Disclaimer » Advertising
- HealthyChildren.org

- Next Article

Practice Gaps
Introduction, epidemiology, pathogenesis, clinical presentations, cf management, therapies to maintain optimal lung health, therapies to maintain optimal nutritional status, cftr modulator therapies, diagnosis and management of common pulmonary and extrapulmonary complications of cf, cystic fibrosis.
AUTHOR DISCLOSURE
Drs Dickinson and Collaco have disclosed no financial disclosures relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of commercial products/devices.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Kimberly M. Dickinson , Joseph M. Collaco; Cystic Fibrosis. Pediatr Rev February 2021; 42 (2): 55–67. https://doi.org/10.1542/pir.2019-0212
Download citation file:
- Ris (Zotero)
- Reference Manager
Cystic fibrosis (CF) is one of the most commonly diagnosed genetic disorders. Clinical characteristics include progressive obstructive lung disease, sinusitis, exocrine pancreatic insufficiency leading to malabsorption and malnutrition, liver and pancreatic dysfunction, and male infertility. Although CF is a life-shortening disease, survival has continued to improve to a median age of 46.2 years due to earlier diagnosis through routine newborn screening, promulgation of evidence-based guidelines to optimize nutritional and pulmonary health, and the development of CF-specific interdisciplinary care centers. Future improvements in health and quality of life for individuals with CF are likely with the recent development of mutation-specific modulator therapies. In this review, we will cover the current understanding of the disease manifestations, diagnosis, and management as well as common complications seen in individuals with CF.
In light of the significant increase in the median survival age for individuals with cystic fibrosis (CF), both pediatric and adult providers should be familiar with the current recommendations related to optimization of lung health and nutritional status.
Providers should also be aware of the development of modulator therapies that target the different basic genetic defects of the disease, which have allowed for personalized therapies that promise continued improvement in outcomes. As of 2019, approximately 90% of individuals with CF have mutations that would benefit from modulator therapy.
Clinicians should be familiar with the clinical presentation, diagnosis, and current management of CF as well as the more common disease-related morbidities.
After completing this article, readers should be able to:
Describe the common clinical manifestations of cystic fibrosis (CF) as well as the laboratory and genetic studies needed to diagnose CF.
Recognize the most common presentations of CF-related morbidities.
Describe the current recommendations for long-term maintenance of optimal lung health, nutritional status, and other involved organ systems in children with CF.
Cystic fibrosis (CF) is a common life-shortening autosomal recessive genetic disorder characterized by pulmonary manifestations, specifically chronic and progressive obstructive lung disease, sinusitis, malabsorption due to pancreatic exocrine insufficiency leading to malnutrition, liver disease (biliary cirrhosis), and CF-related diabetes mellitus (CFRD). Earlier diagnosis through newborn screening (NBS), improved therapies to optimize lung health and nutritional status, as well as aggressive treatment of chronic respiratory infections and lung transplant for end-stage lung disease have led to significant improvements in survival. CF was a uniformly fatal disease in childhood at the time of its initial description in 1938, ( 1 ) but the predicted median survival is currently 46.2 years. Currently, more than 50% of people living with CF are 18 years and older, ( 2 ) resulting in the evolution of the disease from exclusively a disease of childhood to a disease where affected individuals transition to adulthood and adult providers. With the advent of newer therapies targeting the basic genetic defect that causes the disease and the expansion of the age and genetic variants for which these therapies are indicated, there is promise of continued improvement in quality of life as well as overall health and survival. Individuals with CF benefit from coordination of care between their primary care providers and their interdisciplinary CF care team, in addition to routine visits with both. This review covers the current understanding of the disease manifestations, diagnosis, and management as well as common complications seen in individuals with CF.
CF is one of the most common genetic disorders among white people, with an incidence of 1:3,200 individuals. ( 3 ) The incidence does vary significantly by race/ethnicity, with an incidence of 1:13,500 in people of Hispanic background, 1:15,000 in people of African descent, and 1:35,000 in people of Asian descent. ( 4 ) It is estimated that 1 in every 35 Americans is a carrier of CF. ( 5 ) Based on statistics from the 2019 Annual Data Report published by the Cystic Fibrosis Foundation (CFF), there are an estimated 31,000 affected individuals in the United States living with CF. ( 2 ) Worldwide, there are an estimated 70,000 affected people, with the highest prevalence in North America, Europe, and Australia. Annually, approximately 1,000 new cases are diagnosed in the United States (although the rate of new cases may be declining related to preconception screening), ( 6 ) and the incidence is equal in males and females. Since 2010, all 50 states screen neonates for CF, ( 7 ) and, as a result, >60% of new diagnoses occur via NBS. ( 2 )
CF is a multisystem disorder that results from deleterious genetic variants in the CFTR gene located on chromosome 7q31.2, which encodes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Defects in this protein lead to absent or malfunctioning chloride channels in the apical membranes of the lung surface epithelium, resulting in the formation of thick and sticky mucus, leading to chronic lung infections, pancreatic and liver dysfunction, and reduced fertility. CF also results in abnormal chloride channel function in the sweat glands, resulting in excessive salt loss in sweat, an observation first made by Dr Paul di Sant’Agnese after caring for infants with CF presenting with dehydration during a heat wave in New York City in 1948. ( 8 ) This clinical observation paved the way for the pilocarpine iontophoresis sweat test for CF diagnosis. ( 9 )
CF is an autosomal recessive disorder, and for individuals to have CF they must inherit 2 deleterious CFTR variants. To date, there are more than 2,000 different CFTR variants reported, some of which are confirmed to cause CF and others with more putative links to the disease. ( 10 )( 11 ) They are classified into 6 distinct groups that reflect abnormalities of CFTR protein synthesis, structure, and function ( Fig 1 ). The most common CF-causing variant is F508del (p.Phe508del). F508del is a class II mutation, meaning that the CFTR protein is created, but misfolding prevents it from reaching the cell surface (trafficking defect). Overall, 44.4% of individuals with CF are homozygous for F508del , an additional 40.9% have 1 copy of F508del and another variant, and 14.7% have 2 non- F508del variants. ( 2 ) The specific CFTR variants that an individual carries determine the amount of functioning CFTR protein present and are partially correlated with phenotypic severity and organ involvement. ( 12 )

Cystic fibrosis transmembrane conductance regulator (CFTR) mutation classes. CFTR mutations have been grouped into 6 distinct classes based on abnormalities of CFTR synthesis, structure, and function. Reprinted with from: Boyle MP, DeBoeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med . 2013;1(2):158–163.
The classic manifestations of CF include a triad of recurrent sinus and pulmonary infections, steatorrhea, and malnutrition, which in its most severe form presents as failure to thrive. In the lungs, mucous plugging from dehydrated thick secretions results in inflammation, chronic infection, progressive obstruction of the small airways, and the development of bronchiectasis, which is an abnormal, permanent enlargement of the bronchi ( Fig 2 ). Bronchiectasis leads to decreased ability to clear secretions, causing increased rates of infections, which further dilates and damages the airways. In addition, the effects of diminished or absent chloride channel function can result in dysfunction in several other organ systems ( Fig 3 ). Pancreatic involvement includes pancreatic exocrine insufficiency, which results in fat, protein, and carbohydrate malabsorption and subsequent malnutrition, as well as insulin insufficiency and the development of CFRD.

Chest computed tomographic scan of an adolescent girl with cystic fibrosis demonstrates significant bronchiectasis (white open arrows) and mucous plugging (white asterisks). Reprinted from: Paranjape SM, Mogayzel PJ. Cystic fibrosis. Pediatr Rev. 2014;35(5).

Common clinical manifestations of cystic fibrosis. Reprinted with permission from Link Studio LLC.
In infants and young children, other presentations may also be indicative of CF. In utero, ultrasonographic evidence of hyperechogenic or dilated bowel suggests intestinal obstruction, which has been reported in 50% to 78% of fetuses affected with CF. ( 13 )( 14 ) Postnatally, delayed meconium passage or meconium ileus is present in 11.9% of infants younger than 1 year with CF; ( 2 ) it results from thick gastrointestinal secretions that become adherent to the intestinal mucosa, leading to bowel obstruction. Meconium ileus is often accompanied by abdominal distention and dilated loops of bowel on imaging, and a reported 30% of cases of meconium ileus are complicated by intestinal perforation and peritonitis. ( 2 ) Approximately 20% of untreated children (aged 6 months to 3 years) have rectal prolapse, which is secondary to malabsorption, malnutrition, and bulky stools as opposed to constipation. ( 15 ) Other clinical presentations during the neonatal period may include prolonged jaundice secondary to biliary stasis or bile duct obstruction and hemorrhagic disease of the newborn owing to vitamin K deficiency. Throughout infancy and early childhood, individuals may also present with salt depletion syndrome characterized by a hyponatremic, hypochloremic, hypokalemic metabolic alkalosis and edema/acrodermatitis due to hypoproteinemia from malabsorption. ( 16 )
Typical respiratory findings in older children, adolescents, and adults who newly present with CF may include recurrent sinusitis, bronchitis, or pneumonias; asthma that is poorly responsive to standard management; nasal polyposis or digital clubbing on physical examination; and bronchiectasis on lung imaging studies. ( 16 ) Common gastrointestinal symptoms may include malnutrition, poor growth, steatorrhea, intestinal obstruction, chronic constipation, rectal prolapse, and liver disease. ( 17 ) Individuals with pancreatic-sufficient CF (who are more likely to be diagnosed later in life due to appropriate weight gain) can present with pancreatitis secondary to progressive pancreatic inflammation, although the exact etiology is unclear. ( 18 ) Finally, more than 98% of men with CF are infertile as a result of obstructive azotemia secondary to congenital bilateral absence of the vas deferens and may be diagnosed during an infertility evaluation. ( 19 ) NBS is expected to reduce, but not eliminate, late clinical presentations given the expected rate of false-negatives associated with screening. Clinicians should include CF in the differential diagnosis for unexplained recurrent respiratory bacterial infections (pneumonia, bronchitis, persistent cough, and/or sinusitis) and/or failure to thrive.
NBS for CF, first introduced in the 1980s, is performed by measuring an immunoreactive trypsinogen (IRT) level in screening neonatal blood spots. In neonates with CF, mucous plugs partially block the pancreatic ducts that lead into the small intestine, preventing trypsinogen from reaching the intestine and being converted to the pancreatic enzyme trypsin, even in infants with CF who are pancreatic sufficient. If an infant has an elevated IRT level, almost all US state laboratories perform confirmatory CFTR variant testing (IRT/DNA), with 1 state laboratory currently repeating the IRT measurement (IRT/IRT). Screening is considered positive if the IRT level remains persistently elevated between ages 7 and 14 days or if at least 1 deleterious CFTR variant is identified on genetic testing. A positive NBS result will trigger notification of either a neonatal intensive care provider or a primary care provider, and the infant should be referred to a CFF-accredited center for definitive evaluation and sweat testing within 72 hours of a positive screening result. ( 20 ) Care centers may be located on the CFF website ( http://www.cff.org/ccd/ ).
The benefits of diagnosing asymptomatic infants with CF include increased attention to early lung health to slow lung disease progression, optimization of nutritional status with early enzyme replacement and aggressive nutritional counseling, and provision of psychosocial support to families to help prevent or delay serious complications. ( 16 ) Possible risks of NBS include an increased number of medical interventions, earlier exposure to respiratory pathogens through attending a CF clinic, financial hardships given the cost of CF-related therapies, possible iatrogenic complications (eg, early exposure to therapies with adverse effects), and caregiver anxiety or guilt stemming from false-positive screening results due to perinatal asphyxia or other perinatal problems. ( 16 )( 21 ) False-positive rates may also be higher in African American children because they have higher IRT levels than white children, ( 22 ) but a much lower risk of CF. NBS can also be falsely negative, particularly in neonates with meconium ileus ( 23 ) or those screened via IRT/DNA because this testing may be less sensitive for picking up mutations in minority populations. ( 22 ) With current NBS practices, the possibility exists for a positive NBS and the identification of CFTR variants that do not meet clinical criteria for CF diagnosis in individuals with normal or intermediate sweat chloride testing, a syndrome known as CFTR-related metabolic syndrome/CF screen positive, inconclusive diagnosis. ( 24 )
The CFF published consensus guidelines in 2017 establishing that a diagnosis of CF can be made if an individual has a clinical presentation consistent with the disease, ie, 1) a positive NBS result, 2) clinical features consistent with CF (the presence of characteristic phenotypes such as of chronic, recurrent sinus and pulmonary disease, nutritional and gastrointestinal abnormalities, urogenital abnormalities in males [eg, absence of the vas deferens], and/or salt depletion syndromes), or 3) a positive family history of CF and evidence of CFTR dysfunction (eg, a sweat chloride concentration ≥60 mEq/L[≥60 mmol/L]). ( 24 )( 25 )
Although prenatal screening and NBS have allowed for earlier detection of CF in asymptomatic individuals, the quantitative pilocarpine iontophoresis sweat test remains the gold standard for the diagnosis of CF. The sweat test, developed by Drs Lewis Gibson and Robert Cooke in 1959, ( 9 ) specifically measures the amount of chloride in a person’s sweat. Sweat chloride testing should be performed as soon as possible after a positive NBS result. It can be performed as early as 48 hours after birth (because sweat sodium levels are transiently elevated in the first 24 hours) ( 26 ) but should be undertaken as soon as possible after 10 days of age and ideally by 4 weeks of age. Infants should weigh more than 2 kg or be corrected to 36 weeks’ gestation to increase the likelihood of adequate sweat collection. ( 24 ) Infants with meconium ileus and infants and children with symptoms suggestive of CF, such as recurrent bacterial respiratory infections and/or failure to thrive, should receive sweat chloride testing regardless of age or NBS results. Any sweat test with an abnormal result should be repeated on a separate date or confirmed with genetic testing.
Results from sweat chloride testing can be categorized into diagnostic, intermediate, and unlikely. Diagnostic sweat chloride values are 60 mEq/L or greater (≥60 mmol/L) and require a confirmatory second sweat test or 2 identified CF-causing genetic variants to make the diagnosis. Intermediate values are between 30 and 59 mEq/L (30-59 mmol/L); sweat chloride testing should be repeated periodically in these individuals, and further evaluation at a CF center should be considered. A diagnosis of CF can still be made in an individual with an intermediate value if the individual has 2 identified CF-causing genetic variants. Individuals with an intermediate sweat chloride value of 30 to 59 mEq/L (30-59 mmol/L) and 0 to 1 CF-causing genetic variants may be diagnosed as having a CFTR-related disorder depending on clinical presentation and family history. Individuals with sweat chloride values less than 30 mEq/L (<30 mmol/L) are unlikely to have CF, but if 2 CF-causing genetic variants are identified, they should still be diagnosed as having CF. ( 24 )
In conjunction with sweat testing, genetic testing is now widely available to help confirm a diagnosis of CF, particularly for cases with intermediate sweat chloride values. Identifying an individual’s specific CF-causing variants is needed for prescribing CFTR modulator therapies, which are approved for particular CFTR variants. A diagnosis by genotype can be made with the identification of 2 known pathogenic variants on separate chromosomes. Most individuals with CF can be diagnosed through commercial laboratories, which test for the most common CFTR variants, ( 27 ) but complete sequencing of the CFTR gene may be necessary to help confirm the diagnosis in individuals with clinically atypical presentations. ( 16 ) Clinical information related to specific CFTR variants can be found online ( http://cftr2.org ).
The era of genetic testing has expanded our understanding of CFTR dysfunction but has also added complexity to the diagnosis of CF because there are individuals with CF phenotypes without known CF-causing mutations as well as individuals with detected mutations who remain asymptomatic. The limitations of sweat chloride testing and genetic testing may require performing both tests in selected patients where there is strong clinical suspicion of CF. ( 28 )
In the United States, individuals with CF should be evaluated a minimum of quarterly in a CFF-accredited care center, ( 29 ) which can provide interdisciplinary, patient/family-centered care. Infants younger than 6 months should be evaluated monthly and then every 1 to 2 months in the second 6 months after birth. ( 20 ) These regular visits allow for education surrounding airway clearance methods, infection prevention, monitoring of age-appropriate growth and weight gain, and, in older children, assessment of lung function.
CF is characterized by viscous airway secretions that lead to chronic mucous obstruction, inflammation, and recurrent infections that result in long-term damage to the airways (bronchiectasis) and lung parenchyma. ( 16 ) Chronic cough and sputum production are characteristic symptoms. Management of respiratory symptoms focuses on maintaining lung function and preventing the development of bronchiectasis and parenchymal destruction. ( 30 ) In addition to encouraging a smoke-free environment, children with CF are often prescribed several therapies, which will likely be lifelong and should be initiated shortly after diagnosis.
Clearance of Airway Secretions.
A critical aspect of maintaining lung health is airway clearance therapy (ACT). By removing airway mucus, ACT helps decrease the respiratory bacterial load along with irritants, leading to improved gas exchange and a decrease in airway obstruction. ( 16 ) Twice daily ACT as maintenance is recommended for all patients with CF, regardless of symptoms or disease severity, and is increased in frequency during acute CF pulmonary exacerbations (PEx). ( 31 ) Commonly used ACT modalities include manual percussion, positive expiratory pressure devices, and high-frequency chest wall oscillation (achieved through an inflatable vest that performs chest physical therapy by vibrating at a high frequency). No form of ACT has been demonstrated to be superior to any other form, and ACT choice should be personalized. Exercise should be encouraged as an adjunctive therapy but should not be used as a substitute for airway clearance. ( 31 ) Nebulized agents that thin the viscous mucus of CF are commonly used with ACT and include recombinant human rhDNase (dornase alfa [Pulmozyme®; Genentech Inc, South San Francisco, CA]) and hypertonic saline.
Chronic Airway Infections.
In addition to patient education and infection control measures, aggressive management of chronic airway infections has been shown to prevent lung function decline. ( 32 ) Management includes frequent respiratory cultures (oropharyngeal or sputum), including surveilling for Staphylococcus aureus (particularly methicillin-resistant S aureus ) and Pseudomonas aeruginosa . ( 16 ) Receiving microbiology laboratories should be made aware of the patient’s CF diagnosis to assess for the respiratory pathogens commonly seen in CF. The initial acquisition of P aeruginosa is typically treated with antipseudomonal antibiotics, such as nebulized tobramycin, in an attempt to achieve eradication. ( 33 ) Nebulized antibiotics, such as tobramycin or aztreonam (Cayston®; Gilead Sciences Inc, Foster City, CA), can also be used as suppressive therapy for individuals with chronic infection or colonization with P aeruginosa and/or other gram-negative organisms. This suppressive therapy is administered every other month to decrease the risk of antibiotic resistance. Other organisms, including Burkholderia cepacia complex, nontuberculous mycobacteria ( Mycobacterium avium complex and Mycobacterium abscessus ), and fungal pathogens ( Aspergillus fumigatus ), are also monitored because they can have a significant effect on CF lung disease. Of note, individuals with CF are also prone to developing a hypersensitivity reaction to Aspergillus , known as allergic bronchopulmonary aspergillosis (ABPA), which can affect lung function and requires management with corticosteroid therapy. ( 34 )
Chronic Airway Inflammation.
Cystic fibrosis lung disease is caused by a combination of infection and inflammation. The routine use of oral or inhaled corticosteroids in CF is not indicated unless used for another inflammatory comorbidity such as asthma or ABPA. ( 30 ) Chronic airway inflammation is managed with either high-dose ibuprofen or azithromycin. ( 35 ) Although ibuprofen has proven benefits in CF, the risk of gastrointestinal bleeding and the need for monitoring serum levels has limited its use. ( 36 ) Azithromycin therapy has been demonstrated to result in improved lung function and a reduced number of PEx, and it is typically given orally 3 times per week. ( 37 ) However, there is concern that individuals with unrecognized mycobacterial infections receiving azithromycin long-term may develop resistance; screening using mycobacterial cultures is recommended before initiating treatment. ( 30 )
Decline in pulmonary status is the hallmark of CF; however, poor growth is one of its earliest manifestations. The combined effects of decreased intake, malabsorption, and increased metabolic demands contribute to the poor growth seen as early as infancy. Malnutrition has been associated with increased morbidity and mortality in CF. ( 38 ) CFF guidelines recommend that all children achieve a weight-for-length at or above the 50th percentile by 2 years old and that all children and adolescents aged 2 to 20 years maintain a BMI at or above the 50th percentile. ( 20 )( 39 ) Education about the role of enteral tube feeding in optimizing nutritional status should be provided to caregivers and patients throughout their lifetime. ( 40 ) Infants with CF should receive human milk if possible, and otherwise should receive standard infant formula (rather than hydrolyzed protein formulas). ( 20 ) Infants younger than 2 years should be supplemented with table salt, up to ¼ tsp per day by 6 months of age, ( 20 ) due to ongoing salt losses. Infants with CF should have their fecal elastase measured after diagnosis to assess pancreatic functional status because 85% of individuals with CF are exocrine pancreatic insufficient (fecal elastase level <200 μg/g). ( 2 ) Pancreatic insufficiency leads to malabsorption, which presents as bulky, malodorous stools; malnutrition; and, ultimately, failure to thrive.
Pancreatic Enzyme Replacement Therapy.
Pancreatic enzyme replacement therapy (PERT) should be initiated in those with a diagnostic fecal elastase level or 2 CFTR variants associated with pancreatic insufficiency as well as those with unequivocal signs or symptoms of malabsorption. Individuals who have exocrine pancreatic insufficiency require enzyme replacement with every meal, snack, and enteral tube feeding, ranging from 2,000 to 2,500 U/kg of lipase per meal to a maximum of 10,000 U/kg per day. ( 29 )( 41 ) Immobilized lipase cartridges may be used for patients on continuous enteral tube feeding to help hydrolyze fats in enteral formulas. Exceeding recommended dosages generally does not result in improved nutrient absorption, and supratherapeutic dosing is associated with fibrosing colonopathy, an uncommon complication characterized by foreshortening and strictures of the right colon. ( 42 ) Individuals with CF can achieve age-appropriate growth with optimized PERT. ( 43 )
Fat-Soluble Vitamin Replacement Therapy.
Pancreatic insufficiency results in malabsorption of fat and associated fat-soluble vitamins A, D, E, and K. Vitamin A deficiency can be associated with night blindness and ocular xerosis, as well as dermatologic manifestations, such as follicular hyperkeratosis. ( 16 ) Vitamin D deficiency may result in rickets, osteopenia, and osteoporosis, which can result in fractures; recommendations for individuals with CF specify maintaining a goal serum 25-hydroxyvitamin D level of at least 30 ng/mL (≥75 nmol/L). ( 44 ) Vitamin E deficiency may result in peripheral neuropathy, myopathy, and hemolysis, and vitamin K deficiency is associated with coagulopathy and may also contribute to bone disease. ( 16 ) Supplemental vitamin therapy should begin after diagnosis, and annual monitoring of serum vitamin A, D, and E levels by CF providers is recommended. ( 20 )
CFTR modulators are the first therapies to target the basic defect in CF by directly acting on the CFTR protein. They are categorized into 3 types: potentiators, correctors, and amplifiers. ( 45 ) Ivacaftor (Kalydeco®; Vertex Pharmaceuticals Inc, Boston, MA), the first approved modulator therapy, is a potentiator, which helps improve chloride flow through the CFTR protein at the cell surface for patients with class III-V mutations. Correctors such as lumacaftor and tezacaftor help the CFTR protein to form correctly and allow the protein to move, or traffic, to the cell surface. When added to potentiators, correctors such as lumacaftor/ivacaftor (Orkambi®; Vertex Pharmaceuticals Inc) or tezacaftor/ivacaftor (Symdeko®; Vertex Pharmaceuticals Inc) work to improve the amount of protein that reaches the cell surface for patients with class II mutations. Kalydeco is currently Food and Drug Administration (FDA)–approved for individuals 6 months and older, Orkambi for those 2 years and older with homozygous F508del variants, and Symdeko for those 6 years and older with homozygous F508del or several other specific CF variants.
In 2019, the FDA approved the use of a triple-combination therapy, elexacaftor/tezacaftor/ivacaftor (Trikafta®; Vertex Pharmaceuticals Inc), for individuals 12 years and older with at least 1 F508del variant. This new therapy will allow nearly 90% of individuals with CF to have a highly effective therapy for the underlying cause of their disease. Trikafta has been shown in clinical trials to dramatically improve key measures of disease, including increasing lung function, reducing PEx, decreasing sweat chloride values, increasing BMI, and improving patient-reported quality of life. Amplifiers are expected to increase the amount of CFTR protein that a cell makes, but they are under development and not currently available clinically. ( 46 )
Pulmonary Exacerbations.
One of the most common complications of CF lung disease is episodic acute worsening of symptoms, referred to as PEx. PEx are characterized by increased respiratory symptoms, including coughing, sputum production, and/or wheezing; a decline in pulmonary function measures (specifically, forced expiratory volume in 1 second [FEV 1 ]); fatigue; decreased appetite; and weight loss. ( 47 ) Fevers are not commonly seen with PEx. ( 16 ) The frequency of PEx varies among individuals, but contributes to the long-term lung function decline of most people with CF. Treatment typically includes antibiotics and increased frequency of ACT to help clear secretions from the airways. Antibiotic therapy and mode of delivery (enteral, inhaled, and/or intravenous) are dictated by the severity of the exacerbation and previous/current respiratory culture results ( Fig 4 ).

