An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diagnostics (Basel)


A Review of the Recent Advances in Alzheimer’s Disease Research and the Utilization of Network Biology Approaches for Prioritizing Diagnostics and Therapeutics
1 Department of Pharmacy, Faculty of Pharmacy, Al-Zaytoonah University of Jordan, P.O. Box 130, Amman 11733, Jordan
2 Laboratory for Molecular Modeling, Division of Chemical Biology and Medicinal Chemistry, Eshelman School of Pharmacy, The University of North Carlina at Chapel Hill, Chapel Hill, NC 27599, USA
3 National Center for Epidemics and Communicable Disease Control, Amman 11118, Jordan
Dima A. Sabbah
Osama h. abusara, abdel qader al bawab, associated data.
Data supporting the reported results can be requested by contacting the corresponding author directly.
Alzheimer’s disease (AD) is a polygenic multifactorial neurodegenerative disease that, after decades of research and development, is still without a cure. There are some symptomatic treatments to manage the psychological symptoms but none of these drugs can halt disease progression. Additionally, over the last few years, many anti-AD drugs failed in late stages of clinical trials and many hypotheses surfaced to explain these failures, including the lack of clear understanding of disease pathways and processes. Recently, different epigenetic factors have been implicated in AD pathogenesis; thus, they could serve as promising AD diagnostic biomarkers. Additionally, network biology approaches have been suggested as effective tools to study AD on the systems level and discover multi-target-directed ligands as novel treatments for AD. Herein, we provide a comprehensive review on Alzheimer’s disease pathophysiology to provide a better understanding of disease pathogenesis hypotheses and decipher the role of genetic and epigenetic factors in disease development and progression. We also provide an overview of disease biomarkers and drug targets and suggest network biology approaches as new tools for identifying novel biomarkers and drugs. We also posit that the application of machine learning and artificial intelligence to mining Alzheimer’s disease multi-omics data will facilitate drug and biomarker discovery efforts and lead to effective individualized anti-Alzheimer treatments.
1. Introduction
Alzheimer’s disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [ 1 , 2 , 3 , 4 , 5 ]. It is estimated that 6.5 million Americans, 65 years and older, are living with Alzheimer’s dementia, and this number is likely to grow rapidly [ 6 ], reaching 13.8 million by 2060 [ 6 , 7 ]. Additionally, approximately 10 million Americans are currently living with mild cognitive impairment (MCI). In fact, 50% of MCI cases are due to AD based on biomarker evidence [ 8 ]. Eventually, 15% of MCI patients develop dementia after two years, and one-third develop dementia due to AD within five years [ 6 ]. The number of Americans with AD-MCI and AD dementia is approximately 11.2 million [ 6 ]. The healthcare cost for AD patients in 2022 is expected to reach USD 321 billion [ 6 ]. Furthermore, as the global population ages, the number of people suffering from dementia is expected to triple from 50 million to 152 million by 2050 [ 9 ]. Therefore, there is an urgent need to impede or slow down the onset and progress of the disease and ameliorate the disease-debilitating symptoms in hopes of putting an end to this disease and preventing a growing public health crisis [ 10 ].
Multifactorial mechanisms are involved in AD pathogenesis, including genetic, epigenetic, biological, and environmental factors networking with each other [ 11 ]. However, genomic experiments affirmed that no particular gene could be assigned as a potential target for AD pathogenesis. In fact, multiple genetic and non-genetic factors contribute to disease development [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. In the past decade, there has been an increased understanding of potential targets for disease-modifying therapies that delayed or slowed down the clinical course of AD. According to the U.S. National Library of Medicine’s (NLM) ClinicalTrials.gov Beta (beta.clinicaltrials.gov) [ 19 ], there have been 1943 clinical trials of different phases and different designs to investigate new potential molecules in treating AD. Among these studies, 151 clinical trials progressed to Phase III clinical trials, in which safety and monitoring of side effects were investigated.
After decades of AD drug discovery research and billions of dollars spent on clinical trials, we still do not have a single effective anti-AD drug. Promising novel strategies to developing anti-AD drugs keep failing in clinical trials [ 20 , 21 ]. The exact cause of the disease remains the subject of ongoing debate and investigation. At this time, the most plausible hypothesis is that AD is a multifactorial disorder in which genetic and environmental risk factors interact, leading to an acceleration in the rate of normal aging. More than 600 genes contribute to AD pathogenesis, along with environmental factors and epigenetic changes [ 4 , 5 ]. The genetic deficiencies in AD consist of germline mutations, mitochondrial DNA mutations, and single-DNA nucleotide polymorphisms [ 22 , 23 ].
Therapeutic clinical treatment targets of AD aim to enhance behavioral, cognitive, and non-cognitive symptoms of the diseases. In the last two decades, there were no new medication approvals for the treatment or the prevention of AD. Currently, the aim of developing new anti-AD agents is to utilize new disease-modifying agents that delay the onset or slow down the progression of an established disease. Aβ, tau protein, and cell oxidation are the most currently promising targets to modify the pathologic status of Alzheimer’s disease [ 24 ].
In this review, we aim to provide a comprehensive overview of AD pathophysiological hypotheses, classifications, diagnostic biomarkers, and currently approved pharmacological and non-pharmacological treatment options. Moreover, we discuss the emerging role of the microbiome as well as epigenetics in the pathogenesis of AD and discuss the potential of epigenetic and genetic treatment options. We also provide a summary on drugs being developed or currently investigated in clinical trials, their success, failures or drawbacks, newer target approaches, and most important considerations for conducting effective future clinical trials. In addition, we discuss novel approaches for the proper diagnosis and drug-development for AD through exploiting network biology, artificial intelligence (AI), and machine learning (ML).
2. Alzheimer’s Disease Pathophysiology
There are two main pathological hallmarks of AD: (1) the presence of increased extracellular Aβ plaques, formed due to the aggregation and impaired clearance of Aβ oligomers (hydrophobic Aβ aggregates) [ 25 , 26 ], and (2) the formation of NFTs [ 27 ] that are composed of an insoluble intracellular hyperphosphorylated microtubule-associated protein tau [ 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ]. MetaCore’s [ 39 ] pathway map presented in Figure 1 summarizes the most important processes, genes, and proteins linked to AD pathophysiology. We further highlighted which network objects on the map that are known AD biomarkers according the MetaCore TM , and which ones are drug targets for known drugs (i.e., as a proof of their druggability).

Alzheimer’s disease (AD) pathway map highlighting validated diagnostic/prognostic AD biomarkers in addition to genes/gene products involved in the tau pathology pathway. Connections between network objects on the map are referred to as links (or edges). A link identifies an interaction or a logical relation between two nodes. The type of interaction or relation is reflected by an appropriate symbol placed in the middle of the link. B = binding (i.e., physical interaction between molecules); C = cleavage; CM = covalent modification; +P = phosphorylation; −P = dephosphorylation; Tn = transport; TR = transcription regulation; red arrows = inhibition; green arrows = activation; grey arrows = unspecified action; solid purple arrows = emergence in disease; dashed purple arrows = enhancement in disease; arrows with purple x = disruption in disease; light violet text box = normal process; pink text box = pathological processes; white text box with blue outline = notes; grey block = reaction; blue block = normal process; pink block = pathological process; solid purple hexagon = compound; CS = complex subunit; GR = group relation; starred network objects = groups or complex processes; thermometers on pathway map = network object is also a validated diagnostic or prognostic disease biomarker from the Cortellis Drug Discovery Intelligence (CDDI) database [ 40 ]; thermometer 1 is for late-onset AD (LOAD); thermometer 2 is for early-onset AD (EOAD); a pink hexagon with a capital D on the upper left side of the network object indicates an AD biomarker according to MetaCore TM ; a blue hexagon on the upper left side of the network object with a capital R indicates that the network object (i.e., gene/gene product) is a drug target (not necessarily for AD). Map generated using MetaCore TM version 21.4. MetaCore TM , a Cortellis™ solution, 14 October 2022, © 2022 Clarivate. All rights reserved.
2.1. The Amyloid Hypothesis
It was long thought that polymorphism in the amyloid precursor protein (APP) gene ( APP ) causes alterations within and around the Aβ domain of translated APP proteins, altering APP proteolytic processing, and leading to changes in Aβ cleavage and aggregation [ 41 ]. This would favor the amyloidogenic pathway, causing the accumulation of Aβ, which contributes to AD pathogenesis [ 42 ].
The APP protein contains the Aβ domain, which, in healthy individuals, is cleaved and processed into Aβ and other Aβ-related peptides [ 43 , 44 , 45 , 46 , 47 , 48 , 49 ] by α-secretase followed by γ-secretase and later cleared [ 26 , 50 , 51 ]. The APP protein is encoded by the APP gene ( Figure 2 ), which has 17 transcripts (splice variants), 279 orthologues, 2 paralogues, and is associated with 9 phenotypes (ABeta amyloidosis Arctic type, ABeta amyloidosis Dutchr type, ABeta amyloidosis Iowa type, ABeta amyloidosis Italian type, ABetaA21G amyloidosis, ABetaL34V amyloidosis, ADs, cerebral amyloid angiopathy APP-related, and early-onset autosomal dominant AD).

