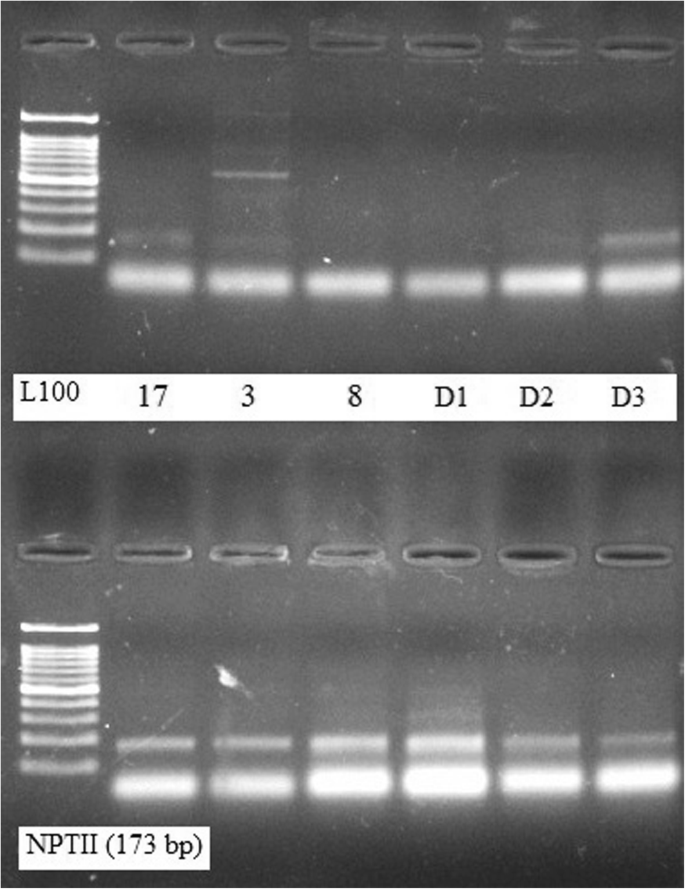
PCR amplification with nptII for detection of GM crops. Quality of the amplification of nptII (173 bp target) in the representative of DNA samples which were extracted by the conventional method (upper lane) and the standardized protocol (lower lane)
The extraction of DNA from plant seeds is an essential step for satisfactory results in molecular studies particularly those involving plant genetics (Junior et al. 2016 ). Different seeds belonging to related genera or different orders contain many components with variable complexities that badly interfere with purity of the extracted DNA and molecular investigations following isolation procedures (Porebski et al. 1997 ; Ribeiro and Lovato 2007 ).
To insure isolation of DNA with better yield and quality from seeds of diverse plant orders, we implemented several steps in the present modified protocol. Liquid nitrogen was used to break the cell wall and disrupt the cell membrane (Clark 1997 ) while keeping cellular enzymes and other undesired chemicals deactivated, thus reducing shearing and damaging of the DNA. Other methods used for disrupting plant tissues, such as digestion with pectinase and cellulose (Manen et al. 2005 ), are not as reproducible or accurate as the use of liquid nitrogen.
High concentration of the 3× CTAB was also used to disrupt the cells and nuclear membranes in order to expose the genetic components (Amani et al. 2011 ). In the present modified method, the 3× CTAB buffer also contains the highest recommended concentration level (0.3%) of 2-β-mercaptoethanol which successfully removed polyphenols (Horne et al. 2004 ; Li et al. 2007 ) giving rise a clear translucent DNA pellet. The CTAB extraction buffer also includes 1.4 M of NaCl which improved the quality of the extracted DNA (Sahu et al. 2012 ).
To remove the remaining polysaccharides during DNA extraction from all plant samples included in the present work, a modification for the precipitation of DNA was also performed by increasing the concentration of sodium chloride and potassium acetate. The concentration of NaCl varied with plant species in a range between 0.7 M (Clark 1997 ) and 6 M (Aljanabi et al. 1999 ; Moreira and Oliveira 2011 ). In the present standardized protocol, we used 6 M NaCl (Moreira and Oliveira 2011 ) and 3 M potassium acetate (Paterson et al. 1993 ). These modifications successfully removed polysaccharides impurities from DNA extracted by this modified protocol from all plant samples and produced pure and high-quality DNA suitable for further molecular analysis. Proteins, most lipids, and cellular debris were removed by binding with non-aqueous compounds and precipitated during the chloroform-isoamyl alcohol step.
Longer incubation of the extracted DNA at − 20 °C also enhanced precipitation of DNA. In general, the quantity and quality of isolated DNA depend on precipitation temperature and duration (Michiels et al. 2003 ). Low-temperature precipitation employed in the present modified protocol increased DNA yield. Extracted DNA were re-suspended in minimum amount of 1× TE buffer since the presence of chelating agents in TE buffer can affect the PCR and other molecular analysis of the extracted DNA.
The method employed in the present work proved to be successful and applicable for extraction of DNA with high yield and purity from 19 different plant species that belong to seven different plant orders. The matrix variation effects on the purity and quality of the isolated genomic DNA were minimized by using the same plant samples as starting materials for both protocols employed in the present investigation.
Electrophoresis separation of DNA extracted by the present protocol showed intense bands very close to the gel wells (Fig. 1 , upper lane) signifying high degree of purity and intact DNA. It is known that the presence of smear could be a sign of degradation of the extracted DNA which easily affects the quality of the subsequent molecular application results (Devi et al. 2013 ).
DNA samples extracted by the present protocol were assessed for successful PCR amplification with RAPD primer (OPZ-09). The presence of clear and well-differentiated band patterns (Fig. 4 ) reflects the efficiency of the protocol to produce genomic DNA with high purity suitable for molecular studies that based on PCR techniques (Devi et al. 2013 ).
Purification of DNA is also an important step for analyzing and measuring genetically modified (GM) food products (Ateş Sönmezoğlu and Keskin 2015 ). The DNA extracted by our standardized protocol yielded detectable and reproducible bands for NPTII (173 bp target) proving its suitability for PCR amplification as well as for the identification of GM crops using the PCR assay.
The A 260 /A 280 purity ratio is an important measure for estimating the polyphenol contamination levels of the extracted DNA. Ratios of A 260 /A 280 below 1.8 render the extracted DNA inappropriate for molecular investigations (Sambrook and Russell 2001 ). Therefore, higher level of 2-β-mercaptoethanol (0.3%) used in the present standardized method successfully removed polyphenols giving rise to translucent final DNA pellets (Suman et al. 1999 ).
In the present modified CTAB-based protocol, although the RNase A enzyme was not used during isolation and purification of DNA, the ratios of absorption A 260 /A 280 of the extracted DNA (Table 2 ) were higher than the recommended optimal limit of DNA purity (Sambrook and Russell 2001 ). Similar results were also observed by Sambrook and Russell ( 2001 ) which were taken to be associated with RNA contamination. In our case, the resulted intact DNA bands, very close to the wells (Fig. 1 , upper lane), indicated high purity of the extracted DNA with no RNA contamination, particularly that the recommended and the most accurate way to determine RNA contamination is to run the sample on an agarose gel where another band of the RNA, if present, will be visible in the gel (Wang et al. 2012 ). Therefore, the higher ratios of absorption A 260 /A 280 in our case may be attributed to slight changes in the pH of the extracted samples (Wilfinger et al. 1997 ).
Polysaccharide contamination was also assessed (Table 2 ) by estimating the absorbance ratio A 260 /A 230 as a secondary measure of nucleic acid purity (Wilson and Walker 2005 ). This ratio is important to evaluate the level of salt residues in the purified DNA. It is recommended to be greater than 1.5 and preferably close to 1.8. The reported values of A 260 /A 230 ratio in most of the DNA plant samples extracted by the present modified protocol are higher than those of the DNA samples extracted by the other classical method.
The principle modifications currently employed for DNA extraction involved the use of higher CTAB concentration and higher levels of 2-β-mercaptoethanol. Additionally, higher concentrations of sodium chloride and potassium acetate were added simultaneously with absolute ice cold isopropanol for the precipitation of DNA free from polysaccharides.
The prescribed modifications in the present method establish a quick and efficient standardized protocol for DNA extraction from different plant orders. These modifications consistently produced pure and high-quality DNA suitable for further molecular analysis. The DNA standardized extraction protocol presented here is important for the assessment of food safety, detection of genetically modified crops, and biodiversity conservation. Therefore, it is of great value for molecular analysis involving large number of different plant samples.
Abbreviations
- Cetyl trimethylammonium bromide
Deoxyribonucleic acid
Genetically modified organisms
Hydrochloric acid
Sodium chloride
Neomycinphosphotransferase II gene
Optical density
Random amplified polymorphic DNA
Aljanabi MS, Forget L, Dookun A (1999) An improved and rapid protocol for the isolation of polysaccharide and polyphenol free sugarcane DNA. Plant Mol Biol Rep 17:1–8
Article Google Scholar
Amani J, Kazemi R, Abbasi AR, Salmanian AH (2011) A simple and rapid leaf genomic DNA extraction method for polymerase chain reaction analysis. Iran J Biotech 9:69
CAS Google Scholar
Ateş Sönmezoğlu Ö, Keskin H (2015) Determination of genetically modified corn and soy in processed food products. J App Biol Biotech 3:032
Google Scholar
Clark MS (ed) (1997) Plant molecular biology- a laboratory manual. Springer, New York, pp 305–328
Devi KD, Punyarani K, Singh S, Devi HS (2013) An efficient protocol for total DNA extraction from the members of order Zingiberales - suitable for diverse PCR based downstream applications. Springer Plus 2:669. https://doi.org/10.1186/2193-180-2-669
Article PubMed Google Scholar
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13
Fang G, Hammar S, Grumet R (1992) A quick and inexpensive method for removing polysaccharides from plant genomic DNA. BioTechniques 13:52–56
CAS PubMed Google Scholar
Haymes KM (1996) Mini-prep method suitable for a plant breeding program. Plant Mol Biol Rep 14:280
Article CAS Google Scholar
Horne EC, Kumpatla SP, Patterson MG, Thompson SA (2004) Improved high-throughput sunflower and cotton genomic DNA extraction and PCR fidelity. Plant Mol Biol Rep. 22:83
Júnior CDS, Teles NMM, Luiz DP, Isabel TF (2016) DNA Extraction from Seeds. In: Micic M, editor. Sample Preparation Techniques for Soil, Plant, and Animal Samples. Springer Protocols Handbooks. Humana Press, New York, pp.265-276. https://doi.org/10.1007/978-1-4939-3185-9_18
Khanuja SPS, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep. 17:1
Li JT, Yang J, Chen DC, Zhang XL, Tang ZS (2007) An optimized mini-preparation method to obtain high-quality genomic DNA from mature leaves of sunflower. Genet Mol Res 6:1064
Loomis MD (1974) Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol 31:528
Manen JF, Sinitsyna O, Aeschbach L, Markov AV, Sinitsyn A (2005) A fully automatable enzymatic method for DNA extraction from plant tissues. BMC Plant Biol 5:23
Michiels A, Van den Ende W, Tucker M, Van Riet L (2003) Extraction of high-quality genomic DNA from latex-containing plants. Anal Biochem 315:85
Mogg RJ, Bond JM (2003) A cheap, reliable and rapid method of extracting high-quality DNA from plants. Mol Ecol Notes 3:666
Moreira PA, Oliveira DA (2011) Leaf age affects the quality of DNA extracted from Dimorphandra mollis (Fabaceae), atropical tree species from the Cerrado region of Brazil. Genet Mol Res 10:353
Novaes RML, Rodrigues JG, Lovato MB (2009) An efficient protocol for tissue sampling and DNA isolation from the stem bark of Leguminosae trees. Genet Mol Res 8:86–96
Paterson AH, Brubaker CL, Wendel JF (1993) A rapid method for extraction of cotton ( Gossypium spp. ) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep. 11:122
Pirttilä MA, Hirsikorpi M, Kämäräinen T, Jaakola L, Hohtola A (2001) DNA isolation methods for medicinal and aromatic plants. Plant Mol Biol Rep. 19:273
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep. 15:8–15
Ribeiro RA, Lovato MB (2007) Comparative analysis of different DNA extraction protocols in fresh and herbarium specimens of the genus Dalbergia. Genet Mol Res 6:173
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA sepacer-length polymorphism in barley: Mendelian inheritance, chromosalmal localtionk, and population dynamic. Proc Natl Acad Sci U S A 81:8014
Article ADS CAS Google Scholar
Sahu SK, Thangaraj M, Kathiresan KDNA (2012) Extraction protocol for plants with high levels of secondary metabolites and polysaccharides without using liquid nitrogen and phenol. Mol Biol 12:1
Sambrook J, Russell DW (2001) Molecular Cloning. A Laboratory Manual, Cold Spring Harbor Laboratory Press, New York
Scott KD, Playford J (1996) DNA extraction technique for PCR in rain forest plant species. Bio Techniques 20:974
Sharma KK, Lavanya M, V A (2000) A method for isolation and purification of peanut genomic DNA suitable for analytical applications. Plant BioTechniques Rep 18:393a
Shepherd M, Cross M, Stokoe RL, Scott LJ (2002) High-throughput DNA extraction from forest trees. Plant Mol Biol Rep. 20:425
Shioda M, Marakami-Muofushi K (1987) Selective inhibition of DNA polymerase by a polysaccharide purified from slime of Physarum polycephalum . Biochem Biophys Res Commun 146:61–66
Silva MN (2010) Extraction of genomic DNA from leaf tissues of mature native species of the Cerrado. Rev. Árvore 34:973–978
Suman PSK, Ajit KS, Darokar MP, Sushil K (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep. 17:1
Wang X, Xiao H, Zhao X, Li C, Ren J, Wang F , Pang L. Isolation of high-quality DNA from a desert plant Reaumuria soongorica, genetic diversity in plants, Mahmut Caliskan (Ed.), ISBN: 978–953–51-0185-7, InTech; 2012. https://doi.org/10.5772/38367
Wilfinger WW, Mackey K, Chomczynski P (1997) Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. BioTechniques 22:474–481
Wilson K, Walker J (2005) Principles and techniques of biochemistry and molecular biology. University Press, Cambridge
Book Google Scholar
Download references
Acknowledgements
Not applicable
The authors declare that this work was funded by the National Research Centre in Egypt (the 11th Research Project Plan, 2016-2019, Project ID: 11040201).
Availability of data and materials
We declare that all data generated or analyzed during this study are included in this article.
Author information
Authors and affiliations.
Cell Biology Department, Genetic Engineering and Biotechnology Research Division, National Research Centre, Cairo, Dokki, 11622, Egypt
Nadia Aboul-Ftooh Aboul-Maaty & Hanaa Abdel-Sadek Oraby
You can also search for this author in PubMed Google Scholar
Contributions
NA-M made substantial contributions to conception and design of the work, involved in conducting the practical section of the work, and also involved in drafting the manuscript. HO made substantial contributions to conception, planning of the work, analysis, and interpretation of results and also involved in drafting the manuscript and revising it critically for important intellectual content, as well as gave the final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Both authors read and approved the final manuscript.
Corresponding author
Correspondence to Hanaa Abdel-Sadek Oraby .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Aboul-Maaty, N.AF., Oraby, H.AS. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull Natl Res Cent 43 , 25 (2019). https://doi.org/10.1186/s42269-019-0066-1
Download citation
Received : 15 August 2018
Accepted : 31 January 2019
Published : 12 February 2019
DOI : https://doi.org/10.1186/s42269-019-0066-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Isolation of nucleic acid
- Plant seeds
- Molecular techniques
- Quality of nucleic acids
- Plants’ genomic DNA
- GMO detection
MIT Technology Review
- Newsletters
Google DeepMind’s new AlphaFold can model a much larger slice of biological life
AlphaFold 3 can predict how DNA, RNA, and other molecules interact, further cementing its leading role in drug discovery and research. Who will benefit?
- James O'Donnell archive page
Google DeepMind has released an improved version of its biology prediction tool, AlphaFold, that can predict the structures not only of proteins but of nearly all the elements of biological life.
It’s a development that could help accelerate drug discovery and other scientific research. The tool is currently being used to experiment with identifying everything from resilient crops to new vaccines.
While the previous model, released in 2020, amazed the research community with its ability to predict proteins structures, researchers have been clamoring for the tool to handle more than just proteins.
Now, DeepMind says, AlphaFold 3 can predict the structures of DNA, RNA, and molecules like ligands, which are essential to drug discovery. DeepMind says the tool provides a more nuanced and dynamic portrait of molecule interactions than anything previously available.
“Biology is a dynamic system,” DeepMind CEO Demis Hassabis told reporters on a call. “Properties of biology emerge through the interactions between different molecules in the cell, and you can think about AlphaFold 3 as our first big sort of step toward [modeling] that.”
AlphaFold 2 helped us better map the human heart , model antimicrobial resistance , and identify the eggs of extinct birds , but we don’t yet know what advances AlphaFold 3 will bring.
Mohammed AlQuraishi, an assistant professor of systems biology at Columbia University who is unaffiliated with DeepMind, thinks the new version of the model will be even better for drug discovery. “The AlphaFold 2 system only knew about amino acids, so it was of very limited utility for biopharma,” he says. “But now, the system can in principle predict where a drug binds a protein.”
Isomorphic Labs, a drug discovery spinoff of DeepMind, is already using the model for exactly that purpose, collaborating with pharmaceutical companies to try to develop new treatments for diseases, according to DeepMind.
AlQuraishi says the release marks a big leap forward. But there are caveats.
“It makes the system much more general, and in particular for drug discovery purposes (in early-stage research), it’s far more useful now than AlphaFold 2,” he says. But as with most models, the impact of AlphaFold will depend on how accurate its predictions are. For some uses, AlphaFold 3 has double the success rate of similar leading models like RoseTTAFold. But for others, like protein-RNA interactions, AlQuraishi says it’s still very inaccurate.
DeepMind says that depending on the interaction being modeled, accuracy can range from 40% to over 80%, and the model will let researchers know how confident it is in its prediction. With less accurate predictions, researchers have to use AlphaFold merely as a starting point before pursuing other methods. Regardless of these ranges in accuracy, if researchers are trying to take the first steps toward answering a question like which enzymes have the potential to break down the plastic in water bottles, it’s vastly more efficient to use a tool like AlphaFold than experimental techniques such as x-ray crystallography.
A revamped model
AlphaFold 3’s larger library of molecules and higher level of complexity required improvements to the underlying model architecture. So DeepMind turned to diffusion techniques, which AI researchers have been steadily improving in recent years and now power image and video generators like OpenAI’s DALL-E 2 and Sora. It works by training a model to start with a noisy image and then reduce that noise bit by bit until an accurate prediction emerges. That method allows AlphaFold 3 to handle a much larger set of inputs.
That marked “a big evolution from the previous model,” says John Jumper, director at Google DeepMind. “It really simplified the whole process of getting all these different atoms to work together.”
It also presented new risks. As the AlphaFold 3 paper details, the use of diffusion techniques made it possible for the model to hallucinate, or generate structures that look plausible but in reality could not exist. Researchers reduced that risk by adding more training data to the areas most prone to hallucination, though that doesn’t eliminate the problem completely.
Restricted access
Part of AlphaFold 3’s impact will depend on how DeepMind divvies up access to the model. For AlphaFold 2, the company released the open-source code , allowing researchers to look under the hood to gain a better understanding of how it worked. It was also available for all purposes, including commercial use by drugmakers. For AlphaFold 3, Hassabis said, there are no current plans to release the full code. The company is instead releasing a public interface for the model called the AlphaFold Server , which imposes limitations on which molecules can be experimented with and can only be used for noncommercial purposes. DeepMind says the interface will lower the technical barrier and broaden the use of the tool to biologists who are less knowledgeable about this technology.
Artificial intelligence
Sam altman says helpful agents are poised to become ai’s killer function.
Open AI’s CEO says we won’t need new hardware or lots more training data to get there.
Is robotics about to have its own ChatGPT moment?
Researchers are using generative AI and other techniques to teach robots new skills—including tasks they could perform in homes.
- Melissa Heikkilä archive page
What’s next for generative video
OpenAI's Sora has raised the bar for AI moviemaking. Here are four things to bear in mind as we wrap our heads around what's coming.
- Will Douglas Heaven archive page
An AI startup made a hyperrealistic deepfake of me that’s so good it’s scary
Synthesia's new technology is impressive but raises big questions about a world where we increasingly can’t tell what’s real.
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Will Knight
Google DeepMind’s Groundbreaking AI for Protein Structure Can Now Model DNA
Google spent much of the past year hustling to build its Gemini chatbot to counter ChatGPT , pitching it as a multifunctional AI assistant that can help with work tasks or the digital chores of personal life. More quietly, the company has been working to enhance a more specialized artificial intelligence tool that is already a must-have for some scientists.
AlphaFold , software developed by Google’s DeepMind AI unit to predict the 3D structure of proteins, has received a significant upgrade. It can now model other molecules of biological importance, including DNA, and the interactions between antibodies produced by the immune system and the molecules of disease organisms. DeepMind added those new capabilities to AlphaFold 3 in part through borrowing techniques from AI image generators.
“This is a big advance for us,” Demis Hassabis , CEO of Google DeepMind, told WIRED ahead of Wednesday’s publication of a paper on AlphaFold 3 in the science journal Nature . “This is exactly what you need for drug discovery: You need to see how a small molecule is going to bind to a drug, how strongly, and also what else it might bind to.”
AlphaFold 3 can model large molecules such as DNA and RNA, which carry genetic code, but also much smaller entities, including metal ions. It can predict with high accuracy how these different molecules will interact with one another, Google’s research paper claims.
The software was developed by Google DeepMind and Isomorphic labs, a sibling company under parent Alphabet working on AI for biotech that is also led by Hassabis. In January, Isomorphic Labs announced that it would work with Eli Lilly and Novartis on drug development.
AlphaFold 3 will be made available via the cloud for outside researchers to access for free, but DeepMind is not releasing the software as open source the way it did for earlier versions of AlphaFold. John Jumper, who leads the Google DeepMind team working on the software, says it could help provide a deeper understanding of how proteins interact and work with DNA inside the body. “How do proteins respond to DNA damage; how do they find, repair it?” Jumper says. “We can start to answer these questions.”
Understanding protein structures used to require painstaking work using electron microscopes and a technique called x-ray crystallography. Several years ago, academic research groups began testing whether deep learning , the technique at the heart of many recent AI advances, could predict the shape of proteins simply from their constituent amino acids, by learning from structures that had been experimentally verified.
In 2018, Google DeepMind revealed it was working on AI software called AlphaFold to accurately predict the shape of proteins. In 2020, AlphaFold 2 produced results accurate enough to set off a storm of excitement in molecular biology. A year later, the company released an open source version of AlphaFold for anyone to use, along with 350,000 predicted protein structures, including for almost every protein known to exist in the human body. In 2022 the company released more than 2 million protein structures.

