

Study at Cambridge
About the university, research at cambridge.
- Events and open days
- Fees and finance
- Student blogs and videos
- Why Cambridge
- Qualifications directory
- How to apply
- Fees and funding
- Frequently asked questions
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Term dates and calendars
- Video and audio
- Find an expert
- Publications
- International Cambridge
- Public engagement
- Giving to Cambridge
- For current students
- For business
- Colleges & departments
- Libraries & facilities
- Museums & collections
- Email & phone search

- About us overview
- Our History
- Key contacts
- News overview
- Grants & Awards
- Public Engagement News
- For Press & the Media
- Research overview
- Research Groups overview
- Aitken Group
- Chapman Group
- MacFarlane Group
- Martins Group
- Mennella Group
- Patil Group
- Sawarkar Group
- Thaventhiran Group
- Willis Group
- Emirali (Selley) Project
- Mukherjee Project
- Mechanisms of Fibre Toxicity
- COVID-19 Unit Research
- Recent publications
- Datasets & Software
- Facilities & Services overview
- Advanced Light Imaging overview
- Bioinformatics overview
- Analytical pipelines
- Co-ordinated translational control
- Next Generation Sequencing
- Translational profiling by microarray
- Electron Microscopy and Ultrastructural Pathology overview
- Array Tomography
- Deep-etch EM
- Electron Tomography
- HPF & FS
- Image Processing
- Immune-gold Labelling
- Negative Staining
- Single Particle Analysis with Cryo EM
- Flow Cytometry overview
- Cell sorting
- Cell analysers
- Histopathology overview
- Digital pathology
- Image analysis
- In situ assays
- Special stains
- Tissue microarrays
- Proteomics overview
- Proteomics Facility Services
- Proteomics Facility Instruments and Software
- Support Services
- People overview
- Principal Investigators
- Research Staff
- Core Facilities Staff
- Support Staff
- Affiliated Members
- PhD Programme overview
- Studentships and Application information
- ITTP overview
- ITTP Summer School Attendees 2023
- MPhil programme
- Upcoming events
- Previous events
- Public Engagement overview
- Festivals & Events
- Community engagement
- For schools & students
- Science-art projects
- On-demand resources
- Impact & Innovation overview
- Partnering with Industry
- Partnering with Academia
- Partnering for Policy
- Commericalisation
- Working with NHS
- KTE Committee

Welcome to the MRC Toxicology Unit
The Medical Research Council (MRC) Toxicology Unit is a leading International Research Institute within the School of Biological Sciences, University of Cambridge. The Unit delivers mechanistic toxicology research, pursuing hypothesis-driven toxicological questions with a particular focus on the study of the causal links between exposure to endogenous and exogenous toxicants, molecular initiating events and adverse outcome pathways. The Unit's overall aims are to carry out pioneering research which leads to improved health and to train and mentor the next generation of toxicologists.
Professor Anne Willis is Director of the MRC Toxicology Unit. Anne was appointed as a member of the European Molecular Biology Organisation in 2015, and in 2017 awarded an OBE for services to biomedical sciences and supporting the careers of women scientists.
anne_willis.jpg

Professor Anne Willis OBE
Unit photo 2023

UK proteostasis community comes together for a successful inaugural event
21 May 2024
The conference marked the inaugural event of the UK Proteostasis Network , an initiative launched by the Babraham Institute and shaped by Network Coordinators: Ritwick Sawarkar from the MRC Toxicology Unit, Della David and Oliver Florey at the Babraham Institute, and Laura Itzhaki at the University of Cambridge.
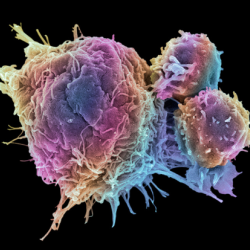
Computational approach to minimise off-target toxicity of CAR T-cell therapy targeting myeloma
Multiple myeloma is a type of bone marrow cancer which impacts a type of white blood cell called plasma cells, which are essential for the production of antibodies. Myeloma remains incurable but a treatment called CAR-T cell therapy - a type of cancer immunotherapy - could be a promising new avenue for treatment, if...

Toxicology Unit returns to Cambridge Festival
27 March 2024
Researchers from the MRC Toxicology Unit were involved in the Cambridge Festival 2024 last weekend to engage families with our research. The Cambridge Festival is an annual event coordinated by the University of Cambridge’s Public Engagement team. It is a multi-disciplinary festival with a mixture of online, on-demand and...
Tweets by MRC_TU
Upcoming Events
- 25 Jun Introduction to printmaking
- 08 Jul Aspiring Scientist Training Programme
- 15 Jul ITTP Summer School
- 13 Aug Printing immunology
- 11 Sep Seminar: Dr Giando Turchiano, AstraZeneca
View all events
Current vacancies
Explore all opportunities currently open across the Unit on our recruitment page.
MRC Toxicology Unit
Gleeson Building Tennis Court Road Cambridge CB2 1QR
View on Google maps
Tel: +44 (0)1223 334176
Email: [email protected]
Site Privacy & Cookie Policies
Connect with us
© 2024 University of Cambridge
- University A-Z
- Contact the University
- Accessibility
- Freedom of information
- Terms and conditions
- Undergraduate
- Spotlight on...
- About research at Cambridge

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections
- Course Directory
- Department directory
MRC Laboratory of Molecular Biology
Postgraduate Study
- Why Cambridge overview
- Chat with our students
- Cambridge explained overview
- The supervision system
- Student life overview
- In and around Cambridge
- Leisure activities
- Student unions
- Music awards
- Student support overview
- Mental health and wellbeing
- Disabled students
- Accommodation
- Language tuition
- Skills training
- Support for refugees
- Courses overview
- Qualification types
- Funded studentships
- Part-time study
- Research degrees
- Visiting students
- Finance overview
- Fees overview
- What is my fee status?
- Part-time fees
- Application fee
- Living costs
- Funding overview
- Funding search
- How to apply for funding
- University funding overview
- Research Councils (UKRI)
- External funding and loans overview
- Funding searches
- External scholarships
- Charities and the voluntary sector
- Funding for disabled students
- Widening participation in funding
- Colleges overview
- What is a College?
- Choosing a College
- Terms of Residence
- Applying overview
- Before you apply
- Entry requirements
- Application deadlines
- How do I apply? overview
- Application fee overview
- Application fee waiver
- Life Science courses
- Terms and conditions
- Continuing students
- Disabled applicants
- Supporting documents overview
- Academic documents
- Finance documents
- Evidence of competence in English
- AI and postgraduate applications
- Terms and Conditions
- Applicant portal and self-service
- After you apply overview
- Confirmation of admission
- Student registry
- Previous criminal convictions
- Deferring an application
- Updating your personal details
- Appeals and Complaints
- Widening participation
- Postgraduate admissions fraud
- International overview
- Immigration overview
- ATAS overview
- Applying for an ATAS certificate
- Current Cambridge students
- International qualifications
- Competence in English overview
- What tests are accepted?
- International events
- International student views overview
- Akhila’s story
- Alex’s story
- Huijie’s story
- Kelsey’s story
- Nilesh’s story
- Get in touch!
- Events overview
- Upcoming events
- Postgraduate Open Days overview
- Discover Cambridge: Master’s and PhD Study webinars
- Virtual tour
- Research Internships
- How we use participant data
- Postgraduate Newsletter
About the MRC Laboratory of Molecular Biology
The MRC Laboratory of Molecular Biology (LMB) is a world-class research laboratory, dedicated to understanding important biological processes at the molecular level – with the goal of using this knowledge to tackle major problems in human health and disease.
The LMB is one of the birthplaces of modern molecular biology. Many techniques were pioneered at the laboratory, including DNA sequencing, methods for determining the three-dimensional structure of proteins and the development of monoclonal antibodies .
Over the years, the work of LMB scientists has attracted 12 Nobel prizes , dozens of Royal Society awards and numerous other scientific honours.
In addition, many of our scientists have succeeded in exploiting their discoveries through technology transfer generating over £700 million of commercial income, to help support UK science.
2 courses offered in the MRC Laboratory of Molecular Biology
Biological science (mrc laboratory of molecular biology) - phd.
The Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) is one of the world's leading research institutes. Discoveries and inventions developed at the LMB such as DNA sequencing or methods to determine the structure of proteins, have revolutionised all areas of biology. Our scientists work to advance the current knowledge of biological processes at the molecular level. This information will help us to understand the workings of complex systems, such as the immune system and the brain, and solve key problems in human health.
Every year the LMB International PhD Programme welcomes 20–30 postgraduate students from universities all over the world to do cutting-edge research. At the LMB, we aim to train the scientific leaders of the future: we seek engaged and motivated students, give them rewarding research projects and provide a supportive environment with access to world-class facilities and experts.
Our building is at the heart of the Cambridge Biomedical Campus, a hub of one of the largest and most internationally competitive concentrations of healthcare-related talent and enterprise in Europe. The LMB building's stunning design is home to a globally competitive research centre with state of the art facilities for making the discoveries of the 21st century.
We have an active student association, founded and run entirely by students, who organise numerous events specifically for postgraduates. Postgraduate students register for their PhD with the University of Cambridge and belong to a Cambridge College.
More Information
Biological Science (MRC Laboratory of Molecular Biology) by thesis - MPhil - Closed
The MRC Laboratory of Molecular Biology occasionally takes on MPhil students providing they have the support of a group leader in the Laboratory. To obtain support from a group leader, students should contact them directly. For information on our faculty members’ research interests, please visit the Research and the Group Leaders pages on the LMB website.
The research MPhil is assessed by submission of a thesis and an oral examination. Students start in October and the thesis is submitted by the end of August.
Applicants will generally have a bioscience background, but applicants with a computational, physics or mathematical background can also apply.
3 courses also advertised in the MRC Laboratory of Molecular Biology
Biological sciences bbsrc dtp - phd - closed.
From the School of the Biological Sciences
The Cambridge Biosciences DTP is a four year fully-funded PhD programme that aims to create highly skilled and employable people. The programme offers training across 23 University Departments/Institutes and 3 Partner Institutes providing access to a wide range of research areas related to the strategic themes of the BBSRC. We offer three types of DTP studentships:
- DTP Standard
During the programme, DTP Standard and Targeted students will undertake two ten-week rotations in different labs before commencing their PhD. They will receive training in a variety of areas including but not limited to statistics, programming, ethics, data analysis, scientific writing and public engagement. Students will also undertake a 12-week internship (PIPS).
iCase students are not required to undertake rotations but may do so if they feel that this training would be useful. They must undertake a placement with their Industrial Partner for a minimum of three months and a maximum of 18 months.
Students will be expected to submit their thesis at the end of the fourth year.
Part-time study, whilst not the norm, may be viable, depending on the project, and will be considered on a case by case basis so please discuss this option with your proposed supervisor before making an application for this mode of study.
Clinical Medicine Wellcome Trust - PhD - Closed
From the Faculty of Clinical Medicine
We provide high-quality research training to clinical health professionals with an aptitude for research to enable them to become future leaders in medical and healthcare science. We offer training in an outstanding environment, spanning basic science, translational medicine, interdisciplinary, behavioural and applied health research.
We take great pride in our track record of successfully training health professionals to undertake the highest quality research across Cambridge and Norwich. We offer one of the most rewarding environments in which you could pursue your research training with world-leading researchers in The Schools of Clinical Medicine and Biological Sciences at the Universities of Cambridge, Wellcome Sanger Institute and other MRC, Wellcome & Cancer Research UK funded Institutes, Centres & Units in the wider Cambridge area, as well as the School of Health Sciences and Norwich Medical School at the University of East Anglia with other partners on the Norwich Research Park. The most important criteria we are looking for are the pursuit of research excellence, hard work and the will to make a difference to health.
The programme faculty provides mentoring and guidance on opportunities to undertake pre-doctoral research placements, enabling successful candidates to make an informed choice of PhD project and supervisor. Bespoke training and support for career development for fellows, together with support to supervisors, ensures a successful research experience. Post-doctorally, we will guide fellows based on their individual progress, to make the transition into higher research fellowships and clinical pathways, enabling ongoing training with continuance of research momentum.
National Institutes of Health Oxford/Cambridge Programme NIH Ox/Cam - PhD - Closed
From the Department of Medicine
This innovative programme was established in 2002 as a collaboration between the University of Cambridge and the National Institutes of Health (NIH) in the US. Its aim is to train outstanding students in biomedical research, taking advantage of the excellent research environments in Cambridge and the US. Students work on collaborative projects organised by co-supervisors in Cambridge and the NIH, spending two years at each institution. Students have access to all NIH facilities and are paid by the NIH. The PhD is awarded by the University of Cambridge.
Department Members
Dr jan löwe head of department, dr cristina rada director of graduate studies.
- 54 Academic Staff
- 200 Postdoctoral Researchers
- 106 Graduate Students
http://www2.mrc-lmb.cam.ac.uk/
Postgraduate admissions office.
- Admissions Statistics
- Start an Application
- Applicant Self-Service
At a glance
- Bringing a family
- Current Postgraduates
- Cambridge Students' Union (SU)
University Policy and Guidelines
Privacy Policy
Information compliance
Equality and Diversity
Terms of Study
About this site
About our website
Privacy policy
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
- Spotlight on...