Visualizing APP gene information using ( a ) Ensembl genome browser 107 (July 2022, GRCh38) and ( b ) NCBI’s gene browser.
APP genetic variants are often observed in Familial Alzheimer’s disease (FAD) [ 52 , 53 , 54 , 55 , 56 , 57 , 58 ]. Such mutations in the amino terminus of the Aβ domain result in the production of better substrates for β-secretase (BACE1) activity [ 55 ], leading to significant increase in Aβ production and Aβ fragments consisting of 40 or 42 amino acid residues (Aβ40 and Aβ42, respectively) [ 51 , 59 , 60 ]. These Aβ fragments are formed via the amyloidogenic pathway in AD patients, as BACE1 cleaves APP followed by γ-secretase [ 50 ]. Enhanced aggregation of altered Aβ is observed due to mutations affecting the mid region of Aβ [ 57 , 58 ]. Amino acid mutations located beyond the carboxyl terminus region of Aβ increase the production of Aβ42 [ 55 , 56 ], which is considered the major neurotoxic Aβ species [ 25 , 42 , 50 , 61 ]. Aβ42 is of particular interest; it is more hydrophobic, more pathogenic, and it aggregates faster than Aβ-40, resulting in an earlier onset of AD.
Furthermore, APP has a substrate inhibitory domain (ASID) that negatively regulates the activity of γ-secretase by decreasing Aβ production [ 62 ]. Alterations of ASID region by deletion or single-nucleotide polymorphism were reported, in which an inhibitory effect is reduced, causing an increase in Aβ generation [ 50 , 62 ]. Presenilin (PSEN), the catalytic subunit of γ-secretase [ 63 , 64 , 65 , 66 ], may also be modified via mutations in PSEN1 and PSEN2 genes ( PSEN1 and PSEN2 , respectively) [ 67 ], resulting in the destabilization of the γ-secretase–APP interactions [ 68 ], causing the generation of longer and more hydrophobic Aβ peptides that contribute to the pathogenesis of AD [ 41 , 68 , 69 , 70 ].
Tau is a microtubule-associated protein, found in axons of neurons [ 71 ], and it functions as a microtubule stabilizer that enables cytoplasmic extensions for the eventual formation of the axon and the dendrites [ 72 , 73 ]. Once tau is phosphorylated, its ability to bind to microtubules is reduced [ 74 , 75 , 76 , 77 ] and self-assembly into tangles/filaments is induced [ 78 ], hence affecting its normal function. It is considered as a main pathological lesion in the brains of patients with AD in addition to Aβ fibrils. In fact, AD is considered one of the neurodegenerative diseases that are termed “tauopathies” [ 79 , 80 , 81 ], where tauopathy indicates a tau-related pathology (i.e., dysfunction and/or tangle formation that contributes to the pathogenesis of AD and other neurodegenerative diseases) [ 27 ]. As for AD, this is driven by the intracellular aggregation of tau proteins via different mechanisms. Tau’s phosphorylation can lead to aggregation via liquid–liquid phase separation mechanism [ 82 ]. Cleavage of tau by several proteases can also lead to aggregation [ 83 , 84 , 85 ]. It is also suggested that tau pathology could be a downstream effect of Aβ aggregation or both could enhance each other’s toxic effects [ 41 , 86 , 87 ]. In vivo, Aβ plaques enhanced the aggregation of human-derived pathological tau injected into mouse brains [ 88 ]. In autopsy specimens from deceased AD patients, the number of NFT-positive cells is positively correlated to AD stages [ 89 , 90 , 91 , 92 , 93 ].
2.1.3. APOE, TERM2, SORL1, and ABCA7 Mutations
Mutations of genes, such as apolipoprotein E ( APOE ) and triggering receptor expressed on myeloid cells 2 ( TREM2 ) [ 70 ], would affect the microglial clearance of Aβ. Briefly, APOE protein, which is a lipoprotein, binds to Aβ plaques forming Aβ-lipoprotein complexes that are engulfed into microglia via TREM2 receptors [ 94 ]. Mutations of APOE and TREM2, resulting in mutated proteins, such as APOE2, APOE4, or TREM2 mutations (R47H, R62H, and D87N), would contribute to AD development [ 94 , 95 , 96 , 97 ]. Additionally, genetic mutations of SORL1 and ABCA7 are also considered as causative or strong risk-increasing variants for AD [ 70 ]. Sortilin-related receptor L1 (SORL1) controls APP cleavage and APOE uptake, while increasing ATP-binding cassette transporter A7 (ABCA7) increases Aβ phagocytosis [ 67 ]. Hence, mutations resulting in alterations of SORL1 and ABCA7 would act as a risk factor for AD [ 67 ]. Other genetic mutations that have low, medium, or high risk for AD are summarized by Scheltens et al. [ 41 , 70 ].
2.2. The Cholinergic Hypothesis
In addition to the amyloid hypothesis described above, other hypotheses have been suggested such as the impairment of cholinergic function, also known as the cholinergic hypothesis [ 98 ]. The cholinergic deficit in AD patients was confirmed in various studies, which highlighted a major deficit in the enzyme responsible for acetylcholine (ACh) synthesis (choline acetyltransferase) [ 99 , 100 , 101 ], reduced choline uptake [ 102 ], reduced ACh release [ 103 ], and loss of ACh (cholinergic) neurons [ 104 ]. Along with the role of ACh in learning and memory [ 105 , 106 ], degeneration of cholinergic neurons and loss of cholinergic neurotransmission contributes to the worsening of cognitive function in patients with AD [ 106 , 107 ].
2.3. The Mitochondrial Cascade (Oxidative Stress) Hypothesis
Neuronal mitochondria are considered the main organelles responsible for the neuronal oxidative stress via the generation of free radicals through their electron transport chain [ 108 ]. In the case of high levels of ATP and diminishing electron transport effect, superoxide would be formed from oxygen via mitochondrial respiration [ 109 ]. Superoxide would then be converted to hydrogen peroxide by superoxide dismutase and later to hydroxyl radicals and anions by the Fenton reaction [ 110 ]. These reactive oxygen species (ROS) would affect redox imbalance, cause neurotoxicity and genomic instability, transcription of pro-inflammatory genes, and cytokine release [ 111 ]. ROS would further damage and inactivate parts of the mitochondrial electron transport chain, leading to the formation of superoxide from the electron reduction of oxygen in a positive feedback cycle [ 109 , 111 , 112 ].
ROS damage mitochondrial DNA (mtDNA) that leads to neuronal functional impairments and damaged mitochondria due to this oxidative stress will not be degraded by mitophagy [ 108 , 111 , 113 ]. Usually, oxidative stress acts as a signal for mitophagy process to degrade damaged mitochondria, as oxidative stress reduces mitochondrial membrane potential [ 108 ]. However, ROS alter parkin, which is a key mitophagy regulator, and inhibits its function, causing the continual presence of mitochondria [ 113 ]. Collectively, ROS, DNA damage, and mitochondria contribute to the aging process [ 114 , 115 ]. Briefly, following DNA damage via ROS, kinases and PARP are activated, leading to the decrease of NAD+ production, which is essential for metabolic pathways and ATP production. Thus, oxygen consumption and ATP production would be required to increase to meet high energy demand. Mitochondrial coupling would occur, which increases membrane potential, increases free radical formation, and decreases mitophagy. Furthermore, as discussed above, free radicals would further cause DNA damage.
Denham Harman proposed the free radical theory of aging, in which free radicals are involved in the changes associated with the aging process [ 116 ]. It was later confirmed that free radicals are involved in the aging process and advanced age diseases, such as AD [ 115 , 117 , 118 ]. Furthermore, the effect of ROS on mitochondria supported the theory that relates mitochondria to the aging process and neurodegenerative diseases, such as AD [ 108 ]. This theory is associated mostly with the central nervous system since it consumes 20% of the body’s oxygen and is susceptible to oxidative stress [ 119 ]. Neurons would have high sensitivity to free radicals since they are non-dividing and post-mitotic cells and cannot be replaced in the event of damage, leading to mitochondrial dysfunction with aging [ 118 , 120 ].
It is observed that mitochondrial dysfunction is prevalent in the aging process [ 108 ]. In addition, healthy aging results in reduced mitochondrial metabolism in terms of α subunit reduction of the mitochondrial F1 ATP synthase, thus interfering with ATP production [ 108 ]. Eventually, ATP production decreases, ROS production increase, and this will cause an increase in DNA, protein, and lipid oxidation [ 118 , 121 , 122 ]. Moreover, apart from mtDNA damage caused by mitochondrial dysfunction, nuclear DNA is also damaged, leading to impairments in vesicular function, synaptic plasticity, and mitochondrial function [ 121 ].
Excess ROS and bioactive metals, such as copper, iron, zinc, and magnesium are present in AD brains that promote Aβ aggregations and NFT formation [ 123 , 124 , 125 , 126 ]. Moreover, the elevated levels of mtDNA oxidation are considered as one of the early markers of AD pathogenesis [ 118 , 122 ]. Moreover, late onset AD pathogenesis may be linked to age-associated mitochondrial decline [ 108 ]. The expression and processing of APP would be altered with age-associated mitochondrial decline, leading to the production of Aβ oligomers that aggregate into plaques in AD [ 127 , 128 ]. These Aβ oligomers are associated with neuronal toxicities and ROS generation. Studies have shown that the hydrophobic 25–35 region of Aβ leads to neuronal toxicity and generates ROS, showing that Aβ itself is a source of oxidative stress [ 108 , 129 , 130 ]. Aβ42 is hydrophobic in nature, and it can reside within the neuronal membrane lipid bilayer and cause lipid peroxidation, identified through 4-hydroxy-2-trans-nonenal (HNE) production that is bound to neuronal proteins [ 131 ]. Studies have also shown that the production of HNE—due to lipid peroxidation associated with the residing of hydrophobic Aβ protein in lipid bilayer—and HNE’s neuronal protein binding are linked to cell death [ 132 , 133 , 134 ]. This are related to neurodegenerative diseases’ pathogenesis, including AD. Furthermore, the oxidative stress triggered by Aβ is also likely to be as result of complexation with redox active metals, such as copper, zinc, and iron [ 108 ], which are highly present in AD brains [ 123 , 124 , 125 , 126 ], promoting Aβ aggregation into plaques [ 108 ]. Copper forms the most stable complex and can generate superoxide and hydrogen peroxide that contribute to AD pathogenesis [ 127 , 135 ].
2.4. Other Factors Affecting Disease Pathogenesis
Other factors that affect the clinical development of AD include vascular pathology of blood–brain barrier that result in leakage and cause dementia [ 136 , 137 ]. Moreover, elevated iron levels in brain and dysregulation of its metabolism are also present in AD patients as it is linked with cellular damage and oxidative stress [ 138 ]. Exosomes may play a part in the spreading of Aβ plaques and NFTs through the brain [ 139 ].
3. Alzheimer’s Disease Classifications
AD is usually classified on the basis of two criteria: age and heredity ( Table 1 ). Based on age, there are two main forms of AD: late onset AD (LOAD) and early onset AD (EOAD). LOAD is diagnosed at age 65 or older [ 140 ], although the degenerative process may begin damaging the brain 20 years before symptoms appear [ 141 ]. This is the most prevalent form of the disease, comprising 95% of patients [ 142 ]. Polymorphism in APOE is a major risk determinant of LOAD, contributing to 60–80% of the disease pathogenesis [ 140 , 143 , 144 ]. APOE encodes for the principal lipid transport protein in the central nervous system (CNS), which has three alleles (E2, E3, and E4). APOE4 surges the risk of LOAD development [ 11 ]. Genome-wide association studies (GWAS) identified >20 LOAD risk genes linked to endocytosis, inborn immunity, and lipid metabolism [ 143 , 145 ]. EOAD is typically diagnosed between 40 and 50 years of age, comprises 5% of the total AD cases [ 146 ], is linked to a defect in chromosome 14 and myoclonus [ 147 , 148 ], and, therefore, it is referred to as a distinctive autosomal dominant inherited form of AD [ 140 ]. Usually, people with Down’s syndrome are more prone to EOAD [ 149 ]. In fact, mutations in APP genes, particularly PSEN1 and PSEN2 , are associated with EOAD development [ 140 ]. These APP variants explain 5–10% of the autosomal dominant inherited cases, leaving the majority of cases totally unexplained [ 150 , 151 ].
Based on heredity, AD can be either familial or sporadic [ 152 ]. Compiling the two diagnostic criteria (i.e., age and hereditability), AD can be classified into EOAD, LOAD, early onset familial (eFAD), late-onset familial, early onset sporadic, and late-onset sporadic. Yet, these classifications overlap in terms of nomenclature, diagnosis, and treatment. FAD is linked to genes [ 153 ].
Main types of Alzheimer’s disease based on age and heredity.
4. Alzheimer’s Disease Diagnosis
AD is a dual clinicopathological condition, which means that two requirements must be met for the definite diagnosis: (1) the presence of a clinical phenotype characterized by symptoms, such as episodic memory impairment or involvement of other cognitive, behavioral, and neuropsychiatric domains and (2) the development of neurological changes, such as the accumulation of NFTs and Aβ plaques in the brain. NFTs and Aβ plaques can be detected only through autopsy; as such, clinical diagnosis relies heavily on the observation of behaviors that are compatible with known clinical features of AD [ 41 , 158 ] and the exclusion of other potential causes [ 9 ]. The guidelines of neuropathological assessment of autopsy samples for the definitive diagnosis of AD have been published by the National Institute on Aging and the Alzheimer’s Association in 2012 [ 159 ].
In fact, it remains challenging to discriminate AD from other neuropathological dementia despite the advances in research protocols and current diagnostic tools [ 11 ]. Currently, AD diagnosis is based on confirming memory loss and cognitive impairments using neurological tests, such as the Montreal Cognitive Assessment (MOCA) [ 160 ] and Mini-Mental Status Examination (MMSE) [ 161 ]. However, the ultimate AD diagnostic protocol can only be performed post-mortem to detect Aβ and tau NFTs in brains of deceased patients [ 11 ]. The current limitations in AD diagnostics burden the development of effective AD treatments since it depends on signs and symptoms, whereas the accurate status of the brain can only be assessed post-death [ 162 ]. Currently, scientists are suggesting epigenetics alterations could be exploited as diagnostic surrogates for AD [ 7 , 11 , 12 , 13 , 14 , 15 , 16 , 18 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 ].
5. Epigenetic Changes in Alzheimer’s Disease
Epigenetic modifications have emerged as significant contributors in AD pathogenesis, mediating promises for AD treatment [ 7 , 11 , 12 , 13 , 14 , 15 , 16 , 18 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 ]. Various epigenetic changes, including mitochondrial epigenetics (i.e., mitoepigenetics), DNA methylation and hydroxymethylation, noncoding RNA translation, and histone post-translational modifications have been implicated in AD development [ 11 ]. Disruption of both DNA methylation and DNA hydroxymethylation processes has been implicated in many diseases that are classified as neuropathologies, including AD [ 172 ]. Interestingly, the key genes involved in AD pathogenesis are regulated by miRNAs and DNA methylation [ 11 , 13 ]. Other studies reported that there is an overlap between distinctively methylated DNA spots in AD and histone signatures in H3K27me3 and H3K4me3 in the Polycomb-repressed (poised) promoter [ 173 ]. Further studies declared that indistinguishable 5-methylcytosine (5mC) models are observed in AD patients associated or not associated with schizophrenia [ 174 ].
5.1. DNA Methylation
DNA methylation retains fundamental cellular functions and synaptic elasticity in the CNS, and it influences cognitive processes [ 175 ]. DNA hydroxymethylation is essential for neurodevelopment and is concentrated in the CNS, which further signifies the importance of DNA methylation [ 175 ]. Some studies showed that overall DNA methylation is decreased in AD patients [ 176 , 177 , 178 , 179 ], while other studies recorded no significant differences in DNA methylation between AD and age-matched healthy individuals [ 180 , 181 ]. DNA methylation patterns that were in connection with AD were investigated for the following genes: glycogen synthase kinase 3 beta ( GSK3b ) [ 182 , 183 ], ankyrin 1 ( ANK1 ) [ 184 ], TREM2 [ 17 ], and brain-derived neurotrophic factor ( BDNF ) [ 185 ]. ANK1 methylation has been increased in AD patients [ 186 , 187 , 188 , 189 ]. An increase in DNA methylation has been also recorded in the dorsolateral prefrontal cortex [ 187 ], entorhinal cortex [ 190 ], temporal cortex [ 190 ], temporal gyrus [ 191 ], and the hippocampus. Contrarily, a decrease in DNA methylation has been reported in locus coeruleus, prefrontal cortex [ 192 , 193 ], and blood samples [ 194 ]. Additionally, studies showed that 13% of noncoding RNA CpG motifs were methylated in AD patients, leading to a significant increase in 5mC levels in these genetic loci in particular [ 190 ].
5.2. Mitochondrial DNA Methylation
Mitochondria generate the energy (ATP) required to vitalize the cell’s reactions, and AD has been postulated to be associated with energetic decrease arising from mitochondrial disorder. Dysfunction of the mitochondrial oxidative phosphorylation and energy-producing cascade increases in reactive oxygen species (ROS) generation and apoptosis such that both are implicated in neurodegeneration and disease development [ 11 , 12 , 13 , 16 , 195 , 196 , 197 ]. Studies reported that multiple considerable deletions are detected in mitochondrial DNA (mtDNA) and are linked with AD pathogenesis [ 198 ]. In addition, mutations of mitochondrial rRNA and tRNAs [ 199 , 200 ], cytochrome C oxidase [ 200 , 201 ], and the regulatory D-loop influence mtDNA copy number, transcription, and translation [ 202 ]. Low levels of mtDNA were observed in AD patients having low Aβ and high tau in the cerebrospinal fluid (CSF) and in presymptomatic patients having PSEN1 mutation [ 203 ]. Low mtDNA copy number and abnormal propagation of mitochondria were associated with low level of mtDNA in CSF that might act as a biomarker for AD in the preclinical phase [ 203 ]. Another study demonstrated a positive correlation between Aβ and CSF mtDNA content but a negative correlation between phosphorylated tau protein and CSF mtDNA levels [ 204 ]. Low levels of CSF mtDNA accompanied by low Aβ and high phosphorylated tau assist in distinctive AD diagnosis against other neurological disabilities [ 204 ]. Studies revealed an increase in methylation of mtDNA at CpG and non-CpG repeats of D-loop of AD entorhinal cortex with Braak stages I to II and III to IV [ 192 , 205 ]. However, a significant decrease in mtDNA methylation in AD blood samples was detected [ 197 ].
5.3. DNA Hydroxymethylation
Studies revealed that thousands of distinguishable hydroxymethylated regions (DhMRs) in AD brains are associated with an increase of 5hmC levels in intragenic regions [ 206 , 207 , 208 ]. Genomic studies reported an increase in 5-hydroxymethylcytosine (5hmC) levels in the F-box and leucine rich motif protein 16 (FBXL16) gene [ 186 ]. FBXL16 was reported as a potential AD-associated gene, showing an encoding decrease in microglia cells of mouse AD models [ 209 ]. Another study reported a decrease in 5hmC levels in four CPG repeats in ANK1 [ 186 ]. Diverse studies confirmed a decrease in 5hmC levels in the AD entorhinal cortex, cerebellum [ 173 ], and CA3 region of the hippocampus [ 210 ] but an increase in 5hmC levels in AD brains’ parahippocampal gyrus [ 211 ], middle frontal gyrus, and middle temporal gyrus [ 212 ]. Further studies reported an increase in tau protein deposition and a decrease in astrocytes location [ 213 ], whereas one investigation recorded that 5hmC is not localized in AD cerebellum and entorhinal cortex [ 214 ]. Another study declared a decrease in 5hmC deposition in AD glial cells of hippocampus CA1 region [ 210 ]. Further study investigated the effect of TREM2 on AD pathogenesis and found there is a positive association between 5hmC repeats in exon 2 of TREM2 and TREM2 expression, postulating that an increase in gene expression might assist in tissue repair [ 215 ]. TREM2 is encoded in microglia cells and is required in tissue repair, homeostasis, and natural immunity reaction [ 215 ].
5.4. Histone Modifications
Histones (H1, H2A, H2B, H3, and H4) are biochemically highly basic proteins rich in arginine and lysine residues. Histones serves as a scaffold, assisting DNA to wrap and condense in eukaryotic nucleus forming nucleosomes [ 216 , 217 ]. Histone modifications are involved in neuronal differentiation and growth, older individuals’ brains homeostasis, and in AD pathology [ 7 , 11 , 12 , 13 , 14 , 16 , 18 , 164 , 165 , 169 , 171 , 204 , 218 ]. A prevalent lack of heterochromatin was detected in human AD, tau transgenic Drosophila, and mice [ 219 ]. Oxidative stress and DNA deterioration were associated with transgenic tau expression and heterochromatin relaxation [ 219 ].
Histone modifications, such as abnormal acetylation, were linked with aberrant signaling, apoptosis, inflammation, immunity, and neuroplasticity [ 220 ]. Histone acetylation was detected in postmortem AD brains [ 221 , 222 , 223 ]. A decrease in histone acetylation was observed in AD temporal lobes [ 224 ]. Acetylation of lysine 16 on histone H4 (H4K16ac) is implicated in aging and DNA damage, and such deterioration was previously observed in AD cortex patients [ 218 , 225 ]. However, acetylation of lysine 12 on histone H4 (H4K12ac) was accompanied with memory disturbance [ 218 ]. Higher H4K12ac content was detected in MCI but not detected in AD, confirming its role in an infant stage of disease development and aggregation deposition [ 15 ]. High levels of acetylated histone as well as H3 and H4 were detected in human post-mortem AD brains [ 225 ]. In addition, higher levels of histone deacetylases (HDACs), particularly class I (HDAC2 and HDAC3), were observed in AD brains’ regions that are involved in memory, learning, and neural plasticity. HDACs are linked with cognitive impairments and synaptic functions [ 171 , 218 ]. On the contrary, other investigations declared a decrease in HDACs in dysfunction brains’ regions that are associated with MCI symptoms [ 11 ]. In addition, class II HDACs are implicated in AD pathogenesis [ 226 ]. An increase in HDAC6 level was discovered in AD brains’ cortex and hippocampus and in AD animal models [ 226 ]. HDAC6 influences tau phosphorylation and degradation as well as tubulin acetylation, and it mediates inflammatory processes [ 169 , 227 ]. A decrease in HDAC6 level results in higher clearance and decrease of tau aggregation and consequently assists in nerve survival [ 220 , 228 ], while an increase in HDAC6 level leads to a decrease in α-tubulin acetylation and subsequently disrupts microtubules’ homeostasis and mitochondrial as well as vesicular transport [ 220 , 228 ]. A decrease in HDAC4 content, another member of class II HDACs, adversely influences learning and memory development [ 229 ]. HDAC4 is involved in neural function, and its increase results in apoptosis, whereas its decrease inhibits nerve cell death [ 229 ]. Class III HDACs, sirtuins (SIRTs), are involved in synaptic elasticity and memory functions as well as AD pathogenesis [ 227 ]. Studies reported that levels of SIRT1 are reduced in the parietal cortex, whereas SIRT1 levels in AD cerebellum are not reduced [ 171 ]. Such expression aberrations, probing Aβ and tau deposition, as well as acetylation of lysine 28 of tau protein, lead to tau aggregation [ 171 , 220 ]. Aberrations in histone methylation have been detected in AD patients as well [ 230 ]. The levels of histone methyltransferases (HMT) and histone demethylases are significant for brain vitality and memory function [ 230 ]. Studies declared that an increase in trimethylation of lysine residue on histone H3 (H3K9), a biomarker of gene silencing and heterochromatin condensation [ 231 ], and overexpression of histone lysine methyltransferase 1 (EHMT1) are observed in post-mortem AD brains [ 232 ]. The G9a HMT, an enzyme responsible for demethylation of lysine 9 on H3 (H3K9), is involved in cognitive function in mice; however, H3K4 demethylase contributes to human memory deficiency [ 230 ]. Studies observed that an increase in phosphorylation of serine 10 on H3 (H3S10), detected in AD hippocampal neurons [ 233 ], and an increase in phosphorylation of serine 139 on H2AX, detected in AD astrocytes, might serve as indicators of DNA damage [ 234 ]. ADP ribosylation of H1 was detected in AD brains [ 235 ]. Altogether, these results shed light on histone aberration in AD pathogenesis and motivate more researchers to explore the complexity of such factor.
5.5. MicroRNA
Diverse microRNAs (miRNAs) target genes are implicated in AD pathogenesis [ 11 , 12 , 13 , 14 , 16 , 18 , 169 , 171 ]. There are approximately 161 miRNAs that could contribute to AD pathogenesis, while ten miRNAs have been linked to AD, including miRNA-9, miRNA-29, miRNA-34, miRNA-107, miRNA-125, miRNA-132/-212, miRNA-146, miRNA-155, miRNA-181, and miRNA-206 [ 190 ]. Additionally, specific miRNAs were related to myelin sheath formation and others were involved in AD development, such as SIRT1, BACE1, and APP [ 190 ]. miRNAs are also involved in APP degradation and Aβ metabolism by modulating the activity of APP-degrading enzymes, such as BACE1 [ 236 ]. Furthermore, many miRNAs were found to regulate BACE1 expression, such as miRNA-124, miRNA-135b, miRNA-195, miRNA-15b, miRNA-29c, and miRNA-399-5p [ 237 , 238 , 239 , 240 ]. Other miRNAs, such as miRNA-219, regulate microtubule-associated protein tau (MAPT) gene ( MAPT ), while others, such as miRNA-124-3p and miRNA-125b, modulate the activity of kinases that are involved in the phosphorylation of tau protein [ 183 , 241 , 242 , 243 ].
BDNF, or abrineurin, expressed by BDNF , is a potential regulator of synaptic elasticity and transmission that induces miRNA-132 expression [ 244 ]. Studies reported that miRNA-132 and miRNA-212 encoding is suppressed in the early AD stage [ 245 , 246 ]. Other studies declared that miRNA-9 modulates neural progenitor cells’ growth, differentiation, and migration [ 247 , 248 ]. In addition, miRNA-9 upregulates ACE1 [ 249 ], and subsequently, increases Aβ formation and accumulation [ 250 ].
It was found that downregulation of miRNA-9 modulates calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) expression [ 251 ], resulting in an increase in phosphorylated tau and Aβ deposition through CAMKK2-cyclic adenosine monophosphate-activated protein kinase (AMPK) cascade [ 183 , 252 ]. Modulation of BACE1 encoding is also carried by miRNA-29, implying that an increased level of BACE1 is associated with a decrease in the level of miRNA-29 [ 253 , 254 ]. Studies declared that miRNA-29 regulates neuron navigator 3 ( NAV3 ) that is overexpressed in AD frontal cortexes [ 255 ]. Studies showed that both miRNA-34 and tau mRNA are upregulated in AD, suggesting a linked mechanism for AD pathogenesis [ 256 ]. Studies demonstrated that miRNA-107 expression is suppressed in AD CNS and blood, particularly at the begging of AD. Further studies exhibited negative association between miRNA-107 expression and BACE1, inferring that BACE1 mRNA could modulate miRNA-107 [ 257 ]. In addition, miRNA-107 regulates cyclin-dependent kinase 5 ( CDK5 ) that is responsible for CNS integrity and function [ 258 ]. Studies showed that higher levels of miRNA-125 stimulate tau hyperphosphorylation, resulting in promoting mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) signaling and increasing p53 expression [ 183 , 241 ]. Studies revealed that miRNA-132/-212 was linked with cognitive function and was suppressed in AD brains [ 259 ]. Studies reported that miRNA-146 expression is modulated by nuclear factor kappa-B (NF-κB), and the overexpression of miRNA-146 paves the way for NF-κB to downregulate the translation of complement factor H (CFH) and subsequently influence the inflammatory reaction in CNS [ 260 ].
Studies showed that the overexpression of some microRNAs (miRNA-155, miRNA-146, and miRNA-124) is associated with over production of APP and Aβ [ 261 ]. Studies revealed that miRNA-181 was suppressed in AD CNS [ 262 ]. Further investigations showed that the downregulation of miRNA-181 is associated with higher level of Aβ expression [ 262 ]. Furthermore, the downregulation of miRNA-181 influences MAPK signaling cascade [ 262 ]. Other investigations reported that miRNA-206 is overexpressed in AD CSF and blood [ 51 , 263 ].
6. Alzheimer’s Disease Biomarkers
Biomarkers are important tools for the accurate diagnosis of many diseases, including AD. Despite the recent advances in diagnostic methodology for Alzheimer’s disease, differentiation of Alzheimer’s dementia from other forms of dementia remains challenging. The analysis of Aβ-42, total tau protein, and phosphorylated tau (p-tau) from cerebrospinal fluid (CSF) is currently considered the best-established biological marker for the diagnosis of AD as well as differentiation from mild cognitive impairment and other types of dementia. The familiar AD biomarkers are the reduced levels of Aβ in CSF and the appearance of Aβ or tau depositions in the brains of AD patients [ 264 , 265 , 266 ]. Additionally, biomarker evidence obtained through PET can be used to attribute the clinical syndrome of dementia or MCI to underlying AD pathology, with varying probability [ 264 ]. In most cases, AD diagnosis in living patients continues to rely on the patient’s clinical history, family members with neuropsychological conditions, and the observance of symptom progression over time [ 41 ].
Before the early 2000s, the only sure way to know whether a person had AD or another form of dementia was after death through autopsy. Today, we have 12,073 biomarkers linked to AD. An overview of these biomarkers is provided in Figure 3 a–d. Approximately 441 biomarkers are either approved or in late-stage clinical studies for AD diagnosis, prognosis, staging, and monitoring of disease progression. The most widely used AD biomarkers are Aβ42 (the major component of amyloid plaques in the brain), tau, and phospho-tau (major components of tau tangles in the brain) [ 267 ]. These biomarkers are measured in CSF, which is the clear fluid that surrounds the brain and spinal cord, providing protection and insulation.

