
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Section 1: Introduction to Toxicology
- Last updated
- Save as PDF
- Page ID 316721

- ToxMSDT Online component
- Toxicology MSDT
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
After completing this lesson, you will be able to:
- Define toxicology and identify adverse effects.
- Recognize the history of toxicology .
- Explain how dose determines whether a substance is a remedy or a poison .
- Differentiate between toxic agents and toxic substances .
In this section...
Topics include:
- 1.1: What is toxicology?
- 1.2: Basic Terminology
What We've Covered
In this section, we covered several important concepts:
- Toxicology is the study of adverse effects of chemicals and physical agents on living organisms.
- A xenobiotic is a foreign substance taken into the body.
- A toxic agent is any chemical, physical, or biological agent that can produce an adverse biological effect.
- Toxic substances can be systemic toxicants , which affect the entire body or multiple organs , or organ toxicants , which affect a specific organ or tissues .
- The dose of a substance is the most important determinant of toxicity .
Coming Up...
In the next section, we will explore the concept of dose and its importance to toxicology in greater detail.
Module One – Lecture Notes
- I. Introduction to Toxicology
- II. Classification of Toxic Agents
- III. Toxicological Information Sources
IV. Contact Information
I. introduction to toxicology (1), a. what is toxicology.
Toxicology is the study of how natural or man-made poisons cause undesirable effects in living organisms.
- What are harmful or adverse effects? Harmful or adverse effects are those that are damaging to either the survival or normal function of the individual.
- What is Toxicity? The word “toxicity” describes the degree to which a substance is poisonous or can cause injury. The toxicity depends on a variety of factors: dose, duration and route of exposure (see Module Two ), shape and structure of the chemical itself, and individual human factors.
Refer to Handout 1-1 General diagram of routes of exposure and Module Two – Routes of Exposure [PDF – 32 KB]
- What is Toxic? This term relates to poisonous or deadly effects on the body by inhalation (breathing), ingestion (eating), or absorption, or by direct contact with a chemical
- What is a Toxicant? A toxicant is any chemical that can injure or kill humans, animals, or plants; a poison. The term “toxicant” is used when talking about toxic substances that are produced by or are a by-product of human-made activities. For example, dioxin (2,3-7,8-tetrachlorodibenzo- p -dioxin {TCDD}), produced as a by-product of certain chlorinated chemicals, is a toxicant. On the other hand, arsenic , a toxic metal, may occur as a natural contaminant of groundwater or may contaminate groundwater as a by-product of industrial activities. If the second case is true, such toxic substances are referred to as toxicants, rather than toxins.
- What is a Toxin? The term “toxin” usually is used when talking about toxic substances produced naturally. A toxin is any poisonous substance of microbial (bacteria or other tiny plants or animals), vegetable, or synthetic chemical origin that reacts with specific cellular components to kill cells, alter growth or development, or kill the organism.
- What is a Toxic Symptom? This term includes any feeling or sign indicating the presence of a poison in the system.
- What are Toxic Effects? This term refers to the health effects that occur due to exposure to a toxic substance; also known as a poisonous effect on the body.
- What is Selective Toxicity? “Selective toxicity” means that a chemical will produce injury to one kind of living matter without harming another form of life, even though the two may exist close together.
- How Does Toxicity Develop? Before toxicity can develop, a substance must come into contact with a body surface such as skin, eye or mucosa of the digestive or respiratory tract. The dose of the chemical, or the amount one comes into contact with, is important when discussing how “toxic” an substance can be.
- What is a dose? The dose is the actual amount of a chemical that enters the body. The dose received may be due to either acute (short) or chronic (long-term) exposure. An acute exposure occurs over a very short period of time, usually 24 hours. Chronic exposures occur over long periods of time such as weeks, months, or years. The amount of exposure and the type of toxin will determine the toxic effect.
- What is dose-response? Dose-response is a relationship between exposure and health effect, that can be established by measuring the response relative to an increasing dose. This relationship is important in determining the toxicity of a particular substance (2). It relies on the concept that a dose, or a time of exposure (to a chemical, drug, or toxic substance), will cause an effect (response) on the exposed organism. Usually, the larger or more intense the dose, the greater the response, or the effect. This is the meaning behind the statement “the dose makes the poison.”
- What is the threshold dose? Given the idea of a dose-response, there should be a dose or exposure level below which the harmful or adverse effects of a substance are not seen in a population. That dose is referred to as the ‘threshold dose’. This dose is also referred to as the no observed adverse effect level (NOAEL), or the no effect level (NEL) . These terms are often used by toxicologists when discussing the relationship between exposure and dose. However, for substances causing cancer (carcinogens) , no safe level of exposure exists, since any exposure could result in cancer.
- What is meant by ‘individual susceptibility?’ This term describes the differences in types of responses to hazardous substances, between people. Each person is unique, and because of that, there may be great differences in the response to exposure. Exposure in one person may have no effect, while a second person may become seriously ill, and a third may develop cancer.
- What is a “sensitive sub-population?” A sensitive sub-population describes those persons who are more at risk from illness due to exposure to hazardous substances than the average, healthy person. These persons usually include the very young, the chronically ill, and the very old. It may also include pregnant women and women of childbearing age. Depending on the type of contaminant, other factors (e.g., age, weight, lifestyle, sex) could be used to describe the population.
B. The Field of Toxicology
Toxicology addresses a variety of questions. For example, in agriculture, toxicology determines the possible health effects from exposure to pesticides or herbicides, or the effect of animal feed additives, such as growth factors, on people. Toxicology is also used in laboratory experiments on animals to establish dose-response relationships. Toxicology also deals with the way chemicals and waste products affect the health of an individual.
C. Sub-disciplines of Toxicology
The field of toxicology can be further divided into the following sub-disciplines or sub-specialities:
- Environmental Toxicology is concerned with the study of chemicals that contaminate food, water, soil, or the atmosphere. It also deals with toxic substances that enter bodies of waters such as lakes, streams, rivers, and oceans. This sub-discipline addresses the question of how various plants, animals, and humans are affected by exposure to toxic substances.
- Occupational (Industrial) Toxicology is concerned with health effects from exposure to chemicals in the workplace. This field grew out of a need to protect workers from toxic substances and to make their work environment safe. Occupational diseases caused by industrial chemicals account for an estimated 50,000 to 70,000 deaths, and 350,000 new cases of illness each year in the United States (1).
- Regulatory Toxicology gathers and evaluates existing toxicological information to establish concentration-based standards of “safe” exposure. The standard is the level of a chemical that a person can be exposed to without any harmful health effects.
- Food Toxicology is involved in delivering a safe and edible supply of food to the consumer. During processing, a number of substances may be added to food to make it look, taste, or smell better. Fats, oils, sugars, starches and other substances may be added to change the texture and taste of food. All of these additives are studied to determine if and at what amount, they may produce adverse effects. A second area of interest includes food allergies. Almost 30% of the American people have some food allergy. For example, many people have trouble digesting milk, and are lactose intolerant. In addition, toxic substances such as pesticides may be applied to a food crop in the field, while lead, arsenic, and cadmium are naturally present in soil and water, and may be absorbed by plants. Toxicologists must determine the acceptable daily intake level for those substances.
- Clinical Toxicology is concerned with diseases and illnesses associated with short term or long term exposure to toxic chemicals. Clinical toxicologists include emergency room physicians who must be familiar with the symptoms associated with exposure to a wide variety of toxic substances in order to administer the appropriate treatment.
- Descriptive Toxicology is concerned with gathering toxicological information from animal experimentation. These types of experiments are used to establish how much of a chemical would cause illness or death. The United States Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), and the Food and Drug Administration (FDA), use information from these studies to set regulatory exposure limits.
- Forensic Toxicology is used to help establish cause and effect relationships between exposure to a drug or chemical and the toxic or lethal effects that result from that exposure.
- Analytical toxicology identifies the toxicant through analysis of body fluids, stomach content, excrement, or skin.
