Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 02 May 2024

Opportunities and challenges for T cell-based influenza vaccines
- Tim R. Mosmann ORCID: orcid.org/0000-0001-9753-6941 1 ,
- Andrew J. McMichael ORCID: orcid.org/0000-0002-9101-7478 2 ,
- Alexandre LeVert 3 ,
- John W. McCauley ORCID: orcid.org/0000-0002-4744-6347 4 &
- Jeffrey W. Almond 5
Nature Reviews Immunology ( 2024 ) Cite this article
343 Accesses
11 Altmetric
Metrics details
- Influenza virus
Vaccination remains our main defence against influenza, which causes substantial annual mortality and poses a serious pandemic threat. Influenza virus evades immunity by rapidly changing its surface antigens but, even when the vaccine is well matched to the current circulating virus strains, influenza vaccines are not as effective as many other vaccines. Influenza vaccine development has traditionally focused on the induction of protective antibodies, but there is mounting evidence that T cell responses are also protective against influenza. Thus, future vaccines designed to promote both broad T cell effector functions and antibodies may provide enhanced protection. As we discuss, such vaccines present several challenges that require new strategic and economic considerations. Vaccine-induced T cells relevant to protection may reside in the lungs or lymphoid tissues, requiring more invasive assays to assess the immunogenicity of vaccine candidates. T cell functions may contain and resolve infection rather than completely prevent infection and early illness, requiring vaccine effectiveness to be assessed based on the prevention of severe disease and death rather than symptomatic infection. It can be complex and costly to measure T cell responses and infrequent clinical outcomes, and thus innovations in clinical trial design are needed for economic reasons. Nevertheless, the goal of more effective influenza vaccines justifies renewed and intensive efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Defining the balance between optimal immunity and immunopathology in influenza virus infection
Assessing the generation of tissue resident memory T cells by vaccines
MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination
Potter, C. W. A history of influenza. J. Appl. Microbiol. 91 , 572–579 (2001).
Article CAS PubMed Google Scholar
Creighton, C. A History of Epidemics in Britain from A.D. 664 to the Extinction of Plague (Cambridge Univ. Press, 1891).
Dowdle, W. R. Influenza A virus recycling revisited. Bull. World Health Organ. 77 , 820–828 (1999).
CAS PubMed PubMed Central Google Scholar
Taubenberger, J. K., Reid, A. H., Krafft, A. E., Bijwaard, K. E. & Fanning, T. G. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 275 , 1793–1796 (1997).
Iuliano, A. D. et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391 , 1285–1300 (2018).
Article PubMed Google Scholar
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2021–2022 northern hemisphere influenza season. Wkly. Epidemiological Rec. 96 , 77–88 (2021).
Google Scholar
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022–2023 northern hemisphere influenza season. Wkly Epidemiological Rec. 97 , 109–132 (2022).
Jackson, L. A. Using surveillance to evaluate influenza vaccine effectiveness. J. Infect. Dis. 199 , 155–158 (2009).
Jackson, M. L. & Nelson, J. C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 31 , 2165–2168 (2013).
Sullivan, S. G., Tchetgen Tchetgen, E. J. & Cowling, B. J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am. J. Epidemiol. 184 , 345–353 (2016).
Article PubMed PubMed Central Google Scholar
Feng, S., Cowling, B. J., Kelly, H. & Sullivan, S. G. Estimating influenza vaccine effectiveness with the test-negative design using alternative control groups: a systematic review and meta-analysis. Am. J. Epidemiol. 187 , 389–397 (2018).
Li, L. et al. Heterogeneity in estimates of the impact of influenza on population mortality: a systematic review. Am. J. Epidemiol. 187 , 378–388 (2018).
Kissling, E. et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: results from the 2021-2022 I-MOVE primary care multicentre study. Influenza Other Respir. Viruses 17 , e13069 (2022).
McLean, H. Q. et al. Interim estimates of 2022-23 seasonal influenza vaccine effectiveness - Wisconsin, October 2022-February 2023. MMWR Morb. Mortal. Wkly Rep. 72 , 201–205 (2023).
Skowronski, D. M. et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Eur. Surveill. 28 , 2300043 (2023).
Article CAS Google Scholar
CDC. Manual for the Surveillance of Vaccine-preventable Disease. Chapter 6, Influenza . https://www.cdc.gov/vaccines/pubs/surv-manual/chpt06-influenza.html (2023).
Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20 , 454–475 (2021).
Article CAS PubMed PubMed Central Google Scholar
Coffman, R. L., Sher, A. & Seder, R. A. Vaccine adjuvants: putting innate immunity to work. Immunity 33 , 492–503 (2010).
Geckin, B., Fohse, F. K., Dominguez-Andres, J. & Netea, M. G. Trained immunity: implications for vaccination. Curr. Opin. Immunol. 77 , 102190 (2022).
Parker, L. et al. Effects of egg-adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J. Gen. Virol. 97 , 1333–1344 (2016).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333 , 850–856 (2011). Description of an antibody that bound to a conserved epitope in the fusion subdomain of haemagglutinin and offered protection in mice against influenza A (H1N1) and influenza A (H3N2) viruses and in ferrets against influenza A (H5N1) virus.
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324 , 246–251 (2009).
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333 , 843–850 (2011).
Kallewaard, N. L. et al. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166 , 596–608 (2016).
Xiao, H. et al. Light chain modulates heavy chain conformation to change protection profile of monoclonal antibodies against influenza A viruses. Cell Discov. 5 , 21 (2019).
Krammer, F., Li, L. & Wilson, P. C. Emerging from the shadow of hemagglutinin: neuraminidase is an important target for influenza vaccination. Cell Host Microbe 26 , 712–713 (2019).
Doyle, T. M. et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res. 100 , 567–574 (2013).
Rijal, P. et al. Broadly inhibiting antineuraminidase monoclonal antibodies induced by trivalent influenza vaccine and H7N9 infection in humans. J. Virol. 94 , e01182-19 (2020).
Moore, K. A. et al. A research and development (R&D) roadmap for influenza vaccines: looking toward the future. Vaccine 39 , 6573–6584 (2021). This describes a pathway to improved and universal influenza vaccines with links to an up-to-date tracking of their research and development.
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27 , 106–114 (2021).
Folschweiller, N. et al. Reactogenicity, safety, and immunogenicity of chimeric haemagglutinin influenza split-virion vaccines, adjuvanted with AS01 or AS03 or non-adjuvanted: a phase 1-2 randomised controlled trial. Lancet Infect. Dis. 22 , 1062–1075 (2022).
Lewis, N. M. et al. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin. Infect. Dis. 75 , 170–175 (2022).
Izurieta, H. S. et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017-2018. J. Infect. Dis. 220 , 1255–1264 (2019).
Izurieta, H. S. et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J. Infect. Dis. 222 , 278–287 (2020).
Izurieta, H. S. et al. Comparative effectiveness of influenza vaccines among US medicare beneficiaries ages 65 years and older during the 2019-2020 season. Clin. Infect. Dis. 73 , e4251–e4259 (2021). The third of a series of reports on the relative vaccine effectiveness of enhanced seasonal influenza vaccines compared with standard egg-based vaccines in preventing hospital encounters among people aged >65 during the last influenza season prior to the emergence of COVID-19.
Boikos, C. et al. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in US adults ≥65 years during the 2017-2018 and 2018-2019 influenza seasons. Clin. Infect. Dis. 73 , 816–823 (2021).
Boikos, C. et al. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018-2019 influenza season in the United States. Clin. Infect. Dis. 73 , e692–e698 (2021).
Whitney, C. G. et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348 , 1737–1746 (2003).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384 , 403–416 (2021).
Rachlin, A. et al. Progress toward polio eradication — worldwide, January 2020-April 2022. MMWR Morb. Mortal. Wkly Rep. 71 , 650–655 (2022).
de Quadros, C. A., Andrus, J. K., Danovaro-Holliday, M. C. & Castillo-Solorzano, C. Feasibility of global measles eradication after interruption of transmission in the Americas. Expert Rev. Vaccines 7 , 355–362 (2008).
Minta, A. A. et al. Progress toward regional measles elimination — worldwide, 2000-2021. MMWR Morb. Mortal. Wkly Rep. 71 , 1489–1495 (2022).
Flannery, B. et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018-2019 season. J. Infect. Dis. 221 , 8–15 (2020).
Francis, T. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 104 , 572–578 (1960). A perspective of the early work done by Francis, Davenport and Hennessey on how the recognition of influenza viruses is affected by an imprint established by the original virus infection governing later antibody responses.
Shanks, G. D., Hussell, T. & Brundage, J. F. Epidemiological isolation causing variable mortality in Island populations during the 1918-1920 influenza pandemic. Influenza Other Respir. Viruses 6 , 417–423 (2012).
Mantle, J. & Tyrrell, D. A. An epidemic of influenza on Tristan da Cunha. J. Hyg. 71 , 89–95 (1973).
Nogales, A., Martinez-Sobrido, L., Topham, D. J. & DeDiego, M. L. Modulation of innate immune responses by the influenza A NS1 and PA-X proteins. Viruses 10 , 708 (2018).
Harper, D. M. et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364 , 1757–1765 (2004).
Villa, L. L. et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24 , 5571–5583 (2006).
Falsey, A. R. et al. Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N. Engl. J. Med. 388 , 609–620 (2023).
Leroux-Roels, I. et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase I/II randomized clinical trial. J. Infect. Dis. 227 , 761–772 (2023).
Tsang, T. K. et al. Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study. BMC Med. 20 , 230 (2022).
Phillips, R. E. et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354 , 453–459 (1991).
GeurtsvanKessel, C. H. et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 7 , eabo2202 (2022).
Deng, N., Weaver, J. M. & Mosmann, T. R. Cytokine diversity in the Th1-dominated human anti-influenza response caused by variable cytokine expression by Th1 cells, and a minor population of uncommitted IL-2 + IFNγ – Thpp cells. PLoS One 9 , e95986 (2014).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12 , 1203–1207 (2006).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50 , 1132–1148 (2019).
Nguyen, T. H. O. et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat. Commun. 12 , 2691 (2021).
Herati, R. S. et al. Vaccine-induced ICOS + CD38 + circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways. Cell Rep. Med. 2 , 100262 (2021).
Koutsakos, M. et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 10 , eaan8405 (2018).
Laidlaw, B. J., Craft, J. E. & Kaech, S. M. The multifaceted role of CD4 + T cells in CD8 + T cell memory. Nat. Rev. Immunol. 16 , 102–111 (2016).
Cullen, J. G. et al. CD4 + T help promotes influenza virus-specific CD8 + T cell memory by limiting metabolic dysfunction. Proc. Natl Acad. Sci. USA 116 , 4481–4488 (2019).
Lu, Y. J. et al. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 36 , 109696 (2021).
Son, Y. M. et al. Tissue-resident CD4 + T helper cells assist the development of protective respiratory B and CD8 + T cell memory responses. Sci. Immunol. 6 , eabb6852 (2021).
Busselaar, J., Tian, S., van Eenennaam, H. & Borst, J. Helpless priming sends CD8 + T cells on the road to exhaustion. Front. Immunol. 11 , 592569 (2020).
Sant, A. J. & McMichael, A. Revealing the role of CD4 + T cells in viral immunity. J. Exp. Med. 209 , 1391–1395 (2012).
Cenerenti, M., Saillard, M., Romero, P. & Jandus, C. The era of cytotoxic CD4 T cells. Front. Immunol. 13 , 867189 (2022).
Wilkinson, T. M. et al. Preexisting influenza-specific CD4 + T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18 , 274–280 (2012).
Brown, D. M., Lampe, A. T. & Workman, A. M. The differentiation and protective function of cytolytic CD4 T cells in influenza infection. Front. Immunol. 7 , 93 (2016).
Hua, L. et al. Cytokine-dependent induction of CD4 + T cells with cytotoxic potential during influenza virus infection. J. Virol. 87 , 11884–11893 (2013).
van Leeuwen, E. M., Remmerswaal, E. B., Heemskerk, M. H., ten Berge, I. J. & van Lier, R. A. Strong selection of virus-specific cytotoxic CD4 + T-cell clones during primary human cytomegalovirus infection. Blood 108 , 3121–3127 (2006).
Chen, M. & Wang, J. Programmed cell death of dendritic cells in immune regulation. Immunol. Rev. 236 , 11–27 (2010).
Zweerink, H. J., Courtneidge, S. A., Skehel, J. J., Crumpton, M. J. & Askonas, B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature 267 , 354–356 (1977).
Doherty, P. C., Biddison, W. E., Bennink, J. R. & Knowles, B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J. Exp. Med. 148 , 534–543 (1978).
Effros, R. B., Doherty, P. C., Gerhard, W. & Bennink, J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 145 , 557–568 (1977).
McMichael, A. J., Ting, A., Zweerink, H. J. & Askonas, B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature 270 , 524–526 (1977).
Bennink, J. R., Yewdell, J. W. & Gerhard, W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature 296 , 75–76 (1982).
Gotch, F., McMichael, A., Smith, G. & Moss, B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 165 , 408–416 (1987).
Townsend, A. R., Skehel, J. J., Taylor, P. M. & Palese, P. Recognition of influenza A virus nucleoprotein by an H-2-restricted cytotoxic T-cell clone. Virology 133 , 456–459 (1984).
Topham, D. J. & Reilly, E. C. Tissue-resident memory CD8 + T cells: from phenotype to function. Front. Immunol. 9 , 515 (2018).
Farber, D. L. Tissues, not blood, are where immune cells function. Nature 593 , 506–509 (2021).
Zheng, M. Z. M. & Wakim, L. M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15 , 379–388 (2022).
Rotrosen, E. & Kupper, T. S. Assessing the generation of tissue resident memory T cells by vaccines. Nat. Rev. Immunol. 23 , 655–665 (2023).
Ray, S. J. et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20 , 167–179 (2004). This study of lung-resident T cells showed that CD49A was required for maintenance in the lungs but not lymphoid tissues, and loss of the lung-resident population after CD49A blockade was associated with reduced resistance to subsequent influenza virus re-infection.
Wu, T. et al. Lung-resident memory CD8 T cells (T RM ) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95 , 215–224 (2014).
Ariotti, S. et al. Tissue-resident memory CD8 + T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl Acad. Sci. USA 109 , 19739–19744 (2012).
Pizzolla, A. et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 128 , 721–733 (2018).
Gaide, O. et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 21 , 647–653 (2015).
Park, S. L. et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19 , 183–191 (2018).
Ely, K. H., Cookenham, T., Roberts, A. D. & Woodland, D. L. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 176 , 537–543 (2006).
Slutter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2 , eaag2031 (2017).
Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 4 , eaav5581 (2019).
Moyron-Quiroz, J. E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10 , 927–934 (2004).
Takamura, S. Niches for the long-term maintenance of tissue-resident memory T cells. Front. Immunol. 9 , 1214 (2018).
Yap, K. L., Ada, G. L. & McKenzie, I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273 , 238–239 (1978).
Taylor, P. M. & Askonas, B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology 58 , 417–420 (1986).
Graham, M. B., Braciale, V. L. & Braciale, T. J. Influenza virus-specific CD4 + T helper type 2T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 180 , 1273–1282 (1994).
McKinstry, K. K. et al. Memory CD4 + T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 122 , 2847–2856 (2012).
Scherle, P. A., Palladino, G. & Gerhard, W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148 , 212–217 (1992).
McMichael, A. J., Gotch, F. M., Noble, G. R. & Beare, P. A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309 , 13–17 (1983).
Paterson, S. et al. Innate-like gene expression of lung-resident memory CD8 + T cells during experimental human influenza: a clinical study. Am. J. Respir. Crit. Care Med. 204 , 826–841 (2021). This human influenza virus challenge study also incorporated local sampling by bronchoalveolar lavage, providing valuable information on T cell responses in the lung.
Omokanye, A. et al. Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity. Mucosal Immunol. 15 , 717–729 (2022).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19 , 1305–1312 (2013).
Hayward, A. C. et al. Natural T cell-mediated protection against seasonal and pandemic influenza. Results of the flu watch cohort study. Am. J. Respir. Crit. Care Med. 191 , 1422–1431 (2015).
Zhao, M. et al. Prolonged evolution of virus-specific memory T cell immunity after severe avian influenza A (H7N9) virus infection. J. Virol. 92 , e01024–18 (2018).
Von Holle, T. A. & Moody, M. A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 10 , 1457 (2019).
Article Google Scholar
Jegaskanda, S. et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J. Infect. Dis. 214 , 945–952 (2016).
Edgar, J. E. et al. Antibodies elicited in humans upon chimeric hemagglutinin-based influenza virus vaccination confer FcγR-dependent protection in vivo. Proc. Natl Acad. Sci. USA 120 , e2314905120 (2023).
Laidlaw, B. J. et al. Cooperativity between CD8 + T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 9 , e1003207 (2013).
Paluch, C., Santos, A. M., Anzilotti, C., Cornall, R. J. & Davis, S. J. Immune checkpoints as therapeutic targets in autoimmunity. Front. Immunol. 9 , 2306 (2018).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361 , 2209–2220 (2009).
Gray, G. E. et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 384 , 1089–1100 (2021).
Allan, W. H., Madeley, C. R. & Kendal, A. P. Studies with avian influenza A viruses: cross protection experiments in chickens. J. Gen. Virol. 12 , 79–84 (1971).
Beutner, K. R. et al. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J. Infect. Dis. 140 , 844–850 (1979).
Couch, R. B., Kasel, J. A., Gerin, J. L., Schulman, J. L. & Kilbourne, E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 129 , 411–420 (1974).
Skehel, J. J. Polypeptide synthesis in influenza virus-infected cells. Virology 49 , 23–36 (1972).
Shapiro, G. I., Gurney, T. Jr & Krug, R. M. Influenza virus gene expression: control mechanisms at early and late times of infection and nuclear-cytoplasmic transport of virus-specific RNAs. J. Virol. 61 , 764–773 (1987).
Hu, Y., Sneyd, H., Dekant, R. & Wang, J. Influenza A virus nucleoprotein: a highly conserved multi-functional viral protein as a hot antiviral drug target. Curr. Top. Med. Chem. 17 , 2271–2285 (2017).
Babar, M. M. & Zaidi, N. U. Protein sequence conservation and stable molecular evolution reveals influenza virus nucleoprotein as a universal druggable target. Infect. Genet. Evol. 34 , 200–210 (2015).
Wraith, D. C., Vessey, A. E. & Askonas, B. A. Purified influenza virus nucleoprotein protects mice from lethal infection. J. Gen. Virol. 68 , 433–440 (1987).
Andrew, M. E., Coupar, B. E., Boyle, D. B. & Ada, G. L. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand. J. Immunol. 25 , 21–28 (1987).
Christensen, J. P., Doherty, P. C., Branum, K. C. & Riberdy, J. M. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8 + T-cell memory. J. Virol. 74 , 11690–11696 (2000).
Tite, J. P. et al. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology 71 , 202–207 (1990).
Ulmer, J. B. et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259 , 1745–1749 (1993).
Ulmer, J. B. et al. Protective CD4 + and CD8 + T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72 , 5648–5653 (1998).
Xie, C., Yao, R. & Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 8 , 162 (2023).
Hochheiser, K. et al. Cutting edge: the RIG-I ligand 3pRNA potently improves CTL cross-priming and facilitates antiviral vaccination. J. Immunol. 196 , 2439–2443 (2016).
Valencia-Hernandez, A. M. et al. Complexing CpG adjuvants with cationic liposomes enhances vaccine-induced formation of liver T RM cells. Vaccine 41 , 1094–1107 (2023).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8 + T cell tolerance. J. Exp. Med. 196 , 1627–1638 (2002).
Iborra, S. et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1 + dendritic cells. Immunity 45 , 847–860 (2016).
Wakim, L. M., Smith, J., Caminschi, I., Lahoud, M. H. & Villadangos, J. A. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 8 , 1060–1071 (2015).
Vatzia, E. et al. Immunization with matrix-, nucleoprotein and neuraminidase protects against H3N2 influenza challenge in pH1N1 pre-exposed pigs. NPJ Vaccines 8 , 19 (2023).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491 , 463–467 (2012).
Pan, Y. et al. Epicutaneous immunization with modified vaccinia Ankara viral vectors generates superior T cell immunity against a respiratory viral challenge. Vaccines 6 , 1 (2021).
Oberhardt, V. et al. Rapid and stable mobilization of CD8 + T cells by SARS-CoV-2 mRNA vaccine. Nature 597 , 268–273 (2021).
Gao, F. et al. Spheromers reveal robust T cell responses to the Pfizer/BioNTech vaccine and attenuated peripheral CD8 + T cell responses post SARS-CoV-2 infection. Immunity 56 , 864–878.e4 (2023).
Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 374 , eabj9853 (2021).
Ssemaganda, A. et al. Expansion of cytotoxic tissue-resident CD8 + T cells and CCR6 + CD161 + CD4 + T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat. Commun. 13 , 3357 (2022).
Mitsi, E. et al. Respiratory mucosal immune memory to SARS-CoV-2 after infection and vaccination. Nat. Commun. 14 , 6815 (2023).
Xiong, F. et al. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 12 , 2256422 (2023).
Lee, I. T. et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis. Nat. Commun. 14 , 3631 (2023).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25 , 1316–1327 (2017).
Feldman, R. A. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37 , 3326–3334 (2019).
Ewer, K. J. et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 27 , 270–278 (2021).
Stephenson, K. E. et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 325 , 1535–1544 (2021).
Knight, F. C. & Wilson, J. T. Engineering vaccines for tissue-resident memory T cells. Adv. Ther. 4 , 2000230 (2021).
Lillie, P. J. et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 55 , 19–25 (2012).
Evans, T. G. et al. Efficacy and safety of a universal influenza A vaccine (MVA-NP+M1) in adults when given after seasonal quadrivalent influenza vaccine immunisation (FLU009): a phase 2b, randomised, double-blind trial. Lancet Infect. Dis. 22 , 857–866 (2022).
Del Campo, J. et al. OVX836 heptameric nucleoprotein vaccine generates lung tissue-resident memory CD8 + T-cells for cross-protection against influenza. Front. Immunol. 12 , 678483 (2021).
Leroux-Roels, I. et al. Immunogenicity, safety, and preliminary efficacy evaluation of OVX836, a nucleoprotein-based universal influenza A vaccine candidate: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Infect. Dis. 22 , 857–866 (2023).
Leroux-Roels, I. et al. Randomized, double-blind, reference-controlled, phase 2a study evaluating the immunogenicity and safety of OVX836, a nucleoprotein-based influenza vaccine. Front. Immunol. 13 , 852904 (2022).
Pleguezuelos, O. et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. Vaccines 5 , 22 (2020).
Taylor, P. M., Esquivel, F. & Askonas, B. A. Murine CD4 + T cell clones vary in function in vitro and in influenza infection in vivo. Int. Immunol. 2 , 323–328 (1990).
Enelow, R. I. et al. Structural and functional consequences of alveolar cell recognition by CD8 + T lymphocytes in experimental lung disease. J. Clin. Invest. 102 , 1653–1661 (1998).
Small, B. A. et al. CD8 + T cell-mediated injury in vivo progresses in the absence of effector T cells. J. Exp. Med. 194 , 1835–1846 (2001).
Hoskins, T. W., Davies, J. R., Smith, A. J., Miller, C. L. & Allchin, A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1 , 33–35 (1979).
Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 23 , 186–193 (2022).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543 , 559–563 (2017).
Zens, K. D., Chen, J. K. & Farber, D. L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1 , e85832 (2016).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 7 , eadd4853 (2022).
Brenna, E. et al. CD4 + T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-Tfh CD4 + cells. Cell Rep. 30 , 137–152.e5 (2020).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27 , 125–135 (2021).
Valkenburg, S. A. & Poon, L. L. M. Universal influenza vaccines are futile when benchmarked against seasonal influenza vaccines. Lancet Infect. Dis. 22 , 750–751 (2022).
Wu, N. et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 11 , 439–452 (2023).
Zheng, X. et al. Mucosal CD8 + T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 15 , e1008036 (2019).
Gouglas, D. et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Health 6 , e1386–e1396 (2018).
Bloom, D. E., Cadarette, D. & Tortorice, D. L. An ounce of prevention: our approach to vaccine finance is ill-suited to addressing epidemic risk. Finance Dev. 57 , 54–57 (2020).
Plotkin, S., Robinson, J. M., Cunningham, G., Iqbal, R. & Larsen, S. The complexity and cost of vaccine manufacturing — an overview. Vaccine 35 , 4064–4071 (2017).
Download references
Acknowledgements
J.W.McC. is supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC001030), the Medical Research Council (FC001030) and the Wellcome Trust (FC001030). A.J.McM. is supported by the CAMS-Oxford Institute.
Author information
Authors and affiliations.
David H. Smith Center for Vaccine Biology and Immunology, University of Rochester Medical Center, Rochester, NY, USA
Tim R. Mosmann
Centre for Immuno-Oncology, Old Road Campus Research Building, University of Oxford, Oxford, UK
Andrew J. McMichael
Osivax, Lyon, France
Alexandre LeVert
The Francis Crick Institute, London, UK
John W. McCauley
The Sir William Dunn School of Pathology, South Parks Road, University of Oxford, Oxford, UK
Jeffrey W. Almond
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Tim R. Mosmann .
Ethics declarations
Competing interests.
T.R.M. is a member of the Osivax scientific advisory board. A.J.McM. is a member of the scientific advisory boards of Osivax, Oxford Vacmedix and T-Cypher Bio. A.L.V. is an employee and shareholder of Osivax. J.W.McC. has provided advice to CSL Seqirus, Sanofi Pasteur, Pfizer and Iceni Diagnostics. J.W.A. has provided scientific advice to Osivax, IosBios Ltd., Mynvax and Blue Lake Biotechnology. He is also a shareholder of Sanofi, Vaxcyte, Moderna and OVO Biomanufacturing Ltd.
Peer review
Peer review information.
Nature Reviews Immunology thanks Katherine Kedzierska and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CDC. About Diphtheria, Tetanus, and Pertussis Vaccines: https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html
CDC. About HPV Vaccines: https://www.cdc.gov/vaccines/vpd/hpv/hcp/vaccines.html
CDC. Past Seasons Estimated Influenza Disease Burden: https://www.cdc.gov/flu/about/burden/past-seasons.html
Influenza Vaccines Roadmap: https://ivr.cidrap.umn.edu/universal-influenza-vaccine-technology-landscape
Measles & Rubella Partnership: https://measlesrubellainitiative.org/measles-rubella-strategic-framework-2021-2030/
Polio Global Eradication Initiative: https://polioeradication.org/
WHO. FluNet Summary: https://www.who.int/tools/flunet/flunet-summary
WHO. Measles: https://www.who.int/news-room/fact-sheets/detail/measles
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Mosmann, T.R., McMichael, A.J., LeVert, A. et al. Opportunities and challenges for T cell-based influenza vaccines. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01030-8
Download citation
Accepted : 27 March 2024
Published : 02 May 2024
DOI : https://doi.org/10.1038/s41577-024-01030-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine brand hesitancy and other challenges to vaccination in the Philippines
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Medicine and Public Health, Ateneo de Manila University, Manila, Philippines
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
Affiliations School of Medicine and Public Health, Ateneo de Manila University, Manila, Philippines, The Medical City, Manila, Philippines
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – review & editing
- Arianna Maever L. Amit,
- Veincent Christian F. Pepito,
- Lourdes Sumpaico-Tanchanco,
- Manuel M. Dayrit

