

Class 9 Science Case Study Questions Chapter 3 Atoms and Molecules
- Post author: studyrate
- Post published:
- Post category: class 9th
- Post comments: 0 Comments
Case study Questions in Class 9 Science Chapter 3 are very important to solve for your exam. Class 9 Science Chapter 3 Class 9 Science Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 3 Atoms and Molecules
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Atoms and Molecules Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 3 Atoms and Molecules
Case Study/Passage-Based Questions
Case Study 1: The knowledge of the valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total negative charge on negative ions (anions) in a molecule. Therefore, in writing the formula of a compound, the positive and negative ions are adjusted in such a way that the total number of positive charges of positive ions (cations) becomes equal to the total number of negative charges of negative ions (anions). There is another simple method for writing the formulae of ionic compounds. In this method, the valencies (or positive or negative charges) of the ions can be ‘crossed over’ to give subscripts. The purpose of crossing over of charges is to find the number of ions required to equalise the number of positive and negative charges.
Element X has two valencies 5 and 3 and Y has valency 2. The elements X and Y are most likely to be respectively (a) copper and sulphur (b) sulphur and iron (c) phosphorus and fluorine (d) nitrogen and iron.
Answer: (d) nitrogen and iron.
The formula of the sulphate of an element X is X 2 (SO 4 ) 3 . The formula of nitride of element X will be (a) X 2 N (b) XN 2 (c) XN (d) X 2 N 3
Answer: (c) XN
The formula of a compound is X 3 Y. The valencies of elements X and Y will be respectively (a) 1 and 3 (b) 3 and 1 (c) 2 and 3 (d) 3 and 2
Answer: (a) 1 and 3
Case Study/Passage Based Questions
Case Study 2: A mole of an atom is a collection of atoms whose total mass is the number of grams equal to the atomic mass. Since an equal number of moles of different elements contain an equal number of atoms it becomes convenient to express the amounts of the elements in terms of moles. A mole represents a definite number of particles viz, atoms, molecules, ions or electrons. This definite number is called the Avogadro number or Avogadro constant which is equal to 6.022 × 1023. Hence a mole represents 6.022 × 1023 particles of the substance. One mole of a substance represents one gram-formula of the substance. One mole of a gas at standard temperature and pressure occupies 22.4 litres.
How many grams of sodium must be taken to get 1 mole of the element? (a) 23 g (b) 35.5 g (c) 63.5 g (d) 46 g
Answer: (a) 23 g
What is the mass in grams of a single atom of chlorine? (Atomic mass of chlorine = 35.5) (a) 6.54 × 10 23 g (b) 5.9 × 10 –23 g (c) 0.0025 g (d) 35.5 g
Answer: (b) 5.9 × 10–23 g
How many number of moles are there in 5.75 g of sodium ? (Atomic mass of sodium = 23) (a) 0.25 (b) 0.5 (c) 1 (d) 2.5
Answer: (a) 0.25
What is the mass in grams of 2.42 mol of zinc? (Atomic mass of Zn = 65.41) (a) 200 g (b) 25 g (c) 85 g (d) 158 g
Answer: (d) 158 g
Case Study 3: According to Dalton’s atomic theory, all matter whether an element, a compound, or a mixture is composed of small particles called atoms which can neither be created nor destroyed during a chemical reaction. Dalton’s theory provides a simple explanation for the laws of chemical combination. He used his theory to explain the law of conservation of masses, the law of constant proportions, and the law of multiple proportions, based on various postulates of the theory. Dalton was the first scientist to use the symbols for the elements in a very specific sense. When he used a symbol for an element he also meant a definite quantity of that element, that is one atom of that element.
Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass? (a) Atoms can neither be created nor destroyed. (b) Each element is composed of extremely small particles called atoms. (c) All the atoms of a given element are identical. (d) During chemical combination, atoms of different elements combine in simple ratios.
Answer: (a) Atoms can neither be created nor destroyed.
Which postulate of Dalton’s atomic theory explains law of definite proportions? (a) Atoms of an element do not change during a chemical reaction. (b) An element consists of atoms having fixed mass and the number and kind of atoms in a given compound is fixed. (c) Different elements have different kind of atoms. (d) Atoms are of various kinds
Answer: (b) An element consists of atoms having fixed mass and the number and kind of atoms in a given compound is fixed.
“If 100 g of calcium carbonate (whether in the form of marble or chalk) is decomposed, 56 g of calcium oxide and 44 g of carbon dioxide are formed.” Which law of chemical combination is illustrated by this statement? (a) Law of constant proportions (b) Law of conservation of mass (c) Law of multiple proportions (d) Law of conservation of energy
Answer: (b) Law of conservation of mass
When 5 g calcium is burnt in 2 g oxygen, 7 g of calcium oxide is produced. When 5 g of calcium is burnt in 20 g of oxygen, then also 7 g of calcium oxide is produced. Which law of chemical combination is being followed? (a) Law of conservation of mass (b) Law of multiple proportions (c) Law of constant proportions (d) No law is being followed.
Answer: (c) Law of constant proportions
Case Study 4: Atoms and molecules are the building blocks of matter. An atom is the smallest unit of an element that retains its chemical properties, while a molecule is a group of two or more atoms held together by chemical bonds. Atoms consist of a positively charged nucleus, which contains protons and neutrons, surrounded by negatively charged electrons in energy levels or shells. The number of protons in an atom determines its atomic number and defines its unique identity as an element. The electrons in an atom occupy specific energy levels, and the outermost shell is known as the valence shell. Atoms gain, lose, or share electrons to achieve a stable electron configuration, forming chemical bonds and giving rise to molecules. Understanding the concept of atoms and molecules is crucial for comprehending various chemical reactions and the composition of substances.
What is the smallest unit of an element that retains its chemical properties? a) Proton b) Electron c) Nucleus d) Atom Answer: d) Atom
What is a group of two or more atoms held together by chemical bonds called? a) Element b) Compound c) Molecule d) Nucleus Answer: c) Molecule
What are the positively charged particles present in the nucleus of an atom called? a) Electrons b) Protons c) Neutrons d) Valence electrons Answer: b) Protons
Which part of an atom contains electrons in energy levels or shells? a) Protons b) Neutrons c) Nucleus d) Valence shell Answer: d) Valence shell
What do atoms do to achieve a stable electron configuration? a) Gain, lose, or share electrons b) Absorb protons c) Increase their atomic number d) Create chemical bonds Answer: a) Gain, lose, or share electrons
Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 3 Atoms and Molecules with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 9 Science Atoms and Molecules Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible By Team Study Rate
You Might Also Like
Class 9 maths case study questions of chapter 1 real numbers, class 9 science case study questions chapter 1 matter in our surroundings.

Class 9 CBSE Science Handwritten Notes by Topper’s: Download PDF FREE
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

- Book Solutions
- State Boards
Case Study Questions Class 9 Science Structure of the Atom
Case study questions class 9 science chapter 4 structure of the atom.
CBSE Class 9 Case Study Questions Science Structure of the Atom. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Structure of the Atom.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks.
CBSE Case Study Questions Class 9 Science – Structure of the Atom
Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. For explaining this, many scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom.
J.J. Thomson (1856- 1940) was a British physicist, He was awarded the Nobel Prize in Physics for his work on the discovery of electrons. Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge. We can also think of a watermelon, the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon. Thomson proposed that: An atom consists of a positively charged sphere and the electrons are embedded in it. The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
(1) Identify the correct statement
Statement 1 – Dalton’s atomic theory suggested that the atom was indivisible and indestructible.
Statement 2 – Electrons and protons are present inside the atom.
Statement 3 – J.J. Thomson was the first one to propose a model for the structure of an atom.
Statement 4 – Protons are positively charged particle.
(b) Both 3 & 4
(c) Both 1 & 2
(d) All of the above
(2) According to Dalton’s Atomic Theory, matter consists of indivisible _______
(a) Molecules
(d) Mixtures
(3) Who was the first to propose atomic theory?
(a) J.J. Thomson
(b) John Dalton
(c) E. Rutherford
(d) Neilsbhore
(4) “Atom is indivisible and indestructible” why this aspect of Dalton’s atomic theory leds to the failure?
(5) Explain the J.J. Thomson’s model for the structure of an atom?
(4) Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory.
(5) Thomson was the first one to propose a model for the structure of an atom:
Postulate 1: An atom consists of a positively charged sphere with electrons embedded in it.
Postulate 2: An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude
Thomson atomic model is compared to watermelon. Where he considered:
- Watermelon seeds as negatively charged particles
- The red part of the watermelon as positively charged
Rutherford (1871-1937) was known as the ‘Father’ of nuclear physics. He is famous for his work on radioactivity and the discovery of the nucleus of an atom with the gold foil experiment. Ernest Rutherford was interested in knowing how the electrons are arranged within an atom. Rutherford designed an experiment for this. In this experiment, fast moving alpha (α)-particles were made to fall on a thin gold foil. On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
- There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
- The electrons revolve around the nucleus in circular paths.
- The size of the nucleus is very small as compared to the size of the atom.
Drawbacks of Rutherford’s model of the atom: The revolution of the electron in a circular orbit is not expected to be stable. Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know. We know that atoms are quite stable.
(1) Which of the following scientist was known as the ‘Father of nuclear physics?
(2) Positively charged centre in an atom is termed as
(a) Nucleus
(b) Molecule
(d) Protons
(3) Identify the correct statement
Statement 1 – Positively charged centre in an atom called the nucleus.
Statement 2 – The electrons revolve around the nucleus in circular paths.
Statement 3 – Nearly all the mass of an atom resides in the nucleus.
Statement 4 – The size of the nucleus is very small as compared to the size of the atom.
(4) Write the features of Rutherford’s nuclear model of an atom?
(5) Define Nucleus.
(4) Rutherford put forward the nuclear model of an atom, which had the following features:
(5) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
Protons are present in the nucleus of an atom. It is the number of protons of an atom, which determines its atomic number. It is denoted by ‘Z’. All atoms of an element have the same atomic number, Z. In fact, elements are defined by the number of protons they possess. For hydrogen, Z = 1, because in hydrogen atom have only one proton is present in the nucleus. Therefore, the atomic number is defined as the total number of protons present in the nucleus of an atom.
The mass of an atom is practically due to protons and neutrons alone. These are present in the nucleus of an atom. Hence protons and neutrons are also called nucleons. Therefore, the mass of an atom resides in its nucleus. For example, mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u. Similarly, the mass of aluminium is 27 u (13 protons+14 neutrons). The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. It is denoted by ‘A’.
(1) Atomic number is denoted by
(2) The sum of the total number of protons and neutrons present in the nucleus of an atom.
(a) Atomic number
(b) Mass number
(c) Atomic weight
(d) None of the above
(3) Mass number is denoted by
(4) Identify the correct statement
Statement 1 – Protons are present in the nucleus of an atom.
Statement 2 – Atomic number is the number of protons of an atom.
Statement 3 – Atomic number is denoted by ‘Z’.
Statement 4 – The mass of an atom is due to protons and neutrons alone.
(5) Why mass of carbon is 12 u give the reason?
(5) Mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u.
A number of atoms of some elements have the same atomic number but different mass numbers. For example, hydrogen atom, it has three atomic species, namely Protium, Deuterium and Tritium. The atomic number of each one is 1, but the mass number is 1, 2 and 3, respectively. On the basis of these examples, isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers. Therefore, we can say that there are three isotopes of hydrogen atom, namely protium, deuterium and tritium.
Many elements consist of a mixture of isotopes. Each isotope of an element is a pure substance. The chemical properties of isotopes are similar but their physical properties are different.
The mass of an atom of any natural element is taken as the average mass of all the naturally occurring atoms of that element. If an element has no isotopes, then the mass of its atom would be the same as the sum of protons and neutrons in it. But if an element occurs in isotopic forms, then we have to know the percentage of each isotopic form and then the average mass is calculated.
Chemical properties of all the isotopes of an element are the same. Some isotopes have special properties which find them useful in various fields. Such as, an isotope of uranium is used as a fuel in nuclear reactors, isotope of cobalt is used in the treatment of cancer, iodine is used in the treatment of goitre.
(1) The atoms of the same element, having the same atomic number but different mass numbers are termed as __________
(a) Isotopes
(b) Protium
(c) Deuterium
(d) Tritium
(2) Which of the following are the isotopes of hydrogen atom.
(a) Protium
(b) Deuterium
(c) Tritium
Statement 1 – Chemical properties of all the isotopes of an element are the same.
Statement 2 – Physical properties are different.
Statement 3 – Chemical properties of all the isotopes of an element are different.
Statement 4 – Physical properties are same.
(b)Both 3 & 4
(4) Give any two uses of isotopes.
(5) Define isotopes.
(4) Isotopes have special properties which find them useful in various fields. Such as,
- An isotope of uranium is used as a fuel in nuclear reactors,
- Isotope of cobalt is used in the treatment of cancer,
- Isotope of iodine is used in the treatment of goitre.
(5) Isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers.

