Advances in Prostate Cancer Research
Nanoparticles are tested as a means to deliver drugs to prostate cancer cells.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat prostate cancer. Most men diagnosed with prostate cancer will live a long time, but challenges remain in choosing the best treatments for individuals at all stages of the disease.
This page highlights some of the latest research in prostate cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.

Studying Early Detection for Men at High Risk
Men with certain inherited genetic traits are at increased risk for developing prostate cancer. Examples of such traits include inherited BRCA gene mutations and Lynch syndrome . No clear guidelines exist for when or how—or if—to screen men at high genetic risk for prostate cancer.
NCI researchers are using magnetic resonance imaging (MRI) of the prostate in men at high risk to learn more about how often and how early these cancers occur. They’re also testing whether regular scans in such men can detect cancers early, before they spread elsewhere in the body ( metastasize ).
Diagnosing Prostate Cancer
Improving biopsies for prostate cancer.
Traditionally, prostate cancer has been diagnosed using needles inserted into the prostate gland in several places under the guidance of transrectal ultrasound (TRUS) imaging to collect samples of tissue. This approach is called systematic biopsy .
However, ultrasound does not generally show the location of cancer within the prostate. It is mainly used to make sure the biopsy needles go into the gland safely. Therefore, biopsy samples using ultrasound guidance can miss cancer altogether. Or they may identify low-grade cancer while missing areas of high-grade , potentially more aggressive cancer.
Some doctors, concerned that a systematic biopsy showing only low-grade cancer could have missed a high-grade cancer, may suggest surgery or radiation. However, in some cases these treatments will be for a cancer that may have never caused a problem, which is considered overtreatment .
Using MRI and ultrasound . Scientists at NCI have developed a procedure that combines magnetic resonance imaging (MRI) with TRUS for more accurate prostate biopsies. MRI can locate potential areas of cancer within the gland but is not practical for real-time imaging to guide a prostate biopsy. The procedure, known as MRI-targeted biopsy, uses computers to fuse an MRI image with an ultrasound image. This lets doctors use ultrasound guidance to take biopsy samples of areas of possible cancer seen on MRI.
NCI researchers have found that combining MRI-targeted biopsy with systematic biopsy can increase the detection of high-grade prostate cancers while decreasing detection of low-grade cancers that are unlikely to progress.
Testing machine learning . Researchers are testing the use of machine learning , also called artificial intelligence (AI), to better recognize suspicious areas in a prostate MRI that should be biopsied. AI is also being developed to help pathologist s who aren't prostate cancer experts accurately assess prostate cancer grade . Cancer grade is the most important factor in determining the need for treatment versus active surveillance .
Finding small amounts of prostate cancer using imaging and PSMA
NCI-supported researchers are developing new imaging techniques to improve the diagnosis of recurrent prostate cancer. A protein called prostate-specific membrane antigen (PSMA) is found in large amounts—and almost exclusively—on prostate cells. By fusing a molecule that binds to PSMA to a compound used in PET imaging, scientists have been able to see tiny deposits of prostate cancer that are too small to be detected by regular imaging.
The Food and Drug Administration (FDA) has approved two such compounds for use in PET imaging of men with prostate cancer. These approvals are for men whose cancer may have spread to other parts of the body but is still considered curable, either with surgery or other treatments.
The ability to detect very small amounts of metastatic prostate cancer could help doctors and patients make better-informed treatment decisions. For example, if metastatic cancer is found when a man is first diagnosed, he may choose an alternative to surgery because the cancer has already spread. Or doctors may be able to treat cancer recurrence—either in the prostate or metastatic disease—earlier. which may lead to better survival. Studies are being done to determine if such early detection can improve outcomes.
As part of the Cancer Moonshot℠ , NCI researchers are testing whether PSMA-PET imaging can also identify men who are at high risk of their cancer recurring. Such imaging may eventually be able to help predict who needs more aggressive treatment—such as radiation therapy in addition to surgery—after diagnosis.
Research teams are also looking at:
- whether certain patterns seen on PSMA tests taken over time may indicate an increased risk of recurrence after initial treatment.
- how small metastases discovered with PSMA change over time , with or without treatment.
New Prostate Cancer Treatments
Standard treatments for prostate cancer that has not spread elsewhere in the body are surgery or radiation therapy (RT), with or without hormone therapy .
Active surveillance is also an option for men who have a low risk of their cancer spreading. This means monitoring the cancer with regular biopsies and holding off on treatment unless there is evidence of progression. Rates of active surveillance more than doubled between 2014 and 2021 , to almost 60% of US men diagnosed with low-risk prostate cancer.
Hormone therapy for prostate cancer
Over the last decade, several new approaches to hormone therapy for advanced or metastatic prostate cancer have been approved for clinical use.
Many prostate cancers that originally respond to treatment with standard hormone therapy become resistant over time, resulting in castrate-resistant prostate cancer (CRPC). Four newer drugs have been shown to extend survival in some groups of men with CRPC. All inhibit the action of hormones that drive CRPC:
- enzalutamide (Xtandi)
- abiraterone (Zytiga)
- darolutamide (Nubeqa)
- apalutamide (Erleada)
These drugs are now also used in some people whose prostate cancer still responds to standard hormone therapies but has spread elsewhere in the body (metastasized).
Scientists are continuing to study novel treatments and drugs, along with new combinations of existing treatments, in men with metastatic and castration-resistant prostate cancer.
PARP inhibitors for prostate cancer
A PARP inhibitor is a substance that blocks an enzyme in cells called PARP. PARP helps repair DNA when it becomes damaged. Some prostate tumors have genetic defects that limit their ability to repair DNA damage. Such tumors may be sensitive to PARP inhibitors.
Two PARP inhibitors, olaparib (Lynparza) and rucaparib (Rubraca) , have been approved for some men whose prostate cancer has such genetic defects and has metastasized , and whose disease has stopped responding to standard hormone treatments. Ongoing studies are looking at combing PARP inhibitors with hormone therapies.
Immunotherapy: vaccines for prostate cancer
Immunotherapies are treatments that harness the power of the immune system to fight cancer. These treatments can either help the immune system attack the cancer directly or stimulate the immune system in a more general way.
Vaccines and checkpoint inhibitors are two types of immunotherapy being tested in prostate cancer. Treatment vaccines are injections that stimulate the immune system to recognize and attack a tumor.
One type of treatment vaccine called sipuleucel-T (Provenge) is approved for men with few or no symptoms from metastatic CRPC.
Immunotherapy: checkpoint inhibitors for prostate cancer
An immune checkpoint inhibitor is a type of drug that blocks proteins on immune cells, making the immune system more effective at killing cancer cells.
Two checkpoint inhibitors, pembrolizumab (Keytruda) and dostarlimab (Jemperli) have been approved for the treatment of tumors, including prostate cancers, that have specific genetic features . Pembrolizumab has also been approved for any tumor that has metastasized and has a high number of genetic mutations .
But relatively few prostate cancers have these features, and prostate cancer in general has largely been resistant to treatment with checkpoint inhibitors and other immunotherapies, such as CAR T-cell therapy .
Research is ongoing to find ways to help the immune system recognize prostate tumors and help immune cells penetrate prostate tumor tissue. Studies are looking at whether combinations of immunotherapy drugs, or immunotherapy drugs given with other types of treatment, may be more effective in treating prostate cancer than single immunotherapies alone.
Targeted radiation therapy and PSMA
Scientists have developed targeted therapies based on PSMA, the same protein that is being tested for imaging prostate cancer. For treatment, the molecule that targets PSMA is chemically linked to a radioactive compound . This new compound can potentially find, bind to, and kill prostate cancer cells throughout the body.
In a recent clinical trial, men with a type of advanced prostate cancer who received a PSMA-targeting drug lived longer than those who received standard therapies . This trial led to FDA approval of the drug, Lu177-PSMA-617 (Pluvicto) , to treat some people with metastatic prostate cancer. Ongoing and planned clinical trials are testing PSMA-targeting drugs in patients with earlier stages of prostate cancer, and in combination with other treatments, including targeted therapies like PARP inhibitors and immunotherapy.
Personalized clinical trials for prostate cancer
Research is uncovering more information about the genetic changes that happen as prostate cancers develop and progress. Although early-stage prostate cancer has relatively few genetic changes compared with other types of cancer, researchers have learned that metastatic prostate cancers usually accumulate more mutations as they spread through the body.
These mutations may make men with metastatic prostate cancers candidates for what are called “basket” clinical trials of new drugs. Such trials enroll participants based on the mutations found in their cancer, not where in the body the cancer arose. In the NCI-MATCH trial , a high percentage of enrolled men with advanced prostate cancer had mutations that could potentially be targeted with investigational drugs.
NCI-Supported Research Programs
Many NCI-funded researchers working at the National Institutes of Health campus, as well as across the United States and world, are seeking ways to address prostate cancer more effectively. Some of this research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in prostate cancer.
- The Cancer Biomarkers Research Group promotes research on cancer biomarkers and manages the Early Detection Research Network (EDRN) . EDRN is a network of NCI-funded institutions that are collaborating to discover and validate early detection biomarkers.
- Within the Center for Cancer Research , the Prostate Cancer Multidisciplinary Clinic (PCMC) provides comprehensive consultations on diagnosis and treatment options to people with newly-diagnosed prostate cancer.
- The Prostate Specialized Programs of Research Excellence (Prostate SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Prostate SPOREs support the development of new therapies and technologies and studies to better understand how to prevent, monitor, and treat prostate cancer.
- The NCI Cancer Intervention and Surveillance Modeling Network (CISNET) focuses on using modeling to improve our understanding of which men are most likely to benefit from PSA-based screening. CISNET also studies treatment strategies for prostate cancer and approaches for reducing prostate cancer disparities.
- The NCI Genitourinary Malignancies Center of Excellence (GUM-COE) brings together scientists studying genitourinary cancers (GU) from across NCI’s Center for Cancer Research and the Division of Cancer Epidemiology and Genetics, as well as investigators who study GU malignancies in other institutes of NIH. The goal is to provide a centralized resource and infrastructure to accelerate the discovery, development, and delivery of interventions for the prevention, diagnosis, and treatment of these cancers.
- The Research on Prostate Cancer in Men with African Ancestry (RESPOND) study is the largest-ever coordinated research effort to study biological and non-biological factors associated with aggressive prostate cancer in African American men. The study , launched by NCI and the National Institute on Minority Health and Health Disparities in partnership with the Prostate Cancer Foundation, is looking at the environmental and genetic factors related to the aggressiveness of prostate cancer in African American men to better understand why they disproportionally experience aggressive disease.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for prostate cancer prevention , screening , and treatment .
Prostate Cancer Research Results
The following are some of our latest news articles on prostate cancer research:
- Enzalutamide Gets Added Approval for Prostate Cancer That Hasn’t Spread
- FDA Approves New Initial Treatment Option for Some Metastatic Prostate Cancers
- Is a Genomic Test Better at Finding Aggressive Prostate Cancer?
- Active Surveillance for Low-Risk Prostate Cancer Continues to Rise
- Darolutamide Extends Survival for Some People with Metastatic Prostate Cancer
- Shorter, More Intensive Radiation Safe after Surgery for Prostate Cancer
View the full list of Prostate Cancer Research Results and Study Updates .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Prostate cancer research: The next generation; report from the 2019 Coffey-Holden Prostate Cancer Academy Meeting
Andrea k. miyahira.
1 Science Department, Prostate Cancer Foundation, Santa Monica, California
2 Division of Clinical Studies, Institute of Cancer Research, London, UK
3 Department of Medicine, The Royal Marsden NHS Foundation Trust, London, UK
Leigh Ellis
4 Department of Oncologic Pathology, Dana-Farber Cancer Institute, Boston, Massachusetts
5 Department of Pathology, Brigham and Womenʼs Hospital, Harvard Medical School, Boston, Massachusetts
6 The Broad Institute of MIT and Harvard University, Cambridge, Massachusetts
Jennifer Jones
7 National Cancer Institute, Center for Cancer Research, National Institutes of Health, Bethesda, Maryland
Salma Kaochar
8 Department of Medicine, Baylor College of Medicine, Houston, Texas
H. Benjamin Larman
9 Division of Immunology, Department of Pathology, The Johns Hopkins School of Medicine, Baltimore, Maryland
David A. Quigley
10 Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, California
11 Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, California
12 Department of Pathology, University of California Los Angeles, Los Angeles, California
13 Department of Urology, University of California Los Angeles, Los Angeles, California
Jonathan W. Simons
Kenneth j. pienta.
14 Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins School of Medicine, Baltimore, Maryland
15 Department of Urology, The James Buchanan Brady Urological Institute, Baltimore, Maryland
16 Department of Pharmacology and Molecular Sciences, The Johns Hopkins School of Medicine, Baltimore, Maryland
Howard R. Soule
Introduction:.
The 2019 Coffey-Holden Prostate Cancer Academy (CHPCA) Meeting, “Prostate Cancer Research: The Next Generation,” was held 20 to 23 June, 2019, in Los Angeles, California.
The CHPCA Meeting is an annual conference held by the Prostate Cancer Foundation, that is uniquely structured to stimulate intense discussion surrounding topics most critical to accelerating prostate cancer research and the discovery of new life-extending treatments for patients. The 7th Annual CHPCA Meeting was attended by 86 investigators and concentrated on many of the most promising new treatment opportunities and next-generation research technologies.
The topics of focus at the meeting included: new treatment strategies and novel agents for targeted therapies and precision medicine, new treatment strategies that may synergize with checkpoint immunotherapy, next-generation technologies that visualize tumor microenvironment (TME) and molecular pathology in situ, multi-omics and tumor heterogeneity using single cells, 3D and TME models, and the role of extracellular vesicles in cancer and their potential as biomarkers.
Discussion:
This meeting report provides a comprehensive summary of the talks and discussions held at the 2019 CHPCA Meeting, for the purpose of globally disseminating this knowledge and ultimately accelerating new treatments and diagnostics for patients with prostate cancer.
1 |. INTRODUCTION
The Prostate Cancer Foundation (PCF) is a non-profit organization that funds global research focused on advancing the understanding of prostate cancer biology and the development of new treatments for men with aggressive and/or advanced disease. In addition to funding research, the PCF extends its service to the research community and patients by convening two annual research conferences and conducting other programs to accelerate the global exchange of knowledge, and create new research partnerships and initiatives in areas of critical unmet need in prostate cancer research.
The Coffey-Holden Prostate Cancer Academy (CHPCA) Meeting is an annual “think tank” conference, that has been held by PCF for seven consecutive years, and is named in honor of Dr. Stuart Holden and the late Dr. Donald Coffey, two great pioneers of prostate cancer research. 1 – 6 This conference convenes approximately 75 researchers, with a policy that the organizing committee and at least half of the attendees must be early-career investigators. The CHPCA meeting is designed to maximize discussion by organizing the agenda as short talks followed by long discussion times, in the model of the former NCI Prouts Neck Meetings on prostate cancer. 7 Each year, the meeting addresses highly innovative and critical topics of unmet need that have a significant potential for driving new discoveries and treatment developments in the prostate cancer field.
The 2019 CHPCA Meeting was held at the UCLA Luskin Conference Center in Los Angeles, California, from 20 to 23 June, 2019, and was themed “Prostate Cancer Research: The Next Generation.” The meeting was attended by 86 investigators, including 49 young investigators (57%). The talks and discussions centered on promising new prostate cancer treatment opportunities in preclinical and early clinical stages, issues, and opportunities for optimizing and combining treatments with checkpoint immunotherapy, next-generation technologies, including in genomics, transcriptomics, epigenomics, digital pathology, artificial intelligence, single-cell technologies, novel multifluidic and 3D methodologies to study tumor biology, tumor heterogeneity and other cells and activities in the tumor microenvironment (TME), and the biology and potential for extracellular vesicles as biomarkers.
2 |. DEFINING DRIVERS OF PROSTATE CANCER MOLECULAR AND CLINICAL SUBTYPES
Prostate cancer evolution depends on the acquisition of genomic alterations that drive disease progression. A number of genomic alterations that drive prostate cancer initiation have been identified, including ERG, SPOP, MYC, and androgen receptor (AR) alterations. 8 – 10 Studies of the molecular landscape of untreated primary prostate cancer have identified distinct molecular subtypes that are defined by underlying molecular aberrations. 9 , 10 SPOP is an E3 ligase adaptor protein and is commonly mutated in clinically localized and metastatic prostate cancers (overall approximately 10%); and defines a distinct molecular subclass of prostate cancer. 9 – 11 The biological significance of SPOP mutations in prostate cancer has been studied in detail. 12 , 13 A conditional mouse model of mutant SPOP (in the setting of PTEN loss) led to the formation of neoplastic lesions confirming SPOP drives prostate tumorigeneses. 12 Mutant SPOP drove increased proliferation and transcriptional signatures consistent with human prostate cancer in mouse prostate organoids. 12 Furthermore, these studies demonstrated that mutant SPOP activates phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling, and upregulates a network of AR associated transcription factors and co-activators. 12 Taken together, mutant SPOP effectively activates two pathways critical to the pathogenesis of prostate cancer.
Critically, a molecular subtype is rarely defined by a single genomic aberration. Consistent with this, deletion of the chromatin remodeler CHD1 commonly co-occurs with SPOP mutations in prostate cancer. 9 – 11 A recent study has interrogated the functional importance of CHD1 loss in prostate cancer. Loss of CHD1 leads to redistribution of the AR cistrome, driving a unique AR transcriptional signature enriched for pro-oncogenic pathways and tumor formation in the murine prostate. 14 This change in genome-wide AR binding was consistent with the oncogenic AR cistrome observed in prostate cancer samples. 14 Collectively, this study implicates CHD1 as a critical tumor suppressor in prostate cancer through regulation of AR function. Gene expression profiles from radical prostatectomies have also been utilized to clinically define these molecular subtypes. 15 Analyses by Barbieri et al found that men with SPOP-mutated prostate cancer have a better clinical outcome (measured by longer metastasis-free survival) compared with SPOP-mutated tumors that have an additional loss of CHD1, consistent with both genomic and clinical progression (Liu et al, manuscript in preparation). Going forward, it is important that studies of distinct molecular subtypes of prostate cancer interrogate the functional and clinical significance of underlying genomic aberrations to best guide treatment strategies and the development of novel therapies, to improve the outcome of men with lethal prostate cancer.
3 |. DRIVERS OF THE BENIGN TO MALIGNANT TRANSITION
While studies have identified a number of alterations that may drive the development of prostate cancer, these alterations alone do not drive or predict tumor aggressiveness. Identifying the genomic alterations that drive the benign to malignant transition in cancer cells is critical, as tumors can develop these at different pathological stages, and having biomarkers to identify which prostate cancers are at high-risk for progression will aid in earlier identification of patients who should receive more aggressive treatments.
Genomic alterations observed at the benign to malignant transition include loss of telomerase activity, telomere erosion, loss of p53, and loss of DNA damage-repair capability, which are associated with the onset of significant chromosomal instability, and select for the amplification of oncogenes and deletion of tumor suppressor genes. 16 Developing methods to identify and understand the biology of these genomic alterations in the setting of multifocal primary prostate tumors is a challenge.
The ability to develop faithful models of disease is critical to advancing the understanding of biology and developing better treatments for prostate cancer. Multiple mouse models as well as diverse human models are needed, due to the complexity and inter- and intra-patient heterogeneity of human prostate cancer.
Studies using genetically engineered mouse models (GEMM) have demonstrated that prostate-specific PTEN-loss results in the development of highly penetrant prostatic intraepithelial neoplasia (PIN) lesions that are poorly progressive, have long latency, and rarely develop into metastatic disease. 17 , 18 These data have led to the hypothesis that PTEN-deletion may activate a cell cycle checkpoint response that constrains progression. Studies in murine PTEN −/− prostate epithelial cells identified significant activation of the PI3K/AKT, p53, bone morphogenetic protein (BMP), and transforming growth factor β (TGFβ)-SMAD2/3/4 signaling pathways. 19 , 20 Mice with prostate-specific co-deletion of PTEN and p53 develop prostate cancer that is locally aggressive but not metastatic. However, mice with prostate-specific co-deletion of PTEN and SMAD4 developed rapidly aggressive, lethal prostate cancer, and 100% of mice developed lymph node and/or lung metastases, although no bone metastases were observed. 19 Expression of cellular movement, cell growth, and proliferation genes were significantly higher in PTEN −/− SMAD4 −/− prostate cancer compared with PTEN −/− p53 −/− prostate cancer cells. Moreover, the expression of PTEN/SMAD4-regulated genes was able to dichotomize prostate cancer patients into those at low vs. high-risk for biochemical recurrence (BCR), whereas a set of PTEN/p53 coregulated genes did not. 19 A four-gene set of direct SMAD4-regulated genes (PTEN, SMAD4, CCND1, and SPP1) was identified that was prognostic for lethal metastatic prostate cancer. 19 The expression of these genes (low PTEN and SMAD4; high CCND1 and SPP1) in prostate biopsy tissue microarray samples outperformed Gleason score in identifying lethal versus indolent cases, while combining the 4-gene PTEN/SMAD4 score with Gleason scoring outperformed either factor alone. 19 Altogether, these data identify the TGFβ-SMAD4 pathway as a major progression barrier in mouse and human prostate cancer and suggest that the expression of SMAD4-regulated genes is prognostic for outcomes. Whether this pathway serves as a treatment target and how to improve the prognostic potential of the 4-gene PTEN/SMAD4 score deserves further investigation.
Telomere erosion and telomere-based crisis may be a major driver of genomic instability and enables the acquisition of genomic alterations that drive cancer progression. GEMM mice able to model telomere erosion and telomere reactivation have been generated (G3/4 LSL - mTert) . When crossed onto the prostate-specific PTEN −/− p53 −/− background, mice developed prostate cancer with lumbar spine metastases (penetrance of 5/20, 25%). 21 PTEN −/− p53 −/− mTERT tumors also exhibited copy number alterations characteristic of human prostate cancer, including frequent MYC amplification and SMAD4 deletion. 21 Fourteen genes were identified that were commonly altered in human prostate cancer and PTEN −/− p53 −/− mTERT mice (SMAD2, SMAD7, RBL2, DCC, PARD3, ERCC3, MBD2, MTERF, ATP5A1, ATP6V1C1, CyC1, CUL2, PTK2, and SMAD4) and were associated with metastatic disease. Expression of these genes, combined with the 4-gene PTEN/SMAD4 score discussed above were highly predictive for BCR-free survival in two independent prostate cancer datasets and outperformed either gene set alone. 21 These findings demonstrate that appropriate mouse models can be useful for identifying genes and pathways that are clinically relevant in human prostate cancer.
Loss of SMAD4 may also drive the development of an immunesuppressive TME, as PTEN −/− SMAD4 −/− prostate tumors have increased numbers of infiltrating CD11b + myeloid-derived suppressor cells (MDSCs) and decreased numbers of CD8 + T cells, compared with PTEN −/− tumors. 22 Recruitment of MDSCs was found to proceed through YAP1-dependent upregulation of the chemokine CXCL5 in PTEN −/− SMAD4 −/− prostate tumors, which promoted recruitment of CXCR2-positive MDSCs. 22 Treatment of these mice with a CXCR2-inhibitor significantly decreased the number of intratumoral MDSCs and slowed tumor growth and progression. 22 Targeting MDSCs may be an effective treatment strategy in prostate cancer. In support of this, in prostate cancer GEMM models, checkpoint immunotherapy (anti-CTLA4 + anti-PD1) was highly synergistic in combination with multi-kinase inhibitors that impact MDSC function, such as cabozantinib and BEZ235, but not with dasatinib, which has strong inhibitory effects on T cells but not MDSCs. 23
Genomic deletion of tumor suppressor genes is a rite of passage for virtually all human cancers. Collateral lethality is a concept in which deletion of tumor suppressor genes can result in collateral deletion of neighboring “housekeeping” genes, but the cancer cells survive though the activities of redundant housekeeping genes. These redundant but essential paralogs may serve as promising cancer-specific therapeutic targets, numerous examples of which are being pursued by academic and pharmaceutical drug developers. 24 – 26 A similar concept is a form of synthetic lethality, termed synthetic essentiality, in which certain gene(s) that are never deleted in the context of a tumor suppressor gene loss, may be essential functional surrogates of tumor suppressor gene deficiencies and thus ideal therapeutic targets. 27 , 28 Synthetic essential gene pairs can also be identified by this mutually exclusively deletion pattern in the cancer genome. For instance, CHD1 was identified as a synthetic essential gene in prostate cancer with PTEN deletion. 28 In this study, CHD1 inhibition led to tumor growth suppression in PTEN-deficient but not in PTEN-intact prostate cancer models. 28 In PTEN-intact cells, CHD1 is constantly degraded, however, upon PTEN-loss CHD1 becomes stabilized and drives a nuclear factor κB (NF-κB)-dependent prostate cancer progression. 28 Studies to validate CHD1 as a therapeutic target and identify optimal combination treatment approaches are ongoing.
4 |. UTILIZING PREDICTIVE MEDICINE TO GUIDE TREATMENT FOR MEN WITH CASTRATION RESISTANT PROSTATE CANCER
The development of precise and accurate predictive biomarkers to guide therapy to clinically benefit men with castration-resistant prostate cancer (CRPC) remains an urgent unmet clinical need. Two promising predictive biomarkers under investigation are the constitutively active AR splice variant-7 (AR-V7; 10% to 75% of cases) that associates with decreased sensitivity to endocrine therapy, and DNA repair defects (20% to 25% of cases) that associate with sensitivity to PARP inhibitor therapy. 11 , 29 – 36 However, the development of these important predictive biomarkers is not without its challenges. The significance of AR-V7 testing may only be fully realized when it is studied with active AR-V7 targeting therapies, converting AR-V7 from a negative to a positive predictive biomarker. 37 , 38 Secondly, not all DNA repair defects confer sensitivity to PARP inhibition, with a recent study demonstrating that men harboring ATM mutations experienced inferior outcomes to PARP inhibitor therapy than those harboring BRCA1/2 mutations (discussed in additional detail below). Further studies will be important to define the optimal predictive biomarker suite for PARP inhibitor sensitivity to provide the greatest clinical benefit for men with lethal prostate cancer. 39 , 40
5 |. ELUCIDATING NOVEL FUNCTIONS OF DNA REPAIR PROTEINS TO SUPPORT NOVEL THERAPEUTIC STRATEGIES IN PROSTATE CANCER
DNA repair alternations are common (20%−25%) in lethal prostate cancer and are significantly enriched when compared with localized prostate cancer. 11 , 29 , 40 Clinical studies of PARP inhibitors (including olaparib and rucaparib) have demonstrated single-agent antitumor activity in men with metastatic CRPC (mCRPC). 31 , 40 The TOPARP-A trial demonstrated eight out of eight (100%) patients with BRCA1/2 alternations responded to olaparib (composite response assessment) with other DNA repair aberrations (such as ATM, PALB2, or FANCA) also being observed in some responding patients. 31 Beyond single-agent PARP inhibition in DNA repair defective prostate cancers, uncovering novel roles of DNA repair proteins in prostate cancer pathogenesis may identify therapeutic strategies to benefit men with lethal prostate cancer.
One potential candidate is DNA dependent protein kinase (DNA-PK) a key DNA repair protein. The AR plays an important role in DNA repair by promoting the resolution of double-strand breaks and resistance to DNA damage. 41 DNA-PK is a key target of AR during DNA damage, regulating AR-mediated DNA repair and cell survival. 41 In addition, DNA-PK functions as a selective modulator of transcriptional networks facilitating cell migration, invasion, and metastasis, and DNA-PK expression is associated with worse outcomes for men with prostate cancer. 42 More recently, pharmacological targeting of DNA-PK has been shown to inhibit prostate cancer cell growth and suppress DNA-PK mediated transcriptional processes, including novel functions, such as the regulation of epithelial-mesenchymal transition, immune responses, and metabolic processes. 43 Consistent with previous studies, specific targeting of DNA-PK suppressed AR signaling. In contrast, dual targeting of DNA-PK/TORK using CC-115, which is currently being evaluated in clinical studies, upregulated AR signaling and this could be repressed by targeting the AR with enzalutamide. 43 Finally, co-targeting of DNA-PK/TORK and AR has synergistic growth inhibitory effects in prostate cancer models, and this combination is currently being evaluated in men with CRPC. Taken together, these data demonstrate how detailed interrogation of protein function can lead to bench-to-bed discoveries that have the potential to impact the management and treatment of men with lethal prostate cancer.
