Morning Rundown: Biden could close border with today's executive action, extreme heat hits the Southwest, police say a revelation enraged ex-mayor before triple shooting

Pfizer’s Lorbrena extends life for patients with rare lung cancer
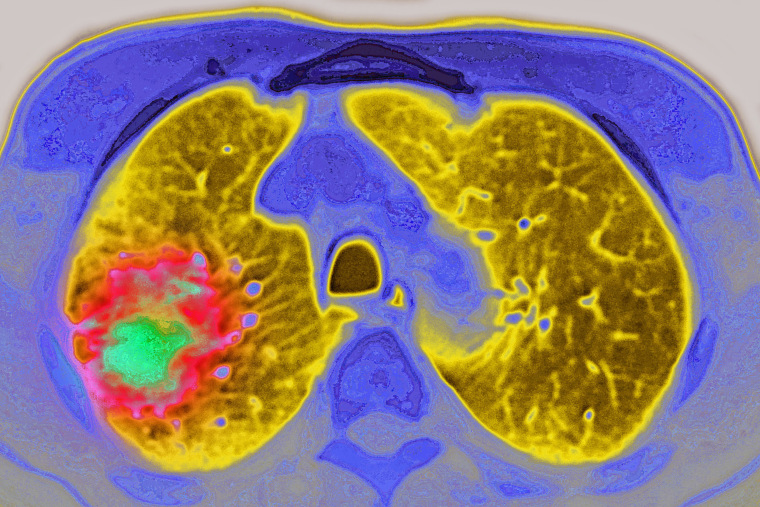
Pfizer’s lung cancer drug Lorbrena can extend life for patients with a rare form of the disease for years longer than other drugs, according to new research published Friday.
The drug treats a type of non-small cell lung cancer with a genetic mutation called ALK. Non-small cell lung cancers account for about 85% of lung cancer diagnoses , and ALK-positive cancers account for about 4% of those diagnoses — more than 70,000 people every year.
The cancer tends to occur in younger patients who are nonsmokers .
It’s also particularly deadly: ALK-positive lung cancers are especially adept at spreading to the brain. About 25% of patients have brain metastasis within the first two years of being diagnosed.
“As time moves on, that increases,” said Dr. Ben Solomon, head of lung medical oncology at the Peter MacCallum Cancer Centre in Australia, who led the Pfizer-supported study.
The new research, a follow-up to a previous phase 3 clinical trial , found that after five years, 60% of patients who got Lorbrena were still alive, compared with 8% of patients who received a different drug, crizotinib (Xalkori, also from Pfizer).
The study was published in the Journal of Clinical Oncology and will be presented Friday at the American Society of Clinical Oncology’s annual meeting in Chicago.
“These results are remarkable,” said Dr. John Heymach, the chair of thoracic and head and neck medical oncology at the MD Anderson Cancer Center in Houston, who was not involved with the study. “For the first time, we are seeing that most patients go more than five years without their cancer progressing, and that is more than any other drug in the space. Previously, the best drugs we had were two to three years.”
There is one caveat: The drug that Lorbrena was compared to, crizotinib, is no longer used in the U.S., said Dr. Julie Gralow, the chief medical officer and executive vice president at the American Society of Clinical Oncology.
Both drugs — part of a class of medications called tyrosine kinase inhibitors — fight tumor cells in generally the same way. However, crizotinib is a first-generation version of this type of drug, whereas lorlatinib (the generic name for Lorbrena) is the third generation.
The current standard of care, meaning the drugs oncologists typically use to treat ALK-positive lung cancers , includes Lorbrena and two second-generation drugs: brigatinib (Takeda’s Alunbrig) and alectinib (Genentech’s Alecensa).
“In an ideal world, we would have compared lorlatinib to one of those, but the study was started before those were approved,” Gralow said.
Still, “this is the best long-term data we’ve ever seen for” this class of drugs, she said. “This is just the most impressive progression-free survival we’ve ever seen in this population.”
The new study also found that the patients taking Lorbrena were nearly 95% less likely to have their cancer spread to their brain than those taking crizotinib. Four of the 114 patients taking Lorbrena developed brain metastasis within about 16 months, compared to 39 out of the 109 taking crizotinib, a huge feat for people living with ALK-positive lung cancer.
“Earlier drugs like crizotinib were not effective at blocking the spread in the brain,” Heymach said. While alectinib and brigatinib do have some ability to block tumors from spreading to the brain, he added, “the activity we’re seeing in the brain for lorlatinib is really unprecedented.”
The reason Lorbrena is so effective at preventing and treating brain metastasis is its ability to cross a membrane called the blood-brian barrier and enter the brain, something not all drugs can do.
“When the cancer spreads to the brain , that often leads to a marked decline in quality of life and really horrible side effects,” Heymach said.
Only a trial that compares all three standard-of-care drugs head to head, rather than to crizotinib can determine which is superior, said Dr. Isabel Preeshagul, a thoracic medical oncologist at Memorial Sloan Kettering Cancer Center in New York City.
However, “it does seem that lorlatinib may have an edge in regards to CNS efficacy,” Preeshagul said, referring to the drug’s ability to treat and prevent spread to the brain. “This was a big unmet need for patients that harbor” the ALK mutation.
With its exceptional ability to cross into the brain comes the potential for more side effects, said Dr. Aaron Mansfield, a medical oncologist at the Mayo Clinic in Rochester, Minnesota.
People taking Lorbrena may be more prone to memory or mood issues compared to those taking similar second-generation drugs.
Solomon, the study leader, said because of this, the drug might not be the best choice for people with psychiatric disorders.
But a lower dose may be able to remedy the drugs’ neurotoxicity.
“We know that reducing the dose is very effective at reducing the cognitive side effects, and in those patients, outcomes were just as good as those that didn’t lower the dose,” Solomon said.
All four tyrosine kinase inhibitors only work in people who have ALK-positive lung cancer, not other types of lung cancer. While most mutations are caused by a single change in a gene, the ALK-positive mutation occurs when two genes break and then fuse together. Because of this, it’s sometimes referred to as ALK fusion.
Preeshagul said it’s important for patients diagnosed with lung cancer to advocate for themselves and ask for further testing to determine if they have the mutation, which is missed without additional tests.
“If you are diagnosed with stage 4 lung cancer, it’s important to ask your oncologist to send off next-generation sequencing,” she said. “If you have an ALK fusion, you should absolutely be on targeted therapy. The treatments that we have are life-altering.”
Kaitlin Sullivan is a contributor for NBCNews.com who has worked with NBC News Investigations. She reports on health, science and the environment and is a graduate of the Craig Newmark Graduate School of Journalism at City University of New York.
Berkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Lung Cancer Research Results and Study Updates
See Advances in Lung Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
FDA has approved alectinib (Alecensa) as adjuvant therapy for people with lung cancer who have ALK-positive tumors. In a clinical trial, alectinib helped people live longer after surgery without their cancer returning than chemotherapy.
The results of the clinical trial that led to FDA’s 2023 approval of repotrectinib (Augtyro) for lung cancers with ROS1 fusions have been published. The drug shrank tumors in 80% of people receiving the drug as an initial treatment.
A collection of material about the ALCHEMIST lung cancer trials that will examine tumor tissue from patients with certain types of early-stage, completely resected non-small cell lung cancer for gene mutations in the EGFR and ALK genes, and assign patients with these gene mutations to treatment trials testing post-surgical use of drugs targeted against these mutations.
Tarlatamab, a new type of targeted immunotherapy, shrank small cell lung cancer (SCLC) tumors in more than 30% of participants in an early-stage clinical trial. Participants had SCLC that had progressed after previous treatments with other drugs.
For people with lung cancer and medullary thyroid cancer whose tumors have changes in the RET gene, selpercatinib improved progression-free survival compared with other common treatments, according to new clinical trial results.
In the ADAURA clinical trial, people with early-stage lung cancer treated with osimertinib (Tagrisso) after surgery lived longer than people treated with a placebo after surgery. Despite some criticisms about its design, the trial is expected to change patient care.
For certain people with early-stage non-small cell lung cancer, sublobar surgery to remove only a piece of the affected lung lobe is as effective as surgery to remove the whole lobe, new research shows.
Pragmatica-Lung is a clinical trial for people with non-small cell lung cancer that has spread beyond the lungs (stage 4 cancer). The trial will help confirm if the combination of pembrolizumab and ramucirumab helps people with advanced lung cancer live longer.
On August 11, the Food and Drug Administration (FDA) gave accelerated approval to trastuzumab deruxtecan (Enhertu) for adults with non-small cell lung cancer (NSCLC) that has a specific mutation in the HER2 gene. Around 3% of people with NSCLC have this kind of HER2 mutation.
Giving people with early-stage lung cancer the immunotherapy drug nivolumab (Opdivo) and chemotherapy before surgery can substantially delay the progression or return of their cancer, a large clinical trial found.
Atezolizumab (Tecentriq) is now the first immunotherapy approved by FDA for use as an additional, or adjuvant, treatment for some patients with non-small cell lung cancer. The approval was based on results of a clinical trial called IMpower010.
Quitting smoking after a diagnosis of early-stage lung cancer may help people live longer, a new study finds. The study, which included more than 500 patients, also found that quitting smoking delayed the cancer from returning or getting worse.
NCI scientists and their international collaborators have found that the majority of lung cancers in never smokers arise when mutations caused by natural processes in the body accumulate. They also identified three subtypes of lung cancer these individuals.
FDA has approved the first KRAS-blocking drug, sotorasib (Lumakras). The approval, which covers the use of sotorasib to treat some patients with advanced lung cancer, sets the stage for other KRAS inhibitors already in development, researchers said.
Combining the chemotherapy drug topotecan and the investigational drug berzosertib shrank tumors in some patients with small cell lung cancer, results from an NCI-supported phase 1 clinical trial show. Two phase 2 trials of the combination are planned.
Mortality rates from the most common lung cancer, non-small cell lung cancer (NSCLC), have fallen sharply in the United States in recent years, due primarily to recent advances in treatment, an NCI study shows.
In a study of more than 50,000 veterans with lung cancer, those with mental illness who received mental health treatment—including for substance use—lived substantially longer than those who didn’t participate in such programs.
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
Osimertinib (Tagrisso) improves survival in people with non-small cell lung cancer with EGFR mutations, updated clinical trial results show. People treated with osimertinib lived longer than those treated with earlier-generation EGFR-targeted drugs.
A large clinical trial showed that adding the immunotherapy drug durvalumab (Imfinzi) to standard chemotherapy can prolong survival in some people with previously untreated advanced small cell lung cancer.
The investigational drug selpercatinib may benefit patients with lung cancer whose tumors have alterations in the RET gene, including fusions with other genes, according to results from a small clinical trial.
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
Clinical recommendations on who should be screened for lung cancer may need to be reviewed when it comes to African Americans who smoke, findings from a new study suggest.
Use of a multipronged approach within hospitals, including community centers, not only eliminated treatment disparities among black and white patients with early-stage lung cancer, it also improved treatment rates for all patients, results from a new study show.
In everyday medical care, there may be more complications from invasive diagnostic procedures performed after lung cancer screening than has been reported in large studies.
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
On December 6, 2018, the Food and Drug Administration (FDA) approved atezolizumab (Tecentriq) in combination with a standard three-drug regimen as an initial treatment for advanced lung cancer that does not have EGFR or ALK mutations.
A new study has identified a potential biomarker of early-stage non–small cell lung cancer (NSCLC). The biomarker, the study’s leaders said, could help diagnose precancerous lung growths and early-stage lung cancers noninvasively and distinguish them from noncancerous growths.
Results from two large clinical trials should cement the value of the drugs brigatinib (Alunbrig) and durvalumab (Imfinzi) in treating non-small cell lung cancer (NSCLC). The trial results, several experts said, confirm that the drugs can improve the outcomes of patients with advanced NSCLC.
Cancer researchers have trained a computer program to scan images of tissue samples to differentiate normal lung tissue from the two most common forms of lung cancer. The program also learned to detect cancer-related genetic mutations in the samples.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Top advances in lung cancer, 2021