In order to overcome the objections raised against Rutherford’s model of the atom, Neil’s Bohr put forward the following postulates about the model of an atom:
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy. These orbits or shells are called energy levels. Energy levels in an atom are shown in Fig. A few energy levels in an atom These orbits or shells are represented by the letters K,L,M,N,… or the numbers, n=1,2,3,4,….
(1) The orbits or shells are represented by
(a) Letters
(b) Numbers
(c) Both a & b
(d) Special symbols
(2) These orbits or shells are called
(a) Energy levels
(b) Discrete orbit
(c) Atomic levels
(3) Which of the following book is written by Professor Bohr’s
(a) The Theory of Spectra and Atomic Constitution
(b) Atomic Theory
(c) The Description of Nature
Statement 1 – The orbits or shells are represented by letters only.
Statement 2 – The orbits or shells are represented by numbers only.
Statement 3 – While revolving in discrete orbits the electrons do not radiate energy.
Statement 4 – Certain special orbits known as discrete orbits of electrons.
(a) Both 1 & 2
(5) Write the postulate of Neil’s Bohr model of an atom?
(5) Neil’s Bohr put forward the following postulates about the model of an atom:
- While revolving in discrete orbits the electrons do not radiate energy.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Tripura board class 6 bengali solutions chapter 6 খোকার আকাঙ্ক্ষা, the bangle sellers class 11 mcq questions and answers for semester 1, the second coming by william butler yeats mcq question and answers, case study questions class 7 maths algebraic expressions.
Sign in to your account
Username or Email Address
Remember Me
Class 9 Science Chapter 3 Case Based Questions - Atoms and Molecules
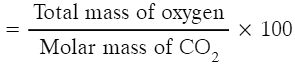
Q3: 0.25 mole of an element ‘X’ is 9.75 g. What is X ? Ans: 0.25 mole of X = 9.75 g 1 mole of X = 9.75 ÷ 0.25 = 39.0 g mol –1 The element is Potassium.
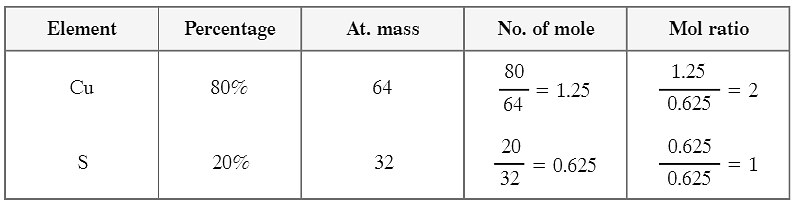
Q3: Write name of (NH 4 ) 2 SO 4 Ans: Ammonium sulphate.
Q4: Give one example of polyatomic anion. Ans: CO 3 2- (Carbonate).
Top Courses for Class 9
Study material, sample paper, viva questions, video lectures, shortcuts and tricks, important questions, extra questions, mock tests for examination, objective type questions, semester notes, past year papers, previous year questions with solutions, practice quizzes.

Case Based Question Answer: Atoms and Molecules Free PDF Download
Importance of case based question answer: atoms and molecules, case based question answer: atoms and molecules notes, case based question answer: atoms and molecules class 9 questions, study case based question answer: atoms and molecules on the app, welcome back, create your account for free.

Forgot Password
Unattempted tests, change country, practice & revise.
myCBSEguide
- Class 9 Science Case...
Class 9 Science Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
If you are wondering how to solve class 9 science case study questions, then myCBSEguide is the best platform to choose. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions.
You can find a wide range of solved case studies on myCBSEguide, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
The rationale behind Science
Science is crucial for Class 9 students’ cognitive, emotional, and psychomotor development. It encourages curiosity, inventiveness, objectivity, and aesthetic sense.
In the upper primary stage, students should be given a variety of opportunities to engage with scientific processes such as observing, recording observations, drawing, tabulating, plotting graphs, and so on, whereas in the secondary stage, abstraction and quantitative reasoning should take a more prominent role in science teaching and learning. As a result, the concept of atoms and molecules as matter’s building units, as well as Newton’s law of gravitation, emerges.
Science is important because it allows Class 9 Science students to understand the world around us. It helps to find out how things work and to find solutions to problems at the Class 9 Science level. Science is also a source of enjoyment for many people. It can be a hobby, a career, or a source of intellectual stimulation.
Case study questions in Class 9 Science
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Examples of Class 9 science class case study questions
Class 9 science case study questions have been prepared by myCBSEguide’s qualified teachers. Class 9 case study questions are meant to evaluate students’ knowledge and comprehension of the material. They are not intended to be difficult, but they will require you to think critically about the material. We hope you find Class 9 science case study questions beneficial and that they assist you in your exam preparation.
The following are a few examples of Class 9 science case study questions.
Class 9 science case study question 1
- due to its high compressibility
- large volumes of a gas can be compressed into a small cylinder
- transported easily
- all of these
- shape, volume
- volume, shape
- shape, size
- size, shape
- the presence of dissolved carbon dioxide in water
- the presence of dissolved oxygen in the water
- the presence of dissolved Nitrogen in the water
- liquid particles move freely
- liquid have greater space between each other
- both (a) and (b)
- none of these
- Only gases behave like fluids
- Gases and solids behave like fluids
- Gases and liquids behave like fluids
- Only liquids are fluids
Answer Key:
- (d) all of these
- (a) shape, volume
- (b) the presence of dissolved oxygen in the water
- (c) both (a) and (b)
- (c) Gases and liquids behave like fluids
Class 9 science case study question 2
- 12/32 times
- 18 g of O 2
- 18 g of CO 2
- 18 g of CH 4
- 1 g of CO 2
- 1 g of CH 4 CH 4
- 2 moles of H2O
- 20 moles of water
- 6.022 × 1023 molecules of water
- 1.2044 × 1025 molecules of water
- (I) and (IV)
- (II) and (III)
- (II) and (IV)
- Sulphate molecule
- Ozone molecule
- Phosphorus molecule
- Methane molecule
- (c) 8/3 times
- (d) 18g of CH 4
- (c) 1g of H 2
- (d) (II) and (IV)
- (c) phosphorus molecule
Class 9 science case study question 3
- collenchyma
- chlorenchyma
- It performs photosynthesis
- It helps the aquatic plant to float
- It provides mechanical support
- Sclerenchyma
- Collenchyma
- Epithelial tissue
- Parenchyma tissues have intercellular spaces.
- Collenchymatous tissues are irregularly thickened at corners.
- Apical and intercalary meristems are permanent tissues.
- Meristematic tissues, in its early stage, lack vacuoles, muscles
- (I) and (II)
- (III) and (I)
- Transpiration
- Provides mechanical support
- Provides strength to the plant parts
- None of these
- (a) Collenchyma
- (b) help aquatic plant to float
- (b) Sclerenchyma
- (d) Only (III)
- (c) provide strength to plant parts
Cracking Class 9 Science Case Study Questions
There is no one definitive answer to Class 9 Science case study questions. Every case study is unique and will necessitate a unique strategy. There are, nevertheless, certain general guidelines to follow while answering case study questions.
- To begin, double-check that you understand the Class 9 science case study questions. Make sure you understand what is being asked by reading it carefully. If you’re unclear, seek clarification from your teacher or tutor.
- It’s critical to read the Class 9 Science case study material thoroughly once you’ve grasped the question. This will provide you with a thorough understanding of the problem as well as the various potential solutions.
- Brainstorming potential solutions with classmates or other students might also be beneficial. This might provide you with multiple viewpoints on the situation and assist you in determining the best solution.
- Finally, make sure your answer is presented simply and concisely. Make sure you clarify your rationale and back up your claim with evidence.
A look at the Class 9 Science Syllabus
The CBSE class 9 science syllabus provides a strong foundation for students who want to pursue a career in science. The topics are chosen in such a way that they build on the concepts learned in the previous classes and provide a strong foundation for further studies in science. The table below lists the topics covered in the Class 9 Science syllabus of the Central Board of Secondary Education (CBSE). As can be seen, the Class 9 science syllabus is divided into three sections: Physics, Chemistry and Biology. Each section contains a number of topics that Class 9 science students must study during the course.
CBSE Class 9 Science (Code No. 086)
Theme: Materials Unit I: Matter-Nature and Behaviour Definition of matter; solid, liquid and gas; characteristics – shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation. Nature of matter: Elements, compounds and mixtures. Heterogeneous and homogenous mixtures, colloids and suspensions. Particle nature and their basic units: Atoms and molecules, Law of constant proportions, Atomic and molecular masses. Mole concept: Relationship of mole to mass of the particles and numbers. Structure of atoms: Electrons, protons and neutrons, valency, the chemical formula of common compounds. Isotopes and Isobars.
Theme: The World of the Living Unit II: Organization in the Living World Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number. Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Theme: Moving Things, People and Ideas Unit III: Motion, Force and Work Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, derivation of equations of motion by graphical method; elementary idea of uniform circular motion. Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum. Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy. Work, energy and power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy. Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Theme: Food Unit IV: Food Production Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRESCRIBED BOOKS:
- Science-Textbook for class IX-NCERT Publication
- Assessment of Practical Skills in Science-Class IX – CBSE Publication
- Laboratory Manual-Science-Class IX, NCERT Publication
- Exemplar Problems Class IX – NCERT Publication
myCBSEguide: A true helper
There are numerous advantages to using myCBSEguide to achieve the highest results in Class 9 Science.
- myCBSEguide offers high-quality study materials that cover all of the topics in the Class 9 Science curriculum.
- myCBSEguide provides practice questions and mock examinations to assist students in the best possible preparation for their exams.
- On our myCBSEguide app, you’ll find a variety of solved Class 9 Science case study questions covering a variety of topics and concepts. These case studies are intended to help you understand how certain principles are applied in real-world settings
- myCBSEguide is that the study material and practice problems are developed by a team of specialists who are always accessible to assist students with any questions they may have. As a result, students may be confident that they will receive the finest possible assistance and support when studying for their exams.
So, if you’re seeking the most effective strategy to study for your Class 9 Science examinations, myCBSEguide is the place to go!
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
Talk to our experts
1800-120-456-456
NCERT Solutions for Class 9 Science Chapter 3 - Atoms And Molecules
- NCERT Solutions
- Chapter 3 Atoms And Molecules

NCERT Solutions for Class 9 Science Chapter 3 - Atoms and Molecules
There is good news for all students struggling with the NCERT problems of Class 9 Atoms and Molecules chapter. The NCERT Solutions for Class 9 Science Chapter 3 PDF file prepared by Vedantu experts are now available for free download. Our experts have followed a very easy approach in preparing these CBSE Solutions for Class 9 Science Atoms and Molecules chapter. Referring to the Class 9 Science Chapter 3 PDF will help you solve and practice all the exercise questions precisely. So, download the PDF and refer to the solutions for effective exam preparation. Maths Students who are looking for the better solutions ,they can download Class 9 Maths NCERT Solutions to help you to revise complete syllabus and score more marks in your examinations.