- Mechanistic Toxicology makes observations on how toxic substances cause their effects. The effects of exposure can depend on a number of factors, including the size of the molecule, the specific tissue type or cellular components affected, whether the substance is easily dissolved in water or fatty tissues, all of which are important when trying to determine the way a toxic substance causes harm, and whether effects seen in animals can be expected in humans.
II. Classification of Toxic Agents (2):
Toxic substances are classified into the following:
A. Heavy Metals
Metals differ from other toxic substances in that they are neither created nor destroyed by humans. Their use by humans plays an important role in determining their potential for health effects. Their effect on health could occur through at least two mechanisms: first, by increasing the presence of heavy metals in air, water, soil, and food, and second, by changing the structure of the chemical. For example, chromium III can be converted to or from chromium VI, the more toxic form of the metal.
B. Solvents and Vapors
Nearly everyone is exposed to solvents. Occupational exposures can range from the use of “white-out” by administrative personnel, to the use of chemicals by technicians in a nail salon. When a solvent evaporates, the vapors may also pose a threat to the exposed population.
� Have participants discuss possible solvents they use ormay be exposed to during the course of a typical day.
C. Radiation and Radioactive Materials
Radiation is the release and propagation of energy in space or through a material medium in the form of waves, the transfer of heat or light by waves of energy, or the stream of particles from a nuclear reactor (3).
� An example for discussion purposes would be the dropping of the atomic bomb during World War II, or the Chernobyl Accident in Russia. These items can be provided by the presenter.
D. Dioxin/Furans
Dioxin, (or TCDD) was originally discovered as a contaminant in the herbicide Agent Orange. Dioxin is also a by-product of chlorine processing in paper producing industries.
E. Pesticides
The EPA defines pesticide as any substance or mixture of substances intended to prevent, destroy, repel, or mitigate any pest. Pesticides may also be described as any physical, chemical, or biological agent that will kill an undesirable plant or animal pest (2).
� Have participants list pesticides they are familiar with either through personal use or in relation to hazardous chemicals in their community.
F. Plant Toxins (2)
Different portions of a plant may contain different concentrations of chemicals. Some chemicals made by plants can be lethal. For example, taxon, used in chemotherapy to kill cancer cells, is produced by a species of the yew plant.
G. Animal Toxins (2)
These toxins can result from venomous or poisonous animal releases. Venomous animals are usually defined as those that are capable of producing a poison in a highly developed gland or group of cells, and can deliver that toxin through biting or stinging. Poisonous animals are generally regarded as those whose tissues, either in part or in their whole, are toxic.
� Trainer may want to provide examples of venomous animals, such as snakes, spiders, etc., and poisonous animals, such as puffer fish, or oysters, which may be toxic to some individuals when contaminated with vibrio vulnificus.
H. Subcategories of Toxic Substance Classifications
All of these substances may also be further classified according to their:
- Effect on target organs (liver, kidney, hematopoietic system),
- Use (pesticide, solvent, food additive),
- Source of the agent (animal and plant toxins),
- Effects (cancer mutation, liver injury),
- Physical state (gas, dust, liquid),
- Labeling requirements (explosive, flammable, oxidizer),
- Chemistry (aromatic amine, halogenated hydrocarbon), or
- Poisoning potential (extremely toxic, very toxic, slightly toxic)
I. General Classifications of Interest to Communities
- Air pollutants
- Occupation-related
- Acute and chronic poisons
All chemicals (or any chemical) may be poisonous at a given dose and through a particular route. For example, breathing too much pure oxygen, drinking excessive amounts of water, or eating too much salt can cause poisoning or death (1).
III. Toxicological Information Sources (4)
A. the agency for toxic substances and disease registry (atsdr).
ATSDR is part of the U.S. Department of Health and Human Services. It was created by Congress in 1980 to provide health-based information for use in the cleanup of chemical waste disposal sites mandated by the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). As the lead agency for implementing the health-related guidelines of CERCLA, ATSDR assesses the presence and nature of health hazards at specific Superfund Sites, to help prevent or reduce further exposure and the illnesses that result from such exposures, and to expand the knowledge-base about health effects from exposure to hazardous substances.
ATSDR is mostly concerned with the health effects that may occur from exposure to toxic chemicals. ATSDR’s Hazardous Substances and Health Effects Database (HazDat) discusses the issue. ATSDR also publishes Toxicological Profiles (which provide information on specific chemicals and possible health effects), Case Studies in Environmental Medicine (which are used to provide information to health care providers about the toxic effects of chemicals), and Public Health Statements (which contain information on toxic chemical exposures)(4).
ATSDR’s Division of Toxicology also produces ToxFAQs™, a series of summaries about hazardous substances. Information for this series is excerpted from the ATSDR Toxicological Profiles and Public Health Statements. Each ToxFAQ summary is quick and easy to understand, and answers the most frequently asked questions (FAQs) about exposure to hazardous substances found around hazardous waste sites and the effects of exposure on human health. Medical Management Guidelines for Acute Chemical Exposures (Guidelines) were developed by ATSDR to aid emergency department physicians and other emergency healthcare professionals who manage acute exposures resulting from chemical incidents. The guidelines are intended to aid healthcare professionals involved in emergency response to effectively decontaminate patients, protect themselves and others from contamination, communicate with other involved personnel, efficiently transport patients to a medical facility, and provide competent medical evaluation and treatment to exposed persons.
B. The United States Environmental Protection Agency (EPA)
EPA is responsible for a number of activities, including enforcing federal laws designed to protect human health and the environment. There are ten regional EPA offices throughout the United States, with EPA headquarters located in Washington, D.C. Each regional office is responsible within selected states for implementing the agency’s programs, considering regional needs and implementing federal environmental laws. Following is a list of the regions and the states they cover.
Refer to Figure 1.1 – Map of EPA Regional Offices [PDF – 2 MB]
- Region 1: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont
- Region 2: New Jersey, New York, Puerto Rico, and the U.S. Virgin Islands
- Region 3: Delaware, Maryland, Pennsylvania, Virginia, West Virginia, the District of Columbia
- Region 4: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, Tennessee
- Region 5: Illinois, Indiana, Michigan, Minnesota, Ohio, Wisconsin
- Region 6: Arkansas, Louisiana, New Mexico, Oklahoma, Texas
- Region 7: Iowa, Kansas, Missouri, Nebraska
- Region 8: Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming
- Region 9: Arizona, California, Hawaii, Nevada, the territories of Guam and American Samoa
- Region 10: Alaska, Idaho, Oregon, Washington
The specific chemicals regulated by EPA and the standards associated with them are found in the Code of Federal Regulations or CFR. The different sections of the CFR are called Titles, and the ones that apply to EPA are in Title 40 (1). EPA has developed rules and regulations that activate the requirements of several environmental laws provided below.
Trainer Note: Refer to Table 1.1 which is a list of selected EPA laws that regulate chemicals in the environment. [PDF – 50 KB]
- In addition, the following EPA Laws regulate chemicals in the environment:
- The Clean Air Act implements regulations that control and abate air emissions from stationary and mobile sources.
- The Clean Water Act regulates discharge of pollutants to surface waters.
- The Safe Drinking Water Act establishes standards for contaminants in drinking water; regulates discharges to underground injection wells, sole source aquifers, and public drinking water systems.
- The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA or Superfund) deals with cleanup of hazardous waste sites and definition of requirements for response to hazardous waste spills.
- The Resource Conservation and Recovery Act (RCRA) deals with identification and regulation of hazardous waste treatment, storage, and disposal.
- The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) requires registration and testing of pesticides, regulates their sale, distribution, and use.
- The Toxic Substances Control Act (TSCA) requires testing and reporting of chemicals prior to manufacturing, distribution, and use; and restricts the use of chemicals that pose a threat to human health and the environment.
- The Emergency Planning and Community Right to Know Act (EPCRA) requires companies to report inventories of hazardous chemicals and toxic releases; and requires state and local governments to develop plans for responding to emergency releases.