Nisha a. mohindra.
1 Division of Hematology and Oncology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA
2 Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, Illinois, USA
Jyoti D. Patel
Despite a global pandemic that continued to inflict chaos and confusion on the world, resulting in fewer cancer screenings and delayed surgeries, remarkable lung cancer treatment advancements were made in 2021. From immunotherapy in the adjuvant setting to the approval of the first-in-class, highly selective inhibitor of KRAS G12C, these treatment advances have significant clinical impact in patients with lung cancer.
INTRODUCTION
The treatment paradigm and outcomes for patients with lung cancer have vastly evolved over the last several decades, largely because the use of immune checkpoint inhibitors and targeted therapies. 1 This past year, we continued to see immense progress for nonsmall cell lung cancer (NSCLC) as immunotherapy entered earlier stages of disease and innovative strategies were developed to effectively target some of our more difficult-to-treat subsets of disease.
IMMUNOTHERAPY IN THE ADJUVANT SETTING
In October 2021, the US Food and Drug Administration approved atezolizumab for adjuvant treatment after resection and platinum-based chemotherapy in patients with stage II–IIIA NSCLC whose tumors have PD-L1 expression on ≥1% of tumor cells. 2 In the IMpower010 trial ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT02486718","term_id":"NCT02486718"}} NCT02486718 ), a randomized, multinational, phase 3 trial, adjuvant atezolizumab was compared with best supportive care in patients with completely resected, stage IB–IIIA NSCLC (according to the American Joint Committee on Cancer AJCC Cancer Staging Manual , seventh edition) after adjuvant platinum-based chemotherapy. 3 In total, 1005 patients were randomized in a 1:1 fashion to receive either atezolizumab for a 1-year duration ( n = 507) or best supportive care ( n = 498). All patients received adjuvant platinum-based chemotherapy before atezolizumab. After a median follow-up of 32 months, adjuvant atezolizumab demonstrated a significant improvement in disease-free survival (DFS) in the stage II–IIIA PD-L1 ≥ 1% population with a hazard ratio (HR) of 0.66 (95% CI, 0.50–0.88; p = .0039) and a 3-year DFS rate of 60% compared with 48% in the best supportive care group. In the overall stage II–IIIA population, a DFS benefit was noted, with an HR of 0.79 (95% CI, 0.64–0.96; p = .020); however, in the intention-to-treat population, which included stage IB–IIIA disease, the boundary for statistical significance for DFS was not crossed (HR, 0.81; 95% CI, 0.67–0.99; p = .040). The magnitude of benefit was most striking in patients who had PD-L1 expression on ≥50% of tumor cells (HR, 0.43; 95% CI, 0.27–0.68). Treatment with adjuvant atezolizumab did not yield any new safety concerns. Although overall survival results from this trial are still immature, we now have options for effective adjuvant therapy with both osimeritinib in resected epidermal growth factor receptor ( EGFR ) mutation–positive disease and atezolizumab in resected stage II and III PD-L1–positive NSCLC. 4
Beyond the adjuvant setting, there remains much interest in the potential of immunotherapy in the neoadjuvant setting. In the preoperative setting, intact tumor and tumor antigens may yield higher T-cell responses from immunotherapy. The CheckMate 816 trial ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT02998528","term_id":"NCT02998528"}} NCT02998528 ) was the first phase 3 trial in NSCLC to report a significant improvement in rates of pathologic complete response with neoadjuvant nivolumab and chemotherapy versus chemotherapy alone (24.0% vs. 2.2%; p < .0001). 5 Patients on the trial were randomized to receive either three cycles of nivolumab and platinum-doublet chemotherapy or three cycles of chemotherapy, followed by surgery. Eighty-three percent of patients on nivolumab/chemotherapy and 75% of patients who received chemotherapy underwent definitive resection, suggesting that the addition of neoadjuvant immunotherapy did not affect the extent or timing of surgical resection. 6 Further data from this trial and others like it are awaited as we see the potential impact of these agents on survival, gain clarity about the impact of a pathologic complete response on lung cancer survival, and learn how to best integrate immunotherapy with surgery.
NOVEL AGENTS
Despite great advancements in targeted therapies for NSCLC, there remain molecular subsets in which effective targeted agents have remained elusive. In 2021, we saw three of these difficult-to-drug subsets, Kirsten rat sarcoma (KRAS ), EGFR exon 20, and human epidermal growth factor receptor 2 ( HER2 ) mutations, achieve meaningful outcomes with novel agents.
Activating mutations in the KRAS oncogene are found in 25%–30% of lung adenocarcinomas; however, there are distinct molecular underpinnings that make this group of mutations difficult to target and lead to various clinical and therapeutic outcomes. 7 , 8 In May 2021, a novel drug, sotorasib, was approved to treat the most frequent variant among KRAS mutations, KRAS p.G12C, which comprises 13% of lung adenocarcinomas. 8 , 9 Sotorasib is a small molecule that irreversibly binds to a pocket of the switch II region of KRAS p.G12C that is only present when KRAS p.G12C is in its inactive conformation. 10 , 11 Therefore, when sotorasib is bound, KRAS p.G12C is blocked from cycling from an inactive state to an activated state.
The CODEBREAK trial ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT04185883","term_id":"NCT04185883"}} NCT04185883 ) was a single-arm, phase 2 trial evaluating the efficacy of sotorasib in patients with KRAS p.G12C–mutant NSCLC that had progressed on prior lines of therapy. 12 Of the 124 evaluable patients treated with sotorasib, 37% had an objective response (95% CI, 28.6%–46.2%), and 81% of patients achieved disease control (95% CI, 72.6%–87.2%).The median progression-free survival (PFS) was 6.8 months (95% CI, 5.1–8.2 months), the median duration of response (DOR) was 11.1 months (95% CI, from 6.9 months to not evaluable), and the median overall survival was 12.5 months (95% CI, from 10.0 months to not evaluable). Treatment-related adverse events (AEs) occurred in 70% of patients, and the most common were diarrhea (32%), fatigue (11%), and emesis (8%). Sotorasib demonstrated durable clinical benefit in the majority of patients on trial and had promising efficacy in some of our most difficult-to-treat subsets of disease. Other novel agents, such as adagrasib, have also demonstrated promising activity in KRAS p.G12C–mutant NSCLC and may become additional agents available to this disease subset in the future. 13
Another subset of NSCLC that has lacked effective targeted therapies is NSCLC with EGFR exon 20 gene insertion mutations, which includes 2% of lung adenocarcinomas. 14 , 15 Exon 20 insertions result in a restricted drug-binding pocket within the EGFR tyrosine kinase domain, making conventional EGFR tyrosine kinase inhibitors largely ineffective. 14 , 16 Prior efforts to develop effective targeted treatments have yielded low response rates (range, 5%–20%) and various degrees of toxicity. 17 – 19 In late 2021, two agents for EGFR exon 20 insertion mutations were approved: amivantamab, a bispecific antibody directed at EGFR and MET, and mobocertinib, an oral tyrosine kinase inhibitor developed to select for EGFR exon 20 insertion mutations.
Amivantinib, a fully human, bispecific antibody, binds to both EGFR and MET extracellular domains, leading to inhibition of ligand binding, endocytosis, and degradation of the receptor–antibody complex, as well as destruction by macrophages and antibody-dependent cellular cytotoxicity by natural killer cells. 20 , 21 In the CHRYSALIS study ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT02609776","term_id":"NCT02609776"}} NCT02609776 ), a first-in-human, phase 1, dose-escalation and dose-expansion study, amivantinib demonstrated a 40% overall response rate among 81 evaluable patients, with a median DOR of 11.1 months (95% CI, from 6.9 months to not evaluable). 22 The most common AEs were rash (86%), infusion-related reaction (66%), paronychia (45%), and constipation (24%).
Mobocertinib is a novel, potent, irreversible EGFR tyrosine kinase inhibitor designed specifically to inhibit activating EGFR exon 20 insertion mutations with relative sparing of wild-type EGFR. 23 The Study 101 trial ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT02716116","term_id":"NCT02716116"}} NCT02716116 ) was a multicohort, nonrandomized, open-label trial evaluating the efficacy of mobocertinib in patients who had locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations. In the 114 patients whose disease had progressed on or after platinum-based chemotherapy, mobocertinib produced an overall response rate of 28% (95% CI, 20%–37%), with a median DOR of 17.5 months (95% CI, 7.4–20.3 months). The median PFS was 7.3 months (95% CI, 5.5–9.2 months), and the median OS was 24.0 months (95% CI, 14.6–28.8 months). The most common AEs were diarrhea (91%) and rash (45%).
Patients with HER2 gene mutations, which are identified in 3% of NSCLC adenocarcinomas, represent another population that has lacked effective targeted agents because previous HER2–directed therapies have only resulted in limited efficacy in NSCLC. 4 , 24 – 27 Trastuzumab deruxtecan is an antibody–drug conjugate of humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor payload that leads to cellular apoptosis when the payload is intracellularly released. 28 DESTINY-Lung01 ( ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT03505710","term_id":"NCT03505710"}} NCT03505710 ) was a single-arm, phase 2 trial that evaluated the efficacy of trastuzumab deruxtecan among 91 pretreated patients with metastatic, HER2 -mutant NSCLC. 29 After 13.1 months of follow-up, 55% of patients had a confirmed overall response (95% CI, 44%–65%), and 92% achieved disease control. The median PFS was 8.2 months (95% CI, 6.0–11.9 months), and the median OS was 17.8 months (95% CI, 13.8–22.1 months). Nearly all patients had at least one AE, and the most common AEs were nausea (73%), fatigue (53%), and anemia (35%). Interstitial lung disease (ILD) occurred in 26% of patients; 7% of patients had grade ≥ 3 ILD, and two patients died from ILD. Trastuzumab deruxtecan is an effective targeted therapy option for patients with metastatic, HER2 -mutant NSCLC and has achieved US Food and Drug Administration breakthrough designation. Despite this efficacy, ongoing research will be needed to understand and mitigate the risk of treatment-related ILD in patients with NSCLC.
The demonstrated efficacy of four different agents to target three different subsets of NSCLC underscores the importance of molecular testing and PD-L1 testing in NSCLC. Current guidelines now recommend testing for numerous biomarkers at the time of diagnosis in advanced stage NSCLC— EGFR , ALK , ROS1 , BRAF , KRAS , NTRK , MET , RET , and HER2 —highlighting the wide breadth of targets and corresponding therapies that can be leveraged for disease management. In early stage disease, testing tumors for EGFR mutations and PD-L1 has become paramount given the advances made in adjuvant treatments. As we look back at the last year, it truly was one marked by innovation and the integration of effective therapies for patients with lung cancer. In particular, the addition of immunotherapy as adjuvant therapy in early stage disease and the ability to develop targeted therapies (bispecific antibodies, antibody–drug conjugates, and small-molecule inhibitors) for new subsets of patients are notable advances that will further improve outcomes for patients with NSCLC.
Lay summary
- There has been tremendous innovation in the treatment of nonsmall cell lung cancer.
- The year 2021 was marked by new approaches to adjuvant therapy and the availability of agents to target new subsets of nonsmall cell lung cancer.
Funding information
Nisha A. Mohindra is supported by grant AHRQ-K12HS02638 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. Jyoti D. Patel is supported by a Cancer Center Core Grant from the National Cancer Institute, National Institutes of Health (3P30CA060553-26S2).
CONFLICTS OF INTEREST
Nisha A. Mohindra reports grants from Pfizer Inc. and personal fees from Astra Zeneca outside the submitted work. Jyoti D. Patel reports grants from Bristol-Myers Squibb and personal fees from AbbVie, AstraZeneca, Eli Lilly & Company, Genentech USA, Inc., and Takeda Oncology outside the submitted work.
Trial registration: ClinicalTrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02486718","term_id":"NCT02486718"}} NCT02486718 .
Advertisement
Recent advances in lung cancer research: unravelling the future of treatment
- Review Article
- Published: 06 April 2024
Cite this article

