An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.8(7); 2019 Jul

Ebola virus: A global public health menace: A narrative review
Shamimul hasan.
1 Department of Oral Medicine and Radiology, Faculty of Dentistry, Jamia Millia Islamia, New Delhi, India
Syed Ansar Ahmad
2 Department of Oral Surgery, Faculty of Dentistry, Jamia Millia Islamia, New Delhi, India
Rahnuma Masood
3 Department of Conservative Dentistry, Faculty of Dentistry, Jamia Millia Islamia, New Delhi, India
Shazina Saeed
4 Department of Amity Institute of Public Health, Amity University, Noida, Uttar Pradesh, India
Ebola virus disease (EVD), a fatal viral hemorrhagic illness, is due to infection with the Ebola virus of the Filoviridae family. The disease has evolved as a global public health menace due to a large immigrant population. Initially, the patients present with nonspecific influenza-like symptoms and eventually terminate into shock and multiorgan failure. There exists no specific treatment protocol for EVD and only supportive and symptomatic therapy is the line of treatment. This review article provides a detailed overview of the Ebola virus; it's clinical and oral manifestations, diagnostic aids, differential diagnosis, preventive aspects, and management protocol.
Introduction
Ebola, earlier termed as Ebola hemorrhagic fever (EHF), is a critically lethal ailment which primarily affects the humans and nonhuman primates. Ebola virus disease (EVD) occurs due to a virus infection which belongs to the family Filoviridae and genus Ebolavirus .[ 1 ] EVDs has posed diagnostic challenges and has been a universal public health threat since its discovery. While investigating an alleged yellow fever case, Dr. Peter Piot in the year 1976 first detected the disease in Zaire, Africa (presently the Democratic Republic of Congo).[ 2 ] The name “Ebola” was termed as the disease was noticed near the Ebola river in Congo.[ 3 ]
Fruit bats of Pteropodidae family, such as Hypsignathus monstrous, Epomops franqueti , and Myonycteris torquata serve as the natural hosts of the EBOV in Africa. Nonhuman primates may develop the infection by eating the partly eaten fruits and may also transmit the infection to humans.[ 4 ] Indian population is an impending threat to EVD, as India falls in the home range of Pteropodidae family of fruit bats.[ 5 ]
Ebola virus transmission primarily takes place through close bodily contact with the infected patient or their fluids, contaminated tissue surfaces, and clothing from alive, infected or deceased individuals. Unsafe traditional burial practices also play a pivotal role in the disease transmission.[ 6 ] There is documented evidence regarding the sexual mode of disease transmission, although transmission through the air is unlikely.[ 7 ]
EVD present with bizarre and atypical manifestations mimicking other viral diseases, especially in the initial disease phase. Constitutional symptoms, such as fever, myalgia, headache, vomiting, and diarrhea are the early presenting features. Hemorrhagic rash, internal and external bleeding are usually the warning manifestations in the late stages.[ 8 ] Bleeding from the body apertures is a distinguishing EVD manifestation.[ 9 ] Gum bleeding, odynophagia, and atypical oral manifestations constitute the oral features of EVD.[ 10 ]
Till date, there is no precise antiviral management or vaccination for EVD. The management protocol mainly relies on supportive and symptomatic therapy, along with monitoring coagulopathies and multiorgan dysfunction.[ 2 ]
The World Health Organization (WHO) affirmed the EVD outbreak as a “Public Health Emergency of International Concern” on August 8 th , 2014.[ 5 ]
With the enormous immigrant population, India is estimating the likelihood of a probable EVD outbreak. The Ministry of Health and Family Welfare, Government of India, in collaboration with other agencies has appraised the situation and recommended travel instructions by air, land, and sea and health care professionals.[ 11 ]
The virus belongs to the Ebola virus genus, Filoviridae family, and Mononegavirales order.[ 12 ] The genus Ebolavirus includes the following species- Zaire ebolavirus (EBOV), Reston ebolavirus (RESTV), Bundibugyo ebolavirus (BDBV), Taï Forest ebolavirus (TAFV), Sudan ebolavirus (SUDV), and the newly identified Bombali ebolavirus (BOMV).[ 13 ] Except for exclusive identification of RESTV in the Philippines, all the other species causes endemic West African EVD.[ 14 ]
EBOV responsible for the EHF causes the highest human mortality (57%–90%), followed by SUDV (41%–65%) and Bundibugyo virus (40%). TAFV has caused only two nonlethal human infections to date, whereas RESTV causes asymptomatic human infections.[ 15 ]
Figure 1 shows the taxonomy of Ebola virus.

Taxonomy of Ebola virus
Transmission
Based on the Centers for Disease Control and Prevention (CDC) classification, Ebola virus is considered as a biosafety level 4 and category A bioterrorism pathogen with an immense likelihood for massive nationwide transmission.[ 16 ]
Source of Infection
Intimate physical contact with the patients in the acute disease stages and contact with the blood/fluids from the dead individuals constitutes the most important modes of transmission.[ 17 ]
The long-established funeral ceremonies in the African countries entail direct handling of the dead bodies, thus significantly contributing to the disease dissemination. Unsafe conventional burial procedures accounted for 68% infected cases in 2014 EVD outburst of Guinea.[ 18 ]
EBOV RNA may be identified for up to a month in rectal, conjunctival, and vaginal discharges and semen specimens may demonstrate the virus presence up to 3 months, thus signifying the presence of EBOV in recuperating patients.[ 14 ] The sexually transmitted case of EVD has been reported between a convalescent patient and close family member. Another study demonstrated a case in a recuperating male patient. The patient's semen specimen tested positive with Ebola viral antigen almost 3 months after the disease onset.[ 19 ]
Asymptomatic EBOV carriers are not infectious and do not have a major role play in the EVD outburst, and the field practice in Western Africa supported this assumption.[ 20 ] However, this presumption was refuted after the documentation of a pioneer asymptomatic carrier case in North Gabon epidemic (1996).[ 21 ]
EBOV has been detected from blood, saliva, semen, and breast milk, while RNA has been isolated from sweat, tears, stool, and on the skin, vaginal, and rectal swabs, thus highlighting that exposure to infected blood and bodily secretions constitute the major means of dissemination.[ 22 ]
Eating uncooked infected animal meat such as bats or chimpanzees account significantly to oral EVD transmission, especially in the African countries.[ 23 ] The demonstration of the Ebola virus in the Filipino pigs in 2008 triggered the likelihood of an extensive range of possible animal hosts.[ 24 ]
EVD dissemination has also been reported with hospital-acquired infections, particularly in areas with poor hygiene conditions. The infected needles usage was responsible for the 1976 EVD outbreak in Sudan and Zaire.[ 25 , 26 ] Improper hygiene and sterilization were the crucial factors for the 1967 Yambuku EVD outburst.[ 27 ]
EVD dissemination may also occur through the inanimate materials with infected body secretions (fomites).[ 19 ] However, disease transmission through the airborne and droplet infection is ambiguous.[ 10 ]
Figure 2 shows the primary and secondary transmission of disease.

Primary and secondary transmission
Table 1 depicts the possible routes of transmission.
Possible routes of transmission
Epidemiology
The vast majority of EVD cases and outbursts have been endemic to African continent ever since the disease detection in 1976,[ 28 ] and 36 such outbreaks have occurred in six African countries.[ 29 ]
Table 2 shows Ebola epidemiological outbreaks between 1976 and 2014.
Ebola outbreaks between 1976 and 2014 (Adapted from WHO 2014)
UVRI: Uganda Virus Research Institute; CDC: Centers for Disease Control and Prevention
The 2014–2016 EVD started in South East Guinea rural surroundings and eventually became a global public health menace by rapidly disseminating to urban localities and other countries.[ 28 ]
Figure 3 depicts the geographical distribution of Ebola virus disease.

Geographic distribution of Ebola virus disease outbreaks
The conducive environmental surroundings of the African continent facilitate EVD endemicity. However, intermittent imported Ebola cases have also been noticed in United States, United Kingdom, Canada, Spain, and Thailand.[ 30 , 31 ]
Figure 4 depicts the distribution of Ebola virus disease in West African Countries.

Distribution of Ebola virus disease in West African Countries
Out of the unparalleled globally reported 28,616 cases and 11,310 casualties, Liberia accounted for almost 11,000 cases and over 4,800 deaths.[ 32 ]
Table 3 shows the statistics of the 2014–16 West African outbreak.
Statistics of 2014-16 West African outbreak
Pathogenesis
Ebola viruses penetrate the human body through mucous membranes, skin lacerations/tear, close contact with infected patients/corpse, or by direct parental dissemination.[ 33 ] EBOV has a predilection to infect various cells of immune system (dendritic cells, monocytes, and macrophages), endothelial and epithelial cells, hepatocytes, and fibroblasts where it actively replicates by gene modulation and apoptosis and demonstrate significantly high viremia.[ 34 ] The virus reaches the regional lymph nodes causing lymphadenopathy and hematogenous spread to the liver and spleen promote an active inflammatory response.[ 35 ] Release of chemical mediators of inflammation (cytokines and chemokines) causes a dysregulated immune response by disrupting the vasculature system harmony, eventually causing disseminated intravascular coagulation and multiple organ dysfunction.[ 36 ]
Figure 5 demonstrates the pathogenesis of Ebola virus disease.