Study at Cambridge
About the university, research at cambridge.
- Events and open days
- Fees and finance
- Student blogs and videos
- Why Cambridge
- Qualifications directory
- How to apply
- Fees and funding
- Frequently asked questions
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Term dates and calendars
- Video and audio
- Find an expert
- Publications
- International Cambridge
- Public engagement
- Giving to Cambridge
- For current students
- For business
- Colleges & departments
- Libraries & facilities
- Museums & collections
- Email & phone search
MRC Cognition and Brain Sciences Unit
Using cognitive theory and innovations in neuroscience to understand and improve mental wellbeing across the lifespan

We investigate fundamental human cognitive processes such as attention, language, memory, and emotion through state of the art brain imaging techniques and clinical approaches to improve our understanding of the brain.
Find out more about by visiting our research page here or visit the people page to look at some of the scientists work more closely.

Would you like to help our research by volunteering? Find out more here

We host 20-30 graduate students at any time and applications are invited from prospective PhD students in experimental cognitive psychology, neuropsychology, computational modeling and neuroimaging using MRI, MEG, and EEG.
Information about places can be found here .

The CBU hosts the Chaucer Club programme of talks from leading scientists in the field of cognitive neuroscience. Find details here .
The CBU Wednesday Lunchtime seminars are open to all and have talks from scientists from in and around Cambridge talking about their current research projects. Find out more here
Online user experience feedback
If you have any feedback about how we could improve the overall user experience for you on our websites and social media platforms, please feel free to get in touch using the contact form located here . We look forward to hearing from you.
Latest news
Getting to grips with an extra thumb
May 30, 2024
An emerging area of future technology is motor augmentation – using motorised wearable devices such as exoskeletons or extra robotic…
Cambridge Fellows announced as Medical Science Fellows
May 21, 2024
Professor Nita Forouhi from the Medical Research Council (MRC) Epidemiology Unit and Professor Susan Gathercole from the Department of Psychiatry…
Lewis Owens sponsored walk, raising funds for the MRC CBU
April 24, 2024
On Friday 21 June 2024, Lewis Owens (Queens’ 1996) will be taking on a sponsored walk in Cambridge, stopping at…
Moataz Assem awarded the prestigious Wellcome Trust Early Career Award
April 22, 2024
Congratulations to Moataz Assem from the MRC Cognition and Brain Sciences Unit, University of Cambridge (MRC CBU), who has been…
Undergraduate Summer Placement Scheme 2024
March 26, 2024
Undergraduate Summer Placement Scheme at the MRC Cognition and Brain Sciences Unit, University of Cambridge Monday 8 July – Friday…
View all news

Quick links
Useful information.
- Cookies and Privacy
- Wireless Access
Our research sites
The Centre for Attention, Learning and Memory (CALM)

The Cambridge Clinical Research Centre in Affective Disorders.

Cambridge Centre for Ageing and Neuroscience.
Email: [email protected] Tel: (+44) (0)1223 766166

© 2024 University of Cambridge
- University A-Z
- Contact the University
- Accessibility
- Freedom of information
- Terms and conditions
- Undergraduate
- Spotlight on...
- About research at Cambridge

Study at Cambridge
About the university, research at cambridge.
- Events and open days
- Fees and finance
- Student blogs and videos
- Why Cambridge
- Qualifications directory
- How to apply
- Fees and funding
- Frequently asked questions
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Term dates and calendars
- Video and audio
- Find an expert
- Publications
- International Cambridge
- Public engagement
- Giving to Cambridge
- For current students
- For business
- Colleges & departments
- Libraries & facilities
- Museums & collections
- Email & phone search
MRC Epidemiology Unit

The MRC Epidemiology Unit is a department at the University of Cambridge. It is working to improve the health of people in the UK and around the world.
Obesity, type 2 diabetes and related metabolic disorders present a major and growing global public health challenge. These disorders result from a complex interplay between genetic, developmental, behavioural and environmental factors that operate throughout life.
The mission of the Unit is to investigate the individual and combined effects of these factors and to develop and evaluate strategies to prevent these diseases and their consequences.
More about who we are
MRC Epidemiology Unit Seminar series
Find a list of upcoming seminars and recordings of previous ones . Receive emails about future seminars and other alerts: subscribe here.

Become a study volunteer
We are involved in many studies into obesity, type 2 diabetes and related metabolic disorders. But you don’t need to have one of these conditions to help us with our research. We are constantly looking for volunteers to enroll in our studies and help us improve our knowledge of how to treat and prevent these diseases.

Data sharing
We are committed to sharing data to maximise the value of our work for the public good. Our Data Sharing pages have details of the principles and processes for accessing and sharing data.

Work and study
We offer a range of opportunities for scientists, research support staff and management professionals. Whether you are a recent graduate or a senior researcher, we want to attract the brightest minds and invest in their futures through structured career development and on-the-job learning.

Making an Impact
We are building research, clinical and public health pathways for the application of our work. This means collaborating across sectors, developing new approaches for healthcare, and informing population approaches to disease prevention and public health promotion.
Our latest news
- Professor Nita Forouhi awarded prestigious Academy of Medical Sciences Fellowship
Congratulations to Professor Nita Forouhi on being elected to the Academy of Medical Sciences Fellowship. Nita is one of 58 exceptional biomedical and…
- Rare gene variants conferring up to 6-fold increase in obesity risk hint at new mechanism affecting appetite control
A study led by Medical Research Council (MRC) researchers has identified genetic variants in two genes that have some of the largest impacts…
- Prof Nick Wareham to play leading role in new national research network for population health
New network established to bring together researchers from across the United Kingdom to boost research into sustainable and equitable population health improvement. The…
- AI predicts healthiness of food menus and highlights ‘double burden’ of unhealthy food environment in deprived areas
Today the Guardian reports on how Unit PhD student Yuru Huang and colleagues have used artificial intelligence to predict the healthiness of cafe,…
Read more news >
Recent Posts
- HERA study to better understand women’s reproductive health receives £5.6 million Wellcome award
Information provided by:
[email protected] Site Privacy & Cookie Policies
Useful Links
Freedom of information, privacy policy, research integrity, website terms and conditions, social media, follow us on twitter, follow us on instagram, follow us on facebook, follow us on linkedin, subscribe on youtube.

© 2024 University of Cambridge
- University A-Z
- Contact the University
- Accessibility
- Freedom of information
- Terms and conditions
- Undergraduate
- Spotlight on...
- About research at Cambridge
Wellcome Trust and MRC invest in world-class Stem Cell Institute
Two of the UK’s largest funders of medical research are to invest £8 million in a new world-leading centre for stem cell biology and medicine. The Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute will advance our understanding of stem cells and their potential to treat a range of life-threatening conditions that currently have no effective cures.
- Share with Facebook
- Share with X
- Share with LinkedIn
- Share with Email
Stem cells can renew themselves almost indefinitely and can develop into any of the cell types in the body. They are an invaluable tool for scientists studying the mechanisms of human disease and could be used as an alternative to animal models by pharmaceutical companies developing new drugs. They also show great promise as potential treatments for devastating conditions such as liver disease, diabetes, blindness and spinal cord injury and neurodegenerative disorders like Parkinson's disease.
The new Institute, at the University of Cambridge, will build on existing investment by the Medical Research Council (MRC) and the Wellcome Trust, uniting 30 leading research teams with expertise across the three main types of stem cell: embryonic, adult and induced pluripotent cells.
Research scientists will work alongside technology specialists and doctors to develop new therapeutic approaches underpinned by a strong base of fundamental stem cell biology. Located in Cambridge, the Institute is near the largest cluster of biotechnology companies in Europe, allowing unrivalled opportunities for industry collaboration.
Professor Austin Smith, Director of the new Institute, said: "The Wellcome Trust-MRC Cambridge Stem Cell Institute will be an invigorating environment for cross-fertilisation between fundamental and translational researchers. Our aim is to close the knowledge gap and drive stem cell research forward towards clinical applications. The world-class facilities will attract the best international talent from the fields of stem cell biology and regenerative medicine to pursue this goal."
Sir Mark Walport, Director of the Wellcome Trust, said: "This strategic collaboration between the UK's two largest funders of medical research has united teams from across Cambridge that work across all types of stem cell research and will enable its director, Austin Smith, to attract outstanding researchers in the field. The new institute will play a vital part in accelerating our understanding health and disease and in the development of new treatments and will cement the UK's position as a world leader in stem cell research."
Professor Sir John Savill, Chief Executive of the MRC, said: "The UK is currently one of the best places in the world to do stem cell research, and we want to make sure that continues to be the case now and for the next generation of scientists. By joining forces with the Wellcome Trust to invest strategically in all areas of stem cell science, embracing both adult and embryonic stem cells, we will create a competitive and attractive environment for future commercial investment in regenerative medicine."
It is intended that the Institute will eventually be housed in a purpose-built 8000-m2 facility to be constructed on the Cambridge Biomedical Research Campus. Key areas of research at the Institute include pluripotency, haematopoiesis, epithelial tissues, and neural and cardiovascular stem cells.
Professor Sir Patrick Sissons, Regius Professor of Physic and Head of the School of Clinical Medicine at the University of Cambridge, said: "This joint funding initiative from the Wellcome Trust and MRC gives us the opportunity to link Cambridge's great strengths in stem cell biology with our strengths in translational clinical research, and thus to give new insights into disease mechanisms - and ultimately to develop new therapies.
"In association with the initiative, we all look forward to the future co-location of stem cell biology and medicine in the new building planned for the Cambridge Biomedical Campus."
About the Medical Research Council
For almost 100 years the Medical Research Council has improved the health of people in the UK and around the world by supporting the highest quality science. The MRC invests in world-class scientists. It has produced 29 Nobel Prize winners and sustains a flourishing environment for internationally recognised research. The MRC focuses on making an impact and provides the financial muscle and scientific expertise behind medical breakthroughs, including one of the first antibiotics (penicillin), the structure of DNA and the lethal link between smoking and cancer. Today MRC funded scientists tackle research into the major health challenges of the 21st century.
About the University of Cambridge
The University of Cambridge’s mission is to contribute to society through the pursuit of education, learning and research at the highest international levels of excellence. Cambridge's reputation for excellence is known internationally and reflects the scholastic achievements of its academics and students, as well as the world-class original research carried out by its staff. Some of the most significant scientific breakthroughs occurred at the University, including the splitting of the atom, invention of the jet engine and the discoveries of stem cells, plate tectonics, pulsars and the structure of DNA. From Isaac Newton to Stephen Hawking, the University has nurtured some of history's greatest minds and has produced more Nobel Prize winners than any other UK institution with over 80 laureates.
About the Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. It supports the brightest minds in biomedical research and the medical humanities. The Trust's breadth of support includes public engagement, education and the application of research to improve health. It is independent of both political and commercial interests.
Share this page

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections
Wellcome-MRC Cambridge Stem Cell Institute
- About us overview
- Our building overview
- How to find us
- Art at JCBC overview
- Anna Brownsted
- Victoria Morton
- Harold Offeh
- Kelly Briggs
- Public engagement overview
- Reaching beyond Cambridge
- Connecting with local communities
- Giving patients a voice
- Creating an open and engaged research culture
- Under the Microscope
- What would you become?
- Equity, diversity & wellbeing
- For current students
- People overview
- Leadership & governance overview
- Postgraduate Education Committee
- Professional services
- Principal investigators overview
- Dr Maria Alcolea
- Professor Roger Barker
- Dr Thorsten Boroviak
- Dr Harry Bulstrode
- Dr Maria Duque-Correa
- Professor Cédric Ghevaert
- Professor Bertie Göttgens
- Professor Tony Green
- Dr Brian Hendrich
- Dr Daniel Hodson
- Professor Brian Huntly
- Professor Thóra Káradóttir
- Professor Walid Khaled
- Professor Elisa Laurenti
- Dr Joo-Hyeon Lee
- Professor Andrew McCaskie
- Professor Simón Méndez-Ferrer
- Dr Jyoti Nangalia
- Professor Anna Philpott
- Professor David Rowitch
- Dr Fotios Sampaziotis
- Professor Ben Simons
- Professor Sanjay Sinha
- Dr Mekayla Storer
- Professor Sarah Teichmann
- Dr Richard Tyser
- Dr Konstantinos Tzelepis
- Professor George Vassiliou
- Professor Matthias Zilbauer
- Affiliated principal investigators overview
- Dr Irving Aye
- Dr Srinjan Basu
- Dr Sumru Bayin
- Professor Serena Best
- Professor Allan Bradley
- Professor Sarah Bray
- Professor Ruth Cameron
- Dr Peter Campbell
- Dr Maria Christophorou
- Dr Ana Cvejic
- Professor Anne Ferguson-Smith
- Professor Sarah Franklin
- Professor Kristian Franze
- Professor Richard Gilbertson
- Dr Namshik Han
- Professor Muzlifah Haniffa
- Dr Phil Jones
- Dr Golnar Kolahgar
- Dr Mark Kotter
- Dr András Lakatos
- Professor Madeline Lancaster
- Professor Ernest Laue
- Professor Paul Lehner
- Dr Mo Lotfollahi
- Dr Florian Merkle
- Professor James Nathan
- Professor Kathy Niakan
- Professor Ewa Paluch
- Dr Manav Pathania
- Dr Emma Rawlins
- Dr Teresa Rayon
- Dr Peter Rugg-Gunn
- Dr Marta Shahbazi
- Professor Azim Surani
- Dr Martin Turner
- Dr Jelle van den Ameele
- Professor Ludovic Vallier
- Professor Alan Warren
- Professor Doug Winton
- Dr Evgeny Zatulovskiy
- Academic & research staff
- Research overview
SCI-TIF - Technology & Innovation Forum
- Stem cell states
- Stem cells in disease
- Stem cells & therapeutics
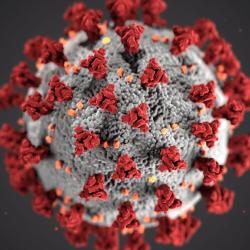
Our COVID-19 research
- Clinical trials
- Interdisciplinary Research Centre overview
- Cambridge Centre for Myelin Repair overview
- CCMR PI and Affiliates
- Research culture and integrity overview
- Open access & Plan S
- Guidelines and Policies
- Pluripotency platforms
- Core facilities overview
- Bioinformatics
- Electron microscopy
- Genomics overview
- Submission guidelines
- Flow cytometry
- Single cell platforms
- Tissue culture
- Events overview
- 2024 CSCI Annual Retreat
- International Seminars overview
- Group Leader Seminars overview
- PhD and Postdoc Seminars overview
- Research Culture & Integrity Seminars overview
- Past Events
- Haematology Event: Cambridge Lymphoma Biology International Symposium
- Learn more overview
- Patient information
- Join us overview
- Students overview
- Wellcome-funded Four Year (MRes + PhD) Programme in Stem Cell Biology & Medicine overview
- Application Process and Eligibility
- Students: Past & Present
- 1+3 Stem Cell Programme Statistics
- MPhil in Biological Science (Stem Cell Biology)
- PhD in Biological Science (Stem Cell Biology)
- Other funding opportunities
- Work experience & internships
- Equality, diversity & wellbeing (students)
- FAQs for Wellcome studentships
- Institute Only overview