In addition, EPA’s Office of Research and Development (ORD) studies the effects of toxic exposure on people and the environment.
C. The Centers for Disease Control and Prevention (CDC)
CDC is an agency in the Department of Health and Human Services, its mission is to promote health and quality of life by preventing and controlling disease, injury, and disability. In the past, the CDC has focused on the study and prevention of infectious diseases such as malaria and smallpox. However, now its responsibilities have enlarged to include environmental and occupational hazards.
Refer participants to Table 1.2 (Handout 1.3) – CDC Organizational Offices [PDF – 33 KB]
The CDC Centers that deal with environmental health are the National Center for Environmental Health (NCEH) and the National Institute for Occupational Safety and Health (NIOSH) (2). NCEH addresses hazards associated with chemical exposure inside and outside the workplace. NIOSH was established by the Occupational Safety and Health Act of 1970. NIOSH has several functions, including investigating potentially hazardous work conditions, and evaluating chemical hazards in the workplace. NIOSH is the only federal institute responsible for conducting research and making recommendations for the prevention of work-related illnesses and injuries. NIOSH’s responsibilities include:
- Investigating potentially hazardous working conditions as requested by employers or employees,
- Evaluating hazards in the workplace, ranging from chemicals to machinery,
- Creating and disseminating methods for preventing disease, injury, and disability,
- Conducting research and providing scientifically valid recommendations for protecting workers; and
- Providing education and training to individuals preparing for or actively working in the field of occupational safety and health. Information gathered from these activities is used to help reduce disease, injury and disability in the workplace. The information is provided to OSHA, which uses it to establish standards to protect health in the workplace.
D. The Nuclear Regulatory Commission (NRC) (1)
NRC, established in 1974, regulates the use of nuclear materials for commercial, industrial, academic, and medical purposes. This includes regulating nuclear power plants, nuclear materials used in the diagnosis and treatment of cancer, and nuclear materials used in smoke detectors. NRC also regulates non-power research, test and training reactors; nuclear fuel cycle facilities (the production of nuclear fuel); and the transport, storage, and disposal of nuclear materials and waste. Like OSHA and EPA, NRC obtains and evaluates information about acceptable exposure levels for workers handling nuclear materials.
E. The Food and Drug Administration (FDA) (5)
FDA promotes and protects the public health by helping safe and effective products reach the market in a timely way, and monitoring products for continued safety after they are in use.
F. The American Conference of Governmental Industrial Hygienists (ACGIH)
ACGIH is a professional organization that produces a listing of Threshold Limit Values (TLV) and Biological Exposure Indices (BEI) for several hundred chemicals, updating them every year. BEIs are recommended maximum concentrations of various types of toxic substances, and are guidelines to evaluate the potential health hazards associated with exposure. The maximum concentration may be measured in blood, urine, or exhaled air. The TLVs are guidelines for occupational exposure to hazardous chemicals and are published in a booklet called Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (6).
G. Electronic Databases
Information on toxic chemicals is available at the following sites:
- Toxicology Data Network (TOXNET – www.toxnet.nlm.nih.gov ). Several databases, such as the Hazardous Substances Data Bank (HSDB) and the Registry of Toxic Effects of Chemical Substances (RTECS), can be found in the TOXNET database. The HSDB has toxicological information on more than 4,000 chemicals, as well as information on emergency handling procedures, environmental data, regulatory status, and human exposure. The RTECS is maintained by NIOSH and contains information on the health effects for more than 90,000 chemicals.
- CHEMTREC (Chemical Transportation Emergency Center – www.chemtrec.org ). CHEMTREC is provided by the American Chemistry Council (formerly known as the Chemical Manufacturer’s Association) and provides information and assistance for emergency incidents involving chemicals and hazardous materials. CHEMTREC also supplies basic information about the production, shipping, and use of chemicals and provides information about medical treatment in response to chemical exposures.
- Material Safety Data Sheets (MSDS) are available on the Occupational Safety and Health Administration website: www.osha.gov . MSDSs provide information such as physical and chemical properties of a substance, first aid information, emergency response, and disposal information.
- Hazardous Substances and Health Effects Database (HazDat), available on ATSDR’s website at www.atsdr.cdc.gov . contains information on hazardous substances found at National Priorities List (NPL) and non-NPL waste sites and emergency events, and on the levels at which health effects from exposure to hazardous substances have been reported in humans and animals. HAZDAT contains environmental contamination and other data on more than 3,000 uncontrolled hazardous waste sites for which ATSDR has conducted public health assessments, prepared health consultations, or provided responses to emergencies involving releases of toxic substances into community environments. It contains toxicity information taken from the ATSDR’s Toxicological Profiles for more than 200 substances most frequently found at sites (4).
Agency for Toxic Substances and Disease Registry (ATSDR) 1600 Clifton Road, NE Atlanta, Georgia 30333 1-888-42-ATSDR or 1-888-422-8737 Web site: https://www.atsdr.cdc.gov
American Conference of Government Industrial Hygienists (ACGIH) 1330 Kemper Meadow Drive, Suite 6000 Cincinnati, OH 45240 513-742-2020 Web site: https://www.acgih.org
Association of Occupational and Environmental Clinics (AOEC) 1010 Vermont Avenue, NW, Suite 513 Washington, DC 20005 (202) 347-4976
Centers for Disease Control and Prevention (CDC) 1600 Clifton Rd. Atlanta, GA 30333 1-800-311-3455 Web site: https://www.cdc.gov
National Institute of Environmental Health Sciences (NIEHS) P.O. Box 12233 Research Triangle Park, NC 27709 or 111 Alexander Drive Research Triangle Park, NC 27709 (919) 541-3345 Web site: https://www.niehs.nih.gov
National Toxicology Program (NTP) P.O. Box 12233 M.D. B2-04 Research Triangle Park, NC 27709 (919) 541-0530 Web site: https://ntp.niehs.nih.gov/
Occupational Safety and Health Administration (OSHA) Directorate of Technical Support Technical Data Center, Room N-2634 200 Constitution Avenue, N.W. Washington, DC 20210 1-800-321-OSHA or 1-800-321-6742 Web site: https://www.osha.gov
U.S. Environmental Protection Agency (EPA) Ariel Rios Building 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 (202) 260-2090 Web site: https://www.epa.gov
U.S. Food and Drug Administration (FDA) 5600 Fishers Lane Rockville, Maryland 20857 1-888-INFO-FDA (1-888-463-6332) Web site: https://www.FDA.Gov
U.S. Nuclear Regulatory Commission (NRC) One White Flint North 11555 Rockville Pike Rockville, MD 20852-2738 (301) 415-7000 Web site: https://www.nrc.gov
« Presentation Outline
Test Your Knowledge Quiz »
- Medical Disclaimer and Disclosure
- Case Studies in Environmental Medicine (CSEM)
- Interaction Profiles
- Managing Hazardous Materials Incidents
- Medical Management Guidelines
- Minimal Risk Levels
- Priority List of Hazardous Substances
- ToxFAQs™
- ToxFAQs™ en Español
- ToxGuides™
- Division of Toxicology and Human Health Sciences
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Introduction to Toxicity Studies
Table of Contents
Introduction
Toxicity studies play a crucial role in assessing the potential adverse effects of chemical substances, pharmaceuticals, cosmetics, pesticides, food additives, and other agents on living organisms, including humans. These studies aim to evaluate the dose-response relationship, identify target organs or systems affected by exposure, and determine the mechanisms underlying toxicity. The introduction of toxicity studies typically outlines the purpose, methods, and significance of the research.
Purpose: The primary objective of toxicity studies is to assess the safety of substances and products that may come into contact with humans, animals, or the environment. These studies help regulatory agencies, such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in Europe, make informed decisions regarding product approval, labeling, and usage guidelines. Additionally, toxicity studies contribute to scientific understanding of the biological effects of various compounds and aid in the development of safer alternatives.
