

Clinical Project Manager
Clinical trial management certification.

Demo Clinical Project Manager Training
Research Project Manager Certification
CCRPS Research Manager Graduates obtained job roles including:
Clinical Trial Project Manager, Research Nurse Manager, Clinical Research Coordinator-Data Manager, Clinical Research Associate, Transdisciplinary Research Project Manager, IT Project Manager in Clinical Research, Publicly Funded Research Project Manager (2024 CCRPS Graduate LinkedIn Survey).
Clinical Research Project Manager Training
Advanced Clinical Research Associate Certification (ACRAC)
Introduction
CME Handout
Common Terminology Used In Clinical Research - Reference Glossary
Commonly Used Abbreviations and Terms in Clinical Research
An Overview of ICH GCP
CFR 21 Part 11
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated
Ethics of Research Involving Pregnant Women and Fetuses
Fundamentals of Project Management
Project Management Fundamentals
PMBOK Summary - Mandatory Project Management Review
Clinical Trial Project Management
Importance of Project Management
Roles and Relationships in Clinical Trials
Role of a Project Sponsor
ICH GCP E6 Section 5 - Sponsor/CRO Responsibilities
Institutional Review Board/Ethics Committee (IRB/EC) (Requirements, sIRB, Application, Exemptions, Expedited Review, Continuation, and Reporting)
Data Safety Monitoring board- DSMB
Stakeholders in Clinical Trials (Sponsor, Project Manager, IRB, PI, CRA, CRC, Site Staff, Data Team/DSMB, Patients)
Contract Research Organizations (Delegation, Responsibilities, Management )
ICH GCP E6 Sections 2-4 Principles, IRB, & Investigator Roles
ICH GCP E6 Section 4 - Reporting Responsibilities of the Investigators
Skills of a Project Manager
Essential skills of a Project Manager
Technical skills for Project Management
Project Team
Managing a Project Team
Project Management Documents
Regulatory Documents
Regulatory Documents in Clinical Trials
Delegation of Authority Log – DOAL
Investigators Brochure (IB)
Trial Master File
Essential Regulatory Documents Binder Tab Organization (Trial Master File)
Trial Master File Reference Guide
New Drug Application
The Investigational New Drug (IND) & New Drug Application (NDA) Process
Investigator Initiated Multi-Center Trials
IND and IDE AE Reporting
Safety Reporting Requirements for Sponsor Investigators of An IND
Problem Solving in Project Management
Problem Solving as a Project Manager
Project Failures and Statistics
Project Reporting Styles
Avoiding Project Failure
Budgeting for Clinical Trials and Projects
Project Budgeting
Payments and Budgeting for Investigators and Site
Advertisement Aid in Subject Recruitment and Retention
Clinical Trial Design
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
Randomized Controlled Trials (Randomization, Allocation Concealment, Validity, Blinding, Controls, Outcomes, Fidelity)
Blinding and Unblinding in Clinical Trials
The Clinical Trial Protocol - Advanced Mastery Review
Inclusion and Exclusion Criteria in Clinical Research (Writing, Assessing for Broad vs. Narrow, Organ Dysfunction, Older Adults, Pediatrics, Pregnant Women)
Protocol Deviations and Violations (Major, Minor, Exceptions, Resolution)
Project Management Scheduling and Tracking
Basics of Project Scheduling
Project Progress Tracking
Project Management Planning Process
Project Management Plan
Closing a Project
Project Delays
Process Mapping
Metric Tracking
Duties of a Successful Project Manager
Roles and Responsibilties of a Project Manager
Project Management Success Factors
Adverse Events
Advanced Review of Adverse Events
Site Selection and Visits
Types of Monitoring Visits (Selection, Initiation, Routine, Close-Out)
Site and Investigator Selection Criteria (Process, Criteria, Investigator Selection, Agreements, Decision-Making)
Site Selection/Qualification Pre-Study Visit (SSV/SQV) (Before, During, After, Letters, Checklists, and Report)
Audit and Inspections
Audits and Inspections in Clinical Trials
Clinical Trial Data Audits
FDA Warning Letter
Quality Control and Safety
Quality Control in Clinical Trials ( QC/QA, KQI, QMS, Checklist)
ICH GCP - Safety of Human Subjects in Clinical Research
Technology in Trials (IVRS, CTMS, EDC)
Clinical Trial Management System-CTMS
ICH GCP - Trial Management, Data Handling, and Record Keeping
An Overview of Remote Monitoring - COVID-19 Update
Centralized Monitoring
Interactive Response Technologies in Clinical Trials (IVRS, IWRS, IRT, RTSM)
Pharmacovigilance and Regulatory Affairs
Advanced Practice of Pharmacovigilance
Regulatory Affairs for Clinical Trials
Investigational Product and Labs
Investigational Product Storage and Dispensing
Investigational Product Accountability in Clinical Trials
Local and Central Labs in Clinical Trials (Local, Regional, Central, GLCP, CLIA Cert, Lab Audit Checklist)
Patient Recruitment, Retention, and Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Project Manager Job Readiness
Project Manager Skills Interview Questions
Interview Questions
Competency Examination
Competency Exam

About this course
- Required: Prior clinical research or project management experience.
- Length: 100 hours. Online, self paced, start anytime.
- ACCRE, Joint Accreditation with AMA, ANCC, ACPE for 17.5 CME. Online certificate. Exam score 70% or higher on 2 attempts.
Enroll Schedule Advising
CCRPS Reviews
Clinical Research Project Manager Certification
Navigating the Course
Natalie johnson.
I was originally intimidated by the materials and all that I had to learn. The syllabus, the instructors and the course material were easy to follow and lear...
I was originally intimidated by the materials and all that I had to learn. The syllabus, the instructors and the course material were easy to follow and learn from. I am very happy I completed the course.
Advanced Clinical Research Project Manager Certification ...
Roger andersen.
There is extensive material in this course. It is highly relevant to managing clinical trials.
Advanced Clinical Research Project Manager
Ellen lyrtzis.
The clinical trial project manager is responsible for different aspects of the clinical trial process, such as setting timelines, developing budgets, and overseeing data analysis. To become a clinical trial manager, you can gain experience in project management or clinical research roles. Clinical trial manager certification will increase your chances of getting hired.
To become a clinical project manager, one must first obtain a Bachelor's degree in a health-related field. Additionally, developing skills for project management and participating in relevant courses are necessary.
Experience can be gained by volunteering or interning in clinical trials with pharmaceutical companies or medical research centers.
Pursuing an advanced degree or getting certified as a Clinical Project Manager with open up more opportunities.
Clinical Trial Manager
Clinical Trial Managers are responsible for planning and overseeing all aspects of clinical research projects. This includes making sure the project is conducted according to regulations and best practices. They also manage budgets, timelines, and resources to ensure the project is completed successfully.
Clinical Project Managers plan and execute clinical research projects by coordinating with internal and external stakeholders, regulatory authorities, and by develop key documents like protocols, consent forms, investigator brochures, budget sheets, study reports, and final reports.
Clinical Research Manager
Clinical Research Managers make sure that data is being collected and analyzed correctly, and that everyone is compliant. Clinical Project Managers design research studies and monitoring projects in terms of cost, budgeting, quality assurance, and risk assessment.
The Clinical Trials Management Certificate program is designed to provide students with the knowledge and skills necessary to design, implement, and manage clinical trial protocols.
This Clinical Trials Design & Management Certificate Program introduces learners to the fundamentals of clinical trials design and management.
The program covers principles and regulations of clinical trial design, analysis techniques for statistical analysis, quality control and assurance, data management and reporting.
Students will also gain an understanding of risk assessment strategies, study site selection, protocol implementation and monitoring, resource management as well as safety requirements for conducting clinical trials.
Clinical Trial Manager Salary
Clinical manager salary.
Clinical trial manager salaries vary based on experience, location, and company size. The average hourly rate for a clinical trial manager is $30-$60 per hour.
Clinical trial manager salary
The monthly salary of a clinical trial manager typically ranges from $5,000 to $10,000. Those with more experience or who work at well-known organizations can earn up to $20,000 per month.
Clinical project manager salary
Annual clinical project manager salary ranges between $60,000 and $120,000 dollars per year. The median salary for a clinical trial manager is approximately $82,500 across all industries and geographies
What does a Research Project Manager do?
Clinical Project Managers are responsible for creating project plans, timelines, budgets, and communication with vendors and stakeholders. They also provide training to personnel involved in the project, establish systems to track project progress, and identify risks associated with the project.
Clinical Project Managers make sure that clinical research projects go well by working groups like contract research organizations (CROs), internal departments, and external vendors. Clinical Project Managers make sure that projects follow the research protocols, good clinical practices (GCPs), applicable regulations, and standards.
Research Project Managers also develop protocols for data collection and analysis, prepare reports for regulatory submissions, coordinate activities related to safety monitoring, and provide support to staff during project-related training sessions or workshops.
Trial Project Managers make sure that data is collected accurately according to guidelines from the FDA or EMA. This includes finding risks associated with the project; assessing their impacts; planning ways to reduce the risks; and also planning how to use resources so that everything runs smoothly.
The most advanced clinical trial project management training available
Take the fast track.
Take the fast track to a lucrative career as a Clinical Research Project Manager to start earning salaries of $100k+
Get advanced training
Get the most advanced training - ACRPM is recognized as a gold standard by many CROs in the industry thanks to its comprehensive training
Work at your own pace
Work at your own pace from wherever you are with flexible online training. The 100+ modules included can be completed in as little as 2 weeks
Requirements
Designed for those holding a minimum of a BA in Science, ACRPM is internationally accredited to ACCRE, ACCME, ACPE, ANCC, and Transcelerate Biopharma. In other words, upon completion of the course and the final exam, you will have a level of knowledge equivalent to (and beyond!) that of a senior CRA.
ACRPM features 100+ modules, or 250 hours, of on-demand online training (worth 17.5 CME credits). The course has been put together by clinical trial project managers, enabling students to build a deep knowledge of the industry.
Certification
This course can be completed in as little as two weeks, with certification and a letter of recommendation awarded after completing a final exam. ACRPM also provides you with tools to help you find a job, including resume and interview guides, giving you a further edge over other applicants for the same position.
Clinical Project Manager Guide
Clinical Project Managers are responsible for clinical research activities and initiatives in the healthcare, pharmaceutical, and biotechnology sectors. This includes creating and implementing project plans, developing timelines, overseeing budgets, managing resources, and ensuring compliance with regulatory requirements. CPMs typically work with cross-functional teams that may include clinicians, scientists, regulatory affairs specialists, data management personnel, software developers/engineers, project sponsors/coordinators.
The career path of a Clinical Research Project Manager is very rewarding. You get to use your clinical research expertise to develop new treatments for existing medical conditions and to create new treatments for future medical needs. Additionally, you get to work with leading doctors, scientists, and other professionals who are working towards improving healthcare outcomes. You also have the opportunity to build relationships with key stakeholders including pharmaceutical companies, governmental agencies, and funding bodies which leads to greater satisfaction in your career.
Research project managers are responsible for planning and executing research studies throughout their entire life cycle. This includes activities such as designing experiments or surveys, selecting appropriate experimental methods or sampling techniques, recruiting participants for studies or surveys, collecting data from multiple sources, analyzing results using statistical methods or software programs, interpreting results and preparing recommendations for further action.
The clinical project manager definition refers to a professional who is in charge of developing clinical research activities from the beginning to the end. This includes making a plan for the scope and timeline of the research project; making sure that the project meets all regulatory requirements; organizing the technical aspects of data collection; coordinating meetings between stakeholders; monitoring progress and deadlines; utilizing risk management processes; providing guidance to staff members; ensuring quality control of data collection processes; providing reports to stakeholders and company executives regarding project status updates as needed.
Working as a clinical project manager can be challenging due to the complexity of the tasks involved. Clinical projects require significant planning, coordination and supervision between multiple stakeholders. Additionally, time management can be difficult due to deadlines that must be met and ever-changing regulatory requirements. Other challenges include managing competing priorities and ensuring proper communication between team members. It is also important for clinical project managers to stay current on trends, technologies and best practices within their field so that they can remain competitive in their work.
The core duties of a clinical project manager vary depending on the industry but typically include planning and organizing activities related to assigned projects; developing budgets; coordinating resources; monitoring progress; overseeing quality control standards; ensuring adherence to safety procedures; providing leadership for teams; communicating with stakeholders throughout the duration of the project; developing plans for corrective action if needed; preparing reports for upper management; analyzing data related to performance metrics; maintaining records of all activities related to assigned projects; and staying informed of changes in regulations relating to their area of expertise.
To be a good clinical project manager, it is important to have experience working in healthcare, like in a hospital. It is also helpful to be good at organizing and communicating, as well as understanding the medical environment and the rules that govern it. Additionally, clinical project managers should know about principles related to project management, like budgeting, scheduling, and risk management. Those who have earned a healthcare-related degree or certificate (like in nursing or health information management) may have a better chance of being successful in the role.
A clinical project manager oversees clinical research studies from design through implementation. This includes working with teams of physicians, pharmacists, nurses, statisticians or other health professionals. Responsibilities include making sure the project runs smoothly while staying within the budget and timeline. As part of their role they must understand FDA regulations when conducting trials in the US or ICH guidelines when conducting international studies. Additionally they must be able to identify potential risks associated with each study and develop strategies for mitigating them throughout all stages of the study process.
The average salary for a Clinical Project Manager is approximately $85,000 per year. Hourly pay for Clinical Project Managers usually ranges from around $41 to $58 per hour, with an average rate of approximately $49.50 per hour. Monthly salaries typically range from around $7,083 to $9,833 per month or more.
•Clinical Project Manager – Research and Development: This person is responsible for making sure clinical research studies are organized and run smoothly. • Senior Clinical Project Manager: This person provides guidance to project teams and management when they are developing clinical research projects. • Global Clinical Trial Program Manager: This person creates global clinical trial plans that meet operational requirements while following regulatory guidelines. • Clinical Data Management Project Manager: This person oversees all aspects of data collection, management, analysis and reporting associated with a clinical research project from start to finish. • Regulatory Affairs/Clinical Project Manager: monitors and reports on the regulatory submissions for clinical trials taking place in different countries. • Clinical Operations Project Manager:: oversees and coordinates the daily operations of clinical research projects to ensure that they meet quality assurance standards set out by IRBs or FDA .
Achieving certification as a Clinical Project Manager is a way to show that you are an expert in project management within the healthcare and medical research industries. This certification allows individuals to demonstrate their understanding of clinical project management concepts and skills, which are necessary for ensuring successful outcomes for initiatives within complex clinical research environments. To obtain a Clinical Project Manager Certification, you need extensive knowledge and experience in clinical project management, which can be obtained through formal education, training courses, and hands-on experience.
Certification as a clinical project manager provides healthcare professionals with the skills and knowledge necessary to effectively manage complex clinical trials, research projects, and quality improvement initiatives. There are several key reasons to get certified, including professional credibility, a competitive edge in the job market, increased knowledge base, improved efficiency in completing tasks related to clinical project management, and networking opportunities.