6 |. TROP2 AS A NOVEL THERAPEUTIC TARGET IN CRPC
In the search to define the molecular mechanisms driving prostate cancer progression and develop new therapeutic approaches for patients with CRPC, Trop2 has emerged as a potential therapeutic target. Trop2 is a transmembrane glycoprotein expressed in a subpopulation of murine and human prostate basal cells with stem cell characteristics 44 – 47 and is overexpressed in human epithelial cancers. New data suggest that Trop2 is highly expressed in CRPC/neuroendocrine prostate cancer (NEPC) and drives the NEPC phenotype. High levels of Trop2 correlate with biochemical recurrence. Overexpression of Trop2 enhances prostate tumor formation, tumor growth, metastasis, and resistance to androgen ablation, while the loss of Trop2 significantly delays tumor formation, tumor growth and prostate cancer metastasis in vivo. Trop2-driven CRPC/NEPC is sensitive to PARP1 inhibition and PARP inhibitors reverse Trop2-driven NEPC features.
These observations lead to novel opportunities for CRPC/NEPC treatment via targeting Trop2 therapeutically using: (a) small molecule inhibitors of Trop2; (b) antibody-mediated targeting; and (c) pharmacological inhibition of Trop2 downstream targets. In particular, monoclonal antibodies against Trop2 have potent anticancer activity in xenograft models of various carcinomas. Sacituzumab govitecan, an antibody-drug conjugate targeting Trop2-positive cells, has yielded durable objective responses in patients with heavily pretreated metastatic triple-negative breast cancer (TNBC). 48 These discoveries have resulted in the recent opening of a clinical trial testing sacituzumab govitecan in patients with mCRPC progressing on abiraterone or enzalutamide ( ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03725761","term_id":"NCT03725761"}} NCT03725761 ).
7 |. IDENTIFYING DRUGGABLE TARGETS IN NEPC
While androgen deprivation therapy (ADT) remains the backbone for prostate cancer treatment, the use of more potent ADT regimens has led to an increased emergence of resistance mechanisms independent of AR activity. This lethal phenotype adapts to ADT via lineage plasticity and is no longer reliant on AR expression and signaling. These tumors can typically display neuroendocrine features, altered kinase signaling, stem and basal cell gene expression patterns, and characteristic epigenetic alterations. 49 – 52 Recent preclinical and clinical data have identified key molecular events driving this phenotype including combinatorial loss-of-function of tumor suppressor genes including PTEN , RB1 , and TP53 . 52 , 53 Also, distinct epigenome rewiring is indicated by DNA methylation patterns, 52 global repositioning of histone marks (L. Ellis et al, unpublished; M. Freedman et al, manuscript submitted) and overexpression of epigenetic regulators including LSD1 54 and EZH2. 49 , 52 Further, inhibition of EZH2 has demonstrated re-sensitization to ADT in preclinical models. 49 , 55
Another emerging driver of CRPC/NEPC is the transcription factor POU3F2 (BRN2). BRN2 is a master transcription factor that controls neuronal differentiation during development and is sufficient to drive neuronal differentiation in embryonic stem cells and fibroblasts. It is highly expressed in neuroendocrine or small cell lung cancer (both de novo and treatment-induced). In prostate cancer, AR directly suppresses BRN2 transcription. As a result, BRN2 expression is the highest in NEPC tumors and significantly increased compared with adenocarcinoma. 56 BRN2 is distributed to chromatin sites enriched with stem cell and neuronal-associated binding motifs and regulates neuronal differentiation programs. Thus, BRN2 is sufficient to induce NEPC and is required for enzalutamide-induced NEPC. BRN2 knockdown suppresses NEPC growth in vitro and in vivo. Therefore, targeting BRN2 is a promising strategy to treat or prevent NEPC.
Zoubeidi et al have used X-ray crystallography of BRN2 and virtual compound screening to identify a BRN2 inhibitor. Their lead compound reduced the affinity of BRN2 for DNA and displaced it from chromatin sites that it regularly occupies in NEPC, resulting in suppressed expression of NEPC drivers and markers (EZH2, ASCL1, SOX2, and PEG10), and increased expression of luminal markers. Treatment with the BRN2 inhibitor also suppressed the expression of NEPC markers and the growth of NEPC cells in vivo. 57 , 58 Collectively, these results provide a promising pathway towards targeting BRN2 and NEPC in the clinic.
8 |. EXPLOITING METABOLIC LIABILITIES IN PROSTATE CANCER
The majority of studies exploring novel therapeutic opportunities in prostate cancer focus on the regulation of gene transcription and messenger RNA (mRNA) synthesis via targeting transcription factors and their complexes. However, an emerging and promising field is examining the regulation of mRNA translation and protein synthesis by growth factors, oncogenic pathways, and stress signals (both internal and external), to unveil specific vulnerabilities that can be exploited for cancer treatment. Cancer cells require rapid protein synthesis to meet their needs for high-speed growth, but this addiction also creates a liability as excessive protein synthesis is bioenergetically demanding and can be toxic to the cell via increased flux of misfolded proteins into the endoplasmic reticulum. Therefore, cancer cells need to be able to handle this form of proteotoxic stress.
Prostate cancer GEMM models driven by either PTEN-loss or hyperactivation of MYC exhibit significantly increased global protein synthesis by about 20%, an expected range for such potent drivers of prostate cancer development. 59 Unexpectedly however, significant suppression in global protein synthesis was observed in mice harboring both genetic events, despite the fact that these mice develop more aggressive prostate cancer. 59 The combination of MYC-gain and PTEN-loss was found to trigger the unfolded protein response, a response to cellular stress that suppresses protein synthesis via phosphorylation of the eukaryotic initiation factor 2-α(P-eIF2α). 59 In support, an inhibitor of P-eIF2α activity, ISRIB 60 , was able to target this adaptive mechanism and selectively trigger cytotoxicity in aggressive patient-derived prostate cancer xenograft models. 59
9 |. EXPLOITING THE AR AXIS AS A CELL CYCLE LIABILITY IN CRPC
While AR inhibition has been the backbone of advanced prostate cancer treatment since the original observations of Huggins and Hodges, 61 there is also substantial preclinical and clinical evidence that overactivation of AR can have inhibitory effects on prostate cancer growth. 62 – 67 A proposed explanation for the latter effect is that, during prostate carcinogenesis, AR gains an oncogenic function in DNA replication licensing during the S-phase and binds to DNA at origins of replication sites as part of the origin of replication complex (ORC), and must be removed via its degradation in late mitosis. As CRPC cells have significantly elevated AR protein levels due to adaptive autoregulation, as part of their adaptation to AR axis inhibition, their exposure to pharmacologic doses of testosterone results in over-stabilized and ORC-bound AR, which inhibits DNA relicensing, resulting in cell death in the subsequent cycle. 62 , 65 This has led to the hypothesis that men with CRPC may benefit from rapid cycling between polar extremes of supraphysiologic and castrate testosterone levels, a treatment strategy known as bipolar androgen therapy (BAT). Clinical trial data suggest that BAT is a safe therapy that can yield responses in asymptomatic men with mCRPC and also may resensitize CRPC to androgen ablative therapies in patients undergoing rechallenge. 67 Ongoing studies are aiming to define the exact role for BAT in CRPC management and the optimal strategy for sequencing between androgen and anti-androgen therapies to maximize patient benefit. Moreover, it has been demonstrated that supraphysiologic levels of testosterone (SupT) induce DNA double-strand breaks in prostate cancer cells and promote autophagic degradation, which can activate cytoplasmic DNA sensing pathways and downstream innate immune signaling. These findings suggest that BAT engages the immune system to inhibit tumor growth and that combination of BAT with existing immunotherapeutics, including immune checkpoint blockade, may prove beneficial for the treatment of CRPC. 68
10 |. NUCLEAR PORE-RELATED MECHANISMS AS POTENTIAL THERAPEUTIC TARGETS
The development of new therapies targeting AR signaling, such as abiraterone acetate and enzalutamide has led to improved outcomes for patients with mCRPC and de novo metastatic castration-sensitive prostate cancer. 69 – 76 However, resistance to abiraterone and enzalutamide remains common, with nearly all cases of the advanced disease being fatal. Therefore, as patients develop resistance to AR-targeting therapies earlier in their disease history, there will be an urgent clinical need to develop novel therapeutic strategies that provide further clinical benefit for this emerging patient population.
Nuclear pores are involved in multiple cellular processes including nucleo-cytoplasmic transport, DNA replication, and DNA damage, mitosis and chromosome stability, and gene expression. 77 , 78 Importantly, nucleoporins from which the nuclear pore complex is derived, are deregulated during prostate cancer disease progression. 79 POM121, a nucleoporin, has been shown to be critical for prostate cancer aggressiveness. POM121 enhances the importin beta function that promotes nuclear import of key oncogenic (E2F1 and MYC) and prostate-specific (AR and GATA2) transcription factors that drive prostate cancer progression. 79 Consistent with this, genomic abrogation of POM121 reduced AR and AR-V7 nuclear localization, and is associated with decreased AR signaling in treatment-resistant prostate cancer models; this may provide an attractive therapeutic strategy to overcome oncogenic AR-V7 signaling. 79 Furthermore, targeting the POM121-importin beta axis decreased tumor growth, re-sensitized to standard therapy, and improved survival in prostate cancer models. 79 Interestingly, studies have shown a novel role for nucleoporins beyond nuclear pore formation and function, in modulating prostate cancer cell biology through potential direct gene expression regulation (Rodriguez-Bravo et al, unpublished). Taken together, these data suggest that targeting nuclear pore-related mechanisms may have potential as a therapeutic strategy for the treatment of lethal prostate cancer. Further studies will be required to provide a deeper understating of these processes to ensure such strategies are not associated with increased toxicity, and to identify those patients most likely to benefit from these therapeutic approaches.
11 |. OPTIMIZING IMMUNOTHERAPY FOR PROSTATE CANCER
Immunotherapy, particularly treatments that target T cell inhibitory molecules (“checkpoints”), such as programmed cell death protein-1 (PD-1) have proven to be an effective treatment for multiple cancer types 80 , 81 For instance, in metastatic melanoma, median overall survival outcomes for patients receiving nivolumab + ipilimumab have been recently reported as >60.0 months (not reached). 82 While a subset of patients receiving these therapies experience favorable responses, many still experience disease progression, 80 , 81 , 83 , 84 high-lighting the complexity of the tumor immune microenvironment and the heterogeneity of antitumor immune responses. In prostate cancer, checkpoint immunotherapy has yet to be optimized.
12 |. MOLECULAR SUBTYPES THAT ARE SENSITIVE TO CHECKPOINT INHIBITORS
One area of intense investigation is the development of predictive biomarkers to identify men with CRPC who will respond to treatment with immunotherapy. Currently, despite evidence of antitumor activity, the overall response rate to pembrolizumab in molecularly unselected CRPC is low. 85 , 86 A recent study of anti-PD-1/PD-L1 therapy in men with microsatellite instability (MSI)-high/mismatch repair deficient (dMMR) (approximately 3% of cases) prostate cancer demonstrated 6 out of 11 patients (54.5%) achieved ≥50% prostate-specific antigen (PSA) response. 87 A further molecular subgroup of patients with CRPC (approximately 6.9% of cases) with potential to benefit from immunotherapy are those harboring biallelic loss of CDK12, which associates with increased gene fusions, neoantigen burden, T cell infiltration, and early ≥50% PSA responses (two out of four patients; 50%) to anti-PD-1 therapy. 88 More recently, a study of 48 men with CRPC who received pembrolizumab demonstrated 18 out of 48 patients (38%) achieved ≥50% PSA response. 89 Furthermore, 4 out of 48 patients (8%) achieved ≥90% PSA response, and two of these four patients had mutations in LRP1b, one of whom also had MSH2 loss and was MSI-high and tumor mutational burden-high. 89 These data provide further evidence of clinical benefit from pembrolizumab therapy in men with CRPC and suggest LRP1b mutations may enrich for anti-PD-1 therapy responsiveness. Taken together, although MSI-high/dMMR, CDK12 loss, and LRP1b mutations are relatively uncommon in CRPC, the enrichment for response to immunotherapy is therapeutically meaningful and requires further evaluation.
13 |. PROSTATE CANCER LACKS STEMNICHES TO SUPPORT EFFECTIVE ANTITUMOR IMMUNITY
Recent studies have shown that stem-like CD8 + cells are associated with response to checkpoint blockade therapy. 90 , 91 In renal and prostate cancer, dendritic cells were observed to closely interact with a stem-like population of CD8 + cells, together constructing a stem-niche where antigen-presenting dendritic cells activate the stem-like CD8 + cells and maintain their proliferation (Kissick, et al, manuscript in press). These stem-niches were enriched in highly immune-infiltrated, that is, “hot,” renal tumors compared with “cold” renal tumors, and may play a key role in reinvigorating and expanding CD8 + cells in response to immune checkpoint blockade. However, these niches were extremely rare in prostate cancer, which may explain their non-responsiveness to anti-PD-1/PD-L1 drugs. Determining the cause of the stem-niche-poor environment of prostate cancer and how to facilitate the seeding of stem-niches in prostate cancer could be of critical importance. In contrast to the rapid T cell activation response and lymphocyte trafficking between lymph nodes and target organs that typically occurs quickly after an acute viral infection, differentiated CD8 + T cells migrate to the tumor to seed the stem-niches in a delayed fashion in response to cancer antigens. A provoking hypothesis to be tested is that poor infiltration of vasculatures (blood vessels and/or lymphatic channels) in prostate cancer significantly compromises the delayed migration of dendritic cells and stem-like CD8 + cells from peripheral blood or lymph nodes into the tumors. Current ongoing studies are aiming to increase vasculature infiltration and test its effects in promoting the establishment of stem-niches and immune response in prostate cancer.
14 |. THE ROLE OF IMMUNOTHERAPY FOLLOWING OR IN COMBINATION WITH ANTI-ANDROGEN THERAPY
The prostate cancer immune microenvironment is complex, and gene expression patterns of single cells likely harbor important prognostic information. Recent single-cell RNA sequencing (sc-RNA-seq) studies of melanoma, 90 breast cancer, 92 , 93 and other solid tumors 94 – 97 have unveiled the remarkable heterogeneity of the tumor-infiltrating immune cell landscape. However, studies of mCRPC have not been adequately performed, due in part to challenges associated with obtaining bone biopsies, the most common site of metastatic disease. 98 , 99
Using the 10X platform, CD45-positive cells were analyzed from metastatic lesions of patients with mCRPC who had progressed on enzalutamide and were subsequently treated with pembrolizumab. T cells were the most abundant cell type across all lesions examined (bone, lymph node, liver) including from both responding and non-responding patients. A more focused analysis of single CD8 + T cell expression profiles revealed a gene signature associated with response to PD-1 blockade in the enzalutamide-resistant setting. Importantly, this signature could be retrospectively validated in an analysis of bulk RNA-seq data. A simplified gene expression signature capable of stratifying patients who could benefit from PD-1 blockade therapy may provide an important biomarker to determine which enzalutamide-resistant patients should move on to immunotherapy treatment.
15 |. COMBINATION TREATMENT APPROACHES TO IMPROVE CHECKPOINT IMMUNOTHERAPY
Another route to improving the efficacy of checkpoint immunotherapies is to identify synergistic treatment combinations. Numerous clinical trials investigating inhibition of PD-1 in combination with a second treatment to enhance responsiveness have been undertaken. Beginning with a single combination trial in 2009, there were over 1100 such registered trials in 2017. 100 As more and more therapies are considered for combination treatments, the possible combinations may soon exceed the number of eligible cancer patients. 100 New approaches are therefore needed to determine rational drug combinations with the highest likelihood of exhibiting synergy.
16 |. CANCER VACCINES IN COMBINATION WITH CHECKPOINT IMMUNOTHERAPY
Despite prostate cancer being viewed as an immunologically “cold” tumor, there has been a sustained interest in vaccines that activate antitumor T cell responses. In particular, DNA vaccines would ideally be used to activate tumor-specific cytolytic CD8 + T cells as a treatment for early recurrent prostate cancer. In murine studies, engineered CD8 epitopes with increased binding to MHC class I molecules induce the upregulation of PD-1 on antigen-specific CD8 + T cells. The activity of these cells negatively impacted antitumor responses. 101 Interference with the expression or function of PD-1 at the time of T cell activation by vaccine improved antitumor immunity. 102 , 103 These preclinical findings were translated into a trial for patients with mCRPC, which tested a DNA vaccine encoding prostatic acid phosphatase (PAP) in combination with pembrolizumab. As in the preclinical model, PD-1 blockade, when used at the time of T cell activation by vaccine, led to PSA declines and objective tumor responses. 104 In follow up studies aimed at deciphering mechanisms of resistance in the setting of combination vaccine and PD-1 checkpoint blockade, increased expression of indoleamine 2,3-dioxygenase (IDO) was observed following treatment, and occurred to the greatest extent in patients that failed to mount a clinical response. 105 In addition to PD-1 checkpoint blockade, concurrent inhibition of IDO may therefore further enhance the activity of engineered prostate cancer vaccines.
The PSA-directed vaccine Prostvac has shown promising results in a few phase II trials through provoking PSA-specific immune responses, however Prostvac failed to meet the primary endpoint of an improvement in overall survival in a randomized double-blinded phase III trial in patients with mCRPC. 106 – 108 To understand this failure and identify alternate therapeutic strategies to move forward with, samples from patients treated with Prostvac have been further evaluated. A recent study demonstrated that Prostvac increased tumor-infiltrating lymphocytes (TILs) in different tumor compartments in localized prostate cancer in a phase II neoadjuvant trial ( {"type":"clinical-trial","attrs":{"text":"NCT02153918","term_id":"NCT02153918"}} NCT02153918 ) (N = 27). Both non-compartmentalized and compartmentalized analyses were utilized to examine the distribution of immune cells in the post-treatment prostatectomy tissue compared with that in the baseline biopsy tissue. The non-compartmentalized analysis revealed a significant increase in CD4 + TILs but not CD8 + TILs in the post-treatment tumors. Using compartmentalized analysis by separating tumor environment into core of tumor (CT), invasive margin (IM), and benign glands (NL), 109 the study discovered a distinct influx of TILs in different spatial compartments. Prostvac increased CD4 + TILs in IM and NL, not in CT, and increased CD8 + TILs in CT but not in IM or NL. Further analysis of spatial heterogeneity revealed that there was increased peak density and heterogeneity of CD4 + and CD8 + TILs in all three compartments post-treatment, indicating an immune stimulation effect. The study also looked into the changes in regulatory T cells (T REG ), activated T cells, and PD-L1 expression post-treatment (Sater et al, unpublished). It is yet unknown if Prostvac changes distributions of immunosuppressive MDSCs or proximity of different T cell subsets in the tumor. The underlying biology and potential therapeutic implications of the spatial-dependent immune modulation by Prostvac also await further investigation.
17 |. TARGETING EZH2 TO ENHANCE RESPONSES TO CHECKPOINT IMMUNOTHERAPY
Across many cancers, two factors that seem most strongly associated with response to checkpoint blockade are tumor mutational burden and an inflammatory gene expression profile. 110 In a pan-cancer study of these parameters, prostate cancer was found to have both low tumor mutational burden and an immunologically “cold” gene expression profile. 111 While the prostate cancer tumor mutational burden is relatively low and cannot easily be therapeutically manipulated, recent data have demonstrated that epigenetic therapy can alter gene expression profiles and potentially reverse resistance to checkpoint blockade. In a recent phase II study testing the efficacy of histone deacetylase (HDAC) inhibition via entinostat in combination with pembrolizumab in 53 men (ENCORE 601), an overall response rate of 19% was observed, with a median duration of the response being 12.5 months. 112 These promising clinical data support additional studies in combining epigenetic modulation with immune checkpoint inhibition.
EZH2 is an essential component of the polycomb repressive complex 2 (PRC2), which maintains epigenetic programs important for development and cellular differentiation. It has recently been reported that EZH2-mediated histone H3 lysine 27 tri-methylation represses the production of T helper 1 (T H 1) chemokines in the ovarian TME, which may provide a mechanism for tumor immune evasion. 113 In further support of this hypothesis, levels of PRC2 components, most notably EZH2, were inversely associated with critical T H 1-type chemokines in colon cancer tissue. 114 Using an EZH2 activity score, prostate cancers with low EZH2 activity demonstrate enrichment of an interferon gene signature. Low EZH2 activity was also found to associate with increased PD-L1 mRNA and protein. Therapeutic inhibition of EZH2 in combination with checkpoint blockade may therefore be a promising approach to the treatment of prostate cancer. 115 In the Hi-MYC mouse model of prostate cancer, chemical inhibition of EZH2 synergized with PD-1 blockade to significantly slow tumor growth. This effect was dependent upon upregulation of tumor PD-L1, and was associated with enhanced CD4 + and CD8 + T cell trafficking, activation of CD8 + T cells, and skewing of the tumor-associated macrophage (TAM) population toward the M1 phenotype. 116 Efforts are now being made to open a clinical trial testing EZH2-inhibitors in combination with anti-PD-1/PD-L1 checkpoint immunotherapy in prostate cancer.
18 |. DDR2 AS A POTENTIAL NEW TARGET TO ENHANCE RESPONSES TO CHECKPOINT IMMUNOTHERAPY
In vivo screens of pooled RNA interference (RNAi) knockdown libraries or CRISPR-mediated gene deletion libraries have been used to identify putative new targets to enhance responsiveness to immunotherapy. 117 In one such screen, the collagen receptor discoidin domain receptor tyrosine kinase 2 (DDR2) was identified as a target to enhance responsiveness to anti-PD-1 immunotherapy. In proof-of-principle studies, DDR2 knockdown synergized with anti-PD-1 therapy in isogenic murine models of bladder cancer, breast cancer, colon cancer, sarcoma, and melanoma. Combination treatment of tumor-bearing mice with anti-PD-1 and dasatinib, a tyrosine kinase inhibitor of DDR2, led to enhanced tumor control and in some cases, complete clearance rarely seen with monotherapy. This work provides a scientific rationale for targeting DDR2 in combination with PD-1 inhibitors, and more generally for synergistic genetic screens using in vivo preclinical models. Studies to evaluate the potential for DDR as a treatment target to enhance immunotherapy responses in prostate cancer are needed.
19 |. B7–H3 AS A POTENTIAL CHECKPOINT IMMUNOTHERAPY TARGET IN PROSTATE CANCER
Prostate cancer expresses a low level of PD-L1 (B7–H1), which is thought to be the main reason for the limited success of anti-PD-1/PD-L1 in prostate cancer patients. Efforts have been focused on identifying alternative immune checkpoint targets in prostate cancer.
A B7 family molecule, B7–H3, has been found to be commonly expressed in benign prostatic tissue, with increased expression in prostate cancer. Further, its higher expression was associated with worse disease outcomes. 118 A humanized Fc-optimized anti-B7–H3 antibody, enoblituzumab, 119 was evaluated in a phase I trial and demonstrated benefits in tumor reduction in prostate cancer among multiple tumor types. 120 Together these results indicate that B7–H3 is a potential immune-modulating target in prostate cancer. A phase I/II neoadjuvant trial, treating patients with intermediate to high-risk localized prostate cancer with enoblituzumab, recently completed enrollment at Johns Hopkins Hospital ( ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT02923180","term_id":"NCT02923180"}} NCT02923180 ), with clinical endpoints maturing and correlative data analysis ongoing. Single-plex immunohistochemistry revealed a significant increase of CD8 + TILs but not T REGS in post-treatment tumor tissue. An expanded high-plex study using the Nanostring DSP platform is ongoing to examine 40 protein targets (mainly immune markers) as well as RNA profiles in treated tumors, using matched untreated tumors as controls. In each tumor, 12 regions of interest (ROIs), including 10 tumor ROIs and two normal ROIs were profiled. Early data revealed a significant upregulation of T cell related immune markers including cytotoxic molecules in treated tumors compared with controls (Shenderov et al, unpublished).
20 |. IL-8 AS A NOVEL TREATMENT TARGET IN COMBINATION WITH CHECKPOINT IMMUNOTHERAPY AND ADT
Resistance to ADT and progression to castration-resistant disease remains a major challenge in the clinic. Recent evidence suggests that ADT can augment the response to immune checkpoint blockade. 85 , 121 In line with this, recent work utilized a Hi-MYC-CaP cell line 122 in vivo transplant model to identify novel vaccination targets that can be induced by ADT. Instead of identifying novel neoantigens, it was observed that a significant shift in the expression of chemokines occurred. The myeloid chemoattractant chemokine, interleukin-8 (IL-8 or CXCL8), has been demonstrated to be upregulated in human prostate cancer and largely mediated by NF-kB (p65) transcriptional activity. 123 Further, a novel AR binding motif was identified in the proximal IL - 8 promoter, such that AR negatively regulates IL-8 gene expression. Thus, ADT increases IL-8 expression in both patient samples and cell lines. Preclinical in vivo modeling convincingly showed that ADT-induced tumor production of IL-8 was associated with increased tumor infiltration of polymorphonuclear MDSCs (PMN-MDSCs). Antagonism of the IL-8 receptor, CXCR2, or genetic knockout of the IL-8 mouse homolog (Cxcl15) demonstrated therapeutic synergy with ADT, decreasing PMN-MDSC tumor infiltration and generating a tumor response to anti-CTLA4 checkpoint blockade. 124 This work has led to the initiation of a Phase Ib/II trial in patients with hormone-sensitive prostate cancer to investigate either nivolumab or nivolumab plus an anti-IL-8 antibody in combination with short-term degarelix (MAGIC-8, {"type":"clinical-trial","attrs":{"text":"NCT03689699","term_id":"NCT03689699"}} NCT03689699 ). Among numerous primary objectives, investigators will utilize single-cell RNAseq (sc-RNAseq) from patient samples to identify and understand necessary alterations of the prostate TME that underlie therapeutic response.
21 |. TARGETING M2-TUMOR-ASSOCIATED MACROPHAGES TO OVERCOME TUMOR IMMUNOSUPPRESSION IN PROSTATE CANCER
Although recent studies of anti-PD-1 therapy with pembrolizumab in unselected patients with CRPC have demonstrated antitumor activity, the overall response rates have been low. 85 , 86 Despite efforts to identify predictive biomarkers and enrich for patients that respond to checkpoint inhibitors, it is also important to consider other immunotherapeutic strategies that may enhance efficacy and improve clinical benefit for men with lethal prostate cancer. 87 – 89 , 125 One potential strategy is to target M2-TAMs, which are consistently being attracted to cancer lesions where they scavenge debris and facilitate the growth and dissemination of the tumor. 126
Studies of homogeneous human populations of M1- and M2-TAMs have characterized cell surface markers enriched on M2-TAMs that could be utilized as therapeutic targets to abrogate their function. 127 , 128 Using solid-phase extraction of N-glycopeptides followed by liquid chromatography-tandem mass spectrometry on homogeneous macrophage populations, CD206 was demonstrated to be enriched on M2-TAMs (compared with M1-TAMs) and N-glycosylated. 127 In addition, infiltration of CD206-positive macrophages increased in a stepwise fashion from normal prostate tissue to primary untreated castration-sensitive disease, to castration-sensitive regional lymph node metastasis, and to metastatic castration-resistant disease. 129 Importantly, a peptide (RP-182) that binds to the fibronectin type II domain of the extracellular portion of CD206 has been shown to decrease the viability of human M2-TAMs, but not M1-TAMs, in vitro (Jaynes, et al., in press). These studies suggest that targeting CD206 has the potential to decrease the viability of immunosuppressive M2-TAMs within the prostate cancer microenvironment, which may provide an attractive method to augment immunotherapeutic responses in lethal prostate cancer.
22 |. HYPOXIA DRIVES TUMOR IMMUNE SUPPRESSION AND IMMUNOTHERAPY RESISTANCE
Despite our best efforts to elicit adaptive immune responses, whether it be by checkpoint blockade therapy, either alone or in combination with an orthogonal therapy, epigenetic modification, or vaccination, important metabolic impediments may also need to be addressed. 130 For instance, hypoxia is known to be prevalent in prostate cancer across preclinical models and in patients. Because T cells fail to accumulate in hypoxic niches, recruitment of suppressive stromal cells tend to dominate these microenvironments. 131 The hypoxia-activated prodrug evofosfamide (TH-302) reduces hypoxia in prostate cancer through a tissue-remodeling process. Interestingly, hypoxia reduction with TH-302 sensitizes otherwise resistant murine models of prostate cancer to blockade of CTLA-4 and PD-1. TH-302 treatment reduces MDSC density in prostate tumor tissue by roughly twofold. In this reprogrammed environment, the combination of TH-302 and checkpoint blockade results in enhanced CD8 + T cell proliferation, cytotoxicity, cell survival, and production of effector cytokines. 132 In a phase I study combining evofosfamide and ipilimumab, patients with hormone-refractory metastatic prostate cancer experienced objective clinical responses. This is important, since this patient population has relatively few therapeutic alternatives. These positive data have generated excitement for a phase II trial combining evofosfamide with dual CTLA-4 and PD-1 blockade, which is planned for enrollment beginning in 2020 at MD Anderson Cancer Center.