- Published: January 13, 2022
- https://doi.org/10.1371/journal.pgph.0000165
- See the preprint
- Peer Review
- Reader Comments
Effective and safe COVID-19 vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths. However, COVID-19 vaccine uptake is challenged by vaccine hesitancy and anti-vaccination sentiments, a global shortage of vaccine supply, and inequitable vaccine distribution especially among low- and middle-income countries including the Philippines. In this paper, we explored vaccination narratives and challenges experienced and observed by Filipinos during the early vaccination period. We interviewed 35 individuals from a subsample of 1,599 survey respondents 18 years and older in the Philippines. The interviews were conducted in Filipino, Cebuano, and/or English via online platforms such as Zoom or via phone call. All interviews were recorded, transcribed verbatim, translated, and analysed using inductive content analysis. To highlight the complex reasons for delaying and/or refusing COVID-19 vaccines, we embedded our findings within the social ecological model. Our analysis showed that individual perceptions play a major role in the decision to vaccinate. Such perceptions are shaped by exposure to (mis)information amplified by the media, the community, and the health system. Social networks may either positively or negatively impact vaccination uptake, depending on their views on vaccines. Political issues contribute to vaccine brand hesitancy, resulting in vaccination delays and refusals. Perceptions about the inefficiency and inflexibility of the system also create additional barriers to the vaccine rollout in the country, especially among vulnerable and marginalised groups. Recognising and addressing concerns at all levels are needed to improve COVID-19 vaccination uptake and reach. Strengthening health literacy is a critical tool to combat misinformation that undermines vaccine confidence. Vaccination systems must also consider the needs of marginalised and vulnerable groups to ensure their access to vaccines. In all these efforts to improve vaccine uptake, governments will need to engage with communities to ‘co-create’ solutions.
Citation: Amit AML, Pepito VCF, Sumpaico-Tanchanco L, Dayrit MM (2022) COVID-19 vaccine brand hesitancy and other challenges to vaccination in the Philippines. PLOS Glob Public Health 2(1): e0000165. https://doi.org/10.1371/journal.pgph.0000165
Editor: Dione Benjumea-Bedoya, Corporacion Universitaria Remington, COLOMBIA
Received: October 27, 2021; Accepted: December 22, 2021; Published: January 13, 2022
Copyright: © 2022 Amit et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data relevant to the study are included in the article.
Funding: AMLA/VCFP/LST/MMD are funded by the Ateneo de Manila University Research Council COVID-19 Research Grant (Grant No. COVID-URC 01 2021). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: We have read the journal’s policy and the authors of this manuscript have the following competing interests: VCFP owns shares of GMA Network, Inc., a Philippine Stock Exchange-listed company with interests in mass media. AMLA, VCFP, and MMD receive funding from Sanofi to conduct research on self-care.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to burden health systems and communities globally, with millions of cases and deaths [ 1 ]. Because of the significant and continued impact of COVID-19, vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths [ 2 ]. Many vaccines have been shown to be safe and effective with high-income countries having vaccinated more than half of their population [ 3 ]. Despite the availability of these vaccines, countries are faced with various challenges including vaccine hesitancy and anti-vaccination sentiments, limited global supply, and inefficient vaccine deployment [ 4 , 5 ]. These issues in vaccine uptake, together with declining community acceptance of other public health interventions, will mean a delayed recovery and prolonged pandemic [ 6 ].
The World Health Organization (WHO) in 2019 identified vaccine hesitancy or the reluctance to vaccinate as one of the top ten threats to global health despite evidence of the important role of vaccines in improving population health outcomes [ 7 ]. Together with weak primary health care and other health challenges, countries especially low- and middle-income countries (LMICs) will struggle to meet the demands of the communities within their health system capacity. With the pandemic, countries are further burdened with many health systems overwhelmed throughout its course. The Philippines presently faces these challenges: vaccine hesitancy and increasing anti-vaccination sentiments, a weak primary health care system with efforts to strengthen it through the recently implemented Universal Health Care Law, and an overwhelmed health system because of the demands of COVID-19 and other public health problems [ 8 – 13 ]. These challenges are further compounded by a global shortage of vaccine supply with inequitable vaccine distributions [ 14 ].
Historically, the Philippines was one of the countries with generally high vaccine confidence rates [ 15 ]. Following the dengue vaccine controversy in 2017 however, confidence levels have dramatically dropped and have impacted succeeding vaccination efforts including the COVID-19 vaccination campaign [ 9 , 12 , 15 – 17 ]. Dengvaxia, the world’s first commercially available dengue vaccine developed by Sanofi Pasteur, was introduced as part of a national school-based immunization programme despite the lack of empirical data on the risks associated with administration of the vaccine among those not previously infected with dengue or seronegative children [ 9 , 12 , 15 – 17 ]. By the time reports were released that the vaccine may cause more severe disease among seronegatives, the Philippines had already inoculated more than 800,000 Filipino school-age children [ 9 ]. This was highly politicised, and damaged trust in vaccines and the health sector [ 9 , 12 , 15 – 17 ]. As a result, immunisation rates dropped and the country saw outbreaks of previously controlled vaccine-preventable diseases such as measles and polio [ 18 , 19 ]. In addition to vaccine hesitancy, the Philippine health system is not prepared for additional health care demands. As early as the first phase of the pandemic, critical care capacity was overwhelmed with the influx of patients in hospitals [ 10 , 11 ]. As of 16 September 2021, the Philippines ranks third among countries with the highest number of newly confirmed cases per one million population [ 1 , 20 ]. Globally, 42.9% of the world population have received one dose of a COVID-19 vaccine, with much lower rates in LMICs like the Philippines [ 20 , 21 ]. Only 55% of Filipinos have expressed willingness to be vaccinated against COVID-19, and as of 16 September 2021, only 30% of the population have been fully vaccinated[ 21 , 22 ].
To end this pandemic, it is critical to implement all possible public health interventions and strategies from face masks, physical distancing, to getting vaccinated [ 4 , 23 ]. However, there is a need to recognise that the adoption of all these interventions is influenced by individual risk perceptions, and these perceptions are shaped by various sources of information and experiences [ 24 ]. Additionally, there are interpersonal and structural factors that influence health decisions of individuals. Recognising the multiple dimensions in which behaviours and decisions occur, theories and models have been proposed to explain how individuals make decisions on their health based on factors that change over time and context [ 25 , 26 ]. The social ecological model provides a useful framework for investigating health behaviours and decisions by recognising that a multiplicity of factors interacts to influence health of individuals [ 26 ]. These include individual factors representing biological or behavioural characteristics, interpersonal factors representing networks and social capital operating within a defined boundary, and structural factors that include health systems and are mediated through laws and policies [ 26 ]. Published studies on vaccination that utilised this model reported that vaccine intentions and attitudes operate along multiple dimensions, with a series of events influencing decisions related to vaccination [ 17 , 27 , 28 ]. Improving adherence to interventions and vaccination rates therefore requires a better understanding of the different reasons behind vaccine mistrust and not just determining their individual beliefs, knowledge, and levels of trust [ 17 , 27 , 28 ]. A recently published scoping review supports the use of the social ecological model in understanding attitudes towards COVID-19 vaccination [ 29 ]. The review showed that influencing factors are embedded within the social ecological model and that multilevel interventions are needed to improve uptake of vaccines [ 29 ]. This scoping review of 50 articles had representation from various countries, but did not include data from the Philippines. We address this gap by exploring the vaccination narratives and challenges experienced and observed by Filipinos during the early COVID-19 vaccination period. We used qualitative data from a mixed-methods study conducted from June to August 2021 that aimed to understand how people in the Philippines view COVID-19 and what influences their behaviours. With these findings, we hope to provide insights to possible avenues of future research and directions for improving COVID-19 vaccine uptake and reach.
Material and methods
Design and setting.
We conducted an online survey among adults ages 18 and older in the Philippines (n = 1,599) from June to August 2021. A subsample participated in the semi-structured interviews (n = 35) with representation from the general population and health workforce from July to August 2021. Data from the interviews informed the findings of this paper.
Participants and recruitment
We aimed to interview participants from different regions in the Philippines, various age groups, socio-economic classes, and vaccination status and attitudes. This allowed us to ensure maximum variation sampling, which aims to capture as many population contexts as possible. We contacted a total of 115 individuals through the information they provided (i.e., mobile number, phone number, e-mail). Out of the 115, 35 participants completed the interviews. The remaining 80 either refused or could not be contacted after a maximum of three attempts. We classified participants according to their vaccination priority group based on the COVID-19 Vaccination Program’s prioritisation framework [ 30 ]. Those in the first priority group (A1) were frontline workers in health facilities; other priority groups (A2 to C) comprised and represented the general population ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0000165.t001
Data collection
We conducted the interviews in Filipino, Cebuano, and/or English via online platforms such as Zoom or via phone call. The interview guide included questions about their views on COVID-19, vaccines, and their risk perceptions and behaviours. We recruited interview participants until saturation was reached (i.e., no new information was being obtained from the interviews) [ 31 ]. The interviews lasted between 60 to 90 minutes with a token amounting to USD 6 provided to each participant. All participants consented to the interview being recorded.
Data analysis
The interviews were digitally recorded, transcribed verbatim, and translated from Filipino or Cebuano to English. The research team are native and/or fluent speakers of the three languages, and checked for linguistic and conceptual equivalence in the translated documents. We de-identified all participants and assigned pseudonyms. We analysed the data using inductive content analysis focusing on the experiences and views towards vaccination [ 32 ]. Our analysis was guided by principles of grounded theory. Transcripts of the interviews were read to identify themes and two investigators (AMLA, VCFP) independently coded the interviews according to emergent themes in Microsoft Excel [ 33 ]. We used coding language that was close to the participants’ terms and phrases to ensure that we were co-constructing accurate categories reflective of their responses [ 34 ]. The codes were reviewed, and areas of disagreement were resolved between the two investigators. Themes from the interviews were further explored through discussions with the other members of the team. We considered reflexivity throughout data collection and analysis, acknowledging that our preconceptions and experiences about vaccination as public health practitioners and health professionals may influence the way we analyse and interpret data. Our use of the grounded theory allowed us to explore the experiences of our participants and our own shared experiences, and avoided being limited by how we view COVID-19 vaccination [ 35 ]. To highlight the complex reasons for delaying and/or refusing COVID-19 vaccination, we embedded our findings within the social ecological model with three broad themes: individual factors (attitudes, beliefs, knowledge, behaviours), interpersonal factors (relationships and social networks), and structural factors (health systems and service delivery; media; and policies, regulations, and laws at the local, national, and global level) [ 26 ] ( Fig 1 ). The quotes presented in this paper are either in the original English or translated from Filipino or Cebuano.
This figure shows the three main tiers of factors influencing vaccination intention and uptake: individual (beliefs, attitudes, knowledge, health literacy), interpersonal (relationships, networks), and structural (health systems and service delivery, media, policies). These three dimensions are jointly or individually impacted by misinformation (white circles).
https://doi.org/10.1371/journal.pgph.0000165.g001
Patients and public involvement
The public were not directly involved in the design, recruitment, conduct, reporting, or dissemination plans of this research. Their only involvement was as research participants.
Ethics statement
This study was approved by the University Research Ethics Office of Ateneo de Manila University (Study No. SMPH CORISK 2021). All participants were informed about the aims and objectives of the study by including the written consent form in the email correspondence. Prior the interview, the research team thoroughly explained the study to them and provided them the opportunity to ask questions they may have. Written digital consent was taken from study participants before the interview.
We interviewed 35 participants with representation from different vaccination priority groups working in various parts of the country. Our participants also had different educational backgrounds, employment status, and vaccination attitude ( Table 2 ). There was an almost equal proportion of females and males (females: 19; males: 16) with a median age of 38 years old (range: 21 to 74 years old) in the overall study population.
https://doi.org/10.1371/journal.pgph.0000165.t002
Participant views on the barriers to COVID-19 vaccination are presented below, organised using the three tiers of the social ecological model. Individual barriers include perceptions; attitudes; and beliefs about the science, about vaccines, about the health system and government. Interpersonal barriers are the networks and social capital that influence health beliefs and decisions. Vaccine procurement, supply, and logistics, together with media- and policy-related issues, comprise the structural barriers. Where there are differences between the general population and health workers, these are highlighted in the text.
Individual barriers
Vaccine brand hesitancy and brand preferences..
Vaccine brand hesitancy or delay in getting the vaccine due to brand preferences was a common theme among the participants. The country’s first administered vaccine was Sinovac-CoronaVac, which is manufactured by a Chinese biopharmaceutical company. This was given to health workers despite lack of published data on effectiveness at the time and initial announcements that these were not recommended for high-risk individuals ( Quote I1, Table 3 ). In addition to concerns about the effectiveness of the vaccine, participants also read and heard information on how this vaccine was made. They believed this specific vaccine was using the same virus to ‘immunise’ an individual’s system, which may have unintended effects ( Quote I2, Table 3 ). Other participants cited that this specific brand was not recognised by other countries, and therefore wanted and waited for other vaccines. Meanwhile, others refused to receive mRNA vaccines due to beliefs about its safety and effectiveness.
https://doi.org/10.1371/journal.pgph.0000165.t003
Negative experiences with the health system as source of vaccine hesitancy and anti-vaccination sentiments.
The participants cited negative experiences in the past, whether these happened recently or decades ago, as causes of their negative attitude towards vaccines. Three participants who identified themselves as COVID-19 ‘anti-vaxxers’ or those opposed to vaccines, had different sources of anti-vaccination sentiments. These three participants belong to different priority groups. One belongs to the A1 or frontliner group and is working as a Barangay Health/Emergency Response Team (BHERT) member who responds to COVID-19 related health care needs in the community. The second is a retired professional (A2 or senior citizen group) while the third is an environmental protection officer who oversees implementation of public health standards in the community (B2 or other government workers). These participants experienced an undesired event related to vaccines and/or medical care from four years to more than three decades prior the pandemic ( Quotes I3-I5, Table 3 ). Except for one anti-vaxxer, no other health worker reported negative experiences that caused mistrust in the COVID-19 vaccines and vaccination campaign.
Vaccines are viewed as unsafe and deadly.
Perceptions on risk of getting infection with and dying from the virus varied among the participants. However, for those who were opposed to the vaccines, their fear of the COVID-19 vaccine and its effects was greater than their fear of the virus and outcomes ( Quote I6, Table 3 ). This fear and their view of vaccines being unsafe and deadly resulted to vaccine refusals or delays. According to them, the deaths observed after administration of the vaccine are caused by the vaccine; however, medical doctors and hospitals report the death as being caused by underlying conditions such as comorbidities ( Quotes I7-I8, Table 3 ). Some participants also believed the circulating theory that the life span of those who are vaccinated is shortened and they only have two to three years to live: “ you are healthy but because of the vaccine , you suddenly die ”. In addition to the belief that vaccines cause death or shorten an individual’s life span, participants also had doubts about the COVID-19 vaccines particularly the mRNA vaccines that use a relatively new technology ( Quote I9, Table 3 ). These concerns about the safety profile of vaccines either caused delays in vaccine acceptance and uptake or refusals. The reverse was reported among most of the health workers and other participants who viewed vaccines positively. They believed that the vaccine protects them from severe illness, hospitalisation, and death, and that vaccines only have minimal risk.
Vaccines are viewed as unnecessary and insufficient to prevent disease.
Vaccines were viewed as unnecessary by some participants, especially those in older age groups who are not allowed to go out ( Quote I10, Table 3 ). Those in lower priority groups felt that others needed the vaccine more than them. Younger participants shared that they were COVID-19 survivors even without the vaccine; but those at high risk especially the elderly and persons with comorbidities will need the vaccine to protect them ( Quote I11, Table 3 ) . The participants also viewed vaccines as insufficient–they expected that getting vaccinated means no longer needing other public health interventions but were disappointed to learn that vaccines are only one part of the solution. Participants therefore questioned the need for the vaccines given the information they have read and/or watched about still being at risk of getting infected despite being vaccinated ( Quote I12, Table 3 ). The lack of clarity in the role of the vaccines has negatively influenced people’s decisions on getting the vaccine.
Skepticism towards vaccine incentives.
Vaccine incentives in the country, such as promotions and offers for those vaccinated, created skepticism among some of the participants. These incentives ‘bothered’ participants and raised questions about the role of vaccines and the intentions of the government. As a result, these incentives ‘disincentivised’ participants from getting the vaccine as participants felt being forced to take it ( Quote I13, Table 3 ).
Use of vaccines not fully approved by the Food and Drug Administration (FDA).
Participants viewed decisions to vaccinate individuals as ‘rash’ and expressed concerns about vaccines not yet being fully approved by the Food and Drug Administration (FDA). Some also shared concerns about the rapid development of vaccines compared to other vaccines that took decades to develop ( Quote I14, Table 3 ). Participants felt that they were being experimented on using an unproven vaccine, relating this with the dengue vaccine controversy ( Quote I15, Table 3 ). This caused delay or refusal in getting the vaccines when it was offered to them.
Low health literacy and lack of critical skills to evaluate health information.