Interdisciplinary research centre
Our Stem Cells IRC facilitates interdisciplinary interactions across the University and the wider community of Cambridge researchers whose work has significance to stem cell research and therapeutics.

Cambridge Stem Cell Institute Technology & Innovation Forum (SCI-TIF) This bespoke new programme brings together scientists with an interest in stem cells and technology in order to foster rich discussions, partnerships and translational opportunities. What is SCI-TIF? A membership programme...

Made The Same Way: developmental biology podcast
This podcast from Reform Radio and the Wellcome-funded Human Developmental Biology Initiative brings together emerging artists and researchers to discuss science, life, and music.
Institute researchers are involved in COVID-19 research projects, working collaboratively with researchers across the University of Cambridge and at other institutions in the UK and beyond.
Latest news
SCI-TIF gather for 2nd Annual Networking Dinner
16 May 2024
This month the Cambridge Stem Cell Institute Technology & Innovation Forum (SCI-TIF ) gathered to celebrate their 2 nd Annual Networking Dinner. SCI-TIF members, CSCI Principal Investigators , and Stem Cell Interdisciplinary Research Centre members got together at SIX Restaurant at the Varsity to meet, discuss...
CSCI Postgraduates take a break from the bench at this year's Postgraduate Away Day
Recently the CSCI PhD and MPhil students got together for their annual Postgraduate Away Day for a day of talks, brainstorming sessions, and a pub quiz. The event, planned by the student committee, took place on 25 April. The 25 new students from the October 2023 intake (pictured) under the supervision of 16 different PIs...
‘Exhausted’ immune cells in healthy women could be target for breast cancer prevention in new study by the Khaled Group
28 March 2024
Researchers at CSCI and University of Cambridge have created the world’s largest catalogue of human breast cells, which has revealed early cell changes in healthy carriers of BRCA1 and BRCA2 gene mutations. The study, published today (28 March) in the journal Nature Genetics from r esearchers in the Khaled Group in the...
CSCI Hot Topic Event covers AI’s impact on stem cell research & medicine
20 March 2024
This week (Monday 18 March) , members of the Cambridge Stem Cell Institute, the Technology and Innovation Forum ( SCI-TIF ) and guests from around Cambridge convened to delve into the pressing subject of AI’s role in advancing stem cell research and medicine ( see the poster here ). The event began with a welcome by...
View all news
The Cambridge Stem Cell Institute is a world-leading centre for stem cell research.
Our mission is to transform human health through a deep understanding of stem cell biology. , we're funded by wellcome and the medical research council..

- 03 Jun CSCI PhD and Postdoc Seminar - Lijiang Fei & Ken To & Bart Theeuwes
- 10 Jun CSCI PhD and Postdoc Seminar - Cortina Chen & Klara Stark
- 12 Jun CSCI Visitor Seminar - Dr. Samantha Morris
- 17 Jun CSCI PhD and Postdoc Seminar - Alexander Anderson & Tomoya Isobe
- 18 Jun CSCI Visitor Seminar - Prof. Jasmin Fisher
View all events
Stay connected
Sign up to our newsletter to stay up to date with news and events from the Cambridge stem cell community.
Log into your account to see
Tweets by SCICambridge
athena-swan-silver-award.png

Jeffrey Cheah Biomedical Centre Puddicombe Way Cambridge Biomedical Campus CB2 0AW
Social media
Quick links
Logo and brand guidelines
Data protection policies
Site privacy and cookie policies
Our funders
Medical Research Council
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
- Spotlight on...
- Skip to main content
- Skip to footer

Jason is a native of the UK. He was an undergraduate at Oxford University, where he worked with Professor John Sutherland on Cephalosporin biosynthesis. He obtained his PhD as a Fulbright grantee from Yale University, working with Professor Alanna Schepartz. He was a Damon Runyon Fellow at The Scripps Research Institute with Professor Peter Schultz where he developed the first approaches to systematically expand the genetic code of eukaryotic cells and pioneered approaches, that are now widely used, for defining protein interactions by genetically encoding photocrosslinking amino acids.
Jason’s work has been recognized by a number of awards, including: the Francis Crick Prize (Royal Society), the Corday Morgan Prize (Royal Society of Chemistry), European Molecular Biology Organization’s (EMBO) Gold Medal, Louis-Jeantet Young Investigator Career Award, Sackler International Prize in the Physical Science. He is in the European Inventors Hall of Fame, a member of EMBO, a Fellow of the Academy of Medical Sciences, and a Fellow of The Royal Society. His current scientific interests are described in the Research pages.
Download Jason’s CV. Read Elizabeth Pain’s profile of Jason in Science Careers. Watch Jason discuss our research with Thomas Lembeger in an interview for EMBO.
Citation analysis, h-index etc.
© Chin Laboratory MRC Laboratory of Molecular Biology Centre for Chemical & Synthetic Biology Division of Protein and Nucleic Acid Chemistry

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections

MRC Mitochondrial Biology Unit
- Director's Introduction
- MBU Past and Present
Key Contacts
- Directions/How to find us
- Research Groups overview
- Hirst Group overview
- Complex I cryoEM
- Complex I mechanism
- Physiological effects and mitochondrial disease models
- Pharmacology & toxicology involving complex I
- Chinnery Group
- Kunji Group overview
- Calcium regulation of mitochondrial carriers
- Diseases caused by mitochondrial transport proteins
- Electron microscopy of mitochondrial transport proteins
- Identification of mitochondrial transport proteins
- Role of cardiolipin in mitochondrial carriers
- Structural and functional analysis of the mitochondrial pyruvate carrier
- Structures of mitochondrial transport proteins
- Substrate binding and proton coupling
- Transport mechanism of mitochondrial carriers
- Minczuk Group overview
- Mito gene expression
- Mitochondrial disease
- mtDNA engineering
- Murphy Group overview
- Mitochondrial Exomarkers
- Mitochondrial Therapies
- Thiol Proteomics
- Petkevicius Group overview
- The biology of TLCD proteins
- Lipotoxicity and mitochondrial phospholipids
- Prudent Group overview
- Mechanisms of mitochondrial dynamics
- Mitochondria-organelle membrane contact sites
- Mitochondrial membrane remodelling and mtDNA dynamics
- Ryan Group overview
- Mitochondrial retrograde signalling
- Transcription factor-driven metabolic reprogramming
- van den Ameele Group overview
- Chromatin and Mitochondrial Disease
- Neural stem cell-niche interactions in mitochondrial disease
- Walker Group overview
- ATP Synthase
- Subunit composition of ATP synthase
- Evolution of the structure of ATP synthase
- The structure and function of ATP synthases
- Molecular animations of ATP synthase
- Regulation of ATP synthases
- The biogenesis and assembly of the human ATP synthase
- Protein methylation
- The ATP synthase and the permeability transition in mitochondria
- Whitworth Group overview
- Cell biology of mitochondrial homeostasis - overview
- Mechanisms of mitophagy and PINK1/Parkin function
- Mechanisms to handle and mitigate mtDNA mutations
- Mitochondrial calcium in health and neurodegeneration
- Redox biology in neurodegeneration
- Regulation of RNA biology in mitochondrial and neuronal homestasis
- Activation of immune signalling upon mitochondrial disruption
- Integration with the Department of Clinical Neurosciences and NHS overview
- Associated Research Groups
- Research Resources and Facilities overview
- Publications
- Postgraduate Studies
- Postdoctoral research
- Current Vacancies
- What are Mitochondria overview
- Mitochondria in Biology
- Mitochondria in Disease
- Public Engagement overview
- Public Engagement Objectives
- Public Engagement Team and Contacts
- Mitochondria and Art
- Teaching Resources for Schools
- Seminars and events announcements overview
- Environment
- Wellbeing and Equality overview
- Wellbeing, Inclusion, Diversity and Equality (WIDE) Committee
- Mentoring Scheme
- Wellbeing Resources
- Reporting issues and concerns
- MBU Postgraduate Student Society
- MBU Postdoctoral Society overview
- Postdocs overview
- Stephen Burr
- Mariangela Dionysopoulou
- Dnyanesh Dubal
- Federica De Lazzari
- Michele Frison
- Denis Lacabanne
- Leonor Miller-Fleming
- Stavroula Petridi
- Hiran Ambelal Prag
- Alvaro Sanchez-Martinez
- Pedro Silva Pinheiro
- Alice Sowton
- Sotiria Tavoulari
- Lindsey Van Haute
- Local Quick Links
The strategic mission of the MBU is to understand mitochondrial biology in health and disease, and to exploit this understanding to develop new therapies and improve human health.


Patrick Chinnery elected as a Fellow of the Royal Society
16 May 2024
Congratulations to Professor Patrick Chinnery , who has been elected as a Fellow of the Royal Society in recognition of his substantial contribution to the advancement of science. Nine of the Royal Society’s newly elected Fellows are from the University of Cambridge. University of Cambridge press release

Official Opening of the Hirst Building at Greenhead College, Huddersfield
24 April 2024
We are delighted to share coverage of the official opening of the Hirst Building at Greenhead College Huddersfield, which has been named after the MBU’s Director - Professor Judy Hirst , who is a former Greenhead College student. The Hirst Building replaces the College’s 1960s Science block with vibrant, modern classrooms...
MBU members involved in new £7.5 million LifeArc Centre for Rare Mitochondrial Diseases
Professor Michal Minczuk , leader of the MBU’s Mitochondrial Genetics programme, Dr Jelle van den Ameele , leader of the Mitochondria in Development group and MBU associates - Professors Rita Horvath and Patrick Yu Wai Man , are excited to be key partners in the LifeArc Centre for Rare Mitochondrial Diseases . The LifeArc...
ISAC/M 2024: Inspiring Scientists at the Cambridge Institute for Medical Research and the MRC Mitochondrial Biology Unit
5 March 2024
This free, widening participation programme for Cambridgeshire-based Year 12 students (16+) took place from 20-22 February (half-term) at the Cambridge Institute for Medical Research (CIMR) and MRC Mitochondrial Biology Unit (MBU), in association with St Catharine's College , Cambridge. This was our second year of...

Visit by delegates from Henan University of Chinese Medicine
20 March 2024
On 13 March we hosted 15 delegates from Henan University of Chinese Medicine. Our visitors received an overview of our research, and were escorted on a tour of the Mitochondrial Neurodegeneration laboratory and the Mass Spectrometry Facility.

MBU at the Cambridge Festival 2024: Biomedical Campus Day
On Saturday, 16 March the MBU participated in the Cambridge Festival's Biomedical Campus Day at the Cambridge Academy of Science and Technology (CAST). Ten members of the Unit showcased our research through poster demonstrations, activitiies including our MITOTrumps card game, Mitochondrial Pinball and two versions of our...
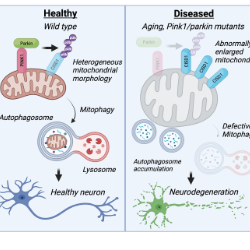
Whitworth group research identifies an important PINK1/Parkin mitophagy regulator
20 February 2024
Mitochondrial dysfunction is a key pathogenic driver of neurodegenerative diseases such as Parkinson’s disease (PD). Extensive research has established that the PD-linked genes, PINK1 and Parkin, are important factors mediating the degradation of dysfunctional mitochondria via mitophagy. Hence, there is intense interest in...
Tweets by MRC_MBU
Interested in doing a PhD at the MBU?
Link to our Postgraduate Studies webpage
Life at the MRC MBU
Seminars and Events
MBU Seminars 2024
5 June - Prof Dr Alessandro Prigione
11 September - Dr Tom MacVicar
9 October - Professor Annie Curtis
23 October - Dr Simon Johnson
13 November - Dr Laura Greaves
11 December - Dr Alex Gould
The Keith Peters Building
Cambridge Biomedical Campus
Tel: +44 1223 252700
HOW TO FIND US
INFORMATION COMPLIANCE
We follow the University's policies on Information compliance .
FIND US ON SOCIAL MEDIA
Institutional links.
School of Clinical Medicine
UKRI/Medical Research Council
Athena Swan
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
- Spotlight on...