23 |. STROMAL REGULATION OF PROSTATE CANCER DEVELOPMENT
The role of nonimmune stromal cells, such as myofibroblasts or fibroblasts within the prostate tumor microenvironment is a critical question. Prostate cancer initiation and progression have been found to involve extensive interaction with surrounding myofibroblasts or fibroblasts that undergo histopathological changes during disease advancement. It was demonstrated that in vivo co-implantation of non-tumorigenic human prostate epithelial (BPH-1) cells with human prostatic carcinoma-associated fibroblasts (CAFs) resulted in the malignant transformation of BPH-1 cells. This work highlighted the potential of stromal fibroblasts to promote prostate cancer initiation and progression. 133 In a recent study, laser capture microdissection on human prostatectomy samples was performed to capture and profile gene expression exclusively from epithelial cells or stroma cells. Interestingly, it was a 29-gene signature from the stromal compartment that predicted the aggressiveness and metastatic potential of these prostatectomy samples. 134 Utilizing a TMPRSS2:ERG-knock-in prostate cancer GEMM model, it was noted that the epithelial compartment exhibited minor changes, whereas significant alterations of stroma adjacent to the epithelial compartment were observed (Pakula et al, unpublished). Use of single-cell RNA-seq identified enrichment of Wnt signaling genes within the prostatic stroma (Pakula et al, unpublished). As this work matures, it will be important to elucidate the extent of Wnt signaling enrichment in stromal cells and its specific role in driving therapeutic resistance and/or metastatic progression. Moreover, identification and validation of therapeutic targets incorporated with the use of a stromal signature may offer patient selection to receive specific stromal-targeted therapies.
24 |. NEXT GENERATION TISSUE-BASED ASSAYS TO VISUALIZE TUMOR MOLECULAR PATHOLOGY IN SITU
Various next-generation tissue-based assays are now available for investigators to study novel biomarker targets or to measure large panels of antibody and nucleic acid probes simultaneously without losing information on spatial relationships. These tools enable us to investigate interactions among different molecular targets or among different cell populations in vivo. Studies using these assays have advanced our understanding of the prostate cancer-specific TME and illustrated therapeutic modulation of the immune microenvironment as exemplified by several studies discussed above. In addition, artificial intelligence has taken a promising step forward, demonstrating a trained computational image analysis algorithm is able to predict disease outcome using plain hematoxylin and eosin (H&E) slides of primary prostate cancer.
Digital image analysis and machine learning can discover hidden microscopic morphological features that are associated with disease aggressiveness. In prostate cancer, neuroendocrine differentiation (NED), and chromosomal instability (CIN) of tumor cells are both associated with an aggressive disease phenotype. A study assessed the ability of a computer algorithm that captures morphological features related to NED and CIN in predicting disease outcomes, using a cohort of high-grade prostate cancers from biopsy tissue of M1 (de novo metastasis; N = 150) and M0 (stable disease 5 years after treatment; N = 102) patients. First, a CIN-feature computational model was built and shown to predict disease outcomes with a similar performance as the classic CIN70 RNA-signature. 135 Next, an algorithm that distinguishes small cell-neuroendocrine and adenocarcinoma based on morphometric features was trained. The algorithm provides a SC-NED score, which is the likelihood of a cancer being small cell carcinoma. Combining SC-NED score, CIN-features, serum PSA, and age, the 4-variable model was able to predict metastasis and prostate cancer-specific survival with an area under the curve (AUC) of 0.9. Interestingly, gene signatures correlative to the SC-NED score included genes involved in radiation and docetaxel-resistance, discohesiveness, proliferation, and stemness. Further, the expression of a gene signature derived from the SC-NED score significantly increased from low to high-grade primary to metastatic prostate cancer (Knudsen et al, unpublished). The study is a convincing example that machine learning will play an integral role in assisting with the assessment of aggressive behavior of prostate cancer in core needle biopsies at the time of diagnosis. In addition, image analysis can extract hidden information from H&E slides that can be combined with genetic analysis to improve personalized patient care.
There is an urgent need for novel assays that go beyond the detection of protein and mRNA expression and allow investigations of epigenetic alterations, protein-protein interactions, and protein-DNA interactions in clinical tumor samples in situ. To this end, two novel assay platforms were developed that allow the detection of DNA repair alterations and site-specific DNA methylation alterations in situ. Both assays are based on the principles of rolling circle amplification (RCA) and the proximity ligation assay (PLA). 136 , 137 Theses assays work in formalin-fixed paraffin-embedded material and rely on the amplification of a circular DNA probe by isothermal amplification to generate a DNA nanoball that can be visualized directly in the tissue. The first assay, termed in situ DNA damage response (IDDR) assay, relies on the combination of two assay components, PLA and the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The TUNEL assay allows for unbiased labeling of DNA breaks using the enzyme terminal transferase. PLA uses isothermal RCA to detect two interacting targets that are in close spatial proximity (<100 nm). The combination of these two assay principles in the IDDR assay allows for the detection of binding of any DNA repair protein to sites of DNA damage in tissue and is currently being evaluated in two PARP inhibitor trials for its predictive value for treatment response (Haffner et al, unpublished). The second assay is an in situ assay to label sequence-specific DNA methylation. Cancer-specific DNA methylation are useful markers for prostate cancer diagnosis (eg, GSTP1 ) 138 and can be used as heritable markers to study cancer clonal evolution and clonal relationships. 139 This assay includes on-slide bisulfite conversion as the first step, coupled with RCA to generate signals that can be scored on a conventional light microscope. Both in situ assays can be multiplexed, therefore enabling the evaluation of a panel of specific biomarker targets simultaneously (Haffner et al, unpublished).
25 |. NOVEL MOLECULAR AND FUNCTIONAL ANALYSES OF SINGLE CELLS FROM THE TME
In an effort to understand how the tumor microenvironment contributes to drug resistance, a multi-disciplinary team at the University of Wisconsin is utilizing PSMA PET/MRI scans to study distinct lesions of multifocal primary prostate cancer. Patients in this study underwent a baseline PSMA PET/MRI scan, followed by three cycles of neoadjuvant chemo-hormonal therapy, a second PSMA PET/MRI, and then prostatectomy. A patient-specific 3D prostate mold was used to perform an image-guided dissection on the prostatectomy specimen and extract distinct tumor foci that appeared sensitive versus resistant to therapy. Evaluation of these distinct tumor foci found increased M2 macrophage infiltration and Trop-2 expression in resistant lesions with concordant decreases in lymphocyte infiltration and MHC class 1 expression on tumor cells (Lang et al, unpublished).
To investigate how these multi-compartment alterations in tumor and immune cells may promote therapeutic resistance, a microfluidic coculture platform that enables testing of up to six different cell types in the same microenvironment, termed STACKS, was developed. 140 STACKS allows for the “stacking” of layers that contain preconditioned microenvironments with three-dimensional tissue models (eg, cancer). This technology allows the investigation of cell-cell interactions, paracrine-signaling events, and analysis of cell behavior including migration and invasion. Because this system requires very few cells, this technique is applicable for use with human samples including patient lymphocytes, monocytes, and tumor organoids. Initial proof-of-concept experiments utilizing prostate cancer cell lines, LNCaP and C4–2B, cocultured with human primary monocytes indicated a differential of monocyte polarization based on gene expression and overall migration towards tumor cells. Additional experiments using DU145 prostate cancer cell lines stacked with patient T cells further demonstrated the ability to track the migration of T cells towards tumor cells at single-cell resolution using confocal microscopy. Additional stacking with patient-matched M2 macrophages inhibited the migration of T cells, indicating the potential for functional assessment of a patient’s tumor-microenvironment interaction (Lang et al, unpublished). 140 – 142
Pushing 3D culture systems to the next level, Lang et al have now begun to utilize exclusive and/or finite liquid repellency (ELR and FLR, respectively) 143 – 145 platforms to investigate tumor cell interactions with immune cells at the single-cell level. ELR/FLR enables the culture of single cells in microbubbles submerged in oil. These platforms permit the fluid exchange of media and free gas exchange while eliminating evaporation, thus allowing cultures down to the single-cell level. To date, proof-of-concept experiments using ELR have demonstrated successful single-cell culture of autologous human monocytes that have demonstrated differential potentials of individual monocytes to proliferate or not. 145 Further, the application of FLR allowed for successful single-cell culturing of human C4–2B prostate cancer cells, which demonstrated contrasting abilities of individual cells to proliferate, indicated by differential gene expression profiles. 144 , 145 ELR-based coculture experiments of human tumor-associated neutrophils with C4–2B tumoroids demonstrated heterogeneous interactions between tumoroid and neutrophil populations, including induction of tumor invasion versus apoptosis. 144 , 145 These studies and technologies will improve our ability to observe and understand exact tumor-microenvironment interactions and tumor potential for metastatic progression and/or response to therapy.
Using these state-of-the-art and innovative technologies, it is anticipated that the re-creation of a prostate tumor microenvironment using STACKS, ELR, and FLR is feasible, using patient-derived tumor organoids and autologous immune cell populations and stromal cells. Overall, these platforms will allow for critical questions to be addressed including determining: (a) the absolute number of MDSCs or M2 macrophages required to drive immune evasion, (b) the properties of T cells that are able to migrate into a hostile environment, (c) the effects of tumor cell genomics on the tumor microenvironment (including downregulation of HLA expression), and (d) discovering therapies that can produce a tumor microenvironment that is conducive towards response to immunotherapy or eradication of cancer cells.
26 |. MASSIVELY MULTIPLEX SINGLE-CELL CHEMICAL TRANSCRIPTOMICS
As next-generation sequencing technologies advance, the potential to multiplex molecular readouts per cell is a reality. Single-cell combinatorial indexing (“sci”) is a novel approach for profiling the genomes of thousands to millions of cells without ever resorting to single-cell isolation. 146 Sci-sequencing relies on split-pool DNA barcoding of cells or nuclei by employing various molecular techniques (eg, transposition, reverse transcription, ligation) to incorporate unique DNA barcodes into populations of intact cells/nuclei in separate reactions, after which they are pooled, redistributed, and then subjected to subsequent rounds of molecular barcoding. 146 The unique “journey” taken by every single-cell in the assay through multiple barcoding reactions allows for the linking of sequenced molecules back to cells-of-origin by analyzing the unique combinations of molecular barcodes (“combinatorial indices”) associated with each sequencing read. This strategy was used to demonstrate proof-of-concept for high-throughput single-cell sequencing of chromatin accessibility (sci-ATAC), chromosome structure (sci-Hi-C), mRNA expression (sci-RNA), and, most recently, co-measurement of multiple genomic phenomena in the same single cells (sci-CAR). 147 – 149 Importantly, the split-pool nature of these protocols enables i) processing of very large numbers of cells in single experiments, and ii) single-cell data collection without ever resorting to single-cell isolation (ie, in wells, droplets, etc.). 147 – 149
The most recent addition to the sci-family of assays is sci-Chem, a platform pairing chemical screening with sci-RNA-seq (Ramani et al, 149 manuscript in revision]. The assay relies on labeling populations of chemically treated cells with a short single-stranded DNA oligo that is read out during the sci-RNA-seq protocol and allows profiling of approximately 10 5 single-cell transcriptomes after approximately 10 3 different chemical perturbations in a single experimental workflow. A major finding from this pilot screen (approximately 600 000 cells sequenced) was a unified mechanism-of-action for 17 different HDAC inhibitors (HDACi). Across three independent cell lines tested, HDACi resulted in cellular upregulation of nontarget genes that serve as lysine deacetylases (ie, sirtuins), and upregulation of metabolic enzymes required to regenerate acetyl-CoA from non-histone cellular substrates. These data are consistent with a model where HDACi does not act via chromatin remodeling but instead serves to starve cells of an essential intermediate metabolite (acetyl-CoA).
27 |. MEASURING CHROMATIN ARCHITECTURE OF NEPC AT THE SINGLE-CELL LEVEL
A new approach to analyzing chromatin architecture apart from histone ChIP-sequencing is assay for transposase-accessible chromatin (ATACseq), which assesses genome-wide chromatin accessibility. 150 Recent advancements have now allowed for ATACseq to be performed at the single-cell level (scATACseq). 151 A proof-of-concept study (Brown et al, unpublished) performed comparative bulk ATACseq and scATACseq analysis utilizing human adenocarcinoma and neuroendocrine prostate cancers from the LUCaP patient-derived xenograft library. 152 Bulk ATACseq analysis revealed key features known to NEPC including biallelic loss of RB1 , and motif enrichment analysis indicated loss of accessible chromatin at the AR gene locus and gain of an important neuronal transcription factor upregulated in neuroendocrine prostate cancer, ASCL1. These data overlay significantly with transcriptional data from preclinical and clinical samples, involving loss of AR and concurrent gain of ASCL1 transcript. 49 , 52 Further, unique differences were observed when bulk ATACseq data was applied to principle component analysis, indicating that chromatin accessibility information can confidently provide information pertaining to tumor cell identity. The application of scATACseq also revealed that NEPC have a distinct chromatin accessibility pattern when compared with adenocarcinoma. Motif enrichment analysis from scATACseq data more distinctly revealed the discrepancy by which adenocarcinomas may acquire important transcription factors that drive NEPC cell identity, including SOX2 and ASCL1. Excitingly, this proof-of-concept study was performed from frozen tumor tissue and infers that scATACseq will be a robust assay that can be performed using patient samples to understand the state of prostate cancer progression from adenocarcinoma to NEPC.
28 |. NOVEL MECHANISMS TO EDIT GENOMES
Understanding the biological and therapeutic consequences of mutations that promote prostate cancer or arise during therapy requires models that recapitulate these mutations. While biologists can routinely affect the activity of a gene in cell culture systems using tools such as expression plasmids and RNAi, until recently it has been difficult and costly to make durable changes to a cellʼs genome without otherwise perturbing the cell. Modifying the genome directly allows investigators to evaluate the effects of specific mutations on protein expression, structure, and function under physiologically relevant conditions. The discovery of CRISPR-Cas9 153 , 154 and subsequent development of protocols employing CRISPR-Cas9 to edit a cellʼs genome have simplified this task. 155 – 157 Genome editing with CRISPR-Cas9 targets the Cas9 endonuclease to generate a DNA break near the edit location. Template DNA containing a novel sequence is also introduced into the cell, in hopes that DNA repair by homologous recombination will employ the new DNA as a template to repair the break. However, these methods are typically inefficient, cell type-specific, and often result in stochastic mixtures of genome editing byproducts, especially when introducing point mutations.
Base editing technology is a newer approach to genome editing that does not require double-strand breaks, which can negatively affect cell viability. 158 Base editing couples Cas9 to engineered versions of naturally occurring enzymes, such as cytidine deaminase or adenosine deaminase that mutate C-G DNA dinucleotide pairs to T-A 159 or A-T pairs to G-C. 160 The Cas9 proteins used in base editing are modified so they cannot break both DNA strands. Instead, when Cas9 binds to DNA, it exposes single-stranded DNA within a narrow window to the bound deaminase protein, efficiently producing the desired mutations. Although the ABE7.10 adenosine editor system is a specific DNA editor, it generates off-target RNA edits. 161 The third generation BE3 cytosine base editor system is associated with off-target effects on DNA and RNA. 161 – 163 These effects may be mitigated by further protein engineering of the base editing proteins used in these system. 161 , 162
29 |. COMBINING CRISPR EDITING WITH RNA-SEQ TO MEASURE COMPLEX PHENOTYPES
Molecular biologists can now use CRISPR-Cas9 systems to systematically test whether inactivation or activation of any single gene in the genome affects the viability of immortalized cell lines. 164 – 168 These screens use CRISPR systems to mutate (CRISPR) or use modified CRISPR systems to inhibit (CRISPRi) or promote (CRISPRa) transcription thereby modulating gene function. Next-generation studies are expanding the scope of what can be discovered through CRISPR screening on several fronts. Screens that modulate pairs of genes in each cell are being used to map functional genetic interactions in human cells, revealing buffering and synergistic relationships that produce synthetic lethality or disease suppression. 169 The number of cells required to screen all possible combinations of gene pairs exceeds present technical limitations, so interaction studies currently employ rationally selected sets of genes. CRISPR-Cas9 screens are also testing phenotypes beyond cell viability, obtaining much more detailed information about cellular response to perturbations through single-cell RNA sequencing methods, such as Perturb-seq. 170 Screening perturbations of gene pairs, with the phenotype read-out assessed by gene expression in individual cells via Perturb-seq, is revealing new aspects of how genes work together. 171
30 |. GENOME EDITING AT SCALE TO SIMULTANEOUSLY ASSESS ALL POSSIBLE MUTATIONS
Protein-altering variants can produce heritable prostate cancer risk by functional inactivation of a tumor suppressor, such as BRCA2 . Some variants, such as introduced stop codons produce functional inactivation that is reliably predictable from sequence analysis alone. Other benign variants do not affect the protein sequence at all and can be reliably predicted not to affect cancer risk. However, many rare variants change the protein sequence in ways that are not clearly either benign or pathogenic. These variants of uncertain significance (VUS) present a dilemma for patients, clinicians, and investigators. 172 Computational approaches that predict the effects of VUS are informative, but these methods do not always agree and they provide insufficient evidence for clinical decision-making. 173
Exhaustively testing the functional consequences of every possible amino acid change in a target gene is now becoming feasible. Multiplexed functional assays can determine the consequences of a large pool of VUSs by employing CRISPR-Cas9 editing to edit thousands of variants in a pool of cells. Each cell receives a single variant, and the pool of cells is subject to a functional assay such that the damaging variation can be detected through subsequent high-throughput sequencing of the cell pool. A recent study tested almost 4000 variants across BRCA1 to predict which were deleterious. 174 This assay exploited the fact that the cell line HAP1, which harbors only a single copy of every gene, cannot tolerate inactivation of the cancer predisposition gene BRCA1 . This method successfully predicted known pathogenic and benign alterations, made new predictions about which variants would be pathogenic, and generated sequence-function maps of how variants in sequence affect protein function. Multiplexed functional assays are being adopted to investigate driver genes in a variety of contexts. 175 – 178
31 |. FUNCTIONAL INTERROGATION OF GENOMIC REGIONS THAT AFFECT PROSTATE CANCER RISK
Functional screening is also revealing how regulatory regions outside of the coding genome affect prostate cancer risk. Commonly occurring inherited genetic variants are associated with small increases in prostate cancer predisposition. 179 , 180 Functional interrogation of these variants is complex, because the variant identified in association studies may be in genetic linkage with the causal variant, meaning it is physically nearby on the genome and co-inherited with the causal variant without being causal itself. Such variants have modest effects, and it is not obvious a priori in which cell type or when during tumor formation they are relevant. In contrast to mutations that inactivate the function of a protein-coding gene, such as BRCA1 , common variants usually occur in noncoding regulatory regions and exert their effects at a distance. 180 – 182 Recent approaches to functionally interrogate these variants include CRISPR-Cas9-mediated inhibition of activity at regulatory regions to identify which loci influence gene expression. 183 , 184 Such expression quantitative trait loci (eQTL) can directly affect the expression of key driver genes, such as MYC . The effects of these risk loci are mediated by the epigenetic activity of the insulator protein CTCF, which binds to DNA regulatory regions. Integrative studies that combine CRISPRi, eQTL analysis, and assessment of CTCF activity are revealing the mechanisms by which germline variants affect prostate cancer risk.
32 |. NON-CODING GENOMIC REGIONS AFFECT PROSTATE CANCER PROGRESSION AND DRUG RESPONSE
Quantitative assays that determine where and when transcription factors bind to regulatory DNA are revealing how these proteins affect prostate cancer progression. Many investigations have assessed how occupancy of DNA by transcription factors relevant to prostate cancer (AR, FOXA1, HOXB13) and histone marks associated with transcriptional control (H3K27ac, H3K4me2/3, H3K27me3) change across different stages of tumor development. 185 , 186 New studies are integrating genome and epigenome data from tissue samples obtained at all stages of human prostate cancer development. Unlike FOXA1 and HOXB13, the AR cistrome is altered between normal prostate, localized disease, and metastatic disease. This reprogramming in tumors largely reflects increased occupancy at sites occupied in normal tissue, rather than de novo binding at sites not observed in normal tissue. Mapping epigenetic reprogramming across human tumors and combining these data with structural variation data can identify key enhancers affecting AR and other genes. 187 , 188 Integrated analysis of how structural alterations, mutations, and epigenetic alterations alter protein function in tumors will reveal how these genetic and epigenetic forces shape tumor development and drug response.
33 |. EXTRACELLULAR VESICLES: AGENTS AND INDICATORS OF TUMOR EVOLUTION
All cellular organisms release or shed extracellular vesicles (EVs). 189 – 191 EVs are not only a mechanism for discarding cellular debris but also a route of cellular communication via the transfer of the cargo components borne by those vesicles. 192 , 193 Due to the ubiquity of EVs in biological systems, and due to the fact that the cargo of EVs reflects their cellular origin, EVs are an emerging area of research for studying tumor biology and for detection of tumors with liquid biopsies.
EVs as indicators of tumor evolution are a growing focus in all oncologic fields. Because most EVs are typically submicron in size, they are able to diffuse from the tumor microenvironment into peripheral biofluids, such as a serum, plasma, and urine. Therefore, EVs are of interest especially in liquid biopsies, with which EV proteins, RNA, and other cargo components are being investigated. The lipid bilayer structure of EVs protects internal cargo RNA and improves the stability of RNAs for testing in liquid biopsies. 194 , 195 Although circulating tumor DNA (ctDNA) and CTCs are associated most directly with certain stages of tumor cell evolution or death, EVs are commonly released as an active process at all stages of tumor progression, and tumor-specific genetic alterations may be detected in EVs. Although these are early days in our understanding of how to study and use EV-related data in the management of prostate cancer, significant progress is being made.
Not only are EVs a rich source of biologically informative data regarding tumor status, EV cargo may participate in the pathogenesis of tumor progression. PD-L1 expression on tumor-derived EVs is associated with immune evasion, for example. 196 In prostate cancer, large EVs, referred to as “large oncosomes,” ranging in size from 1 to 10 microns, appear to carry most of the tumor DNA that is circulating in prostate cancer patient plasma. 197 , 198 These large prostate cancer oncosomes also mediate changes in the tumor microenvironment and prostate fibroblast reprogramming, via AKT1-induced MYC activation. 199
Because EVs in circulation are a heterogeneous mixture of EVs, new methods for identification and analysis of tumor-specific EVs, such as CSPG4-directed immunoaffinity-based isolation of tumor-derived EVs from patientsʼ plasma, 200 are being used to investigate relative immune suppression or immune activation by different tumor-associated EV populations. Methods for performing analyses of individual EVs remain challenging due to the heterogeneity of EV populations and limitations of tools available to characterize EVs accurately. 201 – 203 Selective capture and analysis of specific EV subsets, based on specific surface markers, such as CSPG4 (for certain tumor-derived EVs) or CD3 (for T cell-derived EVs), has proven to be a useful path forward in the field. In general, these studies altogether indicate that tumor-derived EVs may mediate disease and may be used to monitor disease. In the interplay between tumor and immune cells, tumor-derived EVs appear to drive multiple immune-inhibitory pathways 204 ; CD3 + EVs are associated with advanced tumor stage, 205 and EVs can be used to monitor responses to immunotherapy treatments. 206 Additional studies on the role of EVs in tumor progression and treatment responses, and their potential as predictive biomarkers, are warranted.
34 |. CONCLUSION
The discussions at the 2019 CHPCA Meeting were the most active and abundant ever recorded over the history of this meeting (now in its 7th year), with 422 questions asked over 35 talks. We hope that the knowledge exchanged at this meeting and through the dissemination of this meeting report will accelerate novel basic, translational, and clinical research that will deliver new and critical understandings into prostate cancer biology and how to improve treatment and outcomes for all men with prostate cancer.
The theme of the 2020 CHPCA Meeting will be: “Prostate Cancer Research in the 21st Century.”
ACKNOWLEDGMENTS
We would like to thank all of the speakers for presenting their work at the 2019 CHPCA Meeting and for their thoughtful review of this manuscript.
CONFLICT OF INTERESTS
AS is an employee of The Institute of Cancer Research, which has a commercial interest in abiraterone. AS has received travel support from Roche-Genentech and speaker honorarium from Astellas Pharma. HY is on the advisory board of Johnson & Johnson (Janssen). The authors declare no other conflicts of interest.
Prostate cancer research in the 21st century; report from the 2021 Coffey-Holden prostate cancer academy meeting
Affiliations.
- 1 Department of Science, Prostate Cancer Foundation, Santa Monica, California, USA.
- 2 Department of Oncology, Johns Hopkins University School of Medicine and The Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA.
- 3 Bloomberg Kimmel Institute for Cancer Immunotherapy, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
- 4 Division of Hematology, Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
- 5 Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.
- 6 Department of Pharmaceutical Chemistry, University of California, San Francisco, San Francisco, California, USA.
- 7 Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
- 8 Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
- 9 Department of Experimental Radiation Oncology, MD Anderson Cancer Center, Houston, Texas, USA.
- 10 Department of Human Oncology, Carbone Cancer Center, University of Wisconsin, Madison, Wisconsin, USA.
- 11 The James Buchanan Brady Urological Institute, The Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
- PMID: 34734426
- PMCID: PMC8688282
- DOI: 10.1002/pros.24262
Introduction: The 2021 Coffey-Holden Prostate Cancer Academy (CHPCA) Meeting, "Prostate Cancer Research in the 21st Century," was held virtually, from June 24-25, 2021.
Methods: The CHPCA Meeting is organized by the Prostate Cancer Foundation as a unique discussion-oriented meeting focusing on critical topics in prostate cancer research envisioned to bridge the next major advances in prostate cancer biology and treatment. The 2021 CHPCA Meeting was virtually attended by 89 investigators and included 31 talks over nine sessions.
Results: Major topic areas discussed at the meeting included: cancer genomics and sequencing, functional genomic approaches to studying mediators of plasticity, emerging signaling pathways in metastatic castration resistant prostate cancer, Wnt signaling biology and the challenges of targeted therapy, clonal hematopoiesis, neuroendocrine cell plasticity and antitumor immunity, cancer immunotherapy and its synergizers, and imaging the tumor microenvironment and metabolism.
Discussion: This meeting report summarizes the research presented at the 2021 CHPCA Meeting. We hope that publication of this knowledge will accelerate new understandings and the development of new biomarkers and treatments for prostate cancer.
Keywords: cancer immunotherapy; molecular imaging; precision medicine; therapeutics; tumor genomics.
© 2021 Wiley Periodicals LLC.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, U.S. Gov't, Non-P.H.S.
- Congresses as Topic
- Immunotherapy / methods*
- Prostate* / diagnostic imaging
- Prostate* / immunology
- Prostate* / metabolism
- Prostatic Neoplasms* / genetics
- Prostatic Neoplasms* / pathology
- Prostatic Neoplasms* / therapy
- Research / trends
Grants and funding
- P50 CA140388/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- K22 CA237623/CA/NCI NIH HHS/United States
- R37 CA251980/CA/NCI NIH HHS/United States
- K99 CA226360/CA/NCI NIH HHS/United States
Prostate Cancer
Explore the latest in prostate cancer, including recent screening guidelines and advances in management of localized and metastatic disease.

