- Open access
- Published: 27 May 2023

Gastric cancer treatment: recent progress and future perspectives
- Wen-Long Guan 1 , 2 na1 ,
- Ye He 1 , 2 na1 &
- Rui-Hua Xu 1 , 2
Journal of Hematology & Oncology volume 16 , Article number: 57 ( 2023 ) Cite this article
25k Accesses
69 Citations
11 Altmetric
Metrics details
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become the standard treatment. Ongoing investigations are exploring the potential benefits of targeted therapy or immunotherapy in the perioperative or adjuvant setting. For metastatic disease, there have been notable advancements in immunotherapy and biomarker-directed therapies recently. Classification based on molecular biomarkers, such as programmed cell death ligand 1 (PD-L1), microsatellite instability (MSI), and human epidermal growth factor receptor 2 (HER2), provides an opportunity to differentiate patients who may benefit from immunotherapy or targeted therapy. Molecular diagnostic techniques have facilitated the characterization of GC genetic profiles and the identification of new potential molecular targets. This review systematically summarizes the main research progress in systemic treatment for GC, discusses current individualized strategies and presents future perspectives.
Gastric cancer (GC) is the fifth most common malignant tumor and the fourth leading cause of cancer-associated death worldwide [ 1 , 2 ]. The incidence varies geographically across the globe, with the highest incidence in Eastern Asia (Japan and Mongolia) and Eastern Europe, whereas incidence rates in Northern Europe and Northern America are generally low, comparable to African regions [ 2 ]. Notably, the incidence of gastric cancer among young adults (aged < 50 years) in recent years has been progressively rising in both low-risk and high-risk countries. Aside from Helicobacter Pylori infection, the occurrence of GC has been linked to genetic risk factors as well as lifestyle factors, such as alcohol consumption and smoking [ 3 , 4 , 5 , 6 ].
Despite the high incidence of GC, most patients are unfortunately diagnosed at advanced stages with dismal prognoses due to the lack of distinguishing clinical indications [ 7 , 8 ]. Systemic chemotherapy is the mainstay treatment for metastatic GC (mGC), with a median overall survival (OS) of ~ 12 months for patients treated with conventional chemotherapy [ 9 ]. Intratumoral and intertumoral heterogeneity are the prominent features of GC that partly contribute to its poor prognosis. However, histological classifications alone are insufficient to effectively stratify patients for individualized treatment and improve patients’ clinical outcomes [ 10 ]. Therefore, cutting-edge diagnostic techniques and drugs are of fundamental importance for better characterizing molecular profiles and identifying potential novel therapeutic targets for GC patients [ 11 , 12 , 13 ].
Trastuzumab, a monoclonal antibody targeting Human Epidermal Receptor 2 (HER2), was the first approved targeted therapy for GC. However, after the ToGA study, progress in the development of treatments for gastric cancer stalled for nearly a decade [ 14 ]. Emerging advances in immunotherapy, particularly in anti-HER2 therapy, and various biomarker-directed therapies in GC have recently broken this trend. For example, anti-programmed cell death 1 (PD-1) antibodies have demonstrated impressive efficacy and prolonged survival in untreated MSI-H/dMMR mGC patients [ 15 ]. Substantial breakthroughs in the treatment of gastric cancer have been achieved with novel anti-HER2 therapeutic agents, such as T-DXd and disitamab vedotin (RC48) [ 16 ]. In addition, in light of the success of immunotherapy and targeted therapy as first-line treatments for advanced gastric cancer, ongoing research is investigating their potential to advance the treatment of patients with locally advanced stage GC.
The treatment landscape of gastric cancer has evolved significantly in the past few years, with the emergence of new immunotherapy and targeted therapies for patients at various stages of the disease (Fig. 1 ). In this review, we systematically summarize the pivotal clinical trials in GC treatment and provide an update on the management of localized and metastatic gastric cancer. We also discuss the developments in immunotherapy and targeted therapy and highlight current individualized treatments and future perspectives.
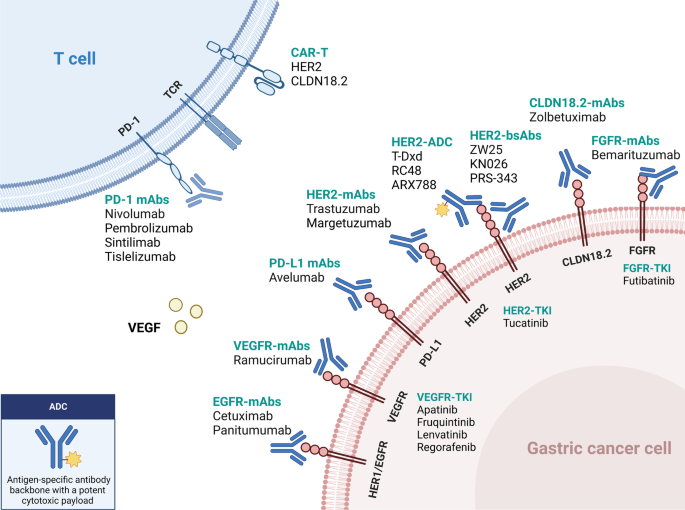
Updated immunotherapy and targeted therapy for gastric cancer. This algorithm provides guidance for selecting currently available immunotherapy and targeted therapy based on different biomarkers
Management for localized GC
Radical surgery is the primary treatment for resectable gastric cancer. Several therapeutic approaches have been established to lower the risk of recurrence and improve long-term survival, including perioperative chemotherapy, adjuvant chemotherapy, and adjuvant chemoradiotherapy (Table 1 ). They are listed as the recommended treatments for resectable localized GC in current guidelines[ 5 , 17 , 18 ]. Further, the addition of targeted therapy and/or immune checkpoint inhibitors (ICIs) is currently being studied in the neoadjuvant/adjuvant setting.
- Perioperative chemotherapy
Perioperative chemotherapy has become the standard treatment for resectable localized GC. Several clinical trials have demonstrated that perioperative chemotherapy could improve the prognosis of patients with resectable GC compared to surgery alone.
The MAGIC trial marked a significant advancement in the field of perioperative chemotherapy for resectable GC. In this phase 3 study, 503 patients were enrolled with resectable gastric, gastroesophageal junction (GEJ), or lower esophageal adenocarcinoma. Patients in the experimental group received three preoperative and three postoperative cycles of epirubicin, cisplatin, and fluorouracil (ECF) [ 19 ]. The results showed that the perioperative ECF regimen could decrease tumor stage and significantly improve progression-free survival (PFS, HR 0.66; 95% CI 0.53–0.81, P < 0.001) and overall survival (OS, HR 0.75; 95% CI 0.60–0.93, P = 0.009). Another phase III trial conducted in 28 French centers compared radical surgery with or without perioperative cisplatin and fluorouracil (CF) chemotherapy and showed that perioperative chemotherapy led to a higher 5-year overall survival rate versus surgery alone (38% versus 24%, respectively; HR 0.69; 95% CI 0.50–0.95, P = 0.02) [ 20 ]. Recently, the randomized phase II/III FLOT4-AIO study compared perioperative FLOT regimen (fluorouracil, leucovorin, oxaliplatin, and docetaxel) with previous standard ECF/ECX (epirubicin, cisplatin, and fluorouracil/capecitabine) regimen in gastric or GEJ cancer patients who had cT2 or higher and nodal positive (cN +) disease [ 21 ]. The results suggested that the FLOT regimen could improve overall survival (50 months versus 35 months), confirming the role of the FLOT regimen as the new standard perioperative treatment for resectable gastric cancer [ 5 , 18 ].
Since most of the clinical trials mentioned above were conducted in western countries, these perioperative regimens (ECF, CF, and FLOT) are less frequently used in Asia. In the phase III PRODIGY trial, 530 Korean patients with cT2-3N + or cT4N any gastric or GEJ cancer were randomly randomized to the neoadjuvant or adjuvant group. Patients in the neoadjuvant arm underwent preoperative DOS (docetaxel, oxaliplatin, and S-1) followed by surgery and S-1 adjuvant chemotherapy, while those in the adjuvant arm received upfront radical surgery followed by S-1 chemotherapy [ 22 ]. The perioperative chemotherapy group had significantly higher rates of R0 resection and pathological complete response (pCR) (95% and 10.4%, respectively). Moreover, PFS was improved in the perioperative arm compared to the adjuvant arm (HR 0.70; 95% CI 0.52–0.95; P = 0.023). The major criticism of this study was that the adjuvant S-1 monotherapy was insufficient for stage III patients, considering another phase III study had demonstrated the superiority of docetaxel plus S-1 to S-1 for 3-year relapse-free survival (RFS) in stage III gastric cancer [ 23 ]. Recently, the phase III RESOLVE trial conducted in China investigated the role of perioperative S-1 plus oxaliplatin (SOX) chemotherapy versus upfront surgery followed by adjuvant chemotherapy [ 24 ]. This study recruited over 1,000 patients with cT4aN + or cT4bN any gastric or GEJ adenocarcinoma, of whom over 60% had gastric cancer. Patients in the intervention group received perioperative SOX (three preoperative cycles and five postoperative cycles followed by three cycles of S-1 monotherapy). The two adjuvant groups received surgery followed by SOX or CAPOX (capecitabine and oxaliplatin) chemotherapy. These results suggested that the perioperative SOX chemotherapy could improve the 3-year disease-free survival (DFS) rate compared to adjuvant CAPOX therapy (59.4% vs. 51.1%, respectively, P = 0.028).
Based on the evidence shown above, perioperative chemotherapy has become the standard treatment in many countries. The FLOT regimen is the most commonly used in Western countries according to the evidence from the FLOT4-AIO study[ 21 ], while the SOX regimen is more recommended in China, based on the results of the RESOLVE study[ 24 ]. However, perioperative chemotherapy is less recommended in Japan, since evidence of the superiority of neoadjuvant chemotherapy is still lacking among Japanese patients[ 25 ].
Adjuvant chemotherapy
Adjuvant chemotherapy is recommended for patients who undergo primary surgery and have stage II or stage III disease due to improvement in survival demonstrated by several clinical trials, particularly in Asian patients. The multi-center phase III CLASSIC trial undertaken in South Korea, China, and Taiwan compared upfront D2 surgery followed by CAPOX adjuvant chemotherapy versus D2 gastrectomy alone in patients with stage II-IIIB gastric cancer [ 26 , 27 ]. Adjuvant CAPOX chemotherapy significantly improved both 5-year DFS (68% vs. 53%; HR 0.58; 95% CI, 0.47 to 0.72; P < 0.0001) and OS (78% vs. 69%; HR 0.66; 95% CI, 0.51 to 0.85; P = 0.0015) compared with surgery alone. Another similar phase III ACTS-GC trial from Japan randomly assigned 1,059 stage II or III GC patients to undergo D2 surgery followed by S-1 monotherapy or D2 surgery alone and showed that adjuvant S-1 monotherapy for one year led to a better 3-year OS than surgery alone (80.1% vs. 70.1%; HR 0.68; 95% CI, 0.52 to 0.87; P = 0.003). The survival benefit persisted after five years of follow-up [ 28 ]. Moreover, the phase III JACCRO GC-07 trial investigated the superiority of adjuvant docetaxel plus S-1 over S-1 monotherapy for pathological stage III gastric cancer [ 23 ]. The addition of docetaxel to S-1 after surgery showed a better 3-year RFS (66% vs. 50%; HR 0.632; 99.99% CI, 0.400 to 0.998; P < 0.001) in the second interim analysis, and the study was terminated as recommended by the independent data and safety monitoring committee. The RESOLVE trial also investigated the non-inferiority of adjuvant SOX chemotherapy compared with the CAPOX regimen. The 3-year DFS was statistically comparable between the two groups (56.5% vs. 51.1%; HR 0.86; 95% CI, 0.68 to 1.07; P = 0.17) [ 24 ]. Based on the results of the phase III trials presented above, several cytotoxic regimens could be used as adjuvant treatments for stage II-III GC after radical surgery, including S-1, CAPOX, SOX, and DS. The choice of regimens depends on many factors, including the pathological staging, patient performance status, and toxicity profile. In general, S-1 monotherapy is more recommended for stage II disease or for patients with poor performance status. Combination therapies such as CAPOX, SOX, or DS are often recommended for pathological stage III disease[ 17 , 25 ].
GC with microsatellite instability-high (MSI-H) or mismatch-repair deficiency (dMMR) is a distinct subtype [ 11 ]. Recently, an individual-patient-data meta-analysis including data from four large phase III studies (CLASSIC, ARTIST, MAGIC, and ITACA-S trial) explored the role of adjuvant chemotherapy in the MSI-H subtype [ 29 ]. It showed that for resectable MSI-H/dMMR GC patients, the prognosis of patients who received surgery alone was better than those who underwent surgery followed by adjuvant chemotherapy, even though the sample size of MSI-H/dMMR in this meta-analysis was very modest (N = 121). Based on this result, adjuvant chemotherapy is not recommended for resectable MSI-H/dMMR GC patients in the latest ESMO guideline [ 5 ]. Additionally, the updated CSCO guidelines suggest that either observation or adjuvant chemotherapy could be considered after a thorough discussion with the patients regarding the possible risks and benefits [ 17 ].
Adjuvant chemoradiotherapy
Unlike chemotherapy, the role of radiotherapy for resectable GC in the adjuvant setting is controversial. Adjuvant chemoradiotherapy (CRT) was once adopted in North America, according to the results of the phase III INT-0116 trial [ 30 ]. In this study, 556 patients with resectable GC or GEJ adenocarcinoma were randomly assigned to the upfront surgery plus adjuvant CRT group or the surgery group. Patients in the experimental arm received adjuvant fluorouracil chemotherapy plus 4500 cGy of radiation (5 × 5). Overall, CRT did prolong the OS compared to surgery alone (36 vs. 27 months, respectively; P = 0.005). However, most patients in this study received D0 or D1 lymphadenectomy and only 10% had D2 lymphadenectomy. The extent of dissection might affect the outcome of the surgery-only group. The phase III ARTIST trial from Korea evaluated the role of postoperative CRT based on the D2 dissection backbone [ 31 ]. Four hundred fifty-eight patients who received D2 lymphadenectomy and R0 resection were enrolled and randomly assigned to the adjuvant chemotherapy arm (capecitabine plus cisplatin, XP) or the adjuvant CRT arm (XP-XRT-XP). Unfortunately, the addition of radiotherapy postoperatively did not improve their DFS ( P = 0.0862). However, in the subgroup analysis, DFS was significantly prolonged in the CRT arm in the patients with lymph node-positive (N +) disease (3-year DFS rate: 77.5% vs.72.3%, HR 0.69, 95% CI 0.474–0.995, P = 0.0365). Based on these findings, the subsequent ARTIST II trial further explored the role of adjuvant CRT in patients with lymph node-positive GC [ 32 ]. Five hundred forty-six patients after D2 dissection were randomly assigned to adjuvant S-1, adjuvant SOX, and adjuvant SOX plus radiotherapy (SOXRT) in a 1:1:1 ratio. However, there was no significant difference in DFS between the adjuvant SOX and SOXRT treatments (3-year DFS rate: 72.8% vs.74.3%; HR 0.97, 95% CI 0.66–1.42, P = 0.879). Therefore, according to current results from these clinical trials, adjuvant CRT is not recommended in patients who received D2 lymphadenectomy and R0 resection.
Novel perioperative therapies
Perioperative targeted therapy.
Anti-HER2 and anti-vascular endothelial growth factor (VEGF) therapies have been recommended as the standard treatments for advanced GC in the first- and second-line setting, respectively. However, the role of targeted therapy in the perioperative or adjuvant setting is still unclear and is currently under investigation.
Anti-HER2 therapy
According to the ToGA study, adding trastuzumab to chemotherapy improved the OS in patients with metastatic HER2-positive GC [ 14 ]. However, the role of anti-HER2 therapy in resectable GC was unclear. In the multicenter phase II HER-FLOT study, patients with HER2-positive esophagogastric adenocarcinoma received perioperative FLOT chemotherapy for four cycles preoperatively and four cycles postoperatively, followed by 9 cycles of trastuzumab monotherapy [ 33 ]. The pCR rate was 21.4%, and the median DFS was 42.5 months. The phase II randomized PETRARCA study investigated the efficacy of adding trastuzumab and pertuzumab to perioperative FLOT chemotherapy in patients with ≥ cT2 or cN + resectable GC [ 34 ]. The pCR rate was significantly improved with trastuzumab and pertuzumab (35% vs. 12%, P = 0.02), and the R0 resection rate and surgical morbidity were comparable between both groups. However, adding targeted therapy to perioperative chemotherapy did not improve DFS or OS and caused more severe adverse events (≥ grade 3), especially diarrhea (41% vs. 5%) and leukopenia (23% vs. 13%). Based on these results, the trial did not proceed to phase III. Another phase II NEOHX study recruited 36 HER2-positive GC patients who received perioperative CAPOX plus trastuzumab treatment, followed by 12 cycles of trastuzumab maintenance therapy [ 35 ]. The pCR rate, 18-month DFS rate, and 5-year OS rate were 9.6%, 71%, and 58%, respectively. The randomized phase II INNOVATION trial assigned patients to 3 groups: perioperative chemotherapy, chemotherapy plus trastuzumab, and chemotherapy plus trastuzumab and pertuzumab [ 36 ]. According to the investigators' choice, the chemotherapy could be FLOT, CAPOX, FOLFOX, or XP. The primary endpoint was major pathological response (MPR) rate, and the result is pending. In general, adding anti-HER2 therapy to chemotherapy showed certain efficacy in the perioperative setting, but the associated survival benefit should be further investigated in a larger randomized trial.
Anti-VEGF therapy
As for anti-VEGF therapy, the randomized, open-label, phase II/III ST03 trial recruited 1,063 resectable esophagogastric adenocarcinoma patients and randomly assigned them to perioperative chemotherapy (ECX) group or perioperative chemotherapy plus bevacizumab group [ 37 ]. The result showed that adding bevacizumab did not improve the 3-year OS (48.1% vs. 50.3% for chemotherapy alone; HR 1.08; 95% CI, 0.91 to 1.29; P = 0.36). Besides, adding bevacizumab was associated with higher rates of postoperative anastomotic leak (24% vs. 10%). Ramucirumab, a VEGF receptor 2 inhibitor, has become one of the standard choices in the second-line treatment of GC [ 5 , 17 , 18 ]. The RAMSES/FLOT7 evaluated the efficacy of adding ramucirumab to perioperative FLOT for resectable GC [ 38 ]. The R0 resection rate in the intervention group was improved compared to the chemotherapy group (96% vs. 82%, P = 0.0093). The median DFS was prolonged in the FLOT plus ramucirumab group (32 months vs. 21 months), while the OS was similar in both groups (46 months vs. 45 months).
Perioperative immunotherapy
Based on several phase III clinical trials, programmed death 1 (PD-1) inhibitors were approved for first- and third-line treatment of unresectable/metastatic GC in different countries [ 5 , 17 , 18 ]. However, the role of ICI in resectable GC remains unclear and is being investigated in various clinical trials. In the randomized phase II DANTE trial, patients with resectable GC were assigned to the experimental arm with the PD-L1 inhibitor atezolizumab plus FLOT chemotherapy and the control arm with standard FLOT chemotherapy [ 39 ]. The R0 resection rate, surgical morbidity and mortality were comparable in both groups. Atezolizumab combined with chemotherapy was associated with tumor downstage and pathological regression, which were more pronounced in patients with a higher PD-L1 combined positive score (CPS).
Several single-arm phase II clinical trials explored the efficacy of perioperative ICIs combined with different treatments (chemotherapy, targeted therapy, or radiotherapy) in resectable GC [ 40 , 41 , 42 , 43 , 44 ]. The pCR rates ranged from 10 to 41%. In the phase III ATTRACTION-5 trial (NCT03006705), the use of nivolumab in the adjuvant setting was investigated. Patients who have undergone D2 surgery will receive either S-1 for one year or CAPOX for six months, with nivolumab added to the adjuvant therapy in the intervention arm. The primary endpoint of the study is relapse-free survival (RFS). The result was announced recently. Unfortunately, the addition of nivolumab did not extend the RFS compared with adjuvant chemotherapy alone. Additionally, the role of pembrolizumab in combination with perioperative chemotherapy for resectable GC is being examined in the phase III clinical trial, KEYNOTE-585 [ 45 ]. The chemotherapy regimens under investigation are XP, FP, or FLOT, and the primary endpoints of the study are OS, event-free survival (EFS), and pCR rate. The potential survival benefits and efficacy of ICI are also being evaluated in the double-blind, randomized phase III MATTERHORN study, which is based on the FLOT backbone [ 46 ]. Patients with resectable GC will receive either perioperative FLOT or FLOT plus durvalumab (a PD-L1 antibody). The primary endpoint of the study is EFS, with secondary endpoints including OS and pCR rate.
For the dMMR/MSI-H subgroup, as discussed above, the value of chemotherapy was controversial. Considering dMMR/MSI-H is a predictive biomarker for immunotherapy in advanced GC, treatment with immune checkpoint inhibitors in the perioperative setting has the potential to improve the response rate and survival. The phase II GERCOR NEONIPIGA study evaluated the response rate and safety of the combination of neoadjuvant nivolumab and low-dose ipilimumab followed by adjuvant nivolumab in patients with dMMR/MSI-H locally advanced G/GEJ adenocarcinoma. Among 29 patients who underwent surgery, 17 (58.6%; 90% CI, 41.8–74.1) achieved pCR[ 47 ]. Similarly, the pCR rate of tremelimumab plus durvalumab was 60% in the neoadjuvant setting (cohort 1) in the phase II INFINITY study[ 48 ]. Based on these encouraging results, it is possible for patients who achieved pCR after neoadjuvant immunotherapy to avoid surgery. Cohort 2 of the INFINITY study has started enrollment to investigate the activity of tremelimumab plus durvalumab as the definitive treatment for dMMR/MSI-H locally advanced GC.
Management for unresectable/metastatic GC
Chemotherapy.
Cytotoxic agents, including fluoropyrimidine, platinum, taxanes and irinotecan, are the main treatment in advanced gastric cancer. Generally, fluoropyrimidine (fluorouracil, capecitabine, and S-1) combined with platinum is used as the backbone therapy in the first line. Oxaliplatin is considered to be as effective as cisplatin. In the phase III SOX-GC trial, the SOX regimen showed improved survival compared to the SP regimen in diffuse or mixed-type GC[ 49 ]. For patients who are not fit for intensive chemotherapy (older age or poor performance status), the phase III GO2 trial showed that a modified dose of two-drug chemotherapy (60% of the full dose) provided a better tolerance but did not compromise the clinical outcome[ 50 ]. Paclitaxel, docetaxel, and irinotecan are commonly used in the second line of chemotherapy. In the ABSOLUTE phase III clinical trial conducted in Japan, weekly use of albumin-bound paclitaxel (nab-paclitaxel) was not inferior to weekly solvent-based paclitaxel in terms of overall survival[ 51 ]. In third-line treatment, trifluridine-tipiracil (TAS-102), an oral cytotoxic agent, has been proven in the phase III TAGS trial to improve overall survival compared with placebo (5.7 vs.3.6 months, HR 0.69, 95% CI 0.56–0.85)[ 52 ].
Immune Checkpoint Inhibitors (ICIs) in unresectable/metastatic GC
Immune checkpoint inhibitors (ICIs) (monotherapy or combined with other treatments) have shown anti-tumor effects across a spectrum of solid tumors, including gastrointestinal tumors. Here, we present an overview of current evidence of ICI treatment in GC (Table 2 ) and discuss different predictive biomarkers for ICIs.
KEYNOTE-062 was the first global, randomized phase III trial to compare the efficacy and safety of immuno-monotherapy (pembrolizumab) or immunotherapy plus chemotherapy versus standard chemotherapy in HER2-negative advanced GC in the first-line setting [ 53 ]. According to the last update in ASCO 2022, it was suggested that pembrolizumab monotherapy was non-inferior to chemotherapy alone (cisplatin and fluorouracil/capecitabine) in patients with PD-L1 CPS ≥ 1 (median OS 10.6 vs. 11.1 months, HR 0.90, 95% CI 0.75–1.08) but was superior in the CPS ≥ 10 population (median OS 17.4 vs. 10.8 months; HR, 0.62; 95% CI, 0.45–0.86) [ 54 ]. However, the combination of pembrolizumab and chemotherapy did not bring OS benefit compared to chemotherapy alone in either CPS ≥ 1 (12.5 vs. 11.1 months; HR, 0.85; 95% CI, 0.71–1.02) or CPS ≥ 10 (12.3 vs. 10.8 months; HR, 0.76; 95% CI, 0.56–1.03) subgroup [ 54 ]. In another double-blind, placebo-controlled phase III KEYNOTE-859 study, the addition of pembrolizumab to chemotherapy (FP or CAPOX) demonstrated slight survival benefit compared with chemotherapy alone (OS 12.9 vs. 11.5 months, HR, 0.78; 95% CI, 0.70–0.87. PFS 6.9 vs. 5.6 months, HR, 0.76; 95% CI, 0.67–0.85). The results were generally consistent in different PD-L1 CPS subgroups[ 55 ].
CheckMate-649 is another global, randomized, phase III trial investigating the effects of ICIs (nivolumab plus ipilimumab, a CTLA-4 inhibitor) or ICI (nivolumab) plus chemotherapy versus chemotherapy (CAPOX or FOLFOX) alone in metastatic HER2-negative GC patients [ 56 ]. One thousand five hundred eighty-one patients were assigned to nivolumab plus chemotherapy arm or chemotherapy arm. The addition of nivolumab to chemotherapy improved the OS (14.4 vs. 11.1 months; HR 0.71; 98.4% CI, 0.59 to 0.86; P < 0.0001) and PFS (7.7 vs. 6.05 months; HR 0.68; 98% CI, 0.56 to 0.81; P < 0.0001) for the patients with PD-L1 CPS ≥ 5; therefore both primary endpoints were met. For all-randomized patients, nivolumab combined with chemotherapy also improved OS (13.8 vs. 11.6 months; HR 0.80; 99.3% CI, 0.68 to 0.94; P = 0.0002). Moreover, all CPS subgroups exhibited an increased objective response rate in the nivo-chemotherapy arm. However, the chemo-free treatment with nivolumab and ipilimumab did not show OS improvement compared to chemotherapy alone [ 57 ]. Based on these findings, nivolumab combined with chemotherapy was listed as one of the recommended first-line treatments in the NCCN, ESMO, and CSCO guidelines [ 5 , 17 , 18 ].
ATTRACTION-04 was a randomized, double-blind, placebo-controlled, multicenter phase II/III trial that evaluated the effects of nivolumab plus chemotherapy (SOX or CAPOX) compared with chemotherapy alone in the first-line treatment for HER2-negative advanced GC in the Asian population, regardless of PD-L1 expression [ 58 ]. The combination therapy significantly improved the PFS (HR 0·68; 98·51% CI 0·51–0·90; P = 0·0007) but not the OS (both groups achieved > 17 months of median OS). One of the possible reasons for the different results of OS between ATTRACTION-04 and CheckMate-649 could be the subsequent anticancer therapies, whereby the proportion of patients who received subsequent anticancer treatments or ICIs therapy was much higher in ATTRACTION-04 (66% vs. 39% in CheckMate-649).
The efficacy of immunotherapy plus chemotherapy was further confirmed in the Asian phase III ORIENT-16 trial, which compared sintilimab plus chemotherapy (CAPOX) to chemotherapy alone as the first-line treatment [ 59 ]. The pre-specified interim result was reported at ESMO 2021. Sintilimab plus chemotherapy showed a survival benefit versus chemotherapy alone in patients with CPS ≥ 5 (18.4 vs. 12.9 months; HR 0.660; 95% CI 0.505–0.864) and all randomized patients (15.2 vs. 12.3 months; HR 0.766; 95% CI 0.626–0.936). Another PD-1 antibody, tislelizumab, is currently being investigated in the phase III RATIONALE-305 trial [ 60 ]. Advanced GC patients are randomized to receive tislelizumab plus chemotherapy (CAPOX/FP regimen) or chemotherapy alone. The primary endpoints are PFS and OS. Results from the interim analysis of the PD-L1 + (i.e., PD-L1 TAP score ≥ 5%) population were represented at 2023 ASCO-GI, showing that tislelizumab plus chemotherapy led to significant OS (17.2 vs. 12.6 months; HR 0·74; 95% CI 0·59–0·94) and PFS (7.2 vs. 5.9 months; HR 0·67; 95% CI 0·55–0·83) improvement compared to chemotherapy alone[ 61 ]. The ITT population outcomes will be reported after the final analysis.
In summary, in first-line treatment for HER2-negative advanced GC, the addition of anti-PD-1 therapy could improve clinical outcomes in patients with high PD-L1 expression, according to the results from CheckMate-649, ORIENT-16, and RATIONALE-305. For patients with low PD-L1 expression or unknown PD-L1 status, the survival benefit of adding PD-1 antibodies is still controversial (discussed below), and the risk–benefit balance of ICIs treatment should be considered, and decisions should be discussed case by case.
The role of maintenance therapy with ICIs after first-line chemotherapy was evaluated in the phase III JAVELIN Gastric 100 trial [ 62 ]. Patients with HER2-negative advanced GC without progression after at least 12 weeks of first-line chemotherapy (oxaliplatin plus fluoropyrimidine) were randomly assigned to avelumab (a PD-L1 inhibitor) maintenance or continued chemotherapy. Avelumab maintenance did not show OS benefit compared to chemotherapy (24-month OS rate: 22.1% versus 15.5%; HR 0.91; 95% CI, 0.74–1.11; P = 0.1779).
Second line and beyond
The randomized, open-label, phase III KEYNOTE-061 trial compared pembrolizumab monotherapy with paclitaxel in patients with advanced GC or GEJ cancer in the second-line setting [ 53 ]. Though the primary endpoints (the OS and PFS in patients with PD-L1 CPS ≥ 1) were not met, it was suggested that the efficacy of pembrolizumab monotherapy was associated with the PD-L1 CPS level. Patients with CPS ≥ 10 had a better outcome in the pembrolizumab group than in the chemotherapy group.