- Luca Bertolaccini ORCID: orcid.org/0000-0002-1153-3334 1 ,
- Monica Casiraghi 1 , 2 ,
- Clarissa Uslenghi 1 ,
- Sebastiano Maiorca 1 &
- Lorenzo Spaggiari 1 , 2
377 Accesses
1 Altmetric
Explore all metrics
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine, enabling targeted therapies that minimise harm to healthy tissues while effectively combating cancer cells. The relationship between pulmonary tuberculosis and lung cancer is examined, shedding light on potential mechanisms linking these two conditions. Early detection methods, notably low-dose computed tomography scans, have significantly improved patient outcomes, emphasising the importance of timely interventions. There has been a growing interest in segmentectomy as a surgical intervention for early-stage lung cancer in recent years. Immunotherapy has emerged as a transformative approach, harnessing the body's immune system to recognise and eliminate cancer cells. Combining immunotherapy with traditional treatments, such as chemotherapy and targeted therapies, has shown enhanced efficacy, addressing the disease's heterogeneity and overcoming drug resistance. Precision medicine, guided by genomic profiling, has enabled the development of targeted therapies like tyrosine kinase inhibitors, offering personalised treatments tailored to individual patients. Challenges such as drug resistance and limited accessibility to advanced therapies persist, emphasising the need for collaborative efforts and innovative technologies like artificial intelligence. Despite challenges, ongoing interdisciplinary collaborations and technological advancements offer hope for a future where lung cancer is treatable and preventable, reducing the burden on patients and healthcare systems worldwide.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Expression patterns of HNF4α, TTF-1, and SMARCA4 in lung adenocarcinomas: impacts on clinicopathological and genetic features
Clinical applications of artificial intelligence and machine learning in cancer diagnosis: looking into the future

SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018)
Data availability.
Not applicable.
Cheng B, Xiong S, Li C, Liang H, Zhao Y, Li J et al (2020) An annual review of the remarkable advances in lung cancer clinical research in 2019. J Thorac Dis 12(3):1056–1069
Article PubMed PubMed Central Google Scholar
Ibodeng GO, Uche IN, Mokua R, Galo M, Odigwe B, Galeas JN, Dasgupta S (2023) A snapshot of lung cancer: where are we now?-a narrative review. Ann Transl Med 11(6):261
Article CAS PubMed PubMed Central Google Scholar
Bertolaccini L, Casiraghi M, Petrella F, Rampinelli C, Tessitore A, Spaggiari L (2022) A methodological quality evaluation of the published guidelines and recommendations about the lung cancer screening. Eur J Cancer Prev 31(1):19–25
Article PubMed Google Scholar
Duma N, Santana-Davila R, Molina JR (2019) Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 94(8):1623–1640
Article CAS PubMed Google Scholar
Hwang SY, Kim JY, Lee HS, Lee S, Kim D, Kim S et al (2022) Pulmonary tuberculosis and risk of lung cancer: a systematic review and meta-analysis. J Clin Med 11(3):765
Yaegashi LB, Baldavira CM, Prieto TG, Machado-Rugolo J, Velosa APP, da Silveira LKR et al (2021) In situ overexpression of matricellular mechanical proteins demands functional immune signature and mitigates non-small cell lung cancer progression. Front Immunol 12:714230
Bourgot I, Primac I, Louis T, Noel A, Maquoi E (2020) Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front Oncol 10:1488
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S et al (2017) Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376(22):2109–2121
Oliver AL (2022) Lung cancer: epidemiology and screening. Surg Clin North Am 102(3):335–344
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547(7662):222–226
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Franzi S, Mattioni G, Rijavec E, Croci GA, Tosi D (2022) Neoadjuvant chemo-immunotherapy for locally advanced non-small-cell lung cancer: a review of the literature. J Clin Med 11(9):2629
Szeto CH, Shalata W, Yakobson A, Agbarya A (2021) Neoadjuvant and adjuvant immunotherapy in early-stage non-small-cell lung cancer, past, present, and future. J Clin Med 10(23):5614
Chai Y, Wu X, Bai H, Duan J (2022) Combined immunotherapy with chemotherapy versus bevacizumab with chemotherapy in first-line treatment of driver-gene-negative non-squamous non-small cell lung cancer: an updated systematic review and network meta-analysis. J Clin Med 11(6):1655
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C et al (2023) Overall Survival with Osimertinib in Resected EGFR-mutated NSCLC. N Engl J Med 389(2):137–147
Dohopolski M, Iyengar P (2021) Oligometastatic non-small cell lung cancer: a narrative review of stereotactic ablative radiotherapy. Ann Palliat Med 10(5):5944–5953
Yuan Z, Wang Y, Zhang J, Zheng J, Li W (2019) A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol 16(3):302–314
Chen Y, Luo H, Liu R, Tan M, Wang Q, Wu X et al (2023) Efficacy and safety of particle therapy for inoperable stage II–III non-small cell lung cancer: a systematic review and meta-analysis. Radiat Oncol 18(1):86
Harada H, Suefuji H, Mori K, Ishikawa H, Nakamura M, Tokumaru S et al (2023) Proton and carbon ion radiotherapy for operable early-stage lung cancer: 3-year results of a prospective nationwide registry. Int J Radiation Oncol Biol Phys 117(2):23
Article Google Scholar
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA et al (2020) reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513
Huo B, Manos D, Xu Z, Matheson K, Chun S, Fris J et al (2023) Screening criteria evaluation for expansion in pulmonary neoplasias (SCREEN). Semin Thorac Cardiovasc Surg 35(4):769–780
Passiglia F, Cinquini M, Bertolaccini L, Del Re M, Facchinetti F, Ferrara R et al (2021) Benefits and harms of lung cancer screening by chest computed tomography: a systematic review and meta-analysis. J Clin Oncol 39(23):2574–2585
Qi SA, Wu Q, Chen Z, Zhang W, Zhou Y, Mao K et al (2021) High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 11(1):11805
Madama D, Martins R, Pires AS, Botelho MF, Alves MG, Abrantes AM, Cordeiro CR (2021) Metabolomic profiling in lung cancer: a systematic review. Metabolites 11(9):630
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F et al (2016) Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17(5):642–650
Araujo DC, Veloso AA, Borges KBG, Carvalho MDG (2022) Prognosing the risk of COVID-19 death through a machine learning-based routine blood panel: a retrospective study in Brazil. Int J Med Inform 165:104835
Chiu HY, Chao HS, Chen YM (2022) Application of artificial intelligence in lung cancer. Cancers (Basel) 14(6):1370
Christie JR, Lang P, Zelko LM, Palma DA, Abdelrazek M, Mattonen SA (2021) Artificial intelligence in lung cancer: bridging the gap between computational power and clinical decision-making. Can Assoc Radiol J 72(1):86–97
Goncalves S, Fong PC, Blokhina M (2022) Artificial intelligence for early diagnosis of lung cancer through incidental nodule detection in low- and middle-income countries-acceleration during the COVID-19 pandemic but here to stay. Am J Cancer Res 12(1):1–16
CAS PubMed PubMed Central Google Scholar
Goldsmith I, Chesterfield-Thomas G, Toghill H (2021) Pre-treatment optimization with pulmonary rehabilitation in lung cancer: making the inoperable patients operable. EClinicalMedicine 31:100663
Shields MD, Chen K, Dutcher G, Patel I, Pellini B (2022) Making the rounds: exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci 23(16):9006
Abbosh C, Frankell AM, Harrison T, Kisistok J, Garnett A, Johnson L et al (2023) Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616(7957):553–562
Zaman FY, Subramaniam A, Afroz A, Samoon Z, Gough D, Arulananda S, Alamgeer M (2023) Circulating tumour DNA (ctDNA) as a predictor of clinical outcome in non-small cell lung cancer undergoing targeted therapies: a systematic review and meta-analysis. Cancers (Basel) 15(9):2425
Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, Ashworth A et al (2017) Future cancer research priorities in the USA: a lancet oncology commission. Lancet Oncol 18(11):e653–e706
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617
Nakada T, Noda Y, Kato D, Shibasaki T, Mori S, Asano H et al (2019) Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 10(10):1945–1952
Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M (2017) Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 9(6):1615–1623
Bertolaccini L, Prisciandaro E, Bardoni C, Cara A, Diotti C, Girelli L, Spaggiari L (2022) Minimally invasive anatomical segmentectomy versus lobectomy in stage IA non-small cell lung cancer: a systematic review and meta-analysis. Cancers (Basel) 14(24):6157
Wang P, Fu YH, Qi HF, He P, Wang HF, Li C, Liu XC (2023) Evaluation of the efficacy and safety of robot-assisted and video assisted thoracic surgery for early non-small cell lung cancer: a meta-analysis. Technol Health Care 32(2):511–523
Casiraghi M, Galetta D, Borri A, Tessitore A, Romano R, Diotti C et al (2019) Ten years’ experience in robotic-assisted thoracic surgery for early stage lung cancer. Thorac Cardiovasc Surg 67(7):564–572
Wang P, Wang S, Liu Z, Sui X, Wang X, Li X et al (2022) Segmentectomy and wedge resection for elderly patients with stage I non-small cell lung cancer: a systematic review and meta-analysis. J Clin Med 11(2):294
Bertolaccini L, Cara A, Chiari M, Diotti C, Glick N, Mohamed S et al (2023) Real-world survival outcomes of wedge resection versus lobectomy for cT1a/b cN0 cM0 non-small cell lung cancer: a single center retrospective analysis. Front Oncol 13:1226429
Bertolaccini L, Spaggiari L (2023) Is it time to cross the pillars of evidence in favor of segmentectomies in early-stage non-small cell lung cancer? Cancers (Basel) 15(7):1993
Zaraca F, Kirschbaum A, Pipitone MD, Bertolaccini L, Group PS (2023) Prospective randomized study on the efficacy of three-dimensional reconstructions of bronchovascular structures on preoperative chest CT scan in patients who are candidates for pulmonary segmentectomy surgery: the patches (prospective randomized study efficacy of three-dimensional reconstructions segmentecomy) study protocol. Trials 24(1):594
Komarnicki P, Musialkiewicz J, Stanska A, Maciejewski A, Gut P, Mastorakos G, Ruchala M (2022) Circulating neuroendocrine tumor biomarkers: past, present and future. J Clin Med 11(19):5542
Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D et al (2018) Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 24(10):1559–1567
Biesinger M, Eicken N, Varga A, Weber M, Brndiar M, Erd G et al (2022) Lymph but not blood vessel invasion is independent prognostic in lung cancer patients treated by VATS-lobectomy and might represent a future upstaging factor for early stages. Cancers 14(8):1893
Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V, et al (2023) IASLC Lung Cancer Staging Project The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 18(5): 564–575
Hardenberg MC, Patel B, Matthews C, Califano R, Garcia Campelo R, Grohe C et al (2022) The value of disease-free survival (DFS) and osimertinib in adjuvant non-small-cell lung cancer (NSCLC): an international Delphi consensus report. ESMO Open 7(5):100572
Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al (2020) Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383(18):1711–1723
Xu H, Baidoo AAH, Su S, Ye J, Chen C, Xie Y et al (2019) A comparison of EGFR mutation status in tissue and plasma cell-free DNA detected by ADx-ARMS in advanced lung adenocarcinoma patients. Transl Lung Cancer Res 8(2):135–143
Zou PC, Wang L, Liu B, Zhang HZ, Liu HC (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Diagnostics 9:38
Google Scholar
Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F et al (2023) Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11(4):354–366
Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K et al (2022) Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naive Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 7(4):100527
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G et al (2020) First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383(21):2018–2029
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Isaacs J, Stinchcombe TE (2022) Neoadjuvant and adjuvant systemic therapy for early-stage non-small-cell lung cancer. Drugs 82(8):855–863
John AO, Ramnath N (2023) Neoadjuvant versus adjuvant systemic therapy for early-stage non-small cell lung cancer: the changing landscape due to immunotherapy. Oncologist 28(9):752–764
Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S et al (2023) Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 389(6):491–503
Kogure Y, Hashimoto H, Oki M (2021) A randomized phase iii study of pembrolizumab versus pembrolizumab-carboplatin-pemetrexed for locally advanced or metastatic nonsquamous non-small-cell lung cancer with PD-L1 50% or more (LAPLACE-50): study protocol. Clin Lung Cancer 11:921–924
Download references
Acknowledgements
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Ministero della Salute, 5 × 1000, Ricerca Corrente.
Author information
Authors and affiliations.
Department of Thoracic Surgery, IEO, European Institute of Oncology IRCCS, Via Ripamonti 435, 20141, Milan, Italy
Luca Bertolaccini, Monica Casiraghi, Clarissa Uslenghi, Sebastiano Maiorca & Lorenzo Spaggiari
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
Monica Casiraghi & Lorenzo Spaggiari
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luca Bertolaccini .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bertolaccini, L., Casiraghi, M., Uslenghi, C. et al. Recent advances in lung cancer research: unravelling the future of treatment. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01841-3
Download citation
Received : 06 March 2024
Accepted : 24 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s13304-024-01841-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Comprehensive review
- Find a journal
- Publish with us
- Track your research
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 40 years of smoking, she survived lung cancer thanks to new treatments

Yuki Noguchi

Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission. Denise Lee hide caption
Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission.
Denise Lee grew up in Detroit in the mid-1970s and went to an all-girls Catholic high school. She smoked her first cigarette at age 14 at school, where cigarettes were a popular way of trying to lose weight.
Instead, her nicotine addiction lasted four decades until she quit in her mid-50s.
"At some point it got up as high as 2.5 packs a day," Lee, 62, recalls.
Yet she didn't think about lung cancer risk — until she saw a billboard urging former smokers to get screened. Lee, a retired lawyer living in Fremont, Calif., used to drive past it on her way to work.
"The thing that caught my attention was the fact that it was an African American female on the front," she recalls.