MRC Metabolic Research Laboratories / MRC Metabolic Diseases
The Medical Research Council (MRC) Metabolic Research Laboratories at the University of Cambridge (MRC MRL) has been developed to improve understanding the mechanisms responsible for obesity and related metabolic diseases, with the eventual goal of developing interventions to prevent and treat them. The MRL also hosts the MRC Metabolic Diseases Unit.
Find out more about the MRC Metabolic Research Laboratories .
Last updated: 31 March 2022
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
- India Election
- T20 WC 2024
Scientists develop robotic 'third thumb' that can greatly boost human efficiency

Story highlights
Scientists at Cambridge University conducted a study and found that a 'third thumb' can really help expand our efficiency and productivity.
How will it enhance human capabilities and productivity if an additional robotic thumb is installed in our hands? Scientists at Cambridge University conducted a study and found that it can really help expand our efficiency. They have also developed a controllable prosthetic extra thumb, which is easy to use and very controllable.
Tamar Makin, a professor from the Medical Research Council (MRC) Cognition and Brain Sciences Unit at the University of Cambridge, said that the new technology can help marginalised communities.
“These technologies open up exciting new opportunities that can benefit society, but it’s vital that we consider how they can help all people equally, especially marginalized communities who are often excluded from innovation research and development,” she said.
Watch: From Big Bang Theory to Toddlerhood: Exploring Universe's early stages
He added that it will greatly help people relying on their hand's manual dexterity to complete their tasks.
"We are also really excited about potential opportunities of using the thumb to enhance productivity in work settings, especially those that are relying on their hand's manual dexterity in order to accomplish their work,” she said.
Also read: Kids more likely to trust robots than humans, reveals study
“This can be anywhere between manual labourers that are trying to solder a complicated kit or even surgeons that have to negotiate between many instruments at the same time," she added.
The device, referred to here as the third thumb, was initially tested by 600 participants at a 2022 exhibition.
Out of the 600 people, only 4 were not able to use the robotic thumb properly due to size issues. It reveals how the device is designed to help most of the people who intend to use it.
The results of the experiment were published in Science Robotics in May which revealed: "98 per cent of participants were able to successfully manipulate objects using the extra thumb during the first minute of use, with no significant influences of gender, handedness, or affinity for hobbies involving the hands”.
(With inputs from agencies)
)
Vikrant Singh
Geopolitical writer at WION, follows Indian foreign policy and world politics, a truth seeker.

Gates Medical Research Institute in Cambridge names new head
A former longtime executive at the Swiss drug giant Novartis will become the new chief executive of the Bill & Melinda Gates Medical Research Institute, a nonprofit biotech in Cambridge working on treatments and vaccines for some of the world’s most common and serious diseases.
Patrice Matchaba will succeed Emilio Emini, who recently announced that he will retire on June 30, according to the institute. Matchaba began his career as a physician in South Africa and worked as a doctor in the mines of Botswana for three years, where he saw the ravages of tuberculosis firsthand.
He spent 23 years at Novartis, starting in drug development and ending up as president of the Novartis US Foundation, which he led for two years until 2023, according to his LinkedIn page. The foundation seeks to improve health in underserved communities.
Trevor Mundel, who chairs the board of the Gates Medical Research Institute, said Matchaba “has seen every aspect of global health drug development.” Matchaba has worked on efforts to treat sickle cell disease in Africa and Chagas disease, which is caused by a parasite, in South America.
Matchaba was born in Zimbabwe and is a citizen of South Africa and the United States.
The institute was spun out of the Bill & Melinda Gates Foundation in 2018, with initial funding of $273 million. It has about 160 staffers, including a number of contractors, according to a spokesperson.
In March of this year, the institute launched a late-stage clinical trial of a tuberculosis vaccine candidate.


Study at Cambridge
About the university, research at cambridge.
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges and Departments
- Email and phone search
- Give to Cambridge
- Museums and collections
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Fees and funding
- Postgraduate events
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
Imperceptible sensors made from ‘electronic spider silk’ can be printed directly on human skin
- Research home
- About research overview
- Animal research overview
- Overseeing animal research overview
- The Animal Welfare and Ethical Review Body
- Animal welfare and ethics
- Report on the allegations and matters raised in the BUAV report
- What types of animal do we use? overview
- Guinea pigs
- Equine species
- Naked mole-rats
- Non-human primates (marmosets)
- Other birds
- Non-technical summaries
- Animal Welfare Policy
- Alternatives to animal use
- Further information
- Funding Agency Committee Members
- Research integrity
- Horizons magazine
- Strategic Initiatives & Networks
- Nobel Prize
- Interdisciplinary Research Centres
- Open access
- Energy sector partnerships
- Podcasts overview
- S2 ep1: What is the future?
- S2 ep2: What did the future look like in the past?
- S2 ep3: What is the future of wellbeing?
- S2 ep4 What would a more just future look like?

Researchers have developed a method to make adaptive and eco-friendly sensors that can be directly and imperceptibly printed onto a wide range of biological surfaces, whether that’s a finger or a flower petal.
The method, developed by researchers from the University of Cambridge, takes its inspiration from spider silk, which can conform and stick to a range of surfaces. These ‘spider silks’ also incorporate bioelectronics, so that different sensing capabilities can be added to the ‘web’.
The fibres, at least 50 times smaller than a human hair, are so lightweight that the researchers printed them directly onto the fluffy seedhead of a dandelion without collapsing its structure. When printed on human skin, the fibre sensors conform to the skin and expose the sweat pores, so the wearer doesn’t detect their presence. Tests of the fibres printed onto a human finger suggest they could be used as continuous health monitors.
This low-waste and low-emission method for augmenting living structures could be used in a range of fields, from healthcare and virtual reality, to electronic textiles and environmental monitoring. The results are reported in the journal Nature Electronics .
Although human skin is remarkably sensitive, augmenting it with electronic sensors could fundamentally change how we interact with the world around us. For example, sensors printed directly onto the skin could be used for continuous health monitoring, for understanding skin sensations, or could improve the sensation of ‘reality’ in gaming or virtual reality application.
While wearable technologies with embedded sensors, such as smartwatches, are widely available, these devices can be uncomfortable, obtrusive and can inhibit the skin’s intrinsic sensations.
“If you want to accurately sense anything on a biological surface like skin or a leaf, the interface between the device and the surface is vital,” said Professor Yan Yan Shery Huang from Cambridge’s Department of Engineering, who led the research. “We also want bioelectronics that are completely imperceptible to the user, so they don’t in any way interfere with how the user interacts with the world, and we want them to be sustainable and low waste.”
There are multiple methods for making wearable sensors, but these all have drawbacks. Flexible electronics, for example, are normally printed on plastic films that don’t allow gas or moisture to pass through, so it would be like wrapping your skin in cling film. Other researchers have recently developed flexible electronics that are gas-permeable, like artificial skins, but these still interfere with normal sensation, and rely on energy- and waste-intensive manufacturing techniques.
3D printing is another potential route for bioelectronics since it is less wasteful than other production methods, but leads to thicker devices that can interfere with normal behaviour. Spinning electronic fibres results in devices that are imperceptible to the user, but don't have a high degree of sensitivity or sophistication, and they’re difficult to transfer onto the object in question.
Now, the Cambridge-led team has developed a new way of making high-performance bioelectronics that can be customised to a wide range of biological surfaces, from a fingertip to the fluffy seedhead of a dandelion, by printing them directly onto that surface. Their technique takes its inspiration in part from spiders, who create sophisticated and strong web structures adapted to their environment, using minimal material.
The researchers spun their bioelectronic ‘spider silk’ from PEDOT:PSS (a biocompatible conducting polymer), hyaluronic acid and polyethylene oxide. The high-performance fibres were produced from water-based solution at room temperature, which enabled the researchers to control the ‘spinnability’ of the fibres. The researchers then designed an orbital spinning approach to allow the fibres to morph to living surfaces, even down to microstructures such as fingerprints.
Tests of the bioelectronic fibres, on surfaces including human fingers and dandelion seedheads, showed that they provided high-quality sensor performance while being imperceptible to the host.
“Our spinning approach allows the bioelectronic fibres to follow the anatomy of different shapes, at both the micro and macro scale, without the need for any image recognition,” said Andy Wang, the first author of the paper. “It opens up a whole different angle in terms of how sustainable electronics and sensors can be made. It’s a much easier way to produce large area sensors.”
Most high-resolution sensors are made in an industrial cleanroom and require the use of toxic chemicals in a multi-step and energy-intensive fabrication process. The Cambridge-developed sensors can be made anywhere and use a tiny fraction of the energy that regular sensors require.
The bioelectronic fibres, which are repairable, can be simply washed away when they have reached the end of their useful lifetime, and generate less than a single milligram of waste: by comparison, a typical single load of laundry produces between 600 and 1500 milligrams of fibre waste.
“Using our simple fabrication technique, we can put sensors almost anywhere and repair them where and when they need it, without needing a big printing machine or a centralised manufacturing facility,” said Huang. “These sensors can be made on-demand, right where they’re needed, and produce minimal waste and emissions.”
The researchers say their devices could be used in applications from health monitoring and virtual reality, to precision agriculture and environmental monitoring. In future, other functional materials could be incorporated into this fibre printing method, to build integrated fibre sensors for augmenting the living systems with display, computation, and energy conversion functions. The research is being commercialised with the support of Cambridge Enterprise, the University’s commercialisation arm.
The research was supported in part by the European Research Council, Wellcome, the Royal Society, and the Biotechnology and Biological Sciences Research Council (BBSRC), part of UK Research and Innovation (UKRI).
Reference: Wenyu Wang et al. ‘ Sustainable and imperceptible augmentation of living structures with organic bioelectronic fibres .’ Nature Electronics (2024). DOI: 10.1038/s41928-024-01174-4

Read this next

Ten Cambridge scientists elected as Fellows of the Royal Society 2024

‘Wraparound’ implants represent new approach to treating spinal cord injuries

Robotic nerve ‘cuffs’ could help treat a range of neurological conditions

Training AI models to answer ‘what if?’ questions could improve medical treatments
Media enquiries.
Sensors printed on human fingers
Credit: Huang Lab, Cambridge
Search research
Sign up to receive our weekly research email.
Our selection of the week's biggest Cambridge research news sent directly to your inbox. Enter your email address, confirm you're happy to receive our emails and then select 'Subscribe'.
I wish to receive a weekly Cambridge research news summary by email.
The University of Cambridge will use your email address to send you our weekly research news email. We are committed to protecting your personal information and being transparent about what information we hold. Please read our email privacy notice for details.
- biotechnology
- electronics
- Engineering
- Yan Yan Shery Huang
- Department of Engineering
- School of Technology
- Cambridge Enterprise
Related organisations
- European Research Council
- Royal Society
- Biotechnology and Biological Sciences Research Council (BBSRC)
- UK Research and Innovation (UKRI)
Connect with us