Publication
Article type.
- Urine-Based Test Could Help Identify Aggressive Prostate Cancer JAMA News May 17, 2024 Reproductive Health Oncology Urology Urologic Cancer Full Text | pdf link PDF free
This review assesses current evidence and emerging treatment strategies for improved survival among patients with metastatic hormone-sensitive prostate cancer and offers recommendations for clinical practice application.
- Prostate Cancer Cases Might Rise to 3 Million Globally by 2040 JAMA News May 3, 2024 Reproductive Health Oncology Urology Urologic Cancer Full Text | pdf link PDF free
This cross-sectional study assesses homologous recombination repair mutation genetic testing and associated characteristics among men with metastatic castration-resistant prostate cancer (mCRPC).
This cohort study examines whether postdiagnostic plant-based dietary patterns are associated with risk of prostate cancer progression or prostate cancer–specific mortality in men with nonmetastatic prostate cancer.
This cross-sectional study examines the association of life expectancy and prostate cancer screening practices among older males using data from a national survey.
This randomized clinical trial compared use of a biomarker-based strategy vs a magnetic resonance imaging–enhanced strategy for detection of clinically significant prostate cancer among men in Sweden.
This report describes the development of an 18-gene urine panel for high-grade prostate cancer and validates its external performance relative to current guideline-endorsed biomarkers.
This secondary analysis of a randomized clinical trial assesses whether screening for prostate-specific antigen reduces prostate cancer mortality at 15-year follow-up.
This preliminary descriptive report compares the detection rates of high-grade and low-grade prostate cancer in men invited for prostate cancer screening vs those of the control group not offered screening.
- A Pragmatic Approach to Prostate Cancer Screening JAMA Opinion April 6, 2024 Oncology Urology Urologic Cancer Cancer Screening, Prevention, Control Full Text | pdf link PDF has multimedia
This systematic review and meta-analysis examines evidence regarding screening pathways incorporating magnetic resonance imaging with targeted biopsy and assess their diagnostic value compared with prostate-specific antigen–based screening with systematic biopsy strategies.
This systematic review and meta-analysis evaluates biopsy decision-making strategies for avoiding unnecessary biopsies and minimizing risk of missing clinically significant prostate cancer.
This cohort study analyzes the association of HSD3B1 status with prostate cancer outcomes among patients in the Veterans Affairs Health System in the US.
- Adrenal-Permissive HSD3B1 Genotype—An Invisible Stimulator of Prostate Cancer Mortality JAMA Network Open Opinion March 20, 2024 Oncology Cancer Genetics Genetics and Genomics Reproductive Health Urologic Cancer Full Text | pdf link PDF open access
This cohort study compares all-cause mortality by neighborhood deprivation and race and ethnicity among individuals with prostate cancer receiving care in the US Department of Veterans Affairs (VA) health care system vs those receiving care outside the VA.
This cohort study uses data from the LATITUDE trial to investigate the association of bone-modifying agent use with time to skeletal-related events and overall survival in patients with high-risk metastatic castration-sensitive prostate cancer treated with abiraterone acetate and prednisone.
This randomized clinical trial aims to determine if hypofractionated postprostatectomy radiotherapy is noninferior to conventionally fractionated postprostatectomy for patient-reported genitourinary (GU) and gastrointestinal (GI) symptoms at 2 years.
This cross-sectional study investigates trends in overall survival among patients with newly diagnosed metastatic prostate cancer in 2 national registries in the United States.
This diagnostic study compares discrimination and calibration for 4 magnetic resonance imaging (MRI)-based prostate cancer risk calculators within independent external cohorts from Europe and North America and a separate cohort with high utilization of an advanced serum biomarker.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Prostate cancer biomarkers: from early diagnosis to precision treatment
- REVIEW ARTICLE
- Published: 14 May 2024
Cite this article