Reece Rogers

Louryn Strampe

Brendan I. Koerner

Scott Gilbertson
The latest AlphaFold’s ability to model different proteins was improved in part through an algorithm called a diffusion model that helps AI image generators like Dall-E and Midjourney create weird and sometimes photo-real imagery. The diffusion model inside AlphaFold 3 sharpens the molecular structures the software generates. The diffusion model is able to generate plausible protein structures based on patterns it picked up from analyzing a collection of verified protein structures, much as an image generator learns from real photographs how to render realistic-looking snapshots.
AlphaFold 3 is not perfect, though, and offers a color-coded confidence scale for its predictions. Areas of a protein structure colored blue indicate high confidence, while red areas show less certainty.
David Baker , a professor at the University of Washington who leads a group working on techniques for protein design, has competed with AlphaFold. In 2021, before DeepMind open sourced its creation, his team released an independent protein-structure prediction inspired by AlphaFold. His own lab recently released a diffusion model to help model a wider range of molecular structures, but he concedes that AlphaFold 3 is more capable. “The structure prediction performance of AlphaFold 3 is very impressive,” Baker says.
Baker adds that it is a shame that the source code for AlphaFold 3 has not been released to the scientific community.
Hassabis, who leads all of Alphabet’s AI initiatives, has long taken a special interest in the potential for AI to accelerate scientific research . But he says the latest techniques being developed for AlphaFold, a highly specialized AI system, could prove useful for building more general systems that aim to exceed human capabilities on many dimensions.
If AI programs like Google’s Gemini become a lot more capable over the next decade, he says, “you could imagine them using things like AlphaFold as tools, to achieve some other goal.”
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
He emptied a crypto exchange onto a thumb drive —then disappeared
The real-time deepfake romance scams have arrived
Boomergasms are booming
Heading outdoors? Here are the best sleeping bags for every adventure

Steven Levy

Lauren Goode

Julian Chokkattu

share this!
May 9, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New DNA origami technique promises advances in medicine
by University of Portsmouth
A new technique in building DNA structures at a microscopic level has the potential to advance drug delivery and disease diagnosis, a study suggests.
A team of scientists, from the universities of Portsmouth and Leicester in the UK, has developed an innovative way to customize and strengthen DNA origami.
DNA origami is the method of creating nanostructures with remarkable precision using DNA strands as building blocks. However, these structures are delicate and can fall apart easily under biological conditions, like changes in temperature or exposure to certain enzymes found in living organisms.
In a paper, published in the Journal of the American Chemical Society , researchers have presented a unique way to make the origami structures stronger and more versatile in a one-pot reaction, via a process known as triplex-directed photo-cross-linking.
By strategically modifying DNA strands during the design process , they were able to introduce additional nucleotide sequences—which are the basic building blocks of DNA—that serve as attachment points for functional molecules.
Attachment of the molecules was achieved by using triplex-forming oligonucleotides carrying a cross-linking agent. They then used a chemical process involving UVA light to permanently link these molecules to the DNA shapes.
A particular benefit of this approach is the generation of "super-staples" that act to weave the structure together. The paper says cross-linking to regions outside of the origami core dramatically reduces the structure's sensitivity to heat and disassembly by enzymes.
Senior author, Dr. David Rusling from the University of Portsmouth's School of Pharmacy and Biomedical Sciences, said, "The potential applications of this technique are far-reaching. The ability to tailor DNA origami structures with specific functionalities holds immense promise for advancing medical treatments and diagnostics.
"We envision a future where DNA origami structures could be used to deliver drugs or DNA directly to diseased cells, or to create highly sensitive diagnostic tools."
Current applications of DNA origami in biomedicine include vaccines, biological nanosensors, drug delivery , structural biology , and delivery vehicles for genetic materials.
Co-author Dr. Andrey Revyakin, formerly from the University of Leicester, said, "My lab has struggled for years to make DNA origami structures that remain functional in real-life biological applications. Dr. Rusling's triplex-based method, which 'upgrades' the classical DNA double-helix with an additional, third strand, stabilizes the DNA shapes, and does so with great precision, without affecting the functional modules of the molecule."
The paper says the new strategy is scalable and cost-effective, as it works with existing origami structures, does not require scaffold redesign, and can be achieved with just one DNA strand.
Dr. Rusling added, "What is really exciting about this technique is that it did not change the underlying origami DNA sequence, offering the ability to use these structures as carriers for synthetic genes."
Journal information: Journal of the American Chemical Society
Provided by University of Portsmouth
Explore further
Feedback to editors

Physicists propose path to faster, more flexible robots
6 hours ago

Scientists develop new geochemical 'fingerprint' to trace contaminants in fertilizer
18 hours ago
Study reveals how a sugar-sensing protein acts as a 'machine' to switch plant growth—and oil production—on and off

Researchers develop world's smallest quantum light detector on a silicon chip

How heat waves are affecting Arctic phytoplankton

Horse remains show Pagan-Christian trade networks supplied horses from overseas for the last horse sacrifices in Europe
Ion irradiation offers promise for 2D material probing
19 hours ago

Furry thieves are running loose in a Maine forest, research shows

Study indicates Earth's earliest sea creatures drove evolution by stirring the water
20 hours ago
Chemists develop new method for introducing fluorinated components into molecules
Relevant physicsforums posts, and now, here comes covid-19 version ba.2, ba.4, ba.5,....
May 17, 2024
Is it usual for vaccine injection site to hurt again during infection?
May 16, 2024

A Brief Biography of Dr Virgina Apgar, creator of the baby APGAR test
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
The cass report (uk).
May 1, 2024
Is 5 milliamps at 240 volts dangerous?
Apr 29, 2024
More from Biology and Medical
Related Stories

Bridge in a box: Unlocking origami's power to produce load-bearing structures
Mar 18, 2024
Capturing DNA origami folding with a new dynamic model
Apr 18, 2024

New method can manipulate the shape and packing of DNA
Jun 16, 2023
Researchers devise genetically encoded DNA origami for targeted and precise gene therapy in vivo
Apr 25, 2023

DNA origami enables fabricating superconducting nanowires
Jan 19, 2021

Researchers craft 'origami DNA' to control virus assembly
Jul 17, 2023
Recommended for you
Nanocarriers loaded with DNA relieve back pain, repairs damaged disk in mice

Engineering a new color palette for single-molecule imaging
May 15, 2024
Scientists from Prague are expanding the possibilities of using RNA in gene medicine
New gel breaks down alcohol in the body
May 13, 2024
Nanoparticle plant virus treatment shows promise in fighting metastatic cancers in mice
Research explores ways to mitigate the environmental toxicity of ubiquitous silver nanoparticles
May 11, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