© 2024 University of Cambridge
- Contact the University
- Accessibility statement
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Cambridge University Press & Assessment
- Research news
- About research at Cambridge
- Spotlight on...
- Open access
- Published: 16 May 2024
Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey
- Mark McJury 1
BMC Health Services Research volume 24 , Article number: 635 ( 2024 ) Cite this article
1024 Accesses
Metrics details
In healthcare, regulation of professions is an important tool to protect the public. With increasing regulation however, professions find themselves under increasing scrutiny. Recently there has also been considerable concern with regulator performance, with high profile reports pointing to cases of inefficiency and bias. Whilst reports have often focused on large staff groups, such as doctors, in the literature there is a dearth of data on the experiences of smaller professional groups such Clinical Scientists with their regulator, the Health and Care Professions Council.
This article reports the findings of a survey from Clinical Scientists (Physical Sciences modality) about their experiences with their regulator, and their perception of the quality and safety of that regulation.
Between July–October 2022, a survey was conducted via the Medical Physics and Engineering mail-base, open to all medical physicists & engineers. Questions covered typical topics of registration, communication, audit and fitness to practice. The questionnaire consisted of open and closed questions. Likert scoring, and thematic analysis were used to assess the quantitative and qualitative data.
Of 146 responses recorded, analysis was based on 143 respondents. Overall survey sentiment was significantly more negative than positive, in terms of regulator performance (negative responses 159; positive 106; significant at p < 0.001). Continuous Professional Development audit was rated median 4; other topics were rated as neutral (fitness to practice, policies & procedures); and some as poor (value).
Conclusions
The Clinical Scientist (Physical Sciences) professional registrants rated the performance of their regulator more negatively than other reported assessments (by the Professional Standards Authority). Survey respondents suggested a variety of performance aspects, such as communication and fitness to practice, would benefit from improvement. Indications from this small dataset, suggest a larger survey of HCPC registrants would be useful.
Peer Review reports
In Healthcare, protection of patients and the public is a core principle. Part the framework of protections, includes regulation of professions [ 1 ]. This aims to mitigate risks such as the risk from bogus practitioners – insufficiently trained people acting as fully-trained professional practitioners, see Fig. 1 .
Recent UK media report on a bogus healthcare practitioner [ 2 ]
Regulation of professions ensures that titles (e.g. Doctor, Dentist, Clinical Scientist) are protected in law. The protected title means someone may only use that title, if they are on the national register, managed by the regulator – the Health and Care Professions Council (HCPC). It is a criminal offence to use a protected title if you are not entitled to do so [ 3 ]. There are a large number of regulators in healthcare – see Table 1 . Most of the regulators manage a register for one profession, except the HCPC which regulates 15 professions.
To be included on the register, a candidate must meet the regulators criteria for knowledge and training, and a key element to remain, is to show evidence of continuous professional development (CPD). Being on the register ensures that a practitioner has met the appropriate level of competence and professional practice.
For many healthcare workers, being on the HCPC register is a compulsory requirement to be appointable to a post. They must pay the necessary annual fees, and abide by the policies drawn-up by the regulator, and generally professions have no choice of regulator – these are statutory bodies, setup by government.
Recently, there has been considerable public dissatisfaction with the activity & performance of some regulators, notably Ofwat [ 4 ], and Ofgem [ 5 ]. Healthcare workers should expect a high level of professionalism, efficiency, and integrity from a regulator, as the regulator’s performance directly affects staff and public safety.
In terms of the regulation of UK Clinical Scientists, there is a dearth of data regarding experiences with the HCPC and views on the quality of regulation provided.
Findings are reported here from a 2022 survey of Medical Physicists and Engineers (one of the 16 job roles or ‘modalities’ under the umbrella of Clinical Scientist). The research aim was to assess experiences, and the level of ‘satisfaction’ with the regulator. For the remainder of this report, the term Clinical Scientist will be taken to mean Clinical Scientist (Medical Physicist/Engineer). The survey was designed to gather & explore data about opinions and experiences regarding several key aspects of how the HCPC performs its role, and perception of the quality & safety of regulation delivered.
A short survey questionnaire was developed, with questions aimed to cover the main regulatory processes, including registration & renewal, CPD audit, and fitness-to-practice. There were also questions relating more generally to HCPC’s performance as an organisation, e.g. handling of personal data. Finally, participants were asked to rate the HCPC’s overall performance and what they felt was the ‘value’ of regulation. The survey questions are listed in the Supplementary file along with this article.
Questions were carefully worded and there was a balance of open and closed questions. A five-point Likert score was used to rate closed questions. The survey was anonymous, and the questions were not compulsory, allowing the responders to skip irrelevant or difficult questions. The survey also aimed to be as short & concise as possible, to be a minimal burden to busy clinical staff & hopefully maximise response rate. There were a small number of questions at the start of the survey, to collect basic demographics on the respondents (role, grade, UK nation etc.).
The survey was advertised on the online JISC-hosted UK Medical Physics and Engineering (UKMPE) mail-base. This offered convenient access for the majority of Clinical Scientists. The survey was advertised twice, to allow for potential work absence, holiday/illness etc. It was active from the end of July 2002 until October 2022, when responses appeared to saturate.
The data is a combination of quantitative rating scores, and qualitative text responses. This allows a mixed-methods approach to data analysis, combining quantitative assessment of the Likert scoring, and (recursive) thematic analysis of the free-text answers [ 6 ]. Thematic analysis is a standard tool, and has been reported as a useful & appropriate for assessing experiences, thoughts, or behaviours in a dataset [ 7 ]. The survey questions addressed the main themes, but further themes were identified using an inductive, data-driven approach. Qualitative data analysis (QDA) was performed using NVivo (QSR International).
Two survey questions attempted to obtain an overall perception of HCPC’s performance: the direct one (Q12), and a further question’Would you recommend HCPC as a regulator…?’. This latter question doesn’t perhaps add anything more, and in fact a few respondents suggested it was a slightly awkward question, given professions do not have a choice of regulator – so that has been excluded from the analysis.
Study conduct was performed in accordance with relevant guidelines and regulations [ 8 , 9 ]. Before conducting the survey of Clinical Scientists, the survey was sent to their professional body, the Institute of Physics and Engineering in Medicine (IPEM). The IPEM Professional Standards Committee reviewed the survey questions [ 10 ]. Written informed consent was obtained from participants.
Data analysis
Data was collected via an MS form, in a single excel sheet and stored on a secure network drive. The respondents were anonymised, and the data checked for errors. The data was then imported into NVivo v12.
Qualitative data was manually coded for themes, and auto-coded for sentiment. An inductive approach was used to develop themes.
The sample size of responses allowed the use of simple parametric tests to establish the level of statistical significance.
Survey demographics
A total of 146 responses were collected. Two respondents noted that they worked as an HCPC Partner (a paid role). They were excluded from the analysis due to potential conflict of interest. One respondent’s responses were all blank aside from the demographic data, so they were also excluded from further analysis.
Analysis is based on 143 responses, which represents ~ 6% of the UK profession [ 11 ]. It is arguable whether it is representative of the profession at this proportion of response – but these responses do offer the only sizeable pool of data currently available. The survey was aimed at those who are on the statutory register as they are most likely to have relevant interactions & experiences of the HCPC, but a small number of responses were also received from Clinical Technologists (Medical Technical Officers-MTOs) and Engineers (CEs) and these have been included in the analysis. Figure 2 shows the breakdown in respondents, by nation.
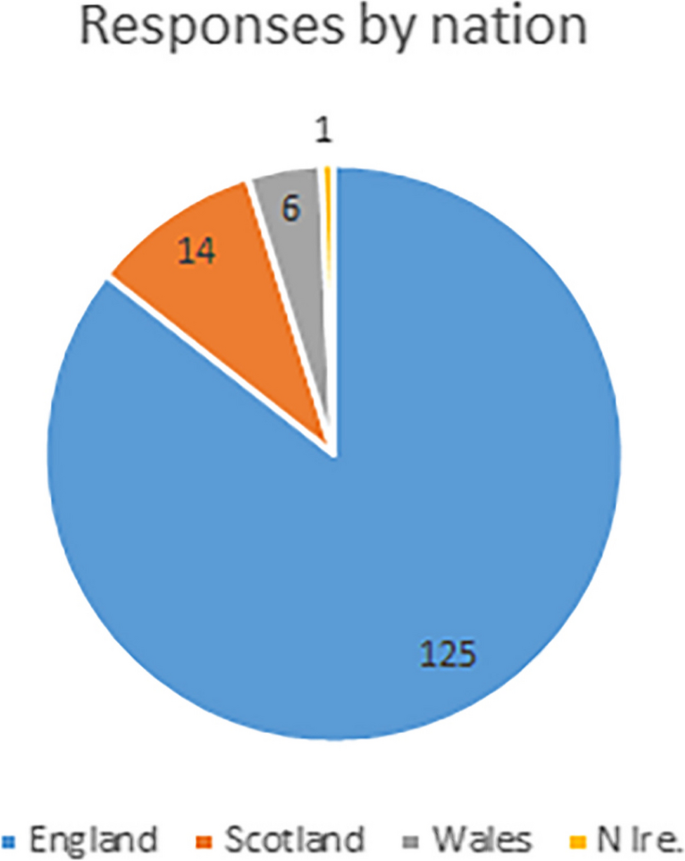
Proportion of respondents, by nation
Of the respondents, 91% are registered Clinical Scientists, and would therefore have a broad range of experience with HCPC and its processes. Mean time on the register was 12 yrs. Respondents show a large range in seniority, and their roles are shown in Fig. 3 (CS-Clinical Scientist; CE-Clinical Engineer; MTO-Medical Technical Officer/Technician; CS-P are those working in private healthcare settings, so not on Agenda for Change (AfC) pay bands).
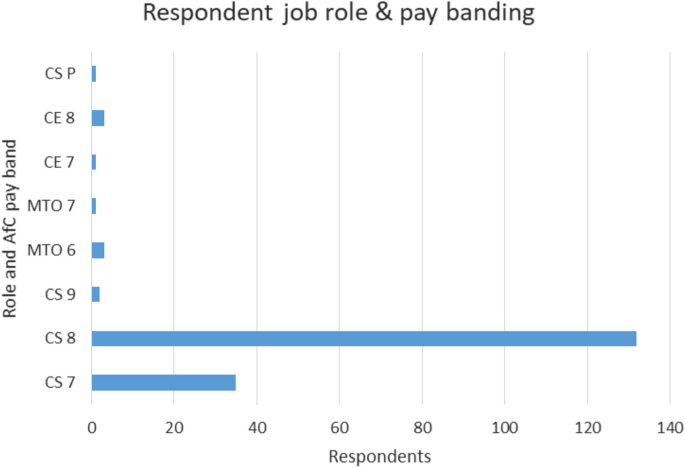
Breakdown in respondents, by role and pay banding
These data can be compared with the most recent HCPC ‘snapshot’ of the CS registrants (find here: Registrants by profession snapshot—1967 to 2019 | ( https://www.hcpc-uk.org/resources/data/2019/registrant-snapshot/ )).
The perception of overall regulator performance, can be assessed in two ways – one interview question directly asked for a rating score, and the overall survey sentiment also offers additional insight.
The score for overall performance was a median of 3 (mean 2.7; response rate 90%) which suggests neutral satisfaction.
Respondents were not asked directly to explain this overall performance rating – themes were extracted from the questionnaire as a whole.
The auto-coded sentiment scores generated in the NVivo software are shown in Table 2 . There is a significantly stronger negative sentiment than positive for HCPC performance – moderate, strong and total sentiment scores are all higher for negative sentiment. The normal test for a single proportion (109), shows the negative and positive sentiment differences have statistical significance with p < 0.001. Whilst the PSA assessment of HCPC performance in 2022–23 shows 100% performance for 4 out of 5 assessment areas, survey data here from regulated professionals suggests considerably less satisfaction with HCPC. This raises associated questions about the relevance and validity of PSA assessment.
A large number of respondents seem to question the value of regulation. Whilst many accepted the value for it in terms of protecting the safety of the public, many questioned its relevance & benefit to themselves. Many respondents also queried the payment model where although the main beneficiaries of regulation are the public & the employer, it is the registrants actually pay the fees for registration. There was very little mention in survey responses, of benefit in terms of protected-title. These issues were amalgamated into Theme 1— Value of regulation , with the two sub-themes Value in monetary terms (value-for-money) and Value in professional terms (benefit and relevance to the individual professional) (see Table 3 ).
In the survey, several aspects of HCPC organisational performance were scored – handling of personal data, registration and renewal, engagement with the profession, audit, and the quality and usefulness of HCPC policies. These formed Theme 2 and its sub-themes.
A third theme Registrant competence and vulnerability , was developed to focus on responses to questions related to the assessment of registrant competence and Fitness To Practice (FTP) processes.
Finally, the survey also directly asked respondents if they could suggest improvements which would have resulted in higher scoring for regulation quality and performance. These were grouped into Theme 4.
Theme 1 – Value of regulation
Value in monetary terms.
The Likert score for value-for-money was a median of 2 (mean 2.3; response rate 100%) which suggests dissatisfaction. This is one of the few survey questions to elicit a 100% response rate – a clear signal of its importance for registrants.
There was a high number of responses suggesting fees are too expensive (and a significantly smaller number suggesting good value). This ties in with some respondents explaining that the ‘benefit’ from registration is mainly for the employer (an assurance of high quality, well-trained staff). Several respondents point to little ‘tangible’ benefit for registrants and query whether the payment model is fair and if the employer should pay registrant fees.
“Expensive fees for what appears to be very little support.” Resp094
“It seems that I pay about £100 per year to have my name written on a list. It is unclear to me what the HCPC actually does in order to justify such a high fee.” Resp014
“I get, quite literally, nothing from it. It’s essentially a tax on work.” Resp008
Several respondents suggested that as registration was mandated by the employer, it was in essence an additional ‘tax’ on their employment, which was highlighted previously by Unison [ 12 ]. A comparator for payment model, are the checks preformed on potential staff who will be working with children and vulnerable adults. In general, these ‘disclosure’ checks are paid for by the employer, however the checks are not recurrent cost for each individual, but done once at recruitment.
Value in professional terms & relevance
This was not a direct question on the questionnaire, but emerged consistently in survey responses. Aside from value-for-money, the value of regulation can also refer to more general benefit and relevance for a professional, for example in protecting a professional title or emphasising the importance of a role. Many respondents commented, in relation to the ‘value’ of regulation, about the relevance of the HCPC to them and their job/role.
The largest number of responses highlighted the lack of clarity about HCPC’s role, and also to note its lack of relevance felt by a significant proportion of respondents.
“Not sure I have seen any value in my registration except that it is a requirement for my role” Resp017
“I really fail to understand what (sic) the benefits of registration.” Resp018
“They do not promote the profession. I see no evidence of supporting the profession. I pay to have the title and I am not aware of any other benefits.” Resp038
Theme 2 – HCPC performance
Communication & handling data.
The survey questionnaire did not have a specific question relating to communication, therefore no specific Likert scores are available. Rather, communication was a sub-theme which emerged in survey responses. The response numbers related to positive (1) and negative experiences (50) clearly suggest an overall experience of poor communication processes (and statistically significant at p < 0.001 for a normal proportion test).
One respondent noted they had ‘given up’ trying to communicate with HCPC electronically. Several respondents also noted issues with conventional communication—letters from HCPC going to old addresses, or being very slow to arrive.
“…I have given up on contacting by electronic means.” Resp134
When trying to renew their registration, communication with HCPC was so difficult that two respondents noted they raised a formal complaint.
A number of respondents noted that when they eventually got through to the HCPC, staff were helpful, so the main communication issue may relate to insufficiently resourced lines of communication (phones & email) or the need for a more focussed first point of contact e.g. some form of helpdesk or triaging system.
“Recently long wait to get through to speak to someone… Once through staff very helpful.” Resp126
This topic overlaps with the next (Processing Registration & renewals) in that both involve online logins, website use etc.
Security & data handling was rated as neutral (median 3, mean 3.4; response rate 91%). Although responses were balanced in terms of satisfaction, a significant number noted a lack of knowledge about HCPC processes. There are almost equal proportions of respondents reporting no issues, some problems with handling of personal data, or insufficient knowledge to express an opinion.
Registration and renewal
The score for processing registrations & renewals, was a median of 4 (mean 3.5; response rate 92%) which suggests modest satisfaction.
The overall rating also suggests that the issues may have been experienced by a comparative minority of registrants and that for most, renewal was straightforward.
“They expected people to call their phone number, which then wasn’t picked up. They didn’t reply to emails except after repeated attempts and finally having to resort to raising a complaint.” Resp023
“Difficult to get a timely response. Difficult to discuss my situation with a human being…” Resp044