Shots - Health News
The american cancer society says more people should get screened for lung cancer.
She eventually got the low-dose CT scan recommended for current and former smokers. When doctors found an early, but dangerous, tumor, Lee cried and panicked. Her mother had cared for her father, who'd died of prostate cancer. "My biggest concern was telling my mom," she says.
But that was six years ago, and Lee is cancer free today. Surgery removed the 2-inch tumor in her lung, then new treatments also boosted her immune system, fighting off any recurrence.
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year – more than breast, prostate and colon cancer combined – which is why many people still think of a diagnosis as synonymous with a death sentence. But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
"If you're gonna have lung cancer, now is a good time," Lee says of the advances that saved her.

Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective. Denise Lee hide caption
Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective.
The key breakthrough, says Robert Winn, a lung cancer specialist at Virginia Commonwealth University, is the ability to better pinpoint the mutations of a patient's particular form of cancer. In the past, treatments were blunt tools that caused lots of collateral damage to healthy parts of the body while treating cancer.
"We've gone from that to molecular characterization of your lung cancer, and it has been a game changer," Winn says. "This is where science and innovation has an impact."
One of those game-changing treatments is called targeted therapy . Scientists identify genetic biomarkers in the mutated cancer cells to target and then deliver drugs that attack those targets, shrinking tumors.

CRISPR gene-editing may boost cancer immunotherapy, new study finds
Another is immunotherapy, usually taken as a pill, which stimulates the body's own defense system to identify foreign cells, then uses the immune system's own power to fight the cancer as if it were a virus.
As scientists identify new cancer genes, they're creating an ever-broader array of these drugs.
Combined, these treatments have helped increase national survival rates by 22% in the past five years – a rapid improvement over a relatively short time, despite the fact that screening rates are very slow to increase. Winn says as these treatments get cheaper and readily available, the benefits are even reaching rural and Black populations with historic challenges accessing health care.
The most remarkable thing about the drugs is their ability to, in some cases, reverse late-stage cancers. Chi-Fu Jeffrey Yang, a thoracic surgeon at Massachusetts General Hospital and faculty at Harvard Medical School, recalls seeing scans where large dark shadows of tumor would disappear: "It was remarkable to see the lung cancer completely melting away."
To Yang, such progress feels personal. He lost his beloved grandfather to the disease when Yang was in college. If he were diagnosed today, he might still be alive.
"Helping to take care of him was a big reason why I wanted to be a doctor," Yang says.
But the work of combating lung cancer is far from over; further progress in lung cancer survival hinges largely on getting more people screened.
Low-dose CT scans are recommended annually for those over 50 who smoked the equivalent of a pack a day for 20 years. But nationally, only 4.5% of those eligible get those scans , compared to rates of more than 75% for mammograms.
Andrea McKee, a radiation oncologist and spokesperson for the American Lung Association, says part of the problem is that lung cancer is associated with the stigma of smoking. Patients often blame themselves for the disease, saying: "'I know I did this to myself. And so I don't I don't think I deserve to get screened.'"
McKee says that's a challenge unique to lung cancer. "And it just boggles my mind when I hear that, because, of course, nobody deserves to die of lung cancer."
Denise Lee acknowledges that fear. "I was afraid of what they would find," she admits. But she urges friends and family to get yearly scans, anyway.
"I'm just so grateful that my diagnosis was early because then I had options," she says. "I could have surgery, I could have chemotherapy, I could be a part of a clinical trial."
And all of that saved her life.
- lung cancer
- immunotherapy
- lung cancer screening
Lung Cancer News
Top headlines, latest headlines.
- Stronger Immunotherapy Against Lung Cancer
- AI May Predict Spread of Lung Cancer to Brain
- Lung Adenocarcinoma: Earliest Cellular Genesis
- Immunotherapy for Non-Small Cell Lung Cancer
- Inhalable Therapy for Lung Cancer
- Hidden Army of Lung Flu Fighters
- One Type of Lung Cancer Can Change Into Another
- Lung Cancer Hijacks Immune Cell Metabolism
- Liquid Biopsy Predicts Immunotherapy Response
- Allergy Medicine for Treating Lung Cancer?
Earlier Headlines
Wednesday, november 29, 2023.
- Drugs Already Licensed Could Be Trialled to Potentially Treat Secondary Brain Cancer
Friday, November 17, 2023
- Lung Cancer Cells' 'memories' Suggest New Strategy for Improving Treatment
Tuesday, November 14, 2023
- High Lung Cancer Rates in Naval Veterans Linked to Asbestos
Wednesday, October 11, 2023
- Pleural Mesothelioma: New Therapeutic Approach to Enhance Sensitivity to Chemo And Radiotherapy
Friday, September 22, 2023
- New Target to Beat Cancer Drug Resistance
Thursday, September 21, 2023
- Small Cell Lung Cancer: New Approach to Overcoming Chemo-Resistance
Tuesday, September 12, 2023
- A Combination of Cancer Inhibitors Shows Success in Slowing Tumor Growth
Monday, September 4, 2023
- New Genes and Natural Toxins Offer Hope for Cancer Patients Unresponsive to Chemotherapy
Thursday, August 31, 2023
- Antioxidants Stimulate Blood Flow in Tumors
Wednesday, August 30, 2023
- Blood Cell Insights Offer Potential Boost to Lung Cancer Therapies
Monday, August 14, 2023
- Research Into Use of Diabetes Medication for Treatment of Metastatic Prostate Cancer
Wednesday, August 9, 2023
- Study May Bring Improved Therapy to Preselected Lung Cancer Patients
Wednesday, July 12, 2023
- Researchers Pinpoint Protein Tied to Drug Resistance in Patients With Lung Cancer
Thursday, June 22, 2023
- Significant Progress in Small-Cell Lung Cancer Research
Tuesday, March 28, 2023
- Fibroblast Inhibitors Assist Anti-Cancer Drugs to Suppress Cancer Growth
Friday, March 17, 2023
- New Combination of Drugs Works Together to Reduce Lung Tumors in Mice
Wednesday, March 15, 2023
- Scientists Discover Key Information About the Function of Mitochondria in Cancer Cells
Monday, March 13, 2023
- Scientists Discover a New Way to Help Prevent Breast Cancer 'time Bomb'
Monday, February 13, 2023
- Researchers Discover New Pathways to Activate Dendritic Cells, Produce Strong Anti-Tumor Immunity
Wednesday, February 1, 2023
- Significant Disease-Free Survival for Patients Diagnosed With Non-Small Cell Lung Cancer, Clinical Trial Finds
Monday, January 9, 2023
- Genetic Code Change Drives Common Lung Cancer Type
Wednesday, January 4, 2023
- Actinidia Arguta (Sarunashi) Juice Inhibits Lung Cancer in Mice, Study Finds
Monday, December 26, 2022
- New Bacterial Therapy Approach to Treat Lung Cancer
Thursday, December 1, 2022
- Muscle Wasting Severity Linked to Type, Size and Location of Tumor in Mice
Tuesday, November 29, 2022
- Carbon Ultrafine Particles Accelerate Lung Cancer Progression
Tuesday, November 15, 2022
- Emphysema More Common in Marijuana Smokers Than Cigarette Smokers
Tuesday, November 8, 2022
- Protein Insights May Boost Lung Cancer Detection and Treatment
Tuesday, October 25, 2022
- Minorities Face Longer Wait Times for Vital Lung Cancer Treatment, Study Finds
Thursday, October 6, 2022
- Zooming in on the Signals of Cancer
Wednesday, September 21, 2022
- Smoking to Blame for Most of England's Socioeconomic Disparity in Cancer Incidence, Study Finds
Friday, September 16, 2022
- The Physics of the Premature Lung: Why Mechanical Ventilation Can Harm Preterm Lungs
Tuesday, September 13, 2022
- Tumor-Infiltrating B Cells and Plasma Cells Influence Early-Stage Lung Cancer Biology, Immunotherapy Responses
- Scientists Discover How Air Pollution May Trigger Lung Cancer in Never-Smokers
Thursday, September 1, 2022
- Structure of Protein RAF1 Revealed: A Key Step in the Development of New Drugs Against Lung Cancer
Wednesday, August 31, 2022
- Drug Combo Therapy in Mice Blocks Drug Resistance, Halts Tumor Growth
Tuesday, August 9, 2022
- Air Pollution as a Risk Factor for Patients With Lung Cancer Who Have Never Smoked
Wednesday, July 20, 2022
- New Combination Therapy Shows Early Promise Against Certain Lung Cancers
Monday, July 18, 2022
- Researchers Develop Liquid Biopsy Technique to Help Detect Cancer in Blood
Thursday, July 14, 2022
- Genetic Discovery to Improve Lung Cancer Treatment
Tuesday, July 12, 2022
- Intensive Telephone-Based Cessation Counseling Results in Improved Smoking Quit Rates
Tuesday, June 14, 2022
- Prolonged, Low-Level Radon Exposure Still a Leading Cause of Lung Cancer
Friday, June 3, 2022
- Targeted Drug Achieves 43% Response Rate in KRAS-Mutated Lung Cancer
Thursday, June 2, 2022
- Joining the Fight Against Non-Small Cell Lung Cancer
- Biomarker in Liquid Biopsy for Lung Cancer Appears More Accurate in Predicting Immunotherapy Response Than Tumor Biopsy
Tuesday, May 17, 2022
- Different Subtypes Defined in Small Cell Lung Cancer
Wednesday, May 4, 2022
- New Target for CAR T Cells in Solid Tumors
Tuesday, May 3, 2022
- Emphysema Severity Associated With Higher Lung Cancer Risk
Monday, April 25, 2022
- Reprogrammed Macrophages Promote Spread of Breast Cancer
Wednesday, April 20, 2022
- A New Pathway to Shrink Cancerous Tumors Through Body's Immune Cells
Tuesday, April 19, 2022
- How Air Pollution Alters Lung Tissue, Increasing Cancer Susceptibility
Monday, April 11, 2022
- Study Suggests Why Most Smokers Don't Get Lung Cancer
Thursday, April 7, 2022
- Evidence in Mice That Bacteria in Tumors Help Cancer Cells Metastasize
Wednesday, March 30, 2022
- Cancer Repair Mechanism Could Be Potential Drug Target
Thursday, March 24, 2022
- Novel Method to Identify and Treat Aggressive Early-Stage Lung Cancers
- Intense Light Protects Against Lung Damage, Research Finds
Wednesday, March 16, 2022
- A Potential New Target for Cancer Immunotherapies