An overview of Alzheimer’s disease biomarkers in all stages of clinical development. Biomarkers are presented by ( a ) type of biomarker, ( b ) highest phase of biomarker development, ( c ) clinical phase of development for clinical biomarkers, and ( d ) according to the biomarker use. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
In May 2022, the US FDA authorized the use of Lumipulse G beta-Amyloid Ratio (1-42/1-40) in vitro diagnostic test for the assessment of beta-amyloid pathology in CSF samples [ 268 ]. The ratio of these two proteins in CSF is indicative of the presence of amyloid plaques. The test is minimally invasive, and it is the first FDA-authorized in vitro diagnostic biomarker for use in individuals being evaluated for AD and other causes of cognitive decline. However, results of the test must be interpreted in conjunction with other patient clinical information. Additionally, Aβ42 levels measured in plasma have been evaluated as a potential biomarker for AD since it is less invasive to sample plasm than CSF. All recommended/approved AD biomarkers for disease diagnosis and prognosis are list in Table 2 .
Highest validity Alzheimer’s disease biomarkers listed according to their biomarker uses in Alzheimer’s disease.
7. Anti-Alzheimer’s Drugs
There are currently 868 anti-Alzheimer’s drugs in different stages of development. However, only 273 drugs are currently under active development by biotech and/or pharma with evidence of active development in the last 6 months, according to Cortellis Drug Discovery Intelligence [ 269 ]. The most effective drugs currently approved for AD management are listed in Table 3 , and they comprise cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the N -methyl-D-aspartate (NMDA) receptor antagonist (glutamate antagonist) memantine [ 41 , 70 ]. All of these drugs offer symptomatic treatments.
Approved symptomatic pharmacological treatments for patients with AD.
AChE: acetylcholine esterase; AD: Alzheimer’s disease; BChE: butyrylcholinesterase; ACh: acetylcholine; and NMDA: N -methyl-D-aspartate.
7.1. Drugs under Active Development
There are currently 273 drugs under active development for the treatment of AD, including small molecules, biotechnology products, peptides, combinations, and herbal materials ( Figure 4 a). The majority of these drugs target Aβ42 precursor protein (24.9%), followed by APP (18.7%), MAPT (10.6%), acetylcholinesterase (AChE) (3.3%), cholinergic receptor muscarinic 1 (CHRM1) (3.3%), NMDA receptor (2.2%), tumor necrosis factor (TNF) (2.2%), 5-hydroxytryptamine receptor 6 (5-HTR6), cholinergic receptor muscarinic 4 (CHRM4) (1.8%), glucagon-like peptide 1 receptor (GLP1R), insulin (1.5%), sigma non-opioid intracellular receptor 1 (SIGMAR1) (1.5%), sodium channel (1.5%), and 5-hydroxytryptamine receptor 4 (5-HTR4) (1.1%). Drug count details are shown in Figure 4 b. Additionally, the top organizations developing these drugs as well as the development status of these drugs are shown in Figure 4 c and Figure 4 d, respectively.