DNA Manipulation and Single-Molecule Imaging
Shunsuke takahashi.
1 Division of Life Science and Engineering, School of Science and Engineering, Tokyo Denki University, Hatoyama-cho, Hiki-gun, Saitama 350-0394, Japan; pj.ca.iadned.liam@ihsahakats
Masahiko Oshige
2 Department of Environmental Engineering Science, Graduate School of Science and Technology, Gunma University, Kiryu, Gunma 376-8515, Japan; pj.ca.u-amnug@egihso
3 Gunma University Center for Food Science and Wellness (GUCFW), Maebashi, Gunma 371-8510, Japan
Shinji Katsura
Associated data.
Not applicable.
DNA replication, repair, and recombination in the cell play a significant role in the regulation of the inheritance, maintenance, and transfer of genetic information. To elucidate the biomolecular mechanism in the cell, some molecular models of DNA replication, repair, and recombination have been proposed. These biological studies have been conducted using bulk assays, such as gel electrophoresis. Because in bulk assays, several millions of biomolecules are subjected to analysis, the results of the biological analysis only reveal the average behavior of a large number of biomolecules. Therefore, revealing the elementary biological processes of a protein acting on DNA (e.g., the binding of protein to DNA, DNA synthesis, the pause of DNA synthesis, and the release of protein from DNA) is difficult. Single-molecule imaging allows the analysis of the dynamic behaviors of individual biomolecules that are hidden during bulk experiments. Thus, the methods for single-molecule imaging have provided new insights into almost all of the aspects of the elementary processes of DNA replication, repair, and recombination. However, in an aqueous solution, DNA molecules are in a randomly coiled state. Thus, the manipulation of the physical form of the single DNA molecules is important. In this review, we provide an overview of the unique studies on DNA manipulation and single-molecule imaging to analyze the dynamic interaction between DNA and protein.
1. Introduction
Single-molecule imaging and DNA manipulation provide new analytical methods in molecular biology and biochemistry [ 1 , 2 , 3 , 4 , 5 ]. The advancements in microscope and fluorescence imaging technology for biomolecules enabled the direct observation of DNA and proteins at the single-molecule level. Unlike electron microscopy (EM) [ 6 ] and atomic force microscopy (AFM) [ 7 ], fluorescence single-molecule imaging technologies allow direct observation of fluorescently labeled biomolecules in an aqueous solution. This makes the determination of the dynamic behavior of individual biomolecules possible [ 2 , 3 ]. Information obtained from single-molecule imaging and DNA manipulation contains not only the average behavior of biomolecules but also the distribution of the dynamic behavior of individual biomolecules. For the past two decades, single-molecule imaging and DNA manipulation have provided insights into biological processes based on the dynamic behavior of individual biomolecules. Here, we describe the features and measurements of single-molecule imaging and DNA manipulation.
1.1. Statistical Average in Biological Fields
In a conventional bulk assay, several millions of biomolecules are submitted for analysis. As a result, the bulk assay provides only the average behavior of molecules. The summation of the behaviors of a large number of molecules converges on a Gaussian distribution due to the central limit theorem in basic statistics, despite the difference between the source distribution of individual molecules and Gaussian distribution. As an example, two model distributions are assumed, whose averages and standard deviations are completely equal. Even if the original distributions, which show the behavior of the individual samples, are completely different, the summation generates quite similar distributions. Thus, distinguishing between the two Gaussian distributions is difficult. This is because the summation of individual distributions of 100 samples corresponds to the average of the behaviors of 100 molecules. Thus, conventional bulk assays are conducted to provide information on only the average behavior of a large number of molecules, but not on the distribution of individual molecules.
1.2. Flexibility of DNA Molecule as a Polymer
The DNA molecule is a long-chain polymer that consists of two antiparallel polynucleotide chains that coil around each other to form a double helix [ 8 , 9 ]. Non-B-DNA structures, such as A-form or Z-form, are induced by a high salt or high ethanol concentration [ 10 , 11 , 12 ], whereas the double helix of DNA in an aqueous solution of low ion concentration forms a B-DNA structure. Both the physical form and positions of the individual DNA molecules are easily changed by external forces in an aqueous solution [ 13 , 14 , 15 ]. However, the physical form of the DNA molecules fluctuates in the absence of external forces due to the Brownian motion derived from the flexibility of the DNA molecules [ 16 , 17 , 18 ]. This is because the DNA molecules tend to increase the variety of physical forms due to the increase in entropy. In particular, DNA molecules in an aqueous solution exhibit a right-handed double-helical B form structure [ 19 ]. Conversely, circular DNA and terminal immobilized linear DNA molecules induce a supercoiled state. In particular, high negative superhelicity locally induces non-B DNA structures, such as interior loops, cruciforms, and Z-forms [ 20 , 21 , 22 ]. Essentially, the energy necessary for DNA supercoiling is almost of the same order as the thermal energy at room temperature. Local DNA supercoiling with various densities can be easily induced by thermal agitation at room temperature in conventional bulk assays [ 23 ]. This indicates that understanding the physiological role of the structure of DNA molecules in biological processes, such as DNA replication, repair, and recombination, is extremely difficult; even the supercoiled state of DNA is controlled in advance. Therefore, it is important to control the physical form of DNA molecules in situ.
1.3. Flexibility of Genomic DNA Molecules
Long-chain DNA molecules over 100 kb are easily broken up by shear stress, along with flow and vortex. Thus, the size of the DNA molecules that can be handled in an aqueous solution is limited to 100 kb. However, in the fields of life science, such as genomic engineering and synthetic biology, it is important to handle genomic DNA without fragmentation (e.g., in the artificial synthesis of genomic DNA and transfection or transformation of synthesized long-chain DNA into cells) [ 24 , 25 , 26 , 27 , 28 , 29 ]. Genomic DNA molecules can be chemically aggregated using condensing agents, such as polyethylene glycol (PEG) and/or low-molecular-weight inorganic salts ( Figure 1 A) [ 30 , 31 , 32 ]. Globular DNA molecules can be prepared via phase transition from a randomly coiled structure to a globular structure ( Figure 1 B) [ 33 , 34 ]. Because the globular DNA molecule is protected from physical stress, it can be manipulated without fragmentation induced by shear stress [ 35 , 36 ]. The reversible transition of the globular structure of the DNA molecules to the randomly coiled structure is performed by removing the PEG and/or salt [ 37 ].

Outline of reversible phase transition between the randomly coiled state and globular state of the DNA molecules. ( A ) The phase transition of the physical form of DNA molecule using condensing agents. By adding condensing agents, the DNA molecule is transformed from a randomly coiled structure to a globular structure. By removing condensing agents, the globular DNA molecule reverts to the randomly coiled structure. The phase transition between the globular and the randomly coiled structures can be reversibly induced in a DNA molecule. ( B ) Relationship of PEG and salt concentrations with the phase transition between the globular and the randomly coiled structures. The dashed line denotes the phase transition between the randomly coiled structure and the equilibrium states, which involve the globular and the randomly coiled structures. The dot chain line denotes the phase transition between the globular structure and the equilibrium states, which involve the globular and the randomly coiled structures.
2. DNA Manipulation
The conformation of DNA molecules in an aqueous solution flexibly fluctuates due to Brownian motion (see Section 1.2 ). The DNA molecules behave as a wormlike chain, and voluntarily shorten the distance between the ends as their entropies increase. Thus, fluorescently stained DNA molecules appear as bright spots under a fluorescence microscopic field. Therefore, measuring the full length of the single DNA molecules under the relaxed state is difficult. Even though bright spots are detected, it is difficult to determine the contour lengths between the bright spots of the protein bound to DNA and the DNA terminus in randomly coiled DNA molecules. For this reason, the manipulation of the physical form of the DNA molecules is necessary for obtaining information on the binding sites of proteins.
Here, we introduce several methods for single DNA molecule manipulation that have been developed: (1) DNA manipulation by orientation forces, (2) DNA manipulation by optical tweezers, (3) the DNA manipulation of terminal immobilized DNA by fluid flow or electric fields, (4) DNA manipulation by magnetic tweezers, (5) DNA manipulation by interface movement, (6) DNA origami, and (7) nanopores and DNA manipulation.
2.1. DNA Manipulation by Orientation Forces
The application of an electric field to linear DNA molecules results in the local induction of charges opposite to the closer electrode on the DNA molecules. Figure 2 presents the orientation force exerted on DNA molecules. Each end of the charged DNA molecules is attracted by the Coulomb force toward the near electrode, which results in the stretching of the DNA molecules toward each electrode [ 38 , 39 ]. However, when a direct current (DC) electric field is applied, fluid flow is generated by bubbles with the electrode reactions, thus preventing the manipulation of DNA molecules. Conversely, when an alternating current (AC) electric field with cycling for the positive half-cycle and negative half-cycle is applied, the fluid flow generation by bubbles with the electrode reactions is significantly suppressed. This is because the electrode reactions generated during the positive half-cycle can be canceled by those generated during the negative half-cycle. Therefore, the application of an AC electric field is suitable for DNA manipulation by orientation forces ( Figure 2 A) [ 40 , 41 ]. The dielectrophoretic force (the interaction between an induced dipole on the molecule and a nonuniform electric field) is generated according to the dielectric properties of the molecule and medium. In a positive dielectrophoretic force, the molecule, which is more polarizable than the medium, is attracted in a direction toward the region of high electric field gradients. On the other hand, in a negative dielectrophoretic force, the molecule, which is less polarizable than the medium, is attracted in a direction away from the region of high electric field gradients. By controlling the dielectrophoresis force, single DNA molecules were manipulated by the application of an AC electric field with a frequency of 1 MHz at a field strength of 1 MV/m ( Figure 2 B) [ 42 ]. The application of an AC electric field enables the efficient alignment of hundreds of individual DNA molecules under fluorescence microscopic fields. The field strength required for DNA manipulation, i.e., 1 MV/m, is quite high; therefore, electrode miniaturization is necessary to generate a sufficient electric field. Electrode miniaturization also suppresses heat generation by Joule heating, thus hindering the manipulation of DNA molecules. For this reason, it should be noted that a higher salt concentration causes significant heat generation and electrode reactions. For DNA manipulation by the electric fields, it is important to keep the solution under a low ionic strength condition in the concentration range 1–100 µM for multivalent cations, such as calcium ions, magnesium ions, zinc ions and aluminum ions, that reduce polarization by binding to the DNA backbone [ 40 ].

Outline of the stretching of the DNA molecule by orientation manipulation. The charge is induced on the single DNA molecule by the electric field. The Coulomb forces are exerted on the induced charges, and then, the DNA molecule is stretched by the Coulomb forces. ( A ) The stretching process for DNA molecule by the application of AC electric field. ( B ) The stretching of DNA molecule by orientational force.
2.2. DNA Manipulation by Optical Tweezers
The physical principles of laser trapping were discovered by Arthur Ashkin in 1970 [ 43 , 44 ]. In laser trapping, an extremely bright and squeezed laser beam is refracted by a particle at the focal point. The photons that have momentum exert a force on the microparticle during refraction. Due to this power, particles are trapped without physical contact around the focal point of the laser beam. The manipulation technology for the particle position is called optical tweezers [ 45 , 46 ]. However, the manipulation of randomly coiled DNA molecules by optical tweezers is extremely difficult. Thus, by attachment to a particle at one end of the linear DNA molecule, the single DNA molecule is indirectly manipulated by the trapped particle by optical tweezers. The indirectly manipulated single DNA molecules can be stretched by the fluid flow or an electric field [ 47 , 48 , 49 ]. However, the fluid flow cannot uniformly stretch the trapped single DNA molecules. To enable the uniform stretching of single DNA molecules, they need to be physically stretched from one end to the other end using particles via laser trapping. Figure 3 demonstrates that one end of a single DNA molecule is immobilized on the surface, whereas the other end is attached to a particle. The manipulation of the particle under this condition enables the uniform stretching of the trapped single DNA molecules [ 50 , 51 ]. The use of dual-beam optical tweezers can successfully stretch DNA molecules whose termini are attached to different particles and control their tension based on the particle position [ 52 , 53 ]. The development of optical tweezers has made it possible to determine the force in the order of piconewtons to nanonewtons, which facilitates progressive analyses of the dynamic interaction between DNA and protein at a single-molecule level [ 54 ].

Physical manipulation of a single DNA molecule attached to microbeads using optical tweezers. Conceptual diagram of DNA manipulation by optical tweezers.
2.3. Manipulation of Genomic DNA by Laser Trapping
As described in Section 2.2 , the application of laser trapping causes the particles to be spatially trapped without physical contact at the focal position of the laser beam. However, the trapping of randomly coiled DNA molecules in an aqueous solution is extremely difficult. This is because there is no significant difference in the refractive index between water molecules and low-density randomly coiled DNA molecules in the solvent. However, high-density globular DNA can be optically trapped using a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser at a wavelength of 1064 nm. Figure 4 presents the trapping of globular DNA by a laser beam [ 55 ]. As can be seen from the figure, the trapped globular DNA is not moved simultaneously with the microscope stage, whereas the nontrapped globular DNA is moved simultaneously with the microscope stage ( Figure 4 A–D). Thus, globular DNA manipulation can be achieved by laser trapping.

Direct laser trapping of the globular DNA molecule. Schematic illustration of the changes in the positions of free and trapped globular DNA molecules induced by the movement of the microscope stage. ( A ) A globular DNA molecule was trapped by laser. ( B ) When the microscope stage moved to the left, the free globular DNA molecule moved to the left position from (a) to (b) with the movement of the microscope stage. On the other hand, the trapped globular DNA molecule stayed at the upper right position of the microscope stage. ( C ) When the microscope stage moved up, the free globular DNA molecule moved to the upper position from (b) to (c) with the movement of the microscope stage. On the other hand, the trapped globular DNA molecules stayed at the upper right position of the microscope stage. ( D ) Merging of steps A, B and C. Refer to [ 55 ] for details.
2.4. DNA Manipulation by Electric Field and Fluid Flow
In a microchannel device, individual linear DNA molecules with one end immobilized on a glass surface can be stretched by the fluid flow or an electric field generated using miniaturized electrodes ( Figure 5 A,B). The application of a DC electric field at 10 V/cm enabled the stretching of single DNA molecules without a fluid flow in a microchannel [ 56 , 57 ]. However, the single DNA molecules were not stretched without both the fluid flow and DC electric field, which resulted in the randomly coiled state [ 57 ]. This indicates that the negative charge of the phosphate backbone of DNA strands was attracted toward the anode upon applying a DC electric field. In terms of the fluid flow, the individual linear DNA molecules can be stretched by manipulating the fluid flow using a microsyringe pump in a microchannel ( Figure 5 B) [ 58 ]. This is because the fluid flow generates an external force to transform the physical form of the single DNA molecules from the randomly coiled state to the stretched state. The solution can be controlled at the reaction field by exchanging with the solution that is newly injected into the microchannel device. Upon applying the fluid flow and electric field, several dozens of stretched single DNA molecules can be observed under the same fluorescence microscopic fields. This enables an efficient analysis of the dynamic interaction between DNA and protein in a high-throughput manner. In particular, by using a simple micro- or nanofabricated glass surface with lipid bilayer coating, a single-molecule technique, called DNA curtains, has been developed to align the arbitrary patterns of thousands of single DNA molecules in a microchannel [ 59 , 60 , 61 , 62 , 63 ]. This technique has provided a powerful experimental platform for the concurrent observation of hundreds or thousands of dynamic interactions between DNA and protein [ 5 ].

Conceptual diagram of the manipulation of single DNA molecules with one end immobilized on a surface. ( A ) Stretching manipulation of single DNA molecules by a DC electric field. The DNA molecule is relaxed without the application of electric field but stretched with the application of it. ( B ) Stretching of single DNA molecule by the fluid flow. The DNA molecule is relaxed without the fluid flow but stretched with the fluid flow.
Contrary to the method using optical tweezers (see Section 2.2 ), stretching methods based on fluid flow or electric fields do not provide uniform stretching of the DNA molecules. This is because the local force for the stretching of the linear DNA molecules is approximately proportional to the contour length from the corresponding point to the free end of the DNA molecule. Thus, the stretching force around the free end region of the linear DNA molecule is much less than that around the immobilized end region of the DNA molecule.
2.5. DNA Manipulation by Magnetic Tweezers
Magnetic tweezers have been utilized to manipulate single DNA molecules and to conduct analysis of the mechanical properties of the interaction between DNA and protein at a single-molecule level [ 64 , 65 ]. A single DNA molecule is tethered to a surface at one end and attached to a magnetic particle at the other end. By generating a magnetic field using external magnets, a force is exerted on the single DNA molecule bound to the magnetic particle. The force applied to the single DNA molecule can be determined from both the applied magnetic force and fluctuations of the magnetic particle position. Thus, by determining the applied force, the force of the action and behavior of protein with the single DNA molecule can be determined by tracking the magnetic particle [ 64 , 65 ]. In addition, magnetic tweezers can generate a rotating magnetic field via a magnet rotation; therefore, they can induce supercoiling of a specified density in the single DNA molecule ( Figure 6 ). For these reasons, magnetic tweezers can be used to analyze the mechanical properties of the various physical forms of DNA, which change in response to DNA-binding protein and enzyme activity [ 66 , 67 , 68 , 69 ]. However, tracking the behavior of magnetic beads makes it difficult to analyze the position of protein bound to a single DNA molecule and that of the non-B DNA structures induced by supercoiling.