Thursday, February 24, 2022
- Cancer Breakthrough Reveals Old Drugs With New Tricks May Limit Spread
- Surprise Small-Cell Lung Cancer Discovery Suggests New Treatment
Thursday, February 3, 2022
- How Long-Term Cannabis Use Can Damage Lungs
Wednesday, January 12, 2022
- New Study Reveals How the Lung's Immune Cells Develop After Birth
Friday, January 7, 2022
- Blood Test Helps Predict Who May Benefit from Lung Cancer Screening
Thursday, December 16, 2021
- Nanoparticle Therapeutic Enhances Cancer Immunotherapy
Monday, December 13, 2021
- Gene Network Changes Associated With Cancer Onset and Progression Identify New Treatment Targets
Thursday, December 9, 2021
- Talk Between Immune Cells Could Lead to New Cancer Vaccine
Tuesday, November 2, 2021
- Chemo Helps Breast Cancer Cells Get Their ‘foot in the Door’ to the Lungs
Thursday, October 14, 2021
- Molecular Atlas of Small Cell Lung Cancer Reveals Unusual Cell Type That Could Explain Why It’s So Aggressive
Wednesday, October 6, 2021
- New Study Reveals Lung Cell Roles in Pulmonary Immunity
Thursday, September 30, 2021
- Most Cases of Never-Smokers’ Lung Cancer Treatable With Mutation-Targeting Drugs
Tuesday, September 28, 2021
- Counting Cells May Shed Light on How Cancer Spreads
Thursday, September 23, 2021
- Different Types of Cancers Are Likely to Spread to Specific Areas of the Brain
Monday, September 20, 2021
- Scientists Find a New Way to Reverse Immune Suppression in Tumors
Thursday, September 16, 2021
- Biologists Identify New Targets for Cancer Vaccines
Tuesday, September 14, 2021
- Long-Term Benefit of SABR for Operable Early-Stage NSCLC Shown in New Study
Monday, September 6, 2021
- Study Illuminates Origins of Lung Cancer in Never Smokers
Friday, September 3, 2021
- Stem-Like T Cells Could Aid Immunotherapy in Cancer Treatment
Monday, August 23, 2021
- Reversal of Lung Fibrosis in Mouse Model Suggests a Novel Therapeutic Target for Pulmonary Fibrosis
Thursday, August 12, 2021
- New Advances for Treating Non-Small Cell Lung Cancer
Tuesday, August 3, 2021
- Novel Therapy Shows Promise for Lung Cancer Patients With Rare EGFR Mutation
Thursday, July 29, 2021
- New Relevant Target for PARP Inhibitor Talazoparib
Tuesday, July 13, 2021
- Preventing Lung Cancer's Unwelcome Return
Thursday, July 8, 2021
- Potential Marker for Success of Immunotherapy in the Treatment of Lung Cancer
Monday, June 28, 2021
- Fast IR Imaging-Based AI Identifies Tumor Type in Lung Cancer
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- More Effective Multipurpose Robots
- CO2 Conversion at a Much Larger Scale
- The Embryo Assembles Itself
- Thawing Permafrost: Not A Tipping Point
- Climate Change Was No Problem for Sharks
- Fungus Breaks Down Ocean Plastic
- Kinship and Ancestry of the Celts
- How Statin Therapy May Prevent Cancer
- Origins of 'Welsh Dragons' Exposed
- Resting Brain: Neurons Rehearse for Future
Trending Topics
Strange & offbeat.
- My View My View
- Following Following
- Saved Saved
Pfizer sees lung cancer drug topping $1 billion in sales following impressive 5-year data
- Medium Text

- Company Pfizer Inc Follow
Sign up here.
Reporting by Michael Erman in New York; Editing by Bill Berkrot
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Annexon Inc said on Tuesday its experimental drug to treat a rare neurological disorder met the main goal of a late-stage trial.

Business Chevron

Roaring Kitty's GameStop options up millions, but cashing in may be tricky
"Roaring Kitty" Keith Gill, the stock influencer behind the 2021 meme stock frenzy, may be sitting on a paper profit of tens of millions of dollars on his position in GameStop options, but reaping those gains might not be easy.

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 3, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Trial reveals benefits of 'stepped' palliative care for patients with advanced lung cancer
by Massachusetts General Hospital

A new study led by investigators from Mass General Cancer Center reveals the effectiveness of more scalable ways of delivering palliative care to patients with advanced lung cancer.
The findings were highlighted at the American Society of Clinical Oncology's annual meeting and are published in JAMA in a paper titled "Stepped Palliative Care for Patients with Advanced Lung Cancer: A Multi-site Randomized Clinical Trial."
The study, led by Jennifer S. Temel, MD of the Mass General Cancer Center, assessed the effectiveness of stepped palliative care , in which all patients receive palliative care for their condition, but with a minimum of required contact with a specialty-trained clinician. More intensive treatment is reserved for those who do not benefit sufficiently from the less intensive care.
This randomized trial included 507 patients with advanced lung cancer who received either early standard palliative care or stepped palliative care.
Studies show that early palliative care, integrated with oncology care from the time of diagnosis of advanced cancer, improves patient and caregiver outcomes. However, this care model has not been widely implemented for two main reasons: the shortage of palliative care clinicians nationwide and challenges in providing palliative care visits throughout the course of cancer treatment, especially as novel therapeutics prolong survival.
Investigators in this study found that stepped palliative care, with visits occurring only at key points in patients' cancer trajectories and using a decrease in quality of life to trigger more visits, resulted in fewer palliative care visits without diminishing the benefits for patients' quality of life.
"To our knowledge, this is the first randomized trial to establish the non-inferiority of a palliative care strategy that's tailored to a patient's needs by triggering more intensive palliative care services based on patient-reported quality of life , compared with resource-intensive early palliative care," said Temel, who co-directs the Cancer Outcomes Research and Education Program and is the clinical director of Thoracic Oncology at Massachusetts General Hospital.
Explore further
Feedback to editors

Study shows that opportunity costs influence when people leave social interactions
48 minutes ago

Bloody insights: Organs-on-chip ready to help snake venom research
2 hours ago

Five-minute test leads to better care for people with dementia in the primary care setting

Researchers develop technology that may allow stroke patients to undergo rehab at home
3 hours ago
Wearable brain imaging provides a precise picture of children's developing brains
4 hours ago
Researchers identify first step in allergic reactions, paving the way for preventative strategies
10 hours ago
Facial thermal imaging and AI can accurately predict presence of coronary artery disease
13 hours ago

Study: High excess death rates in the West for 3 years running since start of pandemic despite containment and vaccines

Inflight alcohol plus cabin pressure can lower blood oxygen and raise heart rate, even in the young and healthy

Personalized oxygenation could improve outcomes for patients on ventilators
14 hours ago
Related Stories

When should patients with dementia receive palliative care?
Mar 3, 2021
Integrated palliative care in ambulatory settings does not relieve symptom burden but improves health care use
Jan 26, 2022
Study: Palliative care provided at point of oncology surgery does not improve patient outcomes
May 10, 2023