Pathogenesis of Ebola virus disease
Clinical Features
Due to the bizarre and atypical manifestations in the initial phase, mimicking dengue fever, typhoid fever, malaria, meningococcemia, and other bacterial infections, EVD poses diagnostic dilemmas.[ 37 ]
The incubation period ranges from 2 to 21 days. However, symptoms usually develop 8–11 days following infection.[ 38 , 39 ]
The initial disease phase is represented by constitutional symptoms.[ 40 ] High-grade fever of >38 o C is the most frequently reported symptom (85–95%), followed by other vague symptoms such as general malaise (85–95%), headaches (52–74%), dysphagia, sore throat (56–58%), and dry cough.[ 41 , 42 ] The progressively advanced disease is accompanied by abdominal pain (62–68%), myalgia (50–79%), nausea, vomiting, and diarrhea (84–86%).[ 41 ]
Variety of hemorrhagic manifestations forms an integral component of the late disease phase.[ 38 ] Gastrointestinal tract bleeding manifests as petechiae, hematuria, melena, conjunctival bleeding, contusion, or intraperitoneal bleeding. Mucous membrane and venipuncture site bleeding, along with excess clot formation may also occur. As the features advances with time, the patients experience dehydration, confusion, stupor, hypotension, and multiorgan dysfunction, resulting in fulminant shock and ultimately death.[ 43 , 44 ]
Maculopapular exanthema constitutes a characteristic manifestation of all Filovirus infection, including EVD.[ 45 ] The rash usually appears during the 5 th to 7 th day of disease and occur in 25–52% of patients in the past EVD outbreaks.[ 46 ]
Table 4 shows the clinical manifestations of Ebola virus disease.
Clinical manifestations of Ebola virus disease
Although EVD has a number of similar features with other viral hemorrhagic fevers (e.g. dengue), there are differences that set them apart.
Table 5 depicts the differentiating features of the Ebola virus and dengue virus infection.
Differentiating features of Ebola and dengue virus infection
Orofacial features
Gum bleeding, atypical mucosal lesions, and odynophagia comprise the distinctive oral manifestations. Epistaxis (nasal bleed), bleeding from venipuncture sites, conjunctivitis, and cutaneous exanthema are the other manifestations.[ 9 ] Bleeding tendencies and gum bleeding is not seen in asymptomatic or initial EBOV patients reporting to the dental hospital.
EVD dissemination in the field of oral and dental health may appear nonsignificant; although, probable situations which may pose a risk to dental health professional have been appraised by Samaranayake et al. [ 21 ] and Galvin et al. [ 10 ]
Table 6 depicts the various orofacial manifestations of Ebola virus disease
Orofacial manifestations of Ebola virus disease
EVD patients usually demonstrate altered laboratory parameters based on the stage of the disease.
Table 7 shows the laboratory findings in Ebola virus disease.
Laboratory findings in Ebola virus disease
The WHO (2014) recommended the sample collection of whole blood or oral swab at suitable centres called Ebola treatment centers.[ 47 ] Reverse transcriptase polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) are the most frequently utilized tests for laboratory affirmation of the EVD.[ 43 ] RT-PCR is capable of detecting viral RNA in the blood samples of infected patients immediately after the commencement of signs and symptoms,[ 42 , 48 ] has a high sensitivity (up to 100%), and gives results within 1–2 days in cases of epidemics. ELISA detects the immunoglobulins G and M in samples of infected patients, has a low sensitivity (91%) and is not suitable for initial affirmation during an outbreak.[ 42 , 49 ]
The most imperative strategy in EVD is to avert the vulnerable population from getting infected and limit the transmission. These preventive strategies entail intensive and rigorous endeavors from the Government, public health amenities, medical units, and personals.[ 50 ]
The most essential aspect to curb EVD transmission is to avert direct bodily contact with infected individuals and their body fluids.[ 51 ]
Health caregivers are extremely vulnerable and experience an augmented professional threat for EVD.[ 52 ] Thus, scrupulous adherence to the universal infection control measures is fundamental in all the hospitals, laboratories, and other health care services.[ 53 ] The U.S. CDC has advocated the appropriate use of various personal protective equipment as a mandate for health care professionals.[ 50 ]
The risk of rapid importation of Ebola virus into human beings can be prevented by averting the direct bush meat and bats contact.[ 54 ]
Unsafe traditional burial procedures, especially in the African continent significantly contributed to the EVD transmission. Hence, it is essential to practice safe and guarded funeral rituals to prevent the disease spread.[ 55 ]
WHO recommends the implementation of safe sex practices to combat the sexual transmission of EVD. Strict abstinence or proper and regular condom use in male EVD survivors at least for a period of 12 months of the symptom onset or until their semen has twice tested negative should be followed.[ 56 ]
Dental health care personals are extremely susceptible to EVD as they are in regular contact with blood and saliva during the routine diagnostic procedures. There is no documented case of EVD through saliva till date. A study on the identification of EBOV in oral fluids affirmed that patients presenting with demonstrable serum levels of EBOV RNA also exhibit identifiable salivary levels.[ 57 ] The incubation period for all body fluids including saliva is 21 days; hence, oral health personals are vulnerable to develop the disease if universal infection control protocol is not followed.[ 58 ]
Table 8 demonstrates the various infection control measures to prevent the Ebola virus spread.
Infection control measures to prevent Ebola virus spread
Box 1 shows the travel guidelines to EBOV affected regions.
Shows the UK Travel guidelines to EBV infested regions.
Till date, there is no precise antiviral management or vaccination for EVD.[ 51 ] The management protocol mainly relies on supportive and symptomatic therapy. Public health strategies emphasizing on epidemiological surveillance, contact tracing, and quarantine of the patient have been recommended to combat the dissemination of EVD.[ 59 ]
Rehydration, adequate nourishment, analgesics, and blood transfusion form a keystone supportive treatment of EVD patient.[ 60 ] Intravenous fluids and oral rehydration solution endow with proper electrolytes substitute and maintain the intravascular volume. Unrelenting vomiting and diarrhea are taken care of by the use of antiemetics and antidiarrheal drugs.[ 35 , 60 , 61 ] Suspected cases of secondary bacterial infections and septicemia are best managed by the use of prophylactic antibiotic regimen (third generation I.V. cephalosporins).[ 62 ] Concurrent parasitic coinfections may also be seen and require prompt investigations and management.[ 63 ]
A number of investigative clinical trials emphasizing on the development of vaccine, antibody therapies, and antiviral drugs have been conducted for EVD.[ 64 ]
Table 9 shows experimental treatment for Ebola virus disease.
Experimental treatment for Ebola virus disease
Various clinical trials in Africa, Europe, and the United States suggest that Ebola vaccines are in various development stages (Phase I–III). A number of candidate vaccines employ diverse platforms, including recombinant viral vectors (most evolved vaccine candidate), DNA vaccines, inactivated viral particles, subunit proteins, recombinant proteins, and virus-like particles. Example of viral vectors expressing ebolavirus glycoproteins include recombinant simian adenovirus (cAd3), recombinant vaccinia virus, recombinant human adenovirus (Ad26), and a live vesicular stomatitis virus used alone or in prime-booster regimens.[ 65 ]
However, Ebola virus having the glycosylated surface proteins and preferentially infecting the immune cells impedes the development of an effective vaccine.[ 66 ]
Dental Management
Dental health care professionals in Europe have not encountered a case of EVD so far. However, health care personals (including dental surgeons) are more prone to EVD while treating patients in West or sub-Saharan Africa. Dental professionals are more likely to encounter asymptomatic EVD patients or those with early-stage vague symptoms.[ 27 ]
Individuals with a travel history to Ebola endemic regions, but with no direct intimate contact with the disease fall in the low-risk category and may undergo any medical/dental health care procedures without restrictions. However, all the nonessential procedures should be postponed for 21 days in individuals with direct exposure to the virus. The regional Health Service Executive Department of Public Health needs to be notified when the exposed patient's treatment cannot be deferred or controlled with pharmacotherapy.[ 10 ]
EVD has emerged as a significant global public health menace due to multiple disease outbreaks in the last 25 years. Recent advancements are being carried out in the form of effective Ebola virus vaccine and anti-Ebola virus drugs. However, rapid geographic dissemination, nonspecific clinical presentation, lack of vaccine, and specific diagnostic test are the possible challenges to combat this dreaded public health menace.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Use of Ebola Vaccines — Worldwide, 2021–2023
Weekly / April 25, 2024 / 73(16);360–364
Ruth Kallay, MPH 1 ; Reena H. Doshi, PhD 2 ; Pierre Muhoza, PhD 1 ; Mary J. Choi, MD 3 ; Anaïs Legand, MPH 4 ; Emma Aberle-Grasse 1 ; Aminata Bagayoko, MD 4 ; Terri B. Hyde, MD 1 ; Pierre Formenty, DVM 4 ; Alejandro Costa, MSc 4 ( View author affiliations )
What is already known about this topic?
The International Coordinating Group on Vaccine Provision established an Ebola vaccine stockpile in 2021 to ensure equitable, rapid access to vaccines during an outbreak.
What is added by this report?
Since 2021, the absence of large Ebola virus disease (Ebola) outbreaks has resulted in fewer vaccine doses being used for outbreak response. Out of the 145,690 doses shipped from the stockpile through 2023, 95% (139,120) have been repurposed for preventive vaccination, and 5% (6,570) were used in outbreak response.
What are the implications for public health practice?
Repurposing doses for preventive vaccination could be prioritized in the absence of Ebola outbreaks to prevent transmission and maximize the cost-efficiency and benefits of the stockpile.
- Article PDF
- Full Issue PDF
Ebola virus disease (Ebola) is a rare but severe illness in humans, with an average case fatality rate of approximately 50%. Two licensed vaccines are currently available against Orthoebolavirus zairense , the virus that causes Ebola: the 1-dose rVSVΔG-ZEBOV-GP (ERVEBO [Merck]) and the 2-dose regimen of Ad26.ZEBOV and MVA-BN-Filo (Zabdeno/Mvabea [Johnson & Johnson]). The Strategic Advisory Group of Experts on Immunization recommends the use of 1-dose ERVEBO during Ebola outbreaks, and in 2021, a global stockpile of ERVEBO was established to ensure equitable, timely, and targeted access to vaccine doses for future Ebola outbreaks. This report describes the use of Ebola vaccines and the role of the stockpile developed and managed by the International Coordinating Group (ICG) on Vaccine Provision during 2021–2023. A total of 145,690 doses have been shipped from the ICG stockpile since 2021. However, because outbreaks since 2021 have been limited and rapidly contained, most doses (139,120; 95%) shipped from the ICG stockpile have been repurposed for preventive vaccination of high-risk groups, compared with 6,570 (5%) used for outbreak response. Repurposing doses for preventive vaccination could be prioritized in the absence of Ebola outbreaks to prevent transmission and maximize the cost-efficiency and benefits of the stockpile.
Introduction
Orthoebolavirus zairense , the virus responsible for Ebola virus disease (Ebola), has caused the largest filovirus outbreaks worldwide; the average Ebola case fatality rate is approximately 50% ( 1 ). Currently, two licensed vaccines are recommended for the prevention of Ebola caused by Orthoebolavirus zairense : the 1-dose rVSVΔG-ZEBOV-GP (ERVEBO [Merck]) and the 2-dose Ad26.ZEBOV and MVA-BN-Filo (Zabdeno/Mvabea [Johnson & Johnson]) ( 2 ). ERVEBO was licensed by the European Medicines Agency and the Food and Drug Administration in 2019 and is indicated for use in persons aged >12 months ( 2 , 3 ). It has a shelf life of 3 years. The vaccine has also been approved in Burundi, Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo (DRC), Ghana, Guinea, Republic of the Congo, Rwanda, Sierra Leone, Uganda, and Zambia (Merck regulatory department, personal communication, December 6, 2023) ( 2 ). In 2021, the Strategic Advisory Group of Experts on Immunization recommended using ERVEBO in ring vaccination during Ebola outbreaks, because it confers protection after 1 dose ( 4 ). Zabdeno/Mvabea is recommended for preventive vaccination in areas at lower risk for Ebola (or areas neighboring an outbreak) because the full regimen requires 2 doses administered 56 days apart ( 4 ).
ERVEBO was shown to be safe and effective during clinical trials and has likely played an important role in limiting Ebola morbidity and mortality during outbreaks since it was first introduced ( 2 ). In a study conducted in Ebola treatment facilities in DRC, 56% of unvaccinated patients died from Ebola, compared with 25% of patients vaccinated before symptom onset ( 5 ). Ensuring timely availability of Ebola vaccine doses in the event of a major Ebola outbreak is crucial to limiting its spread and protecting global health security.
In 2021, a global stockpile of ERVEBO was established under the International Coordinating Group (ICG) on Vaccine Provision to ensure equitable and timely access to vaccine doses for Ebola outbreaks* ( 6 ). Upon the establishment of the ICG stockpile, the global agreement was to maintain the stockpile at 500,000 doses ( 6 ). Gavi, the Vaccine Alliance ( https://www.gavi.org ), supports the procurement of vaccine and operational costs to countries for vaccination ( 6 ). Whereas the availability of doses for outbreak response is the primary objective of the stockpile, ICG has approved requests for targeted preventive vaccination of high-risk groups, including health care workers and frontline workers in countries at risk for Ebola outbreaks. This report describes the use of Ebola vaccines and the role of the ICG vaccine stockpile during 2021–2023.
Data on past Ebola outbreaks were obtained from the World Health Organization (WHO) Regional Office for Africa’s weekly Outbreak and Emergencies situation reports ( 1 ). Information on Ebola vaccine stockpile requests and deliveries during 2021–2023 was obtained from the ICG Secretariat. Data on the stockpile size were obtained from UNICEF Supply Division’s ICG Ebola vaccine stockpile report dated January 19, 2024 ( 7 ). This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy. †
Ebola vaccine was first used during clinical trials in the 2014–2015 West African outbreak, then under a compassionate use protocol in Guinea during 2015, and again in the 2018–2020 eastern DRC outbreak. Since 2015, when Ebola vaccines were first deployed in outbreak response, recorded Ebola outbreaks have varied in frequency, size, and origin, with recent outbreaks more often linked to reintroduction through viral persistence § (four of five outbreaks since 2021) than to zoonotic spillover ( Table 1 ).
The ICG Ebola vaccine stockpile reached the goal of 500,000 doses in 2022 and, as of December 2023, holds 518,890 doses. In total, 208,390 (40%) doses from the current stockpile are scheduled to expire in 2024. Doses from the ICG stockpile were first deployed in 2021 in DRC for outbreak response. During 2021–2023, a total of 145,690 ERVEBO doses were shipped through requests from the ICG stockpile. Among 11 requests to ICG during this period, 10 were approved or partially approved, and one request was declined ¶ ( Table 2 ). All requests to ICG for outbreak response (three of 11) were delivered within 1 week of being received. Longer times to delivery were noted for shipments intended for preventive vaccination because of the additional planning and engagement around those activities.
The number of doses shipped from the stockpile has increased annually, from 4,800 doses in 2021, to 13,870 doses in 2022, and 127,020 doses in 2023. During this period, 42,620 doses expired. Most doses shipped (139,120; 95%) were repurposed for preventive vaccination. Five percent (6,570) of doses were shipped for outbreak response use. DRC has received the largest number of vaccine doses (111,000; 76%), followed by Uganda (23,460; 16%) and Guinea-Bissau (11,170; 8%).
The ICG stockpile provides equitable access to vaccines that can be shipped quickly in the event of an Ebola outbreak. The relatively small number of doses used for outbreak response (6,570; 5% of doses shipped) reflects the smaller size and rapid containment of Ebola outbreaks since 2021. North Kivu, DRC, has received and administered more doses than any other geographic area worldwide since 2018, which might have contributed to the rapid containment of subsequent outbreaks in that area ( 1 ).
After approvals of vaccine for preventive use by ICG in 2022, WHO, in early 2023, circulated an internal memo on behalf of ICG informing at-risk countries of the availability of vaccines for preventive vaccination of health care workers and frontline workers. Preventive vaccination campaigns have targeted health care workers and frontline workers in at-risk countries, given their increased risk for exposure because of their frequent contact with patients ( 8 ). The addition of preventive Ebola vaccination of these workers could reduce total cases, hospitalizations, and deaths in Ebola outbreaks by an estimated 14%–38% compared with nonpharmaceutical interventions and ring vaccination alone ( 8 ).
The variability of Ebola outbreak size and time to containment makes predicting future vaccine needs challenging. Repurposing doses for preventive vaccination of targeted groups can protect high-risk persons as well as make use of doses with a shorter shelf life. More than 200,000 short–shelf-life doses in the ICG stockpile due to expire in 2024 could be redirected for preventive vaccination. In addition to focusing on reactive (outbreak response) vaccination, early planning for preventive vaccination with short–shelf-life doses could be incorporated into future stockpile management strategies. Additional studies accounting for the variability in outbreak size could guide planning to maximize the cost-efficiency of stockpile management.
The frequency of recent outbreaks, especially those linked to viral persistence, highlights the need for innovative strategies to protect Ebola survivors and prevent reintroductions. One such strategy is to offer postoutbreak immunization to close contacts of survivors, including new sex partners and other groups at risk for transmission because of viral persistence ( 9 ). Additional avenues to expand preventive vaccination among high-risk populations could be explored in countries at risk for outbreaks. Demand-generation activities** incorporating findings from community engagement and vaccine acceptance studies in targeted risk groups could accompany vaccination campaigns and help develop targeted engagement plans. Investments and advocacy for preventive vaccination against Ebola are crucial for health system preparedness and resiliency. Currently, Gavi, WHO, and UNICEF are coordinating with other partners to develop a learning agenda †† to help guide research prioritization and funding decisions for Ebola vaccine use.
Limitations
The findings in this report are subject to at least two limitations. First, whereas the Ebola vaccine has reduced morbidity and mortality during outbreaks, the impact of Ebola vaccines on preventing outbreaks is difficult to ascertain because of the infrequent occurrence of the disease. Second, important data are lacking regarding the duration of protection, vaccine effectiveness in outbreak situations, and the need for booster doses. These data will be needed to guide decision-making regarding vaccination strategies and should be a focus for future research.
Implications for Public Health Practice
The availability of licensed Ebola vaccines is an important advancement in Ebola prevention and global health security. In the absence of large-scale outbreaks, the demand for vaccines lags behind the current supply of doses, and preventive vaccination could be considered for high-risk groups. Investments, advocacy, and additional research to inform preventive vaccination are crucial for health system preparedness and resiliency. Focus on working with countries at risk for Ebola outbreaks to identify high-risk groups and generate demand for preventive vaccination is important for optimizing the use of the stockpile. Ensuring the availability of sufficient Ebola vaccine doses for emergency outbreak response remains the priority of ICG.
Corresponding author: Ruth Kallay, [email protected] .
1 Global Immunization Division, Global Health Center, CDC; 2 Emergency Preparedness and Response, World Health Organization Regional Office for Africa, Brazzaville, Republic of the Congo; 3 Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 4 Viral Hemorrhagic Fevers team, Health Emergencies Programme, World Health Organization, Geneva, Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* The ICG Ebola vaccine stockpile is managed by the ICG on Vaccine Provision comprising Médecins sans Frontières, the International Federation of Red Cross and Red Crescent Societies, UNICEF, and the World Health Organization. These organizations support maintenance and decisions regarding vaccine allocations from the ICG on Vaccine Provision’s stockpile of Ebola vaccine. https://www.who.int/groups/icg/about
† 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
§ Person-to-person transmission of Ebola virus that persisted in immunologically privileged sites (sites that are able to tolerate the introduction of antigen without eliciting an inflammatory immune response, including the eyes, placenta, fetus, testicles, and central nervous system) or body fluids after recovery from acute infection in humans, in contrast to outbreaks originating from zoonotic spillover, which is the transmission of virus from an animal to a human.
¶ The request to ICG that was not approved lacked justification that the security forces to be vaccinated were involved in Ebola outbreak response and were at risk. ICG invited the country to resubmit the application prioritizing staff members involved in Ebola response activities.
** Activities that aim to increase public awareness of and coverage with the vaccine and might include public education campaigns, health care worker education and engagement, community outreach, targeted messaging to high-risk groups, and increased access to the vaccine.
†† A set of prioritized vaccine implementation research questions and activities to guide evidence-building and decision-making around the Ebola vaccine.
- World Health Organization Regional Office for Africa. Outbreaks and Emergencies Bulletin. Cité du Djoué, Brazzaville, Republic of the Congo: World Health Organization Regional Office for Africa; 2024. https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates?page=0
- World Health Organization. Ebola virus disease vaccines. Geneva, Switzerland: World Health Organization; 2023. https://www.who.int/news-room/questions-and-answers/item/ebola-vaccines
- Merck. U.S. FDA approves Merck’s ERVEBO (Ebola Zaire Vaccine, Live) for use in children 12 months of age and older. [Press release]. Rahway, NJ: Merck; 2023. https://www.merck.com/news/u-s-fda-approves-mercks-ervebo-ebola-zaire-vaccine-live-for-use-in-children-12-months-of-age-and-older/
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, 22–24 March 2021: conclusions and recommendations. Geneva, Switzerland: World Health Organization; 2021. https://www.who.int/publications/i/item/meeting-of-the-strategic-advisory-group-of-experts-on-immunization-22-24-march-2021-conclusions-and-recommendations
- Coulborn RM, Bastard M, Peyraud N, et al. Case fatality risk among individuals vaccinated with rVSVΔG-ZEBOV-GP: a retrospective cohort analysis of patients with confirmed Ebola virus disease in the Democratic Republic of the Congo. Lancet Infect Dis 2024. Epub February 7, 2024. https://doi.org/10.1016/S1473-3099(23)00819-8 PMID:38340736
- Gavi, The Vaccine Alliance. 500,000 doses of Ebola vaccine to be made available to countries for outbreak response. Geneva, Switzerland: Gavi, The Vaccine Alliance; 2021. https://www.gavi.org/news/media-room/500000-doses-ebola-vaccine-be-made-available-countries-outbreak-response
- UNICEF. Emergency stockpile availability report – Ebola vaccine. New York, NY: UNICEF; 2024. https://www.unicef.org/supply/documents/emergency-stockpile-availability-report-ebola-vaccine
- Bisanzio D, Davis AE, Talbird SE, et al. Targeted preventive vaccination campaigns to reduce Ebola outbreaks: an individual-based modeling study. Vaccine 2023;41:684–93. https://doi.org/10.1016/j.vaccine.2022.11.036 PMID:36526505
- Doshi RH, Fleming M, Mukoka AK, et al. Vaccination of contacts of Ebola virus disease survivors to prevent further transmission. Lancet Glob Health 2020;8:e1455–6. https://doi.org/10.1016/S2214-109X(20)30454-X PMID:33220205
Abbreviations: CFR = case fatality rate; DRC = Democratic Republic of the Congo; NA = not applicable. * Outbreak data obtained from the World Health Organization Regional Office for Africa weekly Outbreak and Emergencies situation reports was compared with data from CDC available online. https://www.cdc.gov/vhf/ebola/history/chronology.html (Accessed January 9, 2024). † Zoonotic spillover is the transmission of virus from an animal to a human. § Person-to-person transmission of Ebola virus from virus that persisted in immunologically privileged sites (sites that are able to tolerate the introduction of antigen without eliciting an inflammatory immune response, including the eyes, placenta, fetus, testicles, and central nervous system) or body fluids after recovery from acute infection.
Abbreviations: DRC = Democratic Republic of the Congo; ICG = International Coordinating Group; NA = not applicable. * Doses shifted from Equateur province to North Kivu province in DRC from previously shipped doses approved by ICG. † Frontline workers are generally considered to be personnel directly involved in essential, public-facing roles related to health services or outbreak response; countries might define this group differently. § The request to ICG that was not approved lacked justification that the security forces to be vaccinated were involved in Ebola outbreak response and were at risk. ICG invited the country to resubmit the application prioritizing staff members involved in Ebola response activities.
Suggested citation for this article: Kallay R, Doshi RH, Muhoza P, et al. Use of Ebola Vaccines — Worldwide, 2021–2023. MMWR Morb Mortal Wkly Rep 2024;73:360–364. DOI: http://dx.doi.org/10.15585/mmwr.mm7316a1 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Reference Manager
- Simple TEXT file
People also looked at
Brief research report article, brief research report: ebola virus differentially infects human iris and retinal pigment epithelial cells.