Methods: Toxicity studies employ a variety of experimental techniques and models to assess different aspects of toxicity, including acute toxicity, subacute toxicity, chronic toxicity, carcinogenicity, mutagenicity, reproductive toxicity, and developmental toxicity. Commonly used methods include:
- Animal Studies: Rodents (e.g., rats, mice) and non-rodents (e.g., rabbits, dogs) are frequently used in toxicity studies due to their physiological similarities to humans. These studies involve administering test substances at varying doses and durations to observe their effects on different organ systems.
- In Vitro Studies: Cell culture techniques allow researchers to investigate the toxic effects of substances on specific cell types or tissues in a controlled laboratory setting. In vitro studies are often used for initial screening and mechanistic studies.
- Epidemiological Studies: These studies analyze human populations to identify associations between exposure to certain substances and adverse health outcomes. Epidemiological data provide valuable insights into long-term health effects and help guide public health policies.
- Computational Toxicology: Computational models and bioinformatics tools are increasingly used to predict toxicity based on chemical structure, molecular interactions, and biological pathways. These approaches accelerate toxicity assessment and reduce reliance on animal testing.
Significance: Toxicity studies are essential for protecting human health and the environment by identifying potential hazards and guiding risk management strategies. Regulatory agencies use toxicity data to establish safe exposure limits, develop guidelines for product labeling and handling, and enforce regulatory standards. Moreover, toxicity studies contribute to scientific knowledge by advancing our understanding of toxicological mechanisms and facilitating the development of safer chemicals and products.

Types of Toxicology Studies
Toxicology studies encompass a range of methodologies and approaches to assess the potential adverse effects of chemical substances on living organisms. These studies are crucial for understanding the toxicity profile of substances and for making informed decisions regarding their safe use. Here are the primary types of toxicology studies:
- Aim: Acute toxicity studies determine the adverse effects of a single or short-term exposure to a substance within a relatively short period, usually 24 to 48 hours.
- Methods: These studies typically involve administering increasing doses of the test substance to experimental animals (often rodents) and observing mortality, clinical signs, and changes in organ function.
- Endpoints: The median lethal dose (LD50) is often used as a measure of acute toxicity, representing the dose at which 50% of the test animals die.
- Aim: Subacute toxicity studies assess the adverse effects of repeated exposure to a substance over several weeks (usually 28 to 90 days).
- Methods: Animals receive daily doses of the test substance, and various parameters such as clinical signs, body weight changes, organ weights, and histopathological examinations are evaluated.
- Endpoints: These studies provide information on target organ toxicity and help establish dose levels for further long-term studies.
- Aim: Chronic toxicity studies investigate the adverse effects of prolonged exposure to a substance over a significant portion of the lifespan of the test animals (often 6 months to 2 years).
- Methods: Animals are exposed to the test substance daily for an extended duration, and comprehensive evaluations of clinical signs, organ function, pathology, and carcinogenic potential are conducted.
- Endpoints: Chronic toxicity studies provide critical data for assessing the long-term health risks associated with exposure to the substance.
- Aim: Carcinogenicity studies assess the potential of a substance to induce cancer in experimental animals.
- Methods: Animals are exposed to varying doses of the test substance for a prolonged period, and tumors are monitored and characterized.
- Endpoints: These studies help determine the carcinogenic potential of the substance and its relevance to human health risk assessment.
- Aim: Reproductive and developmental toxicity studies evaluate the effects of a substance on fertility, pregnancy, embryonic development, and offspring viability.
- Methods: Animals are exposed to the test substance during specific reproductive stages, and parameters such as mating behavior, fertility, gestation length, litter size, and developmental abnormalities are assessed.
- Endpoints: These studies provide insights into potential reproductive hazards and developmental defects associated with exposure to the substance.
- Aim: Genetic toxicity studies assess the ability of a substance to induce mutations or chromosomal damage in cells.
- Methods: Various in vitro and in vivo assays, such as the Ames test, micronucleus assay, and comet assay, are used to evaluate genotoxicity.
- Endpoints: These studies help identify potential mutagens and carcinogens and provide essential data for risk assessment and regulatory decision-making.
You May Like: Introduction to Allergy Testing
Each type of toxicology study serves a specific purpose in characterizing the safety profile of substances and informing risk assessment and regulatory decisions. Together, they contribute to ensuring the protection of human health and the environment from potential hazards posed by chemical agents.
In conclusion, toxicology studies are indispensable tools for assessing the safety of chemical substances and products and for safeguarding human health and the environment. Through a diverse array of methodologies and approaches, these studies enable researchers and regulatory agencies to comprehensively evaluate the potential adverse effects of substances and make informed decisions regarding their use, handling, and regulation.
From acute toxicity studies, which provide insights into the immediate effects of single or short-term exposures, to chronic toxicity and carcinogenicity studies, which investigate the long-term health risks associated with prolonged exposure and potential carcinogenic properties, each type of toxicological assessment serves a critical role in understanding the hazards posed by substances.
Furthermore, reproductive and developmental toxicity studies shed light on the impact of substances on fertility, pregnancy, and offspring viability, while genetic toxicity studies identify potential mutagens and carcinogens by assessing their effects on DNA integrity and chromosomal stability.
The findings from toxicology studies not only inform regulatory decisions and risk management strategies but also contribute to scientific knowledge by advancing our understanding of toxicological mechanisms and facilitating the development of safer alternatives.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Collaborate
General toxicity studies
- Medicines R&D
Table of contents
Last update: 19 November 2015

Introduction
Toxicity studies investigate the safety profile of the candidate compound . They also provide important information about the absorption , distribution, metabolism, and excretion (ADME) of the compound in the body. A candidate compound must be assessed in many different kinds of non-clinical toxicity study before it can be administered to the first human volunteer; even more toxicity studies are required thereafter before the medicine receives marketing authorisation . The following article explores the various types of toxicology study that may be necessary to include in a non-clinical programme.
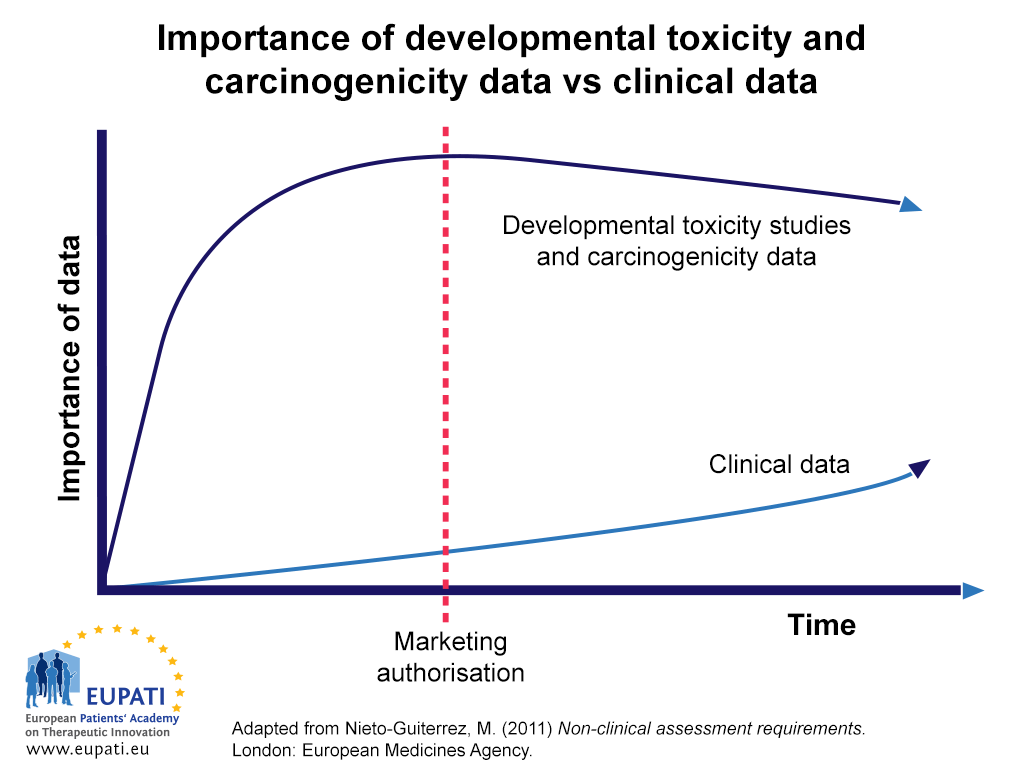
Types of Toxicology Studies
The following kinds of toxicology studies must be performed during non-clinical testing :
- Single- dose studies
- Repeated- dose studies
- Male fertility studies
- Female reproduction and developmental toxicology studies
- Local toxicology studies
- Hypersensitivity studies
Genotoxicity studies
Carcinogenicity studies.