Health literacy or how people acquire, evaluate, and apply health information to inform their decisions, including getting the vaccine, is an important but underestimated tool to combat misinformation. Participants shared that Filipinos seemed to know a lot about vaccines, but only superficially. They shared that those among low-resource communities and older population groups were especially vulnerable to misinformation ( Quote I16, Table 3 ). This lack of awareness and critical skills to evaluate information, together with the rapid spread of misinformation, influences people’s decisions to get their first dose, to return to their second and get fully vaccinated ( Quote I17, Table 3 ). There were also several participants who shared that they were confused with the contradictory information they were reading and hearing ( Quotes I18, Table 3 ).
Religious beliefs do not support vaccines.
‘Antichrist’–this was how one participant described the vaccines against COVID-19. Another participant shared concerns about the vaccines and how they would replace antibodies created by God ( Quote I19, Table 3 ). She mentioned that these vaccines have active chemicals that are causing unintended side effects and deaths.
Interpersonal barriers
Family influence and opposition to vaccines..
Participants recognised the influence of their family on their health decisions including getting vaccinated. One participant who was opposed to COVID-19 vaccines shared that everyone in their family was unvaccinated because they believed her (A1, 51–60 years old, female, Misamis Oriental). Similarly, a mother who had a negative experience related to the dengue vaccine that was administered to her child, refused to have herself and her family vaccinated against COVID-19 (B2, 41–50 years old, female, National Capital Region).
Misinformation spread by networks.
Rumours and misinformation about COVID-19 vaccines are easily spread by networks, whether by word of mouth or through social media. A participant said her “ eyes have been opened only now because of YouTube ” (A2, 61–70 years old, female, Camarines Norte). Participants believed that this affected vaccine uptake, especially among individuals who do not have the opportunity to receive accurate information from official sources including the Department of Health ( Quote IC1, Table 4 ).
https://doi.org/10.1371/journal.pgph.0000165.t004
Perceived conflicts of interest of health professionals.
Participants viewed key figures in the response to the pandemic as having conflicts of interests. This perception of having ‘hidden agenda’ created mistrust in the information provided health professionals, health organisations, and other figures and institutions. These conflicts of interest, whether financial or non-financial, subject evidence and data to bias especially if there are undesired adverse effects to the treatment or vaccine ( Quote IC2, Table 4 ).
Structural barriers: Health systems and service delivery
Inadequate supply of vaccines..
Observations of participants regarding supply of vaccines varied according to location and membership to the vaccine priority groups. Participants, especially those from cities and provinces outside of metropolitan areas, reported that the supply of vaccines was insufficient to meet the demands and needs of the communities ( Quote S-HS1, Table 5 ). However, even within highly urbanised areas, participants shared that there were those who did not get their second doses on time because no vaccines arrived ( Quote S-HS2, Table 5 ). Health workers found that vaccines for them were easily accessible, however those in other groups had to wait longer before getting the vaccine ( Quote S-HS3, Table 5 ).
https://doi.org/10.1371/journal.pgph.0000165.t005
Perceived inefficiencies of the vaccination system.
Participants highlighted issues with the system including the slow rollout of vaccines, long waiting time, inefficient registration systems, and lack of a centralised system. Participants mentioned getting frustrated with the speed at which vaccines are being distributed and administered in the country ( Quote S-HS4, Table 5 ). Participants also mentioned issues with the waiting process to get a slot after registration and the waiting time at the day of the vaccination, with some being asked to stay at vaccination sites for two hours to watch a seminar on COVID-19 and vaccines ( Quotes S-HS5-6, Table 5 ). There was perceived risk of exposure, which could be lessened if the process was faster and more efficient. There were also glitches in the online registration systems used by local governments that caused additional delays in getting people vaccinated ( Quote S-HS7, Table 5 ). Local governments are responsible for the distribution and administration of vaccines among their constituents, and individuals may register with various local governments depending on their place of residence or work. This lack of a centralised system makes it difficult to track who have already been vaccinated and where they have been vaccinated such that those who are still waiting for a slot are unable to secure one ( Quote S-HS8, Table 5 ).
View that the vaccination system is inflexible and excludes vulnerable and marginalised populations.
The current vaccination system of some local governments is viewed as inflexible that excludes vulnerable and marginalised populations. There are individuals who lack access to technology and digital platforms. Especially in rural areas and among the elderly, their exclusion due to access issues is further compounded by their low digital health literacy. These individuals are then unable to register online and get the vaccine ( Quote S-HS9, Table 5 ). While registration is online, even those in older age groups who are part of highly prioritised groups because of their susceptibility to the virus are required to go to the vaccination centre ( Quote S-HS10, Table 5 ). Similarly, those belonging to marginalised groups and communities also encounter considerable challenges to getting the vaccine ( Quote S-HS11, Table 5 ).
Logistical challenges.
A participant recognised that there are also logistical constraints in the distribution of vaccines, in addition to problems with supply. The COVID-19 vaccines have different temperature requirements with some requiring special distribution systems ( S-HS12, Table 5 ). These logistical challenges influence the distribution of vaccine brands to areas that have the capability to store them and affect decisions to delay getting the vaccine especially among those who prefer other brands ( S-HS13, Table 5 ).
Health professionals seen as amplifiers of misinformation.
Misinformation on vaccines and treatment were not only observed within families and social networks, but also within the medical community reported by participants who are health professionals themselves. There have been debates about Ivermectin as treatment for COVID-19, as well as vaccines, which have created factions within the group ( S-HS14, Table 5 ). Some of these health professionals who are anti-vaxxers or opposed to vaccines publicly share their views in media and in their practice ( S-HS15, Table 5 ). Because of the stature and credibility of health professionals, their views, whether backed by science or not, get amplified in the media and communities.
Pandemic response deemed as ineffective affects trust in health institutions.
The response and messaging of health organisations, together with other key figures and institutions in the country, were viewed by participants as ineffective ( S-HS16, Table 5 ). As a result, there is declining trust in these organisations with participants doubting information provided, such that Filipinos no longer take the pandemic seriously ( S-HS17, S-HS18, Table 5 ). In turn, participants turn to other sources of information that they think are more credible and trustworthy.
Structural barriers: Media and policies
Traditional and digital media accelerating the infodemic..
Information on the virus and vaccines are easily and effectively amplified by the media. With the infodemic (portmanteau of information and epidemic) or the exponential production of information whether scientifically accurate or not, traditional media and digital media become drivers of (mis)information or fear towards vaccines ( Quotes S-MP1-S-MP2, Table 6 ). Information that participants were receiving from these sources influenced their health beliefs and vaccine decisions ( Quote S-MP3, Table 6 ).
https://doi.org/10.1371/journal.pgph.0000165.t006
Perceived poor policy implementation and lack of evidence-based policies contributing to loss of confidence in vaccines and health institutions.
The government developed the Philippine “National Deployment and Vaccination Plan for COVID-19 Vaccines” that identifies population groups to be prioritised ensure vaccine equity accounting for different risks and needs [ 36 ]. This plan also stated that only vaccines granted with emergency use authorisation (EUA) or certificate of product registration (CPR) by the Philippine FDA will be purchased by the government. However, this was reported by participants to be poorly implemented with others using connections also known as ‘ palakasan ’ system to get the vaccine ahead of those in the priority list ( Quote S-MP4, Table 6 ). Even within the government, the Presidential Security Group were given vaccines even without EUA and/or CPR registration from the FDA ( Quote S-MP5, Table 6 ). In addition, the government purchased vaccines that did not publish their results, and reportedly had lower efficacy rates but more expensive ( Quote S-MP6, Table 6 ). As a result, participants felt that the government was ‘settling for less’ and that Filipinos deserved better (A4, 21–30 years old, female, National Capital Region). These issues contributed to declining confidence in vaccines and health institutions, with Filipinos questioning the safety of such vaccines and the implementation of these prioritisation frameworks.
National and local political issues.
Past and current political issues contributed to refusals to specific vaccine brands. Together with reports of how the virus emerged from Wuhan, China, these triggered skepticism towards vaccines manufactured in their country. Participants mentioned the dispute of the Philippines and China regarding contested territory at the West Philippine Sea (South China Sea) as a reason for not preferring and/or refusing vaccines from their country, even when donations of Sinovac from China were the first vaccines to be available ( Quote S-MP7, Table 6 ). This dispute also influenced how participants thought about the origins of the virus and why other countries developed their own vaccines ( Quote S-MP8, Table 6 ). Locally, participants viewed politics to have influence on which cities or provinces receive preferred vaccine brands. They mentioned that these ‘favored hospitals and provinces’ were prioritised, which was perceived as unfair and causing further delays in the vaccination rollout ( Quote S-MP9, Table 6 ).
One of the most effective public health strategies, vaccination, has been the focus of false and inaccurate information with rapidly declining rates of acceptance. [ 37 ]. In the Philippines, vaccine confidence plummeted after the Dengue vaccine controversy [ 9 , 12 , 15 – 17 ]. While anti-vaccination views and vaccine hesitancy are not yet the main barrier to vaccination in the Philippines which still struggles with vaccine access and distribution, lessons from other countries indicate that these equally and urgently need to be addressed in addition to other challenges [ 38 ]. Our study supports the findings of other published research that report a host of individual, interpersonal, and structural barriers that work individually or collectively against vaccination uptake and reach [ 29 ]. Therefore, there is a need for a holistic approach to promote COVID-19 vaccination that not only addresses barriers at the individual level, but also at the interpersonal and structural levels [ 38 , 39 ].
Individual perceptions, beliefs, and experiences play a major role on the decision to vaccinate. These are shaped by exposure to (mis)information spread by networks, by key health figures and institutions, and through the media [ 40 – 43 ]. Misinformation regarding vaccines have been present since vaccines were first developed [ 44 – 46 ], but the advent of social media made its propagation much easier [ 43 , 45 , 47 ]. Unique to the Philippine context is vaccine brand hesitancy, specifically towards Chinese manufactured vaccines and mRNA vaccines. This is caused in part by lack of transparency and scientific information, and spread through networks and the media. Further aggravating the issue is how some people attempt to correct misconceptions in a way that alienates people instead of addressing misinformation. People involved in vaccine promotion activities, especially primary care providers, may need to be trained on how to engage with vocal vaccine deniers and promote vaccination. The World Health Organization document outlining how to respond to vaccine misinformation would be an important resource in such an endeavour [ 48 ]. Celebrities and social media influencers may also play a role in promoting vaccination [ 41 ], but it is essential that they disclose conflicts of interest to develop trust with their audience. The media also needs to be trained on how to present news regarding adverse effects following immunsation, and regarding COVID-19 in general, so as not to create unnecessary panic and dissuade people from getting vaccinated. A study reported that there may be a need to use first-person, people-centred narratives to prevent ‘psychic numbing’ and give faces to numbers [ 49 ]. In all these, it is vital to engage with the public, especially those who are vaccine hesitant, in order to promote vaccination using language that is inclusive and applicable to their context [ 48 ].
The health system and one’s interactions with it also contribute to one’s decision to get vaccinated. As in this study, trust in the health system has been found to be a major factor in getting COVID-19 vaccine [ 41 , 50 ]. The Philippine government has instituted several health system confidence-building policies. The recent COVID-19 Vaccination Program Act stipulates the provision of free COVID-19 vaccines to all Filipinos and the establishment of an indemnification fund for people who could possibly develop adverse effects following immunisation [ 51 ]. Perceptions of ‘ palakasan ’ (i.e., use of political connections), stemming from instances during the course of the pandemic where powerful individuals seem to be above the law [ 52 ], contribute to vaccine hesitancy and poor uptake of vaccines. These negative impacts are further compounded by the highly politicised Dengvaxia controversy where individuals, especially parents of school-age children, felt that health institutions and governments were experimenting on them [ 9 , 12 ] with our participants relating the COVID-19 vaccine ‘experiment’ with the dengue vaccine. In addition, inadequate supply, logistical challenges, and perceptions about the inefficiency and inflexibility of the system negatively impact vaccination rates in the country. As of 16 September 2021, only 3 in 10 Filipinos received one dose with significant differences between population groups: almost all frontline and health workers have been vaccinated while only 2 in 5 elderly Filipinos received their first dose [ 21 ]. Those in the third priority group have higher rates than the elderly population group, which were offered the vaccines earlier. Apart from individual reasons, marginalised and vulnerable groups such as the elderly have reported not being able to get their vaccine due to lack of home vaccination services and guidance in using online registration systems. The system will need to consider needs of all population groups to improve vaccination uptake. In all these, trust in the health system needs to be maintained, while disregarding regulations and policies in place can erode trust in the vaccination process.
In the Philippines, the national government has the responsibility to procure, allocate, and distribute the vaccines to the different provinces and municipalities, but it is the local government that is responsible for last-mile transport and actual inoculation. This results in wide variations in client registration and procedures between different localities. This underlines the need to identify best practices in vaccine rollout systems to implement a system that is efficient and inclusive to ensure that access to technology and mobility will not be barriers to vaccination.
There are a number of limitations that need to be considered when interpreting our findings. First, we were not able to have representation from the A5 priority group (indigent population). While we initially were able to get a participant from this group based on the survey response, we later found during the interview that this individual belonged to a different vaccination priority classification. This may point to issues with online data collection where researchers are unable to reach individuals from low-resource households. Second, there may be social desirability bias because we were unable to ensure if the respondent had other people with them that may have caused a change in their responses. Additionally, we did not disclose any political affiliations and interests, but participants may have been cautious in mentioning negative experiences related to vaccination. Participants may also have chosen more positive responses considering our background as health researchers. However, we emphasised that they will remain anonymous and their data treated with utmost confidentiality. Lastly, factors influencing COVID-19 vaccination uptake is context-specific, and this paper does not aim to represent all situations and circumstances.
Challenges to COVID-19 vaccination may be individual, interpersonal, and/or structural, which interact to influence decisions. Individual perceptions play a major role in the decision to vaccinate, and such perceptions are shaped by exposure to (mis)information amplified by the media, the community, and the health system. In the Philippines, vaccine brand hesitancy and misinformation are prevalent due to their rapid spread through social media and sensationalism in traditional media. Information on the effectiveness of safety of vaccines regardless of brand needs to be communicated to the public to increase COVID-19 vaccine confidence. At the interpersonal level, exposure to networks and health workers who are opposed to vaccines heightens public skepticism of vaccination. Structural barriers including political issues and poor implementation further contribute to vaccine refusals. The ongoing infodemic and anti-vaccination sentiments operating at all three levels (individual, interpersonal, structural) require empowering individuals to evaluate health information, and therefore health literacy becomes a critical tool to combat misinformation. Families and peers also need to be involved in these discussions as they influence vaccine uptake. Individuals engaged in vaccine promotion activities may need to be retrained on how to engage with vocal vaccine deniers in public. Given the involvement of traditional media, trainings on public health and science communication may be helpful in reporting vaccination-related news. Public figures need to disclose conflicts of interests and be transparent to the public, laying out the risks and benefits of vaccines. Laws should be well-implemented and equally implemented regardless of socioeconomic class or social position to encourage trust in the health care system and in vaccination initiatives. There is also a need to study best practices in vaccine rollout to implement systems that are efficient and inclusive so that we can vaccinate as many people against COVID-19 as quickly and as inclusively as possible: provide technological support particularly among older populations and allow flexible options for receiving the vaccine such as home vaccination. Given resource limitations, the vaccination rollout could also be improved by increasing the role of the private sector in the rollout and administration of the vaccine. The government and health organisations will need to connect with individuals, communities, and other institutions, including those who are against vaccines or hesitant towards vaccines, to co-create effective and sustainable solutions.
Acknowledgments
We would like to thank Michelle Edillon, Kriselle Abcede, Ryan Molen, and Josef Bondoc for their invaluable support to this project. We provide credit to BioRender.com for the figures illustrated in this paper. Finally, we are grateful to our participants who generously shared their stories with us.
- View Article
- Google Scholar
- PubMed/NCBI
- 34. Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. London, Great Britain: SAGE Publications Ltd; 2006.
- 35. Strauss A, Corbin J. Grounded theory methodology. Handbook of qualitative research. New York: Sage; 1994. pp. 273–85.
- 51. Department of Health (Philippines). Implementing rules and regulations of Republic Act No. 11525 known as "An Act Establishing the COVID-19 Vaccination Program Expediting the Vaccine Procurement and Administration Process, Providing Funds Therefor, and For Other Purposes | Department of Health website. 2021 [Cited 16 Sep 2021]. Available from: https://doh.gov.ph/IRR-of-RA11525-An-Act-Establishing-the-COVID-19-Vaccination-Program-Expediting-the-Vaccine-Procurement-and-Administration-Process-Providing-Funds-Therefor-and-For-Other-Purposes
- Open access
- Published: 14 July 2023
Do peer-based education interventions effectively improve vaccination acceptance? a systematic review
- Elisa L. S. Gobbo 1 ,
- Claudia Hanson 1 ,
- Khadija S. S. Abunnaja 1 &
- Sibylle Herzig van Wees 1
BMC Public Health volume 23 , Article number: 1354 ( 2023 ) Cite this article
1623 Accesses
3 Citations
1 Altmetric
Metrics details
Vaccination efforts are a vital part of controlling the spread of diseases, however, lack of vaccine acceptance undermines the efficacy of this public health effort. Current evidence suggests that the most effective interventions to support vaccination uptake and positive vaccination beliefs are multicomponent, and dialogue based. Peer-based education interventions are such a strategy that involves an individual within the same group to act as the vaccine educator.
