Careers in Biomedical Research
New section.
Learn more about careers in medical research.

If you have an interest in scientific exploration and a desire to break new ground in medical knowledge, a career in medical research might be for you.
MD-PhD programs provide training in both medicine and research. They are specifically designed for those who want to become research physicians.
The AAMC MD-PhD section is committed to recruiting and training a diverse Physician-Scientist workforce and an inclusive learning and working environment.

Biomedical scientists bridge the gap between the basic sciences and medicine. The PhD degree is the gateway to a career in biomedical research.


Medical Scientists
Career, salary and education information.
What They Do : Medical scientists conduct research aimed at improving overall human health.
Work Environment : Medical scientists work in offices and laboratories. Most work full time.
How to Become One : Medical scientists typically have a Ph.D., usually in biology or a related life science. Some medical scientists get a medical degree instead of, or in addition to, a Ph.D.
Salary : The median annual wage for medical scientists is $95,310.
Job Outlook : Employment of medical scientists is projected to grow 17 percent over the next ten years, much faster than the average for all occupations.
Related Careers : Compare the job duties, education, job growth, and pay of medical scientists with similar occupations.
Following is everything you need to know about a career as a medical scientist with lots of details. As a first step, take a look at some of the following jobs, which are real jobs with real employers. You will be able to see the very real job career requirements for employers who are actively hiring. The link will open in a new tab so that you can come back to this page to continue reading about the career:
Top 3 Medical Scientist Jobs
Are you an expert in Basic Medical Science , Anatomy, or Neuroscience? Outlier needs your expertise as we work to improve AI models' ability to reason about the topics! About the Opportunity
Primary Function The Medical Science Liaison is a key contributor to the company's clinical and commercial development with responsibility for establishing, developing, and maintaining relationships ...
The Position We are seeking an experienced cardiometabolic Medical Science Liaison (MSL) to join an exciting opportunity within our Medical Affairs (MA) team and work in a dynamic and collaborative ...
See all Medical Scientist jobs
What Medical Scientists Do [ About this section ] [ To Top ]
Medical scientists conduct research aimed at improving overall human health. They often use clinical trials and other investigative methods to reach their findings.
Duties of Medical Scientists
Medical scientists typically do the following:
- Design and conduct studies that investigate both human diseases and methods to prevent and treat them
- Prepare and analyze medical samples and data to investigate causes and treatment of toxicity, pathogens, or chronic diseases
- Standardize drug potency, doses, and methods to allow for the mass manufacturing and distribution of drugs and medicinal compounds
- Create and test medical devices
- Develop programs that improve health outcomes, in partnership with health departments, industry personnel, and physicians
- Write research grant proposals and apply for funding from government agencies and private funding sources
- Follow procedures to avoid contamination and maintain safety
Many medical scientists form hypotheses and develop experiments, with little supervision. They often lead teams of technicians and, sometimes, students, who perform support tasks. For example, a medical scientist working in a university laboratory may have undergraduate assistants take measurements and make observations for the scientist's research.
Medical scientists study the causes of diseases and other health problems. For example, a medical scientist who does cancer research might put together a combination of drugs that could slow the cancer's progress. A clinical trial may be done to test the drugs. A medical scientist may work with licensed physicians to test the new combination on patients who are willing to participate in the study.
In a clinical trial, patients agree to help determine if a particular drug, a combination of drugs, or some other medical intervention works. Without knowing which group they are in, patients in a drug-related clinical trial receive either the trial drug or a placebo—a pill or injection that looks like the trial drug but does not actually contain the drug.
Medical scientists analyze the data from all of the patients in the clinical trial, to see how the trial drug performed. They compare the results with those obtained from the control group that took the placebo, and they analyze the attributes of the participants. After they complete their analysis, medical scientists may write about and publish their findings.
Medical scientists do research both to develop new treatments and to try to prevent health problems. For example, they may study the link between smoking and lung cancer or between diet and diabetes.
Medical scientists who work in private industry usually have to research the topics that benefit their company the most, rather than investigate their own interests. Although they may not have the pressure of writing grant proposals to get money for their research, they may have to explain their research plans to nonscientist managers or executives.
Medical scientists usually specialize in an area of research within the broad area of understanding and improving human health. Medical scientists may engage in basic and translational research that seeks to improve the understanding of, or strategies for, improving health. They may also choose to engage in clinical research that studies specific experimental treatments.
Work Environment for Medical Scientists [ About this section ] [ To Top ]
Medical scientists hold about 119,200 jobs. The largest employers of medical scientists are as follows:
Medical scientists usually work in offices and laboratories. They spend most of their time studying data and reports. Medical scientists sometimes work with dangerous biological samples and chemicals, but they take precautions that ensure a safe environment.
Medical Scientist Work Schedules
Most medical scientists work full time.
How to Become a Medical Scientist [ About this section ] [ To Top ]
Get the education you need: Find schools for Medical Scientists near you!
Medical scientists typically have a Ph.D., usually in biology or a related life science. Some medical scientists get a medical degree instead of, or in addition to, a Ph.D.
Education for Medical Scientists
Students planning careers as medical scientists generally pursue a bachelor's degree in biology, chemistry, or a related field. Undergraduate students benefit from taking a broad range of classes, including life sciences, physical sciences, and math. Students also typically take courses that develop communication and writing skills, because they must learn to write grants effectively and publish their research findings.
After students have completed their undergraduate studies, they typically enter Ph.D. programs. Dual-degree programs are available that pair a Ph.D. with a range of specialized medical degrees. A few degree programs that are commonly paired with Ph.D. studies are Medical Doctor (M.D.), Doctor of Dental Surgery (D.D.S.), Doctor of Dental Medicine (D.M.D.), Doctor of Osteopathic Medicine (D.O.), and advanced nursing degrees. Whereas Ph.D. studies focus on research methods, such as project design and data interpretation, students in dual-degree programs learn both the clinical skills needed to be a physician and the research skills needed to be a scientist.
Graduate programs emphasize both laboratory work and original research. These programs offer prospective medical scientists the opportunity to develop their experiments and, sometimes, to supervise undergraduates. Ph.D. programs culminate in a dissertation that the candidate presents before a committee of professors. Students may specialize in a particular field, such as gerontology, neurology, or cancer.
Those who go to medical school spend most of the first 2 years in labs and classrooms, taking courses such as anatomy, biochemistry, physiology, pharmacology, psychology, microbiology, pathology, medical ethics, and medical law. They also learn how to record medical histories, examine patients, and diagnose illnesses. They may be required to participate in residency programs, meeting the same requirements that physicians and surgeons have to fulfill.
Medical scientists often continue their education with postdoctoral work. This provides additional and more independent lab experience, including experience in specific processes and techniques, such as gene splicing. Often, that experience is transferable to other research projects.
Licenses, Certifications, and Registrations for Medical Scientists
Medical scientists primarily conduct research and typically do not need licenses or certifications. However, those who administer drugs or gene therapy or who otherwise practice medicine on patients in clinical trials or a private practice need a license to practice as a physician.
Medical Scientist Training
Medical scientists often begin their careers in temporary postdoctoral research positions or in medical residency. During their postdoctoral appointments, they work with experienced scientists as they continue to learn about their specialties or develop a broader understanding of related areas of research. Graduates of M.D. or D.O. programs may enter a residency program in their specialty of interest. A residency usually takes place in a hospital and varies in duration, generally lasting from 3 to 7 years, depending on the specialty. Some fellowships exist that train medical practitioners in research skills. These may take place before or after residency.
Postdoctoral positions frequently offer the opportunity to publish research findings. A solid record of published research is essential to getting a permanent college or university faculty position.
Work Experience in a Related Occupation for Medical Scientists
Although it is not a requirement for entry, many medical scientists become interested in research after working as a physician or surgeon , or in another medical profession, such as dentist .
Important Qualities for Medical Scientists
Communication skills. Communication is critical, because medical scientists must be able to explain their conclusions. In addition, medical scientists write grant proposals, because grants often are required to fund their research.
Critical-thinking skills. Medical scientists must use their expertise to determine the best method for solving a specific research question.
Data-analysis skills. Medical scientists use statistical techniques, so that they can properly quantify and analyze health research questions.
Decisionmaking skills. Medical scientists must determine what research questions to ask, how best to investigate the questions, and what data will best answer the questions.
Observation skills. Medical scientists conduct experiments that require precise observation of samples and other health-related data. Any mistake could lead to inconclusive or misleading results.
Medical Scientist Salaries [ About this section ] [ More salary/earnings info ] [ To Top ]
The median annual wage for medical scientists is $95,310. The median wage is the wage at which half the workers in an occupation earned more than that amount and half earned less. The lowest 10 percent earned less than $50,100, and the highest 10 percent earned more than $166,980.
The median annual wages for medical scientists in the top industries in which they work are as follows:
Job Outlook for Medical Scientists [ About this section ] [ To Top ]
Employment of medical scientists is projected to grow 17 percent over the next ten years, much faster than the average for all occupations.
About 10,000 openings for medical scientists are projected each year, on average, over the decade. Many of those openings are expected to result from the need to replace workers who transfer to different occupations or exit the labor force, such as to retire.
Employment of Medical Scientists
Demand for medical scientists will stem from greater demand for a variety of healthcare services as the population continues to age and rates of chronic disease continue to increase. These scientists will be needed for research into treating diseases, such as Alzheimer’s disease and cancer, and problems related to treatment, such as resistance to antibiotics. In addition, medical scientists will continue to be needed for medical research as a growing population travels globally and facilitates the spread of diseases.
The availability of federal funds for medical research grants also may affect opportunities for these scientists.
Careers Related to Medical Scientists [ About this section ] [ To Top ]
Agricultural and food scientists.
Agricultural and food scientists research ways to improve the efficiency and safety of agricultural establishments and products.
Biochemists and Biophysicists
Biochemists and biophysicists study the chemical and physical principles of living things and of biological processes, such as cell development, growth, heredity, and disease.
Epidemiologists
Epidemiologists are public health professionals who investigate patterns and causes of disease and injury in humans. They seek to reduce the risk and occurrence of negative health outcomes through research, community education, and health policy.
Health Educators and Community Health Workers
Health educators teach people about behaviors that promote wellness. They develop and implement strategies to improve the health of individuals and communities. Community health workers collect data and discuss health concerns with members of specific populations or communities.
Medical and Clinical Laboratory Technologists and Technicians
Medical laboratory technologists (commonly known as medical laboratory scientists) and medical laboratory technicians collect samples and perform tests to analyze body fluids, tissue, and other substances.
Microbiologists
Microbiologists study microorganisms such as bacteria, viruses, algae, fungi, and some types of parasites. They try to understand how these organisms live, grow, and interact with their environments.
Physicians and Surgeons
Physicians and surgeons diagnose and treat injuries or illnesses. Physicians examine patients; take medical histories; prescribe medications; and order, perform, and interpret diagnostic tests. They counsel patients on diet, hygiene, and preventive healthcare. Surgeons operate on patients to treat injuries, such as broken bones; diseases, such as cancerous tumors; and deformities, such as cleft palates.
Postsecondary Teachers
Postsecondary teachers instruct students in a wide variety of academic and technical subjects beyond the high school level. They may also conduct research and publish scholarly papers and books.
Veterinarians
Veterinarians care for the health of animals and work to improve public health. They diagnose, treat, and research medical conditions and diseases of pets, livestock, and other animals.
More Medical Scientist Information [ About this section ] [ To Top ]
For more information about research specialties and opportunities within specialized fields for medical scientists, visit
American Association for Cancer Research
American Society for Biochemistry and Molecular Biology
The American Society for Clinical Laboratory Science
American Society for Clinical Pathology
American Society for Clinical Pharmacology and Therapeutics
The American Society for Pharmacology and Experimental Therapeutics
The Gerontological Society of America
Infectious Diseases Society of America
National Institute of General Medical Sciences
Society for Neuroscience
Society of Toxicology
A portion of the information on this page is used by permission of the U.S. Department of Labor.
Explore more careers: View all Careers or the Top 30 Career Profiles
Search for jobs:.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News Feature
- Published: 07 December 2020
2021: research and medical trends in a post-pandemic world
- Mike May 1
Nature Medicine volume 26 , pages 1808–1809 ( 2020 ) Cite this article
9946 Accesses
3 Citations
25 Altmetric
Metrics details
Goodbye 2020, a year of arguably too many challenges for the world. As tempting as it is to leave this year behind, the biomedical community is forever changed by the pandemic, while business as usual needs to carry on. Looking forward to a new year, experts share six trends for the biomedical community in 2021.
Summing up 2020, Sharon Peacock, director of the COVID-19 Genomics UK Consortium, says “we’ve seen some excellent examples of people working together from academia, industry, and healthcare sectors...I’m hopeful that will stay with us going into 2021.” Nonetheless, we have lost ground and momentum in non-COVID research, she says. “This could have a profound effect on our ability to research other areas in the future.”

The coronavirus SARS-CoV-2 has already revealed weaknesses in medical research and clinical capabilities, as well as opportunities. Although it is too soon to know when countries around the world will control the COVID-19 pandemic, there is already much to be learned.
To explore trends for 2021, we talked to experts from around the world who specialize in medical research. Here is what we learned.
1. The new normal
Marion Koopman, head of the Erasmus MC Department of Viroscience, predicts that emerging-disease experts will overwhelmingly remain focused on SARS-CoV-2, at least for the coming year.
“I really hope we will not go back to life as we used to know it, because that would mean that the risk of emerging diseases and the need for an ambitious preparedness research agenda would go to the back burner,” Koopman says. “That cannot happen.”
Scientists must stay prepared, because the virus keeps changing. Already, Koopman says, “We have seen spillback [of SARS-CoV-2] into mink in our country, and ongoing circulation with accumulation of mutations in the spike and other parts of the genome.”
Juleen R. Zierath, an expert in the physiological mechanisms of metabolic diseases at the Karolinska Institute and the University of Copenhagen, points out that the pandemic “has raised attention to deleterious health consequences of metabolic diseases, including obesity and type 2 diabetes,” because people with these disorders have been “disproportionally affected by COVID-19.” She notes that the coupling of the immune system to metabolism at large probably deserves more attention.
2. Trial by fire for open repositories
The speed of SARS-CoV-2’s spread transformed how scientists disseminate information. “There is an increased use of open repositories such as bioRxiv and medRxiv, enabling faster dissemination of study and trial results,” says Alan Karthikesalingam, Research Lead at Google Health UK. “When paired with the complementary — though necessarily slower — approach of peer review that safeguards rigor and quality, this can result in faster innovation.”
“I suspect that the way in which we communicate ongoing scientific developments from our laboratories will change going forward,” Zierath says. That is already happening, with many meetings going to virtual formats.
Deborah Johnson, president and CEO of the Keystone Symposia on Molecular and Cellular Biology, notes that while virtual events cannot fully replace the networking opportunities that are created with in-person meetings, “virtual events have democratized access to biomedical research conferences, enabling greater participation from young investigators and those from low-and-middle-income countries.” Even when in-person conferences return, she says, “it will be important to continue to offer virtual components that engage these broader audiences.”
3. Leaps and bounds for immunology
Basic research on the immune system, catapulted to the frontlines of the COVID-19 response, has received a boost in attention this year, and more research in that field could pay off big going forward.
Immunobiologist Akiko Iwasaki at the Yale School of Medicine hopes that the pandemic will drive a transformation in immunology. “It has become quite clear over decades of research that mucosal immunity against respiratory, gastrointestinal, and sexually transmitted infections is much more effective in thwarting off invading pathogens than systemic immunity,” she says. “Yet, the vast majority of vaccine efforts are put into parenteral vaccines.”
“It is time for the immunology field to do a deep dive in understanding fundamental mechanisms of protection at the mucosal surfaces, as well as to developing strategies that allow the immune response to be targeted to the mucosal surfaces,” she explains.
“We are discovering that the roles of immune cells extend far beyond what was previously thought, to play underlying roles in health and disease across all human systems, from cancer to mental health,” says Johnson.
She sees this knowledge leading to more engineered immune cells to treat diseases. “Cancer immunotherapies will likely serve as the proving ground for immune-mediated therapies against many other diseases that we are only starting to see through the lens of the immune system.”
4. Rewind time for neurodegeneration
Oskar Hansson, research team manager of Lund University’s Clinical Memory Research, expects the trend of attempting to intervene against neurodegenerative disease before widespread neurodegeneration, and even before symptom onset, to continue next year.
This approach has already shown potential. “Several promising disease-modifying therapies against Alzheimer’s disease are now planned to be evaluated in this early pre-symptomatic disease phase,” he says, “and I think we will have similar developments in other areas like Parkinson’s disease and [amyotrophic lateral sclerosis].”
Delving deeper into such treatments depends on better understanding of how neurodegeneration develops. As Hansson notes, the continued development of cohort studies from around the world will help scientists “study how different factors — genetics, development, lifestyle, etcetera — affect the initiation and evolution of even the pre-symptomatic stages of the disease, which most probably will result in a much deeper understanding of the disease as well as discovery of new drug targets.”
5. Digital still front and center
“As [artificial intelligence] algorithms around the world begin to be released more commonly in regulated medical device software, I think there will be an increasing trend toward prospective research examining algorithmic robustness, safety, credibility and fairness in real-world medical settings,” says Karthikesalingam. “The opportunity for clinical and machine-learning research to improve patient outcomes in this setting is substantial.”
However, more trials are needed to prove which artificial intelligence works in medicine and which does not. Eric Topol, a cardiologist who combines genomic and digital medicine in his work at Scripps Research, says “there are not many big, annotated sets of data on, for example, scans, and you need big datasets to train new algorithms.” Otherwise, only unsupervised learning algorithms can be used, and “that’s trickier,” he says.
Despite today’s bottlenecks in advancing digital health, Topol remains very optimistic. “Over time, we’ll see tremendous progress across all modalities — imaging data, speech data, and text data — to gather important information through patient tests, research articles or reviewing patient chats,” he says.
He envisions that speech-recognition software could, for instance, capture physician–patient talks and turn them into notes. “Doctors will love this,” he says, “and patients will be able to look a doctor in the eye, which enhances the relationship.”
6. ‘Be better prepared’ — a new medical mantra
One trend that every expert interviewed has emphasized is the need for preparation. As Gabriel Leung, a specialist in public-health medicine at the University of Hong Kong, put it, “We need a readiness — not just in technology platforms but also business cases — to have a sustained pipeline of vaccines and therapies, so that we would not be scrambling for some of the solutions in the middle of a pandemic.”
Building social resilience ahead of a crisis is also important. “[SARS-CoV-2] and the resulting pandemic make up the single most important watershed in healthcare,” Leung explains. “The justice issue around infection risk, access to testing and treatment — thus outcomes — already make up the single gravest health inequity in the last century.”
One change that Peacock hopes for in the near future is the sequencing of pathogens on location, instead of more centrally. “For pathogen sequencing, you need to be able to apply it where the problem under investigation is happening,” she explains. “In the UK, COVID-19 has been the catalyst for us to develop a highly collaborative, distributed network of sequencing capabilities.”
Author information
Authors and affiliations.
Freelance writer and editor, Bradenton, FL, USA
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
May, M. 2021: research and medical trends in a post-pandemic world. Nat Med 26 , 1808–1809 (2020). https://doi.org/10.1038/s41591-020-01146-z
Download citation
Published : 07 December 2020
Issue Date : December 2020
DOI : https://doi.org/10.1038/s41591-020-01146-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
December 22, 2021
2021 Research Highlights — Promising Medical Findings
Results with potential for enhancing human health.
With NIH support, scientists across the United States and around the world conduct wide-ranging research to discover ways to enhance health, lengthen life, and reduce illness and disability. Groundbreaking NIH-funded research often receives top scientific honors. In 2021, these honors included Nobel Prizes to five NIH-supported scientists . Here’s just a small sample of the NIH-supported research accomplishments in 2021.
Printer-friendly version of full 2021 NIH Research Highlights
20210615-covid.jpg
Advancing COVID-19 treatment and prevention
Amid the sustained pandemic, researchers continued to develop new drugs and vaccines for COVID-19. They found oral drugs that could inhibit virus replication in hamsters and shut down a key enzyme that the virus needs to replicate. Both drugs are currently in clinical trials. Another drug effectively treated both SARS-CoV-2 and RSV, another serious respiratory virus, in animals. Other researchers used an airway-on-a-chip to screen approved drugs for use against COVID-19. These studies identified oral drugs that could be administered outside of clinical settings. Such drugs could become powerful tools for fighting the ongoing pandemic. Also in development are an intranasal vaccine , which could help prevent virus transmission, and vaccines that can protect against a range of coronaviruses .
202211214-alz.jpg