Prevalence of bacterial pathogens in cystic fibrosis. MDR-PA=multidrug-resistant Pseudomonas aeruginosa, MRSA=methicillin-resistant Staphylococcus aureus . Reprinted from: Cystic Fibrosis Foundation Patient Registry © 2017 Cystic Fibrosis Foundation.
Hemoptysis.
Hemoptysis is reported to occur in 3% of individuals with CF annually. ( 2 ) Although often seen with severe lung disease, it can also be a manifestation of a PEx. ( 48 ) CF-related hemoptysis is most commonly a result of chronic infection and inflammation, leading to erosion of hypertrophied bronchial arteries into the airways. ( 49 ) Vitamin K deficiency, either from malabsorption or liver disease, can contribute to hemoptysis. Managing scant to moderate hemoptysis (≤240 mL) includes evaluation, likely antibiotic therapy for PEx management, and consideration of limiting certain exacerbating therapies, such as ibuprofen, hypertonic saline, DNAse, and ACT. ( 48 ) Massive hemoptysis (>240 mL) is considered life-threatening, and management includes appropriate stabilization and discontinuation of anti-inflammatory and airway clearance measures. The treatment for massive hemoptysis or significant recurrent hemoptysis is bronchial artery embolization, if the site of bleeding can be identified. ( 48 )
Pneumothorax.
Pneumothoraces occur secondary to air trapping. High alveolar pressure forces air into the lower pressure interstitial spaces, leading to air leak into the pleural space, resulting in symptoms of acute chest pain and dyspnea. ( 16 ) The prevalence of having at least 1 pneumothorax is 3.4% among individuals with CF. ( 50 ) Pneumothoraces are more likely in adults and those with advanced lung disease. ( 50 ) The initial diagnostic test is chest radiography, but computed tomography may be required to define the extent of a pneumothorax in severe lung disease. ( 16 ) Small pneumothoraces may be observed or treated with needle aspiration, but large pneumothoraces require chest tube placement and hospitalization. ( 48 ) Pleurodesis is an option for recurrent pneumothoraces but may complicate later lung transplant. After development of a pneumothorax, individuals should refrain from devices that use positive pressure (including vest therapy) as well as pulmonary function testing for at least 2 weeks because these can hinder the resolution of the pneumothorax or lead to recurrence. ( 48 )
Chronic Rhinosinusitis and Nasal Polyposis.
The epithelium of the sinuses also possesses defective CFTR protein, so chronic pan-sinus disease is almost universal in individuals with CF. ( 51 ) In contrast, the prevalence of nasal polyposis is more variable and increases with age. ( 52 ) Both rhinosinusitis and nasal polyposis result from mucous obstruction of the sinus ostia. Clinical presentations may include chronic headache and facial pressure. Long-term findings may include broadening of the nasal bridge and septal deformation from chronic nasal obstruction. ( 16 ) Medical management includes saline nasal irrigation to aid with mucus clearance and nasal steroids to decrease inflammation. Surgical treatment for severe and recurrent disease can improve mucus clearance, but may not necessarily improve lung function. ( 53 )( 54 )
Distal Intestinal Obstruction Syndrome.
Distal intestinal obstruction syndrome (DIOS) is a common gastrointestinal complication in CF. It presents as partial or complete small bowel obstruction secondary to viscous fecal impaction in the distal intestine. Clinical manifestations include abdominal pain and distention, emesis, and a history of decreased stooling. Pathophysiology may be related to CFTR-dependent bile acid secretion and uptake in the distal ileum. ( 55 ) Known risk factors for DIOS include dehydration, dietary changes, suboptimal fat absorption (ie, inadequate PERT dosing), immobilization, bacterial overgrowth, a previous episode of DIOS, and constipating medications. ( 55 )( 56 ) The differential diagnosis for DIOS includes intussusception, constipation, intestinal adhesions from previous abdominal surgery, volvulus, inflammatory bowel disease, and appendicitis. ( 16 )( 56 ) Abdominal radiography, history, and physical examination are usually sufficient to make the diagnosis, but other causes of bowel obstruction should be considered. Management typically includes rehydration and osmotic laxative therapy. More severe cases may require inpatient admission, intravenous fluids, complete bowel rest, and the use of large volumes of polyethylene glycol. Near-complete obstruction may require sodium meglumine diatrizoate (Gastrografin®; Bracco Diagnostics Inc, Monroe Township, NJ) enemas with retrograde lavage and visualization of the terminal ileum by an experienced radiologist. With early diagnosis and implementation of appropriate medical management, surgical interventions are generally not required for DIOS. ( 16 )
CF-Related Diabetes Mellitus.
CF leads to disruption of both the exocrine and endocrine functions of the pancreas. CFRD results from ongoing obstructive damage to the pancreas from thick secretions, which in turn results in fatty infiltration of the pancreas and islet cell destruction. ( 16 ) CFRD typically presents after the first decade and occurs in up to 20% of adolescents and at least 50% of adults with CF. ( 57 ) CFRD is typically diagnosed in patients with pancreatic insufficiency and has been associated with increased morbidity and mortality through worse nutritional status and decreased lung function. ( 58 )( 59 ) CFRD is often asymptomatic, and unexplained decreases in growth, weight, or lung function may be related to occult CFRD. Annual screening conducted via 2-hour oral glucose tolerance test should be initiated at age 10 years. ( 60 ) Studies have found that fasting levels of hyperglycemia or increased levels of glycated hemoglobin are not sufficiently sensitive for the diagnosis of CFRD; therefore, hemoglobin A1c levels should not be used for screening for CFRD. ( 61 ) However, hemoglobin A1c levels can be used to monitor glucose control in individuals with CFRD. Although microvascular complications such as retinopathy, microalbuminuria, and neuropathy may occur with CFRD, similar to other forms of diabetes, ketoacidosis is uncommon. ( 16 )( 60 ) The management of CFRD is focused on glycemic control through insulin therapy; oral antihyperglycemic agents are not as effective as insulin in improving long-term outcomes. Nutritional management remains focused on maintaining a high-calorie diet while attempting to limit intake of processed carbohydrates to avoid hyperglycemia.
CF Liver Disease.
Liver disease accounts for 3.2% of overall CF mortality. ( 2 ) Approximately 3% of individuals develop CF-related cirrhosis (primarily individuals with pancreatic insufficiency), with a median age at diagnosis of 10 years. ( 62 )( 63 ) Clinical manifestations include cholestasis, cholelithiasis, cirrhosis, portal hypertension, and, in severe cases, end-stage liver disease. The pathophysiology is thought to be related to the role of CFTR in promoting bile flow, with abnormal flow leading to cholestasis and biliary fibrosis. ( 64 ) Males as well as carriers of alpha-1 antitrypsin Z allele are at increased risk for advanced liver disease. ( 64 )( 65 ) Periodic screening and evaluation for CF liver disease is critical because many individuals remain asymptomatic even with advanced cirrhosis. Annual screening of individuals should include assessment of liver function (aspartate aminotransferaseAST, ALT, and GGT), an ultrasound evaluation and/or evaluation by a gastroenterologist for other causes of liver disease, as indicated. The presence of CFLD should be considered with at least 2 of the following( 1 ): abnormal physical examination findings such as hepatosplenomegaly, ( 2 ) abnormalities of liver function test results above normal reference ranges on at least 3 consecutive determinations during a 12-month period, ( 3 ) ultrasound with Doppler evidence of abnormal liver echotexture or portal hypertension, or( 4 ) tissue biopsy has ruled out other causes of liver disease. ( 66 While there are currently no proven therapies to prevent development or progression of CF-related cirrhosis, ursodiol is frequently used in the management of hyperbilirubinemia in CFLD.
Bone Disease.
Another complication of CF is cystic fibrosis-related bone disease, which manifests as low bone density and increased rates of fractures. Poor bone health is likely a result of a combination of factors including malabsorption of fat-soluble vitamins such as vitamin D and K, in addition to poor nutritional status and chronic lung inflammation. Prevention includes optimizing nutritional status as well as encouraging weightbearing exercise, while dual X-ray absorptiometry is used for screening at-risk patients. Treatment includes aggressive management of potential pulmonary or endocrine comorbidities, and may include bisphosphonates for those with severe osteopenia. ( 67 )
Depression/Anxiety.
Living with a chronic illness, such as CF, can be both challenging and isolating, placing individuals with CF at higher risk for mental health issues. Approximately 15% of all individuals with CF report having either an anxiety disorder or depression, and 44% of individuals with CF report having both conditions. ( 2 ) It is recommended that all children with CF who are 7-11 years old be clinically assessed for depression and anxiety when a caregiver reports clinically elevated symptoms of depression or anxiety, or when there is significant concern for the child exhibiting symptoms of depression or anxiety. Annual screening for depression and anxiety in individuals with CF should begin at 12 years old using the PHQ-9 and GAD-7, respectively. Annual screening is also recommended for caregivers of children with CF. ( 68 ) Early identification of mental health difficulties is critical to helping ensure individuals receive referrals to appropriate mental health services in order to receive treatment and maintain their overall health and quality of life.
Preventative Care.
Children with CF should receive routine well-child care according to the American Academy of Pediatrics guidelines, including all vaccinations. Annual influenza vaccination is recommended for children 6 months and older, as well as for all household members. The use of palivizumab should be considered in all children with CF younger than 2 years as prophylaxis against respiratory syncytial virus. ( 20 ) Providers should encourage a smoke-free environment for all children with CF, and caregivers should be informed of the health effects associated with second-hand smoke exposure.
Transition from Pediatric to Adult CF Care Centers.
Because most individuals with CF are living into adulthood, the topic of transition from pediatric care centers to adult care centers will continue to be important for patients and their families. One step identified to improve the transition process includes introducing the ideas of self-care skills and transition to adult care during the teenage years. Readiness assessments for self-management skills, including the Transition Readiness Assessment Questionnaire, ( 69 ) and transition tool sets have been used to assess patients’ readiness to transition to more independent management of their disease. These discussions should also include educational/vocational plans, behavioral risk counseling, screening for depression/anxiety, and reproductive health and family planning. Finally, implementation of formal transition-focused visits may be helpful to introduce patients to their new care team in a familiar clinic setting. ( 70 )
Lung Transplant.
Pulmonary disease continues to account for almost 60% of CF-related mortality. ( 2 ) Lung transplant is a surgical option that can extend and improve the quality of life of individuals with CF, but it involves extensive evaluation before transplant as well as adherence with therapies and lifestyle recommendations to optimize the success of transplanted lungs. Lung transplant confers a survival benefit, ( 71 ) with recent reports indicating that individuals with CF are experiencing 9.5-year median survival after lung transplant. ( 72 ) CF providers are recommended to discuss disease trajectory and treatment options, including risk and benefits of lung transplant, with individuals with advanced lung disease, and referral to lung transplant centers should be discussed with individuals whose FEV 1 is less than 50% predicted or is rapidly declining (>20% decline in FEV 1 over 12 months). ( 73 ) Overall, 6.3% of CF transplants performed in 2018 were in individuals younger than 18 years, ( 2 ) and criteria for referral are similar to those for adults older than 18 years. ( 73 ) Discussion about lung transplant may be viewed as another transition by individuals with CF and should be facilitated by education, communication, and support for the individual and his or her family. ( 73 )
Individuals affected by CF are living longer and healthier lives, and survival is expected to continue to improve with earlier diagnosis through routine NBS, promulgation of evidence-based guidelines, interdisciplinary care centers, and the use of mutation-specific modulator therapies. The primary goals of treatment remain optimization of pulmonary function and nutritional status, and incremental advances in these therapies have had a profound effect on health and quality of life for individuals with CF. Building partnerships with individuals and their families requires recognition of the emotional, social, and financial effects of this lifelong disease and effective communication and coordination among primary care physicians and CF care center teams.
On the basis of consensus, ( 22 ) the diagnosis of cystic fibrosis (CF) is based on 1) a positive newborn screening; 2) clinical features consistent with CF (the presence of ≥1 characteristic phenotypic features of chronic, recurrent sinopulmonary disease, nutritional and gastrointestinal abnormalities, male urogenital abnormalities (eg, absence of the vas deferens), and salt depletion syndromes; or 3) a positive family history of CF and laboratory-demonstrated evidence of CFTR dysfunction, such as elevation of sweat chloride concentration (≥60 mEq/L [≥60 mmol/L]).
On the basis of consensus, ( 50 ) annual oral glucose tolerance tests are recommended for people with CF older than 9 years to screen for CF-related diabetes mellitus.
On the basis of research evidence, ( 25 ) the long-term therapies to maintain optimal lung health for children and adults with CF include control of chronic airway infection and inflammation, clearance of mucous secretions, and, where clinically applicable, treatments aimed at the basic CF genetic defect.
To view the Teaching Slides that accompany this article, please see the Supplementary Data at https://doi.org/10.1542/pir.2019-0212.
airway clearance therapy
allergic bronchopulmonary aspergillosis
cystic fibrosis
Cystic Fibrosis Foundation
cystic fibrosis–related diabetes mellitus
cystic fibrosis transmembrane conductance regulator
distal intestinal obstruction syndrome
Food and Drug Administration
forced expiratory volume in 1 second
immunoreactive trypsinogen
newborn screening
pancreatic enzyme replacement therapy
pulmonary exacerbations
Competing Interests
Supplementary data.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- ABP Content Spec Map
- Pediatrics On Call
- Online ISSN 1526-3347
- Print ISSN 0191-9601
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 11
- Cystic fibrosis: a diagnosis in an adolescent
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-9674-0879 Monica Bennett 1 ,
- Andreia Filipa Nogueira 1 ,
- Maria Manuel Flores 2 and
- Teresa Reis Silva 1
- 1 Pediatric , Centro Hospitalar e Universitario de Coimbra EPE , Coimbra , Portugal
- 2 Pediatric , Centro Hospitalar do Baixo Vouga EPE , Aveiro , Aveiro , Portugal
- Correspondence to Dr Monica Bennett; acinomaicila{at}gmail.com
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, antileukotrienes and several courses of empirical oral antibiotic therapy, there was no clinical improvement. There was no reference to gastrointestinal symptoms. Due to clinical worsening, extended investigations were initiated, which revealed Pseudomonas aeruginosa in sputum culture, sweat test with a positive result and heterozygosity for F508del and R334W mutations in genetic study which allowed to confirm the diagnosis of CF. In this case, heterozygosity with a class IV mutation can explain the atypical clinical presentation. It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents.
- cystic fibrosis
- pneumonia (respiratory medicine)
https://doi.org/10.1136/bcr-2021-245971
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
A high degree of diagnostic suspicion is of fundamental importance when chronic symptoms persist, despite optimised therapy for previous diagnoses and in case of isolation of atypical bacterial agents in microbiological studies.
This case describes an adolescent with a chronic cough since the first year of life, adequate weight gain and normal pubertal development, without improvement with optimised therapy for other respiratory pathologies. There was no reference to gastrointestinal symptoms. There was clinical worsening at 13 years of age and isolation of Pseudomonas aeruginosa in sputum culture. After extensive investigation, including sweat test and genetic study, it was possible to confirm the diagnosis of cystic fibrosis (CF).
Case presentation
A 13-year-old female teenager presented with chronic cough and purulent rhinorrhoea with periods of intermittent clinical worsening with associated fever since the first year of life. This was accompanied by various medical specialties, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. She was under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, and antileukotrienes and submitted to several courses of empirical oral antibiotic therapy, without sustained and effective clinical improvement. She presented an adequate height–weight evolution, with a body mass index (BMI) at 50th−85th percentile and normal pubertal development, no reference to gastrointestinal symptoms or previous hospitalisations. Her family background was irrelevant. Due to clinical worsening, with emetising cough associated with intermittent fever and night sweats, a pulmonary CT scan was performed, which revealed parenchymal densification, air bronchogram, thickened bronchi, mucoid impaction and mediastinal adenopathies. Observed in the emergency department, the objective examination highlighted bibasal crackles on pulmonary auscultation, without other alterations. She was treated with clarithromycin, later associated with co-amoxiclav. An extended investigation was initiated, which revealed erythrocyte sedimentation rate of 52 mm/hour, C reactive protein test of 4.10 mg/dL, negative BK and interferon gamma release assay test, and isolation of P. aeruginosa in sputum culture. The antibiotic therapy was changed to ciprofloxacin and sweat tests were performed with positive results on two occasions (102 and 110 mmol/L). Later, a genetic study revealed heterozygosity for the F508del and R334W mutations, which confirmed the diagnosis of CF. Faecal elastase was performed, and the result was normal (>500 µg/g).
After antimicrobial therapy with ciprofloxacin, she maintained P. aeruginosa, and methicillin-sensitive Staphylococcus aureus (MSSA) was now discovered in the sputum. For this reason, she was hospitalised for intravenous eradication. After 2 weeks of antibiotic therapy with meropenem, gentamicin and teicoplanin, P. aeruginosa was eradicated but not MSSA. Linezulide was prescribed for 2 weeks, with a good response, and the microbiological study was negative.
Outcome and follow-up
During the follow-up period (2 years), she continued having frequent respiratory infections, with isolation of P. aeruginosa and MSSA in respiratory secretions intermittently, requiring the need for several courses of antibiotic therapy. The antibiogram of P. aeruginosa has remained sensible. Currently, she continues follow-up in a specialised fibrosis cystic centre, under inhaled therapy with colistin/tobramycin, hypertonic saline, salbutamol, dornase alfa, budesonide/formoterol, chest physiotherapy and oral azithromycin prophylaxis. Her pulmonary function is normal with a currently forced expiratory volume in 1 s of 87% and she shows adequate height−weight evolution, with BMI maintained at P50–85. The sweat chloride test was not repeated after confirmed diagnosis.
CF is one of the most commonly diagnosed genetic disorders 1 and the most common life-shortening autosomal recessive disease among Caucasian populations, with a frequency of 1 in 2000–3000 live births. 2 CF is caused by mutations in a single large gene on chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator ( CFTR ) protein.
There are more than 2000 mutations/variations of the CFTR gene reported and listed in the CFTR mutation database. A small subset are CF disease-causing mutations, of which the majority are associated with pancreatic insufficiency and a smaller subset are associated with pancreatic sufficiency. Most of the known mutations/variations related to CF are described in the CFTR2 database (Clinical and Functional Translation of CFTR). This website provides information about what is currently known about specific genetic variants or variant combination and is a useful resource to correlate clinical measures to the large number of variants identified to date. 3 4
Clinical disease requires disease-causing mutations in both copies of the CFTR gene. Mutations of the CFTR gene have been divided into five different classes. The most common mutation is F508del which is included in category class II mutations—defective protein processing. Approximately 50% of patients with CF are homozygous for this mutation, and 90% will carry at least one copy of this gene. In general, mutations in classes I−III cause more severe disease than those in classes IV and V. Class IV and V mutations are associated with moderate phenotypes and pancreatic sufficiency. 5 The R334W is a rare mutation included in class IV—defective conduction and associated with pancreatic sufficiency. 5 6 Those with less severe mutations present with pancreatic sufficiency and single organ manifestations of CF. Some of these patients would fulfil the diagnostic criteria for CF and some would be classified as having a CFTR-related disorder if the diagnosis of CF cannot be fulfilled. 7
The phenotypic expression of disease varies widely, based on CFTR-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers). Genotype–phenotype correlations are weak for pulmonary disease in CF and somewhat stronger for the pancreatic insufficiency phenotype. 5
Many studies in different individuals heterozygous for CFTR gene mutation have been performed to find out the association of CFTR gene mutation with asthma. The results are inconclusive, as some of the studies have shown positive association, whereas other could find either protective or no association. 8 Also, at this time, there is no evidence for a specific association between CFTR gene mutation and other allergic manifestations.
Clinical manifestations are multisystemic and heterogeneous. 9 The first symptoms of the disease usually appear in the first years of life, and most patients develop a multisystem disease, with predominantly respiratory and digestive symptoms. 2 5 10 The usual presenting symptoms and signs include persistent pulmonary infection, pancreatic insufficiency and elevated sweat chloride levels. However, many patients demonstrate mild or atypical symptoms, and clinicians should remain alert to the possibility of CF even when only a few of the usual features are present. 2 Progressive pulmonary involvement is the main cause of morbidity and mortality. Clinically significant pancreatic insufficiency eventually develops in approximately 85% of individuals with CF. The remaining 10%–15% of patients with CF remain pancreatic sufficient throughout childhood and early adulthood, but these individuals are at risk of pancreatitis. Pancreatic exocrine function may be evaluated indirectly by measurement of faecal elastase, which is clinically practical but has limited accuracy. Low levels of faecal elastase suggest pancreatic insufficiency and support a diagnosis of CF. 2 5 11–13
The diagnosis of CF is based on compatible clinical findings with biochemical or genetic confirmation. The sweat chloride test is the mainstay of laboratory confirmation, although tests for specific mutations, nasal potential difference (NPD), immunoreactive trypsinogen, stool faecal fat or pancreatic enzyme secretion may also be useful in some cases.
Both of the following criteria must be met to diagnose CF: (1) clinical symptoms consistent with CF in at least one organ system, or positive newborn screen or having a sibling with CF; and (2) evidence of cystic CFTR dysfunction (any of the following): elevated sweat chloride ≥60 mmol/L; presence of two disease-causing mutations in the CFTR gene, one from each parental allele; abnormal NPD.
Sweat chloride test ≥60 mmol/L is considered abnormal. If confirmed on a second occasion, this is sufficient to confirm the diagnosis of CF in patients with clinical symptoms of CF. Positive results of sweat testing should be further evaluated by CFTR sequencing. Determining the CFTR genotype is important because the results may affect treatment choices as well as confirm the diagnosis. For patients with inconclusive results of sweat chloride and DNA testing, measurement of NPD can be used to further evaluate for CFTR dysfunction. 5 14
Newborn screening programmes for CF are now performed routinely in several countries, which contributed to a dramatic increase in the number of CF cases identified before presenting with symptoms. The rationale for this screening is that early detection of CF may lead to earlier intervention and improved outcomes because the affected individuals are diagnosed, referred and treated earlier in life compared with individuals who are diagnosed after presenting with symptomatic CF. In Portugal and some other European countries, this programme was implemented less than 10 years ago, contributing to a late diagnosis in older children.
There are different neonatal screening programmes that include biochemical screening and/or DNA assays with panels to test for the most common CFTR mutations in the local population. Most programmes test for between 23 and 40 mutations, and some programmes even perform adjunctive full gene sequencing. Screening for a greater number of mutations increases the likelihood of identifying infants with CF and also increases the identification of rare or unique sequence mutations, making interpretation of the result more complicated. As only a limited number of mutations are evaluated on the genetic screens, it is possible to miss the diagnosis. Thus, it is important to follow such children closely, with particular attention to weight gain and recurrent respiratory infections. Clinicians should consider CF in individuals with suggestive symptoms, even when results of the newborn screen are negative or equivocal. 5 14
In the case described here, heterozygosity with a class IV mutation, usually associated with an intermediate phenotype and pancreatic sufficiency, may explain the atypical clinical presentation and consequent diagnosis only in adolescents. We also hypothesise that this child’s allergic manifestations may have delayed the diagnosis.
As the spectrum of clinical presentation is very variable, it is very important for clinicians from multiple specialties to be vigilant and suspect this diagnosis in conditions such as recurrent pulmonary infection, male infertility, pancreatitis, nasal polyposis and malabsorption even in patients with negative newborn screening. 2 10 13
Learning points
There is a wide spectrum of manifestations of cystic fibrosis (CF). These variations and wide spectrum are based on cystic fibrosis transmembrane conductance regulator (CFTR)-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers).
Most patients with CF develop multisystemic and heterogeneous clinical manifestations, with predominantly respiratory and digestive symptoms.
A minority have mild or atypical symptoms.
Heterozygosity with a class IV mutation usually is associated with an intermediate phenotype and pancreatic sufficiency and can explain the atypical clinical presentation.
It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents in microbiological studies.
Ethics statements
Patient consent for publication.
Consent obtained from parent(s)/guardian(s)
- Dickinson KM ,
- ↵ Cystic fibrosis mutation database . Available: http://www.genet.sickkids.on.ca/Home.html
- ↵ Clinical and functional translation of CFTR . Available: https://cftr2.org/
- Ellis L , et al
- Awasthi S ,
- Gartner S ,
- Salcedo Posadas A ,
- García Hernández G
- Castellani C ,
- Linnane B ,
- Pranke I , et al
- Farrell PM ,
- Ren CL , et al
- Kharrazi M ,
- Bishop T , et al
Contributors MB cared for study patient, planned and wrote the article. AFN collected data. MMF provided and cared for study patient, served as scientific advisors and critically reviewed the study proposal. TRS cared for study patient, served as scientific advisors and critically reviewed the study proposal.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Case Study: Cystic Fibrosis in the Newborn
- PMID: 29789057
- DOI: 10.1891/0730-0832.37.3.164
Cystic fibrosis (CF) is considered one of the most commonly occurring fatal genetic disorders. This disorder is associated with pancreatic insufficiency and pulmonary complications. However, at birth the initial complications are associated with bowel obstruction. Cystic fibrosis management warrants an interdisciplinary team because this disorder affects various organ systems. Effective management of the newborn with CF assists in improving the child's overall prognosis. Family support is critical throughout the prenatal and postnatal periods. The case presented reviews a child born with suspected CF and the clinical course within the NICU.
Publication types
- Case Reports
- Cystic Fibrosis / complications*
- Cystic Fibrosis / diagnosis
- Cystic Fibrosis / genetics
- Cystic Fibrosis / physiopathology
- Cystic Fibrosis Transmembrane Conductance Regulator / genetics
- Family Health
- Infant, Newborn
- Intensive Care, Neonatal / methods
- Intestinal Obstruction* / diagnosis
- Intestinal Obstruction* / etiology
- Intestinal Obstruction* / physiopathology
- Intestinal Obstruction* / therapy
- Neonatal Nursing / methods*
- Patient Care Management / methods*
- Patient Care Team / organization & administration
- Social Support
- Cystic Fibrosis Transmembrane Conductance Regulator
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 April 2024
The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: an 18-year experience
- Jagdev Singh 1 , 2 ,
- Sharon Hunt 1 ,
- Sharon Simonds 1 ,
- Christie Boyton 1 ,
- Anna Middleton 1 ,
- Matthew Elias 2 ,
- Susan Towns 1 , 3 ,
- Chetan Pandit 1 , 3 ,
- Paul Robinson 1 , 3 ,
- Dominic A. Fitzgerald 1 , 3 &
- Hiran Selvadurai 1 , 3
Scientific Reports volume 14 , Article number: 9056 ( 2024 ) Cite this article
298 Accesses
1 Altmetric
Metrics details
- Bacterial infection
- Cystic fibrosis
The impact of evolving treatment regimens, airway clearance strategies, and antibiotic combinations on the incidence and prevalence of respiratory infection in cystic fibrosis (CF) in children and adolescents remains unclear. The incidence, prevalence, and prescription trends from 2002 to 2019 with 18,339 airway samples were analysed. Staphylococcus aureus [− 3.86% (95% CI − 5.28–2.43)] showed the largest annual decline in incidence, followed by Haemophilus influenzae [− 3.46% (95% CI − 4.95–1.96)] and Pseudomonas aeruginosa [− 2.80%95% CI (− 4.26–1.34)]. Non-tuberculous mycobacteria and Burkholderia cepacia showed a non-significant increase in incidence. A similar pattern of change in prevalence was observed. No change in trend was observed in infants < 2 years of age. The mean age of the first isolation of S. aureus ( p < 0.001), P. aeruginosa ( p < 0.001), H. influenza ( p < 0.001), Serratia marcescens ( p = 0.006) and Aspergillus fumigatus ( p = 0.02) have increased. Nebulised amikacin (+ 3.09 ± 2.24 prescription/year, p = 0.003) and colistin (+ 1.95 ± 0.3 prescriptions/year, p = 0.032) were increasingly prescribed, while tobramycin (− 8.46 ± 4.7 prescriptions/year, p < 0.001) showed a decrease in prescription. Dornase alfa and hypertonic saline nebulisation prescription increased by 16.74 ± 4.1 prescriptions/year and 24 ± 4.6 prescriptions/year ( p < 0.001). There is a shift in CF among respiratory pathogens and prescriptions which reflects the evolution of cystic fibrosis treatment strategies over time.
Similar content being viewed by others