These are explained in turn in the sections below.
Systemic toxicity studies
Systemic toxicology studies investigate the toxicity profile of the candidate compound in all of the animal’s tissues and organs. Systemic toxicology studies can be either single- dose or repeated- dose studies.
Reproductive toxicity studies
Reproduction toxicity studies investigate the effect of the candidate compound on the ability to reproduce and develop normally. These studies should be conducted as is appropriate for the population to be exposed to the candidate compound , and according to the following considerations:
- Men can be included in Phase I and II clinical trials before the conduct of the male fertility study, as an evaluation of the male reproductive organs is performed in the repeated- dose toxicity studies, although these studies should happen early in the process whenever possible. In any case, a male fertility study should be completed before the initiation of large scale or long duration clinical trials (for instance, Phase III trials ).
- Women not of childbearing potential (for instance, permanently sterilised or postmenopausal women) can be included in clinical trials without reproduction toxicity studies, if the relevant repeated- dose toxicity studies (which include an evaluation of the female reproductive organs) have been conducted.
- If women of child-bearing potential are identified as a potential user population of the medicine, reproduction toxicity studies need to be done as early as possible.
Local tolerance studies
Local tolerance studies investigate the effect of the compound on the skin or eyes. These local toxicity studies are usually part of the general toxicity studies. To support limited human administration by non-therapeutic routes, e.g. a single intravenous dose for determination of absolute bioavailability, a single dose local tolerance study in a single species is usually sufficient.
Genotoxicity studies investigate the effect of the candidate compound on the chromosomes and genes, and are generally needed to support human safety . Assessment of gene mutation is considered sufficient to support all single- dose clinical trials . For multiple- dose clinical trials , an additional assessment of chromosomal damage in mammalian systems is needed, and a full battery of tests for genotoxicity should be completed before initiation of Phase II clinical trials . If positive findings are observed in genotoxicity tests , the need for additional testing must be considered.
Carcinogenicity studies assess the effect that the candidate compound has on cancer generation. Carcinogenicity studies are generally conducted to support the marketing application of a new medicine. However, if there is a significant cause for concern, carcinogenicity studies should be conducted to bolster safety within clinical trials . In this case, a longer-term clinical trial duration with frequent monitoring, can be carried out. Generally, for medicines indicated for serious diseases in adults or paediatric patients, carcinogenicity testing may be concluded post-approval, based on the assumption that the early access to the medicines for patients outweighs the possible risk , although the earlier these tests can be completed, the better.
Attachments
- Presentation: Non- Clinical Development Size: 478,517 bytes, Format: .pptx Presentation on aspects of non- clinical development , including its aims, background activities, and the different types of non-clinical study .
A2-2.02.4-v1.3
- Term: Reproductive Toxicology
- Term: Marketing Authorisation
- Term: Carcinogenicity studies
- Term: Non-clinical testing
- Term: Systemic toxicology
- Term: Genotoxicity Study
- Term: Clinical development
- Term: Phase III Trials
- Term: Clinical trial
- Term: Gene Mutation
- Term: Intravenous
- Term: Absorption
- Term: Toxicology
- Term: Population
- Term: Significance
- Term: Toxicity
- Term: Compound
- Term: Phase I Trials
- Term: Phase II Trials
- Term: Safety
Article information
Categories: Non-Clinical Studies
Search Toolbox
- Sort by Sort Results By Relevance Title (A-Z) Title (Z-A) Date (Asc) Date (Desc)
- Patient Engagement
- Search the Toolbox
- Advocacy Basics of Medicine R&D Benefit and Risk Assessment Clinical Development / Trials Digital Health Drug Discovery Health Communication HTA Interview Medical Devices Non-Clinical Studies Patient engagement case studies Patient Engagement Toolbox Patient Involvement Personalised Medicine Pharmaceutical Development Pharmacoepidemiology Regulatory Affairs Safety of Medicines Starter Kits Types of Medicines Webinar
- Absorption Active Ingredient Active Pharmaceutical Ingredient Activism Adaptive Design Adaptive Pathways Adherence ADME Administering Medicine Advanced Therapeutic Medicinal Products (ATMPs) Advanced Therapies Adverse Event (AE) Advertising Clinical Trials Advocacy AIDS Analytic Framework Animal models Benefit-Risk Assessment Benefit-Risk Balance Best Pharmaceuticals For Children Act (BPCA) Best Practice Bias Bioavailability Bioequivalence Biologic Medicines Biomarkers Biomedical Research Biosimilar Medicines Blinding Botanicals Breastfeeding Carcinogenicity Centralised Procedure (CP) Certificate Of Suitability (CEP) Children Classification Clinical Development Clinical Effectiveness Clinical Studies Clinical Trial Application Clinical Trial Design Clinical Trial Registries Clinical Trial Results Clinical Trials Cohort Study Companion Diagnostics Comparison Trials Compassionate use Compliance Confirmatory Studies Critical Review Cross-Over Trial Data Capture Data Collection Data monitoring committees Data Protection Demographics Development Phases Diagnosis Digital Health Technologies Distribution Diversity Documentary Dosage Dossier Double Blind Drug-Drug Interaction (DDI) Early Clinical Development Economic Evaluation Education Effectiveness Assessment Efficacy Elderly People Eligibility Endpoints Engagement Epidemiology Epigenetics Equal Access Equity Ethics Ethnic Sub-groups EUPATI European Medicines Agency (EMA) European Public Assessment Report (EPAR) European Public Assessment Reports (EPARs) Example of Patient Involvement Excretion First in Human Food Effect Galenic Genotoxicity Geriatric Group Sequential Design Guidance Guidelines Health Data Health inequity Health Policy Health Systems Health-related Quality of Life (HRQoL) Healthcare Policy Herbal Medicines HIV HTA Hypothesis Immunity Impact Assessment In Silico In Vitro In Vivo Incidence Informed Consent Innovation Lead Compound Lead Generation Lead Molecule Lead Optimisation Legislation Levels of Evidence Life-Cycle Management Local Tolerance Market Access Marketing Mass balance Matched Pair Measurement Bias Medical Devices Medical Technology Medicine Medicine Manufacturing Medicines Discovery and Development Medicines regulation Metabolism Mini-course Multiple Sclerosis Nanotechnology Non-Clinical Testing Off-Label Open Label Orphan Medicine Paediatrics Parallel-Import Participants’ Rights Patient Patient Experience Patient expert Patient inclusion Patient Information Patient Involvement Patient Organisations Patient Participation Patient Reported Outcome (PRO) Patient Role In Digital Health Patient Role In HTA Patient story Patient Training Patient voice Patient-Reported Outcome Measures (PROMs) Patients Involved Patients Involved Case Report Patients’ Rights Pediatric Research Equity Act (PREA) Pediatrics Periodic Safety Update Report (PSUR) Personalised Medicine Pharmacodynamics Pharmacogenetics Pharmacokinetics Pharmacology Pharmacopeidemiology Pharmacopoeia Pharmacovigilance Pharmacovigilance Risk Assessment Committee (PRAC) Phase I Phase II Phase III Phase IV Phytomedicine Placebo Populations Post-Authorisation Efficacy Studies (PAES) Post-Authorisation Safety Studies (PASS) Pre-Clinical Pre-Clinical Safety Studies Pre-Clinical Testing Pre-Discovery Pregnancy Premature termination Prevalence Prevention Product Information Prognosis Proof of Concept Proof of Mechanism Proof of principle Prospective Studies Proteins Protest Protocol Design Public Hearings Public Involvement Publication Publication Bias Qualitative Research Quality Assurance Quality Of Medicines Quality Standards Quantitative Research Randomisation Regulation Reimbursement Research Research Priorities Retrospective Studies Risk Risk Mitigation Strategies Risk-Benefit Safety Sample Population Screening Seamless Phase II/Phase III Design Selection Bias Side Effects Single Ascending Dose Escalation Study Single Blind Small Populations Social Determinant Special Populations Stakeholders Statistics Stratification Surrogate Endpoint Symptoms Target Thorough QT Toxicity Toxicokinetics Toxicology Training Translational Medicine Treatment Trial medicine Triple Blind Unauthorised Medicines Unblinding Vaccine Withdrawal Trial