Developments in Alzheimer’s disease research
One of the hallmarks of Alzheimer’s is an abnormal buildup of amyloid-beta protein. A study in mice suggests that antibody therapies targeting amyloid-beta protein could be more effective after enhancing the brain’s waste drainage system . In another study, irisin, an exercise-induced hormone, was found to improve cognitive performance in mice . New approaches also found two approved drugs (described below) with promise for treating AD. These findings point to potential strategies for treating Alzheimer’s. Meanwhile, researchers found that people who slept six hours or less per night in their 50s and 60s were more likely to develop dementia later in life, suggesting that inadequate sleep duration could increase dementia risk.
20211109-retinal.jpg
New uses for old drugs
Developing new drugs can be costly, and the odds of success can be slim. So, some researchers have turned to repurposing drugs that are already approved for other conditions. Scientists found that two FDA-approved drugs were associated with lower rates of Alzheimer’s disease. One is used for high blood pressure and swelling. The other is FDA-approved to treat erectile dysfunction and pulmonary hypertension. Meanwhile, the antidepressant fluoxetine was associated with reduced risk of age-related macular degeneration. Clinical trials will be needed to confirm these drugs’ effects.
20210713-heart.jpg
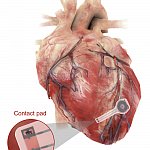
Making a wireless, biodegradable pacemaker
Pacemakers are a vital part of medical care for many people with heart rhythm disorders. Temporary pacemakers currently use wires connected to a power source outside the body. Researchers developed a temporary pacemaker that is powered wirelessly. It also breaks down harmlessly in the body after use. Studies showed that the device can generate enough power to pace a human heart without causing damage or inflammation.
20210330-crohns.jpg

Fungi may impair wound healing in Crohn’s disease
Inflammatory bowel disease develops when immune cells in the gut overreact to a perceived threat to the body. It’s thought that the microbiome plays a role in this process. Researchers found that a fungus called Debaryomyces hansenii impaired gut wound healing in mice and was also found in damaged gut tissue in people with Crohn’s disease, a type of inflammatory bowel disease. Blocking this microbe might encourage tissue repair in Crohn’s disease.
20210406-flu.jpg
Nanoparticle-based flu vaccine
Influenza, or flu, kills an estimated 290,000-650,000 people each year worldwide. The flu virus changes, or mutates, quickly. A single vaccine that conferred protection against a wide variety of strains would provide a major boost to global health. Researchers developed a nanoparticle-based vaccine that protected against a broad range of flu virus strains in animals. The vaccine may prevent flu more effectively than current seasonal vaccines. Researchers are planning a Phase 1 clinical trial to test the vaccine in people.
20211002-lyme.jpg

A targeted antibiotic for treating Lyme disease
Lyme disease cases are becoming more frequent and widespread. Current treatment entails the use of broad-spectrum antibiotics. But these drugs can damage the patient’s gut microbiome and select for resistance in non-target bacteria. Researchers found that a neglected antibiotic called hygromycin A selectively kills the bacteria that cause Lyme disease. The antibiotic was able to treat Lyme disease in mice without disrupting the microbiome and could make an attractive therapeutic candidate.
20211102-back.jpg

Retraining the brain to treat chronic pain
More than 25 million people in the U.S. live with chronic pain. After a treatment called pain reprocessing therapy, two-thirds of people with mild or moderate chronic back pain for which no physical cause could be found were mostly or completely pain-free. The findings suggest that people can learn to reduce the brain activity causing some types of chronic pain that occur in the absence of injury or persist after healing.
2021 Research Highlights — Basic Research Insights >>
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009.

Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research.
- Hardcopy Version at National Academies Press
3 The Value, Importance, and Oversight of Health Research
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2 , the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
In addition to defining health research and delineating its value to individuals and society, this chapter provides an overview and historical perspective of federal research regulations that were in place long before the Privacy Rule was implemented. Because a great deal of medical research falls under the purview of multiple federal regulations, it is important to understand how the various rules overlap or diverge. The chapter also explains how the definition of research has become quite complex under the various federal regulations, which make a distinction between research and some closely related health practice activities that also use health data, such as quality improvement initiatives.
The chapter also reviews the available survey data regarding public perceptions of health research and describes the importance of effective communication about health research with patients and the public.
- CONCEPTS AND VALUE OF HEALTH RESEARCH
Definitions
Under both the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and the Common Rule , “research” is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” This is a broad definition that may include biomedical research, epidemiological studies, 1 and health services research, 2 as well as studies of behavioral, social, and economic factors that affect health.
Perhaps the most familiar form of health research is the clinical trial, in which patients volunteer to participate in studies to test the efficacy and safety of new medical interventions. But an increasingly large portion of health research is now information based. A great deal of research entails the analysis of data and biological samples that were initially collected for diagnostic, treatment, or billing purposes, or that were collected as part of other research projects, and are now being used for new research purposes. This secondary 3 use of data is a common research approach in fields such as epidemiology, health services research, and public health research, and includes analysis of patterns of occurrences, determinants, and natural history of disease; evaluation of health care interventions and services; drug safety surveillance; and some genetic and social studies ( Lowrance, 2002 ; Lowrance and Collins, 2007 ).
The Importance of Health Research
Like privacy, health research has high value to society. It can provide important information about disease trends and risk factors, outcomes of treatment or public health interventions, functional abilities, patterns of care, and health care costs and use. The different approaches to research provide complementary insights. Clinical trials can provide important information about the efficacy and adverse effects of medical interventions by controlling the variables that could impact the results of the study, but feedback from real-world clinical experience is also crucial for comparing and improving the use of drugs, vaccines, medical devices, and diagnostics. For example, Food and Drug Administration (FDA) approval of a drug for a particular indication is based on a series of controlled clinical trials, often with a few hundred to a few thousand patients, but after approval it may be used by millions of people in many different contexts. Therefore, tracking clinical experience with the drug is important for identifying relatively rare adverse effects and for determining the effectiveness in different populations or in various circumstances. It is also vital to record and assess experience in clinical practice in order to develop guidelines for best practices and to ensure high-quality patient care.
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the population contributes greatly to the national economy ( Hatfield et al., 2001 ; Murphy and Topel, 1999 ) in addition to the individual benefits of improved health. If the research enterprise is impeded, or if it is less robust, important societal interests are affected.
The development of Herceptin as a treatment for breast cancer is a prime example of the benefits of research using biological samples and patient records ( Box 3-1 ) ( Slamon et al., 1987 ). Many other examples of findings from medical records research have changed the practice of medicine as well. Such research underlies the estimate that tens of thousands of Americans die each year from medical errors in the hospital, and research has provided valuable information for reducing these medical errors by implementing health information technology, such as e-prescribing ( Bates et al., 1998 ; IOM, 2000b ). This type of research also has documented that disparities in health care and lack of access to care in inner cities and rural areas result in poorer health outcomes ( Mick et al., 1994 ). Furthermore, medical records research has demonstrated that preventive services (e.g., mammography) substantially reduce mortality and morbidity at reasonable costs ( Mandelblatt et al., 2003 ), and has established a causal link between the nursing shortage and patient health outcomes by documenting that patients in hospitals with fewer registered nurses are hospitalized longer and are more likely to suffer complications, such as urinary tract infections and upper gastrointestinal bleeding ( Needleman et al., 2002 ). These findings have all informed and influenced policy decisions at the national level. As the use of electronic medical records increases, the pace of this form of research is accelerating, and the opportunities to generate new knowledge about what works in health care are expanding ( CHSR, 2008 ).
Examples of Important Findings from Medical Database Research. Herceptin and breast cancer: Data were collected from a cohort of more than 9,000 breast cancer patients whose tumor specimens were consecutively received at the University (more...)
Advances in health information technology are enabling a transformation in health research that could facilitate studies that were not feasible in the past, and thus lead to new insights regarding health and disease. As noted by the National Committee on Vital and Health Statistics, “Clinically rich information is now more readily available, in a more structured format, and able to be electronically exchanged throughout the health and health care continuum. As a result, the information can be better used for quality improvement, public health, and research, and can significantly contribute to improvements in health and health care for individuals and populations” ( NCVHS, 2007a ). The informatics grid recently developed with support from the National Cancer Institute (Cancer Biomedical Informatics Grid, or caBIG) is an example of a how information technologies can facilitate health research by enabling broader sharing of health data while still ensuring regulatory compliance and protecting patient privacy ( Box 3-2 ).
caBIG (Cancer Biomedical Informatics Grid). The National Cancer Institute’s caBIG Data Sharing and Intellectual Capital Workspace’s mission is to enable all constituencies in the cancer community—including researchers, physicians, (more...)
Science today is also changing rapidly and becoming more complex, so no single researcher or single site can bring all the expertise to develop and validate medical innovations or to ensure their safety. Thus, efficient sharing of information between institutions has become even more important than in previous eras, when there were fewer new therapies introduced. The expansion of treatment options, as well as the escalating expense of new therapies, mandates greater scrutiny of true effectiveness, 5 once efficacy has been demonstrated. This requires registries of patient characteristics, outcomes, and adverse events. Large populations are required to facilitate comparison of patient populations and to calculate risk/benefit estimates. For example, INTERMACS 6 (Interagency Registry for Mechanically Assisted Circulatory Support) is a national registry for patients who are receiving mechanical circulatory support device therapy to treat advanced heart failure. This registry was devised as a joint effort of the National Heart, Lung and Blood Institute, Centers for Medicare & Medicaid Services, FDA, clinicians, scientists and industry representatives. Analysis of the data collected is expected to facilitate improved patient evaluation and management while aiding in better device development. Registry results are also expected to influence future research and facilitate appropriate regulation and reimbursement of such devices. Similarly, the Extracorporeal Life Support Organization (ELSO), 7 an international consortium of health care professionals and scientists who focus on the development and evaluation of novel therapies for support of failing organ systems, maintains a registry of extracorporeal membrane oxygenation and other novel forms of organ system support. Registry data are used to support clinical practice and research, as well as regulatory agencies. Another example is the database developed by the United Network for Organ Sharing (UNOS) for the collection, storage, analysis and publication of data pertaining to the patient waiting list, organ matching, and transplants. 8 Launched in 1999, this secure Internet-based system contains data regarding every organ donation and transplant event occurring in the United States since 1986.
Information-based research, such as research using health information databases has many advantages (reviewed by Lowrance, 2002 ). It is often faster and less expensive than experimental studies; it can analyze very large sets of data and may detect unexpected phenomena or differences among subpopulations that might not be included in a controlled experimental study; it can often be undertaken when controlled trials are simply not possible for ethical, technical, or other reasons, and it can be used to study effectiveness of a specific test or intervention in clinical practice, rather than just the efficacy as determined by a controlled experimental study. It can also reexamine data accrued in other research studies, such as clinical trials, to answer new questions quickly and inexpensively. However, information-based research does have limitations. Often it has less statistical rigor than controlled clinical studies because it lacks scientific control over the original data collection, quality, and format that prospective experimental research can dictate from the start. In addition to these scientific limitations, because of its relational and often distant physical separation from the data subjects, and the sheer volume of the records involved, obtaining individual consent for the research can be difficult or impossible.
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. The goal of personalized medicine is to tailor prevention strategies and treatments to each individual based on his/her genetic composition and health history. In spite of the strides made in improving health through new treatments, it is widely known that most drugs are effective in only a fraction of patients who have the condition for which the drug is indicated. Moreover, a small percentage of patients are likely to have adverse reactions to drugs that are found to be safe for the majority of the population at the recommended dose. Both of these phenomena are due to variability in the patient population. Revolutionary advances in the study of genetics and other markers of health and disease are now making it possible to identify and study these variations, and are leading to more personalized approaches to health care—that is, the ability to give “the appropriate drug, at the appropriate dose, to the appropriate patient, at the appropriate time.” Achieving the goals of personalized medicine will lead to improvements in both the effectiveness and the safety of medical therapies.
Public Perceptions of Health Research
A number of studies have been undertaken to gauge the public’s attitude toward research and the factors that influence individuals’ willingness to participate in research. The surveys reviewed in this chapter focus on interventional clinical trials. A review of survey questions to gauge the public willingness to allow their medical records to be used in research can be found in Chapter 2 .
The Public Values Health Research
A number of studies suggest that most Americans have a positive view of medical research and believe that research is beneficial to society. A recent Harris poll found that nearly 80 percent of respondents were interested in health research findings, consistent with previous survey results ( Westin, 2007 ). A study in 2005 compiled data from 70 state surveys and 18 national surveys and found that the majority of Americans believe maintaining world leadership in health-related research is important. Seventy-eight percent of respondents said that it is very important, and 17 percent said that it is somewhat important. Only 4 percent of Americans reported that maintaining world leadership in health-related research is not impor tant ( Woolley and Propst, 2005 ). Similar results were found in a 2007 survey—76 percent of respondents reported that science plays a very important role in our health, and 78 percent reported that science plays a very important role in our competitiveness ( Research!America, 2007 ).
The Virginia Commonwealth University 2004 Life Sciences Survey also found that most Americans have a positive view of research. In this study, 90 percent of respondents agreed that developments in science have made society better; 92 percent reported that “scientific research is essential for improving the quality of human lives”; and 84 percent agreed that “the benefits of scientific research outweigh the harmful results” ( NSF, 2006 ).
Overall Experience When Participating in Research
Little is known about the attitudes of individuals who have actually participated in medical research. However, the available evidence suggests that most research participants have positive experiences. A recent Harris Poll found that 13 percent of respondents had participated in some form of health research, and 87 percent of those felt comfortable about their experience ( Westin, 2007 ). In a study focused on cancer, 93 percent of respondents who participated in research reported it as a very positive experience; 76 percent said they would recommend participation in a clinical trial to someone with cancer. Most physicians surveyed in this study stated that they believe clinical trial participants receive the best possible care, and have outcomes at least as good as patients receiving standard cancer treatment ( Comis et al., 2000 ). Another study found that 55 percent of individuals who participated in a research study would be willing to participate again in a future research study ( Trauth et al., 2000 ).
Willingness to Participate in Research
Public opinion surveys indicate that a majority of Americans are willing to participate in clinical research studies. In 2001, a compilation of studies commissioned by Research !America found that 63 percent of Americans would be willing to participate in a clinical research study ( Woolley and Propst, 2005 ). This percentage has remained stable over time. A 2007 Research!America survey also found that 63 percent of Americans would be very likely to participate in a clinical research study if asked ( Research!America, 2007 ); 68 percent of respondents reported that their desire to improve their own health or the health of others was a major factor in deciding whether to participate in a clinical research project ( Research!America, 2007 ).
Other surveys also suggest that willingness to participate in research focused on specific diseases is quite high. In one survey, the percentage of respondents indicating a willingness to participate in a medical research study was 88 percent for cancer, 86 percent for heart disease, 83 percent for a noncurable fatal disease, 79 percent for addiction, 78 percent for depression, and 76 percent for schizophrenia ( Trauth et al., 2000 ). Respondents with greater knowledge of how research is conducted were more willing to participate ( Trauth et al., 2000 ). Another study found that 8 of 10 Americans would consider participating in a clinical trial if faced with cancer. More than two-thirds of respondents said they would be willing to participate in a clinical trial designed to prevent cancer ( Comis et al., 2000 ).
Americans also seem to be very supportive of medical research that relies on genetic data. A 2007 survey found that 93 percent of Americans supported the use of genetic testing if the information collected is used by researchers to find new ways to diagnose, prevent, or treat disease ( Genetics & Public Policy Center, 2007 ). Two separate surveys found that 66 percent of Americans would be willing to donate their genetic material for medical research ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ). However, despite this apparent positive view of genetic research, 92 percent of Americans reported they were concerned about their genetic information being used in a “harmful way” ( Genetics & Public Policy Center, 2007 ).
Many factors, in addition to concerns about privacy and confidentiality ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ), may influence an individual’s willingness to participate in a medical research study. The Trauth survey found that individuals with higher income levels, with a college or graduate degree, or with children were more likely to participate in research. Age affected willingness to participate: 57 percent of respondents ages 18–34 were willing to participate in research, but only 31 percent of respondents ages 65 or older were willing ( Trauth et al., 2000 ).
Other factors that potentially influence an individual’s willingness to participate in research are race and ethnicity. It is well documented that minorities participate in health research at a much lower percentage than white Americans. Many cultural, linguistic, and socioeconomic barriers could be responsible for this difference ( Giuliano et al., 2000 ), and study results have been variable on this issue. Several studies suggest that the low participation rates by racial and ethnic minority groups are due to their strong distrust of the medical research community compared to the general population ( Braunstein et al., 2008 ; Corbie-Smith et al., 1999 ; Farmer et al., 2007 ; Grady et al., 2006 ; Shavers et al., 2002 ).
However, other evidence suggests that the low percentage of minorities participating in research is related to minority groups’ lack of access to the research community ( Brown et al., 2000 ; Wendler et al., 2006 ; Williams and Corbie-Smith, 2006 ). Thus, it is likely that the low number of minority individuals participating in medical research is at least partly due to recruitment techniques that are ineffective for minority populations.
The survey that focused on cancer research suggests that one of the main reasons why individuals do not participate in research is lack of knowledge about the availability of clinical trials. In a survey of nearly 6,000 cancer patients, 85 percent said they were unaware of the opportunity to participate in a clinical trial. Respondents who did participate said they did so because of one of the following beliefs: (1) trials provide access to the best quality of care (76 percent), (2) their participation would benefit future cancer patients (72 percent), (3) they would receive newer and better treatment (63 percent), and (4) participation would get them more care and attention (40 percent) ( Comis et al., 2000 ).
A recommendation from a physician can also impact participation. In the United States, 48 percent of respondents to one survey reported that a physicians’ recommendation would be a major factor in deciding whether to take part in a research study. Nearly three-fourths of respondents also cited an institution’s reputation as a key factor to consider when deciding whether to participate in a study ( Research!America, 2007 ). Twenty percent of respondents in an Italian public survey indicated that the presence of a physician as a reference during a research study influenced their willingness to participate ( Mosconi et al., 2005 ).
In sum, surveys indicate that the vast majority of Americans have a positive view of medical research, believe that research is beneficial to society, and are interested in health research findings. Although little is known about the attitudes of individuals who have actually participated in medical research, the available evidence suggests that most research participants have positive experiences. Surveys also suggest that a majority of Americans are willing to participate in clinical research studies. Similar to the findings in Chapter 2 , surveys indicate that many factors, in addition to concerns about privacy and confidentiality, can potentially influence an individual’s willingness to participate in medical research, including the type of research and personal characteristics such as health status, age, education, and race. Notably, respondents with greater knowledge of how research is conducted were more willing to participate in research.
- OVERSIGHT OF HEALTH RESEARCH
Historical Development of Federal Protections of Health Information in Research
The development of international codes, federal legislation, and federal regulation of human subjects often occurred in response to past abuses in biomedical experiments (reviewed by Pritts, 2008 ) ( Box 3-3 ). The most well-known examples included (1) reported abuses of concentration camp prisoners in Nazi experiments during World War II, and (2) the Tuskegee syphilis study begun in 1932, in which researchers withheld effective treatment from affected African American men long after a cure for syphilis was found. Most of the current principles and standards for conducting human subjects research were developed primarily to protect against the physical and mental harms that can result from these types of biomedical experiments. Therefore, they focus on the principles of autonomy and consent. Although the standards apply to research that uses identifiable health information, research based solely on information is not their primary focus.
The Basis for Human Subjects Protections in Biomedical Research. Nuremberg Code The Nuremberg Code, created by the international community after the Nazi War Crimes Trials, is generally seen as the first codification (more...)
In the United States, perhaps the most influential inquiry into the protection of human subjects in research was the Belmont Report. The Belmont principles have been elaborated on in many settings, and served as the basis for formal regulation of human subjects research in the United States. In general, states do not directly regulate the activity of most researchers ( Burris et al., 2003 ). However, the Belmont Commission’s recommendations were reflected in the Department of Health and Human Services’ (HHS’s) Policy for Protection of Human Subjects Research , Subpart A of 45 C.F.R. 46 (“Subpart A”) in 1979. 9 These protections were considered a benchmark policy for federal agencies, and in December 1981, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research recommended 10 that all federal departments and agencies adopt the HHS regulations. 11
In 1982, the President’s Office of Science and Technology Policy appointed a Committee for the Protection of Human Research Subjects to respond to the recommendations of the President’s commission. The committee agreed that uniformity of federal regulations on human subjects protection is desirable to eliminate unnecessary regulations and to promote increased understanding by institutions that conduct federally supported or regulated research. As a result, in 1991, other federal departments and agencies joined HHS in adopting a uniform set of rules for the protection of human subjects of research, identical to Subpart A of 45 C.F.R. 46, which is now informally known as the “ Common Rule .” Eighteen federal agencies have now adopted the Common Rule as their own respective regulations.
Overview of the Common Rule
The Common Rule governs most federally funded research conducted on human beings and aims to ensure that the rights of human subjects are protected during the course of a research project. The Common Rule stresses the importance of individual autonomy and consent; requires independent review of research by an Institutional Review Board (IRB); and seeks to minimize physical and mental harm. Privacy and confidentiality protections, although not defined in a detailed and prescriptive manner, are included as important components of risk in research.
The framework for achieving the goal of protecting human subjects is based on two foundational requirements: the informed consent of the research participant and the review of proposed research by an IRB. This section describes some of the basic parameters of the Common Rule (reviewed by Pritts, 2008 ). Particular provisions that interact with the HIPAA Privacy Rule are described in more detail in Chapter 4 .
Scope of the Common Rule
In general, the Common Rule applies only to research on human subjects that is supported by the federal government. 12 As noted previously, research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” 13
Under the Common Rule , a “human subject” is defined as “a living individual about whom an investigator … conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information.” Private information is considered to be personally identifiable if the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
The Common Rule applies to most human subjects research conducted using federal funds, but its influence is broader because most institutions that accept federal funds sign an agreement (a Federalwide Assurance or FWA) with HHS to abide by the Common Rule requirements in all research, regardless of funding source. Nonetheless, some privately funded human subjects research is conducted outside the purview of federal regulation ( Goldman and Choy, 2001 ; Williams, 2005 ). Companies and other organizations may voluntarily choose to apply the Common Rule to their research projects, and many do. However, research projects in which compliance is voluntary are not subject to oversight or disciplinary action by HHS ( Goldman and Choy, 2001 ; Williams, 2005 ).
Informed Consent 14
The Common Rule requires that a researcher obtain informed consent (usually in writing) from a person before he/she can be admitted to a study ( Williams, 2005 ). Informed consent is sought through a process in which a person learns key facts about a research study, including the potential risks and benefits, so that he/she can then agree voluntarily to take part or decide against it.
The Common Rule informed consent regulations focus primarily on the elements and documentation of informed consent rather than on the process used to obtain it. As to the process, the regulations require that informed consent be sought only under circumstances that provide the prospective subject with adequate opportunity to consider whether to participate. The Common Rule requires that information pertaining to informed consent be given in language understandable to the subject, and that the consent does not imply that the subject is giving up his/her legal rights or that the investigator is released from liability for negligence during the conduct of the study. 15
The Common Rule also specifies a number of elements that must be provided when informed consent is sought. These elements include:
- an explanation of the purposes of the research,
- the expected duration of the subject’s participation,
- the potential risks and benefits of the research,
- how confidentiality will be maintained,
- the fact that participation is strictly voluntary, and
- who the subject can contact to answer questions about the study or about his/her rights as a research participant.
In certain limited circumstances, the Common Rule allows an informed consent to be for unspecified future research. For example, under the Common Rule an informed consent can be used to obtain a person’s permission to study personally identifiable information maintained in a repository for future, unspecified research purposes ( HHS, 2003 ).
For the most part, the required elements of an informed consent address all types of research, although some are more relevant to biomedical research (e.g., the consent must include a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject). One required element of informed consent is particularly relevant to research involving personally identifiable health information. The Common Rule requires an informed consent to include a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. 16
Institutional Review Boards