WELCOME TO THE CLINICAL RESEARCH PROJECT MANAGEMENT ASSOCIATION
The Clinical Research Project Management Association (CRPM) is a global professional organization dedicated to uniting clinical research professionals who employ project management tools and methodologies to ensure the successful completion of project deliverables on time and within budget. Our mission is to foster connections, promote continuous learning, and enhance the well-being of Clinical Research Project Managers worldwide.
Join us today and become a part of the CRPM community, where you’ll find endless opportunities to connect, learn, and grow.
Welcome to a brighter future in Clinical Research Project Management!

Connect with the CRPM Community
At CRPM, we believe that networking is a vital part of professional growth. We facilitate connections between our members through various channels, including online groups, in-person meet-ups, monthly webinars, and retreats. By expanding the CRPM network, we aim to create a supportive and resourceful community that spans the clinical research industry. We invite you to join us and experience the power of connection and collaboration.
BECOME A MEMBER
Becoming a member of CRPM grants you access to a thriving community of clinical research project management professionals, along with a wealth of resources, networking opportunities, and educational events designed to support your career growth. Joining CRPM empowers you to stay ahead in the industry, expand your professional network, and continuously develop your skills and knowledge.
GET INVOLVED
By getting involved with CRPM as a volunteer or ambassador, you have the opportunity to make a meaningful impact within the clinical research project management community while developing your leadership and collaboration skills. Your contributions will help shape the future of the profession, foster a supportive environment for fellow members, and further establish CRPM as a leading organization in the field.
EVENTS CALENDAR
CRPM events, such as webinars, in-person meet-ups, annual conferences, and retreats, provide members with diverse opportunities to learn, network, and engage with fellow clinical research project management professionals. These events not only foster a sense of community but also help members stay current with industry trends, enhance their skills, and grow professionally.

Empower Your Career through Education and Professional Development
Unlock the full potential of your clinical research project management career by joining CRPM, where you will gain access to a wealth of tools, templates, and cutting-edge resources specifically curated for professionals like you. Don’t miss this opportunity to elevate your expertise and stay ahead in the rapidly evolving field of clinical research project management.
PROFESSIONAL DEVELOPMENT
Professional development articles offer valuable insights and practical advice to help CRPMs stay informed on the latest developments and best practices in their field. By regularly engaging with these articles, professionals can enhance their skills, broaden their knowledge, and ultimately excel in their roles.
CRPM ARTICLES
Our extensive library of articles covers a wide array of topics related to clinical research project management. These articles provide valuable insights and practical advice to help our members stay informed and up-to-date on the latest developments and best practices in the field.
CRPM TOOLKIT
The CRPM toolkit is a carefully curated collection of resources, templates, and best practices designed to help clinical research project managers streamline their work and optimize project outcomes. By utilizing this toolkit, members can enhance their efficiency, reduce project risks, and drive success in their clinical research projects.

Health and Wellness: The Key to Success
CRPM understands that the well-being of our members is essential for their overall success. We recognize that CRPMs are not just productivity engines, but whole individuals with unique needs and challenges. To address this, we feature health and wellness initiatives, such as stress management techniques, work-life balance strategies, and self-care practices tailored to the needs of CRPMs. By promoting a healthy lifestyle and fostering a culture of well-being, we empower our members to become the best versions of themselves, both professionally and personally.
HEALTH & WELLNESS ARTICLES
The health and wellness articles featured by CRPM address the unique challenges faced by clinical research project managers and offer practical advice on maintaining a balanced, healthy lifestyle. These articles promote self-care, stress management techniques, and work-life balance strategies, empowering CRPMs to succeed both professionally and personally.
ANNUAL RETREAT
Our annual retreats offer an immersive experience for CRPMs to connect with fellow professionals in a relaxed and inspiring setting to recharge, grow, and create lasting memories . Through workshops, team-building activities, and networking sessions, attendees can deepen their connections and foster long-term relationships with other members of the CRPM community.
HEALTH & WELLNESS EVENTS
CRPM’s wellness and health webinars and in-person events are designed to address the unique needs of clinical research project managers, promoting a balanced and healthy lifestyle. These programs provide practical guidance on self-care, stress management, and work-life balance strategies, enabling members to maintain their well-being while excelling in their professional roles.

Navigating Project Success with Scrum Methodologies
Scrum has emerged as a beacon of efficiency, collaboration, and adaptability in the ever-evolving landscape of project management methodologies. Originating from the agile framework, Scrum offers a structured approach to managing complex projects while fostering continuous improvement and flexibility. Whether you’re a seasoned project manager or a newcomer to the world of agile methodologies, understanding…
Continue Reading Navigating Project Success with Scrum Methodologies

How to Write a Professional Bio
Welcome to the world of clinical research project management, where your professional bio is more than just a formality—it’s a powerful tool to showcase your expertise and achievements. Whether you’re presenting at a conference, networking, or updating your online profile, crafting an engaging and informative bio is crucial. In this comprehensive guide, we’ll walk you…
Continue Reading How to Write a Professional Bio

What is Project Management?
Project Management is an essential skill set in various fields and industries. This article aims to introduce you to the multifaceted world of project management, providing a comprehensive insight into the roles, responsibilities, and key concepts involved. Whether you are looking to kickstart your career in project management or aiming to enhance your knowledge, this…
Continue Reading What is Project Management?

Understanding EIN: The Backbone of Your Business Identity
Every business needs a unique identifier, much like every individual needs a Social Security number. For businesses, this identifier is known as an Employer Identification Number (EIN), provided by the Internal Revenue Service (IRS). Let’s take a look at what an EIN is, why your business needs one, how to get one, and what to…
Continue Reading Understanding EIN: The Backbone of Your Business Identity

Mastering the Eisenhower Matrix
Keeping up with tasks and responsibilities can be a struggle when everything seems important. If you’re often caught in a whirlwind of to-do lists, it’s time to harness the power of the Eisenhower Matrix. This time management tool will help you decide, prioritize, and conquer your tasks more effectively. Let’s delve into the how-to of…
Continue Reading Mastering the Eisenhower Matrix