This review aims to consolidate the quantitative evidence surrounding the effectiveness and experience of peer-based education initiatives to improve vaccination beliefs and behaviors.
We conducted a systematic search of PubMed, Web of Science, and a hand reference search. The search was conducted between April and June 2022. The inclusion criteria encompassed using peers, being education based, and being an intervention that addresses vaccination beliefs and behaviors (e.g. vaccination uptake).
Systematic screening revealed 16 articles in the final review. Half of the studies focused on students as their study population. The human papillomavirus vaccine was the most common vaccine assessed in the studies, followed by COVID and influenza vaccines. 11 out of 16 of the articles reported a positive impact of the peer intervention and two studies had mixed results. Six studies suggest a mixed peer- healthcare expert approach.
Conclusions
Despite reported positive effects of using peer-education based initiatives to improve vaccine uptake and beliefs, this systematic review reveals that there is limited existing research in support of this strategy. The strategies that initially appear the most effect are those with a combined peer and health-expert approach, and those that have more group specific and long-term peer interventions. More research is needed to confirm these results and to assess the effectiveness of a peer-based education intervention in a wider variety of settings and for other vaccine types.
Peer Review reports
Introduction
Vaccines are indispensable for reducing disease morbidity and mortality. Low vaccine uptake and limited confidence in vaccines harms this endeavor [ 1 ]. The COVID-19 pandemic illustrated this problem. Based on surveys of general public opinions, rates of COVID-19 vaccine acceptance vary from 97.0% in Ecuador to as low as 23.6% in Kuwait [ 2 ]. While the rates of COVID-19 vaccine uptake have been making headlines, limited confidence in vaccines is a well-established phenomenon that applies to almost all vaccines [ 1 ]. This topic has been widely discussed within the vaccine hesitancy literature.
Vaccine hesitancy is a complex phenomenon that MacDonald (2015) described as the delay in acceptance or refusal of vaccines despite availability of vaccination services [ 3 ]. This definition has been challenged by H Larson (2022), M Goldberg (2021), and Bussink-Voorend et al. (2022) who propose that vaccine hesitancy should be defined as “a state of indecisiveness regarding a vaccination decision” [ 4 , 5 , 6 ]. Whilst MacDonald defines vaccine hesitancy as a behavior, the latter highlight it as a process of decision making [ 3 ]. Much research focuses on vaccine confidence or vaccine acceptance as more tangible and positive approaches [ 7 ]. Scholars have formulated a vaccine hesitancy determinants matrix that includes contextual influences, individual and group influences, and vaccine specific issues [ 3 ]. Overcoming these complex, historical, political and socio-cultural factors is not a simple task, and addressing them requires various interventions [ 8 ]. This systematic review includes studies that focus on vaccine hesitancy, vaccine acceptance, vaccine knowledge and beliefs, vaccine confidence and vaccination uptake. We recognize the diversity within these definitions and concepts, and the limits of them to fully capture vaccine knowledge, beliefs, and behavior. For the purpose of this review, we refer to vaccine beliefs and uptake to capture a broad range of widely discussed definitions.
Peer-based education interventions are refined and population specific interventions with the potential to increase vaccine uptake [ 9 , 10 , 11 ]. This strategy allows for improved cultural competencies taking into account many sociocultural and population characteristic factors [ 10 , 11 ]. Prior studies have shown the benefits of peer-based interventions for improving other health behaviors [ 12 , 13 ]. However, despite the mention of peer-based education in several reviews of vaccine beliefs and uptake[ 9 , 10 ] to our knowledge, there is no comprehensive review of the effectiveness of and experience with peer-based education interventions for vaccination. Jama et al. postulate that peer-to-peer interventions could be a strategy for overcoming some of the barriers to vaccine uptake as the peers can lead by example and act as vaccine ambassadors [ 14 ]. There is also some evidence to show that peers in community-based intervention can improve vaccine coverage [ 15 , 16 ]. This review aims to summarize the quantitative evidence surrounding the effectiveness and experience of peer-education initiatives that have been implemented.
Search strategy
For the systematic review, we conducted searches of Web of Science and PubMed. The review search was conducted between April and June 2022, then again in June 2023. We ran the searches with all of the combined search terms using the Boolean Operators. For Web of Science, all terms were searched with the ALL category, and for PubMed the advanced search setting was used with All fields. The search terms used for both databases were (Vaccine), (Vaccin*), (Vaccine hesitancy), (vaccine confidence), (vaccine coverage), (vaccination refusal), (vaccine-preventable diseases), (immunization), (peer education initiatives), (peer group*), (peer education), (Peer-to-peer), (peer-to-peer support), (peer), (health education), (vaccin* education), (education*), (Health knowledge, attitudes, practices), (patient education), (intervention). In addition, to the database searching, a hand search of article references was conducted. In relevant systematic reviews and pertinent articles, a researcher did an initial screening of the references to find other articles for inclusion. From the results of the database search, we first did a duplicate deletion using the EndNote software [ 17 ]. Then two members of the research team determined inclusion for the screening process. Two members of the team screened the articles that were sought for retrieval.
In the literature there is no single definition of a peer-based education intervention. For the purpose of this study, we define a peer as individuals with key shared characteristics, circumstance or experiences, and who do not have professional training [ 18 ]. Simoni et al. (2011) make a distinction between a peer intervener who acts to improve health behavior and has shared characteristics, rather than the colloquial peer as someone with equal standing [ 18 ]. For the purpose of this paper, “peer” will mean a “peer intervener.” In the case of a peer-based health intervention, they frequently collaborate with more qualified service providers [ 18 ]. Peer’s roles might include advocating, connecting people to resources, conveying information, and offering assistance [ 18 ]. Further, the majority of peer-based interventions include some kind of educational delivery. The core idea is that peer education is responsive to the values and objectives of the target group [ 18 ]. Peer-based education interventions allow for an individual affected by the same disease or among the same social group to provide vaccine and health information to peers in a more culturally or socially relevant manner than a health educator or provider [ 19 , 20 ].
The guiding criteria for inclusion was that the articles need to have a peer-based education intervention on vaccination uptake and beliefs. Articles included had the term peer described above [ 18 ]. We defined an education intervention as an effort to improve knowledge or awareness on vaccination by providing some type of education via lecture, paper or digital information, tabling event, group meetings, or other means [ 18 , 21 ]. We included articles that assessed an intervention that focused on addressing vaccination uptake and beliefs as their outcomes. This includes articles that addressed vaccine uptake, willingness-to-vaccine, vaccine hesitancy, and measuring knowledge and beliefs of vaccines.
Each screener assigned the article with a level; definitely fits, probably fits, most likely does not fit, and most likely excluded. This was a self-developed screening tool to guide the screening process. The articles screened as definitely fits and most likely excluded by both screeners were then directly included or excluded. Then based on discussion and input from a third member of the team, articles in probably fits or most likely does not fit were determined for inclusion [ 17 , 21 ]. Having two options in the middle allowed for the researchers to have more nuance in identifying the article’s likelihood of inclusion and assisted with the conversations with the third team member.
In June 2023, the search was rerun on both PubMed and Web of Science to check for articles published in the year since the initial search. The same search terms and inclusion/exclusion criteria were utilized. On PubMed, the filter of article published last year was used to assess for new articles, and for Web of Science the filter for articles published in 2022 or 2023 was used.
Data extraction and data analysis
For the data extraction, we modified a standardized systematic review extraction form [ 22 ]. One researcher conducted the extraction, and a second researcher double-checked the extraction results. Any discrepancies in data extraction were discussed among the authors and adjustments were made based on agreed upon reading or interpretation of the articles.
Quality assessment
We utilized the Effective Public Health Practice Project quality assessment tool for quantitative studies to assess all included articles for their quality [ 23 ]. Articles were scored as 1 – Strong, 2 – Moderate, 3 – Weak based on assessment of study design, methods used, biases, and more (Additional file 1)[ 23 ]. Two researchers conducted the quality assessment and then matched the two scores. Our average quality score was 1.63 (Table 1 ) [ 22 ]. This indicates that there was a a moderate quality of research papers presented in this review. Overall, the many aspects of the study designs and testing were of high quality, but due to the nature of the interventions often participant randomization or blinding were not conducted [ 23 ].
The systematic review revealed 3927 articles from PubMed and Web of Science, and 175 from citation searching (Fig. 1 ). After removing duplicates, and screening for eligibility, 11 articles were included from database searching. In addition, two eligible articles from citation searching and three articles from the June 2023 search rerun were identified making a total of 16 eligible articles included in this review. One article was excluded due to inability to access the full article.
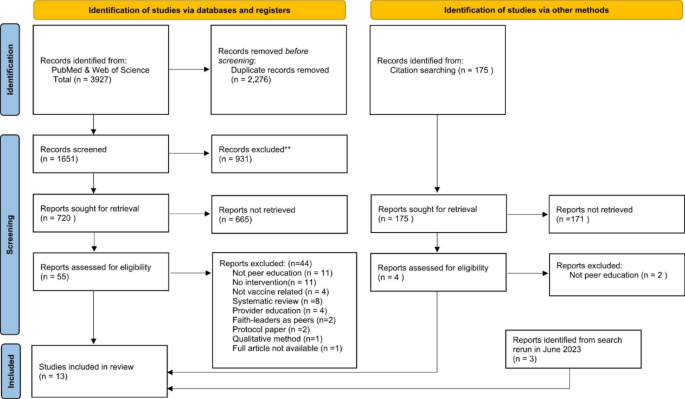
PRISMA flow diagram of systematic review. Strategy PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources From : Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: https://doi.org/10.1136/bmj.n71 . For more information, visit: http://www.prisma-statement.org/
Table 1 summarizes all of the results. For the study populations, eight articles studied students, four of which focused on women, two studied senior citizens, and six studied other populations (parents, Japanese general population, gay men, essential workers, and the Diné/Navajo). Studies took place in five different countries with 11 in the United States, two in Poland, and one study in Japan, Scotland, and Nigeria. The vaccines assessed were COVID-19 (six), followed by HPV (human papilloma virus) (five), influenza (three), then childhood vaccination (one), and hepatis B virus (one). The various study methods utilized in the articles were surveys (seven), randomized control trials (six), population vaccination rate (two), and mixed methods with surveys and interviews (one).
The studies covered a variety of primary outcome measures as a strategy to assess a peer education-based intervention for vaccine beliefs and outcomes. Five of them measured a change in vaccination rate [ 24 , 25 , 26 , 27 , 28 ], four collected vaccination uptake rates [ 29 , 30 , 31 , 32 ], three gathered willingness to vaccinate [ 33 , 34 , 35 ], another three surveyed the knowledge and beliefs of participants [ 36 , 37 , 38 ], and one study assessed the satisfaction with the intervention [ 39 ]. Overall, 13 of 16 studies reported a positive impact of the peer intervention, in the studies’ outcome measure [ 24 , 25 , 26 , 27 , 28 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ]. A positive impact could mean improved knowledge or intention to vaccine or improved vaccination rates or uptake of vaccines. Three studies had mixed results with some results showing improvement, but some outcomes not being statistically significant [ 30 , 32 , 40 ]. Six out of the 16 studies utilized or suggest a mixed peer and healthcare expert approach [ 25 , 28 , 31 , 33 , 36 , 38 , 39 , 40 ].
The different intervention strategies used were grouped into a quick chat with a peer [ 6 ], a workshop/lecture approach [ 5 ], a narrative- onetime approach [ 3 ], and repeated contact with a peer [ 2 ]. The interventions utilizing a quick chat with a peer strategy involved peer education tables or promotions [ 26 , 30 ], having peers provide information and support during an immunization clinic [ 27 , 28 , 31 ], and peer phone-calls to unvaccinated individuals [ 24 ]. Even though this was the least intensive intervention strategy, five studies had a positive impact on vaccination with increased vaccination rates compared with baselines [ 24 , 26 , 27 , 28 , 31 ]. Several other studies used a one-time workshop or lecture by peers to provide information on vaccination[ 34 , 36 , 37 , 38 , 40 ]. These mostly involved a peer training by public health professionals and then an educational session. All studies, except one [ 40 ], had positive impact on either knowledge and beliefs or willingness to vaccinate. The Fenick, et al. study had a mixed impact, but utilized a slightly different intervention strategy with group wellness visits with parents to encourage peer support for childhood vaccination [ 40 ]. Three studies used a narrative-onetime approach that had peer-based messaging provided online and assessed the impact on willingness to vaccinate, all of which had a positive impact [ 33 , 35 , 39 ]. Lastly, two studies used more intensives strategy with repeated peer contact. One was via a two-month long programing among seniors [ 25 ], and one had a peer led Facebook group among essential workers [ 32 ]. Both studies had an increase in vaccination uptake or interest in vaccination information.
Several studies (nine) showed improvements in the uptake of vaccines [ 24 , 25 , 26 , 27 , 28 , 31 , 33 , 34 , 38 ] [ 33 ]. In an intervention at a senior living center the immunization rates for influenza and pneumococcal rose by a greater percentage for those in the intervention building than the control over the two years [ 24 ]. Similarly, in a population of low income and minority adults with a repeated peer multilevel participatory intervention there was a 40.6% increase in the intervention group compared with only an 18% increase in the control group [ 25 ]. A narrative intervention to improve HPV knowledge and vaccination in college aged women, found that the combined peer and health expert intervention had a nearly double rate of HPV vaccination at two months compared with the control [ 39 ]. In a study that utilized peer ambassadors at same-day COVID-19 vaccination clinics, 197 more individuals were vaccinated at centers with peers compared with those without peers [ 31 ].
Peer intervention also appears to improve knowledge and intention to vaccinate. In a study of students regarding COVID-19, the intervention had a slight increased willingness to vaccinate from 31.8 to 35.2% [ 34 ]. After receiving messaging on HPV by trained students, female adolescence in Nigeria mean knowledge score increased from 12.94 to 53.74 [ 37 ]. Unlike the other studies, these two focused on vaccine knowledge and beliefs, rather than changes in vaccination uptake.
Some studies found a mixed result. In a study with a group-based general wellness checkup visit, compared with regular individual visits, they found that there was no difference between the two groups in terms of vaccination timeliness at 2 and 4 months. However, the infants were more likely to be immunized on schedule at 6 months and 12 months, and to attend all 6 visits [ 40 ]. A study conducted at a university among Chinese international students found limited knowledge levels on HPV, but 94.9% were interested in receiving the vaccine [ 28 ]. However, of the first 400 students educated only 80 actually visited the health center for vaccination [ 28 ]. Therefore, whilst the education was arguably successful, it did not lead to significant uptake of the HPV vaccine.
This systematic review of peer-education based interventions to improve vaccine uptake and beliefs revealed several benefits of peer-based interventions. Thirteen studies illustrated some positive effect of the peer-based education intervention on the intended outcomes [ 24 , 25 , 26 , 27 , 28 , 33 , 34 , 35 , 36 , 37 , 39 ]. This shows that the peer-education based interventions could be a useful tool for improving vaccine uptake and beliefs. Of the 13 studies reporting a positive effect, six combined the peer-based education intervention with a health expert intervention. The studies that were using mixed approaches demonstrated a stronger impact on their outcomes, than studies that only used one intervention type [ 25 , 28 , 33 , 36 , 39 , 40 ]. Thus, future studies or interventions should consider using the potential benefits of utilizing a combination health expert and peers’ approach to encourage vaccination.
Furthermore, the different specific intervention strategies appear to affect the efficacy of the intervention. Although unable to directly compare results, there are some trends that can be seen in the review. Studies that only provided a quick chat with a peer intervener through tabling or a bar intervention seem to have the weakest impact on vaccination and vaccine knowledge [ 26 , 27 , 28 , 30 , 31 , 35 ]. However, those at universities are more successful than others [ 27 , 28 ]. The one-time workshop interventions seem to have some limited success in improving vaccine knowledge [ 34 , 36 ]. The one-time narrative approaches seem be even a bit more effective in improving knowledge [ 37 , 38 , 39 ], and one even reported doubling the HPV vaccination rate among students receiving the intervention [ 33 ]. The two studies with repeated peer interventions had statistically significant increases in vaccination rates, indicating the strength of this strategy [ 25 , 32 ]. This indicates that a more targeted and repeated approach, appear to have more potential than more generic, one-time interventions.
It is important to note that the peer education interventions presented in these studies were mostly used to encourage vaccination rather than to address vaccinate hesitancy or refusal. Seven of the studies focused on improving vaccine uptake or a change in vaccination rates among groups that were not yet vaccinated such as international students, children, or seniors [ 24 , 25 , 26 , 27 , 28 , 32 , 40 ]. Thus, the peer-based interventions show a generally positive effect on vaccine uptake and beliefs, but apart from one paper, - on the Diné (Navajo) population which is known to be vaccinate hesitant rooted in governmental mistrust [ 34 ] - there was no research on the effectiveness of peer-based educational interventions in communities that present low levels of vaccine confidence. This suggests that there is limited evidence for the use of peer- based interventions to address vaccine hesitancy, which is rooted in specific communities for example, migrant communities, anthroposophic or religious communities, or online communities.
Limitations
There are limitations to the generalizability of these results to diverse range of population groups and vaccine types. The scope of the results is also restricted as only one study took place in a middle-income country [ 37 ], none in a low-income country, and the majority were set in the United States [ 24 , 25 , 27 , 28 , 30 , 31 , 32 , 33 , 38 , 39 , 40 ]. Several United States-based studies (ten) demonstrated a positive impact, pointing to encouraging benefits of a peer intervention in a high-income setting amongst adults [ 24 , 25 , 27 , 28 , 31 , 32 , 33 , 38 , 39 , 40 ]. However, the limited geographic scope curtails the generalizability of the evidence found in the systematic review. One study in Nigeria showed promising results with an illustrated improvement in awareness and knowledge regarding the HPV vaccine and cervical cancer. The study did not explore the impact on HPV uptake; thus, the effect remains poorly understood [ 37 ]. To understand the potential of peer-based education initiatives for different areas it is necessary to conduct more research in economically and geographically diverse regions.
Additionally, more long-term studies may be required to assess the sustainability of effects of peer-based education intervention. For example, two studies demonstrate the effects of a peer-education on either knowledge or vaccination rates for up to one year [ 24 , 40 ]. For some vaccines that require a repeated yearly dosage, such as influenza, it may be pertinent to assess the effects of peer-based education interventions for more long-term sustainability.
There are several limitations to this systematic review. Publication bias could be a limitation of the results. We found no articles with a negative outcome. Research with less favorable results could have been conducted but not published. Some studies also could have been missed due to the utilization of different terminology other than peer. The quality of the studies found was mixed. One consistent issue throughout the studies was the inability to blind the participants due to the nature of the interventions. We also had to exclude one article due to an inability to access the full article.