Comparison of survival times of advanced cancer patients with palliative care at home and in hospital
May 8, 2023

Palliative care is underused for patients with malignant urinary obstruction, say researchers
Dec 21, 2023

Palliative care improves quality of life for patients with advanced blood cancer
Dec 17, 2020
Recommended for you
Two-pronged attack strategy boosts immunotherapy in preclinical studies
16 hours ago

Scientists develop AI tool to predict how cancer patients will respond to immunotherapy
18 hours ago

Study finds people of color disproportionately dropped from Medicaid
20 hours ago
Researchers create 'chameleon' compound that targets drug-resistant brain cancers
21 hours ago

New study shows vitamin C boosts DNA damage and cell death in melanoma cells
Chromatin openness sheds new light on prostate cancer plasticity
17 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Lung Cancer Was a Death Sentence. Now Drugs Are Saving Lives.
There is more hope than ever for people diagnosed with the deadliest cancer.
Declines in smoking and the advent of screening and newer drugs have transformed the outlook for patients with lung cancer, once considered a death sentence. Progress against the disease has propelled the drop in overall cancer deaths in the U.S. over the past three decades.
And there is more to gain. More patients can fend off the disease for months or years with targeted or immune-boosting drugs, results released this weekend at a top cancer conference showed. That includes patients with forms of the disease that are notoriously tough to treat.
“It had such an abysmal prognosis. And now we have people who are being cured who we never thought would be cured,” said Dr. Angela DeMichele, a medical oncologist at Penn Medicine.
AstraZeneca’s drug Tagrisso can contain lung cancer nearly three years longer than chemotherapy and radiation alone for some stage-three patients, one study released Sunday showed. Another found that some patients with aggressive disease survived nearly two years longer with the company’s immunotherapy drug Imfinzi, the first advance for that lung-cancer subtype in decades.
Another study presented at the American Society of Clinical Oncology conference in Chicago found that 60% of advanced patients were alive without their disease advancing at five years after taking Pfizer’s Lorbrena, a drug that targeted a genetic mutation in their tumors. That compares with just 8% of patients on an older drug with the same target.
“These results are really outstanding,” said Dr. David Spigel, chief scientific officer at Sarah Cannon Research Institute in Tennessee, lead researcher on the Imfinzi trial. “A really major step forward in lung-cancer care.”
Tagrisso, Imfinzi and Lorbrena are all approved by the Food and Drug Administration and in use.
Lorbrena has kept Matt Hiznay’s stage-four lung cancer at bay for nine years. Hiznay, who never smoked, was diagnosed in 2011 after a persistent cough at 24 years old.
“Hearing that, you get old really fast,” he said.
But there was some good news: His tumor tested positive for something called an ALK gene mutation, a rare finding that made him eligible for a targeted drug. Hiznay’s doctor shook his hand and congratulated him on being a mutant.
Hiznay tried a string of drugs and older therapies including chemotherapy and radiation, each of which held off his disease for some time. He joined a clinical trial for Lorbrena in 2015 and has taken the drug ever since. Between treatment, Hiznay earned a doctorate, got married and had a daughter.
“It became a bit easier to see the future again,” said Hiznay, who lives in Brecksville, Ohio. He cherishes each day, even when his infant daughter wakes him up in the middle of the night.
More than 234,000 Americans are diagnosed with lung cancer annually. It is the leading cause of cancer death among men and women, killing some 125,000 Americans each year. The lung-cancer survival rate has increased by some 20% in the past five years, according to the American Lung Association.
Lung cancer has responded to newer drugs such as immunotherapies better than some other cancers, doctors said, in part because its tumors tend to have many mutations that make it easier to find and attack.
Tagrisso targets mutations on the EGFR gene, found in 15% of lung cancers in the U.S. One study presented at the conference added Tagrisso after chemotherapy and radiation for patients with the mutation whose stage-three disease was too far along for surgery. The median time before the disease advanced in those patients was more than three years, compared with just under six months for patients who weren’t on the drug.
Patients in the study were intended to take Tagrisso indefinitely, rather than the three years that many patients take the drug after surgery. A future step would be to study the costs of longer-term treatment and whether some patients could stop it eventually, said Dr. Lecia Sequist, a lung-cancer specialist at Mass General Cancer Center who wasn’t involved in the trial.
The results show how much lung-cancer treatment has changed in the past decade, she said: “It’s like Dorothy looking around and saying we’re not in Kansas anymore.”
Another study showed rare progress against small-cell lung cancer, a less common and more aggressive form of the disease that is harder to treat. AstraZeneca’s Imfinzi increased median survival to around 56 months, compared with 33 months on the standard chemotherapy and radiation alone. The trial included patients with small-cell lung cancer that hadn’t spread.
“To see something where we’re measuring benefit in years versus months is a huge step in the right direction,” said Dr. Lauren Averett Byers, a lung-cancer oncologist at MD Anderson Cancer Center in Houston, who wasn’t involved in the trial.
The FDA in May approved Amgen’s Imdelltra for more advanced small-cell lung cancer. Median survival with the drug was 14 months, with 40% of patients responding to the treatment.
About a quarter of lung-cancer patients are alive five years after diagnosis. The newer treatments can give some patients with advanced disease months or years more to live, but the cancer often comes back and becomes incurable. Many lung cancers are caught late.
“When you look at the disease statistics, you have to be humbled a little bit,” said Dr. Pasi Jänne, a lung-cancer specialist at Dana-Farber Cancer Institute in Boston. “We still have room to go.”
Through Hiznay’s 13 years with lung cancer, he has seen many fellow patients die. “The survivor’s guilt, it’s there, it’s real,” he said.
Five years after his diagnosis, Hiznay rented a brewery basement for a “One Percent” party, celebrating his victory against the odds of survival in 2011. He did it again after 10 years. He plans to be there for 15.
Write to Brianna Abbott at [email protected]

Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Health & Medicine
- Science & Technology
Thousands of NHS patients to access trials of personalised cancer ‘vaccines’

31 May 2024
Today, the NHS announced it has treated its first patient in England with a personalised vaccine against their bowel cancer, in a clinical trial part of NHS England’s new Cancer Vaccine Launch Pad .
As part of the platform, which is run by our Southampton Clinical Trials Unit , thousands of cancer patients in England are set to gain fast-tracked access to trials of personalised cancer vaccines following the launch of a world-leading NHS trial ‘matchmaking’ service to help find new life-saving treatments.
The vaccines being tested as part of the trials aim to help patients with different types of cancer and, if successfully developed, researched and approved, cancer vaccines could become part of standard care.
“It’s incredibly exciting that patients in England are beginning to access personalised cancer vaccines for bowel cancer,” said Iain Foulkes, executive director of research and innovation at Cancer Research UK.
“This technology pioneers the use of mRNA-based vaccines to sensitise people’s immune system and in turn detect and target cancer at its earliest stages.
“Clinical trials like this are vital in helping more people live longer, better lives, free from the fear of cancer. If successful, the vaccine will be a game changer in preventing the onset or return of bowel cancer.”
A UK first trial
Elliot Pfebve, 55, received the developmental jab at University Hospitals Birmingham NHS Foundation Trust, one of several sites taking part in the colorectal cancer vaccine trial sponsored by BioNTech SE.
A higher-education lecturer, Elliot had no cancer symptoms and was diagnosed through a routine health check with his GP.
Following blood tests, he was immediately invited to Manor Hospital in Walsall and triaged to a hospital ward to receive blood transfusions.
A CT scan and a colonoscopy confirmed he had colon cancer and Eliott had surgery to remove the tumour and 30 cm of his large intestine.
He was then referred to the Queen Elizabeth Hospital Birmingham for initial rounds of chemotherapy and to take part in a clinical trial.
“Taking part in this trial tallies with my profession as a lecturer, and as a community-centred person,” he said.
“I want to impact other people’s lives positively and help them realise their potential.
“Through the potential of this trial, if it is successful, it may help thousands, if not millions of people, so they can have hope, and may not experience all I have gone through. I hope this will help other people.”
How do cancer vaccines work?
The vaccine trial Elliot’s taking part in is one of several that will be taking place across the country to treat different types of cancer.
Patients who agree to take part have a sample of their cancer tissue and a blood test taken.
If they meet a clinical trial’s eligibility criteria, they can be referred to their nearest participating NHS site, meaning patients from hospitals across the country will find it easier than ever to take part in groundbreaking research.
The investigational cancer vaccines evaluated in the colorectal cancer trial are based on a molecule called mRNA, the same technology used for the COVID-19 vaccine .
They’re created by analysing a patient’s tumour to identify mutations specific to their own cancer. Using this information, medics can create an individualised cancer vaccine.
The developmental vaccines are designed to induce an immune response that may prevent cancer from returning after surgery by stimulating the patient’s immune system to specifically recognise and potentially destroy any remaining cancer cells.
The investigational cancer vaccines, which are being jointly developed by biopharmaceutical companies BioNTech and Genentech, are still undergoing trials and have not yet been approved by regulators.
If successful, the vaccine will be a game changer in preventing the onset or return of bowel cancer.
The launch pad
19 hospitals in England are already signed up to the Cancer Vaccine Launch Pad, one of the biggest projects of its kind in the world, with more sites joining the platform over the coming months.
Some trials have already enlisted patients, although the majority of participants are expected to be enrolled from 2026 onwards.
The scheme aims to expand and work with a range of partners in the pharmaceutical industry to include patients across many cancer types who could potentially join a vaccine trial, such as those with pancreatic and lung cancer.
“Seeing Elliot receive his first treatment as part of the Cancer Vaccine Launch Pad is a landmark moment for patients and the health service as we seek to develop better and more effective ways to stop this disease,” said Amanda Pritchard, NHS chief executive.
“Thanks to advances in care and treatment, cancer survival is at an all-time high in this country, but these vaccine trials could one day offer us a way of vaccinating people against their own cancer to help save more lives.
“The NHS is in a unique position to deliver this kind of world-leading research at size and scale, and as more of these trials get up and running at hospitals across the country, our national match-making service will ensure as many eligible patients as possible get the opportunity to access them.”
The NHS is working in partnership with Genomics England on the launch pad, with work already helping patients access the latest testing technologies and ensures they are given more targeted precision treatments for their cancer.
This would be amazing. I have ALk Positive lung cancer diagnosed at 52 and having never ever smoked. When the targeted therapy stops working if I have access to these trails that would be incredible
Tell us what you think
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Read our comment policy .
Highlighted content
Thousands of nhs patients to access trials of personalised cancer 'vaccines', sarah harding's legacy: finding women who may have higher breast cancer risks, at-home saliva test could help diagnose prostate cancer sooner, asco 2024: breast cancer relapse test, bowel cancer immunotherapy and more, general election 2024: what does this mean for the tobacco and vapes bill, skin cancer cases reach all-time high, an animal's guide to staying safe in the sun, hpv vaccine slashes cervical cancer rates across society, one to one with penny: volunteering special 1 - that cancer conversation podcast, the ‘mystery’ culprit causing kidney cancer worldwide, related topics.
- Research and trials
- Immune system
- Bowel (colorectal) cancer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Small-cell lung cancer articles from across Nature Portfolio
Small-cell lung cancer is a highly malignant form of lung cancer, also known as oat-cell carcinoma, that accounts for approximately 15% of lung cancer cases. Composed of small, ovoid cells with very little cytoplasm, small-cell lung cancer usually originates in the bronchi and is caused by smoking.
Latest Research and Reviews
CDK9 inhibition as an effective therapy for small cell lung cancer
- L. Valdez Capuccino
FAK-LINC01089 negative regulatory loop controls chemoresistance and progression of small cell lung cancer
- Xianteng Wang