- Versha Dahiya 1 ,
- Sanjana Hans 1 na1 ,
- Ruchi Kumari 1 na1 &
- Gargi Bagchi ORCID: orcid.org/0000-0001-6416-2584 1
53 Accesses
Explore all metrics
Prostate cancer (PCa) is the second most prevalent cancer in men. In 2020, approximately 1,414,259 new cases were reported that accounted for 3,75,324 deaths (Sung et al. in CA 71:209–249, 2021). PCa is often asymptomatic at early stages; hence, routine screening and monitoring based on reliable biomarkers is crucial for early detection and assessment of cancer progression. Early diagnosis of disease is key step in reducing PCa-induced mortality. Biomarkers such as PSA have played vital role in reducing recent PCa deaths. Recent research has identified many other biomarkers and also refined PSA-based tests for non-invasive diagnosis of PCa in patients. Despite progress in screening methods, an important issue that influences treatment is heterogeneity of the cancer in different individuals, necessitating personalized treatment. Currently, focus is to identify biomarkers that can accurately diagnose PCa at early stage, indicate the stage of the disease, metastatic nature and chances of survival based on individual patient profile (Fig. 1 ).
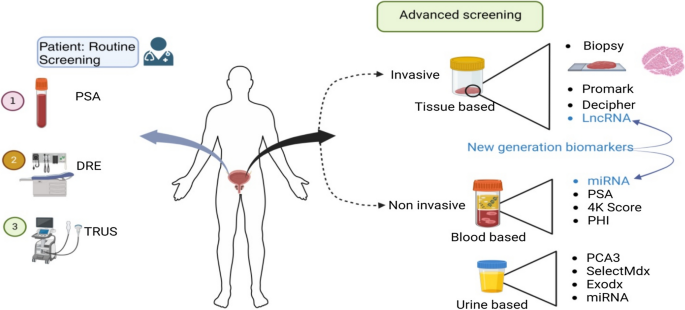
Graphical abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Biomarkers in prostate cancer: new era and prospective