Content Management System (CMS): Selecting the Right Platform
In the realm of business, establishing a strong online presence is as crucial as the research itself, especially for entrepreneurs stepping into this domain. A critical step in this journey is choosing the right Content Management System (CMS) for your website. A CMS is not just a tool to build a website; it’s the foundation…
Continue Reading Content Management System (CMS): Selecting the Right Platform
Graduate Certificate in Clinical Research Design and Management
The Graduate Certificate in Clinical Research Design and Management (a blended program that allows online participation) provides an ideal training platform for those interested in research at a clinical site or in a medical products company.
The Graduate Certificate in Clinical Research Design and Management program is designed to provide the students with a sound understanding of the organization and conduct of clinical trials. They will be able to explain and compare the types of testing that will be required to introduce a new drug or device onto market, and describe the relationship of statistical design and hypothesis development to the validity of the trial and the formulation of claims.
Well-structured and managed clinical trials are an important cornerstone in the development of medical products. The development and execution of such trials requires a knowledge of scientific principles and statistical methods, complemented by a solid grounding in bioethical principles underlying the protection of human research subjects.
This program provides a learning environment in which students will analyze the current policies and guidelines under which clinical projects are organized and regulated. Designed to strengthen the statistical, research and project management skills of clinical researchers and their associated clinical team members, students will also explore the trends in policy development relating to human research.
Our accredited graduate certificates develop specialists for high-demand areas within the broader field of regulatory and quality science. Programs are structured so working professionals can take one or two courses per term. Each certificate can stand alone, or you have the option to build upon your coursework and apply it later toward a master’s degree or our doctoral program.
Earn your Graduate Certificate in Clinical Research Design and Management online. Request more information .
Graduate Certificate in Clinical Research Design Curriculum
The USC Graduate Certificate in Clinical Research Design and Management requires satisfactory completion of 12 units beyond the baccalaureate degree.
Typically, courses offered by the Department of Regulatory and Quality Science can be taken either in-person or online (within the United States). Upon admission, a course plan will be developed to fit your needs.
Career Opportunities for Clinical Research Design Graduates
The FDA offers employment in more than 30 distinct disciplines, and opportunities within the industry can be even more varied. The following are but a few of the broad range of job categories within regulatory and quality sciences:
- Business development/corporate strategy
- Government affairs and policy
- Research and development
- Clinical trials management
- Risk management
- Quality assurance and control
- Good manufacturing processes
- Quality system regulation
- Domestic and international compliance
- Supply chain management
- Project management
- Drug safety
- Medical writing
- Post-marketing surveillance
- Comparative effectiveness
- Reimbursement
Your career in clinical research design and management starts here. Request information today .
Designed for Working Professionals
All courses offered by the Department of Regulatory and Quality Sciences can be taken online or in-person. Created for working professionals who wish to further their education but cannot travel to traditional classrooms, online students participate in the same classes as students who attend onsite. Lectures can be viewed in real time or at another time.
Related Online Graduate Programs
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Clinical Research Management Certificate
Admissions | Curriculum | FAQs
The demand for well-prepared clinical researchers to fulfill roles in academic medical centers, private practice research, contract research organizations and biopharmaceutical companies is growing at an unprecedented rate. Now is the time to break into or expand your career opportunities in the biopharmaceutical and healthcare research industries.
This online graduate-level Clinical Research Management certificate will provide you with a broad foundation of knowledge and skills in human subjects’ research regulations, conduct, management and leadership and meets the internationally recognized Joint Task Force for Clinical Trial Competency framework.
Upon completion of this certificate program, students will be prepared to:
Systematically apply understanding of regulations, processes and management of human subjects’ research, with a focus on clinical trials of drugs and devices.
Apply bioethical standards to medical development and innovations of complex clinical research studies conducted in the healthcare and biopharmaceutical environment.
Apply quality improvement, project or data management to support and monitor clinical research studies.
The Clinical Research Management certificate includes 12 credits of graduate coursework, is offered 100% online and asynchronously and is designed to be able to be completed in three semesters.
At the completion of the program, you will earn an academic certificate from The Ohio State University.
All courses in the Clinical Research Management certificate program are included in our existing Masters of Clinical Research program which allows students the opportunity to explore the content of the degree program prior to applying.
Admission to the certificate program will occur every semester.
Online program availability may vary by state. For more information on state authorization requirements and disclosures related to online programs and state licensing board contact information, please visit the State Authorization website .
Application Deadlines
Autumn Opens: March 2 Deadline: June 18
Spring Opens: June 19 Deadline: November 1
Summer Opens: November 2 Deadline: March 1
Program Info and Updates
Want to learn more about the program and receive updates about overview sessions, applications and more? Request information below.
- Undergraduate
- Master's
- Palliative and End of Life Care Certificate
- Health and Wellness Coaching Certificate
- Healthcare Leadership and Innovation Certificate
- Interprofessional Healthcare Academic Certificate
- Undergraduate Certificates
- Office of Continuing Education
- Program Information
Clinical Research Project/Program Management Conference
Unlock the art and science of clinical trial project and program management with this immersive workshop. Whether you're diving into this dynamic field or seeking to enhance your expertise, this program is designed to introduce, affirm, or elevate your understanding of project management in the field of clinical research. Delve into the regulatory landscape and refine your skills in planning, administering, tracking, evaluating and budgeting through engaging discussions and interactive sessions.
May 16 & 17, 2024 | Scottsdale, AZ
Embassy Suites by Hilton Scottsdale Resort 5001 N Scottsdale Rd Scottsdale, AZ 85250
Hotel Phone: (480) 949-1414 Reservations: (480) 949-1414 Or ONLINE
SOCRA hotel room rate of $204 USD (Includes resort fee), plus applicable taxes, is available until April 25, 2024 or until the room block is filled. You must mention SOCRA to receive room rate.
October 17 & 18, 2024 | Philadelphia, PA
Four Points Sheraton Philadelphia City Center Hotel 1201 Race Street Philadelphia, Pennsylvania 19107
Hotel Phone: 215-496-2700 Reservations: 215-496-2700 Or ONLINE
SOCRA hotel room rate of $229 USD (Includes resort fee), plus applicable taxes, is available until October 2, 2024 or until the room block is filled. You must mention SOCRA to receive room rate.
Registration Fees:
- Member Fee - $655
- Non-Member Fee - $730 Non-Member Fees include a non-refundable one-year membership in SOCRA
Continuing Education Credit Hours:
SOCRA designates this educational activity for a maximum of 14.25 Continuing Education Credits for SOCRA CE and Nurse CNE. SOCRA designates this live activity for a maximum of 14.25 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Accreditation Statements:
CME for Physicians: The Society of Clinical Research Associates is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. CNE for Nurses: Society of Clinical Research Associates is accredited as a provider of nursing continuing professional development by the American Nurses Credentialing Center's Commission on Accreditation.
SOCRA Course Series: 250
- Program Description
- Return to Live Programs
Top 11 Clinical Project Manager Certifications
Updated July 8, 2023 19 min read
Certifications are important for clinical project managers in the job market because employers view them as a sign of commitment to the profession and an indication that the individual has demonstrated knowledge and experience. Certification serves to confirm that the individual has achieved a certain level of expertise and proficiency in clinical project management. Additionally, certifications provide evidence of an individual's commitment to staying current with industry trends and best practices, as well as their dedication to quality service delivery. Finally, certifications can help differentiate qualified candidates from others in a competitive job market.
This article reviews some of the top certifications for Clinical Project Managers and explains how they can help advance a clinical project manager's career.
What are Clinical Project Manager Certifications?
Clinical project manager certification is a professional credential that demonstrates an individual’s knowledge and experience in managing clinical projects. This certification is designed to help individuals demonstrate their expertise and proficiency in managing clinical research, development, implementation, and evaluation of clinical projects. Clinical project managers are responsible for overseeing the entire life cycle of a clinical project from conception to completion. They must ensure that all stakeholders are informed about the project's progress and that the project meets its goals on time and within budget.
The certification program typically requires individuals to have at least three years of experience in the field of clinical research or management. Candidates must also pass an exam covering topics such as risk management, project planning, budgeting, communication, regulatory requirements, data analysis, and more. Upon successful completion of the exam, individuals will receive a certificate attesting to their expertise in this area.
Having a clinical project manager certification can help individuals stand out among their peers when applying for jobs or promotions within the medical field. It provides proof that they possess the necessary knowledge and skills required for success in this role. Additionally, it shows employers that they are committed to continuing their education by taking courses related to this field. Furthermore, a certified individual may be eligible for higher salaries than those without the credential due to their specialized knowledge.
Pro Tip: When considering a clinical project manager certification, research the requirements and be sure to have sufficient experience in clinical project management. Many certifications require a minimum number of years of experience, as well as successful completion of an exam or other assessment. Additionally, many certifications also require that you maintain your certification through continuing education and/or recertification.
Related : What does a Clinical Project Manager do?
Here’s our list of the best certifications available to Clinical Project Managers today.
1. Project Management Professional (PMP)
Project Management Professional (PMP) is a professional certification offered by the Project Management Institute (PMI) that is designed to demonstrate an individual’s knowledge and experience in project management. It is one of the most sought after certifications in the field of project management, and it is recognized worldwide as a symbol of excellence.
In order to obtain the PMP certification, an individual must first meet certain eligibility criteria. This includes having at least three years of project management experience and 4,500 hours leading and directing projects. Additionally, individuals must have at least 35 hours of formal education or training in project management.
Once an individual meets the eligibility requirements, they can apply for the PMP exam. The exam consists of 200 multiple-choice questions that are designed to test an individual’s knowledge and understanding of project management concepts and processes. The exam takes approximately four hours to complete and costs $405 for PMI members or $555 for non-members.
The PMP certification is valid for three years, after which time individuals must recertify by either retaking the exam or completing 60 professional development units (PDUs). PDUs can be earned through activities such as attending conferences or workshops related to project management, teaching courses related to project management, or writing articles related to project management.
Overall, obtaining a PMP certification can be a lengthy process but it can also be very rewarding as it demonstrates expertise in the field of project management and provides individuals with valuable skills that they can use throughout their career.
2. Certified Associate in Project Management (CAPM)
Certified Associate in Project Management (CAPM) is a certification offered by the Project Management Institute (PMI). It is designed to demonstrate an individual’s understanding of the fundamental knowledge, processes, and terminology used in project management. This certification is ideal for those who are new to project management or those who are looking to advance their career in the field.
The CAPM certification requires that applicants have at least 1,500 hours of project experience or 23 hours of formal education related to project management. Those without this experience can still qualify for the exam by completing a PMI-approved training course.
The exam consists of 150 multiple-choice questions and must be completed within three hours. The cost of the exam is $300 for PMI members and $405 for non-members. To maintain the certification, individuals must earn sixty professional development units (PDUs) every three years.
3. Certified Clinical Project Manager (CCPM)
Certified Clinical Project Manager (CCPM) is a professional certification that recognizes an individual’s expertise in managing clinical research projects. It is designed to demonstrate an individual's knowledge and experience in the field of clinical project management, as well as their commitment to the highest standards of ethical practice.
The CCPM credential is offered by the Society of Clinical Research Associates (SOCRA). To become certified, individuals must have at least three years of experience in clinical research project management and must pass a comprehensive examination. The exam consists of 150 multiple-choice questions covering topics such as risk management, budgeting and finance, regulatory compliance, data analysis and reporting, project planning and execution, and quality assurance.
The cost for the CCPM certification exam is $500 USD. The exam can be taken online or in-person at one of SOCRA's testing centers located around the world. The exam typically takes two hours to complete. After successfully passing the exam, individuals will receive their CCPM certification within four weeks.
To maintain their certification status, individuals must participate in continuing education activities every three years to ensure they remain up-to-date on best practices in clinical project management.
4. Agile Certified Practitioner (ACP)
Agile Certified Practitioner (ACP) is a certification program offered by the Project Management Institute (PMI). It is designed to recognize professionals who have demonstrated their knowledge and experience in agile practices. The ACP certification is based on the PMI Agile Practice Guide, which outlines best practices for using agile methods in project management.
The ACP certification requires applicants to pass an exam that covers topics such as agile principles, values, and mindset; agile planning and estimation; product delivery; quality assurance; team dynamics; and stakeholder engagement. The exam consists of multiple-choice questions and takes about three hours to complete.
In order to be eligible for the ACP certification, applicants must have at least 2,000 hours of general project experience in the last five years or 1,500 hours of project experience using agile methods in the last three years. Applicants must also hold either a current PMP or PgMP credential from PMI or a current PMI-ACP credential from another organization.
The cost of the ACP exam varies depending on where you take it, but typically ranges from $400-$600 USD. Additionally, there may be additional fees associated with registering for the exam and taking it at a testing center.
5. Certified ScrumMaster (CSM)
Certified ScrumMaster (CSM) is a certification program offered by Scrum Alliance, an international non-profit organization dedicated to promoting and advancing the use of the Scrum framework in software development. The CSM certification is designed to help professionals understand and apply the principles and practices of Scrum, a popular Agile methodology for managing complex projects.
To become a Certified ScrumMaster, you must attend an official two-day CSM course taught by a Certified Scrum Trainer (CST). During the course, you will learn about the foundations of Scrum, roles and responsibilities within a Scrum team, how to plan and execute sprints, how to facilitate meetings effectively, and more. After completing the course, you will be eligible to take an online assessment to become certified.
The cost of attending a CSM course varies depending on location and instructor but typically ranges from $1,000 - $2,000 USD. Once you have completed the course and passed the online assessment, there is no additional cost associated with becoming certified.
In addition to attending a CSM course, it is recommended that aspiring Certified ScrumMasters have at least six months of experience working on an Agile project prior to taking the assessment. This experience can include participating in daily standups or other Agile ceremonies as well as using tools such as JIRA or Trello for project management.
6. PRINCE2 Foundation & Practitioner
PRINCE2 (Projects IN Controlled Environments) is a project management methodology developed by the UK government in 1989. It is based on seven principles, seven themes and seven processes that provide a framework for managing projects in any organization.
The PRINCE2 Foundation and Practitioner certifications are the two levels of certification available from PRINCE2. The Foundation level provides an understanding of the PRINCE2 methodology and how it can be used to manage projects. The Practitioner level builds on this knowledge to give practitioners the skills needed to apply the method effectively.
To get certified at either level, you must pass an exam administered by PeopleCert, an accredited examination institute. The Foundation exam consists of 75 multiple-choice questions and takes 1 hour 15 minutes to complete. The Practitioner exam consists of 8 scenario-based objective test questions and takes 2 hours 30 minutes to complete.
The cost for each certification varies depending on where you take the exams and who administers them. Generally speaking, both levels cost around $500 USD or more depending on your location.
7. American Academy of Project Management (AAPM)
The American Academy of Project Management (AAPM) is an international professional organization dedicated to advancing the field of project management. The AAPM provides certifications and training programs to help individuals and organizations enhance their knowledge and skills in project management.
The AAPM offers four levels of certification: Certified Associate in Project Management (CAPM), Project Management Professional (PMP), Program Management Professional (PgMP), and Master Project Manager (MPM). Each certification requires a certain level of experience, education, and/or training in order to qualify for the exam.
To get certified by the AAPM, applicants must first meet the prerequisites for a particular certification. Once these requirements are met, applicants can then register for the exam. Exams are administered by Prometric Testing Centers throughout the world. The exams typically take 3-4 hours to complete and cost $400-$500 depending on the certification level.
Once an applicant passes the exam, they will receive their official AAPM certificate within 4-6 weeks. The certificate is valid for three years from the date it was issued and must be renewed every three years in order to maintain its validity.
In addition to providing certifications, the AAPM also offers a variety of educational programs such as online courses, webinars, seminars, conferences, workshops, and more. These programs provide individuals with an opportunity to learn about project management topics such as risk management, budgeting & scheduling, communication & leadership skills, process improvement strategies, etc.
Overall, the American Academy of Project Management is a great resource for individuals looking to advance their career in project management or gain additional knowledge on related topics.
8. Six Sigma Green Belt
Six Sigma Green Belt is a certification program offered by many organizations to recognize individuals who have achieved a certain level of proficiency in Six Sigma methodology. It is designed to help professionals develop their problem-solving and process improvement skills, enabling them to become more effective and efficient in their roles.
The Six Sigma Green Belt certification typically requires two to four weeks of training, depending on the organization offering the program. During this time, participants learn how to identify problems, develop solutions, and implement process improvements using Six Sigma tools and techniques. They also gain an understanding of the basic principles of Lean and Kaizen methodologies.
In order to obtain a Six Sigma Green Belt certification, individuals must pass an exam administered by the organization offering the program. The cost of the exam varies depending on the organization offering it; however, most programs range from $500-$1,000 USD.
Once certified, individuals can use their Six Sigma Green Belt credentials to demonstrate their knowledge and expertise in process improvement projects within their organization or industry.
9. Six Sigma Black Belt
Six Sigma Black Belt is a certification program designed to help individuals master the principles of Six Sigma. It is an advanced level of training and requires a high level of commitment and dedication. The Six Sigma Black Belt certification focuses on the following areas:
1. Lean Six Sigma Methodology: This includes learning how to identify, analyze, and improve processes using Lean Six Sigma tools and techniques.
2. Project Management: This includes learning how to manage projects using the DMAIC (Define, Measure, Analyze, Improve, Control) process and other project management tools.
3. Quality Improvement: This includes learning how to use quality improvement methods such as Design for Six Sigma (DFSS) and Total Quality Management (TQM).
4. Leadership Skills: This includes developing leadership skills such as problem solving, decision making, communication, team building, and conflict resolution.
The average time it takes to complete the Black Belt certification program is between 6-12 months depending on the individual's availability for study and practice time. To get certified as a Six Sigma Black Belt one must pass an exam administered by an accredited organization such as ASQ or IASSC after completing the required coursework in each of the four areas mentioned above.
The cost of getting certified varies depending on which organization you choose to take your exam from but typically ranges from $500-$2000 USD for the entire program including course materials and exam fees.
10. Lean Six Sigma Green Belt
Lean Six Sigma Green Belt is a certification program that provides individuals with the skills and knowledge to become successful in their roles as Lean Six Sigma practitioners. The Green Belt certification is designed to help professionals identify, analyze, and solve problems within their organization.
The Lean Six Sigma Green Belt certification typically takes between four to six weeks to complete. This includes completing coursework, taking exams, and completing projects. During the training process, students will learn how to use Lean Six Sigma tools and techniques such as DMAIC (Define-Measure-Analyze-Improve-Control) methodology, process mapping, value stream mapping, root cause analysis, data collection and analysis techniques, and project management principles.
In order to obtain the Lean Six Sigma Green Belt certification you must meet certain requirements including having a minimum of three years of work experience in a related field or two years of experience with a Lean Six Sigma Yellow Belt certification. Additionally, applicants must successfully complete an approved training course and pass an exam administered by an accredited certifying body such as ASQ (American Society for Quality).
The cost of obtaining a Lean Six Sigma Green Belt certification can vary depending on the provider and type of course taken. Generally speaking however, courses range from $1,500 - $3,000 USD per person.