Adopting the principles of the Belmont Report, the Common Rule requires that protocols for human subjects research be reviewed by an IRB ( Box 3-4 ) before research may begin. 17 The IRB must meet certain membership requirements, including having members with different expertise and at least one member who is not affiliated with the investigator’s institution. The Common Rule specifies which level of IRB review is needed for various types of research and provides criteria for the IRB to consider during the review. Although the Common Rule does not specify the procedures an IRB must follow in its review of protocols, it does require the IRB to have written procedures for how it will review protocols and document IRB decisions.
Institutional Review Boards. According to the Department of Health and Human Services (HHS) Institutional Review Board (IRB) guidebook, “the IRB is an administrative body established to protect the rights and welfare of human research subjects (more...)
The Common Rule requires that an IRB determine the following factors are satisfied to approve proposed research:
- Risks to subjects are minimized;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result;
- The selection of subjects is equitable;
- Informed consent will be sought in accordance with the rules and will be documented;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects; and
- When appropriate, adequate provisions are in place to protect the privacy of subjects and to maintain the confidentiality of data. 18
An IRB may waive the requirement to obtain informed consent or approve an alteration of the consent form for some minimal risk research. The IRB may also waive the requirement for signed consent in certain circumstances. 19
Anonymized Data
As noted above, the Common Rule considers use of “private identifiable information” to be human subjects research. Data are considered personally identifiable if the identity of the subject is or may be readily ascertained by the investigator or associated with the information accessed by the researcher. 20 However, the Common Rule exempts from its requirements research that involves:
[T]he collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. 21
Otherwise identifiable data may be deidentified or “anonymized” for purposes of the Common Rule if it is coded and certain other conditions are met ( HHS, 2004 ). Under Guidance issued by the Office for Human Research Protection, information is “coded” if identifying information (such as name or Social Security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (the code), and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimen.
Research involving only coded private information or specimens is not considered to involve human subjects under the Common Rule if the following conditions are met:
- The private information or specimens were not collected specifically for the currently proposed research project through an interaction or intervention with living individuals; and
- —The key to decipher the code is destroyed before the research begins;
- —The investigators and the holder of the key enter into an agreement prohibiting the release of the key to the investigators under any circumstances, until the individuals are deceased;
- —IRB-approved written policies and operating procedures for a repository or data management center prohibit the release of the key to investigators under any circumstances, until the individuals are deceased; or
- —Other legal requirements prohibit the release of the key to the investigators, until the individuals are deceased.
Under this standard, when a researcher accesses or receives data that have been coded and does not have access to the identifying key, the research is not considered human subjects research and is not subject to the Common Rule ’s requirements of informed consent or IRB review and approval of protocol.
Enforcement of the Common Rule
The Common Rule requirements for informed consent do not preempt any applicable federal, state, or local laws that require additional information to be disclosed to a subject in order for informed consent to be legally effective. 22
Federal funding can be suspended or withdrawn from an institution when it is found to be in material violation of the Common Rule . 23 There is no authority to impose penalties directly on individual researchers for violations. Neither does the Common Rule expressly provide a research participant with a private right of action. It should be noted, however, that recent cases indicate that courts may be willing to hold an institution liable under common law negligence theories where the approved informed consent form is determined to be less than adequate ( Shaul et al., 2005 ). 24
FDA Protection of Human Research Subjects
Some health research is also subject to FDA regulations. The FDA is charged by statute with ensuring the protection of the rights, safety, and welfare of human subjects who participate in clinical investigations 25 involving articles subject to the Federal Food, Drug, and Cosmetic Act 26 (the Act), as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA, including drugs, medical devices, and biological products for human use ( Box 3-5 ).
FDA Protection of Human Subjects Regulations. The Food and Drug Administration (FDA) Protection of Human Subjects Regulations aim to protect the rights of human subjects enrolled in research involving products that the FDA regulates (i.e., drugs, medical (more...)
In January 1981, the FDA adopted regulations governing informed consent of human subjects 27 and regulations establishing standards for the composition, operation, and responsibilities of IRBs that review clinical investigations involving human subjects. 28 At the same time, HHS adopted the Common Rule regulations on the protection of human research subjects. 29 The FDA’s regulations were harmonized with the Common Rule in 1991 to the extent permitted by statute. Key differences between FDA and HHS regulations include that the FDA does not allow for waiver or alteration of informed consent and requires that subjects be informed that the FDA may inspect their medical records. In addition, studies of efficacy based solely on medical records research are not permitted to support registration. Remaining differences in the rules are due to differences in the statutory scope or requirements ( Lee, 2000 ).
- DISTINGUISHING HEALTH RESEARCH FROM PRACTICE
The Common Rule and Privacy Rule make a somewhat artificial distinction between health research and some closely related health care practices, such as public health practice, quality improvement activities, program evaluations, 30 and utilization reviews, 31 all of which may involve collection and analysis of personally identifiable health information. However, determining which activities meet the definition of “research” is a major challenge for IRBs, Privacy Boards , 32 investigators, and health care practitioners because neither the regulations nor their interpretations by HHS provide clear guidance on how to distinguish research from activities that use similar techniques to analyze health information ( IOM, 2000a ).
It is important for IRBs and Privacy Boards to correctly distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule . Only research requires formal IRB or Privacy Board review and informed consent. 33 Inappropriate classification of an activity as research can make it difficult or impossible for important health care activities, such as public health practice and quality improvement, to be undertaken. On the other hand, failure to correctly identify an activity as research could potentially allow improper disclosure of personally identifiable health information without sufficient oversight.
Thus, standard criteria are urgently needed for IRBs and Privacy Boards to use when making distinctions between health research and related activities, and the committee recommends that HHS consult with relevant stake holders to develop such standard criteria. HHS is aware of this need, and created a working document titled “What Is Research ?” However, the work on this project apparently has been delayed for unknown reasons ( NCURA, 2007 ). 34 As described below, a number of other models have already been proposed to help determine whether activities should be classified as research in the fields of public health and quality improvement, and these could be instructive for developing HHS guidance. Any criteria adopted by HHS should be regularly evaluated to ensure that they are helpful and producing the desired outcomes.
The following sections describe some ongoing efforts to develop such criteria in the fields of public health and quality improvement. The intent of the committee is not to endorse these particular models, but rather to illustrate the challenges associated with making these distinctions and establishing standard criteria.
Public Health Practice Versus Public Health Research
The Belmont Report defined health practice as “interventions designed solely to enhance the well-being of the person, patient or client, and which have reasonable expectation of success” ( CDC, 1999 ). To apply this definition to “public” health practice, the targeted beneficiary of the intervention must be expanded to include benefit to the community, rather than just a particular person. Neither the Common Rule nor the Privacy Rule provides a specific definition for public health research; rather public health research is included in the general definition of research. However, the Privacy Rule regulates public health practice differently from public health research (see Chapter 4 ).
An early model for distinguishing public health research from public health practice focused on the intent for which the activity was designed, noting that the intent of public health research is to “contribute to or generate generalizable knowledge,” while the intent of public health practice is to “conduct programs to prevent disease and injury and improve the health of communities” ( Snider and Stroup, 1997 ). The Centers for Disease Control and Prevention developed a similar method with an expanded assessment of intent. For example, the model posits that in public health research, the intended benefits of the project extend beyond the study participants, and the data collected exceed the requirements for the care of the study participants. But for public health practice, the intended benefits of the project are primarily for the participants in the activity, or for the participants’ community, and the only data collected are those needed to assess or improve a public health program or service, or the health of the participants and their community. The model also assumes that public health practice is based on well-established medical interventions and is nonexperimental ( CDC, 1999 ). However, these models both have been criticized as too subjective and too dependent on the opinion of the person conducting the activity ( Gostin, 2008 ; Hodge, 2005 ).
A new, more comprehensive model incorporating much of the previous two was recently proposed as a more objective checklist to be used by IRBs, Privacy Boards , and interested parties ( Hodge, 2005 ; Hodge and Gostin, 2004 ). The foundations for this model are specific definitions of public health research: “the collection and analysis of identifiable health data by a public health authority for the purpose of generating knowledge that will benefit those beyond the participating community who bear the risks of participation,” and public health practice: “the collection and analysis of identifiable health data by a public health authority for the purpose of protecting the health of a particular community, where the benefits and risks are primarily designed to accrue to the participating community.”
The model is based on two primary assumptions. First, the actor performing the activity in question is a governmental public health official, agent, agency, or entity at the federal, tribal, state, or local level. Second, the activity in question involves the acquisition, use, or disclosure of personally identifiable health data. The model is then divided into two stages. Stage 1 is applied to all activities, and can be used to distinguish practice from research in the easiest cases. Stage 2 is only applied to those cases that are hard to distinguish, and where Stage 1 failed to lead to a definitive IRB/ Privacy Board decision ( Box 3-6 ).
A Model for Distinguishing Public Health Practice from Research. Stage 1 Public health practice:
Quality Improvement Versus Health Research
Quality improvement has been defined as “systematic, data-guided activities designed to bring about immediate, positive change in the delivery of health care in a particular setting” ( Baily, 2008 ). Quality improvement activities do not require IRB or Privacy Board approval under the Common Rule or the Privacy Rule, which classify quality improvement as a component of health care operations. 35
However, in many cases, it is difficult for health care providers, IRBs, and Privacy Boards to determine whether a particular activity is purely for quality improvement, or whether it also entails research. One survey 36 exploring opinions in the health care community about the need for IRBs to review various quality-related activities found that physicians conducting quality improvement were less likely than IRB chairs to believe that IRB review was required for a given hypothetical activity, or that informed consent was necessary ( Lindenauer et al., 2002 ). Recently, a highly publicized case has again brought the issue to the forefront for all the stakeholders ( Box 3-7 ).
A Case Study of Quality Improvement and Research. Peter Pronovost of Johns Hopkins University (JHU) led a quality improvement effort at 103 intensive care units (ICUs) in Michigan hospitals to reduce the number of catheter-related bloodstream infections. (more...)
Some members of the health care community have proposed requiring that all prospective quality improvement activities go through external review ( Bellin and Dubler, 2001 ), while others have outlined specific criteria to differentiate quality improvement activities from research.
For example, Casarett and colleagues developed a two-part test to identify quality improvement activities. The first test is whether the majority of patients are expected to benefit directly from “the knowledge to be gained” from the initiative. This means that the patients must actually benefit from the knowledge learned during the evaluation, not just from being a recipient of the protocol itself. If the patients are generally expected to directly benefit from the knowledge gained during the activity, then the activity is quality improvement. If not, the activity is research. The second test is whether the participants would be subjected to additional risks or burdens, including the risk of privacy breach, beyond the usual clinical practice in order to make the results of the initiative generalizable. If yes, then the initiative should be reviewed as research ( Casarett et al., 2000 ).
More recently, the Hastings Center published a report exploring the similarities and differences between research and quality improvement. The report emphasized three fundamental characteristics of quality improvement and three fundamental characteristics of research. The authors argue that individuals have a responsibility to participate in the quality improvement activities because all patients have an interest in receiving high-quality medical care, and the success of a quality improvement activity depends on the cooperation of all patients. In addition, the report notes that quality improvement activities are a low risk to the patient, so there is little justification for not participating. The report also assumes that quality improvement activities are based on existing knowledge about human health and should lead to immediate local improvements in the provision of medical care.
In contrast, the report notes that participation in research should be voluntary, and decisions to participate should be based on researchers’ full disclosure of all the potential risks and benefits. In addition, the authors assert that research is designed to create new knowledge about human health, rather than relying solely on existing knowledge, and that most research does not result in any direct benefit to the institution where the research is being conducted.
The authors concluded that IRBs are not the appropriate body for the ethical oversight of quality improvement activities. They argue that IRBs unnecessarily impose high transaction costs on these activities because of the difference in the way they are conducted compared to research. For example, in research, any changes in methodology require further IRB approval. In contrast, quality improvement activities involve frequent adjustments in the intervention, measurement, and goals of the activity based on the experience of the investigators. Requiring the investigator to revisit an IRB every time a small adjustment is needed in such an activity significantly increases the amount of time and effort required to conduct the initiative and to produce meaningful data. Also, the investigators involved in quality improvement activities ordinarily are already involved in the clinical care of participants and bear responsibility for the quality and safety of an intervention. Thus, the authors argue that there is no need for the additional oversight by an IRB to protect participant safety.
Rather, the report recommended integrating the ethical oversight of quality improvement activities into the ongoing management of an institution’s health care delivery system, suggesting that oversight of quality improvement could be left with the managers of clinical care organizations, and that consent to receive treatment should include consent to participate in any quality improvement project that is minimal risk. However, the report stated that if a project has the characteristics of both quality improvement and research, the project should be reviewed as both human subjects research and quality improvement ( Baily et al., 2006 ; Lynn et al., 2007 ).
In response to the ongoing confusion over when quality improvement rises to the level of research and requires IRB review, the IOM jointly hosted a meeting with the American Board of Internal Medicine in May 2008 to discuss this issue. Key members of the quality improvement community attended, and short- and long-term solutions to this problem were proposed. However, no written report from this meeting was produced and no general consensus was reached.
- THE IMPORTANCE OF EFFECTIVE COMMUNICATION WITH THE PUBLIC
As noted previously in this chapter, surveys indicate that the vast majority of Americans believe that health research is important and are interested in the findings of research studies. The majority of patients also appear to be willing to participate in health research, either by volunteering for a study to test a medical intervention or by allowing access to their medical records or stored biospecimens, under certain conditions. Their willingness to participate depends on trust in researchers to safeguard the rights and well-being of patients, including assurance of privacy and confidentiality, and the belief that it is a worthwhile endeavor that warrants their involvement. Yet patients often lack information about how research is conducted, and are rarely informed about research results that may have a direct impact on their health. The committee’s recommendations in this section are intended to address both the public’s desire for more information about health research and to help fulfill two of the committees overarching goals of the report: (1) improving the privacy and security of health information, and (2) improving the effectiveness of health research.
Disseminating Health Research Results
Ethicists have long suggested greater community involvement in health research studies, including more communication about research results (reviewed by Shalowitz and Miller, 2008a , b ). In addition, the IOM committee identified transparency—the responsibility to disclose clearly how and why personally identifiable information is being collected—as an important component of comprehensive privacy protections. A previous IOM report also recommended improved communication with the public and research participants to ensure that the protection process is open and accessible to all interested parties ( IOM, 2002 ). Effective communication would build the public’s trust of the research community and is consistent with the principles of fair information practices.
When patients consent to the use of their medical records in a particular study, health researchers should make greater efforts at the conclusion of the study to inform study participants about the results, and the relevance and importance of those results. Learning about clinically relevant findings from a study in which a patient has participated could make patients feel more integrated into the process and could encourage more to participate in future studies. A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community ( AMS, 2006 ). Moreover, direct feedback with study participants could lead to improved health care for the individuals if the results indicate that an altered course of care is warranted.
Nonetheless, there are multiple impediments, beyond cost, to providing meaningful feedback to participants. A summary of the results alone, while necessary and reasonable, can be seen as a token, and also raises questions about issues such as how best to write summaries, the stage at which results should be disseminated, and how to present research with uninformative outcomes. For example, one recent study found that sharing results directly with study participants was met with overwhelmingly favorable reactions from patients, but the study also revealed some obstacles ( Partridge et al., 2008 ). In a survey of women who had participated in a randomized trial of breast cancer therapy and had received a summary of the study results by mail, 95 percent reported that they were glad they received the results. Most respondents interpreted the results correctly, although incorrect interpretation of the results was associated with increased anxiety, as was dissatisfaction with treatment.
Although some guidelines for providing and explaining study results to research participants have been proposed, they differ in details because limited data are available on this subject, and thus standards are lacking ( Partridge and Winer, 2002 ; Partridge et al., 2008 ; Shalowitz and Miller, 2008b ; Zarin and Tse, 2008 ). Because transparency is best achieved by providing graded levels of information and guidance to interested parties ( IOM, 2002 ), it will be important to develop effective and efficient ways to communicate with various sectors of the population. A commitment to the principles of “plain language” 37 will be important. Broader adoption of electronic medical records may also be helpful in accomplishing this goal.
Research Registries
One way to make information about research studies more broadly available to the public is through registration of trials and other studies in public databases. HHS should encourage such registration of trials and other studies, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization (see Chapter 4 ). Numerous clinical trial registries already exist, and registration has increased in recent years (reviewed by Zarin and Tse, 2008 ). In 2000, the National Library of Medicine established a clinical trials registry ( ClinicalTrials.gov ), which has expanded to include information from several other trial registries and to serve as the FDA-required site for submissions about clinical trials subject to the FDA databank requirement. The FDA Amendments Act of 2007 38 expanded the scope of required registrations at ClinicalTrials.gov and provided the first federally funded trials results database. It mandates registrations of controlled clinical investigations, except for Phase I trials, of drugs, biologics, and devices subject to FDA regulation.
A policy of the International Committee of Medical Journal Editors (ICMJE), adopted in fall 2005, also requires prospective trial registration as a precondition for publication ( DeAngelis et al., 2004 ). This policy led to a 73 percent increase in trial registrations of all intervention types from around the world ( Zarin et al., 2005 ). Nearly 45,000 trials had been registered by fall 2007.
However, although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, no centralized system currently exists to disseminate information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. The current statutory requirements for registration and data reporting in the United States are not as broad as the transnational policies of the ICMJE or the World Health Organization, which call for the registration of all interventional studies in human beings regardless of intervention type ( Laine et al., 2007 ; Sim et al., 2006 ). Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/ Privacy Board approved waiver of consent or authorization, including those studies in a registry could be an important method for increasing public knowledge of such studies.
Informing the Public About the Methods and Value of Research
As noted previously, clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study subjects will be necessary to derive meaningful results.
However, many patients probably are not aware that their medical records are being used in information-based research. For example, the recent study that used focus groups to examine the views of veterans toward the use of medical records in research found that the majority of participants (75 percent) were not aware that “under some circumstances, [their] medical records could be used in some research studies without [their] permission,” despite the fact that a notice of privacy practices, which included a statement that such research could occur, had been mailed to all participants less than a year prior to the study ( Damschroder et al., 2007 ).
Moreover, surveys show that many patients desire not only notice, but also the opportunity to decide whether to consent to such research with medical records. Those surveys further indicate that patients who wish to be asked for consent for each study are most concerned about the potentially detrimental affects of inappropriate disclosure of their personally identifiable health information, including discrimination in obtaining health or life insurance or employment.
As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported on could also help to ease patient concerns about privacy and increase patients’ trust in the research community, which as noted above is important for the public’s continued participation in health research. For example, datasets are most often provided to researchers without direct identifiers such as name and Social Security number. Furthermore, identifiers are not included in publications about research results. Also, under both the Privacy Rule and the Common Rule , a waiver of consent and authorization is possible only under the supervision of an IRB or Privacy Board , and a waiver is granted only when the research entails minimal risk and when obtaining individual consent and authorization is impracticable (see the previous section and also Chapter 4 ). Finally, professional ethics dictate that researchers safeguard data and respect privacy.
Conveying the value of medical records research to patients will be important. Surveys show that people are more supportive of research that is relevant to them and their loved ones. At the same time, educational efforts should stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research ( Box 3-8 ), but datasets will not represent the entire population if some people withhold access to their health information.
Selection Bias in Health Research. When researchers are required to obtain consent or authorization to access each individual’s medical record for a research study, it is likely that individuals’ willingness to grant access will not be (more...)
In addition, an educated public could also decrease the potential for biased research samples. A universal requirement for consent or authorization in medical records research leads to incomplete datasets, and thus to biased results and inaccurate conclusions. Some large medical institutions with a strong research history and reputation (e.g., Mayo Clinic) can obtain authorization and consent rates as high as 80 percent, but the 20 percent who refuse have distinct demographic and health characteristics. In fact, even a refusal rate of less than 5 percent can create selection bias in the data ( Jacobsen et al., 1999 ; see Chapter 5 for more detail). Conveying to the public the importance of health care improvements derived from medical records research and stressing the negative impact of incomplete datasets on research findings may increase the public’s participation in research and their willingness to support information-based research that is conducted with IRB or Privacy Board oversight, under a waiver of patient consent or authorization.
Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required ( Box 3-1 ). For example, analysis of medical records showed that infants exposed to diethylstilbesterol (DES) during the first trimester of pregnancy had an increased risk of breast, vaginal, and cervical cancer as well as reproductive anomalies as adults. Similarly, studies of medical records led to the discovery that folic acid supplementation during pregnancy can prevent neural tube defects.
Thus, HHS and the health research community should work to edu cate the public about how research is done and the value it provides. All stakeholders, including professional organizations, nonprofit funders, and patient organizations, have different interests and responsibilities to make sure that their constituencies are well informed. For example, the American Society of Clinical Oncology and the American Heart Association already have some online resources to help patients gather information about research that may be relevant to their conditions. But coordination and identification of best practices by HHS would be helpful, and research is needed to identify which segments of the population would be receptive to and benefit from various types of information about how research is done and its value in order to create and implement an effective plan.
Greater use of community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained, 39 could help achieve this goal. These groups help researchers to recruit research participants by using the knowledge of the community to understand health problems and to design activities that the community is likely to value. They also inform community members about how the research is done and what comes out of it, with the goal of providing immediate community benefits from the results when possible.
- CONCLUSIONS AND RECOMMENDATIONS
Based on its review of the information described in this chapter, the committee agreed on a second overarching principle to guide the formation of recommendations. The committee affirms the importance of maintaining and improving health research effectiveness. Research discoveries are central to achieving the goal of extending the quality of healthy lives. Research into causes of disease, methods for prevention, techniques for diagnosis, and new approaches to treatment has increased life expectancy, reduced infant mortality, limited the toll of infectious diseases, and improved outcomes for patients with heart disease, cancer, diabetes, and other chronic diseases. Patient-oriented clinical research that tests new ideas makes rapid medical progress possible. Today, the rate of discovery is accelerating, and we are at the precipice of a remarkable period of investigative promise made possible by new knowledge about the genetic underpinnings of disease. Genomic research is opening new possibilities for preventing illness and for developing safer, more effective medical care that may eventually be tailored for specific individuals. Further advances in relating genetic information to predispositions to disease and responses to treatments will require the use of large amounts of existing health-related information and stored tissue specimens. The increasing use of electronic medical records will further facilitate the generation of new knowledge through research and accelerate the pace of discovery. These efforts will require broad participation of patients in research and broad data sharing to ensure that the results are valid and applicable to different segments of the population. Collaborative partnerships among communities of patients, their physicians, and teams of researchers to gain new scientific knowledge will bring tangible benefits for people in this country and around the world.
Surveys indicate that the majority of Americans believe that health research is important, are interested in the findings of research studies, and are willing to participate in health research. But patients often lack information about how research is conducted and are rarely informed about research results that may have a direct impact on their health. Effective communication could build the public’s trust of the research community, which is important because trust is necessary for the public’s continued participation in research. Moreover, direct feedback could lead to improved health care for study participants if the results indicate that an altered course of care is warranted.
Thus, the committee recommends that when patients consent to the use of their medical records in a particular study, health researchers should make greater efforts when the study ends to inform study participants about the results, and the relevance and importance of those results. Broader adoption of electronic health records may be helpful in accomplishing this goal, but standards and guidelines for providing and explaining study results to research participants or various sectors of the public are needed.
HHS should also encourage registration of trials and other studies in public databases, particularly when research is conducted with an IRB/ Privacy Board approved waiver of consent or authorization, as a way to make information about research studies more broadly available to the public. Numerous clinical trial registries already exist, and registration has increased in recent years, but no centralized system currently exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including such studies in a registry could be an important method for increasing public knowledge of those studies.
Interventional clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic health records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study participants will be necessary to derive meaningful results.
However, many patients are likely not aware that their medical records are being used in information-based research, and surveys show that many patients desire not only notice, but also the opportunity to decide about whether to consent to such research with medical records. As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported could also increase patients’ trust in the research community. Thus, HHS and the health research community should work to educate the public about how research is done.
It will also be important for HHS and researchers to convey the value of health care improvements derived from medical records research, and to stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research, but datasets will not be representative of the entire population if some people withhold access to their health information. A universal requirement for consent or authorization in information-based research may lead to incomplete datasets, and thus to biased results and inaccurate conclusions. Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required.
To ensure that beneficial health research and related activities continue to be undertaken with appropriate oversight under federal regulations, it will be important for HHS to also provide more guidance on how to distinguish the various activities. The Privacy Rule makes a distinction between health research and some closely related endeavors, such as public health and quality improvement activities, which also may involve collection and analysis of personally identifiable health information. Under the Privacy Rule (as well as the Common Rule ), these activities, which aim to protect the public’s health and improve the quality of patient care, are considered health care “practice” rather than health research. Therefore, they can be undertaken without consent or authorization, or an IRB/ Privacy Board waiver of consent or authorization. However, it can be a challenge for IRBs and Privacy Boards to distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule, and inappropriate decisions may prevent important activities from being undertaken or could potentially allow improper disclosure of personally identifiable health information.
To address these difficulties, a number of models have been proposed that outline the criteria IRBs and Privacy Boards should use to distinguish practice and research. For example, one recent model provides a detailed checklist for IRBs and Privacy Boards to use in determining whether an activity is public health research and required to comply with the research provisions of the Privacy Rule, or public health practice that does not need IRB/Privacy Board review. The committee believes that standardizing the criteria is essential to support the conduct of these important health care activities.
Thus, HHS should convene the relevant stakeholders to develop standard criteria for IRBs and Privacy Boards to use when making decisions about whether protocols entail research or practice. There should be flexibility in the regulation to allow important activities to go forward with appropriate levels of oversight. Also, it will be important to evaluate whether these criteria are effective in aiding IRB/Privacy Board reviews of proposed protocols, and whether they lead to appropriate IRB/Privacy Board decisions.
These changes suggested above could be accomplished without any changes to HIPAA by making them a condition of funding from HHS and other research sponsors and by providing some additional funds to cover the cost.
- AMS (Academy of Medical Sciences). Personal data for public good: Using health information in medical research. 2006. [accessed August 28, 2008]. http://www .acmedsci.ac .uk/images/project/Personal.pdf .
- Baily MA. Harming through protection? New England Journal of Medicine. 2008; 258 (8):768–769. [ PubMed : 18287599 ]
- Baily MA, Bottrell M, Lynn J, Jennings B. The ethics of using QI methods to improve health care quality and safety. A Hastings Center Special Report. 2006; 36 (4):S1–S40. [ PubMed : 16898359 ]
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander VM, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998; 280 (15):1311–1316. [ PubMed : 9794308 ]
- Bellin E, Dubler NN. The quality improvement-research divide and the need for external oversight. American Journal of Public Health. 2001; 91 (9):1512–1517. [ PMC free article : PMC1446813 ] [ PubMed : 11527790 ]
- Big Health. Big health consortium. 2008. [accessed October 29, 2008]. http://www .bighealthconsortium .org/about/
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87 (1):1–9. [ PubMed : 18204365 ]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention and treatment trials. Annals of Epidemiology. 2000; 10 :S13–S21. [ PubMed : 11189088 ]
- Burris S, Gable L, Stone L, Lazzarini Z. The role of state law in protecting human subjects of public health research and practice. Journal of Law, Medicine & Ethics. 2003; 31 :654. [ PubMed : 14968667 ]
- Casarett D, Karlawish J, Sugarman J. Determining when quality improvement initiatives should be considered research: Proposed criteria and potential implications. JAMA. 2000; 284 (7):2275–2280. [ PubMed : 10807388 ]
- Casarett D, Karlawish J, Andrews E, Caplan A. Bioethical issues in pharmacoepidemiological research. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 417–432.
- CDC (Centers for Disease Control and Prevention). Guidelines for defining public health research and public health non-research. 1999. [accessed March 4, 2008]. http://www .cdc.gov/od /science/regs/hrpp/researchdefinition .htm .
- CHSR (Coalition for Health Services Research). Framework for health services research policy for 2008. 2008. [accessed August 21, 2008]. http://www .chsr.org/Policy_Priorities .pdf .
- Comis RL, Aldige CR, Stovall EL, Krebs LU, Risher PJ, Taylor HJ. A quantitative survey of public attitudes towards cancer clinical trials. Philadelphia, PA: Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council, and Oncology Nursing Society; 2000.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans towards participation in medical research. Journal of General Internal Medicine. 1999; 14 :537–546. [ PMC free article : PMC1496744 ] [ PubMed : 10491242 ]
- Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Social Science & Medicine. 2007; 64 (1):223–235. [ PubMed : 17045717 ]
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA. 2004; 292 (11):1363–1364. [ PubMed : 15355936 ]
- Farmer D, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. Journal of Health Care for the Poor and Underserved. 2007; 18 :85–99. [ PubMed : 17337800 ]
- FDA (Food and Drug Administration). Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation. Federal Register. 1971; 36 (217):21537–21538.
- FDA. FDA public health advisory, deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/advisory/antipsychotics.htm .
- FDA. FDA alert [6/16/2008]: Information for healthcare professionals. Antipsychotics. 2008. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/infosheets/hcp /antipsychotics_conventional.htm .
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who’s paying? Health Affairs Web Exclusive. 2003. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w3.219v1 . [ PubMed : 14527256 ]
- Furrow BR, Greaney TL, Johnson SH, Jost TS, Schwartz RL. Bioethics: Health care law and ethics. St. Paul, MN: Thomson/West; 2004.
- GAO (Government Accountability Office). Scientific research: Continued vigilance critical to protecting human subjects. Washington, DC: GAO; 1996.
- Genetics & Public Policy Center. U.S. public opinion on uses of genetic information and genetic discrimination. 2007. [accessed August 21, 2008]. http://www .dnapolicy .org/resources/GINAPublic _Opinion_Genetic _Information_Discrimination.pdf .
- Gill S, Bronskill S, Normand S, Anderson G, Sykora K, Lam K, Bell C, Lee P, Fischer H, Herrmann N, Gurwitz J, Rochon P. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine. 2007; 146 :775–786. [ PubMed : 17548409 ]
- Giuliano AR, Mokuau N, Hughes C, Tortelero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000; 10 :S22–S34. [ PubMed : 11189089 ]
- Goldman J, Choy A. Ethical and policy issues in research involving human participants. Bethesda, MD: National Bioethics Advisory Commission; 2001. Privacy and confidentiality in health research; pp. C1–C34.
- Gostin LO. Public health law: Power, duty, restraint. Berkeley, CA: University of California Press; 2008. Surveillance and public health research: Personal privacy and the “right to know.”
- Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American Journal of Public Health. 2006; 96 (11):1996–2001. [ PMC free article : PMC1751807 ] [ PubMed : 17018826 ]
- Hatfield M, Sonnenschein HF, Rosenberg LE. Exceptional returns: The economic value of America’s investment in medical research. 2001. [accessed August 21, 2008]. http://www .laskerfoundation .org/advocacy/pdf/exceptional.pdf .
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971; 284 (15):878–881. [ PubMed : 5549830 ]
- HEW (Department of Health, Education and Welfare). The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. 1979. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/belmont.html . [ PubMed : 25951677 ]
- HHS (Department of Health and Human Services). Institutional review boards and the HIPAA Privacy Rule. 2003. [accessed August 21, 2008]. http: //privacyruleandresearch .nih.gov/pdf/IRB_Factsheet.pdf .
- HHS. Guidance on research involving coded private information or biological specimens. 2004. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /humansubjects/guidance/cdebiol.pdf .
- Hodge JG Jr. An enhanced approach to distinguishing public health practice and human subjects research. Journal of Law, Medicine & Ethics. 2005; 33 (1):125–141. [ PubMed : 15934670 ]
- Hodge JG, Gostin LO. Public health practice vs. research: A report for public health practitioners including cases and guidance for making distinctions. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004.
- IOM (Institute of Medicine). Health services research: Work force and educational issues. Washington, DC: National Academy Press; 1995.
- IOM. Protecting data privacy in health services research. Washington, DC: National Academy Press; 2000a. [ PubMed : 25057723 ]
- IOM. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000b. [ PubMed : 25077248 ]
- IOM. Responsible research: A systems approach to protecting research participants. Washington, DC: The National Academies Press; 2002. [ PubMed : 20669487 ]
- Jacobsen S, Xia Z, Campion M, Darby C, Plevak M, Seltman K, Melton L. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999; 74 :330–338. [ PubMed : 10221460 ]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van Der Weyden MB, Verheugt FWA. Clinical trial registration—looking back and moving ahead. New England Journal of Medicine. 2007; 356 (26):2734–2736. [ PubMed : 17548427 ]
- Lee B. Comparison of FDA and HHS human subject protection regulations. 2000. [accessed August 21, 2008]. http://www .fda.gov/oc/gcp/comparison .html .
- Lindenauer PK, Benjamin EM, Naglieri-Prescod D, Fitzgerald J, Pekow P. The role of the institutional review board in quality improvement: A survey of quality officers, institutional review board chairs, and journal editors. The American Journal of Medicine. 2002; 113 :575–579. [ PubMed : 12459404 ]
- Lowrance WW. Learning from experience, privacy and the secondary use of data in health research. London: The Nuffield Trust; 2002.
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007; 317 :600–602. [ PubMed : 17673640 ]
- Lynn J, Baily MA, Bottrell M, Jennings B, Levine RJ, Davidoff F, Casarett D, Corrigan J, Fox E, Wynia MK, Agich GJ, Speroff T, Schyve P, Batalden P, Tunis S, Berlinger N, Cronenwett L, Fitzmaurice M, Dubler NN, James B. The ethics of using quality improvement methods in health care. Annals of Internal Medicine. 2007; 146 (6):666–674. [ PubMed : 17438310 ]
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, Klein J, Helfand M. The cost-effectiveness of screening mammography beyond age 65: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003; 139 :835–842. [ PubMed : 14623621 ]
- Mick S, Morlock LL, Salkever D, de Lissovoy G, Malitz F, Wise CG, Jones A. Strategic activity and financial performance of U.S. rural hospitals: A national study, 1983 to 1988. Journal of Rural Health. 1994; 10 (3):150–167. [ PubMed : 10138031 ]
- Mosconi P, Poli P, Giolo A, Apolone G. How Italian health consumers feel about clinical research: A questionnaire survey. European Journal of Public Health. 2005; 15 (4):372–379. [ PubMed : 16014662 ]
- Murphy K, Topel R. The economic value of medical research. Chicago, IL: University of Chicago Press; 1999.
- NCI (National Cancer Institute). Getting connected with caBIG: Data sharing and security framework. 2008. [accessed October 27, 2008]. https://cabig .nci.nih .gov/working_groups /DSIC_SLWG/data_sharing_policy/
- NCURA (National Council of University Research Administrators). Report on research compliance. 2007. [accessed March 4, 2008]. http://www .reportonresearchcompliance .com .
- NCVHS (National Committee on Vital and Health Statistics). Enhanced protections for uses of health data: A stewardship framework for “secondary uses” of electronically collected and transmitted health data. 2007a. [accessed December 19, 2007]. http://ncvhs .hhs.gov/071221lt.pdf .
- NCVHS, Ad Hoc Work Group on Secondary Uses of Health Data. Testimony of the cancer Biomedical Informatics Grid (caBIG) Data Sharing and Intellectual Capital (DSIC) workspace. 2007b August 1, 2007
- Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002; 346 (22):1715–1722. [ PubMed : 12037152 ]
- NSF (National Science Foundation). Science and Engineering Indicators 2006. Arlington, VA: National Science Foundation; 2006. Science and technology: Public attitudes and understanding. Chapter 7.
- OHRP (Office for Human Research Protections). IRB guidebook, part I.A. 2008a. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /irb/irb_guidebook.htm .
- OHRP. OHRP concludes case regarding Johns Hopkins University research on hospital infections. 2008b. [accessed August 21, 2008]. http://www .hhs.gov/ohrp/news/recentnews .html#20080215 .
- Partridge A, Winer E. Informing clinical trial participants about study results. JAMA. 2002; 288 :363–365. [ PubMed : 12117402 ]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Research and Treatment. 2008 June 10 [ PubMed : 18543100 ]
- Pitkin R. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007; 85 (1):285S–288S. [ PubMed : 17209211 ]
- Pritts J. The importance and value of protecting the privacy of health information: Roles of HIPAA Privacy Rule and the Common Rule in health research. 2008. [accessed March 15, 2008]. http://www .iom.edu/CMS/3740/43729/53160 .aspx .
- Research!America. America speaks: Poll summary. Vol. 7. Alexandria, VA: United Health Foundation; 2007.
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang P. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal. 2007; 176 :672–632. [ PMC free article : PMC1800321 ] [ PubMed : 17325327 ]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. PLoS Medicine. 2008a May 13; 5 (5):e91. [ PMC free article : PMC2375946 ] [ PubMed : 18479180 ]
- Shalowitz DI, Miller FG. The search for clarity in communicating research results to study participants. Journal of Medical Ethics. 2008b September; 34 (9):e17. [ PubMed : 18757617 ]
- Shaul RZ, Birenbaum S, Evans M. Legal liability in research: Early lessons from North America. BMC Medical Ethics. 2005; 6 (4):1–4. [ PMC free article : PMC1182131 ] [ PubMed : 15953387 ]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002; 12 :248–256. [ PubMed : 11988413 ]
- Sim I, Chan A-W, Gülmezoglu AM, Evans T, Pang T. Clinical trial registration: Transparency is the watchword. The Lancet. 2006; 367 (9523):1631–1633. [ PubMed : 16714166 ]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987; 235 (4785):177–182. [ PubMed : 3798106 ]
- Snider DE, Stroup DF. Defining research when it comes to public health. Public Health Reports. 1997; 112 :29–32. [ PMC free article : PMC1381834 ] [ PubMed : 9018284 ]
- Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity in rising medical spending. Health Affairs Web Exclusive. 2004. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w4.480v1 . [ PubMed : 15496437 ]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health & Social Policy. 2000; 12 (2):23–43. [ PubMed : 11184441 ]
- Veurink M, Koster M, Berg L. The history of DES, lessons to be learned. Pharmacy World & Science. 2005; 27 (3):139–143. [ PubMed : 16096877 ]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3 (2):201–210. [ PMC free article : PMC1298944 ] [ PubMed : 16318411 ]
- Westin A. How the public views privacy and health research. 2007. [accessed November 11, 2008]. http://www .iom.edu/Object .File/Master/48 /528/%20Westin%20IOM %20Srvy%20Rept%2011-1107.pdf .
- Williams ED. Federal protection for human research subjects: An analysis of the Common Rule and its interactions with FDA regulations and the HIPAA Privacy Rule, CRS report for Congress. Washington, DC: Congressional Research Service; 2005.
- Williams IC, Corbie-Smith G. Investigator beliefs and reported success in recruiting minority participants. Contemporary Clinical Trials. 2006; 27 :580–586. [ PubMed : 16839822 ]
- Winston FK, Durbin DR, Kallan MJ, Moll EK. The danger of premature graduation to seat belts for young children. Pediatrics. 2000; 105 (6):1179–1183. [ PubMed : 10835054 ]
- WMA (World Medical Association). Declaration of Helsinki: Ethical principles for medical research involving human subjects. 1964. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/helsinki.html . [ PubMed : 16010903 ]
- Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294 (11):1380–1384. [ PubMed : 16174697 ]
- Zarin DA, Tse T. Moving toward transparency of clinical trials. Science. 2008; 319 :1340–1342. [ PMC free article : PMC2396952 ] [ PubMed : 18323436 ]
- Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine. 2005; 353 (26):2779–2787. [ PMC free article : PMC1568386 ] [ PubMed : 16382064 ]
Epidemiology is the study of the occurrence, distribution, and control of diseases in populations.
Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understanding of the structure, processes, and effects of health services for individuals and populations ( IOM, 1995 ).
The National Committee on Vital and Health Statistics has noted that “secondary uses” of health data is an ill-defined term, and urges abandoning it in favor of precise description of each use ( NCVHS, 2007a ). Thus, the committee chose to minimize use of the term in this report.
See Standards for Privacy of Individually Identifiable Health Information , 64 Fed. Reg. 59918, 59967 (preamble to rule proposed November 3, 1999) for a discussion on the benefits of health records research.
Effectiveness can be defined as the extent to which a specific test or intervention, when used under ordinary circumstances, does what it is intended to do. Efficacy refers to the extent to which a specific test or intervention produces a beneficial result under ideal conditions (e.g., in a clinical trial).
See http://www .intermacs.org .
See http://www .elso.med.umich.edu .
See http://www .unos.org/Data .
The Department of Health, Education and Welfare (now HHS) had previously issued policy and guidance on the protection of human subjects. See Williams (2005) .
In its report “First Biennial Report on the Adequacy and Uniformity of Federal Rules and Policies, and their Implementation, for the Protection of Human Subjects in Biomedical and Behavioral Research , Protecting Human Subjects.”
45 C.F.R. part 46 (2005).
See 45 C.F.R. § 46.101 (2005).
See 45 C.F.R. § 46.102(d) (2005).
This section on informed consent is based largely on a Congressional Research Service report ( Williams, 2005 ), as adapted by Pritts (2008) .
See 45 C.F.R. § 46.116 (2005).
See 45 C.F.R. § 46.116(b) (2005).
See 45 C.F.R. § 46.103 (2005).
See 45 C.F.R. § 46.111 (2005). There are additional factors if the study includes subjects who are likely to be vulnerable to coercion or undue influence.
See 45 C.F.R. § 46.116(d); 46.117(c) (2005).
See 45 C.F.R. § 46.102(f) (2005).
See 45 C.F.R. § 46.101(b)(4) (2005).
See 45 C.F.R. § 46.116(e) (2005).
See 45 C.F.R. § 46.123 (2005).
See also Grimes v. Kennedy Krieger Institute , 782 A. 2d 807 (Md. Ct. App. 2001); Gelsinger v. University of Pennsylvania (Philadelphia County Court of Common Pleas filed September 18, 2000), available at http://www .sskrplaw.com /links/healthcare2.html .
The FDA has defined “clinical investigation” to be synonymous with “research.”
The Food, Drug, and Cosmetic Act Section 505(i), 507(d), or 520(g) of 21 U.S.C. 355(i), 357(d), or 360j(g) (1972).
See 21 C.F.R. part 50 (2008); 46 Fed. Reg. 8942 (1981).
See 21 C.F.R. part 56 (2008); 46 Fed. Reg. 8958 (1981).
See 45 C.F.R. part 46 (2005); 46 Fed. Reg. 8366 (1981).
The Centers for Disease Control and Prevention defines program evaluation as the “systematic investigation of the merit, worth, or significance of organized public health action,” noting that such evaluations are “systematic ways to improve and account for public health actions by involving procedures that are useful, feasible, ethical, and accurate.” They can be based on goals, processes, outcomes, or value ( http://www .cdc.gov/mmwr /preview/mmwrhtml/rr4811a1.htm ).
The Utilization Review Accreditation Commission defines utilization review as “the evaluation of the medical necessity, appropriateness, and efficiency of the use of health care services, procedures, and facilities under the provisions of the applicable health benefits plans” ( http://www .urac.org/about/ ).
Another type of oversight board defined by the Privacy Rule. See Chapter 4 .
Under the Privacy Rule, consent is referred to as authorization. See Chapter 4 .
Personal communication, C. Heide, Office for Civil Rights, HHS, May 29, 2008.
The Privacy Rule defines the term “health care operations” by listing a number of specific activities that qualify as health care operations. These include “conducting quality assessment and improvement activities, population-based activities relating to improving or reducing health care costs, and case management and care coordination.” See 45 C.F.R. § 164.501 (2006).
A total of 444 surveys were mailed to the medical directors of quality improvement and IRB chairs at hospitals with 400 or more beds that belong to the Council of Teaching Hospitals of the Association of American Medical Colleges, and to the editors of all U.S.-based medical journals that publish original research and appear in the Abridged Index Medicus. 236 surveys were returned, for a 53 percent response rate. The survey consisted of six brief scenarios that asked respondents to determine whether the described project needed IRB review and informed consent.
See http: //plainlanguage.gov/index.cfm .
FDA, Public Law 110–85 § 801 (2007).
See http://www .ahrq.gov/research/cbprrole .htm .
- Cite this Page Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009. 3, The Value, Importance, and Oversight of Health Research.
- PDF version of this title (1.6M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Priva... The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Medical Research Courses
- Social Sciences