Role of Molecular Diagnostics in Prostate Cancer

Prostate Cancer
Data availability.
Not applicable.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–49.
PubMed Google Scholar
Rice SM, Oliffe JL, Kelly MT, Cormie P, Chambers S, Ogrodniczuk JS, et al. Depression and prostate cancer: examining comorbidity and male-specific symptoms. Am J Mens Health. 2018;12(6):1864–72.
Article PubMed PubMed Central Google Scholar
Madu CO, Lu Y. Novel diagnostic biomarkers for prostate cancer. J Cancer. 2010;1:150.
Gutman AB, Gutman EB. An “acid” phosphatase occurring in the serum of patients with metastasizing carcinoma of the prostate gland. J Clin Investig. 1938;17(4):473–8.
Article CAS PubMed PubMed Central Google Scholar
Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17(2):159–63.
CAS PubMed Google Scholar
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–16.
Article CAS PubMed Google Scholar
Mettlin C, Lee F, Drago J, Murphy GP. The American cancer society national prostate cancer detection project. Findings on the detection of early prostate cancer in 2425 men. Cancer. 1991;67(12):2949–58.
Basler JW, Thompson IM. Lest we abandon digital rectal examination as a screening test for prostate cancer. J Natl Cancer Inst. 1998;90(23):1761–3.
Mahon SM. Screening for prostate cancer: informing men about their options. Clin J Oncol Nurs. 2005;9(5):625.
Article PubMed Google Scholar
Hara M. Some physiochemical characteristics of gamma-semino-protein: An antigenic component specific for human plasma. Jpn J Legal Med. 1971;25:322–4.
CAS Google Scholar
Rao AR, Motiwala HG, Karim OMA. The discovery of prostate-specific antigen. BJU Int. 2008;101(1):5–10.
Klotz L. Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol. 2013;14:97–108.
Panzone J, Byler T, Bratslavsky G, Goldberg H. Applications of focused ultrasound in the treatment of genitourinary cancers. Cancers. 2022;14(6):1536.
Crawford ED, Scholz MC, Kar AJ, Fegan JE, Haregewoin A, Kaldate RR, et al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr Med Res Opin. 2014;30(6):1025–31.
Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(4):599–608.
Van der Kwast TH. Prognostic prostate tissue biomarkers of potential clinical use. Virchows Arch. 2014;464(3):293–300.
Bazzichetto C, Conciatori F, Pallocca M, Falcone I, Fanciulli M, et al. PTEN as a prognostic/predictive biomarker in cancer: an unfulfilled promise? Cancers. 2019;11(4):435.
Coradduzza D, Solinas T, Balzano F, Culeddu N, Rossi N, Cruciani S, et al. miRNAs as molecular biomarkers for prostate cancer. J Mol Diagn. 2022;24(11):1171–80.
Rana S, Valbuena GN, Curry E, Bevan CL, Keun HC. MicroRNAs as biomarkers for prostate cancer prognosis: a systematic review and a systematic reanalysis of public data. Br J Cancer. 2022;126(3):502–13.
Wang W, Wang M, Wang L, Adams TS, Tian Y, Xu J. Diagnostic ability of% p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep. 2014;4(1):5012.
De La Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy naive men. J Urol. 2015;194(1):65–72.
Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochem Biophys Acta. 2014;1846(1):99–112.
Filella X, Gimenez N. Evaluation of [− 2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013;51(4):729–39.
Hernández J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101(5):894–904.
Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199(6):1459–63.
Hayes VM, Bornman MR. Prostate cancer in Southern Africa: does Africa hold untapped potential to add value to the current understanding of a common disease? J Global Oncol. 2018;4:1–7.
Google Scholar
Voigt JD, Dong Y, Linder V, Zappala S. Use of the 4Kscore test to predict the risk of aggressive prostate cancer prior to prostate biopsy: Overall cost savings and improved quality of care to the us healthcare system. Rev Urol. 2017;19(1):1.
PubMed PubMed Central Google Scholar
Zappala SM, Scardino PT, Okrongly D, Linder V, Dong Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Rev urol. 2017;19(3):149.
Lin DW, Newcomb LF, Brown MD, Sjoberg DD, Dong Y, Brooks JD, et al. Evaluating the four kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary Prostate Active Surveillance Study. Eur Urol. 2017;72(3):448–54.
Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case–control study. Eur Urol. 2015;68(2):207–13.
Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185(5):1650–5.
Porzycki P, Ciszkowicz E. Modern biomarkers in prostate cancer diagnosis. Central Eur J Urol. 2020;73(3):300.
Filella X, Foj L, Augé JM, Molina R, Alcover J. Clinical utility of% p2PSA and prostate health index in the detection of prostate cancer. Clin Chem Lab Med. 2014;52(9):1347–55.
Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193(4):1163–9.
Hussein AA, Baban R, Hussein A. Prostate-specific antigen and free prostate-specific antigen/prostate-specific antigen ratio in patients with benign prostatic hyperplasia and prostate cancer. Baghdad J Biochem Appl Biol Sci. 2020;1(01):18–26.
Article Google Scholar
Eyrich NW, Morgan TM, Tosoian JJ. Biomarkers for detection of clinically significant prostate cancer: contemporary clinical data and future directions. Transl Androl Urol. 2021;10(7):3091.
Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6(1):25776.
Kornberg Z, Cooperberg MR, Spratt DE, Feng FY. Genomic biomarkers in prostate cancer. Transl Androl Urol. 2018;7(3):459.
Tutrone R, Donovan MJ, Torkler P, Tadigotla V, McLain T, Noerholm M, et al. Clinical utility of the exosome based ExoDx Prostate (IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020;23(4):607–14.
Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018;19(10):2877.
Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13(1):12–9.
Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56(2):275–86.
Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6(5):255–61.
Nicholson A, Mahon J, Boland A, Beale S, Dwan K, Fleeman N, et al. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(87):1–31.
Munroz Rodríguez SVM, García-Perdomo HA. Diagnostic accuracy of prostate cancer antigen 3 (PCA3) prior to first prostate biopsy: A systematic review and meta-analysis. Can Urol Assoc J. 2020;14(5):E214.
Kristiansen G. Diagnostic and prognostic molecular biomarkers for prostate cancer. Histopathology. 2012;60(1):125–41.
Kretschmer A, Tilki D. Biomarkers in prostate cancer–current clinical utility and future perspectives. Crit Rev Oncol Hematol. 2017;120:180–93.
Alford AV, Brito JM, Yadav KK, Yadav SS, Tewari AK, Renzulli J. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19(4):221.
Kohaar I, Petrovics G, Srivastava S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(8):1813.
Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25(9):770–9.
Demichelis F, Fall K, Perner S, Andrén O, Schmidt F, Setlur SR, et al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–9.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2(7):882–9.
Humphrey PA. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7(10):a030411.
Basourakos SP, Tzeng M, Lewicki PJ, Patel K, Awamlh BAHA, Venkat S, et al. Tissue-based biomarkers for the risk stratification of men with clinically localized prostate cancer. Front Oncol. 2021;11:676716.
Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol. 2021;18(7):433–42.
Moschini M, Spahn M, Mattei A, Cheville J, Karnes RJ. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med. 2016;14(1):1–7.
Khoo A, Liu LY, Nyalwidhe JO, Semmes OJ, Vesprini D, Downes MR, et al. Proteomic discovery of non-invasive biomarkers of localized prostate cancer using mass spectrometry. Nat Rev Urol. 2021;18(12):707–24.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–29.
Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE. 2013;8(6):e66855.
Spratt DE, Dai DL, Den RB, Troncoso P, Yousefi K, Ross AE, et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate-specific antigen persistence postprostatectomy. Eur Urol. 2018;74(1):107–14.
Behm-Ansmant I, Rehwinkel J, Izaurralde E. MiRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Quant Biol. 2006;71:523–30.
Article CAS Google Scholar
Pedroza-Torres A, Romero-Córdoba SL, Justo-Garrido M, Salido-Guadarrama I, Rodríguez-Bautista R, Montaño S, et al. MicroRNAs in tumor cell metabolism: roles and therapeutic opportunities. Front Oncol. 2019;9:1404.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105(30):10513–8.
Meiri E, Mueller WC, Rosenwald S, Zepeniuk M, Klinke E, Edmonston TB, et al. A second-generation microRNA-based assay for diagnosing tumor tissue origin. Oncologist. 2012;17(6):801–12.
Li D, Hao X, Song Y. Identification of the key MicroRNAs and the miRNA-mRNA regulatory pathways in prostate cancer by bioinformatics methods. Biomed Res Int. 2018. https://doi.org/10.1155/2018/6204128 .
Guo H, Qi RQ, Sheng J, Liu C, Ma H, Wang HX, et al. MiR-155, a potential serum marker of extramammary Paget’s disease. BMC Cancer. 2018;18:1–8.
Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017;259:135–9.
Matin F, Jeet V, Moya L, Selth LA, Chambers S, Yeadon APCB, et al. A plasma biomarker panel of four microRNAs for the diagnosis of prostate cancer. Sci Rep. 2018;8(1):6653.
Leite KR, Morais DR, Reis ST, Viana N, Moura C, Florez MG, et al. MicroRNA 100: a context dependent miRNA in prostate cancer. Clinics. 2013;68:797–802.
Ghamlouche F, Yehya A, Zeid Y, Fakhereddine H, Fawaz J, Liu YN, Abou-Kheir W. MicroRNAs as clinical tools for diagnosis, prognosis, and therapy in prostate cancer. Transl Oncol. 2023;28:101613.
Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9(1):13772.
Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55.
Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67(1):33–41.
Penney KL, Sinnott JA, Fall K, Pawitan Y, Hoshida Y, Kraft P, et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J Clin Oncol. 2011;29(17):2391.
Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45(11):1392–8.
Xiong T, Li J, Chen F, Zhang F. PCAT-1: a novel oncogenic long non-coding RNA in human cancers. Int J Biol Sci. 2019;15(4):847.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208.
Camacho CV, Choudhari R, Gadad SS. Long noncoding RNAs and cancer, an overview. Steroids. 2018;133:93–5.
Qiu M, Xu Y, Wang J, Zhang E, Sun M, Zheng Y, et al. A novel lncRNA, LUADT1, promotes lung adenocarcinoma proliferation via the epigenetic suppression of p27. Cell Death Dis. 2015;6(8):e1858–e1858.
Lemos AEG, Ferreira LB, Batoreu NM, de Freitas PP, Bonamino MH, Gimba ERP. PCA3 long noncoding RNA modulates the expression of key cancer-related genes in LNCaP prostate cancer cells. Tumor Biol. 2016;37:11339–48.
Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan D, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 2017;3(8):1085–93. https://doi.org/10.1001/jamaoncol.2017.0177 .
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407.
Prensner JR, Sahu A, Iyer MK, Malik R, Chandler B, Asangani IA, et al. The lncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget. 2014;5(6):1434.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, et al. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16(12):1121–7.
Cozar JM, Robles-Fernandez I, Rodriguez-Martinez A, Puche-Sanz I, Vazquez-Alonso F, Lorente JA, et al. The role of miRNAs as biomarkers in PCa. Mutat Res Rev Mutat Res. 2019;781:165–74.
Allemailem KS, Almatroudi A, Alrumaihi F, Makki Almansour N, Aldakheel FM, Rather RA, et al. Single nucleotide polymorphisms (SNPs) in prostate cancer: its implications in diagnostics and therapeutics. Am J Transl Res. 2021;13(4):3868–89.
CAS PubMed PubMed Central Google Scholar
Chang HH, Lee CH, Chen YT, Huang CY, Yu CC, Lin VC, et al. Genetic analysis reveals the prognostic significance of the DNA mismatch repair gene MSH2 in advanced prostate cancer. Cancers. 2022;14(1):223.
Lee CH, Pao JB, Lu TL, Lee HZ, Lee YC, Liu CC, et al. Prognostic value of prostaglandin-endoperoxide synthase 2 polymorphisms in prostate cancer recurrence after radical prostatectomy. Int J Med Sci. 2016;13(9):696–700. https://doi.org/10.7150/ijms.16259 .
Mangolini A, Rocca C, Bassi C, Ippolito C, Negrini M, Dell’Atti L, et al. Detection of disease-causing mutations in prostate cancer by NGS sequencing. Cell Biol Int. 2022;46(7):1047–61.
Goel S, Bhatia V, Kundu S, Biswas T, Carskadon S, Gupta N, et al. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat Commun. 2021;12(1):5325.
Singh JP, Dagar M, Dagar G, Kumar S, Rawal S, Bagchi G, et al. Activation of GPR56, a novel adhesion GPCR, is necessary for nuclear androgen receptor signaling in prostate cells. PLoS ONE. 2020;15(9):e0226056.
Sánchez Iglesias Á, Morillo Macías V, Picó Peris A, Fuster-Matanzo A, Nogué Infante A, Muelas Soria R, et al. Prostate region-wise imaging biomarker profiles for risk stratification and biochemical recurrence prediction. Cancers. 2023;15(16):4163.
Padhani AR, Schoots IG. Prostate cancer screening—stepping forward with MRI. Eur Radiol. 2023;33(10):6670–6.
Eklund M, Jäderling F, Discacciati A, Bergman M, Annerstedt M, Aly M, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med. 2021;385(10):908–20.
Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021;22(9):1240–9.
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biol. 2011;32:583–8.
Download references
Acknowledgements
We are thankful to Science & Engineering Research Board (SERB) grant CRG/2019/002583.
Science and Engineering Research Board, CRG/2019/002583, Gargi Bagchi.
Author information
Sanjana Hans and Ruchi Kumari have contributed equally to this work.
Authors and Affiliations
Amity Institute of Biotechnology, Amity University Haryana, Gurgaon, India, 122413
Versha Dahiya, Sanjana Hans, Ruchi Kumari & Gargi Bagchi
You can also search for this author in PubMed Google Scholar
Contributions
Gargi Bagchi: conceptualization, supervision, review and editing. Versha Dahiya: manuscript writing, preparing figures and tables. Sanjana Hans and Ruchi Kumari: manuscript writing.
Corresponding author
Correspondence to Gargi Bagchi .
Ethics declarations
Conflict of interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
“No ethical approval was required as this study did not involve human participants or laboratory animals.”
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Dahiya, V., Hans, S., Kumari, R. et al. Prostate cancer biomarkers: from early diagnosis to precision treatment. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03508-2
Download citation
Received : 26 February 2024
Accepted : 26 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1007/s12094-024-03508-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prostate cancer
- New generation biomarkers
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 5, May 2024 (In Progress)
- Volume 2024, Issue 4, April 2024
- Case of the Year
- MSF Case Reports
- Audiovestibular medicine
- Cardiology and cardiovascular systems
- Critical care medicine
- Dermatology
- Emergency medicine
- Endocrinology and metabolism
- Gastroenterology and hepatology
- Geriatrics and gerontology
- Haematology
- Infectious diseases and tropical medicine
- Medical ophthalmology
- Medical disorders in pregnancy
- Paediatrics
- Palliative medicine
- Pharmacology and pharmacy
- Radiology, nuclear medicine, and medical imaging
- Respiratory disorders
- Rheumatology
- Sexual and reproductive health
- Sports medicine
- Substance abuse
- Author Guidelines
- Submission Site
- Open Access
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case report, acknowledgements, conflict of interest statement, ethical approval.
- < Previous
Adenoid cystic/basal-cell carcinoma of the prostate following high-grade urothelial bladder cancer: a case report
- Article contents
- Figures & tables
- Supplementary Data
Maja Sofronievska Glavinov, Blagica Krsteska, Vita Stojmenova, Tanja Petrovska, Rubens Jovanovic, Adenoid cystic/basal-cell carcinoma of the prostate following high-grade urothelial bladder cancer: a case report, Oxford Medical Case Reports , Volume 2024, Issue 5, May 2024, omae050, https://doi.org/10.1093/omcr/omae050
- Permissions Icon Permissions
Adenoid cystic/Basal-cell carcinoma (ACC/BCC) of the prostate is a rare histological type exhibiting various morphological characteristics and an optimal treatment has not yet been established. We report the case of a 63-year-old patient who complained of incomplete bladder emptying and recurrent urinary infection six months after transurethral resection of a high-grade urothelial bladder tumor. The clinical features, digital rectal examination, serum PSA levels, and multiparametric MRI did not refer to any suspicious prostatic lesions and cystoscopy revealed bladder neck hypertrophy, and yellowish zones in the prostatic urethra. Transurethral resection was performed due to these findings and histopathological analysis showed poorly differentiated ACC/BCC of the prostate. Even though there is no proven mutual correlation between ACC/BCC and urothelial bladder cancer, the appearance of obstructive urinary symptoms, bladder-neck hypertrophy, and macroscopic changes in prostatic urethra should be reconsidered for transurethral resection biopsy considering the possibility of ACC/BCC.
Adenoid cystic/Basal-cell carcinoma of the prostate is a rare histological type accounting for approximately 0.01% of all prostate cancer cases first described in 1974 [ 1 ]. Since its various histologic and immunohistochemical features, outlining the diagnosis, treatment, prognosis, and outcome remains challenging, which results in delayed diagnosis and treatment and suggests poor prognosis. This type of prostate cancer is characterized by low serum PSA value since it arises from the basal cells and its diagnosis is incidental after transurethral resection of the prostate due to obstructive symptoms thus the stage when diagnosed is usually advanced. Even though the current treatment recommendation is primarily surgical resection, other treatment options may benefit the patients with continuous follow-up [ 2 ]. Most of the published case reports relate to the prostate but some cases report ACC of the urethra/Cowper’s glands [ 3 ].
Even though there is no proven mutual correlation between ACC/BCC and urothelial bladder cancer, recently one case of ACC/BCC following urothelial bladder cancer was published [ 4 ].
We report an unusual case of a patient with high-grade urothelial bladder cancer followed by incidentally diagnosed early-stage prostatic ACC/BCC on the follow-up.
A 63-year-old male patient complained of recurrent urinary infections and a feeling of incomplete bladder emptying, otherwise in good general condition, without significant weight or appetite loss. Before six months he underwent transurethral resection of a non-invasive high-grade urothelial carcinoma (pTa) of the bladder without signs of tumor recurrence at a follow-up cystoscopy after three months.
The clinical work-up included a digital rectal prostate examination that did not refer to suspicious prostatic or periprostatic lesions. Prostate-specific antigen serum level was 0,657 ng/ml and MRI showed a preserved morphology and structure of the prostate with a preserved capsule and a diameter of 38.5×25mm with changes in the bladder wall due to previous transurethral resection of tumor but no pathological contrast accumulation was seen in the prostate tissue after administration of contrast or restriction of diffusion with PI-RADS score 1 ( Fig. 1 ).

MRI findings in normal ranges A. T2-weighted imaging (T2WI), B. diffusion-weighted imaging (DWI), C. ADC map and Dynamic contrast enhancement (DCE), D.T1-weighted fast spoiled gradient echo.
During the second follow-up cystoscopy which was performed six months after the resection of the urothelial bladder cancer, moderate bladder neck hypertrophy, and yellowish zones in the prostatic urethra were seen ( Fig. 2A ).

A. Bladder neck endoscopic visual, upper arrow pointing to the place of previously resected bladder tumor; lower arrow pointing to a new lesion of bladder neck before TUR, B. bladder neck and prostate after TUR.
Due to the cystoscopy finding, obstructive symptoms, incomplete bladder emptying, and recurrent urinary infections, the patient underwent transurethral resection of the bladder neck, and prostate ( Fig. 2B ).
The histopathological report showed morphological features of poorly differentiated ACC/BCC of the prostate. The microscopic findings showed an infiltrative tumor composed of focally desmoplastic stroma infiltrated by nests of basaloid cells with hyperchromic nuclei and scant cytoplasm, showing peripheral palisading, admixed with adenoid-cystic and cribriform structures lined by a single to few layers of the neoplastic epithelium ( Fig. 3 ).

Basal-cell/adenoid cystic carcinoma; A. HeEo staining (40×) B. HeEo staining (100×).
Immunohistochemical staining for expression of CKHMW, p63, CK7, AMACR, GATA3, PAP, PSA, Estrogen, and androgen receptors was performed. The tumor was positive for CKHMW and CK7 ( Fig. 4 ). The expression of p63 was partial, predominantly in the peripheral cells of the nests and adenoid-cystic structures and the PSA staining showed weak and partial signal with multifocal distribution ( Fig. 5 ).

Immunohistochemical positive staining for; A. CKHMW (100×) B. CK7 (100×).