11. Lean Six Sigma Black Belt
Lean Six Sigma Black Belt is an advanced certification that provides professionals with the skills and knowledge to lead Lean Six Sigma projects, initiatives, and transformations. It is a highly sought-after certification in the business world as it demonstrates expertise in process improvement methodologies.
A Lean Six Sigma Black Belt typically takes about 6 months to complete. The program consists of three parts: classroom training, project work, and an exam. During classroom training, students learn the fundamentals of Lean Six Sigma such as DMAIC (Define-Measure-Analyze-Improve-Control) methodology, process mapping tools, data analysis techniques, and problem solving strategies. Project work involves applying these skills to real-world problems within a company or organization. The final part is taking the exam which tests knowledge and understanding of the topics covered in the program.
To become a Lean Six Sigma Black Belt, you must have a minimum of three years of experience working on projects using Lean Six Sigma methodology or equivalent experience in a related field. You must also be able to demonstrate mastery of all aspects of Lean Six Sigma including project management and data analysis techniques.
The cost for obtaining a Lean Six Sigma Black Belt certification can vary depending on the provider offering the course. Generally speaking, you can expect to pay anywhere from $2,500 - $5,000 for a full course package that includes classroom instruction and project work as well as access to online materials such as practice exams and study guides.
Do You Really Need a Clinical Project Manager Certificate?
The clinical project manager certificate is a valuable asset for those interested in pursuing a career in clinical project management. It provides proof of the skills and knowledge needed to manage projects in the healthcare industry, and it can be beneficial for those looking to advance their careers.
However, whether or not you need a clinical project manager certificate depends on your individual situation. If you already have experience in the field of project management, then a certificate may not be necessary. On the other hand, if you are just starting out in the field or are looking to move up into higher positions, then obtaining a certification can help demonstrate your expertise and provide an advantage when applying for jobs.
In addition to showing potential employers that you have the necessary skills and knowledge for managing healthcare projects, having a certification can also give you access to specialized training programs and additional resources. This can be especially useful if you are planning on taking on larger-scale projects or working with more complex technologies.
Ultimately, whether or not you need a clinical project manager certificate comes down to your individual goals and needs. If you are looking to gain an edge over other applicants or expand your knowledge base, then obtaining this type of certification may be worth considering.
Related : Clinical Project Manager Resume Examples
FAQs About Clinical Project Manager Certifications
1. What is a Clinical Project Manager Certification?
Answer: A Clinical Project Manager Certification is a professional credential that demonstrates an individual’s expertise in the management of clinical research projects. It is designed to provide project managers with the skills and knowledge necessary to effectively plan, manage, and execute clinical research projects within organizational guidelines.
2. What are the benefits of obtaining a Clinical Project Manager Certification?
Answer: Obtaining a Clinical Project Manager Certification provides project managers with recognition for their hard work, dedication and commitment to the field of clinical research. It also can help enhance career opportunities by demonstrating mastery of clinical project management principles, as well as providing access to additional resources, networking opportunities and job openings.
3. How much does it cost to obtain a Clinical Project Manager Certification?
Answer: The cost of obtaining a Clinical Project Manager Certification varies depending on the institution or certification provider offering it. Generally speaking, costs range from several hundred dollars for online certifications up to several thousand dollars for more advanced certifications such as those offered by major universities or professional associations.
4. What types of courses are required for a Clinical Project Manager Certification?
Answer: The specific courses required for a Clinical Project Manager Certification will depend on the institution or certification provider offering it. Generally speaking, courses may include topics such as project planning and management, risk management and budgeting, clinical trial design and implementation, data collection and analysis methods, regulatory affairs and compliance procedures, quality assurance practices and medical writing techniques.
5. How long does it take to complete a Clinical Project Manager Certification?
Answer: The amount of time needed to complete a Clinical Project Manager Certification can vary greatly depending on the institution or certification provider offering it – some programs may be completed in as little as six months while others may require up two years or more of study time.
Editorial staff
Brenna Goyette
Brenna is a certified professional resume writer, career expert, and the content manager of the ResumeCat team. She has a background in corporate recruiting and human resources and has been writing resumes for over 10 years. Brenna has experience in recruiting for tech, finance, and marketing roles and has a passion for helping people find their dream jobs. She creates expert resources to help job seekers write the best resumes and cover letters, land the job, and succeed in the workplace.
Similar articles
- Top 18 Clinical Project Manager Resume Objective Examples
- What does a Clinical Project Manager do?
- Top 11 Clinical Manager Certifications
- Top 11 Clinical Nurse Manager Certifications
- Top 11 Clinical Trial Manager Certifications
- Top 11 Project Manager Certifications
Connect with us:
7 clinical trial project management courses to consider.