Access NCERT Solutions for Science Class 9 Chapter 3 – Atoms and Molecules
Intext Exercise 1
1. In a reaction, 5.3g of sodium carbonate reacted with 6g of ethanoic acid. The products were 2.2g of carbon dioxide, 0.9g water and 8.2g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Ans: Given,
Mass of sodium carbonate = 5.3 g
Mass of ethanoic acid = 6 g
Mass of sodium ethanoate = 8.2 g
Mass of carbon dioxide = 2.2 g
Mass of water = 0.9 g
Now, total mass before the reaction \[ = {\text{ }}\left( {5.3{\text{ }} + {\text{ }}6} \right){\text{ }}g{\text{ }} = {\text{ }}11.3{\text{ }}g\]
And, total mass after the reaction =\[\;\left( {8.2{\text{ }} + {\text{ }}2.2{\text{ }} + {\text{ }}0.9} \right){\text{ }}g{\text{ }} = {\text{ }}11.3{\text{ }}g\]
∴Total mass before the reaction = Total mass after the reaction
Hence, this is in agreement with the law of conservation of mass.
2. Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Ans: In water, H:O (by mass) =$1\;:\;8$
The mass of oxygen gas required to react completely with 1 g of hydrogen gas = 8 g.
the mass of oxygen gas required to react completely with 3 g of hydrogen gas = \[(8\; \times \;3)\;g\; = \;24\;g\]
3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Ans: The postulate of Dalton’s atomic theory which is based on the law of conservation of mass is: “Atoms are indivisible particles, which can neither be created nor destroyed in a chemical reaction.”
4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Ans: “The elements consist of atoms having fixed mass and that the number and kind of atoms of each element in a given compound is fixed.” This explains the law of definite proportion.
Intext Exercise 2
1. Define atomic mass unit.
Ans: Mass unit equal to exactly one-twelfth the $\left( {\dfrac{1}{{{{12}^{th}}}}} \right)$mass of one atom of carbon-12 is called one atomic mass unit. It is represented by as ‘a.m.u.’ or ‘u’.
2. Why is it not possible to see an atom with naked eyes?
Ans: Due to small size of an atom we cannot see them with naked eyes.
Intext Exercise 3
1. Write down the formulae of
(i) Sodium oxide
Ans: Sodium Oxide: \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\]
(ii) Aluminium chloride
Ans: Aluminium chloride: \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]
(iii) sodium sulphide
Ans: Sodium sulphide: \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}\]
(iv) Magnesium hydroxide
Ans: Magnesium hydroxide: ${\text{Mg(OH}}{{\text{)}}_{\text{2}}}$
2. Write down the names of compounds represented by the following formulae:
i) $\mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right) \mathrm{_3}$
Ans: Aluminium sulphate
ii) ${\text{CaC}}{{\text{l}}_{\text{2}}}$
Ans: Calcium chloride
iii) ${{\text{K}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$
Ans: :Potassium sulphate
iv) ${\text{KN}}{{\text{O}}_3}$
Ans: Potassium nitrate
v) \[{\text{CaC}}{{\text{O}}_{\text{3}}}\]
Ans: : Calcium carbonate
3. What is meant by the term chemical formula?
Ans: The symbolic representation of composition of a compound is known as chemical formula. Chemical formula gives us the idea of number of atoms present.
Example: from the chemical formula ${\text{C}}{{\text{O}}_{\text{2}}}$ of Carbon Dioxide, we come to know that one carbon atom and two oxygens atoms are chemically bonded together to form one molecule of the compound, carbon dioxide.
4. How many atoms are present in a:
i) $\mathrm{H}_{2}$ S molecule
Ans: There are total 3 atoms present in ${{\text{H}}_{\text{2}}}{\text{S}}$ molecule, two hydrogen atoms and one Sulphur atom.
ii) \[{\text{PO}}_4^{3 - }\] ion
Ans: There are total 5 atoms in \[{\text{PO}}_4^{3 - }\] ion, one phosphorus atom and 4 oxygen atoms.
Intext Exercise 4
1. Calculate the molecular masses of \[{{\text{H}}_2},{{\text{O}}_2},{\text{C }}{{\text{l}}_2},{\text{C}}{{\text{O}}_2},{\text{C}}{{\text{H}}_4},{{\text{C}}_2}{{\text{H}}_6},{{\text{C}}_2}{{\text{H}}_4},{\text{N}}{{\text{H}}_3},{\text{C}}{{\text{H}}_3}{\text{OH}}.\]
Ans: Molecular mass of ${{\text{H}}_{\text{2}}}$ = $2\; \times $ Atomic mass of H
$ = \;2\; \times \;1\;{\text{u = }}\;{\text{2 u}}$
Molecular mass of ${{\text{O}}_{\text{2}}}$ = $2\; \times $ Atomic mass of O
$ = \;2\; \times \;16\;{\text{u = }}\;3{\text{2 u}}$
Molecular mass of ${\text{C }}{{\text{l}}_{\text{2}}}$ = $2\; \times $ Atomic mass of Cl
$ = \;2\; \times \;35.5\;{\text{u = }}\;71{\text{ u}}$
Molecular mass of ${\text{C}}{{\text{O}}_{\text{2}}}$ = Atomic mass of C $ + $ $2\; \times $Atomic mass of O
$ = \;(12 + 2\; \times \;16)\;{\text{u = }}\;44{\text{ u}}$
Molecular mass of ${\text{C}}{{\text{H}}_4}$ = Atomic mass of C $ + $ $4\; \times $Atomic mass of H
$ = \;(12 + 4\; \times \;1)\;{\text{u = }}\;16{\text{ u}}$
Molecular mass of ${{\text{C}}_2}{{\text{H}}_6}$ = $2\; \times $Atomic mass of C $ + $ $6\; \times $Atomic mass of H
$ = \;(2 \times 12 + 6\; \times \;1)\;{\text{u = }}\;30{\text{ u}}$
Molecular mass of ${{\text{C}}_2}{{\text{H}}_4}$ = $2\; \times $Atomic mass of C $ + $ $4\; \times $Atomic mass of H
Molecular mass of ${\text{N}}{{\text{H}}_3}$ = Atomic mass of N $ + $ $3\; \times $Atomic mass of H
$ = \;(14 + 3\; \times \;1)\;{\text{u = }}\;17{\text{ u}}$
Molecular mass of ${\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}$ = Atomic mass of C $ + $ $3\; \times $Atomic mass of H $ + $ Atomic mass of O
$ + $ Atomic mass of $ = \;(12 + 4\; \times \;1\; + \;16)\;{\text{u = }}\;32{\text{ u}}$
2. Calculate the formula unit masses of \[{\text{ZnO}},\;{\text{N}}{{\text{a}}_2}{\text{O}},\;{{\text{K}}_2}{\text{C}}{{\text{O}}_3}\]given atomic masses of \[{\text{Z}} = 65{\text{u}},{\text{Na}} = 23\;{\text{u}},\;{\text{K}} = 39\;{\text{u}},\;{\text{C}} = 12{\text{u}},\;{\text{and O}} = 16{\text{u}}{\text{.}}\]
Ans: Formula unit mass of ZnO = Atomic mass of Zn + Atomic mass of O
\[ = \;(65 + 16)\;{\text{u = 81}}\;{\text{u}}\]
Formula unit mass of ${\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}$ = $2\; \times $ Atomic mass of Na + Atomic mass of O
= \[(2\; \times \;23\; + \;16)\;{\text{u}}\; = \;62\;{\text{u}}\]
Formula unit mass of ${{\text{K}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}$ = $2\; \times $ Atomic mass of K + Atomic mass of C + $3\; \times $ Atomic mass O=
$=(2 \times 39+12+3 \times 16) \mathrm{u} $
$=138 \mathrm{u}$
Refer to page 42.
1. If one mole of carbon atoms weighs 12 gram, what is the mass (in gram) of 1 atom of carbon?
Ans: Given mass of One mole of carbon atoms = 12 g
Therefore , Mass of \[6.022 \times {10^{23}}\] number of carbon atoms = 12 g
Mass of 1 atom of carbon will be:
$ = \dfrac{{12}}{{6.022 \times {{10}^{23}}}}g$
$ = 1.9927 \times {{10}^{ - 23}}{\text{g}}$
2. Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given, atomic mass of Na = 23 u, Fe = 56 u)?
Ans: Atomic mass of Na = 23 u (Given)
Then, gram atomic mass of Na = 23 g
Now, 23 g of Na contains = \[6.022 \times {10^{23}}\] number of Na atoms
Thus, 100 g of Na contains = \[\dfrac{{6.022 \times {{10}^{23}}}}{{23}} \times 100\] number of Na atoms
\[ = 2.6182 \times {10^{24}}{\text{number of}}\;{\text{Na}}\;{\text{atoms}}\]
Atomic mass of Fe = 56 u (Given)
Then, gram atomic mass of Fe = 56 g
Now, 56 g of Fe contains = \[6.022 \times {10^{23}}\] number of Fe atoms
Thus, 100 g of Fe contains = \[\dfrac{{6.022 \times {{10}^{23}}}}{{56}} \times 100\] number of Fe atoms
\[ = 1.0753 \times {10^{24}}{\text{number of Fe atoms}}\]
\[2.6182 \times {10^{24}} > 1.0753 \times {10^{24}}\]
Therefore, 100 grams of sodium contain a greater number of atoms than 100 grams of iron.
NCERT QUESTIONS:
1. A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Given,
Mass of boron = 0.096 g
Mass of oxygen = 0.144 g
Mass of sample = 0.24 g
The percentage of boron by weight in the compound \[ = \dfrac{{0.096}}{{0.24}} \times 100\% \; = \;40\;\% \]
And, percentage of oxygen by weight in the compound \[ = \dfrac{{0.144}}{{0.24}} \times 100\% \; = \;60\;\% \]
2. When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combinations will govern your answer?
Ans: Carbon + Oxygen ⎯⎯→ Carbon dioxide
3 g of carbon reacts with 8 g of oxygen to produce 11 g of carbon dioxide.
If 3 g of carbon is burnt in 50 g of oxygen, then 3 g of carbon will react with 8 g of oxygen to form11 g of carbon dioxide.
The remaining (50 –8) = 42 g of oxygen will be left unreacted.
The above answer is governed by the law of constant proportions.
3. What are polyatomic ions? Give examples?
Ans: A polyatomic ion is a group of atoms carrying a charge either positive or negative.
For example,\[\;{\text{ammonium ion}}\;\left( {{\text{NH}}_4^ + } \right),\;{\text{hydroxide ion}}\;\left( {{\text{O}}{{\text{H}}^ - }} \right),\;{\text{carbonate ion}},\;\left( {{\text{CO}}_3^{2 - }} \right){\text{,}}\;\;{\text{sulphate ion}}\;\left( {{\text{SO}}_4^{2 - }} \right)\]
4. Write the chemical formulae of the following:
(a) Magnesium chloride
Ans: \[{\text{MgC}}{{\text{l}}_{\text{2}}}\]
(b) Calcium oxide
(c) Copper nitrate
Ans: \[{\text{Cu}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}}\]
(d) Aluminium chloride
Ans: \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]
(e) Calcium carbonate
Ans: \[{\text{CaC}}{{\text{O}}_{\text{3}}}\]
5. Give the names of the elements present in the following compounds:
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate
6. Calculate the molar mass of the following substances:
a) Ethyne ${{\text{C}}_{_{\text{2}}}}{{\text{H}}_{\text{2}}}$
Ans: Molar mass of \[{{\text{C}}_2}{{\text{H}}_2} = 2 \times 12 + 2 \times 1 = 28{\text{g}}/{\text{mol}}\]
b) Sulphur molecule, ${{\text{S}}_{\text{8}}}$
Ans: Molar mass of \[{{\text{S}}_8} = 8 \times 32 = 256{\text{g}}/{\text{mol}}\]
c) Phosphorus molecule \[{{\text{P}}_{\text{4}}}\] (atomic mass of phosphorus = 31)
Ans: Molar mass of \[{{\text{P}}_4} = 4 \times 31 = 124{\text{g}}/{\text{mol}}\]
c) Hydrochloric acid, HCl
Ans: Molar mass of \[{\text{HCl}} = 1 + 35.5 = 36.5{\text{g}}/{\text{mol}}\]
d) Nitric acid, \[{\text{HN}}{{\text{O}}_{\text{3}}}\]
Ans: Molar mass of \[{\text{HN}}{{\text{O}}_3} = 1 + 14 + 3 \times 16 = 63{\text{g}}/{\text{mol}}\]
7. What is the mass of:
(a) 1 mole of nitrogen atoms?
Ans: The mass of 1 mole of N- atoms = 14 g
(b) 4 moles of aluminium atoms (Atomic mass of aluminium = 27)?
Ans: \[[{\text{Atomic mass of}}\;{\text{Al}} = 27{\text{u}}]\]
The mass of 4 moles of Al-atoms \[ = (4 \times 27){\text{g}}\;{\text{ = }}\;\;{\text{108}}\;{\text{g}}\]
(c) 10 moles of sodium sulphite \[\left( {{\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}} \right)\]?
Ans: Atomic mass of Na = 23 u, Atomic mass of S = 32 u, Atomic mass of O = 16 u
The mass of 10 moles of sodium sulphite \[\left( {{\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}} \right)\]\[ = 10 \times [2 \times 23 + 32 + 3 \times 16]{\text{g}}\]
\[ = 10 \times 126{\text{g}} = 1260{\text{g}}\]
8. Convert into mole.
(a) 12 g of oxygen gas
Ans: 32 g of oxygen gas = 1 mole
Then, 12 g of oxygen gas $ = \dfrac{{12}}{{32}}\;{\text{moles}}\; = \;1.11\;{\text{moles}}$
(b) 20 g of water
Ans: 18 g of Water ${{\text{H}}_{\text{2}}}{\text{O}}$ = 1 mole
Then, 20 g of water ${{\text{H}}_{\text{2}}}{\text{O}}$ $ = \dfrac{{20}}{{18}}\;{\text{moles}}\; = \;0.375\;{\text{moles}}$
(c) 22 g of carbon dioxide
Ans: 44 g of Carbon Dioxide ${\text{C}}{{\text{O}}_{\text{2}}}$ = 1 mole
Then, 22 g of water Carbon Dioxide ${\text{C}}{{\text{O}}_{\text{2}}}$ $ = \dfrac{{22}}{{44}}\;{\text{moles}}\; = \;0.5\;{\text{moles}}$
9. What is the mass of:
(a) 0.2 mole of oxygen atoms?
Ans: Mass of 1 mole of oxygen atoms = 16 g
Then, mass of 0.2 mole of oxygen atoms \[ = {\text{ }}0.2{\text{ }} \times {\text{ }}16g{\text{ }} = {\text{ }}3.2{\text{ }}g\]
(b) 0.5 mole of water molecules?
Ans: Mass of 1 mole of water molecules (${{\text{H}}_{\text{2}}}{\text{O}}$) = 18 g
Then, mass of 0.5 mole of water molecules (${{\text{H}}_{\text{2}}}{\text{O}}$) \[ = 0.5{\text{ }} \times {\text{ }}18{\text{ }}g{\text{ }} = {\text{ }}9{\text{ }}g\]
10. Calculate the number of molecules of sulphur (${{\text{S}}_{\text{8}}}$) present in 16 g of solid sulphur.
Ans: 1 mole of solid sulphur (${{\text{S}}_{\text{8}}}$) = \[8{\text{ }} \times {\text{ }}32{\text{ }}g{\text{ }} = {\text{ }}256{\text{ }}g\]
i.e., 256 g of solid sulphur contains = \[6.022 \times {10^{23}}\] molecules
Then, 16 g of solid sulphur contains = \[\dfrac{{6.022 \times {{10}^{23}}}}{{256}} \times 16\] molecules
= \[ = 3.76 \times {10^{22}}{text{\;molecules}}\]
11. Calculate the number of aluminium ions present in 0.051 g of aluminium oxide. (Hint: The mass of an ion is the same as that of an atom of the same element. Atomic mass of Al = 27 u).
Ans: Given, atomic mass of $Al=27 \mathrm{u}$, atomic mass of $\mathrm{O}=16 \mathrm{u}$
1 mole of aluminium oxide $\left(\mathrm{Al}_{2} \mathrm{O}_{3}\right)=(2 \times 27+3 \times 16)=102 \mathrm{~g}$
i.e., $102 \mathrm{~g}$ of $\mathrm{Al}_{2} \mathrm{O}_{3}=6.022 \times 10^{23}$ molecules of $\mathrm{Al}_{2} \mathrm{O}_{3}$
Then, $0.051 \mathrm{~g}$ of $\mathrm{Al}_{2} \mathrm{O}_{3}$ contains;
$=\frac{6.022 \times 10^{2}}{102} \times 0.051$ molecules of $\mathrm{Al}_{2} \mathrm{O}_{3}$
$=3.011 \times 10^{20}$ molecules of $\mathrm{Al}_{2} \mathrm{O}_{3}$
The number of aluminium ions $\left(\mathrm{Al}^{3+}\right)$ present in 1 molecule of aluminium oxide is 2 .
Therefore, the number of aluminium ions $\left.(A]^{3}\right)$ present in $3.011 \times 10^{20}$ molecules $(0.051 \mathrm{~g})$ of aluminium oxide $\left(\mathrm{Al}_{2} \mathrm{O}_{3}\right)=2 \times 3.011 \times 10^{20}=6.022 \times 10^{20}$
NCERT Solutions for Class 9 Science – Free PDF Download
You can opt for Chapter 3 - Structure of Atom NCERT Solutions for Class 9 Science PDF for Upcoming Exams and also You can Find the Solutions of All the Maths Chapters below.
The NCERT Solutions for Class 9 Science Chapter 3 on Vedantu can be accessed by students online and can also be downloaded for free. Following these NCERT solutions will help you get an insight into all the concepts covered in this chapter.
Chapter 3 – Atoms and Molecules NCERT Solutions for Class 9 Science Chapters
Important topics covered in Class 9 Science Chapter 3- Atoms And Molecules
Atoms And Molecules
Laws of Chemical Combination
Law of Constant Proportions
What is an Atom?
Atomic Mass
What is a Molecule?
Molecules of Elements
What is an Ion?
Writing Chemical Formulae
Formulae of Simple Compounds
Molecular Mass and Mole Concept
Mole Concept
Key points covered in Class 9 Science Chapter 3 NCERT Solutions
The total mass of the reactants and products remains constant during a chemical reaction. This is referred to as the Mass Conservation Law.
Elements are always present in a defined proportion by mass in pure chemical composition. It is called the Law of Definite Proportions.
A molecule is the tiniest particle of an element or compound that may exist on its own under normal conditions. It displays all of the qualities or properties of the compound.
A compound's chemical formula lists its constituent elements as well as the number of atoms in each combining element.
The charge on each ion is used to calculate the chemical formula of an ionic molecule.
The relative atomic mass scale is used by scientists to compare the masses of various atoms in different elements. The relative atomic mass of carbon-12 isotopes is 12, and the relative masses of all other atoms are calculated by comparing them to the mass of a carbon-12 atom.
The number of atoms in exactly 12 g of carbon-12 is specified as the Avogadro constant 6.022 x 10 23 .
A mole is defined as the quantity of an element or a compound that has the same number of particles (atoms, ions, molecules, formula units, and so on) as there are atoms in 12 g of carbon-12.
The molar mass of a material is defined as the mass of one mole of that substance.
3.1 Law of Chemical Combination
There are two laws of chemical combination- the law of conservation of mass, and the law of constant proportion. Both these laws are discussed in detail in chapter 3, science class 9.
There is one important question related to the chemical reaction. Is there any change in the total mass of the system when a chemical reaction takes place? According to the law of conservation of mass, mass can neither be created nor be destroyed. The same logic stands true for chemical reactions as well. Several experiments carried out by Lavoisier standardized this law. This law is discussed in NCERT Solutions for Class 9 Science Chemistry Chapter 3.
The law of chemical combination is based on another law- the law of constant proportion. This law was also the result of experiments conducted by Lavoisier and Joseph L. Proust. According to the law of constant proportion, any compound is made by mixing two or more elements in a constant proportion, and this proportion does not change irrespective of the source of the compounds. For example, in water, the ratio of the mass of Hydrogen to the mass of Oxygen is 1:8. The ratio of the mass is the same for water from different sources. This law is also known as the law of definite proportion. The law of constant proportion is explained thoroughly in Class 9 Science Chapter 3.
3.2 What is an Atom?
The issue that the scientist faced with the law of constant proportion is to provide a possible explanation for this. Eminent scientist John Dalton tried to provide an explanation and introduced the concept of atoms. An atom is the smallest unit of matter. They are so small that thousands of them are stacked in your hair. The atom of one element is different from that of the other elements. Therefore, each of these atoms is depicted by different symbols.
Dalton used different combinations of straight lines and circles for denoting various atoms. However, the modern-day symbols consider the first or the first two letters of the element's name to denote an atom of the element. For example, Hydrogen is denoted by H and Oxygen by O. In some cases, and the Latin names are also considered. For example, the Latin name of Potassium is Kalium, and it is denoted by K. A list of names of elements is shown in Atom and Molecules Class 9.
Every atom has a definite mass, called the atomic mass unit. The atomic mass is the characteristic of the atom. The concept of atomic mass supports the law of constant proportion and the law of conservation of mass. A detailed analysis of atomic mass is provided in the NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules.
However, atoms cannot exist freely in nature. It interacts with the same or different atoms to form molecules. They can also form ions. These ions or molecules aggregate to form elements of compounds that we can see. These concepts are covered in Ch 3 Science Class 9.