Prescription Drug Regulation, Cost, and Access: Current Controversies in Context
Understand how the FDA regulates pharmaceuticals and explore debates on prescription drug costs, marketing, and testing.
Cancer Genomics and Precision Oncology
Learn how cancer treatment is evolving due to advances in genetics..

Gene Therapy
Explore recent advances in gene therapy and learn about the implications for patient care..

Immuno-oncology
See how the immune system is being used to improve cancer treatment..

Clinical Drug Development
Learning about the process of clinical drug development has important implications for anyone working in health care and related sectors.

Foundations of Clinical Research
This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career.

Global Clinical Scholars Research Training
This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods.
- Student/Faculty Portal
- Learning Hub (Brightspace)
- Continuous Professional Development
Careers A-Z
Your healthcare career search starts here. Check out details about more than 40 jobs in healthcare and medicine, with trusted information from Mayo Clinic College of Medicine and Science.
Interested in a Mayo Clinic educational program?
Participating in Health Research Studies
What is health research.
- Is Health Research Safe?
- Is Health Research Right for Me?
- Types of Health Research
The term "health research," sometimes also called "medical research" or "clinical research," refers to research that is done to learn more about human health. Health research also aims to find better ways to prevent and treat disease. Health research is an important way to help improve the care and treatment of people worldwide.
Have you ever wondered how certain drugs can cure or help treat illness? For instance, you might have wondered how aspirin helps reduce pain. Well, health research begins with questions that have not been answered yet such as:
"Does a certain drug improve health?"
To gain more knowledge about illness and how the human body and mind work, volunteers can help researchers answer questions about health in studies of an illness. Studies might involve testing new drugs, vaccines, surgical procedures, or medical devices in clinical trials . For this reason, health research can involve known and unknown risks. To answer questions correctly, safely, and according to the best methods, researchers have detailed plans for the research and procedures that are part of any study. These procedures are called "protocols."
An example of a research protocol includes the process for determining participation in a study. A person might meet certain conditions, called "inclusion criteria," if they have the required characteristics for a study. A study on menopause may require participants to be female. On the other hand, a person might not be able to enroll in a study if they do not meet these criteria based on "exclusion criteria." A male may not be able to enroll in a study on menopause. These criteria are part of all research protocols. Study requirements are listed in the description of the study.
A Brief History
While a few studies of disease were done using a scientific approach as far back as the 14th Century, the era of modern health research started after World War II with early studies of antibiotics. Since then, health research and clinical trials have been essential for the development of more than 1,000 Food and Drug Administration (FDA) approved drugs. These drugs help treat infections, manage long term or chronic illness, and prolong the life of patients with cancer and HIV.
Sound research demands a clear consent process. Public knowledge of the potential abuses of medical research arose after the severe misconduct of research in Germany during World War II. This resulted in rules to ensure that volunteers freely agree, or give "consent," to any study they are involved in. To give consent, one should have clear knowledge about the study process explained by study staff. Additional safeguards for volunteers were also written in the Nuremberg Code and the Declaration of Helsinki .
New rules and regulations to protect research volunteers and to eliminate ethical violations have also been put in to place after the Tuskegee trial . In this unfortunate study, African American patients with syphilis were denied known treatment so that researchers could study the history of the illness. With these added protections, health research has brought new drugs and treatments to patients worldwide. Thus, health research has found cures to many diseases and helped manage many others.
Why is Health Research Important?
The development of new medical treatments and cures would not happen without health research and the active role of research volunteers. Behind every discovery of a new medicine and treatment are thousands of people who were involved in health research. Thanks to the advances in medical care and public health, we now live on average 10 years longer than in the 1960's and 20 years longer than in the 1930's. Without research, many diseases that can now be treated would cripple people or result in early death. New drugs, new ways to treat old and new illnesses, and new ways to prevent diseases in people at risk of developing them, can only result from health research.
Before health research was a part of health care, doctors would choose medical treatments based on their best guesses, and they were often wrong. Now, health research takes the guesswork out. In fact, the Food and Drug Administration (FDA) requires that all new medicines are fully tested before doctors can prescribe them. Many things that we now take for granted are the result of medical studies that have been done in the past. For instance, blood pressure pills, vaccines to prevent infectious diseases, transplant surgery, and chemotherapy are all the result of research.
Medical research often seems much like standard medical care, but it has a distinct goal. Medical care is the way that your doctors treat your illness or injury. Its only purpose is to make you feel better and you receive direct benefits. On the other hand, medical research studies are done to learn about and to improve current treatments. We all benefit from the new knowledge that is gained in the form of new drugs, vaccines, medical devices (such as pacemakers) and surgeries. However, it is crucial to know that volunteers do not always receive any direct benefits from being in a study. It is not known if the treatment or drug being studied is better, the same, or even worse than what is now used. If this was known, there would be no need for any medical studies.
- Next: Is Health Research Safe? >>
- Last Updated: May 27, 2020 3:05 PM
- URL: https://guides.library.harvard.edu/healthresearch
77 interesting medical research topics for 2024
Last updated
25 November 2023
Reviewed by
Brittany Ferri, PhD, OTR/L
Medical research is the gateway to improved patient care and expanding our available treatment options. However, finding a relevant and compelling research topic can be challenging.
Use this article as a jumping-off point to select an interesting medical research topic for your next paper or clinical study.
- How to choose a medical research topic
When choosing a research topic , it’s essential to consider a couple of things. What topics interest you? What unanswered questions do you want to address?
During the decision-making and brainstorming process, here are a few helpful tips to help you pick the right medical research topic:
Focus on a particular field of study
The best medical research is specific to a particular area. Generalized studies are often too broad to produce meaningful results, so we advise picking a specific niche early in the process.
Maybe a certain topic interests you, or your industry knowledge reveals areas of need.
Look into commonly researched topics
Once you’ve chosen your research field, do some preliminary research. What have other academics done in their papers and projects?
From this list, you can focus on specific topics that interest you without accidentally creating a copycat project. This groundwork will also help you uncover any literature gaps—those may be beneficial areas for research.
Get curious and ask questions
Now you can get curious. Ask questions that start with why, how, or what. These questions are the starting point of your project design and will act as your guiding light throughout the process.
For example:
What impact does pollution have on children’s lung function in inner-city neighborhoods?
Why is pollution-based asthma on the rise?
How can we address pollution-induced asthma in young children?
- 77 medical research topics worth exploring in 2023
Need some research inspiration for your upcoming paper or clinical study? We’ve compiled a list of 77 topical and in-demand medical research ideas. Let’s take a look.
- Exciting new medical research topics
If you want to study cutting-edge topics, here are some exciting options:
COVID-19 and long COVID symptoms
Since 2020, COVID-19 has been a hot-button topic in medicine, along with the long-term symptoms in those with a history of COVID-19.
Examples of COVID-19-related research topics worth exploring include:
The long-term impact of COVID-19 on cardiac and respiratory health
COVID-19 vaccination rates
The evolution of COVID-19 symptoms over time
New variants and strains of the COVID-19 virus
Changes in social behavior and public health regulations amid COVID-19
Vaccinations
Finding ways to cure or reduce the disease burden of chronic infectious diseases is a crucial research area. Vaccination is a powerful option and a great topic to research.
Examples of vaccination-related research topics include:
mRNA vaccines for viral infections
Biomaterial vaccination capabilities
Vaccination rates based on location, ethnicity, or age
Public opinion about vaccination safety
Artificial tissues fabrication
With the need for donor organs increasing, finding ways to fabricate artificial bioactive tissues (and possibly organs) is a popular research area.
Examples of artificial tissue-related research topics you can study include:
The viability of artificially printed tissues
Tissue substrate and building block material studies
The ethics and efficacy of artificial tissue creation
- Medical research topics for medical students
For many medical students, research is a big driver for entering healthcare. If you’re a medical student looking for a research topic, here are some great ideas to work from:
Sleep disorders
Poor sleep quality is a growing problem, and it can significantly impact a person’s overall health.
Examples of sleep disorder-related research topics include:
How stress affects sleep quality
The prevalence and impact of insomnia on patients with mental health conditions
Possible triggers for sleep disorder development
The impact of poor sleep quality on psychological and physical health
How melatonin supplements impact sleep quality
Alzheimer’s and dementia
Cognitive conditions like dementia and Alzheimer’s disease are on the rise worldwide. They currently have no cure. As a result, research about these topics is in high demand.
Examples of dementia-related research topics you could explore include:
The prevalence of Alzheimer’s disease in a chosen population
Early onset symptoms of dementia
Possible triggers or causes of cognitive decline with age
Treatment options for dementia-like conditions
The mental and physical burden of caregiving for patients with dementia
- Lifestyle habits and public health
Modern lifestyles have profoundly impacted the average person’s daily habits, and plenty of interesting topics explore its effects.
Examples of lifestyle and public health-related research topics include:
The nutritional intake of college students
The impact of chronic work stress on overall health
The rise of upper back and neck pain from laptop use
Prevalence and cause of repetitive strain injuries (RSI)
- Controversial medical research paper topics
Medical research is a hotbed of controversial topics, content, and areas of study.
If you want to explore a more niche (and attention-grabbing) concept, here are some controversial medical research topics worth looking into:
The benefits and risks of medical cannabis
Depending on where you live, the legalization and use of cannabis for medical conditions is controversial for the general public and healthcare providers.
Examples of medical cannabis-related research topics that might grab your attention include:
The legalization process of medical cannabis
The impact of cannabis use on developmental milestones in youth users
Cannabis and mental health diagnoses
CBD’s impact on chronic pain
Prevalence of cannabis use in young people
The impact of maternal cannabis use on fetal development
Understanding how THC impacts cognitive function
Human genetics
The Human Genome Project identified, mapped, and sequenced all human DNA genes. Its completion in 2003 opened up a world of exciting and controversial studies in human genetics.
Examples of human genetics-related research topics worth delving into include:
Medical genetics and the incidence of genetic-based health disorders
Behavioral genetics differences between identical twins
Genetic risk factors for neurodegenerative disorders
Machine learning technologies for genetic research
Sexual health studies
Human sexuality and sexual health are important (yet often stigmatized) medical topics that need new research and analysis.
As a diverse field ranging from sexual orientation studies to sexual pathophysiology, examples of sexual health-related research topics include:
The incidence of sexually transmitted infections within a chosen population
Mental health conditions within the LGBTQIA+ community
The impact of untreated sexually transmitted infections
Access to safe sex resources (condoms, dental dams, etc.) in rural areas
- Health and wellness research topics
Human wellness and health are trendy topics in modern medicine as more people are interested in finding natural ways to live healthier lifestyles.
If this field of study interests you, here are some big topics in the wellness space:
Gluten sensitivity
Gluten allergies and intolerances have risen over the past few decades. If you’re interested in exploring this topic, your options range in severity from mild gastrointestinal symptoms to full-blown anaphylaxis.
Some examples of gluten sensitivity-related research topics include:
The pathophysiology and incidence of Celiac disease
Early onset symptoms of gluten intolerance
The prevalence of gluten allergies within a set population
Gluten allergies and the incidence of other gastrointestinal health conditions
Pollution and lung health
Living in large urban cities means regular exposure to high levels of pollutants.
As more people become interested in protecting their lung health, examples of impactful lung health and pollution-related research topics include:
The extent of pollution in densely packed urban areas
The prevalence of pollution-based asthma in a set population
Lung capacity and function in young people
The benefits and risks of steroid therapy for asthma
Pollution risks based on geographical location
Plant-based diets
Plant-based diets like vegan and paleo diets are emerging trends in healthcare due to their limited supporting research.
If you’re interested in learning more about the potential benefits or risks of holistic, diet-based medicine, examples of plant-based diet research topics to explore include:
Vegan and plant-based diets as part of disease management
Potential risks and benefits of specific plant-based diets
Plant-based diets and their impact on body mass index
The effect of diet and lifestyle on chronic disease management
Health supplements
Supplements are a multi-billion dollar industry. Many health-conscious people take supplements, including vitamins, minerals, herbal medicine, and more.
Examples of health supplement-related research topics worth investigating include:
Omega-3 fish oil safety and efficacy for cardiac patients
The benefits and risks of regular vitamin D supplementation
Health supplementation regulation and product quality
The impact of social influencer marketing on consumer supplement practices
Analyzing added ingredients in protein powders
- Healthcare research topics
Working within the healthcare industry means you have insider knowledge and opportunity. Maybe you’d like to research the overall system, administration, and inherent biases that disrupt access to quality care.
While these topics are essential to explore, it is important to note that these studies usually require approval and oversight from an Institutional Review Board (IRB). This ensures the study is ethical and does not harm any subjects.
For this reason, the IRB sets protocols that require additional planning, so consider this when mapping out your study’s timeline.
Here are some examples of trending healthcare research areas worth pursuing:
The pros and cons of electronic health records
The rise of electronic healthcare charting and records has forever changed how medical professionals and patients interact with their health data.
Examples of electronic health record-related research topics include:
The number of medication errors reported during a software switch
Nurse sentiment analysis of electronic charting practices
Ethical and legal studies into encrypting and storing personal health data
Inequities within healthcare access
Many barriers inhibit people from accessing the quality medical care they need. These issues result in health disparities and injustices.
Examples of research topics about health inequities include:
The impact of social determinants of health in a set population
Early and late-stage cancer stage diagnosis in urban vs. rural populations
Affordability of life-saving medications
Health insurance limitations and their impact on overall health
Diagnostic and treatment rates across ethnicities
People who belong to an ethnic minority are more likely to experience barriers and restrictions when trying to receive quality medical care. This is due to systemic healthcare racism and bias.
As a result, diagnostic and treatment rates in minority populations are a hot-button field of research. Examples of ethnicity-based research topics include:
Cancer biopsy rates in BIPOC women
The prevalence of diabetes in Indigenous communities
Access inequalities in women’s health preventative screenings
The prevalence of undiagnosed hypertension in Black populations
- Pharmaceutical research topics
Large pharmaceutical companies are incredibly interested in investing in research to learn more about potential cures and treatments for diseases.
If you’re interested in building a career in pharmaceutical research, here are a few examples of in-demand research topics:
Cancer treatment options
Clinical research is in high demand as pharmaceutical companies explore novel cancer treatment options outside of chemotherapy and radiation.
Examples of cancer treatment-related research topics include:
Stem cell therapy for cancer
Oncogenic gene dysregulation and its impact on disease
Cancer-causing viral agents and their risks
Treatment efficacy based on early vs. late-stage cancer diagnosis
Cancer vaccines and targeted therapies
Immunotherapy for cancer
Pain medication alternatives
Historically, opioid medications were the primary treatment for short- and long-term pain. But, with the opioid epidemic getting worse, the need for alternative pain medications has never been more urgent.
Examples of pain medication-related research topics include:
Opioid withdrawal symptoms and risks
Early signs of pain medication misuse
Anti-inflammatory medications for pain control
- Identify trends in your medical research with Dovetail
Are you interested in contributing life-changing research? Today’s medical research is part of the future of clinical patient care.
As your go-to resource for speedy and accurate data analysis , we are proud to partner with healthcare researchers to innovate and improve the future of healthcare.
Should you be using a customer insights hub?
Do you want to discover previous research faster?
Do you share your research findings with others?
Do you analyze research data?
Start for free today, add your research, and get to key insights faster
Editor’s picks
Last updated: 11 January 2024
Last updated: 15 January 2024
Last updated: 17 January 2024
Last updated: 25 November 2023
Last updated: 12 May 2023
Last updated: 30 April 2024
Last updated: 13 May 2024
Latest articles
Related topics, .css-je19u9{-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-flex-direction:row;-ms-flex-direction:row;flex-direction:row;-webkit-box-flex-wrap:wrap;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;-webkit-box-pack:center;-ms-flex-pack:center;-webkit-justify-content:center;justify-content:center;row-gap:0;text-align:center;max-width:671px;}@media (max-width: 1079px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}}@media (max-width: 799px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}} decide what to .css-1kiodld{max-height:56px;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;}@media (max-width: 1079px){.css-1kiodld{display:none;}} build next, decide what to build next.

Users report unexpectedly high data usage, especially during streaming sessions.

Users find it hard to navigate from the home page to relevant playlists in the app.

It would be great to have a sleep timer feature, especially for bedtime listening.