Recommendations
Based on the limitations of the results, we recommend further research to examine the full benefits of a peer-based education approach, particularly for different population groups (not only adults), vaccination types (particularly childhood vaccines), and geographical regions (particularly in low-income settings). Given that we found that the benefits of peer-based education intervention to address vaccine beliefs and uptake are inconclusive, we recommend further studies that apply strong methods such as randomized controlled trial. This would provide greater generalizability of the results and provide clearer guidance for policy making for public health promotion. A peer-based intervention may work better in certain setting than in others, but the positive nature of the results implies that the strategy should be proliferated more in research and interventions [ 8 ]. More studies into peer-based interventions with known vaccine hesitant populations would be useful to determine if they are useful for addressing specific vaccine fears or rumors and not just for encouraging vaccination. Also, conducting a randomized controlled trial comparing the effects of a single peer-based and a combined (health expert and peer) would be beneficial for strengthening the findings.
In conclusion, there is limited existing research on peer-based education interventions to improve vaccine beliefs and uptake. The research that exists illustrates the promise of this approach for certain vaccines and populations. To fully assess the effectiveness of peer-based education further research into this strategy for different peer groups, in different parts of the world, and using different methodological approaches is required. Importantly, further research must examine peer-based education interventions in vaccine hesitant communities [ 8 ]. Implementing vaccine education by peers could help to address socio-cultural barriers through a culturally competent addition to traditional vaccine interventions. Whilst the claim of the value of peer-based education system continues to be made in the literature, more solid evidence on best approaches is needed.
Data Availability
The datasets used and/or analysed during the current study is available from the corresponding author on reasonable request.
Abbreviations
Human Papillomavirus
Ali KA, Celentano LP, ADDRESSING VACCINE HESITANCY, IN THE “POST-TRUTH” ERA. Eurohealth Int Eurohealth. 2017;23(4).
Sallam M. COVID-19 Vaccine Hesitancy Worldwide: a concise systematic review of Vaccine Acceptance Rates. Vaccines (Basel). 2021 Feb 16;9(2):160.
MacDonald NE, SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015 Aug;33(34):4161–4.
Larson HJ, Schulz WS, Tucker JD, Smith DMD. Measuring Vaccine Confidence: Introducing a Global Vaccine Confidence Index. PLoS Curr [Internet]. 2015 Feb 25 [cited 2023 Jul 4];7(OUTBREAKS). Available from: /pmc/articles/PMC4353663/.
Bussink-Voorend D, Hautvast JLA, Vandeberg L, Visser O, Hulscher MEJL. A systematic literature review to clarify the concept of vaccine hesitancy. Nat Hum Behav [Internet]. 2022 Dec 1 [cited 2023 Jul 4];6(12):1634–48. Available from: https://pubmed.ncbi.nlm.nih.gov/35995837/ .
Goldenberg MJ. Vaccine hesitancy - Public Trust, Expertise and the War on Scince. Universty of Pittsburgh Press; 2021.
Dubé È, Ward JK, Verger P, Macdonald NE. Vaccine Hesitancy, Acceptance, and Anti-Vaccination: Trends and Future Prospects for Public Health. Annu Rev Public Health [Internet]. 2021 [cited 2023 Jul 4];42:175–91. Available from: https://doi.org/10.1146/annurev-publhealth .
Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy. http://dx.doi.org/104161/hv24657 [Internet]. 2013 Aug [cited 2023 Mar 5];9(8):1763–73. Available from: https://www.tandfonline.com/doi/abs/10.4161/hv.24657 .
Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ, Eskola J, et al. Strategies for addressing vaccine hesitancy - A systematic review. Volume 33. Vaccine: Elsevier Ltd; 2015. pp. 4180–90.
Google Scholar
Shon EJ, Choe S, Lee L, Ki Y, Influenza Vaccination Among US. College or University students: a systematic review. Am J Health Promotion 2021 Jun 3;35(5):708–19.
Abahussin AA, Albarrak AI. Vaccination adherence: review and proposed model. J Infect Public Health 2016 Nov 1;9(6):781–9.
Posavac EJ, Kattapong KR, Dew DE. Peer-Based Interventions to Influence Health-Related Behaviors and Attitudes: A Meta-Analysis. http://dx.doi.org/102466/pr01999853f1179 [Internet]. 2016 Sep 1 [cited 2023 Mar 5];85(3 PART 2):1179–94. Available from: https://journals.sagepub.com/doi/ 10.2466/pr0.1999.85.3 f.1179?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
Webel AR, Okonsky J, Trompeta J, Holzerner WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. Am J Public Health [Internet]. 2010 Feb 1 [cited 2023 Mar 5];100(2):247–53. Available from: https://doi.org/10.2105/AJPH.2008.149419 .
Jama A, Appelqvist E, Kulane A, Karregård S, Rubin J, Nejat S et al. Design and implementation of tailored intervention to increase vaccine acceptance in a Somali community in Stockholm, Sweden - based on the Tailoring Immunization Programmes approach. Public Health in Practice. 2022 Dec 1;4:100305.
Findley SE, Irigoyen M, Sanchez M, Stockwell MS, Mejia M, Guzman L et al. Effectiveness of a Community Coalition for Improving Child Vaccination Rates in New York City. Am J Public Health [Internet]. 2008 Nov 1 [cited 2023 Mar 13];98(11):1959. Available from: /pmc/articles/PMC2636428/.
Findley SE, Irigoyen M, Sanchez M, Guzman L, Mejia M, Sajous M et al. Community-based strategies to reduce childhood immunization disparities. Health Promot Pract. 2006 Jul;7(3 Suppl).
EndNote. EndNote [Internet]. [cited 2023 Mar 13]. Available from: https://www.myendnoteweb.com/EndNoteWeb.html .
Simoni JM, Franks JC, Lehavot K, Yard SS. Peer interventions to promote health: conceptual considerations. Am J Orthopsychiatry. 2011;81(3):351–9.
Article PubMed PubMed Central Google Scholar
Ramchand R, Ahluwalia SC, Xenakis L, Apaydin E, Raaen L, Grimm G. A systematic review of peer-supported interventions for health promotion and disease prevention. Prev Med (Baltim). 2017 Aug;1:101:156–70.
Nelson KM, Yang JP, Molina Y, Simoni JM. Peer-Based Intervention Approaches. Encyclopedia of AIDS [Internet]. 2014 [cited 2023 Mar 5];1–4. Available from: https://link.springer.com/referenceworkentry/10.1007/ 978-1-4614-9610-6_94-1.
Abdi F, Simbar M. The Peer Education Approach in Adolescents- Narrative Review Article. Iran J Public Health [Internet]. 2013 [cited 2023 Mar 8];42(11):1200. Available from: /pmc/articles/PMC4499060/.
Emily Jones MA, UNC Health Science Library. 2023 [cited 2023 Mar 8]. LibGuides: Systematic Reviews: Step 7: Extract Data from Included Studies. Available from: https://guides.lib.unc.edu/systematic-reviews/extract-data .
Effective Public Healthcare Panacea Project. EPHPP. 2023 [cited 2023 Mar 6]. Quality Assessment Tool for Quantitative Studies. Available from: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/ .
Krieger JW, Castorina JS, Walls ML, Weaver MR, Ciske S. Increasing Influenza and Pneumococcal Immunization Rates: a randomized controlled study of a senior Center-Based intervention. Am J Prev Med. 2000.
Schensul JJ, Radda K, Coman E, Vazquez E. Multi-level intervention to prevent influenza infections in older low income and minority adults. In: Am J Community Psychol. 2009. p. 313–29.
Flowers P, Hart GJ, Williamson LM, Ba F, Der Bsc GJ. Does bar-based, peer-led sexual health promotion have a community-level effect amongst gay men in Scotland? Int J STD AIDS. 2002;13:102–8.
Article CAS PubMed Google Scholar
Huang JJ, Francesconi M, Cooper MH, Covello A, Guo M, Gharib SD. Community health workers on a college campus: Effects on influenza vaccination. J Am Coll Health. 2018 May;19(4):317–23.
Long AG, Roberts CM, Hayney MS. Pharmacy student involvement with increasing human papillomavirus (HPV) vaccination among international college students. J Am Pharmacists Association. 2017 Jan;57(1):127–8.
Fenick AM, Leventhal JM, Gilliam W, Rosenthal MS. A randomized controlled trial of Group Well-Child Care: Improved Attendance and Vaccination Timeliness. Clin Pediatr (Phila). 2020 Jun 1;59(7):686–91.
Esagoff A, Cohen S, Chang G, Equils O, Sarah ;, Orman V et al. 2531. Using peer-to-peer education to increase awareness and uptake of HPV Vaccine among Chinese International Students. Open Forum Infectious Disease. 2022.
Shover CL, Rosen A, Mata J, Robie B, Alvarado J, Frederes A et al. Engaging Same-Day Peer Ambassadors to Increase Coronavirus Disease 2019 Vaccination Among People Experiencing Unsheltered Homelessness in Los Angeles County: A Hybrid Feasibility-Evaluation Study. 2019 [cited 2023 Jun 27]; Available from: https://doi.org/10.1093/infdis/jiac291 .
Ugarte DA, Young S. Effects of an Online Community Peer-support Intervention on COVID-19 Vaccine Misinformation Among Essential Workers: Mixed-methods Analysis. Western Journal of Emergency Medicine [Internet]. 2023 [cited 2023 Jun 27];264(2). Available from: http://escholarship.org/uc/uciem_westjem .
Hopfer S. Effects of a narrative HPV vaccination intervention aimed at reaching College Women: a Randomized Controlled Trial. Prev Sci. 2012 Apr;13(2):173–82.
Pasek O, Michalska J, Piechowicz M, Stoliński M, Ganczak M. Effect of peer-education on willingness to vaccinate against COVID-19 among high school students. European Public Health Conference. 2021.
Sasaki S, Saito T, Ohtake F. Nudges for COVID-19 voluntary vaccination: how to explain peer information? Soc Sci Med. 2022 Jan 1;292.
Ganczak M, Pasek O, Duda-Duma Ł, Komorzycka J, Nowak K, Korzeń M. A peer-based educational intervention effects on SARS-CoV-2 knowledge and attitudes among polish high-school students. Int J Environ Res Public Health. 2021 Nov 1;18(22).
Sadoh AE, Okonkwo C, Nwaneri DU, Ogboghodo BC, Eregie C, Oviawe O, et al. Effect of peer education on knowledge of human papilloma virus and cervical cancer among female adolescent students in Benin city, Nigeria. Ann Glob Health. 2018;84(1):121–8.
Tutt M, Begay C, George S, Dickerson C, Kahn C, Bauer M et al. Diné teachings and public health students informing peers and relatives about vaccine education: providing Diné (Navajo)-centered COVID-19 education materials using student health messengers. Front Public Health. 2022 Dec 14.
Kim M, Lee H, Kiang P, Allison J. Development and acceptability of a peer-paired, cross-cultural and cross-generational storytelling HPV intervention for korean american college women. Health Educ Res. 2019 Oct;34(1):483–94.
Fenick AM, Leventhal JM, Gilliam W, Rosenthal MS. A Randomized Controlled Trial of Group Well-Child Care: Improved Attendance and Vaccination Timeliness. https://doi.org/101177/0009922820908582 [Internet]. 2020 Feb 28 [cited 2023 Mar 8];59(7):686–91. Available from: https://journals.sagepub.com/doi/ https://doi.org/10.1177/0009922820908582 .
Download references
Acknowledgements
none needed.
Sibylle Herzig van Wees is funded by Forte - Forskningsrådet för hälsa, arbetsliv och välfärd (2021 − 01299) to work on vaccine confidence in Sweden.
Open access funding provided by Karolinska Institute.
Author information
Authors and affiliations.
Department of Global Public Health, Karolinska Institutet, Solna, 171 65, Sweden
Elisa L. S. Gobbo, Claudia Hanson, Khadija S. S. Abunnaja & Sibylle Herzig van Wees
You can also search for this author in PubMed Google Scholar
Contributions
E.G. and S.H.vW conceptualized the project and wrote the manuscript. C.H. contributed to the writing of the article. E.G. and K.A. conducted the systematic review under supervision of SHvW, EG and KA conducted the screening and the quality assessment. All authors reviewed the final manuscript.
Corresponding author
Correspondence to Elisa L. S. Gobbo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gobbo, E.L.S., Hanson, C., Abunnaja, K.S.S. et al. Do peer-based education interventions effectively improve vaccination acceptance? a systematic review. BMC Public Health 23 , 1354 (2023). https://doi.org/10.1186/s12889-023-16294-3
Download citation
Received : 23 April 2023
Accepted : 12 July 2023
Published : 14 July 2023
DOI : https://doi.org/10.1186/s12889-023-16294-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vaccine hesitancy
- Vaccine acceptance
- Intervention
- Systematic review
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Expanding a lymph node, boosting a vaccine
A biomaterial vaccine enhances and sustains lymph node expansion following vaccination, boosting anti-tumor immunity in an animal model.
The authors used a method know as high-frequency ultrasound to monitor individual lymph nodes in MPS- and control-vaccinated mice. The top row shows a series of lymph nodes on day 7 following MPS vaccination. All of them were significantly expanded, compared to lymph nodes following control vaccination imaged at the same time and shown in the bottom row. (Credit: Wyss Institute at Harvard University)
Each one of us has around 600 lymph nodes (LNs) – small, bean-shaped organs that house various types of blood cells and filter lymph fluid – scattered throughout our bodies. Many of us have also experienced some of our LNs to temporarily swelling during infections with viruses or other pathogens. This LN expansion and subsequent contraction can also result from vaccines injected nearby, and in fact is thought to reflect the ongoing vaccine immune response. While researchers have studied the early expansion of LNs following vaccination, they have not investigated whether prolonged LN expansion could affect vaccine outcomes.
Now, for the first time, researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), the Wyss Institute for Biologically Inspired Engineering at Harvard University , and Genentech , a member of the Roche Group, found a way to enhance and extend LN expansion, and study how this phenomenon affects both the immune system and efficacy of vaccinations against tumors. Key to their approach was a biomaterial vaccine formulation that enabled greater and more persistent LN expansion than standard control vaccines. While the oversized LNs maintained a normal tissue organization, they displayed altered mechanical features and hosted higher numbers of various immune cell types that commonly are involved in immune responses against pathogens and cancers. Importantly, “jump-starting” lymph node expansion prior to administering a traditional vaccine against a melanoma-specific model antigen led to more effective and sustained anti-tumor responses in mice. The findings are published in Nature Biomedical Engineering .
“By enhancing the initial and sustained expansion of LNs with biomaterial scaffolds, non-invasively monitoring them individually over long time periods, and probing deeply into their tissue architecture and immune cell populations, we tightly correlate a persistent LN expansion with more robust immune and vaccination responses,” said David Mooney , the Robert P. Pinkas Family Professor of Bioengineering at SEAS, who led the study. “This opens a new front of investigation for immunologists, and could have far-reaching implications for future vaccine developments.”
Mooney is also a Wyss Institute Founding Core Faculty member.
Mooney’s team at SEAS and the Wyss Institute had previously developed different biomaterial scaffolds as a matrix for cancer and infection vaccines. The researchers have demonstrated the potential of biomaterial vaccine formulations to successfully fight the growth of tumors in an extensive body of work performed in preclinical animal models and a first clinical trial with cancer patients. But they hadn’t yet investigated how their vaccines and those developed by others could influence the response of LNs draining leaked tissue fluid at vaccine injection sites, and have an impact on the LNs tissue organization, different cell types, and their gene expression, which could in turn affect vaccine efficacy. In their new study, they tested a previously developed vaccine formulation that is based on microscale mesoporous silica (MPS) rods that can be injected close to tumors and form a cell-permeable 3D scaffold structure under the skin. Engineered to release an immune cell-attracting cytokine (GM-CSF), and immune cell-activating adjuvant (CpG), and tumor-antigen molecules, MPS-vaccines are able to reprogram recruited so-called antigen-presenting cells that, upon migrating into nearby LNs, orchestrate complex tumor cell-killing immune responses. Their new study showed that there are more facets to that concept.
“As it turns out, the immune-boosting functions of basic MPS-vaccines actively change the state of LNs by persistently enlarging their whole organ structure, as well as changing their tissue mechanics and immune cell populations and functions,” said first-author Alexander Najibi , who performed his Ph.D. thesis with Mooney.
Probing LNs with ultra-sound and nano-devices
To understand the response of LNs to MPS-vaccines over time, the team applied an ultra-sound imaging technique known as high-frequency ultrasound (HFUS). Similar to monitoring a tiny fetus developing in a mother’s womb by clinical ultra-sound, HFUS, on a much smaller scale, enables non-invasively and non-destructively monitoring of anatomical details of tissues and organs in small animals such as mice. Using HFUS, the team traced individual LNs in MPS-vaccinated mice over 100 days. They identified an initial peak expansion period that lasted until day 20, in which LN volumes increased about 7-fold, significantly greater than in animals that received traditional vaccine formulations. Importantly, the LNs of MPS-vaccinated mice, while decreasing in volumes after this peak expansion, remained significantly more expanded than LNs from traditionally vaccinated mice throughout the 100-day time course.
When Najibi and the team investigated the mechanical responses of the LNs using a nanoindentation device, they found that LNs in MPS-vaccinated animals, although maintaining an overall normal structure, were less stiff and more viscous in certain locations. This was accompanied by a re-organization of a protein that assembles and controls cells’ mechanically active cytoskeleton. Interestingly, Mooney’s group had shown in an earlier biomaterial study that changing mechanical features of immune cells’ environments, especially their viscoelasticity, affects immune cell development and functions.
“It is very well-possible that in order to accommodate the significant growth induced by MPS-vaccines, LNs need to become softer and more viscous, and that this then further impacts immune cell recruitment, proliferation, and differentiation in a feed-forward process,” said Najibi.
From immune cell engagement to vaccine responses
Upon MPS-vaccination, the numbers of “innate immune cells,” including monocytes, neutrophils, macrophages, and other cell types that build up the first wave of immune defenses against pathogens and unwanted cells, peaked first in expanding LNs. Peaking with a delay were dendritic cells (DCs), which normally transfer information in the form of antigens from invading pathogens and cancer cells to “adaptive immune cells” that then launch subsequent waves of highly specific immune responses against the antigen-producing invaders. In fact, along with DCs, also T and B cell types of the adaptive immune system started to reach their highest numbers.
“It was fascinating to see how the distinct changes in immune cell populations that we detected in expanding LNs in response to the MPS-vaccine over time re-enacted a typical immune response to infectious pathogens,” said Najibi.
Innate immune cells and DCs are also known as “myeloid cells,” which are known to interact with LN tissue during early expansion. To further define the impact of myeloid cells on LN expansion, Mooney’s team collaborated with the group of Shannon Turley , the VP of Immunology and Regenerative Medicine at Genentech, and an expert in lymph node biology and tumor immunology.
“The MPS-vaccine led to extraordinary structural and cellular changes within the lymph node that supported potent antigen-specific immunity,” said Turley.
By isolating myeloid cells from LNs and analyzing the gene expression profiles of individual cells (single cell RNA-seq), the groups were able to reconstruct distinct changes in myeloid cell populations during LN expansion, and identified distinct DC populations in durably expanded LNs whose changed gene expression was associated with LN expansion. In addition, the collaborators found that the number of monocytes was increased 80-fold upon MPS-vaccination – the highest increase among all myeloid cell types – and pinpointed subpopulations of “inflammatory and antigen-presenting monocytes” as promising candidates for facilitating LN expansion. In fact, when they depleted specific subpopulations of these types of monocytes from circulating blood of mice after vaccination, the maintenance of LN expansion, and timing of the T cell response to vaccination, was altered.
Finally, the team explored whether LN expansion could enhance the effectiveness of vaccination. “Jump-starting” the immune system in LNs with an antigen-free MPS-vaccine and subsequently administering the antigen in a traditional vaccine format significantly improved anti-tumor immunity and prolonged the survival of melanoma-bearing mice, compared to the traditional vaccine alone.
“The priming of lymph nodes for subsequent vaccinations using various formulations could be a low-hanging fruit for future vaccine developments,” said Mooney.
Other authors on the study are Ryan Lane, Miguel Sobral, Giovanni Bovone, Shawn Kang, Benjamin Freedman, Joel Gutierrez Estupinan, Alberto Elosegui-Artola, Christina Tringides, Maxence Dellacherie, Katherine Williams, Hamza Ijaz, and Sören Müller. The study was funded by the National Institutes of Health/National Cancer Institute (award# U54 CA244726 and R01 CA223255).
Topics: Bioengineering
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles

David Mooney
Robert P. Pinkas Family Professor of Bioengineering
Press Contact
Leah Burrows | 617-496-1351 | [email protected]
Related News
A new glue, potentially also for you
A new bonding method enabling instant and effective adhesion of hydrogels has potential to broadly advance new biomaterials solutions for multiple unmet clinical needs
Bioengineering , Health / Medicine
Repairing patients’ dura more durably
Highly adhesive and mechanically strong Dural Tough Adhesive addresses multiple limitations in the repair of the dural membrane lining the brain and spinal cord after trauma and surgeries

Using suction cups inspired by fish to listen in on whale conversations
Project CETI researchers develop gentle, resilient, and reversible tags
Bioengineering , Materials Science & Mechanical Engineering , Robotics
A positive association found between autism prevalence and childhood vaccination uptake across the U.S. population
Affiliation.
- 1 Department of Economics and Finance, Baruch College/City University of New York, New York, New York, USA. [email protected]
- PMID: 21623535
- DOI: 10.1080/15287394.2011.573736
The reason for the rapid rise of autism in the United States that began in the 1990s is a mystery. Although individuals probably have a genetic predisposition to develop autism, researchers suspect that one or more environmental triggers are also needed. One of those triggers might be the battery of vaccinations that young children receive. Using regression analysis and controlling for family income and ethnicity, the relationship between the proportion of children who received the recommended vaccines by age 2 years and the prevalence of autism (AUT) or speech or language impairment (SLI) in each U.S. state from 2001 and 2007 was determined. A positive and statistically significant relationship was found: The higher the proportion of children receiving recommended vaccinations, the higher was the prevalence of AUT or SLI. A 1% increase in vaccination was associated with an additional 680 children having AUT or SLI. Neither parental behavior nor access to care affected the results, since vaccination proportions were not significantly related (statistically) to any other disability or to the number of pediatricians in a U.S. state. The results suggest that although mercury has been removed from many vaccines, other culprits may link vaccines to autism. Further study into the relationship between vaccines and autism is warranted.
- Autistic Disorder / epidemiology*
- Child, Preschool
- Regression Analysis
- Speech Disorders / epidemiology
- United States / epidemiology
- Vaccination / adverse effects*
- Vaccination / statistics & numerical data
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 6, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Biomaterial vaccine enhances lymph node expansion following vaccination, boosting anti-tumor immunity
by Benjamin Boettner, Harvard University
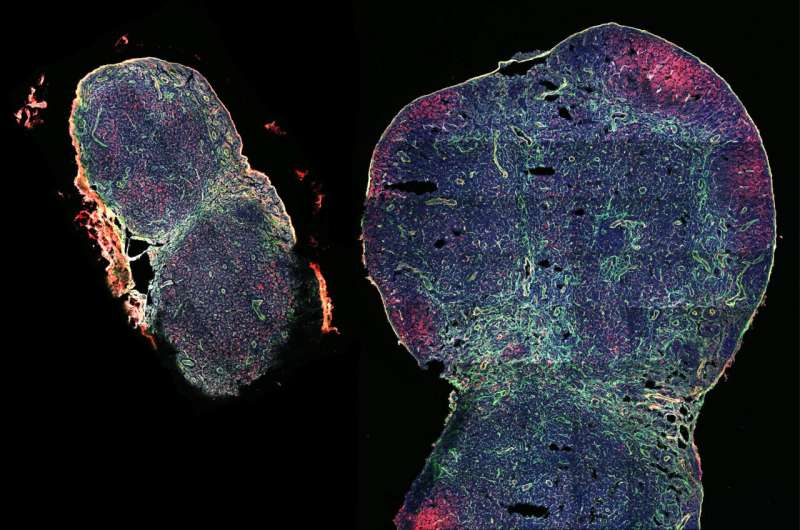
Each one of us has around 600 lymph nodes (LNs)—small, bean-shaped organs that house various types of blood cells and filter lymph fluid—scattered throughout our bodies. Many of us have also experienced some of our LNs to temporarily swelling during infections with viruses or other pathogens.
This LN expansion and subsequent contraction can also result from vaccines injected nearby, and in fact is thought to reflect the ongoing vaccine immune response. While researchers have studied the early expansion of LNs following vaccination, they have not investigated whether prolonged LN expansion could affect vaccine outcomes.
Now, for the first time, researchers from the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and Genentech, a member of the Roche Group found a way to enhance and extend LN expansion, and study how this phenomenon affects both the immune system and efficacy of vaccinations against tumors.
Key to their approach was a biomaterial vaccine formulation that enabled greater and more persistent LN expansion than standard control vaccines.
While the oversized LNs maintained a normal tissue organization, they displayed altered mechanical features and hosted higher numbers of various immune cell types that commonly are involved in immune responses against pathogens and cancers. Importantly, "jump-starting" lymph node expansion prior to administering a traditional vaccine against a melanoma-specific model antigen led to more effective and sustained anti-tumor responses in mice. The findings are published in Nature Biomedical Engineering .
"By enhancing the initial and sustained expansion of LNs with biomaterial scaffolds, non-invasively monitoring them individually over long time periods, and probing deeply into their tissue architecture and immune cell populations, we tightly correlate a persistent LN expansion with more robust immune and vaccination responses," said Wyss Institute Founding Core Faculty member David Mooney, Ph.D., who led the study.
"This opens a new front of investigation for immunologists, and could have far-reaching implications for future vaccine developments." Mooney also is the Robert P. Pinkas Family Professor of Bioengineering at SEAS, and a co-principal investigator of the Wyss-coordinated Immuno-Engineering to Improve Immunotherapy (i3) Center.
Mooney's team at the Wyss Institute and SEAS had previously developed different biomaterial scaffolds as a matrix for cancer and infection vaccines.
The researchers have demonstrated the potential of biomaterial vaccine formulations to successfully fight the growth of tumors in an extensive body of work performed in preclinical animal models and a first clinical trial with cancer patients. But they hadn't yet investigated how their vaccines and those developed by others could influence the response of LNs draining leaked tissue fluid at vaccine injection sites, and have an impact on the LNs tissue organization, different cell types, and their gene expression, which could in turn affect vaccine efficacy.
In their new study, they tested a previously developed vaccine formulation that is based on microscale mesoporous silica (MPS) rods that can be injected close to tumors and form a cell-permeable 3D scaffold structure under the skin.
Engineered to release an immune cell-attracting cytokine (GM-CSF), and immune cell-activating adjuvant (CpG), and tumor-antigen molecules, MPS-vaccines are able to reprogram recruited so-called antigen-presenting cells that, upon migrating into nearby LNs, orchestrate complex tumor cell-killing immune responses. Their new study showed that there are more facets to that concept.
"As it turns out, the immune-boosting functions of basic MPS-vaccines actively change the state of LNs by persistently enlarging their whole organ structure, as well as changing their tissue mechanics and immune cell populations and functions," said first-author Alexander Najibi, Ph.D., who performed his Ph.D. thesis with Mooney.
Probing LNs with ultra-sound and nano-devices
To understand the response of LNs to MPS-vaccines over time, the team applied an ultra-sound imaging technique known as high-frequency ultrasound (HFUS). Similar to monitoring a tiny fetus developing in a mother's womb by clinical ultra-sound, HFUS, on a much smaller scale, enables non-invasively and non-destructively monitoring of anatomical details of tissues and organs in small animals such as mice. Using HFUS, the team traced individual LNs in MPS-vaccinated mice over 100 days.
They identified an initial peak expansion period that lasted until day 20, in which LN volumes increased about seven-fold, significantly greater than in animals that received traditional vaccine formulations. Importantly, the LNs of MPS-vaccinated mice, while decreasing in volumes after this peak expansion, remained significantly more expanded than LNs from traditionally vaccinated mice throughout the 100-day time course.
When Najibi and the team investigated the mechanical responses of the LNs using a nanoindentation device, they found that LNs in MPS-vaccinated animals, although maintaining an overall normal structure, were less stiff and more viscous in certain locations. This was accompanied by a re-organization of a protein that assembles and controls cells' mechanically active cytoskeleton.
Interestingly, Mooney's group had shown in an earlier biomaterial study that changing mechanical features of immune cells' environments, especially their viscoelasticity, affects immune cell development and functions.
"It is very well-possible that in order to accommodate the significant growth induced by MPS-vaccines, LNs need to become softer and more viscous, and that this then further impacts immune cell recruitment, proliferation, and differentiation in a feed-forward process," said Najibi.
From immune cell engagement to vaccine responses
Interestingly, upon MPS-vaccination, the numbers of "innate immune cells," including monocytes, neutrophils, macrophages, and other cell types that build up the first wave of immune defenses against pathogens and unwanted cells, peaked first in expanding LNs.
Peaking with a delay were dendritic cells (DCs), which normally transfer information in the form of antigens from invading pathogens and cancer cells to "adaptive immune cells" that then launch subsequent waves of highly specific immune responses against the antigen-producing invaders. In fact, along with DCs, also T and B cell types of the adaptive immune system started to reach their highest numbers.
"It was fascinating to see how the distinct changes in immune cell populations that we detected in expanding LNs in response to the MPS-vaccine over time re-enacted a typical immune response to infectious pathogens," commented Najibi.
Innate immune cells and DCs are also known as "myeloid cells," which are known to interact with LN tissue during early expansion. To further define the impact of myeloid cells on LN expansion, Mooney's team collaborated with the group of Shannon Turley, Ph.D., the VP of Immunology and Regenerative Medicine at Genentech, and an expert in lymph node biology and tumor immunology.
"The MPS-vaccine led to extraordinary structural and cellular changes within the lymph node that supported potent antigen-specific immunity," said Turley.
By isolating myeloid cells from LNs and analyzing the gene expression profiles of individual cells (single cell RNA-seq), the groups were able to reconstruct distinct changes in myeloid cell populations during LN expansion, and identified distinct DC populations in durably expanded LNs whose changed gene expression was associated with LN expansion.
In addition, the collaborators found that the number of monocytes was increased 80-fold upon MPS-vaccination—the highest increase among all myeloid cell types—and pinpointed subpopulations of "inflammatory and antigen-presenting monocytes" as promising candidates for facilitating LN expansion. In fact, when they depleted specific subpopulations of these types of monocytes from circulating blood of mice after vaccination, the maintenance of LN expansion, and timing of the T cell response to vaccination, was altered.
Finally, the team explored whether LN expansion could enhance the effectiveness of vaccination. "Jump-starting" the immune system in LNs with an antigen-free MPS-vaccine and subsequently administering the antigen in a traditional vaccine format significantly improved anti-tumor immunity and prolonged the survival of melanoma-bearing mice, compared to the traditional vaccine alone.
"The priming of lymph nodes for subsequent vaccinations using various formulations could be a low-hanging fruit for future vaccine developments," said Mooney.
"This newfound ability to physically expand lymph nodes and enhance their various immune activities over longer treatment courses, using cleverly designed and easy-to-administer biomaterials, could provide a tremendous push to immunotherapies in patients. It is also yet another great example of how mechanics plays a key role in regulation of living systems, even immune responses where few would consider physical cues to be important," said Wyss Founding Director Donald Ingber, M.D., Ph.D.
Explore further
Feedback to editors