KEYNOTE-059 was a phase II study that explored the effect of pembrolizumab in patients with advanced GC after progression from ≥ 2 lines of treatment [ 63 ]. Among the 259 patients enrolled, the ORR and median duration of response (DoR) was 11.6% and 8.4 months, respectively. Moreover, pembrolizumab showed higher efficacy in the subgroup with PD-L1-positive cancer (CPS ≥ 1) compared to PD-L1-negative cancers (ORR 15.5% vs. 6.4%; DoR 16.3 vs. 6.9 months, respectively). The phase III ATTRACTION-2 study compared nivolumab monotherapy versus placebo in advanced GC patients after two lines of therapy, regardless of the PD-L1 expression [ 64 ], and survival benefit was observed in the nivolumab group (OS 5.3 vs. 4.1 months; HR 0·63, 95% CI 0·51–0·78; P < 0·0001). Based on the results of this study, nivolumab is recommended as monotherapy in third-line treatment for GC in the CSCO guideline but not in the ESMO or NCCN guidelines due to the patients enrolled being exclusively Asian. The role of avelumab in the third-line treatment for advanced GC was investigated in the phase III JAVELIN Gastric 300 trial [ 65 ]. Though avelumab showed a more manageable safety than the physician's choice of chemotherapy, it did not improve OS (primary endpoint, 4.6 vs. 5.0 months; HR 1.1, 95% CI 0·9–1.4; P = 0.81), PFS, or ORR.
Molecular Biomarkers of Immunotherapy in GC
HER2-positive GC, defined as immunohistochemical (IHC) expression 3+ or 2 + combined with positive fluorescent in situ hybridization (FISH) verification, accounts for approximately 15–20% of gastric or gastroesophageal cancer. The phase III ToGA study has established trastuzumab combined with chemotherapy as the standard first-line treatment for HER2-positive advanced GC [ 14 ]. In preclinical models, HER2 signaling could regulate the recruitment and activation of tumor-infiltrating immune cells [ 66 ]. Besides, trastuzumab has been shown to upregulate the expression of PD-1 and PD-L1 [ 67 , 68 ], and anti-PD-1 antibodies could significantly increase the therapeutic activity of HER2 inhibitors [ 69 ]. Several phase I/II studies demonstrated the promising efficacy of the addition of ICIs to trastuzumab and chemotherapy in HER2-positive GC. In the phase Ib Ni-HIGH study conducted in Japan, patients with HER2-positive advanced GC received nivolumab, trastuzumab, and chemotherapy (CAPOX or SOX regimen) in the first-line setting, and the ORR was 75%, as reported at ASCO 2020 [ 70 ]. The multi-institutional phase Ib/II PANTHERA trial explored the efficacy and safety of the combination of pembrolizumab, trastuzumab and chemotherapy as first-line therapy for HER2-positive advanced GC [ 71 ]. The updated data at ASCO-GI 2021 showed that the ORR was 76.7% (CR 16.3%, PR 60.5%), the PFS was 8.6 months (95% CI 7.2–16.5 months), and the OS was 19.3 months (95% CI 16.5-NR). The striking efficacy was also reported in another phase II study, in which patients with HER2-positive GC received pembrolizumab, trastuzumab and chemotherapy (oxaliplatin/cisplatin + capecitabine/5-FU) [ 72 ]. Overall, the ORR was 91% and DCR was 100%. The median PFS and OS was 13·0 months and 27·3 months, respectively, which was much better than the OS reported in the ToGA study. Recently, the randomized, double-blind, placebo-controlled phase III KEYNOTE-811 trial reported the results of its first interim analysis [ 73 ], in which patients with metastatic HER2-positive GC or GEJ cancer received pembrolizumab or placebo plus trastuzumab and chemotherapy. The results showed that adding pembrolizumab to trastuzumab and chemotherapy could markedly increase the ORR (74.4% vs. 51.9%; the estimated difference between the two groups was 22.7%; 95% CI, 11.2–33.7%; P = 0.00006). Based on this result, the FDA approved pembrolizumab combined with trastuzumab and chemotherapy as the first-line treatment for advanced HER2-positive gastric or GEJ adenocarcinoma. The results of the primary endpoints (PFS and OS) are still immature.
MSI-H tumor is one of the four subtypes of GC according to The Cancer Genome Atlas (TCGA) Research Network [ 11 ]. The incidence of MSI-H status in GC was reported to range from 8 to 25%, which was much lower in metastatic disease [ 74 ]. Mismatch repair (MMR) proteins are supposed to fix the errors that occur during DNA replication. When MMR proteins are deficient, the defects of DNA replication will lead to the accumulation of mutations and the expression of neoantigens, which may act as potential targets of immune cells [ 75 ]. Hence, it is reasonable that tumors with MSI-H/dMMR status may attract more immune cell infiltration and enhance the effect of immune checkpoint inhibitors. A post hoc analysis of KEYNOTE-059 (third-line treatment), KEYNOTE-061 (second-line treatment), and KEYNOTE-062 (first-line treatment) was conducted to evaluate the efficacy of pembrolizumab versus chemotherapy in the patients with MSI-H advanced G/GEJ adenocarcinoma [ 15 ]. Overall, 7 of 174 patients (4.0%) in KEYNOTE-059, 27 of 514 (5.3%) in KEYNOTE-061, and 50 of 682 (7.3%) in KEYNOTE-062 with MSI-H status were enrolled. By the time of analysis, the OS of the patients with MSI-H was not reached for pembrolizumab monotherapy in KEYNOTE-059, 061 and 062, or for pembrolizumab combined with chemotherapy in KEYNOTE-062, compared with an OS of around 8 months for chemotherapy alone. Besides, the ORR was much higher in the immunotherapy groups. In another meta-analysis including four phase III trials (KEYNOTE-062, CheckMate-649, JAVELIN Gastric 100, and KEYNOTE-061), 2545 patients with known MSI status were enrolled, and the proportion of MSI-H was 4.8% [ 76 ]. In the MSI-H group, the HR for OS benefit with immunotherapy was 0.34 (95% CI 0.21–0.54), compared to 0.85 (95% CI 0.71–1.00) for the MSS group. Among the patients with MSI-H status, the HR for PFS was 0.57 (95% CI 0.33–0.97; P = 0.04), and the odds ratio (OR) for ORR was 1.76 (95% CI 1.10–2.83; P = 0.02). Altogether, these findings suggested that MSI-H status was a predictive biomarker for immune checkpoint inhibitor treatments, regardless of the line of therapy.
Epstein-Barr virus-associated GC (EBVaGC) is another distinct molecular subtype of the TCGA classification [ 11 ], accounting for about 9% of GC in the TCGA cohort and approximately 5% in China [ 77 , 78 ]. EBV has been linked to CD8 + T cell infiltration and increased expression of PD-L1 and PD-L2 [ 11 , 79 ], making it a potential biomarker for ICI treatment. While a Korean study with a small sample size (n = 6) once reported a 100% response rate in EBV-positive advanced GC [ 80 ], several other studies did not demonstrate a high response rate [ 81 , 82 , 83 ]. Differences in response rates across studies may be attributed to confounding factors such as tumor mutational burden (TMB) and PD-L1 expression. Therefore, the role of EBV positivity in immunotherapy for GC remains unclear and requires further investigation.
As discussed earlier, the level of PL-L1 expression, especially the CPS score, has been considered a predictive biomarker for response to ICIs. However, the reliable cut-off value to predict the benefit of immunotherapy is needed to be determined. The cut-off points often used in clinical trials are 1, 5 and 10. In the KEYNOTE-059 trial, CPS ≥ 1 was used to separate the patients that could benefit from third-line pembrolizumab treatment [ 63 ]. However, this benefit was not seen compared to chemotherapy in the KEYNOTE-061/062 trials [ 53 , 84 ]. In KEYNOTE-061/062, CPS ≥ 10 effectively differentiated the response to pembrolizumab. Patients with CPS ≥ 10 had better OS benefits than those with CPS ≥ 1. A comprehensive analysis of patients with CPS ≥ 10 in KEYNOTE-059, 061 and 062 also showed consistent improvement toward better outcomes with pembrolizumab in different lines of treatment in this subgroup [ 85 ]. In the CheckMate-649 and ORIENT-16 studies, CPS ≥ 5 was used as the cut-off value for the primary endpoint OS. Though the OS benefit of nivolumab plus chemotherapy was also observed in all randomized patients in CheckMate-649, the subgroup analysis suggested that the benefit was insignificant in the CPS < 5 or < 1 group [ 86 ]. A recent study reconstructed unreported Kaplan–Meier plots of PD-L1 CPS subgroups of three phase III trials (CheckMate-649, KEYNOTE-062, and KEYNOTE-590) and investigated the outcome of low CPS subgroup [ 87 ]. The result suggested that patients with low PD-L1 expression (CPS 1–4 and CPS 1–9) did not benefit from adding ICIs to chemotherapy. In summary, although the predictive role of PD-L1 CPS for immunotherapy efficacy has been demonstrated in multiple clinical trials, there is still a need to determine the optimal cut-off value for CPS and to develop further classifications for patients with low CPS scores. Recently, the result of the phase III RATIONALE-305 trial suggested that the TAP score > 5% also had predictive value for ICI treatment in gastric cancer[ 61 ], and further exploration is needed.
Tumor mutation burden (TMB)
It is hypothesized that a high TMB status results in the high expression of neoantigens, which are immunogenic and can induce the response of the immune system and potentially increase the efficacy of ICI treatment. In a phase Ib/II study that explored the efficacy of the PD-1 antibody toripalimab in patients with advanced GC, patients with TMB-high (TMB-H, TMB ≥ 12 mut/Mb) showed a higher ORR and better OS compared with patients with TMB-L status (ORR 33.3% vs. 7.1%, P = 0.017; OS 14.6 vs. 4.0 months, P = 0.038)[ 88 ]. In the subgroup analysis of the KEYNOTE-061 study, the TMB status (≥ 10 or < 10 mut/Mb) was associated with response rate, PFS, and OS in patients treated with pembrolizumab. In the TMB-H subgroup, pembrolizumab demonstrated a better OS compared with paclitaxel, and this benefit remained even when MSI-H patients were excluded[ 89 ]. Though FDA granted approval for the use of pembrolizumab in patients with TMB-H (i.e., TMB ≥ 10 mutations/Mb) advanced solid tumors that progressed after standard treatments, according to the subgroup analysis of KEYNOTE-158 study[ 90 ], the evidence is still not enough for the use of ICIs in TMB-H gastric cancer, and phase III studies to illustrate the predictive value of TMB are needed.
Molecular targeted therapy in unresectable/metastatic GC
Molecular targeted therapy remains an essential treatment option for patients with advanced GC, aimed to inhibit tumor proliferation and increase survival rates. Targeted therapies, including anti-HER2, anti-angiogenesis, and other biomarker-directed therapies, have demonstrated promising efficacy in treating GC, with significant benefits observed in biomarker-enriched patients (Table 3 ). Therefore, next-generation sequencing or ctDNA detection is crucial for mGC patients to establish a comprehensive molecular profile, including the status of HER2, fibroblast growth factor receptor (FGFR), Claudin18.2 (CLDN18.2), PD-L1 and EGFR.
HER2, also known as ERBB2, is a member of the ERBB protein families that includes the epidermal growth factor receptor (EGFR or HER1), HER3, and HER4 [ 91 ]. HER2 overexpression or amplification has been found in a range of 7.3% to 20.2% in advanced gastric and gastroesophageal junction adenocarcinomas, with the overexpression rate varying globally [ 92 ]. In addition, intestinal-type gastric cancers and those arising from the proximal stomach or gastroesophageal junction are more likely to exhibit HER2 positivity. [ 11 , 93 ].
Trastuzumab is a humanized monoclonal antibody that targets HER2 extracellular domain 4, then inhibits downstream signal activation and cancer cell proliferation. Trastuzumab plus chemotherapy has been established as the standard first-line treatment for HER2-positive advanced GC. The landmark ToGA trial revealed that trastuzumab plus chemotherapy significantly improved the overall survival of patients with advanced GC [ 14 ], especially for patients with HER2 positivity, who were identified as having HER2 immunohistochemistry (IHC) scores of 2 + and fluorescence in situ hybridization (FISH)-positive or HER2 IHC 3 + based on a post-hoc exploratory analysis [ 92 ]. The EVIDENCE trial has demonstrated that combining first-line trastuzumab with chemotherapy was associated with improved clinical outcomes in Chinese patients with HER2-positive metastatic GC, providing real-world evidence. [ 94 ].
However, subsequent attempts of HER2-targeted therapy in advanced GC were not as successful as expected. Even though pertuzumab [ 95 , 96 ], trastuzumab emtansine (T-DM1) [ 97 ], and lapatinib [ 98 , 99 ] were all investigated in several first-line and second-line trials, no survival improvement was observed in any of these trials. Additionally, trastuzumab beyond progression also failed to show a survival benefit in pre-treated HER2-positive GC patients in the T-ACT trial [ 100 ].
Potential resistance mechanisms of HER2-targeted therapy
Primary or acquired resistance is a major impediment to the management of mGC patients, while mechanisms underlying the poor efficacy of HER2-directed therapy in GC are not fully understood. Multiple potential resistance mechanisms have been researched, as listed below, and further studies are warranted to improve treatment resistance in GC patients treated with HER2-targeted therapy in clinical settings.
HER2 heterogeneity
Intratumoral HER2 heterogeneity is observed in 23% to 79% of GC patients and is associated with patients’ survival [ 101 , 102 , 103 ]. Specifically, Shusuke et al. reported prolonged survival in homo-HER2 positive GC patients, defined as all tumor cells overexpressing HER2 in biopsy specimens [ 101 ]. Tumor cells with HER2 overexpression or amplification are killed during HER2-targeted therapy, while residual drug-resistant colonies keep proliferating and eventually take control, leading to tumor recurrence. As a result, resistance to HER2-targeted therapy has been associated with the heterogeneity of HER2 expression [ 101 , 104 , 105 ]. Discordance between next-generation sequencing and FISH/IHC may also indicate intratumoral heterogeneity and result in an unfavorable treatment outcome. In addition, there still exist discrepancies in HER2 status between primary tumor and metastatic sites, which increases the risk of HER2-targeted therapy failure due to false-positive HER2 detection [ 106 , 107 ].
Loss of HER2 expression
For mGC patients experiencing progression on trastuzumab, 29–69% of them may experience loss of HER2 expression, which is an important factor responsible for resistance [ 108 , 109 , 110 ]. Given the risk of HER2 expression loss during treatment, patients should re-evaluate HER2 status upon progression after anti-HER2 therapy to determine the most optimal treatment.
Gene amplification
Receptor tyrosine kinase (RTK) amplification was commonly detected in MET-amplified metastatic GC, with 40% to 50% of cases exhibiting co-amplification of either HER2 or EGFR. These patients did not usually respond to HER2-targeted therapy, but MET and HER2 combination inhibition could sometimes bring extra clinical benefit [ 111 ]. CCNE1, which encodes the cell cycle regulator cyclin E1, is another oncogene co-amplified with HER2 in metastatic GC. CCNE1 co-amplification has been found to be more strongly related to HER2-positive AGC than to HER2-positive breast cancer [ 112 ]. In a phase II study of lapatinib with capecitabine and oxaliplatin in HER2-positive AGC patients, CCNE1 amplification was demonstrated to play a role in resistance to HER2-targeted therapy [ 113 ]. A high level of copy number variation for CCNE1 has also been associated with worse survival in patients with HER2-positive metastatic GC treated with trastuzumab [ 114 ]. Other studies have also reported that deletion of ErbB2 16 exon and co-mutation and/or amplification of KRAS, HER3, EGFR, PI3K or PTEN could contribute to the resistance of anti-HER2 therapy [ 109 , 113 , 115 , 116 ].
Alterations in intracellular signaling
HER2-targeted therapy suppresses downstream signaling pathways by blocking the binding of HER2 receptors and ligands, which inhibits the migration and proliferation of tumor cells and leads to apoptosis. RTK/RAS/PI3K signaling alterations have been shown to be involved in the development of resistance to trastuzumab. [ 109 ]. Furthermore, activation of the bypass pathway might also result in resistance. Sampera et al. discovered that SRC-mediated persistent activation of the MAPK-ERK and PI3K-mTOR pathways was connected to the treatment resistance in HER2-positive GC cell lines [ 117 ]. NRF2 has also been associated with HER2 resistance by activating the PI3K-mTOR signaling pathway [ 118 ].
Newer HER2-targeted agents
To overcome intrinsic and acquired resistance to trastuzumab, various clinical trials have explored newer agents and combinations. The following innovative HER2-targeted agents for advanced metastatic GC are currently under investigation (Table 4 ): monoclonal antibodies (mAbs) (e.g., margetuximab), bispecific antibodies (BsAbs) (e.g., ZW25, KN026), antibody–drug conjugates (ADCs) (e.g., T-DXd, Disitamab vedotin, ARX788), tyrosine kinase inhibitors (TKIs) (e.g., tucatinib), and other novel therapeutic approaches.
Monoclonal antibodies
Margetuximab
Margetuximab is an Fc-engineered anti-HER2 mAb that targets the same epitope as trastuzumab but with a higher affinity for single-nucleotide polymorphisms of the activating Fc receptor (CD16A) [ 119 , 120 ]. Margetuximab can recruit CD16A-expressing natural killer cells, macrophages and monocytes and further promote antibody-dependent cell-mediated cytotoxicity (ADCC) [ 119 ]. The first phase I study of margetuximab in humans illustrated that margetuximab was well-tolerated with promising efficacy in relapsed HER2-overexpressing carcinoma [ 121 ]. Later in the phase Ib/II CP-MGAH22-05 study, patients with previously treated HER2-positive GC responded effectively to a chemotherapy-free treatment consisting of margetuximab plus pembrolizumab. Patients with HER2 IHC3 + and PD-L1 positive (CPS ≥ 1, by IHC) had an ORR of 44% and a DCR of 72% [ 122 ]. More recently, the phase II/III MAHOGANY trial has reported the efficacy of margetuximab plus anti-PD-1 antibody retifanlimab (Cohort A) for the first-line treatment of patients with G/GEJ adenocarcinoma, with an ORR and a DCR of 53% and 73% [ 123 ]. The ORR reported in this trial was superior to the ORR observed with other history chemotherapy-free treatments; nonetheless, given that chemotherapy-based regimens remain the predominant treatment for GC, the MAHOGANY trial has been halted for commercial reasons.
Bispecific antibodies (BsAbs)
Zanidatamab (ZW25)
Zanidatamab (ZW25) is a novel HER2-targeted bispecific antibody that binds to HER2 extracellular domain (ECD) II and IV. According to a phase I study, ZW25 was well tolerated with durable response in heavily pretreated GEA patients (including prior HER2-targeted therapy) [ 86 ]. Later in a phase II trial involving patients with advanced/metastatic HER2-positive GEA, zanidatamab plus chemotherapy (CAPOX or FP) showed a confirmed ORR of 75%, mDOR of 16.4 months and mPFS of 12.0 months in the first-line setting [ 124 ]. Based on these findings, a global phase III study (HERIZON-GEA-01) has been designed to assess the efficacy and safety profiles of zanidatamab plus chemotherapy with or without tislelizumab versus standard of care (trastuzumab plus chemotherapy) for patients with metastatic HER2-positive GEAs in first-line settings [ 125 ].
KN026 mimics the dual effects of trastuzumab and pertuzumab by simultaneously binding to HER2 ECD II and IV [ 126 ]. In a phase II clinical study, KN026 showed favorable results in patients with HER2-overexpressing G/GEJ adenocarcinoma (IHC3 + or IHC 2 + ISH +) with an ORR of 56% [ 127 ]. The ongoing phase II/III trial (KN026-001) is planned to evaluate the survival benefit of KN026 plus chemotherapy in patients with HER2-positive unresectable or advanced G/GEJ adenocarcinoma upon progression after trastuzumab-containing treatment (NCT05427383). Most recently, the preliminary data presented at ESMO 2022 illustrated that KN026 plus KN046, a recombinant humanized PD-L1/CTLA-4 bispecific antibody, had remarkable efficacy and tolerable safety in HER2-positive G/GEJ patients without prior systemic treatment [ 128 ]. In this phase II study, the ORR was 77.8%, and the DCR was 92.6%, indicating the need for a future randomized clinical trial to confirm the efficacy of KN026 plus KN046 treatment versus standard of care.
Other BsAbs
PRS-343 is a BsAb that targets HER2 and the costimulatory immunoreceptor 4-1BB on immune cells. In patients with advanced HER2-positive solid tumors, including GC, PRS-343 showed anticancer efficacy both alone and in combination with the anti-PD-L1 antibody atezolizumab in a phase I clinical study [ 129 ]. A phase II study (NCT05190445) is ongoing to investigate the efficacy of PRS-343 in combination with ramucirumab and paclitaxel in patients who have already received treatment for HER2-high (IHC 3+ or IHC 2+ with HER2/neu gene amplification) G/GEJ adenocarcinoma and in combination with tucatinib in HER2-low (IHC 1+ or IHC 2+ without HER2/neu gene amplification) G/GEJ adenocarcinoma.
Antibody–drug conjugates (ADCs)
Trastuzumab deruxtecan (T-DXd)
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate (ADC) composed of an anti-HER2 antibody connected to a cytotoxic topoisomerase I inhibitor via a cleavable tetrapeptide-based linker [ 130 ]. Different from T-DM1, T-DXd has a bystander effect on nearby cells, including those not expressing HER2, thus greatly enhancing the antitumor effect [ 131 ]. This action method is inspiring, particularly for advanced GC patients with diverse intratumoral HER2 expression. In the Asia DESTINY-Gastric01 trial, T-DXd significantly improved overall survival in patients with HER2 + advanced GC compared with chemotherapy in the later-line settings [ 132 ]. Interestingly, the efficacy and safety of T-DXd were also evaluated in exploratory cohorts of patients with HER2-low G/GEJ cancers in the DESTINY-Gastric01 trial (cohort 1, IHC 2 + /ISH–; cohort 2, IHC 1 +). The confirmed ORR was 26.3% in Cohort 1 and 9.5% in Cohort 2. The median OS was 7.8 months in cohort 1 and 8.5 months in cohort 2[ 133 ]. These results provide initial evidence that T-DXd has clinical benefits in patients with heavily pretreated HER2-low G/GEJ cancers.
Similarly, T-Dxd in the DESTINY-Gastric02 trial also achieved encouraging results in 2L western GC patients with a cORR of 41.8% and a median PFS of 5.6 months [ 134 ]. Other trials, such as phase III 2L DESTINY-Gastric04 and phase III 1L DESTINY-Gastric03, are also in progress (NCT04379596, NCT04704934).
Disitamab vedotin (RC48)
Disitamab vedotin (RC48) is a novel HER2-ADC drug independently developed in China, which is composed of three parts: anti-HER2 extracellular domain antibody, MC-Val-Cit-PAB linker, and cytotoxin monomethyl auristatin E (MMAE) [ 135 ]. This novel antibody has a stronger affinity to HER2 than the standard of care. Unlike T-DM1, disitamab vedotin has a bypass-killing effect on nearby tumor cells regardless of HER2 status, which could help overcome spatial heterogeneity and enhance anti-tumor effects. RC48 was well tolerated and showed promising antitumor activity in patients with HER2-positive advanced GC in a phase I trial [ 136 ]. The phase II RC48-C008 trial revealed a significant benefit of RC48 with HER2-overexpressing GC patients who had undergone at least two prior lines of therapy, in which the ORR was 24.8%, mPFS was 4.1 months and mOS was 7.9 months [ 137 ]. Of note, the ORR of RC48 in patients with HER2 IHC2 + /FISH- was 16.7%, slightly lower than in HER2-positive patients. These findings indicated that RC48 exerted considerable anti-tumor effectiveness and tolerable safety in patients with HER2-positive GC, as well as in those with HER2 low expression GC. In June 2021, disitamab vedotin was approved in China for the treatment of patients with HER2-overexpressing advanced or metastatic G/GEJ adenocarcinoma who received at least two systemic chemotherapy regimens. The ongoing phase III RC48-C007 (NCT04714190) trial aims to evaluate the efficacy and safety of RC48 as a third-line treatment and beyond in patients with advanced HER2-positive GC.
ARX788 is another investigational anti-HER2 antibody–drug conjugate consisting of HER2-targeted monoclonal antibody (mAb) coupled with a highly effective tubulin inhibitor (AS269). ARX788 was well tolerated and had a promising anti-tumor effect in HER2-positive GC patients previously treated with trastuzumab-based regimens in a phase I multicenter dosage expansion trial [ 138 ]. The ORR was confirmed to be 37.9%, and the DCR was 55.2%. With a median follow-up period of 10 months, the mPFS and OS were 4.1 and 10.7 months, respectively. On March 18, 2021, the FDA granted ARX788 as an orphan drug for treating HER2-positive GC. A randomized controlled, multicenter, open-label phase II/III study is underway to assess the efficacy of ARX788 as second-line treatment for HER2-positive advanced G/GEJ adenocarcinoma (Chinadrugtrials.org.cn: CTR20211583).
Tyrosine kinase inhibitors
Tucatinib, a highly selective HER2-directed tyrosine kinase inhibitor (TKI), was approved by FDA for HER2-positive metastatic breast cancer in 2020 and is under exploration in GC. In preclinical studies, tucatinib plus trastuzumab demonstrated superior activity compared to a single agent in GEC xenograft models [ 139 ]. Recently, the phase II/III MOUNTAINEER-02 (NCT04499924) was initiated to evaluate the efficacy of tucatinib, trastuzumab combined with ramucirumab, and paclitaxel in previously treated HER2 + advanced G/GEJ adenocarcinoma [ 140 ].
Other novel therapeutic approaches are being under investigation, including anti-HER2 CAR-T-cell therapy (NCT04511871, NCT04650451), CAR-natural killer cell (NK) therapy [ 141 ], and CAR-macrophage (CAR-M) therapy (NCT04660929), B-cell and monocyte-based immunotherapeutic vaccines (BVAC-B), BAY2701439 and CAM-H2 targeted HER2 radiotherapy (NCT04147819, NCT04467515). These widespread attempts at HER2-targeted CAR cell therapy in solid tumors may hopefully lead to the development of new drug candidates in patients with HER2-positive GC.
Antiangiogenic therapy
Blocking angiogenesis is a key strategy in GC therapy, including anti-VEGF monoclonal antibodies, VEGF-binding proteins, and VEGF receptor TKIs (Table 5 ) [ 142 ]. Ramucirumab, a typical antiangiogenic monoclonal antibody, targets VEGFR-2 and is approved by the FDA for treating advanced GC [ 143 ]. In the second-line REGARD trial, ramucirumab demonstrated significant improvement in patient OS and PFS versus best supportive care in metastatic GC [ 144 ]. In the RAINBOW trial, when coupled with paclitaxel, ramucirumab significantly prolonged overall survival compared to paclitaxel alone [ 145 ]. Similarly, results from RAINBOW-Asia bridging study also supported the application of ramucirumab plus paclitaxel as second-line therapy in a predominantly Chinese population with advanced gastric or GEJ adenocarcinoma [ 146 ]. However, neither ramucirumab nor bevacizumab brought extra survival benefits when added to platinum or fluoropyrimidine chemotherapy in GC patients in the first-line settings [ 147 , 148 ].
Regorafenib is an oral multi-kinase inhibitor targeting angiogenic, stromal and oncogenic receptor tyrosine kinases (RTK). Results from a phase III trial (INTEGRATE IIa) presented at ASCO GI 2023 demonstrated that regorafenib significantly improved OS (4.5 months vs. 4.0 months; HR = 0.52; P = 0.011) in patients with advanced gastro-oesophageal cancer (AGOC) in later-line settings [ 149 ]. Meanwhile, other studies exploring the efficacy of anti-VEGF and anti-PD1 combination in GC populations are also under investigation. The combination of regorafenib and nivolumab had a manageable safety profile and effective antitumor activity in a phase I trial for the GC subgroup [ 150 ]. INTEGRATE IIb ((NCT0487936)), an international randomized phase 3 trial, is ongoing to compare regorafenib plus nivolumab to standard chemotherapy in pre-treated patients with AGOC. Besides, lenvatinib plus pembrolizumab showed promising anti-tumor activity with an ORR of 69% in the first-line and second-line treatment of advanced GC [ 151 ].
Apatinib is a small molecule VEGFR inhibitor with China Food and Drug Administration (CFDA) approval for the treatment of advanced or metastatic chemotherapy-refractory GC. Apatinib improved median PFS and OS versus placebo in Chinese patients with advanced gastric or gastroesophageal junction adenocarcinoma in the third line and beyond[ 152 ]. Most of the patients in this trial did not receive prior antiangiogenic therapies since they were not standard treatments in China at that time, so clinical evidence is still lacking for the use of apatinib in patients who previously received ramucirumab. Unfortunately, no significant improvements were observed in overall survival (OS) in western populations in the phase III ANGEL clinical trial [ 153 ].
Fruquintinib is a highly selective VEGFR family kinase inhibitor that targets VEGFR1, 2 and 3 and is independently developed in China. Fruquintinib was approved in China by the NMPA in September 2018 and commercially launched in late November 2018 as a third-line treatment for patients with metastatic colorectal cancer. In a phase Ib/II study, adding fruquintinib to paclitaxel as second-line treatment for mGC patients at recommended phase 2 dose (RP2D) showed an mPFS of 4 months and mOS of 8.5 months. In the 4 mg dose cohort of 27 patients with evaluable tumor response, the ORR was 25.9% and the DCR was 66.7%[ 154 ]. A randomized phase III FRUTIGA study has investigated fruquintinib plus paclitaxel versus paclitaxel alone in patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma who had progressed after first-line standard chemotherapy (NCT03223376). Initial results from FRUTIGA showed that fruquintinib combined with paclitaxel showed significant improvements in PFS, ORR and DCR. Full detailed results are still being analyzed and will be revealed soon.
Other biomarker-targeted therapy
Novel diagnostic techniques have contributed to characterizing the genetic profile of GC and identifying new potential molecular targets. Recently, researchers have looked into Claudin-18.2-targeted therapy, fibroblast growth receptor (FGFR) pathway inhibitors, and EGFR inhibitors as effective targeted therapies to treat advanced GC (Table 5 ). Although emerging innovative drugs have made remarkable progress in GC treatments, extensive clinical explorations are needed to advance precision medicine.