3.3 What is Molecule?
A group of two or more atoms is joined together to form a molecule. A molecule can exist freely in nature. For example, Hydrogen, Oxygen, Calcium molecules can exist freely in nature. Molecules can be created from the same atoms or different atoms. If the molecules are made from the same kind of atoms, they are called molecules of elements. For example, Sodium and Potassium elements. Examples of molecules of elements are shown in Atoms and Molecules Class 9 NCERT Solutions.
Molecules can also be made from dissimilar atoms. When different atoms come together, they form a compound. The molecules made of different atoms are called molecules of compounds. Atoms and Molecules Class 9 NCERT PDF contains more examples of molecules of compounds.
Sometimes, the atoms can be charged. Charged atoms are called ions. An ion can always be charged positively or negatively charged. Positively charged ones are known as cations, while negative ones are known as anions. Sometimes, a molecule can contain more than one ion. Such molecules make a polyatomic ion. The individual charges on each atom determine the net charge of such ions. To learn more about polyatomic ions, go through Class 9 Science Chapter 3.
3.4 Writing Chemical Formulae
The symbolic representation of the compounds is said to be their chemical formulae. The chemical formula considers the net charge of individual ions in the formula. The charge of individual ions determines the valency of the radical. The valency of the individual ions determines how many ions of a particular kind will take part in the interaction.
For the proper representation of the chemical formula, the valency of individual ions must be balanced. The metal names should come before any non-metal name. In the case of polyatomic ions, the individual elements must be kept in a bracket if their overall valency is more than one. Otherwise, there is no need to use a bracket—for example, NaOH. You will learn more about writing chemical formulas when you go through the NCERT Solutions for Class 9 Science Chapter 3.
3.5 Molecular Mass and Mole Concept
The molecular mass of a compound is the sum of all the atomic masses. Therefore, the molecular mass is represented in atomic mass units. Read NCERT solutions for class 9 science chapter atoms and molecules to learn more about molecular mass.
Formula unit mass is calculated similarly to molecular mass. However, the ions’ mass is considered in calculating the formula unit mass of a compound. Learn more about formula mass units in ch 3 science class 9.
Mole concept is another important concept related to atoms and molecules. It defines the total number of molecules taking part in a chemical reaction. You can learn the mole concept in detail in Atoms and Molecules Class 9.
Exercise 3.5 total Solutions: 11 Questions (6 short question and 5 Long questions).
Key Features of NCERT Solutions for Class 9 Science Chapter 3
NCERT Solutions for Class 9 Science Chapter 3 has the following features.
Chapter 3 Science Class 9, is presented comprehensively.
All the concepts covered in the Atom and Molecules Class 9 chapter are explained with the help of relevant examples in these NCERT Solutions.
The practice exercise of NCERT Class 9 Science Chapter 3 is solved as per the CBSE guidelines so that students can score good marks in the examination.
The pointwise approach of chemistry ch 3 class 9 NCERT solutions help students revise the chapter before the examination.
The topics covered in ch 3 science class 9 become easily understandable when students go through the atoms and molecules class 9 NCERT solutions.
Along with this, students can also view additional study materials provided by Vedantu, for Class 9
NCERT Solutions For Class 9
Revision Notes for Class 9
CBSE Class 9 Syllabus
NCERT Solutions for Class 9 Science
Chapter 1 - Matter in Our Surroundings
Chapter 2 - Is Matter Around us Pure
Chapter 4 - Structure of Atom
Chapter 5 - The Fundamental Unit of Life
Chapter 6 - Tissues
Chapter 7 - Diversity in Living Organisms
Chapter 8 - Motion
Chapter 9 - Force and Laws of Motion
Chapter 10 - Gravitation
Chapter 11 - Work and Energy
Chapter 12 - Sound
Chapter 13 - Why do We Fall ill
Chapter 14 - Natural Resources
Chapter 15 - Improvement in Food Resources
NCERT Solutions by Vedantu are comprehensive and cover all of the important concepts in the CBSE syllabus . They are also aligned with the latest CBSE exam pattern and the types of questions that are asked in the exams. Therefore, students who study NCERT Solutions from Vedantu are more likely to do well in the CBSE Exam. Specifically, for Chapter 3, students should mandatorily study all of the NCERT Solutions in order to get a good understanding of the concepts and the types of questions that are asked in the exam. This is because Chapter 3 is a very important chapter, and it covers a lot of the material that is tested on the CBSE Exam. We encourage students to download NCERT Solutions for Chapter 3 and to use them to prepare for the CBSE Exam.