Although the Likert score is positive, the themes in responses explaining the rating, are more mixed. Many respondents mentioned either having or knowing others who had issues with registration renewal, and its online processes including payments. A few respondents mentioned that the process was unforgiving of small errors. One respondent, for example, missed ticking a box on the renewal form, was removed from the register and experienced significant difficulties (poor communication with HCPC) getting the issue resolved.
Some respondents noted issues related to a long absence from work (e.g. maternity/illness etc.) causing them to miss registration deadlines – for some, this seems to have resulted in additional fees to renew registration. It seems rather easy for small errors (on either side) to result in registrants being removed from the register. For registrants, this can have very serious consequences and it can then be difficult and slow to resolve this, sometimes whilst on no pay. There have also been other reported instances of renewal payment collection errors [ 13 ].
“I had been off work… and had missed their renewal emails…I was told that there would be no allowances for this situation, and I would have to pay an additional fee to re-register…” Resp139.
Some respondents raised the issue of exclusion – certain staff groups not being included on the register—such as Clinical Technologists and Clinical Engineers. This desire for inclusion, also points to a perception of value in being on the register. One respondent raised an issue of very difficult and slow processing of registration for a candidate from outside the UK.
“Staff member who qualified as medical physicist abroad…has had a dreadful, drawn out and fruitless experience.” Resp135
Overall, many respondents noted difficulties in renewing registration and issues with HCPC’s online processes. Some of these issues (e.g. website renewal problems) may have been temporary and are now resolved, but others (e.g. available routes for registration) remain to be resolved.
Audit process & policies
In the survey, 12% respondents reported having been audited by HCPC regarding their CPD (response rate 97%). This is well above the level of 2.5% of each profession, which HCPC aims to review at each renewal [ 14 ], and similar values reported by some professional bodies [ 15 ]. The participants seem representative, although two respondents mentioned their perception of low audit rates. Data on CPD audit is available here: https://www.hcpc-uk.org/about-us/insights-and-data/cpd/cpd-audit-reports/
Respondents rated the process of being audited as a median of 4 (mean 3.7), which is the joint highest score on the survey, pointing to satisfaction with the process. From the responses, the overall perception could be summed up as straight-forward, but time-consuming. Without regular record-keeping, unfortunately most audits will be time-consuming – the HCPC more so, as it is not an annual audit, but covers the two preceding years.
Some respondents did find the process not only straight-forward, but also useful (related to feedback received). However, responses regarding feedback were mixed, with comments on both good, and poor feedback from HCPC.
“Not difficult but quite long-winded” Resp008
“Very stressful and time consuming” Resp081
“While it was a lot of work the process seemed very thorough and well explained.” Resp114
The HCPC’s policies & procedures were rated as a median of 3 (mean 3.2; response rate 98%). This neutral score could suggest a mixture of confidence in HCPC practise. This score may also reflect the fact that the majority of respondents had either not read, or felt they had no need to read the policies, and so are largely unfamiliar with them.
The reasons for this lack of familiarity are also explained by some respondents – four commented that the policies & procedures are rather too generic/vague. Three respondents noted that they felt the policies were not sufficiently relevant to their clinical roles to be useful. This may be due to the policies being written at a level to be applicable to registrants from all 16 modalities – and perhaps a limitation of the nature of HCPC as a very large regulator. Familiarity seemed mainly to be restricted to policies around registration, and CPD. There were slightly lower response levels for positive sentiment (6), than negative sentiment (9).
“I’ve never had cause to read them.” Resp115
“Detached from the real clinical interface for our professions…” Resp083
HCPC split their policies into ‘corporate’- which relate to organisational issues (e.g. equality & diversity; find them here: Our policies and procedures | ( https://www.hcpc-uk.org/about-us/corporate-governance/freedom-of-information/policies/#:~:text=Our%20main%20policies%20and%20procedures%201%20Customer%20feedback,scheme%20...%207%20Freedom%20of%20Information%20Policy%20 )) and those more relevant to professions (e.g. relating to the register; find them here: Resources | ( https://www.hcpc-uk.org/resources/?Query=&Categories=76 )).
One respondent noted not only that the policies were ‘as you might expect’, but felt the policies were less demanding than those from other similar bodies such as the CQC ( https://www.cqc.org.uk/publications ).
“…Other regulatory bodies (such as the CQC for example) have policies and procedures that are a lot more challenging to comply with.” Resp022
Theme 3 – Registrant competence and vulnerability
In this survey, 3.5% (5/143) of respondents noted some involvement with the HCPC’s Fitness to Practice service. These interactions were rated at a median of 3 (mean 2.8) suggesting neutral sentiment.
Firstly, we can immediately see the level of interaction with the FTP team is very small. CS registrants represent approx. 2% of HCPC registrants, and the level of CS referrals to FTP in 2020–21 was 0.2% [ 16 ].
The data is a very small sample, but responses vary strongly, so it is worth digging a little further into the granularity of individual responses. Response scores were 1, 1, 2, 5, 5 – which are mainly at the extremes of the rating spectrum. The majority of respondents described poor experiences with the FTP team: errors, a process which was ‘extremely prolonged’, involved slow/poor communication, and processes which were ‘entirely opaque’.
“It is slow, the process was badly managed… and the system was entirely opaque,” Resp37
“They were hard to contact and I didn't feel they listened…no explanation, apology or assurance it would not happen again. It left my colleague disillusioned and me very angry on their behalf…” Resp044
Some respondents commented that the team were not only difficult to contact, but also didn’t seem to listen. At the end of a process which involved errors from HCPC, one respondent noted were ‘no explanation, apologies or assurance that it would not happen again’, leaving the registrant ‘disillusioned’. These experiences do not fit with the HCPC’s stated goal to be a compassionate regulator, see Fig. 4 . Arguably it is more difficult to change a culture of behaviour and beliefs, than to publish a corporate goal or statement of vision.
HCPC’s vision statement & purpose [ 17 ]
Some survey respondents have noted the necessity of regulation for our profession.
“Ultimately I am very grateful that I can register as a professional.” Resp024
Theme 4 – Suggestions for improved regulation
Following the question relating to overall performance, respondents were invited to suggest things which might improve their rating for HCPC’s performance and value. These suggestions were also combined with those which appeared in earlier survey responses.
Although we are in a current cost-of-living crisis, responses did not query simply high absolute cost of fees, but also queried the value/benefit of HCPC regulation for registrants. Many responses expressed doubt as to the added value & relevance of HCPC registration for them. They seem to point to a desire for more tangible benefit from their fees. Perhaps, given the costs and levels of scrutiny, registrants want some definite benefit to balance the scales .
“Cost less and do more for the people who are on the register.” Resp089
“Vastly reduced cost. Employer paying registrant fees.” Resp074
A significant number of responses pointed out that the main benefits of registration are for the public, and for employers – but that it is the registrants who pay for registration. Many queries why this should be, and whether there should be a different payment model, where for example employers pay.
Similarly, some respondents felt that the HCPC’s unusual position of regulating a large swathe of healthcare professions was not necessarily helpful for their profession or others.
Communication and response times are obviously an issue of concern for registrants, and improvements are needed based on the low satisfaction levels reported here. This is also linked to a wish for increased engagement with the CS profession.
“Engagement with the workforce, specialism specific development, reduced fees” Resp025
Some responses suggested they would be comforted by increased accountability / governance of HCPC including improved FTP efficiency.
“More accountability to registrants” Resp130
Finally, improvement in terms of additional registration routes for Engineers & Technical staff were also suggested. It may be damaging to work-place moral, if two professionals doing roles of a similar nature are not being governanced is the same way and if there is not parity of their gross salary due to mandatory professional fees & reductions.
Value-for-money : This will vary between individuals depending on many variables, such as upbringing & environment, salary, lifestyle priorities, political persuasion, and so on. However, many of these factors should balance in a large sample. In general, it can be suggestive of satisfaction (or lack of) with a service. The score here suggesting dissatisfaction, echoes with other reports on HCPC’s spending, and financial irregularities [ 18 , 19 ].
In the survey findings, respondents have voiced dissatisfaction with registration value for money. In fact, HCPC’s registration fees are not high when compared to the other healthcare professions regulators. Table 1 shows data from 2021–22 for regulator annual registration fees. However, the HCPC has risen from having the lowest regulator fees in 2014–5, to its current position (9 th of 13) slightly higher in the table. Perhaps more concerning than the absolute level of fees, are when large increases are proposed [ 12 , 20 , 21 , 22 ].
However, fees have regularly increased to current figure of £196.48 for a two-year cycle. During a consultation process in 2018, the Academy for Healthcare Clinical Scientists (AHCS) wrote an open letter to the HCPC, disputing what they felt was a disproportionate fee increase [ 23 ]. Further fee rises have also been well above the level of inflation at the time.
HCPC expenditure (which is linked to registration fees) has arguably been even more controversial than fee increases – noted by several respondents. A freedom of information (FOI) request in 2016 showed HCPC’s spending of £17,000 for their Christmas party [ 18 ] – which amounts to just over £76 per person. This cost was close to the annual registration fee (at that time) for registrants.
In 2019, regulation of social workers in England moved from HCPC, to Social Work England. This resulted in a loss of over 100,000 registrants, and a loss in registration fee income. HCPC raised fees to compensate, but a freedom of information (FoI) request in 2020 [ 18 ] showed that even though there was an associated lowering in workload associated with the loss of 100 k registrants, the HCPC had no redundancies, suggesting the loss of income was compensated mainly by the fees increase.
Inherent value & relevance
One of HCPC’s aims is to promote ‘the value of regulation’ [ 24 ]. However, not only is there dissatisfaction with value-for-money, the second highest response suggests a lack of inherent value (or benefit) from regulation to the individual registrant. In some ways, there is a lack of balance – registrants are under increasing scrutiny, but feel there is little direct benefit, to provide balance.
This also suggests that HCPC’s aim or message is not getting through to the CS profession. It’s not clear what the HCPC 2021–22 achieved milestone – ‘Embedded our registrant experiences research into employee learning and development and inductions’ has actually achieved.
A large number of responses pointed to the lack of clarity about HCPC’s role, and also to note its lack of relevance for respondents. Some of this is understandable – until recently, many CS registrants will have little interaction with HCPC. They would typically get one email reminder each year to renew their registration and pay those fees, and hear little else from the HCPC. That is beginning to change, and HCPC have recently begun to send more regular, direct emails/updates to registrants.
However, for many registrants, the HCPC appears not to be clearly communicating its role, or the relevance/importance of regulation. As mentioned above, this also links in to previous mentions of the lack of any tangible benefit for registrants. Some note little more relevance other than the mandatory aspects of regulation.
Finally, relevance is also queried in relation to the limited access for some professional groups to a professional register. The current situation of gaps in registration for some groups, results in two situations – firstly, for Clinical Scientists and Clinical Engineers/Technologists, one group has to compulsorily pay a fee to be allowed/approved to do their job and the other does not; also, the public are routinely helped and assisted by Clinical Scientists and Clinical Engineers/Technologists – but only one group is regulated to ensure public safety.
HCPC Communication
This was highlighted by respondents as often poor. Recently in the media, there has been a concern raised by The College of Paramedics (CoP) about communications issues with HCPC—changes to the HCPC policy on the use of social media [ 25 ]. They raised particular concerns about the use of social media content and ‘historical content’ in the context of investigations of fitness-to practice.
There have previously been some concerns raised on the UKMPE mail-base regarding handling of personal data, and lack of efficiency in addressing the issue [ 26 ]. Several messages detailed HCPC communicating unencrypted registrant passwords in emails and sending personal data to the incorrect registrant. Some on the forum noted that they had reported this problem over a period of several years to HCPC, suggesting HCPC’s response to these serious issues was extremely slow. Several responses noted these previous issues.
Registration processes
Although responses here show some satisfaction, there have been reports in the media of significant issues with registration (such as removing registrants from the register in error) with associated impact for patients and the public [ 27 , 28 ]. Similarly, there were reports on the UKMPE mail-base of significant issues with registration renewals being problematic [ 26 ]. In Scotland, NHS.net email accounts ceased to be supported in July-Sept 2020 and the associated lack of access to email accounts and messages used for HCPC communication and registration, caused a major issue in registration renewal. This coincided with COVID lockdowns and a period of unusually difficult communication with HCPC. If NHS staff lose registration (irrespective of the reason), respondents noted that some Human Resources (HR) departments were quick to suspend staff from work, and in some cases withhold pay. That spike in difficulties is likely the cause of the most common responses suggesting issues with a complicated process.
In safe-guarding public safety, a key task for a healthcare regulator is assessing the competence of registrants. This is done via a small set of related activities. Registrants must return regular evidence of CPD, and these are audited for 2.5% registrants. This process is simple and routine, and as seen in Theme 2 responses here suggest registrants are reasonably satisfied with this process.
More formal and in-depth competence assessment happens when a complaint is raised against a registrant, either by a work colleague/management, a member of the public or occasionally by the HCPC itself. The process is complex, lengthy and can end in a registrant attending a court hearing [ 29 ].
It is usual for registrants to continue in their normal job during FTP investigations – effectively the public remains at risk from a registrant if their competence is eventually proven to be below the regulators standards, so there is a need for investigations to be efficient both in timeliness, and outcome.
Obviously, being under investigation can be highly stressful, and has the potential for the registrant to be ‘struck off’ the register, and lose their job if registration is mandated (e.g. NHS posts). There are many reports of the process & experience either provoking or increasing underlying mental health challenges [ 30 , 31 , 32 ]. Along with efficiency, a regulator needs to behave compassionately. Investigations of highly-skilled professionals engaging in complex work activities, is also necessarily complex and requires a high degree of knowledge and experience from the regulator’s investigational panel.
The Professional Standards Authority (PSA) regulate the HCPC, and publish annual reviews of their performance ( https://www.professionalstandards.org.uk/publications/performance-reviews ) (see Table 4 ). HCPC performance as reported by PSA, seems to be generally higher than noted by survey respondents here. For 2022–23, aside from one area, the HCPC has scored 100% for performance, which seems at odds with these survey responses [ 33 ]. The FTP team is notable in repeatedly performing very poorly compared to most other sections of the HCPC (even though the majority of the HCPC budget goes to FTP activity, see Fig. 4 ). The HCPC Annual Report 2018–9 [ 34 ] highlighted the completion of the first phase of the Fitness-To-Practice Improvement Plan. This delivered “A root and branch review of this regulatory function… a restructure, tightened roles and processes and the introduction of a new Threshold Policy”, but this seems to have no impact on the performance reported by the PSA for the next few years shown in Table 4 . However, the most recent data does suggest improvement, and HCPC continues to develop FTP team practice [ 17 ].