An overview of Alzheimer’s disease drug discovery pipeline under active development by ( a ) drug type, ( b ) top targets, ( c ) top organizations, and ( d ) development status. Under active development, according to Cortellis Drug Discovery Intelligence (CDDI) [ 40 ] database as of 10 October 2022. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
Recently, monoclonal antibodies (mAbs) have revived the hope for AD treatments. Aducanumab, an mAb, targets Aβ aggregates in AD patients’ brains to decrease their formation [ 10 , 277 ]. In 2021, aducanumab was approved for AD and prescribed for individuals with AD-MCI and mild AD dementia [ 10 , 277 ]. It is a humanized recombinant monoclonal antibody to Aβ. In a clinical study on 165 patients, aducanumab demonstrated significant reduction of soluble and insoluble Aβ. Furthermore, aducanumab reduced AD clinical decline measured by Mini-Mental State Examination scores. At 12-month follow-up, cerebral Aβ disappeared from almost 50% of patients diagnosed with mild AD. A Phase III clinical trial on 1638 patients of aducanumab has been terminated due to safety and efficacy issues [ 278 ]. In addition, two monoclonal antibodies, donanemab and lecanemab, are currently under US Food and Drug Administration (FDA) investigations [ 277 , 279 , 280 , 281 ].
The pro-drug of methylene blue, leuco-methylthioninium, is a second-generation tau aggregation inhibitor (TAI) and the only tau-specific agent to undergo Phase III clinical trials. Two Phase III clinical trials were conducted in 2016 to demonstrate the efficacy of different doses of leuco-methylthioninium and to compare the efficacy of monotherapy compared with combination with cholinesterase inhibitors or memantine. A third clinical trial to demonstrate the efficacy of low dose leuco-methylthioninium is still active and recruiting to date [ 282 ]. Anti-tau monoclonal antibody (tau vaccine) is an IgG4 antibody that targets aggregated tau protein. Preclinical and Phase I clinical trial data demonstrated that it was safe and might present a potential agent for treating AD. A 96-week Phase II safety and efficacy trial (453 participants with AD) was conducted. Recruitment has completed (in August 2022), but the final study report is not yet published [ 283 ]. Gosuranemab is a therapeutic mAb for the N-terminal of extracellular tau. Gosuranemab was demonstrated to be safe and effective in a single ascending dose study. Gosuranemab has been investigated through a Phase II clinical trial (654 participants with MCI or mild AD). However, the study was terminated due to lack of efficacy following the placebo-controlled period readout [ 284 ]. Semorinemab is another antibody that targets the extracellular tau. Promising results were concluded from a pilot safety study, and currently, a Phase II clinical trial (272 patients with moderate AD) to investigate cognitive function and functional capacities of patients is still active [ 285 ]. Zagotenemab, an mAb, binds to tau aggregates. Phase I clinical trial (single dose) was conducted on zagotenemab in patients with mild AD [ 277 ].
7.2. Withdrawn, Discontinued, or Suspended Drugs
There are 108 drugs that have been either withdrawn, discontinued, or suspended from use for AD. The majority of these drugs were small molecules, but there were some biotechnology products and few peptides ( Figure 5 a). Many of the drug targets ( Figure 5 b) are similar to drug targets under active development for AD, which may give the impression that those drug targets may not be successful for the treatment of the disease, especially since many of the big pharmaceutical companies have abandoned them, including Pfizer, Sanofi, Lilly, AstraZeneca, and others ( Figure 5 c). Table 4 summarizes the major failures and suggests hypotheses explaining them.

An overview of Alzheimer’s disease drugs that were either suspended, withdrawn, or discontinued by ( a ) drug type, ( b ) top targets, and ( c ) top organizations. Under active development, according to Cortellis Drug Discovery Intelligence (CDDI) [ 40 ] database as of 10 October 2022. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
Most important drug classes that failed as anti-AD treatments in different stages of clinical trials.
8. Exploiting Network Biology Approaches in Alzheimer’s Disease Research
Network biology approaches have been suggested as paradigm-changing approaches for the discovery of disease biomarkers, drug targets, and effective drugs for polygenic multifactorial diseases, including cancer, diabetes, psychological disorders, and AD. However, the typical focus on one single type of omics has been a limiting factor for the success of previous systems biology studies because the findings were explaining only a modest portion of the complex disease, and AD was no exception. Therefore, future studies should study multiple omics data simultaneously and apply new technologies, including machine leaning (ML) and artificial intelligence (AI) to derive novel multi-system and multi-target hypotheses.
8.1. Previous Alzheimer’s Disease Drug Discovery Failures
Misunderstanding of the disease mechanisms coupled with inconsistent drug development protocols that relied on single-target approaches, in addition to the improper management of drug discovery projects, led to the inopportune nomination of drug targets which contributed to many drug failures [ 3 , 286 ]. Additionally, clinical trial design utilized in drug discovery failed due to many reasons, including the delay in initiation of treatments, incorrect drug doses, or lack of good drug-monitoring biomarkers [ 287 , 288 ]. The success rate in progressing AD clinical trials from one phase to the next has been poor, and the number of therapeutic agents approaching FDA approval is low [ 289 ]. Failures in clinical trials might be due to ineffective treatments, drug side effects, or misconducted trials [ 289 ]. The improper selection of methodological parameters in clinical trial design [ 290 ] impeded the success of previous clinical trials [ 287 , 288 , 289 , 291 ]. In fact, the clinical trials dilemma in psychiatry, neurology, and AD has been discussed elsewhere by many researchers [ 292 , 293 , 294 ]. Issues including inaccuracy, incorrectness, and bias hindered clinical trials success [ 295 , 296 ]. Other factors included personal errors, drawbacks in rating scales, and limitations in neuropsychological tests leading to errors regarding the underestimation of the clinical outcome in clinical trials [ 290 , 297 ]. Increasing the number of clinical trials investigating drug effects has been associated with better treatment outcome [ 289 ].
Additionally, limitations in cell-based models to probe neurodegenerative diseases, such as AD, contributes to AD failure treatment [ 298 ]. The complexity of CNS motivates researchers to integrate the molecular basis of neurodegenerative diseases with the unique organization and construction of brain tissue [ 298 ]. This combined approach is displayed via 3D cell models accompanied by microfluidic technology, which are in their early stages and ready for improvement [ 298 ]. Subsequently, this integrative system should enrich the preclinical drug development pipeline [ 298 ]. The biodiversity of AD-drug design and development needs to unify healthcare workers’ and scientists’ efforts [ 289 ]. Other factors that played an important part in the failure of many AD drug development programs were improper diagnostic evaluations, elusive genetic factors, and/or concomitant diseases [ 299 , 300 ].
8.2. Network Biology Approaches Hold the Promise to Revolutionize Alzhiemer’s Disease Research
AD is a complex disease associated with multiple perturbations in biological networks and functional network connectivity that are fundamental for normal physiological function; hence, multi-target treatment approaches seem imperative to treat the disease [ 301 , 302 , 303 ]. Studies have reported that numerous brain functional networks are significantly impaired in AD patients, including the control network (CON), default mode network (DMN), dorsal attention network (DAN), salience network (SAL), and sensory–motor network (SMN) [ 304 ]. In mild AD patients, there is evidence indicating reduced functional network connectivity in the brain is a predisposing factor. Additionally, the DMN is impaired in very mild to mild AD patients, while severe AD patients suffer from disrupted network crosstalk [ 304 ]. Thus, network and systems biology approaches that target multiple disease networks and pathways hold great promise to revolutionize AD drug discovery research.
Furthermore, network biology approaches enable the identification of novel disease biomarkers, including quantitative diagnostic and prognostic biomarkers, imaging, and biochemical tests. Novel validated disease biomarkers could potentially equip AD researchers with the proper tools to accurately differentiate between AD and non-AD dementias, which can positively impact drug discovery efforts, clinical trial design, and patient selection for clinical trials [ 305 ]. There is agreement among scientists [ 1 , 2 , 3 , 4 , 5 , 22 , 306 ] that future AD research should focus on the following: (1) reassessing previous and current prevalent AD pathogenesis hypotheses, (2) identifying effective disease-specific biomarkers, (3) re-evaluating previous disease diagnostic standards, (4) considering new guidelines and procedures for disease control, (5) reorienting drug discovery efforts toward employing approved multi-target approaches and pharmacogenetic hypotheses, (6) updating the managerial requirements for drug design and development, (7) applying pharmacogenomics approaches in biomarker and drug discovery and development, and (8) implementing disease-prevention strategies for susceptible individuals.
8.3. Current Network Biology Efforts
The underlying hypothesis of network medicine has been recruited in the development of multi-target ligands and combination drugs [ 21 ]. The multi-target ligands and combination drugs are considered promising network medicines for challenging and complex diseases [ 307 , 308 ]. Clinical studies showed that multi-target ligands and combined drugs are more effective than single-target drugs in complex diseases treatment, including depression, cancer, and infectious diseases, such as the acquired immunodeficiency syndrome (AIDS) [ 309 , 310 , 311 , 312 ]. Combining donepezil and memantine improve the brain’s cognition function, and patient’s overall status in mild and advanced AD. Additionally, such drugs decrease the rate of clinical decay and are safe and tolerable [ 307 ].
The idea of network medicine is based on the hypothesis that diseases occur due to the disruption of biological networks responsible for homeostasis as a result of activation or deactivation of certain proteins or biochemical reactions, which eventually disturb the balance of normal physiology pathways [ 313 , 314 , 315 ]. Hence, disease networks are complex disease processes that are caused by irregular diverse genes, proteins, and signaling cascades [ 308 ]. Therefore, network medicines intend to restore disrupted disease networks to their default normal physiology status by targeting multiple key effectors in disease pathways [ 308 ].
Recent advances in multi-omics data analysis coupled with advancements in computational chemical biology methods led to better disease understanding. As a result, network medicines have been suggested as potential surrogates for identifying effective treatments for complex diseases, including AD [ 301 , 302 , 303 , 316 ]. Additionally, the application of network approaches to AD research projects has shed light on a crosstalk among diverse signaling pathways involved in AD pathogenesis [ 21 ]. Further work is required to lay the groundwork for the development of the next-generation anti-AD drugs. Furthermore, the diverse disease networks could not be revived through targeting of a single protein and/or signaling cascade because there are numerous active and spare cellular mechanisms in biological systems [ 317 ].
8.4. Multi-Target-Directed Ligands as Network Biology Treatments
The Multi-Target-Directed Ligands (MTDLs) approach is one of the most promising therapeutic interventions for AD patients as well as other complex multifactorial diseases, including cancer, diabetes, and other psychological disorders [ 318 , 319 , 320 ]. The design of MTDL hypothesizes that successful disease-modifying treatments of AD should target systems biology pathways rather than selectively targeting individual proteins or drug targets [ 321 , 322 ]. As such, MTDLs can be defined as drugs and/or technologies designed to interact with more than one target involved in the pathogenesis of a defined disease [ 322 , 323 , 324 , 325 , 326 , 327 , 328 ], surpassing the “one-molecule, one-target” model [ 313 , 319 ]. It has been theorized that potent MTDL should simultaneously target the typical signs of AD, such as the irregular accumulation of Aβ peptides [ 329 , 330 , 331 , 332 , 333 ], tauopathies [ 334 , 335 , 336 , 337 ], and the cholinergic insufficiency in CNS [ 106 , 338 , 339 ]. In addition, the effective MTDL should consider other AD features, such as the oxidative and nitrosative stresses [ 117 , 340 , 341 ], inflammatory response of brain and spinal cord, excitotoxicity [ 342 ], mitochondrial dysfunction [ 327 , 343 , 344 ], aberrances in calcium [ 345 , 346 ] and other metals [ 19 , 267 , 347 ], and irregularities in apolipoproteins [ 348 , 349 ].
It is suggested that the rational design of MTDLs can be achieved by two approaches: (1) drug repurposing, considering drug design methods that take into account the biological fingerprints (or biological spectra) of familiar active drugs against other therapeutic receptors where one or more drugs can modulate several targets [ 269 , 350 , 351 , 352 , 353 ] and (2) fragment-based drug design, which is based on the core structures of active compounds against specific targets to generate a new merged scaffold with dual or multiple activity against two or more targets [ 269 ].
In the first approach, compounds are screened against multiple proteins/drug targets to retrieve hits with the desired biological profiles [ 350 , 351 , 352 ]. The main advantage of this approach is that the investigated compounds are often commercially available and clinically proven to be safe, thus reducing development time and costs [ 269 , 350 , 351 ], and most importantly, the proposed lead might act as a synergistic effector, modulating the disease pathway effectively [ 354 ]. However, the optimization protocol of the biological activity of the lead compound to fit the new disease application has been limited [ 269 , 350 , 351 ]. Therefore, more work is required to improve hit identification and lead optimization. Sometimes the pharmacokinetics properties of the lead hinder the application to new diseases such as AD where drugs have to meet the criteria for CNS drug design [ 355 , 356 ]. The latter, fragment-based, MTDL approach can be designed using three main methods: (1) linking active fragments/compounds using a linker/spacer and keeping known pharmacophoric features [ 269 ], (2) fusing or integrating the active compounds to generate a new chemical entity that shares identical features [ 269 ], and (3) merging/mixing the selected bioactive compounds to yield a scaffold that has the key functionalities of the pharmacophore [ 269 ].
Studies indicated that the major impedance of the MTDL success is the need to maintain or boost the biological activity of the prioritized compounds while preserving drug-like properties [ 40 ]. Many MTDLs may have limitations due to lower selectivity towards some drug targets [ 357 , 358 , 359 , 360 , 361 , 362 ], while drug development efforts focusing on increasing the biological activity of MTDL may increase the risk of drug toxicity [ 357 , 358 , 359 , 360 , 361 , 362 ]. Therefore, MTDLs should be optimized by improving the selectivity towards certain protein targets while reducing drug toxicity [ 359 , 360 , 361 , 362 ]. Additionally, the designed chimeric entities using the fragment approach have higher molecular weights than the parent compounds, which may affect drug-like properties, while at other times, the merging protocol might be a promising solution for developing oral bioavailable drugs [ 363 , 364 , 365 ].
Finally, when considering MTDL, it is crucial to pay special attention to the required physicochemical properties, including pharmacokinetics, pharmacodynamics, hydrophilicity, and hydrophobicity [ 269 ]. MTDL design against neurodegenerative disorders should take into account the drug’s blood–brain barrier permeability [ 355 , 356 ].
8.5. Suggested Disease Biomarkers and Disease Modifying Drugs
Known diagnostic and prognostic biomarkers for AD [ 269 ] significantly enrich pathways involved in inflammation and immune regulation. AD biomarkers can be divided into two groups: 168 EOAD biomarkers [ 154 , 366 ] and 932 LOAD biomarkers [ 367 , 368 ]. There are 69 biomarkers that overlap between EOAD and LOAD: ACO2, ACTB, ACTG1, ADAM10, ADIPOQ, ADRA1A, AIF1, APP, ANG, ACE, APOE, ABCA7, ATP6V1B2, ATP2A2, AURKC, AXL, BACE1, CACNA1G, CD33, CLP1, CLU, CR1, DICER1, DUSP13, DNMBP, FNDC5, GRK5, GBA1, GRN, H3C1, H3C10, H3C11, H3C12, H3C2, H3C3, H3C4, H3C6, H3C7, H3C8, IL1B, IL6, IL6R, KIF5A, HLA-DRA, MTHFR, MAPT, MBP, NSF, NDRG4, NRGN, NCSTN, NSUN2, PAK1, PLD3, PSEN1, RTN3, SLC10A3, SLC12A5, SLC24A4, SORBS2, SORL1, SPARCL1, TCIRG1, TYROBP, TREM2, TNF, YWHAG, VSNL1, and VWA2.
In order to get a better idea of these 69 overlapping biomarkers, we used the compared experiment workflow in Metacore [ 39 ] to compare enrichments results in pathway maps for EOAD and LOAD biomarkers. We found that the top enriched pathway map by common disease biomarkers for EOAD and LOAD is “protein-folding and maturation related to angiotensin system maturation” ( Figure 6 ).