Effective clinical trial project management is vital for keeping studies on time and on budget., and project management skills are key to doing this successfully. While many project management skills can be learned on the job, clinical research project managers may find formal training programs to be highly beneficial.
Below is a list of project management courses that can help project managers get the training they need. Many of the courses are conducted online, or are hybrid programs with both online classes and in-person workshops.
Courses for clinical trial management training
- Project Management for Clinical Research Professionals - ACRP This course, available to organizations, provides training on the specific project management skills needed by study sponsors and CROs. Over the two-day course, enrollees will learn about how to take control of projects, improve timeline design, enhance documentation, and optimize overall trial management.
- Certificate in Pharmaceuticals and Clinical Trials Management - Rutgers University (Online) This year-long certificate program is designed for bioscience and pharmaceutical professionals, in addition to engineering and science graduate students. It incorporates classes about drug development and discovery alongside ones that focus on business, marketing, and project management.
- Drug Development and Lifecycle Management eLearning Program - DIA DIA is a global organization for life science professionals that provides certifications to project managers in the clinical research field. Courses cover general management, conflict management, budgeting, drug development, and adverse event reporting. Additionally, this program offers a group rate for companies interested in purchasing the program for more than 10 users.
- Project Management Certificate Program - The Pharmaceutical Education & Research Institute The Pharmaceutical Education & Research Institute offers this certificate program designed for clinical research professionals interested in exploring project management. It offers courses in drug development, project management in the research-based pharmaceutical industry, statistical concepts used in clinical trials, and other relevant classes.
- Project Management Professional Certification - The Project Management Institute While this certification is not specifically designed for the medical research industry, the Project Management Institute is highly regarded in professional circles. Attendees can apply the skills they learn to clinical trial management, as the courses cover many relevant topics such as project scope, resource management, and communication skills.
- Postgraduate Certificate in Clinical Trial Management - Parexel This program is designed for working professionals who have graduated from the life sciences field (nursing, medicine, and other life sciences) who are interested in pursuing a career in clinical research. It is a six-month course made up of online modules and in-person weekend sessions with the Paraxel team in Berlin, Germany. Courses cover topics such as drug development, regulatory affairs, clinical data management, project management, and more.
- 30-Hour Clinical Project Management Fundamentals Certification Program - Barnett International This online course is designed for anyone looking to gain experience in clinical research project management, whether they have an established career in project management or not. It includes guidance on developing clinical trial project plans, budgeting considerations, trial closure processes, and more.
Clinical trial project management tips for success
When coordinating the details of a clinical trial , there are a few tips that can be helpful in keeping the project on track.
- Start with a project plan. Creating a project plan should be the first step before any new trial launches. This plan will establish a timeline for key milestones, outline which staff members are responsible for each element of the study, provide communication details, and include a risk assessment that addresses potential roadblocks.
- Create a plan for potential risks. While the ideal would be to avoid any delays throughout the trial, it is better to be realistic and create a risk assessment plan. Common challenges include delays with IRB approval, staff turnover, patient recruitment and protocol changes. While this can feel like a lot to consider, planning for it in advance can ensure that challenges can be addressed more efficiently in the moment.
- Create a plan for stakeholders. To ensure the study gets buy-in from the right stakeholders, it’s important to include them in the planning process. By using the RACI method of project management, each part of the plan will note who needs to be involved and in what capacity. The RACI method keeps track of who is: Responsible. This is the person who does the actual work of the task (for example, a marketing lead who gathers outreach material to submit to the IRB). Accountable. This is the person who ensures the work is done thoroughly, accurately, and on time. Consulted. This includes any additional people who may need to weigh in on a piece of the project, such as supporting team members or subject matter experts Informed. The individuals who are kept up-to-date on the progress of the project, but don't need to give approval.
- Schedule time to think. There are many moving pieces involved with running a trial, so it is important to schedule time for thinking and reflection to ensure everything is operating according to plan. This time can be used for anything that’s necessary for continued success, such as establishing project scope, refining objectives, defining courses of action, and analyzing metrics.
- Monitor and analyze your results. Throughout the project, continually monitor results and watch out for inefficiencies that arise early on. Additionally, after the trial is completed, it is important to take time to review the experience before moving on to the next project. This is a good time to solicit feedback from key stakeholders and open the floor for suggestions on how to improve processes for future trials.
Curious about how to fit patient recruitment into your project plan? We can help. Contact us today to learn more about our clinical trial patient recruitment services.
Learn how Antidote connects patients with research.

Get our latest blog posts in your inbox
- Professional Scrum Product Owner (PSPO)
- SAFe for Government
- Professional Scrum Master (PSM)
- Certified ScrumMaster
- PMI-ACP Exam Prep
- Leading SAFe® 6.0 Certification
- SAFe Scrum Master
- Certified Scrum Product Owner (CSPO)
- SAFe for Teams
- Agile Scrum Foundation
- AgilePM Foundation and Practitioner Certification
- Agile Scrum Master (ASM)
- Kanban Training
- Scrum Fundamentals
PMP Certification
Project Management Fundamentals
CAPM Exam Prep
- Change Management Foundation and Practitioner Certification
- PRINCE2 Foundation & Practitioner Certification (7th Edition)
- PRINCE2 Agile Foundation & Practitioner Certification
- Business Analysis Foundation and Practitioner Certification
- Microsoft Project Training
- JIRA Certification Training
- Lean Project Management
- ITIL 4 Foundation
- VeriSM™ Foundation
- SIAM Foundation
- SIAM Professional
- 7 QC Tools Training
- Minitab Essentials
- Lean Six Sigma Yellow Belt
- Six Sigma Awareness
- Lean Six Sigma Green Belt
- Design for Six Sigma
- Lean Six Sigma Black Belt
- Lean Fundamentals
- Value Stream Mapping
- Quality by Design
- Quality Function Deployment
- BPM and Six Sigma
- RCA through Six Sigma
- DevOps Foundation
- DevOps Master
- DevOps Professional
- Continuous Delivery Architecture
- COBIT 5 Certification
- Corporate Group Training
- 1-to-1 Training
- Join as a Trainer

- Best Project Management Blogs
What Does a Clinical Project Manger Do? Roles & Responsibilities