Research shows altered regulation of genes linked to prostate cancer among firefighters
2 hours ago

Biomarker found to help identify cells that can repair damaged blood vessels

Researchers develop reminder system to enhance memory recall

Years after his death, late scientist's work could yield new cancer treatments
4 hours ago

Cannabis, nicotine use during pregnancy found to increase rate of infant death fourfold
5 hours ago

Study finds genetic link between growth during puberty and long-term health conditions

Using AI and social media to track depression in communities could offer more reliable assessments than surveys
6 hours ago

Mouse study shows intermittent fasting protects against liver inflammation and liver cancer

New genetic mutation identified for congenital thyroid condition

A potential treatment for inflammatory bowel disease: Engineered yeast can transport medicines and lower inflammation
Related stories.

Researchers discover key metabolic process responsible for rapid immune responses
Mar 15, 2024

Biomaterial-delivered one-two punch boosts cancer immunotherapy
Jul 13, 2023
DNA origami-based vaccine platform enhances anti-tumor responses through nanometer-precise spacing of molecules

Researchers develop dual anti-tumor vaccine
Mar 14, 2024

Stem cell-based vaccine offers a new approach that may protect against pancreatic cancer
May 6, 2021

Study sheds light on how breast cancer cells evade immune surveillance and survive in lymph nodes
Jun 21, 2023
Recommended for you

Seeking medical insights in the physics of mucus
8 hours ago
Three-dimensional retinal electrodes in a convex Braille shape partially restore sight
9 hours ago

Researchers report exceptionally small implant for future vision correction
10 hours ago

Small pump for kids awaiting heart transplant shows promise in new trial
14 hours ago
CRIPSR gene editing leads to improvements in vision for people with inherited blindness, clinical trial shows
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Still No Evidence COVID-19 Vaccination Increases Cancer Risk, Despite Posts
By Catalina Jaramillo
Posted on May 3, 2024
SciCheck Digest
It has not been shown that COVID-19 vaccines cause or accelerate cancer. Yet opponents of the vaccines say a new review article “has found that COVID-19 mRNA vaccines could aid cancer development.” The review conclusions are mainly based on the misinterpretation of a study on mRNA cancer vaccines in mice.
Messenger RNA, or mRNA, vaccines work by instructing a small number of a person’s cells to make specific proteins. In the case of the approved mRNA vaccines for COVID-19, the cells make spike protein — one component of the virus that causes COVID-19.
For mRNA vaccines to work, it’s not enough to just put mRNA molecules into a vial and then inject them into a person’s muscle . One innovation that made the current mRNA vaccines possible was the use of lipids to encircle the mRNA molecules.
These fatty structures — called lipid nanoparticles — protect the mRNA from being broken down prematurely. They also help the mRNA cross the cell membrane and get into cells. The approved mRNA vaccines for COVID-19 each use a blend of four types of lipids.
Once the lipid nanoparticles make it past the cell membrane, they release the mRNA into the cell’s interior. Called the cytoplasm , this region encompasses the inside of the cell excluding the nucleus, in which the cell’s DNA resides. The mRNA is processed and used to create the spike protein. The body then mounts an immune response to the spike protein, preparing the immune system to respond to the virus that causes COVID-19 in the future.
Link to this
Clinical trials, involving thousands of people, and multiple studies have shown that the mRNA COVID-19 vaccines from Pfizer/BioNTech and Moderna are safe. Hundreds of millions of doses have been administered under close monitoring systems that have found serious side effects are rare . Studies have also shown that the vaccines work very well in preventing severe COVID-19 disease and death, saving millions of lives across the globe.

There is no evidence to support a link between COVID-19 vaccines and cancer, as we’ve reported . Both the National Cancer Institute and the American Cancer Society have stated there’s no information that suggests COVID-19 vaccines cause cancer, make it more aggressive or lead to recurrence of cancer.
Yet, vaccine opponents falsely claim a review article published in April proves the contrary.
“BREAKING: A review in the International Journal of Biological Macromolecules has found that COVID-19 mRNA vaccines could aid cancer development,” reads an April 16 Facebook post by America’s Frontline Doctors , a group that has repeatedly spread misinformation about the pandemic and whose founder was sentenced to 60 days in prison for entering the U.S. Capitol during the Jan. 6 riot.
A separate Facebook post used a Gateway Pundit headline to falsely claim, “Confirmed: Researchers Reveal COVID mRNA Vaccines Contain Component that Suppresses Immune Response and Stimulates Cancer Growth.” Similarly, a post on X said the new “study … confirms what some medical experts have been suspecting for 18 months: The COVID mRNA shots containing N1-methyl-pseudouridine SUPPRESS the immune system and STIMULATE cancer growth!”
Messenger RNA, or mRNA, vaccines work by instructing a small number of a person’s cells to make specific proteins, which then prompt the body to mount an immune response. N1-methylpseudouridine is a modification naturally found in some RNA molecules that’s attached to mRNA in vaccines to allow it to deliver its message to the cell without being destroyed by an innate immune response, as we will explain.
Experts told us the review paper, which is based on other published articles and does not contain original research, misleads by misinterpreting several studies and the role of N1-methylpseudouridine in vaccines. The authors also refer to an unreliable review article , written by authors known for spreading misinformation , that falsely claimed the mRNA COVID-19 vaccines impair the immune system and increase the risk of cancer, as we have explained before.
One of the most important misrepresentations, and one that the authors heavily rely on, is based on the findings of a study on mRNA cancer vaccines in mice. The study looked at the efficacy of mRNA cancer vaccines with different degrees of N1-methylpseudouridine modification in a mouse melanoma model. According to the review, the study found that “adding 100% of N1-methyl-pseudouridine (m1Ψ) to the mRNA vaccine in a melanoma model stimulated cancer growth and metastasis, while non-modified mRNA vaccines induced opposite results, thus suggesting that COVID-19 mRNA vaccines could aid cancer development.”
But that’s not what the study found.
“[O]ur results did not show, suggest or indicate that modified mRNA promotes tumor growth/metastasis,” Tanapat Palaga , professor of microbiology at the Chulalongkorn University in Thailand and the corresponding author of that study, told us in an email.
What the study actually showed is that both unmodified mRNA and modified mRNA induced immune responses against the tumor antigens, but only the unmodified mRNA reduced cancer growth and metastasis, while the modified mRNA didn’t. The study was published in 2022 and co-authored by Drew Weissman , who won the 2023 Nobel Prize with Katalin Karikó for discovering this mRNA modification that eventually led to the mRNA COVID-19 vaccines.
Dr. James A. Hoxie , an emeritus professor of medicine at the University of Pennsylvania and co-director of the Penn Institute of RNA Innovation (directed by Weissman), told us those findings are relevant for scientists who are studying ways in which mRNA cancer vaccines can elicit immune responses needed to prevent or delay cancer progression. (See “ Social Media Posts Misinterpret Biden on mRNA Cancer Vaccines ” for more information about mRNA cancer vaccines.)
“But that is a far cry from saying that the vaccine that was used to prevent COVID-19 disease causes cancer,” he said. Implying that by regulating the innate immune system, which is something scientists working in immunotherapies are trying to understand, “you’re leaving yourself open for cancer risk — that is ludicrous.”
“I believe that the authors of this review article intentionally or [unintentionally] misinterpret our results and tried to twist the conclusion to support their agenda,” Palaga told us.
There are no studies supporting a link between N1-methylpseudouridine and cancer in animals or mice, experts told us.
There is also no evidence mRNA COVID-19 vaccines impair, much less suppress, the immune system, as we’ve reported . In fact, the vaccines enhance immunity by teaching the immune system how to identify and fight the coronavirus.
N1-methylpseudouridine and Its Role in mRNA Vaccines
To understand the role of N1-methylpseudouridine we have to look back at the history of mRNA vaccines.
Normally, when a cell encounters a foreign RNA, a molecule present in most living organisms and viruses, it activates a strong innate immune response against the molecule.

This was a problem for scientists trying to use mRNA as a therapeutic, since the goal was for the cell to receive the instructions carried by the mRNA and produce certain proteins. Until the mid-2000s, Karikó, Weissman and others observed that if they attached certain chemical modifications found in some kinds of natural RNA molecules, such as pseudouridine, into one of the four bases of mRNA, they could blunt that innate immune response and, at the same time, increase the mRNA’s capacity to translate its code for the cell to make the desired proteins.
Later, scientists found N1-methylpseudouridine, another modification naturally found in some kinds of RNA molecules, worked better than pseudouridine.
The modification is not “suppressing” the immune system, Hoxie told us — it just allows for certain parts of the immune system not to activate temporarily “in order to get the desired effect.”
Jordan L. Meier , senior investigator at the National Cancer Institute who has studied the role of N1-methylpseudouridine in COVID-19 vaccines, told us the authors of the review paper misrepresent what N1-methylpseudouridine, which is abbreviated as m1Ψ, does.
The review “incorrectly” confuses “m1Y’s ability to hide from the immune system with an ability to weaken or disable it,” he told us in an email.
To explain it, Meier compared the mRNA modification to a spy using a disguise in order to pass security guards.
“The authors are essentially suggesting that the disguise somehow makes the guards less able to do their jobs going forward,” he wrote. “In reality, once the disguised person is through, the guards remain just as vigilant and capable as before.”
The review, he added, doesn’t provide evidence that N1-methylpseudouridine “leaves the immune system any worse off for future threats.”
Misrepresented Studies in the Review Paper
Similarly, the review misleads by cherry-picking or misrepresenting figures and tables of this and other papers.
For example, in the study by Palaga, Weissman and others using a mouse melanoma model (in which malignant cells from a tumor are given to a mouse), scientists found that relative to mice that received no vaccine (and instead received a saline solution) no increase in tumor growth or decrease in survival occurred when animals were vaccinated with a modified mRNA vaccine. However, when animals received a vaccine containing unmodified mRNA, the study showed a decrease in tumor growth and an increase in survival compared with the control group that received the saline solution. In other words, the study found that the unmodified mRNA generated immune responses that decreased tumor growth and improved survival, while, similar to the control group, the modified mRNA had no effect on the tumor.
Table 1 of the review, however, incorrectly says the study found that the modified mRNA vaccine “increases tumor growth” and “decreases survival.”
“This is simply not true and is a gross misrepresentation of the data that paper actually shows. The modified RNA had no effect on the tumor, and results using that vaccine were the same as using a saline solution,” Hoxie told us.
The tumor growth in mice receiving the modified mRNA was “increased relative to the unmodified vaccine, but it was identical to when there was no intervention,” Hoxie told us. “Animals that received the modified mRNA vaccine died at the same rate and with the same amount of tumor as did animals that received the saline solution. The fact tumor progression in this model was reduced with the unmodified mRNA vaccine is the key point of this paper and indicated that in this model immune responses to unmodified mRNA may have anti-tumor activity, an important finding for the cancer immunotherapy field.”
The review also refers to a study that has been extensively misinterpreted to falsely claim that the Pfizer/BioNTech mRNA COVID-19 vaccine causes what vaccine opponents called “turbo cancer.” The study describes one mouse that died from a lymphoma after 14 mice were given a high dose of the vaccine. The review paper reproduces images from the study that show dissected mice and compares the organs of the mouse that died with one with a normal anatomy.
As we explained , and as the authors of that paper noted in an addendum , there is no such thing as “turbo cancer,” and, more importantly, the case report does not demonstrate a causal relationship between the lymphoma and the vaccine.
Meier told us the review also wrongly refers to a study published in 2016 to support its thesis that modified mRNA vaccines turn off an immune sensor known as RIG-I.
“In reality, this study only showed m1Y mRNAs are unable to activate RIG-I and did not test inhibition. In other words, what was shown was that m1Y is a strong camouflage, not that it is an immune suppressor,” he wrote.
Editor’s note: SciCheck’s articles providing accurate health information and correcting health misinformation are made possible by a grant from the Robert Wood Johnson Foundation. The foundation has no control over FactCheck.org’s editorial decisions, and the views expressed in our articles do not necessarily reflect the views of the foundation.
“ Safety of COVID-19 Vaccines .” CDC website. Updated 3 Nov 2023.
“ Selected Adverse Events Reported after COVID-19 Vaccination .” CDC website. Updated 12 Sep 2023.
Trang, Brittany. “ Covid vaccines averted 3 million deaths in U.S., according to new study .” Stat. 13 Dec 2022.
“ COVID-19 vaccinations have saved more than 1.4 million lives in the WHO European Region, a new study finds .” WHO. Press release. 16 Jan 2024.
Van Beusekom, Mary. “ Global COVID vaccination saved 2.4 million lives in first 8 months, study estimates .” CIDRAP, University of Minnesota. 31 Oct 2023.
Watson, Oliver J., et al. “ Global impact of the first year of COVID-19 vaccination: a mathematical modelling study .” Infectious Diseases. 23 Jun 2022.
Yandell, Kate. “ COVID-19 Vaccines Have Not Been Shown to Cause ‘Turbo Cancer’ .” FactCheck.org. 31 Aug 2023.
Yandell, Kate. “ COVID-19 Vaccines Have Not Been Shown to Alter DNA, Cause Cancer. ” FactCheck.org. 26 Oct 2023.
“ COVID-19 Vaccines and People with Cancer .” National Cancer Institute website. Accessed 2 May 2024.
“ COVID-19 Vaccines in People with Cancer .” American Cancer Society website. Accessed 2 May 2024.
Bergengruen, Vera. “‘ What Price Was My Father’s Life Worth?’ Right-Wing Doctors Are Still Peddling Dubious COVID Drugs .” Time. 15 May 2023.
Van Beusekom, Mary. “ Report spotlights 52 US doctors who posted potentially harmful COVID misinformation online .” CIDRAP. 16 Aug 2023.
Dyer, Owen. “ Founder of America’s Frontline Doctors is sentenced to prison for role in Capitol riot .” BMJ. 22 Jun 2022.
“ Understanding COVID-19 mRNA Vaccines. ” National Human Genome Research Institute website. Accessed 22 Mar 2024.
McDonald, Jessica. “ COVID-19 Vaccination Increases Immunity, Contrary to Immune Suppression Claims .” FactCheck.org. 30 Jul 2022.
Sittplangkoon, Chutamath. “ mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model .” Frontiers in Immunology. 14 Oct 2022.
Palaga, Tanapat . Professor of microbiology at the Chulalongkorn University in Thailand. Email sent to FactCheck.org. 19 April 2024.
“ The Nobel Prize in Physiology or Medicine 2023 .” The Nobel Prize. Accessed 2 May 2024.
Hoxie, James A . Emeritus professor of medicine at the University of Pennsylvania and co-director of the Penn Institute of RNA Innovation. Phone interview with FactCheck.org. 25 Apr 2024.
Jaramillo, Catalina. “ Social Media Posts Misinterpret Biden on mRNA Cancer Vaccines .” FactCheck.org. 22 Mar 2024.
Karikó, Katalin, et al. “ Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA .” Immunity. 23 Aug 2005.
Anderson, Bart R., et al. “ Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation .” Nucleic Acids Research. 1 Sep 2010.
Karikó, Katalin, et al. “ Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability .” Molecular Therapy. Nov 2008.
Andries, Oliwia, et al. “ N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice .” Journal of Controlled Release. 10 Nov 2015.
Meier, Jordan L . Senior investigator at the National Cancer Institute. Email to FactCheck.org. 26 Apr 2024.
Nance, Kellie D, and Jordan L. Meier. “ Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines .” ACS Cent. Sci. 26 May 2021.
Eens, Sander, et al. “ B-cell lymphoblastic lymphoma following intravenous BNT162b2 mRNA booster in a BALB/c mouse: A case report. ” Frontiers in Oncology. 1 May 2023.
Fiegen Durbin, Ann, et al. “ RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling .” mBio. 20 Sep 2016.
Why Vaccinate
On-time vaccination throughout childhood is essential because it helps provide immunity before children are exposed to potentially life-threatening diseases. Vaccines are tested to ensure that they are safe and effective for children to receive at the recommended ages.