CLAUDIN 18.2-targeted therapy
Claudin 18.2 (CLDN18.2), a component of intercellular junctions [ 155 ], is exclusively detected in gastric mucosa and absent from other healthy tissues. Upon malignant transformation, CLDN18.2 expression can be retained in various tumor tissues, including G/GEJ cancer and especially diffuse-type GC [ 156 ]. The prevalence of CLDN18.2 overexpression in GC varies wildly among studies ranging from 14.1% to 72% [ 157 , 158 , 159 ].
Zolbetuximab is a chimeric IgG1 monoclonal antibody that binds to CLDN18.2 and induces antibody-dependent and complement-dependent cytotoxicity [ 160 ]. To date, zolbetuximab has shown great potential to become a valuable target in GC. In the phase II MONO study, single-agent zolbetuximab achieved an ORR of 9% and a disease control rate of 23% in 43 patients with previously treated oesophageal or G/GEJ cancers [ 161 ]. A randomized phase II study (FAST) indicated that zolbetuximab plus first-line chemotherapy significantly improved PFS and OS in patients with CLDN18.2-positive G/GEJ cancer [ 159 ]. Subgroup analysis indicated a correlation between moderate-to-strong CLDN18.2 expression and a better overall survival rate. In the phase III SPOTLIGHT trial, zolbetuximab plus mFOLFOX6 significantly improved mPFS (10.61 vs 8.67 months, HR 0.751, P = 0.0066) and mOS (18.23 vs 15.54 months, HR 0.750, P = 0.0053) in patients with CLDN18.2-positive and HER-2-negative advanced G/GEJ cancer[ 162 ].
GLOW (NCT03653507) is another phase III trial investigating zolbetuximab plus CAPOX as first-line treatment in patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric or GEJ cancer. In this study, zolbetuximab plus CAPOX showed a significant improvement in mPFS (8.21 vs 6.80 months, HR 0.687, P = 0.0007) and mOS (14.39 vs 12.16 months, HR 0.771, P = 0.0118) compared to placebo plus CAPOX[ 163 ]. Additionally, zolbetuximab is also being studied in combination with immunotherapy in patients with CLDN18.2-positive advanced gastric or GEJ cancer in the ILUSTRO study (NCT03505320).
Another promising therapeutic approach targeting CLDN18.2 employs CLDN18.2-specific chimeric antigen receptor (CAR) T cells. CLDN18.2-specific CAR T cells achieved partial or complete tumor regression in CLDN18.2-positive PDX models [ 164 ]. A phase I study of CLDN18.2-specific CAR T cells in gastrointestinal cancers conducted by Prof. Shen Lin's team demonstrated that in GC patients, the ORR and DCR were 57.1% and 75.0%, respectively, and the 6-month overall survival rate was 81.2% [ 165 ]. Claudin 18.2 served as a new target for the later-line treatment of GC, with considerable ORR improvement achieved in Claudin 18.2 CAR-T therapy, which has become a hallmark event for cellular immunotherapy in solid tumors. Currently, several new drugs focusing on Claudin 18.2, such as Claudin 18.2 bispecific antibodies (Claudin 18.2/CD3, Claudin 18.2/PD-L1) and ADC analogs, are being developed. Although these drugs have not been approved for clinical applications, some of them showed promising preclinical data and are being widely studied in different clinical trials. Since Claudin 18.2 is also expressed on the normal gastric mucosal epithelial surface, the risk of adverse reactions and whether ADC drugs may aggravate normal mucosal damage should also be a concern.
FGFR-targeted therapy
FGFR1 mutations, FGFR2 amplifications, and FGFR3 rearrangements are the most common FGFR alterations in GC [ 166 ]. Different types of FGFR targeting agents were explored or developed in GC, including multikinase inhibitors, pan-FGFR inhibitors, FGFR1-3 inhibitors, selective FGFR inhibitors and ADC. Nevertheless, most multikinase inhibitor studies were preclinical or single case reports in GC without robust clinical evidence [ 167 ]. Futibatinib, an irreversible and highly selective FGFR1–4 inhibitor that permanently disables FGFR2, has been tested in a phase II trial involving patients with advanced-stage solid tumors harboring FGFR alterations, including those with FGFR2-amplified G/GEJ cancers [ 168 ]. Although the ORR was reported to be 22.2% in the GC cohort [ 169 ], more data are needed to support the efficacy of multiple FGFR inhibitors in different FGFR gene alterations in GC.
Currently, bemarituzumab has shown some promising results in the treatment of mGC [ 170 ]. It is a first-in-class afucosylated monoclonal antibody against the FGFR2b splice variant frequently overexpressed in FGFR2- amplified G/GEJ cancers. In a phase I trial, 17.9% of patients with FGFR2 amplifications had a confirmed response to bemarituzumab [ 171 ]. Based on the safety and activity profile of bemarituzumab monotherapy in GC, the phase II FIGHT trial was designed to evaluate the efficacy of bemarituzumab plus mFOLFOX6 regimen in previously untreated, FGFR2b-overexpressing advanced-stage G/GEJ cancers [ 172 ]. The trial showed a 2-month improvement in PFS, and the OS was not reached (NR) in the experimental arm (bemarituzumab + mFOLFOX6). However, the experimental arm had a higher incidence of adverse events than the control chemotherapy arm, particularly in regard to ocular toxicity.
EGFR-targeted therapy
Approximately 5–10% of patients with G/GEJ cancers have EGFR amplifications or EGFR overexpression, both of which are associated with poor prognosis [ 173 ]. Previous large randomized clinical trials have failed to demonstrate any significant survival benefit with EGFR-targeted agents [ 92 , 174 ], perhaps because most of the studies were performed in unselected patient populations regardless of EGFR status. Besides, biomarker analysis of the EXPAND and COG trials suggests activity in patients with tumors expressing high levels of EGFR, thus supporting the significance of patient selection for future trials [ 175 , 176 ]. In a prospective cohort, patients with metastatic gastroesophageal adenocarcinoma were screened for EGFR amplification and subsequently treated with anti-EGFR therapy (cetuximab). The ORR was 58% (4 of 7 patients), and the DCR was 100% (7 of 7 patients), implying that EGFR inhibition should be further studied in selected patients [ 177 ]. Many of the ongoing EGFR inhibitor studies should test EGFR alterations in the GC patients prior to enrollment to overcome resistance to EGFR-targeted therapies.
MET/HGF pathway inhibitors
c-Mesenchymal-Epithelial Transition (c-MET) is a tyrosine kinase receptor from MET families, and hepatocyte growth factor (HGF) is the common ligand to c-MET [ 178 ]. MET/HGF pathway activation is associated with tumor invasiveness and poor disease prognosis. The anti-MET monoclonal antibody, onartuzumab, has been studied in a phase III trial of onartuzumab plus mFOLFOX6 vs placebo plus mFOLFOX6 in patients with metastatic HER2-negative G/GEJ cancers. However, the addition of onartuzumab to mFOLFOX6 did not improve clinical outcomes in the ITT population or in the MET-positive population [ 179 ]. Rilotumumab is a humanized monoclonal antibody targeting HGF. Two phase III trials (RILOMET-1 and RILOMET-2) investigated rilotumumab plus chemotherapy in advanced MET-positive G/GEJ cancers. Unfortunately, both studies were terminated due to increased number of deaths in the rilotumumab group[ 180 , 181 ]. Additionally, several selective/non-selective c-MET TKIs, such as tinvatinib, AMG 337 and foretinib, have also been tested in MET-positive GC, but no significant benefit was seen in clinical trials[ 182 , 182 , 184 ].
Challenges and future perspectives
Even though substantial advances have been made in the treatment of GC, further research and development are still necessary. Improving early detection, reducing recurrence and optimizing treatment strategies are the primary challenges and prospects for GC management. To increase GC early detection and promote patients’ overall survival, endoscopic screening programs should be implemented in high-risk regions, and more precise early detection technologies are of great value. In a previous study, we demonstrated an artificial intelligence (AI) diagnostic platform, GRAIDS, to detect upper gastrointestinal cancers using real-world endoscopic imaging data from six Chinese hospitals with varying experience in the endoscopic diagnosis of upper gastrointestinal cancer [ 185 ]. GRAIDS provided both real-time and retrospective assistance for enhancing the effectiveness of upper gastrointestinal cancer screening and diagnosis, with high diagnostic accuracy and sensitivity in detecting upper gastrointestinal cancers. In the near future, the AI system will help many physicians in community-based hospitals identify upper gastrointestinal cancers more efficiently and accurately [ 186 ].
In addition, recurrence of GC remains common despite the multimodality treatment, so many studies in progress aim to identify individuals at risk of recurrence after treatment. Circulating tumor DNA (ctDNA) can be detected in the circulation of cancer patients and has the potential to predict minimal residual disease [ 187 ]. Liquid biopsies can detect a broader spectrum of abnormalities in a heterogeneous tumor compared to conventional tissue biopsies. According to a study investigating perioperative therapies in patients in the CRITICS trial with resectable GC, the presence of ctDNA could predict recurrence when analyzed within nine weeks after preoperative treatment and after surgery in patients eligible for multimodal treatment [ 187 ]. These findings highlight the significance of ctDNA as a biomarker for predicting patient outcomes following perioperative cancer treatment and surgical resection in patients with GC. In another 1630-patient cohort of ctDNA results, genomic alterations were correlated with clinicopathologic characteristics and outcomes and provided prognostic and predictive information [ 188 ]. As for advanced GC, ctDNA also serves as a potential biomarker of immunotherapy response, and its potential role in predicting irAEs is worth further investigation [ 189 ]. Further research aimed at prospectively collecting ctDNA is needed to confirm these findings. The existence of persistent ctDNA following curative-intent treatment of GC may indicate minimal residual disease, and trials are underway to determine whether additional adjuvant therapy can result in the clearance of ctDNA.
Intratumoral, intrapatient, and interpatient heterogeneity in GC is the major barrier to drug development for systemic therapies. Most GC patients are not susceptible to immune checkpoint inhibitor monotherapies. Thus, one of the major challenges in systemic treatments for GC is overcoming resistance to ICI therapy. One strategy is to develop novel ICIs with better efficacy. Recently, many novel immune checkpoint modulators have been widely investigated, including LAG-3, VISTA, TIM-3, TIGIT, CD38, CD39, and CD73[ 190 ]. Another key strategy is combining ICI and other therapies, such as other ICI, targeted therapies, other immune-modulating agents, chemotherapy (as discussed above), and radiotherapy [ 191 ]. As mentioned above, in the CheckMate-649 study, the combination of anti-PD-1 and anti-CTLA-4 agents (nivolumab plus ipilimumab) failed to improve treatment outcomes compared to traditional chemotherapy [ 57 ]. In the EPOC1706 study, lenvatinib, an anti-angiogenic multiple receptor tyrosine kinase inhibitor, combined with pembrolizumab showed an exciting activity with an ORR of 69% in the first-line and second-line treatment of advanced GC[ 151 ]. ICI combined with other anti-immunosuppressive factor agents, such as anti-transforming growth factor-β (TGF-β), is also being investigated in clinical trials (NCT04856774). To fully understand the mechanism of resistance to immunotherapy, factors such as epigenetics, metabolism, immune suppression, and microbiota must be considered. Therefore, the development of combined therapies should be based on understanding the underlying mechanisms of immune modulation and resistance, rather than simply combining available therapies in a haphazard manner.
Rapid developments are ongoing in the clinical use of ADCs and are now considered one of the current hot spots for antitumor drug development. In particular, ADCs have emerged as a new era of targeted therapy in the field of GC treatment. The latest generation of ADCs has expanded the treatment population to include novel targets and demonstrated superior clinical outcomes compared to traditional chemotherapy drugs. Nevertheless, certain aspects of ADCs remain to be addressed. Firstly, it is necessary to explore ways to advance ADCs as first-line therapy to benefit a larger number of patients. Secondly, to make better use of medical resources, a more differentiated target layout needs to be established, moving beyond the focus on distinct targets such as HER2. To address these challenges, optimization of the toxin, linker and toxicity of ADCs is essential, along with the development of ADC-combination therapies to improve efficacy. We anticipate the discovery of more potential ADC drugs and expect a breakthrough in first-line treatment.
Currently, many clinical trials have complex treatment regimens, including mono-immunotherapy, double-checkpoint inhibitors, anti-angiogenic drugs, and biomarker-directed therapies [ 190 , 192 ]. However, the challenge of determining the optimal treatment strategy and the appropriate timing of molecular biomarker screening has yet to be resolved. We expect that extensive translational research, preclinical investigations, and multi-omics-based clinical trials will lead to breakthroughs in the diagnosis and treatment of GC. Therefore, we eagerly anticipate future studies that have the potential to improve clinical practice in the coming years.
Availability of data and materials
Not applicable.
Abbreviations
Antibody–drug conjugates
Antibody-dependent cell-mediated cytotoxicity
Advanced gastro-oesophageal cancer
Artificial intelligence
American Society of Clinical Oncology
Bispecific antibodies
Best supportive care
Capecitabine and oxaliplatin
Chimeric antigen receptor
Cisplatin and fluorouracil
China Food and Drug Administration
Confidence interval
Claudin18.2
Combined positive score
Chemoradiotherapy
Chinese Society for Clinical Oncology
Circulating tumor DNA
Cytotoxic T lymphocyte antigen-4
Disease control rate
Disease-free survival
Mismatch-repair deficiency
Duration of response
Docetaxel, oxaliplatin, and S-1
Epstein-Barr virus
Epstein-Barr virus-associated gastric cancer
Extracellular domain
Epirubicin, cisplatin, and fluorouracil
Event-free survival
Epidermal growth factor receptor
Epirubicin and oxaliplatin
Erythroblastic leukemia viral oncogene homolog
Extracellular regulated protein kinase
European Society for Medical Oncology
Food and Drug Administration
Fibroblast growth factor receptor
Fluorescent in situ hybridization
Fluorouracil, leucovorin, oxaliplatin, and docetaxel
Fluorouracil, leucovorin, and oxaliplatin
Fluorouracil and cisplatin
- Gastric cancer
Gastroesophageal junction adenocarcinoma
Gastroesophageal junction
Human epidermal growth factor receptor 2
Hazard ratio
Immune checkpoint inhibitor
Immunohistochemistry
Kirsten rats sarcomaviral oncogene homolog
Lymphocyte-activation gene 3
Mitogen-activated protein kinase
Mesenchymal epithelial transition
Cytotoxin monomethyl auristatin E
Microsatellite instability
Mammalian target of rapamycin
National Comprehensive Cancer Network
Not evaluable
Natural killer
Objective response rate
Overall survival
Pathological complete response
Programmed cell death 1
Programmed cell death ligand 1
Progression-free survival
Phosphatidylinositol-3-kinase
Phosphatase and tensin homolog
Relapse-free survival
Recommended phase 2 dose
Receptor tyrosine kinase
S-1 and oxaliplatin
S-1, oxaliplatin and radiotherapy
S-1 and cisplatin
Tumor area positivity
The Cancer Genome Atlas
Trastuzumab emtansine
Trastuzumab deruxtecan
Transforming growth factor-β
T cell immunoreceptor with Ig and ITIM domain
T cell immunoglobulin and mucin domain 3
Tumor mutational burden
Vascular endothelial growth factor
V-domain Ig suppressor of T cell activation
Capecitabine and cisplatin
Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Article PubMed Google Scholar
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–62.
Article CAS PubMed Google Scholar
Tramacere I, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28–36.
Lordick F, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Lu L, et al. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). 2021;41(11):1137–51.
Pennathur A, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48.
Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
PubMed Google Scholar
Korfer J, Lordick F, Hacker UT. Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers (Basel), 2021;13(20).
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature, 2014;513(7517): 202–9.
Salem ME, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319–27.
Article CAS PubMed PubMed Central Google Scholar
Wang J, et al. Large-scale analysis of KMT2 mutations defines a distinctive molecular subset with treatment implication in gastric cancer. Oncogene. 2021;40(30):4894–905.
Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Chao J, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021;7(6):895–902.
Article PubMed PubMed Central Google Scholar
Nakamura Y, et al. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18(8):473–87.
Wang FH, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95.
Ajani JA, et al. Gastric cancer, version 2, 2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(2):167–92.
Article CAS Google Scholar
Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Ychou M, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
Al-Batran SE, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Kang YK, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39(26):2903–13.
Yoshida K, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Zhang X, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92.
Japanese Gastric Cancer, A.Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer, 2023;26(1): 1–25.
Bang YJ, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Noh SH, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96.
Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93.
Pietrantonio F, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37(35):3392–400.
Macdonald JS, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Lee J, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–73.
Park SH, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol. 2021;32(3):368–74.
Hofheinz RD, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149(6):1322–31.
Hofheinz RD, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trial of the AIO. J Clin Oncol. 2020;38(15_suppl):4502–4502.
Article Google Scholar
Rivera F, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67.
Wagner AD, et al. EORTC-1203-GITCG - the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19(1):494.
Cunningham D, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017;18(3):357–70.
Goetze TO, et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7) with high rate of signet cell component: final results of the multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J Clin Oncol. 2022;40(16_suppl):4042–4042.
Al-Batran S-E, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022;40(16_suppl):4003–4003.
Liu Y, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: updated results of efficacy and safety. J Clin Oncol. 2021;39(15_suppl):4036.
Li H, et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4050–4050.
Alcindor T, et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: preliminary results. J Clin Oncol. 2021;39(15_suppl):4046–4046.
Li S, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib, S-1 ± oxaliplatin for locally advanced cT4a/bN+ gastric cancer. J Clin Oncol. 2021;39(15_suppl):4061.
Wei J, et al. SHARED: Efficacy and safety of sintilimab in combination with concurrent chemoradiotherapy (cCRT) in patients with locally advanced gastric (G) or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4040–4040.
Bang YJ, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943–52.
Janjigian YY, et al. MATTERHORN: efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer—a randomized, double-blind, placebo-controlled, phase 3 study. J Clin Oncol. 2021;39(15):TPS4151
Andre T, et al. neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41(2):255–65.
Pietrantonio F, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41(4_suppl):358–358.
Xu R-H, et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: a randomized, phase 3 trial. J Clin Oncol. 2019;37(15_suppl):4017–4017.
Hall PS, et al. efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the go2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–77.
Shitara K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–87.
Shitara K, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48.
Shitara K, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80.
Wainberg ZA, et al. Pembrolizumab with or without chemotherapy versus chemotherapy alone for patients with PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma: Update from the phase 3 KEYNOTE-062 trial. J Clin Oncol. 2022;40(4_suppl):243–243.
Rha SY, et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;34(3):319–20.
Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Janjigian YY, et al. LBA7 Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study. Ann Oncol. 2021;32:S1329–30.
Kang YK, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47.
Xu J, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331.
Xu R-h, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. J Clin Oncol 2020;38(4_suppl): TPS458
Moehler MH, et al. Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 2023;41(4):286–286.
Moehler M, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–77.
Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5): e180013.
Kang YK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Bang YJ, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60.
Triulzi T, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. 2019;8(1): e1512942.
Varadan V, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperativetrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22(13):3249–59.
Chaganty BKR, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56.
Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142–7.
Takahari D, et al. A phase Ib study of nivolumab plus trastuzumab with S-1/capecitabine plus oxaliplatin for HER2-positive advanced gastric cancer (Ni-HIGH study): safety evaluation. J Clin Oncol. 2020;38(15_suppl): 4525
Rha SY, et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): Molecular profiling and clinical update. J Clin. Oncol.2021;39(3_suppl):218.
Janjigian YY, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31.
Janjigian YY, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30.
Guan WL, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: a multicenter analysis. Front Oncol. 2021;11: 712760.
Vanderwalde A, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–56.
Pietrantonio F, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6(1): 100036.
Huang SC, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145(12):3218–30.
Qiu MZ, et al. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer. 2020;146(1):272–80.
Derks S, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32.
Kim ST, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Sun YT, et al. PD-1 antibody camrelizumab for Epstein-Barr virus-positive metastatic gastric cancer: a single-arm, open-label, phase 2 trial. Am J Cancer Res. 2021;11(10):5006–15.
CAS PubMed PubMed Central Google Scholar
Xie T, et al. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43(4):139–44.
Mishima S, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7(1):24.
Shitara K, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Wainberg ZA, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score >/=10. Clin Cancer Res. 2021;27(7):1923–31.
Moehler MH, et al. First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): expanded efficacy and safety data from CheckMate 649. J Clin Oncol. 2021;39(15_suppl): 4002
Zhao JJ, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402.
Wang F, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479–86.
Shitara K, et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol. 2020;38(15_suppl): 4537
Marabelle A, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Van Cutsem E, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84.
Qin S, et al. Treatment patterns and outcomes in chinese patients with gastric cancer by HER2 status: a noninterventional registry study (EVIDENCE). Oncologist. 2021;26(9):e1567–80.
Tabernero J, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84.
Liu T, et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond). 2019;39(1):38.
Thuss-Patience PC, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Hecht JR, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol. 2016;34(5):443–51.
Satoh T, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Makiyama A, et al. Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J Clin Oncol. 2020;38(17):1919–27.
Yagi S, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22(3):518–25.
Nishida Y, et al. A novel gene-protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2015;18(3):458–66.
Lee HJ, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2-positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144(4):570–8.
Kim KC, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10):2833–40.
Haffner I, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. 2021;39(13):1468–78.
Palle J, et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15.
Park SR, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Seo S, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22(3):527–35.
Janjigian YY, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8(1):49–58.
Shen L. Liquid biopsy: a powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun (Lond). 2018;38(1):72.
Kwak EL, et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 2015;5(12):1271–81.
Kim J, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124(12):5145–58.
Kim ST, et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29(4):1037–48.
Lee JY, et al. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci Rep. 2015;5:9289.
Sanchez-Vega F, et al. EGFR and MET amplifications determine response to HER2 Inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019;9(2):199–209.
Wang DS, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68(7):1152–61.
Sampera A, et al. HER-family ligands promote acquired resistance to trastuzumab in gastric cancer. Mol Cancer Ther. 2019;18(11):2135–45.
Gambardella V, et al. NRF2 through RPS6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25(5):1639–49.
Nordstrom JL, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123.
Shinde A, et al. Can immunotherapy replace radiotherapy in melanoma brain metastases? J Clin Oncol. 2019;37(12):1030–1.
Bang YJ, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61.
Catenacci DVT, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76.
Catenacci DVT, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7(5)
Ku G, et al. 1380P Phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. 2021;32:S1044–5.
Tabernero J, et al. HERIZON-GEA-01: Zanidatamab + chemo +/- tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18(29):3255–66.
Zhang J, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I Study. Clin Cancer Res. 2022;28(4):618–28.
Xu J, et al. A phase II study evaluating KN026 monotherapy in patients (pts) with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancers (GC/GEJC). J Clin Oncol. 2022;40(16_suppl):4040–4040.
Shen L, et al. 1210P The preliminary efficacy and safety of KN026 combined with KN046 treatment in HER2-positive locally advanced unresectable or metastatic gastric/gastroesophageal junction cancer without prior systemic treatment in a phase II study. Ann Oncol. 2022;33:S1102.
Piha-Paul S, et al. O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J Immunother Cancer. 2020;8(Suppl 1):A1.
Google Scholar
Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14(1):20.
Ogitani Y, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Yamaguchi K, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II Trial. J Clin Oncol. 2023;41(4):816–25.
Ku GY, et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol. 2022;33:S1100.
Dai L, et al. Efficacy of disitamab vedotin in treating HER2 2+/FISH- gastric cancer. Onco Targets Ther. 2022;15:267–75.
Xu Y, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25.
Peng Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41(11):1173–82.
Zhang Y, et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep Med. 2022;3(11): 100814.
Kulukian A, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Catenacci DVT, et al. MOUNTAINEER-02: Phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—Trial in progress. J Clin Oncol. 2022;40(4_suppl):371.
Wu X, Huang S. HER2-specific chimeric antigen receptor-engineered natural killer cells combined with apatinib for the treatment of gastric cancer. Bull Cancer. 2019;106(11):946–58.
Smyth EC, et al. Gastric cancer. Lancet. 2020;396(10251):635–48.
Casak SJ, et al. FDA approval summary: ramucirumab for gastric cancer. Clin Cancer Res. 2015;21(15):3372–6.
Fuchs CS, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
Wilke H, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Xu RH, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1015–24.
Fuchs CS, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–76.
Pavlakis N, et al. INTEGRATE IIa: a randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—a study led by the Australasian Gastro-intestinal Trials Group (AGITG). J Clin. Oncol. 2023;41(4):LBA294.
Fukuoka S, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61.
Kawazoe A, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057–65.
Li J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54.
Kang YK, et al. Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed & 2 prior chemotherapy regimens. Ann Oncol. 2019;30:v877–8.
Zhang Y, et al. A phase Ib/II study of fruquintinib in combination with paclitaxel as the second-line therapy for advanced gastric cancer. Cancer Commun (Lond), 2022.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021
Sahin U, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624–34.
Hong JY, et al. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. 2020;9(5):3367–74.
Rohde C, et al. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–6.
Sahin U, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609–19.
Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10(1):105.
Tureci O, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30(9):1487–95.
Shitara K, et al. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) withclaudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: primary results from phase 3 SPOTLIGHT study. J Clin Oncol 2023;41(4_suppl): LBA292
Shitara K, et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol. 2023;41(36_suppl):405736–405736.
Jiang H, et al. Claudin182-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111(4):409–18.
Qi C, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189–98.
Helsten T, Schwaederle M, Kurzrock R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 2015;34(3):479–96.
Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J Hematol Oncol. 2021;14(1):23.
Bahleda R, et al. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann Oncol. 2020;31(10):1405–12.
Meric-Bernstam F, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12(2):402–15.
Lengyel CG, et al. FGFR Pathway Inhibition in Gastric Cancer: The Golden Era of an Old Target? Life (Basel), 2022;12(1)
Catenacci DVT, et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J Clin Oncol. 2020;38(21):2418–26.
Catenacci DVT, et al. FIGHT: a randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). J Clin Oncol. 2021;39(15_suppl):4010–4010.
Nagatsuma AK, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18(2):227–38.
Dutton SJ, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904.
Petty RD, et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279–87.
Lordick F, et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. J Clin Oncol. 2013;31(15_suppl):4021–4021.
Maron SB, et al. Targeted therapies for targeted populations: anti-EGFR Treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696–713.
Raj S, et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol Cancer. 2022;21(1):31.
Shah MA, et al. A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist. 2016;21(9):1085–90.
Catenacci DVT, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–82.
Doi T, et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the RILOMET-2 trial. J Clin Oncol. 2015;33(3_suppl): TPS226
Kang YK, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32(2):355–61.
Hong DS, et al. Phase I Study of AMG 337, a highly selective small-molecule MET inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2019;25(8):2403–13.
Shah MA, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS ONE. 2013;8(3): e54014.
Luo H, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20(12):1645–54.
Chen ZH, et al. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond). 2021;41(11):1100–15.
Leal A, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020;11(1):525.
Maron SB, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25(23):7098–112.
Jin Y, et al. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19(1):154.
Wang Y, et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol. 2022;15(1):111.
Upadhaya S, et al. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20(3):168–9.
Zhao H, et al. Emerging immunological strategies: recent advances and future directions. Front Med. 2021;15(6):805–28.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82203678 to Y.H.), the Science and Technology Program of Guangdong (Grant No. 2019B020227002 to R.-H.X.), the CAMS Innovation Fund for Medical Sciences (Grant No. 2019-I2M-5-036 to R.-H.X.).
Author information
Wen-Long Guan and Ye He have contributed equally.
Authors and Affiliations
Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China
Wen-Long Guan, Ye He & Rui-Hua Xu
Research Unit of Precision Diagnosis and Treatment for Gastrointestinal Cancer, Chinese Academy of Medical Sciences, Guangzhou, 510060, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Rui-Hua Xu designed this review. Rui-Hua Xu, Wen-Long Guan, and Ye He drafted the manuscript and prepared the figures. Rui-Hua Xu, Wen-Long Guan, and Ye He collected the related references and participated in the discussion. All authors contributed to this manuscript and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rui-Hua Xu .
Ethics declarations
Ethical approval and consent to participate, competing interests.
All authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guan, WL., He, Y. & Xu, RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 16 , 57 (2023). https://doi.org/10.1186/s13045-023-01451-3
Download citation
Received : 13 March 2023
Accepted : 10 May 2023
Published : 27 May 2023
DOI : https://doi.org/10.1186/s13045-023-01451-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- Targeted therapy
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
Stomach Cancer Research
In a recent study, more than 90% of people who’d had their stomach surgically removed to prevent cancer experienced a least one chronic complication 2 years out from their surgery. For some, the complications are life altering.
FDA has changed its 2021 approval of pembrolizumab (Keytruda) along with trastuzumab (Herceptin) and chemotherapy for treating HER2-positive stomach or GEJ cancer. The agency also announced a new approval of pembrolizumab for HER2-negative forms of these same cancers.
For some people with advanced stomach cancer, the drug nivolumab (Opdivo) plus chemotherapy may improve how long they live, results from a large clinical trial show. The trial also included patients with gastric cancers that involve the esophagus.
A type of cancer that occurs in the lower stomach has been increasing among some Americans under the age of 50, even though in the general population the incidence of all stomach cancers has been declining for decades, according to a new study.
The FDA has granted accelerated approval to the immunotherapy drug pembrolizumab (Keytruda®) for use in patients with advanced gastric (stomach) cancer that is PD-L1 positive.
Stomach cancers fall into four distinct molecular subtypes, researchers with The Cancer Genome Atlas (TCGA) Network have found. Scientists report that this discovery could change how researchers think about developing treatments for stomach cancer, also called gastric cancers or gastric adenocarcinomas.
- Open access
- Published: 07 May 2024
Comprehensive analysis of T-cell regulatory factors and tumor immune microenvironment in stomach adenocarcinoma
- Shuchang Wang 1 na1 ,
- Weifeng Zhang 2 na1 ,
- Xinrui Wu 3 na1 ,
- Zhu Zhu 4 ,
- Yuanbiao Chen 5 ,
- Wangrui Liu 6 ,
- Junnfei Xu 7 ,
- Li Chen 3 , 8 &
- Chun Zhuang 1
BMC Cancer volume 24 , Article number: 570 ( 2024 ) Cite this article
19 Accesses
Metrics details
Gastric cancer (GC) is one of the most prevalent malignant tumors worldwide and is associated with high morbidity and mortality rates. However, the specific biomarkers used to predict the postoperative prognosis of patients with gastric cancer remain unknown. Recent research has shown that the tumor microenvironment (TME) has an increasingly positive effect on anti-tumor activity. This study aims to build signatures to study the effect of certain genes on gastric cancer.