Induction of supercoiling in the single DNA molecules by magnetic tweezers.
By using a fluorescence microscope equipped with magnetic tweezers in a microchannel, a single-molecule manipulation system has been developed [ 70 , 71 ]. This system can be used to control the supercoiling density of single DNA molecules. Single DNA molecules are supercoiled by rotating the magnetic field generated by rotating a magnet above the microscope stage. By placing the magnet downstream of the microflow channel, the supercoiled single DNA molecules are stretched, and this can be observed under a microscope. By using magnetic tweezers, the full length of the supercoiled single DNA molecules can be directly observed under a fluorescence microscopic field.
2.6. DNA Manipulation by Interface Movement
Around an interface between a liquid phase and a solid phase, or between a liquid phase and a vapor phase, DNA molecules, which are highly hydrophilic materials, tend to maximize the contact area in an aqueous solution. By moving the interfaces between the vapor, liquid, and solid phases, some DNA molecules in the liquid are left behind the interface, which results in the stretching of the DNA molecules [ 72 , 73 , 74 ]. Based on these properties, the stretching methods for individual DNA molecules have been used in optical mapping. In the molecular combing method, DNA with one end immobilized on a glass surface was stretched by moving interfaces between the vapor, liquid, and solid phases [ 74 , 75 ]. For example, the following molecular combing methods have been developed: (i) the stretching of DNA molecules by sliding another coverslip to a droplet containing DNA molecules on a coverslip [ 72 , 76 ], (ii) the stretching of DNA molecules by the air blowing of a droplet containing DNA molecules on a coverslip [ 77 ], (iii) the stretching of DNA molecules by DNA molecule absorption using filter paper [ 78 ], and (vi) the dipping of a coverslip into a solution containing DNA molecules and then the stretching of the DNA molecules by lifting up the coverslip (dynamic molecular combing) [ 79 , 80 ]. In another attractive approach, droplets containing DNA molecules are slid along a slope of a coverslip modified using 3-aminopropyltriethoxysilane (APTES) ( Figure 7 A) [ 81 , 82 ]. This method is based on the adsorption of the DNA molecules with a negative charge of the phosphate backbone on the amino silane-treated glass surface. The sliding of the droplets enables the movement of the interface between the vapor, liquid, and solid phases, which results in the stretching of the single DNA molecules ( Figure 7 B). In this method, the sliding speed of the droplet can be controlled by the slope angle of the slide glass. Moreover, the sample can be recovered using Parafilm placed under the slide glass, which reduces the amount of droplet that needs to be used. Using molecular combing methods, the sequence-specific positions of stretched DNA molecules can be utilized as optical mapping, facilitating the application of genome-wide analysis (see Section 3.2 ).

Stretching single DNA molecules using the molecular combing method. ( A ) Conceptual diagram of the stretching manipulation of single DNA molecules by using the moving droplet method. ( B ) Image of the stretched single DNA molecules. Refer to [ 81 ] for details.
2.7. DNA Origami and Control of DNA Folding
DNA, the genetic information carrier, has been playing wide roles as an attractive material for the construction of nanoscale structures [ 83 , 84 , 85 ]. Pioneering work by Paul Rothemund has significantly expanded the potential for designing and building arbitrarily shaped, complex, two- and three-dimensional nanostructures with DNA (DNA origami) [ 86 ]. DNA origami is achieved by controlling self-folding based on the molecular DNA sequence. In the DNA-origami technique, a long single-stranded DNA (scaffold DNA) is hybridized with hundreds of short synthetic DNA oligonucleotides around 30 nt in length (staple strands). Each staple strand is designed to be complementary in sequence to different parts of the scaffold DNA [ 87 ]. For this reason, the staple strands are hybridized to distant parts of the scaffold DNA (e.g., crossover points), resulting in inducing the mechanical folding of the scaffold DNA into a designed nanostructure. The development of DNA-origami research has dramatically improved the complexity of DNA designs for building 2D and 3D nanostructures. Thus, DNA-origami-frame-based nanotechnology has been used in various research fields including DNA–protein interactions [ 88 , 89 ], the nanofabrication of nanoelectronics and nanophotonics devices [ 90 , 91 , 92 ], biosensors [ 93 , 94 ], nanopores [ 95 , 96 ], and drug-delivery systems [ 97 , 98 ].
In several previous studies, the dynamic behavior of and interaction between DNA and protein have been directly observed by high-speed AFM (see Section 3.7 ) [ 99 , 100 ]. However, the conformation of DNA molecules in an aqueous solution flexibly fluctuates due to Brownian motion, as described in Section 1.2 . Therefore, it is difficult to obtain the dynamic behavior of and interaction between protein and relaxed DNA. To manipulate single DNA molecules at the nanolevel, a DNA-origami structure, called a DNA-origami frame, was designed and built by the group of Paul Rothemund et al. The DNA origami frame can accommodate two different DNA fragments in the cavity, resulting in incorporating any modified DNA and RNA strands [ 101 ]. The physical form such as tensions, rotations, and the torsion of incorporated DNA can be manipulated by inducing two different DNA strands into four connection sites in the DNA-origami frame [ 102 ]. In addition, the orientations of two dsDNA strands or four ssDNA strands can be manipulated via the DNA-origami frame, resulting in manipulating the arrangement of the incorporated DNA strands. The four different ssDNA strands, three-way branched strand, and four-way branched strands formed in the DNA-origami frame have been used for the formation of the G-quadruplex [ 102 , 103 , 104 ], a substrate of proteins acting on DNA [ 88 , 89 , 105 ].
2.8. Nanopores and DNA Manipulation
Nanopore technology has been used as a platform to identify the structure and sequence of single-molecule DNA or RNA [ 106 ]. Cis and trans electrode chambers are separated from a thin membrane with a nanopore that connects the conductive solution and the target biomolecules [ 107 ]. Upon applying a voltage across the membrane with a nanopore, electrolyte ions flow through the nanopore, resulting in highly sensitively detecting the formed pore current. By excluding the electrolyte ions from the pore current, a translocating biomolecule can be detected by monitoring the changes in the current. Nanopore systems are divided into the two types of solid-state nanopores and protein nanopores [ 108 ]. Solid-state nanopores are functional over wider ranges of temperatures, voltages, and solvent conditions and can be tuned in diameter with subnanometer precision, resulting in advantaging solid-state nanopores as compared to biological/protein nanopores [ 109 ]. However, nanopores have the two major issues of low spatial and low temporal resolutions. In the obstacle of the low spatial, dozens of bases can pass solid-state nanopores at a time because of the thickness of solid based materials, resulting in limitation of the distinguish the four kinds of base [ 109 ]. In the obstacle of the temporal resolution, DNA translocation is too fast under a strong electrical force, which is required in drawing DNA into the nanopore, resulting in the limitation of the acquisition of valid data points. To solve this problem, the speed of DNA translocation through nanopores has been controlled by DNA manipulation techniques such as optical tweezers [ 110 ] and magnetic tweezers [ 111 ]. The force for drawing a single DNA molecule inside a nanopore was measured by combining optical tweezers with ionic-current detection. A force opposing the force for drawing the single DNA molecule inside the pore can be exerted by the optical tweezers. Thus, DNA tension induced by optical tweezers reduced the speed of DNA translocation inside a nanopore [ 110 ]. The speed of the translocating DNA inside a nanopore can be controlled by the balance between the electrical force for drawing a single DNA molecule into a pore and the gradient of the magnetic field on the magnetic bead exerted by magnetic tweezers. Using magnetic tweezers, thus, the DNA tethered to the magnetic beads can be slowly translocated into a nanopore [ 111 ]. AFM can also be applied to controlling DNA translocation in a pore. DNA tethered to the tip of cantilever can be manipulated through an AFM tip. The tension and electrical forces for drawing a single DNA molecule can be measured through manipulating DNA inside a nanopore. These measurements revealed the kinetics for translocating DNA inside nanopores, indicating the appropriate forces necessary for drawing a single DNA molecule [ 112 ].
As a third type of nanopore that is completely different from the protein nanopores and the solid-state nanopores, a combination of nanopores and DNA origami has been developed. Through the manipulation of the mechanical folding of DNA origami, DNA-origami nanoplates and origami nanopore blockages are designed with accurate shapes and sizes. The speeds of translocation in a nanopore can be controlled by controlling the interactions between biomolecules and DNA scaffolds [ 113 , 114 , 115 ]. As an application example of DNA sequencing, the ionic conductivity was monitored using nanopores on graphene docked with layered DNA origami. The four types of DNA bases were distinguished according to the blockade current, according to the specific interactions between the DNA-origami plate layers and the different DNA bases. Thus, the translocation speeds and detection of DNA in a nanopore can be controlled by the interactions between target biomolecules and the DNA scaffold [ 116 , 117 ]. By introducing various modifications (e.g., sensing elements, such as DNA, fluorophores, and FRET (see Section 3.1.3 ), and moving parts) at specific sites of DNA origami, DNA-origami nanopores have provided new insight as a powerful tool for single-molecule detection [ 116 , 117 ]. In the near future, DNA-origami nanopores may be achieved in the development of variable and dynamic nanopore structures.
3. Single-Molecule Imaging for Biological Processes
By manipulating DNA, the randomly coiled state of a single DNA molecule can be controlled toward stretched and supercoiled states. Unlike in the randomly coiled state, the position of a protein bound to single DNA molecules under the stretched state can be determined from the length information of the DNA molecules. Thus, single-molecule techniques combining DNA manipulation and single-molecule imaging have successfully analyzed the dynamic interaction between DNA and protein, thus providing a new insight into the elementary processes of DNA replication, repair, recombination, and others.
Here, we provide a summary of the biological processes on the basis of the combination of both DNA manipulation and single-molecule imaging as follows: (1) the fluorescence labeling of biomolecules for single-molecule imaging, (2) optical mapping on single DNA molecules, (3) single-molecule imaging for ssDNA molecules by fluorescent ssDNA-binding protein, (4) single-molecule imaging for DNA digestion by exonuclease, (5) single-molecule imaging for DNA synthesis by DNA polymerase, (6) single-molecule imaging for supercoiled DNA and DNA secondary structures, (7) single-molecule imaging for DNA origami, (8) single-molecule imaging for the initiation of DNA replication, and (9) single-molecule imaging based on zero-mode waveguides.
3.1. Fluorescence Labeling of Biomolecules for Single-Molecule Imaging
3.1.1. fluorescence staining of double-stranded dna.
Fluorescence microscopy has allowed the analysis of the dynamic behavior of single DNA molecules in an aqueous solution. However, nonlabeled DNA, which has no fluorescent properties, is not captured by fluorescence microscopy techniques. For this reason, DNA must be labeled with a fluorescent compound. To date, a large variety of fluorescent compounds have been developed as DNA-labeling dyes. These fluorescent compounds can be categorized into three types: (i) those binding to the minor/major grooves of double-stranded DNA (dsDNA), (ii) those intercalating between the base pairs of the dsDNA, and (iii) those doing both [ 118 , 119 , 120 , 121 , 122 ]. Among them, fluorescent dyes, such as YOYO-1 and SYTOX Orange, which are intercalated between the base pairs of the dsDNA, have been utilized for the single-molecule imaging of DNA molecules. These intercalating dyes, which are cyanine dyes, are largely nonfluorescent in solution and interact with dsDNA with high affinity [ 123 , 124 , 125 , 126 ], resulting in a 1000-fold increase in fluorescence intensity. Thus, single DNA molecules are easily detected with increases in the signal-to-background ratio. These fluorescent dyes are essential tools for single-molecule imaging, but several studies have revealed that parameters such as the contour length and the persistence length of DNA were significantly changed by the binding of fluorescent dyes, irrespective of their DNA-binding mechanisms [ 127 , 128 ]. These parameters are strongly dependent on concentration and fluorescence dye type; therefore, these should be considered in single-molecule experiments.
3.1.2. Fluorescence Labeling of DNA-Binding Proteins
Analysis of the interactions between DNA and proteins has been an important component of single-molecule approaches for investigating elementary biological processes such as DNA replication, repair, and recombination. Target proteins can be easily fused with a fluorescent protein to the N- or C-terminal of a target protein. To achieve this goal, it is necessary to build an expression vector in which a fluorescent protein gene is inserted either upstream or downstream of the target protein gene. Using a protein expression system from bacterial, insect, and mammalian cultivated cells, the target fusion protein is prepared by extracting it from the cells, followed by purification. However, because the molecular weights of fluorescent proteins are larger than those of peptide tags (e.g., EGFP; 27 kDa), the fusion protein may form an inclusion body due to inappropriate protein folding. In addition, the fusion often impairs the activity of the target protein. To prepare a fusion protein, thus, it is necessary to clearly understand the function and structure of the target proteins.
Because the labeling of DNA-binding proteins with fluorescent protein is quite effective for investigating DNA–protein interactions, fluorescent-labeling technologies for DNA-binding proteins have also been developed [ 129 , 130 ]. The fluorescent-labeling approaches were achieved by fusing self-labeling protein tags (e.g., SNAP-tag, CLIP-tag, or HALO-tag) to a target protein. The fusion protein can be labeled by attaching various colored fluorophores. The bright fluorophores are designed to bind to the self-labeling protein tags. The use of the self-labeling protein tags allows avoiding the steric hindrance effect caused by the fusion of fluorescent protein, resulting in a far brighter and more photostable fluorophore.
In the above methods using a fusion protein for fluorescence protein preparation, the fluorescence activity may be insufficient for single-molecule imaging, as the number ratio of the molecules of the fluorescence protein to those of the target protein is only 1:1. To solve this problem, the methods have been developed such that the surface of the proteins is modified with fluorescence chemicals while protecting the active sites of the proteins [ 131 ]. In particular, DNA-binding proteins catalyze by specifically binding to DNA. This means that the active sites of DNA-binding proteins are protected by the binding of DNA during DNA–protein interaction. By modifying with fluorescent compounds or using a crosslinking agent, thus, it is possible to prepare a highly fluorescently labeled target protein without losing the activity [ 131 , 132 ]. Using this method, a restriction enzyme was labeled with an amine-reactive fluorescent moiety, facilitating the optical mapping of specific positions of DNA sequences (see Section 3.2 ).
3.1.3. Single-Molecule Imaging by Single-Molecule FRET
Förster resonance energy transfer (FRET) has been used as a powerful tool to quantify putative interactions between neighboring biomolecules, such as protein–protein interactions, protein–DNA interactions, and also protein conformational changes [ 133 , 134 ]. For monitoring the interaction between two biomolecules, one of the biomolecules is fluorescently labeled as a donor fluorophore, and the other, as an acceptor one. When the donor and the acceptor biomolecules are separated by less than approximately 10 nm, the excitation energy of the donor, which reaches an excited electronic state, is transferred to the acceptor through the electronic resonance of molecular orbitals [ 133 , 134 ]. By nonradiative energy transfer between a donor and an acceptor molecule, thus, the FRET is able to identify interactions between the labeled complexes.
Single-molecule FRET (smFRET) facilitates measurement for the dynamic interactions and behavior between a fluorescently labeled donor and an acceptor, such as protein–protein and protein–DNA [ 135 , 136 , 137 , 138 , 139 ] interactions. In addition, by labeling with a donor and an acceptor at different sites on the same single molecule, the conformation changes within a single molecule are allowed to be observed [ 140 , 141 ]. However, it is necessary to understand the overall structure of the molecule or complex of molecules and the site-specific attachment of fluorophores and color of the fluorophores.
SmFRET has been applied to the analysis of the DNA-unwinding mechanisms of various DNA helicases acting on single DNA molecules. With one smFRET-based approach, the DNA-unwinding mechanisms of Mcm2–7 helicase in eukaryotic DNA replication have been analyzed [ 142 , 143 , 144 ]. Based on the closed Mcm2–7 ring structure, the Mcm2 and Mcm5 were fluorescently labeled with a donor and an acceptor, respectively [ 144 ]. This is because ATP binding at the Mcm2–Mcm5 interface is purported to close the Mcm2–7 ring by cryo-EM and negative-stain EM studies. For this reason, the opening and closing of S. cerevisiae ring-shaped Mcm2–7 DNA helicases were monitored during the recruitment of the pre-replicative complex (pre-RC) to the origin of replication. The application of smFRET provided new insights into the mechanism of eukaryotic DNA replication. Through another smFRET-based approach, the DNA-unwinding mechanisms of the WRN helicase of Werner syndrome (WRN), which is caused by mutations in the WRN gene encoding WRN helicase, have been analyzed [ 145 ]. To evaluate the DNA-unwinding properties of WRN helicase, the substrates of different DNA structures, including forked DNA, overhanging DNA, and G-quadruplex-containing DNA, were fluorescently labeled as a donor and an acceptor, respectively. The DNA unwinding of WRN helicase was not caused by complete dissociation from and rebinding to substrates or by strand switching, but by the reciprocating of WRN moving along the same ssDNA substrates. The repetitive movements were shown to behave differently for each DNA substrate, such as forked DNA, 3′/5′-overhanging DNA, and G-quadruplex-containing DNA. These functions of WRN helicase assist the access of other enzymes acting during biological processes, such as DNA replication, repair, and recombination, resulting in it being helpful for the inheritance and maintenance of genome stability.
3.2. Optical Mapping on Single DNA Molecules
A restriction enzyme binds and cleaves double-stranded DNA (dsDNA) molecules at specific restriction sites. This characteristic of the restriction enzyme can be employed for the optical mapping of restriction sites on single DNA molecules [ 146 , 147 , 148 , 149 ]. In the optical mapping of a DNA-binding protein, the fluorescent labeling of the target protein is necessary. Because fluorescently labeled reagents often attack the active sites of a protein and impair the protein functions, the active sites should be protected [ 150 , 151 ]. In the absence of magnesium ions, most restriction enzymes bind to the DNA sites of recognition sequences but do not progress to cleavage reactions. Therefore, the active sites of the restriction enzymes are protected by binding the enzyme to the DNA molecules in a buffer solution without magnesium ions. Then, the enzyme bound to the DNA molecules is stained with an amine-reactive fluorescent dye. As a result, the catalytic site of the enzyme is protected from modification by the amine-reactive fluorescent dye. Finally, the fluorescently labeled restriction enzyme can be released by cleaving the bound DNA molecule via the addition of magnesium ions to the solution. In single-molecule imaging, fluorescently labeled Eco RI enzyme was observed at five specific recognition sites on stretched lambda DNA molecules without magnesium ions [ 150 ]. The positions of the Eco RI binding and cutting sites on the lambda DNA molecules are in line with the restriction map of the nucleotide sequence [ 147 , 150 ]. Through optical mapping, the specific binding sites of the DNA-binding protein in the single DNA molecules can be determined from the length information of the DNA molecules.
As another single-molecule approach for optical mapping, DNA methyltransferases (MTases) have been used [ 152 , 153 , 154 ]. In DNA methylation, DNA MTases catalyze the transfers of methyl groups from the ubiquitous cofactor S-adenosyl-L-methionine (AdoMet) to specific positions of DNA sequences [ 155 ]. By using synthetic cofactor analogues, specific positions of DNA sequences can be labeled via engineered DNA MTases [ 156 ]. The optical mapping approaches for DNA sequences by the conjunction with synthetic cofactor analogues and DNA MTases are divided into the two types of the methyltransferase-directed transfer of activated groups (mTAG) [ 157 , 158 ] and methyltransferase-directed labeling [ 159 ]. The fluorescent labeling of DNA sequences using mTAG is a simple, two-step procedure [ 157 , 158 ]. In the first step, the specific positions of DNA sequences were aminated by DNA MTases and synthetic cofactor analogues. In the second step, the aminated DNA was fluorescently labeled by an amine-reactive fluorophore, resulting in optical mapping from single DNA molecules immobilized on a surface. In a methyltransferase-directed labeling approach [ 159 ], the covalent attachment of a fluorophore to a specific DNA sequence was catalyzed by the DNA MTase M.TaqI, from Thermus aquaticus . The fluorescent labeling of MTase on DNA was achieved in a single step by using a synthetic cofactor containing a fluorophore at the transfer position. The labeling positions of the stretched DNA molecules were analyzed from the fluorescence signals, resulting in the accurate genotyping of the genomes of λ and T7 bacteriophages. These methods demonstrated that the analysis of specific positions of genomic DNA can be advantageous for genome-wide analysis [ 160 , 161 , 162 ].
3.3. Single-Molecule Imaging for ssDNA Molecules by Fluorescent ssDNA-Binding Protein
To directly observe DNA molecules under a fluorescence microscopic field, the biomolecules need to be labeled using a fluorescent chemical compound (see Section 3.1.1 ). Intercalating dyes (e.g., YOYO-1 and SYTOX Orange), which are intercalated between the base pairs of dsDNA, can be utilized to directly observe the double-stranded regions of DNA molecules ( Figure 8 A). However, fluorescent intercalating dyes are not applicable for the direct observation of the single-stranded regions of DNA molecules. However, in the elementary processes of DNA replication, repair, and recombination, single-stranded regions of DNA molecules are generated by proteins acting on DNA, such as DNA helicase and DNA exonuclease. Thus, to analyze the elementary biological processes, the single-stranded regions of the DNA molecules need to be directly observed.