Immunohistochemical partially positive staining for A. p63 (100×), B.PSA (100×).
The immunostainings for AMACR, GATA3, PAP, Estrogen, and Androgen receptors were negative.
A metastatic workup was performed using a skeletal scintigraphy with 99Tc-MDP showing the tracer’s inhomogeneous distribution in the spinal column’s thoracic segment, with emphasized activity in the projection of the eight thoracic vertebrae ( Fig. 6 ). The patient had a history of vertebral injury, so the presence of metastasis in the thoracic vertebrae could not be reliably confirmed. According to the TNM classification for adenoid cystic prostatic carcinoma, the patient was in stage I (T1N0Mx).

Skeletal scintigraphy with 99mTc-MDP showing the tracer’s inhomogeneous distribution in the spinal column’s thoracic segment, with emphasized activity in the projection of the eight thoracic vertebrae (arrow).
The patient underwent definitive radiotherapy treatment in 38 fractions, with a total treatment dose of 70.4 Gy. The treatment was carried out according to a controlled diuresis protocol, with the volumetric modulated arc therapy with Halcyon™ accelerator. Serum levels of PSA after radiation were 0,651 ng/ml. In further follow-ups, an enhanced abdominal CT scan and skeletal scintigraphy will be performed after 6 and 12 months.
The presented case is unique in presenting incidental diagnosis of ACC/BCC after transurethral resection of noninvasive high-grade carcinoma (pTa) in a patient with obstructive symptoms and normal serum PSA level. In the literature so far, there is only one reported case of a patient with high-grade muscle-invasive carcinoma, with incidentally diagnosed ACC/BCC [ 4 ]. In the presented case patient denied a family history of cancer and tobacco smoking was the risk factor for urothelial carcinoma.
Regarding the rarity of the ACC/BCC, the natural history of this tumor is not predictable. Case reports show various findings during clinical investigations such as digital rectal examination and ultrasonography, from an enlarged hard nodular prostate [ 5 ] to normal findings as in the case presented. Serum PSA and PAP levels are usually within the normal range. The most common diagnosis is incidental after transurethral prostate resection due to obstructive lower urinary symptoms [ 6 ].
The final diagnosis is based on immunohistochemical analyses, thus showing immunoreactivity for p63, CK7, and 34-beta-E12 stressing that CKHMW stains prostatic basal cells whose presence rules out usual-type prostatic adenocarcinoma [ 7 ], and distinguishes high-grade PIN (intact or fragmented basal cell layer) from adenocarcinoma [ 8 ].
A recent literature review showed that there is no uniformity in the management of such a rare prostate disease due to the lack of knowledge of its clinical and biological characteristics and the presentation of more cases is needed to overcome this challenge [ 1 ]. There are inconclusive data regarding the most effective systemic treatment and the effectiveness of androgen deprivation therapy, since ACC/BCC has demonstrated independence from androgen stimulation [ 9 ].
ACC/BCC in more than half of the cases has a poor prognosis due to prominent perineural and periglandular invasion suggesting aggressive behavior and has high-risk pathologic features or local recurrence, especially in younger patients [ 10 ].
Even though there is no proven mutual correlation between ACC/BCC and urothelial bladder cancer, the appearance of obstructive urinary symptoms, bladder-neck hypertrophy, and macroscopic changes in prostatic urethra should be reconsidered for transurethral resection considering the possibility of ACC/BCC.
None to mention.
No conflict of interest.
The publishing of the case report was approved by the Ethical Board of the institution where the patient was referred.
A written consent of participation and publishing was obtained from the patient.
Rubens Jovanovic (RJ), Institute of Pathology, Medical Faculty, University ‘Ss. Cyril and Methodius University in Skopje’, North Macedonia, 1000, Skopje; email: [email protected] ; ORCID: 0000-0002-6574-9333.
Cozzi S , Bardoscia L , Najafi M , Botti A , Blandino G , Augugliaro M . et al. Adenoid cystic carcinoma/basal cell carcinoma of the prostate: overview and update on rare prostate cancer subtypes . Curr Oncol 2022 ; 29 : 1866 – 76 .
Google Scholar
Julka PK . Adenoid cystic carcinoma of the prostate: an unusual subtype of prostate cancer . J Transl Genet Genom 2020 ; 4 : 455 – 63 .
Ryan P , Kelly C , Shanahan S , Jordan KE , Daly J , Ryan P . Adenoid cystic carcinoma of the prostate-a rare case of genitourinary malignancy . Urol Case Rep 2022 ; 42 : 1020 – 5 .
Taskovska M , Frelih M , Smrkolj T , Volavšek M . Basal cell carcinoma of the prostate misdiagnosed as high-grade urothelial cancer - a case report of a diagnostic pitfall . Res Rep Urol 2023 ; 15 : 187 – 92 .
Chang K , Dai B , Kong Y , Qu Y , Wu J , Ye D . Basal cell carcinoma of the prostate: clinicopathologic analysis of three cases and a review of the literature . World J Surg Oncol 2013 ; 11 : 193 – 3 .
Ahuja A , Das P , Kumar N , Saini AK , Seth A , Ray R . Adenoid cystic carcinoma of the prostate: case report on a rare entity and review of the literature . Pathol Res Pract 2011 ; 207 : 391 – 4 .
Dardik M , Epstein JI . Efficacy of restaining prostate needle biopsies with high-molecular-weight cytokeratin . Hum Pathol 2000 ; 31 : 1155 – 61 .
Bostwick DG , Qian J . High-grade prostatic intraepithelial neoplasia . Mod Pathol 2004 ; 17 : 360 – 79 .
Bishop JA , Yonescu R , Epstein JI , Westra WH . A subset of prostatic basal cell carcinomas harbor the MYB rearrangement of adenoid cystic carcinoma . Hum Pathol 2015 ; 46 : 1204 – 8 .
Simper NB , Jones CL , Maclennan GT , Montironi R , Williamson SR , Osunkoya AO . Basal cell carcinoma of the prostate is an aggressive tumor with frequent loss of PTEN expression and overexpression of EGFR . Hum Pathol 2015 ; 46 : 805 – 12 .
Supplementary data
Email alerts, citing articles via, affiliations.
- Online ISSN 2053-8855
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Posted: 17 December 2021
- Tagged: PCR News
What we achieved in 2020-21
Exponential research growth
Our Impact 2020-21
Every year we report on the progress we've made towards achieving our aim for a world where people are free from the impact of prostate cancer. Our annual impact reports showcase the steps we've made, allow us to check-in with our objectives, and our aims for the future.
Read our full report and annual accounts
Our 2020-21 in Numbers
total projects
shortlisted for a major industry award
research budgets or projects cut due to COVID-19
patient panellists who helped us decide what to fund
Chief Executive's Statement
PCR CEO, Oliver Kemp, discussed the significant progress we made in 2020, despite the COVID-19 pandemic.
This is a particularly nice impact report to write the introduction for. A few years ago, we set a goal to significantly expand our research from 4 to 23 projects by 2023. At the time it felt incredibly ambitious, but possible if we had the right people, culture, and harnessed the power of our community. Then a pandemic ripped through the whole world, and it all seemed much more challenging.
At the beginning of the pandemic, we agreed that we wouldn’t furlough people, we would keep our research going, fund new research and put in place new initiatives that would make us stronger and more capable of achieving our goals. These initiatives are starting to reap significant rewards. The research we decided to fund in 2020 is underway and we are now funding 15 projects, a 375% increase since 2018. Having reached 15 projects, it now feels like our distant dream of 23 by 2023 is within reach.
We have also changed our approach to put much more focus on targeting key needs for patients within the prostate cancer ecosystem, such as bone metastases. We are adding more value along the way by connecting researchers and trying to ensure that more research is funded all the way to the clinic. To that end, we made an investment in a small biotech, Lucida Medical, that makes prostate scans cheaper, more accessible, and more accurate, and which plans to roll out its technology in late 2022.
It now feels that despite the pandemic, the original long-term goal is intact due to the tremendous efforts of everyone connected with the organisation.
“ We also connected more deeply with patients by expanding our patient webinars, carried out a rebrand on a shoe string budget, launched PCR Inc. in the US and investigated how we could launch the first cancer-specific translational fund to help ensure good ideas get translated into real benefits for patients. Oliver Kemp PCR CEO
readers of our booklet Treating Prostate Cancer
people attended our virtual talks
researchers supported
podcast launched - The Prostate Pod
Mitigating the impact of COVID-19
Within research
There is an urgent need to develop breakthrough treatments for men with prostate cancer, and research is how we will achieve this. The COVID-19 pandemic has increased the urgency by causing significant delays to cancer diagnosis and treatment.
We supported our scientists throughout the pandemic, in addition to running a new grant call to provide a funding opportunity during a period when there was little available.
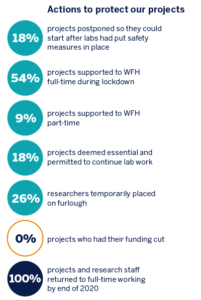
For patients
As prostate cancer patients are at higher risk from COVID-19, we worked hard to quickly provide information to prostate cancer patients regarding their health and safety in the pandemic. We published a patient story from a gentleman who attended a hospital appointment during the pandemic to reassure other patients about safety measures.
We also ran a survey to better understand patient experiences and plan to use this information to build better patient information programmes.
5 Zoom calls, with appearances by 9 scientists and attended by 172 patients and supporters
37 men with prostate cancer engaged in an online research community led by two experienced researchers for 14 days
28 patient panellists reviewed our 2020 shortlist and helped us decide what new research to fund in 2021
Achievements 2020-21 & Targets 2021-22
GOLD STANDARD: Fostering innovation through research
- We completed our analysis of the funding gaps in prostate cancer.
- We will now run grant calls focusing on black men, who are at significantly higher risk and have worse outcomes, on the gaps in the science , and focused on career stages at which scientists most struggle to get funding. We will also make our first investments in translational research, helping move good ideas into the clinic.
ASSEMBLING EXPERTS: Strengthening internal structure and processes
- We made new appointments to our Scientific Advisory Committee and Board of Trustees to fill any gaps in experience and skills.
- We improved our contracts to ensure junior scientists get fairer recognition for their work.
- We will continue to analyse and assess our skills and experience and fill gaps that may exist in our Scientific Advisory Committee, Executive Advisory Group and Board of T rustees.
CONTINOUS LEARNING: Learning and connecting
- We ran internal workshops on agility, strategy, and science to help us respond better to the pandemic and remain connected to our mission.
- We dramatically increased our connections to patients, through live (pre-pandemic) and virtual interactions, increased information provision though our website and booklet, and engaging patients in an online study.
- We will continue to invest in training internally, maintaining expenditure at double the sector average.
LEADING AUTHORITY: Positioning PCR as a thought leader
- We spoke at two large pharmaceutical events about best practice in involving patients.
- We were shortlisted for the Charity of the Year Change Project of the Year award.
- We will continue to create relationships and build partnerships to raise our profile.
FORM PARTNERSHIPS: Leveraging partnerships
- We signed contracts with two complementary prostate cancer charities and established regular catch-ups to align priorities and strategy with two other cancer research funders.
- We will expand our pilot work with biotechs to ensure that the patient voice is translated into the development of new treatments and diagnostics.
SUSTAINABLE GROWTH: Sustainable growth
- We were able to expand to funding 15 projects.
- We adjusted our 2020 income goals, given we couldn’t carry out community fundraising due to the Covid-19 pandemic, and we achieved our modified non-legacy income.
- In 2021/22, we will run new grant calls to increase the number of projects we fund to 18.
website users
PCR staff furloughed or made redundant in 2020
research institutions
years supporting prostate cancer research
What Our Scientists Achieved
Despite COVID-19, our scientists continued to strive forwards, from identifying potential drugs that stop resistant androgen receptors, to confirming aggressive prostate cancer subtypes. In addition, we started funding five new research projects, all focussing on filling vital gaps in prostate cancer research, from bone metastases to co-morbidities.

Meet our newly funded scientists

Partnerships
We are focused on forming partnerships with like-minded organisations. We hope these collaborations will help us to:
- address the areas of unmet need in prostate cancer
- develop drugs through shared knowledge
- place patient voice at the heart of advances in the prostate cancer field
In 2020-21, we formed a partnership with the biotechnology company CellCentric, to help them better understand patient needs in drug development.
We are also working on a number of upcoming partnerships that we are excited to reveal in 2021-22.
How we fund our research
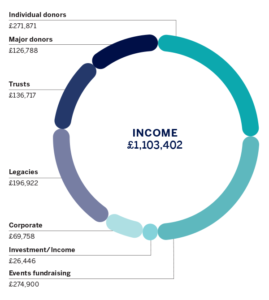
In spite of a challenging year for the whole medical research sector, we are pleased with our overall financial performance. Although events and community activities were postponed or cancelled we have found that donors have remained generous and supportive. Many of our larger donors have retained or increased their donations, and trusts and foundations have continued to give in spite of the uncertainty. We have also invested in growth and expect to be back to above pre-pandemic levels in the next financial year. Although some expenditure on research didn’t happen due to circumstances outside of our control, at no time did we have to reduce grant funding or stop any research. We made a strategic decision to use some of our reserves so that cancer research could continue, despite a challenging financial year for the entire sector because of the pandemic. This is why, for 20/21, our expenditure exceeded our income. We have also spent some of our funds on new initiatives, such as a new patient engagement tool to help shape the latest prostate cancer research and ensure the patient voice is heard and magnified. Finally, we have completed setting up an office in the US, which is projected to make £300,000 to be spent on new research next year.
“ Our existing research projects, along with the new awards that we intend to make later this year, will continue to progress through major milestones, getting ever closer to new treatments for men with prostate cancer. Matthew Ellis PCR Chairman
You can access our full report and accounts here:
Related articles
Pcr partners with gfct in screening push, mark clark appointed as new chair of prostate cancer research, pcr x doc cleaning – london marathon 2024, walk 100 miles in may for prostate cancer research 2024, jog 31 miles in march for prostate cancer, prostate cancer research partners with our future health, pcr submits evidence to the health and social care committee as part of inquiry into men’s health, pcr partners with cac for prostate cancer awareness month.

Research team identifies a new way to treat prostate cancer
T he American Cancer Society estimates there are nearly 300,000 new cases of prostate cancer every year in the U.S., and approximately one in eight men will be diagnosed with prostate cancer in their lifetime. Prostate cancer is often treated with androgen deprivation therapy, which lowers testosterone levels to shrink tumors. However, this treatment has side effects including sexual dysfunction and weight gain, and it eventually results in castrate-resistant prostate cancer, a deadlier form that grows even when testosterone levels are low.
Now, new research published in Science Advances suggests there may be a workaround to improve prostate cancer therapy.
"We want to develop a new therapeutic approach for androgen deprivation in a way that is more patient-friendly," says Marja Nevalainen, MD, Ph.D., a translational medicine physician-scientist and senior author on the study. Using a clever trick of cell signaling, her research curbs tumor growth through a different route.
Androgen deprivation therapy works by decreasing activity of the androgen receptor, a key protein involved in both testosterone signaling and prostate tumor growth. However, the androgen receptor eventually develops mutations that stop androgen deprivation therapy from working. Dr. Nevalainen wondered if she could avoid mutations and target the androgen receptor and associated tumor more directly by blocking activity at Stat5, a protein involved in boosting androgen receptor levels and promoting prostate cancer growth.
Using a drug that inhibits Stat5, Dr. Nevalainen measured androgen receptor levels and prostate cancer growth in cell cultures and human tumors in vitro and grafted into mice. She found that inhibiting Stat5 significantly slowed tumor growth and decreased androgen receptor levels. Because Stat5 inhibition suppresses the androgen receptor, Dr. Nevalainen believes that this approach may carry a lower risk of inducing castrate-resistant prostate cancer because the androgen receptor is less likely to mutate or develop other genetic adaptations.
The next steps will involve translating this research into humans. Nevalainen says a new drug influencing Stat5 activity is beginning to be tested in Phase II clinical trials.
More information: Cristina Maranto et al, Stat5 induces androgen receptor ( AR ) gene transcription in prostate cancer and offers a druggable pathway to target AR signaling, Science Advances (2024). DOI: 10.1126/sciadv.adi2742
Provided by Thomas Jefferson University

Prostate cancer study: More health benefits from plant-based diet
Men with prostate cancer could significantly reduce the chances of the disease worsening by eating more fruits, vegetables, nuts, and olive oil, according to new research by UC San Francisco.
A study of more than 2,000 men with localized prostate cancer found that eating a primarily plant-based diet was associated with a 47% lower risk that their cancer would progress, compared with those who consumed the most animal products.
This amounted to eating just one or two more servings per day of healthy foods, particularly vegetables, fruits, and whole grains, while eating fewer animal products, like dairy and meat. The study followed the men, whose median age was 65 years old, over time to see how dietary factors affected the progression of their cancer.
Plant-based diets include fruits, vegetables, whole grains, nuts, legumes, vegetable oils, tea and coffee. The researchers measured consumption using a plant-based index and compared the men who scored in the highest 20% to those who scored in the lowest 20%.
"These results could guide people to make better, more healthful choices across their whole diet, rather than adding or removing select foods," said Vivian N. Liu, formerly lead clinical research coordinator at the UCSF Osher Center for Integrative Health and first author of the study, which appears in JAMA Network Open .
"Progressing to advanced disease is one of many pivotal concerns among patients with prostate cancer, their family, caregivers and physicians," she said. "This adds to numerous other health benefits associated with consuming a primarily plant-based diet, such as a reduction in diabetes, cardiovascular disease and overall mortality."
Antioxidants and anti-inflammatory compounds
Plant-based diets are becoming increasingly popular in the United States, and evidence is accumulating that they can be beneficial to patients with prostate cancer, the most common cancer among men in the country after non-melanoma skin cancer.
Fruits and vegetables contain antioxidants, as well as anti-inflammatory compounds that have been shown to protect against prostate cancer, and prior research has consistently demonstrated the importance of dietary factors to overall health and well-being.
"Making small changes in one's diet each day is beneficial," said senior author Stacey A. Kenfield, ScD, a UCSF professor of urology and the Helen Diller Family Chair in Population Science for Urologic Cancer. "Greater consumption of plant-based food after a prostate cancer diagnosis has also recently been associated with better quality of life, including sexual function, urinary function and vitality, so it's a win-win on both levels."
Coauthors: From UCSF, other authors are Erin L. Van Blarigan, ScD; Li Zhang, PhD; Rebecca E. Graff, ScD; Crystal S. Langlais, PhD; Janet E. Cowan, MA; Peter R. Carroll, MD, MPH; and June M. Chan, ScD.
- Men's Health
- Prostate Cancer
- Diseases and Conditions
- Agriculture and Food
- Food and Agriculture
- Prostate cancer
- Stomach cancer
- Mediterranean diet
Story Source:
Materials provided by University of California - San Francisco . Original written by Elizabeth Fernandez. Note: Content may be edited for style and length.
Journal Reference :
- Vivian N. Liu, Erin L. Van Blarigan, Li Zhang, Rebecca E. Graff, Stacy Loeb, Crystal S. Langlais, Janet E. Cowan, Peter R. Carroll, June M. Chan, Stacey A. Kenfield. Plant-Based Diets and Disease Progression in Men With Prostate Cancer . JAMA Network Open , 2024; 7 (5): e249053 DOI: 10.1001/jamanetworkopen.2024.9053
Cite This Page :
Explore More
- New AI Accurately Predicts Fly Behavior
- Extreme Impacts: Metals Stronger When Heated
- Rare Earth Element's Secrets Exposed
- Origin of Sun's Magnetic Field
- Measuring Spin of Supermassive Black Hole
- 'Cell Cannibalism' Across Tree of Life
- Very Low Emission Concrete at Scale
- Kangaroos: Fearing Human 'Super Predator'
- Stopping Flu Before It Takes Hold
- Cosmic Rays Illuminate the Past
Trending Topics
Strange & offbeat.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Prostate Cancer Health Tips
- Resources to Share
- What CDC Is Doing
- Talk to Nathan
What CDC Is Doing About Prostate Cancer
What to know.
CDC is working to reduce prostate cancer in several ways.