FAQs on NCERT Solutions for Class 9 Science Chapter 3 - Atoms And Molecules
1.What is an Atom?
When you study ch 3 science class 9, you will have a clear concept about atoms. Atoms are the smallest component of any matter that can exist independently in nature. However, some atoms cannot exist freely and form molecules with the same type of atoms or form compounds with different atoms. Go through the class 9th science chapter 3 for a detailed explanation of the concepts related to atoms and molecules.
2. What is a Molecule, as per the NCERT solutions?
When atoms combine, they form a molecule. A minimum of two atoms can form a molecule. A molecule can be made of the same or different atoms. To completely understand the concept of molecules, refer to the atoms and molecules class 9 solutions.
3. Mention the topics included in Chapter 3 Atoms and Molecules of NCERT Solutions for Class 9 Science.
The topics that are included in Chapter 3 of NCERT Solutions for Class 9 Science are Law of Chemical Combination, What is an Atom, What is Molecule, Writing Chemical Formulae, Molecular Mass and Mole Concept. These topics are in a strategic manner such that they best serve the NCERT and impart the right kind of knowledge at the same time. The questions from these topics are the most frequent in the examination and they are expanded upon in higher classes, so it’s best if the students do them thoroughly.
4. What are the key features of NCERT Solutions for Chapter 3 Atoms and Molecules of Class 9 Science?
There are several features in NCERT Solutions for Class 9 Science Chapter 3.
The information in Chapter 3 Science Class 9 is provided in detail. In these NCERT Solutions, all of the topics discussed in the Atom and Molecules Class 9 chapter are explained with applicable examples. The NCERT Class 9 Science Chapter 3 practise assignment is answered according to CBSE rules so that students may do well in the test. Chemistry Chapter 3 Class 9 NCERT answers use a point-by-point method to assist students to review the material before the test. When students work through the atoms and molecules class 9 NCERT answers, the concepts covered in Chapter 3 Science Class 9 become clear.
These solutions are available on Vedantu's official website( vedantu.com ) and mobile app free of cost.
5. What is the chemical formula of compounds as per the NCERT solutions?
Chemical formulas are believed to be the symbolic representation of substances. The net charge of each ion in the formula is taken into account in the chemical formula. The radical's valency is determined by the charge of individual ions. The valency of individual ions affects how many of each type of ion will participate in the interaction. The valency of individual ions must be balanced in order to properly represent the chemical formula. Metal names should appear first, followed by non-metal names.
6. What is a molecule made up of as per the NCERT solutions?
A molecule is made up of two or more atoms linked together. In nature, a molecule can exist without restriction. Hydrogen, oxygen, and calcium molecules, for example, can exist freely in nature. Molecules can be made from the same atoms or from atoms that are different. When molecules are made up of the same type of atoms, they are referred to as element molecules. Elements such as sodium and potassium, for example. Molecules can also be formed from atoms with different properties. A compound is formed when various atoms join together.
7. What is atomic mass as per the NCERT solutions?
The atomic mass unit is the unit of mass for each atom. The atomic mass is an atom's property. The law of constant proportion and the law of mass conservation are both supported by the idea of atomic mass. The NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules provides a thorough examination of atomic mass. There are a lot of properties of atoms that are discussed in Chapter 3 of Class 9 Science NCERT.
NCERT Solutions for Class 9