Labeling of the single-stranded regions of single DNA molecules with fluorescent ssDNA-binding proteins. ( A ) Staining the double-stranded region of a DNA molecule with an intercalating dye. The single-stranded region is not stained with an intercalating dye. ( B ) Labeling of the single-stranded regions of DNA molecules with fluorescent ssDNA-binding proteins. ( C ) Schematic illustration of the dynamic behavior of the single-stranded DNA molecules labeled with fluorescent ssDNA-binding protein. One end of ssDNA molecule was specifically immobilized on modified glass surface, and then, fluorescent RPA molecules were injected into the flow cell, resulting in the binding of fluorescent RPA to the ssDNA molecules. The dynamic behavior of the physical form of ssDNA molecule was monitored with and without fluid flow. When salt was added at more than 200 mM, the fluorescent RPA molecules were released from the ssDNA molecule. The ssDNA was restained with the fluorescent RPA after removing the salt. Refer to [ 166 ] for details.
To enable the direct observation of the single-stranded regions of DNA molecules, ssDNA-binding proteins are used ( Figure 8 B) [ 82 , 163 ]. In particular, eukaryotic replication protein A (RPA), which is composed of the heterotrimeric subunits of 70, 32, and 14 kDa, is an ssDNA-binding protein that binds to the phosphate group of ssDNA [ 164 , 165 ]. Using a fusion protein between the ssDNA-binding domain of RPA and a fluorescent protein (see Section 3.1.2 ), the single-stranded region of a single DNA molecule has been directly observed under a fluorescence microscopic field [ 82 , 163 ].
In single-molecule imaging using a microchannel device, ssDNA molecules fluorescently labeled with ssDNA-binding proteins are stretched with the fluid flow, whereas are they randomly coiled without the fluid flow [ 166 ]. As an effect of the salt, the fluorescent ssDNA-binding protein, RPA–YFP molecules bound to the ssDNA molecules are released by a high concentration of sodium chloride, and then, the RPA–YFP molecules are rebound to the ssDNA molecules under a salt-free condition [ 166 ] ( Figure 8 C). In the presence of free DNA-binding protein (e.g., RPA, SSB, and Rad51) in the solution, the RPA molecules dissociate from the single-stranded regions of DNA molecules, which results in a rapid exchange between the free and bound states of RPA molecules [ 167 ]. Thus, single-molecule imaging has revealed that the ssDNA-binding affinity of RPA is not strong but that RPA is reversibly bound to ssDNA. These results indicate that RPA protects ssDNA. However, RPA is easily displaced from ssDNA; therefore, other proteins acting on DNA are allowed to access the ssDNA. Therefore, fluorescent ssDNA-binding protein is highly effective for the single-molecule imaging of the elementary process of DNA replication, repair, and recombination and others.
3.4. Single-Molecule Imaging for DNA Digestion by Exonuclease
Exonucleases with DNA digestion activities starting from either the 3′ or the 5′ end play significant roles in the maintenance of genomic stability, such as DNA replication, repair, and recombination [ 168 , 169 ]. In particular, the exonuclease activity exists in an independent protein and protein combined with other enzymatic activities. For instance, DNA polymerase is known to exhibit both DNA synthesis activity and exonuclease activity. The exonuclease activity functions to rectify the incorrect pairing of bases during DNA synthesis [ 170 ]. To analyze the dynamic DNA digestion by exonuclease, it is significantly effective to directly observe it at the single-molecule level [ 56 , 171 , 172 , 173 , 174 ]. Thus, by combining DNA manipulation and single-molecule imaging, the elementary process of DNA digestion by λ exonuclease [ 56 , 171 ], exonuclease III [ 172 , 173 ], and T7 exonuclease [ 174 ] has been successfully captured.
In the direct observation of DNA digestion by T7 exonuclease, the activity of T7 exonuclease was analyzed on the basis of the length of the single DNA molecule stretched by fluid flow [ 174 ]. The single-stranded and double-stranded regions of the single DNA molecule were stained with two different fluorescent dyes. During the observation, the double-stranded region was monotonously shortened by the DNA digestion of T7 exonuclease, whereas the single-stranded region, as a product, was monotonously elongated from the free end ( Figure 9 A). Furthermore, the DNA digestion of T7 exonuclease was directly observed under a pulse-chase condition and continuous buffer flow conditions with T7 exonuclease. In the pulse-chase condition, T7 exonuclease was only supplied when the reaction started. This result indicates that the single DNA molecules were gradually digested by the following process, the cycling of the binding, digestion, and dissociation of T7 exonuclease. The average rate of DNA digestion was estimated to be 5.3 ± 0.6 bases/s, with a processivity of 5072 ± 1554 bases ( Figure 9 B).

Analysis of the single-molecule imaging of the exonuclease activity on single DNA molecules. ( A ) Time course for the length of the double-stranded region and single-stranded region of a single DNA molecule during DNA digestion by T7 exonuclease. White bars indicate the length of the double-stranded region in single DNA molecule stained with SYTOX Orange. Black bars are the length of the single-stranded region digested by T7 exonuclease stained with fluorescently labeled ssDNA-binding protein. Sum of the lengths of single- and double-strand regions agreed with that of the undigested DNA at 40 min of DNA digestion time. ( B ) Histogram of the DNA digestion rates for T7 exonuclease. White bars and black bars indicate the results determined from single-molecule imaging of DNA digestion under pulse-chase conditions and under continuous conditions with T7 exonuclease. Refer to [ 174 ] for details.
3.5. Single-Molecule Imaging for DNA Synthesis by DNA Polymerase
DNA polymerase, which catalyzes the synthesis of DNA strands with complementary nucleotide sequences to ssDNA as a template, plays a significant role in DNA replication and repair. Six types of DNA polymerase have been discovered in prokaryotes (five types in Escherichia coli ) [ 175 ], and 15 types, in eukaryotes [ 176 ], allowing it to perform various functions, such as DNA replication and base-excision repair. Based on the functional analysis of DNA polymerase, DNA synthesis models, such as leading- and lagging-strand synthesis and translation DNA synthesis (TLS), have been proposed. However, in these models, the following processes of DNA synthesis have not been sufficiently elucidated: (i) the dynamic behavior, such as the binding, synthesis, dissociation, and pauses of DNA polymerase with template DNA; (ii) the replacement process between DNA polymerases during DNA synthesis; and (iii) the processivity and speed of DNA polymerase. To demonstrate the process of DNA synthesis, it is significantly effective to directly observe it at the single-molecule level. This is because single-molecule imaging has provided new insights into the elementary process of DNA synthesis by DNA polymerase [ 177 , 178 , 179 , 180 ].
In the single-molecule technique for DNA synthesis by DNA polymerase, DNA synthesis was directly observed using template ssDNA labeled by fluorescent ssDNA-binding protein ( Figure 10 A). An E. coli DNA polymerase I mutant, namely, Klenow fragment (3′–5′ exonuclease minus), lacks the exonuclease activities for both directions (5′–3′ and 3′–5′) and has only the polymerase activity of DNA polymerase I [ 181 , 182 ]. This means that the digestion of the template ssDNA by the exonuclease activity of DNA polymerase I was eliminated. Therefore, only the DNA synthetic reaction was directly observed in this single-molecule experiment [ 183 ]. Figure 10 A shows the scheme of DNA synthesis by DNA polymerase at the single-molecule level. The DNA tensions under the randomly coiled state and stretched state were controlled in the presence or absence of fluid flow. The analyses were conducted on the basis of the length of the stretched single DNA molecule. Under the randomly coiled state, the activity of DNA polymerase was measured via the temporary stretching of the single DNA molecule. Conversely, under the stretched state, the activity of DNA polymerase was measured via the continuous stretching of the single DNA molecule. As a result, the synthesis rate of DNA polymerase for the stretched DNA was approximately 75% higher than that for the randomly coiled DNA molecules (91 nt/s vs. 52 nt/s) ( Figure 10 B). These results indicate that the physical form of DNA significantly affects the activity of DNA polymerase.