I need better filters to find the songs or artists I’m looking for.
Log in or sign up
Get started for free
What Premed Students Should Know About Emerging Fields of Medical Research
Aspiring physician-scientists should bone up on areas such as gene editing, nanotechnology and regenerative medicine.
Premeds and Emerging Medical Research

Getty Images | Image Source
If you find a field that interests you, don't hesitate to join a like-minded laboratory while training.
Premedical students aspiring to become physician-scientists will be tasked with navigating emerging fields in research and translating exciting discoveries into the clinical realm. Understanding the latest trends and breakthroughs in biomedical science is paramount for those hoping to bridge the gap between such cutting-edge research and clinical practice – a career goal for many aspiring physician-scientists.
What are these emerging fields, what should aspiring physician-scientists – including those applying to combined M.D.-Ph.D. programs – know about getting involved in these fields, and are there any pitfalls?
This is an extraordinarily exciting time in scientific research, with recent breakthroughs in diverse fields such as gene editing, immunotherapies, nanotechnology, precision medicine, machine learning and regenerative medicine. Highlights run the gamut of the biomedical spectrum, including evolutionary genomics, novel neurotechnology, advances in cardiovascular imaging, cell-based therapies and therapeutic manipulation of the microbiome, to name a few.
Aspiring physician-scientists will undoubtedly be tempted to ride this wave of exciting discoveries and join laboratories moving the needle in these fields, many of which are still in their infancy.
Premed students should be aware of these emerging fields, as these advances are expected to contribute increasingly to health care throughout the coming decades and will undoubtedly remain important for the duration of a lengthy career in medicine .
These fields are likely to hold long-term career opportunities for students interested in biomedical research. They also represent opportunities to contribute to innovation, be involved in groundbreaking discoveries and help shape the future of science and medicine.
Many emerging fields are exciting in part due to new or newly appreciated applications to clinical practice, with direct implications for patient care . By understanding these emerging fields, premed students will remain informed and up to date regarding novel treatment paradigms, new diagnostic tools and different preventive strategies that could benefit their future patients.
Students’ research interests often evolve during undergraduate, graduate and postgraduate education. Many fascinating fields of biomedical science are neither new nor well known, and they deserve serious consideration. You will have multiple opportunities to change fields should your interests diverge at any point, so you should not feel locked in to the discipline of your first research experience.
However, if you do have a genuine intellectual interest in a popular scientific field at an early phase of training, don’t hesitate to join such a like-minded laboratory.
Finding a Laboratory in Emerging Research Fields
If you are a premed student interested in an exciting field like cancer immunotherapy, genomics, AI-enabled precision medicine , etc., you may struggle to understand which laboratories would be appropriate and rewarding to join and a good fit for your career goals.
To start, assess the research landscape at your home institution through departmental web pages and note which faculty in your field of interest are involved in active research projects. Get in touch with a few faculty members and discuss the possibility of joining their laboratory.
As you learn about their research projects, you can also ask if they know of other labs in the same field that may also be of interest. Often, research faculty themselves are the best resource for understanding the current research landscape of the university, as departmental web pages and related resources can be out of date.
Departmental administrators or undergraduate research coordinators may also be quite helpful in finding a lab in a specific area that would be a good fit for an undergraduate student. If you read a lay press article – especially from a local publication – about an area of exciting, “hot” science, pay attention to which studies and researchers they reference or quote. These investigators are often leading voices in the field.
Use PubMed to find the latest work in a field or by a specific investigator. Explore the "trending articles" section to see which articles have had recent activity – a sign of a field gaining broad interest. If you find investigators doing work that is particularly interesting to you, use the "saved searches" function to get updates about their work directly in your email inbox.
Appreciate that emerging fields are often a result of novel collaboration across disparate disciplines such as distinct subfields in biology and medicine, biomedical engineering or computer science .
Application of a known technology to a new field can also yield exciting advancements. A recent example is cryo-EM-mediated determination of complex structures, such as ligand-bound receptors, which could not previously be accurately determined.
Look for labs that are working in an interdisciplinary manner to tackle an important question in medicine or biology, and you are likely to find stimulating research in an important emerging field.
Pitfalls to Avoid
Avoid presuming that only well-known fields with significant popularity and press attention are the only interesting domains of scientific research. The biggest discoveries often come from unpredictable places, and their genesis can be traced to less well-known fields.
Recent high-profile examples include prokaryotic genomics that spawned CRISPR/Cas9-based gene editing, and nucleoside modifications that advanced mRNA vaccines. This is characteristic of biomedical research and should lead you to explore various fields and meet with a variety of investigators to find the field, research and lab that most interest you.
A few exceedingly popular fields – such as microbiome research, cancer immunotherapy , etc. – run the risk of becoming oversaturated, with many excellent investigators trying to solve similar problems. These fields can thus become quite competitive, with several associated challenges.
If you do join a competitive field, look for opportunities to do novel work that can separate your project from the rest of the crowd. A good strategy when selecting a laboratory is to assess which researchers are pushing the boundaries in these fields and are looking to incorporate interdisciplinary approaches, as they are more likely to be working in their own lane, away from other investigators. Use the same approach when selecting a project within your lab.
Medical School Application Mistakes

Tags: medical school , research , graduate schools , education , students
About Medical School Admissions Doctor
Need a guide through the murky medical school admissions process? Medical School Admissions Doctor offers a roundup of expert and student voices in the field to guide prospective students in their pursuit of a medical education. The blog is currently authored by Dr. Ali Loftizadeh, Dr. Azadeh Salek and Zach Grimmett at Admissions Helpers , a provider of medical school application services; Dr. Renee Marinelli at MedSchoolCoach , a premed and med school admissions consultancy; Dr. Rachel Rizal, co-founder and CEO of the Cracking Med School Admissions consultancy; Dr. Cassie Kosarec at Varsity Tutors , an advertiser with U.S. News & World Report; Dr. Kathleen Franco, a med school emeritus professor and psychiatrist; and Liana Meffert, a fourth-year medical student at the University of Iowa's Carver College of Medicine and a writer for Admissions Helpers. Got a question? Email [email protected] .
Popular Stories
Applying to Graduate School

Best Colleges

Medical School Admissions Doctor

Law Admissions Lowdown

You May Also Like
Avoid procrastinating in medical school.
Kathleen Franco, M.D., M.S. May 21, 2024
Good Law School Recommendation Letters
Gabriel Kuris May 20, 2024
Get Accepted to Multiple Top B-schools
Anayat Durrani May 16, 2024

How to Get a Perfect Score on the LSAT
Gabriel Kuris May 13, 2024

Premeds Take 5 Public Health Courses
Rachel Rizal May 7, 2024

Fortune 500 CEOs With a Law Degree
Cole Claybourn May 7, 2024

Why It's Hard to Get Into Med School
A.R. Cabral May 6, 2024

Pros, Cons of Unaccredited Law Schools
Gabriel Kuris May 6, 2024

An MBA and Management Consulting
Sammy Allen May 2, 2024

Med School Access for Minority Students
Cole Claybourn May 2, 2024

Graduate School
Fyodor d. urnov: pioneering gene editing for medical breakthroughs.
A trailblazer in the field of therapeutic genome editing, Fyodor D. Urnov’s research focuses on developing medicines for devastating genetic diseases.
.jpg)
Fyodor D. Urnov ‘96 Ph.D. is Professor of Molecular Therapeutics in the Department of Molecular and Cell Biology at the University of California, Berkeley and Director of Technology and Translation at the Innovative Genomics Institute (IGI). He co-developed the toolbox for human genome and epigenome editing, co-named the term “genome editing”, and was on the team to advance the first-in-human applications in a clinic.
Urnov also helped identify the genome editing target for the first medicine approved to treat sickle cell disease and beta-thalassemia. A major goal for the field of genome editing and a key focus of Urnov's work is expanding access to CRISPR therapies (which modify genomes) for genetic diseases to those most in need.
He will receive the Horace Mann Medal at the Doctoral Ceremony during Brown University’s Commencement weekend.
Prior to attending Brown, Urnov completed his undergraduate studies in biology at Moscow State University in Russia. He then joined the Molecular and Cell Biology and Biochemistry (MCB) department at Brown where he earned his doctoral degree. His dissertation work focused on the DNA structure and chromatin dynamics of one of the scarce origins of replication that are thoroughly understood, initiating DNA synthesis prior to cellular division. He worked in the lab of Susan Gerbi, the George Eggleston Professor of Biochemistry and founding chair of the MCB department.
Urnov credits his pioneering work on gene editing to the doctoral training he received at Brown. Urnov then completed postdoctoral training at the National Institutes of Health before joining Sangamo BioSciences, a biotech firm in the San Francisco bay area as a Senior Scientist and Team Leader.
At every stage of his career, Urnov’s exceptional work has been marked by medical breakthroughs and awards. One of the most groundbreaking biological advancements in recent years involves the ability to safely and precisely modify DNA sequences within genes - gene editing. This innovation began with the development of proteins designed to selectively bind to specific DNA sequences and enact targeted alterations. These proteins, known as "zinc-finger nucleases" or ZFNs, have paved the way for transformative research in genetic engineering.
In 2005 at Sangamo, Urnov spearheaded a pivotal study showcasing the efficacy of ZFNs to precisely target a disease-causing sequence in the genome and correct it. The study was published in the journal Nature. The field of therapeutic genome editing, which Urnov co-named, was thus born. This paper marked the inaugural instance of mutation correction in human cells. The study demonstrated remarkably efficient repair (i.e. editing) of a mutated gene linked to severe combined immune deficiency, underscoring the potential of gene editing technology in addressing genetic disorders - potential that has recently started to be realized.
After this initial publication, interest in using gene editing technology exploded. Stuart Orkin, the David G. Nathan Distinguished Professor of Pediatrics at Harvard Medical School and Investigator at the Howard Hughes Medical Institute collaborated with Urnov to use gene editing to cure sickle cell disease (SCD) and beta-thalassemia (both inherited blood disorders), ushering in the first CRISPR gene editing clinical trial for a genetic disease, treating both SCD and thalassemia patients. In both of these inherited diseases, the gene for making beta-hemoglobin is disrupted.
“Fyodor Urnov has been a visionary in the field of gene manipulation and editing, and is widely recognized both for his scientific contributions and his remarkable skill in communicating the work to other scientists and the public,” shares Orkin.
The outcome of the clinical trials have thus far been transformative for the around 100 patients involved; all have been symptom-free after gene editing. Based on these results the FDA has approved this approach as the first-ever gene-editing based medicine - a medicine for which a key foundation was the work Urnov did in collaboration with Orkin.
Urnov’s other collaborations at Sangamo led to the deployment of genome editing in human pluripotent stem cells (hPSCs) for basic science and translational applications. Examples include applied gene editing to Down syndrome and in vivo therapeutics for Huntington’s disease and Alzheimer’s dementia.
In 2019 Urnov moved to the University of California, Berkeley, where he took on the challenge of building CRISPR Cures research and development teams for genetic diseases of the blood and the brain, genetic disorders of the immune system, radiation injury, cystic fibrosis, and neurological disorders.
Urnov explains gene editing technology in a New York Times article from December of 2022.
“Gene editing relies on a molecular machine called CRISPR, which can be instructed to repair a mutation in a gene in nearly any organism, right where that “typo” occurs. Impressively versatile, potential applications for CRISPR range from basic science to agriculture and climate change. In medicine, CRISPR gene editing allows physicians to directly fix typos in the patients’ DNA. And so much substantive progress has been made in the field of genetic medicine that it’s clear scientists have now delivered on a remarkable dream: word-processor-like control over DNA.”
As Urnov explains in this piece, a wealth of regulatory hurdles and healthcare economics challenges have, to date, prevented gene editing from making a greater impact. Urnov shares, “the invention of CRISPR gene editing gave us remarkable treatment powers, yet no one should do a victory lap. Scientists can rewrite a person’s DNA on demand. But now what? Unless things change dramatically, the millions of people CRISPR could save will never benefit from it. We must, and we can, build a world with CRISPR for all.”
An effort to bring us closer to that world is now the centerpiece of Urnov’s professional life. His work currently focuses on developing scalable, affordable platforms to engineer gene editing cures on-demand for severe disorders of childhood. Urnov directs a unique academia-industry partnership, the IGI-Danaher Beacon for CRISPR Cures, that is advancing to the clinic innovative treatments for inborn errors of immunity that cause severe diseases of infancy.
Urnov has made an impact at UC Berkeley and IGI beyond his research. As the Covid-19 pandemic commenced, he assumed the task of organizing resources to set up a nonprofit diagnostic clinical laboratory at IGI for swift testing of the SARS-CoV-2 coronavirus. The objective was to offer greater throughput, faster results, and enhanced accuracy compared to existing commercial options - and provide such testing for free to communities most in need.
As described in Walter Isaacson’s best selling book, The Codebreaker , Urnov emerged as a pivotal figure in this initiative, playing a significant role in resource mobilization encompassing equipment, personnel, and funding - and ultimately providing over 500,000 free COVID tests to individuals in socioeconomically disadvantaged communities when for-profit testing laboratories failed at the task.
Not only is Urnov renowned in the field of gene editing, but his list of publications, teaching ability, and public speaking acumen is also exceptional. Urnov has authored more than 100 scientific publications and is an inventor on 87 published patents related to genome editing and targeted gene regulation technology. His 2005 Nature paper has been cited over 2000 times, and a subsequent paper he wrote for Nature Reviews Genetics has been cited over 2500 times. Many of his other papers have been cited over 1000 times.
“Fyodor is a world class researcher at the forefront of arguably the most exciting and important biomedical research advance in our lifetimes – genome editing – because he is perhaps the most engaging orator I have ever heard speak, because he is a scholar of truly extraordinary depth and breadth of knowledge in biomedicine, and because he is a dedicated and highly effective teacher and mentor,“ shares David Drubin, Ernette Comby Chair in Microbiology and a professor of Cell and Development in the Department of Molecular and Cell Biology at UC Berkeley.
Urnov is also known for being a dynamic public speaker and teacher and is much sought after. Urnov credits his experience as a graduate student instructor here at Brown for his interest in teaching, starting with watching faculty at Brown, including George Eggleston Professor of Biochemistry, Susan Gerbi and Professor of Molecular Biology, Cell Biology and Biochemistry Kenneth Miller, in his first stint as a graduate student instructor.
Urnov’s awards, not surprisingly, are quite notable. As far back as his time at Brown he was selected for the Barry J. Rosen Memorial Award For High Achievement In Molecular Biology and the President’s Award for Excellence in Teaching.
In 2014 he was named as one of “The World’s Most Influential Scientific Minds” by Thomson Reuters and received a Fellows Award for Research Excellence from the National Institutes of Health.
More From Forbes
How important is research for bs/md programs.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Direct medical programs, often referred to as BS/MD programs, are some of the most competitive programs in the country. With programs at Baylor University, Brown University and Case Western Reserve University accepting less than 3% of all its applicants, these programs are often more competitive than the Ivy League. They are looking for exceptional students who are completely committed to becoming physicians. That means the students have spent the better part of their high school career pursuing STEM-focused activities, including physician shadowing, volunteering in healthcare settings and leadership positions in clubs.
Many BS/MD hopefuls pursue research as a way to build their resume.
Numerous BS/MD programs like Rensselaer Polytechnic University, like to see students with extensive research experience. Its program, aptly named the Physician-Scientist Program, wants to see students who will not only participate in research during their tenure in the program but also lead and create their own research projects. The University of South Carolina’s Accelerated Undergraduate to M.D. program has an extensive research and thesis component that is required throughout the student’s academic career. The University of Rochester offers funding for summer research for its BS/MD students. Similarly, the University of Illinois at Chicago looks for students who can demonstrate their “research aptitude.”
What Type Of Research Do BS/MD Programs Accept?
High school students have access to a wide array of research opportunities. School-related options could include science fair projects or AP Seminar and AP Research. Students might also choose to pursue camps or programs over the summer, which allows them to dedicate more time to research. Other students find independent research projects with a local professor. Alternatively, others opt to write a literature review paper to get published.
When BS/MD admission officers review applications, they don’t pit one type of experience against another. They know not every student will be able to find a local professor who allows them to research with them or can afford to do a paid summer program that spans numerous weeks or months. Consequently, they typically will consider holistically the depth of a student’s research experience, irrespective of the type of research the student completes.
Virtual Or In-Person Programs?
Both virtual and in-person experiences can add value to a BS/MD application. However, it depends on the program’s learning objectives and deliverables. Some students don’t have the flexibility to travel to an in-person camp and spend multiple weeks or months there. The University of Pittsburgh’s Guaranteed Admission Program says that “while in-person experiences are encouraged, virtual or remote experiences will be considered when evaluating the applicant.” For those students who have other obligations, a virtual camp might be the perfect fit and still offer a valuable experience.
The 10 Best Body Oils To Nourish And Soften Skin According To Experts
Top ceo bets on a shock biden crypto flip as congress hurtles toward a crucial vote that could blow up the price of bitcoin ethereum and xrp, iphone 16 pro max all-new design upgrade promised, insider claims, does the research topic matter.
The research experience doesn’t necessarily have to align with the student’s research interests, but it can often be helpful if it does. However, BS/MD admission officers know that high school students are still exploring their interests, which will likely evolve over the years. An opportunity that doesn’t align with the student’s interest will still be valuable because it allows the student to gain valuable skills that they can leverage to other research experiences in the future.
Summer programs might give students a chance to explore dual interests. Some students interested in medicine might also want to explore computer science or Artificial Intelligence, so finding an opportunity that allows them to blend those interests might be ideal. For example, Rising Researchers , a sister company of Moon Prep, is hosting two five-week summer camps that allow students to practice AI and Machine Learning to study human diseases. Other camps, like Penn Summer Academies, allow students to apply coding skills to other areas of study.
How Long Should The Research Experience Be?
The typical length of a research experience, especially one in the summer, can vary from as short as one week to up to eight weeks. A longer research experience can give students a more comprehensive understanding of the subject matter and, importantly, the opportunity to build meaningful relationships with their mentor and fellow students. However, the duration is not the sole determinant of a meaningful experience. Students should also look to see what the tangible outcomes of the program, such as a research paper, skills gained, letter of recommendation and more.
For students who find an independent research experience, the relationship might span several months or even years. Those experiences might result in more fruitful research results and a strong relationship between the student and the mentor.
Are Publications Required?
An experience resulting in a research publication is an added bonus, but it isn’t a requirement. If a student writes a research paper, even if not published, can still demonstrate the student’s scientific writing ability and add value to their college application.
Every BS/MD program is different, and the admission officers' value of research might vary from program to program. Ultimately, BS/MD programs are looking for students who are passionate about medicine and have had extensive experiences to affirm that passion. The College of New Jersey stated in an interview with Moon Prep that they are looking for passionate students, be it a deep involvement in Boy Scouts, Taekwondo or music. Therefore, students should never feel obligated to research if it does not align with their interests. Being genuine in their activities and demonstrating their passions is how to build a resume that stands out to BS/MD admission officers.
- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
- MTS Biotechnology How Technology is Bridging Brains with Computers
- Anesthesia Technician
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Respiratory Therapist
- Surgical Technologist
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Medical Laboratory Scientist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Patient-Facing Technology Programs
- Laboratory Technology programs
- Natural & Clinical Lab Science
- Medical IT & Administrative
Certification Guides
Career guides, interviews & features, how technology is bridging brains with computers, search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
“It’s all being done with my brain. If y’all can see the cursor moving around the screen, that’s all me. It’s pretty cool, huh?”
Those were the words of Noland Arbaugh, a man paralyzed from the shoulders down, who, with the help of a brain implant created by Neuralink, played chess on a laptop using only his mind. The company shared the moment with the world in a live stream on X in March.
Neuralink, a U.S. startup co-founded by Elon Musk, works on “brain-computing interfaces,” known as BCI, which connect the brain to digital technology. Such devices come in different forms: Some are surgically implanted into peoples’ heads, while others are noninvasive and sit outside of a person’s body. In May 2023, the product received approval from the Food and Drug Administration for clinical trials, but it has yet to hit the market.
So how exactly does the technology work?
The chip transmits neural signals from a person’s brain to a phone or computer that can then execute a function without tapping or swiping. In practice, electrical signals from the brain are converted to real-time data. In simpler terms, Arbaugh reported thinking of his intended movement and then watched it happen on screen.
In addition to changing the game for individuals with paralysis, this class of technology is helping researchers and medical professionals monitor brain activity to track unusual sleep patterns or early signs of epilepsy.
Medical Technology Schools examined academic research and news reports to explore how the technology bridging brains with computers has evolved, a fast-growing and in-demand biotechnology field.
Researchers Have Been Working on This for a Long Time
Countless scientists have attempted to understand how the brain affects the rest of the body. In the late 18th century, Italian physicist and physician Luigi and his partner Lucia Galvani discovered they could induce muscle contractions in frogs by applying electric shocks. The married pair from Bologna theorized they witnessed animal electricity and that the brain was vital in transmitting electrical fluid through nerves.
Over a century later, researchers honed their understanding of the electromagnetic signals sent from the brain. In 1929, German psychiatrist Hans Berger was the first to create an electrocorticogram recording of a surgery patient’s brain waves, an inflection point in the evolution of neuroscience.
Brain wave research did not take off in earnest until the 1960s, with early experiments often focusing on monkeys. In 1973, Jacques Vidal, a computer science professor at the University of California, Los Angeles, published a paper coining the phrase “ brain-computer interface ” and laying the groundwork for the field.
In the early 1990s, Jonathan Wolpaw and a group of other researchers created early brain-computer interfaces that let people move computer cursors from the center of a computer screen to the edges—with their minds.
By 1999, researchers figured out how to build a machine that let rats control a robotic arm well enough to get water, then used their “brain-derived signals” to control it. Studies that once may have read like science fiction now had a higher purpose: to learn about the potential to restore mobility in paralysis patients.
Neuralink’s brain chip reflects the latest advancements in BCI technology. It works wirelessly, meaning people don’t have a wire coming out of their heads as they did with past products. The brain chip also offers users more precise control compared to past devices. Arbaugh reported he could play Civilization VI, a popular computer game, for eight hours, only stopping because his brain implant needed recharging.
Other companies like Blackrock Neurotech and BrainGate are building similar devices, part of a larger influx of interest and funding into embeddable tech, leading to Silicon Valley employing medical professionals such as biotechnologists and biomedical scientists .
Scientific Advancement Can be Risky
As with any new technological frontier, research about BCI is fraught with ethical challenges. The science is still in its infancy, and human trials are limited.
Some critics cite the privacy concerns inherent in machines capable of scanning signals from a person’s brain. Others say machines implanted in the brain can unexpectedly influence decision-making or moods. Should the future of brain-computing interfaces provide, for example, enhanced cognition, it could create another digital divide—if the tech is only available to a few people, it may further entrench social inequality.
Safety and animal welfare concerns have clouded Neuralink in controversy. The company, which tested many of its products on monkeys, was investigated by the Department of Agriculture for animal welfare violations. Last year, Wired reported that as many as a dozen of Neuralink’s test subjects died in captivity . Musk has denied that the monkeys died due to his company’s experiments.
That same year, a special report from Reuters found that company employees were pressured to compromise safety to secure FDA approval quickly. Moreover, members of Congress have called upon the Securities and Exchange Commission to investigate whether Neuralink misled investors about the potential risks of its technology.
Professionals are split on how best to utilize BCI. The neurological community is keen on developing medical advancements to help individuals with neurological disorders and paralysis; some software and engineering “futurists” are eager to enhance human intelligence and augment everyone’s abilities. As Musk put it, the ultimate goal of Neuralink is to “achieve symbiosis with artificial intelligence.”
For Arbaugh, the technology hasn’t created a total mind meld, but it’s helping him push past former limitations: “Every day it seems like we’re learning new stuff, and I just can’t even describe how cool it is to be able to do this.”
As public interest and the technical feasibility of brain-computer interface devices increases, the need for medical professionals specializing in technological fields is rising as a result. Here’s how to land a role in one of these in-demand medical fields .