Expression profiles of 37 T cell-related genes and their TME characteristics were comprehensively analyzed. A risk signature was constructed and validated based on the screened T cell-related genes, and the roles of hub genes in GC were experimentally validated.
A novel T cell-related gene signature was constructed based on CD5, ABCA8, SERPINE2, ESM1, SERPINA5, and NMU. The high-risk group indicated lower overall survival (OS), poorer immune efficacy, and higher drug resistance, with SERPINE2 promoting GC cell proliferation, according to experiments. SERPINE2 and CXCL12 were significantly correlated, indicating poor OS via the Youjiang cohort.
Conclusions
This study identified T cell-related genes in patients with stomach adenocarcinoma (STAD) for prognosis estimation and proposed potential immunotherapeutic targets for STAD.
Peer Review reports
As one of the most prevalent malignant tumors worldwide, gastric cancer (GC) has high morbidity and mortality rates, with stomach adenocarcinoma (STAD) as one of the major pathological subtypes [ 1 ]. STAD accounts for 80–90% of all gastric cases [ 2 ]. The 5-year survival rate of patients with STAD with advanced or metastatic disease is less than 30% [ 3 , 4 ]. Most patients worldwide are not diagnosed until the advanced stage owing to the absence of significant early symptoms and limitations in medical screening [ 5 , 6 ]. Therefore, the accurate prediction of biomarkers is crucial for clinical prognostication and treatment. Currently, surgical resection is the primary treatment for STAD [ 7 ]. However, because of the recurrence and metastasis of STAD after surgery, its prognosis is poor [ 8 ]. Immunoassay inhibitors of PD-L1 have positive effects on immunotherapy and targeted therapy, and they can significantly improve the prognosis of patients with stable microsatellite carcinomas [ 9 ].
Increasing evidence suggests that the tumor microenvironment (TME) plays a role in anti-tumor activity and contributes to the prediction of immune checkpoint blockade (ICB) responses [ 10 , 11 , 12 , 13 ]. However, gaining advantages from STAD is not common [ 14 ]. The TME comprises tumor cells, fibroblasts, extracellular matrix elements, and diffusing cytokines [ 15 , 16 ]. Growing tumor cells can be recognized and destroyed by tumor-infiltrating immune cells. This defensive behavior may involve inflammation as tumor cells evade immune defense mechanisms by selecting more aggressive tumor clones [ 17 ]. High levels of regulatory T cells are commonly found in patients with cancer and are associated with the prognosis of different types of STAD [ 18 ].
Furthermore, treatment is influenced by T-cell therapy [ 19 ]. However, these cellular treatments need to be improved further to improve the cure rate. To date, studies on T-cell function have mainly focused on the negative regulatory factors contributing to functional deficiency [ 20 ]. The US Food and Drug Administration (FDA) approved the first chimeric antigen receptor T-cell (CAR-T) therapy [ 21 ]. Nevertheless, the therapeutic effect of the chimeric antigen receptor on STAD did not meet expectations because of the failure of T cells to perform their effector function within the TME fully. Positive regulators reportedly have a positive effect on T-cell proliferation, activation, and secretion of key cytokines, thereby optimizing and improving T-cell function in STAD [ 22 ], that may further improve the treatment of STAD.
In this work, we aim to build signatures to study the effect of certain genes on gastric cancer using LASSO and multivariate Cox regression analyses. Our findings underscore the significance of considering tumor-infiltrating immune cells and their interactions with the TME in prognostic assessment and treatment planning. Also, the identification of potential therapeutic targets such as CXCL12 and SERPINE2 presents opportunities for developing targeted interventions aimed at mitigating immune exhaustion and enhancing treatment efficacy.
Materials and methods
Stad data source and preprocessing.
Data on STAD-related gene expression, prognosis, and clinicopathological characteristics were collected from comprehensive GEO (GSE38749, GSE84437, GSE34942, and GSE15459; https://www.ncbi.nlm.nih.gov/geo/ ) and TCGA ( https://portal.gdc.cancer.gov/ ) datasets. Following this, the FPKM values were converted to TPM values within the composite matrix. The 37 T cell-related genes were derived from the latest research [ 22 ]. Ten tumors and ten normal single-cell ribonucleic acid (scRNA) samples were obtained from GSE18394.
WGCNA co-expression network construction
The co-representation network of differentially expressed genes (DEG) is built by the “WGCNA”package in the R package (version xx; The R Foundation for Statistical Computing, Vienna, Austria). Subsequently, pairs of genes were subjected to Pearson’s correlation matrix analysis.
To further analyze sample clustering for outliers detection, we calculated the similarity of the characteristic genes within each module, selected the standard tangent value from the module tree, and merged specific modules. The module-feature relationship between modular feature genes and T cells was described within the characteristic gene network, and two modules related to specific traits were found.
DEGs identification and enrichment analyses
The parameter of “limma”in the R package (The R Foundation for Statistical Computing, Vienna, Austria) was set to 1.5, and the P-value was adjusted to be less than 0.05 to screen DEGs across different T-cell clusters. A total of 178 genes were identified as DEGs. Moreover, disease ontology (DO), gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were used to explore the characteristics of the DEGs [ 23 , 24 , 25 ].
Construction of prognostic T cell-related signature
The signature score was calculated to express the properties of each cell type, and DEGs were identified from T-cell clusters using LASSO and multivariate Cox regression analyses. Univariate Cox regression, overall survival (OS) ( p < 0.05), and heatmap analysis were used to verify the signature validity. The GSE38749, GSE84437, GSE34942, GSE15459, and TCGA-STAD cohorts were segmented into training ( n = 522) and test ( n = 521) datasets at a ratio of 1:1, and the former was used to establish risk characteristics. We analyzed the various trajectories of each variable and identified candidate genes using multivariate Cox analysis. The risk score was calculated using the following formula:
Risk score = Σ(Expi × Coefi).
Analysis of TME, immunological checkpoint, mutation, and drug susceptibility
Based on the data from TCIA and TIDE ( http://tide.dfci.harvard.edu/ ), a boxplot was used to visualize the differences between the two groups in the expression levels and treatment diversity of the PD-1 and CTLA-4 immunological checkpoints. Additionally, we examined drug susceptibility and mutation status in the high- and low-risk groups using the prophetic and maftools packages, respectively.
Validation of external cohort
Survival analysis was conducted on the external IMV210 cohort and GSE62254 for validation. This involved analyzing the binary response in risk scores among CR/PR and SD/PD, as well as Kaplan–Meier survival analysis between high-risk and low-risk score groups. For the scRNA data, we created a project using the Seurat package and set the following screening criteria: (1) Each gene was expressed in at least three cells; (2) The total number of molecules detected in each cell was > 1000; (3) The number of genes detected in each cell was > 200 and < 10,000; (4) The proportion of mitochondrial and ribosomal genes was < 20%. The first 2000 highly variable genes were selected for scale analysis, and PCA dimensionality reduction analysis was performed. Twenty PC were selected for unsupervised clustering and labeled according to the marker genes of different cell types. Four methods, Ucell, singscore, ssgsea, and addmoduleScore, were used to score the TME of both normal and tumor tissues. The scores obtained from the UCell, irGSEA, and GSVA packages were utilized to explore the differential expression of SEPRINE2 in T cells from normal and tumor groups.
Youjiang cohort and Immunohistochemical
OS with high and low SEPRINE2 expression in GC was verified using the Youjiang cohort, and immunohistochemistry was conducted to test the content of high or low-expression SEPRINE2 samples in formalin/PFA-fixed paraffin-embedded sections ( n = 93). Human GC tissues were stained for SERPINE2/PN-1 using ab154591 at a dilution of 1/500.
Cell culture and transfection
Human GC cell lines AGS and BGC-823 were purchased from the Shanghai Institutes of Biological Sciences (Shanghai, China). AGS and BGC-823 cells were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 1% penicillin-streptomycin (Gibco, USA) and 10% fetal bovine serum (Gibco, USA). BGC-823 cells were maintained in Dulbecco’s modified DMEM medium (Gibco, USA) supplemented with 1% penicillin-streptomycin (Gibco, USA) and 10% fetal bovine serum (Gibco, USA). All the cells were cultured at 37 °C and 5% CO2. Cell transfections were performed using Lipofectamine 3000 (Invitrogen, USA), with oligonucleotides as a control. After 48 h of transfection, cellular RNA and proteins were extracted.
Cell migration assays and proliferation
Stably transfected AGS and BGC-823 cells were seeded in 96-well plates at a density of 5 × 104 cells/mL. A Cell Counting Kit-8 (Dojindo, Japan) was used to test cellular proliferative capacity. On each of the subsequent 6 days, the optical density was evaluated at 450 nm using a microplate reader (TEAN, Switzerland). Additionally, a transwell migratory assay was conducted to study the migratory response of AGS and BGC-823. The cell density was standardized to 2 × 105 cells/mL, and a volume of 100 µL cell suspension was added to the upper chamber. Medium containing 20% fetal bovine serum was added to the lower chamber. After 24 h, AGS and BGC-823 cells within the lower chamber were washed in 4% polyoxymethylene for 15 min, followed by staining with 0.1% crystal violet and subsequent rinsing with deionized water for 30 min. Finally, the cells were counted under a microscope.
Statistical analyses
R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria) ( https://www.r-project.org/ ) was applied for all analyses, and statistical significance was set at p < 0.05.
Landscape of T cell-related gene modification in STAD
Among the included 37 T cell-related genes, the copy number variations (CNV) were investigated (Fig. 1 a). Notably, significant increases in CNV were observed in genes such as LIG3, ZNF830, ATF6B, CLIC1, and DUPD1, while decreases were most obvious in HOMER1, DCLRE1B, B2M, MRPL51, and LTBR. The distribution profile of CNV modification in the 37 T cell-related genes is illustrated in Fig. 1 b. To investigate the association between genes and their interactions in STAD studies, we generated a T cell-related co-expression visualization protein-protein interaction (PPI) network (Fig. 1 c).
Genetic alterations and tumor microenvironment (TME) of T-cell related genes in stomach adenocarcinoma (STAD). a Frequencies of copy number variations (CNV) gain, loss, and non-CNV among T-cell related genes. b. Locations of CNV alterations in T-cell related genes on 23 pairs of chromosomes. c. The interaction between 37 T-cell related genes in STAD. d. Correlation matrix for all 22 immune cell proportions. The darker the colour, the higher the correlation was. e. Heatmap of the 22 immune cell proportions. f. Module − trait relationships between module eigengenes and T-cells
Distribution of immune infiltration in STAD
The proportions of each T-cell subset were not significantly correlated, as shown in Fig. 1 d. The population quantities with remarkably positive relevance were CD8 + T cells and CD4 + memory-activated T cells (0.5), activated neutrophils and mast cells (0.45), and resting mast cells and activated natural killer cells (0.36). As shown in Fig. 1 e, the levels of macrophage M1, T-cell follicular helper, macrophage M0, and T-cell CD4 memory activation were comparatively high in the tumor samples contained in the heatmap. In the PPI network, key gene pathways such as B2M and HLA have multiple functions and play a central role in co-expression and physical interactions.
WGCNA co-expression analysis of STAD samples
The intercept value was set to 76 to detect outliers, the outlier samples were removed, and the remaining samples were included in the analysis (Additional figure S1 a). As shown in Additional figure S1 b, when power = 5, the scale independence was 0.9, and the mean connectivity was relatively high. Power = 5 was set to build the co-expression module and obtain the result of the preliminary module division. Different modules were represented in different colors in line with the results of WGCNA (Additional figure S1 c). To detect outliers, a tree was built using the eigenvalues of the module and very close distance module merging, with the intercept value set to 0.5 (Additonal figure S1 d). As shown in Additional figure S1 e, a co-expression module was constructed, and the results were obtained after merging similar modules. According to the characteristic value of each sample in each module, correlation analysis was carried out to find out two modules related to specific traits (infiltrated immune cells). Among the seven modules, the green module was highly correlated to T cells CD8 (CD8 + T cells) (R2 = 0.3; p = 2e-08) and activated T cells CD4 memory (R2 = 0.24; p = 5e-06). Furthermore, the green-yellow module showed a higher correlation with activated T cells CD4 memory (R2 = 0.34; p = 8e-11) and T cells CD8 (CD8 + T cells) (R2 = 0.29; p = 6e-08; Fig. 1 f).
Screening genes and survival analysis
From the chart, we identified five genes (IFNL2, IL12B, B2M, HLA-A, and CD19) that were selected from the intersection of the T-cell regulatory factor and the WGCNA gene (Fig. 2 a), with only four (IL12B, B2M, HLA-A, and CD19) expressed in selected samples. Among the T-cell regulatory factors, normal and tumor genes showed prominently distinct Inter-cell Interference Coordination (ICIC), with tumor gene expression higher than normal in RAN, CHK1, and CDK2 cells (Fig. 2 b). Prognostic analysis indicated a significant survival advantage for CD19 and IL12B (Fig. 2 c, d). The distribution of genes encoding T-cell regulatory factors is shown in Fig. 2 e. As shown in Fig. 2 f, B2M and HLA − A exhibited a high correlation, as did IL12B and CD19.
Screening genes and survival analysis. (a) VEEN diagram results. (b) Box plot demonstrating the immune cell-infiltrating characteristics of T-cell regulatory factor. (c) Survival analysis of genes. (d) The interaction between 4 genes in T-cell regulatory factor. (e) Correlation of genes
NMF clustering based on related genes in STAD samples
In the union of the GSE38749, GSE84437, GSE34942, GSE15459, and TCGA-STAD cohorts, the STAD samples were grouped into diverse molecular subtypes using NMF analysis (Fig. 3 a and b). We designated these two clusters as T cell-related genes: Clusters C1 and C2. Cluster C1 displayed marked indigenous survival advantages (Fig. 3 c). Furthermore, these two clusters also displayed notably diverse enrichment characteristics of the KEGG pathway in GSVA enrichment analysis. For instance, in KEGG intestinal immune network for IgA production, the KEGG T-cell receptor signaling pathway and KEGG B cell receptor signaling pathway had high-level gene expression in C1 and low-level gene expression in C2, while KEGG basal transcription factors had high expression levels in C2 and low expression levels in C1 (Fig. 3 d). These two T-cell clusters also displayed marked distinctions in ICIC (Fig. 3 e). Activated CD8 T-cells, activated B cells, and macrophages were amplified in cluster C1, suggesting that they could promote inflammation, while activated CD4 T-cells, type 17 T-helper cells, and neutrophils were abundant in another cluster. The results indicated that the expression of major histocompatibility complex type 1 (MHC-1) in the C1 cluster was higher than that in the other clusters, which may be one of the reasons why the survival rate of CI was higher than that of the other clusters. As shown in Fig. 3 f, the genes were divided into two clusters, C1 and C2, proving that the two groups clustered well.
Clinicopathological and biological characteristics of two distinct T-cell clusters of samples from the combination of GSE38749, GSE84437 and The Cancer Genome Atlas-analysis of stomach adenocarcinoma (TCGA-STAD) cohorts, divided by Non-negative Matrix Factorization (NMF) analysis. (a) NMF analysis heatmap defining two clusters (k = 2) and their correlation area. (b) NMF rank survey performed on the two T-cell clusters. (c) Survival analysis of the two distinct clusters. (d) Heatmap of gene set variation analysis(GSVA) enrichment analysis. (e) Box plot demonstrating the immune cell-infiltrating characteristics of the two clusters. (f) principal components analysis(PCA) of two clusters
GO, KEGG, and DO enrichment analyses of clusters
A total of 178 genes with an absolute value of LogFC greater than 1.5 and p < 0.05 were obtained by analyzing the differences between the C1 and C2 groups. To investigate the association between T cell-related genes and other illnesses, DO analysis was carried out, and the results showed that all genes were strongly correlated with bacterial infectious diseases, leukocyte diseases, and systemic mastocytosis (Fig. 4 a). For each T cell-related gene cluster, a deeper understanding of the characteristics and GO functional enrichment analysis was performed using the cluster package. These genes showed abnormal enrichment related to biological processes, molecular functions, and cellular components, which could also partially account for the high incidence and recurrence rates of malignant gastric cancer (Fig. 4 b). Accumulation of the biological processes was studied by carrying out KEGG analysis, confirming T-cellular receptor signaling transduction pathway, Th1, Th2, and Th17 cell transdifferentiation (Fig. 4 c, d), all of which are related to T-cells and the immune microenvironment.
Disease Ontology (DO), Gene Ontology (GO) and Kyoto encyclopedia of genes and genomes(KEGG) enrichment analysis of T-cell related genes. (a) Bubble chart of DO enrichment analysis of 178 DEGs. (b) Enrichment circle graph of GO terms of 178 DEGs. c-d. Enrichment circle graph of KEGG biological process of 178 DEGs
Establishment of the risk signature
LASSO and multivariate Cox regression analyses were employed, and 13 genes were selected from 178 genes by LASSO to construct signatures; six core genes (CD5, ABCA8, SERPINE2, ESM1, SERPINA5, and NMU) were screened from the DEGs to build the risk signature (Fig. 5 a, b). Patients with STAD ( n = 1043) were stochastically separated into a training group ( n = 522) and a test group ( n = 521) using the caret R package, in which the training group was employed to construct signatures. After the multivariate Cox regression analysis, the process of constructing the risk score was calculated as follows:
Selection of optimal prognostic signatures and constructure of risk signature in the training set. a-b. Least absolute shrinkage and selection operator(LASSO) regression analysis for prognostic genes. c. Differences in risk score among distinct gene clusters. d. Heat map regarding the correlation between the risk signature, molecular, genetic classification, prognosis and clinical features. e. Differences in the expression of Differential Expression (DEG) Analysis among the low-risk group and high-risk groups
Risk score = (-0.3081*expression of CD5) + (0.18156*expression of ABCA8) +.
(0.2309*SERPINE2) + (0.2112*expression of ESM1) + (0.0970*SERPINA5) + (0.1116*NMU).
The outcomes of the risk-scoring system applied to all patients showed significant diversity. Patients with STAD and risk scores below the average standard in the gene cluster were assigned to the low-risk group ( n = 549), whereas those exceeding the average standard were assigned to the high-risk group ( n = 494). Overall, the risk score of patients with STAD in the T cell C2 group was higher than that in the other groups (Fig. 5 c).
A heatmap was created, highlighting that tumor, node, and metastasis staging was elevated in the high-risk group (Fig. 5 d). This conclusion also confirmed that C1 had a significantly better prognosis than C2. Gene expression in the two groups was visually analyzed to thoroughly investigate the association between the risk score and other parameters (Fig. 5 e). When TCGA and GEO cohorts were merged, log2 was used for data with large values to eliminate batch effects (Additional figure S1 f). Survival analysis was carried out in the training and test groups and the initial merged cohort to guarantee that the outcome of the obtained risk signature’s predictive ability could be confirmed. Similar survival advantage results were observed across all analyzed groups (Fig. 6 a-c). In the low-risk group, the expression of T cell-related genes was relatively high, whereas in the other groups, the expression was low. The distribution of risk scores demonstrated that a lower risk score was associated with a higher survival probability and that the survival rate decreased with an increase in the risk score. From the distribution risk score curve, affordable T cell-related genes such as CD5 were higher in the low-risk group, whereas the inverse outcome was observed in the high-risk group. ABCA8, SERPINE2, ESM1, SERPINE2, and NMU expression levels were higher in the high-risk group, whereas an inverse outcome was noted in the low-risk group. The six core genes were distributed in the low- and high-risk groups within the training, testing, and total samples using heat maps. The probability of survival decreased as the risk score increased (Fig. 6 d-f). To verify the veracity of the risk signature, we generated ROC curves (Fig. 6 g-i). Univariate and multivariate Cox regression analyses of the combined cohort also verified that the risk characteristics could be independently applied as a prognostic signature for STAD (Fig. 6 j, k). The 1-, 3-, and 5-year survival rates of patients could also be predicted using a contingency map containing risk scores and clinicopathological parameters (Fig. 6 l). Based on the calibration chart, the previous line chart had characteristics similar to those of the calibration plot (Fig. 6 m). We analyzed the predictive effects of univariate and multivariate Cox regression analyses on individual cohorts (GSE15459, GSE34942 + GSE38749, GSE84437, and TCGA) (Fig. 6 n). A survival analysis was performed to test the predictive effect of the signature on individual cohorts. In all cohorts, survival decreased with increasing risk scores (Fig. 6 o).
Validation of the risk signature in training, testing sets and the two combined. a-c. Kaplan-Meier survival analysis between the high- and low-risk score groups in training, testing sets and the two combined. d-f. Scatter plots showing the survival status of stomach adenocarcinoma (STAD) patients with increasing risk scores. Ranked dot plots indicating the risk score distribution. Heat maps showing the distribution of the six candidate genes. g-i. receiver operating characteristic (ROC) curves predicting the sensitivity and specificity of 1-, 3- and 5-year survival according to the risk signature. j-k. Univariate COX regression analysis in the merged cohort and multivariate COX regression analysis in the merged cohort. l-m. Nomogram and Calibration curves for prediction of 1-, 3-, and 5-year survival rate of STAD patients in the two sets. n. Prediction effects of univariate and multivariate cox regression analyses on individual cohorts. o. Survival analysis in individual cohorts. The cohort order is as follows: GSE15459, GSE34942 + GSE38749, GSE84437, TCGA
Characteristics of TME and results of immunotherapy
The correlation between the clusters and risk was visualized using a scatter diagram and heatmap (Fig. 7 a). The results showed mast cells, monocytes, and type II interferon responses were positively linked to the risk score. T-cell inhibition, activated CD8 + T cells, activated B cells, and other immune cells were significantly negatively correlated. The matrix and estimate scores in the low-risk group were lower; however, there was no significant difference between the two groups (Fig. 7 b). The interstitial and estimate scores showed marked variation between the low- and high-risk subgroups (Fig. 7 c). The survival ratio and degree of purity of tumor cells in the high-risk group were significantly higher than those in the low-risk group. In STAD, the comprehensive risk score and cancer stem cell (CSC) index values were used to comprehensively assess the link between risk markers and CSC (Fig. 7 d). The study displayed that risk score was positively associated with the CSC index ( R = 0.28; p < 2.2e-16), demonstrating that the higher the risk score, the more obvious the differentiation grade of the stem cells. Subsequent analysis to explore the immune infiltration condition of T cell-related core genes was performed by studying the distribution disparity of somatic cell mutations between the low- and high-risk score groups using the maftools package (Fig. 8 a, b). We performed a survival analysis of TMB and risk scores in different groups to forecast the prognosis of patients with STAD, and the outcome illustrated that the low-risk score and high-TMB groups had the highest survival rates (Fig. 8 c, d). We also assessed the degree of association between TMB and the risk score, as well as between T-cell clusters and gene clusters (Fig. 8 e). The results further demonstrated that low-risk scores correlated with high TMB. In addition, the TMB of clusters C2 and B were higher than those of the other two clusters. The low-risk group, compared to the high-risk group with a higher mutation load, was more extensive, which was consistent with the above findings and confirmed by subsequent TMB quantitative analysis (Fig. 8 f). We also analyzed microsatellite instability (MSI) in the low- and high-risk subgroups to further assess the capacity of risk markers to predict ICB responses in patients (Fig. 8 g). The rate of MSI was higher in the low-risk group than in the high-risk group, suggesting that immunotherapy and clinical therapy were more effective in this subgroup. The association between the risk characteristics and MSI was further confirmed (Fig. 8 h).
Immune annotation and correlation between T-cell and
anti-PD-1/L1, anti-CTLA-4 immunotherapy. (a) Relations between tumor purity, ESTIMATE score, immune score, stromal score, and different stomach adenocarcinoma (STAD) phenotypes. (b) Correlation between the abundance of immune cells and six candidate genes. (c) Violin plot illustrating the result of ESTIMATE analysis. d. Relationships between the risk signature and cancer stem cell (CSC) index
Exploration of the association between the tumor somatic
mutation, Microsatellite Instability(MSI) and risk signature. a-b. The waterfall plots of tumor somatic mutation constructed by those with low- and high-risk scores, respectively. c. Survival analysis on stomach adenocarcinoma (STAD) samples with high and low tumor mutational burden(TMB). d. Survival analysis on STAD samples with different TMB and risk score. e. Relationships between TMB and risk score based on T-cell clusters, respectively. f. Distribution of STAD samples with low- and high-risk score in TMB. g-h. Relationships between risk signature and MSI
Immune escape and immunotherapy analysis
The high-risk group exhibited a high immune escape rate and poor immunotherapeutic efficacy (Fig. 9 a). Through the analysis of immune cell differentiation, it can be seen that the immune expression of the PDCD1 and CD274 high-risk group was low. Based on the data obtained, PD1 and PD-L1 were not very effective in the immune treatment (Fig. 9 b). According to the characteristic value of the sample in each module and the characteristics of the sample, correlation analysis was performed to identify two modules related to specific traits: KEGG T-cell receptor signaling pathway and KEGG B-cell receptor signaling pathway were positively correlated in CD8 T cells (Fig. 9 c). The treatment effect was observed using survival analysis in each group. The CR/PR group exhibited the highest probability of survival (Fig. 9 d). Estimate analysis revealed a significant gap between the matrix and estimate score between the low- and high-risk subgroups (Fig. 9 e-h). The results indicated that the survival rate of tumor cells in the high-risk group was significantly higher than that in the low-risk group, accompanied by poorer immune efficacy. The half-maximal inhibitory concentrations (IC50) of paclitaxel, gemcitabine, 5-fluorouracil, and doxorubicin were all higher in the high-risk groups, indicating potential drug resistance (Fig. 9 i-l).
Immunotherapy and Drug sensitivity analysis. (a) Box plot suggesting the difference of response towards immunotherapy between high- and low-risk score group. (b) Differences in the expression of T-cells among the low-risk group and high-risk groups. (c) gene set variation analysis(GSVA) analysis of 6 core genes of the signature. (d) survival after immunotherapy. e-h. The efficacy of immunotherapy for high- or low- risk groups. i-l. Drug sensitivity of Paclitaxel, Gemcitabine, 5-Fluorouracil and Doxorubicin
Reference to an external cohort for validation
Survival analyses were performed on the external IMV210 and GSE62254 cohorts to confirm the predictive power of this risk marker in validating the survival advantage of the low-risk group. Similar results were observed in all pooled analyses (Fig. 10 a, b). The binary response system showed that the SD/PD risk scores were significantly higher than those of CR/PR (Fig. 10 c).
Cohorts of IMV210 and GSE62254 and external signatures for the validation of TRG-signature, and further scRNA analysis based on GSE18394. a, b. Kaplan-Meier survival analysis between the high- and low- risk score groups. In GSE62254 and IMV210,respectively. c. Binary response in risk score among CR/PR and SD/PD. d. C-index of different signatures. e. Restricted Mean Survival (RMS) Curves for different Signature Values. f. The distribution of different cells in gastric cancer and adjacent areas. g. The total gene enrichment score in T cell. h. Expression of SEPRINE2 gene in T cells. i. Ratio map of T cell subsets. j. Gene enrichment score in subset
Signature discrimination
We compared our T cell-related gene signature with other published signatures [ 7 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ], which yielded a C-index of 0.613, which was higher than other signatures published within the last 3 years (Fig. 10 d). According to restricted mean survival, it also achieved a higher accuracy of survival estimation in the validation datasets (HR: 2.361; p < 0.001; Fig. 10 e). ROC curves and survival analyses were generated for comparison (Additional figure S2).
Comprehensive analysis of scRNA
After quality control, 54,274 cells were labeled to show their distribution (Fig. 10 f). Enrichment analysis suggested that the T-cell content in tumors was significantly higher compared to normal tissues (Fig. 10 g). Furthermore, the T-cell subpopulations were divided into gamma delta, CD4+, NK, and CD8 + T cells. SERPINE2 was highly expressed in tumors, mainly in CD4 + T cells and NK cells (Fig. 10 h, i). The signature score was higher for CD4 + T cells and NK cells of tumors and lower for gamma delta T cells (Fig. 10 j).
Immunohistochemistry and Youjiang cohort analysis
Based on immunohistochemistry, the Youjiang cohort was divided into Low SERPINE2 ( n = 44) and High SERPINE2 ( n = 49) groups, and samples with lower SERPINE2 expression exhibited better OS (HR = 3.197; p = 0.007; Fig. 11 a-d). As shown in Fig. 11 e, the SERPIN2 and CXCL12 levels were significantly correlated.
Clinical cohort and experimental exploration of SERPINE2 and CXCL12 in GC. a Youjiang cohort of overall survival. b-d. Low, medium and high expression in immunohistochemistry of samples from Youjiang cohort. e. Relationship between SERPIN2 and CXCL12. f. Down-regulated of CXCL12 in AGS and BGC-823 cells. g. CCK-8 assay, down-regulated level of CXCL12 expression significantly reduce the proliferative ability of GC cells. h. Transwell cell migration assay analyse the down-regulation level of CXCL12 expression of GC cell. i. Survival analyses conducted on patients with different CXCL12 expression level. * P < 0.05, ** P < 0.01, *** P < 0.001
Down-regulation of CXCL12 inhibited the proliferation and migration of AGS and BCG-823 cells
To investigate the effects of CXCL12 in vitro, we first validated the downregulation of CXCL12 expression in AGS and BCG-823 cells (Fig. 11 f). According to the results of the CCK-8 assay, the knockdown of CXCL12 significantly reduced the proliferative viability of AGS and BCG-823 cells compared to the control group (Fig. 11 g). Furthermore, the transwell migration assay indicated that the knockdown of CXCL12 expression markedly suppressed the metastatic ability of AGS and BCG-823 cells (Fig. 11 h). Overall, the downregulation of CXCL12 significantly inhibited the proliferation and migration of AGS and BCG-823 cells.