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study and Passage Based Questions for Class 9 Science Chapter 4 Structure of Atom
- Last modified on: 2 years ago
- Reading Time: 4 Minutes
Case Study Questions for Class 9 Science Chapter 4 Structure of Atom
In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based on it will be asked.
Here, we have provided case based/passage based questions for Class 9 Science Chapter 4 Structure of Atom . Students can practice these questions for their exam.
Case Study/Passage Based Questions
Question 1:
Read the following and answer any four questions from (i) to (iii).
The maximum number of the electrons which are permitted to be assigned to an energy shell of an atom is called the electron capacity of that shell. The distribution of electrons in different orbits or shell is governed by a scheme known as Bohr-Bury scheme. According to this scheme : (a) The maximum number of the electrons that can be present in any shell is given by the formula 2n 2 where, n is the number of energy level. (b) The maximum number of electrons that can be accommodated in the outermost shell is 8. Electrons are filled in the shells in a stepwise manner in increasing order of energy of the energy shell.
(i) What is the maximum electrons capacity of N shell? (a) 24 (b) 8 (c) 18 (d) 32
(ii) Identify the element with the configuration K-2, L-8, M-3. (a) Aluminium (b) Magnesium (c) Sodium (d) Beryllium
(iii) Which of the following configuration represent sodium? (a) 2, 8, 4 (b) 2, 8, 5 (c) 2, 3 (d) 2, 8, 1
You may also like:
Case study and passage based questions for other chapters of class 9 science is given below.
Chapter 3 Atoms and Molecules
Chapter 4 Structure of Atom
Chapter 10 Gravitation
Chapter 11 Work and Energy
Chapter 13 Why Do We Fall Ill?
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Extra Questions for Class 9 Science Chapter 3 Atoms and Molecules
Extra questions for Class 9 Science Chapter 3 Atoms and Molecules with answers is given below. Our subject expert prepared these solutions as per the latest NCERT textbook. These questions will be helpful to revise the all topics and concepts. CBSE Class 9 extra questions are the most simple and conceptual questions that are prepared by subject experts for the students to study well for the final exams. By solving these extra questions, students can be very efficient in their exam preparations.
Atoms and Molecules Class 9 Science Extra Questions and Answers
Very short answer questions.
1: Define law of conservation of mass. Answer: In a chemical reaction mass can neither be created nor destroyed.
2: Explain law of constant proportion. Answer: In a chemical substance the elements are always present in definite proportions by mass. E.g., In water, the ratio of the mass of hydrogen to the mass of oxygen H : O is always 1:8
3: Who coined the term atom? Answer: John Dalton coined the term atom.
4: Define atom. Answer: The smallest particle of matter, which can take part in a chemical reaction is called atom.
5: Define molecule. Answer: The smallest particle of an element or compound which can exist independently is called molecule.
6: Define atomicity. Answer: The number of atoms constituting a molecule is known as its atomicity.
7: What is atomic mass unit? Answer: The sum of the atomic masses of all the atoms in a molecule of the substance is expressed.in atomic mass unit. E.g., H 2 0 = 1 × 2 + 16 = 18 amu
8: How do atoms exist? Answer: Atoms exist in the form of atom, molecule or ions.
9: Give the atomicity of phosphorous and nitrogen. Answer: The atomicity of phosphorus is P 4 i.e., 4. The atomicity of nitrogen is N 2 i.e., 2.
10: What is an ion? Answer: Charged atom is called as an ion. The ion can be positively charged called cation or negatively charged called anion.
11: Give one example of cation and anion. Answer: Cation = Na + Anion = Cl –
12: Give one difference between cation and anion. Answer: Cations are positively charged ion. Anions are negatively charged ion.
13: Give the chemical formula for ammonium sulphate. Answer: Ammonium sulphate – NH 4 + SO 4 2- Chemical formula – (NH 4 ) 2 S0 4 .
14: What is Avogadro’s constant? Answer: The Avogadro’s constant (6.022 x 10 23 ) is defined as the number of atoms that are present in exactly 12 g of carbon-12.
15: Find the molecular mass of H 2 O. Answer: Molecular mass of H 2 O = (2 × 1) + (16) = 2 + 16 = 18 u
Short Answer Type Questions
1: Give the unit to measure size of atom and give size of hydrogen atom. Answer: The unit to measure size of atom, is nanometer, size of hydrogen atom is 10 -10 m.
2: What is IUPAC, give its one function? Answer: IUPAC is International Union for Pure and Applied Chemistry. It approves the names of elements.
3: Give the Latin name for sodium, potassium, gold and mercury. Answer: Sodium → Natrium, Gold → Aurum Potassium → Kalium, Mercury → Hydrargyrum
4: What is the ratio by mass of combining elements in H 2 O, CO 2 and NH 3 ?
Answer: H 2 O ratio by mass of combining elements 2 : 16 →1 : 8 (H : O) CO 2 ratio by mass of combining elements 12 : 32 → 3 : 08 (C : O) NH 3 ratio by mass of combining elements 14 : 3 → 14 : 3 (N : H)
5: Define valency and give the valency for the following elements: Magnesium, Aluminium, Chlorine and Copper.
Answer: Valency: The combining capacity of an element is called its valency. Valency of the following elements: Magnesium – 2 Aluminium – 3 Chlorine – 1 Copper – 2
6: What is polyatomic ton? Give one example.
Answer: A group of atoms carrying a charge is known as a polyatomic ion. E.g., Ammonium – NH 4 + Nitrate – NO 3 –
7: Write down the formula for: Copper nitrate, calcium sulphate and aluminium hydroxide.
Answer: Chemical formula: Copper nitrate → Cu(NO 3 ) Calcium sulphate → CaSO 4 Aluminium hydroxide Al(OH) 3
8: What is formula unit mass? How is it different from molecular mass?
Answer: The formula unit mass of a substance is a sum of the atomic masses of all atoms in a formula unit of a compound. The constituent particles of formula unit mass are ions and the constituent particles of molecular mass are atoms.
9: Find the number of moles in the following: (i) 50 g of H 2 O (ii) 7 g of Na
Answer: (i) Molar mass of H 2 O = 18 g Given mass of H 2 O = 50 g ∴ No. of moles in 50g of H 2 O = 58/18 = 2.78 moles.
(ii) Molar mass of Na = 23 g Given mass of Na = 7 g ∴ No. of moles in 50g of H 2 O = 7/23 = 0.304 moles.
10: Find the number of atoms in the following: (i) 0.5 mole of C atom (ii) 2 mole of N atom
Answer: (i) 0.5 mole of C atom: Number of atoms in 1 mole of C atom = 6.022 × 10 23 atoms Number of atoms in 0.5 mole of C atom = 6.022 × 10 23 × 0.5 = 3.011 × 10 23 atoms
(ii) 2 mole of N atom: Number of atoms in 1 mole of N atom = 6.022 × 10 23 atoms Number of atoms in 2 mole of N atom = 6.022 × 2 × 10 23 = 1.2044 × 10 24 atoms
11: Find the mass of the following: (i) 6.022 × 10 23 number of O 2 molecules (ii) 1.5 mole of CO 2 molecule
Answer: (i) 6.022 × 10 23 number of 02 molecules: Mass of 1 mole of O 2 molecule = 6.022 × 10 23 molecules = 32 g
(ii) 1.5 mole of CO 2 molecule: Mass of 1 mole of CO 2 molecule = 6.022 × 10 23 molecules = 44 g Mass of 1.5 mole CO 2 molecule = 44 × 1.5 = 66 g
12: Show the relationship between mole, Avogadro number and mass. Answer:
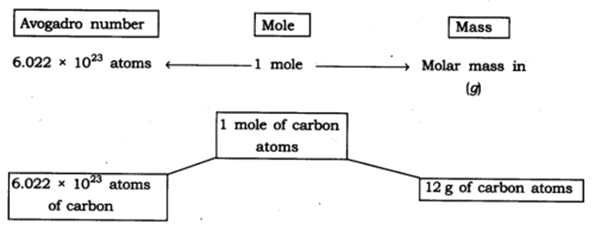
13: What are the rules for writing the symbol of an element?
Answer: IUPAC → International Union of Pure and Applied Chemistry approves name of elements. Symbols are the first one or two letters of the element’s name in English. The first letter of a symbol is always written as a capital letter (upper case) and the second letter as a small letter (lower case). e.g., Hydrogen → H Helium → He Some symbols are taken from the names of elements in Latin, German or Greek. e.g., Symbol of iron is Fe, its Latin name is Ferrum. Symbol of sodium is Na, its Latin name is Natrium. 14: Explain relative atomic mass and relative molecular mass.
Answer: Relative atomic mass: It can be defined as the number of times one atom of given element is heavier than 1/12 th of the mass of an atom of carbon-12. Relative Molecular Mass: It is defined as the number of times one molecule of a substance or given element is heavier than 1/12 th of the mass of one atom of carbon-12. 15: The formula of carbon-dioxide is CO 2 . What information do you get from this formula?
Answer: (i) CO 2 represents carbon-dioxide. (ii) CO 2 is one molecule of carbon-dioxide. (iii) CO 2 is one mole of carbon-dioxide i.e., it contains 6.022 × 10 23 molecules of carbon dioxide. (iv) CO 2 contains 1 atom of carbon and two atoms of oxygen. (v) CO 2 represents 44 g of molar mass.
16: State 3 points of difference between an atom and an ion. Answer:
17: Calculate the formula unit mass of NaCl and CaCl 2 . (Na = 23, Cl = 35.5, Ca = 40)
Answer: Formula unit mass of NaCl = 23 + 35.5 = 58.5 u
Formula unit mass of CaCl 2 = 40 + (2 × 35.5) = 40 + 71 = 111 u
18: The ratio by mass for hydrogen and oxygen in water is given as 1 : 8 respectively. Calculate the ratio by number of atoms for a water molecule. Answer: The ratio by number of atoms for a water molecule are:
Thus, the ratio by number of atoms for water is H : O = 2 : 1.
19: Write down the chemical formula for the following compounds: (a) Aluminium carbonate (b) Calcium sulphide (c) Zinc carbonate (d) Copper phosphate (e) Magnesium bicarbonate (f) Aluminium hydroxide.
Answer: The chemical formula are:
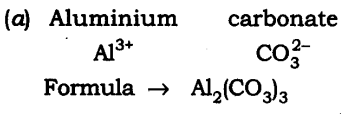
20: Give the atomicity of the following compounds: (a) Ca(OH) 2 (b) Mg(HCO 3 ) 2 (c) Cu 2 O. (d) H 2 SO 4 (e) Al 2 (SO 4 ) 3 (f) MgCl 2
Answer: The atomicity of the molecules are: (a) Ca(OH) 2 → 05 (b) Mg(HCO 3 ) 2 → 11 (c) Cu 2 O → 03 (d) H 2 SO 4 → 07 (e) Al 2 (SO 4 ) 3 → 17 (f) MgCl 2 → 03
21: Explain the difference between 2O, O 2 and O 3 .
Answer: 2O → It represents 2 atoms of oxygen (cannot exist independently). O 2 → It represents one molecule of oxygen (made up of 2 atom) can exist freely. O 3 → It represents one molecule of ozone (made up of 3 atoms) it can exist independently.
Long Answer Type Questions
1: (a) How do atoms exist? (b) What is atomicity? (c) What are polyatomic ions?
Answer: (a) Atoms of some elements are not able to exist independently. For such elements atoms form molecules and ions. In case of metals and inert gases atoms can exist independently.
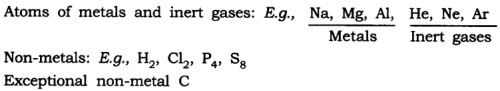
(b) The number of atoms constituting a molecule is known as its atomicity. E.g.,O 3 → atomicity is 3 O 2 → atomicity is 2
(c) Polyatomic ions: When more than two atoms combine together and act like an atom with a charge on it is called polyatomic ion. E.g., OH – , N0 3 – , NH 4 +
2: Calculate (a) the mass of one atom of oxygen (b) the mass of one molecule of oxygen (c) the mass of one mole of oxygen gas (d) the mass of one ion of oxygen (e) the number of atoms in 1 mole of oxygen molecule
Answer: (a) Mass of one atom of oxygen 1 mole of oxygen atom = 16 gm = 6.022 × 10 23 atoms. ∴ Mass of one atom of oxygen = 16/6.022 × 10 23 = 2.65 × 10 23
(b) Mass of one molecule of oxygen 1 molecule of oxygen = O 2 = 2 × 16 = 32 u
(c) Mass of one oxygen gas 1 molecule of oxygen gas is O 2 = 32 u
(d) Mass of one ion of oxygen One mole of oxygen = 6.022 × 10 23 atoms = 16g. 16 Mass of one ion of oxygen = 16/6.022 × 10 23 = 2.65 × 10 23
(e) Number of atoms in one mole of oxygen molecule 1 mole of oxygen molecule i.e. 0 2 = 6.022 × 10 23 molecules. 1 molecule of O 2 = 2 atoms.
∴ Number of atoms in 1 mole of oxygen molecule = 6.022 × 10 23 × 2 atoms = 1.2044 × 10 24 atoms
3: What is meant by atomic mass, gram atomic mass of an element? Why is the mass have different expressions i.e., ‘u’ and ‘g’?
Answer: The atoms are very tiny and their individual mass cannot be calculated as it is negligible. Hence the mass of atoms is expressed in units with respect to a fixed standard. Initially hydrogen atom with mass 1 was taken as standard unit by Dalton. Later, it was replaced by oxygen atom (0=16). But due to the isotopes the masses were found in fractions instead of whole number. Hence, carbon (C=12) isotope was taken as standard unit and was universally accepted. The atomic mass unit is equal to one twelfth (1/12) the mass of an atom of carbon-12, its unit is u.
Gram atomic mass: When the atomic mass of an element is expressed in grams, it is called the gram atomic mass of the element. The mass of atoms, molecules is expressed in ‘u’ and the mass of moles i.e., molar mass is expressed in g.
4: Define a mole. Give the significance of the mole.
Answer: Mole-One mole of any species (atoms, molecules, ions or particles) is that quantity or number having a mass equal to its atomic or molecular mass in grams. 1 mole = 6.022 × 10 23 in number (atoms, molecules, ions or particles)
Significance of the mole 1. A mole gives the number of entities present i.e, 6.022 × 10 23 particles of the substance. 2. Mass of 1 mole is expressed as M grams. 3. Mass of 1 mole = mass of 6.022 × 10 23 atoms of the element.

NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules

Chapter 3 Atoms and Molecules NCERT Solutions for Class 9 Science
Contact form.
- Class 9 Chemistry Mcqs
- Class 9 Chemistry Mcqs Chapter 3 Atoms and Molecules

Class 9 Chemistry Chapter 3 Atoms and Molecules MCQs
Class 9 chemistry MCQs with answers are provided here for chapter 3 Atoms and Molecules. These MCQs are based on CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus. By practicing these Class 9 Multiple choice questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations as well as other entrance exams such as CTET and KVS.
Download Chapter 3 Atoms and Molecules MCQs PDF by clicking on the button below. Download PDF
Recommended Video
Atoms and molecules – one shot (concepts+questions).