As communities open up, it’s important your child goes in for their well-child visit.

Learn all about vaccines and immunity, what vaccines are made of, and how they are kept safe.

Vaccines work with your child’s immune system to prevent various serious diseases.

See the common combination vaccines and how they protect your child against more than one disease with a single shot.
- Vaccines & Immunizations
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Share full article
Advertisement
The Morning
The side effects of covid vaccines.
Thousands of Americans have filed vaccine-injury claims with the federal government.

By David Leonhardt
Let me start with a disclaimer: The subject of today’s newsletter will make some readers uncomfortable. It makes me a little uncomfortable.
The Times has just published an article about Americans who believe they suffered serious side effects from a Covid vaccine. More than 13,000 of them have filed vaccine-injury claims with the federal government.
My colleague Apoorva Mandavilli tells some of their stories in the article , including those of several people who work in medicine and science:
Ilka Warshawsky, a 58-year-old pathologist, said she lost all hearing in her right ear shortly after receiving a Covid booster shot.
Dr. Gregory Poland, 68 — no less than the editor in chief of Vaccine, a scientific journal — said that a loud whooshing sound in his ears had accompanied every moment since his first Covid shot.
Shaun Barcavage, 54, a nurse practitioner in New York City, has experienced a ringing sound in his ears, a racing heart and pain in his eyes, mouth and genitals for more than three years. “I can’t get the government to help me,” Barcavage said. “I am told I’m not real.”
This subject is uncomfortable because it feeds into false stories about the Covid vaccines that many Americans have come to believe — namely, that the vaccines are ineffective or have side effects that exceed their benefits. Robert F. Kennedy Jr., the independent presidential candidate, has promoted these stories, as have some Republican politicians and conservative media figures. “The scale of misinformation,” Dr. Joshua Sharfstein of Johns Hopkins University told Apoorva, “is staggering.”
So let me be clear: The benefits of the Covid vaccines have far outweighed the downsides, according to a voluminous amount of data and scientific studies from around the world. In the U.S. alone, the vaccines have saved at least several hundred thousand lives and perhaps more than one million , studies estimate. Rates of death, hospitalization and serious illness have all been much higher among the unvaccinated than the vaccinated.
Here is data from the C.D.C., in a chart by my colleague Ashley Wu:
Average weekly Covid deaths per 100,000 in the U.S.
From the weeks of Oct. 1, 2022 to April 1, 2023

Unvaccinated
to 4 years old

80 years and older
6 months to 4 years old
Not only are the vaccines’ benefits enormous, but the true toll of the side effects may be lower than the perceived toll: Experts told Apoorva that some people who believe Covid vaccines have harmed them are probably wrong about the cause of their problems.
How so? Human beings suffer mysterious medical ailments all the time. If you happened to begin experiencing one in the weeks after receiving a vaccine, you might blame the shot, too, even if it were a coincidence. So far, federal officials have approved less than 2 percent of the Covid vaccine injury-compensation claims they have reviewed.
Still, some ailments almost certainly do stem from the vaccines. The C.D.C. says some people are allergic (as is the case with any vaccine). Both the C.D.C. and researchers in Israel — which has better medical tracking than the U.S. — have concluded that the vaccines contributed to heart inflammation, especially in young men and boys. Officials in Hong Kong — another place with good health care data — have concluded that the vaccines caused severe shingles in about seven vaccine recipients per million.
Honesty and trust
These side effects are worthy of attention for two main reasons.
First, people who are suffering deserve recognition — and the lack of it can be infuriating. Dr. Janet Woodcock, a former F.D.A. commissioner, told The Times that she regretted not doing more to respond to people who blame the vaccines for harming them while she was in office. “I believe their suffering should be acknowledged, that they have real problems, and they should be taken seriously,” Woodcock said.
The second reason is that public health depends on public trust, and public trust in turn depends on honesty. During the pandemic, as I’ve written in the past, government officials and academic experts sometimes made the mistake of deciding that Americans couldn’t handle the truth.
Instead, experts emphasized evidence that was convenient to their recommendations and buried inconvenient facts. They exaggerated the risk of outdoor Covid transmission , the virus’s danger to children and the benefits of mask mandates , among other things. The goal may have been admirable — fighting a deadly virus — but the strategy backfired. Many people ended up confused, wondering what the truth was.
The overall picture
Here’s my best attempt to summarize the full truth about the Covid vaccines:
They are overwhelmingly safe and effective. They have saved millions of lives and prevented untold misery around the world. They’re so valuable that elderly people and those with underlying health conditions should be vigilant about getting booster shots when they’re eligible. For most children, on the other hand, booster shots seem to have only modest benefits, which is why many countries don’t recommend them .
And, yes, a small fraction of people will experience significant side effects from the vaccines. Eventually, scientific research may be able to better understand and reduce those side effects — which is more reason to pay attention to them.
Overall, Covid vaccines are probably the most beneficial medical breakthrough in years, if not decades.
I encourage you to read Apoorva’s article .
THE LATEST NEWS
Trump on trial.
The jury in Donald Trump’s criminal trial heard audio — secretly recorded by Trump’s former lawyer Michael Cohen — that seemed to show Trump’s involvement in the hush-money payments to two women who allegedly had affairs with him.
In one recording, Cohen claimed that Trump hates “the fact that we did it,” referring to paying off Stormy Daniels. In another, Trump and Cohen discussed the deal with Karen McDougal.
The jury also saw texts from 2016 in which Daniels’s former lawyer acknowledged that the hush money might have helped Trump win the election. “What have we done?” he wrote.
Prosecutors asked the judge to hold Trump in contempt for again violating a gag order .
Jimmy Kimmel joked about texts that mention his show being entered into evidence. “Why was I not asked to testify?” he said.
More on Politics
In North Carolina, a swing state, President Biden announced more funding to replace toxic lead pipes . He also met with the families of police officers killed this week in Charlotte.
Biden, defending America’s history of immigration, called Japan and India — U.S. allies — xenophobic and said that China and Russia “don’t want immigrants.”
Senator Bob Menendez’s lawyers want a psychologist to testify at his corruption trial that traumatic experiences explain the cash he stockpiled .
Campus Protests
In a televised statement, Biden condemned violence and intimidation on college campuses. “There’s the right to protest, but not the right to cause chaos,” he said.
In recent weeks, more than 2,000 demonstrators have been arrested or detained on campuses across the U.S., according to a Times tally.
The House passed a bill that would crack down on antisemitic speech at colleges . About one-third of Democrats voted no, and some far-right Republicans criticized it as a threat to Christian teachings.
A teakettle, sleeping bags and guard shifts: This is what it was like inside a building at Columbia occupied by pro-Palestinian demonstrators .
Universities including Brown agreed to consider ending investments linked to Israel in response to protests. It’s a gamble that risks angering influential donors .
Israel-Hamas War
Hamas’s political leader said the group was studying Israel’s latest cease-fire proposal with a “positive spirit” and would soon resume in-person negotiations.
Some senior Israeli officials are weighing a postwar plan for Gaza in which Israel would share oversight of the enclave with an alliance of Arab nations.
The war strategies of Benjamin Netanyahu and Hamas’s leader, Yahya Sinwar, leave little room for compromise , The Wall Street Journal reports.
More International News
In Taiwan, Times reporters joined the faithful on pilgrimages honoring Mazu , sometimes known as the Goddess of the Sea.
The U.S. accused Russia of using chemical weapons against Ukrainian troops, in violation of a global ban .
At least 29 people died after several days of heavy rain in southern Brazil.
Business and Economy
Oil companies, betting that the world is not yet ready to move past fossil fuels, have expanded drilling into deeper waters . (See photos of life aboard a facility 80 miles out to sea .)
The restaurant chain Dave & Buster’s, which features arcade games like Skee-Ball, announced that it will soon allow customers to gamble on the games .
Sony Pictures and the private equity company Apollo Global Management formally expressed interest in acquiring Paramount for around $26 billion.
Other Big Stories
A judge declared a mistrial in a lawsuit from three Iraqi men who said they were tortured at Abu Ghraib prison two decades ago.
The Kentucky Derby will take place tomorrow. The breakdown of 12 horses after the event last year has led to existential questions about the sport .
New York City police infringed on First Amendment rights when they blocked journalists from witnessing their raid on Columbia University , Mara Gay writes.
Gerrymandering turned Michigan into a bastion of minority rule — until democracy activists fought back and won , Ari Berman writes.
Here are columns by David Brooks on how the protests help Trump , Michelle Cottle on Biden’s wise words about the protests and Michelle Goldberg on Kari Lake’s abortion stance .
MORNING READS
Medals: For decades, the Olympics included art competitions. The winning entries are largely forgotten .
‘Queer food’: Scholars gathered to discuss the role gender and sexuality play in the food space. (Snacks were plentiful.)
Night sky: The Eta Aquarids meteor shower, a result of debris from Halley’s Comet, will be at its peak this weekend. Here’s how to watch .
Lives Lived: Peggy Mellon Hitchcock was born into privilege but enthusiastically supported the 1960s counterculture. She offered Timothy Leary and Richard Alpert her brother’s mansion after they lost their jobs at Harvard for experimenting with psychedelic drugs. Hitchcock died at 90 .
N.B.A.: The New York Knicks defeated the Philadelphia 76ers in a thrilling game on the road. The Knicks will face the Indiana Pacers in the second round of the playoffs.
N.H.L.: The Toronto Maple Leafs fended off elimination and forced a Game 7 against the Boston Bruins in a tense 2-1 win .
Kentucky Derby: Larry Demeritte, the trainer of long-shot West Saratoga, will become the first Black trainer with a Derby entrant since 1989.
ARTS AND IDEAS
A new story by Ben Sisario, The Times’s music industry reporter, explores the surprisingly complicated answer to what seems to be a simple question: What is a song?
When it comes to copyright — and the multimillion-dollar lawsuits that come from it — a song is often defined by only the notes written on a piece of sheet music, and not by the much fuller recording. “It is completely divorced from actual music-making practice,” said Joseph P. Fishman, a professor at Vanderbilt Law School.
More on culture
Times Book Review editors gathered their picks for the best books published since 2000 .
Hip-hop’s popularity is growing in China . Artists there must strike a balance between creative expression and appeasing censors, The A.P. reports.
TikTok and Universal Music Group reached a new licensing deal , ending a three-month stalemate that blocked songs from pop’s biggest stars from the platform.
THE MORNING RECOMMENDS …
Roast simple miso salmon as part of a traditional Japanese breakfast spread.
Snuggle into bed with a comfy duvet .
Buy a gift for an occult enthusiast .
Take our news quiz .
Here is today’s Spelling Bee . Yesterday’s pangram was motorway .
And here are today’s Mini Crossword , Wordle , Sudoku , Connections and Strands .
Thanks for spending part of your morning with The Times. See you tomorrow. — David
P.S. For World Press Freedom Day, A.G. Sulzberger, The Times’s publisher, and Joseph Kahn, the executive editor, wrote a letter calling attention to missing and detained journalists across the globe .
Sign up here to get this newsletter in your inbox . Reach our team at [email protected] .
David Leonhardt runs The Morning , The Times’s flagship daily newsletter. Since joining The Times in 1999, he has been an economics columnist, opinion columnist, head of the Washington bureau and founding editor of the Upshot section, among other roles. More about David Leonhardt

IMAGES
VIDEO
COMMENTS
Introduction "The impact of vaccination on the health of the world's peoples is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth" (Plotkin and Mortimer, 1988).The development of safe and efficacious vaccination against diseases that cause substantial morbidity and mortality has been one of the ...
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
To ensure vaccines benefit the global community, the ethical principles of beneficence, justice, non-maleficence, and autonomy should be examined and adhered to in the process of development, distribution, and implementation. This study, therefore, aimed to examine ethical considerations of vaccine development and vaccination processes.
The achievements of vaccine research and development bring a hope to our societies that we may cope with the COVID‐19 pandemic. There are two aspects that should be maintained in balance: the immediate necessity for speed of vaccine research and the inherent need for protection of research subjects, which is the foremost concern of research ethics.
A study of how people perceive the COVID-19 vaccine compared to other historic vaccines, based on surveys of University of South Dakota students and faculty.
Although these are reasonable endpoints for antibody-focused vaccines, vaccine-induced T cells are more likely to reduce rather than prevent infection. Indeed, in the influenza virus challenge and ...
As the world's population grows older, vaccination is becoming a key strategy for promoting healthy aging. Despite scientific progress in adult vaccine development, obstacles such as immunosenescence and vaccine hesitancy remain. To unlock the potential of adult vaccines fully, we must enhance immunization programs, dispel misinformation, and ...
There is a need to systematically review the existing vaccine candidates and investigate their safety, efficacy, immunogenicity, unwanted events, and limitations. The review was undertaken by searching online databases, i.e., Google Scholar, PubMed, and ScienceDirect, with finally 59 studies selected. Our findings showed several types of ...
Thus, future vaccines designed to promote both broad T cell effector functions and antibodies may provide enhanced protection. As we discuss, such vaccines present several challenges that require new strategic and economic considerations. Vaccine-induced T cells relevant to protection may reside in the lungs or lymphoid tissues, requiring more ...
Introduction. Vaccine hesitancy is a complex and multifaceted problem, and one that is influenced by a range of contextual (e.g. historical, institutional, political) factors, as well as individual-level and vaccine specific factors (e.g. costs or design of a given vaccination program) [].Individual-level factors, include health-system and providers, knowledge and beliefs about health and ...
Introduction Vaccine acceptance is a critical component of sustainable immunization programs, yet rates of vaccine hesitancy are rising. Increased access to misinformation through media and anti-vaccine advocacy is an important contributor to hesitancy in the United States and other high-income nations with robust immunization programs. Little is known about the content and effect of ...
Shortly after the FDA's emergency use authorization of the J&J/Janssen vaccine, leading US organizations advocated that all three vaccines should be regarded as equivalent in preventing major outcomes of public health interest: COVID-19 hospitalizations and mortality ().Early in the vaccination distribution process, when vaccine supplies were limited, the Advisory Committee on Immunization ...
Background By mid 2023, European countries reached 75% of vaccine coverage for COVID-19 and although vaccination rates are quite high, many people are still hesitant. A plethora of studies have investigated factors associated with COVID-19 vaccine hesitancy, however, insufficient attention has been paid to the reasons why people get vaccinated against COVID-19. Our work aims to investigate the ...
Effective and safe COVID-19 vaccines have been developed at a rapid and unprecedented pace to control the spread of the virus, and prevent hospitalisations and deaths. However, COVID-19 vaccine uptake is challenged by vaccine hesitancy and anti-vaccination sentiments, a global shortage of vaccine supply, and inequitable vaccine distribution especially among low- and middle-income countries ...
vaccination all the more challenging is misinformationon vaccinations and the rising anti-vaccination movement. Online platforms and socialmedia have been found to be the main source of misinformation and place of communicationfor the anti-vaccination community (Smith et al., 2020; CCDH, 2020). The online methodsare harnessed to spread distrust,
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety. Vaccines go through various phases of development and testing - there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease, which is called efficacy.
Background Vaccination efforts are a vital part of controlling the spread of diseases, however, lack of vaccine acceptance undermines the efficacy of this public health effort. Current evidence suggests that the most effective interventions to support vaccination uptake and positive vaccination beliefs are multicomponent, and dialogue based. Peer-based education interventions are such a ...
The percentage of COVID-19 vaccine acceptance was not so high (up to 86.1% students or 77.6% general population); for influenza vaccine, the maximum percentage was 69%. Several factors influenced the acceptance or refusal (ethnicity, working status, religiosity, politics, gender, age, education, income, etc.).
A biomaterial vaccine enhances and sustains lymph node expansion following vaccination, boosting anti-tumor immunity in an animal model. The authors used a method know as high-frequency ultrasound to monitor individual lymph nodes in MPS- and control-vaccinated mice. The top row shows a series of lymph nodes on day 7 following MPS vaccination.
Caroline Gutman for The New York Times. 2. Hearing pro-vaccine messages from doctors, friends and relatives. For many people who got vaccinated, messages from politicians, national experts and the ...
A 1% increase in vaccination was associated with an additional 680 children having AUT or SLI. Neither parental behavior nor access to care affected the results, since vaccination proportions were not significantly related (statistically) to any other disability or to the number of pediatricians in a U.S. state. The results suggest that ...
Our children are safer and live longer thanks to the availability of these immunizations. Our government has put into place a stringent, organized system to ensure the vaccinations are safe and the side effects are known (Rhodes, 2013 ). Vaccines are safe, they are needed, and most importantly they save lives. References.
A vaccination is "the injection of a killed or weakened organism that produces immunity in the body against that organism" (vaccines.gov). Vaccines are designed to provide immunization to certain illnesses. People of all ages are encouraged to get vaccinations not only to protect themselves but also for the safety of the public.
This immunofluorescent staining shows a lymph node that has been significantly expanded in mice with the help of the biomaterial MPS-vaccine (on the right), next to a lymph node taken from non ...
For mRNA vaccines to work, ... Meier told us the review also wrongly refers to a study published in 2016 to support its thesis that modified mRNA vaccines turn off an immune sensor known as RIG-I.
Why Vaccinate. Español (Spanish) | Print. On-time vaccination throughout childhood is essential because it helps provide immunity before children are exposed to potentially life-threatening diseases. Vaccines are tested to ensure that they are safe and effective for children to receive at the recommended ages.
The first of the cons is vaccinations can cause serious and fatal side effects. "According to the CDC, all vaccines carry a risk of a life-threatening allergic reaction (anaphylaxis) in about one per million children. The rotavirus vaccination can cause intussusception, a type of bowel blockage that may require hospitalization, in about one ...
Vaccine hesitant - were skeptical about conspiracy theories such as the "mark of the beast" and microchips in vaccines. The sudden pause of vaccine trials also triggered worries among users about the safety of vaccination. Some Twitter users claimed that they would not get vaccinated because of previous experience with vaccination-related ...
Dr. Gregory Poland, 68 — no less than the editor in chief of Vaccine, a scientific journal — said that a loud whooshing sound in his ears had accompanied every moment since his first Covid ...
Importantly, "jump-starting" lymph node expansion prior to administering a traditional vaccine against a melanoma -specific model antigen led to more effective and sustained anti-tumor responses ...