Association between survival and GC subtypes defined by CXCL12 expression
To determine the effect of CXCL12 on patient survival, we analyzed the survival of patients with different CXCL12 expression levels. Survival analysis showed that patients with higher CXCL12 expression had worse OS than those with lower CXCL12 expression (Fig. 11 i). These data suggest that the analysis of CXCL12 expression yields different subtypes of GCs. Specifically, our results suggest that lower CXCL12 expression levels are associated with improved survival in patients with GC.
The incidence of STAD is decreasing in most developed countries [ 34 ]; however, the number of deaths due to the disease is increasing [ 35 ]. Currently, treatment for gastric cancer is not satisfactory [ 36 ]. Moreover, more than half of the patients diagnosed with gastric cancer cannot be treated surgically at the time of diagnosis [ 37 ]. The lack of treatment for gastric cancer also leads to rapid disease progression and increased mortality. Previous studies have investigated the correlation between genes and carcinogenesis in various cancers, including GC [ 38 , 39 ]. Multiple types of genomic damage, including the activation of oncogenes and inactivation of tumor suppressor genes, are factors that cause gastric cancer [ 40 ]. Its anti-tumor effects are characterized by the highly coordinated actions of many genes. Owing to the inadequacy of current medical technology, only one or two genotypes have been evaluated [ 41 ].
The present study aimed to build signatures to study the effect of certain genes on gastric cancer using LASSO and multivariate Cox regression analyses. In this study, LASSO and multivariate Cox regression analyses were applied to build signatures to study the effect of certain genes on gastric cancer. Six core genes (CD5, ABCA8, SERPINE2, ESM1, SERPINA5, and NMU) were selected from the DEGs to establish risk marker signatures. The results showed that The risk score of T cell Cluster C2 was significantly higher than that of T cell Cluster C1, and the prognosis of C2 was significantly better than that of CI. We also performed validation using an external cohort, which further confirmed that our phenotypic classification of T cell-associated gene mutations was meaningful. In addition, we preliminarily found that the signature genes were closely correlated with STAD, which provides valuable clues for further research on immunotherapy targets for STAD.
Initially, we selected four genes (IL12B, B2M, HLA-A, and CD19) for further study, among which CD19 and IL12B showed a significant survival advantage in the predictive analysis. Autologous CD19-targeted CAR T-cells could significantly help treat blood cancer [ 42 ]. Epidemiological studies have shown that IL-12B is associated with an increased incidence of cervical cancer [ 43 ]. The TME cannot be ignored during tumor development [ 44 ] as it contains many different cell types, such as endothelial and fibroblast [ 45 ]. Tumor-infiltrating immune cells can directly or indirectly participate in immune responses, thereby affecting the prognosis of patients with tumors [ 46 ]. For example, dendritic cells can capture antigens emitted by tumors, while Effector T cells (CD8+) and TAMs can lyse and phagocytose tumor cells.
Additionally, helper T cells (CD4+) limit the immune response [ 47 ]. Inhibition of these cytokines can strengthen the anti-tumor effect of tumor-infiltrating lymphocytes and further improve their clinical therapeutic effect [ 48 , 49 ]. A recent study also confirmed that T helper cells are effective prognostic immune cells, which is correlated with further studies on gastric cancer [ 50 ]. Based on immunological and drug sensitivity analyses, we found that the high-risk group had a higher probability of immune escape and was generally resistant to first-line chemotherapy, indicating insensitivity to these treatment methods.
The scRNA results suggested that, compared to normal tissue, T-cell infiltration in GC was more abundant, mainly composed of differentiated CD4 + T cells and NK cells, while gamma delta T cells with higher differentiation potential were fewer, indicating T-cell exhaustion in the tumors. SERPINE2, which had the highest score in the signature, was highly expressed in T cells from GC. We found a significant positive correlation between SERPINE2 and the T cell-related factor CXCL12 in our dataset. Previous studies indicated that CXCL12 interacts with T cells to reduce OS in patients with GC [ 51 ], while SERPINE2 promotes cell proliferation [ 52 ]. Correspondingly, we tested the effects of CXCL12 downregulation on cell proliferation and migration and found that CXCL12 significantly reduces promotional and migration potential in GC cell lines. Survival analyses performed for patients with different CXCL12 expression levels confirmed that patients with high CXCL12 expression levels had poor survival probability. However, our study not only confirmed the effects of CXCL12 on tumor cell promotion and metathesis, but also showed the potential value of CXCL12 in tumor treatment. Therefore, we preliminarily speculated that SERPINE2 affects CXCL12 through a potential pathway, thereby promoting T-cell exhaustion.
Numerous methods were employed to assist our signature in this study; however, there were still some shortcomings. Environmental, racial, economic, predictive, and follow-up factors influence OS [ 22 ]; this is a limitation of our study, and in a follow-up study, we will control for the variables for a further in-depth study.
This work may contribute to the understanding of tumor immunity and provide new ideas for the personalized treatment of STAD.
Data availability
The datasets analysed during the current study are available in the TCGA, GSE15459,
GSE34942, GSE38749, GSE84437, GSE62254 and IMV210 repository.
Abbreviations
Gastric cancer
Stomach adenocarcinoma
Tumor microenvironment
Single-cell ribonucleic acid
Differentially expressed genes
Disease ontology
Gene ontology
Kyoto Encyclopedia of Genes and Genomes
Copy number variations
Protein-protein interaction
Inter-cell Interference Coordination
Cancer stem cell
Microsatellite instability
Half-maximal inhibitory concentrations
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Article PubMed Google Scholar
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. ESMO guidelines Working Group gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v50–4.
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205.
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977–1010.
Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–75.
Jiang K, Li L, Xie Y, Xie D, Xiao Q. High ADAMTS18 expression is associated with poor prognosis in stomach adenocarcinoma. Oncol Lett. 2020;20(5):211.
Article CAS PubMed PubMed Central Google Scholar
Zhang B, Li Y, Yang L, Chen Y. A four-gene-based risk score with High Prognostic Value in Gastric Cancer. Front Oncol. 2021;11:584213.
Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of Advanced Gastric Cancer. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2017;39(7):1010428317714626.
Article Google Scholar
Fuchs CS, Doi T, Jang RW et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer. JAMA Oncol. 2018;4(5).
Zeng D, Zhou R, Yu Y, et al. Gene expression profiles for a prognostic immunoscore in gastric cancer. Br J Surg. 2018;105:1338–48.
Article CAS PubMed Google Scholar
Zeng D, Li M, Zhou R, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7:737–50.
Zeng D, Ye Z, Wu J, et al. Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer. Theranostics. 2020;10:7002–14.
Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34.
Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10:36.
Article PubMed PubMed Central Google Scholar
Traves PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediat Inflamm. 2012;2012:568783.
1.Song W, Ren J, Xiang R, Kong C, Fu T. Identification of pyroptosis-related subtypes, the development of a prognosis signature, and characterization of tumor microenvironment infiltration in colorectal cancer. Oncoimmunology. 2021;10(1):1987636.
Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res. 2020;39:1–21.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Abramson JS, et al. Transcend NHL 001: immunotherapy with the CD19-directed CAR T-cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-hodgkin lymphoma. Blood. 2016;128:4192–4192.
Chen Z, et al. In vivo CD8 + T-cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell. 2021;184:1262–80.
Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T-cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11(1):22.
Legut M, Gajic Z, Guarino M, Daniloski Z, Rahman JA, Xue X, Lu C, Lu L, Mimitou EP, Hao S, Davoli T, Diefenbach C, Smibert P, Sanjana NE. A genome-scale screen for synthetic drivers of T-cell proliferation. Nature. 2022;603(7902):728–35.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. https://doi.org/10.1093/nar/28.1.27 .
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51. https://doi.org/10.1002/pro.3715 . Epub 2019 Sep 9.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–92. https://doi.org/10.1093/nar/gkac963 .
Shao W, Yang Z, Fu Y, Zheng L, Liu F, Chai L, Jia J. The pyroptosis-related signature predicts prognosis and indicates Immune Microenvironment Infiltration in Gastric Cancer. Front Cell Dev Biol. 2021;9:676485.
Wang N, Liu D. Identification and validation a necroptosis–related Prognostic signature and Associated Regulatory Axis in stomach adenocarcinoma. Onco Targets Ther. 2021;14:5373–83.
Xiao R, Wang S, Guo J, Liu S, Ding A, Wang G, Li W, Zhang Y, Bian X, Zhao S, Qiu W. Ferroptosis-related gene NOX4, CHAC1 and HIF1A are valid biomarkers for stomach adenocarcinoma. J Cell Mol Med. 2022;26(4):1183–93.
Xu P, Liu S, Song S, Yao X, Li X, Zhang J, Liu Y, Zheng Y, Gao G, Xu J. Identification and validation of a novel angiogenesis-related gene signature for predicting prognosis in gastric adenocarcinoma. Front Oncol. 2023;12:965102.
Wang X, Zhang W, Guo Y, Zhang Y, Bai X, Xie Y. Identification of critical prognosis signature associated with lymph node metastasis of stomach adenocarcinomas. World J Surg Oncol. 2023;21(1):61.
Chen F, Wang Y, Zhang X, Fang J. Five hub genes contributing to the oncogenesis and trastuzumab-resistance in gastric cancer. Gene. 2023;851:146942.
Wu X, Jian A, Tang H, Liu W, Liu F, Liu S, Wu H. A Multi-omics Study on the Effect of Helicobacter Pylori-related genes in the Tumor immunity on stomach adenocarcinoma. Front Cell Infect Microbiol. 2022;12:880636.
Ding W, Jiang H, Ye N, Zhuang L, Yuan Z, Tan Y, Xue W, Xu X. Identification and analysis of crucial genes in H. Pylori-Associated Gastric Cancer using an Integrated Bioinformatics Approach. J Oncol. 2023;2023:8538240.
Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol. 2006;101(11):2485–92.
Forman D. Helicobacter pylori: the gastric cancer problem. Gut. 1998;43(Suppl 1):S33–4.
Ford AC, Yuan Y, Forman D, Hunt R, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev. 2020;7(7):CD005583.
PubMed Google Scholar
Lello E, Furnes B, Edna TH. Short and long-term survival from gastric cancer. A population-based study from a county hospital during 25 years. Acta Oncol. 2007;46(3):308–15.
Uozaki H, Barua RR, Minhua S, Ushiku T, Hino R, Shinozaki A, et al. Transcriptional factor typing with SOX2, HNF4aP1, and CDX2 closely relates to Tumor Invasion and Epstein-Barr Virus Status in Gastric Cancer. Int J Clin Exp Pathol. 2011;4:230–40.
CAS PubMed PubMed Central Google Scholar
Gomes AR, Zhao F, Lam EW. Role and Regulation of the Forkhead Transcription Factors FOXO3a and FOXM1 in Carcinogenesis and Drug Resistance. Chin J Cancer (2013) 32:365–70.10.5732/cjc.012.10277.
Ji K, Zhang L, Zhang M, Chu Q, Li X, Wang W. Prognostic Value and Clinicopathological significance of p-stat3 among gastric carcinoma patients: a systematic review and Meta-analysis. Med (Baltim). 2016;95(5):e2641.
Article CAS Google Scholar
Song W, Ren J, Xiang R, Kong C, Fu T. Identification of pyroptosis-related subtypes, the development of a prognosis signature, and characterization of tumor microenvironment infiltration in colorectal cancer. Oncoimmunology. 2021;10(1):1987636.
Lim WA, June CH. The principles of Engineering Immune cells to treat Cancer. Cell. 2017;168:724–40. https://doi.org/10.1016/j.cell.2017.01.016 .
Karimi-Zarchi M, Abbasi H, Javaheri A, et al. Association of IL-12B rs3212227 and IL-6 rs1800795 polymorphisms with susceptibility to Cervical Cancer: a systematic review and Meta-analysis. Asian Pac J Cancer Prev. 2020;21(5):1197–206.
Runa F, Hamalian S, Meade K, Shisgal P, Gray PC, Kelber JA. Tumor microenvironment heterogeneity: challenges and opportunities. Curr Mol Biology Rep. 2017;3(4):218–29. https://doi.org/10.1007/s40610-017-0073-7 .
Arneth B. Tumor Microenvironment. Medicina (Kaunas) (2019) 56(1):1–21.10.3390/medicina56010015.
Jiang W, Liu K, Guo Q, et al. Tumor-infiltrating immune cells and prognosis in gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(37):62312–29.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Costa R, Carneiro BA, Agulnik M, Rademaker AW, Pai SG, Villaflor VM, Cristofanilli M, Sosman JA, Giles FJ. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget. 2017;8(5):8910–20.
Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950–6.
Wang M, Li Z, Peng Y, Fang J, Fang T, Wu J, Zhou J. Identification of immune cells and mRNA associated with prognosis of gastric cancer. BMC Cancer. 2020;20(1):206.
Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, Li X, Zhou W, Li J, Li Z, Bai C, Zhao L, Han Q, Zhao RC, Wang X. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022;12(2):620–38.
Chen C, Qin F, Singh S, Tang Y, Li H. CTNNBIP1-CLSTN1 functions as a housekeeping chimeric RNA and regulates cell proliferation through SERPINE2. Cell Death Discov. 2023;9(1):369.
Download references
This project was supported by Shanghai Anticancer Association EYAS PROJECT (SACA-CY22801) and engineering and medical science cooperation project of Shanghai Jiao Tong University (YG2023QNA10).
Author information
Shuchang Wang, Weifeng Zhang, Xinrui Wu contributed equally to this work.
Authors and Affiliations
Department of Gastrointestinal Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Shuchang Wang & Chun Zhuang
Department of Cardiology, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Weifeng Zhang
Department of Urology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Xinrui Wu & Li Chen
Department of Clinical Medicine, Medical School of Nantong University, Nantong, China
Department of Neurosurgery, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
Yuanbiao Chen
Department of Interventional Oncology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Wangrui Liu
Department of General Surgery, Affiliated Hospital of Nantong University, Nantong, 226001, Jiangsu, China
Department of Nursing, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, JX, LC and CZ; Methodology, XW, ZZ, CY and WL; Software, SW; Validation, all authors; Writing, SW, WZ and XW.
Corresponding authors
Correspondence to Junnfei Xu , Li Chen or Chun Zhuang .
Ethics declarations
Ethics approval and consent to participate.
All procedures performed in this study complied with the ethical standards of the institution and the National Commission for Human Experimentation, as well as the 1964 Declaration of Helsinki and its subsequent amendments or equivalent. This study was approved by the Ethics Committee of Youjiang Medical University For Nationalities. We obtained informed consent from all individual patients included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, S., Zhang, W., Wu, X. et al. Comprehensive analysis of T-cell regulatory factors and tumor immune microenvironment in stomach adenocarcinoma. BMC Cancer 24 , 570 (2024). https://doi.org/10.1186/s12885-024-12302-w
Download citation
Received : 11 July 2023
Accepted : 22 April 2024
Published : 07 May 2024
DOI : https://doi.org/10.1186/s12885-024-12302-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prognostic marker
- Gastric Cancer
- Gene Signature
- Immune Infiltration
- Immunotherapy
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Stomach Cancer: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about this type of cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about stomach cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials . Always talk with your doctor about the best diagnostic and treatment options for you.
Chemoprevention. Chemoprevention is the use of drugs or nutrients to lower a person’s risk of developing cancer. Early research suggests that using antibiotics to treat H. pylori infections (see Risk Factors ) can prevent changes to stomach cells that may lead to cancer.
Combination therapy. The combination of chemotherapy, radiation therapy, and surgery may reduce the chance that stomach cancer will return. Doctors may give chemotherapy before surgery, called neoadjuvant therapy, or after surgery, called adjuvant therapy. In addition, doctors may also combine radiation therapy and chemotherapy after surgery. Doctors are also looking at giving both radiation therapy and chemotherapy before surgery.
Newer chemotherapy treatments. Chemotherapy with multiple combinations of drugs is being increasingly used for people with stomach cancer. Drug combinations work slightly better than single drugs. As outlined in the Types of Treatment section, drugs such as 5-FU, paclitaxel, docetaxel, irinotecan, oxaliplatin, as well as oral medications such as capecitabine are being studied in combination with other types of chemotherapy.
Molecular testing of the tumor . Researchers are looking at the genetic changes in tumor cells to identify specific genes, proteins, and other factors unique to the tumor. Patients with different types of tumors with the same genetic change are able to participate in clinical trials, called “basket trials” , with the goal of finding treatments that target that genetic change.
Targeted therapy. Previous research has shown that several types of targeted therapy do not work well for stomach cancer. These include drugs that target the gene c-MET , bevacizumab (Avastin, Mvasi), and drugs that block epidermal growth factor receptor (EGFR). However, research continues on this type of treatment approach (see Molecular testing of the tumor, above).
Immunotherapy. Immunotherapy is an expanding area of research for stomach cancer. Researchers are looking at different types of immunotherapy that block the CTLA4 and/or PD-1 pathways. A tumor can use these pathways to hide from the body’s immune system. Immunotherapy that blocks these pathways allow the immune system to identify and destroy the cancer.
Palliative and supportive care . Clinical trials are underway to find better ways of reducing symptoms and side effects of current stomach cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in stomach cancer, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to read about research announced in ASCO's Clinical Cancer Advances report from 2021 .
Listen to a podcast from ASCO experts discussing highlights from 2022 Congress of the European Society for Medical Oncology (ESMO).
Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Stomach Cancer Guide
Cancer.Net Guide Stomach Cancer
- Introduction
- Medical Illustrations
- Risk Factors
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
Epidemiological research in stomach cancer: progress over the last ten years
Affiliation.
- 1 German Cancer Research Center, Institute of Epidemiology and Biometry, Heidelberg.
- PMID: 2036128
- DOI: 10.1007/BF01613137
In this paper the progress of epidemiological research in stomach cancer during 1980-1990 is reviewed in respect to regional variation, etiology, and formation of carcinogens. The evaluation of 4 cohort and 16 case-control studies revealed a consistently inverse relationship of stomach cancer risk with raw vegetables, fruit, and wholemeal bread consumption and with vitamin C and carotene intake. Milk, cooked vegetables and vitamins A and E were not consistently found to be related to stomach cancer risk. Positive associations of increasing consumption with stomach cancer risk were occasionally found for processed or particularly prepared meat and fish, and for nitrite. Dietary nitrate intake did not appear to be related to stomach cancer risk in these studies. This latter observation is also supported by metabolic studies in high- and low-risk areas for stomach cancer. Consistently among studies, increased risk for stomach cancer was also found for later availability of refrigeration facilities in the household, non-centralized water supply (especially well water), and high salt intake. Prospective studies agreed in an increased risk for stomach cancer for cigarette smoking, but not for alcohol drinking, whereas case-control studies showed divergent results on these factors. Recent metabolic studies in high- and low-risk areas for stomach cancer or in groups with precursor lesions, with the N-nitrosoproline test as a marker for endogenous nitrosation, revealed inconsistent results. Higher nitrite concentration and increased pH in stomach juice were found to be associated with precursor conditions for stomach cancer. It is still not clear whether intake of preformed carcinogens or endogenous formation in the stomach with or without the inclusion of nitrite is the most important source of tumor-initiating or -promoting substances. Preservation or preparation of meat and fish may play an important role in this process, and vitamin C may be an inhibiting substance.
Publication types
- Alcohol Drinking
- Risk Factors
- Stomach Neoplasms / epidemiology*
- Stomach Neoplasms / etiology
Cystatin SA attenuates gastric cancer cells growth and increases sensitivity to oxaliplatin via PI3K/AKT signaling pathway
- Open access
- Published: 08 May 2024
- Volume 150 , article number 244 , ( 2024 )
Cite this article
You have full access to this open access article

- Yida Lu 1 na1 ,
- Huizhen Wang 1 na1 ,
- Sihan Chen 2 ,
- Bo Yang 1 ,
- Yaxian Li 1 &
- Yongxiang Li 1
Cystatin SA (CST2) belongs to the superfamily of cysteine protease inhibitors. Emerging research indicates that CST2 is often dysregulated across various cancers. Its role and molecular mechanisms in gastric cancer remain underexplored. This study aims to explore the expression and function of CST2 in gastric cancer.
CST2 expression was analyzed and validated through Western blot. CST2 overexpression was induced by lentivirus in GC cells, and the correlation between CST2 expression levels and downstream signaling pathways was assessed. In addition, multiple assays, including cell proliferation, colony formation, wound-healing, and transwell migration/invasion, were considered to ascertain the influence of CST2 overexpression on gastric cancer. The cell cycle and apoptosis were detected by flow cytometry.
CST2 expression at the protein level was decreased to be reduced in both gastric cancer tissues and cell lines, and CST2 expression attenuate gastric cancer growth, an effect restricted to gastric cancer cells and absent in gastric epithelial GES-1 cells. Furthermore, CST2 was demonstrated to improve chemosensitivity to Oxaliplatin in gastric cancer cells through the PI3K/AKT signaling pathway.
These findings indicate that CST2 is downregulated at the protein level in gastric cancer tissues and cell lines. Additionally, CST2 was found to attenuate the growth of gastric cancer cells and to enhance sensitivity to Oxaliplatin through the PI3K/AKT signaling pathway, specific to gastric cancer cell lines. CST2 may serve as a tumor suppressor gene increasing sensitivity to Oxaliplatin in gastric cancer.
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most aggressive and lethal malignancies of the digestive system, with a higher incidence in males than in females (Sung et al. 2021 ). The main risk factors include Helicobacter pylori infection, unhealthy diets, high alcohol consumption, and smoking (Campbell et al. 2007 ; Ishaq and Nunn 2015 ; Kim et al. 2014 ). The majority of GC cases are classified as adenocarcinomas, accounting for 80–90% (Casamayor et al. 2018 ). The WHO classifies GC into multiple subtypes, including papillary, tubular, mucinous, poorly cohesive carcinoma (signet-ring cell carcinoma and other variants), and mixed adenocarcinoma. The Lauren classification system consists of intestinal, diffuse, and indeterminate types (Bray et al. 2018 ).
Currently, treatment regimens against GC can be created from a molecular perspective, as many signaling pathways are abnormally regulated in GC cases (Clements et al. 2002 ; Feng et al. 2010 ). Although surgical resection is widely accepted as the main treatment for GC, chemotherapy remains a vital alternative, especially when resection is not feasible or the cancer is in metastatic stages. However, acquired resistance to chemotherapy agents limits their clinical efficacy in GC treatment. Nevertheless, the underlying molecular mechanisms of GC remain elusive. Therefore, further investigation of the unknown molecular mechanisms of GC is essential.
Cystatin SA (CST2) is a specific cysteine proteases inhibitor and a cystatin superfamily member. This superfamily is categorized into three main categories: type 1 (including CSTA, CSTB, etc.), which are predominantly intracellular proteins; type 2 (including CST1-7, etc.), which are primarily secretory proteins; and type 3, which are multi-domain proteins, such as mammalian kininogen (Koblinski et al. 2000 ; Lah et al. 1993 ). Several studies have found that members of the cystatin superfamily play important roles in inflammation and tumorigenesis. For instance, the upregulation of cystatin SN has been shown to promote hepatocellular carcinoma progression and is associated with a poor prognosis (Cui et al. 2019 ). Moreover, CST1 may serve as an independent prognostic factor for patients undergoing esophageal cancer surgery (Chen et al. 2013 ). CST6 exhibits upregulation in triple-negative breast cancer (Li et al. 2018 ). The expression level of CST2 is vital for the diagnosis of prostate cancer and may also influence drug efficacy in breast cancer. One study employed bioinformatics to identify CST2 as a potential prognostic marker in gastric cancer (Bao et al. 2019 ; Cheng et al. 2019 ; Liu et al. 2018 ). However, the specific roles and functions of CST2 in GC remain to be elucidated. Accordingly, in this study, we investigate CST2’s role in GC carcinogenesis and subsequently validated CST2’s functions using normal gastric cells, gastric cancer cells, and samples from GC patients. We ascertained that CST2 possesses the ability to suppress the malignant biological behaviors of gastric cancer cells, including cellular proliferation, migration, and invasion, and to enhance the chemosensitivity of gastric cancer cells to the chemotherapeutic agent oxaliplatin.
Materials and methods
Gc sample collection.
The samples of GC patients and their control samples were collected from surgical specimens from patients with GC between 2013 and 2017. All patients signed informed and received ethical approval to consent the use of human gastric tissue.
GC cell lines culture
Human normal gastric epithelial cell line GES-1 and GC cell lines MGC803, SGC7901, HGC27, BGC823 and AGS were obtained from the Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in (RPMI)-1640 medium (R10-040-CV, Corning, USA), added with 10% FBS (FB25015, CLARK, USA) and 1% Penicillin–Streptomycin Solution (SV30010, HyClone, USA). All cell lines were maintained at 37 °C in an incubator containing a humidified 5% CO 2 atmosphere.
Cell transfection
We constructed a lentivirus overexpressing CST2 and a negative control lentivirus was purchased by Hanbio Technology Company (Shanghai, China). For lentiviral transfections, 5 × 10 4 cells were pre-seeded in a 24-well plate overnight and transduced with the virus. After 48 h of transduction, cells were selected with 2 μg/ml puromycin (ST551, Sangon Biotech, China) for 6 days. The efficiency of overexpression was measured by western blot.
Western blot (WB)
CST2 (Abcam, USA), GAPDH (BBI Life Sciences Corporation, China), PI3K and AKT antibodies (Santa Cruz Biotechnology, USA) were used to detect the expression of corresponding proteins by Western blot. Cell or tissue proteins were extracted using lysis buffer (78,501, Thermo Fisher Scientific, USA). Proteins was electrophoresed on Tris–Glycine SDS Running Buffer, and then transferred onto polyvinylidene fluoride membranes (ISEQ00010, Merck Millipore, Germany). The membranes were blocked with 5% non-fat milk (A600669, Sangon Biotech, China) and maintained overnight at 4 °C with specific antibodies. After incubation with secondary antibodies (1:5000, Thermo Fisher) for one hour at room temperature, The immune binding was detected using the ECL detection system (5200 multi, Tanon, China) (Sun et al. 2014 ).

EdU staining
The BeyoClick™EdU Cell Proliferation Kit (C0078S, Beyotime, China) was used to assess cell proliferative ability. Approximately 5 × 10 4 cells were seeded into 12-well cell culture plates and incubated overnight at 37 °C in an incubator. The cell were then trated with either DMSO or various reagents for 48 h. Subsequently, an equal volume of 20 μM EdU was added to the cell culture medium, and the cells were incubated for 120 min before being fixed with 4% PFA for 20 min. After fixation, the cells were then rinsed three times with 3% bovine serum albumin (BSA) and permeabilized for 15 min with in 0.3% Triton X-100 in PBS. Following permeabilization, the cells were incubated with BeyoClick™ Click Additive Solution at room temperature, protected from light for 30 min. Finally, the cell nuclei were stained with Hoechst 33,342 for 10 min at room temperature. The cells were imaged using a Live cell Imaging System (Cell discoverer 7, Carl Zeiss, Germany), and the number of cells that were positive for EdU in each field was calculated.
Quantitative real-time PCR
Total RNA from both cells and tissues was extracted by using TRIzol reagent (Invitrogen, USA), and then used the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Japan) according to the recommended protocol. Quantitative real-time PCR was conducted using KOD SYBR qPCR Mix (Toyobo, Japan). The primers used in this study were as follows CST2-F: GGAGGACAGGATAATCGAGGG, CST2-R: GTTCGGCCCACCTCTATGTC; GAPDH-F: CTCTGCTCCTCCTGTTCGAC, GAPDH-R: ACGACCAAATCCGTTGACTC.
Cell counting Kit-8 (CCK-8) experiment
In total, 5 × 10 3 cells were seeded on per well of a 96-well plate and cultured overnight for attachment. After a treatment with different concentrations of Oxaliplatin (MedChemExpress, USA) for 48 h, Cell counting Kit-8 (MedChemExpress, USA) was added to each well and cultured for 30, 60 and 90 min. Oxaliplatin crystals were dissolved in DMF before being added (the stock solution was 100 mM, and the working solution ranged from 0.1 to 10 mM). Then a Microplate reader (EXL800, BioTek Instruments, USA) was used to obtain the OD value of cells at 450 nm. The relative cell viability was calculated based on the absorbance of each well.
Colony formation assay
In total, 500 cells were plated per well and maintained in a cell incubator for 12–14 days, with medium changes every 3 days. At the end of the experiment, cells were fixed with 4% formaldehyde for 20 min, stained with 0.1% crystal violet solution for another 20 min, followed by PBS washes several times. Colonies with more than 50 cells were counted. The efficiency of colony formation was calculated as [colonies counted/cells seeded × 100] %. All experiments were conducted in triplicate and repeated at least three times.
Scratch wound assay
Transfected cells were seeded on 6-well plates one day prior and cultured until they reached 90% confluence. A linear wound in ever well was made in each well by scratching the confluent cell layer with 200 μL pipette tip. Cells were then rinsed three times with phosphate-buffered saline (PBS) to remove floating cells and debris. The wounds were photographed and measured at 0, 24 and 48 h using a Live Cell Imaging System (Cell discoverer 7, Carl Zeiss, Germany).
Cell invasion assay
Cells were seeded on the upper chamber of a 24-well plate at a concentration of 5 × 10 5 cells, which was coated with Matrigel (BD Biosciences, USA). RPMI-1640 containing 1–2% FBS was added to the upper chamber, while the lower chamber was filled with RPMI-1640 with 10% FBS. After 24–48 h, the cells that migrated through the upper chamber were fixed in 4% paraformaldehyde (PFA) and stained with 0.1% crystal violet. The stained cells were then photographed and counted under a microscope (DMi1, Leica, Germany).
Immunofluorescence assay
Cells were seeded into a culture plate with pre-added cover glasses (Nest, China) and treated with various concentrations of Oxaliplatin. After 48 h, cells adhered to the cover glasses were fixed with 4% paraformaldehyde for 15 min at room temperature and washed three times with PBS. Cells were then permeabilized with 0.1% PBS-TX (0.1% Triton X-100 in PBS) for 5 min and washed three times with PBS. Cells were blocked in 2% BSA (bovine serum albumin), diluted in 0.1% PBS-TX, for 30 min, followed by the addition of 80 μL of 1X Phalloidin-iFluorTM 594 Conjugate (AAT Bioquest, USA) working solution, diluted 1:1000 in the blocking solution, for 1 h. Cells were then washed with PBS 3 to 5 times to remove excess solution. Subsequently, nuclei were stained with Antifade Mounting Medium with DAPI (P0131, Beyotime Institute of Biotechnology) for 15 min at room temperature. Confocal images were captured using a Live Cell Imaging System (Cell Discoverer 7, Carl Zeiss, Germany).