- 1 Australian Centre for Disease Preparedness, Commonwealth Scientific and Industrial Research Organisation, Geelong, VIC, Australia
- 2 Flinders University College of Medicine and Public Health, Adelaide, SA, Australia
- 3 Departments of Ophthalmology, and Cell, Developmental and Regenerative Biology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 4 Department of Ophthalmology and Visual Sciences, Truhlsen Eye Institute, University of Nebraska Medical Center, Omaha, NE, United States
Uveitis is a common manifestation of post-Ebola syndrome, associated with persistence of Ebola virus (EBOV; Zaire ebolavirus ) inside the eye. The iris and retinal pigment epithelia are key components of the blood-ocular barriers, but have the capacity to act as hosts for microorganisms. We investigated the ability of EBOV to productively infect these cell populations. Donor-matched human iris and retinal pigment epithelial isolates (n = 5) were infected with EBOV at a multiplicity of infection of 1 for up to 72 hours. Parallel cultures were infected with Reston virus (RESTV; Reston ebolavirus ) or Zika virus (ZIKV), or held uninfected under the same conditions. Viral transcript expression by RT-qPCR on total cellular RNA, cytoimmunofluorescence, and assays of 50% tissue culture infected dose of culture supernatant showed that both iris and retinal pigment epithelial isolates were permissive to infection, and supported replication and release of EBOV, as well as RESTV and ZIKV. However, in comparison to cells isolated from iris, those from retina demonstrated obvious EBOV-induced cytopathic effect, had higher intracellular EBOV nucleoprotein transcript, expressed intracellular EBOV protein more widely, and released EBOV at higher titer. Comparable results were obtained for isolates infected with RESTV and ZIKV. Consistent with observations of retinal pigment epithelial scars in Ebola survivors, our results suggest that an early event in post-Ebola uveitis is infection of the retinal pigment epithelium. Relative susceptibility of retinal pigment epithelial cells to infection with RESTV and ZIKV, as well as EBOV, implies this phenomenon may relate to a cell-specific attribute, such as high phagocytic activity.
1 Introduction
The majority of individuals who become infected with Ebola virus (EBOV; Zaire ebolavirus ) and survive the acute hemorrhagic Ebola virus disease, develop a chronic inflammatory condition that is often referred to as ‘post-Ebola syndrome’ ( 1 ). This syndrome is characterized by arthritis, neuro-inflammation, and fatigue, and it has been linked to long-term persistence of live virus in immune-privileged sites ( 2 ). One of the most serious manifestations of post-Ebola syndrome is uveitis, or inflammation inside the eye, seen in up to 33% of Ebola survivors ( 3 ). Uveitis may affect the anterior and/or posterior segments of the eye, and lead to complications that include cataract, glaucoma and macular oedema. The inflammation or its complications cause vision loss in as many as 60% of survivors ( 4 ).
Ocular pigment epithelial cells play central roles in ocular infection and inflammation. These cells form an important component of the blood-ocular barriers that regulate the movement of molecules, cells, microorganisms and other foreign products between the bloodstream and the eye ( 5 , 6 ). They have molecular properties that contribute to ocular immune privilege, which describes the mechanisms that the immune system uses to control inflammation within the eye in order to protect intraocular tissues that are critical for vision ( 7 ). However, these cells are also implicated in ocular pathology, with capacity to produce inflammatory cytokines and other molecules ( 8 , 9 ), and the potential to act as host to a range of microorganisms ( 10 – 12 ).
These cells include the iris pigment epithelium protecting the anterior segment, and the retinal pigment epithelium protecting the posterior segment, which form a continuous layer with the intervening ciliary body epithelium. While the iris and retinal pigment epithelia play similar roles in the anterior and posterior eye, respectively, they are phenotypically distinct cells with different molecular profiles, that interact differently with microorganisms. The ocular pigment epithelial cell line – ARPE-19 ( 13 ) – is susceptible to infection with EBOV when exposed to high titer ( 14 ), but this cell has a different phenotype and often behaves differently to primary cells ( 15 ), including in the setting of ocular infection ( 16 ).
To understand how EBOV infects the human eye, to cause uveitis, we prepared multiple donor-matched, phenotyped iris and retinal pigment epithelial cell isolates from human cadaveric eyes, and infected these isolates in parallel with EBOV. We measured susceptibility of the cells to infection by multiple qualitative and quantitative methods, and we also compared susceptibility to Reston virus (RESTV; Reston ebolavirus , another member of the Ebolavirus genus, but understood to be non-pathogenic in humans) and Zika virus (ZIKV, another single-stranded RNA virus that causes systemic disease and uveitis, but of the family, Flaviviridae ). This work represents the first effort to establish infectious mechanisms of EBOV in human ocular pigment epithelial cells.
2.1 Ocular Pigment Epithelial Cells
Donor-matched iris and retinal pigment epithelial cell isolates were prepared from paired human cadaver eyes, using methods we have previously published ( 10 , 11 ). In brief, irises and retinal pigment epithelium-choroid were dissected from the two posterior eyecups. Irises were digested in 0.25% trypsin (Thermo Fisher Scientific-Gibco, Grand Island, NY), and pigment epithelial cells were brushed from the digested tissue. Cells were plated in Epithelial Cell Medium (ScienCell Research Laboratories, Carlsbad, CA; catalogue number 4101, containing 2% FBS and penicillin-streptomycin). The retinal pigment epithelium-choroid was digested with 0.5 mg/mL collagenase IA and 0.5 mg/mL collagenase IV, scraped off in sheets, and collected by sucrose density gradient centrifugation. Cell sheets were plated in 50% Minimum Essential Medium Eagle alpha modification (with sodium bicarbonate) [MEM], 25% Dulbecco’s Modified Eagle Medium [DMEM] and 25% F-12 with 1x N1 Medium Supplement, 1x Non-Essential Amino Acids Solution, 1x GlutaMAX Supplement, 0.25 mg/mL taurine, 0.02 μg/mL hydrocortisone, 0.013 ng/mL 3,3’,5-triiodo-L-thyronine sodium, 100 U/mL Penicillin-Streptomycin (all obtained from Merck-Sigma Aldrich, St Louis, MO or Thermo Fisher Scientific-GIBCO) and 10% FBS (Bovogen Biologicals, Keilor East, Australia).
The pigment epithelial cells were expanded in plating medium supplemented with 2% FCS, refreshed twice a week, at 37°C and 5% CO 2 in air, and stored frozen in liquid nitrogen. Cell phenotype was verified for all cell isolates by immunocytochemical detection of cytokeratin-8, indicating epithelial lineage, and absence of α-smooth muscle actin, which is expressed during mesenchymal differentiation; expression of retinal pigment epithelial cell specific markers (i.e. cytokeratin-8, retinal pigment epithelium-specific protein 65 [RPE65] and zonula occludens 1 [ZO1]) were also assessed in those isolates (see ‘Cytoimmunofluorescence’). All cell isolates were demonstrated to be free of Mycoplasma species contamination by quantitative real-time polymerase chain reaction (qPCR) of DNA extracted from culture supernatant.
2.2 Viruses
The following viruses were used in this work: EBOV, variant Mayinga; RESTV, Philippines, 2008; and Zika virus (ZIKV), strain PRVABC59. These viruses were amplified in Vero C1008 cells (European Collection of Authenticated Cell Cultures [ECACC], Salisbury, UK), cultured with DMEM supplemented with 10% FBS at 37°C and 5% CO2 in air, and titrated by end-point dilution of culture supernatant in fresh Vero C1008 cell monolayers.
2.3 Viral Infection of Ocular Pigment Epithelial Cells
Ocular pigment epithelial cells suspended in Epithelial Cell Medium or supplemented 50% MEM/25% DMEM/25% F-12, both with 2% FBS, were plated at passage 2, in 6-well (growth area = 10 cm 2 ) or 24-well (growth area = 2 cm 2 ) multi-well plates, and incubated for 2 days at 37°C and 5% CO2 in air. Subconfluent cell monolayers were infected with EBOV, RESTV or ZIKV at a multiplicity of infection (MOI) of 1, or mock-infected, in minimum volumes of DMEM with 2% FBS (250 µL and 100 µL in 6-well or 24 well-plates, respectively). After 30-40 minutes, the medium was added back to standard volumes (2 ml and 1 ml in 6-well or 24 well-plates, respectively) with fresh medium. At intervals of 24, 48 and 72 hours, supernatant was collected and frozen at -80°C, and cells were either fixed or lysed with TRIzol Reagent (Thermo Fisher Scientific-Invitrogen, Carlsbad, CA), and stored at -80°C ahead of RNA extraction for reverse transcription (RT)-qPCR. At 48 hours, 24-well plates were fixed in 10% neutral buffered formalin for 48 hours, and stored at 4°C for cytoimmunofluorescence. All work with live virus was conducted under biosafety level 4 conditions, including the use of positive pressure personnel suits with segregated air supply.
2.4 Cytoimmunofluorescence
For cell phenotyping, 4% paraformaldehyde-fixed cell monolayers were labelled overnight at 4°C with one of following rabbit polyclonal antibodies diluted in 0.05% Triton X-100 and 2% bovine serum albumin in PBS: anti-human cytokeratin 8 (Abcam, Cambridge, United Kingdom; catalogue number ab53280; working dilution, 1:250, equivalent to 0.132 µg/mL), α-smooth muscle actin (Abcam, catalogue number ab5694; working dilution, 1:100, equivalent to 2 µg/mL) and rabbit immunoglobulin (Vector Laboratories, Burlingame, CA; catalogue number I-1000, working concentration, 2 µg/mL). Additional retinal pigment epithelial cell monolayers were labelled with: mouse anti-human RPE65 (Novus Biologicals, Centennial, CO; catalogue number NB100-355; working concentration, 4 µg/mL), rabbit anti-human ZO1 (Thermo Fisher Scientific-Invitrogen, catalogue number 40-2200; working dilution, 1:100, equivalent to 2.5 µg/mL) and mouse immunoglobulin (BD Pharmingen-BD Biosciences, San Diego, CA; catalogue number 555746, working concentration, 4 µg/mL). Cell monolayers were incubated with Alexa Fluor 488-tagged donkey anti-rabbit immunoglobulin antibody or anti-mouse immunoglobulin antibody (Thermo Fisher Scientific-Molecular Probes, Eugene, OR; catalogue numbers A11008 and A11029; working concentration, 1 µg/mL) for 1 hour at room temperature, counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and imaged by fluorescence microscopy at 200x magnification.
To demonstrate viral infection, 10% neutral buffered formalin-fixed virus-infected and uninfected cell monolayers were permeabilized with 0.1% Nonidet P-40 (Merck-Sigma Aldrich), and labelled overnight at room temperature with rabbit anti-ebolavirus nucleoprotein (NP) antiserum ( 17 ), diluted 1:2000 to detect the ebolaviruses, or mouse anti-double stranded (ds)RNA monoclonal antibody (SCICONS, Budapest, Hungary) at 5 µg/mL to detect ZIKV in phosphate buffered saline with 1% bovine serum albumin. Subsequently, monolayers were incubated with Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin or anti-mouse immunoglobulin antibody (Thermo Fisher Scientific-Molecular Probes, catalogue numbers A11008 and A11001; working concentration, 2 µg/mL) for 1 hour at room temperature, counterstained with DAPI, and imaged on the EVOS FL Cell Imaging System (Thermo Fisher Scientific-Invitrogen) at 10x magnification.
2.5 RNA Extraction and Reverse Transcription
RNA was extracted by TRIzol Reagent (Thermo Fisher Scientific-Invitrogen), according to the manufacturer’s instructions, and stored at -80°C. Concentration was measured by spectrophotometry using the Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE). For all samples, the cDNA synthesis reaction was performed using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories, Hercules, CA), with 500 ng of RNA input resulting in 20 μL of cDNA.
2.6 Ebolavirus Primers
Genomic sequences for EBOV and RESTV were obtained from the Nucleotide database of the US National Library of Medicine National Center for Biotechnology Information Nucleotide under the following accession identifiers: EBOV, AF086833.2; RESTV, AB050936.1. The sequences were aligned using the European Molecular Biology Laboratory-European Bioinformatics Institute Clustal Omega multiple sequence alignment web tool ( 18 ). Primers were designed that amplified 194 base pair (bp) of the ebolavirus NP transcript: forward 5’- TGGCAATCTGTCGGACAAATGATG-3’, reverse 5’- AGGATATGATCAAGGACGGTTTTGAC-3’. Primers included intentional mismatches (3 forward and 3 reverse) to ensure that transcript from the two viruses would be amplified with approximately equal efficiency: EBOV, 89.4%; RESTV, 92.8%. Products were sequenced to confirm amplification of the correct transcript.
2.7 Quantitative Real-Time Polymerase Chain Reaction
The qPCR was performed on the CFX Connect Real-Time PCR System (Bio-Rad Laboratories). In addition to SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) and nuclease-free water, each reaction contained 0.375 μM forward and 0.375 μM reverse primer, and 2 μL of undiluted cDNA. The ZIKV envelope (Env) primers were: forward 5’-GCTGGDGCRGACACHGGRACT-3’, reverse 5’-RTCYACYGCCATYTGGRCTG-3’ (304 bp amplicon, 76.5% amplification efficiency) ( 10 ). The GAPDH primers were: forward 5’-AGCTGAACGGGAAGCTCACTGG-3’, reverse 5’-GGAGTGGGTGTCGCTGTTGAAGTC-3’ (209 bp amplicon, 85.1% amplification efficiency) ( 19 ). Cycling conditions were as follows: pre-amplification hold of 95°C for 30 seconds; 45 cycles of denaturation at 95°C for 30 seconds, annealing at 54°C (NP) or 59°C (Env) for 30 seconds, extension; and fluorescence reading at 72°C for 30 seconds. Melting curves were performed from 70°C to 95°C for each run to confirm a single product. Absolute number of NP or Env transcripts was calculated from target starting quantity, which was determined from standard curves generated by serial dilution of purified PCR product in CFX Manager v3.0 (Bio-Rad Laboratories), from the formula: [target starting quantity (ng) x 6.022x10 23 ]/[product length (bp) x 10 9 x 660]. Each result was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript number in the same sample.
2.8 Measurement of Viral Titer
Confluent monolayers of Vero C1008 cells were incubated in triplicate with 10-fold serial dilutions of supernatant collected from virus-infected pigment epithelial cells. After 7 days, cells were fixed for 48 hours with 10% neutral buffered formalin and immunolabeled to detect infected cells, following the method described in ‘Cytoimmunofluorescence’. The 50% tissue culture infective dose (TCID50) was determined according to the method described by Reed and Muench ( 20 ).
2.9 Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). The Mann-Whitney U test was used to compare cellular viral load and supernatant TCID50 between iris and retinal pigment epithelial cells. For all tests, a statistically significant difference was defined by a p-value of less than 0.05.
2.10 Research Compliance
Use of human cadaver donor eyes from the Eye Bank of South Australia (Adelaide, Australia) for this research was approved by the Southern Adelaide Clinical Human Research Ethics Committee (protocol number: 175.13).
Ocular pigment epithelial cells were isolated separately from the posterior eyecups of 5 cadaveric donors (1 man and 4 women), whose ages at death ranged from 56 to 71 years (median = 64 years). Time from death to processing of the eyecups extended from 12 to 41 hours (median = 29 hours). Cytoimmunofluorescent labelling of all 5 paired cell isolates at the passage used for infections demonstrated strong expression cytokeratin-8 and no expression of α-smooth muscle actin, indicating an epithelial phenotype with no mesenchymal differentiation; in addition, all retinal cell isolates expressed the retinal pigment epithelial cell-specific proteins, RPE65 and ZO1 ( Figure 1A ).
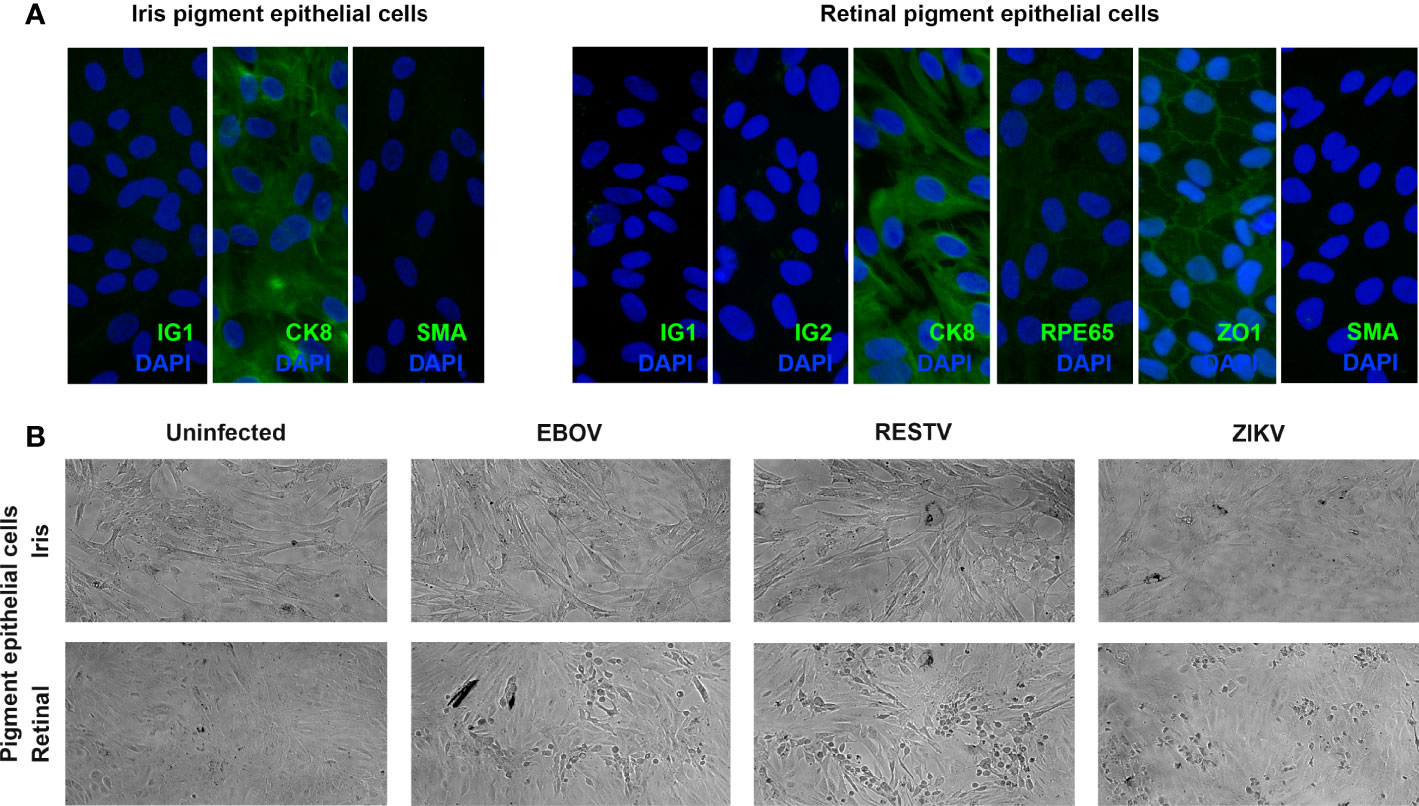
Figure 1 (A) Representative fluorescence photomicrographs of donor-matched human iris and retinal pigment epithelial cells immunolabelled to detect the presence of cytokeratin 8 (CK8) and absence of alpha-smooth muscle actin (SMA), plus human retinal pigment epithelial cells immunolabelled for retinal pigment epithelium-specific protein 65 (RPE65), and zonula occludens 1 (ZO1), with negative controls labelled with species-matched immunoglobulin (IG1 and IG2). Alexa Fluor 488 (green) with DAPI nuclear counterstain (blue). Original magnification: 400x. (B) Representative light photomicrographs of EBOV-, RESTV- and ZIKV-infected, plus uninfected donor-matched human iris and retinal pigment epithelial cell monolayers 72 hours following inoculation at a multiplicity of infection of 1. Original magnification: 10x.
To investigate the susceptibility of human ocular pigment epithelial cells to infection with EBOV, subconfluent donor-matched iris and retinal pigment epithelial cell monolayers were inoculated with virus at the MOI of 1 for intervals of 24, 48 and 72 hours. For comparison, additional cell monolayers from the same donors were inoculated in parallel with the non-pathogenic ebolavirus, RESTV, or the unrelated uveitogenic dsRNA virus, ZIKV, or incubated in parallel without inoculation. By 72 hours, virus-induced cytopathic effect was observed in retinal pigment epithelial cells inoculated with EBOV, as well as RESTV and ZIKV; this effect was not observed in the infected iris pigment epithelial cells ( Figure 1B ). This observation suggests that human retinal pigment epithelial cells are more susceptible to EBOV infection than iris pigment epithelial cells, and that a similar cell differential exists for other viruses.
Viral transcript in human ocular pigmented epithelial cells was quantified over time by RT-qPCR of total RNA extracted from the cell monolayers harvested at 24, 48 and 72 hours post-inoculation ( Figure 2 ). Additional cell monolayers were fixed at 48 hours and immunolabelled for viral antigen: NP for EBOV and RESTV, and dsRNA for ZIKV ( Figure 3 ). Level of viral transcript was similar across the different viruses for the same cell populations and time points; however, for each virus, transcript was higher in the retinal compared to the iris pigment epithelial cells across all time points (EBOV NP, p < 0.05 at 72 hours; RESTV NP and ZIKV Env, p < 0.05 at 48 hours). Immunolabelling of cell monolayers for viral antigen showed a clear difference in cell subset infection across all 5 paired human ocular cell isolates, with more infected cells in the retinal versus the iris pigment epithelial cell monolayers. These two results indicate that both human iris and retinal pigment epithelial cells are susceptible to infection with EBOV, as well as RESTV and ZIKV, but also suggest that retinal pigment epithelial cells are more readily infected.
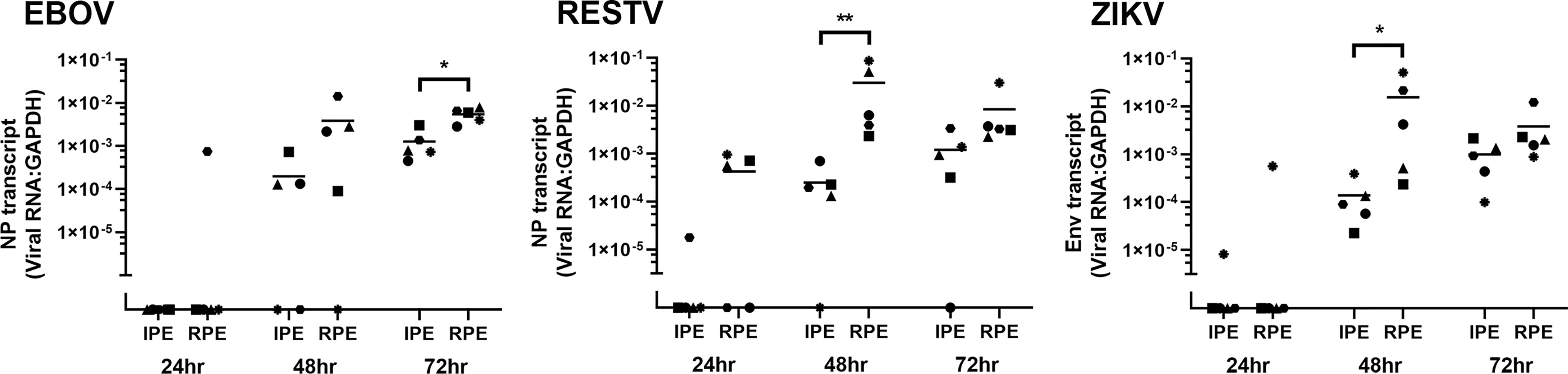