Find Out More
Creative Commons
Resources on medicines research and development, including patient involvement in the medicines lifecycle
Tools and resources to enable meaningful patient engagement around and within medicines R&D
Toxicity: An Introduction
- First Online: 05 May 2022
Cite this chapter

- Shyamasree Ghosh 3 &
- Rathi Dasgupta 4
996 Accesses
Toxicity induced by drug or chemicals may be life-threatening to human lives. Therefore a drug prior to human use should be tested for its toxic effects in preclinical studies in animals. In this chapter we discuss the types of toxicity, pathophysiology associated with drug induced toxicity, and the molecular basis involved. This forms the introductory chapter towards the next chapter which highlights the application of machine learning in detecting toxicity.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Abbreviations
Adverse drug reactions
Carbon monoxide
Cell mediated immunity
Central nervous system
Carbon nanotubes
Hyperthermia
Lethal dose
Multiwalled carbon nanotubes
Receptor–substrate
Stevens–Johnson syndrome
Single walled carbon nanotubes
Toxic epidermal necrolysis
World Health Organization
Araujo OE, Flowers FP (1984) Stevens-Johnson syndrome. J Emerg Med. 2(2):129–135
Article CAS Google Scholar
Baker N, Boobis A, Burgoon L et al (2018) Building a developmental toxicity ontology. Birth Defects Res 110(6):502–518
Behal N, Wong A, Mantara R, Cantrell FL (2016) Human poisoning through atypical routes of exposure. J Community Health 41(1):105–108
Article Google Scholar
Benigni R, Bossa C (2011) Mechanisms of chemical carcinogenicity and mutagenicity: a review with implications for predictive toxicology. Chem Rev 111(4):2507–2536
Cohen SM, Boobis AR, Dellarco VL et al (2019) Chemical carcinogenicity revisited 3: risk assessment of carcinogenic potential based on the current state of knowledge of carcinogenesis in humans. Regul Toxicol Pharmacol 103:100–105
Deaton JG, Nappe TM (2020) Warfarin toxicity. [Updated 2020 Jan 10]. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island. https://www.ncbi.nlm.nih.gov/books/NBK431112/
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet. 356(9237):1255–1259
EFSA Scientific Committee, More S, Bampidis V et al (2018) Genotoxicity assessment of chemical mixtures. EFSA J 17(1):e05519
PubMed Central Google Scholar
Ghiazza M, Fenoglio GV (2014) Chapter 8 - Carbon nanotubes: properties, applications, and toxicity. In: Health and environmental safety of nanomaterials polymer nanocomposites and other materials containing nanoparticles. Woodhead Publishing, Sawston, pp 147–174
Chapter Google Scholar
Harr T, French LE (2012) Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy 97:149–166
Hazelden KP (2013) The developmental toxicity testing of biologics. Methods Mol Biol 947:31–36
Hougaard KS, Hansen AM (2007) Enhancement of developmental toxicity effects of chemicals by gestational stress. A review. Neurotoxicol Teratol 29(4):425–445
Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants (1994) In: Bondurant S, Ernster V, Herdman R (eds) Safety of silicone breast implants, vol 4: Silicone toxicology. National Academies Press (US), Washington, DC. https://www.ncbi.nlm.nih.gov/books/NBK44789/
Google Scholar
Laurence DR (1982) Adverse drug reactions and acute poisoning reviews, vol 1, No. 1. J R Soc Med 75(8):683
Lei T, Chen F, Liu H et al (2017) ADMET evaluation in drug discovery. Part 17: development of quantitative and qualitative prediction models for chemical-induced respiratory toxicity. Mol Pharm 14(7):2407–2421
Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T (2018) Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol 54(1):147–176
Loomis TA, Hayes AW (1996) Chapter 7 - Classification of harmful effects of chemicals. In: Loomis’s essentials of toxicology, 4th edn. Elsevier, pp 101–106
McKinney JD (1985) The molecular basis of chemical toxicity. Environ Health Perspect 61:5–10
Mitchell JR, McMurtry RJ, Statham CN, Nelson SD (1977) Molecular basis for several drug-induced nephropathies. Am J Med 62(4):518–526
Mückter H (2003) What is toxicology and how does toxicity occur? Best Pract Res Clin Anaesthesiol 17(1):5–27
Naas DJ (2005) Quinidine. In: Encyclopedia of toxicology, 2nd edn. Elsevier, Oxford, pp 593–594
Nath R, Prasad R, Palinal VK, Chopra RK (1984) Molecular basis of cadmium toxicity. Prog Food Nutr Sci 8(1-2):109–163
CAS PubMed Google Scholar
Olson K, Smollin C (2008) Carbon monoxide poisoning (acute). BMJ Clin Evid 2008:2103
PubMed PubMed Central Google Scholar
Patel SV, Khan DA (2017) Adverse reactions to biologic therapy. Immunol Allergy Clin North Am. 37(2):397–412
Paton WD (1975) The molecular basis of drug toxicity. J Clin Pathol Suppl (R Coll Pathol). 9:1–6
Patton K, Borshoff DC (2018) Adverse drug reactions. Anaesthesia 73:76–84
Peach MS, Showalter TN, Ohri N (2015) Systematic review of the relationship between acute and late gastrointestinal toxicity after radiotherapy for prostate cancer. Prostate Cancer 2015:624736
Rencuzogullari E, Aydin M (2018) Methodology of genotoxic and teratogenic studies in rats. Methods Mol Biol 1797:555–575
Saeidnia S (2014) Turpentine encyclopedia of toxicology, 3rd edn. Academic, Boston, pp 860–865
Book Google Scholar
Seagrave Z, Bamba S (2017) Adverse drug reactions. Dis Mon 63(2):49–53
Shionogi & Co., Ltd. (1990) Summary of toxicity data on methyl isothiocyanate (MITC). J Pestic Sci 15(2):297–304. Released 5 Aug 2010. Online ISSN 1349-092
Swenberg JA, Beauchamp RO Jr (1997) A review of the chronic toxicity, carcinogenicity, and possible mechanisms of action of inorganic acid mists in animals. Crit Rev Toxicol 27(3):253–259
Wolf DC, Cohen SM, Boobis AR et al (2019) Chemical carcinogenicity revisited 1: a unified theory of carcinogenicity based on contemporary knowledge. Regul Toxicol Pharmacol 103:86
Download references
Author information
Authors and affiliations.
School of Biological Sciences, National Institute of Science Education and Research (NISER), Bhubaneswar, Odisha, India
Shyamasree Ghosh
Heathcare, Data Core Systems, Philadelphia, PA, USA
Rathi Dasgupta
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Ghosh, S., Dasgupta, R. (2022). Toxicity: An Introduction. In: Machine Learning in Biological Sciences. Springer, Singapore. https://doi.org/10.1007/978-981-16-8881-2_20
Download citation
DOI : https://doi.org/10.1007/978-981-16-8881-2_20
Published : 05 May 2022
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-8880-5
Online ISBN : 978-981-16-8881-2
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Environmental Toxicology
(3 reviews)
Kees van Gestel, Vrije Universiteit, Amsterdam
Copyright Year: 2020
Publisher: Environmental Toxicology
Language: English
Formats Available
Conditions of use.