Evolutionary trajectories of small cell lung cancer under therapy
We uncover key processes of the genomic evolution of small cell lung cancer under therapy, identify the common ancestor as the source of clonal diversity at relapse and show central genomic patterns associated with drug response.
- Julie George
- Roman K. Thomas
The pancancer overexpressed NFYC Antisense 1 controls cell cycle mitotic progression through in cis and in trans modes of action
- Cecilia Pandini
- Giulia Pagani
- Paolo Gandellini
A SWI/SNF-dependent transcriptional regulation mediated by POU2AF2/C11orf53 at enhancer
POU2AF2 is a co-activator of POU2F3 in normal and neoplastic tuft cells, such as small cell lung cancer. Here, the authors report that POU2AF2 dictates opposing transcriptional regulation at distal enhance elements.
- Aileen Szczepanski
- Natsumi Tsuboyama

Histopathology images-based deep learning prediction of prognosis and therapeutic response in small cell lung cancer
- Zijian Yang
News and Comment
Pegargiminase improves outcomes in nonepithelioid mpm.
- Peter Sidaway
Deciphering the role of immunoglobulin secreting malignant lineages in the invasive frontiers of small cell lung cancer by scRNA-seq and spatial transcriptomics analysis
Selective regulation of tuft cell-like small cell lung cancer by novel transcriptional co-activators C11orf53 and COLCA2
New capstone of sclc therapy.
- David Killock
Dual PD-L1 and CTLA4 inhibition in ES-SCLC

Back to the beginning to understand metastasis
Yang et al. have used genetically engineered mouse models of small cell lung cancer to show that the same genomic alterations in different cells of origin lead to the formation of tumours that follow distinct evolutionary paths to metastasis.
- Sarah Seton-Rogers
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Pfizer's lung cancer drug Lorbrena can extend life for patients with a rare form of the disease for years longer than other drugs, according to new research published Friday. The drug treats a ...
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for both non-small cell lung cancer treatment and small cell lung cancer treatment. Lung Cancer Research Results. The following are some of our latest news articles on lung cancer research:
The FDA approved sotorasib for treating lung cancers with this specific KRAS mutation. At the American Association for Cancer Research annual meeting in April 2022, researchers presented longer-term follow-up data for the clinical trial that led to sotorasib's approval. Notably, nearly one-third of patients continued to survive after two ...
Under the new approval, sotorasib can be used to treat people with non-small cell lung cancer (NSCLC) that has spread nearby (locally advanced) or to distant locations in the body. Patients must have previously received at least one other systemic cancer treatment, such as chemotherapy, and have a particular KRAS mutation , known as G12C, in ...
Find research articles on lung cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies. ... Quitting smoking after a diagnosis of early-stage lung cancer may help people live longer, a new study finds. The study, which included more than 500 patients, also found that quitting smoking delayed the ...
Lung cancer arises in tissues of the lung, usually in the cells lining air passages. The two main types are small-cell lung cancer and non-small-cell lung cancer, according to the shape of cells ...
Osimertinib is a recommended treatment for advanced non-small-cell lung cancer (NSCLC) with an epidermal growth factor receptor (EGFR) mutation and as adjuvant treatment for resected EGFR ...
Lung cancer is a very aggressive and highly prevalent disease worldwide, with an estimated 2.2 million new cases and 1.8 million deaths in 2020.
In 2022, approximately 236,000 lung cancer diagnoses and 130,000 lung cancer deaths were expected to occur in the United States (US) 1.Every day, roughly 350 patients are expected to die from lung ...
Research round-up: lung cancer Lung-cancer researchers and clinicians must pay more attention to women Better treatments on the way for lung cancer that spreads to the brain
Abstract. Lung cancer remains the leading cause of cancer-related death worldwide. 1 Even in the context of early-stage disease, survival among patients with lung cancer lags behind that among ...
The year 2021 was marked by new approaches to adjuvant therapy and the availability of agents to target new subsets of nonsmall cell lung cancer. Funding information Nisha A. Mohindra is supported by grant AHRQ-K12HS02638 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services.
At 10 years of follow-up, 156 men with a known date of lung-cancer diagnosis in the screening group and 206 in the control group had died from lung cancer (2.50 deaths per 1000 person-years and 3. ...
Pfizer on Friday said its drug for an advanced form of lung cancer showed promising long-term results in a late-stage trial, which could help establish it as the new standard treatment for the ...
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine ...
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed ...
The research team obtained tissue samples from 101 Korean never-smoking lung cancer patients without identified treatment targets among 1,597 patients who visited the National Cancer Center over ...
But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
Nov. 17, 2023 — Research shows that some lung cancer cells retain a 'memory' of the healthy cell where they came from -- one that might be exploited to make an emerging type of lung cancer ...
The more can do at the beginning of treatment to essentially cure the cancer is where our research lies and where these exciting new changes come into play." Screening for Early Stage Lung Cancer. The identification of early stage lung cancer has increased significantly in the past five years because of low-dose CT screening for smokers.
Among the screen-detected lung cancers, only 9.4% were at stage IV, whereas in the non-screened group, 45.7% of lung cancers diagnosed were at stage IV. The authors reported that adherence to ...
NSCLC is the most common type of lung cancer. Boshoff also noted that many patients in Pfizer's clinical trials have been on Lorbrena for more than 5 years, compared to median progression-free ...
C.M. LovlyN Engl J Med 2023;389:560-561. Lung cancer remains the leading cause of cancer-related deaths worldwide. 1 However, the surge of new therapies and the use of these therapies in earlier ...
Screening lowers the risk of death from lung cancer. Ongoing studies are looking at new ways to improve early detection of lung cancer: Ways to use molecular markers from your body fluid (i.e., sputum or blood) for lung cancer screening. Ways to use new forms of bronchoscopies for lung cancer screening, such as autofluorescence bronchoscopy.
A new study led by investigators from Mass General Cancer Center reveals the effectiveness of more scalable ways of delivering palliative care to patients with advanced lung cancer. The study, led ...
Lung cancer is a significant public health concern, causing a considerable number of deaths globally. GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC) show as lung cancer remains the leading cause of cancer death, with an estimated 1.8 million deaths (18%) in 2020.
More than 234,000 Americans are diagnosed with lung cancer annually. It is the leading cause of cancer death among men and women, killing some 125,000 Americans each year. The lung-cancer survival ...
As part of the platform, thousands of cancer patients in England are set to gain fast-tracked access to trials of personalised cancer vaccines following the launch of a world-leading NHS trial 'matchmaking' service to help find new life-saving treatments. The vaccines being tested as part of the trials aim to help patients with different ...
Small-cell lung cancer is a highly malignant form of lung cancer, also known as oat-cell carcinoma, that accounts for approximately 15% of lung cancer cases. ... Research Highlights 04 Mar 2024 ...