Figure 2 Graphs showing viral transcript in EBOV-, RESTV- and ZIKV-infected human iris (IPE) and retinal (RPE) pigment epithelial cell monolayers 24, 48 and 72 hours (hr) following inoculation at a multiplicity of infection of 1 (n = 5 donors/condition). NP = ebolavirus nucleoprotein; Env = ZIKV envelope. Shapes represent individual cell isolates, and crossbars represent means. Statistical comparisons were made between IPE and RPE by Mann-Whitney U test (* p < 0.05; ** p < 0.01).
Figure 3 Representative fluorescence photomicrographs of EBOV-, RESTV- and ZIKV-infected, plus uninfected donor-matched human iris and retinal pigment epithelial cell monolayers 48 hours following inoculation at a multiplicity of infection of 1 immunolabelled to detect ebolavirus nucleoprotein (EBOV and RESTV) or double-stranded RNA (ZIKV). Alexa Fluor 488 (green) with DAPI nuclear counterstain (blue). Original magnification: 10x.
To confirm these observations of differential human ocular pigment cell infectivity with EBOV by another method, viral titer in supernatant collected from the cell monolayers at 24, 48 and 72 hours was determined as TCID50 ( Figure 4 ). For infected cultures, the TCID50 increased across the time intervals, particularly for the retinal pigment epithelial cells, which released significantly more EBOV, plus RESTV and ZIKV, than the iris pigment epithelial cells by 48 hours (p < 0.01). Interestingly, the TCID50 for RESTV-infected pigment epithelial cells was higher than that for EBOV-infected cells across all time points, suggesting the cells have greater capacity to release infectious RESTV than EBOV up to 72 hours. For uninfected cultures, there was consistently no TCID 50 measured. Taken together, these findings demonstrate that human retinal pigment epithelial cells are relatively more susceptible to infection with EBOV than iris pigment epithelial cells, and that this susceptibility extends to other viruses, here RESTV and ZIKV.
Figure 4 Graphs showing the 50% tissue culture infective dose (TCID50) in culture supernatant collected from EBOV-, RESTV- and ZIKV-infected human iris (IPE) and retinal (RPE) pigment epithelial cell monolayers 24, 48 and 72 hours (hr) following inoculation at a multiplicity of infection of 1 (n = 5 donors/condition). Shapes represent individual cell isolates, and crossbars represent means. Statistical comparisons were made between IPE and RPE by Mann-Whitney U test (** p < 0.01).
4 Discussion
Up to one-third of Ebola survivors will develop uveitis, associated with the persistence of live EBOV inside the eye. The ocular pigment epithelial cells are key players in ocular inflammation, and our work represents the first effort to examine the susceptibility of primary human ocular pigment epithelial cells to EBOV infection. Using donor-matched human iris and retinal pigment epithelial cell isolates and EBOV, variant Mayinga, we observed that both cell populations were permissive to infection, but that retinal pigment epithelial cells were substantially more susceptible. In comparison to pigment epithelial cells isolated from the iris, those from the retina demonstrated obvious viral-induced cytopathic effect, had higher intracellular viral transcript and more widely expressed protein, and released the virus at higher titer as the infection progressed. Our study design, with low MOI, was chosen to establish differences in infectivity of these two cell populations, but host cell responses to infection – including production of inflammatory, immunomodulatory and anti-viral cytokines and chemokines – would be of interest and could be addressed in future studies.
In order to access the eye from the blood stream, EBOV must interact with the blood-aqueous barrier or the blood-retinal barrier, placing the virus in contact with the iris pigment epithelium or the retinal pigment epithelium, respectively. The difference in susceptibility of these two cell populations to infection could reflect differences in effectiveness of EBOV entry into, replication within and/or exit from the cells. Overall, EBOV is able to enter a broad range of cells, as its surface protein, glycoprotein (GP)1, interacts with diverse, common cell surface proteins that include lectins, glycosaminoglycans, integrins, receptor tyrosine kinases, folate receptor 1, and T-cell immunoglobulin and mucin domain 1 ( 21 ). Once inside the host cell, EBOV uses cellular transcriptional, translational and post-transcriptional machinery during viral replication, and hijacks the host endosomal sorting complexes required for transport (ESCRT) pathway in order to bud from the cell membrane ( 21 ). Despite molecular promiscuity, differential infectivity by EBOV has been demonstrated amongst leukocyte subsets ( 22 ).
Another explanation for the relative susceptibility of retinal pigment epithelial cells to infection with EBOV may relate to their specialized cellular phenotype. Retinal pigment epithelial cells are considered an epithelial-derived subset of tissue-resident phagocytes ( 23 ). They mediate turn-over of photoreceptor outer segments by phagocytosis, which is essential for vision. However, retinal pigment epithelial cells may ingest other materials including apoptotic cells and microbial antigens ( 24 , 25 ). For example, retinal pigment epithelial cells phagocytose Mycobacterium tuberculosis and permit intracellular replication ( 26 ). The fact that we saw similar differences for two comparison viruses – the closely related ebolavirus strain that is understood to be non-pathogenic in humans, and has a modified and less active GP1, RESTV ( 27 ), and the unrelated single-stranded virus that causes infectious disease and uveitis, ZIKV ( 10 ) – supports this possibility.
Across multiple cohort studies, there are reports of Ebola survivors with retinal scars ( 4 , 28 , 29 ) that by optical coherence tomography involve the outer neural retina and extend to the retinal pigment epithelium ( 29 ). The characteristic appearance of hyperpigmentation with a hypopigmented halo also is consistent with retinal pigment epithelial involvement. Of strong relevance, during a routine eye examination prior to the onset of uveitis, a patient whose clinical course has been described in considerable detail, was found to have retinal pigment epithelial scars; during uveitis extremely high levels of intraocular virus were detected in the affected eye ( 30 ). These findings all would be consistent with a high susceptibility of human retinal pigment epithelial cells to infection, suggesting the disease starts in these cells. Multiple types of post-Ebola uveitis have been reported, including anterior (based at the iris and ciliary body), intermediate (based in the vitreous), posterior (based at the choroid and/or retina) and panuveitis, with relative frequency of these different forms varying across studies by different groups ( 4 , 28 , 29 ); in the largest reported group of 564 Ebola survivors in Liberia (the PREVAIL III longitudinal cohort study), there was a slight predominance of posterior uveitis ( 29 ).
Our research has some limitations. The study necessarily involved in vitro infections of human ocular pigment epithelial cells. There is always potential for phenotypic drift in cultured cells, and retinal pigment epithelial cells in particular are prone to differentiation ( 31 ); thus, we used cells in earliest possible passage, and we confirmed the phenotype with cytoimmunofluorescence. We addressed inter-individual differences by studying isolates from multiple eyes, and using donor-matched iris and retinal pigment epithelial cells. We limited the comparison to iris and retinal pigment epithelial cells, and did not also study ciliary body epithelial cells; while one can readily separately identify iris and retina, given that the boundaries between iris and ciliary body, and ciliary body and retina are blurred, it is difficult to be certain of pure cell populations ( 32 ). Infections were not carried beyond 72 hours, when a virus-induced cytopathic effect in retinal pigment epithelial cells was observed; the infection may continue to progress in iris pigment epithelial cells past this time, to achieve higher intracellular expression of viral RNA and increased release of infectious virus. In the 2014 Ebola outbreak in West Africa, the Makona EBOV strain predominated, and we worked with the Mayinga strain, which was isolated during the 1976 Ebola outbreak in the Democratic Republic of Congo. Although unrelated research with dengue virus shows viral strain may impact ocular pathology ( 12 ), and EBOV strain-specific differences have not been studied, uveitis has been reported in both Congolese and West African Ebola survivors ( 4 , 33 ).
In summary, our work has showed human retinal pigment epithelial cells to be relatively susceptible to infection with EBOV. This suggests that they may be a primary target within the eye, and also suggests that they could potentially be monitored during acute infection to identify patients at highest risk of uveitis: ophthalmic imaging modalities such as ‘fundus autofluorescence’ that demonstrates retinal pigment epithelial activity, which may not be visible clinically ( 34 ), would be particularly valuable in this context.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
The use of human cadaver donor eyes for this research was approved by the Southern Adelaide Clinical Human Research Ethics Committee.
Author Contributions
SY, GM and JS conceptualized the study. ST, YM, LA, BA, MM and TB developed the methodology. ST, YM, LA and JS wrote the original draft. BA, MM, TB and SY reviewed and edited the manuscript. GM and JS supervised the study. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the National Health & Medical Research Council (GNT1139857 to JS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank Ms. Janet Matthews for administrative support in preparing this manuscript.
1. Burki TK. Post-Ebola Syndrome. Lancet Infect Dis (2016) 16:780–1. doi: 10.1016/S1473-3099(15)00259-5
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Jacob ST, Crozier I, Fischer WA 2nd, Hewlett A, Kraft CS, Vega MA, et al. Ebola Virus Disease. Nat Rev Dis Primers. (2020) 6:13. doi: 10.1038/s41572-020-0147-3
3. PREVAIL III Study Group, Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, et al. A Longitudinal Study of Ebola Sequelae in Liberia. N Engl J Med (2019) 380:924–34. doi: 10.1056/NEJMoa1805435
4. Shantha JG, Crozier I, Hayek BR, Bruce BB, Gargu C, Brown J, et al. Ophthalmic Manifestations and Causes of Vision Impairment in Ebola Virus Disease Survivors in Monrovia, Liberia. Ophthalmology. (2017) 124:170–7. doi: 10.1016/j.ophtha.2016.10.011
5. Freddo TF. A Contemporary Concept of the Blood-Aqueous Barrier. Prog Retin Eye Res (2013) 32:181–95. doi: 10.1016/j.preteyeres.2012.10.004
6. O'Leary F, Campbell M. The Blood Retina Barrier in Health and Disease. FEBS J (2021). doi: 10.1111/febs.16330
7. Mochizuki M, Sugita S, Kamoi K. Immunological Homeostasis of the Eye. Prog Retin Eye Res (2013) 33:10–27. doi: 10.1016/j.preteyeres.2012.10.002
8. Chui JJ, Li MW, Di Girolamo N, Chang JH, McCluskey PJ, Wakefield D. Iris Pigment Epithelial Cells Express a Functional Lipopolysaccharide Receptor Complex. Invest Ophthalmol Vis Sci (2010) 51:2558–67. doi: 10.1167/iovs.09-3923
9. Fukuoka Y, Strainic M, Medof ME. Differential Cytokine Expression of Human Retinal Pigment Epithelial Cells in Response to Stimulation by C5a. Clin Exp Immunol (2003) 131:248–53. doi: 10.1046/j.1365-2249.2003.02087.x
10. Ryan FJ, Carr JM, Furtado JM, Ma Y, Ashander LM, Simoes M, et al. Zika Virus Infection of Human Iris Pigment Epithelial Cells. Front Immunol (2021) 12:644153. doi: 10.3389/fimmu.2021.644153
11. Lie S, Rochet E, Segerdell E, Ma Y, Ashander LM, Shadforth AMA, et al. Immunological Molecular Responses of Human Retinal Pigment Epithelial Cells to Infection With Toxoplasma Gondii. Front Immunol (2019) 10:708. doi: 10.3389/fimmu.2019.00708
12. Ashander LM, Lumsden AL, Dawson AC, Ma Y, Ferreira LB, Oliver GF, et al. Infection of Human Retinal Pigment Epithelial Cells With Dengue Virus Strains Isolated During Outbreaks in Singapore. Microorganisms (2022) 10(2):310. doi: 10.3390/microorganisms10020310
13. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a Human Retinal Pigment Epithelial Cell Line With Differentiated Properties. Exp Eye Res (1996) 62:155–69. doi: 10.1006/exer.1996.0020
14. Smith JR, Todd S, Ashander LM, Charitou T, Ma Y, Yeh S, et al. Retinal Pigment Epithelial Cells are a Potential Reservoir for Ebola Virus in the Human Eye. Transl Vis Sci Technol (2017) 6:12. doi: 10.1167/tvst.6.4.12
15. Strunnikova NV, Maminishkis A, Barb JJ, Wang F, Zhi C, Sergeev Y, et al. Transcriptome Analysis and Molecular Signature of Human Retinal Pigment Epithelium. Hum Mol Genet (2010) 19:2468–86. doi: 10.1093/hmg/ddq129
16. Smith JR, Ashander LM, Arruda SL, Cordeiro CA, Lie S, Rochet E, et al. Pathogenesis of Ocular Toxoplasmosis. Prog Retin Eye Res (2021) 81:100882. doi: 10.1016/j.preteyeres.2020.100882
17. Marsh GA, Haining J, Robinson R, Foord A, Yamada M, Barr JA, et al. Ebola Reston Virus Infection of Pigs: Clinical Significance and Transmission Potential. J Infect Dis (2011) 204 Suppl 3:S804–9. doi: 10.1093/infdis/jir300
18. Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI Search and Sequence Analysis Tools APIs in 2019. Nucleic Acids Res (2019) 47(W1):W636–41. doi: 10.1093/nar/gkz268
19. Silverman MD, Zamora DO, Pan Y, Texeira PV, Baek SH, Planck SR, et al. Constitutive and Inflammatory Mediator-Regulated Fractalkine Expression in Human Ocular Tissues and Cultured Cells. Invest Ophthalmol Vis Sci (2003) 44:1608–15. doi: 10.1167/iovs.02-0233
20. Reed LJ, Muench LH. A Simple Method of Estimating Fifty Percent Endpoints. Am J Hyg (1938) 27:493–7. doi: 10.1093/oxfordjournals.aje.a118408
CrossRef Full Text | Google Scholar
21. Rojas M, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramirez-Santana C, Ansari AA, et al. Ebola Virus Disease: An Emerging and Re-Emerging Viral Threat. J Autoimmun (2020) 106:102375. doi: 10.1016/j.jaut.2019.102375
22. Kotliar D, Lin AE, Logue J, Hughes TK, Khoury NM, Raju SS, et al. Single-Cell Profiling of Ebola Virus Disease In Vivo Reveals Viral and Host Dynamics. Cell. (2020) 183:1383–401 e19. doi: 10.1016/j.cell.2020.10.002
23. Kwon W, Freeman SA. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front Immunol (2020) 11:604205. doi: 10.3389/fimmu.2020.604205
24. Mayerson PL, Hall MO. Rat Retinal Pigment Epithelial Cells Show Specificity of Phagocytosis In Vitro . J Cell Biol (1986) 103:299–308. doi: 10.1083/jcb.103.1.299
25. Finnemann SC, Rodriguez-Boulan E. Macrophage and Retinal Pigment Epithelium Phagocytosis: Apoptotic Cells and Photoreceptors Compete for Alphavbeta3 and Alphavbeta5 Integrins, and Protein Kinase C Regulates Alphavbeta5 Binding and Cytoskeletal Linkage. J Exp Med (1999) 190:861–74. doi: 10.1084/jem.190.6.861
26. Nazari H, Karakousis PC, Rao NA. Replication of Mycobacterium Tuberculosis in Retinal Pigment Epithelium. JAMA Ophthalmol (2014) 132:724–9. doi: 10.1001/jamaophthalmol.2014.270
27. Fujihira H, Usami K, Matsuno K, Takeuchi H, Denda-Nagai K, Furukawa JI, et al. A Critical Domain of Ebolavirus Envelope Glycoprotein Determines Glycoform and Infectivity. Sci Rep (2018) 8:5495. doi: 10.1038/s41598-018-23357-8
28. Hereth-Hebert E, Bah MO, Etard JF, Sow MS, Resnikoff S, Fardeau C, et al. Ocular Complications in Survivors of the Ebola Outbreak in Guinea. Am J Ophthalmol (2017) 175:114–21. doi: 10.1016/j.ajo.2016.12.005
29. Eghrari AO, Bishop RJ, Ross RD, Davis B, Larbelee J, Amegashie F, et al. Characterization of Ebola Virus-Associated Eye Disease. JAMA Netw Open (2021) 4:e2032216. doi: 10.1001/jamanetworkopen.2020.32216
30. Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola Virus in Ocular Fluid During Convalescence. N Engl J Med (2015) 372:2423–7. doi: 10.1056/NEJMoa1500306
31. Fronk AH, Vargis E. Methods for Culturing Retinal Pigment Epithelial Cells: A Review of Current Protocols and Future Recommendations. J Tissue Eng. (2016) 7:2041731416650838. doi: 10.1177/2041731416650838
32. Janssen SF, Gorgels TG, Bossers K, Ten Brink JB, Essing AH, Nagtegaal M, et al. Gene Expression and Functional Annotation of the Human Ciliary Body Epithelia. PloS One (2012) 7:e44973. doi: 10.1371/journal.pone.0044973
33. Kibadi K, Mupapa K, Kuvula K, Massamba M, Ndaberey D, Muyembe-Tamfum JJ, et al. Late Ophthalmologic Manifestations in Survivors of the 1995 Ebola Virus Epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis (1999) 179(Suppl 1):S13–4. doi: 10.1086/514288
34. Reznicek L, Seidensticker F, Stumpf C, Kampik A, Thurau S, Kernt M, et al. Systematic Analysis of Wide-Field Fundus Autofluorescence (FAF) Imaging in Posterior Uveitis. Curr Eye Res (2014) 39:164–71. doi: 10.3109/02713683.2013.834938
Keywords: ebola virus, human, retina, iris, pigment epithelium, uveitis
Citation: Todd S, Ma Y, Ashander LM, Appukuttan B, Michael MZ, Blenkinsop TA, Yeh S, Marsh GA and Smith JR (2022) Brief Research Report: Ebola Virus Differentially Infects Human Iris and Retinal Pigment Epithelial Cells. Front.Virol. 2:892394. doi: 10.3389/fviro.2022.892394
Received: 09 March 2022; Accepted: 23 May 2022; Published: 16 June 2022.
Reviewed by:
Copyright © 2022 Todd, Ma, Ashander, Appukuttan, Michael, Blenkinsop, Yeh, Marsh and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justine R. Smith, [email protected]
† These authors have contributed equally to this work and share first authorship
‡ These authors have contributed equally to this work and share senior authorship
This article is part of the Research Topic
Women In Modeling of Viral Replication and Pathogenesis:2021
Genetic discoveries in mice shed light on vulnerability to Ebola virus disease
May 10, 2024
The virus that causes Ebola virus disease (EVD) has seen rare but repeated outbreaks , particularly in African countries. While most outbreaks are limited, the risk of dying from contracting Ebola virus is around 50% on average, making any occurrence of the disease an urgent public health concern.
Data from other outbreaks, like SARS and COVID-19, have helped scientists understand the role that genetics play in susceptibility to viral infections. Researchers at UNC-Chapel Hill have been studying the effects of Ebola virus in a special breed of mice that shares similarities with humans in their viral response patterns and health outcomes.
Graphic abstract: Mapping of susceptibility loci for Ebola virus pathogenesis in mice
In collaboration with researchers at the National Institutes of Health (NIH), this team of researchers have published a new study in Cell Reports identifying two specific genetic regions in these mice that are vulnerable to Ebola virus’ most deadly effects.
These genetic vulnerabilities, found in chromosomes eight and seven, are linked to high amounts of virus in the blood and severe inflammation, particularly in the liver.
“We know the liver is one of the principal target organs for Ebola virus infection,” said Alexandra Schaefer, PhD , who is assistant professor of epidemiology at the UNC Gillings School of Global Public Health. While the virus is transmitted by contact with infected bodily fluids, the liver becomes a dominant site for virus replication and inflammation. In some individuals, this overactive immune response can oftentimes be fatal. “The liver is pretty much where everything starts and ends with Ebola.”