Wade Zhou is an experienced senior data journalist who covers education, economic trends, healthcare, and more.
He previously wrote for The Economist and received his BA from the University of Miami and his MS from Johns Hopkins University.
Related Articles
- How to Become a Biotechnologist
- Online Master's in Bioinformatics & Genomics
- Drug Discovery & Development Career Guide
- What Can You Do with a Biotechnology Degree?
- Bioinformatics vs. Computational Biology
Related Programs

Research involvement of medical students in a medical school of India: exploring knowledge, attitude, practices, and perceived barriers
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Abhinav Jha
- ORCID record for Deepak Dhamnetiya
- For correspondence: [email protected]
- ORCID record for Ravi Prakash Jha
- Info/History
- Preview PDF
Introduction: Research in the medical discipline significantly impacts society by improving the general well-being of the population, through improvements in diagnostic and treatment modalities. However, of 579 Indian medical colleges, 332 (57.3%) did not publish a single paper from the year 2005 to 2014," indicating a limited contribution from medical fraternity In order to probe in to the cause of this a study was conducted to assess the knowledge, attitude, practices (KAP) and perceived barriers to research among students of a medical school in Delhi, India. Methods: A cross-sectional study was conducted among medical students and the data on academic-cum-demographic information, assessment of knowledge, attitude, practices and barriers to research was collected using a pre-tested, semi-structured questionnaire. Chi-square test was used to check the association of various factors with the KAP of research. A p-value less than 0.05 was considered significant. Results: A total of 402 (N) subjects were enrolled in the study. Majority were male (79.6%) and from clinical professional years (57%). Majority (266, 66.2%) of the subjects had adequate knowledge. Of the study subjects (61,15%) having inadequate knowledge of research, sixty percent were from pre- and para- clinical years, while around 70 % of those having good knowledge were from clinical professional years. However, only 16.9% of the participants had participated in a research project, and only 4.72% had authored a publication. Sixty one percent of study subjects having a positive attitude towards research, were from pre- and para- clinical years. Among the study subjects having a positive attitude towards research, over 60% were from pre- and para- clinical years. The barriers for conducting research were mostly; lack of funds/laboratory equipment/infrastructure (85.1%), lack of exposure to opportunities for research in the medical (MBBS) curriculum (83.8%), and lack of time (83.3%). There was a statistically significant association between knowledge and attitude towards research with a professional year of study. Conclusions: The study revealed that while most of the students had a positive attitude towards research as well as an adequate knowledge of research, there was a poor level of participation in research. These challenges can be overcome by incorporating research as a part of the medical school curriculum from early years on, setting aside separate time for research, and establishing student research societies that can actively promote research.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study did not receive any funding.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The ethics committee of Dr Baba Saheb Ambedkar Medical College and Hospital, New Delhi gave ethical approval for this work.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced in the present study are available upon reasonable request to the authors.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
- Addiction Medicine (324)
- Allergy and Immunology (628)
- Anesthesia (165)
- Cardiovascular Medicine (2384)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (380)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (839)
- Epidemiology (11778)
- Forensic Medicine (10)
- Gastroenterology (703)
- Genetic and Genomic Medicine (3752)
- Geriatric Medicine (350)
- Health Economics (635)
- Health Informatics (2401)
- Health Policy (935)
- Health Systems and Quality Improvement (900)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13324)
- Intensive Care and Critical Care Medicine (769)
- Medical Education (366)
- Medical Ethics (105)
- Nephrology (398)
- Neurology (3514)
- Nursing (198)
- Nutrition (528)
- Obstetrics and Gynecology (675)
- Occupational and Environmental Health (665)
- Oncology (1825)
- Ophthalmology (538)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (234)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1035)
- Pharmacology and Therapeutics (426)
- Primary Care Research (422)
- Psychiatry and Clinical Psychology (3181)
- Public and Global Health (6152)
- Radiology and Imaging (1281)
- Rehabilitation Medicine and Physical Therapy (749)
- Respiratory Medicine (830)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (146)

Unlocking the secrets of plant-electromagnetic field interactions: A comprehensive review
A research team has meticulously analyzed the biological impacts of ornamental plants' exposure to electromagnetic fields (EMFs), especially those at high frequencies. They proposed a comprehensive strategy to predict and mitigate these effects by considering various factors such as the source of exposure, material properties, and environmental conditions.
The team successfully forecasted thermal effects and outlined protective measures by leveraging governing equations related to electromagnetic and heat transfer phenomena. This exhaustive approach identifies vulnerable plant parts and suggests avenues for further investigation, including categorizing plants, establishing bio-thermal-EM parameters, and defining exposure thresholds.
The review article is published in the journal Ornamental Plant Research .
Human-plant interactions offer manifold benefits spanning economic, environmental, and health domains, fostering a growing recognition of plants as essential rather than mere luxuries. However, alongside these advantages, human activities generate pollutants that detrimentally affect plant life.
Ornamental plants, crucial for their decorative value, face heightened vulnerability due to their proximity to human habitats, which are increasingly saturated with EMFs emitted by various devices. EMF exposure from artificial sources or natural phenomena like lightning induces biological stress in plants, primarily manifesting as thermal effects.
Existing research on plant stress factors has explored histology and specific species' responses to stress, laying the groundwork for understanding plants' responses to EMF exposure.
The research proposes an approach to study the biological effects of radio frequency EMF exposure on plants, aiming to predict, monitor, and mitigate such effects through an analysis of tissue anatomy, electromagnetic, and heat transfer equations, and strategies for supervising and protecting against EMF radiation effects.
This review delves into the intricate relationship between thermal biological effects (BEs) and tissue anatomy in the context of EMF exposure. With an emphasis on ornamental plants, the analysis highlights how tissue properties and geometries influence vulnerability to EMF exposure, necessitating predictive control measures.
Understanding tissue anatomy, facilitated by histology, unveils the intricate cellular composition of plants and their functional significance. Similar to arteries and veins in animals, vascular tissues play a vital role in transporting essential nutrients and fluids throughout the plant.
Importantly, the review distinguishes between thermal effects induced by EMF exposure and ambient temperature fluctuations. Unlike natural heat sources, EMF-induced heating occurs swiftly within plant tissues, posing unique challenges for thermal regulation.
Governing equations elucidate the complex interplay between EMF exposure and heat transfer, providing a mathematical framework for predictive modeling. Coupled with computational techniques, these equations enable a localized analysis of EMF-induced thermal effects, which is critical for understanding and mitigating potential harm to plant tissues.
Supervisory and protection strategies, including threshold monitoring and shielding technologies, offer avenues for safeguarding ornamental plants against adverse EMF effects.
According to the study's researcher, Adel Razek, "This contribution aims to propose an approach to study the BEs of RF-EMF exposure of plants. Such an approach intends to account for the nature of the exposure source, the properties of the exposed subject, and the exposure conditions."
This comprehensive review underscores the urgent need for further investigations to refine predictive models and establish robust protection measures tailored to diverse plant species and environmental conditions.
More information: Adel Razek, Analysis and control of ornamental plant responses to exposure to electromagnetic fields, Ornamental Plant Research (2024). DOI: 10.48130/opr-0024-0007
Provided by Chinese Academy of Sciences

- Washington State University
- Go to wsu twitter
- Go to wsu facebook
- Go to wsu linkedin
Uniform Medical Plan participants and MultiCare
Premera Blue Cross, which is associated with the Regence third party administrator of the Uniform Medical Plans, is in negotiations with the Spokane MultiCare medical providers. The intent of the two parties is to reach an agreement on their contracts to avoid any disruption in care.
More information including what will happen if an agreement is not reached, and who to contact with questions can be found at hrs.wsu.edu/employees/benefits .
The Notices and Announcements section is provided as a service to the WSU community for sharing events such as lectures, trainings, and other highly transactional types of information related to the university experience. Information provided and opinions expressed may not reflect the understanding or opinion of WSU. Accuracy of the information presented is the responsibility of those who submitted it. The self-uploaded posts are reviewed for compliance with state statutes and ethics guidelines but are not edited for spelling, grammar, or clarity.

Cillay to assume role of interim Pullman campus chancellor
Recent news.

College of Education celebrates faculty and staff success

Reflecting on spring semester

Charles Moore selected as Faculty Senate chair-elect

Inaugural Jordan Awards showcase excellence in veterinary medicine, biomedical sciences

WSU offers expanded equine reproductive care and advanced techniques

Robot-phobia could exacerbate hotel, restaurant labor shortage

IMAGES
VIDEO
COMMENTS
The University of Florida Cancer and Genetics Research Complex is an integrated medical research facility. Medical research (or biomedical research ), also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion ...
10 careers in medical research Here are 10 careers you can pursue in the field of medical research: 1. Clinical laboratory scientist National average salary: $89,291 per year Primary duties: A clinical laboratory scientist is a scientist who specializes in using lab equipment to perform tests on biological specimens. This can involve extracting and testing bodily fluids to look for diseases or ...
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving ...
MD-PhD Dual Degree Training. MD-PhD programs provide training in both medicine and research. They are specifically designed for those who want to become research physicians. The AAMC MD-PhD section is committed to recruiting and training a diverse Physician-Scientist workforce and an inclusive learning and working environment.
The lowest 10 percent earned less than $50,100, and the highest 10 percent earned more than $166,980. The median annual wages for medical scientists in the top industries in which they work are as follows: Research and development in the physical, engineering, and life sciences. $102,210.
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. ... Interventional studies are experimental in character and are further subdivided into field studies (sample from an area ...
PhD investigator, Howard Hughes Medical Institute; core member, Broad Institute of MIT and Harvard; James and Patricia Poitras Professor of Neuroscience, McGovern Institute for Brain Research, MIT.
Physicians, scientists, investigators, pharmacists, and pharmaceutical leaders who have a strong clinical research foundation to build on will play an increasingly significant role in moving the medical field forward in exciting new directions over the next decade, according to Ebrahim Barkoudah, MD, MPH, FACP, Medical Director at Brigham and ...
Here are nine jobs in medical research you can pursue: 1. Medical technologist. National average salary: $59,561 per year Primary duties: A medical technologist is a research professional who works in a diagnostic laboratory and conducts tests to determine a patient's health status. They process and analyze body fluids and tissues as requested ...
Here are the 2023-2024 Best Medical Schools: Research. Harvard University. Johns Hopkins University. University of Pennsylvania (Perelman) Columbia University. Duke University. Stanford University ...
Looking forward to a new year, experts share six trends for the biomedical community in 2021. Summing up 2020, Sharon Peacock, director of the COVID-19 Genomics UK Consortium, says "we've seen ...
Mailing Address: NIH Research Matters. Bldg. 31, Rm. 5B52, MSC 2094. Bethesda, MD 20892-2094. Editor: Assistant Editors: NIH Research Matters Office of Communications and Public Liaison NIH Office of the Director. NIH findings with potential for enhancing human health include new approaches to COVID-19, a universal mosquito vaccine, and ...
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators,
The findings suggest that people can learn to reduce the brain activity causing some types of chronic pain that occur in the absence of injury or persist after healing. 2021 Research Highlights — Basic Research Insights >>. NIH findings with potential for enhancing human health include new drugs and vaccines in development for COVID-19 ...
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. ... Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility ...
Global Clinical Scholars Research Training. This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods. $14,900 - $15,900. Register by Sep 11.
Check out details about more than 40 jobs in healthcare and medicine, with trusted information from Mayo Clinic College of Medicine and Science. Career. Work type. Median salary. Higher education required. Athletic trainer. Patient care. $48,000. 4-6 years.
Medical research often seems much like standard medical care, but it has a distinct goal. Medical care is the way that your doctors treat your illness or injury. Its only purpose is to make you feel better and you receive direct benefits. On the other hand, medical research studies are done to learn about and to improve current treatments.
Senior Clinical Research Associate (SCRA) New. EPM Scientific 3.3. Hybrid work in South San Francisco, CA. $121,000 - $139,000 a year. Full-time. Easily apply. Competitive salary with comprehensive benefits (90% medical coverage). Company Summary: Working with a precision IO biotechnology company to build out their….
Medical research is the gateway to improved patient care and expanding our available treatment options. However, finding a relevant and compelling research topic can be challenging. ... Focus on a particular field of study. The best medical research is specific to a particular area. Generalized studies are often too broad to produce meaningful ...
Premedical students aspiring to become physician-scientists will be tasked with navigating emerging fields in research and translating exciting discoveries into the clinical realm. Understanding ...
A trailblazer in the field of therapeutic genome editing, Fyodor D. Urnov's research focuses on developing medicines for devastating genetic diseases. Join us on Saturday, May 25 at 11 am at Stephen Robert Hall, True North Classroom for his forum presentation, A Gene-Edited Future for Medicine: CRISPR Cures For All In Need .
Many BS/MD hopefuls pursue research as a way to build their resume. Numerous BS/MD programs like Rensselaer Polytechnic University, like to see students with extensive research experience. Its ...
Brain wave research did not take off in earnest until the 1960s, with early experiments often focusing on monkeys. In 1973, Jacques Vidal, a computer science professor at the University of California, Los Angeles, published a paper coining the phrase " brain-computer interface " and laying the groundwork for the field.
1. Laboratory technician. National average salary: $45,059 per year Primary duties: A laboratory technician is a biology and health care specialist who works in a lab to examine and research medical samples, like body fluids, and processes. The technician works with other scientists and managers to perform experiments and operate lab equipment.
Introduction: Research in the medical discipline significantly impacts society by improving the general well-being of the population, through improvements in diagnostic and treatment modalities. However, of 579 Indian medical colleges, 332 (57.3%) did not publish a single paper from the year 2005 to 2014," indicating a limited contribution from medical fraternity In order to probe in to the ...
A research team has meticulously analyzed the biological impacts of ornamental plants' exposure to electromagnetic fields (EMFs), especially those at high frequencies. They proposed a ...
CNN —. A 62-year-old man has died months after becoming the world's first living recipient of a genetically edited pig kidney transplant, hailed as a medical milestone. Rick Slayman received ...
Tour crop research for the drylands at WSU's 106th Lind Field Day, June 13 May 17, 2024 WSU invites grain producers and partners to meet scientists at the Lind Dryland Research Station and learn about current research in one of the nation's driest growing regions at the 106th annual Lind Field Day, Thursday, June 13, 2024.
The top four placers at the D-I district meet at Findlay advanced to this week's regional at Port Clinton, which runs on Wednesday and Friday. Wapakoneta's Olberding, Kohler shine at Findlay ...