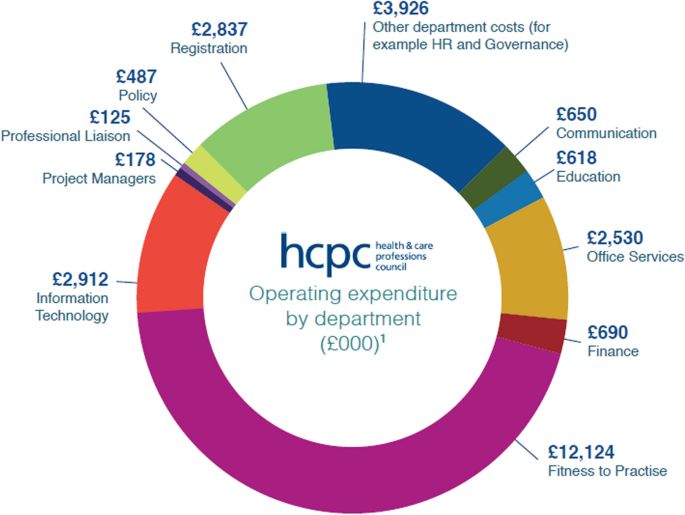
HCPC expenditure for the year 2020–21 [ 17 ]
There are other reports of poor experiences with this team [ 35 , 36 ], and in one report the FTP team’s processes have been noted as being rather inhumane [ 35 ].
Regulation is an important part of public protection, but how effectively it is managed & enforced is also a concern, given it involves increased scrutiny of registrants. A topical comparator is the current dissatisfaction by a large section of the public about several other government regulators allowing seemingly poor performance to go unchecked [ 4 , 5 ].
It is arguable, that registrants remain on the register as long as the HCPC allows them. Several respondents in this survey noted being removed from the register through HCPC administrative error. Removal could also happen through poor judgement/decision-making – the FTP team handle large numbers of very complex investigational cases – 1603 concluded cases for the year 2021–22 and 1024 hearings [ 16 ]. Every justice system is subject to a level of error – guilty parties can be erroneously ‘cleared’, and vice-versa. It is essential therefore, that policies & procedures relating to FTP are fit for purpose—that the FTP team work effectively and humanely, and that there is genuine & effective governance of HCPC to ensure accountability. In this survey, some respondents seem to be saying that currently this seems not to be the case.
It might have been anticipated that the greatest concern is costs, especially in the current cost-of-living crisis. The recent HCPC consultation to increase fees [ 37 ] seems particularly tone-deaf and has caused concern across the professions [ 21 , 22 ].
Above findings show respondents are interested in lower fees, but also increased benefit for their fees. Some respondents pointed out that whilst registrants pay for registration, benefit is mainly for the public and employers. The HCPC is a statutory body, its funding model will have been designed/decided upon by government, and may be unlikely to change. However, there are a variety of potential regulation models [ 38 ], and so change is possible. A review of the financial model for regulation may be welcome.
Regulator size
Some aspects of HCPC performance, policies, and distribution of spending, is related to the nature of it being the largest and only multi-professional regulator in the healthcare sector. Data from the HCPC suggests (see Fig. 5 ) that the majority of spending relates to FTP activity. Data also points to Clinical Scientists having very low levels of FTP investigation compared to others in HCPC [ 16 ]. This suggests that a significant proportion of CS registrant fees are used to investigate other professions. It’s possible (perhaps simplistically) that if, like many other healthcare professions such as doctors & dentists who’s regulator is concerned only with that single profession, if CSs were regulated separately, their registrant fees may be reduced. This model of single-profession regulation may also mitigate against other disadvantages of the HCPC’s practice, such as the ‘generic’ policies aiming to apply to a pool of 15 professions.
Although there is a very low level of data for this topic, the concerned raised by registrants are serious in nature. There also seems to be issues in handling of complaints related to this service and advocacy for registrants. Certainly, there is a clear governance path via PSA, to the Health Secretary. However, this does not offer a route for individual complaints to be raised and addressed. Unlike complaints from the public in other areas, there is no recourse to an ombudsman for registrants. The only option for individual registrants, is the submission of a formal complaint to the HCPC itself, which is dealt with internally. Comments from survey respondents suggest this process does not guarantee satisfaction. Indeed, one of the respondents who mentioned submitting a complaint, made it clear they remained unhappy with HCPC’s response. Overall, there seems to be a lack of clear & effective advocacy for registrants.
“…the HCPC’s stance appeared to be guilty until proven innocent… At no point did I feel the HCPC cared that their (sic) was an individual involved....” Resp044.
FTP processes affect a comparatively small number of CS registrants, compared to other professions. However, it seems clear that the majority of those who have interacted with the FTP team have had poor experiences, and respondents have suggested improvements are needed. The reason for FTP investigations, is protection of staff and the public. If processes are slow, and investigations prolonged, or decisions flawed, the public may be exposed to increased levels of risk, as healthcare practitioners who may be lacking in competence continue to practice. The data in Table 4 shows concerning but improving trends in FTP performance levels.
Limitations
There are two main limitations to this work. Firstly, due to time constraints, there was no pilot work done when designing the survey questionnaire. This may have helped, as noted earlier, a few responses pointed to some awkwardness with one survey question. Although no pilot work was done, the questionnaire was reviewed by the IPEM Professional Standards Committee, as noted in the Acknowledgements section.
The other obvious limitation is the low response rate (~ 6% of UK Medical Physicists). Circulation of the survey was performed via the only online forum for the profession currently available. The survey was advertised multiple times to ensure visibility to staff who may have missed it initially due to leave etc. However, the forum does reach 100% of the profession, and some addressees may have filters set to send specific posts to junk folders etc. The professional body IPEM declined to offer support in circulating the survey (believing the issues involved would affect/be of interest only to a small minority of members.)
The low response rate also has a particular impact on the pool of responses relating to FTP issues, which inherently affect low numbers of registrants.
However, the importance of some of the findings here (e.g. expressed dissatisfaction with regulation in terms of value; the poor experience of some members with the Registration, Communication and FTP teams) and the low sample surveyed, both justify the need for a larger follow-on survey, across all of Clinical Science.
In Healthcare, regulation of professions is a key aspect of protecting the public. However, to be effective, regulation must be performed professionally, impartially, and associated concerns or complaints investigated efficiently and respectfully.
This report presents findings from a survey aimed at collecting a snap-shot of the experiences of Clinical Scientists with their regulator, and their perception of the quality and safety of that regulation performance.
Overall survey sentiment scores showed a significantly more negative responses than positive. Survey comments relate not only to current issues, but to previous problems and controversial issues [ 18 , 26 ]. It seems that some respondents have at some point lost confidence and trust in the HCPC, and survey responses suggest there has not been enough engagement and work done by HCPC to repair and rebuild this trust.
In the midst of a cost of living crisis, costs are a large concern for many. The HCPC fees are neither the highest not lowest amongst the healthcare regulators. Spending is transparent, and details can be found in any of the HCPC’s annual reports.
A repeating sub-theme in responses, was a lack of tangible value for the registrant, and that the employer should pay the costs of registration, where registration is mandated by the job.
Many respondents have suggested that they feel there should be more proactive engagement from HCPC with the profession. Most respondents were not familiar with or felt the HCPC policies are relevant/important to them.
Survey data showed moderate satisfaction with registration processes for the majority of respondents. Some respondents also noted a lack of registration route for engineering & technical healthcare staff. CPD processes also achieved a score indicating registrant satisfaction. This generated the highest ratings in the survey. Communication scored poorly and many respondents suggests there needs to be improved levels of communication in terms of response times and access to support.
The CS profession experiences low levels of interaction with the FTP service. However, those interactions which were recorded in the survey, show some poor experiences for registrants. There also seems to be a lack of advocacy/route for complaints about HCPC from individual registrants. There may need to be more engagement between registrants and their professional body regarding HCPC performance, and more proactivity from the stake-holder, IPEM.
Some of the findings reported here relate to important issues, but the survey data are based on a low response rate. A larger survey across all of Clinical Science is being planned.
Availability of data and materials
To protect confidentiality of survey respondents, the source data is not available publicly, but are available from the author on reasonable request.
Abbreviations
Agenda for Change
Academy for Healthcare Clinical Scientists
Continuous professional development
Clinical Engineer
Clinical Scientist
College of Paramedics
Clinical Technologist
Freedom of Information
Fitness-to-practice
Health and Care Professions Council
Human resources
Institute of Physics and Engineering in Medicine
Joint Information Systems Committee
Medical Technical Officer
Professional Standards Authority
Professional Standards Committee
Qualitative data analysis
UK Medical Physics and Engineering
Professional Standards Authority. Professional healthcare regulation in the UK. https://www.professionalstandards.org.uk/news-and-blog/blog/detail/blog/2018/04/10/professional-healthcare-regulation-explained#:~:text=Regulation%20is%20simply%20a%20way,may%20face%20when%20receiving%20treatment . Accessed 26 Jul 2023
Evening Standard. Bogus surgeon treated hundreds. https://www.standard.co.uk/hp/front/bogus-surgeon-treated-hundreds-6326549.html . Accessed 26 Jul 2023.
HCPC . About registration: protected titles. http://www.hcpc-uk.org/aboutregistration/protectedtitles/ . Accessed 27 Jul 23.
The Guardian. Public patience is wearing thin. Ofwat must wield the big stick | Nils Pratley | https://www.theguardian.com/business/nils-pratley-on-finance/2022/dec/08/public-patience-is-wearing-thin-ofwat-must-wield-the-big-stick . Accessed 19 Jul 2023.
TrustPilot. Reviews of Ofgem. Ofgem Reviews | Read Customer Service Reviews of ofgem.com (trustpilot.com). Accessed 19 Jul 2023.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Article Google Scholar
Kiger ME, Varpio L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach. 2020;42(8):846–54.
Article PubMed Google Scholar
Declaration of Helsinki. 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 12 Sept 2023.
UK Data Protection Act. 2018. https://www.gov.uk/data-protection . Accessed 15 Sept 2023.
Rowbottom C. Private communication on behalf of the IPEM Professional Standards Committee; 2022.
IPEM Workforce Team. Clinical scientist & engineer workforce data. Personal communication. 2022.
Unison. HCPC fee increase is an unjustified ‘tax on practising.’ https://www.unison.org.uk/news/press-release/2019/02/hcpc-fee-increase-unjustified-tax-practising/ . Accessed 27 Jul 2023.
HCPC. Direct debit collection errors. https://www.hcpc-uk.org/news-and-events/news/2020/early-direct-debit-collections/?dm_i=2NJF,141CO,7C0ZNI,4A8IE,1 . Accessed 27 Jul 23.
HCPC. CPD audit rates. https://www.hcpc-uk.org/cpd/cpd-audits/ . Accessed 21 Jul 2023.
IPEM. CPD audit rates. https://www.ipem.ac.uk/your-career/cpd-career-development/cpd-audit/ . Accessed 21 Jul 2023.
HCPC. Fitness to practice annual report 2020–21. https://www.hcpc-uk.org/about-us/insights-and-data/ftp/fitness-to-practise-annual-report-2020-21/ . Accessed 23 Jul 2023.
HCPC. Annual report and accounts, 2020–21. https://www.hcpc-uk.org/resources/reports/2022/annual-report-and-accounts-2020-21/ . Accessed 19 Jul 2023.
Wikipedia. The health and care professions council. https://en.wikipedia.org/wiki/Health_and_Care_Professions_Council . Accessed 2 Jul 23.
HCPC. Annual report 2005–06. https://www.hcpc-uk.org/resources/reports/2006/annual-report-2005-06/ . Accessed 19 Jul 2023.
British Dental Association. BDA very disappointed by HCPC decision to raise registration fees by 18%. https://www.bda.uk.com/resource/bda-very-disappointed-by-hcpc-decision-to-raise-registration-fees-by-18.html . Accessed 27 Jul 2023.
British Psychological Society. HCPC fees consultation – share your views. https://www.bps.org.uk/news/hcpc-fee-consultation-share-your-views . Accessed 27 Jul 23.
IBMS. IBMS response to the HCPC registration fees consultation. https://www.ibms.org/resources/news/ibms-response-to-hcpc-registration-fees-consultation/ . Accessed 17 Jul 23.
Association of HealthCare Scientists. Open letter to HCPC. https://www.ahcs.ac.uk/wp-content/uploads/2018/11/HCPC-Open-Letter.pdf . Accessed 27 Jul 23.
HCPC. Corporate plan 2022–23. https://www.hcpc-uk.org/resources/reports/2022/hcpc-corporate-plan-2022-23/ . Accessed 23 Jul 2023.
College of Paramedics. Our formal response to the HCPC consultation. https://collegeofparamedics.co.uk/COP/News/2023/Our%20formal%20response%20to%20the%20HCPC%20consultation.aspx . Accessed 27 Jul 23.
JISC Mail - MPE mailbase. JISCMail - Medical-physics-engineering list at www.jiscmail.ac.uk . Accessed 19 July 2023.
The Guardian. Thousands miss out on treatment as physiotherapists are taken off UK register. https://www.theguardian.com/society/2022/may/14/thousands-miss-out-on-treatment-as-physiotherapists-are-struck-off-uk-register . Accessed 27 Jul 2023.
HSJJobs.com. https://www.hsjjobs.com/article/thousands-of-clinicians-unable-to-work-after-registration-blunder . Accessed 27 Jul 2023.
HCPC. How we investigate. https://www.hcpc-uk.org/concerns/how-we-investigate/ . Accessed 21 Nov 2023.
Sirriyeh R, Lawton R, Gardner P, Armitage G. Coping with medical error: a systematic review of papers to assess the effects of involvement in medical errors on healthcare professionals’ psychological well-being. Br Med J Qual Saf. 2010;19:6.
Google Scholar
Bourne T, Wynants L, Peters M, van Audenhove C, Timmerman D, van Calster B, et al. The impact of complaints procedures on the welfare, health and clinical practise of 7926 doctors in the UK: a cross-sectional survey. BMJ Open. 2015;5:e006687.
Article PubMed PubMed Central Google Scholar
Jones-Berry S. Suicide risk for nurses during fitness to practice process. Ment Health Pract. 2016;19:8.
Professional Standards Authority. HCPC performance review 2022–23. https://www.professionalstandards.org.uk/publications/performance-review-detail/periodic-review-hcpc-2022-23 . Accessed 25 Jul 2023
HCPC. Annual report and accounts, 2018–19. https://www.hcpc-uk.org/resources/reports/2019/hcpc-annual-report-and-accounts-2018-19/ . Accessed 19 Jul 2023.
Maben J, Hoinville L, Querstret D, Taylor C, Zasada M, Abrams R. Living life in limbo: experiences of healthcare professionals during the HCPC fitness to practice investigation process in the UK. BMC Health Serv Res. 2021;21:839–54.
Leigh J, Worsley A, Richard C, McLaughlin K. An analysis of HCPC fitness to practise hearings: fit to practise or fit for purpose? Ethics Soc Welfare. 2017;11(4):382–96.
HCPC. Consultation changes to fees. https://www.hcpc-uk.org/news-and-events/consultations/2022/consultation-on-changes-to-fees/ . Accessed 27 Jul 23
Department of Health. Review of the regulation of public health professions. London: DoH; 2010.
Download references
Acknowledgements
The author wishes to kindly acknowledge the input of Dr Carl Rowbottom (IPEM Professional Standards Committee), in reviewing the survey questions. Thanks also to Dr Nina Cockton for helpful advice on ethics and recruitment issues.
There were no sources of funding required for this work.
Author information
Authors and affiliations.
University of Glasgow, Level 2, ICE Building, Queen Elizabeth University Hospital Campus, 1345 Govan Road, Glasgow, G51 4TF, UK
Mark McJury
You can also search for this author in PubMed Google Scholar
Contributions
All work to collect, analyse & publish this survey, are the work of the author Dr Mark McJury.
Corresponding author
Correspondence to Mark McJury .
Ethics declarations
Ethics approval and consent to participate.
As this study relates to low risk, survey data, formal ethics committee approval is not required (exemption obtained from NHSGGC REC04 REC Officer Dr Judith Godden [email protected]). As the survey responses were from members of a professional body (The Institute of Medical Physics and Engineering in Medicine (IPEM) it was consulted. Its Professional Standards Committee (PSC) reviewed the survey and raised no objections. The survey questions were assessed for bias and approved unchanged (acknowledged in the manuscript). Written informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
The survey questionnaire has been provided as a supplementary file.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
McJury, M. Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey. BMC Health Serv Res 24 , 635 (2024). https://doi.org/10.1186/s12913-024-10956-7
Download citation
Received : 06 September 2023
Accepted : 05 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12913-024-10956-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Regulation of professions
- Clinical scientists
- Medical physicists
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