Learn more about reviews.
Reviewed by Bailey Bowers, Visiting Assistant Professor, Earlham College on 12/13/22
Overall, the text is very comprehensive and all relevant subjects are covered. I appreciate that both the environmental aspects as well as the toxicology are given similar attention - often there is more focus on one vs. the other in textbooks... read more
Comprehensiveness rating: 4 see less
Overall, the text is very comprehensive and all relevant subjects are covered. I appreciate that both the environmental aspects as well as the toxicology are given similar attention - often there is more focus on one vs. the other in textbooks like this. My only misgiving is that there is a focus on European chemical regulations, but not any mention or detail on non-European regulations. Obviously this is understandable given that the author(s) are European, but I could envision having to supplement this portion of the text when I teach a course.
Content Accuracy rating: 5
All content is accurate and error-free to my knowledge. All content is referenced appropriately.
Relevance/Longevity rating: 5
The book captures the current state of understanding in this field quite well, and I feel it will stand the test of time. The main section that would likely need to be updated is the one that outlines specific classes of chemicals that are used, but this would be straightforward to update.
Clarity rating: 5
The text is written well for an undergraduate science audience - it assumes little prior knowledge of the relevant basic chemistry and biology concepts, and explains these at a good level before discussing their application in environmental toxicology.
Consistency rating: 5
Terminology and framework within the book is internally consistent.
Modularity rating: 5
The text is readily divided into smaller sections, and even includes some short reflection/assessments at the end of each section. This would be really apt for assigning reading in a course. I could envision using portions of this book rather than the entire thing, with little adverse consequence.
Organization/Structure/Flow rating: 5
The text is excellently organized. Beginning with basic environmental concepts and introducing classes of chemical contaminants before delving into toxicology is a great way to organize the text.
Interface rating: 3
The interface and typesetting is the book's biggest weakness. Several tables are split across pages. Mathematical and chemical equations are not formatted properly, which leads to confusion. The latter half of the book is in italic font for some reason. Some chemical structures are rather large, while others are small. Also, the table and figure numbering is not conducive for a book of this page length - each table/figure should really have a unique identifier and ideally, there should be an index of tables and figures.
Grammatical Errors rating: 5
No grammatical errors were observed.
Cultural Relevance rating: 5
The text is not culturally insensitive, and in fact, in the history section, the text made very clear that Indigenous people have important local knowledge relevant to environmental toxicology, and are not in the past, but rather still here in the present.
Overall, I could envision using this book in a course. Many environmental toxicology books miss the mark, and despite the formatting issues here, the content is excellent.
Reviewed by Sarah Commodore, Assistant Professor, Indiana University - Bloomington on 5/18/21
Overall the topics covered in this text are broad and provide a great overview for current relevant topics such as POPs, metals, pharmaceuticals and many more. Some modules have short clips or animations that allow for more illustration of the... read more
Comprehensiveness rating: 5 see less
Overall the topics covered in this text are broad and provide a great overview for current relevant topics such as POPs, metals, pharmaceuticals and many more. Some modules have short clips or animations that allow for more illustration of the topic introduced. The YouTube video with the experts was quite enlightening and unique. The final chapter focuses on regulatory toxicology in Europe - while this is great for students in Europe, there may be the need to consider shorter sections for other continents (even a brief mention will do) to give the text even more international relevance.
The text is quite accurate and there are no unfounded statements. There are appropriate references for the main ideas. The references section is rather short and the reader can check those out for further reading and detailed information.
Certainly, this text covers very relevant topics in the field of environmental toxicology. For instance, primary and secondary microplastics, nanomaterials as well as complex mixtures are current hot topics and this text introduces the reader to current literature on these.
Considering that this work is still in progress, the usage of technical terminology is adequate. The language is easy to read and sections are presented using normal, easy to follow wording.
The text is very consistent in its framework. Terms are defined appropriately. While it may have been helpful to group terms and definitions preferably at the end of each chapter, definitions are interspersed throughout each section and that also works well.
This text is divided into sections that are manageable that can be assigned for reading (even during class) to help address in-class discussions and to answer short answer/1-2 paragraph type questions for homework assignments.
Organization/Structure/Flow rating: 4
This text is well organized and modules are introduced in a decent order. The final section on page 823/824 of this current version seems to be in Dutch and needs to be translated into English to help English readers find other important materials (e.g. the YouTube video appears to be referenced on this page as well)
Interface rating: 4
While the figures are informative and help drive home key points, the labels and clarity can be improved. For instance, some figures seem to be truncated (at the time of this review) and individual chapter labels can be provided to help orient the leader (eg Fig 1 in Chapter 4 can be labelled as Fig 4.1 while figure 1 in chapter 3 can also be labelled as Fig 3.1).
No significant grammatical errors were observed.
The text is not offensive in any way and examples provided are suitable and appropriate.
Excellent book that covers broad relevant and timely topics.
Reviewed by Kan Shao, Associate Professor, Indiana University - Bloomington on 5/16/21
This textbook provides comprehensive coverage on the areas and topics of Environmental Toxicology. Unlike some other textbooks of Environmental Toxicology, which are primarily built upon textbooks of Toxicology with some additional sections on... read more
This textbook provides comprehensive coverage on the areas and topics of Environmental Toxicology. Unlike some other textbooks of Environmental Toxicology, which are primarily built upon textbooks of Toxicology with some additional sections on environmental contaminations and toxicants, this book truly emphasizes both keywords, i.e., “environmental” and “toxicology”, and integrates the contents harmonically. Some chapters, like “environmental chemistry” and “fate to exposure” are very useful and bridge the environment with toxicology.
Content Accuracy rating: 4
The contents are relatively accurate, but a few improvements will make the textbook even better. For example, on Page 60, two of the box plots shown in Figure 2 don’t have the bottom line. Reasons for the missing line should be explained in the caption. Another example is on pages 527 to 529, the table has been cut out by the edge of the page. Moreover, the page numbering at the bottom left corner on each page should be in English. Some of the sections are still under development (e.g., Section 4.3.9).
The textbook should be applauded for its contemporality. Many new but important topics are included in the textbook, including various toxicity testing techniques described in Section 4.3 and regulatory frameworks discussed in Section 6.5. The textbook doesn’t organize the contents based on toxicological effects like many other textbooks in toxicology do, but based on the major components in environmental toxicology (e.g., exposure, toxicity testing, risk assessment, regulations), therefore, it is much easier for the textbook to be updated when the knowledge and technologies evolved.
Clarity rating: 4
The numbering system used in the book for figures and tables should be modified. For a textbook of this size, each table and figure should have a unique number for quick tracking, a similar numbering method should be applied to the equations as well (currently the equations were indexed in some of the chapters).
Consistency rating: 4
Regarding terminology and framework, the text is relatively consistent. However, the book should keep the format and style of the figures with the captions consistent. For example, the color of the words of figure numbers should be consistent. Additionally, for plots that were generated by authors (not directly cited from other publications), the textbook should keep the style as consistent as possible (e.g., the color of the curve, the font of the plot caption).
The textbook did an excellent job separating the contents in a very organized way. Instead of organizing the sections according to various types of toxicities, the book presents the topics based on the related components in environmental toxicology, namely environmental chemistry, environmental exposure sciences, toxicology, population and ecosystem ecotoxicology, risk assessment and regulation. Each of the sections can be used independently or used as supporting materials in other courses/subjects.
The organization of the topics has been appreciated many times in this review. The topics have been organized in an uncommon way but very reasonable way. I would recommend such an organizational method being applied to other specific areas of toxicology.
Tables on pages from 527 to 529 are cut by the page edge. Other than that, everything looks well laid out.