Top enriched pathway map for ‘common’ biomarkers of EOAD and LOAD. The pathway map representing protein-folding and maturation for the angiotensin system maturation. Connections between network objects on the map are referred to as links (or edges). A link identifies an interaction or a logical relation between two nodes. The type of interaction or relation is reflected by an appropriate symbol placed in the middle of the link. B = binding (i.e., physical interaction between molecules); C = cleavage of a protein at a specific site, yielding distinctive peptide fragments and carried out by enzymes or compounds; red arrows = inhibition; green arrows = activation; grey arrows = unspecified action; solid purple arrows = emergence in disease; light violet text box = normal process; pink text box = pathological processes; white text box with blue outline = notes; grey block = reaction; blue block = normal process; pink block = pathological process; starred network objects = groups or complex processes; red thermometers on pathway map = network object is a validated biomarker for AD, according to the Cortellis Drug Discovery Intelligence (CDDI) database [ 40 ]; thermometer 1 for EOAD, and thermometer 2 for LOAD; a pink hexagon with a capital D on the upper left side of the network object indicates an AD biomarker according to MetaCore TM ; a green hexagon on the upper left side of the network object with a capital T indicates that the network object is expressed in the brain. Map generated using MetaCore TM version 21.4. MetaCore TM , a Cortellis™ solution, 14 October 2022, © 2022 Clarivate. All rights reserved.
LOAD biomarkers led to more significant enrichments of immune system and allergic response pathways, apoptosis, tissue remodeling and repair, cell differentiation, cell cycle regulation, and neurofibromatosis [ 366 ], while EOAD led to more significant enrichments of heart failure pathway maps, stem cells, spermatogenesis, lipid biosynthesis regulation, and blood clotting pathways [ 366 ].
9. Artificial Intelligence and Machine Learning Approaches
Machine learning (ML) and artificial intelligence (AI) have been used successfully to extract insight from ‘big’ biological data [ 369 , 370 , 371 ]. Domain expertise from biology, genetics, elderly medicine, psychiatry, psychology, neurology, and neuroscience could be combined with new bioinformatics and statistical analytical tools to gain insight from multi-omics data. Such insight is valuable for providing answers for challenging research questions, and it can be achieved through the use of theoretical modeling [ 372 , 373 ]. In AD research, ML and AI can answer critical questions about combination diagnostic biomarkers, AD patient subgroups, and disease pathogenesis, thus supporting the identification of a personalized treatments for AD patients [ 374 , 375 ]. In fact, the use of AI has been suggested to probe the pathogenesis mechanisms of AD by analyzing big multi-omics data in parallel [ 376 , 377 ]. Additionally, AI has the capability to differentiate AD patients from other patients suffering from non-AD cognition impairment. It can also anticipate the progression from MCI to AD dementia and assign a tailored treatment for each individual patient [ 376 ]. Furthermore, the application of ML and AI approaches to AD research data, can lead to novel hypotheses regarding efficient interventions for AD patients [ 376 ]. AI can also aid in the diagnosis of the early stage of dementia [ 268 ].
Many research efforts focused on utilizing ML and AI approaches to mine data from clinicaltrials.gov records to evaluate anti-AD therapeutics in different stages of clinical development to study their mechanisms of action and important clinical trial characteristics [ 10 , 372 , 378 , 379 , 380 , 381 , 382 ]. AI and ML approaches can lead to important discoveries by learning from the recent advances in clinical trials and anti-Alzheimer’s drug development pipelines [ 383 , 384 , 385 , 386 , 387 , 388 ]. Complex AI-based models could be exploited to inform researchers and health care providers about diverse disease etiologies, effective diagnostic biomarkers [ 375 ], and individualized treatments based on network biology approaches [ 389 , 390 ].
10. Exploring Epigenetic Treatments
Studies showed that DNA methylation/hydroxymethylation is dysregulated in AD patients prior the onset of clinical symptoms [ 11 ]. These were presented in a prospective study on autopsied brains, as level of methylation, in terms of 5mC levels, in presymptomatic patients is similar to those with AD patients [ 189 ]. The levels of 5mC, 5hmC, and ten–eleven translocation 1 (TET1) proteins were elevated in preclinical AD patients and AD patients compared with the control group [ 171 , 211 ]. Although further validation is required, DNA methylation/hydroxymethylation may be used as a biomarker for AD diagnosis [ 11 ].
Histone modifications, particularly acetylation, deacetylation, and methylation dysregulation, play a role AD pathogenesis [ 11 ]. HDACs are highly expressed in patients with AD [ 171 , 218 ], affecting learning, memory, and cognition; hence, HDAC inhibitors (HDACi) are considered a potential treatment option [ 391 ]. Studies on AD patients showing low histone acetylation were reported [ 211 , 224 , 225 ], allowing the potential use of histone acetyltransferases (HATs) [ 211 ]. Increased levels of histone methylation and histone methyltransferase enzyme mRNA were reported in postmortem brains of AD patients [ 11 , 218 ]. Although the loss of histone methyltransferase function would affect learning capabilities in AD patients [ 11 ], the use of partial histone methyltransferase inhibitors [ 211 ] would restore the balance between histone methylation and demethylation in patients with AD to maintain brain integrity and memory [ 230 ]. Inhibitor of histone acetyltransferases (INHAT) is reported to bind to histones and block their access to HATs [ 392 ]. Studies showed that ANP32A, which is a component of INHAT and inhibitor of protein phosphatase-2A, is upregulated in AD patients [ 393 , 394 ]. In an in vivo study, the down regulation of ANP32A would reduce INHAT formation and allow for histone acetylation [ 395 ]. Collectively, drugs from those classes would comprise potential therapeutic options for AD treatment.
HDACi are considered to be non-selective [ 220 ], but they are beneficial, as they reduce AD hallmarks [ 225 ]. The use of HDACi that selectively inhibits HDAC2 and HDAC3 would improve cognition, in contrast to inhibiting HDAC1 that would result in neurotoxicity [ 11 , 225 ]. HDAC6 selective inhibitors were also shown to have neuroprotective effects [ 228 , 229 ]. Sirtuins, which are a class of HDACs, contribute to AD pathogenesis and selective inhibitors would also be beneficial [ 227 , 229 ]. Although some HATs showed better response than non-selective HDACi, their low membrane permeability and solubility limit their use in AD treatment [ 11 ].
The miRNAs are responsible for the regulation of gene expression through post-transcriptional gene silencing [ 396 ]. In relation to AD, several studies summarized by Nikolac Perkovic et al., 2021 [ 11 ] showed that miRNAs would be either downregulated or upregulated, altering proteins and enzymes expression responsible for AD pathology. Hence, the use of miRNA mimics to downregulate the expression of genes or proteins [ 397 ] or anti-miRNA therapies to alter the function of a specific miRNA [ 398 ] are also considered potential treatment options for AD patients.
11. Genetic Treatments
Targeting genetic alterations in AD patients and consequent gene editing and correction is another potential treatment strategy. These include the use of programmable nucleases, such as zinc finger proteins (ZFP), transcription activator-like effectors (TALE), and RNA-guided clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) [ 399 ]. The latter showed more promising results for AD treatment and other neurological diseases than did ZFP and TALE [ 400 , 401 , 402 ]. The presence of the mutant Cas9 protein, dead Cas9 (dCas9), advanced the CRISPR/Cas9 editing tool, resulting in the emergence of CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) technologies, in which dCas9 is fused or interacts with transcriptional repressors or activators, respectively [ 403 ]. With regard to epigenetics, AD, and dCas9 protein, studies showed promising results with targeting histone demethylase [ 404 ], histone acetyltransferase [ 405 ], and histone methyltransferases [ 406 , 407 ].
12. Non-Pharmacological Treatment Options and Preventive Measures
Non-pharmacological treatments encompass several recommendations for various lifestyle modifications, including physical and social activity, tobacco cessation, alcohol consumption, weight management, nutrition, and regular exercise. Other interventions include underlying-disease management (e.g., hypertension, diabetes, dyslipidemia, depression, and hearing loss), as stated in WHO guidelines [ 408 ]. More studies should assess the relationship between vaccines and AD; it was found that flu vaccines reduce the risk of AD development [ 409 ]. However, the protective mechanisms have not yet been elucidated.
Aberrations in the ecosystem of microbiome have been implicated in diverse gastrointestinal and metabolic dysfunction, such as diabetes, insulin resistance, obesity, and inflammatory bowel disease [ 410 ]. In addition, studies showed that changes in gut microbiome is associated with neurological disorders, such as multiple sclerosis (MS), autism, and Parkinson’s disease [ 411 , 412 , 413 ]. Studies recorded a decrease in microbial diversity in gut microbiome of AD patients [ 414 , 415 , 416 , 417 , 418 , 419 , 420 , 421 ]. Further studies in rats suggested that alterations in gut microbiome might proceed Aβ deposition [ 422 ].
13. Special Considerations for Clinical Trials
Aspects to be considered when designing a clinical trial include trial rationale, outcomes of interest, statistical analysis design, sample size and recruitment, and interim monitoring [ 423 ]. Common clinical trial designs include single-arm trials, placebo-controlled trials, crossover trials, and factorial trials [ 424 ]. In AD-related clinical trials, infrastructure and technology, cultures and linguistics, regulatory and reimbursement issues, academia and industry harmonization, availability, and access were considered to be the ultimate challenges that limit the conducting of successful clinical trials [ 425 ].
According to NLM’s ClinicalTrials.gov Beta (beta.clinicaltrials.gov), 109 clinical trials related to AD were terminated in the last ten years [ 19 ]. AD clinical trials were terminated due to the following reasons: unavailability of further funding, halted visits due to COVID-19, feasibility of enrolment, safety issues, slow recruitment of eligible participants (patients), inappropriate study design to achieve the trial’s endpoint, new safety or efficacy data from other studies, unfavourable risk–benefit ratio, and inappropriate dosage settings. Yet, patient recruitment remains the ultimate determinant in AD clinical trials.
Therefore, there is a need for new and advanced clinical trials designs to accelerate passage through the legal authorities’ requirements to register new promising molecules for treatment and/or prevention of AD. However, new investigation approaches need to be fully validated before they can be implemented in clinical trials [ 426 ].
14. Conclusions
AD is a multifactorial and polygenetic disease. Novel disease diagnostic biomarkers and disease-modifying treatments are required to halt or slow the onset and disease progression, decrease behavioral aberrations, and ameliorate cognition in AD patients. The recent advances in network biology approaches coupled with the advances in clinical trial design and protocols, in additional to the availability of powerful machine learning and artificial intelligence algorithms, hold promise to identify novel diagnostic biomarkers, better drug targets, and effective disease-modifying drugs. Herein, we provide a comprehensive review on Alzheimer’s disease highlighting the mainstream hypotheses explaining disease pathophysiology as well as current disease treatments and drug discovery projects. We also emphasize the recent scientific evidence implicating epigenetic mechanisms and the microbiome in AD pathogenesis and progression. We suggest that the application of Al and ML approaches in analyzing AD network biology derived from AD data, including genetic, transcriptomic, epigenetic, and metagenomic data would revolutionize our understanding of the disease pathways and will lead to the discovery of novel biomarkers and drug targets. Ultimately, these studies will increase our chances of identifying validated diagnostic biomarkers and effective disease-modifying cures. Hence, breakthrough discoveries in AD research are more likely to occur in the near future. This review provides a summary of the current hypotheses regarding AD pathogenesis in addition to the most recent advances in the search of effective disease biomarkers and drug targets. This review also details AD drugs in various stages of development and highlights technologies that are expected to accelerate AD drug and biomarker discoveries.
Acknowledgments
The authors acknowledge funding from the Deanship of Scientific Research at Al-Zaytoonah University of Jordan (Grant number 2020-2019/17/03).
Funding Statement
R.H. and D.A.S. acknowledge support from the Deanship of Scientific Research at Al-Zaytoonah University of Jordan (Grant number 2020-2019/17/03).
Author Contributions
Conceptualization, R.H.; software, R.H.; formal analysis, R.H.; investigation, R.H., D.A.S., O.H.A. and A.Q.A.B.; resources, R.H., D.A.S., O.H.A. and A.Q.A.B.; biomarker data curation, R.H.; writing—original draft preparation, R.H., D.A.S., O.H.A. and A.Q.A.B.; writing—review and editing, R.H., D.A.S., O.H.A. and A.Q.A.B.; visualization, R.H.; supervision, R.H.; project administration, R.H.; funding acquisition, R.H. and D.A.S. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Study Suggests Treatments that Unleash Immune Cells in the Brain Could Help Combat Alzheimer’s
Posted on April 25th, 2024 by Dr. Monica M. Bertagnolli
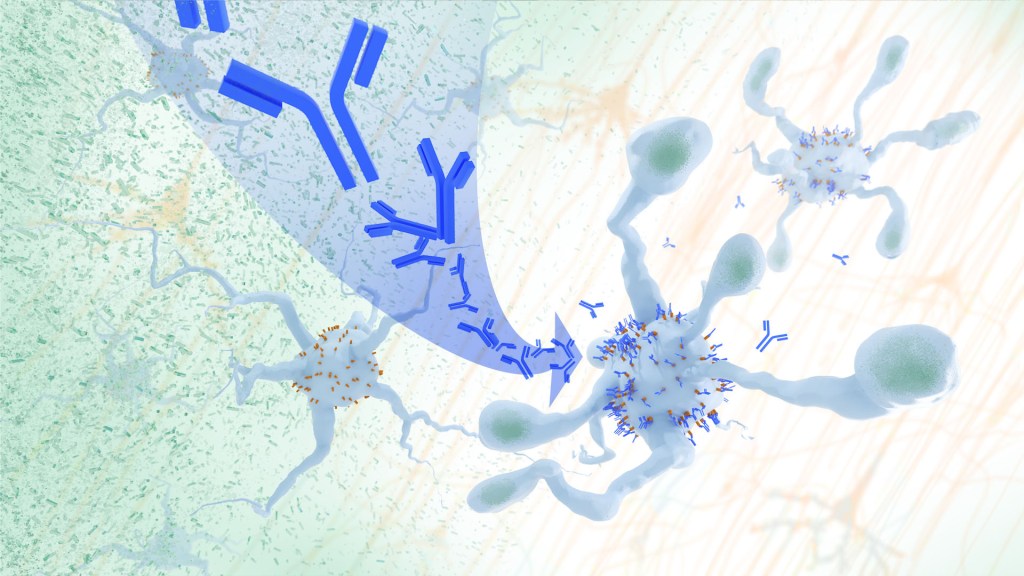
In Alzheimer’s disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place. In fact, the Food and Drug Administration recently approved the first drug for early Alzheimer’s that moderately slows cognitive decline by reducing amyloid plaques. 1 Still, more progress is needed to combat this devastating disease that as many as 6.7 million Americans were living with in 2023.
Recent findings from a study in mice, supported in part by NIH and reported in Science Translational Medicine , offer another potential way to clear amyloid plaques in the brain. The key component of this strategy is using the brain’s built-in cleanup crew for amyloid plaques and other waste products: immune cells known as microglia that naturally help to limit the progression of Alzheimer’s. The findings suggest it may be possible to develop immunotherapies—treatments that use the body’s immune system to fight disease—to activate microglia in the brains of people with Alzheimer’s and clear amyloid plaques more effectively. 2
In their report, the research team—including Marco Colonna , Washington University School of Medicine in St. Louis, and Jinchao Hou, now at Children’s Hospital of Zhejiang University School of Medicine in Zhejiang Province, China—wrote that microglia in the brain surround plaques to create a barrier that controls their spread. Microglia can also destroy amyloid plaques directly. But how microglia work in the brain depends on a fine-tuned balance of signals that activate or inhibit them. In people with Alzheimer’s, microglia don’t do their job well enough.
The researchers suspected this might have something to do with a protein called apolipoprotein E (APOE). This protein normally helps carry cholesterol and other fats in the bloodstream. But the gene encoding the protein is known for its role in influencing a person’s risk for developing Alzheimer’s, and in the Alzheimer’s brain, the protein is a key component of amyloid plaques. The protein can also inactivate microglia by binding to a receptor called LILRB4 found on the immune cells’ surfaces.
Earlier studies in mouse models of Alzheimer’s showed that the LILRB4 receptor is expressed at high levels in microglia when amyloid plaques build up. This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer’s. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer’s.
To do this, the researchers first studied brain tissue samples from people who died with this disease and discovered unusually high amounts of the LILRB4 receptor on the surfaces of microglia, similar to what had been seen in the mouse models. This could help explain why microglia struggle to control amyloid plaques in the Alzheimer’s brain.
Next, the researchers conducted studies of mouse brains with accumulating amyloid plaques that express the LILRB4 receptor to see if an antibody targeting the receptor could lower amyloid levels by boosting activity of immune microglia. Their findings suggest that the antibody treatment blocked the interaction between APOE proteins and LILRB4 receptors and enabled microglia to clear amyloid plaques. Intriguingly, the team’s additional studies found that this clearing process also changed the animals’ behavior, making them less likely to take risks. That’s important because people with Alzheimer’s may engage in risky behaviors as they lack memories of earlier experiences that they could use to make decisions.
There’s plenty more to learn. For instance, the researchers don’t know yet whether this approach will affect the tau protein , which forms damaging tangles inside neurons in the Alzheimer’s brain. They also want to investigate whether this strategy of clearing amyloid plaques might come with other health risks.
But overall, these findings add to evidence that immunotherapies of this kind could be a promising way to treat Alzheimer’s. This strategy may also have implications for treating other neurodegenerative conditions characterized by toxic debris in the brain, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. The hope is that this kind of research will ultimately lead to more effective treatments for Alzheimer’s and other conditions affecting the brain.
References:
[1] FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval . U.S. Food and Drug Administration (2023).
[2] Hou J, et al . Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model . Science Translational Medicine . DOI: 10.1126/scitranslmed.adj9052 (2024).
NIH Support: National Institute of General Medical Sciences, National Institute on Aging
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Health , News , Science
Tags: Alzheimer's treatment , Alzheimer’s disease , amyloid , basic research , brain , microglia , neurons , neuroscience
One Comment
so, whats next? is it available? how to obtain this??
Leave a Comment Cancel reply
@nihdirector on x, nih on social media.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How old is too old to run?