Analysis of the single-molecule imaging of the activity of DNA polymerase on single DNA molecules. ( A ) Schematic illustration of the DNA synthesis by DNA polymerase at a single-molecule level. One end of template ssDNA for DNA synthesis was specifically immobilized on a modified glass surface, and then, fluorescently labeled RPA molecules were injected into the flow cell, resulting in the binding of fluorescently labeled RPA to the ssDNA molecules. During DNA synthesis by DNA polymerase, the DNA tensions under the randomly coiled state and stretched state were controlled in the presence or absence of fluid flow. After the DNA synthesis, the dsDNA synthesized from ssDNA was stained by SYTOX Orange, allowing visualizing under a fluorescence microscopic field. ( B ) Time course for the length of the single-stranded regions of the single DNA molecules under the relaxed and stretched states during DNA synthesis by Klenow fragment (3′–5′ exonuclease). Closed circles and open circles indicate the length of the single-stranded region on stretched ssDNA and that on relaxed ssDNA, respectively. Refer to [ 183 ] for details.
3.6. Single-Molecule Imaging for Supercoiled DNA and DNA Secondary Structures
It has been strongly suggested that the physical form of DNA affects the activity of enzymes acting on DNA [ 51 , 53 , 173 , 183 , 184 ]. This indicates that the physical forms of DNA, such as supercoiling and supercoiling-induced non-B-DNA structures, may play significant roles in the regulation of DNA replication, repair, recombination, and others. To induce supercoiling of a specified density in a single DNA molecule, magnetic tweezers have been used (see Section 2.5 ). The superhelicity of single DNA molecules has been controlled using magnetic tweezers under a fluorescence microscopic field [ 70 ]. Single-molecule imaging has revealed the following: (i) plectonemes were induced by supercoiling; (ii) plectonemes were moved along a single DNA molecule by diffusion; (iii) new plectonemes were induced at a distant position via a fast hopping process. It has been suggested that the dynamic behavior of plectonemes may enhance protein binding and gene expression as follows [ 185 ]. In single-molecule imaging, the plectonemes on single DNA molecules were simply observed by the intercalating of SYTOX Orange between base pairs of DNA. It was shown that plectonemes were induced upstream of promoters in several prokaryotic genomes.
It has been proposed that DNA looping with DNA supercoiling plays critical roles in the spatial organization of chromosomes. Structural maintenance of chromosome (SMC) protein complexes such as condensin and cohesion play key roles in restructuring genomes during the cell cycle [ 186 , 187 ]. The formation and processive extension of DNA loops by yeast condensin on single DNA molecules was directly observed via single-molecule imaging [ 188 ]. The elegant single-molecule experiment demonstrated that by the action of a condensin complex, tens of kilobase pairs of DNA were extruded as a loop structure, at a force-dependent speed of up to 1500 base pairs per second. Single-molecule imaging has shown that the induction of DNA loop extrusion by SMC complexes may be the key principle for the organization of genome architecture.
In single-molecule imaging for DNA secondary structures induced by supercoiling, local structure denaturation of single DNA molecules was frequently induced under a high negative supercoiling density but was almost not induced under a low negative supercoiling density [ 71 ]. Local structure denaturation was often observed at the initiation region for DNA replication on single DNA molecules under a high supercoiling density. These results indicate that negative supercoiling enhances the initiation of DNA replication.
3.7. Single-Molecule Imaging for DNA Origami
As described in Section 2.7 , DNA-origami-based nanotechnology has been developed for the construction of various custom-made nanostructures. To evaluate the nanostructures of DNA origami, high-resolution visualization is necessary. To date, the nanostructures of DNA origami have been imaged by AFM and/or EM, which is commonly used to directly acquire high-resolution images of nonlabeled biomolecules under physiological conditions [ 189 ]. However, the speed performance of AFM imaging was significantly too low to monitor dynamic interactions between DNA and protein. To overcome the limitation, the development of high-speed AFM (HS-AFM) was started around 1993. By extensive improvements of AFM instruments, such as in the cantilevers, detectors, and scanners, HS-AFM has now materialized, resulting in capturing images of biological molecules within 100 ms or less [ 190 , 191 , 192 ]. HS-AFM imaging has captured a wide range of dynamic events in biomolecular processes [ 190 , 191 , 192 , 193 , 194 ]. Combining the DNA-origami frame (see Section 2.7 ) and HS-AFM, the dynamic behavior of biomolecular processes, such as enzymatic reactions [ 88 , 89 , 101 , 195 ] and DNA structural changes [ 103 , 104 , 196 ], has been directly observed at a nanolevel. For example, the conformational changes of the G-quadruplex induced in the DNA-origami frame were visualized in real time with HS-AFM [ 195 ]. The G-quadruple was formed in the presence of salt, whereas it was disrupted by the removal of the salt. The DNA-origami frame is suitable for visualizing various conformational changes in DNA secondary structures. As a result of recent research on the dynamic interaction between enzymes and DNA-origami frames, the digestion of DNA-origami nanostructures by endonuclease DNase I was directly observed at a single-structure level by HS-AFM [ 195 ]. By analyzing the digestion patterns of DNase I for DNA-origami nanostructures, the design of DNase I-resistant DNA-origami nanostructures was suggested as guidelines. The guidelines play an important role in the design for new DNA-origami nanostructures for specific applications such as drug-delivery systems [ 189 ].
In fluorescence-based optical microscopes, the dynamic behavior of biomolecules at the nano level such as DNA-origami nanostructures cannot be observed due to the diffraction limit of light (half the wavelength of the emission light). This is because photons emitted from two fluorophores closer than the resolution limit cannot be distinguished under a fluorescence microscopic field. However, the diffraction limit in optical microscopy has been overcome by the development of super-resolution microscopes [ 197 , 198 ]. One of the typical super-resolution techniques, stochastic super-resolution, can resolve the individual fluorophores in time through light-emitting the several fluorophores neighbored at separate times. The super-resolution techniques include single-molecule localization methods (SMLM), such as PALM, STORM, and PAINT. The super-resolution techniques are achieved by isolating emitters and fitting images with the point-spread function to the fluorescence of single molecules [ 199 , 200 ]. Point accumulation for imaging in nanoscale topography (PAINT), which is the one of SMLM, allows capturing several points of stochastic single-molecule fluorescence emitted by molecular adsorption/absorption and photobleaching/desorption by using fast and transient fluorescence dyes. For this reason, instead of the stochastic photoactivation of a permanently bound fluorophore, PAINT depends exclusively on the stochastic/temporary binding of a fluorescent ligand. Using the characteristics of PAINT, DNA-PAINT, which is an extension of PAINT, has been used as a powerful tool in the characterization of DNA-origami nanostructures (e.g., including tetrahedrons, triangular prisms, cubes, pentagonal prisms, and hexagonal prisms) [ 201 , 202 , 203 ]. In DNA-PAINT imaging, the stochastic blinking of targets is achieved by the transient hybridization of short, dye-labeled DNA oligonucleotides to a complementary target DNA molecule. For this reason, the dye-labeled DNA oligonucleotides unbound to the complementary target DNA strands are freely diffused in solution. Thus, when the dye-labeled DNA oligonucleotides are bound to the complementary target DNA molecule, the acquisition of the image is performed in total internal reflection or oblique illumination, resulting in minimal background. For this reason, DNA-PAINT is significantly suitable for fluorescently visualizing DNA nanostructures, such as the folding of DNA origami and incorporation of DNA strands. Because dye-labeled DNA oligonucleotides, which are parts of origami structure, are constantly replenished from solution, DNA-PAINT resists photobleaching. For this reason, high spatial resolutions are achieved by capturing the maximum number of photons from dye-labeled DNA oligonucleotides bound to a complementary target DNA molecule before unbinding from the complementary target DNA molecule. DNA nanostructures with single binding sites spaced 5 nm apart were observed as the “MPI” and “LMU” logos [ 204 ]. Thus, DNA-PAINT has provided new insight into the design analysis of DNA-origami nanostructures [ 205 , 206 , 207 , 208 , 209 ].
3.8. Single-Molecule Imaging for the Initiation of DNA Replication
DNA replication is initiated from replication origins [ 210 ]. The consensus sequence of replication origin is highly conserved among bacteria and budding yeast; however, it is not completely conserved in some eukaryotes, such as flies, frogs, and mammals [ 210 , 211 , 212 , 213 , 214 ]. However, in bacteria and eukaryotes, the initiation of DNA replication is promoted by the negative supercoiling of DNA [ 215 , 216 , 217 , 218 , 219 ]. It has been suggested that the physical form of DNA plays a significant role in regulating the initiation of DNA replication. The large T antigen (Tag) of Simian virus 40 (SV40) has been utilized as a model of DNA replication in eukaryotic cells [ 220 ]. SV40 Tag is crucial for SV40 DNA replication and is the only replication factor encoded by a viral gene. The multifunctional protein SV40 Tag acts as a replication initiator and simultaneously exhibits a DNA helicase activity during DNA replication. Thus, it is assembled at the SV40 replication origin and unwinds duplex DNA [ 220 ].
The initiation of DNA replication using SV40 Tag has been analyzed via single-molecule imaging [ 221 ]. In the single-molecule technique, the unwound DNA on the single DNA molecules was labeled by fluorescent ssDNA-binding protein, whereas the double-stranded regions of the single DNA molecules were labeled by fluorescent intercalating dyes. SV40 Tag assembled on the SV40 origin efficiently unwound DNA as a single hexamer translocated on the ssDNA of the lagging strand in the 3′-to-5′ direction. The translocation of SV40 Tag is the same as that of the Mcm2-7 helicase [ 222 , 223 , 224 ]. These results indicate that the replicative DNA helicase in eukaryotes unwinds a ssDNA on a lagging strand in the 3′-to-5′ direction. The effect of negative supercoiling on the initiation of DNA replication initiated by SV40 Tag has been analyzed by combining DNA manipulation and single-molecule imaging [ 225 ]. The increase in the negative supercoiling density stimulated the DNA unwinding more strongly. Furthermore, negative supercoiling was associated with an increased probability, from the assembly of Tag on the SV40 origin, of DNA unwinding. These results indicate that negative superhelicity facilitates the initiation of DNA replication.
3.9. Single-Molecule Imaging Based on Zero-Mode Waveguides (ZMWs)
The diffraction of light limits the optical performance of confocal microscopes, resulting in preventing the detection of a single molecule in a crowded environment [ 226 , 227 , 228 ]. To solve the diffraction limit, zero-mode waveguides (ZMWs) have been introduced for confining the fluorescent signal at the nanometer scale [ 229 , 230 ]. The confined fluorescent signal can be monitored via ZMWs [ 229 ]. ZMWs milled in opaque metallic films supported on a glass surface are nanoapertures in nanoscale cylindrical cavities ~100 nm in diameter and height. A ZMW that is an optical waveguide directs light energy into a well, which is smaller than the wavelength of the illuminating light. This allows ZMWs to generate an evanescently decaying intensity profile that provides a detection volume in the range of atto- to zeptoliters, three orders of magnitude below the diffraction-limited confocal volumes [ 231 ]. By enhancing the local fluorescence excitation intensity and modifying the fluorescence decay rate inside the ZMWs, the fluorescence brightness per single molecule close to the bottom of the ZMW is made able to be detected with a high signal [ 232 , 233 ]. Since 2002, ZMWs have been widely used for a large range of biophysical and biochemical applications, including DNA sequencing [ 234 , 235 ], enzymatic reaction monitoring [ 236 , 237 ], studying protein–protein interactions [ 238 , 239 ], nanopore sensing [ 240 , 241 ], and FRET [ 242 , 243 , 244 ]. In particular, single-molecule sequencing technology is commercialized by Pacific Biosystems as single-molecule real-time (SMRT) DNA sequencing [ 245 , 246 , 247 , 248 ]. Single-molecule sequencing uses fluorescent dNTPs phospholinked by four different colored fluorophores. The fluorescent signals generated by the incorporation of each nucleotide with DNA synthesis by DNA polymerase are detected and classified via monitoring in ZMWs. Single-molecule sequencing allows long reading exceeding 10,000 base pairs in length. Over the past decade, the capabilities of single-molecule sequencing technologies have made remarkable progress. However, to make them widely available in medical clinics and research laboratories, key challenges still need to be solved, such as the instrument costs, running costs, handling of the instruments, and data analysis.
4. Conclusions
DNA manipulation and single-molecule imaging provide new analytical methods in biotechnology. In this review, the following single-molecule techniques, which are powerful tools for DNA manipulation and single-molecule imaging, have been introduced:
- DNA manipulation by orientation forces, optical tweezers, fluid flow, and magnetic tweezers;
- Nanopores and DNA manipulation;
- Single-molecule imaging for double- and single-stranded DNA;
- The optical mapping of single DNA molecules;
- Single-molecule imaging for biological processes between DNA and protein;
- Single-molecule imaging for supercoiled DNA and DNA secondary structures;
- Single-molecule imaging for DNA origami and DNA sequencing.
These single-molecule techniques are maximally utilized through integration with microfluidic devices. Microdevices used in single-molecule analysis have many advantages in terms of reduced sample volumes and the reaction field to be controlled. In particular, the detection frequencies for the biological events between DNA and protein are significantly improved by the reduced distance of molecular diffusion. The further improvement of DNA manipulation and single-molecule imaging techniques will significantly enhance the dynamic analysis of the behavior of and interaction between biomolecules and provide new insights into the mechanisms of elementary biological processes such as DNA replication, repair, and recombination. A knowledge of DNA replication, repair, recombination, and others at a single-molecule level is helpful for not only predicting behaviors at the cellular level but also understanding the inheritance and maintenance of genome stability and diseases associated with DNA replication, repair, and recombination (e.g., aging and cancer). Thus, these single-molecule studies will play important roles as bridges to close the gaps between the research at the molecular level and the research at the cellular level in the fields of biochemistry and molecular biology.
Author Contributions
Writing—review and editing, S.T., M.O., and S.K. All authors have read and agreed to the published version of the manuscript.
S.T. was supported by a Grant-in-Aid for Young Scientists, Grant Number JP50778967, and a Naito Foundation Natural Science Scholarship. M.O. was supported by a Grant-in-Aid for Young Scientists, Grant Numbers JP19870003, JP21770183 and JP24770164, and for Scientific Research, Grant Number JP15K06946. S.K. was supported by a Grant-in-Aid for Challenging Exploratory Research, Grant Number JP25650002, and for Scientific Research, Grant Number JP17K07280.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 02 July 2019
Touch DNA: impact of handling time on touch deposit and evaluation of different recovery techniques: An experimental study
- Francesco Sessa ORCID: orcid.org/0000-0003-0357-8791 1 ,
- Monica Salerno 1 ,
- Giuseppe Bertozzi 1 ,
- Giovanni Messina 2 ,
- Pietrantonio Ricci 1 , 3 ,
- Caterina Ledda ORCID: orcid.org/0000-0003-0739-8798 4 ,
- Venerando Rapisarda 4 ,
- Santina Cantatore 1 ,
- Emanuela Turillazzi 5 &
- Cristoforo Pomara 6
Scientific Reports volume 9 , Article number: 9542 ( 2019 ) Cite this article
47k Accesses
37 Citations
8 Altmetric
Metrics details
- DNA sequencing
- PCR-based techniques
“Touch DNA” is DNA obtained from biological material transferred from a donor to an object or a person during physical contact. This particular kind of evidence could play an essential role in forensic laboratory work and is considered an important tool for investigators. Even though the principal aspects of “Touch DNA” have been extensively studied, to date, there are few reports in the research field of DNA retrieval from garments that have been worn. This study aimed to investigate the “handling time”, analyzing particularly the minimum contact time required to deposit a sufficient amount of DNA on a garment to produce an interpretable profile of the “handler”. Moreover, three different sampling techniques were compared (“dry swab”, “cutting out”, and “adhesive tape”) with the aim of defining the technique that guarantees the best recovery of the three methods tested. Analyzing the data of this experimental model, a “handling time” of two seconds is enough to release sufficient DNA on to a garment to obtain a complete profile. Moreover, this study demonstrated that when targeting for foreign DNA, the sample area should be narrowed down as much as possible to the smallest area possible to maximize target DNA recovery.
Similar content being viewed by others

Active self-treatment of a facial wound with a biologically active plant by a male Sumatran orangutan