Therapeutic beta-lactam dosages and broad-spectrum antibiotics are associated with reductions in microbial richness and diversity in persons with cystic fibrosis
Andrea Hahn, Aszia Burrell, … Edith T. Zemanick

The antibiotic resistance reservoir of the lung microbiome expands with age in a population of critically ill patients
Victoria T. Chu, Alexandra Tsitsiklis, … Charles R. Langelier
Long-term macrolide treatment for non-cystic fibrosis bronchiectasis in children: a meta-analysis
Eun Lee, In Suk Sol, … You Hoon Jeon
Introduction
The management of pulmonary infections is critical in the care of individuals with cystic fibrosis (CF). Despite an increase in the median survival age over recent years, chronic pulmonary infection and concomitant airway inflammation leading to respiratory failure still account for 80–95% of deaths in individuals with CF 1 , 2 . This vicious cycle of infection and inflammation begins early in life, resulting in a decline in lung function, poorer nutrition, and structural lung abnormalities 3 .
Assessing long-term epidemiological trends in CF among children poses significant challenges, with studies often limited to registry reports, of a limited timeframe 4 , involve a small number of children and adolescents 5 , focus on specific organisms of interest 6 , 7 , or are derived from results obtained from bronchioalveolar sampling alone 8 , 9 . Furthermore, larger studies conducted before the year 2000 may not reflect recent advancements in CF treatment 10 , 11 , 12 , 13 , 14 , highlighting the need to evaluate any changes in the incidence and prevalence of CF bacterial pathogens to establish a reference point for future therapeutic interventions.
To this end, we conducted a study to investigate the trends in the incidence and prevalence of respiratory pathogens among children and adolescents with CF since the turn of the new millennium. By evaluating long-term longitudinal data within a clinical setting in the modern era of eradication therapy 15 , we would like to determine the changes that may have occurred in different age groups over time.
Methodology
Study population.
Children and adolescents with CF between birth to 18 years of age who were managed within a large CF centre in Australia between January 2002 and December 2019 were included in this study. Universal newborn screening of cystic fibrosis had been well-established before the study period 16 . Data collected from their existing electronic medical record included; the microbiological culture result (method of collection, date during which sample was collected with the corresponding age of the child or adolescent), and hospital pharmacy-based medication prescription data. This study was approved by the Ethics Committee of the Sydney Children’s Hospital Network (2020/ETH00815) and was conducted based on local guidelines and regulations. Exemption from consent was obtained from, and approved by the same committee.
Clinical routine during the study period
In our centre which encompasses a large region in New South Wales, outpatient (CF clinic) reviews occur four times a year, with infants or those who are clinically unwell reviewed on a more frequent basis. During these visits, airway samples are routinely collected regardless of the presence or absence of symptoms either through spontaneous expectoration (typically in older children), oropharyngeal suctioning performed by a trained CF nurse (typically in younger children), or via bronchoalveolar lavage (BAL). Airway samples microbiological cultures are ordered based on either BAL culture order label (samples obtained via BAL) or sputum CF culture order label (samples obtained through either spontaneously expectorated sputum or airway sample obtained from oropharyngeal suctioning).
All infants less than one year of age have been prescribed oral flucloxacillin or occasionally amoxicillin and clavulanic acid from diagnosis as part of our CF clinics’ routine Staphylococcus aureus prophylaxis approach for over 20 years.
In terms of the microbiological practices which has remained consistent during this study period, sputum specimens have been set up on (1) MacConkey agar for gram-negative bacteria e.g., coliforms, Pseudomonas aeruginosa, and Inquilinus limosus , (2) Anaerobically incubated chocolate agar with Bacitracin for Haemophilus influenzae . (3) Mannitol salt agar for S. aureus (4) Horse blood agar for e.g., Streptococcus pneumoniae and Moraxella catarrhalis . (5) Cepacia agar for Burkholderia cepacia and incubated for 7 days. (6) Non-tuberculous mycobacteria (NTM) testing is performed in an external Mycobacterium Reference Laboratory (MRL) using the automated blood culture system (BD BACTEC™) and testing occurs annually. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) has been used since 2015 for the rapid identification of organisms.
The microbiologist's report on the results of the collected airway samples is routinely reviewed by the CF team within 5–7 days after the samples are obtained. Treatment, where applicable following discussion with the primary CF physician is then prescribed. The treatment strategy includes; admission for parenteral antibiotics, a course of oral antibiotics, and/or nebulised antibiotic treatment.
Case definitions and stratification
Incidence was defined as the first time a respiratory pathogen of interest is isolated from the sputum of the child or adolescent with CF. Once the child or adolescent is an incident case for that particular pathogen, they were excluded from the denominator for the subsequent years.
Prevalence was defined as a child or adolescent with a respiratory pathogen isolated from their sputum in a specific year. Once the child or adolescent is a prevalent case for that particular pathogen, any further positive culture of the same pathogen isolated from the same child or adolescent was excluded for the remainder of that year.
Nine organisms of clinical interest in CF were selected for analysis. This includes; S. aureus, P. aeruginosa, H. influenza, Aspergillus fumigatus, Serratia marcescens, NTM, B. cepacia, Achromobacter xylosoxidans , and Stenotrophomonas maltophilia 17 .
The cohort was divided into four age groups: < 2 years, 2–5 years, 6–11 years, and > 12 years. The rationale behind this age group includes (1) biological variability in terms of differences in microbiome composition, immune system development and environmental exposure e.g. home or pre-school (2) management approaches such as methods of physiotherapy, lung function testing or the availability of medications such as dornase alfa (3) to align with existing clinical trials in CF transmembrane conductance regulator (CFTR) and CF registry reports.
In terms of medications prescribed and obtained from the hospital pharmacy, prescription of oral antimicrobials (including amoxicillin and clavulanic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, flucloxacillin, and itraconazole), nebulised antimicrobials (including amikacin, colistin, and tobramycin), and other medications (including dornase-alfa, hypertonic saline nebules, and CFTR modulators and correctors) were reviewed.
Statistical analysis
We used descriptive statistics to summarise the data, reporting organism incidence and prevalence as n (%). To assess changes over time, we calculated the annual incidence and prevalence of each organism based on individual airway samples, and used regression analysis to evaluate these measures. Based on the coefficients obtained from the regression model, the average change in incidence and prevalence was presented. Prescription trends were also analysed on an individual basis. Results are reported as % change (with 95% confidence intervals) for incidence and prevalence, and as number of prescriptions/year ± standard deviation for medications prescribed. Changes in the mean age of first organism isolation were assessed using analysis of variance. All statistical calculations were performed using the SPSS Statistic Data Editor (IBM Version 28, New York, USA, 2021). Statistical significance was defined as p < 0.05.
Study population and bacterial samples
During the study period, 419 children and adolescents with CF were followed up with 206 (49.2%) born on, or after 1st January 2002. A total of 18,339 airway samples were collected during the study period with 401 (2.2%) collected via bronchioalveolar lavage, with the remaining samples obtained from expectorated sputum or oropharyngeal suction.
Out of the total airway samples that were collected, 724 (3.9%) samples met the criteria for incidence and 15,332 (83.6%) samples met the criteria for prevalence as defined in the methodology of this study were included in the analysis.
Incidence and prevalence of respiratory pathogens
Throughout the entire study period, S. aureus (25.1%), P. aeruginosa (26.2%), and H. influenzae (17.9%) exhibited the highest incidence among respiratory pathogens. Together, these pathogens accounted for 70% of the overall incidence over 18 years. In contrast, B. cepacia (0.69%), A. xylosoxidans (2.1%), and NTM (3.7%) had the lowest incidence across the study period, collectively representing 6.5% of the overall incidence over 18 years (Table 1 ).
Throughout the entire study period, S. aureus (47.8%), P. aeruginosa (34.5%), and A. fumigatus (8.4%) exhibited the highest prevalence among respiratory pathogens. Together, these organisms constituted almost 95% of the overall prevalence over 18 years. In contrast, the least prevalent respiratory pathogens were NTM (0.72%), B. cepacia (0.69%), and A. xylosoxidans (0.48%) throughout the study period. Collectively, these organisms represented less than two percent of the overall prevalence over 18 years (Table 2 ).
Changes in age of first isolation of respiratory pathogens
The ages at which these pathogens were first isolated are as follows: S. aureus (3.35 ± 2.1 years), H. influenza (4.28 ± 2.7 years), S. marcescens (5.24 ± 4.09 years), P. aeruginosa (5.27 ± 2.9 years), A. fumigatus (7.31 ± 2.85 years). This is followed by S. maltophilia (8.95 ± 2.95 years), B. cepacia (9.055 ± 2.3 years), NTM (11.38 ± 2.06 years), A. xylosoxidans (11.71 ± 2.86 years).
Over time, respiratory pathogens have shown an increase in the mean age of the first isolation: S. aureus ( p < 0.001), P. aeruginosa ( p < 0.001), H. influenza ( p < 0.001), S. marcescens ( p = 0.006), A. Fumigatus ( p = 0.02), B. cepacia ( p = 0.58), NTM ( p = 0.052), S. marcescens ( p = 0.308), S. maltophilia ( p = 0.47), A. xylosoxidans ( p = 0.80). The changes over years of these respiratory pathogens are illustrated in Fig. 1 .
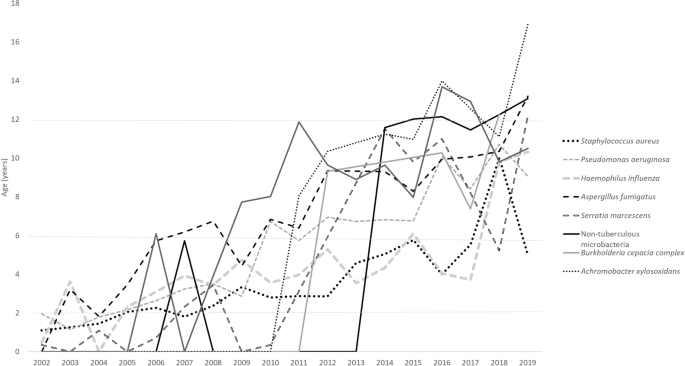
Mean age group of the first culture of CF organisms.
Changes of overall and age-specific incidence and prevalence of CF organisms from 2002 to 2019
Amongst the organisms with the highest incidence, S. aureus showed the largest decline in incidence over time, followed by H. influenza and P. aeruginosa . Meanwhile, NTM and B. cepacia showed a non-significant increase in incidence. A similar pattern of change in prevalence was observed (Tables 1 , 2 ).
With respect to age groups, incidence of S. aureus, P. aeruginosa, H. influenza and A. fumigatus in children < 2 years of age have remained unchanged. A similar pattern of change in prevalence was observed. Meanwhile, NTM showed a significant increase in both incidence and prevalence in children 6–11 years of age.
Throughout this study, a total of 29,203 medications (oral antimicrobials n = 18,367, 62.9%) were prescribed. The antibiotics that were increasingly prescribed include amikacin (3.09 ± 2.24 prescription/year, p = 0.003), amoxicillin/clavulanic acid (8.98 ± 2.17 prescriptions/year, p < 0.001), colistin (1.95 ± 0.3 prescriptions/year, p = 0.032), trimethoprim/sulfamethoxazole (18.1 ± 8.7, p < 0.001). Flucloxacillin (− 4.48 ± 1.073, p < 0.001), tobramycin (− 8.46 ± 4.7, p < 0.001) showed a decrease in prescription. Ciprofloxacin (− 6.049 ± 5.1 prescriptions/year, p = 0.068) and itraconazole (− 4.53 ± 1 prescriptions/year, p = 0.07) did not show any significant change over time.
Dornase alfa prescription increased by 16.74 ± 4.1 prescriptions/year ( p < 0.001). The prescription of hypertonic saline nebulisation increased by 24 ± 4.6 prescriptions/year ( p < 0.001). There were 7 children or adolescents on CFTR corrector or modulator therapy.
This paediatric-focused study evaluates annual changes in the incidence and prevalence rates of respiratory pathogens across different age groups, while also comparing medication prescription trends over an 18-year period. This study provides valuable data from a real-world clinical setting where infants under the age of one receive universal antimicrobial prophylaxis and, standardised respiratory pathogen surveillance is conducted by qualified personals using consistent sampling and microbiological testing protocols. In particular, obtaining samples through sputum and oropharyngeal suctioning is considered to have the highest concordance with BAL samples, rendering them more representative of lower airway infections compared to other sampling methods like throat or cough swabs 18 . The findings contribute to our understanding of the long-term trends in respiratory pathogens and associated clinical management in the paediatric population, particularly in the modern era of eradication therapy 15 .
Our study showed that together, S. aureus and P. aeruginosa make up the majority of respiratory pathogens both in terms of incidence (51.3%) and prevalence (82.3%). Data preceding 2000, report prevalence of these two respiratory pathogens to be higher at 95% 14 .
Registry data taken from 2018 to 2020 showed a prevalence of P. aeruginosa of 20.9% 17 and S. aureus of 55.26% in children and adolescents under the age of 18. In comparison, our data shows a recent prevalence of P. aeruginosa of 17.6% and S. aureus of 45.3%. Of the less frequent respiratory pathogens, NTM prevalence was 4.3% from registry data vs 3.7% from our cohort and B .cepacia was 3.2% vs. 1.3% respectively.
In a recent publication by VanDevanter et al., a trend of decline in P. aeruginosa prevalence was observed, as evidenced by the examination and presentation of registry data within a comparable time frame 19 . Following this, Fischer et al. raised a crucial question regarding whether the observed changes in P. aeruginosa over time were also apparent in other respiratory pathogens of interest in CF 20 . We have demonstrated that over the past 18 years, the incidence and prevalence of the most common respiratory pathogens in CF such as S. aureus , P. aeruginosa , H. influenzae and A. fumigatus have decreased steadily. This significant decline of between 2 and 4% of individual respiratory pathogens are observed both in the incidence and prevalence. Meanwhile, less common organisms such as NTM , B. cepacia and A. xylosoxidans, S. maltophilia showed no significant change in terms of incidence and prevalence.
We also found that the incidence and prevalence of respiratory pathogens remain unchanged for infants up to 2 years of age across all respiratory pathogens. Additionally, we have found that our cohort of children and adolescents with CF are found to have a positive airway sample culture for these respiratory pathogens significantly later that the earlier years of this study.
Our centre has adopted the universal use of S. aureus prophylactic antibiotics in infants diagnosed with CF preceding this study period. In a systematic analysis performed which reviewed four studies, there was a weak indication that P.aeruginosa was isolated less frequently in children under three years and more frequently in children between three to six years in the prophylactic group 21 . In contrast, despite our universal use of prophylactic antibiotics in infants, our study shows (1) a decline in the incidence and prevalence of P. aeruginosa , (2) no significant increase in the incidence and prevalence of organisms such as NTM and B. cepacia (3) an increase in the mean age of first isolation of respiratory pathogens of interest, (4) no change of incidence and prevalence of respiratory pathogen < 2 years of age. A contributing factor in terms of improvements in infection control practices may have helped keep our incidence and prevalence lower than the national average. While being potentially circumstantial, these findings suggest that the use of prophylactic anti-staphylococcal antibiotics is not associated with an increase in P. aeruginosa or increase in prevalence of other less common respiratory pathogen. Prospective studies such as the CF-START study in evaluating outcomes of prophylactic treatments will hopefully provide conclusive proof of its benefits and safety 21 .
By examining prescription trends, we have found that there is a rise in the use of anti-pseudomonal nebulised antibiotics such as amikacin and colistin. This suggests that P.aeruginosa is being more aggressively treated over time as both this antibiotics are considered as second line after tobramycin 22 . However, the increase in use of amikacin could also be attributed to an increase in NTM incidence and prevalence. Encouragingly, we have found that the emphasis on respiratory clearance has increased over time with the significant increase in the prescription of dornase alpha and hypertonic saline in our cohort.
Our study comes with certain limitations that warrant consideration. Firstly, the sputum and prescription data lack representation from external laboratories or pharmacies, potentially limiting the comprehensiveness of our findings. Additionally, we did not culture anaerobic bacteria and did not routinely test for co-infection with respiratory viruses, leading to an omission in addressing potential co-infections among these organisms in our study. Moreover, the annual frequency of NTM testing, as opposed to routine CF airway sample cultures, may result in an underrepresentation of NTM within our study cohort.
Thirdly, our data originated from a single CF centre in Australia, raising concerns about the generalisability of our findings to a broader population. Fourthly, our incidence calculation may involve a small number of children or adolescents intermittently found to have these respiratory pathogens in their airway samples. Finally, the relatively limited sample size of children and adolescents on CFTR modulators or correctors is noteworthy, as our study predates the widespread adoption that followed the approval and government funding of these medications in Australia. Current evidence suggests that while it may more difficult to obtain sputum samples in children on CFTR therapy, its’ impact on the growth of specific bacterial pathogens needs to be closely examined 23 . The low number of children or adolescents on CFTR modulators or correctors is an important aspect of this study as it will enable future comparison in a post-modulator era in the management of CF.
Our study has several strengths. First, we analysed a large number of sputum samples, both overall and in different age groups, providing a longitudinal comparison of changes in CF treatment over the past 18 years. This is the first study of such magnitude in children and adolescents with CF, providing age-specific incidence and prevalence, as well as prescription trends. In particular, our review of incidences of these organisms and the age of first positive culture provides additional information towards our understanding of CF respiratory pathogens over the past two decades.
Second, our study includes a large cohort of children born on or after January 1st, 2002, when newborn screening has already been well-established, allowing us to assess the acquisition of respiratory pathogens from shortly after birth over the past 18 years. Third, the practice of using prophylactic anti-staphylococcus antibiotics universally has given us the opportunity to assess the outcomes of its’ use over a significantly long period of time. While strong conclusions cannot be made without a non-prophylactic control arm, it does provide insight into the long-term impact of its’ implementation on respiratory pathogens in our cohort.
In summary, our study shows a change in the epidemiology of CF pathogens in a single large paediatric clinic that practices universal prophylaxis in children. First, we observed a decline in the incidence and prevalence of the most commonly found CF pathogens such as S. aureus, P. aeruginosa, H. influenzae, and A. fumigatus , as well as a delay in the first acquisition of these pathogens. However, less common pathogens such as S. marcescens , NTM, B. cepacia, A. xylosoxidans , and S. maltophilia did not show significant changes. Second, we found no change in the incidence or prevalence of respiratory pathogens in infants under 2 years of age over time.
Data availability
Data is available from the corresponding author, upon reasonable request.
Flume, P. A. et al. Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 176 , 957–969 (2007).
Article CAS PubMed Google Scholar
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15 , 194–222. https://doi.org/10.1128/CMR.15.2.194-222.2002 (2002).
Article CAS PubMed PubMed Central Google Scholar
Pillarisetti, N. et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am. J. Resp. Crit. Care Med. 184 , 75–81. https://doi.org/10.1164/rccm.201011-1892OC (2011).
Article PubMed Google Scholar
Spicuzza, L. et al. Emerging pathogens in cystic fibrosis: Ten years of follow-up in a cohort of patients. Eur. J. Clin. Microbiol. Infect. Dis. 28 , 191–195 (2009).
Coburn, B. et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 5 , 10241. https://doi.org/10.1038/srep10241 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Abidin, N. Z. et al. Trends in nontuberculous mycobacteria infection in children and young people with cystic fibrosis. J. Cystic Fibrosis 20 , 737–741 (2021).
Article Google Scholar
Miall, L. S., McGinley, N. T., Brownlee, K. G. & Conway, S. P. Methicillin resistant Staphylococcus aureus (MRSA) infection in cystic fibrosis. Arch. Dis. Childhood 84 , 160–162. https://doi.org/10.1136/adc.84.2.160 (2001).
Article CAS Google Scholar
Breuer, O. et al. Changing prevalence of lower airway infections in young children with cystic fibrosis. Am. J. Resp. Crit. Care Med. 200 , 590–599. https://doi.org/10.1164/rccm.201810-1919OC (2019).
Hilliard, T. N. et al. Bronchoscopy following diagnosis with cystic fibrosis. Arch. Dis. Childhood 92 , 898–899. https://doi.org/10.1136/adc.2006.105825 (2007).
Gilligan, P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4 , 35–51 (1991).
Burns, J. L. et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27 , 158–163 (1998).
Govan, J. & Nelson, J. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 48 , 912–930 (1992).
Millar, F., Simmonds, N. & Hodson, M. Trends in pathogens colonising the respiratory tract of adult patients with cystic fibrosis, 1985–2005. J. Cystic Fibrosis 8 , 386–391 (2009).
Razvi, S. et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 136 , 1554–1560. https://doi.org/10.1378/chest.09-0132 (2009).
Ramsay, K. A. et al. The changing prevalence of pulmonary infection in adults with cystic fibrosis: A longitudinal analysis. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. 16 , 70–77. https://doi.org/10.1016/j.jcf.2016.07.010 (2017).
McKay, K. O., Waters, D. L. & Gaskin, K. J. The influence of newborn screening for cystic fibrosis on pulmonary outcomes in New South Wales. J. Pediatrics 147 , S47–S50. https://doi.org/10.1016/j.jpeds.2005.08.013 (2005).
Ahern, S. et al. The Australian cystic fibrosis data registry: Annual report 2019 (2021).
Schultz, A. & Caudri, D. Cough swabs less useful but induced sputum very useful in symptomatic older children with cystic fibrosis. Lancet Resp. Med. 6 , 410–411 (2018).
VanDevanter, D. R., LiPuma, J. J. & Konstan, M. W. Longitudinal bacterial prevalence in cystic fibrosis airways: Fact and artifact. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. https://doi.org/10.1016/j.jcf.2023.09.011 (2023).
Fischer, A. J. & Planet, P. J. A birth cohort approach to understanding cystic fibrosis lung infections. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. https://doi.org/10.1016/j.jcf.2023.10.014 (2023).
Rosenfeld, M., Rayner, O. & Smyth, A. R. Prophylactic anti-staphylococcal antibiotics for cystic fibrosis. Cochrane Database System. Rev. https://doi.org/10.1002/14651858.CD001912.pub5 (2020).
Elborn, J., Hodson, M. & Bertram, C. Implementation of European standards of care for cystic fibrosis—control and treatment of infection. J. Cystic fibrosis 8 , 211–217 (2009).
Nichols, D. P. et al. PROMISE: Working with the CF community to understand emerging clinical and research needs for those treated with highly effective CFTR modulator therapy. J. Cystic Fibrosis 20 , 205–212. https://doi.org/10.1016/j.jcf.2021.02.003 (2021).
Download references
Acknowledgements
We extend our gratitude to The Cure4CF Foundation and The Team Simon Foundation for Cystic Fibrosis for their generous financial support towards this study.
Author information
Authors and affiliations.
Department of Respiratory Medicine, The Children’s Hospital at Westmead, Sydney, NSW, Australia
Jagdev Singh, Sharon Hunt, Sharon Simonds, Christie Boyton, Anna Middleton, Susan Towns, Chetan Pandit, Paul Robinson, Dominic A. Fitzgerald & Hiran Selvadurai
Department of Pharmacy, The Children’s Hospital at Westmead, Sydney, NSW, Australia
Jagdev Singh & Matthew Elias
Discipline of Child and Adolescent Health, Sydney Medical School, University of Sydney, Sydney, NSW, Australia
Susan Towns, Chetan Pandit, Paul Robinson, Dominic A. Fitzgerald & Hiran Selvadurai
You can also search for this author in PubMed Google Scholar
Contributions
H.S. and J.S. conceived the research question. J.S., H.S. and D.F. designed the study and analysis plan. J.S., S.H., S.S., C.B., A.M. and M.E. collected the data. J.S. performed the statistical analysis. H.S., D.F., P.R., S.T. and C.P. reviewed the data. J.S. drafted the initial and final versions of the manuscript. All authors critically reviewed early and final versions of the manuscript.
Corresponding author
Correspondence to Jagdev Singh .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Singh, J., Hunt, S., Simonds, S. et al. The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: an 18-year experience. Sci Rep 14 , 9056 (2024). https://doi.org/10.1038/s41598-024-59658-4
Download citation
Received : 31 August 2023
Accepted : 12 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1038/s41598-024-59658-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 19: Case Study: Cystic Fibrosis
Julie M. Skrzat; Carole A. Tucker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction.
- Examination: Age 2 Months
- Evaluation, Diagnosis, and Prognosis
- Intervention
- Conclusion of Care
- Examination: Age 8 Years
- Examination: Age 16 Years
- Recommended Readings
- Full Chapter
- Supplementary Content
C ystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation ( Cystic Fibrosis Foundation, 2019a ), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease prevalence varies greatly by ethnicity, with the highest prevalence occurring in Western European descendants and within the Ashkenazi Jewish population.
The CF gene, located on chromosome 7, was first identified in 1989. The disease process is caused by a mutation to the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein. This mutation alters the production, structure, and function of cyclic adenosine monophosphate (cAMP), a dependent transmembrane chloride channel carrier protein found in the exocrine mucus glands throughout the body. The mutated carrier protein is unable to transport chloride across the cell membrane, resulting in an electrolyte and charge imbalance. Diffusion of water across the cell membrane is thus impaired, resulting in the development of a viscous layer of mucus. The thick mucus obstructs the cell membranes, traps nearby bacteria, and incites a local inflammatory response. Subsequent bacterial colonization occurs at an early age and ultimately this repetitive infectious process leads to progressive inflammatory damage to the organs involved in individuals with CF.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait

- Previous Article
- Next Article
Case Presentations
Clinical pearls, article information, pediatric case series of cystic fibrosis, diabetes, and islet cell autoimmunity.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Grace J. Kim , Lina Merjaneh; Pediatric Case Series of Cystic Fibrosis, Diabetes, and Islet Cell Autoimmunity. Clin Diabetes 1 October 2018; 36 (4): 331–333. https://doi.org/10.2337/cd18-0018
Download citation file:
- Ris (Zotero)
- Reference Manager
Diabetes is the most common comorbidity in individuals with cystic fibrosis (CF). Among people with CF, the prevalence of CF-related diabetes (CFRD) is estimated to be 2% in children, 19% in adolescents, 40% in individuals in their 20s, and 45–50% in those ≥30 years of age ( 1 ). CFRD shares some aspects with type 1 diabetes in that it is primarily due to insulin deficiency, but it is distinguished from type 1 diabetes because of its insidious onset over years and the persistence of some insulin production long after diagnosis, which makes diabetic ketoacidosis (DKA) very rare in CFRD ( 2 ).
Routine screening for type 1 diabetes autoantibodies is not recommended for patients with CF and hyperglycemia ( 3 ). However, the following three cases illustrate certain instances that may warrant checking for type 1 diabetes autoantibodies (i.e., hyperglycemia before age 10, symptomatic presentation, and DKA; increasing insulin requirement; or presence of other autoimmune diseases or family history of autoimmune diseases in a CF patient).
Patient 1 is a non-Hispanic white male with CF (genotype delta F508 homozygous), pancreatic insufficiency, and CF-related liver disease who presented at 16 years of age with polyuria, polydipsia, dry mouth, and weight loss of 5 kg over 3 months. His family history was positive for hypothyroidism in his father and CF in his sister and negative for diabetes. His height was 152 cm (<1st percentile), his weight was 41.6 kg (<1st percentile), and his BMI was 18.0 kg/m 2 (12th percentile). Physical examination showed normal mental status, no Kussmaul breathing, dry mucous membranes, and clubbing. Laboratory evaluation revealed fasting glucose of 350 mg/dL, pH 7.42, bicarbonate 24 mEq/L, blood urea nitrogen (BUN) 22 mg/dL, and creatinine 0.6 mg/dL. Urinalysis showed +3 glucose and +1 ketones. His C-peptide level was 1.1 ng/mL (normal range 1.1–4.4 ng/mL), and his A1C was >14%. Type 1 diabetes autoantibodies testing was positive for anti-GAD at 147 (normal range ≤25) and negative for islet cell antigen autoantibody 512 (IA-2), zinc transporter 8 (ZnT8), and insulin autoantibodies (IAAs).
He was started on insulin 0.8 units/kg/day (insulin glargine 16 units at night and insulin lispro with a carbohydrate ratio of 1 unit per 15 g carbohydrates, a sensitivity of 1 unit per 50 mg/dL, and a target blood glucose of 120 mg/dL). His current insulin requirement at the age of 23 years is 0.8–0.9 units/kg/day, and his A1C since diagnosis has ranged from 6.7 to 12.0%. He does not have other autoimmune diseases.
Patient 2 is patient 1’s sister, a non-Hispanic white female with CF (genotype delta F508 homozygous) and pancreatic insufficiency. She was initially diagnosed with CFRD at 9 years of age when she had her first oral glucose tolerance test (OGTT) screening. At that time, she was asymptomatic with no weight loss, polyuria, or polydipsia. Her height was 123.5 cm (1st percentile), her weight was 27.4 kg (19th percentile), and her BMI was 18 kg/m 2 (69th percentile). Physical examination was unremarkable. Her OGTT showed a fasting glucose of 92 mg/dL and a 2-hour glucose of 209 mg/dL. Her C-peptide level was 7.1 ng/mL, and her A1C was 6.7%. Type 1 diabetes autoantibodies were negative at presentation. She was started on insulin at 0.4 units/kg/day (insulin glargine 5 units at night and insulin lispro with a carbohydrate ratio of 1 unit per 75 g carbohydrate, a correction factor of 1 unit per 150 mg/dL, and a target blood glucose of 150 mg/dL).
At the age of 14 years, because her insulin requirement was increasing and her A1C had increased from 6.9 to 11.2% over 18 months, type 1 diabetes autoantibodies were retested and found to be positive (anti-GAD 31, IA-2 52 [normal range ≤7], and ZnT8 0.955 [normal range ≤0.030]). Her treatment was intensified, and insulin was dosed at 1 unit/kg/day (insulin glargine 25 units at night and insulin lispro with a carbohydrate ratio of 1 unit per 16 g carbohydrate, a sensitivity of 1 unit per 35 mg/dL, and a target blood glucose of 100 mg/dL). Her current insulin dose at 16 years of age is 0.7 units/kg/day, and her A1C since diagnosis has ranged from 6.7 to 12.7%. No other autoimmune diseases were diagnosed.
Patient 3 is a biracial (non-Hispanic black, non-Hispanic white) female with CF (genotype 1 copy of delta F508 and 1 copy of delta I507), pancreatic insufficiency, and autoimmune thyroiditis with hypothyroidism, on thyroid hormone replacement. Hypothyroidism was diagnosed 4 months before her diabetes presentation. She presented at the age of 9 years with polyuria, polydipsia, fatigue, and weight loss of 4.5 kg over 3 months. Family history was positive for rheumatoid arthritis in her maternal grandmother and hypothyroidism on her mother’s side and negative for diabetes. Her height was 147 cm (97th percentile), her weight was 26.3 kg (20th percentile), and her BMI was 12.2 kg/m 2 (<1st percentile). Physical examination revealed normal mental status and increased work of breathing consistent with Kussmaul breathing in addition to dry mucous membranes. Laboratory evaluation was consistent with DKA, with glucose 686 mg/dL, pH 7.06, bicarbonate 5 mEq/L, beta-hydroxybutyrate 5.4 mmol/L, BUN 15 mg/dL, and creatinine 0.7 mg/dL. Her C-peptide level was 0.1 ng/mL, and her A1C was >14%. Type 1 diabetes autoantibodies testing was positive (anti-GAD 192, IA-2 183, ZnT8 0.177, and IAAs 0.017). There were no concerns regarding cerebral edema.
She was treated with an insulin infusion of 0.1 units/kg/hour and then transitioned to a basal-bolus insulin regimen after DKA resolution with a dose of 0.8 units/kg/day (insulin glargine 10 units at night and insulin lispro with a carbohydrate ratio of 1 unit per 20 g carbohydrate, a sensitivity of 1 unit per 85 mg/dL, and a target blood glucose of 120 mg/dL). Her current insulin dose at the age of 11 years is 0.55 units/kg/day, and her A1C since diagnosis has ranged from 7.7 to 10.2%. The patient has switched from multiple daily injections to continuous subcutaneous insulin infusion (CSII). She reports improved satisfaction with CSII.
None of the three patients described above have developed microvascular complications (i.e., microalbuminuria or retinopathy), hypertension, or dyslipidemia, although their diabetes duration is only 4–7 years.
How common are positive type 1 diabetes autoantibodies in CF patients with hyperglycemia?
How can primary care providers (PCPs) play a role in diabetes screening in CF patients?
When should providers consider screening CF patients with hyperglycemia for diabetes autoantibodies (i.e., autoimmune type 1 diabetes)?
Why is it important to correctly diagnose the type of diabetes associated with CF?
Here, we report three unique cases of CF with diabetes related to islet cell autoimmunity. Type 1 diabetes was suspected because of the severity of presentation (patient 1), increasing insulin requirement and family history of type 1 diabetes (patient 2), and DKA presentation and presence of autoimmune thyroid disease (patient 3). The third example (involving DKA) highlights the need to correctly identify the type of diabetes to prevent the morbidity and mortality associated with type 1 diabetes.
Annual screening for CFRD is recommended for CF patients who are ≥10 years of age. This test appears burdensome because it requires patients to fast and is time-consuming. PCPs can play an important role in advocating for CF patients to undergo an annual OGTT. PCPs should encourage those CF patients who are diagnosed with CFRD to adhere to their insulin regimen because of its benefits on nutrition and pulmonary function. For most patients with CFRD, the A1C goal is ≤7% to reduce the risk for microvascular complications.
Screening CF patients with hyperglycemia for type 1 diabetes autoantibodies is not routinely recommended. Data are conflicting on the frequency of islet cell autoimmunity in CF patients with diabetes. In the general population, 2% of individuals are positive for IA-2, GAD, ZnT8, or IAAs ( 4 ). In type 1 diabetes, 55–98% of patients are positive for at least one of these autoantibodies ( 5 ). Few studies reported no increased rate of islet cell autoimmunity in CFRD patients ( 6 – 8 ). Gottlieb et al. ( 8 ) reported that the presence of autoantibodies associated with type 1 diabetes is no greater in CFRD than in the general population. In a sample of 76 CFRD patients with fasting hyperglycemia, only 5% had type 1 diabetes autoantibodies, including three subjects with antibodies to GAD and one subject with antibodies to IA-2. In contrast, more recently, Konrad at al. ( 9 ) reported that, in a cohort of 837 CFRD patients, 8.5% had positive type 1 diabetes antibodies (64% had IA-2 antibodies, 76% had islet cell antibodies [ICAs], 72% had GAD antibodies, and 83% had IAAs), indicating that the rate of islet cell autoimmunity in CFRD is higher than in the general population.
Our three cases illustrate specific circumstances in which screening for type 1 diabetes autoimmunity should be considered. CFRD is rare in prepubertal children ( 1 ). Also, CF patients are often asymptomatic at the time of diabetes diagnosis because CFRD is subtle and gradual in onset ( 2 ). When comparing CFRD patients with type 1 diabetes autoimmunity to those without autoimmunity, Konrad et al. ( 9 ) found that diabetes onset was earlier, insulin doses were higher, A1C was higher, and thyroid autoimmunity was more common in those with type 1 diabetes autoimmunity. Therefore, hyperglycemia before the age of 10 years, symptomatic presentation, DKA, increasing insulin requirement, the presence of other autoimmune diseases, or a family history of autoimmune diseases in CF patients should prompt a work up to rule out type 1 diabetes autoimmunity.
Treatment of CFRD is different from that of type 1 diabetes because the latter involves more intensive insulin dosing and glucose monitoring given the absolute insulin deficiency associated with it. In CFRD, insulin secretion is rarely totally absent. Therefore, patients usually require lower doses of insulin and rarely develop DKA. The rate of treatment complications such as severe hypoglycemia with coma and DKA are significantly higher in antibody-positive CFRD patients compared to those who are antibody negative (hypoglycemia with coma 8.0 vs. 1.4%, P <0.05; DKA 9.3 vs. 0.9%, P <0.05) ( 9 ).
Correctly diagnosing the type of diabetes in CF is important because it has an impact on diabetes progression, care, and treatment; it helps prevent the short-term complications associated with type 1 diabetes, especially DKA, with its worrisome complication of cerebral edema. It is also important for appropriate screening for long-term micro- and macrovascular complications given that the rate of these complications is higher in type 1 diabetes than in CFRD ( 10 ).
PCPs should ensure that CF patients undergo a yearly OGTT. For CF patients who develop diabetes, PCPs should encourage adherence to their insulin regimen and monitor whether they are meeting the A1C goal of ≤7%.
Type 1 diabetes should be considered in CF patients with hyperglycemia when patients are prepubertal, symptomatic, in DKA, requiring high insulin doses, or have other autoimmune diseases or a family history of autoimmune diseases.
Correct diagnosis allows for proper treatment and helps to prevent short- and long-term diabetes complications.
Acknowledgments
The authors thank Dr. Catherine Pihoker for assistance with this manuscript.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors researched data and co-wrote the manuscript. G.J.K. is the guarantor of this work and, as such, had full access to all data and takes responsibility for the integrity of the data and accuracy of the information presented.

Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account

- Meet the Provost
- Provost Office
- Administration
- University Leadership
- Views of the Campus
- Prospective Students
- College of Allied Health
- College of Dentistry
- Graduate College
- College of Medicine
- Fran and Earl Ziegler College of Nursing
- College of Pharmacy
- Hudson College of Public Health
- College of Professional and Continuing Studies
- Continuing Education Programs
- Update and Preparation of Research Programs at HSC for COVID-19
- Vice President for Research
- Research Administration
- Clinical / Patient
- Researcher Profiles
- Core Facilities
- Human Research Participant Protection (HRPP) and Institutional Review Board (IRB)
- Institutional Animal Care and Use Committee (IACUC)
- Institutional Biosafety Committee (IBC)
- Faculty & Staff

Genetic Testing May Provide Improved Medication Therapy Safety and Efficacy for Pediatric Cystic Fibrosis Patients
Second-year University of Oklahoma pediatric resident Dr. Caroline Thompson has a professional and personal connection to cystic fibrosis patient care for children. A childhood friend had the disease and made a lasting impression on Thompson. Now, Thompson has received the Cystic Fibrosis Foundation’s Medical Resident Research Award for a pilot study evaluating pharmacogenomic-directed therapy for pediatric patients at the Oklahoma Cystic Fibrosis Center Tulsa.
Pharmacogenomics, says Thompson, is the relationship between genes and how they interact with the drugs that we take. “[Pharmacogenomics is] particularly pertinent to the CF population because they have such a complex disease process that incorporates many different organ systems and therefore many different types of medications,” she said. “And so it's really a huge aspect of their life and their treatment.”
For this study, researchers will recruit as many as 40 children with CF and have their genetics tested. Once that information comes back, the findings will be compared against databases of previously researched and established gene and drug interactions, explains Thompson. Then, the patients and their families will be provided with education on current medications as well as any possible drug interactions for future use. To conclude the study, the participants and their families will be asked to share from their perspective whether the information was helpful for current care and how they felt it would work for their child.
This type of genetic testing is a standard of care for other types of disease processes, relates Dr. Michelle Condren, OU-TU School of Community Medicine Department of Pediatrics professor, Thompson’s mentor, and pharmacist for the Oklahoma Cystic Fibrosis Center Tulsa, but genetic testing has not been traditionally used for CF care. Other studies have been done on adult and pediatric patients, but this will be the first known study on a specifically pediatric patient group.
The importance of this work for individuals with CF is paramount, relates Thompson, because “the more drugs you take, statistically the more likely you are to have either the lack of efficacy or certain side effects or interactions between drugs that may happen.” She describes how certain genes and metabolizers in each person’s body can be slightly different and cause everyone to interact with medications differently. This can affect many different systems in the body, including the gastrointestinal tract and the respiratory system, and can even affect mental health.
This award from the Cystic Fibrosis Foundation is specifically designed for residents, as it attempts to get medical professionals interested in CF research earlier in their training, says Condren. She knows first-hand the challenges of care for these special patients.
“While there are people everywhere that are trying to come up with new medications, those of us on the front lines [of CF care] are trying to figure out how to decrease side effects and how to make sure what we're doing is effective,” Condren said.
Thompson and Condren are part of a research team that includes Joseph Walter, M.D., Saint Francis Health System Warren Clinic, Samie Sabet-Sarvestani, PharmD; Amy Hendrix-Dicken, statistician; Alex Chidester, research assistant; and fellow pediatric resident, Jeffrey Frerking, M.D.
REVIEW article
Cystic fibrosis management in pediatric populationfrom clinical features to personalized therapy provisionally accepted.

- 1 Grigore T. Popa University of Medicine and Pharmacy, Romania
- 2 Pediatrics, Grigore T. Popa University of Medicine and Pharmacy, Romania
The final, formatted version of the article will be published soon.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). In 1949, it's been identified as a monogenic disease and was thought to primarily affect individuals of Northern European descent. It was the most prevalent autosomal recessive disease that shortens life. With the availability of multiple testing methodologies nowadays, there is a chance to create novel and enhanced treatment options. Even in the absence of a high sweat chloride test (SCT) result, the discovery of two causal mutations is diagnostic for cystic fibrosis (CF). For a CF diagnosis, however, at least two positive E sweat chloride tests are still required. In order to achieve early and active intervention to manage cystic fibrosis (CF) and its comorbidities, treatment regimens for pediatric patients should be evaluated, improved, and closely monitored. New developments in the treatment of cystic fibrosis (CF) have led to the development of medications derived from molecules that target the pathogenetic pathway of the illness. These options are very efficient and allow pediatric patients to receive individualized care. However, in order to better direct patient care and enhance patient outcomes, it is crucial to research uncommon CF mutations, which can provide crucial information about the prognosis of the disease and the relationships between genotype and phenotype. To ensure the success of creating novel, safer, and more efficient treatment approaches, a deeper understanding of the pathogeny of the illness is required. In the age of customized medicine, genetic research will be essential to improving patient care and quality of life for those with uncommon mutations.
Keywords: Cystic Fibrosis, Children, screening, Mutation, Treatment
Received: 28 Feb 2024; Accepted: 24 Apr 2024.
Copyright: © 2024 AZOICAI, Lupu, Trandafir, Alexoae, Alecsa, STARCEA, Cuciureanu, Knieling, Salaru, Hanganu, Mocanu, Lupu and Ioniuc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dr. Ancuta Lupu, Grigore T. Popa University of Medicine and Pharmacy, Iași, 700115, Iasi, Romania Prof. Vasile Valeriu Lupu, Grigore T. Popa University of Medicine and Pharmacy, Iași, 700115, Iasi, Romania
People also looked at

Enhancing Pediatric Care For Cystic Fibrosis Patients: A Multidisciplinary Approach Involving Anesthesia, Midwifery, Pediatric Nursing, And Physiotherapy In ICU Settings
- Ebraheem Owaif Alzahrani ,Atyaf Mohammed Aqiel Aqiel ,Alkhathami Abdullah Mohammed ,Majdi Ahmed Jandly ,Mashael Hamoud Aljuaid ,Mansor Mogbel Awad Alshammary ,Rami Ramadan Alshehri,
- Wael Suwaylih B Alotaibi ,Nasser Fahd Nasser Alsuroor ,Zahra Mohammed Khobrani ,Aishah Abdow Hadadi ,Fahad Mohammed Al-Husseini ,Fatmah Samet Mhana Alrasheadi
Background: This research paper explores the efficacy of a multidisciplinary approach to pediatric cystic fibrosis (CF) care within intensive care unit (ICU) settings. Through a comprehensive literature review, the study investigates the roles of anesthesia, midwifery, pediatric nursing, and physiotherapy in enhancing patient outcomes and quality of life.
Methods: Key findings demonstrate the significant impact of collaborative teamwork and interdisciplinary communication on optimizing care delivery fo [1] r pediatric CF patients.
Results: underscore the potential of the multidisciplinary approach to improve treatment adherence, reduce hospital stays, and enhance overall well-being.
Discussion: This paper concludes by calling for further research to explore interdisciplinary synergies and refine care delivery strategies for pediatric CF patients in ICU settings.

How to Cite
- Endnote/Zotero/Mendeley (RIS)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License .
CC Attribution-NonCommercial-NoDerivatives 4.0
Old publisher: Transnational Press London
New Publisher: Migration Letters & The London Publishers

- Patient Care
- What is Phage Therapy?
- Patient Stories
- CF Clinical Trial FAQ
- School of Medicine Home
- Administration
COVID-19 Updates
Visit UC San Diego's Coronavirus portal for the latest information for the campus community.
View Details

- Hospital & Clinic Locations
- Find a Provider
- Medical Services
- MyUCSDChart Login
- Clinical Trials
- Refer a Patient
- School of Medicine
- Academic Departments
- Skaggs School of Pharmacy
- Continuing Medical Education
- Altman Clinical and Translational Research Institute
- Moores Cancer Center
- Research Centers
- Research Labs
- Volunteering