Clinical Project Managers (CPM) play a crucial role in advancing the progress of a clinical trial. The significance of CPMs lies in their ability to navigate complex clinical trials, ensuring precision, compliance, and efficiency.
As stewards of the entire research lifecycle, from planning to execution, Clinical Project Managers wield strategic thinking, leadership insight, and a deep understanding of regulatory landscapes.
In a world where the pursuit of groundbreaking therapies intensifies, the demand for skilled managers is reaching new heights, with organizations recognizing their pivotal role in trial success.
This blog explores the pivotal responsibilities of clinical project managers and sheds light on why their expertise is becoming increasingly coveted, underscoring the crucial role they play in shaping the future of healthcare.
Table of Contents:
What is a Clinical Project Manager?
What does a clinical project manager do, skills required to become a clinical project manager.
- Essential Certifications or Degrees Required to become a Clinical Project Manager
How to Become a Clinical Project Manager?
Salary and job outlook for a clinical project manager.
A Clinical Project Manager (CPM) is an experienced expert in clinical research and healthcare management who is responsible for managing and coordinating the different aspects of clinical trials.
This multifaceted role encompasses strategic planning, execution, and monitoring of clinical research projects to ensure they adhere to regulatory standards, timelines, and budgets.
Clinical Project Manager acts as a connecting point between research teams, sponsors, regulatory authorities, and other stakeholders, facilitating effective communication and collaboration.
Clinical Project Manager responsibilities include protocol development, risk management , team leadership, and navigating the complexities of regulatory compliance. By leveraging their expertise in project management, scientific understanding, and regulatory knowledge, they contribute significantly to successful clinical trials, ultimately advancing medical knowledge and bringing novel treatments to needy patients.
A Clinical Project Manager (CPM) is a pivotal figure in clinical trials, overseeing the intricate processes that lead to the successful execution of healthcare research. Their role encompasses many responsibilities, blending scientific expertise with project management skills to ensure the seamless progression of clinical trials.
Other key roles and responsibilities of a Clinical Project Manager:
- Strategic Planning: Develop comprehensive plans for the initiation, execution, and completion of clinical trials, aligning them with project goals and timelines
- Protocol Development: Contribute to the creation and refinement of study protocols, outlining the methodology, objectives, and criteria for participant selection
- Site Selection: Identify and evaluate suitable clinical trial sites, considering factors such as patient demographics, facilities, and regulatory compliance
- Regulatory Compliance: Navigate and ensure adherence to the complex web of regulatory requirements, obtaining necessary approvals and permissions for the clinical trial
- Budget Oversight: Manage the financial aspects of the clinical trial, ensuring adherence to the allocated budget and making informed decisions to optimize resource utilization
- Data Integrity: Oversee data collection and management processes, emphasizing the importance of data accuracy, completeness, and compliance with regulatory standards
- Problem Resolution: Address challenges and obstacles that may arise during the trial, making decisions that safeguard patient safety and ensure the integrity of the study
- Quality Assurance: Maintain a focus on the overall quality of the clinical trial, implementing measures to uphold ethical standards, patient welfare, and the reliability of research outcomes
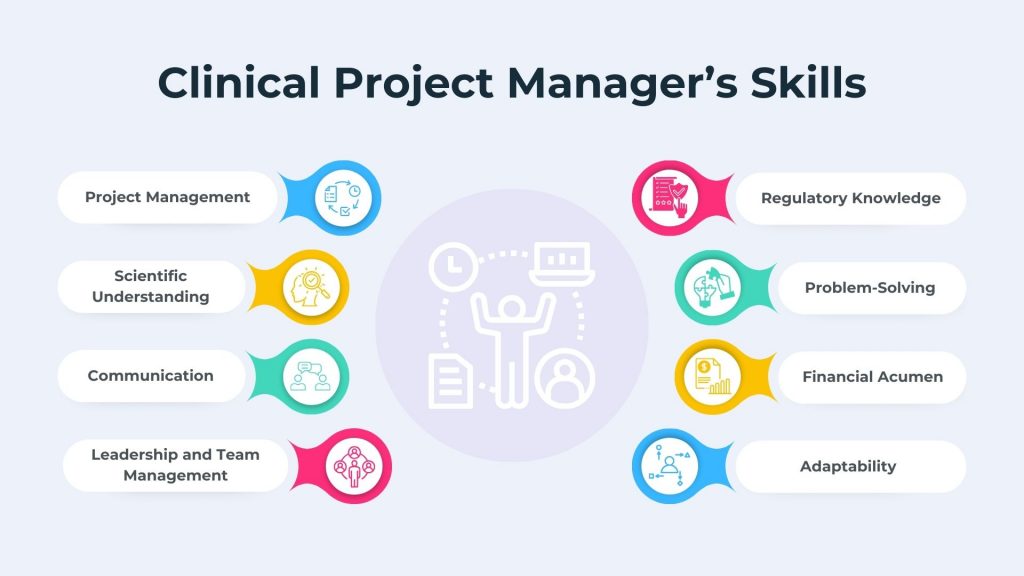
Becoming a successful clinical project manager requires a diverse set of skills that combines scientific knowledge, project management proficiency, and effective communication. Below are some of the key skills that a clinical project manager is required to excel in the role:
1. Project Management Skills
- Planning and Organization: Ability to develop and execute comprehensive project plans, ensuring all aspects of the clinical trial are well-coordinated
- Time Management: Efficiently allocate resources, manage timelines, and prioritize tasks to meet project milestones
- Risk Management: Identify potential risks and proactively implement strategies to mitigate them, ensuring smooth project progression
2. Scientific Understanding Skills
- Clinical Research Knowledge: Familiarity with the principles and processes of clinical research, including study design, protocols, and ethical considerations
- Medical Terminology: Ability to understand and interpret medical and scientific terminology crucial for effective communication with research teams and stakeholders
3. Communication Skills
- Interpersonal Communication: Build strong professional relationships with diverse stakeholders , including research teams, sponsors, regulatory authorities, and site personnel
- Presentation Skills: Effectively convey complex information clearly and concisely, verbally and in written form
4. Leadership and Team Management Skills
- Team Building: Foster collaboration and cohesion within cross-functional teams, inspiring motivation and commitment to project goals
- Decision-Making: Make informed decisions promptly, especially in high-pressure situations, to address challenges and keep the project on track
5. Regulatory Knowledge and Skills
- Regulatory Compliance: Stay updated on and ensure adherence to relevant regulations and guidelines governing clinical trials in different regions
- Ethical Considerations: Understand and navigate the ethical considerations in clinical research, prioritizing patient safety and welfare
6. Problem-Solving Skills
- Critical Thinking: Analyze complex situations, identify root causes of issues, and develop effective solutions to keep the project moving forward
7. Financial Acumen
- Budget Management: Proficiency in managing project budgets, optimizing resource allocation, and ensuring financial accountability throughout the trial
8. Adaptability Skills
- Flexibility: Navigate unforeseen challenges and changes in project scope with adaptability, adjusting strategies and plans as needed
- Learning Agility: Stay abreast of advancements in clinical research, project management methodologies, and regulatory requirements
Essential Certifications or Degrees Required to Become a Clinical Project Manager
Becoming a Clinical Project Manager requires a combination of education, relevant degrees, and professional certifications. The specific requirements may vary based on the employer, industry sector, and the clinical trials complexity.
Here are some essential certifications and degrees that can enhance the qualifications of individuals aspiring to become Clinical Project Managers:
1. Educational Background
- Bachelor’s Degree: A bachelor’s degree in an appropriate field such as life sciences, healthcare, nursing, pharmacy, or a related discipline is frequently the minimum educational requirement
- Advanced Degrees: While not always mandatory, having a master’s degree (e.g., Master of Public Health, Master of Science in Clinical Research) or a Ph.D. can be advantageous, especially for more senior or specialized roles
2. Project Management Professional (PMP) Certification
The PMP certification is offered by the Project Management Institute (PMI), is widely recognized, and demonstrates proficiency in project management principles. It is valuable for Clinical Project Managers as they oversee complex clinical trials.
Achieve global recognition with the PMP certification from Invensis Learning. Benefit from expert trainers, flexible learning options, and success guarantees to propel your career to new heights. Enroll now to access exclusive discounts and become a certified leader in project management.
3. Certified Clinical Research Professional (CCRP) Certification
The Certified Clinical Research Professional (CCRP) certification is a professional designation offered by the Society of Clinical Research Associates (SoCRA). It is a worldwide recognized credential that demonstrates an individual’s skills and understanding of the principles and practices of clinical research.
4. Project Management Fundamentals (PMF) Certification
The Project Management Fundamentals (PMF) Certification is an entry-level credential offered by the Association for Project Management (APM) that validates an individual’s understanding of the fundamental principles and practices of project management. It is designed for those new to the field or wanting to formalize their project management knowledge.
5. Certified Clinical Project Manager (CCPM) Certification
The Certified Clinical Project Manager (CCPM) certification is a professional designation offered by various organizations that demonstrates an individual’s expertise in managing clinical trials and research projects. It validates their ability to effectively plan, execute, monitor, and evaluate clinical research studies, ensuring adherence to regulatory and ethical guidelines.
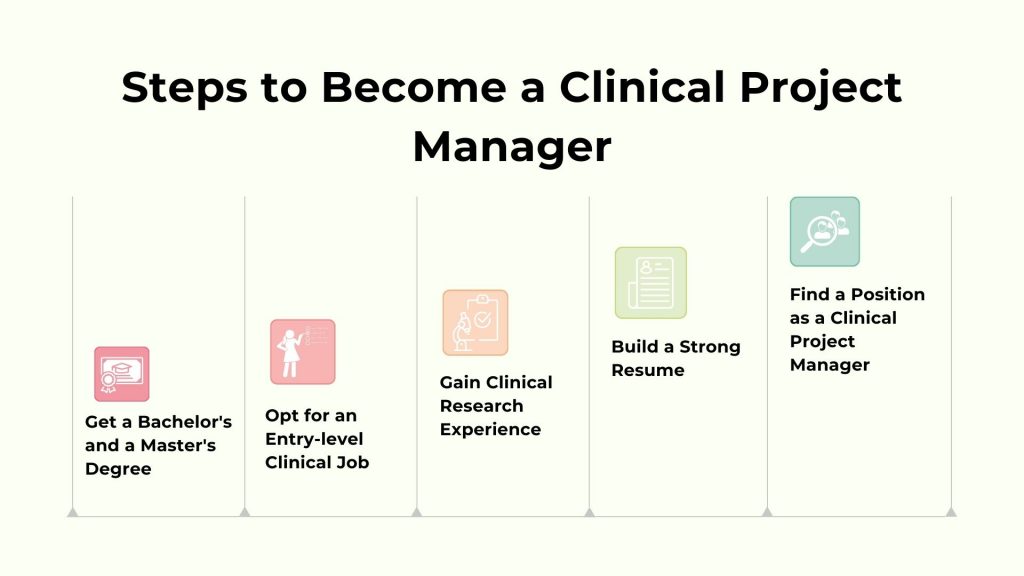
Becoming a Clinical Project Manager involves a strategic combination of education, experience, and professional development. Here’s a step-by-step guide on how to embark on a career as a Clinical Project Manager:
1. Get a Bachelor’s and a Master’s Degree
Embark on your journey by earning a bachelor’s degree in a relevant field, such as life sciences or healthcare. This foundational step equips you with essential knowledge for a career in clinical research.
To enhance your qualifications further, pursue a master’s degree, opting for specialized programs like a Master’s in Public Health (MPH) or a Master’s in Clinical Research.
2. Opt for an Entry-level Clinical Job
Kickstart your career with an entry-level position in clinical research, such as a Clinical Research Assistant or Coordinator. These roles expose you to the day-to-day operations of clinical trials, providing valuable insights into research protocols, data management, and regulatory compliance.
3. Gain Clinical Research Experience
Actively seek hands-on experience in clinical research, engaging in tasks like patient recruitment and study coordination. Develop a strong understanding of Good Clinical Practice (GCP) guidelines and ethical considerations. This practical experience lays the groundwork for a well-rounded skill set and prepares you for more advanced roles.
4. Build a Strong Resume
Create an effective resume that highlights your educational background, relevant coursework, and practical experience. Emphasize key skills such as attention to detail, data management, and knowledge of regulatory standards. Include certifications, like GCP, to underscore your commitment to maintaining high-quality standards in clinical research.
5. Find a Position as a Clinical Project Manager
Progress in your career by applying for roles with increasing responsibilities, focusing on project management within clinical trials. Leverage your educational background, practical experience, and certifications to showcase readiness for a Clinical Project Manager role. Highlight your ability to lead teams, manage timelines, and strictly adhere to regulatory standards.
Before switching any career, individuals should know two main things: one is salary growth and the other one is job opportunities. The salary and job outlook for a Clinical Project Manager (CPM) can vary based on factors such as experience, education, location, and the specific industry within healthcare or clinical research. It’s essential to note that salary trends and job outlook may evolve over time.
Salary of a Clinical Project Manager
The salary prospects for a clinical project manager are generally quite positive. They play a crucial role in the healthcare industry, overseeing the planning, execution, and monitoring of clinical trials and research projects.
Their expertise in project management, clinical research methodology, and regulatory compliance ensures the successful completion of these studies, leading to the development of new drugs, treatments, and medical devices.
Experience is a significant factor in determining salary. The salary ranges for clinical project managers are as follows:
Clinical project managers have the potential to experience significant salary growth throughout their careers. With increasing experience, specialized skills, and advanced certifications, clinical project managers can advance into senior-level positions with higher earning potential.
Additionally, the demand for clinical project managers is expected to grow faster than average in the coming years, further contributing to positive salary prospects.
Job Outlook of a Clinical Project Manager
The job outlook for clinical project managers is exceptionally promising, driven by the increasing demand for clinical trials, the growing complexity of research projects, and the expanding healthcare needs of an aging population.
As per the US Bureau of Labor Statistics (BLS) , employment of medical and health services managers, which includes clinical project managers, will expand by 32% from 2020 to 2030, much faster than the average for all professions. This growth is related to the aging population and the increasing demand for healthcare services.
Here are some specific factors that contribute to the positive job outlook for clinical project managers:
- Increasing demand for clinical trials
- Growing complexity of clinical trials
- The aging population and rising healthcare needs
- Expansion of medical group practices
Clinical project managers can pursue diverse career paths and advance into senior-level positions with increasing responsibilities and higher compensation.
Some potential career trajectories include:
- Clinical Research Associate (CRA)
- Clinical Research Coordinator
- Clinical Research Manager
- Director of Clinical Research
- Clinical Research Program Manager
- Clinical Research Portfolio Manager
- Clinical Project Manager Specialist
- Clinical Project Manager Lead
- Global Clinical Project Manager
- Senior Clinical Project Manager
- Executive Clinical Project Manager
A clinical project manager plays a pivotal role in the healthcare industry, ensuring the successful execution of clinical trials and research projects. Their expertise in project management, clinical research methodology, and regulatory compliance is crucial for bringing new drugs, devices, and therapies to patients, improving healthcare outcomes, and advancing medical knowledge.
If you are passionate about healthcare, have strong organizational skills, and possess a keen eye for detail, a career as a clinical project manager could be a rewarding and fulfilling path. With the right education, experience, and certifications, you can significantly impact the future of healthcare by overseeing the development of life-saving treatments and technologies.
Change Management Foundation and Practiitioner Certification Training
PRINCE2 Foundation and Practitioner Certification Training
EXIN Business Analysis Foundation and Practitioner Training
RELATED ARTICLES MORE FROM AUTHOR

Project Management vs Data Analytics: Complete Overview

What is Quality Assurance (QA) in Project Management?

What is Sensitivity Analysis in Project Management?
Leave a reply.
Save my name, email, and website in this browser for the next time I comment.
- 14,517 Likes
- 444 Followers
- 96,900 Subscribers
- 2,170 Followers
Related Articles

6 Useful Strategies to Dealing with Difficult People at Work

What is Project Resource Management and Why it is Important?