America’s graying. We need to change the way we think about age.

Can we talk?
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.
“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this
Newly identified genetic variant protects against Alzheimer’s
Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.
No such thing, specialist says — but when your body is trying to tell you something, listen

Experts say instead of disability, focus needs to shift to ability, health, with greater participation, economically and socially

Study finds that conversation – even online – could be an effective strategy to help prevent cognitive decline and dementia
When math is the dream
Dora Woodruff was drawn to beauty of numbers as child. Next up: Ph.D. at MIT.
Seem like Lyme disease risk is getting worse? It is.
The risk of Lyme disease has increased due to climate change and warmer temperature. A rheumatologist offers advice on how to best avoid ticks while going outdoors.
Three will receive 2024 Harvard Medal
In recognition of their extraordinary service
As populations age, Alzheimer’s and dementia are becoming more prevalent. A new drug could offer hope

As populations age, the number of cases of dementia rises. Image: Unsplash/centelm
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Charlotte Edmond

.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Global Health is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, global health.
Listen to the article
- A new drug, lecanemab, has been shown to reduce the decline in memory and thinking associated with Alzheimer's.
- As populations age, dementia cases are on the rise, with 10 million new people diagnosed each year.
- Dementia is a collective term for a group of diseases or brain injuries that can lead to a change in cognitive functioning as well as other symptoms like lack of emotional control.
It is one of the biggest diseases of our time: 10 million new cases of dementia are diagnosed every year, according to the World Health Organization (WHO). More than 55 million people worldwide live with a form of dementia and it is the seventh leading cause of death among all diseases.
Now a new drug is offering a glimmer of hope after years of searching for a treatment. In clinical trials, lecanemab has been shown to slow the cognitive decline associated with the disease. The drug attacks the protein clumps in the brain that many think are the cause of the disease.
Although dementia patients are currently offered drugs, none of them affect the progression of the disease which is why scientists in the field are so excited about this latest development. Alzheimer's Research UK called the findings "a major step forwards" .
But while this is undoubtedly positive news, the body also points out that the benefits of the drug were small and came with significant side effects. In addition, lecanemab has been proven to work in the early stages of the disease, so would rely on doctors spotting it before it had progressed too far.
With the number of dementia cases expected to rise to 78 million by 2030 and 139 million in 2050, according to the WHO, the race is on for scientific developments and research that will help us understand, treat and possibly prevent the disease.
A global impact
As populations age, the number of cases of dementia rises. While the deterioration of cognitive functioning is not caused by age itself, it does primarily affect the older generation. For many elderly people it also results in disability and loss of independence - which can have psychological, social and economic implications for them and their families, carers and society more broadly.
The estimated global cost of dementia to society was placed at $1.3 trillion in 2019, and is expected to rise to $2.8 trillion by 2030, WHO says.
Alzheimer’s Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
To catalyse the fight against Alzheimer's, the World Economic Forum is partnering with the Global CEO Initiative (CEOi) to form a coalition of public and private stakeholders – including pharmaceutical manufacturers, biotech companies, governments, international organizations, foundations and research agencies.
The initiative aims to advance pre-clinical research to advance the understanding of the disease, attract more capital by lowering the risks to investment in biomarkers, develop standing clinical trial platforms, and advance healthcare system readiness in the fields of detection, diagnosis, infrastructure and access.
What is dementia?
Dementia is a collective term for a group of diseases or injuries which primarily or secondarily affect the brain. Alzheimer’s is the most common of these and accounts for around 60-70% of cases. Other types include vascular dementia , dementia with Lewy bodies (abnormal protein clumps) and a group of diseases that contribute to frontotemporal dementia. It can also be triggered by strokes, excessive use of alcohol, repetitive head injuries, nutritional deficiencies, or follow some infections like HIV, the Alzheimer’s Society explains.
The different forms of dementia can often be indistinct and can co-exist.
Different people are affected in different ways, depending on the underlying cause. But the syndrome is usually progressive and can affect a range of functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language and judgement.
Changes in mood and ability to control emotions often accompany these cognitive variations.

Can it be treated?
There is no cure for dementia, although there are numerous treatments being worked on and at clinical trial phase. Dementia care currently focuses on early diagnosis, optimizing health and wellbeing and providing long-term support to carers.
Besides age, there are a number of other risk factors, which if avoided, can decrease the chances of dementia and slow its progression. Preventative steps include being physically active, not smoking, avoiding the harmful use of alcohol, as well as maintaining a healthy diet, weight, blood pressure, cholesterol and blood sugar levels.
Other risk factors associated with dementia include depression, social isolation, low educational attainment, cognitive inactivity and even air pollution .

What is the impact?
People with dementia rely heavily on informal care - i.e., friends and family. These carers spent on average five hours a day looking after people living with dementia in 2019, according to WHO figures. Informal care is thought to cover half of the overall financial burden of dementia.
There is also a disproportionate impact on women. They account for 65% of all dementia-related deaths, and also have a greater number of years affected by the disease. Women also typically provide the majority of informal care - covering over two-thirds of the carer hours for people living with dementia.

What are the latest developments?
The fact that dementia is only diagnosed once symptoms appear means that by the time people take part in clinical trials the disease is often quite well advanced. This can hamper the development of drugs. However, research analyzing data from the UK Biobank has indicated there are a collection of signals that could indicate a problem years before dementia is currently being diagnosed .
Other scientists postulate that, rather than being a disease of the brain, Alzheimer’s is in fact a disorder of the immune system within the brain . They believe research should instead focus on drugs targeting auto-immune pathways .
On a less positive note, researchers found that people who have recently received a dementia diagnosis, or diagnosed with the condition at a younger age, are at an increased risk of suicide . This underlines the importance of a strong support network, particularly among those newly diagnosed.
Have you read?
Here’s how exercise alters our brain chemistry – and could prevent dementia, dementia cases are expected to double in many countries over the next 30 years, these 7 simple habits could halve your risk of dementia, don't miss any update on this topic.
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} weekly.
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Wellbeing and Mental Health .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

From 'Quit-Tok' to proximity bias, here are 11 buzzwords from the world of hybrid work
Kate Whiting
April 17, 2024

Young people are becoming unhappier, a new report finds

Promoting healthy habit formation is key to improving public health. Here's why
Adrian Gore
April 15, 2024

What's 'biophilic design' and how can it benefit neurodivergent people?
Fatemeh Aminpour, Ilan Katz and Jennifer Skattebol

A generation adrift: Why young people are less happy and what we can do about it
Andrew Moose and Ruma Bhargava
April 5, 2024

The six drivers of employee health every employer should know
Jacqueline Brassey, Lars Hartenstein, Patrick Simon and Barbara Jeffery
April 3, 2024
Innovative microscopy demystifies metabolism of Alzheimer's
Alzheimer's disease causes significant problems with memory, thinking and behavior and is the most common form of dementia, affecting more than 50 million people around the world each year. This number is expected to triple by the year 2050.
Using their own state-of-the art imaging technologies, scientists at the University of California San Diego have now revealed how the metabolism of lipids, a class of molecule that includes fats, oils and many hormones, is changed in Alzheimer's disease. They also revealed a new strategy to target this metabolic system with new and existing drugs. The findings are published in Cell Metabolism .
"Lipids have been associated with Alzheimer's for as long as we've known about the disease," said senior and co-corresponding author Xu Chen, Ph.D., an assistant professor in the Department of Neurosciences at UC San Diego School of Medicine, referring to the original 1907 report by Alois Alzheimer that described the unusual presence of fat deposits in the brain of the first person to be diagnosed with the disease. "So much of the emphasis since then has been placed on tau and other proteins that the research community has, until the last decade or so, largely overlooked this important aspect of the disease."
"Driven by a keen interest in lipid droplet functions in aging and disease, we initiated this fruitful collaboration to harness cutting-edge SRS technology for studying lipid metabolism in tauopathy mouse brains." Said Yajuan Li, M.D., Ph.D., a postdoctoral researcher in the Shu Chien-Gene Lay Department of Bioengineering at UC San Diego Jacobs School of Engineering. SRS imaging is an approach that analyzes the way molecules in a sample interact with laser light.
In the brain, lipids come in the form of tiny droplets that control a variety of processes, such as energy storage and cellular responses to stress. These processes are tightly regulated in typical brains, but in Alzheimer's or similar diseases, lipid droplet metabolism can malfunction. While scientists understand that there is a relationship between Alzheimer's and lipid metabolism, exactly how they influence one another has remained a mystery.
To answer this question, the team looked directly at lipid droplets in the brains of mice with excess tau protein. They used a state-of-the-art SRS imaging platform developed in Lingyan Shi's lab at the Jacobs School of Engineering. The platform makes it possible to take microscopic images of lipid droplets within cells without the use chemical dyes, which can alter the delicate molecules and compromise the results.
"Intriguingly, the inert lipid droplets observed in tauopathy brains exhibit similar behavior to those found in aging brains," said co-corresponding author Lingyan Shi, Ph.D., assistant professor of bioengineering at the Jacobs School. "We are now focusing on understanding the underlying mechanisms by combing SRS imaging with other utilizing multidisciplinary techniques. Our approach is biologically neutral, so we're able to observe what's happening in the brain at the molecular level with as little interference as possible."
Shi and her team, including Li, pioneered the new approach, which uses a specially modified version of water, called heavy water, as a metabolic probe.
"Instead of using a typical chemical dye to stain lipids, we use heavy water that is naturally participating in the metabolic activities we're interested in," added Shi. "This gives us a much clearer picture of how lipids are formed spatiotemporally, which would not be possible with other approaches. Our current focus is on comprehending the underlying mechanisms of these dynamic changes of lipid metabolism in the context of aging and diseases."
The researchers discovered that in brains with tauopathy, neurons accumulate excess lipids as a result of stress or damage. This influx forces neurons to offload the excess to immune cells in the brain, called microglia. These microglia then mount an inflammatory response that causes further stress to neurons, triggering a repeating and worsening cycle.
In addition to characterizing this process, they were also able to identify a critical enzyme, called adenosine monophosphate-activated protein kinase (AMPK) that orchestrates the cycle. According to the researchers, breaking this cycle could unlock new treatment options for Alzheimer's disease. Chen is particularly optimistic about the possibility of repurposing existing drugs that modify lipid metabolism.
"We don't think this is an incidental phenomenon," said Chen. "The evidence suggests that lipid metabolism is a driving mechanism for Alzheimer's disease. There are many drugs that target lipid metabolism in other body systems, such as in the liver, so we might be able to change this system quite dramatically using tools we already have."
- Alzheimer's Research
- Healthy Aging
- Gene Therapy
- Diseases and Conditions
- Alzheimer's
- Disorders and Syndromes
- Alzheimer's disease
- Dementia with Lewy bodies
- Confocal laser scanning microscopy
- Transmission electron microscopy
- Psychotherapy
- Urinary incontinence
- Erectile dysfunction
- Biological tissue
Story Source:
Materials provided by University of California - San Diego . Original written by Miles Martin. Note: Content may be edited for style and length.
Journal Reference :
- Yajuan Li, Daniel Munoz-Mayorga, Yuhang Nie, Ningxin Kang, Yuren Tao, Jessica Lagerwall, Carla Pernaci, Genevieve Curtin, Nicole G. Coufal, Jerome Mertens, Lingyan Shi, Xu Chen. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK . Cell Metabolism , 2024; DOI: 10.1016/j.cmet.2024.03.014
Cite This Page :
Explore More
- Mice Given Mouse-Rat Brains Can Smell Again
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
Trending Topics
Strange & offbeat.
Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.
Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
In 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (Leqembi) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
A phase 3 clinical trial found that the medicine slowed cognitive decline in people with early Alzheimer's disease. The medicine prevents amyloid plaques in the brain from clumping. The phase 3 trial was the largest so far to study whether clearing clumps of amyloid plaques from the brain can slow the disease.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Also, people taking lecanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. It also recommends being monitored with brain MRI s during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends being tested for this gene before starting treatment with lecanemab.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your health care professional before taking lecanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab. Other research is looking at how effective lecanemab may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
Another medicine being studied is donanemab. It targets and reduces amyloid plaques and tau proteins. It was found to slow declines in thinking and functioning in people with early Alzheimer's disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.

Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Alzheimer's drug development pipeline: Positive results, new insight on biomarkers position 2024 as 'learning year'
by Steven Slivka, John Domol, University of Nevada, Las Vegas

The world of Alzheimer's treatments is at an inflection point as more potential drugs make their way out of clinical trials.
On the heels of newly FDA-approved drugs Aduhelm (aducanumab) in 2021 and Leqembi (lecanemab) in 2023, a UNLV researcher says that 2024 is a "learning year" for Alzheimer's drug development.
"There are a large number of drugs in the pipeline that have very diverse actions on the brain," said Dr. Jeffrey Cummings, a leading Alzheimer's clinician-scientist and research professor in UNLV's School of Integrated Health Sciences.
Cummings leads the Alzheimer's drug development observatory within UNLV's Department of Brain Health, a robust database of all clinical trials results he began in 2016. It is the only observatory of its kind in the world.
While nearly 7 million Americans are living with Alzheimer's disease and are in need of better treatments, Cummings remains encouraged by the results of this year's pipeline developments—including the recent inclusion of biomarkers, which are often found in blood and signal normal or abnormal processes, or a condition or disease. Alzheimer's biomarkers can now be measured in the blood.
"Most of our biomarkers for Alzheimer's and dementia have been discovered only within the past three years. They provide information on the drug's impact," he said. "Biomarkers will be featured in nearly all clinical trials moving forward to guide the process."
Biomarker studies highlight current treatment pipeline
Cummings's annual report is featured in Alzheimer's & Dementia: Translational Research & Clinical Interventions . According to this year's data, there are 164 active trials and 127 unique treatments, a roughly 10% decrease from the previous year that saw a record-high 187 active trials and 141 unique treatments.
Some of this year's results include:
- 76% are disease-modifying treatments that aim to slow the decline of memory
- 34% are biological therapies given intravenously or through some other injection
- 12% are cognitive enhancing agents that are intended to improve memory
- 13% are drugs for behavioral symptoms, such as agitation
- 31% are repurposed agents approved for other diseases, such as cancer or Parkinson's disease
"One prediction we can make with confidence is that we should be prepared for more complex biological therapies that require intravenous infusion and vigilant monitoring for side effects; more like cancer therapies," Cummings said.
Cummings attributes the decrease in overall clinical trials and unique treatments to a lack of funding on the federal level and from less private investment from the biopharmaceutical industry.
"Simply put, we need more investments from the government and from the pharmaceutical companies to fight this trend of declining clinical trials," Cummings said, adding that the purpose of his annual Alzheimer's drug development pipeline report is to keep policymakers, health care systems, and patients apprised of the updates taking place among Alzheimer's clinical trials.
A lack of participants for clinical trials has also inhibited results, as nearly 51,400 participants are needed for all active trials. Still, Cummings said the newest Alzheimer's treatments in the pipeline, including results from 35 phase 2 trials and 12 from phase 3—the final phase before FDA review—will offer critical information as newer drugs continue to be developed.
"Eight of the drugs with reported data from phase 2 for this year are all anti-inflammatory drugs and the biomarkers included in the trials will allow us to dissect the importance of the individual aspects of inflammation," he added. He said that mounting results from multiple drugs focusing on one target will lead to new and potentially helpful information that could advance multiple related trials.
"The pipeline of potential new Alzheimer's treatments is diverse, offering a future with new, safe, and effective treatments and powerful combination therapies," said Maria C. Carrillo, Ph.D., Alzheimer's Association chief science officer. "The recent FDA approvals for Alzheimer's, and the diversity of the pipeline, provide hope to those impacted by this devastating disease."
"The Alzheimer's Association has long been committed to advancing all evidence-based potential treatment avenues, and to combining diverse approaches," Carrillo said.
Hopeful for the future
In addition to the FDA-approved Aduhelm and Leqembi, Cummings is confident that the FDA will approve the use of donanemab, which yielded positive results in its clinical trials before FDA review was halted for further evaluation.
"Development successes are emerging, which is why I think we are experiencing such a breakthrough in the world of Alzheimer's treatment," he said. "It takes a decade to advance a drug from phase 1 to phase 3 and then nearly two more years for FDA review, so these processes take time. We know that most drugs fail, but not all of them. Even drugs that fail in the clinical trial can still tell us a lot."
Explore further
Feedback to editors
Blood samples enhance B-cell lymphoma diagnostics and prognosis, study shows
10 minutes ago

Pandemic fatigue and vaccine hesitancy continue to affect global public health, new 23-country study finds
12 minutes ago

Experimental drug shields pancreas from type 1 diabetes attack
21 minutes ago

Study suggests that stevia is the most brain-compatible sugar substitute
22 minutes ago
Scientists develop new organoid model to study thymus function
34 minutes ago

Cancer screening rates significantly lower in US federally qualified health centers, study finds
42 minutes ago
Study uncovers the mechanism that avoids conflicts in the activity of brain stem cells
Scientists discover key bacterium that maintains protective intestinal mucus barrier under low-fiber diet

Macaque study sheds light on brain's perception of static images
After 25 years, researchers uncover genetic cause of rare neurological disease
6 hours ago
Related Stories

Alzheimer's drug development pipeline: Promising therapies, pharma investment drive momentum in clinical trials
May 25, 2023

Nonprofits, federal government surpass pharma to lead Alzheimer's drug development
May 25, 2021
Alzheimer's drug gantenerumab fails to slow decline in phase III clinical trial
Nov 15, 2022
Cleveland Clinic releases fourth installment of Alzheimer's Disease Drug Pipeline Report
Jul 10, 2019

Leaders in Alzheimer's research herald the dawn of a new era in drug development based on biology of aging
Jul 6, 2022

Biogen pulls controversial Alzheimer's drug Aduhelm
Jan 31, 2024
Recommended for you

Clinical trial evaluates azithromycin for preventing chronic lung disease in premature babies
Apr 26, 2024

Study finds RNA modification is responsible for disruption of mitochondrial protein synthesis in Alzheimer's disease
Apr 25, 2024

Targeting specific protein regions offers a new treatment approach in medulloblastoma

Nanomaterial that mimics proteins could be basis for new neurodegenerative disease treatments

Treatment for deadly superbug C. diff may be weakening

National trial safely scales back prescribing of a powerful antipsychotic for the elderly
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Search Menu
- CNS Injury and Stroke
- Epilepsy and Sleep
- Movement Disorders
- Multiple Sclerosis/Neuroinflammation
- Neuro-oncology
- Neurodegeneration - Cellular & Molecular
- Neuromuscular Disease
- Neuropsychiatry
- Pain and Headache
- Advance articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Why publish with this journal?
- Open Access
- About Brain
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Alzheimer's Collection 2024
Research in Alzheimer’s disease has undergone a rapid transformation over the last few years. Here we bring together some of the exciting new papers published in Brain on this topic. They span commentaries and reviews on anti-amyloid therapies and their potential side effects, through to molecular, genetic, microbiological, imaging and focused ultrasound studies. We invite our readers to enjoy some of the most stimulating and provocative papers on the neuroscience and neurology of Alzheimer’s disease.
Commentaries and reviews

Original papers

Interested in publishing your research?
Brain has published landmark papers in both clinical neurology and translational neuroscience since 1878 and is committed to increasing its scope: from studies that illuminate mechanisms of disease to novel clinical trials for brain disorders. With an easy submission system, a distinguished editorial team, and fast publication speed, make Brain the home for your research.
- Find out more reasons you should publish with Brain .
- Read our author guidelines to find out more about the submissions process.
- Submit your manuscript using our online submission system.
- Visit the author hub to read our tips on the publishing process.
- Contact the editorial office
- Guarantors of Brain
- Recommend to your Library
Affiliations
- Online ISSN 1460-2156
- Print ISSN 0006-8950
- Copyright © 2024 Guarantors of Brain
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
Earlier Diagnosis
- Part the Cloud
- Research Momentum
Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
Can Alzheimer's Disease Be Prevented?
Brain donation.
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
Any Given Moment
New ideas study.
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council
Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

Make an Impact in the Fight to End Alzheimer's
Invest in big, bold ideas with part the cloud.
Be part of an important effort to supercharge the development of genetic-based Alzheimer's therapies. Join the Gene Targeting Challenge.
Research and Progress
This is a time of unprecedented promise in the quest to end alzheimer’s..
Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research – all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer’s disease.
Promising Research and Treatments
In the century since Alzheimer’s was first described, scientists have made remarkable strides in understanding the disease.
What if we could diagnose Alzheimer's before symptoms started? Research around earlier diagnosis is among the most active areas in Alzheimer's science.
While Alzheimer's prevention has no definitive answers yet, research has shown that we can take action to reduce our risk of developing it.
Play a Role in Progress
A donation today supports vital research toward methods of treatment, prevention and ultimately, a cure.
Both healthy brains and brains affected by diseases such as Alzheimer’s are needed to help advance dementia research. Learn about the donation process.
Clinical Trials
Don’t just hope for a cure, help us find one. Participating in a clinical trial, whether you’re living with the disease or not, can make a big difference.
The New IDEAS Study is designed to evaluate the use of PET scans in diverse populations living with dementia and Alzheimer's.
U.S. POINTER
U.S. POINTER is a lifestyle intervention clinical trial to support brain health and prevent cognitive decline. Learn about study locations and eligibility.
Advancing Research Around the Globe
The Alzheimer's Association is the world's largest nonprofit funder of Alzheimer's research, currently investing more than $405 million in over 1,100 active best-of-field projects in 56 countries, spanning six continents.
Our commitment to accelerating the global effort to eliminate Alzheimer's disease and other dementias is at the core of all we do.
Through our many initiatives and worldwide reach, the Alzheimer’s Association leads the charge in Alzheimer’s care, support, research and advocacy.
Discover how the Alzheimer’s Association connects scientists and fuels diverse research in an effort to change the trajectory of the disease.
The Alzheimer’s Association is an unrelenting advocate for public policies that increase critical research and support for all those affected.
Keep Up With Alzheimer’s News and Events
The first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one. Volunteer for a clinical trial.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 19 April 2024
The advent of Alzheimer treatments will change the trajectory of human aging
- Dennis J. Selkoe ORCID: orcid.org/0000-0001-8846-9767 1
Nature Aging volume 4 , pages 453–463 ( 2024 ) Cite this article
500 Accesses
25 Altmetric
Metrics details
- Medical research
Slowing neurodegenerative disorders of late life has lagged behind progress on other chronic diseases. But advances in two areas, biochemical pathology and human genetics, have now identified early pathogenic events, enabling molecular hypotheses and disease-modifying treatments. A salient example is the discovery that antibodies to amyloid ß-protein, long debated as a causative factor in Alzheimer’s disease (AD), clear amyloid plaques, decrease levels of abnormal tau proteins and slow cognitive decline. Approval of amyloid antibodies as the first disease-modifying treatments means a gradually rising fraction of the world’s estimated 60 million people with symptomatic disease may decline less or even stabilize. Society is entering an era in which the unchecked devastation of AD is no longer inevitable. This Perspective considers the impact of slowing AD and other neurodegenerative disorders on the trajectory of aging, allowing people to survive into late life with less functional decline. The implications of this moment for medicine and society are profound.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Emerging diagnostics and therapeutics for Alzheimer disease