- Meet the Directors
- Press Releases
- Scientific Reports
- Seminar Series
- IPATH Media
- Advisor Committees
- Academic Partners
- Industry Partners
- Projects and Focus
- CF Clinical Trial FAQ Currently selected
Cystic Fibrosis Clinical Trial
IPATH's Co-Director, Dr. Robert Schooley, is co-leading the first NIH-sponsored phage therapy clinical trial. This trial is recruiting cystic fibrosis (CF) patients with Pseudomonas aeruginosa infections. Enrolled patients will receive a single dose of a four-phage cocktail that targets P. aeruginosa .
The trial's primary objectives are to assess the safety and microbiological activity of a single intravenous (IV) phage dose and to assess the benefit-to-risk profile for CF patients with P. aeruginosa infections.
If you are interested in participating in this trial, you can discuss your interest with your physician that manages your cystic fibrosis, as well as visit the ClinicalTrials.gov site that provides a list of all enrolling locations and contacts.
Please also feel free to email our center with any questions about this trial, at [email protected].
Frequently Asked Questions
IPATH created the below FAQs for the Phase 1b/2, Multi-Centered, Randomized, Double-Blind, Placebo-Controlled Trial of the Safety and Microbiological Activity of a Single Dose of Bacteriophage Therapy in Cystic Fibrosis Subjects Colonized With Pseudomonas Aeruginosa. This information is from the ClinicalTrials.gov page for this study.
Who is eligible for the study?
Subjects must meet all of the below inclusion criteria to be eligible to participate in the study:
- Adult (>/= 18 years) at the time of screening.
- Confirmed CF diagnosis based on a compatible clinical syndrome confirmed by either an abnormal sweat chloride testing or two CFTR gene variations. (Can be obtained from documentation in medical records; actual test results not necessary.)
- Readily able to produce at least 2 mL of sputum during a 30-minute sputum collection following a hypertonic saline treatment or other approach to increase sputum production. (Determined by investigator or their designee judgment. Approaches for obtaining sputum may include, but are not limited to, inhaled hypertonic saline (e.g. 3%, 7%, or 10%), inhaled hypertonic bicarbonate, inhaled mannitol, or spontaneously expectorated sputum. The same approach should be used for all sputum collections for a given subject, refer to the MOP for further details.)
- P. aeruginosa (regardless of Colony Forming Units (CFU)/mL) isolated from a sputum, throat culture, or other respiratory specimen in the past 12 months.
- Confirmed P. aeruginosa isolation from a sample of expectorated sputum at the screening visit.
- Capable of providing informed consent.
- Capable and willing to complete all study visits and perform all procedures required by this protocol.
Subjects who meet any of the below exclusion criteria will not be enrolled in the study:
- Body weight < 30 kg.
- Forced Expiratory Volume 1< 20% of predicted value at screening, using the Hankinson equations.25
- Elevated LFTs obtained at screening. (a. Alanine aminotransferase (ALT) > 5 x the upper limit of normal (ULN) or aspartate transaminase (AST) > 5 x ULN or total bilirubin > 3 x ULN, OR b. Total bilirubin > 1.5 x ULN combined with either ALT > 3 x ULN or AST > 3 x ULN. ULN reflect local laboratory ranges.)
- Acute clinical illness requiring a new (oral, parenteral), or inhaled antibiotic(s) = 30 days prior to the baseline visit. (Does not include chronic suppressive medications or cyclic dosing medications such as inhaled antibiotics.)
- Women who are pregnant, planning to become pregnant during the study period, or breastfeeding. (Women of childbearing potential must have a negative serum Beta-Human chorionic gonadotropin test during screening and agree to use an effective method of contraception for the duration of the trial.
- A female is considered of childbearing potential unless postmenopausal, or surgically sterilized and at least 3 months has passed since sterilization procedure.)
- Female surgical sterilization procedures include tubal ligation, bilateral salpingectomy, hysterectomy, or bilateral oophorectomy
- Female is considered postmenopausal if she is >45 years old and has gone at least 12 months without a spontaneous menstrual period without other known or suspected cause.
- Effective methods of contraception include (a) abstinence, (b) partner vasectomy, (c) intrauterine devices, (d) hormonal implants (such as Implanon), or (e) other hormonal methods (birth control pills, injections, patches, vaginal rings).
- Active treatment of any nontuberculous mycobacteria or fungal organisms </=30 days prior to baseline. Chronic treatment for suppression of fungal populations is allowable.
- Anticipated need to change chronic antibiotic regimens during the study period. (Subjects on cyclic dosing medications such as inhaled antibiotics, must be able and express willingness to keep the therapies at the time of screening constant for the duration of the follow-up period (approximately 30 days). Subjects on chronic suppressive antimicrobial therapy must be able and express willingness to stay on the therapies for the duration of their follow-up period. This includes chronic azithromycin therapy.)
- Known allergy to any component of the study product.
- Any significant finding that, in the opinion of the investigator, would make it unsafe for the subject to participate in this study.
- Enrolled in a clinical trial within </=30 days of the baseline/dosing visit, or participating in a clinical trial while enrolled in this clinical trial (inclusive of prophylactic vaccine trials).
- Currently or previously enrolled in this trial.
Where are the study sites located?
- University of California San Diego School of Medicine – Pathology; La Jolla, California, United States, 92037
- University of California Davis Medical Center - Internal Medicine - Infectious Disease; Sacramento, California, United States, 95816
- Stanford University School of Medicine; Stanford, California, United States, 94305-2200
- National Jewish Health - Division of Pulmonary, Critical Care and Sleep Medicine; Denver, Colorado, United States, 80206
- University of South Florida Health - Internal Medicine; Tampa, Florida, United States, 33606
- Emory U niversity - Woodruff Health Sciences Center; Atlanta, Georgia, United States, 30324
- University of Iowa Hospitals & Clinics - Department of Internal Medicine; Iowa City, Iowa, United States, 52242
Maryland
- Johns Hopkins Children's Center - Pediatric Infectious Diseases ; Baltimore, Maryland, United States, 21287-0011
- University of Michigan - Infectious Disease Clinic at Taubman Center; Ann Arbor, Michigan, United States, 48109
- University of Minnesota - Pediatric Infectious Disease; Minneapolis, Minnesota, United States, 55455-0341
- University of Minnesota Medical Center, Fairview - Infectious Diseases and International Medicine; Minneapolis, Minnesota, United States, 55455-0341
Missouri
- Washington University in St. Louis; Saint Louis, Missouri, United States, 63110-1010
North Carolina
- Duke University School of Medicine - Duke Clinical Research Institute - Duke Clinical Research Unit; Durham, North Carolina, United States, 27701
- Case Western Reserve University School of Medicine - Medicine - Infectious Diseases and HIV Medicine; Cleveland, Ohio, United States, 44106
Pennsylvania
- Children's Hospital of Pittsburgh of UPMC - Allergy, Immunology and Infectious Diseases; Pittsburgh, Pennsylvania, United States, 15213
- University of Texas Southwestern Medical Center - Internal Medicine - Infectious Diseases; Dallas, Texas, United States, 75390-8884
Wisconsin
- Medical College of Wisconsin; Milwaukee, Wisconsin, United States, 53226
What is involved?
Brief Summary from Clinicaltrials.gov :
This clinical trial is designed to assess the safety and microbiologic activity of bacteriophage product WRAIR_PAM-CF1, directed at Pseudomonas aeruginosa in clinically stable CF individuals. WRAIR_PAM-CF1 is a 4 component anti-pseudomonal bacteriophage containing between >/= 4 x 10^7 and >/= 4 x 10^9 Plaque Forming Units (PFU) in the target dose.
In stage 1, two eligible subjects will be assigned to each of the three dosing arms receiving a single dosage of the IV bacteriophage therapy (4 x 10^7 PFU, 4 x 10^8 PFU, and 4 x 10^9 PFU; total of 6 sentinel subjects), followed by a 96-hour observation period.
Stage 2a will proceed if no serious adverse events (SAEs) related to the study product occur during the observation period of stage 1; 32 subjects will be enrolled into one of 4 arms (placebo IV, 4 x 10^7 PFU, 4 x 10^8 PFU, and 4 x 10^9 PFU) in a 1:1:1:1 allocation. An interim analysis after all subjects have completed follow up visit 5 on Day 30 will be performed to select an IV bacteriophage dose with the most favorable safety and microbiologic activity profile.
During Stage 2b, subjects will be randomized into the bacteriophage (dose selected based on Interim Analysis following Stage 2a) or placebo arm. The final sample size is expected to be up to 72 subjects total with up to 25 subjects in the placebo arm and up to 25 subjects in the Stage 2b bacteriophage dose.
How do I enroll?
If you believe you are eligible for this study, and would like to be screened for enrollment, please reach out to the cystic fibrosis center at the trial site nearest you.
What if I am not eligible for this trial?
You can search https://www.clinicaltrials.gov/ for other clinical trials for which you may be eligible. We also expect additional phage therapy clinical trials in the future, so you are welcome to check our website periodically for future trial announcements.
- UC San Diego School of Medicine
- 9500 Gilman Drive
- About School of Medicine

- Policy Notices
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
Pathophysiology of non-cystic fibrosis bronchiectasis in children and adolescents with asthma: A protocol for systematic review and meta-analysis
Contributed equally to this work with: Natali Caroline da Silva, Beatriz Cocato Malagutti, Joelia Maria Costa Dias Ladeira, Milena Baptistella Grotta, Adyleia Aparecida Dalbo Contrera Toro
Roles Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Postgraduate Program in Child and Adolescent Health of the School of Medical Sciences (FCM), State University of Campinas (UNICAMP), Campinas, São Paulo, Brazil
Affiliation Medical School, Pontifical Catholic University of Campinas, Campinas, São Paulo, Brazil
Affiliation Center of Integration in Pediatrics (CIPED) of the School of Medical Sciences (FCM), State University of Campinas (UNICAMP), Campinas, São Paulo, Brazil
- Natali Caroline da Silva,
- Beatriz Cocato Malagutti,
- Joelia Maria Costa Dias Ladeira,
- Milena Baptistella Grotta,
- Adyleia Aparecida Dalbo Contrera Toro

- Published: April 18, 2024
- https://doi.org/10.1371/journal.pone.0294921
- Reader Comments
The pathophysiological mechanisms by which asthma and bronchiectasis are associated are still unclear. The association of these two diseases can result in more severe symptoms and a greater number of exacerbations.
The aim of this systematic review is to collect evidence of the pathophysiology of non-cystic fibrosis bronchiectasis with associated asthma in children and adolescents, aged 6–18 years old.
A systematic and comprehensive search will be performed using eight main databases, PubMed, PubMed PMC, BVS/BIREME, Scopus, EMBASE, Cochrane Library, Scielo and Web of Science. Articles will be searched from the earliest available time to July 2023. The studied population will be composed of children and adolescents with asthma and non-cystic fibrosis bronchiectasis. From the data obtained, all articles found will be transferred to the Rayyan platform. Study selection will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols Checklist (PRISMA P-2015). In addition, if sufficient data are available, a meta-analysis will be conducted. Two independent reviewers will conduct the studies selection, data extraction, and risk of bias assessment. The outcome measures will be to analyze if non-cystic fibrosis bronchiectasis is related to a specific inflammatory profile.
A systematic review will provide better knowledge about the etiopathogenesis and causes of the association between asthma and bronchiectasis and its role in the severity and control of asthma. Identifying, selecting and critically evaluating studies on asthma and bronchiectasis, would be possible to illuminate the characteristics of children and adolescents with associated diagnoses and provide information to help individualized treatments in order to control and prevent complications. The findings of this study will be published in a peer-reviewed journal.
Systematic review registration
The systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) in July 2023 (registration number CRD42023440355).
Citation: da Silva NC, Malagutti BC, Ladeira JMCD, Grotta MB, Toro AADC (2024) Pathophysiology of non-cystic fibrosis bronchiectasis in children and adolescents with asthma: A protocol for systematic review and meta-analysis. PLoS ONE 19(4): e0294921. https://doi.org/10.1371/journal.pone.0294921
Editor: Pisirai Ndarukwa, Bindura University of Science Education, SOUTH AFRICA
Received: August 23, 2023; Accepted: November 12, 2023; Published: April 18, 2024
Copyright: © 2024 da Silva et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding: This study was financed in part by the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) - Finance Code 001.
Competing interests: The authors have declared that no competing interests exist.
1. Introduction
1.1. rationale.
The Global Initiative for Asthma (GINA) define asthma as “a heterogeneous disease characterized by chronic airway inflammation,” affecting 1–29% of the population worldwide [ 1 ]. In recent years, there has been evidence of an association between asthma and other diseases, including bronchiectasis [ 2 , 3 ].
Bronchiectasis is a chronic lung disease defined by an irreversible widening of the bronchial tree, usually characterized by a vicious cycle of infection and inflammation, leading to structural damage of the small airways and, eventually, of its surrounding parenchyma [ 4 , 5 ]. In the literature, bronchiectasis is divided into those caused by cystic fibrosis and those not associated with cystic fibrosis (non-cystic fibrosis bronchiectasis) [ 6 ].
The major goal for asthma control, especially in cases of severe asthma, would be identify and manage its comorbidities and that the lack of this identification would be the responsible of therapy failures and for the worsening of symptoms [ 2 , 7 , 8 ].
Asthma would be identified as one of the causes of bronchiectasis, due to its prevalence in the disease, both atopic and non-atopic, in the pediatric population [ 2 , 3 ]. However, the pathophysiological mechanisms by which the two diseases are associated are still unclear [ 9 ].
It is believed that non-cystic fibrosis bronchiectasis in children is caused by repeatedly episodes of external agents associated with genetic vulnerability, however, the other possible etiologies are poorly defined, with heterogeneous risk factors [ 4 , 10 ]. Diagnosis consists of evaluating the patient’s clinical condition and the results of computed tomography, but it is possible to observe that children with bronchiectasis also develop similar symptoms to asthma, which can be easily confused [ 4 , 11 – 14 ].
The association of these two diseases can result in more severe symptoms and a greater number of exacerbations [ 13 , 14 ], therefore, this systematic review is justified because we believe that by identifying, selecting and critically evaluating studies on asthma and bronchiectasis, would be possible to enlighten the characteristics of children and adolescents with associated diagnoses and provide information to help individualized treatments in order to control and prevent complications.
1.2. Objectives
The aim of this systematic review is to collect evidence that supports that non-cystic fibrosis bronchiectasis diagnosed in children and adolescents is associated with control and severity of asthma and worsening lung function. To this end, the proposed systematic review will look to answer the following questions:
- Do children and adolescents with associated asthma and non-cystic fibrosis bronchiectasis show a worse lung function profile evidenced by pulmonary function tests?
- Does the presence of both non-cystic fibrosis bronchiectasis and asthma, in children and adolescents, results in worse asthma control and severity?
- Is non-cystic fibrosis bronchiectasis, in asthmatic children and adolescents, related to a specific inflammatory profile?
2.1. Eligibility criteria
Study selection will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols Checklist (PRISMA P-2015) ( S1 Checklist ).
2.1.1. Study designs.
We will include observational studies (including cohort, case-control and cross-sectional studies). Will be excluded letters to editor, case reports, editorials and narrative reviews. Table 1 shows the inclusion and exclusion criteria that will be used in the study screening, first by title and abstract and then by full text. The study population must be composed of children and/or adolescents with asthma with associated non-cystic fibrosis bronchiectasis.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0294921.t001
2.1.2. Participants.
We will include children and adolescents aged 6 to 18 years old, diagnosed with asthma with associated non-cystic fibrosis bronchiectasis, irrespective of gender and ethnicity. All participants must be diagnosed with asthma using clearly defined or internationally recognized criteria and have non-cystic fibrosis bronchiectasis confirmed by computed tomography. Participants with cystic fibrosis and ciliary dyskinesia as the cause of bronchiectasis will not be included.
2.1.3. Interventions.
Studies to be examined will include any article with children and adolescents with asthma and associated non-cystic fibrosis bronchiectasis. Those articles reporting asthma control, asthma severity, inflammation pattern and pulmonary function will be considered.
2.1.4. Comparators.
Given the broad perspective for interventions of interest, comparisons will include the data from groups with no bronchiectasis. It is more likely to appear in experimental studies if they match the inclusion criteria for the review. Comparisons between asthma and non-cystic fibrosis group and asthma without bronchiectasis group may include:
- Asthma severity
- Asthma control
- Pulmonary function
- Inflammation pattern
2.1.5. Outcomes.
Outcomes will be collected individually as reported in the studies, including for those data reported as a composite measure. The outcomes must be reported through a validated tool, in accordance with its particularities.
2.1.6. Timing.
There will be no restrictions related to timing.
2.1.7. Setting.
There will be no restrictions by type of setting.
2.1.8. Language.
There will be no restrictions related to language.
2.2. Information sources
Literature search strategies will be developed using medical subject headings (MeSH) and text words related to asthma and non-cystic fibrosis bronchiectasis. We will search the following electronic bibliographic databases: PubMed, PubMed PMC, BVS-BIREME, EMBASE, COCHRANE, SCOOPUS, Web of Science and Scielo database with use of boolean operators AND and OR. The search did not utilize a year limit or filters.
2.3. Search strategy
This systematic review will use the following search strategy in all databases cited in 2.2: (Child OR Children) OR (Adolescent OR Adolescents OR Adolescence OR Teens OR Teen OR Teenagers OR Teenager OR Youth OR Youths OR "Adolescents, Female" OR "Adolescent, Female" OR "Female Adolescent" OR "Female Adolescents" OR "Adolescents, Male" OR "Adolescent, Male" OR "Male Adolescent" OR "Male Adolescents "") AND (Asthma OR Asthmas OR "Bronchial Asthma" OR "Asthma, Bronchial") AND (Bronchiectasis OR Bronchiectases OR "Saccular Bronchiectasis" OR "Bronchiectasis, Saccular" OR "Saccular Bronchiectases" OR "Cylindrical Bronchiectasis" OR "Bronchiectasis, Cylindrical" OR "Cylindrical Bronchiectases" OR "Varicose Bronchiectasis" OR "Bronchiectasis, Varicose" OR "Varicose Bronchiectases"). ( S1 Appendix )
2.4. Study records
2.4.1. data management..
The selected literature will be managed using Rayyan, a free web and mobile app for systematic review developed by Qatar Computing Research Institute (QCPI) [ 15 ]. Once in Rayyan, duplicate articles will be excluded when identified. Full-text reports will be screen after qualified studies been selected by title and abstract. If necessary, we will provide training to new members of the review team not familiar with the Rayyan platform and the content area prior to the start of the review.
2.4.2. Selection process.
The screening will be performed at Rayyan platform, by two reviewers, who will extract the articles independently, first by title and abstract and then by full text. If any disagreement or inconsistency remains, a third investigator will be involved. After reading the full- text reports, it will be decided whether they meet or not the inclusion criteria.
2.4.3. Data collection process.
We will extract data independently and in pairs from each eligible study. A calibration exercises before starting the review will be conducted to ensure consistency across reviewers. Data abstracted will include demographic information, methodology, intervention details, and all reported patient-important outcomes. Reviewers will resolve disagreements by discussion. In case of incomplete data, the original author will be contacted and if data cannot be obtained, the study will be excluded.
2.5 Data items
The following data will be extracted: study characteristics, type and source of financial support, author names, year of publication, patient characteristics (such as age, gender, symptoms, comorbidities, weight, and height), blood inflammatory biomarkers, pulmonary function tests scores, asthma questionnaires scores, computerized tomography results and other data judge by team as needed for quality assessment and outcomes.
2.6. Outcomes and prioritization
The primary outcome:
Non-cystic fibrosis bronchiectasis is related to asthma control. Since severity and control are assessed in asthma by multiple tools, all of them will be considered and evaluated.
The secondary outcomes:
- Non-cystic fibrosis bronchiectasis, in children and adolescents with associated asthma, is related to asthma severity.
- Non-cystic fibrosis bronchiectasis is related to worse pulmonary function in children and adolescents with associated asthma (assessed by spirometry values).
- Children and adolescents with asthma and non-cystic fibrosis bronchiectasis show a specific inflammatory profile. (assessed by IgE, prick test, serum and sputum inflammatory profile or bronchoalveolar lavage).
2.7. Risk of bias in individual studies
The risk of bias for analytical cross-sectional studies and prevalence in cross-sectional studies will be assessed using the appropriate tool of the Joanna Briggs Institute (JBI) [ 16 ]. For the first one, the tool consists of eight questions that include the possibility of selection bias, measurement bias, and confounding bias.
For prevalence studies, The Critical Assessment Tool for Studies with Prevalence Data is composed of nine questions, which assess from the sample structure of the included study, how it was selected and calculated, to whether the study described in detail how the outcome was evaluated, whether it was evaluated standardized way and with a good response rate, among the study participants included in the prevalence systematic review.
The Newcastle-Ottawa Scale (NOS) [ 17 ] will be used for asses risk of bias in case-control and cohort studies, which assesses three study quality parameters: selection, comparability, and exposure assessment. It assigns a maximum score of four for selection, two for comparability, and three for exposure, for a maximum total score of nine. Studies with a total NOS score of five or more are considered to be of moderate to high quality, while those with a total NOS score of less than five are considered low-quality studies.
The quality of evidence for the clinical outcomes will be assessed according to the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group [ 18 ].
2.8. Data synthesis
2.8.1. quantitative synthesis..
If studies are sufficiently homogeneous in terms of design and comparator, we will conduct meta-analyses using RevMan 5.4 software. according to the statistical guidelines referenced in the current version of the Cochrane Handbook for Systematic Reviews of Interventions. The Mantel-Haenszel method will be used for the fixed effect model if tests of heterogeneity are not significant. If statistical heterogeneity is observed (I2 > = 50% or P < 0.1) the random effects model will be chosen. If heterogeneity is substantial, we will not perform a meta-analysis; a narrative, qualitative summary will be done.
2.8.2. Measures of treatment effect.
- For dichotomous outcomes:
Dichotomous data (asthma control and asthma severity) will be reported as risk ratios (RRs) with 95% confidence intervals (CIs). Statistical significance will be set at P<0.05.
- For continuous outcomes:
Continuous outcomes will be analyzed using weighted mean differences (with 95% CI) or standardized mean differences (95% CI) if different measurement scales are used. Skewed data and non-quantitative data will be presented descriptively.
- Dealing with missing data
When there are missing data, we will attempt to contact the original authors of the study to obtain the relevant missing data. Important numerical data will be carefully evaluated. If missing data cannot be obtained, an imputation method will be used. We will use sensitivity analysis to assess the impact on the overall treatment effects of inclusion of trials which do not report an intention to treat analysis, have high rates of participant attrition, or with other missing data.
- Assessment of heterogeneity
Heterogeneity will be evaluated by the I 2 test. The value of I 2 ranges from 0% to 100%. with 0–% to 40% indicating no major heterogeneity, 40–% to 60% indicating moderate heterogeneity, 60–% to 90% indicating substantial heterogeneity, and >90% indicating considerable heterogeneity.
2.8.3 Additional analyses.
Subgroup analyses or sensitivity analyses will be used to explore sources of heterogeneity. If the results can be analyzed quantitatively a meta-regression prediction will be performed. Sensitivity analyses will considerer quality components and risk of bias previously appraised by specific tools.
Subgroup analyses will be based on the following:
- Patient characteristic (age, sex, body mass index)
- Step of treatment for asthma according to GINA
2.8.4. Narrative synthesis.
If quantitative synthesis is not appropriate, a systematic narrative synthesis will be provided with information presented in the text and tables to summarize and explain the characteristics and findings of the included studies. The narrative synthesis will explore the relationship and findings both within and between the included studies, in line with the guidance from the Centre for Reviews and Dissemination.
2.9. Meta-bias(es)
A funnel plot will be used to evaluate publication bias if more than 10 studies are included. RR from each study is plotted against their variance. Asymmetrical appearance of the plot indicates the presence of publication bias. Egger test will be used to test the asymmetry of the funnel plot.
2.10. Confidence in cumulative evidence
The quality of evidence will be assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. The evidence will be adjusted to 4 levels: high, moderate, low, or very low.
3. Discussion
Both asthma and non-cystic fibrosis bronchiectasis are chronical diseases affecting pediatric population, with great impact in their quality of life, in addition to economic and financial impact to their family and health system [ 12 – 14 , 19 ]. Its association, despite being described in literature, requires better comprehension about the pathophysiology involved. We hope that this systematic review will provide better knowledge about the pathophysiology and causes of the association between asthma and bronchiectasis and its role in the severity and control of asthma. It will be possible to open a new range in the understanding of the diseases, which may result in new research and better management of these diseases.
To achieve this goal, it is necessary to carefully evaluate and summarize the evidence published to date.
Supporting information
S1 checklist. prisma checklist / prisma-p 2015 checklist..
https://doi.org/10.1371/journal.pone.0294921.s001
S1 Appendix. Search strategy/Search strategy for PubMed, PubMed PMC, BVS-BIREME, EMBASE, COCHRANE, SCOOPUS, Web of Science and Scielo databases.
https://doi.org/10.1371/journal.pone.0294921.s002
Acknowledgments
Mrs. Ana Paula Oliveira. Universidade Estadual de Campinas, Campinas SP, Brazil.
- View Article
- Google Scholar
- PubMed/NCBI
- 16. Joanna Briggs Institute (JBI) critical appraisal tools. Available from: https://jbi.global/critical-appraisal-tools .
- 18. The GRADE working group. Available from: https://www.gradeworkinggroup.org/ .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Animal Model Exp Med
- v.4(3); 2021 Sep

A review of cystic fibrosis: Basic and clinical aspects
Qionghua chen.
1 Department No. 2 of Respiratory Medicine, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing China
2 Department of Respiratory Medicine, Quanzhou Children’s Hospital, Fujian Province, Quanzhou China
Yuelin Shen
Jingyang zheng.
Cystic fibrosis is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). Here we summarize, at the basic descriptive level, clinical and genetic characteristics of cystic fibrosis gene mutations, while emphasizing differences between CF mutations found in Chinese pediatric CF patients compared to those found in Caucasian CF patients. In addition, we describe animal models used to study human cystic fibrosis disease and highlight unique features of each model that mimic specific human CF‐associated signs and symptoms. At the clinical level, we summarize CF clinical manifestations and diagnostic, treatment, and prognostic methods to provide clinicians with information toward reducing CF misdiagnosis and missed diagnosis rates.
Cystic fibrosis is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). We summarize clinical and genetic characteristics of cystic fibrosis gene mutations, as well as animal models used to study human cystic fibrosis disease.