Dr. Alexandra Schaefer

Dr. Ralph Baric
Schaefer co-authored the study along with epidemiologists, virologists and geneticists from across Carolina and NIH as part of ongoing research on Ebola led by the lab of Ralph S. Baric, PhD , William R. Kenan, Jr. Distinguished Professor in the Department of Epidemiology at the Gillings School and professor in the Department of Microbiology and Immunology at the UNC School of Medicine.
Baric noted that “Alex’s work has revealed an entirely new understanding of how Ebola virus causes lethal infections, providing new opportunities for diagnostics, therapeutic intervention and control.”
The lab has, for years, been seeking potential treatment strategies for dangerous viral threats by mapping how natural variation in host genes regulates disease severity in mammals using collaborative cross (CC) mice . These innovative mice were bred specifically to resemble the genetic diversity of humans in the hopes of mapping common mammalian genes that influence severe viral disease outcomes. By identifying these host genes, new strategies can be developed to counter disease severity.
In this new study, researchers found one host vulnerability in chromosome eight, which regulates viral load, or the amount of virus in the blood. Higher amounts of viral load typically indicate the progression of an infection.
The team also found a second host vulnerability in chromosome seven, where the presence of a functional genetic region (locus) called TRIM5 was linked to severe liver inflammation, weight loss and eventual death. In this instance, individuals with defective TRIM5 genes are protected from severe disease.
“TRIMs are innate immune genes,” Schaefer explained. “They have a big function in how cells respond to control virus infection and replication.”
Many TRIMs activate critical functions in the body that fight viral infections. However, the researchers found that in the case of Ebola virus infection, the presence of a functional TRIM5 locus actually led to worse outcomes and eventual death because the virus provoked an overactive immune response called a cytokine storm, causing massive cell death, inflammation and liver failure.
The genetic responses the lab observed in CC mice infected with Ebola virus were similar to genetic responses previously observed in those infected with the viruses that cause SARS (SARS-CoV ) and COVID-19 (SARS-CoV-2) . Such findings eventually played a role in the treatment and vaccine development of both diseases.
While very little data is currently available on the biomarkers that identify humans who could be susceptible to lethal Ebola infections, the research team is hopeful that these new results in mice will be similarly valuable in the quest to find effective treatment and prevention strategies.
Schaefer says that because so much of Ebola’s damage is concentrated in the liver, their next goal is to investigate the exact mechanism that TRIM5 genes use to cause liver failure and lethal disease.
“[TRIM5] is a protein that interacts with other proteins in cells to respond effectively to most viral infections. Ebola virus has figured out a strategy to use this basic host defense strategy and subvert it. Understanding the mechanism that drives disease,” she said, “will provide opportunities to do something about it.”
Contact the UNC Gillings School of Global Public Health communications team at [email protected] .
Use this form to submit news, events and announcements to be shared via our newsletter and digital screens.
View and download the visual elements associated with the Gillings School.
For the use of our faculty, staff and students, the School offers the following PowerPoint template, which can be modified as needed.
This form allows faculty and staff to create a new web profile or update a current one.
This form enables Gillings School representatives to submit requests for website edits.
Gillings School’s 84th commencement celebrates Class of 2024
Information for:.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest.

Tracing largely forgotten history of major community

Roger Ware Brockett, 84

Providing community support
So how do you track spread of disease by the numbers.

Kris Snibbe/Harvard Staff Photographer
Anne J. Manning
Harvard Staff Writer
Ivan Specht decided to employ his love of math during pandemic, which led to contact-tracing app, papers, future path
Part of the commencement 2024 series.
A collection of stories covering Harvard University’s 373rd Commencement.
Ivan Specht started at Harvard on track to study pure mathematics. But when COVID-19 sent everyone home, he began wishing the math he was doing had more relevance to what was happening in the world.
Specht, a New York City native, expanded his coursework, arming himself with statistical modeling classes, and began to “fiddle around” with simulating ways diseases spread through populations. He got hooked. During the pandemic, he became one of only two undergraduates to serve on Harvard’s testing and tracing committee, eventually developing a prototype contact-tracing app called CrimsonShield.
Specht took his curiosity for understanding disease propagation to the lab of computational geneticist Pardis Sabeti , professor in Organismic and Evolutionary Biology at Harvard and member of the Broad Institute, known for her work sequencing the Ebola virus in 2014 . Specht, now a senior, has since co-authored several studies around new statistical methods for analyzing the spread of infectious diseases, with plans to continue that work in graduate school.
“Ivan is absolutely brilliant and a joy to work with, and his research accomplishments already as an undergraduate are simply astounding,” Sabeti said. “He is operating at the level of a seasoned postdoc.”
His senior thesis, “Reconstructing Viral Epidemics: A Random Tree Approach,” described a statistical model aimed at tackling one of the most intractable problems that plague infectious disease researchers: determining who transmitted a given pathogen to whom during a viral outbreak. Specht was co-advised by computer science Professor Michael Mitzenmacher, who guided the statistical and computational sections of his thesis, particularly in deriving genomic frequencies within a host using probabilistic methods.
Specht said the pandemic made clear that testing technology could provide valuable information about who got sick, and even what genetic variant of a pathogen made them sick. But mapping paths of transmission was much more challenging because that process was completely invisible. Such information, however, could provide crucial new details into how and where transmission occurred and be used to test things such as vaccine efficacy or the effects of closing schools.
Specht’s work exploited the fact that viruses leave clues about their transmission path in their phylogenetic trees, or lines of evolutionary descent from a common ancestor. “It turns out that genome sequences of viruses provide key insight into that underlying network,” said the joint mathematics and statistics concentrator.
Uncovering this transmission network goes to the heart of how single-stranded RNA pathogens survive: Once they infect their host, they mutate, producing variants that are marked by slightly different genetic barcodes. Specht’s statistical model determines how the virus spreads by tracking the frequencies of different viral variants observed within a host.
As the centerpiece of his thesis, he reconstructed a dataset of about 45,000 SARS-CoV-2 genomes across Massachusetts, providing insights into how outbreaks unfolded across the state.
Specht will take his passion for epidemiological modeling to graduate school at Stanford University, with an eye toward helping both researchers and communities understand and respond to public health crises.
A graphic designer with experience in scientific data visualization, Specht is focused not only on understanding outbreaks, but also creating clear illustrations of them. For example, his thesis contains a creative visual representation of those 45,000 Massachusetts genomes, with colored dots representing cases, positioned nearby other “dots” they are likely to have infected.
Specht’s interest in graphics began in middle school when, as an enthusiast of trains and mass transit, he started designing imagined subway maps for cities that lack actual subways, like Austin, Texas . At Harvard, he designed an interactive “subway map” depicting a viral outbreak.
As a member of the Sabeti lab, Specht taught an infectious disease modeling course to master’s and Ph.D. students at University of Sierra Leone last summer. His outbreak analysis tool is also now being used in an ongoing study of Lassa fever in that region. And he co-authored two chapters of a textbook on outbreak science in collaboration with the Moore Foundation.
Over the past three years, Specht has been lead author of a paper in Scientific Reports and another in Cell Patterns , and co-author on two others, including a cover story in Cell . His first lead-author paper, “The case for altruism in institutional diagnostic testing,” showed that organizations like Harvard should allocate COVID-19 testing capacity to their surrounding communities, rather than monopolize it for themselves. That work was featured in The New York Times .
During his time at Harvard, Specht lived in Quincy House and was design editor of the Harvard Advocate, the University’s undergraduate literary magazine. In his free time he also composes music, and he still considers himself a mass transit enthusiast.
In the acknowledgements section of his thesis, he credited Sabeti with opening his eyes to the “many fascinating problems at the intersection of math, statistics, and computational biology.”
“I could fill this entire thesis with reasons I am grateful for Professor Sabeti, but I think they can be summarized by the sense of wonder and inspiration I feel every time I set foot in her lab.”
Share this article
You might like.
Julia Tellides explored shifts, upheavals of Thessaloniki between two wars

Memorial Minute — Faculty of Arts and Sciences

Harvard Allston Partnership Fund awards grants to 26 Allston-Brighton nonprofits
Epic science inside a cubic millimeter of brain
Researchers publish largest-ever dataset of neural connections
Excited about new diet drug? This procedure seems better choice.
Study finds minimally invasive treatment more cost-effective over time, brings greater weight loss
How far has COVID set back students?
An economist, a policy expert, and a teacher explain why learning losses are worse than many parents realize