IMAGES
COMMENTS
Funding boost for medical research units at University of Cambridge. MRC. 22 May 2024. Fostering translational clinical research via industry partnership. MRC. 18 April 2024. ... Your research can focus on any area of Medical Research Council (MRC)'s remit to improve human health. You must:
The Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) is a research institute in Cambridge, England, involved in the revolution in molecular biology which occurred in the 1950-60s. Since then it has remained a major medical research laboratory at the forefront of scientific discovery, dedicated to improving the understanding of key biological processes at atomic, molecular ...
The MRC Laboratory of Molecular Biology (LMB) is a research institute dedicated to the understanding of important biological processes at the levels of atoms, molecules, cells and organisms. In doing so, we provide knowledge needed to solve key problems in human health. Our scientists tackle fundamental, often difficult and long-term research ...
The Medical Research Committee and Advisory Council was set up in 1913. It was in effect a single research organisation for the whole of the UK, with funds provided under the National Insurance Act for medical research, and not limited to TB. The members organised visits to researchers in laboratories throughout the UK and in 1913 submitted the ...
The Medical Research Council (MRC) is responsible for co-coordinating and funding medical research in the United Kingdom.It is part of United Kingdom Research and Innovation (UKRI), which came into operation 1 April 2018, and brings together the UK's seven research councils, Innovate UK and Research England. UK Research and Innovation is answerable to, although politically independent from ...
Single-cell transcriptomic analysis of human pleura reveals stromal heterogeneity and informs in vitro models of mesothelioma. The pleural lining of the thorax regulates local immunity and wound healing. Disruption of these functions induces fibrosis and in the case of exposure to asbestos, chronic irritation can cause...
This research was funded by the Medical Research Council and the Wellcome LEAP R3 programme, and supported by the NIHR Cambridge BRC. Reference: Mulroney, T E et al: '(N)1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting.' Nature, Dec 23. DOI: 10.1038/s41586-023-06800-3
Professor Patrick Chinnery, Head of the Department of Clinical Neurosciences at the University of Cambridge, has been appointed as the new Executive Chair of the Medical Research Council (MRC). Opportunities to advance human health through research have never been greater, and the UK is in a very strong position globally.
CIMR research is mostly funded by competitively-awarded grants from a range of funding bodies, including: UK statutory funders: UKRI (Medical Research Council, Biological and Biotechnology and Biological Sciences Research Council), National Institute of Health Research (Cambridge Biomedical Research Centre)
The Medical Research Council (MRC) Toxicology Unit is a leading International Research Institute within the School of Biological Sciences, University of Cambridge. The Unit delivers mechanistic toxicology research, pursuing hypothesis-driven toxicological questions with a particular focus on the study of the causal links between exposure to ...
The Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) is one of the world's leading research institutes. ... Its aim is to train outstanding students in biomedical research, taking advantage of the excellent research environments in Cambridge and the US. Students work on collaborative projects organised by co-supervisors in ...
About us. Established as the Applied Psychology Unit by the Medical Research Council in 1944, the Cognition and Brain Sciences Unit is one of the largest and most enduring contributors to the understanding of human cognition and its disorders. Our research investigates fundamental human cognitive processes such as attention, language, memory ...
Welcome to the Cognition and Brain Sciences Unit. The MRC Cognition and Brain Sciences Unit is a research centre for advancing our knowledge of human cognition, with programmes that cover childhood development, mental health, ageing and dementias, neurological and sensory disorders, and fundamental cognitive neuroscience.
The MRC Epidemiology Unit is a department at the University of Cambridge. It is working to improve the health of people in the UK and around the world. Obesity, type 2 diabetes and related metabolic disorders present a major and growing global public health challenge. These disorders result from a complex interplay between genetic ...
The Cognition and Brain Sciences Unit is a branch of the UK Medical Research Council, based in Cambridge, England.The CBSU is a centre for cognitive neuroscience, with a mission to improve human health by understanding and enhancing cognition and behaviour in health, disease and disorder.It is one of the largest and most long-lasting contributors to the development of psychological theory and ...
The new Institute, at the University of Cambridge, will build on existing investment by the Medical Research Council (MRC) and the Wellcome Trust, uniting 30 leading research teams with expertise across the three main types of stem cell: embryonic, adult and induced pluripotent cells. Research scientists will work alongside technology ...
The Cambridge Stem Cell Institute is a world-leading centre for stem cell research. Our mission is to transform human health through a deep understanding of stem cell biology. We're funded by Wellcome and the Medical Research Council.
ason Chin is currently a Programme Leader at the Medical Research Council Laboratory of Molecular Biology (MRC-LMB), where he is also Head of the Centre for Chemical & Synthetic Biology (CCSB). He is Professor of Chemistry & Chemical Biology at the University of Cambridge, and holds a joint appointment at the University of Cambridge Department of Chemistry.
ISAC/M 2024: Inspiring Scientists at the Cambridge Institute for Medical Research and the MRC Mitochondrial Biology Unit. 5 March 2024. This free, widening participation programme for Cambridgeshire-based Year 12 students (16+) took place from 20-22 February (half-term) at the Cambridge Institute for Medical Research (CIMR) and MRC ...
The Medical Research Council (MRC) Metabolic Research Laboratories at the University of Cambridge (MRC MRL) has been developed to improve understanding the mechanisms responsible for obesity and related metabolic diseases, with the eventual goal of developing interventions to prevent and treat them. The MRL also hosts the MRC Metabolic Diseases Unit.
Medical Research Council - Volume 159 Issue 4. To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account.
Medical Research Council Reviews by biologist. Pros "Multicultural working environment," (in 5 reviews) "Good people to work with" (in 4 reviews) Cons "Low salaries for Cambridge and minimum yearly increases." (in 7 reviews) "Poor career progression:" (in 2 reviews)
Scientists at Cambridge University conducted a study and found that it can really help expand our efficiency. They have also developed a controllable prosthetic extra thumb, which is easy to use and very controllable. Tamar Makin, a professor from the Medical Research Council (MRC) Cognition and Brain Sciences Unit at the University of ...
A former longtime executive at the Swiss drug giant Novartis will become the new chief executive of the Bill & Melinda Gates Medical Research Institute, a nonprofit biotech in Cambridge working on ...
The research is being commercialised with the support of Cambridge Enterprise, the University's commercialisation arm. The research was supported in part by the European Research Council, Wellcome, the Royal Society, and the Biotechnology and Biological Sciences Research Council (BBSRC), part of UK Research and Innovation (UKRI). Reference:
Background In healthcare, regulation of professions is an important tool to protect the public. With increasing regulation however, professions find themselves under increasing scrutiny. Recently there has also been considerable concern with regulator performance, with high profile reports pointing to cases of inefficiency and bias. Whilst reports have often focused on large staff groups, such ...