Nicotine pouches: a review for the dental team

High-density, highly sensitive sensor array of spiky carbon nanospheres for strain field mapping
Introduction.
“Touch DNA” is DNA obtained from shed skin cells and other biological material transferred from a donor to an object or a person during physical contact 1 , 2 . This particular kind of evidence could play an essential role in forensic laboratory work and is considered an important tool for investigators 3 . “Touch” DNA refers to the collection of minute biological samples at the crime scene or extracting tiny amounts of material from a sample in a forensic laboratory. Based on Locard’s Exchange Principle, which states, “every contact leaves a trace” 4 , the collection of “touch” DNA, to obtain significant profiles from different surfaces, remains an important procedure in forensic investigations.
Genetic profiles generated from fingermarks were first described in 1997 by RAH van Oorschot et al . 5 . Fingermarks refer to the marks left by the papillary ridge patterns present on fingers, palms, toes, and soles on touched surfaces 6 . Fingermarks are essential forensic evidence used in a wide range of forensic investigations helping to generate a DNA profile for human identification 5 . This kind of evidence is very useful in a wide range of criminal investigations ranging from theft, sexual violence, to murder. For instance, very important evidence could be collected by analyzing the steering wheel of a vehicle used in a theft, or weapons, and clothes in cases of murder or sexual assault. Moreover, the collection of “Touch DNA” with the aim of identifying a person of interest from a crime scene could be very useful especially in the absence of body fluids 7 , 8 , 9 .
Even though the principal aspects of “Touch DNA” have been extensively studied 10 , 11 , 12 , 13 , to date, there are few reports in the research field of DNA retrieval from garments that have been worn 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 . In criminal cases, sampling techniques are very important to collect the best evidence. One of the most common methods for optimal collection of cellular material is the so-called “swab technique”, using sterile cotton swabs on the surface of the object 22 . To improve the quality of the resulting DNA profiles, the double swab technique (wet and dry) is usually applied 23 . Another sampling technique frequently used in a large number of forensic laboratories is “cutting out” the area of interest; this method is applied especially to soft items 24 , 25 . Moreover, the “adhesive tape” lifting technique has been used for years for DNA profiling 26 . This last sampling method is quick and straightforward but the DNA extraction is challenging due to the stickiness, rigidity, and size of the tape 27 .
This study aimed to investigate the “handling time”, analyzing particularly the contact time needed to deposit a sufficient amount of DNA on a garment to produce an interpretable profile of the “handler”. Moreover, three different sampling techniques were compared (“dry swab”, “cutting out”, and “adhesive tape”) with the aim of defining the technique that guarantees the best recovery of the three methods tested.
Substrates and contact simulations
Ten female volunteers (“wearers”) wore a new brassiere (previously irradiated with UV rays for 20 min to remove exogenous DNA) for more than 12 h (from 8 pm to 8/9 am, minimum 12 h and maximum 13 h), under normal conditions (during this time they carried out their usual activities such as walking about, eating, sleeping). Prior to the experiment, the lateral regions of the brassieres were marked in 8 areas (4 for each side).
Each brassiere was then placed in a sterile “acid-free” plastic bag. Subsequently, after 1 h, the brassieres were removed from the sterile bag by 10 male volunteers (“handlers”), each assigned to a specific brassiere. Moreover, they held each region of the brassiere for different time intervals with the same two fingers (thumb and index) for each hand. Particularly, the two fingers moved on two opposed specific region of the brasserie for the established time, depositing DNA on the brassiere. Prior to this, they had washed their hands 2 h before the experiment, to allow for sufficient DNA transfer. Furthermore, after the first touch and between touches, they were invited to avoid contact with other subjects, handling only their own personal objects. Moreover, between each application, an interval of at least 60 min was maintained.
For each handler a set of eight different handling times on brassieres was recorded: 60 s for region A, 45 s for B, 30 s for C, 20 s for D, 10 s for E, 8 s for F, 5 s for G, and 2 s for H. Lateral regions were chosen because they are in full contact with the “wearer’s” skin. Reference profiles of both handler and wearer were obtained with buccal swabs.
For consistency, and for the purpose of this study, the same model of brassieres was used with bright textiles (white and beige) made of cotton (85%) and elastane (15%). The experimental model is illustrated in Fig. 1 .
The experimental model is summarized in this picture. Three simulations were performed, one for each sampling method. Moreover, for each simulation 8 DNA depositions (4 for each side) with different contact times were carried out. At the end of the simulations, 240 samples were collected.
All procedures were approved by the Scientific Committee of University of Foggia (Italy).
All participants gave their written informed consent to take part in the study and signed permission for obtaining DNA profiles and all procedures were performed in accordance with the Declaration of Helsinki. The participants were free to withdraw their participation at any time during the course of the study.
Each experiment was carried out in triplicate using the same couple “handler-wearer”. This condition was applied with the aim of detecting the expected DNA profiles (handler and/or wearer), even if it cannot be ruled out that some extraneous DNA may be picked up by the participants while performing their everyday tasks. Negative controls - unworn brasseries of the same material and brand after irradiation with UV rays and before the experimentation phases - were investigated, obtaining 30 control samples. To collect DNA from the brassieres three recovery methods were used, deposit area ≅ 1.5 cm 2 : 1- sampling with dry-swab (Copan, Brescia, Italy) that was rubbed with moderate pressure and rotation on both surfaces (internal and external). The use of a dry-swab was chosen for this experimental model because it was used on a porous surface; in this manner, a possible contamination of the other deposition areas was avoided (which could have happened using the wet/dry double swabbing method); 2- sampling the selected area by cutting it, using sterile scissors, into small pieces; 3- sampling the internal and external area with adhesive tape (Sirchie® Fingerprint Laboratories, Youngsville, NC, USA) as previously described 26 , 28 . A total of 240 samples were obtained.
DNA analysis
All samples were placed in a 1.5 mL tube and DNA extraction was performed following the QIAmp® DNA Investigator Kit protocol (QIAGEN Vic, AUS), with minor modifications (overnight incubation at 56 °C, mixing the samples several times.). DNA was then eluted into 30 μL of AE buffer. The concentration of all DNA extracted was determined with the use of the Quantifiler Duo DNA Quantification kit (Applied Biosystems, Europe BV), according to the manufacturer’s guidelines. Duplicate Quantifiler standards ranging from 50 ng/μL to 0.023 ng/μL and duplicate negative controls were processed in tandem with the reactions. Both reactions were carried out on the ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Europe BV).
All samples were amplified with the Identifiler Plus Amplification Kit (Applied Biosystems): input trace DNA volume was set to 10, 5, 1 μL at a concentration range of 0–0.062, 0.0625–0.125, 0.500–1.000 ng/μL, respectively, in 25 μL PCR final volume. Samples of higher concentrations were diluted down to 1 ng/μL. Amplification was performed by PCR on an Applied Biosystems (Forest City, CA, USA) 9700 thermal cycler following the manufacturer’s specifications.
A positive control, using the 9947 A DNA template, and a negative control, to monitor amplification success and reagent contamination, were also amplified. The amplified product was analyzed in a 10 μL reaction that consisted of 1 μL amplified product plus 9 μL formamide/GeneScan TM 500 LIZ TM dye size standard (Applied Biosystem) mixture, using capillary electrophoresis on an AB 3130 (Applied Biosystem) instrument with Gene-Mapper.
The DNA profiles obtained were analyzed and labeled as unknown profiles. They were subsequently analyzed using an analytical threshold of 50 RFU. In the mixture interpretation, the criteria used to define major or minor contributors were based on peak proportions and peak height ratios (PHR). Considering that PHRs become more varied and tend to have a lower value as the amount of input DNA decreases, a single PHR expectation was applied (60%).
Statistical analysis
Descriptive analyses were performed using frequency percentages (total number of alleles observed/total number of alleles expected). The alleles shared among the handler and wearer at any of the loci were counted for both profiles. The “drop in” alleles were not considered for analysis (no more than 1–2 alleles were observed). Data were analyzed with the software SPSS 22.0 for Windows. The one-way variance analysis (ANOVA) was used to determine any statistically significant differences among the groups. This test was applied to the ratio (alleles observed/alleles expected) from each subject, standardizing the different number of alleles.
In this study, DNA profiles of “handlers” were frequently found as the major contributor and these findings were consistent with all three methods of sampling: “adhesive tape” (AT), “dry swabbing” (DS) and “cutting out” (CO).
The values of DNA concentration are summarized in Fig. 2 . No statistical differences were reported in the box plot analysis considering both the handler time and the extraction techniques (one-way ANOVA [F(2,21) = 3.46, p = 0.52]).
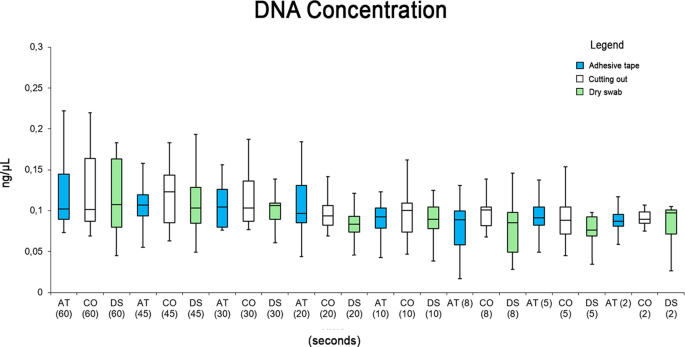
Box plot summarizing the DNA concentrations for all extraction techniques and for handler time.
The “Wearer” DNA profiles were found as the major contributor in the mixture in only 5 cases: these 5 tests were sampled with “adhesive tape” (Table 1 ).
The data are summarized as a percentage (number of alleles observed/number of alleles expected, averages across people and repeats), and are reported in Table 2 . The Amelogenin locus was excluded from this statistical analysis.
Moreover, the data are schematized in a box plot, considering the percentage of profile recovered (Fig. 3 ). There were statistically significant differences among group means as determined by one-way ANOVA [F(1.46) = 920.85, p = <0.05 (4,55 × 10 −32 )]: the handler profiles were recovered in significantly higher number with respect to the wearer profiles.
Box plot summarizing the recovered profiles (data expressed in percentage).
The results obtained were analyzed in relationship to the sampling techniques and to the handling time (Fig. 4 ).
Graph summarizing the recovery percentage (alleles observed/expected) for “adhesive tape” (AT), “cutting out” (CO), and “dry swabbing” (DS), considering the collected profiles “handler” (H) or “wearer” (W) in relation to the handling time.
As summarized in Fig. 5 , the percentage of recovered “handler” profiles decreased at short times (5 and 2 s); moreover, at 60 s there was a great variability in the recovered profiles. This evaluation was obtained without considering the recovery techniques but only the handling time. No statistically significant differences were found among group means with one-way ANOVA [F(7,16) = 2.43, p = 0.066].
Box plot summarizing the recovery percentage (alleles observed/expected) for “handler” profiles recovered related to the handling time.
The same analysis was performed with the “wearer” data (Fig. 6 ): the best recovery for the “wearer” profiles was recorded at the time of two seconds. No statistically significant differences were found among group means with one-way ANOVA [F(7,16) = 1.91, p = 0.134].
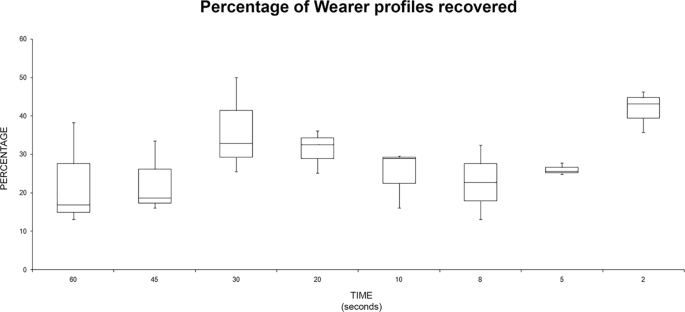
Box plot summarizing the recovery percentage (alleles observed/expected) for “wearer” profiles recovered related to the handling time.
Finally, the data were summarized as percentage, in order to analyze the variation of the sensitivity of each sampling method, observing the “handler” and the “wearer” profiles.
Figure 7 shows that the best values for “handler” profiles were obtained with the “cutting out” technique. However, it is important to note that no statistically significant differences were found among techniques with one-way ANOVA [F(2,21) = 1.52, p = 0.24].
Box plot summarizing the recovery percentage (alleles observed/expected) for “handler” profiles recovered related to the sampling technique.
Figure 8 summarizes the data analyzing the recovery percentage for “wearer” profiles related to the sampling technique: “adhesive tape” was the best technique for “wearer” sampling. There were statistically significant differences among group means as determined by one-way ANOVA [F(2,21) = 4.77, p = 0.019]. Moreover, comparing, singularly, this technique with the others, it was significantly better compared with “dry swabbing” [F (1,14) = 12.3, p = 0.003], while it was not significant when compared with “cutting out” [F (1,14) = 3.37, p = 0.087]. There were no significant differences in the recovering of wearer profiles comparing “cutting out” vs “dry swabbing” [F(1,14) = 4.6, p = 0.32].
Box plot summarizing the recovery percentage (alleles observed/expected) for “wearer” profiles recovered related to the sampling technique.
All negative controls including the unworn brasseries showed no profiles. Finally, 13 profiles showed a “drop in” DNA (1–2 alleles per profile): this result could be linked with the secondary or indirect DNA transfer during the daily activities of the wearer or the handler.
It is a known fact in the forensic community that the recovery of DNA profiles is related to many factors when touch DNA is collected from the clothing of a victim or suspect. Unmanageable factors, such as the type of contact, shedder status of victim and offender, and environmental conditions, can play significant roles in touch DNA recovery. In this study, 10 “handler” males were invited to touch the brassieres worn by 10 “wearer” females for different times: this experimental model was used to enhance the inter-individual variability, considering the presence of good or bad shedders 28 , 29 . The first step in collecting touch DNA is to target the relevant area. Touch DNA on clothing is normally not visible even under a forensic polilight source. Usually, the sampling should be restricted to the area of interest, avoiding the error of sampling the wrong area and thus obtaining a DNA profile not useful for the investigation, with the presence of unknown or “drop in” alleles that could be related to secondary/indirect DNA transfer. On the other hand, the selection of the appropriate recovery method should be standardized to obtain an optimal DNA profile.
In this study, three sampling techniques for detecting touch DNA were compared: “adhesive tape”, “cutting out” the area of interest and “dry swab”. The data reported here show that in recovering the “handler” there were no statistically significant differences among the three techniques analyzed, even if the “cutting out” technique showed the greatest recovery. However, analyzing the data of the “wearer”, the sampling with “adhesive tape” showed the best result among the three techniques: this result was statistically significant. “Dry swab” sampling seems the worst technique for DNA profiling in the proposed experimental model, even though at the shortest periods of contact, this technique yielded the highest number of “handler” profiles recovered compared to the other techniques.
The reported data show that “adhesive tape” and “cutting out” of the area are the two most efficient and sensitive techniques. Nonetheless, each method has its own limitations: the adhesive tape technique is quick and straightforward, however, DNA extraction may require some adjustment, according to Forsberg et al . 19 . On the other hand, “cutting out” the area of interest does not conserve the garment; moreover, it cannot always be applied because not every surface can be cut out. Another problem related to “cutting out” is that both inner and outer surfaces are sampled at the same time increasing the chance of wearer DNA swamping any traces of foreign DNA present and/or generation of uninterpretable complex mixtures.
The low DNA recovery observed with the “dry swabbing” technique could be related to the use of a single swab without a moistening agent, which is required to dissolve epithelial cells from the substrate. Indeed, in other study 30 and in the forensic laboratories, a wet and dry swabbing technique is often used, as it has been accepted as an optimal method for DNA collection when using swabs. Although the use of this sampling technique is undoubtedly a limitation of this experimental model, the dry-swab was applied in this particular experimental design with the aim of avoiding contamination in the contiguous deposition areas, considering the porous surface.
The effects of the handling time were subsequently analyzed to determine if it had any impact on detection of “handler” and/or “wearer” profile. Complete “handler” profiles were found with a high percentage (87.6% to 99.24%) at different touch times, with all sampling methods; the “handler” was the major contributor compared to the “wearer” in all mixed DNA profiles, with the exception of five tests.
The handler was detected in all 240 samples analyzed. In all DNA mixtures, the “wearer” DNA profile was detected when the total DNA concentration was lower than 70 pg. However, in the samples with higher DNA concentrations, we frequently detected only the “handler” profile: it was indicative that the “handler” profile could “overwrite” the “wearer” profile.
The discussed data seem in accordance with Meakin and Jamieson’s review, nevertheless, the friction was not tested 31 . In particular, even if it is commonly thought that the amount of DNA deposited on a surface could be increased with increased handling time, the reviewed data suggest the length of contact is not a significant factor.
This experimental work was performed in particular settings. In fact, the principal limitation of this study design and thus the limitation of the generated results, is related to the sampling area. In routine forensic casework, when sampling for wearer DNA, the target area is usually on the inside and larger than the surface sampled in this experimental model: for examples, Petricevic et al . sampled approximately 3 cm 2 of fabric in their study 32 , while Dong et al . sampled about 4 cm 2 24 . The entire area of such a sample is likely to contain the DNA of the wearer, while the DNA of the handler is likely to be confined to a small area where the garment was touched. For these reasons, in other papers and in real casework, the wearer was frequently detected as a single or a major component 18 , 20 , 21 .