1. INTRODUCTION
Cystic fibrosis (CF), a monogenic disease, is the most common life‐shortening autosomal recessive disease that afflicts people of Northern European descent. It was first formally reported to the worldwide medical community in 1949. According to the American Cystic Fibrosis Foundation patient registry, there are currently more than 30 000 CF patients in the United States and more than 70 000 CF patients throughout the world. Globally, about 1000 cases of CF are newly diagnosed every year, with over 75% of CF patients diagnosed at 2 years of age and an average age at diagnosis of about 3 years of age. CF incidence rates vary around the world, but rates as high as 1 in 2000 to 3000 live births are associated with Caucasian populations with Northern European ancestry. The median predicted survival time of CF patients in the United States is approximately 47.4 years (95% CI, 44.2‐50.3) according to the Cystic Fibrosis Foundation 2018 Registry Report. However, epidemiological data on CF prevalence in China have not yet been reported, aside from observations that the genotypic spectrum of Chinese CF varies widely among resident subpopulations based on their geographical and ethnic origins.
Numerous animal CF models have been established based on specific types of human CFTR mutations, but models differ in their effectiveness in mirroring features of human CF‐specific disease. For example, the mouse CF model differs markedly from human CF at the pathological level, while at the molecular level CFTR genes of pig and human are highly homologous, but their corresponding CFTR protein structures and functions are vastly different. At present, ferret and rabbit CF models hold promise as human CF models, but additional models based on other species should also be evaluated. Meanwhile, the introduction of human CFTR genes harboring CFTR mutations into genomes of animals holds promise as a strategy for creating better animal models for human CF. Nevertheless, current animal models each have their own unique features that are useful for studying particular aspects of human CF disease, as described below.
2. PATHOGENESIS OF CYSTIC FIBROSIS
2.1. characteristics of the human cystic fibrosis gene and encoded cftr protein.
Cystic fibrosis is caused by pathogenic mutations in a single large gene located on human chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein. 1 , 2 , 3 CFTR belongs to the ABC (ATP‐binding cassette) family of proteins, a large group of related proteins that share transmembrane transport functions. The CFTR gene comprises 250 kilobases of genomic sequence that encodes an epithelial cell protein that is composed of 1480 amino acids in its mature state. The CFTR protein forms a cell membrane‐spanning chloride channel whose function is regulated by phosphorylation mediated by cAMP‐dependent phosphokinases. CFTR phosphorylation in the presence of ATP can trigger channel opening to allow migration of about 10 chloride ions to the outside of the cell every minute. Certain CFTR gene mutations lead to generation of defective CFTR proteins that cannot be processed normally by the endoplasmic reticulum for effective transport to the cell membrane. The few mutated CFTR protein molecules that do reach the cell membrane are dysfunctional and thus cannot carry out chloride ion transport, leading to accumulation of chloride ions and associated water molecules in epithelial cells and lack of hydration of extracellular mucus and secretions. The most common pathogenic mutation found in Caucasians of Northern European descent is a deletion mutant designated F508del (with deletion of phenylalanine at site 508 caused by genomic deletion of three nucleotides, designated c.1521_1523delCTT). Indeed, approximately 70% of CF Caucasian patients in the United States harbor this specific CFTR mutation, with severe clinical manifestations observed in patients who are homozygous for the delF508 genotype.
The structure of normal CFTR protein contains two groups of six membrane‐spanning structural motifs, two intracellular nucleotide‐binding folds (NBFs), and a highly charged ‘R domain’ containing multiple phosphorylation sites. Activation of the chloride channel requires phosphokinase A‐mediated phosphorylation of the R domain and sustained ATP levels within the NBFs. 4 , 5
2.2. Genetic mutation types in CFTR
CFTR mutations are currently categorized according to cause of dysfunction, including dysfunctional protein translation, cell processing, or CFTR channel gating. Missense (single amino acid substitution) mutations account for 38.74% of CFTR mutants, frameshift (insertion or deletion) mutations account for 16.25%, splicing (incorrect intron splicing) mutations account for 10.93%, and nonsense (early termination codon) mutations account for 8.41% of all known CFTR mutations detected worldwide. 6
Mutations of the CFTR gene fall into six different classes that roughly correspond to specific types of CFTR dysfunction. 7 , 8 In general, mutations in classes I to III cause more severe disease than those in classes IV to VI. 8 , 9 However, clinical manifestations of CF caused by any particular combination of mutations can vary, perhaps due to effects of gene modifiers. For example, genotype‐phenotype correlations are weak for CF associated with pulmonary disease, but are somewhat stronger for CF types associated with pancreatic insufficiency. Indeed, in most cases specific mutations should not be used to make assumptions about CF severity for an individual patient, while clinical decisions should be guided by patient metrics of growth, lung function, and nutritional status. Nevertheless, characterization of mutations may be useful to guide initial therapy for some patients, as several new therapies have been recently developed that target CF disease caused by specific classes of CFTR mutations. The five types of CFTR mutations are listed below.
Class I mutations: Defective protein production. This type of defect is usually caused by nonsense, frameshift, or splice‐site mutations, leading to premature termination of messenger RNA (mRNA) transcripts and a complete absence of CFTR protein. Examples include G542X, W1282X, R553X, 621+G>T, and 1717‐1G>A. 10
Class II mutations: Defective protein processing. This class of mutation causes abnormal post‐translational processing of the CFTR protein, which prevents the protein from trafficking to the correct cellular location, as exemplified by the F508del mutation that is present in a homozygous state in approximately 50% of CF patients and in at least a heterozygous state in 90% of CF patients. 10
Class III mutations: Defective regulation. These mutations cause diminished channel activity even when ATP levels are adequate. Many mutations alter NBF ATP‐binding regions (designated NBO1 and NBO2), whereby some mutants retain varying degrees of sensitivity to nucleotide binding. The mutation giving rise to CFTR substitution G551D, which abolishes ATP binding, is the most common class III mutation in Caucasian populations. Meanwhile, other CFTR mutations within the region encoding the CFTR R domain may also fall into this category. 10
Class IV mutations: Defective conduction. CTFR protein is produced and transported correctly to the cell surface. However, the rate of ion flow and the duration of channel opening are reduced as compared to normal CFTR protein even though chloride currents are generated in response to cAMP stimulation. A mutation that induces a CFTR protein amino acid substitution (R117H) is the most common class IV mutation in Caucasian populations. 10
Class V mutations: Reduced amounts of functional CFTR protein. This class is not included in some classification schemes. It includes several mutations that alter mRNA stability and other types of mutations that alter stability of the mature CFTR protein (with the latter sometimes classified separately into an additional class, class VI). 8 , 11
Class VI mutations: Decreased CFTR stability. This class causes substantial plasma membrane instability and includes Phe508del when rescued by most correctors (rPhe508del). 8
2.3. Distribution of CF gene mutation
CF is being increasingly detected in regions where it was previously undiagnosed due to a lack of clinicians with knowledge of the disease, including areas of South and East Asia, Africa, and Latin America. Due to increased newborn CF screening and detection of individuals with mild CF or disease limited to one organ system, CF prevalence rates are likely to rise. To date, 2107 mutations have been entered into the Cystic Fibrosis Mutation Database ( www.genet.sickkids.on.ca/StatisticsPage.html ) (Table 1 ).
Statistics by CF mutation type
2.4. Characteristics of mutations in Chinese pediatric CF patients
In order to collect information pertaining to CFTR mutations associated with pediatric CF cases in China, we searched scientific literature repositories that included the Chinese Knowledge Infrastructure Digital Library, Wanfang database, VIP database, and PubMed for reports published from January 1, 1975, to June 1, 2021. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Duplicate reports, reports of undiagnosed cases, and reports lacking CFTR gene mutation information were excluded from our final analysis. From the final set of reports, we detected 106 Chinese pediatric CF cases associated with 101 CFTR gene mutations (Table 2 ). Our findings revealed that the CFTR gene mutation spectrum in Chinese pediatric CF patients differed markedly from the corresponding spectrum for European and American countries. The most common mutation detected in Chinese pediatric CF patients was c.2909G>A, whereas the common mutation c.1766+5G>T found in Chinese CF patients was not found in Caucasians, while the most frequent F508del mutation in Caucasians was rare in China. The majority of mutations in Chinese patients have been found only once or are absent in Caucasians. In the carrier screening panel of CF recommended by the American College of Medical Genetics, the 23 most common CFTR variants cover about 84% of CF‐causing mutations among Caucasians. However, only 4 mutations have been observed in Chinese CF patients. Our results also revealed the value of neonatal screening for achieving early CF detection and treatment in some countries. Moreover, the results also revealed that a CF diagnosis can be confirmed in some cases using sweat chloride‐based tests, while in other cases genetic testing is needed to confirm a CF diagnosis.
CFTR gene mutation in Chinese children with cystic fibrosis
Abbreviations: M, male; F, female; NA, not applicable; Pa , Pseudomonas aeruginosa ; SA , Staphylococcus aureus ; MRSA , methicillin‐resistant Staphylococcus aureus ; Kp , Klebsiella pneumoniae ; Sp, Streptococcus pneumoniae ; Ac , Acinetobacter; BC , Burkholderia cepacia ; HI , Haemophilus influenzae ; MC , Moraxella catarrhalis ; NTM , non‐tuberculosis mycobacterium; PM , Pseudomonas pseudomallei ; SM , Stenotrophomonas maltophilia .
3. CLINICAL FEATURES OF CYSTIC FIBROSIS
CF is caused by dysfunctional transport of chloride and/or other ions (such as sodium and bicarbonate) that leads to generation of thick, viscous secretions (eg mucus) in the lungs, pancreas, liver, intestine, and reproductive tract and increased salt content in sweat gland secretions. Ultimately, progressive lung disease is the main cause of CF complications and patient mortality. 8 The course of disease varies greatly and can begin from a few months after birth to decades after birth, with many patients exhibiting mild or atypical symptoms. Therefore, clinicians should take care to avoid excluding CF as a possible diagnosis in cases where patients exhibit only a few typical CF signs and symptoms.
3.1. Respiratory tract involvement
Typical respiratory manifestations of CF include a persistent productive cough, hyperinflation of lung fields on chest radiograph, and pulmonary function test findings indicative of obstructive airway disease. As the disease progresses, repeated infections associated with inflammatory cell accumulation and release of cell contents damage bronchial walls, leading to loss of bronchial cartilaginous support and muscle tone and eventual bronchiectasis. Disease progression includes acute exacerbations of cough, tachypnea, dyspnea, increased sputum production, malaise, anorexia, and weight loss. These acute events are associated with acute, transient loss of lung function that improves with treatment but that often progresses to permanent loss of lung function over time.
Although CF patients often vary, transient airway infection with pathogenic bacteria often first occurs early in life. After years of CF disease, chronic airway infection with either Staphylococcus aureus or Pseudomonas aeruginosa often becomes established and is often detected based on radiographic evidence of bronchiectasis. In addition, airways of CF patients can be colonized or infected by other species of microbes, including Stenotrophomonas maltophilia , Achromobacter xylosoxidans , Burkholderia cepacia complex, nontuberculous mycobacteria (especially Mycobacterium avium complex and Mycobacterium abscessus ), and the filamentous fungus Aspergillus fumigatus . 31 Continuous airway colonisation and infection by bacteria (especially P aeruginosa ) can enhance the inflammatory response by triggering neutrophils to release large amounts of DNA and matrix proteins into airways. These substances, coupled with CF‐induced impaired airway clearance functions and chronic inflammation, increase airway mucus viscosity. Current research efforts are underway to identify additional bacterial species in CF patient airways, including obligate anaerobes that may be identified using next‐generation sequencing technology. 32 , 33
3.2. Sinus disease
The majority of CF patients develop sinus disease. 34 Sinus disease can present with chronic nasal congestion, headaches, cough caused by chronic postnasal drip, and sleep disturbances. Sinus infections can trigger lower respiratory exacerbations in some patients, although organisms found in sinuses do not always match those recovered from lungs. Meanwhile, some individuals with isolated chronic rhinosinusitis have signs and symptoms suggestive of CFTR dysfunction that do not satisfy CF diagnostic criteria, prompting clinicians to refer to this affliction as CFTR‐related disorder. Notably, in one case‐control study, the single CFTR mutation rate for a group of chronic rhinosinusitis cases was significantly higher than the corresponding rate for the general population (7% versus 2%). 35
3.3. Digestive system diseases
Approximately two‐thirds of CF patients exhibit CF insufficiency of the exocrine pancreas from birth, with an additional 20% to 25% developing this condition during the first several years of life, and most exhibiting signs of fat malabsorption by one year of age. 36 CF‐associated pancreatic disease tends to be progressive; many patients with apparently normal or marginal pancreatic function at birth develop overt evidence of pancreatic insufficiency in childhood or adulthood. Overall, approximately 85% of individuals with CF eventually develop clinically significant pancreatic insufficiency. 37 Common symptoms and signs of pancreatic insufficiency include steatorrhea, characterized by frequent, bulky, foul‐smelling stools that may be oily, as well as failure to thrive or poor weight gain resulting from malabsorption of fat and protein. Infants with severe untreated pancreatic insufficiency occasionally present with edema, hypoproteinemia, electrolyte loss, and anemia due to malabsorption of macro‐ and micronutrients. Some patients also may present with symptoms caused by deficiencies of the fat‐soluble vitamins A, D, E, and K. Vitamin K deficiency can present as a coagulopathy and vitamin D deficiency as rickets. Continued defective ductular and acinar pancreatic secretion functions lead to progressive pancreatic damage that can trigger acute or recurrent pancreatitis. Moreover, patients with exocrine pancreatic insufficiency often develop dysfunction of the endocrine pancreas, leading to glucose intolerance and CF‐related diabetes.
With regard to other CF‐associated digestive system disorders, 10% to 20% of newborns with CF present with meconium ileus characterized by obstruction of the bowel by meconium, which is a risk factor for poor CF prognosis. 38 Rectal prolapse, which previously was rarely detected in children with CF, has been detected frequently in recent years and appears to be associated with constipation and/or malnutrition. Focal biliary cirrhosis caused by inspissated bile is present in many patients and may cause elevated serum alkaline phosphatase and lobular hepatomegaly. A minority of CF patients develop periportal fibrosis, cirrhosis, symptomatic portal hypertension, and variceal bleeding that are associated with progressive liver disease. 37
3.4. Reproductive system diseases
More than 95% of men with CF are infertile because of defects in sperm transport, although spermatogenesis is not affected. Intriguingly, nearly one‐half of all men with congenital bilateral absence of the vas deferens and normal lung function possess two CFTR mutations. 39 Meanwhile, females with CF are less fertile than normal healthy women, due to malnutrition and the production of abnormally tenacious cervical mucus. Nonetheless, females with CF may become pregnant and those who do should be counselled accordingly about contraception and childbearing decisions. 40 Indeed, comprehensive genetic counselling is essential for prospective parents with CF.
3.5. Nutrition and growth disorders
Patients with CF have reduced bone mineral content and increased rates of fractures and kyphoscoliosis. In all age groups, up to 30% of patients present with clinically significantly reduced bone density, while this proportion approaches 75% in adults with CF. 41 , 42 Clubbed fingers (and toes) and hypertrophic osteoarthropathy can also occur in patients, with clubbing of fingers (and toes) found commonly in patients with long‐term disease, while hypertrophic osteoarthropathy is only rarely observed.
4. DIAGNOSIS OF CYSTIC FIBROSIS
4.1. diagnostic criteria of cystic fibrosis.
Both of the following criteria must be met to diagnose CF 43 : (1) Clinical symptoms consistent with CF in at least one organ system, or a positive newborn CF screening result, or a sibling with CF. (2) Evidence of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction (any of the following): Elevated sweat chloride ≥60 mmol/L, or presence of two disease‐causing mutations in the CFTR gene (one from each parental allele), or abnormal nasal potential difference (NPD) result.