Class 9 Atoms and Molecules MCQs
1. The atomic symbol of silver is —————
Solution: The atomic symbol of silver is Ag
2. A box contains some identical red colour balls labelled as A each weighing 2 g. Another box contains identical blue colored balls, labelled as B, each weighing 5 g. In the combinations AB, AB 2 , A 2 B and A 2 B 3 which is applicable?
(a) Law of Definite proportion
(b) Law of multiple proportion
(c) Law of conservation of mass
(d) None of the above
Solution: According to the law of multiple proportion if an element forms more than one compound with another element for a given mass of an element, masses of other elements are in the ratio of the small whole number.
3. What is the value of Avogadro’s number?
(a) 6.02 x 10 -23
(b) 6.02 x 10 23
(c) 6.02 x 10 -22
(d) 6.02 x 10 22
Solution: The value of Avogadro’s number is 6.02 x 10 23
4. What is the chemical formula of sodium carbonate?
(a) Na 2 CO 3
(b) NaHCO 3
(d) Na 2 HCO 3
Solution: The chemical formula of sodium carbonate is Na 2 CO 3
5. Which of the following is the correct pair of atom and its atomic symbol?
(a) Sulphur – Su
(b) Potassium – P
(c) Phosphorus -P
(d) Sodium- S
Solution: The correct pair of atom and its atomic symbol is Phosphorus -P
6. How many moles are present in 40 g of He?
(a) 5 moles
(b) 20 moles
(c) 6 moles
(d) 10 moles
Solution: 1 mole of He = 4 gm. ⇒ 40 gm of He = 10 moles
7. Atomic mass of Chlorine is ————– (u)
Atomic mass of Chlorine is 35.5 u
8. 46 gm of sodium is equal to ————-
(a) 7 moles
(b) 1.5 moles
(c) 2 moles
Solution: 1 mole of sodium = 23gm, 46 gm of sodium = 2 moles
9. The formula of Ammonium Sulphate is —————–
(a) NH 4 SO 4
(b) NH 4 SO 2
(c) (NH 4 ) 2 SO 4
(d) NH 2 SO 4
Solution: The formula of Ammonium Sulphate is (NH 4 ) 2 SO 4 .
10. The atomic symbol of Iron is —————.
Solution: The atomic symbol of Iron is Fe.
Recommended Videos

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

NCERT Solutions for Class 9 Science Chapter 3- Atoms and Molecules
NCERT Solutions for Class 9 Science Chapter 3- Atoms and Molecules is a detailed study material prepared by experts. It provides answers to the questions given in the textbook. NCERT Solutions are very helpful for a better understanding of the concepts and self-analysis.
Questions from all the topics are covered in the solutions. Everything is presented in a way that students can easily understand. It will help them score well in the examination. The answers to all kinds of long and short questions, MCQs, tricks and tips are provided in the NCERT Solutions for Class 9 Science . Try solving the questions after completing the entire syllabus and overcome the shortcomings before the exams arrive.
The chapter Atoms and Molecules form the basis of the upcoming chapters, therefore, it should be dealt with thoroughly. The questions and solutions will help the students clarify all their doubts related to the topic.
The NCERT Solutions for Class 9 carries all the important questions and answers for all the subjects and chapters. The students can refer to these solutions to excel in the examinations.
- Chapter 1 Matter in Our Surroundings
- Chapter 2 Is Matter Around Us Pure
- Chapter 4 Structure Of The Atom
- Chapter 5 The Fundamental Unit Of Life
- Chapter 6 Tissues
- Chapter 7 Diversity in Living Organisms
- Chapter 8 Motion
- Chapter 9 Force And Laws Of Motion
- Chapter 10 Gravitation
- Chapter 11 Work and Energy
- Chapter 12 Sound
- Chapter 13 Why Do We Fall ill
- Chapter 14 Natural Resources
- Chapter 15 Improvement in Food Resources
Download PDF of NCERT Solutions for Class 9 Science Chapter 3- Atoms and Molecules

Access Answers of Science NCERT Class 9 Chapter 3 – Atoms and Molecules (All in text and Exercise Questions solved)
Class 9 science chapter 3 exercise-3.1 questions with answer, exercise-3.1 page: 32.
1. In a reaction, 5.3g of sodium carbonate reacted with 6 g of acetic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium acetate. Show that these observations are in agreement with the law of conservation of mass.
Sodium carbonate + acetic acid → Sodium acetate + carbon dioxide + water
5.3g 6g 8.2g 2.2g 0.9g
As per the law of conservation of mass, the total mass of reactants must be equal to the total mass of
As per the above reaction, LHS = RHS i.e., 5.3g + 6g = 2.2g + 0.9 g + 8.2 g = 11.3 g
Hence the observations are in agreement with the law of conservation of mass.
2. Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
We know hydrogen and water mix in the ratio 1: 8.
For every 1g of hydrogen, it is 8g of oxygen.
Therefore, for 3g of hydrogen, the quantity of oxygen = 3 x 8 = 24g
Hence, 24g of oxygen would be required for the complete reaction with 3g of hydrogen gas.
3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
The postulate of Dalton’s Atomic theory which is a result of the law of conservation of mass is,
“Atoms can neither be created nor destroyed”.
4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
The postulate of Dalton’s atomic theory that can explain the law of definite proportions is – the
relative number and kinds of atoms are equal in given compounds.
Exercise-3.2 Page: 35
1. Define the atomic mass unit?
An atomic mass unit is a unit of mass used to express weights of atoms and molecules where one
atomic mass is equal to 1/12th the mass of one carbon-12 atom.
2. Why is it not possible to see an atom with naked eyes?
Firstly, atoms are miniscule in nature, measured in nanometers. Secondly, except for atoms of noble
gasses, they do not exist independently. Hence, an atom cannot be visible to the naked eyes.
Exercise-3.3-3.4 Page: 39
1. Write down the formulae of
(i) sodium oxide
(ii) aluminium chloride
(iii) sodium sulphide
(iv) magnesium hydroxide
The following are the formulae:
(i) sodium oxide – Na 2 O
(ii) aluminium chloride – AlCl 3
(iii) sodium sulphide – Na 2 S
(iv) magnesium hydroxide – Mg (OH) 2
2. Write down the names of compounds represented by the following formulae:
(i) Al 2 (SO 4 ) 3
(ii) CaCl 2
(iii) K 2 SO 4
(v) CaCO 3 .
Listed below are the names of the compounds for each of the following formulae
(i) Al 2 (SO 4 ) 3 – Aluminium sulphate
(ii) CaCl 2 – Calcium chloride
(iii) K 2 SO 4 – Potassium sulphate
(iv) KNO 3 – Potassium nitrate
(v) CaCO 3 – Calcium carbonate
3. What is meant by the term chemical formula?
Chemical formula is the symbolic representation of a chemical compound. For example: The chemical formula of hydrochloric acid is HCl.
4. How many atoms are present in a
(i) H 2 S molecule and
(ii) PO 4 3- ion?
The number of atoms present are as follows:
(i) H 2 S molecule has 2 atoms of hydrogen and 1 atom of sulphur hence 3 atoms in totality.
(ii) PO 4 3- ion has 1 atom of phosphorus and 4 atoms of oxygen hence 5 atoms in totality.
Exercise-3.5.1-3.5.2 Page: 40
1. Calculate the molecular masses of H 2 , O 2 , Cl 2 , CO 2 , CH 4 , C 2 H 6 , C 2 H 4 , NH 3 , CH 3 OH.
The following are the molecular masses:
The molecular mass of H 2 – 2 x atoms atomic mass of H = 2 x 1u = 2u
The molecular mass of O 2 – 2 x atoms atomic mass of O = 2 x 16u = 32u
The molecular mass of Cl 2 – 2 x atoms atomic mass of Cl = 2 x 35.5u = 71u
The molecular mass of CO 2 – atomic mass of C + 2 x atomic mass of O = 12 + ( 2×16)u = 44u
The molecular mass of CH 4 – atomic mass of C + 4 x atomic mass of H = 12 + ( 4 x 1)u = 16u
The molecular mass of C 2 H 6 – 2 x atomic mass of C + 6 x atomic mass of H = (2 x 12) +
(6 x 1)u=24+6=30u
The molecular mass of C 2 H 4 – 2 x atomic mass of C + 4 x atomic mass of H = (2x 12) +
(4 x 1)u=24+4=28u
The molecular mass of NH 3 – atomic mass of N + 3 x atomic mass of H = (14 +3 x 1)u= 17u
The molecular mass of CH 3 OH – atomic mass of C + 3x atomic mass of H + atomic mass of O + atomic mass of H = (12 + 3×1+16+1)u=(12+3+17)u = 32u
2. Calculate the formula unit masses of ZnO, Na 2 O, K 2 CO 3 , given atomic masses of Zn = 65u,
Na = 23 u, K=39u, C = 12u, and O=16u.
Atomic mass of Zn = 65u
Atomic mass of Na = 23u
Atomic mass of K = 39u
Atomic mass of C = 12u
Atomic mass of O = 16u
The formula unit mass of ZnO= Atomic mass of Zn + Atomic mass of O = 65u + 16u = 81u
The formula unit mass of Na 2 O = 2 x Atomic mass of Na + Atomic mass of O = (2 x 23)u + 16u = 46u + 16u = 62u
The formula unit mass of K 2 CO 3 = 2 x Atomic mass of K + Atomic mass of C + 3 x Atomic mass of O = (2 x 39)u + 12u + (3 x 16)u = 78u + 12u + 48u = 138u
Exercise-3.5.3 Page: 42
1. If one mole of carbon atoms weighs 12grams, what is the mass (in grams) of 1 atom of carbon?
Given: 1 mole of carbon weighs 12g
1 mole of carbon atoms = 6.022 x 10 23
Molecular mass of carbon atoms = 12g = an atom of carbon mass
Hence, mass of 1 carbon atom = 12 / 6.022 x 10 23 = 1.99 x 10 -23 g
2. Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given, atomic mass of Na = 23u, Fe = 56 u)?
Given: Atomic mass of Na=23u, Atomic mass of Fe= 56u
To calculate the number of atoms in 100g of sodium:
23g of Na contains = 6.022 x 10 23 atoms
1g of Na contains = 6.022 x 10 23 atoms / 23
100g of Na contains = 6.022 x 10 23 atoms x 100 / 23
= 2.6182 x 10 24 atoms
56g of Fe contains = 6.022 x 10 23 atoms
1g of Fe contains = 6.022 x 10 23 atoms / 56
100g of Fe contains = 6.022 x 10 23 atoms x 100 / 56
= 1.075 x 10 24 atoms
Hence, through comparison, it is evident that 100g of Na has more atoms.
Exercise Page: 43
1. A 0.24g sample of compound of oxygen and boron was found by analysis to contain 0.096g of boron and 0.144g of oxygen. Calculate the percentage composition of the compound by weight.
Given: Mass of the sample compound = 0.24g, mass of boron = 0.096g, mass of oxygen = 0.144g
To calculate percentage composition of the compound:
Percentage of boron = mass of boron / mass of the compound x 100
= 0.096g / 0.24g x 100 = 40%
Percentage of oxygen = 100 – percentage of boron
= 100 – 40 = 60%
2. When 3.0g of carbon is burnt in 8.00 g of oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
11.00g of carbon dioxide is formed when 3.00g carbon is burnt in 8.00g of oxygen.
Carbon and oxygen are combined in the ratio 3:8 to give carbon dioxide using up all the carbon and
Hence, for 3g of carbon and 50g of oxygen, 8g of oxygen is used and 11g of carbon is formed, the
left oxygen is unused i.e., 50-8=42g of oxygen is unused.
This depicts the law of definite proportions – The combining elements in compounds are present in
definite proportions by mass.
3. What are polyatomic ions? Give examples.
Polyatomic ions are ions that contain more than one atom but they behave as a single unit
Example: CO 3 2- , H 2 PO 4 –
4. Write the chemical formula of the following.
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
The following are the chemical formula of the above-mentioned list:
(a) Magnesium chloride – MgCl 2
(b) Calcium oxide – CaO
(c) Copper nitrate – Cu(NO 3 ) 2
(d) Aluminium chloride – AlCl 3
(e) Calcium carbonate – CaCO 3
5. Give the names of the elements present in the following compounds.
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate.
The following are the names of the elements present in the following compounds:
(a) Quick lime – Calcium and oxygen (CaO)
(b) Hydrogen bromide – Hydrogen and bromine (HBr)
(c) Baking powder – Sodium, Carbon, Hydrogen, Oxygen (NaHCO 3 )
(d) Potassium sulphate – Sulphur, Oxygen, Potassium (K 2 SO 4 )
6. Calculate the molar mass of the following substances.
(a) Ethyne, C 2 H 2
(b) Sulphur molecule, S 8
(c) Phosphorus molecule, P 4 (Atomic mass of phosphorus =31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO 3
Listed below is the molar mass of the following substances:
(a) Molar mass of Ethyne C 2 H 2 = 2 x Mass of C+2 x Mass of H = (2×12)+(2×1)=24+2=26g
(b) Molar mass of Sulphur molecule S 8 = 8 x Mass of S = 8 x 32 = 256g
(c) Molar mass of Phosphorus molecule, P 4 = 4 x Mass of P = 4 x 31 = 124g
(d) Molar mass of Hydrochloric acid, HCl = Mass of H+ Mass of Cl = 1+35.5 = 36.5g
(e) Molar mass of Nitric acid, HNO 3 =Mass of H+ Mass of Nitrogen + 3 x Mass of O = 1 + 14+
7. What is the mass of –
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminium atoms((Atomic mass of aluminium =27)?
(c) 10 moles of sodium sulphite (Na 2 SO 3 )?
The mass of the above mentioned list is as follows:
(a) Atomic mass of nitrogen atoms = 14u
Mass of 1 mole of nitrogen atoms= Atomic mass of nitrogen atoms
Therefore, mass of 1 mole of nitrogen atom is 14g
(b) Atomic mass of aluminium =27u
Mass of 1 mole of aluminium atoms = 27g
1 mole of aluminium atoms = 27g, 4 moles of aluminium atoms = 4 x 27 = 108g
(c) Mass of 1 mole of sodium sulphite Na 2 SO 3 = Molecular mass of sodium sulphite = 2 x Mass of Na + Mass of S + 3 x Mass of O = (2 x 23) + 32 +(3x 16) = 46+32+48 = 126g
Therefore, mass of 10 moles of Na 2 SO 3 = 10 x 126 = 1260g
8. Convert into mole.
(a) 12g of oxygen gas
(b) 20g of water
(c) 22g of carbon dioxide
Conversion of the above-mentioned molecules into moles is as follows:
(a) Given: Mass of oxygen gas=12g
Molar mass of oxygen gas = 2 Mass of Oxygen = 2 x 16 = 32g
Number of moles = Mass given / molar mass of oxygen gas = 12/32 = 0.375 moles
(b) Given: Mass of water = 20g
Molar mass of water = 2 x Mass of Hydrogen + Mass of Oxygen = 2 x 1 + 16 = 18g
Number of moles = Mass given / molar mass of water
= 20/18 = 1.11 moles
(c) Given: Mass of carbon dioxide = 22g
Molar mass of carbon dioxide = Mass of C + 2 x Mass of Oxygen = 12 + 2x 16 = 12+32=44g
Number of moles = Mass given/ molar mass of carbon dioxide = 22/44 = 0.5 moles
9. What is the mass of:
(a) 0.2 mole of oxygen atoms?
(b) 0.5 mole of water molecules?
The mass is as follows:
(a) Mass of 1 mole of oxygen atoms = 16u, hence it weighs 16g
Mass of 0.2 moles of oxygen atoms = 0.2 x 16 = 3.2u
(b) Mass of 1 mole of water molecules = 18u, hence it weighs 18g
Mass of 0.5 moles of water molecules = 0.5 x 18 = 9u
10. Calculate the number of molecules of sulphur (S 8 ) present in 16g of solid sulphur.
To calculate molecular mass of sulphur:
Molecular mass of Sulphur (S 8 ) = 8xMass of Sulphur = 8×32 = 256g
Mass given = 16g
Number of moles = mass given/ molar mass of sulphur
= 16/256 = 0.0625 moles
To calculate the number of molecules of sulphur in 16g of solid sulphur:
Number of molecules = Number of moles x Avogadro number
= 0.0625 x 6.022 x 10²³ molecules
= 3.763 x 10 22 molecules
11. Calculate the number of aluminium ions present in 0.051g of aluminium oxide.
( Hint: The mass of an ion is the same as that of an atom of the same element. Atomic mass of Al = 27u)
To calculate the number of aluminium ions in 0.051g of aluminium oxide:
1 mole of aluminium oxide = 6.022 x 10 23 molecules of aluminium oxide
1 mole of aluminium oxide (Al 2 O 3 ) = 2 x Mass of aluminium + 3 x Mass of Oxygen
= (2x 27) + (3 x16) = 54 +48 = 102g
1 mole of aluminium oxide = 102g = 6.022 x 10 23 molecules of aluminium oxide
Therefore, 0.051g of aluminium oxide has = 0.051 x 6.022 x 10 23 / 102
= 3.011 x 10 20 molecules of aluminium oxide
One molecule of aluminium oxide has 2 aluminium ions, hence number of aluminium ions present in 0.051g of aluminium oxide = 2 x 3.011x 10 20 molecules of aluminium oxide
= 6.022 x 10 20
This chapter holds a weightage of 23 marks in the final examinations. The questions are not specific and can be asked from any topic. The students are therefore advised to be thorough with the entire chapter. The chemical formulae and the numerical should be practised well.
The important topics provided in this chapter include:
3.1 Laws of Chemical Combination
3.2 What is an Atom?
3.3 What is a Molecule?
3.4 Writing Chemical Formulae
3.5 Molecular Mass and Mole Concept
Exercise Solutions 11 Questions (8 numerical, 3 short)
Molecular mass and Mole concept- 8 numerical
Chemical Formula- 2 Questions
What is an Atom- 1 Question
The smallest unit of matter is an atom. It has the properties of an element. An atom comprises of a dense core called nucleus surrounded by a series of outer shells. The electrons are present in these shells. The nucleus contains the protons and neutrons. Protons have a positive charge, while the neutrons are neutral.
Two or more atoms tightly bound together form a molecule. The molecules made up of two atoms are known as diatomic. Oxygen, nitrogen, hydrogen, iodine are diatomic molecules. Earth’s atmosphere is comprised mainly of the diatomic molecules. A molecule is the smallest part of a compound.
Key Features of NCERT Solutions for Class 9 Science Chapter 3- Atoms and Molecules
- The answers are provided by Science experts.
- The answers are non-erroneous.
- The Solutions provide questions from every important topic.
- It gives a thorough understanding of the concepts.
Frequently Asked Questions on NCERT Solutions for Class 9 Science Chapter 3
Mention the topics included in the chapter 3 of ncert solutions for class 9 science., how to solve the numericals present in the chapter 3 of ncert solutions for class 9 science faster, what are the key features of ncert solutions for class 9 science chapter 3.