- Work With Us
South Dakota follows CDC guidance for monitoring persons with potential Ebola virus exposure . There have been no cases of Ebola in South Dakota.
Centers for Disease Control and Prevention Resources
- Countries with active Ebola transmission
- Ebola Virus Disease
- Posters and Fact Sheets
- Information for Healthcare Workers and Settings
Personal Protective Equipment (PPE) Resources
- South Dakota Department of Health PPE Checklists
- N95 and Coverall Checklists: Donning | Doffing
- N95 and Gown Checklists: Donning | Doffing
- PPAR and Coverall Checklists: Donning | Doffing
- PPAR and Gown Checklists: Donning | Doffing
- Web-based Training for Putting On and Removing Personal Protective Equipment (PPE) - CDC, Johns Hopkins Medicine, Salesforce Foundation, Miami University, Association for Professionals in Infection Control and Epidemiology, Society for Healthcare Epidemiology of America
Additional Resources
- Waste Management and Private Property Cleanup - South Dakota Department of Environment and Natural Resources
- Disinfectants for Use Against the Ebola Virus - Environmental Protection Agency
- Information for Hospitals and Critical Access Hospitals Concerning Possible Ebola Virus Disease - Center for Medicare and Medicaid Services
Discover Ebola disease and its causes. FDA-approved ERVEBO® vaccine provides safe and effective prevention against Zaire ebolavirus.
Advertisement
Supported by
U.S. Tightens Rules on Risky Virus Research
A long-awaited new policy broadens the type of regulated viruses, bacteria, fungi and toxins, including those that could threaten crops and livestock.
- Share full article

By Carl Zimmer and Benjamin Mueller
The White House has unveiled tighter rules for research on potentially dangerous microbes and toxins, in an effort to stave off laboratory accidents that could unleash a pandemic.
The new policy, published Monday evening, arrives after years of deliberations by an expert panel and a charged public debate over whether Covid arose from an animal market or a laboratory in China.
A number of researchers worried that the government had been too lax about lab safety in the past, with some even calling for the creation of an independent agency to make decisions about risky experiments that could allow viruses, bacteria or fungi to spread quickly between people or become more deadly. But others warned against creating restrictive rules that would stifle valuable research without making people safer.
The debate grew sharper during the pandemic, as politicians raised questions about the origin of Covid. Those who suggested it came from a lab raised concerns about studies that tweaked pathogens to make them more dangerous — sometimes known as “gain of function” research.
The new policy, which applies to research funded by the federal government, strengthens the government’s oversight by replacing a short list of dangerous pathogens with broad categories into which more pathogens might fall. The policy pays attention not only to human pathogens, but also those that could threaten crops and livestock. And it provides more details about the kinds of experiments that would draw the attention of government regulators.
The rules will take effect in a year, giving government agencies and departments time to update their guidance to meet the new requirements.
“It’s a big and important step forward,” said Dr. Tom Inglesby, the director of the Johns Hopkins Center for Health Security and a longtime proponent of stricter safety regulations. “I think this policy is what any reasonable member of the public would expect is in place in terms of oversight of the world’s most transmissible and lethal organisms.”
Still, the policy does not embrace the most aggressive proposals made by lab safety proponents, such as creating an independent regulatory agency. It also makes exemptions for certain types of research, including disease surveillance and vaccine development. And some parts of the policy are recommendations rather than government-enforced requirements.
“It’s a moderate shift in policy, with a number of more significant signals about how the White House expects the issue to be treated moving forward,” said Nicholas Evans, an ethicist at University of Massachusetts Lowell.
Experts have been waiting for the policy for more than a year. Still, some said they were surprised that it came out at such a politically fraught moment . “I wasn’t expecting anything, especially in an election year,” Dr. Evans said. “I’m pleasantly surprised.”
Under the new policy, scientists who want to carry out experiments will need to run their proposals past their universities or research institutions, which will to determine if the work poses a risk. Potentially dangerous proposals will then be reviewed by government agencies. The most scrutiny will go to experiments that could result in the most dangerous outcomes, such as those tweaking pathogens that could start a pandemic.
In a guidance document , the White House provided examples of research that would be expected to come under such scrutiny. In one case, they envisioned scientists trying to understand the evolutionary steps a pathogen needed to transmit more easily between humans. The researchers might try to produce a transmissible strain to study, for example, by repeatedly infecting human cells in petri dishes, allowing the pathogens to evolve more efficient ways to enter the cells.
Scientists who do not follow the new policy could become ineligible for federal funding for their work. Their entire institution may have its support for life science research cut off as well.
One of the weaknesses of existing policies is that they only apply to funding given out by the federal government. But for years , the National Institutes of Health and other government agencies have struggled with stagnant funding, leading some researchers to turn instead to private sources. In recent years, for example, crypto titans have poured money into pandemic prevention research.
The new policy does not give the government direct regulation of privately funded research. But it does say that research institutions that receive any federal money for life-science research should apply a similar oversight to scientists doing research with support from outside the government.
“This effectively limits them, as the N.I.H. does a lot of work everywhere in the world,” Dr. Evans said.
The new policy takes into account the advances in biotechnology that could lead to new risks. When pathogens become extinct, for example, they can be resurrected by recreating their genomes. Research on extinct pathogens will draw the highest levels of scrutiny.
Dr. Evans also noted that the new rules emphasize the risk that lab research can have on plants and animals. In the 20th century, the United States and Russia both carried out extensive research on crop-destroying pathogens such as wheat-killing fungi as part of their biological weapons programs. “It’s significant as a signal the White House is sending,” Dr. Evans said.
Marc Lipsitch, an epidemiologist at Harvard and a longtime critic of the government’s policy, gave the new one a grade of A minus. “I think it’s a lot clearer and more specific in many ways than the old guidance,” he said. But he was disappointed that the government will not provide detailed information to the public about the risky research it evaluates. “The transparency is far from transparent,” he said.
Scientists who have warned of the dangers of impeding useful virus research were also largely optimistic about the new rules.
Gigi Gronvall, a biosafety specialist at the Johns Hopkins Bloomberg School of Public Health, said the policy’s success would depend on how federal health officials interpreted it, but applauded the way it recognized the value of research needed during a crisis, such as the current bird flu outbreak .
“I was cautiously optimistic in reading through it,” she said of the policy. “It seems like the orientation is for it to be thoughtfully implemented so it doesn’t have a chilling effect on needed research.”
Anice Lowen, an influenza virologist at Emory University, said the expanded scope of the new policy was “reasonable.” She said, for instance, that the decision not to create an entirely new review body helped to alleviate concerns about how unwieldy the process might become.
Still, she said, ambiguities in the instructions for assessing risks in certain experiments made it difficult to know how different university and health officials would police them.
“I think there will be more reviews carried out, and more research will be slowed down because of it,” she said.
Carl Zimmer covers news about science for The Times and writes the Origins column . More about Carl Zimmer
Benjamin Mueller reports on health and medicine. He was previously a U.K. correspondent in London and a police reporter in New York. More about Benjamin Mueller

Chinese scientists create mutant Ebola virus that killed hamsters to 'avoid biosafety rules'
Scientists in China created a mutant Ebola virus in a lab that killed hamsters to "avoid biosafety rules".
Researchers at Hebei Medical University combined a contagious livestock disease and a protein found in Ebola virus. The protein allows the virus to infect cells and spread throughout the human body.
The virus was injected into a group of hamsters who “developed severe systematic diseases similar to those observed in human Ebola patients", including “multi-organ failure,” according to the study.
- Monkeypox warning as New York City sees huge spike in cases with 52 in January alone
- Bird flu outbreak 'poses pandemic potential' as fears of human infection surge with CDC offering stern warning
The team studied five female and five male hamsters that were all three weeks old. The infected hamsters suffered secretions in their eyes, which impaired their vision and scabbed over the surface of the eyeballs. All but two hamsters died within two to three days of the lethal injection.
The infected hamsters could be a model for studying the spread and treatment of Ebola in the future as there are strict Biosafety regulations around Ebola research. It must be handled in a Level 4 facility, which involves a higher level of security than a typical lab.
The last major outbreak of the Ebola virus occurred between 2014 and 2015, in several West African countries, according to a report from the World Health Organization. During those two years, more than 28,600 people were reportedly infected, and about 11,300 died.
For the latest local news and features on Irish America, visit our homepage here .


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Fact Sheet: USDA, HHS Announce New Actions to Reduce Impact and Spread of H5N1
Contact: [email protected] | [email protected]
On March 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle region, USDA and HHS began their work to understand the origin of the emergence and its potential impact in bovines and humans. USDA experts also took swift action to trace animal movements, began sampling to assess the disease prevalence in herds, and initiated a variety of testing activities to confirm the safety of the meat and milk supplies alongside federal partners. On April 1, 2024, Texas reported the first and only confirmed human H5N1 infection associated with this outbreak, after confirmation by CDC. On April 24, 2024, USDA issued a Federal Order, that took effect on April 29, to limit the movement of lactating dairy cattle and to collect and aggregate H5N1 test results to better understand the nature of the outbreak.
Since the detection of H5N1 in dairy cattle, the Federal response has leveraged the latest available scientific data, field epidemiology, and risk assessments to mitigate risks to workers and the general public, to ensure the safety of America’s food supply and to mitigate risk to livestock, owners, and producers. Today, USDA is taking a series of additional steps to help achieve these goals and reduce the impact of H5N1 on affected premises and producers, and HHS is announcing new actions through the CDC and FDA to increase testing and laboratory screening and testing capacity, genomic sequencing, and other interventions to protect the health and safety of dairy and other potentially impacted food items.
Today, USDA is announcing assistance for producers with H5N1 affected premises to improve on-site biosecurity in order to reduce the spread. In addition, USDA is taking steps to make available financial tools for lost milk production in herds affected by H5N1. Building on the Federal Order addressing pre-movement testing, these steps will further equip producers with tools they can use to keep their affected herds and workers healthy and reduce risk of the virus spreading to additional herds.
Protect against the potential for spread between human and animals. Provide financial support (up to $2,000 per affected premises per month) for producers who supply PPE to employees and/or provide outerwear uniform laundering, for producers of affected herds who facilitate the participation of their workers in USDA/CDC workplace and farmworker study.
Complementary to USDA’s new financial support for producers, workers who participate in the study are also eligible for financial incentives to compensate them for their time, regardless of whether the study is led by federal, state, or local public health professionals.
Support producers in biosecurity planning and implementation . Provide support (up to $1,500 per affected premises) to develop biosecurity plans based on existing secure milk supply plans. This includes recommended enhanced biosecurity for individuals that frequently move between dairy farms – milk haulers, veterinarians, feed trucks, AI technicians, etc. In addition, USDA will provide a $100 payment to producers who purchase and use an in-line sampler for their milk system.
Provide funding for heat treatment to dispose of milk in a bio secure fashion. This will provide producers a safe option for disposal of milk. Heat treatment performed in accordance with standards set by FDA is the only currently available method considered to effectively inactivate the virus in milk. If a producer establishes a system to heat treat all waste milk before disposal, USDA will pay the producer up to $2,000 per affected premises per month.
Reimburse producers for veterinarian costs associated with confirmed positive H5N1 premises. This provides support to producers to cover veterinary costs necessarily incurred for treating cattle infected with H5N1, as well as fees for veterinarians to collect samples for testing. This can include veterinary fees and/or specific supplies needed for treatment and sample collection. Veterinary costs are eligible to be covered from the initial date of positive confirmation at NVSL for that farm, up to $10,000 per affected premises.
Offset shipping costs for influenza A testing at laboratories in the National Animal Health Laboratory Network (NAHLN). USDA will pay for the cost of shipping samples to NAHLN labs for testing. USDA will pay actual shipping costs, not to exceed $50 per shipment for up to 2 shipments per month for each affected premises. Testing at NAHLN laboratories for samples associated with this event (e.g., pre-movement, testing of sick/suspect animals, samples from concerned producers) is already being conducted at no-cost to the producer.
Taken together, these tools represent a value of up to $28,000 per premises to support increased biosecurity activities over the next 120 days.
Compensate producers for loss of milk production. USDA is taking steps to make funding available from the Emergency Assistance for Livestock, Honey Bees, and Farm-raised Fish Program (ELAP) to compensate eligible producers with positive herds who experience loss of milk production. While dairy cows that have been infected with H5N1 generally recover well, and there is little mortality associated with the disease, it does dramatically limit milk production, causing economic losses for producers with affected premises. USDA can support farmers with the ELAP program to offset some of these losses. This compensation program is distinct from the strategy to contain the spread.
Work with states to limit movement of lactating cattle . Additionally, USDA will work with and support the actions of States with affected herds as they consider movement restrictions within their borders to further limit the spread of H5N1 between herds to reduce further spread of this virus.
USDA will make $98 million in existing funds available to APHIS to fund these initiatives. If needed, USDA has the authority, with Congressional notification, to make additional funds available.
These additional measures build on a suite of actions USDA has taken to date. This includes implementation of the Federal Order to limit spread of the disease, coordinating with federal partners to share expertise and lab capacity, doubling down on our work with producers to practice good biosecurity measures, continuing to conduct investigations to determine how the virus is spread within and between farms, and analyzing and sharing sequences alongside validated epidemiological information.
The U.S. government is addressing this situation with urgency and through a whole-of-government approach. USDA is working closely with federal partners at FDA, which has the primary responsibility for the safety of milk and dairy products, by assisting with conducting lab testing at USDA labs. USDA is also working closely with federal partners at CDC, which has the primary responsibility for public health, by encouraging producer and industry cooperation with public health officials to get vital information necessary to assess the level of risk to human health.
Additional details on how producers can access and apply for the financial tools is forthcoming.
Today, HHS announced new funding investments through CDC and FDA totaling $101 million to mitigate the risk of H5N1 and continue its work to test, prevent, and treat H5N1. Although the CDC’s assessment of the risk of avian influenza infection for the general public continues to remain low at this time, these investments reflect the Department’s commitment to prioritizing the health and safety of the American public.
Public and animal health experts and agencies have been preparing for avian influenza outbreak for 20 years. Our primary responsibility at HHS is to protect public health and the safety of the food supply, which is why we continue to approach the outbreak with urgency. We stood up a response team which includes four HHS agencies – CDC, FDA, NIH and ASPR – which are working closely with USDA to:
- Ensure we keep communities healthy, safe, and informed;
- Ensure that our Nation’s food supply remains safe;
- Safeguard American agriculture and the livelihood and well-being of American farmers and farmworkers; and
- Monitor any and all trends to mitigate risk and prevent the spread of H5N1 among both people and animals.
Some examples of this work include:
- CDC monitoring of the virus to detect any changes that may increase risk to people, and updated avian flu guidance for workers to ensure people who work with dairy cows and those who work in slaughterhouses have the guides and information they need in both English and Spanish.
- CDC's ongoing discussions with multiple states about field investigations and incentives for workers who participate in these on-site studies. CDC has also asked health departments to distribute existing PPE stocks to farm workers, prioritizing those who work with infected cows. To help states comply with CDC recommendations, ASPR has PPE in the Strategic National Stockpile (SNS) available for states to request if needed.
- FDA’s close coordination with USDA to conduct H5N1 retail milk and dairy sample testing from across the country to ensure the safety of the commercial pasteurized milk supply. NIAID – a part of NIH - is also providing scientific support to this entire effort through six U. S. based Centers for Excellence for Influenza Research and Response, known as CEIRRs.
Today, in light of HHS’ ongoing commitment to ensure the safety of the American people and food supply, HHS announced additional resources to further these efforts through CDC and FDA:
CDC announced it has identified an additional $93 million to support its current response efforts for avian influenza. Building on bipartisan investments in public health, this funding will allow CDC to capitalize on the influenza foundation that has been laid over the last two decades, specifically where CDC has worked domestically and globally to prevent, detect, and respond to avian influenza.
These investments will allow CDC to bolster testing and laboratory capacity, surveillance, genomic sequencing, support jurisdictions and partner efforts to reach high risk populations and initiate a new wastewater surveillance pilot.
- Develop and optimize assays that can be used to sequence virus independent of virus identification.
- Assess circulating H5N1 viruses for any concerning viral changes, including increased transmissibility or severity in humans or decreasing efficacy of diagnostics or antivirals.
- Support the ability of STLT Public Health Labs throughout the country to surge their testing abilities, including support for the additional costs of shipping human avian influenza specimens, which are select agents.
- Through the International Reagent Resource (IRR), support manufacture, storage, and distribution of roughly one thousand additional influenza diagnostic test kits (equaling nearly around one million additional tests) for virologic surveillance. The IRR would also provide influenza reagents for research and development activities on a global scale. This is in addition to current influenza testing capacity at CDC and in STLT public health and DOD labs, which is approximately 490,000 H5-specific tests.
- Address the manufacturer issue detected with current avian flu test kits.
- Initiate avian flu testing in one commercial laboratory.
- Scale up existing efforts to monitor people who are exposed to infected birds and poultry to accommodate workers at likely many more poultry facilities, as well as potentially workers at other agricultural facilities and other people (e.g., hunters) who may be exposed to species that pose a threat.
- Scale up contact tracing efforts and data reporting to accommodate monitoring of contacts of additional sporadic cases.
- Support the collection and characterization of additional clinical specimens through established surveillance systems from regions with large numbers of exposed persons to enhance the ability to detect any unrecognized cases in the community if they occur.
- Expand respiratory virus surveillance to capture more samples from persons with acute respiratory illness in different care settings.
- Support continuation and possible expansion of existing respiratory surveillance platforms and vaccine effectiveness platforms.
- Provide bioinformatics and data analytics support for genomic sequencing at CDC that supports surveillance needs for enhanced monitoring.
- Expand sequencing capacity for HPAI in state-level National Influenza Reference Centers (NIRCs), Influenza Sequencing Center (ISC), and Pathogen Genomic Centers of Excellence.
- Analyze circulating H5N1 viruses to determine whether current Candidate Vaccine Viruses (CVVs) would be effective and develop new ones if necessary.
- Support partner efforts to reach high risk populations.
- Initiate wastewater pilot to evaluate the use case for HPAI in up to 10 livestock-adjacent sites in partnership with state and local public health agencies and utility partners.
- Implement a study to evaluate the use of Influenza A sequencing in wastewater samples for highly pathogenic avian influenza typing. Initiate laboratory evaluation for HA typing and examine animal-specific markers in community wastewater to assess wildlife and livestock contribution and inform interpretation of wastewater data for action.
Additionally, the FDA is announcing an additional $8 million is being made available to support its ongoing response activities to ensure the safety of the commercial milk supply. This funding will support the agency’s ability to validate pasteurization criteria, conduct surveillance at different points in the milk production system, bolster laboratory capacity and provide needed resources to train staff on biosecurity procedures. Additionally, these funds will help support H5N1 activities in partnership with state co-regulatory partners, who administer state programs as part of the federal/state milk safety system. It may also allow the FDA to partner with universities on critical research questions.
Additional Information:
To learn more about USDA’s response to H5N1 in dairy cattle, visit https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock .
To learn more about CDC’s response to H5N1, visit https://www.cdc.gov/flu/avianflu/mammals.htm .
To learn more about FDA’s response to H5N1, visit https://www.fda.gov/food/alerts-advisories-safety-information/updates-highly-pathogenic-avian-influenza-hpai
Sign Up for Email Updates
Receive the latest updates from the Secretary, Blogs, and News Releases
Subscribe to RSS
Receive latest updates

Related News Releases
Biden-harris administration reports significant progress toward protecting children from lead poisoning, readout of hhs secretary xavier becerra’s remarks at the sickle cell disease trailblazers event, final rule to establish first-ever regulations for adult protective services, related blog posts.