4.2. Sweat chloride
The sweat chloride test remains the primary test used for CF diagnosis. If the concentration of chlorine is greater than 60 mmol/L, the diagnosis of CF is confirmed, while a high concentration of 40‐60 mmol/L is suspicious, and a concentration <40 mmol/L is normal (excluding adrenal insufficiency). However, new clinical guidelines 43 indicate that a sweat chloride concentration <30mmol/L is the normal threshold for all age groups (excluding adrenal insufficiency). Importantly, a normal sweat chloride concentration is observed in approximately 1 percent of CF patients with unusual genotypes, such as the c.3717+12191C>T (legacy name: 3849 + 10 kb C‐T) or poly‐T defects. 43
4.3. CF diagnostic challenges
Due to the wide range of clinical phenotypic differences among CF patients, CF can be difficult to diagnose. Apart from the respiratory manifestation, symptoms involving other organs in Chinese CF patients are not as common as in Caucasians, and in particular there are fewer digestive symptoms. This is true for approximately 10%‐15% of CF patients who have mild symptoms, normal pancreatic function, good nutrition, slow decline of lung function, no obvious family history, borderline normal sweat test results, and only one detected CFTR gene‐associated mutation. In addition, if onset of CF is delayed or if the patient has a CF‐associated mutation that is very rare and thus is not included in the scope of routine screening, diagnosing the disease is even more challenging, causing diagnostic delay. At the current time, nearly 70% of typical CF patients are diagnosed before they reach 1 year of age. The median age of CF diagnosis in the United States in 2018 was 3 years of age, while 8% of patients were diagnosed after 10 years of age. 44 With regard to Chinese CF patients, Shen reported a 5.7‐year delay between the first clinical presentation and the eventual CF diagnosis, 45 while another study of pediatric Chinese patients revealed a median age at diagnosis of 8 years of age. 46 Moreover, Chinese CF patients are more likely to have a negative family history, possibly due to the previous one‐child policy. Diagnosis of CF may be less suppressed following the recent implementation of the three‐child policy.
5. ANIMAL MODELS OF CYSTIC FIBROSIS
To date, many animal models of CF have been established that vary according to type of CFTR mutation. Phenotypes of human and animal models of cystic fibrosis are listed in Table 3 .
Phenotypes of human and animal cystic fibrosis models
5.1. Murine models of CF
Murine CF models have been developed, but they do not mirror human disease very well due to differences in lung and pancreas anatomical structures and physiologies between mice and humans. For example, mice with dysfunctional CFTR can present with impaired chloride transport in some types of epithelial cells, but the resulting pathology differs markedly from human CF pathology. By contrast, CF mice harboring the most severe CFTR mutation may exhibit gastrointestinal pathological effects resembling those associated with human CF, including intestinal obstruction, mucus accumulation, goblet cell proliferation, and fat absorption disorder. However, most of these mice die of intestinal obstruction soon after birth if they are not fed a special diet, making use of CF mice model impractical as a human CF model. CF mice also exhibit male sterility, as occurs in human male CF patients. Paradoxically, no CF mice exhibit pancreatic insufficiency, possibly due to low CFTR gene expression in mouse pancreas, as well as other mechanisms unique to mice, including intracellular calcium‐based activation pathways that enable mouse cells to expel water. Moreover, bacterial infections, inflammatory reactions, mucus accumulation, and tissue remodeling have never been detected within CF mice lungs, although the reasons for the absence of lung pathologies are unknown. One possible factor may be related to the absence of ciliated epithelium and submucosal glands in mouse lung compared to human lung. Another possible factor is the presence in mice of other chloride channels, such as ICACCS, which may compensate for CFTR dysfunction; this potential backup system is absent in human CF patients. In the meantime, modifications of the mouse CF model have been conducted to create better CF mouse models. For example, electrophysiological analysis of CF mouse nasal epithelium has revealed increased sodium current in nasal epithelium. Inhibition of this current via treatment of CF mice with amiloride (an agent that blocks cAMP‐regulated chloride transport) generated a CF mouse phenotype mirroring human CF. Nevertheless, this CF mouse model did not develop lung lesions and was not susceptible to spontaneous pulmonary infections that occur in human CF patients. 47 Taken together, these results highlight differences between humans and mice with regard to the effects of CFTR dysfunction that may reflect species differences in airway cell biological characteristics, numbers of submucous glands, and expression and activation of other chloride channels. Moreover, even though a CTFR gene knockout mouse model has been established, results of studies using this model have not yet been reported. This lack of reporting may reflect the fact that the model did not perform well, since CF mice can utilize a CFTR‐independent alternative Cl − channel that enables cells of CFTR‐deficient mice to secrete Cl − to compensate for the lack of a functional CFTR. Notably, ATP12A belongs to the P2‐type ATPase family and shares sequence homologies with both the gastric H,K‐ATPase (ATP4A) and the Na,K‐ATPase (ATP1A). ATP12A mediates the electroneutral exchange of H + for potassium (K + ) but may also function in a Na + /K + exchange mode. 48 In humans lacking CFTR, unchecked H + secretion by the nongastric H + /K + adenosine triphosphatase (ATP12A) acidified airway surface liquid, which impaired airway host defenses. However, the expression of ATP12A is low in murine airways, which may partly explain the very mild pulmonary phenotype in murine models of CF. 49 In any case, mouse models are not helpful for studying the long‐term pathology of human CF disease, due to the short lifespan of mice.
5.2. CF rat models
Compared with mice, rats are appreciably bigger and provide better tissue specimens and blood samples for analysis. Compared with larger animals, rats have a shorter gestation and earlier sexual maturity. Like humans, rats have extensive submucosal glands, which are implicated in the development of CF airway disease. 50
To date, several CF rat models have been generated with interesting phenotypes. The first CF rat model was a CFTR‐knockout rat strain. 51 Recently, two CF rat models of KO and F508del CFTR using CRISPR/Cas9 gene editing have showed encouraging results. 52 , 53 They revealed CF manifestations including reduced survival, intestinal obstruction, bioelectric defects in the nasal epithelium, histopathological changes, and male reproductive abnormalities. Moreover, they represent a novel resource to advance the development of CF therapeutics.
5.3. Porcine CF models
Pigs have a large number of offspring, mature rapidly, and have a long lifespan, enabling researchers to study long‐term pathology and prognosis of CF. In addition, pig anatomy and physiology mimic corresponding human characteristics and porcine CFTR is 92% homologous in nucleotide sequence to human CFTR. In 2008, Rogers et al 54 generated CFTR gene knockout and delF508 pig models using a recombinant adeno‐associated virus (AAV mediated) method. Lack of functional CFTR protein in CFTR −/− piglets led to similar phenotypic effects in lung, liver, pancreas, and gastrointestinal tract tissues as those found in human CF. 55 , 56 , 57 Meconium ileus was present in 100% of CFTR−/− piglets (compared with 15% in CF infants), which in humans can be fatal without early surgical intervention, 56 thus limiting potential use of porcine models in some research settings due to risks associated with surgery (including intestinal atresia). Finally, although CFTR genes of pigs and humans are similar at the nucleotide level, their encoded CFTR proteins differ markedly in structure and function.
5.4. The ferret CF model
The ferret CF model shares many CF pathological characteristics with human CF, especially in newborns. However, considerable effort is needed to produce enough CFTR−/− ferrets to ensure that some animals overcome gastrointestinal pathology and reach puberty so they can be used to model human CF based on similarities of lung pathology. Notably, characteristics of pulmonary infections in CFTR−/− ferrets at the beginning and end of life mirror corresponding pulmonary features associated with human CF, highlighting the potential benefits of this model. Nevertheless, further development of the delF508 and G551D CFTR mutant forms of the ferret CF model are needed to better model human CF.
5.5. The rabbit CF model
Recently, CRISPR/CAS9 has been used to generate CFTR knockout and F508del genomic mutations to create CF rabbit models. 58 Rabbits are considered to be an ideal species for simulating human CF lung disease, as their airway anatomy and inflammatory responses resemble corresponding human characteristics. Preliminary findings of experiments using CF rabbits to model human CF indicate that CF rabbits will likely be useful for modelling human CF disease.
5.6. Prospective animal models for CF research
With the development of rapid and accurate gene editing technologies such as CRISPR/CAS9, better animal models of human CF can now be created by introducing specific CFTR genes and mutations into animal genomes. Development of animal models that accurately mimic human CF will facilitate development of experimental pulmonary therapies, identification of new therapeutic targets, and enable clarification of complex mechanisms underlying initiation and progression of pulmonary CF. 59 Although no perfect CF animal model exists, each animal model has its own unique advantages for use in studying specific CF‐related pathogenic mechanisms.
6. IMPORTANCE OF ANALYZING CF GENOTYPES
The spectrum of CFTR genotypes of Chinese children with CF significantly differs from that of children in European and American countries. The most common CF mutation in Caucasian patients (F508del) is rare in China, while CFTR genotypes detected in Chinese CF patients are more diverse than genotypes of Caucasian CF patients.
To help patients, family members, healthcare providers, and scientists understand the complexity and clinical significance of widely recognized CFTR mutations, a team at Johns Hopkins University has developed a website to provide information regarding specific cystic fibrosis mutations ( http://www.cftr2.org ). By analyzing registered functional data obtained from cellular research studies, researchers can obtain useful information about genotype‐phenotype relationships, especially for individuals with borderline functional CTFR mutations and less severe CF phenotypes.
Although the abovementioned cftr2 website is a valuable tool for studying diagnostic characteristics of patients with CFTR mutations, it cannot replace clinical observations and professional knowledge. Indeed, the use of such a tool may lead to missed diagnoses, especially if the cftr2 database is biased toward common mutation sites in one population, as indicated in new cystic fibrosis guidelines. 43 Importantly, even though the incidence rate of CF in the Chinese population is lower than the CF incidence rate in Caucasians, the absolute number of mutant alleles in Chinese populations may be quite large due to the presence of a large number of undiagnosed CF patients in China. 60 Therefore, whole gene sequencing is advocated in China as an effective method for detecting rare or new CTFR mutations and decreasing the missed diagnosis rate there.
A major challenge for treatment of CF, a rare disease, is that more than 1000 rare mutations have been detected that are each likely carried by no more than five carriers throughout the world. Thus, traditional clinical methods are not suitable for detecting these rare mutations, warranting use of newer methodologies for diagnosing CF in such cases. Nevertheless, it is important to study rare CF mutations, which can reveal important information about disease prognosis and genotype‐phenotype relationships to better guide patient care and improve patient outcomes. In this era of personalized medicine and treatment, such studies will be extremely important for improving care of patients with rare mutations.
7. TREATMENT AND MANAGEMENT OF CYSTIC FIBROSIS
Treatment regimens for CF should be evaluated, improved, and administered in combination with close monitoring to achieve early, active intervention to manage CF. In order to achieve these goals, prospective CF patients should be hospitalized until additional test results and other findings are obtained to support or exclude a CF diagnosis. Once a CF diagnosis has been made, clinicians should immediate initiate patient treatment and educate patients and their families to effectively manage the disease.
7.1. CF treatment regimens
Treatment for CF lung disease includes administration of mucus thinner, airway clearance, and antibiotics. To thin mucus, inhalation therapy consisting of hypertonic saline is administered to hydrate thick mucus within CE patient airways. The high osmotic pressure of the solution draws water out of airway epithelial cells to reconstruct the water‐containing surface layer that is absent in CF patients. To address retention of purulent secretions in CF patients that obstruct airflow and damage airways, chest physiotherapy based on postural drainage and percussion is the standard method for clearing secretions, with bronchoscopic lavage also used for this purpose. Although antibiotics are essential for treatment of chronic infections and acute CF exacerbations, long‐term oral antibiotics are generally not recommended for controlling infection. However, long‐term azithromycin use is recommended for many CF patients, due to its anti‐inflammatory and/or antibacterial properties, while long‐term treatments with aerosolized antibiotics against P aeruginosa (eg tobramycin and aztreonam) are recommended due to their beneficial effects on lung function. Meanwhile, bronchodilator use has been evaluated for CF treatment in many studies, but such treatment does not appear to benefit CF patients.
Notably, the main CF airway pathological feature is severe neutrophil inflammation. In past years, inflammation was considered necessary for preventing spread of infection, but accumulating evidence suggests that excessive inflammation is generally harmful. Thus, in clinical practice, azithromycin is recommended for all CF patients older than 6 years of age with clinical evidence of airway inflammation (eg chronic cough) or any decrease in FEV1, regardless of the status of P aeruginosa infection.
To further support CF patient growth and nutrition, patient diets should be supplemented with pancreatic enzymes, calories, and fat‐soluble vitamins. At present, several new types of drugs 61 , 62 , 63 are under development, of which some are well‐tolerated by patients, including drugs to restore normal function of defective CFTR protein and drugs that have a direct impact on mucociliary clearance. CFTR modulators, such as ivacaftor (Kalydeco), lumacaftor/ivacaftor (Orkambi), tezacaftor/ivacaftor (Symdeko), target different potential CFTR protein defects caused by different gene mutations, thus rendering these drugs effective only for people with specific mutations. Trikafta (tezacaftor plus elexacaftor and ivacaftor) is the third drug approved by FDA that rescues defects caused by F508del, which is superior to its predecessors. Trikafta is also effective in CF patients with one copy of F508del‐CFTR mutation. It demonstrates safety and sustained efficacy for 24 weeks or longer in people with CF and one or more F508del alleles. 64 To prevent long‐term infection and inflammation that eventually cause irreversible bronchiectasis and respiratory failure, lung transplantation is feasible for end‐stage patient treatment depending on the health of the particular patient.
7.2. Management of cystic fibrosis
In addition to timely diagnosis and treatment, long‐term follow‐up and monitoring of CF patient status are also very important. For children, long‐term nutritional assessment should be carried out, including monitoring of height, weight, body mass index, and other indicators, as well as timely nutritional guidance. Moreover, CF patients should receive all recommended routine childhood immunisations, especially the seasonal influenza vaccine. Furthermore, timely evaluation of exacerbations, treatment of severe infections, and maintenance of follow‐up care should all be implemented as necessary components of optimal CF management. Nevertheless, CF chronicity and complexity can seriously impact the mental health of patients and their caregivers by triggering anxiety and depression that can adversely affect treatment compliance and long‐term prognosis. Therefore, psychological counselling is also very important.
8. PROGNOSIS OF CYSTIC FIBROSIS
Although cystic fibrosis is currently incurable and greatly reduces life expectancy, the average CF survival age has increased significantly over the past 50 years and now exceeds 40 years of age. Thus, CF is no longer viewed solely as a childhood disease, but now is recognized as a disease of children and adults. Currently more than half of CF patients are adults as old as 60 years of age, indicating that active treatment can improve prognosis, increase quality of life, and prolong lifespan. Time to diagnosis and treatment, severity of lung disease, nutritional and general conditions, and mental state are key factors that influence prognosis. 44 With regard to pediatric CF patients, attention should be paid to improving awareness and compliance of family members to prevent infection, actively treat acute exacerbations, and comply with recommended care instructions to maximize quality of life and long‐term survival.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Chen Q, Shen Y, Zheng J. A review of cystic fibrosis: Basic and clinical aspects . Anim Models Exp Med . 2021; 4 :220–232. 10.1002/ame2.12180 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
This work was supported by the National Natural Science Foundation of China (81600002), Science and Technology Project of Quanzhou, Fujian Province (2020N050s).