Alzheimer disease

Is Alzheimer disease a disease?
Kang, J. et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325 , 733–736 (1987).
Article CAS PubMed Google Scholar
Wasco, W. et al. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid β protein precursor. Proc. Natl Acad. Sci. USA 89 , 10758–10762 (1992).
Article CAS PubMed PubMed Central Google Scholar
Wasco, W. et al. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer’s associated amyloid β protein precursor. Nat. Genet. 5 , 95–100 (1993).
Sherrington, R. et al. Cloning of a novel gene bearing missense mutations in early onset familial Alzheimer disease. Nature 375 , 754–760 (1995).
Rogaev, E. I. et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376 , 775–778 (1995).
Vassar, R. et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286 , 735–741 (1999).
Sinha, S. et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402 , 537–540 (1999).
Ghosh, A. K. et al. Design of potent inhibitors for human brain memapsin 2 (beta-secretase). J. Am. Chem. Soc. 122 , 3522–3523 (2000).
Wolfe, M. S. et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398 , 513–517 (1999).
Struhl, G. & Greenwald, I. Presenilin is required for activity and nuclear access of Notch in Drosophila . Nature 398 , 522–525 (1999).
De Strooper, B. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398 , 518–522 (1999).
Article PubMed Google Scholar
Katzman, R. Editorial: the prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 33 , 217–218 (1976).
Martens, Y. A. et al. ApoE cascade hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110 , 1304–1317 (2022).
Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Prim. 7 , 33 (2021).
Chen, X. & Holtzman, D. M. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 55 , 2236–2254 (2022).
Alzheimer, A. Ueber eine eigenartige Erkrankung der Hirnrinde. Centralblatt Nervenheilkd. Psychiatr. 30 , 177–179 (1907).
Google Scholar
Davies, P. & Maloney, A. J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2 , 1403 (1976).
White, P. et al. Neocortical cholinergic neurons in elderly people. Lancet 1 , 668–671 (1977).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120 , 885–890 (1984).
Glenner, G. G. & Wong, C. W. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122 , 1131–1135 (1984).
Masters, C. L. et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl Acad. Sci. USA 82 , 4245–4249 (1985).
Levy, E. et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch-type. Science 248 , 1124–1126 (1990).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349 , 704–706 (1991).
Haass, C. et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359 , 322–325 (1992).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373 , 523–527 (1995).
Brion, J., Passareiro, E., Nunez, J. & Flament-Durand, J. Mise en evidence immunologique de la protein tau au niveau des lesions de degenerescence neurofibrillaire de la maladie d’Alzheimer. Arch. Biol. 95 , 229–235 (1985).
Nukina, N. & Ihara, Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J. Biochem. 99 , 1541–1544 (1986).
Kosik, K. S., Joachim, C. L. & Selkoe, D. J. Microtubule-associated protein, tau, is a major antigenic component of paired helical filaments in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 83 , 4044–4048 (1986).
Grundke-Iqbal, I. et al. Abnormal phosphorylation of the microtubule-associated protein t (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA 83 , 4913–4917 (1986).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited FTDP-17. Nature 393 , 702–705 (1998).
Strittmatter, W. J. et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA 90 , 1977–1981 (1993).
Selkoe, D. J. Preventing Alzheimer’s disease. Science 337 , 1488–1492 (2012).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488 , 96–99 (2012).
Prasher, V. P. et al. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 43 , 380–383 (1998).
Lemere, C. A. et al. Sequence of deposition of heterogeneous amyloid β-peptides and Apo E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 3 , 16–32 (1996).
Mann, D. M. & Iwatsubo, T. Diffuse plaques in the cerebellum and corpus striatum in Down’s syndrome contain amyloid beta protein (Aβ) only in the form of Aβ 42(43) . Neurodegeneration 5 , 115–120 (1996).
Rovelet-Lecrux, A. et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 38 , 24–26 (2006).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 , 921–923 (1993).
Castellano, J. M. et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3 , 89ra57 (2011).
Zhang, Q. et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat. Commun. 11 , 4799 (2020).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53 , 1276–1282 (2021).
Kakuda, N. et al. γ-Secretase activity is associated with braak senile plaque stages. Am. J. Pathol. 190 , 1323–1331 (2020).
Kakuda, N. et al. Altered gamma-secretase activity in mild cognitive impairment and Alzheimer’s disease. EMBO Mol. Med. 4 , 344–352 (2012).
Huang, Z. et al. Proteomics of resilience to Alzheimer’s disease identifies brain regional soluble Aβ levels, actin filament processes, and response to injury. Preprint at bioRxiv https://doi.org/10.1101/2022.10.09.511430 (2022).
Glenner, G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (first of two parts). N. Engl. J. Med. 302 , 1283–1292 (1980).
Selkoe, D. J. The molecular pathology of Alzheimer’s disease. Neuron 6 , 487–498 (1991).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256 , 184–185 (1992).
Karran, E. & De Strooper, B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21 , 306–318 (2022).
Haass, C. & Selkoe, D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 20 , e3001694 (2022).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400 , 173–177 (1999).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6 , 916–919 (2000).
Monsonego, A. et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112 , 415–422 (2003).
Serrano-Pozo, A. et al. Beneficial effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. Brain 133 , 1312–1327 (2010).
Article PubMed PubMed Central Google Scholar
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537 , 50–56 (2016).
Lord, A. et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 36 , 425–434 (2009).
Salloway, S. et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73 , 2061–2070 (2009).
Sperling, R. A. et al. Trial of solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 389 , 1096–1107 (2023).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9 , 197–210 (2022).
CAS PubMed Google Scholar
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388 , 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer Disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330 , 512–527 (2023).
Johnson, K. et al. Biomarker assessments from Clarity AD: downstream implications of targeting protofibrils and tau as a predictive biomarker. In 16th Clinical Trialson Alzheimer’s Disease (ed. Shobha, D.) S9–S10 (CTAD, 2023).
Rafii, M. S. et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 19 , 1227–1233 (2023).
Boxer, A. L. & Sperling, R. Accelerating Alzheimer’s therapeutic development: the past and future of clinical trials. Cell 186 , 4757–4772 (2023).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10 , 362–377 (2023).
CAS PubMed PubMed Central Google Scholar
Rosenberg, A., Mangialasche, F., Ngandu, T., Solomon, A. & Kivipelto, M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to world-wide FINGERS. J. Prev. Alzheimers Dis. 7 , 29–36 (2020).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414 , 212–216 (2001).
Ohki, Y. et al. Phenylpiperidine-type gamma-secretase modulators target the transmembrane domain 1 of presenilin 1. EMBO J. 30 , 4815–4824 (2011).
Crump, C. J., Johnson, D. S. & Li, Y. M. Development and mechanism of γ-secretase modulators for Alzheimer’s disease. Biochemistry 52 , 3197–3216 (2013).
Wagner, S. L. et al. Soluble γ-secretase modulators selectively inhibit the production of the 42-amino acid amyloid beta peptide variant and augment the production of multiple carboxy-truncated amyloid beta species. Biochemistry 53 , 702–713 (2014).
Johnson, D. S., Li, Y.-M., Pettersson, M. & St George-Hyslop, P. H. Structural and chemical biology of presenilin complexes. Cold Spring Harb. Perspect. Med . 7 , a024067 (2017).
Liu, L., Lauro, B. M., Wolfe, M. S. & Selkoe, D. J. Hydrophilic loop 1 of presenilin-1 and the APP GxxxG transmembrane motif regulate gamma-secretase function in generating Alzheimer-causing Abeta peptides. J. Biol. Chem. 296 , 100393 (2021).
McGowan, E. et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47 , 191–199 (2005).
Kim, J. et al. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 27 , 627–633 (2007).
Sturm, S. RG6289, A new γ-secretase modulator for the treatment of alzheimer’s disease: results from a phase I healthy volunteer study. In 16th Clinical Trials on Alzheimer ’ s Disease (ed. Shobha, D.) S23-24 (CTAD, 2023).
Rynearson, K. D. et al. Preclinical validation of a potent γ-secretase modulator for Alzheimer’s disease prevention. J. Exp. Med. 218 , e20202560 (2021).
Edwards, A. L. et al. Exploratory tau biomarker results from a multiple ascending-dose study of BIIB080 in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 80 , 1344–1352 (2023).
Frenkel, D. et al. A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann. Neurol. 63 , 591–601 (2008).
He, W. G. D. & Kowal, P. U.S. Census Bureau, International Population Reports P95/16-1, An Aging World: 2015 (US Government Publishing Office, 2016).
US Census Bureau. 2014 National Population Projections: Downloadable Files (2014).
Rajan, K. B. et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 17 , 1966–1975 (2021).
Yaffe, K. et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Br. Med. J. 347 , f7051 (2013).
Article Google Scholar
Plassman, B. L. et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29 , 125–132 (2007).
Alzheimer’s Association. Alzheimer’s Disease Facts and Figures 2024 (Alzheimer’s Association, 2024).
CDC WONDER Online Database: About Underlying Cause of Death, 1999–2020 (US Centers for Disease Control, accessed 9 December 2022).
US Burden of Disease Collaborators Collaborators et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA 319 , 1444–1472 (2018).
Brunnstrom, H. R. & Englund, E. M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 16 , 488–492 (2009).
Arrighi, H. M., Neumann, P. J., Lieberburg, I. M. & Townsend, R. J. Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis. Assoc. Disord. 24 , 90–95 (2010).
Tejada-Vera, B. Mortality from Alzheimer’s disease in the United States: data for 2000 and 2010. NCHS Data Brief 1–8 (2013).
Schrijvers, E. M. et al. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78 , 1456–1463 (2012).
Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars (Alzheimer’s Association, 2015).
Zissimopoulos, J., Crimmins, E. & St Clair, P. The value of delaying Alzheimer’s disease onset. Forum Health Econ. Policy 18 , 25–39 (2014).
Alzheimer’s Association. 2024 Alzheimer ’ s Disease Facts and Figures (Alzheimer’s Association, 2024).
Hall, K. S. et al. Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement. 5 , 227–233 (2009).
Dufour, A. B., Shaffer, M. L., D’Agata, E. M., Habtemariam, D. & Mitchell, S. L. Survival after suspected urinary tract infection in individuals with advanced dementia. J. Am. Geriatr. Soc. 63 , 2472–2477 (2015).
Nicolle, L. E. Urinary tract infection in long-term-care facility residents. Clin. Infect. Dis. 31 , 757–761 (2000).
Lin, P. J., Fillit, H. M., Cohen, J. T. & Neumann, P. J. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 9 , 30–38 (2013).
Skaria, A. P. The economic and societal burden of Alzheimer disease: managed care considerations. Am. J. Manag. Care 28 , S188–S196 (2022).
Albert, S. M. et al. Hospitalization and Alzheimer’s disease: results from a community-based study. J. Gerontol. A Biol. Sci. Med Sci. 54 , M267–M271 (1999).
Fillit, H., Hill, J. W. & Futterman, R. Health care utilization and costs of Alzheimer’s disease: the role of co-morbid conditions, disease stage, and pharmacotherapy. Fam. Med. 34 , 528–535 (2002).
PubMed Google Scholar
Fanning, S. et al. Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for parkinson treatment. Mol. Cell. 73 , 1001–1014(2019).
Nuber, S. et al. A stearoyl-coenzyme a dDesaturase inhibitor prevents multiple Parkinson disease phenotypes in α-synuclein mice. Ann. Neurol. 89 , 74–90 (2021).
Download references
Author information
Authors and affiliations.
Ann Romney Center for Neurologic Diseases Brigham and Women’s Hospital Harvard Medical School, Boston, MA, USA
Dennis J. Selkoe
You can also search for this author in PubMed Google Scholar
Contributions
D.J.S. is the only contributor to the piece.
Corresponding author
Correspondence to Dennis J. Selkoe .
Ethics declarations
Competing interests.
D.J.S. is a director of and consultant to Prothena Biosciences and an ad hoc consultant to Eisai.
Peer review
Peer review information.
Nature Aging thanks Wiesje van der Flier, Todd Golde, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Selkoe, D.J. The advent of Alzheimer treatments will change the trajectory of human aging. Nat Aging 4 , 453–463 (2024). https://doi.org/10.1038/s43587-024-00611-5
Download citation
Received : 21 July 2023
Accepted : 08 March 2024
Published : 19 April 2024
Issue Date : April 2024
DOI : https://doi.org/10.1038/s43587-024-00611-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Alzheimer's drug adoption in US slowed by doctors' skepticism
- Medium Text

'SIGNIFICANT RISKS, MARGINAL BENEFIT'
'your enemy is nihilism', 'peace and quiet'.
Sign up here.
Reporting by Julie Steenhuysen in Chicago; editing by Caroline Humer and Suzanne Goldenberg
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

Sign of intervention? Japan's yen jumps against dollar
The yen jumped against the dollar on Monday, with traders citing yen-buying intervention by Japanese authorities as a trigger for the bounce in a currency languishing at levels last seen over three decades ago.


IMAGES
COMMENTS
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
Beyond Alzheimer's disease, time-saved models could be applied to other progressive conditions, including Parkinson's disease and amyotrophic lateral sclerosis (ALS).
November 8, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
Abstract. Alzheimer disease (AD) is the most common contributor to dementia in the world, but strategies that slow or prevent its clinical progression have largely remained elusive, until recently ...
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent.Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug development, lifestyle interventions, biomarker research, and more.
Office of the Director (OD). NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address ...
The inexorable progression of Alzheimer's disease exerts a huge toll on patients, families, and society, costing approximately $1 trillion annually, an amount that is likely to increase with the ...
In Alzheimer's disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place.
New findings from big-data and open-science research are revealing clues about the molecular mechanisms of Alzheimer's disease and new ways to discover potential therapeutic targets and biomarkers. These new discoveries were made by six research teams participating in the Accelerating Medicines Partnership Alzheimer's Disease (AMP AD) program.
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. ... Use Current Location Use Map Selector. Search Alzheimer's Association. En Español Research. ... Learn how Alzheimer's disease affects the brain. Take the Brain Tour. Don't just hope for a cure. Help ...
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Alzheimer's Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
Alzheimer's disease causes significant problems with memory, thinking and behavior and is the most common form of dementia, affecting more than 50 million people around the world each year. This ...
What thrilled Sperling, who won the award for her work on clinical trials of Alzheimer's treatments, was a sense of hope, which has been conspicuously missing from research into the disease for ...
Alzheimer's & Dementia: Translational Research & Clinical Interventions journal bridges drug discovery research and clinical studies for dementia & Alzheimer's. Abstract INTRODUCTION New therapies to prevent or delay the onset of symptoms, slow progression, or improve cognitive and behavioral symptoms of Alzheimer's disease (AD) are needed.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning. ... the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking ...
Towards the Integrative Theory of Alzheimer's Disease: Linking Molecular Mechanisms of Neurotoxicity, Beta-amyloid Biomarkers, and the Diagnosis. Read the latest articles of Current Alzheimer Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
The research team — located at Emory University School of Medicine, part of the Accelerating Medicines Partnership® Program for Alzheimer's Disease (AMP®-AD) Consortium — used advanced automated techniques to compare the levels of both proteins and RNA molecules in more than 1,000 brain tissue samples. The samples came from the ...
Biomarker studies highlight current treatment pipeline. Cummings's annual report is featured in Alzheimer's & Dementia: Translational Research & Clinical Interventions.According to this year's ...
Alzheimer's Collection 2024. Research in Alzheimer's disease has undergone a rapid transformation over the last few years. Here we bring together some of the exciting new papers published in Brain on this topic.They span commentaries and reviews on anti-amyloid therapies and their potential side effects, through to molecular, genetic, microbiological, imaging and focused ultrasound studies.
Research and Progress This is a time of unprecedented promise in the quest to end Alzheimer's. Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research - all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer's disease.
More than 50% of people diagnosed with Alzheimer's dementia who were studied at Alzheimer's Disease Research Centers had mixed dementia. 22 ... a reversion rate of 26%. 53 Identifying which individuals with MCI are more likely to develop dementia is a major goal of current research. 2.4.3 Dementia due to Alzheimer's disease. Dementia due to ...
Meta-analysis of genome-wide association studies on Alzheimer's disease and related dementias identifies new loci and enables generation of a new genetic risk score associated with the risk of ...
The federal government's Alzheimer's and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field.
Current research projects Current research projects Our research aims to understand the underlying causes of the condition, improve diagnosis and care, identifying ways to prevent dementia and searching for a cure. ... How the humble fruit fly is helping research into Alzheimer's disease Research. Meet Professor Amrit Mudher, whose research ...
Two common habits could be putting you at an increased risk of Alzheimer's and related dementias, neuroscientists have warned. Alzheimer's disease affects roughly 5.8 million Americans, according ...
The advent of plaque-clearing antibodies to the amyloid-β as the first disease-modifying treatment for Alzheimer's disease will change the course of this disease, the most common type of dementia.
Dr. Nathaniel Chin, a geriatrician with the University of Wisconsin's Alzheimer's Disease Research Center, said he was the target of negative comments on social media after he urged geriatricians ...