Preventing and Addressing Sexual Violence Against People with I/DD
Donate blood and help save lives, the u.s. department of health and human services is taking action to strengthen primary care, media inquiries.
For general media inquiries, please contact [email protected] .
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Public health
- Health protection
- Health surveillance and reporting programmes
- Notifiable diseases: causative agents reports for 2024
- UK Health Security Agency
NOIDs causative agents: week 19 (week ending 12 May 2024)
Updated 13 May 2024
Applies to England and Wales

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/notifiable-diseases-causative-agents-reports-for-2024/noids-causative-agents-week-19-week-ending-12-may-2024
Laboratories in England have a statutory duty to notify the UK Health Security Agency (UKHSA) of the identification of the following causative agents:
- Bacillus anthracis
- Bacillus cereus
- Bordetella pertussis
- Borrelia spp
- Brucella spp
- Burkholderia mallei
- Burkholderia pseudomallei
- Campylobacter spp
- Carbapenemase-producing Gram-negative bacteria
- Chikungunya virus
- Chlamydophila psittaci
- Clostridium botulinum
- Clostridium perfringens
- Clostridium tetani
- Corynebacterium diphtheriae
- Corynebacterium ulcerans
- Coxiella burnetii
- Crimean-Congo haemorrhagic fever virus
- Cryptosporidium spp
- Dengue virus
- Ebola virus
- Entamoeba histolytica
- Escherichia coli O 157
- Francisella tularensis
- Giardia lamblia
- Guanarito virus
- Haemophilus influenzae (invasive)
- Hanta virus
- Hepatitis A
- Hepatitis B
- Hepatitis C
- Hepatitis D
- Hepatitis E
- Influenza virus
- Junin virus
- Kyasanur Forest disease virus
- Lassa virus
- Legionella spp
- Leptospira interrogans
- Listeria monocytogenes
- Machupo virus
- Marburg virus
- Measles virus
- Mumps virus
- Mycobacterium tuberculosis complex
- Neisseria meningitidis
- Omsk haemorrhagic fever virus
- Plasmodium falciparum
- Plasmodium Knowlesi
- Plasmodium Malariae
- Plasmodium Ovale
- Plasmodium Vivax
- Polio virus
- Rabies virus
- Rickettsia spp
- Rift Valley fever virus
- Rubella virus
- Sabia virus
- Salmonella spp
- SARS Coronavirus
- Shigella spp
- Streptococcus group A (invasive)
- Streptococcus pneumoniae (invasive)
- Varicella zoster virus
- Variola virus
- Vibrio cholerae
- West Nile virus
- Yellow fever virus
- Yersinia pestis
Statutory notifications of causative agents, grouped by root organism
Totals for the current week compared to the previous 5.
Carbapenemase-producing Enterobacterales ( CPE )
For all carbapenemase-producing Gram-negative organisms, the reports are de-duplicated by first mention of organism species and resistance mechanism by person in a rolling 52-week period.
Note: the numbers presented here do not include specimens that have been referred to the AMRHAI Reference Unit.
Other carbapenemase-producing Gram-negative organisms
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Sign up for alerts
- NEWS FEATURE
- 30 October 2022
Research successfully integrated into the Ebola outbreak response in Africa
- Paul Adepoju
You can also search for this author in PubMed Google Scholar

Uganda Battles Seventh Ebola Outbreak since 2000: Red Cross workers don Personal Personal Protective Equipment (PPE) prior to burying a 3-year-old boy suspected of dying from Ebola on October 13, 2022 in Mubende, Uganda. Emergency response teams, isolation centres and treatment tents have been set up by the Ugandan health authorities around the central Mubende district. Cedit: Luke Dray/Getty Images
Lire en Francais
Clinical trials conducted during Ebola outbreaks in Africa have been crucial in expanding the capacity to combat the disease. The latest Ebola outbreak in Uganda is expected to further improve knowledge on the disease, including testing new tools and vaccines.
On September 20, health authorities in Uganda declared an outbreak of Ebola virus disease (EVD). Several advances have been made regarding vaccines against Ebola. In October 2021, Nature Africa reported the Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimens were safe, well tolerated and produced strong immune responses in children and adults. In November 2019, the WHO prequalified the injectable Ebola vaccine, Ervebo, paving the way for its use in high-risk countries.
Since then, Ervebo has been used for ring vaccination exercises in a number of African countries where Ebola Zaire virus outbreaks, occurred. This vaccine, however, is not effective in the current outbreak in Uganda, which is caused by the Ebola Sudan Virus (species Sudan ebolavirus).
Integrating research into Africa’s Ebola response
For about 30 years, work on the development of filovirus vaccines has advanced with the emergence of different platforms and types of vaccines, in addition to several preclinical studies. The problem, however, is the few outbreaks and the fewer number of infected individuals with the Ebola Sudan Virus in comparison with the Zaire virus.
“But I think there is a very strong foundation since everything we’ve learned from the West African (Ebola) epidemic is also going to help foster the development of vaccines for Sudan,” Cesar Munoz-Fontela, Group Leader at the German Center for Infection Research, told Nature Africa .
Clinical trial plans for Uganda
An approach that has been successfully implemented in Sierra Leone, Liberia, Sudan and other countries now allows the implementation of clinical trials quickly (within weeks) as part of the response to an outbreak says Ana Maria Henao-Restrepo, Implementation Research and Economic Analysis , Initiative for Vaccine Research , World Health Organisation. “We are talking directly with the developers who are also sharing all the vaccines information very quickly. The choice of a candidate vaccine to deploy in the current outbreak will be determined by which one has sufficient data to move on , we also ensure they have sufficient doses of othe clinical grade material available for the study to start.”
There is also the preparatory phase in the country. There is already a core protocol that includes all the critical elements to conduct a robust evaluation of a candidate vaccine based on experience with the Ebola virus. “We have that ready and it only needs to be adjusted to the Ugandan situation with the inputs of Ugandan researchers and with the approval of the national regulatory authority in Uganda. We already have the vaccine supplies ready to be deployed in trials,” Henao-Restrepo says. “These elements are all ready for the triggering of a clinical trial within a few weeks while still ensuring we are adhering to international and national standards. We are not cutting corners.”
doi: https://doi.org/10.1038/d44148-022-00153-1
Reprints and permissions
Assistant/Associate Professor in Sustainable Biobased Products Manufacturing
Lubbock, Texas
Texas Tech University
Professor and Center for Vector-borne and Zoonotic Diseases Director
Assistant scientist/professor in rare disease research, sanford research.
Assistant Scientist/Professor in Rare Disease Research, Sanford Research Sanford Research invites applications for full-time faculty at the rank of...
Sioux Falls, South Dakota
Sanford Research
Postdoctoral Fellow - Boyi Gan lab
New postdoctoral positions are open in a cancer research laboratory located within The University of Texas MD Anderson Cancer Center. The lab curre...
Houston, Texas (US)
The University of Texas MD Anderson Cancer Center - Experimental Radiation Oncology
Assistant Professor
Tenure-track Assistant Professor position in the Cell and Molecular Physiology Department at Loyola University Chicago Stritch School of Medicine.
Maywood, Illinois
Loyola University of Chicago - Cell and Molecular Physiology Department
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Who Had Chinese Ebola Hamsters on Their 2024 Bingo Card?
The researchers assert that the aim has been to reproduce the symptoms of Ebola in a laboratory so as to better understand the condition and prevent its spread.

- Facebook Messenger
The last time we reported on the deadly hemorrhagic disease Ebola, 4 people in Uganda had died during a small, localized outbreak. That was back in 2022 .
Historically, cases of Ebola have been confined to Africa and infections are associated with close contact with the blood and bodily fluids of those suffering from the disease.
However, it appears that Chinese have not learned their lesson about tampering with viruses…especially the potentially lethal ones. Chinese researchers have created another franken-virus with parts of Ebola in a lab, resulting in an exceptionally deadly infection that killed a group of hamsters horrifically.
A team of researchers at Hebei Medical University used a contagious disease of livestock and added a protein found in Ebola, which allows the virus to infect cells and spread throughout the human body. The group of hamsters that received the lethal injection ‘developed severe systemic diseases similar to those observed in human Ebola patients,’ including multi-organ failure,’ the study shared. One particularly horrific symptom saw the infected hamsters develop secretions in their eyes, which impaired their vision and scabbed over the surface of the eyeballs While the experiment may spark fears of another lab leak, the researchers say their goal was to find the right animal models that can safely mimic Ebola symptoms in a lab setting. googletag.cmd.push(function() { googletag.display('div-gpt-ad-1567704208086-0'); }); googletag.cmd.push(function() { googletag.display("div-hre-Legal-Insurrection-2601"); });
The Chinese scientists published their findings in ScienceDirect. The researchers asset that the aim has been to reproduce the symptoms of Ebola in a laboratory so as to better understand the condition and prevent its spread.
Due to the dangers of infection, Ebola needs to be handled in a Biosafety Level 4 (BSL-4) facility and using a BSL-2 the researchers worked around this by using vesicular stomatitis virus to carry the Ebola glycoprotein. There were 10 hamsters, half male and half female, used that was three weeks old and all died bar two of the males. The dead hamsters were found to have seen the virus spread throughout their body including the heart, brain and kidneys, with the highest levels in the liver. “Overall, this surrogate model represents a safe, effective, and economical tool for rapid preclinical evaluation of medical countermeasures against EBOV (Ebola virus) under BSL-2 conditions, which would accelerate technological advances and breakthroughs in confronting Ebola virus disease [EVD],” stated the study. It added: “It is a sign that 3-week-old Syrian hamsters infected with VSV-EBOV/GP have the possibility of playing a role in the study of optic nerve disorders caused by EVD.”
The reason the novel coronavirus that was spread worldwide was supposedly created for was a better understanding of the deadly severe acute respiratory diseases that had been spreading since the 2003 periodically. We know how that went .
The good news is that the route of transmission of this virus still involves blood and bodily fluids. Let’s hope the scientists don’t tamper with it farther, creating one that is airborne.
As an interesting side-note, a new study published in The Lancet Infectious Diseases shows that a promising vaccine (named rVSVΔG-ZEBOV-GP) can cut Ebola mortality numbers in half.
The vaccine is typically administered to those at highest risk of exposure to the virus — a strategy called ring vaccination that targets “people who are contacts of an Ebola case, contacts of contacts and health-care workers,” says [Rebecca Coulborn, an epidemiologist with Epicentre, the medical research arm of Doctors Without Borders]. The vaccine is not yet commercially available. Researchers showed that rVSVΔG-ZEBOV-GP was highly effective at reducing the risk of infection, but no one knew how capable it was of preventing death in someone who was vaccinated after becoming infected during an epidemic. This is what Coulborn and her colleagues set out to determine. They focused their efforts on the second-largest Ebola outbreak ever recorded, which took place in the Democratic Republic of Congo between 2018 and 2020. Despite the outbreak flaring up in the midst of an active conflict zone, meticulous records were kept. “Every single Ebola health facility across the entire Ebola epidemic had a standardized, harmonized and compiled list of all admissions,” says Coulborn. This list included 2,279 confirmed Ebola patients, and it recorded whether or not each person had been vaccinated before they got sick — and if so, when they’d received the vaccine. Coulborn then compared how those two groups fared. The result was striking. Among the unvaccinated, mortality was 56%. But for those who’d received the vaccine, that rate was cut in half.
Let’s hope rVSVΔG-ZEBOV-GP truly works better than the covid vaccines, then.

Donations tax deductible to the full extent allowed by law.
This is appalling.. and besides that, why hamsters? I guess I just don’t understand science. SMH TY Leslie.
It’s not science. It’s biological warfare research. The CCP doesn’t give a hamster’s a$$ about advancing science or helping humanity.
doesn’t give a hamster’s a$$ Annnnd, Richard Gere enters the conversation.
Yes, I will go denounce myself, now.
I think that was gerbil, not a hamster. And almost sure no ebola was involved.
Because you can modify it to infect rats and turn them loose on a population Center. Remember that story a few months ago over in Cali of an illegal lab run by Chinese doing all sorts of dangerous things?
After the monkeypox thing? Yeah, I kinda expected this.
Also, don’t we already pretty well understand how ebola kills and how it spreads?
The purpose of the study is to see if we can make it spread quicker.
3-week-old Syrian hamsters Oh, it was terrorist hamsters? Well, that’s ok, then. /eyeroll/
Gain-of-function?
Ah, This explains why hamsters are now being sold at the wet market down the street from Hebei Medical University.
You hamster, you dirty hamster.
We pour gasoline on a fire to better understand its condition and prevent its spread, reports a team of arsonists posing as scientist.
The very premise of their research – to better understand how it spreads – is laughably fallacious. The medical community understands EXACTLY how and why Ebola outbreaks occur in Africa. First, Africans love themselves some bush meat. And, apparently, they like it on the rarer side. Mission accomplished on the zoonotic transfer. What happens next is also well-understood and predictable. The same people who like to eat bush meat also LOVE, FEEL COMPELLED, to kiss dead people…the ones that haven’t been embalmed. It’s part of their backward culture. So, Uncle Barack finds himself delicious-looking monkey – the slow, sick one that was easier to catch. He ‘cooks’ himself up a feast and contracts Ebola in the process. He then gets sick, dies and everyone in his family run to kiss his dead, still warm body. Ebola outbreak commences. Rinse, repeat.
I envision a future where assassination teams are sent to target these ‘scientists’ and wipe them out before they wipe us out.
I’m seeing a story here…. A Moral Agency whose job is to hunt down rogue scientists, kill them, and “secure” their experiments with nuclear fire (or just regular fire if they’re not gray goo).
Anyone remember the Tom Clancy novel RAINBOW SIX?
Nuke the facilities from space. It’s the only way to be sure.
If it had anything to do with China, Fauci had his fingers in it.
b/c its the same hamsters that monkypox joe uses
Speaking of hamsters and monkey pox, Michael Cohen is a nasty little hamster who is flinging his monkey pox in a NY courtroom this very hour.
any way the wind blows; doesnt really matta to him
msnbc calling
Two thumbs up!
It’s not a bingo card anymore. More like Mad Libs. Fill in the blanks: “We can’t have an election this year because of the (Country/nationality) (disease/manner of death).”
I do! I have that square! Now, the only one I need to fill in to win 2024 is, “Joe Biden revealed to be 80-year long con of Loki’s.
The torture we do on these poor animals Dogs, cats, hamsters, sheep, primates
For you doesn’t remember Fauci s dog experiments where the poor little beagles were murdered by bugs eating their faces and then…
Fu$&er I really thought THAT would be the end of him…
That is spot on gonzo.
Tony Fauci could stand in the middle of Fifth Avenue and torture puppies, and still be an MSM hero.
torture in the name of leftist is
Don’t we have enough computer power these days that we could figure this stuff out electronically?
Models more often reflect the way you want the world to work to prove you right, rather than the way the world actually works.
I wonder if Richard Gere heard about this?/s
Explain this?
https://wegotthiscovered.com/celebrities/what-is-the-richard-gere-gerbil-rumor-and-how-did-it-start/
Bwahahahaha. I’m glad I missed that one
You were late to the game, man. 😉
We’re I president I’d be working to have each employee meet unfortunate ends. This kind of work serves NO PURPOSE.
the dems continue to treat these animals like they did the poc in the tuskeegee syph test
and still do to this day
doesnt matter to a lefty
be it human /animal etc
you are to be their pleb
People’s Republic of China + civil-military fusion + lab + virus = disaster.
Leave a Comment
Leave a reply cancel reply.