IMAGES
VIDEO
COMMENTS
BACKGROUND AND OBJECTIVE:. Cystic fibrosis (CF) is a common life-shortening genetic disease and is associated with poor psychosocial and quality of life outcomes. The objective of this study was to describe the experiences and perspectives of children and adolescents with CF to direct care toward areas that patients regard as important.METHODS:. MEDLINE, Embase, PsycINFO, and Cumulative Index ...
A 9-year-old boy was admitted to the hospital because of fever, cough, respiratory distress, and chest pain. Imaging revealed ill-defined nodular opacities throughout all lung lobes, areas of conso...
Finally, the infant was diagnosed with cystic fibrosis (CF). Review of literature revealed five children (including the infant in this case study) with CF who presented with white stool. All five children had anemia, four had edema and hypoproteinemia, five had changes in stool color (it was pistachio-green color in two patients, pale colored ...
Cystic fibrosis (CF) is one of the most commonly diagnosed genetic disorders. Clinical characteristics include progressive obstructive lung disease, sinusitis, exocrine pancreatic insufficiency leading to malabsorption and malnutrition, liver and pancreatic dysfunction, and male infertility. Although CF is a life-shortening disease, survival has continued to improve to a median age of 46.2 ...
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids ...
Cystic fibrosis (CF) is a multiorgan disease, caused by autosomal recessive (AR) mutations in the cystic fibrosis transmembrane regulator (CFTR) acting primarily as a chloride channel. CF is most commonly diagnosed in Caucasian populations. Common clinical presentations in pediatric patients include chronic cough, respiratory tract infections ...
The objectives of this study were as follows: (1) describe the clinical phenotypes of children and adolescents with CF in a reference center in Brazil; (2) assess the influence of clinical phenotype (pancreatic insufficiency) at the age of diagnosis; and, (3) evaluate the role of neonatal screening test in reducing the age of diagnosis. Go to:
BackgroundUniversal newborn screening changed the way medical providers think about the presentation of cystic fibrosis (CF). Before implementation of universal screening, it was common for children with CF to present with failure to thrive, nutritional deficiencies, and recurrent infections. Now, nearly all cases of CF are diagnosed by newborn screening shortly after birth before significant ...
This case report presents the first case of cystic fibrosis ever reported in Ethiopia and possibly East Africa, that of a 17-year-old female diagnosed with the disease following a CT scan of her abdomen and chest. ... A study comparing HRCT and magnetic resonance imaging (MRI) showed almost equivalent detection rates of bronchiectasis and ...
The studies we included highlighted the negative impact of cystic fibrosis on mental health, like depression and anxiety, as well as on sleep, physical health, and overall quality of life. Several non-medical interventions, such as logotherapy, psychological interventions, complementary and alternative medicine, and many more, have been shown ...
Cystic fibrosis (CF) is an autosomal recessive disease characterized by pancreatic insufficiency and chronic endobronchial airway infection. This latter feature results in progressive bronchiectasis and ultimately respiratory failure, which is the leading cause of death in patients with CF. Other complications include sinusitis, diabetes ...
Cystic fibrosis management warrants an interdisciplinary team because this disorder affects various organ systems. Effective management of the newborn with CF assists in improving the child's overall prognosis. Family support is critical throughout the prenatal and postnatal periods. The case presented reviews a child born with suspected CF and ...
Case Studies in Pediatric Anesthesia - December 2019. ... This chapter reviews the pathophysiology of cystic fibrosis and its effects on a host of organ systems. The author discusses the anesthetic implications of cystic fibrosis allowing the reader to formulate a safe perioperative plan for these patients.
The experience of healthcare transition from pediatric to adult care in cystic fibrosis (CF) remains poorly understood, particularly among racially and ethnically diverse adolescents and young adults (AYAs) with CF. The objective of this qualitative study was to explore the perspectives of a diverse sample of AYAs with CF at one urban academic medical center regarding healthcare transition.
Universal newborn screening of cystic fibrosis had been well-established before the study period 16. Data collected from their existing electronic medical record included; the microbiological ...
Cystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation (Cystic Fibrosis Foundation, 2019a), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease ...
Fewer studies include pediatric patients (12-16) and only four of those studies focused solely on the pediatric population (13 ... Fan LL, Kozinetz CA, et al.: Invasive mechanical ventilation for acute respiratory failure in children with cystic fibrosis: Outcome analysis and case-control study. Pediatr Pulmonol 2002; 34:297-303 ...
Diabetes is the most common comorbidity in individuals with cystic fibrosis (CF). Among people with CF, the prevalence of CF-related diabetes (CFRD) is estimated to be 2% in children, 19% in adolescents, 40% in individuals in their 20s, and 45-50% in those ≥30 years of age ().CFRD shares some aspects with type 1 diabetes in that it is primarily due to insulin deficiency, but it is ...
Second-year University of Oklahoma pediatric resident Dr. Caroline Thompson has a professional and personal connection to cystic fibrosis patient care for children. A childhood friend had the disease and made a lasting impression on Thompson. Now, Thompson has received the Cystic Fibrosis Foundation's Medical Resident Research Award for a pilot study evaluating pharmacogenomic-directed ...
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). In 1949, it's been identified as a monogenic disease and was thought to primarily affect individuals of Northern European descent. It was the most prevalent autosomal recessive disease that shortens life. With the availability of ...
Background: This research paper explores the efficacy of a multidisciplinary approach to pediatric cystic fibrosis (CF) care within intensive care unit (ICU) settings. Through a comprehensive literature review, the study investigates the roles of anesthesia, midwifery, pediatric nursing, and physiotherapy in enhancing patient outcomes and quality of life.
One, some, or all options may be correct.) -Administer pancreatic enzymes in the morning and at night. -Mix the pancreatic enzymes with hot, starchy foods such as macaroni or pasta. -Open the enzyme capsules and mix the beads in a nonprotein food. -While the capusules can be swallowed whole, client is 24 months old.
Cystic Fibrosis case study. unfolding case study. Course. Family Health Nursing (NUR 338) 10 Documents. Students shared 10 documents in this course. University ... Justin is admitted to the pediatric unit for IV antibiotics, and you will be the nurse responsible for his care. Justin has an implanted port for vascular access since he frequently ...
The nurse educator obtained a contact number for a CF clinic near the beach in case an exacerbation occurs. Pamela and Donald now feel confident as they plan their first vacation with Darla. Study with Quizlet and memorize flashcards containing terms like Meet the Client: Darla WilliamsPamela and Donald Williams bring their 24-month-old ...
Between 5% and 10% of patients with cystic fibrosis will develop cirrhosis. Dr. Murray has led several studies in this area, particularly on the use of ultrasound and other imaging to detect liver complications in these patients and identify early those at risk for advancing liver disease.
Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide . Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease.
The study is a retrospective case-control study of PwCF and pwoCF transplanted 2004-2017 at the University of Alberta hospital (Edmonton, Canada) with 1:1 matching to patients transplanted for non-CF diagnoses. PwCF and pwoCF were matched based on age, sex-at-birth, and year of transplantation. Patients were first matched by sex, then each ...
IPATH's Co-Director, Dr. Robert Schooley, is co-leading the first NIH-sponsored phage therapy clinical trial. This trial is recruiting cystic fibrosis (CF) patients with Pseudomonas aeruginosa infections. Enrolled patients will receive a single dose of a four-phage cocktail that targets P. aeruginosa. The trial's primary objectives are to assess the safety and microbiological activity of a ...
The outcome measures will be to analyze if non-cystic fibrosis bronchiectasis is related to a specific inflammatory profile. ... in the pediatric population [2, 3]. However, the pathophysiological mechanisms by which the two diseases are ... We will include observational studies (including cohort, case-control and cross-sectional studies). Will ...
Cystic fibrosis is an autosomal recessive disease caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). We summarize clinical and genetic characteristics of cystic fibrosis gene mutations, as well as animal models used to study human cystic fibrosis disease. 1.