DevOps Release Manager – Roles, Responsibilities, and Salary Insights

How to Crack ITIL 4 Foundation Certification Exam 2024?

Business Analyst Skill Report – Business Analyst Skills, Jobs and Salary...
Popular posts.

The Project Management Life Cycle Explained

Roles and Responsibilities of a Quality Control Inspector

7 Cs of Effective Communication with Example

Top 5 Factors for Project Success

Quality Analyst Job Role and Responsibilities- Explained!
Suggested posts.
- 7 Cs of Effective Communication with Examples
- Project Management Lifecycle
- Project Success Factors
- Quality Control Inspector Job Description
- Risk Management Examples
- QA Manager Job Description
- Quality Management Team Roles and Responsibilities
- Risk Management Tools & Techniques
- Quality Analyst Job Description
- What is Business Value
- Who are Project Stakeholders
- Importance of Project Management
- What is Project Management
- Project Management Skills
- Project Manager Job Description
- Agile Project Manager Interview Questions
- Risk and Compliance Manager Job Description
- Risk Management Process
- Project Scope Management
- Healthcare Project Manager Job Description
- Six Sigma Project Examples
- Risk Analysis Methods
- ITIL Service Lifecycle
- Risk Manager Job Description
POPULAR CATEGORIES
- Best Project Management Blogs 265
- Top Agile Blog Posts 158
- Top Blogs on Quality Management 126
- Latest IT Service Management Blogs 108
- Trending Articles on DevOps 65
- Popular Blogs on IT Security and Governance 55
- Top Blogs on Professional Development 33
- Top Infographics Collection 8
Download E-book Blog
Thank You for submitting your enquiry. One of our training consultants will get in touch with you shortly.
50+ Training and Certification Programs - Upskill Today Learn more about our training programs.

Get extra -20% OFF THE ALL-IN-ONE COURSE with "EXTRA20"
SAVE UP TO -50%
ON YOUR FIRST PURCHASE
Clinical Research Project Manager Academy
- Level: Professional
- Continuing Education Units: up to 150 contact hours, 15.0 CEUs
- Certificates: VIARES
Listen what you can learn in this course:
Academy content.
Clinical Research Project Manager
- Get familiar with project management terminology.
- Define project success criteria.
- Set up project phases correctly.
- Choose fitting tools to manage a project.
- Communicate efficiently while managing a project.
- Understand typical differences between the PM’s role depending on the company type.
- Understand the stakeholder landscape.
- Analyse stakeholders.
- Identify key stakeholders.
- Communicate successfully with different stakeholders choosing adequate style, frequency, and the level of detail.
- Understand the project external and internal environment.
- Assess how the environmental factors affect the project.
- Define requirements to make the project a success story.
- Define the project scope.
- Build a strong project definition.
- Obtain the approval for your project definition.
- Differentiate risks, constraints, and issues.
- Identify project risks and decide on a suitable primary response strategy.
- Analyse project risks in three dimensions – likelihood, impact, and detectability.
- Quantify risks and calculate the Risk Priority Number.
- Optimise conditions for high risks.
- Monitor risks and keep them under control.
- Assess the effectiveness of your risk management process.
- Understand the regulatory framework for risk management in clinical studies.
- Apply general risk management principles and procedures on clinical projects.
- Develop the Clinical Risk Management Plan.
- Use adequate tools to manage clinical study risks efficiently.
- Communicate effectively with functional teams to manage clinical study risks successfully.
- Identify key stages of a project.
- Analyse whether something is missing for a successful delivery (gaps).
- Plan how to bridge the gaps.
- Assign responsibilities to your team members.
- Develop the clinical study budget.
- Prepare a detailed realistic plan (baseline schedule) of the project including milestones, critical path, work breakdown, and allocated resources.
- Use a well-designed baseline schedule to measure progress and manage the project in the execution phase.
- Monitor study progress.
- Identify early signs of the project falling behind or going astray compared to the plan.
- Apply measures to keep the project on track.
- Apply measures to maintain GCP and regulatory compliance.
- Control study costs and handle out-of-scope requests.
- Manage and support your team during audits and inspections.
- Manage CAPA plans effectively and efficiently.
- Contribute to the timely development of the CSR.
- Avoid project drift by proper planning, strong management, and clear communication.
- Set project completion criteria and produce an adequate Project Acceptance Checklist.
- Lead the close-out meeting.
- Evaluate the project – what went well, what went wrong and why, derive lessons learnt.
Application support program
included with one-time payment plan
- Setting clear goals and expectations is the crucial first step when it comes to your job search.
- Understand WHAT are the required skills of your dream job and how to acquire them
- you will address THE most important piece to get your dream job
- As often, it’s more about preparation and practice than any other “miracle tip”. There is no secret – however, there is a good way to prepare correctly for any interview (a method), and this method works well.
- Negotiate and Get What You Truly Deserve.
VIARES CERTIFICATE
Upon enrollment you will receive your first certification “Confirmation of Enrollment”. After successful completion of the course and your exam, you get your personal “Clinical Research Project Manager” including:
- course title
- contact hours
- continuing education units (CEU)
- your overall course score
- date of completion
- personal certificate verification code
We also show you how to best share your certificate on LinkedIn and other social media platforms.
Getting a clinical project manager certificate can provide you with several benefits, including:
- Enhanced skills and knowledge: A clinical project manager certificate program can provide you with the skills and knowledge needed to effectively manage clinical trials, including project planning, regulatory compliance, risk management, and team coordination. You can gain expertise in the latest regulations and trends in the field, and stay up-to-date with the latest industry best practices.
- Career advancement: A clinical project manager certificate can enhance your resume and demonstrate to potential employers that you have the knowledge and skills required for a clinical project manager position. It can also help you stand out in a competitive job market and increase your chances of career advancement.
- Industry recognition: A clinical project manager certificate can provide additional credibility and recognition in the field.
Overall, getting a clinical project manager certificate can provide you with a valuable credential, enhanced skills and knowledge, and opportunities for career advancement and networking in the clinical research field.
DELIVERED BY INDUSTRY EXPERTS

KAMILA NOVAK
Clinical Project Management Guru
Hear from our graduates
It was a great experience to have gone through the VIARIES Clinical Research Talent Program, which was a breath taken opportunity for me as the course gave me the opportunity to broaden my perspective in regards to the dynamics of
clinical research. The online learning platform was very informative, well organized, and very inspiring for the future Clinical Research professionals. I particularly enjoyed the online webinars and assignments, which made the content understandable. I strongly recommend it to all folks interested in the field of clinical Research. Finally, many thanks to Dietmar Eglhofer and team for ensuring to provide quality t raining.
I really enjoyed the VIARES CRA Academy. The course is well structured and covers all relevant aspects to prepare you for a job in clinical research. In addition, guidelines for CV optimization and job applications are included. I’ve completed several
courses in clinical research and I can confirm that VIARES offers the best quality.
Customer Reviews
Following my passion for clinical research and developing my career in this field, VIARES ACADEMY has played an incredible role, with the opportunity to be enrolled in such extraordinary, structured, well-organized, and dynamic training programs, led byClinical Research Professionals. Recently, I have been a graduate in Clinical Research Project Management Program, after CRA Talent program in April 2020. Definitely, from both programs, I have gained the skills of learning and thinking at the same time through assignments and knowledge checks. As Confucius said: “Learning without thinking is useless, thinking without learning is dangerous“
Love the structure and value this course gave me. Will start my job soon as a CRPM.
Very enlightening and well-coordinated!
reviews curated by judge.me

$ 199.90 Original price was: $ 199.90. $ 99.90 Current price is: $ 99.90.
- Language - English
- 1 Year access on any device
- Online Exam & Certificate of Completion
- Contact Hours and CEUs
Courses you may also like
Clinical research career accelerator.
$ 59.90 Original price was: $ 59.90. $ 29.90 Current price is: $ 29.90.
Clinical Study Coordinator Academy
Navigating ich gcp e6(r3).
Get new job posts and all the news about our VIARES clinical research courses in your inbox!
- All Courses
- Verify Certificate
- Privacy Policy
- Term Of Service
- VIARES Trainer
- Contract Sales Specialist
Our support and sales team is available 24 /7 to answer your queries.
Clinical Research Training courses all prices incl. VAT
certified Lead Auditor for ISO 9001:2015
Kamila Novak, MSc, has been involved in clinical research since 1995, having worked in various positions in pharma and CROs. Since 2010, she has been working as an independent consultant in medical writing, auditing, training, project and risk management. She delivered dozens of courses in areas related to clinical research and project management. In August 2019, Kamila became a certified Lead Auditor for ISO 9001:2015. Kamila is a Core Team Member in the DIA GCP & QA, Clinical Research, and Project Management Communities.
Request Course outline
Explore our pricing plans, 12 payments.
- One year access to your training courses
- Personalized certificate upon completion
- VIARES exam – 2 attempts included
- All online-training course material
- Online access to our learning management system
- One year access to your training courses
- VIARES exam – 2 attempts included
- Including the VIAES CAREER ACCELERATOR
- Plan ends automatically after 12 payments
- No training course handouts for offline learning
- No unlimited access
- All benefits from the montly payment plan
- Life-time access to your purchased course
- Training course handouts included
- Life-time support from the VIARES tutors
- And much more...
ACRP Project Management Best Practices™ Training Program
For employers looking to expand project management skills across the entire study team, acrp’s project management best practices™ training program delivers practical, hands-on training specific to clinical research..
ACRP’s Project Management Best Practices™ training program is supported by case studies and real-world scenarios from clinical trials, making it a one-of-a-kind training solution.
- Quickly and Efficiently Onboard New Project Managers
- Ensure Project Management Competency Across the Study Team
- Standardize Project Manager Training
- Customize to Address Organization SOPs
- Receive Certificate of Completion Demonstrating Foundational Knowledge in Clinical Research
Program Specs & Curriculum
- 8-Hour Instructor-Led Training Program
- Flexible Virtual and In-Person Delivery Options
- Program Content Covers: Traits and Competencies of a Good Project Manager; Communication Skills; Time Management; Collaboration and Team Building; Leadership and Taking the Lead; Work-Life Balance
This site is part of the Informa Connect Division of Informa PLC
This site is operated by a business or businesses owned by Informa PLC and all copyright resides with them. Informa PLC's registered office is 5 Howick Place, London SW1P 1WG. Registered in England and Wales. Number 3099067.
- Informa Connect Academy

Live Digital
- Customised Training
- Relevant Courses
- Our Faculty
Clinical Research Project Management
Good project management is key to completing a trial on time and cost effectively, clinical research project management training, effective project management is essential for maintaining data integrity, patient safety, and overall study quality. .

About the Course
This course will demonstrate how a project manager from a single background (e.g. a CRA) can manage a breadth of operational challenges from technical data management issues to strategic interactions with regulatory authorities.
Additionally, this course will cover the difference between doing the work to implement a clinical trial, and project managing the clinical trial team. Offering you tools and techniques, you will learn how to project manage all the different components of a clinical trial, from site selection to study closure. From there, you will learn how to detect drift in the timelines or budget and decide what corrective actions are available to you.

Who Should Attend
- Project Management
- Patient Recruitment specialists
- Site professionals
- Clinical Outsourcing
- Clinical Operations
- Contract Management
- Clinical Quality Control
- Finance/Purchasing/Project Controlling
- Medical Affairs
- Program/Portfolio Management
- CRO Administration and Management

Key Learning Objectives
- Overview of the components of clinical research and understanding the project manager’s role
- Identify the key roles and core competencies needed by a project manager
- Learn how to track and interpret project progress
- Assess & manage clinical project/site risks during Covid-19
- Obtain knowledge on various project management techniques
- Understand how the budget and timelines are structured
Run this course in-house
Informa Connect Academy’s customised training solutions have helped organisations deliver tailored learning in different languages to suit every requirement.
Bespoke training designed for your organisation only, combining traditional classroom setting, blended and online learning models
Next Courses
Learn more about this training course.

Delivered by Ian Stokes
Ian Stokes is a project management specialist, certified facilitator and trainer who delivers process and learning solutions to the pharmaceutical industry. His recent clients include Pfizer, Sanofi Aventis, Nestle, DANONE and Celgene.

Associate Director
Tessa Therapeutics
“It is relevant to the processes we are doing in real world, the trainer is experienced and knows the subject well. Classes are well-organised and engaging with flowcharts, controls, organisational structure”
Secretary of Hospital - - Shenzhen Ho
GCP Office, The University of Hongkong - Shenzhen Hospital
“A lot of group discussion and communications with different people. The course combined basic definitions and useful practical tools, opening the mind to help attendees see the whole picture of clinical trials”
Institute for Applied Medical Research
A pilot plant "Pul-Sar" in Dubna (Special Economic Zone "Dubna"
"Russian Medical School" is an educational institution for postgraduate medical education
If you have any questions or suggestions?
Please contact us
+7 (968) 408 42 - 69
[email protected]
Developments

We cooperate with:
- N.N. Priorov Central Institute of Trauma Surgery (development of the newest methods of hemostasis during spine surgeries)
- M.F. Vladimirsky Moscow Regional Clinical Research Institute (clinical studies of endoscopic methods of hemostasis)
- N.V. Sklifosovsky Research Institute of Emergency Medicine (development of methods of emergency gastroenterological care)
- A.N. Rychikh Scientific Research Center for Coloproctology (colorectal surgery, endoscopy)
- Sechenov Moscow State Medical University
- Pirogov Russian National Research Medical University
- Pavlov Russian State Medical University
- Russian Society of Surgeons
- Children’s City Clinical Hospital named after Z.A. Bashlyaeva

Press service
Stay informed
October 14 21
Dubna, Ulitsa Programmistov 4, building 6 Special Economic Zone “Dubna”
- State registration certificate
- Certificate of registration with the tax authority
- Statute IAMR

- VIEW ALL TOPICS
- Art & Design
- Articles & News Stories
- Arts & Architecture
- Arts & Ideas
- Automobiles
- Biography & Memoir
- Book Excerpts
- Books - Fiction
- Books - Non-fiction
- Business & Economics
- Business & Leadership
- Business/Law
- Children's Literature
- Comic Fiction & Satire
- Computers & Technology
- Contemporary Fiction
- Contemporary Women
- Cooking & Food
- Corporate Finance
- Court Filings
- Court Records
- Creative Writing
- Criminal Procedure
- Current Economy
- Diet & Nutrition
- Economic Conditions
- Economic History & Theory
- Emigration & Immigration Studies
- Environmental Economics
- Essays & Theses
- Ethnic & Minority Studies
- Ethnicity, Race & Gender
- Faith & Spirituality
- Family Sagas
- Fan Fiction
- Fiction & Literature
- Food & Wine
- Games & Puzzles
- Genre Fiction
- Government & Politics
- Government Documents
- Graphic Art
- Health & Lifestyle
- Health & Medicine
- Health & Wellness
- History, Criticism & Theory
- Information Technology & Theory
- Instruction manuals
- Internet & Technology
- Legal forms
- Magazines/Newspapers
- Login / Register
Medical Science Liaison or clinical research manager or clinical
Share & embed.
Medical Science Liaison, clinical research manager, clinical research monitor, project manager with 10 years experience looking for a Supervisor position.
Sponsor Documents
Recommended.

Medical Liaison or Clinical Research Associate or Research Assoc

Medical Science Liaison or medical consultant or clinical consul

Clinical Informatics Liaison or Informatics Manager or Clinical

Medical Science Liaison/Medical Affairs/Pharmaceutical- Clinical

Registered nurse or Case manager or Clinical liaison

Regional Manager or Clinical Director or Clinical Consultant or

MSL (Medical Science Liaison) OR MSM (Medical Science Manager)

Clinical Research Manager, Clinical Research

clinical science specialist or medical science specialist or med

Medical Director or Director or Clinical Research or Psychiatris

Clinical Research Associate or Clinical Trial Associate

Clinician or Counselor or Clinical Liaison or Psychiatric Rehabi

Clinical Trial Associate or Clinical Research Associate

Research Administrator or Clinical Trial Manager

Account Executive or Clinical Manager or Clinical Specialist

clinical study operations or clinical research specialist or reg
Facebook Google Twitter
Password Hide
Remember me
Forgot your password?
I agree to the Terms
Lost your password? Please enter your email address. You will receive a link to create a new password.
Back to log-in

IMAGES
VIDEO
COMMENTS
Clinical trial management certification prepares students by teaching them how to effectively oversee clinical studies, ensuring adherence to protocols, budget, and timelines. Accredited and trusted by over 1,100 students. Salary range 65k-143k+. Complete in 2-4 weeks in 80-100 hours. Free clinical project manager job coaching after completion.
With a 30-year legacy, ACRP Certification is the most reputable credentialing program in clinical research. Since 1992, more than 40,000 professionals and their employers have come to trust ACRP Certification as the mark of excellence in clinical research. "Joining ACRP and becoming certified was the best thing I ever did to jumpstart my ...
ACRP-PM® is a credential for clinical research professionals with specialized knowledge in project management. To be eligible, you must hold one of the following ACRP Certifications: CCRC, CPI, ACRP-CP, or CCRA.
ACRPM is a network of clinical research project managers who use project management tools and methodologies. It offers membership, events, education, resources, and wellness initiatives to support CRPMs' career growth and success.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
Here's a list of six steps that you can follow when trying to pursue a career as a clinical project manager: 1. Earn a bachelor's degree. You can start pursuing a career in clinical project management by earning an undergraduate degree in an applicable field. Many clinical project managers have bachelor's degrees in biology, health, life ...
This is an intensive two-day course which provides detailed and practical guidance on the different project management skills used by study sponsors and Contract Research Organizations (CROs), so helps you decide how to apply these skills in your situation. The course is specifically designed for clinical research project management, with ...
The Clinical Research Project Manager (CRPM) certification offered by CCRPS is a globally-recognized credential that demonstrates advanced clinical research project management skills. Earning this certification requires passing a course that assesses your ability to manage all aspects of clinical research projects, from planning and initiation ...
The USC Graduate Certificate in Clinical Research Design and Management requires satisfactory completion of 12 units beyond the baccalaureate degree. Typically, courses offered by the Department of Regulatory and Quality Science can be taken either in-person or online (within the United States). Upon admission, a course plan will be developed ...
Clinical research management certificate courses. In as little as a year, you can complete the required 15 credits in courses that have a strong focus on research-centric topics as they relate to clinical settings. Upon completion of this graduate certificate, you'll be prepared to pursue advanced roles in various industries of research.
There are 5 modules in this course. In this course, you'll learn about the more advanced elements of managing clinical trials. From anticipating and planning for protocol events to conducting systematic reviews to synthesize evidence, you and your study team need the skills to implement best practices throughout the trial process.
Apply quality improvement, project or data management to support and monitor clinical research studies. The Clinical Research Management certificate includes 12 credits of graduate coursework, is offered 100% online and asynchronously and is designed to be able to be completed in three semesters. At the completion of the program, you will earn ...
Unlock the art and science of clinical trial project and program management with this immersive workshop. Whether you're diving into this dynamic field or seeking to enhance your expertise, this program is designed to introduce, affirm, or elevate your understanding of project management in the field of clinical research.
This specialization is designed for individuals and teams that will be running or interacting with clinical trials. In four courses, learners will develop insights and build the skills they need to design, manage, and monitor clinical trials as well as analyze, document, and communicate the results. Learners will also learn best practices ...
Certified Clinical Project Manager (CCPM) is a professional certification that recognizes an individual's expertise in managing clinical research projects. It is designed to demonstrate an individual's knowledge and experience in the field of clinical project management, as well as their commitment to the highest standards of ethical practice.
It is a six-month course made up of online modules and in-person weekend sessions with the Paraxel team in Berlin, Germany. Courses cover topics such as drug development, regulatory affairs, clinical data management, project management, and more. 30-Hour Clinical Project Management Fundamentals Certification Program - Barnett International.
A Clinical Project Manager (CPM) is a pivotal figure in clinical trials, overseeing the intricate processes that lead to the successful execution of healthcare research. Their role encompasses many responsibilities, blending scientific expertise with project management skills to ensure the seamless progression of clinical trials.
She delivered dozens of courses in areas related to clinical research and project management. In August 2019, Kamila became a certified Lead Auditor for ISO 9001:2015. Kamila is a Core Team Member in the DIA GCP & QA, Clinical Research, and Project Management Communities.
ACRP's Project Management Best Practices™ training program is supported by case studies and real-world scenarios from clinical trials, making it a one-of-a-kind training solution. Benefits: Quickly and Efficiently Onboard New Project Managers. Ensure Project Management Competency Across the Study Team. Standardize Project Manager Training.
The Institute of Clinical Research (ICR) is probably the oldest independent membership-led professional body for global clinical researchers. For over 40 years, The Institute of Clinical Research ...
Expert clinical research project management services ensuring efficient protocol implementation, data integrity, and regulatory compliance. ... Ian Stokes is a project management specialist, certified facilitator and trainer who delivers process and learning solutions to the pharmaceutical industry. His recent clients include Pfizer, Sanofi ...
We were awarded the ISO 9001:2000 certification in 1998 for quality management and have an unusually low staff turnover of less than 5%, many of our key staff having been with the company since ...
Prepare for a career in the high-demand field of project management. The project management skills you learn in this program prepare you for an entry-level role as a project manager. This program also prepares you for a variety of entry-level roles that require goal-driven problem-solving and strategic planning. These roles include: · Project ...
Arsept. Contacts. +7 (968) 408 42 - 69. Dubna, Ulitsa Programmistov 4, building 6 Special Economic Zone "Dubna". Show on map. Documents. State registration certificate. Certificate of registration with the tax authority. Statute IAMR.
Ekaterina Wright 48 Gurley Road * Stamford, CT 06902 (212) 464-7245 * [email protected] Experienced Medical Project Manager and Subject Matter Expert MANAGEMENT PROFILE A strong leader with deep medical expertise in the fields of Dermatology, Cardio vascular, and Rheumatology. Extensive history in managing medical and clinical s tudies, research, development, licensing and market launch of new ...