Nevertheless, this study provides insights into the effect of the duration of deposition of touch DNA and on the techniques used to recover it. As previously described, in another experimental study a handling time of 15 s was successful in releasing enough DNA, deemed useful for detecting the “handler” as the major contributor 10 . In this experimental model, the results show that the person handling the garment last contributes the most even though he/she may touch the garment for merely a few seconds (even only 2 seconds); these findings are not influenced by the couple “handler/wearer”.
The discovery of “touch DNA” has broadened the investigators’ horizon since it is possible to obtain genetic profiles from a crime scene in the absence of biological fluids. On the other hand, uncertainties regarding “touch DNA” remain, as “touch DNA” often fails to provide a straightforward and precise answer to the judge. Taking into consideration that clothes are often used as evidence from a crime scene, this study demonstrates that it could be possible to obtain a DNA profile that belongs to the person who had simply just touched the garment. For these reasons, a new insight into forensic genetics is related to the application of mRNA/miRNA technologies to identify the source origin. In the last years, significant researches into developing more definitive, molecular-based, methods for the conclusive identification of all forensically relevant body fluids were performed 33 , 34 , 35 . Moreover, in order to identify the anatomical region of the skin cells sampled, Lindenbergh et al . 36 described a different expression of the mRNAs set used in their study linked to different areas of skin.
More knowledge on the frequency of detection of wearer and/or handler DNA profiles will allow scientists to evaluate the likelihood of obtaining a matching profile if an individual wore a garment rather than simply touching the garment in disputed case scenarios.
Overall, this study demonstrates that the presence of a single DNA profile or the major contributor to a mixture obtained sampling a worn garment may not necessarily be the wearer. Moreover, it should also affect the common practice of generating profiles for a missing person using their clothes, and warrants the need for checking a number of garments instead of just a single garment. This procedure should eventually be applied when there are no toothbrushes, hairbrushes and/or samples from relatives. In this regard, the results of the present study highlight the importance of the sampling area, related to the aim of forensic investigation. Indeed, in casework, when targeting clothing for wearer DNA (as opposed to foreign DNA) only the inner surface is sampled, specifically to avoid foreign DNA that is known to be present mostly on outer surfaces. On the other hand, these experimental data showed that when targeting for foreign DNA, the sample area should be narrowed down as much as possible to the smallest area possible to maximize target DNA recovery. Moreover, the contact duration does not appear to have an effect on the amount of DNA deposited and when a major contributor is detected on a garment, based on this experimental study, it provides an indication of direct contact as opposed to indirect transfer.
Finally, this study highlights an important aspect: everyone in the medico-legal community - forensic scientists and technicians, DNA analysts, potential jurors, judges and lawyers for both the prosecution and defense - must know the power of touch DNA and understand the potential limitations of this technique.
Hanson, E. K. & Ballantyne, J. ‘getting blood from a stone’: Ultra sensitive forensic DNA profiling of microscopic bio-particles recovered from ‘touch DNA’ evidence . Methods in Molecular Biology 1039 (2013).
Burrill, J., Daniel, B. & Frascione, N. A review of trace “Touch DNA” deposits: Variability factors and an exploration of cellular composition. Forensic Sci. Int. Genet. 39 , 8–18, https://doi.org/10.1016/j.fsigen.2018.11.019 (2019).
Article CAS PubMed Google Scholar
van Oorschot, R. A. H., Ballantyne, K. N. & Mitchell, R. J. Forensic trace DNA: A review. Investig. Genet . 1, https://doi.org/10.1186/2041-2223-1-14 (2010).
Article CAS PubMed PubMed Central Google Scholar
Locard, E. The analysis of dust traces. Part 1. Am J Police Sci 1 , 276–98 (1930).
Article Google Scholar
Van Oorschot, R. A. H. & Jones, M. K. DNA fingerprints from fingerprints [6]. Nature 387 , 767 (1997).
Article ADS PubMed Google Scholar
Meuwly, D. In Encyclopedia of Biometrics (eds Li, S. Z. &Jain, A. K.) 1–15, https://doi.org/10.1007/978-3-642-27733-7_181-3 (Springer US, 2009).
Google Scholar
Buckingham, A. K., Harvey, M. L. & van Oorschot, R. A. H. The origin of unknown source DNA from touched objects. Forensic Sci. Int. Genet. 25 , 26–33 (2016).
Montpetit, S. & O’Donnell, P. An optimized procedure for obtaining DNA from fired and unfired ammunition. Forensic Sci. Int. Genet. 17 , 70–74 (2015).
Kirgiz, I. A. & Calloway, C. Increased recovery of touch DNA evidence using FTA paper compared to conventional collection methods. J. Forensic Leg. Med. 47 , 9–15 (2017).
Article PubMed Google Scholar
Breathnach, M., Williams, L., McKenna, L. & Moore, E. Probability of detection of DNA deposited by habitual wearer and/or the second individual who touched the garment. Forensic Sci. Int. Genet. 20 , 53–60 (2016).
Ehrhardt, C. J., Stanciu, C. E., Philpott, M. K., Kwon, Y. J. & Bustamante, E. E. Optical characterization of epidermal cells and their relationship to DNA recovery from touch samples. F1000 Research 4 (2015).
Goray, M., Fowler, S., Szkuta, B. & Van Oorschot, R. A. H. Shedder status - An analysis of self and non-self DNA in multiple hand prints deposited by the same individuals over time. Forensic Sci. Int. Genet. 23 , 190–196 (2016).
Daly, D. J., Murphy, C. & McDermott, S. D. The transfer of touch DNA from hands to glass, fabric and wood. Forensic Sci. Int. Genet. 6 , 41–46 (2012).
Van Oorschot, R. A. H., Glavich, G. & Mitchell, R. J. Persistence of DNA deposited by the original user on objects after subsequent use by a second person. Forensic Sci. Int. Genet. 8 , 219–225 (2014).
Oldoni, F., Castella, V. & Hall, D. Shedding light on the relative DNA contribution of two persons handling the same object. Forensic Sci. Int. Genet. 24 , 148–157 (2016).
Jones, S. et al . DNA transfer through on intimate social contact. Sci. Justice 56 , 90–95 (2016).
Fonneløp, A. E., Ramse, M., Egeland, T. & Gill, P. The implications of shedder status and background DNA on direct and secondary transfer in an attack scenario. Forensic Sci. Int. Genet. 29 , 48–60 (2017).
Helmus, J., Zorell, S., Bajanowski, T. & Poetsch, M. Persistence of DNA on clothes after exposure to water for different time periods—a study on bathtub, pond, and river. Int. J. Legal Med. 132 , 99–106 (2018).
Farash, K., Hanson, E. K. & Ballantyne, J. Single source DNA profile recovery from single cells isolated from skin and fabric from touch DNA mixtures in mock physical assaults. Sci. Justice 58 , 191–199 (2018).
Hess, S. & Haas, C. Recovery of Trace DNA on Clothing: A Comparison of Mini-tape Lifting and Three Other Forensic Evidence Collection Techniques. J. Forensic Sci. 62 , 187–191 (2017).
Poetsch, M., Pfeifer, M., Konrad, H., Bajanowski, T. & Helmus, J. Impact of several wearers on the persistence of DNA on clothes—a study with experimental scenarios. Int. J. Legal Med. 132 , 117–123 (2018).
Sołtyszewski, I., Szeremeta, M., Skawrońska, M., Niemcunowicz-Janica, A. & Pepiński, W. Type ability of DNA in touch traces deposited on paper and optical data discs. Adv. Clin. Exp. Med. 24 , 437–440 (2015).
Pang, B. C. M. & Cheung, B. K. K. Double swab technique for collecting touched evidence. Leg. Med. 9 , 181–184 (2007).
Article CAS Google Scholar
Dong, H. et al . Comparison of preprocessing methods and storage times for touch DNA samples. Croat. Med. J. 58 , 4–13 (2017).
Gunnarsson, J., Eriksson, H. & Ansell, R. Success rate of a forensic tape-lift method for DNA recovery | Ocenawydajnościodzysku DNA metodaa̧ zbieraniapróbeknataśmȩadhezyjnaa̧. Z Zagadnien Nauk Sadowych. 83 , 243–254 (2010).
CAS Google Scholar
Barash, M., Reshef, A. & Brauner, P. The use of adhesive tape for recovery of dna from crime scene items. J. Forensic Sci. 55 , 1058–1064 (2010).
Forsberg, C., Jansson, L., Ansell, R. & Hedman, J. High-through put DNA extraction of forensic adhesive tapes. Forensic Sci. Int. Genet. 24 , 158–163 (2016).
Lowe, A., Murray, C., Whitaker, J., Tully, G. & Gill, P. The propensity of individuals to deposit DNA and secondary transfer of low level DNA from individuals to inert surfaces. Forensic Sci. Int. 129 , 25–34 (2002).
Phipps, M. & Petricevic, S. The tendency of individuals to transfer DNA to handled items. Forensic Sci. Int. 168 , 162–168 (2007).
Wood, I. et al . Efficiencies of recovery and extraction of trace DNA from non-porous surfaces. Forensic Sci. Int. Genet. Suppl. Ser ., https://doi.org/10.1016/j.fsigss.2017.09.022 (2017).
Meakin, G. & Jamieson, A. DNA transfer: Review and implications for casework. Forensic Sci. Int. Genet. 7 , 434–443 (2013).
Petricevic, S. F., Bright, J. A. & Cockerton, S. L. DNA profiling of trace DNA recovered from bedding. Forensic Sci. Int ., https://doi.org/10.1016/j.forsciint.2005.06.004 (2006).
Johnson, D. J. et al . A Molecular Method to Detect Wound Cells in Bloodstains Resultant of Sharp Force Injuries for Crime Scene Reconstruction. J. Forensic Sci. 63 , 842–848 (2018).
Blackman, S. et al . Developmental validation of the ParaDNA® Body Fluid ID System. Forensic Sci. Int. Genet. Suppl. Ser ., https://doi.org/10.1016/j.fsigss.2017.09.214 (2017).
Blackman, S. et al . Developmental validation of the ParaDNA® Body Fluid ID System—A rapid multiplex mRNA-profiling system for the forensic identification of body fluids. Forensic Sci. Int. Genet ., https://doi.org/10.1016/j.fsigen.2018.08.012 (2018).
Lindenbergh, A. et al . A multiplex (m)RNA-profiling system for the forensic identification of body fluids and contact traces. Forensic Sci. Int. Genet ., https://doi.org/10.1016/j.fsigen.2012.01.009 (2012).
Download references
Acknowledgements
The authors thank B. Tomaiuolo for technical assistance and the Scientific Bureau of the University of Catania for language support.
Author information
Authors and affiliations.
Department of Clinical and Experimental Medicine, Section of Legal Medicine, University of Foggia, Foggia, Italy
Francesco Sessa, Monica Salerno, Giuseppe Bertozzi, Pietrantonio Ricci & Santina Cantatore
Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
Giovanni Messina
Institute of Legal Medicine, Università degli Studi Magna Graecia di Catanzaro, Catanzaro, Italy
Pietrantonio Ricci
Department “G.F. Ingrassia” - Section of Hygiene and Public Health, University of Catania, Catania, Italy
Caterina Ledda & Venerando Rapisarda
Department of Surgical, University of Pisa, Pisa, Italy
Emanuela Turillazzi
Department of Medical and Surgical Sciences and Advanced Technologies GF Ingrassia, University of Catania, Catania, Italy
Cristoforo Pomara
You can also search for this author in PubMed Google Scholar
Contributions
F.S., M.S., G.B., E.T. and C.P. conceived and planned the experiments. F.S., G.M., C.L., V.R., and S.C. carried out the experiments. F.S., P.R., M.S. and S.C. contributed to sample preparation. F.S., M.S., G.B., E.T. and C.P. contributed to the interpretation of the results. C.L. and F.S. performed the statistical analysis. F.S. and C.P. wrote the manuscript in consultation with M.S., G.B. and P.R. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding authors
Correspondence to Francesco Sessa or Cristoforo Pomara .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Sessa, F., Salerno, M., Bertozzi, G. et al. Touch DNA: impact of handling time on touch deposit and evaluation of different recovery techniques: An experimental study. Sci Rep 9 , 9542 (2019). https://doi.org/10.1038/s41598-019-46051-9
Download citation
Received : 09 November 2018
Accepted : 17 June 2019
Published : 02 July 2019
DOI : https://doi.org/10.1038/s41598-019-46051-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
DNA (deoxyribonucleic acid) is the nucleic acid polymer that forms the genetic code for a cell or virus. Most DNA molecules consist of two polymers (double-stranded) of four nucleotides that each ...
DNA Research is an internationally peer-reviewed journal which aims at publishing papers of highest quality in broad aspects of DNA and genome-related research ... Accepting high quality papers on broad aspects of DNA and genome-related research. Submit. Related Titles.
In 1987, the New York Times Magazine characterized the Human Genome Project as the "biggest, costliest, most provocative biomedical research project in history." 2 But in the years between the ...
This comprehensive review paper consolidates research efforts, focusing on DNA repair mechanisms, computational research methods, and associated databases. Our work is a valuable resource for scientists and researchers engaged in computational DNA research, offering the latest insights into DNA-related proteins, diseases, and cutting-edge ...
The information encoded by DNA is both digital - the precise base specifying, for example, amino acid sequences - and analogue. The latter determines the sequence-dependent physicochemical properties of DNA, for example, its stiffness and susceptibility to strand separation. Most importantly, DNA chirality enables the formation of supercoiling ...
Introduction. DNA synthesis occurs during the S phase of the cell cycle and is ensured by the replisome, a molecular machine made of a large number of proteins acting in a coordinated manner to synthesize DNA at many genomic locations, the replication origins 1.Replication origin activation in space and time (or replication program) is set by a sequence of events, starting already at the end ...
The introduction of AlphaFold 21 has spurred a revolution in modelling the structure of proteins and their interactions, enabling a huge range of applications in protein modelling and design2-6 ...
Enzymatic DNA labeling is a powerful tool with applications in biochemistry, molecular biology, biotechnology, medical science, and genomic research. This paper contributes to the evolving field of DNA-based data storage by presenting a formal framework for modeling DNA labeling in strings, specifically tailored for data storage purposes.
Replication errors, DNA base mismatches and topoisomerase-DNA complexes. Every time a human cell replicates, approximately 3 X 10 9 bases are copied over by high fidelity replicative polymerases (δ and ε). However, a battery of other DNA polymerases (α, β, σ, γ, λ, REV1, ζ, η, ι, κ, θ, ν, μ, Tdt and PrimPol) can carry out lower fidelity DNA synthesis during DNA replication or ...
Genetic functions of DNA can be understood in two ways: the nitrogen base sequence, constituting the archive of information encoding the sequences of proteins and RNA, and the double helix ...
Synthetic biology is a research area that is emerging rapidly and impacting extensively on biology, nanofabrication, and medicine. ... A survey paper on DNA-based data storage. 2020 International Conference on Emerging Trends in Information Technology and Engineering (Ic-ETITE), IEEE (2020), pp. 1-4.
So it came as rather a shock when, in several 2012 papers in Nature, he and the rest of the ENCODE team reported that at one time or another, at least 75 percent of the genome gets transcribed ...
In our research, "patchy particles" specifically serve as a representation of wireframe DNA origami nanostructures , where a scaffold is constructed with single-stranded overhangs (sticky sequences), located in controllable positions, which can control the interaction between different patches. We show that our approach, which couples ...
Unlike DNA sequence, genomic methylation patterns are not directly inherited during meiosis [], but are mostly reprogrammed in two waves during embryogenesis [13-15].Following this, DNA methylation modifications can be both stable and dynamic during mitosis events that accumulate over the life course [16, 17].These observations suggest that the environment may be a key driving force behind ...
MLA. APA. Chicago. University of California - Santa Cruz. "Cellular activity hints that recycling is in our DNA." ScienceDaily. ScienceDaily, 10 May 2024. <www.sciencedaily.com / releases / 2024 ...
1. Introduction. The potential benefits of DNA profiling are clear. When two DNA profiles do not match, it suggests that the DNA samples were derived from different individuals; it provides exclusionary evidence [1].Post-conviction DNA comparison has helped to exonerate many from wrongful convictions [2].Forensic DNA analysis, based on the analysis of a single or simple mixed DNA sample, has ...
Biotechnology which is synonymous with genetic engineering or recombinant DNA (rDNA) is an industrial process that uses the scientific research on DNA for practical applications. rDNA is a form of ...
Invert the tube containing the DNA pellet on tissue paper to complete draining off the supernatant. xi. Wash DNA pellet with 500 μl of 70% ethanol and invert once (to dissolve residual salts and to increase purity of the DNA). xii. ... (MJ Research PTC-100 thermocycler) programmed to perform an initial denaturation step of 98 °C for 2 min ...
AlphaFold 3 can predict how DNA, RNA, and other molecules interact, further cementing its leading role in drug discovery and research. Who will benefit? Google DeepMind has released an improved ...
The DNA polymerases that appear most able to tolerate modifications include KOD Dash, ... (GM060005 and GM097489) and the Defense Advanced Research Projects Agency Folded Non-Natural Polymers with Biological Function Fold F(x) Program (Award No. N66001-14-2-4052). ... Papers of particular interest, published within the period of review, have ...
Current research in forensic DNA and genetic analysis have focused on the reconstruction of the physical appearance of individuals—DNA phenotyping (Kayser, ... In this focus paper, we reviewed the major reasons that may limit the effectiveness of forensic DNA analysis, including the scope of its application, significance or usefulness ...
Move over, chatbots. This upgraded AI can model antibodies, DNA, and molecules from disease organisms. This next generation of AlphaFold, from Google Deepmind, is poised to significantly advance ...
New DNA origami technique promises advances in medicine. by University of Portsmouth. Graphical abstract. Credit: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03413. A new ...
1.2. Flexibility of DNA Molecule as a Polymer. The DNA molecule is a long-chain polymer that consists of two antiparallel polynucleotide chains that coil around each other to form a double helix [8,9].Non-B-DNA structures, such as A-form or Z-form, are induced by a high salt or high ethanol concentration [10,11,12], whereas the double helix of DNA in an aqueous solution of low ion ...
In antibacterial applications, chitosan exhibits potent antimicrobial properties by disrupting microbial membranes and DNA, making it a promising natural preservative and agent against bacterial infections. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be ...
Even though the principal aspects of "Touch DNA" have been extensively studied 10,11,12,13, to date, there are few reports in the research field of DNA retrieval from garments that have been ...