No culturally offensive or inappropriate contents or examples were observed in the textbook.
Table of Contents
- Chapter 1: Environmental toxicology
- Chapter 2: Environmental Chemistry, Chemicals
- Chapter 3: Environmental Chemistry, from Fate to Exposure
- Chapter 4: Toxicology
- Chapter 5: Population, Community and Ecosystem Ecotoxicology
- Chapter 6. Risk assessment & regulation
Ancillary Material
About the book.
This open online textbook on Environmental Toxicology aims at covering the field in its full width, including aspects of environmental chemistry, ecotoxicology, toxicology and risk assessment. With that, it will contribute to improving the quality, continuity and transparency of the education in environmental toxicology. We also want to make sure that fundamental insights on fate and effects of chemicals gained in the past are combined with recent approaches of effect assessment and molecular analysis of mechanisms causing toxicity.
The book consists of six chapters, with each chapter being divided into several sub-chapters to enable covering all aspects relevant to the topic. All chapters are designed in a modular way, which each module having clear training goals and being flagged with a number of keywords. Most modules have an average length of 1000-2000 words, a limited number of references, and 3-5 figures and/or tables. A few modules are enlighted with short clips, animations or movies to allow better illustration of the theory. The introduction chapter of the book, for instance, contains a short interview with two key experts reflecting on the development of the field over the past 30 years.
The book contains tools for self-study and training, like a (limited) number of questions at the end of each module. For the future we foresee the addition of separate exercises and other tools that may help the student in understanding the theory.
About the Contributors
Cornelis A.M. (Kees) van Gestel is professor of Ecotoxicology of Soil Ecosystems at the Vrije Universiteit, Amsterdam. He has been working on different aspects of soil ecotoxicology, including toxicity test development, bioavailability, mixture toxicity, toxicokinetics, multigeneration effects and ecosystem level effects. His main interest is in linking bioavailability and ecological effects.
Contribute to this Page
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
Toxicity testing is critical in the evaluation of novel medications before they are used on humans. The goal of toxicity. testing is to identify any potential h azardous consequences that a test ...
toxicity experiments on different species can be combined to assess the adverse effect of the test substance on an ecological community. This chapter is intended to provide a general overview of toxicity studies and an introduction to the topics covered in this book. 1.1.1 Designed Experiments Compared to Observational Studies
toxicological testing. In vitro - research conducted on tissues, cells, or proteins outside of a whole organism. Advantages: Can easily expose cells to a toxicant. Can use bioassays to gauge the effect. More controlled - can more clearly link toxic effects to the toxicant. Faster and less expensive.
Scientific characterization of potential adverse effects resulting from exposures to hazardous agents or situations. Scientific calculation related to the probability that a random adverse effect will occur. Scientific evaluation of the toxicity of a compound.
Toxicity studies are divided into: Acute toxicity studies This is a short term assessment and evaluation of potential hazard test substance or consequences of single dose of a test substance.Acute toxicity testing may be used in risk assessments of chemicals for humans and non-target environmental organisms. Acute toxicity study is better ...
Principles of Toxicology Modules. Toxicology is defined as inves1ga1on of any adverse effects that physical, chemical, or biological agents may have on living organisms and the environment. Toxicity can be acute or chronic; mild or severe. There are a myriad of interconnected issues that researchers and designers face when determining whether a ...
Toxicology is the study of adverse effects of chemicals and physical agents on living organisms. A xenobiotic is a foreign substance taken into the body. A toxic agent is any chemical, physical, or biological agent that can produce an adverse biological effect. Toxic substances can be systemic toxicants, which affect the entire body or multiple ...
2 Introduction to Biochemical and Molecular Methods in Toxicology 13 Ernest Hodgson, Gerald A. LeBlanc, Sharon A. Meyer, and Robert C. Smart 2.1 Introduction 13 2.2 Cell Culture Techniques 13 2.2.1 Suspension Cell Culture 14 2.2.2 Monolayer Cell Culture 14 2.2.3 Indicators of Toxicity in Cultured Cells 14 2.3 Molecular Techniques 16
An introduction is given to the use of historical controls and how these studies relate to regulatory risk assessment of chemicals in the environment. A hierarchy of statistical models is provided that, in broad terms, defines the statistics used in this field of study and, specifically, in this text.
It relies on the concept that a dose, or a time of exposure (to a chemical, drug, or toxic substance), will cause an effect (response) on the exposed organism. Usually, the larger or more intense the dose, the greater the response, or the effect. This is the meaning behind the statement "the dose makes the poison.".
All documents and materials including study plan, raw data, formats, draft and final study reports, paraffin blocks and histological slides should be archived for minimum of 5 years following the introduction of the drug in the market. 1.10.2.1 Systemic Toxicity Studies (Schedule Y(amended version) - CDSCO n.d.) a. Single-Dose Toxicity Studies
Introduction to Toxicology. Ernest Hodgson, Ernest Hodgson. Department of Environmental and Molecular Toxicology, North Carolina State University, Raleigh, NC, USA. Search for more papers by this author. ... Single Chapter PDF Download $42.00. Details. Unlimited viewing of the article/chapter PDF and any associated supplements and figures. ...
Toxicokinetics: It is the study of how a chemical/drug/toxin gets into the body and what the body does to it. Involves 4 main processes: 1. absorption 2. distribution 3. biotransformation 4. elimination or excretion Toxicodynamics: Dynamic means a change or interaction so toxicodynamics is the study of the effects toxins may have on the body.
The introduction of toxicity studies typically outlines the purpose, methods, and significance of the research. Purpose: The primary objective of toxicity studies is to assess the safety of substances and products that may come into contact with humans, animals, or the environment. These studies help regulatory agencies, such as the Food and ...
Introduction. Toxicity studies investigate the safety profile of the candidate compound.They also provide important information about the absorption, distribution, metabolism, and excretion (ADME) of the compound in the body. A candidate compound must be assessed in many different kinds of non-clinical toxicity study before it can be administered to the first human volunteer; even more ...
Toxicology Terminology. Toxicologyis the study of the adverse effects of chemicals or physical agents on living organisms. A toxicologistis a scientist that determines the harmful effects of agents and the cellular, biochemical, and molecular mechanisms responsible for the effects. Toxicant, toxin, and poison are often used interchangeably in ...
Apply the basic principles of toxicology to evaluate the risk that exposure to chemicals in the environment will cause toxicity and disease in humans. Explain how personal characteristics, such as diet and genetics, affect how individuals respond to environmental toxicants. Retrieve toxicology information from public health effects databases ...
20.2.1 Acute Toxicity. Acute exposures to a toxic substance may not last long but may be toxic enough to cause severe harm or death. Like, for example, acute toxicity with suicidal intent in taking of cyanamide through oral or dermal route is known that affects the parasympathetic nervous system in both animals and in humans. Cyanamide exposure through dust or liquid can cause eye irritation ...
This open online textbook on Environmental Toxicology aims at covering the field in its full width, including aspects of environmental chemistry, ecotoxicology, toxicology and risk assessment. With that, it will contribute to improving the quality, continuity and transparency of the education in environmental toxicology. We also want to make sure that fundamental insights on fate and effects ...
D. Dr.Kavitha Vivek. A short seminar on Toxicity studies for post graduates. Health & Medicine. 1 of 30. Preclinical drug development is a stage that begins before clinical trials (testing in humans) during which important safety and pharmacology data are collected. He determined the relationship between poisons and their biological properties ...
Figure 1.5: Snake poison The most common symptoms of all snakebites are overwhelming fear, which may cause symptoms such as nausea and vomiting, diarrhea, vertigo, fainting, tachycardia and cold, clammy skin. Dry snakebites and those inflicted by a non-venomous species, can still cause severe injury.
For the duration of any such detail or assignment, employees will not be subject to the provisions of this part, except this section, or, except as provided in paragraph (d) of this section, to any supplemental agency regulations of their employing agencies, but will remain subject to the conflict of interest prohibitions in title 18 of the ...