Cell cycle distribution assay
The cell cycle distribution for each sample was detected by PI/RNase Staining Buffer Solution (550825, BD PharmingenTM, USA). After treatment with specific drugs, cells were trypsinized and collected in tubes, washed with PBS, and then fixed using 75% pre-cold ethanol at − 20 °C for at least overnight. Then, the cells were centrifuged at 12,000 rcf for 10 min, washed with PBS, and incubated in PI working solution for 20–30 min in the dark at room temperature. Cells were harvested using a flow cytometer at slow flow speed, and analyzed with ModFit LT cell cycle analysis software (Modfit LT 5.0; Verity Software House, Topsham, ME, USA).
Nude mice xenograft experiments
4–6-week-old female nude mice (300–350 g) purchased from LINGCHANG BIOTECH (Shanghai, China) were randomly allocated to four groups: MGC803-CST2 group, MGC803-NC group, SGC7901-CST2 group and SGC7901-NC group. To establish the mouse xenograft model, nude mice were inoculated with 5 × 10 6 GC cells dissolved in PBS into their right flank to induce tumors. Tumor volume was measured from the tenth day after injection and then measured every 3 days in three dimensions using a digital caliper. Tumor volume was calculated according to the formula: tumor volume (mm 3 ) = π/6 × (W) 2 × (L), where L represents the long diameter and W represents the short diameter. After 28 days, we used the IVIS Lumina XR Series III Imaging System (Perkin Elmer, USA) to obtain tumor fluorescence images before euthanizing the mice.. The tumors were then resected and measured.
Ethics statement
All experiments involving animals follow the guidelines of the Animal Center of Anhui Medical University. Experimental protocols were approved by the Experimental Animal Ethical Committee of Anhui Medical University. All animals used in the study were euthanized at the end of this study.
Statistical analysis
All experiments were repeated at least three times independently, and the data were assessed by GraphPad Prism 8 (version 8.0.1, GraphPad Software, La Jolla, CA, USA). Mean values are shown in the figures, and SDs are shown as error bars. Comparisons between treatments were assessed by a two-tailed Student’s t -test. All p values are labeled in the figures for where data were compared or between the experimental group and its control group.
The expression of CST2 in gastric cancer and CST2 inhibits cell proliferation, migration and invasion in GC cells
Three datasets gene expression profiles (GSE51575, GSE65801, and GSE79973) were retrieved from those present in the GEO database including both normal and gastric cancer samples. All differentially expressed genes (DEGs) were compared between normal controls and gastric cancer samples. These genes were further filtered and the Venn diagrams representing these genes were plotted (Fig. S1 ). CST2 was screened out to be further explored its effects on gastric cancer, according to our preliminary experiments. Western blot analysis was used to assess CST2 expression in cells and tissues. CST2 expression was lower in GC cell lines (MKN45, MKN74, SGC7901, MGC803, and AGS) compared to the normal gastric epithelial cell line GES-1 (Fig. 1 A). Furthermore, CST2 protein expression was assessed in 12 pairs of fresh GC tissues and adjacent normal tissues, and a similar result was found, indicating that CST2 expression was decreased in GC tissues (Fig. 1 B). The patients information is listed in Table 1 .
A Protein levels of CST2 in the normal gastric mucosal epithelial cell line GES-1 and various human GC cell lines. B The expression levels of CST2 protein in GC tissues compared to adjacent normal gastric tissues. C The overexpression and knockdown efficiency of CST2 were confirmed using Western blot analysis. D The proliferation curves were employed to compare proliferation rates between CST2 overexpression cells and control cells. E EdU staining was employed to assess the effect of CST2 overexpression and knockdown on GC cell proliferation. F Wound healing in cell monolayers was assessed at 48 h, and wound closure rates were calculated. (G) Cell migration and invasion abilities were evaluated using Transwell assays. Data are displayed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
To further explore CST2’s role in GC carcinogenesis, GES-1, MGC803, and SGC7901 cells were selected for the overexpression and knockdown of CST2. The CST2 overexpression and knockdown efficiency were verified using Western blot (Fig. 1 C).
Initially, 5000 GC cells were seeded per well in 12-well plates, and cell counting was conducted over the following 6 days. The proliferation curves, plotted based on the cell counting data, indicated that CST2-overexpression cells (MGC803-CST2/SGC7901-CST2) had lower proliferation rates compared to the negative control groups (MGC803/SGC7901-NC) (Fig. 1 D). The number of MGC803 and SGC7901 cells decreased by 25.1% and 37.3%, respectively.
To validate the proliferation curves, EdU staining was performed on both CST2 overexpression and knockdown cells. The results also demonstrated that CST2 overexpression significantly inhibited GC cell proliferation (Fig. 1 E), and CST2 knockdown could partially reverse the inhibitory effect. Consistently, both wound healing and cell invasion assays yielded similar results: Cells with CST2 overexpression exhibited reduced migration abilities compared to the control group, with scratch healing rates of MGC803 and SGC7901 cells decreasing by 20.4% and 31.8%, respectively (Fig. 1 F). We then conducted transwell assays to assess cell invasion ability further, and the results similarly indicated that CST2 overexpression attenuated the invasive potential of GC cells. Notably, CST2 knockdown partially enhanced GC cells’ migration and invasion capacities (Fig. 1 G). However, it was apparent that the inhibitory effect of CST2 on cell proliferation, migration, and invasion remained confined to the two GC cell lines and was not detected in the gastric epithelial cell line GES-1.
CST2 suppresses tumor growth through the inhibition of the PI3K/AKT signaling pathway in mouse xenograft model
To further examine CST2’s impact on GC tumor growth in vivo, CST2 overexpression cells (MGC803-CST2 and SGC7901-CST2), along with their corresponding control cells, were used to establish mouse xenograft models. Tumor volume measurements commenced on the 10th day post-injection and were conducted every 3 days after that (Fig. 2 A). After 18 days of measurement, tumor fluorescence imaging was carried out prior to the euthanasia of the mice and tumor resection. Compared to the control group, slower tumor volume increases were noted in the MGC803-CST2 and SGC7901-CST2 groups. The fluorescent intensity readings ([p/s]/[μW/cm 2 ]) provided consistent results. The average fluorescent intensity was reduced from 5.12 × 10 11 to 4.53 × 10 10 in the MGC803 group, and from 7.26 × 10 11 to 7.63 × 10 9 in the SGC7901 group (Fig. 2 B). Additionally, the final tumor weights for the MGC803-NC and SGC7901-NC groups measured 0.632 g and 0.622 g, respectively, while the weights for the MGC803-CST2 and SGC7901-CST2 groups were substantially lower at 0.072 g and 0.077 g, representing reductions of 88.6% and 87.6%, respectively (Fig. 2 C). To elucidate the underlying mechanisms attributed to CST2, protein expression levels within the PI3K/AKT signaling pathway and apoptosis were investigated. Western blot analysis showed that CST2 suppressed the expression of phospho-PI3K and phospho-AKT, two key components of the PI3K/AKT pathway. Furthermore, there was a significant elevation in the levels of pro-apoptotic proteins (BAX, BAD), while conversely, the level of the anti-apoptotic protein (BCL-2) was significantly reduced in the CST2 groups. Collectively, these data suggest that CST2 may suppress tumor growth by inhibiting the PI3K/AKT signaling pathway in vivo (Fig. 2 D).
A Tumor volume for each group was recorded on the indicated days. B Images of the xenograft models were acquired using bioluminescence imaging, accompanied by a statistical analysis of tumor fluorescence intensity. C Tumors were dissected from mice post-euthanasia; subsequently, each tumor was collected and weighed. D Western blot analysis was performed to assess the expression of PI3K, p-PI3K, AKT, p-AKT, Bcl-2, BAX, and BAD in subcutaneous tumor samples of MGC803 and SGC7901 from each group. Data are displayed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
SC79 reverses the effect of CST2 upregulation in gastric cancer cells
To further investigate whether the PI3K/AKT signaling pathway is vital for CST2-mediated antitumor effects, the AKT activator SC79 was administered to MGC803 and SGC7901 cell lines. The results showed that SC79 significantly counteracted the decreased cell migration ability observed in the CST2 overexpression cell lines (Fig. 3 A). The proliferation curves and EdU staining results revealed that SC79 likewise counteracted the decreased cell proliferation associated with CST2 overexpression (Fig. 3 B, C ). Moreover, SC79 treatment increased the phosphorylation levels of PI3K and AKT and resulted in the restoration of the expression levels of Bcl-2, BAX, and BAD in cells overexpressing CST2 (Fig. 3 D).
A Transwell assay to assess cell migration in NC (negative control), CST2-overexpressing, and CST2 + SC79-treated groups. B , C Cell proliferation in the two groups was assessed using cell proliferation curves and EdU staining. D Western blot analysis to assess the expression of PI3K, p-PI3K, AKT, p-AKT, Bcl-2, BAX, and BAD proteins in GC cells, with GAPDH serving as a loading control. Data are displayed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
CST2 upregulation contributes to oxaliplatin sensitivity via PI3K/AKT signaling pathway
Previous studies have shown that CST2 expression can potentially influence the efficacy of chemotherapy in cancer patients (Li et al. 2017 ; Qin et al. 2017 ). Given that oxaliplatin is a commonly used first-line chemotherapeutic agent for GC, we aimed to determine whether CST2 expression affects the therapeutic efficacy of oxaliplatin.
A Cell Counting Kit-8 (CCK-8) assay was conducted to evaluate the cytotoxicity in CST2 overexpression and control cells, showing that CST2 upregulation could enhance chemosensitivity to oxaliplatin in GC cells. The IC 50 of oxaliplatin for MGC803-CST2 cells (12.81 μM) proved to be substantially lower than that for the control cells (27.77 μM). In SGC7901 cells, the IC 50 of oxaliplatin was reduced from 49.42 to 31.17 μM. However, such change was not observed in GES-1 cells (Fig. 4 A).
A The IC 50 for oxaliplatin was determined for GES-1, MGC803, and SGC7901 cell lines using cell viability assays. B Cell colony formation assays were performed to confirm changes in oxaliplatin sensitivity among the three groups. The number of colonies was counted and graphically represented. C Following oxaliplatin treatment, differential immunofluorescence staining of phalloidin was used to visualize changes in microfilaments within GC cells. Data are displayed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
Cell colony formation assays were conducted for MGC803/SGC7901-CST2 and control groups to validate these results further. We plated 500 cells in a 6-well plate and treated them with low doses of oxaliplatin (0.1 μM for MGC803 and 0.25 μM for SGC7901) for 12 days; the results showed that CST2 overexpression significantly inhibited colony formation in GC cells. GC cells also observed that treatment with 10 μg/mL oxaliplatin induced a change to a spindle-shaped morphology and cell scattering in GC cells (Fig. 4 B).
Subsequently, an examination was undertaken to determine whether CST2 could regulate the reassembly of actin filaments in GES-1, MGC803, and SGC7901 cells. Fluorescent phalloidin staining was utilized to visualize the distribution of filamentous actin (F-actin). Oxaliplatin treatment was found to disrupt microfilament formation and to increase the area covered by microfilaments. Additionally, CST2 overexpression further disrupted microfilament formation and significantly enlarged the microfilaments. These findings indicate that CST2 overexpression may induce microfilament disruption following oxaliplatin treatment. However, these effects were not manifested in the gastric epithelial cell line GES-1 (Fig. 4 C).
CST2 facilitates cell cycle arrest and apoptosis induced by oxaliplatin in GC cells
To explore the effects of CST2 on oxaliplatin response, MGC803 and SGC7901 cells were treated with low concentrations of oxaliplatin over 48 h. Flow cytometry analysis indicated that increased CST2 levels improved oxaliplatin-induced apoptosis in GC cells (Fig. 5 A). Furthermore, CST2 overexpression showed favorable modulation in the protein levels of p-AKT, Bcl-2, BAX, and BAD, following oxaliplatin treatment (Fig. 5 B, C ). The impact of oxaliplatin on cell-cycle progression was also assessed using DNA content analysis via flow cytometry. Compared with control cells, cells overexpressing CST2 demonstrated a significant decrease in the percentage of G0/G1-phase cells and a lower proportion of cells in S and G2/M phases (Fig. 5 D). Western blot analysis showed that the increased expression of Cyclin B1 protein following oxaliplatin treatment was significantly reduced in MGC803/SGC7901 cells with CST2 overexpression (Fig. 5 E).
A The effects of CST2 on apoptosis of GC cells under oxaliplatin treatment were assessed by measuring the percentages of apoptotic cells in each group using flow cytometry. B , C Western blot analysis was carried out to measure the expression levels of AKT and apoptosis-related proteins in the representative groups. D Flow cytometry was used to analyze the effects of oxaliplatin on the cell cycle of various GC cell lines. E The expression levels of Cyclin B1 were assessed by Western blot analysis. Data are displayed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001
The development and progression of gastric cancer (GC) involves a complex interplay of numerous steps and genes. Therefore, understanding abnormal gene expression during carcinogenesis holds significant theoretical and practical importance. The high mortality rate associated with gastric cancer and its resistance to current chemotherapeutic agents highlight the urgent need for novel antitumor drugs, a primary focus for clinical researchers. Given the challenges in developing new antitumor agents, it is prudent to aim for reducing tumor resistance and enhancing the efficacy of chemotherapeutic agents (Lordick et al. 2022 ; Qu et al. 2023 ).
In our previous study, we confirmed and substantiated that CST2 RNA levels were inversely correlated with clinical data in the database. Unexpectedly, we discovered that CST2 was markedly expressed in tissues and exerted an anticancer effect (Xie et al. 2021 ). Furthermore, the literature lacks information on CST2 protein expression in GC tissues and cells. This observation could be attributed to the selective tumor inhibitory effect of CST2 on gastric cancer cells, sparing normal gastric epithelial cells. Investigating the potential relationship between these observations is crucial for guiding our subsequent research. If this relationship is linked to clinical drug resistance, it could improve the specificity of chemotherapeutic agents for gastric cancer cells and mitigate their cytotoxic effects on normal gastric epithelial cells, thereby enhancing chemotherapy outcomes for gastric cancer patients and reducing harm.
This study confirms that CST2 expression in gastric cancer tissues and cell lines is significantly lower than in normal gastric tissues and epithelial cells. Additionally, we generated multiple CST2 overexpression and knockdown cell models and validated the impact of CST2 expression levels on biological processes, including proliferation, apoptosis, migration, and invasion in GC cells. This was assessed through cell counting, colony formation, scratch wound healing, transwell migration/invasion assays, and flow cytometric analysis. These findings suggest that CST2 overexpression inhibits cell proliferation, migration, and invasion while enhancing the efficacy of oxaliplatin. Furthermore, we established a xenograft model in nude mice and observed that the CST2 overexpression significantly inhibited GC tumor growth in vivo compared to the control groups. Overall, our results indicate that CST2 overexpression has the potential to attenuate GC aggressiveness and progression both in vitro and in vivo.
The PI3K pathway is frequently dysregulated in human malignancies, regulating fundamental aspects of cancer such as cell proliferation, migration, metastasis, and survival (Alzahrani 2019 ; Gallardo et al. 2012 ). AKT, a serine-threonine kinase and a key target of PI3K, modulates numerous downstream target genes (Altomare and Testa 2005 ; Revathidevi and Munirajan 2019 ). To investigate the involvement of the PI3K/AKT signaling pathway in the oncogenic mechanism of CST2 in gastric cancer, we assessed the relationship between CST2 and p-PI3K, p-AKT, and other apoptosis-related proteins. Compared to the negative control (NC) groups, phosphorylation levels of PI3K and AKT were significantly reduced following CST2 overexpression, indicating CST2’s inhibition of PI3K/AKT pathway activation in gastric cancer. To further elucidate the correlation between CST2 overexpression, inhibition of gastric cancer cell proliferation and migration and decreased AKT phosphorylation, the AKT phosphorylation activator SC79 was utilized in vitro. The results suggest that AKT activation can counteract the inhibitory effects of CST2 overexpression on gastric cancer cells, implying that CST2 exerts its effects through the AKT pathway.
Commonly utilized drugs in gastric cancer chemotherapy induce adriamycin (ADR), platinum-based agents, 5-fluorouracil (5-FU), vincristine (VCR), and paclitaxel (PTX) (Rottenberg et al. 2021 ). Oxaliplatin, a second-generation platinum-based agent, is recommended for both adjuvant and palliative chemotherapy in gastric cancer treatment (Fong et al. 2022 ). Its principal cytotoxic mechanism involves the inhibition of DNA synthesis. Intrinsic or acquired resistance to oxaliplatin may lead to a poor prognosis. Oxaliplatin resistance in gastric cancer has been reported to be related to the PI3K/AKT pathway (Ren et al. 2023 ). Consequently, we explored the relationship between CST2, oxaliplatin sensitivity, and the PI3K/AKT pathway. The results demonstrated a significant enhancement of oxaliplatin sensitivity in gastric cancer cells overexpressing CST2. Upon exposure to equivalent concentrations of oxaliplatin, groups with CST2 overexpression showed significantly reduced levels of p-AKT and apoptosis-antagonizing proteins, as well as increased levels of pro-apoptotic proteins, compared to the control group. These findings suggest that CST2 may augment oxaliplatin sensitivity in gastric cancer cells by suppressing the PI3K/AKT pathway activity.
Previous studies have shown that the PI3K/AKT signaling pathway constitutes a pivotal nexus in cancer cells, governing cell growth, migration, proliferation, and metabolism. Targeting the oncogenic PI3K/AKT signaling pathway is currently considered a highly promising strategy for gastric cancer intervention. Apoptosis plays a crucial role in tumorigenesis, development, and drug resistance (Braicu et al. 2022 ; Yu et al. 2020 ). Suppression of the PI3K/AKT pathway induces cell apoptosis through diverse mechanisms, including modulating the activities of Bcl-2 family members and activating caspase family proteases (Kircher et al. 2019 ). In conclusion, our findings suggest that CST2 may modulate both the activity and the drug resistance of gastric cancer cells by inhibiting the PI3K/AKT signaling pathway. Furthermore, CST2 could suppress GC cell proliferation, migration, and invasion, while also augmenting the efficacy of oxaliplatin through the inhibition of the PI3K/AKT signaling pathway. This positions CST2 as a potential prognostic marker and therapeutic target for treating gastric cancer.
Conclusions
We determined and confirmed that CST2 can suppress the malignant biological behaviors of gastric cancer cells, including cellular proliferation, migration, and invasion. Additionally, CST2 enhances the chemosensitivity of gastric cancer cells to the oxaliplatin, an effect observed specifically in gastric cancer cells without affecting normal gastric epithelial cells. We observed that the effects by CST2 on gastric cancer cells may be mediated by inhibition of PI3K/AKT pathway. To substantiate these obervations, we utilized the AKT activator SC79.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
Cystatin SA
- Gastric cancer
Western blot
Cell counting Kit-8
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455–7464
Article CAS PubMed Google Scholar
Alzahrani AS (2019) PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol 59:125–132
Bao Y, Wang L, Shi L, Yun F, Liu X, Chen Y, Chen C, Ren Y, Jia Y (2019) Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell Mol Biol Lett 24:38
Article PubMed PubMed Central Google Scholar
Braicu C, Zanoaga O, Zimta AA, Tigu AB, Kilpatrick KL, Bishayee A, Nabavi SM, Berindan-Neagoe I (2022) Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Semin Cancer Biol 80:218–236
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Article PubMed Google Scholar
Campbell PT, Sloan M, Kreiger N (2007) Physical activity and stomach cancer risk: the influence of intensity and timing during the lifetime. Eur J Cancer 43:593–600
Casamayor M, Morlock R, Maeda H, Ajani J (2018) Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 12:883
Chen YF, Ma G, Cao X, Luo RZ, He LR, He JH, Huang ZL, Zeng MS, Wen ZS (2013) Overexpression of cystatin SN positively affects survival of patients with surgically resected esophageal squamous cell carcinoma. BMC Surg 13:15
Article CAS PubMed PubMed Central Google Scholar
Cheng A, Zhao S, FitzGerald LM, Wright JL, Kolb S, Karnes RJ, Jenkins RB, Davicioni E, Ostrander EA, Feng Z et al (2019) A four-gene transcript score to predict metastatic-lethal progression in men treated for localized prostate cancer: development and validation studies. Prostate 79:1589–1596
Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM (2002) beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 62:3503–3506
CAS PubMed Google Scholar
Cui Y, Sun D, Song R, Zhang S, Liu X, Wang Y, Meng F, Lan Y, Han J, Pan S et al (2019) Upregulation of cystatin SN promotes hepatocellular carcinoma progression and predicts a poor prognosis. J Cell Physiol 234:22623–22634
Feng R, Chen X, Yu Y, Su L, Yu B, Li J, Cai Q, Yan M, Liu B, Zhu Z (2010) miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett 298:50–63
Fong C, Johnston E, Starling N (2022) Neoadjuvant and adjuvant therapy approaches to gastric cancer. Curr Treat Options Oncol 23:1247–1268
Gallardo A, Lerma E, Escuin D, Tibau A, Muñoz J, Ojeda B, Barnadas A, Adrover E, Sánchez-Tejada L, Giner D et al (2012) Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer 106:1367–1373
Ishaq S, Nunn L (2015) Helicobacter pylori and gastric cancer: a state of the art review. Gastroenterol Hepatol Bed Bench 8:S6–S14
PubMed PubMed Central Google Scholar
Kim J, Cho YA, Choi WJ, Jeong SH (2014) Gene-diet interactions in gastric cancer risk: a systematic review. World J Gastroenterol 20:9600–9610
Kircher DA, Trombetti KA, Silvis MR, Parkman GL, Fischer GM, Angel SN, Stehn CM, Strain SC, Grossmann AH, Duffy KL et al (2019) AKT1(E17K) activates focal adhesion kinase and promotes melanoma brain metastasis. Mol Cancer Res 17:1787–1800
Koblinski JE, Ahram M, Sloane BF (2000) Unraveling the role of proteases in cancer. Clin Chim Acta 291:113–135
Lah TT, Babnik J, Schiffmann E, Turk V, Skaleric U (1993) Cysteine proteinases and inhibitors in inflammation: their role in periodontal disease. J Periodontol 64:485–491
Li P, Zhang X, Wang H, Wang L, Liu T, Du L, Yang Y, Wang C (2017) MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther 16:739–751
Li Q, Zheng ZC, Ni CJ, Jin WX, Jin YX, Chen Y, Zhang XH, Chen ED, Cai YF (2018) Correlation of cystatin E/M with clinicopathological features and prognosis in triple-negative breast cancer. Ann Clin Lab Sci 48:40–44
Liu X, Wu J, Zhang D, Bing Z, Tian J, Ni M, Zhang X, Meng Z, Liu S (2018) Identification of potential key genes associated with the pathogenesis and prognosis of gastric cancer based on integrated bioinformatics analysis. Front Genet 9:265
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC (2022) Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:1005–1020
Qin Y, Yang G, Li M, Liu HJ, Zhong WL, Yan XQ, Qiao KL, Yang JH, Zhai DH, Yang W et al (2017) Dihydroartemisinin inhibits EMT induced by platinum-based drugs via Akt-Snail pathway. Oncotarget 8:103815–103827
Qu X, Liu B, Wang L, Liu L, Zhao W, Liu C, Ding J, Zhao S, Xu B, Yu H et al (2023) Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist Updates 68:100936
Article CAS Google Scholar
Ren J, Hu Z, Niu G, Xia J, Wang X, Hong R, Gu J, Wang D, Ke C (2023) Annexin A1 induces oxaliplatin resistance of gastric cancer through autophagy by targeting PI3K/AKT/mTOR. FASEB J 37:e22790
Revathidevi S, Munirajan AK (2019) Akt in cancer: Mediator and more. Semin Cancer Biol 59:80–91
Rottenberg S, Disler C, Perego P (2021) The rediscovery of platinum-based cancer therapy. Nat Rev Cancer 21:37–50
Sun R, Wu J, Chen Y, Lu M, Zhang S, Lu D, Li Y (2014) Down regulation of Thrombospondin2 predicts poor prognosis in patients with gastric cancer. Mol Cancer 13:225
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Xie Q, Liu L, Chen X, Cheng Y, Li J, Zhang X, Xu N, Han Y, Liu H, Wei L et al (2021) Identification of cysteine protease inhibitor CST2 as a potential biomarker for colorectal cancer. J Cancer 12:5144–5152
Yu X, Li S, Pang M, Du Y, Xu T, Bai T, Yang K, Hu J, Zhu S, Wang L et al (2020) TSPAN7 exerts anti-tumor effects in bladder cancer through the PTEN/PI3K/AKT pathway. Front Oncol 10:613869
Download references
Acknowledgements
The results here are partially based upon data generated by the GEO and TCGA Research Network. We thank Mingliang Wang for his technical supports. We thank Xin Xu for the helpful suggestions for this manuscript. We further thank all volunteers who participated in this study.
This work was supported by the National Natural Science Foundation of China (81874063), the Natural Science Foundation of Anhui Province (2008085QH408) and the Scientific Research Foundation of Education Department of Anhui Province (2023AH053334). The funding bodies had no role in the design of the study or the collection, analysis, and interpretation of data or in writing the manuscript.
Author information
Yida Lu and Huizhen Wang contributed equally to this work.
Authors and Affiliations
Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
Yida Lu, Huizhen Wang, Bo Yang, Yaxian Li & Yongxiang Li
Taikang Ningbo Hospital, Ningbo, Zhejiang, 315000, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Yida Lu and Sihan Chen: experimentation and writing of the manuscript. Bo Yang: clinical data collection and analysis. Yaxian Li: assistance in cell function experiments and clinical data compilation. Huizhen Wang and Yongxiang Li: concept and design, supervision and writing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Yongxiang Li .
Ethics declarations
Conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
The study was conducted in accordance with relevant guidelines and regulations. This present study was approved by the Institute Biomedical Research Ethics Committee of Anhui Medical University and written informed consent was obtained from all patients involved. All experiments involving animals follow the guidelines of the Animal Center of Anhui Medical University, and all animal experimental protocols were approved by the Experimental Animal Ethical Committee of Anhui Medical University. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 2700 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Lu, Y., Wang, H., Chen, S. et al. Cystatin SA attenuates gastric cancer cells growth and increases sensitivity to oxaliplatin via PI3K/AKT signaling pathway. J Cancer Res Clin Oncol 150 , 244 (2024). https://doi.org/10.1007/s00432-024-05780-9
Download citation
Received : 03 March 2024
Accepted : 03 May 2024
Published : 08 May 2024
DOI : https://doi.org/10.1007/s00432-024-05780-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Oxaliplatin
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Gastric cancer articles within Nature Reviews Gastroenterology & Hepatology
In Brief | 21 April 2023
Zolbetuximab treatment in metastatic gastric cancer
- Eleni Kotsiliti
In Brief | 08 February 2023
Effect of bariatric surgery on risk of oesophagogastric cancer
- Jordan Hindson
In Brief | 28 November 2022
A novel pathomics signature for gastric cancer
Review Article | 07 November 2022
Current developments in gastric cancer: from molecular profiling to treatment strategy
Gastric and gastro-oesophageal cancer is a leading cause of cancer-related death worldwide with a poor prognosis. This Review provides a comprehensive overview of current treatment strategies set on a molecular basis, and discusses future therapeutic avenues.
- Maria Alsina
- , Virginia Arrazubi
- & Josep Tabernero
In Brief | 06 June 2022
Promising phase I interim results for CAR T cells in gastrointestinal cancers
- Katrina Ray
Review Article | 14 March 2022
The immune microenvironment in gastric adenocarcinoma
In this Review, the authors describe how the chronic inflammatory microenvironment in the gastric mucosal epithelia during Helicobacter pylori infection can stimulate intracellular signalling pathways that lead to the development of gastric adenocarcinoma.
- Yana Zavros
- & Juanita L. Merchant
In Brief | 11 January 2022
KEYNOTE-811: pembrolizumab in advanced HER2 + gastric cancer
In Brief | 22 June 2021
Nivolumab plus chemotherapy for advanced gastric cancer and oesophageal adenocarcinoma
Research Highlight | 26 February 2020
New markers and models of premalignancy and the early development of gastric cancer
In Brief | 21 February 2020
H. pylori elimination reduces gastric cancer risk
- Iain Dickson
Research Highlight | 07 September 2018
Exploring human gastric cancers through organoid culture
- Hugh Thomas
Research Highlight | 20 August 2018
Immunotherapy-responsive gastric cancers identified
- Peter Sidaway
News & Views | 30 April 2018
Gastric cancer: evidence boosts Helicobacter pylori eradication
The efficacy of Helicobacter pylori eradication therapy for the prevention of preneoplastic lesions in gastric cancer remains controversial. A new placebo-controlled trial and a large-scale observational study tackle this problem and show the positive effects of eradication therapy.
- Hidekazu Suzuki
- & Juntaro Matsuzaki
Review Article | 21 February 2018
Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer
The stomach responds to injury via two main patterns, the superficial response and the glandular response. In this Review, Sáenz and Mills discuss cellular plasticity and reprogramming in the stomach in the context of disease (such as gastric cancer) and during repair and homeostasis.
- José B. Sáenz
- & Jason C. Mills
In Brief | 02 November 2017
The ATTRACTION of nivolumab for gastric and gastro-oesophageal junction cancer
News & Views | 18 October 2017
The gastric microbiota — bacterial diversity and implications
Although Helicobacter pylori is associated with gastric cancer, bacterial communities that reside in the stomach are mostly unacknowledged. A new study shows that some gastric bacterial communities have emigrated from our mouth, prefer certain neighbours and prefer certain environments. By understanding the interactions of these bacteria, we hope to understand the environment most conducive to gastric cancer carcinogenesis.
- Manish A. Shah
Research Highlight | 06 September 2017
R-spondin 3 is a critical regulator of gastric antral stem cell homeostasis
Research Highlight | 21 June 2017
A chief role for Lgr5 + cells
Review Article | 17 May 2017
How to stomach an epigenetic insult: the gastric cancer epigenome
Gastric cancer is a deadly malignancy and accumulating evidence suggests that epigenetic abnormalities promote carcinogenesis. Here, the authors summarize the gastric cancer epigenome, highlighting key advances from studies of DNA methylation and histone modifications, and how these findings might lead to therapeutic opportunities.