IMAGES
VIDEO
COMMENTS
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 3 Atoms and Molecules. Case Study/Passage-Based Questions. Case Study 1: The knowledge of the valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total ...
The formula of the sulphate of an element X is X2(SO4)3. The formula of nitride of element X will be. The formula of a compound is X3Y. The valencies of elements X and Y will be respectively. Case Study/Passage Based Questions. A mole of an atom is a collection of atoms whose total mass is the number of grams equal to the atomic mass.
Statement 1 - Dalton's atomic theory suggested that the atom was indivisible and indestructible. Statement 2 - Electrons and protons are present inside the atom. Statement 3 - J.J. Thomson was the first one to propose a model for the structure of an atom. Statement 4 - Protons are positively charged particle.
Case Study Questions for Class 9 Science Chapter 3 Atoms and Molecules In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based on it … Continue reading Case Study and Passage Based Questions for Class 9 ...
Class 9 chemistry important questions with answers are provided here for Chapter 3 Atoms and Molecules. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus. By practising these Class 9 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual ...
Introduction of Case Based Question Answer: Atoms and Molecules in English is available as part of our Science Class 9 for Class 9 & Case Based Question Answer: Atoms and Molecules in Hindi for Science Class 9 course. Download more important topics related with notes, lectures and mock test series for Class 9 Exam by signing up for free. Class 9: Class 9 Science Chapter 3 Case Based Questions ...
Class 9th is one of the most challenging years in a student's life as there are so many things they need to learn in a single year. As a subject science has always been on a stricter side of things and thus we at Vedantu came up with a solution to provide students with important questions chapter-wise. In this article, we will talk about the fundamentals of atoms and molecules and how they are ...
In this amazing video session of Subjective Case Based Questions: Atoms and Molecules [L-1] |CBSE Class 9 Chemistry | Term 2 | Vedantu by Shilpi Mam is reall...
Case Study Based Class 9 Science | Atoms and Molecules MCQs | NCERT Solutions for Class 9 Science Chapter 3 | CBSE Class 9 Chemistry. Hey Students, In this Video Shilpi Mam takes you to an Amazing ...
Class 9 Science Chapter 3 Textbook Questions and Answers. Question 1: In a reaction 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Here we have given Value Based Questions in Science for Class 9 Chapter 3 Atoms and Molecules. Question 1. Kamla prepared aqueous solutions of barium chloride and sodium sulphate. She weighed them separately and then mixed them in a beaker. A white precipitate was immediately formed.
Questions from all the topics are covered in the solutions. Everything is presented in a way that students can easily understand. It will help them score well in the CBSE examination. The answers to all kinds of long and short questions, MCQs, tricks and tips are provided in the NCERT Solutions for Class 9 Science. Try solving the questions after completing the entire syllabus and overcoming ...
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis ...
Important Questions for Class 9 Science Chapter 3 Atoms and Molecules. Get important questions for Class 9 Science Chapter 3 Atoms and Molecules with PDF. Our subject expert prepared these important questions and answers as per the latest NCERT textbook. These important questions will be helpful to revise the important topics and concepts.
The atomic mass is the characteristic of the atom. The concept of atomic mass supports the law of constant proportion and the law of conservation of mass. A detailed analysis of atomic mass is provided in the NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules. However, atoms cannot exist freely in nature.
NCERT Solutions Class 9 Science Chapter 3 Atoms and Molecules - Here are all the NCERT solutions for Class 9 Science Chapter 3. This solution contains questions, answers, images, step by step explanations of the complete Chapter 3 titled Atoms and Molecules of Science taught in class 9. If you are a student of class 9 who is using NCERT Textbook to study Science, then you must come across ...
Question. 2. (a) Give one point of difference between an atom and an ion. (b) Give one example each of a polyatomic cation and an anion. (c) Identify the correct chemical name of FeSO3: Ferrous sulphate, Ferrous sulphide, Ferrous sulphite. (d) Write the chemical formula for the chloride of magnesium.
The Chapter 3 - Atoms and Molecules important questions are prepared by professional subject experts. These CBSE Class 9 Science Chapter 3 important questions help students in learning the essential concepts of the chapter. We at BYJU'S provide these important questions so that students don't need to put extra effort in noting down important concepts. Solving these important questions of ...
Case Study Questions for Class 9 Science Chapter 4 Structure of Atom In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based on it … Continue reading Case Study and Passage Based Questions for Class 9 ...
1. A mole gives the number of entities present i.e, 6.022 × 10 23 particles of the substance. 2. Mass of 1 mole is expressed as M grams. 3. Mass of 1 mole = mass of 6.022 × 10 23 atoms of the element. Extra questions for Class 9 Science Chapter 3 Atoms and Molecules with answers is given below. Our subject expert prepared these solutions as ...
Answer. (a) Mass of one mole of oxygen atoms = 16 g. Then, mass of 0.2 mole of oxygen atoms = 0.2 × 16g = 3.2 g. (b) Mass of one mole of water molecule = 18 g. Then, mass of 0.5 mole of water molecules = 0.5 × 18 g = 9 g. 10. Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur. Answer.
Class 9 chemistry MCQs with answers are provided here for chapter 3 Atoms and Molecules. These MCQs are based on CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus. By practicing these Class 9 Multiple choice questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations as well as other ...
Questions from all the topics are covered in the solutions. Everything is presented in a way that students can easily understand. It will help them score well in the examination. The answers to all kinds of long and short questions, MCQs, tricks and tips are provided in the NCERT Solutions for Class 9 Science. Try solving the questions after completing the entire syllabus and overcome the ...