CDC's prostate cancer activities include:
- Conducting research and developing materials to communicate about prostate cancer screening and treatment. See CDC's prostate cancer research.
- Enhancing prostate cancer data in cancer registries, especially information about the grade and stage of disease at the time of diagnosis, patterns of care, and the race and ethnicity of men with prostate cancer.
- Developing communication tools and decision aids related to prostate cancer. See Talk to Nathan.
- Sponsoring research on men's and health care providers' knowledge and awareness of prostate cancer screening.
- Monitoring the prostate cancer activities identified in local comprehensive cancer control plans.
- Participating in conferences, workshops, and seminars to foster collaboration with partners.
These prevention activities advance CDC's overarching goal of helping everyone live better, longer lives.
Prostate Cancer
Most prostate cancers grow slowly and don't cause health problems. Talk to your doctor before you get tested for prostate cancer.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Browse Articles
Letter to the editor: “incidence of prostate cancer in transgender women in the us: a large database analysis.”.
- Wayne R. Lawrence
- Carl G. Streed Jr
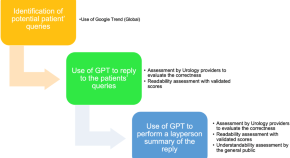
Accuracy, readability, and understandability of large language models for prostate cancer information to the public
- Jacob S. Hershenhouse
- Daniel Mokhtar
- Giovanni E. Cacciamani
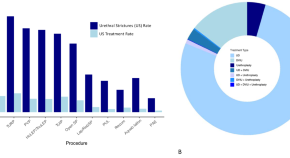
Incidence and management of BPH surgery-related urethral stricture: results from a large U.S. database
- Leslie Claire Licari
- Eugenio Bologna
- Riccardo Autorino
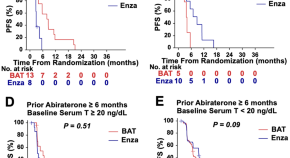
Baseline serum testosterone and differential efficacy of bipolar androgen therapy and enzalutamide in the randomized TRANSFORMER trial
- Mayuko Kanayama
- Hua-Ling Tsai
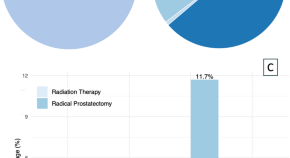
Benign prostatic hyperplasia during active surveillance for prostate cancer: is it time to define management strategies?
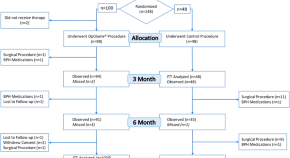
Two-year long-term follow-up of treatment with the Optilume BPH catheter system in a randomized controlled trial for benign prostatic hyperplasia (The PINNACLE Study)
- Steven A. Kaplan
- Jared L. Moss
- Sheldon J. Freedman
Editorial comment on “Reevaluating ‘Top-Down’ HoLEP: the case for anterior fibromuscular stroma as a surgical landmark”
- Hazem Elmansy
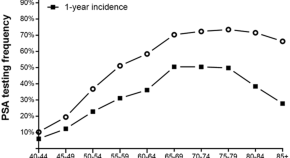
Prevalence and determinants of shared decision-making for PSA testing in the United States
- Naeem Bhojani
- Larry E. Miller
- Ben H. Chew
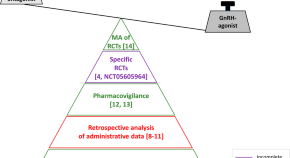
Cardiovascular risk in ADT recipients: does the type of ADT matter?
- Jehonathan H. Pinthus
- Wilhelmina C. M. Duivenvoorden
Methodological considerations in the use of a large database to estimate incidence of prostate cancer in transgender women in the US
- Alison M. Berner
- Devon Buchanan
- Stewart O’Callaghan
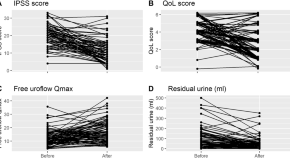
Real-world experience of water vapour therapy (Rezum) in patients with benign prostatic enlargement: a retrospective single-center study
- Mathias Wolters
- Martin Krastel
- Christoph-A. J. von Klot
Correction: A multidisciplinary approach to address unmet needs in the management of patients with non-metastatic castration-resistant prostate cancer
- Neal D. Shore
- Emmanuel S. Antonarakis
- Marina D. Kaymakcalan
Prostatectomy in oligometastatic prostate cancer: a call for high-quality evidence
- Tanya Dorff
- Sheetal R. Kashid
- Cosimo De Nunzio
Transrectal prostate biopsy: easy, effective and safe
- Romain Diamand
- Alexandre Peltier
- Simone Albisinni
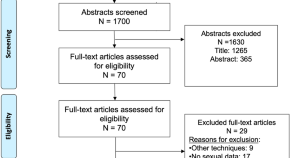
Ejaculation sparing of classic and minimally invasive surgical treatments of LUTS/BPH
- Gian Maria Busetto
- Riccardo Lombardo
- Thomas R. W. Herrmann

Addressing racial disparities in prostate cancer pathology prediction models: external validation and comparison of four models of pathological outcome prediction before radical prostatectomy in the multiethnic SEARCH cohort
- Mahdi Mottaghi
- Thomas J. Polascik
Propensity score-matched evaluation of palliative transurethral resection and holmium laser enucleation of the prostate for bladder outlet obstruction in patients with prostate cancer
- Alexander Tamalunas
- Patrick Keller
- Giuseppe Magistro
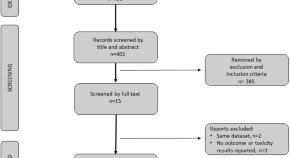
Role of enzalutamide in primary and recurrent non-metastatic hormone sensitive prostate cancer: a systematic review of prospective clinical trials
- Mohamed Shelan
- Vérane Achard
- Richard Cathomas
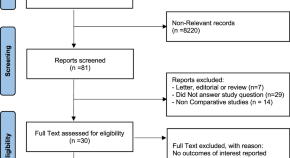
Prostate cancer detection and complications of MRI-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: a systematic review and meta-analysis of comparative studies
- Ugo Giovanni Falagario
- Francesco Pellegrino
- Gianluca Giannarini
The times have changed. Let the urologists change!
- Luca Cindolo
- Feras Al Jaafari
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

2024 ANNUAL REPORT / FACULTY HIGHLIGHT
Translating disparities research into action for men with prostate cancer
Alexander P. Cole, MD and Quoc-Dien Trinh, MD, MBA
Prostate cancer affects over 200,000 people in the U.S. annually, making up almost 30% of all cancer cases in men, and is the most frequently diagnosed type of cancer in men in Massachusetts. However, the impact of prostate cancer is not experienced equally. “Despite having near-universal insurance coverage in Massachusetts, men of color are 22% less likely to receive treatment for prostate cancer when treatment is warranted,” Quoc-Dien Trinh, MD, MBA , a urologist at Brigham and Women’s Hospital, explained to Brigham On a Mission in November 2022.
"Despite having near-universal insurance coverage in Massachusetts, men of color are 22% less likely to receive treatment for prostate cancer when treatment is warranted," Quoc-Dien Trinh, MD, MBA, a urologist at Brigham and Women’s Hospital, explained.
CSPH researchers, Dr. Trinh and Alexander P. Cole, MD , along with their research fellows at CSPH, have produced significant research on prostate cancer disparities. They have worked closely with members of the local community to translate this research to design interventions to improve access to life-saving treatment.
Dr. Cole’s work has shown that racial disparities in prostate cancer outcomes can be substantially reduced in equal access health systems and that including shared decision-making can lessen disparities by race in prostate-specific antigen (PSA) testing, the primary screening test used to detect prostate cancer. In June 2023, he was awarded a $1.3 million Physician Research Award from the Department of Defense to remove barriers to advanced testing and imaging services for Black men at risk for prostate cancer. In October 2023, he received a Young Investigator Award from the Prostate Cancer Foundation and the American Cancer Society for the “ Rural Outpatient Advanced Diagnostics to Maximize Access to Prostate Health” (ROADMAP) study. This study will investigate the nature of disparities in access to prostate cancer care in rural settings.
Serving the Community through the Prostate Cancer Outreach Clinic
In late 2021, Dr. Trinh and colleagues received funding from Mass General Brigham’s United Against Racism campaign, which supports research into health inequities and seeks to build more equitable patient care across the MGB system. These funds helped create the Prostate Cancer Outreach Clinic (PCOC) , which improves access to prostate cancer screening, diagnosis, and treatment for Black men in Massachusetts and provides insights into barriers to care and why men avoid medical care at academic medical centers like the Brigham and Mass General Hospital. Early insights from Dr. Trinh’s and Dr. Cole’s work informed the development of the clinic, which centers its work around proactive outreach and education initiatives.
Since its inception, the clinic has seen over 250 patients. Dr. Trinh engages in direct community outreach at churches and community centers to encourage patients to get screened and visit the clinic. At the clinic, if a PSA test comes back with abnormal results, the information is sent to a community health worker, who works directly with the patient to ensure appropriate follow-up. Dr. Trinh was recently awarded a $1.5 million Health Disparity Award from the Department of Defense Prostate Cancer Research Program to explore the burden of transportation as a barrier to cancer treatment and whether providing ridesharing services is an effective means to reduce missed appointments.
Adapted from the 2024 CSPH Annual Report

Alexander P. Cole, MD
Assistant Professor of Surgery, Harvard Medical School
Associate Surgeon, Brigham and Women’s Hospital
Junior Core Faculty, Center for Surgery and Public Health

Quoc-Dien Trinh, MD, MBA
Chief of Urology, Brigham and Women’s Faulkner Hospital
Co-Director, Prostate Cancer Program, Dana-Farber Brigham Cancer Center
Associate Professor of Surgery, Harvard Medical School
Lead Faculty, Center for Surgery and Public Health
Center for Surgery and Public Health
One Brigham Circle 1620 Tremont Street, Suite 2-016 Boston, MA 02120
617.525.7300
A Division of the Department of Surgery at Brigham and Women’s Hospital
© 2021 Brigham and Women’s Hospital | All Rights Reserved | Privacy Policy

COMMENTS
Prostate cancer is the most common cancer in men in 112 countries, and accounts for 15% of cancers. In this Commission, we report projections of prostate cancer cases in 2040 on the basis of data for demographic changes worldwide and rising life expectancy. Our findings suggest that the number of new cases annually will rise from 1·4 million in 2020 to 2·9 million by 2040.
The Prostate Cancer Prevention Trial showed that finasteride can reduce the risk of prostate cancer, but might increase the risk of aggressive disease. NCI's Howard Parnes talks about subsequent findings and what they mean for men aged 55 and older. Darolutamide Delays the Spread of Some Prostate Cancers.
Read the latest Research articles from Prostate Cancer and Prostatic Diseases. ... Case Report (25) Keyword Index (5) Original Article (606) Paper (384) Point of Technique (1)
Life expectancy for men with localized prostate cancer can be as high as 99% over 10 years if diagnosed at an early stage 15. This long survival can largely be attributed to improvements in lead ...
The Research on Prostate Cancer in Men with African Ancestry (RESPOND) study is the largest-ever coordinated research effort to study biological and non-biological factors associated with aggressive prostate cancer in African American men. The study, launched by NCI and the National Institute on Minority Health and Health Disparities in ...
Read the latest Research articles in Prostate cancer from Scientific Reports. ... Prostate cancer articles within Scientific Reports. Featured. Article 17 May 2024 | Open Access.
Introduction. The Prostate Cancer Foundation (PCF) is a 501(c)(3) charitable organization that globally funds academic research focused on biology, biomarkers and treatments for aggressive and/or advanced prostate cancer.
In this review, we describe the recent advances in prostate cancer research. ... Estrogens also contribute to the development of prostate cancer . In a report from Ellem and Risbridger using aromatase knockout (KO) mice, the knockout mice could not metabolize androgens to estrogens, and it was observed that high levels of testosterone led to ...
In addition to funding research, the PCF extends its service to the research community and patients by convening two annual research conferences and conducting other programs to accelerate the global exchange of knowledge, and create new research partnerships and initiatives in areas of critical unmet need in prostate cancer research. The ...
Introduction: The 2021 Coffey-Holden Prostate Cancer Academy (CHPCA) Meeting, "Prostate Cancer Research in the 21st Century," was held virtually, from June 24-25, 2021. Methods: The CHPCA Meeting is organized by the Prostate Cancer Foundation as a unique discussion-oriented meeting focusing on critical topics in prostate cancer research envisioned to bridge the next major advances in prostate ...
JAMA Network Open. Research. March 19, 2024. This cohort study compares all-cause mortality by neighborhood deprivation and race and ethnicity among individuals with prostate cancer receiving care in the US Department of Veterans Affairs (VA) health care system vs those receiving care outside the VA.
Dr. Morris played a leading role in clinical trials testing a particular tracer that gained FDA approval in 2021. "This is the biggest diagnostic advance for prostate cancer since the 1980s, when the PSA test was introduced," he says. "Imaging has been the Achilles' heel of prostate cancer, forcing many treatment choices to be based on ...
Prostate cancer (PCa) is the second most prevalent cancer in men. ... Research studies demonstrate that these panel-based tests often achieve an area under the ROC curve (AUC) higher than 0.7. ... Studies by Manholini et al. report that variants of ATM (rs587781894), TP53 (rs786203436 and rs587780075), SPOP, ...
The CHPCA Meeting is an annual conference held by the Prostate Cancer Foundation, that is uniquely structured to stimulate intense discussion surrounding topics most critical to accelerating prostate cancer research and the discovery of new life-extending treatments for patients.
As a result, the proportion of prostate cancers diagnosed at an advanced stage has more than doubled over the past 10 years, fr om 3.9% to 8.2%. ACS researchers note that "controversy remains about the underutilized potential of the PSA test" to reduce deaths from prostate cancer by detecting potentially fatal disease earlier.
Zhu et al. summarize recent AI advances for prostate cancer management, including automated diagnosis, grading, patient outcome prediction, and molecular subtyping. This review highlights collaborative human-AI systems, public resources, and challenges such as model interpretability, generalization, and bias. AI pathology tools can assist clinicians to enhance efficiency and improve patient care.
1Department of Biological Sciences, Boler-Parseghian Center for Rare and Neglected Diseases, Harper Cancer Research Institute, University of Notre Dame, Notre Dame, Indiana. Search for other works by this author on: 1Department of Biological Sciences, Boler-Parseghian Center for Rare and Neglected ...
Biomarkers in Prostate Cancer. Sheng-Fei Oon used The Urology Foundation funding to help research new and more accurate ways of testing for prostate cancer - with promising early results.
INTRODUCTION. Adenoid cystic/Basal-cell carcinoma of the prostate is a rare histological type accounting for approximately 0.01% of all prostate cancer cases first described in 1974 [].Since its various histologic and immunohistochemical features, outlining the diagnosis, treatment, prognosis, and outcome remains challenging, which results in delayed diagnosis and treatment and suggests poor ...
Part of Nature Outlook: Prostate cancer. The researchers developed CAR-T cells that target prostate-specific membrane antigen (PSMA) — a protein that is highly expressed in prostate cancer cells ...
0/1000. One-time donation $0.00 USD. I'd like to cover the fees associated with my donation so more of my donation goes directly to American Institute for Cancer Research. This is an edition of the Continuous Update Project (CUP) with the intention of providing the most updated information possible regarding Prostate Cancer.
Metabolic reprogramming of tumor-associated macrophages using glutamine antagonist JHU083 drives tumor immunity in myeloid-rich prostate and bladder cancer tumors. Cancer Immunology Research ...
address the areas of unmet need in prostate cancer. develop drugs through shared knowledge. place patient voice at the heart of advances in the prostate cancer field. In 2020-21, we formed a partnership with the biotechnology company CellCentric, to help them better understand patient needs in drug development.
Now, new research published in Science Advances suggests there may be a workaround to improve prostate cancer therapy. "We want to develop a new therapeutic approach for androgen deprivation in a ...
We want to stop prostate cancer being a killer. We fund groundbreaking research, drive improvements in treatment, and fight injustice in care. ... Annual Reports Annual Report and Accounts 2022/2023;
After a first test, the guidelines strongly recommend offering regular prostate cancer screening every 2-4 years from age 50-69. Again, I want to point out that the interval of every 2-4 years ...
Men with prostate cancer could significantly reduce the chances of the disease worsening by eating more fruits, vegetables, nuts, and olive oil, according to new research by UC San Francisco. A ...
CDC's prostate cancer activities include: Conducting research and developing materials to communicate about prostate cancer screening and treatment. See CDC's prostate cancer research. Enhancing prostate cancer data in cancer registries, especially information about the grade and stage of disease at the time of diagnosis, patterns of care, and ...
Browse the archive of articles on Prostate Cancer and Prostatic Diseases. ... Meeting Report (6) News (27) Original Article (606) ... Research articles
2024 ANNUAL REPORT / FACULTY HIGHLIGHT Translating disparities research into action for men with prostate cancer Alexander P. Cole, MD and Quoc-Dien Trinh, MD, MBA Prostate cancer affects over 200,000 people in the U.S. annually, making up almost 30% of all cancer cases in men, and is the most frequently diagnosed type of cancer in […]