- Nisha Padmanabhan
- , Toshikazu Ushijima
- & Patrick Tan
Review Article | 05 January 2017
Population screening and treatment of Helicobacter pylori infection
Helicobacter pylori remains an important human pathogen with links to both malignant (gastric cancer) and non-malignant diseases (such as peptic ulcer). Here, the authors discuss issues related to implementation of population screening and eradication of H. pylori infection.
- Anthony O'Connor
- , Colm A. O'Morain
- & Alexander C. Ford
Research Highlight | 12 October 2016
Settling the stomach — tracing gastric stem cells
Research Highlight | 21 September 2016
DARPP-32 : a link between infection and gastric cancer
- Charlotte Ridler
Research Highlight | 20 July 2016
Dysregulation of RNA editing in gastric cancer
News & Views | 05 May 2016
Pathogenic enablers — toxic relationships in the stomach
Chronic infection with Helicobacter pylori is the strongest known risk factor for the development of gastric cancer. Saju et al . shed new light on mechanisms by which Epstein–Barr virus, a viral initiator of gastric cancer, potentiates the oncogenic effects of Helicobacter pylori in the stomach.
- Lydia E. Wroblewski
- & Richard M. Peek Jr
Research Highlight | 28 April 2015
Breathing a sigh of relief for noninvasive cancer detection
- Gillian Patman
Research Highlight | 27 January 2015
Metformin improves survival and recurrence rate in patients with diabetes and gastric cancer
- Claire Greenhill
In Brief | 20 January 2015
New model for Helicobacter pylori infection developed
In Brief | 25 November 2014
Distinct tumour signatures observed in Asian and non-Asian patients with gastric cancer
Review Article | 19 August 2014
Genetics of gastric cancer
Gastric cancer accounts for a notable proportion of cancer mortality around the world. Over the past few decades, advances in technology and high-throughput analysis have improved understanding of the molecular aspects of the pathogenesis of gastric cancer. This Review discusses these genetic aspects of the pathogenesis of gastric cancer.
- Mairi H. McLean
- & Emad M. El-Omar
Research Highlight | 12 August 2014
New molecular classification of gastric adenocarcinoma proposed by The Cancer Genome Atlas
- Mina Razzak
Research Highlight | 27 May 2014
Revealing the genomic landscape of gastric cancer
In Brief | 15 April 2014
Helicobacter pylori infection improves response to cisplatin
Research Highlight | 15 October 2013
New biologic therapy effective as second-line treatment in gastric cancer
- Katherine Smith
In Brief | 09 April 2013
Breath test for gastric cancer
Research Highlight | 22 May 2012
Integrated epigenomic analysis sheds light on role of BMP4 in regulating cisplatin sensitivity in gastric cancer
- Natalie J. Wood
News & Views | 06 March 2012
Localized gastric cancer—a CLASSIC shift in the paradigm?
The management of localized gastric cancer has evolved globally to region-specific approaches with little convergence. The unifying theme, however, is that surgery alone is insufficient for best outcomes, and adjunctive therapy must be offered. Multidisciplinary evaluation before therapy is highly recommended, but postoperative approaches are not conducive to this scenario.
- Mariela A. Blum
- & Jaffer A. Ajani
Research Highlight | 14 February 2012
Folate prevents gastric cancer
- Andy McLarnon
News & Views | 14 February 2012
Secondary prevention of gastric cancer
The natural history of 'epidemic' intestinal-type gastric cancer provides consistent data that enable clinicians to design multidisciplinary strategies for secondary prevention. A recent paper by Dinis-Ribeiro et al . reports guidelines for the management of patients with precancerous stomach lesions.
- Massimo Rugge
- , Matteo Fassan
- & David Y. Graham
Research Highlight | 13 December 2011
Bone-marrow-derived cells could cause gastric preneoplasia in chronic Helicobacter pylori infection
Research Highlight | 05 October 2011
ESD is associated with a moderate risk of deep vein thrombosis that may be determined by D-dimer levels
Review Article | 04 July 2011
Anti-HER agents in gastric cancer: from bench to bedside
Despite some advances, the search for effective treatment modalities for advanced gastric and gastro-esophageal junction cancer (GEJC) is far from over. However, using biologic agents to target key molecular pathways, such as those regulated by human epidermal growth factor receptor (HER) family members, may be an effective approach. This Review briefly describes HER biology, summarizes available data regarding the clinical activity of anti-HER agents and their use in gastric cancer and GEJC, and provides insight into treatment personalization strategies.
- Lorenzo Fornaro
- , Maurizio Lucchesi
- & Alfredo Falcone
Research Highlight | 06 June 2011
Gene fusion identified in gastric cancer
Research Highlight | 05 April 2011
Helicobacter pylori ancestry associated with cancer risk
Research Highlight | 07 March 2011
Value of CLE for gastric cancer detection
- Rachel Thompson
Research Highlight | 01 November 2010
FAK autophosphorylation independently predicts recurrence of gastric cancer
- Shreeya Nanda
Review Article | 12 October 2010
Mechanisms of disease: Helicobacter pylori virulence factors
Helicobacter pylori has an essential role in the development of various gastroduodenal diseases, but only a small proportion of people infected with H. pylori develop these diseases. In this Review, Yoshio Yamaoka discusses current knowledge of the H. pylori virulence factors CagA, VacA, OipA and DupA. In particular, he considers how these virulence factors contribute to differing geographic gastric cancer disease patterns and to the development of both gastric cancer and duodenal ulcer.
- Yoshio Yamaoka
Browse broader subjects
- Stomach diseases
- Gastrointestinal cancer
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Gastric cancer.
Shiva Kumar R. Mukkamalla ; Alejandro Recio-Boiles ; Hani M. Babiker .
Affiliations
Last Update: July 4, 2023 .
- Continuing Education Activity
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide. The only potentially curative treatment approach for patients with gastric cancer is surgical resection with adequate lymphadenectomy. Regrettably, patients with an unresectable, locally advanced, or metastatic disease can only be offered life-prolonging palliative therapy regimens. This activity discusses the etiology, epidemiology, evaluation, and treatment modalities for gastric cancer and outlines the interprofessional team's role in providing the optimal management for patients with gastric cancer.
- Review the epidemiology and etiology of gastric cancer.
- Outline the typical clinical presentation and evaluation of a patient with gastric cancer.
- Summarize the treatment modalities and palliative care strategies for gastric cancer.
- Explain the significance of improving care coordination among the interprofessional team to enhance care delivery for patients with gastric cancer.
- Introduction
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide, albeit there has been a global decline since the last mid-century. In the United States, gastric cancer incidence has decreased during the past few decades, although the incidence of gastroesophageal cancer has concomitantly increased. There are two distinct types of gastric adenocarcinoma, intestinal (well-differentiated) and diffuse (undifferentiated), which have a distinct morphologic appearance, pathogenesis, and genetic profiles. The only potentially curative treatment approach for patients with gastric cancer is surgical resection with adequate lymphadenectomy. Current evidence supports perioperative therapies to improve a patient’s survival. Regrettably, patients with an unresectable, locally advanced, or metastatic disease could solely be offered life-prolonging palliative therapy regimens. [1]
Factors linked with an increased risk of gastric cancer include nutritional factors such as high-salt (salt-preserved food), N-nitroso compounds consumption (dietary source), smoking, a low vitamin A and C diet, consuming large amounts of smoked or cured foods, a deficit of refrigerated foods, and contaminated drinking water. High body mass index (BMI), increased calorie consumption, gastroesophageal reflux, and smoking are associated with an increased risk of adenocarcinomas of the distal esophagus, proximal stomach, and junction. Occupational exposure to rubber manufacturing, tin mining, metal processing, and coal also increases the risk. Helicobacter pylori infection has an attributable risk of 46% to 63%, while Epstein-Barr virus infection is an estimated 5% to 10% worldwide. Radiation exposure and prior gastric surgery also have been implicated as risk factors.
Diverse meta-analysis has shown that high consumption of fiber (relative risk (RR) 0.58 95% CI 0.49-0.67), fruits (RR 0.90, 95% CI 0.83-0.98 and vegetables (RR 0.96, 95% CI 0.88-1.06) have a probable protective benefit against gastric cancer. Aspirin and other non-steroidal anti-inflammatory agents have been associated with a lower risk of cancer of the gastroesophageal junction and other gastrointestinal tumors (HR 0.79 for each year of NSAID use). Alcohol consumption has not been demonstrated as a risk factor, and in fact, some data suggest daily wine intake may be protective despite insufficient evidence. Chronic “iatrogenic” histamine-2-receptor antagonist or proton pump inhibitor has not been associated with gastric cancer.
Host factors include type A blood with an approximate 20% more gastric cancer cases than in blood groups O, B, or AB and are particularly associated with the diffuse type. Pernicious anemia, an autoimmune chronic atrophic gastritis, has up to six-fold increased risk of intestinal-type gastric cancer. Benign gastric ulcers, hypertrophic gastropathy, and gastric polyps are risk factors associated with an increased risk of stomach cancer.
Most gastric cancers are sporadic, but 5% to 10% of cases have a family history of gastric cancer. Hereditary diffuse gastric cancer (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal gastric cancer (FIGC) are three major syndromes accounting for up to 3% to 5% of hereditary familial gastric cancer. Other hereditary cancer syndromes are:
- Hereditary non-polyposis colon cancer (HNPCC 13% lifetime risk, predominantly intestinal type)
- Familial adenomatous syndrome (FAP, 10% risk)
- Peutz Jeghers syndrome (PJS, 29% risk)
- Juvenile polyposis syndrome (JPS, 21%)
- Li-Fraumeni syndrome
- Hereditary breast and ovarian cancer syndrome
- Phosphatase and tensin homolog (PTEN) or hamartoma tumor (Cowden's) syndrome.
However, all these are rare causes of gastric cancer. It is recommended to follow screening guidelines on hereditary syndromes associated with gastric cancer according to their risk. Certain polymorphisms have been associated with gastric cancer. Carriers of IL-1B-511*T/*T or IL-1B-511*T/*C that are up-regulated by H. pylori infection could cause a pro-inflammation and acid inhibition leading to malignancy. Intestinal-type gastric carcinogenesis may have overexpressed oncogenes (K-ras and c-met) or tumor suppressors (TP53, APC, TTF, and CDKN1B,p27); although, not consistently present.
For cancer research, the World Health Organization has classified H. pylori as a definite gastric carcinogen and likewise concluded a positive association between the consumption of processed meat and stomach cancer. [2] [3] [4]
- Epidemiology
Gastric cancer rates are significantly declining worldwide. However, the pace has been variable in different regions such as China and Japan. An estimated 28,000 new cases will be diagnosed with gastric cancer in the United States, with an expected 10,960 new deaths during 2017. Gastric cancer decline may be due to identification and treatment of infectious causes in addition to lifestyle modifications of dietary and environmental risk factors, but it is still common in regions of the world where the storage of fresh foods and the quality of water are poor. The vast majority of gastric cancer occurs in developing countries, twice more common in men than women, more frequent in black men than in white men. The lowest incidence is in white western society with higher socioeconomic status.
Migration studies have supported evidence for the effect of lifestyle changes on gastric cancer development, as the second and third generation born in the United States have lower rates. Prior concepts of gastric cancer strongly support the notion that nutritional, socioeconomic, and medical factors rather than genetic predisposition have been demonstrated on Japanese migrants. Gastric cancer’s histological patterns have also seen an epidemiological change in the pattern; whereas, the intestinal gastric type is on a gradual decline but continues to be more common (70%). Characteristically, it is seen in males above their fifth decade with linked environmental factors. Contrarily, the diffuse or infiltrative type is less frequent (30%) but diagnosed at a younger age in both sexes and carries a worse prognosis. A major anatomical shift from distal to proximal stomach cancer parallels the increasing incidence of distal esophageal carcinoma in the United States. The proximal lesser curvature, cardiac, and esophagogastric junction (EGJ) are the most common sites in Western countries, whereas non-proximal continues to predominate in Japan. In Japan, gastric cancer is associated with a distinctly better prognosis than in the United States, largely due to endoscopic screening programs contributing to detecting early lesions and potentially curable stage. [5] [6] [7]
- Pathophysiology
There are two main histologic variants of gastric adenocarcinoma based on Lauren's histopathologic classification. The most frequent is the "intestinal type," so-called because of its morphologic similarity to adenocarcinomas arising in the intestinal tract. The less common diffuse-type gastric cancer is characterized by a lack of intercellular adhesions, which disrupt the formation of glandular structures.
In patients with an inherited form of diffuse-type gastric cancer, the absence of intercellular adhesions is caused by a germline mutation (HDGC) in the cell adhesion protein E-cadherin (CDH1). HDGC is inherited as an autosomal dominant trait with high penetrance, highly invasive, late presentation (average age 38), and poor prognosis. CDH1 asymptomatic carriers may warrant prophylactic gastrectomy before age 30, and women are also at risk of early breast cancer. The diffuse type has no clear precancerous lesions. One model for the "intestinal-type" of gastric cancer describes a progression from chronic gastritis caused by H. pylori , pernicious anemia, or high-salt diets leading to a loss of parietal cells, therefore, reducing acid production causing chronic atrophic gastritis. Atrophic gastritis compensatory hypergastrinemia induces chronic inflammation resulting in intestinal metaplasia, dysplasia, and, eventually, adenocarcinoma. It was estimated that approximately 1 in 39 patients with intestinal metaplasia (hazard ratio [HR] 6.2, 95% CI 4.7-8.2) and 1 in 19 with dysplasia (HR 10.9, 95% CI 7.7-15.4) would develop gastric cancer within 20 years. As opposed to the diffuse-type cell lines, the intestinal-type cancer cell lines are significantly more sensitive to 5-fluorouracil and oxaliplatin but more resistant to cisplatin.
Results from several studies have demonstrated an approximately sixfold increase in H. pylori infection in patients with gastric cancer, particularly adenocarcinoma of the distal stomach, including both intestinal and diffuse types. As mentioned above, H. pylori trigger inflammation, resulting in stomach atrophy and subsequent metaplasia culminating in carcinoma. Moreover, most patients with H. pylori infection develop ulcers and not a malignancy. No definitive evidence of bacterial virulence (vacAs1-, vacAm1-, and cagA-positive) or widespread mass eradication could increase or reduce gastric cancer incidence. As per current guidelines, screening and treatment of H. pylori infections are recommended. [8] [9]
- History and Physical
In the United States, most patients have symptoms of an advanced stage at the time of presentation. The most common presenting symptoms for gastric cancers are non-specific weight loss, persistent abdominal pain, dysphagia, hematemesis, anorexia, nausea, early satiety, and dyspepsia. Patients presenting with a locally-advanced or metastatic disease usually present with significant abdominal pain, potential ascites, weight loss, fatigue, and have visceral metastasis on scans, and can have a gastric-outlet obstruction.
The most common physical examination finding is a palpable abdominal mass indicating advanced disease. The patient may also present with signs of metastatic lymphatic spread distribution, including Virchow’s node (left supraclavicular adenopathy), Sister Mary Joseph node (peri-umbilical nodule), and Irish node (left axillary node). Direct metastasis to the peritoneum can present as Krukenberg’s tumor (ovary mass), Blumer’s shelf (cul-de-sac mass), ascites (peritoneal carcinomatosis), and hepatomegaly (often diffuse disease burden).
Paraneoplastic manifestations may include dermatological (diffuse seborrheic keratosis or acanthosis nigricans), hematological (microangiopathic hemolytic anemia and hypercoagulable state [Trousseau’s syndrome]), renal (membranous nephropathy), and autoimmune (polyarteritis nodosa) are rare clinical findings, and none is specific to gastric cancer. [10]
Patients presenting with any symptoms suspicious for gastric cancer should undergo an upper endoscopy over barium study (except for limited plastic presenting as leather-flask appearance). Although upper endoscopy is more invasive and costly, it offers tissue diagnosis by direct biopsy of esophageal, gastric, or duodenal lesions. Any suspicious gastric ulcer should be biopsied multiple times for higher diagnostic accuracy (one (70%) versus seven (98%) sensitivity). Gastric cancer screening by upper endoscopy has successfully detected early stages with higher curable rates after resection only in areas of high cancer incidence (Japan).
The American Joint Committee on Cancer/ Union for International Cancer Control (AJCC/UICC) Eight Edition 2017 has outlined a new staging scheme based on tumor, node, metastasis (TNM) with 5-year overall survival (5-y OS) according to pathological stage and intervention (surgery only IA-93.6%, IIA-81.8%, and IIIA-54.2% or with neoadjuvant I-76.5%, II-46.3%, III-18.3%, and IV-5.7%).
Staging pre-preoperative evaluations include chest and abdominal imaging to rule out metastasis and to determine surgical resectability. Abdominopelvic computerized tomography is performed early to rule out gross metastatic disease but does not accurately assess T, N, and small peritoneal metastases with an overall accuracy of 42% to 82%. Endoscopic ultrasound has a better diagnostic accuracy of tumor depth (57% to 88%) and lymph node status (30% to 90%), and hence, helps with accurate staging but is operator dependent. Biopsies should confirm suspicious solitary or oligometastatic sites; similarly, paracentesis should be performed if malignant ascites is suspected. Chest computerized tomography (CT) is preferred over plain radiograph. If prior staging evaluation is negative for the metastatic disease, positron emission tomography combined with computerized tomography imaging may help determine the resectability of gastric cancers in select cases (T2N0). Serum markers (carcinoembryonic antigen, glycoprotein CA 125 antigen, carbohydrate antigen 19-9, and cancer antigen 72-4) have limited utility and may be elevated due to other causes. Staging laparoscopy with peritoneal cytology analysis is indicated before surgery in the absence of visible spread, particularly for clinical stages with higher than T1b, and it is recommended for patients receiving preoperative therapy. Positive peritoneal cytology in the absence of identifiable peritoneal spread is an independent predictor of high recurrence after curative resection, and hence, surgery is not recommended.
Gastric cancer has reported human epidermal growth factor receptor 2 ( HER2 ) gene amplification in 12% to 27% of cases and protein overexpression in 9% to 23% of cases. The impact of HER2 positivity remains largely unclear, but it has been implicated in tumor invasion and lymph node metastasis and is associated with poorer survival. HER2 positivity is more frequently found in intestinal subtype (33%) than diffuse (8%) with lower rates in the United States (19% and 6%, respectively). HER2 testing is recommended for all metastatic gastric cancer, first using immunohistochemistry score; negative for 0 or 1+ and positive for 3+, with reflex, fluorescent, in situ hybridization for an equivocal 2+ score to confirm. The United States Food and Drug Administration (FDA) has granted approval of immunotherapy for patients with microsatellite instability in solid tumors, including gastric cancer, and can evaluate the potential of immunotherapy in patients with metastatic disease who progressed on standard therapy. Gastric tumors positive for Epstein-Barr virus (EBV) have a better prognosis; however, staining for EBV is not yet recommended in routine clinical care. [11] [12] [13] [14] [15]
- Treatment / Management
Treatment modality for gastric cancer depends on accurate preoperative staging. Therapeutic approach can be endoscopic resection for superficial, limited mucosa disease (< T1b, N0), upfront surgical resection with lymphadenectomy (< T3, any N), neoadjuvant (> T2) / adjuvant (> T1N1 or > T3N0) chemotherapy, radiation therapy, or combined with resectable lesions or palliative systemic therapy for those with locally advanced unresectable or metastatic disease (T4, any N, or M1).
Endoscopic Resection for Early Local Disease
Endoscopic resection either by endoscopic mucosal resection or endoscopic submucosal resection is offered to select patients with early gastric cancer with negative lymph nodes who meet selection criteria at centers of expertise. Standard selection criteria have a high- probability of en bloc resection, intestinal-type adenocarcinoma confined to the mucosa/submucosal, and absent venous or lymphatic invasion and tumors with diameters less than 20 mm without ulceration or 10 mm nonpolypoid flat or depressed lesions. Expanded criteria are under active investigation. Ten percent of mucosal and 20% of submucosal lesions will have lymph node metastasis and should be investigated carefully. If prior criteria are not met or an incomplete resection is performed, patients are referred for gastrectomy with regional lymph node resection. Successful endoscopic resection may offer a 5-year overall survival of 84% to 96% depending on the tumor's depth compared to gastrectomy survival rates up to 98%, but no randomized trials have compared both. Synchronous or metachronous gastric cancers can be found within 5 years in up to 9.2% of patients. H. pylori have been associated with metachronous gastric lesions, and eradication is recommended. Surveillance after endoscopic resection is the same strategy as for advanced cancer (detailed below). [16] [17] [18]
Surgical Resection for Resectable Disease
Patients with localized, resectable gastric cancer have the best chance of long-term survival with surgery alone. The main goal of surgery is complete resection with adequate margins (more than 4 cm), and only 50% of patients will obtain R0. Unresectability criteria are an invasion of major vasculature structure (aorta, hepatic artery, celiac axis, or proximal splenic artery), bulky adenopathy outside the surgical field, and the presence of linitis plastica; although, the latter is debatable. Most surgeons prefer total gastrectomy, but the technique depends on location, with proximal lesions requiring total resection and some distal lesions partial resection. Large mid-gastric lesions or diffuse disease should be offered a total gastrectomy. Routine or prophylactic splenectomy should be avoided. Standard surgical techniques in Japan, characterized by a better cancer survival, includes D2 resection (meticulous resection of all regional lymph nodes), which differs from the conservative type of lymphadenectomy performed in the United States, which carries less operative morbidity and mortality (standard D1 resection, removal of only perigastric lymph nodes). Two large trials by the Dutch Cancer Group and the Medical Research Council, comparing D1 with D2 lymphadenectomy, were flawed and underpowered to show D2 benefit. However, after a median 15-year follow-up of a 1078-patients in the randomized Dutch trial, D2 lymphadenectomy was associated with lower locoregional recurrence (12% versus 22%), regional recurrence (13% versus 19%), and gastric cancer–related death rates (37% versus 48%) than D1 surgery. Although D2 dissection was associated with significantly higher operative morbidity (10% versus 4%), complication rate (43% versus 25%), and higher reoperation rate (18% versus 8%) than D1 surgery. Considering a safer spleen-preserving D2 resection technique is currently available in high-volume centers, D2 lymphadenectomy is the recommended surgical approach for patients with resectable (curable) gastric cancer. D3 super-extended lymphadenectomy, including periaortic dissection, showed no added survival benefit with significantly worse perioperative complication rate in the multicenter Japan Clinical Oncology Group (JCOG) study 9501. While the optimal extent of lymphadenectomy is debated, current guidelines recommend 15 lymph nodes or more sampling, which showed a survival benefit. A high-volume center of excellence may offer laparoscopic resection instead of open gastrectomy, with a 5-year overall survival (OS) of 58.9% and 55.7%, respectively. Palliative resection, even with positive margins, is acceptable for symptomatic disease (obstruction or uncontrolled bleeding). [19] [20]
Neoadjuvant and Adjuvant Therapy for Locally Advanced Resectable Disease
Surgical resection alone is potentially curative but only in early gastric cancer stages as seen in long-term survival rates on reported 5-year overall survival. It significantly declines from 75% for stage I to 35% for stage II and 25% or less for stage III, pushing research efforts to improve results using neoadjuvant (preoperative) or adjuvant (postoperative) therapies.
Neoadjuvant chemotherapy has been shown to downstage primary tumors and regional lymph nodes to attempt higher long-term curative resections. Neoadjuvant therapy should be offered to patients at high risk of developing distant metastases (bulky T3/T4, perigastric nodes, linitis plastica, or positive peritoneal cytology), sparing unnecessary surgery in case an emerging metastasis appears. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial was the first well-powered randomized clinical trial with 503 patients with resectable stomach cancer (74%), GEJ (15%), and distal esophagus (11%). Inclusion criteria were performance status 0-1, histological adenocarcinoma, and T2 or higher without distant metastasis. The MAGIC trial compared perioperative chemotherapy (consisted of three preoperative and three postoperative cycles of intravenous epirubicin [E: 50 mg per square meter of body-surface area] and cisplatin [C: 60 mg per square meter] on day 1, and a continuous intravenous infusion of fluorouracil [F: 200 mg per square meter per day] for 21 days) with surgery alone. Perioperative ECF chemotherapy significantly improved median overall survival (mOS HR, 0.75; 95% CI; 0.60, 0.93 [p = 0.009], 5-year overall survival rate, 36% versus 23%) and progression-free survival (PFS HR, 0.66; 95% CI; 0.53, 0.81 [p = < 0.001]) for patients. Worth noting, only about 55% of patients in the perioperative chemotherapy group received post-resection therapy. This suggests that the main therapy component responsible for the improved outcome was the preoperative treatment phase. Similar benefits for perioperative chemotherapy for CF was reported in the French FNLCC/FFCD trial that compared two to three cycles of preoperative and three to four postoperative chemotherapy (infusional FU 800 mg/m2 daily for 5 days plus cisplatin 100 mg/m2 on day 1 or 2, every 4 weeks) or surgery alone, resulting in a significantly better R0 (84% versus 73%), 35% reduction in disease-free survival (DSF) and 31% lower risk of death (5-year overall survival 38% versus 21%). EORTC trial 40954 failed to show a survival benefit of CF in the perioperative setting due to underpowered-limited accrual. Most recently, the FLOT4-AIO phase II/III trial compared the FLOT regimen (docetaxel [D: 50 mg/m2] plus oxaliplatin [O: 85 mg/m2] and leucovorin [L: 200 mg/m2] with short-term infusional fluorouracil [F: 2600 mg/m2 as a 24-hour infusion], all on day 1 and administered every 2 weeks) to an epirubicin-containing regimen (such as ECF [Magic trial protocol] or ECX [capecitabine X: 1250 mg/m by mouth, daily days 1 to 21 equivalent substitution REAL-2 trial]). In the phase, II FLOT regimen proved to have a higher pathologic complete response rate and less toxicity profile except for neutropenia. The phase III component enrolled 716 patients with resectable gastric cancer (44%) and GEJ (56%) with a preliminary report favoring FLOT mOS 50 months versus ECF/ECX 35 months (2017 annual meeting of the American Society of Clinical Oncology [ASCO]). There is no strong evidence regarding neoadjuvant chemoradiation over chemotherapy, becoming the focus of future research. The CALGB 80101, TROG 03.02, and TOPGEAR studies will evaluate whether chemoradiation therapy, in addition to perioperative chemotherapy, will be superior to the MAGIC approach alone.
Patients who have received potential curative resection without neoadjuvant therapy but are at high risk of recurrence should be offered adjuvant chemoradiation rather than surgery alone. The SWOG 9008/INT0116, a randomized phase III trial, assigned 556 patients with stages I to III gastroesophageal (20%) or gastric cancers (80%) to receive either surgery alone or surgery followed by adjuvant combined chemoradiotherapy. The combined therapy included the Macdonald regimen (one cycle of F; 425 mg/m2 per day and L; 20 mg/m2 per day) daily for 5 days followed 1 month later by 45 Gy (1.8 Gy/day) of radiation therapy (XRT), given with F; 400 mg/m2 per day and L; 20 mg/m2 per day on days 1 through 4 and the last 3 days of XRT, followed by two more 5-day cycles of F; 425 mg/m2 per day and L; 20 mg/m2 per day given at monthly intervals beginning 1 month after XRT completion). There was an approximate 20% improvement in survival for the group receiving the combined-modality therapy. The mOS in the surgery-only group was 27 months compared with 36 months in the chemoradiation group; the HR for death in the surgery-only arm was 1.35 (95% CI; 1.09, 1.66; p = 0.005) and relapse HR was 1.52 (95% CI; 1.23, 1.86; p < 0.001). The study has been criticized for the very low rate of D1 (< 54%) or D2 (< 10%) lymph node dissection, which was mandated per protocol. It is noticeable that the rate of distant metastasis was not reduced in the adjuvant chemoradiation group (16% versus 18%), suggesting that adjuvant therapy could have mainly compensated for inferior surgery. Despite the aforementioned, INT01116 established continued to demonstrate a persistent benefit after 10 years and became a new standard of care for patients with this disease in the United States in the adjuvant setting. CALGB Intergroup C80101 tried to improve the results obtained with INT0116 protocol regimen therapy by randomly selecting patients with resected gastric or GEJ cancer to receive chemoradiotherapy with ECF. This study was not adequately powered to assess non-inferiority and did not demonstrate any difference in outcomes between the two arms. The Korean Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) trial provided further combined therapy evidence. This trial evaluated the addition of radiation therapy to adjuvant chemotherapy in patients who underwent gastrectomy with D2 lymph node dissection. Patients were randomly selected to receive six cycles of chemotherapy with CX (X; 2,000 mg/m2 per day on days 1 to 14 and C; 60 mg/m2 on day 1, repeated every 3 weeks) or two cycles of CX followed by chemoradiation (45 Gy XRT with concurrent daily X; 825 mg/m2 twice daily), followed by two more cycles of CX. At a median follow-up of 84 months, neither DFS nor OS was different between the two arms, although unplanned subsets of patients with node-positive disease and intestinal-type gastric cancer did have a significantly improved DFS with the addition of radiation therapy. The ongoing ARTIST2 trial will try to determine whether the addition of radiation therapy to adjuvant chemotherapy improves DFS in these subsets of patients at higher risk for recurrent disease.
In the United States, adjuvant chemotherapy is not frequently accepted and has not replaced combined therapy. Adjuvant chemotherapy alone was evaluated in the Japanese ACTS-GC phase III trial with S1 (an oral fluoropyrimidine 80 to 120 mg daily for 4 weeks, repeated every 6 weeks for 1 year) as postoperative D2-resection adjuvant therapy compared to surgery alone in 1059 patients with stage II or III gastric cancer and demonstrated a survival benefit with a 5-year OS rate of 72% in the S-1 group and 61% in the surgery only group (HR, 0.669; 95% CI, 0.540 to 0.828) and RFS (HR, 0.653; 95% CI, 0.537 to 0.793). By indirect comparison, the ACT-GC trial has better 5-year survival rates than INT0116 and MAGIC trials. The Korean phase III multicenter CLASSIC trial enrolled 1,035 patients with D2-resected stage II/III gastric cancer randomly assigned to adjuvant therapy with OX (eight 21-day cycles of X; 1000 mg/m2 twice daily on days 1 to 14 plus O; 130 mg/m2 on day 1) or surgery alone. Adjuvant chemotherapy had significant improvement in 3-year DFS (74% versus 59%; HR, 0.56 95% CI 0.44-0.72) and 5-year OS (78% versus 69%; HR, 0.66; 95% CI; 0.51, 0.85). In addition to these individual trials, an analysis of 34 trials with 724 patients confirmed that adjuvant chemotherapy without radiation after gastric cancer resection was associated with a significant OS benefit with an HR of 0.85 (95% CI; 0.80, 0.90) and an improvement in DFS (HR 0.79; 95% CI 0.72 to 0.87) versus surgery alone.