CONTRIBUTORS
Sr. Contrib Editor
Contrib Editor
Weekend Editor
Editor Emerita
- Learn more about the Contributors

Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 6—June 2024
Research Letter
Effect of myxoma virus species jump on iberian hare populations.
Suggested citation for this article
The myxoma virus species jump from European rabbits ( Oryctolagus cuniculus ) to Iberian hares ( Lepus granatensis ) has raised concerns. We assess the decline suffered by Iberian hare populations on the Iberian Peninsula and discuss the association between the effect of myxomatosis and the average abundance index, which we estimated by using hunting bags.
In July 2018, after 60 years of endemic circulation in European wild rabbits ( Oryctolagus cuniculus ), myxoma virus (MYXV) jumped to the Iberian hare ( Lepus granatensis ) ( 1 ). This species jump resulted from the emergence of a recombinant strain of MYXV, named ha-MYXV, containing a 2.8-kb insertion derived from an unknown poxvirus ( 2 , 3 ). Outbreak notifications rapidly spread across the Iberian Peninsula, resulting in an estimated mean mortality rate of 55.4% (median 70%) in hares ( 4 ). Concerns were raised about the effect of myxomatosis on the Iberian hare populations ( 4 ). We investigated those concerns and determined how myxomatosis affected Iberian hares by evaluating hare abundance indexes before and after the emergence of ha-MYXV.
We used hunting bag data to approximate population abundance ( 5 ). We collected information on hunting yields from hunting grounds in Portugal and the most affected regions of Spain, Andalusia, and Castilla-La Mancha during the hunting seasons (October–February) spanning from 2007–08 to 2020–21. Our study period includes 11 seasons before ha-MYXV emergence (premyxomatosis), from 2007–08 to 2017–18, and 3 after (postmyxomatosis), from 2018–19 until 2020–21. For each hunting ground and season, we estimated the abundance index as the number of hares hunted per square kilometer. We used analysis of variance tests to evaluate the differences between abundance indexes. We gathered data on myxomatosis outbreaks from nationwide passive surveillance efforts conducted after the first case reports. We used the coefficient (−1 to 1) obtained from the linear regression between hunting yields and hunting seasons to compute population trends ( 6 ) for the study period and the premyxomatosis period. Because the postmyxomatosis period was too short to estimate population trends accurately, we calculated the disease effect as the difference between the global and the premyxomatosis trends. We estimated the threshold of premyxomatosis abundance index from which >50% of populations were negatively affected by the disease.
Figure . Evidence of the effect of myxomatosis outbreaks on Iberian hare ( Lepus granatensis ) populations and the link to the abundance index, in the Iberian Peninsula, after the initial species...
We found a reduction of 77.2% in hares hunted during the study period ( Figure , panel A). In the decade preceding the first myxomatosis outbreak, a smooth negative population trend was noted ( https://www.intechopen.com/chapters/71640 ), with a mean annual reduction of 3.2% and a total decline of 29.6% in the number of hunted Iberian hares ( Figure , panel A). Coinciding with the emergence of ha-MYXV, the highest annual decline of 51.5% occurred from 2017–18 to 2018–19 ( Figure , panel A). This decrease was 57.1% in Andalusia and 50.9% in Castilla-La Mancha. In Portugal, the decrease was only 10.0% but increased to 30.9% in the following hunting season (2019–20). This abrupt population decline could result from the rapid spread of ha-MYXV in the Iberian Peninsula ( 4 ). The number of hunted hares remained low after 2018, which is not suggestive of a postmyxomatosis recovery ( Figure , panel A). Nevertheless, the evolution of hare population trends needs to be monitored over a longer period for more accurate inferences.
We found significant differences (p < 0.05) between the mean abundance indexes in the premyxomatosis versus postmyxomatosis periods, demonstrating further evidence of the myxomatosis-related decrease in hare populations ( Figure , panel B). Areas with confirmed cases showed higher premyxomatosis abundance indexes compared with the overall average in the same period ( Figure , panel B). We found concordant results when investigating the association between the premyxomatosis hare abundance index and the estimated disease effect. We found that, above a threshold of abundance index, the estimated disease effect is likely negative ( Figure , panel C). Lower abundances may act as a barrier to virus dispersal, limiting the effect of myxomatosis, as previously described in wild rabbits ( 7 ). Of note, the abundance index threshold estimated for the study area is low (1.5 hares hunted/km 2 ) ( Figure , panel C), meaning most hunting grounds have surpassing abundance indexes (76.4% in Spain and 51.0% in Portugal). This finding suggests ha-MYXV is highly effective in establishing itself in Iberian hare populations. The comparatively lower abundance indexes in Portugal may explain the lesser effect of myxomatosis in the Iberian Peninsula region.
The future evolution of myxomatosis in Iberian hare populations is uncertain, and concerns remain if myxomatosis will mimic the evolution documented in European wild rabbits. Hare populations were already in decline during the decade before the first myxomatosis outbreak. Information on hare population status was and still is scarce. To ensure the future sustainability of Iberian hares, long-term and holistic conservation, management, and monitoring programs are needed, especially when worldwide viral emergence events have become increasingly more frequent in lagomorph species over the past decade ( 8 , 9 ). The conservation status of the Iberian hare across its distribution range should be continuously monitored and reassessed as needed. Our results indicate the decline suffered by Iberian hare populations in the past few years can be linked to the emergence of ha-MYXV.
Ms. Cardoso is a PhD candidate developing her doctoral research project at the Research Centre in Biodiversity and Genetic Resources, and the Spanish Game and Wildlife Research Institute at the University of Castilla-La Mancha. Her research interests focus on the epidemiology of myxomatosis and other lagomorph diseases, as well as their impact on the hosts’ populations.
Acknowledgments
We thank Emídio Santos and the Regional Government of Andalusia for their collaboration.
This research was funded in part from a research grant provided by the Spanish Ministry of Science and Innovation (project LagoHealth; reference no. PID2019-111080RB-C21). This research was partially funded by the Sub-modality 2.4. “UCOLIDERA” of the “Enrique Aguilar Benítez de Lugo” Research Plan of the University of Cordoba and CIBER-Consorcio Centro de Investigación Biomédica en Red (grant no. CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea-Next Generation EU. B.C. was funded by the Fundação para a Ciência e Tecnologia, FCT (grant no. 2020.04872.BD). J.F.-L. was funded by the Margarita Salas grant from the European Union, NextGenerationEU, through Complutense University.
- García-Bocanegra I , Camacho-Sillero L , Risalde MA , Dalton KP , Caballero-Gómez J , Agüero M , et al. First outbreak of myxomatosis in Iberian hares ( Lepus granatensis ). Transbound Emerg Dis . 2019 ; 66 : 2204 – 8 . DOI PubMed Google Scholar
- Águeda-Pinto A , Lemos de Matos A , Abrantes M , Kraberger S , Risalde MA , Gortázar C , et al. Genetic characterization of a recombinant myxoma virus in the iberian hare ( Lepus granatensis ). Viruses . 2019 ; 11 : 1 – 16 . DOI PubMed Google Scholar
- Dalton KP , Martín JM , Nicieza I , Podadera A , de Llano D , Casais R , et al. Myxoma virus jumps species to the Iberian hare. Transbound Emerg Dis . 2019 ; 66 : 2218 – 26 . DOI PubMed Google Scholar
- García-Bocanegra I , Camacho-Sillero L , Caballero-Gómez J , Agüero M , Gómez-Guillamón F , Manuel Ruiz-Casas J , et al. Monitoring of emerging myxoma virus epidemics in Iberian hares ( Lepus granatensis ) in Spain, 2018-2020. Transbound Emerg Dis . 2021 ; 68 : 1275 – 82 . DOI PubMed Google Scholar
- Imperio S , Ferrante M , Grignetti A , Santini G , Focardi S . Investigating population dynamics in ungulates: do hunting statistics make up a good index of population abundance? Wildl Biol . 2010 ; 16 : 205 – 14 . DOI Google Scholar
- Williams D , Acevedo P , Gortázar C , Escudero MA , Labarta JL , Marco J , et al. Hunting for answers: rabbit ( Oryctolagus cuniculus ) population trends in northeastern Spain. Eur J Wildl Res . 2007 ; 53 : 19 – 28 . DOI Google Scholar
- Villafuerte R , Castro F , Ramírez E , Cotilla I , Parra F , Delibes-Mateos M , et al. Large-scale assessment of myxomatosis prevalence in European wild rabbits ( Oryctolagus cuniculus ) 60years after first outbreak in Spain. Res Vet Sci . 2017 ; 114 : 281 – 6 . DOI PubMed Google Scholar
- Asin J , Nyaoke AC , Moore JD , Gonzalez-Astudillo V , Clifford DL , Lantz EL , et al. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J Vet Diagn Invest . 2021 ; 33 : 728 – 31 . DOI PubMed Google Scholar
- Velarde R , Abrantes J , Lopes AM , Estruch J , Côrte-Real JV , Esteves PJ , et al. Spillover event of recombinant Lagovirus europaeus /GI.2 into the Iberian hare ( Lepus granatensis ) in Spain. Transbound Emerg Dis . 2021 ; 68 : 3187 – 93 . DOI PubMed Google Scholar
- Figure . Evidence of the effect of myxomatosis outbreaks on Iberian hare (Lepus granatensis) populations and the link to the abundance index, in the Iberian Peninsula, after the initial species jump...
Suggested citation for this article : Cardoso B, García-Bocanegra I, Queirós J, Fernández-López J, Alves PC, Acevedo P. Effect of myxoma virus species jump on Iberian hare populations. Emerg Infect Dis. 2024 Jun [ date cited ]. https://doi.org/10.3201/eid3006.231280
DOI: 10.3201/eid3006.231280
Table of Contents – Volume 30, Number 6—June 2024
Please use the form below to submit correspondence to the authors or contact them at the following address:
Pelayo Acevedo, Instituto de Investigación en Recursos Cinegético, Ronda de Toledo, s/n, 13071 Ciudad Real, Spain
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
What is the altmetric attention score.
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.

IMAGES
COMMENTS
Introduction. Ebola, earlier termed as Ebola hemorrhagic fever (EHF), is a critically lethal ailment which primarily affects the humans and nonhuman primates. Ebola virus disease (EVD) occurs due to a virus infection which belongs to the family Filoviridae and genus Ebolavirus . [ 1] EVDs has posed diagnostic challenges and has been a universal ...
A study reports the three-dimensional structure of the Ebola virus polymerase in complex with VP35 and RNA, and reveals features required for initiation of viral replication. Qi Peng Bin Yuan
The mean CFRs for each ebolavirus are 33.65 ± 8.38% (BDBV), 43.92 ± 0.7% (EBOV) and 53.72 ± 4.456% (SUDV) 4; that is, a CFR of ~40-50% overall, with the remaining difference between the ...
N Engl J Med 2020;382: 1832 - 1842. DOI: 10.1056/NEJMra1901594. VOL. 382 NO. 19. Ebola virus (EBOV) was the best-known and most extensively studied member of the Filoviridae family ...
The PREVAIL III Study GroupN Engl J Med 2019; 380:924-934. Ebola virus causes severe illness, often leading to death. Little is known about the health sequelae of those who survive infection. In ...
BMC Genomics (2020) Filoviruses such as Ebola virus continue to pose a substantial health risk to humans. Advances in the sequencing and functional characterization of both pathogen and host ...
Ebola RNA persistence in semen of Ebola virus disease survivors—final report. N Engl J Med. 2017; 377: 1428-1437. ... Insights from clinical research completed during the west Africa Ebola virus disease epidemic. Lancet Infect Dis. 2017; 17: e280-e292. Summary; Full Text; Full Text PDF; PubMed;
Symptoms. The symptoms of Ebola infection can be sudden and include fever, fatigue, muscle pain, headache and sore throat. These are followed by vomiting, diarrhoea, rash, and internal and external bleeding. The time from when someone gets infected to having symptoms is usually from 2 to 21 days.
ZMapp was chosen as the control on the basis of data from the Partnership for Research on Ebola Virus in Liberia II (PREVAIL II) trial. 11 The current trial was originally designed in November ...
Ebola virus disease (EVD), formerly known as Ebola haemorrhagic fever, is a severe, often fatal illness affecting humans and other primates. The virus is transmitted to people from wild animals (such as fruit bats, porcupines and non-human primates) and then spreads in the human population through direct contact with the blood, secretions, organs or other bodily fluids of infected people, and ...
The 2022 Sudan virus disease (SVD) outbreak in Uganda is the latest reported outbreak caused by an Ebola virus species, and highlights the importance of the continued development and deployment of new tools (diagnostic tests, vaccines, and therapeutics) as part of a multifaceted approach to controlling such outbreaks. Since the start of the 2013-16 west Africa Ebola virus disease (EVD ...
This report describes the use of Ebola vaccines and the role of the stockpile developed and managed by the International Coordinating Group (ICG) on Vaccine Provision during 2021-2023. A total of 145,690 doses have been shipped from the ICG stockpile since 2021. However, because outbreaks since 2021 have been limited and rapidly contained ...
Read the paper: Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks ... Perhaps most important will be future research to determine whether vaccinating survivors can ...
Uveitis is a common manifestation of post-Ebola syndrome, associated with persistence of Ebola virus (EBOV; Zaire ebolavirus) inside the eye. The iris and retinal pigment epithelia are key components of the blood-ocular barriers, but have the capacity to act as hosts for microorganisms. We investigated the ability of EBOV to productively infect these cell populations. Donor-matched human iris ...
In collaboration with researchers at the National Institutes of Health (NIH), this team of researchers have published a new study in Cell Reports identifying two specific genetic regions in these mice that are vulnerable to Ebola virus' most deadly effects.. These genetic vulnerabilities, found in chromosomes eight and seven, are linked to high amounts of virus in the blood and severe ...
Specht took his curiosity for understanding disease propagation to the lab of computational geneticist Pardis Sabeti, professor in Organismic and Evolutionary Biology at Harvard and member of the Broad Institute, known for her work sequencing the Ebola virus in 2014. Specht, now a senior, has since co-authored several studies around new ...
These findings suggest that health-care workers should prioritise the use of REGN-EB3 and mAb114 for patients with Ebola virus disease during future outbreaks. ... Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada ... We report this systematic review and network meta-analysis according to the ...
South Dakota follows CDC guidance for monitoring persons with potential Ebola virus exposure. There have been no cases of Ebola in South Dakota. Centers for Disease Control and Prevention Resources. Countries with active Ebola transmission; Ebola Virus Disease; Posters and Fact Sheets; Information for Healthcare Workers and Settings
Effect of anti-Ebola virus monoclonal antibodies on endogenous antibody production in survivors of Ebola virus disease in the Democratic Republic of the Congo: an observational cohort study, The ...
U.S. Tightens Rules on Risky Virus Research A long-awaited new policy broadens the type of regulated viruses, bacteria, fungi and toxins, including those that could threaten crops and livestock ...
The last major outbreak of the Ebola virus occurred between 2014 and 2015, in several West African countries, according to a report from the World Health Organization. During those two years, more ...
Develop and optimize assays that can be used to sequence virus independent of virus identification. Assess circulating H5N1 viruses for any concerning viral changes, including increased transmissibility or severity in humans or decreasing efficacy of diagnostics or antivirals.
Research and statistics. Reports, analysis and official statistics. Policy papers and consultations. Consultations and strategy. Transparency. ... Ebola virus: 1-----Entamoeba ...
Integrating research into Africa's Ebola response For about 30 years, work on the development of filovirus vaccines has advanced with the emergence of different platforms and types of vaccines ...
Due to the dangers of infection, Ebola needs to be handled in a Biosafety Level 4 (BSL-4) facility and using a BSL-2 the researchers worked around this by using vesicular stomatitis virus to carry the Ebola glycoprotein. There were 10 hamsters, half male and half female, used that was three weeks old and all died bar two of the males.
In July 2018, after 60 years of endemic circulation in European wild rabbits (Oryctolagus cuniculus), myxoma virus (MYXV) jumped to the Iberian hare (Lepus granatensis) ().This species jump resulted from the emergence of a recombinant strain of MYXV, named ha-MYXV, containing a 2.8-kb insertion derived from an unknown poxvirus (2,3).Outbreak notifications rapidly spread across the Iberian ...
West Africa is currently experiencing the largest outbreak of Ebola virus disease (EVD) in history. ... The example of ZMapp, Antiviral Research, 226 ... Brief Report: A Case of Severe Ebola Virus ...