Standard of care varies according to regional preferences with postoperative therapy; chemoradiotherapy in the United States based on INT0116 trial, pre- and postoperative chemotherapy in the United Kingdom-based on MAGIC trial, or adjuvant chemotherapy alone after D2-resection in Korea and Japan based on ACT-GC and CLASSIC trial. Recently presented at ASCO 2016, the DUTCH CRITICS trial attempted to provide clarification by assigning 788 patients with potentially resectable stage Ib-IVa gastric cancers to receive three cycles of ECX or EOX followed by surgery later randomly allocated to three postoperative cycles of chemotherapy or chemoradiotherapy (45 Gy in 25 fractions with weekly cisplatin and daily capecitabine). Preliminary results showed no significant 5-year OS difference between chemotherapy and chemoradiotherapy (40.8 versus 40.9%). Of note, all patients had D1 or better resection, only 50% of patients completed full postoperative treatment, and toxicity profile was preferable in the chemotherapy alone group. Future enrollment in clinical trials is encouraged. [21] [22] [23] [24] [25] [26] [27] [28] [29]
Palliative Therapy for Locally Advanced Unresectable and Advanced Metastatic Disease
Unresectable locally advanced gastric cancer is often treated with advanced metastatic disease therapy regimens. The goals of medical treatment of advanced gastric cancer are primarily palliative symptoms, improve quality of life, and modest life-prolonging effect of weeks to months. Multiple agents are active in gastric cancer, including fluoropyrimidines (fluorouracil, capecitabine, and S1), anthracyclines (epirubicin), platinum agents (cisplatin and oxaliplatin), taxanes (paclitaxel and docetaxel), irinotecan, and other targeted therapies, including trastuzumab for HER2 -overexpressing gastric cancers and ramucirumab, a VEGFR2 antibody. Combination regimens are associated with an increased response rate of up to 65% compared with single-agent therapies up to 40%.
All patients with advanced gastric cancer who are chemotherapy candidates should have their tumor evaluated for HER2 overexpression. HER2 -positive patients can have trastuzumab added to a cytotoxic chemotherapy doublet backbone (most commonly fluoropyrimidine plus platinum). HER2 -negative healthy patients can be offered doublet therapy (e.g., oxaliplatin plus leucovorin and infusional FU [FOLFOX], oxaliplatin plus capecitabine [XELOX], irinotecan plus leucovorin and FU [FOLFIRI] or FU plus cisplatin [CF]) over a triplet regimen (e.g., epirubicin plus cisplatin and infusional FU [ECF], epirubicin, cisplatin and capecitabine [ECX], epirubicin plus cisplatin and capecitabine [EOX], docetaxel, cisplatin, infusional FU [DCF] or modified DCF) due to treatment toxicities reserved only for highly selected patients with excellent performance status. Poor performance or elderly patients may be appropriate for single agents (e.g., leucovorin-modulated fluorouracil alone, single-agent capecitabine, single-agent irinotecan, or low-dose weekly taxanes). Docetaxel and irinotecan have shown a survival benefit for second-line chemotherapy. Nevertheless, there is no optimal standard of care.
Various fluorouracil-backbone combination trials established the additive benefit of anthracyclines (epirubicin), platinum agents (cisplatin and oxaliplatin), or taxanes (docetaxel) over older agents (mitomycin [FAM or MCF] or methotrexate [FAMTX]). A phase III German study trial showed similar survival benefit between the FLP regimen (fluorouracil, leucovorin, and cisplatin) and the FLO regimen (fluorouracil, leucovorin, and oxaliplatin); the later regimen had a significantly superior survival benefit in patients older than 65 compared to FLP. TAX-325 was a phase III clinical trial involving 445 chemotherapy-naïve patients with gastric cancer demonstrated the superiority of the addition of docetaxel to cisplatin and fluorouracil (DCF; 21-day cycles of C; 75 mg/m2 on day 1 plus F; 750 mg/m2 daily, days 1 to 5 and D; 75 mg/m2 on day 1) compared with cisplatin and fluorouracil alone (CF; 28-day cycles of C; 100 mg/m on day 1 plus F; 1000 mg/m per day days 1 to 5). DCF has a higher response rate (ORR, 37% versus 25%, p = 0.01), time to tumor progression (TTP, 5.6 months versus 3.7 months, p < 0.001), and mOS (9.2 months versus 8.6 months, p = 0.02). However, the addition of docetaxel is associated with significant toxicities, most notably, a high rate of febrile neutropenia (30% versus 14%) requiring supportive granulocyte-colony stimulating factor; therefore, this regimen is not advisable for patients with gastric cancer who have a poor performance status. The REAL-2 trial was a landmark large randomized phase III trial including 1,002 patients that compared four different chemotherapy regimens by substituting oral capecitabine (X) for continuous-infusion fluorouracil (F) and by using the non-nephrotoxic compound oxaliplatin (O) instead of cisplatin (C). The mOS in the ECF (control arm), ECX, EOF, and EOX groups were 9.9 months, 9.9 months, 9.3 months, and 11.2 months, respectively; survival rates at 1 year were 37.7%, 40.8%, 40.4%, and 46.8%, respectively. The combination of EOX was found to be less toxic and with significantly modestly longer mOS benefit compared to ECF (HR, 0.80; 95% CI; 0.66, 0.97 [p = 0.02]). Despite the previous trial results, the added value of an anthracycline (E) or taxane (D) component in the triplet treatment of advanced gastric cancer has been questioned; thus, a fluoropyrimidine /platinum chemotherapy doublet is still considered the standard of care by most experts. S1, when available, in combination with cisplatin, has also shown to be safe and effective (SPIRITS trial and FLAGS trial), but it remains investigational in The United States.
Irinotecan-based regimens (with fluoropyrimidine, cisplatin, or docetaxel) for advanced gastric cancer has shown no difference in outcome measures (ORR, PFS, and OS) when compared to non-irinotecan regimens (mainly FC or ECF). On comparison meta-analysis, the irinotecan-based regimen (FOLFIRI) was found to be less toxic, and there was a non-significant trend toward better survival with irinotecan (HR for death 0.86, 95% CI 0.73-1.02); thus, FOLFIRI could be offered as an alternative for those patients who are not considered candidates or unable to tolerate a platinum-based treatment regimen.
The role of different targeted agents added to cytotoxic backbone therapy has been investigated in several clinical trials. The first targeted agent with documented efficacy in advanced gastric and GEJ cancer was trastuzumab, the humanized monoclonal antibody against HER2 . The phase III ToGA (trastuzumab in gastric cancer) trial compared six courses of cisplatin (80 mg/m2 on day 1) plus either infusional fluorouracil (F; 800 mg/m2/day on days 1 to 5) or capecitabine (X; 1,000 mg/m2 twice daily on days 1 to 14) based on physician choice with and without trastuzumab. Patients received trastuzumab 8 mg/kg loading dose, then 6 mg/kg every three weeks until disease progression on HER2 -positive either by IHC 3+ or FISH + on a majority gastric cancer (80%) and GEJ (18%). Of the 3,807 tumors from patients with gastric cancer tested, 810 (22.1%) were positive for HER2 overexpression using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) analysis. The addition of trastuzumab to FX increased mOS from 11.1 months to 13.8 months (HR, 0.74; 95% CI; 0.60, 0.91; p = 0.0046). In addition, secondary endpoints such as PFS (6.7 months vs. 5.5 months, p = 0.0002) and ORR (47.3% vs. 34.5%, p = 0.0017) were also improved in the trastuzumab arm. There were no significant differences in toxicity between the two treatment arms. The asymptomatic decrease in ejection fraction occurred in 4.6% in the trastuzumab arms, requiring baseline echocardiography and clinical monitoring. As a result of the ToGA trial, trastuzumab added to standard chemotherapy is the standard of care in patients with metastatic, HER2-overexpressing gastric, and gastroesophageal cancers. A higher trastuzumab dose (10mg/kg) did not improve efficacy in a phase III HELOISE trial. Contrary to the results obtains with trastuzumab, lapatinib (an oral HER2/EGFR kinase inhibitor) added to chemotherapy in HER2-overexpressing gastroesophageal cancers failed to meet survival benefit in the first-line (TRIO-01/LOGIC ) and second-line (TyTAN) settings. However, two pre-specified subgroups of the LOGIC trial patients, Asians, and those younger than 60 years, exhibited significant survival benefit with the addition of lapatinib to OX versus placebo/OX in the first-line therapy. Likewise, in the second-line setting evaluated in the TyTAN trial, patients with IHC3+ had significantly lower DFS and risk of death than those who received lapatinib plus paclitaxel. It is unclear if lapatinib can play a role in gastric cancer, given that trastuzumab has become the standard of care for HER2 -positive gastroesophageal cancers.
Antibodies targeting the EGFR, a member of the HER family of receptor tyrosine kinases, such as cetuximab (EXPAND trial) and panitumumab (REAL-3 trial), have also been added to a chemotherapy backbone (CX and EOC, respectively) in gastroesophageal adenocarcinoma with worse toxicity profile and a detrimental effect on overall survival. The consistently negative data from two large phase III trials confirmed that cetuximab and panitumumab cannot be recommended in the first-line therapy advanced gastroesophageal adenocarcinoma.
Angiogenesis inhibitors in gastric and esophageal cancers have demonstrated inconsistent results. Promising data was obtained from a phase II study of bevacizumab added to cisplatin plus irinotecan with an improved response rate and median survival when compared to historical controls. However, in the first phase III trial (AVAGAST), bevacizumab failed to demonstrate an overall survival benefit (12.1 versus 10.1 months; HR, 0.87; 95% CI; 0.73, 1.03; p = 0.10), although, significantly improved PFS (6.7 versus 5.3 months; HR, 0.80; 95% CI; 0.68, 0.93; p = 0.0037) and ORR (46.0% versus 37.4%; p = 0.0315) when added to CF in patients with gastric adenocarcinomas. Due to noticeable geographical difference benefits in the Asian population, AVATAR study failed to improve mOS and PFS with the AVAGAST protocol in selected Chinese patients.
Ramucirumab, a different anti-angiogenesis VEGFR2 monoclonal antibody, was investigated in the second-line setting of advanced gastroesophageal cancer. Ramucirumab dosed at 8 mg/kg intravenously (IV) every 2 weeks showed a modest survival benefit of 9.6 months when combined with paclitaxel versus paclitaxel dosed at 80 mg/m on days 1, 8, and 15 of each 28-day cycle alone of 7.4 months at the RAINBOW trial (HR, 0.807; 95% CI; 0.678, 0.962; p = 0.017), and when used as a single agent (5.2 months) versus best supportive care (3.8 months) at the REGARD trial (HR, 0.776; 95% CI; 0.603, 0.998; p = 0.047), both in phase III clinical trial for patients with advanced gastroesophageal cancers who progressed first-line chemotherapy with a fluoropyrimidine and platinum agent. Of note, the REGARD trial had fewer Asian patients and higher GEJ in comparison to AVAGAST. Despite the benefit of ramucirumab seen in second-line trials, a randomized phase II study did not show a benefit to adding ramucirumab to mFOLFOX6 in the first-line setting for advanced gastroesophageal tumors.
After the failure of first-line fluoropyrimidine/platinum, the choice of second-line chemotherapy in the palliative management of gastric cancer can be either irinotecan or paclitaxel therapy with three randomized trials of chemotherapy versus best supportive care clearly demonstrated improvement in overall survival or neither is superior. Radiation therapy alone can be effective for symptomatic control, but it is rarely used alone to treat primary advanced unresectable gastric cancer.
Several novel agents are currently being investigated in advanced gastric cancer. A phase I clinical trial selected patients with low expression of ataxia-telangiectasia protein had a significant survival benefit from paclitaxel plus olaparib (an oral active poly-ADP ribose polymerase inhibitor) with mOS not reached when compared to paclitaxel plus placebo of 8.2 months, pending an undergoing phase III trial to confirm results. Other promising agents are immune checkpoint inhibitors, particularly anti-PD-1 monoclonal antibody pembrolizumab. KEYNOTE-012 trial evaluated pembrolizumab in patients PD-L1 positive tumors on heavily treated metastatic gastric cancer (and other advanced carcinomas) showing median response duration of 40 weeks and mOS 11.4 months, higher when compared to historical 5.2 months REGARD study. Advanced Gastric and GEJ PD-L1 expressing carcinoma were explored by the KEYNOTE-059 cohort showing an ORR of 13% (95% CI: 8.2,20.0) with a 58% having a response duration of 6 months or longer, obtaining an FDA accelerated approval. The frequency of microsatellite instability-high (MSI-H) and deficient mismatch repair (dMMR), which are susceptible to immunotherapy, has been reported as high as 16% in gastric adenocarcinomas. Eighty-six selected dMMR patients with 12 different tumor types (including refractory advanced or metastatic gastroesophageal carcinoma) who received pembrolizumab achieved a 53% objective response rate and a 21% complete response, further resulting in a subsequent FDA approval for any advanced solid tumor with these genetic features and non-satisfactory alternative. Other second-line targeted therapy agents with clinically improved survival outcomes in clinical trials are apatinib (an oral VEGFR-2 inhibitor with median 6.5 versus 4.7 months placebo) and regorafenib (an oral multikinase inhibitor with median 5.8 versus 4.5 months placebo), demonstrating promise as salvage therapy for this patient population. [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40]
- Differential Diagnosis
- Acute gastritis
- Atrophic gastritis
- Bacterial gastroenteritis
- Chronic gastritis
- Esophageal cancer
- Esophageal stricture
- Esophagitis
- Non-Hodgkin lymphoma
- Peptic ulcer disease
- Viral gastroenteritis
The prognosis of gastric cancer correlates with the extent of the tumor and includes both nodal involvement and direct tumor extension beyond the gastric wall. [41] Tumor grade may also provide some prognostic information. [42]
Over 50% of patients can achieve cure from localized distal gastric cancer, but early-stage disease only accounts for between 10% and 20% of all cases diagnosed in the United States. The remaining gastric cancer patients present with metastatic disease in either regional or distant sites. The overall 5-year survival rate for these patients ranges from almost zero for patients with disseminated disease to nearly 50% for patients with distal, resectable regional disease. Even apparent localized disease only shows a 5-year survival rate in patients with proximal gastric cancer of only 10% to 15%. While therapy for patients with disseminated gastric cancer may result in symptomatic palliation and some prolongation of life, extended remissions remain uncommon.
- Complications
Gastric cancer can lead to loss of appetite and weight loss. Cancer in the stomach can lead to ascites, causing fluid build-up in the abdomen, leading to the patient feeling pressure on their abdomen and shortness of breath. Metastases are also a complication of advanced gastric cancer. Common sites for gastric cancer metastases include the lungs, liver, or bones. Radiation therapy and chemotherapy can cause adverse effects for some patients.
- Deterrence and Patient Education
Patients need to understand that gastric cancer is a lethal condition with a poor prognosis and low survival rate. They must understand the nature of the disease, the treatments and their adverse effects, their chances for survival, and be prepared for possible hospice care if they do not respond. This will also include family counseling and support.
- Enhancing Healthcare Team Outcomes
The incidence of gastric cancer correlates with socioeconomic status and is clearly dependent on environmental/geographical factors. The management of gastric cancer patients requires the medical expertise of an interprofessional team in addition to a supportive team (nutritionist, social worker, nurses, geneticists, and palliative care providers). Members and patients should discuss the controversy surrounding the benefit of perioperative chemotherapy or radiotherapy alone or combined with competing standards of care before choosing the best operation for gastric cancer and extension lymph node dissection. HER2 testing is recommended in all advanced unresectable and metastatic disease, and if HER2 -overexpressing, trastuzumab should be added to a fluoropyrimidine/platinum combination chemotherapy (triplets reserved for selected patients) regimens over single-agent therapy. The VEGFR antibody ramucirumab has the best efficacy in the second-line as single or combined with paclitaxel. Novel target therapies are under investigation; noticeable, the immune checkpoint inhibitor pembrolizumab has shown promising results obtaining FDA-accelerated approval for advanced or metastatic cancer patients whose tumors express with PD-L1, MSI, or dMMR. Future enrollment in clinical trials is encouraged. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Gastric carcinoma, 10x H/E Contributed by Fabiola Farci, MD
Gastric carcinoma, signet ring type, diffuse gastric cancer. 4x H/E Contributed by Fabiola Farci, MD
Signet ring carcinoma. 10x H/E Contributed by Fabiola Farci, MD
Gastric cancer, signet ring type. Diffuse carcinoma of the stomach. 4x H/E Contributed by Fabiola Farci, MD
Prognostic and predictive HER2 analysis is crucial for gastric cancer treatment. Correct HER2 evaluation can select patients for target-treatment. Here, immunohistochemistry is performed and graded as 1+ ("staining is weak or detected in only one part (more...)
Disclosure: Shiva Kumar Mukkamalla declares no relevant financial relationships with ineligible companies.
Disclosure: Alejandro Recio-Boiles declares no relevant financial relationships with ineligible companies.
Disclosure: Hani Babiker declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Mukkamalla SKR, Recio-Boiles A, Babiker HM. Gastric Cancer. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Palliative Management of Unresectable Pancreas Cancer. [Surg Oncol Clin N Am. 2016] Review Palliative Management of Unresectable Pancreas Cancer. Poruk KE, Wolfgang CL. Surg Oncol Clin N Am. 2016 Apr; 25(2):327-37. Epub 2016 Feb 17.
- Review Surgical Management of Gastric Cancer: A Systematic Review. [J Clin Med. 2021] Review Surgical Management of Gastric Cancer: A Systematic Review. Mocan L. J Clin Med. 2021 Jun 9; 10(12). Epub 2021 Jun 9.
- [Epidemiology of the two types of gastric adenocarcinoma in Iceland according to the Laurén histological classification 1990-2009]. [Laeknabladid. 2016] [Epidemiology of the two types of gastric adenocarcinoma in Iceland according to the Laurén histological classification 1990-2009]. Olafsdottir HS, Alexiusdottir KK, Lund SH, Jonasson JG, Jonsson T, Skuladottir H. Laeknabladid. 2016 Mar; 102(3):125-30.
- Gastric Resection. [StatPearls. 2024] Gastric Resection. Marsh AM, Buicko Lopez JL. StatPearls. 2024 Jan
- Docetaxel, Oxaliplatin, and 5-Fluorouracil (DOF) in Metastatic and Unresectable Gastric/Gastroesophageal Junction Adenocarcinoma: A Phase II Study with Long-Term Follow-Up. [Oncologist. 2019] Docetaxel, Oxaliplatin, and 5-Fluorouracil (DOF) in Metastatic and Unresectable Gastric/Gastroesophageal Junction Adenocarcinoma: A Phase II Study with Long-Term Follow-Up. Rosenberg AJ, Rademaker A, Hochster HS, Ryan T, Hensing T, Shankaran V, Baddi L, Mahalingam D, Mulcahy MF, Benson AB 3rd. Oncologist. 2019 Aug; 24(8):1039-e642. Epub 2019 May 28.
Recent Activity
- Gastric Cancer - StatPearls Gastric Cancer - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
ORIGINAL RESEARCH article
Proteomic profiling and biomarker discovery for predicting response to pd-1 inhibitor immunotherapy in gastric cancer patients.

- 1 Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
- 2 Department of Pharmacy, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
- 3 School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment; however, a significant proportion of gastric cancer (GC) patients do not respond to this therapy.Consequently, there is an urgent need to elucidate the mechanisms underlying resistance to ICIs and to identify robust biomarkers capable of predicting the response to ICIs at treatment initiation.In this study, we collected GC tissues from 28 patients prior to the administration of anti-programmed death 1 (PD-1) immunotherapy and conducted protein quantification using high-resolution mass spectrometry (MS). Subsequently, we analyzed differences in protein expression, pathways, and the tumor microenvironment (TME) between responders and non-responders. Furthermore, we explored the potential of these differences as predictive indicators. Finally, utilizing machine learning algorithms, we screened for biomarkers and constructed a predictive model.Our proteomics-based analysis revealed that low activity in the complement and coagulation cascades pathway (CCCP) and a high abundance of activated CD8 T cells are positive signals corresponding to ICIs. Utilizing machine learning, we successfully identified a set of 10 protein biomarkers, and the constructed model demonstrated excellent performance in predicting response on an independent validation set (N = 14, area under the curve (AUC) = 0.959).In summary, our proteomic analyses unveiled unique potential biomarkers for predicting the response to PD-1 Inhibitor Immunotherapy in GC patients, which may provide the impetus for precision immunotherapy.
Keywords: gastric cancer, Immune checkpoint inhibitor, Proteomics, machine learning, biomarkers
Received: 04 Dec 2023; Accepted: 08 May 2024.
Copyright: © 2024 Sun, Li, Wang, Chen, Zhao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jiangang Sun, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China Xiaojing Li, Department of Pharmacy, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China Peng Chen, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China Longfei Zhao, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China Yongshun Gao, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- Search forum
All Categories
- Create a post
What Options are there for second opinions?
Hi - my sister has advanced stage 4 bowel cancer that has spread from her bowel to the lining of her abdomen / stomach. It seems to be growing at quite a speed - she is young, about 50. She already has a stent in but there is a further partial blockage due to the growth. They are planning to put another stent in and her treatment has changed from cure to contain. Surgery is no longer an option for us according to the latest meeting of clinical professionals. I am not doubting their diagnosis, but what options do I have to get a second opinion. To explain, I imagine decisions are made on the basis of risk vs reward vs resources treatment takes up. I want to know if there are any options available to me that give us a chance - any chance - that might have been dismissed for whatever reason. I have one focus which is on my family member whereas medical professionals have multiple focuses that they have to balance. I would just like to be as informed as possible before the fact rather than finding out options once it’s … too late.
Yours, clutching at straws. :(
- Join to reply
- Sign in to reply
From some of the people, my wife met along the way, getting opinions after the 2nd or 3rd time is easier if you do research via the internet and go armed with valid data. Trials etc are also worth asking about. Or the biggie being, willing to go private.
- Join to reply Sign in to reply
Speak to a nurse
- Nurse Helpline 0808 800 4040
- Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
Quick links
- Find local shops
- Shop online
- About our information
- Terms and conditions
- Modern Slavery Statement
- Accessibility


IMAGES
VIDEO
COMMENTS
Background. Gastric cancer is a deadly disease with poor overall survival statistics throughout the world. The majority of new diagnoses per year of gastric cancer occur mainly in Asian and South American countries ().Within the United States, there are a projected 27,000 new cases to be diagnosed in 2020 ().It is only recently that researchers started to understand how heterogenous gastric ...
Gastric stump cancer. Gastric stump cancer (GSC) is a separate subtype of GC, defined as a carcinoma that occurs in the gastric remnant at least 5 years after the surgery for peptic ulcer. 64 GSC represents from 1.1% to 7% of all GCs (Figure 1), and males are more prone to them than woman. 55, 65, 66 Gastrectomy is a well-established risk factor for GSC, even long time after the initial ...
Introduction. Although gastric cancer is not in the top 10 malignancies ranked by either incidence or mortality in the United States, it does represent the second most common cause of cancer death worldwide. 1, 2 Therefore, the advances we make in gastric cancer treatment, even in low-incidence countries, can have global implications. Caution must be exercised, however, in applying findings ...
However, patients with stage III tumors undergoing surgery have a dismal 5-year survival rate between 18 and 50% depending on the dataset. These figures indicate the need for more effective molecularly driven treatment strategies. This review discusses the molecular profile of gastric tumors, the success, and challenges with available ...
Stomach cancer is a common malignant tumor that poses a serious disease burden worldwide 1.As the fourth leading cause of cancer-related death and the fifth most commonly diagnosed cancer in the ...
2. Epidemiology and Risk Factors. Gastric Cancer is the 5th most common and the 3rd deadliest cancer worldwide, according to the latest report of the International Agency for research on Cancer [].Its geographic incidence remains heterogenous with most cases occurring in Eastern Asia (619,226 in 2018), and men being twice as much affected as women.
Globally, gastric cancer is a common and highly fatal cancer with two anatomical subtypes, non-cardia and cardia gastric cancer. Helicobacter pylori causes almost 90% of distal gastric cancers ...
The molecular characterization of gastric cancer was first described by Deng et al. 13 and Dulak et al. 14 in papers published in different journals. They similarly observed that ~40% of these ...
Gastric cancer (GC) is a significant global public health problem. It is the third leading cause of cancer-related mortality despite its decline in incidence since the past five decades. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial ...
Systemic chemotherapy, radiotherapy, surgery, immunotherapy, and targeted therapy all have proven efficacy in gastric adenocarcinoma; therefore, multidisciplinary treatment is paramount to treatment selection. Triplet chemotherapy for resectable gastric cancer is now accepted and could represent a plateau of standard cytotoxic chemotherapy for ...
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become ...
We searched for the keywords "gastric cancer" AND "deep learning" OR "artificial intelligence", with no restrictions on language or publication date. We learned that gastric cancer (GC) was the fifth most common type of malignant disease, and it ranks as the third leading cause of cancer-related deaths worldwide.
Overview. Gastric Cancer is a high-level global forum dedicated to all areas of research, treatment, and biology relevant to gastric cancer. Welcomes a variety of content such as original articles, case reports, short communications, and technical notes. Encourages Letters to the Editor on articles published or expressing views on gastric ...
Stomach Cancer Research For People with Inherited Risk of Stomach Cancer, Gastrectomy Has Lasting Consequences. Posted: January 12, 2024. In a recent study, more than 90% of people who'd had their stomach surgically removed to prevent cancer experienced a least one chronic complication 2 years out from their surgery. For some, the ...
Gastric cancer (GC) is one of the most prevalent malignant tumors worldwide and is associated with high morbidity and mortality rates. However, the specific biomarkers used to predict the postoperative prognosis of patients with gastric cancer remain unknown. Recent research has shown that the tumor microenvironment (TME) has an increasingly positive effect on anti-tumor activity.
Gastric cancer (GC) is a prevalent malignant tumor of the gastrointestinal (GI) system. However, the lack of reliable biomarkers has made its diagnosis, prognosis, and treatment challenging. Immunogenic cell death (ICD) is a type of programmed cell death that is strongly related to the immune system.
Immunotherapy. Immunotherapy is an expanding area of research for stomach cancer. Researchers are looking at different types of immunotherapy that block the CTLA4 and/or PD-1 pathways. A tumor can use these pathways to hide from the body's immune system. Immunotherapy that blocks these pathways allow the immune system to identify and destroy ...
Gastric cancer, or stomach cancer, is a type of cancer that begins in the mucus-producing cells on the inside lining of the stomach. ... Research Highlights 03 May 2023 Nature Reviews Clinical ...
Abstract. In this paper the progress of epidemiological research in stomach cancer during 1980-1990 is reviewed in respect to regional variation, etiology, and formation of carcinogens. The evaluation of 4 cohort and 16 case-control studies revealed a consistently inverse relationship of stomach cancer risk with raw vegetables, fruit, and ...
Purpose Cystatin SA (CST2) belongs to the superfamily of cysteine protease inhibitors. Emerging research indicates that CST2 is often dysregulated across various cancers. Its role and molecular mechanisms in gastric cancer remain underexplored. This study aims to explore the expression and function of CST2 in gastric cancer. Methods CST2 expression was analyzed and validated through Western ...
Gastric cancer remains one of the most common and deadly cancers worldwide, especially among older males. Based on GLOBOCAN 2018 data, stomach cancer is the 5 th most common neoplasm and the 3 rd most deadly cancer, with an estimated 783,000 deaths in 2018. Gastric cancer incidence and mortality are highly variable by region and highly dependent on diet and Helicobacter pylori infection.
Cancer Medicine is an open access, broad-scope oncology journal covering clinical cancer research, cancer biology, cancer prevention, & bioinformatics. Abstract Aims To investigate the effect of Self-designed Metabolic Equivalent Exercises (SMEE) on cancer-related fatigue in patients with gastric cancer.
Drugs that target VEGF (or the VEGF receptors on the surface of cells) can help stop some stomach cancers from growing. Ramucirumab (Cyramza), a drug that blocks VEGF receptors, can be used to treat some advanced stomach cancers. Other targeted drugs that target VEGF receptors, such as apatinib, are also being studied.
In the list of the most common types of cancer worldwide, stomach cancer is in fifth place. The risk of this tumor disease increases with age, but the latest statistics paint a worrying picture of ...
This revealed that people who said they always or frequently added salt to their food were 39% more likely to develop stomach cancer over an observation period of about 11 years than those who ...
Gastric and gastro-oesophageal cancer is a leading cause of cancer-related death worldwide with a poor prognosis. This Review provides a comprehensive overview of current treatment strategies set ...
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide, albeit there has been a global decline since the last mid-century. In the United States, gastric cancer incidence has decreased during the past few decades, although the incidence of gastroesophageal cancer has concomitantly increased. There are two distinct types of gastric ...
Watch for these red-flag stomach cancer symptoms. Other stomach cancer symptoms include unintentional weight loss, feeling full quickly, and losing your appetite. But if you experience any of the following, seek medical attention right away. Vomiting blood: Any amount is considered too much. Bloody stools: These are often described as looking ...
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment; however, a significant proportion of gastric cancer (GC) patients do not respond to this therapy.Consequently, there is an urgent need to elucidate the mechanisms underlying resistance to ICIs and to identify robust biomarkers capable of predicting the response to ICIs at treatment initiation.In this study, we collected ...
Hi - my sister has advanced stage 4 bowel cancer that has spread from her bowel to the lining of her abdomen / stomach. It seems to be growing at quite a speed - she is young, about 50. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited ...